User login
‘Uptake is only the first step’ for effective HIV PrEP protection
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
Half of women treated for gynecologic cancers miss or skip doses of oral drugs
Oral agents are taking on an ever-greater role in the management of gynecologic cancers. However, women being treated for these cancers have less than ideal adherence to such medications, a new study has found – with just over half reporting taking them exactly as prescribed.
The findings reported in Obstetrics & Gynecology are consistent with reports from other populations taking oral anticancer agents, in which adherence is generally lower than with intravenous therapies.
For their research, Catherine Watson, MD, and colleagues at Duke University, Durham, N.C., recruited 100 women at their institution taking a variety of oral anticancer agents for uterine or ovarian cancer for 30 days or more (median time, 6 months). The women answered a questionnaire that measured adherence as well as health literacy, quality of life, and distress. The researchers also collected information on the subjects’ race, age, insurance type, medication burden, and medication costs.
Fourteen of the women in the study additionally underwent qualitative interviews about their experiences with oral anticancer drugs. The researchers also queried physicians and nurse practitioners about their thoughts on adherence.
Dr. Watson and colleagues reported that 54% of women self-reported perfect adherence to their medication in the previous week, while 21% had missed or skipped one dose, and 25% reported skipping or missing more than one dose.
The researchers saw no significant differences between the adherent and nonadherent groups corresponding with race, age, or other demographic or clinical characteristics, but they noted that their study was not powered to detect such associations. The small sample size and self-reported data were among this study’s limitations, Dr. Watson and colleagues acknowledged.
Interviews with patients revealed some surprising reasons for the less-than-optimal adherence, with 43% of women reporting feeling anxiety about the burden of administering medication at home. While patients acknowledged the convenience of oral regimens, some also expressed a wish for more physician contact and support. Some women who were nonadherent said they perceived the efficacy of oral agents to be less than intravenous therapies.
Physicians and nurse practitioners interviewed by the researchers “tended to assume that their patients were adherent to oral anticancer therapy because of the therapy’s importance, and many did not routinely ask their patients about adherence,” Dr. Watson and colleagues wrote.
Emma Rossi, MD, a gynecologic oncologist at the University of North Carolina at Chapel Hill, commented in an interview that the study highlighted “the importance of not generalizing our perception of patients. As providers we have to spend the time asking patients where they’re at and how they’re thinking about taking an oral medication, with special attention to their fears, their expectations, and their concerns. We should be touching base with them not just before starting drugs, but during the course of treatment.”
Dr. Rossi stressed that compliance in real-life settings is likely to be different from that seen in clinical trials of oral anticancer drugs. “It’s important to recognize that real-world efficacy of treatments may be different from trial efficacy. We see these differences a lot in medicine where studies show a large magnitude of effect that doesn’t play out in real life practice – because of factors like this.”
Some 57% of the women in the study were taking their medications as maintenance, while the rest were taking the medications as active treatment. This too might have an effect on adherence, she said, with the active-treatment group potentially more motivated to maintain perfect adherence.
“You see in the interviews that doctors assume patients would be compliant because of the seriousness of the disease,” Dr. Rossi said. “But some patients said they perceived oral drugs as less strong or effective. If a patient is cancer free on a scan and doesn’t have measurable disease, prescribing an oral medication may be sending the subliminal message that it’s not as important.”
Physicians “may need to take the extra steps to individualize our counseling of patients – especially with therapies that they’re responsible for administering,” Dr. Rossi continued. “As this study shows, every patient sees treatment through her own individual lens. We really need to meet them where they’re at to make them comfortable with their treatment and optimize compliance.”
Dr. Watson and colleagues’ study was supported by their institution. One coauthor reported financial ties with drug manufacturers in the form of grant and clinical trial support and honoraria. Another coauthor is the member of various boards and steering committees, which are uncompensated. Dr. Rossi reported no financial conflicts of interest.
SOURCE: Watson C et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004170.
Oral agents are taking on an ever-greater role in the management of gynecologic cancers. However, women being treated for these cancers have less than ideal adherence to such medications, a new study has found – with just over half reporting taking them exactly as prescribed.
The findings reported in Obstetrics & Gynecology are consistent with reports from other populations taking oral anticancer agents, in which adherence is generally lower than with intravenous therapies.
For their research, Catherine Watson, MD, and colleagues at Duke University, Durham, N.C., recruited 100 women at their institution taking a variety of oral anticancer agents for uterine or ovarian cancer for 30 days or more (median time, 6 months). The women answered a questionnaire that measured adherence as well as health literacy, quality of life, and distress. The researchers also collected information on the subjects’ race, age, insurance type, medication burden, and medication costs.
Fourteen of the women in the study additionally underwent qualitative interviews about their experiences with oral anticancer drugs. The researchers also queried physicians and nurse practitioners about their thoughts on adherence.
Dr. Watson and colleagues reported that 54% of women self-reported perfect adherence to their medication in the previous week, while 21% had missed or skipped one dose, and 25% reported skipping or missing more than one dose.
The researchers saw no significant differences between the adherent and nonadherent groups corresponding with race, age, or other demographic or clinical characteristics, but they noted that their study was not powered to detect such associations. The small sample size and self-reported data were among this study’s limitations, Dr. Watson and colleagues acknowledged.
Interviews with patients revealed some surprising reasons for the less-than-optimal adherence, with 43% of women reporting feeling anxiety about the burden of administering medication at home. While patients acknowledged the convenience of oral regimens, some also expressed a wish for more physician contact and support. Some women who were nonadherent said they perceived the efficacy of oral agents to be less than intravenous therapies.
Physicians and nurse practitioners interviewed by the researchers “tended to assume that their patients were adherent to oral anticancer therapy because of the therapy’s importance, and many did not routinely ask their patients about adherence,” Dr. Watson and colleagues wrote.
Emma Rossi, MD, a gynecologic oncologist at the University of North Carolina at Chapel Hill, commented in an interview that the study highlighted “the importance of not generalizing our perception of patients. As providers we have to spend the time asking patients where they’re at and how they’re thinking about taking an oral medication, with special attention to their fears, their expectations, and their concerns. We should be touching base with them not just before starting drugs, but during the course of treatment.”
Dr. Rossi stressed that compliance in real-life settings is likely to be different from that seen in clinical trials of oral anticancer drugs. “It’s important to recognize that real-world efficacy of treatments may be different from trial efficacy. We see these differences a lot in medicine where studies show a large magnitude of effect that doesn’t play out in real life practice – because of factors like this.”
Some 57% of the women in the study were taking their medications as maintenance, while the rest were taking the medications as active treatment. This too might have an effect on adherence, she said, with the active-treatment group potentially more motivated to maintain perfect adherence.
“You see in the interviews that doctors assume patients would be compliant because of the seriousness of the disease,” Dr. Rossi said. “But some patients said they perceived oral drugs as less strong or effective. If a patient is cancer free on a scan and doesn’t have measurable disease, prescribing an oral medication may be sending the subliminal message that it’s not as important.”
Physicians “may need to take the extra steps to individualize our counseling of patients – especially with therapies that they’re responsible for administering,” Dr. Rossi continued. “As this study shows, every patient sees treatment through her own individual lens. We really need to meet them where they’re at to make them comfortable with their treatment and optimize compliance.”
Dr. Watson and colleagues’ study was supported by their institution. One coauthor reported financial ties with drug manufacturers in the form of grant and clinical trial support and honoraria. Another coauthor is the member of various boards and steering committees, which are uncompensated. Dr. Rossi reported no financial conflicts of interest.
SOURCE: Watson C et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004170.
Oral agents are taking on an ever-greater role in the management of gynecologic cancers. However, women being treated for these cancers have less than ideal adherence to such medications, a new study has found – with just over half reporting taking them exactly as prescribed.
The findings reported in Obstetrics & Gynecology are consistent with reports from other populations taking oral anticancer agents, in which adherence is generally lower than with intravenous therapies.
For their research, Catherine Watson, MD, and colleagues at Duke University, Durham, N.C., recruited 100 women at their institution taking a variety of oral anticancer agents for uterine or ovarian cancer for 30 days or more (median time, 6 months). The women answered a questionnaire that measured adherence as well as health literacy, quality of life, and distress. The researchers also collected information on the subjects’ race, age, insurance type, medication burden, and medication costs.
Fourteen of the women in the study additionally underwent qualitative interviews about their experiences with oral anticancer drugs. The researchers also queried physicians and nurse practitioners about their thoughts on adherence.
Dr. Watson and colleagues reported that 54% of women self-reported perfect adherence to their medication in the previous week, while 21% had missed or skipped one dose, and 25% reported skipping or missing more than one dose.
The researchers saw no significant differences between the adherent and nonadherent groups corresponding with race, age, or other demographic or clinical characteristics, but they noted that their study was not powered to detect such associations. The small sample size and self-reported data were among this study’s limitations, Dr. Watson and colleagues acknowledged.
Interviews with patients revealed some surprising reasons for the less-than-optimal adherence, with 43% of women reporting feeling anxiety about the burden of administering medication at home. While patients acknowledged the convenience of oral regimens, some also expressed a wish for more physician contact and support. Some women who were nonadherent said they perceived the efficacy of oral agents to be less than intravenous therapies.
Physicians and nurse practitioners interviewed by the researchers “tended to assume that their patients were adherent to oral anticancer therapy because of the therapy’s importance, and many did not routinely ask their patients about adherence,” Dr. Watson and colleagues wrote.
Emma Rossi, MD, a gynecologic oncologist at the University of North Carolina at Chapel Hill, commented in an interview that the study highlighted “the importance of not generalizing our perception of patients. As providers we have to spend the time asking patients where they’re at and how they’re thinking about taking an oral medication, with special attention to their fears, their expectations, and their concerns. We should be touching base with them not just before starting drugs, but during the course of treatment.”
Dr. Rossi stressed that compliance in real-life settings is likely to be different from that seen in clinical trials of oral anticancer drugs. “It’s important to recognize that real-world efficacy of treatments may be different from trial efficacy. We see these differences a lot in medicine where studies show a large magnitude of effect that doesn’t play out in real life practice – because of factors like this.”
Some 57% of the women in the study were taking their medications as maintenance, while the rest were taking the medications as active treatment. This too might have an effect on adherence, she said, with the active-treatment group potentially more motivated to maintain perfect adherence.
“You see in the interviews that doctors assume patients would be compliant because of the seriousness of the disease,” Dr. Rossi said. “But some patients said they perceived oral drugs as less strong or effective. If a patient is cancer free on a scan and doesn’t have measurable disease, prescribing an oral medication may be sending the subliminal message that it’s not as important.”
Physicians “may need to take the extra steps to individualize our counseling of patients – especially with therapies that they’re responsible for administering,” Dr. Rossi continued. “As this study shows, every patient sees treatment through her own individual lens. We really need to meet them where they’re at to make them comfortable with their treatment and optimize compliance.”
Dr. Watson and colleagues’ study was supported by their institution. One coauthor reported financial ties with drug manufacturers in the form of grant and clinical trial support and honoraria. Another coauthor is the member of various boards and steering committees, which are uncompensated. Dr. Rossi reported no financial conflicts of interest.
SOURCE: Watson C et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004170.
FROM OBSTETRICS & GYNECOLOGY
Sleep apnea may correlate with anxiety, depression in patients with PCOS
a study suggests.
This finding could have implications for screening and treatment, Diana Xiaojie Zhou, MD, said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“Routine OSA screening in women with PCOS should be considered in the setting of existing depression and anxiety,” said Dr. Zhou, a reproductive endocrinology and infertility fellow at the University of California, San Francisco. “Referral for OSA diagnosis and treatment in those who screen positive may have added psychological benefits in this population, as has been seen in the general population.”
Patients with PCOS experience a range of comorbidities, including higher rates of psychological disorders and OSA, she said.
OSA has been associated with depression and anxiety in the general population, and research indicates that treatment, such as with continuous positive airway pressure (CPAP), may have psychological benefits, such as reduced depression symptoms.
PCOS guidelines recommend screening for OSA to identify and alleviate symptoms such as fatigue that may to contribute to mood disorders. “However, there is a lack of studies assessing the relationship between OSA and depression and anxiety specifically in women with PCOS,” Dr. Zhou said.
A cross-sectional study
To evaluate whether OSA is associated with depression and anxiety in women with PCOS, Dr. Zhou and colleagues conducted a cross-sectional study of all women seen at a multidisciplinary PCOS clinic at university between June 2017 and June 2020.
Participants had a diagnosis of PCOS clinically confirmed by the Rotterdam criteria. Researchers determined OSA risk using the Berlin questionnaire, which is divided into three domains. A positive score in two or more domains indicates a high risk of OSA.
The investigators used the Patient Health Questionnaire-9 (PHQ-9) to assess depression symptoms, and they used the Generalized Anxiety Disorder-7 (GAD-7) to assess anxiety symptoms.
Researchers used two-sided t-test, chi-square test, and Fisher’s exact test to evaluate for differences in patient characteristics. They performed multivariate logistic regression analyses to determine the odds of moderate to severe symptoms of depression (that is, a PHQ-9 score of 10 or greater) and anxiety (a GAD-7 score of 10 or greater) among patients with a high risk of OSA, compared with patients with a low risk of OSA. They adjusted for age, body mass index, free testosterone level, and insulin resistance using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
The researchers examined data from 201 patients: 125 with a low risk of OSA and 76 with a high risk of OSA. The average age of the patients was 28 years.
On average, patients in the high-risk OSA group had a greater body mass index (37.9 vs. 26.5), a higher level of free testosterone (6.5 ng/dL vs. 4.5 ng/dL), and a higher HOMA-IR score (7 vs. 3.1), relative to those with a low risk of OSA. In addition, a greater percentage of patients with a high risk of OSA experienced oligomenorrhea (84.9% vs. 70.5%).
The average PHQ-9 score was significantly higher in the high-risk OSA group (12 vs. 8.3), as was the average GAD-7 score (8.9 vs. 6.1).
In univariate analyses, having a high risk of OSA increased the likelihood of moderate or severe depression or anxiety approximately threefold.
In multivariate analyses, a high risk of OSA remained significantly associated with moderate or severe depression or anxiety, with an odds ratio of about 2.5. “Of note, BMI was a statistically significant predictor in the univariate analyses, but not so in the multivariate analyses,” Dr. Zhou said.
Although the investigators assessed OSA, depression, and anxiety using validated questionnaires, a study with clinically confirmed diagnoses of those conditions would strengthen these findings, she said.
Various possible links
Investigators have proposed various links between PCOS, OSA, and depression and anxiety, Dr. Zhou noted. Features of PCOS such as insulin resistance, obesity, and hyperandrogenemia increase the risk of OSA. “The sleep loss and fragmentation and hypoxia that define OSA then serve to increase sympathetic tone and oxidative stress, which then potentially can lead to an increase in depression and anxiety,” Dr. Zhou said.
The results suggests that treating OSA “may have added psychological benefits for women with PCOS and highlights the broad health implications of this condition,” Marla Lujan, PhD, chair of the ASRM’s androgen excess special interest group, said in a society news release.
“The cause of PCOS is still not well understood, but we do know that 1 in 10 women in their childbearing years suffer from PCOS,” said Dr. Lujan, of Cornell University, Ithaca, N.Y. “In addition to infertility, PCOS is also associated with type 2 diabetes and cardiovascular complications such as hypertension and abnormal blood lipids.”
In a discussion following Dr. Zhou’s presentation, Alice D. Domar, PhD, said the study was eye opening.
Dr. Domar, director of integrative care at Boston IVF and associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, said that she does not typically discuss sleep apnea with patients. “For those of us who routinely work with PCOS patients, we are always looking for more information.”
Although PCOS guidelines mention screening for OSA, Dr. Zhou expects that few generalists who see PCOS patients or even subspecialists actually do.
Nevertheless, the potential for intervention is fascinating, she said. And if treating OSA also reduced a patient’s need for psychiatric medications, there could be added benefit in PCOS due to the metabolic side effects that accompany some of the drugs.
Dr. Zhou and Dr. Lujan had no relevant disclosures. Dr. Domar is a co-owner of FertiCalm, FertiStrong, and Aliz Health Apps, and a speaker for Ferring, EMD Serono, Merck, and Abbott.
SOURCE: Zhou DX et al. ASRM 2020. Abstract O-146.
a study suggests.
This finding could have implications for screening and treatment, Diana Xiaojie Zhou, MD, said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“Routine OSA screening in women with PCOS should be considered in the setting of existing depression and anxiety,” said Dr. Zhou, a reproductive endocrinology and infertility fellow at the University of California, San Francisco. “Referral for OSA diagnosis and treatment in those who screen positive may have added psychological benefits in this population, as has been seen in the general population.”
Patients with PCOS experience a range of comorbidities, including higher rates of psychological disorders and OSA, she said.
OSA has been associated with depression and anxiety in the general population, and research indicates that treatment, such as with continuous positive airway pressure (CPAP), may have psychological benefits, such as reduced depression symptoms.
PCOS guidelines recommend screening for OSA to identify and alleviate symptoms such as fatigue that may to contribute to mood disorders. “However, there is a lack of studies assessing the relationship between OSA and depression and anxiety specifically in women with PCOS,” Dr. Zhou said.
A cross-sectional study
To evaluate whether OSA is associated with depression and anxiety in women with PCOS, Dr. Zhou and colleagues conducted a cross-sectional study of all women seen at a multidisciplinary PCOS clinic at university between June 2017 and June 2020.
Participants had a diagnosis of PCOS clinically confirmed by the Rotterdam criteria. Researchers determined OSA risk using the Berlin questionnaire, which is divided into three domains. A positive score in two or more domains indicates a high risk of OSA.
The investigators used the Patient Health Questionnaire-9 (PHQ-9) to assess depression symptoms, and they used the Generalized Anxiety Disorder-7 (GAD-7) to assess anxiety symptoms.
Researchers used two-sided t-test, chi-square test, and Fisher’s exact test to evaluate for differences in patient characteristics. They performed multivariate logistic regression analyses to determine the odds of moderate to severe symptoms of depression (that is, a PHQ-9 score of 10 or greater) and anxiety (a GAD-7 score of 10 or greater) among patients with a high risk of OSA, compared with patients with a low risk of OSA. They adjusted for age, body mass index, free testosterone level, and insulin resistance using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
The researchers examined data from 201 patients: 125 with a low risk of OSA and 76 with a high risk of OSA. The average age of the patients was 28 years.
On average, patients in the high-risk OSA group had a greater body mass index (37.9 vs. 26.5), a higher level of free testosterone (6.5 ng/dL vs. 4.5 ng/dL), and a higher HOMA-IR score (7 vs. 3.1), relative to those with a low risk of OSA. In addition, a greater percentage of patients with a high risk of OSA experienced oligomenorrhea (84.9% vs. 70.5%).
The average PHQ-9 score was significantly higher in the high-risk OSA group (12 vs. 8.3), as was the average GAD-7 score (8.9 vs. 6.1).
In univariate analyses, having a high risk of OSA increased the likelihood of moderate or severe depression or anxiety approximately threefold.
In multivariate analyses, a high risk of OSA remained significantly associated with moderate or severe depression or anxiety, with an odds ratio of about 2.5. “Of note, BMI was a statistically significant predictor in the univariate analyses, but not so in the multivariate analyses,” Dr. Zhou said.
Although the investigators assessed OSA, depression, and anxiety using validated questionnaires, a study with clinically confirmed diagnoses of those conditions would strengthen these findings, she said.
Various possible links
Investigators have proposed various links between PCOS, OSA, and depression and anxiety, Dr. Zhou noted. Features of PCOS such as insulin resistance, obesity, and hyperandrogenemia increase the risk of OSA. “The sleep loss and fragmentation and hypoxia that define OSA then serve to increase sympathetic tone and oxidative stress, which then potentially can lead to an increase in depression and anxiety,” Dr. Zhou said.
The results suggests that treating OSA “may have added psychological benefits for women with PCOS and highlights the broad health implications of this condition,” Marla Lujan, PhD, chair of the ASRM’s androgen excess special interest group, said in a society news release.
“The cause of PCOS is still not well understood, but we do know that 1 in 10 women in their childbearing years suffer from PCOS,” said Dr. Lujan, of Cornell University, Ithaca, N.Y. “In addition to infertility, PCOS is also associated with type 2 diabetes and cardiovascular complications such as hypertension and abnormal blood lipids.”
In a discussion following Dr. Zhou’s presentation, Alice D. Domar, PhD, said the study was eye opening.
Dr. Domar, director of integrative care at Boston IVF and associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, said that she does not typically discuss sleep apnea with patients. “For those of us who routinely work with PCOS patients, we are always looking for more information.”
Although PCOS guidelines mention screening for OSA, Dr. Zhou expects that few generalists who see PCOS patients or even subspecialists actually do.
Nevertheless, the potential for intervention is fascinating, she said. And if treating OSA also reduced a patient’s need for psychiatric medications, there could be added benefit in PCOS due to the metabolic side effects that accompany some of the drugs.
Dr. Zhou and Dr. Lujan had no relevant disclosures. Dr. Domar is a co-owner of FertiCalm, FertiStrong, and Aliz Health Apps, and a speaker for Ferring, EMD Serono, Merck, and Abbott.
SOURCE: Zhou DX et al. ASRM 2020. Abstract O-146.
a study suggests.
This finding could have implications for screening and treatment, Diana Xiaojie Zhou, MD, said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“Routine OSA screening in women with PCOS should be considered in the setting of existing depression and anxiety,” said Dr. Zhou, a reproductive endocrinology and infertility fellow at the University of California, San Francisco. “Referral for OSA diagnosis and treatment in those who screen positive may have added psychological benefits in this population, as has been seen in the general population.”
Patients with PCOS experience a range of comorbidities, including higher rates of psychological disorders and OSA, she said.
OSA has been associated with depression and anxiety in the general population, and research indicates that treatment, such as with continuous positive airway pressure (CPAP), may have psychological benefits, such as reduced depression symptoms.
PCOS guidelines recommend screening for OSA to identify and alleviate symptoms such as fatigue that may to contribute to mood disorders. “However, there is a lack of studies assessing the relationship between OSA and depression and anxiety specifically in women with PCOS,” Dr. Zhou said.
A cross-sectional study
To evaluate whether OSA is associated with depression and anxiety in women with PCOS, Dr. Zhou and colleagues conducted a cross-sectional study of all women seen at a multidisciplinary PCOS clinic at university between June 2017 and June 2020.
Participants had a diagnosis of PCOS clinically confirmed by the Rotterdam criteria. Researchers determined OSA risk using the Berlin questionnaire, which is divided into three domains. A positive score in two or more domains indicates a high risk of OSA.
The investigators used the Patient Health Questionnaire-9 (PHQ-9) to assess depression symptoms, and they used the Generalized Anxiety Disorder-7 (GAD-7) to assess anxiety symptoms.
Researchers used two-sided t-test, chi-square test, and Fisher’s exact test to evaluate for differences in patient characteristics. They performed multivariate logistic regression analyses to determine the odds of moderate to severe symptoms of depression (that is, a PHQ-9 score of 10 or greater) and anxiety (a GAD-7 score of 10 or greater) among patients with a high risk of OSA, compared with patients with a low risk of OSA. They adjusted for age, body mass index, free testosterone level, and insulin resistance using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
The researchers examined data from 201 patients: 125 with a low risk of OSA and 76 with a high risk of OSA. The average age of the patients was 28 years.
On average, patients in the high-risk OSA group had a greater body mass index (37.9 vs. 26.5), a higher level of free testosterone (6.5 ng/dL vs. 4.5 ng/dL), and a higher HOMA-IR score (7 vs. 3.1), relative to those with a low risk of OSA. In addition, a greater percentage of patients with a high risk of OSA experienced oligomenorrhea (84.9% vs. 70.5%).
The average PHQ-9 score was significantly higher in the high-risk OSA group (12 vs. 8.3), as was the average GAD-7 score (8.9 vs. 6.1).
In univariate analyses, having a high risk of OSA increased the likelihood of moderate or severe depression or anxiety approximately threefold.
In multivariate analyses, a high risk of OSA remained significantly associated with moderate or severe depression or anxiety, with an odds ratio of about 2.5. “Of note, BMI was a statistically significant predictor in the univariate analyses, but not so in the multivariate analyses,” Dr. Zhou said.
Although the investigators assessed OSA, depression, and anxiety using validated questionnaires, a study with clinically confirmed diagnoses of those conditions would strengthen these findings, she said.
Various possible links
Investigators have proposed various links between PCOS, OSA, and depression and anxiety, Dr. Zhou noted. Features of PCOS such as insulin resistance, obesity, and hyperandrogenemia increase the risk of OSA. “The sleep loss and fragmentation and hypoxia that define OSA then serve to increase sympathetic tone and oxidative stress, which then potentially can lead to an increase in depression and anxiety,” Dr. Zhou said.
The results suggests that treating OSA “may have added psychological benefits for women with PCOS and highlights the broad health implications of this condition,” Marla Lujan, PhD, chair of the ASRM’s androgen excess special interest group, said in a society news release.
“The cause of PCOS is still not well understood, but we do know that 1 in 10 women in their childbearing years suffer from PCOS,” said Dr. Lujan, of Cornell University, Ithaca, N.Y. “In addition to infertility, PCOS is also associated with type 2 diabetes and cardiovascular complications such as hypertension and abnormal blood lipids.”
In a discussion following Dr. Zhou’s presentation, Alice D. Domar, PhD, said the study was eye opening.
Dr. Domar, director of integrative care at Boston IVF and associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, said that she does not typically discuss sleep apnea with patients. “For those of us who routinely work with PCOS patients, we are always looking for more information.”
Although PCOS guidelines mention screening for OSA, Dr. Zhou expects that few generalists who see PCOS patients or even subspecialists actually do.
Nevertheless, the potential for intervention is fascinating, she said. And if treating OSA also reduced a patient’s need for psychiatric medications, there could be added benefit in PCOS due to the metabolic side effects that accompany some of the drugs.
Dr. Zhou and Dr. Lujan had no relevant disclosures. Dr. Domar is a co-owner of FertiCalm, FertiStrong, and Aliz Health Apps, and a speaker for Ferring, EMD Serono, Merck, and Abbott.
SOURCE: Zhou DX et al. ASRM 2020. Abstract O-146.
FROM ASRM 2020
Studies gauge toll of pausing fertility treatment during pandemic
More than 60% of patients at a center for reproductive medicine in Utah who had fertility treatments canceled because of the COVID-19 pandemic opted to resume treatment once the suspension was lifted about 7 weeks later.
At another fertility center in New York, a survey found that 96% of respondents who had a cycle canceled because of the pandemic found it upsetting, and 22% found it extremely upsetting, with extremely upsetting defined as equivalent to the loss of a child.
The indefinite time frame for resuming treatment when the New York survey was conducted may have been a major source of distress for patients, one of the researchers said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“They don’t know when they might have that chance again,” said Jenna M. Turocy, MD, of Columbia University Fertility Center, New York.
COVID-19 guidelines published by ASRM on March 17 recommended the suspension of new treatment cycles, including ovulation induction, intrauterine inseminations, and in vitro fertilization (IVF).
An ASRM COVID-19 task force has since supported “the measured resumption of fertility care following the easing of restrictions,” said Paul C. Lin, MD, president of the Society for Assisted Reproductive Technology and a member of the task force.
“Over the past several months, significant knowledge has been gained regarding the COVID-19 virus and its impact on patients and the medical system,” he said in a news release about the two studies that assessed the pandemic’s effects.
Certain precautions remain. “It has become clear that we will need to be practicing COVID-19 protocols at least until an effective and safe vaccine or broadly effective treatment becomes widely available,” Dr. Lin said.
Desire to proceed during a pandemic
The center continued to offer IVF cycles for oncofertility patients on an urgent basis.
In early May, patients whose cycles had been suspended had the option to receive treatment.
“Upon reopening, every patient received standardized counseling from their primary IVF physician,” Lauren Verrilli, MD, a reproductive endocrinology and infertility fellow at the University of Utah, Salt Lake City, said at the virtual meeting.
Doctors explained that much remained unknown about COVID-19 in pregnancy, and that it was unclear whether the clinic would need to shut down again. In addition, patients had to undergo COVID-19 testing.
To identify factors associated with proceeding with treatment after the suspension, the researchers compared patients who resumed treatment with patients who did not.
Their analysis included 278 patients who had planned an IVF cycle or frozen embryo transfer (FET) prior to the shutdown. The researchers examined factors such as age, parity, anti-Müllerian hormone, antral follicle count, history of prior IVF cycles or FET, number of frozen blastocysts, gamete source, and use of a gestational carrier.
In all, 62% of patients opted to receive treatment once restrictions were lifted, including 69 of the 133 (52%) patients with planned fresh cycles and 104 of the 145 (72%) patients with planned FET cycles.
Among those with planned fresh cycles, those who opted to resume treatment tended to be older than those who did not resume treatment, with a median age of 37 years versus 35 years, but the difference was not statistically significant.
Among patients with planned FET cycles, those who did not resume treatment were more likely to have a gestational carrier, compared with those who resumed treatment (7% vs. 1%). In some cases, gestational carriers lived in another state and the pandemic complicated travel arrangements, which contributed to delays, Dr. Verrilli said.
The analysis did not include information about income or socioeconomic status, which may play a role in patients’ decisions, Dr. Verrilli said.
Emotional impact of indefinite delay
Fertility treatment is often time sensitive, particularly for patients with advanced reproductive age or diminished ovarian reserve, and indefinite postponement of fertility treatment potentially could lead some patients to lose the ability to conceive with their own gametes, Dr. Turocy said.
In early April, Dr. Turocy and colleagues surveyed patients at their academic fertility center in New York City to assess patients’ reactions to the ASRM recommendations. They decided to conduct the study after they realized that treatment cancellations were having a significant emotional effect.
Investigators emailed an 18-item survey to more than 3,000 patients.
In all, 518 patients completed the survey, a response rate of 17%. Patients had an average age of 37 years (range, 23-52 years), and 92% were female. About 24% had children, and 66% had received at least one fertility treatment.
Half had a cycle canceled because of the COVID-19 pandemic, including timed intercourse cycles (5%), intrauterine insemination cycles (23%), IVF cycles with a planned fresh embryo transfer (10%), IVF with all frozen embryos (27%), egg freeze cycles (3%), and FET cycles (30%).
In response to survey questions about whether they agreed with ASRM recommendations, “the reactions were mixed,” Dr. Turocy said.
About 36% of patients agreed all fertility cycles should be canceled, 22% were unsure, and 43% disagreed with the recommendation. Patients who had a cycle canceled were slightly more likely to agree with the cancellations (40% agreed) than those who did not have a cycle canceled (30% agreed), Dr. Turocy said.
Most respondents would have preferred an option to start a treatment cycle in consultation with their doctor. Half “would have chosen to start a new cycle during the height of the pandemic in New York City,” Dr. Turocy said.
Patient opinions may vary by region and depend on the severity of COVID-19 outbreaks there, and they also might change over time, Dr. Turocy suggested. In addition, the opinions and characteristics of patients who responded to the anonymous survey may differ from those of patients who did not respond.
Dr. Verrilli, Dr. Turocy, and Dr. Lin had no relevant financial disclosures.
More than 60% of patients at a center for reproductive medicine in Utah who had fertility treatments canceled because of the COVID-19 pandemic opted to resume treatment once the suspension was lifted about 7 weeks later.
At another fertility center in New York, a survey found that 96% of respondents who had a cycle canceled because of the pandemic found it upsetting, and 22% found it extremely upsetting, with extremely upsetting defined as equivalent to the loss of a child.
The indefinite time frame for resuming treatment when the New York survey was conducted may have been a major source of distress for patients, one of the researchers said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“They don’t know when they might have that chance again,” said Jenna M. Turocy, MD, of Columbia University Fertility Center, New York.
COVID-19 guidelines published by ASRM on March 17 recommended the suspension of new treatment cycles, including ovulation induction, intrauterine inseminations, and in vitro fertilization (IVF).
An ASRM COVID-19 task force has since supported “the measured resumption of fertility care following the easing of restrictions,” said Paul C. Lin, MD, president of the Society for Assisted Reproductive Technology and a member of the task force.
“Over the past several months, significant knowledge has been gained regarding the COVID-19 virus and its impact on patients and the medical system,” he said in a news release about the two studies that assessed the pandemic’s effects.
Certain precautions remain. “It has become clear that we will need to be practicing COVID-19 protocols at least until an effective and safe vaccine or broadly effective treatment becomes widely available,” Dr. Lin said.
Desire to proceed during a pandemic
The center continued to offer IVF cycles for oncofertility patients on an urgent basis.
In early May, patients whose cycles had been suspended had the option to receive treatment.
“Upon reopening, every patient received standardized counseling from their primary IVF physician,” Lauren Verrilli, MD, a reproductive endocrinology and infertility fellow at the University of Utah, Salt Lake City, said at the virtual meeting.
Doctors explained that much remained unknown about COVID-19 in pregnancy, and that it was unclear whether the clinic would need to shut down again. In addition, patients had to undergo COVID-19 testing.
To identify factors associated with proceeding with treatment after the suspension, the researchers compared patients who resumed treatment with patients who did not.
Their analysis included 278 patients who had planned an IVF cycle or frozen embryo transfer (FET) prior to the shutdown. The researchers examined factors such as age, parity, anti-Müllerian hormone, antral follicle count, history of prior IVF cycles or FET, number of frozen blastocysts, gamete source, and use of a gestational carrier.
In all, 62% of patients opted to receive treatment once restrictions were lifted, including 69 of the 133 (52%) patients with planned fresh cycles and 104 of the 145 (72%) patients with planned FET cycles.
Among those with planned fresh cycles, those who opted to resume treatment tended to be older than those who did not resume treatment, with a median age of 37 years versus 35 years, but the difference was not statistically significant.
Among patients with planned FET cycles, those who did not resume treatment were more likely to have a gestational carrier, compared with those who resumed treatment (7% vs. 1%). In some cases, gestational carriers lived in another state and the pandemic complicated travel arrangements, which contributed to delays, Dr. Verrilli said.
The analysis did not include information about income or socioeconomic status, which may play a role in patients’ decisions, Dr. Verrilli said.
Emotional impact of indefinite delay
Fertility treatment is often time sensitive, particularly for patients with advanced reproductive age or diminished ovarian reserve, and indefinite postponement of fertility treatment potentially could lead some patients to lose the ability to conceive with their own gametes, Dr. Turocy said.
In early April, Dr. Turocy and colleagues surveyed patients at their academic fertility center in New York City to assess patients’ reactions to the ASRM recommendations. They decided to conduct the study after they realized that treatment cancellations were having a significant emotional effect.
Investigators emailed an 18-item survey to more than 3,000 patients.
In all, 518 patients completed the survey, a response rate of 17%. Patients had an average age of 37 years (range, 23-52 years), and 92% were female. About 24% had children, and 66% had received at least one fertility treatment.
Half had a cycle canceled because of the COVID-19 pandemic, including timed intercourse cycles (5%), intrauterine insemination cycles (23%), IVF cycles with a planned fresh embryo transfer (10%), IVF with all frozen embryos (27%), egg freeze cycles (3%), and FET cycles (30%).
In response to survey questions about whether they agreed with ASRM recommendations, “the reactions were mixed,” Dr. Turocy said.
About 36% of patients agreed all fertility cycles should be canceled, 22% were unsure, and 43% disagreed with the recommendation. Patients who had a cycle canceled were slightly more likely to agree with the cancellations (40% agreed) than those who did not have a cycle canceled (30% agreed), Dr. Turocy said.
Most respondents would have preferred an option to start a treatment cycle in consultation with their doctor. Half “would have chosen to start a new cycle during the height of the pandemic in New York City,” Dr. Turocy said.
Patient opinions may vary by region and depend on the severity of COVID-19 outbreaks there, and they also might change over time, Dr. Turocy suggested. In addition, the opinions and characteristics of patients who responded to the anonymous survey may differ from those of patients who did not respond.
Dr. Verrilli, Dr. Turocy, and Dr. Lin had no relevant financial disclosures.
More than 60% of patients at a center for reproductive medicine in Utah who had fertility treatments canceled because of the COVID-19 pandemic opted to resume treatment once the suspension was lifted about 7 weeks later.
At another fertility center in New York, a survey found that 96% of respondents who had a cycle canceled because of the pandemic found it upsetting, and 22% found it extremely upsetting, with extremely upsetting defined as equivalent to the loss of a child.
The indefinite time frame for resuming treatment when the New York survey was conducted may have been a major source of distress for patients, one of the researchers said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“They don’t know when they might have that chance again,” said Jenna M. Turocy, MD, of Columbia University Fertility Center, New York.
COVID-19 guidelines published by ASRM on March 17 recommended the suspension of new treatment cycles, including ovulation induction, intrauterine inseminations, and in vitro fertilization (IVF).
An ASRM COVID-19 task force has since supported “the measured resumption of fertility care following the easing of restrictions,” said Paul C. Lin, MD, president of the Society for Assisted Reproductive Technology and a member of the task force.
“Over the past several months, significant knowledge has been gained regarding the COVID-19 virus and its impact on patients and the medical system,” he said in a news release about the two studies that assessed the pandemic’s effects.
Certain precautions remain. “It has become clear that we will need to be practicing COVID-19 protocols at least until an effective and safe vaccine or broadly effective treatment becomes widely available,” Dr. Lin said.
Desire to proceed during a pandemic
The center continued to offer IVF cycles for oncofertility patients on an urgent basis.
In early May, patients whose cycles had been suspended had the option to receive treatment.
“Upon reopening, every patient received standardized counseling from their primary IVF physician,” Lauren Verrilli, MD, a reproductive endocrinology and infertility fellow at the University of Utah, Salt Lake City, said at the virtual meeting.
Doctors explained that much remained unknown about COVID-19 in pregnancy, and that it was unclear whether the clinic would need to shut down again. In addition, patients had to undergo COVID-19 testing.
To identify factors associated with proceeding with treatment after the suspension, the researchers compared patients who resumed treatment with patients who did not.
Their analysis included 278 patients who had planned an IVF cycle or frozen embryo transfer (FET) prior to the shutdown. The researchers examined factors such as age, parity, anti-Müllerian hormone, antral follicle count, history of prior IVF cycles or FET, number of frozen blastocysts, gamete source, and use of a gestational carrier.
In all, 62% of patients opted to receive treatment once restrictions were lifted, including 69 of the 133 (52%) patients with planned fresh cycles and 104 of the 145 (72%) patients with planned FET cycles.
Among those with planned fresh cycles, those who opted to resume treatment tended to be older than those who did not resume treatment, with a median age of 37 years versus 35 years, but the difference was not statistically significant.
Among patients with planned FET cycles, those who did not resume treatment were more likely to have a gestational carrier, compared with those who resumed treatment (7% vs. 1%). In some cases, gestational carriers lived in another state and the pandemic complicated travel arrangements, which contributed to delays, Dr. Verrilli said.
The analysis did not include information about income or socioeconomic status, which may play a role in patients’ decisions, Dr. Verrilli said.
Emotional impact of indefinite delay
Fertility treatment is often time sensitive, particularly for patients with advanced reproductive age or diminished ovarian reserve, and indefinite postponement of fertility treatment potentially could lead some patients to lose the ability to conceive with their own gametes, Dr. Turocy said.
In early April, Dr. Turocy and colleagues surveyed patients at their academic fertility center in New York City to assess patients’ reactions to the ASRM recommendations. They decided to conduct the study after they realized that treatment cancellations were having a significant emotional effect.
Investigators emailed an 18-item survey to more than 3,000 patients.
In all, 518 patients completed the survey, a response rate of 17%. Patients had an average age of 37 years (range, 23-52 years), and 92% were female. About 24% had children, and 66% had received at least one fertility treatment.
Half had a cycle canceled because of the COVID-19 pandemic, including timed intercourse cycles (5%), intrauterine insemination cycles (23%), IVF cycles with a planned fresh embryo transfer (10%), IVF with all frozen embryos (27%), egg freeze cycles (3%), and FET cycles (30%).
In response to survey questions about whether they agreed with ASRM recommendations, “the reactions were mixed,” Dr. Turocy said.
About 36% of patients agreed all fertility cycles should be canceled, 22% were unsure, and 43% disagreed with the recommendation. Patients who had a cycle canceled were slightly more likely to agree with the cancellations (40% agreed) than those who did not have a cycle canceled (30% agreed), Dr. Turocy said.
Most respondents would have preferred an option to start a treatment cycle in consultation with their doctor. Half “would have chosen to start a new cycle during the height of the pandemic in New York City,” Dr. Turocy said.
Patient opinions may vary by region and depend on the severity of COVID-19 outbreaks there, and they also might change over time, Dr. Turocy suggested. In addition, the opinions and characteristics of patients who responded to the anonymous survey may differ from those of patients who did not respond.
Dr. Verrilli, Dr. Turocy, and Dr. Lin had no relevant financial disclosures.
FROM ASRM 2020
Employment protections now include sexual orientation, but our role in LGBTQIA+ equality continues
The state of Tennessee, where I worked and attended medical school, did not have legislation in place prohibiting termination of employment based on sexual orientation alone. As a lesbian, I never felt safe at work knowing that I could be fired at any time simply because of who I loved and how I identified. When I started medical school in rural Appalachia, I decided I would be “out” but remained cautious. That meant inspecting everyone I encountered for signs of acceptance and safety before sharing details about my life. As a third-year medical student, I started wearing a rainbow triangle on my white coat. One of the first patients I cared for cried and thanked me for wearing the pin. She then proceeded to tell me about her partner, her own struggles with depression, and the secrets she had to keep from her community. It was overwhelming and, yet, so familiar. I was struck by how wearing this pin, a small gesture, made this patient feel safe enough to come out to me and seek help for her depression. Although I found a supportive community in Tennessee, it was only after I moved to Massachusetts for residency—where antidiscrimination laws protected lesbian, gay, bisexual, transgender, queer/questioning, intersex, asexual, plus all other gender and sexual minority (LGBTQIA+) identified people—did I feel safe to freely share about my partner and our life together.
A landmark decision in the Supreme Court
This past June, in a 6 to 3 decision, the US Supreme Court ruled in the case of Bostock v Clayton County that Title VII’s ban on discrimination also protects LGBTQIA+ employees. Title VII is a federal law that protects employees from discrimination based on race, color, national origin, sex, and religion.1 In this decision, the court determined that “sex” cannot be differentiated from sexual orientation. Justice Neil Gorsuch, who wrote the majority opinion, stated, “It is impossible… to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex.”2 Title VII not only protects employees in hiring and firing practices but also protects against harassment and retaliation. Prior to this ruling, there were no federal antidiscrimination laws for LGBTQIA+ individuals, and only 22 states and the District of Columbia had laws in place that specified antidiscrimination protection for this community.3 Because of this landmark decision, Title VII now protects all employees in all states from discrimination, including due to an individual’s sexual orientation.
This is a huge victory in the battle for equality; however, the fight is not over. Justice Gorsuch stated, “We do not purport to address bathrooms, locker rooms or anything else of the kind…whether other policies and practices might or might not qualify as unlawful discrimination or find justifications under other provisions of Title VII are questions for future cases, not these.”2 This victory sets a new precedent and will continue to be further defined with more court cases as states and employers push back against these protections.
Continue to: A worrying shift in the Court...
A worrying shift in the Court
We have already started to see the repercussions of this ruling from Supreme Court justices themselves. Justice Clarence Thomas, who dissented in the Obergefell v Hodges decision in 2015, which established the constitutional right for marriage equality, recently wrote a petition to have the Supreme Court reconsider that ruling. He wrote “Obergefell enables courts and governments to brand religious adherents who believe that marriage is between one man and one woman as bigots, making their religious liberty concerns that much easier to dismiss.”3 After the passing of Justice Ruth Bader Ginsburg, the Supreme Court became decidedly more conservative with the appointment of Judge Amy Coney Barrett, whose mentor was the late Justice Antonin Scalia, who also dissented in the 2015 case.
As we celebrate this huge win for equality in this June decision, we also must recognize that LGBTQIA+ rights are still at risk.
LGBTQIA+ patients at higher risk for litany of conditions
Even with the Bostock v Clayton County ruling, we must not forget that discrimination will continue to exist. As health care providers, we have a responsibility to advocate on behalf of our LGBTQIA+ colleagues and patients. According to the Healthy People 2020 survey, there are higher rates of obesity, tobacco dependence, and sexually transmitted infection, as well as lower adherence to cancer screening recommendations in the LGBTQIA+ community.4 These disparities are a result of systemic, legal, and social factors, including limited access to affirming and inclusive health care.5 The LGBTQIA+ community deserves better.
Take action
In the coming months and years, as the US Supreme Court hears more cases that will threaten the rights of the LGBTQIA+ community, I challenge all clinicians to take action. Even the smallest of gestures, such as wearing a rainbow pin, can be transformative for our patients and within our communities.
- Advocate for your state to enact nondiscrimination laws protecting the LGBTQIA+ community. Find out if your state has a law.
- Support your LGBTQIA+ colleagues by establishing an employee support group.
- Educate yourself and your colleagues on LGBTQIA+ inclusive medical practices.
- US Equal Employment Opportunity Commission. Title VII of the Civil Rights Act of 1964. https://www.eeoc.gov/statutes/title-vii-civil-rights-act-1964. Accessed November 4, 2020.
- Bostock v Clayton County, 590 US ___ (2020).
- Petition for Writ of Certiorari, Clarence Thomas. October 2020. https://www.supremecourt.gov/orders/courtorders/100520zor_3204.pdf. Accessed November 11, 2020.
- US Department of Health and Human Services. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Accessed November 4, 2020.
- Ard KL, Makadon HJ. Improving the health of lesbian, gay, bisexual and transgender people: understanding and eliminating health disparities. The National LGBT Health Education Center website. https://www.lgbtqiahealtheducation.org/wp-content/uploads/Improving-the-Health-of-LGBT-People.pdf. Accessed November 4, 2020.
The state of Tennessee, where I worked and attended medical school, did not have legislation in place prohibiting termination of employment based on sexual orientation alone. As a lesbian, I never felt safe at work knowing that I could be fired at any time simply because of who I loved and how I identified. When I started medical school in rural Appalachia, I decided I would be “out” but remained cautious. That meant inspecting everyone I encountered for signs of acceptance and safety before sharing details about my life. As a third-year medical student, I started wearing a rainbow triangle on my white coat. One of the first patients I cared for cried and thanked me for wearing the pin. She then proceeded to tell me about her partner, her own struggles with depression, and the secrets she had to keep from her community. It was overwhelming and, yet, so familiar. I was struck by how wearing this pin, a small gesture, made this patient feel safe enough to come out to me and seek help for her depression. Although I found a supportive community in Tennessee, it was only after I moved to Massachusetts for residency—where antidiscrimination laws protected lesbian, gay, bisexual, transgender, queer/questioning, intersex, asexual, plus all other gender and sexual minority (LGBTQIA+) identified people—did I feel safe to freely share about my partner and our life together.
A landmark decision in the Supreme Court
This past June, in a 6 to 3 decision, the US Supreme Court ruled in the case of Bostock v Clayton County that Title VII’s ban on discrimination also protects LGBTQIA+ employees. Title VII is a federal law that protects employees from discrimination based on race, color, national origin, sex, and religion.1 In this decision, the court determined that “sex” cannot be differentiated from sexual orientation. Justice Neil Gorsuch, who wrote the majority opinion, stated, “It is impossible… to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex.”2 Title VII not only protects employees in hiring and firing practices but also protects against harassment and retaliation. Prior to this ruling, there were no federal antidiscrimination laws for LGBTQIA+ individuals, and only 22 states and the District of Columbia had laws in place that specified antidiscrimination protection for this community.3 Because of this landmark decision, Title VII now protects all employees in all states from discrimination, including due to an individual’s sexual orientation.
This is a huge victory in the battle for equality; however, the fight is not over. Justice Gorsuch stated, “We do not purport to address bathrooms, locker rooms or anything else of the kind…whether other policies and practices might or might not qualify as unlawful discrimination or find justifications under other provisions of Title VII are questions for future cases, not these.”2 This victory sets a new precedent and will continue to be further defined with more court cases as states and employers push back against these protections.
Continue to: A worrying shift in the Court...
A worrying shift in the Court
We have already started to see the repercussions of this ruling from Supreme Court justices themselves. Justice Clarence Thomas, who dissented in the Obergefell v Hodges decision in 2015, which established the constitutional right for marriage equality, recently wrote a petition to have the Supreme Court reconsider that ruling. He wrote “Obergefell enables courts and governments to brand religious adherents who believe that marriage is between one man and one woman as bigots, making their religious liberty concerns that much easier to dismiss.”3 After the passing of Justice Ruth Bader Ginsburg, the Supreme Court became decidedly more conservative with the appointment of Judge Amy Coney Barrett, whose mentor was the late Justice Antonin Scalia, who also dissented in the 2015 case.
As we celebrate this huge win for equality in this June decision, we also must recognize that LGBTQIA+ rights are still at risk.
LGBTQIA+ patients at higher risk for litany of conditions
Even with the Bostock v Clayton County ruling, we must not forget that discrimination will continue to exist. As health care providers, we have a responsibility to advocate on behalf of our LGBTQIA+ colleagues and patients. According to the Healthy People 2020 survey, there are higher rates of obesity, tobacco dependence, and sexually transmitted infection, as well as lower adherence to cancer screening recommendations in the LGBTQIA+ community.4 These disparities are a result of systemic, legal, and social factors, including limited access to affirming and inclusive health care.5 The LGBTQIA+ community deserves better.
Take action
In the coming months and years, as the US Supreme Court hears more cases that will threaten the rights of the LGBTQIA+ community, I challenge all clinicians to take action. Even the smallest of gestures, such as wearing a rainbow pin, can be transformative for our patients and within our communities.
- Advocate for your state to enact nondiscrimination laws protecting the LGBTQIA+ community. Find out if your state has a law.
- Support your LGBTQIA+ colleagues by establishing an employee support group.
- Educate yourself and your colleagues on LGBTQIA+ inclusive medical practices.
The state of Tennessee, where I worked and attended medical school, did not have legislation in place prohibiting termination of employment based on sexual orientation alone. As a lesbian, I never felt safe at work knowing that I could be fired at any time simply because of who I loved and how I identified. When I started medical school in rural Appalachia, I decided I would be “out” but remained cautious. That meant inspecting everyone I encountered for signs of acceptance and safety before sharing details about my life. As a third-year medical student, I started wearing a rainbow triangle on my white coat. One of the first patients I cared for cried and thanked me for wearing the pin. She then proceeded to tell me about her partner, her own struggles with depression, and the secrets she had to keep from her community. It was overwhelming and, yet, so familiar. I was struck by how wearing this pin, a small gesture, made this patient feel safe enough to come out to me and seek help for her depression. Although I found a supportive community in Tennessee, it was only after I moved to Massachusetts for residency—where antidiscrimination laws protected lesbian, gay, bisexual, transgender, queer/questioning, intersex, asexual, plus all other gender and sexual minority (LGBTQIA+) identified people—did I feel safe to freely share about my partner and our life together.
A landmark decision in the Supreme Court
This past June, in a 6 to 3 decision, the US Supreme Court ruled in the case of Bostock v Clayton County that Title VII’s ban on discrimination also protects LGBTQIA+ employees. Title VII is a federal law that protects employees from discrimination based on race, color, national origin, sex, and religion.1 In this decision, the court determined that “sex” cannot be differentiated from sexual orientation. Justice Neil Gorsuch, who wrote the majority opinion, stated, “It is impossible… to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex.”2 Title VII not only protects employees in hiring and firing practices but also protects against harassment and retaliation. Prior to this ruling, there were no federal antidiscrimination laws for LGBTQIA+ individuals, and only 22 states and the District of Columbia had laws in place that specified antidiscrimination protection for this community.3 Because of this landmark decision, Title VII now protects all employees in all states from discrimination, including due to an individual’s sexual orientation.
This is a huge victory in the battle for equality; however, the fight is not over. Justice Gorsuch stated, “We do not purport to address bathrooms, locker rooms or anything else of the kind…whether other policies and practices might or might not qualify as unlawful discrimination or find justifications under other provisions of Title VII are questions for future cases, not these.”2 This victory sets a new precedent and will continue to be further defined with more court cases as states and employers push back against these protections.
Continue to: A worrying shift in the Court...
A worrying shift in the Court
We have already started to see the repercussions of this ruling from Supreme Court justices themselves. Justice Clarence Thomas, who dissented in the Obergefell v Hodges decision in 2015, which established the constitutional right for marriage equality, recently wrote a petition to have the Supreme Court reconsider that ruling. He wrote “Obergefell enables courts and governments to brand religious adherents who believe that marriage is between one man and one woman as bigots, making their religious liberty concerns that much easier to dismiss.”3 After the passing of Justice Ruth Bader Ginsburg, the Supreme Court became decidedly more conservative with the appointment of Judge Amy Coney Barrett, whose mentor was the late Justice Antonin Scalia, who also dissented in the 2015 case.
As we celebrate this huge win for equality in this June decision, we also must recognize that LGBTQIA+ rights are still at risk.
LGBTQIA+ patients at higher risk for litany of conditions
Even with the Bostock v Clayton County ruling, we must not forget that discrimination will continue to exist. As health care providers, we have a responsibility to advocate on behalf of our LGBTQIA+ colleagues and patients. According to the Healthy People 2020 survey, there are higher rates of obesity, tobacco dependence, and sexually transmitted infection, as well as lower adherence to cancer screening recommendations in the LGBTQIA+ community.4 These disparities are a result of systemic, legal, and social factors, including limited access to affirming and inclusive health care.5 The LGBTQIA+ community deserves better.
Take action
In the coming months and years, as the US Supreme Court hears more cases that will threaten the rights of the LGBTQIA+ community, I challenge all clinicians to take action. Even the smallest of gestures, such as wearing a rainbow pin, can be transformative for our patients and within our communities.
- Advocate for your state to enact nondiscrimination laws protecting the LGBTQIA+ community. Find out if your state has a law.
- Support your LGBTQIA+ colleagues by establishing an employee support group.
- Educate yourself and your colleagues on LGBTQIA+ inclusive medical practices.
- US Equal Employment Opportunity Commission. Title VII of the Civil Rights Act of 1964. https://www.eeoc.gov/statutes/title-vii-civil-rights-act-1964. Accessed November 4, 2020.
- Bostock v Clayton County, 590 US ___ (2020).
- Petition for Writ of Certiorari, Clarence Thomas. October 2020. https://www.supremecourt.gov/orders/courtorders/100520zor_3204.pdf. Accessed November 11, 2020.
- US Department of Health and Human Services. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Accessed November 4, 2020.
- Ard KL, Makadon HJ. Improving the health of lesbian, gay, bisexual and transgender people: understanding and eliminating health disparities. The National LGBT Health Education Center website. https://www.lgbtqiahealtheducation.org/wp-content/uploads/Improving-the-Health-of-LGBT-People.pdf. Accessed November 4, 2020.
- US Equal Employment Opportunity Commission. Title VII of the Civil Rights Act of 1964. https://www.eeoc.gov/statutes/title-vii-civil-rights-act-1964. Accessed November 4, 2020.
- Bostock v Clayton County, 590 US ___ (2020).
- Petition for Writ of Certiorari, Clarence Thomas. October 2020. https://www.supremecourt.gov/orders/courtorders/100520zor_3204.pdf. Accessed November 11, 2020.
- US Department of Health and Human Services. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Accessed November 4, 2020.
- Ard KL, Makadon HJ. Improving the health of lesbian, gay, bisexual and transgender people: understanding and eliminating health disparities. The National LGBT Health Education Center website. https://www.lgbtqiahealtheducation.org/wp-content/uploads/Improving-the-Health-of-LGBT-People.pdf. Accessed November 4, 2020.
Syphilis: Cutting risk through primary prevention and prenatal screening
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
Which hormonal management approach for women with premature ovarian insufficiency is best for bone?
Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
EXPERT COMMENTARY
Premature ovarian insufficiency (POI) refers to a condition in women in whom ovarian function ceases prior to age 40 years. Although hormone therapy (HT) is a mainstay of treatment for women with POI, it is uncertain which approach to HT is most effective in terms of bone mineral density (BMD). Investigators recently published their results of an observational study that aimed to evaluate the use of combined oral contraceptives (COCs) for preserving BMD in women with POI.
Details of the study
At an academic center in Brazil, Carvalho Gazarra and colleagues identified women with POI who had undergone 2 or more BMD assessments performed 2 or more years apart.1 HT regimens (all of which were taken continuously) employed the following: a COC with ethinyl estradiol (EE) 30 µg and levonorgestrel; low-dose estrogen plus progestin therapy (EPT, conjugated equine estrogen [CEE] 0.625 mg with medroxyprogesterone acetate or estradiol 1.0 mg with norethindrone acetate); or high-dose estrogen plus progestin (CEE 1.25 mg or estradiol 2.0 mg combined with the same progestins).
Results. Among 119 evaluable women with POI (mean age, 30.3 years), the use of COC was associated with the most positive BMD trends. For women using COC or high-dose EPT, BMD at the lumbar spine increased. By contrast, BMD of the lumbar spine declined in women who used no treatment or low-dose EPT.1
Other studies’ take on dose, route of administration, and cost considerations
Sequelae of POI include infertility, bothersome hot flashes, vaginal dryness, sexual dysfunction, mood disorders, and an elevated risk of cardiovascular disease, dementia, Parkinson’s disease, and osteoporosis. Importantly, clinicians and patients need to understand that the results from the Women’s Health Initiative studies do not apply to women with POI.2 Physiologic doses of HT (that is, doses higher than those used to treat menopausal symptoms in women with normal/spontaneous menopause) are appropriate for women with POI, at least until they reach the normal age of menopause (51 to 52 years).
A clinical trial conducted in Scotland in women with POI found that high-dose transdermal estrogen (application of one to two 0.1-mg estradiol patches) daily had an impact on BMD that was more positive than that of an oral contraceptive formulated with EE 30 µg.3 Likewise, a trial in the United States found that, among oligo-amenorrheic athletes, a hormone replacement regimen using a 0.1-mg estradiol patch had a more positive impact on BMD than an oral contraceptive formulated with EE 30 µg.4
Although Carvalho Gazarra and colleagues acknowledged awareness of reports suggesting the skeletal health benefits of high-dose estradiol patches, in the Brazilian public health system oral hormone therapy is less expensive and oral contraceptives are available at no charge.1 ●
When replacing estrogen and progestin in young women who lack ovarian function, it is appropriate to use considerably higher doses than those used to treat bothersome vasomotor symptoms in women with normal/spontaneous menopause. From the perspective of venous thromboembolism risk, the transdermal route of administration is safer than the oral route,5 and the Scottish and US studies discussed here indicate that transdermal estradiol is an effective approach to maintaining skeletal health in young women without ovarian function. Accordingly, hormonal management with high-dose transdermal estradiol with a progestin (such as progesterone 200–300 mg at bedtime or medroxyprogesterone 5–10 mg daily) represents an appropriate strategy. In situations where transdermal estradiol plus oral progestin treatment is not covered by health insurance or acceptable to the patient, an oral estrogen-progestin contraceptive formulated with EE 30 or 35 µg will provide protection against bone loss.
- Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
- Jiang XD. Bone health and beyond in women with primary ovarian insufficiency: time to narrow the knowledge-action gap in care. Menopause. 2020;27:1101-1103.
- Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf). 2010;73:707-714.
- Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53:229-236.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
EXPERT COMMENTARY
Premature ovarian insufficiency (POI) refers to a condition in women in whom ovarian function ceases prior to age 40 years. Although hormone therapy (HT) is a mainstay of treatment for women with POI, it is uncertain which approach to HT is most effective in terms of bone mineral density (BMD). Investigators recently published their results of an observational study that aimed to evaluate the use of combined oral contraceptives (COCs) for preserving BMD in women with POI.
Details of the study
At an academic center in Brazil, Carvalho Gazarra and colleagues identified women with POI who had undergone 2 or more BMD assessments performed 2 or more years apart.1 HT regimens (all of which were taken continuously) employed the following: a COC with ethinyl estradiol (EE) 30 µg and levonorgestrel; low-dose estrogen plus progestin therapy (EPT, conjugated equine estrogen [CEE] 0.625 mg with medroxyprogesterone acetate or estradiol 1.0 mg with norethindrone acetate); or high-dose estrogen plus progestin (CEE 1.25 mg or estradiol 2.0 mg combined with the same progestins).
Results. Among 119 evaluable women with POI (mean age, 30.3 years), the use of COC was associated with the most positive BMD trends. For women using COC or high-dose EPT, BMD at the lumbar spine increased. By contrast, BMD of the lumbar spine declined in women who used no treatment or low-dose EPT.1
Other studies’ take on dose, route of administration, and cost considerations
Sequelae of POI include infertility, bothersome hot flashes, vaginal dryness, sexual dysfunction, mood disorders, and an elevated risk of cardiovascular disease, dementia, Parkinson’s disease, and osteoporosis. Importantly, clinicians and patients need to understand that the results from the Women’s Health Initiative studies do not apply to women with POI.2 Physiologic doses of HT (that is, doses higher than those used to treat menopausal symptoms in women with normal/spontaneous menopause) are appropriate for women with POI, at least until they reach the normal age of menopause (51 to 52 years).
A clinical trial conducted in Scotland in women with POI found that high-dose transdermal estrogen (application of one to two 0.1-mg estradiol patches) daily had an impact on BMD that was more positive than that of an oral contraceptive formulated with EE 30 µg.3 Likewise, a trial in the United States found that, among oligo-amenorrheic athletes, a hormone replacement regimen using a 0.1-mg estradiol patch had a more positive impact on BMD than an oral contraceptive formulated with EE 30 µg.4
Although Carvalho Gazarra and colleagues acknowledged awareness of reports suggesting the skeletal health benefits of high-dose estradiol patches, in the Brazilian public health system oral hormone therapy is less expensive and oral contraceptives are available at no charge.1 ●
When replacing estrogen and progestin in young women who lack ovarian function, it is appropriate to use considerably higher doses than those used to treat bothersome vasomotor symptoms in women with normal/spontaneous menopause. From the perspective of venous thromboembolism risk, the transdermal route of administration is safer than the oral route,5 and the Scottish and US studies discussed here indicate that transdermal estradiol is an effective approach to maintaining skeletal health in young women without ovarian function. Accordingly, hormonal management with high-dose transdermal estradiol with a progestin (such as progesterone 200–300 mg at bedtime or medroxyprogesterone 5–10 mg daily) represents an appropriate strategy. In situations where transdermal estradiol plus oral progestin treatment is not covered by health insurance or acceptable to the patient, an oral estrogen-progestin contraceptive formulated with EE 30 or 35 µg will provide protection against bone loss.
Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
EXPERT COMMENTARY
Premature ovarian insufficiency (POI) refers to a condition in women in whom ovarian function ceases prior to age 40 years. Although hormone therapy (HT) is a mainstay of treatment for women with POI, it is uncertain which approach to HT is most effective in terms of bone mineral density (BMD). Investigators recently published their results of an observational study that aimed to evaluate the use of combined oral contraceptives (COCs) for preserving BMD in women with POI.
Details of the study
At an academic center in Brazil, Carvalho Gazarra and colleagues identified women with POI who had undergone 2 or more BMD assessments performed 2 or more years apart.1 HT regimens (all of which were taken continuously) employed the following: a COC with ethinyl estradiol (EE) 30 µg and levonorgestrel; low-dose estrogen plus progestin therapy (EPT, conjugated equine estrogen [CEE] 0.625 mg with medroxyprogesterone acetate or estradiol 1.0 mg with norethindrone acetate); or high-dose estrogen plus progestin (CEE 1.25 mg or estradiol 2.0 mg combined with the same progestins).
Results. Among 119 evaluable women with POI (mean age, 30.3 years), the use of COC was associated with the most positive BMD trends. For women using COC or high-dose EPT, BMD at the lumbar spine increased. By contrast, BMD of the lumbar spine declined in women who used no treatment or low-dose EPT.1
Other studies’ take on dose, route of administration, and cost considerations
Sequelae of POI include infertility, bothersome hot flashes, vaginal dryness, sexual dysfunction, mood disorders, and an elevated risk of cardiovascular disease, dementia, Parkinson’s disease, and osteoporosis. Importantly, clinicians and patients need to understand that the results from the Women’s Health Initiative studies do not apply to women with POI.2 Physiologic doses of HT (that is, doses higher than those used to treat menopausal symptoms in women with normal/spontaneous menopause) are appropriate for women with POI, at least until they reach the normal age of menopause (51 to 52 years).
A clinical trial conducted in Scotland in women with POI found that high-dose transdermal estrogen (application of one to two 0.1-mg estradiol patches) daily had an impact on BMD that was more positive than that of an oral contraceptive formulated with EE 30 µg.3 Likewise, a trial in the United States found that, among oligo-amenorrheic athletes, a hormone replacement regimen using a 0.1-mg estradiol patch had a more positive impact on BMD than an oral contraceptive formulated with EE 30 µg.4
Although Carvalho Gazarra and colleagues acknowledged awareness of reports suggesting the skeletal health benefits of high-dose estradiol patches, in the Brazilian public health system oral hormone therapy is less expensive and oral contraceptives are available at no charge.1 ●
When replacing estrogen and progestin in young women who lack ovarian function, it is appropriate to use considerably higher doses than those used to treat bothersome vasomotor symptoms in women with normal/spontaneous menopause. From the perspective of venous thromboembolism risk, the transdermal route of administration is safer than the oral route,5 and the Scottish and US studies discussed here indicate that transdermal estradiol is an effective approach to maintaining skeletal health in young women without ovarian function. Accordingly, hormonal management with high-dose transdermal estradiol with a progestin (such as progesterone 200–300 mg at bedtime or medroxyprogesterone 5–10 mg daily) represents an appropriate strategy. In situations where transdermal estradiol plus oral progestin treatment is not covered by health insurance or acceptable to the patient, an oral estrogen-progestin contraceptive formulated with EE 30 or 35 µg will provide protection against bone loss.
- Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
- Jiang XD. Bone health and beyond in women with primary ovarian insufficiency: time to narrow the knowledge-action gap in care. Menopause. 2020;27:1101-1103.
- Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf). 2010;73:707-714.
- Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53:229-236.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
- Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
- Jiang XD. Bone health and beyond in women with primary ovarian insufficiency: time to narrow the knowledge-action gap in care. Menopause. 2020;27:1101-1103.
- Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf). 2010;73:707-714.
- Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53:229-236.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
9vHPV vaccine: Prevention of oropharyngeal cancer
Surprisingly, in the United States, the most common cancer associated with human papillomavirus (HPV) is oropharyngeal squamous cell cancer (SCC), with one study reporting 15,479 cases among men and 3,428 cases among women in 2015.1 In the same year, the investigators reported 11,788 cases of cervical cancer.1 A public health concern is that cases of oropharyngeal SCC are increasing, while cases of cervical cancer are decreasing. From 1999 to 2015, the rate of oropharyngeal SCC increased annually among both men and women, at rates of 2.7% and 0.8% per year, respectively. By contrast, the rate of cervical cancer decreased by 1.6% per year.1
Although the incidence of HPV-negative oropharyngeal SCC (cases associated with cigarette smoking) has declined by 50% from 1988 to 2004, the incidence of HPV-positive oropharyngeal SCC has increased by 225%, with much of the increase occurring among young, white men.2 HPV infection is a major cause of oropharyngeal SCC at the base of the tongue and tonsils, but not in the soft palate or oropharyngeal walls.3
Most physicians and parents recognize that the 9-valent (9v)HPV vaccine prevents the majority of cervical cancers and precancers in women. Far fewer people realize that there is an important opportunity to prevent a large number of oropharyngeal cancers by improving 9vHPV vaccination in men and women.
Which HPV types are associated with oropharyngeal cancer?
HPV16 is the most common HPV type associated with oropharyngeal SCC. Among these cancer types, greater than 80% harbor HPV16, with greater than 90% harboring HPV16 or 18 and less than 10% of tumors associated with HPV types 31, 33, 45, 52, or 58.4-7
The high prevalence of HPV16 in patients with oropharyngeal cancer raises the question of the HPV status of the intimate partner of the index patient. In one study of 164 people with HPV detected in their oropharyngeal, the partner of the index patient had a low prevalence of high-risk HPV types (1.2%) in oral rinse and gargle samples, similar to the rate in the general population (1.3%).7 This finding is reassuring and suggests that intimate partners of patients with HPV-positive oropharyngeal cancer effectively clear high-risk HPV virus from the oropharynx. The HPV status of the genital tissue of the intimate partner of an index patient with oropharyngeal SCC has not been adequately studied.
Men are more likely than women to harbor oral HPV
Among a sample of 5,501 men and women aged 14 to 69 years from the National Health and Nutrition Examination Survey, oral rinses were obtained and analyzed for the presence of HPV.8 The prevalence of any oral HPV and any oral high-risk HPV was 6.9% and 3.7%, respectively. Oral HPV-16 was detected in 1.6% of men and 0.3% of women. The prevalence of HPV was higher among current smokers, heavy alcohol drinkers, and people with a history of a greater number of sexual partners. In men and women reporting more than 20 lifetime sexual partners, the prevalence of oral HPV was 20%.
In a study of 2,627 men and women aged 18 to 33 years, the prevalence of oral HPV 16/18/6/11 was lower among those vaccinated versus those unvaccinated (0.11% and 1.6%, respectively; P = .008).9 Among men, oral HPV 16/18/6/11 was lower among those vaccinated versus unvaccinated (0.0% and 2.13%, respectively; P = .007).9 The results of this observational study support the important role of vaccination in reducing oral HPV infection.
In 2020, the US Food and Drug Administration (FDA) approved the 9-valent human papillomavirus (9vHPV) vaccine for the prevention of oropharyngeal cancer. The 9vHPV vaccine contains inactive L1 capsid proteins for 9 HPV types, including types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The vaccine stimulates the production of neutralizing antibodies to the capsid protein.
9vHPV is approved for females aged 9 to 45 years to prevent cancers and precancers of the cervix, vulva, vagina, and anus caused by HPV types 16, 18, 31, 33, 45, 52, and 58.1 It is also approved for males aged 9 to 45 years to prevent cancer and precancers of the anus caused by those viral types. In 2020 the 9vHPV vaccine was approved by the FDA to prevent oropharyngeal cancer in males and females. Of note, the FDA reported that, “the oropharyngeal and head and neck cancer indication is approved under accelerated approval based on effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.”2
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls and boys, 11 to 12 years of age.1 Children with a history of sexual abuse or assault can start the vaccine at 9 years of age. Catch-up vaccination is recommended for all females and males through age 26 years. The ACIP recommends shared clinical decision-making regarding vaccination for some adults 27 to 45 years of age. Gynecologists with routine exposure to HPV may have occupational risk that warrants HPV vaccination3 (see “As a gynecologist, should you receive the 9vHPV vaccine?”).
For most individuals who start the vaccine series before age 15, two doses of 9vHPV vaccine are recommended, with the second dose 6 to 12 months following the first dose. For teens and adults aged 15 to 26 years, 3 doses of 9vHPV vaccine are recommended, with the second dose 1 to 2 months later and the third dose 6 months following the first dose. Immunocompromised individuals 9 to 26 years of age, including those with HIV infection, should receive 3 doses of the vaccine.
References
1. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
2. Gardasil 9 [package insert]. Whitehouse Station, NJ: Merck & Co. Inc; 2020.
3. Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. https://www.asccp.org/Assets/d3abdb05-25c5-4e58-9cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
Continue to: Vaccinate boys and girls to prevent cancer...
Vaccinate boys and girls to prevent cancer
Most population studies report that males are less likely to receive an HPV vaccine than females. For example, based on the National Health Interview Survey of people aged 18 to 26, the percentage of women who self-reported receiving at least one dose of HPV vaccine was 37% in 2013 and 54% in 2018.10 By contrast, among men, the rates of self-reported vaccination were much lower—8% in 2013 and 27% in 2018.10
The percentage of women who received the recommended number of doses of HPV vaccine (see “9vHPV vaccine: Indications and immunization schedule”) was 26% in 2013 and 35% in 2018.10 For men, these percentages were 2% in 2013 and 9% in 2018.10 These data indicate that, compared with women, men are less likely to receive an HPV vaccination and far less likely to have received the recommended number of doses.
It is heartening that there has been a slow and steady increase in the prevalence of HPV vaccination. In fact, increasing the HPV vaccination rate among both boys and girls has the potential to markedly reduce the incidence of oropharyngeal cancer.
The reasons for the female-male gap in vaccination rates are not fully characterized. For one, parental awareness of the importance of HPV vaccination to prevent cancer among men is limited, and represents an important opportunity for additional public health education. In a qualitative interview study of mothers with children aged 11 to 19, the investigators reported that most mothers were aware that HPV vaccination could prevent cervical cancer in women, but most mothers did not know that HPV causes cancer of the mouth and that vaccination could prevent oropharyngeal cancer in boys and girls.11 Because of this lack of knowledge, the mothers did not think their sons needed to have an HPV vaccine. The research report is aptly titled, “I don’t think he needs the HPV vaccine cause boys can’t have cervical cancer.”11
Clinicians are highly influential in guiding parents to accept HPV vaccination of their children. Offering consistent messaging to parents that HPV vaccination prevents cancer in both women and men, and reducing the out-of-pocket cost of vaccination surely will result in an increase in the vaccination rate of boys and girls. ●
Surgical treatment of tissues infected with human papillomavirus (HPV) often involves the use of laser or electrosurgical devices that generate smoke, which is known to contain HPV nucleic acid sequences and may contain infective virions.1 It is known that HPV nucleic acid sequences are present in surgical smoke. In one study plantar warts were treated with a carbon dioxide laser or electrocoagulation. The vapor produced from the surgery was collected with a dry filter apparatus. Five of 8 laser-derived vapors and 4 of 7 electrocoagulation-derived vapors were positive for HPV DNA. The concentration of HPV DNA was greater with laser than with electrocoagulation treatment.2
It is not known if surgical smoke derived from treatment of HPV-infected tissues contains infective HPV virions. In an experimental bovine model, smoke generated by laser ablation of fibropapillomas was collected. Injection of the contents of the smoke caused cutaneous papillomavirus lesions when inoculated into calves, suggesting that the smoke contained infective HPV virions.3 Although this animal experiment is a proof of principle that surgical smoke generated from treatment of HPVinfected tissue contain virions, it is unclear if surgical smoke generated in gynecologic practice contains HPV virions.
To investigate the prevalence of nasal HPV DNA among gynecologists, 700 physicians in Zhejiang Province, China, completed a questionnaire and provided a nasal swab for HPV DNA analysis.4 Among gynecologists who performed or did not perform LEEP, the prevalence of HPV DNA in the nose was 10% and 3%, respectively. The most common HPV types detected were HPV16 (76%), HPV31 (10%), HPV58 (5%), HPV55 (5%), HPV56 (2%), and HPV59 (2%).4 Among gynecologists who performed LEEP procedures, the prevalence of HPV DNA was 19% for those who did not use a surgical mask, 8% for clinicians who used a standard surgical mask, and 0% for those who used an N95 filtering facepiece respirator, suggesting that an N95 respirator provides the greatest protection from surgical smoke.4 Over 24 months of follow-up, all the gynecologists who had initially tested positive for HPV DNA no longer had detectable nasal HPV DNA. In this study, no gynecologist was diagnosed with an HPV-associated oropharyngeal disease. The investigators concluded that surgical masks, especially an N95 respirator, should be used by gynecologists performing LEEP procedures.
Investigators also have evaluated for the presence of HPV DNA in matched samples from the cervix of 134 patients undergoing loop electrosurgical excision procedure (LEEP) for cervical dysplasia, as well as the smoke generated during the procedure and nasal swabs from the surgeon performing the LEEP.5 HPV DNA was detected in 95% of the cervical samples, 30% of the surgical smoke samples, and 1.5% of the surgeons’ nasal swabs.5 At 6 months of follow-up, the two surgeons who initially had HPV-positive nasal swabs no longer had detected HPV DNA.
Of concern is that otolaryngologists have reported sporadic cases of oropharyngeal squamous cell cancer6 and laryngeal papillomatosis7 in health care workers with frequent and repetitive exposure to HPVs. For example, in one case report, a 53-year-old male gynecologist, nonsmoker, presented to his physician with a lump on the neck.6 The gynecologist had performed more than 3,000 laser ablation or LEEP procedures of dysplastic cervical, vaginal, and vulvar lesions over a span of 20 years.6 Most of the procedures were performed without wearing a mask and in a poorly ventilated procedure room. A computed tomography scan demonstrated a 2.2-cm soft tissue lesion in the right tonsil extending to the right soft palate and a level-2 lymph node. A biopsy of the tonsil confirmed invasive squamous cell carcinoma containing HPV16. He was treated with 35 fractions of radiotherapy and adjuvant cisplatin. Treatment adverse effects included dysphagia and xerostomia, and the patient lost 40 pounds.
Available interventions to reduce exposure of clinicians to HPV virions that may be present in surgical smoke include:
- wearing a fit-tested N95 respirator
- routinely using a smoke evacuation device, and
- ensuring sufficient ventilation in the procedure room.
A new recommendation is to consider 9vHPV vaccination for clinicians who are routinely exposed to HPV virions.8,9 In February 2020, the American Society for Colposcopy and Cervical Pathology recommended that clinicians who are routinely exposed to HPVs consider 9vHPV vaccination.8 This recommendation pertains to all members of the clinical team in the procedure room, including physicians, nurses, and staff. Based on the available data, gynecologists who have not been vaccinated will need to weigh the benefits and costs of receiving a 9vHPV vaccine to protect themselves against an occupational exposure that may adversely impact their health.
References
- Liu Y, Song Y, Hu X, et al. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among gynecologists. J Cancer. 2019;10:2788-2799.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Garden JM, O’Banion MK, Bakus AD, et al. Viral transmitted by laser-generated plume (aerosol). Arch Dermatol. 2002;138:1303-1307.
- Hu X, Zhou Q, Yu J, et al. Prevalence of HPV infections in surgical smoke exposed gynecologists. Int Arch Occup Environ Health. 2020; Epub September 1. doi: 10.1007 /s00420-020-01568-9.
- Zhou Q, Hu X, Zhou J, et al. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019;11:3643-3654.
- Rioux M, Garland A, Webster D, et al. HPV-positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54-57.
- Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425-427.
Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. www.asccp.org/Assets/d3abdb05-25c5-4e58-%209cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
- Harrison R, Huh W. Occupational exposure to human papillomavirus and vaccination for health care workers. Obstet Gynecol. 2020;136:663-665
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers--United States, 1999-2015. MMWR. 2018;67:918-924.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Haeggblom L, Ramqvist T, Tommasino M, et al. Time to change perspective on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Research. 2017;4:1-11.
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-475.
- D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664-670.
- D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408-2415.
- Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693.
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262-267.
- Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18 to 26, 2013-2018. NCHS Data Brief. 2020:1-8.
- Lindsay AC, Delgado D, Valdez MJ, et al. "I don't think he needs the HPV vaccine cause boys can't have cervical cancer": a qualitative study of Latina mothers' (Mis) understandings about human papillomavirus transmission, associated cancers and the vaccine. J Cancer Educ. July 11, 2020. doi: 10.1007/s13187-020-01824-z.
Surprisingly, in the United States, the most common cancer associated with human papillomavirus (HPV) is oropharyngeal squamous cell cancer (SCC), with one study reporting 15,479 cases among men and 3,428 cases among women in 2015.1 In the same year, the investigators reported 11,788 cases of cervical cancer.1 A public health concern is that cases of oropharyngeal SCC are increasing, while cases of cervical cancer are decreasing. From 1999 to 2015, the rate of oropharyngeal SCC increased annually among both men and women, at rates of 2.7% and 0.8% per year, respectively. By contrast, the rate of cervical cancer decreased by 1.6% per year.1
Although the incidence of HPV-negative oropharyngeal SCC (cases associated with cigarette smoking) has declined by 50% from 1988 to 2004, the incidence of HPV-positive oropharyngeal SCC has increased by 225%, with much of the increase occurring among young, white men.2 HPV infection is a major cause of oropharyngeal SCC at the base of the tongue and tonsils, but not in the soft palate or oropharyngeal walls.3
Most physicians and parents recognize that the 9-valent (9v)HPV vaccine prevents the majority of cervical cancers and precancers in women. Far fewer people realize that there is an important opportunity to prevent a large number of oropharyngeal cancers by improving 9vHPV vaccination in men and women.
Which HPV types are associated with oropharyngeal cancer?
HPV16 is the most common HPV type associated with oropharyngeal SCC. Among these cancer types, greater than 80% harbor HPV16, with greater than 90% harboring HPV16 or 18 and less than 10% of tumors associated with HPV types 31, 33, 45, 52, or 58.4-7
The high prevalence of HPV16 in patients with oropharyngeal cancer raises the question of the HPV status of the intimate partner of the index patient. In one study of 164 people with HPV detected in their oropharyngeal, the partner of the index patient had a low prevalence of high-risk HPV types (1.2%) in oral rinse and gargle samples, similar to the rate in the general population (1.3%).7 This finding is reassuring and suggests that intimate partners of patients with HPV-positive oropharyngeal cancer effectively clear high-risk HPV virus from the oropharynx. The HPV status of the genital tissue of the intimate partner of an index patient with oropharyngeal SCC has not been adequately studied.
Men are more likely than women to harbor oral HPV
Among a sample of 5,501 men and women aged 14 to 69 years from the National Health and Nutrition Examination Survey, oral rinses were obtained and analyzed for the presence of HPV.8 The prevalence of any oral HPV and any oral high-risk HPV was 6.9% and 3.7%, respectively. Oral HPV-16 was detected in 1.6% of men and 0.3% of women. The prevalence of HPV was higher among current smokers, heavy alcohol drinkers, and people with a history of a greater number of sexual partners. In men and women reporting more than 20 lifetime sexual partners, the prevalence of oral HPV was 20%.
In a study of 2,627 men and women aged 18 to 33 years, the prevalence of oral HPV 16/18/6/11 was lower among those vaccinated versus those unvaccinated (0.11% and 1.6%, respectively; P = .008).9 Among men, oral HPV 16/18/6/11 was lower among those vaccinated versus unvaccinated (0.0% and 2.13%, respectively; P = .007).9 The results of this observational study support the important role of vaccination in reducing oral HPV infection.
In 2020, the US Food and Drug Administration (FDA) approved the 9-valent human papillomavirus (9vHPV) vaccine for the prevention of oropharyngeal cancer. The 9vHPV vaccine contains inactive L1 capsid proteins for 9 HPV types, including types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The vaccine stimulates the production of neutralizing antibodies to the capsid protein.
9vHPV is approved for females aged 9 to 45 years to prevent cancers and precancers of the cervix, vulva, vagina, and anus caused by HPV types 16, 18, 31, 33, 45, 52, and 58.1 It is also approved for males aged 9 to 45 years to prevent cancer and precancers of the anus caused by those viral types. In 2020 the 9vHPV vaccine was approved by the FDA to prevent oropharyngeal cancer in males and females. Of note, the FDA reported that, “the oropharyngeal and head and neck cancer indication is approved under accelerated approval based on effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.”2
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls and boys, 11 to 12 years of age.1 Children with a history of sexual abuse or assault can start the vaccine at 9 years of age. Catch-up vaccination is recommended for all females and males through age 26 years. The ACIP recommends shared clinical decision-making regarding vaccination for some adults 27 to 45 years of age. Gynecologists with routine exposure to HPV may have occupational risk that warrants HPV vaccination3 (see “As a gynecologist, should you receive the 9vHPV vaccine?”).
For most individuals who start the vaccine series before age 15, two doses of 9vHPV vaccine are recommended, with the second dose 6 to 12 months following the first dose. For teens and adults aged 15 to 26 years, 3 doses of 9vHPV vaccine are recommended, with the second dose 1 to 2 months later and the third dose 6 months following the first dose. Immunocompromised individuals 9 to 26 years of age, including those with HIV infection, should receive 3 doses of the vaccine.
References
1. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
2. Gardasil 9 [package insert]. Whitehouse Station, NJ: Merck & Co. Inc; 2020.
3. Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. https://www.asccp.org/Assets/d3abdb05-25c5-4e58-9cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
Continue to: Vaccinate boys and girls to prevent cancer...
Vaccinate boys and girls to prevent cancer
Most population studies report that males are less likely to receive an HPV vaccine than females. For example, based on the National Health Interview Survey of people aged 18 to 26, the percentage of women who self-reported receiving at least one dose of HPV vaccine was 37% in 2013 and 54% in 2018.10 By contrast, among men, the rates of self-reported vaccination were much lower—8% in 2013 and 27% in 2018.10
The percentage of women who received the recommended number of doses of HPV vaccine (see “9vHPV vaccine: Indications and immunization schedule”) was 26% in 2013 and 35% in 2018.10 For men, these percentages were 2% in 2013 and 9% in 2018.10 These data indicate that, compared with women, men are less likely to receive an HPV vaccination and far less likely to have received the recommended number of doses.
It is heartening that there has been a slow and steady increase in the prevalence of HPV vaccination. In fact, increasing the HPV vaccination rate among both boys and girls has the potential to markedly reduce the incidence of oropharyngeal cancer.
The reasons for the female-male gap in vaccination rates are not fully characterized. For one, parental awareness of the importance of HPV vaccination to prevent cancer among men is limited, and represents an important opportunity for additional public health education. In a qualitative interview study of mothers with children aged 11 to 19, the investigators reported that most mothers were aware that HPV vaccination could prevent cervical cancer in women, but most mothers did not know that HPV causes cancer of the mouth and that vaccination could prevent oropharyngeal cancer in boys and girls.11 Because of this lack of knowledge, the mothers did not think their sons needed to have an HPV vaccine. The research report is aptly titled, “I don’t think he needs the HPV vaccine cause boys can’t have cervical cancer.”11
Clinicians are highly influential in guiding parents to accept HPV vaccination of their children. Offering consistent messaging to parents that HPV vaccination prevents cancer in both women and men, and reducing the out-of-pocket cost of vaccination surely will result in an increase in the vaccination rate of boys and girls. ●
Surgical treatment of tissues infected with human papillomavirus (HPV) often involves the use of laser or electrosurgical devices that generate smoke, which is known to contain HPV nucleic acid sequences and may contain infective virions.1 It is known that HPV nucleic acid sequences are present in surgical smoke. In one study plantar warts were treated with a carbon dioxide laser or electrocoagulation. The vapor produced from the surgery was collected with a dry filter apparatus. Five of 8 laser-derived vapors and 4 of 7 electrocoagulation-derived vapors were positive for HPV DNA. The concentration of HPV DNA was greater with laser than with electrocoagulation treatment.2
It is not known if surgical smoke derived from treatment of HPV-infected tissues contains infective HPV virions. In an experimental bovine model, smoke generated by laser ablation of fibropapillomas was collected. Injection of the contents of the smoke caused cutaneous papillomavirus lesions when inoculated into calves, suggesting that the smoke contained infective HPV virions.3 Although this animal experiment is a proof of principle that surgical smoke generated from treatment of HPVinfected tissue contain virions, it is unclear if surgical smoke generated in gynecologic practice contains HPV virions.
To investigate the prevalence of nasal HPV DNA among gynecologists, 700 physicians in Zhejiang Province, China, completed a questionnaire and provided a nasal swab for HPV DNA analysis.4 Among gynecologists who performed or did not perform LEEP, the prevalence of HPV DNA in the nose was 10% and 3%, respectively. The most common HPV types detected were HPV16 (76%), HPV31 (10%), HPV58 (5%), HPV55 (5%), HPV56 (2%), and HPV59 (2%).4 Among gynecologists who performed LEEP procedures, the prevalence of HPV DNA was 19% for those who did not use a surgical mask, 8% for clinicians who used a standard surgical mask, and 0% for those who used an N95 filtering facepiece respirator, suggesting that an N95 respirator provides the greatest protection from surgical smoke.4 Over 24 months of follow-up, all the gynecologists who had initially tested positive for HPV DNA no longer had detectable nasal HPV DNA. In this study, no gynecologist was diagnosed with an HPV-associated oropharyngeal disease. The investigators concluded that surgical masks, especially an N95 respirator, should be used by gynecologists performing LEEP procedures.
Investigators also have evaluated for the presence of HPV DNA in matched samples from the cervix of 134 patients undergoing loop electrosurgical excision procedure (LEEP) for cervical dysplasia, as well as the smoke generated during the procedure and nasal swabs from the surgeon performing the LEEP.5 HPV DNA was detected in 95% of the cervical samples, 30% of the surgical smoke samples, and 1.5% of the surgeons’ nasal swabs.5 At 6 months of follow-up, the two surgeons who initially had HPV-positive nasal swabs no longer had detected HPV DNA.
Of concern is that otolaryngologists have reported sporadic cases of oropharyngeal squamous cell cancer6 and laryngeal papillomatosis7 in health care workers with frequent and repetitive exposure to HPVs. For example, in one case report, a 53-year-old male gynecologist, nonsmoker, presented to his physician with a lump on the neck.6 The gynecologist had performed more than 3,000 laser ablation or LEEP procedures of dysplastic cervical, vaginal, and vulvar lesions over a span of 20 years.6 Most of the procedures were performed without wearing a mask and in a poorly ventilated procedure room. A computed tomography scan demonstrated a 2.2-cm soft tissue lesion in the right tonsil extending to the right soft palate and a level-2 lymph node. A biopsy of the tonsil confirmed invasive squamous cell carcinoma containing HPV16. He was treated with 35 fractions of radiotherapy and adjuvant cisplatin. Treatment adverse effects included dysphagia and xerostomia, and the patient lost 40 pounds.
Available interventions to reduce exposure of clinicians to HPV virions that may be present in surgical smoke include:
- wearing a fit-tested N95 respirator
- routinely using a smoke evacuation device, and
- ensuring sufficient ventilation in the procedure room.
A new recommendation is to consider 9vHPV vaccination for clinicians who are routinely exposed to HPV virions.8,9 In February 2020, the American Society for Colposcopy and Cervical Pathology recommended that clinicians who are routinely exposed to HPVs consider 9vHPV vaccination.8 This recommendation pertains to all members of the clinical team in the procedure room, including physicians, nurses, and staff. Based on the available data, gynecologists who have not been vaccinated will need to weigh the benefits and costs of receiving a 9vHPV vaccine to protect themselves against an occupational exposure that may adversely impact their health.
References
- Liu Y, Song Y, Hu X, et al. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among gynecologists. J Cancer. 2019;10:2788-2799.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Garden JM, O’Banion MK, Bakus AD, et al. Viral transmitted by laser-generated plume (aerosol). Arch Dermatol. 2002;138:1303-1307.
- Hu X, Zhou Q, Yu J, et al. Prevalence of HPV infections in surgical smoke exposed gynecologists. Int Arch Occup Environ Health. 2020; Epub September 1. doi: 10.1007 /s00420-020-01568-9.
- Zhou Q, Hu X, Zhou J, et al. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019;11:3643-3654.
- Rioux M, Garland A, Webster D, et al. HPV-positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54-57.
- Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425-427.
Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. www.asccp.org/Assets/d3abdb05-25c5-4e58-%209cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
- Harrison R, Huh W. Occupational exposure to human papillomavirus and vaccination for health care workers. Obstet Gynecol. 2020;136:663-665
Surprisingly, in the United States, the most common cancer associated with human papillomavirus (HPV) is oropharyngeal squamous cell cancer (SCC), with one study reporting 15,479 cases among men and 3,428 cases among women in 2015.1 In the same year, the investigators reported 11,788 cases of cervical cancer.1 A public health concern is that cases of oropharyngeal SCC are increasing, while cases of cervical cancer are decreasing. From 1999 to 2015, the rate of oropharyngeal SCC increased annually among both men and women, at rates of 2.7% and 0.8% per year, respectively. By contrast, the rate of cervical cancer decreased by 1.6% per year.1
Although the incidence of HPV-negative oropharyngeal SCC (cases associated with cigarette smoking) has declined by 50% from 1988 to 2004, the incidence of HPV-positive oropharyngeal SCC has increased by 225%, with much of the increase occurring among young, white men.2 HPV infection is a major cause of oropharyngeal SCC at the base of the tongue and tonsils, but not in the soft palate or oropharyngeal walls.3
Most physicians and parents recognize that the 9-valent (9v)HPV vaccine prevents the majority of cervical cancers and precancers in women. Far fewer people realize that there is an important opportunity to prevent a large number of oropharyngeal cancers by improving 9vHPV vaccination in men and women.
Which HPV types are associated with oropharyngeal cancer?
HPV16 is the most common HPV type associated with oropharyngeal SCC. Among these cancer types, greater than 80% harbor HPV16, with greater than 90% harboring HPV16 or 18 and less than 10% of tumors associated with HPV types 31, 33, 45, 52, or 58.4-7
The high prevalence of HPV16 in patients with oropharyngeal cancer raises the question of the HPV status of the intimate partner of the index patient. In one study of 164 people with HPV detected in their oropharyngeal, the partner of the index patient had a low prevalence of high-risk HPV types (1.2%) in oral rinse and gargle samples, similar to the rate in the general population (1.3%).7 This finding is reassuring and suggests that intimate partners of patients with HPV-positive oropharyngeal cancer effectively clear high-risk HPV virus from the oropharynx. The HPV status of the genital tissue of the intimate partner of an index patient with oropharyngeal SCC has not been adequately studied.
Men are more likely than women to harbor oral HPV
Among a sample of 5,501 men and women aged 14 to 69 years from the National Health and Nutrition Examination Survey, oral rinses were obtained and analyzed for the presence of HPV.8 The prevalence of any oral HPV and any oral high-risk HPV was 6.9% and 3.7%, respectively. Oral HPV-16 was detected in 1.6% of men and 0.3% of women. The prevalence of HPV was higher among current smokers, heavy alcohol drinkers, and people with a history of a greater number of sexual partners. In men and women reporting more than 20 lifetime sexual partners, the prevalence of oral HPV was 20%.
In a study of 2,627 men and women aged 18 to 33 years, the prevalence of oral HPV 16/18/6/11 was lower among those vaccinated versus those unvaccinated (0.11% and 1.6%, respectively; P = .008).9 Among men, oral HPV 16/18/6/11 was lower among those vaccinated versus unvaccinated (0.0% and 2.13%, respectively; P = .007).9 The results of this observational study support the important role of vaccination in reducing oral HPV infection.
In 2020, the US Food and Drug Administration (FDA) approved the 9-valent human papillomavirus (9vHPV) vaccine for the prevention of oropharyngeal cancer. The 9vHPV vaccine contains inactive L1 capsid proteins for 9 HPV types, including types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The vaccine stimulates the production of neutralizing antibodies to the capsid protein.
9vHPV is approved for females aged 9 to 45 years to prevent cancers and precancers of the cervix, vulva, vagina, and anus caused by HPV types 16, 18, 31, 33, 45, 52, and 58.1 It is also approved for males aged 9 to 45 years to prevent cancer and precancers of the anus caused by those viral types. In 2020 the 9vHPV vaccine was approved by the FDA to prevent oropharyngeal cancer in males and females. Of note, the FDA reported that, “the oropharyngeal and head and neck cancer indication is approved under accelerated approval based on effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.”2
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls and boys, 11 to 12 years of age.1 Children with a history of sexual abuse or assault can start the vaccine at 9 years of age. Catch-up vaccination is recommended for all females and males through age 26 years. The ACIP recommends shared clinical decision-making regarding vaccination for some adults 27 to 45 years of age. Gynecologists with routine exposure to HPV may have occupational risk that warrants HPV vaccination3 (see “As a gynecologist, should you receive the 9vHPV vaccine?”).
For most individuals who start the vaccine series before age 15, two doses of 9vHPV vaccine are recommended, with the second dose 6 to 12 months following the first dose. For teens and adults aged 15 to 26 years, 3 doses of 9vHPV vaccine are recommended, with the second dose 1 to 2 months later and the third dose 6 months following the first dose. Immunocompromised individuals 9 to 26 years of age, including those with HIV infection, should receive 3 doses of the vaccine.
References
1. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
2. Gardasil 9 [package insert]. Whitehouse Station, NJ: Merck & Co. Inc; 2020.
3. Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. https://www.asccp.org/Assets/d3abdb05-25c5-4e58-9cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
Continue to: Vaccinate boys and girls to prevent cancer...
Vaccinate boys and girls to prevent cancer
Most population studies report that males are less likely to receive an HPV vaccine than females. For example, based on the National Health Interview Survey of people aged 18 to 26, the percentage of women who self-reported receiving at least one dose of HPV vaccine was 37% in 2013 and 54% in 2018.10 By contrast, among men, the rates of self-reported vaccination were much lower—8% in 2013 and 27% in 2018.10
The percentage of women who received the recommended number of doses of HPV vaccine (see “9vHPV vaccine: Indications and immunization schedule”) was 26% in 2013 and 35% in 2018.10 For men, these percentages were 2% in 2013 and 9% in 2018.10 These data indicate that, compared with women, men are less likely to receive an HPV vaccination and far less likely to have received the recommended number of doses.
It is heartening that there has been a slow and steady increase in the prevalence of HPV vaccination. In fact, increasing the HPV vaccination rate among both boys and girls has the potential to markedly reduce the incidence of oropharyngeal cancer.
The reasons for the female-male gap in vaccination rates are not fully characterized. For one, parental awareness of the importance of HPV vaccination to prevent cancer among men is limited, and represents an important opportunity for additional public health education. In a qualitative interview study of mothers with children aged 11 to 19, the investigators reported that most mothers were aware that HPV vaccination could prevent cervical cancer in women, but most mothers did not know that HPV causes cancer of the mouth and that vaccination could prevent oropharyngeal cancer in boys and girls.11 Because of this lack of knowledge, the mothers did not think their sons needed to have an HPV vaccine. The research report is aptly titled, “I don’t think he needs the HPV vaccine cause boys can’t have cervical cancer.”11
Clinicians are highly influential in guiding parents to accept HPV vaccination of their children. Offering consistent messaging to parents that HPV vaccination prevents cancer in both women and men, and reducing the out-of-pocket cost of vaccination surely will result in an increase in the vaccination rate of boys and girls. ●
Surgical treatment of tissues infected with human papillomavirus (HPV) often involves the use of laser or electrosurgical devices that generate smoke, which is known to contain HPV nucleic acid sequences and may contain infective virions.1 It is known that HPV nucleic acid sequences are present in surgical smoke. In one study plantar warts were treated with a carbon dioxide laser or electrocoagulation. The vapor produced from the surgery was collected with a dry filter apparatus. Five of 8 laser-derived vapors and 4 of 7 electrocoagulation-derived vapors were positive for HPV DNA. The concentration of HPV DNA was greater with laser than with electrocoagulation treatment.2
It is not known if surgical smoke derived from treatment of HPV-infected tissues contains infective HPV virions. In an experimental bovine model, smoke generated by laser ablation of fibropapillomas was collected. Injection of the contents of the smoke caused cutaneous papillomavirus lesions when inoculated into calves, suggesting that the smoke contained infective HPV virions.3 Although this animal experiment is a proof of principle that surgical smoke generated from treatment of HPVinfected tissue contain virions, it is unclear if surgical smoke generated in gynecologic practice contains HPV virions.
To investigate the prevalence of nasal HPV DNA among gynecologists, 700 physicians in Zhejiang Province, China, completed a questionnaire and provided a nasal swab for HPV DNA analysis.4 Among gynecologists who performed or did not perform LEEP, the prevalence of HPV DNA in the nose was 10% and 3%, respectively. The most common HPV types detected were HPV16 (76%), HPV31 (10%), HPV58 (5%), HPV55 (5%), HPV56 (2%), and HPV59 (2%).4 Among gynecologists who performed LEEP procedures, the prevalence of HPV DNA was 19% for those who did not use a surgical mask, 8% for clinicians who used a standard surgical mask, and 0% for those who used an N95 filtering facepiece respirator, suggesting that an N95 respirator provides the greatest protection from surgical smoke.4 Over 24 months of follow-up, all the gynecologists who had initially tested positive for HPV DNA no longer had detectable nasal HPV DNA. In this study, no gynecologist was diagnosed with an HPV-associated oropharyngeal disease. The investigators concluded that surgical masks, especially an N95 respirator, should be used by gynecologists performing LEEP procedures.
Investigators also have evaluated for the presence of HPV DNA in matched samples from the cervix of 134 patients undergoing loop electrosurgical excision procedure (LEEP) for cervical dysplasia, as well as the smoke generated during the procedure and nasal swabs from the surgeon performing the LEEP.5 HPV DNA was detected in 95% of the cervical samples, 30% of the surgical smoke samples, and 1.5% of the surgeons’ nasal swabs.5 At 6 months of follow-up, the two surgeons who initially had HPV-positive nasal swabs no longer had detected HPV DNA.
Of concern is that otolaryngologists have reported sporadic cases of oropharyngeal squamous cell cancer6 and laryngeal papillomatosis7 in health care workers with frequent and repetitive exposure to HPVs. For example, in one case report, a 53-year-old male gynecologist, nonsmoker, presented to his physician with a lump on the neck.6 The gynecologist had performed more than 3,000 laser ablation or LEEP procedures of dysplastic cervical, vaginal, and vulvar lesions over a span of 20 years.6 Most of the procedures were performed without wearing a mask and in a poorly ventilated procedure room. A computed tomography scan demonstrated a 2.2-cm soft tissue lesion in the right tonsil extending to the right soft palate and a level-2 lymph node. A biopsy of the tonsil confirmed invasive squamous cell carcinoma containing HPV16. He was treated with 35 fractions of radiotherapy and adjuvant cisplatin. Treatment adverse effects included dysphagia and xerostomia, and the patient lost 40 pounds.
Available interventions to reduce exposure of clinicians to HPV virions that may be present in surgical smoke include:
- wearing a fit-tested N95 respirator
- routinely using a smoke evacuation device, and
- ensuring sufficient ventilation in the procedure room.
A new recommendation is to consider 9vHPV vaccination for clinicians who are routinely exposed to HPV virions.8,9 In February 2020, the American Society for Colposcopy and Cervical Pathology recommended that clinicians who are routinely exposed to HPVs consider 9vHPV vaccination.8 This recommendation pertains to all members of the clinical team in the procedure room, including physicians, nurses, and staff. Based on the available data, gynecologists who have not been vaccinated will need to weigh the benefits and costs of receiving a 9vHPV vaccine to protect themselves against an occupational exposure that may adversely impact their health.
References
- Liu Y, Song Y, Hu X, et al. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among gynecologists. J Cancer. 2019;10:2788-2799.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Garden JM, O’Banion MK, Bakus AD, et al. Viral transmitted by laser-generated plume (aerosol). Arch Dermatol. 2002;138:1303-1307.
- Hu X, Zhou Q, Yu J, et al. Prevalence of HPV infections in surgical smoke exposed gynecologists. Int Arch Occup Environ Health. 2020; Epub September 1. doi: 10.1007 /s00420-020-01568-9.
- Zhou Q, Hu X, Zhou J, et al. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019;11:3643-3654.
- Rioux M, Garland A, Webster D, et al. HPV-positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54-57.
- Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425-427.
Stockdale CK, Einstein MH, Huh WK. ASCCP recommends HPV vaccination for providers. February 19, 2020. www.asccp.org/Assets/d3abdb05-25c5-4e58-%209cec-05c11fb2b920/637177876310030000/hpv-vaccinemember-announcment-02-19-20-pdf. Accessed October 23, 2020.
- Harrison R, Huh W. Occupational exposure to human papillomavirus and vaccination for health care workers. Obstet Gynecol. 2020;136:663-665
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers--United States, 1999-2015. MMWR. 2018;67:918-924.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Haeggblom L, Ramqvist T, Tommasino M, et al. Time to change perspective on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Research. 2017;4:1-11.
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-475.
- D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664-670.
- D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408-2415.
- Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693.
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262-267.
- Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18 to 26, 2013-2018. NCHS Data Brief. 2020:1-8.
- Lindsay AC, Delgado D, Valdez MJ, et al. "I don't think he needs the HPV vaccine cause boys can't have cervical cancer": a qualitative study of Latina mothers' (Mis) understandings about human papillomavirus transmission, associated cancers and the vaccine. J Cancer Educ. July 11, 2020. doi: 10.1007/s13187-020-01824-z.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers--United States, 1999-2015. MMWR. 2018;67:918-924.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Haeggblom L, Ramqvist T, Tommasino M, et al. Time to change perspective on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Research. 2017;4:1-11.
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-475.
- D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664-670.
- D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408-2415.
- Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693.
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262-267.
- Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18 to 26, 2013-2018. NCHS Data Brief. 2020:1-8.
- Lindsay AC, Delgado D, Valdez MJ, et al. "I don't think he needs the HPV vaccine cause boys can't have cervical cancer": a qualitative study of Latina mothers' (Mis) understandings about human papillomavirus transmission, associated cancers and the vaccine. J Cancer Educ. July 11, 2020. doi: 10.1007/s13187-020-01824-z.
Apps for applying to ObGyn residency programs in the era of virtual interviews
The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.
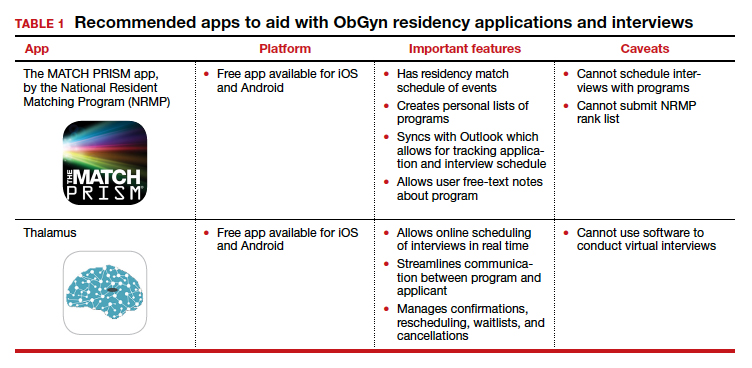
We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●
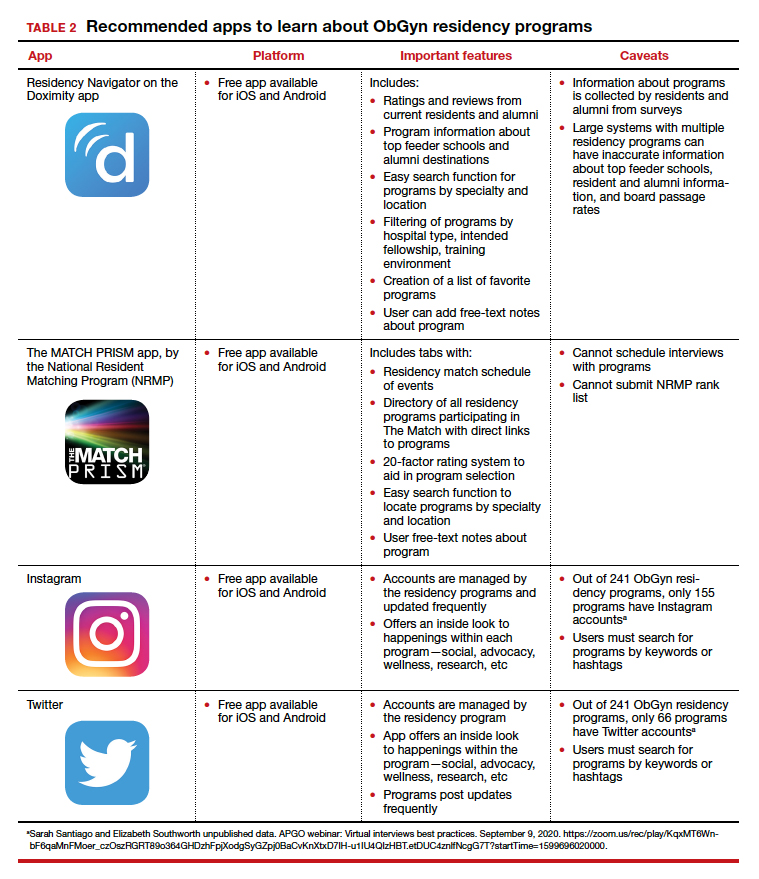
- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.

We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●

The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.

We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●

- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
Adenomyosis: An update on imaging, medical, and surgical treatment
Adenomyosis is a benign disorder, present in 20%-35% of women and characterized by the presence of endometrial glands and stroma within the myometrium. The ectopic endometrial tissue appears to cause hypertrophy in the myometrium, resulting in an enlarged globular uterus.
Adenomyosis may present as diffuse or focal involvement within the uterus. When the focal lesion appears to be well defined, it is referred to as an adenomyoma. It is not encapsulated like a fibroid. There may be involvement of the junctional zone of the myometrium – the area between the subendometrial myometrium and the outer myometrium. While the pathogenesis of adenomyosis is unknown, two rigorous theories exist: endomyometrial invagination of the endometrium and de novo from Müllerian rests.
For this installment of the Master Class in Gynecologic Surgery, I have enlisted Keith B. Isaacson, MD, to discuss the clinical presentation, diagnosis, and medical and surgical treatment of adenomyosis.
Dr. Isaacson is the director of minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, Newton, Mass., and associate professor of obstetrics and gynecology at Harvard Medical School, Boston. He is currently in practice specializing in minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, where he is the director of the AAGL Fellowship in Minimally Invasive Gynecologic Surgery. Dr. Isaacson is a past president of both the AAGL and the Society of Reproductive Surgeons, as well as a published clinical researcher and surgical innovator.
It is a true honor to welcome Dr. Isaacson to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is professor of obstetrics & gynecology in the Department of Clinical Sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Dr. Miller reported that he has no relevant disclosures. Email him at [email protected].
Adenomyosis is a benign disorder, present in 20%-35% of women and characterized by the presence of endometrial glands and stroma within the myometrium. The ectopic endometrial tissue appears to cause hypertrophy in the myometrium, resulting in an enlarged globular uterus.
Adenomyosis may present as diffuse or focal involvement within the uterus. When the focal lesion appears to be well defined, it is referred to as an adenomyoma. It is not encapsulated like a fibroid. There may be involvement of the junctional zone of the myometrium – the area between the subendometrial myometrium and the outer myometrium. While the pathogenesis of adenomyosis is unknown, two rigorous theories exist: endomyometrial invagination of the endometrium and de novo from Müllerian rests.
For this installment of the Master Class in Gynecologic Surgery, I have enlisted Keith B. Isaacson, MD, to discuss the clinical presentation, diagnosis, and medical and surgical treatment of adenomyosis.
Dr. Isaacson is the director of minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, Newton, Mass., and associate professor of obstetrics and gynecology at Harvard Medical School, Boston. He is currently in practice specializing in minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, where he is the director of the AAGL Fellowship in Minimally Invasive Gynecologic Surgery. Dr. Isaacson is a past president of both the AAGL and the Society of Reproductive Surgeons, as well as a published clinical researcher and surgical innovator.
It is a true honor to welcome Dr. Isaacson to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is professor of obstetrics & gynecology in the Department of Clinical Sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Dr. Miller reported that he has no relevant disclosures. Email him at [email protected].
Adenomyosis is a benign disorder, present in 20%-35% of women and characterized by the presence of endometrial glands and stroma within the myometrium. The ectopic endometrial tissue appears to cause hypertrophy in the myometrium, resulting in an enlarged globular uterus.
Adenomyosis may present as diffuse or focal involvement within the uterus. When the focal lesion appears to be well defined, it is referred to as an adenomyoma. It is not encapsulated like a fibroid. There may be involvement of the junctional zone of the myometrium – the area between the subendometrial myometrium and the outer myometrium. While the pathogenesis of adenomyosis is unknown, two rigorous theories exist: endomyometrial invagination of the endometrium and de novo from Müllerian rests.
For this installment of the Master Class in Gynecologic Surgery, I have enlisted Keith B. Isaacson, MD, to discuss the clinical presentation, diagnosis, and medical and surgical treatment of adenomyosis.
Dr. Isaacson is the director of minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, Newton, Mass., and associate professor of obstetrics and gynecology at Harvard Medical School, Boston. He is currently in practice specializing in minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, where he is the director of the AAGL Fellowship in Minimally Invasive Gynecologic Surgery. Dr. Isaacson is a past president of both the AAGL and the Society of Reproductive Surgeons, as well as a published clinical researcher and surgical innovator.
It is a true honor to welcome Dr. Isaacson to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is professor of obstetrics & gynecology in the Department of Clinical Sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Dr. Miller reported that he has no relevant disclosures. Email him at [email protected].