User login
Impact of a Hospitalist-Run Procedure Service on Time to Paracentesis and Length of Stay
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
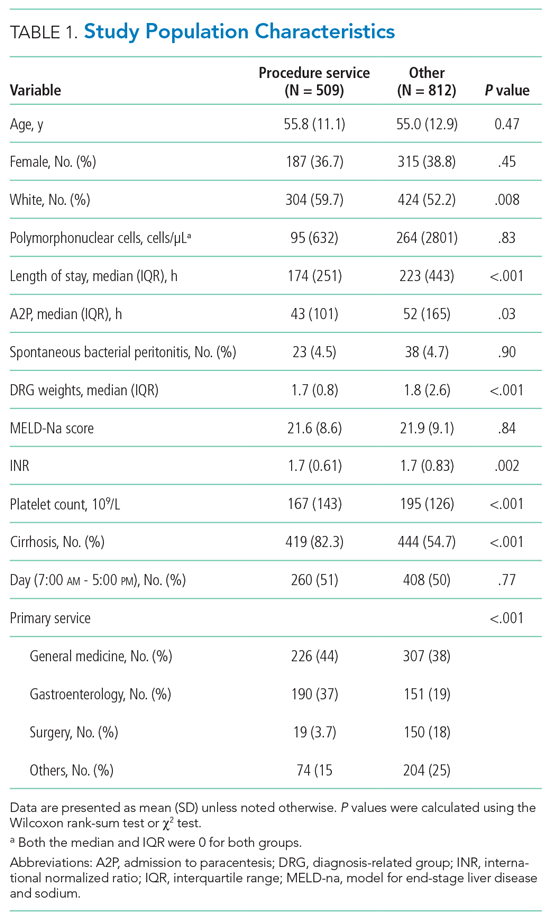
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.
In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.

In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.

In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
© Society of Hospital Medicine
Methodological Progress Note: Interrupted Time Series
Hospital medicine research often asks the question whether an intervention, such as a policy or guideline, has improved quality of care and/or whether there were any unintended consequences. Alternatively, investigators may be interested in understanding the impact of an event, such as a natural disaster or a pandemic, on hospital care. The study design that provides the best estimate of the causal effect of the intervention is the randomized controlled trial (RCT). The goal of randomization, which can be implemented at the patient or cluster level (eg, hospitals), is attaining a balance of the known and unknown confounders between study groups.
However, an RCT may not be feasible for several reasons: complexity, insufficient setup time or funding, ethical barriers to randomization, unwillingness of funders or payers to withhold the intervention from patients (ie, the control group), or anticipated contamination of the intervention into the control group (eg, provider practice change interventions). In addition, it may be impossible to conduct an RCT because the investigator does not have control over the design of an intervention or because they are studying an event, such as a pandemic.
In the June 2020 issue of the Journal of Hospital Medicine, Coon et al1 use a type of quasi-experimental design (QED)—specifically, the interrupted time series (ITS)—to examine the impact of the adoption of ward-based high-flow nasal cannula protocols on intensive care unit (ICU) admission for bronchiolitis at children’s hospitals. In this methodologic progress note, we discuss QEDs for evaluating the impact of healthcare interventions or events and focus on ITS, one of the strongest QEDs.
WHAT IS A QUASI-EXPERIMENTAL DESIGN?
Quasi-experimental design refers to a broad range of nonrandomized or partially randomized pre- vs postintervention studies.2 In order to test a causal hypothesis without randomization, QEDs define a comparison group or a time period in which an intervention has not been implemented, as well as at least one group or time period in which an intervention has been implemented. In a QED, the control may lack similarity with the intervention group or time period because of differences in the patients, sites, or time period (sometimes referred to as having a “nonequivalent control group”). Several design and analytic approaches are available to enhance the extent to which the study is able to make conclusions about the causal impact of the intervention.2,3 Because randomization is not necessary, QEDs allow for inclusion of a broader population than that which is feasible by RCTs, which increases the applicability and generalizability of the results. Therefore, they are a powerful research design to test the effectiveness of interventions in real-world settings.
The choice of which QED depends on whether the investigators are conducting a prospective evaluation and have control over the study design (ie, the ordering of the intervention, selection of sites or individuals, and/or timing and frequency of the data collection) or whether the investigators do not have control over the intervention, which is also known as a “natural experiment.”4,5 Some studies may also incorporate two QEDs in tandem.6 The Table provides a brief summary of different QEDs, ordered by methodologic strength, and distinguishes those that can be used to study natural experiments. In the study by Coon et al,1 an ITS is used as opposed to a methodologically stronger QED, such as the stepped-wedge design, because the investigators did not have control over the rollout of heated high-flow nasal canula protocols across hospitals.
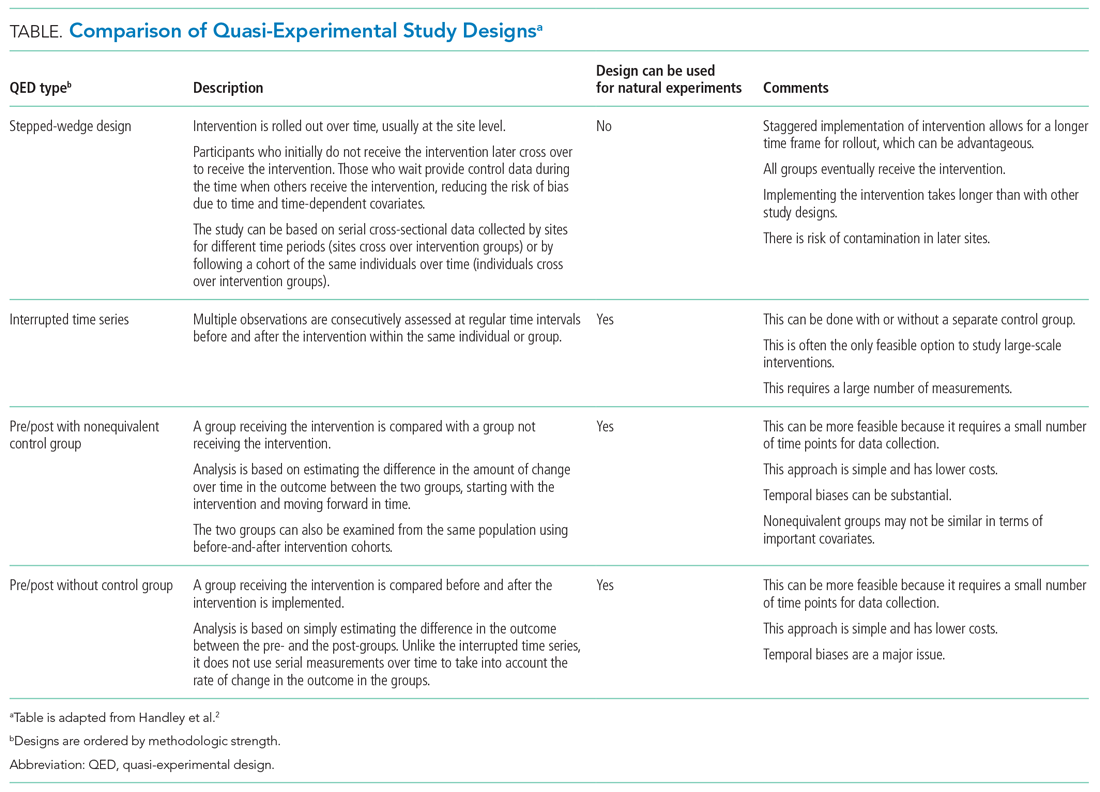
WHAT IS AN INTERRUPTED TIME SERIES?
Interrupted time series designs use repeated observations of an outcome over time. This method then divides, or “interrupts,” the series of data into two time periods: before the intervention or event and after. Using data from the preintervention period, an underlying trend in the outcome is estimated and assumed to continue forward into the postintervention period to estimate what would have occurred without the intervention. Any significant change in the outcome at the beginning of the postintervention period or change in the trend in the postintervention is then attributed to the intervention.
There are several important methodologic considerations when designing an ITS study, as detailed in other review papers.2,3,7,8 An ITS design can be retrospective or prospective. It can be of a single center or include multiple sites, as in Coon et al. It can be conducted with or without a control. The inclusion of a control, when appropriately chosen, improves the strength of the study design because it can account for seasonal trends and potential confounders that vary over time. The control can be a different group of hospitals or participants that are similar but did not receive the intervention, or it can be a different outcome in the same group of hospitals or participants that are not expected to be affected by the intervention. The ITS design may also be set up to estimate the individual effects of multicomponent interventions. If the different components are phased in sequentially over time, then it may be possible to interrupt the time series at these points and estimate the impact of each intervention component.
Other examples of ITS studies in hospital medicine include those that evaluated the impact of a readmission-reduction program,9 of state sepsis regulations on in-hospital mortality,10 of resident duty-hour reform on mortality among hospitalized patients,11 of a quality-improvement initiative on early discharge,12 and of national guidelines on pediatric pneumonia antibiotic selection.13 There are several types of ITS analysis, and in this article, we focus on segmented regression without a control group.7,8
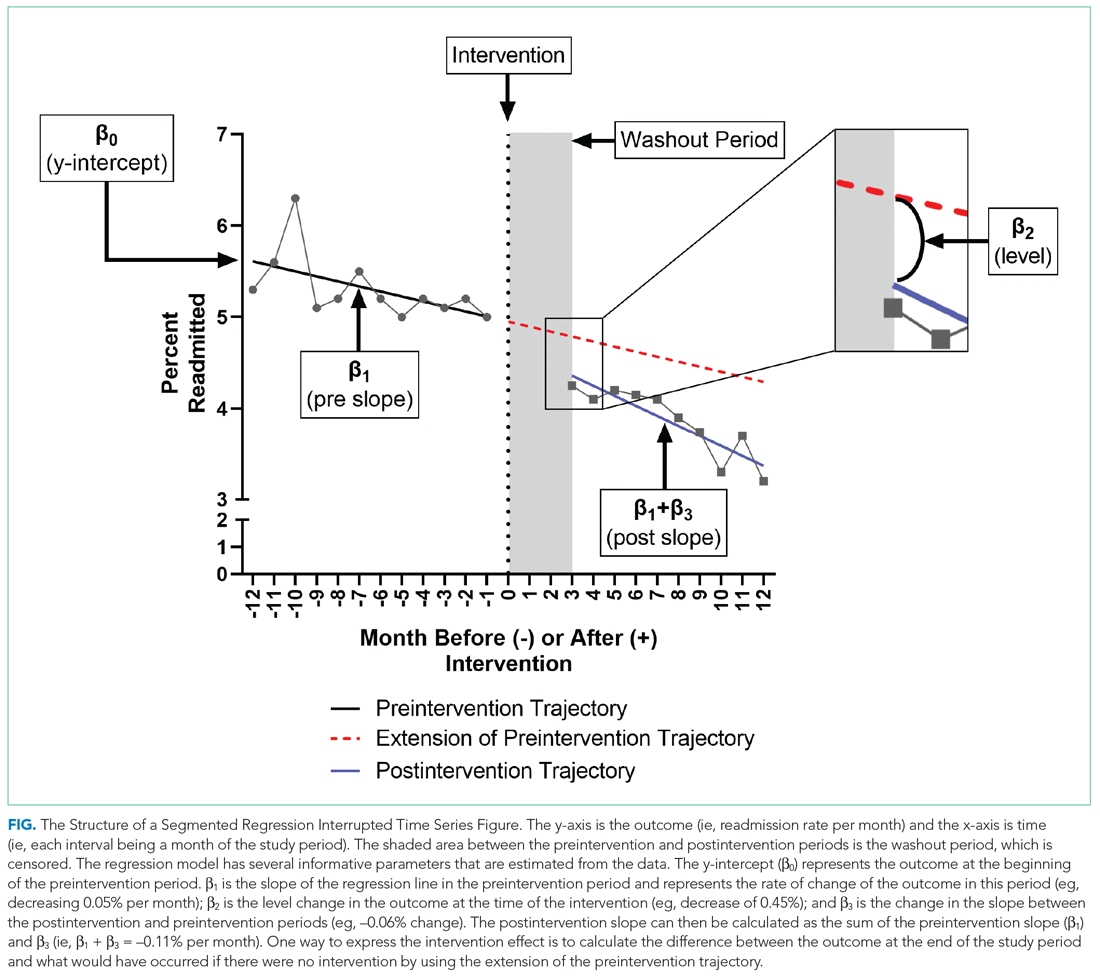
WHAT IS A SEGMENTED REGRESSION ITS?
Segmented regression is the statistical model used to measure (a) the immediate change in the outcome (level) at the start of the intervention and (b) the change in the trend of the outcome (slope) in the postintervention period vs that in the preintervention period. Therefore, the intervention effect size is expressed in terms of the level change and the slope change. To function properly, the models require several repeated (eg, monthly) measurements of the outcome before and after the intervention. Some experts suggest a minimum of 4 to 12 observations, depending on a number of factors including the stability of the outcome and seasonal variations.7,8 If changes before and after more than one intervention are being examined, there should be the minimum number of observations separating them. Unlike typical regression models, time-series models can correct for autocorrelation if it is present in the data. Autocorrelation is the type of correlation that arises when data are collected over time, with those closest in time being more strongly correlated (there are also other types of autocorrelation, such as seasonal patterns). Using available statistical software, autocorrelation can be detected and, if present, it can be controlled for in the segmented regression models.
HOW ARE SEGMENTED REGRESSION RESULTS PRESENTED?
Coon et al present results of their ITS analysis in a panel of figures detailing each study outcome, ICU admission, ICU length of stay, total length of stay, and rates of mechanical ventilation. Each panel shows the rate of change in the outcome per season across hospitals, before and after adoption of heated high-flow nasal cannula protocols, and the level change at the time of adoption.
To further explain how segmented regression results are presented, in the Figure we detail the structure of a segmented regression figure evaluating the impact of an intervention without a control group. In addition to the regression figure, authors typically provide 95% CIs around the rates, level change, and the difference between the postintervention and preintervention periods, along with P values demonstrating whether the rates, level change, and the differences between period slopes differ significantly from zero.
WHAT ARE THE UNDERLYING ASSUMPTIONS OF THE SEGMENTED REGRESSION ITS?
Segmented regression models assume a linear trend in the outcome. If the outcome follows a nonlinear pattern (eg, exponential spread of a disease during a pandemic), then using different distributions in the modeling or transformations of the data may be necessary. The validity of the comparison between the pre- and postintervention groups relies on the similarity between the populations. When there is imbalance, investigators can consider matching based on important characteristics or applying risk adjustment as necessary. Another important assumption is that the outcome of interest is unchanged in the absence of the intervention. Finally, the analysis assumes that the intervention is fully implemented at the time the postintervention period begins. Often, there is a washout period during which the old approach is stopped and the new approach (the intervention) is being implemented and can easily be taken into account.
WHAT ARE THE STRENGTHS OF THE SEGMENTED REGRESSION ITS?
There are several strengths of the ITS analysis and segmented regression.7,8 First, this approach accounts for a possible secular trend in the outcome measure that may have been present prior to the intervention. For example, investigators might conclude that a readmissions program was effective in reducing readmissions if they found that the mean readmission percentage in the period after the intervention was significantly lower than before using a simple pre/post study design. However, what if the readmission rate was already going down prior to the intervention? Using an ITS approach, they may have found that the rate of readmissions simply continued to decrease after the intervention at the same rate that it was decreasing prior to the intervention and, therefore, conclude that the intervention was not effective. Second, because the ITS approach evaluates changes in rates of an outcome at a population level, confounding by individual-level variables will not introduce serious bias unless the confounding occurred at the same time as the intervention. Third, ITS can be used to measure the unintended consequences of interventions or events, and investigators can construct separate time-series analyses for different outcomes. Fourth, ITS can be used to evaluate the impact of the intervention on subpopulations (eg, those grouped by age, sex, race) by conducting stratified analysis. Fifth, ITS provides simple and clear graphical results that can be easily understood by various audiences.
WHAT ARE THE IMPORTANT LIMITATIONS OF AN ITS?
By accounting for preintervention trends, ITS studies permit stronger causal inference than do cross-sectional or simple pre/post QEDs, but they may by prone to confounding by cointerventions or by changes in the population composition. Causal inference based on the ITS analysis is only valid to the extent to which the intervention was the only thing that changed at the point in time between the preintervention and postintervention periods. It is important for investigators to consider this in the design and discuss any coincident interventions. If there are multiple interventions over time, it is possible to account for these changes in the study design by creating multiple points of interruption provided there are sufficient measurements of the outcome between interventions. If the composition of the population changes at the same time as the intervention, this introduces bias. Changes in the ability to measure the outcome or changes to its definition also threaten the validity of the study’s inferences. Finally, it is also important to remember that when the outcome is a population-level measurement, inferences about individual-level outcomes are inappropriate due to ecological fallacies (ie, when inferences about individuals are deduced from inferences about the group to which those individuals belong). For example, Coon et al found that infants with bronchiolitis in the ward-based high-flow nasal cannula protocol group had greater ICU admission rates. It would be inappropriate to conclude that, based on this, an individual infant in a hospital on a ward-based protocol is more likely to be admitted to the ICU.
CONCLUSION
Studies evaluating interventions and events are important for informing healthcare practice, policy, and public health. While an RCT is the preferred method for such evaluations, investigators must often consider alternative study designs when an RCT is not feasible or when more real-world outcome evaluation is desired. Quasi-experimental designs are employed in studies that do not use randomization to study the impact of interventions in real-world settings, and an interrupted time series is a strong QED for the evaluation of interventions and natural experiments.
1. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
2. Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5-25. https://doi.org/10.1146/annurev-publhealth-040617-014128
3. Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;38:39-56. https://doi.org/10.1146/annurev-publhealth-031816-044327
4. Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health. 2012;66(12):1182-1186. https://doi.org/10.1136/jech-2011-200375
5. Coly A, Parry G. Evaluating Complex Health Interventions: A Guide to Rigorous Research Designs. AcademyHealth; 2017.
6. Orenstein EW, Rasooly IR, Mai MV, et al. Influence of simulation on electronic health record use patterns among pediatric residents. J Am Med Inform Assoc. 2018;25(11):1501-1506. https://doi.org/10.1093/jamia/ocy105
7. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
8. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. https://doi.org/10.1046/j.1365-2710.2002.00430.x
9. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533
10. Kahn JM, Davis BS, Yabes JG, et al. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322(3):240-250. https://doi.org/10.1001/jama.2019.9021
11. Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):975-983. https://doi.org/10.1001/jama.298.9.975
12. Destino L, Bennett D, Wood M, et al. Improving patient flow: analysis of an initiative to improve early discharge. J Hosp Med. 2019;14(1):22-27. https://doi.org/10.12788/jhm.3133
13. Williams DJ, Hall M, Gerber JS, et al; Pediatric Research in Inpatient Settings Network. Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics. 2017;139(4):e20163231. https://doi.org/10.1542/peds.2016-3231
Hospital medicine research often asks the question whether an intervention, such as a policy or guideline, has improved quality of care and/or whether there were any unintended consequences. Alternatively, investigators may be interested in understanding the impact of an event, such as a natural disaster or a pandemic, on hospital care. The study design that provides the best estimate of the causal effect of the intervention is the randomized controlled trial (RCT). The goal of randomization, which can be implemented at the patient or cluster level (eg, hospitals), is attaining a balance of the known and unknown confounders between study groups.
However, an RCT may not be feasible for several reasons: complexity, insufficient setup time or funding, ethical barriers to randomization, unwillingness of funders or payers to withhold the intervention from patients (ie, the control group), or anticipated contamination of the intervention into the control group (eg, provider practice change interventions). In addition, it may be impossible to conduct an RCT because the investigator does not have control over the design of an intervention or because they are studying an event, such as a pandemic.
In the June 2020 issue of the Journal of Hospital Medicine, Coon et al1 use a type of quasi-experimental design (QED)—specifically, the interrupted time series (ITS)—to examine the impact of the adoption of ward-based high-flow nasal cannula protocols on intensive care unit (ICU) admission for bronchiolitis at children’s hospitals. In this methodologic progress note, we discuss QEDs for evaluating the impact of healthcare interventions or events and focus on ITS, one of the strongest QEDs.
WHAT IS A QUASI-EXPERIMENTAL DESIGN?
Quasi-experimental design refers to a broad range of nonrandomized or partially randomized pre- vs postintervention studies.2 In order to test a causal hypothesis without randomization, QEDs define a comparison group or a time period in which an intervention has not been implemented, as well as at least one group or time period in which an intervention has been implemented. In a QED, the control may lack similarity with the intervention group or time period because of differences in the patients, sites, or time period (sometimes referred to as having a “nonequivalent control group”). Several design and analytic approaches are available to enhance the extent to which the study is able to make conclusions about the causal impact of the intervention.2,3 Because randomization is not necessary, QEDs allow for inclusion of a broader population than that which is feasible by RCTs, which increases the applicability and generalizability of the results. Therefore, they are a powerful research design to test the effectiveness of interventions in real-world settings.
The choice of which QED depends on whether the investigators are conducting a prospective evaluation and have control over the study design (ie, the ordering of the intervention, selection of sites or individuals, and/or timing and frequency of the data collection) or whether the investigators do not have control over the intervention, which is also known as a “natural experiment.”4,5 Some studies may also incorporate two QEDs in tandem.6 The Table provides a brief summary of different QEDs, ordered by methodologic strength, and distinguishes those that can be used to study natural experiments. In the study by Coon et al,1 an ITS is used as opposed to a methodologically stronger QED, such as the stepped-wedge design, because the investigators did not have control over the rollout of heated high-flow nasal canula protocols across hospitals.

WHAT IS AN INTERRUPTED TIME SERIES?
Interrupted time series designs use repeated observations of an outcome over time. This method then divides, or “interrupts,” the series of data into two time periods: before the intervention or event and after. Using data from the preintervention period, an underlying trend in the outcome is estimated and assumed to continue forward into the postintervention period to estimate what would have occurred without the intervention. Any significant change in the outcome at the beginning of the postintervention period or change in the trend in the postintervention is then attributed to the intervention.
There are several important methodologic considerations when designing an ITS study, as detailed in other review papers.2,3,7,8 An ITS design can be retrospective or prospective. It can be of a single center or include multiple sites, as in Coon et al. It can be conducted with or without a control. The inclusion of a control, when appropriately chosen, improves the strength of the study design because it can account for seasonal trends and potential confounders that vary over time. The control can be a different group of hospitals or participants that are similar but did not receive the intervention, or it can be a different outcome in the same group of hospitals or participants that are not expected to be affected by the intervention. The ITS design may also be set up to estimate the individual effects of multicomponent interventions. If the different components are phased in sequentially over time, then it may be possible to interrupt the time series at these points and estimate the impact of each intervention component.
Other examples of ITS studies in hospital medicine include those that evaluated the impact of a readmission-reduction program,9 of state sepsis regulations on in-hospital mortality,10 of resident duty-hour reform on mortality among hospitalized patients,11 of a quality-improvement initiative on early discharge,12 and of national guidelines on pediatric pneumonia antibiotic selection.13 There are several types of ITS analysis, and in this article, we focus on segmented regression without a control group.7,8
WHAT IS A SEGMENTED REGRESSION ITS?
Segmented regression is the statistical model used to measure (a) the immediate change in the outcome (level) at the start of the intervention and (b) the change in the trend of the outcome (slope) in the postintervention period vs that in the preintervention period. Therefore, the intervention effect size is expressed in terms of the level change and the slope change. To function properly, the models require several repeated (eg, monthly) measurements of the outcome before and after the intervention. Some experts suggest a minimum of 4 to 12 observations, depending on a number of factors including the stability of the outcome and seasonal variations.7,8 If changes before and after more than one intervention are being examined, there should be the minimum number of observations separating them. Unlike typical regression models, time-series models can correct for autocorrelation if it is present in the data. Autocorrelation is the type of correlation that arises when data are collected over time, with those closest in time being more strongly correlated (there are also other types of autocorrelation, such as seasonal patterns). Using available statistical software, autocorrelation can be detected and, if present, it can be controlled for in the segmented regression models.
HOW ARE SEGMENTED REGRESSION RESULTS PRESENTED?
Coon et al present results of their ITS analysis in a panel of figures detailing each study outcome, ICU admission, ICU length of stay, total length of stay, and rates of mechanical ventilation. Each panel shows the rate of change in the outcome per season across hospitals, before and after adoption of heated high-flow nasal cannula protocols, and the level change at the time of adoption.
To further explain how segmented regression results are presented, in the Figure we detail the structure of a segmented regression figure evaluating the impact of an intervention without a control group. In addition to the regression figure, authors typically provide 95% CIs around the rates, level change, and the difference between the postintervention and preintervention periods, along with P values demonstrating whether the rates, level change, and the differences between period slopes differ significantly from zero.

WHAT ARE THE UNDERLYING ASSUMPTIONS OF THE SEGMENTED REGRESSION ITS?
Segmented regression models assume a linear trend in the outcome. If the outcome follows a nonlinear pattern (eg, exponential spread of a disease during a pandemic), then using different distributions in the modeling or transformations of the data may be necessary. The validity of the comparison between the pre- and postintervention groups relies on the similarity between the populations. When there is imbalance, investigators can consider matching based on important characteristics or applying risk adjustment as necessary. Another important assumption is that the outcome of interest is unchanged in the absence of the intervention. Finally, the analysis assumes that the intervention is fully implemented at the time the postintervention period begins. Often, there is a washout period during which the old approach is stopped and the new approach (the intervention) is being implemented and can easily be taken into account.
WHAT ARE THE STRENGTHS OF THE SEGMENTED REGRESSION ITS?
There are several strengths of the ITS analysis and segmented regression.7,8 First, this approach accounts for a possible secular trend in the outcome measure that may have been present prior to the intervention. For example, investigators might conclude that a readmissions program was effective in reducing readmissions if they found that the mean readmission percentage in the period after the intervention was significantly lower than before using a simple pre/post study design. However, what if the readmission rate was already going down prior to the intervention? Using an ITS approach, they may have found that the rate of readmissions simply continued to decrease after the intervention at the same rate that it was decreasing prior to the intervention and, therefore, conclude that the intervention was not effective. Second, because the ITS approach evaluates changes in rates of an outcome at a population level, confounding by individual-level variables will not introduce serious bias unless the confounding occurred at the same time as the intervention. Third, ITS can be used to measure the unintended consequences of interventions or events, and investigators can construct separate time-series analyses for different outcomes. Fourth, ITS can be used to evaluate the impact of the intervention on subpopulations (eg, those grouped by age, sex, race) by conducting stratified analysis. Fifth, ITS provides simple and clear graphical results that can be easily understood by various audiences.
WHAT ARE THE IMPORTANT LIMITATIONS OF AN ITS?
By accounting for preintervention trends, ITS studies permit stronger causal inference than do cross-sectional or simple pre/post QEDs, but they may by prone to confounding by cointerventions or by changes in the population composition. Causal inference based on the ITS analysis is only valid to the extent to which the intervention was the only thing that changed at the point in time between the preintervention and postintervention periods. It is important for investigators to consider this in the design and discuss any coincident interventions. If there are multiple interventions over time, it is possible to account for these changes in the study design by creating multiple points of interruption provided there are sufficient measurements of the outcome between interventions. If the composition of the population changes at the same time as the intervention, this introduces bias. Changes in the ability to measure the outcome or changes to its definition also threaten the validity of the study’s inferences. Finally, it is also important to remember that when the outcome is a population-level measurement, inferences about individual-level outcomes are inappropriate due to ecological fallacies (ie, when inferences about individuals are deduced from inferences about the group to which those individuals belong). For example, Coon et al found that infants with bronchiolitis in the ward-based high-flow nasal cannula protocol group had greater ICU admission rates. It would be inappropriate to conclude that, based on this, an individual infant in a hospital on a ward-based protocol is more likely to be admitted to the ICU.
CONCLUSION
Studies evaluating interventions and events are important for informing healthcare practice, policy, and public health. While an RCT is the preferred method for such evaluations, investigators must often consider alternative study designs when an RCT is not feasible or when more real-world outcome evaluation is desired. Quasi-experimental designs are employed in studies that do not use randomization to study the impact of interventions in real-world settings, and an interrupted time series is a strong QED for the evaluation of interventions and natural experiments.
Hospital medicine research often asks the question whether an intervention, such as a policy or guideline, has improved quality of care and/or whether there were any unintended consequences. Alternatively, investigators may be interested in understanding the impact of an event, such as a natural disaster or a pandemic, on hospital care. The study design that provides the best estimate of the causal effect of the intervention is the randomized controlled trial (RCT). The goal of randomization, which can be implemented at the patient or cluster level (eg, hospitals), is attaining a balance of the known and unknown confounders between study groups.
However, an RCT may not be feasible for several reasons: complexity, insufficient setup time or funding, ethical barriers to randomization, unwillingness of funders or payers to withhold the intervention from patients (ie, the control group), or anticipated contamination of the intervention into the control group (eg, provider practice change interventions). In addition, it may be impossible to conduct an RCT because the investigator does not have control over the design of an intervention or because they are studying an event, such as a pandemic.
In the June 2020 issue of the Journal of Hospital Medicine, Coon et al1 use a type of quasi-experimental design (QED)—specifically, the interrupted time series (ITS)—to examine the impact of the adoption of ward-based high-flow nasal cannula protocols on intensive care unit (ICU) admission for bronchiolitis at children’s hospitals. In this methodologic progress note, we discuss QEDs for evaluating the impact of healthcare interventions or events and focus on ITS, one of the strongest QEDs.
WHAT IS A QUASI-EXPERIMENTAL DESIGN?
Quasi-experimental design refers to a broad range of nonrandomized or partially randomized pre- vs postintervention studies.2 In order to test a causal hypothesis without randomization, QEDs define a comparison group or a time period in which an intervention has not been implemented, as well as at least one group or time period in which an intervention has been implemented. In a QED, the control may lack similarity with the intervention group or time period because of differences in the patients, sites, or time period (sometimes referred to as having a “nonequivalent control group”). Several design and analytic approaches are available to enhance the extent to which the study is able to make conclusions about the causal impact of the intervention.2,3 Because randomization is not necessary, QEDs allow for inclusion of a broader population than that which is feasible by RCTs, which increases the applicability and generalizability of the results. Therefore, they are a powerful research design to test the effectiveness of interventions in real-world settings.
The choice of which QED depends on whether the investigators are conducting a prospective evaluation and have control over the study design (ie, the ordering of the intervention, selection of sites or individuals, and/or timing and frequency of the data collection) or whether the investigators do not have control over the intervention, which is also known as a “natural experiment.”4,5 Some studies may also incorporate two QEDs in tandem.6 The Table provides a brief summary of different QEDs, ordered by methodologic strength, and distinguishes those that can be used to study natural experiments. In the study by Coon et al,1 an ITS is used as opposed to a methodologically stronger QED, such as the stepped-wedge design, because the investigators did not have control over the rollout of heated high-flow nasal canula protocols across hospitals.

WHAT IS AN INTERRUPTED TIME SERIES?
Interrupted time series designs use repeated observations of an outcome over time. This method then divides, or “interrupts,” the series of data into two time periods: before the intervention or event and after. Using data from the preintervention period, an underlying trend in the outcome is estimated and assumed to continue forward into the postintervention period to estimate what would have occurred without the intervention. Any significant change in the outcome at the beginning of the postintervention period or change in the trend in the postintervention is then attributed to the intervention.
There are several important methodologic considerations when designing an ITS study, as detailed in other review papers.2,3,7,8 An ITS design can be retrospective or prospective. It can be of a single center or include multiple sites, as in Coon et al. It can be conducted with or without a control. The inclusion of a control, when appropriately chosen, improves the strength of the study design because it can account for seasonal trends and potential confounders that vary over time. The control can be a different group of hospitals or participants that are similar but did not receive the intervention, or it can be a different outcome in the same group of hospitals or participants that are not expected to be affected by the intervention. The ITS design may also be set up to estimate the individual effects of multicomponent interventions. If the different components are phased in sequentially over time, then it may be possible to interrupt the time series at these points and estimate the impact of each intervention component.
Other examples of ITS studies in hospital medicine include those that evaluated the impact of a readmission-reduction program,9 of state sepsis regulations on in-hospital mortality,10 of resident duty-hour reform on mortality among hospitalized patients,11 of a quality-improvement initiative on early discharge,12 and of national guidelines on pediatric pneumonia antibiotic selection.13 There are several types of ITS analysis, and in this article, we focus on segmented regression without a control group.7,8
WHAT IS A SEGMENTED REGRESSION ITS?
Segmented regression is the statistical model used to measure (a) the immediate change in the outcome (level) at the start of the intervention and (b) the change in the trend of the outcome (slope) in the postintervention period vs that in the preintervention period. Therefore, the intervention effect size is expressed in terms of the level change and the slope change. To function properly, the models require several repeated (eg, monthly) measurements of the outcome before and after the intervention. Some experts suggest a minimum of 4 to 12 observations, depending on a number of factors including the stability of the outcome and seasonal variations.7,8 If changes before and after more than one intervention are being examined, there should be the minimum number of observations separating them. Unlike typical regression models, time-series models can correct for autocorrelation if it is present in the data. Autocorrelation is the type of correlation that arises when data are collected over time, with those closest in time being more strongly correlated (there are also other types of autocorrelation, such as seasonal patterns). Using available statistical software, autocorrelation can be detected and, if present, it can be controlled for in the segmented regression models.
HOW ARE SEGMENTED REGRESSION RESULTS PRESENTED?
Coon et al present results of their ITS analysis in a panel of figures detailing each study outcome, ICU admission, ICU length of stay, total length of stay, and rates of mechanical ventilation. Each panel shows the rate of change in the outcome per season across hospitals, before and after adoption of heated high-flow nasal cannula protocols, and the level change at the time of adoption.
To further explain how segmented regression results are presented, in the Figure we detail the structure of a segmented regression figure evaluating the impact of an intervention without a control group. In addition to the regression figure, authors typically provide 95% CIs around the rates, level change, and the difference between the postintervention and preintervention periods, along with P values demonstrating whether the rates, level change, and the differences between period slopes differ significantly from zero.

WHAT ARE THE UNDERLYING ASSUMPTIONS OF THE SEGMENTED REGRESSION ITS?
Segmented regression models assume a linear trend in the outcome. If the outcome follows a nonlinear pattern (eg, exponential spread of a disease during a pandemic), then using different distributions in the modeling or transformations of the data may be necessary. The validity of the comparison between the pre- and postintervention groups relies on the similarity between the populations. When there is imbalance, investigators can consider matching based on important characteristics or applying risk adjustment as necessary. Another important assumption is that the outcome of interest is unchanged in the absence of the intervention. Finally, the analysis assumes that the intervention is fully implemented at the time the postintervention period begins. Often, there is a washout period during which the old approach is stopped and the new approach (the intervention) is being implemented and can easily be taken into account.
WHAT ARE THE STRENGTHS OF THE SEGMENTED REGRESSION ITS?
There are several strengths of the ITS analysis and segmented regression.7,8 First, this approach accounts for a possible secular trend in the outcome measure that may have been present prior to the intervention. For example, investigators might conclude that a readmissions program was effective in reducing readmissions if they found that the mean readmission percentage in the period after the intervention was significantly lower than before using a simple pre/post study design. However, what if the readmission rate was already going down prior to the intervention? Using an ITS approach, they may have found that the rate of readmissions simply continued to decrease after the intervention at the same rate that it was decreasing prior to the intervention and, therefore, conclude that the intervention was not effective. Second, because the ITS approach evaluates changes in rates of an outcome at a population level, confounding by individual-level variables will not introduce serious bias unless the confounding occurred at the same time as the intervention. Third, ITS can be used to measure the unintended consequences of interventions or events, and investigators can construct separate time-series analyses for different outcomes. Fourth, ITS can be used to evaluate the impact of the intervention on subpopulations (eg, those grouped by age, sex, race) by conducting stratified analysis. Fifth, ITS provides simple and clear graphical results that can be easily understood by various audiences.
WHAT ARE THE IMPORTANT LIMITATIONS OF AN ITS?
By accounting for preintervention trends, ITS studies permit stronger causal inference than do cross-sectional or simple pre/post QEDs, but they may by prone to confounding by cointerventions or by changes in the population composition. Causal inference based on the ITS analysis is only valid to the extent to which the intervention was the only thing that changed at the point in time between the preintervention and postintervention periods. It is important for investigators to consider this in the design and discuss any coincident interventions. If there are multiple interventions over time, it is possible to account for these changes in the study design by creating multiple points of interruption provided there are sufficient measurements of the outcome between interventions. If the composition of the population changes at the same time as the intervention, this introduces bias. Changes in the ability to measure the outcome or changes to its definition also threaten the validity of the study’s inferences. Finally, it is also important to remember that when the outcome is a population-level measurement, inferences about individual-level outcomes are inappropriate due to ecological fallacies (ie, when inferences about individuals are deduced from inferences about the group to which those individuals belong). For example, Coon et al found that infants with bronchiolitis in the ward-based high-flow nasal cannula protocol group had greater ICU admission rates. It would be inappropriate to conclude that, based on this, an individual infant in a hospital on a ward-based protocol is more likely to be admitted to the ICU.
CONCLUSION
Studies evaluating interventions and events are important for informing healthcare practice, policy, and public health. While an RCT is the preferred method for such evaluations, investigators must often consider alternative study designs when an RCT is not feasible or when more real-world outcome evaluation is desired. Quasi-experimental designs are employed in studies that do not use randomization to study the impact of interventions in real-world settings, and an interrupted time series is a strong QED for the evaluation of interventions and natural experiments.
1. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
2. Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5-25. https://doi.org/10.1146/annurev-publhealth-040617-014128
3. Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;38:39-56. https://doi.org/10.1146/annurev-publhealth-031816-044327
4. Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health. 2012;66(12):1182-1186. https://doi.org/10.1136/jech-2011-200375
5. Coly A, Parry G. Evaluating Complex Health Interventions: A Guide to Rigorous Research Designs. AcademyHealth; 2017.
6. Orenstein EW, Rasooly IR, Mai MV, et al. Influence of simulation on electronic health record use patterns among pediatric residents. J Am Med Inform Assoc. 2018;25(11):1501-1506. https://doi.org/10.1093/jamia/ocy105
7. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
8. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. https://doi.org/10.1046/j.1365-2710.2002.00430.x
9. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533
10. Kahn JM, Davis BS, Yabes JG, et al. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322(3):240-250. https://doi.org/10.1001/jama.2019.9021
11. Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):975-983. https://doi.org/10.1001/jama.298.9.975
12. Destino L, Bennett D, Wood M, et al. Improving patient flow: analysis of an initiative to improve early discharge. J Hosp Med. 2019;14(1):22-27. https://doi.org/10.12788/jhm.3133
13. Williams DJ, Hall M, Gerber JS, et al; Pediatric Research in Inpatient Settings Network. Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics. 2017;139(4):e20163231. https://doi.org/10.1542/peds.2016-3231
1. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
2. Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5-25. https://doi.org/10.1146/annurev-publhealth-040617-014128
3. Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;38:39-56. https://doi.org/10.1146/annurev-publhealth-031816-044327
4. Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health. 2012;66(12):1182-1186. https://doi.org/10.1136/jech-2011-200375
5. Coly A, Parry G. Evaluating Complex Health Interventions: A Guide to Rigorous Research Designs. AcademyHealth; 2017.
6. Orenstein EW, Rasooly IR, Mai MV, et al. Influence of simulation on electronic health record use patterns among pediatric residents. J Am Med Inform Assoc. 2018;25(11):1501-1506. https://doi.org/10.1093/jamia/ocy105
7. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
8. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. https://doi.org/10.1046/j.1365-2710.2002.00430.x
9. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533
10. Kahn JM, Davis BS, Yabes JG, et al. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322(3):240-250. https://doi.org/10.1001/jama.2019.9021
11. Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):975-983. https://doi.org/10.1001/jama.298.9.975
12. Destino L, Bennett D, Wood M, et al. Improving patient flow: analysis of an initiative to improve early discharge. J Hosp Med. 2019;14(1):22-27. https://doi.org/10.12788/jhm.3133
13. Williams DJ, Hall M, Gerber JS, et al; Pediatric Research in Inpatient Settings Network. Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics. 2017;139(4):e20163231. https://doi.org/10.1542/peds.2016-3231
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: NPO After Midnight
Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™ ” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 18-year-old man for newly diagnosed granulomatosis with polyangiitis to receive expedited pulse-dose steroids and plasma exchange. After consulting interventional radiology for catheter placement the following day, the hospitalist places a “strict” nil per os (nothing by mouth, NPO) after midnight order. During rounds the following morning, the patient reports that he wants to eat. At 9
BACKGROUND
Hospitalists commonly order “NPO after midnight” diets in anticipation of procedures requiring sedation or general anesthesia. Typically, NPO refers to no food or drink, but in some instances, NPO includes no oral medications. Up to half of medical patients experience some time of fasting while hospitalized.1 However, NPO practices vary widely across institutions.2,3 A study from 2014 notes that, on average, patients fast preprocedure for approximately 13.5 hours for solids and 9.6 hours for liquids.2 Prolonged fasting times offer little benefit to patients and may lead to frequent patient dissatisfaction and complaints.
WHY YOU MIGHT THINK THAT MAKING PATIENTS NPO AFTER MIDNIGHT IS APPROPRIATE
In 1883, Sir Joseph Lister described 19th century NPO practices distinguishing solids from liquids, allowing patients “tea or beef tea” until 2 to 3 hours prior to surgery.4 However, in 1946, Mendelson published an influential account of 66 pregnant women who aspirated during delivery under general anesthesia.5 Two of the 66 patients, both of whom had eaten a full meal 6 to 8 hours prior to general anesthesia, died. The study not only increased awareness of the risk of aspiration with general anesthesia in pregnancy, but it influenced the care for the nonpregnant population of patients as well. By the 1960s, anesthesia texts recommended “NPO after midnight” for both liquids and solids in all patients, regardless of pregnancy status.4 To minimize the risk to patients, we have continued to pass down the practice of NPO after midnight to subsequent generations.
Additionally, medical centers and hospitals feel pressure to provide efficient, patient-centered, high-value care. Given the complexity of procedural scheduling and the penalties associated with delays, keeping patients NPO ensures their availability for the next open procedural slot. NPO after midnight orders aim to prevent potential delays in treatment that occur when inadvertent ingestion of food and drink leads to cancellation of procedures.
WHY THE INDISCRIMINATE USE OF NPO AFTER MIDNIGHT IS UNNECESSARY
Recent studies have led to a more sophisticated understanding of gastric emptying and the risks of aspiration during sedation and intubation. Gastric emptying studies routinely show that transit of clear liquids out of the stomach is virtually complete within two hours of drinking.6 Age, body mass index, and alcohol have no effect on gastric emptying time, and almost all patients return to preingestion gastric residual volumes within 2 hours of clear liquid consumption.6,7 While morbidly obese patients tend to have higher gastric fluid volumes after 9 hours of fasting, their stomachs empty at rates similar to nonobese individuals.6 Note that, regardless of fasting times, morbid obesity predisposes patients to a higher overall gastric volume and lower pH of gastric contents, which may increase risk of aspiration.8 A Cochrane review found no statistical difference in gastric volumes or stomach pH in patients on a standard fast vs shortened (<180 minutes) liquid fast.9 The review included nine studies that found patients who consumed a clear liquid beverage had reduced gastric volumes, compared with patients in a fasting state (P < .001).9
In a pediatric retrospective study of pulmonary aspiration events, the researchers demonstrated that clinically significant aspiration (presence of bilious secretions in the tracheobronchial airways) occurred at a rate of 0.04% with emergency surgery.10 Bowel obstruction or ileus accounted for approximately 54% of those cases. Importantly, the reported aspiration rate approximates the rate of pregnant patients from the 1946 Mendelson study of 0.14% (66 out of 44,016), which originally prompted the use of the prolonged NPO status. Based on the Cochrane review of perioperative fasting recommendations for those older than 18 years, consuming fluids more than 90 minutes preoperatively confers a negligible (0 adverse events reported in 9 studies) risk for aspiration or regurgitation events.9
In 1998, as a result of these and other similar studies, the American Society of Anesthesiologists (ASA) along with global anesthesia partners adopted guidelines that allowed clear liquids up until 2 hours prior to anesthesia or sedation in low-aspiration-risk patients undergoing elective cases.11 The guidelines allowed for other beverages and food based on their standard transit times (Table). The ASA guidelines do not define low-aspiration-risk patients. Anesthesiologists generally exclude from the low-risk category patients who may have delayed gastric emptying from medical or iatrogenic causes. The updated 2017 ASA guidelines remain unchanged regarding fasting guidelines.12 Studies suggest that approximately 10% to 20% of NPO after midnight orders are avoidable.1,3 For those instances, procedures are often deemed not necessary or do not require NPO status.1
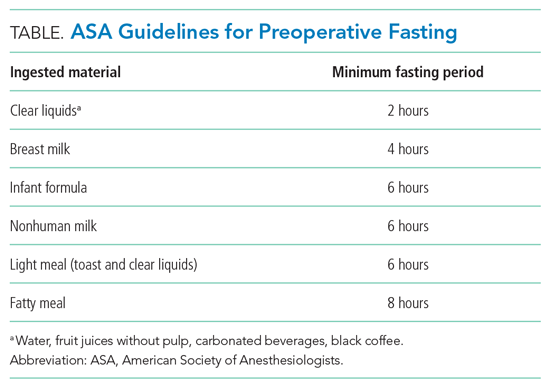
In a study evaluating the reasons that necessary procedures are canceled, only 0.5% of inpatient procedures are cancelled due to the inappropriate ingestion of food or drink.3 In addition, NPO status creates risk. Patients with prolonged NPO status report greater hunger, thirst, tiredness, and weakness prior to surgery when compared with patients receiving a carbohydrate-rich drink 2 hours prior to procedures.9,13,14 In fact, multiple studies have suggested that preoperative carbohydrate-rich drinks 2 hours before surgery can be associated with decreased insulin resistance in the perioperative period, decreased length of stay, and improvement in perioperative metabolic, cardiac, and psychosomatic status.9,13-15 These types of studies have informed the enhanced recovery after surgery program, which recommends a carbohydrate beverage 2 to 3 hours prior to surgery.
WHEN TO ORDER LONGER PREPROCEDURAL NPO TIMES
Prescribe the minimum recommended fasting times only for low-aspiration-risk patients undergoing elective procedures. Risk for regurgitation or aspiration increases for patients with conditions resulting in decreased gastric emptying, gastric or bowel obstruction, or lower esophageal sphincter incompetence. Those patients may require longer NPO time periods.8 Higher-risk diagnoses and clinical conditions include gastroparesis, trauma, and pregnancy.5,8,16 Specific risk factors for aspiration in children may include trauma, bowel obstruction, depressed consciousness, shock, or ileus.10 For surgical emergencies, balance the risk of surgical delay vs perceived aspiration risk.
WHAT WE SHOULD DO INSTEAD OF ROUTINELY ORDERING NPO AFTER MIDNIGHT
Use evidence-based guidelines to assess periprocedural aspiration risk. The ASA guidelines suggest that healthy, nonpregnant patients should fast for 8 hours after heavy meals, 6 hours after a light, nonfatty meal, and 2 hours after clear liquids (eg, water, fruit juices without pulp, carbonated beverages, black coffee).12 Focus on the type of food or drink rather than the volume ingested.12 Additionally, patients should ingest, with small amounts of clear fluids, appropriate home medications for acute and chronic conditions regardless of NPO status.
While procedure delays or cancellations for any reason upset patients and families and can disrupt the flow of the operating room and procedural suite, we can achieve the delicate balance between efficiency and patient safety and comfort. Since complex inpatient procedural scheduling may not allow for liberalization of solids requiring 6 to 8 hours of fasting time, focus on liberalizing liquids 2 hours prior to anesthesia. This allows staff to minimize the time low-risk patients fast while still maintaining flexibility for operating room case scheduling. We must promote communication between operating room and floor staff to anticipate timing of procedures each day. Healthcare facilities should aim to achieve time-based preprocedural NPO status as opposed to an arbitrary starting time like midnight.4
RECOMMENDATIONS
- Risk stratify patients for anesthesia-related aspiration with the aim of identifying those at low aspiration risk.
- For low-risk patients, adhere to recommended fasting times: 2 hours for a clear carbohydrate beverage, 4 hours for breast milk, 6 hours for a light meal or formula, and 8 hours for a fatty meal.
- For patients not deemed low risk, determine the appropriate length of preprocedural fasting by consulting with the anesthesia and surgical teams.
CONCLUSION
NPO after midnight represents a low-value and arbitrary practice that leaves patients fasting longer than necessary.2,3,12 In addition to the 2017 ASA guidelines, newer studies and protocols are improving patient satisfaction, minimizing patient dehydration and electrolyte disturbances, and incorporating enhanced recovery after surgery factors into a better patient experience. Returning to the clinical scenario, the hospitalist team can increase patient satisfaction by focusing on liberalizing clear fluids with a carbohydrate beverage up to 2 hours prior to elective surgery while still allowing for schedule flexibility. For this patient, a 3
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Disclaimer: The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs. The Veterans Affairs Quality Scholars Program is supported by the Veterans Affairs Office of Academic Affiliations, Washington, DC.
1. Sorita A, Thongprayoon C, Ahmed A, et al. Frequency and appropriateness of fasting orders in the hospital. Mayo Clin Proc. 2015;90(9):1225-1232. https://doi.org/10.1016/j.mayocp.2015.07.013
2. Falconer R, Skouras C, Carter T, Greenway L, Paisley AM. Preoperative fasting: current practice and areas for improvement. Updates Surg. 2014;66(1):31-39. https://doi.org/10.1007/s13304-013-0242-z
3. Sorita A, Thongprayoon C, Ratelle JT, et al. Characteristics and outcomes of fasting orders among medical inpatients. J Hosp Med. 2017;12(1):36-39. https://doi.org/10.1002/jhm.2674
4. Maltby JR. Fasting from midnight–the history behind the dogma. Best Pract Res Clin Anaesthesiol. 2006;20(3):363-378. https://doi.org/10.1016/j.bpa.2006.02.001
5. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205. https://doi.org/10.1016/s0002-9378(16)39829-5
6. Shiraishi T, Kurosaki D, Nakamura M, et al. Gastric fluid volume change after oral rehydration solution intake in morbidly obese and normal controls: a magnetic resonance imaging-based analysis. Anesth Analg. 2017;124(4):1174-1178. https://doi.org/10.1213/ane.0000000000001886
7. Vasavid P, Chaiwatanarat T, Pusuwan P, et al. Normal solid gastric emptying values measured by scintigraphy using Asian-style meal: a multicenter study in healthy volunteers. J Neurogastroenterol Motil. 2014;20(3):371-378. https://doi.org/10.5056/jnm13114
8. Mahajan V, Hashmi J, Singh R, Samra T, Aneja S. Comparative evaluation of gastric pH and volume in morbidly obese and lean patients undergoing elective surgery and effect of aspiration prophylaxis. J Clin Anesth. 2015;27(5):396-400. https://doi.org/10.1016/j.jclinane.2015.03.004
9. Brady MC, Kinn S, Stuart P, Ness V. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423. https://doi.org/10.1002/14651858.cd004423
10. Warner MA, Warner ME, Warner DO, Warner LO, Warner EJ. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999;90(1):66-71. https://doi.org/10.1097/00000542-199901000-00011
11. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90(3):896-905. https://doi.org/10.1097/00000542-199903000-00034
12. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376-393. https://doi.org/10.1097/aln.0000000000001452
13. Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344-1350. https://doi.org/10.1097/00000539-200111000-00063
14. Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32(1):34-44. https://doi.org/10.1016/j.clnu.2012.10.011
15. Kaška M, Grosmanová T, Havel E, et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery–a randomized controlled trial. Wien Klin Wochenschr. 2010;122(1-2):23-30. https://doi.org/10.1007/s00508-009-1291-7
16. Tokumine J, Sugahara K, Fuchigami T, Teruya K, Nitta K, Satou K. Unanticipated full stomach at anesthesia induction in a type I diabetic patient with asymptomatic gastroparesis. J Anesth. 2005;19(3):247-248. https://doi.org/10.1007/s00540-005-0321-5
Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™ ” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 18-year-old man for newly diagnosed granulomatosis with polyangiitis to receive expedited pulse-dose steroids and plasma exchange. After consulting interventional radiology for catheter placement the following day, the hospitalist places a “strict” nil per os (nothing by mouth, NPO) after midnight order. During rounds the following morning, the patient reports that he wants to eat. At 9
BACKGROUND
Hospitalists commonly order “NPO after midnight” diets in anticipation of procedures requiring sedation or general anesthesia. Typically, NPO refers to no food or drink, but in some instances, NPO includes no oral medications. Up to half of medical patients experience some time of fasting while hospitalized.1 However, NPO practices vary widely across institutions.2,3 A study from 2014 notes that, on average, patients fast preprocedure for approximately 13.5 hours for solids and 9.6 hours for liquids.2 Prolonged fasting times offer little benefit to patients and may lead to frequent patient dissatisfaction and complaints.
WHY YOU MIGHT THINK THAT MAKING PATIENTS NPO AFTER MIDNIGHT IS APPROPRIATE
In 1883, Sir Joseph Lister described 19th century NPO practices distinguishing solids from liquids, allowing patients “tea or beef tea” until 2 to 3 hours prior to surgery.4 However, in 1946, Mendelson published an influential account of 66 pregnant women who aspirated during delivery under general anesthesia.5 Two of the 66 patients, both of whom had eaten a full meal 6 to 8 hours prior to general anesthesia, died. The study not only increased awareness of the risk of aspiration with general anesthesia in pregnancy, but it influenced the care for the nonpregnant population of patients as well. By the 1960s, anesthesia texts recommended “NPO after midnight” for both liquids and solids in all patients, regardless of pregnancy status.4 To minimize the risk to patients, we have continued to pass down the practice of NPO after midnight to subsequent generations.
Additionally, medical centers and hospitals feel pressure to provide efficient, patient-centered, high-value care. Given the complexity of procedural scheduling and the penalties associated with delays, keeping patients NPO ensures their availability for the next open procedural slot. NPO after midnight orders aim to prevent potential delays in treatment that occur when inadvertent ingestion of food and drink leads to cancellation of procedures.
WHY THE INDISCRIMINATE USE OF NPO AFTER MIDNIGHT IS UNNECESSARY
Recent studies have led to a more sophisticated understanding of gastric emptying and the risks of aspiration during sedation and intubation. Gastric emptying studies routinely show that transit of clear liquids out of the stomach is virtually complete within two hours of drinking.6 Age, body mass index, and alcohol have no effect on gastric emptying time, and almost all patients return to preingestion gastric residual volumes within 2 hours of clear liquid consumption.6,7 While morbidly obese patients tend to have higher gastric fluid volumes after 9 hours of fasting, their stomachs empty at rates similar to nonobese individuals.6 Note that, regardless of fasting times, morbid obesity predisposes patients to a higher overall gastric volume and lower pH of gastric contents, which may increase risk of aspiration.8 A Cochrane review found no statistical difference in gastric volumes or stomach pH in patients on a standard fast vs shortened (<180 minutes) liquid fast.9 The review included nine studies that found patients who consumed a clear liquid beverage had reduced gastric volumes, compared with patients in a fasting state (P < .001).9
In a pediatric retrospective study of pulmonary aspiration events, the researchers demonstrated that clinically significant aspiration (presence of bilious secretions in the tracheobronchial airways) occurred at a rate of 0.04% with emergency surgery.10 Bowel obstruction or ileus accounted for approximately 54% of those cases. Importantly, the reported aspiration rate approximates the rate of pregnant patients from the 1946 Mendelson study of 0.14% (66 out of 44,016), which originally prompted the use of the prolonged NPO status. Based on the Cochrane review of perioperative fasting recommendations for those older than 18 years, consuming fluids more than 90 minutes preoperatively confers a negligible (0 adverse events reported in 9 studies) risk for aspiration or regurgitation events.9
In 1998, as a result of these and other similar studies, the American Society of Anesthesiologists (ASA) along with global anesthesia partners adopted guidelines that allowed clear liquids up until 2 hours prior to anesthesia or sedation in low-aspiration-risk patients undergoing elective cases.11 The guidelines allowed for other beverages and food based on their standard transit times (Table). The ASA guidelines do not define low-aspiration-risk patients. Anesthesiologists generally exclude from the low-risk category patients who may have delayed gastric emptying from medical or iatrogenic causes. The updated 2017 ASA guidelines remain unchanged regarding fasting guidelines.12 Studies suggest that approximately 10% to 20% of NPO after midnight orders are avoidable.1,3 For those instances, procedures are often deemed not necessary or do not require NPO status.1

In a study evaluating the reasons that necessary procedures are canceled, only 0.5% of inpatient procedures are cancelled due to the inappropriate ingestion of food or drink.3 In addition, NPO status creates risk. Patients with prolonged NPO status report greater hunger, thirst, tiredness, and weakness prior to surgery when compared with patients receiving a carbohydrate-rich drink 2 hours prior to procedures.9,13,14 In fact, multiple studies have suggested that preoperative carbohydrate-rich drinks 2 hours before surgery can be associated with decreased insulin resistance in the perioperative period, decreased length of stay, and improvement in perioperative metabolic, cardiac, and psychosomatic status.9,13-15 These types of studies have informed the enhanced recovery after surgery program, which recommends a carbohydrate beverage 2 to 3 hours prior to surgery.
WHEN TO ORDER LONGER PREPROCEDURAL NPO TIMES
Prescribe the minimum recommended fasting times only for low-aspiration-risk patients undergoing elective procedures. Risk for regurgitation or aspiration increases for patients with conditions resulting in decreased gastric emptying, gastric or bowel obstruction, or lower esophageal sphincter incompetence. Those patients may require longer NPO time periods.8 Higher-risk diagnoses and clinical conditions include gastroparesis, trauma, and pregnancy.5,8,16 Specific risk factors for aspiration in children may include trauma, bowel obstruction, depressed consciousness, shock, or ileus.10 For surgical emergencies, balance the risk of surgical delay vs perceived aspiration risk.
WHAT WE SHOULD DO INSTEAD OF ROUTINELY ORDERING NPO AFTER MIDNIGHT
Use evidence-based guidelines to assess periprocedural aspiration risk. The ASA guidelines suggest that healthy, nonpregnant patients should fast for 8 hours after heavy meals, 6 hours after a light, nonfatty meal, and 2 hours after clear liquids (eg, water, fruit juices without pulp, carbonated beverages, black coffee).12 Focus on the type of food or drink rather than the volume ingested.12 Additionally, patients should ingest, with small amounts of clear fluids, appropriate home medications for acute and chronic conditions regardless of NPO status.
While procedure delays or cancellations for any reason upset patients and families and can disrupt the flow of the operating room and procedural suite, we can achieve the delicate balance between efficiency and patient safety and comfort. Since complex inpatient procedural scheduling may not allow for liberalization of solids requiring 6 to 8 hours of fasting time, focus on liberalizing liquids 2 hours prior to anesthesia. This allows staff to minimize the time low-risk patients fast while still maintaining flexibility for operating room case scheduling. We must promote communication between operating room and floor staff to anticipate timing of procedures each day. Healthcare facilities should aim to achieve time-based preprocedural NPO status as opposed to an arbitrary starting time like midnight.4
RECOMMENDATIONS
- Risk stratify patients for anesthesia-related aspiration with the aim of identifying those at low aspiration risk.
- For low-risk patients, adhere to recommended fasting times: 2 hours for a clear carbohydrate beverage, 4 hours for breast milk, 6 hours for a light meal or formula, and 8 hours for a fatty meal.
- For patients not deemed low risk, determine the appropriate length of preprocedural fasting by consulting with the anesthesia and surgical teams.
CONCLUSION
NPO after midnight represents a low-value and arbitrary practice that leaves patients fasting longer than necessary.2,3,12 In addition to the 2017 ASA guidelines, newer studies and protocols are improving patient satisfaction, minimizing patient dehydration and electrolyte disturbances, and incorporating enhanced recovery after surgery factors into a better patient experience. Returning to the clinical scenario, the hospitalist team can increase patient satisfaction by focusing on liberalizing clear fluids with a carbohydrate beverage up to 2 hours prior to elective surgery while still allowing for schedule flexibility. For this patient, a 3
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Disclaimer: The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs. The Veterans Affairs Quality Scholars Program is supported by the Veterans Affairs Office of Academic Affiliations, Washington, DC.
Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™ ” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 18-year-old man for newly diagnosed granulomatosis with polyangiitis to receive expedited pulse-dose steroids and plasma exchange. After consulting interventional radiology for catheter placement the following day, the hospitalist places a “strict” nil per os (nothing by mouth, NPO) after midnight order. During rounds the following morning, the patient reports that he wants to eat. At 9
BACKGROUND
Hospitalists commonly order “NPO after midnight” diets in anticipation of procedures requiring sedation or general anesthesia. Typically, NPO refers to no food or drink, but in some instances, NPO includes no oral medications. Up to half of medical patients experience some time of fasting while hospitalized.1 However, NPO practices vary widely across institutions.2,3 A study from 2014 notes that, on average, patients fast preprocedure for approximately 13.5 hours for solids and 9.6 hours for liquids.2 Prolonged fasting times offer little benefit to patients and may lead to frequent patient dissatisfaction and complaints.
WHY YOU MIGHT THINK THAT MAKING PATIENTS NPO AFTER MIDNIGHT IS APPROPRIATE
In 1883, Sir Joseph Lister described 19th century NPO practices distinguishing solids from liquids, allowing patients “tea or beef tea” until 2 to 3 hours prior to surgery.4 However, in 1946, Mendelson published an influential account of 66 pregnant women who aspirated during delivery under general anesthesia.5 Two of the 66 patients, both of whom had eaten a full meal 6 to 8 hours prior to general anesthesia, died. The study not only increased awareness of the risk of aspiration with general anesthesia in pregnancy, but it influenced the care for the nonpregnant population of patients as well. By the 1960s, anesthesia texts recommended “NPO after midnight” for both liquids and solids in all patients, regardless of pregnancy status.4 To minimize the risk to patients, we have continued to pass down the practice of NPO after midnight to subsequent generations.
Additionally, medical centers and hospitals feel pressure to provide efficient, patient-centered, high-value care. Given the complexity of procedural scheduling and the penalties associated with delays, keeping patients NPO ensures their availability for the next open procedural slot. NPO after midnight orders aim to prevent potential delays in treatment that occur when inadvertent ingestion of food and drink leads to cancellation of procedures.
WHY THE INDISCRIMINATE USE OF NPO AFTER MIDNIGHT IS UNNECESSARY
Recent studies have led to a more sophisticated understanding of gastric emptying and the risks of aspiration during sedation and intubation. Gastric emptying studies routinely show that transit of clear liquids out of the stomach is virtually complete within two hours of drinking.6 Age, body mass index, and alcohol have no effect on gastric emptying time, and almost all patients return to preingestion gastric residual volumes within 2 hours of clear liquid consumption.6,7 While morbidly obese patients tend to have higher gastric fluid volumes after 9 hours of fasting, their stomachs empty at rates similar to nonobese individuals.6 Note that, regardless of fasting times, morbid obesity predisposes patients to a higher overall gastric volume and lower pH of gastric contents, which may increase risk of aspiration.8 A Cochrane review found no statistical difference in gastric volumes or stomach pH in patients on a standard fast vs shortened (<180 minutes) liquid fast.9 The review included nine studies that found patients who consumed a clear liquid beverage had reduced gastric volumes, compared with patients in a fasting state (P < .001).9
In a pediatric retrospective study of pulmonary aspiration events, the researchers demonstrated that clinically significant aspiration (presence of bilious secretions in the tracheobronchial airways) occurred at a rate of 0.04% with emergency surgery.10 Bowel obstruction or ileus accounted for approximately 54% of those cases. Importantly, the reported aspiration rate approximates the rate of pregnant patients from the 1946 Mendelson study of 0.14% (66 out of 44,016), which originally prompted the use of the prolonged NPO status. Based on the Cochrane review of perioperative fasting recommendations for those older than 18 years, consuming fluids more than 90 minutes preoperatively confers a negligible (0 adverse events reported in 9 studies) risk for aspiration or regurgitation events.9
In 1998, as a result of these and other similar studies, the American Society of Anesthesiologists (ASA) along with global anesthesia partners adopted guidelines that allowed clear liquids up until 2 hours prior to anesthesia or sedation in low-aspiration-risk patients undergoing elective cases.11 The guidelines allowed for other beverages and food based on their standard transit times (Table). The ASA guidelines do not define low-aspiration-risk patients. Anesthesiologists generally exclude from the low-risk category patients who may have delayed gastric emptying from medical or iatrogenic causes. The updated 2017 ASA guidelines remain unchanged regarding fasting guidelines.12 Studies suggest that approximately 10% to 20% of NPO after midnight orders are avoidable.1,3 For those instances, procedures are often deemed not necessary or do not require NPO status.1

In a study evaluating the reasons that necessary procedures are canceled, only 0.5% of inpatient procedures are cancelled due to the inappropriate ingestion of food or drink.3 In addition, NPO status creates risk. Patients with prolonged NPO status report greater hunger, thirst, tiredness, and weakness prior to surgery when compared with patients receiving a carbohydrate-rich drink 2 hours prior to procedures.9,13,14 In fact, multiple studies have suggested that preoperative carbohydrate-rich drinks 2 hours before surgery can be associated with decreased insulin resistance in the perioperative period, decreased length of stay, and improvement in perioperative metabolic, cardiac, and psychosomatic status.9,13-15 These types of studies have informed the enhanced recovery after surgery program, which recommends a carbohydrate beverage 2 to 3 hours prior to surgery.
WHEN TO ORDER LONGER PREPROCEDURAL NPO TIMES
Prescribe the minimum recommended fasting times only for low-aspiration-risk patients undergoing elective procedures. Risk for regurgitation or aspiration increases for patients with conditions resulting in decreased gastric emptying, gastric or bowel obstruction, or lower esophageal sphincter incompetence. Those patients may require longer NPO time periods.8 Higher-risk diagnoses and clinical conditions include gastroparesis, trauma, and pregnancy.5,8,16 Specific risk factors for aspiration in children may include trauma, bowel obstruction, depressed consciousness, shock, or ileus.10 For surgical emergencies, balance the risk of surgical delay vs perceived aspiration risk.
WHAT WE SHOULD DO INSTEAD OF ROUTINELY ORDERING NPO AFTER MIDNIGHT
Use evidence-based guidelines to assess periprocedural aspiration risk. The ASA guidelines suggest that healthy, nonpregnant patients should fast for 8 hours after heavy meals, 6 hours after a light, nonfatty meal, and 2 hours after clear liquids (eg, water, fruit juices without pulp, carbonated beverages, black coffee).12 Focus on the type of food or drink rather than the volume ingested.12 Additionally, patients should ingest, with small amounts of clear fluids, appropriate home medications for acute and chronic conditions regardless of NPO status.
While procedure delays or cancellations for any reason upset patients and families and can disrupt the flow of the operating room and procedural suite, we can achieve the delicate balance between efficiency and patient safety and comfort. Since complex inpatient procedural scheduling may not allow for liberalization of solids requiring 6 to 8 hours of fasting time, focus on liberalizing liquids 2 hours prior to anesthesia. This allows staff to minimize the time low-risk patients fast while still maintaining flexibility for operating room case scheduling. We must promote communication between operating room and floor staff to anticipate timing of procedures each day. Healthcare facilities should aim to achieve time-based preprocedural NPO status as opposed to an arbitrary starting time like midnight.4
RECOMMENDATIONS
- Risk stratify patients for anesthesia-related aspiration with the aim of identifying those at low aspiration risk.
- For low-risk patients, adhere to recommended fasting times: 2 hours for a clear carbohydrate beverage, 4 hours for breast milk, 6 hours for a light meal or formula, and 8 hours for a fatty meal.
- For patients not deemed low risk, determine the appropriate length of preprocedural fasting by consulting with the anesthesia and surgical teams.
CONCLUSION
NPO after midnight represents a low-value and arbitrary practice that leaves patients fasting longer than necessary.2,3,12 In addition to the 2017 ASA guidelines, newer studies and protocols are improving patient satisfaction, minimizing patient dehydration and electrolyte disturbances, and incorporating enhanced recovery after surgery factors into a better patient experience. Returning to the clinical scenario, the hospitalist team can increase patient satisfaction by focusing on liberalizing clear fluids with a carbohydrate beverage up to 2 hours prior to elective surgery while still allowing for schedule flexibility. For this patient, a 3
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Disclaimer: The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs. The Veterans Affairs Quality Scholars Program is supported by the Veterans Affairs Office of Academic Affiliations, Washington, DC.
1. Sorita A, Thongprayoon C, Ahmed A, et al. Frequency and appropriateness of fasting orders in the hospital. Mayo Clin Proc. 2015;90(9):1225-1232. https://doi.org/10.1016/j.mayocp.2015.07.013
2. Falconer R, Skouras C, Carter T, Greenway L, Paisley AM. Preoperative fasting: current practice and areas for improvement. Updates Surg. 2014;66(1):31-39. https://doi.org/10.1007/s13304-013-0242-z
3. Sorita A, Thongprayoon C, Ratelle JT, et al. Characteristics and outcomes of fasting orders among medical inpatients. J Hosp Med. 2017;12(1):36-39. https://doi.org/10.1002/jhm.2674
4. Maltby JR. Fasting from midnight–the history behind the dogma. Best Pract Res Clin Anaesthesiol. 2006;20(3):363-378. https://doi.org/10.1016/j.bpa.2006.02.001
5. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205. https://doi.org/10.1016/s0002-9378(16)39829-5
6. Shiraishi T, Kurosaki D, Nakamura M, et al. Gastric fluid volume change after oral rehydration solution intake in morbidly obese and normal controls: a magnetic resonance imaging-based analysis. Anesth Analg. 2017;124(4):1174-1178. https://doi.org/10.1213/ane.0000000000001886
7. Vasavid P, Chaiwatanarat T, Pusuwan P, et al. Normal solid gastric emptying values measured by scintigraphy using Asian-style meal: a multicenter study in healthy volunteers. J Neurogastroenterol Motil. 2014;20(3):371-378. https://doi.org/10.5056/jnm13114
8. Mahajan V, Hashmi J, Singh R, Samra T, Aneja S. Comparative evaluation of gastric pH and volume in morbidly obese and lean patients undergoing elective surgery and effect of aspiration prophylaxis. J Clin Anesth. 2015;27(5):396-400. https://doi.org/10.1016/j.jclinane.2015.03.004
9. Brady MC, Kinn S, Stuart P, Ness V. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423. https://doi.org/10.1002/14651858.cd004423
10. Warner MA, Warner ME, Warner DO, Warner LO, Warner EJ. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999;90(1):66-71. https://doi.org/10.1097/00000542-199901000-00011
11. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90(3):896-905. https://doi.org/10.1097/00000542-199903000-00034
12. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376-393. https://doi.org/10.1097/aln.0000000000001452
13. Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344-1350. https://doi.org/10.1097/00000539-200111000-00063
14. Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32(1):34-44. https://doi.org/10.1016/j.clnu.2012.10.011
15. Kaška M, Grosmanová T, Havel E, et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery–a randomized controlled trial. Wien Klin Wochenschr. 2010;122(1-2):23-30. https://doi.org/10.1007/s00508-009-1291-7
16. Tokumine J, Sugahara K, Fuchigami T, Teruya K, Nitta K, Satou K. Unanticipated full stomach at anesthesia induction in a type I diabetic patient with asymptomatic gastroparesis. J Anesth. 2005;19(3):247-248. https://doi.org/10.1007/s00540-005-0321-5
1. Sorita A, Thongprayoon C, Ahmed A, et al. Frequency and appropriateness of fasting orders in the hospital. Mayo Clin Proc. 2015;90(9):1225-1232. https://doi.org/10.1016/j.mayocp.2015.07.013
2. Falconer R, Skouras C, Carter T, Greenway L, Paisley AM. Preoperative fasting: current practice and areas for improvement. Updates Surg. 2014;66(1):31-39. https://doi.org/10.1007/s13304-013-0242-z
3. Sorita A, Thongprayoon C, Ratelle JT, et al. Characteristics and outcomes of fasting orders among medical inpatients. J Hosp Med. 2017;12(1):36-39. https://doi.org/10.1002/jhm.2674
4. Maltby JR. Fasting from midnight–the history behind the dogma. Best Pract Res Clin Anaesthesiol. 2006;20(3):363-378. https://doi.org/10.1016/j.bpa.2006.02.001
5. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205. https://doi.org/10.1016/s0002-9378(16)39829-5
6. Shiraishi T, Kurosaki D, Nakamura M, et al. Gastric fluid volume change after oral rehydration solution intake in morbidly obese and normal controls: a magnetic resonance imaging-based analysis. Anesth Analg. 2017;124(4):1174-1178. https://doi.org/10.1213/ane.0000000000001886
7. Vasavid P, Chaiwatanarat T, Pusuwan P, et al. Normal solid gastric emptying values measured by scintigraphy using Asian-style meal: a multicenter study in healthy volunteers. J Neurogastroenterol Motil. 2014;20(3):371-378. https://doi.org/10.5056/jnm13114
8. Mahajan V, Hashmi J, Singh R, Samra T, Aneja S. Comparative evaluation of gastric pH and volume in morbidly obese and lean patients undergoing elective surgery and effect of aspiration prophylaxis. J Clin Anesth. 2015;27(5):396-400. https://doi.org/10.1016/j.jclinane.2015.03.004
9. Brady MC, Kinn S, Stuart P, Ness V. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423. https://doi.org/10.1002/14651858.cd004423
10. Warner MA, Warner ME, Warner DO, Warner LO, Warner EJ. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999;90(1):66-71. https://doi.org/10.1097/00000542-199901000-00011
11. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90(3):896-905. https://doi.org/10.1097/00000542-199903000-00034
12. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376-393. https://doi.org/10.1097/aln.0000000000001452
13. Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344-1350. https://doi.org/10.1097/00000539-200111000-00063
14. Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32(1):34-44. https://doi.org/10.1016/j.clnu.2012.10.011
15. Kaška M, Grosmanová T, Havel E, et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery–a randomized controlled trial. Wien Klin Wochenschr. 2010;122(1-2):23-30. https://doi.org/10.1007/s00508-009-1291-7
16. Tokumine J, Sugahara K, Fuchigami T, Teruya K, Nitta K, Satou K. Unanticipated full stomach at anesthesia induction in a type I diabetic patient with asymptomatic gastroparesis. J Anesth. 2005;19(3):247-248. https://doi.org/10.1007/s00540-005-0321-5
© 2021 Society of Hospital Medicine
A Painful Coincidence?
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.
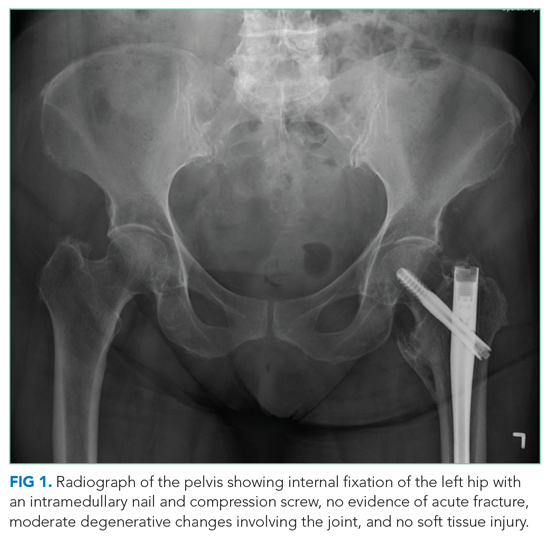
The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170
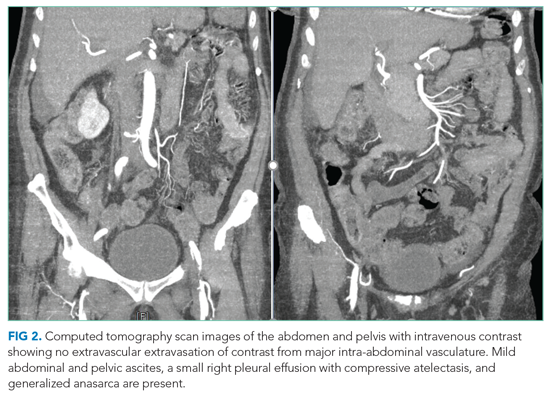
The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.
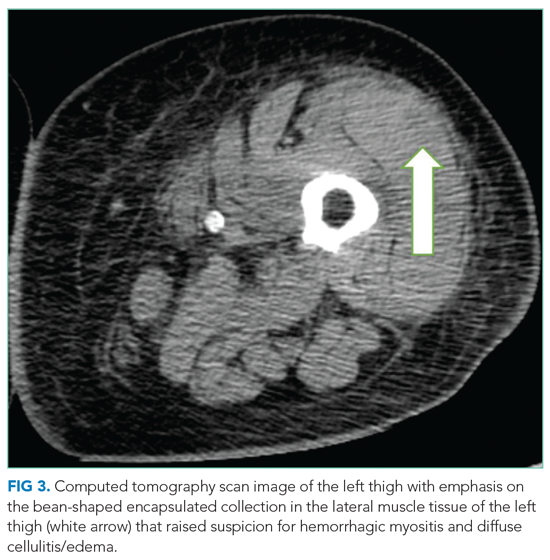
Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.
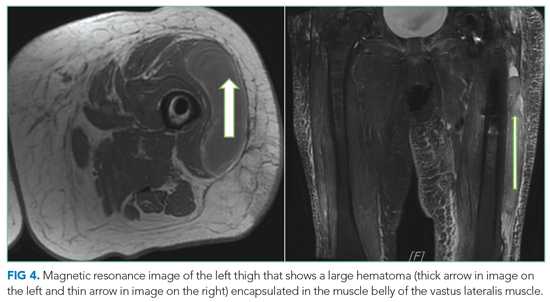
DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
© 2021 Society of Hospital Medicine
End the Routine Shackling of Incarcerated Inpatients
The police shooting of Jacob Blake, an unarmed Wisconsin man, during an arrest in August 2020, led to more protests in a summer filled with calls against the unequal application of police force. Outrage grew as it was revealed that Blake, paralyzed from his waist down and not yet convicted of a crime, was still handcuffed to his hospital bed while receiving treatment.1 To many this seemed unusually cruel, but to those tasked with caring for incarcerated patients, it is all too familiar. Given the high rates of incarceration in the United States and the increased medical needs of this population, caring for those in custody is unavoidable for many physicians and hospitals. Though safety should be paramount, the universal application of metal handcuffs or leg cuffs by law enforcement officials, a process known as shackling, can lead to a variety of harms and should be abandoned.
BACKGROUND
The United States incarcerates more individuals both in total numbers and per capita than any other country in the world. This is currently believed to be more than two million people on any given day or more than 650 persons per 100,000 population.2 Incarceration occurs in jails, which are locally run facilities holding individuals on short sentences or those not yet convicted who are unable to afford bail before their trials (pretrial), or prisons, which are state and federally run facilities that house those with long sentences. When an incarcerated person experiences a medical emergency requiring hospitalization, they are either treated in the correctional facility or transferred to a local hospital for a higher level of care. Some hospitals are equipped with security measures similar to those of a correctional facility, with secure floors or wings dedicated solely to the care of the incarcerated. Secure units are more commonly seen in hospitals associated with prisons rather than local jails. Other hospitals house incarcerated patients in the same rooms as the public population, and thus movement is restricted by other means.3 Most commonly, this is done with a hard metal shackle resembling a handcuff with one end attached to the leg or wrist and the other end attached to the bed. Some agencies require more restraints, often requiring the use of wrist cuffs and leg cuffs concurrently for the entire duration of a patient’s hospitalization.4 In our experience, agencies apply these restraints universally, regardless of age, illness, mobility, or pretrial status.
Restraint practices are rooted in a concern for practitioner and public safety and bear merit. A patient from a correctional facility is usually guarded by just one officer in lieu of the multiple security measures at a jail or prison facility. Nonsecured hospitals have become sites of multiple escapes by incarcerated inpatients, given the lack of secured doors and the multiple movements during the admission and discharge processes.5 Furthermore, violence against hospital staff is now a focus issue in many hospitals and is no longer accepted as just “part of the job.” In several high-profile incidents, incarcerated inpatients have harmed staff, including one at our own institution, when an incarcerated patient held a makeshift weapon to a student’s throat.6
LEGAL CHALLENGES
The use of shackles during hospital visits has been challenged in US courts and routinely upheld. In one case, an incarcerated patient with renal failure received injuries after his leg edema was so severe that “at one point the shackles themselves were barely visible.”7 Though he was injured, the shackles were determined to have served a penological purpose outside of punishment, such as preventing escape, and the injuries were the result of the patient’s guards not following protocol. British courts have taken a different stance, ruling for an incarcerated patient who challenged the use of cuffs during three outpatient appointments and one inpatient admission.8 While the cuffs in the outpatient setting were deemed acceptable (as they were removed during the medical visit itself), they remained during the duration of the inpatient stay. This was deemed in violation of Title I/Article 3 of the Charter of Fundamental Rights of the European Union, Dignity/The right to integrity of the person. One area in US healthcare where shackling has been roundly condemned is the peripartum shackling of pregnant women. Though courts have had a mixed record to challenges, activism and advocacy have led to the banning of the practice in 23 states, though in most states significant exemptions exist.9 Through the First Step Act of 2018, the federal government banned peripartum shackling for all federal prisoners, but as most incarcerations are under state or local control, a considerable number of incarcerated pregnant women can legally be shackled during their deliveries.
RISKS OF SHACKLING
Legal and safety concerns aside, the shackling of incarcerated patients carries enormous risk. The use of medical restraints in hospitals has decreased over the past few decades, given their proven harms in increasing falls, exacerbating delirium, and increasing the risk of in-hospital death.10 There is no reason to believe that trading a soft medical restraint for a metal leg or wrist cuff would not confer the same risk. Additionally, metal law enforcement cuffs are not designed with patient safety in mind and have been known to cause specific nerve injuries, or handcuff neuropathy. This can occur when placement is too tight or when a patient struggles against them, as could happen with an agitated or delirious patient. The bar for removal, even briefly for an exam, is also much higher than that of a medical restraint, leading to a greater likelihood that certain aspects of the physical exam, such as gait or strength assessment, may not be adequately performed. In one small survey, British physicians reported often performing an exam while the patient was cuffed and with a guard in the room, despite country guidelines against both practices.11
Additionally, marginalized communities are disproportionately incarcerated and have a fraught and tenuous relationship with the healthcare system. Black patients routinely report greater mistrust than White patients in the outcomes of care and the motivations of physicians, in large part due to past and current discrimination and the medical community’s history of experimentation.12 A shackled patient may view a treating physician and hospital as complicit with the practice, rather than seeing the practice as something outside of their control. If a patient’s sole interaction with inpatient medicine involves shackling, it risks damaging whatever fragile physician-patient relationship may exist and could delay or limit care even further.
While the universal application of metal handcuffs or leg cuffs ensures low rates of escape or attacks on workers, it does so at the expense of vulnerable individuals. We have cared for an incarcerated elderly woman arrested for multiple traffic violations, a man with severe autism who slipped through the cracks of mental health diversion protocols and ended up in jail, and an arrested delirious man with severe alcohol withdrawal, all shackled with hard shackles on the wrists, legs, or in the final case, both. Safety and the rights of the vulnerable are not mutually exclusive, and we feel the following measures can protect both.
A WAY FORWARD
First, the universal application of shackles in the hospitalized incarcerated patient should end. If no alternative security measures are available for high-risk patients, correctional facilities must document their necessity as physicians and nurses are required to do for medical restraints. Hospitals should have processes in place for providers who feel unsafe with an unshackled patient or think a patient is unnecessarily shackled, and collegial discussions about shackling with law enforcement should be the norm. If safe to do so, shackles should routinely be removed for physical exams without question. Since law enforcement officials, rather than the hospitals, make the rules for shackling, this will take some degree of physician and administrative advocacy at the hospital level and legislative advocacy at the local and state levels.
Second, vulnerable populations, such as the elderly, those experiencing a mental health crisis, or others at risk for in-hospital delirium, should never be restrained with hard law enforcement cuffs. Restraint procedures should follow standard medical restraint procedures, and soft restraints should be used if at all possible. Given the high rates of psychiatric illness amongst the incarcerated and the role jails play in filling gaps in psychiatric care, medical admissions for those with mental illness are not rare occasions.
Finally, hospitals routinely taking care of an incarcerated population should seek to build secure units, a move that would dramatically reduce the need for shackling. In several cities, the primary referral hospitals for some of the largest jails in the country do not have units with the proper security to allow for freedom of movement, and thus, shackling persists. Creating secure units will take significant investment on the part of hospital and local authorities, but there is potential for decreasing costs due to consolidating supervision, which would lead to better patient outcomes given the above risks.
Advocating for the health of the incarcerated, even those who have not yet been convicted, is typically not a high priority for the general public. As inpatient physicians, we see the impact universal shackling has on some of our most vulnerable patients and should be their voice where they have none. Advocating for and implementing the above procedures will be a step toward improving patient care while maintaining safety.
1. Proctor C. Jacob Blake handcuffed to hospital bed, father says. Chicago Sun-Times. Updated August 27, 2020. Accessed December 29, 2020. chicago.suntimes.com/2020/8/27/21404463/jacob-blake-father-kenosha-police-shooting-hospital-bed-handcuffs
2. Maruschak LM, Minton TD. Correctional populations in the United States, 2017-2018. Bureau of Justice Statistics. August 2020. Accessed September 30, 2020. https://www.bjs.gov/content/pub/pdf/cpus1718.pdf
3. Huh K, Boucher A, Fehr S, McGaffey F, McKillop M, Schiff M. State prisons and the delivery of hospital care: how states set up and finance off-site care for incarcerated individuals. The Pew Charitable Trusts. July 2018. Accessed September 30, 2020. https://www.pewtrusts.org/-/media/assets/2018/07/prisons-and-hospital-care_report.pdf
4. Haber LA, Erickson HP, Ranji SR, Ortiz GM, Pratt LA. Acute care for patients who are incarcerated: a review. JAMA Intern Med. 2019;179(11):1561-1567. https://doi.org/10.1001/jamainternmed.2019.3881
5. Mikow-Porto VA, Smith TA. The IHSSF 2011 Prisoner Escape Study. J Healthc Prot Manage. 2011;27(2):38-58.
6. Lezon D, Blakinger K. Inmate shot by deputy after holding medical student at Ben Taub. Houston Chronicle. October 6, 2016. Accessed December 29, 2020. https://www.chron.com/news/houston-texas/article/Deputy-shoots-suspect-at-Ben-Taub-hopsital-9873972.php
7. Yearwood LT. Pregnant and shackled: why inmates are still giving birth cuffed and bound. The Guardian. January 24, 2020. Accessed December 29, 2020. theguardian.com/us-news/2020/jan/24/shackled-pregnant-women-prisoners-birth
8. Haslar v Megerman, 104 F.3d 178 (8th Cir. 1997).
9. FGP v Serco Plc and SSHD, EWHC 1804 (Admin) (2012).
10. Cleary K, Prescott K. The use of physical restraints in acute and long-term care: an updated review of the evidence, regulations, ethics, and legality. J Acute Care Phys Ther. 2015;6(1):8-15. https://doi.org/10.1097/JAT.0000000000000005
11. Tuite H, Browne K, O’Neill D. Prisoners in general hospitals: doctors’ attitudes and practice. BMJ. 2006;332(7540):548-549. https://doi.org/10.1136/bmj.332.7540.548-b
12. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146-161. https://doi.org/10.1177/1077558700057001S07
The police shooting of Jacob Blake, an unarmed Wisconsin man, during an arrest in August 2020, led to more protests in a summer filled with calls against the unequal application of police force. Outrage grew as it was revealed that Blake, paralyzed from his waist down and not yet convicted of a crime, was still handcuffed to his hospital bed while receiving treatment.1 To many this seemed unusually cruel, but to those tasked with caring for incarcerated patients, it is all too familiar. Given the high rates of incarceration in the United States and the increased medical needs of this population, caring for those in custody is unavoidable for many physicians and hospitals. Though safety should be paramount, the universal application of metal handcuffs or leg cuffs by law enforcement officials, a process known as shackling, can lead to a variety of harms and should be abandoned.
BACKGROUND
The United States incarcerates more individuals both in total numbers and per capita than any other country in the world. This is currently believed to be more than two million people on any given day or more than 650 persons per 100,000 population.2 Incarceration occurs in jails, which are locally run facilities holding individuals on short sentences or those not yet convicted who are unable to afford bail before their trials (pretrial), or prisons, which are state and federally run facilities that house those with long sentences. When an incarcerated person experiences a medical emergency requiring hospitalization, they are either treated in the correctional facility or transferred to a local hospital for a higher level of care. Some hospitals are equipped with security measures similar to those of a correctional facility, with secure floors or wings dedicated solely to the care of the incarcerated. Secure units are more commonly seen in hospitals associated with prisons rather than local jails. Other hospitals house incarcerated patients in the same rooms as the public population, and thus movement is restricted by other means.3 Most commonly, this is done with a hard metal shackle resembling a handcuff with one end attached to the leg or wrist and the other end attached to the bed. Some agencies require more restraints, often requiring the use of wrist cuffs and leg cuffs concurrently for the entire duration of a patient’s hospitalization.4 In our experience, agencies apply these restraints universally, regardless of age, illness, mobility, or pretrial status.
Restraint practices are rooted in a concern for practitioner and public safety and bear merit. A patient from a correctional facility is usually guarded by just one officer in lieu of the multiple security measures at a jail or prison facility. Nonsecured hospitals have become sites of multiple escapes by incarcerated inpatients, given the lack of secured doors and the multiple movements during the admission and discharge processes.5 Furthermore, violence against hospital staff is now a focus issue in many hospitals and is no longer accepted as just “part of the job.” In several high-profile incidents, incarcerated inpatients have harmed staff, including one at our own institution, when an incarcerated patient held a makeshift weapon to a student’s throat.6
LEGAL CHALLENGES
The use of shackles during hospital visits has been challenged in US courts and routinely upheld. In one case, an incarcerated patient with renal failure received injuries after his leg edema was so severe that “at one point the shackles themselves were barely visible.”7 Though he was injured, the shackles were determined to have served a penological purpose outside of punishment, such as preventing escape, and the injuries were the result of the patient’s guards not following protocol. British courts have taken a different stance, ruling for an incarcerated patient who challenged the use of cuffs during three outpatient appointments and one inpatient admission.8 While the cuffs in the outpatient setting were deemed acceptable (as they were removed during the medical visit itself), they remained during the duration of the inpatient stay. This was deemed in violation of Title I/Article 3 of the Charter of Fundamental Rights of the European Union, Dignity/The right to integrity of the person. One area in US healthcare where shackling has been roundly condemned is the peripartum shackling of pregnant women. Though courts have had a mixed record to challenges, activism and advocacy have led to the banning of the practice in 23 states, though in most states significant exemptions exist.9 Through the First Step Act of 2018, the federal government banned peripartum shackling for all federal prisoners, but as most incarcerations are under state or local control, a considerable number of incarcerated pregnant women can legally be shackled during their deliveries.
RISKS OF SHACKLING
Legal and safety concerns aside, the shackling of incarcerated patients carries enormous risk. The use of medical restraints in hospitals has decreased over the past few decades, given their proven harms in increasing falls, exacerbating delirium, and increasing the risk of in-hospital death.10 There is no reason to believe that trading a soft medical restraint for a metal leg or wrist cuff would not confer the same risk. Additionally, metal law enforcement cuffs are not designed with patient safety in mind and have been known to cause specific nerve injuries, or handcuff neuropathy. This can occur when placement is too tight or when a patient struggles against them, as could happen with an agitated or delirious patient. The bar for removal, even briefly for an exam, is also much higher than that of a medical restraint, leading to a greater likelihood that certain aspects of the physical exam, such as gait or strength assessment, may not be adequately performed. In one small survey, British physicians reported often performing an exam while the patient was cuffed and with a guard in the room, despite country guidelines against both practices.11
Additionally, marginalized communities are disproportionately incarcerated and have a fraught and tenuous relationship with the healthcare system. Black patients routinely report greater mistrust than White patients in the outcomes of care and the motivations of physicians, in large part due to past and current discrimination and the medical community’s history of experimentation.12 A shackled patient may view a treating physician and hospital as complicit with the practice, rather than seeing the practice as something outside of their control. If a patient’s sole interaction with inpatient medicine involves shackling, it risks damaging whatever fragile physician-patient relationship may exist and could delay or limit care even further.
While the universal application of metal handcuffs or leg cuffs ensures low rates of escape or attacks on workers, it does so at the expense of vulnerable individuals. We have cared for an incarcerated elderly woman arrested for multiple traffic violations, a man with severe autism who slipped through the cracks of mental health diversion protocols and ended up in jail, and an arrested delirious man with severe alcohol withdrawal, all shackled with hard shackles on the wrists, legs, or in the final case, both. Safety and the rights of the vulnerable are not mutually exclusive, and we feel the following measures can protect both.
A WAY FORWARD
First, the universal application of shackles in the hospitalized incarcerated patient should end. If no alternative security measures are available for high-risk patients, correctional facilities must document their necessity as physicians and nurses are required to do for medical restraints. Hospitals should have processes in place for providers who feel unsafe with an unshackled patient or think a patient is unnecessarily shackled, and collegial discussions about shackling with law enforcement should be the norm. If safe to do so, shackles should routinely be removed for physical exams without question. Since law enforcement officials, rather than the hospitals, make the rules for shackling, this will take some degree of physician and administrative advocacy at the hospital level and legislative advocacy at the local and state levels.
Second, vulnerable populations, such as the elderly, those experiencing a mental health crisis, or others at risk for in-hospital delirium, should never be restrained with hard law enforcement cuffs. Restraint procedures should follow standard medical restraint procedures, and soft restraints should be used if at all possible. Given the high rates of psychiatric illness amongst the incarcerated and the role jails play in filling gaps in psychiatric care, medical admissions for those with mental illness are not rare occasions.
Finally, hospitals routinely taking care of an incarcerated population should seek to build secure units, a move that would dramatically reduce the need for shackling. In several cities, the primary referral hospitals for some of the largest jails in the country do not have units with the proper security to allow for freedom of movement, and thus, shackling persists. Creating secure units will take significant investment on the part of hospital and local authorities, but there is potential for decreasing costs due to consolidating supervision, which would lead to better patient outcomes given the above risks.
Advocating for the health of the incarcerated, even those who have not yet been convicted, is typically not a high priority for the general public. As inpatient physicians, we see the impact universal shackling has on some of our most vulnerable patients and should be their voice where they have none. Advocating for and implementing the above procedures will be a step toward improving patient care while maintaining safety.
The police shooting of Jacob Blake, an unarmed Wisconsin man, during an arrest in August 2020, led to more protests in a summer filled with calls against the unequal application of police force. Outrage grew as it was revealed that Blake, paralyzed from his waist down and not yet convicted of a crime, was still handcuffed to his hospital bed while receiving treatment.1 To many this seemed unusually cruel, but to those tasked with caring for incarcerated patients, it is all too familiar. Given the high rates of incarceration in the United States and the increased medical needs of this population, caring for those in custody is unavoidable for many physicians and hospitals. Though safety should be paramount, the universal application of metal handcuffs or leg cuffs by law enforcement officials, a process known as shackling, can lead to a variety of harms and should be abandoned.
BACKGROUND
The United States incarcerates more individuals both in total numbers and per capita than any other country in the world. This is currently believed to be more than two million people on any given day or more than 650 persons per 100,000 population.2 Incarceration occurs in jails, which are locally run facilities holding individuals on short sentences or those not yet convicted who are unable to afford bail before their trials (pretrial), or prisons, which are state and federally run facilities that house those with long sentences. When an incarcerated person experiences a medical emergency requiring hospitalization, they are either treated in the correctional facility or transferred to a local hospital for a higher level of care. Some hospitals are equipped with security measures similar to those of a correctional facility, with secure floors or wings dedicated solely to the care of the incarcerated. Secure units are more commonly seen in hospitals associated with prisons rather than local jails. Other hospitals house incarcerated patients in the same rooms as the public population, and thus movement is restricted by other means.3 Most commonly, this is done with a hard metal shackle resembling a handcuff with one end attached to the leg or wrist and the other end attached to the bed. Some agencies require more restraints, often requiring the use of wrist cuffs and leg cuffs concurrently for the entire duration of a patient’s hospitalization.4 In our experience, agencies apply these restraints universally, regardless of age, illness, mobility, or pretrial status.
Restraint practices are rooted in a concern for practitioner and public safety and bear merit. A patient from a correctional facility is usually guarded by just one officer in lieu of the multiple security measures at a jail or prison facility. Nonsecured hospitals have become sites of multiple escapes by incarcerated inpatients, given the lack of secured doors and the multiple movements during the admission and discharge processes.5 Furthermore, violence against hospital staff is now a focus issue in many hospitals and is no longer accepted as just “part of the job.” In several high-profile incidents, incarcerated inpatients have harmed staff, including one at our own institution, when an incarcerated patient held a makeshift weapon to a student’s throat.6
LEGAL CHALLENGES
The use of shackles during hospital visits has been challenged in US courts and routinely upheld. In one case, an incarcerated patient with renal failure received injuries after his leg edema was so severe that “at one point the shackles themselves were barely visible.”7 Though he was injured, the shackles were determined to have served a penological purpose outside of punishment, such as preventing escape, and the injuries were the result of the patient’s guards not following protocol. British courts have taken a different stance, ruling for an incarcerated patient who challenged the use of cuffs during three outpatient appointments and one inpatient admission.8 While the cuffs in the outpatient setting were deemed acceptable (as they were removed during the medical visit itself), they remained during the duration of the inpatient stay. This was deemed in violation of Title I/Article 3 of the Charter of Fundamental Rights of the European Union, Dignity/The right to integrity of the person. One area in US healthcare where shackling has been roundly condemned is the peripartum shackling of pregnant women. Though courts have had a mixed record to challenges, activism and advocacy have led to the banning of the practice in 23 states, though in most states significant exemptions exist.9 Through the First Step Act of 2018, the federal government banned peripartum shackling for all federal prisoners, but as most incarcerations are under state or local control, a considerable number of incarcerated pregnant women can legally be shackled during their deliveries.
RISKS OF SHACKLING
Legal and safety concerns aside, the shackling of incarcerated patients carries enormous risk. The use of medical restraints in hospitals has decreased over the past few decades, given their proven harms in increasing falls, exacerbating delirium, and increasing the risk of in-hospital death.10 There is no reason to believe that trading a soft medical restraint for a metal leg or wrist cuff would not confer the same risk. Additionally, metal law enforcement cuffs are not designed with patient safety in mind and have been known to cause specific nerve injuries, or handcuff neuropathy. This can occur when placement is too tight or when a patient struggles against them, as could happen with an agitated or delirious patient. The bar for removal, even briefly for an exam, is also much higher than that of a medical restraint, leading to a greater likelihood that certain aspects of the physical exam, such as gait or strength assessment, may not be adequately performed. In one small survey, British physicians reported often performing an exam while the patient was cuffed and with a guard in the room, despite country guidelines against both practices.11
Additionally, marginalized communities are disproportionately incarcerated and have a fraught and tenuous relationship with the healthcare system. Black patients routinely report greater mistrust than White patients in the outcomes of care and the motivations of physicians, in large part due to past and current discrimination and the medical community’s history of experimentation.12 A shackled patient may view a treating physician and hospital as complicit with the practice, rather than seeing the practice as something outside of their control. If a patient’s sole interaction with inpatient medicine involves shackling, it risks damaging whatever fragile physician-patient relationship may exist and could delay or limit care even further.
While the universal application of metal handcuffs or leg cuffs ensures low rates of escape or attacks on workers, it does so at the expense of vulnerable individuals. We have cared for an incarcerated elderly woman arrested for multiple traffic violations, a man with severe autism who slipped through the cracks of mental health diversion protocols and ended up in jail, and an arrested delirious man with severe alcohol withdrawal, all shackled with hard shackles on the wrists, legs, or in the final case, both. Safety and the rights of the vulnerable are not mutually exclusive, and we feel the following measures can protect both.
A WAY FORWARD
First, the universal application of shackles in the hospitalized incarcerated patient should end. If no alternative security measures are available for high-risk patients, correctional facilities must document their necessity as physicians and nurses are required to do for medical restraints. Hospitals should have processes in place for providers who feel unsafe with an unshackled patient or think a patient is unnecessarily shackled, and collegial discussions about shackling with law enforcement should be the norm. If safe to do so, shackles should routinely be removed for physical exams without question. Since law enforcement officials, rather than the hospitals, make the rules for shackling, this will take some degree of physician and administrative advocacy at the hospital level and legislative advocacy at the local and state levels.
Second, vulnerable populations, such as the elderly, those experiencing a mental health crisis, or others at risk for in-hospital delirium, should never be restrained with hard law enforcement cuffs. Restraint procedures should follow standard medical restraint procedures, and soft restraints should be used if at all possible. Given the high rates of psychiatric illness amongst the incarcerated and the role jails play in filling gaps in psychiatric care, medical admissions for those with mental illness are not rare occasions.
Finally, hospitals routinely taking care of an incarcerated population should seek to build secure units, a move that would dramatically reduce the need for shackling. In several cities, the primary referral hospitals for some of the largest jails in the country do not have units with the proper security to allow for freedom of movement, and thus, shackling persists. Creating secure units will take significant investment on the part of hospital and local authorities, but there is potential for decreasing costs due to consolidating supervision, which would lead to better patient outcomes given the above risks.
Advocating for the health of the incarcerated, even those who have not yet been convicted, is typically not a high priority for the general public. As inpatient physicians, we see the impact universal shackling has on some of our most vulnerable patients and should be their voice where they have none. Advocating for and implementing the above procedures will be a step toward improving patient care while maintaining safety.
1. Proctor C. Jacob Blake handcuffed to hospital bed, father says. Chicago Sun-Times. Updated August 27, 2020. Accessed December 29, 2020. chicago.suntimes.com/2020/8/27/21404463/jacob-blake-father-kenosha-police-shooting-hospital-bed-handcuffs
2. Maruschak LM, Minton TD. Correctional populations in the United States, 2017-2018. Bureau of Justice Statistics. August 2020. Accessed September 30, 2020. https://www.bjs.gov/content/pub/pdf/cpus1718.pdf
3. Huh K, Boucher A, Fehr S, McGaffey F, McKillop M, Schiff M. State prisons and the delivery of hospital care: how states set up and finance off-site care for incarcerated individuals. The Pew Charitable Trusts. July 2018. Accessed September 30, 2020. https://www.pewtrusts.org/-/media/assets/2018/07/prisons-and-hospital-care_report.pdf
4. Haber LA, Erickson HP, Ranji SR, Ortiz GM, Pratt LA. Acute care for patients who are incarcerated: a review. JAMA Intern Med. 2019;179(11):1561-1567. https://doi.org/10.1001/jamainternmed.2019.3881
5. Mikow-Porto VA, Smith TA. The IHSSF 2011 Prisoner Escape Study. J Healthc Prot Manage. 2011;27(2):38-58.
6. Lezon D, Blakinger K. Inmate shot by deputy after holding medical student at Ben Taub. Houston Chronicle. October 6, 2016. Accessed December 29, 2020. https://www.chron.com/news/houston-texas/article/Deputy-shoots-suspect-at-Ben-Taub-hopsital-9873972.php
7. Yearwood LT. Pregnant and shackled: why inmates are still giving birth cuffed and bound. The Guardian. January 24, 2020. Accessed December 29, 2020. theguardian.com/us-news/2020/jan/24/shackled-pregnant-women-prisoners-birth
8. Haslar v Megerman, 104 F.3d 178 (8th Cir. 1997).
9. FGP v Serco Plc and SSHD, EWHC 1804 (Admin) (2012).
10. Cleary K, Prescott K. The use of physical restraints in acute and long-term care: an updated review of the evidence, regulations, ethics, and legality. J Acute Care Phys Ther. 2015;6(1):8-15. https://doi.org/10.1097/JAT.0000000000000005
11. Tuite H, Browne K, O’Neill D. Prisoners in general hospitals: doctors’ attitudes and practice. BMJ. 2006;332(7540):548-549. https://doi.org/10.1136/bmj.332.7540.548-b
12. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146-161. https://doi.org/10.1177/1077558700057001S07
1. Proctor C. Jacob Blake handcuffed to hospital bed, father says. Chicago Sun-Times. Updated August 27, 2020. Accessed December 29, 2020. chicago.suntimes.com/2020/8/27/21404463/jacob-blake-father-kenosha-police-shooting-hospital-bed-handcuffs
2. Maruschak LM, Minton TD. Correctional populations in the United States, 2017-2018. Bureau of Justice Statistics. August 2020. Accessed September 30, 2020. https://www.bjs.gov/content/pub/pdf/cpus1718.pdf
3. Huh K, Boucher A, Fehr S, McGaffey F, McKillop M, Schiff M. State prisons and the delivery of hospital care: how states set up and finance off-site care for incarcerated individuals. The Pew Charitable Trusts. July 2018. Accessed September 30, 2020. https://www.pewtrusts.org/-/media/assets/2018/07/prisons-and-hospital-care_report.pdf
4. Haber LA, Erickson HP, Ranji SR, Ortiz GM, Pratt LA. Acute care for patients who are incarcerated: a review. JAMA Intern Med. 2019;179(11):1561-1567. https://doi.org/10.1001/jamainternmed.2019.3881
5. Mikow-Porto VA, Smith TA. The IHSSF 2011 Prisoner Escape Study. J Healthc Prot Manage. 2011;27(2):38-58.
6. Lezon D, Blakinger K. Inmate shot by deputy after holding medical student at Ben Taub. Houston Chronicle. October 6, 2016. Accessed December 29, 2020. https://www.chron.com/news/houston-texas/article/Deputy-shoots-suspect-at-Ben-Taub-hopsital-9873972.php
7. Yearwood LT. Pregnant and shackled: why inmates are still giving birth cuffed and bound. The Guardian. January 24, 2020. Accessed December 29, 2020. theguardian.com/us-news/2020/jan/24/shackled-pregnant-women-prisoners-birth
8. Haslar v Megerman, 104 F.3d 178 (8th Cir. 1997).
9. FGP v Serco Plc and SSHD, EWHC 1804 (Admin) (2012).
10. Cleary K, Prescott K. The use of physical restraints in acute and long-term care: an updated review of the evidence, regulations, ethics, and legality. J Acute Care Phys Ther. 2015;6(1):8-15. https://doi.org/10.1097/JAT.0000000000000005
11. Tuite H, Browne K, O’Neill D. Prisoners in general hospitals: doctors’ attitudes and practice. BMJ. 2006;332(7540):548-549. https://doi.org/10.1136/bmj.332.7540.548-b
12. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146-161. https://doi.org/10.1177/1077558700057001S07
© 2021 Society of Hospital Medicine
Albuterol, Acidosis, and Aneurysms
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
Risk Factors and Antipsychotic Usage Patterns Associated With Terminal Delirium in a Veteran Long-Term Care Hospice Population
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
Photographic Confirmation of Biopsy Sites Saves Lives
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
Predictors of COVID-19 Seropositivity Among Healthcare Workers: An Important Piece of an Incomplete Puzzle
SARS-CoV-2 seroprevalence studies of healthcare workers (HCWs) provide valuable insights into the excess risk of infection in this population and indirect evidence supporting the value of personal protective equipment (PPE) use. Seroprevalence estimates are composite measures of exposure risk and transmission mitigation both in the healthcare and community environments. The challenge of interpreting these studies arises from the diversity of HCW vocational roles and work settings in juxtaposition to heterogeneous community exposure risks. In this issue, two studies untangle some of these competing factors.
Investigators from Kashmir, India, assessed the relationship between seropositivity and specific HCW roles and work sites.1 They found a lower seroprevalence among HCWs at hospitals dedicated to COVID patients, relative to non-COVID hospitals. This seemingly paradoxical finding likely results from a combination of vigilant PPE adherence enforced through a buddy system, restrictive visitation policies, HCW residential dormitories reducing community exposure, and a spillover effect of careful in-hospital exposure avoidance practices on out-of-hospital behavior. A similar spillover effect has been hypothesized for low HCW seroprevalence relative to the surrounding community in California.2
In complement, researchers at a large New York City (NYC) hospital found higher overall HCW seropositivity rates compared with the community, though estimates were strikingly variable after detailed stratification by job function and location.3 The gradient of seroprevalence showed the highest risk among nurses and those in nonclinical, low-wage jobs (eg, patient transport, housekeeping), a finding also seen in another US study prior to adjustment for demographic and community factors.4 This finding highlights the association between socioeconomic status, structural community exposure risk factors such as multiple essential workers living within multigenerational households, and the challenges of sickness absenteeism. High seroprevalence among nurses and emergency department HCWs (who expeditiously evaluate many undifferentiated patients) may reflect both greater aggregate duration of exposure to infected patients and increased frequency of PPE donning and doffing, resulting in fatigue and diminished vigilance.5
A NYC-based study similarly showed high HCW seroprevalence, although no consistent associations with job function (albeit measured with less granularity) or community-based exposures were identified.6 Several studies comparing HCW to local community seropositivity rates have reached disparate conclusions.2,7 These contrasting data may result from variability in vigilance of PPE use, mask use in work rooms or during meals/breaktimes, sick leave policies driven by staffing demands, and neighborhood factors. In addition, selection biases and timing of blood sampling relative to viral transmission peaks (with differing degrees of temporal antibody waning) may contribute to the apparent discordance. In particular, comparative community-based samples vary greatly in their inclusion of asymptomatic patients, which can substantially affect such estimates by changing the denominator population.
We draw three conclusions: (1) Evidence for HCW exposure often tracks with community infection rates, suggesting that nonworkplace exposures are a dominant source of HCW seropositivity; (2) vigilant PPE use and assertively implemented protective measures unrelated to patient encounters can dramatically reduce infection risk, even among those with frequent exposures; and (3) HCW infection risk during future peaks can be effectively restrained with adequate resources and support, even in the presence of variants for which no effective vaccination or preventive pharmacotherapy exists. Given the divergent seroprevalence rates found in these studies after detailed stratification by job function and location, it is important for future studies to evaluate their relationship with infectious risk. Accurately quantifying the excess risks borne by HCWs may remain an elusive objective, but experiential knowledge offers numerous strategies worthy of proactive implementation to preserve HCW safety and well-being.
1. Khan M, Haq I, Qurieshi MA, et al. SARS-CoV-2 seroprevalence among healthcare workers by workplace exposure risk in Kashmir, India. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3609
2. Brant-Zawadzki M, Fridman D, Robinson PA, et al. Prevalence and longevity of SARS-CoV-2 antibodies among health care workers. Open Forum Infect Dis. 2021;8(2):ofab015. https://doi.org/10.1093/ofid/ofab015
3. Purswani MU, Bucciarelli J, Tiburcio J. SARS-CoV-2 seroprevalence among healthcare workers by job function and work location in a New York inner-city hospital. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3627
4. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):e211283. https://doi.org/10.1001/jamanetworkopen.2021.1283
5. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
6. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021(1);102:63-69. https://doi.org/10.1016/j.ijid.2020.10.0367. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120-134. https://doi.org/10.1016/j.jhin.2020.11.008
SARS-CoV-2 seroprevalence studies of healthcare workers (HCWs) provide valuable insights into the excess risk of infection in this population and indirect evidence supporting the value of personal protective equipment (PPE) use. Seroprevalence estimates are composite measures of exposure risk and transmission mitigation both in the healthcare and community environments. The challenge of interpreting these studies arises from the diversity of HCW vocational roles and work settings in juxtaposition to heterogeneous community exposure risks. In this issue, two studies untangle some of these competing factors.
Investigators from Kashmir, India, assessed the relationship between seropositivity and specific HCW roles and work sites.1 They found a lower seroprevalence among HCWs at hospitals dedicated to COVID patients, relative to non-COVID hospitals. This seemingly paradoxical finding likely results from a combination of vigilant PPE adherence enforced through a buddy system, restrictive visitation policies, HCW residential dormitories reducing community exposure, and a spillover effect of careful in-hospital exposure avoidance practices on out-of-hospital behavior. A similar spillover effect has been hypothesized for low HCW seroprevalence relative to the surrounding community in California.2
In complement, researchers at a large New York City (NYC) hospital found higher overall HCW seropositivity rates compared with the community, though estimates were strikingly variable after detailed stratification by job function and location.3 The gradient of seroprevalence showed the highest risk among nurses and those in nonclinical, low-wage jobs (eg, patient transport, housekeeping), a finding also seen in another US study prior to adjustment for demographic and community factors.4 This finding highlights the association between socioeconomic status, structural community exposure risk factors such as multiple essential workers living within multigenerational households, and the challenges of sickness absenteeism. High seroprevalence among nurses and emergency department HCWs (who expeditiously evaluate many undifferentiated patients) may reflect both greater aggregate duration of exposure to infected patients and increased frequency of PPE donning and doffing, resulting in fatigue and diminished vigilance.5
A NYC-based study similarly showed high HCW seroprevalence, although no consistent associations with job function (albeit measured with less granularity) or community-based exposures were identified.6 Several studies comparing HCW to local community seropositivity rates have reached disparate conclusions.2,7 These contrasting data may result from variability in vigilance of PPE use, mask use in work rooms or during meals/breaktimes, sick leave policies driven by staffing demands, and neighborhood factors. In addition, selection biases and timing of blood sampling relative to viral transmission peaks (with differing degrees of temporal antibody waning) may contribute to the apparent discordance. In particular, comparative community-based samples vary greatly in their inclusion of asymptomatic patients, which can substantially affect such estimates by changing the denominator population.
We draw three conclusions: (1) Evidence for HCW exposure often tracks with community infection rates, suggesting that nonworkplace exposures are a dominant source of HCW seropositivity; (2) vigilant PPE use and assertively implemented protective measures unrelated to patient encounters can dramatically reduce infection risk, even among those with frequent exposures; and (3) HCW infection risk during future peaks can be effectively restrained with adequate resources and support, even in the presence of variants for which no effective vaccination or preventive pharmacotherapy exists. Given the divergent seroprevalence rates found in these studies after detailed stratification by job function and location, it is important for future studies to evaluate their relationship with infectious risk. Accurately quantifying the excess risks borne by HCWs may remain an elusive objective, but experiential knowledge offers numerous strategies worthy of proactive implementation to preserve HCW safety and well-being.
SARS-CoV-2 seroprevalence studies of healthcare workers (HCWs) provide valuable insights into the excess risk of infection in this population and indirect evidence supporting the value of personal protective equipment (PPE) use. Seroprevalence estimates are composite measures of exposure risk and transmission mitigation both in the healthcare and community environments. The challenge of interpreting these studies arises from the diversity of HCW vocational roles and work settings in juxtaposition to heterogeneous community exposure risks. In this issue, two studies untangle some of these competing factors.
Investigators from Kashmir, India, assessed the relationship between seropositivity and specific HCW roles and work sites.1 They found a lower seroprevalence among HCWs at hospitals dedicated to COVID patients, relative to non-COVID hospitals. This seemingly paradoxical finding likely results from a combination of vigilant PPE adherence enforced through a buddy system, restrictive visitation policies, HCW residential dormitories reducing community exposure, and a spillover effect of careful in-hospital exposure avoidance practices on out-of-hospital behavior. A similar spillover effect has been hypothesized for low HCW seroprevalence relative to the surrounding community in California.2
In complement, researchers at a large New York City (NYC) hospital found higher overall HCW seropositivity rates compared with the community, though estimates were strikingly variable after detailed stratification by job function and location.3 The gradient of seroprevalence showed the highest risk among nurses and those in nonclinical, low-wage jobs (eg, patient transport, housekeeping), a finding also seen in another US study prior to adjustment for demographic and community factors.4 This finding highlights the association between socioeconomic status, structural community exposure risk factors such as multiple essential workers living within multigenerational households, and the challenges of sickness absenteeism. High seroprevalence among nurses and emergency department HCWs (who expeditiously evaluate many undifferentiated patients) may reflect both greater aggregate duration of exposure to infected patients and increased frequency of PPE donning and doffing, resulting in fatigue and diminished vigilance.5
A NYC-based study similarly showed high HCW seroprevalence, although no consistent associations with job function (albeit measured with less granularity) or community-based exposures were identified.6 Several studies comparing HCW to local community seropositivity rates have reached disparate conclusions.2,7 These contrasting data may result from variability in vigilance of PPE use, mask use in work rooms or during meals/breaktimes, sick leave policies driven by staffing demands, and neighborhood factors. In addition, selection biases and timing of blood sampling relative to viral transmission peaks (with differing degrees of temporal antibody waning) may contribute to the apparent discordance. In particular, comparative community-based samples vary greatly in their inclusion of asymptomatic patients, which can substantially affect such estimates by changing the denominator population.
We draw three conclusions: (1) Evidence for HCW exposure often tracks with community infection rates, suggesting that nonworkplace exposures are a dominant source of HCW seropositivity; (2) vigilant PPE use and assertively implemented protective measures unrelated to patient encounters can dramatically reduce infection risk, even among those with frequent exposures; and (3) HCW infection risk during future peaks can be effectively restrained with adequate resources and support, even in the presence of variants for which no effective vaccination or preventive pharmacotherapy exists. Given the divergent seroprevalence rates found in these studies after detailed stratification by job function and location, it is important for future studies to evaluate their relationship with infectious risk. Accurately quantifying the excess risks borne by HCWs may remain an elusive objective, but experiential knowledge offers numerous strategies worthy of proactive implementation to preserve HCW safety and well-being.
1. Khan M, Haq I, Qurieshi MA, et al. SARS-CoV-2 seroprevalence among healthcare workers by workplace exposure risk in Kashmir, India. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3609
2. Brant-Zawadzki M, Fridman D, Robinson PA, et al. Prevalence and longevity of SARS-CoV-2 antibodies among health care workers. Open Forum Infect Dis. 2021;8(2):ofab015. https://doi.org/10.1093/ofid/ofab015
3. Purswani MU, Bucciarelli J, Tiburcio J. SARS-CoV-2 seroprevalence among healthcare workers by job function and work location in a New York inner-city hospital. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3627
4. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):e211283. https://doi.org/10.1001/jamanetworkopen.2021.1283
5. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
6. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021(1);102:63-69. https://doi.org/10.1016/j.ijid.2020.10.0367. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120-134. https://doi.org/10.1016/j.jhin.2020.11.008
1. Khan M, Haq I, Qurieshi MA, et al. SARS-CoV-2 seroprevalence among healthcare workers by workplace exposure risk in Kashmir, India. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3609
2. Brant-Zawadzki M, Fridman D, Robinson PA, et al. Prevalence and longevity of SARS-CoV-2 antibodies among health care workers. Open Forum Infect Dis. 2021;8(2):ofab015. https://doi.org/10.1093/ofid/ofab015
3. Purswani MU, Bucciarelli J, Tiburcio J. SARS-CoV-2 seroprevalence among healthcare workers by job function and work location in a New York inner-city hospital. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3627
4. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):e211283. https://doi.org/10.1001/jamanetworkopen.2021.1283
5. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
6. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021(1);102:63-69. https://doi.org/10.1016/j.ijid.2020.10.0367. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120-134. https://doi.org/10.1016/j.jhin.2020.11.008
© 2021 Society of Hospital Medicine
Bronchiolitis: Less Is More, but Different Is Better
Bronchiolitis, the most common cause of hospital admission for infants, is responsible for more than $500 million in direct medical costs in the United States yearly. Recent efforts have focused on what can be safely avoided when caring for patients with bronchiolitis (eg, continuous pulse oximetry, bronchodilator administration). While there remains substantial room for improvement in avoiding such low-value (or no-value) practices, the incremental improvements from these de-escalations will reach an asymptote over time. Further improvements in care and value must occur by doing things differently—not just simply doing less.
In this month’s Journal of Hospital Medicine, Ohlsen et al1 describe an intervention to decrease length of stay (LOS) for patients with bronchiolitis They employed an interrupted time series analysis to evaluate implementation of an observation unit and home oxygen therapy (OU-HOT) model of care and found that LOS dramatically decreased immediately following implementation. This reduction was maintained over 9 years. Use of home oxygen decreased over the study period, while LOS remained low, suggesting that the most important intervention was a structural one—the admission of patients to a unit dedicated to efficient discharge.
Observation units, staffed 24/7 with attending physicians, are well adapted to care for patients with illnesses like bronchiolitis, where hospitalization, though often needed, may be brief.2 These units are designed more like an emergency department than an inpatient unit, with protocolized care and the expectation of rapid turnover.
Multiple studies have shown that physician-related delays are a primary driver of delayed discharge from inpatient units. Such delays include delayed or variable clinical decision-making, inadequate communication of discharge criteria, and waiting to staff patients with an attending physician.3-5 Addressing these issues could allow inpatient units to function more like observation units for specific diagnoses. Standardization of care around specific diagnoses can make decision-making and discharge more efficient. In 2014, White et al4 showed that standardizing discharge criteria for specific diagnoses (including bronchiolitis) and embedding these criteria in admission order sets resulted in a significant decrease in LOS without affecting readmission rates or patient satisfaction.
To address the issues of attending availability, we may need to rethink rounding. The daily structure of inpatient rounding has not meaningfully changed since the 1950s. While there has been a push for increased morning discharges, this approach misses many patients whose illness course is evolving and who may be ready for discharge in the afternoon or evening.6 The current structure of morning rounds on medical teams is based on the need for resident education, supervision, and time available for attendings to complete administrative tasks and teaching in the afternoons. Structural change in patient care requires academic institutions to rethink what “being on service” actually means. Since LOS in these cases is brief, multiple days of clinical continuity may not be as beneficial as with other diagnoses. Further, there is no reason that daytime rounding teams are the only teams that can discharge patients. Telemedicine could also offer an opportunity for attending physicians to remotely determine whether a patient is discharge appropriate. Standardization of discharge criteria at admission could allow for trainees to discharge patients when they meet those criteria.
Perhaps we should begin to adapt our work structure to our patients’ needs, rather than the other way around. In pediatrics, we have already made traditional rounding more patient-focused through the practice of family-centered rounding. We should identify, as the authors have, ways to do things differently to make further improvements in care.
Ultimately, the success of this OU-HOT protocol demonstrates the power of structural interventions aimed at changing how we do things rather than just doing more (or less) of the same.
1. Ohlsen T, Knudson A, Korgenski EK, et al. Nine seasons of a bronchiolitis observation unit and home oxygen therapy protocol. J Hosp Med. 2021;16(5):261-267.
2. Plamann JM, Zedreck-Gonzalez J, Fennimore L. Creation of an adult observation unit: improving outcomes. J Nurs Care Qual. 2018;33(1):72-78. https://doi.org/10.1097/NCQ.0000000000000267
3. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
5. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. https://doi.org/10.1002/jhm.490
6. Gordon SA, Garber D, Taufique Z, et al. Improving on-time discharge in otolaryngology admissions. Otolaryngol Head Neck Surg. 2020;163(2):188-193. https://doi.org/10.1177/0194599819898910
Bronchiolitis, the most common cause of hospital admission for infants, is responsible for more than $500 million in direct medical costs in the United States yearly. Recent efforts have focused on what can be safely avoided when caring for patients with bronchiolitis (eg, continuous pulse oximetry, bronchodilator administration). While there remains substantial room for improvement in avoiding such low-value (or no-value) practices, the incremental improvements from these de-escalations will reach an asymptote over time. Further improvements in care and value must occur by doing things differently—not just simply doing less.
In this month’s Journal of Hospital Medicine, Ohlsen et al1 describe an intervention to decrease length of stay (LOS) for patients with bronchiolitis They employed an interrupted time series analysis to evaluate implementation of an observation unit and home oxygen therapy (OU-HOT) model of care and found that LOS dramatically decreased immediately following implementation. This reduction was maintained over 9 years. Use of home oxygen decreased over the study period, while LOS remained low, suggesting that the most important intervention was a structural one—the admission of patients to a unit dedicated to efficient discharge.
Observation units, staffed 24/7 with attending physicians, are well adapted to care for patients with illnesses like bronchiolitis, where hospitalization, though often needed, may be brief.2 These units are designed more like an emergency department than an inpatient unit, with protocolized care and the expectation of rapid turnover.
Multiple studies have shown that physician-related delays are a primary driver of delayed discharge from inpatient units. Such delays include delayed or variable clinical decision-making, inadequate communication of discharge criteria, and waiting to staff patients with an attending physician.3-5 Addressing these issues could allow inpatient units to function more like observation units for specific diagnoses. Standardization of care around specific diagnoses can make decision-making and discharge more efficient. In 2014, White et al4 showed that standardizing discharge criteria for specific diagnoses (including bronchiolitis) and embedding these criteria in admission order sets resulted in a significant decrease in LOS without affecting readmission rates or patient satisfaction.
To address the issues of attending availability, we may need to rethink rounding. The daily structure of inpatient rounding has not meaningfully changed since the 1950s. While there has been a push for increased morning discharges, this approach misses many patients whose illness course is evolving and who may be ready for discharge in the afternoon or evening.6 The current structure of morning rounds on medical teams is based on the need for resident education, supervision, and time available for attendings to complete administrative tasks and teaching in the afternoons. Structural change in patient care requires academic institutions to rethink what “being on service” actually means. Since LOS in these cases is brief, multiple days of clinical continuity may not be as beneficial as with other diagnoses. Further, there is no reason that daytime rounding teams are the only teams that can discharge patients. Telemedicine could also offer an opportunity for attending physicians to remotely determine whether a patient is discharge appropriate. Standardization of discharge criteria at admission could allow for trainees to discharge patients when they meet those criteria.
Perhaps we should begin to adapt our work structure to our patients’ needs, rather than the other way around. In pediatrics, we have already made traditional rounding more patient-focused through the practice of family-centered rounding. We should identify, as the authors have, ways to do things differently to make further improvements in care.
Ultimately, the success of this OU-HOT protocol demonstrates the power of structural interventions aimed at changing how we do things rather than just doing more (or less) of the same.
Bronchiolitis, the most common cause of hospital admission for infants, is responsible for more than $500 million in direct medical costs in the United States yearly. Recent efforts have focused on what can be safely avoided when caring for patients with bronchiolitis (eg, continuous pulse oximetry, bronchodilator administration). While there remains substantial room for improvement in avoiding such low-value (or no-value) practices, the incremental improvements from these de-escalations will reach an asymptote over time. Further improvements in care and value must occur by doing things differently—not just simply doing less.
In this month’s Journal of Hospital Medicine, Ohlsen et al1 describe an intervention to decrease length of stay (LOS) for patients with bronchiolitis They employed an interrupted time series analysis to evaluate implementation of an observation unit and home oxygen therapy (OU-HOT) model of care and found that LOS dramatically decreased immediately following implementation. This reduction was maintained over 9 years. Use of home oxygen decreased over the study period, while LOS remained low, suggesting that the most important intervention was a structural one—the admission of patients to a unit dedicated to efficient discharge.
Observation units, staffed 24/7 with attending physicians, are well adapted to care for patients with illnesses like bronchiolitis, where hospitalization, though often needed, may be brief.2 These units are designed more like an emergency department than an inpatient unit, with protocolized care and the expectation of rapid turnover.
Multiple studies have shown that physician-related delays are a primary driver of delayed discharge from inpatient units. Such delays include delayed or variable clinical decision-making, inadequate communication of discharge criteria, and waiting to staff patients with an attending physician.3-5 Addressing these issues could allow inpatient units to function more like observation units for specific diagnoses. Standardization of care around specific diagnoses can make decision-making and discharge more efficient. In 2014, White et al4 showed that standardizing discharge criteria for specific diagnoses (including bronchiolitis) and embedding these criteria in admission order sets resulted in a significant decrease in LOS without affecting readmission rates or patient satisfaction.
To address the issues of attending availability, we may need to rethink rounding. The daily structure of inpatient rounding has not meaningfully changed since the 1950s. While there has been a push for increased morning discharges, this approach misses many patients whose illness course is evolving and who may be ready for discharge in the afternoon or evening.6 The current structure of morning rounds on medical teams is based on the need for resident education, supervision, and time available for attendings to complete administrative tasks and teaching in the afternoons. Structural change in patient care requires academic institutions to rethink what “being on service” actually means. Since LOS in these cases is brief, multiple days of clinical continuity may not be as beneficial as with other diagnoses. Further, there is no reason that daytime rounding teams are the only teams that can discharge patients. Telemedicine could also offer an opportunity for attending physicians to remotely determine whether a patient is discharge appropriate. Standardization of discharge criteria at admission could allow for trainees to discharge patients when they meet those criteria.
Perhaps we should begin to adapt our work structure to our patients’ needs, rather than the other way around. In pediatrics, we have already made traditional rounding more patient-focused through the practice of family-centered rounding. We should identify, as the authors have, ways to do things differently to make further improvements in care.
Ultimately, the success of this OU-HOT protocol demonstrates the power of structural interventions aimed at changing how we do things rather than just doing more (or less) of the same.
1. Ohlsen T, Knudson A, Korgenski EK, et al. Nine seasons of a bronchiolitis observation unit and home oxygen therapy protocol. J Hosp Med. 2021;16(5):261-267.
2. Plamann JM, Zedreck-Gonzalez J, Fennimore L. Creation of an adult observation unit: improving outcomes. J Nurs Care Qual. 2018;33(1):72-78. https://doi.org/10.1097/NCQ.0000000000000267
3. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
5. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. https://doi.org/10.1002/jhm.490
6. Gordon SA, Garber D, Taufique Z, et al. Improving on-time discharge in otolaryngology admissions. Otolaryngol Head Neck Surg. 2020;163(2):188-193. https://doi.org/10.1177/0194599819898910
1. Ohlsen T, Knudson A, Korgenski EK, et al. Nine seasons of a bronchiolitis observation unit and home oxygen therapy protocol. J Hosp Med. 2021;16(5):261-267.
2. Plamann JM, Zedreck-Gonzalez J, Fennimore L. Creation of an adult observation unit: improving outcomes. J Nurs Care Qual. 2018;33(1):72-78. https://doi.org/10.1097/NCQ.0000000000000267
3. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
5. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. https://doi.org/10.1002/jhm.490
6. Gordon SA, Garber D, Taufique Z, et al. Improving on-time discharge in otolaryngology admissions. Otolaryngol Head Neck Surg. 2020;163(2):188-193. https://doi.org/10.1177/0194599819898910
© 2021 Society of Hospital Medicine