User login
Polycystic Ovary Syndrome Associated With Midlife Memory, Thinking Problems
TOPLINE:
People with polycystic ovary syndrome (PCOS) may score lower on cognitive tests than people without the condition, a research showed. They also may have worse integrity of brain tissue as evident on an MRI.
METHODOLOGY:
- Researchers used data from the Coronary Artery Risk Development in Young Adults Women’s Study; individuals were 18-30 years old at the beginning of the study and were followed over 30 years.
- A little over 900 women were included in the study, of which 66 had PCOS, which was defined as having elevated androgen levels or self-reported hirsutism and irregular menstrual cycles more than 32 days apart.
- Study participants completed tests measuring verbal learning and memory, processing speed and executive function, attention and cognitive control, and semantics and attention.
- Researchers analyzed brain white matter integrity for 291 of the individuals, including 25 with PCOS, who underwent MRI.
TAKEAWAY:
- Individuals with PCOS had worse memory, attention, and verbal ability scores than those without the disorder.
- MRI scans showed that those with PCOS had lower white matter integrity, an indicator of cognitive deficits, including poorer decision-making abilities.
- Those in the PCOS group were more likely to be White and have diabetes than those in the control group.
IN PRACTICE:
“This report of midlife cognition in PCOS raises a new concern about another potential comorbidity for individuals with this common disorder; given that up to 10% of women may be affected by PCOS, these results have important implications for public health at large,” the authors concluded.
SOURCE:
Heather G. Huddleston, MD, director of the PCOS Clinic at the UCSF Health, San Francisco, California, is the lead author of the study published in Neurology.
LIMITATIONS:
PCOS was determined on the basis of serum androgen levels and self-reporting of hirsutism and oligomenorrhea, so some cases may have been misclassified without the official diagnosis of a clinician.
DISCLOSURES:
The authors did not report any relevant financial conflicts. The study was funded by a grant from the University of California, San Francisco, California.
A version of this article appeared on Medscape.com.
TOPLINE:
People with polycystic ovary syndrome (PCOS) may score lower on cognitive tests than people without the condition, a research showed. They also may have worse integrity of brain tissue as evident on an MRI.
METHODOLOGY:
- Researchers used data from the Coronary Artery Risk Development in Young Adults Women’s Study; individuals were 18-30 years old at the beginning of the study and were followed over 30 years.
- A little over 900 women were included in the study, of which 66 had PCOS, which was defined as having elevated androgen levels or self-reported hirsutism and irregular menstrual cycles more than 32 days apart.
- Study participants completed tests measuring verbal learning and memory, processing speed and executive function, attention and cognitive control, and semantics and attention.
- Researchers analyzed brain white matter integrity for 291 of the individuals, including 25 with PCOS, who underwent MRI.
TAKEAWAY:
- Individuals with PCOS had worse memory, attention, and verbal ability scores than those without the disorder.
- MRI scans showed that those with PCOS had lower white matter integrity, an indicator of cognitive deficits, including poorer decision-making abilities.
- Those in the PCOS group were more likely to be White and have diabetes than those in the control group.
IN PRACTICE:
“This report of midlife cognition in PCOS raises a new concern about another potential comorbidity for individuals with this common disorder; given that up to 10% of women may be affected by PCOS, these results have important implications for public health at large,” the authors concluded.
SOURCE:
Heather G. Huddleston, MD, director of the PCOS Clinic at the UCSF Health, San Francisco, California, is the lead author of the study published in Neurology.
LIMITATIONS:
PCOS was determined on the basis of serum androgen levels and self-reporting of hirsutism and oligomenorrhea, so some cases may have been misclassified without the official diagnosis of a clinician.
DISCLOSURES:
The authors did not report any relevant financial conflicts. The study was funded by a grant from the University of California, San Francisco, California.
A version of this article appeared on Medscape.com.
TOPLINE:
People with polycystic ovary syndrome (PCOS) may score lower on cognitive tests than people without the condition, a research showed. They also may have worse integrity of brain tissue as evident on an MRI.
METHODOLOGY:
- Researchers used data from the Coronary Artery Risk Development in Young Adults Women’s Study; individuals were 18-30 years old at the beginning of the study and were followed over 30 years.
- A little over 900 women were included in the study, of which 66 had PCOS, which was defined as having elevated androgen levels or self-reported hirsutism and irregular menstrual cycles more than 32 days apart.
- Study participants completed tests measuring verbal learning and memory, processing speed and executive function, attention and cognitive control, and semantics and attention.
- Researchers analyzed brain white matter integrity for 291 of the individuals, including 25 with PCOS, who underwent MRI.
TAKEAWAY:
- Individuals with PCOS had worse memory, attention, and verbal ability scores than those without the disorder.
- MRI scans showed that those with PCOS had lower white matter integrity, an indicator of cognitive deficits, including poorer decision-making abilities.
- Those in the PCOS group were more likely to be White and have diabetes than those in the control group.
IN PRACTICE:
“This report of midlife cognition in PCOS raises a new concern about another potential comorbidity for individuals with this common disorder; given that up to 10% of women may be affected by PCOS, these results have important implications for public health at large,” the authors concluded.
SOURCE:
Heather G. Huddleston, MD, director of the PCOS Clinic at the UCSF Health, San Francisco, California, is the lead author of the study published in Neurology.
LIMITATIONS:
PCOS was determined on the basis of serum androgen levels and self-reporting of hirsutism and oligomenorrhea, so some cases may have been misclassified without the official diagnosis of a clinician.
DISCLOSURES:
The authors did not report any relevant financial conflicts. The study was funded by a grant from the University of California, San Francisco, California.
A version of this article appeared on Medscape.com.
Restricted Abortion Access Tied to Mental Health Harm
, which revoked a woman’s constitutional right to an abortion, new research shows.
This could be due to a variety of factors, investigators led by Benjamin Thornburg, Johns Hopkins Bloomberg School of Public Health, Baltimore, noted. These include fear about the imminent risk of being denied an abortion, uncertainty around future limitations on abortion and other related rights such as contraception, worry over the ability to receive lifesaving medical care during pregnancy, and a general sense of violation and powerlessness related to loss of the right to reproductive autonomy.
The study was published online on January 23, 2024, in JAMA.
Mental Health Harm
In June 2022, the US Supreme Court overturned Roe vs Wade, removing federal protections for abortion rights. Thirteen states had “trigger laws” that immediately banned or severely restricted abortion — raising concerns this could negatively affect mental health.
The researchers used data from the Household Pulse Survey to estimate changes in anxiety and depression symptoms after vs before the Dobbs decision in nearly 160,000 adults living in 13 states with trigger laws compared with roughly 559,000 adults living in 37 states without trigger laws.
The mean age of respondents was 48 years, and 51% were women. Anxiety and depression symptoms were measured via the Patient Health Questionnaire-4 (PHQ-4).
In trigger states, the mean PHQ-4 score at baseline (before Dobbs) was 3.51 (out of 12) and increased to 3.81 after the Dobbs decision. In nontrigger states, the mean PHQ-4 score at baseline was 3.31 and increased to 3.49 after Dobbs.
Living in a trigger state was associated with a small but statistically significant worsening (0.11-point; P < .001) in anxiety/depression symptoms following the Dobbs decision vs living in a nontrigger state, the investigators report.
Women aged 18-45 years faced greater worsening of anxiety and depression symptoms following Dobbs in trigger vs nontrigger states, whereas men of a similar age experienced minimal or negligible changes.
Implications for Care
In an accompanying editorial, Julie Steinberg, PhD, with University of Maryland in College Park, notes the study results provide “emerging evidence that at an individual level taking away reproductive autonomy (by not having legal access to an abortion) may increase symptoms of anxiety and depression in all people and particularly females of reproductive age.”
These results add to findings from two other studies that examined abortion restrictions and mental health outcomes. Both found that limiting access to abortion was associated with more mental health symptoms among females of reproductive age than among others,” Dr. Steinberg pointed out.
“Together these findings highlight the need for clinicians who practice in states where abortion is banned to be aware that female patients of reproductive age may be experiencing significantly more distress than before the Dobbs decision,” Dr. Steinberg added.
The study received no specific funding. The authors had no relevant conflicts of interest. Dr. Steinberg reported serving as a paid expert scientist on abortion and mental health in seven cases challenging abortion policies.
A version of this article appeared on Medscape.com.
, which revoked a woman’s constitutional right to an abortion, new research shows.
This could be due to a variety of factors, investigators led by Benjamin Thornburg, Johns Hopkins Bloomberg School of Public Health, Baltimore, noted. These include fear about the imminent risk of being denied an abortion, uncertainty around future limitations on abortion and other related rights such as contraception, worry over the ability to receive lifesaving medical care during pregnancy, and a general sense of violation and powerlessness related to loss of the right to reproductive autonomy.
The study was published online on January 23, 2024, in JAMA.
Mental Health Harm
In June 2022, the US Supreme Court overturned Roe vs Wade, removing federal protections for abortion rights. Thirteen states had “trigger laws” that immediately banned or severely restricted abortion — raising concerns this could negatively affect mental health.
The researchers used data from the Household Pulse Survey to estimate changes in anxiety and depression symptoms after vs before the Dobbs decision in nearly 160,000 adults living in 13 states with trigger laws compared with roughly 559,000 adults living in 37 states without trigger laws.
The mean age of respondents was 48 years, and 51% were women. Anxiety and depression symptoms were measured via the Patient Health Questionnaire-4 (PHQ-4).
In trigger states, the mean PHQ-4 score at baseline (before Dobbs) was 3.51 (out of 12) and increased to 3.81 after the Dobbs decision. In nontrigger states, the mean PHQ-4 score at baseline was 3.31 and increased to 3.49 after Dobbs.
Living in a trigger state was associated with a small but statistically significant worsening (0.11-point; P < .001) in anxiety/depression symptoms following the Dobbs decision vs living in a nontrigger state, the investigators report.
Women aged 18-45 years faced greater worsening of anxiety and depression symptoms following Dobbs in trigger vs nontrigger states, whereas men of a similar age experienced minimal or negligible changes.
Implications for Care
In an accompanying editorial, Julie Steinberg, PhD, with University of Maryland in College Park, notes the study results provide “emerging evidence that at an individual level taking away reproductive autonomy (by not having legal access to an abortion) may increase symptoms of anxiety and depression in all people and particularly females of reproductive age.”
These results add to findings from two other studies that examined abortion restrictions and mental health outcomes. Both found that limiting access to abortion was associated with more mental health symptoms among females of reproductive age than among others,” Dr. Steinberg pointed out.
“Together these findings highlight the need for clinicians who practice in states where abortion is banned to be aware that female patients of reproductive age may be experiencing significantly more distress than before the Dobbs decision,” Dr. Steinberg added.
The study received no specific funding. The authors had no relevant conflicts of interest. Dr. Steinberg reported serving as a paid expert scientist on abortion and mental health in seven cases challenging abortion policies.
A version of this article appeared on Medscape.com.
, which revoked a woman’s constitutional right to an abortion, new research shows.
This could be due to a variety of factors, investigators led by Benjamin Thornburg, Johns Hopkins Bloomberg School of Public Health, Baltimore, noted. These include fear about the imminent risk of being denied an abortion, uncertainty around future limitations on abortion and other related rights such as contraception, worry over the ability to receive lifesaving medical care during pregnancy, and a general sense of violation and powerlessness related to loss of the right to reproductive autonomy.
The study was published online on January 23, 2024, in JAMA.
Mental Health Harm
In June 2022, the US Supreme Court overturned Roe vs Wade, removing federal protections for abortion rights. Thirteen states had “trigger laws” that immediately banned or severely restricted abortion — raising concerns this could negatively affect mental health.
The researchers used data from the Household Pulse Survey to estimate changes in anxiety and depression symptoms after vs before the Dobbs decision in nearly 160,000 adults living in 13 states with trigger laws compared with roughly 559,000 adults living in 37 states without trigger laws.
The mean age of respondents was 48 years, and 51% were women. Anxiety and depression symptoms were measured via the Patient Health Questionnaire-4 (PHQ-4).
In trigger states, the mean PHQ-4 score at baseline (before Dobbs) was 3.51 (out of 12) and increased to 3.81 after the Dobbs decision. In nontrigger states, the mean PHQ-4 score at baseline was 3.31 and increased to 3.49 after Dobbs.
Living in a trigger state was associated with a small but statistically significant worsening (0.11-point; P < .001) in anxiety/depression symptoms following the Dobbs decision vs living in a nontrigger state, the investigators report.
Women aged 18-45 years faced greater worsening of anxiety and depression symptoms following Dobbs in trigger vs nontrigger states, whereas men of a similar age experienced minimal or negligible changes.
Implications for Care
In an accompanying editorial, Julie Steinberg, PhD, with University of Maryland in College Park, notes the study results provide “emerging evidence that at an individual level taking away reproductive autonomy (by not having legal access to an abortion) may increase symptoms of anxiety and depression in all people and particularly females of reproductive age.”
These results add to findings from two other studies that examined abortion restrictions and mental health outcomes. Both found that limiting access to abortion was associated with more mental health symptoms among females of reproductive age than among others,” Dr. Steinberg pointed out.
“Together these findings highlight the need for clinicians who practice in states where abortion is banned to be aware that female patients of reproductive age may be experiencing significantly more distress than before the Dobbs decision,” Dr. Steinberg added.
The study received no specific funding. The authors had no relevant conflicts of interest. Dr. Steinberg reported serving as a paid expert scientist on abortion and mental health in seven cases challenging abortion policies.
A version of this article appeared on Medscape.com.
FROM JAMA
Time to rethink endometrial ablation: A gyn oncology perspective on the sequelae of an overused procedure
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
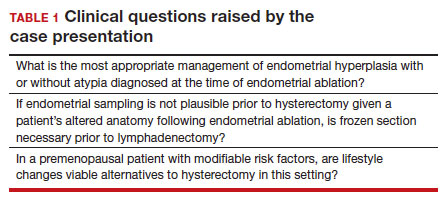
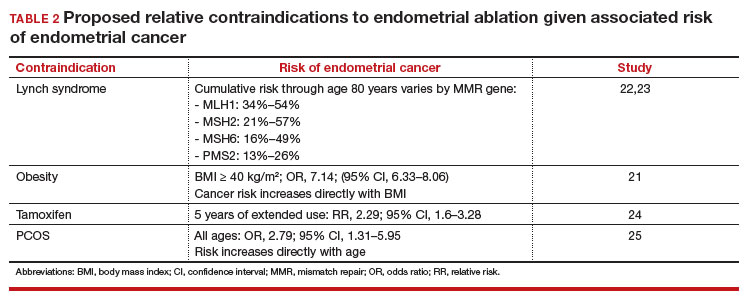
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.
Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
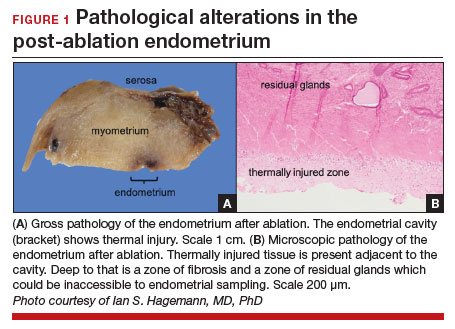
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.
Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.
Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
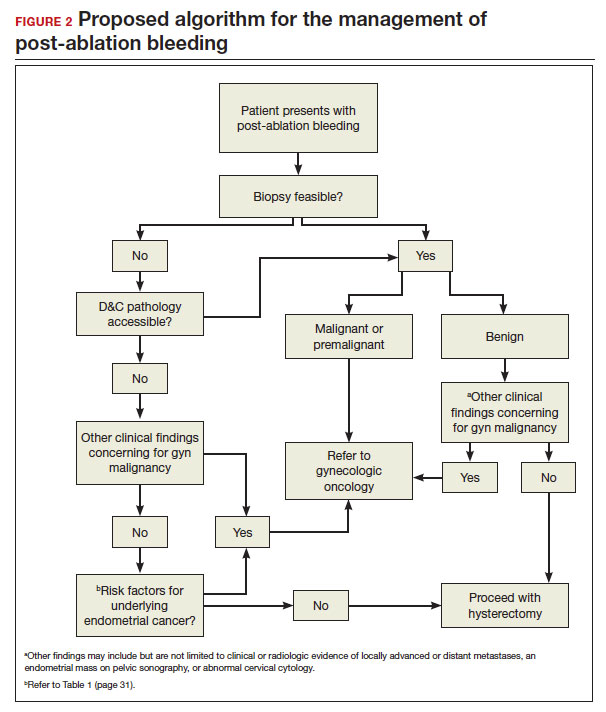
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.
CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
Elagolix curbs heavy bleeding linked to uterine leiomyomas
Uterine leiomyomas are common in premenopausal women, and 60% experience heavy menstrual bleeding, but nonsurgical options as an alternative to hysterectomy are limited, wrote Eric Brown, MD, of Gyn-Care, Atlanta, Georgia, and colleagues.
Elagolix sodium, an oral, short-acting nonpeptide, gonadotropin-releasing hormone antagonist, has been approved by the Food and Drug Administration at a dose of 300 mg twice daily with add-back therapy for up to 24 months of use. However, this treatment protocol is contraindicated or not preferable for some patients, the researchers said.
In a study published in Obstetrics & Gynecology , the researchers randomized 54 women to 150 mg of oral elagolix once daily, and 28 to a placebo for 6 months to investigate the safety and efficacy of the lower dose. The study population included women aged 18-51 years with a history of heavy menstrual bleeding association with uterine leiomyomas. Approximately two-thirds (65.9%) were Black.
The primary endpoint was the proportion of patients who met the criteria of menstrual blood loss volume less than 80 mL during the final month of treatment and menstrual blood loss volume reduction of 50% or more from baseline to the final month of treatment.
After 6 months, nearly half (49.4%) of the elagolix group met the study endpoint compared with 23.3% of the placebo group (P = .035).
Elagolix patients showed significantly greater reductions in both mean and median menstrual blood loss volumes compared with the placebo patients over the study period, and significant differences between the groups in the mean reduction of menstrual blood loss were evident after 1 month of treatment (P < .05 for months 1, 2, 3, and 5).
Results were similar in a further sensitivity analysis in which patients with incomplete final month data were considered nonresponders; 44.4% of elagolix patients and 21.4% of patients met the primary endpoint.
Overall, 51.9% of elagolix patients and 39.3% of placebo patients reported adverse events; the most common were headache and hot flush. Three patients (5.6%) in the elagolix group discontinued the drug because of adverse events. No serious or severe adverse events were reported in the elagolix group; both cases of reported serious adverse events (COVID-19 and an enlarged uvula) occurred in placebo patients.
Patient-reported outcomes were significantly greater in the elagolix patients, based on symptom severity score, 5 of 6 Uterine Fibroid Symptom and Quality of Life (UFS-QOL) health-related quality of life subscales, and the HRQOL total score at the end of the study.
The findings were limited by several factors including the small study population and lenient eligibility criteria that may have led to a higher placebo response rate, and the study did not monitor bone mineral density, the researchers noted.
However, the results suggest that elagolix at a 150-mg dose was well tolerated, with a safety profile similar to that seen in women who took the drug for endometriosis pain, and may be an option for women with contraindications to other therapy or for those who prefer once-daily dosing, they concluded.
The study was funded by AbbVie. Lead author Dr. Brown had no additional financial conflicts to disclose, but several coauthors disclosed relationships with AbbVie and other companies.
Uterine leiomyomas are common in premenopausal women, and 60% experience heavy menstrual bleeding, but nonsurgical options as an alternative to hysterectomy are limited, wrote Eric Brown, MD, of Gyn-Care, Atlanta, Georgia, and colleagues.
Elagolix sodium, an oral, short-acting nonpeptide, gonadotropin-releasing hormone antagonist, has been approved by the Food and Drug Administration at a dose of 300 mg twice daily with add-back therapy for up to 24 months of use. However, this treatment protocol is contraindicated or not preferable for some patients, the researchers said.
In a study published in Obstetrics & Gynecology , the researchers randomized 54 women to 150 mg of oral elagolix once daily, and 28 to a placebo for 6 months to investigate the safety and efficacy of the lower dose. The study population included women aged 18-51 years with a history of heavy menstrual bleeding association with uterine leiomyomas. Approximately two-thirds (65.9%) were Black.
The primary endpoint was the proportion of patients who met the criteria of menstrual blood loss volume less than 80 mL during the final month of treatment and menstrual blood loss volume reduction of 50% or more from baseline to the final month of treatment.
After 6 months, nearly half (49.4%) of the elagolix group met the study endpoint compared with 23.3% of the placebo group (P = .035).
Elagolix patients showed significantly greater reductions in both mean and median menstrual blood loss volumes compared with the placebo patients over the study period, and significant differences between the groups in the mean reduction of menstrual blood loss were evident after 1 month of treatment (P < .05 for months 1, 2, 3, and 5).
Results were similar in a further sensitivity analysis in which patients with incomplete final month data were considered nonresponders; 44.4% of elagolix patients and 21.4% of patients met the primary endpoint.
Overall, 51.9% of elagolix patients and 39.3% of placebo patients reported adverse events; the most common were headache and hot flush. Three patients (5.6%) in the elagolix group discontinued the drug because of adverse events. No serious or severe adverse events were reported in the elagolix group; both cases of reported serious adverse events (COVID-19 and an enlarged uvula) occurred in placebo patients.
Patient-reported outcomes were significantly greater in the elagolix patients, based on symptom severity score, 5 of 6 Uterine Fibroid Symptom and Quality of Life (UFS-QOL) health-related quality of life subscales, and the HRQOL total score at the end of the study.
The findings were limited by several factors including the small study population and lenient eligibility criteria that may have led to a higher placebo response rate, and the study did not monitor bone mineral density, the researchers noted.
However, the results suggest that elagolix at a 150-mg dose was well tolerated, with a safety profile similar to that seen in women who took the drug for endometriosis pain, and may be an option for women with contraindications to other therapy or for those who prefer once-daily dosing, they concluded.
The study was funded by AbbVie. Lead author Dr. Brown had no additional financial conflicts to disclose, but several coauthors disclosed relationships with AbbVie and other companies.
Uterine leiomyomas are common in premenopausal women, and 60% experience heavy menstrual bleeding, but nonsurgical options as an alternative to hysterectomy are limited, wrote Eric Brown, MD, of Gyn-Care, Atlanta, Georgia, and colleagues.
Elagolix sodium, an oral, short-acting nonpeptide, gonadotropin-releasing hormone antagonist, has been approved by the Food and Drug Administration at a dose of 300 mg twice daily with add-back therapy for up to 24 months of use. However, this treatment protocol is contraindicated or not preferable for some patients, the researchers said.
In a study published in Obstetrics & Gynecology , the researchers randomized 54 women to 150 mg of oral elagolix once daily, and 28 to a placebo for 6 months to investigate the safety and efficacy of the lower dose. The study population included women aged 18-51 years with a history of heavy menstrual bleeding association with uterine leiomyomas. Approximately two-thirds (65.9%) were Black.
The primary endpoint was the proportion of patients who met the criteria of menstrual blood loss volume less than 80 mL during the final month of treatment and menstrual blood loss volume reduction of 50% or more from baseline to the final month of treatment.
After 6 months, nearly half (49.4%) of the elagolix group met the study endpoint compared with 23.3% of the placebo group (P = .035).
Elagolix patients showed significantly greater reductions in both mean and median menstrual blood loss volumes compared with the placebo patients over the study period, and significant differences between the groups in the mean reduction of menstrual blood loss were evident after 1 month of treatment (P < .05 for months 1, 2, 3, and 5).
Results were similar in a further sensitivity analysis in which patients with incomplete final month data were considered nonresponders; 44.4% of elagolix patients and 21.4% of patients met the primary endpoint.
Overall, 51.9% of elagolix patients and 39.3% of placebo patients reported adverse events; the most common were headache and hot flush. Three patients (5.6%) in the elagolix group discontinued the drug because of adverse events. No serious or severe adverse events were reported in the elagolix group; both cases of reported serious adverse events (COVID-19 and an enlarged uvula) occurred in placebo patients.
Patient-reported outcomes were significantly greater in the elagolix patients, based on symptom severity score, 5 of 6 Uterine Fibroid Symptom and Quality of Life (UFS-QOL) health-related quality of life subscales, and the HRQOL total score at the end of the study.
The findings were limited by several factors including the small study population and lenient eligibility criteria that may have led to a higher placebo response rate, and the study did not monitor bone mineral density, the researchers noted.
However, the results suggest that elagolix at a 150-mg dose was well tolerated, with a safety profile similar to that seen in women who took the drug for endometriosis pain, and may be an option for women with contraindications to other therapy or for those who prefer once-daily dosing, they concluded.
The study was funded by AbbVie. Lead author Dr. Brown had no additional financial conflicts to disclose, but several coauthors disclosed relationships with AbbVie and other companies.
FROM OBSTETRICS & GYNECOLOGY
Early age at first period raises type 2 diabetes risk
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Despite effective therapies, fibroid care still lacking
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Bipolar disorder may raise risk of polycystic ovarian syndrome
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
New at-home test approved for chlamydia and gonorrhea
Called Simple 2, it’s the first test approved by the Food and Drug Administration that uses a sample collected at home to test for an STD, other than tests for HIV. The test can be purchased over-the-counter in stores or ordered online and delivered in discreet packaging. A vaginal swab or urine sample is collected and then sent for laboratory testing using a prepaid shipping label.
The FDA issued the final needed approval on Nov. 15, and the product is already for sale on the website of the manufacturer, LetsGetChecked. The listed price is $99 with free shipping for a single test kit, and the site offers a discounted subscription to receive a kit every 3 months for $69.30 per kit.
Gonorrhea cases have surged 28% since 2017, reaching 700,000 cases during 2021, Centers for Disease Control and Prevention data show. Chlamydia has also been on the rise, up 4% from 2020 to 2021, with 1.6 million annual infections.
Previously, tests for the two STDs required that samples be taken at a health care location such as a doctor’s office. The Simple 2 test results can be retrieved online, and a health care provider will reach out to people whose tests are positive or invalid. Results are typically received in 2-5 days, according to a press release from LetsGetChecked, which also offers treatment services.
“This authorization marks an important public health milestone, giving patients more information about their health from the privacy of their own home,” said Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a statement. “We are eager to continue supporting greater consumer access to diagnostic tests, which helps further our goal of bringing more health care into the home.”
A version of this article first appeared on WebMD.com.
Called Simple 2, it’s the first test approved by the Food and Drug Administration that uses a sample collected at home to test for an STD, other than tests for HIV. The test can be purchased over-the-counter in stores or ordered online and delivered in discreet packaging. A vaginal swab or urine sample is collected and then sent for laboratory testing using a prepaid shipping label.
The FDA issued the final needed approval on Nov. 15, and the product is already for sale on the website of the manufacturer, LetsGetChecked. The listed price is $99 with free shipping for a single test kit, and the site offers a discounted subscription to receive a kit every 3 months for $69.30 per kit.
Gonorrhea cases have surged 28% since 2017, reaching 700,000 cases during 2021, Centers for Disease Control and Prevention data show. Chlamydia has also been on the rise, up 4% from 2020 to 2021, with 1.6 million annual infections.
Previously, tests for the two STDs required that samples be taken at a health care location such as a doctor’s office. The Simple 2 test results can be retrieved online, and a health care provider will reach out to people whose tests are positive or invalid. Results are typically received in 2-5 days, according to a press release from LetsGetChecked, which also offers treatment services.
“This authorization marks an important public health milestone, giving patients more information about their health from the privacy of their own home,” said Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a statement. “We are eager to continue supporting greater consumer access to diagnostic tests, which helps further our goal of bringing more health care into the home.”
A version of this article first appeared on WebMD.com.
Called Simple 2, it’s the first test approved by the Food and Drug Administration that uses a sample collected at home to test for an STD, other than tests for HIV. The test can be purchased over-the-counter in stores or ordered online and delivered in discreet packaging. A vaginal swab or urine sample is collected and then sent for laboratory testing using a prepaid shipping label.
The FDA issued the final needed approval on Nov. 15, and the product is already for sale on the website of the manufacturer, LetsGetChecked. The listed price is $99 with free shipping for a single test kit, and the site offers a discounted subscription to receive a kit every 3 months for $69.30 per kit.
Gonorrhea cases have surged 28% since 2017, reaching 700,000 cases during 2021, Centers for Disease Control and Prevention data show. Chlamydia has also been on the rise, up 4% from 2020 to 2021, with 1.6 million annual infections.
Previously, tests for the two STDs required that samples be taken at a health care location such as a doctor’s office. The Simple 2 test results can be retrieved online, and a health care provider will reach out to people whose tests are positive or invalid. Results are typically received in 2-5 days, according to a press release from LetsGetChecked, which also offers treatment services.
“This authorization marks an important public health milestone, giving patients more information about their health from the privacy of their own home,” said Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a statement. “We are eager to continue supporting greater consumer access to diagnostic tests, which helps further our goal of bringing more health care into the home.”
A version of this article first appeared on WebMD.com.
A nurse’s view: Women desperately need information about pelvic floor disorders
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Is the 9-valent HPV vaccine safe and effective long term?
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
