User login
Osteoporosis drugs may extend life after fracture
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: Residence Characteristics and Nursing Home Compare Quality Measures
Relationships Between Residence Characteristics and Nursing Home Compare Database Quality Measures
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
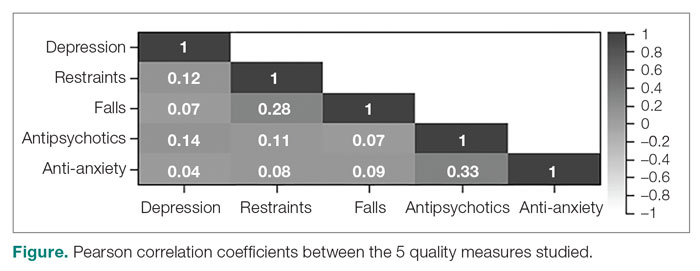
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18
Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).
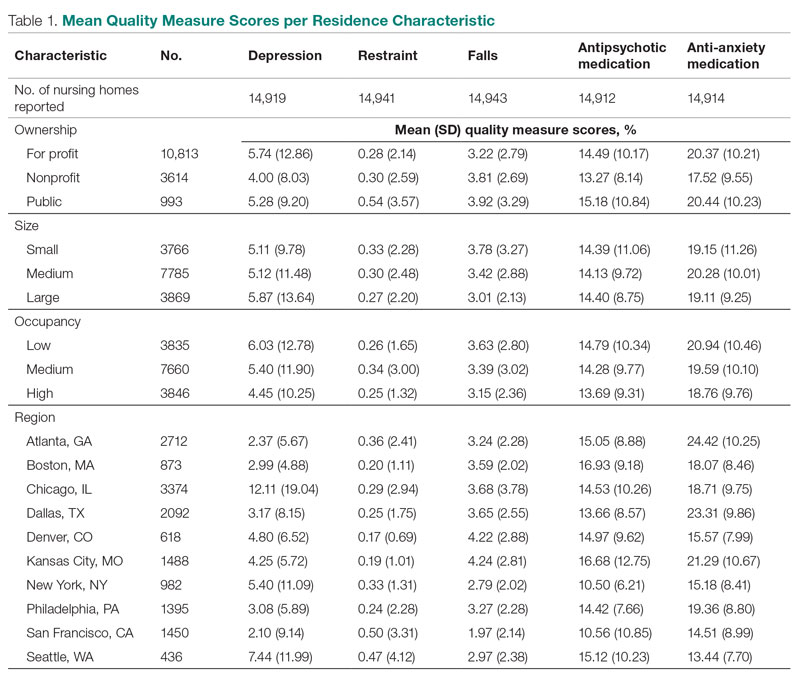
Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.
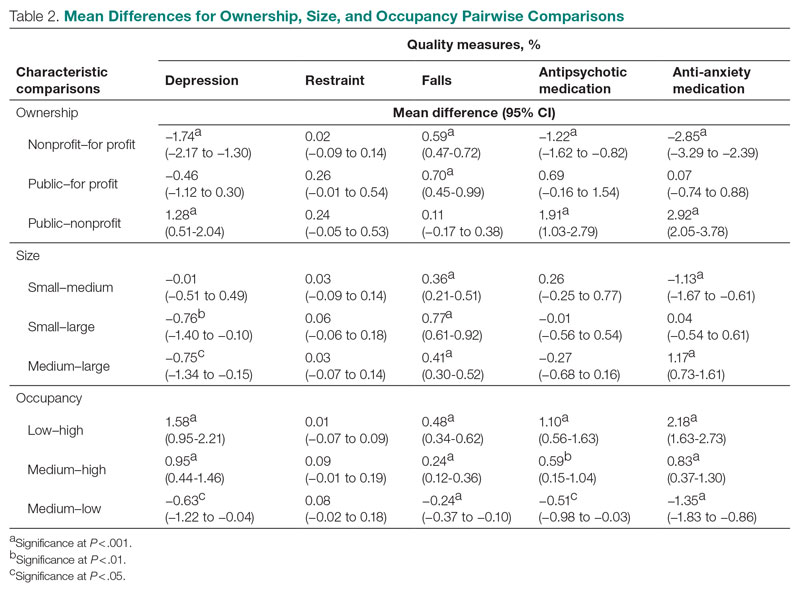
Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.
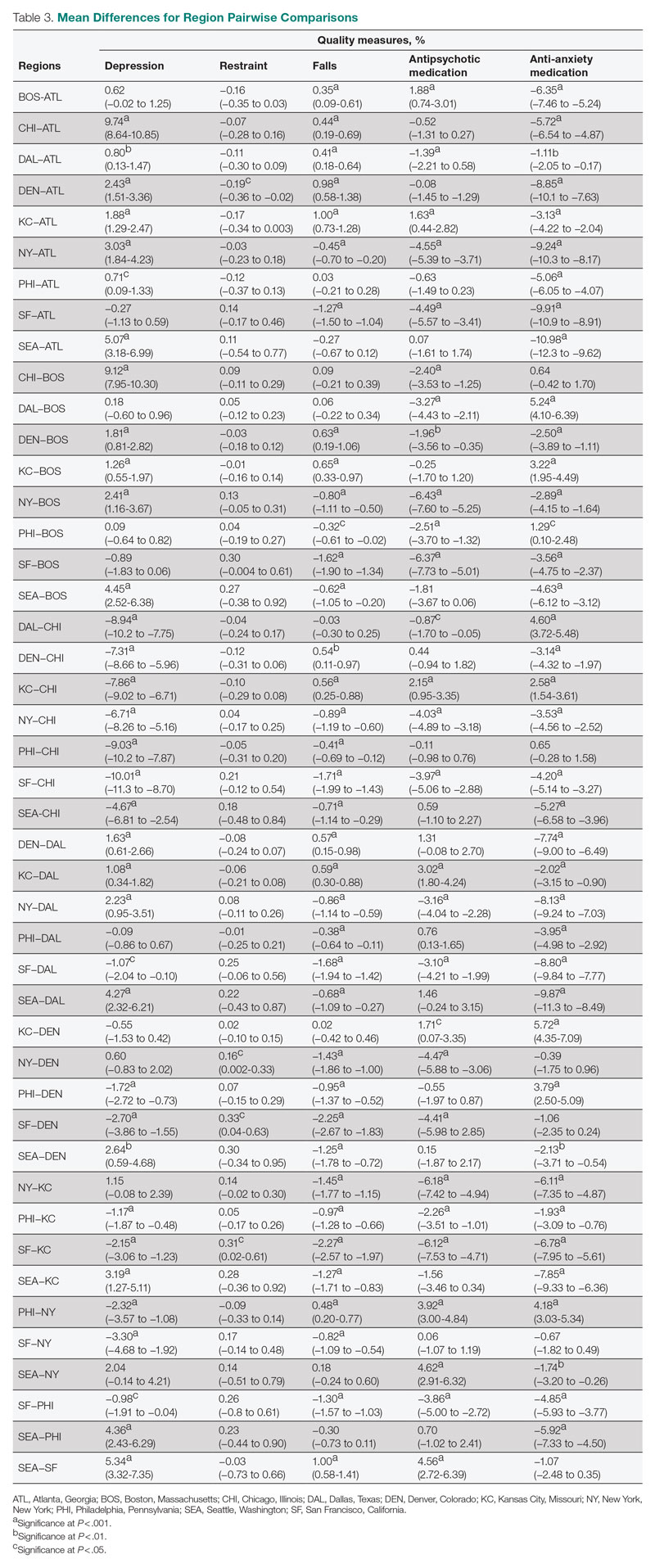
Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
Tooth loss and diabetes together hasten mental decline
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF DENTAL RESEARCH
Surgery for early breast cancer can worsen frailty in older women
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Restless legs a new modifiable risk factor for dementia?
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S RESEARCH AND THERAPY
Bruce Willis’ frontotemporal dementia is not your grandpa’s dementia
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
Older men more at risk as dangerous falls rise for all seniors
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
What’s driving the "world’s fastest-growing brain disease"?
An international team of researchers reviewed previous research and cited data that suggest the chemical trichloroethylene (TCE) is associated with as much as a 500% increased risk for Parkinson’s disease (PD).
Lead investigator Ray Dorsey, MD, professor of neurology, University of Rochester, N.Y., called PD “the world’s fastest-growing brain disease,” and told this news organization that it “may be largely preventable.”
“Countless people have died over generations from cancer and other disease linked to TCE [and] Parkinson’s may be the latest,” he said. “Banning these chemicals, containing contaminated sites, and protecting homes, schools, and buildings at risk may all create a world where Parkinson’s is increasingly rare, not common.”
The paper was published online in the Journal of Parkinson’s Disease.
Invisible, ubiquitous
TCE was first synthesized in a lab in 1864, with commercial production beginning in 1920, the researchers noted.
“Because of its unique properties, TCE has had countless industrial, commercial, military, and medical applications,” including producing refrigerants, cleaning electronics, and degreasing engine parts.
In addition, it’s been used in dry cleaning, although a similar chemical (perchloroethylene [PCE]) is currently more widely used for that purpose. Nevertheless, the authors noted, in anaerobic conditions, perchloroethylene often transforms into TCE “and their toxicity may be similar.”
Consumer products in which TCE is found include typewriter correction fluid, paint removers, gun cleaners, and aerosol cleaning products. Up until the 1970s, it was used to decaffeinate coffee.
TCE exposure isn’t confined to those who work with it. It also pollutes outdoor air, taints groundwater, and contaminates indoor air. It’s present in a substantial amount of groundwater in the United States and it “evaporates from underlying soil and groundwater and enters homes, workplaces, or schools, often undetected,” the researchers noted.
“Exposure can come via occupation or the environment and is often largely unknown at the time it occurs,” Dr. Dorsey said.
He noted that the rapid increase in PD incidence cannot be explained by genetic factors alone, which affect only about 15% of patients with PD, nor can it be explained by aging alone. “Certain pesticides ... are likely causes but would not explain the high prevalence of PD in urban areas, as is the case in the U.S.” Rather, “other factors” are involved, and “TCE is likely one such factor.”
Yet, “despite widespread contamination and increasing industrial, commercial, and military use, clinical investigations of TCE and PD have been limited.”
To fill this knowledge gap, Dr. Dorsey and his coauthors of the book, “Ending Parkinson’s Disease: A Prescription for Action,” took a deep dive into studies focusing on the potential association of TCE and PD and presented seven cases to illustrate the association.
“Like many genetic mutations (e.g., Parkin) and other environmental toxicants ... TCE damages the energy-producing parts of cells, i.e., the mitochondria,” said Dr. Dorsey.
TCE and PCE “likely mediate their toxicity through a common metabolite.” Because both are lipophilic, they “readily distribute in the brain and body tissues and appear to cause mitochondrial dysfunction at high doses,” the researchers hypothesized.
Dopaminergic neurons are particularly sensitive to mitochondrial neurotoxicants, so this might “partially explain the link to PD.”
Animal studies have shown that TCE “caused selective loss of dopaminergic neurons.” Moreover, PD-related neuropathology was found in the substantia nigra of rodents exposed to TCE over time. In addition, studies as early as 1960 were showing an association between TCE and parkinsonism.
The authors describe TCE as “ubiquitous” in the 1970s, with 10 million Americans working with the chemical or other organic solvents daily. The review details an extensive list of industries and occupations in which TCE exposure continues to occur.
People working with TCE might inhale it or touch it; but “millions more encounter the chemical unknowingly through outdoor air, contaminated groundwater, and indoor air pollution.”
They noted that TCE contaminates up to one-third of U.S. drinking water, has polluted the groundwater in more than 20 different countries on five continents, and is found in half of the 1,300 most toxic “Superfund” sites that are “part of a federal clean-up program, including 15 in California’s Silicon Valley, where TCE was used to clean electronics.”
Although the U.S. military stopped using TCE, numerous sites have been contaminated, including Marine Corps Base Camp Lejeune in North Carolina, where TCE and PCE were found in drinking water at 280 times the recommended safety standards.
The researchers highlighted seven cases of individuals who developed PD after likely exposure to TCE, including NBA basketball player Brian Grant, who developed symptoms of PD in 2006 at the age of 34.
Mr. Grant and his family had lived in Camp Lejeune when he was a child, during which time he drank, bathed, and swam in contaminated water, “unaware of its toxicity.” His father also died of esophageal cancer, “which is linked to TCE,” the authors of the study wrote. Mr. Grant has created a foundation to inspire and support patients with PD.
All of the individuals either grew up in or spent time in an area where they were extensively exposed to TCE, PCE, or other chemicals, or experienced occupational exposure.
The authors acknowledged that the role of TCE in PD, as illustrated by the cases, is “far from definitive.” For example, exposure to TCE is often combined with exposure to other toxins, or with unmeasured genetic risk factors.
They highlighted the need for more research and called for cleaning and containing contaminated sites, monitoring TCE levels, and publicly communicating risk and a ban on TCE.
Recall bias?
Commenting for this news organization, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association (APDA), noted that the authors “are very frank about the limitations of this approach [illustrative cases] as proof of causation between PD and TCE exposure.”
Another limitation is that TCE exposure is very common, “as argued in the paper.” But “most people with exposure do not develop PD,” Dr. Gilbert pointed out. “By probing the TCE exposure of those who already have PD, there is a danger of recall bias.”
Dr. Gilbert, associate professor of neurology at NYU Langone Health, who was not involved with the study, acknowledged that the authors “present their work as hypothesis and clearly state that more work is needed to understand the connection between TCE and PD.”
In the meantime, however, there are “well-established health risks of TCE exposure, including development of various cancers,” she said. Therefore, the authors’ goals appear to be educating the public about known health risks, working to clean up known sites of contamination, and advocating to ban future use of TCE.
These goals “do not need to wait for [proof of] firm causation between TCE and PD,” she stated.
Dr. Dorsey reported he has received honoraria for speaking at the American Academy of Neurology and at multiple other societies and foundations and has received compensation for consulting services from pharmaceutical companies, foundations, medical education companies, and medical publications; he owns stock in several companies. The other authors’ disclosures can be found in the original paper. Dr. Gilbert is employed by the American Parkinson Disease Association and Bellevue Hospital Center in New York City.
A version of this article first appeared on Medscape.com.
An international team of researchers reviewed previous research and cited data that suggest the chemical trichloroethylene (TCE) is associated with as much as a 500% increased risk for Parkinson’s disease (PD).
Lead investigator Ray Dorsey, MD, professor of neurology, University of Rochester, N.Y., called PD “the world’s fastest-growing brain disease,” and told this news organization that it “may be largely preventable.”
“Countless people have died over generations from cancer and other disease linked to TCE [and] Parkinson’s may be the latest,” he said. “Banning these chemicals, containing contaminated sites, and protecting homes, schools, and buildings at risk may all create a world where Parkinson’s is increasingly rare, not common.”
The paper was published online in the Journal of Parkinson’s Disease.
Invisible, ubiquitous
TCE was first synthesized in a lab in 1864, with commercial production beginning in 1920, the researchers noted.
“Because of its unique properties, TCE has had countless industrial, commercial, military, and medical applications,” including producing refrigerants, cleaning electronics, and degreasing engine parts.
In addition, it’s been used in dry cleaning, although a similar chemical (perchloroethylene [PCE]) is currently more widely used for that purpose. Nevertheless, the authors noted, in anaerobic conditions, perchloroethylene often transforms into TCE “and their toxicity may be similar.”
Consumer products in which TCE is found include typewriter correction fluid, paint removers, gun cleaners, and aerosol cleaning products. Up until the 1970s, it was used to decaffeinate coffee.
TCE exposure isn’t confined to those who work with it. It also pollutes outdoor air, taints groundwater, and contaminates indoor air. It’s present in a substantial amount of groundwater in the United States and it “evaporates from underlying soil and groundwater and enters homes, workplaces, or schools, often undetected,” the researchers noted.
“Exposure can come via occupation or the environment and is often largely unknown at the time it occurs,” Dr. Dorsey said.
He noted that the rapid increase in PD incidence cannot be explained by genetic factors alone, which affect only about 15% of patients with PD, nor can it be explained by aging alone. “Certain pesticides ... are likely causes but would not explain the high prevalence of PD in urban areas, as is the case in the U.S.” Rather, “other factors” are involved, and “TCE is likely one such factor.”
Yet, “despite widespread contamination and increasing industrial, commercial, and military use, clinical investigations of TCE and PD have been limited.”
To fill this knowledge gap, Dr. Dorsey and his coauthors of the book, “Ending Parkinson’s Disease: A Prescription for Action,” took a deep dive into studies focusing on the potential association of TCE and PD and presented seven cases to illustrate the association.
“Like many genetic mutations (e.g., Parkin) and other environmental toxicants ... TCE damages the energy-producing parts of cells, i.e., the mitochondria,” said Dr. Dorsey.
TCE and PCE “likely mediate their toxicity through a common metabolite.” Because both are lipophilic, they “readily distribute in the brain and body tissues and appear to cause mitochondrial dysfunction at high doses,” the researchers hypothesized.
Dopaminergic neurons are particularly sensitive to mitochondrial neurotoxicants, so this might “partially explain the link to PD.”
Animal studies have shown that TCE “caused selective loss of dopaminergic neurons.” Moreover, PD-related neuropathology was found in the substantia nigra of rodents exposed to TCE over time. In addition, studies as early as 1960 were showing an association between TCE and parkinsonism.
The authors describe TCE as “ubiquitous” in the 1970s, with 10 million Americans working with the chemical or other organic solvents daily. The review details an extensive list of industries and occupations in which TCE exposure continues to occur.
People working with TCE might inhale it or touch it; but “millions more encounter the chemical unknowingly through outdoor air, contaminated groundwater, and indoor air pollution.”
They noted that TCE contaminates up to one-third of U.S. drinking water, has polluted the groundwater in more than 20 different countries on five continents, and is found in half of the 1,300 most toxic “Superfund” sites that are “part of a federal clean-up program, including 15 in California’s Silicon Valley, where TCE was used to clean electronics.”
Although the U.S. military stopped using TCE, numerous sites have been contaminated, including Marine Corps Base Camp Lejeune in North Carolina, where TCE and PCE were found in drinking water at 280 times the recommended safety standards.
The researchers highlighted seven cases of individuals who developed PD after likely exposure to TCE, including NBA basketball player Brian Grant, who developed symptoms of PD in 2006 at the age of 34.
Mr. Grant and his family had lived in Camp Lejeune when he was a child, during which time he drank, bathed, and swam in contaminated water, “unaware of its toxicity.” His father also died of esophageal cancer, “which is linked to TCE,” the authors of the study wrote. Mr. Grant has created a foundation to inspire and support patients with PD.
All of the individuals either grew up in or spent time in an area where they were extensively exposed to TCE, PCE, or other chemicals, or experienced occupational exposure.
The authors acknowledged that the role of TCE in PD, as illustrated by the cases, is “far from definitive.” For example, exposure to TCE is often combined with exposure to other toxins, or with unmeasured genetic risk factors.
They highlighted the need for more research and called for cleaning and containing contaminated sites, monitoring TCE levels, and publicly communicating risk and a ban on TCE.
Recall bias?
Commenting for this news organization, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association (APDA), noted that the authors “are very frank about the limitations of this approach [illustrative cases] as proof of causation between PD and TCE exposure.”
Another limitation is that TCE exposure is very common, “as argued in the paper.” But “most people with exposure do not develop PD,” Dr. Gilbert pointed out. “By probing the TCE exposure of those who already have PD, there is a danger of recall bias.”
Dr. Gilbert, associate professor of neurology at NYU Langone Health, who was not involved with the study, acknowledged that the authors “present their work as hypothesis and clearly state that more work is needed to understand the connection between TCE and PD.”
In the meantime, however, there are “well-established health risks of TCE exposure, including development of various cancers,” she said. Therefore, the authors’ goals appear to be educating the public about known health risks, working to clean up known sites of contamination, and advocating to ban future use of TCE.
These goals “do not need to wait for [proof of] firm causation between TCE and PD,” she stated.
Dr. Dorsey reported he has received honoraria for speaking at the American Academy of Neurology and at multiple other societies and foundations and has received compensation for consulting services from pharmaceutical companies, foundations, medical education companies, and medical publications; he owns stock in several companies. The other authors’ disclosures can be found in the original paper. Dr. Gilbert is employed by the American Parkinson Disease Association and Bellevue Hospital Center in New York City.
A version of this article first appeared on Medscape.com.
An international team of researchers reviewed previous research and cited data that suggest the chemical trichloroethylene (TCE) is associated with as much as a 500% increased risk for Parkinson’s disease (PD).
Lead investigator Ray Dorsey, MD, professor of neurology, University of Rochester, N.Y., called PD “the world’s fastest-growing brain disease,” and told this news organization that it “may be largely preventable.”
“Countless people have died over generations from cancer and other disease linked to TCE [and] Parkinson’s may be the latest,” he said. “Banning these chemicals, containing contaminated sites, and protecting homes, schools, and buildings at risk may all create a world where Parkinson’s is increasingly rare, not common.”
The paper was published online in the Journal of Parkinson’s Disease.
Invisible, ubiquitous
TCE was first synthesized in a lab in 1864, with commercial production beginning in 1920, the researchers noted.
“Because of its unique properties, TCE has had countless industrial, commercial, military, and medical applications,” including producing refrigerants, cleaning electronics, and degreasing engine parts.
In addition, it’s been used in dry cleaning, although a similar chemical (perchloroethylene [PCE]) is currently more widely used for that purpose. Nevertheless, the authors noted, in anaerobic conditions, perchloroethylene often transforms into TCE “and their toxicity may be similar.”
Consumer products in which TCE is found include typewriter correction fluid, paint removers, gun cleaners, and aerosol cleaning products. Up until the 1970s, it was used to decaffeinate coffee.
TCE exposure isn’t confined to those who work with it. It also pollutes outdoor air, taints groundwater, and contaminates indoor air. It’s present in a substantial amount of groundwater in the United States and it “evaporates from underlying soil and groundwater and enters homes, workplaces, or schools, often undetected,” the researchers noted.
“Exposure can come via occupation or the environment and is often largely unknown at the time it occurs,” Dr. Dorsey said.
He noted that the rapid increase in PD incidence cannot be explained by genetic factors alone, which affect only about 15% of patients with PD, nor can it be explained by aging alone. “Certain pesticides ... are likely causes but would not explain the high prevalence of PD in urban areas, as is the case in the U.S.” Rather, “other factors” are involved, and “TCE is likely one such factor.”
Yet, “despite widespread contamination and increasing industrial, commercial, and military use, clinical investigations of TCE and PD have been limited.”
To fill this knowledge gap, Dr. Dorsey and his coauthors of the book, “Ending Parkinson’s Disease: A Prescription for Action,” took a deep dive into studies focusing on the potential association of TCE and PD and presented seven cases to illustrate the association.
“Like many genetic mutations (e.g., Parkin) and other environmental toxicants ... TCE damages the energy-producing parts of cells, i.e., the mitochondria,” said Dr. Dorsey.
TCE and PCE “likely mediate their toxicity through a common metabolite.” Because both are lipophilic, they “readily distribute in the brain and body tissues and appear to cause mitochondrial dysfunction at high doses,” the researchers hypothesized.
Dopaminergic neurons are particularly sensitive to mitochondrial neurotoxicants, so this might “partially explain the link to PD.”
Animal studies have shown that TCE “caused selective loss of dopaminergic neurons.” Moreover, PD-related neuropathology was found in the substantia nigra of rodents exposed to TCE over time. In addition, studies as early as 1960 were showing an association between TCE and parkinsonism.
The authors describe TCE as “ubiquitous” in the 1970s, with 10 million Americans working with the chemical or other organic solvents daily. The review details an extensive list of industries and occupations in which TCE exposure continues to occur.
People working with TCE might inhale it or touch it; but “millions more encounter the chemical unknowingly through outdoor air, contaminated groundwater, and indoor air pollution.”
They noted that TCE contaminates up to one-third of U.S. drinking water, has polluted the groundwater in more than 20 different countries on five continents, and is found in half of the 1,300 most toxic “Superfund” sites that are “part of a federal clean-up program, including 15 in California’s Silicon Valley, where TCE was used to clean electronics.”
Although the U.S. military stopped using TCE, numerous sites have been contaminated, including Marine Corps Base Camp Lejeune in North Carolina, where TCE and PCE were found in drinking water at 280 times the recommended safety standards.
The researchers highlighted seven cases of individuals who developed PD after likely exposure to TCE, including NBA basketball player Brian Grant, who developed symptoms of PD in 2006 at the age of 34.
Mr. Grant and his family had lived in Camp Lejeune when he was a child, during which time he drank, bathed, and swam in contaminated water, “unaware of its toxicity.” His father also died of esophageal cancer, “which is linked to TCE,” the authors of the study wrote. Mr. Grant has created a foundation to inspire and support patients with PD.
All of the individuals either grew up in or spent time in an area where they were extensively exposed to TCE, PCE, or other chemicals, or experienced occupational exposure.
The authors acknowledged that the role of TCE in PD, as illustrated by the cases, is “far from definitive.” For example, exposure to TCE is often combined with exposure to other toxins, or with unmeasured genetic risk factors.
They highlighted the need for more research and called for cleaning and containing contaminated sites, monitoring TCE levels, and publicly communicating risk and a ban on TCE.
Recall bias?
Commenting for this news organization, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association (APDA), noted that the authors “are very frank about the limitations of this approach [illustrative cases] as proof of causation between PD and TCE exposure.”
Another limitation is that TCE exposure is very common, “as argued in the paper.” But “most people with exposure do not develop PD,” Dr. Gilbert pointed out. “By probing the TCE exposure of those who already have PD, there is a danger of recall bias.”
Dr. Gilbert, associate professor of neurology at NYU Langone Health, who was not involved with the study, acknowledged that the authors “present their work as hypothesis and clearly state that more work is needed to understand the connection between TCE and PD.”
In the meantime, however, there are “well-established health risks of TCE exposure, including development of various cancers,” she said. Therefore, the authors’ goals appear to be educating the public about known health risks, working to clean up known sites of contamination, and advocating to ban future use of TCE.
These goals “do not need to wait for [proof of] firm causation between TCE and PD,” she stated.
Dr. Dorsey reported he has received honoraria for speaking at the American Academy of Neurology and at multiple other societies and foundations and has received compensation for consulting services from pharmaceutical companies, foundations, medical education companies, and medical publications; he owns stock in several companies. The other authors’ disclosures can be found in the original paper. Dr. Gilbert is employed by the American Parkinson Disease Association and Bellevue Hospital Center in New York City.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PARKINSON’S DISEASE
Strong support for CBT as first-line treatment for insomnia in seniors
NEW ORLEANS –
“The lack of awareness among clinicians who take care of older adults that CBT for insomnia (CBT-I) is an effective treatment for insomnia is an issue,” Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry, Creighton University, Omaha, Neb., told this news organization.
Dr. Tampi was among the speakers during a session as part of the American Association for Geriatric Psychiatry annual meeting addressing the complex challenges of treating insomnia in older patients, who tend to have higher rates of insomnia than their younger counterparts.
The prevalence of insomnia in older adults is estimated to be 20%-40%, and medication is frequently the first treatment choice, a less than ideal approach, said Dr. Tampi.
“Prescribing sedatives and hypnotics, which can cause severe adverse effects, without a thorough assessment that includes comorbidities that may be causing the insomnia” is among the biggest mistakes clinicians make in the treatment of insomnia in older patients, Dr. Tampi said in an interview.
“It’s our duty as providers to first take a good assessment, talk about polymorbidity, and try to address those conditions, and judiciously use medications in conjunction with at least components of CBT-I,” he said.
Long-term safety, efficacy unclear
About one-third of older adults take at least one form of pharmacological treatment for insomnia symptoms, said Ebony Dix, MD, assistant professor of psychiatry at Yale University, New Haven, Conn., in a separate talk during the session. This, despite the low-risk profile of CBT and recommendations from various medical societies that CBT should be tried first.
Dr. Dix noted that medications approved for insomnia by the U.S. Food and Drug Administration, including melatonin receptor agonists, heterocyclics, and dual orexin receptor antagonists (DORAs), can play an important role in the short-term management of insomnia, but their long-term effects are unknown.
“Pharmacotherapeutic agents may be effective in the short term, but there is a lack of sufficient, statistically significant data to support the long-term safety and efficacy of any [sleep] medication, especially in aging adults, due to the impact of hypnotic drugs on sleep architecture, the impact of aging on pharmacokinetics, as well as polypharmacy and drug-to-drug interactions,” Dr. Dix said. She noted that clinical trials of insomnia drugs rarely include geriatric patients.
The American Academy of Sleep Medicine recommends CBT-I as first-line treatment for insomnia, with the key benefit being its exemplary safety profile, said Shilpa Srinivasan, MD, a professor of clinical psychiatry at the University of South Carolina, Columbia, who also presented during the session.
“The biggest [attribute] of CBT-I management strategies is the low risk of side effects,” she said. “How many medications can we say that about?”
The CBT-I intervention includes a focus on key components of lifestyle and mental health issues to improve sleep. These include the following:
- Strictly restricting sleep hours for bedtime and arising (with napping discouraged).
- Control of stimulus to disrupt falling asleep.
- Cognitive therapy to identify and replace maladaptive beliefs.
- Control of sleep hygiene for optimal sleep.
- Relaxation training.
Keys to success
Dr. Srinivasan noted one recent study of CBT-I among patients aged 60 and older with insomnia and depression. The 156 participants randomized to receive weekly 120-minute CBT-I sessions over 2 months were significantly less likely to develop new or recurrent major depression versus their counterparts randomized to receive sleep education (hazard ratio, 0.51; P = .02).
However, CBT-I is more labor intensive than medication and requires provider training and motivation, and commitment on the part of the patient, to be successful.
“We really need to ensure that even when patients are receiving pharmacologic interventions for insomnia that we provide psychoeducation. At the end of the day, some of these nonpharmacologic components can make or break the success of pharmacotherapy,” said Dr. Srinivasan.
Whether using CBT-I alone or in combination with pharmacotherapy, the intervention does not necessarily have to include all components to be beneficial, she said.
“I think one of the challenges in incorporating CBT-I is the misconception that it is an all-or-nothing approach wherein every modality must be utilized,” she said. “While multicomponent CBT-I has been shown to be effective, the individual components can be incorporated into patient encounters in a stepped approach.”
Informing patients that they have options other than medications and involving them in decision-making is key, she added.
“In the case of insomnia, this is particularly relevant because of the physical and emotional distress that it causes,” Dr. Srinivasan said. “Patients often seek over-the-counter medications or other nonprescribed agents to try to obtain relief even before seeking treatment in a health care setting. There is less awareness about evidence-based and effective nonpharmacologic treatments such as CBT-I.”
Dr. Tampi, Dr. Dix, and Dr. Srinivasan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“The lack of awareness among clinicians who take care of older adults that CBT for insomnia (CBT-I) is an effective treatment for insomnia is an issue,” Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry, Creighton University, Omaha, Neb., told this news organization.
Dr. Tampi was among the speakers during a session as part of the American Association for Geriatric Psychiatry annual meeting addressing the complex challenges of treating insomnia in older patients, who tend to have higher rates of insomnia than their younger counterparts.
The prevalence of insomnia in older adults is estimated to be 20%-40%, and medication is frequently the first treatment choice, a less than ideal approach, said Dr. Tampi.
“Prescribing sedatives and hypnotics, which can cause severe adverse effects, without a thorough assessment that includes comorbidities that may be causing the insomnia” is among the biggest mistakes clinicians make in the treatment of insomnia in older patients, Dr. Tampi said in an interview.
“It’s our duty as providers to first take a good assessment, talk about polymorbidity, and try to address those conditions, and judiciously use medications in conjunction with at least components of CBT-I,” he said.
Long-term safety, efficacy unclear
About one-third of older adults take at least one form of pharmacological treatment for insomnia symptoms, said Ebony Dix, MD, assistant professor of psychiatry at Yale University, New Haven, Conn., in a separate talk during the session. This, despite the low-risk profile of CBT and recommendations from various medical societies that CBT should be tried first.
Dr. Dix noted that medications approved for insomnia by the U.S. Food and Drug Administration, including melatonin receptor agonists, heterocyclics, and dual orexin receptor antagonists (DORAs), can play an important role in the short-term management of insomnia, but their long-term effects are unknown.
“Pharmacotherapeutic agents may be effective in the short term, but there is a lack of sufficient, statistically significant data to support the long-term safety and efficacy of any [sleep] medication, especially in aging adults, due to the impact of hypnotic drugs on sleep architecture, the impact of aging on pharmacokinetics, as well as polypharmacy and drug-to-drug interactions,” Dr. Dix said. She noted that clinical trials of insomnia drugs rarely include geriatric patients.
The American Academy of Sleep Medicine recommends CBT-I as first-line treatment for insomnia, with the key benefit being its exemplary safety profile, said Shilpa Srinivasan, MD, a professor of clinical psychiatry at the University of South Carolina, Columbia, who also presented during the session.
“The biggest [attribute] of CBT-I management strategies is the low risk of side effects,” she said. “How many medications can we say that about?”
The CBT-I intervention includes a focus on key components of lifestyle and mental health issues to improve sleep. These include the following:
- Strictly restricting sleep hours for bedtime and arising (with napping discouraged).
- Control of stimulus to disrupt falling asleep.
- Cognitive therapy to identify and replace maladaptive beliefs.
- Control of sleep hygiene for optimal sleep.
- Relaxation training.
Keys to success
Dr. Srinivasan noted one recent study of CBT-I among patients aged 60 and older with insomnia and depression. The 156 participants randomized to receive weekly 120-minute CBT-I sessions over 2 months were significantly less likely to develop new or recurrent major depression versus their counterparts randomized to receive sleep education (hazard ratio, 0.51; P = .02).
However, CBT-I is more labor intensive than medication and requires provider training and motivation, and commitment on the part of the patient, to be successful.
“We really need to ensure that even when patients are receiving pharmacologic interventions for insomnia that we provide psychoeducation. At the end of the day, some of these nonpharmacologic components can make or break the success of pharmacotherapy,” said Dr. Srinivasan.
Whether using CBT-I alone or in combination with pharmacotherapy, the intervention does not necessarily have to include all components to be beneficial, she said.
“I think one of the challenges in incorporating CBT-I is the misconception that it is an all-or-nothing approach wherein every modality must be utilized,” she said. “While multicomponent CBT-I has been shown to be effective, the individual components can be incorporated into patient encounters in a stepped approach.”
Informing patients that they have options other than medications and involving them in decision-making is key, she added.
“In the case of insomnia, this is particularly relevant because of the physical and emotional distress that it causes,” Dr. Srinivasan said. “Patients often seek over-the-counter medications or other nonprescribed agents to try to obtain relief even before seeking treatment in a health care setting. There is less awareness about evidence-based and effective nonpharmacologic treatments such as CBT-I.”
Dr. Tampi, Dr. Dix, and Dr. Srinivasan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“The lack of awareness among clinicians who take care of older adults that CBT for insomnia (CBT-I) is an effective treatment for insomnia is an issue,” Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry, Creighton University, Omaha, Neb., told this news organization.
Dr. Tampi was among the speakers during a session as part of the American Association for Geriatric Psychiatry annual meeting addressing the complex challenges of treating insomnia in older patients, who tend to have higher rates of insomnia than their younger counterparts.
The prevalence of insomnia in older adults is estimated to be 20%-40%, and medication is frequently the first treatment choice, a less than ideal approach, said Dr. Tampi.
“Prescribing sedatives and hypnotics, which can cause severe adverse effects, without a thorough assessment that includes comorbidities that may be causing the insomnia” is among the biggest mistakes clinicians make in the treatment of insomnia in older patients, Dr. Tampi said in an interview.
“It’s our duty as providers to first take a good assessment, talk about polymorbidity, and try to address those conditions, and judiciously use medications in conjunction with at least components of CBT-I,” he said.
Long-term safety, efficacy unclear
About one-third of older adults take at least one form of pharmacological treatment for insomnia symptoms, said Ebony Dix, MD, assistant professor of psychiatry at Yale University, New Haven, Conn., in a separate talk during the session. This, despite the low-risk profile of CBT and recommendations from various medical societies that CBT should be tried first.
Dr. Dix noted that medications approved for insomnia by the U.S. Food and Drug Administration, including melatonin receptor agonists, heterocyclics, and dual orexin receptor antagonists (DORAs), can play an important role in the short-term management of insomnia, but their long-term effects are unknown.
“Pharmacotherapeutic agents may be effective in the short term, but there is a lack of sufficient, statistically significant data to support the long-term safety and efficacy of any [sleep] medication, especially in aging adults, due to the impact of hypnotic drugs on sleep architecture, the impact of aging on pharmacokinetics, as well as polypharmacy and drug-to-drug interactions,” Dr. Dix said. She noted that clinical trials of insomnia drugs rarely include geriatric patients.
The American Academy of Sleep Medicine recommends CBT-I as first-line treatment for insomnia, with the key benefit being its exemplary safety profile, said Shilpa Srinivasan, MD, a professor of clinical psychiatry at the University of South Carolina, Columbia, who also presented during the session.
“The biggest [attribute] of CBT-I management strategies is the low risk of side effects,” she said. “How many medications can we say that about?”
The CBT-I intervention includes a focus on key components of lifestyle and mental health issues to improve sleep. These include the following:
- Strictly restricting sleep hours for bedtime and arising (with napping discouraged).
- Control of stimulus to disrupt falling asleep.
- Cognitive therapy to identify and replace maladaptive beliefs.
- Control of sleep hygiene for optimal sleep.
- Relaxation training.
Keys to success
Dr. Srinivasan noted one recent study of CBT-I among patients aged 60 and older with insomnia and depression. The 156 participants randomized to receive weekly 120-minute CBT-I sessions over 2 months were significantly less likely to develop new or recurrent major depression versus their counterparts randomized to receive sleep education (hazard ratio, 0.51; P = .02).
However, CBT-I is more labor intensive than medication and requires provider training and motivation, and commitment on the part of the patient, to be successful.
“We really need to ensure that even when patients are receiving pharmacologic interventions for insomnia that we provide psychoeducation. At the end of the day, some of these nonpharmacologic components can make or break the success of pharmacotherapy,” said Dr. Srinivasan.
Whether using CBT-I alone or in combination with pharmacotherapy, the intervention does not necessarily have to include all components to be beneficial, she said.
“I think one of the challenges in incorporating CBT-I is the misconception that it is an all-or-nothing approach wherein every modality must be utilized,” she said. “While multicomponent CBT-I has been shown to be effective, the individual components can be incorporated into patient encounters in a stepped approach.”
Informing patients that they have options other than medications and involving them in decision-making is key, she added.
“In the case of insomnia, this is particularly relevant because of the physical and emotional distress that it causes,” Dr. Srinivasan said. “Patients often seek over-the-counter medications or other nonprescribed agents to try to obtain relief even before seeking treatment in a health care setting. There is less awareness about evidence-based and effective nonpharmacologic treatments such as CBT-I.”
Dr. Tampi, Dr. Dix, and Dr. Srinivasan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAGP 2023


