User login
VHA Practice Guideline Recommendations for Diffuse Gliomas (FULL)
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
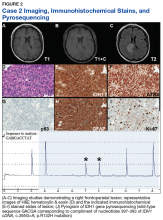
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
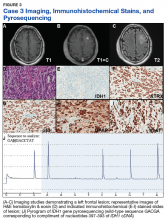
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
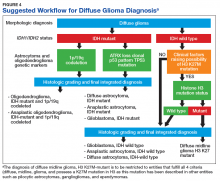
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
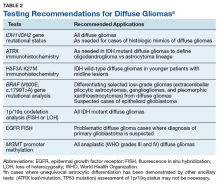
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.
Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
FDA approves Keytruda for metastatic HNSCC
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the first-line treatment of patients with metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC).
FDA approval was based on results of the randomized, multicenter, three-arm, open‑label, active‑controlled KEYNOTE-048 trial. The 882 patients in the trial had metastatic HNSCC, and they received either single-agent pembrolizumab; pembrolizumab, carboplatin or cisplatin, and platinum and fluorouracil; or cetuximab, carboplatin or cisplatin, and platinum and fluorouracil.
Patients who received pembrolizumab plus chemotherapy had a mean overall survival of 13.0 months, compared with 10.7 months in the cetuximab plus chemotherapy group (hazard ratio, 0.77; 95% CI, 0.63-0.93; P = .0067).
In the patient subgroups that received single-agent pembrolizumab, overall survival was 12.3 months in patients with a Combined Positive Score of at least 1, compared with 10.3 months for the cetuximab plus chemotherapy group (HR, 0.78; 95% CI, 0.64-0.96; P = .0171). In patients with a Combined Positive Score of at least 20, overall survival was 14.9 months in the pembrolizumab-only group and 10.7 months in the cetuximab plus chemotherapy group (HR, 0.61; 95% CI, 0.45-0.83; P = .0015).
The most common adverse events in patients who received single-agent pembrolizumab were fatigue, constipation, and rash. In patients who received pembrolizumab plus chemotherapy, the most common adverse events were nausea, fatigue, constipation, vomiting, mucosal inflammation, diarrhea, decreased appetite, stomatitis, and cough.
Pembrolizumab is approved for use in combination with platinum and fluorouracil for all patients and as a single agent for patients whose tumors express programmed death–ligand 1, the FDA said.
The FDA also expanded the intended use for the PD-L1 IHC 22C3 pharmDx kit to include use as a companion diagnostic device for selecting patients with HNSCC for treatment with pembrolizumab as a single agent.
Find the full press release on the FDA website.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the first-line treatment of patients with metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC).
FDA approval was based on results of the randomized, multicenter, three-arm, open‑label, active‑controlled KEYNOTE-048 trial. The 882 patients in the trial had metastatic HNSCC, and they received either single-agent pembrolizumab; pembrolizumab, carboplatin or cisplatin, and platinum and fluorouracil; or cetuximab, carboplatin or cisplatin, and platinum and fluorouracil.
Patients who received pembrolizumab plus chemotherapy had a mean overall survival of 13.0 months, compared with 10.7 months in the cetuximab plus chemotherapy group (hazard ratio, 0.77; 95% CI, 0.63-0.93; P = .0067).
In the patient subgroups that received single-agent pembrolizumab, overall survival was 12.3 months in patients with a Combined Positive Score of at least 1, compared with 10.3 months for the cetuximab plus chemotherapy group (HR, 0.78; 95% CI, 0.64-0.96; P = .0171). In patients with a Combined Positive Score of at least 20, overall survival was 14.9 months in the pembrolizumab-only group and 10.7 months in the cetuximab plus chemotherapy group (HR, 0.61; 95% CI, 0.45-0.83; P = .0015).
The most common adverse events in patients who received single-agent pembrolizumab were fatigue, constipation, and rash. In patients who received pembrolizumab plus chemotherapy, the most common adverse events were nausea, fatigue, constipation, vomiting, mucosal inflammation, diarrhea, decreased appetite, stomatitis, and cough.
Pembrolizumab is approved for use in combination with platinum and fluorouracil for all patients and as a single agent for patients whose tumors express programmed death–ligand 1, the FDA said.
The FDA also expanded the intended use for the PD-L1 IHC 22C3 pharmDx kit to include use as a companion diagnostic device for selecting patients with HNSCC for treatment with pembrolizumab as a single agent.
Find the full press release on the FDA website.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the first-line treatment of patients with metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC).
FDA approval was based on results of the randomized, multicenter, three-arm, open‑label, active‑controlled KEYNOTE-048 trial. The 882 patients in the trial had metastatic HNSCC, and they received either single-agent pembrolizumab; pembrolizumab, carboplatin or cisplatin, and platinum and fluorouracil; or cetuximab, carboplatin or cisplatin, and platinum and fluorouracil.
Patients who received pembrolizumab plus chemotherapy had a mean overall survival of 13.0 months, compared with 10.7 months in the cetuximab plus chemotherapy group (hazard ratio, 0.77; 95% CI, 0.63-0.93; P = .0067).
In the patient subgroups that received single-agent pembrolizumab, overall survival was 12.3 months in patients with a Combined Positive Score of at least 1, compared with 10.3 months for the cetuximab plus chemotherapy group (HR, 0.78; 95% CI, 0.64-0.96; P = .0171). In patients with a Combined Positive Score of at least 20, overall survival was 14.9 months in the pembrolizumab-only group and 10.7 months in the cetuximab plus chemotherapy group (HR, 0.61; 95% CI, 0.45-0.83; P = .0015).
The most common adverse events in patients who received single-agent pembrolizumab were fatigue, constipation, and rash. In patients who received pembrolizumab plus chemotherapy, the most common adverse events were nausea, fatigue, constipation, vomiting, mucosal inflammation, diarrhea, decreased appetite, stomatitis, and cough.
Pembrolizumab is approved for use in combination with platinum and fluorouracil for all patients and as a single agent for patients whose tumors express programmed death–ligand 1, the FDA said.
The FDA also expanded the intended use for the PD-L1 IHC 22C3 pharmDx kit to include use as a companion diagnostic device for selecting patients with HNSCC for treatment with pembrolizumab as a single agent.
Find the full press release on the FDA website.
Ado-trastuzumab highly efficacious for rare HER2-amplified SGCs
CHICAGO – Ado-trastuzumab emtansine, previously known as T-DM1, is highly efficacious in patients with HER2-amplified salivary gland cancers, according to findings from an ongoing phase 2 multi-histology basket trial.
In fact, nine of 10 patients with this rare tumor responded to treatment with the HER2-targeted antibody drug conjugate after prior trastuzumab, pertuzumab, and anti-androgen therapy, and 5 of those had a complete response, Bob T. Li, MD, said at the annual meeting of the American Society of Clinical Oncology.
“We are reporting this study early because it has already met its primary endpoint,” said Dr. Li of Memorial Sloan Kettering Cancer Center, N.Y.
The 90% response rate was based on either Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 or Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) criteria; the latter was used because many patients with HER-amplified salivary gland cancer aren’t “RECIST measurable” due to presentation with only lymph node- and bone-only metastasis, he explained, adding that “many of the responses were quite durable, with some lasting 2 years.”
Even at a median of 12 months, neither duration of response nor median progression-free survival have been reached, he said.
Study subjects included nine men and one woman with salivary gland cancers (SGCs) and HER2 amplification identified by next-generation sequencing (NGS). They had a median age of 65 years and a median of 2 prior lines of systemic therapy.
Dr. Li described one patient who had bone and vertebral metastases.
“After just two doses, he had a complete metabolic response,” he said. “His symptoms improved, his pain went away, he feels well, and just recently he celebrated his 92nd birthday.”
Treatment, which included 3.6 mg/kg delivered intravenously every 3 weeks until disease progression or unacceptable toxicity, was well tolerated; toxicities included grade 1 or 2 infusion reactions, thrombocytopenia, and transaminitis. Two dose reductions were required, but no treatment-related deaths occurred, Dr. Li said.
SGCs are rare tumors accounting for only about 0.8% of malignancies. There is no approved therapy for metastatic disease, and due to the rarity of the disease there is no established standard of care, he said, noting, however, that chemotherapy and anti-androgen therapy are considered treatment options based on some retrospective case series.
“Now, coming in from a molecular angle, HER2 amplification turns out to be very common in this rare tumor,” he said.
In fact, NGS of more than 40,000 tumors using the MSK-IMPACT 468-gene oncopanel showed that HER2 amplification occurs in 8% of all SGC histologies, and additional published data show that it occurs in about 30% of those with “the very aggressive salivary duct carcinoma histologic subtype,” he said, adding that case reports and a phase 2 study reported at ASCO 2018 showed encouraging response rates with chemotherapy plus trastuzumab.
Ado-trastuzumab emtansine is a Food and Drug Administration-approved agent for the treatment HER2-positive breast cancer.
“It’s got the trastuzumab antibody, it has a linker which attaches the highly toxic DM1 chemotherapy to it, and ... it binds to the over-expressed HER2 receptor and uses that receptor to internalize the drug into the cancer cell and by lysosome deregulation release the highly toxic DM1 into the cell to cause cancer cell kill,” he explained. “We hypothesized that this drug, as a single agent, would be efficacious in HER2-amplified SGC tumors, and it turns out [that] recently there was a nice case series published from the University of Pennsylvania supporting this hypothesis in a group of patients.”
Indeed, the findings are encouraging and warrant cohort expansion to confirm the results, he said.
Of note, HER2 amplification by NGS (fold change 2.8 to 22.8) correlated with findings on fluorescence in situ hybridization (FISH) in 8 of 8 patients tested, and with immunohistochemistry (IHC) 3+ in 10 of 10 patients tested, thereby confirming the validity of this testing method for the biomarker and as a study entry criterion, he said, adding that ongoing correlative analyses are focusing on cell-free DNA NGS to look for acquired resistance, quantitative HER2 protein analysis by mass spectrometry, and also a dimerization assay looking at the degree of HER2-HER3 dimerization, which leads to receptor internalization that may predict response to HER2 antibody drug conjugates.
“We wanted to see why [HER2-amplified SGC patients] respond so well in contrast to the other diseases in the basket trial,” Dr. Li said, explaining that the trial also includes lung, bladder and urinary tract, endometrial, and colorectal cancer cohorts.
“However, to me as an oncologist, the most pressing thing is that with these kind of results and with this kind of response rate and progression-free survival ... there are patients in need of this treatment, so that is certainly the priority–to further accrue patients, complete the trial, publish the data, and hopefully have this new treatment approved to benefit all patients,” he concluded.
Discussant Vanita Noronha, MD, noted that the survival data are immature but “very clinically relevant and clinically significant,” and that they fulfill an unmet need.
“As much as we would like to have randomized trial, this is really a challenge in these kind of rare tumors,” said Dr. Noronha, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India. “So my take-home message ... is that HER2neu is an important molecule driver in salivary gland tumors [and] all patients with salivary gland cancers should be tested for HER2neu amplification.”
Ado trastuzumab emtansine appears to be a good treatment option in those with HER2 amplified SGC, she added.
“Is this a practice changing study? Yes, potentially it is,” she said, noting that in patients with recurrent/metastatic SGC not amenable to radical therapy who are found to have HER2neu amplification, treatment options include either ado trastuzumab emtansine or the combination of trastuzumab and chemotherapy.
Dr. Li reported consulting or advisory roles with Biosceptre International, Guardant Health, Hengrui Therapeutics, Mersana, Roche, and Thermo Fisher Scientific. He reported research funding to his institution from AstraZeneca, BioMed Valley Discoveries, Daiichi Sankyo, GRAIL, Guardant Health, Hengrui Therapeutics, Illumina, and Roche/Genentech. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: Li B et al., ASCO 2019: Abstract 6001.
CHICAGO – Ado-trastuzumab emtansine, previously known as T-DM1, is highly efficacious in patients with HER2-amplified salivary gland cancers, according to findings from an ongoing phase 2 multi-histology basket trial.
In fact, nine of 10 patients with this rare tumor responded to treatment with the HER2-targeted antibody drug conjugate after prior trastuzumab, pertuzumab, and anti-androgen therapy, and 5 of those had a complete response, Bob T. Li, MD, said at the annual meeting of the American Society of Clinical Oncology.
“We are reporting this study early because it has already met its primary endpoint,” said Dr. Li of Memorial Sloan Kettering Cancer Center, N.Y.
The 90% response rate was based on either Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 or Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) criteria; the latter was used because many patients with HER-amplified salivary gland cancer aren’t “RECIST measurable” due to presentation with only lymph node- and bone-only metastasis, he explained, adding that “many of the responses were quite durable, with some lasting 2 years.”
Even at a median of 12 months, neither duration of response nor median progression-free survival have been reached, he said.
Study subjects included nine men and one woman with salivary gland cancers (SGCs) and HER2 amplification identified by next-generation sequencing (NGS). They had a median age of 65 years and a median of 2 prior lines of systemic therapy.
Dr. Li described one patient who had bone and vertebral metastases.
“After just two doses, he had a complete metabolic response,” he said. “His symptoms improved, his pain went away, he feels well, and just recently he celebrated his 92nd birthday.”
Treatment, which included 3.6 mg/kg delivered intravenously every 3 weeks until disease progression or unacceptable toxicity, was well tolerated; toxicities included grade 1 or 2 infusion reactions, thrombocytopenia, and transaminitis. Two dose reductions were required, but no treatment-related deaths occurred, Dr. Li said.
SGCs are rare tumors accounting for only about 0.8% of malignancies. There is no approved therapy for metastatic disease, and due to the rarity of the disease there is no established standard of care, he said, noting, however, that chemotherapy and anti-androgen therapy are considered treatment options based on some retrospective case series.
“Now, coming in from a molecular angle, HER2 amplification turns out to be very common in this rare tumor,” he said.
In fact, NGS of more than 40,000 tumors using the MSK-IMPACT 468-gene oncopanel showed that HER2 amplification occurs in 8% of all SGC histologies, and additional published data show that it occurs in about 30% of those with “the very aggressive salivary duct carcinoma histologic subtype,” he said, adding that case reports and a phase 2 study reported at ASCO 2018 showed encouraging response rates with chemotherapy plus trastuzumab.
Ado-trastuzumab emtansine is a Food and Drug Administration-approved agent for the treatment HER2-positive breast cancer.
“It’s got the trastuzumab antibody, it has a linker which attaches the highly toxic DM1 chemotherapy to it, and ... it binds to the over-expressed HER2 receptor and uses that receptor to internalize the drug into the cancer cell and by lysosome deregulation release the highly toxic DM1 into the cell to cause cancer cell kill,” he explained. “We hypothesized that this drug, as a single agent, would be efficacious in HER2-amplified SGC tumors, and it turns out [that] recently there was a nice case series published from the University of Pennsylvania supporting this hypothesis in a group of patients.”
Indeed, the findings are encouraging and warrant cohort expansion to confirm the results, he said.
Of note, HER2 amplification by NGS (fold change 2.8 to 22.8) correlated with findings on fluorescence in situ hybridization (FISH) in 8 of 8 patients tested, and with immunohistochemistry (IHC) 3+ in 10 of 10 patients tested, thereby confirming the validity of this testing method for the biomarker and as a study entry criterion, he said, adding that ongoing correlative analyses are focusing on cell-free DNA NGS to look for acquired resistance, quantitative HER2 protein analysis by mass spectrometry, and also a dimerization assay looking at the degree of HER2-HER3 dimerization, which leads to receptor internalization that may predict response to HER2 antibody drug conjugates.
“We wanted to see why [HER2-amplified SGC patients] respond so well in contrast to the other diseases in the basket trial,” Dr. Li said, explaining that the trial also includes lung, bladder and urinary tract, endometrial, and colorectal cancer cohorts.
“However, to me as an oncologist, the most pressing thing is that with these kind of results and with this kind of response rate and progression-free survival ... there are patients in need of this treatment, so that is certainly the priority–to further accrue patients, complete the trial, publish the data, and hopefully have this new treatment approved to benefit all patients,” he concluded.
Discussant Vanita Noronha, MD, noted that the survival data are immature but “very clinically relevant and clinically significant,” and that they fulfill an unmet need.
“As much as we would like to have randomized trial, this is really a challenge in these kind of rare tumors,” said Dr. Noronha, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India. “So my take-home message ... is that HER2neu is an important molecule driver in salivary gland tumors [and] all patients with salivary gland cancers should be tested for HER2neu amplification.”
Ado trastuzumab emtansine appears to be a good treatment option in those with HER2 amplified SGC, she added.
“Is this a practice changing study? Yes, potentially it is,” she said, noting that in patients with recurrent/metastatic SGC not amenable to radical therapy who are found to have HER2neu amplification, treatment options include either ado trastuzumab emtansine or the combination of trastuzumab and chemotherapy.
Dr. Li reported consulting or advisory roles with Biosceptre International, Guardant Health, Hengrui Therapeutics, Mersana, Roche, and Thermo Fisher Scientific. He reported research funding to his institution from AstraZeneca, BioMed Valley Discoveries, Daiichi Sankyo, GRAIL, Guardant Health, Hengrui Therapeutics, Illumina, and Roche/Genentech. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: Li B et al., ASCO 2019: Abstract 6001.
CHICAGO – Ado-trastuzumab emtansine, previously known as T-DM1, is highly efficacious in patients with HER2-amplified salivary gland cancers, according to findings from an ongoing phase 2 multi-histology basket trial.
In fact, nine of 10 patients with this rare tumor responded to treatment with the HER2-targeted antibody drug conjugate after prior trastuzumab, pertuzumab, and anti-androgen therapy, and 5 of those had a complete response, Bob T. Li, MD, said at the annual meeting of the American Society of Clinical Oncology.
“We are reporting this study early because it has already met its primary endpoint,” said Dr. Li of Memorial Sloan Kettering Cancer Center, N.Y.
The 90% response rate was based on either Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 or Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) criteria; the latter was used because many patients with HER-amplified salivary gland cancer aren’t “RECIST measurable” due to presentation with only lymph node- and bone-only metastasis, he explained, adding that “many of the responses were quite durable, with some lasting 2 years.”
Even at a median of 12 months, neither duration of response nor median progression-free survival have been reached, he said.
Study subjects included nine men and one woman with salivary gland cancers (SGCs) and HER2 amplification identified by next-generation sequencing (NGS). They had a median age of 65 years and a median of 2 prior lines of systemic therapy.
Dr. Li described one patient who had bone and vertebral metastases.
“After just two doses, he had a complete metabolic response,” he said. “His symptoms improved, his pain went away, he feels well, and just recently he celebrated his 92nd birthday.”
Treatment, which included 3.6 mg/kg delivered intravenously every 3 weeks until disease progression or unacceptable toxicity, was well tolerated; toxicities included grade 1 or 2 infusion reactions, thrombocytopenia, and transaminitis. Two dose reductions were required, but no treatment-related deaths occurred, Dr. Li said.
SGCs are rare tumors accounting for only about 0.8% of malignancies. There is no approved therapy for metastatic disease, and due to the rarity of the disease there is no established standard of care, he said, noting, however, that chemotherapy and anti-androgen therapy are considered treatment options based on some retrospective case series.
“Now, coming in from a molecular angle, HER2 amplification turns out to be very common in this rare tumor,” he said.
In fact, NGS of more than 40,000 tumors using the MSK-IMPACT 468-gene oncopanel showed that HER2 amplification occurs in 8% of all SGC histologies, and additional published data show that it occurs in about 30% of those with “the very aggressive salivary duct carcinoma histologic subtype,” he said, adding that case reports and a phase 2 study reported at ASCO 2018 showed encouraging response rates with chemotherapy plus trastuzumab.
Ado-trastuzumab emtansine is a Food and Drug Administration-approved agent for the treatment HER2-positive breast cancer.
“It’s got the trastuzumab antibody, it has a linker which attaches the highly toxic DM1 chemotherapy to it, and ... it binds to the over-expressed HER2 receptor and uses that receptor to internalize the drug into the cancer cell and by lysosome deregulation release the highly toxic DM1 into the cell to cause cancer cell kill,” he explained. “We hypothesized that this drug, as a single agent, would be efficacious in HER2-amplified SGC tumors, and it turns out [that] recently there was a nice case series published from the University of Pennsylvania supporting this hypothesis in a group of patients.”
Indeed, the findings are encouraging and warrant cohort expansion to confirm the results, he said.
Of note, HER2 amplification by NGS (fold change 2.8 to 22.8) correlated with findings on fluorescence in situ hybridization (FISH) in 8 of 8 patients tested, and with immunohistochemistry (IHC) 3+ in 10 of 10 patients tested, thereby confirming the validity of this testing method for the biomarker and as a study entry criterion, he said, adding that ongoing correlative analyses are focusing on cell-free DNA NGS to look for acquired resistance, quantitative HER2 protein analysis by mass spectrometry, and also a dimerization assay looking at the degree of HER2-HER3 dimerization, which leads to receptor internalization that may predict response to HER2 antibody drug conjugates.
“We wanted to see why [HER2-amplified SGC patients] respond so well in contrast to the other diseases in the basket trial,” Dr. Li said, explaining that the trial also includes lung, bladder and urinary tract, endometrial, and colorectal cancer cohorts.
“However, to me as an oncologist, the most pressing thing is that with these kind of results and with this kind of response rate and progression-free survival ... there are patients in need of this treatment, so that is certainly the priority–to further accrue patients, complete the trial, publish the data, and hopefully have this new treatment approved to benefit all patients,” he concluded.
Discussant Vanita Noronha, MD, noted that the survival data are immature but “very clinically relevant and clinically significant,” and that they fulfill an unmet need.
“As much as we would like to have randomized trial, this is really a challenge in these kind of rare tumors,” said Dr. Noronha, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India. “So my take-home message ... is that HER2neu is an important molecule driver in salivary gland tumors [and] all patients with salivary gland cancers should be tested for HER2neu amplification.”
Ado trastuzumab emtansine appears to be a good treatment option in those with HER2 amplified SGC, she added.
“Is this a practice changing study? Yes, potentially it is,” she said, noting that in patients with recurrent/metastatic SGC not amenable to radical therapy who are found to have HER2neu amplification, treatment options include either ado trastuzumab emtansine or the combination of trastuzumab and chemotherapy.
Dr. Li reported consulting or advisory roles with Biosceptre International, Guardant Health, Hengrui Therapeutics, Mersana, Roche, and Thermo Fisher Scientific. He reported research funding to his institution from AstraZeneca, BioMed Valley Discoveries, Daiichi Sankyo, GRAIL, Guardant Health, Hengrui Therapeutics, Illumina, and Roche/Genentech. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: Li B et al., ASCO 2019: Abstract 6001.
REPORTING FROM ASCO 2019
Pembro with or without chemo superior to EXTREME for advanced HNSCC
CHICAGO – Pembrolizumab with and without chemotherapy proved superior for overall survival compared with the EXTREME regimen when used first line in certain subgroups of patients with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC), according to “practice-changing” final results from the randomized phase 3 KEYNOTE-048 study.
Compared with 300 patients randomized to receive the EXTREME regimen (a certuximab loading dose followed by carboplatin or cisplatin and 5-fluorouracil), 281 who received pembrolizumab plus chemotherapy (P+C) had superior overall survival (OS) with comparable safety–including both those with programmed death-Ligand 1 (PD-L1) combined positive score (CPS) of 20 or greater (median 14.7 vs 11.0 months; hazard ratio, 0.60) and with CPS of 1 or greater (median, 13.6 vs. 10.4 months; HR, 0.65), Danny Rischin, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The differences were highly statistically significant, said Dr. Rischin, a professor and director of the Division of Cancer Medicine and head of the Department of Medical Oncology at Peter MacCallum Cancer Centre, Melbourne, Australia.
“And this benefit in overall survival in CPS greater than or equal to 20 and greater than or equal to 1 appeared to be present across all the subgroups that we looked at,” he added.
The response rates did not differ between P+C and EXTREME groups, but the median duration of response was significantly greater with P+C vs. EXTREME in both the CPS of 20 or greater and 1 or greater (7.1 vs. 4.2 months and 6.7 vs. 4.3 months, respectively).
Additionally, the final results of the study showed an OS benefit with P+C vs. EXTREME in the total population (13.0 vs. 10.7 months; HR, 0.72), Dr. Rischin said.
The difference between the groups with respect to progression-free survival (PFS), however, was not statistically significant and did not reach the superiority threshold, he noted.
In the 301 patients who received pembrolizumab alone, OS was superior in the CPS 20 or greater and 1 or greater populations (median, 14.8 vs. 10.7 months; HR, 0.58 and 12.4 vs. 10.3 months; HR, 0.74, respectively), compared with EXTREME, but was noninferior in the total population (median 11.5 vs. 10.7 months; HR, 0.83), and safety was favorable .
Again, PFS did not differ between the groups (median, 2.3 vs. 5.2 months; HR, 1.34), and while the overall response rates did not differ significantly, the median duration of response was substantially longer with pembrolizumab at 22.6 vs. 4.5 months with EXTREME, he said.
Study participants had locally incurable R/M HNSCC and no prior systemic therapy in the R/M setting. Those in he P+C arm received pembrolizumab at 200 mg plus 6 cycles of cisplatin at 100 mg/m2 or carboplatin AUC 5, and 5-fluorouracil at a dose of 1000 mg/m2/day for 4 days every 3 weeks; those in the pembrolizumab alone arm received 200 mg every 3 weeks for up to 35 cycles, and those in the EXTREME arm received certuximab at a 400 mg/m2 loading dose followed by 250 mg/m2 weekly with carboplatin AUC 5 or cisplatin at 100 mg/m2, and 5-FU at 1000 mg/m2/day for 4 days for 6 cycles.
“The data from KEYNOTE-048 support pembrolizumab plus platinum-based chemotherapy and pembrolizumab monotherapy as new standard of care monotherapies for recurrent/metastatic head and neck squamous cell carcinoma,” he concluded.
Discussant Vanita Noronha, MD, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India, said that while the findings are practice changing, they also raise a number of questions, such as which patients should get pembrolizumab and which should get P+C, why there is a differential effect of pembrolizumab based on PD-L1 by CPS–and what about those with CPS of 0 or 1-20, and why the response rates and PFS rates were not improved in the pembrolizumab groups.
Other important questions include whether there are predictive biomarkers for response, and whether sequential therapy would be of benefit, she added.
While these and other questions remain to be addressed, the KEYNOTE-048 findings have implications for practice going forward; based on the current data, her approach to treating patients with R/M HNSCC not amenable to radical therapy is to treat with pembrolizumab alone in those with disease-free interval of 6 months or less, she said.
For those with disease-free interval greater than 6 months and good performance status who have controlled comorbidities, are platinum eligible, and for whom the treatment is reimbursable/affordable, treatment depends on symptom severity; she would treat those with mild/moderate symptoms and CPS of 20 or greater with pembrolizumab alone, those with CPS of 1 or greater with P+C or pembrolizumab alone, and those with CPS of 0 or unknown CPS with EXTREME or a similar regimen or with P+C, and she would treat those with severe symptoms with P+C.
“If the patient were a bit borderline, had multiple comorbidities, could not receive platinum, or had financial constraints, I would treat the patient with singe-agent intravenous chemotherapy or with oral metronomic chemotherapy, single-agent targeted therapy or with best supportive care,” she said.
Dr. Rischin has received research funding from Amgen, Bristol-Myers Squibb, Genentech/Roche, GSK, Merck, and Regeneron. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: D Rischin et al., ASCO 2019: Abstract 6000.
CHICAGO – Pembrolizumab with and without chemotherapy proved superior for overall survival compared with the EXTREME regimen when used first line in certain subgroups of patients with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC), according to “practice-changing” final results from the randomized phase 3 KEYNOTE-048 study.
Compared with 300 patients randomized to receive the EXTREME regimen (a certuximab loading dose followed by carboplatin or cisplatin and 5-fluorouracil), 281 who received pembrolizumab plus chemotherapy (P+C) had superior overall survival (OS) with comparable safety–including both those with programmed death-Ligand 1 (PD-L1) combined positive score (CPS) of 20 or greater (median 14.7 vs 11.0 months; hazard ratio, 0.60) and with CPS of 1 or greater (median, 13.6 vs. 10.4 months; HR, 0.65), Danny Rischin, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The differences were highly statistically significant, said Dr. Rischin, a professor and director of the Division of Cancer Medicine and head of the Department of Medical Oncology at Peter MacCallum Cancer Centre, Melbourne, Australia.
“And this benefit in overall survival in CPS greater than or equal to 20 and greater than or equal to 1 appeared to be present across all the subgroups that we looked at,” he added.
The response rates did not differ between P+C and EXTREME groups, but the median duration of response was significantly greater with P+C vs. EXTREME in both the CPS of 20 or greater and 1 or greater (7.1 vs. 4.2 months and 6.7 vs. 4.3 months, respectively).
Additionally, the final results of the study showed an OS benefit with P+C vs. EXTREME in the total population (13.0 vs. 10.7 months; HR, 0.72), Dr. Rischin said.
The difference between the groups with respect to progression-free survival (PFS), however, was not statistically significant and did not reach the superiority threshold, he noted.
In the 301 patients who received pembrolizumab alone, OS was superior in the CPS 20 or greater and 1 or greater populations (median, 14.8 vs. 10.7 months; HR, 0.58 and 12.4 vs. 10.3 months; HR, 0.74, respectively), compared with EXTREME, but was noninferior in the total population (median 11.5 vs. 10.7 months; HR, 0.83), and safety was favorable .
Again, PFS did not differ between the groups (median, 2.3 vs. 5.2 months; HR, 1.34), and while the overall response rates did not differ significantly, the median duration of response was substantially longer with pembrolizumab at 22.6 vs. 4.5 months with EXTREME, he said.
Study participants had locally incurable R/M HNSCC and no prior systemic therapy in the R/M setting. Those in he P+C arm received pembrolizumab at 200 mg plus 6 cycles of cisplatin at 100 mg/m2 or carboplatin AUC 5, and 5-fluorouracil at a dose of 1000 mg/m2/day for 4 days every 3 weeks; those in the pembrolizumab alone arm received 200 mg every 3 weeks for up to 35 cycles, and those in the EXTREME arm received certuximab at a 400 mg/m2 loading dose followed by 250 mg/m2 weekly with carboplatin AUC 5 or cisplatin at 100 mg/m2, and 5-FU at 1000 mg/m2/day for 4 days for 6 cycles.
“The data from KEYNOTE-048 support pembrolizumab plus platinum-based chemotherapy and pembrolizumab monotherapy as new standard of care monotherapies for recurrent/metastatic head and neck squamous cell carcinoma,” he concluded.
Discussant Vanita Noronha, MD, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India, said that while the findings are practice changing, they also raise a number of questions, such as which patients should get pembrolizumab and which should get P+C, why there is a differential effect of pembrolizumab based on PD-L1 by CPS–and what about those with CPS of 0 or 1-20, and why the response rates and PFS rates were not improved in the pembrolizumab groups.
Other important questions include whether there are predictive biomarkers for response, and whether sequential therapy would be of benefit, she added.
While these and other questions remain to be addressed, the KEYNOTE-048 findings have implications for practice going forward; based on the current data, her approach to treating patients with R/M HNSCC not amenable to radical therapy is to treat with pembrolizumab alone in those with disease-free interval of 6 months or less, she said.
For those with disease-free interval greater than 6 months and good performance status who have controlled comorbidities, are platinum eligible, and for whom the treatment is reimbursable/affordable, treatment depends on symptom severity; she would treat those with mild/moderate symptoms and CPS of 20 or greater with pembrolizumab alone, those with CPS of 1 or greater with P+C or pembrolizumab alone, and those with CPS of 0 or unknown CPS with EXTREME or a similar regimen or with P+C, and she would treat those with severe symptoms with P+C.
“If the patient were a bit borderline, had multiple comorbidities, could not receive platinum, or had financial constraints, I would treat the patient with singe-agent intravenous chemotherapy or with oral metronomic chemotherapy, single-agent targeted therapy or with best supportive care,” she said.
Dr. Rischin has received research funding from Amgen, Bristol-Myers Squibb, Genentech/Roche, GSK, Merck, and Regeneron. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: D Rischin et al., ASCO 2019: Abstract 6000.
CHICAGO – Pembrolizumab with and without chemotherapy proved superior for overall survival compared with the EXTREME regimen when used first line in certain subgroups of patients with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC), according to “practice-changing” final results from the randomized phase 3 KEYNOTE-048 study.
Compared with 300 patients randomized to receive the EXTREME regimen (a certuximab loading dose followed by carboplatin or cisplatin and 5-fluorouracil), 281 who received pembrolizumab plus chemotherapy (P+C) had superior overall survival (OS) with comparable safety–including both those with programmed death-Ligand 1 (PD-L1) combined positive score (CPS) of 20 or greater (median 14.7 vs 11.0 months; hazard ratio, 0.60) and with CPS of 1 or greater (median, 13.6 vs. 10.4 months; HR, 0.65), Danny Rischin, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The differences were highly statistically significant, said Dr. Rischin, a professor and director of the Division of Cancer Medicine and head of the Department of Medical Oncology at Peter MacCallum Cancer Centre, Melbourne, Australia.
“And this benefit in overall survival in CPS greater than or equal to 20 and greater than or equal to 1 appeared to be present across all the subgroups that we looked at,” he added.
The response rates did not differ between P+C and EXTREME groups, but the median duration of response was significantly greater with P+C vs. EXTREME in both the CPS of 20 or greater and 1 or greater (7.1 vs. 4.2 months and 6.7 vs. 4.3 months, respectively).
Additionally, the final results of the study showed an OS benefit with P+C vs. EXTREME in the total population (13.0 vs. 10.7 months; HR, 0.72), Dr. Rischin said.
The difference between the groups with respect to progression-free survival (PFS), however, was not statistically significant and did not reach the superiority threshold, he noted.
In the 301 patients who received pembrolizumab alone, OS was superior in the CPS 20 or greater and 1 or greater populations (median, 14.8 vs. 10.7 months; HR, 0.58 and 12.4 vs. 10.3 months; HR, 0.74, respectively), compared with EXTREME, but was noninferior in the total population (median 11.5 vs. 10.7 months; HR, 0.83), and safety was favorable .
Again, PFS did not differ between the groups (median, 2.3 vs. 5.2 months; HR, 1.34), and while the overall response rates did not differ significantly, the median duration of response was substantially longer with pembrolizumab at 22.6 vs. 4.5 months with EXTREME, he said.
Study participants had locally incurable R/M HNSCC and no prior systemic therapy in the R/M setting. Those in he P+C arm received pembrolizumab at 200 mg plus 6 cycles of cisplatin at 100 mg/m2 or carboplatin AUC 5, and 5-fluorouracil at a dose of 1000 mg/m2/day for 4 days every 3 weeks; those in the pembrolizumab alone arm received 200 mg every 3 weeks for up to 35 cycles, and those in the EXTREME arm received certuximab at a 400 mg/m2 loading dose followed by 250 mg/m2 weekly with carboplatin AUC 5 or cisplatin at 100 mg/m2, and 5-FU at 1000 mg/m2/day for 4 days for 6 cycles.
“The data from KEYNOTE-048 support pembrolizumab plus platinum-based chemotherapy and pembrolizumab monotherapy as new standard of care monotherapies for recurrent/metastatic head and neck squamous cell carcinoma,” he concluded.
Discussant Vanita Noronha, MD, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India, said that while the findings are practice changing, they also raise a number of questions, such as which patients should get pembrolizumab and which should get P+C, why there is a differential effect of pembrolizumab based on PD-L1 by CPS–and what about those with CPS of 0 or 1-20, and why the response rates and PFS rates were not improved in the pembrolizumab groups.
Other important questions include whether there are predictive biomarkers for response, and whether sequential therapy would be of benefit, she added.
While these and other questions remain to be addressed, the KEYNOTE-048 findings have implications for practice going forward; based on the current data, her approach to treating patients with R/M HNSCC not amenable to radical therapy is to treat with pembrolizumab alone in those with disease-free interval of 6 months or less, she said.
For those with disease-free interval greater than 6 months and good performance status who have controlled comorbidities, are platinum eligible, and for whom the treatment is reimbursable/affordable, treatment depends on symptom severity; she would treat those with mild/moderate symptoms and CPS of 20 or greater with pembrolizumab alone, those with CPS of 1 or greater with P+C or pembrolizumab alone, and those with CPS of 0 or unknown CPS with EXTREME or a similar regimen or with P+C, and she would treat those with severe symptoms with P+C.
“If the patient were a bit borderline, had multiple comorbidities, could not receive platinum, or had financial constraints, I would treat the patient with singe-agent intravenous chemotherapy or with oral metronomic chemotherapy, single-agent targeted therapy or with best supportive care,” she said.
Dr. Rischin has received research funding from Amgen, Bristol-Myers Squibb, Genentech/Roche, GSK, Merck, and Regeneron. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: D Rischin et al., ASCO 2019: Abstract 6000.
REPORTING FROM ASCO 2019
Novel anti-PD-1 antibody can be given subcutaneously
based on results from an ongoing phase 1 trial.
“We assessed feasibility of monthly subcutaneous administration of PF-06801591, a humanized immunoglobulin G4 monoclonal antibody that binds to the programmed cell death [PD-1] receptor,” Melissa L. Johnson, MD, of the Sarah Cannon Research Institute, Nashville, Tenn., and colleagues wrote in JAMA Oncology. “Subcutaneous administration of an anti-PD-1 antibody in patients with advanced solid tumors appears to be feasible.”
The researchers evaluated the efficacy, safety, and pharmacokinetics of the novel anti-PD-1 therapy in a group of 40 patients with locally advanced or metastatic solid tumors. The antibody was administered in both subcutaneous and intravenous forms.
Study participants were administered the subcutaneous form of the antibody at 300 mg every 4 weeks or the intravenous form at 0.5, 1, 3, or 10 mg/kg every 3 weeks.
“Dose escalation occurred after two to four patients were enrolled per dose level, with additional patients enrolled in each cohort for further assessment,” the researchers wrote.
The primary endpoints were safety and dose-limiting adverse effects. Efficacy, immunogenicity, pharmacokinetics, and PD-1 receptor occupancy were secondary endpoints.
After analysis, Dr. Johnson and colleagues reported that the overall response rate was 18.4%. In addition, no dose-limiting toxicities were detected in both intravenous and subcutaneous groups, but grade 3 or greater adverse events were reported in 6.7% and 16% of patients treated with subcutaneous and intravenous dosage forms, respectively.
“No dose–adverse event associations were observed during intravenous dose escalation, and no serious skin toxic effects occurred with subcutaneous delivery,” they reported.
Based on the findings, the team selected the subcutaneous form (300 mg) of the therapy for additional assessment in the second half of this ongoing trial.
The study was funded by Pfizer. The authors reported financial affiliations with BerGenBio, EMD Serono, Janssen, Mirati Therapeutics, Pfizer, and several others.
SOURCE: Johnson ML et al. JAMA Oncol. 2019 May 30. doi: 10.1001/jamaoncol.2019.0836.
based on results from an ongoing phase 1 trial.
“We assessed feasibility of monthly subcutaneous administration of PF-06801591, a humanized immunoglobulin G4 monoclonal antibody that binds to the programmed cell death [PD-1] receptor,” Melissa L. Johnson, MD, of the Sarah Cannon Research Institute, Nashville, Tenn., and colleagues wrote in JAMA Oncology. “Subcutaneous administration of an anti-PD-1 antibody in patients with advanced solid tumors appears to be feasible.”
The researchers evaluated the efficacy, safety, and pharmacokinetics of the novel anti-PD-1 therapy in a group of 40 patients with locally advanced or metastatic solid tumors. The antibody was administered in both subcutaneous and intravenous forms.
Study participants were administered the subcutaneous form of the antibody at 300 mg every 4 weeks or the intravenous form at 0.5, 1, 3, or 10 mg/kg every 3 weeks.
“Dose escalation occurred after two to four patients were enrolled per dose level, with additional patients enrolled in each cohort for further assessment,” the researchers wrote.
The primary endpoints were safety and dose-limiting adverse effects. Efficacy, immunogenicity, pharmacokinetics, and PD-1 receptor occupancy were secondary endpoints.
After analysis, Dr. Johnson and colleagues reported that the overall response rate was 18.4%. In addition, no dose-limiting toxicities were detected in both intravenous and subcutaneous groups, but grade 3 or greater adverse events were reported in 6.7% and 16% of patients treated with subcutaneous and intravenous dosage forms, respectively.
“No dose–adverse event associations were observed during intravenous dose escalation, and no serious skin toxic effects occurred with subcutaneous delivery,” they reported.
Based on the findings, the team selected the subcutaneous form (300 mg) of the therapy for additional assessment in the second half of this ongoing trial.
The study was funded by Pfizer. The authors reported financial affiliations with BerGenBio, EMD Serono, Janssen, Mirati Therapeutics, Pfizer, and several others.
SOURCE: Johnson ML et al. JAMA Oncol. 2019 May 30. doi: 10.1001/jamaoncol.2019.0836.
based on results from an ongoing phase 1 trial.
“We assessed feasibility of monthly subcutaneous administration of PF-06801591, a humanized immunoglobulin G4 monoclonal antibody that binds to the programmed cell death [PD-1] receptor,” Melissa L. Johnson, MD, of the Sarah Cannon Research Institute, Nashville, Tenn., and colleagues wrote in JAMA Oncology. “Subcutaneous administration of an anti-PD-1 antibody in patients with advanced solid tumors appears to be feasible.”
The researchers evaluated the efficacy, safety, and pharmacokinetics of the novel anti-PD-1 therapy in a group of 40 patients with locally advanced or metastatic solid tumors. The antibody was administered in both subcutaneous and intravenous forms.
Study participants were administered the subcutaneous form of the antibody at 300 mg every 4 weeks or the intravenous form at 0.5, 1, 3, or 10 mg/kg every 3 weeks.
“Dose escalation occurred after two to four patients were enrolled per dose level, with additional patients enrolled in each cohort for further assessment,” the researchers wrote.
The primary endpoints were safety and dose-limiting adverse effects. Efficacy, immunogenicity, pharmacokinetics, and PD-1 receptor occupancy were secondary endpoints.
After analysis, Dr. Johnson and colleagues reported that the overall response rate was 18.4%. In addition, no dose-limiting toxicities were detected in both intravenous and subcutaneous groups, but grade 3 or greater adverse events were reported in 6.7% and 16% of patients treated with subcutaneous and intravenous dosage forms, respectively.
“No dose–adverse event associations were observed during intravenous dose escalation, and no serious skin toxic effects occurred with subcutaneous delivery,” they reported.
Based on the findings, the team selected the subcutaneous form (300 mg) of the therapy for additional assessment in the second half of this ongoing trial.
The study was funded by Pfizer. The authors reported financial affiliations with BerGenBio, EMD Serono, Janssen, Mirati Therapeutics, Pfizer, and several others.
SOURCE: Johnson ML et al. JAMA Oncol. 2019 May 30. doi: 10.1001/jamaoncol.2019.0836.
FROM JAMA ONCOLOGY
Study finds inconsistent links with aspirin, nonaspirin NSAIDs and reduced skin cancer risk
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Skip metastasis rate low in node-negative oral cancers
In patients with clinically node-negative oral cavity cancers, the rate of skip metastasis to neck level IV is extremely low, according to authors of a recent meta-analysis.
The rate of level IV involvement was about 2.5%, and the rate of skip metastasis was 0.5% in the analysis, which comprised 11 retrospective studies and 2 randomized clinical trials including a total of 1,359 patients with clinically node-negative oral cavity squamous cell carcinomas.
Encountering a suspected positive lymph node during neck dissection does not appear to be an indicator of high rates of level IV involvement, according to Anton Warshavsky, MD, and colleagues in the department of otolaryngology–head and neck surgery and maxillofacial surgery at Tel Aviv Sourasky Medical Center, Israel.
“Supraomohyoid neck dissection [SOHND] is adequate for this subset of patients,” Dr. Warshavsky and coauthors wrote in a report on the study that appears in JAMA Otolaryngology–Head & Neck Surgery.
SOHND, a type of selective neck dissection, refers to removal of lymph nodes in levels I-III. This approach is now frequently used in managing clinically node-negative oral cavity squamous cell carcinoma and provides control rates similar to those associated with more extensive neck dissections, Dr. Warshavsky and colleagues wrote.
However, concern regarding the risk of skip metastases, or involvement of neck level IV without involvement of lower levels, has stirred controversy. SOHND might not be adequate in these patients because of the possibility of occult metastasis to neck level IV.
Accordingly, Dr. Warshavsky and colleagues combed the available medical literature to find relevant articles for a meta-analysis to better characterize the rate of skip metastasis to level IV in patients who had undergone neck dissection.
Level IV involvement rates in clinically node-negative patients ranged from 0% to 11.4% in the 13 included studies. Based on fixed-effects modeling, the rate of involvement was 2.53% (95% CI, 1.64-3.55%), according to the published report.
The rate of skip metastasis was “extremely low,” wrote Dr. Warshavsky and coauthors. Rates ranged from 0% to 5.50%, with a fixed-effects model of 0.50% (95% CI, 0.09%-1.11%).
Cases involving higher levels of the neck did not impact the rate of level IV metastasis, results of a subgroup analysis found. Likewise, an analysis based on T stage showed that rates of level 4 involvement were comparable and low for T stages I-II and III-IV.
These findings are limited, however, not only by the retrospective nature of this study, but also by the fact that many studies reported limited data, hampering the investigators’ ability to run statistics and perform subgroup analyses.
“Unfortunately, data in almost all of the analyzed articles failed to report the relations between the primary tumor site and the neck levels involved by metastatic tumor,” they wrote. “Only primary lesions of the tongue could be accurately assessed.”
Dr. Warshavsky and coauthors reported no conflicts of interest related to the research.
SOURCE: Warshavsky A et al. JAMA Otolaryngol Head Neck Surg. 2019 May 9. doi: 10.1001/jamaoto.2019.0784.
Although this meta-analysis shows that the risk of level IV involvement is less than 5% in patients with clinically node-negative (cN0) oral cavity cancers, going beyond standard supraomohyoid neck dissection (SOHND) may still be warranted for specific patients.
While the authors conclude that elective treatment of level IV is not required in patients with cN0 oral cavity cancer, there are two situations in which clinicians should consider adding level IV to standard SOHND.
The first is when a patient has gross macroscopic disease in upper levels, particularly level III. Most studies in the meta-analysis had insufficient data to determine whether involvement of upper levels increased risk of level IV involvement. When encountering gross disease during an elective neck dissection, some researchers have recommended including level IV and V.
The second scenario is when a posterolateral oral tongue cancer is near or at the tongue base, since oropharyngeal cancers are known to drain to levels II-IV.
The decision to make exceptions in these two situations should be based on the combination of clinical judgment and evidence-based medicine in certain situations. That said, for most patients with cNO oral cavity cancer, SOHND is enough.
Arun Sharma, MD, MS , is with the division of otolaryngology–head and neck surgery at Southern Illinois University, Springfield. He had no conflict of interest related to his editorial, which appears in JAMA Otolaryngology–Head & Neck Surgery .
Although this meta-analysis shows that the risk of level IV involvement is less than 5% in patients with clinically node-negative (cN0) oral cavity cancers, going beyond standard supraomohyoid neck dissection (SOHND) may still be warranted for specific patients.
While the authors conclude that elective treatment of level IV is not required in patients with cN0 oral cavity cancer, there are two situations in which clinicians should consider adding level IV to standard SOHND.
The first is when a patient has gross macroscopic disease in upper levels, particularly level III. Most studies in the meta-analysis had insufficient data to determine whether involvement of upper levels increased risk of level IV involvement. When encountering gross disease during an elective neck dissection, some researchers have recommended including level IV and V.
The second scenario is when a posterolateral oral tongue cancer is near or at the tongue base, since oropharyngeal cancers are known to drain to levels II-IV.
The decision to make exceptions in these two situations should be based on the combination of clinical judgment and evidence-based medicine in certain situations. That said, for most patients with cNO oral cavity cancer, SOHND is enough.
Arun Sharma, MD, MS , is with the division of otolaryngology–head and neck surgery at Southern Illinois University, Springfield. He had no conflict of interest related to his editorial, which appears in JAMA Otolaryngology–Head & Neck Surgery .
Although this meta-analysis shows that the risk of level IV involvement is less than 5% in patients with clinically node-negative (cN0) oral cavity cancers, going beyond standard supraomohyoid neck dissection (SOHND) may still be warranted for specific patients.
While the authors conclude that elective treatment of level IV is not required in patients with cN0 oral cavity cancer, there are two situations in which clinicians should consider adding level IV to standard SOHND.
The first is when a patient has gross macroscopic disease in upper levels, particularly level III. Most studies in the meta-analysis had insufficient data to determine whether involvement of upper levels increased risk of level IV involvement. When encountering gross disease during an elective neck dissection, some researchers have recommended including level IV and V.
The second scenario is when a posterolateral oral tongue cancer is near or at the tongue base, since oropharyngeal cancers are known to drain to levels II-IV.
The decision to make exceptions in these two situations should be based on the combination of clinical judgment and evidence-based medicine in certain situations. That said, for most patients with cNO oral cavity cancer, SOHND is enough.
Arun Sharma, MD, MS , is with the division of otolaryngology–head and neck surgery at Southern Illinois University, Springfield. He had no conflict of interest related to his editorial, which appears in JAMA Otolaryngology–Head & Neck Surgery .
In patients with clinically node-negative oral cavity cancers, the rate of skip metastasis to neck level IV is extremely low, according to authors of a recent meta-analysis.
The rate of level IV involvement was about 2.5%, and the rate of skip metastasis was 0.5% in the analysis, which comprised 11 retrospective studies and 2 randomized clinical trials including a total of 1,359 patients with clinically node-negative oral cavity squamous cell carcinomas.
Encountering a suspected positive lymph node during neck dissection does not appear to be an indicator of high rates of level IV involvement, according to Anton Warshavsky, MD, and colleagues in the department of otolaryngology–head and neck surgery and maxillofacial surgery at Tel Aviv Sourasky Medical Center, Israel.
“Supraomohyoid neck dissection [SOHND] is adequate for this subset of patients,” Dr. Warshavsky and coauthors wrote in a report on the study that appears in JAMA Otolaryngology–Head & Neck Surgery.
SOHND, a type of selective neck dissection, refers to removal of lymph nodes in levels I-III. This approach is now frequently used in managing clinically node-negative oral cavity squamous cell carcinoma and provides control rates similar to those associated with more extensive neck dissections, Dr. Warshavsky and colleagues wrote.
However, concern regarding the risk of skip metastases, or involvement of neck level IV without involvement of lower levels, has stirred controversy. SOHND might not be adequate in these patients because of the possibility of occult metastasis to neck level IV.
Accordingly, Dr. Warshavsky and colleagues combed the available medical literature to find relevant articles for a meta-analysis to better characterize the rate of skip metastasis to level IV in patients who had undergone neck dissection.
Level IV involvement rates in clinically node-negative patients ranged from 0% to 11.4% in the 13 included studies. Based on fixed-effects modeling, the rate of involvement was 2.53% (95% CI, 1.64-3.55%), according to the published report.
The rate of skip metastasis was “extremely low,” wrote Dr. Warshavsky and coauthors. Rates ranged from 0% to 5.50%, with a fixed-effects model of 0.50% (95% CI, 0.09%-1.11%).
Cases involving higher levels of the neck did not impact the rate of level IV metastasis, results of a subgroup analysis found. Likewise, an analysis based on T stage showed that rates of level 4 involvement were comparable and low for T stages I-II and III-IV.
These findings are limited, however, not only by the retrospective nature of this study, but also by the fact that many studies reported limited data, hampering the investigators’ ability to run statistics and perform subgroup analyses.
“Unfortunately, data in almost all of the analyzed articles failed to report the relations between the primary tumor site and the neck levels involved by metastatic tumor,” they wrote. “Only primary lesions of the tongue could be accurately assessed.”
Dr. Warshavsky and coauthors reported no conflicts of interest related to the research.
SOURCE: Warshavsky A et al. JAMA Otolaryngol Head Neck Surg. 2019 May 9. doi: 10.1001/jamaoto.2019.0784.
In patients with clinically node-negative oral cavity cancers, the rate of skip metastasis to neck level IV is extremely low, according to authors of a recent meta-analysis.
The rate of level IV involvement was about 2.5%, and the rate of skip metastasis was 0.5% in the analysis, which comprised 11 retrospective studies and 2 randomized clinical trials including a total of 1,359 patients with clinically node-negative oral cavity squamous cell carcinomas.
Encountering a suspected positive lymph node during neck dissection does not appear to be an indicator of high rates of level IV involvement, according to Anton Warshavsky, MD, and colleagues in the department of otolaryngology–head and neck surgery and maxillofacial surgery at Tel Aviv Sourasky Medical Center, Israel.
“Supraomohyoid neck dissection [SOHND] is adequate for this subset of patients,” Dr. Warshavsky and coauthors wrote in a report on the study that appears in JAMA Otolaryngology–Head & Neck Surgery.
SOHND, a type of selective neck dissection, refers to removal of lymph nodes in levels I-III. This approach is now frequently used in managing clinically node-negative oral cavity squamous cell carcinoma and provides control rates similar to those associated with more extensive neck dissections, Dr. Warshavsky and colleagues wrote.
However, concern regarding the risk of skip metastases, or involvement of neck level IV without involvement of lower levels, has stirred controversy. SOHND might not be adequate in these patients because of the possibility of occult metastasis to neck level IV.
Accordingly, Dr. Warshavsky and colleagues combed the available medical literature to find relevant articles for a meta-analysis to better characterize the rate of skip metastasis to level IV in patients who had undergone neck dissection.
Level IV involvement rates in clinically node-negative patients ranged from 0% to 11.4% in the 13 included studies. Based on fixed-effects modeling, the rate of involvement was 2.53% (95% CI, 1.64-3.55%), according to the published report.
The rate of skip metastasis was “extremely low,” wrote Dr. Warshavsky and coauthors. Rates ranged from 0% to 5.50%, with a fixed-effects model of 0.50% (95% CI, 0.09%-1.11%).
Cases involving higher levels of the neck did not impact the rate of level IV metastasis, results of a subgroup analysis found. Likewise, an analysis based on T stage showed that rates of level 4 involvement were comparable and low for T stages I-II and III-IV.
These findings are limited, however, not only by the retrospective nature of this study, but also by the fact that many studies reported limited data, hampering the investigators’ ability to run statistics and perform subgroup analyses.
“Unfortunately, data in almost all of the analyzed articles failed to report the relations between the primary tumor site and the neck levels involved by metastatic tumor,” they wrote. “Only primary lesions of the tongue could be accurately assessed.”
Dr. Warshavsky and coauthors reported no conflicts of interest related to the research.
SOURCE: Warshavsky A et al. JAMA Otolaryngol Head Neck Surg. 2019 May 9. doi: 10.1001/jamaoto.2019.0784.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Key clinical point: In patients with clinically node-negative oral cavity cancers, rates of skip metastasis to neck level IV are extremely low, meaning that supraomohyoid neck dissection is likely sufficient for most cases.
Major finding: The rate of level IV involvement was about 2.5%, and the rate of skip metastasis was 0.5%.
Study details: A meta-analysis of 11 retrospective studies and 2 randomized clinical trials, including a total of 1,359 patients who had undergone neck dissection.
Disclosures: The study authors reported no conflicts of interest.
Source: Warshavsky A et al. JAMA Otolaryngol Head Neck Surg. 2019 May 9. doi: 10.1001/jamaoto.2019.0784.
PET imaging at diagnosis improves oropharyngeal cancer outcomes
For patients with oropharyngeal squamous cell carcinoma (OPSCC), initial staging with PET is associated with better cancer-specific survival (CSS) than staging with other imaging modalities, based on a retrospective analysis of more than 1,700 patients.
PET was associated with a better 3-year overall survival rate than either MRI without PET or CT alone, reported lead author Rustain L. Morgan, MD, of the University of Colorado at Denver, Aurora, and colleagues.
“To our knowledge, there have been no prospective, randomized, controlled trials to date to evaluate the effect of different imaging modalities at the time of initial staging on cancer-specific survival,” the investigators wrote in Cancer. “A population-based data source such as the Surveillance, Epidemiology, and End Results [SEER]–Medicare database provides an excellent opportunity to compare the impact of imaging modality differences on survival in patients with OPSCC.”
Using SEER data, the investigators identified more than 3,704 patients with oropharyngeal cancer; following exclusions, 1,765 patients were involved in the final analysis based on various factors, including survival beyond 2 months after diagnosis and squamous cell carcinoma histology. A Cox proportional hazards model was used to assess relationships between the primary outcome and 3-year CSS rate and imaging, sex, age, region, race, and education.
Results showed that most patients had PET imaging upon diagnosis (83.3%), while fewer had CT alone (11.4%) or MRI without PET (5.2%). Several underlying trends were found: Patients in the West were more likely to undergo PET than patients in the Midwest, South, or East; patients younger than 75 years were more likely to have PET than older patients; and men were more likely to be staged with PET than women. The 3-year CSS was longest for patients who underwent PET (56.8%), followed by MRI without PET (50.1%) and CT alone (47.3%). Controlling for treatment and stage of disease, multivariate analysis also suggested that PET is associated with better CSS; patients staged with MRI without PET had a hazard ratio of 1.748 (P = .0036) and those imaged with CT alone had an HR of 1.337 (P = .0491). Although the Cox proportional hazards model for overall survival revealed numerical trends, these lacked statistical significance for MRI without PET (HR, 1.365; P =.0683) and CT alone (HR, 1.213; P = .114).
“The current study demonstrated a significant difference in CSS based on initial imaging,” the investigators wrote. “These findings are consistent with a prior study that reported that PET imaging can improve the staging of patients with head and neck cancers, particularly as it relates to radiotherapy planning.
“The data from the current study suggest the need for further prospective research to evaluate whether CT or MRI should be considered adequate for the initial staging of patients with OPSCC,” the investigators concluded.
The study was funded by the University of Colorado Cancer Center. One coauthor reported relationships with the American Heart Association and the National Heart, Lung, and Blood Institute.
SOURCE: Morgan RL et al. Cancer. 2019 May 1. doi:10.1002/cncr.32148.
For patients with oropharyngeal squamous cell carcinoma (OPSCC), initial staging with PET is associated with better cancer-specific survival (CSS) than staging with other imaging modalities, based on a retrospective analysis of more than 1,700 patients.
PET was associated with a better 3-year overall survival rate than either MRI without PET or CT alone, reported lead author Rustain L. Morgan, MD, of the University of Colorado at Denver, Aurora, and colleagues.
“To our knowledge, there have been no prospective, randomized, controlled trials to date to evaluate the effect of different imaging modalities at the time of initial staging on cancer-specific survival,” the investigators wrote in Cancer. “A population-based data source such as the Surveillance, Epidemiology, and End Results [SEER]–Medicare database provides an excellent opportunity to compare the impact of imaging modality differences on survival in patients with OPSCC.”
Using SEER data, the investigators identified more than 3,704 patients with oropharyngeal cancer; following exclusions, 1,765 patients were involved in the final analysis based on various factors, including survival beyond 2 months after diagnosis and squamous cell carcinoma histology. A Cox proportional hazards model was used to assess relationships between the primary outcome and 3-year CSS rate and imaging, sex, age, region, race, and education.
Results showed that most patients had PET imaging upon diagnosis (83.3%), while fewer had CT alone (11.4%) or MRI without PET (5.2%). Several underlying trends were found: Patients in the West were more likely to undergo PET than patients in the Midwest, South, or East; patients younger than 75 years were more likely to have PET than older patients; and men were more likely to be staged with PET than women. The 3-year CSS was longest for patients who underwent PET (56.8%), followed by MRI without PET (50.1%) and CT alone (47.3%). Controlling for treatment and stage of disease, multivariate analysis also suggested that PET is associated with better CSS; patients staged with MRI without PET had a hazard ratio of 1.748 (P = .0036) and those imaged with CT alone had an HR of 1.337 (P = .0491). Although the Cox proportional hazards model for overall survival revealed numerical trends, these lacked statistical significance for MRI without PET (HR, 1.365; P =.0683) and CT alone (HR, 1.213; P = .114).
“The current study demonstrated a significant difference in CSS based on initial imaging,” the investigators wrote. “These findings are consistent with a prior study that reported that PET imaging can improve the staging of patients with head and neck cancers, particularly as it relates to radiotherapy planning.
“The data from the current study suggest the need for further prospective research to evaluate whether CT or MRI should be considered adequate for the initial staging of patients with OPSCC,” the investigators concluded.
The study was funded by the University of Colorado Cancer Center. One coauthor reported relationships with the American Heart Association and the National Heart, Lung, and Blood Institute.
SOURCE: Morgan RL et al. Cancer. 2019 May 1. doi:10.1002/cncr.32148.
For patients with oropharyngeal squamous cell carcinoma (OPSCC), initial staging with PET is associated with better cancer-specific survival (CSS) than staging with other imaging modalities, based on a retrospective analysis of more than 1,700 patients.
PET was associated with a better 3-year overall survival rate than either MRI without PET or CT alone, reported lead author Rustain L. Morgan, MD, of the University of Colorado at Denver, Aurora, and colleagues.
“To our knowledge, there have been no prospective, randomized, controlled trials to date to evaluate the effect of different imaging modalities at the time of initial staging on cancer-specific survival,” the investigators wrote in Cancer. “A population-based data source such as the Surveillance, Epidemiology, and End Results [SEER]–Medicare database provides an excellent opportunity to compare the impact of imaging modality differences on survival in patients with OPSCC.”
Using SEER data, the investigators identified more than 3,704 patients with oropharyngeal cancer; following exclusions, 1,765 patients were involved in the final analysis based on various factors, including survival beyond 2 months after diagnosis and squamous cell carcinoma histology. A Cox proportional hazards model was used to assess relationships between the primary outcome and 3-year CSS rate and imaging, sex, age, region, race, and education.
Results showed that most patients had PET imaging upon diagnosis (83.3%), while fewer had CT alone (11.4%) or MRI without PET (5.2%). Several underlying trends were found: Patients in the West were more likely to undergo PET than patients in the Midwest, South, or East; patients younger than 75 years were more likely to have PET than older patients; and men were more likely to be staged with PET than women. The 3-year CSS was longest for patients who underwent PET (56.8%), followed by MRI without PET (50.1%) and CT alone (47.3%). Controlling for treatment and stage of disease, multivariate analysis also suggested that PET is associated with better CSS; patients staged with MRI without PET had a hazard ratio of 1.748 (P = .0036) and those imaged with CT alone had an HR of 1.337 (P = .0491). Although the Cox proportional hazards model for overall survival revealed numerical trends, these lacked statistical significance for MRI without PET (HR, 1.365; P =.0683) and CT alone (HR, 1.213; P = .114).
“The current study demonstrated a significant difference in CSS based on initial imaging,” the investigators wrote. “These findings are consistent with a prior study that reported that PET imaging can improve the staging of patients with head and neck cancers, particularly as it relates to radiotherapy planning.
“The data from the current study suggest the need for further prospective research to evaluate whether CT or MRI should be considered adequate for the initial staging of patients with OPSCC,” the investigators concluded.
The study was funded by the University of Colorado Cancer Center. One coauthor reported relationships with the American Heart Association and the National Heart, Lung, and Blood Institute.
SOURCE: Morgan RL et al. Cancer. 2019 May 1. doi:10.1002/cncr.32148.
FROM CANCER
Key clinical point: For patients with oropharyngeal cancer, initial staging with PET imaging is associated with better cancer-specific survival than staging with other imaging modalities.
Major finding: Patients who underwent PET at diagnosis had a 3-year overall survival rate of 56.8%, compared with those who had MRI without PET (50.1%) or CT alone (47.3%).
Study details: A retrospective analysis of 1,765 patients with oropharyngeal cancer who had imaging performed at diagnosis.
Disclosures: The study was funded by the University of Colorado Cancer Center. One coauthor reported relationships with the American Heart Association and the National Heart, Lung, and Blood Institute.
Source: Morgan RL et al. Cancer. 2019 May 1. doi:10.1002/cncr.32148.
Post-treatment persistence of oral HPV in HNSCC predicts recurrence, death
Persistence of oral human papillomavirus (HPV) DNA after primary treatment of HPV-positive oral cavity or oropharyngeal head and neck squamous cell carcinoma (HNSCC) is a strong risk factor for poor outcomes, finds a prospective cohort study.
Investigators working under senior author Maura L. Gillison, MD, PhD, of the department of thoracic head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston, collected serial oral rinses from 396 patients with HNSCC (217 with oropharyngeal cancer, 170 with oral cavity cancer, 9 with unknown primary cancer) treated at two institutions. Overall, 51% had HPV-positive tumors.
Patients with HPV-positive tumors were much more likely to have detectable oral HPV at diagnosis than their counterparts with HPV-negative tumors (84.2% vs 12.4%; P less than .001). Detection of oral HPV-16 DNA had good sensitivity (81%) and excellent specificity (100%) for HPV-16-positive tumors, Dr. Gillison and colleagues reported in JAMA Oncology.
Patients’ odds of having detectable tumor-type HPV fell during primary therapy (odds ratio per each postdiagnosis month, 0.41; P less than .001); in contrast, their odds of having of having detectable nontumor types did not. Current smokers were about half as likely to achieve clearing of tumor-type HPV DNA (hazard ratio, 0.49; P = .01).
Compared with counterparts who no longer had detectable tumor-type DNA after therapy, HPV-positive patients who did had dramatically poorer recurrence-free survival (55% vs. 88%; adjusted hazard ratio, 3.72; P less than .001) and overall survival (68% vs. 95%; adjusted hazard ratio, 6.61; P = .003).
In contrast, persistence of nontumor-type HPV DNA did not predict these outcomes among either patients with HPV-positive tumors or patients with HPV-negative tumors.
“Analysis of tumor type HPV DNA has considerable promise as a biomarker for treatment response and risk of progression,” Dr. Gillison and coinvestigators maintain.
“Our data suggest that a subset of patients with HPV-positive HNSCC at high-risk for locoregional recurrence can be identified by detection of persistent, oral HPV after treatment. However, the clinical utility may be constrained by a need to identify the tumor-type infection, a low-moderate positive predictive value for recurrence, and weak associations with risk of distant metastases,” they conclude. “Ongoing studies will evaluate whether multiplexed detection of plasma HPV DNA can improve these limitations.”
Dr. Gillison disclosed consulting for Roche Holding AG, Bristol-Myers Squibb, Merck & Co Inc., Celgene Corporation, Amgen, AstraZeneca, Rakuten Aspyrian Inc. (now known as Rakuten Medical), EMD Serono Inc., NewLink Genetics Corporation, and Genocea Biosciences. The study was supported by the Oral Cancer Foundation and The Ohio State University Comprehensive Cancer Center. Dr. Gillison is a Cancer Prevention and Research Institute of Texas Scholar.
SOURCE: Fakhry C et al. JAMA Oncol. 2019 May 2. doi: 10.1001/jamaoncol.2019.0439.
Persistence of oral human papillomavirus (HPV) DNA after primary treatment of HPV-positive oral cavity or oropharyngeal head and neck squamous cell carcinoma (HNSCC) is a strong risk factor for poor outcomes, finds a prospective cohort study.
Investigators working under senior author Maura L. Gillison, MD, PhD, of the department of thoracic head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston, collected serial oral rinses from 396 patients with HNSCC (217 with oropharyngeal cancer, 170 with oral cavity cancer, 9 with unknown primary cancer) treated at two institutions. Overall, 51% had HPV-positive tumors.
Patients with HPV-positive tumors were much more likely to have detectable oral HPV at diagnosis than their counterparts with HPV-negative tumors (84.2% vs 12.4%; P less than .001). Detection of oral HPV-16 DNA had good sensitivity (81%) and excellent specificity (100%) for HPV-16-positive tumors, Dr. Gillison and colleagues reported in JAMA Oncology.
Patients’ odds of having detectable tumor-type HPV fell during primary therapy (odds ratio per each postdiagnosis month, 0.41; P less than .001); in contrast, their odds of having of having detectable nontumor types did not. Current smokers were about half as likely to achieve clearing of tumor-type HPV DNA (hazard ratio, 0.49; P = .01).
Compared with counterparts who no longer had detectable tumor-type DNA after therapy, HPV-positive patients who did had dramatically poorer recurrence-free survival (55% vs. 88%; adjusted hazard ratio, 3.72; P less than .001) and overall survival (68% vs. 95%; adjusted hazard ratio, 6.61; P = .003).
In contrast, persistence of nontumor-type HPV DNA did not predict these outcomes among either patients with HPV-positive tumors or patients with HPV-negative tumors.
“Analysis of tumor type HPV DNA has considerable promise as a biomarker for treatment response and risk of progression,” Dr. Gillison and coinvestigators maintain.
“Our data suggest that a subset of patients with HPV-positive HNSCC at high-risk for locoregional recurrence can be identified by detection of persistent, oral HPV after treatment. However, the clinical utility may be constrained by a need to identify the tumor-type infection, a low-moderate positive predictive value for recurrence, and weak associations with risk of distant metastases,” they conclude. “Ongoing studies will evaluate whether multiplexed detection of plasma HPV DNA can improve these limitations.”
Dr. Gillison disclosed consulting for Roche Holding AG, Bristol-Myers Squibb, Merck & Co Inc., Celgene Corporation, Amgen, AstraZeneca, Rakuten Aspyrian Inc. (now known as Rakuten Medical), EMD Serono Inc., NewLink Genetics Corporation, and Genocea Biosciences. The study was supported by the Oral Cancer Foundation and The Ohio State University Comprehensive Cancer Center. Dr. Gillison is a Cancer Prevention and Research Institute of Texas Scholar.
SOURCE: Fakhry C et al. JAMA Oncol. 2019 May 2. doi: 10.1001/jamaoncol.2019.0439.
Persistence of oral human papillomavirus (HPV) DNA after primary treatment of HPV-positive oral cavity or oropharyngeal head and neck squamous cell carcinoma (HNSCC) is a strong risk factor for poor outcomes, finds a prospective cohort study.
Investigators working under senior author Maura L. Gillison, MD, PhD, of the department of thoracic head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston, collected serial oral rinses from 396 patients with HNSCC (217 with oropharyngeal cancer, 170 with oral cavity cancer, 9 with unknown primary cancer) treated at two institutions. Overall, 51% had HPV-positive tumors.
Patients with HPV-positive tumors were much more likely to have detectable oral HPV at diagnosis than their counterparts with HPV-negative tumors (84.2% vs 12.4%; P less than .001). Detection of oral HPV-16 DNA had good sensitivity (81%) and excellent specificity (100%) for HPV-16-positive tumors, Dr. Gillison and colleagues reported in JAMA Oncology.
Patients’ odds of having detectable tumor-type HPV fell during primary therapy (odds ratio per each postdiagnosis month, 0.41; P less than .001); in contrast, their odds of having of having detectable nontumor types did not. Current smokers were about half as likely to achieve clearing of tumor-type HPV DNA (hazard ratio, 0.49; P = .01).
Compared with counterparts who no longer had detectable tumor-type DNA after therapy, HPV-positive patients who did had dramatically poorer recurrence-free survival (55% vs. 88%; adjusted hazard ratio, 3.72; P less than .001) and overall survival (68% vs. 95%; adjusted hazard ratio, 6.61; P = .003).
In contrast, persistence of nontumor-type HPV DNA did not predict these outcomes among either patients with HPV-positive tumors or patients with HPV-negative tumors.
“Analysis of tumor type HPV DNA has considerable promise as a biomarker for treatment response and risk of progression,” Dr. Gillison and coinvestigators maintain.
“Our data suggest that a subset of patients with HPV-positive HNSCC at high-risk for locoregional recurrence can be identified by detection of persistent, oral HPV after treatment. However, the clinical utility may be constrained by a need to identify the tumor-type infection, a low-moderate positive predictive value for recurrence, and weak associations with risk of distant metastases,” they conclude. “Ongoing studies will evaluate whether multiplexed detection of plasma HPV DNA can improve these limitations.”
Dr. Gillison disclosed consulting for Roche Holding AG, Bristol-Myers Squibb, Merck & Co Inc., Celgene Corporation, Amgen, AstraZeneca, Rakuten Aspyrian Inc. (now known as Rakuten Medical), EMD Serono Inc., NewLink Genetics Corporation, and Genocea Biosciences. The study was supported by the Oral Cancer Foundation and The Ohio State University Comprehensive Cancer Center. Dr. Gillison is a Cancer Prevention and Research Institute of Texas Scholar.
SOURCE: Fakhry C et al. JAMA Oncol. 2019 May 2. doi: 10.1001/jamaoncol.2019.0439.
FROM JAMA ONCOLOGY
Key clinical point: Persistence of detectable tumor-type HPV DNA in oral rinses after primary treatment of HPV-positive HNSCC identifies patients at high risk for poor outcomes.
Major finding: HPV-positive patients with persistent oral tumor-type HPV DNA had sharply higher risks of 2-year recurrence-free survival events (hazard ratio, 3.72; P less than .001) and death (hazard ratio, 6.61; P = .003).
Study details: Prospective cohort study of 396 patients with newly diagnosed oral cavity or oropharyngeal HNSCC.
Disclosures: Dr. Gillison disclosed consulting for Roche Holding AG, Bristol-Myers Squibb, Merck & Co Inc., Celgene Corporation, Amgen, AstraZeneca, Rakuten Aspyrian Inc. (now known as Rakuten Medical), EMD Serono Inc., NewLink Genetics Corporation, and Genocea Biosciences. The study was supported by the Oral Cancer Foundation and The Ohio State University Comprehensive Cancer Center. Dr. Gillison is a Cancer Prevention and Research Institute of Texas Scholar.
Source: Gillison ML et al. JAMA Oncol. 2019 May 2. doi: 10.1001/jamaoncol.2019.0439.
Ultrasound offers advantages for long-term lymph node surveillance in high-grade SCC patients
BALTIMORE – Ultrasound can be a very effective way to track early nodal metastasis in patients with high-stage cutaneous squamous cell carcinomas, and at a fraction of the cost of other imaging modalities.
The technique shows not only abnormal variations in the shape of nodes, but changes in the core and outer density, and vascular patterns, Emily Ruiz, MD, said at the annual meeting of the American College of Mohs Surgery. And over a 2-year surveillance period, this costs thousands less than radiation-based imaging.
Dr. Ruiz, director of the High-Risk Skin Cancer Clinic at Dana-Farber/Brigham and Women’s Cancer Center, Boston, said the standard imaging technique at that center used to be serial CT scans performed at diagnosis and every 6 months thereafter, for 2 years. But recently, the protocol changed: Ultrasound is now the preferred technique.
“The big problem with CT in this earlier disease, is that it can only identify the nodes that are enlarged, and doesn’t tell us anything about the etiology. Ultrasound, on the other hand, looks at a number of different features of the node.”
Tracking high-risk squamous cell carcinoma patients is a must, she said. “About 4% of people diagnosed with high-risk SCC will develop nodal metastases, and 1.5% of those will die from disease-specific death,” most often from locoregional disease. “So it’s critical to identify nodal diseases early as possible. Earlier identification leads to better outcomes.” Ultrasound simply provides more information about nodal metastasis, Dr. Ruiz added.
“The first thing we look at is the general architecture of the node. Resting and reactive nodes have a hypoechoic hilus and a hyperechoic cortex. As they become infiltrated with tumor, the hilus becomes more hyperechoic, and areas of metastasis stand out as much more hyperechoic than the surrounding node.”
Another tip-off is overall shape. If the ratio of the long axis to short axis diameter is less than 2, the lymph node is more likely to be malignant, she said.
“One more important factor that can’t be seen on CT is the node’s vascular pattern. Both resting and reactive nodes tend to have a centralized vascular pattern in the hilus. With tumor infiltration you start to see an asymmetrical vascularization as the nodes are replaced by tumor. The perfusion becomes much more peripheral.”
Cost is another consideration, Dr. Ruiz said. Five CT scans conducted over the recommended 2 years of follow-up will run about $5,000. Five scans with magnetic resonance imaging come in at about $6,500. PET CT is, of course, the most expensive, racking up a national average cost of $28,500 for five scans.
Ultrasound is amazingly inexpensive, Dr. Ruiz said. The national average cost of one scan is around $180, bringing the 2-year cost of five surveillance scans to $900.
Finally, clinicians and patients should consider the potential impact of repeated radiation exposure. “This can really add up over the follow-up period. Because there’s a 10-year latency period for these cancers, this might not be an issue for our older patients, but it really is something to consider in younger ones. “
However, she acknowledged that it’s not a completely rosy picture.
“Ultrasound is very user dependent, but we do think that by putting this in the hands of dermatologists with special training, we can solve this issue. In Europe, ultrasound’s very high sensitivity and specificity, combined with clinical exams, really improves disease detection.”
Unfortunately, at this point, anyone who wants to learn the technique has to go to Europe. “I trained in Germany, where I took a standard 3-day course, did 250 supervised scans, and completed an exam. I realize that’s unrealistic for most people,” she said. But a training protocol is being developed at Brigham and Women’s, under the auspices of the institution’s imaging experts, who felt that 3 days and 250 supervised scans was excessive. The Brigham and Women’s program comprises 8 hours of didactic training and at least 30 supervised scans with at least three abnormalities correctly identified, and will be put into place soon, Dr. Ruiz said.
The biggest obstacle to large-scale adoption of this protocol is data – there are not a lot, at least now.
“We are working on that, too. In conjunction with the Skin Cancer Foundation, we’re launching a prospective study. We want to recruit 80 patients with T2B/T3 cutaneous SCCs. They get both and ultrasound and a CT scan at diagnosis and every 6 months for 2 years,” she said.
BALTIMORE – Ultrasound can be a very effective way to track early nodal metastasis in patients with high-stage cutaneous squamous cell carcinomas, and at a fraction of the cost of other imaging modalities.
The technique shows not only abnormal variations in the shape of nodes, but changes in the core and outer density, and vascular patterns, Emily Ruiz, MD, said at the annual meeting of the American College of Mohs Surgery. And over a 2-year surveillance period, this costs thousands less than radiation-based imaging.
Dr. Ruiz, director of the High-Risk Skin Cancer Clinic at Dana-Farber/Brigham and Women’s Cancer Center, Boston, said the standard imaging technique at that center used to be serial CT scans performed at diagnosis and every 6 months thereafter, for 2 years. But recently, the protocol changed: Ultrasound is now the preferred technique.
“The big problem with CT in this earlier disease, is that it can only identify the nodes that are enlarged, and doesn’t tell us anything about the etiology. Ultrasound, on the other hand, looks at a number of different features of the node.”
Tracking high-risk squamous cell carcinoma patients is a must, she said. “About 4% of people diagnosed with high-risk SCC will develop nodal metastases, and 1.5% of those will die from disease-specific death,” most often from locoregional disease. “So it’s critical to identify nodal diseases early as possible. Earlier identification leads to better outcomes.” Ultrasound simply provides more information about nodal metastasis, Dr. Ruiz added.
“The first thing we look at is the general architecture of the node. Resting and reactive nodes have a hypoechoic hilus and a hyperechoic cortex. As they become infiltrated with tumor, the hilus becomes more hyperechoic, and areas of metastasis stand out as much more hyperechoic than the surrounding node.”
Another tip-off is overall shape. If the ratio of the long axis to short axis diameter is less than 2, the lymph node is more likely to be malignant, she said.
“One more important factor that can’t be seen on CT is the node’s vascular pattern. Both resting and reactive nodes tend to have a centralized vascular pattern in the hilus. With tumor infiltration you start to see an asymmetrical vascularization as the nodes are replaced by tumor. The perfusion becomes much more peripheral.”
Cost is another consideration, Dr. Ruiz said. Five CT scans conducted over the recommended 2 years of follow-up will run about $5,000. Five scans with magnetic resonance imaging come in at about $6,500. PET CT is, of course, the most expensive, racking up a national average cost of $28,500 for five scans.
Ultrasound is amazingly inexpensive, Dr. Ruiz said. The national average cost of one scan is around $180, bringing the 2-year cost of five surveillance scans to $900.
Finally, clinicians and patients should consider the potential impact of repeated radiation exposure. “This can really add up over the follow-up period. Because there’s a 10-year latency period for these cancers, this might not be an issue for our older patients, but it really is something to consider in younger ones. “
However, she acknowledged that it’s not a completely rosy picture.
“Ultrasound is very user dependent, but we do think that by putting this in the hands of dermatologists with special training, we can solve this issue. In Europe, ultrasound’s very high sensitivity and specificity, combined with clinical exams, really improves disease detection.”
Unfortunately, at this point, anyone who wants to learn the technique has to go to Europe. “I trained in Germany, where I took a standard 3-day course, did 250 supervised scans, and completed an exam. I realize that’s unrealistic for most people,” she said. But a training protocol is being developed at Brigham and Women’s, under the auspices of the institution’s imaging experts, who felt that 3 days and 250 supervised scans was excessive. The Brigham and Women’s program comprises 8 hours of didactic training and at least 30 supervised scans with at least three abnormalities correctly identified, and will be put into place soon, Dr. Ruiz said.
The biggest obstacle to large-scale adoption of this protocol is data – there are not a lot, at least now.
“We are working on that, too. In conjunction with the Skin Cancer Foundation, we’re launching a prospective study. We want to recruit 80 patients with T2B/T3 cutaneous SCCs. They get both and ultrasound and a CT scan at diagnosis and every 6 months for 2 years,” she said.
BALTIMORE – Ultrasound can be a very effective way to track early nodal metastasis in patients with high-stage cutaneous squamous cell carcinomas, and at a fraction of the cost of other imaging modalities.
The technique shows not only abnormal variations in the shape of nodes, but changes in the core and outer density, and vascular patterns, Emily Ruiz, MD, said at the annual meeting of the American College of Mohs Surgery. And over a 2-year surveillance period, this costs thousands less than radiation-based imaging.
Dr. Ruiz, director of the High-Risk Skin Cancer Clinic at Dana-Farber/Brigham and Women’s Cancer Center, Boston, said the standard imaging technique at that center used to be serial CT scans performed at diagnosis and every 6 months thereafter, for 2 years. But recently, the protocol changed: Ultrasound is now the preferred technique.
“The big problem with CT in this earlier disease, is that it can only identify the nodes that are enlarged, and doesn’t tell us anything about the etiology. Ultrasound, on the other hand, looks at a number of different features of the node.”
Tracking high-risk squamous cell carcinoma patients is a must, she said. “About 4% of people diagnosed with high-risk SCC will develop nodal metastases, and 1.5% of those will die from disease-specific death,” most often from locoregional disease. “So it’s critical to identify nodal diseases early as possible. Earlier identification leads to better outcomes.” Ultrasound simply provides more information about nodal metastasis, Dr. Ruiz added.
“The first thing we look at is the general architecture of the node. Resting and reactive nodes have a hypoechoic hilus and a hyperechoic cortex. As they become infiltrated with tumor, the hilus becomes more hyperechoic, and areas of metastasis stand out as much more hyperechoic than the surrounding node.”
Another tip-off is overall shape. If the ratio of the long axis to short axis diameter is less than 2, the lymph node is more likely to be malignant, she said.
“One more important factor that can’t be seen on CT is the node’s vascular pattern. Both resting and reactive nodes tend to have a centralized vascular pattern in the hilus. With tumor infiltration you start to see an asymmetrical vascularization as the nodes are replaced by tumor. The perfusion becomes much more peripheral.”
Cost is another consideration, Dr. Ruiz said. Five CT scans conducted over the recommended 2 years of follow-up will run about $5,000. Five scans with magnetic resonance imaging come in at about $6,500. PET CT is, of course, the most expensive, racking up a national average cost of $28,500 for five scans.
Ultrasound is amazingly inexpensive, Dr. Ruiz said. The national average cost of one scan is around $180, bringing the 2-year cost of five surveillance scans to $900.
Finally, clinicians and patients should consider the potential impact of repeated radiation exposure. “This can really add up over the follow-up period. Because there’s a 10-year latency period for these cancers, this might not be an issue for our older patients, but it really is something to consider in younger ones. “
However, she acknowledged that it’s not a completely rosy picture.
“Ultrasound is very user dependent, but we do think that by putting this in the hands of dermatologists with special training, we can solve this issue. In Europe, ultrasound’s very high sensitivity and specificity, combined with clinical exams, really improves disease detection.”
Unfortunately, at this point, anyone who wants to learn the technique has to go to Europe. “I trained in Germany, where I took a standard 3-day course, did 250 supervised scans, and completed an exam. I realize that’s unrealistic for most people,” she said. But a training protocol is being developed at Brigham and Women’s, under the auspices of the institution’s imaging experts, who felt that 3 days and 250 supervised scans was excessive. The Brigham and Women’s program comprises 8 hours of didactic training and at least 30 supervised scans with at least three abnormalities correctly identified, and will be put into place soon, Dr. Ruiz said.
The biggest obstacle to large-scale adoption of this protocol is data – there are not a lot, at least now.
“We are working on that, too. In conjunction with the Skin Cancer Foundation, we’re launching a prospective study. We want to recruit 80 patients with T2B/T3 cutaneous SCCs. They get both and ultrasound and a CT scan at diagnosis and every 6 months for 2 years,” she said.
EXPERT ANALYSIS FROM the ACMS Annual Meeting