User login
A Smoky Dilemma
A 23-year-old woman presented to the emergency department complaining of “feeling terrible” for the past week. She described subjective fevers, chills, nonproductive cough, myalgias, and nausea. Her symptoms worsened on the day of presentation, with drenching night sweats, worsening myalgias, and generalized fatigue. She was unable to tolerate oral intake due to persistent nausea and had one episode of emesis.
While the initial constellation of symptoms suggests a viral syndrome, its progression over a week raises concern for something more ominous. Of her relatively nonspecific symptoms, prominent myalgias accompanied by a febrile illness may be most helpful. Fever, myalgias, and nonproductive cough are typical of seasonal influenza, although the presence of nausea and vomiting is atypical in adults. (Though this patient presented for care prior to the coronavirus disease 2019 [COVID-19] pandemic, depending on the timing of this presentation, COVID-19 should be considered.) Acute viral myositis can complicate many viral illnesses, such as influenza, coxsackie, and Epstein-Barr virus infections. Other infectious causes of myositis include systemic bacterial infections, spirochete diseases, and other viral infections, including dengue fever. Myalgias can also be a prominent feature of noninfectious systemic inflammatory conditions, such as systemic lupus erythematosus, rheumatoid arthritis, polymyositis, and systemic vasculitis. Night sweats, while concerning, can be present in myriad conditions, and are not usually a discriminating symptom.
Her past medical history included depression, nephrolithiasis, frequent urinary tract infections, bladder spasms, and recurrent genital herpes simplex virus infection. Her medications included bupropion, microgestin, mirabegron, and valacyclovir. Her father had emphysema.
The patient was employed as a physical therapy assistant in a geriatric care center. Two weeks prior to presentation, she traveled from her home in North Carolina to visit a friend in Atlanta, Georgia. Shortly after the patient returned home, her friend in Atlanta became ill and was treated empirically for Legionella infection because of a recent outbreak in the area. One week prior to presentation, the patient and her boyfriend went on a day hike in the Smoky Mountains in North Carolina, but the patient did not recall any insect or tick bites. Her boyfriend had not been ill.
This history elucidates several potentially relevant medication and environmental exposures. Although bupropion can cause myalgias, neither it nor the other medications she is taking are likely to cause her constellation of symptoms. Her travel history to Atlanta suggests possible, though unconfirmed, exposure to Legionella pneumophila. Notably, she would have had to be exposed to the same source as her friend, since transmission of Legionella occurs via contaminated water and soil, not by human-to-human contact. Legionella infection typically causes a pneumonic process as described here, but her prominent myalgias would not be typical.
Her hike in the Smoky Mountains could have exposed her to several vector-borne diseases. Mosquito-borne dengue in North Carolina is extremely rare, but West Nile virus and eastern equine virus are found within that region. West Nile virus could cause a similar illness, although the cough and lack of neurologic symptoms would be unusual. Eastern equine virus can also cause similar symptoms but is quite rare.
Tick-borne illnesses that should be considered for this region include Lyme disease, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and babesiosis. These tend to present with nonspecific symptoms, but myalgias and fever are consistent features. Lyme disease this close to tick exposure usually presents with the characteristic erythema migrans rash, present in 80% of cases, with or without an influenza-like illness. Approximately 80% of patients do not recall a tick bite, even though a tick must be attached for 36 to 48 hours to transmit the spirochete. RMSF often presents with fever and myalgias, with arthralgias and headache, which are lacking in this case. The common, characteristic rash of blanching erythematous macules that convert to petechiae, starting at the ankles and wrists and spreading to the trunk, is often absent at presentation, showing up at days 3 to 5 in most patients.
Ehrlichiosis presents with an influenza-like illness, but up to half of patients also have nausea and cough. It can also present with a macular and petechial rash in a minority of patients. Lastly, babesiosis presents with an influenza-like illness and less often with cough or nausea. At this juncture, RMSF and ehrlichiosis are possibilities given the hiking history and symptoms, although the absence of a rash points more to ehrlichiosis.
The patient did not smoke cigarettes but had used a JUUL© vaporizer daily for the prior 2 years. Her last use was 1 week prior to admission. She used tetrahydrocannabinol (THC) pods purchased online in the vaporizer on a few occasions 1month prior but had not used THC since that time. She denied alcohol or other drug use.
Until recently, this important detail about vaping use would have been passed over without much consideration. Though reports of acute lung injury from vaping were published as early as 2017, it first came to national attention in August 2019 when the Centers for Disease Control and Prevention posted a Health Advisory about severe lung injury associated with e-cigarette use. Of note, this advisory and subsequent published case series outline that e-cigarette, or vaping, use-associated lung injury (EVALI) may present with more than just respiratory symptoms. Most patients have respiratory symptoms such as shortness of breath, cough, or pleurisy, but many have gastrointestinal symptoms which may include abdominal pain, nausea, vomiting, and diarrhea.1 Constitutional symptoms, including fever, chills, or weight loss, may also predominate.2 In some cases, the gastrointestinal symptoms precede the pulmonary symptoms. This patient’s symptoms warrant consideration of EVALI starting with a chest x-ray (CXR), which is usually abnormal in this disease.2
Physical examination revealed that the patient was alert, diaphoretic, and in mild respiratory distress. Temperature was 103.6 °F, blood pressure 129/75 mm Hg, pulse 130 beats per minute, respiratory rate 20 per minute, and oxygen saturation 97% while breathing ambient air. Cardiac examination revealed tachycardia without murmurs, rubs, or gallops. Lung exam revealed scattered rhonchi over the left posterior lower chest without egophony or dullness to percussion. Findings from abdominal, skin, neurologic, lymph node, and musculoskeletal exams were unremarkable.
Her fever, tachycardia, and respiratory distress point to a pulmonary process such as pneumonia or EVALI, even though she does not have definitive physical exam evidence of pneumonia. She presents with systemic inflammatory response syndrome without significant hypoxia and with borderline tachypnea, which could be related to sepsis or lactic acidosis from a systemic infection other than pneumonia. Her symptom complex could also be compatible with severe influenza infection. The absence of rash makes RMSF less likely.
Results of a complete blood count demonstrated a white blood cell count of 12,600/µL with 87% neutrophils. Results of a metabolic panel were normal, and a urine pregnancy test was negative. The electrocardiogram revealed sinus tachycardia without other abnormalities. A CXR showed no evidence of acute cardiopulmonary abnormalities.
Her lab studies lack thrombocytopenia, which is often found in ehrlichiosis and RMSF. Leukopenia is also absent, which can be seen in Lyme disease and ehrlichiosis. The mild leukocytosis could be consistent with pneumonia, influenza, and EVALI and is not discriminating. The normal CXR goes against pneumonia or EVALI; however, 9% of patients with EVALI in one case series had a normal CXR, while computed tomography (CT) of the chest demonstrated bilateral ground-glass opacities.3 Chest CT is indicated in this case given the poor correlation of the CXR findings and this patient’s pronounced respiratory symptoms.
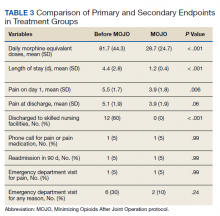
CT of the chest with contrast did not show a pulmonary embolism but revealed diffuse ground-glass opacities, predominantly in the dependent lower lobes (Figure 1).
Acute conditions with diffuse ground-glass opacities include mycoplasma, Pneumocystis jiroveci and viral pneumonias, pulmonary hemorrhage and edema, acute interstitial pneumonia, eosinophilic lung diseases, and hypersensitivity pneumonitis. Diffuse ground-glass opacities are also seen in almost all patients with EVALI. Though less likely, RMSF, babesiosis, and ehrlichiosis are not ruled out by these chest CT findings, since these disease entities can sometimes cause pulmonary manifestations, including pneumonia, pulmonary edema, and acute respiratory distress syndrome (ARDS).4
In addition to Legionella and pneumococcal urinary antigen tests, respiratory viral panel, and blood cultures, it would be judicious to obtain HIV, C-reactive protein, and erythrocyte sedimentation rate (ESR) testing; these last two tests are often markedly elevated in EVALI. The utility of bronchoalveolar lavage (BAL) in suspected EVALI cases is not clearly defined, but should be considered in this case to ensure that infectious etiologies are not missed.2 Because of her potential environmental exposures, serologic testing for RMSF and ehrlichiosis should be sent.
Given the overlap in signs and symptoms of EVALI with various, potentially life-threatening infections, she should be empirically treated with antibiotics to cover for community-acquired pneumonia. Adding or even substituting doxycycline for a macrolide antibiotic in this regimen should be considered given that it would treat both RMSF and ehrlichiosis pending further test results. Delay in treating RMSF is associated with worse outcomes. If she is presenting during influenza season, she should also be treated with a neuraminidase inhibitor while awaiting influenza test results. Though the pathophysiology of EVALI is not entirely known, it appears to be inflammatory in nature. Most presumed cases have responded to corticosteroids, with improvement in oxygenation.2 Therefore, treatment with corticosteroids may be warranted to improve oxygenation while ruling out infectious processes.
The patient was admitted to the general medicine wards and started on ceftriaxone and azithromycin for empiric treatment of community-acquired pneumonia. On hospital day 2, a respiratory viral panel returned negative. Procalcitonin, HIV, and blood cultures all returned negative. An ESR was elevated at 86 mm/h. The patient continued to have daily fevers and developed erythematous, blanching macules on the neck, chest, back, and arms, which were noted to occur during febrile periods. Ceftriaxone and azithromycin were discontinued, and doxycycline was started. By hospital day 4, the patient’s oxygen saturation worsened to 86% on ambient air. She continued to have fevers and her cough worsened, with occasional blood-streaked sputum. The patient was transferred to the intensive care unit for closer monitoring.
On hospital day 5, she required intubation for worsening hypoxia. Bronchoscopy was performed, which revealed small mucosal crypts along the left mainstem bronchus. A small amount of bleeding after transbronchial biopsy of the left lower lobe was noted, which resolved with occlusion using the bronchoscope. BAL was performed, which revealed red, cloudy aspirate with 1,100 white blood cells (85% neutrophils) and 22,400 red blood cells. No bacteria were identified.
The patient has developed hypoxic respiratory failure despite appropriate antibiotics and negative cultures, increasing the likelihood of a noninfectious etiology. Her rash is not typical for RMSF, which usually starts as a macular or petechial rash at the ankles and wrists, and spreads centrally to the trunk. Rash is not typically associated with EVALI, and in this case, may represent miliaria caused by her fever.
The mucosal crypts seen on bronchoscopy are nonspecific, likely indicating inflammation from vaping. The BAL otherwise suggests diffuse alveolar hemorrhage (DAH), although sequential BAL aliquots are needed to confirm this diagnosis. DAH is usually caused by pulmonary capillaritis from vasculitis, Goodpasture disease, rheumatic diseases, or diffuse alveolar damage from toxins, infections, rheumatic diseases, or interstitial or organizing pneumonias. Diffuse alveolar damage is the pathologic finding of ARDS, which can be seen in severe cases of many of the conditions discussed, including EVALI, ehrlichiosis, babesiosis, sepsis, and community-acquired pneumonia.4
The BAL is most consistent with EVALI, which often shows elevated neutrophils. DAH due to vaping has also been reported.5 In patients with EVALI, varied pathologic findings of acute lung injury have been reported, including diffuse alveolar damage.6 At this point, laboratory evaluation for rheumatologic diseases and vasculitis should be obtained, and lung biopsy results reviewed. Given her clinical deterioration, treatment with intravenous corticosteroids for presumed EVALI is warranted.
Urine Legionella and Streptococcal pneumoniae antigen tests were negative. The patient was started on methylprednisolone 40 mg intravenously every 8 hours. Further testing included antinuclear antibodies, which was positive at 1:320, with a dense, fine speckled pattern. Perinuclear antineutrophilic cytoplasmic autoantibody, cytoplasmic antineutrophilic cytoplasmic autoantibody, myeloperoxidase, proteinase 3, double-stranded DNA, and glomerular basement membrane IgG were all negative. Transbronchial lung biopsy revealed severe acute lung injury consistent with diffuse alveolar damage. The pulmonary interstitium was mildly expanded by edema, with a moderate number of eosinophilic hyaline membranes. There were no eosinophils or evidence of hemorrhage, granulomas, or giant cells. These changes, within this clinical context, were diagnostic for EVALI.
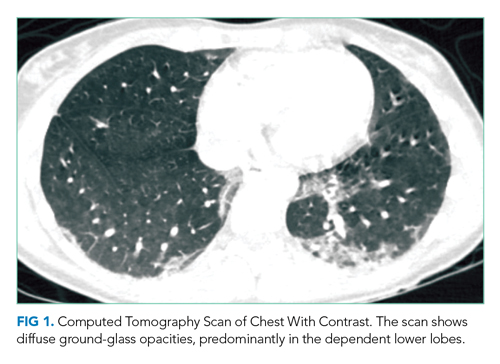
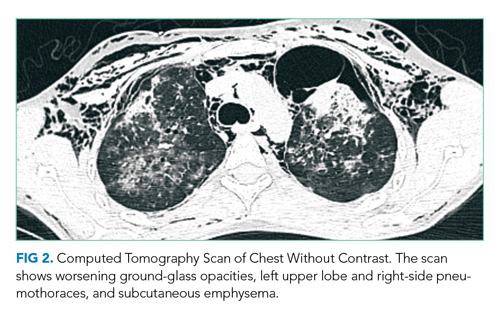
The patient was intubated for 4 days and completed a course of empiric antibiotics as well as a 10-day course of prednisone. She was discharged on hospital day 17 on 2 L continuous oxygen via nasal cannula. Two days after discharge, she developed worsening dyspnea and chest pain and was readmitted with worsening ground-glass opacities, left upper lobe and right- sided pneumothoraces, and subcutaneous emphysema (Figure 2). She was treated with continuous oxygen to maintain oxygen saturation at 100% and eventually discharged home 3 days later on 3 L continuous oxygen. She attended pulmonary rehabilitation and was weaned off oxygen 2 months later, with marked improvement in aeration of both lungs (Figure 3). She continued to abstain from tobacco and THC products.
DISCUSSION
The first electronic cigarette (e-cigarette) device was developed in 2003 by a Chinese pharmacist and introduced to the American market in 2007.7 E-cigarettes produce an inhalable aerosol by heating a liquid containing a variety of chemicals, nicotine, and flavors, with or without other additives. Originally promoted as a safer nontobacco and cessation device by producers, e-cigarette sales grew at an annual rate of 115% between 2009 and 2012.8 E-cigarettes can also be used to deliver THC, the psychoactive component of cannabis.
Since the advent of e-cigarettes, their safety has been a topic of concern. In August 2019, the CDC announced 215 possible cases of severe pulmonary disease associated with the use of e-cigarette products that were reported by 25 state health departments.1 By February 2020, EVALI had affected more than 2,800 patients hospitalized across the United States.9
The presenting symptoms of EVALI are varied and nonspecific. The largest EVALI case series, published by Layden et al in 2020, included 98 patients who had a median duration of 6 days of symptoms prior to presentation.3 Respiratory symptoms occurred in 97% of patients, including shortness of breath, any chest pain, pleuritic chest pain, cough, and hemoptysis.3 Presentations also included a variety of gastrointestinal (77%) and constitutional (100%) symptoms, which most commonly included nausea, vomiting, and fever.3 Additional case series have supported a specific pattern of presentation, most commonly including pleuritic chest pain, nonproductive cough, or shortness of breath occurring days to weeks prior to presentation. Associated fatigue, fever, and tachycardia may be present, as well as nausea, vomiting, diarrhea and abdominal pain, and in some cases, these have preceded respiratory symptoms.3,10,11
The vital signs and physical examination, laboratory, and imaging results associated with EVALI are also fairly nonspecific. The most common reason for hospitalization in EVALI is hypoxia, which can progress to acute respiratory failure requiring supplemental oxygen or, as in this case, mechanical ventilation. The most common laboratory finding is leukocytosis greater than 11,000/µL, with more than 80% neutrophils and an ESR greater than 30 mm/hr. In the Layden et al case series, 83% of patients had an abnormal CXR. All patients who underwent CT scan of the chest had bilateral ground-glass opacities, often with subpleural sparing.3 A minority of patients were found to have a pneumothorax, generally a late finding.3,12 Accordingly, the CDC now defines confirmed EVALI as use of e-cigarettes during the 90 days before symptom onset with the presence of pulmonary infiltrates (opacities on CXR or ground-glass opacities on chest CT), negative results on testing for all clinically indicated respiratory infections including respiratory viral panel and influenza PCR, and no alternative plausible diagnoses.13
The presumed etiology of EVALI is chemical exposure because no consistent infectious etiology has been identified.6 No consistent e-cigarette product, substance, or additive has been identified in all cases, nor has one product been directly linked to EVALI. However, the CDC recently announced that vitamin E acetate in vaping products appears to be associated with EVALI.9 In December 2019, Blount et al identified vitamin E acetate in BAL fluid samples from 48 of 51 EVALI patients.14 Additionally, while no other toxins were identified, 94% of samples contained THC or its metabolites or patients had reported vaping THC within 90 days preceding illness.14
The most effective treatment strategy for EVALI is still unknown. It is recommended to treat with empiric antibiotics for at least 48 hours (and antivirals during influenza season) if the history is unclear or if the patient is intubated or has severe hypoxemia.2 If antibiotic and/or antiviral therapies do not lead to clinical improvement, corticosteroids should be added, as they lead to improved oxygenation in many patients.2 Kalininskiy et al recommend initial administration of methylprednisolone 40 mg every 8 hours, with transition to oral prednisone to complete a 2-week course.2 Given rates of rehospitalization (2.7%) and death (2%) in EVALI, the CDC advises that patients should be clinically stable for 24 to 48 hours prior to discharge; that follow-up visits should be arranged within 48 hours of discharge; and that cases of EVALI should be reported to the state and local health departments.15 As seen in the case presented here, with time and continued abstinence from e-cigarette use, the pulmonary effects of EVALI can improve, but long-term outcomes remain unclear. Clinicians must now consider EVALI in patients presenting with respiratory, constitutional, and gastrointestinal complaints when a history of e-cigarette use is present.
KEY TEACHING POINTS
- EVALI presents most commonly with a combination of respiratory, gastrointestinal, and constitutional symptoms. including shortness of breath, cough, nausea, vomiting, and fever.
- When considering EVALI, evaluate and treat for potential infectious causes of disease first.
- Corticosteroids are the mainstay of therapy in EVALI, leading to improvement in oxygenation in many patients.
- Most of the reported cases of EVALI have occurred in patients who have vaped THC-containing products.
1. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use – Interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68(36):787-790. https://doi.org/10.15585/mmwr.mm6836e2
2. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/s2213-2600(19)30415-1
3. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illiniois and Wisconsin – final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/nejmoa1911614
4. Faul JL, Doyle RL, Kao PN, Ruoss SJ. Tick-borne pulmonary disease: update on diagnosis and management. Chest. 1999;116(1):222-230. https://doi.org/10.1378/chest.116.1.222
5. Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. https://doi.org/10.1155/2018/9724530
6. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780-1781. https://doi.org/10.1056/nejmc1913069
7. Office of the Surgeon General. E-Cigarette Use Among Youth and Young Adults. Chapter 1. Public Health Service, U.S. Department of Health & Human Services; 2016. Accessed January 22, 2020. https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
8. Grana R, Benowitz N, Glantz SA. Background Paper on E-cigarettes (Electronic Nicotine Delivery Systems). UCSF: Center for Tobacco Control Research and Education; 2013. https://escholarship.org/uc/item/13p2b72n
9. Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. December 12, 2019. Updated February 25, 2020. Accessed January 22, 2020 and July 16, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
10. Davidson K, Brancato A, Heetkerks P, et al. Outbreak of e-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36);784-786. https://doi.org/10.15585/mmwr.mm6836e1
11. Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488-1489. https://doi.org/10.1056/nejmc1912038
12. Henry TS, Kanne JP, Klingerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486-1487. https://doi.org/10.1056/nejmc1911995
13. Smoking and Tobacco Use: For State, Local, Territorial, and Tribal Health Departments. Centers for Disease Control and Prevention. Accessed Jan 24, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html
14. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/nejmoa1916433
15. Evans ME, Twentyman E, Click ES, et al. Update: Interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use–associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge — United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
A 23-year-old woman presented to the emergency department complaining of “feeling terrible” for the past week. She described subjective fevers, chills, nonproductive cough, myalgias, and nausea. Her symptoms worsened on the day of presentation, with drenching night sweats, worsening myalgias, and generalized fatigue. She was unable to tolerate oral intake due to persistent nausea and had one episode of emesis.
While the initial constellation of symptoms suggests a viral syndrome, its progression over a week raises concern for something more ominous. Of her relatively nonspecific symptoms, prominent myalgias accompanied by a febrile illness may be most helpful. Fever, myalgias, and nonproductive cough are typical of seasonal influenza, although the presence of nausea and vomiting is atypical in adults. (Though this patient presented for care prior to the coronavirus disease 2019 [COVID-19] pandemic, depending on the timing of this presentation, COVID-19 should be considered.) Acute viral myositis can complicate many viral illnesses, such as influenza, coxsackie, and Epstein-Barr virus infections. Other infectious causes of myositis include systemic bacterial infections, spirochete diseases, and other viral infections, including dengue fever. Myalgias can also be a prominent feature of noninfectious systemic inflammatory conditions, such as systemic lupus erythematosus, rheumatoid arthritis, polymyositis, and systemic vasculitis. Night sweats, while concerning, can be present in myriad conditions, and are not usually a discriminating symptom.
Her past medical history included depression, nephrolithiasis, frequent urinary tract infections, bladder spasms, and recurrent genital herpes simplex virus infection. Her medications included bupropion, microgestin, mirabegron, and valacyclovir. Her father had emphysema.
The patient was employed as a physical therapy assistant in a geriatric care center. Two weeks prior to presentation, she traveled from her home in North Carolina to visit a friend in Atlanta, Georgia. Shortly after the patient returned home, her friend in Atlanta became ill and was treated empirically for Legionella infection because of a recent outbreak in the area. One week prior to presentation, the patient and her boyfriend went on a day hike in the Smoky Mountains in North Carolina, but the patient did not recall any insect or tick bites. Her boyfriend had not been ill.
This history elucidates several potentially relevant medication and environmental exposures. Although bupropion can cause myalgias, neither it nor the other medications she is taking are likely to cause her constellation of symptoms. Her travel history to Atlanta suggests possible, though unconfirmed, exposure to Legionella pneumophila. Notably, she would have had to be exposed to the same source as her friend, since transmission of Legionella occurs via contaminated water and soil, not by human-to-human contact. Legionella infection typically causes a pneumonic process as described here, but her prominent myalgias would not be typical.
Her hike in the Smoky Mountains could have exposed her to several vector-borne diseases. Mosquito-borne dengue in North Carolina is extremely rare, but West Nile virus and eastern equine virus are found within that region. West Nile virus could cause a similar illness, although the cough and lack of neurologic symptoms would be unusual. Eastern equine virus can also cause similar symptoms but is quite rare.
Tick-borne illnesses that should be considered for this region include Lyme disease, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and babesiosis. These tend to present with nonspecific symptoms, but myalgias and fever are consistent features. Lyme disease this close to tick exposure usually presents with the characteristic erythema migrans rash, present in 80% of cases, with or without an influenza-like illness. Approximately 80% of patients do not recall a tick bite, even though a tick must be attached for 36 to 48 hours to transmit the spirochete. RMSF often presents with fever and myalgias, with arthralgias and headache, which are lacking in this case. The common, characteristic rash of blanching erythematous macules that convert to petechiae, starting at the ankles and wrists and spreading to the trunk, is often absent at presentation, showing up at days 3 to 5 in most patients.
Ehrlichiosis presents with an influenza-like illness, but up to half of patients also have nausea and cough. It can also present with a macular and petechial rash in a minority of patients. Lastly, babesiosis presents with an influenza-like illness and less often with cough or nausea. At this juncture, RMSF and ehrlichiosis are possibilities given the hiking history and symptoms, although the absence of a rash points more to ehrlichiosis.
The patient did not smoke cigarettes but had used a JUUL© vaporizer daily for the prior 2 years. Her last use was 1 week prior to admission. She used tetrahydrocannabinol (THC) pods purchased online in the vaporizer on a few occasions 1month prior but had not used THC since that time. She denied alcohol or other drug use.
Until recently, this important detail about vaping use would have been passed over without much consideration. Though reports of acute lung injury from vaping were published as early as 2017, it first came to national attention in August 2019 when the Centers for Disease Control and Prevention posted a Health Advisory about severe lung injury associated with e-cigarette use. Of note, this advisory and subsequent published case series outline that e-cigarette, or vaping, use-associated lung injury (EVALI) may present with more than just respiratory symptoms. Most patients have respiratory symptoms such as shortness of breath, cough, or pleurisy, but many have gastrointestinal symptoms which may include abdominal pain, nausea, vomiting, and diarrhea.1 Constitutional symptoms, including fever, chills, or weight loss, may also predominate.2 In some cases, the gastrointestinal symptoms precede the pulmonary symptoms. This patient’s symptoms warrant consideration of EVALI starting with a chest x-ray (CXR), which is usually abnormal in this disease.2
Physical examination revealed that the patient was alert, diaphoretic, and in mild respiratory distress. Temperature was 103.6 °F, blood pressure 129/75 mm Hg, pulse 130 beats per minute, respiratory rate 20 per minute, and oxygen saturation 97% while breathing ambient air. Cardiac examination revealed tachycardia without murmurs, rubs, or gallops. Lung exam revealed scattered rhonchi over the left posterior lower chest without egophony or dullness to percussion. Findings from abdominal, skin, neurologic, lymph node, and musculoskeletal exams were unremarkable.
Her fever, tachycardia, and respiratory distress point to a pulmonary process such as pneumonia or EVALI, even though she does not have definitive physical exam evidence of pneumonia. She presents with systemic inflammatory response syndrome without significant hypoxia and with borderline tachypnea, which could be related to sepsis or lactic acidosis from a systemic infection other than pneumonia. Her symptom complex could also be compatible with severe influenza infection. The absence of rash makes RMSF less likely.
Results of a complete blood count demonstrated a white blood cell count of 12,600/µL with 87% neutrophils. Results of a metabolic panel were normal, and a urine pregnancy test was negative. The electrocardiogram revealed sinus tachycardia without other abnormalities. A CXR showed no evidence of acute cardiopulmonary abnormalities.
Her lab studies lack thrombocytopenia, which is often found in ehrlichiosis and RMSF. Leukopenia is also absent, which can be seen in Lyme disease and ehrlichiosis. The mild leukocytosis could be consistent with pneumonia, influenza, and EVALI and is not discriminating. The normal CXR goes against pneumonia or EVALI; however, 9% of patients with EVALI in one case series had a normal CXR, while computed tomography (CT) of the chest demonstrated bilateral ground-glass opacities.3 Chest CT is indicated in this case given the poor correlation of the CXR findings and this patient’s pronounced respiratory symptoms.
CT of the chest with contrast did not show a pulmonary embolism but revealed diffuse ground-glass opacities, predominantly in the dependent lower lobes (Figure 1).
Acute conditions with diffuse ground-glass opacities include mycoplasma, Pneumocystis jiroveci and viral pneumonias, pulmonary hemorrhage and edema, acute interstitial pneumonia, eosinophilic lung diseases, and hypersensitivity pneumonitis. Diffuse ground-glass opacities are also seen in almost all patients with EVALI. Though less likely, RMSF, babesiosis, and ehrlichiosis are not ruled out by these chest CT findings, since these disease entities can sometimes cause pulmonary manifestations, including pneumonia, pulmonary edema, and acute respiratory distress syndrome (ARDS).4

In addition to Legionella and pneumococcal urinary antigen tests, respiratory viral panel, and blood cultures, it would be judicious to obtain HIV, C-reactive protein, and erythrocyte sedimentation rate (ESR) testing; these last two tests are often markedly elevated in EVALI. The utility of bronchoalveolar lavage (BAL) in suspected EVALI cases is not clearly defined, but should be considered in this case to ensure that infectious etiologies are not missed.2 Because of her potential environmental exposures, serologic testing for RMSF and ehrlichiosis should be sent.
Given the overlap in signs and symptoms of EVALI with various, potentially life-threatening infections, she should be empirically treated with antibiotics to cover for community-acquired pneumonia. Adding or even substituting doxycycline for a macrolide antibiotic in this regimen should be considered given that it would treat both RMSF and ehrlichiosis pending further test results. Delay in treating RMSF is associated with worse outcomes. If she is presenting during influenza season, she should also be treated with a neuraminidase inhibitor while awaiting influenza test results. Though the pathophysiology of EVALI is not entirely known, it appears to be inflammatory in nature. Most presumed cases have responded to corticosteroids, with improvement in oxygenation.2 Therefore, treatment with corticosteroids may be warranted to improve oxygenation while ruling out infectious processes.
The patient was admitted to the general medicine wards and started on ceftriaxone and azithromycin for empiric treatment of community-acquired pneumonia. On hospital day 2, a respiratory viral panel returned negative. Procalcitonin, HIV, and blood cultures all returned negative. An ESR was elevated at 86 mm/h. The patient continued to have daily fevers and developed erythematous, blanching macules on the neck, chest, back, and arms, which were noted to occur during febrile periods. Ceftriaxone and azithromycin were discontinued, and doxycycline was started. By hospital day 4, the patient’s oxygen saturation worsened to 86% on ambient air. She continued to have fevers and her cough worsened, with occasional blood-streaked sputum. The patient was transferred to the intensive care unit for closer monitoring.
On hospital day 5, she required intubation for worsening hypoxia. Bronchoscopy was performed, which revealed small mucosal crypts along the left mainstem bronchus. A small amount of bleeding after transbronchial biopsy of the left lower lobe was noted, which resolved with occlusion using the bronchoscope. BAL was performed, which revealed red, cloudy aspirate with 1,100 white blood cells (85% neutrophils) and 22,400 red blood cells. No bacteria were identified.
The patient has developed hypoxic respiratory failure despite appropriate antibiotics and negative cultures, increasing the likelihood of a noninfectious etiology. Her rash is not typical for RMSF, which usually starts as a macular or petechial rash at the ankles and wrists, and spreads centrally to the trunk. Rash is not typically associated with EVALI, and in this case, may represent miliaria caused by her fever.
The mucosal crypts seen on bronchoscopy are nonspecific, likely indicating inflammation from vaping. The BAL otherwise suggests diffuse alveolar hemorrhage (DAH), although sequential BAL aliquots are needed to confirm this diagnosis. DAH is usually caused by pulmonary capillaritis from vasculitis, Goodpasture disease, rheumatic diseases, or diffuse alveolar damage from toxins, infections, rheumatic diseases, or interstitial or organizing pneumonias. Diffuse alveolar damage is the pathologic finding of ARDS, which can be seen in severe cases of many of the conditions discussed, including EVALI, ehrlichiosis, babesiosis, sepsis, and community-acquired pneumonia.4
The BAL is most consistent with EVALI, which often shows elevated neutrophils. DAH due to vaping has also been reported.5 In patients with EVALI, varied pathologic findings of acute lung injury have been reported, including diffuse alveolar damage.6 At this point, laboratory evaluation for rheumatologic diseases and vasculitis should be obtained, and lung biopsy results reviewed. Given her clinical deterioration, treatment with intravenous corticosteroids for presumed EVALI is warranted.
Urine Legionella and Streptococcal pneumoniae antigen tests were negative. The patient was started on methylprednisolone 40 mg intravenously every 8 hours. Further testing included antinuclear antibodies, which was positive at 1:320, with a dense, fine speckled pattern. Perinuclear antineutrophilic cytoplasmic autoantibody, cytoplasmic antineutrophilic cytoplasmic autoantibody, myeloperoxidase, proteinase 3, double-stranded DNA, and glomerular basement membrane IgG were all negative. Transbronchial lung biopsy revealed severe acute lung injury consistent with diffuse alveolar damage. The pulmonary interstitium was mildly expanded by edema, with a moderate number of eosinophilic hyaline membranes. There were no eosinophils or evidence of hemorrhage, granulomas, or giant cells. These changes, within this clinical context, were diagnostic for EVALI.
The patient was intubated for 4 days and completed a course of empiric antibiotics as well as a 10-day course of prednisone. She was discharged on hospital day 17 on 2 L continuous oxygen via nasal cannula. Two days after discharge, she developed worsening dyspnea and chest pain and was readmitted with worsening ground-glass opacities, left upper lobe and right- sided pneumothoraces, and subcutaneous emphysema (Figure 2). She was treated with continuous oxygen to maintain oxygen saturation at 100% and eventually discharged home 3 days later on 3 L continuous oxygen. She attended pulmonary rehabilitation and was weaned off oxygen 2 months later, with marked improvement in aeration of both lungs (Figure 3). She continued to abstain from tobacco and THC products.

DISCUSSION
The first electronic cigarette (e-cigarette) device was developed in 2003 by a Chinese pharmacist and introduced to the American market in 2007.7 E-cigarettes produce an inhalable aerosol by heating a liquid containing a variety of chemicals, nicotine, and flavors, with or without other additives. Originally promoted as a safer nontobacco and cessation device by producers, e-cigarette sales grew at an annual rate of 115% between 2009 and 2012.8 E-cigarettes can also be used to deliver THC, the psychoactive component of cannabis.

Since the advent of e-cigarettes, their safety has been a topic of concern. In August 2019, the CDC announced 215 possible cases of severe pulmonary disease associated with the use of e-cigarette products that were reported by 25 state health departments.1 By February 2020, EVALI had affected more than 2,800 patients hospitalized across the United States.9
The presenting symptoms of EVALI are varied and nonspecific. The largest EVALI case series, published by Layden et al in 2020, included 98 patients who had a median duration of 6 days of symptoms prior to presentation.3 Respiratory symptoms occurred in 97% of patients, including shortness of breath, any chest pain, pleuritic chest pain, cough, and hemoptysis.3 Presentations also included a variety of gastrointestinal (77%) and constitutional (100%) symptoms, which most commonly included nausea, vomiting, and fever.3 Additional case series have supported a specific pattern of presentation, most commonly including pleuritic chest pain, nonproductive cough, or shortness of breath occurring days to weeks prior to presentation. Associated fatigue, fever, and tachycardia may be present, as well as nausea, vomiting, diarrhea and abdominal pain, and in some cases, these have preceded respiratory symptoms.3,10,11
The vital signs and physical examination, laboratory, and imaging results associated with EVALI are also fairly nonspecific. The most common reason for hospitalization in EVALI is hypoxia, which can progress to acute respiratory failure requiring supplemental oxygen or, as in this case, mechanical ventilation. The most common laboratory finding is leukocytosis greater than 11,000/µL, with more than 80% neutrophils and an ESR greater than 30 mm/hr. In the Layden et al case series, 83% of patients had an abnormal CXR. All patients who underwent CT scan of the chest had bilateral ground-glass opacities, often with subpleural sparing.3 A minority of patients were found to have a pneumothorax, generally a late finding.3,12 Accordingly, the CDC now defines confirmed EVALI as use of e-cigarettes during the 90 days before symptom onset with the presence of pulmonary infiltrates (opacities on CXR or ground-glass opacities on chest CT), negative results on testing for all clinically indicated respiratory infections including respiratory viral panel and influenza PCR, and no alternative plausible diagnoses.13
The presumed etiology of EVALI is chemical exposure because no consistent infectious etiology has been identified.6 No consistent e-cigarette product, substance, or additive has been identified in all cases, nor has one product been directly linked to EVALI. However, the CDC recently announced that vitamin E acetate in vaping products appears to be associated with EVALI.9 In December 2019, Blount et al identified vitamin E acetate in BAL fluid samples from 48 of 51 EVALI patients.14 Additionally, while no other toxins were identified, 94% of samples contained THC or its metabolites or patients had reported vaping THC within 90 days preceding illness.14
The most effective treatment strategy for EVALI is still unknown. It is recommended to treat with empiric antibiotics for at least 48 hours (and antivirals during influenza season) if the history is unclear or if the patient is intubated or has severe hypoxemia.2 If antibiotic and/or antiviral therapies do not lead to clinical improvement, corticosteroids should be added, as they lead to improved oxygenation in many patients.2 Kalininskiy et al recommend initial administration of methylprednisolone 40 mg every 8 hours, with transition to oral prednisone to complete a 2-week course.2 Given rates of rehospitalization (2.7%) and death (2%) in EVALI, the CDC advises that patients should be clinically stable for 24 to 48 hours prior to discharge; that follow-up visits should be arranged within 48 hours of discharge; and that cases of EVALI should be reported to the state and local health departments.15 As seen in the case presented here, with time and continued abstinence from e-cigarette use, the pulmonary effects of EVALI can improve, but long-term outcomes remain unclear. Clinicians must now consider EVALI in patients presenting with respiratory, constitutional, and gastrointestinal complaints when a history of e-cigarette use is present.
KEY TEACHING POINTS
- EVALI presents most commonly with a combination of respiratory, gastrointestinal, and constitutional symptoms. including shortness of breath, cough, nausea, vomiting, and fever.
- When considering EVALI, evaluate and treat for potential infectious causes of disease first.
- Corticosteroids are the mainstay of therapy in EVALI, leading to improvement in oxygenation in many patients.
- Most of the reported cases of EVALI have occurred in patients who have vaped THC-containing products.
A 23-year-old woman presented to the emergency department complaining of “feeling terrible” for the past week. She described subjective fevers, chills, nonproductive cough, myalgias, and nausea. Her symptoms worsened on the day of presentation, with drenching night sweats, worsening myalgias, and generalized fatigue. She was unable to tolerate oral intake due to persistent nausea and had one episode of emesis.
While the initial constellation of symptoms suggests a viral syndrome, its progression over a week raises concern for something more ominous. Of her relatively nonspecific symptoms, prominent myalgias accompanied by a febrile illness may be most helpful. Fever, myalgias, and nonproductive cough are typical of seasonal influenza, although the presence of nausea and vomiting is atypical in adults. (Though this patient presented for care prior to the coronavirus disease 2019 [COVID-19] pandemic, depending on the timing of this presentation, COVID-19 should be considered.) Acute viral myositis can complicate many viral illnesses, such as influenza, coxsackie, and Epstein-Barr virus infections. Other infectious causes of myositis include systemic bacterial infections, spirochete diseases, and other viral infections, including dengue fever. Myalgias can also be a prominent feature of noninfectious systemic inflammatory conditions, such as systemic lupus erythematosus, rheumatoid arthritis, polymyositis, and systemic vasculitis. Night sweats, while concerning, can be present in myriad conditions, and are not usually a discriminating symptom.
Her past medical history included depression, nephrolithiasis, frequent urinary tract infections, bladder spasms, and recurrent genital herpes simplex virus infection. Her medications included bupropion, microgestin, mirabegron, and valacyclovir. Her father had emphysema.
The patient was employed as a physical therapy assistant in a geriatric care center. Two weeks prior to presentation, she traveled from her home in North Carolina to visit a friend in Atlanta, Georgia. Shortly after the patient returned home, her friend in Atlanta became ill and was treated empirically for Legionella infection because of a recent outbreak in the area. One week prior to presentation, the patient and her boyfriend went on a day hike in the Smoky Mountains in North Carolina, but the patient did not recall any insect or tick bites. Her boyfriend had not been ill.
This history elucidates several potentially relevant medication and environmental exposures. Although bupropion can cause myalgias, neither it nor the other medications she is taking are likely to cause her constellation of symptoms. Her travel history to Atlanta suggests possible, though unconfirmed, exposure to Legionella pneumophila. Notably, she would have had to be exposed to the same source as her friend, since transmission of Legionella occurs via contaminated water and soil, not by human-to-human contact. Legionella infection typically causes a pneumonic process as described here, but her prominent myalgias would not be typical.
Her hike in the Smoky Mountains could have exposed her to several vector-borne diseases. Mosquito-borne dengue in North Carolina is extremely rare, but West Nile virus and eastern equine virus are found within that region. West Nile virus could cause a similar illness, although the cough and lack of neurologic symptoms would be unusual. Eastern equine virus can also cause similar symptoms but is quite rare.
Tick-borne illnesses that should be considered for this region include Lyme disease, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and babesiosis. These tend to present with nonspecific symptoms, but myalgias and fever are consistent features. Lyme disease this close to tick exposure usually presents with the characteristic erythema migrans rash, present in 80% of cases, with or without an influenza-like illness. Approximately 80% of patients do not recall a tick bite, even though a tick must be attached for 36 to 48 hours to transmit the spirochete. RMSF often presents with fever and myalgias, with arthralgias and headache, which are lacking in this case. The common, characteristic rash of blanching erythematous macules that convert to petechiae, starting at the ankles and wrists and spreading to the trunk, is often absent at presentation, showing up at days 3 to 5 in most patients.
Ehrlichiosis presents with an influenza-like illness, but up to half of patients also have nausea and cough. It can also present with a macular and petechial rash in a minority of patients. Lastly, babesiosis presents with an influenza-like illness and less often with cough or nausea. At this juncture, RMSF and ehrlichiosis are possibilities given the hiking history and symptoms, although the absence of a rash points more to ehrlichiosis.
The patient did not smoke cigarettes but had used a JUUL© vaporizer daily for the prior 2 years. Her last use was 1 week prior to admission. She used tetrahydrocannabinol (THC) pods purchased online in the vaporizer on a few occasions 1month prior but had not used THC since that time. She denied alcohol or other drug use.
Until recently, this important detail about vaping use would have been passed over without much consideration. Though reports of acute lung injury from vaping were published as early as 2017, it first came to national attention in August 2019 when the Centers for Disease Control and Prevention posted a Health Advisory about severe lung injury associated with e-cigarette use. Of note, this advisory and subsequent published case series outline that e-cigarette, or vaping, use-associated lung injury (EVALI) may present with more than just respiratory symptoms. Most patients have respiratory symptoms such as shortness of breath, cough, or pleurisy, but many have gastrointestinal symptoms which may include abdominal pain, nausea, vomiting, and diarrhea.1 Constitutional symptoms, including fever, chills, or weight loss, may also predominate.2 In some cases, the gastrointestinal symptoms precede the pulmonary symptoms. This patient’s symptoms warrant consideration of EVALI starting with a chest x-ray (CXR), which is usually abnormal in this disease.2
Physical examination revealed that the patient was alert, diaphoretic, and in mild respiratory distress. Temperature was 103.6 °F, blood pressure 129/75 mm Hg, pulse 130 beats per minute, respiratory rate 20 per minute, and oxygen saturation 97% while breathing ambient air. Cardiac examination revealed tachycardia without murmurs, rubs, or gallops. Lung exam revealed scattered rhonchi over the left posterior lower chest without egophony or dullness to percussion. Findings from abdominal, skin, neurologic, lymph node, and musculoskeletal exams were unremarkable.
Her fever, tachycardia, and respiratory distress point to a pulmonary process such as pneumonia or EVALI, even though she does not have definitive physical exam evidence of pneumonia. She presents with systemic inflammatory response syndrome without significant hypoxia and with borderline tachypnea, which could be related to sepsis or lactic acidosis from a systemic infection other than pneumonia. Her symptom complex could also be compatible with severe influenza infection. The absence of rash makes RMSF less likely.
Results of a complete blood count demonstrated a white blood cell count of 12,600/µL with 87% neutrophils. Results of a metabolic panel were normal, and a urine pregnancy test was negative. The electrocardiogram revealed sinus tachycardia without other abnormalities. A CXR showed no evidence of acute cardiopulmonary abnormalities.
Her lab studies lack thrombocytopenia, which is often found in ehrlichiosis and RMSF. Leukopenia is also absent, which can be seen in Lyme disease and ehrlichiosis. The mild leukocytosis could be consistent with pneumonia, influenza, and EVALI and is not discriminating. The normal CXR goes against pneumonia or EVALI; however, 9% of patients with EVALI in one case series had a normal CXR, while computed tomography (CT) of the chest demonstrated bilateral ground-glass opacities.3 Chest CT is indicated in this case given the poor correlation of the CXR findings and this patient’s pronounced respiratory symptoms.
CT of the chest with contrast did not show a pulmonary embolism but revealed diffuse ground-glass opacities, predominantly in the dependent lower lobes (Figure 1).
Acute conditions with diffuse ground-glass opacities include mycoplasma, Pneumocystis jiroveci and viral pneumonias, pulmonary hemorrhage and edema, acute interstitial pneumonia, eosinophilic lung diseases, and hypersensitivity pneumonitis. Diffuse ground-glass opacities are also seen in almost all patients with EVALI. Though less likely, RMSF, babesiosis, and ehrlichiosis are not ruled out by these chest CT findings, since these disease entities can sometimes cause pulmonary manifestations, including pneumonia, pulmonary edema, and acute respiratory distress syndrome (ARDS).4

In addition to Legionella and pneumococcal urinary antigen tests, respiratory viral panel, and blood cultures, it would be judicious to obtain HIV, C-reactive protein, and erythrocyte sedimentation rate (ESR) testing; these last two tests are often markedly elevated in EVALI. The utility of bronchoalveolar lavage (BAL) in suspected EVALI cases is not clearly defined, but should be considered in this case to ensure that infectious etiologies are not missed.2 Because of her potential environmental exposures, serologic testing for RMSF and ehrlichiosis should be sent.
Given the overlap in signs and symptoms of EVALI with various, potentially life-threatening infections, she should be empirically treated with antibiotics to cover for community-acquired pneumonia. Adding or even substituting doxycycline for a macrolide antibiotic in this regimen should be considered given that it would treat both RMSF and ehrlichiosis pending further test results. Delay in treating RMSF is associated with worse outcomes. If she is presenting during influenza season, she should also be treated with a neuraminidase inhibitor while awaiting influenza test results. Though the pathophysiology of EVALI is not entirely known, it appears to be inflammatory in nature. Most presumed cases have responded to corticosteroids, with improvement in oxygenation.2 Therefore, treatment with corticosteroids may be warranted to improve oxygenation while ruling out infectious processes.
The patient was admitted to the general medicine wards and started on ceftriaxone and azithromycin for empiric treatment of community-acquired pneumonia. On hospital day 2, a respiratory viral panel returned negative. Procalcitonin, HIV, and blood cultures all returned negative. An ESR was elevated at 86 mm/h. The patient continued to have daily fevers and developed erythematous, blanching macules on the neck, chest, back, and arms, which were noted to occur during febrile periods. Ceftriaxone and azithromycin were discontinued, and doxycycline was started. By hospital day 4, the patient’s oxygen saturation worsened to 86% on ambient air. She continued to have fevers and her cough worsened, with occasional blood-streaked sputum. The patient was transferred to the intensive care unit for closer monitoring.
On hospital day 5, she required intubation for worsening hypoxia. Bronchoscopy was performed, which revealed small mucosal crypts along the left mainstem bronchus. A small amount of bleeding after transbronchial biopsy of the left lower lobe was noted, which resolved with occlusion using the bronchoscope. BAL was performed, which revealed red, cloudy aspirate with 1,100 white blood cells (85% neutrophils) and 22,400 red blood cells. No bacteria were identified.
The patient has developed hypoxic respiratory failure despite appropriate antibiotics and negative cultures, increasing the likelihood of a noninfectious etiology. Her rash is not typical for RMSF, which usually starts as a macular or petechial rash at the ankles and wrists, and spreads centrally to the trunk. Rash is not typically associated with EVALI, and in this case, may represent miliaria caused by her fever.
The mucosal crypts seen on bronchoscopy are nonspecific, likely indicating inflammation from vaping. The BAL otherwise suggests diffuse alveolar hemorrhage (DAH), although sequential BAL aliquots are needed to confirm this diagnosis. DAH is usually caused by pulmonary capillaritis from vasculitis, Goodpasture disease, rheumatic diseases, or diffuse alveolar damage from toxins, infections, rheumatic diseases, or interstitial or organizing pneumonias. Diffuse alveolar damage is the pathologic finding of ARDS, which can be seen in severe cases of many of the conditions discussed, including EVALI, ehrlichiosis, babesiosis, sepsis, and community-acquired pneumonia.4
The BAL is most consistent with EVALI, which often shows elevated neutrophils. DAH due to vaping has also been reported.5 In patients with EVALI, varied pathologic findings of acute lung injury have been reported, including diffuse alveolar damage.6 At this point, laboratory evaluation for rheumatologic diseases and vasculitis should be obtained, and lung biopsy results reviewed. Given her clinical deterioration, treatment with intravenous corticosteroids for presumed EVALI is warranted.
Urine Legionella and Streptococcal pneumoniae antigen tests were negative. The patient was started on methylprednisolone 40 mg intravenously every 8 hours. Further testing included antinuclear antibodies, which was positive at 1:320, with a dense, fine speckled pattern. Perinuclear antineutrophilic cytoplasmic autoantibody, cytoplasmic antineutrophilic cytoplasmic autoantibody, myeloperoxidase, proteinase 3, double-stranded DNA, and glomerular basement membrane IgG were all negative. Transbronchial lung biopsy revealed severe acute lung injury consistent with diffuse alveolar damage. The pulmonary interstitium was mildly expanded by edema, with a moderate number of eosinophilic hyaline membranes. There were no eosinophils or evidence of hemorrhage, granulomas, or giant cells. These changes, within this clinical context, were diagnostic for EVALI.
The patient was intubated for 4 days and completed a course of empiric antibiotics as well as a 10-day course of prednisone. She was discharged on hospital day 17 on 2 L continuous oxygen via nasal cannula. Two days after discharge, she developed worsening dyspnea and chest pain and was readmitted with worsening ground-glass opacities, left upper lobe and right- sided pneumothoraces, and subcutaneous emphysema (Figure 2). She was treated with continuous oxygen to maintain oxygen saturation at 100% and eventually discharged home 3 days later on 3 L continuous oxygen. She attended pulmonary rehabilitation and was weaned off oxygen 2 months later, with marked improvement in aeration of both lungs (Figure 3). She continued to abstain from tobacco and THC products.

DISCUSSION
The first electronic cigarette (e-cigarette) device was developed in 2003 by a Chinese pharmacist and introduced to the American market in 2007.7 E-cigarettes produce an inhalable aerosol by heating a liquid containing a variety of chemicals, nicotine, and flavors, with or without other additives. Originally promoted as a safer nontobacco and cessation device by producers, e-cigarette sales grew at an annual rate of 115% between 2009 and 2012.8 E-cigarettes can also be used to deliver THC, the psychoactive component of cannabis.

Since the advent of e-cigarettes, their safety has been a topic of concern. In August 2019, the CDC announced 215 possible cases of severe pulmonary disease associated with the use of e-cigarette products that were reported by 25 state health departments.1 By February 2020, EVALI had affected more than 2,800 patients hospitalized across the United States.9
The presenting symptoms of EVALI are varied and nonspecific. The largest EVALI case series, published by Layden et al in 2020, included 98 patients who had a median duration of 6 days of symptoms prior to presentation.3 Respiratory symptoms occurred in 97% of patients, including shortness of breath, any chest pain, pleuritic chest pain, cough, and hemoptysis.3 Presentations also included a variety of gastrointestinal (77%) and constitutional (100%) symptoms, which most commonly included nausea, vomiting, and fever.3 Additional case series have supported a specific pattern of presentation, most commonly including pleuritic chest pain, nonproductive cough, or shortness of breath occurring days to weeks prior to presentation. Associated fatigue, fever, and tachycardia may be present, as well as nausea, vomiting, diarrhea and abdominal pain, and in some cases, these have preceded respiratory symptoms.3,10,11
The vital signs and physical examination, laboratory, and imaging results associated with EVALI are also fairly nonspecific. The most common reason for hospitalization in EVALI is hypoxia, which can progress to acute respiratory failure requiring supplemental oxygen or, as in this case, mechanical ventilation. The most common laboratory finding is leukocytosis greater than 11,000/µL, with more than 80% neutrophils and an ESR greater than 30 mm/hr. In the Layden et al case series, 83% of patients had an abnormal CXR. All patients who underwent CT scan of the chest had bilateral ground-glass opacities, often with subpleural sparing.3 A minority of patients were found to have a pneumothorax, generally a late finding.3,12 Accordingly, the CDC now defines confirmed EVALI as use of e-cigarettes during the 90 days before symptom onset with the presence of pulmonary infiltrates (opacities on CXR or ground-glass opacities on chest CT), negative results on testing for all clinically indicated respiratory infections including respiratory viral panel and influenza PCR, and no alternative plausible diagnoses.13
The presumed etiology of EVALI is chemical exposure because no consistent infectious etiology has been identified.6 No consistent e-cigarette product, substance, or additive has been identified in all cases, nor has one product been directly linked to EVALI. However, the CDC recently announced that vitamin E acetate in vaping products appears to be associated with EVALI.9 In December 2019, Blount et al identified vitamin E acetate in BAL fluid samples from 48 of 51 EVALI patients.14 Additionally, while no other toxins were identified, 94% of samples contained THC or its metabolites or patients had reported vaping THC within 90 days preceding illness.14
The most effective treatment strategy for EVALI is still unknown. It is recommended to treat with empiric antibiotics for at least 48 hours (and antivirals during influenza season) if the history is unclear or if the patient is intubated or has severe hypoxemia.2 If antibiotic and/or antiviral therapies do not lead to clinical improvement, corticosteroids should be added, as they lead to improved oxygenation in many patients.2 Kalininskiy et al recommend initial administration of methylprednisolone 40 mg every 8 hours, with transition to oral prednisone to complete a 2-week course.2 Given rates of rehospitalization (2.7%) and death (2%) in EVALI, the CDC advises that patients should be clinically stable for 24 to 48 hours prior to discharge; that follow-up visits should be arranged within 48 hours of discharge; and that cases of EVALI should be reported to the state and local health departments.15 As seen in the case presented here, with time and continued abstinence from e-cigarette use, the pulmonary effects of EVALI can improve, but long-term outcomes remain unclear. Clinicians must now consider EVALI in patients presenting with respiratory, constitutional, and gastrointestinal complaints when a history of e-cigarette use is present.
KEY TEACHING POINTS
- EVALI presents most commonly with a combination of respiratory, gastrointestinal, and constitutional symptoms. including shortness of breath, cough, nausea, vomiting, and fever.
- When considering EVALI, evaluate and treat for potential infectious causes of disease first.
- Corticosteroids are the mainstay of therapy in EVALI, leading to improvement in oxygenation in many patients.
- Most of the reported cases of EVALI have occurred in patients who have vaped THC-containing products.
1. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use – Interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68(36):787-790. https://doi.org/10.15585/mmwr.mm6836e2
2. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/s2213-2600(19)30415-1
3. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illiniois and Wisconsin – final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/nejmoa1911614
4. Faul JL, Doyle RL, Kao PN, Ruoss SJ. Tick-borne pulmonary disease: update on diagnosis and management. Chest. 1999;116(1):222-230. https://doi.org/10.1378/chest.116.1.222
5. Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. https://doi.org/10.1155/2018/9724530
6. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780-1781. https://doi.org/10.1056/nejmc1913069
7. Office of the Surgeon General. E-Cigarette Use Among Youth and Young Adults. Chapter 1. Public Health Service, U.S. Department of Health & Human Services; 2016. Accessed January 22, 2020. https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
8. Grana R, Benowitz N, Glantz SA. Background Paper on E-cigarettes (Electronic Nicotine Delivery Systems). UCSF: Center for Tobacco Control Research and Education; 2013. https://escholarship.org/uc/item/13p2b72n
9. Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. December 12, 2019. Updated February 25, 2020. Accessed January 22, 2020 and July 16, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
10. Davidson K, Brancato A, Heetkerks P, et al. Outbreak of e-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36);784-786. https://doi.org/10.15585/mmwr.mm6836e1
11. Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488-1489. https://doi.org/10.1056/nejmc1912038
12. Henry TS, Kanne JP, Klingerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486-1487. https://doi.org/10.1056/nejmc1911995
13. Smoking and Tobacco Use: For State, Local, Territorial, and Tribal Health Departments. Centers for Disease Control and Prevention. Accessed Jan 24, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html
14. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/nejmoa1916433
15. Evans ME, Twentyman E, Click ES, et al. Update: Interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use–associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge — United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
1. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use – Interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68(36):787-790. https://doi.org/10.15585/mmwr.mm6836e2
2. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/s2213-2600(19)30415-1
3. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illiniois and Wisconsin – final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/nejmoa1911614
4. Faul JL, Doyle RL, Kao PN, Ruoss SJ. Tick-borne pulmonary disease: update on diagnosis and management. Chest. 1999;116(1):222-230. https://doi.org/10.1378/chest.116.1.222
5. Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. https://doi.org/10.1155/2018/9724530
6. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780-1781. https://doi.org/10.1056/nejmc1913069
7. Office of the Surgeon General. E-Cigarette Use Among Youth and Young Adults. Chapter 1. Public Health Service, U.S. Department of Health & Human Services; 2016. Accessed January 22, 2020. https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
8. Grana R, Benowitz N, Glantz SA. Background Paper on E-cigarettes (Electronic Nicotine Delivery Systems). UCSF: Center for Tobacco Control Research and Education; 2013. https://escholarship.org/uc/item/13p2b72n
9. Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. December 12, 2019. Updated February 25, 2020. Accessed January 22, 2020 and July 16, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
10. Davidson K, Brancato A, Heetkerks P, et al. Outbreak of e-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36);784-786. https://doi.org/10.15585/mmwr.mm6836e1
11. Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488-1489. https://doi.org/10.1056/nejmc1912038
12. Henry TS, Kanne JP, Klingerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486-1487. https://doi.org/10.1056/nejmc1911995
13. Smoking and Tobacco Use: For State, Local, Territorial, and Tribal Health Departments. Centers for Disease Control and Prevention. Accessed Jan 24, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html
14. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/nejmoa1916433
15. Evans ME, Twentyman E, Click ES, et al. Update: Interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use–associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge — United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
© 2021 Society of Hospital Medicine
Point: Healthcare Providers Should Receive Treatment Priority During a Pandemic
Potential catastrophic surges in coronavirus disease 2019 (COVID-19) are leading to more patients requiring intensive care unit beds than are available, prompting hospitals to prepare to activate crisis standards of care (CSC).1,2 These guidelines manage the sobering process of determining which gravely ill patients will have access to limited ventilators, critical care specialists, and other essential hospital personnel. As a member of the CSC triage team at Brigham and Women’s Hospital, Boston, Massachusetts, during the initial surge,1 I was taught how to follow procedures that assign each patient a priority score that ranged from 1 to 8, with lower scores representing higher priority. Scoring decisions were largely based on current status of organ systems and major medical illnesses (predictive of short-term and longer-term survival, respectively), consistent with the objective of maximizing lives and life-years saved.1,3-7 Other parameters included improving the priority score of a pregnant woman with a viable fetus and breaking ties in favor of younger patients who had not lived through life’s major stages.4,7 One issue that elicited sharp disagreement among my colleagues was whether healthcare providers (HCPs; eg, physicians, nurses) should be treated any differently than other individuals.
I believe that HCPs should receive treatment priority during a pandemic because the community has a special obligation to those workers willing to risk serious illness by providing care to potentially infected patients.
THE UTILITARIAN CASE FOR TREATMENT PRIORITIZATION
The most common argument for prioritizing HCPs has been made on utilitarian grounds: save individuals who can save others.3,4,6 Such an approach is not founded on the claim that HCPs have higher intrinsic worth, but is based on the instrumental value of HCPs to keep others alive.4,6 An abiding concern for human life demands systems to ensure individuals with clinical expertise are protected so that they can use their skills to maximize the number of lives saved. A similar case has been made to justify prioritizing HCPs for early access to vaccines during a pandemic.5 To underscore these issues, imagine a scenario in which, because of serious illness among HCPs, there were not enough workers with requisite expertise to care for the rest of the community in which a virus was rapidly spreading. Prioritizing HCPs could mitigate this sequence of events by preventing them from becoming infected through early access to vaccinations or promoting their recovery from the illness, which might allow them to return to work caring for others.
THE ROLE OF SPECIAL OBLIGATIONS
Although the utilitarian argument has merit, my primary reason for advocating the prioritization of HCPs reflects a different ethical framework that emphasizes the reciprocal obligations between HCPs and the community. Obligations of physicians have been framed in terms of the commitments made to their self-chosen profession and the putative social contract that has been constructed with the community.8-10 These principles are well articulated in the American Medical Association’s (AMA’s) Code of Medical Ethics, which states, “Because of their commitment to care for the sick and injured, individual physicians have an obligation to provide urgent medical care during disasters…even in the face of greater than usual risks to their own safety, health, or life.”10,11 Although the AMA qualified its position by indicating that this obligation is not unconditional, it still formulated exceptions within the overarching structure of professional duty, allowing physicians to “balance immediate benefits to individual patients with ability to care for patients in the future.”11,12
If one accepts that HCPs have a professional obligation to take care of sick members of the community, even in perilous situations, what, if any, reciprocal obligation does the community have to its HCPs? Reciprocity is a fundamental ethical principle,13 serving as a foundation for the Golden Rule, which is a component of almost every ethical tradition.14 At its core, reciprocity asks us to treat other people as we would want to be treated. It requires endeavoring to take the perspective of others. Within this framework, a strategy for generating a just policy about treatment prioritization is to develop it under the assumption of not knowing which role one would end up playing in a situation. It is critical that if the positions of the individuals involved were reversed, the same rules and obligations would be accepted as fair.13 I suggest that if members of the community put themselves in the shoes of HCPs who are willing to risk exposure to a potentially deadly virus, they would acknowledge the legitimate expectation of HCPs to receive prioritized care if they became ill from the infection.
In most cases, reciprocity is not construed as requiring an identical exchange, but a fair one in which, for instance, sacrifice is returned in kind. Obligations can be viewed as debts that we either owe or are entitled to receive.15 In the current context, reciprocal obligations are derived from the relationship between HCPs and the community in which they serve. HCPs have a special set of obligations to carry out their work with a high degree of professionalism. If circumstances demand they take on substantial risk for their community, the community, in turn, has a special obligation to take care of them.
To highlight this perspective, imagine HCPs who become ill with COVID-19 and make claims for treatment priority despite having been unwilling to work with patients who are sick with COVID-19. We would consider such claims to be unjust because our moral intuition suggests that individuals are owed a debt for the actual risks they have taken, not for the potential ones they have avoided. A corollary of this view is that HCPs who have demonstrated a willingness to risk their lives contracting COVID-19 have a legitimate claim for prioritization.
Implementation
Acknowledgment of the community’s special obligation to HCPs does not negate competing claims for prioritization, such as trying to save the most lives or accounting for a patient’s pregnancy status and stage of life. Rather, there is a need for CSC guidelines to also include recognition of the special obligations owed HCPs by improving their priority score in the calculus used to triage care. Operationalizing the process would need to be worked out. One possibility would be for HCPs directly caring for patients ill from COVID-19 to have their priority score improve by 2 points, and HCPs directly caring for patients without known disease (but who could still be infectious) to benefit by 1 point. At a minimum, recognition of the risks taken should serve as a tiebreaker in favor of these workers.
To Whom Does the Community Have a Special Obligation?
If we acknowledge that during a pandemic, the community has a special obligation to HCPs because of the risks they are taking to serve others, by the same logic, this commitment should be extended to any personnel linked to the healthcare system (eg, employees in environmental services) or frontline workers providing essential services (eg, grocery store workers) who are taking similar risks that involve exposure to potentially infected individuals. Conversely, HCPs who are working exclusively from home via telemedicine should not receive treatment priority. An approach that extends treatment prioritization to other relevant workers mitigates concerns raised about prioritizing scarce critical care resources to an already advantaged class of individuals (ie, HCPs) as well as the negative optics of a committee of “deciders” in a hospital who are privileging care to their own members.12
CONCLUSION
Reciprocity, a critical component of our notion of justice, should be incorporated into CSC guidelines. The community’s reciprocity to HCPs and frontline workers needs to be commensurate with the sacrifice made by these groups. Although public demonstrations of gratitude may be much appreciated, such displays alone are not adequate for honoring the community’s special obligations. If, during a pandemic, HCPs or frontline workers deliver direct care or services to members of the community, despite serious risk to their own lives, the community has a reciprocal obligation to these individuals to prioritize their access to critical care. HCPs and frontline workers should be prioritized not because their lives have higher intrinsic worth or solely as a reflection of their instrumental value to the community, but out of recognition of the special debt owed them. This is not an unconditional obligation, but one that should be built into the complex, multifaceted decision-making process4,6,16 underlying the allocation of scarce medical resources in a pandemic.
Acknowledgments
The author deeply appreciates the thoughtful comments on the essay from William Snyder, PhD, Melissa Frumin, MD, Brittany McFeeley, BS, Lise Bliss, MBA, and especially Seth Gales, MD, and remains grateful for the guidance and support he received early in his academic career from his first mentors, Carol Gilligan, PhD, and Michael Walzer, PhD.
1. Milliken A, Jurchak M, Sadovnikoff N, et al. Addressing challenges associated with operationalizing a crisis standards of care protocol for the Covid-19 pandemic. NEJM Catalyst. 2020:1-14. https://doi.org/10.1056/CAT.20.0384
2. Paquette ET, Derrington S, Fry JT, et al. Shifting duties of children’s hospitals during the COVID-19 pandemic. J Hosp Med. 2020;15(10):631-633. https://doi.org/10.12788/jhm.3490
3. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19.N Engl J Med.2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
4. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773-1774. https://doi.org/10.1001/jama.2020.5046
5. Emanuel EJ, Wetheimer A. Who should get influenza vaccine when not all can? Science. 2006;312(5775):854-855. https://doi.org/10.1126/science.1125347
6. White DB, Katz MH, Luce JM, Lo B. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150(2):132-138. https://doi.org/10.7326/0003-4819-150-2-200901200-00011
7. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care: Planning Guidance for the COVID-19 Pandemic. Accessed August 1, 2020. https://www.mass.gov/doc/statewide-advisory-committee-recommendations-forstandards-of-care/download?_ga=2.55928739.940920097.159299949-195847297.1590861397
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5
10. Huber SJ, Wynia MK. When pestilence prevails . . . physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-11. https://doi.org/10.1162/152651604773067497
11. AMA Council on Ethical and Judicial Affairs. Opinion 9.067 Physician Obligation in Disaster Preparedness and Response. Virtual Mentor. 2010;12(6):459. d10.1001/virtualmentor.2010.12.6.coet1-1006
12. Rothstein MA. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010;38(2):412-419. https://doi.org/10.1111/j.1748-720X.2010.00499.x
13. Rawls J. A Theory of Justice. The Belknap Press: an imprint of Harvard University Press; 1971.
14. Green WS. Parsing reciprocity: questions for the Golden Rule. In: Neusner J, Chilton BD, eds. The Golden Rule: The Ethics of Reciprocity in World Religions. Continuum International Publishing Group; 2008:1-8.
15. Walzer M. Obligations: Essays on Disobedience, War and Citizenship. Harvard University Press; 1970.
16. Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373(9661):423-431. https://doi.org/10.1016/S0140-6736(09)60137-9
Potential catastrophic surges in coronavirus disease 2019 (COVID-19) are leading to more patients requiring intensive care unit beds than are available, prompting hospitals to prepare to activate crisis standards of care (CSC).1,2 These guidelines manage the sobering process of determining which gravely ill patients will have access to limited ventilators, critical care specialists, and other essential hospital personnel. As a member of the CSC triage team at Brigham and Women’s Hospital, Boston, Massachusetts, during the initial surge,1 I was taught how to follow procedures that assign each patient a priority score that ranged from 1 to 8, with lower scores representing higher priority. Scoring decisions were largely based on current status of organ systems and major medical illnesses (predictive of short-term and longer-term survival, respectively), consistent with the objective of maximizing lives and life-years saved.1,3-7 Other parameters included improving the priority score of a pregnant woman with a viable fetus and breaking ties in favor of younger patients who had not lived through life’s major stages.4,7 One issue that elicited sharp disagreement among my colleagues was whether healthcare providers (HCPs; eg, physicians, nurses) should be treated any differently than other individuals.
I believe that HCPs should receive treatment priority during a pandemic because the community has a special obligation to those workers willing to risk serious illness by providing care to potentially infected patients.
THE UTILITARIAN CASE FOR TREATMENT PRIORITIZATION
The most common argument for prioritizing HCPs has been made on utilitarian grounds: save individuals who can save others.3,4,6 Such an approach is not founded on the claim that HCPs have higher intrinsic worth, but is based on the instrumental value of HCPs to keep others alive.4,6 An abiding concern for human life demands systems to ensure individuals with clinical expertise are protected so that they can use their skills to maximize the number of lives saved. A similar case has been made to justify prioritizing HCPs for early access to vaccines during a pandemic.5 To underscore these issues, imagine a scenario in which, because of serious illness among HCPs, there were not enough workers with requisite expertise to care for the rest of the community in which a virus was rapidly spreading. Prioritizing HCPs could mitigate this sequence of events by preventing them from becoming infected through early access to vaccinations or promoting their recovery from the illness, which might allow them to return to work caring for others.
THE ROLE OF SPECIAL OBLIGATIONS
Although the utilitarian argument has merit, my primary reason for advocating the prioritization of HCPs reflects a different ethical framework that emphasizes the reciprocal obligations between HCPs and the community. Obligations of physicians have been framed in terms of the commitments made to their self-chosen profession and the putative social contract that has been constructed with the community.8-10 These principles are well articulated in the American Medical Association’s (AMA’s) Code of Medical Ethics, which states, “Because of their commitment to care for the sick and injured, individual physicians have an obligation to provide urgent medical care during disasters…even in the face of greater than usual risks to their own safety, health, or life.”10,11 Although the AMA qualified its position by indicating that this obligation is not unconditional, it still formulated exceptions within the overarching structure of professional duty, allowing physicians to “balance immediate benefits to individual patients with ability to care for patients in the future.”11,12
If one accepts that HCPs have a professional obligation to take care of sick members of the community, even in perilous situations, what, if any, reciprocal obligation does the community have to its HCPs? Reciprocity is a fundamental ethical principle,13 serving as a foundation for the Golden Rule, which is a component of almost every ethical tradition.14 At its core, reciprocity asks us to treat other people as we would want to be treated. It requires endeavoring to take the perspective of others. Within this framework, a strategy for generating a just policy about treatment prioritization is to develop it under the assumption of not knowing which role one would end up playing in a situation. It is critical that if the positions of the individuals involved were reversed, the same rules and obligations would be accepted as fair.13 I suggest that if members of the community put themselves in the shoes of HCPs who are willing to risk exposure to a potentially deadly virus, they would acknowledge the legitimate expectation of HCPs to receive prioritized care if they became ill from the infection.
In most cases, reciprocity is not construed as requiring an identical exchange, but a fair one in which, for instance, sacrifice is returned in kind. Obligations can be viewed as debts that we either owe or are entitled to receive.15 In the current context, reciprocal obligations are derived from the relationship between HCPs and the community in which they serve. HCPs have a special set of obligations to carry out their work with a high degree of professionalism. If circumstances demand they take on substantial risk for their community, the community, in turn, has a special obligation to take care of them.
To highlight this perspective, imagine HCPs who become ill with COVID-19 and make claims for treatment priority despite having been unwilling to work with patients who are sick with COVID-19. We would consider such claims to be unjust because our moral intuition suggests that individuals are owed a debt for the actual risks they have taken, not for the potential ones they have avoided. A corollary of this view is that HCPs who have demonstrated a willingness to risk their lives contracting COVID-19 have a legitimate claim for prioritization.
Implementation
Acknowledgment of the community’s special obligation to HCPs does not negate competing claims for prioritization, such as trying to save the most lives or accounting for a patient’s pregnancy status and stage of life. Rather, there is a need for CSC guidelines to also include recognition of the special obligations owed HCPs by improving their priority score in the calculus used to triage care. Operationalizing the process would need to be worked out. One possibility would be for HCPs directly caring for patients ill from COVID-19 to have their priority score improve by 2 points, and HCPs directly caring for patients without known disease (but who could still be infectious) to benefit by 1 point. At a minimum, recognition of the risks taken should serve as a tiebreaker in favor of these workers.
To Whom Does the Community Have a Special Obligation?
If we acknowledge that during a pandemic, the community has a special obligation to HCPs because of the risks they are taking to serve others, by the same logic, this commitment should be extended to any personnel linked to the healthcare system (eg, employees in environmental services) or frontline workers providing essential services (eg, grocery store workers) who are taking similar risks that involve exposure to potentially infected individuals. Conversely, HCPs who are working exclusively from home via telemedicine should not receive treatment priority. An approach that extends treatment prioritization to other relevant workers mitigates concerns raised about prioritizing scarce critical care resources to an already advantaged class of individuals (ie, HCPs) as well as the negative optics of a committee of “deciders” in a hospital who are privileging care to their own members.12
CONCLUSION
Reciprocity, a critical component of our notion of justice, should be incorporated into CSC guidelines. The community’s reciprocity to HCPs and frontline workers needs to be commensurate with the sacrifice made by these groups. Although public demonstrations of gratitude may be much appreciated, such displays alone are not adequate for honoring the community’s special obligations. If, during a pandemic, HCPs or frontline workers deliver direct care or services to members of the community, despite serious risk to their own lives, the community has a reciprocal obligation to these individuals to prioritize their access to critical care. HCPs and frontline workers should be prioritized not because their lives have higher intrinsic worth or solely as a reflection of their instrumental value to the community, but out of recognition of the special debt owed them. This is not an unconditional obligation, but one that should be built into the complex, multifaceted decision-making process4,6,16 underlying the allocation of scarce medical resources in a pandemic.
Acknowledgments
The author deeply appreciates the thoughtful comments on the essay from William Snyder, PhD, Melissa Frumin, MD, Brittany McFeeley, BS, Lise Bliss, MBA, and especially Seth Gales, MD, and remains grateful for the guidance and support he received early in his academic career from his first mentors, Carol Gilligan, PhD, and Michael Walzer, PhD.
Potential catastrophic surges in coronavirus disease 2019 (COVID-19) are leading to more patients requiring intensive care unit beds than are available, prompting hospitals to prepare to activate crisis standards of care (CSC).1,2 These guidelines manage the sobering process of determining which gravely ill patients will have access to limited ventilators, critical care specialists, and other essential hospital personnel. As a member of the CSC triage team at Brigham and Women’s Hospital, Boston, Massachusetts, during the initial surge,1 I was taught how to follow procedures that assign each patient a priority score that ranged from 1 to 8, with lower scores representing higher priority. Scoring decisions were largely based on current status of organ systems and major medical illnesses (predictive of short-term and longer-term survival, respectively), consistent with the objective of maximizing lives and life-years saved.1,3-7 Other parameters included improving the priority score of a pregnant woman with a viable fetus and breaking ties in favor of younger patients who had not lived through life’s major stages.4,7 One issue that elicited sharp disagreement among my colleagues was whether healthcare providers (HCPs; eg, physicians, nurses) should be treated any differently than other individuals.
I believe that HCPs should receive treatment priority during a pandemic because the community has a special obligation to those workers willing to risk serious illness by providing care to potentially infected patients.
THE UTILITARIAN CASE FOR TREATMENT PRIORITIZATION
The most common argument for prioritizing HCPs has been made on utilitarian grounds: save individuals who can save others.3,4,6 Such an approach is not founded on the claim that HCPs have higher intrinsic worth, but is based on the instrumental value of HCPs to keep others alive.4,6 An abiding concern for human life demands systems to ensure individuals with clinical expertise are protected so that they can use their skills to maximize the number of lives saved. A similar case has been made to justify prioritizing HCPs for early access to vaccines during a pandemic.5 To underscore these issues, imagine a scenario in which, because of serious illness among HCPs, there were not enough workers with requisite expertise to care for the rest of the community in which a virus was rapidly spreading. Prioritizing HCPs could mitigate this sequence of events by preventing them from becoming infected through early access to vaccinations or promoting their recovery from the illness, which might allow them to return to work caring for others.
THE ROLE OF SPECIAL OBLIGATIONS
Although the utilitarian argument has merit, my primary reason for advocating the prioritization of HCPs reflects a different ethical framework that emphasizes the reciprocal obligations between HCPs and the community. Obligations of physicians have been framed in terms of the commitments made to their self-chosen profession and the putative social contract that has been constructed with the community.8-10 These principles are well articulated in the American Medical Association’s (AMA’s) Code of Medical Ethics, which states, “Because of their commitment to care for the sick and injured, individual physicians have an obligation to provide urgent medical care during disasters…even in the face of greater than usual risks to their own safety, health, or life.”10,11 Although the AMA qualified its position by indicating that this obligation is not unconditional, it still formulated exceptions within the overarching structure of professional duty, allowing physicians to “balance immediate benefits to individual patients with ability to care for patients in the future.”11,12
If one accepts that HCPs have a professional obligation to take care of sick members of the community, even in perilous situations, what, if any, reciprocal obligation does the community have to its HCPs? Reciprocity is a fundamental ethical principle,13 serving as a foundation for the Golden Rule, which is a component of almost every ethical tradition.14 At its core, reciprocity asks us to treat other people as we would want to be treated. It requires endeavoring to take the perspective of others. Within this framework, a strategy for generating a just policy about treatment prioritization is to develop it under the assumption of not knowing which role one would end up playing in a situation. It is critical that if the positions of the individuals involved were reversed, the same rules and obligations would be accepted as fair.13 I suggest that if members of the community put themselves in the shoes of HCPs who are willing to risk exposure to a potentially deadly virus, they would acknowledge the legitimate expectation of HCPs to receive prioritized care if they became ill from the infection.
In most cases, reciprocity is not construed as requiring an identical exchange, but a fair one in which, for instance, sacrifice is returned in kind. Obligations can be viewed as debts that we either owe or are entitled to receive.15 In the current context, reciprocal obligations are derived from the relationship between HCPs and the community in which they serve. HCPs have a special set of obligations to carry out their work with a high degree of professionalism. If circumstances demand they take on substantial risk for their community, the community, in turn, has a special obligation to take care of them.
To highlight this perspective, imagine HCPs who become ill with COVID-19 and make claims for treatment priority despite having been unwilling to work with patients who are sick with COVID-19. We would consider such claims to be unjust because our moral intuition suggests that individuals are owed a debt for the actual risks they have taken, not for the potential ones they have avoided. A corollary of this view is that HCPs who have demonstrated a willingness to risk their lives contracting COVID-19 have a legitimate claim for prioritization.
Implementation
Acknowledgment of the community’s special obligation to HCPs does not negate competing claims for prioritization, such as trying to save the most lives or accounting for a patient’s pregnancy status and stage of life. Rather, there is a need for CSC guidelines to also include recognition of the special obligations owed HCPs by improving their priority score in the calculus used to triage care. Operationalizing the process would need to be worked out. One possibility would be for HCPs directly caring for patients ill from COVID-19 to have their priority score improve by 2 points, and HCPs directly caring for patients without known disease (but who could still be infectious) to benefit by 1 point. At a minimum, recognition of the risks taken should serve as a tiebreaker in favor of these workers.
To Whom Does the Community Have a Special Obligation?
If we acknowledge that during a pandemic, the community has a special obligation to HCPs because of the risks they are taking to serve others, by the same logic, this commitment should be extended to any personnel linked to the healthcare system (eg, employees in environmental services) or frontline workers providing essential services (eg, grocery store workers) who are taking similar risks that involve exposure to potentially infected individuals. Conversely, HCPs who are working exclusively from home via telemedicine should not receive treatment priority. An approach that extends treatment prioritization to other relevant workers mitigates concerns raised about prioritizing scarce critical care resources to an already advantaged class of individuals (ie, HCPs) as well as the negative optics of a committee of “deciders” in a hospital who are privileging care to their own members.12
CONCLUSION
Reciprocity, a critical component of our notion of justice, should be incorporated into CSC guidelines. The community’s reciprocity to HCPs and frontline workers needs to be commensurate with the sacrifice made by these groups. Although public demonstrations of gratitude may be much appreciated, such displays alone are not adequate for honoring the community’s special obligations. If, during a pandemic, HCPs or frontline workers deliver direct care or services to members of the community, despite serious risk to their own lives, the community has a reciprocal obligation to these individuals to prioritize their access to critical care. HCPs and frontline workers should be prioritized not because their lives have higher intrinsic worth or solely as a reflection of their instrumental value to the community, but out of recognition of the special debt owed them. This is not an unconditional obligation, but one that should be built into the complex, multifaceted decision-making process4,6,16 underlying the allocation of scarce medical resources in a pandemic.
Acknowledgments
The author deeply appreciates the thoughtful comments on the essay from William Snyder, PhD, Melissa Frumin, MD, Brittany McFeeley, BS, Lise Bliss, MBA, and especially Seth Gales, MD, and remains grateful for the guidance and support he received early in his academic career from his first mentors, Carol Gilligan, PhD, and Michael Walzer, PhD.
1. Milliken A, Jurchak M, Sadovnikoff N, et al. Addressing challenges associated with operationalizing a crisis standards of care protocol for the Covid-19 pandemic. NEJM Catalyst. 2020:1-14. https://doi.org/10.1056/CAT.20.0384
2. Paquette ET, Derrington S, Fry JT, et al. Shifting duties of children’s hospitals during the COVID-19 pandemic. J Hosp Med. 2020;15(10):631-633. https://doi.org/10.12788/jhm.3490
3. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19.N Engl J Med.2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
4. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773-1774. https://doi.org/10.1001/jama.2020.5046
5. Emanuel EJ, Wetheimer A. Who should get influenza vaccine when not all can? Science. 2006;312(5775):854-855. https://doi.org/10.1126/science.1125347
6. White DB, Katz MH, Luce JM, Lo B. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150(2):132-138. https://doi.org/10.7326/0003-4819-150-2-200901200-00011
7. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care: Planning Guidance for the COVID-19 Pandemic. Accessed August 1, 2020. https://www.mass.gov/doc/statewide-advisory-committee-recommendations-forstandards-of-care/download?_ga=2.55928739.940920097.159299949-195847297.1590861397
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5
10. Huber SJ, Wynia MK. When pestilence prevails . . . physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-11. https://doi.org/10.1162/152651604773067497
11. AMA Council on Ethical and Judicial Affairs. Opinion 9.067 Physician Obligation in Disaster Preparedness and Response. Virtual Mentor. 2010;12(6):459. d10.1001/virtualmentor.2010.12.6.coet1-1006
12. Rothstein MA. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010;38(2):412-419. https://doi.org/10.1111/j.1748-720X.2010.00499.x
13. Rawls J. A Theory of Justice. The Belknap Press: an imprint of Harvard University Press; 1971.
14. Green WS. Parsing reciprocity: questions for the Golden Rule. In: Neusner J, Chilton BD, eds. The Golden Rule: The Ethics of Reciprocity in World Religions. Continuum International Publishing Group; 2008:1-8.
15. Walzer M. Obligations: Essays on Disobedience, War and Citizenship. Harvard University Press; 1970.
16. Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373(9661):423-431. https://doi.org/10.1016/S0140-6736(09)60137-9
1. Milliken A, Jurchak M, Sadovnikoff N, et al. Addressing challenges associated with operationalizing a crisis standards of care protocol for the Covid-19 pandemic. NEJM Catalyst. 2020:1-14. https://doi.org/10.1056/CAT.20.0384
2. Paquette ET, Derrington S, Fry JT, et al. Shifting duties of children’s hospitals during the COVID-19 pandemic. J Hosp Med. 2020;15(10):631-633. https://doi.org/10.12788/jhm.3490
3. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19.N Engl J Med.2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
4. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773-1774. https://doi.org/10.1001/jama.2020.5046
5. Emanuel EJ, Wetheimer A. Who should get influenza vaccine when not all can? Science. 2006;312(5775):854-855. https://doi.org/10.1126/science.1125347
6. White DB, Katz MH, Luce JM, Lo B. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150(2):132-138. https://doi.org/10.7326/0003-4819-150-2-200901200-00011
7. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care: Planning Guidance for the COVID-19 Pandemic. Accessed August 1, 2020. https://www.mass.gov/doc/statewide-advisory-committee-recommendations-forstandards-of-care/download?_ga=2.55928739.940920097.159299949-195847297.1590861397
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5
10. Huber SJ, Wynia MK. When pestilence prevails . . . physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-11. https://doi.org/10.1162/152651604773067497
11. AMA Council on Ethical and Judicial Affairs. Opinion 9.067 Physician Obligation in Disaster Preparedness and Response. Virtual Mentor. 2010;12(6):459. d10.1001/virtualmentor.2010.12.6.coet1-1006
12. Rothstein MA. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010;38(2):412-419. https://doi.org/10.1111/j.1748-720X.2010.00499.x
13. Rawls J. A Theory of Justice. The Belknap Press: an imprint of Harvard University Press; 1971.
14. Green WS. Parsing reciprocity: questions for the Golden Rule. In: Neusner J, Chilton BD, eds. The Golden Rule: The Ethics of Reciprocity in World Religions. Continuum International Publishing Group; 2008:1-8.
15. Walzer M. Obligations: Essays on Disobedience, War and Citizenship. Harvard University Press; 1970.
16. Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373(9661):423-431. https://doi.org/10.1016/S0140-6736(09)60137-9
© 2021 Society of Hospital Medicine
Counterpoint: Prioritizing Healthcare Workers for Scarce Critical Care Resources Is Impractical and Unjust
The impact of the coronavirus disease 2019 (COVID-19) pandemic has been far reaching and devastating. As the pandemic reaches its 1-year mark, there have been more cases and deaths than most of us can comprehend: nearly 28 million cases and 497,000 deaths in the United States1 and more than 111 million cases and 2.4 million deaths globally.2 Frontline healthcare workers (HCWs) have struggled to provide compassionate care in the face of heavy workloads and risks to themselves and their loved ones. Sadly, more than 1,700 US HCWs have died from COVID-19.3 The pandemic has also taken a heavy emotional and psychological toll: HCWs have died by suicide, and others are leaving the profession in which they invested so much and formerly loved. Caring for ill colleagues and dying patients whose family members cannot visit has been particularly difficult. It is, therefore, understandable that some HCWs have called for their prioritization if it becomes necessary to implement crisis standards of care. Although Daffner’s4 reciprocity argument—HCWs should receive priority because of the risks that they have voluntarily accepted—has some appeal, it disregards several important considerations. First, it fails to consider the changing dynamics of viral transmission during the pandemic or alternative ways in which the duty of reciprocity may be fulfilled that do not involve prioritizing HCWs over others. Second, this position is both over- and underinclusive in ways that make it difficult to implement. Third, and most important, the inordinate attention to the prioritization of HCWs ignores the issues the pandemic raises regarding racism and inequity.
LIMITS OF RECIPROCITY AND ALTERNATIVES TO PRIORITIZATION
Although the reciprocity argument has some conceptual merit, there are several different ways that the duty of reciprocity can be fulfilled. One fundamental obligation of government agencies and healthcare systems is providing a safe work environment, including adequate personal protective equipment (PPE) and physical distancing. Before we understood the extent of the pandemic, modes of transmission, and effective preventative measures, hospital transmission was significant. For example, a single-center case series at Zhongan Hospital of Wuhan University, China, from January 1, 2020, to January 28, 2020, found that 29% (40 of 138) of hospitalized patients with COVID-19 were health professionals who were presumed to have been infected by patients.5 There were also significant shortages of PPE, and a number of frontline HCWs reported being dismissed for calling attention to unsafe conditions. Although professionals have an obligation to expose themselves to risk, they are not obligated to expose themselves to inordinate risk. Prioritizing HCWs in ventilator triage may have been justified during the initial surge.
The use of surgical masks by all employees and patients has substantially reduced hospital transmission. A study at Duke Health, Raleigh, North Carolina, of HCWs who tested positive for SARS-CoV-2 between March 15, 2020, and June 6, 2020, found 22% of cases were healthcare acquired, 38% were community acquired, and 40% were of unknown acquisition route. Of the healthcare-acquired cases, 30% were thought to be secondary to direct patient care and 70% to exposure to another worker. The cumulative incidence rate of healthcare-acquired infections among workers decreased significantly 1 week after universal masking was implemented on March 31, 2020. The cumulative incidence rates of community-acquired cases and those with unknown acquisition routes continued to mirror incidence rates in the community.6 There is substantially less justification for prioritizing HCWs during the current phase of the pandemic; reciprocity does not justify granting HCWs infected via community spread greater priority than non-HCWs similarly infected.
There are other means of reciprocating that do not involve prioritization. COVID-19 has exacted an immense toll on the mental well-being of frontline HCWs. They should be provided robust, comprehensive, and accessible mental health services. Additionally, reciprocity can be expressed by providing alternative housing options for HCWs who are concerned about infecting their family members, especially family members at higher risk of morbidity or mortality from COVID-19. Many HCWs have also died from COVID-193; providing life insurance would recognize the sacrifice of HCWs and support their survivors. None of these interventions would require prioritizing HCWs over others.
OVER- AND UNDERINCLUSIVENESS
As Daffner4 acknowledges, the category of “healthcare provider” is both over- and underinclusive. Healthcare providers are exposed to variable risks. Some physicians, for example, are no longer involved in direct patient care. It is unclear how triage teams will identify frontline HCWs or validate claims to being a frontline HCW, especially for individuals not employed by the hospital at which they are seeking care. Hence, triage protocols prioritizing healthcare providers are likely to be substantially overinclusive, which raises significant issues of fairness.
Moreover, the category “healthcare provider” is also underinclusive. Many essential, nonclinical hospital employees expose themselves to risk, including custodial and food service staff. As Daffner4 recognizes, there are also many other occupations outside of healthcare in which individuals voluntarily expose themselves to risks for the benefit of others, including police officers, firefighters, and clerks in grocery stores. We would add that workers in the food-supply system, transportation, and education face similar risks.7 Identifying the types of jobs that should confer priority and validating an individual’s employment also makes implementation difficult and risks injustice.
EQUITY AND JUSTICE
The COVID-19 pandemic and the murder of Black people by police have brought substantial attention to racism and racial inequities in the United States. We must, however, move from merely acknowledging existing inequities to dismantling structures that perpetuate them. The prioritization of HCWs may further privilege those who already have substantial advantages. This is especially true for physicians. For example, although state and federal laws pose limitations, physicians have historically extended one another professional courtesy by providing free or discounted services. Furthermore, HCWs and their family members are more likely to receive VIP treatment. For instance, when taken to the emergency department, children of physicians are less likely to have medical students and residents involved in their care and more likely to see attending physicians and consultants.8
In contrast, other categories of essential workers do not have such advantages. These workers are more likely to be members of marginalized racial and ethnic minority groups, have substantially lower wages, have less access to PPE, and work in more crowded conditions, and are less likely to have paid sick leave compared with HCWs.7 These workers are also more likely to lack access to quality healthcare. In fact, many safety net hospitals that provide care to marginalized communities have faced significant financial hardships as a result of the pandemic, and without additional support, some may close. Prioritizing HCWs will likely widen the gaps in health, economic, and social status among these groups.
With respect to allocation criteria, Black, Latinx, and Native American communities have more severe morbidity and mortality from COVID-19 as a result of racism and its interaction with other social determinants of health. Members of marginalized communities of color have a higher likelihood of becoming infected with COVID-19, a higher prevalence of comorbidities, and less access to treatment.7 Before her untimely death, Dr Susan Moore, a Black family physician, painfully described the racism to which she was subjected while being treated for COVID-19.9 The economic devastation caused by the pandemic, including unemployment, evictions, and food insecurity, compounds the impact of social determinants of health and disproportionately affects minority communities. Purely race- and ethnicity-based approaches to allocation to redress these inequities have potential limitations and obstacles, such as omission of other social determinants of health and legal challenges.7 While currently proposed for allocation of medications or vaccines, alternatives include using the Centers for Disease Control and Prevention’s Social Vulnerability Index8 or the Area Deprivation Index10 as a priority criterion. Most importantly, healthcare systems should more broadly demonstrate themselves trustworthy and assure that marginalized communities of color have access to quality healthcare services.
CONCLUSION
The United States has failed to adequately control the COVID-19 pandemic, and increasing numbers of admissions and staffing shortages have renewed concerns that hospitals will need to implement crisis standards of care. Daffner4 argues that healthcare providers should be prioritized in the allocation of critical care based on reciprocity. In the current phase of the pandemic, HCWs are more likely to be infected by one another or in the community than by patients. There are also other ways that hospitals can discharge this duty that do not require prioritizing HCWs over patients. The category of HCW is both over- and underinclusive, and Daffner4 has not shown that prioritization can be implemented fairly. Finally, inordinate attention has been paid to this topic. Much more attention should be focused on how to redress the ways in which the pandemic has exacerbated existing racial and ethnic inequities.
1. COVID data tracker: United States COVID-19 cases and deaths by state. Centers for Disease Control and Prevention . Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
2. WHO coronavirus disease (COVID-19) dashboard: overview. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
3. Sins of omission: how government failures to track Covid-19 data have led to more than 1,700 health care worker deaths and jeopardize public health. National Nurses United. September 2020. Accessed November 23, 2020. https://act.nationalnursesunited.org/page/-/files/graphics/0920_Covid19_SinsOfOmission_Data_Report.pdf
4. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
6. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
7. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine. The National Academies Press; 2020. https://doi.org/10.17226/25917
8. Diekema DS, Cummings P, Quan L. Physicians’ children are treated differently in the emergency department. Am J Emerg Med. 1996;14(1):6-9. https://doi.org/10.1016/S0735-6757(96)90002-9
9. Maybank A, Jones CP, Blackstock U, Perry JC. Say her name: Dr. Susan Moore. The Washington Post. December 26, 2020. Accessed January 6, 2021. https://www.washingtonpost.com/opinions/2020/12/26/say-her-name-dr-susan-moore/
10. White DB, Schmidhofer M, McCreary E, et al. Model hospital policy for fair allocation of scarce medications to treat COVID-19. University of Pittsburgh. May 28, 2020. Accessed November 23, 2020. https://ccm.pitt.edu/sites/default/files/2020-05-28b%20Model%20hospital%20policy%20for%20allocating%20scarce%20COVID%20meds.pdf
The impact of the coronavirus disease 2019 (COVID-19) pandemic has been far reaching and devastating. As the pandemic reaches its 1-year mark, there have been more cases and deaths than most of us can comprehend: nearly 28 million cases and 497,000 deaths in the United States1 and more than 111 million cases and 2.4 million deaths globally.2 Frontline healthcare workers (HCWs) have struggled to provide compassionate care in the face of heavy workloads and risks to themselves and their loved ones. Sadly, more than 1,700 US HCWs have died from COVID-19.3 The pandemic has also taken a heavy emotional and psychological toll: HCWs have died by suicide, and others are leaving the profession in which they invested so much and formerly loved. Caring for ill colleagues and dying patients whose family members cannot visit has been particularly difficult. It is, therefore, understandable that some HCWs have called for their prioritization if it becomes necessary to implement crisis standards of care. Although Daffner’s4 reciprocity argument—HCWs should receive priority because of the risks that they have voluntarily accepted—has some appeal, it disregards several important considerations. First, it fails to consider the changing dynamics of viral transmission during the pandemic or alternative ways in which the duty of reciprocity may be fulfilled that do not involve prioritizing HCWs over others. Second, this position is both over- and underinclusive in ways that make it difficult to implement. Third, and most important, the inordinate attention to the prioritization of HCWs ignores the issues the pandemic raises regarding racism and inequity.
LIMITS OF RECIPROCITY AND ALTERNATIVES TO PRIORITIZATION
Although the reciprocity argument has some conceptual merit, there are several different ways that the duty of reciprocity can be fulfilled. One fundamental obligation of government agencies and healthcare systems is providing a safe work environment, including adequate personal protective equipment (PPE) and physical distancing. Before we understood the extent of the pandemic, modes of transmission, and effective preventative measures, hospital transmission was significant. For example, a single-center case series at Zhongan Hospital of Wuhan University, China, from January 1, 2020, to January 28, 2020, found that 29% (40 of 138) of hospitalized patients with COVID-19 were health professionals who were presumed to have been infected by patients.5 There were also significant shortages of PPE, and a number of frontline HCWs reported being dismissed for calling attention to unsafe conditions. Although professionals have an obligation to expose themselves to risk, they are not obligated to expose themselves to inordinate risk. Prioritizing HCWs in ventilator triage may have been justified during the initial surge.
The use of surgical masks by all employees and patients has substantially reduced hospital transmission. A study at Duke Health, Raleigh, North Carolina, of HCWs who tested positive for SARS-CoV-2 between March 15, 2020, and June 6, 2020, found 22% of cases were healthcare acquired, 38% were community acquired, and 40% were of unknown acquisition route. Of the healthcare-acquired cases, 30% were thought to be secondary to direct patient care and 70% to exposure to another worker. The cumulative incidence rate of healthcare-acquired infections among workers decreased significantly 1 week after universal masking was implemented on March 31, 2020. The cumulative incidence rates of community-acquired cases and those with unknown acquisition routes continued to mirror incidence rates in the community.6 There is substantially less justification for prioritizing HCWs during the current phase of the pandemic; reciprocity does not justify granting HCWs infected via community spread greater priority than non-HCWs similarly infected.
There are other means of reciprocating that do not involve prioritization. COVID-19 has exacted an immense toll on the mental well-being of frontline HCWs. They should be provided robust, comprehensive, and accessible mental health services. Additionally, reciprocity can be expressed by providing alternative housing options for HCWs who are concerned about infecting their family members, especially family members at higher risk of morbidity or mortality from COVID-19. Many HCWs have also died from COVID-193; providing life insurance would recognize the sacrifice of HCWs and support their survivors. None of these interventions would require prioritizing HCWs over others.
OVER- AND UNDERINCLUSIVENESS
As Daffner4 acknowledges, the category of “healthcare provider” is both over- and underinclusive. Healthcare providers are exposed to variable risks. Some physicians, for example, are no longer involved in direct patient care. It is unclear how triage teams will identify frontline HCWs or validate claims to being a frontline HCW, especially for individuals not employed by the hospital at which they are seeking care. Hence, triage protocols prioritizing healthcare providers are likely to be substantially overinclusive, which raises significant issues of fairness.
Moreover, the category “healthcare provider” is also underinclusive. Many essential, nonclinical hospital employees expose themselves to risk, including custodial and food service staff. As Daffner4 recognizes, there are also many other occupations outside of healthcare in which individuals voluntarily expose themselves to risks for the benefit of others, including police officers, firefighters, and clerks in grocery stores. We would add that workers in the food-supply system, transportation, and education face similar risks.7 Identifying the types of jobs that should confer priority and validating an individual’s employment also makes implementation difficult and risks injustice.
EQUITY AND JUSTICE
The COVID-19 pandemic and the murder of Black people by police have brought substantial attention to racism and racial inequities in the United States. We must, however, move from merely acknowledging existing inequities to dismantling structures that perpetuate them. The prioritization of HCWs may further privilege those who already have substantial advantages. This is especially true for physicians. For example, although state and federal laws pose limitations, physicians have historically extended one another professional courtesy by providing free or discounted services. Furthermore, HCWs and their family members are more likely to receive VIP treatment. For instance, when taken to the emergency department, children of physicians are less likely to have medical students and residents involved in their care and more likely to see attending physicians and consultants.8
In contrast, other categories of essential workers do not have such advantages. These workers are more likely to be members of marginalized racial and ethnic minority groups, have substantially lower wages, have less access to PPE, and work in more crowded conditions, and are less likely to have paid sick leave compared with HCWs.7 These workers are also more likely to lack access to quality healthcare. In fact, many safety net hospitals that provide care to marginalized communities have faced significant financial hardships as a result of the pandemic, and without additional support, some may close. Prioritizing HCWs will likely widen the gaps in health, economic, and social status among these groups.
With respect to allocation criteria, Black, Latinx, and Native American communities have more severe morbidity and mortality from COVID-19 as a result of racism and its interaction with other social determinants of health. Members of marginalized communities of color have a higher likelihood of becoming infected with COVID-19, a higher prevalence of comorbidities, and less access to treatment.7 Before her untimely death, Dr Susan Moore, a Black family physician, painfully described the racism to which she was subjected while being treated for COVID-19.9 The economic devastation caused by the pandemic, including unemployment, evictions, and food insecurity, compounds the impact of social determinants of health and disproportionately affects minority communities. Purely race- and ethnicity-based approaches to allocation to redress these inequities have potential limitations and obstacles, such as omission of other social determinants of health and legal challenges.7 While currently proposed for allocation of medications or vaccines, alternatives include using the Centers for Disease Control and Prevention’s Social Vulnerability Index8 or the Area Deprivation Index10 as a priority criterion. Most importantly, healthcare systems should more broadly demonstrate themselves trustworthy and assure that marginalized communities of color have access to quality healthcare services.
CONCLUSION
The United States has failed to adequately control the COVID-19 pandemic, and increasing numbers of admissions and staffing shortages have renewed concerns that hospitals will need to implement crisis standards of care. Daffner4 argues that healthcare providers should be prioritized in the allocation of critical care based on reciprocity. In the current phase of the pandemic, HCWs are more likely to be infected by one another or in the community than by patients. There are also other ways that hospitals can discharge this duty that do not require prioritizing HCWs over patients. The category of HCW is both over- and underinclusive, and Daffner4 has not shown that prioritization can be implemented fairly. Finally, inordinate attention has been paid to this topic. Much more attention should be focused on how to redress the ways in which the pandemic has exacerbated existing racial and ethnic inequities.
The impact of the coronavirus disease 2019 (COVID-19) pandemic has been far reaching and devastating. As the pandemic reaches its 1-year mark, there have been more cases and deaths than most of us can comprehend: nearly 28 million cases and 497,000 deaths in the United States1 and more than 111 million cases and 2.4 million deaths globally.2 Frontline healthcare workers (HCWs) have struggled to provide compassionate care in the face of heavy workloads and risks to themselves and their loved ones. Sadly, more than 1,700 US HCWs have died from COVID-19.3 The pandemic has also taken a heavy emotional and psychological toll: HCWs have died by suicide, and others are leaving the profession in which they invested so much and formerly loved. Caring for ill colleagues and dying patients whose family members cannot visit has been particularly difficult. It is, therefore, understandable that some HCWs have called for their prioritization if it becomes necessary to implement crisis standards of care. Although Daffner’s4 reciprocity argument—HCWs should receive priority because of the risks that they have voluntarily accepted—has some appeal, it disregards several important considerations. First, it fails to consider the changing dynamics of viral transmission during the pandemic or alternative ways in which the duty of reciprocity may be fulfilled that do not involve prioritizing HCWs over others. Second, this position is both over- and underinclusive in ways that make it difficult to implement. Third, and most important, the inordinate attention to the prioritization of HCWs ignores the issues the pandemic raises regarding racism and inequity.
LIMITS OF RECIPROCITY AND ALTERNATIVES TO PRIORITIZATION
Although the reciprocity argument has some conceptual merit, there are several different ways that the duty of reciprocity can be fulfilled. One fundamental obligation of government agencies and healthcare systems is providing a safe work environment, including adequate personal protective equipment (PPE) and physical distancing. Before we understood the extent of the pandemic, modes of transmission, and effective preventative measures, hospital transmission was significant. For example, a single-center case series at Zhongan Hospital of Wuhan University, China, from January 1, 2020, to January 28, 2020, found that 29% (40 of 138) of hospitalized patients with COVID-19 were health professionals who were presumed to have been infected by patients.5 There were also significant shortages of PPE, and a number of frontline HCWs reported being dismissed for calling attention to unsafe conditions. Although professionals have an obligation to expose themselves to risk, they are not obligated to expose themselves to inordinate risk. Prioritizing HCWs in ventilator triage may have been justified during the initial surge.
The use of surgical masks by all employees and patients has substantially reduced hospital transmission. A study at Duke Health, Raleigh, North Carolina, of HCWs who tested positive for SARS-CoV-2 between March 15, 2020, and June 6, 2020, found 22% of cases were healthcare acquired, 38% were community acquired, and 40% were of unknown acquisition route. Of the healthcare-acquired cases, 30% were thought to be secondary to direct patient care and 70% to exposure to another worker. The cumulative incidence rate of healthcare-acquired infections among workers decreased significantly 1 week after universal masking was implemented on March 31, 2020. The cumulative incidence rates of community-acquired cases and those with unknown acquisition routes continued to mirror incidence rates in the community.6 There is substantially less justification for prioritizing HCWs during the current phase of the pandemic; reciprocity does not justify granting HCWs infected via community spread greater priority than non-HCWs similarly infected.
There are other means of reciprocating that do not involve prioritization. COVID-19 has exacted an immense toll on the mental well-being of frontline HCWs. They should be provided robust, comprehensive, and accessible mental health services. Additionally, reciprocity can be expressed by providing alternative housing options for HCWs who are concerned about infecting their family members, especially family members at higher risk of morbidity or mortality from COVID-19. Many HCWs have also died from COVID-193; providing life insurance would recognize the sacrifice of HCWs and support their survivors. None of these interventions would require prioritizing HCWs over others.
OVER- AND UNDERINCLUSIVENESS
As Daffner4 acknowledges, the category of “healthcare provider” is both over- and underinclusive. Healthcare providers are exposed to variable risks. Some physicians, for example, are no longer involved in direct patient care. It is unclear how triage teams will identify frontline HCWs or validate claims to being a frontline HCW, especially for individuals not employed by the hospital at which they are seeking care. Hence, triage protocols prioritizing healthcare providers are likely to be substantially overinclusive, which raises significant issues of fairness.
Moreover, the category “healthcare provider” is also underinclusive. Many essential, nonclinical hospital employees expose themselves to risk, including custodial and food service staff. As Daffner4 recognizes, there are also many other occupations outside of healthcare in which individuals voluntarily expose themselves to risks for the benefit of others, including police officers, firefighters, and clerks in grocery stores. We would add that workers in the food-supply system, transportation, and education face similar risks.7 Identifying the types of jobs that should confer priority and validating an individual’s employment also makes implementation difficult and risks injustice.
EQUITY AND JUSTICE
The COVID-19 pandemic and the murder of Black people by police have brought substantial attention to racism and racial inequities in the United States. We must, however, move from merely acknowledging existing inequities to dismantling structures that perpetuate them. The prioritization of HCWs may further privilege those who already have substantial advantages. This is especially true for physicians. For example, although state and federal laws pose limitations, physicians have historically extended one another professional courtesy by providing free or discounted services. Furthermore, HCWs and their family members are more likely to receive VIP treatment. For instance, when taken to the emergency department, children of physicians are less likely to have medical students and residents involved in their care and more likely to see attending physicians and consultants.8
In contrast, other categories of essential workers do not have such advantages. These workers are more likely to be members of marginalized racial and ethnic minority groups, have substantially lower wages, have less access to PPE, and work in more crowded conditions, and are less likely to have paid sick leave compared with HCWs.7 These workers are also more likely to lack access to quality healthcare. In fact, many safety net hospitals that provide care to marginalized communities have faced significant financial hardships as a result of the pandemic, and without additional support, some may close. Prioritizing HCWs will likely widen the gaps in health, economic, and social status among these groups.
With respect to allocation criteria, Black, Latinx, and Native American communities have more severe morbidity and mortality from COVID-19 as a result of racism and its interaction with other social determinants of health. Members of marginalized communities of color have a higher likelihood of becoming infected with COVID-19, a higher prevalence of comorbidities, and less access to treatment.7 Before her untimely death, Dr Susan Moore, a Black family physician, painfully described the racism to which she was subjected while being treated for COVID-19.9 The economic devastation caused by the pandemic, including unemployment, evictions, and food insecurity, compounds the impact of social determinants of health and disproportionately affects minority communities. Purely race- and ethnicity-based approaches to allocation to redress these inequities have potential limitations and obstacles, such as omission of other social determinants of health and legal challenges.7 While currently proposed for allocation of medications or vaccines, alternatives include using the Centers for Disease Control and Prevention’s Social Vulnerability Index8 or the Area Deprivation Index10 as a priority criterion. Most importantly, healthcare systems should more broadly demonstrate themselves trustworthy and assure that marginalized communities of color have access to quality healthcare services.
CONCLUSION
The United States has failed to adequately control the COVID-19 pandemic, and increasing numbers of admissions and staffing shortages have renewed concerns that hospitals will need to implement crisis standards of care. Daffner4 argues that healthcare providers should be prioritized in the allocation of critical care based on reciprocity. In the current phase of the pandemic, HCWs are more likely to be infected by one another or in the community than by patients. There are also other ways that hospitals can discharge this duty that do not require prioritizing HCWs over patients. The category of HCW is both over- and underinclusive, and Daffner4 has not shown that prioritization can be implemented fairly. Finally, inordinate attention has been paid to this topic. Much more attention should be focused on how to redress the ways in which the pandemic has exacerbated existing racial and ethnic inequities.
1. COVID data tracker: United States COVID-19 cases and deaths by state. Centers for Disease Control and Prevention . Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
2. WHO coronavirus disease (COVID-19) dashboard: overview. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
3. Sins of omission: how government failures to track Covid-19 data have led to more than 1,700 health care worker deaths and jeopardize public health. National Nurses United. September 2020. Accessed November 23, 2020. https://act.nationalnursesunited.org/page/-/files/graphics/0920_Covid19_SinsOfOmission_Data_Report.pdf
4. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
6. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
7. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine. The National Academies Press; 2020. https://doi.org/10.17226/25917
8. Diekema DS, Cummings P, Quan L. Physicians’ children are treated differently in the emergency department. Am J Emerg Med. 1996;14(1):6-9. https://doi.org/10.1016/S0735-6757(96)90002-9
9. Maybank A, Jones CP, Blackstock U, Perry JC. Say her name: Dr. Susan Moore. The Washington Post. December 26, 2020. Accessed January 6, 2021. https://www.washingtonpost.com/opinions/2020/12/26/say-her-name-dr-susan-moore/
10. White DB, Schmidhofer M, McCreary E, et al. Model hospital policy for fair allocation of scarce medications to treat COVID-19. University of Pittsburgh. May 28, 2020. Accessed November 23, 2020. https://ccm.pitt.edu/sites/default/files/2020-05-28b%20Model%20hospital%20policy%20for%20allocating%20scarce%20COVID%20meds.pdf
1. COVID data tracker: United States COVID-19 cases and deaths by state. Centers for Disease Control and Prevention . Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
2. WHO coronavirus disease (COVID-19) dashboard: overview. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
3. Sins of omission: how government failures to track Covid-19 data have led to more than 1,700 health care worker deaths and jeopardize public health. National Nurses United. September 2020. Accessed November 23, 2020. https://act.nationalnursesunited.org/page/-/files/graphics/0920_Covid19_SinsOfOmission_Data_Report.pdf
4. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
6. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
7. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine. The National Academies Press; 2020. https://doi.org/10.17226/25917
8. Diekema DS, Cummings P, Quan L. Physicians’ children are treated differently in the emergency department. Am J Emerg Med. 1996;14(1):6-9. https://doi.org/10.1016/S0735-6757(96)90002-9
9. Maybank A, Jones CP, Blackstock U, Perry JC. Say her name: Dr. Susan Moore. The Washington Post. December 26, 2020. Accessed January 6, 2021. https://www.washingtonpost.com/opinions/2020/12/26/say-her-name-dr-susan-moore/
10. White DB, Schmidhofer M, McCreary E, et al. Model hospital policy for fair allocation of scarce medications to treat COVID-19. University of Pittsburgh. May 28, 2020. Accessed November 23, 2020. https://ccm.pitt.edu/sites/default/files/2020-05-28b%20Model%20hospital%20policy%20for%20allocating%20scarce%20COVID%20meds.pdf
© 2021 Society of Hospital Medicine
Rebuttal: Accounting for the Community’s Reciprocal Obligations to Healthcare Workers During a Pandemic
In their thoughtful response to the thesis that healthcare workers (HCWs) should be prioritized for scarce resources during a pandemic,1 Antommaria and Unaka offer compelling reasons for opposing this position.2 Common ground can be found in our shared recognition that the community has a reciprocal obligation to HCWs because of their willingness to accept the increased risk of being exposed to serious illness in caring for patients. We disagree on the most appropriate way to honor this obligation and whether HCWs currently have a greater risk of infection than others.
Antommaria and Unaka2 indicate that “prioritizing HCWs …may have been justified during the initial surge” of coronavirus disease 2019 (COVID-19), when risk was excessive. They suggest that, with universal masking and other measures, infection rates among HCWs now mirror those in the community. However, this assessment is questionable. Personal protective equipment is still inadequate in numerous healthcare settings,3,4 and many reports, including one by the National Academies, indicate that the threat to HCWs remains higher.5 In the absence of certainty, I favor erring on the side of continuing to recognize the special obligation to HCWs. Fortunately, COVID-19 vaccines should further reduce the danger of infection, and my article provides justification for prioritizing HCWs to receive them.
Antommaria and Unaka2 seem to support special obligations to HCWs based on reciprocity, but suggest alternatives to critical care prioritization, such as mental health services and life insurance. In my view, mental health care should be universal and not a means of recognizing the sacrifice of HCWs. Providing life insurance for HCWs reflects a tacit acknowledgment of the increased threat they face. However, given governmental delays approving basic COVID-19 relief, it is unlikely that resources will be appropriated for life insurance, which has not occurred since Antommaria et al made this suggestion in 2011.6
Although there may be challenges to identifying and verifying frontline HCWs at risk for exposure to COVID-19, there are always gaps between the principles underlying policies and the way they are implemented. For example, according to guidelines from the Centers for Disease Control and Prevention,7 the first wave of individuals to receive COVID-19 vaccinations should include “frontline essential workers.” Defining and identifying this group of individuals provoke similar concerns to those raised by Antommaria and Unaka2 about my proposal.
I concur that the narrow category of HCWs fails to include nonclinical and other frontline workers who are at a higher risk of being exposed to COVID-19. My article addresses this issue by suggesting the community has a similar set of obligations to these workers.1 Nonclinical hospital workers are disproportionately non-White and have substantially lower median incomes than the average US wage earner.4 Moreover, among HCWs, people of color account for a disproportionate number of COVID-19 cases and deaths.4 Inclusion of at-risk nonclinical and other frontline workers in treatment prioritization is consistent with concerns about fairness that animate Antommaria and Unaka’s article.2
The importance of directing attention to the pandemic’s exacerbation of racial and ethnic inequalities, as highlighted by Antommaria and Unaka,2 does not preclude also carefully examining whether special obligations are owed to HCWs and frontline workers. Thoughtful discussions about weighty ethical questions do not represent a zero-sum game, and, as in the current case, the issues raised during such deliberations often have much broader implications. Of note, social justice can be framed in terms of reciprocity, and efforts to confront societal inequities can reflect the special obligations owed Black Americans to address our long history of systemic racism.
In summary, fairness includes accounting for reciprocity and the duties resulting from it. Special obligations are owed HCWs and frontline workers until they are no longer at higher risk for infection. Hypothetical offers of life insurance or mental health benefits are inadequate ways to demonstrate reciprocity. The challenge of identifying HCWs and other frontline workers ought not preclude efforts to do so. HCWs and frontline workers should not automatically move to the head of the line to receive limited critical care resources. However, recognition of their willingness to risk serious infection should be included in the multidimensional calculus for triaging critical care.
1. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
2. Antommaria AHM, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical care resources is impractical and unjust. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3597
3. Erdman SL. As Covid-19 cases surge, health care workers say PPE is still a struggle. CNN. Updated November 24, 2020. Accessed January 6, 2021. https://www.cnn.com/2020/11/24/health/covid-surge-ppe-availability/index.html
4. Artiga S, Rae M, Pham O, Hamel L, Muñana C. COVID-19 risks and impacts among health care workers by race/ethnicity. November 11, 2020. Accessed January 6, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/covid-19-risks-impacts-health-care-workers-race-ethnicity/
5. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies Press; 2020. https://doi.org/10.17226/25917
6. Antommaria AHM, Powell T, Miller JE, Christian MD, for the Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi.org/10.1097/PCC.0b013e318234a88b
7. Dooling K. Phased allocation of COVID-19 vaccines. Presented at the Advisory Committee on Immunization Practices meeting. December 19-20, 2020. Atlanta, GA. Accessed February 2, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-20/02-COVID-Dooling.pdf
In their thoughtful response to the thesis that healthcare workers (HCWs) should be prioritized for scarce resources during a pandemic,1 Antommaria and Unaka offer compelling reasons for opposing this position.2 Common ground can be found in our shared recognition that the community has a reciprocal obligation to HCWs because of their willingness to accept the increased risk of being exposed to serious illness in caring for patients. We disagree on the most appropriate way to honor this obligation and whether HCWs currently have a greater risk of infection than others.
Antommaria and Unaka2 indicate that “prioritizing HCWs …may have been justified during the initial surge” of coronavirus disease 2019 (COVID-19), when risk was excessive. They suggest that, with universal masking and other measures, infection rates among HCWs now mirror those in the community. However, this assessment is questionable. Personal protective equipment is still inadequate in numerous healthcare settings,3,4 and many reports, including one by the National Academies, indicate that the threat to HCWs remains higher.5 In the absence of certainty, I favor erring on the side of continuing to recognize the special obligation to HCWs. Fortunately, COVID-19 vaccines should further reduce the danger of infection, and my article provides justification for prioritizing HCWs to receive them.
Antommaria and Unaka2 seem to support special obligations to HCWs based on reciprocity, but suggest alternatives to critical care prioritization, such as mental health services and life insurance. In my view, mental health care should be universal and not a means of recognizing the sacrifice of HCWs. Providing life insurance for HCWs reflects a tacit acknowledgment of the increased threat they face. However, given governmental delays approving basic COVID-19 relief, it is unlikely that resources will be appropriated for life insurance, which has not occurred since Antommaria et al made this suggestion in 2011.6
Although there may be challenges to identifying and verifying frontline HCWs at risk for exposure to COVID-19, there are always gaps between the principles underlying policies and the way they are implemented. For example, according to guidelines from the Centers for Disease Control and Prevention,7 the first wave of individuals to receive COVID-19 vaccinations should include “frontline essential workers.” Defining and identifying this group of individuals provoke similar concerns to those raised by Antommaria and Unaka2 about my proposal.
I concur that the narrow category of HCWs fails to include nonclinical and other frontline workers who are at a higher risk of being exposed to COVID-19. My article addresses this issue by suggesting the community has a similar set of obligations to these workers.1 Nonclinical hospital workers are disproportionately non-White and have substantially lower median incomes than the average US wage earner.4 Moreover, among HCWs, people of color account for a disproportionate number of COVID-19 cases and deaths.4 Inclusion of at-risk nonclinical and other frontline workers in treatment prioritization is consistent with concerns about fairness that animate Antommaria and Unaka’s article.2
The importance of directing attention to the pandemic’s exacerbation of racial and ethnic inequalities, as highlighted by Antommaria and Unaka,2 does not preclude also carefully examining whether special obligations are owed to HCWs and frontline workers. Thoughtful discussions about weighty ethical questions do not represent a zero-sum game, and, as in the current case, the issues raised during such deliberations often have much broader implications. Of note, social justice can be framed in terms of reciprocity, and efforts to confront societal inequities can reflect the special obligations owed Black Americans to address our long history of systemic racism.
In summary, fairness includes accounting for reciprocity and the duties resulting from it. Special obligations are owed HCWs and frontline workers until they are no longer at higher risk for infection. Hypothetical offers of life insurance or mental health benefits are inadequate ways to demonstrate reciprocity. The challenge of identifying HCWs and other frontline workers ought not preclude efforts to do so. HCWs and frontline workers should not automatically move to the head of the line to receive limited critical care resources. However, recognition of their willingness to risk serious infection should be included in the multidimensional calculus for triaging critical care.
In their thoughtful response to the thesis that healthcare workers (HCWs) should be prioritized for scarce resources during a pandemic,1 Antommaria and Unaka offer compelling reasons for opposing this position.2 Common ground can be found in our shared recognition that the community has a reciprocal obligation to HCWs because of their willingness to accept the increased risk of being exposed to serious illness in caring for patients. We disagree on the most appropriate way to honor this obligation and whether HCWs currently have a greater risk of infection than others.
Antommaria and Unaka2 indicate that “prioritizing HCWs …may have been justified during the initial surge” of coronavirus disease 2019 (COVID-19), when risk was excessive. They suggest that, with universal masking and other measures, infection rates among HCWs now mirror those in the community. However, this assessment is questionable. Personal protective equipment is still inadequate in numerous healthcare settings,3,4 and many reports, including one by the National Academies, indicate that the threat to HCWs remains higher.5 In the absence of certainty, I favor erring on the side of continuing to recognize the special obligation to HCWs. Fortunately, COVID-19 vaccines should further reduce the danger of infection, and my article provides justification for prioritizing HCWs to receive them.
Antommaria and Unaka2 seem to support special obligations to HCWs based on reciprocity, but suggest alternatives to critical care prioritization, such as mental health services and life insurance. In my view, mental health care should be universal and not a means of recognizing the sacrifice of HCWs. Providing life insurance for HCWs reflects a tacit acknowledgment of the increased threat they face. However, given governmental delays approving basic COVID-19 relief, it is unlikely that resources will be appropriated for life insurance, which has not occurred since Antommaria et al made this suggestion in 2011.6
Although there may be challenges to identifying and verifying frontline HCWs at risk for exposure to COVID-19, there are always gaps between the principles underlying policies and the way they are implemented. For example, according to guidelines from the Centers for Disease Control and Prevention,7 the first wave of individuals to receive COVID-19 vaccinations should include “frontline essential workers.” Defining and identifying this group of individuals provoke similar concerns to those raised by Antommaria and Unaka2 about my proposal.
I concur that the narrow category of HCWs fails to include nonclinical and other frontline workers who are at a higher risk of being exposed to COVID-19. My article addresses this issue by suggesting the community has a similar set of obligations to these workers.1 Nonclinical hospital workers are disproportionately non-White and have substantially lower median incomes than the average US wage earner.4 Moreover, among HCWs, people of color account for a disproportionate number of COVID-19 cases and deaths.4 Inclusion of at-risk nonclinical and other frontline workers in treatment prioritization is consistent with concerns about fairness that animate Antommaria and Unaka’s article.2
The importance of directing attention to the pandemic’s exacerbation of racial and ethnic inequalities, as highlighted by Antommaria and Unaka,2 does not preclude also carefully examining whether special obligations are owed to HCWs and frontline workers. Thoughtful discussions about weighty ethical questions do not represent a zero-sum game, and, as in the current case, the issues raised during such deliberations often have much broader implications. Of note, social justice can be framed in terms of reciprocity, and efforts to confront societal inequities can reflect the special obligations owed Black Americans to address our long history of systemic racism.
In summary, fairness includes accounting for reciprocity and the duties resulting from it. Special obligations are owed HCWs and frontline workers until they are no longer at higher risk for infection. Hypothetical offers of life insurance or mental health benefits are inadequate ways to demonstrate reciprocity. The challenge of identifying HCWs and other frontline workers ought not preclude efforts to do so. HCWs and frontline workers should not automatically move to the head of the line to receive limited critical care resources. However, recognition of their willingness to risk serious infection should be included in the multidimensional calculus for triaging critical care.
1. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
2. Antommaria AHM, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical care resources is impractical and unjust. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3597
3. Erdman SL. As Covid-19 cases surge, health care workers say PPE is still a struggle. CNN. Updated November 24, 2020. Accessed January 6, 2021. https://www.cnn.com/2020/11/24/health/covid-surge-ppe-availability/index.html
4. Artiga S, Rae M, Pham O, Hamel L, Muñana C. COVID-19 risks and impacts among health care workers by race/ethnicity. November 11, 2020. Accessed January 6, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/covid-19-risks-impacts-health-care-workers-race-ethnicity/
5. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies Press; 2020. https://doi.org/10.17226/25917
6. Antommaria AHM, Powell T, Miller JE, Christian MD, for the Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi.org/10.1097/PCC.0b013e318234a88b
7. Dooling K. Phased allocation of COVID-19 vaccines. Presented at the Advisory Committee on Immunization Practices meeting. December 19-20, 2020. Atlanta, GA. Accessed February 2, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-20/02-COVID-Dooling.pdf
1. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
2. Antommaria AHM, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical care resources is impractical and unjust. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3597
3. Erdman SL. As Covid-19 cases surge, health care workers say PPE is still a struggle. CNN. Updated November 24, 2020. Accessed January 6, 2021. https://www.cnn.com/2020/11/24/health/covid-surge-ppe-availability/index.html
4. Artiga S, Rae M, Pham O, Hamel L, Muñana C. COVID-19 risks and impacts among health care workers by race/ethnicity. November 11, 2020. Accessed January 6, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/covid-19-risks-impacts-health-care-workers-race-ethnicity/
5. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies Press; 2020. https://doi.org/10.17226/25917
6. Antommaria AHM, Powell T, Miller JE, Christian MD, for the Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi.org/10.1097/PCC.0b013e318234a88b
7. Dooling K. Phased allocation of COVID-19 vaccines. Presented at the Advisory Committee on Immunization Practices meeting. December 19-20, 2020. Atlanta, GA. Accessed February 2, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-20/02-COVID-Dooling.pdf
© 2021 Society of Hospital Medicine
Dearth of Hospitalist Investigators in Academic Medicine: A Call to Action
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
In their report celebrating the increase in the number of hospitalists from a few hundred in the 1990s to more than 50,000 in 2016, Drs Robert Wachter and Lee Goldman also noted the stunted growth of productive hospital medicine research programs, which presents a challenge to academic credibility in hospital medicine.1 Given the substantial increase in the number of hospitalists over the past two decades, we surveyed adult academic hospital medicine groups to quantify the number of hospitalist clinician investigators and identify gaps in resources for researchers. The number of clinician investigators supported at academic medical centers (AMCs) remains disturbingly low despite the rapid growth of our specialty. Some programs also reported a lack of access to fundamental research services. We report selected results from our survey and provide recommendations to support and facilitate the development of clinician investigators in hospital medicine.
DEARTH OF CLINICIAN INVESTIGATORS IN HOSPITAL MEDICINE
We performed a survey of hospital medicine programs at AMCs in the United States through the Hospital Medicine Reengineering Network (HOMERuN), a hospital medicine research collaborative that facilitates and conducts multisite research studies.2 The purpose of this survey was to obtain a profile of adult academic hospital medicine groups. Surveys were distributed via email to directors and/or senior leaders of each hospital medicine group between January and August 2019. In the survey, a clinician investigator was defined as “faculty whose primary nonclinical focus is scientific papers and grant writing.”
We received responses from 43 of the 86 invitees (50%), each of whom represented a unique hospital medicine group; 41 of the representatives responded to the questions concerning available research services. Collectively, these 43 programs represented 2,503 hospitalists. There were 79 clinician investigators reported among all surveyed hospital medicine groups (3.1% of all hospitalists). The median number of clinician investigators per hospital medicine group was 0 (range 0-12) (Appendix Figure 1), and 22 of 43 (51.2%) hospital medicine groups reported having no clinician investigators. Two of the hospital medicine groups, however, reported having 12 clinician investigators at their respective institutions, comprising nearly one third of the total number of clinician investigators reported in the survey.
Many of the programs reported lack of access to resources such as research assistants (56.1%) and dedicated research fellowships (53.7%) (Appendix Figure 2). A number of groups reported a need for more support for various junior faculty development activities, including research mentoring (53.5%), networking with other researchers (60.5%), and access to clinical data from multiple sites (62.8%).
One of the limitations of this survey was the manner in which the participating hospital medicine groups were chosen. Selection was based on groups affiliated with HOMERuN; among those chosen were highly visible US AMCs, including 70% of the top 20 AMCs based on National Institutes of Health (NIH) funding.3 Therefore, our results likely overestimate the research presence of hospital medicine across all AMCs in the United States.
LACK OF GROWTH OVER TIME: CONTEXTUALIZATION AND IMPLICATIONS
Despite the substantial growth of hospital medicine over the past 2 decades, there has been no proportional increase in the number of hospitalist clinician investigators, with earlier surveys also demonstrating low numbers.4,5 Along with the survey by Chopra and colleagues published in 2019,6 our survey provides an additional contemporary appraisal of research activities for adult academic hospital medicine groups. In the survey by Chopra et al, only 54% (15 of 28) of responding programs reported having any faculty with research as their major activity (ie, >50% effort), and 3% of total faculty reported having funding for >50% effort toward research.6 Our study expands upon these findings by providing more detailed data on the number of clinician investigators per hospital medicine group. Results of our survey showed a concentration of hospitalists within a small number of programs, which may have contributed to the observed lack of growth. We also expand on prior work by identifying a lack of resources and services to support hospitalist researchers.
The findings of our survey have important implications for the field of hospital medicine. Without a critical mass of hospitalist clinician investigators, the quality of research that addresses important questions in our field will suffer. It will also limit academic credibility of the field, as well as individual academic achievement; previous studies have consistently demonstrated that few hospitalists at AMCs achieve the rank of associate or full professor.5-9
POTENTIAL EXPLANATIONS FOR LACK OF RESEARCH GROWTH
The results of our study additionally offer possible explanations for the dearth of clinician investigators in hospital medicine. The limited access to research resources and fellowship training identified in our survey are critical domains that must be addressed in order to develop successful academic hospital medicine programs.4,6,8,10
Regarding dedicated hospital medicine research fellowships, there are only a handful across the country. The small number of existing research fellowships only have one or two fellows per year, and these positions often go unfilled because of a lack of applicants and lower salaries compared to full-time clinical positions.11 The lack of applicants for adult hospital medicine fellowship positions is also integrally linked to board certification requirements. Unlike pediatric hospital medicine where additional fellowship training is required to become board-certified, no such fellowship is required in adult hospital medicine. In pediatrics, this requirement has led to a rapid increase in the number of fellowships with scholarly work requirements (more than 60 fellowships, plus additional programs in development) and greater standardization among training experiences.12,13
The lack of fellowship applicants may also stem from the fact that many trainees are not aware of a potential career as a hospitalist clinician investigator due to limited exposure to this career at most AMCs. Our results revealed that nearly half of sites in our survey had zero clinician investigators, depriving trainees at these programs of role models and thus perpetuating a negative feedback loop. Lastly, although unfilled fellowship positions may indicate that demand is a larger problem than supply, it is also true that fellowship programs generate their own demand through recruitment efforts and the gradual establishment of a positive reputation.
Another potential explanation could relate to the development of hospital medicine in response to rising clinical demands at hospitals: compared with other medical specialties, AMCs may regard hospitalists as being clinicians first and academicians second.1,7,10 Also, hospitalists may be perceived as being beholden to hospitals and less engaged with their surrounding communities than other general medicine fields. With a small footprint in health equity research, academic hospital medicine may be less of a draw to generalists interested in pursuing this area of research. Further, there are very few underrepresented in medicine (URiM) hospital medicine research faculty.5
Another challenge to the career development of hospitalist researchers is the lack of available funding for the type of research typically conducted by hospitalists (eg, rigorous quality improvement implementation and evaluation, optimizing best evidence-based care delivery models, evaluation of patient safety in the hospital setting). As hospitalists tend to be system-level thinkers, this lack of funding may steer potential researchers away from externally funded research careers and into hospital operations and quality improvement positions. Also, unlike other medical specialties, there is no dedicated NIH funding source for hospital medicine research (eg, cardiology and the National Heart, Lung, and Blood Institute), placing hospitalists at a disadvantage in seeking funding compared to subspecialists.
STRATEGIES TO ENHANCE RESEARCH PRESENCE
We recommend several approaches—ones that should be pursued simultaneously—to increase the number of clinician investigators in hospital medicine. First, hospital medicine groups and their respective divisions, departments, and hospitals should allocate funding to support research resources; this includes investing in research assistants, data analysts, statisticians, and administrative support. Through the funding of such research infrastructure programs, AMCs could incentivize hospitalists to research best approaches to improve the value of healthcare delivery, ultimately leading to cost savings.
With 60% of respondents identifying the need for improved access to data across multiple sites, our survey also emphasizes the requirement for further collaboration among hospital medicine groups. Such collaboration could lead to high-powered observational studies and the evaluation of interventions across multiple sites, thus improving the generalizability of study findings.
The Society of Hospital Medicine (SHM) and its research committee can continue to expand the research footprint of hospital medicine. To date, the committee has achieved this by highlighting hospitalist research activity at the SHM Annual Conference Scientific Abstract and Poster Competition and developing a visiting professorship exchange program. In addition to these efforts, SHM could foster collaboration and networking between institutions, as well as take advantage of the current political push for expanded Medicare access by lobbying for robust funding for the Agency for Healthcare Research and Quality, which could provide more opportunities for hospitalists to study the effects of healthcare policy reform on the delivery of inpatient care.
Another strategy to increase the number of hospitalist clinician investigators is to expand hospital medicine research fellowships and recruit trainees for these programs. Fellowships could be internally funded wherein a fellow’s clinical productivity is used to offset the costs associated with obtaining advanced degrees. As an incentive to encourage applicants to temporarily forego a full-time clinical salary during fellowship, hospital medicine groups could offer expanded moonlighting opportunities and contribute to repayment of medical school loans. Hospital medicine groups should also advocate for NIH-funded T32 or K12 training grants for hospital medicine. (There are, however, challenges with this approach because the number of T32 spots per NIH institute is usually fixed). The success of academic emergency medicine offers a precedent for such efforts: After the development of a K12 research training program in emergency medicine, the number of NIH-sponsored principal investigators in this specialty increased by 40% in 6 years.14 Additionally, now that fellowships are required for the pediatric hospital medicine clinician investigators, it would be revealing to track the growth of this workforce.12,13
Structured and formalized mentorship is an essential part of the development of clinician investigators in hospital medicine.4,7,8,10 One successful strategy for mentorship has been the partnering of hospital medicine groups with faculty of general internal medicine and other subspecialty divisions with robust research programs.7,8,15 In addition to developing sustainable mentorship programs, hospital medicine researchers must increase their visibility to trainees. Therefore, it is essential that the majority of academic hospital medicine groups not only hire clinician investigators but also invest in their development, rather than rely on the few programs that have several such faculty members. With this strategy, we could dramatically increase the number of hospitalist clinician investigators from a diverse background of training institutions.
SHM could also play a greater role in organizing events for networking and mentoring for trainees and medical students interested in pursuing a career in hospital medicine research. It is also critically important that hospital medicine groups actively recruit, retain, and develop URiM hospital medicine research faculty in order to attract talented researchers and actively participate in the necessary effort to mitigate the inequities prevalent throughout our healthcare system.
CONCLUSION
Despite the growth of hospital medicine over the past decade, there remains a dearth of hospitalist clinician investigators at major AMCs in the United States. This may be due in part to lack of research resources and mentorship within hospital medicine groups. We believe that investment in these resources, expanded funding opportunities, mentorship development, research fellowship programs, and greater exposure of trainees to hospitalist researchers are solutions that should be strongly considered to develop hospitalist clinician investigators.
Acknowledgments
The authors thank HOMERuN executive committee members, including Grant Fletcher, MD, James Harrison, PhD, BSC, MPH, Peter K. Lindenauer, MD, Melissa Mattison, MD, David Meltzer, MD, PhD, Joshua Metlay, MD, PhD, Jennifer Myers, MD, Sumant Ranji, MD, Gregory Ruhnke, MD, MPH, Edmondo Robinson, MD, MBA, and Neil Sehgal, MPH PhD, for their assistance in developing the survey. They also thank Tiffany Lee, MA, for her project management assistance for HOMERuN.
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
1. Wachter RM, Goldman L. Zero to 50,000 – The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
3. Roskoski R Jr, Parslow TG. Ranking Tables of NIH funding to US medical schools in 2019. Blue Ridge Institute for Medical Research. Published 2020. Updated July 14, 2020. Accessed July 30, 2020. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
6. Chopra V, Burden M, Jones CD, et al; Society of Hospital Medicine Research Committee. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
7. Seymann GB, Southern W, Burger A, et al. Features of successful academic hospitalist programs: insights from the SCHOLAR (SuCcessful HOspitaLists in academics and research) project. J Hosp Med. 2016;11(10):708-713. https://doi.org/10.1002/jhm.2603
8. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
9. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
10. Sehgal NL, Sharpe BA, Auerbach AA, Wachter RM. Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161-166. https://doi.org/10.1002/jhm.845
11. Ranji SR, Rosenman DJ, Amin AN, Kripalani S. Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1-72.e7. https://doi.org/10.1016/j.amjmed.2005.07.061
12. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: a survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571
13. Jerardi KE, Fisher E, Rassbach C, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. Lewis RJ, Neumar RW. Research in emergency medicine: building the investigator pipeline. Ann Emerg Med. 2018;72(6):691-695. https://doi.org/10.1016/j.annemergmed.2018.10.019
15. Flanders SA, Kaufman SR, Nallamothu BK, Saint S. The University of Michigan Specialist-Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308-313. https://doi.org/10.1002/jhm.342
© 2021 Society of Hospital Medicine
Unmasked: How the COVID-19 Pandemic Exacerbates Disparities for People With Communication-Based Disabilities
Adults with communication-based disabilities struggle with healthcare inequities,1-4 largely secondary to poor healthcare provider-patient communication. The prevalence of communication-based disabilities, which include speech, language, voice, and/or hearing disabilities, is relatively high yet difficult to ascertain. Ten percent of adults in the United States report having had a speech, language, or voice disability within the past year,5 and hearing loss also affects 17% of the US population.6 These individuals’ collective communication difficulties have been exacerbated by the coronavirus disease 2019 (COVID-19) pandemic, with healthcare systems mandating personal protective equipment (PPE), including face masks, to ensure the safety of workers and patients. This change has placed patients with communication-based disabilities at even greater risk for communication breakdowns.7,8
Hospitals pose challenging communicative environments due to multiple factors (eg, noisy equipment alarms, harried healthcare teams spending less time with patients, PPE use obstructing faces and muffling sounds). Adverse communication among those with communication-based disabilities results in poorer healthcare outcomes, including higher rates of readmission and preventable adverse medical events, as well as lower healthcare satisfaction.7,9,10 Ineffective communication leads to reduced adherence, longer hospitalizations, and worse health outcomes in general.11-13 This is problematic because those with communication-based disabilities are more likely to require hospitalization due to higher rates of associated comorbidities, including frailty, cardiovascular disease, cognitive decline, and falls.4,14-16 Yet hospitals rarely screen and implement best practices to ensure effective and accessible communication for those with communication-based disabilities. The COVID-19 pandemic has exacerbated existing barriers, despite feasible solutions. Importantly, the Americans with Disabilities Act (ADA) remains in effect despite the pandemic. Therefore, hospitals should review existing policies and approaches to ensure adherence to ADA mandates. We address commonly encountered COVID-19-related communication barriers and recommend potential solutions.17
KEY COMMUNICATION BARRIERS
Limited Time or Support
Patients with communication-based disabilities may need more time than others to communicate their needs, values, and preferences effectively, whether due to slower articulation (eg, movement disorders) or communicating via an intermediary (eg, family member who understands them well) or an interpreter. Due to capacity or patient acuity issues, or even concerns about minimizing time in the room of a patient infected with COVID-19, hospital staff may inadvertently spend less time than needed to develop the necessary therapeutic relationships. This concern is magnified when restrictive visitor policies limit the availability of caregivers, such as loved ones, who assist at the bedside with communication.18
Universal Masking and Face Shields
Standard face masks, now required for all in-person encounters regardless of the patient’s COVID-19 status, obstruct the view of the lips and many facial expressions. Facial cues are an important form of nonverbal communication and are critical to conveying meaning in sign language. Face masks, particularly N95 respirators, substantially degrade speech perception.19 Masking increases the difficulty of acoustically and visually understanding patients who have disorders that decrease speech intelligibility, such as dysphonia, dysarthria, or
Interpreters
For deaf and hard-of-hearing people who use American Sign Language (ASL) as their preferred healthcare communication method, interpreters play a critical role in ensuring accessible healthcare communication. Signed language interpretation can occur in person or remotely by video. For in-person interpretation, interpreters must likewise wear PPE. The use of PPE, including face masks, can obscure many of the facial cues important to ASL grammar. Similarly, patients’ face masks can make it more challenging for interpreters to interpret effectively. With remote video interpretation, technological difficulties (eg, dropped WiFi connections) and the loss of environmental cues (eg, interpreter at a remote location unable to see or hear patient surroundings) often mar opportunities for accessible and effective communication. For the DeafBlind community, the use of remote video interpretation is not feasible. DeafBlind people rely on tactile forms of ASL, requiring interpreters’ physical touch throughout the communication encounter. This potentially increases COVID-19 transmission risk.
POTENTIAL OR IMPLEMENTED SOLUTIONS
While some of the solutions listed below also apply to communication in nonpandemic times, identifying high-risk patients and anticipatory planning for communication has become even more important during the COVID-19 outbreak.
Identification and Assessment of
Hospital staff should systematically review admission and transfer protocols to ensure every patient is asked about their communication preferences, necessary accommodations, and specific needs. Any communication needs or accommodation requests (eg, interpreters, communication boards) should be documented and flagged in highly visible areas of the electronic health record. These patients should be assessed regularly to ensure their communication needs are being met and documented throughout their hospital stay.
Assistive Communication Steps
Some steps can be performed in advance. Careful consideration should be given to healthcare providers’ ability to spend additional time with patients with communication-based disabilities. Even if providers are limited physically in the room, they can still work to optimize mindful, high-quality communication by calling into the patient’s room by phone or video. The additional time is important especially when establishing rapport with patients and identifying their preferred communication approaches, as well as engaging their support networks. Patients with communication-based disabilities and their support team often have expertise on their ideal communication strategies. Healthcare providers and staff should inquire about communication preferences. Patients should also be oriented to hospital team structure and members, which could include simple solutions such as legible name tags. Hearing aids, batteries, and other assistive technology should have designated places to prevent loss and ensure ongoing working status. In addition, nurse stations should have a communication toolbox that includes replacement batteries for hearing aids along with other assistive technology devices, such as a personal sound amplification product.
Communication Strategies
Healthcare teams should be trained and reminded to use patient-centered communication strategies, including assessing their comprehension of shared health information through teach-back principles. Strategies vary by patient and may require teams’ flexibility in meeting the patient’s needs and preferences. Examples include ensuring one has the patient’s attention and uses good eye contact. Using a projected “radio voice,” which emphasizes clarity and articulation rather than volume, is helpful for those with hearing loss. For some, meaningful gestures (eg, pointing to one’s own head when asking about headaches) can aid communication. Another strategy when having difficulty understanding patients with decreased speech intelligibility is to repeat the audible speech so that the patient only needs to repeat the inaudible portions that were missed. Patients should have secure access to personal assistive devices, such as hearing aids and even smartphones with communication apps (eg, speech-to-text apps) to facilitate interpersonal communication.
Clear Face Masks
Face masks with transparent windows have been developed. Deaf and hard of hearing people’s speech perception increases when speakers use transparent versus conventional masks. The Food and Drug Administration has approved two clear face masks as American Society for Testing Materials Level 1 (Table). These two masks have limited utility for high-risk situations, such as aerosolizing procedures; in such cases, a powered air purifying respirator with a clear viewing window will be needed instead. Notably, clear mask supply has lagged behind demand, creating limited mask availability during the pandemic; their use may need to be restricted to those working with patients with communication-based disabilities.
Tools for Communicating Within the Patient’s Room
Erasable whiteboards and communication boards are useful tools for simple exchanges as long as patients’ literacy and fluency are adequate. “PocketTalkers” or personalized sound amplification products may allow providers to speak into a microphone, providing amplified speech via a patient’s headphones. These amplification products are typically useful for those with mild to moderate hearing loss who are not using a hearing aid. Automatic speech recognition apps are device-based apps for converting speech to text. Speakers hold the device near the mouth to maximize accuracy while the patient reads the captions on their screen. With social distancing, lavalier microphones can increase text accuracy, but higher rates of error may still occur due to background noises or accents. For increased reliability and accuracy, Computer Access Realtime Translation stenographers can provide live speech to text on a computer screen from off-site via a computer or smartphone.
Tools for Isolation-Limited Communication
Team members can call an intermediary service to communicate with the patient via the patient’s smartphone or hospital-provided remote video interpreting service, depending on the patient’s preferred communication modality. For oral and spoken language, some services (Table) use remote stenographers to convert speech to text or sign language interpreters for those who use sign language. For both communication modes, smartphone-based videoconferencing may be beneficial while maintaining isolation precautions.
Interpreter Accessibility
Conceptualize interpreters as consulting healthcare team members. They should receive the same PPE training and monitoring as other healthcare workers. For patients using remote video interpretation, this technology needs to be optimized for best results. The room should be in a location with a strong Wi-Fi signal. Equipment should be consistently charged when not in use and rapidly accessible, even remaining in the patients’ room if possible. Healthcare teams need training to appropriately locate and set up the equipment with appropriate support from information technology staff.
Signage
Signage is useful to remind healthcare teams of the patients’ and/or caregivers’ communication-based disability. The most commonly used disability signage shows a line across an ear to indicate hearing loss (Appendix Figure).22 Appropriate signage use, even simple printed sheets documenting a communication issue, can remind healthcare team members of patients’ needs to ensure that communication is accessible and avoid misconceptions toward the patient (eg, noncompliance or cognitive issues). Chart banners, patient room doorways, and over the patients’ beds are good signage locations.
Systematic Noise Reduction
Consistent with previous calls to reduce inpatient noise,23 hospitals should systematically review and monitor protocols to reduce noise pollution. If intra-unit noise varies, patients relying on acoustic-based communication due to hearing loss or speech, language, or voice disability should be placed in quieter rooms.
Communication Concordance
Healthcare professionals and staff with disabilities are an increasingly recognized workforce segment,24 and often are experienced innovators in communicating effectively with patients with communication-based disabilities. Healthcare systems can explore whether they have healthcare team members, employees, disability resource professionals, and/or trainees with these backgrounds and, if they are available, recruit them into developing effective inpatient communication policies and processes.
CONCLUSION
People with communication disabilities experience significant healthcare disparities, now further exacerbated by COVID-19. As clinicians, staff and hospitals work to fuse safety with high-quality communication and care, we should capitalize on multipronged opportunities at the system and individual levels to identify barriers and ensure accessible and effective communication with patients who have communication-based disabilities.
1. McKee MM, Moreland C, Atcherson SR, Zazove P. Hearing loss: communicating with the patient who is deaf or hard of hearing. FP Essent. 2015;434:24-28.
2. Morris MA, Dudgeon BJ, Yorkston K. A qualitative study of adult AAC users’ experiences communicating with medical providers. Disabil Rehabil Assist Technol. 2013;8(6):472-481. https://doi.org/10.3109/17483107.2012.746398
3. Steinberg AG, Barnett S, Meador HE, Wiggins EA, Zazove P. Health care system accessibility. experiences and perceptions of deaf people. J Gen Intern Med. 2006;21(3):260-266. https://doi.org/10.1111/j.1525-1497.2006.00340.x
4. Stransky ML, Jensen KM, Morris MA. Adults with communication disabilities experience poorer health and healthcare outcomes compared to persons without communication disabilities. J Gen Intern Med. 2018;33(12):2147-2155. https://doi.org/10.1007/s11606-018-4625-1
5. Morris MA, Meier SK, Griffin JM, Branda ME, Phelan SM. Prevalence and etiologies of adult communication disabilities in the United States: results from the 2012 National Health Interview Survey. Disabil Health J. 2016;9(1):140-144. https://doi.org/10.1016/j.dhjo.2015.07.004
6. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. Vital Health Stat. 2014(260):1-161.
7. Chang JE, Weinstein B, Chodosh J, Blustein J. Hospital readmission risk for patients with self-reported hearing loss and communication trouble. J Am Geriatr Soc. 2018;66(11):2227-2228. https://doi.org/10.1111/jgs.15545
8. McKee M, Moran C, Zazove P. Overcoming additional barriers to care for deaf and hard of hearing patients during COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(9):781-782. https://doi.org/10.1001/jamaoto.2020.1705
9. Bartlett G, Blais R, Tamblyn R, Clermont RJ, MacGibbon B. Impact of patient communication problems on the risk of preventable adverse events in acute care settings. CMAJ. 2008;178(12):1555-1562. https://doi.org/10.1503/cmaj.070690
10. Hoffman JM, Yorkston KM, Shumway-Cook A, Ciol MA, Dudgeon BJ, Chan L. Effect of communication disability on satisfaction with health care: a survey of medicare beneficiaries. Am J Speech Lang Pathol. 2005;14(3):221-228. https://doi.org/10.1044/1058-0360(2005/022)
11. Kelley JM, Kraft-Todd G, Schapira L, Kossowsky J, Riess H. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(4):e94207. https://doi.org/10.1371/journal.pone.0094207
12. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005
13. Street RL Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295-301. https://doi.org/10.1016/j.pec.2008.11.015
14. Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309(22):2322-2324. https://doi.org/10.1001/jama.2013.5912
15. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299. https://doi.org/10.1001/jamainternmed.2013.1868
16. McKee MM, Stransky ML, Reichard A. Hearing loss and associated medical conditions among individuals 65 years and older. Disabil Health J. 2018;11(1):122-125. https://doi.org/10.1016/j.dhjo.2017.05.007
17. ADA requirements: effective communication. U.S. Department of Justice. January 31, 2014. Accessed February 12, 2021. https://www.ada.gov/effective-comm.htm
18. OCR resolves complaints after State of Connecticut and private hospital safeguard the rights of persons with disabilities to have reasonable access to support persons in hospital settings during COVID-19. Press Release. US Department of Health and Human Services; June 9, 2020. Accessed September 19, 2020. https://www.hhs.gov/about/news/2020/06/09/ocr-resolves-complaints-after-state-connecticut-private-hospital-safeguard-rights-persons.html
19. Goldin A, Weinstein B, Shiman N. How do medical masks degrade speech perception? The Hearing Review. April 1, 2020. Accessed September 30, 2020. https://www.hearingreview.com/hearing-loss/health-wellness/how-do-medical-masks-degrade-speech-reception
20. Mendel LL, Gardino JA, Atcherson SR. Speech understanding using surgical masks: a problem in health care? J Am Acad Audiol. 2008;19(9):686-695. https://doi.org/10.3766/jaaa.19.9.4
21. Atcherson SR, Finley ET, McDowell BR, Watson C. More speech degradations and considerations in the search for transparent face coverings during the COVID-19 pandemic. American Academy of Audiology. November/December 2020. Accessed September 19, 2020. https://www.audiology.org/audiology-today-julyaugust-2020/online-feature-more-speech-degradations-and-considerations-search
22. Hearing Loss. Wikipedia. Accessed October 28, 2020. https://en.wikipedia.org/wiki/Hearing_loss
23. Kamdar BB, Martin JL, Needham DM. Noise and light pollution in the hospital: a call for action. J Hosp Med. 2017;12(10):861-862. https://doi.org/10.12788/jhm.2838
24. Meeks LM, Herzer K, Jain NR. Removing barriers and facilitating access: increasing the number of physicians with disabilities. Acad Med. 2018;93(4):540-543. https://doi.org/10.1097/acm.0000000000002112
25. Communication Access Plan (CAP): Instructions for Patients and Providers. Hearing Loss Association of America (HLAA). Accessed September 30, 2020. https://www.hearingloss.org/wp-content/uploads/HLAA_HC_CAP_Form_and_Instructions.pdf
26. How Do I Communicate with Doctors, Nurses, and Staff at the Hospital During COVID-19? Hearing Loss Association of America (HLAA). May 8, 2020. Accessed September 30, 2020. https://www.hearingloss.org/communication-access-recommendations-hospitals-covid-19/
Adults with communication-based disabilities struggle with healthcare inequities,1-4 largely secondary to poor healthcare provider-patient communication. The prevalence of communication-based disabilities, which include speech, language, voice, and/or hearing disabilities, is relatively high yet difficult to ascertain. Ten percent of adults in the United States report having had a speech, language, or voice disability within the past year,5 and hearing loss also affects 17% of the US population.6 These individuals’ collective communication difficulties have been exacerbated by the coronavirus disease 2019 (COVID-19) pandemic, with healthcare systems mandating personal protective equipment (PPE), including face masks, to ensure the safety of workers and patients. This change has placed patients with communication-based disabilities at even greater risk for communication breakdowns.7,8
Hospitals pose challenging communicative environments due to multiple factors (eg, noisy equipment alarms, harried healthcare teams spending less time with patients, PPE use obstructing faces and muffling sounds). Adverse communication among those with communication-based disabilities results in poorer healthcare outcomes, including higher rates of readmission and preventable adverse medical events, as well as lower healthcare satisfaction.7,9,10 Ineffective communication leads to reduced adherence, longer hospitalizations, and worse health outcomes in general.11-13 This is problematic because those with communication-based disabilities are more likely to require hospitalization due to higher rates of associated comorbidities, including frailty, cardiovascular disease, cognitive decline, and falls.4,14-16 Yet hospitals rarely screen and implement best practices to ensure effective and accessible communication for those with communication-based disabilities. The COVID-19 pandemic has exacerbated existing barriers, despite feasible solutions. Importantly, the Americans with Disabilities Act (ADA) remains in effect despite the pandemic. Therefore, hospitals should review existing policies and approaches to ensure adherence to ADA mandates. We address commonly encountered COVID-19-related communication barriers and recommend potential solutions.17
KEY COMMUNICATION BARRIERS
Limited Time or Support
Patients with communication-based disabilities may need more time than others to communicate their needs, values, and preferences effectively, whether due to slower articulation (eg, movement disorders) or communicating via an intermediary (eg, family member who understands them well) or an interpreter. Due to capacity or patient acuity issues, or even concerns about minimizing time in the room of a patient infected with COVID-19, hospital staff may inadvertently spend less time than needed to develop the necessary therapeutic relationships. This concern is magnified when restrictive visitor policies limit the availability of caregivers, such as loved ones, who assist at the bedside with communication.18
Universal Masking and Face Shields
Standard face masks, now required for all in-person encounters regardless of the patient’s COVID-19 status, obstruct the view of the lips and many facial expressions. Facial cues are an important form of nonverbal communication and are critical to conveying meaning in sign language. Face masks, particularly N95 respirators, substantially degrade speech perception.19 Masking increases the difficulty of acoustically and visually understanding patients who have disorders that decrease speech intelligibility, such as dysphonia, dysarthria, or
Interpreters
For deaf and hard-of-hearing people who use American Sign Language (ASL) as their preferred healthcare communication method, interpreters play a critical role in ensuring accessible healthcare communication. Signed language interpretation can occur in person or remotely by video. For in-person interpretation, interpreters must likewise wear PPE. The use of PPE, including face masks, can obscure many of the facial cues important to ASL grammar. Similarly, patients’ face masks can make it more challenging for interpreters to interpret effectively. With remote video interpretation, technological difficulties (eg, dropped WiFi connections) and the loss of environmental cues (eg, interpreter at a remote location unable to see or hear patient surroundings) often mar opportunities for accessible and effective communication. For the DeafBlind community, the use of remote video interpretation is not feasible. DeafBlind people rely on tactile forms of ASL, requiring interpreters’ physical touch throughout the communication encounter. This potentially increases COVID-19 transmission risk.
POTENTIAL OR IMPLEMENTED SOLUTIONS
While some of the solutions listed below also apply to communication in nonpandemic times, identifying high-risk patients and anticipatory planning for communication has become even more important during the COVID-19 outbreak.
Identification and Assessment of
Hospital staff should systematically review admission and transfer protocols to ensure every patient is asked about their communication preferences, necessary accommodations, and specific needs. Any communication needs or accommodation requests (eg, interpreters, communication boards) should be documented and flagged in highly visible areas of the electronic health record. These patients should be assessed regularly to ensure their communication needs are being met and documented throughout their hospital stay.
Assistive Communication Steps
Some steps can be performed in advance. Careful consideration should be given to healthcare providers’ ability to spend additional time with patients with communication-based disabilities. Even if providers are limited physically in the room, they can still work to optimize mindful, high-quality communication by calling into the patient’s room by phone or video. The additional time is important especially when establishing rapport with patients and identifying their preferred communication approaches, as well as engaging their support networks. Patients with communication-based disabilities and their support team often have expertise on their ideal communication strategies. Healthcare providers and staff should inquire about communication preferences. Patients should also be oriented to hospital team structure and members, which could include simple solutions such as legible name tags. Hearing aids, batteries, and other assistive technology should have designated places to prevent loss and ensure ongoing working status. In addition, nurse stations should have a communication toolbox that includes replacement batteries for hearing aids along with other assistive technology devices, such as a personal sound amplification product.
Communication Strategies
Healthcare teams should be trained and reminded to use patient-centered communication strategies, including assessing their comprehension of shared health information through teach-back principles. Strategies vary by patient and may require teams’ flexibility in meeting the patient’s needs and preferences. Examples include ensuring one has the patient’s attention and uses good eye contact. Using a projected “radio voice,” which emphasizes clarity and articulation rather than volume, is helpful for those with hearing loss. For some, meaningful gestures (eg, pointing to one’s own head when asking about headaches) can aid communication. Another strategy when having difficulty understanding patients with decreased speech intelligibility is to repeat the audible speech so that the patient only needs to repeat the inaudible portions that were missed. Patients should have secure access to personal assistive devices, such as hearing aids and even smartphones with communication apps (eg, speech-to-text apps) to facilitate interpersonal communication.
Clear Face Masks
Face masks with transparent windows have been developed. Deaf and hard of hearing people’s speech perception increases when speakers use transparent versus conventional masks. The Food and Drug Administration has approved two clear face masks as American Society for Testing Materials Level 1 (Table). These two masks have limited utility for high-risk situations, such as aerosolizing procedures; in such cases, a powered air purifying respirator with a clear viewing window will be needed instead. Notably, clear mask supply has lagged behind demand, creating limited mask availability during the pandemic; their use may need to be restricted to those working with patients with communication-based disabilities.

Tools for Communicating Within the Patient’s Room
Erasable whiteboards and communication boards are useful tools for simple exchanges as long as patients’ literacy and fluency are adequate. “PocketTalkers” or personalized sound amplification products may allow providers to speak into a microphone, providing amplified speech via a patient’s headphones. These amplification products are typically useful for those with mild to moderate hearing loss who are not using a hearing aid. Automatic speech recognition apps are device-based apps for converting speech to text. Speakers hold the device near the mouth to maximize accuracy while the patient reads the captions on their screen. With social distancing, lavalier microphones can increase text accuracy, but higher rates of error may still occur due to background noises or accents. For increased reliability and accuracy, Computer Access Realtime Translation stenographers can provide live speech to text on a computer screen from off-site via a computer or smartphone.
Tools for Isolation-Limited Communication
Team members can call an intermediary service to communicate with the patient via the patient’s smartphone or hospital-provided remote video interpreting service, depending on the patient’s preferred communication modality. For oral and spoken language, some services (Table) use remote stenographers to convert speech to text or sign language interpreters for those who use sign language. For both communication modes, smartphone-based videoconferencing may be beneficial while maintaining isolation precautions.
Interpreter Accessibility
Conceptualize interpreters as consulting healthcare team members. They should receive the same PPE training and monitoring as other healthcare workers. For patients using remote video interpretation, this technology needs to be optimized for best results. The room should be in a location with a strong Wi-Fi signal. Equipment should be consistently charged when not in use and rapidly accessible, even remaining in the patients’ room if possible. Healthcare teams need training to appropriately locate and set up the equipment with appropriate support from information technology staff.
Signage
Signage is useful to remind healthcare teams of the patients’ and/or caregivers’ communication-based disability. The most commonly used disability signage shows a line across an ear to indicate hearing loss (Appendix Figure).22 Appropriate signage use, even simple printed sheets documenting a communication issue, can remind healthcare team members of patients’ needs to ensure that communication is accessible and avoid misconceptions toward the patient (eg, noncompliance or cognitive issues). Chart banners, patient room doorways, and over the patients’ beds are good signage locations.
Systematic Noise Reduction
Consistent with previous calls to reduce inpatient noise,23 hospitals should systematically review and monitor protocols to reduce noise pollution. If intra-unit noise varies, patients relying on acoustic-based communication due to hearing loss or speech, language, or voice disability should be placed in quieter rooms.
Communication Concordance
Healthcare professionals and staff with disabilities are an increasingly recognized workforce segment,24 and often are experienced innovators in communicating effectively with patients with communication-based disabilities. Healthcare systems can explore whether they have healthcare team members, employees, disability resource professionals, and/or trainees with these backgrounds and, if they are available, recruit them into developing effective inpatient communication policies and processes.
CONCLUSION
People with communication disabilities experience significant healthcare disparities, now further exacerbated by COVID-19. As clinicians, staff and hospitals work to fuse safety with high-quality communication and care, we should capitalize on multipronged opportunities at the system and individual levels to identify barriers and ensure accessible and effective communication with patients who have communication-based disabilities.
Adults with communication-based disabilities struggle with healthcare inequities,1-4 largely secondary to poor healthcare provider-patient communication. The prevalence of communication-based disabilities, which include speech, language, voice, and/or hearing disabilities, is relatively high yet difficult to ascertain. Ten percent of adults in the United States report having had a speech, language, or voice disability within the past year,5 and hearing loss also affects 17% of the US population.6 These individuals’ collective communication difficulties have been exacerbated by the coronavirus disease 2019 (COVID-19) pandemic, with healthcare systems mandating personal protective equipment (PPE), including face masks, to ensure the safety of workers and patients. This change has placed patients with communication-based disabilities at even greater risk for communication breakdowns.7,8
Hospitals pose challenging communicative environments due to multiple factors (eg, noisy equipment alarms, harried healthcare teams spending less time with patients, PPE use obstructing faces and muffling sounds). Adverse communication among those with communication-based disabilities results in poorer healthcare outcomes, including higher rates of readmission and preventable adverse medical events, as well as lower healthcare satisfaction.7,9,10 Ineffective communication leads to reduced adherence, longer hospitalizations, and worse health outcomes in general.11-13 This is problematic because those with communication-based disabilities are more likely to require hospitalization due to higher rates of associated comorbidities, including frailty, cardiovascular disease, cognitive decline, and falls.4,14-16 Yet hospitals rarely screen and implement best practices to ensure effective and accessible communication for those with communication-based disabilities. The COVID-19 pandemic has exacerbated existing barriers, despite feasible solutions. Importantly, the Americans with Disabilities Act (ADA) remains in effect despite the pandemic. Therefore, hospitals should review existing policies and approaches to ensure adherence to ADA mandates. We address commonly encountered COVID-19-related communication barriers and recommend potential solutions.17
KEY COMMUNICATION BARRIERS
Limited Time or Support
Patients with communication-based disabilities may need more time than others to communicate their needs, values, and preferences effectively, whether due to slower articulation (eg, movement disorders) or communicating via an intermediary (eg, family member who understands them well) or an interpreter. Due to capacity or patient acuity issues, or even concerns about minimizing time in the room of a patient infected with COVID-19, hospital staff may inadvertently spend less time than needed to develop the necessary therapeutic relationships. This concern is magnified when restrictive visitor policies limit the availability of caregivers, such as loved ones, who assist at the bedside with communication.18
Universal Masking and Face Shields
Standard face masks, now required for all in-person encounters regardless of the patient’s COVID-19 status, obstruct the view of the lips and many facial expressions. Facial cues are an important form of nonverbal communication and are critical to conveying meaning in sign language. Face masks, particularly N95 respirators, substantially degrade speech perception.19 Masking increases the difficulty of acoustically and visually understanding patients who have disorders that decrease speech intelligibility, such as dysphonia, dysarthria, or
Interpreters
For deaf and hard-of-hearing people who use American Sign Language (ASL) as their preferred healthcare communication method, interpreters play a critical role in ensuring accessible healthcare communication. Signed language interpretation can occur in person or remotely by video. For in-person interpretation, interpreters must likewise wear PPE. The use of PPE, including face masks, can obscure many of the facial cues important to ASL grammar. Similarly, patients’ face masks can make it more challenging for interpreters to interpret effectively. With remote video interpretation, technological difficulties (eg, dropped WiFi connections) and the loss of environmental cues (eg, interpreter at a remote location unable to see or hear patient surroundings) often mar opportunities for accessible and effective communication. For the DeafBlind community, the use of remote video interpretation is not feasible. DeafBlind people rely on tactile forms of ASL, requiring interpreters’ physical touch throughout the communication encounter. This potentially increases COVID-19 transmission risk.
POTENTIAL OR IMPLEMENTED SOLUTIONS
While some of the solutions listed below also apply to communication in nonpandemic times, identifying high-risk patients and anticipatory planning for communication has become even more important during the COVID-19 outbreak.
Identification and Assessment of
Hospital staff should systematically review admission and transfer protocols to ensure every patient is asked about their communication preferences, necessary accommodations, and specific needs. Any communication needs or accommodation requests (eg, interpreters, communication boards) should be documented and flagged in highly visible areas of the electronic health record. These patients should be assessed regularly to ensure their communication needs are being met and documented throughout their hospital stay.
Assistive Communication Steps
Some steps can be performed in advance. Careful consideration should be given to healthcare providers’ ability to spend additional time with patients with communication-based disabilities. Even if providers are limited physically in the room, they can still work to optimize mindful, high-quality communication by calling into the patient’s room by phone or video. The additional time is important especially when establishing rapport with patients and identifying their preferred communication approaches, as well as engaging their support networks. Patients with communication-based disabilities and their support team often have expertise on their ideal communication strategies. Healthcare providers and staff should inquire about communication preferences. Patients should also be oriented to hospital team structure and members, which could include simple solutions such as legible name tags. Hearing aids, batteries, and other assistive technology should have designated places to prevent loss and ensure ongoing working status. In addition, nurse stations should have a communication toolbox that includes replacement batteries for hearing aids along with other assistive technology devices, such as a personal sound amplification product.
Communication Strategies
Healthcare teams should be trained and reminded to use patient-centered communication strategies, including assessing their comprehension of shared health information through teach-back principles. Strategies vary by patient and may require teams’ flexibility in meeting the patient’s needs and preferences. Examples include ensuring one has the patient’s attention and uses good eye contact. Using a projected “radio voice,” which emphasizes clarity and articulation rather than volume, is helpful for those with hearing loss. For some, meaningful gestures (eg, pointing to one’s own head when asking about headaches) can aid communication. Another strategy when having difficulty understanding patients with decreased speech intelligibility is to repeat the audible speech so that the patient only needs to repeat the inaudible portions that were missed. Patients should have secure access to personal assistive devices, such as hearing aids and even smartphones with communication apps (eg, speech-to-text apps) to facilitate interpersonal communication.
Clear Face Masks
Face masks with transparent windows have been developed. Deaf and hard of hearing people’s speech perception increases when speakers use transparent versus conventional masks. The Food and Drug Administration has approved two clear face masks as American Society for Testing Materials Level 1 (Table). These two masks have limited utility for high-risk situations, such as aerosolizing procedures; in such cases, a powered air purifying respirator with a clear viewing window will be needed instead. Notably, clear mask supply has lagged behind demand, creating limited mask availability during the pandemic; their use may need to be restricted to those working with patients with communication-based disabilities.

Tools for Communicating Within the Patient’s Room
Erasable whiteboards and communication boards are useful tools for simple exchanges as long as patients’ literacy and fluency are adequate. “PocketTalkers” or personalized sound amplification products may allow providers to speak into a microphone, providing amplified speech via a patient’s headphones. These amplification products are typically useful for those with mild to moderate hearing loss who are not using a hearing aid. Automatic speech recognition apps are device-based apps for converting speech to text. Speakers hold the device near the mouth to maximize accuracy while the patient reads the captions on their screen. With social distancing, lavalier microphones can increase text accuracy, but higher rates of error may still occur due to background noises or accents. For increased reliability and accuracy, Computer Access Realtime Translation stenographers can provide live speech to text on a computer screen from off-site via a computer or smartphone.
Tools for Isolation-Limited Communication
Team members can call an intermediary service to communicate with the patient via the patient’s smartphone or hospital-provided remote video interpreting service, depending on the patient’s preferred communication modality. For oral and spoken language, some services (Table) use remote stenographers to convert speech to text or sign language interpreters for those who use sign language. For both communication modes, smartphone-based videoconferencing may be beneficial while maintaining isolation precautions.
Interpreter Accessibility
Conceptualize interpreters as consulting healthcare team members. They should receive the same PPE training and monitoring as other healthcare workers. For patients using remote video interpretation, this technology needs to be optimized for best results. The room should be in a location with a strong Wi-Fi signal. Equipment should be consistently charged when not in use and rapidly accessible, even remaining in the patients’ room if possible. Healthcare teams need training to appropriately locate and set up the equipment with appropriate support from information technology staff.
Signage
Signage is useful to remind healthcare teams of the patients’ and/or caregivers’ communication-based disability. The most commonly used disability signage shows a line across an ear to indicate hearing loss (Appendix Figure).22 Appropriate signage use, even simple printed sheets documenting a communication issue, can remind healthcare team members of patients’ needs to ensure that communication is accessible and avoid misconceptions toward the patient (eg, noncompliance or cognitive issues). Chart banners, patient room doorways, and over the patients’ beds are good signage locations.
Systematic Noise Reduction
Consistent with previous calls to reduce inpatient noise,23 hospitals should systematically review and monitor protocols to reduce noise pollution. If intra-unit noise varies, patients relying on acoustic-based communication due to hearing loss or speech, language, or voice disability should be placed in quieter rooms.
Communication Concordance
Healthcare professionals and staff with disabilities are an increasingly recognized workforce segment,24 and often are experienced innovators in communicating effectively with patients with communication-based disabilities. Healthcare systems can explore whether they have healthcare team members, employees, disability resource professionals, and/or trainees with these backgrounds and, if they are available, recruit them into developing effective inpatient communication policies and processes.
CONCLUSION
People with communication disabilities experience significant healthcare disparities, now further exacerbated by COVID-19. As clinicians, staff and hospitals work to fuse safety with high-quality communication and care, we should capitalize on multipronged opportunities at the system and individual levels to identify barriers and ensure accessible and effective communication with patients who have communication-based disabilities.
1. McKee MM, Moreland C, Atcherson SR, Zazove P. Hearing loss: communicating with the patient who is deaf or hard of hearing. FP Essent. 2015;434:24-28.
2. Morris MA, Dudgeon BJ, Yorkston K. A qualitative study of adult AAC users’ experiences communicating with medical providers. Disabil Rehabil Assist Technol. 2013;8(6):472-481. https://doi.org/10.3109/17483107.2012.746398
3. Steinberg AG, Barnett S, Meador HE, Wiggins EA, Zazove P. Health care system accessibility. experiences and perceptions of deaf people. J Gen Intern Med. 2006;21(3):260-266. https://doi.org/10.1111/j.1525-1497.2006.00340.x
4. Stransky ML, Jensen KM, Morris MA. Adults with communication disabilities experience poorer health and healthcare outcomes compared to persons without communication disabilities. J Gen Intern Med. 2018;33(12):2147-2155. https://doi.org/10.1007/s11606-018-4625-1
5. Morris MA, Meier SK, Griffin JM, Branda ME, Phelan SM. Prevalence and etiologies of adult communication disabilities in the United States: results from the 2012 National Health Interview Survey. Disabil Health J. 2016;9(1):140-144. https://doi.org/10.1016/j.dhjo.2015.07.004
6. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. Vital Health Stat. 2014(260):1-161.
7. Chang JE, Weinstein B, Chodosh J, Blustein J. Hospital readmission risk for patients with self-reported hearing loss and communication trouble. J Am Geriatr Soc. 2018;66(11):2227-2228. https://doi.org/10.1111/jgs.15545
8. McKee M, Moran C, Zazove P. Overcoming additional barriers to care for deaf and hard of hearing patients during COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(9):781-782. https://doi.org/10.1001/jamaoto.2020.1705
9. Bartlett G, Blais R, Tamblyn R, Clermont RJ, MacGibbon B. Impact of patient communication problems on the risk of preventable adverse events in acute care settings. CMAJ. 2008;178(12):1555-1562. https://doi.org/10.1503/cmaj.070690
10. Hoffman JM, Yorkston KM, Shumway-Cook A, Ciol MA, Dudgeon BJ, Chan L. Effect of communication disability on satisfaction with health care: a survey of medicare beneficiaries. Am J Speech Lang Pathol. 2005;14(3):221-228. https://doi.org/10.1044/1058-0360(2005/022)
11. Kelley JM, Kraft-Todd G, Schapira L, Kossowsky J, Riess H. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(4):e94207. https://doi.org/10.1371/journal.pone.0094207
12. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005
13. Street RL Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295-301. https://doi.org/10.1016/j.pec.2008.11.015
14. Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309(22):2322-2324. https://doi.org/10.1001/jama.2013.5912
15. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299. https://doi.org/10.1001/jamainternmed.2013.1868
16. McKee MM, Stransky ML, Reichard A. Hearing loss and associated medical conditions among individuals 65 years and older. Disabil Health J. 2018;11(1):122-125. https://doi.org/10.1016/j.dhjo.2017.05.007
17. ADA requirements: effective communication. U.S. Department of Justice. January 31, 2014. Accessed February 12, 2021. https://www.ada.gov/effective-comm.htm
18. OCR resolves complaints after State of Connecticut and private hospital safeguard the rights of persons with disabilities to have reasonable access to support persons in hospital settings during COVID-19. Press Release. US Department of Health and Human Services; June 9, 2020. Accessed September 19, 2020. https://www.hhs.gov/about/news/2020/06/09/ocr-resolves-complaints-after-state-connecticut-private-hospital-safeguard-rights-persons.html
19. Goldin A, Weinstein B, Shiman N. How do medical masks degrade speech perception? The Hearing Review. April 1, 2020. Accessed September 30, 2020. https://www.hearingreview.com/hearing-loss/health-wellness/how-do-medical-masks-degrade-speech-reception
20. Mendel LL, Gardino JA, Atcherson SR. Speech understanding using surgical masks: a problem in health care? J Am Acad Audiol. 2008;19(9):686-695. https://doi.org/10.3766/jaaa.19.9.4
21. Atcherson SR, Finley ET, McDowell BR, Watson C. More speech degradations and considerations in the search for transparent face coverings during the COVID-19 pandemic. American Academy of Audiology. November/December 2020. Accessed September 19, 2020. https://www.audiology.org/audiology-today-julyaugust-2020/online-feature-more-speech-degradations-and-considerations-search
22. Hearing Loss. Wikipedia. Accessed October 28, 2020. https://en.wikipedia.org/wiki/Hearing_loss
23. Kamdar BB, Martin JL, Needham DM. Noise and light pollution in the hospital: a call for action. J Hosp Med. 2017;12(10):861-862. https://doi.org/10.12788/jhm.2838
24. Meeks LM, Herzer K, Jain NR. Removing barriers and facilitating access: increasing the number of physicians with disabilities. Acad Med. 2018;93(4):540-543. https://doi.org/10.1097/acm.0000000000002112
25. Communication Access Plan (CAP): Instructions for Patients and Providers. Hearing Loss Association of America (HLAA). Accessed September 30, 2020. https://www.hearingloss.org/wp-content/uploads/HLAA_HC_CAP_Form_and_Instructions.pdf
26. How Do I Communicate with Doctors, Nurses, and Staff at the Hospital During COVID-19? Hearing Loss Association of America (HLAA). May 8, 2020. Accessed September 30, 2020. https://www.hearingloss.org/communication-access-recommendations-hospitals-covid-19/
1. McKee MM, Moreland C, Atcherson SR, Zazove P. Hearing loss: communicating with the patient who is deaf or hard of hearing. FP Essent. 2015;434:24-28.
2. Morris MA, Dudgeon BJ, Yorkston K. A qualitative study of adult AAC users’ experiences communicating with medical providers. Disabil Rehabil Assist Technol. 2013;8(6):472-481. https://doi.org/10.3109/17483107.2012.746398
3. Steinberg AG, Barnett S, Meador HE, Wiggins EA, Zazove P. Health care system accessibility. experiences and perceptions of deaf people. J Gen Intern Med. 2006;21(3):260-266. https://doi.org/10.1111/j.1525-1497.2006.00340.x
4. Stransky ML, Jensen KM, Morris MA. Adults with communication disabilities experience poorer health and healthcare outcomes compared to persons without communication disabilities. J Gen Intern Med. 2018;33(12):2147-2155. https://doi.org/10.1007/s11606-018-4625-1
5. Morris MA, Meier SK, Griffin JM, Branda ME, Phelan SM. Prevalence and etiologies of adult communication disabilities in the United States: results from the 2012 National Health Interview Survey. Disabil Health J. 2016;9(1):140-144. https://doi.org/10.1016/j.dhjo.2015.07.004
6. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. Vital Health Stat. 2014(260):1-161.
7. Chang JE, Weinstein B, Chodosh J, Blustein J. Hospital readmission risk for patients with self-reported hearing loss and communication trouble. J Am Geriatr Soc. 2018;66(11):2227-2228. https://doi.org/10.1111/jgs.15545
8. McKee M, Moran C, Zazove P. Overcoming additional barriers to care for deaf and hard of hearing patients during COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(9):781-782. https://doi.org/10.1001/jamaoto.2020.1705
9. Bartlett G, Blais R, Tamblyn R, Clermont RJ, MacGibbon B. Impact of patient communication problems on the risk of preventable adverse events in acute care settings. CMAJ. 2008;178(12):1555-1562. https://doi.org/10.1503/cmaj.070690
10. Hoffman JM, Yorkston KM, Shumway-Cook A, Ciol MA, Dudgeon BJ, Chan L. Effect of communication disability on satisfaction with health care: a survey of medicare beneficiaries. Am J Speech Lang Pathol. 2005;14(3):221-228. https://doi.org/10.1044/1058-0360(2005/022)
11. Kelley JM, Kraft-Todd G, Schapira L, Kossowsky J, Riess H. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(4):e94207. https://doi.org/10.1371/journal.pone.0094207
12. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005
13. Street RL Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295-301. https://doi.org/10.1016/j.pec.2008.11.015
14. Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309(22):2322-2324. https://doi.org/10.1001/jama.2013.5912
15. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299. https://doi.org/10.1001/jamainternmed.2013.1868
16. McKee MM, Stransky ML, Reichard A. Hearing loss and associated medical conditions among individuals 65 years and older. Disabil Health J. 2018;11(1):122-125. https://doi.org/10.1016/j.dhjo.2017.05.007
17. ADA requirements: effective communication. U.S. Department of Justice. January 31, 2014. Accessed February 12, 2021. https://www.ada.gov/effective-comm.htm
18. OCR resolves complaints after State of Connecticut and private hospital safeguard the rights of persons with disabilities to have reasonable access to support persons in hospital settings during COVID-19. Press Release. US Department of Health and Human Services; June 9, 2020. Accessed September 19, 2020. https://www.hhs.gov/about/news/2020/06/09/ocr-resolves-complaints-after-state-connecticut-private-hospital-safeguard-rights-persons.html
19. Goldin A, Weinstein B, Shiman N. How do medical masks degrade speech perception? The Hearing Review. April 1, 2020. Accessed September 30, 2020. https://www.hearingreview.com/hearing-loss/health-wellness/how-do-medical-masks-degrade-speech-reception
20. Mendel LL, Gardino JA, Atcherson SR. Speech understanding using surgical masks: a problem in health care? J Am Acad Audiol. 2008;19(9):686-695. https://doi.org/10.3766/jaaa.19.9.4
21. Atcherson SR, Finley ET, McDowell BR, Watson C. More speech degradations and considerations in the search for transparent face coverings during the COVID-19 pandemic. American Academy of Audiology. November/December 2020. Accessed September 19, 2020. https://www.audiology.org/audiology-today-julyaugust-2020/online-feature-more-speech-degradations-and-considerations-search
22. Hearing Loss. Wikipedia. Accessed October 28, 2020. https://en.wikipedia.org/wiki/Hearing_loss
23. Kamdar BB, Martin JL, Needham DM. Noise and light pollution in the hospital: a call for action. J Hosp Med. 2017;12(10):861-862. https://doi.org/10.12788/jhm.2838
24. Meeks LM, Herzer K, Jain NR. Removing barriers and facilitating access: increasing the number of physicians with disabilities. Acad Med. 2018;93(4):540-543. https://doi.org/10.1097/acm.0000000000002112
25. Communication Access Plan (CAP): Instructions for Patients and Providers. Hearing Loss Association of America (HLAA). Accessed September 30, 2020. https://www.hearingloss.org/wp-content/uploads/HLAA_HC_CAP_Form_and_Instructions.pdf
26. How Do I Communicate with Doctors, Nurses, and Staff at the Hospital During COVID-19? Hearing Loss Association of America (HLAA). May 8, 2020. Accessed September 30, 2020. https://www.hearingloss.org/communication-access-recommendations-hospitals-covid-19/
© 2021 Society of Hospital Medicine
Glucosuria Is Not Always Due to Diabetes
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Management of Do Not Resuscitate Orders Before Invasive Procedures
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
Can Using an Intensive Management Program Improve Primary Care Staff Experiences With Caring for High-Risk Patients?
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
Minimizing Opioids After Joint Operation: Protocol to Decrease Postoperative Opioid Use After Primary Total Knee Arthroplasty
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049