User login
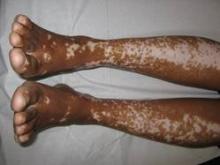
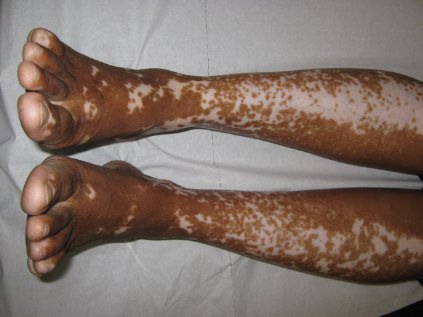
Vitiligo, alopecia more likely in GVHD when donor is female and recipient is male
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
FROM JAMA DERMATOLOGY
Key clinical point: Female-to-male stem cell donation ups the risk for vitiligo in GVHD.
Major finding: 14 of the 15 patients who developed vitiligo or alopecia areata had female donors (the gender of the 15th donor was unknown), and 9 of these 14 recipients were male.
Data source: A retrospective cross-sectional analysis involving 282 adults and children with chronic GVHD, 15 of whom developed vitiligo or alopecia areata.
Disclosures: This study was supported by the National Institutes of Health and the National Cancer Institute. Dr. Zuo and her associates reported no financial conflicts of interest.
Study explains BCR-ABL-independent imatinib resistance

A new study helps explain why some chronic myeloid leukemia (CML) patients develop resistance to imatinib despite the absence of BCR-ABL mutations.
Researchers discovered that a signaling pathway associated with cell division and growth acts as an alternative survival signal underlying imatinib resistance.
But blocking this pathway with an inhibitor known as trametinib can prevent resistance to imatinib and increase survival in mice.
The researchers recounted these discoveries in Science Translational Medicine.
Michael R. Green, MD, PhD, of the University of Massachusetts Medical School in Worcester, and his colleagues began this research with a large-scale RNA interference screen. This revealed a set of genes that promote imatinib sensitivity.
The team then set out to identify the regulatory pathways through which these genes promote imatinib sensitivity. They found that knocking down the genes in BCR-ABL+ cells results in sustained RAF/MEK/ERK signaling after treatment with imatinib.
Further investigation revealed it is PRKCH, which encodes the protein kinase C family member PKCη, that increases RAF/MEK/ERK signaling through phosphorylation and activation of CRAF.
Dr Green and his colleagues also found that PRKCH is upregulated in CML cell lines and patient samples that exhibit BCR-ABL-independent imatinib resistance. Experiments in mice revealed that PRKCH modulates the proliferation of BCR-ABL+ cells, CML progression, and imatinib sensitivity.
Furthermore, imatinib-resistant murine and human CML stem cells contained high levels of PRKCH. And experiments confirmed that high PRKCH expression contributed to the imatinib resistance observed in these cells.
Fortunately, the researchers discovered that combining imatinib with the MEK inhibitor trametinib can overcome BCR-ABL-independent imatinib resistance in CML cells. The combination also prolonged survival in mouse models of imatinib-resistant CML.
Dr Green and his colleagues said these results reveal a mechanism of BCR-ABL-independent imatinib resistance that can be targeted with therapy. And, as treatment with trametinib and imatinib kills CML stem cells but spares normal hematopoietic stem cells, it may be a feasible treatment option for CML patients. ![]()

A new study helps explain why some chronic myeloid leukemia (CML) patients develop resistance to imatinib despite the absence of BCR-ABL mutations.
Researchers discovered that a signaling pathway associated with cell division and growth acts as an alternative survival signal underlying imatinib resistance.
But blocking this pathway with an inhibitor known as trametinib can prevent resistance to imatinib and increase survival in mice.
The researchers recounted these discoveries in Science Translational Medicine.
Michael R. Green, MD, PhD, of the University of Massachusetts Medical School in Worcester, and his colleagues began this research with a large-scale RNA interference screen. This revealed a set of genes that promote imatinib sensitivity.
The team then set out to identify the regulatory pathways through which these genes promote imatinib sensitivity. They found that knocking down the genes in BCR-ABL+ cells results in sustained RAF/MEK/ERK signaling after treatment with imatinib.
Further investigation revealed it is PRKCH, which encodes the protein kinase C family member PKCη, that increases RAF/MEK/ERK signaling through phosphorylation and activation of CRAF.
Dr Green and his colleagues also found that PRKCH is upregulated in CML cell lines and patient samples that exhibit BCR-ABL-independent imatinib resistance. Experiments in mice revealed that PRKCH modulates the proliferation of BCR-ABL+ cells, CML progression, and imatinib sensitivity.
Furthermore, imatinib-resistant murine and human CML stem cells contained high levels of PRKCH. And experiments confirmed that high PRKCH expression contributed to the imatinib resistance observed in these cells.
Fortunately, the researchers discovered that combining imatinib with the MEK inhibitor trametinib can overcome BCR-ABL-independent imatinib resistance in CML cells. The combination also prolonged survival in mouse models of imatinib-resistant CML.
Dr Green and his colleagues said these results reveal a mechanism of BCR-ABL-independent imatinib resistance that can be targeted with therapy. And, as treatment with trametinib and imatinib kills CML stem cells but spares normal hematopoietic stem cells, it may be a feasible treatment option for CML patients. ![]()

A new study helps explain why some chronic myeloid leukemia (CML) patients develop resistance to imatinib despite the absence of BCR-ABL mutations.
Researchers discovered that a signaling pathway associated with cell division and growth acts as an alternative survival signal underlying imatinib resistance.
But blocking this pathway with an inhibitor known as trametinib can prevent resistance to imatinib and increase survival in mice.
The researchers recounted these discoveries in Science Translational Medicine.
Michael R. Green, MD, PhD, of the University of Massachusetts Medical School in Worcester, and his colleagues began this research with a large-scale RNA interference screen. This revealed a set of genes that promote imatinib sensitivity.
The team then set out to identify the regulatory pathways through which these genes promote imatinib sensitivity. They found that knocking down the genes in BCR-ABL+ cells results in sustained RAF/MEK/ERK signaling after treatment with imatinib.
Further investigation revealed it is PRKCH, which encodes the protein kinase C family member PKCη, that increases RAF/MEK/ERK signaling through phosphorylation and activation of CRAF.
Dr Green and his colleagues also found that PRKCH is upregulated in CML cell lines and patient samples that exhibit BCR-ABL-independent imatinib resistance. Experiments in mice revealed that PRKCH modulates the proliferation of BCR-ABL+ cells, CML progression, and imatinib sensitivity.
Furthermore, imatinib-resistant murine and human CML stem cells contained high levels of PRKCH. And experiments confirmed that high PRKCH expression contributed to the imatinib resistance observed in these cells.
Fortunately, the researchers discovered that combining imatinib with the MEK inhibitor trametinib can overcome BCR-ABL-independent imatinib resistance in CML cells. The combination also prolonged survival in mouse models of imatinib-resistant CML.
Dr Green and his colleagues said these results reveal a mechanism of BCR-ABL-independent imatinib resistance that can be targeted with therapy. And, as treatment with trametinib and imatinib kills CML stem cells but spares normal hematopoietic stem cells, it may be a feasible treatment option for CML patients. ![]()
Toward better treatment of CML

Credit: UC San Diego
In vitro experiments have revealed new insight into tyrosine kinase inhibitor (TKI) resistance among patients with chronic myeloid leukemia (CML).
Though it’s now possible to overcome TKI resistance resulting from single BCR-ABL1 mutants, targeting compound mutants remains a challenge.
So researchers tested several TKIs on various BCR-ABL1 compound mutants to determine which drug, if any, would be most effective for each combination.
The results appear in Cancer Cell.
Thomas O’Hare, PhD, of the Huntsman Cancer Institute at the University of Utah, and his colleagues first took an inventory of clinical BCR-ABL1 compound mutations associated with TKI resistance that had been reported in the literature.
The team identified 12 kinase domain positions that account for most clinical BCR-ABL1 TKI resistance—M244, G250, Q252, Y253, E255, V299, F311, T315, F317, M351, F359, and H396.
All of the clinically reported compound mutations include at least 1 of the 12 key positions, and most (65%) include 2. Each position has been implicated in resistance to 1 or more TKIs, including imatinib, nilotinib, dasatinib, bosutinib, rebastinib, and ponatinib.
The researchers found that some of the compound mutations they studied conferred resistance several-fold higher than that of either contributing mutation alone.
“We were able to sequence about 100 clinical samples, which gave us a very large body of data to shed light on the number of compound mutations and how they develop,” said Michael Deininger, MD, PhD, also of the Huntsman Cancer Institute.
“One key finding was that compound mutations containing an already known mutation called T315I tend to confer complete resistance to all available TKIs.”
The researchers had focused their testing on ponatinib, as the drug has proven effective against resistant CML, particularly cases with the T315I mutation. Unfortunately, ponatinib was often no match for compound mutations including T315I.
Tests did suggest that a 30 mg/day dose of ponatinib would maintain efficacy against 7 of the 8 non-T315I compound mutants tested, though the Y253H/E255V mutant proved resistant.
The researchers also found that a 15 mg/day dose of ponatinib could pre-empt outgrowth of 5 of the 8 non-T315I compound mutants, though Y253H/E255V, E255V/V299L, and F317L/F359V might be problematic.
But ponatinib proved substantially less effective against T315I-inclusive compound mutants. Nine of 10 T315I-inclusive compound mutants showed little or no sensitivity to ponatinib or any of the other TKIs tested. M244V/T315I was the only compound mutant not resistant to ponatinib.
The researchers noted that because ponatinib has proven effective against the T315I mutant in isolation, many patients treated with ponatinib are likely to have this mutation.
So it may be necessary to perform more sensitive screening on these patients to determine whether they might have T315I-inclusive compound mutants that could confer resistance.
“Fortunately, the problems we are studying affect a minority of CML patients,” Dr O’Hare said. “[S]till, this leaves some patients with no good treatment option at all. Our goal is to have a TKI option for every patient.”
According to Dr O’Hare, it’s only a matter of time until analogous compound mutations emerge in many other cancers, including acute myeloid leukemia and non-small cell lung cancer.
“Our findings in CML will provide a blueprint for contending with resistance in these highly aggressive diseases as well,” he concluded. ![]()

Credit: UC San Diego
In vitro experiments have revealed new insight into tyrosine kinase inhibitor (TKI) resistance among patients with chronic myeloid leukemia (CML).
Though it’s now possible to overcome TKI resistance resulting from single BCR-ABL1 mutants, targeting compound mutants remains a challenge.
So researchers tested several TKIs on various BCR-ABL1 compound mutants to determine which drug, if any, would be most effective for each combination.
The results appear in Cancer Cell.
Thomas O’Hare, PhD, of the Huntsman Cancer Institute at the University of Utah, and his colleagues first took an inventory of clinical BCR-ABL1 compound mutations associated with TKI resistance that had been reported in the literature.
The team identified 12 kinase domain positions that account for most clinical BCR-ABL1 TKI resistance—M244, G250, Q252, Y253, E255, V299, F311, T315, F317, M351, F359, and H396.
All of the clinically reported compound mutations include at least 1 of the 12 key positions, and most (65%) include 2. Each position has been implicated in resistance to 1 or more TKIs, including imatinib, nilotinib, dasatinib, bosutinib, rebastinib, and ponatinib.
The researchers found that some of the compound mutations they studied conferred resistance several-fold higher than that of either contributing mutation alone.
“We were able to sequence about 100 clinical samples, which gave us a very large body of data to shed light on the number of compound mutations and how they develop,” said Michael Deininger, MD, PhD, also of the Huntsman Cancer Institute.
“One key finding was that compound mutations containing an already known mutation called T315I tend to confer complete resistance to all available TKIs.”
The researchers had focused their testing on ponatinib, as the drug has proven effective against resistant CML, particularly cases with the T315I mutation. Unfortunately, ponatinib was often no match for compound mutations including T315I.
Tests did suggest that a 30 mg/day dose of ponatinib would maintain efficacy against 7 of the 8 non-T315I compound mutants tested, though the Y253H/E255V mutant proved resistant.
The researchers also found that a 15 mg/day dose of ponatinib could pre-empt outgrowth of 5 of the 8 non-T315I compound mutants, though Y253H/E255V, E255V/V299L, and F317L/F359V might be problematic.
But ponatinib proved substantially less effective against T315I-inclusive compound mutants. Nine of 10 T315I-inclusive compound mutants showed little or no sensitivity to ponatinib or any of the other TKIs tested. M244V/T315I was the only compound mutant not resistant to ponatinib.
The researchers noted that because ponatinib has proven effective against the T315I mutant in isolation, many patients treated with ponatinib are likely to have this mutation.
So it may be necessary to perform more sensitive screening on these patients to determine whether they might have T315I-inclusive compound mutants that could confer resistance.
“Fortunately, the problems we are studying affect a minority of CML patients,” Dr O’Hare said. “[S]till, this leaves some patients with no good treatment option at all. Our goal is to have a TKI option for every patient.”
According to Dr O’Hare, it’s only a matter of time until analogous compound mutations emerge in many other cancers, including acute myeloid leukemia and non-small cell lung cancer.
“Our findings in CML will provide a blueprint for contending with resistance in these highly aggressive diseases as well,” he concluded. ![]()

Credit: UC San Diego
In vitro experiments have revealed new insight into tyrosine kinase inhibitor (TKI) resistance among patients with chronic myeloid leukemia (CML).
Though it’s now possible to overcome TKI resistance resulting from single BCR-ABL1 mutants, targeting compound mutants remains a challenge.
So researchers tested several TKIs on various BCR-ABL1 compound mutants to determine which drug, if any, would be most effective for each combination.
The results appear in Cancer Cell.
Thomas O’Hare, PhD, of the Huntsman Cancer Institute at the University of Utah, and his colleagues first took an inventory of clinical BCR-ABL1 compound mutations associated with TKI resistance that had been reported in the literature.
The team identified 12 kinase domain positions that account for most clinical BCR-ABL1 TKI resistance—M244, G250, Q252, Y253, E255, V299, F311, T315, F317, M351, F359, and H396.
All of the clinically reported compound mutations include at least 1 of the 12 key positions, and most (65%) include 2. Each position has been implicated in resistance to 1 or more TKIs, including imatinib, nilotinib, dasatinib, bosutinib, rebastinib, and ponatinib.
The researchers found that some of the compound mutations they studied conferred resistance several-fold higher than that of either contributing mutation alone.
“We were able to sequence about 100 clinical samples, which gave us a very large body of data to shed light on the number of compound mutations and how they develop,” said Michael Deininger, MD, PhD, also of the Huntsman Cancer Institute.
“One key finding was that compound mutations containing an already known mutation called T315I tend to confer complete resistance to all available TKIs.”
The researchers had focused their testing on ponatinib, as the drug has proven effective against resistant CML, particularly cases with the T315I mutation. Unfortunately, ponatinib was often no match for compound mutations including T315I.
Tests did suggest that a 30 mg/day dose of ponatinib would maintain efficacy against 7 of the 8 non-T315I compound mutants tested, though the Y253H/E255V mutant proved resistant.
The researchers also found that a 15 mg/day dose of ponatinib could pre-empt outgrowth of 5 of the 8 non-T315I compound mutants, though Y253H/E255V, E255V/V299L, and F317L/F359V might be problematic.
But ponatinib proved substantially less effective against T315I-inclusive compound mutants. Nine of 10 T315I-inclusive compound mutants showed little or no sensitivity to ponatinib or any of the other TKIs tested. M244V/T315I was the only compound mutant not resistant to ponatinib.
The researchers noted that because ponatinib has proven effective against the T315I mutant in isolation, many patients treated with ponatinib are likely to have this mutation.
So it may be necessary to perform more sensitive screening on these patients to determine whether they might have T315I-inclusive compound mutants that could confer resistance.
“Fortunately, the problems we are studying affect a minority of CML patients,” Dr O’Hare said. “[S]till, this leaves some patients with no good treatment option at all. Our goal is to have a TKI option for every patient.”
According to Dr O’Hare, it’s only a matter of time until analogous compound mutations emerge in many other cancers, including acute myeloid leukemia and non-small cell lung cancer.
“Our findings in CML will provide a blueprint for contending with resistance in these highly aggressive diseases as well,” he concluded. ![]()
Depth of molecular response factors into safe TKI withdrawal in CML
MILAN – A full 61.5% of patients with chronic myeloid leukemia in deep molecular remission for more than 1 year on long-term tyrosine kinase inhibitor therapy remained alive and free of relapse 6 months after stopping their TKI in the EURO-SKI trial.
Molecular relapse-free survival at 9 months was 58% and 55% at 12 months.
The preplanned interim analysis after 200 patients allowed the trialists to discard the study’s null hypothesis that molecular relapse-free survival at 6 months would be 40% or less (P less than .0001).
"With less strict inclusion and relapse criteria than the A-STIM study and other trials, stopping is safe and we can continue with the trial," Dr. Susanne Saussele said in a late-breaking abstract session at the annual meeting of the European Hematology Association.
Importantly, interim results from the EURO-SKI (European Stop TKI) study also suggest that the level of molecular response (MR) achieved prior to TKI withdrawal affects molecular relapse-free survival.
Among 197 patients with molecular laboratory results available for exact classification, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
"In the setting of standardized molecular testing within a CML [chronic myeloid leukemia] stopping trial, it seems that molecular remission has an impact on molecular free-survival," said Dr. Saussele of University Medical Centre Mannheim, Germany.
As no statistical test was performed and this was an interim analysis, MR4.5 or MR5 cannot yet be used as a criterion to select patients to withdraw from treatment, she said in an interview.
The findings do confirm results from the recent A-STIM (According to Stop Imatinib) study showing that loss of major molecular response can be used as a practical criterion for restarting imatinib (J. Clin. Oncol. 2014;32:424-30).
Several studies including the STIM (Stop Imatinib) trial and the STOP 2G-TKI (Stop Second Generation Tyrosine Kinase Inhibitors) study have shown that imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna) can be safely withdrawn in a substantial proportion of patients with CML in deep MR.
A number of questions remain open, however, such as which molecular level has to be reached before stopping TKI therapy, the minimal duration of TKI pretreatment or MR4 before stopping, and which prognostic factors influence molecular relapse-free survival, she said.
EURO-SKI was set up to define prognostic markers to increase the rate of patients in durable deep MR after stopping TKI treatment. Other aims are to evaluate methods of molecular monitoring, quality of life, and saved treatment costs per country.
Patients with chronic-phase CML from eight countries were eligible if they were on a TKI for at least 3 years and had a confirmed deep MR, defined as more than a 4 log reduction in BCR-ABL (breakpoint cluster region–Abelson) transcripts for more than 12 months confirmed by three consecutive polymerase chain reaction results.
MR4 status also had to be confirmed in an MR4-standardized laboratory according to criteria by Cross et al. (Leukemia 2012;26:2172-5)
Patients with previous or planned allogeneic stem cell transplantation or a prior TKI failure were excluded.
A total of 103 patients received pretreatment before TKI therapy, mostly with hydroxyurea alone (n = 71) or with interferon (n = 22). Their median age was 53 years.
First-line TKI was imatinib in 194 patients, nilotinib in 3, and dasatinib in 3.
The median duration of TKI therapy was 8 years and median MR4 duration before stopping TKI therapy was 5 years.
Dr. Saussele cautioned that adverse events and quality of life must be taken into account when considering TKI withdrawal in these patients.
A total of 222 adverse events were reported in 98 patients, with 57 events in 37 patients related to treatment stop. None were grade 4.
The most common adverse event was musculoskeletal or joint pain (39 events of all grades and 6 grade 3/4 events). This was first described in the Swedish EURO-SKI patients in 15-20% of patients after TKI withdrawal, prompting the investigators to send an advisory letter to all participating physicians, she remarked.
Other adverse events included sweating, skin disorders, folliculitis, depressive episodes, fatigue, urticaria, and weight loss.
The EURO-SKI trial was recently expanded to enroll 700 patients, with a quality of life analysis expected later this year. The next report on the primary endpoint is expected at the American Society of Hematology annual meeting, Dr. Saussele said.
Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
MILAN – A full 61.5% of patients with chronic myeloid leukemia in deep molecular remission for more than 1 year on long-term tyrosine kinase inhibitor therapy remained alive and free of relapse 6 months after stopping their TKI in the EURO-SKI trial.
Molecular relapse-free survival at 9 months was 58% and 55% at 12 months.
The preplanned interim analysis after 200 patients allowed the trialists to discard the study’s null hypothesis that molecular relapse-free survival at 6 months would be 40% or less (P less than .0001).
"With less strict inclusion and relapse criteria than the A-STIM study and other trials, stopping is safe and we can continue with the trial," Dr. Susanne Saussele said in a late-breaking abstract session at the annual meeting of the European Hematology Association.
Importantly, interim results from the EURO-SKI (European Stop TKI) study also suggest that the level of molecular response (MR) achieved prior to TKI withdrawal affects molecular relapse-free survival.
Among 197 patients with molecular laboratory results available for exact classification, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
"In the setting of standardized molecular testing within a CML [chronic myeloid leukemia] stopping trial, it seems that molecular remission has an impact on molecular free-survival," said Dr. Saussele of University Medical Centre Mannheim, Germany.
As no statistical test was performed and this was an interim analysis, MR4.5 or MR5 cannot yet be used as a criterion to select patients to withdraw from treatment, she said in an interview.
The findings do confirm results from the recent A-STIM (According to Stop Imatinib) study showing that loss of major molecular response can be used as a practical criterion for restarting imatinib (J. Clin. Oncol. 2014;32:424-30).
Several studies including the STIM (Stop Imatinib) trial and the STOP 2G-TKI (Stop Second Generation Tyrosine Kinase Inhibitors) study have shown that imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna) can be safely withdrawn in a substantial proportion of patients with CML in deep MR.
A number of questions remain open, however, such as which molecular level has to be reached before stopping TKI therapy, the minimal duration of TKI pretreatment or MR4 before stopping, and which prognostic factors influence molecular relapse-free survival, she said.
EURO-SKI was set up to define prognostic markers to increase the rate of patients in durable deep MR after stopping TKI treatment. Other aims are to evaluate methods of molecular monitoring, quality of life, and saved treatment costs per country.
Patients with chronic-phase CML from eight countries were eligible if they were on a TKI for at least 3 years and had a confirmed deep MR, defined as more than a 4 log reduction in BCR-ABL (breakpoint cluster region–Abelson) transcripts for more than 12 months confirmed by three consecutive polymerase chain reaction results.
MR4 status also had to be confirmed in an MR4-standardized laboratory according to criteria by Cross et al. (Leukemia 2012;26:2172-5)
Patients with previous or planned allogeneic stem cell transplantation or a prior TKI failure were excluded.
A total of 103 patients received pretreatment before TKI therapy, mostly with hydroxyurea alone (n = 71) or with interferon (n = 22). Their median age was 53 years.
First-line TKI was imatinib in 194 patients, nilotinib in 3, and dasatinib in 3.
The median duration of TKI therapy was 8 years and median MR4 duration before stopping TKI therapy was 5 years.
Dr. Saussele cautioned that adverse events and quality of life must be taken into account when considering TKI withdrawal in these patients.
A total of 222 adverse events were reported in 98 patients, with 57 events in 37 patients related to treatment stop. None were grade 4.
The most common adverse event was musculoskeletal or joint pain (39 events of all grades and 6 grade 3/4 events). This was first described in the Swedish EURO-SKI patients in 15-20% of patients after TKI withdrawal, prompting the investigators to send an advisory letter to all participating physicians, she remarked.
Other adverse events included sweating, skin disorders, folliculitis, depressive episodes, fatigue, urticaria, and weight loss.
The EURO-SKI trial was recently expanded to enroll 700 patients, with a quality of life analysis expected later this year. The next report on the primary endpoint is expected at the American Society of Hematology annual meeting, Dr. Saussele said.
Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
MILAN – A full 61.5% of patients with chronic myeloid leukemia in deep molecular remission for more than 1 year on long-term tyrosine kinase inhibitor therapy remained alive and free of relapse 6 months after stopping their TKI in the EURO-SKI trial.
Molecular relapse-free survival at 9 months was 58% and 55% at 12 months.
The preplanned interim analysis after 200 patients allowed the trialists to discard the study’s null hypothesis that molecular relapse-free survival at 6 months would be 40% or less (P less than .0001).
"With less strict inclusion and relapse criteria than the A-STIM study and other trials, stopping is safe and we can continue with the trial," Dr. Susanne Saussele said in a late-breaking abstract session at the annual meeting of the European Hematology Association.
Importantly, interim results from the EURO-SKI (European Stop TKI) study also suggest that the level of molecular response (MR) achieved prior to TKI withdrawal affects molecular relapse-free survival.
Among 197 patients with molecular laboratory results available for exact classification, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
"In the setting of standardized molecular testing within a CML [chronic myeloid leukemia] stopping trial, it seems that molecular remission has an impact on molecular free-survival," said Dr. Saussele of University Medical Centre Mannheim, Germany.
As no statistical test was performed and this was an interim analysis, MR4.5 or MR5 cannot yet be used as a criterion to select patients to withdraw from treatment, she said in an interview.
The findings do confirm results from the recent A-STIM (According to Stop Imatinib) study showing that loss of major molecular response can be used as a practical criterion for restarting imatinib (J. Clin. Oncol. 2014;32:424-30).
Several studies including the STIM (Stop Imatinib) trial and the STOP 2G-TKI (Stop Second Generation Tyrosine Kinase Inhibitors) study have shown that imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna) can be safely withdrawn in a substantial proportion of patients with CML in deep MR.
A number of questions remain open, however, such as which molecular level has to be reached before stopping TKI therapy, the minimal duration of TKI pretreatment or MR4 before stopping, and which prognostic factors influence molecular relapse-free survival, she said.
EURO-SKI was set up to define prognostic markers to increase the rate of patients in durable deep MR after stopping TKI treatment. Other aims are to evaluate methods of molecular monitoring, quality of life, and saved treatment costs per country.
Patients with chronic-phase CML from eight countries were eligible if they were on a TKI for at least 3 years and had a confirmed deep MR, defined as more than a 4 log reduction in BCR-ABL (breakpoint cluster region–Abelson) transcripts for more than 12 months confirmed by three consecutive polymerase chain reaction results.
MR4 status also had to be confirmed in an MR4-standardized laboratory according to criteria by Cross et al. (Leukemia 2012;26:2172-5)
Patients with previous or planned allogeneic stem cell transplantation or a prior TKI failure were excluded.
A total of 103 patients received pretreatment before TKI therapy, mostly with hydroxyurea alone (n = 71) or with interferon (n = 22). Their median age was 53 years.
First-line TKI was imatinib in 194 patients, nilotinib in 3, and dasatinib in 3.
The median duration of TKI therapy was 8 years and median MR4 duration before stopping TKI therapy was 5 years.
Dr. Saussele cautioned that adverse events and quality of life must be taken into account when considering TKI withdrawal in these patients.
A total of 222 adverse events were reported in 98 patients, with 57 events in 37 patients related to treatment stop. None were grade 4.
The most common adverse event was musculoskeletal or joint pain (39 events of all grades and 6 grade 3/4 events). This was first described in the Swedish EURO-SKI patients in 15-20% of patients after TKI withdrawal, prompting the investigators to send an advisory letter to all participating physicians, she remarked.
Other adverse events included sweating, skin disorders, folliculitis, depressive episodes, fatigue, urticaria, and weight loss.
The EURO-SKI trial was recently expanded to enroll 700 patients, with a quality of life analysis expected later this year. The next report on the primary endpoint is expected at the American Society of Hematology annual meeting, Dr. Saussele said.
Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
AT THE EHA CONGRESS
Key clinical point: Long-time TKI therapy can be stopped in chronic myeloid leukemia in deep molecular remission. The level of molecular response prior to TKI withdrawal affects molecular relapse-free survival.
Major finding: After TKI withdrawal, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
Data source: First interim analysis from a prospective study in 200 CML patients in deep molecular remission.
Disclosures: Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
Depth of molecular response factors into safe TKI withdrawal in CML
MILAN – A full 61.5% of patients with chronic myeloid leukemia in deep molecular remission for more than 1 year on long-term tyrosine kinase inhibitor therapy remained alive and free of relapse 6 months after stopping their TKI in the EURO-SKI trial.
Molecular relapse-free survival at 9 months was 58% and 55% at 12 months.
The preplanned interim analysis after 200 patients allowed the trialists to discard the study’s null hypothesis that molecular relapse-free survival at 6 months would be 40% or less (P less than .0001).
"With less strict inclusion and relapse criteria than the A-STIM study and other trials, stopping is safe and we can continue with the trial," Dr. Susanne Saussele said in a late-breaking abstract session at the annual meeting of the European Hematology Association.
Importantly, interim results from the EURO-SKI (European Stop TKI) study also suggest that the level of molecular response (MR) achieved prior to TKI withdrawal affects molecular relapse-free survival.
Among 197 patients with molecular laboratory results available for exact classification, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
"In the setting of standardized molecular testing within a CML [chronic myeloid leukemia] stopping trial, it seems that molecular remission has an impact on molecular free-survival," said Dr. Saussele of University Medical Centre Mannheim, Germany.
As no statistical test was performed and this was an interim analysis, MR4.5 or MR5 cannot yet be used as a criterion to select patients to withdraw from treatment, she said in an interview.
The findings do confirm results from the recent A-STIM (According to Stop Imatinib) study showing that loss of major molecular response can be used as a practical criterion for restarting imatinib (J. Clin. Oncol. 2014;32:424-30).
Several studies including the STIM (Stop Imatinib) trial and the STOP 2G-TKI (Stop Second Generation Tyrosine Kinase Inhibitors) study have shown that imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna) can be safely withdrawn in a substantial proportion of patients with CML in deep MR.
A number of questions remain open, however, such as which molecular level has to be reached before stopping TKI therapy, the minimal duration of TKI pretreatment or MR4 before stopping, and which prognostic factors influence molecular relapse-free survival, she said.
EURO-SKI was set up to define prognostic markers to increase the rate of patients in durable deep MR after stopping TKI treatment. Other aims are to evaluate methods of molecular monitoring, quality of life, and saved treatment costs per country.
Patients with chronic-phase CML from eight countries were eligible if they were on a TKI for at least 3 years and had a confirmed deep MR, defined as more than a 4 log reduction in BCR-ABL (breakpoint cluster region–Abelson) transcripts for more than 12 months confirmed by three consecutive polymerase chain reaction results.
MR4 status also had to be confirmed in an MR4-standardized laboratory according to criteria by Cross et al. (Leukemia 2012;26:2172-5)
Patients with previous or planned allogeneic stem cell transplantation or a prior TKI failure were excluded.
A total of 103 patients received pretreatment before TKI therapy, mostly with hydroxyurea alone (n = 71) or with interferon (n = 22). Their median age was 53 years.
First-line TKI was imatinib in 194 patients, nilotinib in 3, and dasatinib in 3.
The median duration of TKI therapy was 8 years and median MR4 duration before stopping TKI therapy was 5 years.
Dr. Saussele cautioned that adverse events and quality of life must be taken into account when considering TKI withdrawal in these patients.
A total of 222 adverse events were reported in 98 patients, with 57 events in 37 patients related to treatment stop. None were grade 4.
The most common adverse event was musculoskeletal or joint pain (39 events of all grades and 6 grade 3/4 events). This was first described in the Swedish EURO-SKI patients in 15-20% of patients after TKI withdrawal, prompting the investigators to send an advisory letter to all participating physicians, she remarked.
Other adverse events included sweating, skin disorders, folliculitis, depressive episodes, fatigue, urticaria, and weight loss.
The EURO-SKI trial was recently expanded to enroll 700 patients, with a quality of life analysis expected later this year. The next report on the primary endpoint is expected at the American Society of Hematology annual meeting, Dr. Saussele said.
Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
MILAN – A full 61.5% of patients with chronic myeloid leukemia in deep molecular remission for more than 1 year on long-term tyrosine kinase inhibitor therapy remained alive and free of relapse 6 months after stopping their TKI in the EURO-SKI trial.
Molecular relapse-free survival at 9 months was 58% and 55% at 12 months.
The preplanned interim analysis after 200 patients allowed the trialists to discard the study’s null hypothesis that molecular relapse-free survival at 6 months would be 40% or less (P less than .0001).
"With less strict inclusion and relapse criteria than the A-STIM study and other trials, stopping is safe and we can continue with the trial," Dr. Susanne Saussele said in a late-breaking abstract session at the annual meeting of the European Hematology Association.
Importantly, interim results from the EURO-SKI (European Stop TKI) study also suggest that the level of molecular response (MR) achieved prior to TKI withdrawal affects molecular relapse-free survival.
Among 197 patients with molecular laboratory results available for exact classification, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
"In the setting of standardized molecular testing within a CML [chronic myeloid leukemia] stopping trial, it seems that molecular remission has an impact on molecular free-survival," said Dr. Saussele of University Medical Centre Mannheim, Germany.
As no statistical test was performed and this was an interim analysis, MR4.5 or MR5 cannot yet be used as a criterion to select patients to withdraw from treatment, she said in an interview.
The findings do confirm results from the recent A-STIM (According to Stop Imatinib) study showing that loss of major molecular response can be used as a practical criterion for restarting imatinib (J. Clin. Oncol. 2014;32:424-30).
Several studies including the STIM (Stop Imatinib) trial and the STOP 2G-TKI (Stop Second Generation Tyrosine Kinase Inhibitors) study have shown that imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna) can be safely withdrawn in a substantial proportion of patients with CML in deep MR.
A number of questions remain open, however, such as which molecular level has to be reached before stopping TKI therapy, the minimal duration of TKI pretreatment or MR4 before stopping, and which prognostic factors influence molecular relapse-free survival, she said.
EURO-SKI was set up to define prognostic markers to increase the rate of patients in durable deep MR after stopping TKI treatment. Other aims are to evaluate methods of molecular monitoring, quality of life, and saved treatment costs per country.
Patients with chronic-phase CML from eight countries were eligible if they were on a TKI for at least 3 years and had a confirmed deep MR, defined as more than a 4 log reduction in BCR-ABL (breakpoint cluster region–Abelson) transcripts for more than 12 months confirmed by three consecutive polymerase chain reaction results.
MR4 status also had to be confirmed in an MR4-standardized laboratory according to criteria by Cross et al. (Leukemia 2012;26:2172-5)
Patients with previous or planned allogeneic stem cell transplantation or a prior TKI failure were excluded.
A total of 103 patients received pretreatment before TKI therapy, mostly with hydroxyurea alone (n = 71) or with interferon (n = 22). Their median age was 53 years.
First-line TKI was imatinib in 194 patients, nilotinib in 3, and dasatinib in 3.
The median duration of TKI therapy was 8 years and median MR4 duration before stopping TKI therapy was 5 years.
Dr. Saussele cautioned that adverse events and quality of life must be taken into account when considering TKI withdrawal in these patients.
A total of 222 adverse events were reported in 98 patients, with 57 events in 37 patients related to treatment stop. None were grade 4.
The most common adverse event was musculoskeletal or joint pain (39 events of all grades and 6 grade 3/4 events). This was first described in the Swedish EURO-SKI patients in 15-20% of patients after TKI withdrawal, prompting the investigators to send an advisory letter to all participating physicians, she remarked.
Other adverse events included sweating, skin disorders, folliculitis, depressive episodes, fatigue, urticaria, and weight loss.
The EURO-SKI trial was recently expanded to enroll 700 patients, with a quality of life analysis expected later this year. The next report on the primary endpoint is expected at the American Society of Hematology annual meeting, Dr. Saussele said.
Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
MILAN – A full 61.5% of patients with chronic myeloid leukemia in deep molecular remission for more than 1 year on long-term tyrosine kinase inhibitor therapy remained alive and free of relapse 6 months after stopping their TKI in the EURO-SKI trial.
Molecular relapse-free survival at 9 months was 58% and 55% at 12 months.
The preplanned interim analysis after 200 patients allowed the trialists to discard the study’s null hypothesis that molecular relapse-free survival at 6 months would be 40% or less (P less than .0001).
"With less strict inclusion and relapse criteria than the A-STIM study and other trials, stopping is safe and we can continue with the trial," Dr. Susanne Saussele said in a late-breaking abstract session at the annual meeting of the European Hematology Association.
Importantly, interim results from the EURO-SKI (European Stop TKI) study also suggest that the level of molecular response (MR) achieved prior to TKI withdrawal affects molecular relapse-free survival.
Among 197 patients with molecular laboratory results available for exact classification, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
"In the setting of standardized molecular testing within a CML [chronic myeloid leukemia] stopping trial, it seems that molecular remission has an impact on molecular free-survival," said Dr. Saussele of University Medical Centre Mannheim, Germany.
As no statistical test was performed and this was an interim analysis, MR4.5 or MR5 cannot yet be used as a criterion to select patients to withdraw from treatment, she said in an interview.
The findings do confirm results from the recent A-STIM (According to Stop Imatinib) study showing that loss of major molecular response can be used as a practical criterion for restarting imatinib (J. Clin. Oncol. 2014;32:424-30).
Several studies including the STIM (Stop Imatinib) trial and the STOP 2G-TKI (Stop Second Generation Tyrosine Kinase Inhibitors) study have shown that imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna) can be safely withdrawn in a substantial proportion of patients with CML in deep MR.
A number of questions remain open, however, such as which molecular level has to be reached before stopping TKI therapy, the minimal duration of TKI pretreatment or MR4 before stopping, and which prognostic factors influence molecular relapse-free survival, she said.
EURO-SKI was set up to define prognostic markers to increase the rate of patients in durable deep MR after stopping TKI treatment. Other aims are to evaluate methods of molecular monitoring, quality of life, and saved treatment costs per country.
Patients with chronic-phase CML from eight countries were eligible if they were on a TKI for at least 3 years and had a confirmed deep MR, defined as more than a 4 log reduction in BCR-ABL (breakpoint cluster region–Abelson) transcripts for more than 12 months confirmed by three consecutive polymerase chain reaction results.
MR4 status also had to be confirmed in an MR4-standardized laboratory according to criteria by Cross et al. (Leukemia 2012;26:2172-5)
Patients with previous or planned allogeneic stem cell transplantation or a prior TKI failure were excluded.
A total of 103 patients received pretreatment before TKI therapy, mostly with hydroxyurea alone (n = 71) or with interferon (n = 22). Their median age was 53 years.
First-line TKI was imatinib in 194 patients, nilotinib in 3, and dasatinib in 3.
The median duration of TKI therapy was 8 years and median MR4 duration before stopping TKI therapy was 5 years.
Dr. Saussele cautioned that adverse events and quality of life must be taken into account when considering TKI withdrawal in these patients.
A total of 222 adverse events were reported in 98 patients, with 57 events in 37 patients related to treatment stop. None were grade 4.
The most common adverse event was musculoskeletal or joint pain (39 events of all grades and 6 grade 3/4 events). This was first described in the Swedish EURO-SKI patients in 15-20% of patients after TKI withdrawal, prompting the investigators to send an advisory letter to all participating physicians, she remarked.
Other adverse events included sweating, skin disorders, folliculitis, depressive episodes, fatigue, urticaria, and weight loss.
The EURO-SKI trial was recently expanded to enroll 700 patients, with a quality of life analysis expected later this year. The next report on the primary endpoint is expected at the American Society of Hematology annual meeting, Dr. Saussele said.
Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
AT THE EHA CONGRESS
Key clinical point: Long-time TKI therapy can be stopped in chronic myeloid leukemia in deep molecular remission. The level of molecular response prior to TKI withdrawal affects molecular relapse-free survival.
Major finding: After TKI withdrawal, 49% of patients in MR4 relapsed, compared with 39% in MR4.5 and 39% in MR5.
Data source: First interim analysis from a prospective study in 200 CML patients in deep molecular remission.
Disclosures: Dr. Saussele reported honoraria and research and travel support from BMS, Novartis, and Pfizer.
Switch to nilotinib improves deep molecular response in CML

©ASCO/Phil McCarten
CHICAGO—Patients with chronic myeloid leukemia (CML) are more likely to achieve a deep molecular response if they switch to nilotinib rather than remain on imatinib, updated research suggests.
Patients with detectable disease who crossed over from imatinib to nilotinib after 24 months on the ENESTcmr study were able to achieve deep molecular responses (MR 4.5) by 36 months.
But none of the patients who remained on imatinib achieved undetectable BCR-ABL transcripts.
“Longer follow-up supports switching from imatinib to nilotinib to attain deep molecular responses,” said study investigator Nelson Spector, MD, of Federal University of Rio de Janeiro in Brazil.
“Achievement of deeper molecular responses with nilotinib therapy may increase patient eligibility for treatment-free remission trials.”
Dr Spector presented these results—an update of the ongoing, phase 3 ENESTcmr study—at the 2014 ASCO Annual Meeting (abstract 7025).
The study included 207 patients with Philadelphia-chromosome-positive CML in chronic phase who were treated with imatinib for at least 2 years and achieved complete cytogenetic response but had detectable BCR-ABL transcripts.
With 36 months’ follow-up, rates of MR 4.5 remained higher with nilotinib vs imatinib (47% vs 33%). The median time to achievement of MR 4.5 was 24 months with nilotinib and was not reached with imatinib after the 36-month follow-up.
“Patients experienced a rapid reduction in median BCR-ABL levels within the first 3 months after crossover from imatinib to nilotinib,” Dr Spector said. “No patient with detectable BCR-ABL at 24 months who remained on imatinib achieved this response with another year of follow-up.”
MR 4.5 rates were higher by 12 months in patients randomized to nilotinib (33%) than patients who crossed over from imatinib to nilotinib (21%).
“Three years follow-up shows the ability to achieve undetectable BCR-ABL status with nilotinib as we strive to achieve deep molecular responses with the potential to stop therapy,” said ASCO discussant Michael Mauro, MD, of the Memorial Sloan-Kettering Cancer Center in New York.
However, he questioned whether MR 4.5, “the last, deepest milestone of relevance, is enough to assess stopping.”
By 3 years of follow-up, 93 patients (92.1%) on nilotinib and 72 patients (69.9%) on imatinib had drug-related adverse events.
The most common events with nilotinib were headache, rash, and pruritus. With imatinib, the most common events were muscle spasms, nausea, and diarrhea. More cardiovascular events were reported in patients randomized to nilotinib compared with imatinib.
“Nilotinib-treated patients experienced adverse events early after switching from long-term imatinib therapy,” Dr Spector said. “These adverse events were expected and consistent with the safety profile of nilotinib observed in other studies.” ![]()

©ASCO/Phil McCarten
CHICAGO—Patients with chronic myeloid leukemia (CML) are more likely to achieve a deep molecular response if they switch to nilotinib rather than remain on imatinib, updated research suggests.
Patients with detectable disease who crossed over from imatinib to nilotinib after 24 months on the ENESTcmr study were able to achieve deep molecular responses (MR 4.5) by 36 months.
But none of the patients who remained on imatinib achieved undetectable BCR-ABL transcripts.
“Longer follow-up supports switching from imatinib to nilotinib to attain deep molecular responses,” said study investigator Nelson Spector, MD, of Federal University of Rio de Janeiro in Brazil.
“Achievement of deeper molecular responses with nilotinib therapy may increase patient eligibility for treatment-free remission trials.”
Dr Spector presented these results—an update of the ongoing, phase 3 ENESTcmr study—at the 2014 ASCO Annual Meeting (abstract 7025).
The study included 207 patients with Philadelphia-chromosome-positive CML in chronic phase who were treated with imatinib for at least 2 years and achieved complete cytogenetic response but had detectable BCR-ABL transcripts.
With 36 months’ follow-up, rates of MR 4.5 remained higher with nilotinib vs imatinib (47% vs 33%). The median time to achievement of MR 4.5 was 24 months with nilotinib and was not reached with imatinib after the 36-month follow-up.
“Patients experienced a rapid reduction in median BCR-ABL levels within the first 3 months after crossover from imatinib to nilotinib,” Dr Spector said. “No patient with detectable BCR-ABL at 24 months who remained on imatinib achieved this response with another year of follow-up.”
MR 4.5 rates were higher by 12 months in patients randomized to nilotinib (33%) than patients who crossed over from imatinib to nilotinib (21%).
“Three years follow-up shows the ability to achieve undetectable BCR-ABL status with nilotinib as we strive to achieve deep molecular responses with the potential to stop therapy,” said ASCO discussant Michael Mauro, MD, of the Memorial Sloan-Kettering Cancer Center in New York.
However, he questioned whether MR 4.5, “the last, deepest milestone of relevance, is enough to assess stopping.”
By 3 years of follow-up, 93 patients (92.1%) on nilotinib and 72 patients (69.9%) on imatinib had drug-related adverse events.
The most common events with nilotinib were headache, rash, and pruritus. With imatinib, the most common events were muscle spasms, nausea, and diarrhea. More cardiovascular events were reported in patients randomized to nilotinib compared with imatinib.
“Nilotinib-treated patients experienced adverse events early after switching from long-term imatinib therapy,” Dr Spector said. “These adverse events were expected and consistent with the safety profile of nilotinib observed in other studies.” ![]()

©ASCO/Phil McCarten
CHICAGO—Patients with chronic myeloid leukemia (CML) are more likely to achieve a deep molecular response if they switch to nilotinib rather than remain on imatinib, updated research suggests.
Patients with detectable disease who crossed over from imatinib to nilotinib after 24 months on the ENESTcmr study were able to achieve deep molecular responses (MR 4.5) by 36 months.
But none of the patients who remained on imatinib achieved undetectable BCR-ABL transcripts.
“Longer follow-up supports switching from imatinib to nilotinib to attain deep molecular responses,” said study investigator Nelson Spector, MD, of Federal University of Rio de Janeiro in Brazil.
“Achievement of deeper molecular responses with nilotinib therapy may increase patient eligibility for treatment-free remission trials.”
Dr Spector presented these results—an update of the ongoing, phase 3 ENESTcmr study—at the 2014 ASCO Annual Meeting (abstract 7025).
The study included 207 patients with Philadelphia-chromosome-positive CML in chronic phase who were treated with imatinib for at least 2 years and achieved complete cytogenetic response but had detectable BCR-ABL transcripts.
With 36 months’ follow-up, rates of MR 4.5 remained higher with nilotinib vs imatinib (47% vs 33%). The median time to achievement of MR 4.5 was 24 months with nilotinib and was not reached with imatinib after the 36-month follow-up.
“Patients experienced a rapid reduction in median BCR-ABL levels within the first 3 months after crossover from imatinib to nilotinib,” Dr Spector said. “No patient with detectable BCR-ABL at 24 months who remained on imatinib achieved this response with another year of follow-up.”
MR 4.5 rates were higher by 12 months in patients randomized to nilotinib (33%) than patients who crossed over from imatinib to nilotinib (21%).
“Three years follow-up shows the ability to achieve undetectable BCR-ABL status with nilotinib as we strive to achieve deep molecular responses with the potential to stop therapy,” said ASCO discussant Michael Mauro, MD, of the Memorial Sloan-Kettering Cancer Center in New York.
However, he questioned whether MR 4.5, “the last, deepest milestone of relevance, is enough to assess stopping.”
By 3 years of follow-up, 93 patients (92.1%) on nilotinib and 72 patients (69.9%) on imatinib had drug-related adverse events.
The most common events with nilotinib were headache, rash, and pruritus. With imatinib, the most common events were muscle spasms, nausea, and diarrhea. More cardiovascular events were reported in patients randomized to nilotinib compared with imatinib.
“Nilotinib-treated patients experienced adverse events early after switching from long-term imatinib therapy,” Dr Spector said. “These adverse events were expected and consistent with the safety profile of nilotinib observed in other studies.” ![]()
Imatinib appears safe, effective for the long haul
CHICAGO – After a decade on therapy with imatinib, a majority of patients with chronic myeloid leukemia will experience an adverse drug reaction, but most reactions are mild and manageable, according to results from a study presented at the annual meeting of the American Society of Clinical Oncology.
Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy at some point, 1,018 (74%) had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities, and there were no deaths attributed to imatinib, reported Dr. Rüdiger Hehlmann of the University of Heidelberg, Germany, and his colleagues.
Adverse drug reactions were manageable even when imatinib was combined with interferon-alfa (IFN-alfa), the investigators from the German CML Study Group reported in a poster at the meeting.
"After 10 years, imatinib continues to be an excellent choice for most patients with CML," they wrote.
In the 13 years that have elapsed since imatinib was approved in the United States as the first-in-class tyrosine kinase inhibitor, second-generation TKIs and other targeted agents have emerged, drawing attention to the safety of the older regimen.
The investigators evaluated long-term follow-up data and analyzed adverse drug reaction data for 1,501 patients treated with imatinib monotherapy in doses of 400 or 800 mg/day, as well as imatinib 400 mg in combination with IFN-alfa.
At the most recent evaluation, in November 2013, 164 patients had died, 1,003 were still on imatinib, 275 had been switched to a second-generation TKI, and 106 underwent bone marrow transplant (some patients received more than one therapy, accounting for the difference in total numbers).
The median follow-up time was 6.5 years, with some patients on study for as long as 11.5 years.
The probability of 10-year survival was 84%, and of 10-year progression-free survival was 81%.
An analysis of survival by molecular response rates showed an overall survival rate of 89% for those who achieved a major molecular response (MR, defined as a BCR-ABL RNA level of 0.1% or less), and 74% for those who achieved MR 4.5 (a 4.5 log10reduction or greater in BCR-ABL transcripts).
The 8-year probabilities for all grades of adverse events among the patients who received imatinib monotherapy were 41% for edema or fluid overload, 38% for gastrointestinal toxicities, 25% for myalgia/arthralgia, 20% for rash, 17% for musculoskeletal events, 17% for fatigue, 11% for neurological toxicities, and 10% for flulike symptoms.
Five patients had grade 2 or 3 peripheral arterial occlusive disease, but it was not clear whether these events were associated with imatinib.
For most patients the first adverse drug reaction occurred within 3 years of starting on imatinib, with the frequency of reactions decreasing thereafter.
Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
CHICAGO – After a decade on therapy with imatinib, a majority of patients with chronic myeloid leukemia will experience an adverse drug reaction, but most reactions are mild and manageable, according to results from a study presented at the annual meeting of the American Society of Clinical Oncology.
Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy at some point, 1,018 (74%) had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities, and there were no deaths attributed to imatinib, reported Dr. Rüdiger Hehlmann of the University of Heidelberg, Germany, and his colleagues.
Adverse drug reactions were manageable even when imatinib was combined with interferon-alfa (IFN-alfa), the investigators from the German CML Study Group reported in a poster at the meeting.
"After 10 years, imatinib continues to be an excellent choice for most patients with CML," they wrote.
In the 13 years that have elapsed since imatinib was approved in the United States as the first-in-class tyrosine kinase inhibitor, second-generation TKIs and other targeted agents have emerged, drawing attention to the safety of the older regimen.
The investigators evaluated long-term follow-up data and analyzed adverse drug reaction data for 1,501 patients treated with imatinib monotherapy in doses of 400 or 800 mg/day, as well as imatinib 400 mg in combination with IFN-alfa.
At the most recent evaluation, in November 2013, 164 patients had died, 1,003 were still on imatinib, 275 had been switched to a second-generation TKI, and 106 underwent bone marrow transplant (some patients received more than one therapy, accounting for the difference in total numbers).
The median follow-up time was 6.5 years, with some patients on study for as long as 11.5 years.
The probability of 10-year survival was 84%, and of 10-year progression-free survival was 81%.
An analysis of survival by molecular response rates showed an overall survival rate of 89% for those who achieved a major molecular response (MR, defined as a BCR-ABL RNA level of 0.1% or less), and 74% for those who achieved MR 4.5 (a 4.5 log10reduction or greater in BCR-ABL transcripts).
The 8-year probabilities for all grades of adverse events among the patients who received imatinib monotherapy were 41% for edema or fluid overload, 38% for gastrointestinal toxicities, 25% for myalgia/arthralgia, 20% for rash, 17% for musculoskeletal events, 17% for fatigue, 11% for neurological toxicities, and 10% for flulike symptoms.
Five patients had grade 2 or 3 peripheral arterial occlusive disease, but it was not clear whether these events were associated with imatinib.
For most patients the first adverse drug reaction occurred within 3 years of starting on imatinib, with the frequency of reactions decreasing thereafter.
Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
CHICAGO – After a decade on therapy with imatinib, a majority of patients with chronic myeloid leukemia will experience an adverse drug reaction, but most reactions are mild and manageable, according to results from a study presented at the annual meeting of the American Society of Clinical Oncology.
Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy at some point, 1,018 (74%) had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities, and there were no deaths attributed to imatinib, reported Dr. Rüdiger Hehlmann of the University of Heidelberg, Germany, and his colleagues.
Adverse drug reactions were manageable even when imatinib was combined with interferon-alfa (IFN-alfa), the investigators from the German CML Study Group reported in a poster at the meeting.
"After 10 years, imatinib continues to be an excellent choice for most patients with CML," they wrote.
In the 13 years that have elapsed since imatinib was approved in the United States as the first-in-class tyrosine kinase inhibitor, second-generation TKIs and other targeted agents have emerged, drawing attention to the safety of the older regimen.
The investigators evaluated long-term follow-up data and analyzed adverse drug reaction data for 1,501 patients treated with imatinib monotherapy in doses of 400 or 800 mg/day, as well as imatinib 400 mg in combination with IFN-alfa.
At the most recent evaluation, in November 2013, 164 patients had died, 1,003 were still on imatinib, 275 had been switched to a second-generation TKI, and 106 underwent bone marrow transplant (some patients received more than one therapy, accounting for the difference in total numbers).
The median follow-up time was 6.5 years, with some patients on study for as long as 11.5 years.
The probability of 10-year survival was 84%, and of 10-year progression-free survival was 81%.
An analysis of survival by molecular response rates showed an overall survival rate of 89% for those who achieved a major molecular response (MR, defined as a BCR-ABL RNA level of 0.1% or less), and 74% for those who achieved MR 4.5 (a 4.5 log10reduction or greater in BCR-ABL transcripts).
The 8-year probabilities for all grades of adverse events among the patients who received imatinib monotherapy were 41% for edema or fluid overload, 38% for gastrointestinal toxicities, 25% for myalgia/arthralgia, 20% for rash, 17% for musculoskeletal events, 17% for fatigue, 11% for neurological toxicities, and 10% for flulike symptoms.
Five patients had grade 2 or 3 peripheral arterial occlusive disease, but it was not clear whether these events were associated with imatinib.
For most patients the first adverse drug reaction occurred within 3 years of starting on imatinib, with the frequency of reactions decreasing thereafter.
Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
AT THE ASCO ANNUAL MEETING 2014
Key clinical finding: Imatinib is safe and effective for treating patients with chronic myeloid leukemia over the course of a decade.
Major finding: Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy, 74% had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities.
Data source: Review of prospectively collected 10-year follow-up data from a phase II trial of imatinib in 1,501 patients with CML.
Disclosures: Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
Molecular monitoring and minimal residual disease in the management of chronic myelogenous leukemia
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
FDA approves CML drug for home administration

The US Food and Drug Administration (FDA) has expanded the approval of omacetaxine mepesuccinate (Synribo) to include home administration.
The drug is already FDA-approved to treat adults with chronic or accelerated phase chronic myeloid leukemia (CML) who do not respond to or cannot tolerate 2 or more tyrosine kinase inhibitors.
The new approval allows CML patients to self-administer subcutaneous injections of omacetaxine mepesuccinate at home.
“It had been necessary for adults living with chronic or accelerated phase CML who are prescribed Synribo to travel to their doctor’s office twice a day for 2 weeks, which can be extremely burdensome and inconvenient to both patients and their caregivers,” said Meir Wetzler, MD, FACP, Chief of the Leukemia Section at Roswell Park Cancer Institute in Buffalo, New York.
“Now, physicians can decide if their patients are candidates for self-administration and, if so, provide their patients with guidance on how to properly administer reconstituted Synribo in the home.”
The drug’s maker, Teva Pharmaceutical Industries, Ltd., is working to finalize a pharmacy support program that will help facilitate successful home administration of omacetaxine mepesuccinate. The program is expected to “go live” this month or next.
About omacetaxine mepesuccinate
Omacetaxine mepesuccinate is a protein synthesis inhibitor. Although the drug’s mechanism of action is not fully understood, it is known to prevent the production of Bcr-Abl and Mcl-1, which help drive CML.
In October 2012, the FDA granted omacetaxine mepesuccinate accelerated approval for the treatment of adult patients with chronic or accelerated phase CML with resistance and/or intolerance to 2 or more tyrosine kinase inhibitors. Omacetaxine mepesuccinate gained full FDA approval in February.
The drug has been associated with severe and fatal myelosuppression, including thrombocytopenia, neutropenia, and anemia in some patients. So healthcare professionals should monitor patients’ complete blood counts weekly during induction and initial maintenance cycles and every 2 weeks during later maintenance cycles, as clinically indicated.
Omacetaxine mepesuccinate has been known to cause severe thrombocytopenia, which increases the risk of hemorrhage. Fatalities from cerebral hemorrhage have occurred. And severe, non-fatal gastrointestinal hemorrhages have occurred.
So healthcare professionals should monitor platelet counts as part of the complete blood count as recommended. Patients should not receive anticoagulants, aspirin, or non-steroidal anti-inflammatory drugs when their platelet counts are <50,000/μL, as these drugs may increase the risk of bleeding.
Omacetaxine mepesuccinate can induce glucose intolerance as well. So healthcare professionals should monitor blood glucose levels frequently, especially in patients with diabetes or risk factors for diabetes. Patients with poorly controlled diabetes mellitus should not receive omacetaxine mepesuccinate until good glycemic control has been established.
Omacetaxine mepesuccinate can cause fetal harm when administered to a pregnant woman. So women should be advised to avoid becoming pregnant while using the drug.
For more details on omacetaxine mepesuccinate, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has expanded the approval of omacetaxine mepesuccinate (Synribo) to include home administration.
The drug is already FDA-approved to treat adults with chronic or accelerated phase chronic myeloid leukemia (CML) who do not respond to or cannot tolerate 2 or more tyrosine kinase inhibitors.
The new approval allows CML patients to self-administer subcutaneous injections of omacetaxine mepesuccinate at home.
“It had been necessary for adults living with chronic or accelerated phase CML who are prescribed Synribo to travel to their doctor’s office twice a day for 2 weeks, which can be extremely burdensome and inconvenient to both patients and their caregivers,” said Meir Wetzler, MD, FACP, Chief of the Leukemia Section at Roswell Park Cancer Institute in Buffalo, New York.
“Now, physicians can decide if their patients are candidates for self-administration and, if so, provide their patients with guidance on how to properly administer reconstituted Synribo in the home.”
The drug’s maker, Teva Pharmaceutical Industries, Ltd., is working to finalize a pharmacy support program that will help facilitate successful home administration of omacetaxine mepesuccinate. The program is expected to “go live” this month or next.
About omacetaxine mepesuccinate
Omacetaxine mepesuccinate is a protein synthesis inhibitor. Although the drug’s mechanism of action is not fully understood, it is known to prevent the production of Bcr-Abl and Mcl-1, which help drive CML.
In October 2012, the FDA granted omacetaxine mepesuccinate accelerated approval for the treatment of adult patients with chronic or accelerated phase CML with resistance and/or intolerance to 2 or more tyrosine kinase inhibitors. Omacetaxine mepesuccinate gained full FDA approval in February.
The drug has been associated with severe and fatal myelosuppression, including thrombocytopenia, neutropenia, and anemia in some patients. So healthcare professionals should monitor patients’ complete blood counts weekly during induction and initial maintenance cycles and every 2 weeks during later maintenance cycles, as clinically indicated.
Omacetaxine mepesuccinate has been known to cause severe thrombocytopenia, which increases the risk of hemorrhage. Fatalities from cerebral hemorrhage have occurred. And severe, non-fatal gastrointestinal hemorrhages have occurred.
So healthcare professionals should monitor platelet counts as part of the complete blood count as recommended. Patients should not receive anticoagulants, aspirin, or non-steroidal anti-inflammatory drugs when their platelet counts are <50,000/μL, as these drugs may increase the risk of bleeding.
Omacetaxine mepesuccinate can induce glucose intolerance as well. So healthcare professionals should monitor blood glucose levels frequently, especially in patients with diabetes or risk factors for diabetes. Patients with poorly controlled diabetes mellitus should not receive omacetaxine mepesuccinate until good glycemic control has been established.
Omacetaxine mepesuccinate can cause fetal harm when administered to a pregnant woman. So women should be advised to avoid becoming pregnant while using the drug.
For more details on omacetaxine mepesuccinate, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has expanded the approval of omacetaxine mepesuccinate (Synribo) to include home administration.
The drug is already FDA-approved to treat adults with chronic or accelerated phase chronic myeloid leukemia (CML) who do not respond to or cannot tolerate 2 or more tyrosine kinase inhibitors.
The new approval allows CML patients to self-administer subcutaneous injections of omacetaxine mepesuccinate at home.
“It had been necessary for adults living with chronic or accelerated phase CML who are prescribed Synribo to travel to their doctor’s office twice a day for 2 weeks, which can be extremely burdensome and inconvenient to both patients and their caregivers,” said Meir Wetzler, MD, FACP, Chief of the Leukemia Section at Roswell Park Cancer Institute in Buffalo, New York.
“Now, physicians can decide if their patients are candidates for self-administration and, if so, provide their patients with guidance on how to properly administer reconstituted Synribo in the home.”
The drug’s maker, Teva Pharmaceutical Industries, Ltd., is working to finalize a pharmacy support program that will help facilitate successful home administration of omacetaxine mepesuccinate. The program is expected to “go live” this month or next.
About omacetaxine mepesuccinate
Omacetaxine mepesuccinate is a protein synthesis inhibitor. Although the drug’s mechanism of action is not fully understood, it is known to prevent the production of Bcr-Abl and Mcl-1, which help drive CML.
In October 2012, the FDA granted omacetaxine mepesuccinate accelerated approval for the treatment of adult patients with chronic or accelerated phase CML with resistance and/or intolerance to 2 or more tyrosine kinase inhibitors. Omacetaxine mepesuccinate gained full FDA approval in February.
The drug has been associated with severe and fatal myelosuppression, including thrombocytopenia, neutropenia, and anemia in some patients. So healthcare professionals should monitor patients’ complete blood counts weekly during induction and initial maintenance cycles and every 2 weeks during later maintenance cycles, as clinically indicated.
Omacetaxine mepesuccinate has been known to cause severe thrombocytopenia, which increases the risk of hemorrhage. Fatalities from cerebral hemorrhage have occurred. And severe, non-fatal gastrointestinal hemorrhages have occurred.
So healthcare professionals should monitor platelet counts as part of the complete blood count as recommended. Patients should not receive anticoagulants, aspirin, or non-steroidal anti-inflammatory drugs when their platelet counts are <50,000/μL, as these drugs may increase the risk of bleeding.
Omacetaxine mepesuccinate can induce glucose intolerance as well. So healthcare professionals should monitor blood glucose levels frequently, especially in patients with diabetes or risk factors for diabetes. Patients with poorly controlled diabetes mellitus should not receive omacetaxine mepesuccinate until good glycemic control has been established.
Omacetaxine mepesuccinate can cause fetal harm when administered to a pregnant woman. So women should be advised to avoid becoming pregnant while using the drug.
For more details on omacetaxine mepesuccinate, see the full prescribing information. ![]()
Cell adherence linked to treatment resistance in CML

Credit: UC San Diego
Preclinical research in chronic myeloid leukemia (CML) has pointed to a relationship between cell adherence and treatment resistance.
Investigators found that a population of plastic-adherent K562 cells with increased expression of BCR-ABL exhibited greater resistance to the tyrosine kinase inhibitor imatinib than nonadherent K562 cells.
“Previous studies have linked high levels of the BCR-ABL mutation with drug resistance,” said Richard Byers, PhD, of The University of Manchester in the UK.
“We wanted to see how expression of BCR-ABL differed across groups of CML cells and, in particular, whether there were differences between adherent and nonadherent populations.”
Dr Byers and his colleagues described this investigation in Experimental Hematology.
The researchers evaluated the heterogeneity of BCR-ABL expression at DNA, messenger RNA, and protein levels, using the CML-derived K562 cell line.
They grew cells in suspension and found that some cells adhered to the plastic dish. The investigators then separated the plastic-adherent and nonadherent cell populations and studied them as single cells and in bulk.
The first discovery was that adherent and nonadherent cells had similar BCR-ABL fusion gene copy numbers.
In bulk-cell analysis, the mean relative normalized ratio for genomic ABL DNA copy number was 47.73 for adherent cells and 53.40 for nonadherent cells (P=0.11). In single-cell analysis, the mean copy numbers were 13.83 and 14.22, respectively (P=0.63).
On the other hand, there was a significant difference in BCR-ABL messenger RNA expression between adherent and nonadherent cells.
In bulk cells, the level of BCR-ABL messenger RNA transcripts was 11-fold higher in adherent cells than in nondherent cells (P=0.022). And single-cell analysis revealed the mean BCR-ABL copy number was 53.11 for adherent cells and 14.06 for nonadherent cells (P=0.0013).
Adherent cells also exhibited significantly upregulated phosphorylation of BCR protein compared to nonadherent cells.
Flow cytometry showed that a mean of 61.9% of adherent cells were positive for phosphor-BCR, compared to 14.5% of nonadherent cells (P=0.0074). And single-cell analysis revealed a mean signal number per cell of 8.23 among adherent cells and 3.02 among nonadherent cells (P<0.0001).
In addition, whole-genome microRNA profiling showed that adherent and nonadherent cell populations expressed significantly different microRNA species.
Finally, the researchers found that treatment with imatinib reduced cell viability more rapidly in nonadherent cells than in adherent cells (P<0.005). The adherent cells showed a decrease in cell viability at 24 hours, compared to 4 hours for nonadherent cells.
The investigators said this research suggests that CML patients may have a similar adherent cell population that mediates resistance to imatinib. And the study highlights the importance of single-cell analysis.
“The small number of cells that show high levels of BCR-ABL may not be detectable through bulk analysis of large samples,” Dr Byers said. “It looks like it is important to look at protein levels in single cells.”
“In future, it may be possible to measure BCR-ABL levels in individual cells in the clinic. This will help us identify the resistant, high-BCR-ABL cells and better understand how patients develop resistance to imatinib treatment, with the aim of combating this resistance to make response more durable and the treatment more effective.” ![]()

Credit: UC San Diego
Preclinical research in chronic myeloid leukemia (CML) has pointed to a relationship between cell adherence and treatment resistance.
Investigators found that a population of plastic-adherent K562 cells with increased expression of BCR-ABL exhibited greater resistance to the tyrosine kinase inhibitor imatinib than nonadherent K562 cells.
“Previous studies have linked high levels of the BCR-ABL mutation with drug resistance,” said Richard Byers, PhD, of The University of Manchester in the UK.
“We wanted to see how expression of BCR-ABL differed across groups of CML cells and, in particular, whether there were differences between adherent and nonadherent populations.”
Dr Byers and his colleagues described this investigation in Experimental Hematology.
The researchers evaluated the heterogeneity of BCR-ABL expression at DNA, messenger RNA, and protein levels, using the CML-derived K562 cell line.
They grew cells in suspension and found that some cells adhered to the plastic dish. The investigators then separated the plastic-adherent and nonadherent cell populations and studied them as single cells and in bulk.
The first discovery was that adherent and nonadherent cells had similar BCR-ABL fusion gene copy numbers.
In bulk-cell analysis, the mean relative normalized ratio for genomic ABL DNA copy number was 47.73 for adherent cells and 53.40 for nonadherent cells (P=0.11). In single-cell analysis, the mean copy numbers were 13.83 and 14.22, respectively (P=0.63).
On the other hand, there was a significant difference in BCR-ABL messenger RNA expression between adherent and nonadherent cells.
In bulk cells, the level of BCR-ABL messenger RNA transcripts was 11-fold higher in adherent cells than in nondherent cells (P=0.022). And single-cell analysis revealed the mean BCR-ABL copy number was 53.11 for adherent cells and 14.06 for nonadherent cells (P=0.0013).
Adherent cells also exhibited significantly upregulated phosphorylation of BCR protein compared to nonadherent cells.
Flow cytometry showed that a mean of 61.9% of adherent cells were positive for phosphor-BCR, compared to 14.5% of nonadherent cells (P=0.0074). And single-cell analysis revealed a mean signal number per cell of 8.23 among adherent cells and 3.02 among nonadherent cells (P<0.0001).
In addition, whole-genome microRNA profiling showed that adherent and nonadherent cell populations expressed significantly different microRNA species.
Finally, the researchers found that treatment with imatinib reduced cell viability more rapidly in nonadherent cells than in adherent cells (P<0.005). The adherent cells showed a decrease in cell viability at 24 hours, compared to 4 hours for nonadherent cells.
The investigators said this research suggests that CML patients may have a similar adherent cell population that mediates resistance to imatinib. And the study highlights the importance of single-cell analysis.
“The small number of cells that show high levels of BCR-ABL may not be detectable through bulk analysis of large samples,” Dr Byers said. “It looks like it is important to look at protein levels in single cells.”
“In future, it may be possible to measure BCR-ABL levels in individual cells in the clinic. This will help us identify the resistant, high-BCR-ABL cells and better understand how patients develop resistance to imatinib treatment, with the aim of combating this resistance to make response more durable and the treatment more effective.” ![]()

Credit: UC San Diego
Preclinical research in chronic myeloid leukemia (CML) has pointed to a relationship between cell adherence and treatment resistance.
Investigators found that a population of plastic-adherent K562 cells with increased expression of BCR-ABL exhibited greater resistance to the tyrosine kinase inhibitor imatinib than nonadherent K562 cells.
“Previous studies have linked high levels of the BCR-ABL mutation with drug resistance,” said Richard Byers, PhD, of The University of Manchester in the UK.
“We wanted to see how expression of BCR-ABL differed across groups of CML cells and, in particular, whether there were differences between adherent and nonadherent populations.”
Dr Byers and his colleagues described this investigation in Experimental Hematology.
The researchers evaluated the heterogeneity of BCR-ABL expression at DNA, messenger RNA, and protein levels, using the CML-derived K562 cell line.
They grew cells in suspension and found that some cells adhered to the plastic dish. The investigators then separated the plastic-adherent and nonadherent cell populations and studied them as single cells and in bulk.
The first discovery was that adherent and nonadherent cells had similar BCR-ABL fusion gene copy numbers.
In bulk-cell analysis, the mean relative normalized ratio for genomic ABL DNA copy number was 47.73 for adherent cells and 53.40 for nonadherent cells (P=0.11). In single-cell analysis, the mean copy numbers were 13.83 and 14.22, respectively (P=0.63).
On the other hand, there was a significant difference in BCR-ABL messenger RNA expression between adherent and nonadherent cells.
In bulk cells, the level of BCR-ABL messenger RNA transcripts was 11-fold higher in adherent cells than in nondherent cells (P=0.022). And single-cell analysis revealed the mean BCR-ABL copy number was 53.11 for adherent cells and 14.06 for nonadherent cells (P=0.0013).
Adherent cells also exhibited significantly upregulated phosphorylation of BCR protein compared to nonadherent cells.
Flow cytometry showed that a mean of 61.9% of adherent cells were positive for phosphor-BCR, compared to 14.5% of nonadherent cells (P=0.0074). And single-cell analysis revealed a mean signal number per cell of 8.23 among adherent cells and 3.02 among nonadherent cells (P<0.0001).
In addition, whole-genome microRNA profiling showed that adherent and nonadherent cell populations expressed significantly different microRNA species.
Finally, the researchers found that treatment with imatinib reduced cell viability more rapidly in nonadherent cells than in adherent cells (P<0.005). The adherent cells showed a decrease in cell viability at 24 hours, compared to 4 hours for nonadherent cells.
The investigators said this research suggests that CML patients may have a similar adherent cell population that mediates resistance to imatinib. And the study highlights the importance of single-cell analysis.
“The small number of cells that show high levels of BCR-ABL may not be detectable through bulk analysis of large samples,” Dr Byers said. “It looks like it is important to look at protein levels in single cells.”
“In future, it may be possible to measure BCR-ABL levels in individual cells in the clinic. This will help us identify the resistant, high-BCR-ABL cells and better understand how patients develop resistance to imatinib treatment, with the aim of combating this resistance to make response more durable and the treatment more effective.” ![]()