User login
Acute cardiorenal syndrome: Mechanisms and clinical implications
As the heart goes, so go the kidneys—and vice versa. Cardiac and renal function are intricately interdependent, and failure of either organ causes injury to the other in a vicious circle of worsening function.1
Here, we discuss acute cardiorenal syndrome, ie, acute exacerbation of heart failure leading to acute kidney injury, a common cause of hospitalization and admission to the intensive care unit. We examine its clinical definition, pathophysiology, hemodynamic derangements, clues that help in diagnosing it, and its treatment.
A GROUP OF LINKED DISORDERS
Two types of acute cardiac dysfunction
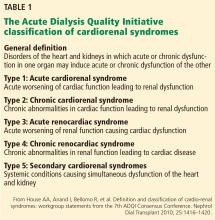
Although these definitions offer a good general description, further clarification of the nature of organ dysfunction is needed. Acute renal dysfunction can be unambiguously defined using the AKIN (Acute Kidney Injury Network) and RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) classifications.3 Acute cardiac dysfunction, on the other hand, is an ambiguous term that encompasses 2 clinically and pathophysiologically distinct conditions: cardiogenic shock and acute heart failure.
Cardiogenic shock is characterized by a catastrophic compromise of cardiac pump function leading to global hypoperfusion severe enough to cause systemic organ damage.4 The cardiac index at which organs start to fail varies in different cases, but a value of less than 1.8 L/min/m2 is typically used to define cardiogenic shock.4
Acute heart failure, on the other hand, is defined as gradually or rapidly worsening signs and symptoms of congestive heart failure due to worsening pulmonary or systemic congestion.5 Hypervolemia is the hallmark of acute heart failure, whereas patients with cardiogenic shock may be hypervolemic, normovolemic, or hypovolemic. Although cardiac output may be mildly reduced in some cases of acute heart failure, systemic perfusion is enough to maintain organ function.
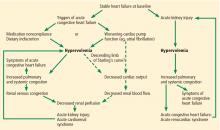
These two conditions cause renal injury by distinct mechanisms and have entirely different therapeutic implications. As we discuss later, reduced renal perfusion due to renal venous congestion is now believed to be the major hemodynamic mechanism of renal injury in acute heart failure. On the other hand, in cardiogenic shock, renal perfusion is reduced due to a critical decline of cardiac pump function.
The ideal definition of acute cardiorenal syndrome should describe a distinct pathophysiology of the syndrome and offer distinct therapeutic options that counteract it. Hence, we propose that renal injury from cardiogenic shock should not be included in its definition, an approach that has been adopted in some of the recent reviews as well.6 Our discussion of acute cardiorenal syndrome is restricted to renal injury caused by acute heart failure.
PATHOPHYSIOLOGY OF ACUTE CARDIORENAL SYNDROME
Multiple mechanisms have been implicated in the pathophysiology of cardiorenal syndrome.7,8
Sympathetic hyperactivity is a compensatory mechanism in heart failure and may be aggravated if cardiac output is further reduced. Its effects include constriction of afferent and efferent arterioles, causing reduced renal perfusion and increased tubular sodium and water reabsorption.7
The renin-angiotensin-aldosterone system is activated in patients with stable congestive heart failure and may be further stimulated in a state of reduced renal perfusion, which is a hallmark of acute cardiorenal syndrome. Its activation can cause further salt and water retention.
However, direct hemodynamic mechanisms likely play the most important role and have obvious diagnostic and therapeutic implications.
Elevated venous pressure, not reduced cardiac output, drives kidney injury
The classic view was that renal dysfunction in acute heart failure is caused by reduced renal blood flow due to failing cardiac pump function. Cardiac output may be reduced in acute heart failure for various reasons, such as atrial fibrillation, myocardial infarction, or other processes, but reduced cardiac output has a minimal role, if any, in the pathogenesis of renal injury in acute heart failure.
As evidence of this, acute heart failure is not always associated with reduced cardiac output.5 Even if the cardiac index (cardiac output divided by body surface area) is mildly reduced, renal blood flow is largely unaffected, thanks to effective renal autoregulatory mechanisms. Not until the mean arterial pressure falls below 70 mm Hg do these mechanisms fail and renal blood flow starts to drop.9 Hence, unless cardiac performance is compromised enough to cause cardiogenic shock, renal blood flow usually does not change significantly with mild reduction in cardiac output.
Hanberg et al10 performed a post hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheter Effectiveness (ESCAPE) trial, in which 525 patients with advanced heart failure underwent pulmonary artery catheterization to measure their cardiac index. The authors found no association between the cardiac index and renal function in these patients.
How venous congestion impairs the kidney
In view of the current clinical evidence, the focus has shifted to renal venous congestion. According to Poiseuille’s law, blood flow through the kidneys depends on the pressure gradient—high pressure on the arterial side, low pressure on the venous side.8 Increased renal venous pressure causes reduced renal perfusion pressure, thereby affecting renal perfusion. This is now recognized as an important hemodynamic mechanism of acute cardiorenal syndrome.
Renal congestion can also affect renal function through indirect mechanisms. For example, it can cause renal interstitial edema that may then increase the intratubular pressure, thereby reducing the transglomerular pressure gradient.11
Firth et al,14 in experiments in animals, found that increasing the renal venous pressure above 18.75 mm Hg significantly reduced the glomerular filtration rate, which completely resolved when renal venous pressure was restored to basal levels.
Mullens et al,15 in a study of 145 patients admitted with acute heart failure, reported that 58 (40%) developed acute kidney injury. Pulmonary artery catheterization revealed that elevated central venous pressure, rather than reduced cardiac index, was the primary hemodynamic factor driving renal dysfunction.
DIAGNOSIS AND CLINICAL ASSESSMENT
Patients with acute cardiorenal syndrome present with clinical features of pulmonary or systemic congestion (or both) and acute kidney injury.
Elevated left-sided pressures are usually but not always associated with elevated right-sided pressures. In a study of 1,000 patients with advanced heart failure, a pulmonary capillary wedge pressure of 22 mm Hg or higher had a positive predictive value of 88% for a right atrial pressure of 10 mm Hg or higher.16 Hence, the clinical presentation may vary depending on the location (pulmonary, systemic, or both) and degree of congestion.
Symptoms of pulmonary congestion include worsening exertional dyspnea and orthopnea; bilateral crackles may be heard on physical examination if pulmonary edema is present.
Systemic congestion can cause significant peripheral edema and weight gain. Jugular venous distention may be noted. Oliguria may be present due to renal dysfunction; patients on maintenance diuretic therapy often note its lack of efficacy.
Signs of acute heart failure
Wang et al,17 in a meta-analysis of 22 studies, concluded that the features that most strongly suggested acute heart failure were:
- History of paroxysmal nocturnal dyspnea
- A third heart sound
- Evidence of pulmonary venous congestion on chest radiography.
Features that most strongly suggested the patient did not have acute heart failure were:
- Absence of exertional dyspnea
- Absence of rales
- Absence of radiographic evidence of cardiomegaly.
Patients may present without some of these classic clinical features, and the diagnosis of acute heart failure may be challenging. For example, even if left-sided pressures are very high, pulmonary edema may be absent because of pulmonary vascular remodeling in chronic heart failure.18 Pulmonary artery catheterization reveals elevated cardiac filling pressures and can be used to guide therapy, but clinical evidence argues against its routine use.19
Urine electrolytes (fractional excretion of sodium < 1% and fractional excretion of urea < 35%) often suggest a prerenal form of acute kidney injury, since the hemodynamic derangements in acute cardiorenal syndrome reduce renal perfusion.
Biomarkers of cell-cycle arrest such as urine insulinlike growth factor-binding protein 7 and tissue inhibitor of metalloproteinase 2 have recently been shown to identify patients with acute heart failure at risk of developing acute cardiorenal syndrome.20
Acute cardiorenal syndrome vs renal injury due to hypovolemia
The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Patients with stable heart failure usually have mild hypervolemia at baseline, but they can become hypovolemic due to overaggressive diuretic therapy, severe diarrhea, or other causes.
Although the fluid status of patients in these 2 conditions is opposite, they can be difficult to distinguish. In both conditions, urine electrolytes suggest a prerenal acute kidney injury. A history of recent fluid losses or diuretic overuse may help identify hypovolemia. If available, analysis of the recent trend in weight can be vital in making the right diagnosis.
Misdiagnosis of acute cardiorenal syndrome as hypovolemia-induced acute kidney injury can be catastrophic. If volume depletion is erroneously judged to be the cause of acute kidney injury, fluid administration can further worsen both cardiac and renal function. This can perpetuate the vicious circle that is already in play. Lack of renal recovery may invite further fluid administration.
TREATMENT
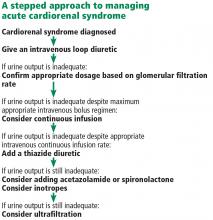
Fluid removal with diuresis or ultrafiltration is the cornerstone of treatment. Other treatments such as inotropes are reserved for patients with resistant disease.
Diuretics
The goal of therapy in acute cardiorenal syndrome is to achieve aggressive diuresis, typically using intravenous diuretics. Loop diuretics are the most potent class of diuretics and are the first-line drugs for this purpose. Other classes of diuretics can be used in conjunction with loop diuretics; however, using them by themselves is neither effective nor recommended.
Resistance to diuretics at usual doses is common in patients with acute cardiorenal syndrome. Several mechanisms contribute to diuretic resistance in these patients.21
Oral bioavailability of diuretics may be reduced due to intestinal edema.
Diuretic pharmacokinetics are significantly deranged in cardiorenal syndrome. All diuretics except mineralocorticoid antagonists (ie, spironolactone and eplerenone) act on targets on the luminal side of renal tubules, but are highly protein-bound and are hence not filtered at the glomerulus. Loop diuretics, thiazides, and carbonic anhydrase inhibitors are secreted in the proximal convoluted tubule via the organic anion transporter,22 whereas epithelial sodium channel inhibitors (amiloride and triamterene) are secreted via the organic cation transporter 2.23 In renal dysfunction, various uremic toxins accumulate in the body and compete with diuretics for secretion into the proximal convoluted tubule via these transporters.24
Finally, activation of the sympathetic nervous system and renin-angiotensin-aldosterone system leads to increased tubular sodium and water retention, thereby also blunting the diuretic response.
Diuretic dosage. In patients whose creatinine clearance is less than 15 mL/min, only 10% to 20% as much loop diuretic is secreted into the renal tubule as in normal individuals.25 This effect warrants dose adjustment of diuretics during uremia.
Continuous infusion or bolus? Continuous infusion of loop diuretics is another strategy to optimize drug delivery. Compared with bolus therapy, continuous infusion provides more sustained and uniform drug delivery and prevents postdiuretic sodium retention.
The Diuretic Optimization Strategies Evaluation (DOSE) trial compared the efficacy and safety of continuous vs bolus furosemide therapy in 308 patients admitted with acute decompensated heart failure.26 There was no difference in symptom control or net fluid loss at 72 hours in either group. Other studies have shown more diuresis with continuous infusion than with a similarly dosed bolus regimen.27 However, definitive clinical evidence is lacking at this point to support routine use of continuous loop diuretic therapy.
Combination diuretic therapy. Sequential nephron blockade with combination diuretic therapy is an important therapeutic strategy against diuretic resistance. Notably, urine output-guided diuretic therapy has been shown to be superior to standard diuretic therapy.28 Such therapeutic protocols may employ combination diuretic therapy as a next step when the desired diuretic response is not obtained with high doses of loop diuretic monotherapy.
The desired diuretic response depends on the clinical situation. For example, in patients with severe congestion, we would like the net fluid output to be at least 2 to 3 L more than the fluid intake after the first 24 hours. Sometimes, patients in the intensive care unit are on several essential drug infusions, so that their net intake amounts to 1 to 2 L. In these patients, the desired urine output would be even more than in patients not on these drug infusions.
Loop diuretics block sodium reabsorption at the thick ascending loop of Henle. This disrupts the countercurrent exchange mechanism and reduces renal medullary interstitial osmolarity; these effects prevent water reabsorption. However, the unresorbed sodium can be taken up by the sodium-chloride cotransporter and the epithelial sodium channel in the distal nephron, thereby blunting the diuretic effect. This is the rationale for combining loop diuretics with thiazides or potassium-sparing diuretics.
Similarly, carbonic anhydrase inhibitors (eg, acetazolamide) reduce sodium reabsorption from the proximal convoluted tubule, but most of this sodium is then reabsorbed distally. Hence, the combination of a loop diuretic and acetazolamide can also have a synergistic diuretic effect.
The most popular combination is a loop diuretic plus a thiazide, although no large-scale placebo-controlled trials have been performed.29 Metolazone (a thiazidelike diuretic) is typically used due to its low cost and availability.30 Metolazone has also been shown to block sodium reabsorption at the proximal tubule, which may contribute to its synergistic effect. Chlorothiazide is available in an intravenous formulation and has a faster onset of action than metolazone. However, studies have failed to detect any benefit of one over the other.31
The potential benefit of combining a loop diuretic with acetazolamide is a lower tendency to develop metabolic alkalosis, a potential side effect of loop diuretics and thiazides. Although data are limited, a recent study showed that adding acetazolamide to bumetanide led to significantly increased natriuresis.32
In the Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, adding spironolactone in high doses to usual therapy was not found to cause any significant change in N-terminal pro-B-type natriuretic peptide level or net urine output.33
Ultrafiltration
Venovenous ultrafiltration (or aquapheresis) employs an extracorporeal circuit, similar to the one used in hemodialysis, which removes iso-osmolar fluid at a fixed rate.34 Newer ultrafiltration systems are more portable, can be used with peripheral venous access, and require minimal nursing supervision.35
Although ultrafiltration seems an attractive alternative to diuresis in acute heart failure, studies have been inconclusive. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial compared ultrafiltration and diuresis in 188 patients with acute heart failure and acute cardiorenal syndrome.36 Diuresis, performed according to an algorithm, was found to be superior to ultrafiltration in terms of a bivariate end point of change in weight and change in serum creatinine level at 96 hours. However, the level of cystatin C is thought to be a more accurate indicator of renal function, and the change in cystatin C level from baseline did not differ between the two treatment groups. Also, the ultrafiltration rate was 200 mL per hour, which, some argue, may have been excessive and may have caused intravascular depletion.
Although the ideal rate of fluid removal is unknown, it should be individualized and adjusted based on the patient’s renal function, volume status, and hemodynamic status. The initial rate should be based on the degree of fluid overload and the anticipated plasma refill rate from the interstitial fluid.37 For example, a malnourished patient may have low serum oncotic pressure and hence have low plasma refill upon ultrafiltration. Disturbance of this delicate balance between the rates of ultrafiltration and plasma refill may lead to intravascular volume contraction.
In summary, although ultrafiltration is a valuable alternative to diuretics in resistant cases, its use as a primary decongestive therapy cannot be endorsed in view of the current data.
Inotropes
Inotropes such as dobutamine and milrinone are typically used in cases of cardiogenic shock to maintain organ perfusion. There is a physiologic rationale to using inotropes in acute cardiorenal syndrome as well, especially when the aforementioned strategies fail to overcome diuretic resistance.7
Inotropes increase cardiac output, improve renal blood flow, improve right ventricular output, and thereby relieve systemic congestion. These hemodynamic effects may improve renal perfusion and response to diuretics. However, clinical evidence to support this is lacking.
The Renal Optimization Strategies Evaluation (ROSE) trial enrolled 360 patients with acute heart failure and renal dysfunction. Adding dopamine in a low dose (2 μg/kg/min) to diuretic therapy had no significant effect on 72-hour cumulative urine output or renal function as measured by cystatin C levels.38 However, acute kidney injury was not identified in this trial, and the renal function of many of these patients may have been at its baseline when they were admitted. In other words, this trial did not necessarily include patients with acute kidney injury along with acute heart failure. Hence, it did not necessarily include patients with acute cardiorenal syndrome.
Vasodilators
Vasodilators such as nitroglycerin, sodium nitroprusside, and hydralazine are commonly used in patients with acute heart failure, although the clinical evidence supporting their use is weak.
Physiologically, arterial dilation reduces afterload and can help relieve pulmonary congestion, and venodilation increases capacitance and reduces preload. In theory, venodilators such as nitroglycerin can relieve renal venous congestion in patients with acute cardiorenal syndrome, thereby improving renal perfusion.
However, the use of vasodilators is often limited by their adverse effects, the most important being hypotension. This is especially relevant in light of recent data identifying reduction in blood pressure during treatment of acute heart failure as an independent risk factor for worsening renal function.39,40 It is important to note that in these studies, changes in cardiac index did not affect the propensity for developing worsening renal function. The precise mechanism of this finding is unclear but it is plausible that systemic vasodilation redistributes the cardiac output to nonrenal tissues, thereby overriding the renal autoregulatory mechanisms that are normally employed in low output states.
Preventive strategies
Various strategies can be used to prevent acute cardiorenal syndrome. An optimal outpatient diuretic regimen to avoid hypervolemia is essential. Patients with advanced congestive heart failure should be followed up closely in dedicated heart failure clinics until their diuretic regimen is optimized. Patients should be advised to check their weight on a regular basis and seek medical advice if they notice an increase in their weight or a reduction in their urine output.
TAKE-HOME POINTS
- A robust clinical definition of cardiorenal syndrome is lacking. Hence, recognition of this condition can be challenging.
- Volume overload is central to its pathogenesis, and accurate assessment of volume status is critical.
- Renal venous congestion is the major mechanism of type 1 cardiorenal syndrome.
- Misdiagnosis can have devastating consequences, as it may lead to an opposite therapeutic approach.
- Fluid removal by various strategies is the mainstay of treatment.
- Temporary inotropic support should be saved for the last resort.
- Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Cleve Clin J Med 2006; 73:485–491.
- House AA, Anand I, Bellomo R, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Chang CH, Lin CY, Tian YC, et al. Acute kidney injury classification: comparison of AKIN and RIFLE criteria. Shock 2010; 33:247-252.
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008; 117:686–697.
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009; 53:557–573.
- ter Maaten JM, Valente MA, Damman K, et al. Diuretic response in acute heart failure—pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015; 12:184–192.
- Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 2013; 9:99–111.
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121:2592–2600.
- Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Afsar B, Ortiz A, Covic A, et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9:39–47.
- Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013; 62:485–495.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1988; 1:1033–1035.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18:1126–1132.
- Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 2004; 125:669–682.
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294:1625–1633.
- Schanz M, Shi J , Wasser C , Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 2017; doi.org/10.1002/clc.22683.
- Bowman BN, Nawarskas JJ, Anderson JR. Treating diuretic resistance: an overview. Cardiol Rev 2016; 24:256–260.
- Uwai Y, Saito H, Hashimoto Y, Inui KI. Interaction and transport of thiazide diuretics, loop diuretics, and acetazolamide via rat renal organic anion transporter rOAT1. J Pharmacol Exp Ther 2000; 295:261–265.
- Hacker K, Maas R, Kornhuber J, et al. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One 2015; 10:e0136451.
- Schophuizen CM, Wilmer MJ, Jansen J, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch 2013; 465:1701–1714.
- Brater DC. Diuretic therapy. N Engl J Med 1998; 339:387–395.
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail 2010; 16:188–193.
- Grodin JL, Stevens SR, de Las Fuentes L, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail 2016; 22:26–32.
- Ng TM, Konopka E, Hyderi AF, et al. Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther 2013; 18:345–353.
- Sica DA. Metolazone and its role in edema management. Congest Heart Fail 2003; 9:100–105.
- Moranville MP, Choi S, Hogg J, Anderson AS, Rich JD. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015; 33:42–49.
- Verbrugge FH, Dupont M, Bertrand PB, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 2015; 70:265–373.
- Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017 Jul 12. doi: 10.1001/jamacardio.2017.2198. [Epub ahead of print]
- Pourafshar N, Karimi A, Kazory A. Extracorporeal ultrafiltration therapy for acute decompensated heart failure. Expert Rev Cardiovasc Ther 2016; 14:5–13.
- Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail 2003; 9:227–231.
- Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Kazory A. Cardiorenal syndrome: ultrafiltration therapy for heart failure—trials and tribulations. Clin J Am Soc Nephrol 2013; 8:1816–1828.
- Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Testani JM, Coca SG, McCauley BD, et al. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011; 13:877–884.
- Dupont M, Mullens W, Finucan M, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013; 15:433–440.
As the heart goes, so go the kidneys—and vice versa. Cardiac and renal function are intricately interdependent, and failure of either organ causes injury to the other in a vicious circle of worsening function.1
Here, we discuss acute cardiorenal syndrome, ie, acute exacerbation of heart failure leading to acute kidney injury, a common cause of hospitalization and admission to the intensive care unit. We examine its clinical definition, pathophysiology, hemodynamic derangements, clues that help in diagnosing it, and its treatment.
A GROUP OF LINKED DISORDERS
Two types of acute cardiac dysfunction
Although these definitions offer a good general description, further clarification of the nature of organ dysfunction is needed. Acute renal dysfunction can be unambiguously defined using the AKIN (Acute Kidney Injury Network) and RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) classifications.3 Acute cardiac dysfunction, on the other hand, is an ambiguous term that encompasses 2 clinically and pathophysiologically distinct conditions: cardiogenic shock and acute heart failure.
Cardiogenic shock is characterized by a catastrophic compromise of cardiac pump function leading to global hypoperfusion severe enough to cause systemic organ damage.4 The cardiac index at which organs start to fail varies in different cases, but a value of less than 1.8 L/min/m2 is typically used to define cardiogenic shock.4
Acute heart failure, on the other hand, is defined as gradually or rapidly worsening signs and symptoms of congestive heart failure due to worsening pulmonary or systemic congestion.5 Hypervolemia is the hallmark of acute heart failure, whereas patients with cardiogenic shock may be hypervolemic, normovolemic, or hypovolemic. Although cardiac output may be mildly reduced in some cases of acute heart failure, systemic perfusion is enough to maintain organ function.
These two conditions cause renal injury by distinct mechanisms and have entirely different therapeutic implications. As we discuss later, reduced renal perfusion due to renal venous congestion is now believed to be the major hemodynamic mechanism of renal injury in acute heart failure. On the other hand, in cardiogenic shock, renal perfusion is reduced due to a critical decline of cardiac pump function.
The ideal definition of acute cardiorenal syndrome should describe a distinct pathophysiology of the syndrome and offer distinct therapeutic options that counteract it. Hence, we propose that renal injury from cardiogenic shock should not be included in its definition, an approach that has been adopted in some of the recent reviews as well.6 Our discussion of acute cardiorenal syndrome is restricted to renal injury caused by acute heart failure.
PATHOPHYSIOLOGY OF ACUTE CARDIORENAL SYNDROME
Multiple mechanisms have been implicated in the pathophysiology of cardiorenal syndrome.7,8
Sympathetic hyperactivity is a compensatory mechanism in heart failure and may be aggravated if cardiac output is further reduced. Its effects include constriction of afferent and efferent arterioles, causing reduced renal perfusion and increased tubular sodium and water reabsorption.7
The renin-angiotensin-aldosterone system is activated in patients with stable congestive heart failure and may be further stimulated in a state of reduced renal perfusion, which is a hallmark of acute cardiorenal syndrome. Its activation can cause further salt and water retention.
However, direct hemodynamic mechanisms likely play the most important role and have obvious diagnostic and therapeutic implications.
Elevated venous pressure, not reduced cardiac output, drives kidney injury
The classic view was that renal dysfunction in acute heart failure is caused by reduced renal blood flow due to failing cardiac pump function. Cardiac output may be reduced in acute heart failure for various reasons, such as atrial fibrillation, myocardial infarction, or other processes, but reduced cardiac output has a minimal role, if any, in the pathogenesis of renal injury in acute heart failure.
As evidence of this, acute heart failure is not always associated with reduced cardiac output.5 Even if the cardiac index (cardiac output divided by body surface area) is mildly reduced, renal blood flow is largely unaffected, thanks to effective renal autoregulatory mechanisms. Not until the mean arterial pressure falls below 70 mm Hg do these mechanisms fail and renal blood flow starts to drop.9 Hence, unless cardiac performance is compromised enough to cause cardiogenic shock, renal blood flow usually does not change significantly with mild reduction in cardiac output.
Hanberg et al10 performed a post hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheter Effectiveness (ESCAPE) trial, in which 525 patients with advanced heart failure underwent pulmonary artery catheterization to measure their cardiac index. The authors found no association between the cardiac index and renal function in these patients.
How venous congestion impairs the kidney
In view of the current clinical evidence, the focus has shifted to renal venous congestion. According to Poiseuille’s law, blood flow through the kidneys depends on the pressure gradient—high pressure on the arterial side, low pressure on the venous side.8 Increased renal venous pressure causes reduced renal perfusion pressure, thereby affecting renal perfusion. This is now recognized as an important hemodynamic mechanism of acute cardiorenal syndrome.
Renal congestion can also affect renal function through indirect mechanisms. For example, it can cause renal interstitial edema that may then increase the intratubular pressure, thereby reducing the transglomerular pressure gradient.11
Firth et al,14 in experiments in animals, found that increasing the renal venous pressure above 18.75 mm Hg significantly reduced the glomerular filtration rate, which completely resolved when renal venous pressure was restored to basal levels.
Mullens et al,15 in a study of 145 patients admitted with acute heart failure, reported that 58 (40%) developed acute kidney injury. Pulmonary artery catheterization revealed that elevated central venous pressure, rather than reduced cardiac index, was the primary hemodynamic factor driving renal dysfunction.
DIAGNOSIS AND CLINICAL ASSESSMENT
Patients with acute cardiorenal syndrome present with clinical features of pulmonary or systemic congestion (or both) and acute kidney injury.
Elevated left-sided pressures are usually but not always associated with elevated right-sided pressures. In a study of 1,000 patients with advanced heart failure, a pulmonary capillary wedge pressure of 22 mm Hg or higher had a positive predictive value of 88% for a right atrial pressure of 10 mm Hg or higher.16 Hence, the clinical presentation may vary depending on the location (pulmonary, systemic, or both) and degree of congestion.
Symptoms of pulmonary congestion include worsening exertional dyspnea and orthopnea; bilateral crackles may be heard on physical examination if pulmonary edema is present.
Systemic congestion can cause significant peripheral edema and weight gain. Jugular venous distention may be noted. Oliguria may be present due to renal dysfunction; patients on maintenance diuretic therapy often note its lack of efficacy.
Signs of acute heart failure
Wang et al,17 in a meta-analysis of 22 studies, concluded that the features that most strongly suggested acute heart failure were:
- History of paroxysmal nocturnal dyspnea
- A third heart sound
- Evidence of pulmonary venous congestion on chest radiography.
Features that most strongly suggested the patient did not have acute heart failure were:
- Absence of exertional dyspnea
- Absence of rales
- Absence of radiographic evidence of cardiomegaly.
Patients may present without some of these classic clinical features, and the diagnosis of acute heart failure may be challenging. For example, even if left-sided pressures are very high, pulmonary edema may be absent because of pulmonary vascular remodeling in chronic heart failure.18 Pulmonary artery catheterization reveals elevated cardiac filling pressures and can be used to guide therapy, but clinical evidence argues against its routine use.19
Urine electrolytes (fractional excretion of sodium < 1% and fractional excretion of urea < 35%) often suggest a prerenal form of acute kidney injury, since the hemodynamic derangements in acute cardiorenal syndrome reduce renal perfusion.
Biomarkers of cell-cycle arrest such as urine insulinlike growth factor-binding protein 7 and tissue inhibitor of metalloproteinase 2 have recently been shown to identify patients with acute heart failure at risk of developing acute cardiorenal syndrome.20
Acute cardiorenal syndrome vs renal injury due to hypovolemia
The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Patients with stable heart failure usually have mild hypervolemia at baseline, but they can become hypovolemic due to overaggressive diuretic therapy, severe diarrhea, or other causes.
Although the fluid status of patients in these 2 conditions is opposite, they can be difficult to distinguish. In both conditions, urine electrolytes suggest a prerenal acute kidney injury. A history of recent fluid losses or diuretic overuse may help identify hypovolemia. If available, analysis of the recent trend in weight can be vital in making the right diagnosis.
Misdiagnosis of acute cardiorenal syndrome as hypovolemia-induced acute kidney injury can be catastrophic. If volume depletion is erroneously judged to be the cause of acute kidney injury, fluid administration can further worsen both cardiac and renal function. This can perpetuate the vicious circle that is already in play. Lack of renal recovery may invite further fluid administration.
TREATMENT
Fluid removal with diuresis or ultrafiltration is the cornerstone of treatment. Other treatments such as inotropes are reserved for patients with resistant disease.
Diuretics
The goal of therapy in acute cardiorenal syndrome is to achieve aggressive diuresis, typically using intravenous diuretics. Loop diuretics are the most potent class of diuretics and are the first-line drugs for this purpose. Other classes of diuretics can be used in conjunction with loop diuretics; however, using them by themselves is neither effective nor recommended.
Resistance to diuretics at usual doses is common in patients with acute cardiorenal syndrome. Several mechanisms contribute to diuretic resistance in these patients.21
Oral bioavailability of diuretics may be reduced due to intestinal edema.
Diuretic pharmacokinetics are significantly deranged in cardiorenal syndrome. All diuretics except mineralocorticoid antagonists (ie, spironolactone and eplerenone) act on targets on the luminal side of renal tubules, but are highly protein-bound and are hence not filtered at the glomerulus. Loop diuretics, thiazides, and carbonic anhydrase inhibitors are secreted in the proximal convoluted tubule via the organic anion transporter,22 whereas epithelial sodium channel inhibitors (amiloride and triamterene) are secreted via the organic cation transporter 2.23 In renal dysfunction, various uremic toxins accumulate in the body and compete with diuretics for secretion into the proximal convoluted tubule via these transporters.24
Finally, activation of the sympathetic nervous system and renin-angiotensin-aldosterone system leads to increased tubular sodium and water retention, thereby also blunting the diuretic response.
Diuretic dosage. In patients whose creatinine clearance is less than 15 mL/min, only 10% to 20% as much loop diuretic is secreted into the renal tubule as in normal individuals.25 This effect warrants dose adjustment of diuretics during uremia.
Continuous infusion or bolus? Continuous infusion of loop diuretics is another strategy to optimize drug delivery. Compared with bolus therapy, continuous infusion provides more sustained and uniform drug delivery and prevents postdiuretic sodium retention.
The Diuretic Optimization Strategies Evaluation (DOSE) trial compared the efficacy and safety of continuous vs bolus furosemide therapy in 308 patients admitted with acute decompensated heart failure.26 There was no difference in symptom control or net fluid loss at 72 hours in either group. Other studies have shown more diuresis with continuous infusion than with a similarly dosed bolus regimen.27 However, definitive clinical evidence is lacking at this point to support routine use of continuous loop diuretic therapy.
Combination diuretic therapy. Sequential nephron blockade with combination diuretic therapy is an important therapeutic strategy against diuretic resistance. Notably, urine output-guided diuretic therapy has been shown to be superior to standard diuretic therapy.28 Such therapeutic protocols may employ combination diuretic therapy as a next step when the desired diuretic response is not obtained with high doses of loop diuretic monotherapy.
The desired diuretic response depends on the clinical situation. For example, in patients with severe congestion, we would like the net fluid output to be at least 2 to 3 L more than the fluid intake after the first 24 hours. Sometimes, patients in the intensive care unit are on several essential drug infusions, so that their net intake amounts to 1 to 2 L. In these patients, the desired urine output would be even more than in patients not on these drug infusions.
Loop diuretics block sodium reabsorption at the thick ascending loop of Henle. This disrupts the countercurrent exchange mechanism and reduces renal medullary interstitial osmolarity; these effects prevent water reabsorption. However, the unresorbed sodium can be taken up by the sodium-chloride cotransporter and the epithelial sodium channel in the distal nephron, thereby blunting the diuretic effect. This is the rationale for combining loop diuretics with thiazides or potassium-sparing diuretics.
Similarly, carbonic anhydrase inhibitors (eg, acetazolamide) reduce sodium reabsorption from the proximal convoluted tubule, but most of this sodium is then reabsorbed distally. Hence, the combination of a loop diuretic and acetazolamide can also have a synergistic diuretic effect.
The most popular combination is a loop diuretic plus a thiazide, although no large-scale placebo-controlled trials have been performed.29 Metolazone (a thiazidelike diuretic) is typically used due to its low cost and availability.30 Metolazone has also been shown to block sodium reabsorption at the proximal tubule, which may contribute to its synergistic effect. Chlorothiazide is available in an intravenous formulation and has a faster onset of action than metolazone. However, studies have failed to detect any benefit of one over the other.31
The potential benefit of combining a loop diuretic with acetazolamide is a lower tendency to develop metabolic alkalosis, a potential side effect of loop diuretics and thiazides. Although data are limited, a recent study showed that adding acetazolamide to bumetanide led to significantly increased natriuresis.32
In the Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, adding spironolactone in high doses to usual therapy was not found to cause any significant change in N-terminal pro-B-type natriuretic peptide level or net urine output.33
Ultrafiltration
Venovenous ultrafiltration (or aquapheresis) employs an extracorporeal circuit, similar to the one used in hemodialysis, which removes iso-osmolar fluid at a fixed rate.34 Newer ultrafiltration systems are more portable, can be used with peripheral venous access, and require minimal nursing supervision.35
Although ultrafiltration seems an attractive alternative to diuresis in acute heart failure, studies have been inconclusive. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial compared ultrafiltration and diuresis in 188 patients with acute heart failure and acute cardiorenal syndrome.36 Diuresis, performed according to an algorithm, was found to be superior to ultrafiltration in terms of a bivariate end point of change in weight and change in serum creatinine level at 96 hours. However, the level of cystatin C is thought to be a more accurate indicator of renal function, and the change in cystatin C level from baseline did not differ between the two treatment groups. Also, the ultrafiltration rate was 200 mL per hour, which, some argue, may have been excessive and may have caused intravascular depletion.
Although the ideal rate of fluid removal is unknown, it should be individualized and adjusted based on the patient’s renal function, volume status, and hemodynamic status. The initial rate should be based on the degree of fluid overload and the anticipated plasma refill rate from the interstitial fluid.37 For example, a malnourished patient may have low serum oncotic pressure and hence have low plasma refill upon ultrafiltration. Disturbance of this delicate balance between the rates of ultrafiltration and plasma refill may lead to intravascular volume contraction.
In summary, although ultrafiltration is a valuable alternative to diuretics in resistant cases, its use as a primary decongestive therapy cannot be endorsed in view of the current data.
Inotropes
Inotropes such as dobutamine and milrinone are typically used in cases of cardiogenic shock to maintain organ perfusion. There is a physiologic rationale to using inotropes in acute cardiorenal syndrome as well, especially when the aforementioned strategies fail to overcome diuretic resistance.7
Inotropes increase cardiac output, improve renal blood flow, improve right ventricular output, and thereby relieve systemic congestion. These hemodynamic effects may improve renal perfusion and response to diuretics. However, clinical evidence to support this is lacking.
The Renal Optimization Strategies Evaluation (ROSE) trial enrolled 360 patients with acute heart failure and renal dysfunction. Adding dopamine in a low dose (2 μg/kg/min) to diuretic therapy had no significant effect on 72-hour cumulative urine output or renal function as measured by cystatin C levels.38 However, acute kidney injury was not identified in this trial, and the renal function of many of these patients may have been at its baseline when they were admitted. In other words, this trial did not necessarily include patients with acute kidney injury along with acute heart failure. Hence, it did not necessarily include patients with acute cardiorenal syndrome.
Vasodilators
Vasodilators such as nitroglycerin, sodium nitroprusside, and hydralazine are commonly used in patients with acute heart failure, although the clinical evidence supporting their use is weak.
Physiologically, arterial dilation reduces afterload and can help relieve pulmonary congestion, and venodilation increases capacitance and reduces preload. In theory, venodilators such as nitroglycerin can relieve renal venous congestion in patients with acute cardiorenal syndrome, thereby improving renal perfusion.
However, the use of vasodilators is often limited by their adverse effects, the most important being hypotension. This is especially relevant in light of recent data identifying reduction in blood pressure during treatment of acute heart failure as an independent risk factor for worsening renal function.39,40 It is important to note that in these studies, changes in cardiac index did not affect the propensity for developing worsening renal function. The precise mechanism of this finding is unclear but it is plausible that systemic vasodilation redistributes the cardiac output to nonrenal tissues, thereby overriding the renal autoregulatory mechanisms that are normally employed in low output states.
Preventive strategies
Various strategies can be used to prevent acute cardiorenal syndrome. An optimal outpatient diuretic regimen to avoid hypervolemia is essential. Patients with advanced congestive heart failure should be followed up closely in dedicated heart failure clinics until their diuretic regimen is optimized. Patients should be advised to check their weight on a regular basis and seek medical advice if they notice an increase in their weight or a reduction in their urine output.
TAKE-HOME POINTS
- A robust clinical definition of cardiorenal syndrome is lacking. Hence, recognition of this condition can be challenging.
- Volume overload is central to its pathogenesis, and accurate assessment of volume status is critical.
- Renal venous congestion is the major mechanism of type 1 cardiorenal syndrome.
- Misdiagnosis can have devastating consequences, as it may lead to an opposite therapeutic approach.
- Fluid removal by various strategies is the mainstay of treatment.
- Temporary inotropic support should be saved for the last resort.
As the heart goes, so go the kidneys—and vice versa. Cardiac and renal function are intricately interdependent, and failure of either organ causes injury to the other in a vicious circle of worsening function.1
Here, we discuss acute cardiorenal syndrome, ie, acute exacerbation of heart failure leading to acute kidney injury, a common cause of hospitalization and admission to the intensive care unit. We examine its clinical definition, pathophysiology, hemodynamic derangements, clues that help in diagnosing it, and its treatment.
A GROUP OF LINKED DISORDERS
Two types of acute cardiac dysfunction
Although these definitions offer a good general description, further clarification of the nature of organ dysfunction is needed. Acute renal dysfunction can be unambiguously defined using the AKIN (Acute Kidney Injury Network) and RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) classifications.3 Acute cardiac dysfunction, on the other hand, is an ambiguous term that encompasses 2 clinically and pathophysiologically distinct conditions: cardiogenic shock and acute heart failure.
Cardiogenic shock is characterized by a catastrophic compromise of cardiac pump function leading to global hypoperfusion severe enough to cause systemic organ damage.4 The cardiac index at which organs start to fail varies in different cases, but a value of less than 1.8 L/min/m2 is typically used to define cardiogenic shock.4
Acute heart failure, on the other hand, is defined as gradually or rapidly worsening signs and symptoms of congestive heart failure due to worsening pulmonary or systemic congestion.5 Hypervolemia is the hallmark of acute heart failure, whereas patients with cardiogenic shock may be hypervolemic, normovolemic, or hypovolemic. Although cardiac output may be mildly reduced in some cases of acute heart failure, systemic perfusion is enough to maintain organ function.
These two conditions cause renal injury by distinct mechanisms and have entirely different therapeutic implications. As we discuss later, reduced renal perfusion due to renal venous congestion is now believed to be the major hemodynamic mechanism of renal injury in acute heart failure. On the other hand, in cardiogenic shock, renal perfusion is reduced due to a critical decline of cardiac pump function.
The ideal definition of acute cardiorenal syndrome should describe a distinct pathophysiology of the syndrome and offer distinct therapeutic options that counteract it. Hence, we propose that renal injury from cardiogenic shock should not be included in its definition, an approach that has been adopted in some of the recent reviews as well.6 Our discussion of acute cardiorenal syndrome is restricted to renal injury caused by acute heart failure.
PATHOPHYSIOLOGY OF ACUTE CARDIORENAL SYNDROME
Multiple mechanisms have been implicated in the pathophysiology of cardiorenal syndrome.7,8
Sympathetic hyperactivity is a compensatory mechanism in heart failure and may be aggravated if cardiac output is further reduced. Its effects include constriction of afferent and efferent arterioles, causing reduced renal perfusion and increased tubular sodium and water reabsorption.7
The renin-angiotensin-aldosterone system is activated in patients with stable congestive heart failure and may be further stimulated in a state of reduced renal perfusion, which is a hallmark of acute cardiorenal syndrome. Its activation can cause further salt and water retention.
However, direct hemodynamic mechanisms likely play the most important role and have obvious diagnostic and therapeutic implications.
Elevated venous pressure, not reduced cardiac output, drives kidney injury
The classic view was that renal dysfunction in acute heart failure is caused by reduced renal blood flow due to failing cardiac pump function. Cardiac output may be reduced in acute heart failure for various reasons, such as atrial fibrillation, myocardial infarction, or other processes, but reduced cardiac output has a minimal role, if any, in the pathogenesis of renal injury in acute heart failure.
As evidence of this, acute heart failure is not always associated with reduced cardiac output.5 Even if the cardiac index (cardiac output divided by body surface area) is mildly reduced, renal blood flow is largely unaffected, thanks to effective renal autoregulatory mechanisms. Not until the mean arterial pressure falls below 70 mm Hg do these mechanisms fail and renal blood flow starts to drop.9 Hence, unless cardiac performance is compromised enough to cause cardiogenic shock, renal blood flow usually does not change significantly with mild reduction in cardiac output.
Hanberg et al10 performed a post hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheter Effectiveness (ESCAPE) trial, in which 525 patients with advanced heart failure underwent pulmonary artery catheterization to measure their cardiac index. The authors found no association between the cardiac index and renal function in these patients.
How venous congestion impairs the kidney
In view of the current clinical evidence, the focus has shifted to renal venous congestion. According to Poiseuille’s law, blood flow through the kidneys depends on the pressure gradient—high pressure on the arterial side, low pressure on the venous side.8 Increased renal venous pressure causes reduced renal perfusion pressure, thereby affecting renal perfusion. This is now recognized as an important hemodynamic mechanism of acute cardiorenal syndrome.
Renal congestion can also affect renal function through indirect mechanisms. For example, it can cause renal interstitial edema that may then increase the intratubular pressure, thereby reducing the transglomerular pressure gradient.11
Firth et al,14 in experiments in animals, found that increasing the renal venous pressure above 18.75 mm Hg significantly reduced the glomerular filtration rate, which completely resolved when renal venous pressure was restored to basal levels.
Mullens et al,15 in a study of 145 patients admitted with acute heart failure, reported that 58 (40%) developed acute kidney injury. Pulmonary artery catheterization revealed that elevated central venous pressure, rather than reduced cardiac index, was the primary hemodynamic factor driving renal dysfunction.
DIAGNOSIS AND CLINICAL ASSESSMENT
Patients with acute cardiorenal syndrome present with clinical features of pulmonary or systemic congestion (or both) and acute kidney injury.
Elevated left-sided pressures are usually but not always associated with elevated right-sided pressures. In a study of 1,000 patients with advanced heart failure, a pulmonary capillary wedge pressure of 22 mm Hg or higher had a positive predictive value of 88% for a right atrial pressure of 10 mm Hg or higher.16 Hence, the clinical presentation may vary depending on the location (pulmonary, systemic, or both) and degree of congestion.
Symptoms of pulmonary congestion include worsening exertional dyspnea and orthopnea; bilateral crackles may be heard on physical examination if pulmonary edema is present.
Systemic congestion can cause significant peripheral edema and weight gain. Jugular venous distention may be noted. Oliguria may be present due to renal dysfunction; patients on maintenance diuretic therapy often note its lack of efficacy.
Signs of acute heart failure
Wang et al,17 in a meta-analysis of 22 studies, concluded that the features that most strongly suggested acute heart failure were:
- History of paroxysmal nocturnal dyspnea
- A third heart sound
- Evidence of pulmonary venous congestion on chest radiography.
Features that most strongly suggested the patient did not have acute heart failure were:
- Absence of exertional dyspnea
- Absence of rales
- Absence of radiographic evidence of cardiomegaly.
Patients may present without some of these classic clinical features, and the diagnosis of acute heart failure may be challenging. For example, even if left-sided pressures are very high, pulmonary edema may be absent because of pulmonary vascular remodeling in chronic heart failure.18 Pulmonary artery catheterization reveals elevated cardiac filling pressures and can be used to guide therapy, but clinical evidence argues against its routine use.19
Urine electrolytes (fractional excretion of sodium < 1% and fractional excretion of urea < 35%) often suggest a prerenal form of acute kidney injury, since the hemodynamic derangements in acute cardiorenal syndrome reduce renal perfusion.
Biomarkers of cell-cycle arrest such as urine insulinlike growth factor-binding protein 7 and tissue inhibitor of metalloproteinase 2 have recently been shown to identify patients with acute heart failure at risk of developing acute cardiorenal syndrome.20
Acute cardiorenal syndrome vs renal injury due to hypovolemia
The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Patients with stable heart failure usually have mild hypervolemia at baseline, but they can become hypovolemic due to overaggressive diuretic therapy, severe diarrhea, or other causes.
Although the fluid status of patients in these 2 conditions is opposite, they can be difficult to distinguish. In both conditions, urine electrolytes suggest a prerenal acute kidney injury. A history of recent fluid losses or diuretic overuse may help identify hypovolemia. If available, analysis of the recent trend in weight can be vital in making the right diagnosis.
Misdiagnosis of acute cardiorenal syndrome as hypovolemia-induced acute kidney injury can be catastrophic. If volume depletion is erroneously judged to be the cause of acute kidney injury, fluid administration can further worsen both cardiac and renal function. This can perpetuate the vicious circle that is already in play. Lack of renal recovery may invite further fluid administration.
TREATMENT
Fluid removal with diuresis or ultrafiltration is the cornerstone of treatment. Other treatments such as inotropes are reserved for patients with resistant disease.
Diuretics
The goal of therapy in acute cardiorenal syndrome is to achieve aggressive diuresis, typically using intravenous diuretics. Loop diuretics are the most potent class of diuretics and are the first-line drugs for this purpose. Other classes of diuretics can be used in conjunction with loop diuretics; however, using them by themselves is neither effective nor recommended.
Resistance to diuretics at usual doses is common in patients with acute cardiorenal syndrome. Several mechanisms contribute to diuretic resistance in these patients.21
Oral bioavailability of diuretics may be reduced due to intestinal edema.
Diuretic pharmacokinetics are significantly deranged in cardiorenal syndrome. All diuretics except mineralocorticoid antagonists (ie, spironolactone and eplerenone) act on targets on the luminal side of renal tubules, but are highly protein-bound and are hence not filtered at the glomerulus. Loop diuretics, thiazides, and carbonic anhydrase inhibitors are secreted in the proximal convoluted tubule via the organic anion transporter,22 whereas epithelial sodium channel inhibitors (amiloride and triamterene) are secreted via the organic cation transporter 2.23 In renal dysfunction, various uremic toxins accumulate in the body and compete with diuretics for secretion into the proximal convoluted tubule via these transporters.24
Finally, activation of the sympathetic nervous system and renin-angiotensin-aldosterone system leads to increased tubular sodium and water retention, thereby also blunting the diuretic response.
Diuretic dosage. In patients whose creatinine clearance is less than 15 mL/min, only 10% to 20% as much loop diuretic is secreted into the renal tubule as in normal individuals.25 This effect warrants dose adjustment of diuretics during uremia.
Continuous infusion or bolus? Continuous infusion of loop diuretics is another strategy to optimize drug delivery. Compared with bolus therapy, continuous infusion provides more sustained and uniform drug delivery and prevents postdiuretic sodium retention.
The Diuretic Optimization Strategies Evaluation (DOSE) trial compared the efficacy and safety of continuous vs bolus furosemide therapy in 308 patients admitted with acute decompensated heart failure.26 There was no difference in symptom control or net fluid loss at 72 hours in either group. Other studies have shown more diuresis with continuous infusion than with a similarly dosed bolus regimen.27 However, definitive clinical evidence is lacking at this point to support routine use of continuous loop diuretic therapy.
Combination diuretic therapy. Sequential nephron blockade with combination diuretic therapy is an important therapeutic strategy against diuretic resistance. Notably, urine output-guided diuretic therapy has been shown to be superior to standard diuretic therapy.28 Such therapeutic protocols may employ combination diuretic therapy as a next step when the desired diuretic response is not obtained with high doses of loop diuretic monotherapy.
The desired diuretic response depends on the clinical situation. For example, in patients with severe congestion, we would like the net fluid output to be at least 2 to 3 L more than the fluid intake after the first 24 hours. Sometimes, patients in the intensive care unit are on several essential drug infusions, so that their net intake amounts to 1 to 2 L. In these patients, the desired urine output would be even more than in patients not on these drug infusions.
Loop diuretics block sodium reabsorption at the thick ascending loop of Henle. This disrupts the countercurrent exchange mechanism and reduces renal medullary interstitial osmolarity; these effects prevent water reabsorption. However, the unresorbed sodium can be taken up by the sodium-chloride cotransporter and the epithelial sodium channel in the distal nephron, thereby blunting the diuretic effect. This is the rationale for combining loop diuretics with thiazides or potassium-sparing diuretics.
Similarly, carbonic anhydrase inhibitors (eg, acetazolamide) reduce sodium reabsorption from the proximal convoluted tubule, but most of this sodium is then reabsorbed distally. Hence, the combination of a loop diuretic and acetazolamide can also have a synergistic diuretic effect.
The most popular combination is a loop diuretic plus a thiazide, although no large-scale placebo-controlled trials have been performed.29 Metolazone (a thiazidelike diuretic) is typically used due to its low cost and availability.30 Metolazone has also been shown to block sodium reabsorption at the proximal tubule, which may contribute to its synergistic effect. Chlorothiazide is available in an intravenous formulation and has a faster onset of action than metolazone. However, studies have failed to detect any benefit of one over the other.31
The potential benefit of combining a loop diuretic with acetazolamide is a lower tendency to develop metabolic alkalosis, a potential side effect of loop diuretics and thiazides. Although data are limited, a recent study showed that adding acetazolamide to bumetanide led to significantly increased natriuresis.32
In the Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, adding spironolactone in high doses to usual therapy was not found to cause any significant change in N-terminal pro-B-type natriuretic peptide level or net urine output.33
Ultrafiltration
Venovenous ultrafiltration (or aquapheresis) employs an extracorporeal circuit, similar to the one used in hemodialysis, which removes iso-osmolar fluid at a fixed rate.34 Newer ultrafiltration systems are more portable, can be used with peripheral venous access, and require minimal nursing supervision.35
Although ultrafiltration seems an attractive alternative to diuresis in acute heart failure, studies have been inconclusive. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial compared ultrafiltration and diuresis in 188 patients with acute heart failure and acute cardiorenal syndrome.36 Diuresis, performed according to an algorithm, was found to be superior to ultrafiltration in terms of a bivariate end point of change in weight and change in serum creatinine level at 96 hours. However, the level of cystatin C is thought to be a more accurate indicator of renal function, and the change in cystatin C level from baseline did not differ between the two treatment groups. Also, the ultrafiltration rate was 200 mL per hour, which, some argue, may have been excessive and may have caused intravascular depletion.
Although the ideal rate of fluid removal is unknown, it should be individualized and adjusted based on the patient’s renal function, volume status, and hemodynamic status. The initial rate should be based on the degree of fluid overload and the anticipated plasma refill rate from the interstitial fluid.37 For example, a malnourished patient may have low serum oncotic pressure and hence have low plasma refill upon ultrafiltration. Disturbance of this delicate balance between the rates of ultrafiltration and plasma refill may lead to intravascular volume contraction.
In summary, although ultrafiltration is a valuable alternative to diuretics in resistant cases, its use as a primary decongestive therapy cannot be endorsed in view of the current data.
Inotropes
Inotropes such as dobutamine and milrinone are typically used in cases of cardiogenic shock to maintain organ perfusion. There is a physiologic rationale to using inotropes in acute cardiorenal syndrome as well, especially when the aforementioned strategies fail to overcome diuretic resistance.7
Inotropes increase cardiac output, improve renal blood flow, improve right ventricular output, and thereby relieve systemic congestion. These hemodynamic effects may improve renal perfusion and response to diuretics. However, clinical evidence to support this is lacking.
The Renal Optimization Strategies Evaluation (ROSE) trial enrolled 360 patients with acute heart failure and renal dysfunction. Adding dopamine in a low dose (2 μg/kg/min) to diuretic therapy had no significant effect on 72-hour cumulative urine output or renal function as measured by cystatin C levels.38 However, acute kidney injury was not identified in this trial, and the renal function of many of these patients may have been at its baseline when they were admitted. In other words, this trial did not necessarily include patients with acute kidney injury along with acute heart failure. Hence, it did not necessarily include patients with acute cardiorenal syndrome.
Vasodilators
Vasodilators such as nitroglycerin, sodium nitroprusside, and hydralazine are commonly used in patients with acute heart failure, although the clinical evidence supporting their use is weak.
Physiologically, arterial dilation reduces afterload and can help relieve pulmonary congestion, and venodilation increases capacitance and reduces preload. In theory, venodilators such as nitroglycerin can relieve renal venous congestion in patients with acute cardiorenal syndrome, thereby improving renal perfusion.
However, the use of vasodilators is often limited by their adverse effects, the most important being hypotension. This is especially relevant in light of recent data identifying reduction in blood pressure during treatment of acute heart failure as an independent risk factor for worsening renal function.39,40 It is important to note that in these studies, changes in cardiac index did not affect the propensity for developing worsening renal function. The precise mechanism of this finding is unclear but it is plausible that systemic vasodilation redistributes the cardiac output to nonrenal tissues, thereby overriding the renal autoregulatory mechanisms that are normally employed in low output states.
Preventive strategies
Various strategies can be used to prevent acute cardiorenal syndrome. An optimal outpatient diuretic regimen to avoid hypervolemia is essential. Patients with advanced congestive heart failure should be followed up closely in dedicated heart failure clinics until their diuretic regimen is optimized. Patients should be advised to check their weight on a regular basis and seek medical advice if they notice an increase in their weight or a reduction in their urine output.
TAKE-HOME POINTS
- A robust clinical definition of cardiorenal syndrome is lacking. Hence, recognition of this condition can be challenging.
- Volume overload is central to its pathogenesis, and accurate assessment of volume status is critical.
- Renal venous congestion is the major mechanism of type 1 cardiorenal syndrome.
- Misdiagnosis can have devastating consequences, as it may lead to an opposite therapeutic approach.
- Fluid removal by various strategies is the mainstay of treatment.
- Temporary inotropic support should be saved for the last resort.
- Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Cleve Clin J Med 2006; 73:485–491.
- House AA, Anand I, Bellomo R, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Chang CH, Lin CY, Tian YC, et al. Acute kidney injury classification: comparison of AKIN and RIFLE criteria. Shock 2010; 33:247-252.
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008; 117:686–697.
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009; 53:557–573.
- ter Maaten JM, Valente MA, Damman K, et al. Diuretic response in acute heart failure—pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015; 12:184–192.
- Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 2013; 9:99–111.
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121:2592–2600.
- Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Afsar B, Ortiz A, Covic A, et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9:39–47.
- Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013; 62:485–495.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1988; 1:1033–1035.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18:1126–1132.
- Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 2004; 125:669–682.
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294:1625–1633.
- Schanz M, Shi J , Wasser C , Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 2017; doi.org/10.1002/clc.22683.
- Bowman BN, Nawarskas JJ, Anderson JR. Treating diuretic resistance: an overview. Cardiol Rev 2016; 24:256–260.
- Uwai Y, Saito H, Hashimoto Y, Inui KI. Interaction and transport of thiazide diuretics, loop diuretics, and acetazolamide via rat renal organic anion transporter rOAT1. J Pharmacol Exp Ther 2000; 295:261–265.
- Hacker K, Maas R, Kornhuber J, et al. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One 2015; 10:e0136451.
- Schophuizen CM, Wilmer MJ, Jansen J, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch 2013; 465:1701–1714.
- Brater DC. Diuretic therapy. N Engl J Med 1998; 339:387–395.
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail 2010; 16:188–193.
- Grodin JL, Stevens SR, de Las Fuentes L, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail 2016; 22:26–32.
- Ng TM, Konopka E, Hyderi AF, et al. Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther 2013; 18:345–353.
- Sica DA. Metolazone and its role in edema management. Congest Heart Fail 2003; 9:100–105.
- Moranville MP, Choi S, Hogg J, Anderson AS, Rich JD. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015; 33:42–49.
- Verbrugge FH, Dupont M, Bertrand PB, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 2015; 70:265–373.
- Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017 Jul 12. doi: 10.1001/jamacardio.2017.2198. [Epub ahead of print]
- Pourafshar N, Karimi A, Kazory A. Extracorporeal ultrafiltration therapy for acute decompensated heart failure. Expert Rev Cardiovasc Ther 2016; 14:5–13.
- Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail 2003; 9:227–231.
- Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Kazory A. Cardiorenal syndrome: ultrafiltration therapy for heart failure—trials and tribulations. Clin J Am Soc Nephrol 2013; 8:1816–1828.
- Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Testani JM, Coca SG, McCauley BD, et al. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011; 13:877–884.
- Dupont M, Mullens W, Finucan M, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013; 15:433–440.
- Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Cleve Clin J Med 2006; 73:485–491.
- House AA, Anand I, Bellomo R, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Chang CH, Lin CY, Tian YC, et al. Acute kidney injury classification: comparison of AKIN and RIFLE criteria. Shock 2010; 33:247-252.
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008; 117:686–697.
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009; 53:557–573.
- ter Maaten JM, Valente MA, Damman K, et al. Diuretic response in acute heart failure—pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015; 12:184–192.
- Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 2013; 9:99–111.
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121:2592–2600.
- Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Afsar B, Ortiz A, Covic A, et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9:39–47.
- Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013; 62:485–495.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1988; 1:1033–1035.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18:1126–1132.
- Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 2004; 125:669–682.
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294:1625–1633.
- Schanz M, Shi J , Wasser C , Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 2017; doi.org/10.1002/clc.22683.
- Bowman BN, Nawarskas JJ, Anderson JR. Treating diuretic resistance: an overview. Cardiol Rev 2016; 24:256–260.
- Uwai Y, Saito H, Hashimoto Y, Inui KI. Interaction and transport of thiazide diuretics, loop diuretics, and acetazolamide via rat renal organic anion transporter rOAT1. J Pharmacol Exp Ther 2000; 295:261–265.
- Hacker K, Maas R, Kornhuber J, et al. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One 2015; 10:e0136451.
- Schophuizen CM, Wilmer MJ, Jansen J, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch 2013; 465:1701–1714.
- Brater DC. Diuretic therapy. N Engl J Med 1998; 339:387–395.
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail 2010; 16:188–193.
- Grodin JL, Stevens SR, de Las Fuentes L, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail 2016; 22:26–32.
- Ng TM, Konopka E, Hyderi AF, et al. Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther 2013; 18:345–353.
- Sica DA. Metolazone and its role in edema management. Congest Heart Fail 2003; 9:100–105.
- Moranville MP, Choi S, Hogg J, Anderson AS, Rich JD. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015; 33:42–49.
- Verbrugge FH, Dupont M, Bertrand PB, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 2015; 70:265–373.
- Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017 Jul 12. doi: 10.1001/jamacardio.2017.2198. [Epub ahead of print]
- Pourafshar N, Karimi A, Kazory A. Extracorporeal ultrafiltration therapy for acute decompensated heart failure. Expert Rev Cardiovasc Ther 2016; 14:5–13.
- Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail 2003; 9:227–231.
- Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Kazory A. Cardiorenal syndrome: ultrafiltration therapy for heart failure—trials and tribulations. Clin J Am Soc Nephrol 2013; 8:1816–1828.
- Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Testani JM, Coca SG, McCauley BD, et al. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011; 13:877–884.
- Dupont M, Mullens W, Finucan M, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013; 15:433–440.
KEY POINTS
- Acute cardiorenal syndrome is the acute worsening of renal function due to acute decompensated heart failure.
- The most important mechanism of acute cardiorenal syndrome is now believed to be systemic congestion leading to increased renal venous pressure, which in turn reduces renal perfusion.
- The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Differentiating the 2 may be challenging if signs of systemic and pulmonary congestion are not obvious.
- Diuretic resistance is common in acute cardiorenal syndrome but may be overcome by using higher doses of diuretics and combinations of diuretics that block reabsorption at different segments of the renal tubules.
Hemodynamically, the kidney is at the heart of cardiorenal syndrome
In heart failure, the heart and the kidneys share a rocky relationship. Cardiac dysfunction can heighten renal dysfunction and vice versa—appropriately dubbed “cardiorenal syndrome.”
Although classically defined by a reduction in the glomerular filtration rate (GFR),1 cardiorenal syndrome also encompasses complex neurohormonal, pharmacologic, and metabolic interactions affecting or affected by both glomerular and tubular function. Unfortunately, all of these maladaptive processes occur in heart failure and perpetuate a vicious circle of continued dual-organ dysfunction.
The central insult here is hemodynamic disarray from acute or chronic cardiac dysfunction, which can directly influence glomerular function. However, to understand the hemodynamic ramifications for glomerular function, we focus on the determinants of glomerular filtration.
DETERMINANTS OF GFR
The GFR is the rate of fluid flow between the glomerular capillaries and the Bowman capsule and is classically represented by the following equations2:
GFR = Kf × (PG – PB – πG + πB)
Kf = N × Lp × S
Kf is the filtration constant, N the number of functional nephrons, Lp the hydraulic conductivity of the glomerular capillary, S the filtration area, PG the hydrostatic pressure in the glomerular capillaries, PB the hydrostatic pressure in the Bowman capsule, and πG and πB the colloid osmotic pressures within the glomerular capillaries and Bowman space, respectively.
Based on this relationship, the GFR is reduced when PG is reduced in the setting of hypovolemia, hypotension, or renin-angiotensin system antagonist use or when PB is increased in the setting of elevated central venous pressure or elevated abdominal pressure—all common in heart failure. With this understanding, one would assume that strategies to increase PG (improve perfusion) and reduce PB (reduce congestion) might ameliorate ongoing renal dysfunction and improve the GFR in heart failure.
In this issue, Thind et al3 highlight the impact of hemodynamic derangements in heart failure with acute cardiorenal syndrome and provide an overview of its treatment. They review the complex relationship between progressive cardiac failure translating into accelerated neurohormonal responses (increases in sympathetic nervous system and renin-angiotensin-aldosterone system activation) and the impact of increased central venous pressure on progressive renal dysfunction. They also provide an overview of efforts to mitigate cardiorenal syndrome, after careful appraisal of volume status, through diuretic-mediated decongestion with aggressive use of loop diuretics (either in isolation or in the form of sequential nephron blockade with a thiazide or acetazolamide), and they highlight the lingering uncertainty regarding inotrope use.
VENOUS CONGESTION VS DECREASED CARDIAC OUTPUT
Returning to the GFR equation, it is clear that an imbalance in PG and PB can worsen glomerular function. Because cardiac dysfunction can lead to both venous congestion and decreased cardiac output, this leads to the question, “Of these, which is the more important driver of this imbalance and its effects on renal function?”
A compelling argument can be made for each side. On one hand, experiments over a half-century old in human models of venous congestion highlighted the profound impact of elevated venous pressure, which decreases electrolyte excretion (sodium included) and diminishes urine flow.4,5 This has been replicated in more-contemporary decompensated heart failure cohorts in which worsening renal function was more closely associated with elevated central venous pressure rather than cardiac output.6,7 On the other hand, early landmark experiments and more recent cohorts with heart failure have also shown that reductions in effective arterial blood volume, renal blood flow, and cardiac output are also associated with reductions in GFR.5,8,9
How then shall we reconcile whether cardiorenal syndrome is a “backward failure” (from central venous pressure) or a “forward failure” (from decreased perfusion) phenomenon?
The answer is complicated and is likely “both,” with the major component being increased central venous pressure. To understand this construct, we must first exclude frank cardiogenic shock—when the hydraulic function of the heart fails to provide enough flow, leading to a catastrophic drop in mean arterial pressure that supersedes the kidney’s ability to autoregulate renal blood flow.10,11
In patients with chronic heart failure and congestion who are not in shock, historical observations suggest that both intra-abdominal pressure (which increases renal venous pressure) and central venous pressure lead to reduced renal blood flow and increased renal vasomotor resistance (increase in afferent, intrarenal, and efferent vascular tone).12–14 More recent observations from epidemiologic studies have largely replicated these findings. Central venous pressure remains essential to impacting renal function in heart failure,6,15 and the impact of cardiac output on renal function remains uncertain.16
The relationship of intracardiac hemodynamics may also play a role in modifying renal function. Several reports recently described the relationship between both right- and left-sided filling pressures as being associated with worse renal function in heart failure.17–19 Patients with a disproportionately higher right atrial pressure to pulmonary capillary wedge pressure have higher serum creatinine during and after decongestive therapies. Therefore, the concept of “right-sided heart failure” expands beyond the simple representation of “backward congestion” at the level of venous return. In fact, a higher ratio of right atrial pressure to pulmonary capillary wedge pressure may point to an inability of the venous and pulmonary circulations to provide adequate left ventricular preload. Therefore, a relatively underfilled left ventricle in the face of biventricular dysfunction may result in worsening renal function.
TREATMENT IS CHALLENGING
The treatment of cardiorenal syndrome is challenging. It is often accompanied by heightened azotemia, diuretic resistance, electrolyte abnormalities, and a spectrum of hemodynamic disarray. As Thind et al point out, there is, unfortunately, no firmly established treatment. While “sequential nephron blockade” (pharmacologically blocking multiple sites on the nephron simultaneously) is theoretically promising, there are no rigorously studied therapeutic strategies with proven efficacy.
On the other hand, mechanical removal of isotonic fluid with ultrafiltration showed early promise in decompensated heart failure, but enthusiasm diminished with results from the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial.20 Ultrafiltration was roughly equivalent to aggressive pharmacologic therapy for fluid loss, was associated with higher serum creatinine levels, and was more challenging to administer.
Equally uncertain is the benefit of inotropic or vasoactive therapy, which directly alters cardiac hemodynamics. Low-dose dopamine or low-dose nesiritide is of no benefit toward enhancement of decongestion or renal protection when added to standard diuretic therapy.21 Furthermore, routine use of inotropes is fraught with more arrhythmias and hypotension and is associated with dismal long-term outcomes.22,23
Alternative therapies that act directly on renal physiology—eg, rolofylline, a selective adenosine A1 receptor antagonist that may enhance renal blood flow, augment natriuresis, and break diuretic resistance—have been similarly disappointing.24
With so much uncertainty, more investigation into novel treatments for cardiorenal syndrome is clearly warranted.
However, because venous congestion is the hemodynamic hallmark of acute cardiorenal syndrome (increasing PB), reducing central venous pressure remains the cornerstone treatment for cardiorenal syndrome. Additionally, efforts to preserve renal perfusion and avoid hypotension are prudent to maintain glomerular capillary hydrostatic pressure (PG).
In light of these considerations, there is no “one size fits all” for the treatment of cardiorenal syndrome. Treatment should be based on thoughtful individualized strategies tailored to the underlying cardiorenal pathophysiology, and with the understanding that the kidney is at the heart of the matter.
- House AA, Anand I, Bellomo R, et al; Acute Dialysis Quality Initiative Consensus Group. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Tucker BJ, Blantz RC. An analysis of the determinants of nephron filtration rate. Am J Physiol 1977; 232:F477–F483.
- Thind GS, Loehrke M, Wilt JL. Acute cardiorenal syndrome: mechanisms and clinical implications. Cleve Clin J Med 2018; 85:231–239.
- Wilkins RW, Tinsley CM, Culbertson JW, et al. The effects of venous congestion of the limbs upon renal clearances and the excretion of water and salt. I. Studies in normal subjects and in hypertensive patients before and after splanchnicectomy. J Clin Invest 1953; 32:1101–1116.
- Judson WE, Hatcher JD, Hollander W, Halperin MH, Wilkins RW. The effects of venous congestion of the limbs and phlebotomy upon renal clearances and the excretion of water and salt. II. Studies in patients with congestive failure. J Clin Invest 1955; 34:1591–1599.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53:582–588.
- Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990; 39(suppl 4):10–21; discussion 22–24.
- Damman K, Navis G, Smilde TD, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9:872–878.
- Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004; 44:340–348.
- Adams PL, Adams FF, Bell PD, Navar LG. Impaired renal blood flow autoregulation in ischemic acute renal failure. Kidney Int 1980; 18:68–76.
- Maxwell MH, Breed ES, Schwartz IL. Renal venous pressure in chronic congestive heart failure. J Clin Invest 1950; 29:342–348.
- Blake WD, Wégria R, Keating RP, Ward HP. Effect of increased renal venous pressure on renal function. Am J Physiol 1949; 157:1–13.
- Bradley SE, Bradley GP. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest 1947; 26:1010–1022.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant 2012; 31:67–72.
- Drazner MH, Velez-Martinez M, Ayers CR, et al. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail 2013; 6:264–270.
- Grodin JL, Drazner MH, Dupont M, et al. A disproportionate elevation in right ventricular filling pressure, in relation to left ventricular filling pressure, is associated with renal impairment and increased mortality in advanced decompensated heart failure. Am Heart J 2015; 169:806–812.
- Bart BA, Goldsmith SR, Lee KL, et al, for the Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Chen HH, Anstrom KJ, Givertz MM, et al; NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324.
- Cuffe MS, Califf RM, Adams KF Jr, et al, for the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002; 287:1541–1547.
- Massie BM, O’Connor CM, Metra M, et al, for the PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010; 363:1419–1428.
In heart failure, the heart and the kidneys share a rocky relationship. Cardiac dysfunction can heighten renal dysfunction and vice versa—appropriately dubbed “cardiorenal syndrome.”
Although classically defined by a reduction in the glomerular filtration rate (GFR),1 cardiorenal syndrome also encompasses complex neurohormonal, pharmacologic, and metabolic interactions affecting or affected by both glomerular and tubular function. Unfortunately, all of these maladaptive processes occur in heart failure and perpetuate a vicious circle of continued dual-organ dysfunction.
The central insult here is hemodynamic disarray from acute or chronic cardiac dysfunction, which can directly influence glomerular function. However, to understand the hemodynamic ramifications for glomerular function, we focus on the determinants of glomerular filtration.
DETERMINANTS OF GFR
The GFR is the rate of fluid flow between the glomerular capillaries and the Bowman capsule and is classically represented by the following equations2:
GFR = Kf × (PG – PB – πG + πB)
Kf = N × Lp × S
Kf is the filtration constant, N the number of functional nephrons, Lp the hydraulic conductivity of the glomerular capillary, S the filtration area, PG the hydrostatic pressure in the glomerular capillaries, PB the hydrostatic pressure in the Bowman capsule, and πG and πB the colloid osmotic pressures within the glomerular capillaries and Bowman space, respectively.
Based on this relationship, the GFR is reduced when PG is reduced in the setting of hypovolemia, hypotension, or renin-angiotensin system antagonist use or when PB is increased in the setting of elevated central venous pressure or elevated abdominal pressure—all common in heart failure. With this understanding, one would assume that strategies to increase PG (improve perfusion) and reduce PB (reduce congestion) might ameliorate ongoing renal dysfunction and improve the GFR in heart failure.
In this issue, Thind et al3 highlight the impact of hemodynamic derangements in heart failure with acute cardiorenal syndrome and provide an overview of its treatment. They review the complex relationship between progressive cardiac failure translating into accelerated neurohormonal responses (increases in sympathetic nervous system and renin-angiotensin-aldosterone system activation) and the impact of increased central venous pressure on progressive renal dysfunction. They also provide an overview of efforts to mitigate cardiorenal syndrome, after careful appraisal of volume status, through diuretic-mediated decongestion with aggressive use of loop diuretics (either in isolation or in the form of sequential nephron blockade with a thiazide or acetazolamide), and they highlight the lingering uncertainty regarding inotrope use.
VENOUS CONGESTION VS DECREASED CARDIAC OUTPUT
Returning to the GFR equation, it is clear that an imbalance in PG and PB can worsen glomerular function. Because cardiac dysfunction can lead to both venous congestion and decreased cardiac output, this leads to the question, “Of these, which is the more important driver of this imbalance and its effects on renal function?”
A compelling argument can be made for each side. On one hand, experiments over a half-century old in human models of venous congestion highlighted the profound impact of elevated venous pressure, which decreases electrolyte excretion (sodium included) and diminishes urine flow.4,5 This has been replicated in more-contemporary decompensated heart failure cohorts in which worsening renal function was more closely associated with elevated central venous pressure rather than cardiac output.6,7 On the other hand, early landmark experiments and more recent cohorts with heart failure have also shown that reductions in effective arterial blood volume, renal blood flow, and cardiac output are also associated with reductions in GFR.5,8,9
How then shall we reconcile whether cardiorenal syndrome is a “backward failure” (from central venous pressure) or a “forward failure” (from decreased perfusion) phenomenon?
The answer is complicated and is likely “both,” with the major component being increased central venous pressure. To understand this construct, we must first exclude frank cardiogenic shock—when the hydraulic function of the heart fails to provide enough flow, leading to a catastrophic drop in mean arterial pressure that supersedes the kidney’s ability to autoregulate renal blood flow.10,11
In patients with chronic heart failure and congestion who are not in shock, historical observations suggest that both intra-abdominal pressure (which increases renal venous pressure) and central venous pressure lead to reduced renal blood flow and increased renal vasomotor resistance (increase in afferent, intrarenal, and efferent vascular tone).12–14 More recent observations from epidemiologic studies have largely replicated these findings. Central venous pressure remains essential to impacting renal function in heart failure,6,15 and the impact of cardiac output on renal function remains uncertain.16
The relationship of intracardiac hemodynamics may also play a role in modifying renal function. Several reports recently described the relationship between both right- and left-sided filling pressures as being associated with worse renal function in heart failure.17–19 Patients with a disproportionately higher right atrial pressure to pulmonary capillary wedge pressure have higher serum creatinine during and after decongestive therapies. Therefore, the concept of “right-sided heart failure” expands beyond the simple representation of “backward congestion” at the level of venous return. In fact, a higher ratio of right atrial pressure to pulmonary capillary wedge pressure may point to an inability of the venous and pulmonary circulations to provide adequate left ventricular preload. Therefore, a relatively underfilled left ventricle in the face of biventricular dysfunction may result in worsening renal function.
TREATMENT IS CHALLENGING
The treatment of cardiorenal syndrome is challenging. It is often accompanied by heightened azotemia, diuretic resistance, electrolyte abnormalities, and a spectrum of hemodynamic disarray. As Thind et al point out, there is, unfortunately, no firmly established treatment. While “sequential nephron blockade” (pharmacologically blocking multiple sites on the nephron simultaneously) is theoretically promising, there are no rigorously studied therapeutic strategies with proven efficacy.
On the other hand, mechanical removal of isotonic fluid with ultrafiltration showed early promise in decompensated heart failure, but enthusiasm diminished with results from the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial.20 Ultrafiltration was roughly equivalent to aggressive pharmacologic therapy for fluid loss, was associated with higher serum creatinine levels, and was more challenging to administer.
Equally uncertain is the benefit of inotropic or vasoactive therapy, which directly alters cardiac hemodynamics. Low-dose dopamine or low-dose nesiritide is of no benefit toward enhancement of decongestion or renal protection when added to standard diuretic therapy.21 Furthermore, routine use of inotropes is fraught with more arrhythmias and hypotension and is associated with dismal long-term outcomes.22,23
Alternative therapies that act directly on renal physiology—eg, rolofylline, a selective adenosine A1 receptor antagonist that may enhance renal blood flow, augment natriuresis, and break diuretic resistance—have been similarly disappointing.24
With so much uncertainty, more investigation into novel treatments for cardiorenal syndrome is clearly warranted.
However, because venous congestion is the hemodynamic hallmark of acute cardiorenal syndrome (increasing PB), reducing central venous pressure remains the cornerstone treatment for cardiorenal syndrome. Additionally, efforts to preserve renal perfusion and avoid hypotension are prudent to maintain glomerular capillary hydrostatic pressure (PG).
In light of these considerations, there is no “one size fits all” for the treatment of cardiorenal syndrome. Treatment should be based on thoughtful individualized strategies tailored to the underlying cardiorenal pathophysiology, and with the understanding that the kidney is at the heart of the matter.
In heart failure, the heart and the kidneys share a rocky relationship. Cardiac dysfunction can heighten renal dysfunction and vice versa—appropriately dubbed “cardiorenal syndrome.”
Although classically defined by a reduction in the glomerular filtration rate (GFR),1 cardiorenal syndrome also encompasses complex neurohormonal, pharmacologic, and metabolic interactions affecting or affected by both glomerular and tubular function. Unfortunately, all of these maladaptive processes occur in heart failure and perpetuate a vicious circle of continued dual-organ dysfunction.
The central insult here is hemodynamic disarray from acute or chronic cardiac dysfunction, which can directly influence glomerular function. However, to understand the hemodynamic ramifications for glomerular function, we focus on the determinants of glomerular filtration.
DETERMINANTS OF GFR
The GFR is the rate of fluid flow between the glomerular capillaries and the Bowman capsule and is classically represented by the following equations2:
GFR = Kf × (PG – PB – πG + πB)
Kf = N × Lp × S
Kf is the filtration constant, N the number of functional nephrons, Lp the hydraulic conductivity of the glomerular capillary, S the filtration area, PG the hydrostatic pressure in the glomerular capillaries, PB the hydrostatic pressure in the Bowman capsule, and πG and πB the colloid osmotic pressures within the glomerular capillaries and Bowman space, respectively.
Based on this relationship, the GFR is reduced when PG is reduced in the setting of hypovolemia, hypotension, or renin-angiotensin system antagonist use or when PB is increased in the setting of elevated central venous pressure or elevated abdominal pressure—all common in heart failure. With this understanding, one would assume that strategies to increase PG (improve perfusion) and reduce PB (reduce congestion) might ameliorate ongoing renal dysfunction and improve the GFR in heart failure.
In this issue, Thind et al3 highlight the impact of hemodynamic derangements in heart failure with acute cardiorenal syndrome and provide an overview of its treatment. They review the complex relationship between progressive cardiac failure translating into accelerated neurohormonal responses (increases in sympathetic nervous system and renin-angiotensin-aldosterone system activation) and the impact of increased central venous pressure on progressive renal dysfunction. They also provide an overview of efforts to mitigate cardiorenal syndrome, after careful appraisal of volume status, through diuretic-mediated decongestion with aggressive use of loop diuretics (either in isolation or in the form of sequential nephron blockade with a thiazide or acetazolamide), and they highlight the lingering uncertainty regarding inotrope use.
VENOUS CONGESTION VS DECREASED CARDIAC OUTPUT
Returning to the GFR equation, it is clear that an imbalance in PG and PB can worsen glomerular function. Because cardiac dysfunction can lead to both venous congestion and decreased cardiac output, this leads to the question, “Of these, which is the more important driver of this imbalance and its effects on renal function?”
A compelling argument can be made for each side. On one hand, experiments over a half-century old in human models of venous congestion highlighted the profound impact of elevated venous pressure, which decreases electrolyte excretion (sodium included) and diminishes urine flow.4,5 This has been replicated in more-contemporary decompensated heart failure cohorts in which worsening renal function was more closely associated with elevated central venous pressure rather than cardiac output.6,7 On the other hand, early landmark experiments and more recent cohorts with heart failure have also shown that reductions in effective arterial blood volume, renal blood flow, and cardiac output are also associated with reductions in GFR.5,8,9
How then shall we reconcile whether cardiorenal syndrome is a “backward failure” (from central venous pressure) or a “forward failure” (from decreased perfusion) phenomenon?
The answer is complicated and is likely “both,” with the major component being increased central venous pressure. To understand this construct, we must first exclude frank cardiogenic shock—when the hydraulic function of the heart fails to provide enough flow, leading to a catastrophic drop in mean arterial pressure that supersedes the kidney’s ability to autoregulate renal blood flow.10,11
In patients with chronic heart failure and congestion who are not in shock, historical observations suggest that both intra-abdominal pressure (which increases renal venous pressure) and central venous pressure lead to reduced renal blood flow and increased renal vasomotor resistance (increase in afferent, intrarenal, and efferent vascular tone).12–14 More recent observations from epidemiologic studies have largely replicated these findings. Central venous pressure remains essential to impacting renal function in heart failure,6,15 and the impact of cardiac output on renal function remains uncertain.16
The relationship of intracardiac hemodynamics may also play a role in modifying renal function. Several reports recently described the relationship between both right- and left-sided filling pressures as being associated with worse renal function in heart failure.17–19 Patients with a disproportionately higher right atrial pressure to pulmonary capillary wedge pressure have higher serum creatinine during and after decongestive therapies. Therefore, the concept of “right-sided heart failure” expands beyond the simple representation of “backward congestion” at the level of venous return. In fact, a higher ratio of right atrial pressure to pulmonary capillary wedge pressure may point to an inability of the venous and pulmonary circulations to provide adequate left ventricular preload. Therefore, a relatively underfilled left ventricle in the face of biventricular dysfunction may result in worsening renal function.
TREATMENT IS CHALLENGING
The treatment of cardiorenal syndrome is challenging. It is often accompanied by heightened azotemia, diuretic resistance, electrolyte abnormalities, and a spectrum of hemodynamic disarray. As Thind et al point out, there is, unfortunately, no firmly established treatment. While “sequential nephron blockade” (pharmacologically blocking multiple sites on the nephron simultaneously) is theoretically promising, there are no rigorously studied therapeutic strategies with proven efficacy.
On the other hand, mechanical removal of isotonic fluid with ultrafiltration showed early promise in decompensated heart failure, but enthusiasm diminished with results from the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial.20 Ultrafiltration was roughly equivalent to aggressive pharmacologic therapy for fluid loss, was associated with higher serum creatinine levels, and was more challenging to administer.
Equally uncertain is the benefit of inotropic or vasoactive therapy, which directly alters cardiac hemodynamics. Low-dose dopamine or low-dose nesiritide is of no benefit toward enhancement of decongestion or renal protection when added to standard diuretic therapy.21 Furthermore, routine use of inotropes is fraught with more arrhythmias and hypotension and is associated with dismal long-term outcomes.22,23
Alternative therapies that act directly on renal physiology—eg, rolofylline, a selective adenosine A1 receptor antagonist that may enhance renal blood flow, augment natriuresis, and break diuretic resistance—have been similarly disappointing.24
With so much uncertainty, more investigation into novel treatments for cardiorenal syndrome is clearly warranted.
However, because venous congestion is the hemodynamic hallmark of acute cardiorenal syndrome (increasing PB), reducing central venous pressure remains the cornerstone treatment for cardiorenal syndrome. Additionally, efforts to preserve renal perfusion and avoid hypotension are prudent to maintain glomerular capillary hydrostatic pressure (PG).
In light of these considerations, there is no “one size fits all” for the treatment of cardiorenal syndrome. Treatment should be based on thoughtful individualized strategies tailored to the underlying cardiorenal pathophysiology, and with the understanding that the kidney is at the heart of the matter.
- House AA, Anand I, Bellomo R, et al; Acute Dialysis Quality Initiative Consensus Group. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Tucker BJ, Blantz RC. An analysis of the determinants of nephron filtration rate. Am J Physiol 1977; 232:F477–F483.
- Thind GS, Loehrke M, Wilt JL. Acute cardiorenal syndrome: mechanisms and clinical implications. Cleve Clin J Med 2018; 85:231–239.
- Wilkins RW, Tinsley CM, Culbertson JW, et al. The effects of venous congestion of the limbs upon renal clearances and the excretion of water and salt. I. Studies in normal subjects and in hypertensive patients before and after splanchnicectomy. J Clin Invest 1953; 32:1101–1116.
- Judson WE, Hatcher JD, Hollander W, Halperin MH, Wilkins RW. The effects of venous congestion of the limbs and phlebotomy upon renal clearances and the excretion of water and salt. II. Studies in patients with congestive failure. J Clin Invest 1955; 34:1591–1599.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53:582–588.
- Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990; 39(suppl 4):10–21; discussion 22–24.
- Damman K, Navis G, Smilde TD, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9:872–878.
- Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004; 44:340–348.
- Adams PL, Adams FF, Bell PD, Navar LG. Impaired renal blood flow autoregulation in ischemic acute renal failure. Kidney Int 1980; 18:68–76.
- Maxwell MH, Breed ES, Schwartz IL. Renal venous pressure in chronic congestive heart failure. J Clin Invest 1950; 29:342–348.
- Blake WD, Wégria R, Keating RP, Ward HP. Effect of increased renal venous pressure on renal function. Am J Physiol 1949; 157:1–13.
- Bradley SE, Bradley GP. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest 1947; 26:1010–1022.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant 2012; 31:67–72.
- Drazner MH, Velez-Martinez M, Ayers CR, et al. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail 2013; 6:264–270.
- Grodin JL, Drazner MH, Dupont M, et al. A disproportionate elevation in right ventricular filling pressure, in relation to left ventricular filling pressure, is associated with renal impairment and increased mortality in advanced decompensated heart failure. Am Heart J 2015; 169:806–812.
- Bart BA, Goldsmith SR, Lee KL, et al, for the Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Chen HH, Anstrom KJ, Givertz MM, et al; NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324.
- Cuffe MS, Califf RM, Adams KF Jr, et al, for the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002; 287:1541–1547.
- Massie BM, O’Connor CM, Metra M, et al, for the PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010; 363:1419–1428.
- House AA, Anand I, Bellomo R, et al; Acute Dialysis Quality Initiative Consensus Group. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Tucker BJ, Blantz RC. An analysis of the determinants of nephron filtration rate. Am J Physiol 1977; 232:F477–F483.
- Thind GS, Loehrke M, Wilt JL. Acute cardiorenal syndrome: mechanisms and clinical implications. Cleve Clin J Med 2018; 85:231–239.
- Wilkins RW, Tinsley CM, Culbertson JW, et al. The effects of venous congestion of the limbs upon renal clearances and the excretion of water and salt. I. Studies in normal subjects and in hypertensive patients before and after splanchnicectomy. J Clin Invest 1953; 32:1101–1116.
- Judson WE, Hatcher JD, Hollander W, Halperin MH, Wilkins RW. The effects of venous congestion of the limbs and phlebotomy upon renal clearances and the excretion of water and salt. II. Studies in patients with congestive failure. J Clin Invest 1955; 34:1591–1599.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53:582–588.
- Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990; 39(suppl 4):10–21; discussion 22–24.
- Damman K, Navis G, Smilde TD, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9:872–878.
- Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004; 44:340–348.
- Adams PL, Adams FF, Bell PD, Navar LG. Impaired renal blood flow autoregulation in ischemic acute renal failure. Kidney Int 1980; 18:68–76.
- Maxwell MH, Breed ES, Schwartz IL. Renal venous pressure in chronic congestive heart failure. J Clin Invest 1950; 29:342–348.
- Blake WD, Wégria R, Keating RP, Ward HP. Effect of increased renal venous pressure on renal function. Am J Physiol 1949; 157:1–13.
- Bradley SE, Bradley GP. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest 1947; 26:1010–1022.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant 2012; 31:67–72.
- Drazner MH, Velez-Martinez M, Ayers CR, et al. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail 2013; 6:264–270.
- Grodin JL, Drazner MH, Dupont M, et al. A disproportionate elevation in right ventricular filling pressure, in relation to left ventricular filling pressure, is associated with renal impairment and increased mortality in advanced decompensated heart failure. Am Heart J 2015; 169:806–812.
- Bart BA, Goldsmith SR, Lee KL, et al, for the Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Chen HH, Anstrom KJ, Givertz MM, et al; NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324.
- Cuffe MS, Califf RM, Adams KF Jr, et al, for the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002; 287:1541–1547.
- Massie BM, O’Connor CM, Metra M, et al, for the PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010; 363:1419–1428.
Disruptive Physician Behavior: The Importance of Recognition and Intervention and Its Impact on Patient Safety
Dramatic stories of disruptive physician behavior (DPB) appear occasionally in the news, such as the physician who shot and killed a colleague within hospital confines or the gynecologist who secretly took photographs using a camera disguised as a pen during pelvic examinations. More common in hospitals, however, are incidents of inappropriate behavior that may generate complaints from patients or other providers and at times snowball into administrative or legal challenges.
“Professionalism” is one of the six competencies listed by the Accreditation Council for Graduate Medical Education (ACGME)1 and the American Board of Medical Specialties. Unfortunately, incidents of disruptive behavior can result in violation of the tenets of professionalism in the healthcare environment. These behaviors fall along a continuum ranging from outwardly aggressive and uncivil to overly passive and insidious. Although these behaviors can occur across all healthcare disciplines and settings and are not just limited to physicians, the behaviors of physicians often have a much greater impact on the healthcare system as a whole because of their positions of relative “power” within the system.2 Hence, this problem requires greater awareness and education. In this context, the aim of this article is to discuss disruptive behaviors in physicians
The AMA defines DPB as “personal conduct, verbal or physical that has the potential to negatively affect patient care or the ability to work with other members of the healthcare team.”3 The definition of DPB by the Joint Commission includes “all behaviors that undermine a culture of safety.”4 Both the Joint Commission and the AMA recognize the significance and patient safety implications of such behavior. Policy statements by both these organizations underscore the importance of confronting and remedying these potentially dangerous interpersonal behaviors.
Data regarding the prevalence of DPB have been inconsistent. One study estimated that 3%–5% of physicians demonstrate this behavior,5 whereas another study reported a DPB prevalence of 97% among physicians and nurses in the workplace.6 According to a 2004 survey of physician executives, more than 95% of them reported regular encounters of DPB.7
The etiology of such disruptive behaviors is multifactorial and complex. Explanations associated with ‘nature versus nurture’ have ranged from physician psychopathology to unhealthy modeling during training. Both extrinsic and intrinsic factors may also contribute to DPB. External stressors and negative experiences–professional and/or personal–can provoke disruptive behaviors. Overwork, fatigue, strife, and a dysfunctional environment that can arise in both work and home environments can contribute to the development of mental health problems. Stress, burnout, and depression have increasingly become prevalent among physicians and can play a significant role in causing impaired patterns of professional conduct.8, 9 These mental health problems can cause physicians to acquire maladaptive coping strategies such as substance abuse and drug or alcohol dependence. However, it is important to note that physician impairment and substance abuse are not the most frequent causes of DPB. In fact, fewer than 10% of physician behavior issues have been related to substance abuse.2, 5
Psychiatric disorders such as major depression and bipolar and anxiety disorders may also contribute to DPB.10 Most of these disorders (except for schizophrenia) are likely as common among physicians as among the general public.9 An essential clarification is that although DPB can be a manifestation of personality disorders or psychiatric disorders, it does not always stem from underlying psychopathology. Clarifying these distinctions is important for managing the problem and calls for expert professional evaluation in some cases.10
A person’s behavior is shaped by character, values, perceptions, and attitudes. Individuals who engage in DPB typically lack insight and justify their behaviors as a means to achieve a goal. Disrespectful behavior is rooted, in part, in characteristics such as insecurity, immaturity, and aggressiveness; however, it can also be learned, tolerated, and reinforced in the hierarchical hospital culture.11
Other intrinsic factors that may contribute to DPB include lack of emotional intelligence, poor social skills, cultural and ethnic issues, and generation and gender bias.12 Identifying the root causes of DPB can be challenging due to the complexity of the interaction between the healthcare environment and the key players within it; nevertheless, awareness of the contributing factors and early recognition are important. Those who take on the mantle of leadership within hospitals should be educated in this regard.
Repercussions of Disruptive Physician Behavior
An institution’s organizational culture often has an impact on how DPB is addressed. Tolerance of such behavior can have far-reaching consequences. The central tenets of a “culture of safety and respect”–teamwork across disciplines and a blame-free environment in which every member of the healthcare team feels equally empowered to report errors and openly discuss safety issues–would be negatively impacted.
DPB can diminish the quality of care provided, increase the risk of medical errors, and adversely affect patient safety and satisfaction.11-13 Such behavior can cause erosion of relationships and communication between individuals and contribute to a hostile work environment. For instance, nurses or trainees may be afraid to question a physician because of the fear of getting yelled at or being humiliated. Consequently, improperly written orders may be overlooked or a potentially “wrong-site” surgical procedure may not be questioned for fear of provoking a hostile response.
DPB can increase litigation risk and financial costs to institutions. Provider retention may be adversely affected; valued staff may leave hospitals and need to be replaced, and productivity may suffer. When physicians in training observe how their superiors model disruptive behaviors with impunity, a concerning problem that arises is that DPB becomes normalized in the workplace culture, especially if such behaviors are tolerated and result in a perceived gain.
Proposed Interventions
Confrontation of DPB can be challenging without appropriate infrastructure. Healthcare facilities should have a fair system in place for reliable reporting and monitoring of DPB, including a complaints’ verification process, appeals process, and an option for fair hearing.
It is best to initially address the issue in a direct, timely, yet informal manner through counseling or a verbal warning. In several situations, such informal counseling opportunities create a mindful awareness of the problem and the problematic behavior ceases without the need for further action.
When informal intervention is either not appropriate (eg, if the alleged event involved an assault or other illegal behavior) or has already been offered in the past, more formal intervention is required. Institutional progressive disciplinary polices should be in place and adhered to. For example, repeat offenders may be issued written warnings or even temporary suspension of privileges.
Institutional resources such as human resources departments, office of general counsel, office of medical affairs, and the hospital’s medical board may be consulted. Some medical centers have “employee assistance programs” staffed with clinicians skilled in dealing with DPB. Individuals diagnosed with substance abuse or a mental health disorder may require consultation with mental health professionals.14
Special “Professionalism Committees” can be instituted and tasked with investigating complaints and making recommendations for the involvement of resources outside the institution, such as a state medical society.15
Conclusion
Although the vast majority of physicians are well-behaved, it is important to acknowledge that disruptive behaviors can occur in the healthcare environment. Such behaviors have a major impact on workplace culture and patient safety and must be recognized early. Hospital executives and leaders must ensure that appropriate interventions are undertaken—before the quality of patient care is affected and before lives are endangered.
Acknowledgment
The authors would like to thank Ansu John for providing editorial assistance with the manuscript.
Disclosures
The authors have nothing to disclose (Conflict of Interest Form submitted as separate PDF document). Dr. Heitt consults with local hospitals, medical practices, and licensing boards regarding physicians and other healthcare practitioners who have been accused of engaging in disruptive behavior. In these situations he may be paid by the board, medical society, hospital, practice or the professional (patient).
1. Accreditation Council for Graduate Medical Education. Common program requirements: general competencies. https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed July 25, 2017.
2. Porto G, Lauve R. Disruptive clinician behavior: a persistent threat to patient safety. Patient safety and quality healthcare. Lionheart Publishing, Inc. 2006;3:16-24 https://www.psqh.com/julaug06/disruptive.html. Accessed October 1, 2017.
3. American Medical Association. Opinion E- 9.045–Physicians with disruptive behavior. Chicago, IL American Medical Association 2008.
4. Joint Commission: Behaviors that undermine a culture of safety. Sentinel event alert, July 9, 2008:40. http://www.jointcommission.org/sentinel_event_alert_issue_40_behaviors_that_undermine_a_culture_of_safety/. Accessed October 1, 2017.
5. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Int Med. 2006;144:107-115. PubMed
6. Rosenstein AH, O’Daniel M. A survey of the impact of disruptive behaviors and communication defects on patient safety. Jt Comm J Qual Patient Saf. 2008;34(8):464-471. PubMed
7. Weber DO. Poll Results: Doctors’ disruptive behavior disturbs physician leaders. The Physician Executive. 2004;30(5):6. PubMed
8. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166. PubMed
9. Brown S, Goske M, Johnson C. Beyond substance abuse: stress, burnout and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009 6;(7):479-485. PubMed
10. Reynolds NT. Disruptive physician behavior: use and misuse of the label. J Med Regulation. 2012;98(1):8-19.
11. Leape LL, Shore MF, Dienstag JL, et al. Perspective: a culture of respect, part 1: the nature and causes of disrespectful behavior by physicians. Acad Med. 2012;87(7):845-852. PubMed
12. Rosenstein AH, O’Daniel M. Impact and implications of disruptive behavior in the perioperative arena. J Am Coll Surg. 2006;203(1):96-105. PubMed
13. Patient Safety Primer: Disruptive and unprofessional behavior. Available at AHRQ Patient Safety Network: https://psnet.ahrq.gov/primers/primer/15/disruptive-and-unprofessional-behavior(Accessed October 1, 2017.
14. Williams BW, Williams MV. The disruptive physician: conceptual organization. JMed Licensure Discipline. 2008;94(3):12-19.
15. Speck R, Foster J, Mulhem V, et al. Development of a professionalism committee approach to address unprofessional medical staff behavior at an academic medical center. Jt Comm J Qual Patient Saf. 2004;40(4):161-167. PubMed
Dramatic stories of disruptive physician behavior (DPB) appear occasionally in the news, such as the physician who shot and killed a colleague within hospital confines or the gynecologist who secretly took photographs using a camera disguised as a pen during pelvic examinations. More common in hospitals, however, are incidents of inappropriate behavior that may generate complaints from patients or other providers and at times snowball into administrative or legal challenges.
“Professionalism” is one of the six competencies listed by the Accreditation Council for Graduate Medical Education (ACGME)1 and the American Board of Medical Specialties. Unfortunately, incidents of disruptive behavior can result in violation of the tenets of professionalism in the healthcare environment. These behaviors fall along a continuum ranging from outwardly aggressive and uncivil to overly passive and insidious. Although these behaviors can occur across all healthcare disciplines and settings and are not just limited to physicians, the behaviors of physicians often have a much greater impact on the healthcare system as a whole because of their positions of relative “power” within the system.2 Hence, this problem requires greater awareness and education. In this context, the aim of this article is to discuss disruptive behaviors in physicians
The AMA defines DPB as “personal conduct, verbal or physical that has the potential to negatively affect patient care or the ability to work with other members of the healthcare team.”3 The definition of DPB by the Joint Commission includes “all behaviors that undermine a culture of safety.”4 Both the Joint Commission and the AMA recognize the significance and patient safety implications of such behavior. Policy statements by both these organizations underscore the importance of confronting and remedying these potentially dangerous interpersonal behaviors.
Data regarding the prevalence of DPB have been inconsistent. One study estimated that 3%–5% of physicians demonstrate this behavior,5 whereas another study reported a DPB prevalence of 97% among physicians and nurses in the workplace.6 According to a 2004 survey of physician executives, more than 95% of them reported regular encounters of DPB.7
The etiology of such disruptive behaviors is multifactorial and complex. Explanations associated with ‘nature versus nurture’ have ranged from physician psychopathology to unhealthy modeling during training. Both extrinsic and intrinsic factors may also contribute to DPB. External stressors and negative experiences–professional and/or personal–can provoke disruptive behaviors. Overwork, fatigue, strife, and a dysfunctional environment that can arise in both work and home environments can contribute to the development of mental health problems. Stress, burnout, and depression have increasingly become prevalent among physicians and can play a significant role in causing impaired patterns of professional conduct.8, 9 These mental health problems can cause physicians to acquire maladaptive coping strategies such as substance abuse and drug or alcohol dependence. However, it is important to note that physician impairment and substance abuse are not the most frequent causes of DPB. In fact, fewer than 10% of physician behavior issues have been related to substance abuse.2, 5
Psychiatric disorders such as major depression and bipolar and anxiety disorders may also contribute to DPB.10 Most of these disorders (except for schizophrenia) are likely as common among physicians as among the general public.9 An essential clarification is that although DPB can be a manifestation of personality disorders or psychiatric disorders, it does not always stem from underlying psychopathology. Clarifying these distinctions is important for managing the problem and calls for expert professional evaluation in some cases.10
A person’s behavior is shaped by character, values, perceptions, and attitudes. Individuals who engage in DPB typically lack insight and justify their behaviors as a means to achieve a goal. Disrespectful behavior is rooted, in part, in characteristics such as insecurity, immaturity, and aggressiveness; however, it can also be learned, tolerated, and reinforced in the hierarchical hospital culture.11
Other intrinsic factors that may contribute to DPB include lack of emotional intelligence, poor social skills, cultural and ethnic issues, and generation and gender bias.12 Identifying the root causes of DPB can be challenging due to the complexity of the interaction between the healthcare environment and the key players within it; nevertheless, awareness of the contributing factors and early recognition are important. Those who take on the mantle of leadership within hospitals should be educated in this regard.
Repercussions of Disruptive Physician Behavior
An institution’s organizational culture often has an impact on how DPB is addressed. Tolerance of such behavior can have far-reaching consequences. The central tenets of a “culture of safety and respect”–teamwork across disciplines and a blame-free environment in which every member of the healthcare team feels equally empowered to report errors and openly discuss safety issues–would be negatively impacted.
DPB can diminish the quality of care provided, increase the risk of medical errors, and adversely affect patient safety and satisfaction.11-13 Such behavior can cause erosion of relationships and communication between individuals and contribute to a hostile work environment. For instance, nurses or trainees may be afraid to question a physician because of the fear of getting yelled at or being humiliated. Consequently, improperly written orders may be overlooked or a potentially “wrong-site” surgical procedure may not be questioned for fear of provoking a hostile response.
DPB can increase litigation risk and financial costs to institutions. Provider retention may be adversely affected; valued staff may leave hospitals and need to be replaced, and productivity may suffer. When physicians in training observe how their superiors model disruptive behaviors with impunity, a concerning problem that arises is that DPB becomes normalized in the workplace culture, especially if such behaviors are tolerated and result in a perceived gain.
Proposed Interventions
Confrontation of DPB can be challenging without appropriate infrastructure. Healthcare facilities should have a fair system in place for reliable reporting and monitoring of DPB, including a complaints’ verification process, appeals process, and an option for fair hearing.
It is best to initially address the issue in a direct, timely, yet informal manner through counseling or a verbal warning. In several situations, such informal counseling opportunities create a mindful awareness of the problem and the problematic behavior ceases without the need for further action.
When informal intervention is either not appropriate (eg, if the alleged event involved an assault or other illegal behavior) or has already been offered in the past, more formal intervention is required. Institutional progressive disciplinary polices should be in place and adhered to. For example, repeat offenders may be issued written warnings or even temporary suspension of privileges.
Institutional resources such as human resources departments, office of general counsel, office of medical affairs, and the hospital’s medical board may be consulted. Some medical centers have “employee assistance programs” staffed with clinicians skilled in dealing with DPB. Individuals diagnosed with substance abuse or a mental health disorder may require consultation with mental health professionals.14
Special “Professionalism Committees” can be instituted and tasked with investigating complaints and making recommendations for the involvement of resources outside the institution, such as a state medical society.15
Conclusion
Although the vast majority of physicians are well-behaved, it is important to acknowledge that disruptive behaviors can occur in the healthcare environment. Such behaviors have a major impact on workplace culture and patient safety and must be recognized early. Hospital executives and leaders must ensure that appropriate interventions are undertaken—before the quality of patient care is affected and before lives are endangered.
Acknowledgment
The authors would like to thank Ansu John for providing editorial assistance with the manuscript.
Disclosures
The authors have nothing to disclose (Conflict of Interest Form submitted as separate PDF document). Dr. Heitt consults with local hospitals, medical practices, and licensing boards regarding physicians and other healthcare practitioners who have been accused of engaging in disruptive behavior. In these situations he may be paid by the board, medical society, hospital, practice or the professional (patient).
Dramatic stories of disruptive physician behavior (DPB) appear occasionally in the news, such as the physician who shot and killed a colleague within hospital confines or the gynecologist who secretly took photographs using a camera disguised as a pen during pelvic examinations. More common in hospitals, however, are incidents of inappropriate behavior that may generate complaints from patients or other providers and at times snowball into administrative or legal challenges.
“Professionalism” is one of the six competencies listed by the Accreditation Council for Graduate Medical Education (ACGME)1 and the American Board of Medical Specialties. Unfortunately, incidents of disruptive behavior can result in violation of the tenets of professionalism in the healthcare environment. These behaviors fall along a continuum ranging from outwardly aggressive and uncivil to overly passive and insidious. Although these behaviors can occur across all healthcare disciplines and settings and are not just limited to physicians, the behaviors of physicians often have a much greater impact on the healthcare system as a whole because of their positions of relative “power” within the system.2 Hence, this problem requires greater awareness and education. In this context, the aim of this article is to discuss disruptive behaviors in physicians
The AMA defines DPB as “personal conduct, verbal or physical that has the potential to negatively affect patient care or the ability to work with other members of the healthcare team.”3 The definition of DPB by the Joint Commission includes “all behaviors that undermine a culture of safety.”4 Both the Joint Commission and the AMA recognize the significance and patient safety implications of such behavior. Policy statements by both these organizations underscore the importance of confronting and remedying these potentially dangerous interpersonal behaviors.
Data regarding the prevalence of DPB have been inconsistent. One study estimated that 3%–5% of physicians demonstrate this behavior,5 whereas another study reported a DPB prevalence of 97% among physicians and nurses in the workplace.6 According to a 2004 survey of physician executives, more than 95% of them reported regular encounters of DPB.7
The etiology of such disruptive behaviors is multifactorial and complex. Explanations associated with ‘nature versus nurture’ have ranged from physician psychopathology to unhealthy modeling during training. Both extrinsic and intrinsic factors may also contribute to DPB. External stressors and negative experiences–professional and/or personal–can provoke disruptive behaviors. Overwork, fatigue, strife, and a dysfunctional environment that can arise in both work and home environments can contribute to the development of mental health problems. Stress, burnout, and depression have increasingly become prevalent among physicians and can play a significant role in causing impaired patterns of professional conduct.8, 9 These mental health problems can cause physicians to acquire maladaptive coping strategies such as substance abuse and drug or alcohol dependence. However, it is important to note that physician impairment and substance abuse are not the most frequent causes of DPB. In fact, fewer than 10% of physician behavior issues have been related to substance abuse.2, 5
Psychiatric disorders such as major depression and bipolar and anxiety disorders may also contribute to DPB.10 Most of these disorders (except for schizophrenia) are likely as common among physicians as among the general public.9 An essential clarification is that although DPB can be a manifestation of personality disorders or psychiatric disorders, it does not always stem from underlying psychopathology. Clarifying these distinctions is important for managing the problem and calls for expert professional evaluation in some cases.10
A person’s behavior is shaped by character, values, perceptions, and attitudes. Individuals who engage in DPB typically lack insight and justify their behaviors as a means to achieve a goal. Disrespectful behavior is rooted, in part, in characteristics such as insecurity, immaturity, and aggressiveness; however, it can also be learned, tolerated, and reinforced in the hierarchical hospital culture.11
Other intrinsic factors that may contribute to DPB include lack of emotional intelligence, poor social skills, cultural and ethnic issues, and generation and gender bias.12 Identifying the root causes of DPB can be challenging due to the complexity of the interaction between the healthcare environment and the key players within it; nevertheless, awareness of the contributing factors and early recognition are important. Those who take on the mantle of leadership within hospitals should be educated in this regard.
Repercussions of Disruptive Physician Behavior
An institution’s organizational culture often has an impact on how DPB is addressed. Tolerance of such behavior can have far-reaching consequences. The central tenets of a “culture of safety and respect”–teamwork across disciplines and a blame-free environment in which every member of the healthcare team feels equally empowered to report errors and openly discuss safety issues–would be negatively impacted.
DPB can diminish the quality of care provided, increase the risk of medical errors, and adversely affect patient safety and satisfaction.11-13 Such behavior can cause erosion of relationships and communication between individuals and contribute to a hostile work environment. For instance, nurses or trainees may be afraid to question a physician because of the fear of getting yelled at or being humiliated. Consequently, improperly written orders may be overlooked or a potentially “wrong-site” surgical procedure may not be questioned for fear of provoking a hostile response.
DPB can increase litigation risk and financial costs to institutions. Provider retention may be adversely affected; valued staff may leave hospitals and need to be replaced, and productivity may suffer. When physicians in training observe how their superiors model disruptive behaviors with impunity, a concerning problem that arises is that DPB becomes normalized in the workplace culture, especially if such behaviors are tolerated and result in a perceived gain.
Proposed Interventions
Confrontation of DPB can be challenging without appropriate infrastructure. Healthcare facilities should have a fair system in place for reliable reporting and monitoring of DPB, including a complaints’ verification process, appeals process, and an option for fair hearing.
It is best to initially address the issue in a direct, timely, yet informal manner through counseling or a verbal warning. In several situations, such informal counseling opportunities create a mindful awareness of the problem and the problematic behavior ceases without the need for further action.
When informal intervention is either not appropriate (eg, if the alleged event involved an assault or other illegal behavior) or has already been offered in the past, more formal intervention is required. Institutional progressive disciplinary polices should be in place and adhered to. For example, repeat offenders may be issued written warnings or even temporary suspension of privileges.
Institutional resources such as human resources departments, office of general counsel, office of medical affairs, and the hospital’s medical board may be consulted. Some medical centers have “employee assistance programs” staffed with clinicians skilled in dealing with DPB. Individuals diagnosed with substance abuse or a mental health disorder may require consultation with mental health professionals.14
Special “Professionalism Committees” can be instituted and tasked with investigating complaints and making recommendations for the involvement of resources outside the institution, such as a state medical society.15
Conclusion
Although the vast majority of physicians are well-behaved, it is important to acknowledge that disruptive behaviors can occur in the healthcare environment. Such behaviors have a major impact on workplace culture and patient safety and must be recognized early. Hospital executives and leaders must ensure that appropriate interventions are undertaken—before the quality of patient care is affected and before lives are endangered.
Acknowledgment
The authors would like to thank Ansu John for providing editorial assistance with the manuscript.
Disclosures
The authors have nothing to disclose (Conflict of Interest Form submitted as separate PDF document). Dr. Heitt consults with local hospitals, medical practices, and licensing boards regarding physicians and other healthcare practitioners who have been accused of engaging in disruptive behavior. In these situations he may be paid by the board, medical society, hospital, practice or the professional (patient).
1. Accreditation Council for Graduate Medical Education. Common program requirements: general competencies. https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed July 25, 2017.
2. Porto G, Lauve R. Disruptive clinician behavior: a persistent threat to patient safety. Patient safety and quality healthcare. Lionheart Publishing, Inc. 2006;3:16-24 https://www.psqh.com/julaug06/disruptive.html. Accessed October 1, 2017.
3. American Medical Association. Opinion E- 9.045–Physicians with disruptive behavior. Chicago, IL American Medical Association 2008.
4. Joint Commission: Behaviors that undermine a culture of safety. Sentinel event alert, July 9, 2008:40. http://www.jointcommission.org/sentinel_event_alert_issue_40_behaviors_that_undermine_a_culture_of_safety/. Accessed October 1, 2017.
5. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Int Med. 2006;144:107-115. PubMed
6. Rosenstein AH, O’Daniel M. A survey of the impact of disruptive behaviors and communication defects on patient safety. Jt Comm J Qual Patient Saf. 2008;34(8):464-471. PubMed
7. Weber DO. Poll Results: Doctors’ disruptive behavior disturbs physician leaders. The Physician Executive. 2004;30(5):6. PubMed
8. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166. PubMed
9. Brown S, Goske M, Johnson C. Beyond substance abuse: stress, burnout and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009 6;(7):479-485. PubMed
10. Reynolds NT. Disruptive physician behavior: use and misuse of the label. J Med Regulation. 2012;98(1):8-19.
11. Leape LL, Shore MF, Dienstag JL, et al. Perspective: a culture of respect, part 1: the nature and causes of disrespectful behavior by physicians. Acad Med. 2012;87(7):845-852. PubMed
12. Rosenstein AH, O’Daniel M. Impact and implications of disruptive behavior in the perioperative arena. J Am Coll Surg. 2006;203(1):96-105. PubMed
13. Patient Safety Primer: Disruptive and unprofessional behavior. Available at AHRQ Patient Safety Network: https://psnet.ahrq.gov/primers/primer/15/disruptive-and-unprofessional-behavior(Accessed October 1, 2017.
14. Williams BW, Williams MV. The disruptive physician: conceptual organization. JMed Licensure Discipline. 2008;94(3):12-19.
15. Speck R, Foster J, Mulhem V, et al. Development of a professionalism committee approach to address unprofessional medical staff behavior at an academic medical center. Jt Comm J Qual Patient Saf. 2004;40(4):161-167. PubMed
1. Accreditation Council for Graduate Medical Education. Common program requirements: general competencies. https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed July 25, 2017.
2. Porto G, Lauve R. Disruptive clinician behavior: a persistent threat to patient safety. Patient safety and quality healthcare. Lionheart Publishing, Inc. 2006;3:16-24 https://www.psqh.com/julaug06/disruptive.html. Accessed October 1, 2017.
3. American Medical Association. Opinion E- 9.045–Physicians with disruptive behavior. Chicago, IL American Medical Association 2008.
4. Joint Commission: Behaviors that undermine a culture of safety. Sentinel event alert, July 9, 2008:40. http://www.jointcommission.org/sentinel_event_alert_issue_40_behaviors_that_undermine_a_culture_of_safety/. Accessed October 1, 2017.
5. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Int Med. 2006;144:107-115. PubMed
6. Rosenstein AH, O’Daniel M. A survey of the impact of disruptive behaviors and communication defects on patient safety. Jt Comm J Qual Patient Saf. 2008;34(8):464-471. PubMed
7. Weber DO. Poll Results: Doctors’ disruptive behavior disturbs physician leaders. The Physician Executive. 2004;30(5):6. PubMed
8. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166. PubMed
9. Brown S, Goske M, Johnson C. Beyond substance abuse: stress, burnout and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009 6;(7):479-485. PubMed
10. Reynolds NT. Disruptive physician behavior: use and misuse of the label. J Med Regulation. 2012;98(1):8-19.
11. Leape LL, Shore MF, Dienstag JL, et al. Perspective: a culture of respect, part 1: the nature and causes of disrespectful behavior by physicians. Acad Med. 2012;87(7):845-852. PubMed
12. Rosenstein AH, O’Daniel M. Impact and implications of disruptive behavior in the perioperative arena. J Am Coll Surg. 2006;203(1):96-105. PubMed
13. Patient Safety Primer: Disruptive and unprofessional behavior. Available at AHRQ Patient Safety Network: https://psnet.ahrq.gov/primers/primer/15/disruptive-and-unprofessional-behavior(Accessed October 1, 2017.
14. Williams BW, Williams MV. The disruptive physician: conceptual organization. JMed Licensure Discipline. 2008;94(3):12-19.
15. Speck R, Foster J, Mulhem V, et al. Development of a professionalism committee approach to address unprofessional medical staff behavior at an academic medical center. Jt Comm J Qual Patient Saf. 2004;40(4):161-167. PubMed
© 2018 Society of Hospital Medicine
Denah Joseph: “In the Hospital”
We recently spoke with Denah Joseph, a clinical chaplain who works with the Palliative Care team to provide spiritual services to patients with serious illness. In addition, Denah leads efforts to address burnout among healthcare providers.
Denah, tell us about yourself.
My first career was actually in clinical psychology, but I’ve been a Palliative Care chaplain for 15 years. I also teach skill-building for providers around burnout and resilience.
What brought you to Palliative Care?
I’ve lost three sisters and a partner to breast cancer, and my dad died when I was quite young, so I’ve had a lot of exposure to loss. The other big thread in my life has been my spiritual practice. My father was an Orthodox Jew, but exceptionally ecumenical for his time. His first wife was Irish Catholic, and my father used to go to church, sit, kneel, and say the rosary, and light candles for his Catholic friends. Three hundred nuns from the local diocese all came to my dad’s funeral. It was really remarkable.
I’ve been a practicing Buddhist since I was 19. When I went back to school to become a chaplain I wanted to bring more of my spiritual interest into counseling work, so chaplaincy seemed like a really interesting way to do that.
Tell us more about what a chaplain actually does.
As a field, healthcare chaplaincy is relatively new. The old model was if a person was religious, somebody would arrange for a rabbi or an imam or a priest to come into the hospital and take care of the pastoral needs of that patient. In the last 10 to 15 years, the consensus guidelines for quality patient care now include addressing the spiritual dimension of patients’ lives. Instead of relying on volunteers from the community with no quality assurance, it’s required that any hospital over 200 beds have spiritual care available. In order to be a board-certified chaplain, you need to be endorsed by a faith community, and have an advanced degree in either Pastoral Counseling or Theology.
Everybody has spiritual needs even if they don’t use that word “spiritual.” We define it in terms of meaning, relationships, impact on one’s life, hope, fears, reconciliation issues, legacy issues, etc. Approximately 80% of patients want their physicians to understand a little bit about their spiritual/existential/emotional world, and only 20% of doctors ask—so there’s a really big gap. This can be a 5-minute conversation about who are you, what’s important to you, what’s the biggest struggle with your illness that is not medically oriented.
Can you share a patient encounter where you learned something?
Recently I cared for a patient whose wish was to survive to see his only son graduate from college. His wife and son both were like, “You’ve got to hang in there, Dad. You’ve got to hang in there.” He had very advanced pancreatic cancer, and the chances of him making it to graduation were exceedingly small, but nobody was dealing with this.
During the hospitalization, I went to the patient and his wife and I said, “We’re all hoping that you’re going to make it until the graduation but in the event you don’t, would you like to write a letter to your son?” In the Jewish tradition, it is called an ethical will. It’s the idea of legacy work. Just like you would make a will for your material possessions, an ethical will expresses what you value, what you hope for and dream for your beloved. He wanted to do it. His wife said, “Absolutely not, that’s like believing you’re not going to make it.” He was a very gentle guy. He would generally completely defer to his wife, but this time he said, “No, I want to do this.”
So I met with the patient and asked questions like, “What are the things you would hope to be remembered for? What are you most proud of that you want your son to know? What would you want your son to know if he became a father?”
I had him just talk, while I took notes. Later on, I wrote it up on official stationery and gave it to the patient.
What was his reaction when you gave the letter to him?
He started to cry. He said it was perfect. I usually read it to them so they can make edits if they want to. It sort of brings the grief forward when you imagine talking to a beloved that you’re leaving behind.
A few days later the patient died in the hospital surrounded by family members.
His wife, who had advocated so strongly against the letter, hugged me. She said, “That letter is the most important thing that happened here in the hospital.” I was shocked she said that, I had no idea he even shared it with her.
If people have the opportunity to share what’s important to them, particularly generationally, it could address a very deep need to be remembered.
Reflecting on it, I actually see myself as a healer and all my work is in healing, whether it’s working with physicians or working with patients or working with students or working with people in my private practice. It’s a theme that runs through everything. It’s not a word we hear often enough in medicine.
Why not?
The culture of medicine has lost its roots, in that sense. I hear a lot of people say, “There’s nothing we can do medically, so we’re just supporting them through this.” Supporting people through the experience is often seen as less valuable, but I think, particularly for serious, terminal illness, supporting people is not optional.
Switching gears a bit, tell us about the skill-building and resilience work you’ve done.
I think if you don’t proactively care for the rest of your life then your work life takes over. Although it’s pronounced in medicine, it’s in all fields. The pace and stress of our contemporary culture can be contrary to well-being in general.
When I first came to UCSF, I saw a culture of silence around stress, anxiety, and burnout. I started reading about burnout and the numbers of people who qualified to be burnt out at any given time, which may be at least 50% and trending upward. It just seemed to me that in any other profession if half the workforce was impaired, somebody would be doing something. I’ve really become passionate about this in the last couple of years.
So I developed a burnout prevention and resilience skills training class for providers. We work on mindfulness, social connection and support, positive psychology emotions like gratitude, appreciation, self-compassion, and humor, and delve into the sources of meaning in our work.
Based on your work, what would you say are the key stressors in medicine, generally?
Well there’s research on the electronic medical record and the increasing focus on metrics and “value-driven medicine,” which can lead to reduced connection with patients. I hope that what I’m doing makes some difference, but fundamentally, I believe there needs to be a real commitment on the part of the health system, to understand and make the changes that need to happen.
What is the fundamental problem? How do you define that?
Well I don’t think anybody knows. I think that’s what we’re saying. How can it be that so many people aren’t happy in such privileged work? It’s not clinical. It’s the system. It’s yet another flow sheet that you have to fill out; the actual amount of time spent with patients is low. No wonder we get burned out. We’re just doing orders all the time and answering phone calls.
It’s the loss of interconnectedness.
Yes, it’s the loss of connection. That goes back to even why chaplains may not be recognized as adding value. You can’t put a metric on connection. You can’t say, “I made 5 connections.”
Anything else you would like to share?
I don’t know how you feel about it, but I feel so grateful to have the opportunity to be in people’s lives in the intimate way we get to be and I, especially, get to be in a way sometimes even more than doctors. You get to be there, and you may even want to talk about the things that we were mentioning, but they’re asking you about their creatinine and their platelets and their urinary incontinence, so that’s what you’re having to talk about. I don’t have to do that, so I feel like I get the best seat in the house that way.
I think the seriously ill have so much to share and often are wise, particularly the young ones, from having dealt with illness. I’m really interested in that idea of wisdom and how you develop wisdom. Traditionally wisdom is associated with being an elder and having lived a long time and having a lot of experience. I think our work gives us that opportunity. We don’t have to necessarily live through everything to develop that kind of wisdom, but just to be with people who are living through these things.
So here I am, almost 70. I’m working harder than I’ve ever worked in my life. My partner is retired. She’s like, “Come on, let’s play.” She rides bikes, takes the dog out, cooks, reads. But I just can’t stop. I think it’s because I feel like, what else would I want to be doing with my time? I think that’s an amazing thing to be given that gift that I learn from my patients all the time and learn about what’s important. Obviously people are different, but it all boils down to relationships in the end.
That’s the promise of medicine, and I think that’s the great sadness of what’s going on with the epidemic of burnout. People lose connection to that.
There is some element to being present in these hard and difficult times that can bring perspective to life; and to know the sadness, in some ways
…is to know the joy.
Thank you, Denah, for sharing your thoughts with us.
We recently spoke with Denah Joseph, a clinical chaplain who works with the Palliative Care team to provide spiritual services to patients with serious illness. In addition, Denah leads efforts to address burnout among healthcare providers.
Denah, tell us about yourself.
My first career was actually in clinical psychology, but I’ve been a Palliative Care chaplain for 15 years. I also teach skill-building for providers around burnout and resilience.
What brought you to Palliative Care?
I’ve lost three sisters and a partner to breast cancer, and my dad died when I was quite young, so I’ve had a lot of exposure to loss. The other big thread in my life has been my spiritual practice. My father was an Orthodox Jew, but exceptionally ecumenical for his time. His first wife was Irish Catholic, and my father used to go to church, sit, kneel, and say the rosary, and light candles for his Catholic friends. Three hundred nuns from the local diocese all came to my dad’s funeral. It was really remarkable.
I’ve been a practicing Buddhist since I was 19. When I went back to school to become a chaplain I wanted to bring more of my spiritual interest into counseling work, so chaplaincy seemed like a really interesting way to do that.
Tell us more about what a chaplain actually does.
As a field, healthcare chaplaincy is relatively new. The old model was if a person was religious, somebody would arrange for a rabbi or an imam or a priest to come into the hospital and take care of the pastoral needs of that patient. In the last 10 to 15 years, the consensus guidelines for quality patient care now include addressing the spiritual dimension of patients’ lives. Instead of relying on volunteers from the community with no quality assurance, it’s required that any hospital over 200 beds have spiritual care available. In order to be a board-certified chaplain, you need to be endorsed by a faith community, and have an advanced degree in either Pastoral Counseling or Theology.
Everybody has spiritual needs even if they don’t use that word “spiritual.” We define it in terms of meaning, relationships, impact on one’s life, hope, fears, reconciliation issues, legacy issues, etc. Approximately 80% of patients want their physicians to understand a little bit about their spiritual/existential/emotional world, and only 20% of doctors ask—so there’s a really big gap. This can be a 5-minute conversation about who are you, what’s important to you, what’s the biggest struggle with your illness that is not medically oriented.
Can you share a patient encounter where you learned something?
Recently I cared for a patient whose wish was to survive to see his only son graduate from college. His wife and son both were like, “You’ve got to hang in there, Dad. You’ve got to hang in there.” He had very advanced pancreatic cancer, and the chances of him making it to graduation were exceedingly small, but nobody was dealing with this.
During the hospitalization, I went to the patient and his wife and I said, “We’re all hoping that you’re going to make it until the graduation but in the event you don’t, would you like to write a letter to your son?” In the Jewish tradition, it is called an ethical will. It’s the idea of legacy work. Just like you would make a will for your material possessions, an ethical will expresses what you value, what you hope for and dream for your beloved. He wanted to do it. His wife said, “Absolutely not, that’s like believing you’re not going to make it.” He was a very gentle guy. He would generally completely defer to his wife, but this time he said, “No, I want to do this.”
So I met with the patient and asked questions like, “What are the things you would hope to be remembered for? What are you most proud of that you want your son to know? What would you want your son to know if he became a father?”
I had him just talk, while I took notes. Later on, I wrote it up on official stationery and gave it to the patient.
What was his reaction when you gave the letter to him?
He started to cry. He said it was perfect. I usually read it to them so they can make edits if they want to. It sort of brings the grief forward when you imagine talking to a beloved that you’re leaving behind.
A few days later the patient died in the hospital surrounded by family members.
His wife, who had advocated so strongly against the letter, hugged me. She said, “That letter is the most important thing that happened here in the hospital.” I was shocked she said that, I had no idea he even shared it with her.
If people have the opportunity to share what’s important to them, particularly generationally, it could address a very deep need to be remembered.
Reflecting on it, I actually see myself as a healer and all my work is in healing, whether it’s working with physicians or working with patients or working with students or working with people in my private practice. It’s a theme that runs through everything. It’s not a word we hear often enough in medicine.
Why not?
The culture of medicine has lost its roots, in that sense. I hear a lot of people say, “There’s nothing we can do medically, so we’re just supporting them through this.” Supporting people through the experience is often seen as less valuable, but I think, particularly for serious, terminal illness, supporting people is not optional.
Switching gears a bit, tell us about the skill-building and resilience work you’ve done.
I think if you don’t proactively care for the rest of your life then your work life takes over. Although it’s pronounced in medicine, it’s in all fields. The pace and stress of our contemporary culture can be contrary to well-being in general.
When I first came to UCSF, I saw a culture of silence around stress, anxiety, and burnout. I started reading about burnout and the numbers of people who qualified to be burnt out at any given time, which may be at least 50% and trending upward. It just seemed to me that in any other profession if half the workforce was impaired, somebody would be doing something. I’ve really become passionate about this in the last couple of years.
So I developed a burnout prevention and resilience skills training class for providers. We work on mindfulness, social connection and support, positive psychology emotions like gratitude, appreciation, self-compassion, and humor, and delve into the sources of meaning in our work.
Based on your work, what would you say are the key stressors in medicine, generally?
Well there’s research on the electronic medical record and the increasing focus on metrics and “value-driven medicine,” which can lead to reduced connection with patients. I hope that what I’m doing makes some difference, but fundamentally, I believe there needs to be a real commitment on the part of the health system, to understand and make the changes that need to happen.
What is the fundamental problem? How do you define that?
Well I don’t think anybody knows. I think that’s what we’re saying. How can it be that so many people aren’t happy in such privileged work? It’s not clinical. It’s the system. It’s yet another flow sheet that you have to fill out; the actual amount of time spent with patients is low. No wonder we get burned out. We’re just doing orders all the time and answering phone calls.
It’s the loss of interconnectedness.
Yes, it’s the loss of connection. That goes back to even why chaplains may not be recognized as adding value. You can’t put a metric on connection. You can’t say, “I made 5 connections.”
Anything else you would like to share?
I don’t know how you feel about it, but I feel so grateful to have the opportunity to be in people’s lives in the intimate way we get to be and I, especially, get to be in a way sometimes even more than doctors. You get to be there, and you may even want to talk about the things that we were mentioning, but they’re asking you about their creatinine and their platelets and their urinary incontinence, so that’s what you’re having to talk about. I don’t have to do that, so I feel like I get the best seat in the house that way.
I think the seriously ill have so much to share and often are wise, particularly the young ones, from having dealt with illness. I’m really interested in that idea of wisdom and how you develop wisdom. Traditionally wisdom is associated with being an elder and having lived a long time and having a lot of experience. I think our work gives us that opportunity. We don’t have to necessarily live through everything to develop that kind of wisdom, but just to be with people who are living through these things.
So here I am, almost 70. I’m working harder than I’ve ever worked in my life. My partner is retired. She’s like, “Come on, let’s play.” She rides bikes, takes the dog out, cooks, reads. But I just can’t stop. I think it’s because I feel like, what else would I want to be doing with my time? I think that’s an amazing thing to be given that gift that I learn from my patients all the time and learn about what’s important. Obviously people are different, but it all boils down to relationships in the end.
That’s the promise of medicine, and I think that’s the great sadness of what’s going on with the epidemic of burnout. People lose connection to that.
There is some element to being present in these hard and difficult times that can bring perspective to life; and to know the sadness, in some ways
…is to know the joy.
Thank you, Denah, for sharing your thoughts with us.
We recently spoke with Denah Joseph, a clinical chaplain who works with the Palliative Care team to provide spiritual services to patients with serious illness. In addition, Denah leads efforts to address burnout among healthcare providers.
Denah, tell us about yourself.
My first career was actually in clinical psychology, but I’ve been a Palliative Care chaplain for 15 years. I also teach skill-building for providers around burnout and resilience.
What brought you to Palliative Care?
I’ve lost three sisters and a partner to breast cancer, and my dad died when I was quite young, so I’ve had a lot of exposure to loss. The other big thread in my life has been my spiritual practice. My father was an Orthodox Jew, but exceptionally ecumenical for his time. His first wife was Irish Catholic, and my father used to go to church, sit, kneel, and say the rosary, and light candles for his Catholic friends. Three hundred nuns from the local diocese all came to my dad’s funeral. It was really remarkable.
I’ve been a practicing Buddhist since I was 19. When I went back to school to become a chaplain I wanted to bring more of my spiritual interest into counseling work, so chaplaincy seemed like a really interesting way to do that.
Tell us more about what a chaplain actually does.
As a field, healthcare chaplaincy is relatively new. The old model was if a person was religious, somebody would arrange for a rabbi or an imam or a priest to come into the hospital and take care of the pastoral needs of that patient. In the last 10 to 15 years, the consensus guidelines for quality patient care now include addressing the spiritual dimension of patients’ lives. Instead of relying on volunteers from the community with no quality assurance, it’s required that any hospital over 200 beds have spiritual care available. In order to be a board-certified chaplain, you need to be endorsed by a faith community, and have an advanced degree in either Pastoral Counseling or Theology.
Everybody has spiritual needs even if they don’t use that word “spiritual.” We define it in terms of meaning, relationships, impact on one’s life, hope, fears, reconciliation issues, legacy issues, etc. Approximately 80% of patients want their physicians to understand a little bit about their spiritual/existential/emotional world, and only 20% of doctors ask—so there’s a really big gap. This can be a 5-minute conversation about who are you, what’s important to you, what’s the biggest struggle with your illness that is not medically oriented.
Can you share a patient encounter where you learned something?
Recently I cared for a patient whose wish was to survive to see his only son graduate from college. His wife and son both were like, “You’ve got to hang in there, Dad. You’ve got to hang in there.” He had very advanced pancreatic cancer, and the chances of him making it to graduation were exceedingly small, but nobody was dealing with this.
During the hospitalization, I went to the patient and his wife and I said, “We’re all hoping that you’re going to make it until the graduation but in the event you don’t, would you like to write a letter to your son?” In the Jewish tradition, it is called an ethical will. It’s the idea of legacy work. Just like you would make a will for your material possessions, an ethical will expresses what you value, what you hope for and dream for your beloved. He wanted to do it. His wife said, “Absolutely not, that’s like believing you’re not going to make it.” He was a very gentle guy. He would generally completely defer to his wife, but this time he said, “No, I want to do this.”
So I met with the patient and asked questions like, “What are the things you would hope to be remembered for? What are you most proud of that you want your son to know? What would you want your son to know if he became a father?”
I had him just talk, while I took notes. Later on, I wrote it up on official stationery and gave it to the patient.
What was his reaction when you gave the letter to him?
He started to cry. He said it was perfect. I usually read it to them so they can make edits if they want to. It sort of brings the grief forward when you imagine talking to a beloved that you’re leaving behind.
A few days later the patient died in the hospital surrounded by family members.
His wife, who had advocated so strongly against the letter, hugged me. She said, “That letter is the most important thing that happened here in the hospital.” I was shocked she said that, I had no idea he even shared it with her.
If people have the opportunity to share what’s important to them, particularly generationally, it could address a very deep need to be remembered.
Reflecting on it, I actually see myself as a healer and all my work is in healing, whether it’s working with physicians or working with patients or working with students or working with people in my private practice. It’s a theme that runs through everything. It’s not a word we hear often enough in medicine.
Why not?
The culture of medicine has lost its roots, in that sense. I hear a lot of people say, “There’s nothing we can do medically, so we’re just supporting them through this.” Supporting people through the experience is often seen as less valuable, but I think, particularly for serious, terminal illness, supporting people is not optional.
Switching gears a bit, tell us about the skill-building and resilience work you’ve done.
I think if you don’t proactively care for the rest of your life then your work life takes over. Although it’s pronounced in medicine, it’s in all fields. The pace and stress of our contemporary culture can be contrary to well-being in general.
When I first came to UCSF, I saw a culture of silence around stress, anxiety, and burnout. I started reading about burnout and the numbers of people who qualified to be burnt out at any given time, which may be at least 50% and trending upward. It just seemed to me that in any other profession if half the workforce was impaired, somebody would be doing something. I’ve really become passionate about this in the last couple of years.
So I developed a burnout prevention and resilience skills training class for providers. We work on mindfulness, social connection and support, positive psychology emotions like gratitude, appreciation, self-compassion, and humor, and delve into the sources of meaning in our work.
Based on your work, what would you say are the key stressors in medicine, generally?
Well there’s research on the electronic medical record and the increasing focus on metrics and “value-driven medicine,” which can lead to reduced connection with patients. I hope that what I’m doing makes some difference, but fundamentally, I believe there needs to be a real commitment on the part of the health system, to understand and make the changes that need to happen.
What is the fundamental problem? How do you define that?
Well I don’t think anybody knows. I think that’s what we’re saying. How can it be that so many people aren’t happy in such privileged work? It’s not clinical. It’s the system. It’s yet another flow sheet that you have to fill out; the actual amount of time spent with patients is low. No wonder we get burned out. We’re just doing orders all the time and answering phone calls.
It’s the loss of interconnectedness.
Yes, it’s the loss of connection. That goes back to even why chaplains may not be recognized as adding value. You can’t put a metric on connection. You can’t say, “I made 5 connections.”
Anything else you would like to share?
I don’t know how you feel about it, but I feel so grateful to have the opportunity to be in people’s lives in the intimate way we get to be and I, especially, get to be in a way sometimes even more than doctors. You get to be there, and you may even want to talk about the things that we were mentioning, but they’re asking you about their creatinine and their platelets and their urinary incontinence, so that’s what you’re having to talk about. I don’t have to do that, so I feel like I get the best seat in the house that way.
I think the seriously ill have so much to share and often are wise, particularly the young ones, from having dealt with illness. I’m really interested in that idea of wisdom and how you develop wisdom. Traditionally wisdom is associated with being an elder and having lived a long time and having a lot of experience. I think our work gives us that opportunity. We don’t have to necessarily live through everything to develop that kind of wisdom, but just to be with people who are living through these things.
So here I am, almost 70. I’m working harder than I’ve ever worked in my life. My partner is retired. She’s like, “Come on, let’s play.” She rides bikes, takes the dog out, cooks, reads. But I just can’t stop. I think it’s because I feel like, what else would I want to be doing with my time? I think that’s an amazing thing to be given that gift that I learn from my patients all the time and learn about what’s important. Obviously people are different, but it all boils down to relationships in the end.
That’s the promise of medicine, and I think that’s the great sadness of what’s going on with the epidemic of burnout. People lose connection to that.
There is some element to being present in these hard and difficult times that can bring perspective to life; and to know the sadness, in some ways
…is to know the joy.
Thank you, Denah, for sharing your thoughts with us.
© 2018 Society of Hospital Medicine
In the Hospital: Series Introduction
The only real voyage of discovery consists not in seeking new landscapes but in having new eyes.
—Marcel Proust
Hospitals can be complex, challenging, and dehumanizing for both patients and practitioners. In a national survey, up to half of hospitalists were affected by burnout and scored highly on emotional exhaustion and depersonalization scales.1
Yet hospitals are also ripe with meaningful stories. In addition to patients’ narratives, the stories of multidisciplinary team members who make quality patient care possible reveal that we are bound together in more ways than we realize. Now, we have the opportunity to tell these stories.
This issue of Journal of Hospital Medicine introduces a new series: In the Hospital. Through selected interviews we explore the day-to-day lives of members of our hospital team. Highlighting the “team”
We invite readers to appreciate the common threads that bind these pieces together. These stories will introduce us to individuals who have discrete and often disparate job descriptions, but all of them care about patients and want the best for them. Some are frustrated with the health care system and the constraints it places on our efficiency. Many of them worry about how to balance the demands of work with the need to be available for their families and friends. Many are trying their best to maintain their humanism, build resilience, and sustain themselves in ways that meet their personal goals for excellence, empathy, and fulfillment.
This series begins with the story of a palliative-care clinical chaplain whose life experience and perspective brings to light issues of resilience, meaning, and purpose. Future stories in this series will include a variety of providers across a spectrum of practice environments. We look forward to engaging you in this journey and welcome feedback and contributions.
Disclosures
The authors have nothing to disclose.
1. Roberts DL, Shanafelt TD, Dyrbye LN, West CP. A national comparison of burnout and work-life balance among internal medicine hospitalists and out- patient general internists. J Hosp Med. 2014;9(3),176-181. PubMed
The only real voyage of discovery consists not in seeking new landscapes but in having new eyes.
—Marcel Proust
Hospitals can be complex, challenging, and dehumanizing for both patients and practitioners. In a national survey, up to half of hospitalists were affected by burnout and scored highly on emotional exhaustion and depersonalization scales.1
Yet hospitals are also ripe with meaningful stories. In addition to patients’ narratives, the stories of multidisciplinary team members who make quality patient care possible reveal that we are bound together in more ways than we realize. Now, we have the opportunity to tell these stories.
This issue of Journal of Hospital Medicine introduces a new series: In the Hospital. Through selected interviews we explore the day-to-day lives of members of our hospital team. Highlighting the “team”
We invite readers to appreciate the common threads that bind these pieces together. These stories will introduce us to individuals who have discrete and often disparate job descriptions, but all of them care about patients and want the best for them. Some are frustrated with the health care system and the constraints it places on our efficiency. Many of them worry about how to balance the demands of work with the need to be available for their families and friends. Many are trying their best to maintain their humanism, build resilience, and sustain themselves in ways that meet their personal goals for excellence, empathy, and fulfillment.
This series begins with the story of a palliative-care clinical chaplain whose life experience and perspective brings to light issues of resilience, meaning, and purpose. Future stories in this series will include a variety of providers across a spectrum of practice environments. We look forward to engaging you in this journey and welcome feedback and contributions.
Disclosures
The authors have nothing to disclose.
The only real voyage of discovery consists not in seeking new landscapes but in having new eyes.
—Marcel Proust
Hospitals can be complex, challenging, and dehumanizing for both patients and practitioners. In a national survey, up to half of hospitalists were affected by burnout and scored highly on emotional exhaustion and depersonalization scales.1
Yet hospitals are also ripe with meaningful stories. In addition to patients’ narratives, the stories of multidisciplinary team members who make quality patient care possible reveal that we are bound together in more ways than we realize. Now, we have the opportunity to tell these stories.
This issue of Journal of Hospital Medicine introduces a new series: In the Hospital. Through selected interviews we explore the day-to-day lives of members of our hospital team. Highlighting the “team”
We invite readers to appreciate the common threads that bind these pieces together. These stories will introduce us to individuals who have discrete and often disparate job descriptions, but all of them care about patients and want the best for them. Some are frustrated with the health care system and the constraints it places on our efficiency. Many of them worry about how to balance the demands of work with the need to be available for their families and friends. Many are trying their best to maintain their humanism, build resilience, and sustain themselves in ways that meet their personal goals for excellence, empathy, and fulfillment.
This series begins with the story of a palliative-care clinical chaplain whose life experience and perspective brings to light issues of resilience, meaning, and purpose. Future stories in this series will include a variety of providers across a spectrum of practice environments. We look forward to engaging you in this journey and welcome feedback and contributions.
Disclosures
The authors have nothing to disclose.
1. Roberts DL, Shanafelt TD, Dyrbye LN, West CP. A national comparison of burnout and work-life balance among internal medicine hospitalists and out- patient general internists. J Hosp Med. 2014;9(3),176-181. PubMed
1. Roberts DL, Shanafelt TD, Dyrbye LN, West CP. A national comparison of burnout and work-life balance among internal medicine hospitalists and out- patient general internists. J Hosp Med. 2014;9(3),176-181. PubMed
© 2018 Society of Hospital Medicine
Relationship between Hospital 30-Day Mortality Rates for Heart Failure and Patterns of Early Inpatient Comfort Care
In an effort to improve the quality of care delivered to heart failure (HF) patients, the Centers for Medicare & Medicaid Services (CMS) publish hospitals’ 30-day risk-standardized mortality rates (RSMRs) for HF.1 These mortality rates are also used by CMS to determine the financial penalties and bonuses that hospitals receive as part of the national Hospital Value-based Purchasing program.2 Whether or not these efforts effectively direct patients towards high-quality providers or motivate hospitals to provide better care, few would disagree with the overarching goal of decreasing the number of patients who die from HF.
However, for some patients with chronic disease at the end of life, goals of care may change. The quality of days lived may become more important than the quantity of days lived. As a consequence, high-quality care for some patients at the end of life is associated with withdrawing life-sustaining or life-extending therapies. Over time, this therapeutic perspective has become more common, with use of hospice care doubling from 23% to 47% between 2000 and 2012 among Medicare beneficiaries who died.3 For a national cohort of older patients admitted with HF—not just those patients who died in that same year—hospitals’ rates of referral to hospice are considerably lower, averaging 2.9% in 2010 in a national study.4 Nevertheless, it is possible that hospitals that more faithfully follow their dying patients’ wishes and withdraw life-prolonging interventions and provide comfort-focused care at the end of life might be unfairly penalized if such efforts resulted in higher mortality rates than other hospitals.
Therefore, we used Medicare data linked to a national HF registry with information about end-of-life care, to address 3 questions: (1) How much do hospitals vary in their rates of early comfort care and how has this changed over time; (2) What hospital and patient factors are associated with higher early comfort care rates; and (3) Is there a correlation between 30-day risk-adjusted mortality rates for HF with hospital rates of early comfort care?
METHODS
Data Sources
We used data from the American Heart Association’s Get With The Guidelines-Heart Failure (GWTG-HF) registry. GWTG-HF is a voluntary, inpatient, quality improvement registry5-7 that uses web-based tools and standard questionnaires to collect data on patients with HF admitted to participating hospitals nationwide. The data include information from admission (eg, sociodemographic characteristics, symptoms, medical history, and initial laboratory and test results), the inpatient stay (eg, therapies), and discharge (eg, discharge destination, whether and when comfort care was initiated). We linked the GWTG-HF registry data to Medicare claims data in order to obtain information about Medicare eligibility and patient comorbidities. Additionally, we used data from the American Hospital Association (2008) for hospital characteristics. Quintiles Real-World & Late Phase Research (Cambridge, MA) serves as the data coordinating center for GWTG-HF and the Duke Clinical Research Institute (Durham, NC) serves as the statistical analytic center. GWTG-HF participating sites have a waiver of informed consent because the data are de-identified and primarily used for quality improvement. All analyses performed on this data have been approved by the Duke Medical Center Institutional Review Board.
Study Population
Study Outcomes
Our outcome of interest was the correlation between a hospital’s rate of initiating early CMO for admitted HF patients and a hospital’s 30-day RSMR for HF. The GWTG-HF questionnaire8 asks “When is the earliest physician/advanced practice nurse/physician assistant documentation of comfort measures only?” and permits 4 responses: day 0 or 1, day 2 or after, timing unclear, or not documented/unable to determine. We defined early CMO as CMO on day 0 or 1, and late/no CMO as any other response. We chose to examine early comfort care because many hospitalized patients transition to comfort care before they die if the death is in any way predictable. Thus, if comfort care is measured at any time during the hospitalization, hospitals that have high mortality rates are likely to have high comfort care rates. Therefore, we chose to use the more precise measure of early comfort care. We created hospital-level, risk-standardized early comfort care rates using the same risk-adjustment model used for RSMRs but with the outcome of early comfort care instead of mortality.9,10
RSMRs were calculated using a validated GWTG-HF 30-day risk-standardized mortality model9 with additional variables identified from other GWTG-HF analyses.10 The 30 days are measured as the 30 days after the index admission date.
Statistical Analyses
We described trends in early comfort care rates over time, from February 17, 2008, to February 17, 2014, using the Cochran-Armitage test for trend. We then grouped hospitals into quintiles based on their unadjusted early comfort care rates. We described patient and hospital characteristics for each quintile, using χ2 tests to test for differences across quintiles for categorical variables and Wilcoxon rank sum tests to assess for differences across quintiles for continuous variables. We then examined the Spearman’s rank correlation between hospitals’ RSMR and risk-adjusted comfort care rates. Finally, we compared hospital-level RSMRs before and after adjusting for early comfort care.
We performed risk-adjustment for these last 2 analyses as follows. For each patient, covariates were obtained from the GWTG-HF registry. Clinical data captured for the index admission were utilized in the risk-adjustment model (for both RSMRs and risk-adjusted comfort care rates). Included covariates were as follows: age (per 10 years); race (black vs non-black); systolic blood pressure at admission ≤170 (per 10 mm Hg); respiratory rate (per 5 respirations/min); heart rate ≤105 (per 10 beats/min); weight ≤100 (per 5 kg); weight >100 (per 5 kg); blood urea nitrogen (per 10 mg/dl); brain natriuretic peptide ≤2000 (per 500 pg/ml); hemoglobin 10-14 (per 1 g/dl); troponin abnormal (vs normal); creatinine ≤1 (per 1 mg/dl); sodium 130-140 (per 5 mEq/l); and chronic obstructive pulmonary disease or asthma.
Hierarchical logistic regression modeling was used to calculate the hospital-specific RSMR. A predicted/expected ratio similar to an observed/expected (O/E) ratio was calculated using the following modifications: (1) instead of the observed (crude) number of deaths, the numerator is the number of deaths predicted by the hierarchical model among a hospital’s patients given the patients’ risk factors and the hospital-specific effect; (2) the denominator is the expected number of deaths among the hospital’s patients given the patients’ risk factors and the average of all hospital-specific effects overall; and (3) the ratio of the numerator and denominator are then multiplied by the observed overall mortality rate (same as O/E). This calculation is the method used by CMS to derive RSMRs.11 Multiple imputation was used to handle missing data in the models; 25 imputed datasets using the fully conditional specification method were created. Patients with missing prior comorbidities were assumed to not have those conditions. Hospital characteristics were not imputed; therefore, for analyses that required construction of risk-adjusted comfort care rates or RSMRs, we excluded 18,867 patients cared for at 82 hospitals missing hospital characteristics. We ran 2 sets of models for risk-adjusted comfort care rates and RSMRs: the first adjusted only for patient characteristics, and the second adjusted for both patient and hospital characteristics. Results from the 2 models were similar, so we present only results from the latter. Variance inflation factors were all <2, indicating the collinearity between covariates was not an issue.
All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Cary, NC). We tested for statistical significance by using 2-tailed tests and considered P values <.05 to be statistically significant.
RESULTS
Of the 272 hospitals included in our final study cohort, the observed median overall rate of early comfort care in this study was 1.9% (25th to 75th percentile: 0.9% to 4.0%); hospitals varied widely in unadjusted early comfort care rates (0.00% to 0.46% in the lowest quintile, and 4.60% to 39.91% in the highest quintile; Table 1).
DISCUSSION
Among a national sample of US hospitals, we found wide variation in how frequently health care providers deliver comfort care within the first 2 days of admission for HF. A minority of hospitals reported no early comfort care on any patients throughout the 6-year study period, but hospitals in the highest quintile initiated early comfort care rates for at least 1 in 20 HF patients. Hospitals that were more likely to initiate early comfort care had a higher proportion of female and white patients and were less likely to have the capacity to deliver aggressive surgical interventions such as heart transplants. Hospital-level 30-day RSMRs were not correlated with rates of early comfort care.
While the appropriate rate of early comfort care for patients hospitalized with HF is unknown, given that the average hospital RSMR is approximately 12% for fee-for-service Medicare patients hospitalized with HF,12 it is surprising that some hospitals initiated early comfort care on none or very few of their HF patients. It is quite possible that many of these hospitals initiated comfort care for some of their patients after 48 hours of hospitalization. We were unable to estimate the average period of time patients received comfort care prior to dying, the degree to which this varies across hospitals or why it might vary, and whether the length of time between comfort care initiation and death is related to satisfaction with end-of-life care. Future research on these topics would help inform providers seeking to deliver better end-of-life care. In this study, we also were unable to estimate how often early comfort care was not initiated because patients had a good prognosis. However, prior studies have suggested low rates of comfort care or hospice referral even among patients at very high estimated mortality risk.4 It is also possible that providers and families had concerns about the ability to accurately prognosticate, although several models have been shown to perform acceptably for patients hospitalized with HF.13
We found that comfort care rates did not increase over time, even though use of hospice care doubled among Medicare beneficiaries between 2000 and 2012. By way of context, cancer—the second leading cause of death in the US—was responsible for 38% of hospice admissions in 2013, whereas heart disease (including but not limited to HF)—the leading cause of death— was responsible for 13% of hospice admissions.14 The 2013 American College of Cardiology Foundation and the American Heart Association guidelines for HF recommend consideration of hospice or palliative care for inpatient and transitional care.15 In future work, it would be important to better understand the drivers behind decisions around comfort care for patients hospitalized with HF.
With regards to the policy implications of our study, we found that on average, adjusting 30-day mortality rates for early comfort care was not associated with a change in hospital mortality rankings. For those hospitals with high comfort care rates, adjusting for comfort care did lower mortality rates, but the change was so small as to be clinically insignificant. CMS’ RSMR for HF excludes patients enrolled in hospice during the 12 months prior to index admission, including the first day of the index admission, acknowledging that death may not be an untoward outcome for such patients.16 Fee-for-service Medicare beneficiaries excluded for hospice enrollment comprised 1.29% of HF admissions from July 2012 to June 201516 and are likely a subset of early comfort care patients in our sample, both because of the inclusiveness of chart review (vs claims-based identification) and because we defined early comfort care as comfort care initiated on day 0 or 1 of hospitalization. Nevertheless, with our data we cannot assess to what degree our findings were due solely to hospice patients excluded from CMS’ current estimates.
Prior research has described the underuse of palliative care among patients with HF17 and the association of palliative care with better patient and family experiences at the end of life.18-20 We add to this literature by describing the epidemiology—prevalence, changes over time, and associated factors—of early comfort care for HF in a national sample of hospitals. This serves as a baseline for future work on end-of-life care among patients hospitalized for HF. Our findings also contribute to ongoing discussion about how best to risk-adjust mortality metrics used to assess hospital quality in pay-for-performance programs. Recent research on stroke and pneumonia based on California data suggests that not accounting for do-not-resuscitate (DNR) status biases hospital mortality rates.21,22 Earlier research examined the impact of adjusting hospital mortality rates for DNR for a broader range of conditions.23,24 We expand this line of inquiry by examining the hospital-level association of early comfort care with mortality rates for HF, utilizing a national, contemporary cohort of inpatient stays. In addition, while studies have found that DNR rates within the first 24 hours of admission are relatively high (median 15.8% for pneumonia; 13.3% for stroke),21,22 comfort care is distinct from DNR.
Our findings should be interpreted in the context of several potential limitations. First, we did not have any information about patient or family wishes regarding end-of-life care, or the exact timing of early comfort care (eg, day 0 or day 1). The initiation of comfort care usually follows conversations about end-of-life care involving a patient, his or her family, and the medical team. Thus, we do not know if low early comfort care rates represent the lack of such a conversation (and thus poor-quality care) or the desire by most patients not to initiate early comfort care (and thus high-quality care). This would be an important area for future research. Second, we included only patients admitted to hospitals that participate in GWTG-HF, a voluntary quality improvement initiative. This may limit the generalizability of our findings, but it is unclear how our sample might bias our findings. Hospitals engaged in quality improvement may be more likely to initiate early comfort care aligned with patients’ wishes; on the other hand, hospitals with advanced surgical capabilities are over-represented in our sample and these hospitals are less likely to initiate early comfort care. Third, we examined associations and cannot make conclusions about causality. Residual measured and unmeasured confounding may influence these findings.
In summary, we found that early comfort care rates for fee-for-service Medicare beneficiaries admitted for HF varies widely among hospitals, but median rates of early comfort care have not changed over time. On average, there was no correlation between hospital-level, 30-day, RSMRs and rates of early comfort care. This suggests that current efforts to lower mortality rates have not had unintended consequences for hospitals that institute early comfort care more commonly than their peers.
Acknowledgments
Dr. Chen and Ms. Cox take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Chen, Levine, and Hayward are responsible for the study concept and design. Drs. Chen and Fonarow acquired the data. Dr. Chen drafted the manuscript. Drs. Chen, Levin, Hayward, Cox, Fonarow, DeVore, Hernandez, Heidenreich, and Yancy revised the manuscript for important intellectual content. Drs. Chen, Hayward, Cox, and Schulte performed the statistical analysis. Drs. Chen and Fonarow obtained funding for the study. Drs. Hayward and Fonarow supervised the study. The authors thank Bailey Green, MPH, for the research assistance she provided. She was compensated for her work.
Disclosure
Dr. Fonarow reports research support from the National Institutes of Health, and consulting for Amgen, Janssen, Novartis, Medtronic, and St Jude Medical. Dr. DeVore reports research support from the American Heart Association, Amgen, and Novartis, and consulting for Amgen. The other authors have no relevant conflicts of interest. Dr. Chen was supported by a Career Development Grant Award (K08HS020671) from the Agency for Healthcare Research and Quality when the manuscript was being prepared. She currently receives support from the Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation for her work there. She also receives support from the Blue Cross Blue Shield of Michigan Foundation’s Investigator Initiated Research Program, the Agency for Healthcare Research and Quality (R01 HS024698), and the National Institute on Aging (P01 AG019783). These funding sources had no role in the preparation, review, or approval of the manuscript. The GWTG-HF program is provided by the American Heart Association. GWTG-HF has been funded in the past through support from Amgen, Medtronic, GlaxoSmithKline, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable. These sponsors had no role in the study design, data analysis or manuscript preparation and revision.
1. Centers for Medicare & Medicaid Services. Hospital Compare. https://www.medicare.gov/hospitalcompare/. Accessed on November 27, 2016.
2. Centers for Medicare & Medicaid Services. Hospital Value-based Purchasing. https://www.medicare.gov/hospitalcompare/data/hospital-vbp.html. Accessed August 30, 2017.
3. Medicare Payment Advisory Comission. Report to the Congress: Medicare payment policy. 2014. http://www.medpac.gov/docs/default-source/reports/mar14_entirereport.pdf. Accessed August 31, 2017.
4. Whellan DJ, Cox M, Hernandez AF, et al. Utilization of hospice and predicted mortality risk among older patients hospitalized with heart failure: findings from GWTG-HF. J Card Fail. 2012;18(6):471-477. PubMed
5. Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5(4):179-186. PubMed
6. LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “get with the guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29(10):539-550. PubMed
7. Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298(13):1525-1532. PubMed
8. Get With The Guidelines-Heart Failure. HF Patient Management Tool, October 2016.
9. Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. 2013;1(3):245-251. PubMed
10. Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3(1):25-32. PubMed
11. Frequently Asked Questions (FAQs): Implementation and Maintenance of CMS Mortality Measures for AMI & HF. 2007. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalMortalityAboutAMI_HF.pdf. Accessed August 30, 2017.
12. Suter LG, Li SX, Grady JN, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333-1340. PubMed
13. Lagu T, Pekow PS, Shieh MS, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. Aug 2016;9(8):e002912. PubMed
14. National Hospice and Palliative Care Organization. NHPCO’s facts and figures: hospice care in america. 2015. https://www.nhpco.org/sites/default/files/public/Statistics_Research/2015_Facts_Figures.pdf. Accessed August 30, 2017.
15. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852. PubMed
16. Centers for Medicare & Medicaid Services. 2016 Condition-Specific Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standardized Mortality Measures. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier3&cid=1228774398696. Accessed August 30, 2017.
17. Bakitas M, Macmartin M, Trzepkowski K, et al. Palliative care consultations for heart failure patients: how many, when, and why? J Card Fail. 2013;19(3):193-201. PubMed
18. Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of End-of-Life Care Provided to Patients With Different Serious Illnesses. JAMA Intern Med. 2016;176(8):1095-1102. PubMed
19. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. PubMed
20. Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: results of a randomized, controlled clinical trial. J Card Fail. 2016;22(11):940. PubMed
21. Kelly AG, Zahuranec DB, Holloway RG, Morgenstern LB, Burke JF. Variation in do-not-resuscitate orders for patients with ischemic stroke: implications for national hospital comparisons. Stroke. 2014;45(3):822-827. PubMed
22. Walkey AJ, Weinberg J, Wiener RS, Cooke CR, Lindenauer PK. Association of Do-Not-Resuscitate Orders and Hospital Mortality Rate Among Patients With Pneumonia. JAMA Intern Med. 2016;176(1):97-104. PubMed
23. Bardach N, Zhao S, Pantilat S, Johnston SC. Adjustment for do-not-resuscitate orders reverses the apparent in-hospital mortality advantage for minorities. Am J Med. 2005;118(4):400-408. PubMed
24. Tabak YP, Johannes RS, Silber JH, Kurtz SG. Should Do-Not-Resuscitate status be included as a mortality risk adjustor? The impact of DNR variations on performance reporting. Med Care. 2005;43(7):658-666. PubMed
In an effort to improve the quality of care delivered to heart failure (HF) patients, the Centers for Medicare & Medicaid Services (CMS) publish hospitals’ 30-day risk-standardized mortality rates (RSMRs) for HF.1 These mortality rates are also used by CMS to determine the financial penalties and bonuses that hospitals receive as part of the national Hospital Value-based Purchasing program.2 Whether or not these efforts effectively direct patients towards high-quality providers or motivate hospitals to provide better care, few would disagree with the overarching goal of decreasing the number of patients who die from HF.
However, for some patients with chronic disease at the end of life, goals of care may change. The quality of days lived may become more important than the quantity of days lived. As a consequence, high-quality care for some patients at the end of life is associated with withdrawing life-sustaining or life-extending therapies. Over time, this therapeutic perspective has become more common, with use of hospice care doubling from 23% to 47% between 2000 and 2012 among Medicare beneficiaries who died.3 For a national cohort of older patients admitted with HF—not just those patients who died in that same year—hospitals’ rates of referral to hospice are considerably lower, averaging 2.9% in 2010 in a national study.4 Nevertheless, it is possible that hospitals that more faithfully follow their dying patients’ wishes and withdraw life-prolonging interventions and provide comfort-focused care at the end of life might be unfairly penalized if such efforts resulted in higher mortality rates than other hospitals.
Therefore, we used Medicare data linked to a national HF registry with information about end-of-life care, to address 3 questions: (1) How much do hospitals vary in their rates of early comfort care and how has this changed over time; (2) What hospital and patient factors are associated with higher early comfort care rates; and (3) Is there a correlation between 30-day risk-adjusted mortality rates for HF with hospital rates of early comfort care?
METHODS
Data Sources
We used data from the American Heart Association’s Get With The Guidelines-Heart Failure (GWTG-HF) registry. GWTG-HF is a voluntary, inpatient, quality improvement registry5-7 that uses web-based tools and standard questionnaires to collect data on patients with HF admitted to participating hospitals nationwide. The data include information from admission (eg, sociodemographic characteristics, symptoms, medical history, and initial laboratory and test results), the inpatient stay (eg, therapies), and discharge (eg, discharge destination, whether and when comfort care was initiated). We linked the GWTG-HF registry data to Medicare claims data in order to obtain information about Medicare eligibility and patient comorbidities. Additionally, we used data from the American Hospital Association (2008) for hospital characteristics. Quintiles Real-World & Late Phase Research (Cambridge, MA) serves as the data coordinating center for GWTG-HF and the Duke Clinical Research Institute (Durham, NC) serves as the statistical analytic center. GWTG-HF participating sites have a waiver of informed consent because the data are de-identified and primarily used for quality improvement. All analyses performed on this data have been approved by the Duke Medical Center Institutional Review Board.
Study Population
Study Outcomes
Our outcome of interest was the correlation between a hospital’s rate of initiating early CMO for admitted HF patients and a hospital’s 30-day RSMR for HF. The GWTG-HF questionnaire8 asks “When is the earliest physician/advanced practice nurse/physician assistant documentation of comfort measures only?” and permits 4 responses: day 0 or 1, day 2 or after, timing unclear, or not documented/unable to determine. We defined early CMO as CMO on day 0 or 1, and late/no CMO as any other response. We chose to examine early comfort care because many hospitalized patients transition to comfort care before they die if the death is in any way predictable. Thus, if comfort care is measured at any time during the hospitalization, hospitals that have high mortality rates are likely to have high comfort care rates. Therefore, we chose to use the more precise measure of early comfort care. We created hospital-level, risk-standardized early comfort care rates using the same risk-adjustment model used for RSMRs but with the outcome of early comfort care instead of mortality.9,10
RSMRs were calculated using a validated GWTG-HF 30-day risk-standardized mortality model9 with additional variables identified from other GWTG-HF analyses.10 The 30 days are measured as the 30 days after the index admission date.
Statistical Analyses
We described trends in early comfort care rates over time, from February 17, 2008, to February 17, 2014, using the Cochran-Armitage test for trend. We then grouped hospitals into quintiles based on their unadjusted early comfort care rates. We described patient and hospital characteristics for each quintile, using χ2 tests to test for differences across quintiles for categorical variables and Wilcoxon rank sum tests to assess for differences across quintiles for continuous variables. We then examined the Spearman’s rank correlation between hospitals’ RSMR and risk-adjusted comfort care rates. Finally, we compared hospital-level RSMRs before and after adjusting for early comfort care.
We performed risk-adjustment for these last 2 analyses as follows. For each patient, covariates were obtained from the GWTG-HF registry. Clinical data captured for the index admission were utilized in the risk-adjustment model (for both RSMRs and risk-adjusted comfort care rates). Included covariates were as follows: age (per 10 years); race (black vs non-black); systolic blood pressure at admission ≤170 (per 10 mm Hg); respiratory rate (per 5 respirations/min); heart rate ≤105 (per 10 beats/min); weight ≤100 (per 5 kg); weight >100 (per 5 kg); blood urea nitrogen (per 10 mg/dl); brain natriuretic peptide ≤2000 (per 500 pg/ml); hemoglobin 10-14 (per 1 g/dl); troponin abnormal (vs normal); creatinine ≤1 (per 1 mg/dl); sodium 130-140 (per 5 mEq/l); and chronic obstructive pulmonary disease or asthma.
Hierarchical logistic regression modeling was used to calculate the hospital-specific RSMR. A predicted/expected ratio similar to an observed/expected (O/E) ratio was calculated using the following modifications: (1) instead of the observed (crude) number of deaths, the numerator is the number of deaths predicted by the hierarchical model among a hospital’s patients given the patients’ risk factors and the hospital-specific effect; (2) the denominator is the expected number of deaths among the hospital’s patients given the patients’ risk factors and the average of all hospital-specific effects overall; and (3) the ratio of the numerator and denominator are then multiplied by the observed overall mortality rate (same as O/E). This calculation is the method used by CMS to derive RSMRs.11 Multiple imputation was used to handle missing data in the models; 25 imputed datasets using the fully conditional specification method were created. Patients with missing prior comorbidities were assumed to not have those conditions. Hospital characteristics were not imputed; therefore, for analyses that required construction of risk-adjusted comfort care rates or RSMRs, we excluded 18,867 patients cared for at 82 hospitals missing hospital characteristics. We ran 2 sets of models for risk-adjusted comfort care rates and RSMRs: the first adjusted only for patient characteristics, and the second adjusted for both patient and hospital characteristics. Results from the 2 models were similar, so we present only results from the latter. Variance inflation factors were all <2, indicating the collinearity between covariates was not an issue.
All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Cary, NC). We tested for statistical significance by using 2-tailed tests and considered P values <.05 to be statistically significant.
RESULTS
Of the 272 hospitals included in our final study cohort, the observed median overall rate of early comfort care in this study was 1.9% (25th to 75th percentile: 0.9% to 4.0%); hospitals varied widely in unadjusted early comfort care rates (0.00% to 0.46% in the lowest quintile, and 4.60% to 39.91% in the highest quintile; Table 1).
DISCUSSION
Among a national sample of US hospitals, we found wide variation in how frequently health care providers deliver comfort care within the first 2 days of admission for HF. A minority of hospitals reported no early comfort care on any patients throughout the 6-year study period, but hospitals in the highest quintile initiated early comfort care rates for at least 1 in 20 HF patients. Hospitals that were more likely to initiate early comfort care had a higher proportion of female and white patients and were less likely to have the capacity to deliver aggressive surgical interventions such as heart transplants. Hospital-level 30-day RSMRs were not correlated with rates of early comfort care.
While the appropriate rate of early comfort care for patients hospitalized with HF is unknown, given that the average hospital RSMR is approximately 12% for fee-for-service Medicare patients hospitalized with HF,12 it is surprising that some hospitals initiated early comfort care on none or very few of their HF patients. It is quite possible that many of these hospitals initiated comfort care for some of their patients after 48 hours of hospitalization. We were unable to estimate the average period of time patients received comfort care prior to dying, the degree to which this varies across hospitals or why it might vary, and whether the length of time between comfort care initiation and death is related to satisfaction with end-of-life care. Future research on these topics would help inform providers seeking to deliver better end-of-life care. In this study, we also were unable to estimate how often early comfort care was not initiated because patients had a good prognosis. However, prior studies have suggested low rates of comfort care or hospice referral even among patients at very high estimated mortality risk.4 It is also possible that providers and families had concerns about the ability to accurately prognosticate, although several models have been shown to perform acceptably for patients hospitalized with HF.13
We found that comfort care rates did not increase over time, even though use of hospice care doubled among Medicare beneficiaries between 2000 and 2012. By way of context, cancer—the second leading cause of death in the US—was responsible for 38% of hospice admissions in 2013, whereas heart disease (including but not limited to HF)—the leading cause of death— was responsible for 13% of hospice admissions.14 The 2013 American College of Cardiology Foundation and the American Heart Association guidelines for HF recommend consideration of hospice or palliative care for inpatient and transitional care.15 In future work, it would be important to better understand the drivers behind decisions around comfort care for patients hospitalized with HF.
With regards to the policy implications of our study, we found that on average, adjusting 30-day mortality rates for early comfort care was not associated with a change in hospital mortality rankings. For those hospitals with high comfort care rates, adjusting for comfort care did lower mortality rates, but the change was so small as to be clinically insignificant. CMS’ RSMR for HF excludes patients enrolled in hospice during the 12 months prior to index admission, including the first day of the index admission, acknowledging that death may not be an untoward outcome for such patients.16 Fee-for-service Medicare beneficiaries excluded for hospice enrollment comprised 1.29% of HF admissions from July 2012 to June 201516 and are likely a subset of early comfort care patients in our sample, both because of the inclusiveness of chart review (vs claims-based identification) and because we defined early comfort care as comfort care initiated on day 0 or 1 of hospitalization. Nevertheless, with our data we cannot assess to what degree our findings were due solely to hospice patients excluded from CMS’ current estimates.
Prior research has described the underuse of palliative care among patients with HF17 and the association of palliative care with better patient and family experiences at the end of life.18-20 We add to this literature by describing the epidemiology—prevalence, changes over time, and associated factors—of early comfort care for HF in a national sample of hospitals. This serves as a baseline for future work on end-of-life care among patients hospitalized for HF. Our findings also contribute to ongoing discussion about how best to risk-adjust mortality metrics used to assess hospital quality in pay-for-performance programs. Recent research on stroke and pneumonia based on California data suggests that not accounting for do-not-resuscitate (DNR) status biases hospital mortality rates.21,22 Earlier research examined the impact of adjusting hospital mortality rates for DNR for a broader range of conditions.23,24 We expand this line of inquiry by examining the hospital-level association of early comfort care with mortality rates for HF, utilizing a national, contemporary cohort of inpatient stays. In addition, while studies have found that DNR rates within the first 24 hours of admission are relatively high (median 15.8% for pneumonia; 13.3% for stroke),21,22 comfort care is distinct from DNR.
Our findings should be interpreted in the context of several potential limitations. First, we did not have any information about patient or family wishes regarding end-of-life care, or the exact timing of early comfort care (eg, day 0 or day 1). The initiation of comfort care usually follows conversations about end-of-life care involving a patient, his or her family, and the medical team. Thus, we do not know if low early comfort care rates represent the lack of such a conversation (and thus poor-quality care) or the desire by most patients not to initiate early comfort care (and thus high-quality care). This would be an important area for future research. Second, we included only patients admitted to hospitals that participate in GWTG-HF, a voluntary quality improvement initiative. This may limit the generalizability of our findings, but it is unclear how our sample might bias our findings. Hospitals engaged in quality improvement may be more likely to initiate early comfort care aligned with patients’ wishes; on the other hand, hospitals with advanced surgical capabilities are over-represented in our sample and these hospitals are less likely to initiate early comfort care. Third, we examined associations and cannot make conclusions about causality. Residual measured and unmeasured confounding may influence these findings.
In summary, we found that early comfort care rates for fee-for-service Medicare beneficiaries admitted for HF varies widely among hospitals, but median rates of early comfort care have not changed over time. On average, there was no correlation between hospital-level, 30-day, RSMRs and rates of early comfort care. This suggests that current efforts to lower mortality rates have not had unintended consequences for hospitals that institute early comfort care more commonly than their peers.
Acknowledgments
Dr. Chen and Ms. Cox take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Chen, Levine, and Hayward are responsible for the study concept and design. Drs. Chen and Fonarow acquired the data. Dr. Chen drafted the manuscript. Drs. Chen, Levin, Hayward, Cox, Fonarow, DeVore, Hernandez, Heidenreich, and Yancy revised the manuscript for important intellectual content. Drs. Chen, Hayward, Cox, and Schulte performed the statistical analysis. Drs. Chen and Fonarow obtained funding for the study. Drs. Hayward and Fonarow supervised the study. The authors thank Bailey Green, MPH, for the research assistance she provided. She was compensated for her work.
Disclosure
Dr. Fonarow reports research support from the National Institutes of Health, and consulting for Amgen, Janssen, Novartis, Medtronic, and St Jude Medical. Dr. DeVore reports research support from the American Heart Association, Amgen, and Novartis, and consulting for Amgen. The other authors have no relevant conflicts of interest. Dr. Chen was supported by a Career Development Grant Award (K08HS020671) from the Agency for Healthcare Research and Quality when the manuscript was being prepared. She currently receives support from the Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation for her work there. She also receives support from the Blue Cross Blue Shield of Michigan Foundation’s Investigator Initiated Research Program, the Agency for Healthcare Research and Quality (R01 HS024698), and the National Institute on Aging (P01 AG019783). These funding sources had no role in the preparation, review, or approval of the manuscript. The GWTG-HF program is provided by the American Heart Association. GWTG-HF has been funded in the past through support from Amgen, Medtronic, GlaxoSmithKline, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable. These sponsors had no role in the study design, data analysis or manuscript preparation and revision.
In an effort to improve the quality of care delivered to heart failure (HF) patients, the Centers for Medicare & Medicaid Services (CMS) publish hospitals’ 30-day risk-standardized mortality rates (RSMRs) for HF.1 These mortality rates are also used by CMS to determine the financial penalties and bonuses that hospitals receive as part of the national Hospital Value-based Purchasing program.2 Whether or not these efforts effectively direct patients towards high-quality providers or motivate hospitals to provide better care, few would disagree with the overarching goal of decreasing the number of patients who die from HF.
However, for some patients with chronic disease at the end of life, goals of care may change. The quality of days lived may become more important than the quantity of days lived. As a consequence, high-quality care for some patients at the end of life is associated with withdrawing life-sustaining or life-extending therapies. Over time, this therapeutic perspective has become more common, with use of hospice care doubling from 23% to 47% between 2000 and 2012 among Medicare beneficiaries who died.3 For a national cohort of older patients admitted with HF—not just those patients who died in that same year—hospitals’ rates of referral to hospice are considerably lower, averaging 2.9% in 2010 in a national study.4 Nevertheless, it is possible that hospitals that more faithfully follow their dying patients’ wishes and withdraw life-prolonging interventions and provide comfort-focused care at the end of life might be unfairly penalized if such efforts resulted in higher mortality rates than other hospitals.
Therefore, we used Medicare data linked to a national HF registry with information about end-of-life care, to address 3 questions: (1) How much do hospitals vary in their rates of early comfort care and how has this changed over time; (2) What hospital and patient factors are associated with higher early comfort care rates; and (3) Is there a correlation between 30-day risk-adjusted mortality rates for HF with hospital rates of early comfort care?
METHODS
Data Sources
We used data from the American Heart Association’s Get With The Guidelines-Heart Failure (GWTG-HF) registry. GWTG-HF is a voluntary, inpatient, quality improvement registry5-7 that uses web-based tools and standard questionnaires to collect data on patients with HF admitted to participating hospitals nationwide. The data include information from admission (eg, sociodemographic characteristics, symptoms, medical history, and initial laboratory and test results), the inpatient stay (eg, therapies), and discharge (eg, discharge destination, whether and when comfort care was initiated). We linked the GWTG-HF registry data to Medicare claims data in order to obtain information about Medicare eligibility and patient comorbidities. Additionally, we used data from the American Hospital Association (2008) for hospital characteristics. Quintiles Real-World & Late Phase Research (Cambridge, MA) serves as the data coordinating center for GWTG-HF and the Duke Clinical Research Institute (Durham, NC) serves as the statistical analytic center. GWTG-HF participating sites have a waiver of informed consent because the data are de-identified and primarily used for quality improvement. All analyses performed on this data have been approved by the Duke Medical Center Institutional Review Board.
Study Population
Study Outcomes
Our outcome of interest was the correlation between a hospital’s rate of initiating early CMO for admitted HF patients and a hospital’s 30-day RSMR for HF. The GWTG-HF questionnaire8 asks “When is the earliest physician/advanced practice nurse/physician assistant documentation of comfort measures only?” and permits 4 responses: day 0 or 1, day 2 or after, timing unclear, or not documented/unable to determine. We defined early CMO as CMO on day 0 or 1, and late/no CMO as any other response. We chose to examine early comfort care because many hospitalized patients transition to comfort care before they die if the death is in any way predictable. Thus, if comfort care is measured at any time during the hospitalization, hospitals that have high mortality rates are likely to have high comfort care rates. Therefore, we chose to use the more precise measure of early comfort care. We created hospital-level, risk-standardized early comfort care rates using the same risk-adjustment model used for RSMRs but with the outcome of early comfort care instead of mortality.9,10
RSMRs were calculated using a validated GWTG-HF 30-day risk-standardized mortality model9 with additional variables identified from other GWTG-HF analyses.10 The 30 days are measured as the 30 days after the index admission date.
Statistical Analyses
We described trends in early comfort care rates over time, from February 17, 2008, to February 17, 2014, using the Cochran-Armitage test for trend. We then grouped hospitals into quintiles based on their unadjusted early comfort care rates. We described patient and hospital characteristics for each quintile, using χ2 tests to test for differences across quintiles for categorical variables and Wilcoxon rank sum tests to assess for differences across quintiles for continuous variables. We then examined the Spearman’s rank correlation between hospitals’ RSMR and risk-adjusted comfort care rates. Finally, we compared hospital-level RSMRs before and after adjusting for early comfort care.
We performed risk-adjustment for these last 2 analyses as follows. For each patient, covariates were obtained from the GWTG-HF registry. Clinical data captured for the index admission were utilized in the risk-adjustment model (for both RSMRs and risk-adjusted comfort care rates). Included covariates were as follows: age (per 10 years); race (black vs non-black); systolic blood pressure at admission ≤170 (per 10 mm Hg); respiratory rate (per 5 respirations/min); heart rate ≤105 (per 10 beats/min); weight ≤100 (per 5 kg); weight >100 (per 5 kg); blood urea nitrogen (per 10 mg/dl); brain natriuretic peptide ≤2000 (per 500 pg/ml); hemoglobin 10-14 (per 1 g/dl); troponin abnormal (vs normal); creatinine ≤1 (per 1 mg/dl); sodium 130-140 (per 5 mEq/l); and chronic obstructive pulmonary disease or asthma.
Hierarchical logistic regression modeling was used to calculate the hospital-specific RSMR. A predicted/expected ratio similar to an observed/expected (O/E) ratio was calculated using the following modifications: (1) instead of the observed (crude) number of deaths, the numerator is the number of deaths predicted by the hierarchical model among a hospital’s patients given the patients’ risk factors and the hospital-specific effect; (2) the denominator is the expected number of deaths among the hospital’s patients given the patients’ risk factors and the average of all hospital-specific effects overall; and (3) the ratio of the numerator and denominator are then multiplied by the observed overall mortality rate (same as O/E). This calculation is the method used by CMS to derive RSMRs.11 Multiple imputation was used to handle missing data in the models; 25 imputed datasets using the fully conditional specification method were created. Patients with missing prior comorbidities were assumed to not have those conditions. Hospital characteristics were not imputed; therefore, for analyses that required construction of risk-adjusted comfort care rates or RSMRs, we excluded 18,867 patients cared for at 82 hospitals missing hospital characteristics. We ran 2 sets of models for risk-adjusted comfort care rates and RSMRs: the first adjusted only for patient characteristics, and the second adjusted for both patient and hospital characteristics. Results from the 2 models were similar, so we present only results from the latter. Variance inflation factors were all <2, indicating the collinearity between covariates was not an issue.
All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Cary, NC). We tested for statistical significance by using 2-tailed tests and considered P values <.05 to be statistically significant.
RESULTS
Of the 272 hospitals included in our final study cohort, the observed median overall rate of early comfort care in this study was 1.9% (25th to 75th percentile: 0.9% to 4.0%); hospitals varied widely in unadjusted early comfort care rates (0.00% to 0.46% in the lowest quintile, and 4.60% to 39.91% in the highest quintile; Table 1).
DISCUSSION
Among a national sample of US hospitals, we found wide variation in how frequently health care providers deliver comfort care within the first 2 days of admission for HF. A minority of hospitals reported no early comfort care on any patients throughout the 6-year study period, but hospitals in the highest quintile initiated early comfort care rates for at least 1 in 20 HF patients. Hospitals that were more likely to initiate early comfort care had a higher proportion of female and white patients and were less likely to have the capacity to deliver aggressive surgical interventions such as heart transplants. Hospital-level 30-day RSMRs were not correlated with rates of early comfort care.
While the appropriate rate of early comfort care for patients hospitalized with HF is unknown, given that the average hospital RSMR is approximately 12% for fee-for-service Medicare patients hospitalized with HF,12 it is surprising that some hospitals initiated early comfort care on none or very few of their HF patients. It is quite possible that many of these hospitals initiated comfort care for some of their patients after 48 hours of hospitalization. We were unable to estimate the average period of time patients received comfort care prior to dying, the degree to which this varies across hospitals or why it might vary, and whether the length of time between comfort care initiation and death is related to satisfaction with end-of-life care. Future research on these topics would help inform providers seeking to deliver better end-of-life care. In this study, we also were unable to estimate how often early comfort care was not initiated because patients had a good prognosis. However, prior studies have suggested low rates of comfort care or hospice referral even among patients at very high estimated mortality risk.4 It is also possible that providers and families had concerns about the ability to accurately prognosticate, although several models have been shown to perform acceptably for patients hospitalized with HF.13
We found that comfort care rates did not increase over time, even though use of hospice care doubled among Medicare beneficiaries between 2000 and 2012. By way of context, cancer—the second leading cause of death in the US—was responsible for 38% of hospice admissions in 2013, whereas heart disease (including but not limited to HF)—the leading cause of death— was responsible for 13% of hospice admissions.14 The 2013 American College of Cardiology Foundation and the American Heart Association guidelines for HF recommend consideration of hospice or palliative care for inpatient and transitional care.15 In future work, it would be important to better understand the drivers behind decisions around comfort care for patients hospitalized with HF.
With regards to the policy implications of our study, we found that on average, adjusting 30-day mortality rates for early comfort care was not associated with a change in hospital mortality rankings. For those hospitals with high comfort care rates, adjusting for comfort care did lower mortality rates, but the change was so small as to be clinically insignificant. CMS’ RSMR for HF excludes patients enrolled in hospice during the 12 months prior to index admission, including the first day of the index admission, acknowledging that death may not be an untoward outcome for such patients.16 Fee-for-service Medicare beneficiaries excluded for hospice enrollment comprised 1.29% of HF admissions from July 2012 to June 201516 and are likely a subset of early comfort care patients in our sample, both because of the inclusiveness of chart review (vs claims-based identification) and because we defined early comfort care as comfort care initiated on day 0 or 1 of hospitalization. Nevertheless, with our data we cannot assess to what degree our findings were due solely to hospice patients excluded from CMS’ current estimates.
Prior research has described the underuse of palliative care among patients with HF17 and the association of palliative care with better patient and family experiences at the end of life.18-20 We add to this literature by describing the epidemiology—prevalence, changes over time, and associated factors—of early comfort care for HF in a national sample of hospitals. This serves as a baseline for future work on end-of-life care among patients hospitalized for HF. Our findings also contribute to ongoing discussion about how best to risk-adjust mortality metrics used to assess hospital quality in pay-for-performance programs. Recent research on stroke and pneumonia based on California data suggests that not accounting for do-not-resuscitate (DNR) status biases hospital mortality rates.21,22 Earlier research examined the impact of adjusting hospital mortality rates for DNR for a broader range of conditions.23,24 We expand this line of inquiry by examining the hospital-level association of early comfort care with mortality rates for HF, utilizing a national, contemporary cohort of inpatient stays. In addition, while studies have found that DNR rates within the first 24 hours of admission are relatively high (median 15.8% for pneumonia; 13.3% for stroke),21,22 comfort care is distinct from DNR.
Our findings should be interpreted in the context of several potential limitations. First, we did not have any information about patient or family wishes regarding end-of-life care, or the exact timing of early comfort care (eg, day 0 or day 1). The initiation of comfort care usually follows conversations about end-of-life care involving a patient, his or her family, and the medical team. Thus, we do not know if low early comfort care rates represent the lack of such a conversation (and thus poor-quality care) or the desire by most patients not to initiate early comfort care (and thus high-quality care). This would be an important area for future research. Second, we included only patients admitted to hospitals that participate in GWTG-HF, a voluntary quality improvement initiative. This may limit the generalizability of our findings, but it is unclear how our sample might bias our findings. Hospitals engaged in quality improvement may be more likely to initiate early comfort care aligned with patients’ wishes; on the other hand, hospitals with advanced surgical capabilities are over-represented in our sample and these hospitals are less likely to initiate early comfort care. Third, we examined associations and cannot make conclusions about causality. Residual measured and unmeasured confounding may influence these findings.
In summary, we found that early comfort care rates for fee-for-service Medicare beneficiaries admitted for HF varies widely among hospitals, but median rates of early comfort care have not changed over time. On average, there was no correlation between hospital-level, 30-day, RSMRs and rates of early comfort care. This suggests that current efforts to lower mortality rates have not had unintended consequences for hospitals that institute early comfort care more commonly than their peers.
Acknowledgments
Dr. Chen and Ms. Cox take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Chen, Levine, and Hayward are responsible for the study concept and design. Drs. Chen and Fonarow acquired the data. Dr. Chen drafted the manuscript. Drs. Chen, Levin, Hayward, Cox, Fonarow, DeVore, Hernandez, Heidenreich, and Yancy revised the manuscript for important intellectual content. Drs. Chen, Hayward, Cox, and Schulte performed the statistical analysis. Drs. Chen and Fonarow obtained funding for the study. Drs. Hayward and Fonarow supervised the study. The authors thank Bailey Green, MPH, for the research assistance she provided. She was compensated for her work.
Disclosure
Dr. Fonarow reports research support from the National Institutes of Health, and consulting for Amgen, Janssen, Novartis, Medtronic, and St Jude Medical. Dr. DeVore reports research support from the American Heart Association, Amgen, and Novartis, and consulting for Amgen. The other authors have no relevant conflicts of interest. Dr. Chen was supported by a Career Development Grant Award (K08HS020671) from the Agency for Healthcare Research and Quality when the manuscript was being prepared. She currently receives support from the Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation for her work there. She also receives support from the Blue Cross Blue Shield of Michigan Foundation’s Investigator Initiated Research Program, the Agency for Healthcare Research and Quality (R01 HS024698), and the National Institute on Aging (P01 AG019783). These funding sources had no role in the preparation, review, or approval of the manuscript. The GWTG-HF program is provided by the American Heart Association. GWTG-HF has been funded in the past through support from Amgen, Medtronic, GlaxoSmithKline, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable. These sponsors had no role in the study design, data analysis or manuscript preparation and revision.
1. Centers for Medicare & Medicaid Services. Hospital Compare. https://www.medicare.gov/hospitalcompare/. Accessed on November 27, 2016.
2. Centers for Medicare & Medicaid Services. Hospital Value-based Purchasing. https://www.medicare.gov/hospitalcompare/data/hospital-vbp.html. Accessed August 30, 2017.
3. Medicare Payment Advisory Comission. Report to the Congress: Medicare payment policy. 2014. http://www.medpac.gov/docs/default-source/reports/mar14_entirereport.pdf. Accessed August 31, 2017.
4. Whellan DJ, Cox M, Hernandez AF, et al. Utilization of hospice and predicted mortality risk among older patients hospitalized with heart failure: findings from GWTG-HF. J Card Fail. 2012;18(6):471-477. PubMed
5. Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5(4):179-186. PubMed
6. LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “get with the guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29(10):539-550. PubMed
7. Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298(13):1525-1532. PubMed
8. Get With The Guidelines-Heart Failure. HF Patient Management Tool, October 2016.
9. Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. 2013;1(3):245-251. PubMed
10. Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3(1):25-32. PubMed
11. Frequently Asked Questions (FAQs): Implementation and Maintenance of CMS Mortality Measures for AMI & HF. 2007. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalMortalityAboutAMI_HF.pdf. Accessed August 30, 2017.
12. Suter LG, Li SX, Grady JN, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333-1340. PubMed
13. Lagu T, Pekow PS, Shieh MS, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. Aug 2016;9(8):e002912. PubMed
14. National Hospice and Palliative Care Organization. NHPCO’s facts and figures: hospice care in america. 2015. https://www.nhpco.org/sites/default/files/public/Statistics_Research/2015_Facts_Figures.pdf. Accessed August 30, 2017.
15. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852. PubMed
16. Centers for Medicare & Medicaid Services. 2016 Condition-Specific Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standardized Mortality Measures. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier3&cid=1228774398696. Accessed August 30, 2017.
17. Bakitas M, Macmartin M, Trzepkowski K, et al. Palliative care consultations for heart failure patients: how many, when, and why? J Card Fail. 2013;19(3):193-201. PubMed
18. Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of End-of-Life Care Provided to Patients With Different Serious Illnesses. JAMA Intern Med. 2016;176(8):1095-1102. PubMed
19. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. PubMed
20. Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: results of a randomized, controlled clinical trial. J Card Fail. 2016;22(11):940. PubMed
21. Kelly AG, Zahuranec DB, Holloway RG, Morgenstern LB, Burke JF. Variation in do-not-resuscitate orders for patients with ischemic stroke: implications for national hospital comparisons. Stroke. 2014;45(3):822-827. PubMed
22. Walkey AJ, Weinberg J, Wiener RS, Cooke CR, Lindenauer PK. Association of Do-Not-Resuscitate Orders and Hospital Mortality Rate Among Patients With Pneumonia. JAMA Intern Med. 2016;176(1):97-104. PubMed
23. Bardach N, Zhao S, Pantilat S, Johnston SC. Adjustment for do-not-resuscitate orders reverses the apparent in-hospital mortality advantage for minorities. Am J Med. 2005;118(4):400-408. PubMed
24. Tabak YP, Johannes RS, Silber JH, Kurtz SG. Should Do-Not-Resuscitate status be included as a mortality risk adjustor? The impact of DNR variations on performance reporting. Med Care. 2005;43(7):658-666. PubMed
1. Centers for Medicare & Medicaid Services. Hospital Compare. https://www.medicare.gov/hospitalcompare/. Accessed on November 27, 2016.
2. Centers for Medicare & Medicaid Services. Hospital Value-based Purchasing. https://www.medicare.gov/hospitalcompare/data/hospital-vbp.html. Accessed August 30, 2017.
3. Medicare Payment Advisory Comission. Report to the Congress: Medicare payment policy. 2014. http://www.medpac.gov/docs/default-source/reports/mar14_entirereport.pdf. Accessed August 31, 2017.
4. Whellan DJ, Cox M, Hernandez AF, et al. Utilization of hospice and predicted mortality risk among older patients hospitalized with heart failure: findings from GWTG-HF. J Card Fail. 2012;18(6):471-477. PubMed
5. Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5(4):179-186. PubMed
6. LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “get with the guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29(10):539-550. PubMed
7. Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298(13):1525-1532. PubMed
8. Get With The Guidelines-Heart Failure. HF Patient Management Tool, October 2016.
9. Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. 2013;1(3):245-251. PubMed
10. Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3(1):25-32. PubMed
11. Frequently Asked Questions (FAQs): Implementation and Maintenance of CMS Mortality Measures for AMI & HF. 2007. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalMortalityAboutAMI_HF.pdf. Accessed August 30, 2017.
12. Suter LG, Li SX, Grady JN, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333-1340. PubMed
13. Lagu T, Pekow PS, Shieh MS, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. Aug 2016;9(8):e002912. PubMed
14. National Hospice and Palliative Care Organization. NHPCO’s facts and figures: hospice care in america. 2015. https://www.nhpco.org/sites/default/files/public/Statistics_Research/2015_Facts_Figures.pdf. Accessed August 30, 2017.
15. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852. PubMed
16. Centers for Medicare & Medicaid Services. 2016 Condition-Specific Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standardized Mortality Measures. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier3&cid=1228774398696. Accessed August 30, 2017.
17. Bakitas M, Macmartin M, Trzepkowski K, et al. Palliative care consultations for heart failure patients: how many, when, and why? J Card Fail. 2013;19(3):193-201. PubMed
18. Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of End-of-Life Care Provided to Patients With Different Serious Illnesses. JAMA Intern Med. 2016;176(8):1095-1102. PubMed
19. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. PubMed
20. Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: results of a randomized, controlled clinical trial. J Card Fail. 2016;22(11):940. PubMed
21. Kelly AG, Zahuranec DB, Holloway RG, Morgenstern LB, Burke JF. Variation in do-not-resuscitate orders for patients with ischemic stroke: implications for national hospital comparisons. Stroke. 2014;45(3):822-827. PubMed
22. Walkey AJ, Weinberg J, Wiener RS, Cooke CR, Lindenauer PK. Association of Do-Not-Resuscitate Orders and Hospital Mortality Rate Among Patients With Pneumonia. JAMA Intern Med. 2016;176(1):97-104. PubMed
23. Bardach N, Zhao S, Pantilat S, Johnston SC. Adjustment for do-not-resuscitate orders reverses the apparent in-hospital mortality advantage for minorities. Am J Med. 2005;118(4):400-408. PubMed
24. Tabak YP, Johannes RS, Silber JH, Kurtz SG. Should Do-Not-Resuscitate status be included as a mortality risk adjustor? The impact of DNR variations on performance reporting. Med Care. 2005;43(7):658-666. PubMed
© 2018 Society of Hospital Medicine
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
© 2018 Society of Hospital Medicine
The Harm We Do: The Environmental Impact of Medicine
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
© 2018 Society of Hospital Medicine
Omalizumab Helps Relieve Food Allergies
More than 80% of children who were given omalizumab with oral immunotherapy (OIT) for 36 weeks could safely consume portions of at least 2 foods they were causing an allergic reaction, according to findings from a phase 2 study funded by the National Institute of Allergy and Infectious Diseases. Omalizumab, an injectable antibody drug approved for moderate-to-severe allergic asthma, blocks the activity of IgE.
Researchers from Stanford University School of Medicine in California enrolled 48 children aged 4 years to 15 years with confirmed allergy to multiple foods, such as milk, egg, wheat, soy, sesame seeds, peanuts, and tree nuts. The children received omalizumab or placebo injections for the first 16 weeks. At week 8, all participants began eating small, gradually increasing amounts of an allergenic food. They continued OIT until week 36, when they underwent an oral food challenge.
Of the 36 children who received omalizumab, 30 were able to eat at least 2 grams of ≥ 2 allergenic foods, compared with that of only 4 of 12 children (33%) who received placebo. Children who received omalizumab also had fewer adverse events from OIT
More than 80% of children who were given omalizumab with oral immunotherapy (OIT) for 36 weeks could safely consume portions of at least 2 foods they were causing an allergic reaction, according to findings from a phase 2 study funded by the National Institute of Allergy and Infectious Diseases. Omalizumab, an injectable antibody drug approved for moderate-to-severe allergic asthma, blocks the activity of IgE.
Researchers from Stanford University School of Medicine in California enrolled 48 children aged 4 years to 15 years with confirmed allergy to multiple foods, such as milk, egg, wheat, soy, sesame seeds, peanuts, and tree nuts. The children received omalizumab or placebo injections for the first 16 weeks. At week 8, all participants began eating small, gradually increasing amounts of an allergenic food. They continued OIT until week 36, when they underwent an oral food challenge.
Of the 36 children who received omalizumab, 30 were able to eat at least 2 grams of ≥ 2 allergenic foods, compared with that of only 4 of 12 children (33%) who received placebo. Children who received omalizumab also had fewer adverse events from OIT
More than 80% of children who were given omalizumab with oral immunotherapy (OIT) for 36 weeks could safely consume portions of at least 2 foods they were causing an allergic reaction, according to findings from a phase 2 study funded by the National Institute of Allergy and Infectious Diseases. Omalizumab, an injectable antibody drug approved for moderate-to-severe allergic asthma, blocks the activity of IgE.
Researchers from Stanford University School of Medicine in California enrolled 48 children aged 4 years to 15 years with confirmed allergy to multiple foods, such as milk, egg, wheat, soy, sesame seeds, peanuts, and tree nuts. The children received omalizumab or placebo injections for the first 16 weeks. At week 8, all participants began eating small, gradually increasing amounts of an allergenic food. They continued OIT until week 36, when they underwent an oral food challenge.
Of the 36 children who received omalizumab, 30 were able to eat at least 2 grams of ≥ 2 allergenic foods, compared with that of only 4 of 12 children (33%) who received placebo. Children who received omalizumab also had fewer adverse events from OIT
Joint Outpatient Experience Gets an A
The results are in: 93% of soldiers, retirees, and family members report very high overall satisfaction with their experience at Army medical treatment facilities.
Survey responses were for the DoD’s 2017 Joint Outpatient Experience Survey (JOES), which also asked about ease of access to Army providers (83% positive response) and overall experience with Army pharmacies (78% positive).
The results showed an increase in satisfaction of about 2% for those 3 questions compared with the results of 2016, the first time the Army participated in the survey, according to Melissa Gliner, senior health policy analyst with the Office of the Army Surgeon General, in an article for Defense.gov. The survey goes to about 10% of patients who have visited a military health facility.
Besides sharing the survey results with the facilities, Gliner advises them on how to improve the patient experience. For instance, she looks at civilian treatment facilities to see what works. One insight she culled was that it helps to have staff members circulate in the waiting area to chat with patients so they do not feel they are being ignored. Another was that facilities should retrain scheduling clerks to set up appointments without making the patient call back.
Gliner says the U.S. Army Medical Command also is working on a website that will help military health facilities share their ideas and “further elevate patient experience and survey scores.”
The results are in: 93% of soldiers, retirees, and family members report very high overall satisfaction with their experience at Army medical treatment facilities.
Survey responses were for the DoD’s 2017 Joint Outpatient Experience Survey (JOES), which also asked about ease of access to Army providers (83% positive response) and overall experience with Army pharmacies (78% positive).
The results showed an increase in satisfaction of about 2% for those 3 questions compared with the results of 2016, the first time the Army participated in the survey, according to Melissa Gliner, senior health policy analyst with the Office of the Army Surgeon General, in an article for Defense.gov. The survey goes to about 10% of patients who have visited a military health facility.
Besides sharing the survey results with the facilities, Gliner advises them on how to improve the patient experience. For instance, she looks at civilian treatment facilities to see what works. One insight she culled was that it helps to have staff members circulate in the waiting area to chat with patients so they do not feel they are being ignored. Another was that facilities should retrain scheduling clerks to set up appointments without making the patient call back.
Gliner says the U.S. Army Medical Command also is working on a website that will help military health facilities share their ideas and “further elevate patient experience and survey scores.”
The results are in: 93% of soldiers, retirees, and family members report very high overall satisfaction with their experience at Army medical treatment facilities.
Survey responses were for the DoD’s 2017 Joint Outpatient Experience Survey (JOES), which also asked about ease of access to Army providers (83% positive response) and overall experience with Army pharmacies (78% positive).
The results showed an increase in satisfaction of about 2% for those 3 questions compared with the results of 2016, the first time the Army participated in the survey, according to Melissa Gliner, senior health policy analyst with the Office of the Army Surgeon General, in an article for Defense.gov. The survey goes to about 10% of patients who have visited a military health facility.
Besides sharing the survey results with the facilities, Gliner advises them on how to improve the patient experience. For instance, she looks at civilian treatment facilities to see what works. One insight she culled was that it helps to have staff members circulate in the waiting area to chat with patients so they do not feel they are being ignored. Another was that facilities should retrain scheduling clerks to set up appointments without making the patient call back.
Gliner says the U.S. Army Medical Command also is working on a website that will help military health facilities share their ideas and “further elevate patient experience and survey scores.”