User login
Guidelines Aren’t For Everybody
An 88-year-old man comes for clinic follow up. He has a medical history of type 2 diabetes, hypertension, heart failure with reduced ejection fraction, and chronic kidney disease. He recently had laboratory tests done: BUN, 32 mg/dL; creatinine, 2.3 mg/dL; potassium, 4.5 mmol/L; bicarbonate, 22 Eq/L; and A1c, 8.2%.
He checks his blood glucose daily (alternating between fasting blood glucose and before dinner) and his fasting blood glucose levels are around 130 mg/dL. His highest glucose reading was 240 mg/dL. He does not have polyuria or visual changes. Current medications: atorvastatin, irbesartan, empagliflozin, and amlodipine. On physical exam his blood pressure is 130/70 mm Hg, pulse is 80, and his BMI 20.
What medication adjustments would you recommend?
A. Begin insulin glargine at bedtime
B. Begin mealtime insulin aspart
C. Begin semaglutide
D. Begin metformin
E. No changes
I think the correct approach here would be no changes. Most physicians know guideline recommendations for A1c of less than 7% are used for patients with diabetes with few comorbid conditions, normal cognition, and functional status. Many of our elderly patients do not meet these criteria and the goal of intense medical treatment of diabetes is different in those patients. The American Diabetes Association has issued a thoughtful paper on treatment of diabetes in elderly people, stressing that patients should have very individualized goals, and that there is no one-size-fits all A1c goal.1
In this patient I would avoid adding insulin, given hypoglycemia risk. A GLP-1 agonist might appear attractive given his multiple cardiovascular risk factors, but his low BMI is a major concern for frailty that may well be worsened with reduced nutrient intake. Diabetes is the chronic condition that probably has the most guidance for management in elderly patients.
I recently saw a 92-year-old man with heart failure with reduced ejection fraction and atrial fibrillation who had been losing weight and becoming weaker. He had suffered several falls in the previous 2 weeks. His medication list included amiodarone, apixaban, sacubitril/valsartan, carvedilol, empagliflozin, spironolactone, and furosemide. He was extremely frail and had stopped eating. He was receiving all guideline-directed therapies, yet he was miserable and dying. Falls in this population are potentially as fatal as decompensated heart disease.
I stopped his amiodarone, furosemide, and spironolactone, and reduced his doses of sacubitril/valsartan and carvedilol. His appetite returned and his will to live returned. Heart failure guidelines do not include robust studies of very elderly patients because few studies exist in this population. Frailty assessment is crucial in decision making in your elderly patients.2,3 and frequent check-ins to make sure that they are not suffering from the effects of polypharmacy are crucial. Our goal in our very elderly patients is quality life-years. Polypharmacy has the potential to decrease the quality of life, as well as potentially shorten life.
The very elderly are at risk of the negative consequences of polypharmacy, especially if they have several diseases like diabetes, congestive heart failure, and hypertension that may require multiple medications. Gutierrez-Valencia and colleagues performed a systematic review of 25 articles on frailty and polypharmacy.4 Their findings demonstrated a significant association between an increased number of medications and frailty. They postulated that polypharmacy could actually be a contributor to frailty. There just isn’t enough evidence for the benefit of guidelines in the very aged and the risks of polypharmacy are real. We should use the lowest possible doses of medications in this population, frequently reassess goals, and monitor closely for side effects.
Pearl: Always consider the risks of polypharmacy when considering therapies for your elderly patients.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Older Adults: Standards of Medical Care in Diabetes — 2021. Diabetes Care 2021;44(Suppl 1):S168–S179.
2. Gaur A et al. Cardiogeriatrics: The current state of the art. Heart. 2024 Jan 11:heartjnl-2022-322117.
3. Denfeld QE et al. Assessing and managing frailty in advanced heart failure: An International Society for Heart and Lung Transplantation consensus statement. J Heart Lung Transplant. 2023 Nov 29:S1053-2498(23)02028-4.
4. Gutiérrez-Valencia M et al. The relationship between frailty and polypharmacy in older people: A systematic review. Br J Clin Pharmacol. 2018 Jul;84(7):1432-44.
An 88-year-old man comes for clinic follow up. He has a medical history of type 2 diabetes, hypertension, heart failure with reduced ejection fraction, and chronic kidney disease. He recently had laboratory tests done: BUN, 32 mg/dL; creatinine, 2.3 mg/dL; potassium, 4.5 mmol/L; bicarbonate, 22 Eq/L; and A1c, 8.2%.
He checks his blood glucose daily (alternating between fasting blood glucose and before dinner) and his fasting blood glucose levels are around 130 mg/dL. His highest glucose reading was 240 mg/dL. He does not have polyuria or visual changes. Current medications: atorvastatin, irbesartan, empagliflozin, and amlodipine. On physical exam his blood pressure is 130/70 mm Hg, pulse is 80, and his BMI 20.
What medication adjustments would you recommend?
A. Begin insulin glargine at bedtime
B. Begin mealtime insulin aspart
C. Begin semaglutide
D. Begin metformin
E. No changes
I think the correct approach here would be no changes. Most physicians know guideline recommendations for A1c of less than 7% are used for patients with diabetes with few comorbid conditions, normal cognition, and functional status. Many of our elderly patients do not meet these criteria and the goal of intense medical treatment of diabetes is different in those patients. The American Diabetes Association has issued a thoughtful paper on treatment of diabetes in elderly people, stressing that patients should have very individualized goals, and that there is no one-size-fits all A1c goal.1
In this patient I would avoid adding insulin, given hypoglycemia risk. A GLP-1 agonist might appear attractive given his multiple cardiovascular risk factors, but his low BMI is a major concern for frailty that may well be worsened with reduced nutrient intake. Diabetes is the chronic condition that probably has the most guidance for management in elderly patients.
I recently saw a 92-year-old man with heart failure with reduced ejection fraction and atrial fibrillation who had been losing weight and becoming weaker. He had suffered several falls in the previous 2 weeks. His medication list included amiodarone, apixaban, sacubitril/valsartan, carvedilol, empagliflozin, spironolactone, and furosemide. He was extremely frail and had stopped eating. He was receiving all guideline-directed therapies, yet he was miserable and dying. Falls in this population are potentially as fatal as decompensated heart disease.
I stopped his amiodarone, furosemide, and spironolactone, and reduced his doses of sacubitril/valsartan and carvedilol. His appetite returned and his will to live returned. Heart failure guidelines do not include robust studies of very elderly patients because few studies exist in this population. Frailty assessment is crucial in decision making in your elderly patients.2,3 and frequent check-ins to make sure that they are not suffering from the effects of polypharmacy are crucial. Our goal in our very elderly patients is quality life-years. Polypharmacy has the potential to decrease the quality of life, as well as potentially shorten life.
The very elderly are at risk of the negative consequences of polypharmacy, especially if they have several diseases like diabetes, congestive heart failure, and hypertension that may require multiple medications. Gutierrez-Valencia and colleagues performed a systematic review of 25 articles on frailty and polypharmacy.4 Their findings demonstrated a significant association between an increased number of medications and frailty. They postulated that polypharmacy could actually be a contributor to frailty. There just isn’t enough evidence for the benefit of guidelines in the very aged and the risks of polypharmacy are real. We should use the lowest possible doses of medications in this population, frequently reassess goals, and monitor closely for side effects.
Pearl: Always consider the risks of polypharmacy when considering therapies for your elderly patients.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Older Adults: Standards of Medical Care in Diabetes — 2021. Diabetes Care 2021;44(Suppl 1):S168–S179.
2. Gaur A et al. Cardiogeriatrics: The current state of the art. Heart. 2024 Jan 11:heartjnl-2022-322117.
3. Denfeld QE et al. Assessing and managing frailty in advanced heart failure: An International Society for Heart and Lung Transplantation consensus statement. J Heart Lung Transplant. 2023 Nov 29:S1053-2498(23)02028-4.
4. Gutiérrez-Valencia M et al. The relationship between frailty and polypharmacy in older people: A systematic review. Br J Clin Pharmacol. 2018 Jul;84(7):1432-44.
An 88-year-old man comes for clinic follow up. He has a medical history of type 2 diabetes, hypertension, heart failure with reduced ejection fraction, and chronic kidney disease. He recently had laboratory tests done: BUN, 32 mg/dL; creatinine, 2.3 mg/dL; potassium, 4.5 mmol/L; bicarbonate, 22 Eq/L; and A1c, 8.2%.
He checks his blood glucose daily (alternating between fasting blood glucose and before dinner) and his fasting blood glucose levels are around 130 mg/dL. His highest glucose reading was 240 mg/dL. He does not have polyuria or visual changes. Current medications: atorvastatin, irbesartan, empagliflozin, and amlodipine. On physical exam his blood pressure is 130/70 mm Hg, pulse is 80, and his BMI 20.
What medication adjustments would you recommend?
A. Begin insulin glargine at bedtime
B. Begin mealtime insulin aspart
C. Begin semaglutide
D. Begin metformin
E. No changes
I think the correct approach here would be no changes. Most physicians know guideline recommendations for A1c of less than 7% are used for patients with diabetes with few comorbid conditions, normal cognition, and functional status. Many of our elderly patients do not meet these criteria and the goal of intense medical treatment of diabetes is different in those patients. The American Diabetes Association has issued a thoughtful paper on treatment of diabetes in elderly people, stressing that patients should have very individualized goals, and that there is no one-size-fits all A1c goal.1
In this patient I would avoid adding insulin, given hypoglycemia risk. A GLP-1 agonist might appear attractive given his multiple cardiovascular risk factors, but his low BMI is a major concern for frailty that may well be worsened with reduced nutrient intake. Diabetes is the chronic condition that probably has the most guidance for management in elderly patients.
I recently saw a 92-year-old man with heart failure with reduced ejection fraction and atrial fibrillation who had been losing weight and becoming weaker. He had suffered several falls in the previous 2 weeks. His medication list included amiodarone, apixaban, sacubitril/valsartan, carvedilol, empagliflozin, spironolactone, and furosemide. He was extremely frail and had stopped eating. He was receiving all guideline-directed therapies, yet he was miserable and dying. Falls in this population are potentially as fatal as decompensated heart disease.
I stopped his amiodarone, furosemide, and spironolactone, and reduced his doses of sacubitril/valsartan and carvedilol. His appetite returned and his will to live returned. Heart failure guidelines do not include robust studies of very elderly patients because few studies exist in this population. Frailty assessment is crucial in decision making in your elderly patients.2,3 and frequent check-ins to make sure that they are not suffering from the effects of polypharmacy are crucial. Our goal in our very elderly patients is quality life-years. Polypharmacy has the potential to decrease the quality of life, as well as potentially shorten life.
The very elderly are at risk of the negative consequences of polypharmacy, especially if they have several diseases like diabetes, congestive heart failure, and hypertension that may require multiple medications. Gutierrez-Valencia and colleagues performed a systematic review of 25 articles on frailty and polypharmacy.4 Their findings demonstrated a significant association between an increased number of medications and frailty. They postulated that polypharmacy could actually be a contributor to frailty. There just isn’t enough evidence for the benefit of guidelines in the very aged and the risks of polypharmacy are real. We should use the lowest possible doses of medications in this population, frequently reassess goals, and monitor closely for side effects.
Pearl: Always consider the risks of polypharmacy when considering therapies for your elderly patients.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Older Adults: Standards of Medical Care in Diabetes — 2021. Diabetes Care 2021;44(Suppl 1):S168–S179.
2. Gaur A et al. Cardiogeriatrics: The current state of the art. Heart. 2024 Jan 11:heartjnl-2022-322117.
3. Denfeld QE et al. Assessing and managing frailty in advanced heart failure: An International Society for Heart and Lung Transplantation consensus statement. J Heart Lung Transplant. 2023 Nov 29:S1053-2498(23)02028-4.
4. Gutiérrez-Valencia M et al. The relationship between frailty and polypharmacy in older people: A systematic review. Br J Clin Pharmacol. 2018 Jul;84(7):1432-44.
Reducing or Discontinuing Insulin or Sulfonylurea When Initiating a Glucagon-like Peptide-1 Agonist
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
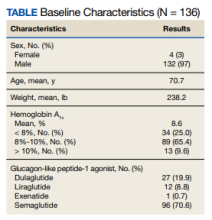
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
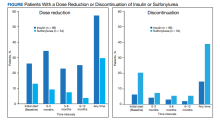
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
Top 5 Medications That Can Increase Blood Glucose Levels
It’s that time of the year, when social media is rife with many top 5 and top 10 lists. Let’s revisit some of the most commonly used medications known to increase glucose levels and look at some practical tips on overcoming these.
1. Glucocorticoids
Without a doubt, corticosteroids are at the top of the list when it comes to the potential for increasing blood glucose levels. High-dose glucocorticoid therapy is known to lead to new-onset diabetes (steroid-induced diabetes). Similarly, people with preexisting diabetes may notice significant worsening of glycemic control when they start on glucocorticoid therapy. The extent of glucose elevation depends on their glycemic status prior to initiation on steroids, the dose and duration of glucocorticoid therapy, and comorbid conditions, among other factors.
Management tip: For those with previously well-controlled diabetes or borderline diabetes, glucocorticoid-induced hyperglycemia may be managed by metformin with or without sulfonylurea therapy, especially if corticosteroid treatment is low-dose and for a shorter duration. However, for many individuals with preexisting poorly controlled diabetes or those initiated on high-dose corticosteroids, insulin therapy would perhaps be the treatment of choice. Glucocorticoid therapy generally leads to more pronounced postprandial hyperglycemia compared with fasting hyperglycemia; hence, the use of short-acting insulin therapy or perhaps NPH insulin in the morning might be a better option for many individuals. Dietary modification plays an important role in limiting the extent of postprandial hyperglycemia. Use of continuous glucose monitoring (CGM) devices may also be very helpful for understanding glycemic excursions and how to adjust insulin. In individuals for whom glucocorticoid therapy is tapered down, it is important to adjust the dose of medications with potential to cause hypoglycemia, such as insulin/sulfonylurea therapy, as the degree of hyperglycemia may decrease with decreased dose of the glucocorticoid therapy.
2. Antipsychotic Therapy
Antipsychotic medications can be obesogenic; between 15% and 72% of people who take second-generation antipsychotics experience weight gain of 7% or more. Increases in weight are not the only factor contributing to an elevated risk of developing type 2 diabetes. Antipsychotics are thought to cause downregulation of intracellular insulin signaling, leading to insulin resistance. At the same time, there seems to be a direct effect on the pancreatic beta cells. Antagonism of the dopamine D2, serotonin 5-HT2C, and muscarinic M3 receptors impairs beta-cell response to changes in blood glucose. In addition to the pharmacologic effects, cell culture experiments have shown that antipsychotics increase apoptosis of beta cells. Increased weight and concomitant development of type 2 diabetes is seen particularly in agents that exhibit high muscarinic M3 and histamine H1 receptor blockade. The effect on glucose metabolism is seen the most with agents such as clozapine, olanzapine, and haloperidol and the least with agents such as ziprasidone.
Management tip: Given the ongoing change in the understanding of increases in weight and their association with the risk of developing type 2 diabetes, a metabolically safer approach involves starting with medications that have a lower propensity for weight gain, and the partial agonists/third-generation antipsychotics as a family presently have the best overall data.
3. Thiazide Diuretics
Thiazide diuretics are commonly used for the management of hypertension and are associated with metabolic complications including hypokalemia; higher cholesterol, triglycerides, and other circulating lipids; and elevated glucose. It’s thought that the reduced potassium level occurring as a result of these medications might contribute to new-onset diabetes. The hypokalemia occurring from these medications is thought to lead to a decrease in insulin secretion and sensitivity, which is dose dependent. Studies show that the number needed to harm for chlorthalidone-induced diabetes is 29 over 1 year. There is believed to be no additional risk beyond 1 year.
Management tip: It’s important to monitor potassium levels for those initiated on thiazide diuretics. If hypokalemia occurs, it would be pertinent to correct the hypokalemia with potassium supplements to mitigate the risk for new-onset diabetes.
4. Statin Therapy
Statin therapy is thought to be associated with decreased insulin sensitivity and impairment in insulin secretion. The overall incidence of diabetes is pegged to be between 9% and 12% on statin therapy on the basis of meta-analysis studies, and higher on the basis of population-based studies. Overall, the estimated number needed to harm is: 1 out of every 255 patients on statin therapy for 4 years may develop new-onset diabetes. Compare this with the extremely strong evidence for number needed to treat being 39 for 5 years with statin therapy in patients with preexisting heart disease to prevent one occurrence of a nonfatal myocardial infarction.
Management tip: Although statins are associated with a small incident increase in the risk of developing diabetes, the potential benefits of using statin therapy for both primary and secondary prevention of cardiovascular disease significantly outweigh any of the potential risks associated with hyperglycemia. This is an important discussion to have with patients who are reluctant to use statin therapy because of the potential risk for new-onset diabetes as a side effect.
5. Beta-Blockers
Beta-blockers are another commonly used group of medications for managing hypertension, heart failure, coronary artery disease, and arrhythmia. Nonvasodilating beta-blockers such as metoprolol and atenolol are more likely to be associated with increases in A1c, mean plasma glucose, body weight, and triglycerides compared with vasodilating beta-blockers such as carvedilol, nebivolol, and labetalol (Bakris GL et al; Giugliano D et al). Similarly, studies have also shown that atenolol and metoprolol are associated with increased odds of hypoglycemia compared with carvedilol. People on beta-blockers may have masking of some of the symptoms of hypoglycemia, such as tremor, irritability, and palpitations, while other symptoms such as diaphoresis may remain unaffected on beta-blockers.
Management tip: Education on recognizing and managing hypoglycemia would be important when starting patients on beta-blockers if they are on preexisting insulin/sulfonylurea therapy. Use of CGM devices may be helpful if there is a high risk for hypoglycemia, especially as symptoms of hypoglycemia are often masked.
Honorable Mention
Several other medications — including antiretroviral therapy, tyrosine kinase inhibitors, mechanistic target of rapamycin (mTOR) inhibitors, immunosuppressants, and interferon alpha — are associated with worsening glycemic control and new-onset diabetes. Consider these agents’ effects on blood glucose, especially in people with an elevated risk of developing diabetes or those with preexisting diabetes, when prescribing.
A special mention should also be made of androgen deprivation therapy. These include treatment options like goserelin and leuprolide, which are gonadotropin-releasing hormone (GnRH) agonist therapies and are commonly used for prostate cancer management. Depending on the patient, these agents may be used for prolonged duration. Androgen deprivation therapy, by definition, decreases testosterone levels in men, thereby leading to worsening insulin resistance. Increase in fat mass and concomitant muscle wasting have been associated with the use of these medications; these, in turn, lead to peripheral insulin resistance. Nearly 1 out of every 5 men treated with long-term androgen deprivation therapy may be prone to developing worsening of A1c by 1% or more.
Management tip: Men on androgen deprivation therapy should be encouraged to participate in regular physical activity to reduce the burden of insulin resistance and to promote cardiovascular health.
Drug-induced diabetes is potentially reversible in many cases. Similarly, worsening of glycemic control due to medications in people with preexisting diabetes may also attenuate once the effect of the drug wears off. Blood glucose should be monitored on an ongoing basis so that diabetes medications can be adjusted. For some individuals, however, the worsening of glycemic status may be more chronic and may require long-term use of antihyperglycemic agents, especially if the benefits of continuation of the medication leading to hyperglycemia far exceed any potential risks.
Dr. Jain is Clinical Instructor, Department of Endocrinology, University of British Columbia; Endocrinologist, Fraser River Endocrinology, Vancouver, British Columbia, Canada. He disclosed ties with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk.
A version of this article appeared on Medscape.com.
It’s that time of the year, when social media is rife with many top 5 and top 10 lists. Let’s revisit some of the most commonly used medications known to increase glucose levels and look at some practical tips on overcoming these.
1. Glucocorticoids
Without a doubt, corticosteroids are at the top of the list when it comes to the potential for increasing blood glucose levels. High-dose glucocorticoid therapy is known to lead to new-onset diabetes (steroid-induced diabetes). Similarly, people with preexisting diabetes may notice significant worsening of glycemic control when they start on glucocorticoid therapy. The extent of glucose elevation depends on their glycemic status prior to initiation on steroids, the dose and duration of glucocorticoid therapy, and comorbid conditions, among other factors.
Management tip: For those with previously well-controlled diabetes or borderline diabetes, glucocorticoid-induced hyperglycemia may be managed by metformin with or without sulfonylurea therapy, especially if corticosteroid treatment is low-dose and for a shorter duration. However, for many individuals with preexisting poorly controlled diabetes or those initiated on high-dose corticosteroids, insulin therapy would perhaps be the treatment of choice. Glucocorticoid therapy generally leads to more pronounced postprandial hyperglycemia compared with fasting hyperglycemia; hence, the use of short-acting insulin therapy or perhaps NPH insulin in the morning might be a better option for many individuals. Dietary modification plays an important role in limiting the extent of postprandial hyperglycemia. Use of continuous glucose monitoring (CGM) devices may also be very helpful for understanding glycemic excursions and how to adjust insulin. In individuals for whom glucocorticoid therapy is tapered down, it is important to adjust the dose of medications with potential to cause hypoglycemia, such as insulin/sulfonylurea therapy, as the degree of hyperglycemia may decrease with decreased dose of the glucocorticoid therapy.
2. Antipsychotic Therapy
Antipsychotic medications can be obesogenic; between 15% and 72% of people who take second-generation antipsychotics experience weight gain of 7% or more. Increases in weight are not the only factor contributing to an elevated risk of developing type 2 diabetes. Antipsychotics are thought to cause downregulation of intracellular insulin signaling, leading to insulin resistance. At the same time, there seems to be a direct effect on the pancreatic beta cells. Antagonism of the dopamine D2, serotonin 5-HT2C, and muscarinic M3 receptors impairs beta-cell response to changes in blood glucose. In addition to the pharmacologic effects, cell culture experiments have shown that antipsychotics increase apoptosis of beta cells. Increased weight and concomitant development of type 2 diabetes is seen particularly in agents that exhibit high muscarinic M3 and histamine H1 receptor blockade. The effect on glucose metabolism is seen the most with agents such as clozapine, olanzapine, and haloperidol and the least with agents such as ziprasidone.
Management tip: Given the ongoing change in the understanding of increases in weight and their association with the risk of developing type 2 diabetes, a metabolically safer approach involves starting with medications that have a lower propensity for weight gain, and the partial agonists/third-generation antipsychotics as a family presently have the best overall data.
3. Thiazide Diuretics
Thiazide diuretics are commonly used for the management of hypertension and are associated with metabolic complications including hypokalemia; higher cholesterol, triglycerides, and other circulating lipids; and elevated glucose. It’s thought that the reduced potassium level occurring as a result of these medications might contribute to new-onset diabetes. The hypokalemia occurring from these medications is thought to lead to a decrease in insulin secretion and sensitivity, which is dose dependent. Studies show that the number needed to harm for chlorthalidone-induced diabetes is 29 over 1 year. There is believed to be no additional risk beyond 1 year.
Management tip: It’s important to monitor potassium levels for those initiated on thiazide diuretics. If hypokalemia occurs, it would be pertinent to correct the hypokalemia with potassium supplements to mitigate the risk for new-onset diabetes.
4. Statin Therapy
Statin therapy is thought to be associated with decreased insulin sensitivity and impairment in insulin secretion. The overall incidence of diabetes is pegged to be between 9% and 12% on statin therapy on the basis of meta-analysis studies, and higher on the basis of population-based studies. Overall, the estimated number needed to harm is: 1 out of every 255 patients on statin therapy for 4 years may develop new-onset diabetes. Compare this with the extremely strong evidence for number needed to treat being 39 for 5 years with statin therapy in patients with preexisting heart disease to prevent one occurrence of a nonfatal myocardial infarction.
Management tip: Although statins are associated with a small incident increase in the risk of developing diabetes, the potential benefits of using statin therapy for both primary and secondary prevention of cardiovascular disease significantly outweigh any of the potential risks associated with hyperglycemia. This is an important discussion to have with patients who are reluctant to use statin therapy because of the potential risk for new-onset diabetes as a side effect.
5. Beta-Blockers
Beta-blockers are another commonly used group of medications for managing hypertension, heart failure, coronary artery disease, and arrhythmia. Nonvasodilating beta-blockers such as metoprolol and atenolol are more likely to be associated with increases in A1c, mean plasma glucose, body weight, and triglycerides compared with vasodilating beta-blockers such as carvedilol, nebivolol, and labetalol (Bakris GL et al; Giugliano D et al). Similarly, studies have also shown that atenolol and metoprolol are associated with increased odds of hypoglycemia compared with carvedilol. People on beta-blockers may have masking of some of the symptoms of hypoglycemia, such as tremor, irritability, and palpitations, while other symptoms such as diaphoresis may remain unaffected on beta-blockers.
Management tip: Education on recognizing and managing hypoglycemia would be important when starting patients on beta-blockers if they are on preexisting insulin/sulfonylurea therapy. Use of CGM devices may be helpful if there is a high risk for hypoglycemia, especially as symptoms of hypoglycemia are often masked.
Honorable Mention
Several other medications — including antiretroviral therapy, tyrosine kinase inhibitors, mechanistic target of rapamycin (mTOR) inhibitors, immunosuppressants, and interferon alpha — are associated with worsening glycemic control and new-onset diabetes. Consider these agents’ effects on blood glucose, especially in people with an elevated risk of developing diabetes or those with preexisting diabetes, when prescribing.
A special mention should also be made of androgen deprivation therapy. These include treatment options like goserelin and leuprolide, which are gonadotropin-releasing hormone (GnRH) agonist therapies and are commonly used for prostate cancer management. Depending on the patient, these agents may be used for prolonged duration. Androgen deprivation therapy, by definition, decreases testosterone levels in men, thereby leading to worsening insulin resistance. Increase in fat mass and concomitant muscle wasting have been associated with the use of these medications; these, in turn, lead to peripheral insulin resistance. Nearly 1 out of every 5 men treated with long-term androgen deprivation therapy may be prone to developing worsening of A1c by 1% or more.
Management tip: Men on androgen deprivation therapy should be encouraged to participate in regular physical activity to reduce the burden of insulin resistance and to promote cardiovascular health.
Drug-induced diabetes is potentially reversible in many cases. Similarly, worsening of glycemic control due to medications in people with preexisting diabetes may also attenuate once the effect of the drug wears off. Blood glucose should be monitored on an ongoing basis so that diabetes medications can be adjusted. For some individuals, however, the worsening of glycemic status may be more chronic and may require long-term use of antihyperglycemic agents, especially if the benefits of continuation of the medication leading to hyperglycemia far exceed any potential risks.
Dr. Jain is Clinical Instructor, Department of Endocrinology, University of British Columbia; Endocrinologist, Fraser River Endocrinology, Vancouver, British Columbia, Canada. He disclosed ties with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk.
A version of this article appeared on Medscape.com.
It’s that time of the year, when social media is rife with many top 5 and top 10 lists. Let’s revisit some of the most commonly used medications known to increase glucose levels and look at some practical tips on overcoming these.
1. Glucocorticoids
Without a doubt, corticosteroids are at the top of the list when it comes to the potential for increasing blood glucose levels. High-dose glucocorticoid therapy is known to lead to new-onset diabetes (steroid-induced diabetes). Similarly, people with preexisting diabetes may notice significant worsening of glycemic control when they start on glucocorticoid therapy. The extent of glucose elevation depends on their glycemic status prior to initiation on steroids, the dose and duration of glucocorticoid therapy, and comorbid conditions, among other factors.
Management tip: For those with previously well-controlled diabetes or borderline diabetes, glucocorticoid-induced hyperglycemia may be managed by metformin with or without sulfonylurea therapy, especially if corticosteroid treatment is low-dose and for a shorter duration. However, for many individuals with preexisting poorly controlled diabetes or those initiated on high-dose corticosteroids, insulin therapy would perhaps be the treatment of choice. Glucocorticoid therapy generally leads to more pronounced postprandial hyperglycemia compared with fasting hyperglycemia; hence, the use of short-acting insulin therapy or perhaps NPH insulin in the morning might be a better option for many individuals. Dietary modification plays an important role in limiting the extent of postprandial hyperglycemia. Use of continuous glucose monitoring (CGM) devices may also be very helpful for understanding glycemic excursions and how to adjust insulin. In individuals for whom glucocorticoid therapy is tapered down, it is important to adjust the dose of medications with potential to cause hypoglycemia, such as insulin/sulfonylurea therapy, as the degree of hyperglycemia may decrease with decreased dose of the glucocorticoid therapy.
2. Antipsychotic Therapy
Antipsychotic medications can be obesogenic; between 15% and 72% of people who take second-generation antipsychotics experience weight gain of 7% or more. Increases in weight are not the only factor contributing to an elevated risk of developing type 2 diabetes. Antipsychotics are thought to cause downregulation of intracellular insulin signaling, leading to insulin resistance. At the same time, there seems to be a direct effect on the pancreatic beta cells. Antagonism of the dopamine D2, serotonin 5-HT2C, and muscarinic M3 receptors impairs beta-cell response to changes in blood glucose. In addition to the pharmacologic effects, cell culture experiments have shown that antipsychotics increase apoptosis of beta cells. Increased weight and concomitant development of type 2 diabetes is seen particularly in agents that exhibit high muscarinic M3 and histamine H1 receptor blockade. The effect on glucose metabolism is seen the most with agents such as clozapine, olanzapine, and haloperidol and the least with agents such as ziprasidone.
Management tip: Given the ongoing change in the understanding of increases in weight and their association with the risk of developing type 2 diabetes, a metabolically safer approach involves starting with medications that have a lower propensity for weight gain, and the partial agonists/third-generation antipsychotics as a family presently have the best overall data.
3. Thiazide Diuretics
Thiazide diuretics are commonly used for the management of hypertension and are associated with metabolic complications including hypokalemia; higher cholesterol, triglycerides, and other circulating lipids; and elevated glucose. It’s thought that the reduced potassium level occurring as a result of these medications might contribute to new-onset diabetes. The hypokalemia occurring from these medications is thought to lead to a decrease in insulin secretion and sensitivity, which is dose dependent. Studies show that the number needed to harm for chlorthalidone-induced diabetes is 29 over 1 year. There is believed to be no additional risk beyond 1 year.
Management tip: It’s important to monitor potassium levels for those initiated on thiazide diuretics. If hypokalemia occurs, it would be pertinent to correct the hypokalemia with potassium supplements to mitigate the risk for new-onset diabetes.
4. Statin Therapy
Statin therapy is thought to be associated with decreased insulin sensitivity and impairment in insulin secretion. The overall incidence of diabetes is pegged to be between 9% and 12% on statin therapy on the basis of meta-analysis studies, and higher on the basis of population-based studies. Overall, the estimated number needed to harm is: 1 out of every 255 patients on statin therapy for 4 years may develop new-onset diabetes. Compare this with the extremely strong evidence for number needed to treat being 39 for 5 years with statin therapy in patients with preexisting heart disease to prevent one occurrence of a nonfatal myocardial infarction.
Management tip: Although statins are associated with a small incident increase in the risk of developing diabetes, the potential benefits of using statin therapy for both primary and secondary prevention of cardiovascular disease significantly outweigh any of the potential risks associated with hyperglycemia. This is an important discussion to have with patients who are reluctant to use statin therapy because of the potential risk for new-onset diabetes as a side effect.
5. Beta-Blockers
Beta-blockers are another commonly used group of medications for managing hypertension, heart failure, coronary artery disease, and arrhythmia. Nonvasodilating beta-blockers such as metoprolol and atenolol are more likely to be associated with increases in A1c, mean plasma glucose, body weight, and triglycerides compared with vasodilating beta-blockers such as carvedilol, nebivolol, and labetalol (Bakris GL et al; Giugliano D et al). Similarly, studies have also shown that atenolol and metoprolol are associated with increased odds of hypoglycemia compared with carvedilol. People on beta-blockers may have masking of some of the symptoms of hypoglycemia, such as tremor, irritability, and palpitations, while other symptoms such as diaphoresis may remain unaffected on beta-blockers.
Management tip: Education on recognizing and managing hypoglycemia would be important when starting patients on beta-blockers if they are on preexisting insulin/sulfonylurea therapy. Use of CGM devices may be helpful if there is a high risk for hypoglycemia, especially as symptoms of hypoglycemia are often masked.
Honorable Mention
Several other medications — including antiretroviral therapy, tyrosine kinase inhibitors, mechanistic target of rapamycin (mTOR) inhibitors, immunosuppressants, and interferon alpha — are associated with worsening glycemic control and new-onset diabetes. Consider these agents’ effects on blood glucose, especially in people with an elevated risk of developing diabetes or those with preexisting diabetes, when prescribing.
A special mention should also be made of androgen deprivation therapy. These include treatment options like goserelin and leuprolide, which are gonadotropin-releasing hormone (GnRH) agonist therapies and are commonly used for prostate cancer management. Depending on the patient, these agents may be used for prolonged duration. Androgen deprivation therapy, by definition, decreases testosterone levels in men, thereby leading to worsening insulin resistance. Increase in fat mass and concomitant muscle wasting have been associated with the use of these medications; these, in turn, lead to peripheral insulin resistance. Nearly 1 out of every 5 men treated with long-term androgen deprivation therapy may be prone to developing worsening of A1c by 1% or more.
Management tip: Men on androgen deprivation therapy should be encouraged to participate in regular physical activity to reduce the burden of insulin resistance and to promote cardiovascular health.
Drug-induced diabetes is potentially reversible in many cases. Similarly, worsening of glycemic control due to medications in people with preexisting diabetes may also attenuate once the effect of the drug wears off. Blood glucose should be monitored on an ongoing basis so that diabetes medications can be adjusted. For some individuals, however, the worsening of glycemic status may be more chronic and may require long-term use of antihyperglycemic agents, especially if the benefits of continuation of the medication leading to hyperglycemia far exceed any potential risks.
Dr. Jain is Clinical Instructor, Department of Endocrinology, University of British Columbia; Endocrinologist, Fraser River Endocrinology, Vancouver, British Columbia, Canada. He disclosed ties with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk.
A version of this article appeared on Medscape.com.
Once-Weekly Insulin Better Than Daily in Type 2 Diabetes
TOPLINE:
Once-weekly insulin icodec shows a higher glycated A1c reduction than once-daily basal insulin analogs in patients with type 2 diabetes (T2D), without major safety concerns.
METHODOLOGY:
- A meta-analysis of five phase 3 ONWARDS randomized controlled trials included 3764 patients with T2D.
- The trials compared the effects of the weekly insulin icodec with those of the daily basal insulin analogs glargine and degludec over 26-78 months.
- The primary outcome was the change in A1c levels.
- Secondary outcomes included fasting plasma glucose levels, A1c levels < 7%, time in target glycemic range, body weight changes, insulin dose, hypoglycemia events, and adverse events.
TAKEAWAY:
- A1c levels < 7% were observed in a higher percentage of patients in the insulin icodec group than in the comparator group (odds ratio, 1.51; P = .004).
- In subgroup analyses, insulin icodec was superior to insulin degludec by several measures but comparatively similar to glargine.
- Insulin icodec was associated with no major safety concerns and had a slightly higher incidence of levels 1, 2, and combined 2/3 than degludec but no significant differences compared with glargine.
IN PRACTICE:
“Sustained glycemic control with once-weekly injections of insulin icodec would lead to better patient acceptance and treatment satisfaction,” the authors wrote.
SOURCE:
This study, authored by Sahana Shetty, MD, and Renuka Suvarna, MSc, Manipal Academy of Higher Education, Department of Endocrinology, Kasturba Medical College, Manipal, Karnataka, was published online on January 8, 2024, in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The comparator group included individuals who used different basal insulin analogs. This heterogeneity in the comparator group introduced a potential source of variability, making it challenging to isolate the specific effects of insulin icodec compared with a standardized comparator. Blinding or masking of participants was performed in only one of the five trials.
DISCLOSURES:
The authors declared no conflicts of interest. All five clinical trials in the meta-analysis were sponsored by Novo Nordisk.
A version of this article appeared on Medscape.com.
TOPLINE:
Once-weekly insulin icodec shows a higher glycated A1c reduction than once-daily basal insulin analogs in patients with type 2 diabetes (T2D), without major safety concerns.
METHODOLOGY:
- A meta-analysis of five phase 3 ONWARDS randomized controlled trials included 3764 patients with T2D.
- The trials compared the effects of the weekly insulin icodec with those of the daily basal insulin analogs glargine and degludec over 26-78 months.
- The primary outcome was the change in A1c levels.
- Secondary outcomes included fasting plasma glucose levels, A1c levels < 7%, time in target glycemic range, body weight changes, insulin dose, hypoglycemia events, and adverse events.
TAKEAWAY:
- A1c levels < 7% were observed in a higher percentage of patients in the insulin icodec group than in the comparator group (odds ratio, 1.51; P = .004).
- In subgroup analyses, insulin icodec was superior to insulin degludec by several measures but comparatively similar to glargine.
- Insulin icodec was associated with no major safety concerns and had a slightly higher incidence of levels 1, 2, and combined 2/3 than degludec but no significant differences compared with glargine.
IN PRACTICE:
“Sustained glycemic control with once-weekly injections of insulin icodec would lead to better patient acceptance and treatment satisfaction,” the authors wrote.
SOURCE:
This study, authored by Sahana Shetty, MD, and Renuka Suvarna, MSc, Manipal Academy of Higher Education, Department of Endocrinology, Kasturba Medical College, Manipal, Karnataka, was published online on January 8, 2024, in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The comparator group included individuals who used different basal insulin analogs. This heterogeneity in the comparator group introduced a potential source of variability, making it challenging to isolate the specific effects of insulin icodec compared with a standardized comparator. Blinding or masking of participants was performed in only one of the five trials.
DISCLOSURES:
The authors declared no conflicts of interest. All five clinical trials in the meta-analysis were sponsored by Novo Nordisk.
A version of this article appeared on Medscape.com.
TOPLINE:
Once-weekly insulin icodec shows a higher glycated A1c reduction than once-daily basal insulin analogs in patients with type 2 diabetes (T2D), without major safety concerns.
METHODOLOGY:
- A meta-analysis of five phase 3 ONWARDS randomized controlled trials included 3764 patients with T2D.
- The trials compared the effects of the weekly insulin icodec with those of the daily basal insulin analogs glargine and degludec over 26-78 months.
- The primary outcome was the change in A1c levels.
- Secondary outcomes included fasting plasma glucose levels, A1c levels < 7%, time in target glycemic range, body weight changes, insulin dose, hypoglycemia events, and adverse events.
TAKEAWAY:
- A1c levels < 7% were observed in a higher percentage of patients in the insulin icodec group than in the comparator group (odds ratio, 1.51; P = .004).
- In subgroup analyses, insulin icodec was superior to insulin degludec by several measures but comparatively similar to glargine.
- Insulin icodec was associated with no major safety concerns and had a slightly higher incidence of levels 1, 2, and combined 2/3 than degludec but no significant differences compared with glargine.
IN PRACTICE:
“Sustained glycemic control with once-weekly injections of insulin icodec would lead to better patient acceptance and treatment satisfaction,” the authors wrote.
SOURCE:
This study, authored by Sahana Shetty, MD, and Renuka Suvarna, MSc, Manipal Academy of Higher Education, Department of Endocrinology, Kasturba Medical College, Manipal, Karnataka, was published online on January 8, 2024, in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The comparator group included individuals who used different basal insulin analogs. This heterogeneity in the comparator group introduced a potential source of variability, making it challenging to isolate the specific effects of insulin icodec compared with a standardized comparator. Blinding or masking of participants was performed in only one of the five trials.
DISCLOSURES:
The authors declared no conflicts of interest. All five clinical trials in the meta-analysis were sponsored by Novo Nordisk.
A version of this article appeared on Medscape.com.
Colchicine May Benefit Patients With Diabetes and Recent MI
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
Bone Mineral Density Higher in Children Living Near Green Areas
A recently published prospective study in JAMA Network Open identified a significant association between children’s bone health and their proximity to green areas.
The literature emphasized the benefits of childhood exposure to green spaces for neurocognitive, social, behavioral, and mental development, as well as well-being. In addition, such exposure is linked to lower body mass index, increased physical activity, and reduced risks for overweight, obesity, and hypertension. However, specific data on bone mineral density implications are limited.
To address this gap, Hanne Sleurs, PhD, a researcher at the Universiteit Hasselt in Belgium, and colleagues followed the bone health of 327 participants from birth to 4-6 years and examined correlations with individuals’ exposure to green areas. Data collection occurred from October 2014 to July 2021.
Green spaces were categorized as high (vegetation height > 3 m), low (vegetation height ≤ 3 m), and mixed (combination of both). The distances of green spaces from participants’ residences ranged from a radius of 100 m to 3 km. Radial bone mineral density assessment was conducted using quantitative ultrasound during follow-up consultations.
The scientists found that participants frequently exposed to high and mixed vegetation areas within a 500-m radius of their homes had significantly higher bone mineral density than those at other distances or those frequenting spaces with different vegetation. In addition, access to larger green spaces with mixed and high vegetation within a 1-km radius was significantly associated with a lower likelihood of low bone density in children.
“These findings illustrate the positive impact on bone health of early childhood exposure to green areas near their homes during critical growth and development periods, with long-term implications,” wrote the researchers.
The results aligned with those of a prior study in which authors noted factors contributing to families’ frequent park visits, including shorter distances, safety, and park organization, as well as the natural diversity and activities offered.
One hypothesis explaining improved bone density in children visiting green areas was increased physical activity practiced in these locations. The mechanical load from exercise can activate signaling pathways favoring bone development. Literature also gathered data on the influence of green areas on young populations engaging in physical activities, showing positive outcomes.
According to the study authors, the findings are crucial for public health because they emphasize the need for urban investments in accessible green spaces as a strategy for fracture and osteoporosis prevention. In the long term, such initiatives translate to reduced public health expenses, along with physical and emotional gains in communities adopting environmental strategies, they concluded.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
A recently published prospective study in JAMA Network Open identified a significant association between children’s bone health and their proximity to green areas.
The literature emphasized the benefits of childhood exposure to green spaces for neurocognitive, social, behavioral, and mental development, as well as well-being. In addition, such exposure is linked to lower body mass index, increased physical activity, and reduced risks for overweight, obesity, and hypertension. However, specific data on bone mineral density implications are limited.
To address this gap, Hanne Sleurs, PhD, a researcher at the Universiteit Hasselt in Belgium, and colleagues followed the bone health of 327 participants from birth to 4-6 years and examined correlations with individuals’ exposure to green areas. Data collection occurred from October 2014 to July 2021.
Green spaces were categorized as high (vegetation height > 3 m), low (vegetation height ≤ 3 m), and mixed (combination of both). The distances of green spaces from participants’ residences ranged from a radius of 100 m to 3 km. Radial bone mineral density assessment was conducted using quantitative ultrasound during follow-up consultations.
The scientists found that participants frequently exposed to high and mixed vegetation areas within a 500-m radius of their homes had significantly higher bone mineral density than those at other distances or those frequenting spaces with different vegetation. In addition, access to larger green spaces with mixed and high vegetation within a 1-km radius was significantly associated with a lower likelihood of low bone density in children.
“These findings illustrate the positive impact on bone health of early childhood exposure to green areas near their homes during critical growth and development periods, with long-term implications,” wrote the researchers.
The results aligned with those of a prior study in which authors noted factors contributing to families’ frequent park visits, including shorter distances, safety, and park organization, as well as the natural diversity and activities offered.
One hypothesis explaining improved bone density in children visiting green areas was increased physical activity practiced in these locations. The mechanical load from exercise can activate signaling pathways favoring bone development. Literature also gathered data on the influence of green areas on young populations engaging in physical activities, showing positive outcomes.
According to the study authors, the findings are crucial for public health because they emphasize the need for urban investments in accessible green spaces as a strategy for fracture and osteoporosis prevention. In the long term, such initiatives translate to reduced public health expenses, along with physical and emotional gains in communities adopting environmental strategies, they concluded.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
A recently published prospective study in JAMA Network Open identified a significant association between children’s bone health and their proximity to green areas.
The literature emphasized the benefits of childhood exposure to green spaces for neurocognitive, social, behavioral, and mental development, as well as well-being. In addition, such exposure is linked to lower body mass index, increased physical activity, and reduced risks for overweight, obesity, and hypertension. However, specific data on bone mineral density implications are limited.
To address this gap, Hanne Sleurs, PhD, a researcher at the Universiteit Hasselt in Belgium, and colleagues followed the bone health of 327 participants from birth to 4-6 years and examined correlations with individuals’ exposure to green areas. Data collection occurred from October 2014 to July 2021.
Green spaces were categorized as high (vegetation height > 3 m), low (vegetation height ≤ 3 m), and mixed (combination of both). The distances of green spaces from participants’ residences ranged from a radius of 100 m to 3 km. Radial bone mineral density assessment was conducted using quantitative ultrasound during follow-up consultations.
The scientists found that participants frequently exposed to high and mixed vegetation areas within a 500-m radius of their homes had significantly higher bone mineral density than those at other distances or those frequenting spaces with different vegetation. In addition, access to larger green spaces with mixed and high vegetation within a 1-km radius was significantly associated with a lower likelihood of low bone density in children.
“These findings illustrate the positive impact on bone health of early childhood exposure to green areas near their homes during critical growth and development periods, with long-term implications,” wrote the researchers.
The results aligned with those of a prior study in which authors noted factors contributing to families’ frequent park visits, including shorter distances, safety, and park organization, as well as the natural diversity and activities offered.
One hypothesis explaining improved bone density in children visiting green areas was increased physical activity practiced in these locations. The mechanical load from exercise can activate signaling pathways favoring bone development. Literature also gathered data on the influence of green areas on young populations engaging in physical activities, showing positive outcomes.
According to the study authors, the findings are crucial for public health because they emphasize the need for urban investments in accessible green spaces as a strategy for fracture and osteoporosis prevention. In the long term, such initiatives translate to reduced public health expenses, along with physical and emotional gains in communities adopting environmental strategies, they concluded.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
Insulin Resistance Doesn’t Affect Finerenone’s Efficacy
TOPLINE:
In patients with chronic kidney disease (CKD) and type 2 diabetes, baseline insulin resistance was associated with increased cardiovascular (CV) but not kidney risk and did not affect the efficacy of finerenone.
METHODOLOGY:
- Insulin resistance is implicated in CV disease in patients with CKD, but its role in CKD progression is less clear.
- This post hoc analysis of FIDELITY, a pooled analysis of the and trials, randomly assigned patients with type 2 diabetes and CKD (who received optimized renin-angiotensin system blockade) to receive finerenone (10 mg or 20 mg) once daily or placebo and followed them for a median of 3 years.
- An estimated glucose disposal rate (eGDR), a measure of insulin resistance, was calculated for 12,964 patients (median age, 65 years), using waist circumference, hypertension status, and glycated hemoglobin.
- Outcomes included a CV composite (time to CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and a kidney composite (time to renal failure, a sustained decrease ≥ 57% in the initial estimated glomerular filtration rate, or renal death).
TAKEAWAY:
- The median eGDR was 4.1 mg/kg/min. The 50% of patients with a lower eGDR were considered insulin resistant, whereas the remaining half with a higher eGDR were considered insulin sensitive.
- The incidence rate of CV outcomes was higher among patients with insulin resistance in both the finerenone group (incidence rate per 100 patient-years, 5.18 vs 3.47 among insulin-sensitive patients) and the placebo group (6.34 vs 3.76), but eGDR showed no association with kidney outcomes.
- The efficacy of finerenone vs placebo on CV (Wald test P = .063) and kidney outcomes (Wald test P = .51) did not change significantly across the range of baseline eGDR values.
- The incidences of treatment-emergent adverse events and severe adverse events with finerenone were similar between the insulin-resistant and insulin-sensitive subgroups.
IN PRACTICE:
“The efficacy and safety of finerenone were not modified by baseline insulin resistance. A higher risk of CV — but not kidney outcomes was observed in patients with CKD and T2D with greater insulin resistance,” the authors wrote.
SOURCE:
This study was led by Thomas Ebert of the Medical Department III — Endocrinology, Nephrology, Rheumatology, University of Leipzig Medical Center, Leipzig, Germany, and published online in Diabetes Care.
LIMITATIONS:
This study was not adequately powered to evaluate the statistical significance of the association of eGDR with CV and kidney outcomes and was hypothesis-generating. Further studies are needed to examine whether the effects of insulin resistance differ between individuals with diabetes vs those with advanced CKD with or without diabetes.
DISCLOSURES:
The FIDELIO-DKD and FIGARO-DKD trials were conducted and sponsored by Bayer AG. Three authors declared being full-time employees of Bayer. Several authors declared receiving personal fees, consulting fees, grants, or research support from; holding patents with; or having ownership interests in various pharmaceutical companies, including Bayer.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with chronic kidney disease (CKD) and type 2 diabetes, baseline insulin resistance was associated with increased cardiovascular (CV) but not kidney risk and did not affect the efficacy of finerenone.
METHODOLOGY:
- Insulin resistance is implicated in CV disease in patients with CKD, but its role in CKD progression is less clear.
- This post hoc analysis of FIDELITY, a pooled analysis of the and trials, randomly assigned patients with type 2 diabetes and CKD (who received optimized renin-angiotensin system blockade) to receive finerenone (10 mg or 20 mg) once daily or placebo and followed them for a median of 3 years.
- An estimated glucose disposal rate (eGDR), a measure of insulin resistance, was calculated for 12,964 patients (median age, 65 years), using waist circumference, hypertension status, and glycated hemoglobin.
- Outcomes included a CV composite (time to CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and a kidney composite (time to renal failure, a sustained decrease ≥ 57% in the initial estimated glomerular filtration rate, or renal death).
TAKEAWAY:
- The median eGDR was 4.1 mg/kg/min. The 50% of patients with a lower eGDR were considered insulin resistant, whereas the remaining half with a higher eGDR were considered insulin sensitive.
- The incidence rate of CV outcomes was higher among patients with insulin resistance in both the finerenone group (incidence rate per 100 patient-years, 5.18 vs 3.47 among insulin-sensitive patients) and the placebo group (6.34 vs 3.76), but eGDR showed no association with kidney outcomes.
- The efficacy of finerenone vs placebo on CV (Wald test P = .063) and kidney outcomes (Wald test P = .51) did not change significantly across the range of baseline eGDR values.
- The incidences of treatment-emergent adverse events and severe adverse events with finerenone were similar between the insulin-resistant and insulin-sensitive subgroups.
IN PRACTICE:
“The efficacy and safety of finerenone were not modified by baseline insulin resistance. A higher risk of CV — but not kidney outcomes was observed in patients with CKD and T2D with greater insulin resistance,” the authors wrote.
SOURCE:
This study was led by Thomas Ebert of the Medical Department III — Endocrinology, Nephrology, Rheumatology, University of Leipzig Medical Center, Leipzig, Germany, and published online in Diabetes Care.
LIMITATIONS:
This study was not adequately powered to evaluate the statistical significance of the association of eGDR with CV and kidney outcomes and was hypothesis-generating. Further studies are needed to examine whether the effects of insulin resistance differ between individuals with diabetes vs those with advanced CKD with or without diabetes.
DISCLOSURES:
The FIDELIO-DKD and FIGARO-DKD trials were conducted and sponsored by Bayer AG. Three authors declared being full-time employees of Bayer. Several authors declared receiving personal fees, consulting fees, grants, or research support from; holding patents with; or having ownership interests in various pharmaceutical companies, including Bayer.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with chronic kidney disease (CKD) and type 2 diabetes, baseline insulin resistance was associated with increased cardiovascular (CV) but not kidney risk and did not affect the efficacy of finerenone.
METHODOLOGY:
- Insulin resistance is implicated in CV disease in patients with CKD, but its role in CKD progression is less clear.
- This post hoc analysis of FIDELITY, a pooled analysis of the and trials, randomly assigned patients with type 2 diabetes and CKD (who received optimized renin-angiotensin system blockade) to receive finerenone (10 mg or 20 mg) once daily or placebo and followed them for a median of 3 years.
- An estimated glucose disposal rate (eGDR), a measure of insulin resistance, was calculated for 12,964 patients (median age, 65 years), using waist circumference, hypertension status, and glycated hemoglobin.
- Outcomes included a CV composite (time to CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and a kidney composite (time to renal failure, a sustained decrease ≥ 57% in the initial estimated glomerular filtration rate, or renal death).
TAKEAWAY:
- The median eGDR was 4.1 mg/kg/min. The 50% of patients with a lower eGDR were considered insulin resistant, whereas the remaining half with a higher eGDR were considered insulin sensitive.
- The incidence rate of CV outcomes was higher among patients with insulin resistance in both the finerenone group (incidence rate per 100 patient-years, 5.18 vs 3.47 among insulin-sensitive patients) and the placebo group (6.34 vs 3.76), but eGDR showed no association with kidney outcomes.
- The efficacy of finerenone vs placebo on CV (Wald test P = .063) and kidney outcomes (Wald test P = .51) did not change significantly across the range of baseline eGDR values.
- The incidences of treatment-emergent adverse events and severe adverse events with finerenone were similar between the insulin-resistant and insulin-sensitive subgroups.
IN PRACTICE:
“The efficacy and safety of finerenone were not modified by baseline insulin resistance. A higher risk of CV — but not kidney outcomes was observed in patients with CKD and T2D with greater insulin resistance,” the authors wrote.
SOURCE:
This study was led by Thomas Ebert of the Medical Department III — Endocrinology, Nephrology, Rheumatology, University of Leipzig Medical Center, Leipzig, Germany, and published online in Diabetes Care.
LIMITATIONS:
This study was not adequately powered to evaluate the statistical significance of the association of eGDR with CV and kidney outcomes and was hypothesis-generating. Further studies are needed to examine whether the effects of insulin resistance differ between individuals with diabetes vs those with advanced CKD with or without diabetes.
DISCLOSURES:
The FIDELIO-DKD and FIGARO-DKD trials were conducted and sponsored by Bayer AG. Three authors declared being full-time employees of Bayer. Several authors declared receiving personal fees, consulting fees, grants, or research support from; holding patents with; or having ownership interests in various pharmaceutical companies, including Bayer.
A version of this article appeared on Medscape.com.
AI Boosts Diabetic Eye Screening and Follow-Up in Youth
TOPLINE:
Artificial intelligence (AI) boosts the screening rate for potentially blinding diabetes eye disorders in a diabetes clinic compared with referral to an eye care provider (ECP) in a racially and ethnically diverse youth population with diabetes.
METHODOLOGY:
- Although early screening and treatment can prevent diabetic eye diseases (DEDs), many people with diabetes in the United States lack access to and knowledge about diabetic eye exams.
- The trial included 164 patients aged 8-21 years (58% female, 35% Black, and 6% Hispanic) with type 1 or 2 diabetes with no known DED and no diabetic eye exam in the last 6 months.
- In a diabetes clinic, patients were randomly assigned to an AI diabetic eye exam (intervention arm) then and there or to standard of care, referred to an ECP with scripted educational material (control).
- Participants in the intervention arm underwent the 5- to 10-minute autonomous AI diabetic eye exam without pharmacologic dilation. The results were generated immediately as either “DED present” or “DED absent.”
- The primary outcome was the completion rate of documented diabetic eye exams within 6 months (“primary gap closure rate”), either by AI or going to the ECP. The secondary outcome was ECP follow-up by intervention participants with DED (intervention) and all control patients.
TAKEAWAY:
- Within 6 months, all the participants (100%) in the intervention arm completed their diabetic eye exam, a primary care gap closure rate of 100% (95% CI, 96%-100%).
- The rate of primary care gap closure was significantly higher in the intervention vs control arm (100% vs 22%; P < .001).
- In the intervention arm, 64% of patients with DED followed up with an eye care provider within 6 months compared with a mere 22% participants in the control arm (P < .001).
- Participants reported high levels of satisfaction with autonomous AI, with 92.5% expressing satisfaction with the exam’s duration and 96% expressing satisfaction with the whole experience.
IN PRACTICE:
“Autonomous AI increases diabetic eye exam completion rates and closes this care gap in a racially and ethnically diverse population of youth with diabetes, compared to standard of care,” the authors wrote.
SOURCE:
This study, which was led by Risa M. Wolf, MD, department of pediatrics, division of endocrinology, Johns Hopkins School of Medicine, Baltimore, was published online on January 11, 2024, in Nature Communications.
LIMITATIONS:
This study used autonomous AI in the youth although it’s not approved by the US Food and Drug Administration for use in individuals aged 21 years and younger. Some of the participants in this study were already familiar with autonomous AI diabetic eye exams, which might have contributed to their willingness to participate in the current study. The autonomous AI used in the study was shown to have a lack of racial and ethnic bias, but any AI bias caused by differences in retinal pigment has potential to increase rather than decrease health disparities.
DISCLOSURES:
The clinical trial was supported by the National Eye Institute of the National Institutes of Health and the Diabetes Research Connection. Wolf, the lead author, declared receiving research support from Boehringer Ingelheim and Novo Nordisk outside the submitted work. Coauthor Michael D. Abramoff, MD, declared serving in various roles such as investor, director, and consultant for Digital Diagnostics Inc., as well as other ties with many sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Artificial intelligence (AI) boosts the screening rate for potentially blinding diabetes eye disorders in a diabetes clinic compared with referral to an eye care provider (ECP) in a racially and ethnically diverse youth population with diabetes.
METHODOLOGY:
- Although early screening and treatment can prevent diabetic eye diseases (DEDs), many people with diabetes in the United States lack access to and knowledge about diabetic eye exams.
- The trial included 164 patients aged 8-21 years (58% female, 35% Black, and 6% Hispanic) with type 1 or 2 diabetes with no known DED and no diabetic eye exam in the last 6 months.
- In a diabetes clinic, patients were randomly assigned to an AI diabetic eye exam (intervention arm) then and there or to standard of care, referred to an ECP with scripted educational material (control).
- Participants in the intervention arm underwent the 5- to 10-minute autonomous AI diabetic eye exam without pharmacologic dilation. The results were generated immediately as either “DED present” or “DED absent.”
- The primary outcome was the completion rate of documented diabetic eye exams within 6 months (“primary gap closure rate”), either by AI or going to the ECP. The secondary outcome was ECP follow-up by intervention participants with DED (intervention) and all control patients.
TAKEAWAY:
- Within 6 months, all the participants (100%) in the intervention arm completed their diabetic eye exam, a primary care gap closure rate of 100% (95% CI, 96%-100%).
- The rate of primary care gap closure was significantly higher in the intervention vs control arm (100% vs 22%; P < .001).
- In the intervention arm, 64% of patients with DED followed up with an eye care provider within 6 months compared with a mere 22% participants in the control arm (P < .001).
- Participants reported high levels of satisfaction with autonomous AI, with 92.5% expressing satisfaction with the exam’s duration and 96% expressing satisfaction with the whole experience.
IN PRACTICE:
“Autonomous AI increases diabetic eye exam completion rates and closes this care gap in a racially and ethnically diverse population of youth with diabetes, compared to standard of care,” the authors wrote.
SOURCE:
This study, which was led by Risa M. Wolf, MD, department of pediatrics, division of endocrinology, Johns Hopkins School of Medicine, Baltimore, was published online on January 11, 2024, in Nature Communications.
LIMITATIONS:
This study used autonomous AI in the youth although it’s not approved by the US Food and Drug Administration for use in individuals aged 21 years and younger. Some of the participants in this study were already familiar with autonomous AI diabetic eye exams, which might have contributed to their willingness to participate in the current study. The autonomous AI used in the study was shown to have a lack of racial and ethnic bias, but any AI bias caused by differences in retinal pigment has potential to increase rather than decrease health disparities.
DISCLOSURES:
The clinical trial was supported by the National Eye Institute of the National Institutes of Health and the Diabetes Research Connection. Wolf, the lead author, declared receiving research support from Boehringer Ingelheim and Novo Nordisk outside the submitted work. Coauthor Michael D. Abramoff, MD, declared serving in various roles such as investor, director, and consultant for Digital Diagnostics Inc., as well as other ties with many sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Artificial intelligence (AI) boosts the screening rate for potentially blinding diabetes eye disorders in a diabetes clinic compared with referral to an eye care provider (ECP) in a racially and ethnically diverse youth population with diabetes.
METHODOLOGY:
- Although early screening and treatment can prevent diabetic eye diseases (DEDs), many people with diabetes in the United States lack access to and knowledge about diabetic eye exams.
- The trial included 164 patients aged 8-21 years (58% female, 35% Black, and 6% Hispanic) with type 1 or 2 diabetes with no known DED and no diabetic eye exam in the last 6 months.
- In a diabetes clinic, patients were randomly assigned to an AI diabetic eye exam (intervention arm) then and there or to standard of care, referred to an ECP with scripted educational material (control).
- Participants in the intervention arm underwent the 5- to 10-minute autonomous AI diabetic eye exam without pharmacologic dilation. The results were generated immediately as either “DED present” or “DED absent.”
- The primary outcome was the completion rate of documented diabetic eye exams within 6 months (“primary gap closure rate”), either by AI or going to the ECP. The secondary outcome was ECP follow-up by intervention participants with DED (intervention) and all control patients.
TAKEAWAY:
- Within 6 months, all the participants (100%) in the intervention arm completed their diabetic eye exam, a primary care gap closure rate of 100% (95% CI, 96%-100%).
- The rate of primary care gap closure was significantly higher in the intervention vs control arm (100% vs 22%; P < .001).
- In the intervention arm, 64% of patients with DED followed up with an eye care provider within 6 months compared with a mere 22% participants in the control arm (P < .001).
- Participants reported high levels of satisfaction with autonomous AI, with 92.5% expressing satisfaction with the exam’s duration and 96% expressing satisfaction with the whole experience.
IN PRACTICE:
“Autonomous AI increases diabetic eye exam completion rates and closes this care gap in a racially and ethnically diverse population of youth with diabetes, compared to standard of care,” the authors wrote.
SOURCE:
This study, which was led by Risa M. Wolf, MD, department of pediatrics, division of endocrinology, Johns Hopkins School of Medicine, Baltimore, was published online on January 11, 2024, in Nature Communications.
LIMITATIONS:
This study used autonomous AI in the youth although it’s not approved by the US Food and Drug Administration for use in individuals aged 21 years and younger. Some of the participants in this study were already familiar with autonomous AI diabetic eye exams, which might have contributed to their willingness to participate in the current study. The autonomous AI used in the study was shown to have a lack of racial and ethnic bias, but any AI bias caused by differences in retinal pigment has potential to increase rather than decrease health disparities.
DISCLOSURES:
The clinical trial was supported by the National Eye Institute of the National Institutes of Health and the Diabetes Research Connection. Wolf, the lead author, declared receiving research support from Boehringer Ingelheim and Novo Nordisk outside the submitted work. Coauthor Michael D. Abramoff, MD, declared serving in various roles such as investor, director, and consultant for Digital Diagnostics Inc., as well as other ties with many sources.
A version of this article appeared on Medscape.com.
Weight Loss Not Enough to Sustain Type 2 Diabetes Remission
Very few patients with type 2 diabetes (T2D) achieve and sustain diabetes remission via weight loss alone, new research suggests.
Among more than 37,000 people with T2D in Hong Kong, only 6% had achieved and sustained diabetes remission solely through weight loss up to 8 years after diagnosis. Among those who initially achieved remission, 67% had hyperglycemia at 3 years.
People who lost the most weight (10% of their body weight or more) in the first year after diagnosis were most likely to have sustained remission.
The study “helped to confirm the low rate of diabetes remission and high rate of returning to hyperglycemia in real-world practice,” Andrea Luk, MD, of the Chinese University of Hong Kong, told this news organization. “Over 80% of diabetes remission occurred within the first 5 years of a diabetes diagnosis. This is in line with our understanding that beta cell function will gradually decline over time, making diabetes remission increasingly difficult even with weight reduction.”
The study was published in PLOS Medicine.
Early Weight Management Works
Recent clinical trials have demonstrated that T2D remission can be achieved following sustained weight loss through bariatric surgery or lifestyle interventions, the authors noted. In this study, they investigated the association of weight change at 1 year after a diabetes diagnosis with the long-term incidence and sustainability of T2D remission in real-world settings, using data from the territory-wide Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM).
A total of 37,326 people with newly diagnosed T2D who were enrolled in the RAMP-DM between 2000 and 2017 were included and followed until 2019.
At baseline, participants’ mean age was 56.6 years, mean body mass index (BMI) was 26.4 kg/m2, and mean A1c was 7.7%, and 65% were using glucose-lowering drugs (GLDs).
T2D remission was defined as two consecutive A1c < 6.5% measurements at least 6 months apart without GLDs currently or in the previous 3 months.
During a median follow-up of 7.9 years, 6.1% of people achieved remission, with an incidence rate of 7.8 per 1000 person-years. The proportion was higher among those with greater weight loss: 14.4% of people who lost 10% of their body weight or more achieved remission compared with 9.9% of those with 5%-9.9% weight loss, 6.5% of those with 0%-4.9% weight loss, and 4.5% of those who gained weight.
After adjustment for age at diagnosis, sex, assessment year, BMI, other metabolic indices, smoking, alcohol drinking, and medication use, the hazard ratio (HR) for diabetes remission was 3.28 for those with 10% or greater weight loss within 1 year of diagnosis, 2.29 for 5%-9.9% weight loss, and 1.34 for 0%-4.9% weight loss compared to weight gain.
The incidence of diabetes remission in the study was significantly lower than that in clinical trials, possibly because trial participants were in structured programs that included intensive lifestyle interventions, regular monitoring and feedback, and reinforcement of a holistic approach to managing diabetes, the authors noted. Real-world settings may or may not include such interventions.
Further analyses showed that within a median follow-up of 3.1 years, 67.2% of people who had achieved diabetes remission returned to hyperglycemia — an incidence rate of 184.8 per 1000 person-years.
The adjusted HR for returning to hyperglycemia was 0.52 for people with 10% or greater weight loss, 0.78 for those with 5%-9.9% weight loss, and 0.90 for those with 0%-4.9% weight loss compared to people with weight gain.
In addition, diabetes remission was associated with a 31% (HR, 0.69) decreased risk for all-cause mortality.
The study “provides evidence for policymakers to design and implement early weight management interventions” for people diagnosed with T2D, the authors concluded.
Clinicians also have a role to play, Dr. Luk said. “At the first encounter with an individual with newly diagnosed T2D, clinicians should emphasize the importance of weight reduction and guide the individual on how this can be achieved through making healthy lifestyle choices. Pharmacotherapy and metabolic surgery for weight management can be considered in appropriate individuals.”
Overall, she added, “clinicians should be informed that the likelihood of achieving and maintaining diabetes remission is low, and patients should be counseled accordingly.”
Similar to US Experience
Mona Mshayekhi, MD, PhD, an assistant professor of medicine in the division of Diabetes, Endocrinology and Metabolism at Vanderbilt University Medical Center, Nashville, Tennessee, commented on the study for this news organization.
“These findings mirror clinical experience in the US very well,” she said. “We know that sustained weight loss without the use of medications or surgery is extremely difficult in the real-world setting due to the hormonal drivers of obesity, in combination with socioeconomic challenges.”
The study was done before newer weight-management strategies such as glucagon-like peptide 1 receptor agonists were widely available, she noted. “This actually strengthens the finding that weight loss without the routine use of medications has a multitude of benefits, including diabetes remission and reduction of all-cause mortality.”
That said, she added, “I suspect that future studies with more modern cohorts will reveal much higher rates of diabetes remission with the use of newer medications.”
“Our ability to help our patients lose meaningful weight has been limited until recently,” she said. “With new tools in our armamentarium, clinicians need to take the lead in helping patients address and treat obesity and fight the stigma that prevents many from even discussing it with their providers.”
The study did not receive funding. Dr. Luk has received research grants or contracts from Amgen, AstraZeneca, Bayer, Biogen, Boehringer Ingelheim, Eli Lilly, Junshi, Lee Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Shanghai Junshi Biosciences, Sugardown, and Takeda and received travel grants and honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and MSD. Dr. Mshayekhi reported no conflicts of interest.
A version of this article appeared on Medscape.com.
Very few patients with type 2 diabetes (T2D) achieve and sustain diabetes remission via weight loss alone, new research suggests.
Among more than 37,000 people with T2D in Hong Kong, only 6% had achieved and sustained diabetes remission solely through weight loss up to 8 years after diagnosis. Among those who initially achieved remission, 67% had hyperglycemia at 3 years.
People who lost the most weight (10% of their body weight or more) in the first year after diagnosis were most likely to have sustained remission.
The study “helped to confirm the low rate of diabetes remission and high rate of returning to hyperglycemia in real-world practice,” Andrea Luk, MD, of the Chinese University of Hong Kong, told this news organization. “Over 80% of diabetes remission occurred within the first 5 years of a diabetes diagnosis. This is in line with our understanding that beta cell function will gradually decline over time, making diabetes remission increasingly difficult even with weight reduction.”
The study was published in PLOS Medicine.
Early Weight Management Works
Recent clinical trials have demonstrated that T2D remission can be achieved following sustained weight loss through bariatric surgery or lifestyle interventions, the authors noted. In this study, they investigated the association of weight change at 1 year after a diabetes diagnosis with the long-term incidence and sustainability of T2D remission in real-world settings, using data from the territory-wide Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM).
A total of 37,326 people with newly diagnosed T2D who were enrolled in the RAMP-DM between 2000 and 2017 were included and followed until 2019.
At baseline, participants’ mean age was 56.6 years, mean body mass index (BMI) was 26.4 kg/m2, and mean A1c was 7.7%, and 65% were using glucose-lowering drugs (GLDs).
T2D remission was defined as two consecutive A1c < 6.5% measurements at least 6 months apart without GLDs currently or in the previous 3 months.
During a median follow-up of 7.9 years, 6.1% of people achieved remission, with an incidence rate of 7.8 per 1000 person-years. The proportion was higher among those with greater weight loss: 14.4% of people who lost 10% of their body weight or more achieved remission compared with 9.9% of those with 5%-9.9% weight loss, 6.5% of those with 0%-4.9% weight loss, and 4.5% of those who gained weight.
After adjustment for age at diagnosis, sex, assessment year, BMI, other metabolic indices, smoking, alcohol drinking, and medication use, the hazard ratio (HR) for diabetes remission was 3.28 for those with 10% or greater weight loss within 1 year of diagnosis, 2.29 for 5%-9.9% weight loss, and 1.34 for 0%-4.9% weight loss compared to weight gain.
The incidence of diabetes remission in the study was significantly lower than that in clinical trials, possibly because trial participants were in structured programs that included intensive lifestyle interventions, regular monitoring and feedback, and reinforcement of a holistic approach to managing diabetes, the authors noted. Real-world settings may or may not include such interventions.
Further analyses showed that within a median follow-up of 3.1 years, 67.2% of people who had achieved diabetes remission returned to hyperglycemia — an incidence rate of 184.8 per 1000 person-years.
The adjusted HR for returning to hyperglycemia was 0.52 for people with 10% or greater weight loss, 0.78 for those with 5%-9.9% weight loss, and 0.90 for those with 0%-4.9% weight loss compared to people with weight gain.
In addition, diabetes remission was associated with a 31% (HR, 0.69) decreased risk for all-cause mortality.
The study “provides evidence for policymakers to design and implement early weight management interventions” for people diagnosed with T2D, the authors concluded.
Clinicians also have a role to play, Dr. Luk said. “At the first encounter with an individual with newly diagnosed T2D, clinicians should emphasize the importance of weight reduction and guide the individual on how this can be achieved through making healthy lifestyle choices. Pharmacotherapy and metabolic surgery for weight management can be considered in appropriate individuals.”
Overall, she added, “clinicians should be informed that the likelihood of achieving and maintaining diabetes remission is low, and patients should be counseled accordingly.”
Similar to US Experience
Mona Mshayekhi, MD, PhD, an assistant professor of medicine in the division of Diabetes, Endocrinology and Metabolism at Vanderbilt University Medical Center, Nashville, Tennessee, commented on the study for this news organization.
“These findings mirror clinical experience in the US very well,” she said. “We know that sustained weight loss without the use of medications or surgery is extremely difficult in the real-world setting due to the hormonal drivers of obesity, in combination with socioeconomic challenges.”
The study was done before newer weight-management strategies such as glucagon-like peptide 1 receptor agonists were widely available, she noted. “This actually strengthens the finding that weight loss without the routine use of medications has a multitude of benefits, including diabetes remission and reduction of all-cause mortality.”
That said, she added, “I suspect that future studies with more modern cohorts will reveal much higher rates of diabetes remission with the use of newer medications.”
“Our ability to help our patients lose meaningful weight has been limited until recently,” she said. “With new tools in our armamentarium, clinicians need to take the lead in helping patients address and treat obesity and fight the stigma that prevents many from even discussing it with their providers.”
The study did not receive funding. Dr. Luk has received research grants or contracts from Amgen, AstraZeneca, Bayer, Biogen, Boehringer Ingelheim, Eli Lilly, Junshi, Lee Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Shanghai Junshi Biosciences, Sugardown, and Takeda and received travel grants and honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and MSD. Dr. Mshayekhi reported no conflicts of interest.
A version of this article appeared on Medscape.com.
Very few patients with type 2 diabetes (T2D) achieve and sustain diabetes remission via weight loss alone, new research suggests.
Among more than 37,000 people with T2D in Hong Kong, only 6% had achieved and sustained diabetes remission solely through weight loss up to 8 years after diagnosis. Among those who initially achieved remission, 67% had hyperglycemia at 3 years.
People who lost the most weight (10% of their body weight or more) in the first year after diagnosis were most likely to have sustained remission.
The study “helped to confirm the low rate of diabetes remission and high rate of returning to hyperglycemia in real-world practice,” Andrea Luk, MD, of the Chinese University of Hong Kong, told this news organization. “Over 80% of diabetes remission occurred within the first 5 years of a diabetes diagnosis. This is in line with our understanding that beta cell function will gradually decline over time, making diabetes remission increasingly difficult even with weight reduction.”
The study was published in PLOS Medicine.
Early Weight Management Works
Recent clinical trials have demonstrated that T2D remission can be achieved following sustained weight loss through bariatric surgery or lifestyle interventions, the authors noted. In this study, they investigated the association of weight change at 1 year after a diabetes diagnosis with the long-term incidence and sustainability of T2D remission in real-world settings, using data from the territory-wide Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM).
A total of 37,326 people with newly diagnosed T2D who were enrolled in the RAMP-DM between 2000 and 2017 were included and followed until 2019.
At baseline, participants’ mean age was 56.6 years, mean body mass index (BMI) was 26.4 kg/m2, and mean A1c was 7.7%, and 65% were using glucose-lowering drugs (GLDs).
T2D remission was defined as two consecutive A1c < 6.5% measurements at least 6 months apart without GLDs currently or in the previous 3 months.
During a median follow-up of 7.9 years, 6.1% of people achieved remission, with an incidence rate of 7.8 per 1000 person-years. The proportion was higher among those with greater weight loss: 14.4% of people who lost 10% of their body weight or more achieved remission compared with 9.9% of those with 5%-9.9% weight loss, 6.5% of those with 0%-4.9% weight loss, and 4.5% of those who gained weight.
After adjustment for age at diagnosis, sex, assessment year, BMI, other metabolic indices, smoking, alcohol drinking, and medication use, the hazard ratio (HR) for diabetes remission was 3.28 for those with 10% or greater weight loss within 1 year of diagnosis, 2.29 for 5%-9.9% weight loss, and 1.34 for 0%-4.9% weight loss compared to weight gain.
The incidence of diabetes remission in the study was significantly lower than that in clinical trials, possibly because trial participants were in structured programs that included intensive lifestyle interventions, regular monitoring and feedback, and reinforcement of a holistic approach to managing diabetes, the authors noted. Real-world settings may or may not include such interventions.
Further analyses showed that within a median follow-up of 3.1 years, 67.2% of people who had achieved diabetes remission returned to hyperglycemia — an incidence rate of 184.8 per 1000 person-years.
The adjusted HR for returning to hyperglycemia was 0.52 for people with 10% or greater weight loss, 0.78 for those with 5%-9.9% weight loss, and 0.90 for those with 0%-4.9% weight loss compared to people with weight gain.
In addition, diabetes remission was associated with a 31% (HR, 0.69) decreased risk for all-cause mortality.
The study “provides evidence for policymakers to design and implement early weight management interventions” for people diagnosed with T2D, the authors concluded.
Clinicians also have a role to play, Dr. Luk said. “At the first encounter with an individual with newly diagnosed T2D, clinicians should emphasize the importance of weight reduction and guide the individual on how this can be achieved through making healthy lifestyle choices. Pharmacotherapy and metabolic surgery for weight management can be considered in appropriate individuals.”
Overall, she added, “clinicians should be informed that the likelihood of achieving and maintaining diabetes remission is low, and patients should be counseled accordingly.”
Similar to US Experience
Mona Mshayekhi, MD, PhD, an assistant professor of medicine in the division of Diabetes, Endocrinology and Metabolism at Vanderbilt University Medical Center, Nashville, Tennessee, commented on the study for this news organization.
“These findings mirror clinical experience in the US very well,” she said. “We know that sustained weight loss without the use of medications or surgery is extremely difficult in the real-world setting due to the hormonal drivers of obesity, in combination with socioeconomic challenges.”
The study was done before newer weight-management strategies such as glucagon-like peptide 1 receptor agonists were widely available, she noted. “This actually strengthens the finding that weight loss without the routine use of medications has a multitude of benefits, including diabetes remission and reduction of all-cause mortality.”
That said, she added, “I suspect that future studies with more modern cohorts will reveal much higher rates of diabetes remission with the use of newer medications.”
“Our ability to help our patients lose meaningful weight has been limited until recently,” she said. “With new tools in our armamentarium, clinicians need to take the lead in helping patients address and treat obesity and fight the stigma that prevents many from even discussing it with their providers.”
The study did not receive funding. Dr. Luk has received research grants or contracts from Amgen, AstraZeneca, Bayer, Biogen, Boehringer Ingelheim, Eli Lilly, Junshi, Lee Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Shanghai Junshi Biosciences, Sugardown, and Takeda and received travel grants and honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and MSD. Dr. Mshayekhi reported no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM PLOS MEDICINE
Corticosteroid Injections Don’t Move Blood Sugar for Most
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.