User login
In the rush to curtail abortion, states adopt a jumbled stew of definitions for human life
As life-preserving medical technology advanced in the second half of the 20th century, doctors and families were faced with a thorny decision, one with weighty legal and moral implications: How should we define when life ends? Cardiopulmonary bypass machines could keep the blood pumping and ventilators could maintain breathing long after a patient’s natural ability to perform those vital functions had ceased.
After decades of deliberations involving physicians, bioethicists, attorneys, and theologians, a U.S. presidential commission in 1981 settled on a scientifically derived dividing line between life and death that has endured, more or less, ever since: A person was considered dead when the entire brain – including the brain stem, its most primitive portion – was no longer functioning, even if other vital functions could be maintained indefinitely through artificial life support.
In the decades since, the committee’s criteria have served as a foundation for laws in most states adopting brain death as a standard for legal death.
Now, with the overturning of Roe v. Wade and dozens of states rushing to impose abortion restrictions, At conception, the hint of a heartbeat, a first breath, the ability to survive outside the womb with the help of the latest technology?
That we’ve been able to devise and apply uniform clinical standards for when life ends, but not when it begins, is due largely to the legal and political maelstrom around abortion. And in the 2 months since the U.S. Supreme Court issued its opinion in Dobbs v. Jackson Women’s Health Organization, eliminating a longstanding federal right to abortion, state legislators are eagerly bounding into that void, looking to codify into law assorted definitions of life that carry profound repercussions for abortion rights, birth control, and assisted reproduction, as well as civil and criminal law.
“The court said that when life begins is up to whoever is running your state – whether they are wrong or not, or you agree with them or not,” said Mary Ziegler, a law professor at the University of California, Davis, who has written several books on the history of abortion.
Unlike the debate over death, which delved into exquisite medical and scientific detail, the legislative scramble to determine when life’s building blocks reach a threshold that warrants government protection as human life has generally ignored the input of mainstream medical professionals.
Instead, red states across much of the South and portions of the Midwest are adopting language drafted by elected officials that is informed by conservative Christian doctrine, often with little scientific underpinning.
A handful of Republican-led states, including Arkansas, Kentucky, Missouri, and Oklahoma, have passed laws declaring that life begins at fertilization, a contention that opens the door to a host of pregnancy-related litigation. This includes wrongful death lawsuits brought on behalf of the estate of an embryo by disgruntled ex-partners against physicians and women who end a pregnancy or even miscarry. (One such lawsuit is underway in Arizona. Another reached the Alabama Supreme Court.)
In Kentucky, the law outlawing abortion uses morally explosive terms to define pregnancy as “the human female reproductive condition of having a living unborn human being within her body throughout the entire embryonic and fetal stages of the unborn child from fertilization to full gestation and childbirth.”
Several other states, including Georgia, have adopted measures equating life with the point at which an embryo’s nascent cardiac activity can be detected by an ultrasound, at around 6 weeks of gestation. Many such laws mischaracterize the flickering electrical impulses detectable at that stage as a heartbeat, including in Georgia, whose Department of Revenue recently announced that “any unborn child with a detectable human heartbeat” can be claimed as a dependent.
The Supreme Court’s 1973 decision in Roe v. Wade that established a constitutional right to abortion did not define a moment when life begins. The opinion, written by Justice Harry Blackmun, observed that the Constitution does not provide a definition of “person,” though it extends protections to those born or naturalized in the United States. The court majority made note of the many disparate views among religions and scientists on when life begins, and concluded it was not up to the states to adopt one theory of life.
Instead, Roe created a framework intended to balance a pregnant woman’s right to make decisions about her body with a public interest in protecting potential human life. That decision and a key ruling that followed generally recognized a woman’s right to abortion up to the point medical professionals judge a fetus viable to survive outside the uterus, at about 24 weeks of gestation.
In decisively overturning Roe in June, the Supreme Court’s conservative majority drew on legal arguments that have shaped another contentious end-of-life issue. The legal standard employed in Dobbs – that there is no right to abortion in the federal Constitution and that states can decide on their own – is the same rationale used in 1997 when the Supreme Court said terminally ill people did not have a constitutional right to medically assisted death. That decision, Washington v. Glucksberg, is mentioned 15 times in the majority opinion for Dobbs and a concurrence by Justice Clarence Thomas.
Often, the same groups that have led the fight to outlaw abortion have also challenged medical aid-in-dying laws. Even after Dobbs, so-called right-to-die laws remain far less common than those codifying state abortion rights. Ten states allow physicians to prescribe lethal doses of medicine for terminally ill patients. Doctors are still prohibited from administering the drugs.
James Bopp, general counsel for the National Right to Life Committee who has been central to the efforts to outlaw abortion, said that both abortion and medically assisted death, which he refers to as physician-assisted suicide, endanger society.
“Every individual human life has inherent value and is sacred,” said Mr. Bopp. “The government has the duty to protect that life.”
Both issues raise profound societal questions: Can the government keep a patient on life support against his wishes, or force a woman to give birth? Can states bar their own residents from going to other states to end a pregnancy, or prohibit out-of-state patients from coming in to seek medically assisted death? And who gets to decide, particularly if the answer imposes a singular religious viewpoint?
Just as there are legal implications that flow from determining a person’s death, from organ donation to inheritance, the implied rights held by a legally recognized zygote are potentially vast. Will death certificates be issued for every lost pregnancy? Will miscarriages be investigated? When will Social Security numbers be issued? How will census counts be tallied and congressional districts drawn?
Medical professionals and bioethicists caution that both the beginning and end of life are complicated biological processes that are not defined by a single identifiable moment – and are ill suited to the political arena.
“Unfortunately, biological occurrences are not events, they are processes,” said David Magnus, PhD, director of the Stanford (Calif.) Center for Biomedical Ethics.
Moreover, asking doctors “What is life?” or “What is death?” may miss the point, said Dr. Magnus: “Medicine can answer the question ‘When does a biological organism cease to exist?’ But they can’t answer the question ‘When does a person begin or end?’ because those are metaphysical issues.”
Ben Sarbey, a doctoral candidate in the department of philosophy at Duke University, Durham, N.C., who studies medical ethics, echoed that perspective, recounting the Paradox of the Heap, a thought experiment that involves placing grains of sand one on top of the next. The philosophical quandary is this: At what point do those grains of sand become something more – a heap?
“We’re going to have a rough time placing a dividing line that this counts as a person and this does not count as a person,” he said. “Many things count as life – a sperm counts as life, a person in a persistent vegetative state counts as life – but does that constitute a person that we should be protecting?”
Even as debate over the court’s abortion decision percolates, the 1981 federal statute that grew out of the presidential committee’s findings, the Uniform Determination of Death Act, is also under review. In 2022, the Uniform Law Commission, a nonpartisan group of legal experts that drafts laws intended for adoption in multiple states, has taken up the work to revisit the definition of death.
The group will consider sharpening the medical standards for brain death in light of advances in the understanding of brain function. And they will look to address lingering questions raised in recent years as families and religious groups have waged heated legal battles over terminating artificial life support for patients with no brain wave activity.
Mr. Bopp, with the National Right to Life Committee, is among those serving on advisory panels for the effort, along with an array of doctors, philosophers, and medical ethicists. The concept of “personhood” that infuses the antiabortion movement’s broader push for fetal rights is expected to be an underlying topic, albeit in mirror image: When does a life form cease being a person?
Dr. Magnus, who is also serving on an advisory panel, has no doubt the commission will reach a consensus, a sober resolution rooted in science. What’s less clear, he said, is whether in today’s political environment that updated definition will hold the same sway, an enduring legal standard embraced across states.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As life-preserving medical technology advanced in the second half of the 20th century, doctors and families were faced with a thorny decision, one with weighty legal and moral implications: How should we define when life ends? Cardiopulmonary bypass machines could keep the blood pumping and ventilators could maintain breathing long after a patient’s natural ability to perform those vital functions had ceased.
After decades of deliberations involving physicians, bioethicists, attorneys, and theologians, a U.S. presidential commission in 1981 settled on a scientifically derived dividing line between life and death that has endured, more or less, ever since: A person was considered dead when the entire brain – including the brain stem, its most primitive portion – was no longer functioning, even if other vital functions could be maintained indefinitely through artificial life support.
In the decades since, the committee’s criteria have served as a foundation for laws in most states adopting brain death as a standard for legal death.
Now, with the overturning of Roe v. Wade and dozens of states rushing to impose abortion restrictions, At conception, the hint of a heartbeat, a first breath, the ability to survive outside the womb with the help of the latest technology?
That we’ve been able to devise and apply uniform clinical standards for when life ends, but not when it begins, is due largely to the legal and political maelstrom around abortion. And in the 2 months since the U.S. Supreme Court issued its opinion in Dobbs v. Jackson Women’s Health Organization, eliminating a longstanding federal right to abortion, state legislators are eagerly bounding into that void, looking to codify into law assorted definitions of life that carry profound repercussions for abortion rights, birth control, and assisted reproduction, as well as civil and criminal law.
“The court said that when life begins is up to whoever is running your state – whether they are wrong or not, or you agree with them or not,” said Mary Ziegler, a law professor at the University of California, Davis, who has written several books on the history of abortion.
Unlike the debate over death, which delved into exquisite medical and scientific detail, the legislative scramble to determine when life’s building blocks reach a threshold that warrants government protection as human life has generally ignored the input of mainstream medical professionals.
Instead, red states across much of the South and portions of the Midwest are adopting language drafted by elected officials that is informed by conservative Christian doctrine, often with little scientific underpinning.
A handful of Republican-led states, including Arkansas, Kentucky, Missouri, and Oklahoma, have passed laws declaring that life begins at fertilization, a contention that opens the door to a host of pregnancy-related litigation. This includes wrongful death lawsuits brought on behalf of the estate of an embryo by disgruntled ex-partners against physicians and women who end a pregnancy or even miscarry. (One such lawsuit is underway in Arizona. Another reached the Alabama Supreme Court.)
In Kentucky, the law outlawing abortion uses morally explosive terms to define pregnancy as “the human female reproductive condition of having a living unborn human being within her body throughout the entire embryonic and fetal stages of the unborn child from fertilization to full gestation and childbirth.”
Several other states, including Georgia, have adopted measures equating life with the point at which an embryo’s nascent cardiac activity can be detected by an ultrasound, at around 6 weeks of gestation. Many such laws mischaracterize the flickering electrical impulses detectable at that stage as a heartbeat, including in Georgia, whose Department of Revenue recently announced that “any unborn child with a detectable human heartbeat” can be claimed as a dependent.
The Supreme Court’s 1973 decision in Roe v. Wade that established a constitutional right to abortion did not define a moment when life begins. The opinion, written by Justice Harry Blackmun, observed that the Constitution does not provide a definition of “person,” though it extends protections to those born or naturalized in the United States. The court majority made note of the many disparate views among religions and scientists on when life begins, and concluded it was not up to the states to adopt one theory of life.
Instead, Roe created a framework intended to balance a pregnant woman’s right to make decisions about her body with a public interest in protecting potential human life. That decision and a key ruling that followed generally recognized a woman’s right to abortion up to the point medical professionals judge a fetus viable to survive outside the uterus, at about 24 weeks of gestation.
In decisively overturning Roe in June, the Supreme Court’s conservative majority drew on legal arguments that have shaped another contentious end-of-life issue. The legal standard employed in Dobbs – that there is no right to abortion in the federal Constitution and that states can decide on their own – is the same rationale used in 1997 when the Supreme Court said terminally ill people did not have a constitutional right to medically assisted death. That decision, Washington v. Glucksberg, is mentioned 15 times in the majority opinion for Dobbs and a concurrence by Justice Clarence Thomas.
Often, the same groups that have led the fight to outlaw abortion have also challenged medical aid-in-dying laws. Even after Dobbs, so-called right-to-die laws remain far less common than those codifying state abortion rights. Ten states allow physicians to prescribe lethal doses of medicine for terminally ill patients. Doctors are still prohibited from administering the drugs.
James Bopp, general counsel for the National Right to Life Committee who has been central to the efforts to outlaw abortion, said that both abortion and medically assisted death, which he refers to as physician-assisted suicide, endanger society.
“Every individual human life has inherent value and is sacred,” said Mr. Bopp. “The government has the duty to protect that life.”
Both issues raise profound societal questions: Can the government keep a patient on life support against his wishes, or force a woman to give birth? Can states bar their own residents from going to other states to end a pregnancy, or prohibit out-of-state patients from coming in to seek medically assisted death? And who gets to decide, particularly if the answer imposes a singular religious viewpoint?
Just as there are legal implications that flow from determining a person’s death, from organ donation to inheritance, the implied rights held by a legally recognized zygote are potentially vast. Will death certificates be issued for every lost pregnancy? Will miscarriages be investigated? When will Social Security numbers be issued? How will census counts be tallied and congressional districts drawn?
Medical professionals and bioethicists caution that both the beginning and end of life are complicated biological processes that are not defined by a single identifiable moment – and are ill suited to the political arena.
“Unfortunately, biological occurrences are not events, they are processes,” said David Magnus, PhD, director of the Stanford (Calif.) Center for Biomedical Ethics.
Moreover, asking doctors “What is life?” or “What is death?” may miss the point, said Dr. Magnus: “Medicine can answer the question ‘When does a biological organism cease to exist?’ But they can’t answer the question ‘When does a person begin or end?’ because those are metaphysical issues.”
Ben Sarbey, a doctoral candidate in the department of philosophy at Duke University, Durham, N.C., who studies medical ethics, echoed that perspective, recounting the Paradox of the Heap, a thought experiment that involves placing grains of sand one on top of the next. The philosophical quandary is this: At what point do those grains of sand become something more – a heap?
“We’re going to have a rough time placing a dividing line that this counts as a person and this does not count as a person,” he said. “Many things count as life – a sperm counts as life, a person in a persistent vegetative state counts as life – but does that constitute a person that we should be protecting?”
Even as debate over the court’s abortion decision percolates, the 1981 federal statute that grew out of the presidential committee’s findings, the Uniform Determination of Death Act, is also under review. In 2022, the Uniform Law Commission, a nonpartisan group of legal experts that drafts laws intended for adoption in multiple states, has taken up the work to revisit the definition of death.
The group will consider sharpening the medical standards for brain death in light of advances in the understanding of brain function. And they will look to address lingering questions raised in recent years as families and religious groups have waged heated legal battles over terminating artificial life support for patients with no brain wave activity.
Mr. Bopp, with the National Right to Life Committee, is among those serving on advisory panels for the effort, along with an array of doctors, philosophers, and medical ethicists. The concept of “personhood” that infuses the antiabortion movement’s broader push for fetal rights is expected to be an underlying topic, albeit in mirror image: When does a life form cease being a person?
Dr. Magnus, who is also serving on an advisory panel, has no doubt the commission will reach a consensus, a sober resolution rooted in science. What’s less clear, he said, is whether in today’s political environment that updated definition will hold the same sway, an enduring legal standard embraced across states.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As life-preserving medical technology advanced in the second half of the 20th century, doctors and families were faced with a thorny decision, one with weighty legal and moral implications: How should we define when life ends? Cardiopulmonary bypass machines could keep the blood pumping and ventilators could maintain breathing long after a patient’s natural ability to perform those vital functions had ceased.
After decades of deliberations involving physicians, bioethicists, attorneys, and theologians, a U.S. presidential commission in 1981 settled on a scientifically derived dividing line between life and death that has endured, more or less, ever since: A person was considered dead when the entire brain – including the brain stem, its most primitive portion – was no longer functioning, even if other vital functions could be maintained indefinitely through artificial life support.
In the decades since, the committee’s criteria have served as a foundation for laws in most states adopting brain death as a standard for legal death.
Now, with the overturning of Roe v. Wade and dozens of states rushing to impose abortion restrictions, At conception, the hint of a heartbeat, a first breath, the ability to survive outside the womb with the help of the latest technology?
That we’ve been able to devise and apply uniform clinical standards for when life ends, but not when it begins, is due largely to the legal and political maelstrom around abortion. And in the 2 months since the U.S. Supreme Court issued its opinion in Dobbs v. Jackson Women’s Health Organization, eliminating a longstanding federal right to abortion, state legislators are eagerly bounding into that void, looking to codify into law assorted definitions of life that carry profound repercussions for abortion rights, birth control, and assisted reproduction, as well as civil and criminal law.
“The court said that when life begins is up to whoever is running your state – whether they are wrong or not, or you agree with them or not,” said Mary Ziegler, a law professor at the University of California, Davis, who has written several books on the history of abortion.
Unlike the debate over death, which delved into exquisite medical and scientific detail, the legislative scramble to determine when life’s building blocks reach a threshold that warrants government protection as human life has generally ignored the input of mainstream medical professionals.
Instead, red states across much of the South and portions of the Midwest are adopting language drafted by elected officials that is informed by conservative Christian doctrine, often with little scientific underpinning.
A handful of Republican-led states, including Arkansas, Kentucky, Missouri, and Oklahoma, have passed laws declaring that life begins at fertilization, a contention that opens the door to a host of pregnancy-related litigation. This includes wrongful death lawsuits brought on behalf of the estate of an embryo by disgruntled ex-partners against physicians and women who end a pregnancy or even miscarry. (One such lawsuit is underway in Arizona. Another reached the Alabama Supreme Court.)
In Kentucky, the law outlawing abortion uses morally explosive terms to define pregnancy as “the human female reproductive condition of having a living unborn human being within her body throughout the entire embryonic and fetal stages of the unborn child from fertilization to full gestation and childbirth.”
Several other states, including Georgia, have adopted measures equating life with the point at which an embryo’s nascent cardiac activity can be detected by an ultrasound, at around 6 weeks of gestation. Many such laws mischaracterize the flickering electrical impulses detectable at that stage as a heartbeat, including in Georgia, whose Department of Revenue recently announced that “any unborn child with a detectable human heartbeat” can be claimed as a dependent.
The Supreme Court’s 1973 decision in Roe v. Wade that established a constitutional right to abortion did not define a moment when life begins. The opinion, written by Justice Harry Blackmun, observed that the Constitution does not provide a definition of “person,” though it extends protections to those born or naturalized in the United States. The court majority made note of the many disparate views among religions and scientists on when life begins, and concluded it was not up to the states to adopt one theory of life.
Instead, Roe created a framework intended to balance a pregnant woman’s right to make decisions about her body with a public interest in protecting potential human life. That decision and a key ruling that followed generally recognized a woman’s right to abortion up to the point medical professionals judge a fetus viable to survive outside the uterus, at about 24 weeks of gestation.
In decisively overturning Roe in June, the Supreme Court’s conservative majority drew on legal arguments that have shaped another contentious end-of-life issue. The legal standard employed in Dobbs – that there is no right to abortion in the federal Constitution and that states can decide on their own – is the same rationale used in 1997 when the Supreme Court said terminally ill people did not have a constitutional right to medically assisted death. That decision, Washington v. Glucksberg, is mentioned 15 times in the majority opinion for Dobbs and a concurrence by Justice Clarence Thomas.
Often, the same groups that have led the fight to outlaw abortion have also challenged medical aid-in-dying laws. Even after Dobbs, so-called right-to-die laws remain far less common than those codifying state abortion rights. Ten states allow physicians to prescribe lethal doses of medicine for terminally ill patients. Doctors are still prohibited from administering the drugs.
James Bopp, general counsel for the National Right to Life Committee who has been central to the efforts to outlaw abortion, said that both abortion and medically assisted death, which he refers to as physician-assisted suicide, endanger society.
“Every individual human life has inherent value and is sacred,” said Mr. Bopp. “The government has the duty to protect that life.”
Both issues raise profound societal questions: Can the government keep a patient on life support against his wishes, or force a woman to give birth? Can states bar their own residents from going to other states to end a pregnancy, or prohibit out-of-state patients from coming in to seek medically assisted death? And who gets to decide, particularly if the answer imposes a singular religious viewpoint?
Just as there are legal implications that flow from determining a person’s death, from organ donation to inheritance, the implied rights held by a legally recognized zygote are potentially vast. Will death certificates be issued for every lost pregnancy? Will miscarriages be investigated? When will Social Security numbers be issued? How will census counts be tallied and congressional districts drawn?
Medical professionals and bioethicists caution that both the beginning and end of life are complicated biological processes that are not defined by a single identifiable moment – and are ill suited to the political arena.
“Unfortunately, biological occurrences are not events, they are processes,” said David Magnus, PhD, director of the Stanford (Calif.) Center for Biomedical Ethics.
Moreover, asking doctors “What is life?” or “What is death?” may miss the point, said Dr. Magnus: “Medicine can answer the question ‘When does a biological organism cease to exist?’ But they can’t answer the question ‘When does a person begin or end?’ because those are metaphysical issues.”
Ben Sarbey, a doctoral candidate in the department of philosophy at Duke University, Durham, N.C., who studies medical ethics, echoed that perspective, recounting the Paradox of the Heap, a thought experiment that involves placing grains of sand one on top of the next. The philosophical quandary is this: At what point do those grains of sand become something more – a heap?
“We’re going to have a rough time placing a dividing line that this counts as a person and this does not count as a person,” he said. “Many things count as life – a sperm counts as life, a person in a persistent vegetative state counts as life – but does that constitute a person that we should be protecting?”
Even as debate over the court’s abortion decision percolates, the 1981 federal statute that grew out of the presidential committee’s findings, the Uniform Determination of Death Act, is also under review. In 2022, the Uniform Law Commission, a nonpartisan group of legal experts that drafts laws intended for adoption in multiple states, has taken up the work to revisit the definition of death.
The group will consider sharpening the medical standards for brain death in light of advances in the understanding of brain function. And they will look to address lingering questions raised in recent years as families and religious groups have waged heated legal battles over terminating artificial life support for patients with no brain wave activity.
Mr. Bopp, with the National Right to Life Committee, is among those serving on advisory panels for the effort, along with an array of doctors, philosophers, and medical ethicists. The concept of “personhood” that infuses the antiabortion movement’s broader push for fetal rights is expected to be an underlying topic, albeit in mirror image: When does a life form cease being a person?
Dr. Magnus, who is also serving on an advisory panel, has no doubt the commission will reach a consensus, a sober resolution rooted in science. What’s less clear, he said, is whether in today’s political environment that updated definition will hold the same sway, an enduring legal standard embraced across states.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
New ovulatory disorder classifications from FIGO replace 50-year-old system
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
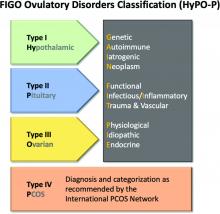
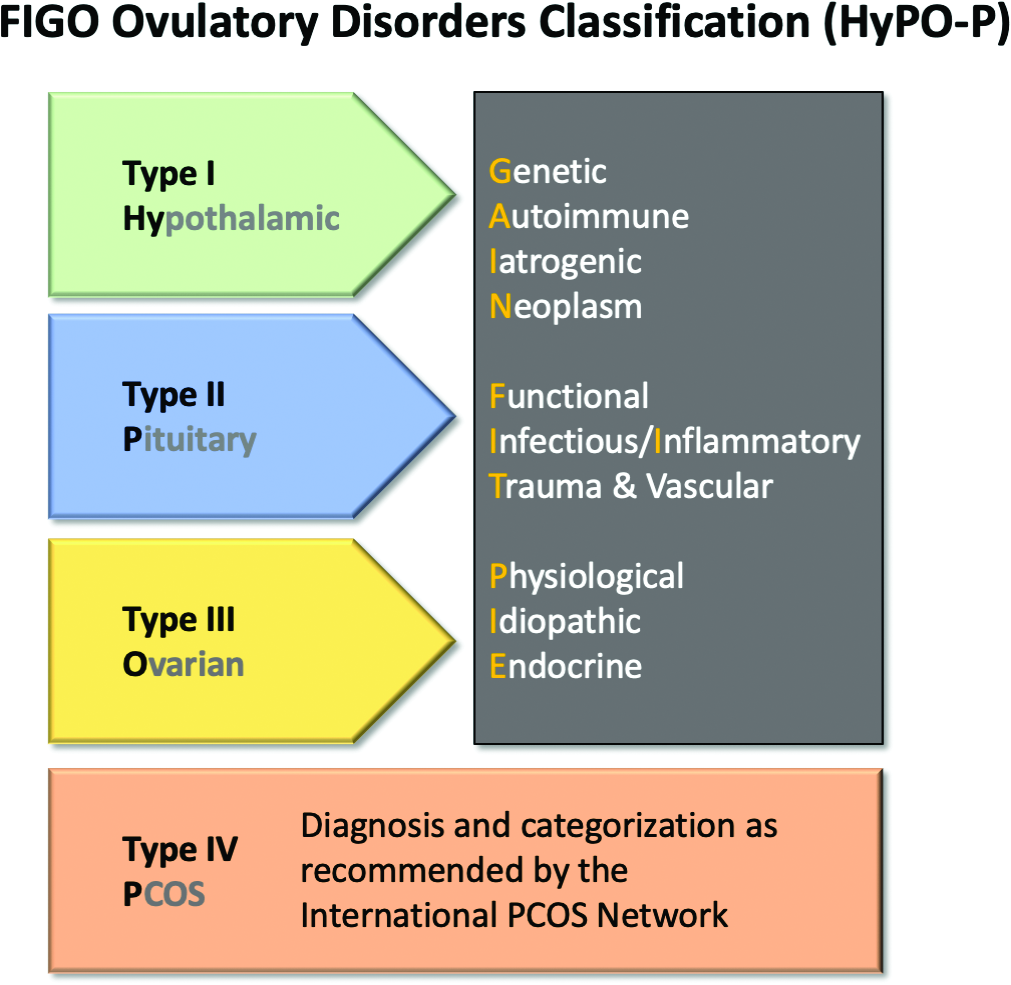
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
FROM INTERNATIONAL JOURNAL OF GYNECOLOGY AND OBSTETRICS
State of the science in PCOS: Emerging neuroendocrine involvement driving research
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
At what age do ObGyns recommend their patients begin cervical cancer screening?
In their peer-to-peer interview, “Cervical cancer: A path to eradication,” (OBG Manag. May 2022;34:30-34.) David G. Mutch, MD, and Warner Huh, MD, discussed the varying guidelines for cervical cancer screening. Dr. Huh pointed out that the 2020 guidelines for the American Cancer Society recommend cervical cancer screening to begin at age 25 years, although the current guidelines for the American College of Obstetricians and Gynecologists continue to recommend age 21. He noted that “the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already” screen beginning at age 25. OBG Management followed up with a poll for readers to ask: “Guidelines vary on what age to begin screening for cervical cancer. What age do you typically recommend for patients?”
A total of 187 readers cast their vote:
82.4% (154 readers) said age 21
8.0% (15 readers) said age 25
9.6% (18 readers) said other age
In their peer-to-peer interview, “Cervical cancer: A path to eradication,” (OBG Manag. May 2022;34:30-34.) David G. Mutch, MD, and Warner Huh, MD, discussed the varying guidelines for cervical cancer screening. Dr. Huh pointed out that the 2020 guidelines for the American Cancer Society recommend cervical cancer screening to begin at age 25 years, although the current guidelines for the American College of Obstetricians and Gynecologists continue to recommend age 21. He noted that “the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already” screen beginning at age 25. OBG Management followed up with a poll for readers to ask: “Guidelines vary on what age to begin screening for cervical cancer. What age do you typically recommend for patients?”
A total of 187 readers cast their vote:
82.4% (154 readers) said age 21
8.0% (15 readers) said age 25
9.6% (18 readers) said other age
In their peer-to-peer interview, “Cervical cancer: A path to eradication,” (OBG Manag. May 2022;34:30-34.) David G. Mutch, MD, and Warner Huh, MD, discussed the varying guidelines for cervical cancer screening. Dr. Huh pointed out that the 2020 guidelines for the American Cancer Society recommend cervical cancer screening to begin at age 25 years, although the current guidelines for the American College of Obstetricians and Gynecologists continue to recommend age 21. He noted that “the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already” screen beginning at age 25. OBG Management followed up with a poll for readers to ask: “Guidelines vary on what age to begin screening for cervical cancer. What age do you typically recommend for patients?”
A total of 187 readers cast their vote:
82.4% (154 readers) said age 21
8.0% (15 readers) said age 25
9.6% (18 readers) said other age
Postpartum depression risk higher with family psych history
Mothers who have a family history of any psychiatric disorder have almost two times the risk of postpartum depression as do mothers without such history, according to a new study.
Mette-Marie Zacher Kjeldsen, MSc, with the National Centre for Register-based Research at Aarhus (Denmark) University, led the study, a meta-analysis that included 26 studies with information on 100,877 women.
Findings were published online in JAMA Psychiatry.
When mothers had a family history of psychiatric disorders, the odds ratio for PPD was 2.08 (95% confidence interval, 1.67-2.59). That corresponds to a risk ratio of 1.79 (95% CI, 1.52-2.09), assuming a 15% postpartum depression prevalence in the general population.
Not doomed to develop PPD
Polina Teslyar, MD, a perinatal psychiatrist at Brigham and Women’s Hospital in Boston told this news organization it’s important to point out that though the risk is higher, women with a family psychiatric history should not feel as though they are destined to develop PPD.
“You are still more likely to not have postpartum depression, but it is important to be aware of personal risk factors so that if a person is experiencing that, they ask for help quickly rather than suffering and not knowing something is amiss,” she emphasized. Dr. Teslyar says she does see the higher risk for PPD, which is preventable and treatable, in her own practice when women have had a family history of psychiatric disorders.
The association makes sense, but literature on why that is has been varied, she said, and likely involves both genetics and socioeconomic factors. It’s difficult to tease apart how big a part each plays.
In her perinatal practice she sees women even before they are pregnant to discuss risk factors for PPD so she does ask about family history of psychiatric disorders, specifically about history of PPD and anxiety.
The researchers suggest routine perinatal care should include an easy low-cost, two-part question about both personal and family history of psychiatric disorders.
“As the assessment is possible even prior to conception, this would leave time for planning preventive efforts, such as psychosocial and psychological interventions targeting these at-risk women,” the authors write.
Asking about family history a challenge
Dr. Teslyar noted though that one of the challenges in asking about family history is that families may not have openly shared psychiatric history details with offspring. Family members may also report conditions they suspect a family member had rather than having a documented diagnosis.
In places where there is universal health care, she noted, finding documented diagnoses is easier, but otherwise “you’re really taking a subjective interpretation.”
The researchers found that subgroup, sensitivity, and meta–regression analyses aligned with the primary findings. The overall certainty of evidence was graded as moderate.
This study was not able to make clear how the specific diagnoses of family members affect the risk of developing PPD because much of the data from the studies came from self-report and questions were not consistent across the studies.
For instance, only 7 studies asked specifically about first-degree family members and 10 asked about specific diagnoses. Diagnoses ranged from mild affective disorders to more intrusive disorders, such as schizophrenia.
And while this study doesn’t seek to determine why the family history and risk of PPD appear to be connected, the authors offer some possible explanations.
“Growing up in an environment with parents struggling with mental health problems potentially influences the social support received from these parents when going into motherhood,” the authors write. “This particular explanation is supported by umbrella reviews concluding that lack of social support is a significant PPD risk factor.”
Screening, extraction, and assessment of studies included was done independently by two reviewers, increasing validity, the authors note.
The authors state that approximately 10%-15% of new mothers experience PPD, but Dr. Teslyar points out the numbers in the United States are typically quoted at up to 20%-30%. PPD ranges from mild to severe episodes and includes symptoms like those for major depression outside the postpartum period.
Study authors received funding from The Lundbeck Foundation and the European Union’s Horizon 2020 Research and Innovation Programme. A coauthor, Vibe G. Frokjaer, MD, PhD, has served as consultant and lecturer for H. Lundbeck and Sage Therapeutics. No other disclosures were reported. Dr. Teslyar reports no relevant financial relationships.
Mothers who have a family history of any psychiatric disorder have almost two times the risk of postpartum depression as do mothers without such history, according to a new study.
Mette-Marie Zacher Kjeldsen, MSc, with the National Centre for Register-based Research at Aarhus (Denmark) University, led the study, a meta-analysis that included 26 studies with information on 100,877 women.
Findings were published online in JAMA Psychiatry.
When mothers had a family history of psychiatric disorders, the odds ratio for PPD was 2.08 (95% confidence interval, 1.67-2.59). That corresponds to a risk ratio of 1.79 (95% CI, 1.52-2.09), assuming a 15% postpartum depression prevalence in the general population.
Not doomed to develop PPD
Polina Teslyar, MD, a perinatal psychiatrist at Brigham and Women’s Hospital in Boston told this news organization it’s important to point out that though the risk is higher, women with a family psychiatric history should not feel as though they are destined to develop PPD.
“You are still more likely to not have postpartum depression, but it is important to be aware of personal risk factors so that if a person is experiencing that, they ask for help quickly rather than suffering and not knowing something is amiss,” she emphasized. Dr. Teslyar says she does see the higher risk for PPD, which is preventable and treatable, in her own practice when women have had a family history of psychiatric disorders.
The association makes sense, but literature on why that is has been varied, she said, and likely involves both genetics and socioeconomic factors. It’s difficult to tease apart how big a part each plays.
In her perinatal practice she sees women even before they are pregnant to discuss risk factors for PPD so she does ask about family history of psychiatric disorders, specifically about history of PPD and anxiety.
The researchers suggest routine perinatal care should include an easy low-cost, two-part question about both personal and family history of psychiatric disorders.
“As the assessment is possible even prior to conception, this would leave time for planning preventive efforts, such as psychosocial and psychological interventions targeting these at-risk women,” the authors write.
Asking about family history a challenge
Dr. Teslyar noted though that one of the challenges in asking about family history is that families may not have openly shared psychiatric history details with offspring. Family members may also report conditions they suspect a family member had rather than having a documented diagnosis.
In places where there is universal health care, she noted, finding documented diagnoses is easier, but otherwise “you’re really taking a subjective interpretation.”
The researchers found that subgroup, sensitivity, and meta–regression analyses aligned with the primary findings. The overall certainty of evidence was graded as moderate.
This study was not able to make clear how the specific diagnoses of family members affect the risk of developing PPD because much of the data from the studies came from self-report and questions were not consistent across the studies.
For instance, only 7 studies asked specifically about first-degree family members and 10 asked about specific diagnoses. Diagnoses ranged from mild affective disorders to more intrusive disorders, such as schizophrenia.
And while this study doesn’t seek to determine why the family history and risk of PPD appear to be connected, the authors offer some possible explanations.
“Growing up in an environment with parents struggling with mental health problems potentially influences the social support received from these parents when going into motherhood,” the authors write. “This particular explanation is supported by umbrella reviews concluding that lack of social support is a significant PPD risk factor.”
Screening, extraction, and assessment of studies included was done independently by two reviewers, increasing validity, the authors note.
The authors state that approximately 10%-15% of new mothers experience PPD, but Dr. Teslyar points out the numbers in the United States are typically quoted at up to 20%-30%. PPD ranges from mild to severe episodes and includes symptoms like those for major depression outside the postpartum period.
Study authors received funding from The Lundbeck Foundation and the European Union’s Horizon 2020 Research and Innovation Programme. A coauthor, Vibe G. Frokjaer, MD, PhD, has served as consultant and lecturer for H. Lundbeck and Sage Therapeutics. No other disclosures were reported. Dr. Teslyar reports no relevant financial relationships.
Mothers who have a family history of any psychiatric disorder have almost two times the risk of postpartum depression as do mothers without such history, according to a new study.
Mette-Marie Zacher Kjeldsen, MSc, with the National Centre for Register-based Research at Aarhus (Denmark) University, led the study, a meta-analysis that included 26 studies with information on 100,877 women.
Findings were published online in JAMA Psychiatry.
When mothers had a family history of psychiatric disorders, the odds ratio for PPD was 2.08 (95% confidence interval, 1.67-2.59). That corresponds to a risk ratio of 1.79 (95% CI, 1.52-2.09), assuming a 15% postpartum depression prevalence in the general population.
Not doomed to develop PPD
Polina Teslyar, MD, a perinatal psychiatrist at Brigham and Women’s Hospital in Boston told this news organization it’s important to point out that though the risk is higher, women with a family psychiatric history should not feel as though they are destined to develop PPD.
“You are still more likely to not have postpartum depression, but it is important to be aware of personal risk factors so that if a person is experiencing that, they ask for help quickly rather than suffering and not knowing something is amiss,” she emphasized. Dr. Teslyar says she does see the higher risk for PPD, which is preventable and treatable, in her own practice when women have had a family history of psychiatric disorders.
The association makes sense, but literature on why that is has been varied, she said, and likely involves both genetics and socioeconomic factors. It’s difficult to tease apart how big a part each plays.
In her perinatal practice she sees women even before they are pregnant to discuss risk factors for PPD so she does ask about family history of psychiatric disorders, specifically about history of PPD and anxiety.
The researchers suggest routine perinatal care should include an easy low-cost, two-part question about both personal and family history of psychiatric disorders.
“As the assessment is possible even prior to conception, this would leave time for planning preventive efforts, such as psychosocial and psychological interventions targeting these at-risk women,” the authors write.
Asking about family history a challenge
Dr. Teslyar noted though that one of the challenges in asking about family history is that families may not have openly shared psychiatric history details with offspring. Family members may also report conditions they suspect a family member had rather than having a documented diagnosis.
In places where there is universal health care, she noted, finding documented diagnoses is easier, but otherwise “you’re really taking a subjective interpretation.”
The researchers found that subgroup, sensitivity, and meta–regression analyses aligned with the primary findings. The overall certainty of evidence was graded as moderate.
This study was not able to make clear how the specific diagnoses of family members affect the risk of developing PPD because much of the data from the studies came from self-report and questions were not consistent across the studies.
For instance, only 7 studies asked specifically about first-degree family members and 10 asked about specific diagnoses. Diagnoses ranged from mild affective disorders to more intrusive disorders, such as schizophrenia.
And while this study doesn’t seek to determine why the family history and risk of PPD appear to be connected, the authors offer some possible explanations.
“Growing up in an environment with parents struggling with mental health problems potentially influences the social support received from these parents when going into motherhood,” the authors write. “This particular explanation is supported by umbrella reviews concluding that lack of social support is a significant PPD risk factor.”
Screening, extraction, and assessment of studies included was done independently by two reviewers, increasing validity, the authors note.
The authors state that approximately 10%-15% of new mothers experience PPD, but Dr. Teslyar points out the numbers in the United States are typically quoted at up to 20%-30%. PPD ranges from mild to severe episodes and includes symptoms like those for major depression outside the postpartum period.
Study authors received funding from The Lundbeck Foundation and the European Union’s Horizon 2020 Research and Innovation Programme. A coauthor, Vibe G. Frokjaer, MD, PhD, has served as consultant and lecturer for H. Lundbeck and Sage Therapeutics. No other disclosures were reported. Dr. Teslyar reports no relevant financial relationships.
FROM JAMA PSYCHIATRY
Are single-incision mini-slings the new gold standard for stress urinary incontinence?
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Monkeypox: Another emerging threat?
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16
Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●
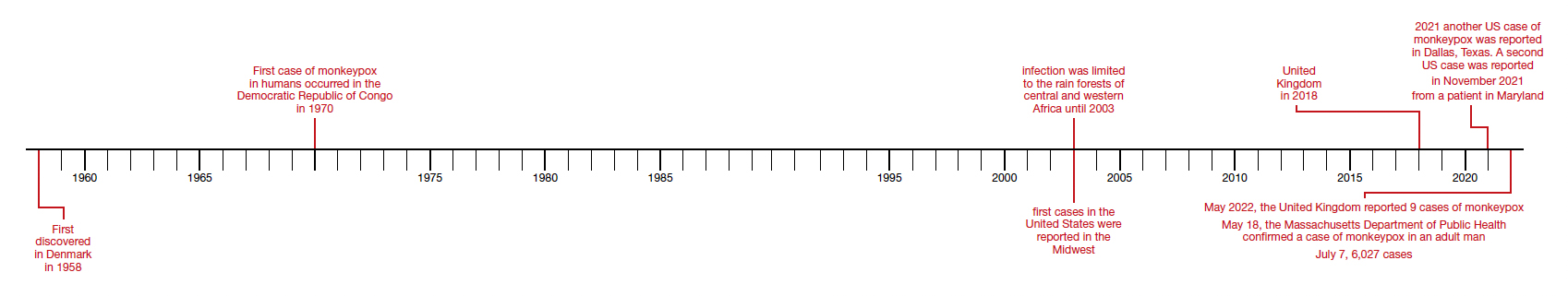
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
Antibiotic-resistant bacteria emerging in community settings
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
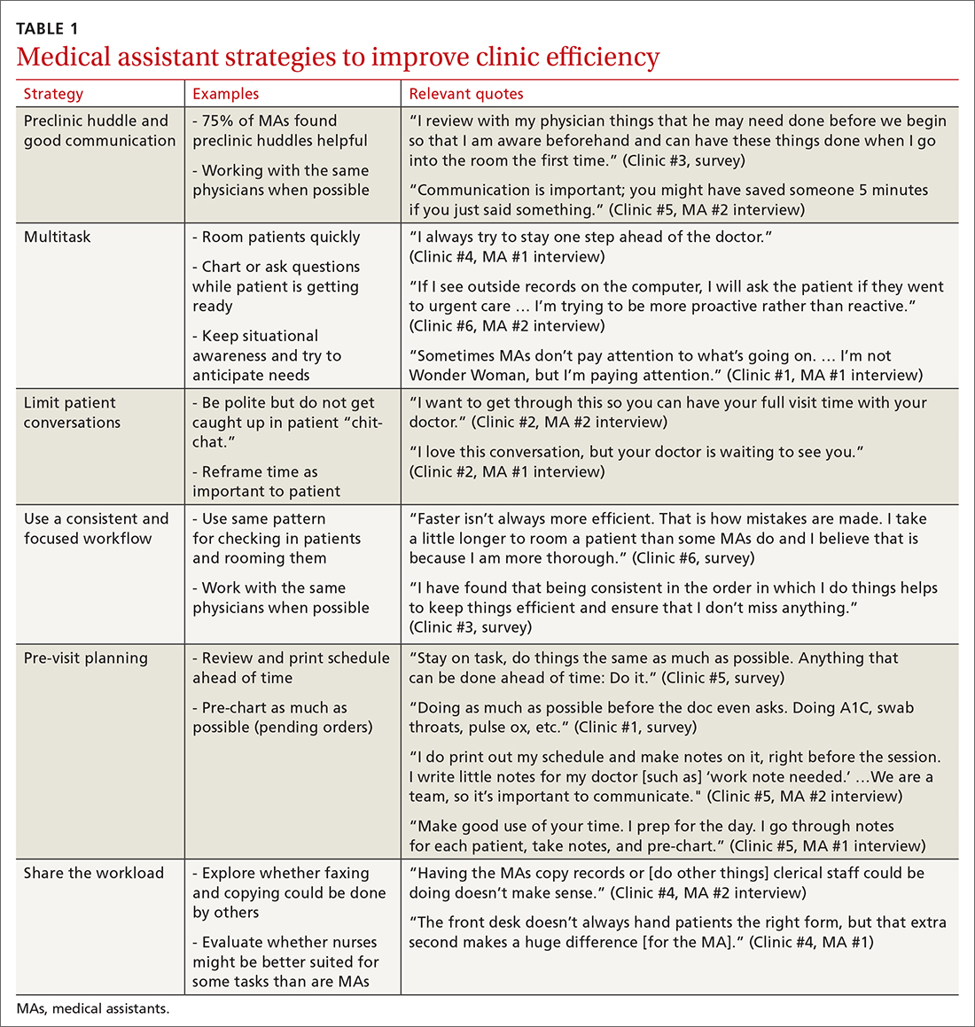
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
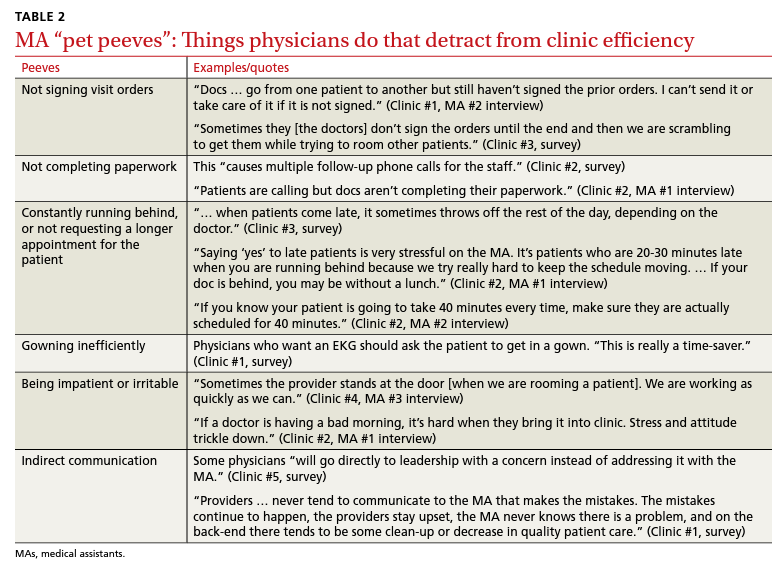
Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
Nurses’ cohort study: Endometriosis elevates stroke risk
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
FROM STROKE