User login
Supreme Court Case: Dobbs v Jackson Women’s Health Organization: What you need to know
This fall, the Supreme Court of the United States (SCOTUS) will announce when they will hear oral arguments for Dobbs v Jackson Women’s Health Organization. The court will examine a Mississippi law, known as the “Gestational Age Act,” originally passed in 2018, that sought to “limit abortions to fifteen weeks’ gestation except in a medical emergency or in cases of severe fetal abnormality.”1 This sets the stage for SCOTUS to make a major ruling on abortion, one which could affirm or upend landmark decisions and nearly 50 years of abortion legislative precedent. Additionally, SCOTUS’ recent decision to not intervene on Texas’ Senate Bill 8 (SB8), which essentially bans all abortions after 6 weeks’ gestational age, may foreshadow how this case will be decided. The current abortion restrictions in Texas and the implications of SB8 will be discussed in a forthcoming column.
SCOTUS and abortion rights
The decision to hear this case comes on the heels of another recent decision regarding a Louisiana law in June Medical Services v Russo. This case examined Louisiana Act 620, which would have required physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.2 The law was deemed constitutionally invalid, with the majority noting the law would have drastically burdened a woman’s right to access abortion services. The Court ruled similarly in 2016 in Whole Women’s Health (WWH) v Hellerstedt, in which WWH challenged Texas House Bill 2, a nearly identical law requiring admitting privileges for abortion care providers. In both of these cases, SCOTUS pointed to precedent set by Southeastern Pennsylvania v Casey, which established that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 The precedent to this, Roe v Wade, and 5 decades of abortion legislation set may be upended by a SCOTUS decision this next term.
Dobbs v Jackson
On March 19, 2018, Mississippi enacted the “Gestational Age Act” into law. The newly enacted law would limit abortions to 15 weeks’ gestation except in a medical emergency or in cases of severe fetal anomalies. Jackson Women’s Health Organization, the only licensed abortion provider in the state, challenged the constitutionality of the law with legal support from Center for Reproductive Rights (CRR). The US District Court for the Southern District of Mississippi granted summary judgement in favor of the clinic and placed an injunction on the law’s enforcement. The state appealed to the Fifth Circuit Court of Appeals, which upheld the district court decision in a 3-0 decision in November 2019. Mississippi appealed to the Supreme Court, with their petition focusing on multiple questions from the appeals process. After repeatedly rescheduling the case, and multiple reviews in conference, SCOTUS agreed to hear the case. Most recently, the state has narrowed its argument, changing course, and attacking Roe v Wade directly. In a brief submitted in July 2021, the state argues the court should hold that all pre-viability prohibitions on elective abortions are constitutional.
Interestingly, during this time the Mississippi legislature also passed a law, House Bill 2116, also known as the “fetal heartbeat bill,” banning abortion with gestational ages after detection of a fetal heartbeat. This was also challenged, deemed unconstitutional, and affirmed on appeal by the Fifth US Circuit Court.
While recent challenges have focused on the “undue burden” state laws placed on those trying to access abortion care, this case will bring the issue of “viability” and gestational age limits to the forefront.4,5 In addition to Roe v Wade, the Court will have the opportunity to reexamine other relevant precedent, such as Southeastern Pennsylvania v Casey, in considering the most recent arguments of the state. In this most recent brief, the state argues that the Court should, “reject viability as a barrier to prohibiting elective abortions” and that a “viability rule has no constitutional basis.” The state goes on to argue the “Constitution does not protect a right to abortion or limit States’ authority to restrict it.”6 The language and tone in this brief are more direct and aggressive than the states’ petition submitted last June.
However, the composition of the Court is different than in the past. This case will be argued with Justice Amy Coney Barrett seated in place of Justice Ruth Bader Ginsburg, who was a strong advocate for women’s rights.7 She joins Justices Neil Gorsuch and Brett Kavanaugh, also appointed by President Donald Trump and widely viewed as conservative judges, tipping the scales to a more conservative Supreme Court. This case will also be argued in a polarized political environment.8,9 Given the conservative Supreme Court in the setting of an increasingly politically charged environment, reproductive right advocates are understandably worried that members of the anti-abortion movement view this as an opportunity to weaken or remove federal constitutional protections for abortion.
Continue to: Potential outcome of Dobbs v Jackson...
Potential outcome of Dobbs v Jackson
Should SCOTUS choose to rule in favor of Mississippi, it could severely weaken, or even overturn Roe v Wade. This would leave a legal path for states with pre-Roe abortion bans and currently unenforced post-Roe bans to take effect. These “trigger” laws are bans or severe restrictions on abortion providers and patients intended to take effect if Roe were to be overturned. Alternatively, the Court may overturn Southeastern Pennsylvania v Casey, but maintain Roe v Wade, essentially leaving the regulation of pre-viability abortion care to individual states. Currently 21 states have laws that would restrict the legal status of abortion.10 In addition, state legislatures are aggressively introducing abortion restrictions. As of June 2021, there have been 561 abortion restrictions, including 165 abortion bans, introduced across 47 states, putting 2021 on course to be the most devastating anti-abortion state legislative session in decades.11
The damage caused by such restriction on abortion care would be significant. It would block or push access out of reach for many. The negative effects of such legislative action would most heavily burden those already marginalized by systemic, structural inequalities including those of low socioeconomic status, people of color, young people, those in rural communities, and members of the LGBTQ community. The medical community has long recognized the harm caused by restricting access to abortion care. Restriction of access to safe abortion care paradoxically has been shown not to decrease the incidence of abortion, but rather increases the number of unsafe abortions.12 The American College of Obstetricians and Gynecologists (ACOG) acknowledge “individuals require access to safe, legal abortion” and that this represents “a necessary component for comprehensive health care.”13,14 They joined the American Medical Association and other professional groups in a 2019 amicus brief to SCOTUS opposing restrictions on abortion access.15 In addition, government laws restricting access to abortion care undermine the fundamental relationship between a person and their physician, limiting a physician’s obligation to honor patient autonomy and provide appropriate medical care.
By taking up the question whether all pre-viability bans on elective abortions violate the Constitution, SCOTUS is indicating a possible willingness to revisit the central holding of abortion jurisprudence. Their decision regarding this case will likely be the most significant ruling regarding the legal status of abortion care in decades, and will significantly affect the delivery of abortion care in the future.
Action items
- Reach out to your representatives to support the Women’s Health Protection Act, an initiative introduced to Congress to protect access to abortion care. If you reside in a state where your federal representatives support the Women’s Health Protection Act, reach out to friends and colleagues in states without supportive elected officials and ask them to call their representatives and ask them to support the bill.
- Get involved with local grassroots groups fighting to protect abortion access.
- Continue to speak out against laws and policies designed to limit access to safe abortion care.
- Connect with your local ACOG chapter for more ways to become involved.
- As always, make sure you are registered to vote, and exercise your right whenever you can.
- HB1510 (As Introduced) - 2018 Regular Session. http://billstatus.ls.state.ms.us/documents/2018/html/HB/1500-1599/HB1510IN.htm Accessed August 13, 2021.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed August 13, 2021.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed August 13, 2021.
- 15-274 Whole Woman’s Health v. Hellerstedt (06/27/2016). Published online 2016:107.
- 18-1323 June Medical Services L. L. C. v. Russo (06/29/2020). Published online 2020:138.
- 19-1392 Dobbs v. Jackson Women’s Health Organization (07/22/2021). Published online 2021.
- What Ruth Bader Ginsburg said about abortion and Roe v. Wade. Time. August 2, 2018. https://time.com/5354490/ruth-bader-ginsburg-roe-v-wade/. Accessed August 13, 2021.
- Montanaro D. Poll: majority want to keep abortion legal, but they also want restrictions. NPR. June 7, 2019. https://www.npr.org/2019/06/07/730183531/poll-majority-want-to-keep-abortion-legal-but-they-also-want-restrictions. Accessed August 13, 2021.
- Abortion support remains steady despite growing partisan divide, survey finds. Washington Post. August 13, 2019. https://www.washingtonpost.com/health/2019/08/13/one-largest-ever-abortion-surveys-shows-growing-partisan-divide/. Accessed August 13, 2021.
- Abortion policy in the absence of Roe. Guttmacher Institute. September 1, 2021. https://www.guttmacher.org/state-policy/explore/abortion-policy-absence-roe#. Accessed September 8, 2021.
- 2021 is on track to become the most devastating antiabortion state legislative session in decades. Guttmacher Institute. Published April 30, 2021. Updated June 14, 2021. https://www.guttmacher.org/article/2021/04/2021-track-become-most-devastating-antiabortion-state-legislative-session-decades. Accessed August 13, 2021.
- Facts and consequences: legality, incidence and safety of abortion worldwide. Guttmacher Institute. November 20, 2009. https://www.guttmacher.org/gpr/2009/11/facts-and-consequences-legality-incidence-and-safety-abortion-worldwide. Accessed August 13, 2021.
- Increasing access to abortion. https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2020/12/increasing-access-to-abortion. Accessed August 13, 2021.
- ACOG statement on Dobbs vs. Jackson Women’s Health. May 17, 2021. https://www.acog.org/en/news/news-releases/2021/05/acog-statement-dobbs-vs-jackson-womens-health. Accessed August 13, 2021.
- Perryman SL, Parker KA, Hickman SA. Brief of amici curiae American College of Obstetricians and Gynecologists, American Medical Associations, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, et al. In support of June Medical Services, LLC, et al. https://www.supremecourt.gov/
DocketPDF/18/18-1323/124091/ . Accessed August 13, 2021.20191202145531124_18-1323% 2018-1460%20tsac%20American% 20College%20of% 20Obstetricians%20and% 20Gynecologists%20et%20al.pdf
This fall, the Supreme Court of the United States (SCOTUS) will announce when they will hear oral arguments for Dobbs v Jackson Women’s Health Organization. The court will examine a Mississippi law, known as the “Gestational Age Act,” originally passed in 2018, that sought to “limit abortions to fifteen weeks’ gestation except in a medical emergency or in cases of severe fetal abnormality.”1 This sets the stage for SCOTUS to make a major ruling on abortion, one which could affirm or upend landmark decisions and nearly 50 years of abortion legislative precedent. Additionally, SCOTUS’ recent decision to not intervene on Texas’ Senate Bill 8 (SB8), which essentially bans all abortions after 6 weeks’ gestational age, may foreshadow how this case will be decided. The current abortion restrictions in Texas and the implications of SB8 will be discussed in a forthcoming column.
SCOTUS and abortion rights
The decision to hear this case comes on the heels of another recent decision regarding a Louisiana law in June Medical Services v Russo. This case examined Louisiana Act 620, which would have required physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.2 The law was deemed constitutionally invalid, with the majority noting the law would have drastically burdened a woman’s right to access abortion services. The Court ruled similarly in 2016 in Whole Women’s Health (WWH) v Hellerstedt, in which WWH challenged Texas House Bill 2, a nearly identical law requiring admitting privileges for abortion care providers. In both of these cases, SCOTUS pointed to precedent set by Southeastern Pennsylvania v Casey, which established that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 The precedent to this, Roe v Wade, and 5 decades of abortion legislation set may be upended by a SCOTUS decision this next term.
Dobbs v Jackson
On March 19, 2018, Mississippi enacted the “Gestational Age Act” into law. The newly enacted law would limit abortions to 15 weeks’ gestation except in a medical emergency or in cases of severe fetal anomalies. Jackson Women’s Health Organization, the only licensed abortion provider in the state, challenged the constitutionality of the law with legal support from Center for Reproductive Rights (CRR). The US District Court for the Southern District of Mississippi granted summary judgement in favor of the clinic and placed an injunction on the law’s enforcement. The state appealed to the Fifth Circuit Court of Appeals, which upheld the district court decision in a 3-0 decision in November 2019. Mississippi appealed to the Supreme Court, with their petition focusing on multiple questions from the appeals process. After repeatedly rescheduling the case, and multiple reviews in conference, SCOTUS agreed to hear the case. Most recently, the state has narrowed its argument, changing course, and attacking Roe v Wade directly. In a brief submitted in July 2021, the state argues the court should hold that all pre-viability prohibitions on elective abortions are constitutional.
Interestingly, during this time the Mississippi legislature also passed a law, House Bill 2116, also known as the “fetal heartbeat bill,” banning abortion with gestational ages after detection of a fetal heartbeat. This was also challenged, deemed unconstitutional, and affirmed on appeal by the Fifth US Circuit Court.
While recent challenges have focused on the “undue burden” state laws placed on those trying to access abortion care, this case will bring the issue of “viability” and gestational age limits to the forefront.4,5 In addition to Roe v Wade, the Court will have the opportunity to reexamine other relevant precedent, such as Southeastern Pennsylvania v Casey, in considering the most recent arguments of the state. In this most recent brief, the state argues that the Court should, “reject viability as a barrier to prohibiting elective abortions” and that a “viability rule has no constitutional basis.” The state goes on to argue the “Constitution does not protect a right to abortion or limit States’ authority to restrict it.”6 The language and tone in this brief are more direct and aggressive than the states’ petition submitted last June.
However, the composition of the Court is different than in the past. This case will be argued with Justice Amy Coney Barrett seated in place of Justice Ruth Bader Ginsburg, who was a strong advocate for women’s rights.7 She joins Justices Neil Gorsuch and Brett Kavanaugh, also appointed by President Donald Trump and widely viewed as conservative judges, tipping the scales to a more conservative Supreme Court. This case will also be argued in a polarized political environment.8,9 Given the conservative Supreme Court in the setting of an increasingly politically charged environment, reproductive right advocates are understandably worried that members of the anti-abortion movement view this as an opportunity to weaken or remove federal constitutional protections for abortion.
Continue to: Potential outcome of Dobbs v Jackson...
Potential outcome of Dobbs v Jackson
Should SCOTUS choose to rule in favor of Mississippi, it could severely weaken, or even overturn Roe v Wade. This would leave a legal path for states with pre-Roe abortion bans and currently unenforced post-Roe bans to take effect. These “trigger” laws are bans or severe restrictions on abortion providers and patients intended to take effect if Roe were to be overturned. Alternatively, the Court may overturn Southeastern Pennsylvania v Casey, but maintain Roe v Wade, essentially leaving the regulation of pre-viability abortion care to individual states. Currently 21 states have laws that would restrict the legal status of abortion.10 In addition, state legislatures are aggressively introducing abortion restrictions. As of June 2021, there have been 561 abortion restrictions, including 165 abortion bans, introduced across 47 states, putting 2021 on course to be the most devastating anti-abortion state legislative session in decades.11
The damage caused by such restriction on abortion care would be significant. It would block or push access out of reach for many. The negative effects of such legislative action would most heavily burden those already marginalized by systemic, structural inequalities including those of low socioeconomic status, people of color, young people, those in rural communities, and members of the LGBTQ community. The medical community has long recognized the harm caused by restricting access to abortion care. Restriction of access to safe abortion care paradoxically has been shown not to decrease the incidence of abortion, but rather increases the number of unsafe abortions.12 The American College of Obstetricians and Gynecologists (ACOG) acknowledge “individuals require access to safe, legal abortion” and that this represents “a necessary component for comprehensive health care.”13,14 They joined the American Medical Association and other professional groups in a 2019 amicus brief to SCOTUS opposing restrictions on abortion access.15 In addition, government laws restricting access to abortion care undermine the fundamental relationship between a person and their physician, limiting a physician’s obligation to honor patient autonomy and provide appropriate medical care.
By taking up the question whether all pre-viability bans on elective abortions violate the Constitution, SCOTUS is indicating a possible willingness to revisit the central holding of abortion jurisprudence. Their decision regarding this case will likely be the most significant ruling regarding the legal status of abortion care in decades, and will significantly affect the delivery of abortion care in the future.
Action items
- Reach out to your representatives to support the Women’s Health Protection Act, an initiative introduced to Congress to protect access to abortion care. If you reside in a state where your federal representatives support the Women’s Health Protection Act, reach out to friends and colleagues in states without supportive elected officials and ask them to call their representatives and ask them to support the bill.
- Get involved with local grassroots groups fighting to protect abortion access.
- Continue to speak out against laws and policies designed to limit access to safe abortion care.
- Connect with your local ACOG chapter for more ways to become involved.
- As always, make sure you are registered to vote, and exercise your right whenever you can.
This fall, the Supreme Court of the United States (SCOTUS) will announce when they will hear oral arguments for Dobbs v Jackson Women’s Health Organization. The court will examine a Mississippi law, known as the “Gestational Age Act,” originally passed in 2018, that sought to “limit abortions to fifteen weeks’ gestation except in a medical emergency or in cases of severe fetal abnormality.”1 This sets the stage for SCOTUS to make a major ruling on abortion, one which could affirm or upend landmark decisions and nearly 50 years of abortion legislative precedent. Additionally, SCOTUS’ recent decision to not intervene on Texas’ Senate Bill 8 (SB8), which essentially bans all abortions after 6 weeks’ gestational age, may foreshadow how this case will be decided. The current abortion restrictions in Texas and the implications of SB8 will be discussed in a forthcoming column.
SCOTUS and abortion rights
The decision to hear this case comes on the heels of another recent decision regarding a Louisiana law in June Medical Services v Russo. This case examined Louisiana Act 620, which would have required physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.2 The law was deemed constitutionally invalid, with the majority noting the law would have drastically burdened a woman’s right to access abortion services. The Court ruled similarly in 2016 in Whole Women’s Health (WWH) v Hellerstedt, in which WWH challenged Texas House Bill 2, a nearly identical law requiring admitting privileges for abortion care providers. In both of these cases, SCOTUS pointed to precedent set by Southeastern Pennsylvania v Casey, which established that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 The precedent to this, Roe v Wade, and 5 decades of abortion legislation set may be upended by a SCOTUS decision this next term.
Dobbs v Jackson
On March 19, 2018, Mississippi enacted the “Gestational Age Act” into law. The newly enacted law would limit abortions to 15 weeks’ gestation except in a medical emergency or in cases of severe fetal anomalies. Jackson Women’s Health Organization, the only licensed abortion provider in the state, challenged the constitutionality of the law with legal support from Center for Reproductive Rights (CRR). The US District Court for the Southern District of Mississippi granted summary judgement in favor of the clinic and placed an injunction on the law’s enforcement. The state appealed to the Fifth Circuit Court of Appeals, which upheld the district court decision in a 3-0 decision in November 2019. Mississippi appealed to the Supreme Court, with their petition focusing on multiple questions from the appeals process. After repeatedly rescheduling the case, and multiple reviews in conference, SCOTUS agreed to hear the case. Most recently, the state has narrowed its argument, changing course, and attacking Roe v Wade directly. In a brief submitted in July 2021, the state argues the court should hold that all pre-viability prohibitions on elective abortions are constitutional.
Interestingly, during this time the Mississippi legislature also passed a law, House Bill 2116, also known as the “fetal heartbeat bill,” banning abortion with gestational ages after detection of a fetal heartbeat. This was also challenged, deemed unconstitutional, and affirmed on appeal by the Fifth US Circuit Court.
While recent challenges have focused on the “undue burden” state laws placed on those trying to access abortion care, this case will bring the issue of “viability” and gestational age limits to the forefront.4,5 In addition to Roe v Wade, the Court will have the opportunity to reexamine other relevant precedent, such as Southeastern Pennsylvania v Casey, in considering the most recent arguments of the state. In this most recent brief, the state argues that the Court should, “reject viability as a barrier to prohibiting elective abortions” and that a “viability rule has no constitutional basis.” The state goes on to argue the “Constitution does not protect a right to abortion or limit States’ authority to restrict it.”6 The language and tone in this brief are more direct and aggressive than the states’ petition submitted last June.
However, the composition of the Court is different than in the past. This case will be argued with Justice Amy Coney Barrett seated in place of Justice Ruth Bader Ginsburg, who was a strong advocate for women’s rights.7 She joins Justices Neil Gorsuch and Brett Kavanaugh, also appointed by President Donald Trump and widely viewed as conservative judges, tipping the scales to a more conservative Supreme Court. This case will also be argued in a polarized political environment.8,9 Given the conservative Supreme Court in the setting of an increasingly politically charged environment, reproductive right advocates are understandably worried that members of the anti-abortion movement view this as an opportunity to weaken or remove federal constitutional protections for abortion.
Continue to: Potential outcome of Dobbs v Jackson...
Potential outcome of Dobbs v Jackson
Should SCOTUS choose to rule in favor of Mississippi, it could severely weaken, or even overturn Roe v Wade. This would leave a legal path for states with pre-Roe abortion bans and currently unenforced post-Roe bans to take effect. These “trigger” laws are bans or severe restrictions on abortion providers and patients intended to take effect if Roe were to be overturned. Alternatively, the Court may overturn Southeastern Pennsylvania v Casey, but maintain Roe v Wade, essentially leaving the regulation of pre-viability abortion care to individual states. Currently 21 states have laws that would restrict the legal status of abortion.10 In addition, state legislatures are aggressively introducing abortion restrictions. As of June 2021, there have been 561 abortion restrictions, including 165 abortion bans, introduced across 47 states, putting 2021 on course to be the most devastating anti-abortion state legislative session in decades.11
The damage caused by such restriction on abortion care would be significant. It would block or push access out of reach for many. The negative effects of such legislative action would most heavily burden those already marginalized by systemic, structural inequalities including those of low socioeconomic status, people of color, young people, those in rural communities, and members of the LGBTQ community. The medical community has long recognized the harm caused by restricting access to abortion care. Restriction of access to safe abortion care paradoxically has been shown not to decrease the incidence of abortion, but rather increases the number of unsafe abortions.12 The American College of Obstetricians and Gynecologists (ACOG) acknowledge “individuals require access to safe, legal abortion” and that this represents “a necessary component for comprehensive health care.”13,14 They joined the American Medical Association and other professional groups in a 2019 amicus brief to SCOTUS opposing restrictions on abortion access.15 In addition, government laws restricting access to abortion care undermine the fundamental relationship between a person and their physician, limiting a physician’s obligation to honor patient autonomy and provide appropriate medical care.
By taking up the question whether all pre-viability bans on elective abortions violate the Constitution, SCOTUS is indicating a possible willingness to revisit the central holding of abortion jurisprudence. Their decision regarding this case will likely be the most significant ruling regarding the legal status of abortion care in decades, and will significantly affect the delivery of abortion care in the future.
Action items
- Reach out to your representatives to support the Women’s Health Protection Act, an initiative introduced to Congress to protect access to abortion care. If you reside in a state where your federal representatives support the Women’s Health Protection Act, reach out to friends and colleagues in states without supportive elected officials and ask them to call their representatives and ask them to support the bill.
- Get involved with local grassroots groups fighting to protect abortion access.
- Continue to speak out against laws and policies designed to limit access to safe abortion care.
- Connect with your local ACOG chapter for more ways to become involved.
- As always, make sure you are registered to vote, and exercise your right whenever you can.
- HB1510 (As Introduced) - 2018 Regular Session. http://billstatus.ls.state.ms.us/documents/2018/html/HB/1500-1599/HB1510IN.htm Accessed August 13, 2021.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed August 13, 2021.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed August 13, 2021.
- 15-274 Whole Woman’s Health v. Hellerstedt (06/27/2016). Published online 2016:107.
- 18-1323 June Medical Services L. L. C. v. Russo (06/29/2020). Published online 2020:138.
- 19-1392 Dobbs v. Jackson Women’s Health Organization (07/22/2021). Published online 2021.
- What Ruth Bader Ginsburg said about abortion and Roe v. Wade. Time. August 2, 2018. https://time.com/5354490/ruth-bader-ginsburg-roe-v-wade/. Accessed August 13, 2021.
- Montanaro D. Poll: majority want to keep abortion legal, but they also want restrictions. NPR. June 7, 2019. https://www.npr.org/2019/06/07/730183531/poll-majority-want-to-keep-abortion-legal-but-they-also-want-restrictions. Accessed August 13, 2021.
- Abortion support remains steady despite growing partisan divide, survey finds. Washington Post. August 13, 2019. https://www.washingtonpost.com/health/2019/08/13/one-largest-ever-abortion-surveys-shows-growing-partisan-divide/. Accessed August 13, 2021.
- Abortion policy in the absence of Roe. Guttmacher Institute. September 1, 2021. https://www.guttmacher.org/state-policy/explore/abortion-policy-absence-roe#. Accessed September 8, 2021.
- 2021 is on track to become the most devastating antiabortion state legislative session in decades. Guttmacher Institute. Published April 30, 2021. Updated June 14, 2021. https://www.guttmacher.org/article/2021/04/2021-track-become-most-devastating-antiabortion-state-legislative-session-decades. Accessed August 13, 2021.
- Facts and consequences: legality, incidence and safety of abortion worldwide. Guttmacher Institute. November 20, 2009. https://www.guttmacher.org/gpr/2009/11/facts-and-consequences-legality-incidence-and-safety-abortion-worldwide. Accessed August 13, 2021.
- Increasing access to abortion. https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2020/12/increasing-access-to-abortion. Accessed August 13, 2021.
- ACOG statement on Dobbs vs. Jackson Women’s Health. May 17, 2021. https://www.acog.org/en/news/news-releases/2021/05/acog-statement-dobbs-vs-jackson-womens-health. Accessed August 13, 2021.
- Perryman SL, Parker KA, Hickman SA. Brief of amici curiae American College of Obstetricians and Gynecologists, American Medical Associations, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, et al. In support of June Medical Services, LLC, et al. https://www.supremecourt.gov/
DocketPDF/18/18-1323/124091/ . Accessed August 13, 2021.20191202145531124_18-1323% 2018-1460%20tsac%20American% 20College%20of% 20Obstetricians%20and% 20Gynecologists%20et%20al.pdf
- HB1510 (As Introduced) - 2018 Regular Session. http://billstatus.ls.state.ms.us/documents/2018/html/HB/1500-1599/HB1510IN.htm Accessed August 13, 2021.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed August 13, 2021.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed August 13, 2021.
- 15-274 Whole Woman’s Health v. Hellerstedt (06/27/2016). Published online 2016:107.
- 18-1323 June Medical Services L. L. C. v. Russo (06/29/2020). Published online 2020:138.
- 19-1392 Dobbs v. Jackson Women’s Health Organization (07/22/2021). Published online 2021.
- What Ruth Bader Ginsburg said about abortion and Roe v. Wade. Time. August 2, 2018. https://time.com/5354490/ruth-bader-ginsburg-roe-v-wade/. Accessed August 13, 2021.
- Montanaro D. Poll: majority want to keep abortion legal, but they also want restrictions. NPR. June 7, 2019. https://www.npr.org/2019/06/07/730183531/poll-majority-want-to-keep-abortion-legal-but-they-also-want-restrictions. Accessed August 13, 2021.
- Abortion support remains steady despite growing partisan divide, survey finds. Washington Post. August 13, 2019. https://www.washingtonpost.com/health/2019/08/13/one-largest-ever-abortion-surveys-shows-growing-partisan-divide/. Accessed August 13, 2021.
- Abortion policy in the absence of Roe. Guttmacher Institute. September 1, 2021. https://www.guttmacher.org/state-policy/explore/abortion-policy-absence-roe#. Accessed September 8, 2021.
- 2021 is on track to become the most devastating antiabortion state legislative session in decades. Guttmacher Institute. Published April 30, 2021. Updated June 14, 2021. https://www.guttmacher.org/article/2021/04/2021-track-become-most-devastating-antiabortion-state-legislative-session-decades. Accessed August 13, 2021.
- Facts and consequences: legality, incidence and safety of abortion worldwide. Guttmacher Institute. November 20, 2009. https://www.guttmacher.org/gpr/2009/11/facts-and-consequences-legality-incidence-and-safety-abortion-worldwide. Accessed August 13, 2021.
- Increasing access to abortion. https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2020/12/increasing-access-to-abortion. Accessed August 13, 2021.
- ACOG statement on Dobbs vs. Jackson Women’s Health. May 17, 2021. https://www.acog.org/en/news/news-releases/2021/05/acog-statement-dobbs-vs-jackson-womens-health. Accessed August 13, 2021.
- Perryman SL, Parker KA, Hickman SA. Brief of amici curiae American College of Obstetricians and Gynecologists, American Medical Associations, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, et al. In support of June Medical Services, LLC, et al. https://www.supremecourt.gov/
DocketPDF/18/18-1323/124091/ . Accessed August 13, 2021.20191202145531124_18-1323% 2018-1460%20tsac%20American% 20College%20of% 20Obstetricians%20and% 20Gynecologists%20et%20al.pdf
Expert shares vulvovaginal candidiasis treatment pearls
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
FROM PDA 2021
Telehealth abortions are 95% effective, similar to in-person care
Telehealth abortion may be just as safe and effective as in-person care, according to a small study published online in JAMA Network Open.
Of the 110 women from whom researchers collected remote abortion outcome data, 95% had a complete abortion without additional medical interventions, such as aspiration or surgery, and none experienced adverse events. Researchers said this efficacy rate is similar to in-person visits.
“There was no reason to expect that the medications prescribed [via telemedicine] and delivered through the mail would have different outcomes from when a patient traveled to a clinic,” study author Ushma D. Upadhyay, PhD, MPH, associate professor in the department of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco, said in an interview.
Medication abortion, which usually involves taking mifepristone (Mifeprex) followed by misoprostol (Cytotec) during the first 10 weeks of pregnancy, has been available in the United States since 2000. The Food and Drug Administration’s Risk Evaluation and Mitigation Strategy requires that mifepristone be dispensed in a medical office, clinic, or hospital, prohibiting dispensing from pharmacies in an effort to reduce potential risk for complications.
In April 2021, the FDA lifted the in-person dispensing requirement for mifepristone for the duration of the COVID-19 pandemic. However, Dr. Upadhyay hopes the findings of her current study will make this suspension permanent.
For the study, Dr. Upadhyay and colleagues examined the safety and efficacy of fully remote, medication abortion care. Eligibility for the medication was assessed using an online form that relies on patient history, or patients recalling their last period, to assess pregnancy duration and screen for ectopic pregnancy risks. Nurse practitioners reviewed the form and referred patients with unknown last menstrual period date or ectopic pregnancy risk factors for ultrasonography. A mail-order pharmacy delivered medications to eligible patients. The protocol involved three follow-up contacts: confirmation of medication administration, a 3-day assessment of symptoms, and a home pregnancy test after 4 weeks. Follow-up interactions were conducted by text, secure messaging, or telephone.
Researchers found that in addition to the 95% of the patients having a complete abortion without intervention, 5% (five) of patients required addition medical care to complete the abortion. Two of those patients were treated in EDs.
Gillian Burkhardt, MD, who was not involved in the study, said Dr. Upadhyay’s study proves what has been known all along, that medication is super safe and that women “can help to determine their own eligibility as well as in conjunction with the provider.”
“I hope that this will be one more study that the FDA can use when thinking about changing the risk evaluation administration strategy so that it’s removing the requirement that a person be in the dispensing medical office,” Dr. Burkhardt, assistant professor of family planning in the department of obstetrics & gynecology at the University of New Mexico Hospital, Albuquerque, said in an interview. “I hope it also makes providers feel more comfortable as well, because I think there’s some hesitancy among providers to provide abortion without doing an ultrasound or without seeing the patient typically in front of them.”
This isn’t the first study to suggest the safety of telemedicine abortion. A 2019 study published in Obstetrics & Gynecology, which analyzed records from nearly 6,000 patients receiving medication abortion either through telemedicine or in person at 26 Planned Parenthood health centers in four states found that ongoing pregnancy and aspiration procedures were less common among telemedicine patients. Another 2017 study published in BMJ found that women who used an online consultation service and self-sourced medical abortion during a 3-year period were able to successfully end their pregnancies with few adverse events.
Dr. Upadhyay said one limitation of the current study is its sample size, so more studies should be conducted to prove telemedicine abortion’s safety.
“I think that we need continued research on this model of care just so we have more multiple studies that contribute to the evidence that can convince providers as well that they don’t need a lot of tests and that they can mail,” Dr. Upadhyay said.
Neither Dr. Upadhyay nor Dr. Burkhardt reported conflicts of interests.
Telehealth abortion may be just as safe and effective as in-person care, according to a small study published online in JAMA Network Open.
Of the 110 women from whom researchers collected remote abortion outcome data, 95% had a complete abortion without additional medical interventions, such as aspiration or surgery, and none experienced adverse events. Researchers said this efficacy rate is similar to in-person visits.
“There was no reason to expect that the medications prescribed [via telemedicine] and delivered through the mail would have different outcomes from when a patient traveled to a clinic,” study author Ushma D. Upadhyay, PhD, MPH, associate professor in the department of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco, said in an interview.
Medication abortion, which usually involves taking mifepristone (Mifeprex) followed by misoprostol (Cytotec) during the first 10 weeks of pregnancy, has been available in the United States since 2000. The Food and Drug Administration’s Risk Evaluation and Mitigation Strategy requires that mifepristone be dispensed in a medical office, clinic, or hospital, prohibiting dispensing from pharmacies in an effort to reduce potential risk for complications.
In April 2021, the FDA lifted the in-person dispensing requirement for mifepristone for the duration of the COVID-19 pandemic. However, Dr. Upadhyay hopes the findings of her current study will make this suspension permanent.
For the study, Dr. Upadhyay and colleagues examined the safety and efficacy of fully remote, medication abortion care. Eligibility for the medication was assessed using an online form that relies on patient history, or patients recalling their last period, to assess pregnancy duration and screen for ectopic pregnancy risks. Nurse practitioners reviewed the form and referred patients with unknown last menstrual period date or ectopic pregnancy risk factors for ultrasonography. A mail-order pharmacy delivered medications to eligible patients. The protocol involved three follow-up contacts: confirmation of medication administration, a 3-day assessment of symptoms, and a home pregnancy test after 4 weeks. Follow-up interactions were conducted by text, secure messaging, or telephone.
Researchers found that in addition to the 95% of the patients having a complete abortion without intervention, 5% (five) of patients required addition medical care to complete the abortion. Two of those patients were treated in EDs.
Gillian Burkhardt, MD, who was not involved in the study, said Dr. Upadhyay’s study proves what has been known all along, that medication is super safe and that women “can help to determine their own eligibility as well as in conjunction with the provider.”
“I hope that this will be one more study that the FDA can use when thinking about changing the risk evaluation administration strategy so that it’s removing the requirement that a person be in the dispensing medical office,” Dr. Burkhardt, assistant professor of family planning in the department of obstetrics & gynecology at the University of New Mexico Hospital, Albuquerque, said in an interview. “I hope it also makes providers feel more comfortable as well, because I think there’s some hesitancy among providers to provide abortion without doing an ultrasound or without seeing the patient typically in front of them.”
This isn’t the first study to suggest the safety of telemedicine abortion. A 2019 study published in Obstetrics & Gynecology, which analyzed records from nearly 6,000 patients receiving medication abortion either through telemedicine or in person at 26 Planned Parenthood health centers in four states found that ongoing pregnancy and aspiration procedures were less common among telemedicine patients. Another 2017 study published in BMJ found that women who used an online consultation service and self-sourced medical abortion during a 3-year period were able to successfully end their pregnancies with few adverse events.
Dr. Upadhyay said one limitation of the current study is its sample size, so more studies should be conducted to prove telemedicine abortion’s safety.
“I think that we need continued research on this model of care just so we have more multiple studies that contribute to the evidence that can convince providers as well that they don’t need a lot of tests and that they can mail,” Dr. Upadhyay said.
Neither Dr. Upadhyay nor Dr. Burkhardt reported conflicts of interests.
Telehealth abortion may be just as safe and effective as in-person care, according to a small study published online in JAMA Network Open.
Of the 110 women from whom researchers collected remote abortion outcome data, 95% had a complete abortion without additional medical interventions, such as aspiration or surgery, and none experienced adverse events. Researchers said this efficacy rate is similar to in-person visits.
“There was no reason to expect that the medications prescribed [via telemedicine] and delivered through the mail would have different outcomes from when a patient traveled to a clinic,” study author Ushma D. Upadhyay, PhD, MPH, associate professor in the department of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco, said in an interview.
Medication abortion, which usually involves taking mifepristone (Mifeprex) followed by misoprostol (Cytotec) during the first 10 weeks of pregnancy, has been available in the United States since 2000. The Food and Drug Administration’s Risk Evaluation and Mitigation Strategy requires that mifepristone be dispensed in a medical office, clinic, or hospital, prohibiting dispensing from pharmacies in an effort to reduce potential risk for complications.
In April 2021, the FDA lifted the in-person dispensing requirement for mifepristone for the duration of the COVID-19 pandemic. However, Dr. Upadhyay hopes the findings of her current study will make this suspension permanent.
For the study, Dr. Upadhyay and colleagues examined the safety and efficacy of fully remote, medication abortion care. Eligibility for the medication was assessed using an online form that relies on patient history, or patients recalling their last period, to assess pregnancy duration and screen for ectopic pregnancy risks. Nurse practitioners reviewed the form and referred patients with unknown last menstrual period date or ectopic pregnancy risk factors for ultrasonography. A mail-order pharmacy delivered medications to eligible patients. The protocol involved three follow-up contacts: confirmation of medication administration, a 3-day assessment of symptoms, and a home pregnancy test after 4 weeks. Follow-up interactions were conducted by text, secure messaging, or telephone.
Researchers found that in addition to the 95% of the patients having a complete abortion without intervention, 5% (five) of patients required addition medical care to complete the abortion. Two of those patients were treated in EDs.
Gillian Burkhardt, MD, who was not involved in the study, said Dr. Upadhyay’s study proves what has been known all along, that medication is super safe and that women “can help to determine their own eligibility as well as in conjunction with the provider.”
“I hope that this will be one more study that the FDA can use when thinking about changing the risk evaluation administration strategy so that it’s removing the requirement that a person be in the dispensing medical office,” Dr. Burkhardt, assistant professor of family planning in the department of obstetrics & gynecology at the University of New Mexico Hospital, Albuquerque, said in an interview. “I hope it also makes providers feel more comfortable as well, because I think there’s some hesitancy among providers to provide abortion without doing an ultrasound or without seeing the patient typically in front of them.”
This isn’t the first study to suggest the safety of telemedicine abortion. A 2019 study published in Obstetrics & Gynecology, which analyzed records from nearly 6,000 patients receiving medication abortion either through telemedicine or in person at 26 Planned Parenthood health centers in four states found that ongoing pregnancy and aspiration procedures were less common among telemedicine patients. Another 2017 study published in BMJ found that women who used an online consultation service and self-sourced medical abortion during a 3-year period were able to successfully end their pregnancies with few adverse events.
Dr. Upadhyay said one limitation of the current study is its sample size, so more studies should be conducted to prove telemedicine abortion’s safety.
“I think that we need continued research on this model of care just so we have more multiple studies that contribute to the evidence that can convince providers as well that they don’t need a lot of tests and that they can mail,” Dr. Upadhyay said.
Neither Dr. Upadhyay nor Dr. Burkhardt reported conflicts of interests.
FROM JAMA NETWORK OPEN
How is a woman determined to have dense breast tissue?
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
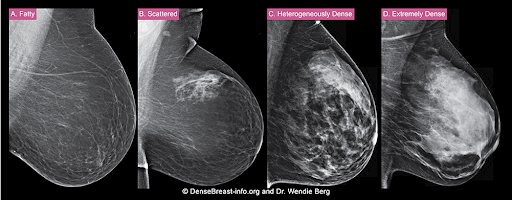
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.
A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
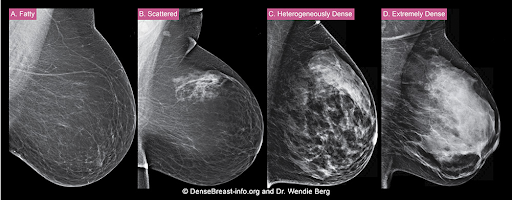
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.

A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.

A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
Recommendations from a gynecologic oncologist to a general ob.gyn., part 2
In this month’s column we continue to discuss recommendations from the gynecologic oncologist to the general gynecologist.
Don’t screen average-risk women for ovarian cancer.
Ovarian cancer is most often diagnosed at an advanced stage, which limits the curability of the disease. Consequently, there is a strong focus on attempting to diagnose the disease at earlier, more curable stages. This leads to the impulse by some well-intentioned providers to implement screening tests, such as ultrasounds and tumor markers, for all women. Unfortunately, the screening of “average risk” women for ovarian cancer is not recommended. Randomized controlled trials of tens of thousands of women have not observed a clinically significant decrease in ovarian cancer mortality with the addition of screening with tumor markers and ultrasound.1 These studies did observe a false-positive rate of 5%. While that may seem like a low rate of false-positive testing, the definitive diagnostic test which follows is a major abdominal surgery (oophorectomy) and serious complications are encountered in 15% of patients undergoing surgery for false-positive ovarian cancer screening.1 Therefore, quite simply, the harms are not balanced by benefits.
The key to offering patients appropriate and effective screening is case selection. It is important to identify which patients are at higher risk for ovarian cancer and offer those women testing for germline mutations and screening strategies. An important component of a well-woman visit is to take a thorough family history of cancer. Women are considered at high risk for having hereditary predisposition to ovarian cancer if they have a first- or second-degree relative with breast cancer younger than 45-50 years, or any age if Ashkenazi Jewish, triple-negative breast cancer younger than 60 years of age, two or more primary breast cancers with the first diagnosed at less than 50 years of age, male breast cancer, ovarian cancer, pancreatic cancer, a known BRCA 1/2 mutation, or a personal history of those same conditions. These women should be recommended to undergo genetic testing for BRCA 1, 2, and Lynch syndrome. They should not automatically be offered ovarian cancer screening. If a patient has a more remote family history for ovarian cancer, their personal risk may be somewhat elevated above the baseline population risk, however, not substantially enough to justify implementing screening in the absence of a confirmed genetic mutation.
While screening tests may not be appropriate for all patients, all patients should be asked about the early symptoms of ovarian cancer because these are consistently present, and frequently overlooked, prior to the eventual diagnosis of advanced disease. Those symptoms include abdominal discomfort, abdominal swelling and bloating, and urinary urgency.2 Consider offering all patients a dedicated ovarian cancer specific review of systems that includes inquiries about these symptoms at their annual wellness visits.
Opt for vertical midline incisions when surgery is anticipated to be complex
What is the first thing gynecologic oncologists do when called in to assist in a difficult gynecologic procedure? Get better exposure. Exposure is the cornerstone of safe, effective surgery. Sometimes this simply means placing a more effective retractor. In other cases, it might mean extending the incision. However, if the incision is a low transverse incision (the go-to for many gynecologists because of its favorable cosmetic and pain-producing profile) this proves to be difficult. Attempting to assist in a complicated case, such as a frozen pelvis, severed ureter or rectal injury, through a pfannensteil incision can be extraordinarily difficult, and while these incisions can be extended by incising the rectus muscle bellies, upper abdominal visualization remains elusive in most patients. This is particularly problematic if the ureter or splenic flexure need to be mobilized, or if extensive lysis of adhesions is necessary to ensure there is no occult enterotomy. As my mentor Dr. John Soper once described to me: “It’s like trying to scratch your armpit by reaching through your fly.”
While pfannensteil incisions come naturally, and comfortably, to most gynecologists, likely because of their frequent application during cesarean section, all gynecologists should be confident in the steps and anatomy for vertical midline, or paramedian incisions. This is not only beneficial for complex gynecologic cases, but also in the event of vascular emergency. In the hands of an experienced abdominal/pelvic surgeon, the vertical midline incision is the quickest way to safely enter the abdomen, and provides the kind of exposure that may be critical in safely repairing or controlling hemorrhage from a major vessel.
While low transverse incisions may be more cosmetic, less painful, and associated with fewer wound complications, our first concern as surgeons should be mitigating complications. In situations where risks of complications are high, it is best to not handicap ourselves with the incision location. And always remember, wound complications are highest when a transverse incision needs to be converted to a vertical one with a “T.”
It’s not just about diagnosis of cancer, it’s also prevention
Detection of cancer is an important role of the obstetrician gynecologist. However, equally important is being able to seize opportunities for cancer prevention. Cervical, vulvar, endometrial and ovarian cancer are all known to have preventative strategies.
All patients up to the age of 45 should be offered vaccination against HPV. Initial indications for HPV vaccination were for women up to age 26; however, recent data support the safety and efficacy of the vaccine in older women.3 HPV vaccination is most effective at preventing cancer when administered prior to exposure (ideally age 9-11), leaving this in the hands of our pediatrician colleagues. However, we must be vigilant to inquire about vaccination status for all our patients and encourage vaccines for those who were missed earlier in their life.
Patients should be counseled regarding the significant risk reduction for cancer that is gained from use of oral hormonal contraceptives and progestin-releasing IUDs (especially for endometrial and ovarian cancers). Providing them with knowledge of this information when considering options for contraception or menstrual cycle management is important in their decision-making process.
Endometrial cancer incidence is sadly on the rise in the United States, likely secondary to increasing rates of obesity. Pregnancy is a time when many women begin to gain, and accumulate, weight and therefore obstetric providers have a unique opportunity to assist patients in strategies to normalize their weight after pregnancy. Many of my patients with endometrial cancer state that they have never heard that it is associated with obesity. This suggests that more can be done to educate patients on the carcinogenic effect of obesity (for both endometrial and breast cancer), which may aid in motivating change of modifiable behaviors.
The fallopian tubes are the source of many ovarian cancers and knowledge of this has led to the recommendation to perform opportunistic salpingectomy as a cancer risk-reducing strategy. Hysterectomy and sterilization procedures are most apropos for this modification. While prospective data to confirm a reduced risk of ovarian cancer with opportunistic salpingectomy are lacking, a reduced incidence of cancer has been observed when the tubes have been removed for indicated surgeries; there appear to be no significant deleterious sequelae.4,5 A focus should be made on removal of the entire distal third of the tube, particularly the fimbriated ends, as this is the portion most implicated in malignancy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant disclosures. Contact her at [email protected].
References
1. Buys SS et al. JAMA. 2011;305(22):2295.
2. Goff BA et al. JAMA. 2004;291(22):2705.
3. Castellsagué X et al. Br J Cancer. 2011;105(1):28.
4. Yoon SH et al. Eur J Cancer. 2016 Mar;55:38-46.
5. Hanley GE et al. Am J Obstet Gynecol. 2018;219(2):172.
In this month’s column we continue to discuss recommendations from the gynecologic oncologist to the general gynecologist.
Don’t screen average-risk women for ovarian cancer.
Ovarian cancer is most often diagnosed at an advanced stage, which limits the curability of the disease. Consequently, there is a strong focus on attempting to diagnose the disease at earlier, more curable stages. This leads to the impulse by some well-intentioned providers to implement screening tests, such as ultrasounds and tumor markers, for all women. Unfortunately, the screening of “average risk” women for ovarian cancer is not recommended. Randomized controlled trials of tens of thousands of women have not observed a clinically significant decrease in ovarian cancer mortality with the addition of screening with tumor markers and ultrasound.1 These studies did observe a false-positive rate of 5%. While that may seem like a low rate of false-positive testing, the definitive diagnostic test which follows is a major abdominal surgery (oophorectomy) and serious complications are encountered in 15% of patients undergoing surgery for false-positive ovarian cancer screening.1 Therefore, quite simply, the harms are not balanced by benefits.
The key to offering patients appropriate and effective screening is case selection. It is important to identify which patients are at higher risk for ovarian cancer and offer those women testing for germline mutations and screening strategies. An important component of a well-woman visit is to take a thorough family history of cancer. Women are considered at high risk for having hereditary predisposition to ovarian cancer if they have a first- or second-degree relative with breast cancer younger than 45-50 years, or any age if Ashkenazi Jewish, triple-negative breast cancer younger than 60 years of age, two or more primary breast cancers with the first diagnosed at less than 50 years of age, male breast cancer, ovarian cancer, pancreatic cancer, a known BRCA 1/2 mutation, or a personal history of those same conditions. These women should be recommended to undergo genetic testing for BRCA 1, 2, and Lynch syndrome. They should not automatically be offered ovarian cancer screening. If a patient has a more remote family history for ovarian cancer, their personal risk may be somewhat elevated above the baseline population risk, however, not substantially enough to justify implementing screening in the absence of a confirmed genetic mutation.
While screening tests may not be appropriate for all patients, all patients should be asked about the early symptoms of ovarian cancer because these are consistently present, and frequently overlooked, prior to the eventual diagnosis of advanced disease. Those symptoms include abdominal discomfort, abdominal swelling and bloating, and urinary urgency.2 Consider offering all patients a dedicated ovarian cancer specific review of systems that includes inquiries about these symptoms at their annual wellness visits.
Opt for vertical midline incisions when surgery is anticipated to be complex
What is the first thing gynecologic oncologists do when called in to assist in a difficult gynecologic procedure? Get better exposure. Exposure is the cornerstone of safe, effective surgery. Sometimes this simply means placing a more effective retractor. In other cases, it might mean extending the incision. However, if the incision is a low transverse incision (the go-to for many gynecologists because of its favorable cosmetic and pain-producing profile) this proves to be difficult. Attempting to assist in a complicated case, such as a frozen pelvis, severed ureter or rectal injury, through a pfannensteil incision can be extraordinarily difficult, and while these incisions can be extended by incising the rectus muscle bellies, upper abdominal visualization remains elusive in most patients. This is particularly problematic if the ureter or splenic flexure need to be mobilized, or if extensive lysis of adhesions is necessary to ensure there is no occult enterotomy. As my mentor Dr. John Soper once described to me: “It’s like trying to scratch your armpit by reaching through your fly.”
While pfannensteil incisions come naturally, and comfortably, to most gynecologists, likely because of their frequent application during cesarean section, all gynecologists should be confident in the steps and anatomy for vertical midline, or paramedian incisions. This is not only beneficial for complex gynecologic cases, but also in the event of vascular emergency. In the hands of an experienced abdominal/pelvic surgeon, the vertical midline incision is the quickest way to safely enter the abdomen, and provides the kind of exposure that may be critical in safely repairing or controlling hemorrhage from a major vessel.
While low transverse incisions may be more cosmetic, less painful, and associated with fewer wound complications, our first concern as surgeons should be mitigating complications. In situations where risks of complications are high, it is best to not handicap ourselves with the incision location. And always remember, wound complications are highest when a transverse incision needs to be converted to a vertical one with a “T.”
It’s not just about diagnosis of cancer, it’s also prevention
Detection of cancer is an important role of the obstetrician gynecologist. However, equally important is being able to seize opportunities for cancer prevention. Cervical, vulvar, endometrial and ovarian cancer are all known to have preventative strategies.
All patients up to the age of 45 should be offered vaccination against HPV. Initial indications for HPV vaccination were for women up to age 26; however, recent data support the safety and efficacy of the vaccine in older women.3 HPV vaccination is most effective at preventing cancer when administered prior to exposure (ideally age 9-11), leaving this in the hands of our pediatrician colleagues. However, we must be vigilant to inquire about vaccination status for all our patients and encourage vaccines for those who were missed earlier in their life.
Patients should be counseled regarding the significant risk reduction for cancer that is gained from use of oral hormonal contraceptives and progestin-releasing IUDs (especially for endometrial and ovarian cancers). Providing them with knowledge of this information when considering options for contraception or menstrual cycle management is important in their decision-making process.
Endometrial cancer incidence is sadly on the rise in the United States, likely secondary to increasing rates of obesity. Pregnancy is a time when many women begin to gain, and accumulate, weight and therefore obstetric providers have a unique opportunity to assist patients in strategies to normalize their weight after pregnancy. Many of my patients with endometrial cancer state that they have never heard that it is associated with obesity. This suggests that more can be done to educate patients on the carcinogenic effect of obesity (for both endometrial and breast cancer), which may aid in motivating change of modifiable behaviors.
The fallopian tubes are the source of many ovarian cancers and knowledge of this has led to the recommendation to perform opportunistic salpingectomy as a cancer risk-reducing strategy. Hysterectomy and sterilization procedures are most apropos for this modification. While prospective data to confirm a reduced risk of ovarian cancer with opportunistic salpingectomy are lacking, a reduced incidence of cancer has been observed when the tubes have been removed for indicated surgeries; there appear to be no significant deleterious sequelae.4,5 A focus should be made on removal of the entire distal third of the tube, particularly the fimbriated ends, as this is the portion most implicated in malignancy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant disclosures. Contact her at [email protected].
References
1. Buys SS et al. JAMA. 2011;305(22):2295.
2. Goff BA et al. JAMA. 2004;291(22):2705.
3. Castellsagué X et al. Br J Cancer. 2011;105(1):28.
4. Yoon SH et al. Eur J Cancer. 2016 Mar;55:38-46.
5. Hanley GE et al. Am J Obstet Gynecol. 2018;219(2):172.
In this month’s column we continue to discuss recommendations from the gynecologic oncologist to the general gynecologist.
Don’t screen average-risk women for ovarian cancer.
Ovarian cancer is most often diagnosed at an advanced stage, which limits the curability of the disease. Consequently, there is a strong focus on attempting to diagnose the disease at earlier, more curable stages. This leads to the impulse by some well-intentioned providers to implement screening tests, such as ultrasounds and tumor markers, for all women. Unfortunately, the screening of “average risk” women for ovarian cancer is not recommended. Randomized controlled trials of tens of thousands of women have not observed a clinically significant decrease in ovarian cancer mortality with the addition of screening with tumor markers and ultrasound.1 These studies did observe a false-positive rate of 5%. While that may seem like a low rate of false-positive testing, the definitive diagnostic test which follows is a major abdominal surgery (oophorectomy) and serious complications are encountered in 15% of patients undergoing surgery for false-positive ovarian cancer screening.1 Therefore, quite simply, the harms are not balanced by benefits.
The key to offering patients appropriate and effective screening is case selection. It is important to identify which patients are at higher risk for ovarian cancer and offer those women testing for germline mutations and screening strategies. An important component of a well-woman visit is to take a thorough family history of cancer. Women are considered at high risk for having hereditary predisposition to ovarian cancer if they have a first- or second-degree relative with breast cancer younger than 45-50 years, or any age if Ashkenazi Jewish, triple-negative breast cancer younger than 60 years of age, two or more primary breast cancers with the first diagnosed at less than 50 years of age, male breast cancer, ovarian cancer, pancreatic cancer, a known BRCA 1/2 mutation, or a personal history of those same conditions. These women should be recommended to undergo genetic testing for BRCA 1, 2, and Lynch syndrome. They should not automatically be offered ovarian cancer screening. If a patient has a more remote family history for ovarian cancer, their personal risk may be somewhat elevated above the baseline population risk, however, not substantially enough to justify implementing screening in the absence of a confirmed genetic mutation.
While screening tests may not be appropriate for all patients, all patients should be asked about the early symptoms of ovarian cancer because these are consistently present, and frequently overlooked, prior to the eventual diagnosis of advanced disease. Those symptoms include abdominal discomfort, abdominal swelling and bloating, and urinary urgency.2 Consider offering all patients a dedicated ovarian cancer specific review of systems that includes inquiries about these symptoms at their annual wellness visits.
Opt for vertical midline incisions when surgery is anticipated to be complex
What is the first thing gynecologic oncologists do when called in to assist in a difficult gynecologic procedure? Get better exposure. Exposure is the cornerstone of safe, effective surgery. Sometimes this simply means placing a more effective retractor. In other cases, it might mean extending the incision. However, if the incision is a low transverse incision (the go-to for many gynecologists because of its favorable cosmetic and pain-producing profile) this proves to be difficult. Attempting to assist in a complicated case, such as a frozen pelvis, severed ureter or rectal injury, through a pfannensteil incision can be extraordinarily difficult, and while these incisions can be extended by incising the rectus muscle bellies, upper abdominal visualization remains elusive in most patients. This is particularly problematic if the ureter or splenic flexure need to be mobilized, or if extensive lysis of adhesions is necessary to ensure there is no occult enterotomy. As my mentor Dr. John Soper once described to me: “It’s like trying to scratch your armpit by reaching through your fly.”
While pfannensteil incisions come naturally, and comfortably, to most gynecologists, likely because of their frequent application during cesarean section, all gynecologists should be confident in the steps and anatomy for vertical midline, or paramedian incisions. This is not only beneficial for complex gynecologic cases, but also in the event of vascular emergency. In the hands of an experienced abdominal/pelvic surgeon, the vertical midline incision is the quickest way to safely enter the abdomen, and provides the kind of exposure that may be critical in safely repairing or controlling hemorrhage from a major vessel.
While low transverse incisions may be more cosmetic, less painful, and associated with fewer wound complications, our first concern as surgeons should be mitigating complications. In situations where risks of complications are high, it is best to not handicap ourselves with the incision location. And always remember, wound complications are highest when a transverse incision needs to be converted to a vertical one with a “T.”
It’s not just about diagnosis of cancer, it’s also prevention
Detection of cancer is an important role of the obstetrician gynecologist. However, equally important is being able to seize opportunities for cancer prevention. Cervical, vulvar, endometrial and ovarian cancer are all known to have preventative strategies.
All patients up to the age of 45 should be offered vaccination against HPV. Initial indications for HPV vaccination were for women up to age 26; however, recent data support the safety and efficacy of the vaccine in older women.3 HPV vaccination is most effective at preventing cancer when administered prior to exposure (ideally age 9-11), leaving this in the hands of our pediatrician colleagues. However, we must be vigilant to inquire about vaccination status for all our patients and encourage vaccines for those who were missed earlier in their life.
Patients should be counseled regarding the significant risk reduction for cancer that is gained from use of oral hormonal contraceptives and progestin-releasing IUDs (especially for endometrial and ovarian cancers). Providing them with knowledge of this information when considering options for contraception or menstrual cycle management is important in their decision-making process.
Endometrial cancer incidence is sadly on the rise in the United States, likely secondary to increasing rates of obesity. Pregnancy is a time when many women begin to gain, and accumulate, weight and therefore obstetric providers have a unique opportunity to assist patients in strategies to normalize their weight after pregnancy. Many of my patients with endometrial cancer state that they have never heard that it is associated with obesity. This suggests that more can be done to educate patients on the carcinogenic effect of obesity (for both endometrial and breast cancer), which may aid in motivating change of modifiable behaviors.
The fallopian tubes are the source of many ovarian cancers and knowledge of this has led to the recommendation to perform opportunistic salpingectomy as a cancer risk-reducing strategy. Hysterectomy and sterilization procedures are most apropos for this modification. While prospective data to confirm a reduced risk of ovarian cancer with opportunistic salpingectomy are lacking, a reduced incidence of cancer has been observed when the tubes have been removed for indicated surgeries; there appear to be no significant deleterious sequelae.4,5 A focus should be made on removal of the entire distal third of the tube, particularly the fimbriated ends, as this is the portion most implicated in malignancy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant disclosures. Contact her at [email protected].
References
1. Buys SS et al. JAMA. 2011;305(22):2295.
2. Goff BA et al. JAMA. 2004;291(22):2705.
3. Castellsagué X et al. Br J Cancer. 2011;105(1):28.
4. Yoon SH et al. Eur J Cancer. 2016 Mar;55:38-46.
5. Hanley GE et al. Am J Obstet Gynecol. 2018;219(2):172.
A sizzling hybrid meeting of the Society of Gynecologic Surgeons
The 47th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS), like so many things in our modern world, endured many changes and had to stay nimble and evolve to changing times. In the end, however, SGS was able to adapt and succeed, just like a skilled gynecologic surgeon in the operating room, to deliver a fresh new type of meeting.
When we chose the meeting theme, “Working together: How collaboration enables us to better help our patients,” we anticipated a meeting discussing medical colleagues and consultants. In our forever-changed world, we knew we needed to reinterpret this to a broader social context. Our special lectures and panel discussions sought to open attendees’ eyes to disparities in health care for people of color and women.
While we highlighted the realities faced by colleagues in medicine, the topics addressed also were designed to grow awareness about struggles our patients encounter as well. Social disparities are sobering, long-standing, and sometimes require creative collaborations to achieve successful outcomes for all patients. The faculty of one of our postgraduate courses reviews in this special 2-part section to
The meeting also kicked off with a postgraduate course on fibroid management, with workshops on harnessing the power of social media and lessons on leadership from a female Fortune 500 CEO, Lori Ryerkerk, offered as well. As the scientific program launched, we were once again treated to strong science on gynecologic surgery, with only a small dip in abstract submissions, despite the challenges of research during a pandemic. Mark Walters, MD, gave the inaugural lecture in his name on the crucial topic of surgical education and teaching. We also heard a special report from the SGS SOCOVID research group, led by Dr. Rosanne Kho, on gynecologic surgery during the pandemic. We also convened a virtual panel for our hybrid attendees on the benefits to patients of a multidisciplinary approach to gynecologic surgery, presented here by Cecile Ferrando, MD.
As our practices continue to grow and evolve, the introduction of innovative technologies can pose a new challenge, as Miles Murphy, MD, and members of the panel on novel gynecologic office procedures will present in this series next month.
The TeLinde keynote speaker was Janet Dombrowski, who works as a coach for many surgeons in various disciplines across the country. She spoke to the resilience gained through community and collaboration.
While our meeting theme dated to the “before” pandemic era, those who were able to be in attendance in person can attest to the value we can all place now on community and personal interactions. With experience strengthened by science, I hope this meeting summary serves to highlight the many ways in which we can collaborate to improve outcomes for ourselves in medicine and for patients.
The 47th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS), like so many things in our modern world, endured many changes and had to stay nimble and evolve to changing times. In the end, however, SGS was able to adapt and succeed, just like a skilled gynecologic surgeon in the operating room, to deliver a fresh new type of meeting.
When we chose the meeting theme, “Working together: How collaboration enables us to better help our patients,” we anticipated a meeting discussing medical colleagues and consultants. In our forever-changed world, we knew we needed to reinterpret this to a broader social context. Our special lectures and panel discussions sought to open attendees’ eyes to disparities in health care for people of color and women.
While we highlighted the realities faced by colleagues in medicine, the topics addressed also were designed to grow awareness about struggles our patients encounter as well. Social disparities are sobering, long-standing, and sometimes require creative collaborations to achieve successful outcomes for all patients. The faculty of one of our postgraduate courses reviews in this special 2-part section to
The meeting also kicked off with a postgraduate course on fibroid management, with workshops on harnessing the power of social media and lessons on leadership from a female Fortune 500 CEO, Lori Ryerkerk, offered as well. As the scientific program launched, we were once again treated to strong science on gynecologic surgery, with only a small dip in abstract submissions, despite the challenges of research during a pandemic. Mark Walters, MD, gave the inaugural lecture in his name on the crucial topic of surgical education and teaching. We also heard a special report from the SGS SOCOVID research group, led by Dr. Rosanne Kho, on gynecologic surgery during the pandemic. We also convened a virtual panel for our hybrid attendees on the benefits to patients of a multidisciplinary approach to gynecologic surgery, presented here by Cecile Ferrando, MD.
As our practices continue to grow and evolve, the introduction of innovative technologies can pose a new challenge, as Miles Murphy, MD, and members of the panel on novel gynecologic office procedures will present in this series next month.
The TeLinde keynote speaker was Janet Dombrowski, who works as a coach for many surgeons in various disciplines across the country. She spoke to the resilience gained through community and collaboration.
While our meeting theme dated to the “before” pandemic era, those who were able to be in attendance in person can attest to the value we can all place now on community and personal interactions. With experience strengthened by science, I hope this meeting summary serves to highlight the many ways in which we can collaborate to improve outcomes for ourselves in medicine and for patients.
The 47th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS), like so many things in our modern world, endured many changes and had to stay nimble and evolve to changing times. In the end, however, SGS was able to adapt and succeed, just like a skilled gynecologic surgeon in the operating room, to deliver a fresh new type of meeting.
When we chose the meeting theme, “Working together: How collaboration enables us to better help our patients,” we anticipated a meeting discussing medical colleagues and consultants. In our forever-changed world, we knew we needed to reinterpret this to a broader social context. Our special lectures and panel discussions sought to open attendees’ eyes to disparities in health care for people of color and women.
While we highlighted the realities faced by colleagues in medicine, the topics addressed also were designed to grow awareness about struggles our patients encounter as well. Social disparities are sobering, long-standing, and sometimes require creative collaborations to achieve successful outcomes for all patients. The faculty of one of our postgraduate courses reviews in this special 2-part section to
The meeting also kicked off with a postgraduate course on fibroid management, with workshops on harnessing the power of social media and lessons on leadership from a female Fortune 500 CEO, Lori Ryerkerk, offered as well. As the scientific program launched, we were once again treated to strong science on gynecologic surgery, with only a small dip in abstract submissions, despite the challenges of research during a pandemic. Mark Walters, MD, gave the inaugural lecture in his name on the crucial topic of surgical education and teaching. We also heard a special report from the SGS SOCOVID research group, led by Dr. Rosanne Kho, on gynecologic surgery during the pandemic. We also convened a virtual panel for our hybrid attendees on the benefits to patients of a multidisciplinary approach to gynecologic surgery, presented here by Cecile Ferrando, MD.
As our practices continue to grow and evolve, the introduction of innovative technologies can pose a new challenge, as Miles Murphy, MD, and members of the panel on novel gynecologic office procedures will present in this series next month.
The TeLinde keynote speaker was Janet Dombrowski, who works as a coach for many surgeons in various disciplines across the country. She spoke to the resilience gained through community and collaboration.
While our meeting theme dated to the “before” pandemic era, those who were able to be in attendance in person can attest to the value we can all place now on community and personal interactions. With experience strengthened by science, I hope this meeting summary serves to highlight the many ways in which we can collaborate to improve outcomes for ourselves in medicine and for patients.
A multidisciplinary approach to gyn care: A single center’s experience
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
Mayo Clinic again named best hospital in U.S. for gynecology
This year, the Mayo Clinic, Rochester, Minn. again ranked as the top hospital for gynecology, according to U.S. News and World Report, which released its annual rankings today.
The top five hospitals for gynecology were the same this year and were in the same order. In second place again this year was Memorial Sloan Kettering Cancer Center, New York, followed by the Cleveland Clinic, Brigham and Women’s Hospital, Boston, and the University of Texas MD Anderson Cancer Center, Houston.
Rounding out the top 10 were (6) Inova Fairfax Hospital, Falls Church, Virginia; (7) the University of Alabama at Birmingham Hospital; (8) Johns Hopkins Hospital, Baltimore, Maryland; and Massachusetts General Hospital, Boston, and Stanford Health Care–Stanford Hospital, Palo Alto, California, which tied for the ninth spot.
U.S. News compared more than 4,750 medical centers nationwide in 15 specialties. Of those, 531 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care.
In 12 of the specialties, including gynecology, rankings are determined by a data-driven analysis that combines performance measures in structure, process, and outcomes. Rankings in three other specialties – ophthalmology, psychiatry, and rheumatology – rely on expert opinion alone, according to the U.S. News methodology report.
The top 20 hospitals overall were also named to the Honor Roll.
Mayo Clinic was again no. 1 on the honor roll, a ranking it has held for 6 years in a row, according to a press release.
In other top specialties, the University of Texas MD Anderson Cancer Center ranked no. 1 in cancer; the Cleveland Clinic is no. 1 in cardiology and heart surgery; and the Hospital for Special Surgery, New York, is no. 1 in orthopedics.
A full list of rankings is available on the magazine’s website.
A version of this article first appeared on Medscape.com.
This year, the Mayo Clinic, Rochester, Minn. again ranked as the top hospital for gynecology, according to U.S. News and World Report, which released its annual rankings today.
The top five hospitals for gynecology were the same this year and were in the same order. In second place again this year was Memorial Sloan Kettering Cancer Center, New York, followed by the Cleveland Clinic, Brigham and Women’s Hospital, Boston, and the University of Texas MD Anderson Cancer Center, Houston.
Rounding out the top 10 were (6) Inova Fairfax Hospital, Falls Church, Virginia; (7) the University of Alabama at Birmingham Hospital; (8) Johns Hopkins Hospital, Baltimore, Maryland; and Massachusetts General Hospital, Boston, and Stanford Health Care–Stanford Hospital, Palo Alto, California, which tied for the ninth spot.
U.S. News compared more than 4,750 medical centers nationwide in 15 specialties. Of those, 531 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care.
In 12 of the specialties, including gynecology, rankings are determined by a data-driven analysis that combines performance measures in structure, process, and outcomes. Rankings in three other specialties – ophthalmology, psychiatry, and rheumatology – rely on expert opinion alone, according to the U.S. News methodology report.
The top 20 hospitals overall were also named to the Honor Roll.
Mayo Clinic was again no. 1 on the honor roll, a ranking it has held for 6 years in a row, according to a press release.
In other top specialties, the University of Texas MD Anderson Cancer Center ranked no. 1 in cancer; the Cleveland Clinic is no. 1 in cardiology and heart surgery; and the Hospital for Special Surgery, New York, is no. 1 in orthopedics.
A full list of rankings is available on the magazine’s website.
A version of this article first appeared on Medscape.com.
This year, the Mayo Clinic, Rochester, Minn. again ranked as the top hospital for gynecology, according to U.S. News and World Report, which released its annual rankings today.
The top five hospitals for gynecology were the same this year and were in the same order. In second place again this year was Memorial Sloan Kettering Cancer Center, New York, followed by the Cleveland Clinic, Brigham and Women’s Hospital, Boston, and the University of Texas MD Anderson Cancer Center, Houston.
Rounding out the top 10 were (6) Inova Fairfax Hospital, Falls Church, Virginia; (7) the University of Alabama at Birmingham Hospital; (8) Johns Hopkins Hospital, Baltimore, Maryland; and Massachusetts General Hospital, Boston, and Stanford Health Care–Stanford Hospital, Palo Alto, California, which tied for the ninth spot.
U.S. News compared more than 4,750 medical centers nationwide in 15 specialties. Of those, 531 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care.
In 12 of the specialties, including gynecology, rankings are determined by a data-driven analysis that combines performance measures in structure, process, and outcomes. Rankings in three other specialties – ophthalmology, psychiatry, and rheumatology – rely on expert opinion alone, according to the U.S. News methodology report.
The top 20 hospitals overall were also named to the Honor Roll.
Mayo Clinic was again no. 1 on the honor roll, a ranking it has held for 6 years in a row, according to a press release.
In other top specialties, the University of Texas MD Anderson Cancer Center ranked no. 1 in cancer; the Cleveland Clinic is no. 1 in cardiology and heart surgery; and the Hospital for Special Surgery, New York, is no. 1 in orthopedics.
A full list of rankings is available on the magazine’s website.
A version of this article first appeared on Medscape.com.
Laparoscopic abdominal cerclage: An effective, patient-sought approach for cervical insufficiency
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.
We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.
The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.
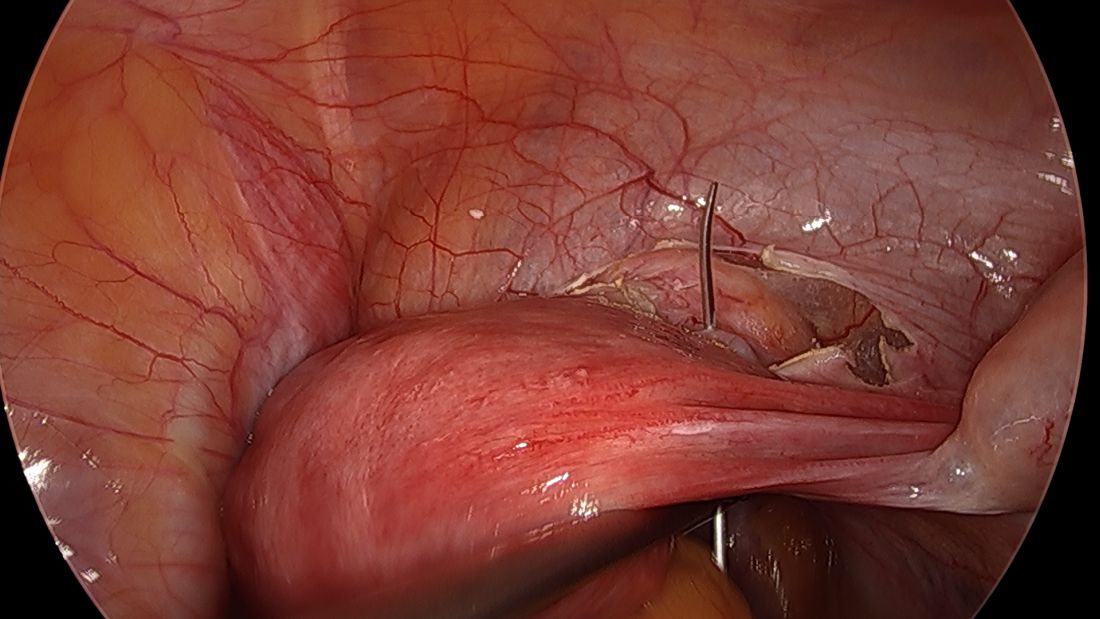
Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.
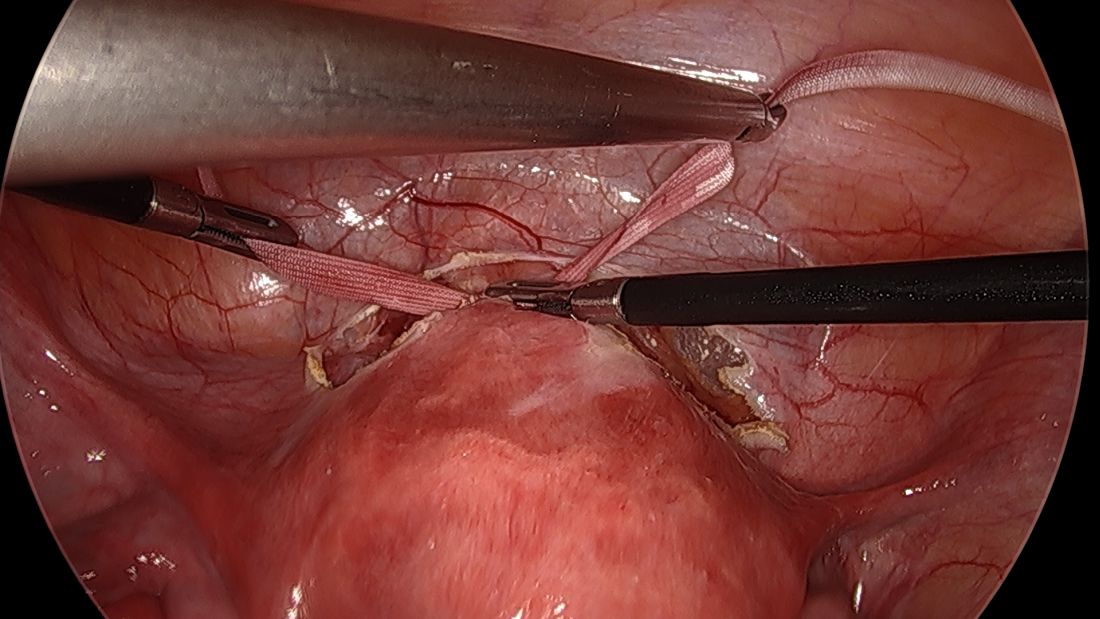
I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.
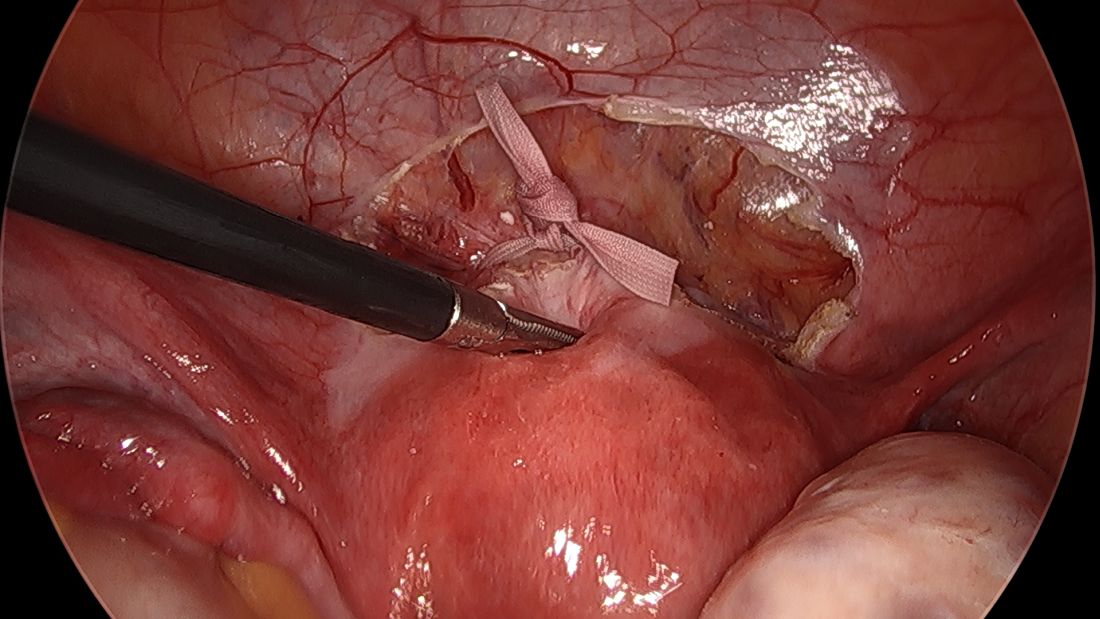
The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.
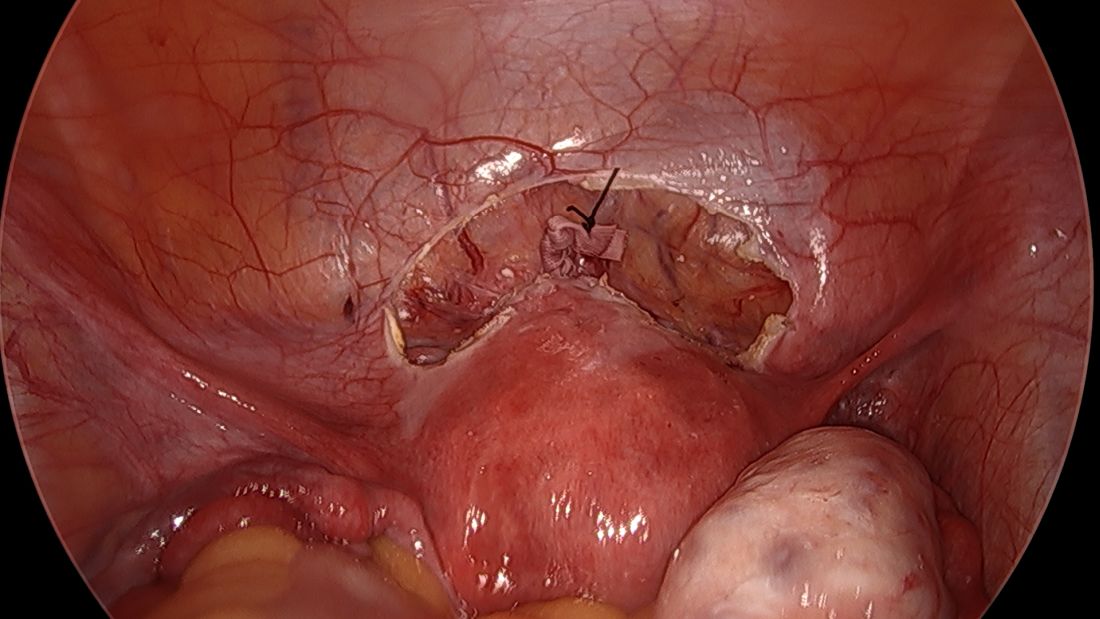
We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.
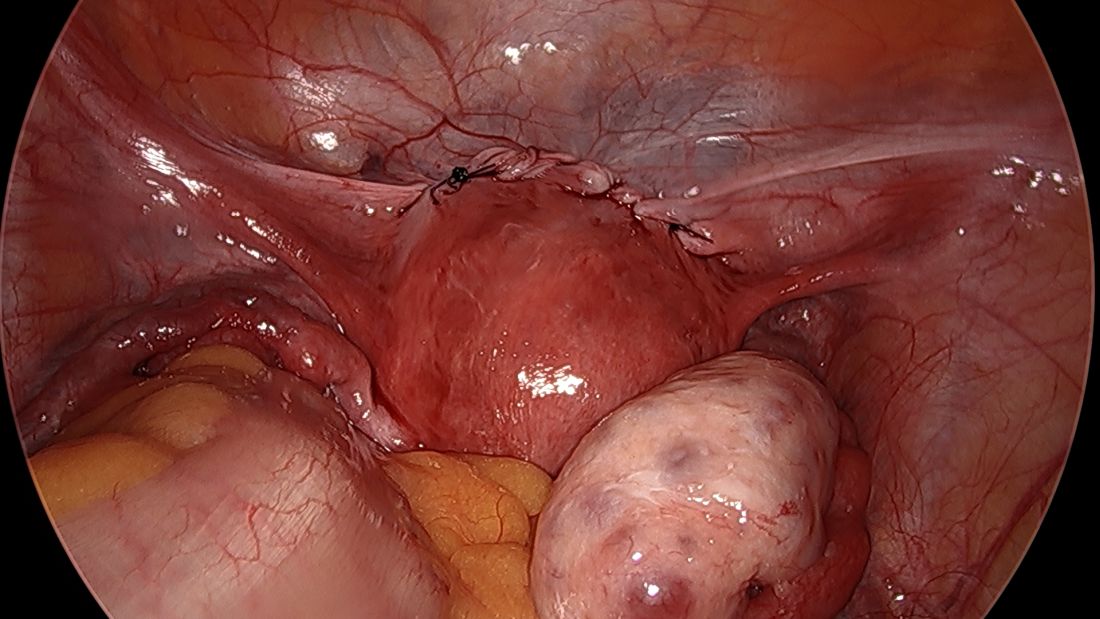
We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.

We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.

The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.

Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.

I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.

The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.

We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.

We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.

We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.

The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.

Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.

I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.

The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.

We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.

We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
When it comes to young women, regular check-ins support ongoing PrEP use
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.