User login
Discharge by Noon: Toward a Better Understanding of Benefits and Costs
Targeting “discharge before noon” (DBN) for hospitalized patients has been proposed as a way to improve hospital throughput and patient safety by reducing emergency department (ED) boarding and crowding. In this issue, Kirubarajan et al1 report no association between morning discharge and length of stay (LOS) for either the ED or hospitalization.1 This large (189,781 patients) 7-year study from seven quite different Canadian hospitals adds important data to a literature that remains divided about whether DBN helps or hurts hospital LOS and ED boarding.
Unlike trials reporting interventions to encourage DBN, this observational study was unique in that it took each day as the unit of observation. This method cleverly allowed the authors to examine whether days with more discharges before noon conferred a lower mean ED and inpatient LOS among patients admitted on those days. Their approach appropriately reframes the central issue as one of patient flow.
Kirubarajan et al’s most notable, and perhaps surprising, finding is the lack of association between morning discharge and ED LOS. Computer modeling supports the hypothesis that ED throughput will improve on days with earlier inpatient bed availability.2 Several studies have also noted earlier ED departure times and decreased ED wait times after implementing interventions to promote DBN.3 Why might the authors’ findings contradict previous studies? Their outcomes may in part be due to high ED LOS (>14 hours), exceeding Canadian published targets and reports from the United States.4,5 Problems relating to ED resources, practice, and hospital census may have overwhelmed DBN as factors in boarding. The interpretation of their findings is limited by the authors’ decision to report only ED LOS, rather than including the time between a decision to admit and ED departure (boarding time).
While early studies that focused on interventions to promote DBN noted decreased inpatient LOS after their implementation, later studies found no effect or even an increase in LOS for general internal medicine patients. Concerns have been raised about the confounding effect of concurrent initiatives aimed at improving LOS as well as misaligned incentives to delay discharge to the following morning. As the number of conflicting studies mounts, and with the current report in hand, it is tempting to conclude that for the DBN evidence base as a whole, we are observing random variation around no effect.
With growing doubt about benefits of morning discharge, perhaps we should turn our attention away from the question of how to increase DBN and consider instead why and at what cost. Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others. Does the effort to discharge before noon consume valuable morning hours and detract from the care of other patients? Are patients held overnight unnecessarily to comply with DBN? Are there consequences in patient, nursing, or trainee satisfaction? Is bedside teaching affected?
And as concepts of patient-centered care are increasingly valued, we may ask whether DBN is such a concept, or is it rather an increasingly dubious strategy aimed at regularizing hospital operations? The need for a more holistic assessment of “discharge quality” is apparent. Instead of focusing on a particular hour, initiatives should determine the “best, earliest discharge time” for each patient and align multidisciplinary efforts toward this patient-centered goal. Such efforts are already underway in pediatric hospitals, where fixed discharge times are being replaced by discharge milestones embedded into the electronic medical record.6 An instrument to track “discharge readiness” such as this one, paired with ongoing analysis of the barriers to timely discharge, might better facilitate throughput by targeting the entire admission, rather than concentrating pressure on its final hours.
1. Kirubarajan A, Shin S, Fralick M, Kwan Jet al. Morning discharges and patient length-of-stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):334-338. https://doi.org/ 10.12788/jhm.3605
2. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. https://doi.org/10.1016/j.jemermed.2010.06.028
3. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. https://doi.org/10.1002/jhm.2412
4. Fee C, Burstin H, Maselli JH, Hsia RY. Association of emergency department length of stay with safety-net status. JAMA. 2012;307(5):476-482. https://doi.org/10.1001/jama.2012.41
5. Ontario wait times. Ontario Ministry of Health and Ministry of Long-Term Care. Accessed February 17, 2021. http://www.health.gov.on.ca/en/pro/programs/waittimes/edrs/targets.aspx
6. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
Targeting “discharge before noon” (DBN) for hospitalized patients has been proposed as a way to improve hospital throughput and patient safety by reducing emergency department (ED) boarding and crowding. In this issue, Kirubarajan et al1 report no association between morning discharge and length of stay (LOS) for either the ED or hospitalization.1 This large (189,781 patients) 7-year study from seven quite different Canadian hospitals adds important data to a literature that remains divided about whether DBN helps or hurts hospital LOS and ED boarding.
Unlike trials reporting interventions to encourage DBN, this observational study was unique in that it took each day as the unit of observation. This method cleverly allowed the authors to examine whether days with more discharges before noon conferred a lower mean ED and inpatient LOS among patients admitted on those days. Their approach appropriately reframes the central issue as one of patient flow.
Kirubarajan et al’s most notable, and perhaps surprising, finding is the lack of association between morning discharge and ED LOS. Computer modeling supports the hypothesis that ED throughput will improve on days with earlier inpatient bed availability.2 Several studies have also noted earlier ED departure times and decreased ED wait times after implementing interventions to promote DBN.3 Why might the authors’ findings contradict previous studies? Their outcomes may in part be due to high ED LOS (>14 hours), exceeding Canadian published targets and reports from the United States.4,5 Problems relating to ED resources, practice, and hospital census may have overwhelmed DBN as factors in boarding. The interpretation of their findings is limited by the authors’ decision to report only ED LOS, rather than including the time between a decision to admit and ED departure (boarding time).
While early studies that focused on interventions to promote DBN noted decreased inpatient LOS after their implementation, later studies found no effect or even an increase in LOS for general internal medicine patients. Concerns have been raised about the confounding effect of concurrent initiatives aimed at improving LOS as well as misaligned incentives to delay discharge to the following morning. As the number of conflicting studies mounts, and with the current report in hand, it is tempting to conclude that for the DBN evidence base as a whole, we are observing random variation around no effect.
With growing doubt about benefits of morning discharge, perhaps we should turn our attention away from the question of how to increase DBN and consider instead why and at what cost. Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others. Does the effort to discharge before noon consume valuable morning hours and detract from the care of other patients? Are patients held overnight unnecessarily to comply with DBN? Are there consequences in patient, nursing, or trainee satisfaction? Is bedside teaching affected?
And as concepts of patient-centered care are increasingly valued, we may ask whether DBN is such a concept, or is it rather an increasingly dubious strategy aimed at regularizing hospital operations? The need for a more holistic assessment of “discharge quality” is apparent. Instead of focusing on a particular hour, initiatives should determine the “best, earliest discharge time” for each patient and align multidisciplinary efforts toward this patient-centered goal. Such efforts are already underway in pediatric hospitals, where fixed discharge times are being replaced by discharge milestones embedded into the electronic medical record.6 An instrument to track “discharge readiness” such as this one, paired with ongoing analysis of the barriers to timely discharge, might better facilitate throughput by targeting the entire admission, rather than concentrating pressure on its final hours.
Targeting “discharge before noon” (DBN) for hospitalized patients has been proposed as a way to improve hospital throughput and patient safety by reducing emergency department (ED) boarding and crowding. In this issue, Kirubarajan et al1 report no association between morning discharge and length of stay (LOS) for either the ED or hospitalization.1 This large (189,781 patients) 7-year study from seven quite different Canadian hospitals adds important data to a literature that remains divided about whether DBN helps or hurts hospital LOS and ED boarding.
Unlike trials reporting interventions to encourage DBN, this observational study was unique in that it took each day as the unit of observation. This method cleverly allowed the authors to examine whether days with more discharges before noon conferred a lower mean ED and inpatient LOS among patients admitted on those days. Their approach appropriately reframes the central issue as one of patient flow.
Kirubarajan et al’s most notable, and perhaps surprising, finding is the lack of association between morning discharge and ED LOS. Computer modeling supports the hypothesis that ED throughput will improve on days with earlier inpatient bed availability.2 Several studies have also noted earlier ED departure times and decreased ED wait times after implementing interventions to promote DBN.3 Why might the authors’ findings contradict previous studies? Their outcomes may in part be due to high ED LOS (>14 hours), exceeding Canadian published targets and reports from the United States.4,5 Problems relating to ED resources, practice, and hospital census may have overwhelmed DBN as factors in boarding. The interpretation of their findings is limited by the authors’ decision to report only ED LOS, rather than including the time between a decision to admit and ED departure (boarding time).
While early studies that focused on interventions to promote DBN noted decreased inpatient LOS after their implementation, later studies found no effect or even an increase in LOS for general internal medicine patients. Concerns have been raised about the confounding effect of concurrent initiatives aimed at improving LOS as well as misaligned incentives to delay discharge to the following morning. As the number of conflicting studies mounts, and with the current report in hand, it is tempting to conclude that for the DBN evidence base as a whole, we are observing random variation around no effect.
With growing doubt about benefits of morning discharge, perhaps we should turn our attention away from the question of how to increase DBN and consider instead why and at what cost. Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others. Does the effort to discharge before noon consume valuable morning hours and detract from the care of other patients? Are patients held overnight unnecessarily to comply with DBN? Are there consequences in patient, nursing, or trainee satisfaction? Is bedside teaching affected?
And as concepts of patient-centered care are increasingly valued, we may ask whether DBN is such a concept, or is it rather an increasingly dubious strategy aimed at regularizing hospital operations? The need for a more holistic assessment of “discharge quality” is apparent. Instead of focusing on a particular hour, initiatives should determine the “best, earliest discharge time” for each patient and align multidisciplinary efforts toward this patient-centered goal. Such efforts are already underway in pediatric hospitals, where fixed discharge times are being replaced by discharge milestones embedded into the electronic medical record.6 An instrument to track “discharge readiness” such as this one, paired with ongoing analysis of the barriers to timely discharge, might better facilitate throughput by targeting the entire admission, rather than concentrating pressure on its final hours.
1. Kirubarajan A, Shin S, Fralick M, Kwan Jet al. Morning discharges and patient length-of-stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):334-338. https://doi.org/ 10.12788/jhm.3605
2. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. https://doi.org/10.1016/j.jemermed.2010.06.028
3. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. https://doi.org/10.1002/jhm.2412
4. Fee C, Burstin H, Maselli JH, Hsia RY. Association of emergency department length of stay with safety-net status. JAMA. 2012;307(5):476-482. https://doi.org/10.1001/jama.2012.41
5. Ontario wait times. Ontario Ministry of Health and Ministry of Long-Term Care. Accessed February 17, 2021. http://www.health.gov.on.ca/en/pro/programs/waittimes/edrs/targets.aspx
6. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
1. Kirubarajan A, Shin S, Fralick M, Kwan Jet al. Morning discharges and patient length-of-stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):334-338. https://doi.org/ 10.12788/jhm.3605
2. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. https://doi.org/10.1016/j.jemermed.2010.06.028
3. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. https://doi.org/10.1002/jhm.2412
4. Fee C, Burstin H, Maselli JH, Hsia RY. Association of emergency department length of stay with safety-net status. JAMA. 2012;307(5):476-482. https://doi.org/10.1001/jama.2012.41
5. Ontario wait times. Ontario Ministry of Health and Ministry of Long-Term Care. Accessed February 17, 2021. http://www.health.gov.on.ca/en/pro/programs/waittimes/edrs/targets.aspx
6. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
© 2021 Society of Hospital Medicine
Are You Thinking What I’m Thinking? The Case for Shared Mental Models in Hospital Discharges
Hospital discharge is a complex, multi-stakeholder event, and evidence suggests that the quality of that transition directly relates to mortality, readmissions, and postdischarge quality of life and functional status.1 The Centers for Medicare & Medicaid Services call for team-based and patient-centered discharge planning,2 yet the process for achieving this is poorly defined.
In this issue of the Journal of Hospital Medicine, Manges et al3 use shared mental models (SMM) as a conceptual framework to describe differences in how care team members and patients perceive hospital discharge readiness. While our understanding of factors associated with safe and patient-centered hospital discharges is still growing, the authors focus on one critical component: lack of agreement between patients and interprofessional teams regarding discharge readiness.
Manges et al3 measured whether interprofessional team members agree, or converge, on their assessment of a patient’s discharge readiness (team-SMM convergence) and whether that assessment converges with the patient’s self-assessment (team-patient SMM convergence). They found good team-SMM convergence regarding the patient’s discharge readiness, yet teams overestimated readiness compared with the patient’s self-assessment nearly half (48.4%) of the time. A clinical trial found that clinician assessments of discharge readiness were poorly predictive of readmissions unless they were combined with a patient’s self-assessment.4 Manges et al’s study findings, while of limited generalizability, enhance our understanding of a potential gap in achieving patient-centered care as outlined in the Institute of Medicine’s Crossing the Quality Chasm,5 which urges clinicians to see patients and families as partners in improving care.
The authors also found that higher team-patient convergence was associated with teams that reported high-quality teamwork and those having more baccalaureate degree−educated nurses (BSN). While Manges et al3 did not elucidate the mechanism by which this occurs, their findings align with existing literature showing that patients receiving care from a higher proportion of BSN-prepared nurses experience an 18.7% reduction in odds of readmission.6 Further research investigating the link between team communication, registered nurse education, and discharge outcomes may reveal additional opportunities for interventions to improve discharge quality.
The lack of patient outcomes and the limited diversity of the patient population are substantial limitations of the study. The authors did not assess the relationship between SMMs and important outcomes like readmission or adverse events. Furthermore, most of the patients were White and English-speaking, precluding assessment of factors that disproportionately impact patient populations that already experience disparities in a multitude of health outcomes.
In summary, Manges et al3 highlight challenges and opportunities in optimizing clinician communication and ensuring that the team’s and the patient’s self-assessments align and inform discharge planning. Their findings suggest the theoretical framework of SMM holds promise in identifying and evaluating some of the complex determinants involved in high-quality, patient-centered hospital discharges.
1. Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x
2. Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; revisions to requirements for discharge planning for hospitals, critical access hospitals, and home health agencies, and hospital and critical access hospital changes to promote innovation, flexibility, and improvement in patient care. Fed Regist. 2019;84(189):51836-51884. https://www.govinfo.gov/content/pkg/FR-2019-09-30/pdf/2019-20732.pdf
3. Manges KA, Wallace AS, Groves PS, Schapira MM, Burke RE. Ready to go home? Assessment of shared mental models of the patient and discharging team regarding readiness for hospital discharge. J Hosp Med. 2020;16(6):326-332. https://doi.org/10.12788/jhm.3464
4. Weiss ME, Yakusheva O, Bobay KL, et al. Effect of implementing discharge readiness assessment in adult medical-surgical units on 30-day return to hospital: the READI randomized clinical trial. JAMA Netw open. 2019;2(1):e187387. https://doi.org/10.1001/jamanetworkopen.2018.7387
5. Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001.
6. Yakusheva O, Lindrooth R, Weiss M. Economic evaluation of the 80% baccalaureate nurse workforce recommendation: a patient-level analysis. Med Care. 2014;52(10):864-869. https://doi.org/10.1097/MLR.0000000000000189
Hospital discharge is a complex, multi-stakeholder event, and evidence suggests that the quality of that transition directly relates to mortality, readmissions, and postdischarge quality of life and functional status.1 The Centers for Medicare & Medicaid Services call for team-based and patient-centered discharge planning,2 yet the process for achieving this is poorly defined.
In this issue of the Journal of Hospital Medicine, Manges et al3 use shared mental models (SMM) as a conceptual framework to describe differences in how care team members and patients perceive hospital discharge readiness. While our understanding of factors associated with safe and patient-centered hospital discharges is still growing, the authors focus on one critical component: lack of agreement between patients and interprofessional teams regarding discharge readiness.
Manges et al3 measured whether interprofessional team members agree, or converge, on their assessment of a patient’s discharge readiness (team-SMM convergence) and whether that assessment converges with the patient’s self-assessment (team-patient SMM convergence). They found good team-SMM convergence regarding the patient’s discharge readiness, yet teams overestimated readiness compared with the patient’s self-assessment nearly half (48.4%) of the time. A clinical trial found that clinician assessments of discharge readiness were poorly predictive of readmissions unless they were combined with a patient’s self-assessment.4 Manges et al’s study findings, while of limited generalizability, enhance our understanding of a potential gap in achieving patient-centered care as outlined in the Institute of Medicine’s Crossing the Quality Chasm,5 which urges clinicians to see patients and families as partners in improving care.
The authors also found that higher team-patient convergence was associated with teams that reported high-quality teamwork and those having more baccalaureate degree−educated nurses (BSN). While Manges et al3 did not elucidate the mechanism by which this occurs, their findings align with existing literature showing that patients receiving care from a higher proportion of BSN-prepared nurses experience an 18.7% reduction in odds of readmission.6 Further research investigating the link between team communication, registered nurse education, and discharge outcomes may reveal additional opportunities for interventions to improve discharge quality.
The lack of patient outcomes and the limited diversity of the patient population are substantial limitations of the study. The authors did not assess the relationship between SMMs and important outcomes like readmission or adverse events. Furthermore, most of the patients were White and English-speaking, precluding assessment of factors that disproportionately impact patient populations that already experience disparities in a multitude of health outcomes.
In summary, Manges et al3 highlight challenges and opportunities in optimizing clinician communication and ensuring that the team’s and the patient’s self-assessments align and inform discharge planning. Their findings suggest the theoretical framework of SMM holds promise in identifying and evaluating some of the complex determinants involved in high-quality, patient-centered hospital discharges.
Hospital discharge is a complex, multi-stakeholder event, and evidence suggests that the quality of that transition directly relates to mortality, readmissions, and postdischarge quality of life and functional status.1 The Centers for Medicare & Medicaid Services call for team-based and patient-centered discharge planning,2 yet the process for achieving this is poorly defined.
In this issue of the Journal of Hospital Medicine, Manges et al3 use shared mental models (SMM) as a conceptual framework to describe differences in how care team members and patients perceive hospital discharge readiness. While our understanding of factors associated with safe and patient-centered hospital discharges is still growing, the authors focus on one critical component: lack of agreement between patients and interprofessional teams regarding discharge readiness.
Manges et al3 measured whether interprofessional team members agree, or converge, on their assessment of a patient’s discharge readiness (team-SMM convergence) and whether that assessment converges with the patient’s self-assessment (team-patient SMM convergence). They found good team-SMM convergence regarding the patient’s discharge readiness, yet teams overestimated readiness compared with the patient’s self-assessment nearly half (48.4%) of the time. A clinical trial found that clinician assessments of discharge readiness were poorly predictive of readmissions unless they were combined with a patient’s self-assessment.4 Manges et al’s study findings, while of limited generalizability, enhance our understanding of a potential gap in achieving patient-centered care as outlined in the Institute of Medicine’s Crossing the Quality Chasm,5 which urges clinicians to see patients and families as partners in improving care.
The authors also found that higher team-patient convergence was associated with teams that reported high-quality teamwork and those having more baccalaureate degree−educated nurses (BSN). While Manges et al3 did not elucidate the mechanism by which this occurs, their findings align with existing literature showing that patients receiving care from a higher proportion of BSN-prepared nurses experience an 18.7% reduction in odds of readmission.6 Further research investigating the link between team communication, registered nurse education, and discharge outcomes may reveal additional opportunities for interventions to improve discharge quality.
The lack of patient outcomes and the limited diversity of the patient population are substantial limitations of the study. The authors did not assess the relationship between SMMs and important outcomes like readmission or adverse events. Furthermore, most of the patients were White and English-speaking, precluding assessment of factors that disproportionately impact patient populations that already experience disparities in a multitude of health outcomes.
In summary, Manges et al3 highlight challenges and opportunities in optimizing clinician communication and ensuring that the team’s and the patient’s self-assessments align and inform discharge planning. Their findings suggest the theoretical framework of SMM holds promise in identifying and evaluating some of the complex determinants involved in high-quality, patient-centered hospital discharges.
1. Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x
2. Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; revisions to requirements for discharge planning for hospitals, critical access hospitals, and home health agencies, and hospital and critical access hospital changes to promote innovation, flexibility, and improvement in patient care. Fed Regist. 2019;84(189):51836-51884. https://www.govinfo.gov/content/pkg/FR-2019-09-30/pdf/2019-20732.pdf
3. Manges KA, Wallace AS, Groves PS, Schapira MM, Burke RE. Ready to go home? Assessment of shared mental models of the patient and discharging team regarding readiness for hospital discharge. J Hosp Med. 2020;16(6):326-332. https://doi.org/10.12788/jhm.3464
4. Weiss ME, Yakusheva O, Bobay KL, et al. Effect of implementing discharge readiness assessment in adult medical-surgical units on 30-day return to hospital: the READI randomized clinical trial. JAMA Netw open. 2019;2(1):e187387. https://doi.org/10.1001/jamanetworkopen.2018.7387
5. Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001.
6. Yakusheva O, Lindrooth R, Weiss M. Economic evaluation of the 80% baccalaureate nurse workforce recommendation: a patient-level analysis. Med Care. 2014;52(10):864-869. https://doi.org/10.1097/MLR.0000000000000189
1. Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x
2. Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; revisions to requirements for discharge planning for hospitals, critical access hospitals, and home health agencies, and hospital and critical access hospital changes to promote innovation, flexibility, and improvement in patient care. Fed Regist. 2019;84(189):51836-51884. https://www.govinfo.gov/content/pkg/FR-2019-09-30/pdf/2019-20732.pdf
3. Manges KA, Wallace AS, Groves PS, Schapira MM, Burke RE. Ready to go home? Assessment of shared mental models of the patient and discharging team regarding readiness for hospital discharge. J Hosp Med. 2020;16(6):326-332. https://doi.org/10.12788/jhm.3464
4. Weiss ME, Yakusheva O, Bobay KL, et al. Effect of implementing discharge readiness assessment in adult medical-surgical units on 30-day return to hospital: the READI randomized clinical trial. JAMA Netw open. 2019;2(1):e187387. https://doi.org/10.1001/jamanetworkopen.2018.7387
5. Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001.
6. Yakusheva O, Lindrooth R, Weiss M. Economic evaluation of the 80% baccalaureate nurse workforce recommendation: a patient-level analysis. Med Care. 2014;52(10):864-869. https://doi.org/10.1097/MLR.0000000000000189
© 2021 Society of Hospital Medicine
Trust in a Time of Uncertainty: A Call for Articles
A functioning healthcare system requires trust on many levels. In its simplest form, this is the trust between an individual patient and their physician that allows for candor, autonomy, informed decisions, and compassionate care. Trust is a central component of medical education, as trainees gradually earn the trust of their supervisors to achieve autonomy. And, on a much larger scale, societal trust in science, the facts, and the medical system influences individual and group decisions that can have far-reaching consequences.
Defining trust is challenging. Trust is relational, an often subconscious decision “by one individual to depend on another,” but it can also be as broad as trust in an institution or a national system.1 Trust also requires vulnerability—trusting another person or system means ceding some level of personal control and accepting risk. Thus, to ask patients and society to trust in physicians, the healthcare system, or public health institutions, though essential, is no small request.
Physicians and the medical system at large have not always behaved in ways that warrant trust. Medical research on vulnerable populations (historically marginalized communities, prisoners, residents of institutions) has occurred within living memory. Systemic racism within medicine has led to marked disparities in access and outcomes between White and minoritized communities.2 These disparities have been accentuated by the pandemic. Black and Brown patients have higher infection rates and higher mortality rates but less access to healthcare.3 Vaccine distribution, which has been complicated by historic earned distrust from Black and Brown communities, revealed systemic racism. For example, many early mass vaccination sites, such as Dodger Stadium in Los Angeles, could only be easily reached by car. Online appointment scheduling platforms were opaque and required access to technology.4
Public trust in institutions has been eroding over the past several decades, but healthcare has unfortunately seen the largest decline.5 Individual healthcare decisions have also been increasingly politicized; the net result is the creation of laws, such as those limiting discussions of firearm safety or banning gender-affirming treatments for transgender children, that influence patient-physician interactions. This combination of erosion of trust and politicization of medical decisions has been harshly highlighted by the global pandemic, complicating public health policy and doctor-patient discussions. Public health measures such as masking and vaccination have become polarized.6 Further, there is diminishing trust in medical recommendations, brought about by the current media landscape and by frequent modifications to public health recommendations. Science and medicine are constantly changing, and knowledge in these fields is ultimately provisional. Unfortunately, when new data are published that contradict prior information or report new or dramatic findings, it can appear that the medical system was somehow obscuring the truth in the past, rather than simply advancing its knowledge in the present.
How do we build trust? How do we function in a healthcare system where trust has been eroded? Trust is ultimately a fragile thing. The process of earning it is not swift or straightforward, but it can be lost in a moment.
In partnership with the ABIM Foundation, the Journal of Hospital Medicine will explore the concept of trust in all facets of healthcare and medical education, including understanding the drivers of trust in a multitude of settings and in different relationships (patient-clinician, clinician-trainee, clinician- or trainee-organization, health system-community), interventions to build trust, and the enablers of those interventions. To this end, we are seeking articles that explore or evaluate trust. These include original research, brief reports, perspectives, and Leadership & Professional Development articles. Articles focusing on trust should be submitted by December 31, 2021.
1. Hendren EM, Kumagai AK. A matter of trust. Acad Med. 2019;94(9):1270-1272. https://doi.org/10.1097/ACM.0000000000002846
2. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(7):572-573. https://doi.org/10.12788/jhm.3492
3. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
4. Dembosky A. It’s not Tuskegee. Current medical racism fuels Black Americans’ vaccine hesitancy. Los Angeles Times. March 25, 2021.
5. Lynch TJ, Wolfson DB, Baron RJ. A trust initiative in health care: why and why now? Acad Med. 2019;94(4):463-465. https://doi.org/10.1097/ACM.0000000000002599
6. Sherling DH, Bell M. Masks, seat belts, and the politicization of public health. J Hosp Med. 2020;15(11):692-693. https://doi.org/10.12788/jhm.3524
A functioning healthcare system requires trust on many levels. In its simplest form, this is the trust between an individual patient and their physician that allows for candor, autonomy, informed decisions, and compassionate care. Trust is a central component of medical education, as trainees gradually earn the trust of their supervisors to achieve autonomy. And, on a much larger scale, societal trust in science, the facts, and the medical system influences individual and group decisions that can have far-reaching consequences.
Defining trust is challenging. Trust is relational, an often subconscious decision “by one individual to depend on another,” but it can also be as broad as trust in an institution or a national system.1 Trust also requires vulnerability—trusting another person or system means ceding some level of personal control and accepting risk. Thus, to ask patients and society to trust in physicians, the healthcare system, or public health institutions, though essential, is no small request.
Physicians and the medical system at large have not always behaved in ways that warrant trust. Medical research on vulnerable populations (historically marginalized communities, prisoners, residents of institutions) has occurred within living memory. Systemic racism within medicine has led to marked disparities in access and outcomes between White and minoritized communities.2 These disparities have been accentuated by the pandemic. Black and Brown patients have higher infection rates and higher mortality rates but less access to healthcare.3 Vaccine distribution, which has been complicated by historic earned distrust from Black and Brown communities, revealed systemic racism. For example, many early mass vaccination sites, such as Dodger Stadium in Los Angeles, could only be easily reached by car. Online appointment scheduling platforms were opaque and required access to technology.4
Public trust in institutions has been eroding over the past several decades, but healthcare has unfortunately seen the largest decline.5 Individual healthcare decisions have also been increasingly politicized; the net result is the creation of laws, such as those limiting discussions of firearm safety or banning gender-affirming treatments for transgender children, that influence patient-physician interactions. This combination of erosion of trust and politicization of medical decisions has been harshly highlighted by the global pandemic, complicating public health policy and doctor-patient discussions. Public health measures such as masking and vaccination have become polarized.6 Further, there is diminishing trust in medical recommendations, brought about by the current media landscape and by frequent modifications to public health recommendations. Science and medicine are constantly changing, and knowledge in these fields is ultimately provisional. Unfortunately, when new data are published that contradict prior information or report new or dramatic findings, it can appear that the medical system was somehow obscuring the truth in the past, rather than simply advancing its knowledge in the present.
How do we build trust? How do we function in a healthcare system where trust has been eroded? Trust is ultimately a fragile thing. The process of earning it is not swift or straightforward, but it can be lost in a moment.
In partnership with the ABIM Foundation, the Journal of Hospital Medicine will explore the concept of trust in all facets of healthcare and medical education, including understanding the drivers of trust in a multitude of settings and in different relationships (patient-clinician, clinician-trainee, clinician- or trainee-organization, health system-community), interventions to build trust, and the enablers of those interventions. To this end, we are seeking articles that explore or evaluate trust. These include original research, brief reports, perspectives, and Leadership & Professional Development articles. Articles focusing on trust should be submitted by December 31, 2021.
A functioning healthcare system requires trust on many levels. In its simplest form, this is the trust between an individual patient and their physician that allows for candor, autonomy, informed decisions, and compassionate care. Trust is a central component of medical education, as trainees gradually earn the trust of their supervisors to achieve autonomy. And, on a much larger scale, societal trust in science, the facts, and the medical system influences individual and group decisions that can have far-reaching consequences.
Defining trust is challenging. Trust is relational, an often subconscious decision “by one individual to depend on another,” but it can also be as broad as trust in an institution or a national system.1 Trust also requires vulnerability—trusting another person or system means ceding some level of personal control and accepting risk. Thus, to ask patients and society to trust in physicians, the healthcare system, or public health institutions, though essential, is no small request.
Physicians and the medical system at large have not always behaved in ways that warrant trust. Medical research on vulnerable populations (historically marginalized communities, prisoners, residents of institutions) has occurred within living memory. Systemic racism within medicine has led to marked disparities in access and outcomes between White and minoritized communities.2 These disparities have been accentuated by the pandemic. Black and Brown patients have higher infection rates and higher mortality rates but less access to healthcare.3 Vaccine distribution, which has been complicated by historic earned distrust from Black and Brown communities, revealed systemic racism. For example, many early mass vaccination sites, such as Dodger Stadium in Los Angeles, could only be easily reached by car. Online appointment scheduling platforms were opaque and required access to technology.4
Public trust in institutions has been eroding over the past several decades, but healthcare has unfortunately seen the largest decline.5 Individual healthcare decisions have also been increasingly politicized; the net result is the creation of laws, such as those limiting discussions of firearm safety or banning gender-affirming treatments for transgender children, that influence patient-physician interactions. This combination of erosion of trust and politicization of medical decisions has been harshly highlighted by the global pandemic, complicating public health policy and doctor-patient discussions. Public health measures such as masking and vaccination have become polarized.6 Further, there is diminishing trust in medical recommendations, brought about by the current media landscape and by frequent modifications to public health recommendations. Science and medicine are constantly changing, and knowledge in these fields is ultimately provisional. Unfortunately, when new data are published that contradict prior information or report new or dramatic findings, it can appear that the medical system was somehow obscuring the truth in the past, rather than simply advancing its knowledge in the present.
How do we build trust? How do we function in a healthcare system where trust has been eroded? Trust is ultimately a fragile thing. The process of earning it is not swift or straightforward, but it can be lost in a moment.
In partnership with the ABIM Foundation, the Journal of Hospital Medicine will explore the concept of trust in all facets of healthcare and medical education, including understanding the drivers of trust in a multitude of settings and in different relationships (patient-clinician, clinician-trainee, clinician- or trainee-organization, health system-community), interventions to build trust, and the enablers of those interventions. To this end, we are seeking articles that explore or evaluate trust. These include original research, brief reports, perspectives, and Leadership & Professional Development articles. Articles focusing on trust should be submitted by December 31, 2021.
1. Hendren EM, Kumagai AK. A matter of trust. Acad Med. 2019;94(9):1270-1272. https://doi.org/10.1097/ACM.0000000000002846
2. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(7):572-573. https://doi.org/10.12788/jhm.3492
3. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
4. Dembosky A. It’s not Tuskegee. Current medical racism fuels Black Americans’ vaccine hesitancy. Los Angeles Times. March 25, 2021.
5. Lynch TJ, Wolfson DB, Baron RJ. A trust initiative in health care: why and why now? Acad Med. 2019;94(4):463-465. https://doi.org/10.1097/ACM.0000000000002599
6. Sherling DH, Bell M. Masks, seat belts, and the politicization of public health. J Hosp Med. 2020;15(11):692-693. https://doi.org/10.12788/jhm.3524
1. Hendren EM, Kumagai AK. A matter of trust. Acad Med. 2019;94(9):1270-1272. https://doi.org/10.1097/ACM.0000000000002846
2. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(7):572-573. https://doi.org/10.12788/jhm.3492
3. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
4. Dembosky A. It’s not Tuskegee. Current medical racism fuels Black Americans’ vaccine hesitancy. Los Angeles Times. March 25, 2021.
5. Lynch TJ, Wolfson DB, Baron RJ. A trust initiative in health care: why and why now? Acad Med. 2019;94(4):463-465. https://doi.org/10.1097/ACM.0000000000002599
6. Sherling DH, Bell M. Masks, seat belts, and the politicization of public health. J Hosp Med. 2020;15(11):692-693. https://doi.org/10.12788/jhm.3524
© 2021 Society of Hospital Medicine
Microaggressions, Accountability, and Our Commitment to Doing Better
We recently published an article in our Leadership & Professional Development series titled “Tribalism: The Good, the Bad, and the Future.” Despite pre- and post-acceptance manuscript review and discussion by a diverse and thoughtful team of editors, we did not appreciate how particular language in this article would be hurtful to some communities. We also promoted the article using the hashtag “tribalism” in a journal tweet. Shortly after we posted the tweet, several readers on social media reached out with constructive feedback on the prejudicial nature of this terminology. Within hours of receiving this feedback, our editorial team met to better understand our error, and we made the decision to immediately retract the manuscript. We also deleted the tweet and issued an apology referencing a screenshot of the original tweet.1,2 We have republished the original article with appropriate language.3 Tweets promoting the new article will incorporate this new language.
From this experience, we learned that the words “tribe” and “tribalism” have no consistent meaning, are associated with negative historical and cultural assumptions, and can promote misleading stereotypes.4 The term “tribe” became popular as a colonial construct to describe forms of social organization considered ”uncivilized” or ”primitive.“5 In using the term “tribe” to describe members of medical communities, we ignored the complex and dynamic identities of Native American, African, and other Indigenous Peoples and the history of their oppression.
The intent of the original article was to highlight how being part of a distinct medical discipline, such as hospital medicine or emergency medicine, conferred benefits, such as shared identity and social support structure, and caution how this group identity could also lead to nonconstructive partisan behaviors that might not best serve our patients. We recognize that other words more accurately convey our intent and do not cause harm. We used “tribe” when we meant “group,” “discipline,” or “specialty.” We used “tribalism” when we meant “siloed” or “factional.”
This misstep underscores how, even with the best intentions and diverse teams, microaggressions can happen. We accept responsibility for this mistake, and we will continue to do the work of respecting and advocating for all members of our community. To minimize the likelihood of future errors, we are developing a systematic process to identify language within manuscripts accepted for publication that may be racist, sexist, ableist, homophobic, or otherwise harmful. As we embrace a growth mindset, we vow to remain transparent, responsive, and welcoming of feedback. We are grateful to our readers for helping us learn.
1. Shah SS [@SamirShahMD]. We are still learning. Despite review by a diverse group of team members, we did not appreciate how language in…. April 30, 2021. Accessed May 5, 2021. https://twitter.com/SamirShahMD/status/1388228974573244431
2. Journal of Hospital Medicine [@JHospMedicine]. We want to apologize. We used insensitive language that may be hurtful to Indigenous Americans & others. We are learning…. April 30, 2021. Accessed May 5, 2021. https://twitter.com/JHospMedicine/status/1388227448962052097
3. Kanjee Z, Bilello L. Specialty silos in medicine: the good, the bad, and the future. J Hosp Med. Published online May 21, 2021. https://doi.org/10.12788/jhm.3647
4. Lowe C. The trouble with tribe: How a common word masks complex African realities. Learning for Justice. Spring 2001. Accessed May 5, 2021. https://www.learningforjustice.org/magazine/spring-2001/the-trouble-with-tribe
5. Mungai C. Pundits who decry ‘tribalism’ know nothing about real tribes. Washington Post. January 30, 2019. Accessed May 6, 2021. https://www.washingtonpost.com/outlook/pundits-who-decry-tribalism-know-nothing-about-real-tribes/2019/01/29/8d14eb44-232f-11e9-90cd-dedb0c92dc17_story.html
We recently published an article in our Leadership & Professional Development series titled “Tribalism: The Good, the Bad, and the Future.” Despite pre- and post-acceptance manuscript review and discussion by a diverse and thoughtful team of editors, we did not appreciate how particular language in this article would be hurtful to some communities. We also promoted the article using the hashtag “tribalism” in a journal tweet. Shortly after we posted the tweet, several readers on social media reached out with constructive feedback on the prejudicial nature of this terminology. Within hours of receiving this feedback, our editorial team met to better understand our error, and we made the decision to immediately retract the manuscript. We also deleted the tweet and issued an apology referencing a screenshot of the original tweet.1,2 We have republished the original article with appropriate language.3 Tweets promoting the new article will incorporate this new language.
From this experience, we learned that the words “tribe” and “tribalism” have no consistent meaning, are associated with negative historical and cultural assumptions, and can promote misleading stereotypes.4 The term “tribe” became popular as a colonial construct to describe forms of social organization considered ”uncivilized” or ”primitive.“5 In using the term “tribe” to describe members of medical communities, we ignored the complex and dynamic identities of Native American, African, and other Indigenous Peoples and the history of their oppression.
The intent of the original article was to highlight how being part of a distinct medical discipline, such as hospital medicine or emergency medicine, conferred benefits, such as shared identity and social support structure, and caution how this group identity could also lead to nonconstructive partisan behaviors that might not best serve our patients. We recognize that other words more accurately convey our intent and do not cause harm. We used “tribe” when we meant “group,” “discipline,” or “specialty.” We used “tribalism” when we meant “siloed” or “factional.”
This misstep underscores how, even with the best intentions and diverse teams, microaggressions can happen. We accept responsibility for this mistake, and we will continue to do the work of respecting and advocating for all members of our community. To minimize the likelihood of future errors, we are developing a systematic process to identify language within manuscripts accepted for publication that may be racist, sexist, ableist, homophobic, or otherwise harmful. As we embrace a growth mindset, we vow to remain transparent, responsive, and welcoming of feedback. We are grateful to our readers for helping us learn.
We recently published an article in our Leadership & Professional Development series titled “Tribalism: The Good, the Bad, and the Future.” Despite pre- and post-acceptance manuscript review and discussion by a diverse and thoughtful team of editors, we did not appreciate how particular language in this article would be hurtful to some communities. We also promoted the article using the hashtag “tribalism” in a journal tweet. Shortly after we posted the tweet, several readers on social media reached out with constructive feedback on the prejudicial nature of this terminology. Within hours of receiving this feedback, our editorial team met to better understand our error, and we made the decision to immediately retract the manuscript. We also deleted the tweet and issued an apology referencing a screenshot of the original tweet.1,2 We have republished the original article with appropriate language.3 Tweets promoting the new article will incorporate this new language.
From this experience, we learned that the words “tribe” and “tribalism” have no consistent meaning, are associated with negative historical and cultural assumptions, and can promote misleading stereotypes.4 The term “tribe” became popular as a colonial construct to describe forms of social organization considered ”uncivilized” or ”primitive.“5 In using the term “tribe” to describe members of medical communities, we ignored the complex and dynamic identities of Native American, African, and other Indigenous Peoples and the history of their oppression.
The intent of the original article was to highlight how being part of a distinct medical discipline, such as hospital medicine or emergency medicine, conferred benefits, such as shared identity and social support structure, and caution how this group identity could also lead to nonconstructive partisan behaviors that might not best serve our patients. We recognize that other words more accurately convey our intent and do not cause harm. We used “tribe” when we meant “group,” “discipline,” or “specialty.” We used “tribalism” when we meant “siloed” or “factional.”
This misstep underscores how, even with the best intentions and diverse teams, microaggressions can happen. We accept responsibility for this mistake, and we will continue to do the work of respecting and advocating for all members of our community. To minimize the likelihood of future errors, we are developing a systematic process to identify language within manuscripts accepted for publication that may be racist, sexist, ableist, homophobic, or otherwise harmful. As we embrace a growth mindset, we vow to remain transparent, responsive, and welcoming of feedback. We are grateful to our readers for helping us learn.
1. Shah SS [@SamirShahMD]. We are still learning. Despite review by a diverse group of team members, we did not appreciate how language in…. April 30, 2021. Accessed May 5, 2021. https://twitter.com/SamirShahMD/status/1388228974573244431
2. Journal of Hospital Medicine [@JHospMedicine]. We want to apologize. We used insensitive language that may be hurtful to Indigenous Americans & others. We are learning…. April 30, 2021. Accessed May 5, 2021. https://twitter.com/JHospMedicine/status/1388227448962052097
3. Kanjee Z, Bilello L. Specialty silos in medicine: the good, the bad, and the future. J Hosp Med. Published online May 21, 2021. https://doi.org/10.12788/jhm.3647
4. Lowe C. The trouble with tribe: How a common word masks complex African realities. Learning for Justice. Spring 2001. Accessed May 5, 2021. https://www.learningforjustice.org/magazine/spring-2001/the-trouble-with-tribe
5. Mungai C. Pundits who decry ‘tribalism’ know nothing about real tribes. Washington Post. January 30, 2019. Accessed May 6, 2021. https://www.washingtonpost.com/outlook/pundits-who-decry-tribalism-know-nothing-about-real-tribes/2019/01/29/8d14eb44-232f-11e9-90cd-dedb0c92dc17_story.html
1. Shah SS [@SamirShahMD]. We are still learning. Despite review by a diverse group of team members, we did not appreciate how language in…. April 30, 2021. Accessed May 5, 2021. https://twitter.com/SamirShahMD/status/1388228974573244431
2. Journal of Hospital Medicine [@JHospMedicine]. We want to apologize. We used insensitive language that may be hurtful to Indigenous Americans & others. We are learning…. April 30, 2021. Accessed May 5, 2021. https://twitter.com/JHospMedicine/status/1388227448962052097
3. Kanjee Z, Bilello L. Specialty silos in medicine: the good, the bad, and the future. J Hosp Med. Published online May 21, 2021. https://doi.org/10.12788/jhm.3647
4. Lowe C. The trouble with tribe: How a common word masks complex African realities. Learning for Justice. Spring 2001. Accessed May 5, 2021. https://www.learningforjustice.org/magazine/spring-2001/the-trouble-with-tribe
5. Mungai C. Pundits who decry ‘tribalism’ know nothing about real tribes. Washington Post. January 30, 2019. Accessed May 6, 2021. https://www.washingtonpost.com/outlook/pundits-who-decry-tribalism-know-nothing-about-real-tribes/2019/01/29/8d14eb44-232f-11e9-90cd-dedb0c92dc17_story.html
© 2021 Society of Hospital Medicine
Leadership & Professional Development: Specialty Silos in Medicine
Siloed, adj.:
Kept in isolation in a way that hinders communication and cooperation . . .
—Merriam-Webster’s Dictionary
Humans naturally separate into groups, and the medical field is no exception. Being a member of a likeminded group, such as one’s specialty, can improve self-esteem and provide social organization: it feels good to identify with people we admire. Through culture, these specialty-based groups implicitly and explicitly guide and encourage positive attributes or behaviors like a hospitalist’s thoroughness or an emergency medicine physician’s steady management of unstable patients. Our specialties also provide support and understanding in challenging times.
Despite these positive aspects, such divisions can negatively affect interprofessional relationships when our specialties become siloed. A potential side-effect of building up ourselves and our own groups is that we can implicitly put others down. For example, a hospitalist who spends extra time on the phone regularly updating each patient’s family will appropriately take pride in their practice, but over time this can also lead to an unreasonable assumption that physicians in other departments with different routines are not as committed to outstanding communication.
These rigid separations facilitate the fundamental attribution error, the tendency to ascribe a problem or disagreement to a colleague’s substandard character or ability. Imagine that the aforementioned hospitalist’s phone call delays a response to an admission page from the emergency room. The emergency medicine physician, who is waiting to sign out the admission while simultaneously managing many sick and complex patients, could assume the hospitalist is being disrespectful, rather than also working hard to provide the best care. Our siloed specialty identities can lead us to imagine the worst in each other and exacerbate intergroup conflict.1
Silos in medicine also adversely affect patients. Poor communication and lack of information-sharing across disciplines can lead to medical error2 and stifle dissemination of safer practices.3 Further, the unintentional disparaging of other medical specialties undermines the confidence our patients have in all of us; a patient within earshot of the hospitalist expressing annoyance at the “impatient” emergency medicine physician who “won’t stop paging,” or the emergency medicine physician complaining about the hospitalist who “refuses to call back,” will lose trust in each of their providers.
We suggest three steps to reduce the negative impact of specialty silos in medicine:
- Get to know each other personally. Friendly conversation during work hours and social interaction outside the hospital can inoculate against interspecialty conflict by putting a human face on our colleagues. The resultant relationships make it easier to work together and see things from another’s perspective.
- Emphasize our shared affiliations.4 The greater the salience of a mutual identity as “healthcare providers,” the more likely we are to recognize each other’s unique contributions and question the stereotypes we imagine about one another.
- Consider projects across specialties. Interdepartmental data-sharing and joint meetings, including educational conferences, can facilitate situational awareness, synergy, and efficient problem-solving.
Our medical specialties will continue to group together. While these groups can be a source of strength and meaning, silos can interfere with professional alliances and effective patient care. Mitigating the harmful effects of silos can benefit all of us and our patients.
Authors’ note: This article was previously published using the term “tribalism,” which we have since learned is derogatory to Indigenous Americans and others. We apologize for any harm. We have retracted and republished the article without this language. We appreciate readers teaching us how to choose better words so all people feel respected and valued.
1. Fiol CM, Pratt MG, O’Connor EJ. Managing intractable identity conflicts. Acad Management Rev. 2009;34(1):32-55. https://doi.org/10.5465/amr.2009.35713276
2. Horowitz LI, Meredith T, Schuur JD, et al. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6): 701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
3. Paine, LA, Baker DR, Rosenstein B, Pronovost PJ. The Johns Hopkins Hospital: identifying and addressing risks and safety issues. JT Comm J Qual Saf. 2004;30(10):543-550. https://doi.org/10.1016/s1549-3741(04)30064-x
4. Burford B. Group processes in medical education: learning from social identity theory. Med Educ. 2012;46(2):143-152. https://doi.org/10.1111/j.1365-2923.2011.04099.x
Siloed, adj.:
Kept in isolation in a way that hinders communication and cooperation . . .
—Merriam-Webster’s Dictionary
Humans naturally separate into groups, and the medical field is no exception. Being a member of a likeminded group, such as one’s specialty, can improve self-esteem and provide social organization: it feels good to identify with people we admire. Through culture, these specialty-based groups implicitly and explicitly guide and encourage positive attributes or behaviors like a hospitalist’s thoroughness or an emergency medicine physician’s steady management of unstable patients. Our specialties also provide support and understanding in challenging times.
Despite these positive aspects, such divisions can negatively affect interprofessional relationships when our specialties become siloed. A potential side-effect of building up ourselves and our own groups is that we can implicitly put others down. For example, a hospitalist who spends extra time on the phone regularly updating each patient’s family will appropriately take pride in their practice, but over time this can also lead to an unreasonable assumption that physicians in other departments with different routines are not as committed to outstanding communication.
These rigid separations facilitate the fundamental attribution error, the tendency to ascribe a problem or disagreement to a colleague’s substandard character or ability. Imagine that the aforementioned hospitalist’s phone call delays a response to an admission page from the emergency room. The emergency medicine physician, who is waiting to sign out the admission while simultaneously managing many sick and complex patients, could assume the hospitalist is being disrespectful, rather than also working hard to provide the best care. Our siloed specialty identities can lead us to imagine the worst in each other and exacerbate intergroup conflict.1
Silos in medicine also adversely affect patients. Poor communication and lack of information-sharing across disciplines can lead to medical error2 and stifle dissemination of safer practices.3 Further, the unintentional disparaging of other medical specialties undermines the confidence our patients have in all of us; a patient within earshot of the hospitalist expressing annoyance at the “impatient” emergency medicine physician who “won’t stop paging,” or the emergency medicine physician complaining about the hospitalist who “refuses to call back,” will lose trust in each of their providers.
We suggest three steps to reduce the negative impact of specialty silos in medicine:
- Get to know each other personally. Friendly conversation during work hours and social interaction outside the hospital can inoculate against interspecialty conflict by putting a human face on our colleagues. The resultant relationships make it easier to work together and see things from another’s perspective.
- Emphasize our shared affiliations.4 The greater the salience of a mutual identity as “healthcare providers,” the more likely we are to recognize each other’s unique contributions and question the stereotypes we imagine about one another.
- Consider projects across specialties. Interdepartmental data-sharing and joint meetings, including educational conferences, can facilitate situational awareness, synergy, and efficient problem-solving.
Our medical specialties will continue to group together. While these groups can be a source of strength and meaning, silos can interfere with professional alliances and effective patient care. Mitigating the harmful effects of silos can benefit all of us and our patients.
Authors’ note: This article was previously published using the term “tribalism,” which we have since learned is derogatory to Indigenous Americans and others. We apologize for any harm. We have retracted and republished the article without this language. We appreciate readers teaching us how to choose better words so all people feel respected and valued.
Siloed, adj.:
Kept in isolation in a way that hinders communication and cooperation . . .
—Merriam-Webster’s Dictionary
Humans naturally separate into groups, and the medical field is no exception. Being a member of a likeminded group, such as one’s specialty, can improve self-esteem and provide social organization: it feels good to identify with people we admire. Through culture, these specialty-based groups implicitly and explicitly guide and encourage positive attributes or behaviors like a hospitalist’s thoroughness or an emergency medicine physician’s steady management of unstable patients. Our specialties also provide support and understanding in challenging times.
Despite these positive aspects, such divisions can negatively affect interprofessional relationships when our specialties become siloed. A potential side-effect of building up ourselves and our own groups is that we can implicitly put others down. For example, a hospitalist who spends extra time on the phone regularly updating each patient’s family will appropriately take pride in their practice, but over time this can also lead to an unreasonable assumption that physicians in other departments with different routines are not as committed to outstanding communication.
These rigid separations facilitate the fundamental attribution error, the tendency to ascribe a problem or disagreement to a colleague’s substandard character or ability. Imagine that the aforementioned hospitalist’s phone call delays a response to an admission page from the emergency room. The emergency medicine physician, who is waiting to sign out the admission while simultaneously managing many sick and complex patients, could assume the hospitalist is being disrespectful, rather than also working hard to provide the best care. Our siloed specialty identities can lead us to imagine the worst in each other and exacerbate intergroup conflict.1
Silos in medicine also adversely affect patients. Poor communication and lack of information-sharing across disciplines can lead to medical error2 and stifle dissemination of safer practices.3 Further, the unintentional disparaging of other medical specialties undermines the confidence our patients have in all of us; a patient within earshot of the hospitalist expressing annoyance at the “impatient” emergency medicine physician who “won’t stop paging,” or the emergency medicine physician complaining about the hospitalist who “refuses to call back,” will lose trust in each of their providers.
We suggest three steps to reduce the negative impact of specialty silos in medicine:
- Get to know each other personally. Friendly conversation during work hours and social interaction outside the hospital can inoculate against interspecialty conflict by putting a human face on our colleagues. The resultant relationships make it easier to work together and see things from another’s perspective.
- Emphasize our shared affiliations.4 The greater the salience of a mutual identity as “healthcare providers,” the more likely we are to recognize each other’s unique contributions and question the stereotypes we imagine about one another.
- Consider projects across specialties. Interdepartmental data-sharing and joint meetings, including educational conferences, can facilitate situational awareness, synergy, and efficient problem-solving.
Our medical specialties will continue to group together. While these groups can be a source of strength and meaning, silos can interfere with professional alliances and effective patient care. Mitigating the harmful effects of silos can benefit all of us and our patients.
Authors’ note: This article was previously published using the term “tribalism,” which we have since learned is derogatory to Indigenous Americans and others. We apologize for any harm. We have retracted and republished the article without this language. We appreciate readers teaching us how to choose better words so all people feel respected and valued.
1. Fiol CM, Pratt MG, O’Connor EJ. Managing intractable identity conflicts. Acad Management Rev. 2009;34(1):32-55. https://doi.org/10.5465/amr.2009.35713276
2. Horowitz LI, Meredith T, Schuur JD, et al. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6): 701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
3. Paine, LA, Baker DR, Rosenstein B, Pronovost PJ. The Johns Hopkins Hospital: identifying and addressing risks and safety issues. JT Comm J Qual Saf. 2004;30(10):543-550. https://doi.org/10.1016/s1549-3741(04)30064-x
4. Burford B. Group processes in medical education: learning from social identity theory. Med Educ. 2012;46(2):143-152. https://doi.org/10.1111/j.1365-2923.2011.04099.x
1. Fiol CM, Pratt MG, O’Connor EJ. Managing intractable identity conflicts. Acad Management Rev. 2009;34(1):32-55. https://doi.org/10.5465/amr.2009.35713276
2. Horowitz LI, Meredith T, Schuur JD, et al. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6): 701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
3. Paine, LA, Baker DR, Rosenstein B, Pronovost PJ. The Johns Hopkins Hospital: identifying and addressing risks and safety issues. JT Comm J Qual Saf. 2004;30(10):543-550. https://doi.org/10.1016/s1549-3741(04)30064-x
4. Burford B. Group processes in medical education: learning from social identity theory. Med Educ. 2012;46(2):143-152. https://doi.org/10.1111/j.1365-2923.2011.04099.x
© 2021 Society of Hospital Medicine
Morning Discharges and Patient Length of Stay in Inpatient General Internal Medicine
There is substantial interest in improving patient flow and reducing hospital length of stay (LOS).1-4 Impaired hospital flow may negatively impact both patient satisfaction and safety through, for example, emergency department (ED) overcrowding.5,6 Impaired hospital flow is associated with downstream effects on patient care, hospital costs, and availability of beds.7-9
A number of quality-improvement interventions aim to improve patient flow, including efforts to increase the number of discharges that occur before noon.10,11 Morning discharges have been hypothesized to free hospital beds earlier, thus reducing ED wait times for incoming patients and increasing beds for elective surgeries.11 Morning discharges may also be more predictable for staff and patients. However, it is unclear whether efforts to increase the number of morning discharges have a negative impact on inpatient LOS by incentivizing physicians to keep patients in the hospital for an extra night to facilitate discharge in the early morning rather than the late afternoon. Morning discharges have been associated with both increased12 and decreased LOS.10,11,13-15
The purpose of this study was to examine the associations between morning discharges and ED LOS and hospital LOS in general internal medicine (GIM) at seven hospitals. GIM patients represent nearly 40% of ED admissions to a hospital,16 and thus are an important determinant of patient flow through the ED and hospital. We hypothesized that patients who were admitted to GIM on days with more morning discharges would have shorter ED LOS and hospital LOS.
METHODS
Design, Setting, and Participants
This was a retrospective cohort study conducted using the General Medicine Inpatient Initiative (GEMINI) clinical dataset.16 The dataset includes all GIM admissions at seven large hospital sites in Toronto and Mississauga, Ontario, Canada. These include five academic hospitals and two community-based teaching hospitals. Each hospital is publicly funded and provides tertiary and/or quaternary care to diverse multiethnic populations. Research ethics board approval was obtained from all participating sites.
GIM care is delivered by several interdisciplinary clinical teams functioning in parallel. Attending physicians are predominantly internists who practice as hospitalists in discrete service blocks, typically lasting 2 weeks at a time. Although GIM patients are preferentially admitted to GIM wards, participating hospitals did not have strict policies regarding cohorting GIM patients to specific wards (ie, holding patients in ED until a specific bed becomes available) that would confound the association between morning discharge and ED wait times. Approximately 75% of GIM patients are cared for on dedicated GIM wards at participating hospitals, with the remainder cared for on other medical or surgical wards.
We included all hospitalized patients who were admitted to hospital and discharged from GIM between April 1, 2010, and October 31, 2017, from the seven GEMINI hospitals. We included only patients admitted through the ED. As such, we did not include elective admissions or interfacility transfers who would not experience ED wait times. We excluded patients who were discharged without a provincial health insurance number (N = 2,169; 1.1% of total sample) because they could not be linked across visits to measure readmissions.
Data Source
The GEMINI dataset has been rigorously validated and previously described in detail.16 GEMINI collects both administrative health data reported to the Canadian Institute for Health Information (including data about patient demographics, comorbidities, and discharge destination) as well as electronic clinical data extracted from hospital computer systems (including attending physicians, in-hospital patient room transfers, and laboratory test results). Data are collected for each individual hospital encounter, and the provincial health insurance number is used to link patients across encounters.
Exposures and Outcomes
The two primary outcomes were ED LOS and hospital LOS. ED LOS was calculated as the difference between the time from triage by nursing staff to a patient’s exit from the ED, measured in hours. We also examined 30-day readmission to GIM at any participating hospital as a balancing measure against premature discharges and inpatient mortality because it could modify hospital LOS.
Patient Characteristics
Baseline patient characteristics were measured, including age, sex, Charlson Comorbidity Index score,17 day of admission (categorized as weekend/holiday or weekday), time of admission to hospital (
Statistical Analysis
The study population and physician characteristics were summarized with descriptive statistics. The balance of baseline patient characteristics across morning discharge quartiles was assessed using standardized differences. A standardized difference of less than 0.1 reflects good balance.20
Unadjusted estimates of patient outcomes were reported across morning discharge quartiles. To model the overall association between morning discharge and outcomes, the number of morning GIM discharges on the day of admission was subtracted from the mean number of morning discharges at each hospital and considered as a continuous exposure. We used generalized linear mixed models to estimate the effect of morning discharges on patient outcomes. We fit negative binomial regression models with log link to examine the association between the number of morning discharges (centered by subtracting the hospital mean) and the two main outcomes, ED LOS and hospital LOS. Given the overdispersion of the study population due to the unequal mean and variance, a negative binomial model was preferred over a Poisson regression, as the mean and variance were not equal.21 For our secondary outcomes of binary measures (30-day readmission and morality), we fit logistic regression models. Adjustment for multiple comparisons was not performed.
Multivariable analysis was conducted to adjust for the baseline characteristics described above as well as the total number of GIM discharges on the day of admission and GIM census on the day of admission. Hospital and study month (to account for secular time trends) were included as fixed effects, and patients and admitting physicians were included as crossed random effects to account for the nested structure of admissions within patients and admissions within physicians within hospitals.
A sensitivity analysis was performed to assess for nonlinear associations between morning discharges and the four outcomes (hospital LOS, ED LOS, in-hospital mortality, and readmission) by inputting the term as a restricted cubic spline, with up to five knots
RESULTS
Study Population and Patient Characteristics
The study population consisted of 189,781 hospitalizations involving 115,630 unique patients. The median patient age was 73 years (interquartile range [IQR], 57-84), 50.3% were female, 43.8% had a high Charlson Comorbidity Index score, and 11.1% were admitted to GIM in the prior 30 days (Table 1). The median ED LOS was 14.5 hours (IQR, 10.0-23.1), and the mean was 18.1 hours (SD, 12.2). The median hospital LOS was 4.6 days (IQR, 2.4-9.0), and the mean was 8.6 days (SD, 18.7).
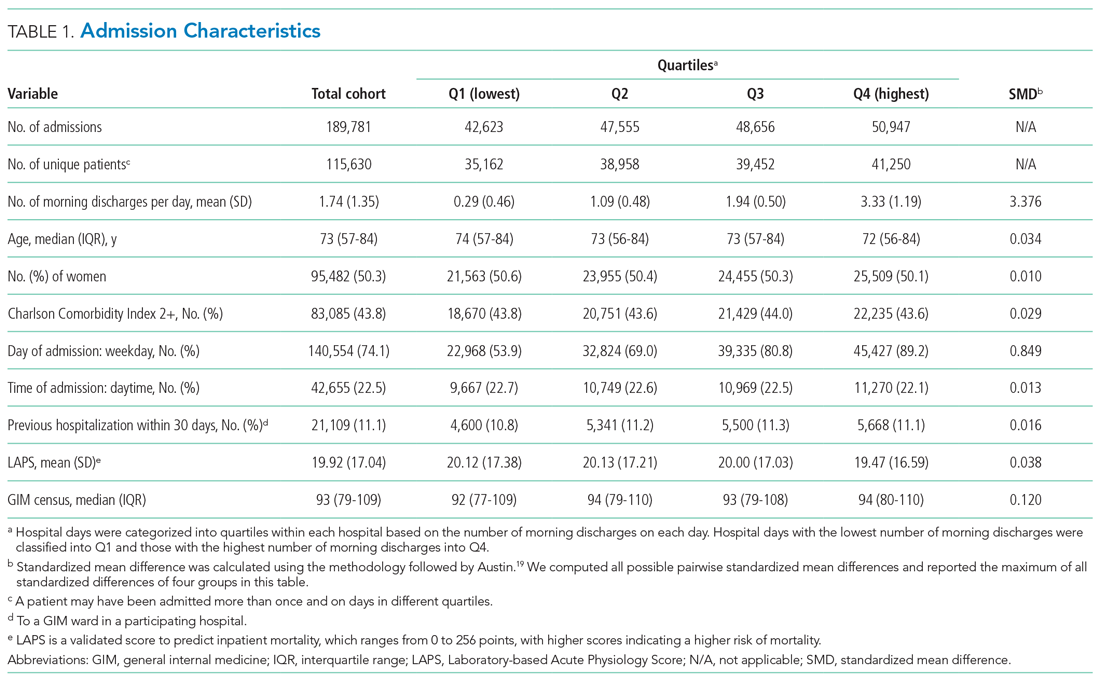
In total, 36,043 (19.0%) discharges occurred between 8:00
Outcomes
Unadjusted clinical outcomes by number of morning discharges are presented in Table 2. The median unadjusted ED LOS was 14.4 (SD, 14.1), 14.3 (SD, 13.2), 14.5 (SD, 13.0), and 14.8 (SD, 13.0) hours for the first to fourth quartiles (fewest to largest number of morning discharges), respectively. The median unadjusted hospital LOS was 4.6 (SD, 6.5), 4.6 (SD, 6.9), 4.7 (SD, 6.4), and 4.6 (SD, 6.4) days for the first to fourth quartiles, respectively.

Unadjusted inpatient mortality was 6.1%, 5.5%, 5.5%, and 5.2% across the first to fourth quartiles, respectively. Unadjusted 30-day readmission to GIM was 12.2%, 12.6%, 12.6%, and 12.5% across the first to fourth quartiles, respectively.
After multivariable adjustment, there was no significant association between morning discharge and hospital LOS (aRR, 1.000; 95% CI, 0.996-1.000; P = .997), ED LOS (aRR, 0.999; 95% CI, 0.997-1.000; P = .307), in-hospital mortality (aRR, 0.967; 95% CI, 0.920-1.020; P =.183), or 30-day readmission (aRR, 1.010; 95% CI, 0.991-1.020; P = .471) (Table 3, Appendix Table 2, Appendix Table 3, Appendix Table 4, Appendix Table 5). When examining each hospital separately, we found that morning discharge was significantly associated with hospital LOS at only one hospital (Hospital D; aRR, 0.981; 95% CI, 0.966-0.996; P = .013). Morning discharge was statistically significantly associated with ED LOS at three hospitals (A, B, and C), but the aRR was at least 0.99 in all three cases (Table 4).
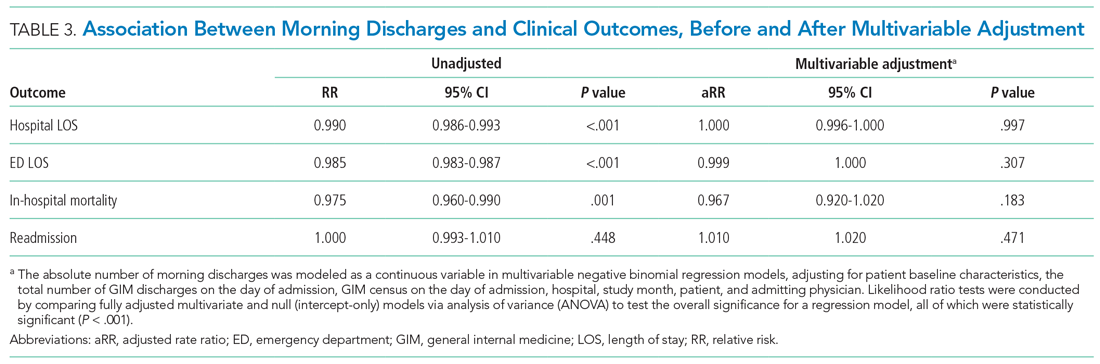
In sensitivity analyses, we found no improvements in model fit when adding spline terms to the model, suggesting no significant nonlinear associations between morning discharges and the outcomes of interest.
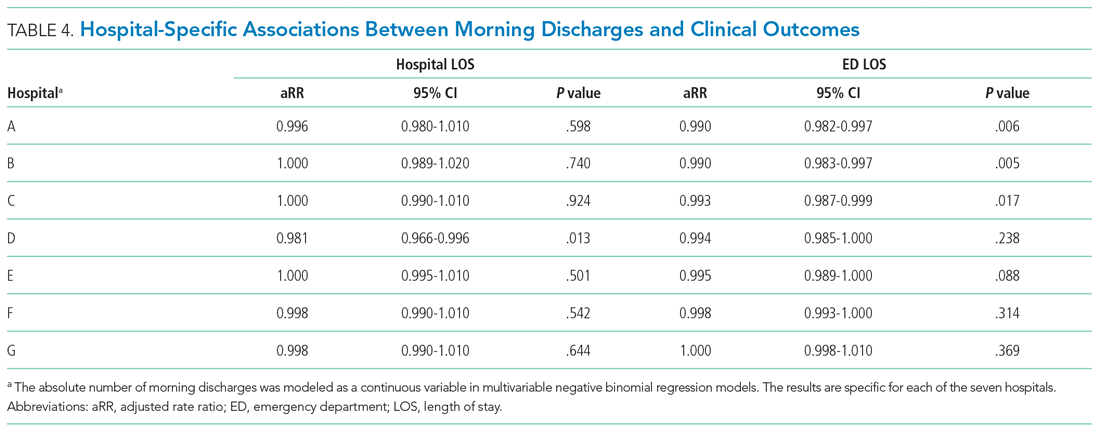
DISCUSSION
This large multicenter cohort study found no significant overall association between the number of morning discharges and ED or hospital LOS in GIM. At one hospital, there was a 1.9% reduction in adjusted ED LOS for every additional morning discharge, but no difference in hospital LOS. We also did not observe differences in readmission or inpatient mortality associated with the number of morning discharges. Our observational findings suggest that there is unlikely to be a strong association between morning discharge and patient throughput in GIM. Given that there may be other downstream benefits of morning discharge, such as freeing beds for daytime surgeries,23 further research is needed to determine the effectiveness of specific interventions.
Several studies have posited morning discharge as a method of improving both patient care and hospital flow metrics.10,11,13-15,23 Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creating deadlines for discharge orders.24-29 Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust. Werthemier et al10 found a 9.0% reduction of observed to expected LOS associated with increasing the number of early discharges. However, a response article suggested that their findings were confounded by other hospital initiatives, such as allocation of medical and social services to weekends.30 Other observational studies have concluded that hospital LOS is not affected by the number of morning discharges, but this research has been limited by single-center analysis and relatively smaller sample sizes.12 Our study further calls into question the association between morning discharge and patient throughput.
An additional reason for the controversy is that physicians may actively work to discharge patients late in the day to avoid an additional night in hospital. A qualitative study by Minichiello et al31 evaluated staff perceptions regarding afternoon discharges. Physicians and medical students believed that afternoon discharges were a result of waiting for test results and procedures, with staff aiming to discharge patients immediately after obtaining results or finishing necessary procedures. As such, there are concerns that incentivizing morning discharge may lead physicians in the opposite direction, to consciously or unconsciously keep patients overnight in order to facilitate an early morning discharge.30
Our study’s greatest strength was the large sample size over 7 years at seven hospitals in two cities, including both academic and community hospitals with different models of care. To our knowledge, this is the first cohort study that has analyzed the association between early discharge and LOS using multiple centers. To avoid the confounding and reverse causality that may exist when examining the relationship between LOS and morning discharge at the patient level (eg, patients who stay in hospital longer may have more “planned” discharges and leave in the morning), we examined the association based on variation across different days within the GIM service of each hospital. Further, we included robust risk adjustment using clinical and laboratory data. Finally, since our study included a diverse patient population served by participating centers in a system with universal insurance for hospital care, our findings are likely generalizable to other urban and suburban hospitals.
There are several important limitations of our analysis. First, we could only include GIM patients, who represent nearly 40% of ED admissions to hospital at participating centers. A more holistic analysis across all hospital services could be justified; however, given that many quality improvement initiatives occur at the level of a single hospital service, we felt our approach would be informative for future research and improvement efforts. Approximately 75% of GIM patients at participating hospitals were cared for on a GIM ward, with 25% cared for on off-service units. We were unable to include the total hospital census in our models, and this could affect LOS and waiting times for GIM patients, particularly those admitted to off-service units. GIM census is likely highly correlated with hospital census, and we were able to adjust for this. Nevertheless, this remains an important potential source of unmeasured confounding. Second, we did not model the effects of morning discharges from GIM on patient-flow measures for non-GIM patients. Given the lack of effects for GIM patients, who would be more likely to be directly affected, it is unlikely that large effects would be seen for other hospital patients, but we did not measure effects on surgical delays or cancellations, for example.23 Third, we report 30-day readmission to GIM at participating hospitals only, rather than all readmissions. However, prior research in our region demonstrated that 82% of hospital readmissions occur to the same site.32 Thus, our measure, which includes admission to any participating hospital, likely captures more than 80% of all readmissions, and this was a secondary outcome in our analysis. Finally, qualitative metrics, such as patient or provider satisfaction, were not measured in our study. Earlier discharge may impact patient care in other ways by being more predictable for staff, improving bed allocation for daytime procedures, making medication pick-ups easier to arrange, or making consultations with allied health services more convenient.11,28,33 Conversely, if pressured to discharge before noon, providers may feel rushed to complete tasks and may face disruptions to typical workflow.24 As such, future research is needed to provide a more complete understanding of the impact of early-morning discharge beyond hospital flow.
CONCLUSION
The number of morning discharges was not significantly associated with shorter ED LOS or hospital LOS for GIM patients. Our observational findings suggest that increasing morning discharges alone may not substantially improve patient flow in GIM. Further research is needed to evaluate specific morning discharge interventions and assess hospital-wide effects.
1. Trzeciak S, Rivers EP. Emergency department overcrowding in the United States: an emerging threat to patient safety and public health. Emerg Med J. 2003;20(5):402-405. https://doi.org/10.1136/emj.20.5.402
2. McKenna P, Heslin SM, Viccellio P, Mallon WK, Hernandez C, Morley EJ. Emergency department and hospital crowding: causes, consequences, and cures. Clin Exp Emerg Med. 2019;6(3):189-195. https://doi.org/10.15441/ceem.18.022
3. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. https://doi.org/10.1111/j.1553-2712.2008.00295.x
4. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. https://doi.org/10.1016/s0196-0644(00)70105-3
5. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. https://doi.org/10.1111/j.1553-2712.2008.00200.x
6. Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106-115. https://doi.org/10.1111/jnu.1205
7. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. https://doi.org/10.1001/jama.2010.748
8. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. https://doi.org/ 10.1002/14651858.CD006632.pub2
9. Zodda D, Underwood J. Improving emergency department throughput: evidence-based strategies aimed at reducing boarding and overcrowding. Phys Leadership J. 2019;6(3):70-73.
10. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. https://doi.org/10.1002/jhm.2154
11. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. https://doi.org/10.1097/NNA.0000000000000418
12. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. https://doi.org/10.1002/jhm.2529
13. Patel H, Morduchowicz S, Mourad M. Using a systematic framework of interventions to improve early discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. https://doi.org/10.1016/j.jcjq.2016.12.003
14. El-Eid GR, Kaddoum R, Tamim H, Hitti EA. Improving hospital discharge time: a successful implementation of Six Sigma methodology. Medicine (Baltimore). 2015;94(12):e633. https://doi.org/10.1097/MD.0000000000000633
15. Mathews KS, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
16. Verma AA, Pasricha SV, Jung HY, et al. Assessing the quality of clinical and administrative data extracted from hospitals: the General Medicine Inpatient Initiative (GEMINI) experience. J Am Med Inform Assoc. 2021; 28(3):578-587. doi: 10.1093/jamia/ocaa225.
17. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(60:676-682. https://doi.org/10.1093/aje/kwq433
18. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6
19. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(60:1228-1234. https://doi.org/10.1080/03610910902859574
20. van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798-803. https://doi.org/10.1016/j.jclinepi.2009.08.020
21. Hilbe JM. Negative binomial regression. In: Modeling Count Data. Cambridge University Press. 2014:126-160.
22. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer; 2015.
23. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. https://doi.org/10.1097/QMH.0000000000000049
24. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
25. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. https://doi.org/10.1080/21548331.2016.1254559
26. Karling A, Tang KW. Discharge before noon: a study in a medical emergency ward. 2015. Accessed February 11, 2021. http://publications.lib.chalmers.se/records/fulltext/231873/231873.pdf
27. Mathews K, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
28. Goodson AS, DeGuzman, PB, Honeycutt A, Summy C, Manly F. Total joint replacement discharge brunch: meeting patient education needs and a hospital initiative of discharge by noon. Orthop Nurs. 2014;33(3):159-162. https://doi.org/10.1097/NOR.0000000000000048
29. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26(2):142-146. https://doi.org/10.1097/01.HCM.0000268617.33491.60
30. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. https://doi.org/10.1016/j.amjmed.2014.12.011
31. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255.
32. Staples JA, Thiruchelvam D, Redelmeier DA. Site of hospital readmission and mortality: a population-based retrospective cohort study. CMAJ Open. 2014;2:E77-E85. https://doi.org/10.9778/cmajo.20130053
33. Bowles KH, Foust JB, Naylor MD. Hospital discharge referral decision making: a multidisciplinary perspective. Appl Nurs Res. 2003;16(3):134-143. https://doi.org/10.1016/s0897-1897(03)00048-x
There is substantial interest in improving patient flow and reducing hospital length of stay (LOS).1-4 Impaired hospital flow may negatively impact both patient satisfaction and safety through, for example, emergency department (ED) overcrowding.5,6 Impaired hospital flow is associated with downstream effects on patient care, hospital costs, and availability of beds.7-9
A number of quality-improvement interventions aim to improve patient flow, including efforts to increase the number of discharges that occur before noon.10,11 Morning discharges have been hypothesized to free hospital beds earlier, thus reducing ED wait times for incoming patients and increasing beds for elective surgeries.11 Morning discharges may also be more predictable for staff and patients. However, it is unclear whether efforts to increase the number of morning discharges have a negative impact on inpatient LOS by incentivizing physicians to keep patients in the hospital for an extra night to facilitate discharge in the early morning rather than the late afternoon. Morning discharges have been associated with both increased12 and decreased LOS.10,11,13-15
The purpose of this study was to examine the associations between morning discharges and ED LOS and hospital LOS in general internal medicine (GIM) at seven hospitals. GIM patients represent nearly 40% of ED admissions to a hospital,16 and thus are an important determinant of patient flow through the ED and hospital. We hypothesized that patients who were admitted to GIM on days with more morning discharges would have shorter ED LOS and hospital LOS.
METHODS
Design, Setting, and Participants
This was a retrospective cohort study conducted using the General Medicine Inpatient Initiative (GEMINI) clinical dataset.16 The dataset includes all GIM admissions at seven large hospital sites in Toronto and Mississauga, Ontario, Canada. These include five academic hospitals and two community-based teaching hospitals. Each hospital is publicly funded and provides tertiary and/or quaternary care to diverse multiethnic populations. Research ethics board approval was obtained from all participating sites.
GIM care is delivered by several interdisciplinary clinical teams functioning in parallel. Attending physicians are predominantly internists who practice as hospitalists in discrete service blocks, typically lasting 2 weeks at a time. Although GIM patients are preferentially admitted to GIM wards, participating hospitals did not have strict policies regarding cohorting GIM patients to specific wards (ie, holding patients in ED until a specific bed becomes available) that would confound the association between morning discharge and ED wait times. Approximately 75% of GIM patients are cared for on dedicated GIM wards at participating hospitals, with the remainder cared for on other medical or surgical wards.
We included all hospitalized patients who were admitted to hospital and discharged from GIM between April 1, 2010, and October 31, 2017, from the seven GEMINI hospitals. We included only patients admitted through the ED. As such, we did not include elective admissions or interfacility transfers who would not experience ED wait times. We excluded patients who were discharged without a provincial health insurance number (N = 2,169; 1.1% of total sample) because they could not be linked across visits to measure readmissions.
Data Source
The GEMINI dataset has been rigorously validated and previously described in detail.16 GEMINI collects both administrative health data reported to the Canadian Institute for Health Information (including data about patient demographics, comorbidities, and discharge destination) as well as electronic clinical data extracted from hospital computer systems (including attending physicians, in-hospital patient room transfers, and laboratory test results). Data are collected for each individual hospital encounter, and the provincial health insurance number is used to link patients across encounters.
Exposures and Outcomes
The two primary outcomes were ED LOS and hospital LOS. ED LOS was calculated as the difference between the time from triage by nursing staff to a patient’s exit from the ED, measured in hours. We also examined 30-day readmission to GIM at any participating hospital as a balancing measure against premature discharges and inpatient mortality because it could modify hospital LOS.
Patient Characteristics
Baseline patient characteristics were measured, including age, sex, Charlson Comorbidity Index score,17 day of admission (categorized as weekend/holiday or weekday), time of admission to hospital (
Statistical Analysis
The study population and physician characteristics were summarized with descriptive statistics. The balance of baseline patient characteristics across morning discharge quartiles was assessed using standardized differences. A standardized difference of less than 0.1 reflects good balance.20
Unadjusted estimates of patient outcomes were reported across morning discharge quartiles. To model the overall association between morning discharge and outcomes, the number of morning GIM discharges on the day of admission was subtracted from the mean number of morning discharges at each hospital and considered as a continuous exposure. We used generalized linear mixed models to estimate the effect of morning discharges on patient outcomes. We fit negative binomial regression models with log link to examine the association between the number of morning discharges (centered by subtracting the hospital mean) and the two main outcomes, ED LOS and hospital LOS. Given the overdispersion of the study population due to the unequal mean and variance, a negative binomial model was preferred over a Poisson regression, as the mean and variance were not equal.21 For our secondary outcomes of binary measures (30-day readmission and morality), we fit logistic regression models. Adjustment for multiple comparisons was not performed.
Multivariable analysis was conducted to adjust for the baseline characteristics described above as well as the total number of GIM discharges on the day of admission and GIM census on the day of admission. Hospital and study month (to account for secular time trends) were included as fixed effects, and patients and admitting physicians were included as crossed random effects to account for the nested structure of admissions within patients and admissions within physicians within hospitals.
A sensitivity analysis was performed to assess for nonlinear associations between morning discharges and the four outcomes (hospital LOS, ED LOS, in-hospital mortality, and readmission) by inputting the term as a restricted cubic spline, with up to five knots
RESULTS
Study Population and Patient Characteristics
The study population consisted of 189,781 hospitalizations involving 115,630 unique patients. The median patient age was 73 years (interquartile range [IQR], 57-84), 50.3% were female, 43.8% had a high Charlson Comorbidity Index score, and 11.1% were admitted to GIM in the prior 30 days (Table 1). The median ED LOS was 14.5 hours (IQR, 10.0-23.1), and the mean was 18.1 hours (SD, 12.2). The median hospital LOS was 4.6 days (IQR, 2.4-9.0), and the mean was 8.6 days (SD, 18.7).

In total, 36,043 (19.0%) discharges occurred between 8:00
Outcomes
Unadjusted clinical outcomes by number of morning discharges are presented in Table 2. The median unadjusted ED LOS was 14.4 (SD, 14.1), 14.3 (SD, 13.2), 14.5 (SD, 13.0), and 14.8 (SD, 13.0) hours for the first to fourth quartiles (fewest to largest number of morning discharges), respectively. The median unadjusted hospital LOS was 4.6 (SD, 6.5), 4.6 (SD, 6.9), 4.7 (SD, 6.4), and 4.6 (SD, 6.4) days for the first to fourth quartiles, respectively.

Unadjusted inpatient mortality was 6.1%, 5.5%, 5.5%, and 5.2% across the first to fourth quartiles, respectively. Unadjusted 30-day readmission to GIM was 12.2%, 12.6%, 12.6%, and 12.5% across the first to fourth quartiles, respectively.
After multivariable adjustment, there was no significant association between morning discharge and hospital LOS (aRR, 1.000; 95% CI, 0.996-1.000; P = .997), ED LOS (aRR, 0.999; 95% CI, 0.997-1.000; P = .307), in-hospital mortality (aRR, 0.967; 95% CI, 0.920-1.020; P =.183), or 30-day readmission (aRR, 1.010; 95% CI, 0.991-1.020; P = .471) (Table 3, Appendix Table 2, Appendix Table 3, Appendix Table 4, Appendix Table 5). When examining each hospital separately, we found that morning discharge was significantly associated with hospital LOS at only one hospital (Hospital D; aRR, 0.981; 95% CI, 0.966-0.996; P = .013). Morning discharge was statistically significantly associated with ED LOS at three hospitals (A, B, and C), but the aRR was at least 0.99 in all three cases (Table 4).

In sensitivity analyses, we found no improvements in model fit when adding spline terms to the model, suggesting no significant nonlinear associations between morning discharges and the outcomes of interest.

DISCUSSION
This large multicenter cohort study found no significant overall association between the number of morning discharges and ED or hospital LOS in GIM. At one hospital, there was a 1.9% reduction in adjusted ED LOS for every additional morning discharge, but no difference in hospital LOS. We also did not observe differences in readmission or inpatient mortality associated with the number of morning discharges. Our observational findings suggest that there is unlikely to be a strong association between morning discharge and patient throughput in GIM. Given that there may be other downstream benefits of morning discharge, such as freeing beds for daytime surgeries,23 further research is needed to determine the effectiveness of specific interventions.
Several studies have posited morning discharge as a method of improving both patient care and hospital flow metrics.10,11,13-15,23 Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creating deadlines for discharge orders.24-29 Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust. Werthemier et al10 found a 9.0% reduction of observed to expected LOS associated with increasing the number of early discharges. However, a response article suggested that their findings were confounded by other hospital initiatives, such as allocation of medical and social services to weekends.30 Other observational studies have concluded that hospital LOS is not affected by the number of morning discharges, but this research has been limited by single-center analysis and relatively smaller sample sizes.12 Our study further calls into question the association between morning discharge and patient throughput.
An additional reason for the controversy is that physicians may actively work to discharge patients late in the day to avoid an additional night in hospital. A qualitative study by Minichiello et al31 evaluated staff perceptions regarding afternoon discharges. Physicians and medical students believed that afternoon discharges were a result of waiting for test results and procedures, with staff aiming to discharge patients immediately after obtaining results or finishing necessary procedures. As such, there are concerns that incentivizing morning discharge may lead physicians in the opposite direction, to consciously or unconsciously keep patients overnight in order to facilitate an early morning discharge.30
Our study’s greatest strength was the large sample size over 7 years at seven hospitals in two cities, including both academic and community hospitals with different models of care. To our knowledge, this is the first cohort study that has analyzed the association between early discharge and LOS using multiple centers. To avoid the confounding and reverse causality that may exist when examining the relationship between LOS and morning discharge at the patient level (eg, patients who stay in hospital longer may have more “planned” discharges and leave in the morning), we examined the association based on variation across different days within the GIM service of each hospital. Further, we included robust risk adjustment using clinical and laboratory data. Finally, since our study included a diverse patient population served by participating centers in a system with universal insurance for hospital care, our findings are likely generalizable to other urban and suburban hospitals.
There are several important limitations of our analysis. First, we could only include GIM patients, who represent nearly 40% of ED admissions to hospital at participating centers. A more holistic analysis across all hospital services could be justified; however, given that many quality improvement initiatives occur at the level of a single hospital service, we felt our approach would be informative for future research and improvement efforts. Approximately 75% of GIM patients at participating hospitals were cared for on a GIM ward, with 25% cared for on off-service units. We were unable to include the total hospital census in our models, and this could affect LOS and waiting times for GIM patients, particularly those admitted to off-service units. GIM census is likely highly correlated with hospital census, and we were able to adjust for this. Nevertheless, this remains an important potential source of unmeasured confounding. Second, we did not model the effects of morning discharges from GIM on patient-flow measures for non-GIM patients. Given the lack of effects for GIM patients, who would be more likely to be directly affected, it is unlikely that large effects would be seen for other hospital patients, but we did not measure effects on surgical delays or cancellations, for example.23 Third, we report 30-day readmission to GIM at participating hospitals only, rather than all readmissions. However, prior research in our region demonstrated that 82% of hospital readmissions occur to the same site.32 Thus, our measure, which includes admission to any participating hospital, likely captures more than 80% of all readmissions, and this was a secondary outcome in our analysis. Finally, qualitative metrics, such as patient or provider satisfaction, were not measured in our study. Earlier discharge may impact patient care in other ways by being more predictable for staff, improving bed allocation for daytime procedures, making medication pick-ups easier to arrange, or making consultations with allied health services more convenient.11,28,33 Conversely, if pressured to discharge before noon, providers may feel rushed to complete tasks and may face disruptions to typical workflow.24 As such, future research is needed to provide a more complete understanding of the impact of early-morning discharge beyond hospital flow.
CONCLUSION
The number of morning discharges was not significantly associated with shorter ED LOS or hospital LOS for GIM patients. Our observational findings suggest that increasing morning discharges alone may not substantially improve patient flow in GIM. Further research is needed to evaluate specific morning discharge interventions and assess hospital-wide effects.
There is substantial interest in improving patient flow and reducing hospital length of stay (LOS).1-4 Impaired hospital flow may negatively impact both patient satisfaction and safety through, for example, emergency department (ED) overcrowding.5,6 Impaired hospital flow is associated with downstream effects on patient care, hospital costs, and availability of beds.7-9
A number of quality-improvement interventions aim to improve patient flow, including efforts to increase the number of discharges that occur before noon.10,11 Morning discharges have been hypothesized to free hospital beds earlier, thus reducing ED wait times for incoming patients and increasing beds for elective surgeries.11 Morning discharges may also be more predictable for staff and patients. However, it is unclear whether efforts to increase the number of morning discharges have a negative impact on inpatient LOS by incentivizing physicians to keep patients in the hospital for an extra night to facilitate discharge in the early morning rather than the late afternoon. Morning discharges have been associated with both increased12 and decreased LOS.10,11,13-15
The purpose of this study was to examine the associations between morning discharges and ED LOS and hospital LOS in general internal medicine (GIM) at seven hospitals. GIM patients represent nearly 40% of ED admissions to a hospital,16 and thus are an important determinant of patient flow through the ED and hospital. We hypothesized that patients who were admitted to GIM on days with more morning discharges would have shorter ED LOS and hospital LOS.
METHODS
Design, Setting, and Participants
This was a retrospective cohort study conducted using the General Medicine Inpatient Initiative (GEMINI) clinical dataset.16 The dataset includes all GIM admissions at seven large hospital sites in Toronto and Mississauga, Ontario, Canada. These include five academic hospitals and two community-based teaching hospitals. Each hospital is publicly funded and provides tertiary and/or quaternary care to diverse multiethnic populations. Research ethics board approval was obtained from all participating sites.
GIM care is delivered by several interdisciplinary clinical teams functioning in parallel. Attending physicians are predominantly internists who practice as hospitalists in discrete service blocks, typically lasting 2 weeks at a time. Although GIM patients are preferentially admitted to GIM wards, participating hospitals did not have strict policies regarding cohorting GIM patients to specific wards (ie, holding patients in ED until a specific bed becomes available) that would confound the association between morning discharge and ED wait times. Approximately 75% of GIM patients are cared for on dedicated GIM wards at participating hospitals, with the remainder cared for on other medical or surgical wards.
We included all hospitalized patients who were admitted to hospital and discharged from GIM between April 1, 2010, and October 31, 2017, from the seven GEMINI hospitals. We included only patients admitted through the ED. As such, we did not include elective admissions or interfacility transfers who would not experience ED wait times. We excluded patients who were discharged without a provincial health insurance number (N = 2,169; 1.1% of total sample) because they could not be linked across visits to measure readmissions.
Data Source
The GEMINI dataset has been rigorously validated and previously described in detail.16 GEMINI collects both administrative health data reported to the Canadian Institute for Health Information (including data about patient demographics, comorbidities, and discharge destination) as well as electronic clinical data extracted from hospital computer systems (including attending physicians, in-hospital patient room transfers, and laboratory test results). Data are collected for each individual hospital encounter, and the provincial health insurance number is used to link patients across encounters.
Exposures and Outcomes
The two primary outcomes were ED LOS and hospital LOS. ED LOS was calculated as the difference between the time from triage by nursing staff to a patient’s exit from the ED, measured in hours. We also examined 30-day readmission to GIM at any participating hospital as a balancing measure against premature discharges and inpatient mortality because it could modify hospital LOS.
Patient Characteristics
Baseline patient characteristics were measured, including age, sex, Charlson Comorbidity Index score,17 day of admission (categorized as weekend/holiday or weekday), time of admission to hospital (
Statistical Analysis
The study population and physician characteristics were summarized with descriptive statistics. The balance of baseline patient characteristics across morning discharge quartiles was assessed using standardized differences. A standardized difference of less than 0.1 reflects good balance.20
Unadjusted estimates of patient outcomes were reported across morning discharge quartiles. To model the overall association between morning discharge and outcomes, the number of morning GIM discharges on the day of admission was subtracted from the mean number of morning discharges at each hospital and considered as a continuous exposure. We used generalized linear mixed models to estimate the effect of morning discharges on patient outcomes. We fit negative binomial regression models with log link to examine the association between the number of morning discharges (centered by subtracting the hospital mean) and the two main outcomes, ED LOS and hospital LOS. Given the overdispersion of the study population due to the unequal mean and variance, a negative binomial model was preferred over a Poisson regression, as the mean and variance were not equal.21 For our secondary outcomes of binary measures (30-day readmission and morality), we fit logistic regression models. Adjustment for multiple comparisons was not performed.
Multivariable analysis was conducted to adjust for the baseline characteristics described above as well as the total number of GIM discharges on the day of admission and GIM census on the day of admission. Hospital and study month (to account for secular time trends) were included as fixed effects, and patients and admitting physicians were included as crossed random effects to account for the nested structure of admissions within patients and admissions within physicians within hospitals.
A sensitivity analysis was performed to assess for nonlinear associations between morning discharges and the four outcomes (hospital LOS, ED LOS, in-hospital mortality, and readmission) by inputting the term as a restricted cubic spline, with up to five knots
RESULTS
Study Population and Patient Characteristics
The study population consisted of 189,781 hospitalizations involving 115,630 unique patients. The median patient age was 73 years (interquartile range [IQR], 57-84), 50.3% were female, 43.8% had a high Charlson Comorbidity Index score, and 11.1% were admitted to GIM in the prior 30 days (Table 1). The median ED LOS was 14.5 hours (IQR, 10.0-23.1), and the mean was 18.1 hours (SD, 12.2). The median hospital LOS was 4.6 days (IQR, 2.4-9.0), and the mean was 8.6 days (SD, 18.7).

In total, 36,043 (19.0%) discharges occurred between 8:00
Outcomes
Unadjusted clinical outcomes by number of morning discharges are presented in Table 2. The median unadjusted ED LOS was 14.4 (SD, 14.1), 14.3 (SD, 13.2), 14.5 (SD, 13.0), and 14.8 (SD, 13.0) hours for the first to fourth quartiles (fewest to largest number of morning discharges), respectively. The median unadjusted hospital LOS was 4.6 (SD, 6.5), 4.6 (SD, 6.9), 4.7 (SD, 6.4), and 4.6 (SD, 6.4) days for the first to fourth quartiles, respectively.

Unadjusted inpatient mortality was 6.1%, 5.5%, 5.5%, and 5.2% across the first to fourth quartiles, respectively. Unadjusted 30-day readmission to GIM was 12.2%, 12.6%, 12.6%, and 12.5% across the first to fourth quartiles, respectively.
After multivariable adjustment, there was no significant association between morning discharge and hospital LOS (aRR, 1.000; 95% CI, 0.996-1.000; P = .997), ED LOS (aRR, 0.999; 95% CI, 0.997-1.000; P = .307), in-hospital mortality (aRR, 0.967; 95% CI, 0.920-1.020; P =.183), or 30-day readmission (aRR, 1.010; 95% CI, 0.991-1.020; P = .471) (Table 3, Appendix Table 2, Appendix Table 3, Appendix Table 4, Appendix Table 5). When examining each hospital separately, we found that morning discharge was significantly associated with hospital LOS at only one hospital (Hospital D; aRR, 0.981; 95% CI, 0.966-0.996; P = .013). Morning discharge was statistically significantly associated with ED LOS at three hospitals (A, B, and C), but the aRR was at least 0.99 in all three cases (Table 4).

In sensitivity analyses, we found no improvements in model fit when adding spline terms to the model, suggesting no significant nonlinear associations between morning discharges and the outcomes of interest.

DISCUSSION
This large multicenter cohort study found no significant overall association between the number of morning discharges and ED or hospital LOS in GIM. At one hospital, there was a 1.9% reduction in adjusted ED LOS for every additional morning discharge, but no difference in hospital LOS. We also did not observe differences in readmission or inpatient mortality associated with the number of morning discharges. Our observational findings suggest that there is unlikely to be a strong association between morning discharge and patient throughput in GIM. Given that there may be other downstream benefits of morning discharge, such as freeing beds for daytime surgeries,23 further research is needed to determine the effectiveness of specific interventions.
Several studies have posited morning discharge as a method of improving both patient care and hospital flow metrics.10,11,13-15,23 Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creating deadlines for discharge orders.24-29 Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust. Werthemier et al10 found a 9.0% reduction of observed to expected LOS associated with increasing the number of early discharges. However, a response article suggested that their findings were confounded by other hospital initiatives, such as allocation of medical and social services to weekends.30 Other observational studies have concluded that hospital LOS is not affected by the number of morning discharges, but this research has been limited by single-center analysis and relatively smaller sample sizes.12 Our study further calls into question the association between morning discharge and patient throughput.
An additional reason for the controversy is that physicians may actively work to discharge patients late in the day to avoid an additional night in hospital. A qualitative study by Minichiello et al31 evaluated staff perceptions regarding afternoon discharges. Physicians and medical students believed that afternoon discharges were a result of waiting for test results and procedures, with staff aiming to discharge patients immediately after obtaining results or finishing necessary procedures. As such, there are concerns that incentivizing morning discharge may lead physicians in the opposite direction, to consciously or unconsciously keep patients overnight in order to facilitate an early morning discharge.30
Our study’s greatest strength was the large sample size over 7 years at seven hospitals in two cities, including both academic and community hospitals with different models of care. To our knowledge, this is the first cohort study that has analyzed the association between early discharge and LOS using multiple centers. To avoid the confounding and reverse causality that may exist when examining the relationship between LOS and morning discharge at the patient level (eg, patients who stay in hospital longer may have more “planned” discharges and leave in the morning), we examined the association based on variation across different days within the GIM service of each hospital. Further, we included robust risk adjustment using clinical and laboratory data. Finally, since our study included a diverse patient population served by participating centers in a system with universal insurance for hospital care, our findings are likely generalizable to other urban and suburban hospitals.
There are several important limitations of our analysis. First, we could only include GIM patients, who represent nearly 40% of ED admissions to hospital at participating centers. A more holistic analysis across all hospital services could be justified; however, given that many quality improvement initiatives occur at the level of a single hospital service, we felt our approach would be informative for future research and improvement efforts. Approximately 75% of GIM patients at participating hospitals were cared for on a GIM ward, with 25% cared for on off-service units. We were unable to include the total hospital census in our models, and this could affect LOS and waiting times for GIM patients, particularly those admitted to off-service units. GIM census is likely highly correlated with hospital census, and we were able to adjust for this. Nevertheless, this remains an important potential source of unmeasured confounding. Second, we did not model the effects of morning discharges from GIM on patient-flow measures for non-GIM patients. Given the lack of effects for GIM patients, who would be more likely to be directly affected, it is unlikely that large effects would be seen for other hospital patients, but we did not measure effects on surgical delays or cancellations, for example.23 Third, we report 30-day readmission to GIM at participating hospitals only, rather than all readmissions. However, prior research in our region demonstrated that 82% of hospital readmissions occur to the same site.32 Thus, our measure, which includes admission to any participating hospital, likely captures more than 80% of all readmissions, and this was a secondary outcome in our analysis. Finally, qualitative metrics, such as patient or provider satisfaction, were not measured in our study. Earlier discharge may impact patient care in other ways by being more predictable for staff, improving bed allocation for daytime procedures, making medication pick-ups easier to arrange, or making consultations with allied health services more convenient.11,28,33 Conversely, if pressured to discharge before noon, providers may feel rushed to complete tasks and may face disruptions to typical workflow.24 As such, future research is needed to provide a more complete understanding of the impact of early-morning discharge beyond hospital flow.
CONCLUSION
The number of morning discharges was not significantly associated with shorter ED LOS or hospital LOS for GIM patients. Our observational findings suggest that increasing morning discharges alone may not substantially improve patient flow in GIM. Further research is needed to evaluate specific morning discharge interventions and assess hospital-wide effects.
1. Trzeciak S, Rivers EP. Emergency department overcrowding in the United States: an emerging threat to patient safety and public health. Emerg Med J. 2003;20(5):402-405. https://doi.org/10.1136/emj.20.5.402
2. McKenna P, Heslin SM, Viccellio P, Mallon WK, Hernandez C, Morley EJ. Emergency department and hospital crowding: causes, consequences, and cures. Clin Exp Emerg Med. 2019;6(3):189-195. https://doi.org/10.15441/ceem.18.022
3. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. https://doi.org/10.1111/j.1553-2712.2008.00295.x
4. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. https://doi.org/10.1016/s0196-0644(00)70105-3
5. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. https://doi.org/10.1111/j.1553-2712.2008.00200.x
6. Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106-115. https://doi.org/10.1111/jnu.1205
7. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. https://doi.org/10.1001/jama.2010.748
8. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. https://doi.org/ 10.1002/14651858.CD006632.pub2
9. Zodda D, Underwood J. Improving emergency department throughput: evidence-based strategies aimed at reducing boarding and overcrowding. Phys Leadership J. 2019;6(3):70-73.
10. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. https://doi.org/10.1002/jhm.2154
11. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. https://doi.org/10.1097/NNA.0000000000000418
12. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. https://doi.org/10.1002/jhm.2529
13. Patel H, Morduchowicz S, Mourad M. Using a systematic framework of interventions to improve early discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. https://doi.org/10.1016/j.jcjq.2016.12.003
14. El-Eid GR, Kaddoum R, Tamim H, Hitti EA. Improving hospital discharge time: a successful implementation of Six Sigma methodology. Medicine (Baltimore). 2015;94(12):e633. https://doi.org/10.1097/MD.0000000000000633
15. Mathews KS, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
16. Verma AA, Pasricha SV, Jung HY, et al. Assessing the quality of clinical and administrative data extracted from hospitals: the General Medicine Inpatient Initiative (GEMINI) experience. J Am Med Inform Assoc. 2021; 28(3):578-587. doi: 10.1093/jamia/ocaa225.
17. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(60:676-682. https://doi.org/10.1093/aje/kwq433
18. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6
19. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(60:1228-1234. https://doi.org/10.1080/03610910902859574
20. van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798-803. https://doi.org/10.1016/j.jclinepi.2009.08.020
21. Hilbe JM. Negative binomial regression. In: Modeling Count Data. Cambridge University Press. 2014:126-160.
22. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer; 2015.
23. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. https://doi.org/10.1097/QMH.0000000000000049
24. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
25. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. https://doi.org/10.1080/21548331.2016.1254559
26. Karling A, Tang KW. Discharge before noon: a study in a medical emergency ward. 2015. Accessed February 11, 2021. http://publications.lib.chalmers.se/records/fulltext/231873/231873.pdf
27. Mathews K, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
28. Goodson AS, DeGuzman, PB, Honeycutt A, Summy C, Manly F. Total joint replacement discharge brunch: meeting patient education needs and a hospital initiative of discharge by noon. Orthop Nurs. 2014;33(3):159-162. https://doi.org/10.1097/NOR.0000000000000048
29. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26(2):142-146. https://doi.org/10.1097/01.HCM.0000268617.33491.60
30. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. https://doi.org/10.1016/j.amjmed.2014.12.011
31. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255.
32. Staples JA, Thiruchelvam D, Redelmeier DA. Site of hospital readmission and mortality: a population-based retrospective cohort study. CMAJ Open. 2014;2:E77-E85. https://doi.org/10.9778/cmajo.20130053
33. Bowles KH, Foust JB, Naylor MD. Hospital discharge referral decision making: a multidisciplinary perspective. Appl Nurs Res. 2003;16(3):134-143. https://doi.org/10.1016/s0897-1897(03)00048-x
1. Trzeciak S, Rivers EP. Emergency department overcrowding in the United States: an emerging threat to patient safety and public health. Emerg Med J. 2003;20(5):402-405. https://doi.org/10.1136/emj.20.5.402
2. McKenna P, Heslin SM, Viccellio P, Mallon WK, Hernandez C, Morley EJ. Emergency department and hospital crowding: causes, consequences, and cures. Clin Exp Emerg Med. 2019;6(3):189-195. https://doi.org/10.15441/ceem.18.022
3. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. https://doi.org/10.1111/j.1553-2712.2008.00295.x
4. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. https://doi.org/10.1016/s0196-0644(00)70105-3
5. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. https://doi.org/10.1111/j.1553-2712.2008.00200.x
6. Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106-115. https://doi.org/10.1111/jnu.1205
7. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. https://doi.org/10.1001/jama.2010.748
8. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. https://doi.org/ 10.1002/14651858.CD006632.pub2
9. Zodda D, Underwood J. Improving emergency department throughput: evidence-based strategies aimed at reducing boarding and overcrowding. Phys Leadership J. 2019;6(3):70-73.
10. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. https://doi.org/10.1002/jhm.2154
11. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. https://doi.org/10.1097/NNA.0000000000000418
12. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. https://doi.org/10.1002/jhm.2529
13. Patel H, Morduchowicz S, Mourad M. Using a systematic framework of interventions to improve early discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. https://doi.org/10.1016/j.jcjq.2016.12.003
14. El-Eid GR, Kaddoum R, Tamim H, Hitti EA. Improving hospital discharge time: a successful implementation of Six Sigma methodology. Medicine (Baltimore). 2015;94(12):e633. https://doi.org/10.1097/MD.0000000000000633
15. Mathews KS, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
16. Verma AA, Pasricha SV, Jung HY, et al. Assessing the quality of clinical and administrative data extracted from hospitals: the General Medicine Inpatient Initiative (GEMINI) experience. J Am Med Inform Assoc. 2021; 28(3):578-587. doi: 10.1093/jamia/ocaa225.
17. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(60:676-682. https://doi.org/10.1093/aje/kwq433
18. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6
19. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(60:1228-1234. https://doi.org/10.1080/03610910902859574
20. van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798-803. https://doi.org/10.1016/j.jclinepi.2009.08.020
21. Hilbe JM. Negative binomial regression. In: Modeling Count Data. Cambridge University Press. 2014:126-160.
22. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer; 2015.
23. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. https://doi.org/10.1097/QMH.0000000000000049
24. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
25. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. https://doi.org/10.1080/21548331.2016.1254559
26. Karling A, Tang KW. Discharge before noon: a study in a medical emergency ward. 2015. Accessed February 11, 2021. http://publications.lib.chalmers.se/records/fulltext/231873/231873.pdf
27. Mathews K, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
28. Goodson AS, DeGuzman, PB, Honeycutt A, Summy C, Manly F. Total joint replacement discharge brunch: meeting patient education needs and a hospital initiative of discharge by noon. Orthop Nurs. 2014;33(3):159-162. https://doi.org/10.1097/NOR.0000000000000048
29. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26(2):142-146. https://doi.org/10.1097/01.HCM.0000268617.33491.60
30. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. https://doi.org/10.1016/j.amjmed.2014.12.011
31. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255.
32. Staples JA, Thiruchelvam D, Redelmeier DA. Site of hospital readmission and mortality: a population-based retrospective cohort study. CMAJ Open. 2014;2:E77-E85. https://doi.org/10.9778/cmajo.20130053
33. Bowles KH, Foust JB, Naylor MD. Hospital discharge referral decision making: a multidisciplinary perspective. Appl Nurs Res. 2003;16(3):134-143. https://doi.org/10.1016/s0897-1897(03)00048-x
© 2021 Society of Hospital Medicine
A Resident-Led Intervention to Increase Initiation of Buprenorphine Maintenance for Hospitalized Patients With Opioid Use Disorder
Nearly 48,000 Americans died from overdoses involving opioids in 2018, continuing a national crisis that has led to 446,000 deaths since 1999.1 Annually, opioids are responsible for more than 500,000 admissions, approximately 1% of all hospitalizations, costing the United States nearly $15 billion.2,3 Among hospitalized patients, chronic opioid use is associated with increased mortality, severe infectious complications, and higher rates of readmission.4 Opioid use disorder (OUD) is a chronic, relapsing medical condition with biopsychosocial origins and significant morbidity and mortality.5 Opioid agonist therapy (OAT) with buprenorphine or methadone maintenance, the evidence-based standard of treatment, reduces the mortality rate by half, decreases overdoses and hospital readmissions, and improves retention in care.6-10
OAT maintenance refers to using buprenorphine or methadone for long-term treatment of OUD rather than for acute treatment of opioid withdrawal. Despite evidence supporting OAT maintenance, clinicians start medications for only 11% to 15% of hospitalized patients with OUD, depending on practice contexts.11,12 Three significant barriers—stigma, insufficient clinician education, and restrictive regulations—prevent clinicians from starting OAT.13 Clinicians who do not have the Drug Enforcement Administration (DEA)–issued DATA-2000 waiver (X-waiver) for outpatient prescribing can order buprenorphine for admitted patients but cannot prescribe it at discharge.14 In hospitals where they exist, addiction medicine consult services offer primary teams guidance on pharmacotherapy, leading to reduced hospital readmissions and increased engagement in outpatient addiction treatment.15-17 However, in most hospitals around the country, such specialty services do not exist.18 In some hospitals without addiction medicine consult services, hospitalists with expertise in OUD have started assisting primary teams in starting OAT, but to our knowledge, no prior studies have described the impact of these interventions on patients or clinician experience with OAT.19
This quality improvement project aimed to increase the rate at which internal medicine resident teams at Johns Hopkins Hospital (JHH) in Baltimore, Maryland, started hospitalized patients with OUD on buprenorphine maintenance. We hypothesized that resident education and measures to increase the availability of X-waivered physicians would increase the rate of initiating buprenorphine maintenance. We additionally hypothesized that these interventions would increase knowledge about and comfort with buprenorphine across the residency. This represents the first study to examine the effects of clinician education and a team of X-waivered residents and hospitalists who assist in starting buprenorphine maintenance in a hospital without an addiction medicine consult service.
METHODS
Setting
This study took place from July 2018 to June 2019 at JHH, a large, academic, urban hospital in Baltimore. Prior to the intervention, internal medicine residents at JHH commonly used short courses of buprenorphine to treat withdrawal, but they did not have access to hospital-specific resources to assist with starting maintenance OAT. During the study period, JHH had a Substance Use Disorders team staffed by peer recovery specialists that could be consulted by hospitalists and residents to provide psychosocial support and link admitted patients to treatment after discharge. There were no providers on the team to guide pharmacotherapy or to write discharge buprenorphine prescriptions. The Osler Medical Residency Training Program at JHH has 140 internal medicine residents and 16 combined medicine-pediatrics residents. All residents receive 1 hour of formal education about opioid use disorder annually. In addition, 28 of those 156 residents, those in the Urban Health Primary Care track, spend 1 month on an Addiction Medicine rotation in which they complete the 8-hour training required to receive the X-waiver. Those residents are encouraged to apply for the X-waiver once they obtain a medical license subsidized by a Health Resources & Services Administration (HRSA) grant. Four internal medicine attending physicians on teaching services and one resident had X-waivers prior to the intervention.
Intervention
In November 2018, we administered a survey to residents to identify barriers to starting buprenorphine maintenance and to measure knowledge and confidence with using buprenorphine for OUD (Appendix Figure 1 and Figure 2). We focused on buprenorphine because providers at JHH were familiar with this medication and because Baltimore has widespread access to buprenorphine, with more than 490 local buprenorphine providers.20 Five residents piloted the survey and provided feedback. We then administered the survey to all internal medicine and medicine-pediatrics residents. Based on the results, we developed a targeted educational conference and also created the Buprenorphine Bridge Team (BBT).
In January 2019, we presented the educational conference for residents devoted to the use of buprenorphine for OUD and introduced the BBT. The conference started with a patient testimonial and included peer recovery specialists, pharmacists, nurses, and social workers. We summarized the evidence for buprenorphine and offered a practical guide to start treatment in a one-page protocol. This protocol included guidance on selecting patients, shared decision-making around OUD treatment, avoiding precipitated withdrawal, dosing buprenorphine, and establishing follow-up (Appendix Figure 3). We asked for input on this protocol from nursing leadership, social work teams, and peer recovery specialists. Dosing was adapted from the Guidelines from the American Society of Addiction Medicine, with expert input from physicians from the Addiction Medicine Consult service at Johns Hopkins Bayview Medical Center, also in Baltimore.5 We instructed residents to obtain discharge buprenorphine prescriptions from an X-waivered physician on their team or from the newly established BBT. We asked resident teams to set up a postdischarge appointment for patients with an X-waivered provider, either in a community practice or at the JHH After Care Clinic, a transitional care clinic for discharged patients.21
The BBT is a resident-led group of X-waivered JHH residents and hospitalists who volunteer to write discharge buprenorphine prescriptions for patients. The BBT serves to ensure primary teams have access to an X-waivered prescriber. It is not a consult service. We asked primary teams to contact the BBT after initiating buprenorphine and after securing a follow-up appointment. In response to each request, a member of the BBT reviews the patient chart, confirms the follow-up plan, writes a prescription for buprenorphine along with intranasal naloxone, and leaves a brief note. During the 6-month postintervention period, the team consisted of three residents and three hospitalist attendings. Each week, two members (residents or attendings) staffed the team Monday to Friday, 8
In May 2019, 5 months after the education session and implementation of the BBT, we administered a follow-up survey.
Outcomes
As a secondary outcome, we measured engagement in OUD treatment after discharge by calculating the proportion of patients started on buprenorphine who filled a buprenorphine prescription within 30 days after discharge. We chose 30 days based on the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS) measure for engagement of treatment for alcohol and other drugs.23 We obtained the data from the Chesapeake Regional Information System for our Patients (CRISP) Prescription Drug Monitoring Program, which monitors all prescriptions for controlled substances dispensed in Maryland and five neighboring states. As a balancing measure, we counted patients newly started on methadone maintenance for OUD before and after the intervention. Additional secondary process outcomes included frequency of BBT requests, the volume of buprenorphine prescriptions written by the team, and time required to complete a BBT request.
Clinician-level outcomes, measured with electronically administered pre- and postintervention surveys to residents, included knowledge about and comfort with buprenorphine. Of the 16 questions in the pre- and postimplementation surveys, we analyzed the 6 questions concerning knowledge and comfort that remained identical in the pre- and postintervention surveys and used 5-point Likert scale responses. As an incentive, we randomly distributed three $50 gift cards to survey completers.
Analysis
We used an interrupted time series analysis to evaluate the association between the intervention bundle and a change in the rate that medical teams started patients with OUD on buprenorphine maintenance. This approach allowed us to control for preintervention trends. To evaluate the impact of our interventions, our pre- and postintervention periods include the same residents during the 2018-2019 academic year. Both periods consisted of twelve 2-week intervals (preintervention: July 26, 2019, to January 9, 2019; postintervention: January 10, 2019, to June 26, 2019).
To evaluate for changes in engagement in OUD treatment after discharge, we used two-sample t tests. To evaluate for changes in resident-reported comfort and knowledge with initiating buprenorphine maintenance, we used Wilcoxon rank sum tests for survey data and Wilcoxon signed rank tests for paired data. All analyses employed two-sided P values with statistical significance evaluated at the .05 alpha level. We analyzed data using R version 3.6.3 (Foundation for Statistical Computing). The Institutional Review Board at JHH reviewed and approved the study protocol as a quality improvement project (IRB00193365).
Before the intervention, 13 of the 30 patients (40%) newly started on buprenorphine maintenance during their admission filled a follow-up buprenorphine prescription within 30 days of discharge. After the intervention, 31 of 64 patients (46%) filled a buprenorphine prescription within 30 days (P = .612). Two patients were started on methadone maintenance, one prior to and one after the intervention.
During the 6-month postintervention period, the BBT received 75 requests and wrote 70 prescriptions for buprenorphine. The median time required to complete a BBT request was 15 minutes (minimum, 5 minutes; maximum, 60 minutes).
Of 156 internal medicine and medicine-pediatrics residents, 89 residents (57%) completed the baseline survey and 66 residents (42%) completed the follow-up survey. Forty residents completed both surveys. After the intervention, residents were significantly more likely to feel comfortable dosing buprenorphine (P < .0001) and counseling patients about its use (P = .0237) and were more likely to report ease of establishing follow-up (P < .0001). Self-reported knowledge about preventing precipitated withdrawal increased significantly (P = .0191), as did knowledge about the effectiveness of buprenorphine (P = .0003) independent of formal drug counseling (P = .0066) (Table). Paired survey data also found statistically significant results for all questions except those about preventing precipitated withdrawal and efficacy. For the latter, respondents who completed both surveys were more knowledgeable before the intervention than the overall group that completed the baseline survey (Appendix Table).
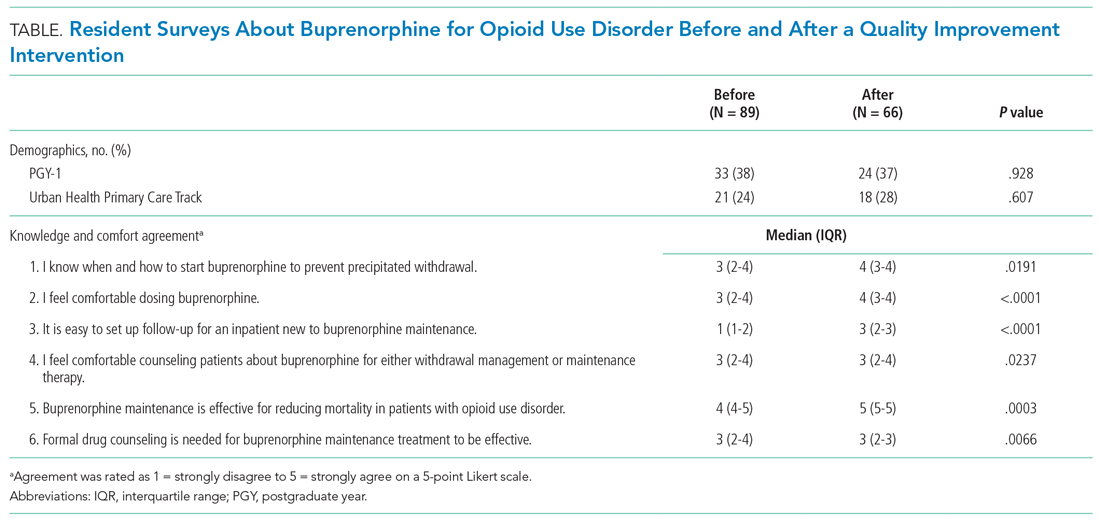
This study shows how a resident-led quality improvement project comprising clinician education and implementation of a novel BBT was associated with an increased rate of starting buprenorphine maintenance in hospitalized patients with OUD and improved resident knowledge about and comfort with buprenorphine. To our knowledge, this is the first study demonstrating how education and a team of X-waivered generalists can help primary teams initiate and discharge patients on buprenorphine maintenance in a hospital without an addiction medicine consult service.
Prior to the intervention, resident internal medicine teams at JHH started 10% of hospitalized patients with OUD on buprenorphine maintenance, consistent with prior studies showing rates of 11% to 15% for initiating OAT for hospitalized patients.11,12 After the intervention, the rate of initiating buprenorphine maintenance more than doubled, rising to 24% of eligible patients. Resident internal medicine teams at JHH started buprenorphine maintenance for 37 more patients over the 24-week postintervention period than would have been predicted prior to the intervention, or an additional three patients every 2 weeks.
Between 40% and 46% of hospitalized patients newly started on buprenorphine maintenance filled an outpatient buprenorphine prescription within 30 days of discharge. We are not aware of comparative data for 30-day follow-up for hospitalized patients newly started on buprenorphine maintenance. Data from other contexts show 5% to 10% of veterans were engaged in addiction treatment 30 days after initiation from inpatient or outpatient encounters. An analysis of an academic medical center in Oregon found engagement with an addiction medicine consult service increased after hospital engagement for patients with any substance use disorder from 23% to 39% using the 34-day HEDIS measure for engagement.17,24,25
The BBT required approximately 15 minutes per request and wrote an average of three prescriptions per week, demonstrating the feasibility of this approach and the high demand for this service. One strength of our approach is that residents gained experience starting buprenorphine independently using the aforementioned protocol instead of deferring to a full consult service. It is likely that this resident engagement in initiating longitudinal OUD care contributed to the success of this initiative, as did existing resident familiarity with using buprenorphine for opioid withdrawal.
This approach to resident education—promoting direct, first-person experience with medications in a clinical context—aligns with recommendations from a recent review about substance use disorder education for health professionals.26 Our interventions increased resident knowledge and comfort with buprenorphine, consistent with prior studies showing increased resident confidence in management of substance use disorders after curricular innovations.24,25
A few contextual features were essential for this project’s viability. Maryland allows American medical graduates to obtain a medical license after 1 year of postgraduate training. This allowed three residents to obtain X-waivers. These residents had access to HRSA funding to subsidize the expenses of applying for state licensure and DEA registration. BBT members volunteered their time while working on other services. Last, we were able to take advantage of buprenorphine-providing clinics in Baltimore, including the JHH After Care Clinic, to accept patients for follow-up appointments after discharge.
Limitations
The BBT required motivated clinicians willing to volunteer for additional clinical responsibilities during inpatient rotations and supportive faculty and residency leadership. Attending physicians, nurse practitioners, or physician assistants could staff a similar BBT in hospitals without residents or in hospitals where residents cannot obtain DEA registration. Crucially, other hospitals may not have access to practices with X-waivered physicians for outpatient follow-up. A recent study found X-waivered primary care physicians were less likely to be affiliated with hospital health systems. Other studies have shown limitations in access to buprenorphine at the county level based on geography and racial/ethnic segregation.27-29
Most patients hospitalized with OUD did not have ICD-10 codes associated with OUD. We addressed this by assuming patients had OUD if buprenorphine or methadone was ordered during their hospitalization, even if the medication was never administered. This may have overcounted patients prescribed these medications for indications other than OUD, and it may have undercounted patients with OUD for whom buprenorphine or methadone were never considered. The opioid withdrawal order set at JHH automatically offers an option to use buprenorphine to treat withdrawal. Patients with OUD for whom buprenorphine or methadone were never ordered likely did not experience withdrawal or were in withdrawal so mild that it escaped the attention of the team, which limits the generalizability of our intervention.
We identified several limitations to the internal validity of our study. First, we used a before-and-after study design without a control group. We could not ethically withhold access to evidence-based, mortality-reducing medications from patients. Without a control group, we cannot rule out the possibility that underlying temporal trends made residents more likely to start buprenorphine maintenance independent of our intervention. We attempted to control for unmeasured confounders by using an interrupted time series analysis to control for preintervention trends, comparing the same group of residents before and after our interventions, and selecting an intervention period during which residents were given only educational sessions and materials provided by our team. Our results may be biased by clustered data because certain residents may have been more likely to initiate buprenorphine, but these effects are likely marginal because resident schedules are balanced between outpatient and inpatient rotations during each 6-month period.
Finally, this project focused on buprenorphine, not on other medications for OUD, including methadone or naltrexone, or nonpharmacologic treatments for OUD.
Sustainability and Next Steps
Since the start of the BBT in January 2019, five additional PGY-2 residents obtained their medical licenses and X-waivers. These residents, with the support of two attending hospitalists, led the BBT and coordinated education sessions that were incorporated into the curriculum during the 2019-2020 academic year. These educational sessions will continue indefinitely. In 2020, JHH started an Addiction Medicine Consult Service staffed by physicians, NPs, and a pharmacist. The BBT continues to operate in conjunction with this service.
We found substantial variability in the rate of buprenorphine maintenance initiation despite our interventions. This is an area for future improvement. In a free-response prompt in our follow-up survey, residents requested additional education sessions and an order set to assist with initiation of buprenorphine. To address these gaps, three educational sessions were added, one of which included education on starting methadone maintenance therapy. We also added a new order set for starting buprenorphine maintenance. We hypothesize that these interventions will improve consistency.
In order for a similar program to be disseminated to other institutions, educational initiatives and a team of dedicated X-waivered prescribers are key. Materials to assist with this process are available in the Appendix.
CONCLUSION
This study shows how a resident-led intervention comprising clinician education and a team of X-waivered generalists was associated with improved treatment of OUD for hospitalized patients. We encourage residents and all clinicians at other hospitals without addiction medicine consult services to design, implement, and study similar interventions that directly increase the use of buprenorphine or methadone maintenance to treat OUD.
Preliminary results from this project were presented at the AMERSA National Conference on November 7, 2019.
1. Wilson N, Kariisa M, Seth P, Iv HS, Davis NL. Drug and opioid-involved overdose deaths – United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. http://dx.doi.org/10.15585/mmwr.mm6911a4
2. Berk J, Rogers KM, Wilson DJ, Thakrar A, Feldman L. Missed opportunities for treatment of opioid use disorder in the hospital setting: updating an outdated policy. J Hosp Med. 2020;15(10):619-621. https://doi.org/10.12788/jhm.3352
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
4. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. https://doi.org/10.1002/jhm.2113
5. Crotty K, Freedman KI, Kampman KM. Executive summary of the focused update of the ASAM national practice guideline for the treatment of opioid use disorder. J Addict Med. 2020;14(2):99-112. https://doi.org/10.1097/adm.0000000000000635
6. Leshner AI, Mancher M, eds. Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. https://www.nap.edu/catalog/25310
7. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357: j1550. https://doi.org/10.1136/bmj.j1550
8. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169(3):137-145. https://dx.doi.org/10.7326%2FM17-3107
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/nejmra1604339
10. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
11. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
12. Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: a pragmatic retrospective cohort analysis. J Gen Intern Med. 2020;35(8):2365-2374. https://doi.org/10.1007/s11606-020-05815-0
13. Madras BK, Ahmad NJ, Wen J, Sharfstein J; Prevention, Treatment, and Recovery Working Group of the Action Collaborative on Countering the U.S. Opioid Epidemic. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. NAM Perspectives. April 27, 2020. https://doi.org/10.31478/202004b
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the X Waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685
15. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/adm.0000000000000496
16. Weimer M, Morford K, Donroe J. Treatment of opioid use disorder in the acute hospital setting: a critical review of the literature (2014–2019). Curr Addict Rep. 2019;6(4):339-354.
17. Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J Gen Intern Med. 2019;34(12):2796-2803. https://doi.org/10.1007/s11606-019-05251-9
18. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;14(3):E1-E4. https://doi.org/10.12788/jhm.3311
19. Bottner R, Moriates C, Tirado C. The role of hospitalists in treating opioid use disorder. J Addict Med. 2020;14(2):178. https://doi.org/10.1097/adm.0000000000000545
20. Behavioral health treatment services locator. Substance Abuse and Mental Health Services Administration. Accessed May 14, 2020. https://findtreatment.samhsa.gov/
21. Groesbeck K, Whiteman LN, Stewart RW. Reducing readmission rates by improving transitional care. South Med J. 2015;108(12):758-760. https://doi.org/10.14423/smj.0000000000000376
22. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015 Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
23. Initiation and engagement of alcohol and other drug abuse or dependence treatment (IET). NCQA. Accessed April 20, 2020. https://www.ncqa.org/hedis/measures/initiation-and-engagement-of-alcohol-and-other-drug-abuse-or-dependence-treatment/
24. Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70-75. https://doi.org/10.1016/j.drugalcdep.2019.01.038
25. Harris AHS, Humphreys K, Finney JW. Veterans Affairs facility performance on Washington Circle indicators and casemix-adjusted effectiveness. J Subst Abuse Treat. 2007;33(4):333-339. https://doi.org/10.1016/j.jsat.2006.12.015
26. Muzyk A, Smothers ZPW, Andolsek KM, et al. Interprofessional substance use disorder education in health professions education programs: a scoping review. Acad Med. 2020;95(3):470-480. https://doi.org/10.1097/acm.0000000000003053
27. Saloner B, Lin L, Simon K. Geographic location of buprenorphine-waivered physicians and integration with health systems. J Subst Abuse Treat. 2020;115:108034. https://doi.org/10.1016/j.jsat.2020.108034
28. Jones CW, Christman Z, Smith CM, et al. Comparison between buprenorphine provider availability and opioid deaths among US counties. J Subst Abuse Treat. 2018;93:19-25. https://doi.org/10.1016/j.jsat.2018.07.008
29. Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. https://doi.org/10.1001/jamanetworkopen.2020.3711
Nearly 48,000 Americans died from overdoses involving opioids in 2018, continuing a national crisis that has led to 446,000 deaths since 1999.1 Annually, opioids are responsible for more than 500,000 admissions, approximately 1% of all hospitalizations, costing the United States nearly $15 billion.2,3 Among hospitalized patients, chronic opioid use is associated with increased mortality, severe infectious complications, and higher rates of readmission.4 Opioid use disorder (OUD) is a chronic, relapsing medical condition with biopsychosocial origins and significant morbidity and mortality.5 Opioid agonist therapy (OAT) with buprenorphine or methadone maintenance, the evidence-based standard of treatment, reduces the mortality rate by half, decreases overdoses and hospital readmissions, and improves retention in care.6-10
OAT maintenance refers to using buprenorphine or methadone for long-term treatment of OUD rather than for acute treatment of opioid withdrawal. Despite evidence supporting OAT maintenance, clinicians start medications for only 11% to 15% of hospitalized patients with OUD, depending on practice contexts.11,12 Three significant barriers—stigma, insufficient clinician education, and restrictive regulations—prevent clinicians from starting OAT.13 Clinicians who do not have the Drug Enforcement Administration (DEA)–issued DATA-2000 waiver (X-waiver) for outpatient prescribing can order buprenorphine for admitted patients but cannot prescribe it at discharge.14 In hospitals where they exist, addiction medicine consult services offer primary teams guidance on pharmacotherapy, leading to reduced hospital readmissions and increased engagement in outpatient addiction treatment.15-17 However, in most hospitals around the country, such specialty services do not exist.18 In some hospitals without addiction medicine consult services, hospitalists with expertise in OUD have started assisting primary teams in starting OAT, but to our knowledge, no prior studies have described the impact of these interventions on patients or clinician experience with OAT.19
This quality improvement project aimed to increase the rate at which internal medicine resident teams at Johns Hopkins Hospital (JHH) in Baltimore, Maryland, started hospitalized patients with OUD on buprenorphine maintenance. We hypothesized that resident education and measures to increase the availability of X-waivered physicians would increase the rate of initiating buprenorphine maintenance. We additionally hypothesized that these interventions would increase knowledge about and comfort with buprenorphine across the residency. This represents the first study to examine the effects of clinician education and a team of X-waivered residents and hospitalists who assist in starting buprenorphine maintenance in a hospital without an addiction medicine consult service.
METHODS
Setting
This study took place from July 2018 to June 2019 at JHH, a large, academic, urban hospital in Baltimore. Prior to the intervention, internal medicine residents at JHH commonly used short courses of buprenorphine to treat withdrawal, but they did not have access to hospital-specific resources to assist with starting maintenance OAT. During the study period, JHH had a Substance Use Disorders team staffed by peer recovery specialists that could be consulted by hospitalists and residents to provide psychosocial support and link admitted patients to treatment after discharge. There were no providers on the team to guide pharmacotherapy or to write discharge buprenorphine prescriptions. The Osler Medical Residency Training Program at JHH has 140 internal medicine residents and 16 combined medicine-pediatrics residents. All residents receive 1 hour of formal education about opioid use disorder annually. In addition, 28 of those 156 residents, those in the Urban Health Primary Care track, spend 1 month on an Addiction Medicine rotation in which they complete the 8-hour training required to receive the X-waiver. Those residents are encouraged to apply for the X-waiver once they obtain a medical license subsidized by a Health Resources & Services Administration (HRSA) grant. Four internal medicine attending physicians on teaching services and one resident had X-waivers prior to the intervention.
Intervention
In November 2018, we administered a survey to residents to identify barriers to starting buprenorphine maintenance and to measure knowledge and confidence with using buprenorphine for OUD (Appendix Figure 1 and Figure 2). We focused on buprenorphine because providers at JHH were familiar with this medication and because Baltimore has widespread access to buprenorphine, with more than 490 local buprenorphine providers.20 Five residents piloted the survey and provided feedback. We then administered the survey to all internal medicine and medicine-pediatrics residents. Based on the results, we developed a targeted educational conference and also created the Buprenorphine Bridge Team (BBT).
In January 2019, we presented the educational conference for residents devoted to the use of buprenorphine for OUD and introduced the BBT. The conference started with a patient testimonial and included peer recovery specialists, pharmacists, nurses, and social workers. We summarized the evidence for buprenorphine and offered a practical guide to start treatment in a one-page protocol. This protocol included guidance on selecting patients, shared decision-making around OUD treatment, avoiding precipitated withdrawal, dosing buprenorphine, and establishing follow-up (Appendix Figure 3). We asked for input on this protocol from nursing leadership, social work teams, and peer recovery specialists. Dosing was adapted from the Guidelines from the American Society of Addiction Medicine, with expert input from physicians from the Addiction Medicine Consult service at Johns Hopkins Bayview Medical Center, also in Baltimore.5 We instructed residents to obtain discharge buprenorphine prescriptions from an X-waivered physician on their team or from the newly established BBT. We asked resident teams to set up a postdischarge appointment for patients with an X-waivered provider, either in a community practice or at the JHH After Care Clinic, a transitional care clinic for discharged patients.21
The BBT is a resident-led group of X-waivered JHH residents and hospitalists who volunteer to write discharge buprenorphine prescriptions for patients. The BBT serves to ensure primary teams have access to an X-waivered prescriber. It is not a consult service. We asked primary teams to contact the BBT after initiating buprenorphine and after securing a follow-up appointment. In response to each request, a member of the BBT reviews the patient chart, confirms the follow-up plan, writes a prescription for buprenorphine along with intranasal naloxone, and leaves a brief note. During the 6-month postintervention period, the team consisted of three residents and three hospitalist attendings. Each week, two members (residents or attendings) staffed the team Monday to Friday, 8
In May 2019, 5 months after the education session and implementation of the BBT, we administered a follow-up survey.
Outcomes
As a secondary outcome, we measured engagement in OUD treatment after discharge by calculating the proportion of patients started on buprenorphine who filled a buprenorphine prescription within 30 days after discharge. We chose 30 days based on the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS) measure for engagement of treatment for alcohol and other drugs.23 We obtained the data from the Chesapeake Regional Information System for our Patients (CRISP) Prescription Drug Monitoring Program, which monitors all prescriptions for controlled substances dispensed in Maryland and five neighboring states. As a balancing measure, we counted patients newly started on methadone maintenance for OUD before and after the intervention. Additional secondary process outcomes included frequency of BBT requests, the volume of buprenorphine prescriptions written by the team, and time required to complete a BBT request.
Clinician-level outcomes, measured with electronically administered pre- and postintervention surveys to residents, included knowledge about and comfort with buprenorphine. Of the 16 questions in the pre- and postimplementation surveys, we analyzed the 6 questions concerning knowledge and comfort that remained identical in the pre- and postintervention surveys and used 5-point Likert scale responses. As an incentive, we randomly distributed three $50 gift cards to survey completers.
Analysis
We used an interrupted time series analysis to evaluate the association between the intervention bundle and a change in the rate that medical teams started patients with OUD on buprenorphine maintenance. This approach allowed us to control for preintervention trends. To evaluate the impact of our interventions, our pre- and postintervention periods include the same residents during the 2018-2019 academic year. Both periods consisted of twelve 2-week intervals (preintervention: July 26, 2019, to January 9, 2019; postintervention: January 10, 2019, to June 26, 2019).
To evaluate for changes in engagement in OUD treatment after discharge, we used two-sample t tests. To evaluate for changes in resident-reported comfort and knowledge with initiating buprenorphine maintenance, we used Wilcoxon rank sum tests for survey data and Wilcoxon signed rank tests for paired data. All analyses employed two-sided P values with statistical significance evaluated at the .05 alpha level. We analyzed data using R version 3.6.3 (Foundation for Statistical Computing). The Institutional Review Board at JHH reviewed and approved the study protocol as a quality improvement project (IRB00193365).

Before the intervention, 13 of the 30 patients (40%) newly started on buprenorphine maintenance during their admission filled a follow-up buprenorphine prescription within 30 days of discharge. After the intervention, 31 of 64 patients (46%) filled a buprenorphine prescription within 30 days (P = .612). Two patients were started on methadone maintenance, one prior to and one after the intervention.
During the 6-month postintervention period, the BBT received 75 requests and wrote 70 prescriptions for buprenorphine. The median time required to complete a BBT request was 15 minutes (minimum, 5 minutes; maximum, 60 minutes).
Of 156 internal medicine and medicine-pediatrics residents, 89 residents (57%) completed the baseline survey and 66 residents (42%) completed the follow-up survey. Forty residents completed both surveys. After the intervention, residents were significantly more likely to feel comfortable dosing buprenorphine (P < .0001) and counseling patients about its use (P = .0237) and were more likely to report ease of establishing follow-up (P < .0001). Self-reported knowledge about preventing precipitated withdrawal increased significantly (P = .0191), as did knowledge about the effectiveness of buprenorphine (P = .0003) independent of formal drug counseling (P = .0066) (Table). Paired survey data also found statistically significant results for all questions except those about preventing precipitated withdrawal and efficacy. For the latter, respondents who completed both surveys were more knowledgeable before the intervention than the overall group that completed the baseline survey (Appendix Table).

This study shows how a resident-led quality improvement project comprising clinician education and implementation of a novel BBT was associated with an increased rate of starting buprenorphine maintenance in hospitalized patients with OUD and improved resident knowledge about and comfort with buprenorphine. To our knowledge, this is the first study demonstrating how education and a team of X-waivered generalists can help primary teams initiate and discharge patients on buprenorphine maintenance in a hospital without an addiction medicine consult service.
Prior to the intervention, resident internal medicine teams at JHH started 10% of hospitalized patients with OUD on buprenorphine maintenance, consistent with prior studies showing rates of 11% to 15% for initiating OAT for hospitalized patients.11,12 After the intervention, the rate of initiating buprenorphine maintenance more than doubled, rising to 24% of eligible patients. Resident internal medicine teams at JHH started buprenorphine maintenance for 37 more patients over the 24-week postintervention period than would have been predicted prior to the intervention, or an additional three patients every 2 weeks.
Between 40% and 46% of hospitalized patients newly started on buprenorphine maintenance filled an outpatient buprenorphine prescription within 30 days of discharge. We are not aware of comparative data for 30-day follow-up for hospitalized patients newly started on buprenorphine maintenance. Data from other contexts show 5% to 10% of veterans were engaged in addiction treatment 30 days after initiation from inpatient or outpatient encounters. An analysis of an academic medical center in Oregon found engagement with an addiction medicine consult service increased after hospital engagement for patients with any substance use disorder from 23% to 39% using the 34-day HEDIS measure for engagement.17,24,25
The BBT required approximately 15 minutes per request and wrote an average of three prescriptions per week, demonstrating the feasibility of this approach and the high demand for this service. One strength of our approach is that residents gained experience starting buprenorphine independently using the aforementioned protocol instead of deferring to a full consult service. It is likely that this resident engagement in initiating longitudinal OUD care contributed to the success of this initiative, as did existing resident familiarity with using buprenorphine for opioid withdrawal.
This approach to resident education—promoting direct, first-person experience with medications in a clinical context—aligns with recommendations from a recent review about substance use disorder education for health professionals.26 Our interventions increased resident knowledge and comfort with buprenorphine, consistent with prior studies showing increased resident confidence in management of substance use disorders after curricular innovations.24,25
A few contextual features were essential for this project’s viability. Maryland allows American medical graduates to obtain a medical license after 1 year of postgraduate training. This allowed three residents to obtain X-waivers. These residents had access to HRSA funding to subsidize the expenses of applying for state licensure and DEA registration. BBT members volunteered their time while working on other services. Last, we were able to take advantage of buprenorphine-providing clinics in Baltimore, including the JHH After Care Clinic, to accept patients for follow-up appointments after discharge.
Limitations
The BBT required motivated clinicians willing to volunteer for additional clinical responsibilities during inpatient rotations and supportive faculty and residency leadership. Attending physicians, nurse practitioners, or physician assistants could staff a similar BBT in hospitals without residents or in hospitals where residents cannot obtain DEA registration. Crucially, other hospitals may not have access to practices with X-waivered physicians for outpatient follow-up. A recent study found X-waivered primary care physicians were less likely to be affiliated with hospital health systems. Other studies have shown limitations in access to buprenorphine at the county level based on geography and racial/ethnic segregation.27-29
Most patients hospitalized with OUD did not have ICD-10 codes associated with OUD. We addressed this by assuming patients had OUD if buprenorphine or methadone was ordered during their hospitalization, even if the medication was never administered. This may have overcounted patients prescribed these medications for indications other than OUD, and it may have undercounted patients with OUD for whom buprenorphine or methadone were never considered. The opioid withdrawal order set at JHH automatically offers an option to use buprenorphine to treat withdrawal. Patients with OUD for whom buprenorphine or methadone were never ordered likely did not experience withdrawal or were in withdrawal so mild that it escaped the attention of the team, which limits the generalizability of our intervention.
We identified several limitations to the internal validity of our study. First, we used a before-and-after study design without a control group. We could not ethically withhold access to evidence-based, mortality-reducing medications from patients. Without a control group, we cannot rule out the possibility that underlying temporal trends made residents more likely to start buprenorphine maintenance independent of our intervention. We attempted to control for unmeasured confounders by using an interrupted time series analysis to control for preintervention trends, comparing the same group of residents before and after our interventions, and selecting an intervention period during which residents were given only educational sessions and materials provided by our team. Our results may be biased by clustered data because certain residents may have been more likely to initiate buprenorphine, but these effects are likely marginal because resident schedules are balanced between outpatient and inpatient rotations during each 6-month period.
Finally, this project focused on buprenorphine, not on other medications for OUD, including methadone or naltrexone, or nonpharmacologic treatments for OUD.
Sustainability and Next Steps
Since the start of the BBT in January 2019, five additional PGY-2 residents obtained their medical licenses and X-waivers. These residents, with the support of two attending hospitalists, led the BBT and coordinated education sessions that were incorporated into the curriculum during the 2019-2020 academic year. These educational sessions will continue indefinitely. In 2020, JHH started an Addiction Medicine Consult Service staffed by physicians, NPs, and a pharmacist. The BBT continues to operate in conjunction with this service.
We found substantial variability in the rate of buprenorphine maintenance initiation despite our interventions. This is an area for future improvement. In a free-response prompt in our follow-up survey, residents requested additional education sessions and an order set to assist with initiation of buprenorphine. To address these gaps, three educational sessions were added, one of which included education on starting methadone maintenance therapy. We also added a new order set for starting buprenorphine maintenance. We hypothesize that these interventions will improve consistency.
In order for a similar program to be disseminated to other institutions, educational initiatives and a team of dedicated X-waivered prescribers are key. Materials to assist with this process are available in the Appendix.
CONCLUSION
This study shows how a resident-led intervention comprising clinician education and a team of X-waivered generalists was associated with improved treatment of OUD for hospitalized patients. We encourage residents and all clinicians at other hospitals without addiction medicine consult services to design, implement, and study similar interventions that directly increase the use of buprenorphine or methadone maintenance to treat OUD.
Preliminary results from this project were presented at the AMERSA National Conference on November 7, 2019.
Nearly 48,000 Americans died from overdoses involving opioids in 2018, continuing a national crisis that has led to 446,000 deaths since 1999.1 Annually, opioids are responsible for more than 500,000 admissions, approximately 1% of all hospitalizations, costing the United States nearly $15 billion.2,3 Among hospitalized patients, chronic opioid use is associated with increased mortality, severe infectious complications, and higher rates of readmission.4 Opioid use disorder (OUD) is a chronic, relapsing medical condition with biopsychosocial origins and significant morbidity and mortality.5 Opioid agonist therapy (OAT) with buprenorphine or methadone maintenance, the evidence-based standard of treatment, reduces the mortality rate by half, decreases overdoses and hospital readmissions, and improves retention in care.6-10
OAT maintenance refers to using buprenorphine or methadone for long-term treatment of OUD rather than for acute treatment of opioid withdrawal. Despite evidence supporting OAT maintenance, clinicians start medications for only 11% to 15% of hospitalized patients with OUD, depending on practice contexts.11,12 Three significant barriers—stigma, insufficient clinician education, and restrictive regulations—prevent clinicians from starting OAT.13 Clinicians who do not have the Drug Enforcement Administration (DEA)–issued DATA-2000 waiver (X-waiver) for outpatient prescribing can order buprenorphine for admitted patients but cannot prescribe it at discharge.14 In hospitals where they exist, addiction medicine consult services offer primary teams guidance on pharmacotherapy, leading to reduced hospital readmissions and increased engagement in outpatient addiction treatment.15-17 However, in most hospitals around the country, such specialty services do not exist.18 In some hospitals without addiction medicine consult services, hospitalists with expertise in OUD have started assisting primary teams in starting OAT, but to our knowledge, no prior studies have described the impact of these interventions on patients or clinician experience with OAT.19
This quality improvement project aimed to increase the rate at which internal medicine resident teams at Johns Hopkins Hospital (JHH) in Baltimore, Maryland, started hospitalized patients with OUD on buprenorphine maintenance. We hypothesized that resident education and measures to increase the availability of X-waivered physicians would increase the rate of initiating buprenorphine maintenance. We additionally hypothesized that these interventions would increase knowledge about and comfort with buprenorphine across the residency. This represents the first study to examine the effects of clinician education and a team of X-waivered residents and hospitalists who assist in starting buprenorphine maintenance in a hospital without an addiction medicine consult service.
METHODS
Setting
This study took place from July 2018 to June 2019 at JHH, a large, academic, urban hospital in Baltimore. Prior to the intervention, internal medicine residents at JHH commonly used short courses of buprenorphine to treat withdrawal, but they did not have access to hospital-specific resources to assist with starting maintenance OAT. During the study period, JHH had a Substance Use Disorders team staffed by peer recovery specialists that could be consulted by hospitalists and residents to provide psychosocial support and link admitted patients to treatment after discharge. There were no providers on the team to guide pharmacotherapy or to write discharge buprenorphine prescriptions. The Osler Medical Residency Training Program at JHH has 140 internal medicine residents and 16 combined medicine-pediatrics residents. All residents receive 1 hour of formal education about opioid use disorder annually. In addition, 28 of those 156 residents, those in the Urban Health Primary Care track, spend 1 month on an Addiction Medicine rotation in which they complete the 8-hour training required to receive the X-waiver. Those residents are encouraged to apply for the X-waiver once they obtain a medical license subsidized by a Health Resources & Services Administration (HRSA) grant. Four internal medicine attending physicians on teaching services and one resident had X-waivers prior to the intervention.
Intervention
In November 2018, we administered a survey to residents to identify barriers to starting buprenorphine maintenance and to measure knowledge and confidence with using buprenorphine for OUD (Appendix Figure 1 and Figure 2). We focused on buprenorphine because providers at JHH were familiar with this medication and because Baltimore has widespread access to buprenorphine, with more than 490 local buprenorphine providers.20 Five residents piloted the survey and provided feedback. We then administered the survey to all internal medicine and medicine-pediatrics residents. Based on the results, we developed a targeted educational conference and also created the Buprenorphine Bridge Team (BBT).
In January 2019, we presented the educational conference for residents devoted to the use of buprenorphine for OUD and introduced the BBT. The conference started with a patient testimonial and included peer recovery specialists, pharmacists, nurses, and social workers. We summarized the evidence for buprenorphine and offered a practical guide to start treatment in a one-page protocol. This protocol included guidance on selecting patients, shared decision-making around OUD treatment, avoiding precipitated withdrawal, dosing buprenorphine, and establishing follow-up (Appendix Figure 3). We asked for input on this protocol from nursing leadership, social work teams, and peer recovery specialists. Dosing was adapted from the Guidelines from the American Society of Addiction Medicine, with expert input from physicians from the Addiction Medicine Consult service at Johns Hopkins Bayview Medical Center, also in Baltimore.5 We instructed residents to obtain discharge buprenorphine prescriptions from an X-waivered physician on their team or from the newly established BBT. We asked resident teams to set up a postdischarge appointment for patients with an X-waivered provider, either in a community practice or at the JHH After Care Clinic, a transitional care clinic for discharged patients.21
The BBT is a resident-led group of X-waivered JHH residents and hospitalists who volunteer to write discharge buprenorphine prescriptions for patients. The BBT serves to ensure primary teams have access to an X-waivered prescriber. It is not a consult service. We asked primary teams to contact the BBT after initiating buprenorphine and after securing a follow-up appointment. In response to each request, a member of the BBT reviews the patient chart, confirms the follow-up plan, writes a prescription for buprenorphine along with intranasal naloxone, and leaves a brief note. During the 6-month postintervention period, the team consisted of three residents and three hospitalist attendings. Each week, two members (residents or attendings) staffed the team Monday to Friday, 8
In May 2019, 5 months after the education session and implementation of the BBT, we administered a follow-up survey.
Outcomes
As a secondary outcome, we measured engagement in OUD treatment after discharge by calculating the proportion of patients started on buprenorphine who filled a buprenorphine prescription within 30 days after discharge. We chose 30 days based on the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS) measure for engagement of treatment for alcohol and other drugs.23 We obtained the data from the Chesapeake Regional Information System for our Patients (CRISP) Prescription Drug Monitoring Program, which monitors all prescriptions for controlled substances dispensed in Maryland and five neighboring states. As a balancing measure, we counted patients newly started on methadone maintenance for OUD before and after the intervention. Additional secondary process outcomes included frequency of BBT requests, the volume of buprenorphine prescriptions written by the team, and time required to complete a BBT request.
Clinician-level outcomes, measured with electronically administered pre- and postintervention surveys to residents, included knowledge about and comfort with buprenorphine. Of the 16 questions in the pre- and postimplementation surveys, we analyzed the 6 questions concerning knowledge and comfort that remained identical in the pre- and postintervention surveys and used 5-point Likert scale responses. As an incentive, we randomly distributed three $50 gift cards to survey completers.
Analysis
We used an interrupted time series analysis to evaluate the association between the intervention bundle and a change in the rate that medical teams started patients with OUD on buprenorphine maintenance. This approach allowed us to control for preintervention trends. To evaluate the impact of our interventions, our pre- and postintervention periods include the same residents during the 2018-2019 academic year. Both periods consisted of twelve 2-week intervals (preintervention: July 26, 2019, to January 9, 2019; postintervention: January 10, 2019, to June 26, 2019).
To evaluate for changes in engagement in OUD treatment after discharge, we used two-sample t tests. To evaluate for changes in resident-reported comfort and knowledge with initiating buprenorphine maintenance, we used Wilcoxon rank sum tests for survey data and Wilcoxon signed rank tests for paired data. All analyses employed two-sided P values with statistical significance evaluated at the .05 alpha level. We analyzed data using R version 3.6.3 (Foundation for Statistical Computing). The Institutional Review Board at JHH reviewed and approved the study protocol as a quality improvement project (IRB00193365).

Before the intervention, 13 of the 30 patients (40%) newly started on buprenorphine maintenance during their admission filled a follow-up buprenorphine prescription within 30 days of discharge. After the intervention, 31 of 64 patients (46%) filled a buprenorphine prescription within 30 days (P = .612). Two patients were started on methadone maintenance, one prior to and one after the intervention.
During the 6-month postintervention period, the BBT received 75 requests and wrote 70 prescriptions for buprenorphine. The median time required to complete a BBT request was 15 minutes (minimum, 5 minutes; maximum, 60 minutes).
Of 156 internal medicine and medicine-pediatrics residents, 89 residents (57%) completed the baseline survey and 66 residents (42%) completed the follow-up survey. Forty residents completed both surveys. After the intervention, residents were significantly more likely to feel comfortable dosing buprenorphine (P < .0001) and counseling patients about its use (P = .0237) and were more likely to report ease of establishing follow-up (P < .0001). Self-reported knowledge about preventing precipitated withdrawal increased significantly (P = .0191), as did knowledge about the effectiveness of buprenorphine (P = .0003) independent of formal drug counseling (P = .0066) (Table). Paired survey data also found statistically significant results for all questions except those about preventing precipitated withdrawal and efficacy. For the latter, respondents who completed both surveys were more knowledgeable before the intervention than the overall group that completed the baseline survey (Appendix Table).

This study shows how a resident-led quality improvement project comprising clinician education and implementation of a novel BBT was associated with an increased rate of starting buprenorphine maintenance in hospitalized patients with OUD and improved resident knowledge about and comfort with buprenorphine. To our knowledge, this is the first study demonstrating how education and a team of X-waivered generalists can help primary teams initiate and discharge patients on buprenorphine maintenance in a hospital without an addiction medicine consult service.
Prior to the intervention, resident internal medicine teams at JHH started 10% of hospitalized patients with OUD on buprenorphine maintenance, consistent with prior studies showing rates of 11% to 15% for initiating OAT for hospitalized patients.11,12 After the intervention, the rate of initiating buprenorphine maintenance more than doubled, rising to 24% of eligible patients. Resident internal medicine teams at JHH started buprenorphine maintenance for 37 more patients over the 24-week postintervention period than would have been predicted prior to the intervention, or an additional three patients every 2 weeks.
Between 40% and 46% of hospitalized patients newly started on buprenorphine maintenance filled an outpatient buprenorphine prescription within 30 days of discharge. We are not aware of comparative data for 30-day follow-up for hospitalized patients newly started on buprenorphine maintenance. Data from other contexts show 5% to 10% of veterans were engaged in addiction treatment 30 days after initiation from inpatient or outpatient encounters. An analysis of an academic medical center in Oregon found engagement with an addiction medicine consult service increased after hospital engagement for patients with any substance use disorder from 23% to 39% using the 34-day HEDIS measure for engagement.17,24,25
The BBT required approximately 15 minutes per request and wrote an average of three prescriptions per week, demonstrating the feasibility of this approach and the high demand for this service. One strength of our approach is that residents gained experience starting buprenorphine independently using the aforementioned protocol instead of deferring to a full consult service. It is likely that this resident engagement in initiating longitudinal OUD care contributed to the success of this initiative, as did existing resident familiarity with using buprenorphine for opioid withdrawal.
This approach to resident education—promoting direct, first-person experience with medications in a clinical context—aligns with recommendations from a recent review about substance use disorder education for health professionals.26 Our interventions increased resident knowledge and comfort with buprenorphine, consistent with prior studies showing increased resident confidence in management of substance use disorders after curricular innovations.24,25
A few contextual features were essential for this project’s viability. Maryland allows American medical graduates to obtain a medical license after 1 year of postgraduate training. This allowed three residents to obtain X-waivers. These residents had access to HRSA funding to subsidize the expenses of applying for state licensure and DEA registration. BBT members volunteered their time while working on other services. Last, we were able to take advantage of buprenorphine-providing clinics in Baltimore, including the JHH After Care Clinic, to accept patients for follow-up appointments after discharge.
Limitations
The BBT required motivated clinicians willing to volunteer for additional clinical responsibilities during inpatient rotations and supportive faculty and residency leadership. Attending physicians, nurse practitioners, or physician assistants could staff a similar BBT in hospitals without residents or in hospitals where residents cannot obtain DEA registration. Crucially, other hospitals may not have access to practices with X-waivered physicians for outpatient follow-up. A recent study found X-waivered primary care physicians were less likely to be affiliated with hospital health systems. Other studies have shown limitations in access to buprenorphine at the county level based on geography and racial/ethnic segregation.27-29
Most patients hospitalized with OUD did not have ICD-10 codes associated with OUD. We addressed this by assuming patients had OUD if buprenorphine or methadone was ordered during their hospitalization, even if the medication was never administered. This may have overcounted patients prescribed these medications for indications other than OUD, and it may have undercounted patients with OUD for whom buprenorphine or methadone were never considered. The opioid withdrawal order set at JHH automatically offers an option to use buprenorphine to treat withdrawal. Patients with OUD for whom buprenorphine or methadone were never ordered likely did not experience withdrawal or were in withdrawal so mild that it escaped the attention of the team, which limits the generalizability of our intervention.
We identified several limitations to the internal validity of our study. First, we used a before-and-after study design without a control group. We could not ethically withhold access to evidence-based, mortality-reducing medications from patients. Without a control group, we cannot rule out the possibility that underlying temporal trends made residents more likely to start buprenorphine maintenance independent of our intervention. We attempted to control for unmeasured confounders by using an interrupted time series analysis to control for preintervention trends, comparing the same group of residents before and after our interventions, and selecting an intervention period during which residents were given only educational sessions and materials provided by our team. Our results may be biased by clustered data because certain residents may have been more likely to initiate buprenorphine, but these effects are likely marginal because resident schedules are balanced between outpatient and inpatient rotations during each 6-month period.
Finally, this project focused on buprenorphine, not on other medications for OUD, including methadone or naltrexone, or nonpharmacologic treatments for OUD.
Sustainability and Next Steps
Since the start of the BBT in January 2019, five additional PGY-2 residents obtained their medical licenses and X-waivers. These residents, with the support of two attending hospitalists, led the BBT and coordinated education sessions that were incorporated into the curriculum during the 2019-2020 academic year. These educational sessions will continue indefinitely. In 2020, JHH started an Addiction Medicine Consult Service staffed by physicians, NPs, and a pharmacist. The BBT continues to operate in conjunction with this service.
We found substantial variability in the rate of buprenorphine maintenance initiation despite our interventions. This is an area for future improvement. In a free-response prompt in our follow-up survey, residents requested additional education sessions and an order set to assist with initiation of buprenorphine. To address these gaps, three educational sessions were added, one of which included education on starting methadone maintenance therapy. We also added a new order set for starting buprenorphine maintenance. We hypothesize that these interventions will improve consistency.
In order for a similar program to be disseminated to other institutions, educational initiatives and a team of dedicated X-waivered prescribers are key. Materials to assist with this process are available in the Appendix.
CONCLUSION
This study shows how a resident-led intervention comprising clinician education and a team of X-waivered generalists was associated with improved treatment of OUD for hospitalized patients. We encourage residents and all clinicians at other hospitals without addiction medicine consult services to design, implement, and study similar interventions that directly increase the use of buprenorphine or methadone maintenance to treat OUD.
Preliminary results from this project were presented at the AMERSA National Conference on November 7, 2019.
1. Wilson N, Kariisa M, Seth P, Iv HS, Davis NL. Drug and opioid-involved overdose deaths – United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. http://dx.doi.org/10.15585/mmwr.mm6911a4
2. Berk J, Rogers KM, Wilson DJ, Thakrar A, Feldman L. Missed opportunities for treatment of opioid use disorder in the hospital setting: updating an outdated policy. J Hosp Med. 2020;15(10):619-621. https://doi.org/10.12788/jhm.3352
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
4. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. https://doi.org/10.1002/jhm.2113
5. Crotty K, Freedman KI, Kampman KM. Executive summary of the focused update of the ASAM national practice guideline for the treatment of opioid use disorder. J Addict Med. 2020;14(2):99-112. https://doi.org/10.1097/adm.0000000000000635
6. Leshner AI, Mancher M, eds. Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. https://www.nap.edu/catalog/25310
7. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357: j1550. https://doi.org/10.1136/bmj.j1550
8. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169(3):137-145. https://dx.doi.org/10.7326%2FM17-3107
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/nejmra1604339
10. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
11. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
12. Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: a pragmatic retrospective cohort analysis. J Gen Intern Med. 2020;35(8):2365-2374. https://doi.org/10.1007/s11606-020-05815-0
13. Madras BK, Ahmad NJ, Wen J, Sharfstein J; Prevention, Treatment, and Recovery Working Group of the Action Collaborative on Countering the U.S. Opioid Epidemic. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. NAM Perspectives. April 27, 2020. https://doi.org/10.31478/202004b
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the X Waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685
15. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/adm.0000000000000496
16. Weimer M, Morford K, Donroe J. Treatment of opioid use disorder in the acute hospital setting: a critical review of the literature (2014–2019). Curr Addict Rep. 2019;6(4):339-354.
17. Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J Gen Intern Med. 2019;34(12):2796-2803. https://doi.org/10.1007/s11606-019-05251-9
18. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;14(3):E1-E4. https://doi.org/10.12788/jhm.3311
19. Bottner R, Moriates C, Tirado C. The role of hospitalists in treating opioid use disorder. J Addict Med. 2020;14(2):178. https://doi.org/10.1097/adm.0000000000000545
20. Behavioral health treatment services locator. Substance Abuse and Mental Health Services Administration. Accessed May 14, 2020. https://findtreatment.samhsa.gov/
21. Groesbeck K, Whiteman LN, Stewart RW. Reducing readmission rates by improving transitional care. South Med J. 2015;108(12):758-760. https://doi.org/10.14423/smj.0000000000000376
22. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015 Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
23. Initiation and engagement of alcohol and other drug abuse or dependence treatment (IET). NCQA. Accessed April 20, 2020. https://www.ncqa.org/hedis/measures/initiation-and-engagement-of-alcohol-and-other-drug-abuse-or-dependence-treatment/
24. Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70-75. https://doi.org/10.1016/j.drugalcdep.2019.01.038
25. Harris AHS, Humphreys K, Finney JW. Veterans Affairs facility performance on Washington Circle indicators and casemix-adjusted effectiveness. J Subst Abuse Treat. 2007;33(4):333-339. https://doi.org/10.1016/j.jsat.2006.12.015
26. Muzyk A, Smothers ZPW, Andolsek KM, et al. Interprofessional substance use disorder education in health professions education programs: a scoping review. Acad Med. 2020;95(3):470-480. https://doi.org/10.1097/acm.0000000000003053
27. Saloner B, Lin L, Simon K. Geographic location of buprenorphine-waivered physicians and integration with health systems. J Subst Abuse Treat. 2020;115:108034. https://doi.org/10.1016/j.jsat.2020.108034
28. Jones CW, Christman Z, Smith CM, et al. Comparison between buprenorphine provider availability and opioid deaths among US counties. J Subst Abuse Treat. 2018;93:19-25. https://doi.org/10.1016/j.jsat.2018.07.008
29. Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. https://doi.org/10.1001/jamanetworkopen.2020.3711
1. Wilson N, Kariisa M, Seth P, Iv HS, Davis NL. Drug and opioid-involved overdose deaths – United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. http://dx.doi.org/10.15585/mmwr.mm6911a4
2. Berk J, Rogers KM, Wilson DJ, Thakrar A, Feldman L. Missed opportunities for treatment of opioid use disorder in the hospital setting: updating an outdated policy. J Hosp Med. 2020;15(10):619-621. https://doi.org/10.12788/jhm.3352
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
4. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. https://doi.org/10.1002/jhm.2113
5. Crotty K, Freedman KI, Kampman KM. Executive summary of the focused update of the ASAM national practice guideline for the treatment of opioid use disorder. J Addict Med. 2020;14(2):99-112. https://doi.org/10.1097/adm.0000000000000635
6. Leshner AI, Mancher M, eds. Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. https://www.nap.edu/catalog/25310
7. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357: j1550. https://doi.org/10.1136/bmj.j1550
8. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169(3):137-145. https://dx.doi.org/10.7326%2FM17-3107
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/nejmra1604339
10. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
11. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
12. Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: a pragmatic retrospective cohort analysis. J Gen Intern Med. 2020;35(8):2365-2374. https://doi.org/10.1007/s11606-020-05815-0
13. Madras BK, Ahmad NJ, Wen J, Sharfstein J; Prevention, Treatment, and Recovery Working Group of the Action Collaborative on Countering the U.S. Opioid Epidemic. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. NAM Perspectives. April 27, 2020. https://doi.org/10.31478/202004b
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the X Waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685
15. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/adm.0000000000000496
16. Weimer M, Morford K, Donroe J. Treatment of opioid use disorder in the acute hospital setting: a critical review of the literature (2014–2019). Curr Addict Rep. 2019;6(4):339-354.
17. Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J Gen Intern Med. 2019;34(12):2796-2803. https://doi.org/10.1007/s11606-019-05251-9
18. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;14(3):E1-E4. https://doi.org/10.12788/jhm.3311
19. Bottner R, Moriates C, Tirado C. The role of hospitalists in treating opioid use disorder. J Addict Med. 2020;14(2):178. https://doi.org/10.1097/adm.0000000000000545
20. Behavioral health treatment services locator. Substance Abuse and Mental Health Services Administration. Accessed May 14, 2020. https://findtreatment.samhsa.gov/
21. Groesbeck K, Whiteman LN, Stewart RW. Reducing readmission rates by improving transitional care. South Med J. 2015;108(12):758-760. https://doi.org/10.14423/smj.0000000000000376
22. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015 Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
23. Initiation and engagement of alcohol and other drug abuse or dependence treatment (IET). NCQA. Accessed April 20, 2020. https://www.ncqa.org/hedis/measures/initiation-and-engagement-of-alcohol-and-other-drug-abuse-or-dependence-treatment/
24. Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70-75. https://doi.org/10.1016/j.drugalcdep.2019.01.038
25. Harris AHS, Humphreys K, Finney JW. Veterans Affairs facility performance on Washington Circle indicators and casemix-adjusted effectiveness. J Subst Abuse Treat. 2007;33(4):333-339. https://doi.org/10.1016/j.jsat.2006.12.015
26. Muzyk A, Smothers ZPW, Andolsek KM, et al. Interprofessional substance use disorder education in health professions education programs: a scoping review. Acad Med. 2020;95(3):470-480. https://doi.org/10.1097/acm.0000000000003053
27. Saloner B, Lin L, Simon K. Geographic location of buprenorphine-waivered physicians and integration with health systems. J Subst Abuse Treat. 2020;115:108034. https://doi.org/10.1016/j.jsat.2020.108034
28. Jones CW, Christman Z, Smith CM, et al. Comparison between buprenorphine provider availability and opioid deaths among US counties. J Subst Abuse Treat. 2018;93:19-25. https://doi.org/10.1016/j.jsat.2018.07.008
29. Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. https://doi.org/10.1001/jamanetworkopen.2020.3711
© 2021 Society of Hospital Medicine
Hospital Buprenorphine Program for Opioid Use Disorder Is Associated With Increased Inpatient and Outpatient Addiction Treatment
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased
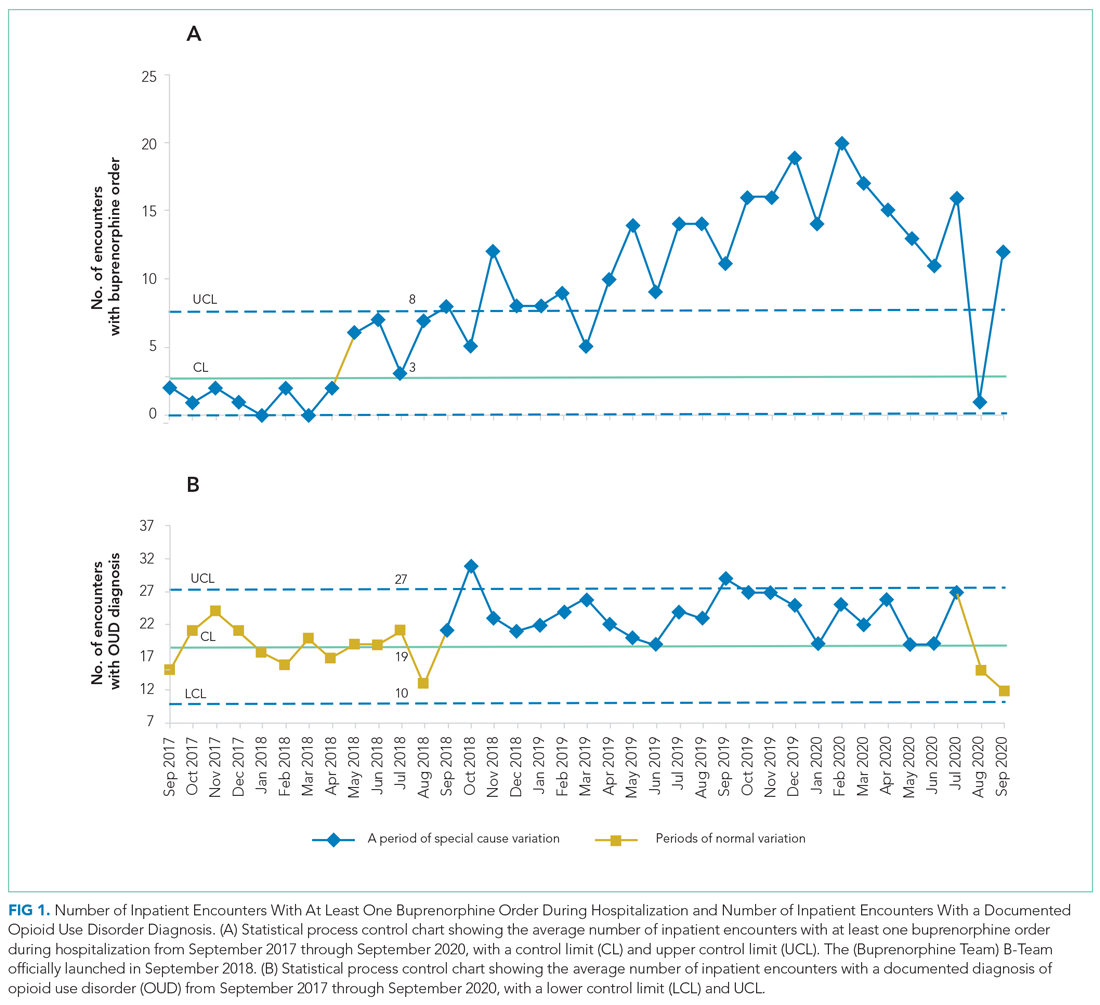
The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).
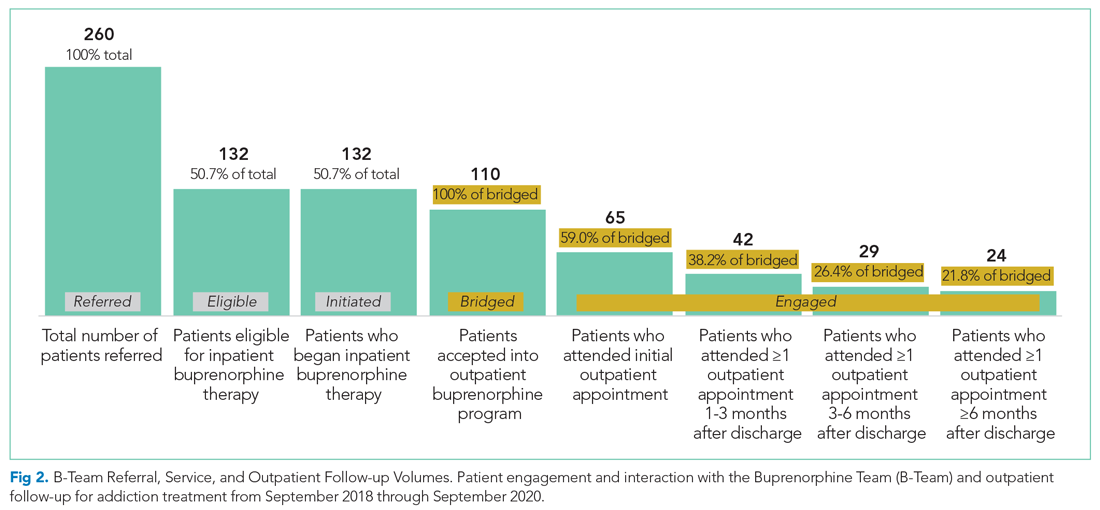
Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
© 2021 Society of Hospital Medicine
Gender Differences in the Presentation and Outcomes of Hospitalized Patients With COVID-19
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.
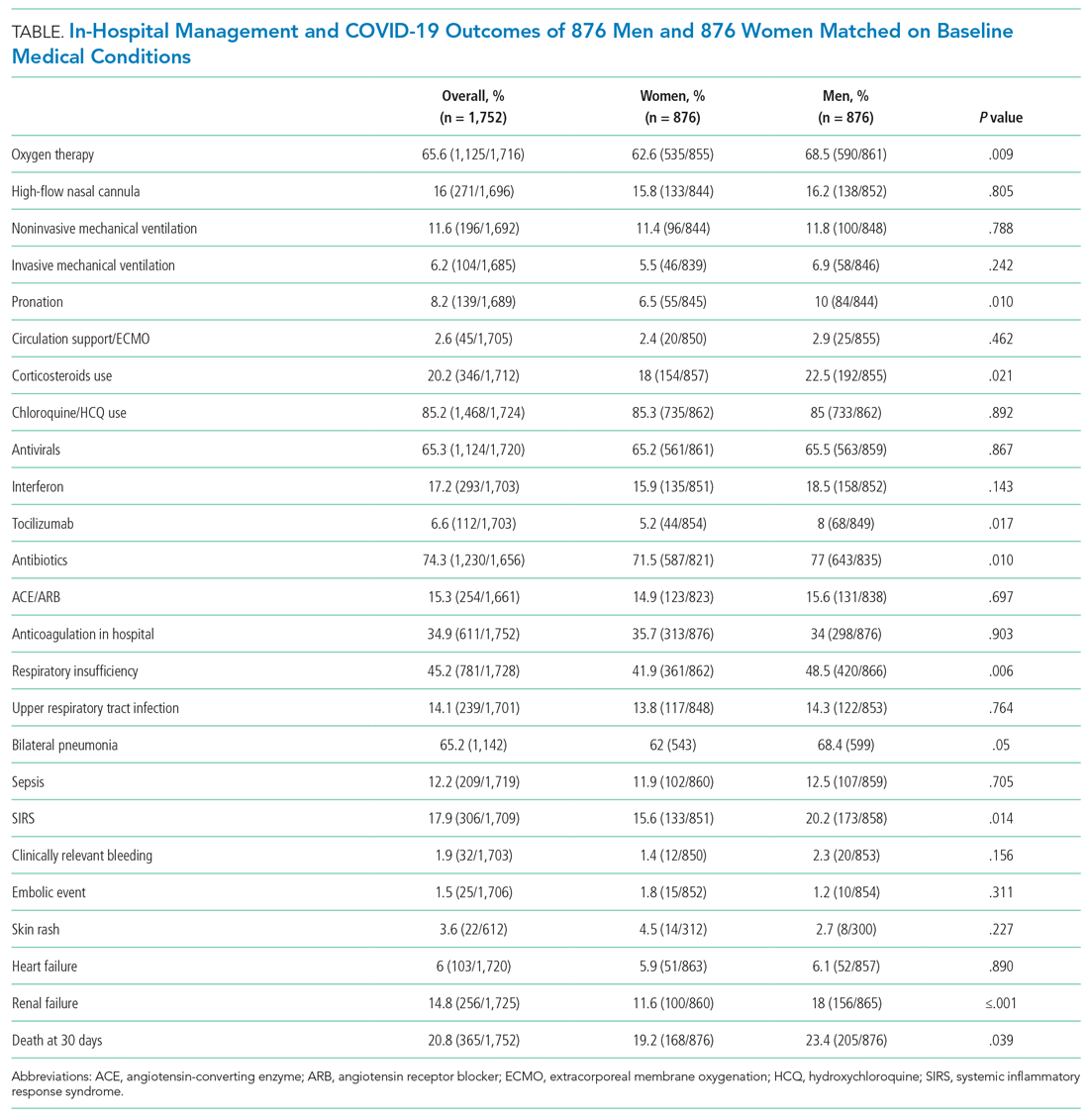
Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).
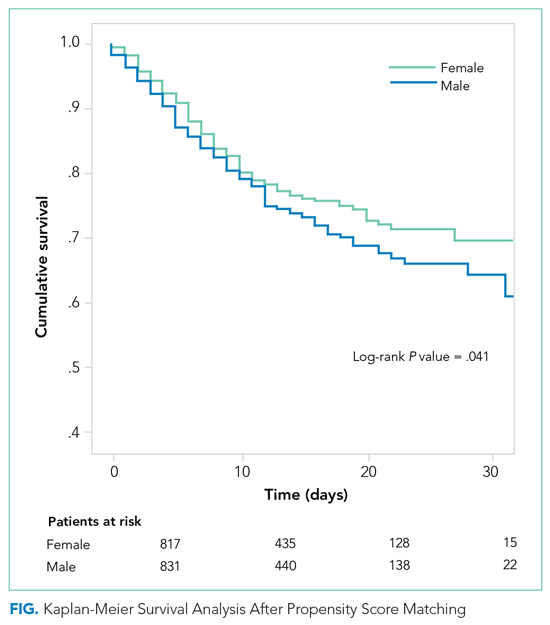
DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.

Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.

Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
© 2021 Society of Hospital Medicine
Mapping the Clinical Experience of a New York City Residency Program During the COVID-19 Pandemic
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).
Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).
DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).

Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).

DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).

Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).

DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
© 2021 Society of Hospital Medicine