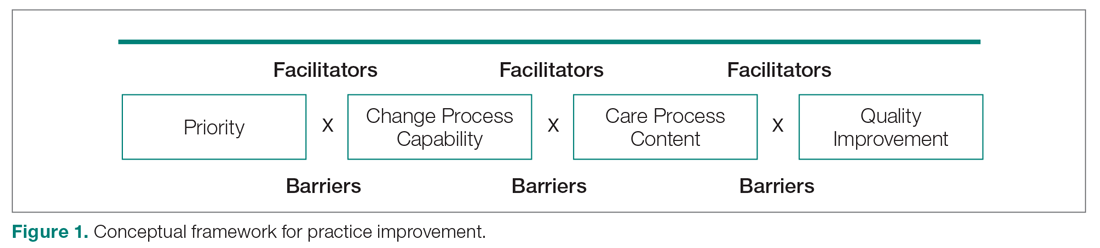
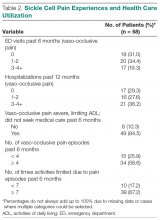
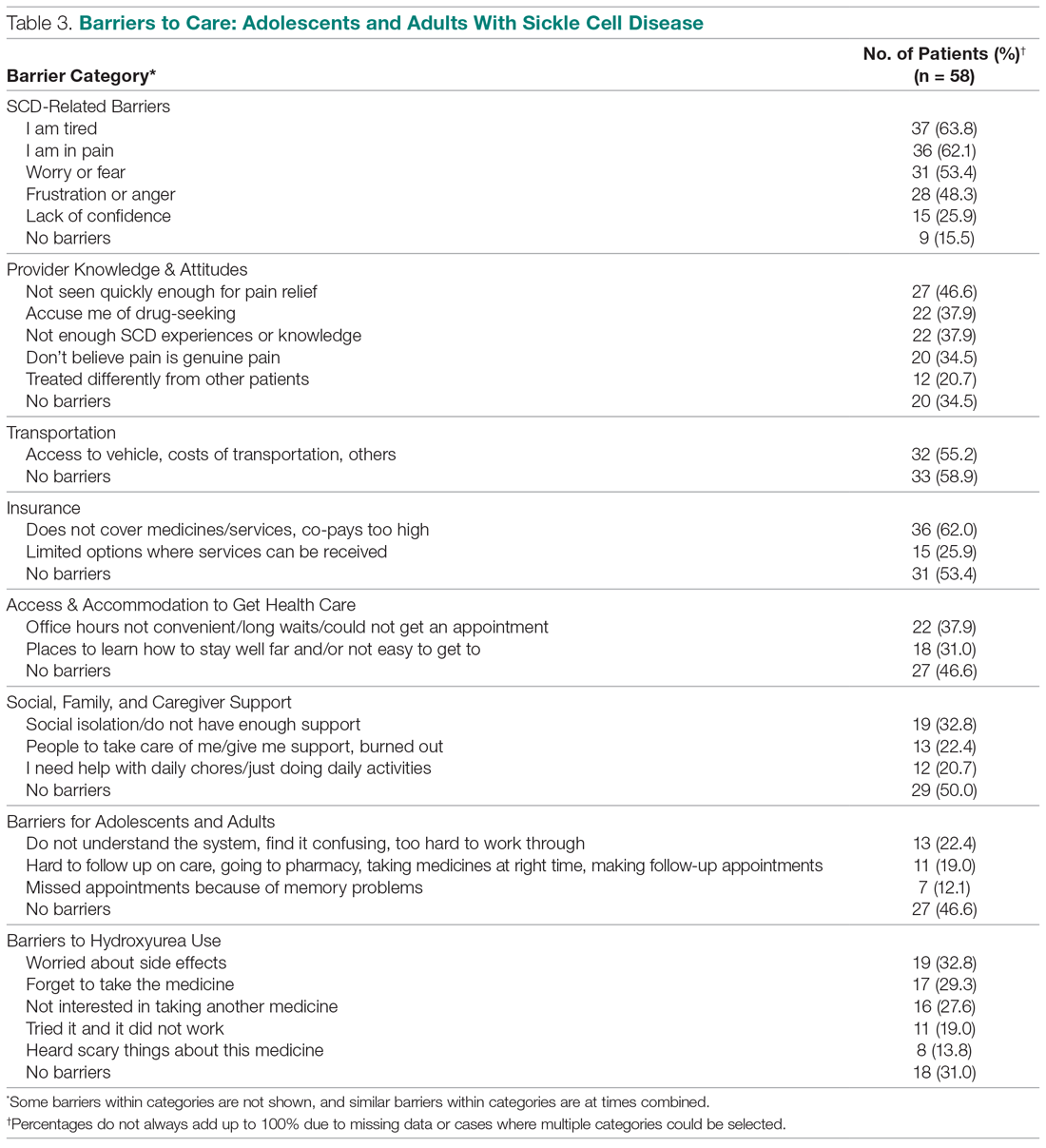
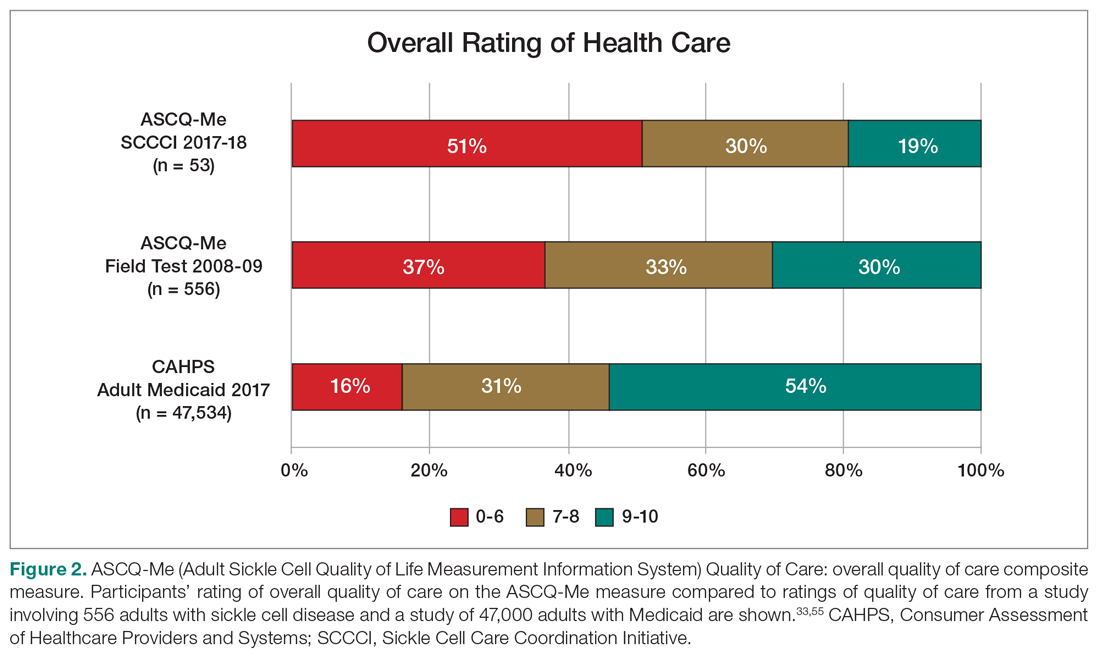
User login
Medical Communities Go Virtual
Throughout history, physicians have formed communities to aid in the dissemination of knowledge, skills, and professional norms. From local physician groups to international societies and conferences, this drive to connect with members of our profession across the globe is timeless. We do so to learn from each other and continue to move the field of medicine forward.
Yet, these communities are being strained by necessary physical distancing required during the COVID-19 pandemic. Many physicians accustomed to a sense of community are now finding themselves surprisingly isolated and alone. Into this distanced landscape, however, new digital groups—specifically social media (SoMe), online learning communities, and virtual conferences—have emerged. We are all active members in virtual communities; all of the authors are team members of The Clinical Problem Solvers podcast and one author of this paper, A.P., has previously served as the medical education lead for the Human Diagnosis Project. Both entities are described later in this article. Here, we provide an overview of these virtual communities and discuss how they have the potential to more equitably and effectively disseminate medical knowledge and education both during and after the COVID-19 pandemic (Table).
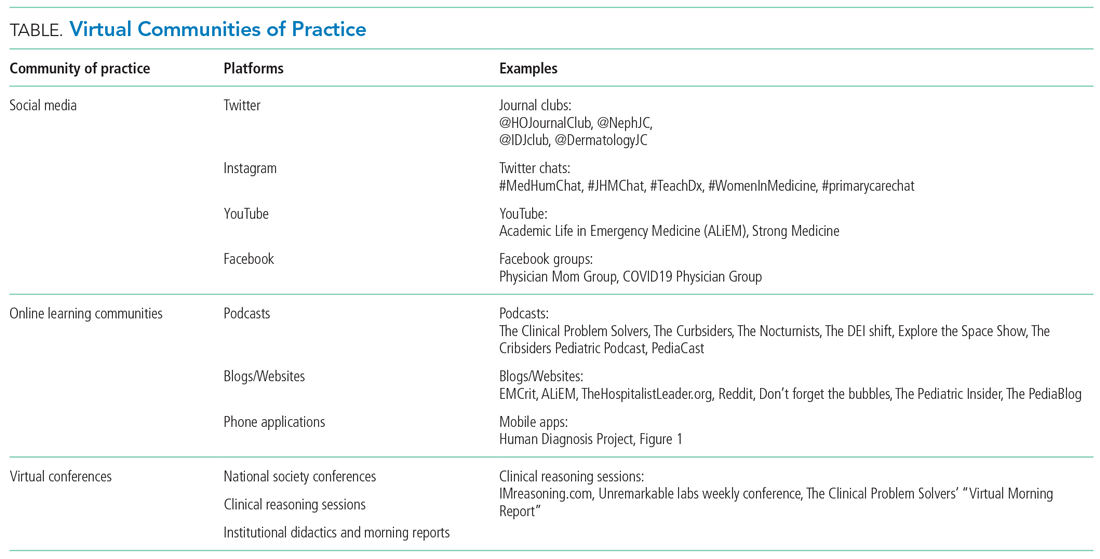
SOCIAL MEDIA
Even prior to the COVID-19 pandemic, SoMe—especially Twitter—had become a virtual gathering place where digital colleagues exchange Twitter handles like business cards.1,2 They celebrate each other’s achievements and provide support during difficult times.
Importantly, the format of Twitter tends toward a flattened hierarchy. It is this egalitarian nature that has served SoMe well in its position as a modern learning community. Users from across the experience spectrum engage with and create novel educational content. This often occurs in the form of Tweetorials, or short lessons conveyed over a series of linked tweets. These have gained immense popularity on the platform and are becoming increasingly recognized forms of scholarship.3 Further, case-based lessons have become ubiquitous and are valuable opportunities for users to learn from other members of their digital communities. During the current pandemic, SoMe has become extremely important in the early dissemination and critique of the slew of research on the COVID-19 crisis.4
Beyond its role as an educational platform, SoMe functions as a virtual gathering place for members of the medical community to discuss topics relevant to the field. Subspecialists and researchers have gathered in digital journal clubs (eg, #NephJC, #IDJClub, #BloodandBone) and a number of journals have hosted live Twitter chats covering topics like controversies in clinical practice or professional development (eg, #JHMChat). More recently, social issues affecting the medical field, such as gender equity and the growing antiracism movement, have led to robust discussion on this medium.
Beyond Twitter, many medical professionals gather and exchange ideas on other platforms. Virtual networking and educational groups have arisen using Slack and Facebook.5-7 Trainees and faculty members alike consume and produce content on YouTube, which often serve to teach technical skills.8 Given widespread use of SoMe, we anticipate that the range of platforms utilized by medical professionals will continue to expand in the future.
ONLINE LEARNING COMMUNITIES
There have long existed multiple print and online forums dedicated to the development of clinical skills. These include clinical challenges in medical journals, interactive online cases, and more formal diagnostic education curricula at academic centers.9-11 With the COVID-19 pandemic, it has become more difficult to ensure that trainees have an in-person learning community to discuss and receive feedback. This has led to a wider adoption of application-based clinical exercises, educational podcasts, and curricular innovations to support these virtual efforts.
The Human Diagnosis Project (Human Dx) is a smart-phone application that provides a platform for individuals to submit clinical cases that can be rapidly peer-reviewed and disseminated to the larger user pool. Human Dx is notable for fostering a strong sense of community amongst its users.12,13 Case consumers and case creators are able to engage in further discussion after solving a case, and opportunities for feedback and growth are ample.
Medical education podcasts have taken on greater importance during the pandemic.14,15 Many educators have begun referring their learners towards certain podcasts as in-person learning communities have been put on hold. Medical professionals may appreciate the up-to-date and candid conversations held on many podcasts, which can provide both educationally useful and emotionally sympathetic connections to their distanced peers. Similarly, while academic clinicians previously benefitted from invited grand rounds speakers, they may now find that such expert discussants are most easily accessible through their appearances on podcasts.
As institutions suspended clerkships during the pandemic, many created virtual communities for trainees to engage in diagnostic reasoning and education. They built novel curricula that meld asynchronous learning with online community-based learning.14 Gamified learning tools and quizzes have also been incorporated into these hybrid curricula to help ensure participation of learners within their virtual communities.16,17
VIRTUAL CONFERENCES
Perhaps the most notable advance in digital communities catalyzed by the COVID-19 pandemic has been the increasing reliance on and comfort with video-based software. While many of our clinical, administrative, and social activities have migrated toward these virtual environments, they have also been used for a variety of activities related to education and professional development.
As institutions struggled to adapt to physical distancing, many medical schools and residency programs have moved their regular meetings and conferences to virtual platforms. Similar free and open-access conferences have also emerged, including the “Virtual Morning Report” (VMR) series from The Clinical Problem Solvers podcast, wherein a few individuals are invited to discuss a case on the video conference, with the remainder of the audience contributing via the chat feature.
Beyond the growing popularity of video conferencing for education, these virtual sessions have become their own community. On The Clinical Problem Solvers VMR, many participants, ranging from preclinical students to seasoned attendings, show up on a daily basis and interact with each other as close friends, as do members of more insular institutional sessions (eg, residency run reports). In these strangely isolating times, many of us have experienced comfort in seeing the faces of our friends and colleagues joining us to listen and discuss cases.
Separately, many professional societies have struggled with how to replace their large yearly in-person conferences, which would pose substantial infectious risks were they to be held in person. While many of those scheduled to occur during the early days of the pandemic were canceled or held limited online sessions, the trend towards virtual conference platforms seems to be accelerating. Organizers of the 2020 Conference on Retroviruses and Opportunistic Infections (March 8-11, 2020) decided to convert from an in-person to entirely virtual conference 48 hours before it started. With the benefit of more forewarning, other conferences are planning and exploring best practices to promote networking and advancement of research goals at future academic meetings.18,19
BENEFITS OF VIRTUAL COMMUNITIES
The growing importance of these new digital communities could be viewed as a necessary evolution in the way that we gather and learn from each other. Traditional physician communities were inherently restricted by location, specialty, and hierarchy, thereby limiting the dissemination of knowledge and changes to professional norms. These restrictions could conceivably insulate and promote elite institutions in a fashion that perpetuates the inequalities within global medical systems. Unrestricted and open-access virtual communities, in contrast, have the potential to remove historical barriers and connect first-class mentors with trainees they would never have met otherwise.
Beyond promoting a more equitable distribution of knowledge and resources, these virtual communities are well suited to harness the benefits of group learning. The concept of communities of practice (CoP) refers to groupings of individuals involved in a personal or professional endeavor, with the community facilitating advancement of their own knowledge and skill set. Members of the CoP learn from each other, with more established members passing down essential knowledge and cultural norms. The three main components of CoP are maintaining a social network, a mutual enterprise (eg, a common goal), and a shared repertoire (eg, experiences, languages, etc).
Designing virtual learning spaces with these aspects in mind may allow these communities to function as CoPs. Some strategies include use of chat functions in videoconferences (to promote further dialogue) and development of dedicated sessions for specific subgroups or aims (eg, professional mentorship). The anticipated benefits of integrating virtual CoPs into medical education are notable, as a number of studies have already suggested that they are effective for disseminating knowledge, enhancing social learning, and aiding with professional development.7,20-23 These virtual CoPs continue to evolve, however, and further research is warranted to clarify how best to utilize them in medical education and professional societies.
CONCLUSION
Amidst the tragic loss of lives and financial calamity, the COVID-19 pandemic has also spurred innovation and change in the way health professionals learn and communicate. Going forward, the medical establishment should capitalize on these recent innovations and work to further build, recognize, and foster such digital gathering spaces in order to more equitably and effectively disseminate knowledge and educational resources.
Despite physical distancing, health professionals have grown closer during these past few months. Innovations spurred by the pandemic have made us stronger and more united. Our experience with social media, online learning communities, and virtual conferences suggests the opportunity to grow and evolve from this experience. As Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in March 2020, “...life is not going to be how it used to be [after the pandemic]…” Let’s hope he’s right.
ACKNOWLEDGMENTS
We thank Reza Manesh, MD, Rabih Geha, MD, and Jack Penner, MD, for their careful review of the manuscript.
1. Markham MJ, Gentile D, Graham DL. Social media for networking, professional development, and patient engagement. Am Soc Clin Oncol Educ Book. 2017;37:782-787. https://doi.org/10.1200/EDBK_180077
2. Melvin L, Chan T. Using Twitter in clinical education and practice. J Grad Med Educ. 2014;6(3):581-582. https://doi.org/10.4300/JGME-D-14-00342.1
3. Breu AC. Why is a cow? Curiosity, Tweetorials, and the return to why. N Engl J Med. 2019;381(12):1097-1098. https://doi.org/10.1056/NEJMp1906790
4. Chan AKM, Nickson CP, Rudolph JW, Lee A, Joynt GM. Social media for rapid knowledge dissemination: early experience from the COVID-19 pandemic. Anaesthesia. 2020:10.1111/anae.15057. https://doi.org/10.1111/anae.15057
5. Pander T, Pinilla S, Dimitriadis K, Fischer MR. The use of Facebook in medical education--a literature review. GMS Z Med Ausbild. 2014;31(3):Doc33. https://doi.org/10.3205/zma000925
6. Cree-Green M, Carreau AM, Davis SM, et al. Peer mentoring for professional and personal growth in academic medicine. J Investig Med. 2020;68(6):1128-1134. https://doi.org/10.1136/jim-2020-001391
7. Yarris LM, Chan TM, Gottlieb M, Juve AM. Finding your people in the digital age: virtual communities of practice to promote education scholarship. J Grad Med Educ. 2019;11(1):1-5. https://doi.org/10.4300/JGME-D-18-01093.1
8. Sterling M, Leung P, Wright D, Bishop TF. The use of social media in graduate medical education: a systematic review. Acad Med. 2017;92(7):1043-1056. https://doi.org/10.1097/ACM.0000000000001617
9. Manesh R, Dhaliwal G. Digital tools to enhance clinical reasoning. Med Clin North Am. 2018;102(3):559-565. https://doi.org/10.1016/j.mcna.2017.12.015
10. Subramanian A, Connor DM, Berger G, et al. A curriculum for diagnostic reasoning: JGIM’s exercises in clinical reasoning. J Gen Intern Med. 2019;34(3):344-345. https://doi.org/10.1007/s11606-018-4689-y
11. Olson APJ, Singhal G, Dhaliwal G. Diagnosis education - an emerging field. Diagnosis (Berl). 2019;6(2):75-77. https://doi.org/10.1515/dx-2019-0029
12. Chatterjee S, Desai S, Manesh R, Sun J, Nundy S, Wright SM. Assessment of a simulated case-based measurement of physician diagnostic performance. JAMA Netw Open. 2019;2(1):e187006. https://doi.org/10.1001/jamanetworkopen.2018.7006
13. Russell SW, Desai SV, O’Rourke P, et al. The genealogy of teaching clinical reasoning and diagnostic skill: the GEL Study. Diagnosis (Berl). 2020;7(3):197-203. https://doi.org/10.1515/dx-2019-0107
14. Geha R, Dhaliwal G. Pilot virtual clerkship curriculum during the COVID-19 pandemic: podcasts, peers, and problem-solving. Med Educ. 2020;54(9):855-856. https://doi.org/10.1111/medu.14246
15. AlGaeed M, Grewal M, Richardson PK, Leon Guerrero CR. COVID-19: Neurology residents’ perspective. J Clin Neurosci. 2020;78:452-453. https://doi.org/10.1016/j.jocn.2020.05.032
16. Moro C, Stromberga Z. Enhancing variety through gamified, interactive learning experiences. Med Educ. 2020. Online ahead of print. https://doi.org/10.1111/medu.14251
17. Morawo A, Sun C, Lowden M. Enhancing engagement during live virtual learning using interactive quizzes. Med Educ. 2020. Online ahead of print. https://doi.org/10.1111/medu.14253
18. Rubinger L, Gazendam A, Ekhtiari S, et al. Maximizing virtual meetings and conferences: a review of best practices. Int Orthop. 2020;44(8):1461-1466. https://doi.org/10.1007/s00264-020-04615-9
19. Woolston C. Learning to love virtual conferences in the coronavirus era. Nature. 2020;582(7810):135-136. https://doi.org/10.1038/d41586-020-01489-0
20. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
21. McLoughlin C, Patel KD, O’Callaghan T, Reeves S. The use of virtual communities of practice to improve interprofessional collaboration and education: findings from an integrated review. J Interprof Care. 2018;32(2):136-142. https://doi.org/10.1080/13561820.2017.1377692
22. Barnett S, Jones SC, Caton T, Iverson D, Bennett S, Robinson L. Implementing a virtual community of practice for family physician training: a mixed-methods case study. J Med Internet Res. 2014;16(3):e83. https://doi.org/10.2196/jmir.3083
23. Healy MG, Traeger LN, Axelsson CGS, et al. NEJM Knowledge+ Question of the Week: a novel virtual learning community effectively utilizing an online discussion forum. Med Teach. 2019;41(11):1270-1276. https://doi.org/10.1080/0142159X.2019.1635685
Throughout history, physicians have formed communities to aid in the dissemination of knowledge, skills, and professional norms. From local physician groups to international societies and conferences, this drive to connect with members of our profession across the globe is timeless. We do so to learn from each other and continue to move the field of medicine forward.
Yet, these communities are being strained by necessary physical distancing required during the COVID-19 pandemic. Many physicians accustomed to a sense of community are now finding themselves surprisingly isolated and alone. Into this distanced landscape, however, new digital groups—specifically social media (SoMe), online learning communities, and virtual conferences—have emerged. We are all active members in virtual communities; all of the authors are team members of The Clinical Problem Solvers podcast and one author of this paper, A.P., has previously served as the medical education lead for the Human Diagnosis Project. Both entities are described later in this article. Here, we provide an overview of these virtual communities and discuss how they have the potential to more equitably and effectively disseminate medical knowledge and education both during and after the COVID-19 pandemic (Table).

SOCIAL MEDIA
Even prior to the COVID-19 pandemic, SoMe—especially Twitter—had become a virtual gathering place where digital colleagues exchange Twitter handles like business cards.1,2 They celebrate each other’s achievements and provide support during difficult times.
Importantly, the format of Twitter tends toward a flattened hierarchy. It is this egalitarian nature that has served SoMe well in its position as a modern learning community. Users from across the experience spectrum engage with and create novel educational content. This often occurs in the form of Tweetorials, or short lessons conveyed over a series of linked tweets. These have gained immense popularity on the platform and are becoming increasingly recognized forms of scholarship.3 Further, case-based lessons have become ubiquitous and are valuable opportunities for users to learn from other members of their digital communities. During the current pandemic, SoMe has become extremely important in the early dissemination and critique of the slew of research on the COVID-19 crisis.4
Beyond its role as an educational platform, SoMe functions as a virtual gathering place for members of the medical community to discuss topics relevant to the field. Subspecialists and researchers have gathered in digital journal clubs (eg, #NephJC, #IDJClub, #BloodandBone) and a number of journals have hosted live Twitter chats covering topics like controversies in clinical practice or professional development (eg, #JHMChat). More recently, social issues affecting the medical field, such as gender equity and the growing antiracism movement, have led to robust discussion on this medium.
Beyond Twitter, many medical professionals gather and exchange ideas on other platforms. Virtual networking and educational groups have arisen using Slack and Facebook.5-7 Trainees and faculty members alike consume and produce content on YouTube, which often serve to teach technical skills.8 Given widespread use of SoMe, we anticipate that the range of platforms utilized by medical professionals will continue to expand in the future.
ONLINE LEARNING COMMUNITIES
There have long existed multiple print and online forums dedicated to the development of clinical skills. These include clinical challenges in medical journals, interactive online cases, and more formal diagnostic education curricula at academic centers.9-11 With the COVID-19 pandemic, it has become more difficult to ensure that trainees have an in-person learning community to discuss and receive feedback. This has led to a wider adoption of application-based clinical exercises, educational podcasts, and curricular innovations to support these virtual efforts.
The Human Diagnosis Project (Human Dx) is a smart-phone application that provides a platform for individuals to submit clinical cases that can be rapidly peer-reviewed and disseminated to the larger user pool. Human Dx is notable for fostering a strong sense of community amongst its users.12,13 Case consumers and case creators are able to engage in further discussion after solving a case, and opportunities for feedback and growth are ample.
Medical education podcasts have taken on greater importance during the pandemic.14,15 Many educators have begun referring their learners towards certain podcasts as in-person learning communities have been put on hold. Medical professionals may appreciate the up-to-date and candid conversations held on many podcasts, which can provide both educationally useful and emotionally sympathetic connections to their distanced peers. Similarly, while academic clinicians previously benefitted from invited grand rounds speakers, they may now find that such expert discussants are most easily accessible through their appearances on podcasts.
As institutions suspended clerkships during the pandemic, many created virtual communities for trainees to engage in diagnostic reasoning and education. They built novel curricula that meld asynchronous learning with online community-based learning.14 Gamified learning tools and quizzes have also been incorporated into these hybrid curricula to help ensure participation of learners within their virtual communities.16,17
VIRTUAL CONFERENCES
Perhaps the most notable advance in digital communities catalyzed by the COVID-19 pandemic has been the increasing reliance on and comfort with video-based software. While many of our clinical, administrative, and social activities have migrated toward these virtual environments, they have also been used for a variety of activities related to education and professional development.
As institutions struggled to adapt to physical distancing, many medical schools and residency programs have moved their regular meetings and conferences to virtual platforms. Similar free and open-access conferences have also emerged, including the “Virtual Morning Report” (VMR) series from The Clinical Problem Solvers podcast, wherein a few individuals are invited to discuss a case on the video conference, with the remainder of the audience contributing via the chat feature.
Beyond the growing popularity of video conferencing for education, these virtual sessions have become their own community. On The Clinical Problem Solvers VMR, many participants, ranging from preclinical students to seasoned attendings, show up on a daily basis and interact with each other as close friends, as do members of more insular institutional sessions (eg, residency run reports). In these strangely isolating times, many of us have experienced comfort in seeing the faces of our friends and colleagues joining us to listen and discuss cases.
Separately, many professional societies have struggled with how to replace their large yearly in-person conferences, which would pose substantial infectious risks were they to be held in person. While many of those scheduled to occur during the early days of the pandemic were canceled or held limited online sessions, the trend towards virtual conference platforms seems to be accelerating. Organizers of the 2020 Conference on Retroviruses and Opportunistic Infections (March 8-11, 2020) decided to convert from an in-person to entirely virtual conference 48 hours before it started. With the benefit of more forewarning, other conferences are planning and exploring best practices to promote networking and advancement of research goals at future academic meetings.18,19
BENEFITS OF VIRTUAL COMMUNITIES
The growing importance of these new digital communities could be viewed as a necessary evolution in the way that we gather and learn from each other. Traditional physician communities were inherently restricted by location, specialty, and hierarchy, thereby limiting the dissemination of knowledge and changes to professional norms. These restrictions could conceivably insulate and promote elite institutions in a fashion that perpetuates the inequalities within global medical systems. Unrestricted and open-access virtual communities, in contrast, have the potential to remove historical barriers and connect first-class mentors with trainees they would never have met otherwise.
Beyond promoting a more equitable distribution of knowledge and resources, these virtual communities are well suited to harness the benefits of group learning. The concept of communities of practice (CoP) refers to groupings of individuals involved in a personal or professional endeavor, with the community facilitating advancement of their own knowledge and skill set. Members of the CoP learn from each other, with more established members passing down essential knowledge and cultural norms. The three main components of CoP are maintaining a social network, a mutual enterprise (eg, a common goal), and a shared repertoire (eg, experiences, languages, etc).
Designing virtual learning spaces with these aspects in mind may allow these communities to function as CoPs. Some strategies include use of chat functions in videoconferences (to promote further dialogue) and development of dedicated sessions for specific subgroups or aims (eg, professional mentorship). The anticipated benefits of integrating virtual CoPs into medical education are notable, as a number of studies have already suggested that they are effective for disseminating knowledge, enhancing social learning, and aiding with professional development.7,20-23 These virtual CoPs continue to evolve, however, and further research is warranted to clarify how best to utilize them in medical education and professional societies.
CONCLUSION
Amidst the tragic loss of lives and financial calamity, the COVID-19 pandemic has also spurred innovation and change in the way health professionals learn and communicate. Going forward, the medical establishment should capitalize on these recent innovations and work to further build, recognize, and foster such digital gathering spaces in order to more equitably and effectively disseminate knowledge and educational resources.
Despite physical distancing, health professionals have grown closer during these past few months. Innovations spurred by the pandemic have made us stronger and more united. Our experience with social media, online learning communities, and virtual conferences suggests the opportunity to grow and evolve from this experience. As Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in March 2020, “...life is not going to be how it used to be [after the pandemic]…” Let’s hope he’s right.
ACKNOWLEDGMENTS
We thank Reza Manesh, MD, Rabih Geha, MD, and Jack Penner, MD, for their careful review of the manuscript.
Throughout history, physicians have formed communities to aid in the dissemination of knowledge, skills, and professional norms. From local physician groups to international societies and conferences, this drive to connect with members of our profession across the globe is timeless. We do so to learn from each other and continue to move the field of medicine forward.
Yet, these communities are being strained by necessary physical distancing required during the COVID-19 pandemic. Many physicians accustomed to a sense of community are now finding themselves surprisingly isolated and alone. Into this distanced landscape, however, new digital groups—specifically social media (SoMe), online learning communities, and virtual conferences—have emerged. We are all active members in virtual communities; all of the authors are team members of The Clinical Problem Solvers podcast and one author of this paper, A.P., has previously served as the medical education lead for the Human Diagnosis Project. Both entities are described later in this article. Here, we provide an overview of these virtual communities and discuss how they have the potential to more equitably and effectively disseminate medical knowledge and education both during and after the COVID-19 pandemic (Table).

SOCIAL MEDIA
Even prior to the COVID-19 pandemic, SoMe—especially Twitter—had become a virtual gathering place where digital colleagues exchange Twitter handles like business cards.1,2 They celebrate each other’s achievements and provide support during difficult times.
Importantly, the format of Twitter tends toward a flattened hierarchy. It is this egalitarian nature that has served SoMe well in its position as a modern learning community. Users from across the experience spectrum engage with and create novel educational content. This often occurs in the form of Tweetorials, or short lessons conveyed over a series of linked tweets. These have gained immense popularity on the platform and are becoming increasingly recognized forms of scholarship.3 Further, case-based lessons have become ubiquitous and are valuable opportunities for users to learn from other members of their digital communities. During the current pandemic, SoMe has become extremely important in the early dissemination and critique of the slew of research on the COVID-19 crisis.4
Beyond its role as an educational platform, SoMe functions as a virtual gathering place for members of the medical community to discuss topics relevant to the field. Subspecialists and researchers have gathered in digital journal clubs (eg, #NephJC, #IDJClub, #BloodandBone) and a number of journals have hosted live Twitter chats covering topics like controversies in clinical practice or professional development (eg, #JHMChat). More recently, social issues affecting the medical field, such as gender equity and the growing antiracism movement, have led to robust discussion on this medium.
Beyond Twitter, many medical professionals gather and exchange ideas on other platforms. Virtual networking and educational groups have arisen using Slack and Facebook.5-7 Trainees and faculty members alike consume and produce content on YouTube, which often serve to teach technical skills.8 Given widespread use of SoMe, we anticipate that the range of platforms utilized by medical professionals will continue to expand in the future.
ONLINE LEARNING COMMUNITIES
There have long existed multiple print and online forums dedicated to the development of clinical skills. These include clinical challenges in medical journals, interactive online cases, and more formal diagnostic education curricula at academic centers.9-11 With the COVID-19 pandemic, it has become more difficult to ensure that trainees have an in-person learning community to discuss and receive feedback. This has led to a wider adoption of application-based clinical exercises, educational podcasts, and curricular innovations to support these virtual efforts.
The Human Diagnosis Project (Human Dx) is a smart-phone application that provides a platform for individuals to submit clinical cases that can be rapidly peer-reviewed and disseminated to the larger user pool. Human Dx is notable for fostering a strong sense of community amongst its users.12,13 Case consumers and case creators are able to engage in further discussion after solving a case, and opportunities for feedback and growth are ample.
Medical education podcasts have taken on greater importance during the pandemic.14,15 Many educators have begun referring their learners towards certain podcasts as in-person learning communities have been put on hold. Medical professionals may appreciate the up-to-date and candid conversations held on many podcasts, which can provide both educationally useful and emotionally sympathetic connections to their distanced peers. Similarly, while academic clinicians previously benefitted from invited grand rounds speakers, they may now find that such expert discussants are most easily accessible through their appearances on podcasts.
As institutions suspended clerkships during the pandemic, many created virtual communities for trainees to engage in diagnostic reasoning and education. They built novel curricula that meld asynchronous learning with online community-based learning.14 Gamified learning tools and quizzes have also been incorporated into these hybrid curricula to help ensure participation of learners within their virtual communities.16,17
VIRTUAL CONFERENCES
Perhaps the most notable advance in digital communities catalyzed by the COVID-19 pandemic has been the increasing reliance on and comfort with video-based software. While many of our clinical, administrative, and social activities have migrated toward these virtual environments, they have also been used for a variety of activities related to education and professional development.
As institutions struggled to adapt to physical distancing, many medical schools and residency programs have moved their regular meetings and conferences to virtual platforms. Similar free and open-access conferences have also emerged, including the “Virtual Morning Report” (VMR) series from The Clinical Problem Solvers podcast, wherein a few individuals are invited to discuss a case on the video conference, with the remainder of the audience contributing via the chat feature.
Beyond the growing popularity of video conferencing for education, these virtual sessions have become their own community. On The Clinical Problem Solvers VMR, many participants, ranging from preclinical students to seasoned attendings, show up on a daily basis and interact with each other as close friends, as do members of more insular institutional sessions (eg, residency run reports). In these strangely isolating times, many of us have experienced comfort in seeing the faces of our friends and colleagues joining us to listen and discuss cases.
Separately, many professional societies have struggled with how to replace their large yearly in-person conferences, which would pose substantial infectious risks were they to be held in person. While many of those scheduled to occur during the early days of the pandemic were canceled or held limited online sessions, the trend towards virtual conference platforms seems to be accelerating. Organizers of the 2020 Conference on Retroviruses and Opportunistic Infections (March 8-11, 2020) decided to convert from an in-person to entirely virtual conference 48 hours before it started. With the benefit of more forewarning, other conferences are planning and exploring best practices to promote networking and advancement of research goals at future academic meetings.18,19
BENEFITS OF VIRTUAL COMMUNITIES
The growing importance of these new digital communities could be viewed as a necessary evolution in the way that we gather and learn from each other. Traditional physician communities were inherently restricted by location, specialty, and hierarchy, thereby limiting the dissemination of knowledge and changes to professional norms. These restrictions could conceivably insulate and promote elite institutions in a fashion that perpetuates the inequalities within global medical systems. Unrestricted and open-access virtual communities, in contrast, have the potential to remove historical barriers and connect first-class mentors with trainees they would never have met otherwise.
Beyond promoting a more equitable distribution of knowledge and resources, these virtual communities are well suited to harness the benefits of group learning. The concept of communities of practice (CoP) refers to groupings of individuals involved in a personal or professional endeavor, with the community facilitating advancement of their own knowledge and skill set. Members of the CoP learn from each other, with more established members passing down essential knowledge and cultural norms. The three main components of CoP are maintaining a social network, a mutual enterprise (eg, a common goal), and a shared repertoire (eg, experiences, languages, etc).
Designing virtual learning spaces with these aspects in mind may allow these communities to function as CoPs. Some strategies include use of chat functions in videoconferences (to promote further dialogue) and development of dedicated sessions for specific subgroups or aims (eg, professional mentorship). The anticipated benefits of integrating virtual CoPs into medical education are notable, as a number of studies have already suggested that they are effective for disseminating knowledge, enhancing social learning, and aiding with professional development.7,20-23 These virtual CoPs continue to evolve, however, and further research is warranted to clarify how best to utilize them in medical education and professional societies.
CONCLUSION
Amidst the tragic loss of lives and financial calamity, the COVID-19 pandemic has also spurred innovation and change in the way health professionals learn and communicate. Going forward, the medical establishment should capitalize on these recent innovations and work to further build, recognize, and foster such digital gathering spaces in order to more equitably and effectively disseminate knowledge and educational resources.
Despite physical distancing, health professionals have grown closer during these past few months. Innovations spurred by the pandemic have made us stronger and more united. Our experience with social media, online learning communities, and virtual conferences suggests the opportunity to grow and evolve from this experience. As Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in March 2020, “...life is not going to be how it used to be [after the pandemic]…” Let’s hope he’s right.
ACKNOWLEDGMENTS
We thank Reza Manesh, MD, Rabih Geha, MD, and Jack Penner, MD, for their careful review of the manuscript.
1. Markham MJ, Gentile D, Graham DL. Social media for networking, professional development, and patient engagement. Am Soc Clin Oncol Educ Book. 2017;37:782-787. https://doi.org/10.1200/EDBK_180077
2. Melvin L, Chan T. Using Twitter in clinical education and practice. J Grad Med Educ. 2014;6(3):581-582. https://doi.org/10.4300/JGME-D-14-00342.1
3. Breu AC. Why is a cow? Curiosity, Tweetorials, and the return to why. N Engl J Med. 2019;381(12):1097-1098. https://doi.org/10.1056/NEJMp1906790
4. Chan AKM, Nickson CP, Rudolph JW, Lee A, Joynt GM. Social media for rapid knowledge dissemination: early experience from the COVID-19 pandemic. Anaesthesia. 2020:10.1111/anae.15057. https://doi.org/10.1111/anae.15057
5. Pander T, Pinilla S, Dimitriadis K, Fischer MR. The use of Facebook in medical education--a literature review. GMS Z Med Ausbild. 2014;31(3):Doc33. https://doi.org/10.3205/zma000925
6. Cree-Green M, Carreau AM, Davis SM, et al. Peer mentoring for professional and personal growth in academic medicine. J Investig Med. 2020;68(6):1128-1134. https://doi.org/10.1136/jim-2020-001391
7. Yarris LM, Chan TM, Gottlieb M, Juve AM. Finding your people in the digital age: virtual communities of practice to promote education scholarship. J Grad Med Educ. 2019;11(1):1-5. https://doi.org/10.4300/JGME-D-18-01093.1
8. Sterling M, Leung P, Wright D, Bishop TF. The use of social media in graduate medical education: a systematic review. Acad Med. 2017;92(7):1043-1056. https://doi.org/10.1097/ACM.0000000000001617
9. Manesh R, Dhaliwal G. Digital tools to enhance clinical reasoning. Med Clin North Am. 2018;102(3):559-565. https://doi.org/10.1016/j.mcna.2017.12.015
10. Subramanian A, Connor DM, Berger G, et al. A curriculum for diagnostic reasoning: JGIM’s exercises in clinical reasoning. J Gen Intern Med. 2019;34(3):344-345. https://doi.org/10.1007/s11606-018-4689-y
11. Olson APJ, Singhal G, Dhaliwal G. Diagnosis education - an emerging field. Diagnosis (Berl). 2019;6(2):75-77. https://doi.org/10.1515/dx-2019-0029
12. Chatterjee S, Desai S, Manesh R, Sun J, Nundy S, Wright SM. Assessment of a simulated case-based measurement of physician diagnostic performance. JAMA Netw Open. 2019;2(1):e187006. https://doi.org/10.1001/jamanetworkopen.2018.7006
13. Russell SW, Desai SV, O’Rourke P, et al. The genealogy of teaching clinical reasoning and diagnostic skill: the GEL Study. Diagnosis (Berl). 2020;7(3):197-203. https://doi.org/10.1515/dx-2019-0107
14. Geha R, Dhaliwal G. Pilot virtual clerkship curriculum during the COVID-19 pandemic: podcasts, peers, and problem-solving. Med Educ. 2020;54(9):855-856. https://doi.org/10.1111/medu.14246
15. AlGaeed M, Grewal M, Richardson PK, Leon Guerrero CR. COVID-19: Neurology residents’ perspective. J Clin Neurosci. 2020;78:452-453. https://doi.org/10.1016/j.jocn.2020.05.032
16. Moro C, Stromberga Z. Enhancing variety through gamified, interactive learning experiences. Med Educ. 2020. Online ahead of print. https://doi.org/10.1111/medu.14251
17. Morawo A, Sun C, Lowden M. Enhancing engagement during live virtual learning using interactive quizzes. Med Educ. 2020. Online ahead of print. https://doi.org/10.1111/medu.14253
18. Rubinger L, Gazendam A, Ekhtiari S, et al. Maximizing virtual meetings and conferences: a review of best practices. Int Orthop. 2020;44(8):1461-1466. https://doi.org/10.1007/s00264-020-04615-9
19. Woolston C. Learning to love virtual conferences in the coronavirus era. Nature. 2020;582(7810):135-136. https://doi.org/10.1038/d41586-020-01489-0
20. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
21. McLoughlin C, Patel KD, O’Callaghan T, Reeves S. The use of virtual communities of practice to improve interprofessional collaboration and education: findings from an integrated review. J Interprof Care. 2018;32(2):136-142. https://doi.org/10.1080/13561820.2017.1377692
22. Barnett S, Jones SC, Caton T, Iverson D, Bennett S, Robinson L. Implementing a virtual community of practice for family physician training: a mixed-methods case study. J Med Internet Res. 2014;16(3):e83. https://doi.org/10.2196/jmir.3083
23. Healy MG, Traeger LN, Axelsson CGS, et al. NEJM Knowledge+ Question of the Week: a novel virtual learning community effectively utilizing an online discussion forum. Med Teach. 2019;41(11):1270-1276. https://doi.org/10.1080/0142159X.2019.1635685
1. Markham MJ, Gentile D, Graham DL. Social media for networking, professional development, and patient engagement. Am Soc Clin Oncol Educ Book. 2017;37:782-787. https://doi.org/10.1200/EDBK_180077
2. Melvin L, Chan T. Using Twitter in clinical education and practice. J Grad Med Educ. 2014;6(3):581-582. https://doi.org/10.4300/JGME-D-14-00342.1
3. Breu AC. Why is a cow? Curiosity, Tweetorials, and the return to why. N Engl J Med. 2019;381(12):1097-1098. https://doi.org/10.1056/NEJMp1906790
4. Chan AKM, Nickson CP, Rudolph JW, Lee A, Joynt GM. Social media for rapid knowledge dissemination: early experience from the COVID-19 pandemic. Anaesthesia. 2020:10.1111/anae.15057. https://doi.org/10.1111/anae.15057
5. Pander T, Pinilla S, Dimitriadis K, Fischer MR. The use of Facebook in medical education--a literature review. GMS Z Med Ausbild. 2014;31(3):Doc33. https://doi.org/10.3205/zma000925
6. Cree-Green M, Carreau AM, Davis SM, et al. Peer mentoring for professional and personal growth in academic medicine. J Investig Med. 2020;68(6):1128-1134. https://doi.org/10.1136/jim-2020-001391
7. Yarris LM, Chan TM, Gottlieb M, Juve AM. Finding your people in the digital age: virtual communities of practice to promote education scholarship. J Grad Med Educ. 2019;11(1):1-5. https://doi.org/10.4300/JGME-D-18-01093.1
8. Sterling M, Leung P, Wright D, Bishop TF. The use of social media in graduate medical education: a systematic review. Acad Med. 2017;92(7):1043-1056. https://doi.org/10.1097/ACM.0000000000001617
9. Manesh R, Dhaliwal G. Digital tools to enhance clinical reasoning. Med Clin North Am. 2018;102(3):559-565. https://doi.org/10.1016/j.mcna.2017.12.015
10. Subramanian A, Connor DM, Berger G, et al. A curriculum for diagnostic reasoning: JGIM’s exercises in clinical reasoning. J Gen Intern Med. 2019;34(3):344-345. https://doi.org/10.1007/s11606-018-4689-y
11. Olson APJ, Singhal G, Dhaliwal G. Diagnosis education - an emerging field. Diagnosis (Berl). 2019;6(2):75-77. https://doi.org/10.1515/dx-2019-0029
12. Chatterjee S, Desai S, Manesh R, Sun J, Nundy S, Wright SM. Assessment of a simulated case-based measurement of physician diagnostic performance. JAMA Netw Open. 2019;2(1):e187006. https://doi.org/10.1001/jamanetworkopen.2018.7006
13. Russell SW, Desai SV, O’Rourke P, et al. The genealogy of teaching clinical reasoning and diagnostic skill: the GEL Study. Diagnosis (Berl). 2020;7(3):197-203. https://doi.org/10.1515/dx-2019-0107
14. Geha R, Dhaliwal G. Pilot virtual clerkship curriculum during the COVID-19 pandemic: podcasts, peers, and problem-solving. Med Educ. 2020;54(9):855-856. https://doi.org/10.1111/medu.14246
15. AlGaeed M, Grewal M, Richardson PK, Leon Guerrero CR. COVID-19: Neurology residents’ perspective. J Clin Neurosci. 2020;78:452-453. https://doi.org/10.1016/j.jocn.2020.05.032
16. Moro C, Stromberga Z. Enhancing variety through gamified, interactive learning experiences. Med Educ. 2020. Online ahead of print. https://doi.org/10.1111/medu.14251
17. Morawo A, Sun C, Lowden M. Enhancing engagement during live virtual learning using interactive quizzes. Med Educ. 2020. Online ahead of print. https://doi.org/10.1111/medu.14253
18. Rubinger L, Gazendam A, Ekhtiari S, et al. Maximizing virtual meetings and conferences: a review of best practices. Int Orthop. 2020;44(8):1461-1466. https://doi.org/10.1007/s00264-020-04615-9
19. Woolston C. Learning to love virtual conferences in the coronavirus era. Nature. 2020;582(7810):135-136. https://doi.org/10.1038/d41586-020-01489-0
20. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
21. McLoughlin C, Patel KD, O’Callaghan T, Reeves S. The use of virtual communities of practice to improve interprofessional collaboration and education: findings from an integrated review. J Interprof Care. 2018;32(2):136-142. https://doi.org/10.1080/13561820.2017.1377692
22. Barnett S, Jones SC, Caton T, Iverson D, Bennett S, Robinson L. Implementing a virtual community of practice for family physician training: a mixed-methods case study. J Med Internet Res. 2014;16(3):e83. https://doi.org/10.2196/jmir.3083
23. Healy MG, Traeger LN, Axelsson CGS, et al. NEJM Knowledge+ Question of the Week: a novel virtual learning community effectively utilizing an online discussion forum. Med Teach. 2019;41(11):1270-1276. https://doi.org/10.1080/0142159X.2019.1635685
© 2020 Society of Hospital Medicine
ERRATUM TO: Myocardial Injury Among Postoperative Patients: Where Is the Wisdom in Our Knowledge?
The author would like to make the following correction to the Editorial, originally published in the July issue of the Journal of Hospital Medicine 2020;15(7):447-448. DOI 10.12788/jhm.3468. In the third paragraph, MINS was described as an “umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI).” This is not fully accurate: MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. The correction to the paragraph is as follows, indicated in bold type:
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
The author would like to make the following correction to the Editorial, originally published in the July issue of the Journal of Hospital Medicine 2020;15(7):447-448. DOI 10.12788/jhm.3468. In the third paragraph, MINS was described as an “umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI).” This is not fully accurate: MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. The correction to the paragraph is as follows, indicated in bold type:
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
The author would like to make the following correction to the Editorial, originally published in the July issue of the Journal of Hospital Medicine 2020;15(7):447-448. DOI 10.12788/jhm.3468. In the third paragraph, MINS was described as an “umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI).” This is not fully accurate: MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. The correction to the paragraph is as follows, indicated in bold type:
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
© 2020 Society of Hospital Medicine
Assessing Individual Hospitalist Performance: Domains and Attribution
When asked by friend or family “Which hospital did you go to?” or “Which doctor did you see?” most are likely to answer with a single institution or clinician. Yet for hospital stays the patient’s experience and outcomes are a product of many individuals and an entire system of care, so measuring performance at the group, or “team,” level is appropriate.
Assessing and managing performance of individuals in healthcare is also important. In this regard, though, healthcare may be more like assessing individual baseball players prior to the widespread adoption of detailed statistics, a transition to what is often referred to as sabermetrics (and popularized by the 2004 book Moneyball).1 An individual player’s performance and future potential went from being assessed largely by the opinion of expert talent scouts to including, or even principally relying on, a wide array of measurements and statistics.
It sometimes seems healthcare has arrived at its “sabermetrics moment.” There is a rapidly growing set of measures for individual clinicians, and nearly every week, hospitalists will open a new report of their performance sent by a payer, a government agency, their own hospitals, or other organizations. But most of these metrics suffer from problems with attributing performance to a single clinician; for example, many or most metrics attribute performance to the attending at the time of a patient’s discharge according to the clinical record. Yet while clinical metrics (eg, administer beta-blocker when indicated, length of stay (LOS), readmissions), patient experience, financial metrics (eg, cost per case), and others are vital to understanding performance at an aggregate level such as a hospital or physician group, they are potentially confusing or even misleading when attributed entirely to the discharging provider. So healthcare leaders still tend to rely meaningfully on expert opinion—“talent scouts”—to identify high performers.
In this issue of the Journal of Hospital Medicine, Dow and colleagues have advanced our understanding of the current state of individual- rather than group-level hospitalist performance measurement.2 This scoping review identified 43 studies published over the last 25 years reporting individual adult or pediatric hospitalist performance across one or more of the STEEEP framework domains of performance: Safe, Timely, Effective, Efficient, Equitable, Patient Centered.3
The most common domain assessed in the studies was Patient Centered (20 studies), and in descending order from there were Safe (16), Efficient (13), Timely (10), Effective (9). No studies reported individual hospitalist performance on Equitable care. This distribution of studied domains is likely a function of readily available data and processes for study more than level of interest or importance attached to each domain. Their research was not designed to assess the quality of each study, and some—or even many—might have weaknesses in both determining which clinicians met the definition of hospitalist and how performance was attributed to individuals. The authors appropriately conclude that “further defining and refining approaches to assess individual performance is necessary to ensure the highest quality.”
Their findings should help guide research priorities regarding measurement of individual hospitalist performance. Yet each hospitalist group and individual hospitalist still faces decisions about managing their own group and personal performance and must navigate without the benefit of research providing clear direction. Many hospitalist metrics are tracked and reported to meet regulatory requirements such as those from Centers for Medicare & Medicaid Services, financial metrics for the local hospital and hospitalist group, and for use as components of hospitalist compensation. (The biennial State of Hospital Medicine Report captures extensive data regarding the latter.4)
Many people and processes across an entire healthcare system influence performance on every metric, but it is useful and practical to attribute some metrics entirely to a single hospitalist provider, such as timely documentation and the time of day the discharge order is entered. And arguably, it is useful to attribute readmission rate entirely to the discharging provider—the last hospital provider who can influence readmission risk. But for most other metrics individual attribution is problematic or misleading and collective experience and expert opinion are helpful here. Two examples come to mind of relatively simple approaches that have gained some popularity in teasing out individual contribution to hospitalist performance.
One can estimate individual hospitalist contribution to patient LOS by calculating the ratio of current procedural terminology (CPT) codes for all follow-up services to all discharge codes. For each hospitalist in the group who cares for a similar population, those with the highest ratios likely manage patients in ways associated with longer LOS. It is relatively simple to use billing data to calculate the ratio, and some groups report it for all providers monthly.
Many metrics that aggregate performance across an entire hospital stay, such as patient experience surveys, can be apportioned to each hospitalist who had a billed encounter with the patient. For example, if a hospitalist has 4 of a patient’s 10 billed encounters within the same group, then 40% of the patient’s survey score could be attributed to that hospitalist. It’s still imperfect, but it’s likely more meaningful than attributing the entire survey result to only the discharging provider.
These approaches have value but still leave us unsatisfied and unable to assess performance as effectively as we would like. Advancements in measurement have been slow and incremental, but they are likely to accelerate with maturation of electronic health records paired with machine learning or artificial intelligence, wearable devices, and sensors in patient rooms, which collectively may make capturing a robust set of metrics trivially easy (and raise questions regarding privacy and so forth). For example, it is already possible to capture via a smart speaker all conversations between patient, loved ones, and clinician.5 Imagine you are presented with a word cloud summary of all conversations you had with all patients over a year. Did you use empathy words often enough? How reliably did you address all appropriate discharge-related topics?
As performance metrics become more numerous and ubiquitous, the challenge will be to ensure they accurately capture what they appear to measure, are appropriately attributed to individuals or groups, and provide insights into important domains of performance. Significant opportunity for improvement remains.
Disclosure
Dr Nelson has no conflict of interest to disclose.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. W.W. Norton & Company; 2004.
2. Dow AW, Chopski B, Cyrus JW, et al. A STEEEP hill to climb: a scoping review of assessments of individual hospitalist performance. J Hosp Med. 2020;15:599-605. https://doi.org/10.12788/jhm.3445
3. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press (US); 2001. https://doi.org/10.17226/10027
4. 2018 State of Hospital Medicine Report. Society of Hospital Medicine. Accessed May 19, 2020. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
5. Chiu CC, Tripathi A, Chou K, et al. Speech recognition for medical conversations. arXiv. Preprint posted online November 20, 2017. Revised June 20, 2018. https://arxiv.org/pdf/1711.07274.pdf
When asked by friend or family “Which hospital did you go to?” or “Which doctor did you see?” most are likely to answer with a single institution or clinician. Yet for hospital stays the patient’s experience and outcomes are a product of many individuals and an entire system of care, so measuring performance at the group, or “team,” level is appropriate.
Assessing and managing performance of individuals in healthcare is also important. In this regard, though, healthcare may be more like assessing individual baseball players prior to the widespread adoption of detailed statistics, a transition to what is often referred to as sabermetrics (and popularized by the 2004 book Moneyball).1 An individual player’s performance and future potential went from being assessed largely by the opinion of expert talent scouts to including, or even principally relying on, a wide array of measurements and statistics.
It sometimes seems healthcare has arrived at its “sabermetrics moment.” There is a rapidly growing set of measures for individual clinicians, and nearly every week, hospitalists will open a new report of their performance sent by a payer, a government agency, their own hospitals, or other organizations. But most of these metrics suffer from problems with attributing performance to a single clinician; for example, many or most metrics attribute performance to the attending at the time of a patient’s discharge according to the clinical record. Yet while clinical metrics (eg, administer beta-blocker when indicated, length of stay (LOS), readmissions), patient experience, financial metrics (eg, cost per case), and others are vital to understanding performance at an aggregate level such as a hospital or physician group, they are potentially confusing or even misleading when attributed entirely to the discharging provider. So healthcare leaders still tend to rely meaningfully on expert opinion—“talent scouts”—to identify high performers.
In this issue of the Journal of Hospital Medicine, Dow and colleagues have advanced our understanding of the current state of individual- rather than group-level hospitalist performance measurement.2 This scoping review identified 43 studies published over the last 25 years reporting individual adult or pediatric hospitalist performance across one or more of the STEEEP framework domains of performance: Safe, Timely, Effective, Efficient, Equitable, Patient Centered.3
The most common domain assessed in the studies was Patient Centered (20 studies), and in descending order from there were Safe (16), Efficient (13), Timely (10), Effective (9). No studies reported individual hospitalist performance on Equitable care. This distribution of studied domains is likely a function of readily available data and processes for study more than level of interest or importance attached to each domain. Their research was not designed to assess the quality of each study, and some—or even many—might have weaknesses in both determining which clinicians met the definition of hospitalist and how performance was attributed to individuals. The authors appropriately conclude that “further defining and refining approaches to assess individual performance is necessary to ensure the highest quality.”
Their findings should help guide research priorities regarding measurement of individual hospitalist performance. Yet each hospitalist group and individual hospitalist still faces decisions about managing their own group and personal performance and must navigate without the benefit of research providing clear direction. Many hospitalist metrics are tracked and reported to meet regulatory requirements such as those from Centers for Medicare & Medicaid Services, financial metrics for the local hospital and hospitalist group, and for use as components of hospitalist compensation. (The biennial State of Hospital Medicine Report captures extensive data regarding the latter.4)
Many people and processes across an entire healthcare system influence performance on every metric, but it is useful and practical to attribute some metrics entirely to a single hospitalist provider, such as timely documentation and the time of day the discharge order is entered. And arguably, it is useful to attribute readmission rate entirely to the discharging provider—the last hospital provider who can influence readmission risk. But for most other metrics individual attribution is problematic or misleading and collective experience and expert opinion are helpful here. Two examples come to mind of relatively simple approaches that have gained some popularity in teasing out individual contribution to hospitalist performance.
One can estimate individual hospitalist contribution to patient LOS by calculating the ratio of current procedural terminology (CPT) codes for all follow-up services to all discharge codes. For each hospitalist in the group who cares for a similar population, those with the highest ratios likely manage patients in ways associated with longer LOS. It is relatively simple to use billing data to calculate the ratio, and some groups report it for all providers monthly.
Many metrics that aggregate performance across an entire hospital stay, such as patient experience surveys, can be apportioned to each hospitalist who had a billed encounter with the patient. For example, if a hospitalist has 4 of a patient’s 10 billed encounters within the same group, then 40% of the patient’s survey score could be attributed to that hospitalist. It’s still imperfect, but it’s likely more meaningful than attributing the entire survey result to only the discharging provider.
These approaches have value but still leave us unsatisfied and unable to assess performance as effectively as we would like. Advancements in measurement have been slow and incremental, but they are likely to accelerate with maturation of electronic health records paired with machine learning or artificial intelligence, wearable devices, and sensors in patient rooms, which collectively may make capturing a robust set of metrics trivially easy (and raise questions regarding privacy and so forth). For example, it is already possible to capture via a smart speaker all conversations between patient, loved ones, and clinician.5 Imagine you are presented with a word cloud summary of all conversations you had with all patients over a year. Did you use empathy words often enough? How reliably did you address all appropriate discharge-related topics?
As performance metrics become more numerous and ubiquitous, the challenge will be to ensure they accurately capture what they appear to measure, are appropriately attributed to individuals or groups, and provide insights into important domains of performance. Significant opportunity for improvement remains.
Disclosure
Dr Nelson has no conflict of interest to disclose.
When asked by friend or family “Which hospital did you go to?” or “Which doctor did you see?” most are likely to answer with a single institution or clinician. Yet for hospital stays the patient’s experience and outcomes are a product of many individuals and an entire system of care, so measuring performance at the group, or “team,” level is appropriate.
Assessing and managing performance of individuals in healthcare is also important. In this regard, though, healthcare may be more like assessing individual baseball players prior to the widespread adoption of detailed statistics, a transition to what is often referred to as sabermetrics (and popularized by the 2004 book Moneyball).1 An individual player’s performance and future potential went from being assessed largely by the opinion of expert talent scouts to including, or even principally relying on, a wide array of measurements and statistics.
It sometimes seems healthcare has arrived at its “sabermetrics moment.” There is a rapidly growing set of measures for individual clinicians, and nearly every week, hospitalists will open a new report of their performance sent by a payer, a government agency, their own hospitals, or other organizations. But most of these metrics suffer from problems with attributing performance to a single clinician; for example, many or most metrics attribute performance to the attending at the time of a patient’s discharge according to the clinical record. Yet while clinical metrics (eg, administer beta-blocker when indicated, length of stay (LOS), readmissions), patient experience, financial metrics (eg, cost per case), and others are vital to understanding performance at an aggregate level such as a hospital or physician group, they are potentially confusing or even misleading when attributed entirely to the discharging provider. So healthcare leaders still tend to rely meaningfully on expert opinion—“talent scouts”—to identify high performers.
In this issue of the Journal of Hospital Medicine, Dow and colleagues have advanced our understanding of the current state of individual- rather than group-level hospitalist performance measurement.2 This scoping review identified 43 studies published over the last 25 years reporting individual adult or pediatric hospitalist performance across one or more of the STEEEP framework domains of performance: Safe, Timely, Effective, Efficient, Equitable, Patient Centered.3
The most common domain assessed in the studies was Patient Centered (20 studies), and in descending order from there were Safe (16), Efficient (13), Timely (10), Effective (9). No studies reported individual hospitalist performance on Equitable care. This distribution of studied domains is likely a function of readily available data and processes for study more than level of interest or importance attached to each domain. Their research was not designed to assess the quality of each study, and some—or even many—might have weaknesses in both determining which clinicians met the definition of hospitalist and how performance was attributed to individuals. The authors appropriately conclude that “further defining and refining approaches to assess individual performance is necessary to ensure the highest quality.”
Their findings should help guide research priorities regarding measurement of individual hospitalist performance. Yet each hospitalist group and individual hospitalist still faces decisions about managing their own group and personal performance and must navigate without the benefit of research providing clear direction. Many hospitalist metrics are tracked and reported to meet regulatory requirements such as those from Centers for Medicare & Medicaid Services, financial metrics for the local hospital and hospitalist group, and for use as components of hospitalist compensation. (The biennial State of Hospital Medicine Report captures extensive data regarding the latter.4)
Many people and processes across an entire healthcare system influence performance on every metric, but it is useful and practical to attribute some metrics entirely to a single hospitalist provider, such as timely documentation and the time of day the discharge order is entered. And arguably, it is useful to attribute readmission rate entirely to the discharging provider—the last hospital provider who can influence readmission risk. But for most other metrics individual attribution is problematic or misleading and collective experience and expert opinion are helpful here. Two examples come to mind of relatively simple approaches that have gained some popularity in teasing out individual contribution to hospitalist performance.
One can estimate individual hospitalist contribution to patient LOS by calculating the ratio of current procedural terminology (CPT) codes for all follow-up services to all discharge codes. For each hospitalist in the group who cares for a similar population, those with the highest ratios likely manage patients in ways associated with longer LOS. It is relatively simple to use billing data to calculate the ratio, and some groups report it for all providers monthly.
Many metrics that aggregate performance across an entire hospital stay, such as patient experience surveys, can be apportioned to each hospitalist who had a billed encounter with the patient. For example, if a hospitalist has 4 of a patient’s 10 billed encounters within the same group, then 40% of the patient’s survey score could be attributed to that hospitalist. It’s still imperfect, but it’s likely more meaningful than attributing the entire survey result to only the discharging provider.
These approaches have value but still leave us unsatisfied and unable to assess performance as effectively as we would like. Advancements in measurement have been slow and incremental, but they are likely to accelerate with maturation of electronic health records paired with machine learning or artificial intelligence, wearable devices, and sensors in patient rooms, which collectively may make capturing a robust set of metrics trivially easy (and raise questions regarding privacy and so forth). For example, it is already possible to capture via a smart speaker all conversations between patient, loved ones, and clinician.5 Imagine you are presented with a word cloud summary of all conversations you had with all patients over a year. Did you use empathy words often enough? How reliably did you address all appropriate discharge-related topics?
As performance metrics become more numerous and ubiquitous, the challenge will be to ensure they accurately capture what they appear to measure, are appropriately attributed to individuals or groups, and provide insights into important domains of performance. Significant opportunity for improvement remains.
Disclosure
Dr Nelson has no conflict of interest to disclose.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. W.W. Norton & Company; 2004.
2. Dow AW, Chopski B, Cyrus JW, et al. A STEEEP hill to climb: a scoping review of assessments of individual hospitalist performance. J Hosp Med. 2020;15:599-605. https://doi.org/10.12788/jhm.3445
3. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press (US); 2001. https://doi.org/10.17226/10027
4. 2018 State of Hospital Medicine Report. Society of Hospital Medicine. Accessed May 19, 2020. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
5. Chiu CC, Tripathi A, Chou K, et al. Speech recognition for medical conversations. arXiv. Preprint posted online November 20, 2017. Revised June 20, 2018. https://arxiv.org/pdf/1711.07274.pdf
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. W.W. Norton & Company; 2004.
2. Dow AW, Chopski B, Cyrus JW, et al. A STEEEP hill to climb: a scoping review of assessments of individual hospitalist performance. J Hosp Med. 2020;15:599-605. https://doi.org/10.12788/jhm.3445
3. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press (US); 2001. https://doi.org/10.17226/10027
4. 2018 State of Hospital Medicine Report. Society of Hospital Medicine. Accessed May 19, 2020. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
5. Chiu CC, Tripathi A, Chou K, et al. Speech recognition for medical conversations. arXiv. Preprint posted online November 20, 2017. Revised June 20, 2018. https://arxiv.org/pdf/1711.07274.pdf
© 2020 Society of Hospital Medicine
Hospital Star Ratings and Sociodemographics: A Scoring System in Need of Revision
Still in its infancy, the Hospital Compare overall hospital quality star rating program introduced by the Centers for Medicare & Medicaid Services (CMS) has generated intense industry debate. Individual health systems are microcosms of the challenges of ratings and measurement design. Sibley Memorial Hospital, a member of Johns Hopkins Medicine, is a well-run, 288-bed, community hospital located in a wealthy section of northwest District of Columbia with a five-star rating. In contrast, its academic partner, the Johns Hopkins Hospital, a 1,162-bed hospital with a century-long history of innovation situated in an impoverished Baltimore, Maryland, neighborhood, received a three-star rating.
Hospital ratings are the product of an industry in transition: As care delivery has shifted from an individual provider-driven industry to an increasingly scaled systems enterprise, policymakers implemented regulatory standards targeting quality measurement. Subsequent to the National Academy of Medicine’s 1999 report To Err is Human, policy efforts brought public reporting of quality ratings to multiple market segments, including dialysis facilities (2001), nursing homes (2003), Medicare Advantage plans (2007), and physicians (2015). The hospital industry was no exception, and in 2016—with much controversy1—CMS launched the hospital star ratings program.
CMS Star Ratings for hospitals are based on seven measure groups: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Both industry and researchers have decried the challenges of star ratings, noting that hospitals with a narrower scope of services are more likely to receive higher ratings.2 Measure groupings may be further flawed as shown by recent work demonstrating that larger, safety net, or academic hospitals, as well as hospitals offering transplant services, have higher readmission rates,3 which may be caused by differences in patient complexity. Other research has demonstrated that overall quality ratings inappropriately pool all hospitals together, when it may be fairer to initially categorize hospitals and then score them.4
It is within this maelstrom of debate that, in this month’s issue of the Journal of Hospital Medicine, Shi and colleagues explore the relationship between hospital star ratings and the socioeconomic features of the surrounding communities.5 Conducting their analysis by linking multiple reputable government and industry sources, Shi and colleagues found that counties with higher education attainment and a lower proportion of dual Medicare-Medicaid–eligible populations had higher hospital star ratings. Furthermore, a county’s minority population percentage negatively correlated with hospital ratings. Validating the experience of many rural hospital executives—who frequently experience financial challenges—Shi and colleagues noted that rural hospitals were less likely to receive five-star ratings.
Do these findings reflect a true disparity and lack of access to high-quality hospitals, or are they artifactual—secondary to a flawed construct of hospital quality measurement? Many lower-ranking hospitals are urban academic centers frequently providing services not offered at their five-star community counterparts, such as neurosurgery, comprehensive cancer care, and organ transplants, while simultaneously serving as safety net hospitals, research institutions, trauma centers, and national referral centers.
Sociodemographics factor significantly in self-care management for hospital aftercare. Health literacy, access to primary and behavioral healthcare, and transportation all affect star indicators. Recent work6 demonstrated that comprehensive investments in transitional care strategies and the social determinants of health were ineffective at reducing readmissions, which suggests that high readmission rates for hospitals in impoverished areas are not only common, but also may not accurately reflect hospital quality and local investment.
Patient experience is also complicating, with research demonstrating that patient perceptions vary significantly by education, age, primary language, ethnicity, and overall health. For example, one-third of average-ranked hospitals would have rankings vary by at least 18 percentile points when evaluated by Spanish-speaking patients. Star ratings fail to capture and communicate this granularity.7
More concerning is that star ratings inherently assume that hospital performance is being compared across the same tasks, regardless of patient characteristics, local resources, or the scope of services provided, the latter of which may vary between hospitals. For example, communication may differ in both complexity and time intensity: Explaining an antibiotic to the uncomplicated patient with pneumonia differs from prescribing an antibiotic to a patient who is legally blind from optic neuritis, walks with a cane because of multiple sclerosis, and has 24 other prescription medications. Similar challenges exist for differences in local neighborhood resources and for facilities with differing service scope.
Although one strategy to handle these “disparities” in star ratings might be to risk-adjust for social determinants of health, patients may be better served by first rethinking how star ratings are constructed. Clustering hospitals by scope of services provided and geographic region prior to determining star ratings would provide consumers with meaningful information by helping patients compare and make choices among either local or regional hospitals; national quality rankings are unhelpful for patients.
Arguably one of the most complex and person-dependent service enterprises, care delivery presents unique challenges for evaluation of customer experience and medical quality. Hospital star ratings are no exception: We must rethink their construction so they can be more meaningful for both patients and physicians.
Acknowledgments
The authors would like to acknowledge Daniel J Brotman, MD, for his editorial advice and input.
Disclosures
Dr Miller reported consulting for the Federal Trade Commission and serving as a member of the Centers for Medicare & Medicaid Services Medicare Evidence Development Coverage Advisory Committee. Drs Siddiqui and Deutschendorf have nothing to disclose.
1. Whitman E. CMS releases star ratings for hospitals. Modern Healthcare. July 27, 2016. Accessed April 27, 2020. https://www.modernhealthcare.com/article/20160727/NEWS/160729910/cms-releases-star-ratings-for-hospitals
2. Siddiqui ZK, Abusamaan M, Bertram A, et al. Comparison of services available in 5-star and non-5-star patient experience hospital. JAMA Intern Med. 2019;179(10):1429-1430. https://doi.org/10.1001/jamainternmed.2019.1285
3. Hoyer EH, Padula WV, Brotman DJ, et al. Patterns of hospital performance on the hospital-wide 30-day readmission metric: is the playing field level? J Gen Intern Med. 2018;33(1):57-64. https://doi.org/10.1007/s11606-017-4193-9
4. Chung JW, Dahlke AR, Barnard C, DeLancey JO, Merkow RP, Bilimoria KY. The Centers for Medicare and Medicaid Services hospital ratings: pitfalls of grading on a single curve. Health Aff (Millwood). 2019;38(9):1523-1529. https://doi.org/10.1377/hlthaff.2018.05345
5. Shi B, King C, Huang SS. Relationship of hospital star ratings to race, education, and community income. J Hosp Med. 2020;15:588-593. https://doi.org/10.12788/jhm.3393
6. Finkelstein A, Zhou A, Taubman S, Doyle J. Health care hotspotting—a randomized controlled trial. N Engl J Med. 2020;382:152-162. https://doi.org/10.1056/NEJMsa1906848
7. Elliott MN, Lehrman WG, Goldstein E, Hambarsoomian K, Beckett MK, Giordano LA. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56-73. https://doi.org/10.1177/1077558709339066
Still in its infancy, the Hospital Compare overall hospital quality star rating program introduced by the Centers for Medicare & Medicaid Services (CMS) has generated intense industry debate. Individual health systems are microcosms of the challenges of ratings and measurement design. Sibley Memorial Hospital, a member of Johns Hopkins Medicine, is a well-run, 288-bed, community hospital located in a wealthy section of northwest District of Columbia with a five-star rating. In contrast, its academic partner, the Johns Hopkins Hospital, a 1,162-bed hospital with a century-long history of innovation situated in an impoverished Baltimore, Maryland, neighborhood, received a three-star rating.
Hospital ratings are the product of an industry in transition: As care delivery has shifted from an individual provider-driven industry to an increasingly scaled systems enterprise, policymakers implemented regulatory standards targeting quality measurement. Subsequent to the National Academy of Medicine’s 1999 report To Err is Human, policy efforts brought public reporting of quality ratings to multiple market segments, including dialysis facilities (2001), nursing homes (2003), Medicare Advantage plans (2007), and physicians (2015). The hospital industry was no exception, and in 2016—with much controversy1—CMS launched the hospital star ratings program.
CMS Star Ratings for hospitals are based on seven measure groups: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Both industry and researchers have decried the challenges of star ratings, noting that hospitals with a narrower scope of services are more likely to receive higher ratings.2 Measure groupings may be further flawed as shown by recent work demonstrating that larger, safety net, or academic hospitals, as well as hospitals offering transplant services, have higher readmission rates,3 which may be caused by differences in patient complexity. Other research has demonstrated that overall quality ratings inappropriately pool all hospitals together, when it may be fairer to initially categorize hospitals and then score them.4
It is within this maelstrom of debate that, in this month’s issue of the Journal of Hospital Medicine, Shi and colleagues explore the relationship between hospital star ratings and the socioeconomic features of the surrounding communities.5 Conducting their analysis by linking multiple reputable government and industry sources, Shi and colleagues found that counties with higher education attainment and a lower proportion of dual Medicare-Medicaid–eligible populations had higher hospital star ratings. Furthermore, a county’s minority population percentage negatively correlated with hospital ratings. Validating the experience of many rural hospital executives—who frequently experience financial challenges—Shi and colleagues noted that rural hospitals were less likely to receive five-star ratings.
Do these findings reflect a true disparity and lack of access to high-quality hospitals, or are they artifactual—secondary to a flawed construct of hospital quality measurement? Many lower-ranking hospitals are urban academic centers frequently providing services not offered at their five-star community counterparts, such as neurosurgery, comprehensive cancer care, and organ transplants, while simultaneously serving as safety net hospitals, research institutions, trauma centers, and national referral centers.
Sociodemographics factor significantly in self-care management for hospital aftercare. Health literacy, access to primary and behavioral healthcare, and transportation all affect star indicators. Recent work6 demonstrated that comprehensive investments in transitional care strategies and the social determinants of health were ineffective at reducing readmissions, which suggests that high readmission rates for hospitals in impoverished areas are not only common, but also may not accurately reflect hospital quality and local investment.
Patient experience is also complicating, with research demonstrating that patient perceptions vary significantly by education, age, primary language, ethnicity, and overall health. For example, one-third of average-ranked hospitals would have rankings vary by at least 18 percentile points when evaluated by Spanish-speaking patients. Star ratings fail to capture and communicate this granularity.7
More concerning is that star ratings inherently assume that hospital performance is being compared across the same tasks, regardless of patient characteristics, local resources, or the scope of services provided, the latter of which may vary between hospitals. For example, communication may differ in both complexity and time intensity: Explaining an antibiotic to the uncomplicated patient with pneumonia differs from prescribing an antibiotic to a patient who is legally blind from optic neuritis, walks with a cane because of multiple sclerosis, and has 24 other prescription medications. Similar challenges exist for differences in local neighborhood resources and for facilities with differing service scope.
Although one strategy to handle these “disparities” in star ratings might be to risk-adjust for social determinants of health, patients may be better served by first rethinking how star ratings are constructed. Clustering hospitals by scope of services provided and geographic region prior to determining star ratings would provide consumers with meaningful information by helping patients compare and make choices among either local or regional hospitals; national quality rankings are unhelpful for patients.
Arguably one of the most complex and person-dependent service enterprises, care delivery presents unique challenges for evaluation of customer experience and medical quality. Hospital star ratings are no exception: We must rethink their construction so they can be more meaningful for both patients and physicians.
Acknowledgments
The authors would like to acknowledge Daniel J Brotman, MD, for his editorial advice and input.
Disclosures
Dr Miller reported consulting for the Federal Trade Commission and serving as a member of the Centers for Medicare & Medicaid Services Medicare Evidence Development Coverage Advisory Committee. Drs Siddiqui and Deutschendorf have nothing to disclose.
Still in its infancy, the Hospital Compare overall hospital quality star rating program introduced by the Centers for Medicare & Medicaid Services (CMS) has generated intense industry debate. Individual health systems are microcosms of the challenges of ratings and measurement design. Sibley Memorial Hospital, a member of Johns Hopkins Medicine, is a well-run, 288-bed, community hospital located in a wealthy section of northwest District of Columbia with a five-star rating. In contrast, its academic partner, the Johns Hopkins Hospital, a 1,162-bed hospital with a century-long history of innovation situated in an impoverished Baltimore, Maryland, neighborhood, received a three-star rating.
Hospital ratings are the product of an industry in transition: As care delivery has shifted from an individual provider-driven industry to an increasingly scaled systems enterprise, policymakers implemented regulatory standards targeting quality measurement. Subsequent to the National Academy of Medicine’s 1999 report To Err is Human, policy efforts brought public reporting of quality ratings to multiple market segments, including dialysis facilities (2001), nursing homes (2003), Medicare Advantage plans (2007), and physicians (2015). The hospital industry was no exception, and in 2016—with much controversy1—CMS launched the hospital star ratings program.
CMS Star Ratings for hospitals are based on seven measure groups: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Both industry and researchers have decried the challenges of star ratings, noting that hospitals with a narrower scope of services are more likely to receive higher ratings.2 Measure groupings may be further flawed as shown by recent work demonstrating that larger, safety net, or academic hospitals, as well as hospitals offering transplant services, have higher readmission rates,3 which may be caused by differences in patient complexity. Other research has demonstrated that overall quality ratings inappropriately pool all hospitals together, when it may be fairer to initially categorize hospitals and then score them.4
It is within this maelstrom of debate that, in this month’s issue of the Journal of Hospital Medicine, Shi and colleagues explore the relationship between hospital star ratings and the socioeconomic features of the surrounding communities.5 Conducting their analysis by linking multiple reputable government and industry sources, Shi and colleagues found that counties with higher education attainment and a lower proportion of dual Medicare-Medicaid–eligible populations had higher hospital star ratings. Furthermore, a county’s minority population percentage negatively correlated with hospital ratings. Validating the experience of many rural hospital executives—who frequently experience financial challenges—Shi and colleagues noted that rural hospitals were less likely to receive five-star ratings.
Do these findings reflect a true disparity and lack of access to high-quality hospitals, or are they artifactual—secondary to a flawed construct of hospital quality measurement? Many lower-ranking hospitals are urban academic centers frequently providing services not offered at their five-star community counterparts, such as neurosurgery, comprehensive cancer care, and organ transplants, while simultaneously serving as safety net hospitals, research institutions, trauma centers, and national referral centers.
Sociodemographics factor significantly in self-care management for hospital aftercare. Health literacy, access to primary and behavioral healthcare, and transportation all affect star indicators. Recent work6 demonstrated that comprehensive investments in transitional care strategies and the social determinants of health were ineffective at reducing readmissions, which suggests that high readmission rates for hospitals in impoverished areas are not only common, but also may not accurately reflect hospital quality and local investment.
Patient experience is also complicating, with research demonstrating that patient perceptions vary significantly by education, age, primary language, ethnicity, and overall health. For example, one-third of average-ranked hospitals would have rankings vary by at least 18 percentile points when evaluated by Spanish-speaking patients. Star ratings fail to capture and communicate this granularity.7
More concerning is that star ratings inherently assume that hospital performance is being compared across the same tasks, regardless of patient characteristics, local resources, or the scope of services provided, the latter of which may vary between hospitals. For example, communication may differ in both complexity and time intensity: Explaining an antibiotic to the uncomplicated patient with pneumonia differs from prescribing an antibiotic to a patient who is legally blind from optic neuritis, walks with a cane because of multiple sclerosis, and has 24 other prescription medications. Similar challenges exist for differences in local neighborhood resources and for facilities with differing service scope.
Although one strategy to handle these “disparities” in star ratings might be to risk-adjust for social determinants of health, patients may be better served by first rethinking how star ratings are constructed. Clustering hospitals by scope of services provided and geographic region prior to determining star ratings would provide consumers with meaningful information by helping patients compare and make choices among either local or regional hospitals; national quality rankings are unhelpful for patients.
Arguably one of the most complex and person-dependent service enterprises, care delivery presents unique challenges for evaluation of customer experience and medical quality. Hospital star ratings are no exception: We must rethink their construction so they can be more meaningful for both patients and physicians.
Acknowledgments
The authors would like to acknowledge Daniel J Brotman, MD, for his editorial advice and input.
Disclosures
Dr Miller reported consulting for the Federal Trade Commission and serving as a member of the Centers for Medicare & Medicaid Services Medicare Evidence Development Coverage Advisory Committee. Drs Siddiqui and Deutschendorf have nothing to disclose.
1. Whitman E. CMS releases star ratings for hospitals. Modern Healthcare. July 27, 2016. Accessed April 27, 2020. https://www.modernhealthcare.com/article/20160727/NEWS/160729910/cms-releases-star-ratings-for-hospitals
2. Siddiqui ZK, Abusamaan M, Bertram A, et al. Comparison of services available in 5-star and non-5-star patient experience hospital. JAMA Intern Med. 2019;179(10):1429-1430. https://doi.org/10.1001/jamainternmed.2019.1285
3. Hoyer EH, Padula WV, Brotman DJ, et al. Patterns of hospital performance on the hospital-wide 30-day readmission metric: is the playing field level? J Gen Intern Med. 2018;33(1):57-64. https://doi.org/10.1007/s11606-017-4193-9
4. Chung JW, Dahlke AR, Barnard C, DeLancey JO, Merkow RP, Bilimoria KY. The Centers for Medicare and Medicaid Services hospital ratings: pitfalls of grading on a single curve. Health Aff (Millwood). 2019;38(9):1523-1529. https://doi.org/10.1377/hlthaff.2018.05345
5. Shi B, King C, Huang SS. Relationship of hospital star ratings to race, education, and community income. J Hosp Med. 2020;15:588-593. https://doi.org/10.12788/jhm.3393
6. Finkelstein A, Zhou A, Taubman S, Doyle J. Health care hotspotting—a randomized controlled trial. N Engl J Med. 2020;382:152-162. https://doi.org/10.1056/NEJMsa1906848
7. Elliott MN, Lehrman WG, Goldstein E, Hambarsoomian K, Beckett MK, Giordano LA. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56-73. https://doi.org/10.1177/1077558709339066
1. Whitman E. CMS releases star ratings for hospitals. Modern Healthcare. July 27, 2016. Accessed April 27, 2020. https://www.modernhealthcare.com/article/20160727/NEWS/160729910/cms-releases-star-ratings-for-hospitals
2. Siddiqui ZK, Abusamaan M, Bertram A, et al. Comparison of services available in 5-star and non-5-star patient experience hospital. JAMA Intern Med. 2019;179(10):1429-1430. https://doi.org/10.1001/jamainternmed.2019.1285
3. Hoyer EH, Padula WV, Brotman DJ, et al. Patterns of hospital performance on the hospital-wide 30-day readmission metric: is the playing field level? J Gen Intern Med. 2018;33(1):57-64. https://doi.org/10.1007/s11606-017-4193-9
4. Chung JW, Dahlke AR, Barnard C, DeLancey JO, Merkow RP, Bilimoria KY. The Centers for Medicare and Medicaid Services hospital ratings: pitfalls of grading on a single curve. Health Aff (Millwood). 2019;38(9):1523-1529. https://doi.org/10.1377/hlthaff.2018.05345
5. Shi B, King C, Huang SS. Relationship of hospital star ratings to race, education, and community income. J Hosp Med. 2020;15:588-593. https://doi.org/10.12788/jhm.3393
6. Finkelstein A, Zhou A, Taubman S, Doyle J. Health care hotspotting—a randomized controlled trial. N Engl J Med. 2020;382:152-162. https://doi.org/10.1056/NEJMsa1906848
7. Elliott MN, Lehrman WG, Goldstein E, Hambarsoomian K, Beckett MK, Giordano LA. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56-73. https://doi.org/10.1177/1077558709339066
© 2020 Society of Hospital Medicine
Leadership & Professional Development: Breaking the Silence as a Bystander
“In the end, we will remember not the words of our enemies, but the silence of our friends.”
—Martin Luther King, Jr.
"Code Blue, Emergency Department Code Team to PACU.” A female senior resident dons her personal protective equipment and assembles her team. An enthusiastic male junior resident asks if he can accompany her, and off they go. They encounter a frantic scene in the post-anesthesia care unit (PACU). Before the senior resident can lead the rapid response, a PACU nurse addresses the junior resident: “You are leading the code, correct? What medications would you like?”
“Microaggressions” are subtle, commonplace exchanges that—whether intentional or unintentional—communicate disparaging messages to members of marginalized groups.1 These groups often include women, members of racial/ethnic groups that are underrepresented in medicine, and lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) individuals. Although an individual may not intend to cause harm, their words may still negatively impact the receiving party, who regularly experiences differential treatment based on sex, race, ethnicity, or other social identities. The effects of microaggressions extend beyond personal offense to include anxiety, depression, and even hypertension.1,2
Addressing microaggressions can be challenging. Given that the recipients of microaggressions are often burdened with responding to them, it is important for bystanders to be empowered to respond as well. A bystander witnesses and recognizes the microaggression and can address it. Based on the work of Sue et al,3 we suggest that bystanders adopt the following strategies:
- Make the “invisible” visible. Many people do not perceive their actions as biased or prejudiced. It is therefore important to bring the implicit bias to the forefront by asking for clarification, naming the implication, or challenging the stereotype.
- Disarm the microaggression. Don’t be afraid to stop, deflect, disagree, or challenge what was said or done, thereby highlighting its potentially harmful impact. Another option is to interrupt the comment as it’s being said and redirect the conversation.
- Educate the speaker. Create a nonpunitive discussion by appealing to common values, promoting empathy, and increasing awareness of societal benefits. The speaker may become defensive and emphasize that their intent was not to cause harm. You must emphasize that, regardless of intent, the impact was hurtful. You may refocus the discussion with a simple statement such as, “I know you meant well, and…”
- Seek external support when needed. Addressing microaggressions can be emotionally taxing. Don’t be afraid to utilize community services, find a support group, or seek advice from professionals.
By virtue of being a neutral third party, bystanders who intervene may have greater success at explaining the impact of the microaggression. In doing so, the bystander also relieves the recipient of the microaggression of a burdensome response. In the above example, another provider in the PACU might pull the nurse aside later and say, “When you asked the junior resident if he was leading the code, you unintentionally indicated that he was the most experienced, which made it more challenging for the female senior resident to lead the response.” In this way, the “invisible” implication of the nurse’s words—that the male resident was the most knowledgeable physician in the room—is made visible, and the female resident is relieved of responding.
Microaggressions do not occur in a vacuum; context matters. Before employing these strategies, consider when, where, and how you address microaggressions. These strategies validate and support those on the receiving end of microaggressions, and thus counteract their deleterious effects. The onus is on us: we must not be silent.
Disclosures
The authors have nothing to disclose.
1. Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62(4):271-286. https://doi.org/10.1037/0003-066x.62.4.271
2. Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154(9):868-872. https://doi.org/10.1001/jamasurg.2019.1648
3. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ, Mendez N. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. https://doi.org/10.1037/amp0000296
“In the end, we will remember not the words of our enemies, but the silence of our friends.”
—Martin Luther King, Jr.
"Code Blue, Emergency Department Code Team to PACU.” A female senior resident dons her personal protective equipment and assembles her team. An enthusiastic male junior resident asks if he can accompany her, and off they go. They encounter a frantic scene in the post-anesthesia care unit (PACU). Before the senior resident can lead the rapid response, a PACU nurse addresses the junior resident: “You are leading the code, correct? What medications would you like?”
“Microaggressions” are subtle, commonplace exchanges that—whether intentional or unintentional—communicate disparaging messages to members of marginalized groups.1 These groups often include women, members of racial/ethnic groups that are underrepresented in medicine, and lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) individuals. Although an individual may not intend to cause harm, their words may still negatively impact the receiving party, who regularly experiences differential treatment based on sex, race, ethnicity, or other social identities. The effects of microaggressions extend beyond personal offense to include anxiety, depression, and even hypertension.1,2
Addressing microaggressions can be challenging. Given that the recipients of microaggressions are often burdened with responding to them, it is important for bystanders to be empowered to respond as well. A bystander witnesses and recognizes the microaggression and can address it. Based on the work of Sue et al,3 we suggest that bystanders adopt the following strategies:
- Make the “invisible” visible. Many people do not perceive their actions as biased or prejudiced. It is therefore important to bring the implicit bias to the forefront by asking for clarification, naming the implication, or challenging the stereotype.
- Disarm the microaggression. Don’t be afraid to stop, deflect, disagree, or challenge what was said or done, thereby highlighting its potentially harmful impact. Another option is to interrupt the comment as it’s being said and redirect the conversation.
- Educate the speaker. Create a nonpunitive discussion by appealing to common values, promoting empathy, and increasing awareness of societal benefits. The speaker may become defensive and emphasize that their intent was not to cause harm. You must emphasize that, regardless of intent, the impact was hurtful. You may refocus the discussion with a simple statement such as, “I know you meant well, and…”
- Seek external support when needed. Addressing microaggressions can be emotionally taxing. Don’t be afraid to utilize community services, find a support group, or seek advice from professionals.
By virtue of being a neutral third party, bystanders who intervene may have greater success at explaining the impact of the microaggression. In doing so, the bystander also relieves the recipient of the microaggression of a burdensome response. In the above example, another provider in the PACU might pull the nurse aside later and say, “When you asked the junior resident if he was leading the code, you unintentionally indicated that he was the most experienced, which made it more challenging for the female senior resident to lead the response.” In this way, the “invisible” implication of the nurse’s words—that the male resident was the most knowledgeable physician in the room—is made visible, and the female resident is relieved of responding.
Microaggressions do not occur in a vacuum; context matters. Before employing these strategies, consider when, where, and how you address microaggressions. These strategies validate and support those on the receiving end of microaggressions, and thus counteract their deleterious effects. The onus is on us: we must not be silent.
Disclosures
The authors have nothing to disclose.
“In the end, we will remember not the words of our enemies, but the silence of our friends.”
—Martin Luther King, Jr.
"Code Blue, Emergency Department Code Team to PACU.” A female senior resident dons her personal protective equipment and assembles her team. An enthusiastic male junior resident asks if he can accompany her, and off they go. They encounter a frantic scene in the post-anesthesia care unit (PACU). Before the senior resident can lead the rapid response, a PACU nurse addresses the junior resident: “You are leading the code, correct? What medications would you like?”
“Microaggressions” are subtle, commonplace exchanges that—whether intentional or unintentional—communicate disparaging messages to members of marginalized groups.1 These groups often include women, members of racial/ethnic groups that are underrepresented in medicine, and lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) individuals. Although an individual may not intend to cause harm, their words may still negatively impact the receiving party, who regularly experiences differential treatment based on sex, race, ethnicity, or other social identities. The effects of microaggressions extend beyond personal offense to include anxiety, depression, and even hypertension.1,2
Addressing microaggressions can be challenging. Given that the recipients of microaggressions are often burdened with responding to them, it is important for bystanders to be empowered to respond as well. A bystander witnesses and recognizes the microaggression and can address it. Based on the work of Sue et al,3 we suggest that bystanders adopt the following strategies:
- Make the “invisible” visible. Many people do not perceive their actions as biased or prejudiced. It is therefore important to bring the implicit bias to the forefront by asking for clarification, naming the implication, or challenging the stereotype.
- Disarm the microaggression. Don’t be afraid to stop, deflect, disagree, or challenge what was said or done, thereby highlighting its potentially harmful impact. Another option is to interrupt the comment as it’s being said and redirect the conversation.
- Educate the speaker. Create a nonpunitive discussion by appealing to common values, promoting empathy, and increasing awareness of societal benefits. The speaker may become defensive and emphasize that their intent was not to cause harm. You must emphasize that, regardless of intent, the impact was hurtful. You may refocus the discussion with a simple statement such as, “I know you meant well, and…”
- Seek external support when needed. Addressing microaggressions can be emotionally taxing. Don’t be afraid to utilize community services, find a support group, or seek advice from professionals.
By virtue of being a neutral third party, bystanders who intervene may have greater success at explaining the impact of the microaggression. In doing so, the bystander also relieves the recipient of the microaggression of a burdensome response. In the above example, another provider in the PACU might pull the nurse aside later and say, “When you asked the junior resident if he was leading the code, you unintentionally indicated that he was the most experienced, which made it more challenging for the female senior resident to lead the response.” In this way, the “invisible” implication of the nurse’s words—that the male resident was the most knowledgeable physician in the room—is made visible, and the female resident is relieved of responding.
Microaggressions do not occur in a vacuum; context matters. Before employing these strategies, consider when, where, and how you address microaggressions. These strategies validate and support those on the receiving end of microaggressions, and thus counteract their deleterious effects. The onus is on us: we must not be silent.
Disclosures
The authors have nothing to disclose.
1. Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62(4):271-286. https://doi.org/10.1037/0003-066x.62.4.271
2. Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154(9):868-872. https://doi.org/10.1001/jamasurg.2019.1648
3. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ, Mendez N. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. https://doi.org/10.1037/amp0000296
1. Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62(4):271-286. https://doi.org/10.1037/0003-066x.62.4.271
2. Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154(9):868-872. https://doi.org/10.1001/jamasurg.2019.1648
3. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ, Mendez N. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. https://doi.org/10.1037/amp0000296
© 2020 Society of Hospital Medicine
Health Care Disparities Among Adolescents and Adults With Sickle Cell Disease: A Community-Based Needs Assessment to Inform Intervention Strategies
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23
The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.
Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.
Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”
On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.
Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).
Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23
The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.
Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.
Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”
On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.
Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).
Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23
The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.
Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.
Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”
On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.
Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).
Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
Improving Identification of Patients at Low Risk for Major Cardiac Events After Noncardiac Surgery Using Intraoperative Data
Annually, more than 40 million noncardiac surgeries take place in the US,1 with 1%-3% of patients experiencing a major adverse cardiovascular event (MACE) such as acute myocardial infarction (AMI) or cardiac arrest postoperatively.2 Such patients are at markedly increased risk of both perioperative and long-term death.2-5
Over the past 40 years, efforts to model the risk of cardiac complications after noncardiac surgery have examined relationships between preoperative risk factors and postoperative cardiovascular events. The resulting risk-stratification tools, such as the Lee Revised Cardiac Risk Index (RCRI), have been used to inform perioperative care, including strategies for risk factor management prior to surgery, testing for cardiac events after surgery, and decisions regarding postoperative disposition.6 However, tools used in practice have not incorporated intraoperative data on hemodynamics or medication administration in the transition to postoperative care, which is often provided by nonsurgical clinicians such as hospitalists. Presently, there is active debate about the optimal approach to postoperative evaluation and management of MACE, particularly with regard to indications for cardiac biomarker testing after surgery in patients without signs or symptoms of acute cardiac syndromes. The lack of consensus is reflected in differences among guidelines for postoperative cardiac biomarker testing across professional societies in Europe, Canada, and the United States.7-9
In this study, we examined whether the addition of intraoperative data to preoperative data (together, perioperative data) improved prediction of MACE after noncardiac surgery when compared with RCRI. Additionally, to investigate how such a model could be applied in practice, we compared risk stratification based on our model to a published risk factor–based guideline algorithm for postoperative cardiac biomarker testing.7 In particular, we evaluated to what extent patients recommended for postoperative cardiac biomarkers under the risk factor–based guideline algorithm would be reclassified as low risk by the model using perioperative data. Conducting biomarker tests on these patients would potentially represent low-value care. We hypothesized that adding intraoperative data would (a) lead to improved prediction of MACE complications when compared with RCRI and (b) more effectively identify, compared with a risk factor–based guideline algorithm, patients for whom cardiac biomarker testing would or would not be clinically meaningful.
METHODS
We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.10
Study Data
Baseline, preoperative, and intraoperative data were collected for patients undergoing surgery between January 2014 and April 2018 within the University of Pennsylvania Health System (UPHS) electronic health record (EHR), and these data were then integrated into a comprehensive perioperative dataset (data containing administrative, preoperative, intraoperative, and postoperative information related to surgeries) created through a collaboration with the Multicenter Perioperative Outcomes Group.11 The University of Pennsylvania Institutional Review Board approved this study.
Study Population
Patients aged 18 years or older who underwent inpatient major noncardiac surgery across four tertiary academic medical centers within UPHS in Pennsylvania during the study period were included in the cohort (see Appendix for inclusion/exclusion criteria).12,13 Noncardiac surgery was identified using primary Current Procedural Terminology (CPT) code specification ranges for noncardiac surgeries 10021-32999 and 34001-69990. The study sample was divided randomly into a training set (60%), validation (20%), and test set (20%),14 with similar rates of MACE in the resulting sets. We used a holdout test set for all final analyses to avoid overfitting during model selection.
Outcomes
The composite outcome used to develop the risk-stratification models was in-hospital MACE after major noncardiac surgery. Following prior literature, MACE was defined using billing codes for ST-elevation/non–ST-elevation myocardial infarction (STEMI/NSTEMI, ICD-9-CM 410.xx, ICD-10-CM I21.xx), cardiac arrest (ICD-9-CM 427.5, ICD-10-CM I46.x, I97.121), or all-cause in-hospital death.2,15-17
Variables
Variables were selected from baseline administrative, preoperative clinical, and intraoperative clinical data sources (full list in Appendix). Baseline variables included demographics, insurance type, and Elixhauser comorbidities.18,19 Preoperative variables included surgery type, laboratory results, and American Society of Anesthesiologists (ASA) Physical Status classification.20 Intraoperative variables included vital signs, estimated blood loss, fluid administration, and vasopressor use. We winsorized outlier values and used multiple imputation to address missingness. Rates of missing data can be found in Appendix Table 1.
Risk-Stratification Models Used as Comparisons
Briefly, RCRI variables include the presence of high-risk surgery,21 comorbid cardiovascular diseases (ie, ischemic heart disease, congestive heart failure, and cerebrovascular disease), preoperative use of insulin, and elevated preoperative serum creatinine.6 RCRI uses the inputs to calculate a point score that equates to different risk strata and is based on a stepwise logistic regression model with postoperative cardiovascular complications as the dependent outcome variable. For this study, we implemented the weighted version of the RCRI algorithm and computed the point scores (Appendix).6,7,22
We also applied a risk factor–based algorithm for postoperative cardiac biomarker testing published in 2017 by the Canadian Cardiovascular Society (CCS) guidelines to each patient in the study sample.7 Specifically, this algorithm recommends daily troponin surveillance for 48 to 72 hours after surgery among patients who have (1) an elevated NT-proBNP/BNP measurement or no NT-proBNP/BNP measurement before surgery, (2) have a Revised Cardiac Risk Index score of 1 or greater, (3) are aged 65 years and older, (4) are aged 45 to 64 years with significant cardiovascular disease undergoing elective surgery, or (5) are aged 18 to 64 years with significant cardiovascular disease undergoing semiurgent, urgent, or emergent surgery.
Statistical Analysis
We compared patient characteristics and outcomes between those who did and those who did not experience MACE during hospitalization. Chi-square tests were used to compare categorical variables and Mann Whitney tests were used to compare continuous variables.
To create the perioperative risk-stratification model based on baseline, preoperative, and intraoperative data, we used a logistic regression with elastic net selection using a dichotomous dependent variable indicating MACE and independent variables described earlier. This perioperative model was fit on the training set and the model coefficients were then applied to the patients in the test set. The area under the receiver operating characteristic curve (AUC) was reported and the outcomes were reported by predicted risk decile, with higher deciles indicating higher risk (ie, higher numbers of patients with MACE outcomes in higher deciles implied better risk stratification). Because predicted risk of postoperative MACE may not have been distributed evenly across deciles, we also examined the distribution of the predicted probability of MACE and examined the number of patients below thresholds of risk corresponding to 0.1% or less, 0.25% or less, 0.5% or less, and 1% or less. These thresholds were chosen because they were close to the overall rate of MACE within our cohort.
We tested for differences in predictive performance between the RCRI logistic regression model AUC and the perioperative model AUC using DeLong’s test.23 Additionally, we illustrated differences between the perioperative and RCRI models’ performance in two ways by stratifying patients into deciles based on predicted risk. First, we compared rates of MACE and MACE component events by predicted decile of the perioperative and RCRI models. Second, we further classified patients as RCRI high or low risk (per RCRI score classification in which RCRI score of 1 or greater is high risk and RCRI score of 0 is low risk) and examined numbers of surgical cases and MACE complications within these categories stratified by perioperative model predicted decile.
To compare the perioperative model’s performance with that of a risk factor–based guideline algorithm, we classified patients according to CCS guidelines as high risk (those for whom the CCS guidelines algorithm would recommend postoperative troponin surveillance testing) and low risk (those for whom the CCS guidelines algorithm would not recommend surveillance testing). We also used a logistic regression to examine if the predicted risk from our model was independently associated with MACE above and beyond the testing recommendation of the CCS guidelines algorithm. This model used MACE as the dependent variable and model-predicted risk and a CCS guidelines–defined high-risk indicator as predictors. We computed the association between a 10 percentage–point increase in predicted risk on observed MACE outcome rates.24
In sensitivity analyses, we used a random forest machine learning classifier to test an alternate model specification, used complete case analysis, varied RCRI thresholds, and limited to patients aged 50 years or older. We also varied the penalty parameter in the elastic net model and plotted AUC versus the number of variables included to examine parsimonious models. SAS v9.4 (SAS Institute Inc) was used for main analyses. Data preparations and sensitivity analysis were done in Python v3.6 with Pandas v0.24.2 and Scikit-learn v0.19.1.
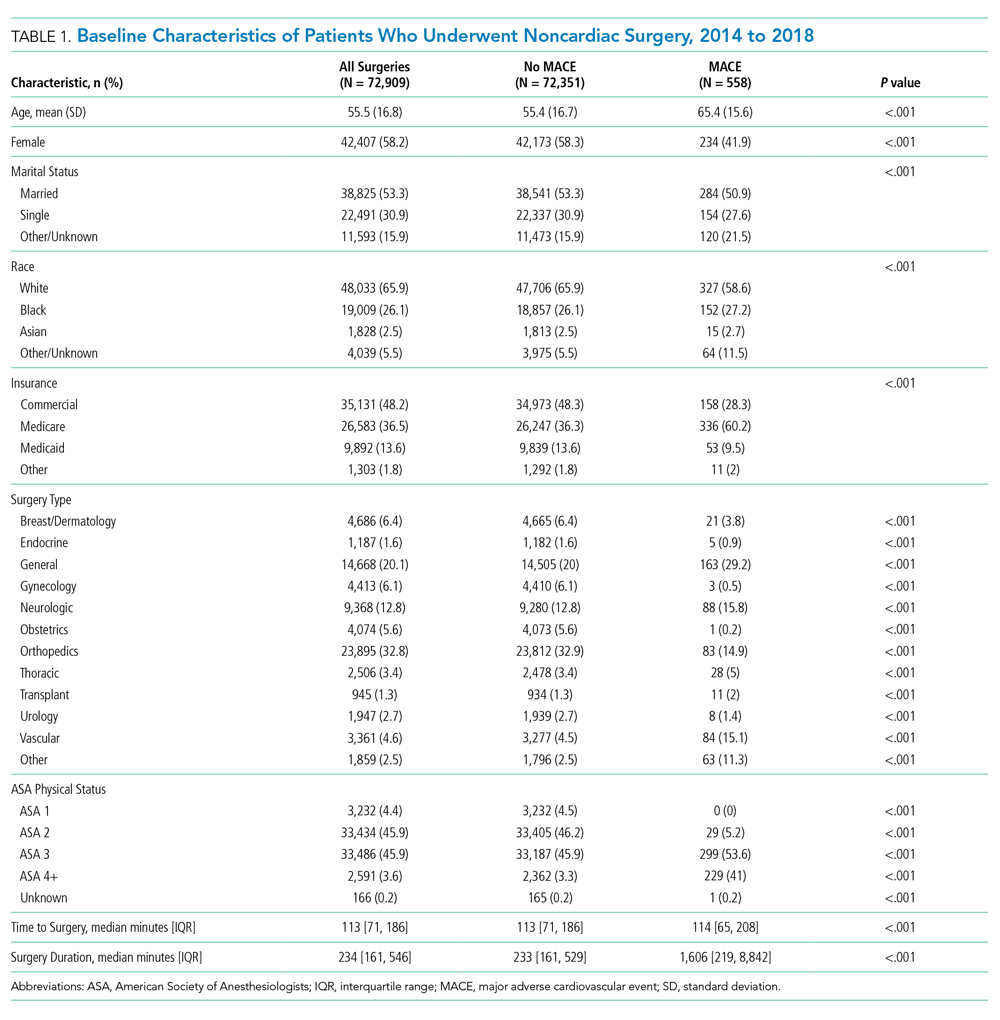
RESULTS
Study Sample
Patients who underwent major noncardiac surgery in our sample (n = 72,909) were approximately a mean age of 56 years, 58% female, 66% of White race and 26% of Black race, and most likely to have received orthopedic surgery (33%) or general surgery (20%). Those who experienced MACE (n = 558; 0.77%) differed along several characteristics (Table 1). For example, those with MACE were older (mean age, 65.4 vs 55.4 years; P < .001) and less likely to be female (41.9% vs 58.3%; P < .001).
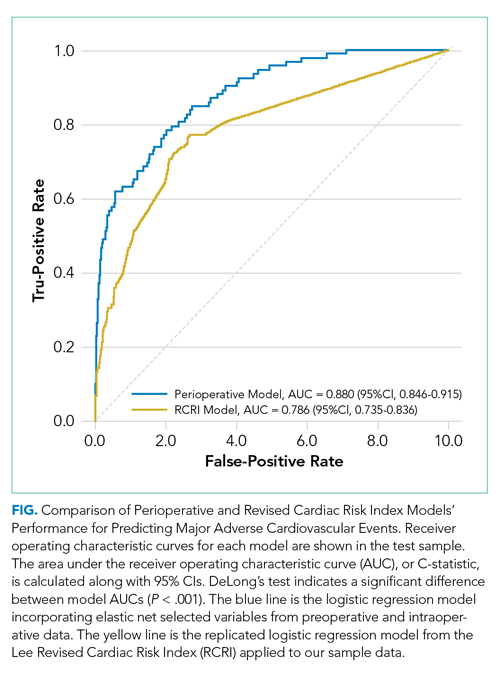
Model Performance After Intraoperative Data Inclusion
In the perioperative model combining preoperative and intraoperative data, 26 variables were included after elastic net selection (Appendix Table 2). Model discrimination in the test set of patients demonstrated an AUC of 0.88 (95% CI, 0.85-0.92; Figure). When examining outcome rates by predicted decile, the outcome rates of in-hospital MACE complications were higher in the highest decile than in the lowest decile, notably with 58 of 92 (63%) cases with MACE complications within the top decile of predicted risk (Table 2). The majority of patients had low predicted risk of MACE, with 5,309 (36.1%), 8,796 (59.7%), 11,335 (77.0%), and 12,972 (88.1%) below the risk thresholds of to 0.1%, 0.25%, 0.5%, and 1.0% respectively. The associated MACE rates were 0.04%, 0.10%, 0.17%, and 0.25% (average rate in sample was 0.63%) (Appendix Table 3).
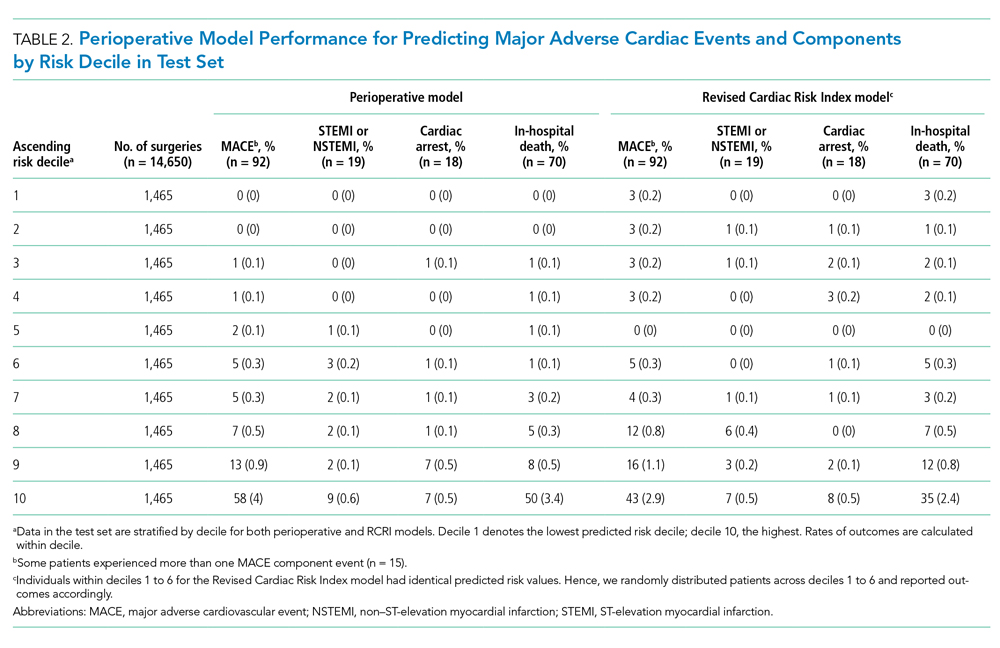
Model Performance Comparisons
The perioperative model AUC of 0.88 was higher when compared with RCRI’s AUC of 0.79 (95% CI, 0.74-0.84; P < .001). The number of MACE complications was more concentrated in the top decile of predicted risk of the perioperative model than it was in that of the RCRI model (58 vs 43 of 92 events, respectively; 63% vs 47%; Table 2). Furthermore, there were fewer cases with MACE complications in the low-risk deciles (ie, deciles 1 to 5) of the perioperative model than in the those of the RCRI model. These relative differences were consistent for MACE component outcomes of STEMI/NSTEMI, cardiac arrest, and in-hospital death, as well.
There was substantial heterogeneity in the perioperative model predicted risk of patients classified as either RCRI low risk or high risk (ie, each category included patients with low and high predicted risk) categories (Table 3). Patients in the bottom (low-risk) five deciles of the perioperative model’s predicted risk who were in the RCRI model’s high-risk group were very unlikely to experience MACE complications (3 out of 722 cases; 0.42%). Furthermore, among those classified as low risk by the RCRI model but were in the top decile of the perioperative model’s predicted risk, the MACE complication rate was 3.5% (8 out of 229), which was 6 times the sample mean MACE complication rate.
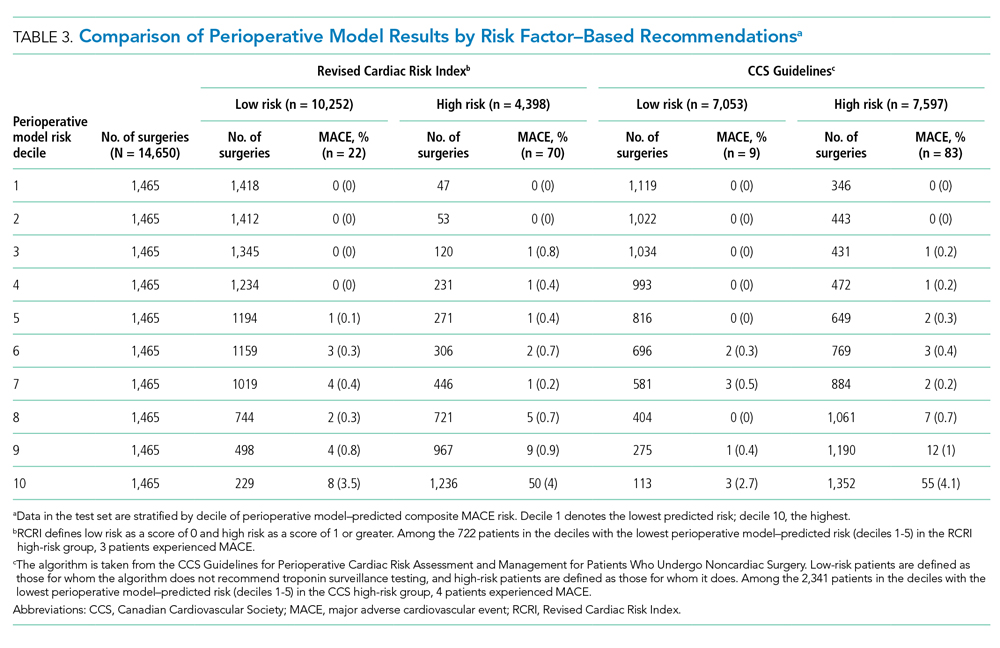
The perioperative model identified more patients as low risk than did the CCS guidelines’ risk factor–based algorithm (Table 3). For example, 2,341 of the patients the CCS guidelines algorithm identified as high risk were in the bottom 50% of the perioperative model’s predicted risk for experiencing MACE (below a 0.18% chance of a MACE complication); only four of these patients (0.17%) actually experienced MACE. This indicates that the 2,341 of 7,597 (31%) high-risk patients identified as low risk in the perioperative model would have been recommended for postoperative troponin testing by CCS guidelines based on preoperative risk factors alone—but did not go on to experience a MACE. Regression results indicated that both CCS guidelines risk-factor classification and the perioperative model’s predicted risk were predictive of MACE outcomes. A change in the perioperative model’s predicted risk of 10 percentage points was associated with an increase in the probability of a MACE outcomes of 0.45 percentage points (95% CI, 0.35-0.55 percentage points; P < .001) and moving from CCS guidelines’ low- to high-risk categories was associated with an increased probability of MACE by 0.96 percentage points (95% CI, 0.75-1.16 percentage points; P < .001).
Results were consistent with the main analysis across all sensitivity analyses (Appendix Tables 4-7). Parsimonious models with variables as few as eight variables retained strong predictive power (AUC, 0.870; Appendix Figure 1 and Table 8).
DISCUSSION
In this study, the addition of intraoperative data improved risk stratification for MACE complications when compared with standard risk tools such as RCRI. This approach also outperformed a guidelines-based approach and identified additional patients at low risk of cardiovascular complications. This study has three main implications.
First, this study demonstrated the additional value of combining intraoperative data with preoperative data in risk prediction for postoperative cardiovascular events. The intraoperative data most strongly associated with MACE, which likely were responsible for the performance improvement, included administration of medications (eg, sodium bicarbonate or calcium chloride) and blood products (eg, platelets and packed red blood cells), vitals (ie, heart rate), and intraoperative procedures (ie, arterial line placement); all model variables and coefficients are reported in Appendix Table 9. The risk-stratification model using intraoperative clinical data outperformed validated standard models such as RCRI. While this model should not be used in causal inference and cannot be used to inform decisions about risk-benefit tradeoffs of undergoing surgery, its improved performance relative to prior models highlights the potential in using real-time data. Preliminary illustrative analysis demonstrated that parsimonious models with as few as eight variables perform well, whose implementation as risk scores in EHRs is likely straightforward (Appendix Table 8). This is particularly important for longitudinal care in the hospital, in which patients frequently are cared for by multiple clinical services and experience handoffs. For example, many orthopedic surgery patients with significant medical comorbidity are managed postoperatively by hospitalist physicians after initial surgical care.
Second, our study aligns well with the cardiac risk-stratification literature more broadly. For example, the patient characteristics and clinical variables most associated with cardiovascular complications were age, history of ischemic heart disease, American Society of Anesthesiologists physical status, use of intraoperative sodium bicarbonate or vasopressors, lowest intraoperative heart rate measured, and lowest intraoperative mean arterial pressure measured. While many of these variables overlap with those included in the RCRI model, others (such as American Society of Anesthesiologists physical status) are not included in RCRI but have been shown to be important in risk prediction in other studies using different data variables.6,25,26
Third, we illustrated a clinical application of this model in identifying patients at low risk of cardiovascular complications, although benefit may extend to other patients as well. This is particularly germane to clinicians who frequently manage patients in the postsurgical or postprocedural setting. Moreover, the clinical relevance to these clinicians is underscored by the lack of consensus among professional societies across Europe, Canada, and the United States about which subgroups of patients undergoing noncardiac surgery should receive postoperative cardiac biomarker surveillance testing in the 48 to 72 hours after surgery.6-9 This may be in part caused by differences in clinical objectives. For example, the CCS guidelines in part aim to detect myocardial injury after noncardiac surgery (MINS) up to 30 days after surgery, which may be more sensitive to myocardial injury but less strongly associated with outcomes like MACE. The results of this study suggest that adopting such risk factor–based testing would likely lead to additional testing of low risk patients, which may represent low value surveillance tests. For example, there were 2,257 patients without postoperative cardiac biomarker testing in our data who would have been categorized as high risk by risk factor guidelines and therefore recommended to receive at least one postoperative cardiac biomarker surveillance test but were classified as low-risk individuals using a predicted probability of MACE less than 0.18% per our perioperative risk stratification model (Appendix Table 4). If each of these patients received one troponin biomarker test, the associated cost increase would be $372,405 (using the $165 cost per test reported at our institution). These costs would multiply if daily surveillance troponin biomarker tests were ordered for 48 to 72 hours after surgery, as recommended by the risk factor–based testing guidelines. This would be a departure from testing among patients using clinician discretion that may avoid low-value testing.
Applying the perioperative model developed in this paper to clinical practice still requires several steps. The technical aspects of finding a parsimonious model that can be implemented in the EHR is likely quite straightforward. Our preliminary analysis illustrates that doing so will not require accessing large numbers of intraoperative variables. Perhaps more important steps include prospective validation of the safety, usability, and clinical benefit of such an algorithm-based risk score.27
The study has several limitations. First, it was an observational study using EHR data subject to missingness and data quality issues that may have persisted despite our methods. Furthermore, EHR data is not generated randomly, and unmeasured variables observed by clinicians but not by researchers could confound the results. However, our approach used the statistical model to examine risk, not causal inference. Second, this is a single institution study and the availability of EHR data, as well as practice patterns, may vary at other institutions. Furthermore, it is possible that performance of the RCRI score, the model fitting RCRI classification of high vs low risk on the sample data, and our model’s performance may not generalize to other clinical settings. However, we utilized data from multiple hospitals within a health system with different surgery and anesthesia groups and providers, and a similar AUC was reported for RCRI in original validation study.6 Third, our follow up period was limited to the hospital setting and we do not capture longitudinal outcomes, such as 30-day MACE. This may impact the ability to risk stratify for other important longer-term outcomes, limit clinical utility, and hinder comparability to other studies. Fourth, results may vary for other important cardiovascular outcomes that may be more sensitive to myocardial injury, such as MINS. Fifth, we used a limited number of modeling strategies.
CONCLUSION
Addition of intraoperative data to preoperative data improves prediction of cardiovascular complications after noncardiac surgery. Improving the identification of patients at low risk for such complications could potentially be applied to reduce unnecessary postoperative cardiac biomarker testing after noncardiac surgery, but it will require further validation in prospective clinical settings.
Disclosures
Dr Navathe reports grants from the following entities: Hawaii Medical Service Association, Anthem Public Policy Institute, Commonwealth Fund, Oscar Health, Cigna Corporation, Robert Wood Johnson Foundation, Donaghue Foundation, Pennsylvania Department of Health, Ochsner Health System, United Healthcare, Blue Cross Blue Shield of NC, Blue Shield of CA; personal fees from the following: Navvis Healthcare, Agathos, Inc, Navahealth, YNHHSC/CORE, Maine Health Accountable Care Organization, Maine Department of Health and Human Services, National University Health System - Singapore, Ministry of Health - Singapore, Social Security Administration - France, Elsevier Press, Medicare Payment Advisory Commission, Cleveland Clinic, Embedded Healthcare; and other support from Integrated Services, Inc, outside of the submitted work. Dr Volpp reports grants from Humana during the conduct of the study; grants from Hawaii Medical Services Agency, Discovery (South Africa), Merck, Weight Watchers, and CVS outside of the submitted work; he has received consulting income from CVS and VALHealth and is a principal in VALHealth, a behavioral economics consulting firm. Dr Holmes receives funding from the Pennsylvania Department of Health, US Public Health Service, and the Cardiovascular Medicine Research and Education Foundation. All other authors declare no conflicts of interest.
Prior Presentations
2019 Academy Health Annual Research Meeting, Poster Abstract Presentation, June 2 to June 4, 2019, Washington, DC.
Funding
This project was funded, in part, under a grant with the Pennsylvania Department of Health. This research was independent from the funder. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
1. National Center for Health Statistics. National Hospital Discharge Survey: 2010 Table, Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. Centers for Disease Control and Prevention; 2010. Accessed November 11, 2018. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
2. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627-634. https://doi.org/10.1503/cmaj.050011
3. Charlson M, Peterson J, Szatrowski TP, MacKenzie R, Gold J. Long-term prognosis after peri-operative cardiac complications. J Clin Epidemiol. 1994;47(12):1389-1400. https://doi.org/10.1016/0895-4356(94)90083-3
4. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. https://doi.org/10.1056/nejmra1502824
5. Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99(2):259-269. https://doi.org/10.1097/00000542-200308000-00006
6. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. https://doi.org/10.1161/01.cir.100.10.1043
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008
8. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944
9. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Euro Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282
10. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. https://doi.org/10.7326/m14-0697
11. Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25(4):489-498. https://doi.org/10.1016/j.bpa.2011.08.008
12. CPT® (Current Procedural Terminology). American Medical Association. 2018. Accessed November 11, 2018. https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology
13. Surgery Flag Software for ICD-9-CM. AHRQ Healthcare Cost and Utilization Project; 2017. Accessed November 11, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp
14. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; 2009. https://www.springer.com/gp/book/9780387848570
15. Bucy R, Hanisko KA, Ewing LA, et al. Abstract 281: Validity of in-hospital cardiac arrest ICD-9-CM codes in veterans. Circ Cardiovasc Qual Outcomes. 2015;8(suppl_2):A281-A281.
16. Institute of Medicine; Board on Health Sciences Policy; Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions. Graham R, McCoy MA, Schultz AM, eds. Strategies to Improve Cardiac Arrest Survival: A Time to Act. The National Academies Press; 2015. https://doi.org/10.17226/21723
17. Pladevall M, Goff DC, Nichaman MZ, et al. An assessment of the validity of ICD Code 410 to identify hospital admissions for myocardial infarction: The Corpus Christi Heart Project. Int J Epidemiol. 1996;25(5):948-952. https://doi.org/10.1093/ije/25.5.948
18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
20. Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49(4):233-236. https://doi.org/10.1097/00000542-197810000-00001
21. Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150(4):325-331. https://doi.org/10.1001/jamasurg.2014.1819
22. VISION Pilot Study Investigators, Devereaux PJ, Bradley D, et al. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med. 2011;5(4):e193-e200.
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
24. Norton EC, Dowd BE, Maciejewski ML. Marginal effects-quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304‐1305. https://doi.org/10.1001/jama.2019.1954
25. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
26. Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204(2):201-208. https://doi.org/10.1016/j.jamcollsurg.2006.11.011
27. Parikh RB, Obermeyer Z, Navathe AS. Regulation of predictive analytics in medicine. Science. 2019;363(6429):810-812. https://doi.org/10.1126/science.aaw0029
Annually, more than 40 million noncardiac surgeries take place in the US,1 with 1%-3% of patients experiencing a major adverse cardiovascular event (MACE) such as acute myocardial infarction (AMI) or cardiac arrest postoperatively.2 Such patients are at markedly increased risk of both perioperative and long-term death.2-5
Over the past 40 years, efforts to model the risk of cardiac complications after noncardiac surgery have examined relationships between preoperative risk factors and postoperative cardiovascular events. The resulting risk-stratification tools, such as the Lee Revised Cardiac Risk Index (RCRI), have been used to inform perioperative care, including strategies for risk factor management prior to surgery, testing for cardiac events after surgery, and decisions regarding postoperative disposition.6 However, tools used in practice have not incorporated intraoperative data on hemodynamics or medication administration in the transition to postoperative care, which is often provided by nonsurgical clinicians such as hospitalists. Presently, there is active debate about the optimal approach to postoperative evaluation and management of MACE, particularly with regard to indications for cardiac biomarker testing after surgery in patients without signs or symptoms of acute cardiac syndromes. The lack of consensus is reflected in differences among guidelines for postoperative cardiac biomarker testing across professional societies in Europe, Canada, and the United States.7-9
In this study, we examined whether the addition of intraoperative data to preoperative data (together, perioperative data) improved prediction of MACE after noncardiac surgery when compared with RCRI. Additionally, to investigate how such a model could be applied in practice, we compared risk stratification based on our model to a published risk factor–based guideline algorithm for postoperative cardiac biomarker testing.7 In particular, we evaluated to what extent patients recommended for postoperative cardiac biomarkers under the risk factor–based guideline algorithm would be reclassified as low risk by the model using perioperative data. Conducting biomarker tests on these patients would potentially represent low-value care. We hypothesized that adding intraoperative data would (a) lead to improved prediction of MACE complications when compared with RCRI and (b) more effectively identify, compared with a risk factor–based guideline algorithm, patients for whom cardiac biomarker testing would or would not be clinically meaningful.
METHODS
We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.10
Study Data
Baseline, preoperative, and intraoperative data were collected for patients undergoing surgery between January 2014 and April 2018 within the University of Pennsylvania Health System (UPHS) electronic health record (EHR), and these data were then integrated into a comprehensive perioperative dataset (data containing administrative, preoperative, intraoperative, and postoperative information related to surgeries) created through a collaboration with the Multicenter Perioperative Outcomes Group.11 The University of Pennsylvania Institutional Review Board approved this study.
Study Population
Patients aged 18 years or older who underwent inpatient major noncardiac surgery across four tertiary academic medical centers within UPHS in Pennsylvania during the study period were included in the cohort (see Appendix for inclusion/exclusion criteria).12,13 Noncardiac surgery was identified using primary Current Procedural Terminology (CPT) code specification ranges for noncardiac surgeries 10021-32999 and 34001-69990. The study sample was divided randomly into a training set (60%), validation (20%), and test set (20%),14 with similar rates of MACE in the resulting sets. We used a holdout test set for all final analyses to avoid overfitting during model selection.
Outcomes
The composite outcome used to develop the risk-stratification models was in-hospital MACE after major noncardiac surgery. Following prior literature, MACE was defined using billing codes for ST-elevation/non–ST-elevation myocardial infarction (STEMI/NSTEMI, ICD-9-CM 410.xx, ICD-10-CM I21.xx), cardiac arrest (ICD-9-CM 427.5, ICD-10-CM I46.x, I97.121), or all-cause in-hospital death.2,15-17
Variables
Variables were selected from baseline administrative, preoperative clinical, and intraoperative clinical data sources (full list in Appendix). Baseline variables included demographics, insurance type, and Elixhauser comorbidities.18,19 Preoperative variables included surgery type, laboratory results, and American Society of Anesthesiologists (ASA) Physical Status classification.20 Intraoperative variables included vital signs, estimated blood loss, fluid administration, and vasopressor use. We winsorized outlier values and used multiple imputation to address missingness. Rates of missing data can be found in Appendix Table 1.
Risk-Stratification Models Used as Comparisons
Briefly, RCRI variables include the presence of high-risk surgery,21 comorbid cardiovascular diseases (ie, ischemic heart disease, congestive heart failure, and cerebrovascular disease), preoperative use of insulin, and elevated preoperative serum creatinine.6 RCRI uses the inputs to calculate a point score that equates to different risk strata and is based on a stepwise logistic regression model with postoperative cardiovascular complications as the dependent outcome variable. For this study, we implemented the weighted version of the RCRI algorithm and computed the point scores (Appendix).6,7,22
We also applied a risk factor–based algorithm for postoperative cardiac biomarker testing published in 2017 by the Canadian Cardiovascular Society (CCS) guidelines to each patient in the study sample.7 Specifically, this algorithm recommends daily troponin surveillance for 48 to 72 hours after surgery among patients who have (1) an elevated NT-proBNP/BNP measurement or no NT-proBNP/BNP measurement before surgery, (2) have a Revised Cardiac Risk Index score of 1 or greater, (3) are aged 65 years and older, (4) are aged 45 to 64 years with significant cardiovascular disease undergoing elective surgery, or (5) are aged 18 to 64 years with significant cardiovascular disease undergoing semiurgent, urgent, or emergent surgery.
Statistical Analysis
We compared patient characteristics and outcomes between those who did and those who did not experience MACE during hospitalization. Chi-square tests were used to compare categorical variables and Mann Whitney tests were used to compare continuous variables.
To create the perioperative risk-stratification model based on baseline, preoperative, and intraoperative data, we used a logistic regression with elastic net selection using a dichotomous dependent variable indicating MACE and independent variables described earlier. This perioperative model was fit on the training set and the model coefficients were then applied to the patients in the test set. The area under the receiver operating characteristic curve (AUC) was reported and the outcomes were reported by predicted risk decile, with higher deciles indicating higher risk (ie, higher numbers of patients with MACE outcomes in higher deciles implied better risk stratification). Because predicted risk of postoperative MACE may not have been distributed evenly across deciles, we also examined the distribution of the predicted probability of MACE and examined the number of patients below thresholds of risk corresponding to 0.1% or less, 0.25% or less, 0.5% or less, and 1% or less. These thresholds were chosen because they were close to the overall rate of MACE within our cohort.
We tested for differences in predictive performance between the RCRI logistic regression model AUC and the perioperative model AUC using DeLong’s test.23 Additionally, we illustrated differences between the perioperative and RCRI models’ performance in two ways by stratifying patients into deciles based on predicted risk. First, we compared rates of MACE and MACE component events by predicted decile of the perioperative and RCRI models. Second, we further classified patients as RCRI high or low risk (per RCRI score classification in which RCRI score of 1 or greater is high risk and RCRI score of 0 is low risk) and examined numbers of surgical cases and MACE complications within these categories stratified by perioperative model predicted decile.
To compare the perioperative model’s performance with that of a risk factor–based guideline algorithm, we classified patients according to CCS guidelines as high risk (those for whom the CCS guidelines algorithm would recommend postoperative troponin surveillance testing) and low risk (those for whom the CCS guidelines algorithm would not recommend surveillance testing). We also used a logistic regression to examine if the predicted risk from our model was independently associated with MACE above and beyond the testing recommendation of the CCS guidelines algorithm. This model used MACE as the dependent variable and model-predicted risk and a CCS guidelines–defined high-risk indicator as predictors. We computed the association between a 10 percentage–point increase in predicted risk on observed MACE outcome rates.24
In sensitivity analyses, we used a random forest machine learning classifier to test an alternate model specification, used complete case analysis, varied RCRI thresholds, and limited to patients aged 50 years or older. We also varied the penalty parameter in the elastic net model and plotted AUC versus the number of variables included to examine parsimonious models. SAS v9.4 (SAS Institute Inc) was used for main analyses. Data preparations and sensitivity analysis were done in Python v3.6 with Pandas v0.24.2 and Scikit-learn v0.19.1.

RESULTS
Study Sample
Patients who underwent major noncardiac surgery in our sample (n = 72,909) were approximately a mean age of 56 years, 58% female, 66% of White race and 26% of Black race, and most likely to have received orthopedic surgery (33%) or general surgery (20%). Those who experienced MACE (n = 558; 0.77%) differed along several characteristics (Table 1). For example, those with MACE were older (mean age, 65.4 vs 55.4 years; P < .001) and less likely to be female (41.9% vs 58.3%; P < .001).

Model Performance After Intraoperative Data Inclusion
In the perioperative model combining preoperative and intraoperative data, 26 variables were included after elastic net selection (Appendix Table 2). Model discrimination in the test set of patients demonstrated an AUC of 0.88 (95% CI, 0.85-0.92; Figure). When examining outcome rates by predicted decile, the outcome rates of in-hospital MACE complications were higher in the highest decile than in the lowest decile, notably with 58 of 92 (63%) cases with MACE complications within the top decile of predicted risk (Table 2). The majority of patients had low predicted risk of MACE, with 5,309 (36.1%), 8,796 (59.7%), 11,335 (77.0%), and 12,972 (88.1%) below the risk thresholds of to 0.1%, 0.25%, 0.5%, and 1.0% respectively. The associated MACE rates were 0.04%, 0.10%, 0.17%, and 0.25% (average rate in sample was 0.63%) (Appendix Table 3).

Model Performance Comparisons
The perioperative model AUC of 0.88 was higher when compared with RCRI’s AUC of 0.79 (95% CI, 0.74-0.84; P < .001). The number of MACE complications was more concentrated in the top decile of predicted risk of the perioperative model than it was in that of the RCRI model (58 vs 43 of 92 events, respectively; 63% vs 47%; Table 2). Furthermore, there were fewer cases with MACE complications in the low-risk deciles (ie, deciles 1 to 5) of the perioperative model than in the those of the RCRI model. These relative differences were consistent for MACE component outcomes of STEMI/NSTEMI, cardiac arrest, and in-hospital death, as well.
There was substantial heterogeneity in the perioperative model predicted risk of patients classified as either RCRI low risk or high risk (ie, each category included patients with low and high predicted risk) categories (Table 3). Patients in the bottom (low-risk) five deciles of the perioperative model’s predicted risk who were in the RCRI model’s high-risk group were very unlikely to experience MACE complications (3 out of 722 cases; 0.42%). Furthermore, among those classified as low risk by the RCRI model but were in the top decile of the perioperative model’s predicted risk, the MACE complication rate was 3.5% (8 out of 229), which was 6 times the sample mean MACE complication rate.

The perioperative model identified more patients as low risk than did the CCS guidelines’ risk factor–based algorithm (Table 3). For example, 2,341 of the patients the CCS guidelines algorithm identified as high risk were in the bottom 50% of the perioperative model’s predicted risk for experiencing MACE (below a 0.18% chance of a MACE complication); only four of these patients (0.17%) actually experienced MACE. This indicates that the 2,341 of 7,597 (31%) high-risk patients identified as low risk in the perioperative model would have been recommended for postoperative troponin testing by CCS guidelines based on preoperative risk factors alone—but did not go on to experience a MACE. Regression results indicated that both CCS guidelines risk-factor classification and the perioperative model’s predicted risk were predictive of MACE outcomes. A change in the perioperative model’s predicted risk of 10 percentage points was associated with an increase in the probability of a MACE outcomes of 0.45 percentage points (95% CI, 0.35-0.55 percentage points; P < .001) and moving from CCS guidelines’ low- to high-risk categories was associated with an increased probability of MACE by 0.96 percentage points (95% CI, 0.75-1.16 percentage points; P < .001).
Results were consistent with the main analysis across all sensitivity analyses (Appendix Tables 4-7). Parsimonious models with variables as few as eight variables retained strong predictive power (AUC, 0.870; Appendix Figure 1 and Table 8).
DISCUSSION
In this study, the addition of intraoperative data improved risk stratification for MACE complications when compared with standard risk tools such as RCRI. This approach also outperformed a guidelines-based approach and identified additional patients at low risk of cardiovascular complications. This study has three main implications.
First, this study demonstrated the additional value of combining intraoperative data with preoperative data in risk prediction for postoperative cardiovascular events. The intraoperative data most strongly associated with MACE, which likely were responsible for the performance improvement, included administration of medications (eg, sodium bicarbonate or calcium chloride) and blood products (eg, platelets and packed red blood cells), vitals (ie, heart rate), and intraoperative procedures (ie, arterial line placement); all model variables and coefficients are reported in Appendix Table 9. The risk-stratification model using intraoperative clinical data outperformed validated standard models such as RCRI. While this model should not be used in causal inference and cannot be used to inform decisions about risk-benefit tradeoffs of undergoing surgery, its improved performance relative to prior models highlights the potential in using real-time data. Preliminary illustrative analysis demonstrated that parsimonious models with as few as eight variables perform well, whose implementation as risk scores in EHRs is likely straightforward (Appendix Table 8). This is particularly important for longitudinal care in the hospital, in which patients frequently are cared for by multiple clinical services and experience handoffs. For example, many orthopedic surgery patients with significant medical comorbidity are managed postoperatively by hospitalist physicians after initial surgical care.
Second, our study aligns well with the cardiac risk-stratification literature more broadly. For example, the patient characteristics and clinical variables most associated with cardiovascular complications were age, history of ischemic heart disease, American Society of Anesthesiologists physical status, use of intraoperative sodium bicarbonate or vasopressors, lowest intraoperative heart rate measured, and lowest intraoperative mean arterial pressure measured. While many of these variables overlap with those included in the RCRI model, others (such as American Society of Anesthesiologists physical status) are not included in RCRI but have been shown to be important in risk prediction in other studies using different data variables.6,25,26
Third, we illustrated a clinical application of this model in identifying patients at low risk of cardiovascular complications, although benefit may extend to other patients as well. This is particularly germane to clinicians who frequently manage patients in the postsurgical or postprocedural setting. Moreover, the clinical relevance to these clinicians is underscored by the lack of consensus among professional societies across Europe, Canada, and the United States about which subgroups of patients undergoing noncardiac surgery should receive postoperative cardiac biomarker surveillance testing in the 48 to 72 hours after surgery.6-9 This may be in part caused by differences in clinical objectives. For example, the CCS guidelines in part aim to detect myocardial injury after noncardiac surgery (MINS) up to 30 days after surgery, which may be more sensitive to myocardial injury but less strongly associated with outcomes like MACE. The results of this study suggest that adopting such risk factor–based testing would likely lead to additional testing of low risk patients, which may represent low value surveillance tests. For example, there were 2,257 patients without postoperative cardiac biomarker testing in our data who would have been categorized as high risk by risk factor guidelines and therefore recommended to receive at least one postoperative cardiac biomarker surveillance test but were classified as low-risk individuals using a predicted probability of MACE less than 0.18% per our perioperative risk stratification model (Appendix Table 4). If each of these patients received one troponin biomarker test, the associated cost increase would be $372,405 (using the $165 cost per test reported at our institution). These costs would multiply if daily surveillance troponin biomarker tests were ordered for 48 to 72 hours after surgery, as recommended by the risk factor–based testing guidelines. This would be a departure from testing among patients using clinician discretion that may avoid low-value testing.
Applying the perioperative model developed in this paper to clinical practice still requires several steps. The technical aspects of finding a parsimonious model that can be implemented in the EHR is likely quite straightforward. Our preliminary analysis illustrates that doing so will not require accessing large numbers of intraoperative variables. Perhaps more important steps include prospective validation of the safety, usability, and clinical benefit of such an algorithm-based risk score.27
The study has several limitations. First, it was an observational study using EHR data subject to missingness and data quality issues that may have persisted despite our methods. Furthermore, EHR data is not generated randomly, and unmeasured variables observed by clinicians but not by researchers could confound the results. However, our approach used the statistical model to examine risk, not causal inference. Second, this is a single institution study and the availability of EHR data, as well as practice patterns, may vary at other institutions. Furthermore, it is possible that performance of the RCRI score, the model fitting RCRI classification of high vs low risk on the sample data, and our model’s performance may not generalize to other clinical settings. However, we utilized data from multiple hospitals within a health system with different surgery and anesthesia groups and providers, and a similar AUC was reported for RCRI in original validation study.6 Third, our follow up period was limited to the hospital setting and we do not capture longitudinal outcomes, such as 30-day MACE. This may impact the ability to risk stratify for other important longer-term outcomes, limit clinical utility, and hinder comparability to other studies. Fourth, results may vary for other important cardiovascular outcomes that may be more sensitive to myocardial injury, such as MINS. Fifth, we used a limited number of modeling strategies.
CONCLUSION
Addition of intraoperative data to preoperative data improves prediction of cardiovascular complications after noncardiac surgery. Improving the identification of patients at low risk for such complications could potentially be applied to reduce unnecessary postoperative cardiac biomarker testing after noncardiac surgery, but it will require further validation in prospective clinical settings.
Disclosures
Dr Navathe reports grants from the following entities: Hawaii Medical Service Association, Anthem Public Policy Institute, Commonwealth Fund, Oscar Health, Cigna Corporation, Robert Wood Johnson Foundation, Donaghue Foundation, Pennsylvania Department of Health, Ochsner Health System, United Healthcare, Blue Cross Blue Shield of NC, Blue Shield of CA; personal fees from the following: Navvis Healthcare, Agathos, Inc, Navahealth, YNHHSC/CORE, Maine Health Accountable Care Organization, Maine Department of Health and Human Services, National University Health System - Singapore, Ministry of Health - Singapore, Social Security Administration - France, Elsevier Press, Medicare Payment Advisory Commission, Cleveland Clinic, Embedded Healthcare; and other support from Integrated Services, Inc, outside of the submitted work. Dr Volpp reports grants from Humana during the conduct of the study; grants from Hawaii Medical Services Agency, Discovery (South Africa), Merck, Weight Watchers, and CVS outside of the submitted work; he has received consulting income from CVS and VALHealth and is a principal in VALHealth, a behavioral economics consulting firm. Dr Holmes receives funding from the Pennsylvania Department of Health, US Public Health Service, and the Cardiovascular Medicine Research and Education Foundation. All other authors declare no conflicts of interest.
Prior Presentations
2019 Academy Health Annual Research Meeting, Poster Abstract Presentation, June 2 to June 4, 2019, Washington, DC.
Funding
This project was funded, in part, under a grant with the Pennsylvania Department of Health. This research was independent from the funder. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Annually, more than 40 million noncardiac surgeries take place in the US,1 with 1%-3% of patients experiencing a major adverse cardiovascular event (MACE) such as acute myocardial infarction (AMI) or cardiac arrest postoperatively.2 Such patients are at markedly increased risk of both perioperative and long-term death.2-5
Over the past 40 years, efforts to model the risk of cardiac complications after noncardiac surgery have examined relationships between preoperative risk factors and postoperative cardiovascular events. The resulting risk-stratification tools, such as the Lee Revised Cardiac Risk Index (RCRI), have been used to inform perioperative care, including strategies for risk factor management prior to surgery, testing for cardiac events after surgery, and decisions regarding postoperative disposition.6 However, tools used in practice have not incorporated intraoperative data on hemodynamics or medication administration in the transition to postoperative care, which is often provided by nonsurgical clinicians such as hospitalists. Presently, there is active debate about the optimal approach to postoperative evaluation and management of MACE, particularly with regard to indications for cardiac biomarker testing after surgery in patients without signs or symptoms of acute cardiac syndromes. The lack of consensus is reflected in differences among guidelines for postoperative cardiac biomarker testing across professional societies in Europe, Canada, and the United States.7-9
In this study, we examined whether the addition of intraoperative data to preoperative data (together, perioperative data) improved prediction of MACE after noncardiac surgery when compared with RCRI. Additionally, to investigate how such a model could be applied in practice, we compared risk stratification based on our model to a published risk factor–based guideline algorithm for postoperative cardiac biomarker testing.7 In particular, we evaluated to what extent patients recommended for postoperative cardiac biomarkers under the risk factor–based guideline algorithm would be reclassified as low risk by the model using perioperative data. Conducting biomarker tests on these patients would potentially represent low-value care. We hypothesized that adding intraoperative data would (a) lead to improved prediction of MACE complications when compared with RCRI and (b) more effectively identify, compared with a risk factor–based guideline algorithm, patients for whom cardiac biomarker testing would or would not be clinically meaningful.
METHODS
We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.10
Study Data
Baseline, preoperative, and intraoperative data were collected for patients undergoing surgery between January 2014 and April 2018 within the University of Pennsylvania Health System (UPHS) electronic health record (EHR), and these data were then integrated into a comprehensive perioperative dataset (data containing administrative, preoperative, intraoperative, and postoperative information related to surgeries) created through a collaboration with the Multicenter Perioperative Outcomes Group.11 The University of Pennsylvania Institutional Review Board approved this study.
Study Population
Patients aged 18 years or older who underwent inpatient major noncardiac surgery across four tertiary academic medical centers within UPHS in Pennsylvania during the study period were included in the cohort (see Appendix for inclusion/exclusion criteria).12,13 Noncardiac surgery was identified using primary Current Procedural Terminology (CPT) code specification ranges for noncardiac surgeries 10021-32999 and 34001-69990. The study sample was divided randomly into a training set (60%), validation (20%), and test set (20%),14 with similar rates of MACE in the resulting sets. We used a holdout test set for all final analyses to avoid overfitting during model selection.
Outcomes
The composite outcome used to develop the risk-stratification models was in-hospital MACE after major noncardiac surgery. Following prior literature, MACE was defined using billing codes for ST-elevation/non–ST-elevation myocardial infarction (STEMI/NSTEMI, ICD-9-CM 410.xx, ICD-10-CM I21.xx), cardiac arrest (ICD-9-CM 427.5, ICD-10-CM I46.x, I97.121), or all-cause in-hospital death.2,15-17
Variables
Variables were selected from baseline administrative, preoperative clinical, and intraoperative clinical data sources (full list in Appendix). Baseline variables included demographics, insurance type, and Elixhauser comorbidities.18,19 Preoperative variables included surgery type, laboratory results, and American Society of Anesthesiologists (ASA) Physical Status classification.20 Intraoperative variables included vital signs, estimated blood loss, fluid administration, and vasopressor use. We winsorized outlier values and used multiple imputation to address missingness. Rates of missing data can be found in Appendix Table 1.
Risk-Stratification Models Used as Comparisons
Briefly, RCRI variables include the presence of high-risk surgery,21 comorbid cardiovascular diseases (ie, ischemic heart disease, congestive heart failure, and cerebrovascular disease), preoperative use of insulin, and elevated preoperative serum creatinine.6 RCRI uses the inputs to calculate a point score that equates to different risk strata and is based on a stepwise logistic regression model with postoperative cardiovascular complications as the dependent outcome variable. For this study, we implemented the weighted version of the RCRI algorithm and computed the point scores (Appendix).6,7,22
We also applied a risk factor–based algorithm for postoperative cardiac biomarker testing published in 2017 by the Canadian Cardiovascular Society (CCS) guidelines to each patient in the study sample.7 Specifically, this algorithm recommends daily troponin surveillance for 48 to 72 hours after surgery among patients who have (1) an elevated NT-proBNP/BNP measurement or no NT-proBNP/BNP measurement before surgery, (2) have a Revised Cardiac Risk Index score of 1 or greater, (3) are aged 65 years and older, (4) are aged 45 to 64 years with significant cardiovascular disease undergoing elective surgery, or (5) are aged 18 to 64 years with significant cardiovascular disease undergoing semiurgent, urgent, or emergent surgery.
Statistical Analysis
We compared patient characteristics and outcomes between those who did and those who did not experience MACE during hospitalization. Chi-square tests were used to compare categorical variables and Mann Whitney tests were used to compare continuous variables.
To create the perioperative risk-stratification model based on baseline, preoperative, and intraoperative data, we used a logistic regression with elastic net selection using a dichotomous dependent variable indicating MACE and independent variables described earlier. This perioperative model was fit on the training set and the model coefficients were then applied to the patients in the test set. The area under the receiver operating characteristic curve (AUC) was reported and the outcomes were reported by predicted risk decile, with higher deciles indicating higher risk (ie, higher numbers of patients with MACE outcomes in higher deciles implied better risk stratification). Because predicted risk of postoperative MACE may not have been distributed evenly across deciles, we also examined the distribution of the predicted probability of MACE and examined the number of patients below thresholds of risk corresponding to 0.1% or less, 0.25% or less, 0.5% or less, and 1% or less. These thresholds were chosen because they were close to the overall rate of MACE within our cohort.
We tested for differences in predictive performance between the RCRI logistic regression model AUC and the perioperative model AUC using DeLong’s test.23 Additionally, we illustrated differences between the perioperative and RCRI models’ performance in two ways by stratifying patients into deciles based on predicted risk. First, we compared rates of MACE and MACE component events by predicted decile of the perioperative and RCRI models. Second, we further classified patients as RCRI high or low risk (per RCRI score classification in which RCRI score of 1 or greater is high risk and RCRI score of 0 is low risk) and examined numbers of surgical cases and MACE complications within these categories stratified by perioperative model predicted decile.
To compare the perioperative model’s performance with that of a risk factor–based guideline algorithm, we classified patients according to CCS guidelines as high risk (those for whom the CCS guidelines algorithm would recommend postoperative troponin surveillance testing) and low risk (those for whom the CCS guidelines algorithm would not recommend surveillance testing). We also used a logistic regression to examine if the predicted risk from our model was independently associated with MACE above and beyond the testing recommendation of the CCS guidelines algorithm. This model used MACE as the dependent variable and model-predicted risk and a CCS guidelines–defined high-risk indicator as predictors. We computed the association between a 10 percentage–point increase in predicted risk on observed MACE outcome rates.24
In sensitivity analyses, we used a random forest machine learning classifier to test an alternate model specification, used complete case analysis, varied RCRI thresholds, and limited to patients aged 50 years or older. We also varied the penalty parameter in the elastic net model and plotted AUC versus the number of variables included to examine parsimonious models. SAS v9.4 (SAS Institute Inc) was used for main analyses. Data preparations and sensitivity analysis were done in Python v3.6 with Pandas v0.24.2 and Scikit-learn v0.19.1.

RESULTS
Study Sample
Patients who underwent major noncardiac surgery in our sample (n = 72,909) were approximately a mean age of 56 years, 58% female, 66% of White race and 26% of Black race, and most likely to have received orthopedic surgery (33%) or general surgery (20%). Those who experienced MACE (n = 558; 0.77%) differed along several characteristics (Table 1). For example, those with MACE were older (mean age, 65.4 vs 55.4 years; P < .001) and less likely to be female (41.9% vs 58.3%; P < .001).

Model Performance After Intraoperative Data Inclusion
In the perioperative model combining preoperative and intraoperative data, 26 variables were included after elastic net selection (Appendix Table 2). Model discrimination in the test set of patients demonstrated an AUC of 0.88 (95% CI, 0.85-0.92; Figure). When examining outcome rates by predicted decile, the outcome rates of in-hospital MACE complications were higher in the highest decile than in the lowest decile, notably with 58 of 92 (63%) cases with MACE complications within the top decile of predicted risk (Table 2). The majority of patients had low predicted risk of MACE, with 5,309 (36.1%), 8,796 (59.7%), 11,335 (77.0%), and 12,972 (88.1%) below the risk thresholds of to 0.1%, 0.25%, 0.5%, and 1.0% respectively. The associated MACE rates were 0.04%, 0.10%, 0.17%, and 0.25% (average rate in sample was 0.63%) (Appendix Table 3).

Model Performance Comparisons
The perioperative model AUC of 0.88 was higher when compared with RCRI’s AUC of 0.79 (95% CI, 0.74-0.84; P < .001). The number of MACE complications was more concentrated in the top decile of predicted risk of the perioperative model than it was in that of the RCRI model (58 vs 43 of 92 events, respectively; 63% vs 47%; Table 2). Furthermore, there were fewer cases with MACE complications in the low-risk deciles (ie, deciles 1 to 5) of the perioperative model than in the those of the RCRI model. These relative differences were consistent for MACE component outcomes of STEMI/NSTEMI, cardiac arrest, and in-hospital death, as well.
There was substantial heterogeneity in the perioperative model predicted risk of patients classified as either RCRI low risk or high risk (ie, each category included patients with low and high predicted risk) categories (Table 3). Patients in the bottom (low-risk) five deciles of the perioperative model’s predicted risk who were in the RCRI model’s high-risk group were very unlikely to experience MACE complications (3 out of 722 cases; 0.42%). Furthermore, among those classified as low risk by the RCRI model but were in the top decile of the perioperative model’s predicted risk, the MACE complication rate was 3.5% (8 out of 229), which was 6 times the sample mean MACE complication rate.

The perioperative model identified more patients as low risk than did the CCS guidelines’ risk factor–based algorithm (Table 3). For example, 2,341 of the patients the CCS guidelines algorithm identified as high risk were in the bottom 50% of the perioperative model’s predicted risk for experiencing MACE (below a 0.18% chance of a MACE complication); only four of these patients (0.17%) actually experienced MACE. This indicates that the 2,341 of 7,597 (31%) high-risk patients identified as low risk in the perioperative model would have been recommended for postoperative troponin testing by CCS guidelines based on preoperative risk factors alone—but did not go on to experience a MACE. Regression results indicated that both CCS guidelines risk-factor classification and the perioperative model’s predicted risk were predictive of MACE outcomes. A change in the perioperative model’s predicted risk of 10 percentage points was associated with an increase in the probability of a MACE outcomes of 0.45 percentage points (95% CI, 0.35-0.55 percentage points; P < .001) and moving from CCS guidelines’ low- to high-risk categories was associated with an increased probability of MACE by 0.96 percentage points (95% CI, 0.75-1.16 percentage points; P < .001).
Results were consistent with the main analysis across all sensitivity analyses (Appendix Tables 4-7). Parsimonious models with variables as few as eight variables retained strong predictive power (AUC, 0.870; Appendix Figure 1 and Table 8).
DISCUSSION
In this study, the addition of intraoperative data improved risk stratification for MACE complications when compared with standard risk tools such as RCRI. This approach also outperformed a guidelines-based approach and identified additional patients at low risk of cardiovascular complications. This study has three main implications.
First, this study demonstrated the additional value of combining intraoperative data with preoperative data in risk prediction for postoperative cardiovascular events. The intraoperative data most strongly associated with MACE, which likely were responsible for the performance improvement, included administration of medications (eg, sodium bicarbonate or calcium chloride) and blood products (eg, platelets and packed red blood cells), vitals (ie, heart rate), and intraoperative procedures (ie, arterial line placement); all model variables and coefficients are reported in Appendix Table 9. The risk-stratification model using intraoperative clinical data outperformed validated standard models such as RCRI. While this model should not be used in causal inference and cannot be used to inform decisions about risk-benefit tradeoffs of undergoing surgery, its improved performance relative to prior models highlights the potential in using real-time data. Preliminary illustrative analysis demonstrated that parsimonious models with as few as eight variables perform well, whose implementation as risk scores in EHRs is likely straightforward (Appendix Table 8). This is particularly important for longitudinal care in the hospital, in which patients frequently are cared for by multiple clinical services and experience handoffs. For example, many orthopedic surgery patients with significant medical comorbidity are managed postoperatively by hospitalist physicians after initial surgical care.
Second, our study aligns well with the cardiac risk-stratification literature more broadly. For example, the patient characteristics and clinical variables most associated with cardiovascular complications were age, history of ischemic heart disease, American Society of Anesthesiologists physical status, use of intraoperative sodium bicarbonate or vasopressors, lowest intraoperative heart rate measured, and lowest intraoperative mean arterial pressure measured. While many of these variables overlap with those included in the RCRI model, others (such as American Society of Anesthesiologists physical status) are not included in RCRI but have been shown to be important in risk prediction in other studies using different data variables.6,25,26
Third, we illustrated a clinical application of this model in identifying patients at low risk of cardiovascular complications, although benefit may extend to other patients as well. This is particularly germane to clinicians who frequently manage patients in the postsurgical or postprocedural setting. Moreover, the clinical relevance to these clinicians is underscored by the lack of consensus among professional societies across Europe, Canada, and the United States about which subgroups of patients undergoing noncardiac surgery should receive postoperative cardiac biomarker surveillance testing in the 48 to 72 hours after surgery.6-9 This may be in part caused by differences in clinical objectives. For example, the CCS guidelines in part aim to detect myocardial injury after noncardiac surgery (MINS) up to 30 days after surgery, which may be more sensitive to myocardial injury but less strongly associated with outcomes like MACE. The results of this study suggest that adopting such risk factor–based testing would likely lead to additional testing of low risk patients, which may represent low value surveillance tests. For example, there were 2,257 patients without postoperative cardiac biomarker testing in our data who would have been categorized as high risk by risk factor guidelines and therefore recommended to receive at least one postoperative cardiac biomarker surveillance test but were classified as low-risk individuals using a predicted probability of MACE less than 0.18% per our perioperative risk stratification model (Appendix Table 4). If each of these patients received one troponin biomarker test, the associated cost increase would be $372,405 (using the $165 cost per test reported at our institution). These costs would multiply if daily surveillance troponin biomarker tests were ordered for 48 to 72 hours after surgery, as recommended by the risk factor–based testing guidelines. This would be a departure from testing among patients using clinician discretion that may avoid low-value testing.
Applying the perioperative model developed in this paper to clinical practice still requires several steps. The technical aspects of finding a parsimonious model that can be implemented in the EHR is likely quite straightforward. Our preliminary analysis illustrates that doing so will not require accessing large numbers of intraoperative variables. Perhaps more important steps include prospective validation of the safety, usability, and clinical benefit of such an algorithm-based risk score.27
The study has several limitations. First, it was an observational study using EHR data subject to missingness and data quality issues that may have persisted despite our methods. Furthermore, EHR data is not generated randomly, and unmeasured variables observed by clinicians but not by researchers could confound the results. However, our approach used the statistical model to examine risk, not causal inference. Second, this is a single institution study and the availability of EHR data, as well as practice patterns, may vary at other institutions. Furthermore, it is possible that performance of the RCRI score, the model fitting RCRI classification of high vs low risk on the sample data, and our model’s performance may not generalize to other clinical settings. However, we utilized data from multiple hospitals within a health system with different surgery and anesthesia groups and providers, and a similar AUC was reported for RCRI in original validation study.6 Third, our follow up period was limited to the hospital setting and we do not capture longitudinal outcomes, such as 30-day MACE. This may impact the ability to risk stratify for other important longer-term outcomes, limit clinical utility, and hinder comparability to other studies. Fourth, results may vary for other important cardiovascular outcomes that may be more sensitive to myocardial injury, such as MINS. Fifth, we used a limited number of modeling strategies.
CONCLUSION
Addition of intraoperative data to preoperative data improves prediction of cardiovascular complications after noncardiac surgery. Improving the identification of patients at low risk for such complications could potentially be applied to reduce unnecessary postoperative cardiac biomarker testing after noncardiac surgery, but it will require further validation in prospective clinical settings.
Disclosures
Dr Navathe reports grants from the following entities: Hawaii Medical Service Association, Anthem Public Policy Institute, Commonwealth Fund, Oscar Health, Cigna Corporation, Robert Wood Johnson Foundation, Donaghue Foundation, Pennsylvania Department of Health, Ochsner Health System, United Healthcare, Blue Cross Blue Shield of NC, Blue Shield of CA; personal fees from the following: Navvis Healthcare, Agathos, Inc, Navahealth, YNHHSC/CORE, Maine Health Accountable Care Organization, Maine Department of Health and Human Services, National University Health System - Singapore, Ministry of Health - Singapore, Social Security Administration - France, Elsevier Press, Medicare Payment Advisory Commission, Cleveland Clinic, Embedded Healthcare; and other support from Integrated Services, Inc, outside of the submitted work. Dr Volpp reports grants from Humana during the conduct of the study; grants from Hawaii Medical Services Agency, Discovery (South Africa), Merck, Weight Watchers, and CVS outside of the submitted work; he has received consulting income from CVS and VALHealth and is a principal in VALHealth, a behavioral economics consulting firm. Dr Holmes receives funding from the Pennsylvania Department of Health, US Public Health Service, and the Cardiovascular Medicine Research and Education Foundation. All other authors declare no conflicts of interest.
Prior Presentations
2019 Academy Health Annual Research Meeting, Poster Abstract Presentation, June 2 to June 4, 2019, Washington, DC.
Funding
This project was funded, in part, under a grant with the Pennsylvania Department of Health. This research was independent from the funder. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
1. National Center for Health Statistics. National Hospital Discharge Survey: 2010 Table, Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. Centers for Disease Control and Prevention; 2010. Accessed November 11, 2018. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
2. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627-634. https://doi.org/10.1503/cmaj.050011
3. Charlson M, Peterson J, Szatrowski TP, MacKenzie R, Gold J. Long-term prognosis after peri-operative cardiac complications. J Clin Epidemiol. 1994;47(12):1389-1400. https://doi.org/10.1016/0895-4356(94)90083-3
4. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. https://doi.org/10.1056/nejmra1502824
5. Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99(2):259-269. https://doi.org/10.1097/00000542-200308000-00006
6. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. https://doi.org/10.1161/01.cir.100.10.1043
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008
8. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944
9. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Euro Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282
10. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. https://doi.org/10.7326/m14-0697
11. Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25(4):489-498. https://doi.org/10.1016/j.bpa.2011.08.008
12. CPT® (Current Procedural Terminology). American Medical Association. 2018. Accessed November 11, 2018. https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology
13. Surgery Flag Software for ICD-9-CM. AHRQ Healthcare Cost and Utilization Project; 2017. Accessed November 11, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp
14. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; 2009. https://www.springer.com/gp/book/9780387848570
15. Bucy R, Hanisko KA, Ewing LA, et al. Abstract 281: Validity of in-hospital cardiac arrest ICD-9-CM codes in veterans. Circ Cardiovasc Qual Outcomes. 2015;8(suppl_2):A281-A281.
16. Institute of Medicine; Board on Health Sciences Policy; Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions. Graham R, McCoy MA, Schultz AM, eds. Strategies to Improve Cardiac Arrest Survival: A Time to Act. The National Academies Press; 2015. https://doi.org/10.17226/21723
17. Pladevall M, Goff DC, Nichaman MZ, et al. An assessment of the validity of ICD Code 410 to identify hospital admissions for myocardial infarction: The Corpus Christi Heart Project. Int J Epidemiol. 1996;25(5):948-952. https://doi.org/10.1093/ije/25.5.948
18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
20. Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49(4):233-236. https://doi.org/10.1097/00000542-197810000-00001
21. Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150(4):325-331. https://doi.org/10.1001/jamasurg.2014.1819
22. VISION Pilot Study Investigators, Devereaux PJ, Bradley D, et al. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med. 2011;5(4):e193-e200.
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
24. Norton EC, Dowd BE, Maciejewski ML. Marginal effects-quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304‐1305. https://doi.org/10.1001/jama.2019.1954
25. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
26. Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204(2):201-208. https://doi.org/10.1016/j.jamcollsurg.2006.11.011
27. Parikh RB, Obermeyer Z, Navathe AS. Regulation of predictive analytics in medicine. Science. 2019;363(6429):810-812. https://doi.org/10.1126/science.aaw0029
1. National Center for Health Statistics. National Hospital Discharge Survey: 2010 Table, Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. Centers for Disease Control and Prevention; 2010. Accessed November 11, 2018. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
2. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627-634. https://doi.org/10.1503/cmaj.050011
3. Charlson M, Peterson J, Szatrowski TP, MacKenzie R, Gold J. Long-term prognosis after peri-operative cardiac complications. J Clin Epidemiol. 1994;47(12):1389-1400. https://doi.org/10.1016/0895-4356(94)90083-3
4. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. https://doi.org/10.1056/nejmra1502824
5. Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99(2):259-269. https://doi.org/10.1097/00000542-200308000-00006
6. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. https://doi.org/10.1161/01.cir.100.10.1043
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008
8. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944
9. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Euro Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282
10. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. https://doi.org/10.7326/m14-0697
11. Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25(4):489-498. https://doi.org/10.1016/j.bpa.2011.08.008
12. CPT® (Current Procedural Terminology). American Medical Association. 2018. Accessed November 11, 2018. https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology
13. Surgery Flag Software for ICD-9-CM. AHRQ Healthcare Cost and Utilization Project; 2017. Accessed November 11, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp
14. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; 2009. https://www.springer.com/gp/book/9780387848570
15. Bucy R, Hanisko KA, Ewing LA, et al. Abstract 281: Validity of in-hospital cardiac arrest ICD-9-CM codes in veterans. Circ Cardiovasc Qual Outcomes. 2015;8(suppl_2):A281-A281.
16. Institute of Medicine; Board on Health Sciences Policy; Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions. Graham R, McCoy MA, Schultz AM, eds. Strategies to Improve Cardiac Arrest Survival: A Time to Act. The National Academies Press; 2015. https://doi.org/10.17226/21723
17. Pladevall M, Goff DC, Nichaman MZ, et al. An assessment of the validity of ICD Code 410 to identify hospital admissions for myocardial infarction: The Corpus Christi Heart Project. Int J Epidemiol. 1996;25(5):948-952. https://doi.org/10.1093/ije/25.5.948
18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
20. Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49(4):233-236. https://doi.org/10.1097/00000542-197810000-00001
21. Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150(4):325-331. https://doi.org/10.1001/jamasurg.2014.1819
22. VISION Pilot Study Investigators, Devereaux PJ, Bradley D, et al. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med. 2011;5(4):e193-e200.
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
24. Norton EC, Dowd BE, Maciejewski ML. Marginal effects-quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304‐1305. https://doi.org/10.1001/jama.2019.1954
25. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
26. Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204(2):201-208. https://doi.org/10.1016/j.jamcollsurg.2006.11.011
27. Parikh RB, Obermeyer Z, Navathe AS. Regulation of predictive analytics in medicine. Science. 2019;363(6429):810-812. https://doi.org/10.1126/science.aaw0029
© 2020 Society of Hospital Medicine
Trends in Use of Postdischarge Intravenous Antibiotic Therapy for Children
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.
DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.
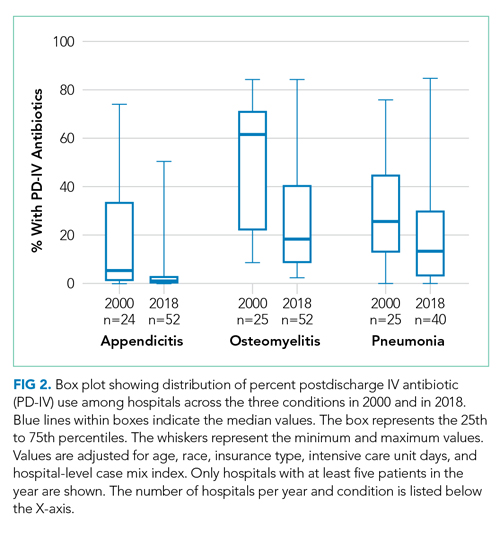
The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.
DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.

The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.
DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.

The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
© 2020 Society of Hospital Medicine
A STEEEP Hill to Climb: A Scoping Review of Assessments of Individual Hospitalist Performance
Healthcare quality is defined as the extent to which healthcare services result in desired outcomes.1 Quality of care depends on how the healthcare system’s various components, including healthcare practitioners, interact to meet each patient’s needs.2 These components can be shaped to achieve desired outcomes through rules, incentives, and other approaches, but influencing the behaviors of each component, such as the performance of hospitalists, requires defining goals for performance and implementing measurement approaches to assess progress toward these goals.
One set of principles to define goals for quality and guide assessment of desired behaviors is the multidimensional STEEEP framework. This framework, created by the Institute of Medicine, identifies six domains of quality: Safe, Timely, Effective, Efficient, Equitable, and Patient Centered.2 Briefly, “Safe” means avoiding injuries to patients, “Timely” means reducing waits and delays in care, “Effective” means providing care based on evidence, “Efficient” means avoiding waste, “Equitable” means ensuring quality does not vary based on personal characteristics such as race and gender, and “Patient Centered” means providing care that is responsive to patients’ values and preferences. The STEEEP domains are not coequal; rather, they ensure that quality is considered broadly, while avoiding errors such as measuring only an intervention’s impact on effectiveness but not assessing its impact on multiple domains of quality, such as how patient centered, efficient (cost effective), or equitable the resulting care is.
Based on our review of the literature, a multidimensional framework like STEEEP has not been used in defining and assessing the quality of individual hospitalists’ performance. Some quality metrics at the hospital level impact several dimensions simultaneously, such as door to balloon time for acute myocardial infarction, which measures effectiveness and timeliness of care. Programs like pay-for-performance, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), and the Merit-Based Incentive Payment System (MIPS) have tied reimbursement to assessments aligned with several STEEEP domains at both individual and institutional levels but lack a holistic approach to quality.3-6 The every-other-year State of Hospital Medicine Report, the most widely used description of individual hospitalist performance, reports group-level performance including relative value units and whether groups are accountable for measures of quality such as performance on core measures, timely documentation, and “citizenship” (eg, committee participation or academic work).7 While these are useful benchmarks, the report focuses on performance at the group level. Concurrently, several academic groups have described more complete dashboards or scorecards to assess individual hospitalist performance, primarily designed to facilitate comparison across hospitalist groups or to incentivize overall group performance.8-10 However, these efforts are not guided by an overarching framework and are structured after traditional academic models with components related to teaching and scholarship, which may not translate to nonacademic environments. Finally, the Core Competencies for Hospital Medicine outlines some goals for hospitalist performance but does not speak to specific measurement approaches.11
Overall, assessing individual hospitalist performance is hindered by lack of consensus on important concepts to measure, a limited number of valid measures, and challenges in data collection such as resource limitations and feasibility. Developing and refining measures grounded in the STEEEP framework may provide a more comprehensive assessment of hospitalist quality and identify approaches to improve overall health outcomes. Comparative data could help individual hospitalists improve performance; leaders of hospitalist groups could use this data to guide faculty development and advancement as they ensure quality care at the individual, group, and system levels.
To better inform quality measurement of individual hospitalists, we sought to identify existing publications on individual hospitalist quality. Our goal was to define the published literature about quality measurement at the individual hospitalist level, relate these publications to domains of quality defined by the STEEEP framework, and identify directions for assessment or further research that could affect the overall quality of care.
METHODS
We conducted a scoping review following methods outlined by Arksey and O’Malley12 and Tricco.13 The goal of a scoping review is to map the extent of research within a specific field. This methodology is well suited to characterizing the existing research related to the quality of hospitalist care at the individual level. A protocol for the scoping review was not registered.
Evidence Search
A systematic search for published, English-language literature on hospitalist care was conducted in Medline (Ovid; 1946 - June 4, 2019) on June 5, 2019. The search used a combination of keywords and controlled vocabulary for the concept of hospitalists or hospital medicine. The search strategy used in this review is described in the Appendix. In addition, a hand search of reference lists of articles was used to discover publications not identified in the database searches.
Study Selection
All references were uploaded to Covidence systematic review software (www.covidence.org; Covidence), and duplicates were removed. Four reviewers (A.D., B.C., L.H., R.Q.) conducted title and abstract, as well as full-text, review to identify studies that measured differences in the performance of hospitalists at the individual level. Any disagreements among reviewers were resolved by consensus. Articles included both adult and pediatric populations. Articles that focused on group-level outcomes could be included if nonpooled data at the individual level was also reported. Studies were excluded if they did not focus on individual quality of care indicators or were not published in English.
Data Charting and Synthesis
We extracted the following information using a standardized data collection form: author, title, year of publication, study design, intervention, and outcome measures. Original manuscripts were accessed as needed to supplement analysis. Critical appraisal of individual studies was not conducted in this review because the goal of this review was to analyze which quality indicators have been studied and how they were measured. Articles were then coded for their alignment to the STEEEP framework by two reviewers (AD and BC). After initial coding was conducted, the reviewers met to consolidate codes and resolve any disagreement by consensus. The results of the analysis were summarized in both text and tabular format with studies grouped by focus of assessment with each one’s methods of assessment listed.
RESULTS
Results of the search strategy are shown in the Figure. The search retrieved a total of 2,363 references of which 113 were duplicates, leaving 2,250 to be screened. After title and abstract and full-text screening, 42 studies were included in the review. The final 42 studies were coded for alignment with the STEEEP framework. The Table displays the focus of assessment and methods of assessment within each STEEEP domain.
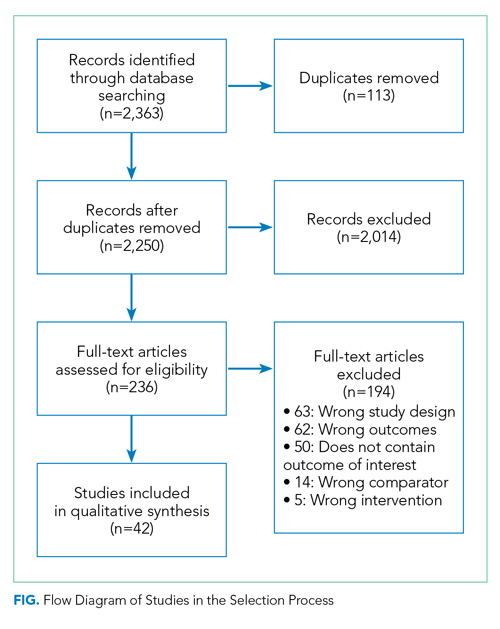
Eighteen studies were coded into a single domain while the rest were coded into at least two domains. The domain Patient Centered was coded as having the most studies (n = 23), followed by the domain of Safe (n = 15). Timely, Effective, and Efficient domains had 11, 9, and 12 studies, respectively. No studies were coded into the domain of Equitable.
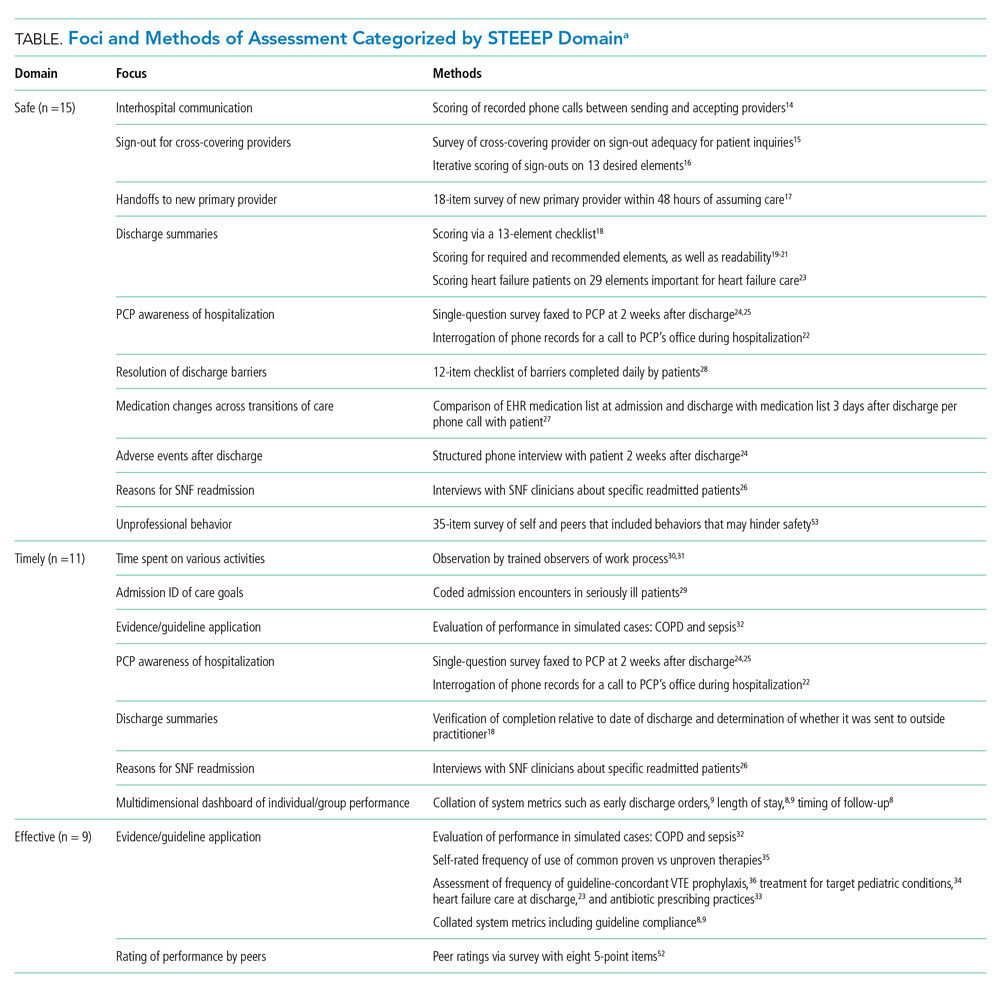
Safe
Nearly all studies coded into the Safe domain focused on transitions of care. These included transfers into a hospital from other hospitals,14 transitions of care to cross-covering providers15,16 and new primary providers,17 and transition out from the acute care setting.18-28 Measures of hospital discharge included measures of both processes18-22 and outcomes.23-27 Methods of assessment varied from use of trained observers or scorers to surveys of individuals and colleagues about performance. Though a few leveraged informatics,22,27 all approaches relied on human interaction, and none were automated.

Timely
All studies coded into the Timely domain were coded into at least one other domain. For example, Anderson et al looked at how hospitalists communicated about potential life-limiting illness at the time of hospital admission and the subsequent effects on plans of care29; this was coded as both Timely and Patient Centered. Likewise, another group of studies centered on application of evidence-based guidelines, such as giving antibiotics within a certain time interval for sepsis and were coded as both Timely and Effective. Another set of authors described dashboards or scorecards that captured a number of group-level metrics of processes of care that span STEEEP domains and may be applicable to individuals, including Fox et al for pediatrics8 and Hwa et al for an adult academic hospitalist group.9 Methods of assessment varied widely across studies and included observations in the clinical environment,28,30,31 performance in simulations,32 and surveys about performance.22-26 A handful of approaches were more automated and made use of informatics8,9,22 or data collected for other health system purposes.8,9
Effective
Effectiveness was most often assessed through adherence to consensus and evidence-based guidelines. Examples included processes of care related to sepsis, venous thromboembolism prophylaxis, COPD, heart failure, pediatric asthma, and antibiotic appropriateness.8,9,23,32-36 Through the review, multiple other studies that included group-level measures of effectiveness for a variety of health conditions were excluded because data on individual-level variation were not reported. Methods of assessment included expert review of cases or discharge summaries, compliance with core measures, performance in simulation, and self-assessment on practice behaviors. Other than those efforts aligned with institutional data collection, most approaches were resource intensive.
Efficient
As with those in the Timely domain, most studies coded into the Efficient domain were coded into at least one other domain. One exception measured unnecessary daily lab work and both showed provider-level variation and demonstrated improvement in quality based on an intervention.37 Another paper coded into the Effective domain evaluated adherence to components of the Choosing Wisely® recommendations.34 In addition to these two studies focusing on cost efficacy, other studies coded to this domain assessed concepts such as ensuring more efficient care from other providers by optimizing transitions of care15-17 and clarifying patients’ goals for care.38 Although integrating insurer information into care plans is emphasized in the Core Competencies of Hospital Medicine,11 this concept was not represented in any of the identified articles. Methods of assessment varied and mostly relied on observation of behaviors or survey of providers. Several approaches were more automated or used Medicare claims data to assess the efficiency of individual providers relative to peers.34,37,39
Equitable
Among the studies reviewed, none were coded into the Equitable domain despite care of vulnerable populations being identified as a core competency of hospital medicine.40
Patient Centered
Studies coded to the Patient Centered domain assessed hospitalist performance through ratings of patient satisfaction,8,9,41-44 rating of communication between hospitalists and patients,19-21,29,45-51 identification of patient preferences,38,52 outcomes of patient-centered care activities,27,28 and peer ratings.53,54 Authors applied several theoretical constructs to these assessments including shared decision-making,50 etiquette-based medicine,47,48 empathetic responsiveness,45 agreement about the goals of care between the patient and healthcare team members,52 and lapses in professionalism.53 Studies often crossed STEEEP domains, such as those assessing quality of discharge information provided to patients, which were coded as both Safe and Patient Centered.19-21 In addition to coded or observed performance in the clinical setting, studies in this domain also used patient ratings as a method of assessment.8,9,28,41-44,49,50 Only a few of these approaches aligned with existing performance measures of health systems and were more automated.8,9
DISCUSSION
This scoping review of performance data for individual hospitalists coded to the STEEEP framework identified robust areas in the published literature, as well as opportunities to develop new approaches or refine existing measures. Transitions of care, both intrahospital and at discharge, and adherence to evidence-based guidelines are areas for which current research has created a foundation for care that is Safe, Timely, Effective, and Efficient. The Patient Centered domain also has several measures described, though the conceptual underpinnings are heterogeneous, and consensus appears necessary to compare performance across groups. No studies were coded to the Equitable domain. Across domains, approaches to measurement varied in resource intensity from simple ones, like integrating existing data collected by hospitals, to more complex ones, like shadowing physicians or coding interactions.
Methods of assessment coded into the Safe domain focused on communication and, less so, patient outcomes around transitions of care. Transitions of care that were evaluated included transfer of patients into a new facility, sign-out to new physicians for both cross-cover responsibilities and for newly assuming the role of primary attending, and discharge from the hospital. Most measures rated the quality of communication, although several23-27 examined patient outcomes. Approaches that survey individuals downstream from a transition of care15,17,24-26 may be the simplest and most feasible approach to implement in the future but, as described to date, do not include all transitions of care and may miss patient outcomes. Important core competencies for hospital medicine under the Safe domain that were not identified in this review include areas such as diagnostic error, hospital-acquired infections, error reporting, and medication safety.11 These are potential areas for future measure development.
The assessments in many studies were coded across more than one domain; for example, measures of the application of evidence-based guidelines were coded into domains of Effective, Timely, Efficient, and others. Applying the six domains of the STEEEP framework revealed the multidimensional outcomes of hospitalist work and could guide more meaningful quality assessments of individual hospitalist performance. For example, assessing adherence to evidence-based guidelines, as well as consideration of the Core Competencies of Hospital Medicine and recommendations of the Choosing Wisely® campaign, are promising areas for measurement and may align with existing hospital metrics. Notably, several reviewed studies measured group-level adherence to guidelines but were excluded because they did not examine variation at the individual level. Future measures based on evidence-based guidelines could center on the Effective domain while also integrating assessment of domains such as Efficient, Timely, and Patient Centered and, in so doing, provide a richer assessment of the diverse aspects of quality.
Several other approaches in the domains of Timely, Effective, and Efficient were described only in a few studies yet deserve consideration for further development. Two time-motion studies30,31 were coded into the domains of Timely and Efficient and would be cumbersome in regular practice but, with advances in wearable technology and electronic health records, could become more feasible in the future. Another approach used Medicare payment data to detect provider-level variation.39 Potentially, “big data” could be analyzed in other ways to compare the performance of individual hospitalists.
The lack of studies coded into the Equitable domain may seem surprising, but the Institute for Healthcare Improvement identifies Equitable as the “forgotten aim” of the STEEEP framework. This organization has developed a guide for health care organizations to promote equitable care.55 While this guide focuses mostly on organizational-level actions, some are focused on individual providers, such as training in implicit bias. Future research should seek to identify disparities in care by individual providers and develop interventions to address any discovered gaps.
The “Patient Centered” domain was the most frequently coded and had the most heterogeneous underpinnings for assessment. Studies varied widely in terminology and conceptual foundations. The field would benefit from future work to identify how “Patient Centered” care might be more clearly conceptualized, guided by comparative studies among different assessment approaches to define those most valid and feasible.
The overarching goal for measuring individual hospitalist quality should be to improve the delivery of patient care in a supportive and formative way. To further this goal, adding or expanding on metrics identified in this article may provide a more complete description of performance. As a future direction, groups should consider partnering with one another to define measurement approaches, collaborate with existing data sources, and even share deidentified individual data to establish performance benchmarks at the individual and group levels.
While this study used broad search terms to support completeness, the search process could have missed important studies. Grey literature, non–English language studies, and industry reports were not included in this review. Groups may also be using other assessments of individual hospitalist performance that are not published in the peer-reviewed literature. Coding of study assessments was achieved through consensus reconciliation; other coders might have classified studies differently.
CONCLUSION
This scoping review describes the peer-reviewed literature of individual hospitalist performance and is the first to link it to the STEEEP quality framework. Assessments of transitions of care, evidence-based care, and cost-effective care are exemplars in the published literature. Patient-centered care is well studied but assessed in a heterogeneous fashion. Assessments of equity in care are notably absent. The STEEEP framework provides a model to structure assessment of individual performance. Future research should build on this framework to define meaningful assessment approaches that are actionable and improve the welfare of our patients and our system.
Disclosures
The authors have nothing to disclose.
1. Quality of Care: A Process for Making Strategic Choices in Health Systems. World Health Organization; 2006.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. Accessed December 20, 2019. http://www.ncbi.nlm.nih.gov/books/NBK222274/
3. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232
4. Kondo KK, Damberg CL, Mendelson A, et al. Implementation processes and pay for performance in healthcare: a systematic review. J Gen Intern Med. 2016;31(Suppl 1):61-69. https://doi.org/10.1007/s11606-015-3567-0
5. Fung CH, Lim Y-W, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148(2):111-123. https://doi.org/10.7326/0003-4819-148-2-200801150-00006
6. Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637-2645. https://doi.org/10.1056/NEJMsa0904859
7. Society of Hospital Medicine. State of Hospital Medicine Report; 2018. Accessed December 20, 2019. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
8. Hwa M, Sharpe BA, Wachter RM. Development and implementation of a balanced scorecard in an academic hospitalist group. J Hosp Med. 2013;8(3):148-153. https://doi.org/10.1002/jhm.2006
9. Fox LA, Walsh KE, Schainker EG. The creation of a pediatric hospital medicine dashboard: performance assessment for improvement. Hosp Pediatr. 2016;6(7):412-419. https://doi.org/10.1542/hpeds.2015-0222
10. Hain PD, Daru J, Robbins E, et al. A proposed dashboard for pediatric hospital medicine groups. Hosp Pediatr. 2012;2(2):59-68. https://doi.org/10.1542/hpeds.2012-0004
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
12. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. https://doi.org/10.1080/1364557032000119616
13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/m18-0850
14. Borofsky JS, Bartsch JC, Howard AB, Repp AB. Quality of interhospital transfer communication practices and association with adverse events on an internal medicine hospitalist service. J Healthc Qual. 2017;39(3):177-185. https://doi.org/10.1097/01.JHQ.0000462682.32512.ad
15. Fogerty RL, Schoenfeld A, Salim Al-Damluji M, Horwitz LI. Effectiveness of written hospitalist sign-outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609-614. https://doi.org10.1002/jhm.2090
16. Miller DM, Schapira MM, Visotcky AM, et al. Changes in written sign-out composition across hospitalization. J Hosp Med. 2015;10(8):534-536. https://doi.org/10.1002/jhm.2390
17. Hinami K, Farnan JM, Meltzer DO, Arora VM. Understanding communication during hospitalist service changes: a mixed methods study. J Hosp Med. 2009;4(9):535-540. https://doi.org/10.1002/jhm.523
18. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. https://doi.org10.1002/jhm.2021
19. Sarzynski E, Hashmi H, Subramanian J, et al. Opportunities to improve clinical summaries for patients at hospital discharge. BMJ Qual Saf. 2017;26(5):372-380. https://doi.org/10.1136/bmjqs-2015-005201
20. Unaka NI, Statile A, Haney J, Beck AF, Brady PW, Jerardi KE. Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(2):98-101. https://doi.org/10.12788/jhm.2688
21. Unaka N, Statile A, Jerardi K, et al. Improving the readability of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(7):551-557. https://doi.org/10.12788/jhm.2770
22. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119
23. Salata BM, Sterling MR, Beecy AN, et al. Discharge processes and 30-day readmission rates of patients hospitalized for heart failure on general medicine and cardiology services. Am J Cardiol. 2018;121(9):1076-1080. https://doi.org/10.1016/j.amjcard.2018.01.027
24. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. https://doi.org/10.1002/jhm.668
25. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. https://doi.org/10.1007/s11606-008-0882-8
26. Clark B, Baron K, Tynan-McKiernan K, Britton M, Minges K, Chaudhry S. Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: a qualitative study. J Hosp Med. 2017;12(8):632-638. https://doi.org/10.12788/jhm.2785
27. Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150-153. https://doi.org/10.1097/PTS.0b013e318286f87d
28. Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set...discharge: patient-reported barriers to discharge readiness at an academic medical center. J Hosp Med. 2016;11(9):610-614. https://doi.org/10.1002/jhm.2591
29. Anderson WG, Kools S, Lyndon A. Dancing around death: hospitalist-patient communication about serious illness. Qual Health Res. 2013;23(1):3-13. https://doi.org/10.1177/1049732312461728
30. Tipping MD, Forth VE, Magill DB, Englert K, Williams MV. Systematic review of time studies evaluating physicians in the hospital setting. J Hosp Med. 2010;5(6):353-359. https://doi.org/10.1002/jhm.647
31. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?--a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. https://doi.org/10.1002/jhm.790
32. Bergmann S, Tran M, Robison K, et al. Standardising hospitalist practice in sepsis and COPD care. BMJ Qual Saf. 2019;28(10):800-808. https://doi.org/10.1136/bmjqs-2018-008829
33. Kisuule F, Wright S, Barreto J, Zenilman J. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med. 2008;3(1):64-70. https://doi.org/10.1002/jhm.278
34. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029
35. Landrigan CP, Conway PH, Stucky ER, et al. Variation in pediatric hospitalists’ use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network. J Hosp Med. 2008;3(4):292-298. https://doi.org/10.1002/jhm.347
36. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboprophylaxis. J Hosp Med. 2015;10(3):172-178. https://doi.org/10.1002/jhm.2303
37. Johnson DP, Lind C, Parker SE, et al. Toward high-value care: a quality improvement initiative to reduce unnecessary repeat complete blood counts and basic metabolic panels on a pediatric hospitalist service. Hosp Pediatr. 2016;6(1):1-8. https://doi.org/10.1542/hpeds.2015-0099
38. Auerbach AD, Katz R, Pantilat SZ, et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437-445. https://doi.org/10.1002/jhm.369
39. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
40. Nichani S, Fitterman N, Lukela M, Crocker J. Equitable allocation of resources. 2017 hospital medicine revised core competencies. J Hosp Med. 2017;12(4):S62. https://doi.org/10.12788/jhm.3016
41. Blanden AR, Rohr RE. Cognitive interview techniques reveal specific behaviors and issues that could affect patient satisfaction relative to hospitalists. J Hosp Med. 2009;4(9):E1-E6. https://doi.org/10.1002/jhm.524
42. Torok H, Ghazarian SR, Kotwal S, Landis R, Wright S, Howell E. Development and validation of the tool to assess inpatient satisfaction with care from hospitalists. J Hosp Med. 2014;9(9):553-558. https://doi.org/10.1002/jhm.2220
43. Torok H, Kotwal S, Landis R, Ozumba U, Howell E, Wright S. Providing feedback on clinical performance to hospitalists: Experience using a new metric tool to assess inpatient satisfaction with care from hospitalists. J Contin Educ Health Prof. 2016;36(1):61-68. https://doi.org/10.1097/CEH.0000000000000060
44. Indovina K, Keniston A, Reid M, et al. Real-time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. https://doi.org/10.1002/jhm.2533
45. Weiss R, Vittinghoff E, Fang MC, et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-810. https://doi.org/10.12788/jhm.2828
46. Apker J, Baker M, Shank S, Hatten K, VanSweden S. Optimizing hospitalist-patient communication: an observation study of medical encounter quality. Jt Comm J Qual Patient Saf. 2018;44(4):196-203. https://doi.org/10.1016/j.jcjq.2017.08.011
47. Kotwal S, Torok H, Khaliq W, Landis R, Howell E, Wright S. Comportment and communication patterns among hospitalist physicians: insight gleaned through observation. South Med J. 2015;108(8):496-501. https://doi.org/10.14423/SMJ.0000000000000328
48. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. https://doi.org/10.1007/s11606-012-2328-6
49. Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527. https://doi.org/10.1002/jhm.787
50. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909
51. Chang D, Mann M, Sommer T, Fallar R, Weinberg A, Friedman E. Using standardized patients to assess hospitalist communication skills. J Hosp Med. 2017;12(7):562-566. https://doi.org/10.12788/jhm.2772
52. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? J Hosp Med. 2016;11(9):615-619. https://doi.org/10.1002/jhm.2569
53. Reddy ST, Iwaz JA, Didwania AK, et al. Participation in unprofessional behaviors among hospitalists: a multicenter study. J Hosp Med. 2012;7(7):543-550. https://doi.org/10.1002/jhm.1946
54. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258. https://doi.org/10.1097/SMJ.0b013e318252d602
55. Wyatt R, Laderman M, Botwinick L, Mate K, Whittington J. Achieving health equity: a guide for health care organizations. IHI White Paper. Institute for Healthcare Improvement; 2016. https://www.ihi.org
Healthcare quality is defined as the extent to which healthcare services result in desired outcomes.1 Quality of care depends on how the healthcare system’s various components, including healthcare practitioners, interact to meet each patient’s needs.2 These components can be shaped to achieve desired outcomes through rules, incentives, and other approaches, but influencing the behaviors of each component, such as the performance of hospitalists, requires defining goals for performance and implementing measurement approaches to assess progress toward these goals.
One set of principles to define goals for quality and guide assessment of desired behaviors is the multidimensional STEEEP framework. This framework, created by the Institute of Medicine, identifies six domains of quality: Safe, Timely, Effective, Efficient, Equitable, and Patient Centered.2 Briefly, “Safe” means avoiding injuries to patients, “Timely” means reducing waits and delays in care, “Effective” means providing care based on evidence, “Efficient” means avoiding waste, “Equitable” means ensuring quality does not vary based on personal characteristics such as race and gender, and “Patient Centered” means providing care that is responsive to patients’ values and preferences. The STEEEP domains are not coequal; rather, they ensure that quality is considered broadly, while avoiding errors such as measuring only an intervention’s impact on effectiveness but not assessing its impact on multiple domains of quality, such as how patient centered, efficient (cost effective), or equitable the resulting care is.
Based on our review of the literature, a multidimensional framework like STEEEP has not been used in defining and assessing the quality of individual hospitalists’ performance. Some quality metrics at the hospital level impact several dimensions simultaneously, such as door to balloon time for acute myocardial infarction, which measures effectiveness and timeliness of care. Programs like pay-for-performance, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), and the Merit-Based Incentive Payment System (MIPS) have tied reimbursement to assessments aligned with several STEEEP domains at both individual and institutional levels but lack a holistic approach to quality.3-6 The every-other-year State of Hospital Medicine Report, the most widely used description of individual hospitalist performance, reports group-level performance including relative value units and whether groups are accountable for measures of quality such as performance on core measures, timely documentation, and “citizenship” (eg, committee participation or academic work).7 While these are useful benchmarks, the report focuses on performance at the group level. Concurrently, several academic groups have described more complete dashboards or scorecards to assess individual hospitalist performance, primarily designed to facilitate comparison across hospitalist groups or to incentivize overall group performance.8-10 However, these efforts are not guided by an overarching framework and are structured after traditional academic models with components related to teaching and scholarship, which may not translate to nonacademic environments. Finally, the Core Competencies for Hospital Medicine outlines some goals for hospitalist performance but does not speak to specific measurement approaches.11
Overall, assessing individual hospitalist performance is hindered by lack of consensus on important concepts to measure, a limited number of valid measures, and challenges in data collection such as resource limitations and feasibility. Developing and refining measures grounded in the STEEEP framework may provide a more comprehensive assessment of hospitalist quality and identify approaches to improve overall health outcomes. Comparative data could help individual hospitalists improve performance; leaders of hospitalist groups could use this data to guide faculty development and advancement as they ensure quality care at the individual, group, and system levels.
To better inform quality measurement of individual hospitalists, we sought to identify existing publications on individual hospitalist quality. Our goal was to define the published literature about quality measurement at the individual hospitalist level, relate these publications to domains of quality defined by the STEEEP framework, and identify directions for assessment or further research that could affect the overall quality of care.
METHODS
We conducted a scoping review following methods outlined by Arksey and O’Malley12 and Tricco.13 The goal of a scoping review is to map the extent of research within a specific field. This methodology is well suited to characterizing the existing research related to the quality of hospitalist care at the individual level. A protocol for the scoping review was not registered.
Evidence Search
A systematic search for published, English-language literature on hospitalist care was conducted in Medline (Ovid; 1946 - June 4, 2019) on June 5, 2019. The search used a combination of keywords and controlled vocabulary for the concept of hospitalists or hospital medicine. The search strategy used in this review is described in the Appendix. In addition, a hand search of reference lists of articles was used to discover publications not identified in the database searches.
Study Selection
All references were uploaded to Covidence systematic review software (www.covidence.org; Covidence), and duplicates were removed. Four reviewers (A.D., B.C., L.H., R.Q.) conducted title and abstract, as well as full-text, review to identify studies that measured differences in the performance of hospitalists at the individual level. Any disagreements among reviewers were resolved by consensus. Articles included both adult and pediatric populations. Articles that focused on group-level outcomes could be included if nonpooled data at the individual level was also reported. Studies were excluded if they did not focus on individual quality of care indicators or were not published in English.
Data Charting and Synthesis
We extracted the following information using a standardized data collection form: author, title, year of publication, study design, intervention, and outcome measures. Original manuscripts were accessed as needed to supplement analysis. Critical appraisal of individual studies was not conducted in this review because the goal of this review was to analyze which quality indicators have been studied and how they were measured. Articles were then coded for their alignment to the STEEEP framework by two reviewers (AD and BC). After initial coding was conducted, the reviewers met to consolidate codes and resolve any disagreement by consensus. The results of the analysis were summarized in both text and tabular format with studies grouped by focus of assessment with each one’s methods of assessment listed.
RESULTS
Results of the search strategy are shown in the Figure. The search retrieved a total of 2,363 references of which 113 were duplicates, leaving 2,250 to be screened. After title and abstract and full-text screening, 42 studies were included in the review. The final 42 studies were coded for alignment with the STEEEP framework. The Table displays the focus of assessment and methods of assessment within each STEEEP domain.

Eighteen studies were coded into a single domain while the rest were coded into at least two domains. The domain Patient Centered was coded as having the most studies (n = 23), followed by the domain of Safe (n = 15). Timely, Effective, and Efficient domains had 11, 9, and 12 studies, respectively. No studies were coded into the domain of Equitable.

Safe
Nearly all studies coded into the Safe domain focused on transitions of care. These included transfers into a hospital from other hospitals,14 transitions of care to cross-covering providers15,16 and new primary providers,17 and transition out from the acute care setting.18-28 Measures of hospital discharge included measures of both processes18-22 and outcomes.23-27 Methods of assessment varied from use of trained observers or scorers to surveys of individuals and colleagues about performance. Though a few leveraged informatics,22,27 all approaches relied on human interaction, and none were automated.

Timely
All studies coded into the Timely domain were coded into at least one other domain. For example, Anderson et al looked at how hospitalists communicated about potential life-limiting illness at the time of hospital admission and the subsequent effects on plans of care29; this was coded as both Timely and Patient Centered. Likewise, another group of studies centered on application of evidence-based guidelines, such as giving antibiotics within a certain time interval for sepsis and were coded as both Timely and Effective. Another set of authors described dashboards or scorecards that captured a number of group-level metrics of processes of care that span STEEEP domains and may be applicable to individuals, including Fox et al for pediatrics8 and Hwa et al for an adult academic hospitalist group.9 Methods of assessment varied widely across studies and included observations in the clinical environment,28,30,31 performance in simulations,32 and surveys about performance.22-26 A handful of approaches were more automated and made use of informatics8,9,22 or data collected for other health system purposes.8,9
Effective
Effectiveness was most often assessed through adherence to consensus and evidence-based guidelines. Examples included processes of care related to sepsis, venous thromboembolism prophylaxis, COPD, heart failure, pediatric asthma, and antibiotic appropriateness.8,9,23,32-36 Through the review, multiple other studies that included group-level measures of effectiveness for a variety of health conditions were excluded because data on individual-level variation were not reported. Methods of assessment included expert review of cases or discharge summaries, compliance with core measures, performance in simulation, and self-assessment on practice behaviors. Other than those efforts aligned with institutional data collection, most approaches were resource intensive.
Efficient
As with those in the Timely domain, most studies coded into the Efficient domain were coded into at least one other domain. One exception measured unnecessary daily lab work and both showed provider-level variation and demonstrated improvement in quality based on an intervention.37 Another paper coded into the Effective domain evaluated adherence to components of the Choosing Wisely® recommendations.34 In addition to these two studies focusing on cost efficacy, other studies coded to this domain assessed concepts such as ensuring more efficient care from other providers by optimizing transitions of care15-17 and clarifying patients’ goals for care.38 Although integrating insurer information into care plans is emphasized in the Core Competencies of Hospital Medicine,11 this concept was not represented in any of the identified articles. Methods of assessment varied and mostly relied on observation of behaviors or survey of providers. Several approaches were more automated or used Medicare claims data to assess the efficiency of individual providers relative to peers.34,37,39
Equitable
Among the studies reviewed, none were coded into the Equitable domain despite care of vulnerable populations being identified as a core competency of hospital medicine.40
Patient Centered
Studies coded to the Patient Centered domain assessed hospitalist performance through ratings of patient satisfaction,8,9,41-44 rating of communication between hospitalists and patients,19-21,29,45-51 identification of patient preferences,38,52 outcomes of patient-centered care activities,27,28 and peer ratings.53,54 Authors applied several theoretical constructs to these assessments including shared decision-making,50 etiquette-based medicine,47,48 empathetic responsiveness,45 agreement about the goals of care between the patient and healthcare team members,52 and lapses in professionalism.53 Studies often crossed STEEEP domains, such as those assessing quality of discharge information provided to patients, which were coded as both Safe and Patient Centered.19-21 In addition to coded or observed performance in the clinical setting, studies in this domain also used patient ratings as a method of assessment.8,9,28,41-44,49,50 Only a few of these approaches aligned with existing performance measures of health systems and were more automated.8,9
DISCUSSION
This scoping review of performance data for individual hospitalists coded to the STEEEP framework identified robust areas in the published literature, as well as opportunities to develop new approaches or refine existing measures. Transitions of care, both intrahospital and at discharge, and adherence to evidence-based guidelines are areas for which current research has created a foundation for care that is Safe, Timely, Effective, and Efficient. The Patient Centered domain also has several measures described, though the conceptual underpinnings are heterogeneous, and consensus appears necessary to compare performance across groups. No studies were coded to the Equitable domain. Across domains, approaches to measurement varied in resource intensity from simple ones, like integrating existing data collected by hospitals, to more complex ones, like shadowing physicians or coding interactions.
Methods of assessment coded into the Safe domain focused on communication and, less so, patient outcomes around transitions of care. Transitions of care that were evaluated included transfer of patients into a new facility, sign-out to new physicians for both cross-cover responsibilities and for newly assuming the role of primary attending, and discharge from the hospital. Most measures rated the quality of communication, although several23-27 examined patient outcomes. Approaches that survey individuals downstream from a transition of care15,17,24-26 may be the simplest and most feasible approach to implement in the future but, as described to date, do not include all transitions of care and may miss patient outcomes. Important core competencies for hospital medicine under the Safe domain that were not identified in this review include areas such as diagnostic error, hospital-acquired infections, error reporting, and medication safety.11 These are potential areas for future measure development.
The assessments in many studies were coded across more than one domain; for example, measures of the application of evidence-based guidelines were coded into domains of Effective, Timely, Efficient, and others. Applying the six domains of the STEEEP framework revealed the multidimensional outcomes of hospitalist work and could guide more meaningful quality assessments of individual hospitalist performance. For example, assessing adherence to evidence-based guidelines, as well as consideration of the Core Competencies of Hospital Medicine and recommendations of the Choosing Wisely® campaign, are promising areas for measurement and may align with existing hospital metrics. Notably, several reviewed studies measured group-level adherence to guidelines but were excluded because they did not examine variation at the individual level. Future measures based on evidence-based guidelines could center on the Effective domain while also integrating assessment of domains such as Efficient, Timely, and Patient Centered and, in so doing, provide a richer assessment of the diverse aspects of quality.
Several other approaches in the domains of Timely, Effective, and Efficient were described only in a few studies yet deserve consideration for further development. Two time-motion studies30,31 were coded into the domains of Timely and Efficient and would be cumbersome in regular practice but, with advances in wearable technology and electronic health records, could become more feasible in the future. Another approach used Medicare payment data to detect provider-level variation.39 Potentially, “big data” could be analyzed in other ways to compare the performance of individual hospitalists.
The lack of studies coded into the Equitable domain may seem surprising, but the Institute for Healthcare Improvement identifies Equitable as the “forgotten aim” of the STEEEP framework. This organization has developed a guide for health care organizations to promote equitable care.55 While this guide focuses mostly on organizational-level actions, some are focused on individual providers, such as training in implicit bias. Future research should seek to identify disparities in care by individual providers and develop interventions to address any discovered gaps.
The “Patient Centered” domain was the most frequently coded and had the most heterogeneous underpinnings for assessment. Studies varied widely in terminology and conceptual foundations. The field would benefit from future work to identify how “Patient Centered” care might be more clearly conceptualized, guided by comparative studies among different assessment approaches to define those most valid and feasible.
The overarching goal for measuring individual hospitalist quality should be to improve the delivery of patient care in a supportive and formative way. To further this goal, adding or expanding on metrics identified in this article may provide a more complete description of performance. As a future direction, groups should consider partnering with one another to define measurement approaches, collaborate with existing data sources, and even share deidentified individual data to establish performance benchmarks at the individual and group levels.
While this study used broad search terms to support completeness, the search process could have missed important studies. Grey literature, non–English language studies, and industry reports were not included in this review. Groups may also be using other assessments of individual hospitalist performance that are not published in the peer-reviewed literature. Coding of study assessments was achieved through consensus reconciliation; other coders might have classified studies differently.
CONCLUSION
This scoping review describes the peer-reviewed literature of individual hospitalist performance and is the first to link it to the STEEEP quality framework. Assessments of transitions of care, evidence-based care, and cost-effective care are exemplars in the published literature. Patient-centered care is well studied but assessed in a heterogeneous fashion. Assessments of equity in care are notably absent. The STEEEP framework provides a model to structure assessment of individual performance. Future research should build on this framework to define meaningful assessment approaches that are actionable and improve the welfare of our patients and our system.
Disclosures
The authors have nothing to disclose.
Healthcare quality is defined as the extent to which healthcare services result in desired outcomes.1 Quality of care depends on how the healthcare system’s various components, including healthcare practitioners, interact to meet each patient’s needs.2 These components can be shaped to achieve desired outcomes through rules, incentives, and other approaches, but influencing the behaviors of each component, such as the performance of hospitalists, requires defining goals for performance and implementing measurement approaches to assess progress toward these goals.
One set of principles to define goals for quality and guide assessment of desired behaviors is the multidimensional STEEEP framework. This framework, created by the Institute of Medicine, identifies six domains of quality: Safe, Timely, Effective, Efficient, Equitable, and Patient Centered.2 Briefly, “Safe” means avoiding injuries to patients, “Timely” means reducing waits and delays in care, “Effective” means providing care based on evidence, “Efficient” means avoiding waste, “Equitable” means ensuring quality does not vary based on personal characteristics such as race and gender, and “Patient Centered” means providing care that is responsive to patients’ values and preferences. The STEEEP domains are not coequal; rather, they ensure that quality is considered broadly, while avoiding errors such as measuring only an intervention’s impact on effectiveness but not assessing its impact on multiple domains of quality, such as how patient centered, efficient (cost effective), or equitable the resulting care is.
Based on our review of the literature, a multidimensional framework like STEEEP has not been used in defining and assessing the quality of individual hospitalists’ performance. Some quality metrics at the hospital level impact several dimensions simultaneously, such as door to balloon time for acute myocardial infarction, which measures effectiveness and timeliness of care. Programs like pay-for-performance, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), and the Merit-Based Incentive Payment System (MIPS) have tied reimbursement to assessments aligned with several STEEEP domains at both individual and institutional levels but lack a holistic approach to quality.3-6 The every-other-year State of Hospital Medicine Report, the most widely used description of individual hospitalist performance, reports group-level performance including relative value units and whether groups are accountable for measures of quality such as performance on core measures, timely documentation, and “citizenship” (eg, committee participation or academic work).7 While these are useful benchmarks, the report focuses on performance at the group level. Concurrently, several academic groups have described more complete dashboards or scorecards to assess individual hospitalist performance, primarily designed to facilitate comparison across hospitalist groups or to incentivize overall group performance.8-10 However, these efforts are not guided by an overarching framework and are structured after traditional academic models with components related to teaching and scholarship, which may not translate to nonacademic environments. Finally, the Core Competencies for Hospital Medicine outlines some goals for hospitalist performance but does not speak to specific measurement approaches.11
Overall, assessing individual hospitalist performance is hindered by lack of consensus on important concepts to measure, a limited number of valid measures, and challenges in data collection such as resource limitations and feasibility. Developing and refining measures grounded in the STEEEP framework may provide a more comprehensive assessment of hospitalist quality and identify approaches to improve overall health outcomes. Comparative data could help individual hospitalists improve performance; leaders of hospitalist groups could use this data to guide faculty development and advancement as they ensure quality care at the individual, group, and system levels.
To better inform quality measurement of individual hospitalists, we sought to identify existing publications on individual hospitalist quality. Our goal was to define the published literature about quality measurement at the individual hospitalist level, relate these publications to domains of quality defined by the STEEEP framework, and identify directions for assessment or further research that could affect the overall quality of care.
METHODS
We conducted a scoping review following methods outlined by Arksey and O’Malley12 and Tricco.13 The goal of a scoping review is to map the extent of research within a specific field. This methodology is well suited to characterizing the existing research related to the quality of hospitalist care at the individual level. A protocol for the scoping review was not registered.
Evidence Search
A systematic search for published, English-language literature on hospitalist care was conducted in Medline (Ovid; 1946 - June 4, 2019) on June 5, 2019. The search used a combination of keywords and controlled vocabulary for the concept of hospitalists or hospital medicine. The search strategy used in this review is described in the Appendix. In addition, a hand search of reference lists of articles was used to discover publications not identified in the database searches.
Study Selection
All references were uploaded to Covidence systematic review software (www.covidence.org; Covidence), and duplicates were removed. Four reviewers (A.D., B.C., L.H., R.Q.) conducted title and abstract, as well as full-text, review to identify studies that measured differences in the performance of hospitalists at the individual level. Any disagreements among reviewers were resolved by consensus. Articles included both adult and pediatric populations. Articles that focused on group-level outcomes could be included if nonpooled data at the individual level was also reported. Studies were excluded if they did not focus on individual quality of care indicators or were not published in English.
Data Charting and Synthesis
We extracted the following information using a standardized data collection form: author, title, year of publication, study design, intervention, and outcome measures. Original manuscripts were accessed as needed to supplement analysis. Critical appraisal of individual studies was not conducted in this review because the goal of this review was to analyze which quality indicators have been studied and how they were measured. Articles were then coded for their alignment to the STEEEP framework by two reviewers (AD and BC). After initial coding was conducted, the reviewers met to consolidate codes and resolve any disagreement by consensus. The results of the analysis were summarized in both text and tabular format with studies grouped by focus of assessment with each one’s methods of assessment listed.
RESULTS
Results of the search strategy are shown in the Figure. The search retrieved a total of 2,363 references of which 113 were duplicates, leaving 2,250 to be screened. After title and abstract and full-text screening, 42 studies were included in the review. The final 42 studies were coded for alignment with the STEEEP framework. The Table displays the focus of assessment and methods of assessment within each STEEEP domain.

Eighteen studies were coded into a single domain while the rest were coded into at least two domains. The domain Patient Centered was coded as having the most studies (n = 23), followed by the domain of Safe (n = 15). Timely, Effective, and Efficient domains had 11, 9, and 12 studies, respectively. No studies were coded into the domain of Equitable.

Safe
Nearly all studies coded into the Safe domain focused on transitions of care. These included transfers into a hospital from other hospitals,14 transitions of care to cross-covering providers15,16 and new primary providers,17 and transition out from the acute care setting.18-28 Measures of hospital discharge included measures of both processes18-22 and outcomes.23-27 Methods of assessment varied from use of trained observers or scorers to surveys of individuals and colleagues about performance. Though a few leveraged informatics,22,27 all approaches relied on human interaction, and none were automated.

Timely
All studies coded into the Timely domain were coded into at least one other domain. For example, Anderson et al looked at how hospitalists communicated about potential life-limiting illness at the time of hospital admission and the subsequent effects on plans of care29; this was coded as both Timely and Patient Centered. Likewise, another group of studies centered on application of evidence-based guidelines, such as giving antibiotics within a certain time interval for sepsis and were coded as both Timely and Effective. Another set of authors described dashboards or scorecards that captured a number of group-level metrics of processes of care that span STEEEP domains and may be applicable to individuals, including Fox et al for pediatrics8 and Hwa et al for an adult academic hospitalist group.9 Methods of assessment varied widely across studies and included observations in the clinical environment,28,30,31 performance in simulations,32 and surveys about performance.22-26 A handful of approaches were more automated and made use of informatics8,9,22 or data collected for other health system purposes.8,9
Effective
Effectiveness was most often assessed through adherence to consensus and evidence-based guidelines. Examples included processes of care related to sepsis, venous thromboembolism prophylaxis, COPD, heart failure, pediatric asthma, and antibiotic appropriateness.8,9,23,32-36 Through the review, multiple other studies that included group-level measures of effectiveness for a variety of health conditions were excluded because data on individual-level variation were not reported. Methods of assessment included expert review of cases or discharge summaries, compliance with core measures, performance in simulation, and self-assessment on practice behaviors. Other than those efforts aligned with institutional data collection, most approaches were resource intensive.
Efficient
As with those in the Timely domain, most studies coded into the Efficient domain were coded into at least one other domain. One exception measured unnecessary daily lab work and both showed provider-level variation and demonstrated improvement in quality based on an intervention.37 Another paper coded into the Effective domain evaluated adherence to components of the Choosing Wisely® recommendations.34 In addition to these two studies focusing on cost efficacy, other studies coded to this domain assessed concepts such as ensuring more efficient care from other providers by optimizing transitions of care15-17 and clarifying patients’ goals for care.38 Although integrating insurer information into care plans is emphasized in the Core Competencies of Hospital Medicine,11 this concept was not represented in any of the identified articles. Methods of assessment varied and mostly relied on observation of behaviors or survey of providers. Several approaches were more automated or used Medicare claims data to assess the efficiency of individual providers relative to peers.34,37,39
Equitable
Among the studies reviewed, none were coded into the Equitable domain despite care of vulnerable populations being identified as a core competency of hospital medicine.40
Patient Centered
Studies coded to the Patient Centered domain assessed hospitalist performance through ratings of patient satisfaction,8,9,41-44 rating of communication between hospitalists and patients,19-21,29,45-51 identification of patient preferences,38,52 outcomes of patient-centered care activities,27,28 and peer ratings.53,54 Authors applied several theoretical constructs to these assessments including shared decision-making,50 etiquette-based medicine,47,48 empathetic responsiveness,45 agreement about the goals of care between the patient and healthcare team members,52 and lapses in professionalism.53 Studies often crossed STEEEP domains, such as those assessing quality of discharge information provided to patients, which were coded as both Safe and Patient Centered.19-21 In addition to coded or observed performance in the clinical setting, studies in this domain also used patient ratings as a method of assessment.8,9,28,41-44,49,50 Only a few of these approaches aligned with existing performance measures of health systems and were more automated.8,9
DISCUSSION
This scoping review of performance data for individual hospitalists coded to the STEEEP framework identified robust areas in the published literature, as well as opportunities to develop new approaches or refine existing measures. Transitions of care, both intrahospital and at discharge, and adherence to evidence-based guidelines are areas for which current research has created a foundation for care that is Safe, Timely, Effective, and Efficient. The Patient Centered domain also has several measures described, though the conceptual underpinnings are heterogeneous, and consensus appears necessary to compare performance across groups. No studies were coded to the Equitable domain. Across domains, approaches to measurement varied in resource intensity from simple ones, like integrating existing data collected by hospitals, to more complex ones, like shadowing physicians or coding interactions.
Methods of assessment coded into the Safe domain focused on communication and, less so, patient outcomes around transitions of care. Transitions of care that were evaluated included transfer of patients into a new facility, sign-out to new physicians for both cross-cover responsibilities and for newly assuming the role of primary attending, and discharge from the hospital. Most measures rated the quality of communication, although several23-27 examined patient outcomes. Approaches that survey individuals downstream from a transition of care15,17,24-26 may be the simplest and most feasible approach to implement in the future but, as described to date, do not include all transitions of care and may miss patient outcomes. Important core competencies for hospital medicine under the Safe domain that were not identified in this review include areas such as diagnostic error, hospital-acquired infections, error reporting, and medication safety.11 These are potential areas for future measure development.
The assessments in many studies were coded across more than one domain; for example, measures of the application of evidence-based guidelines were coded into domains of Effective, Timely, Efficient, and others. Applying the six domains of the STEEEP framework revealed the multidimensional outcomes of hospitalist work and could guide more meaningful quality assessments of individual hospitalist performance. For example, assessing adherence to evidence-based guidelines, as well as consideration of the Core Competencies of Hospital Medicine and recommendations of the Choosing Wisely® campaign, are promising areas for measurement and may align with existing hospital metrics. Notably, several reviewed studies measured group-level adherence to guidelines but were excluded because they did not examine variation at the individual level. Future measures based on evidence-based guidelines could center on the Effective domain while also integrating assessment of domains such as Efficient, Timely, and Patient Centered and, in so doing, provide a richer assessment of the diverse aspects of quality.
Several other approaches in the domains of Timely, Effective, and Efficient were described only in a few studies yet deserve consideration for further development. Two time-motion studies30,31 were coded into the domains of Timely and Efficient and would be cumbersome in regular practice but, with advances in wearable technology and electronic health records, could become more feasible in the future. Another approach used Medicare payment data to detect provider-level variation.39 Potentially, “big data” could be analyzed in other ways to compare the performance of individual hospitalists.
The lack of studies coded into the Equitable domain may seem surprising, but the Institute for Healthcare Improvement identifies Equitable as the “forgotten aim” of the STEEEP framework. This organization has developed a guide for health care organizations to promote equitable care.55 While this guide focuses mostly on organizational-level actions, some are focused on individual providers, such as training in implicit bias. Future research should seek to identify disparities in care by individual providers and develop interventions to address any discovered gaps.
The “Patient Centered” domain was the most frequently coded and had the most heterogeneous underpinnings for assessment. Studies varied widely in terminology and conceptual foundations. The field would benefit from future work to identify how “Patient Centered” care might be more clearly conceptualized, guided by comparative studies among different assessment approaches to define those most valid and feasible.
The overarching goal for measuring individual hospitalist quality should be to improve the delivery of patient care in a supportive and formative way. To further this goal, adding or expanding on metrics identified in this article may provide a more complete description of performance. As a future direction, groups should consider partnering with one another to define measurement approaches, collaborate with existing data sources, and even share deidentified individual data to establish performance benchmarks at the individual and group levels.
While this study used broad search terms to support completeness, the search process could have missed important studies. Grey literature, non–English language studies, and industry reports were not included in this review. Groups may also be using other assessments of individual hospitalist performance that are not published in the peer-reviewed literature. Coding of study assessments was achieved through consensus reconciliation; other coders might have classified studies differently.
CONCLUSION
This scoping review describes the peer-reviewed literature of individual hospitalist performance and is the first to link it to the STEEEP quality framework. Assessments of transitions of care, evidence-based care, and cost-effective care are exemplars in the published literature. Patient-centered care is well studied but assessed in a heterogeneous fashion. Assessments of equity in care are notably absent. The STEEEP framework provides a model to structure assessment of individual performance. Future research should build on this framework to define meaningful assessment approaches that are actionable and improve the welfare of our patients and our system.
Disclosures
The authors have nothing to disclose.
1. Quality of Care: A Process for Making Strategic Choices in Health Systems. World Health Organization; 2006.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. Accessed December 20, 2019. http://www.ncbi.nlm.nih.gov/books/NBK222274/
3. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232
4. Kondo KK, Damberg CL, Mendelson A, et al. Implementation processes and pay for performance in healthcare: a systematic review. J Gen Intern Med. 2016;31(Suppl 1):61-69. https://doi.org/10.1007/s11606-015-3567-0
5. Fung CH, Lim Y-W, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148(2):111-123. https://doi.org/10.7326/0003-4819-148-2-200801150-00006
6. Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637-2645. https://doi.org/10.1056/NEJMsa0904859
7. Society of Hospital Medicine. State of Hospital Medicine Report; 2018. Accessed December 20, 2019. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
8. Hwa M, Sharpe BA, Wachter RM. Development and implementation of a balanced scorecard in an academic hospitalist group. J Hosp Med. 2013;8(3):148-153. https://doi.org/10.1002/jhm.2006
9. Fox LA, Walsh KE, Schainker EG. The creation of a pediatric hospital medicine dashboard: performance assessment for improvement. Hosp Pediatr. 2016;6(7):412-419. https://doi.org/10.1542/hpeds.2015-0222
10. Hain PD, Daru J, Robbins E, et al. A proposed dashboard for pediatric hospital medicine groups. Hosp Pediatr. 2012;2(2):59-68. https://doi.org/10.1542/hpeds.2012-0004
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
12. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. https://doi.org/10.1080/1364557032000119616
13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/m18-0850
14. Borofsky JS, Bartsch JC, Howard AB, Repp AB. Quality of interhospital transfer communication practices and association with adverse events on an internal medicine hospitalist service. J Healthc Qual. 2017;39(3):177-185. https://doi.org/10.1097/01.JHQ.0000462682.32512.ad
15. Fogerty RL, Schoenfeld A, Salim Al-Damluji M, Horwitz LI. Effectiveness of written hospitalist sign-outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609-614. https://doi.org10.1002/jhm.2090
16. Miller DM, Schapira MM, Visotcky AM, et al. Changes in written sign-out composition across hospitalization. J Hosp Med. 2015;10(8):534-536. https://doi.org/10.1002/jhm.2390
17. Hinami K, Farnan JM, Meltzer DO, Arora VM. Understanding communication during hospitalist service changes: a mixed methods study. J Hosp Med. 2009;4(9):535-540. https://doi.org/10.1002/jhm.523
18. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. https://doi.org10.1002/jhm.2021
19. Sarzynski E, Hashmi H, Subramanian J, et al. Opportunities to improve clinical summaries for patients at hospital discharge. BMJ Qual Saf. 2017;26(5):372-380. https://doi.org/10.1136/bmjqs-2015-005201
20. Unaka NI, Statile A, Haney J, Beck AF, Brady PW, Jerardi KE. Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(2):98-101. https://doi.org/10.12788/jhm.2688
21. Unaka N, Statile A, Jerardi K, et al. Improving the readability of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(7):551-557. https://doi.org/10.12788/jhm.2770
22. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119
23. Salata BM, Sterling MR, Beecy AN, et al. Discharge processes and 30-day readmission rates of patients hospitalized for heart failure on general medicine and cardiology services. Am J Cardiol. 2018;121(9):1076-1080. https://doi.org/10.1016/j.amjcard.2018.01.027
24. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. https://doi.org/10.1002/jhm.668
25. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. https://doi.org/10.1007/s11606-008-0882-8
26. Clark B, Baron K, Tynan-McKiernan K, Britton M, Minges K, Chaudhry S. Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: a qualitative study. J Hosp Med. 2017;12(8):632-638. https://doi.org/10.12788/jhm.2785
27. Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150-153. https://doi.org/10.1097/PTS.0b013e318286f87d
28. Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set...discharge: patient-reported barriers to discharge readiness at an academic medical center. J Hosp Med. 2016;11(9):610-614. https://doi.org/10.1002/jhm.2591
29. Anderson WG, Kools S, Lyndon A. Dancing around death: hospitalist-patient communication about serious illness. Qual Health Res. 2013;23(1):3-13. https://doi.org/10.1177/1049732312461728
30. Tipping MD, Forth VE, Magill DB, Englert K, Williams MV. Systematic review of time studies evaluating physicians in the hospital setting. J Hosp Med. 2010;5(6):353-359. https://doi.org/10.1002/jhm.647
31. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?--a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. https://doi.org/10.1002/jhm.790
32. Bergmann S, Tran M, Robison K, et al. Standardising hospitalist practice in sepsis and COPD care. BMJ Qual Saf. 2019;28(10):800-808. https://doi.org/10.1136/bmjqs-2018-008829
33. Kisuule F, Wright S, Barreto J, Zenilman J. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med. 2008;3(1):64-70. https://doi.org/10.1002/jhm.278
34. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029
35. Landrigan CP, Conway PH, Stucky ER, et al. Variation in pediatric hospitalists’ use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network. J Hosp Med. 2008;3(4):292-298. https://doi.org/10.1002/jhm.347
36. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboprophylaxis. J Hosp Med. 2015;10(3):172-178. https://doi.org/10.1002/jhm.2303
37. Johnson DP, Lind C, Parker SE, et al. Toward high-value care: a quality improvement initiative to reduce unnecessary repeat complete blood counts and basic metabolic panels on a pediatric hospitalist service. Hosp Pediatr. 2016;6(1):1-8. https://doi.org/10.1542/hpeds.2015-0099
38. Auerbach AD, Katz R, Pantilat SZ, et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437-445. https://doi.org/10.1002/jhm.369
39. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
40. Nichani S, Fitterman N, Lukela M, Crocker J. Equitable allocation of resources. 2017 hospital medicine revised core competencies. J Hosp Med. 2017;12(4):S62. https://doi.org/10.12788/jhm.3016
41. Blanden AR, Rohr RE. Cognitive interview techniques reveal specific behaviors and issues that could affect patient satisfaction relative to hospitalists. J Hosp Med. 2009;4(9):E1-E6. https://doi.org/10.1002/jhm.524
42. Torok H, Ghazarian SR, Kotwal S, Landis R, Wright S, Howell E. Development and validation of the tool to assess inpatient satisfaction with care from hospitalists. J Hosp Med. 2014;9(9):553-558. https://doi.org/10.1002/jhm.2220
43. Torok H, Kotwal S, Landis R, Ozumba U, Howell E, Wright S. Providing feedback on clinical performance to hospitalists: Experience using a new metric tool to assess inpatient satisfaction with care from hospitalists. J Contin Educ Health Prof. 2016;36(1):61-68. https://doi.org/10.1097/CEH.0000000000000060
44. Indovina K, Keniston A, Reid M, et al. Real-time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. https://doi.org/10.1002/jhm.2533
45. Weiss R, Vittinghoff E, Fang MC, et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-810. https://doi.org/10.12788/jhm.2828
46. Apker J, Baker M, Shank S, Hatten K, VanSweden S. Optimizing hospitalist-patient communication: an observation study of medical encounter quality. Jt Comm J Qual Patient Saf. 2018;44(4):196-203. https://doi.org/10.1016/j.jcjq.2017.08.011
47. Kotwal S, Torok H, Khaliq W, Landis R, Howell E, Wright S. Comportment and communication patterns among hospitalist physicians: insight gleaned through observation. South Med J. 2015;108(8):496-501. https://doi.org/10.14423/SMJ.0000000000000328
48. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. https://doi.org/10.1007/s11606-012-2328-6
49. Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527. https://doi.org/10.1002/jhm.787
50. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909
51. Chang D, Mann M, Sommer T, Fallar R, Weinberg A, Friedman E. Using standardized patients to assess hospitalist communication skills. J Hosp Med. 2017;12(7):562-566. https://doi.org/10.12788/jhm.2772
52. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? J Hosp Med. 2016;11(9):615-619. https://doi.org/10.1002/jhm.2569
53. Reddy ST, Iwaz JA, Didwania AK, et al. Participation in unprofessional behaviors among hospitalists: a multicenter study. J Hosp Med. 2012;7(7):543-550. https://doi.org/10.1002/jhm.1946
54. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258. https://doi.org/10.1097/SMJ.0b013e318252d602
55. Wyatt R, Laderman M, Botwinick L, Mate K, Whittington J. Achieving health equity: a guide for health care organizations. IHI White Paper. Institute for Healthcare Improvement; 2016. https://www.ihi.org
1. Quality of Care: A Process for Making Strategic Choices in Health Systems. World Health Organization; 2006.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. Accessed December 20, 2019. http://www.ncbi.nlm.nih.gov/books/NBK222274/
3. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232
4. Kondo KK, Damberg CL, Mendelson A, et al. Implementation processes and pay for performance in healthcare: a systematic review. J Gen Intern Med. 2016;31(Suppl 1):61-69. https://doi.org/10.1007/s11606-015-3567-0
5. Fung CH, Lim Y-W, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148(2):111-123. https://doi.org/10.7326/0003-4819-148-2-200801150-00006
6. Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637-2645. https://doi.org/10.1056/NEJMsa0904859
7. Society of Hospital Medicine. State of Hospital Medicine Report; 2018. Accessed December 20, 2019. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
8. Hwa M, Sharpe BA, Wachter RM. Development and implementation of a balanced scorecard in an academic hospitalist group. J Hosp Med. 2013;8(3):148-153. https://doi.org/10.1002/jhm.2006
9. Fox LA, Walsh KE, Schainker EG. The creation of a pediatric hospital medicine dashboard: performance assessment for improvement. Hosp Pediatr. 2016;6(7):412-419. https://doi.org/10.1542/hpeds.2015-0222
10. Hain PD, Daru J, Robbins E, et al. A proposed dashboard for pediatric hospital medicine groups. Hosp Pediatr. 2012;2(2):59-68. https://doi.org/10.1542/hpeds.2012-0004
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
12. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. https://doi.org/10.1080/1364557032000119616
13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/m18-0850
14. Borofsky JS, Bartsch JC, Howard AB, Repp AB. Quality of interhospital transfer communication practices and association with adverse events on an internal medicine hospitalist service. J Healthc Qual. 2017;39(3):177-185. https://doi.org/10.1097/01.JHQ.0000462682.32512.ad
15. Fogerty RL, Schoenfeld A, Salim Al-Damluji M, Horwitz LI. Effectiveness of written hospitalist sign-outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609-614. https://doi.org10.1002/jhm.2090
16. Miller DM, Schapira MM, Visotcky AM, et al. Changes in written sign-out composition across hospitalization. J Hosp Med. 2015;10(8):534-536. https://doi.org/10.1002/jhm.2390
17. Hinami K, Farnan JM, Meltzer DO, Arora VM. Understanding communication during hospitalist service changes: a mixed methods study. J Hosp Med. 2009;4(9):535-540. https://doi.org/10.1002/jhm.523
18. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. https://doi.org10.1002/jhm.2021
19. Sarzynski E, Hashmi H, Subramanian J, et al. Opportunities to improve clinical summaries for patients at hospital discharge. BMJ Qual Saf. 2017;26(5):372-380. https://doi.org/10.1136/bmjqs-2015-005201
20. Unaka NI, Statile A, Haney J, Beck AF, Brady PW, Jerardi KE. Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(2):98-101. https://doi.org/10.12788/jhm.2688
21. Unaka N, Statile A, Jerardi K, et al. Improving the readability of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(7):551-557. https://doi.org/10.12788/jhm.2770
22. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119
23. Salata BM, Sterling MR, Beecy AN, et al. Discharge processes and 30-day readmission rates of patients hospitalized for heart failure on general medicine and cardiology services. Am J Cardiol. 2018;121(9):1076-1080. https://doi.org/10.1016/j.amjcard.2018.01.027
24. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. https://doi.org/10.1002/jhm.668
25. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. https://doi.org/10.1007/s11606-008-0882-8
26. Clark B, Baron K, Tynan-McKiernan K, Britton M, Minges K, Chaudhry S. Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: a qualitative study. J Hosp Med. 2017;12(8):632-638. https://doi.org/10.12788/jhm.2785
27. Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150-153. https://doi.org/10.1097/PTS.0b013e318286f87d
28. Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set...discharge: patient-reported barriers to discharge readiness at an academic medical center. J Hosp Med. 2016;11(9):610-614. https://doi.org/10.1002/jhm.2591
29. Anderson WG, Kools S, Lyndon A. Dancing around death: hospitalist-patient communication about serious illness. Qual Health Res. 2013;23(1):3-13. https://doi.org/10.1177/1049732312461728
30. Tipping MD, Forth VE, Magill DB, Englert K, Williams MV. Systematic review of time studies evaluating physicians in the hospital setting. J Hosp Med. 2010;5(6):353-359. https://doi.org/10.1002/jhm.647
31. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?--a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. https://doi.org/10.1002/jhm.790
32. Bergmann S, Tran M, Robison K, et al. Standardising hospitalist practice in sepsis and COPD care. BMJ Qual Saf. 2019;28(10):800-808. https://doi.org/10.1136/bmjqs-2018-008829
33. Kisuule F, Wright S, Barreto J, Zenilman J. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med. 2008;3(1):64-70. https://doi.org/10.1002/jhm.278
34. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029
35. Landrigan CP, Conway PH, Stucky ER, et al. Variation in pediatric hospitalists’ use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network. J Hosp Med. 2008;3(4):292-298. https://doi.org/10.1002/jhm.347
36. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboprophylaxis. J Hosp Med. 2015;10(3):172-178. https://doi.org/10.1002/jhm.2303
37. Johnson DP, Lind C, Parker SE, et al. Toward high-value care: a quality improvement initiative to reduce unnecessary repeat complete blood counts and basic metabolic panels on a pediatric hospitalist service. Hosp Pediatr. 2016;6(1):1-8. https://doi.org/10.1542/hpeds.2015-0099
38. Auerbach AD, Katz R, Pantilat SZ, et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437-445. https://doi.org/10.1002/jhm.369
39. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
40. Nichani S, Fitterman N, Lukela M, Crocker J. Equitable allocation of resources. 2017 hospital medicine revised core competencies. J Hosp Med. 2017;12(4):S62. https://doi.org/10.12788/jhm.3016
41. Blanden AR, Rohr RE. Cognitive interview techniques reveal specific behaviors and issues that could affect patient satisfaction relative to hospitalists. J Hosp Med. 2009;4(9):E1-E6. https://doi.org/10.1002/jhm.524
42. Torok H, Ghazarian SR, Kotwal S, Landis R, Wright S, Howell E. Development and validation of the tool to assess inpatient satisfaction with care from hospitalists. J Hosp Med. 2014;9(9):553-558. https://doi.org/10.1002/jhm.2220
43. Torok H, Kotwal S, Landis R, Ozumba U, Howell E, Wright S. Providing feedback on clinical performance to hospitalists: Experience using a new metric tool to assess inpatient satisfaction with care from hospitalists. J Contin Educ Health Prof. 2016;36(1):61-68. https://doi.org/10.1097/CEH.0000000000000060
44. Indovina K, Keniston A, Reid M, et al. Real-time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. https://doi.org/10.1002/jhm.2533
45. Weiss R, Vittinghoff E, Fang MC, et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-810. https://doi.org/10.12788/jhm.2828
46. Apker J, Baker M, Shank S, Hatten K, VanSweden S. Optimizing hospitalist-patient communication: an observation study of medical encounter quality. Jt Comm J Qual Patient Saf. 2018;44(4):196-203. https://doi.org/10.1016/j.jcjq.2017.08.011
47. Kotwal S, Torok H, Khaliq W, Landis R, Howell E, Wright S. Comportment and communication patterns among hospitalist physicians: insight gleaned through observation. South Med J. 2015;108(8):496-501. https://doi.org/10.14423/SMJ.0000000000000328
48. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. https://doi.org/10.1007/s11606-012-2328-6
49. Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527. https://doi.org/10.1002/jhm.787
50. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909
51. Chang D, Mann M, Sommer T, Fallar R, Weinberg A, Friedman E. Using standardized patients to assess hospitalist communication skills. J Hosp Med. 2017;12(7):562-566. https://doi.org/10.12788/jhm.2772
52. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? J Hosp Med. 2016;11(9):615-619. https://doi.org/10.1002/jhm.2569
53. Reddy ST, Iwaz JA, Didwania AK, et al. Participation in unprofessional behaviors among hospitalists: a multicenter study. J Hosp Med. 2012;7(7):543-550. https://doi.org/10.1002/jhm.1946
54. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258. https://doi.org/10.1097/SMJ.0b013e318252d602
55. Wyatt R, Laderman M, Botwinick L, Mate K, Whittington J. Achieving health equity: a guide for health care organizations. IHI White Paper. Institute for Healthcare Improvement; 2016. https://www.ihi.org
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routine Correction of Elevated INR and Thrombocytopenia Prior to Paracentesis in Patients with Cirrhosis

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
© 2020 Society of Hospital Medicine