User login
Glucosuria Is Not Always Due to Diabetes
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
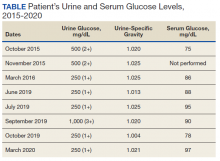
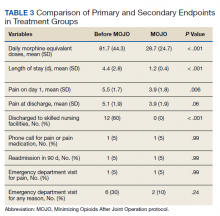
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
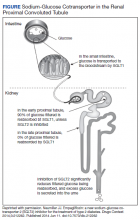
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Management of Do Not Resuscitate Orders Before Invasive Procedures
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
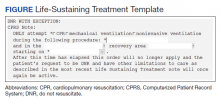
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
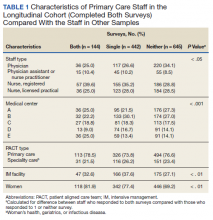
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
Can Using an Intensive Management Program Improve Primary Care Staff Experiences With Caring for High-Risk Patients?
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
Minimizing Opioids After Joint Operation: Protocol to Decrease Postoperative Opioid Use After Primary Total Knee Arthroplasty
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
Trends in Risk-Adjusted 28-Day Mortality Rates for Patients Hospitalized with COVID-19 in England
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 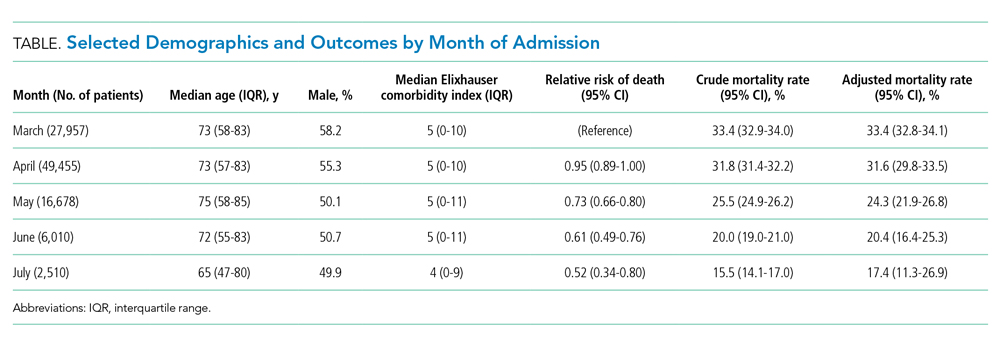
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.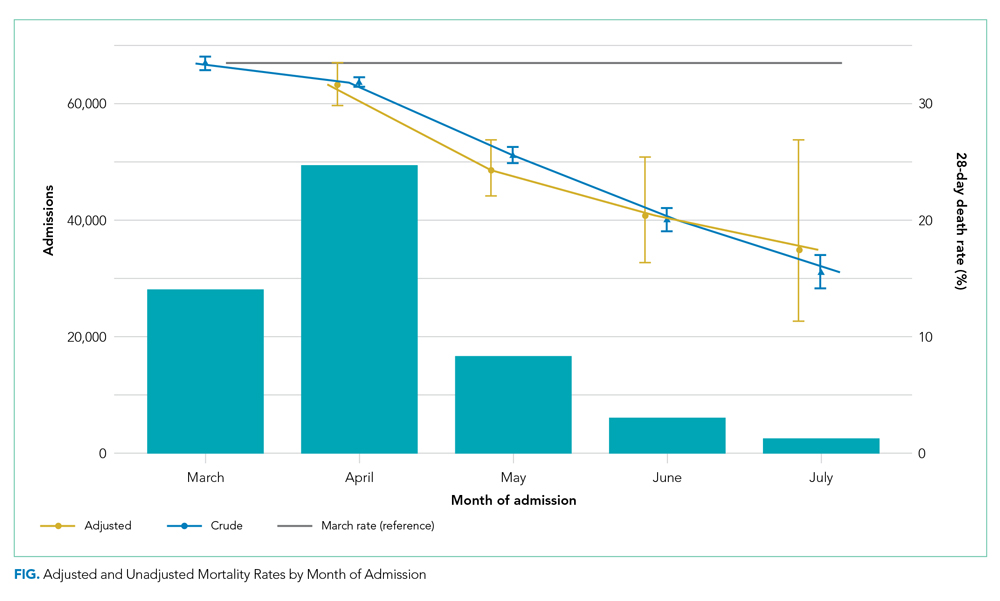
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
© 2021 Society of Hospital Medicine
New Author Guidelines for Addressing Race and Racism in the Journal of Hospital Medicine
We are committed to using our platform at the Journal of Hospital Medicine (JHM) to address inequities in healthcare delivery, policy, and research. Race was conceived as a mechanism of social division, leading to the false belief, propagated over time, of race as a biological variable.1 As a result, racism has contributed to the medical abuse and exploitation of Black and Brown communities and inequities in health status among racialized groups. We must abandon practices that perpetuate inequities and champion practices that resolve them. Racial health equity—the absence of unjust and avoidable health disparities among racialized groups—is unattainable if we continue to simply identify inequities without naming racism as a determinant of health. As a journal, our responsibility is to disseminate evidence-based manuscripts that reflect an understanding of race, racism, and health.
We have modified our author guidelines. First, we now require authors to clearly define race and provide justification for its inclusion in clinical case descriptions and study analyses. We aim to contribute to the necessary course correction as well as promote self-reflection on study design choices that propagate false notions of race as a biological concept and conclusions that reinforce race-based rather than race-conscious practices in medicine.2 Second, we expect authors to explicitly name racism and make a concerted effort to explore its role, identify its specific forms, and examine mutually reinforcing mechanisms of inequity that potentially contributed to study findings. Finally, we instruct authors to avoid the use of phrases like “patient mistrust,” which places blame for inequities on patients and their families and decouples mistrust from the fraught history of racism in medicine.
We must also acknowledge and reflect on our previous contributions to such inequity as authors, reviewers, and editors in order to learn and grow. Among the more than 2,000 articles published in JHM since its inception, only four included the term “racism.” Three of these articles are perspectives published in June 2020 and beyond. The only original research manuscript that directly addressed racism was a qualitative study of adults with sickle cell disease.3 The authors described study participants’ perspectives: “In contrast, the hospital experience during adulthood was often punctuated by bitter relationships with staff, and distrust over possible excessive use of opioids. Moreover, participants raised the possibility of racism in their interactions with hospital staff.” In this example, patients called out racism and its impact on their experience. We know JHM is not alone in falling woefully short in advancing our understanding of racism and racial health inequities. Each of us should identify missed opportunities to call out racism as a driver of racial health disparities in our own publications. We must act on these lessons regarding the ways in which racism infiltrates scientific publishing. We must use this awareness, along with our influence, voice, and collective power, to enact change for the betterment of our patients, their families, and the medical community.
We at JHM will contribute to uncovering and disseminating solutions to health inequities that result from racism. We are grateful to Boyd et al for their call to action and for providing a blueprint for improvement to those of us who write, review, and publish scholarly work.4
1. Roberts D. Fatal Invention: How Science, Politics, and Big Business Re-Create Race in the Twenty-First Century. 2nd ed. The New Press; 2012.
2. Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet. 2020;396:1125-1128. https://doi:10.1016/S0140-6736(20)32076-6
3. Weisberg D, Balf-Soran G, Becker W, et al. “I’m talking about pain”: sickle cell disease patients with extremely high hospital use. J Hosp Med. 2013;8:42-46. https://doi:10.1002/jhm.1987
4. Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs Blog. July 2, 2020. Accessed January 22, 2021. https://doi:10.1377/hblog20200630.939347 https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/
We are committed to using our platform at the Journal of Hospital Medicine (JHM) to address inequities in healthcare delivery, policy, and research. Race was conceived as a mechanism of social division, leading to the false belief, propagated over time, of race as a biological variable.1 As a result, racism has contributed to the medical abuse and exploitation of Black and Brown communities and inequities in health status among racialized groups. We must abandon practices that perpetuate inequities and champion practices that resolve them. Racial health equity—the absence of unjust and avoidable health disparities among racialized groups—is unattainable if we continue to simply identify inequities without naming racism as a determinant of health. As a journal, our responsibility is to disseminate evidence-based manuscripts that reflect an understanding of race, racism, and health.
We have modified our author guidelines. First, we now require authors to clearly define race and provide justification for its inclusion in clinical case descriptions and study analyses. We aim to contribute to the necessary course correction as well as promote self-reflection on study design choices that propagate false notions of race as a biological concept and conclusions that reinforce race-based rather than race-conscious practices in medicine.2 Second, we expect authors to explicitly name racism and make a concerted effort to explore its role, identify its specific forms, and examine mutually reinforcing mechanisms of inequity that potentially contributed to study findings. Finally, we instruct authors to avoid the use of phrases like “patient mistrust,” which places blame for inequities on patients and their families and decouples mistrust from the fraught history of racism in medicine.
We must also acknowledge and reflect on our previous contributions to such inequity as authors, reviewers, and editors in order to learn and grow. Among the more than 2,000 articles published in JHM since its inception, only four included the term “racism.” Three of these articles are perspectives published in June 2020 and beyond. The only original research manuscript that directly addressed racism was a qualitative study of adults with sickle cell disease.3 The authors described study participants’ perspectives: “In contrast, the hospital experience during adulthood was often punctuated by bitter relationships with staff, and distrust over possible excessive use of opioids. Moreover, participants raised the possibility of racism in their interactions with hospital staff.” In this example, patients called out racism and its impact on their experience. We know JHM is not alone in falling woefully short in advancing our understanding of racism and racial health inequities. Each of us should identify missed opportunities to call out racism as a driver of racial health disparities in our own publications. We must act on these lessons regarding the ways in which racism infiltrates scientific publishing. We must use this awareness, along with our influence, voice, and collective power, to enact change for the betterment of our patients, their families, and the medical community.
We at JHM will contribute to uncovering and disseminating solutions to health inequities that result from racism. We are grateful to Boyd et al for their call to action and for providing a blueprint for improvement to those of us who write, review, and publish scholarly work.4
We are committed to using our platform at the Journal of Hospital Medicine (JHM) to address inequities in healthcare delivery, policy, and research. Race was conceived as a mechanism of social division, leading to the false belief, propagated over time, of race as a biological variable.1 As a result, racism has contributed to the medical abuse and exploitation of Black and Brown communities and inequities in health status among racialized groups. We must abandon practices that perpetuate inequities and champion practices that resolve them. Racial health equity—the absence of unjust and avoidable health disparities among racialized groups—is unattainable if we continue to simply identify inequities without naming racism as a determinant of health. As a journal, our responsibility is to disseminate evidence-based manuscripts that reflect an understanding of race, racism, and health.
We have modified our author guidelines. First, we now require authors to clearly define race and provide justification for its inclusion in clinical case descriptions and study analyses. We aim to contribute to the necessary course correction as well as promote self-reflection on study design choices that propagate false notions of race as a biological concept and conclusions that reinforce race-based rather than race-conscious practices in medicine.2 Second, we expect authors to explicitly name racism and make a concerted effort to explore its role, identify its specific forms, and examine mutually reinforcing mechanisms of inequity that potentially contributed to study findings. Finally, we instruct authors to avoid the use of phrases like “patient mistrust,” which places blame for inequities on patients and their families and decouples mistrust from the fraught history of racism in medicine.
We must also acknowledge and reflect on our previous contributions to such inequity as authors, reviewers, and editors in order to learn and grow. Among the more than 2,000 articles published in JHM since its inception, only four included the term “racism.” Three of these articles are perspectives published in June 2020 and beyond. The only original research manuscript that directly addressed racism was a qualitative study of adults with sickle cell disease.3 The authors described study participants’ perspectives: “In contrast, the hospital experience during adulthood was often punctuated by bitter relationships with staff, and distrust over possible excessive use of opioids. Moreover, participants raised the possibility of racism in their interactions with hospital staff.” In this example, patients called out racism and its impact on their experience. We know JHM is not alone in falling woefully short in advancing our understanding of racism and racial health inequities. Each of us should identify missed opportunities to call out racism as a driver of racial health disparities in our own publications. We must act on these lessons regarding the ways in which racism infiltrates scientific publishing. We must use this awareness, along with our influence, voice, and collective power, to enact change for the betterment of our patients, their families, and the medical community.
We at JHM will contribute to uncovering and disseminating solutions to health inequities that result from racism. We are grateful to Boyd et al for their call to action and for providing a blueprint for improvement to those of us who write, review, and publish scholarly work.4
1. Roberts D. Fatal Invention: How Science, Politics, and Big Business Re-Create Race in the Twenty-First Century. 2nd ed. The New Press; 2012.
2. Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet. 2020;396:1125-1128. https://doi:10.1016/S0140-6736(20)32076-6
3. Weisberg D, Balf-Soran G, Becker W, et al. “I’m talking about pain”: sickle cell disease patients with extremely high hospital use. J Hosp Med. 2013;8:42-46. https://doi:10.1002/jhm.1987
4. Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs Blog. July 2, 2020. Accessed January 22, 2021. https://doi:10.1377/hblog20200630.939347 https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/
1. Roberts D. Fatal Invention: How Science, Politics, and Big Business Re-Create Race in the Twenty-First Century. 2nd ed. The New Press; 2012.
2. Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet. 2020;396:1125-1128. https://doi:10.1016/S0140-6736(20)32076-6
3. Weisberg D, Balf-Soran G, Becker W, et al. “I’m talking about pain”: sickle cell disease patients with extremely high hospital use. J Hosp Med. 2013;8:42-46. https://doi:10.1002/jhm.1987
4. Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs Blog. July 2, 2020. Accessed January 22, 2021. https://doi:10.1377/hblog20200630.939347 https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/
© 2021 Society of Hospital Medicine
A Preoperative Transthoracic Echocardiography Protocol to Reduce Time to Hip Fracture Surgery
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
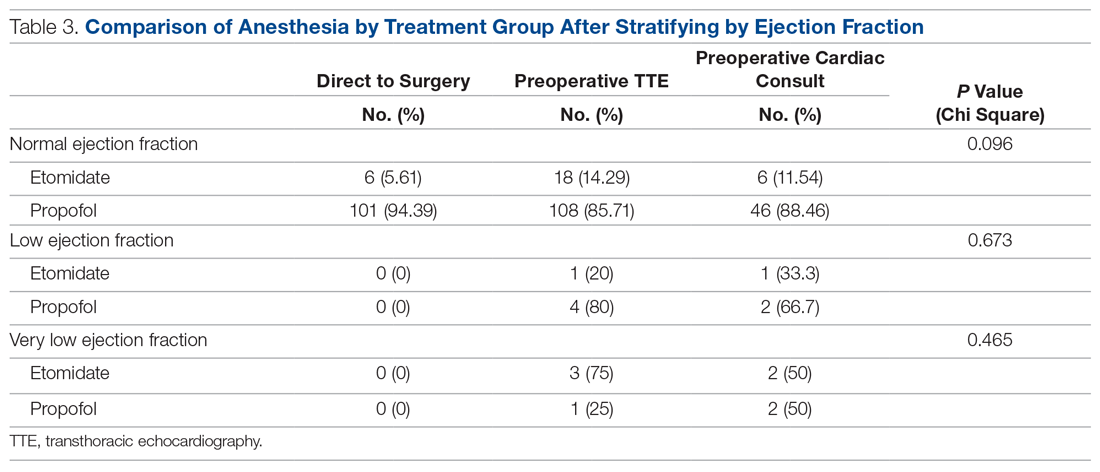
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
A Multi-Membership Approach for Attributing Patient-Level Outcomes to Providers in an Inpatient Setting
From Banner Health Corporation, Phoenix, AZ.
Background: Health care providers are routinely incentivized with pay-for-performance (P4P) metrics to increase the quality of care. In an inpatient setting, P4P models typically measure quality by attributing each patient’s outcome to a single provider even though many providers routinely care for the patient. This study investigates a new attribution approach aiming to distribute each outcome across all providers who provided care.
Methods: The methodology relies on a multi-membership model and is demonstrated in the Banner Health system using 3 clinical outcome measures (length of stay, 30-day readmissions, and mortality) and responses to 3 survey questions that measure a patient’s perception of their care. The new approach is compared to the “standard” method, which attributes each patient to only 1 provider.
Results: When ranking by clinical outcomes, both methods were concordant 72.1% to 82.1% of the time for top-half/bottom-half rankings, with a median percentile difference between 7 and 15. When ranking by survey scores, there was more agreement, with concordance between 84.1% and 86.6% and a median percentile difference between 11 and 13. Last, Pearson correlation coefficients of the paired percentiles ranged from 0.56 to 0.78.
Conclusion: The new approach provides a fairer solution when measuring provider performance.
Keywords: patient attribution; PAMM; PAPR; random effect model; pay for performance.
Providers practicing in hospitals are routinely evaluated based on their performance and, in many cases, are financially incentivized for a better-than-average performance within a pay-for-performance (P4P) model. The use of P4P models is based on the belief that they will “improve, motivate, and enhance providers to pursue aggressively and ultimately achieve the quality performance targets thus decreasing the number of medical errors with less malpractice events.”1 Although P4P models continue to be a movement in health care, they have been challenging to implement.
One concern involves the general quality of implementation, such as defining metrics and targets, setting payout amounts, managing technology and market conditions, and gauging the level of transparency to the provider.2 Another challenge, and the focus of this project, are concerns around measuring performance to avoid perceptions of unfairness. This concern can be minimized if the attribution is handled in a fairer way, by spreading it across all providers who affected the outcome, both in a positive or negative direction.3
To implement these models, the performance of providers needs to be measured and tracked periodically. This requires linking, or attributing, a patient’s outcome to a provider, which is almost always the attending or discharging provider (ie, a single provider).3 In this single-provider attribution approach, one provider will receive all the credit (good or bad) for their respective patients’ outcomes, even though the provider may have seen the patient only a fraction of the time during the hospitalization. Attributing outcomes—for example, length of stay (LOS), readmission rate, mortality rate, net promoter score (NPS)—using this approach reduces the validity of metrics designed to measure provider performance, especially in a rotating provider environment where many providers interact with and care for a patient. For example, the quality of providers’ interpersonal skills and competence were among the strongest determinants of patient satisfaction,4 but it is not credible that this is solely based on the last provider during a hospitalization.
Proportionally distributing the attribution of an outcome has been used successfully in other contexts. Typically, a statistical modeling approach using a multi-membership framework is used because it can handle the sometimes-complicated relationships within the hierarchy. It also allows for auxiliary variables to be introduced, which can help explain and control for exogenous effects.5-7 For example, in the education setting, standardized testing is administered to students at defined years of schooling: at grades 4, 8, and 10, for instance. The progress of students, measured as the academic gains between test years, are proportionally attributed to all the teachers who the student has had between the test years. These partial attributions are combined to evaluate an overall teacher performance.8,9
Although the multi-membership framework has been used in other industries, it has yet to be applied in measuring provider performance. The purpose of this project is to investigate the impact of using a multi-provider approach compared to the standard single-provider approach. The findings may lead to modifications in the way a provider’s performance is measured and, thus, how providers are compensated. A similar study investigated the impact of proportionally distributing patients’ outcomes across all rotating providers using a weighting method based on billing practices to measure the partial impact of each provider.3
This study is different in 2 fundamental ways. First, attribution is weighted based on the number of clinically documented interactions (via clinical notes) between a patient and all rotating providers during the hospitalization. Second, performance is measured via multi-membership models, which can estimate the effect (both positive and negative) that a provider has on an outcome, even when caring for a patient a fraction of the time during the hospitalization.
Methods
Setting
Banner Health is a non-profit, multi-hospital health care system across 6 states in the western United States that is uniquely positioned to study provider quality attribution models. It not only has a large number of providers and serves a broad patient population, but Banner Health also uses an instance of Cerner (Kansas City, MO), an enterprise-level electronic health record (EHR) system that connects all its facilities and allows for advanced analytics across its system.
For this study, we included only general medicine and surgery patients admitted and discharged from the inpatient setting between January 1, 2018, and December 31, 2018, who were between 18 and 89 years old at admission, and who had a LOS between 1 and 14 days. Visit- and patient-level data were collected from Cerner, while outcome data, and corresponding expected outcome data, were obtained from Premier, Inc. (Charlotte, NC) using their CareScience methodologies.10 To measure patient experience, response data were extracted from post-discharge surveys administered by InMoment (Salt Lake City, UT).
Provider Attribution Models
Provider Attribution by Physician of Record (PAPR). In the standard approach, denoted here as the PAPR model, 1 provider—typically the attending or discharging provider, which may be the same person—is attributed to the entire hospitalization. This provider is responsible for the patient’s care, and all patient outcomes are aggregated and attributed to the provider to gauge his or her performance. The PAPR model is the most popular form of attribution across many health care systems and is routinely used for P4P incentives.
In this study, the discharging provider was used when attributing hospitalizations using the PAPR model. Providers responsible for fewer than 12 discharges in the calendar year were excluded. Because of the directness of this type of attribution, the performance of 1 provider does not account for the performance of the other rotating providers during hospitalizations.
Provider Attribution by Multiple Membership (PAMM). In contrast, we introduce another attribution approach here that is designed to assign partial attribution to each provider who cares for the patient during the hospitalization. To aggregate the partial attributions, and possibly control for any exogenous or risk-based factors, a multiple-membership, or multi-member (MM), model is used. The MM model can measure the effect of a provider on an outcome even when the patient-to-provider relationship is complex, such as in a rotating provider environment.8
The purpose of this study is to compare attribution models and to determine whether there are meaningful differences between them. Therefore, for comparison purposes, the same discharging providers using the PAPR approach are eligible for the PAMM approach, so that both attribution models are using the same set of providers. All other providers are excluded because their performance would not be comparable to the PAPR approach.
While there are many ways to document provider-to-patient interactions, 2 methods are available in almost all health care systems. The first method is to link a provider’s billing charges to each patient-day combination. This approach limits the attribution to 1 provider per patient per day because multiple rotating providers cannot charge for the same patient-day combination.3 However, many providers interact with a patient on the same day, so using this approach excludes non-billed provider-to-patient interactions.
The second method, which was used in this study, relies on documented clinical notes within the EHR to determine how attribution is shared. In this approach, attribution is weighted based on the authorship of 3 types of eligible clinical notes: admitting history/physical notes (during admission), progress notes (during subsequent days), and discharge summary notes (during final discharge). This will (likely) result in many providers being linked to a patient on each day, which better reflects the clinical setting (Figure). Recently, clinical notes were used to attribute care of patients in an inpatient setting, and it was found that this approach provides a reliable way of tracking interactions and assigning ownership.11
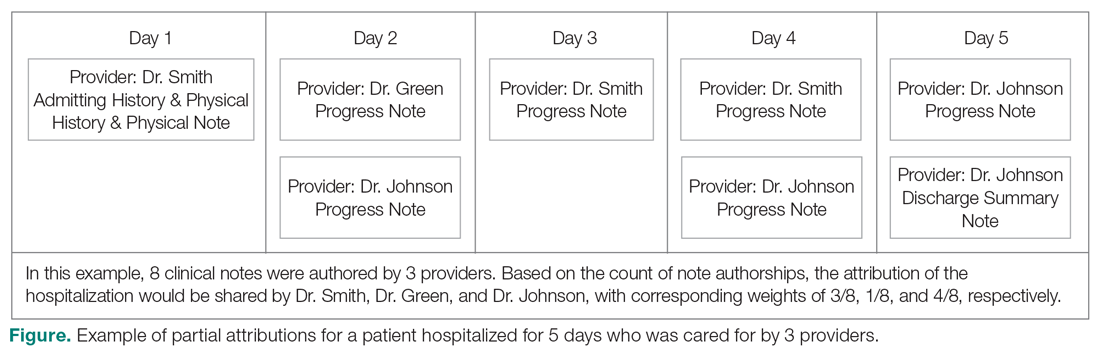
The provider-level attribution weights are based on the share of authorships of eligible note types. Specifically, for each provider j, let aij be the total count of eligible note types for hospitalization i authored by provider j, and let ai be the overall total count of eligible note types for hospitalization i. Then the attribution weight is
(Eq. 1)
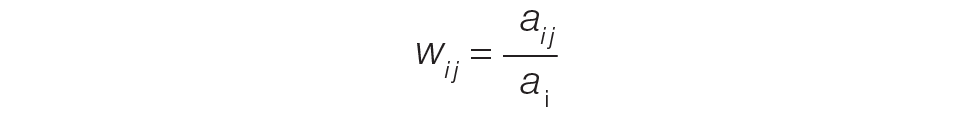
for hospitalization i and provider j. Note that ∑jwij = 1: in other words, the total attribution, summed across all providers, is constrained to be 1 for each hospitalization.
Patient Outcomes
Outcomes were chosen based on their routine use in health care systems as standards when evaluating provider performance. This study included 6 outcomes: inpatient LOS, inpatient mortality, 30-day inpatient readmission, and patient responses from 3 survey questions. These outcomes can be collected without any manual chart reviews, and therefore are viewed as objective outcomes of provider performance.
Each outcome was aggregated for each provider using both attribution methods independently. For the PAPR method, observed-to-expected (OE) indices for LOS, mortality, and readmissions were calculated along with average patient survey scores. For the PAMM method, provider-level random effects from the fitted models were used. In both cases, the calculated measures were used for ranking purposes when determining top (or bottom) providers for each outcome.
Individual Provider Metrics for the PAPR Method
Inpatient LOS Index. Hospital inpatient LOS was measured as the number of days between admission date and discharge date. For each hospital visit, an expected LOS was determined using Premier’s CareScience Analytics (CSA) risk-adjustment methodology.10 The CSA methodology for LOS incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let oij and eij be the observed and expected LOS, respectively, for hospitalization i = 1,…,nj attributed to provider j. Then the inpatient LOS index for provider j is Lj = ∑ioij⁄∑ieij.
Inpatient Mortality Index. Inpatient mortality was defined as the death of the patient during hospitalization. For each hospitalization, an expected mortality probability was determined using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for mortality incorporates a patient’s demographics and comorbidities.
Just as before, let nj be the number of hospitalizations attributed to provider j. Let mij = 1 if the patient died during hospitalization i = 1, … , nj attributed to provider j; mij = 0 otherwise. Let pij(m) be the corresponding expected mortality probability. Then the inpatient mortality index for provider j is Mj = ∑imij⁄∑ipij(m).
30-Day Inpatient Readmission Index. A 30-day inpatient readmission was defined as the event when a patient is discharged and readmits back into the inpatient setting within 30 days. The inclusion criteria defined by the Centers for Medicare and Medicaid Services (CMS) all-cause hospital-wide readmission measure was used and, consequently, planned readmissions were excluded.12 Readmissions could occur at any Banner hospital, including the same hospital. For each hospital visit, an expected readmission probability was derived using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for readmissions incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let rij = 1 if the patient had a readmission following hospitalization i = 1, … , nj attributed to provider j; rij = 0 otherwise. Let pij(r) be the corresponding expected readmission probability. Then the 30-day inpatient readmission index for provider j is Rj = ∑irij ⁄∑ipij(r).
Patient Survey Scores. The satisfaction of the patient’s experience during hospitalization was measured via post-discharge surveys administered by InMoment. Two survey questions were selected because they related directly to a provider’s interaction with the patient: “My interactions with doctors were excellent” (Doctor) and “I received the best possible care” (Care). A third question, “I would recommend this hospital to my family and friends,” was selected as a proxy measure of the overall experience and, in the aggregate, is referred to as the net promoter score (NPS).13,14 The responses were measured on an 11-point Likert scale, ranging from “Strongly Disagree” (0) to “Strongly Agree” (10); “N/A” or missing responses were excluded.
The Likert responses were coded to 3 discrete values as follows: if the value was between 0 and 6, then -1 (ie, detractor); between 7 and 8 (ie, neutral), then 0; otherwise 1 (ie, promoter). Averaging these coded responses results in a patient survey score for each question. Specifically, let nj be the number of hospitalizations attributed to provider j in which the patient responded to the survey question. Let sij ∈{−1, 0, 1} be the coded response linked to hospitalization i = 1, … , nj attributed to provider j. Then the patient experience score for provider j is Sj = ∑isij⁄nj.
Handling Ties in Provider Performance Measures. Because ties can occur in the PAPR approach for all measures, a tie-breaking strategy is needed. For LOS indices, ties are less likely because their numerator is strictly greater than 0, and expected LOS values are typically distinct enough. Indeed, no ties were found in this study for LOS indices. However, mortality and readmission indices can routinely result in ties when the best possible index is achieved, such as 0 deaths or readmissions among attributed hospitalizations. To help differentiate between those indices in the PAPR approach, the total estimated risk (denominator) was utilized as a secondary scoring criterion.
Mortality and readmission metrics were addressed by sorting first by the outcome (mortality index), and second by the denominator (total estimated risk). For example, if provider A has the same mortality rate as provider B, then provider A would be ranked higher if the denominator was larger, indicating a higher risk for mortality.
Similarly, it was very common for providers to have the same overall average rating for a survey question. Therefore, the denominator (number of respondents) was used to break ties. However, the denominator sorting was bidirectional. For example, if the tied score was positive (more promoters than detractors) for providers A and B, then provider A would be ranked higher if the denominator was larger. Conversely, if the tied score between providers A and B was neutral or negative (more detractors than promoters), then provider A would be ranked lower if the denominator was larger.
Individual Provider Metrics for the PAMM Method
For the PAMM method, model-based metrics were derived using a MM model.8 Specifically, let J be the number of rotating providers in a health care system. Let Yi be an outcome of interest from hospitalization i, X1i, …, Xpi be fixed effects or covariates, and ß1, …, ßp be the coefficients for the respective covariates. Then the generalized MM statistical model is
(Eq. 2)
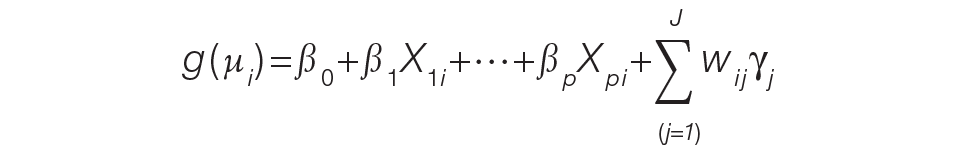
where g(μi ) is a link function between the mean of the outcome, μi, and its linear predictor, ß0, is the marginal intercept, wij represents the attribution weight of provider j on hospitalization i (described in Equation 1), and γj represents the random effect of provider j on the outcome with γj~N(0,σγ2).
For the mortality and readmission binary outcomes, logistic regression was performed using a logit link function, with the corresponding expected probability as the only fixed covariate. The expected probabilities were first converted into odds and then log-transformed before entering the model. For LOS, Poisson regression was performed using a log link function with the log-transformed expected LOS as the only fixed covariate. For coded patient experience responses, an ordered logistic regression was performed using a cumulative logit link function (no fixed effects were added).
MM Model-based Metrics. Each fitted MM model produces a predicted random effect for each provider. The provider-specific random effects can be interpreted as the unobserved influence of each provider on the outcome after controlling for any fixed effect included in the model. Therefore, the provider-specific random effects were used to evaluate the relative provider performance, which is analogous to the individual provider-level metrics used in the PAPR method.
Measuring provider performance using a MM model is more flexible and robust to outliers compared to the standard approach using OE indices or simple averages. First, although not investigated here, the effect of patient-, visit-, provider-, and/or temporal-level covariates can be controlled when evaluating provider performance. For example, a patient’s socioeconomic status, a provider’s workload, and seasonal factors can be added to the MM model. These external factors are not accounted for in OE indices.
Another advantage of using predicted random effects is the concept of “shrinkage.” The process of estimating random effects inherently accounts for small sample sizes (when providers do not treat a large enough sample of patients) and/or when there is a large ratio of patient variance to provider variance (for instance, when patient outcome variability is much higher compared to provider performance variability). In both cases, the estimation of the random effect is pulled ever closer to 0, signaling that the provider performance is closer to the population average. See Henderson15 and Mood16 for further details.
In contrast, OE indices can result in unreliable estimates when a provider has not cared for many patients. This is especially prevalent when the outcome is binary with a low probability of occurring, such as mortality. Indeed, provider-level mortality OE indices are routinely 0 when the patient counts are low, which skews performance rankings unfairly. Finally, OE indices also ignore the magnitude of the variance of an outcome between providers and patients, which can be large.
Comparison Methodology
In this study, we seek to compare the 2 methods of attribution, PAPR and PAMM, to determine whether there are meaningful differences between them when measuring provider performance. Using retrospective data described in the next section, each attribution method was used independently to derive provider-level metrics. To assess relative performance, percentiles were assigned to each provider based on their metric values so that, in the end, there were 2 percentile ranks for each provider for each metric.
Using these paired percentiles, we derived the following measures of concordance, similar to Herzke, Michtalik3: (1) the percent concordance measure—defined as the number of providers who landed in the top half (greater than the median) or bottom half under both attribution models—divided by the total number of providers; (2) the median of the absolute difference in percentiles under both attribution models; and (3) the Pearson correlation coefficient of the paired provider ranks. The first measure is a global measure of concordance between the 2 approaches and would be expected to be 50% by chance. The second measure gauges how an individual provider’s rank is affected by the change in attribution methodologies. The third measure is a statistical measure of linear correlation of the paired percentiles and was not included in the Herzke, Michtalik3 study.
All statistical analyses were performed on SAS (version 9.4; Cary, NC) and the MM models were fitted using PROC GLIMMIX with the EFFECT statement. The Banner Health Institutional Review Board approved this study.
Results
Descriptive Statistics
A total of
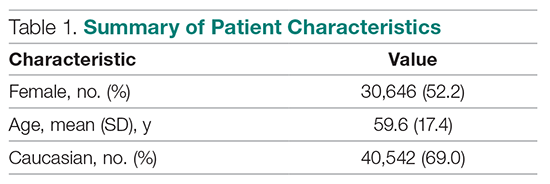
Multi-Membership Model Results
Table 3 displays the results after independently fitting MM models to each of the 3 clinical outcomes. Along with a marginal intercept, the only covariate in each model was the corresponding expected value after a transformation. This was added to use the same information that is typically used in OE indices, therefore allowing for a proper comparison between the 2 attribution methods. The provider-level variance represents the between-provider variation and measures the amount of influence providers have on the corresponding outcome after controlling for any covariates in the model. A provider-level variance of 0 would indicate that providers do not have any influence on the outcome. While the mortality and readmission model results can be compared to each other, the LOS model cannot given its different scale and transformation altogether.
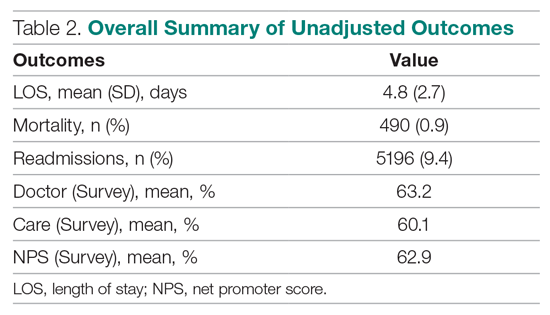
The results in Table 3 suggest that each expected value covariate is highly correlated with its corresponding outcome, which is the anticipated conclusion given that they are constructed in this fashion. The estimated provider-level variances indicate that, after including an expected value in the model, providers have less of an influence on a patient’s LOS and likelihood of being readmitted. On the other hand, the results suggest that providers have much more influence on the likelihood of a patient dying in the hospital, even after controlling for an expected mortality covariate.
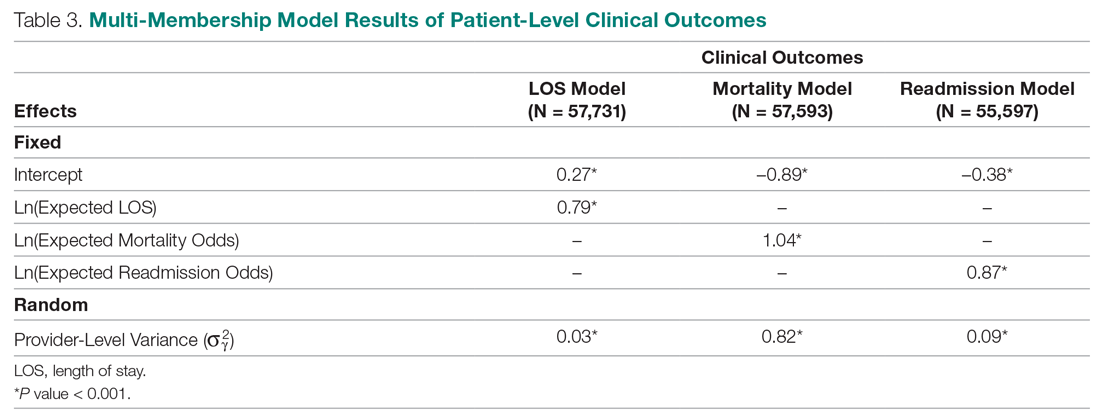
Table 4 shows the results after independently fitting MM-ordered logistic models to each of the 3 survey questions. The similar provider-level variances suggest that providers have the same influence on the patient’s perception of the quality of their interactions with the doctor (Doctor), the quality of the care they received (Care), and their likelihood to recommend a friend or family member to the hospital (NPS).
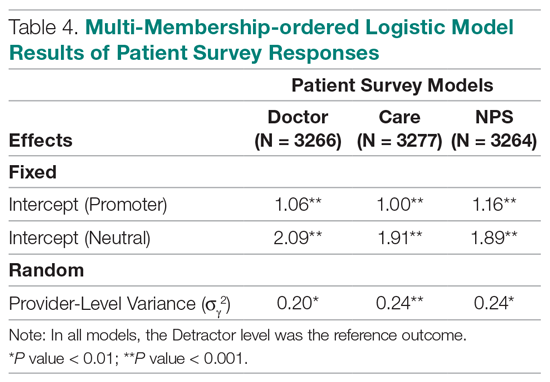
Comparison Results Between Both Attribution Methods
Table 5 compares the 2 attribution methods when ranking providers based on their performance on each outcome measure. The comparison metrics gauge how well the 2 methods agree overall (percent concordance), agree at the provider level (absolute percentile difference and interquartile range [IQR]), and how the paired percentiles linearly correlate to each other (Pearson correlation coefficient).
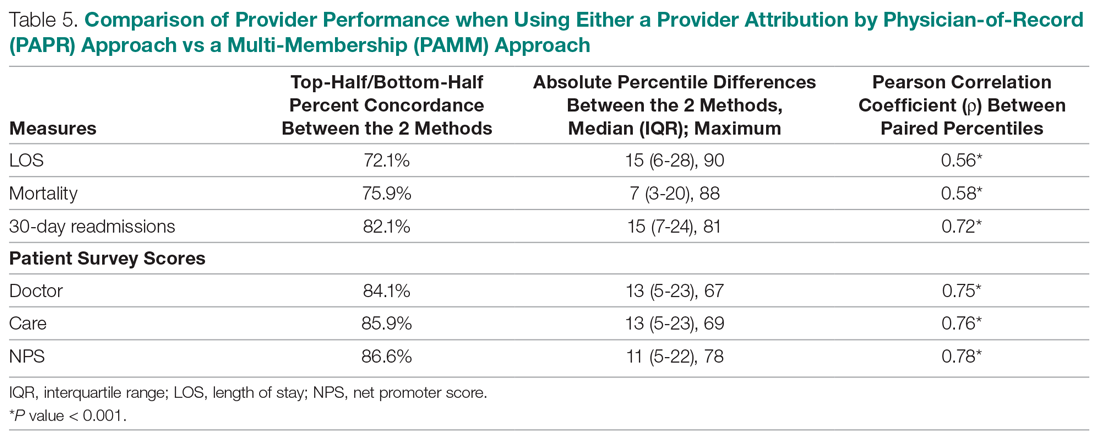
LOS, by a small margin, had the lowest concordance of clinical outcomes (72.1%), followed by mortality (75.9%) and readmissions (82.1%). Generally, the survey scores had higher percent concordance than the clinical outcome measures, with Doctor at 84.1%, Care at 85.9%, and NPS having the highest percent concordance at 86.6%. Given that by chance the percent concordance is expected to be 50%, there was notable discordance, especially with the clinical outcome measures. Using LOS performance as an example, one attribution methodology would rank a provider in the top half or bottom half, while the other attribution methodology would rank the same provider exactly the opposite way about 28% of the time.
The median absolute percentile difference between the 2 methods was more modest (between 7 and 15). Still, there were some providers whose performance ranking was heavily impacted by the attribution methodology that was used. This was especially true when evaluating performance for certain clinical measures, where the attribution method that was used could change the provider performance percentile by up to 90 levels.
The paired percentiles were positively correlated when ranking performance using any of the 6 measures. This suggests that both methodologies assess performance generally in the same direction, irrespective of the methodology and measure. We did not investigate more complex correlation measures and left this for future research.
It should be noted that ties occurred much more frequently with the PAPR method than when using PAMM and therefore required tie-breaking rules to be designed. Given the nature of OE indices, PAPR methodology is especially sensitive to ties whenever the measure includes counting the number of events (for example, mortality and readmissions) and whenever there are many providers with very few attributed patients. On the other hand, using the PAMM method is much more robust against ties given that the summation of all the weighted attributed outcomes will rarely result in ties, even with a nominal set of providers.
Discussion
In this study, the PAMM methodology was introduced and was used to assess relative provider performance on 3 clinical outcome measures and 3 patient survey scores. The new approach aims to distribute each outcome among all providers who provided care for a patient in an inpatient setting. Clinical notes were used to account for patient-to-provider interactions, and fitted MM statistical models were used to compute the effects that each provider had on each outcome. The provider effect was introduced as a random effect, and the set of predicted random effects was used to rank the performance of each provider.
The PAMM approach was compared to the more traditional methodology, PAPR, where each patient is attributed to only 1 provider: the discharging physician in this study. Using this approach, OE indices of clinical outcomes and averages of survey scores were used to rank the performance of each provider. This approach resulted in many ties, which were broken based on the number of hospitalizations, although other tie-breaking methods may be used in practice.
Both methodologies showed modest concordance with each other for the clinical outcomes, but higher concordance for the patient survey scores. This was also true when using the Pearson correlation coefficient to assess agreement. The 1 outcome measure that showed the least concordance and least linear correlation between methods was LOS, which would suggest that LOS performance is more sensitive to the attribution methodology that is used. However, it was the least concordant by a small margin.
Furthermore, although the medians of the absolute percentile differences were small, there were some providers who had large deviations, suggesting that some providers would move from being shown as high-performers to low-performers and vice versa based on the chosen attribution method. We investigated examples of this and determined that the root cause was the difference in effective sample sizes for a provider. For the PAPR method, the effective sample size is simply the number of hospitalizations attributed to the provider. For the PAMM method, the effective sample size is the sum of all non-zero weights across all hospitalizations where the provider cared for a patient. By and large, the PAMM methodology provides more information of the provider effect on an outcome than the PAPR approach because every provider-patient interaction is considered. For example, providers who do not routinely discharge patients, but often care for patients, will have rankings that differ dramatically between the 2 methods.
The PAMM methodology has many statistical advantages that were not fully utilized in this comparative study. For example, we did not include any covariates in the MM models except for the expected value of the outcome, when it was available. Still, it is known that other covariates can impact an outcome as well, such as the patient’s age, socioeconomic indicators, existing chronic conditions, and severity of hospitalization, which can be added to the MM models as fixed effects. In this way, the PAMM approach can control for these other covariates, which are typically outside of the control of providers but typically ignored using OE indices. Therefore, using the PAMM approach would provide a fairer comparison of provider performance.
Using the PAMM method, most providers had a large sample size to assess their performance once all the weighted interactions were included. Still, there were a few who did not care for many patients for a variety of reasons. In these scenarios, MM models “borrow” strength from other providers to produce a more robust predicted provider effect by using a weighted average between the overall population trend and the specific provider outcomes (see Rao and Molina17). As a result, PAMM is a more suitable approach when the sample sizes of patients attributed to providers can be small.
One of the most interesting findings of this study was the relative size of the provider-level variance to the size of the fixed effect in each model (Table 3). Except for mortality, these variances suggest that there is a small difference in performance from one provider to another. However, these should be interpreted as the variance when only 1 provider is involved in the care of a patient. When multiple providers are involved, using basic statistical theory, the overall provider-level variance will be σγ2 ∑wij2 (see Equation 2). For example, the estimated variance among providers for LOS was 0.03 (on a log scale), but, using the scenario in the Figure, the overall provider-level variance for this hospitalization will be 0.03 (0.3752 + 0.1252 + 0.52) = 0.012. Hence, the combined effect of providers on LOS is less than would be expected. Indeed, as more providers are involved with a patient’s care, the more their combined influence on an outcome is diluted.
In this study, the PAMM approach placed an equal weight on all provider-patient interactions via clinical note authorship, but that may not be optimal in some settings. For example, it may make more sense to set a higher weight on the provider who admitted or discharged the patient while placing less (or 0) weight on all other interactions. In the extreme, if the full weight were placed on 1 provider interaction (eg, during discharge, then the MM model would be reduced to a one-way random effects model. The flexibility of weighting interactions is a feature of the PAMM approach, but any weighting framework must be transparent to the providers before implementation.
Conclusion
This study demonstrates that the PAMM approach is a feasible option within a large health care organization. For P4P programs to be successful, providers must be able to trust that their performance will be fairly assessed and that all provider-patient interactions are captured to provide a full comparison amongst their peers. The PAMM methodology is one solution to spread the positive (and negative) outcomes across all providers who cared for a patient and therefore, if implemented, would add trust and fairness when measuring and assessing provider performance.
Acknowledgments: The authors thank Barrie Bradley for his support in the initial stages of this research and Dr. Syed Ismail Jafri for his help and support on the standard approaches of assessing and measuring provider performances.
Corresponding author: Rachel Ginn, MS, Banner Health Corporation, 2901 N. Central Ave., Phoenix, AZ 85012; [email protected].
Financial disclosures: None.
1. Abduljawad A, Al-Assaf AF. Incentives for better performance in health care. Sultan Qaboos Univ Med J. 2011;11:201-206.
2. Milstein R, Schreyoegg J. Pay for performance in the inpatient sector: a review of 34 P4P programs in 14 OECD countries. Health Policy. 2016;120:1125-1140.
3. Herzke CA, Michtalik HJ, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. 2018;13:470-475.
4. Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health. 2017;137:89-101.
5. Ballou D, Sanders W, Wright P. Controlling for student background in value-added assessment of teachers. J Educ Behav Stat. 2004;29:37-65.
6. Hill PW, Goldstein H. Multilevel modeling of educational data with cross-classification and missing identification for units. J Educ Behav Stat. 1998;23:117-128.
7. Rasbash J, Browne WJ. Handbook of Multilevel Analysis. Springer; 2007.
8. Brown WJ, Goldstein H, Rasbash J. Multiple membership multiple classification (MMMC) models. Statistical Modeling. 2001;1:103-124.
9. Sanders WL, Horn SP. The Tennessee Value-Added Assessment System (TVAAS)—mixed-model methodology in educational assessment. J Pers Eval Educ. 1994;8:299-311.
10. Kroch EA, Duan M. CareScience Risk Assessment Model: Hospital Performance Measurement. Premier, Inc., 2008. http://www.ahrq.gov/qual/mortality/KrochRisk.htm
11. Schumacher DJ, Wu DTY, Meganathan K, et al. A feasibility study to attribute patients to primary interns on inpatient ward teams using electronic health record data. Acad Med. 2019;94:1376-1383.
12. Simoes J, Krumholz HM, Lin Z. Hospital-level 30-day risk-standardized readmission measure. Centers for Medicare & Medicaid Services, 2018. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Hospital-Wide-All-Cause-Readmission-Updates.zip
13. Krol MW, de Boer D, Delnoij DM, Rademakers JJDJM. The Net Promoter Score: an asset to patient experience surveys? Health Expect. 2015;18:3099-3109.
14. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3:e001570.
15. Henderson CR. Sire evaluation and genetic trends. J Anim Sci. 1973;1973:10-41.
16. Mood AM. Introduction to the Theory of Statistics. McGraw-Hill; 1950:xiii, 433-xiii.
17. Rao JNK, Molina I. Small Area Estimation. Wiley; 2015.
From Banner Health Corporation, Phoenix, AZ.
Background: Health care providers are routinely incentivized with pay-for-performance (P4P) metrics to increase the quality of care. In an inpatient setting, P4P models typically measure quality by attributing each patient’s outcome to a single provider even though many providers routinely care for the patient. This study investigates a new attribution approach aiming to distribute each outcome across all providers who provided care.
Methods: The methodology relies on a multi-membership model and is demonstrated in the Banner Health system using 3 clinical outcome measures (length of stay, 30-day readmissions, and mortality) and responses to 3 survey questions that measure a patient’s perception of their care. The new approach is compared to the “standard” method, which attributes each patient to only 1 provider.
Results: When ranking by clinical outcomes, both methods were concordant 72.1% to 82.1% of the time for top-half/bottom-half rankings, with a median percentile difference between 7 and 15. When ranking by survey scores, there was more agreement, with concordance between 84.1% and 86.6% and a median percentile difference between 11 and 13. Last, Pearson correlation coefficients of the paired percentiles ranged from 0.56 to 0.78.
Conclusion: The new approach provides a fairer solution when measuring provider performance.
Keywords: patient attribution; PAMM; PAPR; random effect model; pay for performance.
Providers practicing in hospitals are routinely evaluated based on their performance and, in many cases, are financially incentivized for a better-than-average performance within a pay-for-performance (P4P) model. The use of P4P models is based on the belief that they will “improve, motivate, and enhance providers to pursue aggressively and ultimately achieve the quality performance targets thus decreasing the number of medical errors with less malpractice events.”1 Although P4P models continue to be a movement in health care, they have been challenging to implement.
One concern involves the general quality of implementation, such as defining metrics and targets, setting payout amounts, managing technology and market conditions, and gauging the level of transparency to the provider.2 Another challenge, and the focus of this project, are concerns around measuring performance to avoid perceptions of unfairness. This concern can be minimized if the attribution is handled in a fairer way, by spreading it across all providers who affected the outcome, both in a positive or negative direction.3
To implement these models, the performance of providers needs to be measured and tracked periodically. This requires linking, or attributing, a patient’s outcome to a provider, which is almost always the attending or discharging provider (ie, a single provider).3 In this single-provider attribution approach, one provider will receive all the credit (good or bad) for their respective patients’ outcomes, even though the provider may have seen the patient only a fraction of the time during the hospitalization. Attributing outcomes—for example, length of stay (LOS), readmission rate, mortality rate, net promoter score (NPS)—using this approach reduces the validity of metrics designed to measure provider performance, especially in a rotating provider environment where many providers interact with and care for a patient. For example, the quality of providers’ interpersonal skills and competence were among the strongest determinants of patient satisfaction,4 but it is not credible that this is solely based on the last provider during a hospitalization.
Proportionally distributing the attribution of an outcome has been used successfully in other contexts. Typically, a statistical modeling approach using a multi-membership framework is used because it can handle the sometimes-complicated relationships within the hierarchy. It also allows for auxiliary variables to be introduced, which can help explain and control for exogenous effects.5-7 For example, in the education setting, standardized testing is administered to students at defined years of schooling: at grades 4, 8, and 10, for instance. The progress of students, measured as the academic gains between test years, are proportionally attributed to all the teachers who the student has had between the test years. These partial attributions are combined to evaluate an overall teacher performance.8,9
Although the multi-membership framework has been used in other industries, it has yet to be applied in measuring provider performance. The purpose of this project is to investigate the impact of using a multi-provider approach compared to the standard single-provider approach. The findings may lead to modifications in the way a provider’s performance is measured and, thus, how providers are compensated. A similar study investigated the impact of proportionally distributing patients’ outcomes across all rotating providers using a weighting method based on billing practices to measure the partial impact of each provider.3
This study is different in 2 fundamental ways. First, attribution is weighted based on the number of clinically documented interactions (via clinical notes) between a patient and all rotating providers during the hospitalization. Second, performance is measured via multi-membership models, which can estimate the effect (both positive and negative) that a provider has on an outcome, even when caring for a patient a fraction of the time during the hospitalization.
Methods
Setting
Banner Health is a non-profit, multi-hospital health care system across 6 states in the western United States that is uniquely positioned to study provider quality attribution models. It not only has a large number of providers and serves a broad patient population, but Banner Health also uses an instance of Cerner (Kansas City, MO), an enterprise-level electronic health record (EHR) system that connects all its facilities and allows for advanced analytics across its system.
For this study, we included only general medicine and surgery patients admitted and discharged from the inpatient setting between January 1, 2018, and December 31, 2018, who were between 18 and 89 years old at admission, and who had a LOS between 1 and 14 days. Visit- and patient-level data were collected from Cerner, while outcome data, and corresponding expected outcome data, were obtained from Premier, Inc. (Charlotte, NC) using their CareScience methodologies.10 To measure patient experience, response data were extracted from post-discharge surveys administered by InMoment (Salt Lake City, UT).
Provider Attribution Models
Provider Attribution by Physician of Record (PAPR). In the standard approach, denoted here as the PAPR model, 1 provider—typically the attending or discharging provider, which may be the same person—is attributed to the entire hospitalization. This provider is responsible for the patient’s care, and all patient outcomes are aggregated and attributed to the provider to gauge his or her performance. The PAPR model is the most popular form of attribution across many health care systems and is routinely used for P4P incentives.
In this study, the discharging provider was used when attributing hospitalizations using the PAPR model. Providers responsible for fewer than 12 discharges in the calendar year were excluded. Because of the directness of this type of attribution, the performance of 1 provider does not account for the performance of the other rotating providers during hospitalizations.
Provider Attribution by Multiple Membership (PAMM). In contrast, we introduce another attribution approach here that is designed to assign partial attribution to each provider who cares for the patient during the hospitalization. To aggregate the partial attributions, and possibly control for any exogenous or risk-based factors, a multiple-membership, or multi-member (MM), model is used. The MM model can measure the effect of a provider on an outcome even when the patient-to-provider relationship is complex, such as in a rotating provider environment.8
The purpose of this study is to compare attribution models and to determine whether there are meaningful differences between them. Therefore, for comparison purposes, the same discharging providers using the PAPR approach are eligible for the PAMM approach, so that both attribution models are using the same set of providers. All other providers are excluded because their performance would not be comparable to the PAPR approach.
While there are many ways to document provider-to-patient interactions, 2 methods are available in almost all health care systems. The first method is to link a provider’s billing charges to each patient-day combination. This approach limits the attribution to 1 provider per patient per day because multiple rotating providers cannot charge for the same patient-day combination.3 However, many providers interact with a patient on the same day, so using this approach excludes non-billed provider-to-patient interactions.
The second method, which was used in this study, relies on documented clinical notes within the EHR to determine how attribution is shared. In this approach, attribution is weighted based on the authorship of 3 types of eligible clinical notes: admitting history/physical notes (during admission), progress notes (during subsequent days), and discharge summary notes (during final discharge). This will (likely) result in many providers being linked to a patient on each day, which better reflects the clinical setting (Figure). Recently, clinical notes were used to attribute care of patients in an inpatient setting, and it was found that this approach provides a reliable way of tracking interactions and assigning ownership.11

The provider-level attribution weights are based on the share of authorships of eligible note types. Specifically, for each provider j, let aij be the total count of eligible note types for hospitalization i authored by provider j, and let ai be the overall total count of eligible note types for hospitalization i. Then the attribution weight is
(Eq. 1)
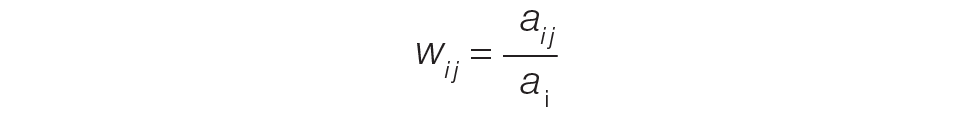
for hospitalization i and provider j. Note that ∑jwij = 1: in other words, the total attribution, summed across all providers, is constrained to be 1 for each hospitalization.
Patient Outcomes
Outcomes were chosen based on their routine use in health care systems as standards when evaluating provider performance. This study included 6 outcomes: inpatient LOS, inpatient mortality, 30-day inpatient readmission, and patient responses from 3 survey questions. These outcomes can be collected without any manual chart reviews, and therefore are viewed as objective outcomes of provider performance.
Each outcome was aggregated for each provider using both attribution methods independently. For the PAPR method, observed-to-expected (OE) indices for LOS, mortality, and readmissions were calculated along with average patient survey scores. For the PAMM method, provider-level random effects from the fitted models were used. In both cases, the calculated measures were used for ranking purposes when determining top (or bottom) providers for each outcome.
Individual Provider Metrics for the PAPR Method
Inpatient LOS Index. Hospital inpatient LOS was measured as the number of days between admission date and discharge date. For each hospital visit, an expected LOS was determined using Premier’s CareScience Analytics (CSA) risk-adjustment methodology.10 The CSA methodology for LOS incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let oij and eij be the observed and expected LOS, respectively, for hospitalization i = 1,…,nj attributed to provider j. Then the inpatient LOS index for provider j is Lj = ∑ioij⁄∑ieij.
Inpatient Mortality Index. Inpatient mortality was defined as the death of the patient during hospitalization. For each hospitalization, an expected mortality probability was determined using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for mortality incorporates a patient’s demographics and comorbidities.
Just as before, let nj be the number of hospitalizations attributed to provider j. Let mij = 1 if the patient died during hospitalization i = 1, … , nj attributed to provider j; mij = 0 otherwise. Let pij(m) be the corresponding expected mortality probability. Then the inpatient mortality index for provider j is Mj = ∑imij⁄∑ipij(m).
30-Day Inpatient Readmission Index. A 30-day inpatient readmission was defined as the event when a patient is discharged and readmits back into the inpatient setting within 30 days. The inclusion criteria defined by the Centers for Medicare and Medicaid Services (CMS) all-cause hospital-wide readmission measure was used and, consequently, planned readmissions were excluded.12 Readmissions could occur at any Banner hospital, including the same hospital. For each hospital visit, an expected readmission probability was derived using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for readmissions incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let rij = 1 if the patient had a readmission following hospitalization i = 1, … , nj attributed to provider j; rij = 0 otherwise. Let pij(r) be the corresponding expected readmission probability. Then the 30-day inpatient readmission index for provider j is Rj = ∑irij ⁄∑ipij(r).
Patient Survey Scores. The satisfaction of the patient’s experience during hospitalization was measured via post-discharge surveys administered by InMoment. Two survey questions were selected because they related directly to a provider’s interaction with the patient: “My interactions with doctors were excellent” (Doctor) and “I received the best possible care” (Care). A third question, “I would recommend this hospital to my family and friends,” was selected as a proxy measure of the overall experience and, in the aggregate, is referred to as the net promoter score (NPS).13,14 The responses were measured on an 11-point Likert scale, ranging from “Strongly Disagree” (0) to “Strongly Agree” (10); “N/A” or missing responses were excluded.
The Likert responses were coded to 3 discrete values as follows: if the value was between 0 and 6, then -1 (ie, detractor); between 7 and 8 (ie, neutral), then 0; otherwise 1 (ie, promoter). Averaging these coded responses results in a patient survey score for each question. Specifically, let nj be the number of hospitalizations attributed to provider j in which the patient responded to the survey question. Let sij ∈{−1, 0, 1} be the coded response linked to hospitalization i = 1, … , nj attributed to provider j. Then the patient experience score for provider j is Sj = ∑isij⁄nj.
Handling Ties in Provider Performance Measures. Because ties can occur in the PAPR approach for all measures, a tie-breaking strategy is needed. For LOS indices, ties are less likely because their numerator is strictly greater than 0, and expected LOS values are typically distinct enough. Indeed, no ties were found in this study for LOS indices. However, mortality and readmission indices can routinely result in ties when the best possible index is achieved, such as 0 deaths or readmissions among attributed hospitalizations. To help differentiate between those indices in the PAPR approach, the total estimated risk (denominator) was utilized as a secondary scoring criterion.
Mortality and readmission metrics were addressed by sorting first by the outcome (mortality index), and second by the denominator (total estimated risk). For example, if provider A has the same mortality rate as provider B, then provider A would be ranked higher if the denominator was larger, indicating a higher risk for mortality.
Similarly, it was very common for providers to have the same overall average rating for a survey question. Therefore, the denominator (number of respondents) was used to break ties. However, the denominator sorting was bidirectional. For example, if the tied score was positive (more promoters than detractors) for providers A and B, then provider A would be ranked higher if the denominator was larger. Conversely, if the tied score between providers A and B was neutral or negative (more detractors than promoters), then provider A would be ranked lower if the denominator was larger.
Individual Provider Metrics for the PAMM Method
For the PAMM method, model-based metrics were derived using a MM model.8 Specifically, let J be the number of rotating providers in a health care system. Let Yi be an outcome of interest from hospitalization i, X1i, …, Xpi be fixed effects or covariates, and ß1, …, ßp be the coefficients for the respective covariates. Then the generalized MM statistical model is
(Eq. 2)
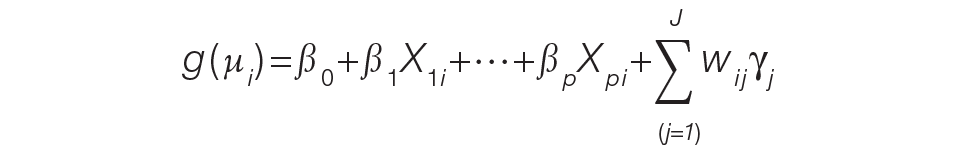
where g(μi ) is a link function between the mean of the outcome, μi, and its linear predictor, ß0, is the marginal intercept, wij represents the attribution weight of provider j on hospitalization i (described in Equation 1), and γj represents the random effect of provider j on the outcome with γj~N(0,σγ2).
For the mortality and readmission binary outcomes, logistic regression was performed using a logit link function, with the corresponding expected probability as the only fixed covariate. The expected probabilities were first converted into odds and then log-transformed before entering the model. For LOS, Poisson regression was performed using a log link function with the log-transformed expected LOS as the only fixed covariate. For coded patient experience responses, an ordered logistic regression was performed using a cumulative logit link function (no fixed effects were added).
MM Model-based Metrics. Each fitted MM model produces a predicted random effect for each provider. The provider-specific random effects can be interpreted as the unobserved influence of each provider on the outcome after controlling for any fixed effect included in the model. Therefore, the provider-specific random effects were used to evaluate the relative provider performance, which is analogous to the individual provider-level metrics used in the PAPR method.
Measuring provider performance using a MM model is more flexible and robust to outliers compared to the standard approach using OE indices or simple averages. First, although not investigated here, the effect of patient-, visit-, provider-, and/or temporal-level covariates can be controlled when evaluating provider performance. For example, a patient’s socioeconomic status, a provider’s workload, and seasonal factors can be added to the MM model. These external factors are not accounted for in OE indices.
Another advantage of using predicted random effects is the concept of “shrinkage.” The process of estimating random effects inherently accounts for small sample sizes (when providers do not treat a large enough sample of patients) and/or when there is a large ratio of patient variance to provider variance (for instance, when patient outcome variability is much higher compared to provider performance variability). In both cases, the estimation of the random effect is pulled ever closer to 0, signaling that the provider performance is closer to the population average. See Henderson15 and Mood16 for further details.
In contrast, OE indices can result in unreliable estimates when a provider has not cared for many patients. This is especially prevalent when the outcome is binary with a low probability of occurring, such as mortality. Indeed, provider-level mortality OE indices are routinely 0 when the patient counts are low, which skews performance rankings unfairly. Finally, OE indices also ignore the magnitude of the variance of an outcome between providers and patients, which can be large.
Comparison Methodology
In this study, we seek to compare the 2 methods of attribution, PAPR and PAMM, to determine whether there are meaningful differences between them when measuring provider performance. Using retrospective data described in the next section, each attribution method was used independently to derive provider-level metrics. To assess relative performance, percentiles were assigned to each provider based on their metric values so that, in the end, there were 2 percentile ranks for each provider for each metric.
Using these paired percentiles, we derived the following measures of concordance, similar to Herzke, Michtalik3: (1) the percent concordance measure—defined as the number of providers who landed in the top half (greater than the median) or bottom half under both attribution models—divided by the total number of providers; (2) the median of the absolute difference in percentiles under both attribution models; and (3) the Pearson correlation coefficient of the paired provider ranks. The first measure is a global measure of concordance between the 2 approaches and would be expected to be 50% by chance. The second measure gauges how an individual provider’s rank is affected by the change in attribution methodologies. The third measure is a statistical measure of linear correlation of the paired percentiles and was not included in the Herzke, Michtalik3 study.
All statistical analyses were performed on SAS (version 9.4; Cary, NC) and the MM models were fitted using PROC GLIMMIX with the EFFECT statement. The Banner Health Institutional Review Board approved this study.
Results
Descriptive Statistics
A total of

Multi-Membership Model Results
Table 3 displays the results after independently fitting MM models to each of the 3 clinical outcomes. Along with a marginal intercept, the only covariate in each model was the corresponding expected value after a transformation. This was added to use the same information that is typically used in OE indices, therefore allowing for a proper comparison between the 2 attribution methods. The provider-level variance represents the between-provider variation and measures the amount of influence providers have on the corresponding outcome after controlling for any covariates in the model. A provider-level variance of 0 would indicate that providers do not have any influence on the outcome. While the mortality and readmission model results can be compared to each other, the LOS model cannot given its different scale and transformation altogether.

The results in Table 3 suggest that each expected value covariate is highly correlated with its corresponding outcome, which is the anticipated conclusion given that they are constructed in this fashion. The estimated provider-level variances indicate that, after including an expected value in the model, providers have less of an influence on a patient’s LOS and likelihood of being readmitted. On the other hand, the results suggest that providers have much more influence on the likelihood of a patient dying in the hospital, even after controlling for an expected mortality covariate.

Table 4 shows the results after independently fitting MM-ordered logistic models to each of the 3 survey questions. The similar provider-level variances suggest that providers have the same influence on the patient’s perception of the quality of their interactions with the doctor (Doctor), the quality of the care they received (Care), and their likelihood to recommend a friend or family member to the hospital (NPS).

Comparison Results Between Both Attribution Methods
Table 5 compares the 2 attribution methods when ranking providers based on their performance on each outcome measure. The comparison metrics gauge how well the 2 methods agree overall (percent concordance), agree at the provider level (absolute percentile difference and interquartile range [IQR]), and how the paired percentiles linearly correlate to each other (Pearson correlation coefficient).

LOS, by a small margin, had the lowest concordance of clinical outcomes (72.1%), followed by mortality (75.9%) and readmissions (82.1%). Generally, the survey scores had higher percent concordance than the clinical outcome measures, with Doctor at 84.1%, Care at 85.9%, and NPS having the highest percent concordance at 86.6%. Given that by chance the percent concordance is expected to be 50%, there was notable discordance, especially with the clinical outcome measures. Using LOS performance as an example, one attribution methodology would rank a provider in the top half or bottom half, while the other attribution methodology would rank the same provider exactly the opposite way about 28% of the time.
The median absolute percentile difference between the 2 methods was more modest (between 7 and 15). Still, there were some providers whose performance ranking was heavily impacted by the attribution methodology that was used. This was especially true when evaluating performance for certain clinical measures, where the attribution method that was used could change the provider performance percentile by up to 90 levels.
The paired percentiles were positively correlated when ranking performance using any of the 6 measures. This suggests that both methodologies assess performance generally in the same direction, irrespective of the methodology and measure. We did not investigate more complex correlation measures and left this for future research.
It should be noted that ties occurred much more frequently with the PAPR method than when using PAMM and therefore required tie-breaking rules to be designed. Given the nature of OE indices, PAPR methodology is especially sensitive to ties whenever the measure includes counting the number of events (for example, mortality and readmissions) and whenever there are many providers with very few attributed patients. On the other hand, using the PAMM method is much more robust against ties given that the summation of all the weighted attributed outcomes will rarely result in ties, even with a nominal set of providers.
Discussion
In this study, the PAMM methodology was introduced and was used to assess relative provider performance on 3 clinical outcome measures and 3 patient survey scores. The new approach aims to distribute each outcome among all providers who provided care for a patient in an inpatient setting. Clinical notes were used to account for patient-to-provider interactions, and fitted MM statistical models were used to compute the effects that each provider had on each outcome. The provider effect was introduced as a random effect, and the set of predicted random effects was used to rank the performance of each provider.
The PAMM approach was compared to the more traditional methodology, PAPR, where each patient is attributed to only 1 provider: the discharging physician in this study. Using this approach, OE indices of clinical outcomes and averages of survey scores were used to rank the performance of each provider. This approach resulted in many ties, which were broken based on the number of hospitalizations, although other tie-breaking methods may be used in practice.
Both methodologies showed modest concordance with each other for the clinical outcomes, but higher concordance for the patient survey scores. This was also true when using the Pearson correlation coefficient to assess agreement. The 1 outcome measure that showed the least concordance and least linear correlation between methods was LOS, which would suggest that LOS performance is more sensitive to the attribution methodology that is used. However, it was the least concordant by a small margin.
Furthermore, although the medians of the absolute percentile differences were small, there were some providers who had large deviations, suggesting that some providers would move from being shown as high-performers to low-performers and vice versa based on the chosen attribution method. We investigated examples of this and determined that the root cause was the difference in effective sample sizes for a provider. For the PAPR method, the effective sample size is simply the number of hospitalizations attributed to the provider. For the PAMM method, the effective sample size is the sum of all non-zero weights across all hospitalizations where the provider cared for a patient. By and large, the PAMM methodology provides more information of the provider effect on an outcome than the PAPR approach because every provider-patient interaction is considered. For example, providers who do not routinely discharge patients, but often care for patients, will have rankings that differ dramatically between the 2 methods.
The PAMM methodology has many statistical advantages that were not fully utilized in this comparative study. For example, we did not include any covariates in the MM models except for the expected value of the outcome, when it was available. Still, it is known that other covariates can impact an outcome as well, such as the patient’s age, socioeconomic indicators, existing chronic conditions, and severity of hospitalization, which can be added to the MM models as fixed effects. In this way, the PAMM approach can control for these other covariates, which are typically outside of the control of providers but typically ignored using OE indices. Therefore, using the PAMM approach would provide a fairer comparison of provider performance.
Using the PAMM method, most providers had a large sample size to assess their performance once all the weighted interactions were included. Still, there were a few who did not care for many patients for a variety of reasons. In these scenarios, MM models “borrow” strength from other providers to produce a more robust predicted provider effect by using a weighted average between the overall population trend and the specific provider outcomes (see Rao and Molina17). As a result, PAMM is a more suitable approach when the sample sizes of patients attributed to providers can be small.
One of the most interesting findings of this study was the relative size of the provider-level variance to the size of the fixed effect in each model (Table 3). Except for mortality, these variances suggest that there is a small difference in performance from one provider to another. However, these should be interpreted as the variance when only 1 provider is involved in the care of a patient. When multiple providers are involved, using basic statistical theory, the overall provider-level variance will be σγ2 ∑wij2 (see Equation 2). For example, the estimated variance among providers for LOS was 0.03 (on a log scale), but, using the scenario in the Figure, the overall provider-level variance for this hospitalization will be 0.03 (0.3752 + 0.1252 + 0.52) = 0.012. Hence, the combined effect of providers on LOS is less than would be expected. Indeed, as more providers are involved with a patient’s care, the more their combined influence on an outcome is diluted.
In this study, the PAMM approach placed an equal weight on all provider-patient interactions via clinical note authorship, but that may not be optimal in some settings. For example, it may make more sense to set a higher weight on the provider who admitted or discharged the patient while placing less (or 0) weight on all other interactions. In the extreme, if the full weight were placed on 1 provider interaction (eg, during discharge, then the MM model would be reduced to a one-way random effects model. The flexibility of weighting interactions is a feature of the PAMM approach, but any weighting framework must be transparent to the providers before implementation.
Conclusion
This study demonstrates that the PAMM approach is a feasible option within a large health care organization. For P4P programs to be successful, providers must be able to trust that their performance will be fairly assessed and that all provider-patient interactions are captured to provide a full comparison amongst their peers. The PAMM methodology is one solution to spread the positive (and negative) outcomes across all providers who cared for a patient and therefore, if implemented, would add trust and fairness when measuring and assessing provider performance.
Acknowledgments: The authors thank Barrie Bradley for his support in the initial stages of this research and Dr. Syed Ismail Jafri for his help and support on the standard approaches of assessing and measuring provider performances.
Corresponding author: Rachel Ginn, MS, Banner Health Corporation, 2901 N. Central Ave., Phoenix, AZ 85012; [email protected].
Financial disclosures: None.
From Banner Health Corporation, Phoenix, AZ.
Background: Health care providers are routinely incentivized with pay-for-performance (P4P) metrics to increase the quality of care. In an inpatient setting, P4P models typically measure quality by attributing each patient’s outcome to a single provider even though many providers routinely care for the patient. This study investigates a new attribution approach aiming to distribute each outcome across all providers who provided care.
Methods: The methodology relies on a multi-membership model and is demonstrated in the Banner Health system using 3 clinical outcome measures (length of stay, 30-day readmissions, and mortality) and responses to 3 survey questions that measure a patient’s perception of their care. The new approach is compared to the “standard” method, which attributes each patient to only 1 provider.
Results: When ranking by clinical outcomes, both methods were concordant 72.1% to 82.1% of the time for top-half/bottom-half rankings, with a median percentile difference between 7 and 15. When ranking by survey scores, there was more agreement, with concordance between 84.1% and 86.6% and a median percentile difference between 11 and 13. Last, Pearson correlation coefficients of the paired percentiles ranged from 0.56 to 0.78.
Conclusion: The new approach provides a fairer solution when measuring provider performance.
Keywords: patient attribution; PAMM; PAPR; random effect model; pay for performance.
Providers practicing in hospitals are routinely evaluated based on their performance and, in many cases, are financially incentivized for a better-than-average performance within a pay-for-performance (P4P) model. The use of P4P models is based on the belief that they will “improve, motivate, and enhance providers to pursue aggressively and ultimately achieve the quality performance targets thus decreasing the number of medical errors with less malpractice events.”1 Although P4P models continue to be a movement in health care, they have been challenging to implement.
One concern involves the general quality of implementation, such as defining metrics and targets, setting payout amounts, managing technology and market conditions, and gauging the level of transparency to the provider.2 Another challenge, and the focus of this project, are concerns around measuring performance to avoid perceptions of unfairness. This concern can be minimized if the attribution is handled in a fairer way, by spreading it across all providers who affected the outcome, both in a positive or negative direction.3
To implement these models, the performance of providers needs to be measured and tracked periodically. This requires linking, or attributing, a patient’s outcome to a provider, which is almost always the attending or discharging provider (ie, a single provider).3 In this single-provider attribution approach, one provider will receive all the credit (good or bad) for their respective patients’ outcomes, even though the provider may have seen the patient only a fraction of the time during the hospitalization. Attributing outcomes—for example, length of stay (LOS), readmission rate, mortality rate, net promoter score (NPS)—using this approach reduces the validity of metrics designed to measure provider performance, especially in a rotating provider environment where many providers interact with and care for a patient. For example, the quality of providers’ interpersonal skills and competence were among the strongest determinants of patient satisfaction,4 but it is not credible that this is solely based on the last provider during a hospitalization.
Proportionally distributing the attribution of an outcome has been used successfully in other contexts. Typically, a statistical modeling approach using a multi-membership framework is used because it can handle the sometimes-complicated relationships within the hierarchy. It also allows for auxiliary variables to be introduced, which can help explain and control for exogenous effects.5-7 For example, in the education setting, standardized testing is administered to students at defined years of schooling: at grades 4, 8, and 10, for instance. The progress of students, measured as the academic gains between test years, are proportionally attributed to all the teachers who the student has had between the test years. These partial attributions are combined to evaluate an overall teacher performance.8,9
Although the multi-membership framework has been used in other industries, it has yet to be applied in measuring provider performance. The purpose of this project is to investigate the impact of using a multi-provider approach compared to the standard single-provider approach. The findings may lead to modifications in the way a provider’s performance is measured and, thus, how providers are compensated. A similar study investigated the impact of proportionally distributing patients’ outcomes across all rotating providers using a weighting method based on billing practices to measure the partial impact of each provider.3
This study is different in 2 fundamental ways. First, attribution is weighted based on the number of clinically documented interactions (via clinical notes) between a patient and all rotating providers during the hospitalization. Second, performance is measured via multi-membership models, which can estimate the effect (both positive and negative) that a provider has on an outcome, even when caring for a patient a fraction of the time during the hospitalization.
Methods
Setting
Banner Health is a non-profit, multi-hospital health care system across 6 states in the western United States that is uniquely positioned to study provider quality attribution models. It not only has a large number of providers and serves a broad patient population, but Banner Health also uses an instance of Cerner (Kansas City, MO), an enterprise-level electronic health record (EHR) system that connects all its facilities and allows for advanced analytics across its system.
For this study, we included only general medicine and surgery patients admitted and discharged from the inpatient setting between January 1, 2018, and December 31, 2018, who were between 18 and 89 years old at admission, and who had a LOS between 1 and 14 days. Visit- and patient-level data were collected from Cerner, while outcome data, and corresponding expected outcome data, were obtained from Premier, Inc. (Charlotte, NC) using their CareScience methodologies.10 To measure patient experience, response data were extracted from post-discharge surveys administered by InMoment (Salt Lake City, UT).
Provider Attribution Models
Provider Attribution by Physician of Record (PAPR). In the standard approach, denoted here as the PAPR model, 1 provider—typically the attending or discharging provider, which may be the same person—is attributed to the entire hospitalization. This provider is responsible for the patient’s care, and all patient outcomes are aggregated and attributed to the provider to gauge his or her performance. The PAPR model is the most popular form of attribution across many health care systems and is routinely used for P4P incentives.
In this study, the discharging provider was used when attributing hospitalizations using the PAPR model. Providers responsible for fewer than 12 discharges in the calendar year were excluded. Because of the directness of this type of attribution, the performance of 1 provider does not account for the performance of the other rotating providers during hospitalizations.
Provider Attribution by Multiple Membership (PAMM). In contrast, we introduce another attribution approach here that is designed to assign partial attribution to each provider who cares for the patient during the hospitalization. To aggregate the partial attributions, and possibly control for any exogenous or risk-based factors, a multiple-membership, or multi-member (MM), model is used. The MM model can measure the effect of a provider on an outcome even when the patient-to-provider relationship is complex, such as in a rotating provider environment.8
The purpose of this study is to compare attribution models and to determine whether there are meaningful differences between them. Therefore, for comparison purposes, the same discharging providers using the PAPR approach are eligible for the PAMM approach, so that both attribution models are using the same set of providers. All other providers are excluded because their performance would not be comparable to the PAPR approach.
While there are many ways to document provider-to-patient interactions, 2 methods are available in almost all health care systems. The first method is to link a provider’s billing charges to each patient-day combination. This approach limits the attribution to 1 provider per patient per day because multiple rotating providers cannot charge for the same patient-day combination.3 However, many providers interact with a patient on the same day, so using this approach excludes non-billed provider-to-patient interactions.
The second method, which was used in this study, relies on documented clinical notes within the EHR to determine how attribution is shared. In this approach, attribution is weighted based on the authorship of 3 types of eligible clinical notes: admitting history/physical notes (during admission), progress notes (during subsequent days), and discharge summary notes (during final discharge). This will (likely) result in many providers being linked to a patient on each day, which better reflects the clinical setting (Figure). Recently, clinical notes were used to attribute care of patients in an inpatient setting, and it was found that this approach provides a reliable way of tracking interactions and assigning ownership.11

The provider-level attribution weights are based on the share of authorships of eligible note types. Specifically, for each provider j, let aij be the total count of eligible note types for hospitalization i authored by provider j, and let ai be the overall total count of eligible note types for hospitalization i. Then the attribution weight is
(Eq. 1)
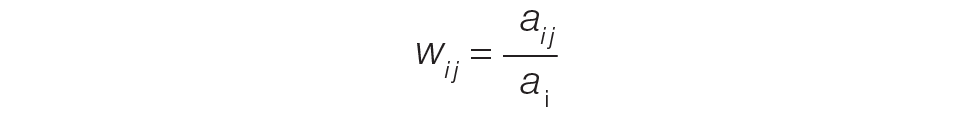
for hospitalization i and provider j. Note that ∑jwij = 1: in other words, the total attribution, summed across all providers, is constrained to be 1 for each hospitalization.
Patient Outcomes
Outcomes were chosen based on their routine use in health care systems as standards when evaluating provider performance. This study included 6 outcomes: inpatient LOS, inpatient mortality, 30-day inpatient readmission, and patient responses from 3 survey questions. These outcomes can be collected without any manual chart reviews, and therefore are viewed as objective outcomes of provider performance.
Each outcome was aggregated for each provider using both attribution methods independently. For the PAPR method, observed-to-expected (OE) indices for LOS, mortality, and readmissions were calculated along with average patient survey scores. For the PAMM method, provider-level random effects from the fitted models were used. In both cases, the calculated measures were used for ranking purposes when determining top (or bottom) providers for each outcome.
Individual Provider Metrics for the PAPR Method
Inpatient LOS Index. Hospital inpatient LOS was measured as the number of days between admission date and discharge date. For each hospital visit, an expected LOS was determined using Premier’s CareScience Analytics (CSA) risk-adjustment methodology.10 The CSA methodology for LOS incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let oij and eij be the observed and expected LOS, respectively, for hospitalization i = 1,…,nj attributed to provider j. Then the inpatient LOS index for provider j is Lj = ∑ioij⁄∑ieij.
Inpatient Mortality Index. Inpatient mortality was defined as the death of the patient during hospitalization. For each hospitalization, an expected mortality probability was determined using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for mortality incorporates a patient’s demographics and comorbidities.
Just as before, let nj be the number of hospitalizations attributed to provider j. Let mij = 1 if the patient died during hospitalization i = 1, … , nj attributed to provider j; mij = 0 otherwise. Let pij(m) be the corresponding expected mortality probability. Then the inpatient mortality index for provider j is Mj = ∑imij⁄∑ipij(m).
30-Day Inpatient Readmission Index. A 30-day inpatient readmission was defined as the event when a patient is discharged and readmits back into the inpatient setting within 30 days. The inclusion criteria defined by the Centers for Medicare and Medicaid Services (CMS) all-cause hospital-wide readmission measure was used and, consequently, planned readmissions were excluded.12 Readmissions could occur at any Banner hospital, including the same hospital. For each hospital visit, an expected readmission probability was derived using Premier’s CSA risk-adjustment methodology.10 The CSA methodology for readmissions incorporates a patient’s clinical history, demographics, and visit-related administrative information.
Let nj be the number of hospitalizations attributed to provider j. Let rij = 1 if the patient had a readmission following hospitalization i = 1, … , nj attributed to provider j; rij = 0 otherwise. Let pij(r) be the corresponding expected readmission probability. Then the 30-day inpatient readmission index for provider j is Rj = ∑irij ⁄∑ipij(r).
Patient Survey Scores. The satisfaction of the patient’s experience during hospitalization was measured via post-discharge surveys administered by InMoment. Two survey questions were selected because they related directly to a provider’s interaction with the patient: “My interactions with doctors were excellent” (Doctor) and “I received the best possible care” (Care). A third question, “I would recommend this hospital to my family and friends,” was selected as a proxy measure of the overall experience and, in the aggregate, is referred to as the net promoter score (NPS).13,14 The responses were measured on an 11-point Likert scale, ranging from “Strongly Disagree” (0) to “Strongly Agree” (10); “N/A” or missing responses were excluded.
The Likert responses were coded to 3 discrete values as follows: if the value was between 0 and 6, then -1 (ie, detractor); between 7 and 8 (ie, neutral), then 0; otherwise 1 (ie, promoter). Averaging these coded responses results in a patient survey score for each question. Specifically, let nj be the number of hospitalizations attributed to provider j in which the patient responded to the survey question. Let sij ∈{−1, 0, 1} be the coded response linked to hospitalization i = 1, … , nj attributed to provider j. Then the patient experience score for provider j is Sj = ∑isij⁄nj.
Handling Ties in Provider Performance Measures. Because ties can occur in the PAPR approach for all measures, a tie-breaking strategy is needed. For LOS indices, ties are less likely because their numerator is strictly greater than 0, and expected LOS values are typically distinct enough. Indeed, no ties were found in this study for LOS indices. However, mortality and readmission indices can routinely result in ties when the best possible index is achieved, such as 0 deaths or readmissions among attributed hospitalizations. To help differentiate between those indices in the PAPR approach, the total estimated risk (denominator) was utilized as a secondary scoring criterion.
Mortality and readmission metrics were addressed by sorting first by the outcome (mortality index), and second by the denominator (total estimated risk). For example, if provider A has the same mortality rate as provider B, then provider A would be ranked higher if the denominator was larger, indicating a higher risk for mortality.
Similarly, it was very common for providers to have the same overall average rating for a survey question. Therefore, the denominator (number of respondents) was used to break ties. However, the denominator sorting was bidirectional. For example, if the tied score was positive (more promoters than detractors) for providers A and B, then provider A would be ranked higher if the denominator was larger. Conversely, if the tied score between providers A and B was neutral or negative (more detractors than promoters), then provider A would be ranked lower if the denominator was larger.
Individual Provider Metrics for the PAMM Method
For the PAMM method, model-based metrics were derived using a MM model.8 Specifically, let J be the number of rotating providers in a health care system. Let Yi be an outcome of interest from hospitalization i, X1i, …, Xpi be fixed effects or covariates, and ß1, …, ßp be the coefficients for the respective covariates. Then the generalized MM statistical model is
(Eq. 2)
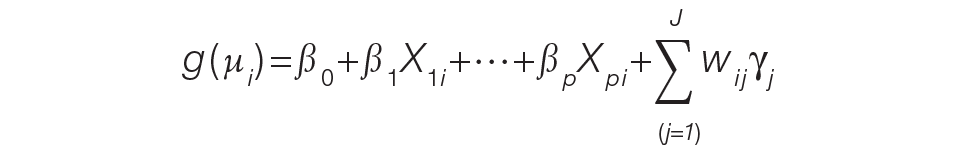
where g(μi ) is a link function between the mean of the outcome, μi, and its linear predictor, ß0, is the marginal intercept, wij represents the attribution weight of provider j on hospitalization i (described in Equation 1), and γj represents the random effect of provider j on the outcome with γj~N(0,σγ2).
For the mortality and readmission binary outcomes, logistic regression was performed using a logit link function, with the corresponding expected probability as the only fixed covariate. The expected probabilities were first converted into odds and then log-transformed before entering the model. For LOS, Poisson regression was performed using a log link function with the log-transformed expected LOS as the only fixed covariate. For coded patient experience responses, an ordered logistic regression was performed using a cumulative logit link function (no fixed effects were added).
MM Model-based Metrics. Each fitted MM model produces a predicted random effect for each provider. The provider-specific random effects can be interpreted as the unobserved influence of each provider on the outcome after controlling for any fixed effect included in the model. Therefore, the provider-specific random effects were used to evaluate the relative provider performance, which is analogous to the individual provider-level metrics used in the PAPR method.
Measuring provider performance using a MM model is more flexible and robust to outliers compared to the standard approach using OE indices or simple averages. First, although not investigated here, the effect of patient-, visit-, provider-, and/or temporal-level covariates can be controlled when evaluating provider performance. For example, a patient’s socioeconomic status, a provider’s workload, and seasonal factors can be added to the MM model. These external factors are not accounted for in OE indices.
Another advantage of using predicted random effects is the concept of “shrinkage.” The process of estimating random effects inherently accounts for small sample sizes (when providers do not treat a large enough sample of patients) and/or when there is a large ratio of patient variance to provider variance (for instance, when patient outcome variability is much higher compared to provider performance variability). In both cases, the estimation of the random effect is pulled ever closer to 0, signaling that the provider performance is closer to the population average. See Henderson15 and Mood16 for further details.
In contrast, OE indices can result in unreliable estimates when a provider has not cared for many patients. This is especially prevalent when the outcome is binary with a low probability of occurring, such as mortality. Indeed, provider-level mortality OE indices are routinely 0 when the patient counts are low, which skews performance rankings unfairly. Finally, OE indices also ignore the magnitude of the variance of an outcome between providers and patients, which can be large.
Comparison Methodology
In this study, we seek to compare the 2 methods of attribution, PAPR and PAMM, to determine whether there are meaningful differences between them when measuring provider performance. Using retrospective data described in the next section, each attribution method was used independently to derive provider-level metrics. To assess relative performance, percentiles were assigned to each provider based on their metric values so that, in the end, there were 2 percentile ranks for each provider for each metric.
Using these paired percentiles, we derived the following measures of concordance, similar to Herzke, Michtalik3: (1) the percent concordance measure—defined as the number of providers who landed in the top half (greater than the median) or bottom half under both attribution models—divided by the total number of providers; (2) the median of the absolute difference in percentiles under both attribution models; and (3) the Pearson correlation coefficient of the paired provider ranks. The first measure is a global measure of concordance between the 2 approaches and would be expected to be 50% by chance. The second measure gauges how an individual provider’s rank is affected by the change in attribution methodologies. The third measure is a statistical measure of linear correlation of the paired percentiles and was not included in the Herzke, Michtalik3 study.
All statistical analyses were performed on SAS (version 9.4; Cary, NC) and the MM models were fitted using PROC GLIMMIX with the EFFECT statement. The Banner Health Institutional Review Board approved this study.
Results
Descriptive Statistics
A total of

Multi-Membership Model Results
Table 3 displays the results after independently fitting MM models to each of the 3 clinical outcomes. Along with a marginal intercept, the only covariate in each model was the corresponding expected value after a transformation. This was added to use the same information that is typically used in OE indices, therefore allowing for a proper comparison between the 2 attribution methods. The provider-level variance represents the between-provider variation and measures the amount of influence providers have on the corresponding outcome after controlling for any covariates in the model. A provider-level variance of 0 would indicate that providers do not have any influence on the outcome. While the mortality and readmission model results can be compared to each other, the LOS model cannot given its different scale and transformation altogether.

The results in Table 3 suggest that each expected value covariate is highly correlated with its corresponding outcome, which is the anticipated conclusion given that they are constructed in this fashion. The estimated provider-level variances indicate that, after including an expected value in the model, providers have less of an influence on a patient’s LOS and likelihood of being readmitted. On the other hand, the results suggest that providers have much more influence on the likelihood of a patient dying in the hospital, even after controlling for an expected mortality covariate.

Table 4 shows the results after independently fitting MM-ordered logistic models to each of the 3 survey questions. The similar provider-level variances suggest that providers have the same influence on the patient’s perception of the quality of their interactions with the doctor (Doctor), the quality of the care they received (Care), and their likelihood to recommend a friend or family member to the hospital (NPS).

Comparison Results Between Both Attribution Methods
Table 5 compares the 2 attribution methods when ranking providers based on their performance on each outcome measure. The comparison metrics gauge how well the 2 methods agree overall (percent concordance), agree at the provider level (absolute percentile difference and interquartile range [IQR]), and how the paired percentiles linearly correlate to each other (Pearson correlation coefficient).

LOS, by a small margin, had the lowest concordance of clinical outcomes (72.1%), followed by mortality (75.9%) and readmissions (82.1%). Generally, the survey scores had higher percent concordance than the clinical outcome measures, with Doctor at 84.1%, Care at 85.9%, and NPS having the highest percent concordance at 86.6%. Given that by chance the percent concordance is expected to be 50%, there was notable discordance, especially with the clinical outcome measures. Using LOS performance as an example, one attribution methodology would rank a provider in the top half or bottom half, while the other attribution methodology would rank the same provider exactly the opposite way about 28% of the time.
The median absolute percentile difference between the 2 methods was more modest (between 7 and 15). Still, there were some providers whose performance ranking was heavily impacted by the attribution methodology that was used. This was especially true when evaluating performance for certain clinical measures, where the attribution method that was used could change the provider performance percentile by up to 90 levels.
The paired percentiles were positively correlated when ranking performance using any of the 6 measures. This suggests that both methodologies assess performance generally in the same direction, irrespective of the methodology and measure. We did not investigate more complex correlation measures and left this for future research.
It should be noted that ties occurred much more frequently with the PAPR method than when using PAMM and therefore required tie-breaking rules to be designed. Given the nature of OE indices, PAPR methodology is especially sensitive to ties whenever the measure includes counting the number of events (for example, mortality and readmissions) and whenever there are many providers with very few attributed patients. On the other hand, using the PAMM method is much more robust against ties given that the summation of all the weighted attributed outcomes will rarely result in ties, even with a nominal set of providers.
Discussion
In this study, the PAMM methodology was introduced and was used to assess relative provider performance on 3 clinical outcome measures and 3 patient survey scores. The new approach aims to distribute each outcome among all providers who provided care for a patient in an inpatient setting. Clinical notes were used to account for patient-to-provider interactions, and fitted MM statistical models were used to compute the effects that each provider had on each outcome. The provider effect was introduced as a random effect, and the set of predicted random effects was used to rank the performance of each provider.
The PAMM approach was compared to the more traditional methodology, PAPR, where each patient is attributed to only 1 provider: the discharging physician in this study. Using this approach, OE indices of clinical outcomes and averages of survey scores were used to rank the performance of each provider. This approach resulted in many ties, which were broken based on the number of hospitalizations, although other tie-breaking methods may be used in practice.
Both methodologies showed modest concordance with each other for the clinical outcomes, but higher concordance for the patient survey scores. This was also true when using the Pearson correlation coefficient to assess agreement. The 1 outcome measure that showed the least concordance and least linear correlation between methods was LOS, which would suggest that LOS performance is more sensitive to the attribution methodology that is used. However, it was the least concordant by a small margin.
Furthermore, although the medians of the absolute percentile differences were small, there were some providers who had large deviations, suggesting that some providers would move from being shown as high-performers to low-performers and vice versa based on the chosen attribution method. We investigated examples of this and determined that the root cause was the difference in effective sample sizes for a provider. For the PAPR method, the effective sample size is simply the number of hospitalizations attributed to the provider. For the PAMM method, the effective sample size is the sum of all non-zero weights across all hospitalizations where the provider cared for a patient. By and large, the PAMM methodology provides more information of the provider effect on an outcome than the PAPR approach because every provider-patient interaction is considered. For example, providers who do not routinely discharge patients, but often care for patients, will have rankings that differ dramatically between the 2 methods.
The PAMM methodology has many statistical advantages that were not fully utilized in this comparative study. For example, we did not include any covariates in the MM models except for the expected value of the outcome, when it was available. Still, it is known that other covariates can impact an outcome as well, such as the patient’s age, socioeconomic indicators, existing chronic conditions, and severity of hospitalization, which can be added to the MM models as fixed effects. In this way, the PAMM approach can control for these other covariates, which are typically outside of the control of providers but typically ignored using OE indices. Therefore, using the PAMM approach would provide a fairer comparison of provider performance.
Using the PAMM method, most providers had a large sample size to assess their performance once all the weighted interactions were included. Still, there were a few who did not care for many patients for a variety of reasons. In these scenarios, MM models “borrow” strength from other providers to produce a more robust predicted provider effect by using a weighted average between the overall population trend and the specific provider outcomes (see Rao and Molina17). As a result, PAMM is a more suitable approach when the sample sizes of patients attributed to providers can be small.
One of the most interesting findings of this study was the relative size of the provider-level variance to the size of the fixed effect in each model (Table 3). Except for mortality, these variances suggest that there is a small difference in performance from one provider to another. However, these should be interpreted as the variance when only 1 provider is involved in the care of a patient. When multiple providers are involved, using basic statistical theory, the overall provider-level variance will be σγ2 ∑wij2 (see Equation 2). For example, the estimated variance among providers for LOS was 0.03 (on a log scale), but, using the scenario in the Figure, the overall provider-level variance for this hospitalization will be 0.03 (0.3752 + 0.1252 + 0.52) = 0.012. Hence, the combined effect of providers on LOS is less than would be expected. Indeed, as more providers are involved with a patient’s care, the more their combined influence on an outcome is diluted.
In this study, the PAMM approach placed an equal weight on all provider-patient interactions via clinical note authorship, but that may not be optimal in some settings. For example, it may make more sense to set a higher weight on the provider who admitted or discharged the patient while placing less (or 0) weight on all other interactions. In the extreme, if the full weight were placed on 1 provider interaction (eg, during discharge, then the MM model would be reduced to a one-way random effects model. The flexibility of weighting interactions is a feature of the PAMM approach, but any weighting framework must be transparent to the providers before implementation.
Conclusion
This study demonstrates that the PAMM approach is a feasible option within a large health care organization. For P4P programs to be successful, providers must be able to trust that their performance will be fairly assessed and that all provider-patient interactions are captured to provide a full comparison amongst their peers. The PAMM methodology is one solution to spread the positive (and negative) outcomes across all providers who cared for a patient and therefore, if implemented, would add trust and fairness when measuring and assessing provider performance.
Acknowledgments: The authors thank Barrie Bradley for his support in the initial stages of this research and Dr. Syed Ismail Jafri for his help and support on the standard approaches of assessing and measuring provider performances.
Corresponding author: Rachel Ginn, MS, Banner Health Corporation, 2901 N. Central Ave., Phoenix, AZ 85012; [email protected].
Financial disclosures: None.
1. Abduljawad A, Al-Assaf AF. Incentives for better performance in health care. Sultan Qaboos Univ Med J. 2011;11:201-206.
2. Milstein R, Schreyoegg J. Pay for performance in the inpatient sector: a review of 34 P4P programs in 14 OECD countries. Health Policy. 2016;120:1125-1140.
3. Herzke CA, Michtalik HJ, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. 2018;13:470-475.
4. Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health. 2017;137:89-101.
5. Ballou D, Sanders W, Wright P. Controlling for student background in value-added assessment of teachers. J Educ Behav Stat. 2004;29:37-65.
6. Hill PW, Goldstein H. Multilevel modeling of educational data with cross-classification and missing identification for units. J Educ Behav Stat. 1998;23:117-128.
7. Rasbash J, Browne WJ. Handbook of Multilevel Analysis. Springer; 2007.
8. Brown WJ, Goldstein H, Rasbash J. Multiple membership multiple classification (MMMC) models. Statistical Modeling. 2001;1:103-124.
9. Sanders WL, Horn SP. The Tennessee Value-Added Assessment System (TVAAS)—mixed-model methodology in educational assessment. J Pers Eval Educ. 1994;8:299-311.
10. Kroch EA, Duan M. CareScience Risk Assessment Model: Hospital Performance Measurement. Premier, Inc., 2008. http://www.ahrq.gov/qual/mortality/KrochRisk.htm
11. Schumacher DJ, Wu DTY, Meganathan K, et al. A feasibility study to attribute patients to primary interns on inpatient ward teams using electronic health record data. Acad Med. 2019;94:1376-1383.
12. Simoes J, Krumholz HM, Lin Z. Hospital-level 30-day risk-standardized readmission measure. Centers for Medicare & Medicaid Services, 2018. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Hospital-Wide-All-Cause-Readmission-Updates.zip
13. Krol MW, de Boer D, Delnoij DM, Rademakers JJDJM. The Net Promoter Score: an asset to patient experience surveys? Health Expect. 2015;18:3099-3109.
14. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3:e001570.
15. Henderson CR. Sire evaluation and genetic trends. J Anim Sci. 1973;1973:10-41.
16. Mood AM. Introduction to the Theory of Statistics. McGraw-Hill; 1950:xiii, 433-xiii.
17. Rao JNK, Molina I. Small Area Estimation. Wiley; 2015.
1. Abduljawad A, Al-Assaf AF. Incentives for better performance in health care. Sultan Qaboos Univ Med J. 2011;11:201-206.
2. Milstein R, Schreyoegg J. Pay for performance in the inpatient sector: a review of 34 P4P programs in 14 OECD countries. Health Policy. 2016;120:1125-1140.
3. Herzke CA, Michtalik HJ, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. 2018;13:470-475.
4. Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health. 2017;137:89-101.
5. Ballou D, Sanders W, Wright P. Controlling for student background in value-added assessment of teachers. J Educ Behav Stat. 2004;29:37-65.
6. Hill PW, Goldstein H. Multilevel modeling of educational data with cross-classification and missing identification for units. J Educ Behav Stat. 1998;23:117-128.
7. Rasbash J, Browne WJ. Handbook of Multilevel Analysis. Springer; 2007.
8. Brown WJ, Goldstein H, Rasbash J. Multiple membership multiple classification (MMMC) models. Statistical Modeling. 2001;1:103-124.
9. Sanders WL, Horn SP. The Tennessee Value-Added Assessment System (TVAAS)—mixed-model methodology in educational assessment. J Pers Eval Educ. 1994;8:299-311.
10. Kroch EA, Duan M. CareScience Risk Assessment Model: Hospital Performance Measurement. Premier, Inc., 2008. http://www.ahrq.gov/qual/mortality/KrochRisk.htm
11. Schumacher DJ, Wu DTY, Meganathan K, et al. A feasibility study to attribute patients to primary interns on inpatient ward teams using electronic health record data. Acad Med. 2019;94:1376-1383.
12. Simoes J, Krumholz HM, Lin Z. Hospital-level 30-day risk-standardized readmission measure. Centers for Medicare & Medicaid Services, 2018. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Hospital-Wide-All-Cause-Readmission-Updates.zip
13. Krol MW, de Boer D, Delnoij DM, Rademakers JJDJM. The Net Promoter Score: an asset to patient experience surveys? Health Expect. 2015;18:3099-3109.
14. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3:e001570.
15. Henderson CR. Sire evaluation and genetic trends. J Anim Sci. 1973;1973:10-41.
16. Mood AM. Introduction to the Theory of Statistics. McGraw-Hill; 1950:xiii, 433-xiii.
17. Rao JNK, Molina I. Small Area Estimation. Wiley; 2015.
Noninvasive Ventilation Use Among Medicare Beneficiaries at the End of Life
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Physician-Driven Discretionary Utilization: Measuring Overuse and Choosing Wisely
Overutilization and low-value care are important clinical and policy problems. Their measurement is challenging because it requires detailed clinical information. Additionally, there are inherent difficulties in identifying discretionary services likely to be inappropriate or low-value and demonstrating that certain services produce little/no health benefit. Quantifying “ideal” expected testing rates—ones that would reflect minimization of inappropriate/low-value care without excluding essential, high-yield diagnostic services—presents additional challenges. Consequently, of 521 unique measures specified by national measurement programs and professional guidelines, 91.6% targeted underuse, while only 6.5% targeted overuse.1
The potential for unintended consequences of implementing measures to eliminate overuse are a barrier to incorporating such measures into practice.2 For example, measuring, reporting, and penalizing overuse of inappropriate bone scanning may lead to underuse in patients for whom scanning is crucial.2 Most overuse measures based on inappropriate or low-value indications relate to imaging and medications.1 However, there is increasing interest in overutilization measures based on a broad set of health services. Identifying low-value testing or treatments often requires a substantial degree of clinical detail to avoid the damaging inclusion of beneficial services, which may lead to unintended negative outcomes, creating skepticism among clinicians. Ultimately, getting measurement of low-value care wrong would undermine adoption of interventions to reduce overuse.
To reduce low-value care through expansive measures of provider ordering behavior,3 Ellenbogen et al4 derived a novel index to identify hospitals with high rates of low-yield diagnostic testing. This index is based on the concept that, in the presence of nonspecific, symptom-based principal diagnoses, a substantial proportion of (apparently) non-diagnostic related studies were probably ordered despite a low pretest probability of serious disease. Since such symptom-based diagnoses reflect the absence of a more specific diagnosis, the examinations observed are markers of physician-driven decisions leading to discretionary utilization likely to be of low-value to patients. This study fills a critical gap in dual measures of appropriateness and yield, rather than simply utilization, to advance the Choosing Wisely campaign.3
Advantages of this overuse index include its derivation from administrative data, obviating the need for electronic health records, and incorporation of diagnostic yield at the inpatient-encounter level. One study selected procedures identifiable solely with claims from a set deemed overused by professional/consumer groups.5 However, the yield of physician decisions in specific cases was not measured. In contrast, this novel index is derived from an assessment of diagnostic yield.4 Although test results are not known with certainty, the absence of a specific discharge diagnosis serves as a test result proxy. Measurement of diagnostic examination yield at the patient-level (aggregated to the hospital-level) may be applicable across hospitals with varied patient populations, which include large differences in patient and/or family preferences to seek medical attention and engage in shared decision-making. The role that patient preferences play in decisions creates a limitation in this index—while decisions for the candidate diagnostic tests are physician driven, patient demand may be a confounding factor. This index cannot therefore be considered purely a measure of physician-induced intensity of diagnostic services. Patient-reported data would enhance future analyses by more fully capturing all dimensions of care necessary to identify low-value services. Subjective outcomes are critical in completely measuring the aggregate benefits of tests and interventions judged low-value based on objective metrics. Such data would also aid in quantifying the relative contributions of patient and physician preferences in driving discretionary utilization.
Finally, the derived index is restricted to diagnostic decision-making and may not be applicable to treatment-related practice patterns. However, the literature suggests strong correlations between diagnostic and therapeutic intensity. Application of this novel index will play an important role in reducing low-value discretionary utilization.
1. Newton EH, Zazzera EA, Van Moorsel G, Sirovich BE. Undermeasuring overuse--an examination of national clinical performance measures. JAMA Intern Med. 2015;175(10):1709-1711. https://doi.org/10.1001/jamainternmed.2015.4025
2. Mathias JS, Baker DW. Developing quality measures to address overuse. JAMA. 2013;309(18):1897-1898. https://doi.org/10.1001/jama.2013.3588
3. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24(8):523-531. https://doi.org/10.1136/bmjqs-2015-004070
4. Ellenbogen MI, Prichett L, Johnson PT, Brotman DJ. Development of a simple index to measure overuse of diagnostic testing at the hospital level using administrative data. J Hosp Med. 2021;16:xxx-xxx. https://doi.org/10.12788/jhm.3547
5. Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52(2):157-163. https://doi.org/10.1097/MLR.0000000000000052
Overutilization and low-value care are important clinical and policy problems. Their measurement is challenging because it requires detailed clinical information. Additionally, there are inherent difficulties in identifying discretionary services likely to be inappropriate or low-value and demonstrating that certain services produce little/no health benefit. Quantifying “ideal” expected testing rates—ones that would reflect minimization of inappropriate/low-value care without excluding essential, high-yield diagnostic services—presents additional challenges. Consequently, of 521 unique measures specified by national measurement programs and professional guidelines, 91.6% targeted underuse, while only 6.5% targeted overuse.1
The potential for unintended consequences of implementing measures to eliminate overuse are a barrier to incorporating such measures into practice.2 For example, measuring, reporting, and penalizing overuse of inappropriate bone scanning may lead to underuse in patients for whom scanning is crucial.2 Most overuse measures based on inappropriate or low-value indications relate to imaging and medications.1 However, there is increasing interest in overutilization measures based on a broad set of health services. Identifying low-value testing or treatments often requires a substantial degree of clinical detail to avoid the damaging inclusion of beneficial services, which may lead to unintended negative outcomes, creating skepticism among clinicians. Ultimately, getting measurement of low-value care wrong would undermine adoption of interventions to reduce overuse.
To reduce low-value care through expansive measures of provider ordering behavior,3 Ellenbogen et al4 derived a novel index to identify hospitals with high rates of low-yield diagnostic testing. This index is based on the concept that, in the presence of nonspecific, symptom-based principal diagnoses, a substantial proportion of (apparently) non-diagnostic related studies were probably ordered despite a low pretest probability of serious disease. Since such symptom-based diagnoses reflect the absence of a more specific diagnosis, the examinations observed are markers of physician-driven decisions leading to discretionary utilization likely to be of low-value to patients. This study fills a critical gap in dual measures of appropriateness and yield, rather than simply utilization, to advance the Choosing Wisely campaign.3
Advantages of this overuse index include its derivation from administrative data, obviating the need for electronic health records, and incorporation of diagnostic yield at the inpatient-encounter level. One study selected procedures identifiable solely with claims from a set deemed overused by professional/consumer groups.5 However, the yield of physician decisions in specific cases was not measured. In contrast, this novel index is derived from an assessment of diagnostic yield.4 Although test results are not known with certainty, the absence of a specific discharge diagnosis serves as a test result proxy. Measurement of diagnostic examination yield at the patient-level (aggregated to the hospital-level) may be applicable across hospitals with varied patient populations, which include large differences in patient and/or family preferences to seek medical attention and engage in shared decision-making. The role that patient preferences play in decisions creates a limitation in this index—while decisions for the candidate diagnostic tests are physician driven, patient demand may be a confounding factor. This index cannot therefore be considered purely a measure of physician-induced intensity of diagnostic services. Patient-reported data would enhance future analyses by more fully capturing all dimensions of care necessary to identify low-value services. Subjective outcomes are critical in completely measuring the aggregate benefits of tests and interventions judged low-value based on objective metrics. Such data would also aid in quantifying the relative contributions of patient and physician preferences in driving discretionary utilization.
Finally, the derived index is restricted to diagnostic decision-making and may not be applicable to treatment-related practice patterns. However, the literature suggests strong correlations between diagnostic and therapeutic intensity. Application of this novel index will play an important role in reducing low-value discretionary utilization.
Overutilization and low-value care are important clinical and policy problems. Their measurement is challenging because it requires detailed clinical information. Additionally, there are inherent difficulties in identifying discretionary services likely to be inappropriate or low-value and demonstrating that certain services produce little/no health benefit. Quantifying “ideal” expected testing rates—ones that would reflect minimization of inappropriate/low-value care without excluding essential, high-yield diagnostic services—presents additional challenges. Consequently, of 521 unique measures specified by national measurement programs and professional guidelines, 91.6% targeted underuse, while only 6.5% targeted overuse.1
The potential for unintended consequences of implementing measures to eliminate overuse are a barrier to incorporating such measures into practice.2 For example, measuring, reporting, and penalizing overuse of inappropriate bone scanning may lead to underuse in patients for whom scanning is crucial.2 Most overuse measures based on inappropriate or low-value indications relate to imaging and medications.1 However, there is increasing interest in overutilization measures based on a broad set of health services. Identifying low-value testing or treatments often requires a substantial degree of clinical detail to avoid the damaging inclusion of beneficial services, which may lead to unintended negative outcomes, creating skepticism among clinicians. Ultimately, getting measurement of low-value care wrong would undermine adoption of interventions to reduce overuse.
To reduce low-value care through expansive measures of provider ordering behavior,3 Ellenbogen et al4 derived a novel index to identify hospitals with high rates of low-yield diagnostic testing. This index is based on the concept that, in the presence of nonspecific, symptom-based principal diagnoses, a substantial proportion of (apparently) non-diagnostic related studies were probably ordered despite a low pretest probability of serious disease. Since such symptom-based diagnoses reflect the absence of a more specific diagnosis, the examinations observed are markers of physician-driven decisions leading to discretionary utilization likely to be of low-value to patients. This study fills a critical gap in dual measures of appropriateness and yield, rather than simply utilization, to advance the Choosing Wisely campaign.3
Advantages of this overuse index include its derivation from administrative data, obviating the need for electronic health records, and incorporation of diagnostic yield at the inpatient-encounter level. One study selected procedures identifiable solely with claims from a set deemed overused by professional/consumer groups.5 However, the yield of physician decisions in specific cases was not measured. In contrast, this novel index is derived from an assessment of diagnostic yield.4 Although test results are not known with certainty, the absence of a specific discharge diagnosis serves as a test result proxy. Measurement of diagnostic examination yield at the patient-level (aggregated to the hospital-level) may be applicable across hospitals with varied patient populations, which include large differences in patient and/or family preferences to seek medical attention and engage in shared decision-making. The role that patient preferences play in decisions creates a limitation in this index—while decisions for the candidate diagnostic tests are physician driven, patient demand may be a confounding factor. This index cannot therefore be considered purely a measure of physician-induced intensity of diagnostic services. Patient-reported data would enhance future analyses by more fully capturing all dimensions of care necessary to identify low-value services. Subjective outcomes are critical in completely measuring the aggregate benefits of tests and interventions judged low-value based on objective metrics. Such data would also aid in quantifying the relative contributions of patient and physician preferences in driving discretionary utilization.
Finally, the derived index is restricted to diagnostic decision-making and may not be applicable to treatment-related practice patterns. However, the literature suggests strong correlations between diagnostic and therapeutic intensity. Application of this novel index will play an important role in reducing low-value discretionary utilization.
1. Newton EH, Zazzera EA, Van Moorsel G, Sirovich BE. Undermeasuring overuse--an examination of national clinical performance measures. JAMA Intern Med. 2015;175(10):1709-1711. https://doi.org/10.1001/jamainternmed.2015.4025
2. Mathias JS, Baker DW. Developing quality measures to address overuse. JAMA. 2013;309(18):1897-1898. https://doi.org/10.1001/jama.2013.3588
3. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24(8):523-531. https://doi.org/10.1136/bmjqs-2015-004070
4. Ellenbogen MI, Prichett L, Johnson PT, Brotman DJ. Development of a simple index to measure overuse of diagnostic testing at the hospital level using administrative data. J Hosp Med. 2021;16:xxx-xxx. https://doi.org/10.12788/jhm.3547
5. Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52(2):157-163. https://doi.org/10.1097/MLR.0000000000000052
1. Newton EH, Zazzera EA, Van Moorsel G, Sirovich BE. Undermeasuring overuse--an examination of national clinical performance measures. JAMA Intern Med. 2015;175(10):1709-1711. https://doi.org/10.1001/jamainternmed.2015.4025
2. Mathias JS, Baker DW. Developing quality measures to address overuse. JAMA. 2013;309(18):1897-1898. https://doi.org/10.1001/jama.2013.3588
3. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24(8):523-531. https://doi.org/10.1136/bmjqs-2015-004070
4. Ellenbogen MI, Prichett L, Johnson PT, Brotman DJ. Development of a simple index to measure overuse of diagnostic testing at the hospital level using administrative data. J Hosp Med. 2021;16:xxx-xxx. https://doi.org/10.12788/jhm.3547
5. Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52(2):157-163. https://doi.org/10.1097/MLR.0000000000000052
© 2021Society of Hospital Medicine