User login
Hearing loss strongly tied to increased dementia risk
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
Early retirement and the terrible, horrible, no good, very bad cognitive decline
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
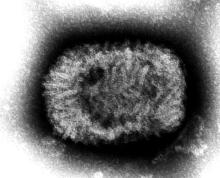
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
Frail ADHF patients benefit more from early rehab
Patients with acute decompensated heart failure who were frail at baseline improved more with targeted, early physical rehabilitation than those who were prefrail, a new analysis of the REHAB-HF study suggests.
“The robust response to the intervention by frail patients exceeded our expectations,” Gordon R. Reeves, MD, PT, of Novant Health Heart and Vascular Institute, Charlotte, N.C., told this news organization. “The effect size from improvement in physical function among frail patients was very large, with at least four times the minimal meaningful improvement, based on the Short Physical Performance Battery (SPPB).”
Furthermore, the interaction between baseline frailty status and treatment in REHAB-HF was such that a 2.6-fold larger improvement in SPPB was seen among frail versus prefrail patients.
However, Dr. Reeves noted, “We need to further evaluate safety and efficacy as it relates to adverse clinical events. Specifically, we observed a numerically higher number of deaths with the REHAB-HF intervention, which warrants further investigation before the intervention is implemented in clinical practice.”
The study was published online in JAMA Cardiology.
Interpret with caution
Dr. Reeves and colleagues conducted a prespecified secondary analysis of the previously published Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial, a multicenter, randomized controlled trial that showed that a 3-month early, transitional, tailored, multidomain physical rehabilitation intervention improved physical function and quality of life (QoL), compared with usual care. The secondary analysis aimed to evaluate whether baseline frailty altered the benefits of the intervention or was associated with risk of adverse outcomes.
According to Dr. Reeves, REHAB-HF differs from more traditional cardiac rehab programs in several ways.
- The intervention targets patients with acute HF, including HF with preserved ejection fraction (HFpEF). Medicare policy limits standard cardiac rehabilitation in HF to long-term patients with HF with reduced ejection fraction (HFrEF) only who have been stabilized for 6 weeks or longer after a recent hospitalization.
- It addresses multiple physical function domains, including balance, mobility, functional strength, and endurance. Standard cardiac rehab is primarily focused on endurance training, which can result in injuries and falls if deficits in balance, mobility, and strength are not addressed first.
- It is delivered one to one rather than in a group setting and primarily by physical therapists who are experts in the rehabilitation of medically complex patients.
- It is transitional, beginning in the hospital, then moving to the outpatient setting, then to home and includes a home assessment.
For the analysis, the Fried phenotype model was used to assess baseline frailty across five domains: unintentional weight loss during the past year; self-reported exhaustion; grip strength; slowness, as assessed by gait speed; and low physical activity, as assessed by the Short Form-12 Physical Composite Score.
At the baseline visit, patients were categorized as frail if they met three or more of these criteria. They were categorized as prefrail if they met one or two criteria and as nonfrail if they met none of the criteria. Because of the small number of nonfrail participants, the analysis included only frail and prefrail participants.
The analysis included 337 participants (mean age, 72; 54%, women; 50%, Black). At baseline, 57% were frail, and 43% were prefrail.
A significant interaction was seen between baseline frailty and the intervention for the primary trial endpoint of overall SPPB score, with a 2.6-fold larger improvement in SPPB among frail (2.1) versus prefrail (0.8) patients.
Trends favored a larger intervention effect size, with significant improvement among frail versus prefrail participants for 6-minute walk distance, QoL, and the geriatric depression score.
“However, we must interpret these findings with caution,” the authors write. “The REHAB-HF trial was not adequately powered to determine the effect of the intervention on clinical events.” This plus the number of deaths “underscore the need for additional research, including prospective clinical trials, investigating the effect of physical function interventions on clinical events among frail patients with HF.”
To address this need, the researchers recently launched a larger clinical trial, called REHAB-HFpEF, which is powered to assess the impact of the intervention on clinical events, according to Dr. Reeves. “As the name implies,” he said, “this trial is focused on older patients recently hospitalized with HFpEF, who, [compared with HFrEF] also showed a more robust response to the intervention, with worse physical function and very high prevalence of frailty near the time of hospital discharge.”
‘Never too old or sick to benefit’
Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation, said, “We have seen in clinical practice and in other (non–heart failure) clinical areas that frail older patients do improve proportionally more than younger and less frail patients with rehabilitation programs. Encouragingly, this very much supports the practice that patients are never too old or too sick to benefit from an individualized multidisciplinary rehabilitation program.”
However, he noted, “patients had to be independent with basic activities of daily living to be included in the study,” so many frail, elderly patients with heart failure who are not independent were not included in the study. It also wasn’t clear whether patients who received postacute care at a rehab facility before going home were included in the trial.
Furthermore, he said, outcomes over 1 to 5 years are needed to understand the long-term impact of the intervention.
On the other hand, he added, the fact that about half of participants were Black and were women is a “tremendous strength.”
“Repeating this study in population groups at high risk for frailty with different diagnoses, such as chronic lung diseases, interstitial lung diseases, chronic kidney disease, and rheumatologic disorders will further support the value of rehabilitation in improving patient health, function, quality of life, and reducing rehospitalizations and health care costs,” Dr. Whiteson concluded.
The study was supported by grants from the National Key R&D program. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with acute decompensated heart failure who were frail at baseline improved more with targeted, early physical rehabilitation than those who were prefrail, a new analysis of the REHAB-HF study suggests.
“The robust response to the intervention by frail patients exceeded our expectations,” Gordon R. Reeves, MD, PT, of Novant Health Heart and Vascular Institute, Charlotte, N.C., told this news organization. “The effect size from improvement in physical function among frail patients was very large, with at least four times the minimal meaningful improvement, based on the Short Physical Performance Battery (SPPB).”
Furthermore, the interaction between baseline frailty status and treatment in REHAB-HF was such that a 2.6-fold larger improvement in SPPB was seen among frail versus prefrail patients.
However, Dr. Reeves noted, “We need to further evaluate safety and efficacy as it relates to adverse clinical events. Specifically, we observed a numerically higher number of deaths with the REHAB-HF intervention, which warrants further investigation before the intervention is implemented in clinical practice.”
The study was published online in JAMA Cardiology.
Interpret with caution
Dr. Reeves and colleagues conducted a prespecified secondary analysis of the previously published Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial, a multicenter, randomized controlled trial that showed that a 3-month early, transitional, tailored, multidomain physical rehabilitation intervention improved physical function and quality of life (QoL), compared with usual care. The secondary analysis aimed to evaluate whether baseline frailty altered the benefits of the intervention or was associated with risk of adverse outcomes.
According to Dr. Reeves, REHAB-HF differs from more traditional cardiac rehab programs in several ways.
- The intervention targets patients with acute HF, including HF with preserved ejection fraction (HFpEF). Medicare policy limits standard cardiac rehabilitation in HF to long-term patients with HF with reduced ejection fraction (HFrEF) only who have been stabilized for 6 weeks or longer after a recent hospitalization.
- It addresses multiple physical function domains, including balance, mobility, functional strength, and endurance. Standard cardiac rehab is primarily focused on endurance training, which can result in injuries and falls if deficits in balance, mobility, and strength are not addressed first.
- It is delivered one to one rather than in a group setting and primarily by physical therapists who are experts in the rehabilitation of medically complex patients.
- It is transitional, beginning in the hospital, then moving to the outpatient setting, then to home and includes a home assessment.
For the analysis, the Fried phenotype model was used to assess baseline frailty across five domains: unintentional weight loss during the past year; self-reported exhaustion; grip strength; slowness, as assessed by gait speed; and low physical activity, as assessed by the Short Form-12 Physical Composite Score.
At the baseline visit, patients were categorized as frail if they met three or more of these criteria. They were categorized as prefrail if they met one or two criteria and as nonfrail if they met none of the criteria. Because of the small number of nonfrail participants, the analysis included only frail and prefrail participants.
The analysis included 337 participants (mean age, 72; 54%, women; 50%, Black). At baseline, 57% were frail, and 43% were prefrail.
A significant interaction was seen between baseline frailty and the intervention for the primary trial endpoint of overall SPPB score, with a 2.6-fold larger improvement in SPPB among frail (2.1) versus prefrail (0.8) patients.
Trends favored a larger intervention effect size, with significant improvement among frail versus prefrail participants for 6-minute walk distance, QoL, and the geriatric depression score.
“However, we must interpret these findings with caution,” the authors write. “The REHAB-HF trial was not adequately powered to determine the effect of the intervention on clinical events.” This plus the number of deaths “underscore the need for additional research, including prospective clinical trials, investigating the effect of physical function interventions on clinical events among frail patients with HF.”
To address this need, the researchers recently launched a larger clinical trial, called REHAB-HFpEF, which is powered to assess the impact of the intervention on clinical events, according to Dr. Reeves. “As the name implies,” he said, “this trial is focused on older patients recently hospitalized with HFpEF, who, [compared with HFrEF] also showed a more robust response to the intervention, with worse physical function and very high prevalence of frailty near the time of hospital discharge.”
‘Never too old or sick to benefit’
Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation, said, “We have seen in clinical practice and in other (non–heart failure) clinical areas that frail older patients do improve proportionally more than younger and less frail patients with rehabilitation programs. Encouragingly, this very much supports the practice that patients are never too old or too sick to benefit from an individualized multidisciplinary rehabilitation program.”
However, he noted, “patients had to be independent with basic activities of daily living to be included in the study,” so many frail, elderly patients with heart failure who are not independent were not included in the study. It also wasn’t clear whether patients who received postacute care at a rehab facility before going home were included in the trial.
Furthermore, he said, outcomes over 1 to 5 years are needed to understand the long-term impact of the intervention.
On the other hand, he added, the fact that about half of participants were Black and were women is a “tremendous strength.”
“Repeating this study in population groups at high risk for frailty with different diagnoses, such as chronic lung diseases, interstitial lung diseases, chronic kidney disease, and rheumatologic disorders will further support the value of rehabilitation in improving patient health, function, quality of life, and reducing rehospitalizations and health care costs,” Dr. Whiteson concluded.
The study was supported by grants from the National Key R&D program. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with acute decompensated heart failure who were frail at baseline improved more with targeted, early physical rehabilitation than those who were prefrail, a new analysis of the REHAB-HF study suggests.
“The robust response to the intervention by frail patients exceeded our expectations,” Gordon R. Reeves, MD, PT, of Novant Health Heart and Vascular Institute, Charlotte, N.C., told this news organization. “The effect size from improvement in physical function among frail patients was very large, with at least four times the minimal meaningful improvement, based on the Short Physical Performance Battery (SPPB).”
Furthermore, the interaction between baseline frailty status and treatment in REHAB-HF was such that a 2.6-fold larger improvement in SPPB was seen among frail versus prefrail patients.
However, Dr. Reeves noted, “We need to further evaluate safety and efficacy as it relates to adverse clinical events. Specifically, we observed a numerically higher number of deaths with the REHAB-HF intervention, which warrants further investigation before the intervention is implemented in clinical practice.”
The study was published online in JAMA Cardiology.
Interpret with caution
Dr. Reeves and colleagues conducted a prespecified secondary analysis of the previously published Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial, a multicenter, randomized controlled trial that showed that a 3-month early, transitional, tailored, multidomain physical rehabilitation intervention improved physical function and quality of life (QoL), compared with usual care. The secondary analysis aimed to evaluate whether baseline frailty altered the benefits of the intervention or was associated with risk of adverse outcomes.
According to Dr. Reeves, REHAB-HF differs from more traditional cardiac rehab programs in several ways.
- The intervention targets patients with acute HF, including HF with preserved ejection fraction (HFpEF). Medicare policy limits standard cardiac rehabilitation in HF to long-term patients with HF with reduced ejection fraction (HFrEF) only who have been stabilized for 6 weeks or longer after a recent hospitalization.
- It addresses multiple physical function domains, including balance, mobility, functional strength, and endurance. Standard cardiac rehab is primarily focused on endurance training, which can result in injuries and falls if deficits in balance, mobility, and strength are not addressed first.
- It is delivered one to one rather than in a group setting and primarily by physical therapists who are experts in the rehabilitation of medically complex patients.
- It is transitional, beginning in the hospital, then moving to the outpatient setting, then to home and includes a home assessment.
For the analysis, the Fried phenotype model was used to assess baseline frailty across five domains: unintentional weight loss during the past year; self-reported exhaustion; grip strength; slowness, as assessed by gait speed; and low physical activity, as assessed by the Short Form-12 Physical Composite Score.
At the baseline visit, patients were categorized as frail if they met three or more of these criteria. They were categorized as prefrail if they met one or two criteria and as nonfrail if they met none of the criteria. Because of the small number of nonfrail participants, the analysis included only frail and prefrail participants.
The analysis included 337 participants (mean age, 72; 54%, women; 50%, Black). At baseline, 57% were frail, and 43% were prefrail.
A significant interaction was seen between baseline frailty and the intervention for the primary trial endpoint of overall SPPB score, with a 2.6-fold larger improvement in SPPB among frail (2.1) versus prefrail (0.8) patients.
Trends favored a larger intervention effect size, with significant improvement among frail versus prefrail participants for 6-minute walk distance, QoL, and the geriatric depression score.
“However, we must interpret these findings with caution,” the authors write. “The REHAB-HF trial was not adequately powered to determine the effect of the intervention on clinical events.” This plus the number of deaths “underscore the need for additional research, including prospective clinical trials, investigating the effect of physical function interventions on clinical events among frail patients with HF.”
To address this need, the researchers recently launched a larger clinical trial, called REHAB-HFpEF, which is powered to assess the impact of the intervention on clinical events, according to Dr. Reeves. “As the name implies,” he said, “this trial is focused on older patients recently hospitalized with HFpEF, who, [compared with HFrEF] also showed a more robust response to the intervention, with worse physical function and very high prevalence of frailty near the time of hospital discharge.”
‘Never too old or sick to benefit’
Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation, said, “We have seen in clinical practice and in other (non–heart failure) clinical areas that frail older patients do improve proportionally more than younger and less frail patients with rehabilitation programs. Encouragingly, this very much supports the practice that patients are never too old or too sick to benefit from an individualized multidisciplinary rehabilitation program.”
However, he noted, “patients had to be independent with basic activities of daily living to be included in the study,” so many frail, elderly patients with heart failure who are not independent were not included in the study. It also wasn’t clear whether patients who received postacute care at a rehab facility before going home were included in the trial.
Furthermore, he said, outcomes over 1 to 5 years are needed to understand the long-term impact of the intervention.
On the other hand, he added, the fact that about half of participants were Black and were women is a “tremendous strength.”
“Repeating this study in population groups at high risk for frailty with different diagnoses, such as chronic lung diseases, interstitial lung diseases, chronic kidney disease, and rheumatologic disorders will further support the value of rehabilitation in improving patient health, function, quality of life, and reducing rehospitalizations and health care costs,” Dr. Whiteson concluded.
The study was supported by grants from the National Key R&D program. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some BP meds tied to significantly lower risk for dementia, Alzheimer’s
Antihypertensive medications that stimulate rather than inhibit type 2 and 4 angiotensin II receptors can lower the rate of dementia among new users of these medications, new research suggests.
Results from a cohort study of more than 57,000 older Medicare beneficiaries showed that the initiation of antihypertensives that stimulate the receptors was linked to a 16% lower risk for incident Alzheimer’s disease and related dementia (ADRD) and an 18% lower risk for vascular dementia compared with those that inhibit the receptors.
“Achieving appropriate blood pressure control is essential for maximizing brain health, and this promising research suggests certain antihypertensives could yield brain benefit compared to others,” lead study author Zachary A. Marcum, PharmD, PhD, associate professor, University of Washington School of Pharmacy, Seattle, told this news organization.
The findings were published online in JAMA Network Open.
Medicare beneficiaries
Previous observational studies showed that antihypertensive medications that stimulate type 2 and 4 angiotensin II receptors, in comparison with those that don’t, were associated with lower rates of dementia. However, those studies included individuals with prevalent hypertension and were relatively small.
The new retrospective cohort study included a random sample of 57,773 Medicare beneficiaries aged at least 65 years with new-onset hypertension. The mean age of participants was 73.8 years, 62.9% were women, and 86.9% were White.
Over the course of the study, some participants filled at least one prescription for a stimulating angiotensin II receptor type 2 and 4, such as angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Others participants filled a prescription for an inhibiting type 2 and 4 angiotensin II receptors, including angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and nondihydropyridine calcium channel blockers.
“All these medications lower blood pressure, but they do it in different ways,” said Dr. Marcum.
The researchers were interested in the varying activity of these drugs at the type 2 and 4 angiotensin II receptors.
For each 30-day interval, they categorized beneficiaries into four groups: a stimulating medication group (n = 4,879) consisting of individuals mostly taking stimulating antihypertensives; an inhibiting medication group (n = 10,303) that mostly included individuals prescribed this type of antihypertensive; a mixed group (n = 2,179) that included a combination of the first two classifications; and a nonuser group (n = 40,413) of individuals who were not using either type of drug.
The primary outcome was time to first occurrence of ADRD. The secondary outcome was time to first occurrence of vascular dementia.
Researchers controlled for cardiovascular risk factors and sociodemographic characteristics, such as age, sex, race/ethnicity, and receipt of low-income subsidy.
Unanswered questions
After adjustments, results showed that initiation of an antihypertensive medication regimen that exclusively stimulates, rather than inhibits, type 2 and 4 angiotensin II receptors was associated with a 16% lower risk for incident ADRD over a follow-up of just under 7 years (hazard ratio, 0.84; 95% confidence interval, 0.79-0.90; P < .001).
The mixed regimen was also associated with statistically significant (P = .001) reduced odds of ADRD compared with the inhibiting medications.
As for vascular dementia, use of stimulating vs. inhibiting medications was associated with an 18% lower risk (HR, 0.82; 95% CI, 0.69-0.96; P = .02).
Again, use of the mixed regimen was associated with reduced risk of vascular dementia compared with the inhibiting medications (P = .03).
A variety of potential mechanisms might explain the superiority of stimulating agents when it comes to dementia risk, said Dr. Marcum. These could include, for example, increased blood flow to the brain and reduced amyloid.
“But more mechanistic work is needed as well as evaluation of dose responses, because that’s not something we looked at in this study,” Dr. Marcum said. “There are still a lot of unanswered questions.”
Stimulators instead of inhibitors?
The results of the current analysis come on the heels of some previous work showing the benefits of lowering blood pressure. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure below 120 mm Hg significantly reduces risk for heart disease, stroke, and death from these diseases.
But in contrast to previous research, the current study included only beneficiaries with incident hypertension and new use of antihypertensive medications, and it adjusted for time-varying confounding.
Prescribing stimulating instead of inhibiting treatments could make a difference at the population level, Dr. Marcum noted.
“If we could shift the prescribing a little bit from inhibiting to stimulating, that could possibly reduce dementia risk,” he said.
However, “we’re not suggesting [that all patients] have their regimen switched,” he added.
That’s because inhibiting medications still have an important place in the antihypertensive treatment armamentarium, Dr. Marcum noted. As an example, beta-blockers are used post heart attack.
As well, factors such as cost and side effects should be taken into consideration when prescribing an antihypertensive drug.
The new results could be used to set up a comparison in a future randomized controlled trial that would provide the strongest evidence for estimating causal effects of treatments, said Dr. Marcum.
‘More convincing’
Carlos G. Santos-Gallego, MD, Icahn School of Medicine at Mount Sinai, New York, said the study is “more convincing” than previous related research, as it has a larger sample size and a longer follow-up.
“And the exquisite statistical analysis gives more robustness, more solidity, to the hypothesis that drugs that stimulate type 2 and 4 angiotensin II receptors might be protective for dementia,” said Dr. Santos-Gallego, who was not involved with the research.
However, he noted that the retrospective study had some limitations, including the underdiagnosis of dementia. “The diagnosis of dementia is, honestly, very poorly done in the clinical setting,” he said.
As well, the study could be subject to “confounding by indication,” Dr. Santos-Gallego said. “There could be a third variable, another confounding factor, that’s responsible both for the dementia and for the prescription of these drugs,” he added.
For example, he noted that comorbidities such as atrial fibrillation, myocardial infarction, and heart failure might increase the risk of dementia.
He agreed with the investigators that a randomized clinical trial would address these limitations. “All comorbidities would be equally shared” in the randomized groups, and all participants would be given “a specific test for dementia at the same time,” Dr. Santos-Gallego said.
Still, he noted that the new results are in keeping with hypertension guidelines that recommend stimulating drugs.
“This trial definitely shows that the current hypertension guidelines are good treatment for our patients, not only to control blood pressure and not only to prevent infarction to prevent stroke but also to prevent dementia,” said Dr. Santos-Gallego.
Also commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the new data provide “clarity” on why previous research had differing results on the effect of antihypertensives on cognition.
Among the caveats of this new analysis is that “it’s unclear if the demographics in this study are fully representative of Medicare beneficiaries,” said Dr. Snyder.
She, too, said a clinical trial is important “to understand if there is a preventative and/or treatment potential in the medications that stimulate type 2 and 4 angiotensin II receptors.”
The study received funding from the National Institute on Aging. Dr. Marcum and Dr. Santos-Gallego have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antihypertensive medications that stimulate rather than inhibit type 2 and 4 angiotensin II receptors can lower the rate of dementia among new users of these medications, new research suggests.
Results from a cohort study of more than 57,000 older Medicare beneficiaries showed that the initiation of antihypertensives that stimulate the receptors was linked to a 16% lower risk for incident Alzheimer’s disease and related dementia (ADRD) and an 18% lower risk for vascular dementia compared with those that inhibit the receptors.
“Achieving appropriate blood pressure control is essential for maximizing brain health, and this promising research suggests certain antihypertensives could yield brain benefit compared to others,” lead study author Zachary A. Marcum, PharmD, PhD, associate professor, University of Washington School of Pharmacy, Seattle, told this news organization.
The findings were published online in JAMA Network Open.
Medicare beneficiaries
Previous observational studies showed that antihypertensive medications that stimulate type 2 and 4 angiotensin II receptors, in comparison with those that don’t, were associated with lower rates of dementia. However, those studies included individuals with prevalent hypertension and were relatively small.
The new retrospective cohort study included a random sample of 57,773 Medicare beneficiaries aged at least 65 years with new-onset hypertension. The mean age of participants was 73.8 years, 62.9% were women, and 86.9% were White.
Over the course of the study, some participants filled at least one prescription for a stimulating angiotensin II receptor type 2 and 4, such as angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Others participants filled a prescription for an inhibiting type 2 and 4 angiotensin II receptors, including angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and nondihydropyridine calcium channel blockers.
“All these medications lower blood pressure, but they do it in different ways,” said Dr. Marcum.
The researchers were interested in the varying activity of these drugs at the type 2 and 4 angiotensin II receptors.
For each 30-day interval, they categorized beneficiaries into four groups: a stimulating medication group (n = 4,879) consisting of individuals mostly taking stimulating antihypertensives; an inhibiting medication group (n = 10,303) that mostly included individuals prescribed this type of antihypertensive; a mixed group (n = 2,179) that included a combination of the first two classifications; and a nonuser group (n = 40,413) of individuals who were not using either type of drug.
The primary outcome was time to first occurrence of ADRD. The secondary outcome was time to first occurrence of vascular dementia.
Researchers controlled for cardiovascular risk factors and sociodemographic characteristics, such as age, sex, race/ethnicity, and receipt of low-income subsidy.
Unanswered questions
After adjustments, results showed that initiation of an antihypertensive medication regimen that exclusively stimulates, rather than inhibits, type 2 and 4 angiotensin II receptors was associated with a 16% lower risk for incident ADRD over a follow-up of just under 7 years (hazard ratio, 0.84; 95% confidence interval, 0.79-0.90; P < .001).
The mixed regimen was also associated with statistically significant (P = .001) reduced odds of ADRD compared with the inhibiting medications.
As for vascular dementia, use of stimulating vs. inhibiting medications was associated with an 18% lower risk (HR, 0.82; 95% CI, 0.69-0.96; P = .02).
Again, use of the mixed regimen was associated with reduced risk of vascular dementia compared with the inhibiting medications (P = .03).
A variety of potential mechanisms might explain the superiority of stimulating agents when it comes to dementia risk, said Dr. Marcum. These could include, for example, increased blood flow to the brain and reduced amyloid.
“But more mechanistic work is needed as well as evaluation of dose responses, because that’s not something we looked at in this study,” Dr. Marcum said. “There are still a lot of unanswered questions.”
Stimulators instead of inhibitors?
The results of the current analysis come on the heels of some previous work showing the benefits of lowering blood pressure. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure below 120 mm Hg significantly reduces risk for heart disease, stroke, and death from these diseases.
But in contrast to previous research, the current study included only beneficiaries with incident hypertension and new use of antihypertensive medications, and it adjusted for time-varying confounding.
Prescribing stimulating instead of inhibiting treatments could make a difference at the population level, Dr. Marcum noted.
“If we could shift the prescribing a little bit from inhibiting to stimulating, that could possibly reduce dementia risk,” he said.
However, “we’re not suggesting [that all patients] have their regimen switched,” he added.
That’s because inhibiting medications still have an important place in the antihypertensive treatment armamentarium, Dr. Marcum noted. As an example, beta-blockers are used post heart attack.
As well, factors such as cost and side effects should be taken into consideration when prescribing an antihypertensive drug.
The new results could be used to set up a comparison in a future randomized controlled trial that would provide the strongest evidence for estimating causal effects of treatments, said Dr. Marcum.
‘More convincing’
Carlos G. Santos-Gallego, MD, Icahn School of Medicine at Mount Sinai, New York, said the study is “more convincing” than previous related research, as it has a larger sample size and a longer follow-up.
“And the exquisite statistical analysis gives more robustness, more solidity, to the hypothesis that drugs that stimulate type 2 and 4 angiotensin II receptors might be protective for dementia,” said Dr. Santos-Gallego, who was not involved with the research.
However, he noted that the retrospective study had some limitations, including the underdiagnosis of dementia. “The diagnosis of dementia is, honestly, very poorly done in the clinical setting,” he said.
As well, the study could be subject to “confounding by indication,” Dr. Santos-Gallego said. “There could be a third variable, another confounding factor, that’s responsible both for the dementia and for the prescription of these drugs,” he added.
For example, he noted that comorbidities such as atrial fibrillation, myocardial infarction, and heart failure might increase the risk of dementia.
He agreed with the investigators that a randomized clinical trial would address these limitations. “All comorbidities would be equally shared” in the randomized groups, and all participants would be given “a specific test for dementia at the same time,” Dr. Santos-Gallego said.
Still, he noted that the new results are in keeping with hypertension guidelines that recommend stimulating drugs.
“This trial definitely shows that the current hypertension guidelines are good treatment for our patients, not only to control blood pressure and not only to prevent infarction to prevent stroke but also to prevent dementia,” said Dr. Santos-Gallego.
Also commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the new data provide “clarity” on why previous research had differing results on the effect of antihypertensives on cognition.
Among the caveats of this new analysis is that “it’s unclear if the demographics in this study are fully representative of Medicare beneficiaries,” said Dr. Snyder.
She, too, said a clinical trial is important “to understand if there is a preventative and/or treatment potential in the medications that stimulate type 2 and 4 angiotensin II receptors.”
The study received funding from the National Institute on Aging. Dr. Marcum and Dr. Santos-Gallego have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antihypertensive medications that stimulate rather than inhibit type 2 and 4 angiotensin II receptors can lower the rate of dementia among new users of these medications, new research suggests.
Results from a cohort study of more than 57,000 older Medicare beneficiaries showed that the initiation of antihypertensives that stimulate the receptors was linked to a 16% lower risk for incident Alzheimer’s disease and related dementia (ADRD) and an 18% lower risk for vascular dementia compared with those that inhibit the receptors.
“Achieving appropriate blood pressure control is essential for maximizing brain health, and this promising research suggests certain antihypertensives could yield brain benefit compared to others,” lead study author Zachary A. Marcum, PharmD, PhD, associate professor, University of Washington School of Pharmacy, Seattle, told this news organization.
The findings were published online in JAMA Network Open.
Medicare beneficiaries
Previous observational studies showed that antihypertensive medications that stimulate type 2 and 4 angiotensin II receptors, in comparison with those that don’t, were associated with lower rates of dementia. However, those studies included individuals with prevalent hypertension and were relatively small.
The new retrospective cohort study included a random sample of 57,773 Medicare beneficiaries aged at least 65 years with new-onset hypertension. The mean age of participants was 73.8 years, 62.9% were women, and 86.9% were White.
Over the course of the study, some participants filled at least one prescription for a stimulating angiotensin II receptor type 2 and 4, such as angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Others participants filled a prescription for an inhibiting type 2 and 4 angiotensin II receptors, including angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and nondihydropyridine calcium channel blockers.
“All these medications lower blood pressure, but they do it in different ways,” said Dr. Marcum.
The researchers were interested in the varying activity of these drugs at the type 2 and 4 angiotensin II receptors.
For each 30-day interval, they categorized beneficiaries into four groups: a stimulating medication group (n = 4,879) consisting of individuals mostly taking stimulating antihypertensives; an inhibiting medication group (n = 10,303) that mostly included individuals prescribed this type of antihypertensive; a mixed group (n = 2,179) that included a combination of the first two classifications; and a nonuser group (n = 40,413) of individuals who were not using either type of drug.
The primary outcome was time to first occurrence of ADRD. The secondary outcome was time to first occurrence of vascular dementia.
Researchers controlled for cardiovascular risk factors and sociodemographic characteristics, such as age, sex, race/ethnicity, and receipt of low-income subsidy.
Unanswered questions
After adjustments, results showed that initiation of an antihypertensive medication regimen that exclusively stimulates, rather than inhibits, type 2 and 4 angiotensin II receptors was associated with a 16% lower risk for incident ADRD over a follow-up of just under 7 years (hazard ratio, 0.84; 95% confidence interval, 0.79-0.90; P < .001).
The mixed regimen was also associated with statistically significant (P = .001) reduced odds of ADRD compared with the inhibiting medications.
As for vascular dementia, use of stimulating vs. inhibiting medications was associated with an 18% lower risk (HR, 0.82; 95% CI, 0.69-0.96; P = .02).
Again, use of the mixed regimen was associated with reduced risk of vascular dementia compared with the inhibiting medications (P = .03).
A variety of potential mechanisms might explain the superiority of stimulating agents when it comes to dementia risk, said Dr. Marcum. These could include, for example, increased blood flow to the brain and reduced amyloid.
“But more mechanistic work is needed as well as evaluation of dose responses, because that’s not something we looked at in this study,” Dr. Marcum said. “There are still a lot of unanswered questions.”
Stimulators instead of inhibitors?
The results of the current analysis come on the heels of some previous work showing the benefits of lowering blood pressure. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure below 120 mm Hg significantly reduces risk for heart disease, stroke, and death from these diseases.
But in contrast to previous research, the current study included only beneficiaries with incident hypertension and new use of antihypertensive medications, and it adjusted for time-varying confounding.
Prescribing stimulating instead of inhibiting treatments could make a difference at the population level, Dr. Marcum noted.
“If we could shift the prescribing a little bit from inhibiting to stimulating, that could possibly reduce dementia risk,” he said.
However, “we’re not suggesting [that all patients] have their regimen switched,” he added.
That’s because inhibiting medications still have an important place in the antihypertensive treatment armamentarium, Dr. Marcum noted. As an example, beta-blockers are used post heart attack.
As well, factors such as cost and side effects should be taken into consideration when prescribing an antihypertensive drug.
The new results could be used to set up a comparison in a future randomized controlled trial that would provide the strongest evidence for estimating causal effects of treatments, said Dr. Marcum.
‘More convincing’
Carlos G. Santos-Gallego, MD, Icahn School of Medicine at Mount Sinai, New York, said the study is “more convincing” than previous related research, as it has a larger sample size and a longer follow-up.
“And the exquisite statistical analysis gives more robustness, more solidity, to the hypothesis that drugs that stimulate type 2 and 4 angiotensin II receptors might be protective for dementia,” said Dr. Santos-Gallego, who was not involved with the research.
However, he noted that the retrospective study had some limitations, including the underdiagnosis of dementia. “The diagnosis of dementia is, honestly, very poorly done in the clinical setting,” he said.
As well, the study could be subject to “confounding by indication,” Dr. Santos-Gallego said. “There could be a third variable, another confounding factor, that’s responsible both for the dementia and for the prescription of these drugs,” he added.
For example, he noted that comorbidities such as atrial fibrillation, myocardial infarction, and heart failure might increase the risk of dementia.
He agreed with the investigators that a randomized clinical trial would address these limitations. “All comorbidities would be equally shared” in the randomized groups, and all participants would be given “a specific test for dementia at the same time,” Dr. Santos-Gallego said.
Still, he noted that the new results are in keeping with hypertension guidelines that recommend stimulating drugs.
“This trial definitely shows that the current hypertension guidelines are good treatment for our patients, not only to control blood pressure and not only to prevent infarction to prevent stroke but also to prevent dementia,” said Dr. Santos-Gallego.
Also commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the new data provide “clarity” on why previous research had differing results on the effect of antihypertensives on cognition.
Among the caveats of this new analysis is that “it’s unclear if the demographics in this study are fully representative of Medicare beneficiaries,” said Dr. Snyder.
She, too, said a clinical trial is important “to understand if there is a preventative and/or treatment potential in the medications that stimulate type 2 and 4 angiotensin II receptors.”
The study received funding from the National Institute on Aging. Dr. Marcum and Dr. Santos-Gallego have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hearing loss strongly tied to increased dementia risk
Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore, Md., told this news organization.
The findings were published online in JAMA.
Dose-dependent effect
For the study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study were collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than those with normal hearing (prevalence ratio, 1.61; 95% confidence interval, 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate/severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assistive devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). This news organization last month reported on similar data published in JAMA Neurology suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore, Md., told this news organization.
The findings were published online in JAMA.
Dose-dependent effect
For the study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study were collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than those with normal hearing (prevalence ratio, 1.61; 95% confidence interval, 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate/severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assistive devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). This news organization last month reported on similar data published in JAMA Neurology suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore, Md., told this news organization.
The findings were published online in JAMA.
Dose-dependent effect
For the study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study were collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than those with normal hearing (prevalence ratio, 1.61; 95% confidence interval, 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate/severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assistive devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). This news organization last month reported on similar data published in JAMA Neurology suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Age competency exams for physicians – yes or no?
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
What to know about newly approved Alzheimer’s drug
, offering hope where there has been little for patients and their families affected by the devastating disease.
More than 6 million people in the United States live with Alzheimer’s.
It’s not a cure, but the drug, given intravenously every 2 weeks, has shown moderate positive effects in clinical trials in slowing early-stage disease.
But many are wary. As explained in an editorial in the journal The Lancet, “The Alzheimer’s disease community has become accustomed to false hope, disappointment, and controversy.”
Some worry about lecanemab’s safety as some people in clinical trials experienced serious side effects of bleeding and swelling in the brain. Scientists recently attributed a third death to lecanemab, brand name Leqembi, though the drugmaker disputed the medication was the cause.
So what should patients and their families make of this news? Here we answer some of the top questions surrounding the drug.
What does the FDA action mean?
The FDA granted accelerated approval to Leqembi after it showed positive trial results in slowing the progression of early-stage disease.
The FDA can grant accelerated approval for drugs that treat serious conditions and fill an unmet medical need while drugs continue to be studied in larger trials.
With the FDA approval in hand, doctors can now prescribe the medication.
Rebecca Edelmayer, PhD, the Alzheimer’s Association senior director of scientific engagement, says that with the FDA’s move, ramping up manufacturing – and eventually nationwide distribution and implementation – will take some time.
“Ask your doctor about availability,” she says. “The main issue is that, without insurance and Medicare coverage of this class of treatments, access for those who could benefit from the newly approved treatment will only be available to those who can pay out-of-pocket. Without coverage, people simply won’t be able to get the treatment.”
The Washington Post reports that with accelerated approval, drugmaker Eisai is expected to immediately apply for full FDA approval, which wouldn’t be likely to come before later this year. Full approval could help clear the path for Medicare coverage of the drug.
Potential benefit?
Those who got Leqembi in a clinical trial for 18 months experienced 27% less decline in memory and thinking relative to the group who got a placebo. It also reduced amyloid in the brain, the sticky protein that builds up in the brains of people with Alzheimer’s and is considered a hallmark of the disease.
Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, says, “It’s the first phase 3 study in our field of a disease-modifying drug where the clinical efficacy was very clear.”
Concerns about side effects
The drug has raised safety concerns as it has been linked with certain serious adverse events, including brain swelling and bleeding. In the trial, 14% of patients who received the drug experienced side effects that included brain swelling and bleeding, compared with about 11% in the placebo group.
Scientists have reportedly linked three deaths during the clinical trial to lecanemab, though it is unclear whether it caused the deaths.
Dr. Fillit notes that the first two people who died were on blood thinners when they received lecanemab.
“There are things about the use of the drug in the real world that we need to work out, especially in the context of people with comorbidities,” he says.
The third death is a little different, Dr. Fillit says. The patient, who had a stroke, showed signs of vasculitis, or inflammation of the blood vessels.
“We don’t know exactly what happened, but we do know it was very, very rare” among the people involved in the trials, he says.
Dr. Edelmayer says that the most common reported side effects during the trials were infusion-related reactions, headache, and amyloid-related imaging abnormalities (ARIA). According to the FDA, these abnormalities “are known to occur with antibodies of this class. ARIA usually does not have symptoms, although serious and life-threatening events rarely may occur.”
The FDA has added these as warnings to the drug’s label, describing the possible infusion-related reactions as flu-like symptoms, nausea, vomiting, and changes in blood pressure.
How much will it cost?
Eisai says that lecanemab will cost $26,500 a year.
In a draft report released in December, the Institute for Clinical and Economic Review said a price ranging from $8,500 to $20,600 a year would make the drug cost-effective. While the group has no authority to set prices, many large health insurers consider its reports when they negotiate prices and some drugmakers take into account ICER’s recommendations when setting prices.
An editorial in The Lancet last month warns that the cost will likely be “prohibitive” for low- and middle-income countries and many health systems don’t have the infrastructure for a widespread rollout.
Will Medicare cover it?
The Centers for Medicare & Medicaid Services, which runs Medicare, which covers most people with Alzheimer’s, has indicated it won’t broadly cover amyloid-lowering drugs until the drug gets full U.S. approval based on clinical benefits, as opposed to accelerated approval.
That means people would have to pay thousands out of pocket at first to get it.
The CMS decision effectively denies Medicare coverage of fast-tracked FDA-approved medications for Alzheimer’s disease unless the person is enrolled in an approved clinical trial.
On Dec. 19, the Alzheimer’s Association filed a formal request asking CMS to remove the trial-only requirement and provide full and unrestricted coverage for FDA-approved Alzheimer’s treatments.
CMS says in a statement issued after the announcement: “Because Eisai’s product, lecanemab, was granted accelerated approval by the FDA, it falls under CMS’s existing national coverage determination. CMS is examining available information and may reconsider its current coverage based on this review.”
“If lecanemab subsequently receives traditional FDA approval, CMS would provide broader coverage,” the statement says.
Who benefits most from this drug?
Lecanemab is a treatment for people with early-stage Alzheimer’s disease who have amyloid in their brain. This means people with other types of dementia, or those in the later stages of Alzheimer’s disease, are not likely to improve with this drug.
Who makes lecanemab?
Japan-based Eisai is developing the drug, a monoclonal antibody, in collaboration with the U.S. company Biogen.
What’s the Alzheimer’s Association’s view?
The association urged accelerated FDA approval. In a statement, it says it “welcomes and is further encouraged” by the clinical trial results.
It says data published in the New England Journal of Medicine confirms lecanemab “can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease.”
“We are energized at the progress we are seeing in the research pipeline. The science is telling us that although antiamyloid treatments are not a cure – they are not going to be the end of treating Alzheimer’s – they are certainly the beginning,” Dr. Edelmayer says.
Are there alternatives?
The FDA gave accelerated approval to Biogen to produce another drug for Alzheimer’s, Aduhelm (aducanemab), in 2021, but the move was controversial as the drug’s effectiveness was widely questioned. It has since largely been pulled from the market.
Aduhelm had been the first approved early-stage Alzheimer’s treatment since 2003.
A version of this article first appeared on WebMD.com.
, offering hope where there has been little for patients and their families affected by the devastating disease.
More than 6 million people in the United States live with Alzheimer’s.
It’s not a cure, but the drug, given intravenously every 2 weeks, has shown moderate positive effects in clinical trials in slowing early-stage disease.
But many are wary. As explained in an editorial in the journal The Lancet, “The Alzheimer’s disease community has become accustomed to false hope, disappointment, and controversy.”
Some worry about lecanemab’s safety as some people in clinical trials experienced serious side effects of bleeding and swelling in the brain. Scientists recently attributed a third death to lecanemab, brand name Leqembi, though the drugmaker disputed the medication was the cause.
So what should patients and their families make of this news? Here we answer some of the top questions surrounding the drug.
What does the FDA action mean?
The FDA granted accelerated approval to Leqembi after it showed positive trial results in slowing the progression of early-stage disease.
The FDA can grant accelerated approval for drugs that treat serious conditions and fill an unmet medical need while drugs continue to be studied in larger trials.
With the FDA approval in hand, doctors can now prescribe the medication.
Rebecca Edelmayer, PhD, the Alzheimer’s Association senior director of scientific engagement, says that with the FDA’s move, ramping up manufacturing – and eventually nationwide distribution and implementation – will take some time.
“Ask your doctor about availability,” she says. “The main issue is that, without insurance and Medicare coverage of this class of treatments, access for those who could benefit from the newly approved treatment will only be available to those who can pay out-of-pocket. Without coverage, people simply won’t be able to get the treatment.”
The Washington Post reports that with accelerated approval, drugmaker Eisai is expected to immediately apply for full FDA approval, which wouldn’t be likely to come before later this year. Full approval could help clear the path for Medicare coverage of the drug.
Potential benefit?
Those who got Leqembi in a clinical trial for 18 months experienced 27% less decline in memory and thinking relative to the group who got a placebo. It also reduced amyloid in the brain, the sticky protein that builds up in the brains of people with Alzheimer’s and is considered a hallmark of the disease.
Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, says, “It’s the first phase 3 study in our field of a disease-modifying drug where the clinical efficacy was very clear.”
Concerns about side effects
The drug has raised safety concerns as it has been linked with certain serious adverse events, including brain swelling and bleeding. In the trial, 14% of patients who received the drug experienced side effects that included brain swelling and bleeding, compared with about 11% in the placebo group.
Scientists have reportedly linked three deaths during the clinical trial to lecanemab, though it is unclear whether it caused the deaths.
Dr. Fillit notes that the first two people who died were on blood thinners when they received lecanemab.
“There are things about the use of the drug in the real world that we need to work out, especially in the context of people with comorbidities,” he says.
The third death is a little different, Dr. Fillit says. The patient, who had a stroke, showed signs of vasculitis, or inflammation of the blood vessels.
“We don’t know exactly what happened, but we do know it was very, very rare” among the people involved in the trials, he says.
Dr. Edelmayer says that the most common reported side effects during the trials were infusion-related reactions, headache, and amyloid-related imaging abnormalities (ARIA). According to the FDA, these abnormalities “are known to occur with antibodies of this class. ARIA usually does not have symptoms, although serious and life-threatening events rarely may occur.”
The FDA has added these as warnings to the drug’s label, describing the possible infusion-related reactions as flu-like symptoms, nausea, vomiting, and changes in blood pressure.
How much will it cost?
Eisai says that lecanemab will cost $26,500 a year.
In a draft report released in December, the Institute for Clinical and Economic Review said a price ranging from $8,500 to $20,600 a year would make the drug cost-effective. While the group has no authority to set prices, many large health insurers consider its reports when they negotiate prices and some drugmakers take into account ICER’s recommendations when setting prices.
An editorial in The Lancet last month warns that the cost will likely be “prohibitive” for low- and middle-income countries and many health systems don’t have the infrastructure for a widespread rollout.
Will Medicare cover it?
The Centers for Medicare & Medicaid Services, which runs Medicare, which covers most people with Alzheimer’s, has indicated it won’t broadly cover amyloid-lowering drugs until the drug gets full U.S. approval based on clinical benefits, as opposed to accelerated approval.
That means people would have to pay thousands out of pocket at first to get it.
The CMS decision effectively denies Medicare coverage of fast-tracked FDA-approved medications for Alzheimer’s disease unless the person is enrolled in an approved clinical trial.
On Dec. 19, the Alzheimer’s Association filed a formal request asking CMS to remove the trial-only requirement and provide full and unrestricted coverage for FDA-approved Alzheimer’s treatments.
CMS says in a statement issued after the announcement: “Because Eisai’s product, lecanemab, was granted accelerated approval by the FDA, it falls under CMS’s existing national coverage determination. CMS is examining available information and may reconsider its current coverage based on this review.”
“If lecanemab subsequently receives traditional FDA approval, CMS would provide broader coverage,” the statement says.
Who benefits most from this drug?
Lecanemab is a treatment for people with early-stage Alzheimer’s disease who have amyloid in their brain. This means people with other types of dementia, or those in the later stages of Alzheimer’s disease, are not likely to improve with this drug.
Who makes lecanemab?
Japan-based Eisai is developing the drug, a monoclonal antibody, in collaboration with the U.S. company Biogen.
What’s the Alzheimer’s Association’s view?
The association urged accelerated FDA approval. In a statement, it says it “welcomes and is further encouraged” by the clinical trial results.
It says data published in the New England Journal of Medicine confirms lecanemab “can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease.”
“We are energized at the progress we are seeing in the research pipeline. The science is telling us that although antiamyloid treatments are not a cure – they are not going to be the end of treating Alzheimer’s – they are certainly the beginning,” Dr. Edelmayer says.
Are there alternatives?
The FDA gave accelerated approval to Biogen to produce another drug for Alzheimer’s, Aduhelm (aducanemab), in 2021, but the move was controversial as the drug’s effectiveness was widely questioned. It has since largely been pulled from the market.
Aduhelm had been the first approved early-stage Alzheimer’s treatment since 2003.
A version of this article first appeared on WebMD.com.
, offering hope where there has been little for patients and their families affected by the devastating disease.
More than 6 million people in the United States live with Alzheimer’s.
It’s not a cure, but the drug, given intravenously every 2 weeks, has shown moderate positive effects in clinical trials in slowing early-stage disease.
But many are wary. As explained in an editorial in the journal The Lancet, “The Alzheimer’s disease community has become accustomed to false hope, disappointment, and controversy.”
Some worry about lecanemab’s safety as some people in clinical trials experienced serious side effects of bleeding and swelling in the brain. Scientists recently attributed a third death to lecanemab, brand name Leqembi, though the drugmaker disputed the medication was the cause.
So what should patients and their families make of this news? Here we answer some of the top questions surrounding the drug.
What does the FDA action mean?
The FDA granted accelerated approval to Leqembi after it showed positive trial results in slowing the progression of early-stage disease.
The FDA can grant accelerated approval for drugs that treat serious conditions and fill an unmet medical need while drugs continue to be studied in larger trials.
With the FDA approval in hand, doctors can now prescribe the medication.
Rebecca Edelmayer, PhD, the Alzheimer’s Association senior director of scientific engagement, says that with the FDA’s move, ramping up manufacturing – and eventually nationwide distribution and implementation – will take some time.
“Ask your doctor about availability,” she says. “The main issue is that, without insurance and Medicare coverage of this class of treatments, access for those who could benefit from the newly approved treatment will only be available to those who can pay out-of-pocket. Without coverage, people simply won’t be able to get the treatment.”
The Washington Post reports that with accelerated approval, drugmaker Eisai is expected to immediately apply for full FDA approval, which wouldn’t be likely to come before later this year. Full approval could help clear the path for Medicare coverage of the drug.
Potential benefit?
Those who got Leqembi in a clinical trial for 18 months experienced 27% less decline in memory and thinking relative to the group who got a placebo. It also reduced amyloid in the brain, the sticky protein that builds up in the brains of people with Alzheimer’s and is considered a hallmark of the disease.
Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, says, “It’s the first phase 3 study in our field of a disease-modifying drug where the clinical efficacy was very clear.”
Concerns about side effects
The drug has raised safety concerns as it has been linked with certain serious adverse events, including brain swelling and bleeding. In the trial, 14% of patients who received the drug experienced side effects that included brain swelling and bleeding, compared with about 11% in the placebo group.
Scientists have reportedly linked three deaths during the clinical trial to lecanemab, though it is unclear whether it caused the deaths.
Dr. Fillit notes that the first two people who died were on blood thinners when they received lecanemab.
“There are things about the use of the drug in the real world that we need to work out, especially in the context of people with comorbidities,” he says.
The third death is a little different, Dr. Fillit says. The patient, who had a stroke, showed signs of vasculitis, or inflammation of the blood vessels.
“We don’t know exactly what happened, but we do know it was very, very rare” among the people involved in the trials, he says.
Dr. Edelmayer says that the most common reported side effects during the trials were infusion-related reactions, headache, and amyloid-related imaging abnormalities (ARIA). According to the FDA, these abnormalities “are known to occur with antibodies of this class. ARIA usually does not have symptoms, although serious and life-threatening events rarely may occur.”
The FDA has added these as warnings to the drug’s label, describing the possible infusion-related reactions as flu-like symptoms, nausea, vomiting, and changes in blood pressure.
How much will it cost?
Eisai says that lecanemab will cost $26,500 a year.
In a draft report released in December, the Institute for Clinical and Economic Review said a price ranging from $8,500 to $20,600 a year would make the drug cost-effective. While the group has no authority to set prices, many large health insurers consider its reports when they negotiate prices and some drugmakers take into account ICER’s recommendations when setting prices.
An editorial in The Lancet last month warns that the cost will likely be “prohibitive” for low- and middle-income countries and many health systems don’t have the infrastructure for a widespread rollout.
Will Medicare cover it?
The Centers for Medicare & Medicaid Services, which runs Medicare, which covers most people with Alzheimer’s, has indicated it won’t broadly cover amyloid-lowering drugs until the drug gets full U.S. approval based on clinical benefits, as opposed to accelerated approval.
That means people would have to pay thousands out of pocket at first to get it.
The CMS decision effectively denies Medicare coverage of fast-tracked FDA-approved medications for Alzheimer’s disease unless the person is enrolled in an approved clinical trial.
On Dec. 19, the Alzheimer’s Association filed a formal request asking CMS to remove the trial-only requirement and provide full and unrestricted coverage for FDA-approved Alzheimer’s treatments.
CMS says in a statement issued after the announcement: “Because Eisai’s product, lecanemab, was granted accelerated approval by the FDA, it falls under CMS’s existing national coverage determination. CMS is examining available information and may reconsider its current coverage based on this review.”
“If lecanemab subsequently receives traditional FDA approval, CMS would provide broader coverage,” the statement says.
Who benefits most from this drug?
Lecanemab is a treatment for people with early-stage Alzheimer’s disease who have amyloid in their brain. This means people with other types of dementia, or those in the later stages of Alzheimer’s disease, are not likely to improve with this drug.
Who makes lecanemab?
Japan-based Eisai is developing the drug, a monoclonal antibody, in collaboration with the U.S. company Biogen.
What’s the Alzheimer’s Association’s view?
The association urged accelerated FDA approval. In a statement, it says it “welcomes and is further encouraged” by the clinical trial results.
It says data published in the New England Journal of Medicine confirms lecanemab “can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease.”
“We are energized at the progress we are seeing in the research pipeline. The science is telling us that although antiamyloid treatments are not a cure – they are not going to be the end of treating Alzheimer’s – they are certainly the beginning,” Dr. Edelmayer says.
Are there alternatives?
The FDA gave accelerated approval to Biogen to produce another drug for Alzheimer’s, Aduhelm (aducanemab), in 2021, but the move was controversial as the drug’s effectiveness was widely questioned. It has since largely been pulled from the market.
Aduhelm had been the first approved early-stage Alzheimer’s treatment since 2003.
A version of this article first appeared on WebMD.com.
Is the FDA serotonin syndrome warning unnecessary?
Results from a study that included more than 1,100 patients who were prescribed linezolid, about 20% of whom were also taking antidepressants, showed that serotonin syndrome occurred in fewer than 0.5% of participants – and that the percentage was actually lower among those who took antidepressants, compared with those who did not.
A comparison of participants who took antidepressants to propensity-matched patients who did not take antidepressants showed similar rates of altered mental status, hospitalization, and death between the two groups.
“In this cohort study of older patients who were prescribed linezolid, serotonin syndrome occurred rarely [and] concurrent antidepressants did not significantly increase the risk of serotonin syndrome,” Anthony Bai, MD, division of infectious diseases, department of medicine, Queen’s University, Kingston, Ont., and colleagues write.
“These findings suggested that linezolid is likely safe for patients receiving antidepressants. Nevertheless, prescribers should remain vigilant for this potential drug interaction,” they warn.
The findings were published online in JAMA Network Open.
Scarce data
Linezolid, a synthetic oxazolidinone antibiotic active against resistant gram-positive bacteria, has bioavailability of 100%, “making it ideal as first-line or step-down oral antibiotic therapy for bacteremia and pneumonia as well as skin and soft tissue infections,” the researchers write.
However, they note its use has been “limited because of concerns of drug interactions,” since it can reversibly inhibit monoamine oxidase (MAO).
Thus, “coadministration with antidepressants, such as nonselective MAO inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion, may precipitate serotonin syndrome,” they write.
The investigators note that many patients who were taking antidepressants and who also needed linezolid for an infection “could not receive it because of this relative contraindication.” They add that data on the risk of serotonin syndrome associated with linezolid are “scarce” and are based largely on case reports or case series from passive surveillance.
Although a previous review of linezolid trials found “no conclusive evidence” that it increased risk for serotonin syndrome in patients taking serotonergic medication, data on patients outside of trials “are lacking.” In addition, an observational study suggested that an increased risk had a small sample size that “likely led to imprecise estimates with a wide CI and inconclusive results,” the researchers write.
Therefore, they sought to fill the knowledge gap by retrospectively analyzing data drawn from the ICES database, an independent nonprofit research institute funded by the Ontario Ministry of Health. This was done in order to “estimate the incidence of serotonin syndrome and how this risk changes because of concomitant antidepressant use in patients receiving linezolid treatment,” they write.
The study included a convenience sample of Ontario-based adults (n = 1,134, 52.5% men) who were dispensed oral linezolid 600 mg twice daily between Oct. 1, 2014, and Jan. 1, 2021. All patients were followed for 30 days.
Of these participants, 19% were also taking antidepressants. Close to half (47.9%) were taking an SSRI, 16.7% were taking an SNRI, 7% were taking a tricyclic antidepressant, and 3.3% were taking a norepinephrine and dopamine reuptake inhibitor.
Patients were divided into groups on the basis of age: 66-69 years (19.8%), 70-79 years (41.7%), and 80 years or older (38.4%).
Reassuring findings
Serotonin syndrome occurred in fewer than six patients (< .5%), although the exact numbers were not reported, owing to patient privacy concerns. However, on the basis of fewer than six events, the investigators calculated the risk difference for serotonin syndrome as ranging from −0.5% to 2.3%.
Fewer patients who were taking antidepressants experienced serotonin syndrome, compared with those who were not taking antidepressants.
The investigators estimated a propensity score for antidepressant use that incorporated several patient baseline characteristics, including age, sex, rural home address, Charlson Comorbidity Index, estimated glomerular filtration rate, history of substance use disorder, and days of use of linezolid and other serotonergic medications. They then matched patients who were not taking antidepressants with those who were taking antidepressants (n = 166 each).
The adjusted risk difference for serotonin syndrome was lower in the antidepressant group than in the no-antidepressant group (−1.2%; 95% confidence interval, −2.9% to 0.5%).
“Within this 95% CI, the worst-case scenario would be a 0.5% increase in the risk of serotonin syndrome due to antidepressants, which is equivalent to a number needed to harm of 200,” the researchers write.
For secondary outcomes, they found “similar rates” of altered mental status or confusion, hospitalization, and death within 30 days between the two propensity score–matched groups.
The investigators note that their findings have “limitations, due to the nature of retrospective observational studies.” Moreover, these types of studies are “not efficient because they often focus on a particular adverse event.”
Future research should move beyond observational studies to phase 4 studies, which would “prospectively monitor for all types of adverse events,” they write.
Still, “while waiting for higher-quality evidence, our study adds to the existing evidence for the safety of linezolid even in the context of concomitant antidepressants,” the researchers note.
“Based on the existing evidence, clinicians should be reassured that it appears safe to prescribe oral linezolid to patients taking antidepressants, especially if there are limited antibiotic options or alternative antibiotic options would be inferior,” they add.
‘Consequential relevance’
Commenting on the study, Ipsit Vahia, MD, associate chief of geriatric psychiatry and director of digital psychiatry translation at McLean Hospital, Boston, noted that although studies of drug interactions across age groups “may not accurately reflect the rates of risk for older adults,” the current study focused on linezolid use among older patients.
“One may expect higher rates of serotonin syndrome in older adults, who generally tend to be more sensitive to adverse reactions,” said Dr. Vahia, who is also director of the Technology and Aging Lab at McLean and was not involved with the current research.
“However, the study finds the risk to be low with a number needed to harm of 200,” Dr. Vahia said.
“This retrospective epidemiologic study does not shed light on why this number may be lower than expected, but it has consequential relevance in clinical practice for the management of severe infections among older adults using antidepressants,” he added.
The study was funded by a Queen’s University Research Initiation Grant. Dr. Bai and three of the four other investigators report no relevant financial relationships. Coinvestigator Mark Loeb, MD, reports having received personal fees from the Paladin Labs Advisory Committee, the International Centre for Professional Development in Health and Medicine Advisory Committee, and the Sunovion Advisory Committee outside the submitted work. Dr. Vahia serves as a consultant for Otsuka, has a research collaboration with Emerald Innovations, and receives honorarium as editor for The American Journal of Geriatric Psychiatry.
A version of this article first appeared on Medscape.com.
Results from a study that included more than 1,100 patients who were prescribed linezolid, about 20% of whom were also taking antidepressants, showed that serotonin syndrome occurred in fewer than 0.5% of participants – and that the percentage was actually lower among those who took antidepressants, compared with those who did not.
A comparison of participants who took antidepressants to propensity-matched patients who did not take antidepressants showed similar rates of altered mental status, hospitalization, and death between the two groups.
“In this cohort study of older patients who were prescribed linezolid, serotonin syndrome occurred rarely [and] concurrent antidepressants did not significantly increase the risk of serotonin syndrome,” Anthony Bai, MD, division of infectious diseases, department of medicine, Queen’s University, Kingston, Ont., and colleagues write.
“These findings suggested that linezolid is likely safe for patients receiving antidepressants. Nevertheless, prescribers should remain vigilant for this potential drug interaction,” they warn.
The findings were published online in JAMA Network Open.
Scarce data
Linezolid, a synthetic oxazolidinone antibiotic active against resistant gram-positive bacteria, has bioavailability of 100%, “making it ideal as first-line or step-down oral antibiotic therapy for bacteremia and pneumonia as well as skin and soft tissue infections,” the researchers write.
However, they note its use has been “limited because of concerns of drug interactions,” since it can reversibly inhibit monoamine oxidase (MAO).
Thus, “coadministration with antidepressants, such as nonselective MAO inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion, may precipitate serotonin syndrome,” they write.
The investigators note that many patients who were taking antidepressants and who also needed linezolid for an infection “could not receive it because of this relative contraindication.” They add that data on the risk of serotonin syndrome associated with linezolid are “scarce” and are based largely on case reports or case series from passive surveillance.
Although a previous review of linezolid trials found “no conclusive evidence” that it increased risk for serotonin syndrome in patients taking serotonergic medication, data on patients outside of trials “are lacking.” In addition, an observational study suggested that an increased risk had a small sample size that “likely led to imprecise estimates with a wide CI and inconclusive results,” the researchers write.
Therefore, they sought to fill the knowledge gap by retrospectively analyzing data drawn from the ICES database, an independent nonprofit research institute funded by the Ontario Ministry of Health. This was done in order to “estimate the incidence of serotonin syndrome and how this risk changes because of concomitant antidepressant use in patients receiving linezolid treatment,” they write.
The study included a convenience sample of Ontario-based adults (n = 1,134, 52.5% men) who were dispensed oral linezolid 600 mg twice daily between Oct. 1, 2014, and Jan. 1, 2021. All patients were followed for 30 days.
Of these participants, 19% were also taking antidepressants. Close to half (47.9%) were taking an SSRI, 16.7% were taking an SNRI, 7% were taking a tricyclic antidepressant, and 3.3% were taking a norepinephrine and dopamine reuptake inhibitor.
Patients were divided into groups on the basis of age: 66-69 years (19.8%), 70-79 years (41.7%), and 80 years or older (38.4%).
Reassuring findings
Serotonin syndrome occurred in fewer than six patients (< .5%), although the exact numbers were not reported, owing to patient privacy concerns. However, on the basis of fewer than six events, the investigators calculated the risk difference for serotonin syndrome as ranging from −0.5% to 2.3%.
Fewer patients who were taking antidepressants experienced serotonin syndrome, compared with those who were not taking antidepressants.
The investigators estimated a propensity score for antidepressant use that incorporated several patient baseline characteristics, including age, sex, rural home address, Charlson Comorbidity Index, estimated glomerular filtration rate, history of substance use disorder, and days of use of linezolid and other serotonergic medications. They then matched patients who were not taking antidepressants with those who were taking antidepressants (n = 166 each).
The adjusted risk difference for serotonin syndrome was lower in the antidepressant group than in the no-antidepressant group (−1.2%; 95% confidence interval, −2.9% to 0.5%).
“Within this 95% CI, the worst-case scenario would be a 0.5% increase in the risk of serotonin syndrome due to antidepressants, which is equivalent to a number needed to harm of 200,” the researchers write.
For secondary outcomes, they found “similar rates” of altered mental status or confusion, hospitalization, and death within 30 days between the two propensity score–matched groups.
The investigators note that their findings have “limitations, due to the nature of retrospective observational studies.” Moreover, these types of studies are “not efficient because they often focus on a particular adverse event.”
Future research should move beyond observational studies to phase 4 studies, which would “prospectively monitor for all types of adverse events,” they write.
Still, “while waiting for higher-quality evidence, our study adds to the existing evidence for the safety of linezolid even in the context of concomitant antidepressants,” the researchers note.
“Based on the existing evidence, clinicians should be reassured that it appears safe to prescribe oral linezolid to patients taking antidepressants, especially if there are limited antibiotic options or alternative antibiotic options would be inferior,” they add.
‘Consequential relevance’
Commenting on the study, Ipsit Vahia, MD, associate chief of geriatric psychiatry and director of digital psychiatry translation at McLean Hospital, Boston, noted that although studies of drug interactions across age groups “may not accurately reflect the rates of risk for older adults,” the current study focused on linezolid use among older patients.
“One may expect higher rates of serotonin syndrome in older adults, who generally tend to be more sensitive to adverse reactions,” said Dr. Vahia, who is also director of the Technology and Aging Lab at McLean and was not involved with the current research.
“However, the study finds the risk to be low with a number needed to harm of 200,” Dr. Vahia said.
“This retrospective epidemiologic study does not shed light on why this number may be lower than expected, but it has consequential relevance in clinical practice for the management of severe infections among older adults using antidepressants,” he added.
The study was funded by a Queen’s University Research Initiation Grant. Dr. Bai and three of the four other investigators report no relevant financial relationships. Coinvestigator Mark Loeb, MD, reports having received personal fees from the Paladin Labs Advisory Committee, the International Centre for Professional Development in Health and Medicine Advisory Committee, and the Sunovion Advisory Committee outside the submitted work. Dr. Vahia serves as a consultant for Otsuka, has a research collaboration with Emerald Innovations, and receives honorarium as editor for The American Journal of Geriatric Psychiatry.
A version of this article first appeared on Medscape.com.
Results from a study that included more than 1,100 patients who were prescribed linezolid, about 20% of whom were also taking antidepressants, showed that serotonin syndrome occurred in fewer than 0.5% of participants – and that the percentage was actually lower among those who took antidepressants, compared with those who did not.
A comparison of participants who took antidepressants to propensity-matched patients who did not take antidepressants showed similar rates of altered mental status, hospitalization, and death between the two groups.
“In this cohort study of older patients who were prescribed linezolid, serotonin syndrome occurred rarely [and] concurrent antidepressants did not significantly increase the risk of serotonin syndrome,” Anthony Bai, MD, division of infectious diseases, department of medicine, Queen’s University, Kingston, Ont., and colleagues write.
“These findings suggested that linezolid is likely safe for patients receiving antidepressants. Nevertheless, prescribers should remain vigilant for this potential drug interaction,” they warn.
The findings were published online in JAMA Network Open.
Scarce data
Linezolid, a synthetic oxazolidinone antibiotic active against resistant gram-positive bacteria, has bioavailability of 100%, “making it ideal as first-line or step-down oral antibiotic therapy for bacteremia and pneumonia as well as skin and soft tissue infections,” the researchers write.
However, they note its use has been “limited because of concerns of drug interactions,” since it can reversibly inhibit monoamine oxidase (MAO).
Thus, “coadministration with antidepressants, such as nonselective MAO inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion, may precipitate serotonin syndrome,” they write.
The investigators note that many patients who were taking antidepressants and who also needed linezolid for an infection “could not receive it because of this relative contraindication.” They add that data on the risk of serotonin syndrome associated with linezolid are “scarce” and are based largely on case reports or case series from passive surveillance.
Although a previous review of linezolid trials found “no conclusive evidence” that it increased risk for serotonin syndrome in patients taking serotonergic medication, data on patients outside of trials “are lacking.” In addition, an observational study suggested that an increased risk had a small sample size that “likely led to imprecise estimates with a wide CI and inconclusive results,” the researchers write.
Therefore, they sought to fill the knowledge gap by retrospectively analyzing data drawn from the ICES database, an independent nonprofit research institute funded by the Ontario Ministry of Health. This was done in order to “estimate the incidence of serotonin syndrome and how this risk changes because of concomitant antidepressant use in patients receiving linezolid treatment,” they write.
The study included a convenience sample of Ontario-based adults (n = 1,134, 52.5% men) who were dispensed oral linezolid 600 mg twice daily between Oct. 1, 2014, and Jan. 1, 2021. All patients were followed for 30 days.
Of these participants, 19% were also taking antidepressants. Close to half (47.9%) were taking an SSRI, 16.7% were taking an SNRI, 7% were taking a tricyclic antidepressant, and 3.3% were taking a norepinephrine and dopamine reuptake inhibitor.
Patients were divided into groups on the basis of age: 66-69 years (19.8%), 70-79 years (41.7%), and 80 years or older (38.4%).
Reassuring findings
Serotonin syndrome occurred in fewer than six patients (< .5%), although the exact numbers were not reported, owing to patient privacy concerns. However, on the basis of fewer than six events, the investigators calculated the risk difference for serotonin syndrome as ranging from −0.5% to 2.3%.
Fewer patients who were taking antidepressants experienced serotonin syndrome, compared with those who were not taking antidepressants.
The investigators estimated a propensity score for antidepressant use that incorporated several patient baseline characteristics, including age, sex, rural home address, Charlson Comorbidity Index, estimated glomerular filtration rate, history of substance use disorder, and days of use of linezolid and other serotonergic medications. They then matched patients who were not taking antidepressants with those who were taking antidepressants (n = 166 each).
The adjusted risk difference for serotonin syndrome was lower in the antidepressant group than in the no-antidepressant group (−1.2%; 95% confidence interval, −2.9% to 0.5%).
“Within this 95% CI, the worst-case scenario would be a 0.5% increase in the risk of serotonin syndrome due to antidepressants, which is equivalent to a number needed to harm of 200,” the researchers write.
For secondary outcomes, they found “similar rates” of altered mental status or confusion, hospitalization, and death within 30 days between the two propensity score–matched groups.
The investigators note that their findings have “limitations, due to the nature of retrospective observational studies.” Moreover, these types of studies are “not efficient because they often focus on a particular adverse event.”
Future research should move beyond observational studies to phase 4 studies, which would “prospectively monitor for all types of adverse events,” they write.
Still, “while waiting for higher-quality evidence, our study adds to the existing evidence for the safety of linezolid even in the context of concomitant antidepressants,” the researchers note.
“Based on the existing evidence, clinicians should be reassured that it appears safe to prescribe oral linezolid to patients taking antidepressants, especially if there are limited antibiotic options or alternative antibiotic options would be inferior,” they add.
‘Consequential relevance’
Commenting on the study, Ipsit Vahia, MD, associate chief of geriatric psychiatry and director of digital psychiatry translation at McLean Hospital, Boston, noted that although studies of drug interactions across age groups “may not accurately reflect the rates of risk for older adults,” the current study focused on linezolid use among older patients.
“One may expect higher rates of serotonin syndrome in older adults, who generally tend to be more sensitive to adverse reactions,” said Dr. Vahia, who is also director of the Technology and Aging Lab at McLean and was not involved with the current research.
“However, the study finds the risk to be low with a number needed to harm of 200,” Dr. Vahia said.
“This retrospective epidemiologic study does not shed light on why this number may be lower than expected, but it has consequential relevance in clinical practice for the management of severe infections among older adults using antidepressants,” he added.
The study was funded by a Queen’s University Research Initiation Grant. Dr. Bai and three of the four other investigators report no relevant financial relationships. Coinvestigator Mark Loeb, MD, reports having received personal fees from the Paladin Labs Advisory Committee, the International Centre for Professional Development in Health and Medicine Advisory Committee, and the Sunovion Advisory Committee outside the submitted work. Dr. Vahia serves as a consultant for Otsuka, has a research collaboration with Emerald Innovations, and receives honorarium as editor for The American Journal of Geriatric Psychiatry.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA approves second antiamyloid for Alzheimer’s disease
Like its controversial cousin aducanumab (Aduhelm, Biogen/Eisai), lecanemab was approved under the FDA’s accelerated approval pathway, which can be used to fast-track a drug that provides a meaningful therapeutic advantage over existing treatments for a serious or life-threatening illness.
Unlike aducanumab, however, there was no formal FDA advisory committee meeting on lecanemab prior to approval.
“Alzheimer’s disease immeasurably incapacitates the lives of those who suffer from it and has devastating effects on their loved ones,” Billy Dunn, MD, director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a press release.
“This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s, instead of only treating the symptoms of the disease,” Dr. Dunn added.
Eisai has reported that lecanemab will cost $26,500 a year.
Modest benefit, adverse events
The FDA noted, “The labeling states that treatment with Leqembi should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was studied in clinical trials.”
The agency approved the treatment on the basis of findings from the CLARITY AD trial, which showed modest cognitive benefit for patients with early AD – but at a cost of increased risk for amyloid-related edema and effusions.
The trial enrolled 1,795 adults with mild cognitive impairment or early Alzheimer’s disease in whom amyloid pathology in the brain had been confirmed. Treatment consisted of lecanemab 10 mg/kg biweekly or matching placebo.
After 18 months of treatment, lecanemab slowed cognitive decline by 27%, compared with placebo, as measured by the Clinical Dementia Rating–Sum of Boxes (CDR-SB). This was an absolute difference of 0.45 points (change from baseline, 1.21 for lecanemab vs. 1.66 with placebo; P < .001).
While the results are “welcome news,” a 0.45-point difference on the CDR-SB might not be clinically meaningful, authors of a recent editorial in The Lancet cautioned.
Amyloid-related imaging abnormalities that manifest as edema or microhemorrhages also occurred in one in five patients taking lecanemab.
In addition, a newly published case report in The New England Journal of Medicine describes a patient with Alzheimer’s disease who was taking lecanemab and who died after experiencing numerous intracerebral hemorrhages during treatment with tissue plasminogen activator (tPA) for acute ischemic stroke.
“The findings raise the possibility of cerebral hemorrhages and necrotizing vasculopathy associated with tPA infusion in a patient with cerebrovascular amyloid who had received lecanemab,” the authors wrote.
Alzheimer’s Association reaction
Still, in anticipation of accelerated approval of lecanemab and the antiamyloid drug donanemab (Eli Lilly), which the FDA has also fast-tracked, the Alzheimer’s Association filed a formal request last month with the Centers for Medicare & Medicaid Services asking that it provide full and unrestricted coverage for FDA-approved Alzheimer’s disease treatments.
In a letter addressed to CMS administrator Chiquita Brooks-LaSure, the association asked the agency to remove the requirements for “coverage with evidence development” in its national coverage determination for FDA-approved antiamyloid monoclonal antibodies.
“Each day matters when it comes to slowing the progression of this disease,” Joanne Pike, DrPH, president and CEO for the Alzheimer’s Association, noted in a news release at the time.
“The current CMS policy to severely limit access to these treatments eliminates people’s options, is resulting in continued irreversible disease progression, and contributes to greater health inequities. That’s not acceptable,” Dr. Pike added.
After news of today’s approval was released, Dr. Pike noted in a new release, “The Alzheimer’s Association welcomes and celebrates this action by the FDA. We now have a second approved treatment that changes the course of Alzheimer’s disease in a meaningful way for people in the early stages of the disease.”
Maria C. Carrillo, PhD, chief science officer at the Alzheimer’s Association, called today’s approval “a milestone achievement.”
“The progress we’ve seen in not only this class of treatments but also in the diversification of treatment types and targets over the past few years is exciting and provides real hope to those impacted by this devastating disease,” Dr. Carrillo said.
Critical issues
Commenting on the approval, Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School, Boston, and chief medical officer at Linus Health, said FDA approval of lecanemab and its adoption in the clinic represent a “very exciting development and prospect; but arguably some critical issues need to be considered.”
He noted that the health care system “is not currently prepared to cope with the challenges and demands of lecanemab,” as well as future pharmacologic agents.
“First, we need better workflows to identify suitable patients who can most benefit from this treatment,” said Dr. Pascual-Leone. He added that beyond identification of cognitive difficulties, amyloid status will need to be determined.
“Presently, this requires expensive and invasive tests,” such as positron-emission tomography scans or lumbar punctures for cerebrospinal fluid analysis. However, these are not fully covered by insurance companies and would be challenging to fully scale, he noted.
“In addition to screening, health systems will need to resolve the logistics challenges around the administration of lecanemab with twice-monthly infusions and the need for careful longitudinal evaluations for potential side effects,” said Dr. Pascual-Leone.
“While lecanemab may represent the first disease-modifying therapy widely available for early Alzheimer’s disease, the likely more promising approach is the addition of other therapies to lecanemab as part of a multi-intervention strategy combining pharmacologic and nonpharmacologic interventions,” he added.
Dr. Pascual-Leone has served as a paid member on scientific advisory boards for Neuroelectrics, Magstim, TetraNeuron, Skin2Neuron, MedRhythms, and Hearts Radiant and is a cofounder of TI Solutions and Linus Health.
A version of this article first appeared on Medscape.com.
This article was updated 1/9/23.
Like its controversial cousin aducanumab (Aduhelm, Biogen/Eisai), lecanemab was approved under the FDA’s accelerated approval pathway, which can be used to fast-track a drug that provides a meaningful therapeutic advantage over existing treatments for a serious or life-threatening illness.
Unlike aducanumab, however, there was no formal FDA advisory committee meeting on lecanemab prior to approval.
“Alzheimer’s disease immeasurably incapacitates the lives of those who suffer from it and has devastating effects on their loved ones,” Billy Dunn, MD, director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a press release.
“This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s, instead of only treating the symptoms of the disease,” Dr. Dunn added.
Eisai has reported that lecanemab will cost $26,500 a year.
Modest benefit, adverse events
The FDA noted, “The labeling states that treatment with Leqembi should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was studied in clinical trials.”
The agency approved the treatment on the basis of findings from the CLARITY AD trial, which showed modest cognitive benefit for patients with early AD – but at a cost of increased risk for amyloid-related edema and effusions.
The trial enrolled 1,795 adults with mild cognitive impairment or early Alzheimer’s disease in whom amyloid pathology in the brain had been confirmed. Treatment consisted of lecanemab 10 mg/kg biweekly or matching placebo.
After 18 months of treatment, lecanemab slowed cognitive decline by 27%, compared with placebo, as measured by the Clinical Dementia Rating–Sum of Boxes (CDR-SB). This was an absolute difference of 0.45 points (change from baseline, 1.21 for lecanemab vs. 1.66 with placebo; P < .001).
While the results are “welcome news,” a 0.45-point difference on the CDR-SB might not be clinically meaningful, authors of a recent editorial in The Lancet cautioned.
Amyloid-related imaging abnormalities that manifest as edema or microhemorrhages also occurred in one in five patients taking lecanemab.
In addition, a newly published case report in The New England Journal of Medicine describes a patient with Alzheimer’s disease who was taking lecanemab and who died after experiencing numerous intracerebral hemorrhages during treatment with tissue plasminogen activator (tPA) for acute ischemic stroke.
“The findings raise the possibility of cerebral hemorrhages and necrotizing vasculopathy associated with tPA infusion in a patient with cerebrovascular amyloid who had received lecanemab,” the authors wrote.
Alzheimer’s Association reaction
Still, in anticipation of accelerated approval of lecanemab and the antiamyloid drug donanemab (Eli Lilly), which the FDA has also fast-tracked, the Alzheimer’s Association filed a formal request last month with the Centers for Medicare & Medicaid Services asking that it provide full and unrestricted coverage for FDA-approved Alzheimer’s disease treatments.
In a letter addressed to CMS administrator Chiquita Brooks-LaSure, the association asked the agency to remove the requirements for “coverage with evidence development” in its national coverage determination for FDA-approved antiamyloid monoclonal antibodies.
“Each day matters when it comes to slowing the progression of this disease,” Joanne Pike, DrPH, president and CEO for the Alzheimer’s Association, noted in a news release at the time.
“The current CMS policy to severely limit access to these treatments eliminates people’s options, is resulting in continued irreversible disease progression, and contributes to greater health inequities. That’s not acceptable,” Dr. Pike added.
After news of today’s approval was released, Dr. Pike noted in a new release, “The Alzheimer’s Association welcomes and celebrates this action by the FDA. We now have a second approved treatment that changes the course of Alzheimer’s disease in a meaningful way for people in the early stages of the disease.”
Maria C. Carrillo, PhD, chief science officer at the Alzheimer’s Association, called today’s approval “a milestone achievement.”
“The progress we’ve seen in not only this class of treatments but also in the diversification of treatment types and targets over the past few years is exciting and provides real hope to those impacted by this devastating disease,” Dr. Carrillo said.
Critical issues
Commenting on the approval, Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School, Boston, and chief medical officer at Linus Health, said FDA approval of lecanemab and its adoption in the clinic represent a “very exciting development and prospect; but arguably some critical issues need to be considered.”
He noted that the health care system “is not currently prepared to cope with the challenges and demands of lecanemab,” as well as future pharmacologic agents.
“First, we need better workflows to identify suitable patients who can most benefit from this treatment,” said Dr. Pascual-Leone. He added that beyond identification of cognitive difficulties, amyloid status will need to be determined.
“Presently, this requires expensive and invasive tests,” such as positron-emission tomography scans or lumbar punctures for cerebrospinal fluid analysis. However, these are not fully covered by insurance companies and would be challenging to fully scale, he noted.
“In addition to screening, health systems will need to resolve the logistics challenges around the administration of lecanemab with twice-monthly infusions and the need for careful longitudinal evaluations for potential side effects,” said Dr. Pascual-Leone.
“While lecanemab may represent the first disease-modifying therapy widely available for early Alzheimer’s disease, the likely more promising approach is the addition of other therapies to lecanemab as part of a multi-intervention strategy combining pharmacologic and nonpharmacologic interventions,” he added.
Dr. Pascual-Leone has served as a paid member on scientific advisory boards for Neuroelectrics, Magstim, TetraNeuron, Skin2Neuron, MedRhythms, and Hearts Radiant and is a cofounder of TI Solutions and Linus Health.
A version of this article first appeared on Medscape.com.
This article was updated 1/9/23.
Like its controversial cousin aducanumab (Aduhelm, Biogen/Eisai), lecanemab was approved under the FDA’s accelerated approval pathway, which can be used to fast-track a drug that provides a meaningful therapeutic advantage over existing treatments for a serious or life-threatening illness.
Unlike aducanumab, however, there was no formal FDA advisory committee meeting on lecanemab prior to approval.
“Alzheimer’s disease immeasurably incapacitates the lives of those who suffer from it and has devastating effects on their loved ones,” Billy Dunn, MD, director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a press release.
“This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s, instead of only treating the symptoms of the disease,” Dr. Dunn added.
Eisai has reported that lecanemab will cost $26,500 a year.
Modest benefit, adverse events
The FDA noted, “The labeling states that treatment with Leqembi should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was studied in clinical trials.”
The agency approved the treatment on the basis of findings from the CLARITY AD trial, which showed modest cognitive benefit for patients with early AD – but at a cost of increased risk for amyloid-related edema and effusions.
The trial enrolled 1,795 adults with mild cognitive impairment or early Alzheimer’s disease in whom amyloid pathology in the brain had been confirmed. Treatment consisted of lecanemab 10 mg/kg biweekly or matching placebo.
After 18 months of treatment, lecanemab slowed cognitive decline by 27%, compared with placebo, as measured by the Clinical Dementia Rating–Sum of Boxes (CDR-SB). This was an absolute difference of 0.45 points (change from baseline, 1.21 for lecanemab vs. 1.66 with placebo; P < .001).
While the results are “welcome news,” a 0.45-point difference on the CDR-SB might not be clinically meaningful, authors of a recent editorial in The Lancet cautioned.
Amyloid-related imaging abnormalities that manifest as edema or microhemorrhages also occurred in one in five patients taking lecanemab.
In addition, a newly published case report in The New England Journal of Medicine describes a patient with Alzheimer’s disease who was taking lecanemab and who died after experiencing numerous intracerebral hemorrhages during treatment with tissue plasminogen activator (tPA) for acute ischemic stroke.
“The findings raise the possibility of cerebral hemorrhages and necrotizing vasculopathy associated with tPA infusion in a patient with cerebrovascular amyloid who had received lecanemab,” the authors wrote.
Alzheimer’s Association reaction
Still, in anticipation of accelerated approval of lecanemab and the antiamyloid drug donanemab (Eli Lilly), which the FDA has also fast-tracked, the Alzheimer’s Association filed a formal request last month with the Centers for Medicare & Medicaid Services asking that it provide full and unrestricted coverage for FDA-approved Alzheimer’s disease treatments.
In a letter addressed to CMS administrator Chiquita Brooks-LaSure, the association asked the agency to remove the requirements for “coverage with evidence development” in its national coverage determination for FDA-approved antiamyloid monoclonal antibodies.
“Each day matters when it comes to slowing the progression of this disease,” Joanne Pike, DrPH, president and CEO for the Alzheimer’s Association, noted in a news release at the time.
“The current CMS policy to severely limit access to these treatments eliminates people’s options, is resulting in continued irreversible disease progression, and contributes to greater health inequities. That’s not acceptable,” Dr. Pike added.
After news of today’s approval was released, Dr. Pike noted in a new release, “The Alzheimer’s Association welcomes and celebrates this action by the FDA. We now have a second approved treatment that changes the course of Alzheimer’s disease in a meaningful way for people in the early stages of the disease.”
Maria C. Carrillo, PhD, chief science officer at the Alzheimer’s Association, called today’s approval “a milestone achievement.”
“The progress we’ve seen in not only this class of treatments but also in the diversification of treatment types and targets over the past few years is exciting and provides real hope to those impacted by this devastating disease,” Dr. Carrillo said.
Critical issues
Commenting on the approval, Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School, Boston, and chief medical officer at Linus Health, said FDA approval of lecanemab and its adoption in the clinic represent a “very exciting development and prospect; but arguably some critical issues need to be considered.”
He noted that the health care system “is not currently prepared to cope with the challenges and demands of lecanemab,” as well as future pharmacologic agents.
“First, we need better workflows to identify suitable patients who can most benefit from this treatment,” said Dr. Pascual-Leone. He added that beyond identification of cognitive difficulties, amyloid status will need to be determined.
“Presently, this requires expensive and invasive tests,” such as positron-emission tomography scans or lumbar punctures for cerebrospinal fluid analysis. However, these are not fully covered by insurance companies and would be challenging to fully scale, he noted.
“In addition to screening, health systems will need to resolve the logistics challenges around the administration of lecanemab with twice-monthly infusions and the need for careful longitudinal evaluations for potential side effects,” said Dr. Pascual-Leone.
“While lecanemab may represent the first disease-modifying therapy widely available for early Alzheimer’s disease, the likely more promising approach is the addition of other therapies to lecanemab as part of a multi-intervention strategy combining pharmacologic and nonpharmacologic interventions,” he added.
Dr. Pascual-Leone has served as a paid member on scientific advisory boards for Neuroelectrics, Magstim, TetraNeuron, Skin2Neuron, MedRhythms, and Hearts Radiant and is a cofounder of TI Solutions and Linus Health.
A version of this article first appeared on Medscape.com.
This article was updated 1/9/23.
Gene associated with vision loss also linked to COVID: Study
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF CLINICAL MEDICINE