User login
FLU/SAL inhalers for COPD carry greater pneumonia risk
For well over a decade the elevated risk of pneumonia from inhaled corticosteroids for moderate to very severe COPD has been well documented, although the pneumonia risks from different types of ICSs have not been well understood.
Researchers from Taiwan have taken a step in to investigate this question with a nationwide cohort study that reported inhalers with budesonide and beclomethasone may have a lower pneumonia risk than that of fluticasone propionate/salmeterol inhalers (CHEST. 2020;157:117-29).
The study is the first to include beclomethasone-containing inhalers in a comparison of ICS/long-acting beta2-agonist (LABA) fixed combinations to evaluate pneumonia risk, along with dose and drug properties, wrote Ting-Yu Chang, MS, of the Graduate Institute of Clinical Pharmacology at the College of Medicine, National Taiwan University in Taipei, and colleagues.
The study evaluated 42,393 people with COPD in the National Health Insurance Research Database who got at least two continuous prescriptions for three different types of inhalers:
- Budesonide/formoterol (BUD/FOR).
- Beclomethasone/formoterol (BEC/FOR).
- Fluticasone propionate/salmeterol (FLU/SAL).
The study included patients aged 40 years and older who used a metered-dose inhaler (MDI) or dry-powder inhaler (DPI) between January 2011 and June 2015.
Patient experience with adverse events (AEs) was a factor in risk stratification, Mr. Chang and colleagues noted. “For the comparison between the BEC/FOR MDI and FLU/SAL MDI, the lower risk associated with the BEC/FOR MDI was more prominent in patients without severe AE in the past year,” they wrote.
The study found that BUD/FOR DPI users had a 17% lower risk of severe pneumonia and a 12% lower risk of severe AEs than that of FLU/SAL DPI users. The risk difference in pneumonia remained significant after adjustment for the ICS-equivalent daily dose, but the spread for AEs didn’t.
BEC/FOR MDI users were 31% less likely to get severe pneumonia and 18% less likely to have severe AEs than were FLU/SAL MDI users, but that difference declined and became nonsignificant after adjustment for the ICS-equivalent daily dose.
The study also found that a high average daily dose (> 500 mcg/d) of FLU/SAL MDI carried a 66% greater risk of severe pneumonia, compared with that of low-dose users. Also, medium-dose BEC/FOR MDI users (FLU equivalent 299-499 mcg/d) had a 38% greater risk of severe pneumonia than low-dose (< 200 mcg/d) users.
The variable pneumonia risks may be linked to each ICS’s pharmacokinetics, specifically their distinct lipophilic properties, Mr. Chang and colleagues wrote. Fluticasone propionate is known to be more lipophilic than budesonide, and while beclomethasone is more lipophilic than both, as a prodrug it rapidly converts to lower lipophilicity upon contact with bronchial secretions. “In general, a lipophilic ICS has a longer retention time within the airway or lung tissue to exert local immunosuppression and reduce inflammation,” Mr. Chang and colleagues stated.
The Taiwan Ministry of Science and Technology provided partial support for the study. Mr. Chang and colleagues have no relationships to disclose.
SOURCE: Chang TY et al. CHEST. 2020;157:117-29.
For well over a decade the elevated risk of pneumonia from inhaled corticosteroids for moderate to very severe COPD has been well documented, although the pneumonia risks from different types of ICSs have not been well understood.
Researchers from Taiwan have taken a step in to investigate this question with a nationwide cohort study that reported inhalers with budesonide and beclomethasone may have a lower pneumonia risk than that of fluticasone propionate/salmeterol inhalers (CHEST. 2020;157:117-29).
The study is the first to include beclomethasone-containing inhalers in a comparison of ICS/long-acting beta2-agonist (LABA) fixed combinations to evaluate pneumonia risk, along with dose and drug properties, wrote Ting-Yu Chang, MS, of the Graduate Institute of Clinical Pharmacology at the College of Medicine, National Taiwan University in Taipei, and colleagues.
The study evaluated 42,393 people with COPD in the National Health Insurance Research Database who got at least two continuous prescriptions for three different types of inhalers:
- Budesonide/formoterol (BUD/FOR).
- Beclomethasone/formoterol (BEC/FOR).
- Fluticasone propionate/salmeterol (FLU/SAL).
The study included patients aged 40 years and older who used a metered-dose inhaler (MDI) or dry-powder inhaler (DPI) between January 2011 and June 2015.
Patient experience with adverse events (AEs) was a factor in risk stratification, Mr. Chang and colleagues noted. “For the comparison between the BEC/FOR MDI and FLU/SAL MDI, the lower risk associated with the BEC/FOR MDI was more prominent in patients without severe AE in the past year,” they wrote.
The study found that BUD/FOR DPI users had a 17% lower risk of severe pneumonia and a 12% lower risk of severe AEs than that of FLU/SAL DPI users. The risk difference in pneumonia remained significant after adjustment for the ICS-equivalent daily dose, but the spread for AEs didn’t.
BEC/FOR MDI users were 31% less likely to get severe pneumonia and 18% less likely to have severe AEs than were FLU/SAL MDI users, but that difference declined and became nonsignificant after adjustment for the ICS-equivalent daily dose.
The study also found that a high average daily dose (> 500 mcg/d) of FLU/SAL MDI carried a 66% greater risk of severe pneumonia, compared with that of low-dose users. Also, medium-dose BEC/FOR MDI users (FLU equivalent 299-499 mcg/d) had a 38% greater risk of severe pneumonia than low-dose (< 200 mcg/d) users.
The variable pneumonia risks may be linked to each ICS’s pharmacokinetics, specifically their distinct lipophilic properties, Mr. Chang and colleagues wrote. Fluticasone propionate is known to be more lipophilic than budesonide, and while beclomethasone is more lipophilic than both, as a prodrug it rapidly converts to lower lipophilicity upon contact with bronchial secretions. “In general, a lipophilic ICS has a longer retention time within the airway or lung tissue to exert local immunosuppression and reduce inflammation,” Mr. Chang and colleagues stated.
The Taiwan Ministry of Science and Technology provided partial support for the study. Mr. Chang and colleagues have no relationships to disclose.
SOURCE: Chang TY et al. CHEST. 2020;157:117-29.
For well over a decade the elevated risk of pneumonia from inhaled corticosteroids for moderate to very severe COPD has been well documented, although the pneumonia risks from different types of ICSs have not been well understood.
Researchers from Taiwan have taken a step in to investigate this question with a nationwide cohort study that reported inhalers with budesonide and beclomethasone may have a lower pneumonia risk than that of fluticasone propionate/salmeterol inhalers (CHEST. 2020;157:117-29).
The study is the first to include beclomethasone-containing inhalers in a comparison of ICS/long-acting beta2-agonist (LABA) fixed combinations to evaluate pneumonia risk, along with dose and drug properties, wrote Ting-Yu Chang, MS, of the Graduate Institute of Clinical Pharmacology at the College of Medicine, National Taiwan University in Taipei, and colleagues.
The study evaluated 42,393 people with COPD in the National Health Insurance Research Database who got at least two continuous prescriptions for three different types of inhalers:
- Budesonide/formoterol (BUD/FOR).
- Beclomethasone/formoterol (BEC/FOR).
- Fluticasone propionate/salmeterol (FLU/SAL).
The study included patients aged 40 years and older who used a metered-dose inhaler (MDI) or dry-powder inhaler (DPI) between January 2011 and June 2015.
Patient experience with adverse events (AEs) was a factor in risk stratification, Mr. Chang and colleagues noted. “For the comparison between the BEC/FOR MDI and FLU/SAL MDI, the lower risk associated with the BEC/FOR MDI was more prominent in patients without severe AE in the past year,” they wrote.
The study found that BUD/FOR DPI users had a 17% lower risk of severe pneumonia and a 12% lower risk of severe AEs than that of FLU/SAL DPI users. The risk difference in pneumonia remained significant after adjustment for the ICS-equivalent daily dose, but the spread for AEs didn’t.
BEC/FOR MDI users were 31% less likely to get severe pneumonia and 18% less likely to have severe AEs than were FLU/SAL MDI users, but that difference declined and became nonsignificant after adjustment for the ICS-equivalent daily dose.
The study also found that a high average daily dose (> 500 mcg/d) of FLU/SAL MDI carried a 66% greater risk of severe pneumonia, compared with that of low-dose users. Also, medium-dose BEC/FOR MDI users (FLU equivalent 299-499 mcg/d) had a 38% greater risk of severe pneumonia than low-dose (< 200 mcg/d) users.
The variable pneumonia risks may be linked to each ICS’s pharmacokinetics, specifically their distinct lipophilic properties, Mr. Chang and colleagues wrote. Fluticasone propionate is known to be more lipophilic than budesonide, and while beclomethasone is more lipophilic than both, as a prodrug it rapidly converts to lower lipophilicity upon contact with bronchial secretions. “In general, a lipophilic ICS has a longer retention time within the airway or lung tissue to exert local immunosuppression and reduce inflammation,” Mr. Chang and colleagues stated.
The Taiwan Ministry of Science and Technology provided partial support for the study. Mr. Chang and colleagues have no relationships to disclose.
SOURCE: Chang TY et al. CHEST. 2020;157:117-29.
FROM CHEST
Low IgG levels in COPD patients linked to increased risk of hospitalization
Among patients with COPD, the presence of hypogammaglobulinemia confers a nearly 30% increased risk of hospitalization, results from a pooled analysis of four studies showed.
“Mechanistic studies are still warranted to better elucidate how IgG and other immunoglobulins, in particular IgA, may contribute to the local airway host defense,” researchers led by Fernando Sergio Leitao Filho, MD, PhD, wrote in a study published in Chest (2020 May 18. doi: 10.1016/j.chest.2020.04.058). “Nevertheless, our results raise the possibility that, in select COPD patients, IgG replacement therapy may be effective in reducing the risk of COPD hospitalizations. Given the growing rate of COPD hospitalization in the U.S. and elsewhere, there is a pressing need for a large well-designed trial to test this hypothesis.”
In an effort to evaluate the effect of IgG levels on the cumulative incidence of COPD hospitalizations, Dr. Leitao Filho, of the University of British Columbia, Vancouver, and colleagues drew from 2,259 patients who participated in four different trials: Azithromycin for Prevention of Exacerbations of COPD (MACRO), Simvastatin for the Prevention of Exacerbations in Moderate and Severe COPD (STATCOPE), the Long-Term Oxygen Treatment Trial (LOTT), and COPD Activity: Serotonin Transporter, Cytokines and Depression (CASCADE). The mean baseline age of study participants was 66 years, and 641 (28.4%) had hypogammaglobulinemia, which was defined as having a serum IgG levels of less than 7.0 g/L, while the remainder had normal IgG levels.
The pooled meta-analysis, which is believed to be the largest of its kind, revealed that the presence of hypogammaglobulinemia was associated with an incidence of COPD hospitalizations that was 1.29-fold higher than that observed among participants who had normal IgG levels (P = .01). The incidence was even higher among patients with prior COPD admissions (pooled subdistribution hazard ratio, 1.58; P < .01), yet the risk of COPD admissions was similar between IgG groups in patients with no prior hospitalizations (pooled SHR, 1.15; P = .34). Patients with hypogammaglobulinemia also showed significantly higher rates of COPD hospitalizations per person-year, compared with their counterparts who had normal IgG levels (0.48 vs. 0.29, respectively; P < .001.)
The authors acknowledged certain limitations of the study, including the fact that they measured serum IgG levels only at baseline “when participants were clinically stable; thus, the variability of IgG levels in a given individual over time and during the course of an AECOPD [severe acute exacerbation of COPD] is uncertain. Secondly, clinical data on corticosteroid use (formulations, dose, and length of use) were not readily available. However, systemic steroid use (one or more courses due to AECOPD prior to study entry) was accounted for in our analyses.”
The MACRO, STATCOPE, LOTT trials, and the CASCADE cohort were supported by the National Heart, Lung, and Blood Institute; National Institutes of Health; and Department of Health & Human Services. The current study was funded by the Canadian Institutes of Health Research and BC Lung Association. The authors reported having no relevant disclosures.
SOURCE: Leitao Filho SF et al. Chest. 2020 May 18. doi: 10.1016/j.chest.2020.04.058.
Among patients with COPD, the presence of hypogammaglobulinemia confers a nearly 30% increased risk of hospitalization, results from a pooled analysis of four studies showed.
“Mechanistic studies are still warranted to better elucidate how IgG and other immunoglobulins, in particular IgA, may contribute to the local airway host defense,” researchers led by Fernando Sergio Leitao Filho, MD, PhD, wrote in a study published in Chest (2020 May 18. doi: 10.1016/j.chest.2020.04.058). “Nevertheless, our results raise the possibility that, in select COPD patients, IgG replacement therapy may be effective in reducing the risk of COPD hospitalizations. Given the growing rate of COPD hospitalization in the U.S. and elsewhere, there is a pressing need for a large well-designed trial to test this hypothesis.”
In an effort to evaluate the effect of IgG levels on the cumulative incidence of COPD hospitalizations, Dr. Leitao Filho, of the University of British Columbia, Vancouver, and colleagues drew from 2,259 patients who participated in four different trials: Azithromycin for Prevention of Exacerbations of COPD (MACRO), Simvastatin for the Prevention of Exacerbations in Moderate and Severe COPD (STATCOPE), the Long-Term Oxygen Treatment Trial (LOTT), and COPD Activity: Serotonin Transporter, Cytokines and Depression (CASCADE). The mean baseline age of study participants was 66 years, and 641 (28.4%) had hypogammaglobulinemia, which was defined as having a serum IgG levels of less than 7.0 g/L, while the remainder had normal IgG levels.
The pooled meta-analysis, which is believed to be the largest of its kind, revealed that the presence of hypogammaglobulinemia was associated with an incidence of COPD hospitalizations that was 1.29-fold higher than that observed among participants who had normal IgG levels (P = .01). The incidence was even higher among patients with prior COPD admissions (pooled subdistribution hazard ratio, 1.58; P < .01), yet the risk of COPD admissions was similar between IgG groups in patients with no prior hospitalizations (pooled SHR, 1.15; P = .34). Patients with hypogammaglobulinemia also showed significantly higher rates of COPD hospitalizations per person-year, compared with their counterparts who had normal IgG levels (0.48 vs. 0.29, respectively; P < .001.)
The authors acknowledged certain limitations of the study, including the fact that they measured serum IgG levels only at baseline “when participants were clinically stable; thus, the variability of IgG levels in a given individual over time and during the course of an AECOPD [severe acute exacerbation of COPD] is uncertain. Secondly, clinical data on corticosteroid use (formulations, dose, and length of use) were not readily available. However, systemic steroid use (one or more courses due to AECOPD prior to study entry) was accounted for in our analyses.”
The MACRO, STATCOPE, LOTT trials, and the CASCADE cohort were supported by the National Heart, Lung, and Blood Institute; National Institutes of Health; and Department of Health & Human Services. The current study was funded by the Canadian Institutes of Health Research and BC Lung Association. The authors reported having no relevant disclosures.
SOURCE: Leitao Filho SF et al. Chest. 2020 May 18. doi: 10.1016/j.chest.2020.04.058.
Among patients with COPD, the presence of hypogammaglobulinemia confers a nearly 30% increased risk of hospitalization, results from a pooled analysis of four studies showed.
“Mechanistic studies are still warranted to better elucidate how IgG and other immunoglobulins, in particular IgA, may contribute to the local airway host defense,” researchers led by Fernando Sergio Leitao Filho, MD, PhD, wrote in a study published in Chest (2020 May 18. doi: 10.1016/j.chest.2020.04.058). “Nevertheless, our results raise the possibility that, in select COPD patients, IgG replacement therapy may be effective in reducing the risk of COPD hospitalizations. Given the growing rate of COPD hospitalization in the U.S. and elsewhere, there is a pressing need for a large well-designed trial to test this hypothesis.”
In an effort to evaluate the effect of IgG levels on the cumulative incidence of COPD hospitalizations, Dr. Leitao Filho, of the University of British Columbia, Vancouver, and colleagues drew from 2,259 patients who participated in four different trials: Azithromycin for Prevention of Exacerbations of COPD (MACRO), Simvastatin for the Prevention of Exacerbations in Moderate and Severe COPD (STATCOPE), the Long-Term Oxygen Treatment Trial (LOTT), and COPD Activity: Serotonin Transporter, Cytokines and Depression (CASCADE). The mean baseline age of study participants was 66 years, and 641 (28.4%) had hypogammaglobulinemia, which was defined as having a serum IgG levels of less than 7.0 g/L, while the remainder had normal IgG levels.
The pooled meta-analysis, which is believed to be the largest of its kind, revealed that the presence of hypogammaglobulinemia was associated with an incidence of COPD hospitalizations that was 1.29-fold higher than that observed among participants who had normal IgG levels (P = .01). The incidence was even higher among patients with prior COPD admissions (pooled subdistribution hazard ratio, 1.58; P < .01), yet the risk of COPD admissions was similar between IgG groups in patients with no prior hospitalizations (pooled SHR, 1.15; P = .34). Patients with hypogammaglobulinemia also showed significantly higher rates of COPD hospitalizations per person-year, compared with their counterparts who had normal IgG levels (0.48 vs. 0.29, respectively; P < .001.)
The authors acknowledged certain limitations of the study, including the fact that they measured serum IgG levels only at baseline “when participants were clinically stable; thus, the variability of IgG levels in a given individual over time and during the course of an AECOPD [severe acute exacerbation of COPD] is uncertain. Secondly, clinical data on corticosteroid use (formulations, dose, and length of use) were not readily available. However, systemic steroid use (one or more courses due to AECOPD prior to study entry) was accounted for in our analyses.”
The MACRO, STATCOPE, LOTT trials, and the CASCADE cohort were supported by the National Heart, Lung, and Blood Institute; National Institutes of Health; and Department of Health & Human Services. The current study was funded by the Canadian Institutes of Health Research and BC Lung Association. The authors reported having no relevant disclosures.
SOURCE: Leitao Filho SF et al. Chest. 2020 May 18. doi: 10.1016/j.chest.2020.04.058.
FROM CHEST
Masks, fear, and loss of connection in the era of COVID-19
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
The third surge: Are we prepared for the non-COVID crisis?
Over the last several weeks, hospitals and health systems have focused on the COVID-19 epidemic, preparing and expanding bed capacities for the surge of admissions both in intensive care and medical units. An indirect impact of this has been the reduction in outpatient staffing and resources, with the shifting of staff for inpatient care. Many areas seem to have passed the peak in the number of cases and are now seeing a plateau or downward trend in the admissions to acute care facilities.
During this period, there has been a noticeable downtrend in patients being evaluated in the ED, or admitted for decompensation of chronic conditions like heart failure, COPD and diabetes mellitus, or such acute conditions as stroke and MI. Studies from Italy and Spain, and closer to home from Atlanta and Boston, point to a significant decrease in numbers of ST-elevation myocardial infarction (STEMI) admissions.1 Duke Health saw a decrease in stroke admissions in their hospitals by 34%.2
One could argue that these patients are in fact presenting with COVID-19 or similar symptoms as is evidenced by the studies linking the severity of SARS-Co-V2 infection to chronic conditions like diabetes mellitus and obesity.2 On the other hand, the message of social isolation and avoidance of nonurgent visits could lead to delays in care resulting in patients presenting sicker and in advanced stages.3 Also, this has not been limited to the adult population. For example, reports indicate that visits to WakeMed’s pediatric emergency rooms in Wake County, N.C., were down by 60%.2
We could well be seeing a calm before the storm. While it is anticipated that there may be a second surge of COVID-19 cases, health systems would do well to be prepared for the “third surge,” consisting of patients coming in with chronic medical conditions for which they have been, so far, avoiding follow-up and managing at home, and acute medical conditions with delayed diagnoses. The impact could likely be more in the subset of patients with limited access to health care, including medications and follow-up, resulting in a disproportionate burden on safety-net hospitals.
Compounding this issue would be the economic impact of the current crisis on health systems, their staffing, and resources. Several major organizations have already proposed budget cuts and reduction of the workforce, raising significant concerns about the future of health care workers who put their lives at risk during this pandemic.4 There is no guarantee that the federal funding provided by the stimulus packages will save jobs in the health care industry. This problem needs new leadership thinking, and every organization that puts employees over profits margins will have a long-term impact on communities.
Another area of concern is a shift in resources and workflow from ambulatory to inpatient settings for the COVID-19 pandemic, and the need for revamping the ambulatory services with reshifting the workforce. As COVID-19 cases plateau, the resurgence of non-COVID–related admissions will require additional help in inpatient settings. Prioritizing the ambulatory services based on financial benefits versus patient outcomes is also a major challenge to leadership.5
Lastly, the current health care crisis has led to significant stress, both emotional and physical, among frontline caregivers, increasing the risk of burnout.6 How leadership helps health care workers to cope with these stressors, and the resources they provide, is going to play a key role in long term retention of their talent, and will reflect on the organizational culture. Though it might seem trivial, posttraumatic stress disorder related to this is already obvious, and health care leadership needs to put every effort in providing the resources to help prevent burnout, in partnership with national organizations like the Society of Hospital Medicine and the American College of Physicians.
The expansion of telemedicine has provided a unique opportunity to address several of these issues while maintaining the nonpharmacologic interventions to fight the epidemic, and keeping the cost curve as low as possible.7 Extension of these services to all ambulatory service lines, including home health and therapy, is the next big step in the new health care era. Virtual check-ins by physicians, advance practice clinicians, and home care nurses could help alleviate the concerns regarding delays in care of patients with chronic conditions, and help identify those at risk. This would also be of help with staffing shortages, and possibly provide much needed support to frontline providers.
Dr. Prasad is currently medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He was previously quality and utilization officer and chief of the medical staff at Aurora Sinai Medical Center. Dr. Prasad is cochair of SHM’s IT Special Interest Group, sits on the HQPS Committee, and is president of SHM’s Wisconsin Chapter. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Wood S. TCTMD. 2020 Apr 2. “The mystery of the missing STEMIs during the COVID-19 pandemic.”
2. Stradling R. The News & Observer. 2020 Apr 21. “Fewer people are going to Triangle [N.C.] emergency rooms, and that could be a bad thing.”
3. Kasanagottu K. USA Today. 2020 Apr 15. “Don’t delay care for chronic illness over coronavirus. It’s bad for you and for hospitals.”
4. Snowbeck C. The Star Tribune. 2020 Apr 11. “Mayo Clinic cutting pay for more than 20,000 workers.”
5. LaPointe J. RevCycle Intelligence. 2020 Mar 31. “How much will the COVID-19 pandemic cost hospitals?”
6. Gavidia M. AJMC. 2020 Mar 31. “Sleep, physician burnout linked amid COVID-19 pandemic.”
7. Hollander JE and Carr BG. N Engl J Med. 2020 Apr 30;382(18):1679-81. “Virtually perfect? Telemedicine for COVID-19.”
Over the last several weeks, hospitals and health systems have focused on the COVID-19 epidemic, preparing and expanding bed capacities for the surge of admissions both in intensive care and medical units. An indirect impact of this has been the reduction in outpatient staffing and resources, with the shifting of staff for inpatient care. Many areas seem to have passed the peak in the number of cases and are now seeing a plateau or downward trend in the admissions to acute care facilities.
During this period, there has been a noticeable downtrend in patients being evaluated in the ED, or admitted for decompensation of chronic conditions like heart failure, COPD and diabetes mellitus, or such acute conditions as stroke and MI. Studies from Italy and Spain, and closer to home from Atlanta and Boston, point to a significant decrease in numbers of ST-elevation myocardial infarction (STEMI) admissions.1 Duke Health saw a decrease in stroke admissions in their hospitals by 34%.2
One could argue that these patients are in fact presenting with COVID-19 or similar symptoms as is evidenced by the studies linking the severity of SARS-Co-V2 infection to chronic conditions like diabetes mellitus and obesity.2 On the other hand, the message of social isolation and avoidance of nonurgent visits could lead to delays in care resulting in patients presenting sicker and in advanced stages.3 Also, this has not been limited to the adult population. For example, reports indicate that visits to WakeMed’s pediatric emergency rooms in Wake County, N.C., were down by 60%.2
We could well be seeing a calm before the storm. While it is anticipated that there may be a second surge of COVID-19 cases, health systems would do well to be prepared for the “third surge,” consisting of patients coming in with chronic medical conditions for which they have been, so far, avoiding follow-up and managing at home, and acute medical conditions with delayed diagnoses. The impact could likely be more in the subset of patients with limited access to health care, including medications and follow-up, resulting in a disproportionate burden on safety-net hospitals.
Compounding this issue would be the economic impact of the current crisis on health systems, their staffing, and resources. Several major organizations have already proposed budget cuts and reduction of the workforce, raising significant concerns about the future of health care workers who put their lives at risk during this pandemic.4 There is no guarantee that the federal funding provided by the stimulus packages will save jobs in the health care industry. This problem needs new leadership thinking, and every organization that puts employees over profits margins will have a long-term impact on communities.
Another area of concern is a shift in resources and workflow from ambulatory to inpatient settings for the COVID-19 pandemic, and the need for revamping the ambulatory services with reshifting the workforce. As COVID-19 cases plateau, the resurgence of non-COVID–related admissions will require additional help in inpatient settings. Prioritizing the ambulatory services based on financial benefits versus patient outcomes is also a major challenge to leadership.5
Lastly, the current health care crisis has led to significant stress, both emotional and physical, among frontline caregivers, increasing the risk of burnout.6 How leadership helps health care workers to cope with these stressors, and the resources they provide, is going to play a key role in long term retention of their talent, and will reflect on the organizational culture. Though it might seem trivial, posttraumatic stress disorder related to this is already obvious, and health care leadership needs to put every effort in providing the resources to help prevent burnout, in partnership with national organizations like the Society of Hospital Medicine and the American College of Physicians.
The expansion of telemedicine has provided a unique opportunity to address several of these issues while maintaining the nonpharmacologic interventions to fight the epidemic, and keeping the cost curve as low as possible.7 Extension of these services to all ambulatory service lines, including home health and therapy, is the next big step in the new health care era. Virtual check-ins by physicians, advance practice clinicians, and home care nurses could help alleviate the concerns regarding delays in care of patients with chronic conditions, and help identify those at risk. This would also be of help with staffing shortages, and possibly provide much needed support to frontline providers.
Dr. Prasad is currently medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He was previously quality and utilization officer and chief of the medical staff at Aurora Sinai Medical Center. Dr. Prasad is cochair of SHM’s IT Special Interest Group, sits on the HQPS Committee, and is president of SHM’s Wisconsin Chapter. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Wood S. TCTMD. 2020 Apr 2. “The mystery of the missing STEMIs during the COVID-19 pandemic.”
2. Stradling R. The News & Observer. 2020 Apr 21. “Fewer people are going to Triangle [N.C.] emergency rooms, and that could be a bad thing.”
3. Kasanagottu K. USA Today. 2020 Apr 15. “Don’t delay care for chronic illness over coronavirus. It’s bad for you and for hospitals.”
4. Snowbeck C. The Star Tribune. 2020 Apr 11. “Mayo Clinic cutting pay for more than 20,000 workers.”
5. LaPointe J. RevCycle Intelligence. 2020 Mar 31. “How much will the COVID-19 pandemic cost hospitals?”
6. Gavidia M. AJMC. 2020 Mar 31. “Sleep, physician burnout linked amid COVID-19 pandemic.”
7. Hollander JE and Carr BG. N Engl J Med. 2020 Apr 30;382(18):1679-81. “Virtually perfect? Telemedicine for COVID-19.”
Over the last several weeks, hospitals and health systems have focused on the COVID-19 epidemic, preparing and expanding bed capacities for the surge of admissions both in intensive care and medical units. An indirect impact of this has been the reduction in outpatient staffing and resources, with the shifting of staff for inpatient care. Many areas seem to have passed the peak in the number of cases and are now seeing a plateau or downward trend in the admissions to acute care facilities.
During this period, there has been a noticeable downtrend in patients being evaluated in the ED, or admitted for decompensation of chronic conditions like heart failure, COPD and diabetes mellitus, or such acute conditions as stroke and MI. Studies from Italy and Spain, and closer to home from Atlanta and Boston, point to a significant decrease in numbers of ST-elevation myocardial infarction (STEMI) admissions.1 Duke Health saw a decrease in stroke admissions in their hospitals by 34%.2
One could argue that these patients are in fact presenting with COVID-19 or similar symptoms as is evidenced by the studies linking the severity of SARS-Co-V2 infection to chronic conditions like diabetes mellitus and obesity.2 On the other hand, the message of social isolation and avoidance of nonurgent visits could lead to delays in care resulting in patients presenting sicker and in advanced stages.3 Also, this has not been limited to the adult population. For example, reports indicate that visits to WakeMed’s pediatric emergency rooms in Wake County, N.C., were down by 60%.2
We could well be seeing a calm before the storm. While it is anticipated that there may be a second surge of COVID-19 cases, health systems would do well to be prepared for the “third surge,” consisting of patients coming in with chronic medical conditions for which they have been, so far, avoiding follow-up and managing at home, and acute medical conditions with delayed diagnoses. The impact could likely be more in the subset of patients with limited access to health care, including medications and follow-up, resulting in a disproportionate burden on safety-net hospitals.
Compounding this issue would be the economic impact of the current crisis on health systems, their staffing, and resources. Several major organizations have already proposed budget cuts and reduction of the workforce, raising significant concerns about the future of health care workers who put their lives at risk during this pandemic.4 There is no guarantee that the federal funding provided by the stimulus packages will save jobs in the health care industry. This problem needs new leadership thinking, and every organization that puts employees over profits margins will have a long-term impact on communities.
Another area of concern is a shift in resources and workflow from ambulatory to inpatient settings for the COVID-19 pandemic, and the need for revamping the ambulatory services with reshifting the workforce. As COVID-19 cases plateau, the resurgence of non-COVID–related admissions will require additional help in inpatient settings. Prioritizing the ambulatory services based on financial benefits versus patient outcomes is also a major challenge to leadership.5
Lastly, the current health care crisis has led to significant stress, both emotional and physical, among frontline caregivers, increasing the risk of burnout.6 How leadership helps health care workers to cope with these stressors, and the resources they provide, is going to play a key role in long term retention of their talent, and will reflect on the organizational culture. Though it might seem trivial, posttraumatic stress disorder related to this is already obvious, and health care leadership needs to put every effort in providing the resources to help prevent burnout, in partnership with national organizations like the Society of Hospital Medicine and the American College of Physicians.
The expansion of telemedicine has provided a unique opportunity to address several of these issues while maintaining the nonpharmacologic interventions to fight the epidemic, and keeping the cost curve as low as possible.7 Extension of these services to all ambulatory service lines, including home health and therapy, is the next big step in the new health care era. Virtual check-ins by physicians, advance practice clinicians, and home care nurses could help alleviate the concerns regarding delays in care of patients with chronic conditions, and help identify those at risk. This would also be of help with staffing shortages, and possibly provide much needed support to frontline providers.
Dr. Prasad is currently medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He was previously quality and utilization officer and chief of the medical staff at Aurora Sinai Medical Center. Dr. Prasad is cochair of SHM’s IT Special Interest Group, sits on the HQPS Committee, and is president of SHM’s Wisconsin Chapter. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Wood S. TCTMD. 2020 Apr 2. “The mystery of the missing STEMIs during the COVID-19 pandemic.”
2. Stradling R. The News & Observer. 2020 Apr 21. “Fewer people are going to Triangle [N.C.] emergency rooms, and that could be a bad thing.”
3. Kasanagottu K. USA Today. 2020 Apr 15. “Don’t delay care for chronic illness over coronavirus. It’s bad for you and for hospitals.”
4. Snowbeck C. The Star Tribune. 2020 Apr 11. “Mayo Clinic cutting pay for more than 20,000 workers.”
5. LaPointe J. RevCycle Intelligence. 2020 Mar 31. “How much will the COVID-19 pandemic cost hospitals?”
6. Gavidia M. AJMC. 2020 Mar 31. “Sleep, physician burnout linked amid COVID-19 pandemic.”
7. Hollander JE and Carr BG. N Engl J Med. 2020 Apr 30;382(18):1679-81. “Virtually perfect? Telemedicine for COVID-19.”
States vary in vulnerability to COVID-19 impact
West Virginia’s large elderly population and high rates of chronic kidney disease, cardiovascular disease, diabetes, and COPD make it the most vulnerable state to the coronavirus, according to a new analysis.

Vulnerability to the virus “isn’t just health related, though, as many people are harmed by the economic effects of the pandemic,” personal finance website WalletHub said May 12.
“It’s important for the U.S. to dedicate a large portion of its resources to providing medical support during the coronavirus pandemic, but we should also support people who don’t have adequate housing or enough money to survive the pandemic,” said WalletHub analyst Jill Gonzalez.
WalletHub graded each state on 28 measures – including share of obese adults, share of homes lacking access to basic hygienic facilities, and biggest increases in unemployment because of COVID-19 – grouped into three dimensions of vulnerability: medical (60% of the total score), housing (15%), and financial (25%).
Using those measures, Louisiana is the most vulnerable state after West Virginia, followed by Mississippi, Arkansas, and Alabama. All 5 states finished in the top 6 for medical vulnerability, and 4 were in the top 10 for financial vulnerability, but only 1 (Arkansas) was in the top 10 for housing vulnerability, WalletHub said.
Among the three vulnerability dimensions, West Virginia was first in medical, Hawaii (33rd overall) was first in housing, and Louisiana was first in financial. Utah is the least vulnerable state, overall, and the least vulnerable states in each dimension are, respectively, Colorado (50th overall), the District of Columbia (29th overall), and Iowa (45th overall), the report showed.
A look at the individual metrics WalletHub used shows some serious disparities:
- New Jersey’s unemployment recipiency rate of 57.2%, the highest in the country, is 6.1 times higher than North Carolina’s 9.3%.
- The highest uninsured rate, 17.4% in Texas, is 6.2 times higher than in Massachusetts, which is the lowest at 2.8%.
- In California, the share of the homeless population that is unsheltered (71.7%) is more than 33 times higher than in North Dakota (2.2%).
“The financial damage caused by COVID-19 is leaving many Americans without the means to pay their bills and purchase necessities. … The U.S. must continue to support its financially vulnerable populations even after the virus has subsided,” Ms. Gonzalez said.
West Virginia’s large elderly population and high rates of chronic kidney disease, cardiovascular disease, diabetes, and COPD make it the most vulnerable state to the coronavirus, according to a new analysis.

Vulnerability to the virus “isn’t just health related, though, as many people are harmed by the economic effects of the pandemic,” personal finance website WalletHub said May 12.
“It’s important for the U.S. to dedicate a large portion of its resources to providing medical support during the coronavirus pandemic, but we should also support people who don’t have adequate housing or enough money to survive the pandemic,” said WalletHub analyst Jill Gonzalez.
WalletHub graded each state on 28 measures – including share of obese adults, share of homes lacking access to basic hygienic facilities, and biggest increases in unemployment because of COVID-19 – grouped into three dimensions of vulnerability: medical (60% of the total score), housing (15%), and financial (25%).
Using those measures, Louisiana is the most vulnerable state after West Virginia, followed by Mississippi, Arkansas, and Alabama. All 5 states finished in the top 6 for medical vulnerability, and 4 were in the top 10 for financial vulnerability, but only 1 (Arkansas) was in the top 10 for housing vulnerability, WalletHub said.
Among the three vulnerability dimensions, West Virginia was first in medical, Hawaii (33rd overall) was first in housing, and Louisiana was first in financial. Utah is the least vulnerable state, overall, and the least vulnerable states in each dimension are, respectively, Colorado (50th overall), the District of Columbia (29th overall), and Iowa (45th overall), the report showed.
A look at the individual metrics WalletHub used shows some serious disparities:
- New Jersey’s unemployment recipiency rate of 57.2%, the highest in the country, is 6.1 times higher than North Carolina’s 9.3%.
- The highest uninsured rate, 17.4% in Texas, is 6.2 times higher than in Massachusetts, which is the lowest at 2.8%.
- In California, the share of the homeless population that is unsheltered (71.7%) is more than 33 times higher than in North Dakota (2.2%).
“The financial damage caused by COVID-19 is leaving many Americans without the means to pay their bills and purchase necessities. … The U.S. must continue to support its financially vulnerable populations even after the virus has subsided,” Ms. Gonzalez said.
West Virginia’s large elderly population and high rates of chronic kidney disease, cardiovascular disease, diabetes, and COPD make it the most vulnerable state to the coronavirus, according to a new analysis.

Vulnerability to the virus “isn’t just health related, though, as many people are harmed by the economic effects of the pandemic,” personal finance website WalletHub said May 12.
“It’s important for the U.S. to dedicate a large portion of its resources to providing medical support during the coronavirus pandemic, but we should also support people who don’t have adequate housing or enough money to survive the pandemic,” said WalletHub analyst Jill Gonzalez.
WalletHub graded each state on 28 measures – including share of obese adults, share of homes lacking access to basic hygienic facilities, and biggest increases in unemployment because of COVID-19 – grouped into three dimensions of vulnerability: medical (60% of the total score), housing (15%), and financial (25%).
Using those measures, Louisiana is the most vulnerable state after West Virginia, followed by Mississippi, Arkansas, and Alabama. All 5 states finished in the top 6 for medical vulnerability, and 4 were in the top 10 for financial vulnerability, but only 1 (Arkansas) was in the top 10 for housing vulnerability, WalletHub said.
Among the three vulnerability dimensions, West Virginia was first in medical, Hawaii (33rd overall) was first in housing, and Louisiana was first in financial. Utah is the least vulnerable state, overall, and the least vulnerable states in each dimension are, respectively, Colorado (50th overall), the District of Columbia (29th overall), and Iowa (45th overall), the report showed.
A look at the individual metrics WalletHub used shows some serious disparities:
- New Jersey’s unemployment recipiency rate of 57.2%, the highest in the country, is 6.1 times higher than North Carolina’s 9.3%.
- The highest uninsured rate, 17.4% in Texas, is 6.2 times higher than in Massachusetts, which is the lowest at 2.8%.
- In California, the share of the homeless population that is unsheltered (71.7%) is more than 33 times higher than in North Dakota (2.2%).
“The financial damage caused by COVID-19 is leaving many Americans without the means to pay their bills and purchase necessities. … The U.S. must continue to support its financially vulnerable populations even after the virus has subsided,” Ms. Gonzalez said.
Sleep quality may affect COPD risk in African American smokers
African American smokers who logged more total sleep time and greater sleep efficacy performed better on a functional walk test than did those with poorer sleep, based on data from 209 adults.
African American smokers tend to develop COPD sooner and also report more sleep problems, compared with white smokers, wrote Andrew J. Gangemi, MD, of Temple University Hospital, Philadelphia, and colleagues.
In addition, African Americans tend to develop COPD at a younger age and with lower levels of smoking than do non-Hispanic whites, they said. “Sleep health may be a contributing factor to the lung and cardiovascular health disparity experienced by AA smokers,” in part because data suggest that insufficient sleep may be associated with increased risk of COPD exacerbation in smokers in general, they said.
In a study published in Chest, the researchers reviewed data from 209 African American adults aged 40-65 years who had smoked at least one cigarette in the past month. The average age of the participants was 55 years, 59% were women, and the average smoking habit was nine cigarettes per day.
The researchers measured functional exercise capacity of the participants using the 6-minute walk test (6MWT). Total sleep time (TST) and sleep efficacy (SE) were measured by way of a finger-based device.
Smokers of at least 10 cigarettes per day gained an additional 0.05-0.58 meters in distance covered on the 6MWT for every added minute of total sleep time in a multivariable regression analysis. Similarly, smokers of at least 10 cigarettes per day gained an additional 0.84-6.17–meter increase in distance covered on the 6MWT for every added percentage of sleep efficacy.
The reasons for the impact of SE and TST on functional exercise capacity in smokers remain unclear, the researchers said. “Heavier smokers have higher levels of autonomic imbalance, including higher resting heart rate and heart rate variability, impaired 24-hour cardiovascular sympathetic tone, and blunted cerebrovascular autonomic regulation and baroreflex response to hypercapnia,” they said.
Also unclear is the reason for the large magnitude of the association between SE and smoking vs. the lesser association between TST and smoking on 6MWT results, the researchers wrote. “Poor sleep efficiency, outside of traditional OSA scoring, is predictive of myocardial infarction, stroke, and cardiovascular-related mortality risk. Moreover, deficits in sleep efficiency have been consistently demonstrated in smokers versus nonsmokers,” they said.
The study findings were limited by several factors including inability to extrapolate data to other demographic groups and the cross-sectional design, the researchers noted. In addition, they did not address how TST and SE may relate to lung function.
However, the results “extend current knowledge about the potential role of improved sleep health to functional exercise capacity in AA smokers,” and set the stage for future studies of how changes in sleep health may affect lung and functional exercise capacity in smokers over time, as well as effects on inflammation and autonomic imbalance, the researchers concluded.
The study was supported by the National Institute on Minority Health and Health Disparities and by the National Institute of General Medical Sciences, both part of the National Institutes Health. The researchers had no financial conflicts to disclose.
SOURCE: Gangemi A et al. Chest 2020 Apr 23. doi: 10.1016/j.chest.2020.03.070.
African American smokers who logged more total sleep time and greater sleep efficacy performed better on a functional walk test than did those with poorer sleep, based on data from 209 adults.
African American smokers tend to develop COPD sooner and also report more sleep problems, compared with white smokers, wrote Andrew J. Gangemi, MD, of Temple University Hospital, Philadelphia, and colleagues.
In addition, African Americans tend to develop COPD at a younger age and with lower levels of smoking than do non-Hispanic whites, they said. “Sleep health may be a contributing factor to the lung and cardiovascular health disparity experienced by AA smokers,” in part because data suggest that insufficient sleep may be associated with increased risk of COPD exacerbation in smokers in general, they said.
In a study published in Chest, the researchers reviewed data from 209 African American adults aged 40-65 years who had smoked at least one cigarette in the past month. The average age of the participants was 55 years, 59% were women, and the average smoking habit was nine cigarettes per day.
The researchers measured functional exercise capacity of the participants using the 6-minute walk test (6MWT). Total sleep time (TST) and sleep efficacy (SE) were measured by way of a finger-based device.
Smokers of at least 10 cigarettes per day gained an additional 0.05-0.58 meters in distance covered on the 6MWT for every added minute of total sleep time in a multivariable regression analysis. Similarly, smokers of at least 10 cigarettes per day gained an additional 0.84-6.17–meter increase in distance covered on the 6MWT for every added percentage of sleep efficacy.
The reasons for the impact of SE and TST on functional exercise capacity in smokers remain unclear, the researchers said. “Heavier smokers have higher levels of autonomic imbalance, including higher resting heart rate and heart rate variability, impaired 24-hour cardiovascular sympathetic tone, and blunted cerebrovascular autonomic regulation and baroreflex response to hypercapnia,” they said.
Also unclear is the reason for the large magnitude of the association between SE and smoking vs. the lesser association between TST and smoking on 6MWT results, the researchers wrote. “Poor sleep efficiency, outside of traditional OSA scoring, is predictive of myocardial infarction, stroke, and cardiovascular-related mortality risk. Moreover, deficits in sleep efficiency have been consistently demonstrated in smokers versus nonsmokers,” they said.
The study findings were limited by several factors including inability to extrapolate data to other demographic groups and the cross-sectional design, the researchers noted. In addition, they did not address how TST and SE may relate to lung function.
However, the results “extend current knowledge about the potential role of improved sleep health to functional exercise capacity in AA smokers,” and set the stage for future studies of how changes in sleep health may affect lung and functional exercise capacity in smokers over time, as well as effects on inflammation and autonomic imbalance, the researchers concluded.
The study was supported by the National Institute on Minority Health and Health Disparities and by the National Institute of General Medical Sciences, both part of the National Institutes Health. The researchers had no financial conflicts to disclose.
SOURCE: Gangemi A et al. Chest 2020 Apr 23. doi: 10.1016/j.chest.2020.03.070.
African American smokers who logged more total sleep time and greater sleep efficacy performed better on a functional walk test than did those with poorer sleep, based on data from 209 adults.
African American smokers tend to develop COPD sooner and also report more sleep problems, compared with white smokers, wrote Andrew J. Gangemi, MD, of Temple University Hospital, Philadelphia, and colleagues.
In addition, African Americans tend to develop COPD at a younger age and with lower levels of smoking than do non-Hispanic whites, they said. “Sleep health may be a contributing factor to the lung and cardiovascular health disparity experienced by AA smokers,” in part because data suggest that insufficient sleep may be associated with increased risk of COPD exacerbation in smokers in general, they said.
In a study published in Chest, the researchers reviewed data from 209 African American adults aged 40-65 years who had smoked at least one cigarette in the past month. The average age of the participants was 55 years, 59% were women, and the average smoking habit was nine cigarettes per day.
The researchers measured functional exercise capacity of the participants using the 6-minute walk test (6MWT). Total sleep time (TST) and sleep efficacy (SE) were measured by way of a finger-based device.
Smokers of at least 10 cigarettes per day gained an additional 0.05-0.58 meters in distance covered on the 6MWT for every added minute of total sleep time in a multivariable regression analysis. Similarly, smokers of at least 10 cigarettes per day gained an additional 0.84-6.17–meter increase in distance covered on the 6MWT for every added percentage of sleep efficacy.
The reasons for the impact of SE and TST on functional exercise capacity in smokers remain unclear, the researchers said. “Heavier smokers have higher levels of autonomic imbalance, including higher resting heart rate and heart rate variability, impaired 24-hour cardiovascular sympathetic tone, and blunted cerebrovascular autonomic regulation and baroreflex response to hypercapnia,” they said.
Also unclear is the reason for the large magnitude of the association between SE and smoking vs. the lesser association between TST and smoking on 6MWT results, the researchers wrote. “Poor sleep efficiency, outside of traditional OSA scoring, is predictive of myocardial infarction, stroke, and cardiovascular-related mortality risk. Moreover, deficits in sleep efficiency have been consistently demonstrated in smokers versus nonsmokers,” they said.
The study findings were limited by several factors including inability to extrapolate data to other demographic groups and the cross-sectional design, the researchers noted. In addition, they did not address how TST and SE may relate to lung function.
However, the results “extend current knowledge about the potential role of improved sleep health to functional exercise capacity in AA smokers,” and set the stage for future studies of how changes in sleep health may affect lung and functional exercise capacity in smokers over time, as well as effects on inflammation and autonomic imbalance, the researchers concluded.
The study was supported by the National Institute on Minority Health and Health Disparities and by the National Institute of General Medical Sciences, both part of the National Institutes Health. The researchers had no financial conflicts to disclose.
SOURCE: Gangemi A et al. Chest 2020 Apr 23. doi: 10.1016/j.chest.2020.03.070.
FROM CHEST
Increased risk of lung cancer with COPD, even in never smokers
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.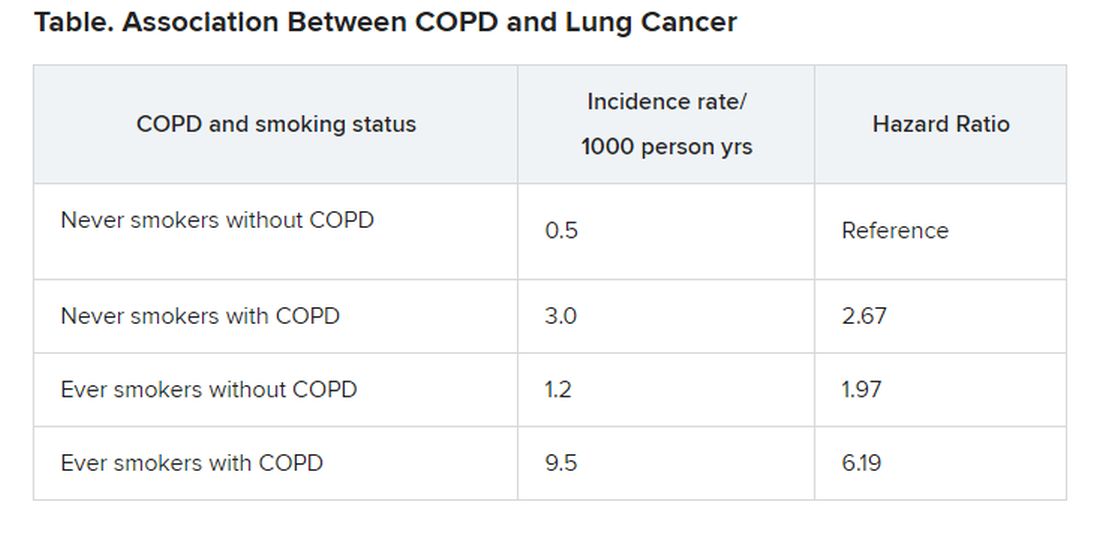
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
European COVID-19 insights: Try helmet CPAP
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
DLCO found to predict outcomes in subset of COPD patients
Use of diffusing capacity of the lung for carbon monoxide may be a useful prognostic tool in patients with chronic pulmonary disease who develop pulmonary hypertension, results from a single-center retrospective cohort study found.
“Historically, COPD-PH was thought to develop as the severity of airflow obstruction, measured by Forced Expiratory Volume in one second (FEV1), and subsequent chronic hypoxemia progressed,” authors led by Aparna Balasubramanian, MD, wrote in a study published online in CHEST. “However, airflow obstruction has increasingly been noted to be insufficient in predicting clinical outcomes in the general COPD population.”
Dr. Balasubramanian of the Johns Hopkins University Division of Pulmonary and Critical Care, Baltimore, and colleagues went on to note that, while studies in COPD-PH have identified hemodynamic measures as better predictors of prognosis, these metrics require right-heart catheterization (RHC), an invasive procedure that carries its own risks. “An alternative noninvasive measure of interest is diffusing capacity of the lung for carbon monoxide (DLCO). DLCO is a measure of gas exchange reflective of the complex interactions occurring at the alveolar-capillary interface, including morphologic changes in the pulmonary vasculature,” they wrote. “Recent work by our group in a large COPD cohort has demonstrated that DLCO is an indicator of disease morbidity beyond that represented by airflow obstruction or by CT evidence of emphysema alone. This may be particularly relevant for those with COPD-PH.”
The study population consisted of 71 patients enrolled in the Johns Hopkins Pulmonary Hypertension Registry between January 2000 and January 2018, all of whom had right-heart catheterization (RHC)–proven PH and pulmonary function testing (PFT) data within 1 year of diagnostic RHC. The researchers calculated transplant-free survival from index RHC and used Cox proportional hazard methods to determine transplant-free survival with age, pulmonary vascular resistance, FEV1, oxygen use, and N-terminal pro-brain natriuretic peptide included as covariates.
The average age of patients was 65 years, 66% were female, their average body mass index was 28.3 kg/m2, and the mean number of pack-years smoked was 44. On unadjusted analysis, the transplant-free survival was 87% at 1 year, 60% at 3 years, and 51% at 5 years. Survival was associated with reduced DLCO across the observed range of pulmonary artery pressures and pulmonary vascular resistance. The researchers found that severe DLCO impairment was associated with poorer survival (P less than .001), and when they adjusted for covariates, they found that mortality increased by 4% for every percent predicted decrease in DLCO (hazard ratio, 1.04).
“This study demonstrates that DLCO, a readily available, inexpensive, noninvasive measurement, is a strong independent predictor of mortality in COPD patients with PH,” the authors concluded. “The presented findings suggest that DLCO should be considered for inclusion in prognostic tools for COPD-PH.”
Dr. Balasubramanian and associates acknowledged certain limitations of the study, including its modest sample size and single-center design and the fact that the cohort underwent subspecialty referral and invasive testing, thereby limiting its generalizability to the larger COPD population. “The findings do, however, offer insight into clinical and physiologic characteristics at one extreme of the pulmonary vascular disease spectrum among COPD patients, and generate hypotheses regarding measures that warrant further exploration in the larger COPD population,” they wrote.
The study was supported by National Heart, Lung and Blood Institute. One of the study authors has served as a consultant to GlaxoSmithKline and Celgene and receives royalties from UpToDate for authorship. Another study author has served as a consultant for Arena, Actelion, Liquidia, and United Therapeutics, and has served on the Scientific Leadership Council of the Pulmonary Hypertension Association. He also serves on the Rare Disease Advisory Panel of the Patient Centered Outcomes Research Institute. The other study authors reported having no disclosures.
SOURCE: Balasubramanian A et al. CHEST. 2020 Mar 14. doi: 10.1016/j.chest.2020.02.047.
Use of diffusing capacity of the lung for carbon monoxide may be a useful prognostic tool in patients with chronic pulmonary disease who develop pulmonary hypertension, results from a single-center retrospective cohort study found.
“Historically, COPD-PH was thought to develop as the severity of airflow obstruction, measured by Forced Expiratory Volume in one second (FEV1), and subsequent chronic hypoxemia progressed,” authors led by Aparna Balasubramanian, MD, wrote in a study published online in CHEST. “However, airflow obstruction has increasingly been noted to be insufficient in predicting clinical outcomes in the general COPD population.”
Dr. Balasubramanian of the Johns Hopkins University Division of Pulmonary and Critical Care, Baltimore, and colleagues went on to note that, while studies in COPD-PH have identified hemodynamic measures as better predictors of prognosis, these metrics require right-heart catheterization (RHC), an invasive procedure that carries its own risks. “An alternative noninvasive measure of interest is diffusing capacity of the lung for carbon monoxide (DLCO). DLCO is a measure of gas exchange reflective of the complex interactions occurring at the alveolar-capillary interface, including morphologic changes in the pulmonary vasculature,” they wrote. “Recent work by our group in a large COPD cohort has demonstrated that DLCO is an indicator of disease morbidity beyond that represented by airflow obstruction or by CT evidence of emphysema alone. This may be particularly relevant for those with COPD-PH.”
The study population consisted of 71 patients enrolled in the Johns Hopkins Pulmonary Hypertension Registry between January 2000 and January 2018, all of whom had right-heart catheterization (RHC)–proven PH and pulmonary function testing (PFT) data within 1 year of diagnostic RHC. The researchers calculated transplant-free survival from index RHC and used Cox proportional hazard methods to determine transplant-free survival with age, pulmonary vascular resistance, FEV1, oxygen use, and N-terminal pro-brain natriuretic peptide included as covariates.
The average age of patients was 65 years, 66% were female, their average body mass index was 28.3 kg/m2, and the mean number of pack-years smoked was 44. On unadjusted analysis, the transplant-free survival was 87% at 1 year, 60% at 3 years, and 51% at 5 years. Survival was associated with reduced DLCO across the observed range of pulmonary artery pressures and pulmonary vascular resistance. The researchers found that severe DLCO impairment was associated with poorer survival (P less than .001), and when they adjusted for covariates, they found that mortality increased by 4% for every percent predicted decrease in DLCO (hazard ratio, 1.04).
“This study demonstrates that DLCO, a readily available, inexpensive, noninvasive measurement, is a strong independent predictor of mortality in COPD patients with PH,” the authors concluded. “The presented findings suggest that DLCO should be considered for inclusion in prognostic tools for COPD-PH.”
Dr. Balasubramanian and associates acknowledged certain limitations of the study, including its modest sample size and single-center design and the fact that the cohort underwent subspecialty referral and invasive testing, thereby limiting its generalizability to the larger COPD population. “The findings do, however, offer insight into clinical and physiologic characteristics at one extreme of the pulmonary vascular disease spectrum among COPD patients, and generate hypotheses regarding measures that warrant further exploration in the larger COPD population,” they wrote.
The study was supported by National Heart, Lung and Blood Institute. One of the study authors has served as a consultant to GlaxoSmithKline and Celgene and receives royalties from UpToDate for authorship. Another study author has served as a consultant for Arena, Actelion, Liquidia, and United Therapeutics, and has served on the Scientific Leadership Council of the Pulmonary Hypertension Association. He also serves on the Rare Disease Advisory Panel of the Patient Centered Outcomes Research Institute. The other study authors reported having no disclosures.
SOURCE: Balasubramanian A et al. CHEST. 2020 Mar 14. doi: 10.1016/j.chest.2020.02.047.
Use of diffusing capacity of the lung for carbon monoxide may be a useful prognostic tool in patients with chronic pulmonary disease who develop pulmonary hypertension, results from a single-center retrospective cohort study found.
“Historically, COPD-PH was thought to develop as the severity of airflow obstruction, measured by Forced Expiratory Volume in one second (FEV1), and subsequent chronic hypoxemia progressed,” authors led by Aparna Balasubramanian, MD, wrote in a study published online in CHEST. “However, airflow obstruction has increasingly been noted to be insufficient in predicting clinical outcomes in the general COPD population.”
Dr. Balasubramanian of the Johns Hopkins University Division of Pulmonary and Critical Care, Baltimore, and colleagues went on to note that, while studies in COPD-PH have identified hemodynamic measures as better predictors of prognosis, these metrics require right-heart catheterization (RHC), an invasive procedure that carries its own risks. “An alternative noninvasive measure of interest is diffusing capacity of the lung for carbon monoxide (DLCO). DLCO is a measure of gas exchange reflective of the complex interactions occurring at the alveolar-capillary interface, including morphologic changes in the pulmonary vasculature,” they wrote. “Recent work by our group in a large COPD cohort has demonstrated that DLCO is an indicator of disease morbidity beyond that represented by airflow obstruction or by CT evidence of emphysema alone. This may be particularly relevant for those with COPD-PH.”
The study population consisted of 71 patients enrolled in the Johns Hopkins Pulmonary Hypertension Registry between January 2000 and January 2018, all of whom had right-heart catheterization (RHC)–proven PH and pulmonary function testing (PFT) data within 1 year of diagnostic RHC. The researchers calculated transplant-free survival from index RHC and used Cox proportional hazard methods to determine transplant-free survival with age, pulmonary vascular resistance, FEV1, oxygen use, and N-terminal pro-brain natriuretic peptide included as covariates.
The average age of patients was 65 years, 66% were female, their average body mass index was 28.3 kg/m2, and the mean number of pack-years smoked was 44. On unadjusted analysis, the transplant-free survival was 87% at 1 year, 60% at 3 years, and 51% at 5 years. Survival was associated with reduced DLCO across the observed range of pulmonary artery pressures and pulmonary vascular resistance. The researchers found that severe DLCO impairment was associated with poorer survival (P less than .001), and when they adjusted for covariates, they found that mortality increased by 4% for every percent predicted decrease in DLCO (hazard ratio, 1.04).
“This study demonstrates that DLCO, a readily available, inexpensive, noninvasive measurement, is a strong independent predictor of mortality in COPD patients with PH,” the authors concluded. “The presented findings suggest that DLCO should be considered for inclusion in prognostic tools for COPD-PH.”
Dr. Balasubramanian and associates acknowledged certain limitations of the study, including its modest sample size and single-center design and the fact that the cohort underwent subspecialty referral and invasive testing, thereby limiting its generalizability to the larger COPD population. “The findings do, however, offer insight into clinical and physiologic characteristics at one extreme of the pulmonary vascular disease spectrum among COPD patients, and generate hypotheses regarding measures that warrant further exploration in the larger COPD population,” they wrote.
The study was supported by National Heart, Lung and Blood Institute. One of the study authors has served as a consultant to GlaxoSmithKline and Celgene and receives royalties from UpToDate for authorship. Another study author has served as a consultant for Arena, Actelion, Liquidia, and United Therapeutics, and has served on the Scientific Leadership Council of the Pulmonary Hypertension Association. He also serves on the Rare Disease Advisory Panel of the Patient Centered Outcomes Research Institute. The other study authors reported having no disclosures.
SOURCE: Balasubramanian A et al. CHEST. 2020 Mar 14. doi: 10.1016/j.chest.2020.02.047.
FROM CHEST
Researchers investigate impact of smoking on COVID-19 risk
but quitting smoking is likely to lower the risk of developing more severe or fatal cases of the infection, according to research from several recent papers.
Interest in how tobacco use affects COVID-19 infection rates stems from research showing that men at the epicenter of the outbreak in China having a higher early mortality rate. Early reports from China showed a case fatality rate of 4.7% for men, compared with 2.8% for women, according to the World Health Organization. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2, is suspected to enter a cell using the ACE2 receptor. Since smoking up-regulates this receptor, one popular theory is that smoking can increase the risk of COVID-19 or exacerbate symptoms of an existing infection (Eur Respir J. 2020 Apr 8. doi: 10.1183/13993003.00688-2020). In China, about half of men are active smokers, compared with 2.7% of women (Transl Lung Cancer Res. 2019;8[Suppl 1]:S21-30), so this association would explain the severe cases and increased mortality in this group. In response to potential risk for public health, the World Health Organization, Centers for Disease Control and Prevention, the Attorney General of Massachusetts, and other organizations have warned that smoking may increase one’s risk of transmitting and developing COVID-19 or may worsen the infection.
“While it is easy to jump to the conclusion that more ACE2 means more susceptibility to severe infection, there is no evidence to support this,” Brandon Michael Henry, MD, of the cardiac intensive care unit and the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in an interview. “Moreover, some would argue (including myself) that increased ACE2 may in fact be protective, as ACE2 decreases the levels of angiotensin-2 which likely plays a significant role in the pathophysiology of ARDS.”
Some researchers have examined the limited evidence of smoking on COVID-19 risk and come to preliminary conclusions. In a letter to the editor recently published in the European Journal of Internal Medicine, Dr. Henry and Giuseppe Lippi, MD, of the section of clinical biochemistry in the department of neuroscience, biomedicine, and movement at the University of Verona (Italy), performed a meta-analysis of papers examining smoking and COVID-19 up to March 9, 2020 and identified five articles with 1,399 COVID-19 cases (Eur J Intern Med. 2020 Mar 16. doi: 10.1016/j.ejim.2020.03.014).
“Given the fact that COVID-19 is a primarily respiratory illness, smoking was one of first risk factors we examined,” Dr. Henry said.
They noted that a study by Liu et al. in the Chinese Medical Journal was the only paper that showed a significant association between smoking status and COVID-19 case severity (Chin Med J [Engl]. 2020 Feb 28. doi: 10.1097/CM9.0000000000000775), while the four other studies showed no significant association. The pooled data of all five studies showed an association that was not statistically significant (odds ratio, 1.69; 95% confidence interval, 0.41-6.92; P = .254). When Dr. Lippi and Dr. Henry performed the analysis again after removing a paper by Guan et al. (N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032) comprising 89.5% of patients in the pooled analysis, there was no significant association (OR, 4.35; 95% CI, 0.86-21.86; P = .129).
Constantine I. Vardavas, MD, FCCP, of the department of oral health policy and epidemiology at Harvard School of Dental Medicine, Boston, and Katerina Nikitara, of the University of Crete in Heraklion, Greece, also published a systematic review in Tobacco Induced Diseases of five studies evaluating smoking and COVID-19 (Tob Induc Dis. 2020. doi: 10.18332/tid/119324). Of the studies chosen for the review, four were shared with the paper by Dr. Lippi and Dr. Henry. They found “a higher percentage of smokers” made up severe COVID-19 cases, but acknowledged the majority of these were from the largest study by Guan et al. Overall, they calculated smokers carried a risk ratio of 1.4 (95% CI, 0.98-2.00) for developing severe COVID-19 symptoms, and were over twice as likely to be admitted to an ICU, require a mechanical ventilator, or die from COVID-19, compared with patients who did not smoke (RR, 2.4; 95% CI, 1.43-4.04).
“Although further research is warranted as the weight of the evidence increases, with the limited available data, and although the above results are unadjusted for other factors that may impact disease progression, smoking is most likely associated with the negative progression and adverse outcomes of COVID-19,” Dr. Vardavas and Ms. Nikitara concluded.
However, the association between smoking and severe disease was not significant, and it is not immediately clear how the analysis was performed based on the details in the editorial. “Both of our reports were limited by a lack of data adjusted for age, sex, and comorbidities which may influence any analysis on smoking,” Dr. Henry said.
Some researchers have proposed collecting information on smoking status and conducting further research on whether vaping devices like e-cigarettes also impact COVID-19 cases. An editorial by Samuel Brake and colleagues published in the Journal of Clinical Medicine proposed the ACE2-receptor binding site as an area of interest for COVID-19 and as a potential therapeutic target (J Clin Med. 2020 Mar 20. doi: 10.3390/jcm9030841).
Ultimately, whether smoking itself is associated with COVID-19 is still an open question. Nonetheless, encouraging patients to quit smoking should be a priority because long-term sequelae of smoking have been linked to worsened or fatal COVID-19 cases, said Dr. Henry.
“There is a lack of definitive data on smoking to date. Nonetheless, we do know that many illnesses associated with smoking, such as [chronic obstructive pulmonary disease, hypertension, and heart disease are all strong risk factors for severe and fatal COVID-19,” he said. “Thus, absolutely we should encourage the public to quit smoking, especially for older individuals and those with comorbidities.”
The papers by Lippi et al., Vardavas et al., and Brake et al. had no funding source, and the authors reported no relevant conflicts of interest.
but quitting smoking is likely to lower the risk of developing more severe or fatal cases of the infection, according to research from several recent papers.
Interest in how tobacco use affects COVID-19 infection rates stems from research showing that men at the epicenter of the outbreak in China having a higher early mortality rate. Early reports from China showed a case fatality rate of 4.7% for men, compared with 2.8% for women, according to the World Health Organization. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2, is suspected to enter a cell using the ACE2 receptor. Since smoking up-regulates this receptor, one popular theory is that smoking can increase the risk of COVID-19 or exacerbate symptoms of an existing infection (Eur Respir J. 2020 Apr 8. doi: 10.1183/13993003.00688-2020). In China, about half of men are active smokers, compared with 2.7% of women (Transl Lung Cancer Res. 2019;8[Suppl 1]:S21-30), so this association would explain the severe cases and increased mortality in this group. In response to potential risk for public health, the World Health Organization, Centers for Disease Control and Prevention, the Attorney General of Massachusetts, and other organizations have warned that smoking may increase one’s risk of transmitting and developing COVID-19 or may worsen the infection.
“While it is easy to jump to the conclusion that more ACE2 means more susceptibility to severe infection, there is no evidence to support this,” Brandon Michael Henry, MD, of the cardiac intensive care unit and the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in an interview. “Moreover, some would argue (including myself) that increased ACE2 may in fact be protective, as ACE2 decreases the levels of angiotensin-2 which likely plays a significant role in the pathophysiology of ARDS.”
Some researchers have examined the limited evidence of smoking on COVID-19 risk and come to preliminary conclusions. In a letter to the editor recently published in the European Journal of Internal Medicine, Dr. Henry and Giuseppe Lippi, MD, of the section of clinical biochemistry in the department of neuroscience, biomedicine, and movement at the University of Verona (Italy), performed a meta-analysis of papers examining smoking and COVID-19 up to March 9, 2020 and identified five articles with 1,399 COVID-19 cases (Eur J Intern Med. 2020 Mar 16. doi: 10.1016/j.ejim.2020.03.014).
“Given the fact that COVID-19 is a primarily respiratory illness, smoking was one of first risk factors we examined,” Dr. Henry said.
They noted that a study by Liu et al. in the Chinese Medical Journal was the only paper that showed a significant association between smoking status and COVID-19 case severity (Chin Med J [Engl]. 2020 Feb 28. doi: 10.1097/CM9.0000000000000775), while the four other studies showed no significant association. The pooled data of all five studies showed an association that was not statistically significant (odds ratio, 1.69; 95% confidence interval, 0.41-6.92; P = .254). When Dr. Lippi and Dr. Henry performed the analysis again after removing a paper by Guan et al. (N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032) comprising 89.5% of patients in the pooled analysis, there was no significant association (OR, 4.35; 95% CI, 0.86-21.86; P = .129).
Constantine I. Vardavas, MD, FCCP, of the department of oral health policy and epidemiology at Harvard School of Dental Medicine, Boston, and Katerina Nikitara, of the University of Crete in Heraklion, Greece, also published a systematic review in Tobacco Induced Diseases of five studies evaluating smoking and COVID-19 (Tob Induc Dis. 2020. doi: 10.18332/tid/119324). Of the studies chosen for the review, four were shared with the paper by Dr. Lippi and Dr. Henry. They found “a higher percentage of smokers” made up severe COVID-19 cases, but acknowledged the majority of these were from the largest study by Guan et al. Overall, they calculated smokers carried a risk ratio of 1.4 (95% CI, 0.98-2.00) for developing severe COVID-19 symptoms, and were over twice as likely to be admitted to an ICU, require a mechanical ventilator, or die from COVID-19, compared with patients who did not smoke (RR, 2.4; 95% CI, 1.43-4.04).
“Although further research is warranted as the weight of the evidence increases, with the limited available data, and although the above results are unadjusted for other factors that may impact disease progression, smoking is most likely associated with the negative progression and adverse outcomes of COVID-19,” Dr. Vardavas and Ms. Nikitara concluded.
However, the association between smoking and severe disease was not significant, and it is not immediately clear how the analysis was performed based on the details in the editorial. “Both of our reports were limited by a lack of data adjusted for age, sex, and comorbidities which may influence any analysis on smoking,” Dr. Henry said.
Some researchers have proposed collecting information on smoking status and conducting further research on whether vaping devices like e-cigarettes also impact COVID-19 cases. An editorial by Samuel Brake and colleagues published in the Journal of Clinical Medicine proposed the ACE2-receptor binding site as an area of interest for COVID-19 and as a potential therapeutic target (J Clin Med. 2020 Mar 20. doi: 10.3390/jcm9030841).
Ultimately, whether smoking itself is associated with COVID-19 is still an open question. Nonetheless, encouraging patients to quit smoking should be a priority because long-term sequelae of smoking have been linked to worsened or fatal COVID-19 cases, said Dr. Henry.
“There is a lack of definitive data on smoking to date. Nonetheless, we do know that many illnesses associated with smoking, such as [chronic obstructive pulmonary disease, hypertension, and heart disease are all strong risk factors for severe and fatal COVID-19,” he said. “Thus, absolutely we should encourage the public to quit smoking, especially for older individuals and those with comorbidities.”
The papers by Lippi et al., Vardavas et al., and Brake et al. had no funding source, and the authors reported no relevant conflicts of interest.
but quitting smoking is likely to lower the risk of developing more severe or fatal cases of the infection, according to research from several recent papers.
Interest in how tobacco use affects COVID-19 infection rates stems from research showing that men at the epicenter of the outbreak in China having a higher early mortality rate. Early reports from China showed a case fatality rate of 4.7% for men, compared with 2.8% for women, according to the World Health Organization. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2, is suspected to enter a cell using the ACE2 receptor. Since smoking up-regulates this receptor, one popular theory is that smoking can increase the risk of COVID-19 or exacerbate symptoms of an existing infection (Eur Respir J. 2020 Apr 8. doi: 10.1183/13993003.00688-2020). In China, about half of men are active smokers, compared with 2.7% of women (Transl Lung Cancer Res. 2019;8[Suppl 1]:S21-30), so this association would explain the severe cases and increased mortality in this group. In response to potential risk for public health, the World Health Organization, Centers for Disease Control and Prevention, the Attorney General of Massachusetts, and other organizations have warned that smoking may increase one’s risk of transmitting and developing COVID-19 or may worsen the infection.
“While it is easy to jump to the conclusion that more ACE2 means more susceptibility to severe infection, there is no evidence to support this,” Brandon Michael Henry, MD, of the cardiac intensive care unit and the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in an interview. “Moreover, some would argue (including myself) that increased ACE2 may in fact be protective, as ACE2 decreases the levels of angiotensin-2 which likely plays a significant role in the pathophysiology of ARDS.”
Some researchers have examined the limited evidence of smoking on COVID-19 risk and come to preliminary conclusions. In a letter to the editor recently published in the European Journal of Internal Medicine, Dr. Henry and Giuseppe Lippi, MD, of the section of clinical biochemistry in the department of neuroscience, biomedicine, and movement at the University of Verona (Italy), performed a meta-analysis of papers examining smoking and COVID-19 up to March 9, 2020 and identified five articles with 1,399 COVID-19 cases (Eur J Intern Med. 2020 Mar 16. doi: 10.1016/j.ejim.2020.03.014).
“Given the fact that COVID-19 is a primarily respiratory illness, smoking was one of first risk factors we examined,” Dr. Henry said.
They noted that a study by Liu et al. in the Chinese Medical Journal was the only paper that showed a significant association between smoking status and COVID-19 case severity (Chin Med J [Engl]. 2020 Feb 28. doi: 10.1097/CM9.0000000000000775), while the four other studies showed no significant association. The pooled data of all five studies showed an association that was not statistically significant (odds ratio, 1.69; 95% confidence interval, 0.41-6.92; P = .254). When Dr. Lippi and Dr. Henry performed the analysis again after removing a paper by Guan et al. (N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032) comprising 89.5% of patients in the pooled analysis, there was no significant association (OR, 4.35; 95% CI, 0.86-21.86; P = .129).
Constantine I. Vardavas, MD, FCCP, of the department of oral health policy and epidemiology at Harvard School of Dental Medicine, Boston, and Katerina Nikitara, of the University of Crete in Heraklion, Greece, also published a systematic review in Tobacco Induced Diseases of five studies evaluating smoking and COVID-19 (Tob Induc Dis. 2020. doi: 10.18332/tid/119324). Of the studies chosen for the review, four were shared with the paper by Dr. Lippi and Dr. Henry. They found “a higher percentage of smokers” made up severe COVID-19 cases, but acknowledged the majority of these were from the largest study by Guan et al. Overall, they calculated smokers carried a risk ratio of 1.4 (95% CI, 0.98-2.00) for developing severe COVID-19 symptoms, and were over twice as likely to be admitted to an ICU, require a mechanical ventilator, or die from COVID-19, compared with patients who did not smoke (RR, 2.4; 95% CI, 1.43-4.04).
“Although further research is warranted as the weight of the evidence increases, with the limited available data, and although the above results are unadjusted for other factors that may impact disease progression, smoking is most likely associated with the negative progression and adverse outcomes of COVID-19,” Dr. Vardavas and Ms. Nikitara concluded.
However, the association between smoking and severe disease was not significant, and it is not immediately clear how the analysis was performed based on the details in the editorial. “Both of our reports were limited by a lack of data adjusted for age, sex, and comorbidities which may influence any analysis on smoking,” Dr. Henry said.
Some researchers have proposed collecting information on smoking status and conducting further research on whether vaping devices like e-cigarettes also impact COVID-19 cases. An editorial by Samuel Brake and colleagues published in the Journal of Clinical Medicine proposed the ACE2-receptor binding site as an area of interest for COVID-19 and as a potential therapeutic target (J Clin Med. 2020 Mar 20. doi: 10.3390/jcm9030841).
Ultimately, whether smoking itself is associated with COVID-19 is still an open question. Nonetheless, encouraging patients to quit smoking should be a priority because long-term sequelae of smoking have been linked to worsened or fatal COVID-19 cases, said Dr. Henry.
“There is a lack of definitive data on smoking to date. Nonetheless, we do know that many illnesses associated with smoking, such as [chronic obstructive pulmonary disease, hypertension, and heart disease are all strong risk factors for severe and fatal COVID-19,” he said. “Thus, absolutely we should encourage the public to quit smoking, especially for older individuals and those with comorbidities.”
The papers by Lippi et al., Vardavas et al., and Brake et al. had no funding source, and the authors reported no relevant conflicts of interest.