User login
Inhaled vasodilator type has no impact on outcomes
based on data from more than 11,000 patients.
Mechanically ventilated patients with severe acute respiratory failure may be treated with inhaled vasodilators using nitric oxide or epoprostenol to improve oxygenation, but data on practice patterns and head-to-head comparisons of effectiveness for the two options are limited, wrote Nicholas A. Bosch, MD, of Boston University, and colleagues.
In a study published in the journal Chest, the researchers used the Premier Healthcare Database to emulate a cluster randomized trial. The study population included 11,200 patients aged 18 years and older who were hospitalized at one of 303 hospitals with acute respiratory failure or acute respiratory distress between 2016 and 2020.
The patients received either nitric oxide (iNO) or epoprostenol (iEpo) during a hospital stay. A total of 6,366 patients received iNO first, 4,720 received iEpo first, and 114 received both on the same day. The median age of the patients was 58 years, and 64.6% of patients received neuromuscular blockades on the day they began vasodilator therapy. The primary outcome for effectiveness was successful extubation within 28 days of receiving a vasodilator. The outcomes for evaluating practice patterns included the choice of first inhaled vasodilator, days of invasive mechanical ventilation before starting a vasodilator, duration of use, proportion of patients who switched between iNO and iEpo, and the proportion who received each type of vasodilator.
A total of 104 hospitals (34.3%) used iNO exclusively, and 118 hospitals (38.9%) used iEpo exclusively. No differences in successful extubation rates appeared between these iNO and iEpo groups (37.0% vs. 34.7%; hazard ratio, 0.97). In addition, no differences were observed between the iNO and iEpo hospitals in total hospital costs or patient deaths or discharge to hospice, and the results persisted in a multivariate analysis.
Overall, the results were similar in a subgroup analysis, although patients receiving iNO were more likely to have successful extubation after controlling for organ dysfunction, the researchers noted.
“Our study provides stronger and more robust evidence that there are no differences in patient outcomes based on inhaled vasodilator type,” and suggest that either type may be used for patients whom clinicians think would benefit, the researchers wrote in their discussion. However, neither vasodilator type has been shown to significantly improve mortality, they noted.
The findings were limited by several factors including the observational design, lack of data on medication dose, and the use of nonrandom samples of hospitalizations and patients with laboratory and vital signs data, the researchers noted. The study also did not identify the specific indication for inhaled vasodilator therapy, and did not adjust for other therapies such as prone positioning or adherence to lung protective ventilation, they said.
However, the results were strengthened by the large sample size and more precise estimates of effectiveness than previous smaller studies, and suggest similar outcomes for patients and costs for hospitals, they concluded.
The study was funded by the National Institutes of Health National Center for Advancing Translational Sciences. Lead author Dr. Bosch also was supported by NIH/NCATS, the National Heart, Lung, and Blood Institute, and the Department of Defense. The researchers had no financial conflicts to disclose.
based on data from more than 11,000 patients.
Mechanically ventilated patients with severe acute respiratory failure may be treated with inhaled vasodilators using nitric oxide or epoprostenol to improve oxygenation, but data on practice patterns and head-to-head comparisons of effectiveness for the two options are limited, wrote Nicholas A. Bosch, MD, of Boston University, and colleagues.
In a study published in the journal Chest, the researchers used the Premier Healthcare Database to emulate a cluster randomized trial. The study population included 11,200 patients aged 18 years and older who were hospitalized at one of 303 hospitals with acute respiratory failure or acute respiratory distress between 2016 and 2020.
The patients received either nitric oxide (iNO) or epoprostenol (iEpo) during a hospital stay. A total of 6,366 patients received iNO first, 4,720 received iEpo first, and 114 received both on the same day. The median age of the patients was 58 years, and 64.6% of patients received neuromuscular blockades on the day they began vasodilator therapy. The primary outcome for effectiveness was successful extubation within 28 days of receiving a vasodilator. The outcomes for evaluating practice patterns included the choice of first inhaled vasodilator, days of invasive mechanical ventilation before starting a vasodilator, duration of use, proportion of patients who switched between iNO and iEpo, and the proportion who received each type of vasodilator.
A total of 104 hospitals (34.3%) used iNO exclusively, and 118 hospitals (38.9%) used iEpo exclusively. No differences in successful extubation rates appeared between these iNO and iEpo groups (37.0% vs. 34.7%; hazard ratio, 0.97). In addition, no differences were observed between the iNO and iEpo hospitals in total hospital costs or patient deaths or discharge to hospice, and the results persisted in a multivariate analysis.
Overall, the results were similar in a subgroup analysis, although patients receiving iNO were more likely to have successful extubation after controlling for organ dysfunction, the researchers noted.
“Our study provides stronger and more robust evidence that there are no differences in patient outcomes based on inhaled vasodilator type,” and suggest that either type may be used for patients whom clinicians think would benefit, the researchers wrote in their discussion. However, neither vasodilator type has been shown to significantly improve mortality, they noted.
The findings were limited by several factors including the observational design, lack of data on medication dose, and the use of nonrandom samples of hospitalizations and patients with laboratory and vital signs data, the researchers noted. The study also did not identify the specific indication for inhaled vasodilator therapy, and did not adjust for other therapies such as prone positioning or adherence to lung protective ventilation, they said.
However, the results were strengthened by the large sample size and more precise estimates of effectiveness than previous smaller studies, and suggest similar outcomes for patients and costs for hospitals, they concluded.
The study was funded by the National Institutes of Health National Center for Advancing Translational Sciences. Lead author Dr. Bosch also was supported by NIH/NCATS, the National Heart, Lung, and Blood Institute, and the Department of Defense. The researchers had no financial conflicts to disclose.
based on data from more than 11,000 patients.
Mechanically ventilated patients with severe acute respiratory failure may be treated with inhaled vasodilators using nitric oxide or epoprostenol to improve oxygenation, but data on practice patterns and head-to-head comparisons of effectiveness for the two options are limited, wrote Nicholas A. Bosch, MD, of Boston University, and colleagues.
In a study published in the journal Chest, the researchers used the Premier Healthcare Database to emulate a cluster randomized trial. The study population included 11,200 patients aged 18 years and older who were hospitalized at one of 303 hospitals with acute respiratory failure or acute respiratory distress between 2016 and 2020.
The patients received either nitric oxide (iNO) or epoprostenol (iEpo) during a hospital stay. A total of 6,366 patients received iNO first, 4,720 received iEpo first, and 114 received both on the same day. The median age of the patients was 58 years, and 64.6% of patients received neuromuscular blockades on the day they began vasodilator therapy. The primary outcome for effectiveness was successful extubation within 28 days of receiving a vasodilator. The outcomes for evaluating practice patterns included the choice of first inhaled vasodilator, days of invasive mechanical ventilation before starting a vasodilator, duration of use, proportion of patients who switched between iNO and iEpo, and the proportion who received each type of vasodilator.
A total of 104 hospitals (34.3%) used iNO exclusively, and 118 hospitals (38.9%) used iEpo exclusively. No differences in successful extubation rates appeared between these iNO and iEpo groups (37.0% vs. 34.7%; hazard ratio, 0.97). In addition, no differences were observed between the iNO and iEpo hospitals in total hospital costs or patient deaths or discharge to hospice, and the results persisted in a multivariate analysis.
Overall, the results were similar in a subgroup analysis, although patients receiving iNO were more likely to have successful extubation after controlling for organ dysfunction, the researchers noted.
“Our study provides stronger and more robust evidence that there are no differences in patient outcomes based on inhaled vasodilator type,” and suggest that either type may be used for patients whom clinicians think would benefit, the researchers wrote in their discussion. However, neither vasodilator type has been shown to significantly improve mortality, they noted.
The findings were limited by several factors including the observational design, lack of data on medication dose, and the use of nonrandom samples of hospitalizations and patients with laboratory and vital signs data, the researchers noted. The study also did not identify the specific indication for inhaled vasodilator therapy, and did not adjust for other therapies such as prone positioning or adherence to lung protective ventilation, they said.
However, the results were strengthened by the large sample size and more precise estimates of effectiveness than previous smaller studies, and suggest similar outcomes for patients and costs for hospitals, they concluded.
The study was funded by the National Institutes of Health National Center for Advancing Translational Sciences. Lead author Dr. Bosch also was supported by NIH/NCATS, the National Heart, Lung, and Blood Institute, and the Department of Defense. The researchers had no financial conflicts to disclose.
FROM CHEST
Former nurse charged with murder in death of 97-year-old war veteran
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
Guidelines: Convalescent plasma not recommended for most hospitalized with COVID
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Death risk doubles for Black infants with bronchopulmonary dysplasia
Infants with bronchopulmonary dysplasia (BPD) who were born to Black mothers were significantly more likely to die or to have a longer hospital stay than infants of other ethnicities, based on data from more than 800 infants.
The overall incidence of BPD is rising, in part because of improved survival for extremely preterm infants, wrote Tamorah R. Lewis, MD, of the University of Missouri, Kansas City, and colleagues.
Previous studies suggest that racial disparities may affect outcomes for preterm infants with a range of neonatal morbidities during neonatal ICU (NICU) hospitalization, including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis. However, the association of racial disparities with outcomes for preterm infants with BPD remains unclear, they said.
In a study published in JAMA Pediatrics, the researchers, on behalf of the Bronchopulmonary Dysplasia Collaborative, reviewed data from 834 preterm infants enrolled in the BPD Collaborative registry from Jan. 1, 2015, to July 19, 2021, at eight centers in the United States.
The study infants were born at less than 32 weeks’ gestation and were diagnosed with severe BPD according to the 2001 National Institutes of Health Consensus Criteria. The study population included 276 Black infants and 558 white infants. The median gestational age was 24 weeks, and 41% of the infants were female.
The primary outcomes were infant death and length of hospital stay.
Although death was infrequent (4% overall), Black maternal race was significantly associated with an increased risk of death from BPD (adjusted odds ratio, 2.1). Black maternal race also was significantly associated with a longer hospital stay for the infants, with an adjusted between-group difference of 10 days.
Infants of Black mothers also were more likely than those with White mothers to receive invasive respiratory support at the time of delivery. Black infants were more likely than White infants to have lower gestational age, lower birth weight and length, and smaller head circumference.
However, the proportions of cesarean deliveries, gender distribution, and infants small for gestational age were similar between Black and White infant groups. Medication exposure at 36 weeks postmenstrual age (PMA) also was similar for Black and White infants, and 50% of patients overall were treated with nasal continuous positive airway pressure at 36 weeks’ PMA. Awareness of the increased risk of death and longer hospital stay for Black infants is critical, “given the highly variable outcomes for patients with BPD and the uncertainty regarding demographic factors that contribute to late respiratory morbidity in severe BPD,” the researchers wrote.
The study findings were limited by several factors including variations among study centers in the identification and recording of maternal race, lack of data on paternal race, and the focus specifically on Black maternal race and not other ethnicities. Given the documented health disparities for Black individuals in the United States, “we restricted our cohort to only those patients born to Black or White mothers to estimate the association of Black maternal race and adverse in-hospital outcomes in infants with severe BPD,” the researchers wrote
Other limitations include the lack of data surrounding infant death and inability to adjust for all potential modifiers of BPD pathogenesis and progression, such as BPD comorbidities.
Prospective studies are needed to identify the sociodemographic mechanisms that may contribute to health outcome disparities for Black infants with severe BPD, the researchers emphasized.
In the meantime, the results highlight the need for more attention to variations in care for infants with BPD of different races, and approaches to family-centered care should consider “the precise needs of high-risk, structurally disadvantaged families while informing the design of prospective trials that improve outcomes for high-risk subgroups of children with severe BPD,” they concluded.
Data raise questions about the origin of disparities
The current study findings contribute to the knowledge and awareness of disparities in the high-risk NICU population, Nicolas A. Bamat, MD, and colleagues wrote in an accompanying editorial. “Further, their findings oppose the central tendency in the literature: that infants of Black mothers have less severe lung disease of prematurity during the birth hospitalization.”
The editorial authors noted that the study’s inclusion of racial characteristics as confounding variables to assess the effect of race on health “can imply questionable assumptions about where in a causal pathway racism begins to exert an effect,” whether after a diagnosis of BPD, during pregnancy in response to inequitable obstetric care, or “centuries ago, propagating forward through the shared experience of communities oppressed by the legacy of racism and its ongoing contemporary manifestations.”
The editorial authors added that, “in lung disease of prematurity, few variables are reliable antecedents to race as an exposure. Complex adjustment is necessary to reduce bias in targeted research questions.” However, the current study findings highlight the need to move toward more equitable neonatal care, and to prioritize interventions to reduce racial health disparities at the level of the NICU as well as at the hospital and government policy levels.
Consider range of contributing factors and confounders
The current study is important because “it is imperative to measure racial outcomes in health care in order to highlight and address disparities and biases,” Tim Joos, MD, said in an interview. However, “it can be difficult to determine how much race is a factor in itself versus a proxy for other important characteristics, such as socioeconomic status and level of education, that can confound the results.”
In the current study, the twofold-increased death rate in the premature infants of Black mothers is concerning and deserves further attention, Dr. Joos said. “The 10-day longer length of stay for infants of Black mothers seems quite shocking at first glance, but because of the long hospital stays for these extremely premature infants in general, it is about 7% longer than the infants born to White mothers.”
The take-home message is that this difference is still significant, and can reflect many factors including disease severity and complications, need for feeding assistance, teaching, and setting up home supports, said Dr. Joos.
As for additional research, “it would be useful for hospitals to break down why the differences exist, although I worry a provider or institution will feel they need to discharge Black families sooner to avoid being biased. Family preference and comfort level should be given high priority,” he emphasized.
The study received no outside funding, but lead author Dr. Lewis was supported by the National Institute on Child Health and Development and the Robert Wood Johnson Foundation. Several coauthors were supported by other grants from the National Institutes of Health. Dr. Barnat and one coauthor were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Joos had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Infants with bronchopulmonary dysplasia (BPD) who were born to Black mothers were significantly more likely to die or to have a longer hospital stay than infants of other ethnicities, based on data from more than 800 infants.
The overall incidence of BPD is rising, in part because of improved survival for extremely preterm infants, wrote Tamorah R. Lewis, MD, of the University of Missouri, Kansas City, and colleagues.
Previous studies suggest that racial disparities may affect outcomes for preterm infants with a range of neonatal morbidities during neonatal ICU (NICU) hospitalization, including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis. However, the association of racial disparities with outcomes for preterm infants with BPD remains unclear, they said.
In a study published in JAMA Pediatrics, the researchers, on behalf of the Bronchopulmonary Dysplasia Collaborative, reviewed data from 834 preterm infants enrolled in the BPD Collaborative registry from Jan. 1, 2015, to July 19, 2021, at eight centers in the United States.
The study infants were born at less than 32 weeks’ gestation and were diagnosed with severe BPD according to the 2001 National Institutes of Health Consensus Criteria. The study population included 276 Black infants and 558 white infants. The median gestational age was 24 weeks, and 41% of the infants were female.
The primary outcomes were infant death and length of hospital stay.
Although death was infrequent (4% overall), Black maternal race was significantly associated with an increased risk of death from BPD (adjusted odds ratio, 2.1). Black maternal race also was significantly associated with a longer hospital stay for the infants, with an adjusted between-group difference of 10 days.
Infants of Black mothers also were more likely than those with White mothers to receive invasive respiratory support at the time of delivery. Black infants were more likely than White infants to have lower gestational age, lower birth weight and length, and smaller head circumference.
However, the proportions of cesarean deliveries, gender distribution, and infants small for gestational age were similar between Black and White infant groups. Medication exposure at 36 weeks postmenstrual age (PMA) also was similar for Black and White infants, and 50% of patients overall were treated with nasal continuous positive airway pressure at 36 weeks’ PMA. Awareness of the increased risk of death and longer hospital stay for Black infants is critical, “given the highly variable outcomes for patients with BPD and the uncertainty regarding demographic factors that contribute to late respiratory morbidity in severe BPD,” the researchers wrote.
The study findings were limited by several factors including variations among study centers in the identification and recording of maternal race, lack of data on paternal race, and the focus specifically on Black maternal race and not other ethnicities. Given the documented health disparities for Black individuals in the United States, “we restricted our cohort to only those patients born to Black or White mothers to estimate the association of Black maternal race and adverse in-hospital outcomes in infants with severe BPD,” the researchers wrote
Other limitations include the lack of data surrounding infant death and inability to adjust for all potential modifiers of BPD pathogenesis and progression, such as BPD comorbidities.
Prospective studies are needed to identify the sociodemographic mechanisms that may contribute to health outcome disparities for Black infants with severe BPD, the researchers emphasized.
In the meantime, the results highlight the need for more attention to variations in care for infants with BPD of different races, and approaches to family-centered care should consider “the precise needs of high-risk, structurally disadvantaged families while informing the design of prospective trials that improve outcomes for high-risk subgroups of children with severe BPD,” they concluded.
Data raise questions about the origin of disparities
The current study findings contribute to the knowledge and awareness of disparities in the high-risk NICU population, Nicolas A. Bamat, MD, and colleagues wrote in an accompanying editorial. “Further, their findings oppose the central tendency in the literature: that infants of Black mothers have less severe lung disease of prematurity during the birth hospitalization.”
The editorial authors noted that the study’s inclusion of racial characteristics as confounding variables to assess the effect of race on health “can imply questionable assumptions about where in a causal pathway racism begins to exert an effect,” whether after a diagnosis of BPD, during pregnancy in response to inequitable obstetric care, or “centuries ago, propagating forward through the shared experience of communities oppressed by the legacy of racism and its ongoing contemporary manifestations.”
The editorial authors added that, “in lung disease of prematurity, few variables are reliable antecedents to race as an exposure. Complex adjustment is necessary to reduce bias in targeted research questions.” However, the current study findings highlight the need to move toward more equitable neonatal care, and to prioritize interventions to reduce racial health disparities at the level of the NICU as well as at the hospital and government policy levels.
Consider range of contributing factors and confounders
The current study is important because “it is imperative to measure racial outcomes in health care in order to highlight and address disparities and biases,” Tim Joos, MD, said in an interview. However, “it can be difficult to determine how much race is a factor in itself versus a proxy for other important characteristics, such as socioeconomic status and level of education, that can confound the results.”
In the current study, the twofold-increased death rate in the premature infants of Black mothers is concerning and deserves further attention, Dr. Joos said. “The 10-day longer length of stay for infants of Black mothers seems quite shocking at first glance, but because of the long hospital stays for these extremely premature infants in general, it is about 7% longer than the infants born to White mothers.”
The take-home message is that this difference is still significant, and can reflect many factors including disease severity and complications, need for feeding assistance, teaching, and setting up home supports, said Dr. Joos.
As for additional research, “it would be useful for hospitals to break down why the differences exist, although I worry a provider or institution will feel they need to discharge Black families sooner to avoid being biased. Family preference and comfort level should be given high priority,” he emphasized.
The study received no outside funding, but lead author Dr. Lewis was supported by the National Institute on Child Health and Development and the Robert Wood Johnson Foundation. Several coauthors were supported by other grants from the National Institutes of Health. Dr. Barnat and one coauthor were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Joos had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Infants with bronchopulmonary dysplasia (BPD) who were born to Black mothers were significantly more likely to die or to have a longer hospital stay than infants of other ethnicities, based on data from more than 800 infants.
The overall incidence of BPD is rising, in part because of improved survival for extremely preterm infants, wrote Tamorah R. Lewis, MD, of the University of Missouri, Kansas City, and colleagues.
Previous studies suggest that racial disparities may affect outcomes for preterm infants with a range of neonatal morbidities during neonatal ICU (NICU) hospitalization, including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis. However, the association of racial disparities with outcomes for preterm infants with BPD remains unclear, they said.
In a study published in JAMA Pediatrics, the researchers, on behalf of the Bronchopulmonary Dysplasia Collaborative, reviewed data from 834 preterm infants enrolled in the BPD Collaborative registry from Jan. 1, 2015, to July 19, 2021, at eight centers in the United States.
The study infants were born at less than 32 weeks’ gestation and were diagnosed with severe BPD according to the 2001 National Institutes of Health Consensus Criteria. The study population included 276 Black infants and 558 white infants. The median gestational age was 24 weeks, and 41% of the infants were female.
The primary outcomes were infant death and length of hospital stay.
Although death was infrequent (4% overall), Black maternal race was significantly associated with an increased risk of death from BPD (adjusted odds ratio, 2.1). Black maternal race also was significantly associated with a longer hospital stay for the infants, with an adjusted between-group difference of 10 days.
Infants of Black mothers also were more likely than those with White mothers to receive invasive respiratory support at the time of delivery. Black infants were more likely than White infants to have lower gestational age, lower birth weight and length, and smaller head circumference.
However, the proportions of cesarean deliveries, gender distribution, and infants small for gestational age were similar between Black and White infant groups. Medication exposure at 36 weeks postmenstrual age (PMA) also was similar for Black and White infants, and 50% of patients overall were treated with nasal continuous positive airway pressure at 36 weeks’ PMA. Awareness of the increased risk of death and longer hospital stay for Black infants is critical, “given the highly variable outcomes for patients with BPD and the uncertainty regarding demographic factors that contribute to late respiratory morbidity in severe BPD,” the researchers wrote.
The study findings were limited by several factors including variations among study centers in the identification and recording of maternal race, lack of data on paternal race, and the focus specifically on Black maternal race and not other ethnicities. Given the documented health disparities for Black individuals in the United States, “we restricted our cohort to only those patients born to Black or White mothers to estimate the association of Black maternal race and adverse in-hospital outcomes in infants with severe BPD,” the researchers wrote
Other limitations include the lack of data surrounding infant death and inability to adjust for all potential modifiers of BPD pathogenesis and progression, such as BPD comorbidities.
Prospective studies are needed to identify the sociodemographic mechanisms that may contribute to health outcome disparities for Black infants with severe BPD, the researchers emphasized.
In the meantime, the results highlight the need for more attention to variations in care for infants with BPD of different races, and approaches to family-centered care should consider “the precise needs of high-risk, structurally disadvantaged families while informing the design of prospective trials that improve outcomes for high-risk subgroups of children with severe BPD,” they concluded.
Data raise questions about the origin of disparities
The current study findings contribute to the knowledge and awareness of disparities in the high-risk NICU population, Nicolas A. Bamat, MD, and colleagues wrote in an accompanying editorial. “Further, their findings oppose the central tendency in the literature: that infants of Black mothers have less severe lung disease of prematurity during the birth hospitalization.”
The editorial authors noted that the study’s inclusion of racial characteristics as confounding variables to assess the effect of race on health “can imply questionable assumptions about where in a causal pathway racism begins to exert an effect,” whether after a diagnosis of BPD, during pregnancy in response to inequitable obstetric care, or “centuries ago, propagating forward through the shared experience of communities oppressed by the legacy of racism and its ongoing contemporary manifestations.”
The editorial authors added that, “in lung disease of prematurity, few variables are reliable antecedents to race as an exposure. Complex adjustment is necessary to reduce bias in targeted research questions.” However, the current study findings highlight the need to move toward more equitable neonatal care, and to prioritize interventions to reduce racial health disparities at the level of the NICU as well as at the hospital and government policy levels.
Consider range of contributing factors and confounders
The current study is important because “it is imperative to measure racial outcomes in health care in order to highlight and address disparities and biases,” Tim Joos, MD, said in an interview. However, “it can be difficult to determine how much race is a factor in itself versus a proxy for other important characteristics, such as socioeconomic status and level of education, that can confound the results.”
In the current study, the twofold-increased death rate in the premature infants of Black mothers is concerning and deserves further attention, Dr. Joos said. “The 10-day longer length of stay for infants of Black mothers seems quite shocking at first glance, but because of the long hospital stays for these extremely premature infants in general, it is about 7% longer than the infants born to White mothers.”
The take-home message is that this difference is still significant, and can reflect many factors including disease severity and complications, need for feeding assistance, teaching, and setting up home supports, said Dr. Joos.
As for additional research, “it would be useful for hospitals to break down why the differences exist, although I worry a provider or institution will feel they need to discharge Black families sooner to avoid being biased. Family preference and comfort level should be given high priority,” he emphasized.
The study received no outside funding, but lead author Dr. Lewis was supported by the National Institute on Child Health and Development and the Robert Wood Johnson Foundation. Several coauthors were supported by other grants from the National Institutes of Health. Dr. Barnat and one coauthor were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Joos had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Author Q&A: Intravenous Immunoglobulin for Treatment of COVID-19 in Select Patients
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Improving Epistaxis Knowledge and Management Among Nursing Staff
From the University of Chicago Medical Center, Chicago, IL.
Abstract
Background: Epistaxis is a common chief complaint addressed by otolaryngologists. A review of the literature showed that there is a deficit in epistaxis education within the nursing community. Conversations with our nursing colleagues confirmed this unmet demand.
Objective: This quality improvement project aimed to increase general epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds among our nursing staff.
Methods: Data were collected through a survey administered before and after our intervention. The survey tested general epistaxis knowledge and assessed comfort and confidence in stopping epistaxis. Our intervention was an educational session covering pertinent epistaxis etiology and management. Quality improvement principles were used to optimize delivery of the intervention.
Results: A total of 51 nurses participated in the project. After participating in the in-service educational session, nurses answered significantly more epistaxis general knowledge questions correctly (mean [SD] difference, 2.07 [1.10] questions; 95% CI, 1.74-2.39; P < .001). There was no statistically significant difference in additional correct questions when stratified by clinical experience or clinical setting (P = .128 and P = 0.446, respectively). Nurses also reported feeling significantly more comfortable and significantly more confident in managing nosebleeds after the in-service (P = .007 and P < 0.001, respectively); 74.46% of nurses had an improvement in comfort level in managing epistaxis and 43.90% of nurses had an improvement in confidence in stopping epistaxis. After we moved the educational session from mid-shift to shift change, the nursing staff reported more satisfaction while maintaining similar improvements in knowledge and confidence.
Conclusion: We were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. Nurses of varying clinical experience and different clinical settings benefitted equally from our intervention.
Keywords: nosebleed; in-service; quality improvement.
Epistaxis, or nosebleed, is estimated to be the chief complaint in 1 in 200 emergency department visits in the United States.1 Additionally, it represents up to one-third of otolaryngology-related emergency room admissions.2 There is no existing literature, to our best knowledge, specifically investigating the incidence of epistaxis after a patient is admitted. Anecdotally, inpatients who develop epistaxis account for an appreciable number of consults to otolaryngology (ENT). Epistaxis is a cross-disciplinary issue, occurring in a range of clinical settings. For example, patients with epistaxis can present to the emergency department or to an outpatient primary care clinic before being referred to ENT. Additionally, inpatients on many different services can develop spontaneous epistaxis due to a variety of environmental and iatrogenic factors, such as dry air, use of nasal cannula, and initiation of anticoagulation. Based on the experience of our ENT providers and discussions with our nursing colleagues, we concluded that there was an interest in epistaxis management training among our nursing workforce.
The presence of unmet demand for epistaxis education among our nursing colleagues was supported by our literature review. A study performed in England surveyed emergency department nurses on first aid measures for management of epistaxis, including ideal head positioning, location of pressure application, and duration of pressure application.3 Overall, only 12% to 14% of the nursing staff answered all 3 questions correctly.3 Additionally, 73% to 78% of the nursing staff felt that their training in epistaxis management was inadequate, and 88% desired further training in epistaxis management.3 If generalized, this study confirms the demand for further epistaxis education among nurses.
In-services have previously been shown to be effective educational tools within the nursing community. A study in Ethiopia that evaluated pain management knowledge and attitudes before and after an in-service found a significant improvement in mean rank score of nurses’ knowledge and attitudes regarding pain management after they participated in the in-service.4 Scores on the knowledge survey improved from 41.4% before the intervention to 63.0% post intervention.4 A study in Connecticut evaluated nurses’ confidence in discussing suicidal ideation with patients and knowledge surrounding suicide precautions.5 After participating in an in-service, nurses were significantly more confident in discussing suicidal ideation with patients; application of appropriate suicide precautions also increased after the in-service.5
Our aim was for nurses to have an improvement in overall epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds after attending our in-service. Additionally, an overarching priority was to provide high-quality epistaxis education based on the literature and best practice guidelines.
Methods
Setting
This study was carried out at an 811-bed quaternary care center located in Chicago, Illinois. In fiscal year 2021, there were 91 643 emergency department visits and 33 805 hospital admissions. At our flagship hospital, 2658 patients were diagnosed with epistaxis during fiscal year 2021. The emergency department saw 533 patients with epistaxis, with 342 requiring admission and 191 being discharged. Separately, 566 inpatients received a diagnosis of epistaxis during their admission. The remainder of the patients with epistaxis were seen on an outpatient basis.
Data Collection
Data were collected from nurses on 5 different inpatient units. An email with information about the in-service was sent to the nurse managers of the inpatient units. These 5 units were included because the nurse managers responded to the email and facilitated delivery of the in-service. Data collection took place from August to December 2020.
Intervention
A quality improvement team composed of a resident physician champion, nurse educators, and nurse managers was formed. The physician champion was a senior otolaryngology resident who was responsible for designing and administering the pre-test, in-service, and post test. The nurse educators and nurse managers helped coordinate times for the in-service and promoted the in-service for their staff.
Our intervention was an educational in-service, a technique that is commonly used at our institution for nurse education. In-services typically involve delivering a lecture on a clinically relevant topic to a group of nurses on a unit. In developing the in-service, a top priority was to present high-quality evidence-based material. There is an abundance of information in the literature surrounding epistaxis management. The clinical practice guideline published by the American Academy of Otolaryngology lists nasal compression, application of vasoconstrictors, nasal packing, and nasal cautery as first-line treatments for the management of epistaxis.6 Nasal packing and nasal cautery tend to be perceived as interventions that require a certain level of expertise and specialized supplies. As such, these interventions are not often performed by floor nurses. In contrast, nasal compression and application of vasoconstrictors require only a few easily accessible supplies, and the risks are relatively minimal. When performing nasal compression, the clinical practice guidelines recommend firm, sustained compression to the lower third of the nose for 5 minutes or longer.6 Topical vasoconstrictors are generally underutilized in epistaxis management. In a study looking at a random sample of all US emergency department visits from 1992 to 2001, only 18% of visits used an epistaxis-related medication.2 Oxymetazoline hydrochloride is a topical vasoconstrictor that is commonly used as a nasal decongestant. However, its vasoconstrictor properties also make it a useful tool for controlling epistaxis. In a study looking at emergency department visits at the University of Texas Health Science Center, 65% of patients had resolution of nosebleed with application of oxymetazoline hydrochloride as the only intervention, with another 18% experiencing resolution of nosebleed with a combination of oxymetazoline hydrochloride and silver nitrate cautery.7 Based on review of the literature, nasal compression and application of vasoconstrictors seemed to be low-resource interventions with minimal morbidity. Therefore, management centered around nasal compression and use of topical vasoconstrictors seemed appropriate for our nursing staff.
The in-service included information about the etiology and management of epistaxis. Particular emphasis was placed on addressing and debunking common misconceptions about nosebleed management. With regards to management, our presentation focused on the use of topical vasoconstrictors and firm pressure to the lower third of the nose for at least 5 minutes. Nasal packing and nasal cautery were presented as procedures that ENT would perform. After the in-service, questions from the nurses were answered as time permitted.
Testing and Outcomes
A pre-test was administered before each in-service. The pre-test components comprised a knowledge survey and a descriptive survey. The general epistaxis knowledge questions on the pre-test included the location of blood vessels most commonly responsible for nosebleeds, the ideal positioning of a patient during a nosebleed, the appropriate location to hold pressure during a nosebleed, and the appropriate duration to hold pressure during a nosebleed. The descriptive survey portion asked nurses to rate whether they felt “very comfortable,” “comfortable,” “uncomfortable,” or “very uncomfortable” managing nosebleeds. It also asked whether nurses thought they would be able to “always,” “usually,” “rarely,” or “never” stop nosebleeds on the floor. We collected demographic information, including gender identity, years of clinical experience, and primary clinical environment.
The post test asked the same questions as the pre-test and was administered immediately after the in-service in order to assess its impact. We also established an ongoing dialogue with our nursing colleagues to obtain feedback on the sessions.
Primary outcomes of interest were the difference in general epistaxis knowledge questions answered correctly between the pre-test and the post test; the difference in comfort level in managing epistaxis before and after the in-service; and the difference in confidence to stop nosebleeds before and after the in-service. A secondary outcome was determining the audience for the in-service. Specifically, we wanted to determine whether there were different outcomes based on clinical setting or years of clinical experience. If nurses in a certain clinical environment or beyond a certain experience level did not show significant improvement from pre-test to post test, we would not target them for the in-service. Another secondary outcome was determining optimal timing for delivery of the in-service. We wanted to determine if there was a nursing preference for delivering the in-service at mid-shift vs shift change.
Analysis
Statistical calculations were performed using Stata 15 (StataCorp LLC). A P value < .05 was considered to be statistically significant. Where applicable, 95% confidence intervals (CI) were calculated. T-test was used to determine whether there was a statistically significant difference between pre-test and post-test epistaxis knowledge question scores. T-test was also used to determine whether there was a statistically significant difference in test scores between nurses receiving the in-service at mid-shift vs shift change. Pearson chi-squared tests were used to determine if there was a statistically significant difference between pre-test and post-test perceptions of epistaxis management, and to investigate outcomes between different subsets of nurses.
SQUIRE 2.0 guidelines were utilized to provide a framework for this project and to structure the manuscript.8 This study met criteria for exemption from institutional review board approval.
Results
Fifty-one nurses took part in this project (Table). The majority of participants identified as female (88.24%), and just over half worked on medical floors (52.94%), with most of the remainder working in intensive care (25.49%) and surgical (15.69%) settings. There was a wide range of clinical experience, with 1.96% reporting 0 to 1 years of experience, 29.41% reporting 2 to 5 years, 23.53% reporting 5 to 10 years, 25.49% reporting 10 to 20 years, and 17.65% reporting more than 20 years.
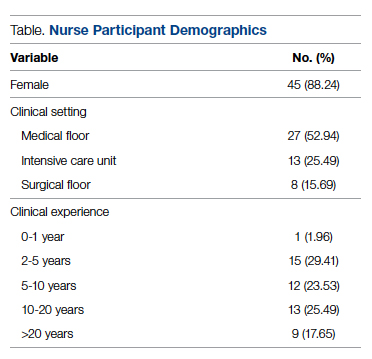
There were unanswered questions on both the pre-test and post test. There was no consistently unanswered question. Omitted answers on the epistaxis knowledge questions were recorded as an “incorrect” answer. Omitted answers on the perception questions were considered null values and not considered in final analysis.
Primary Measures
General epistaxis knowledge (Figure, part A) improved from the pre-test, where out of 4 questions, the mean (SD) score was 1.74 (1.02) correct questions, to the post-test, where out of 4 questions, the mean score was 3.80 (0.40) correct questions. After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (mean difference, 2.07 [1.10]; 95% CI, 1.74-2.39; P < .001), and 80.43% of them got a perfect score on the epistaxis knowledge questions.
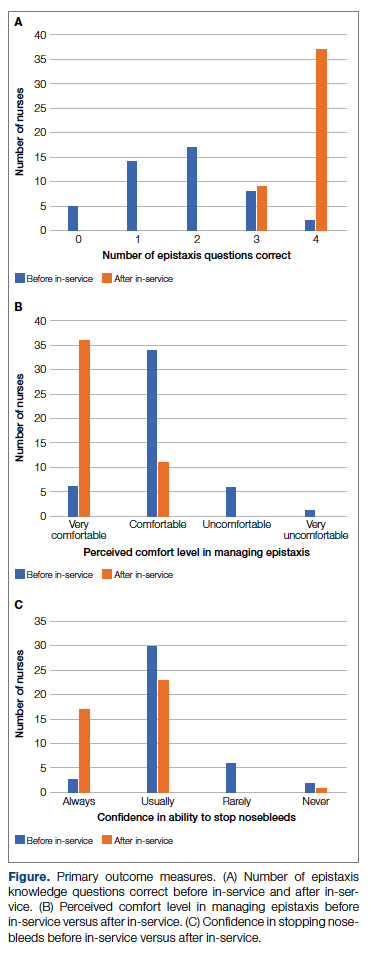
The second primary measure was the difference in comfort level in managing nosebleed. After participating in the in-service, nurses felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), with 74.46% of nurses having an improved comfort level managing nosebleeds. Before the in-service, 12.76% of nurses felt “very comfortable” in managing nosebleeds vs more than three-quarters (76.59%) after the in-service. Of those who answered that they felt “comfortable” managing nosebleeds on the pre-test, 82.35% improved to feeling “very comfortable” in managing nosebleeds. Before the in-service, 14.89% of nurses felt “uncomfortable” or “very uncomfortable” in managing nosebleeds, and this decreased to 0 post intervention. After the in-service, 100.00% of nurses felt “comfortable” or “very comfortable” in managing nosebleeds.
After receiving the in-service, nurses felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001), with 43.90% of them having an improvement in confidence in stopping epistaxis. Before the in-service, 7.31% of nurses felt that they would “always” be able to stop a nose-bleed, and this increased to 41.46% after the in-service. Of those who answered that they felt that they would “usually” be able to stop a nosebleed on the pre-test, 36.67% changed their answer to state that they would “always” be able to stop a nosebleed on the post test. Before the in-service, 19.51% of nurses felt that they would “rarely” or “never” be able to stop a nosebleed, and this decreased to 2.44% after the in-service.
Secondary Measures
All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. However, to determine whether there was a population who would benefit most from the in-service, we stratified the data by years of clinical experience. There was no statistically significant difference in whether nurses with varying clinical experience learned something new (P = .148): 100% of nurses with 0-1 years of experience, 80.00% of nurses with 2-5 years of experience, 100% of nurses with 5-10 years of experience, 69.23% of nurses with 10-20 years of experience, and 100% of nurses with >20 years of experience “strongly agreed” that they learned something new from this in-service. There was no statistically significant difference on the post test compared to the pre-test in additional correct questions when stratified by clinical experience (P = .128). Second, when we stratified by clinical setting, we did not find a statistically significant difference in whether nurses in different clinical settings learned something new (P = .929): 88.89% of nurses in the medical setting, 87.50% of nurses in the surgical setting, and 84.62% of nurses in the intensive care setting “strongly agreed” that they learned something new from this presentation. On investigating additional questions correct on the post test compared to the pre-test, there was no statistically significant difference in additional correct questions when stratified by clinical setting (P = .446).
Optimal timing of the in-service was another important outcome. Initially, the in-service was administered at mid-shift, with 9 nurses participating at mid-shift, but our nursing colleagues gave unanimous feedback that this was a suboptimal time for delivery of an in-service. We changed the timing of the in-service to shift change; 42 nurses received the in-service at shift-change. There was no statistically significant difference in scores on the epistaxis knowledge questions between these two groups (P = .123). This indicated to us that changing the timing of the delivery resulted in similarly improved outcomes while having the added benefit of being preferred by our nursing colleagues.
Discussion
In undertaking this project, our primary aims were to improve epistaxis knowledge and perceived management in our nursing staff. Among our nursing staff, we were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. We also found that nurses of varying clinical experience and different clinical settings benefited equally from our intervention. Using quality improvement principles, we optimized our delivery. Our in-service focused on educating nurses to use epistaxis management techniques that were resource-efficient and low risk.
After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (Figure, part A; mean difference, 2.07 questions [1.10]; 95% CI, 1.74-2.39; P < .001), felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), and felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001). Based on these results, we successfully achieved our primary aims.
Our secondary aim was to determine the audience that would benefit the most from the in-service. All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. There was no statistically significant difference in whether nurses of varying clinical experience learned something new (P = .148) or in additional correct questions when stratified by clinical experience (P =.128). Also, there was no statistically significant difference in whether nurses in different clinical settings learned something new (P = .929) or in additional correct questions when stratified by clinical setting (P = .446). These results indicated to us that all participants learned something new and that there was no specific target audience, but rather that all participants benefitted from our session.
Our nursing colleagues gave us feedback that the timing of the in-service during mid-shift was not ideal. It was difficult to gather nurses mid-shift due to pressing patient-care duties. Nurses also found it difficult to give their full attention at this time. Nurses, nurse educators, and nurse managers suggested that we conduct the in-service at shift change in order to capture a larger population and take advantage of time relatively free of clinical duties. Giving the in-service at a time with relatively fewer clinical responsibilities allowed for a more robust question-and-answer session. It also allowed our nursing colleagues to pay full attention to the in-service. There was no statistically significant difference in epistaxis general knowledge questions answered correctly; this indicates that the quality of the education session did not vary greatly. However, our nursing colleagues strongly preferred the in-service at shift change. By making this modification to our intervention, we were able to optimize our intervention.
The previously mentioned study in England reported that only 12% to 14% of their nursing staff got a perfect score on epistaxis knowledge questions. Prior to our study, there was no literature investigating the impact of an in-service on epistaxis knowledge. After our intervention, 80.43% of our nurses got a perfect score on the epistaxis knowledge questions. We believe that this is a fair comparison because our post-test questions were identical to the survey questions used in the previously mentioned study in England, with the addition of one question.3 Further, the findings of our study are consistent with other studies regarding the positive effect of in-service education on knowledge and attitudes surrounding clinical topics. Similar to the study in Ethiopia investigating nurses’ knowledge surrounding pain management, our study noted a significant improvement in nurses’ knowledge after participating in the in-service.4 Also, when comparing our study to the study performed in Connecticut investigating nurses’ confidence surrounding suicide precautions, we found a similar significant improvement in confidence in management after participating in the in-service.5
Given our reliance on a survey as a tool to collect information, our study was subject to nonresponse bias. For each main outcome question, there was a handful of nonresponders. While this likely indicated either overlooking a question or deferring to answer due to clinical inexperience or nonapplicable clinical role, it is possible that this may have represented a respondent who did not benefit from the in-service. Another source of possible bias is sampling bias. Attempts were made to capture a wide range of nurses at the in-service. However, if a nurse was not interested in the topic material, whether due to abundant clinical experience or disinterest, it is possible that they may not have attended. Additionally, the cohort was selected purely based on responses from nursing managers to the initial email. It is possible that nonresponding units may have benefitted differently from this in-service.
There were several limitations within our analysis. We did not collect data assessing the long-term retention of epistaxis knowledge and management techniques. It is possible that epistaxis knowledge, comfort in managing nosebleeds, and perceived confidence in stopping nosebleeds decreased back to baseline several months after the in-service. Ideally, we would have been able to collect this data to assess retention of the in-service information. Unfortunately, a significant number of nurses who initially participated in the project became lost to follow-up, making such data collection impossible. Additionally, there was no assessment of actual ability to stop nosebleeds before vs after this in-service. Perceived management of epistaxis vs actual management of epistaxis are 2 vastly different things. However, this data would have been difficult to collect, and it likely would not have been in the best interest of patients, especially before the in-service was administered. As an improvement to this project, we could have assessed how many nosebleeds nurses had seen and successfully stopped after the in-service. As previously mentioned, this was not possible due to losing a significant number of nurses to follow-up. Finally, we did not collect objective data on preference for administration of in-service at mid-shift vs shift change. We relied on subjective data from conversations with our colleagues. By collecting objective data, we could have supported this change to our intervention with data.
The primary challenge to sustainability for this intervention is nursing turnover. With each wave of departing nurses and new nursing hires, the difficulty of ensuring a consistent knowledge base and management standards within our nursing workforce became clearer. After optimizing our intervention, our solution was to provide a hospital-wide in-service, which was recorded and uploaded to an institution-wide in-service library. In this way, a nurse with the desire to learn about epistaxis management could access the material at any point in time. Another solution would have been to appoint champions for epistaxis management within each major department to deliver the epistaxis in-service to new hires and new rotators within the department. However, given the turnover witnessed in our study cohort, this may not be sustainable long term.
Conclusion
Epistaxis is a chief complaint that can present in many different clinical settings and situations. Therefore, the ability to stop epistaxis in a timely and effective fashion is valuable. Our study demonstrated that in-services can improve epistaxis knowledge and improve perceived epistaxis management. Ideally, this intervention will lead to improved patient care. Given that epistaxis is a ubiquitous issue, this study may benefit other institutions who want to improve care for patients with epistaxis.
Next steps for this intervention include utilizing in-services for epistaxis education at other institutions and collecting long-term data within our own institution. Collecting long-term data would allow us to assess the retention of epistaxis knowledge from our in-service.
Acknowledgments: The author thanks the nurse managers, nurse educators, and staff nurses involved in this project, as well as Dr. Louis Portugal for providing mentorship throughout this process and Dr. Dara Adams for assisting with statistical analysis.
Corresponding author: Avery Nelson, MD, University of Chicago Medical Center, 5841 S Maryland Ave, MC 1035, Chicago, IL 60637; [email protected]
Disclosures: None reported.
1. Pallin DJ, Chng Y-M, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46(1):77-81. doi:10.1016/j.annemergmed.2004.12.014
2. Walker TWM, Macfarlane TV, McGarry GW. The epidemiology and chronobiology of epistaxis: An investigation of Scottish hospital admissions 1995-2004. Clin Otolaryngol. 2007;32(5):361-365. doi:10.1111/j.1749-4486.2007.01530.x
3. Hakim N, Mummadi SM, Jolly K, et al. Nurse-led epistaxis management within the emergency department. Br J Nurs. 2018;27(1):41-46. doi:10.12968/bjon.2018.27.1.41
4. Germossa GN, Sjetne IS, Hellesø R. The impact of an in-service educational program on nurses’ knowledge and attitudes regarding pain management in an Ethiopian University Hospital. Front Public Health. 2018;6:229. doi:10.3389/fpubh.2018.00229
5. Manister NN, Murray S, Burke JM, Finegan M, McKiernan ME. Effectiveness of nursing education to prevent inpatient suicide. J Contin Educ Nurs. 2017;48(9):413-419. doi:10.3928/00220124-20170816-07
6. Tunkel DE, Anne S, Payne SC, et al. Clinical practice guideline: nosebleed (epistaxis) executive summary. Otolaryngol Head Neck Surg. 2020;162(1):S1-S38. doi:10.1177/0194599819890327
7. Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol. 1995;104(9 Part 1):704-706. doi:10.1177/000348949510400906
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0—standards for quality improvement reporting excellence—revised publication guidelines from a detailed consensus process. J Am Coll Surg. 2016;222(3):317-323. doi:10.1016/j.jamcollsurg.2015.07.456
From the University of Chicago Medical Center, Chicago, IL.
Abstract
Background: Epistaxis is a common chief complaint addressed by otolaryngologists. A review of the literature showed that there is a deficit in epistaxis education within the nursing community. Conversations with our nursing colleagues confirmed this unmet demand.
Objective: This quality improvement project aimed to increase general epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds among our nursing staff.
Methods: Data were collected through a survey administered before and after our intervention. The survey tested general epistaxis knowledge and assessed comfort and confidence in stopping epistaxis. Our intervention was an educational session covering pertinent epistaxis etiology and management. Quality improvement principles were used to optimize delivery of the intervention.
Results: A total of 51 nurses participated in the project. After participating in the in-service educational session, nurses answered significantly more epistaxis general knowledge questions correctly (mean [SD] difference, 2.07 [1.10] questions; 95% CI, 1.74-2.39; P < .001). There was no statistically significant difference in additional correct questions when stratified by clinical experience or clinical setting (P = .128 and P = 0.446, respectively). Nurses also reported feeling significantly more comfortable and significantly more confident in managing nosebleeds after the in-service (P = .007 and P < 0.001, respectively); 74.46% of nurses had an improvement in comfort level in managing epistaxis and 43.90% of nurses had an improvement in confidence in stopping epistaxis. After we moved the educational session from mid-shift to shift change, the nursing staff reported more satisfaction while maintaining similar improvements in knowledge and confidence.
Conclusion: We were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. Nurses of varying clinical experience and different clinical settings benefitted equally from our intervention.
Keywords: nosebleed; in-service; quality improvement.
Epistaxis, or nosebleed, is estimated to be the chief complaint in 1 in 200 emergency department visits in the United States.1 Additionally, it represents up to one-third of otolaryngology-related emergency room admissions.2 There is no existing literature, to our best knowledge, specifically investigating the incidence of epistaxis after a patient is admitted. Anecdotally, inpatients who develop epistaxis account for an appreciable number of consults to otolaryngology (ENT). Epistaxis is a cross-disciplinary issue, occurring in a range of clinical settings. For example, patients with epistaxis can present to the emergency department or to an outpatient primary care clinic before being referred to ENT. Additionally, inpatients on many different services can develop spontaneous epistaxis due to a variety of environmental and iatrogenic factors, such as dry air, use of nasal cannula, and initiation of anticoagulation. Based on the experience of our ENT providers and discussions with our nursing colleagues, we concluded that there was an interest in epistaxis management training among our nursing workforce.
The presence of unmet demand for epistaxis education among our nursing colleagues was supported by our literature review. A study performed in England surveyed emergency department nurses on first aid measures for management of epistaxis, including ideal head positioning, location of pressure application, and duration of pressure application.3 Overall, only 12% to 14% of the nursing staff answered all 3 questions correctly.3 Additionally, 73% to 78% of the nursing staff felt that their training in epistaxis management was inadequate, and 88% desired further training in epistaxis management.3 If generalized, this study confirms the demand for further epistaxis education among nurses.
In-services have previously been shown to be effective educational tools within the nursing community. A study in Ethiopia that evaluated pain management knowledge and attitudes before and after an in-service found a significant improvement in mean rank score of nurses’ knowledge and attitudes regarding pain management after they participated in the in-service.4 Scores on the knowledge survey improved from 41.4% before the intervention to 63.0% post intervention.4 A study in Connecticut evaluated nurses’ confidence in discussing suicidal ideation with patients and knowledge surrounding suicide precautions.5 After participating in an in-service, nurses were significantly more confident in discussing suicidal ideation with patients; application of appropriate suicide precautions also increased after the in-service.5
Our aim was for nurses to have an improvement in overall epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds after attending our in-service. Additionally, an overarching priority was to provide high-quality epistaxis education based on the literature and best practice guidelines.
Methods
Setting
This study was carried out at an 811-bed quaternary care center located in Chicago, Illinois. In fiscal year 2021, there were 91 643 emergency department visits and 33 805 hospital admissions. At our flagship hospital, 2658 patients were diagnosed with epistaxis during fiscal year 2021. The emergency department saw 533 patients with epistaxis, with 342 requiring admission and 191 being discharged. Separately, 566 inpatients received a diagnosis of epistaxis during their admission. The remainder of the patients with epistaxis were seen on an outpatient basis.
Data Collection
Data were collected from nurses on 5 different inpatient units. An email with information about the in-service was sent to the nurse managers of the inpatient units. These 5 units were included because the nurse managers responded to the email and facilitated delivery of the in-service. Data collection took place from August to December 2020.
Intervention
A quality improvement team composed of a resident physician champion, nurse educators, and nurse managers was formed. The physician champion was a senior otolaryngology resident who was responsible for designing and administering the pre-test, in-service, and post test. The nurse educators and nurse managers helped coordinate times for the in-service and promoted the in-service for their staff.
Our intervention was an educational in-service, a technique that is commonly used at our institution for nurse education. In-services typically involve delivering a lecture on a clinically relevant topic to a group of nurses on a unit. In developing the in-service, a top priority was to present high-quality evidence-based material. There is an abundance of information in the literature surrounding epistaxis management. The clinical practice guideline published by the American Academy of Otolaryngology lists nasal compression, application of vasoconstrictors, nasal packing, and nasal cautery as first-line treatments for the management of epistaxis.6 Nasal packing and nasal cautery tend to be perceived as interventions that require a certain level of expertise and specialized supplies. As such, these interventions are not often performed by floor nurses. In contrast, nasal compression and application of vasoconstrictors require only a few easily accessible supplies, and the risks are relatively minimal. When performing nasal compression, the clinical practice guidelines recommend firm, sustained compression to the lower third of the nose for 5 minutes or longer.6 Topical vasoconstrictors are generally underutilized in epistaxis management. In a study looking at a random sample of all US emergency department visits from 1992 to 2001, only 18% of visits used an epistaxis-related medication.2 Oxymetazoline hydrochloride is a topical vasoconstrictor that is commonly used as a nasal decongestant. However, its vasoconstrictor properties also make it a useful tool for controlling epistaxis. In a study looking at emergency department visits at the University of Texas Health Science Center, 65% of patients had resolution of nosebleed with application of oxymetazoline hydrochloride as the only intervention, with another 18% experiencing resolution of nosebleed with a combination of oxymetazoline hydrochloride and silver nitrate cautery.7 Based on review of the literature, nasal compression and application of vasoconstrictors seemed to be low-resource interventions with minimal morbidity. Therefore, management centered around nasal compression and use of topical vasoconstrictors seemed appropriate for our nursing staff.
The in-service included information about the etiology and management of epistaxis. Particular emphasis was placed on addressing and debunking common misconceptions about nosebleed management. With regards to management, our presentation focused on the use of topical vasoconstrictors and firm pressure to the lower third of the nose for at least 5 minutes. Nasal packing and nasal cautery were presented as procedures that ENT would perform. After the in-service, questions from the nurses were answered as time permitted.
Testing and Outcomes
A pre-test was administered before each in-service. The pre-test components comprised a knowledge survey and a descriptive survey. The general epistaxis knowledge questions on the pre-test included the location of blood vessels most commonly responsible for nosebleeds, the ideal positioning of a patient during a nosebleed, the appropriate location to hold pressure during a nosebleed, and the appropriate duration to hold pressure during a nosebleed. The descriptive survey portion asked nurses to rate whether they felt “very comfortable,” “comfortable,” “uncomfortable,” or “very uncomfortable” managing nosebleeds. It also asked whether nurses thought they would be able to “always,” “usually,” “rarely,” or “never” stop nosebleeds on the floor. We collected demographic information, including gender identity, years of clinical experience, and primary clinical environment.
The post test asked the same questions as the pre-test and was administered immediately after the in-service in order to assess its impact. We also established an ongoing dialogue with our nursing colleagues to obtain feedback on the sessions.
Primary outcomes of interest were the difference in general epistaxis knowledge questions answered correctly between the pre-test and the post test; the difference in comfort level in managing epistaxis before and after the in-service; and the difference in confidence to stop nosebleeds before and after the in-service. A secondary outcome was determining the audience for the in-service. Specifically, we wanted to determine whether there were different outcomes based on clinical setting or years of clinical experience. If nurses in a certain clinical environment or beyond a certain experience level did not show significant improvement from pre-test to post test, we would not target them for the in-service. Another secondary outcome was determining optimal timing for delivery of the in-service. We wanted to determine if there was a nursing preference for delivering the in-service at mid-shift vs shift change.
Analysis
Statistical calculations were performed using Stata 15 (StataCorp LLC). A P value < .05 was considered to be statistically significant. Where applicable, 95% confidence intervals (CI) were calculated. T-test was used to determine whether there was a statistically significant difference between pre-test and post-test epistaxis knowledge question scores. T-test was also used to determine whether there was a statistically significant difference in test scores between nurses receiving the in-service at mid-shift vs shift change. Pearson chi-squared tests were used to determine if there was a statistically significant difference between pre-test and post-test perceptions of epistaxis management, and to investigate outcomes between different subsets of nurses.
SQUIRE 2.0 guidelines were utilized to provide a framework for this project and to structure the manuscript.8 This study met criteria for exemption from institutional review board approval.
Results
Fifty-one nurses took part in this project (Table). The majority of participants identified as female (88.24%), and just over half worked on medical floors (52.94%), with most of the remainder working in intensive care (25.49%) and surgical (15.69%) settings. There was a wide range of clinical experience, with 1.96% reporting 0 to 1 years of experience, 29.41% reporting 2 to 5 years, 23.53% reporting 5 to 10 years, 25.49% reporting 10 to 20 years, and 17.65% reporting more than 20 years.

There were unanswered questions on both the pre-test and post test. There was no consistently unanswered question. Omitted answers on the epistaxis knowledge questions were recorded as an “incorrect” answer. Omitted answers on the perception questions were considered null values and not considered in final analysis.
Primary Measures
General epistaxis knowledge (Figure, part A) improved from the pre-test, where out of 4 questions, the mean (SD) score was 1.74 (1.02) correct questions, to the post-test, where out of 4 questions, the mean score was 3.80 (0.40) correct questions. After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (mean difference, 2.07 [1.10]; 95% CI, 1.74-2.39; P < .001), and 80.43% of them got a perfect score on the epistaxis knowledge questions.

The second primary measure was the difference in comfort level in managing nosebleed. After participating in the in-service, nurses felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), with 74.46% of nurses having an improved comfort level managing nosebleeds. Before the in-service, 12.76% of nurses felt “very comfortable” in managing nosebleeds vs more than three-quarters (76.59%) after the in-service. Of those who answered that they felt “comfortable” managing nosebleeds on the pre-test, 82.35% improved to feeling “very comfortable” in managing nosebleeds. Before the in-service, 14.89% of nurses felt “uncomfortable” or “very uncomfortable” in managing nosebleeds, and this decreased to 0 post intervention. After the in-service, 100.00% of nurses felt “comfortable” or “very comfortable” in managing nosebleeds.
After receiving the in-service, nurses felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001), with 43.90% of them having an improvement in confidence in stopping epistaxis. Before the in-service, 7.31% of nurses felt that they would “always” be able to stop a nose-bleed, and this increased to 41.46% after the in-service. Of those who answered that they felt that they would “usually” be able to stop a nosebleed on the pre-test, 36.67% changed their answer to state that they would “always” be able to stop a nosebleed on the post test. Before the in-service, 19.51% of nurses felt that they would “rarely” or “never” be able to stop a nosebleed, and this decreased to 2.44% after the in-service.
Secondary Measures
All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. However, to determine whether there was a population who would benefit most from the in-service, we stratified the data by years of clinical experience. There was no statistically significant difference in whether nurses with varying clinical experience learned something new (P = .148): 100% of nurses with 0-1 years of experience, 80.00% of nurses with 2-5 years of experience, 100% of nurses with 5-10 years of experience, 69.23% of nurses with 10-20 years of experience, and 100% of nurses with >20 years of experience “strongly agreed” that they learned something new from this in-service. There was no statistically significant difference on the post test compared to the pre-test in additional correct questions when stratified by clinical experience (P = .128). Second, when we stratified by clinical setting, we did not find a statistically significant difference in whether nurses in different clinical settings learned something new (P = .929): 88.89% of nurses in the medical setting, 87.50% of nurses in the surgical setting, and 84.62% of nurses in the intensive care setting “strongly agreed” that they learned something new from this presentation. On investigating additional questions correct on the post test compared to the pre-test, there was no statistically significant difference in additional correct questions when stratified by clinical setting (P = .446).
Optimal timing of the in-service was another important outcome. Initially, the in-service was administered at mid-shift, with 9 nurses participating at mid-shift, but our nursing colleagues gave unanimous feedback that this was a suboptimal time for delivery of an in-service. We changed the timing of the in-service to shift change; 42 nurses received the in-service at shift-change. There was no statistically significant difference in scores on the epistaxis knowledge questions between these two groups (P = .123). This indicated to us that changing the timing of the delivery resulted in similarly improved outcomes while having the added benefit of being preferred by our nursing colleagues.
Discussion
In undertaking this project, our primary aims were to improve epistaxis knowledge and perceived management in our nursing staff. Among our nursing staff, we were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. We also found that nurses of varying clinical experience and different clinical settings benefited equally from our intervention. Using quality improvement principles, we optimized our delivery. Our in-service focused on educating nurses to use epistaxis management techniques that were resource-efficient and low risk.
After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (Figure, part A; mean difference, 2.07 questions [1.10]; 95% CI, 1.74-2.39; P < .001), felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), and felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001). Based on these results, we successfully achieved our primary aims.
Our secondary aim was to determine the audience that would benefit the most from the in-service. All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. There was no statistically significant difference in whether nurses of varying clinical experience learned something new (P = .148) or in additional correct questions when stratified by clinical experience (P =.128). Also, there was no statistically significant difference in whether nurses in different clinical settings learned something new (P = .929) or in additional correct questions when stratified by clinical setting (P = .446). These results indicated to us that all participants learned something new and that there was no specific target audience, but rather that all participants benefitted from our session.
Our nursing colleagues gave us feedback that the timing of the in-service during mid-shift was not ideal. It was difficult to gather nurses mid-shift due to pressing patient-care duties. Nurses also found it difficult to give their full attention at this time. Nurses, nurse educators, and nurse managers suggested that we conduct the in-service at shift change in order to capture a larger population and take advantage of time relatively free of clinical duties. Giving the in-service at a time with relatively fewer clinical responsibilities allowed for a more robust question-and-answer session. It also allowed our nursing colleagues to pay full attention to the in-service. There was no statistically significant difference in epistaxis general knowledge questions answered correctly; this indicates that the quality of the education session did not vary greatly. However, our nursing colleagues strongly preferred the in-service at shift change. By making this modification to our intervention, we were able to optimize our intervention.
The previously mentioned study in England reported that only 12% to 14% of their nursing staff got a perfect score on epistaxis knowledge questions. Prior to our study, there was no literature investigating the impact of an in-service on epistaxis knowledge. After our intervention, 80.43% of our nurses got a perfect score on the epistaxis knowledge questions. We believe that this is a fair comparison because our post-test questions were identical to the survey questions used in the previously mentioned study in England, with the addition of one question.3 Further, the findings of our study are consistent with other studies regarding the positive effect of in-service education on knowledge and attitudes surrounding clinical topics. Similar to the study in Ethiopia investigating nurses’ knowledge surrounding pain management, our study noted a significant improvement in nurses’ knowledge after participating in the in-service.4 Also, when comparing our study to the study performed in Connecticut investigating nurses’ confidence surrounding suicide precautions, we found a similar significant improvement in confidence in management after participating in the in-service.5
Given our reliance on a survey as a tool to collect information, our study was subject to nonresponse bias. For each main outcome question, there was a handful of nonresponders. While this likely indicated either overlooking a question or deferring to answer due to clinical inexperience or nonapplicable clinical role, it is possible that this may have represented a respondent who did not benefit from the in-service. Another source of possible bias is sampling bias. Attempts were made to capture a wide range of nurses at the in-service. However, if a nurse was not interested in the topic material, whether due to abundant clinical experience or disinterest, it is possible that they may not have attended. Additionally, the cohort was selected purely based on responses from nursing managers to the initial email. It is possible that nonresponding units may have benefitted differently from this in-service.
There were several limitations within our analysis. We did not collect data assessing the long-term retention of epistaxis knowledge and management techniques. It is possible that epistaxis knowledge, comfort in managing nosebleeds, and perceived confidence in stopping nosebleeds decreased back to baseline several months after the in-service. Ideally, we would have been able to collect this data to assess retention of the in-service information. Unfortunately, a significant number of nurses who initially participated in the project became lost to follow-up, making such data collection impossible. Additionally, there was no assessment of actual ability to stop nosebleeds before vs after this in-service. Perceived management of epistaxis vs actual management of epistaxis are 2 vastly different things. However, this data would have been difficult to collect, and it likely would not have been in the best interest of patients, especially before the in-service was administered. As an improvement to this project, we could have assessed how many nosebleeds nurses had seen and successfully stopped after the in-service. As previously mentioned, this was not possible due to losing a significant number of nurses to follow-up. Finally, we did not collect objective data on preference for administration of in-service at mid-shift vs shift change. We relied on subjective data from conversations with our colleagues. By collecting objective data, we could have supported this change to our intervention with data.
The primary challenge to sustainability for this intervention is nursing turnover. With each wave of departing nurses and new nursing hires, the difficulty of ensuring a consistent knowledge base and management standards within our nursing workforce became clearer. After optimizing our intervention, our solution was to provide a hospital-wide in-service, which was recorded and uploaded to an institution-wide in-service library. In this way, a nurse with the desire to learn about epistaxis management could access the material at any point in time. Another solution would have been to appoint champions for epistaxis management within each major department to deliver the epistaxis in-service to new hires and new rotators within the department. However, given the turnover witnessed in our study cohort, this may not be sustainable long term.
Conclusion
Epistaxis is a chief complaint that can present in many different clinical settings and situations. Therefore, the ability to stop epistaxis in a timely and effective fashion is valuable. Our study demonstrated that in-services can improve epistaxis knowledge and improve perceived epistaxis management. Ideally, this intervention will lead to improved patient care. Given that epistaxis is a ubiquitous issue, this study may benefit other institutions who want to improve care for patients with epistaxis.
Next steps for this intervention include utilizing in-services for epistaxis education at other institutions and collecting long-term data within our own institution. Collecting long-term data would allow us to assess the retention of epistaxis knowledge from our in-service.
Acknowledgments: The author thanks the nurse managers, nurse educators, and staff nurses involved in this project, as well as Dr. Louis Portugal for providing mentorship throughout this process and Dr. Dara Adams for assisting with statistical analysis.
Corresponding author: Avery Nelson, MD, University of Chicago Medical Center, 5841 S Maryland Ave, MC 1035, Chicago, IL 60637; [email protected]
Disclosures: None reported.
From the University of Chicago Medical Center, Chicago, IL.
Abstract
Background: Epistaxis is a common chief complaint addressed by otolaryngologists. A review of the literature showed that there is a deficit in epistaxis education within the nursing community. Conversations with our nursing colleagues confirmed this unmet demand.
Objective: This quality improvement project aimed to increase general epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds among our nursing staff.
Methods: Data were collected through a survey administered before and after our intervention. The survey tested general epistaxis knowledge and assessed comfort and confidence in stopping epistaxis. Our intervention was an educational session covering pertinent epistaxis etiology and management. Quality improvement principles were used to optimize delivery of the intervention.
Results: A total of 51 nurses participated in the project. After participating in the in-service educational session, nurses answered significantly more epistaxis general knowledge questions correctly (mean [SD] difference, 2.07 [1.10] questions; 95% CI, 1.74-2.39; P < .001). There was no statistically significant difference in additional correct questions when stratified by clinical experience or clinical setting (P = .128 and P = 0.446, respectively). Nurses also reported feeling significantly more comfortable and significantly more confident in managing nosebleeds after the in-service (P = .007 and P < 0.001, respectively); 74.46% of nurses had an improvement in comfort level in managing epistaxis and 43.90% of nurses had an improvement in confidence in stopping epistaxis. After we moved the educational session from mid-shift to shift change, the nursing staff reported more satisfaction while maintaining similar improvements in knowledge and confidence.
Conclusion: We were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. Nurses of varying clinical experience and different clinical settings benefitted equally from our intervention.
Keywords: nosebleed; in-service; quality improvement.
Epistaxis, or nosebleed, is estimated to be the chief complaint in 1 in 200 emergency department visits in the United States.1 Additionally, it represents up to one-third of otolaryngology-related emergency room admissions.2 There is no existing literature, to our best knowledge, specifically investigating the incidence of epistaxis after a patient is admitted. Anecdotally, inpatients who develop epistaxis account for an appreciable number of consults to otolaryngology (ENT). Epistaxis is a cross-disciplinary issue, occurring in a range of clinical settings. For example, patients with epistaxis can present to the emergency department or to an outpatient primary care clinic before being referred to ENT. Additionally, inpatients on many different services can develop spontaneous epistaxis due to a variety of environmental and iatrogenic factors, such as dry air, use of nasal cannula, and initiation of anticoagulation. Based on the experience of our ENT providers and discussions with our nursing colleagues, we concluded that there was an interest in epistaxis management training among our nursing workforce.
The presence of unmet demand for epistaxis education among our nursing colleagues was supported by our literature review. A study performed in England surveyed emergency department nurses on first aid measures for management of epistaxis, including ideal head positioning, location of pressure application, and duration of pressure application.3 Overall, only 12% to 14% of the nursing staff answered all 3 questions correctly.3 Additionally, 73% to 78% of the nursing staff felt that their training in epistaxis management was inadequate, and 88% desired further training in epistaxis management.3 If generalized, this study confirms the demand for further epistaxis education among nurses.
In-services have previously been shown to be effective educational tools within the nursing community. A study in Ethiopia that evaluated pain management knowledge and attitudes before and after an in-service found a significant improvement in mean rank score of nurses’ knowledge and attitudes regarding pain management after they participated in the in-service.4 Scores on the knowledge survey improved from 41.4% before the intervention to 63.0% post intervention.4 A study in Connecticut evaluated nurses’ confidence in discussing suicidal ideation with patients and knowledge surrounding suicide precautions.5 After participating in an in-service, nurses were significantly more confident in discussing suicidal ideation with patients; application of appropriate suicide precautions also increased after the in-service.5
Our aim was for nurses to have an improvement in overall epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds after attending our in-service. Additionally, an overarching priority was to provide high-quality epistaxis education based on the literature and best practice guidelines.
Methods
Setting
This study was carried out at an 811-bed quaternary care center located in Chicago, Illinois. In fiscal year 2021, there were 91 643 emergency department visits and 33 805 hospital admissions. At our flagship hospital, 2658 patients were diagnosed with epistaxis during fiscal year 2021. The emergency department saw 533 patients with epistaxis, with 342 requiring admission and 191 being discharged. Separately, 566 inpatients received a diagnosis of epistaxis during their admission. The remainder of the patients with epistaxis were seen on an outpatient basis.
Data Collection
Data were collected from nurses on 5 different inpatient units. An email with information about the in-service was sent to the nurse managers of the inpatient units. These 5 units were included because the nurse managers responded to the email and facilitated delivery of the in-service. Data collection took place from August to December 2020.
Intervention
A quality improvement team composed of a resident physician champion, nurse educators, and nurse managers was formed. The physician champion was a senior otolaryngology resident who was responsible for designing and administering the pre-test, in-service, and post test. The nurse educators and nurse managers helped coordinate times for the in-service and promoted the in-service for their staff.
Our intervention was an educational in-service, a technique that is commonly used at our institution for nurse education. In-services typically involve delivering a lecture on a clinically relevant topic to a group of nurses on a unit. In developing the in-service, a top priority was to present high-quality evidence-based material. There is an abundance of information in the literature surrounding epistaxis management. The clinical practice guideline published by the American Academy of Otolaryngology lists nasal compression, application of vasoconstrictors, nasal packing, and nasal cautery as first-line treatments for the management of epistaxis.6 Nasal packing and nasal cautery tend to be perceived as interventions that require a certain level of expertise and specialized supplies. As such, these interventions are not often performed by floor nurses. In contrast, nasal compression and application of vasoconstrictors require only a few easily accessible supplies, and the risks are relatively minimal. When performing nasal compression, the clinical practice guidelines recommend firm, sustained compression to the lower third of the nose for 5 minutes or longer.6 Topical vasoconstrictors are generally underutilized in epistaxis management. In a study looking at a random sample of all US emergency department visits from 1992 to 2001, only 18% of visits used an epistaxis-related medication.2 Oxymetazoline hydrochloride is a topical vasoconstrictor that is commonly used as a nasal decongestant. However, its vasoconstrictor properties also make it a useful tool for controlling epistaxis. In a study looking at emergency department visits at the University of Texas Health Science Center, 65% of patients had resolution of nosebleed with application of oxymetazoline hydrochloride as the only intervention, with another 18% experiencing resolution of nosebleed with a combination of oxymetazoline hydrochloride and silver nitrate cautery.7 Based on review of the literature, nasal compression and application of vasoconstrictors seemed to be low-resource interventions with minimal morbidity. Therefore, management centered around nasal compression and use of topical vasoconstrictors seemed appropriate for our nursing staff.
The in-service included information about the etiology and management of epistaxis. Particular emphasis was placed on addressing and debunking common misconceptions about nosebleed management. With regards to management, our presentation focused on the use of topical vasoconstrictors and firm pressure to the lower third of the nose for at least 5 minutes. Nasal packing and nasal cautery were presented as procedures that ENT would perform. After the in-service, questions from the nurses were answered as time permitted.
Testing and Outcomes
A pre-test was administered before each in-service. The pre-test components comprised a knowledge survey and a descriptive survey. The general epistaxis knowledge questions on the pre-test included the location of blood vessels most commonly responsible for nosebleeds, the ideal positioning of a patient during a nosebleed, the appropriate location to hold pressure during a nosebleed, and the appropriate duration to hold pressure during a nosebleed. The descriptive survey portion asked nurses to rate whether they felt “very comfortable,” “comfortable,” “uncomfortable,” or “very uncomfortable” managing nosebleeds. It also asked whether nurses thought they would be able to “always,” “usually,” “rarely,” or “never” stop nosebleeds on the floor. We collected demographic information, including gender identity, years of clinical experience, and primary clinical environment.
The post test asked the same questions as the pre-test and was administered immediately after the in-service in order to assess its impact. We also established an ongoing dialogue with our nursing colleagues to obtain feedback on the sessions.
Primary outcomes of interest were the difference in general epistaxis knowledge questions answered correctly between the pre-test and the post test; the difference in comfort level in managing epistaxis before and after the in-service; and the difference in confidence to stop nosebleeds before and after the in-service. A secondary outcome was determining the audience for the in-service. Specifically, we wanted to determine whether there were different outcomes based on clinical setting or years of clinical experience. If nurses in a certain clinical environment or beyond a certain experience level did not show significant improvement from pre-test to post test, we would not target them for the in-service. Another secondary outcome was determining optimal timing for delivery of the in-service. We wanted to determine if there was a nursing preference for delivering the in-service at mid-shift vs shift change.
Analysis
Statistical calculations were performed using Stata 15 (StataCorp LLC). A P value < .05 was considered to be statistically significant. Where applicable, 95% confidence intervals (CI) were calculated. T-test was used to determine whether there was a statistically significant difference between pre-test and post-test epistaxis knowledge question scores. T-test was also used to determine whether there was a statistically significant difference in test scores between nurses receiving the in-service at mid-shift vs shift change. Pearson chi-squared tests were used to determine if there was a statistically significant difference between pre-test and post-test perceptions of epistaxis management, and to investigate outcomes between different subsets of nurses.
SQUIRE 2.0 guidelines were utilized to provide a framework for this project and to structure the manuscript.8 This study met criteria for exemption from institutional review board approval.
Results
Fifty-one nurses took part in this project (Table). The majority of participants identified as female (88.24%), and just over half worked on medical floors (52.94%), with most of the remainder working in intensive care (25.49%) and surgical (15.69%) settings. There was a wide range of clinical experience, with 1.96% reporting 0 to 1 years of experience, 29.41% reporting 2 to 5 years, 23.53% reporting 5 to 10 years, 25.49% reporting 10 to 20 years, and 17.65% reporting more than 20 years.

There were unanswered questions on both the pre-test and post test. There was no consistently unanswered question. Omitted answers on the epistaxis knowledge questions were recorded as an “incorrect” answer. Omitted answers on the perception questions were considered null values and not considered in final analysis.
Primary Measures
General epistaxis knowledge (Figure, part A) improved from the pre-test, where out of 4 questions, the mean (SD) score was 1.74 (1.02) correct questions, to the post-test, where out of 4 questions, the mean score was 3.80 (0.40) correct questions. After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (mean difference, 2.07 [1.10]; 95% CI, 1.74-2.39; P < .001), and 80.43% of them got a perfect score on the epistaxis knowledge questions.

The second primary measure was the difference in comfort level in managing nosebleed. After participating in the in-service, nurses felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), with 74.46% of nurses having an improved comfort level managing nosebleeds. Before the in-service, 12.76% of nurses felt “very comfortable” in managing nosebleeds vs more than three-quarters (76.59%) after the in-service. Of those who answered that they felt “comfortable” managing nosebleeds on the pre-test, 82.35% improved to feeling “very comfortable” in managing nosebleeds. Before the in-service, 14.89% of nurses felt “uncomfortable” or “very uncomfortable” in managing nosebleeds, and this decreased to 0 post intervention. After the in-service, 100.00% of nurses felt “comfortable” or “very comfortable” in managing nosebleeds.
After receiving the in-service, nurses felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001), with 43.90% of them having an improvement in confidence in stopping epistaxis. Before the in-service, 7.31% of nurses felt that they would “always” be able to stop a nose-bleed, and this increased to 41.46% after the in-service. Of those who answered that they felt that they would “usually” be able to stop a nosebleed on the pre-test, 36.67% changed their answer to state that they would “always” be able to stop a nosebleed on the post test. Before the in-service, 19.51% of nurses felt that they would “rarely” or “never” be able to stop a nosebleed, and this decreased to 2.44% after the in-service.
Secondary Measures
All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. However, to determine whether there was a population who would benefit most from the in-service, we stratified the data by years of clinical experience. There was no statistically significant difference in whether nurses with varying clinical experience learned something new (P = .148): 100% of nurses with 0-1 years of experience, 80.00% of nurses with 2-5 years of experience, 100% of nurses with 5-10 years of experience, 69.23% of nurses with 10-20 years of experience, and 100% of nurses with >20 years of experience “strongly agreed” that they learned something new from this in-service. There was no statistically significant difference on the post test compared to the pre-test in additional correct questions when stratified by clinical experience (P = .128). Second, when we stratified by clinical setting, we did not find a statistically significant difference in whether nurses in different clinical settings learned something new (P = .929): 88.89% of nurses in the medical setting, 87.50% of nurses in the surgical setting, and 84.62% of nurses in the intensive care setting “strongly agreed” that they learned something new from this presentation. On investigating additional questions correct on the post test compared to the pre-test, there was no statistically significant difference in additional correct questions when stratified by clinical setting (P = .446).
Optimal timing of the in-service was another important outcome. Initially, the in-service was administered at mid-shift, with 9 nurses participating at mid-shift, but our nursing colleagues gave unanimous feedback that this was a suboptimal time for delivery of an in-service. We changed the timing of the in-service to shift change; 42 nurses received the in-service at shift-change. There was no statistically significant difference in scores on the epistaxis knowledge questions between these two groups (P = .123). This indicated to us that changing the timing of the delivery resulted in similarly improved outcomes while having the added benefit of being preferred by our nursing colleagues.
Discussion
In undertaking this project, our primary aims were to improve epistaxis knowledge and perceived management in our nursing staff. Among our nursing staff, we were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. We also found that nurses of varying clinical experience and different clinical settings benefited equally from our intervention. Using quality improvement principles, we optimized our delivery. Our in-service focused on educating nurses to use epistaxis management techniques that were resource-efficient and low risk.
After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (Figure, part A; mean difference, 2.07 questions [1.10]; 95% CI, 1.74-2.39; P < .001), felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), and felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001). Based on these results, we successfully achieved our primary aims.
Our secondary aim was to determine the audience that would benefit the most from the in-service. All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. There was no statistically significant difference in whether nurses of varying clinical experience learned something new (P = .148) or in additional correct questions when stratified by clinical experience (P =.128). Also, there was no statistically significant difference in whether nurses in different clinical settings learned something new (P = .929) or in additional correct questions when stratified by clinical setting (P = .446). These results indicated to us that all participants learned something new and that there was no specific target audience, but rather that all participants benefitted from our session.
Our nursing colleagues gave us feedback that the timing of the in-service during mid-shift was not ideal. It was difficult to gather nurses mid-shift due to pressing patient-care duties. Nurses also found it difficult to give their full attention at this time. Nurses, nurse educators, and nurse managers suggested that we conduct the in-service at shift change in order to capture a larger population and take advantage of time relatively free of clinical duties. Giving the in-service at a time with relatively fewer clinical responsibilities allowed for a more robust question-and-answer session. It also allowed our nursing colleagues to pay full attention to the in-service. There was no statistically significant difference in epistaxis general knowledge questions answered correctly; this indicates that the quality of the education session did not vary greatly. However, our nursing colleagues strongly preferred the in-service at shift change. By making this modification to our intervention, we were able to optimize our intervention.
The previously mentioned study in England reported that only 12% to 14% of their nursing staff got a perfect score on epistaxis knowledge questions. Prior to our study, there was no literature investigating the impact of an in-service on epistaxis knowledge. After our intervention, 80.43% of our nurses got a perfect score on the epistaxis knowledge questions. We believe that this is a fair comparison because our post-test questions were identical to the survey questions used in the previously mentioned study in England, with the addition of one question.3 Further, the findings of our study are consistent with other studies regarding the positive effect of in-service education on knowledge and attitudes surrounding clinical topics. Similar to the study in Ethiopia investigating nurses’ knowledge surrounding pain management, our study noted a significant improvement in nurses’ knowledge after participating in the in-service.4 Also, when comparing our study to the study performed in Connecticut investigating nurses’ confidence surrounding suicide precautions, we found a similar significant improvement in confidence in management after participating in the in-service.5
Given our reliance on a survey as a tool to collect information, our study was subject to nonresponse bias. For each main outcome question, there was a handful of nonresponders. While this likely indicated either overlooking a question or deferring to answer due to clinical inexperience or nonapplicable clinical role, it is possible that this may have represented a respondent who did not benefit from the in-service. Another source of possible bias is sampling bias. Attempts were made to capture a wide range of nurses at the in-service. However, if a nurse was not interested in the topic material, whether due to abundant clinical experience or disinterest, it is possible that they may not have attended. Additionally, the cohort was selected purely based on responses from nursing managers to the initial email. It is possible that nonresponding units may have benefitted differently from this in-service.
There were several limitations within our analysis. We did not collect data assessing the long-term retention of epistaxis knowledge and management techniques. It is possible that epistaxis knowledge, comfort in managing nosebleeds, and perceived confidence in stopping nosebleeds decreased back to baseline several months after the in-service. Ideally, we would have been able to collect this data to assess retention of the in-service information. Unfortunately, a significant number of nurses who initially participated in the project became lost to follow-up, making such data collection impossible. Additionally, there was no assessment of actual ability to stop nosebleeds before vs after this in-service. Perceived management of epistaxis vs actual management of epistaxis are 2 vastly different things. However, this data would have been difficult to collect, and it likely would not have been in the best interest of patients, especially before the in-service was administered. As an improvement to this project, we could have assessed how many nosebleeds nurses had seen and successfully stopped after the in-service. As previously mentioned, this was not possible due to losing a significant number of nurses to follow-up. Finally, we did not collect objective data on preference for administration of in-service at mid-shift vs shift change. We relied on subjective data from conversations with our colleagues. By collecting objective data, we could have supported this change to our intervention with data.
The primary challenge to sustainability for this intervention is nursing turnover. With each wave of departing nurses and new nursing hires, the difficulty of ensuring a consistent knowledge base and management standards within our nursing workforce became clearer. After optimizing our intervention, our solution was to provide a hospital-wide in-service, which was recorded and uploaded to an institution-wide in-service library. In this way, a nurse with the desire to learn about epistaxis management could access the material at any point in time. Another solution would have been to appoint champions for epistaxis management within each major department to deliver the epistaxis in-service to new hires and new rotators within the department. However, given the turnover witnessed in our study cohort, this may not be sustainable long term.
Conclusion
Epistaxis is a chief complaint that can present in many different clinical settings and situations. Therefore, the ability to stop epistaxis in a timely and effective fashion is valuable. Our study demonstrated that in-services can improve epistaxis knowledge and improve perceived epistaxis management. Ideally, this intervention will lead to improved patient care. Given that epistaxis is a ubiquitous issue, this study may benefit other institutions who want to improve care for patients with epistaxis.
Next steps for this intervention include utilizing in-services for epistaxis education at other institutions and collecting long-term data within our own institution. Collecting long-term data would allow us to assess the retention of epistaxis knowledge from our in-service.
Acknowledgments: The author thanks the nurse managers, nurse educators, and staff nurses involved in this project, as well as Dr. Louis Portugal for providing mentorship throughout this process and Dr. Dara Adams for assisting with statistical analysis.
Corresponding author: Avery Nelson, MD, University of Chicago Medical Center, 5841 S Maryland Ave, MC 1035, Chicago, IL 60637; [email protected]
Disclosures: None reported.
1. Pallin DJ, Chng Y-M, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46(1):77-81. doi:10.1016/j.annemergmed.2004.12.014
2. Walker TWM, Macfarlane TV, McGarry GW. The epidemiology and chronobiology of epistaxis: An investigation of Scottish hospital admissions 1995-2004. Clin Otolaryngol. 2007;32(5):361-365. doi:10.1111/j.1749-4486.2007.01530.x
3. Hakim N, Mummadi SM, Jolly K, et al. Nurse-led epistaxis management within the emergency department. Br J Nurs. 2018;27(1):41-46. doi:10.12968/bjon.2018.27.1.41
4. Germossa GN, Sjetne IS, Hellesø R. The impact of an in-service educational program on nurses’ knowledge and attitudes regarding pain management in an Ethiopian University Hospital. Front Public Health. 2018;6:229. doi:10.3389/fpubh.2018.00229
5. Manister NN, Murray S, Burke JM, Finegan M, McKiernan ME. Effectiveness of nursing education to prevent inpatient suicide. J Contin Educ Nurs. 2017;48(9):413-419. doi:10.3928/00220124-20170816-07
6. Tunkel DE, Anne S, Payne SC, et al. Clinical practice guideline: nosebleed (epistaxis) executive summary. Otolaryngol Head Neck Surg. 2020;162(1):S1-S38. doi:10.1177/0194599819890327
7. Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol. 1995;104(9 Part 1):704-706. doi:10.1177/000348949510400906
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0—standards for quality improvement reporting excellence—revised publication guidelines from a detailed consensus process. J Am Coll Surg. 2016;222(3):317-323. doi:10.1016/j.jamcollsurg.2015.07.456
1. Pallin DJ, Chng Y-M, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46(1):77-81. doi:10.1016/j.annemergmed.2004.12.014
2. Walker TWM, Macfarlane TV, McGarry GW. The epidemiology and chronobiology of epistaxis: An investigation of Scottish hospital admissions 1995-2004. Clin Otolaryngol. 2007;32(5):361-365. doi:10.1111/j.1749-4486.2007.01530.x
3. Hakim N, Mummadi SM, Jolly K, et al. Nurse-led epistaxis management within the emergency department. Br J Nurs. 2018;27(1):41-46. doi:10.12968/bjon.2018.27.1.41
4. Germossa GN, Sjetne IS, Hellesø R. The impact of an in-service educational program on nurses’ knowledge and attitudes regarding pain management in an Ethiopian University Hospital. Front Public Health. 2018;6:229. doi:10.3389/fpubh.2018.00229
5. Manister NN, Murray S, Burke JM, Finegan M, McKiernan ME. Effectiveness of nursing education to prevent inpatient suicide. J Contin Educ Nurs. 2017;48(9):413-419. doi:10.3928/00220124-20170816-07
6. Tunkel DE, Anne S, Payne SC, et al. Clinical practice guideline: nosebleed (epistaxis) executive summary. Otolaryngol Head Neck Surg. 2020;162(1):S1-S38. doi:10.1177/0194599819890327
7. Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol. 1995;104(9 Part 1):704-706. doi:10.1177/000348949510400906
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0—standards for quality improvement reporting excellence—revised publication guidelines from a detailed consensus process. J Am Coll Surg. 2016;222(3):317-323. doi:10.1016/j.jamcollsurg.2015.07.456
Comorbidity Coding and Its Impact on Hospital Complexity: Reply
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).
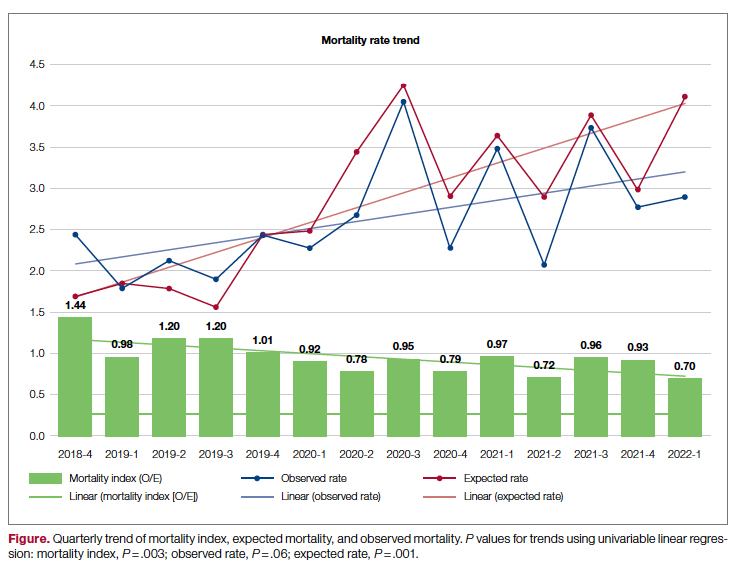
We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
[email protected]
Disclosures: None reported.
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).

We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
[email protected]
Disclosures: None reported.
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).

We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
[email protected]
Disclosures: None reported.
Comorbidity Coding and Its Impact on Hospital Complexity
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
[email protected]
Disclosures: None reported.
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
[email protected]
Disclosures: None reported.
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
[email protected]
Disclosures: None reported.
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
ICU stays linked to a doubling of dementia risk
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAIC 2022
Linezolid succeeds against gram-positive bacterial infections in ICU
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INTENSIVE MEDICINE