User login
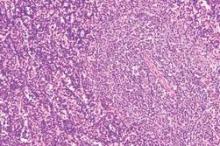
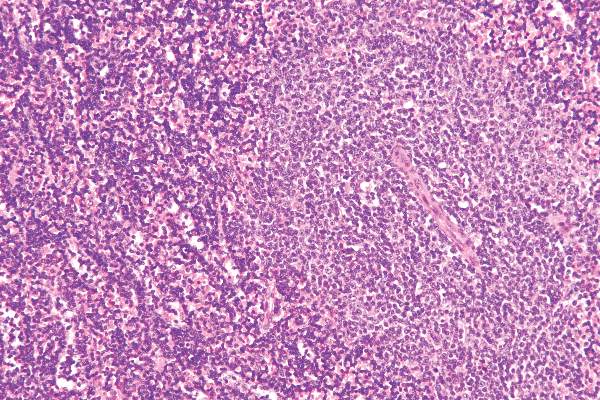
New agents effectively target CLL’s molecular Achilles
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
EXPERT ANALYSIS FROM NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
Anti-BCL2, CD20 combo safe, effective in untreated CLL
ORLANDO – An experimental combination of obinutuzumab and venetoclax appears safe as frontline therapy for patients with active, untreated chronic lymphocytic leukemia and comorbidities.
In the safety run-in portion of a phase 3, open-label trial comparing the combination of obinutuzumab (Gazyva) and the investigational Bcl-2 inhibitor venetoclax with obinutuzumab and its usual partner chlorambucil in 12 patients, only two of seven patients classified as being at high risk for the tumor lysis syndrome (TLS) developed laboratory-defined TLS, and no patients had clinical TLS.
The combination did not meet any of the pre-specified stopping criteria, and early data hinted at efficacy for the combination, said Dr. Kirsten Fischer, from the Center for Integrated Oncology at University Hospital Cologne in Germany.
“As with previous reports, our data confirm rapid and profound reduction in lymphocyte counts after the first dose obinutuzumab in all 12 [evaluable] patients,” she said at the American Society of Hematology annual meeting.
In the CLL 11 trial, investigators in the German CLL group previously showed that the combination of the anti-CD20 antibody obinutuzumab and chlorambucil resulted in improved overall survival compared to chlorambucil alone in patients with previously untreated CLL and coexisting medical conditions. The combination is approved in the United States for adults with treatment-naïve CLL.
Venetoclax has been shown to have good efficacy against relapsed/refractory CLL both as monotherapy and in combination with rituximab, prompting the investigators to explore it in combination with the rituximab follow-on drug obinutuzumab.
In the safety run-in phase of the CLL 14 trial, the investigators enrolled 13 adults (median age 75, range 59-88 years) with newly diagnosed, active confirmed CLL and coexisting medical conditions as determined either by a score greater 6 on the cumulative illness rating scale (CIRS) or by estimated creatinine clearance less than 70 mL/min.
The patients were treated with 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously 100 mg on day 1 and 900 mg on day 2, with the option to deliver 1,000 mg on day 1 instead, then 1,000 mg on days 8 and 15 of cycle 1, and 1,000 mg on day 1 for cycles 2-6.
The dose of venetoclax was titrated upward gradually, with doses of 20 mg, 50 mg, 100 mg, 200 mg, and up to 400 mg administered starting on day 22 of cycle 1.
Planned stopping criteria were one treatment-related death or a grade 4 adverse event related to clinical TLS despite prophylaxis as specified by the protocol.
At the time of data cutoff (October 2015), 12 patients had been on treatment for at least 4 weeks and had reached the maximum venetoclax dose. Two patients had reached 11 cycles, three had reached 10 cycles, and seven had reached 8 cycles.
Grade 1 or 2 adverse events in all 13 enrolled patients included infusion-related reactions in 8; infections in 6; diarrhea, hyperkalemia, and constipation in 5; nausea, dizziness and cough in 4; and fatigue, headache and pruritus in 3.
Grade 3 or 4 adverse events were neutropenia in five patients; infusion related reactions; syncope, thrombocytopenia and laboratory-defined TLS in two patients; and bradycardia, hyperglycemia, influenza, leucopenia, pyrexia, respiratory tract infection, and elevated transaminases in one patient each.
As noted, all 12 evaluable patients had rapid drops in absolute lymphocyte counts, and all but one had complete resolution of lymphadenopathy after three cycles, with the improvement maintained after six cycles. The remaining patient had a decrease to near normal after both three and six cycles.
Of the 12 patients, 11 had a partial response after three cycles, and the remaining patient had stable disease, for an overall response rate of 92%. The overall response rate after six cycles was 100%, with all patients having a partial response.
The data were sufficiently good to justify continuing with the randomized phase, which began in August 2015, Dr. Fischer noted.
The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
ORLANDO – An experimental combination of obinutuzumab and venetoclax appears safe as frontline therapy for patients with active, untreated chronic lymphocytic leukemia and comorbidities.
In the safety run-in portion of a phase 3, open-label trial comparing the combination of obinutuzumab (Gazyva) and the investigational Bcl-2 inhibitor venetoclax with obinutuzumab and its usual partner chlorambucil in 12 patients, only two of seven patients classified as being at high risk for the tumor lysis syndrome (TLS) developed laboratory-defined TLS, and no patients had clinical TLS.
The combination did not meet any of the pre-specified stopping criteria, and early data hinted at efficacy for the combination, said Dr. Kirsten Fischer, from the Center for Integrated Oncology at University Hospital Cologne in Germany.
“As with previous reports, our data confirm rapid and profound reduction in lymphocyte counts after the first dose obinutuzumab in all 12 [evaluable] patients,” she said at the American Society of Hematology annual meeting.
In the CLL 11 trial, investigators in the German CLL group previously showed that the combination of the anti-CD20 antibody obinutuzumab and chlorambucil resulted in improved overall survival compared to chlorambucil alone in patients with previously untreated CLL and coexisting medical conditions. The combination is approved in the United States for adults with treatment-naïve CLL.
Venetoclax has been shown to have good efficacy against relapsed/refractory CLL both as monotherapy and in combination with rituximab, prompting the investigators to explore it in combination with the rituximab follow-on drug obinutuzumab.
In the safety run-in phase of the CLL 14 trial, the investigators enrolled 13 adults (median age 75, range 59-88 years) with newly diagnosed, active confirmed CLL and coexisting medical conditions as determined either by a score greater 6 on the cumulative illness rating scale (CIRS) or by estimated creatinine clearance less than 70 mL/min.
The patients were treated with 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously 100 mg on day 1 and 900 mg on day 2, with the option to deliver 1,000 mg on day 1 instead, then 1,000 mg on days 8 and 15 of cycle 1, and 1,000 mg on day 1 for cycles 2-6.
The dose of venetoclax was titrated upward gradually, with doses of 20 mg, 50 mg, 100 mg, 200 mg, and up to 400 mg administered starting on day 22 of cycle 1.
Planned stopping criteria were one treatment-related death or a grade 4 adverse event related to clinical TLS despite prophylaxis as specified by the protocol.
At the time of data cutoff (October 2015), 12 patients had been on treatment for at least 4 weeks and had reached the maximum venetoclax dose. Two patients had reached 11 cycles, three had reached 10 cycles, and seven had reached 8 cycles.
Grade 1 or 2 adverse events in all 13 enrolled patients included infusion-related reactions in 8; infections in 6; diarrhea, hyperkalemia, and constipation in 5; nausea, dizziness and cough in 4; and fatigue, headache and pruritus in 3.
Grade 3 or 4 adverse events were neutropenia in five patients; infusion related reactions; syncope, thrombocytopenia and laboratory-defined TLS in two patients; and bradycardia, hyperglycemia, influenza, leucopenia, pyrexia, respiratory tract infection, and elevated transaminases in one patient each.
As noted, all 12 evaluable patients had rapid drops in absolute lymphocyte counts, and all but one had complete resolution of lymphadenopathy after three cycles, with the improvement maintained after six cycles. The remaining patient had a decrease to near normal after both three and six cycles.
Of the 12 patients, 11 had a partial response after three cycles, and the remaining patient had stable disease, for an overall response rate of 92%. The overall response rate after six cycles was 100%, with all patients having a partial response.
The data were sufficiently good to justify continuing with the randomized phase, which began in August 2015, Dr. Fischer noted.
The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
ORLANDO – An experimental combination of obinutuzumab and venetoclax appears safe as frontline therapy for patients with active, untreated chronic lymphocytic leukemia and comorbidities.
In the safety run-in portion of a phase 3, open-label trial comparing the combination of obinutuzumab (Gazyva) and the investigational Bcl-2 inhibitor venetoclax with obinutuzumab and its usual partner chlorambucil in 12 patients, only two of seven patients classified as being at high risk for the tumor lysis syndrome (TLS) developed laboratory-defined TLS, and no patients had clinical TLS.
The combination did not meet any of the pre-specified stopping criteria, and early data hinted at efficacy for the combination, said Dr. Kirsten Fischer, from the Center for Integrated Oncology at University Hospital Cologne in Germany.
“As with previous reports, our data confirm rapid and profound reduction in lymphocyte counts after the first dose obinutuzumab in all 12 [evaluable] patients,” she said at the American Society of Hematology annual meeting.
In the CLL 11 trial, investigators in the German CLL group previously showed that the combination of the anti-CD20 antibody obinutuzumab and chlorambucil resulted in improved overall survival compared to chlorambucil alone in patients with previously untreated CLL and coexisting medical conditions. The combination is approved in the United States for adults with treatment-naïve CLL.
Venetoclax has been shown to have good efficacy against relapsed/refractory CLL both as monotherapy and in combination with rituximab, prompting the investigators to explore it in combination with the rituximab follow-on drug obinutuzumab.
In the safety run-in phase of the CLL 14 trial, the investigators enrolled 13 adults (median age 75, range 59-88 years) with newly diagnosed, active confirmed CLL and coexisting medical conditions as determined either by a score greater 6 on the cumulative illness rating scale (CIRS) or by estimated creatinine clearance less than 70 mL/min.
The patients were treated with 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously 100 mg on day 1 and 900 mg on day 2, with the option to deliver 1,000 mg on day 1 instead, then 1,000 mg on days 8 and 15 of cycle 1, and 1,000 mg on day 1 for cycles 2-6.
The dose of venetoclax was titrated upward gradually, with doses of 20 mg, 50 mg, 100 mg, 200 mg, and up to 400 mg administered starting on day 22 of cycle 1.
Planned stopping criteria were one treatment-related death or a grade 4 adverse event related to clinical TLS despite prophylaxis as specified by the protocol.
At the time of data cutoff (October 2015), 12 patients had been on treatment for at least 4 weeks and had reached the maximum venetoclax dose. Two patients had reached 11 cycles, three had reached 10 cycles, and seven had reached 8 cycles.
Grade 1 or 2 adverse events in all 13 enrolled patients included infusion-related reactions in 8; infections in 6; diarrhea, hyperkalemia, and constipation in 5; nausea, dizziness and cough in 4; and fatigue, headache and pruritus in 3.
Grade 3 or 4 adverse events were neutropenia in five patients; infusion related reactions; syncope, thrombocytopenia and laboratory-defined TLS in two patients; and bradycardia, hyperglycemia, influenza, leucopenia, pyrexia, respiratory tract infection, and elevated transaminases in one patient each.
As noted, all 12 evaluable patients had rapid drops in absolute lymphocyte counts, and all but one had complete resolution of lymphadenopathy after three cycles, with the improvement maintained after six cycles. The remaining patient had a decrease to near normal after both three and six cycles.
Of the 12 patients, 11 had a partial response after three cycles, and the remaining patient had stable disease, for an overall response rate of 92%. The overall response rate after six cycles was 100%, with all patients having a partial response.
The data were sufficiently good to justify continuing with the randomized phase, which began in August 2015, Dr. Fischer noted.
The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
AT ASH 2015
Key clinical point: Patients with CLL and comorbidities were able to tolerate an experimental regimen of obinutuzumab and venetoclax.
Major finding: Two of 12 evaluable patients had evidence of laboratory-defined but not clinical tumor lysis syndrome.
Data source: Safety run-in phase of a randomized phase 3 trial with 13 patients with chronic lymphocytic leukemia and comorbidities.
Disclosures: The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
FDA gives nod to rapid-infusion bendamustine for CLL
The Food and Drug Administration has approved a new rapid-infusion form of bendamustine hydrochloride for patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma.
The new formulation of bendamustine (Bendeka) is a 50-mL liquid designed for 10-minute infusion. It was granted orphan drug status for both the leukemia and lymphoma indications.
Bendeka is approved as primary therapy for chronic lymphocytic leukemia (CLL) and also for indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
According to the prescribing information, the recommended dosing regimen for CLL is 100 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 28-day cycle, up to six cycles. For NHL, the regimen calls for 120 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 21-day cycle, up to eight cycles.
The most common hematologic adverse reactions are lymphopenia, anemia, leukopenia, thrombocytopenia, and neutropenia. Bendamustine has been associated with severe – and even fatal – myelosuppression.
Bendeka is manufactured by Teva Pharmaceutical Industries and succeeds Teva’s previously approved form of bendamustine, Treanda, approved in 2008. “Since 2008, Treanda has played a valuable role in the treatment of patients with CLL or indolent B-cell NHL that has progressed,” Paul Rittman, Teva Oncology’s vice president and general manager, said in a press statement. Treanda’s orphan drug exclusivity status for the NHL indication was set to expire in October, and its pediatric exclusivity status for that indication, next April. Its CLL exclusivity status expired in September.
Teva purchased the new formulation from Eagle Pharmaceuticals last February. The deal was closed with an upfront payment of $30 million, and potential for up to $90 million in additional payments, as well as double-digit royalties on net sales.
At the time of purchase, Eagle had secured orphan drug designations for Bendeka in both CLL and NHL and had submitted the New Drug Application under priority review. Bendeka may be eligible for a 7-year exclusivity status.
The drug is scheduled to be available during the first quarter of 2016.
The Food and Drug Administration has approved a new rapid-infusion form of bendamustine hydrochloride for patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma.
The new formulation of bendamustine (Bendeka) is a 50-mL liquid designed for 10-minute infusion. It was granted orphan drug status for both the leukemia and lymphoma indications.
Bendeka is approved as primary therapy for chronic lymphocytic leukemia (CLL) and also for indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
According to the prescribing information, the recommended dosing regimen for CLL is 100 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 28-day cycle, up to six cycles. For NHL, the regimen calls for 120 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 21-day cycle, up to eight cycles.
The most common hematologic adverse reactions are lymphopenia, anemia, leukopenia, thrombocytopenia, and neutropenia. Bendamustine has been associated with severe – and even fatal – myelosuppression.
Bendeka is manufactured by Teva Pharmaceutical Industries and succeeds Teva’s previously approved form of bendamustine, Treanda, approved in 2008. “Since 2008, Treanda has played a valuable role in the treatment of patients with CLL or indolent B-cell NHL that has progressed,” Paul Rittman, Teva Oncology’s vice president and general manager, said in a press statement. Treanda’s orphan drug exclusivity status for the NHL indication was set to expire in October, and its pediatric exclusivity status for that indication, next April. Its CLL exclusivity status expired in September.
Teva purchased the new formulation from Eagle Pharmaceuticals last February. The deal was closed with an upfront payment of $30 million, and potential for up to $90 million in additional payments, as well as double-digit royalties on net sales.
At the time of purchase, Eagle had secured orphan drug designations for Bendeka in both CLL and NHL and had submitted the New Drug Application under priority review. Bendeka may be eligible for a 7-year exclusivity status.
The drug is scheduled to be available during the first quarter of 2016.
The Food and Drug Administration has approved a new rapid-infusion form of bendamustine hydrochloride for patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma.
The new formulation of bendamustine (Bendeka) is a 50-mL liquid designed for 10-minute infusion. It was granted orphan drug status for both the leukemia and lymphoma indications.
Bendeka is approved as primary therapy for chronic lymphocytic leukemia (CLL) and also for indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
According to the prescribing information, the recommended dosing regimen for CLL is 100 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 28-day cycle, up to six cycles. For NHL, the regimen calls for 120 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 21-day cycle, up to eight cycles.
The most common hematologic adverse reactions are lymphopenia, anemia, leukopenia, thrombocytopenia, and neutropenia. Bendamustine has been associated with severe – and even fatal – myelosuppression.
Bendeka is manufactured by Teva Pharmaceutical Industries and succeeds Teva’s previously approved form of bendamustine, Treanda, approved in 2008. “Since 2008, Treanda has played a valuable role in the treatment of patients with CLL or indolent B-cell NHL that has progressed,” Paul Rittman, Teva Oncology’s vice president and general manager, said in a press statement. Treanda’s orphan drug exclusivity status for the NHL indication was set to expire in October, and its pediatric exclusivity status for that indication, next April. Its CLL exclusivity status expired in September.
Teva purchased the new formulation from Eagle Pharmaceuticals last February. The deal was closed with an upfront payment of $30 million, and potential for up to $90 million in additional payments, as well as double-digit royalties on net sales.
At the time of purchase, Eagle had secured orphan drug designations for Bendeka in both CLL and NHL and had submitted the New Drug Application under priority review. Bendeka may be eligible for a 7-year exclusivity status.
The drug is scheduled to be available during the first quarter of 2016.
ASH: Idelalisib plus standard therapy boosts survival in relapsed CLL
ORLANDO – Adding the PI3K inhibitor idelalisib to a standard regimen of bendamustine and rituximab significantly reduced the risk of both disease progression and death for patients with relapsed and/or refractory chronic lymphocytic leukemia, results of a phase III randomized trial showed.
At a median follow-up of 12 months, the primary endpoint of median progression-free survival was 23.1 months for patients treated with idelalisib (Zydelig), bendamustine, and rituximab (idel+BR), compared with 11.1 months for bendamustine and rituximab (BR) plus a placebo, reported Dr. Andrew Zelenetz of Memorial Sloan Kettering Cancer Center, New York.
“Median overall survival was not reached in either arm. However, there was a significant improvement in overall survival, with a 45% reduction in the risk of death [with idel+BR],” he said in a late-breaking abstract session at the annual meeting of the American Society of Hematology.
The trial was stopped early after a data review at the first planned interim analysis showed significant superiority for the three-drug combination.
The results were consistent across subgroups, including patients with high-risk features such as deletion 17p and mutated TP53 (del[17p]/TP53), unmutated immunoglobulin heavy chain variable region (IgHV), and treatment-refractory disease.
The rationale behind adding idelalisib, an inhibitor of the phosphatidylinositol-3 kinase (PI3K), is that signaling via the PI3K pathway is hyperactive and can be targeted, Dr. Zelenetz explained.
Study 115 was a phase III trial with accrual from June 2012 through August 2015. Investigators enrolled 416 patients with relapsed /refractory CLL and randomly assigned them to receive BR for six 28-day cycles of bendamustine (70 mg/m2 on days 1 and 2 of each cycle) and rituximab (375 mg/m2 for cycle 1, and 500 mg/m2 for cycles 2 through 6), plus either idelalisib 150 mg b.i.d. or placebo, each administered continuously until disease progression, intolerable toxicity, withdrawal of consent, or death.
The patients were stratified by mutational and disease status (refractory defined as CLL progression less than 6 months from completion of prior therapy, or relapsed CLL progression 6 months or more from completion of prior therapy.
The trial was halted early after the first planned interim analysis, which was conducted after 75% of the total number of 260 planned events of CLL progression or death from any cause had occurred. The data cutoff was June 15, 2015.
The intention-to-treat analysis included 207 patients assigned to idelalisib and 209 assigned to placebo. Three-fourths (76%) of the patients were male.
In all, 46% of patients had Rai stage III/IV disease. The median time since the completion of the last therapy was 16 months.
The proportions of patients with high-risk features included del(17p)/p53 mutation in 32.9%, unmutated IgHV in 83.2%, and treatment-refractory disease in 29.8%.
As noted, the median progression-free survival with idelalisib at a median follow-up of 12 months was 23.1 months vs. 11.1 months for placebo. That translated into a hazard ratio of 0.33 (P less than .0001).
Among patients with neither del(17p) nor TP53 mutations, the HR for progression was 0.22. Among patients with either del(17p) or a TP53 mutation, the HR was 0.50 (95% confidence intervals show statistical significance for both).
Overall response rates were 68% among patients who received idelalisib, and 45% for those who received placebo. There were five complete responses (2%) in the idelalisib group and none in the placebo group.
The idelalisib group also had a higher proportion of patients with a greater than 50% reduction in involved lymph nodes (96% vs. 61%), and had better organomegaly responses (spleen and liver) and hematologic responses (hemoglobin, neutrophils, and platelets).
Grade 3 or greater adverse events occurred in 93% of patients on idelalisib, compared with 76% of those on placebo. The proportion of patients with any serious adverse event was 66% vs. 44%, respectively.
Adverse events leading to drug dose reduction were seen in 11% of idelalisib-treated patients, compared with 6% of placebo controls, and therapy was discontinued in 26% vs. 13%, respectively.
Ten patients in the idelalisib arm and seven in the placebo arm died during the study.
Adverse events that occurred more commonly with idelalisib included neutropenia, pyrexia, diarrhea, febrile neutropenia, pneumonia, rash, and elevated liver enzymes.
Session moderator Dr. David P. Steensma of the Dana-Farber Cancer Institute in Boston asked Dr. Zelenetz how idelalisib plus BR stacked up to ibrutinib (Imbruvica) plus BR in this population.
Dr. Zelenetz noted that patients were excluded from the HELIOS trial of ibrutinib plus BR if they had del(17p). Comparing the subset of patients in Study 115 without del(17p) with patients in the ibrutinib study, “the results are virtually superimposable,” Dr. Zelenetz said, and “the two treatments are really remarkably similar.”
The overall survival benefit was larger in the HELIOS trial, Dr. Zelenetz noted, but that was largely because the trial allowed patients to cross over from placebo to the active drug.
Gilead Sciences funded Study 115. Dr. Zelenetz disclosed receiving research funding from the company and discussing off-label use of idelalisib for relapsed/refractory CLL.
ORLANDO – Adding the PI3K inhibitor idelalisib to a standard regimen of bendamustine and rituximab significantly reduced the risk of both disease progression and death for patients with relapsed and/or refractory chronic lymphocytic leukemia, results of a phase III randomized trial showed.
At a median follow-up of 12 months, the primary endpoint of median progression-free survival was 23.1 months for patients treated with idelalisib (Zydelig), bendamustine, and rituximab (idel+BR), compared with 11.1 months for bendamustine and rituximab (BR) plus a placebo, reported Dr. Andrew Zelenetz of Memorial Sloan Kettering Cancer Center, New York.
“Median overall survival was not reached in either arm. However, there was a significant improvement in overall survival, with a 45% reduction in the risk of death [with idel+BR],” he said in a late-breaking abstract session at the annual meeting of the American Society of Hematology.
The trial was stopped early after a data review at the first planned interim analysis showed significant superiority for the three-drug combination.
The results were consistent across subgroups, including patients with high-risk features such as deletion 17p and mutated TP53 (del[17p]/TP53), unmutated immunoglobulin heavy chain variable region (IgHV), and treatment-refractory disease.
The rationale behind adding idelalisib, an inhibitor of the phosphatidylinositol-3 kinase (PI3K), is that signaling via the PI3K pathway is hyperactive and can be targeted, Dr. Zelenetz explained.
Study 115 was a phase III trial with accrual from June 2012 through August 2015. Investigators enrolled 416 patients with relapsed /refractory CLL and randomly assigned them to receive BR for six 28-day cycles of bendamustine (70 mg/m2 on days 1 and 2 of each cycle) and rituximab (375 mg/m2 for cycle 1, and 500 mg/m2 for cycles 2 through 6), plus either idelalisib 150 mg b.i.d. or placebo, each administered continuously until disease progression, intolerable toxicity, withdrawal of consent, or death.
The patients were stratified by mutational and disease status (refractory defined as CLL progression less than 6 months from completion of prior therapy, or relapsed CLL progression 6 months or more from completion of prior therapy.
The trial was halted early after the first planned interim analysis, which was conducted after 75% of the total number of 260 planned events of CLL progression or death from any cause had occurred. The data cutoff was June 15, 2015.
The intention-to-treat analysis included 207 patients assigned to idelalisib and 209 assigned to placebo. Three-fourths (76%) of the patients were male.
In all, 46% of patients had Rai stage III/IV disease. The median time since the completion of the last therapy was 16 months.
The proportions of patients with high-risk features included del(17p)/p53 mutation in 32.9%, unmutated IgHV in 83.2%, and treatment-refractory disease in 29.8%.
As noted, the median progression-free survival with idelalisib at a median follow-up of 12 months was 23.1 months vs. 11.1 months for placebo. That translated into a hazard ratio of 0.33 (P less than .0001).
Among patients with neither del(17p) nor TP53 mutations, the HR for progression was 0.22. Among patients with either del(17p) or a TP53 mutation, the HR was 0.50 (95% confidence intervals show statistical significance for both).
Overall response rates were 68% among patients who received idelalisib, and 45% for those who received placebo. There were five complete responses (2%) in the idelalisib group and none in the placebo group.
The idelalisib group also had a higher proportion of patients with a greater than 50% reduction in involved lymph nodes (96% vs. 61%), and had better organomegaly responses (spleen and liver) and hematologic responses (hemoglobin, neutrophils, and platelets).
Grade 3 or greater adverse events occurred in 93% of patients on idelalisib, compared with 76% of those on placebo. The proportion of patients with any serious adverse event was 66% vs. 44%, respectively.
Adverse events leading to drug dose reduction were seen in 11% of idelalisib-treated patients, compared with 6% of placebo controls, and therapy was discontinued in 26% vs. 13%, respectively.
Ten patients in the idelalisib arm and seven in the placebo arm died during the study.
Adverse events that occurred more commonly with idelalisib included neutropenia, pyrexia, diarrhea, febrile neutropenia, pneumonia, rash, and elevated liver enzymes.
Session moderator Dr. David P. Steensma of the Dana-Farber Cancer Institute in Boston asked Dr. Zelenetz how idelalisib plus BR stacked up to ibrutinib (Imbruvica) plus BR in this population.
Dr. Zelenetz noted that patients were excluded from the HELIOS trial of ibrutinib plus BR if they had del(17p). Comparing the subset of patients in Study 115 without del(17p) with patients in the ibrutinib study, “the results are virtually superimposable,” Dr. Zelenetz said, and “the two treatments are really remarkably similar.”
The overall survival benefit was larger in the HELIOS trial, Dr. Zelenetz noted, but that was largely because the trial allowed patients to cross over from placebo to the active drug.
Gilead Sciences funded Study 115. Dr. Zelenetz disclosed receiving research funding from the company and discussing off-label use of idelalisib for relapsed/refractory CLL.
ORLANDO – Adding the PI3K inhibitor idelalisib to a standard regimen of bendamustine and rituximab significantly reduced the risk of both disease progression and death for patients with relapsed and/or refractory chronic lymphocytic leukemia, results of a phase III randomized trial showed.
At a median follow-up of 12 months, the primary endpoint of median progression-free survival was 23.1 months for patients treated with idelalisib (Zydelig), bendamustine, and rituximab (idel+BR), compared with 11.1 months for bendamustine and rituximab (BR) plus a placebo, reported Dr. Andrew Zelenetz of Memorial Sloan Kettering Cancer Center, New York.
“Median overall survival was not reached in either arm. However, there was a significant improvement in overall survival, with a 45% reduction in the risk of death [with idel+BR],” he said in a late-breaking abstract session at the annual meeting of the American Society of Hematology.
The trial was stopped early after a data review at the first planned interim analysis showed significant superiority for the three-drug combination.
The results were consistent across subgroups, including patients with high-risk features such as deletion 17p and mutated TP53 (del[17p]/TP53), unmutated immunoglobulin heavy chain variable region (IgHV), and treatment-refractory disease.
The rationale behind adding idelalisib, an inhibitor of the phosphatidylinositol-3 kinase (PI3K), is that signaling via the PI3K pathway is hyperactive and can be targeted, Dr. Zelenetz explained.
Study 115 was a phase III trial with accrual from June 2012 through August 2015. Investigators enrolled 416 patients with relapsed /refractory CLL and randomly assigned them to receive BR for six 28-day cycles of bendamustine (70 mg/m2 on days 1 and 2 of each cycle) and rituximab (375 mg/m2 for cycle 1, and 500 mg/m2 for cycles 2 through 6), plus either idelalisib 150 mg b.i.d. or placebo, each administered continuously until disease progression, intolerable toxicity, withdrawal of consent, or death.
The patients were stratified by mutational and disease status (refractory defined as CLL progression less than 6 months from completion of prior therapy, or relapsed CLL progression 6 months or more from completion of prior therapy.
The trial was halted early after the first planned interim analysis, which was conducted after 75% of the total number of 260 planned events of CLL progression or death from any cause had occurred. The data cutoff was June 15, 2015.
The intention-to-treat analysis included 207 patients assigned to idelalisib and 209 assigned to placebo. Three-fourths (76%) of the patients were male.
In all, 46% of patients had Rai stage III/IV disease. The median time since the completion of the last therapy was 16 months.
The proportions of patients with high-risk features included del(17p)/p53 mutation in 32.9%, unmutated IgHV in 83.2%, and treatment-refractory disease in 29.8%.
As noted, the median progression-free survival with idelalisib at a median follow-up of 12 months was 23.1 months vs. 11.1 months for placebo. That translated into a hazard ratio of 0.33 (P less than .0001).
Among patients with neither del(17p) nor TP53 mutations, the HR for progression was 0.22. Among patients with either del(17p) or a TP53 mutation, the HR was 0.50 (95% confidence intervals show statistical significance for both).
Overall response rates were 68% among patients who received idelalisib, and 45% for those who received placebo. There were five complete responses (2%) in the idelalisib group and none in the placebo group.
The idelalisib group also had a higher proportion of patients with a greater than 50% reduction in involved lymph nodes (96% vs. 61%), and had better organomegaly responses (spleen and liver) and hematologic responses (hemoglobin, neutrophils, and platelets).
Grade 3 or greater adverse events occurred in 93% of patients on idelalisib, compared with 76% of those on placebo. The proportion of patients with any serious adverse event was 66% vs. 44%, respectively.
Adverse events leading to drug dose reduction were seen in 11% of idelalisib-treated patients, compared with 6% of placebo controls, and therapy was discontinued in 26% vs. 13%, respectively.
Ten patients in the idelalisib arm and seven in the placebo arm died during the study.
Adverse events that occurred more commonly with idelalisib included neutropenia, pyrexia, diarrhea, febrile neutropenia, pneumonia, rash, and elevated liver enzymes.
Session moderator Dr. David P. Steensma of the Dana-Farber Cancer Institute in Boston asked Dr. Zelenetz how idelalisib plus BR stacked up to ibrutinib (Imbruvica) plus BR in this population.
Dr. Zelenetz noted that patients were excluded from the HELIOS trial of ibrutinib plus BR if they had del(17p). Comparing the subset of patients in Study 115 without del(17p) with patients in the ibrutinib study, “the results are virtually superimposable,” Dr. Zelenetz said, and “the two treatments are really remarkably similar.”
The overall survival benefit was larger in the HELIOS trial, Dr. Zelenetz noted, but that was largely because the trial allowed patients to cross over from placebo to the active drug.
Gilead Sciences funded Study 115. Dr. Zelenetz disclosed receiving research funding from the company and discussing off-label use of idelalisib for relapsed/refractory CLL.
AT ASH 2015
Key clinical point: Adding the PI3K inhibitor idelalisib to bendamustine and rituximab significantly improved survival of patients with relapsed/refractory CLL.
Major finding: Median progression-free survival was 23.1 months for patients treated with idelalisib, bendamustine, and rituximab, compared with 11.1 months for bendamustine and rituximab plus placebo.
Data source: Randomized, controlled trial in 416 patients with relapsed/refractory CLL. The trial was halted early for superior efficacy with idelalisib.
Disclosures: Gilead Sciences funded Study 115. Dr. Zelenetz disclosed receiving research funding from the company and discussing off-label use of idelalisib for relapsed/refractory CLL.
Upfront idelalisib carries high risk for acute liver toxicity
ORLANDO – Idelalisib given as first-line therapy for patients with chronic lymphocytic leukemia carries a high risk of early fulminant hepatotoxicity requiring drug interruption and steroids, investigators reported.
Among 24 patients who received idelalisib (Zydelig) monotherapy in a phase II trial of a combination of idelalisib followed by idelalisib concurrent with ofatumumab (Arzerra) as first-line therapy for chronic lymphocytic leukemia (CLL), 12 patients developed acute hepatotoxicity, marked by rapidly soaring levels of transaminase within about 28 days of starting therapy. An additional four patients developed hepatotoxicity at around 130 days, noted Dr. Benjamin L. Lampson, a clinical fellow in medicine at the Dana-Farber Cancer Institute in Boston.
“Multiple lines of evidence suggest that this early hepatotoxicity is immune mediated. The proportion of regulatory T cells in the peripheral blood decrease on idelalisib therapy, providing a possible explanation for the development of early hepatotoxicity,” he said at the annual meeting of the American Society of Hematology.
The toxicities occur more frequently among younger and less heavily pretreated patients, and are likely due to on-target, immune-mediated effects, he noted.
Dr. Lampson presented data on the first 24 patients in an ongoing phase II trial. Patients with previously untreated CLL receive idelalisib 150 mg twice daily for 56 days, in an attempt to mobilize neoplastic B cells from the peripheral lymphoid tissues and into the bloodstream.
Following the monotherapy phase, patients are given ofatumumab in an attempt to clear the disease from peripheral blood.
“This dosing strategy is slightly different than what has been previously been used in trials combining these particular drugs. Specifically, previously reported trials have started these agents simultaneously without a lead-in period of monotherapy,” Dr. Lampson explained.
When the lead-in phase is completed, patients receive idelalisib plus ofatumumab infusions once weekly for 8 weeks, followed by once-monthly infusions for 4 months. Patients then continue on idelalisib indefinitely. The primary endpoint is the overall response rate assessed 2 months after the completion of the combination therapy.
For the first 24 patients treated as of Nov. 9, 2015, the median time on therapy was 7.7 months and median follow-up was 14.7 months.
The median patient age was 67.4 years (range 57.6-84.9). CLL genetics showed that 13 patients had unmutated immunoglobulin heavy chain variable region (IgHV) disease, 4 had the 17p deletion and TP53 mutation, 1 had deletion 11q, and 13 had deletion 13q; some patients had more than one mutation.
“What we began to notice after enrolling just a few subjects on the trial was that severe hepatotoxicity was occurring shortly after initiating idelalisib,” Dr. Lampson said.
He presented one case, a 58-year-old man who was in the idelalisib monotherapy phase of the study. He developed grade 3 hepatotoxicity 28 days after starting the drug, despite having a normal liver function test just 1 day earlier. The drug was stopped, but his liver function tests continued to rise, suggesting a self-perpetuating or self-sustaining process.
On day 32, the patient was admitted to the hospital, and on day 33 he was started on steroids, based on the hypothesis that the hepatotoxicity might have been immune mediated. Two days after initiation of steroids, his liver function tests continued to rise, whereupon he was started on mycophenolate mofetil.
“With these two forms of immunosuppression, the [liver function tests] did eventually normalize, although the steroids and mycophenolate had to be tapered over a period of many weeks. And this patient was not the only patient to experience toxicity; in fact, hepatotoxicity was frequent and often severe,” he said.
At the time of maximum incidence, week 4, the percentage of patients with any hepatotoxicity was 46%, with 13% at grade 4, and 21% at grade 3.
“The median time to initial development of hepatotoxicity is 28 days. This suggests that the mechanism of hepatotoxicity is not immediate, but takes time to develop, consistent with an adaptive immune response. Furthermore, hepatotoxicity is typically occurring before the first dose of ofatumumab is occurring at week 8, suggesting idelalisib alone is the cause of the hepatotoxicity,” Dr. Lampson said.
A comparison of data from the ongoing study and from three previous studies – two with idelalisib in relapsed refractory disease, and one as first-line therapy in patients 65 and older – showed that grade 3 or greater hepatotoxicity was lowest in a phase I trial of idelalisib in which patients had received a median of five prior lines of therapy, occurring in only 1.9% of patients. In contrast, in the current study, 52% of patients experienced grade 3 or 4 transaminitis at some point in the trial.
Evidence for the hepatotoxicity being an on-target immune-mediated effect comes from lymphocytic infiltrate on liver biopsy and lymphocytic colitis in idelalisib-treated patients. Additional evidence comes from the fact that the toxicity is both treatable and preventable with steroids, he said.
He cautioned that hepatotoxicity can recur rapidly when the drug is reintroduced.
“In general, our experience has been if idelalisib is resumed while the subject remains on steroids, the drug is more likely to be tolerated and the subject eventually can be tapered off steroids,” he said.
Asked by an audience member whether patients who are receiving idelalisib in the first-line setting should also receive steroids, Dr. Lampson said that they closely monitor patient liver enzymes around 28 days, and if grade 1 transaminitis is detected, patients are automatically started on low-dose steroids.
The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
ORLANDO – Idelalisib given as first-line therapy for patients with chronic lymphocytic leukemia carries a high risk of early fulminant hepatotoxicity requiring drug interruption and steroids, investigators reported.
Among 24 patients who received idelalisib (Zydelig) monotherapy in a phase II trial of a combination of idelalisib followed by idelalisib concurrent with ofatumumab (Arzerra) as first-line therapy for chronic lymphocytic leukemia (CLL), 12 patients developed acute hepatotoxicity, marked by rapidly soaring levels of transaminase within about 28 days of starting therapy. An additional four patients developed hepatotoxicity at around 130 days, noted Dr. Benjamin L. Lampson, a clinical fellow in medicine at the Dana-Farber Cancer Institute in Boston.
“Multiple lines of evidence suggest that this early hepatotoxicity is immune mediated. The proportion of regulatory T cells in the peripheral blood decrease on idelalisib therapy, providing a possible explanation for the development of early hepatotoxicity,” he said at the annual meeting of the American Society of Hematology.
The toxicities occur more frequently among younger and less heavily pretreated patients, and are likely due to on-target, immune-mediated effects, he noted.
Dr. Lampson presented data on the first 24 patients in an ongoing phase II trial. Patients with previously untreated CLL receive idelalisib 150 mg twice daily for 56 days, in an attempt to mobilize neoplastic B cells from the peripheral lymphoid tissues and into the bloodstream.
Following the monotherapy phase, patients are given ofatumumab in an attempt to clear the disease from peripheral blood.
“This dosing strategy is slightly different than what has been previously been used in trials combining these particular drugs. Specifically, previously reported trials have started these agents simultaneously without a lead-in period of monotherapy,” Dr. Lampson explained.
When the lead-in phase is completed, patients receive idelalisib plus ofatumumab infusions once weekly for 8 weeks, followed by once-monthly infusions for 4 months. Patients then continue on idelalisib indefinitely. The primary endpoint is the overall response rate assessed 2 months after the completion of the combination therapy.
For the first 24 patients treated as of Nov. 9, 2015, the median time on therapy was 7.7 months and median follow-up was 14.7 months.
The median patient age was 67.4 years (range 57.6-84.9). CLL genetics showed that 13 patients had unmutated immunoglobulin heavy chain variable region (IgHV) disease, 4 had the 17p deletion and TP53 mutation, 1 had deletion 11q, and 13 had deletion 13q; some patients had more than one mutation.
“What we began to notice after enrolling just a few subjects on the trial was that severe hepatotoxicity was occurring shortly after initiating idelalisib,” Dr. Lampson said.
He presented one case, a 58-year-old man who was in the idelalisib monotherapy phase of the study. He developed grade 3 hepatotoxicity 28 days after starting the drug, despite having a normal liver function test just 1 day earlier. The drug was stopped, but his liver function tests continued to rise, suggesting a self-perpetuating or self-sustaining process.
On day 32, the patient was admitted to the hospital, and on day 33 he was started on steroids, based on the hypothesis that the hepatotoxicity might have been immune mediated. Two days after initiation of steroids, his liver function tests continued to rise, whereupon he was started on mycophenolate mofetil.
“With these two forms of immunosuppression, the [liver function tests] did eventually normalize, although the steroids and mycophenolate had to be tapered over a period of many weeks. And this patient was not the only patient to experience toxicity; in fact, hepatotoxicity was frequent and often severe,” he said.
At the time of maximum incidence, week 4, the percentage of patients with any hepatotoxicity was 46%, with 13% at grade 4, and 21% at grade 3.
“The median time to initial development of hepatotoxicity is 28 days. This suggests that the mechanism of hepatotoxicity is not immediate, but takes time to develop, consistent with an adaptive immune response. Furthermore, hepatotoxicity is typically occurring before the first dose of ofatumumab is occurring at week 8, suggesting idelalisib alone is the cause of the hepatotoxicity,” Dr. Lampson said.
A comparison of data from the ongoing study and from three previous studies – two with idelalisib in relapsed refractory disease, and one as first-line therapy in patients 65 and older – showed that grade 3 or greater hepatotoxicity was lowest in a phase I trial of idelalisib in which patients had received a median of five prior lines of therapy, occurring in only 1.9% of patients. In contrast, in the current study, 52% of patients experienced grade 3 or 4 transaminitis at some point in the trial.
Evidence for the hepatotoxicity being an on-target immune-mediated effect comes from lymphocytic infiltrate on liver biopsy and lymphocytic colitis in idelalisib-treated patients. Additional evidence comes from the fact that the toxicity is both treatable and preventable with steroids, he said.
He cautioned that hepatotoxicity can recur rapidly when the drug is reintroduced.
“In general, our experience has been if idelalisib is resumed while the subject remains on steroids, the drug is more likely to be tolerated and the subject eventually can be tapered off steroids,” he said.
Asked by an audience member whether patients who are receiving idelalisib in the first-line setting should also receive steroids, Dr. Lampson said that they closely monitor patient liver enzymes around 28 days, and if grade 1 transaminitis is detected, patients are automatically started on low-dose steroids.
The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
ORLANDO – Idelalisib given as first-line therapy for patients with chronic lymphocytic leukemia carries a high risk of early fulminant hepatotoxicity requiring drug interruption and steroids, investigators reported.
Among 24 patients who received idelalisib (Zydelig) monotherapy in a phase II trial of a combination of idelalisib followed by idelalisib concurrent with ofatumumab (Arzerra) as first-line therapy for chronic lymphocytic leukemia (CLL), 12 patients developed acute hepatotoxicity, marked by rapidly soaring levels of transaminase within about 28 days of starting therapy. An additional four patients developed hepatotoxicity at around 130 days, noted Dr. Benjamin L. Lampson, a clinical fellow in medicine at the Dana-Farber Cancer Institute in Boston.
“Multiple lines of evidence suggest that this early hepatotoxicity is immune mediated. The proportion of regulatory T cells in the peripheral blood decrease on idelalisib therapy, providing a possible explanation for the development of early hepatotoxicity,” he said at the annual meeting of the American Society of Hematology.
The toxicities occur more frequently among younger and less heavily pretreated patients, and are likely due to on-target, immune-mediated effects, he noted.
Dr. Lampson presented data on the first 24 patients in an ongoing phase II trial. Patients with previously untreated CLL receive idelalisib 150 mg twice daily for 56 days, in an attempt to mobilize neoplastic B cells from the peripheral lymphoid tissues and into the bloodstream.
Following the monotherapy phase, patients are given ofatumumab in an attempt to clear the disease from peripheral blood.
“This dosing strategy is slightly different than what has been previously been used in trials combining these particular drugs. Specifically, previously reported trials have started these agents simultaneously without a lead-in period of monotherapy,” Dr. Lampson explained.
When the lead-in phase is completed, patients receive idelalisib plus ofatumumab infusions once weekly for 8 weeks, followed by once-monthly infusions for 4 months. Patients then continue on idelalisib indefinitely. The primary endpoint is the overall response rate assessed 2 months after the completion of the combination therapy.
For the first 24 patients treated as of Nov. 9, 2015, the median time on therapy was 7.7 months and median follow-up was 14.7 months.
The median patient age was 67.4 years (range 57.6-84.9). CLL genetics showed that 13 patients had unmutated immunoglobulin heavy chain variable region (IgHV) disease, 4 had the 17p deletion and TP53 mutation, 1 had deletion 11q, and 13 had deletion 13q; some patients had more than one mutation.
“What we began to notice after enrolling just a few subjects on the trial was that severe hepatotoxicity was occurring shortly after initiating idelalisib,” Dr. Lampson said.
He presented one case, a 58-year-old man who was in the idelalisib monotherapy phase of the study. He developed grade 3 hepatotoxicity 28 days after starting the drug, despite having a normal liver function test just 1 day earlier. The drug was stopped, but his liver function tests continued to rise, suggesting a self-perpetuating or self-sustaining process.
On day 32, the patient was admitted to the hospital, and on day 33 he was started on steroids, based on the hypothesis that the hepatotoxicity might have been immune mediated. Two days after initiation of steroids, his liver function tests continued to rise, whereupon he was started on mycophenolate mofetil.
“With these two forms of immunosuppression, the [liver function tests] did eventually normalize, although the steroids and mycophenolate had to be tapered over a period of many weeks. And this patient was not the only patient to experience toxicity; in fact, hepatotoxicity was frequent and often severe,” he said.
At the time of maximum incidence, week 4, the percentage of patients with any hepatotoxicity was 46%, with 13% at grade 4, and 21% at grade 3.
“The median time to initial development of hepatotoxicity is 28 days. This suggests that the mechanism of hepatotoxicity is not immediate, but takes time to develop, consistent with an adaptive immune response. Furthermore, hepatotoxicity is typically occurring before the first dose of ofatumumab is occurring at week 8, suggesting idelalisib alone is the cause of the hepatotoxicity,” Dr. Lampson said.
A comparison of data from the ongoing study and from three previous studies – two with idelalisib in relapsed refractory disease, and one as first-line therapy in patients 65 and older – showed that grade 3 or greater hepatotoxicity was lowest in a phase I trial of idelalisib in which patients had received a median of five prior lines of therapy, occurring in only 1.9% of patients. In contrast, in the current study, 52% of patients experienced grade 3 or 4 transaminitis at some point in the trial.
Evidence for the hepatotoxicity being an on-target immune-mediated effect comes from lymphocytic infiltrate on liver biopsy and lymphocytic colitis in idelalisib-treated patients. Additional evidence comes from the fact that the toxicity is both treatable and preventable with steroids, he said.
He cautioned that hepatotoxicity can recur rapidly when the drug is reintroduced.
“In general, our experience has been if idelalisib is resumed while the subject remains on steroids, the drug is more likely to be tolerated and the subject eventually can be tapered off steroids,” he said.
Asked by an audience member whether patients who are receiving idelalisib in the first-line setting should also receive steroids, Dr. Lampson said that they closely monitor patient liver enzymes around 28 days, and if grade 1 transaminitis is detected, patients are automatically started on low-dose steroids.
The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
AT ASH 2015
Key clinical point: Idelalisib in the first-line setting is associated with significant risk of hepatotoxicity, with a peak incidence at about 28 days of therapy.
Major finding: More than half (52%) of patients with newly diagnosed chronic lymphocytic leukemia had grade 3 or 4 hepatotoxicity with idelalisib monotherapy.
Data source: Ongoing phase II clinical trial with data on 24 patients.
Disclosures: The study is sponsored by the Dana-Farber Cancer Institute in collaboration with Gilead Sciences and GlaxoSmithKline. Dr. Lampson and colleagues declared no relevant conflicts of interest.
ASH: First-line ibrutinib beats standard chemo for CLL/SLL in older patients
ORLANDO – Monotherapy with ibrutinib (Imbruvica) prolonged survival longer than did standard chemotherapy using chlorambucil (Leukeran) in the front-line treatment of older patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in the phase III RESONATE-2 study.
Co–drug developers Pharmacyclics and Janssen Biotech announced this summer that ibrutinib, an orally bioavailable, small-molecule inhibitor of Bruton’s tyrosine kinase, had achieved its primary and secondary endpoints.
But the first full look at the data at the annual meeting of the American Society of Hematology showed ibrutinib reduced the risk of progression or death by 84% by independent review compared with chlorambucil, which has been a standard first-line therapy in older CLL patients.
The results were simultaneously published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1509388).
With a median follow-up of 18.4 months, median progression-free survival (PFS) had not been reached with ibrutinib vs. 19 months with chlorambucil (hazard ratio, 0.16; P less than .001).
By investigator assessment, ibrutinib reduced the risk of progression by 91%, with an 18-month PFS rate of 94% vs. 45% with chlorambucil (HR, 0.09; P less than .001).
The PFS benefit with ibrutinib was consistent regardless of patient age, Rai stage, ECOG (Eastern Cooperative Oncology Group) status, bulky disease, and importantly, such high-risk markers as chromosome 11q deletion and unmutated immunoglobulin heavy chain variable (IGHV) mutation status, study author Dr. Alessandra Tedeschi, of Hospital Niguarda Cà Granda, Milan, , said at a press briefing highlighting the study (Ab. 485).
In addition, ibrutinib led to an 84% reduction in the risk of death compared with chlorambucil (HR, 0.16; P = .001). The 24-month overall survival rate was 98% with ibrutinib versus 85% with chlorambucil.
Single-agent ibrutinib was approved in 2014 for patients with CLL who had received at least one prior therapy and for all patients with the deleterious 17p deletion on the basis of the phase III RESONATE trial in relapsed or refractory CLL.
Three-year follow-up in the phase II PCYC-1102 study signaled a benefit with ibrutinib in treatment-naive CLL, showing an overall response rate of 84%, 30-month PFS of 96%, and overall survival rate of 97% in a subset of 31 patients at least 65 years old (Blood. 2015 Apr 16;125[16]:2497-506).
“The phase III RESONATE-2 trial confirms the efficacy of ibrutinib in treatment-naive CLL patients, leading to a 91% reduction in risk of progression and 84% reduction in risk of death when compared to chlorambucil,” Dr. Tedeschi said.
In all, 269 patients, median age of 73 years, were evenly randomized to once-daily ibrutinib 420 mg until progression or unacceptable toxicity or chlorambucil 0.5 mg/kg (up to a maximum of 0.8 mg/kg) on days 1 and 15 of a 28-day cycle for up to 12 cycles. Patients with the deleterious 17p deletion were excluded, as single-agent chlorambucil is not effective in this population.
Ibrutinib significantly improved bone marrow function, as reflected by a sustained increase in hemoglobin and platelets.
“This is very important in this category of elderly patients, in whom bone marrow failure is the most common cause of morbidity,” Dr. Tedeschi said.
There were 3 deaths on the ibrutinib arm and 17 on the chlorambucil arm.
The majority of patients (87%) in this older population with frequent comorbidities was able to continue on oral, once-daily ibrutinib with a median of 1.5 years of follow-up, she said.
The most common adverse events on ibrutinib were grade one diarrhea, fatigue, cough, and nausea that did not result in treatment discontinuation. On the chlorambucil arm, fatigue nausea, vomiting, and cytopenias occurred more frequently than with ibrutinib.
Grade 3 maculopapular rash occurred in 3% with ibrutinib and 2% with chlorambucil, she said.
Ibrutinib was associated with higher and not insignificant rates of atrial fibrillation and major hemorrhage compared with chlorambucil, said Dr. Brian T. Hill of the Taussig Cancer Institute at the Cleveland Clinic, who was not involved in the study. In our interview, Dr. Hill also questions the relevance today of chlorambucil monotherapy as the comparator arm in RESONATE-2.
Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Monotherapy with ibrutinib (Imbruvica) prolonged survival longer than did standard chemotherapy using chlorambucil (Leukeran) in the front-line treatment of older patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in the phase III RESONATE-2 study.
Co–drug developers Pharmacyclics and Janssen Biotech announced this summer that ibrutinib, an orally bioavailable, small-molecule inhibitor of Bruton’s tyrosine kinase, had achieved its primary and secondary endpoints.
But the first full look at the data at the annual meeting of the American Society of Hematology showed ibrutinib reduced the risk of progression or death by 84% by independent review compared with chlorambucil, which has been a standard first-line therapy in older CLL patients.
The results were simultaneously published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1509388).
With a median follow-up of 18.4 months, median progression-free survival (PFS) had not been reached with ibrutinib vs. 19 months with chlorambucil (hazard ratio, 0.16; P less than .001).
By investigator assessment, ibrutinib reduced the risk of progression by 91%, with an 18-month PFS rate of 94% vs. 45% with chlorambucil (HR, 0.09; P less than .001).
The PFS benefit with ibrutinib was consistent regardless of patient age, Rai stage, ECOG (Eastern Cooperative Oncology Group) status, bulky disease, and importantly, such high-risk markers as chromosome 11q deletion and unmutated immunoglobulin heavy chain variable (IGHV) mutation status, study author Dr. Alessandra Tedeschi, of Hospital Niguarda Cà Granda, Milan, , said at a press briefing highlighting the study (Ab. 485).
In addition, ibrutinib led to an 84% reduction in the risk of death compared with chlorambucil (HR, 0.16; P = .001). The 24-month overall survival rate was 98% with ibrutinib versus 85% with chlorambucil.
Single-agent ibrutinib was approved in 2014 for patients with CLL who had received at least one prior therapy and for all patients with the deleterious 17p deletion on the basis of the phase III RESONATE trial in relapsed or refractory CLL.
Three-year follow-up in the phase II PCYC-1102 study signaled a benefit with ibrutinib in treatment-naive CLL, showing an overall response rate of 84%, 30-month PFS of 96%, and overall survival rate of 97% in a subset of 31 patients at least 65 years old (Blood. 2015 Apr 16;125[16]:2497-506).
“The phase III RESONATE-2 trial confirms the efficacy of ibrutinib in treatment-naive CLL patients, leading to a 91% reduction in risk of progression and 84% reduction in risk of death when compared to chlorambucil,” Dr. Tedeschi said.
In all, 269 patients, median age of 73 years, were evenly randomized to once-daily ibrutinib 420 mg until progression or unacceptable toxicity or chlorambucil 0.5 mg/kg (up to a maximum of 0.8 mg/kg) on days 1 and 15 of a 28-day cycle for up to 12 cycles. Patients with the deleterious 17p deletion were excluded, as single-agent chlorambucil is not effective in this population.
Ibrutinib significantly improved bone marrow function, as reflected by a sustained increase in hemoglobin and platelets.
“This is very important in this category of elderly patients, in whom bone marrow failure is the most common cause of morbidity,” Dr. Tedeschi said.
There were 3 deaths on the ibrutinib arm and 17 on the chlorambucil arm.
The majority of patients (87%) in this older population with frequent comorbidities was able to continue on oral, once-daily ibrutinib with a median of 1.5 years of follow-up, she said.
The most common adverse events on ibrutinib were grade one diarrhea, fatigue, cough, and nausea that did not result in treatment discontinuation. On the chlorambucil arm, fatigue nausea, vomiting, and cytopenias occurred more frequently than with ibrutinib.
Grade 3 maculopapular rash occurred in 3% with ibrutinib and 2% with chlorambucil, she said.
Ibrutinib was associated with higher and not insignificant rates of atrial fibrillation and major hemorrhage compared with chlorambucil, said Dr. Brian T. Hill of the Taussig Cancer Institute at the Cleveland Clinic, who was not involved in the study. In our interview, Dr. Hill also questions the relevance today of chlorambucil monotherapy as the comparator arm in RESONATE-2.
Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Monotherapy with ibrutinib (Imbruvica) prolonged survival longer than did standard chemotherapy using chlorambucil (Leukeran) in the front-line treatment of older patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in the phase III RESONATE-2 study.
Co–drug developers Pharmacyclics and Janssen Biotech announced this summer that ibrutinib, an orally bioavailable, small-molecule inhibitor of Bruton’s tyrosine kinase, had achieved its primary and secondary endpoints.
But the first full look at the data at the annual meeting of the American Society of Hematology showed ibrutinib reduced the risk of progression or death by 84% by independent review compared with chlorambucil, which has been a standard first-line therapy in older CLL patients.
The results were simultaneously published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1509388).
With a median follow-up of 18.4 months, median progression-free survival (PFS) had not been reached with ibrutinib vs. 19 months with chlorambucil (hazard ratio, 0.16; P less than .001).
By investigator assessment, ibrutinib reduced the risk of progression by 91%, with an 18-month PFS rate of 94% vs. 45% with chlorambucil (HR, 0.09; P less than .001).
The PFS benefit with ibrutinib was consistent regardless of patient age, Rai stage, ECOG (Eastern Cooperative Oncology Group) status, bulky disease, and importantly, such high-risk markers as chromosome 11q deletion and unmutated immunoglobulin heavy chain variable (IGHV) mutation status, study author Dr. Alessandra Tedeschi, of Hospital Niguarda Cà Granda, Milan, , said at a press briefing highlighting the study (Ab. 485).
In addition, ibrutinib led to an 84% reduction in the risk of death compared with chlorambucil (HR, 0.16; P = .001). The 24-month overall survival rate was 98% with ibrutinib versus 85% with chlorambucil.
Single-agent ibrutinib was approved in 2014 for patients with CLL who had received at least one prior therapy and for all patients with the deleterious 17p deletion on the basis of the phase III RESONATE trial in relapsed or refractory CLL.
Three-year follow-up in the phase II PCYC-1102 study signaled a benefit with ibrutinib in treatment-naive CLL, showing an overall response rate of 84%, 30-month PFS of 96%, and overall survival rate of 97% in a subset of 31 patients at least 65 years old (Blood. 2015 Apr 16;125[16]:2497-506).
“The phase III RESONATE-2 trial confirms the efficacy of ibrutinib in treatment-naive CLL patients, leading to a 91% reduction in risk of progression and 84% reduction in risk of death when compared to chlorambucil,” Dr. Tedeschi said.
In all, 269 patients, median age of 73 years, were evenly randomized to once-daily ibrutinib 420 mg until progression or unacceptable toxicity or chlorambucil 0.5 mg/kg (up to a maximum of 0.8 mg/kg) on days 1 and 15 of a 28-day cycle for up to 12 cycles. Patients with the deleterious 17p deletion were excluded, as single-agent chlorambucil is not effective in this population.
Ibrutinib significantly improved bone marrow function, as reflected by a sustained increase in hemoglobin and platelets.
“This is very important in this category of elderly patients, in whom bone marrow failure is the most common cause of morbidity,” Dr. Tedeschi said.
There were 3 deaths on the ibrutinib arm and 17 on the chlorambucil arm.
The majority of patients (87%) in this older population with frequent comorbidities was able to continue on oral, once-daily ibrutinib with a median of 1.5 years of follow-up, she said.
The most common adverse events on ibrutinib were grade one diarrhea, fatigue, cough, and nausea that did not result in treatment discontinuation. On the chlorambucil arm, fatigue nausea, vomiting, and cytopenias occurred more frequently than with ibrutinib.
Grade 3 maculopapular rash occurred in 3% with ibrutinib and 2% with chlorambucil, she said.
Ibrutinib was associated with higher and not insignificant rates of atrial fibrillation and major hemorrhage compared with chlorambucil, said Dr. Brian T. Hill of the Taussig Cancer Institute at the Cleveland Clinic, who was not involved in the study. In our interview, Dr. Hill also questions the relevance today of chlorambucil monotherapy as the comparator arm in RESONATE-2.
Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
Key clinical point: First-line ibrutinib significantly extends survival in older patients with untreated chronic lymphocytic leukemia or small lymphocytic lymphoma, compared with chlorambucil chemotherapy.
Major finding: Median progression-free survival was not reached with ibrutinib vs. 19 months with chlorambucil (HR, 0.16; P less than .001).
Data source: Prospective, phase III study of 269 patients 65 years or older with treatment-naive CLL or SLL.
Disclosures: Pharmacyclics, which is jointly developing ibrutinib with Janssen Biotech, sponsored the study. Dr. Tedeschi reported having nothing to disclose. Several coauthors reported relationships with Pharmacyclics and Janssen.
ASH: Donor CAR-T cells elicit responses in mixture of progressive B-cell cancers
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
AT ASH 2015
Key clinical point: Allogeneic anti-CD19 CAR T-cell therapy showed promise in a treatment approach for B-cell malignancies persisting after allogeneic transplantation.
Major finding: Nine of 20 patients achieved remission with anti-CD19 CAR T-cell therapy.
Data source: Phase I study in 20 patients with CD19-positive B-cell malignancies progressing after allogeneic transplant.
Disclosures: Dr. Kochenderfer reported research funding from Bluebird bio, the study sponsor.
Medical Roundtable: The Changing Pharmacologic Treatment Landscape in Chronic Lymphocytic Leukemia
Moderated by: Jennifer R. Brown, MD, PhD1
Discussants: Jeffrey A. Jones, MD, MPH2; Jacqueline C. Barrientos, MD3
From the Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA1; Ohio State University, Columbus, OH2; Hofstra North Shore-LIJ School of Medicine, Lake Success, NY
Address for correspondence: Jennifer R. Brown, MD, PhD, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215
E-mail: [email protected]
Biographical Sketch
From Dana-Farber Cancer Institute and Harvard Medical School:
Jennifer R. Brown, MD, PhD is the Director of the CLL Center of the Division of Hematologic Malignancies at Dana-Farber Cancer Institute and an Associate Professor of Medicine at Harvard Medical School in Boston, Massachusetts. Dr. Brown completed a BS and MS simultaneously in molecular biophysics and biochemistry (MB&B) at Yale, graduating summa cum laude with distinction in MB&B. She proceeded to Harvard Medical School where she received her MD and PhD in molecular genetics in 1998 and was awarded the James Tolbert Shipley Prize for research accomplishment in the graduating class. She then served as an intern and resident in Internal Medicine at Massachusetts General Hospital followed by fellowship in Hematology and Medical Oncology at the Dana-Farber Cancer Institute. Dr. Brown joined the faculty of DFCI and Harvard Medical School in 2004, where she has an active clinical-translational research program in CLL.
Her particular interests include the development of novel targeted therapeutics for CLL, as well as the genomics of CLL. She has been instrumental in the clinical development of both idelalisib and ibrutinib, leading to their regulatory approvals in CLL.
In the area of genomics she has been instrumental in the description of the somatic mutation profile of CLL, and is now particularly interested in the implementation of genomic technology in the clinic, including for prognosis and targeted therapy. She also has a longstanding research interest and focus on the inherited predisposition to CLL.
To date she has published over 130 papers in the scientific literature, predominantly in CLL. She is an active member of the CLL Research Consortium and serves on the Alliance Leukemia and Leukemia Correlative Science Committees as well as the NIH Cancer Biomarkers Study Section. In 2014 she was the recipient of two awards from Dana-Farber Cancer Institute, the Clinical Innovation Award, as well as the George Canellos Award for Excellence in Clinical Investigation and Patient Care. She enjoys a worldwide reputation as a CLL expert and is in much demand as an international speaker.
From Hofstra North Shore-LIJ School of Medicine:
Jacqueline C. Barrientos, MD, is Attending Physician at the Chronic Lymphocytic Leukemia (CLL) Research & Treatment Program of the Division of Hematology and Medical Oncology, Department of Medicine, in the North Shore – LIJ Cancer Institute in Lake Success, New York. She is also Assistant Professor of Medicine at the Hofstra North Shore-LIJ School of Medicine. Dr. Barrientos works in close collaboration with her mentors, Dr. Kanti R. Rai and Dr. Nicholas Chiorazzi of the Feinstein Institute for Medical Research.
Dr. Barrientos received her medical degree at the Ponce School of Medicine in Puerto Rico, where she was elected vice-president of Alpha Omega Alpha Honor Medical Society. During her medical studies, she was the recipient of two Research Fellowship Awards from the Howard Hughes Medical Institute. She completed her internship and residency in internal medicine at Yale-New Haven Hospital of the Yale School of Medicine, and her fellowship in Hematology/Oncology at New York Presbyterian Hospital of Weill Cornell Medical College in New York City, where she also served as Chief Fellow. She is board certified in internal medicine, hematology and oncology.
Dr. Barrientos’ research focus is on chronic lymphocytic leukemia and lymphoma. She has extensive experience with the new promising agents targeting the B-cell receptor signaling pathway in B-cell malignancies, serving as Principal Investigator on several phase I-III clinical trials.
Dr. Barrientos actively participates in multi-institutional clinical trials with the Chronic Lymphocytic Leukemia Research Consortium (CRC) and the Alliance for Clinical Trials in Oncology. She is a cadre member of the Leukemia Committee of the Alliance for Clinical Trials in Oncology and in this capacity is co-chair of a study comparing chemoimmunotherapy against a combination of targeted agents. She is a member of the American Society of Clinical Oncology (ASCO) and the American Society of Hematology (ASH).
She has been an invited speaker for ASCO University “CLL Tumor Board”, ASH “State of the Art Symposium”, and “Highlights of ASH in Latin America”. Dr. Barrientos is the recipient of a 2015 American Society of Hematology-Harold Amos Medical Faculty Development Program (ASH-AMFDP) Fellowship award.
DR. BROWN: I am Jennifer Brown, Director of the Chronic Lymphocytic Leukemia (CLL) Center at Dana-Farber Cancer Institute, and Associate Professor of Medicine at Harvard Medical School. Today, I will be speaking with two of my esteemed CLL colleagues, Drs. Jeffrey Jones and Jacqueline Barrientos, about the new drug approvals in CLL.
DR. BARRIENTOS: I’m Jacqueline Barrientos, Assistant Professor of Hematology/Oncology at the Hofstra North Shore-LIJ School of Medicine, and Attending Hematologist at the CLL Research and Treatment Program in Long Island, NY. Our center participates in clinical trials and we perform correlative basic research. I’m very happy to participate in this expert roundtable discussion.
DR. JONES: I’m Dr. Jeffrey Jones, Associate Professor of Internal Medicine and Section Chief for CLL in the Division of Hematology at The Ohio State University.
DR. BROWN: Thank you Jeff and Jacquie for joining me today. I think we’re all aware what an exciting time this is in CLL with the approvals last year of the targeted inhibitors ibrutinib and idelalisib as well as the new antibody approval obinutuzumab as well as the additional indication for ofatumumab. Let’s start our discussion with ibrutinib and idelalisib. Jeff, please introduce the approvals that these inhibitors received and get us started.
DR. JONES: February 2014 marked a really important time in CLL medicine with the approval of the first oral kinase inhibitor, ibrutinib, for the treatment of CLL after one prior therapy.1,2 This ushered in an entirely new era of molecularly-targeted therapy for CLL. Later that year, ibrutinib received approval for deletion 17p CLL, the highest risk genetic subtype of CLL, whether previously untreated or relapsed disease. The drug has rapidly entered the clinic, although I think most of us are still trying to determine how best to incorporate them into our practice.
DR. BROWN: Jacquie, please comment on how you’re using ibrutinib now in your practice.
DR. BARRIENTOS: In CLL patients with the presence of a mutation of TP53 or deletion 17p, we use ibrutinib. We essentially do not use chemotherapy on this particular set of patients. If, for any reason, they are not able to tolerate the drug, then we consider idelalisib, which is not approved separately for this 17p deletion indication. Idelalisib is approved for use in combination with rituximab for the treatment of relapsed or refractory CLL patients. Idelalisib has shown clinical activity in several clinical trials in patients with deletion 17p.
At this moment, we mainly are using ibrutinib or idelalisib for our relapsed or refractory CLL patients. Clinical trials are underway in the frontline setting and we hope to see the results of the frontline use of ibrutinib in elderly patients soon. As of right now, we don’t use ibrutinib as a frontline therapy unless there is a reason, and usually it’s that they carry the 17p deletion or they are participating in a clinical trial.
DR. JONES: Outside of clinical trials our practice has really been to follow the label indications for ibrutinib. For previously untreated patients, our use has been limited to patients with deletion 17p or TP53 mutated disease, as Jacquie said, since that is the group for which the drug has been approved in the frontline.
DR. BROWN: I would agree. That’s been my practice as well. We should perhaps review the data from the registration trial that led to the ibrutinib approval for relapsed refractory CLL. The initial approval was from the stage IB2 study and was an accelerated approval.1 The confirmatory registration trial, RESONATE, randomized relapsed refractory CLL patients to ibrutinib versus the anti-CD20 antibody ofatumumab.2 Ibrutinib was found to be significantly better in improving both progression free and overall survival, although there was crossover later. As a result, this has moved into our relapse refractory use very rapidly. Although we still use chemoimmunotherapy for upfront therapy for patients without 17p deletion, for those in relapse we have moved entirely to targeted inhibitors. Would you both agree?
DR. JONES: For sure. I think it is very hard in 2015 to think of the patient for whom chemo-immunotherapy is the better choice than ibrutinib for relapsed disease.I think it is very hard in 2015 to think of the patient for whom chemoimmunotherapy is the better choice than ibrutinib for relapsed disease. The benefit is most marked for the group with higher-risk disease as characterized by genetic risk features, not just deletion 17p, but patients with complex abnormal karyotype or deletions of chromosome 11q. All of these patients particularly benefit from treatment with ibrutinib in the second line vs chemoimmunotherapy, as do patients who had either a suboptimal response to frontline chemoimmunotherapy or a brief duration of first remission. All of us are sometimes asked, “Well, who is the patient with relapsed CLL for whom ibrutinib is the best choice?” Right now, in most clinical situations, my response is, “For which patient is ibrutinib not the best choice in first relapse?”
DR. BROWN: That’s actually a good question. Jacquie, how would you answer that? Are there patients for whom you would not choose ibrutinib in first relapse?
DR. BARRIENTOS: I feel a hesitant to use ibrutinib in some patients with a particular comorbidity or medical history. For example, patients with a previous intracranial bleed or a recent history of bleeding, I would prefer to avoid using ibrutinib because there have been rare cases of spontaneous intracranial bleed or severe bleeding after trauma. The other type of patient where I would be cautious is a patient with uncontrolled atrial fibrillation because there are data that in the minority of patients (up to 10% of patients), atrial fibrillation has been an issue. We have some patients that are so frail that they couldn’t tolerate another episode of uncontrolled atrial fibrillation and as such they would not be ideal candidates for the drug. For that type of patient, I would probably abstain from using ibrutinib and consider the use of another therapy. Finally, I would be careful in patients on antiplatelet and anticoagulation therapy because ibrutinib affects platelet functions increasing the risk of bleeding. The bleeding events seen with ibrutinib are mostly grade 1 or grade 2. If the patients have had a serious bleed or serious gastrointestinal bleed or a recent surgery, then I would preferably use another agent.
DR. BROWN:Yes, so that gets to the toxicities of ibrutinib. The more medically significant ones do include perhaps a 5% to 10% risk of atrial fibrillation as well as bleeding risks, which as Jacquie points out are low and usually low grade, but there are occasional higher-risk bleeds. I personally still try to avoid combining anticoagulation with ibrutinib, as we don’t fully understand the mechanism or the risk factors for the more serious bleeds. Jeff, please comment.
DR. JONES: I think the data from the randomized study are actually the most helpful since, as you say, mild bleeding events (grade 1 or 2) were indeed more common amongst the group of patients who were treated with ibrutinib.2 Major bleeding events—which are typically defined as intracranial hemorrhage, bleeding requiring transfusion, or inpatient management—were actually similar between the two arms of the trial. An important caveat in interpreting these data is to know that patients in this trial were excluded if they were anticoagulated with warfarin, if they had an antecedent history of intracranial hemorrhage or recent bleeding, or recent surgery. In line with those exclusions, we will often consider other options. If there is any specific concern for bleeding, such as a patient who has experienced bleeding complications during routine anticoagulation, which is also a patient for whom ibrutinib may not be the best choice. In these clinical situations, it is important to involve the patient in discussing the balance of risks and benefits.
DR. BROWN: Yes. Jacquie, please comment on some of the side effects the patients on ibrutinib have, and how you manage those.
DR. BARRIENTOS: I usually mention to my patients that over the first 2 or 3 months about half of them will have a possible change in their bowel movements. Usually they report some diarrhea or loose stools. Usually these episodes are mild, nothing that requires hospitalization. In any case, if it becomes severe, I definitely make sure that it’s not an infection. We all know that our patients with CLL are prone to infections. The other thing I tell the patients is that in some cases patients may develop a rash on the skin. Many times it may look like a rash, but it’s actually ecchymosis—an effect from the drug on the platelets. Essentially, they are grade 1 and don’t require intervention. I just tell them that eventually they will go away. It can be scary for the patients if they are not expecting these. We have had patients with large areas of hematomas in the arms or in the legs. That is unexpected with a drug that they are taking by mouth. They usually expect that with other drugs like warfarin, but not with ibrutinib, so it is important to mention before they start the drug.
Last but not least, I mention the fact that they may get arthralgias—joint pain—in different areas of their bodies. I would say that I see that in about 20% to 30% of patients. Usually it’s very mild, but on occasion I’ve had patients with arthritis so severe that we’ve had to hold the drug and give them some steroids to help them improve their ability to maneuver their hands or move their joints. I’m sure you have seen some of those same side effects.
DR. BROWN: Yes, definitely. In general, it’s pretty well tolerated but it’s best to warn the patients, then there are no surprises. Let’s turn our attention for a moment back to the highest risk genetic subgroup, the 17p deleted patients—which Jeff had mentioned get particularly strong benefit from ibrutinib. This is certainly true, although it’s also the case that it appears, depending on the data set you look at, that they may relapse earlier than other patients on ibrutinib. In the original phase IB2, the median progression survival for the 17p deleted patients was 28 months. More recent data from Ohio State and MD Anderson suggest that complex karyotype may be a risk factor.3,4 Given these data, how are you two handling the question of allogeneic stem cell transplantation for these patients in this new era?
DR. BARRIENTOS: At our center, if the patient is young and they have access and are fit to tolerate a reduced-intensity allogeneic transplant, we recommend that they be evaluated for a transplant. Unfortunately, if they lose the response to the best drug available for their particular genetic mutation, then we have limited options of salvage therapy. It’s risky to think that they will not relapse at some point, and then what do we do at the time of relapse? We can use other targeted agents that are available, like idelalisib, with the knowledge that they may not always respond to the salvage therapy. Promising clinical activity has been reported for patients with 17p deletion treated with venetoclax in clinical trials. Venetoclax is a new targeted agent in development stages but the drug is only available in clinical trials.Promising clinical activity has been reported for patients with 17p deletion treated with venetoclax in clinical trials. Venetoclax is a new targeted agent in development stages but the drug is only available in clinical trials. One problem is that in order to participate in a clinical trial the patient needs to be able to get to the center to get the drug. Additionally, the patient needs to satisfy certain eligibility criteria for study entry. For these patients that stop responding to ibrutinib, the options of care are very limited at this time. This is the reason why I send all my young patients with a 17p deletion for a transplant evaluation.
At the end of the day it is tough to convince the patients to go for a transplant when they’re feeling in excellent shape. It’s still difficult to make a case to go for a procedure that may have its complications on its own. It is well known that there are some increased mortality risks and infection risks that can arise as a result of a transplant. They may not want to do it because they are feeling so great with their routine. I still sit down and have a long frank talk with the patients, especially if they have complex karyotype and 17p deletion. I am concerned that at some point they’re going to stop responding to ibrutinib.
DR. BROWN: That’s generally my practice as well. What about you, Jeff?
DR. JONES: Until there is greater clarity regarding which of the newer agents can salvage patients progressing after ibrutinib, I think it is still important for younger, transplant eligible patients with deletion 17p disease to undergo evaluation for allograft. It remains potentially curative therapy, and I think the availability of ibrutinib has not really changed the importance of that evaluation.
DR. BROWN: Yes, I would agree. I think that was a good discussion on ibrutinib. Why don’t we turn our attention now to idelalisib, the phosphoinositide 3-kinase (PI3K) inhibitor. How are you using idelalisib in your practices? Is this after ibrutinib in general?
DR. JONES: Published data regarding the sequencing of the new agents are relatively limited since all of the registration trials for idelalisib excluded patients who had received prior therapy with an inhibitor of B-cell receptor signaling, including Bruton’s tyrosine kinase inhibitors like ibrutinib.5,6 A small number of patients enrolled on the phase IB2 trial of ibrutinib, as well as the subsequent randomized trial, had received prior therapy with idelalisib and responded similarly to patients who had not received prior idelalisib.1,2 In our practice, the use of idelalisib has pretty much been limited to patients who have either received prior ibrutinib or patients who are not eligible to receive ibrutinib because of some important contraindication, such as an inherited bleeding defect, perceived increased bleeding, or history of difficult to control atrial fibrillation, since that event also seems to be more likely among patients treated with ibrutinib.
DR. BROWN: How about you, Jacquie?
DR. BARRIENTOS: The same type of patient with the addition of patients with kidney disease. The rationale for this is based on the phase III trial for idelalisib and rituximab, the enrollment allowed participation of patients with decreased renal function, that was one of the entry criteria for eligibility to participate in the trial.6 In most of the ibrutinib trials the creatinine clearance needed to be adequate, whereas this was allowed to be lower on the idelalisib trials. For those patients with severe renal impairment, I tend to prefer idelalisib rather than ibrutinib—only because I feel more comfortable and have more experience treating patients with impaired kidney function with idelalisib.
DR. BROWN: I have seen some episodic elevations in creatinine in patients on ibrutinib, but they’re fairly sporadic and it’s a little hard to assess the direct drug relationship. It is true that the patients in the idelalisib studies had a high level of comorbidity deliberately on the initial registration trial and generally did reasonably well with idelalisib. The toxicity profile of idelalisib is pretty characteristic, and is potentially harder to manage than that of ibrutinib. I think it also dictates some of how it’s being used in later line therapy. Does one of you wish to comment on the pattern of the key toxicities?
DR. BARRIENTOS: One key toxicity that is very particular to this drug that may happen overnight and is very striking is transaminitis. It usually happens more with non-Hodgkin lymphoma patients compared to relapsed CLL patients, but transaminitis can still be very severe. Patients can develop transaminitis even after more than a cycle on therapy even if they were tolerating the drug well without other issues. It’s very important to educate physicians and healthcare providers about the need to monitor the liver function tests, at least every 2 weeks for the first 2 months. Transaminitis events can be very prompt, very rapid, and usually asymptomatic. My patients that developed transaminitis never complained and had we not been cautious about it, we may have missed it.
DR. BROWN: Yes, I even check weekly. The recent safety analysis said the overall incidence of grade 3 to 4 transaminitis is about 15% in relapse patients.7 That’s pretty significant.
DR. JONES: I think it’s important to know that the transaminitis, if monitored carefully and managed with drug interruption and/or dose reduction upon reintroduction, need not lead to discontinuation. Discontinuations for transaminitis are actually the minority of patients who experience the side effect.
DR. BROWN: Absolutely. Do you want to comment on some of the other side effects that may more often lead to discontinuation?
DR. JONES: We should mention that there are some preclinical animal data suggesting that the molecular target of idelalisib, the PI3K delta isoform, is an important signaling molecule in regulatory T cells important for self-tolerance. While it has efficacy in treating B-cell disorders, inhibiting PI3K-delta may also be impairing T regulatory cell function. That may be what leads to the more characteristic later side effects of idelalisib, including pneumonitis and colitis. Pneumonitis is relatively rare, but because it can masquerade as other respiratory ailments in an older patient population with comorbid medical illnesses like chronic obstructive pulmonary disease and preexisting immune dysfunction because of CLL or prior therapy, inflammatory pneumonitis can be misdiagnosed. This rare but potentially life-threatening complication of idelalisib treatment requires prompt recognition, discontinuation of the drug, and appears to be most effectively managed with corticosteroids.
The other commonly occurring late toxicity, colitis, is often one that also eludes prompt recognition since many times patients are seen by primary care practitioners between oncology visits, and these doctors may not yet be aware that colitis can occur as a late side effect of idelalisib. Sometimes the colitis is misdiagnosed as gastroenteritis or Clostridium difficile colitis and eludes initial management. Like the pneumonitis, this problem, which may occur in more than a quarter of patients, is really best managed by prompt recognition and, in many cases, interruption of the drug. In some cases, patients have been managed with interruption of the drug and perhaps rechallenge at a lower dose, but in many other cases, colitis has been a treatment-limiting side effect and is a leading cause of drug discontinuation for toxicity.
DR. BROWN: Yes, I would agree. It can occur even at much later times in people who have tolerated the drug for even a couple of years, which is surprising compared to typical drug-related diarrhea.
DR. JONES: Right. With many other drugs, a patient starts taking the drug and expects the treatment-related side effects to become manifest very early. The diarrhea and rash associated with ibrutinib, for instance, are really timed very close to drug initiation, similar to antibiotics and other medications that we commonly prescribe. When side effects occur late in the course of treatment, I think it is just not on anyone’s radar to suspect that they could be related to a drug that they have been receiving for some time. That is an important message to communicate to patients, as well as to doctors who are just beginning to prescribe these new drugs for the first time.
DR. BROWN: Exactly. Why don’t we turn our attention now to the approval of obinutuzumab, and review the registration trial data there and then how you’re using that in practice. Jacquie?
DR. BARRIENTOS: Obinutuzumab is a third generation monoclonal antibody targeting the CD20 receptor on B cells. It was approved in November of 2013 by the US Food and Drug Administration for use in combination with chlorambucil to treat patients with previously untreated CLL.8 The trial enrolled patients with comorbidities as measured by the Cumulative Index Rating Scale, the scale helps define fitness. The patients that participated in the registration trial were patients that due to their comorbidities would not tolerate well a chemoimmunotherapy regimen like fludarabine, cyclophosphamide, and rituximab (FCR), and possibly the combination of bendamustine and rituximab. In patients older than age 65 with multiple comorbidities, chlorambucil monotherapy is widely used worldwide due to concerns of complications from the use of other chemoimmunotherapy regimens like the ones mentioned above. In the United States, we usually see that physicians prefer to use rituximab as a single agent in frail patients with multiple comorbidities.
The combination of obinutuzumab with chlorambucil compared to chlorambucil as a single agent showed that the patients treated with the combination therapy had a higher rate of response, a higher rate of progression free survival, and an improved overall survival. The main issue with obinutuzumab is the fact that the infusion reactions are much greater than what we traditionally see with rituximab. Severe and life-threatening infusion reactions have been reported. The reactions can also be more abrupt, although they typically occur very early in infusion, so they are more predictable. If the patient develops an infusion reaction or can’t tolerate the drug, the infusion needs to be interrupted. If the patient does not experience any further infusion reaction symptoms, the infusion may be restarted at a lower rate. I believe grade 3 to grade 4 events were higher than 10% in the registration trial, with infusion reactions of any grade seen in 50%–70%, so it can be common—usually within the first day. By the third infusion, the rate of reaction decreases significantly. Most of the time after that third infusion, most patients won’t have any more issues with tolerability.
Who are the patients that develop these infusion reactions? It has been noted that the level of interleukin 6 is elevated in patients that develop an infusion reaction. That’s the reason why all patients should be premedicated with potent steroids (methylprednisolone or dexamethasone, not hydrocortisone). In addition, patients need to be premedicated with acetaminophen and an antihistamine. In the future hopefully we will be able to use other agents like tocilizumab to lessen the risk of infusion reactions, this is currently being tested in clinical trials as its use is theoretical at this point based on the observation of the elevated interleukin 6 levels.
There are other important side effects with this combination regimen that were noted in the registration trial. There was a higher rate of neutropenia in the patients receiving obinutuzumab and chlorambucil, although this did not correlate with a higher rate of grade 3 or grade 4 infections. The rate of grade 3 or 4 infections was the same all across the board in patients that received chlorambucil, chlorambucil in combination with rituximab, or chlorambucil in combination with obinutuzumab.
DR. BROWN: Are you using much obinutuzumab chlorambucil in your practice?
DR. BARRIENTOS: In select patients, yes. For untreated patients with comorbidities that are not participating in a clinical trial, we discuss with them data from the frontline bendamustine and rituximab combination and obinutuzumab and chlorambucil combination. For the most part, most patients prefer obinutuzumab with chlorambucil because the obinutuzumab chlorambucil combination might be better tolerated and possibly less myelosuppressive than the bendamustine rituximab combination. Unfortunately, most of my patients have already been treated by the time we see them. We have a minority of patients that come recently diagnosed, we just don’t see that many untreated patients.
DR. BROWN: How about you, Jeff? Are you using it?
DR. JONES: Yes, it is a consideration for frontline therapy in patients who don’t have deletion 17p. As we discussed before, most of us have already adopted ibrutinib as our first choice in that 17p deleted population outside of clinical trial. For the remainder of patients, I think the first question remains whether their age and health are permissive to safely give FCR, since that regimen has been associated with the best survival outcomes, even some really long survival, in a group of patients with IgVH mutated, favorable cytogenetic risk disease.
For patients who are not eligible or willing to receive FCR, I think the choice between bendumustine and rituximab (BR) and chlorambucil and obinutuzumab is a relatively challenging one. Part of the reason is that while the overall response rates and complete response rates are lower with obinutuzumab and chlorambucil, the toxicity is also a bit lower. That makes it an appealing choice, particularly when we have the availability of drugs like ibrutinib and idelalisib in the second line. For older patients with comorbid medical illnesses in particular, it may be that the duration of first remission after chemotherapy may not matter as much when we have more effective second line options.
DR. BROWN: Yes, I think that’s definitely true. I just want to highlight two points. Your point about the long-term efficacy of FCR, particularly in the IgVH mutated patients—it is important to note that we now have data from both MD Anderson and the German CLL Study Group. The MD Anderson data with 10 year follow up, 60% of that genetic subgroup are progression free after FCR suggesting that a subset of them may in fact be cured. We don’t want to forget that with the excitement of the new inhibitors. I would second your point also about the potential toxicities of BR which can be as myelosuppressive as FCR even though it is not in every case. Again, it’s very important to assess the comorbidities of the patient not just for FCR but also for BR, particularly when FCR has this chance of very long-term remission which is not seen with BR.
DR. JONES: Yes, and there’s also a risk for opportunistic infections with both regimens. Like fludarabine-treated patients, there are patients treated with bendumustine who experience pneumocystis pneumonia or viral reactivation from immune suppression beyond just the neutropenia.
DR. BROWN: Yes, absolutely. Let’s talk briefly about where we see CLL therapy going in the next few years given these exciting new drugs. I’ll just leave that open and see what you have to say. Jacquie?
DR. BARRIENTOS: Some of the possible developments that we may see over the next couple of years are the use of these targeted agents or small molecules as initial therapy either as monotherapy or in combination regimens. We are expecting to see the data of the clinical trial of frontline ibrutinib against chlorambucil in patients that are older than age 65. Idelalisib has other ongoing clinical trials in the frontline setting as monotherapy and in combination therapy. Data have been presented of idelalisib in combination with rituximab as frontline therapy. It was interesting to note that some of these side effects that we saw in the relapsed or refractory setting occurred more often in patients in the frontline setting, although efficacy was very high. These promising data may eventually lead to a change in the way that we treat patients in the frontline, not only as monotherapy. There are several clinical trials that incorporate chemoimmunotherapy with these new targeted agents to see if maybe we will obtain deeper remissions or longer duration of response.
DR. JONES: What preliminary data exist in small phase 1 or phase 2 studies suggest that the new agents may be even more effective in previously untreated disease, with higher overall response rates, higher complete response rates, and more durable remissions than observed among patients with relapsed and refractory disease.9,10 These results underscore that the individual agents are among the most effective drugs that have been developed for CLL in terms of their single-agent activity. If you include the oral BCL-2 inhibitor in development, venetoclax, these drugs have really had remarkable single-agent efficacy. If these newer agents are like older cytotoxic chemotherapy agents, like fludarabine, they may become superstars when used in combination. While we will soon see these drugs move into the frontline setting as single agents, I think the real potential for magic is when they get combined. There we may see the kinds of deep remissions that we only achieve now with chemoimmunotherapy, remissions that will allow similar long-term treatment-free survival without cytotoxic chemotherapy. I’d like nothing more than to see a 60% 10 year survival after a nonchemotherapy-containing combination that emerges when we use these new drugs in ways that maximize their benefit in combination.
DR. BROWN: I would certainly agree. I think that although we have remarkable single-agent activity of these drugs, we know that in the context of single-agent activity, resistance is likely to develop over time. For a subset of patients that may not matter. If they’re older and have comorbidities, they may get enough durability of response from their first single agent that it doesn’t matter, particularly the patients with lower risk CLL. For our younger patients, I think the combinations will have the opportunity to minimize the development of resistance and also allow shorter courses of therapy so that patients can be off treatment still with deep remissions. That is what most excites me about the future of these agents.
Let’s just talk about the future of watch and wait. We now have great drugs and great therapies. Are you considering treatment earlier in any of your patients at this point, Jacquie?
DR. BARRIENTOS: I have been very hesitant to start our patients on any drug before they develop symptoms from the disease. I still wait to initiate therapy according to the International Workshop on CLL (IWCLL) guidelines.11 The reason is that anytime that we start a new agent, the patients may develop some mutation that is driven by these new agents. At this point, there are no data for us to start therapy before symptoms develop. The German CLL study group is currently doing a high risk study in patients that are asymptomatic but have a high risk profile like 17p deletion to see if maybe a drug like ibrutinib could have a benefit. I think that will be very interesting once the data come out. There are certain patients with whom you are always wondering, “Am I doing more harm by withholding therapy at this moment?” So far, early intervention with chemotherapy before symptoms has not shown any additional benefit. We still do the watch and wait for the time being, but this may change in the future for certain patients with certain high-risk characteristics.
DR. BROWN: Yes, I share your concerns about the possibility of evolution of the disease in the context of any treatment. Even though we hope that there will be less clonal evolution with these targeted inhibitors, there is some increasing evidence that some adverse clones like TP53 mutated or 17p deleted clones are preexisting in many cases. Then, under the influence of treatment, these mutations become more evident, ie a higher percentage of the disease. Personally, I would like to see overall survival data before we start treating patients earlier.
DR. JONES: I would absolutely agree. I think if you want to undertake the systematic treatment of patients before they actually progress clinically, those are the kind of data that you want. You want to know whether you are impacting the natural history of the disease. I’ll take a slightly contrarian point of view in talking about elderly patients in particular. Some of our colleagues who treat low-grade lymphoma—where watch and wait is often employed in the initial asymptomatic setting—have argued that there is a strong rationale to treat earlier rather than later because you may find that toxicity becomes more prohibitive if you wait until the patients become ill. There’s a somewhat perverse logic underlying our current approach to therapy—we don’t treat to maintain health, we treat when patients become sick. I think there is room for a slightly different approach still operating within current consensus guidelines. There is a group of elderly patients with comorbid medical illnesses that as it seems their disease is starting to progress, I am inclined to consider—at least discuss—the feasibility of treatment then as a way of limiting both the morbidity from the disease, as well as the morbidity of treatment. When the only available treatments were chemotherapy drugs like fludarabine, which has not clearly resulted in survival benefits for elderly patients, that was as feasible as when the treatment is perhaps obinutuzumab and chlorambucil, or maybe in the near future drugs like ibrutinib and idelalisib. Therefore I think we may all want to start rethinking our approach, cautiously. Ultimately, this is a research question.
DR. BROWN: That’s interesting. I certainly agree that in the setting of chemotherapy or chemoimmunotherapy patients with a higher disease burden have a lot harder time getting started on therapy. If in fact the targeted inhibitors move to upfront therapy, it’s not so clear to me that those drugs have more initial toxicity in patients with a greater disease burden—at least for ibrutinib. Do you disagree?
DR. JONES: No, I think that’s true. You will even hear an argument sometimes that a single-agent rituximab for follicular lymphoma or obinutuzumab and chlorambucil would be better tolerated, and you have more room for management of toxicity when you give them to patients who are healthier at baseline. Part of that is with less extensive disease, but you’re right. I agree that there is no indication right now that the novel, targeted agents are more toxic in older patients. However, I will say that our own retrospective analysis from Ohio State suggested that age was one of the factors associated with early discontinuation among our patients.4
DR. BROWN: Right, but to me, the fact that age is a predictor of less tolerability of therapy suggests that maybe we should save the therapy until the patient really needs it. The toxicities of ibrutinib are not as clearly disease-burden related necessarily.
DR. JONES: Yeah, I think that our disagreement really suggests that it’s a question to study.
As the treatment becomes more manageable and potentially more effective, you start to question whether our goal is to treat patients as they become ill, or to prevent them from ever becoming ill in the first place.DR. BROWN: Oh, absolutely.
DR. JONES: These are important questions that we will necessarily revisit. As the treatment becomes more manageable and potentially more effective, you start to question whether our goal is to treat patients as they become ill, or to prevent them from ever becoming ill in the first place.
DR. BROWN: Right, absolutely. I would say that I feel that we don’t always let the patients become symptomatically ill even in following IWCLL criteria. For example, their counts may be relatively poor, requiring treatment, but the patients are not yet suffering from that.
DR. JONES: Right.
DR. BROWN: I think this was a great discussion. It’s obviously an extremely exciting time in CLL research as we learn how to use our targeted inhibitors, our new antibodies, and hopefully soon we’ll have another targeted inhibitor with ABT199 the BCL-2 inhibitor. Jacquie or Jeff, do you have any points you would like to add before we wrap up?
DR. BARRIENTOS: No. I think we covered most of the important concepts.
DR. JONES: I will just say that with analogy to a cousin disease, chronic myeloid leukemia, after imatinib and the subsequent oral kinase inhibitors were introduced in that disease people thought that the final chapter of the story had been. I think we’re going to find the same thing in CLL medicine. These phenomenally effective agents, safer than the ones we have had available to employ before, are going to open up a whole new range of investigations that we will continue innovating over the next decade.
DR. BROWN: To summarize, in 2014 we saw four new drug approvals for CLL, including two new antibodies for upfront therapy, obinutuzumab and ofatumumab, and two new targeted inhibitors for relapsed therapy, ibrutinib and idelalisib. These innovations are starting to revolutionize the treatment of CLL for the benefit of our patients. However, many questions remain about how best to use each of these drugs, about toxicity, and about resistance. The next 5 years in CLL research will be a very exciting time as we start to answer these questions. Hopefully, ultimately, we will cure more and more of our patients, maybe eventually all of them.
References
1. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
2. Byrd JC, Brown JR, O’Brien S, et al. for the RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223.
3. Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067.
4. Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80–87.
5. Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397.
6. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed in chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007.
7. Coutre S, Leonard J, Flowers C, et al. Idelalisib monotherapy results in durable responses in patients with relapsed or refractory Waldenstrom’s macroglobulinemia (WM). Poster presented at: 20th Congress of European Hematology Association; June 11–14, 2015; Vienna, Austria. Abstract P690.
8. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110.
9. O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58.
10. O’Brien S, Lamanna N, Kipps TJ, et al. Update of a phase 2 study of idelalisib in combination with rituximab in treatment-naïve patients ≥65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, CA. Abstract 1994.
11. Hallek M, Cheson BD, Catovsky D, et al. for the International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456.
Moderated by: Jennifer R. Brown, MD, PhD1
Discussants: Jeffrey A. Jones, MD, MPH2; Jacqueline C. Barrientos, MD3
From the Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA1; Ohio State University, Columbus, OH2; Hofstra North Shore-LIJ School of Medicine, Lake Success, NY
Address for correspondence: Jennifer R. Brown, MD, PhD, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215
E-mail: [email protected]
Biographical Sketch
From Dana-Farber Cancer Institute and Harvard Medical School:
Jennifer R. Brown, MD, PhD is the Director of the CLL Center of the Division of Hematologic Malignancies at Dana-Farber Cancer Institute and an Associate Professor of Medicine at Harvard Medical School in Boston, Massachusetts. Dr. Brown completed a BS and MS simultaneously in molecular biophysics and biochemistry (MB&B) at Yale, graduating summa cum laude with distinction in MB&B. She proceeded to Harvard Medical School where she received her MD and PhD in molecular genetics in 1998 and was awarded the James Tolbert Shipley Prize for research accomplishment in the graduating class. She then served as an intern and resident in Internal Medicine at Massachusetts General Hospital followed by fellowship in Hematology and Medical Oncology at the Dana-Farber Cancer Institute. Dr. Brown joined the faculty of DFCI and Harvard Medical School in 2004, where she has an active clinical-translational research program in CLL.
Her particular interests include the development of novel targeted therapeutics for CLL, as well as the genomics of CLL. She has been instrumental in the clinical development of both idelalisib and ibrutinib, leading to their regulatory approvals in CLL.
In the area of genomics she has been instrumental in the description of the somatic mutation profile of CLL, and is now particularly interested in the implementation of genomic technology in the clinic, including for prognosis and targeted therapy. She also has a longstanding research interest and focus on the inherited predisposition to CLL.
To date she has published over 130 papers in the scientific literature, predominantly in CLL. She is an active member of the CLL Research Consortium and serves on the Alliance Leukemia and Leukemia Correlative Science Committees as well as the NIH Cancer Biomarkers Study Section. In 2014 she was the recipient of two awards from Dana-Farber Cancer Institute, the Clinical Innovation Award, as well as the George Canellos Award for Excellence in Clinical Investigation and Patient Care. She enjoys a worldwide reputation as a CLL expert and is in much demand as an international speaker.
From Hofstra North Shore-LIJ School of Medicine:
Jacqueline C. Barrientos, MD, is Attending Physician at the Chronic Lymphocytic Leukemia (CLL) Research & Treatment Program of the Division of Hematology and Medical Oncology, Department of Medicine, in the North Shore – LIJ Cancer Institute in Lake Success, New York. She is also Assistant Professor of Medicine at the Hofstra North Shore-LIJ School of Medicine. Dr. Barrientos works in close collaboration with her mentors, Dr. Kanti R. Rai and Dr. Nicholas Chiorazzi of the Feinstein Institute for Medical Research.
Dr. Barrientos received her medical degree at the Ponce School of Medicine in Puerto Rico, where she was elected vice-president of Alpha Omega Alpha Honor Medical Society. During her medical studies, she was the recipient of two Research Fellowship Awards from the Howard Hughes Medical Institute. She completed her internship and residency in internal medicine at Yale-New Haven Hospital of the Yale School of Medicine, and her fellowship in Hematology/Oncology at New York Presbyterian Hospital of Weill Cornell Medical College in New York City, where she also served as Chief Fellow. She is board certified in internal medicine, hematology and oncology.
Dr. Barrientos’ research focus is on chronic lymphocytic leukemia and lymphoma. She has extensive experience with the new promising agents targeting the B-cell receptor signaling pathway in B-cell malignancies, serving as Principal Investigator on several phase I-III clinical trials.
Dr. Barrientos actively participates in multi-institutional clinical trials with the Chronic Lymphocytic Leukemia Research Consortium (CRC) and the Alliance for Clinical Trials in Oncology. She is a cadre member of the Leukemia Committee of the Alliance for Clinical Trials in Oncology and in this capacity is co-chair of a study comparing chemoimmunotherapy against a combination of targeted agents. She is a member of the American Society of Clinical Oncology (ASCO) and the American Society of Hematology (ASH).
She has been an invited speaker for ASCO University “CLL Tumor Board”, ASH “State of the Art Symposium”, and “Highlights of ASH in Latin America”. Dr. Barrientos is the recipient of a 2015 American Society of Hematology-Harold Amos Medical Faculty Development Program (ASH-AMFDP) Fellowship award.
DR. BROWN: I am Jennifer Brown, Director of the Chronic Lymphocytic Leukemia (CLL) Center at Dana-Farber Cancer Institute, and Associate Professor of Medicine at Harvard Medical School. Today, I will be speaking with two of my esteemed CLL colleagues, Drs. Jeffrey Jones and Jacqueline Barrientos, about the new drug approvals in CLL.
DR. BARRIENTOS: I’m Jacqueline Barrientos, Assistant Professor of Hematology/Oncology at the Hofstra North Shore-LIJ School of Medicine, and Attending Hematologist at the CLL Research and Treatment Program in Long Island, NY. Our center participates in clinical trials and we perform correlative basic research. I’m very happy to participate in this expert roundtable discussion.
DR. JONES: I’m Dr. Jeffrey Jones, Associate Professor of Internal Medicine and Section Chief for CLL in the Division of Hematology at The Ohio State University.
DR. BROWN: Thank you Jeff and Jacquie for joining me today. I think we’re all aware what an exciting time this is in CLL with the approvals last year of the targeted inhibitors ibrutinib and idelalisib as well as the new antibody approval obinutuzumab as well as the additional indication for ofatumumab. Let’s start our discussion with ibrutinib and idelalisib. Jeff, please introduce the approvals that these inhibitors received and get us started.
DR. JONES: February 2014 marked a really important time in CLL medicine with the approval of the first oral kinase inhibitor, ibrutinib, for the treatment of CLL after one prior therapy.1,2 This ushered in an entirely new era of molecularly-targeted therapy for CLL. Later that year, ibrutinib received approval for deletion 17p CLL, the highest risk genetic subtype of CLL, whether previously untreated or relapsed disease. The drug has rapidly entered the clinic, although I think most of us are still trying to determine how best to incorporate them into our practice.
DR. BROWN: Jacquie, please comment on how you’re using ibrutinib now in your practice.
DR. BARRIENTOS: In CLL patients with the presence of a mutation of TP53 or deletion 17p, we use ibrutinib. We essentially do not use chemotherapy on this particular set of patients. If, for any reason, they are not able to tolerate the drug, then we consider idelalisib, which is not approved separately for this 17p deletion indication. Idelalisib is approved for use in combination with rituximab for the treatment of relapsed or refractory CLL patients. Idelalisib has shown clinical activity in several clinical trials in patients with deletion 17p.
At this moment, we mainly are using ibrutinib or idelalisib for our relapsed or refractory CLL patients. Clinical trials are underway in the frontline setting and we hope to see the results of the frontline use of ibrutinib in elderly patients soon. As of right now, we don’t use ibrutinib as a frontline therapy unless there is a reason, and usually it’s that they carry the 17p deletion or they are participating in a clinical trial.
DR. JONES: Outside of clinical trials our practice has really been to follow the label indications for ibrutinib. For previously untreated patients, our use has been limited to patients with deletion 17p or TP53 mutated disease, as Jacquie said, since that is the group for which the drug has been approved in the frontline.
DR. BROWN: I would agree. That’s been my practice as well. We should perhaps review the data from the registration trial that led to the ibrutinib approval for relapsed refractory CLL. The initial approval was from the stage IB2 study and was an accelerated approval.1 The confirmatory registration trial, RESONATE, randomized relapsed refractory CLL patients to ibrutinib versus the anti-CD20 antibody ofatumumab.2 Ibrutinib was found to be significantly better in improving both progression free and overall survival, although there was crossover later. As a result, this has moved into our relapse refractory use very rapidly. Although we still use chemoimmunotherapy for upfront therapy for patients without 17p deletion, for those in relapse we have moved entirely to targeted inhibitors. Would you both agree?
DR. JONES: For sure. I think it is very hard in 2015 to think of the patient for whom chemo-immunotherapy is the better choice than ibrutinib for relapsed disease.I think it is very hard in 2015 to think of the patient for whom chemoimmunotherapy is the better choice than ibrutinib for relapsed disease. The benefit is most marked for the group with higher-risk disease as characterized by genetic risk features, not just deletion 17p, but patients with complex abnormal karyotype or deletions of chromosome 11q. All of these patients particularly benefit from treatment with ibrutinib in the second line vs chemoimmunotherapy, as do patients who had either a suboptimal response to frontline chemoimmunotherapy or a brief duration of first remission. All of us are sometimes asked, “Well, who is the patient with relapsed CLL for whom ibrutinib is the best choice?” Right now, in most clinical situations, my response is, “For which patient is ibrutinib not the best choice in first relapse?”
DR. BROWN: That’s actually a good question. Jacquie, how would you answer that? Are there patients for whom you would not choose ibrutinib in first relapse?
DR. BARRIENTOS: I feel a hesitant to use ibrutinib in some patients with a particular comorbidity or medical history. For example, patients with a previous intracranial bleed or a recent history of bleeding, I would prefer to avoid using ibrutinib because there have been rare cases of spontaneous intracranial bleed or severe bleeding after trauma. The other type of patient where I would be cautious is a patient with uncontrolled atrial fibrillation because there are data that in the minority of patients (up to 10% of patients), atrial fibrillation has been an issue. We have some patients that are so frail that they couldn’t tolerate another episode of uncontrolled atrial fibrillation and as such they would not be ideal candidates for the drug. For that type of patient, I would probably abstain from using ibrutinib and consider the use of another therapy. Finally, I would be careful in patients on antiplatelet and anticoagulation therapy because ibrutinib affects platelet functions increasing the risk of bleeding. The bleeding events seen with ibrutinib are mostly grade 1 or grade 2. If the patients have had a serious bleed or serious gastrointestinal bleed or a recent surgery, then I would preferably use another agent.
DR. BROWN:Yes, so that gets to the toxicities of ibrutinib. The more medically significant ones do include perhaps a 5% to 10% risk of atrial fibrillation as well as bleeding risks, which as Jacquie points out are low and usually low grade, but there are occasional higher-risk bleeds. I personally still try to avoid combining anticoagulation with ibrutinib, as we don’t fully understand the mechanism or the risk factors for the more serious bleeds. Jeff, please comment.
DR. JONES: I think the data from the randomized study are actually the most helpful since, as you say, mild bleeding events (grade 1 or 2) were indeed more common amongst the group of patients who were treated with ibrutinib.2 Major bleeding events—which are typically defined as intracranial hemorrhage, bleeding requiring transfusion, or inpatient management—were actually similar between the two arms of the trial. An important caveat in interpreting these data is to know that patients in this trial were excluded if they were anticoagulated with warfarin, if they had an antecedent history of intracranial hemorrhage or recent bleeding, or recent surgery. In line with those exclusions, we will often consider other options. If there is any specific concern for bleeding, such as a patient who has experienced bleeding complications during routine anticoagulation, which is also a patient for whom ibrutinib may not be the best choice. In these clinical situations, it is important to involve the patient in discussing the balance of risks and benefits.
DR. BROWN: Yes. Jacquie, please comment on some of the side effects the patients on ibrutinib have, and how you manage those.
DR. BARRIENTOS: I usually mention to my patients that over the first 2 or 3 months about half of them will have a possible change in their bowel movements. Usually they report some diarrhea or loose stools. Usually these episodes are mild, nothing that requires hospitalization. In any case, if it becomes severe, I definitely make sure that it’s not an infection. We all know that our patients with CLL are prone to infections. The other thing I tell the patients is that in some cases patients may develop a rash on the skin. Many times it may look like a rash, but it’s actually ecchymosis—an effect from the drug on the platelets. Essentially, they are grade 1 and don’t require intervention. I just tell them that eventually they will go away. It can be scary for the patients if they are not expecting these. We have had patients with large areas of hematomas in the arms or in the legs. That is unexpected with a drug that they are taking by mouth. They usually expect that with other drugs like warfarin, but not with ibrutinib, so it is important to mention before they start the drug.
Last but not least, I mention the fact that they may get arthralgias—joint pain—in different areas of their bodies. I would say that I see that in about 20% to 30% of patients. Usually it’s very mild, but on occasion I’ve had patients with arthritis so severe that we’ve had to hold the drug and give them some steroids to help them improve their ability to maneuver their hands or move their joints. I’m sure you have seen some of those same side effects.
DR. BROWN: Yes, definitely. In general, it’s pretty well tolerated but it’s best to warn the patients, then there are no surprises. Let’s turn our attention for a moment back to the highest risk genetic subgroup, the 17p deleted patients—which Jeff had mentioned get particularly strong benefit from ibrutinib. This is certainly true, although it’s also the case that it appears, depending on the data set you look at, that they may relapse earlier than other patients on ibrutinib. In the original phase IB2, the median progression survival for the 17p deleted patients was 28 months. More recent data from Ohio State and MD Anderson suggest that complex karyotype may be a risk factor.3,4 Given these data, how are you two handling the question of allogeneic stem cell transplantation for these patients in this new era?
DR. BARRIENTOS: At our center, if the patient is young and they have access and are fit to tolerate a reduced-intensity allogeneic transplant, we recommend that they be evaluated for a transplant. Unfortunately, if they lose the response to the best drug available for their particular genetic mutation, then we have limited options of salvage therapy. It’s risky to think that they will not relapse at some point, and then what do we do at the time of relapse? We can use other targeted agents that are available, like idelalisib, with the knowledge that they may not always respond to the salvage therapy. Promising clinical activity has been reported for patients with 17p deletion treated with venetoclax in clinical trials. Venetoclax is a new targeted agent in development stages but the drug is only available in clinical trials.Promising clinical activity has been reported for patients with 17p deletion treated with venetoclax in clinical trials. Venetoclax is a new targeted agent in development stages but the drug is only available in clinical trials. One problem is that in order to participate in a clinical trial the patient needs to be able to get to the center to get the drug. Additionally, the patient needs to satisfy certain eligibility criteria for study entry. For these patients that stop responding to ibrutinib, the options of care are very limited at this time. This is the reason why I send all my young patients with a 17p deletion for a transplant evaluation.
At the end of the day it is tough to convince the patients to go for a transplant when they’re feeling in excellent shape. It’s still difficult to make a case to go for a procedure that may have its complications on its own. It is well known that there are some increased mortality risks and infection risks that can arise as a result of a transplant. They may not want to do it because they are feeling so great with their routine. I still sit down and have a long frank talk with the patients, especially if they have complex karyotype and 17p deletion. I am concerned that at some point they’re going to stop responding to ibrutinib.
DR. BROWN: That’s generally my practice as well. What about you, Jeff?
DR. JONES: Until there is greater clarity regarding which of the newer agents can salvage patients progressing after ibrutinib, I think it is still important for younger, transplant eligible patients with deletion 17p disease to undergo evaluation for allograft. It remains potentially curative therapy, and I think the availability of ibrutinib has not really changed the importance of that evaluation.
DR. BROWN: Yes, I would agree. I think that was a good discussion on ibrutinib. Why don’t we turn our attention now to idelalisib, the phosphoinositide 3-kinase (PI3K) inhibitor. How are you using idelalisib in your practices? Is this after ibrutinib in general?
DR. JONES: Published data regarding the sequencing of the new agents are relatively limited since all of the registration trials for idelalisib excluded patients who had received prior therapy with an inhibitor of B-cell receptor signaling, including Bruton’s tyrosine kinase inhibitors like ibrutinib.5,6 A small number of patients enrolled on the phase IB2 trial of ibrutinib, as well as the subsequent randomized trial, had received prior therapy with idelalisib and responded similarly to patients who had not received prior idelalisib.1,2 In our practice, the use of idelalisib has pretty much been limited to patients who have either received prior ibrutinib or patients who are not eligible to receive ibrutinib because of some important contraindication, such as an inherited bleeding defect, perceived increased bleeding, or history of difficult to control atrial fibrillation, since that event also seems to be more likely among patients treated with ibrutinib.
DR. BROWN: How about you, Jacquie?
DR. BARRIENTOS: The same type of patient with the addition of patients with kidney disease. The rationale for this is based on the phase III trial for idelalisib and rituximab, the enrollment allowed participation of patients with decreased renal function, that was one of the entry criteria for eligibility to participate in the trial.6 In most of the ibrutinib trials the creatinine clearance needed to be adequate, whereas this was allowed to be lower on the idelalisib trials. For those patients with severe renal impairment, I tend to prefer idelalisib rather than ibrutinib—only because I feel more comfortable and have more experience treating patients with impaired kidney function with idelalisib.
DR. BROWN: I have seen some episodic elevations in creatinine in patients on ibrutinib, but they’re fairly sporadic and it’s a little hard to assess the direct drug relationship. It is true that the patients in the idelalisib studies had a high level of comorbidity deliberately on the initial registration trial and generally did reasonably well with idelalisib. The toxicity profile of idelalisib is pretty characteristic, and is potentially harder to manage than that of ibrutinib. I think it also dictates some of how it’s being used in later line therapy. Does one of you wish to comment on the pattern of the key toxicities?
DR. BARRIENTOS: One key toxicity that is very particular to this drug that may happen overnight and is very striking is transaminitis. It usually happens more with non-Hodgkin lymphoma patients compared to relapsed CLL patients, but transaminitis can still be very severe. Patients can develop transaminitis even after more than a cycle on therapy even if they were tolerating the drug well without other issues. It’s very important to educate physicians and healthcare providers about the need to monitor the liver function tests, at least every 2 weeks for the first 2 months. Transaminitis events can be very prompt, very rapid, and usually asymptomatic. My patients that developed transaminitis never complained and had we not been cautious about it, we may have missed it.
DR. BROWN: Yes, I even check weekly. The recent safety analysis said the overall incidence of grade 3 to 4 transaminitis is about 15% in relapse patients.7 That’s pretty significant.
DR. JONES: I think it’s important to know that the transaminitis, if monitored carefully and managed with drug interruption and/or dose reduction upon reintroduction, need not lead to discontinuation. Discontinuations for transaminitis are actually the minority of patients who experience the side effect.
DR. BROWN: Absolutely. Do you want to comment on some of the other side effects that may more often lead to discontinuation?
DR. JONES: We should mention that there are some preclinical animal data suggesting that the molecular target of idelalisib, the PI3K delta isoform, is an important signaling molecule in regulatory T cells important for self-tolerance. While it has efficacy in treating B-cell disorders, inhibiting PI3K-delta may also be impairing T regulatory cell function. That may be what leads to the more characteristic later side effects of idelalisib, including pneumonitis and colitis. Pneumonitis is relatively rare, but because it can masquerade as other respiratory ailments in an older patient population with comorbid medical illnesses like chronic obstructive pulmonary disease and preexisting immune dysfunction because of CLL or prior therapy, inflammatory pneumonitis can be misdiagnosed. This rare but potentially life-threatening complication of idelalisib treatment requires prompt recognition, discontinuation of the drug, and appears to be most effectively managed with corticosteroids.
The other commonly occurring late toxicity, colitis, is often one that also eludes prompt recognition since many times patients are seen by primary care practitioners between oncology visits, and these doctors may not yet be aware that colitis can occur as a late side effect of idelalisib. Sometimes the colitis is misdiagnosed as gastroenteritis or Clostridium difficile colitis and eludes initial management. Like the pneumonitis, this problem, which may occur in more than a quarter of patients, is really best managed by prompt recognition and, in many cases, interruption of the drug. In some cases, patients have been managed with interruption of the drug and perhaps rechallenge at a lower dose, but in many other cases, colitis has been a treatment-limiting side effect and is a leading cause of drug discontinuation for toxicity.
DR. BROWN: Yes, I would agree. It can occur even at much later times in people who have tolerated the drug for even a couple of years, which is surprising compared to typical drug-related diarrhea.
DR. JONES: Right. With many other drugs, a patient starts taking the drug and expects the treatment-related side effects to become manifest very early. The diarrhea and rash associated with ibrutinib, for instance, are really timed very close to drug initiation, similar to antibiotics and other medications that we commonly prescribe. When side effects occur late in the course of treatment, I think it is just not on anyone’s radar to suspect that they could be related to a drug that they have been receiving for some time. That is an important message to communicate to patients, as well as to doctors who are just beginning to prescribe these new drugs for the first time.
DR. BROWN: Exactly. Why don’t we turn our attention now to the approval of obinutuzumab, and review the registration trial data there and then how you’re using that in practice. Jacquie?
DR. BARRIENTOS: Obinutuzumab is a third generation monoclonal antibody targeting the CD20 receptor on B cells. It was approved in November of 2013 by the US Food and Drug Administration for use in combination with chlorambucil to treat patients with previously untreated CLL.8 The trial enrolled patients with comorbidities as measured by the Cumulative Index Rating Scale, the scale helps define fitness. The patients that participated in the registration trial were patients that due to their comorbidities would not tolerate well a chemoimmunotherapy regimen like fludarabine, cyclophosphamide, and rituximab (FCR), and possibly the combination of bendamustine and rituximab. In patients older than age 65 with multiple comorbidities, chlorambucil monotherapy is widely used worldwide due to concerns of complications from the use of other chemoimmunotherapy regimens like the ones mentioned above. In the United States, we usually see that physicians prefer to use rituximab as a single agent in frail patients with multiple comorbidities.
The combination of obinutuzumab with chlorambucil compared to chlorambucil as a single agent showed that the patients treated with the combination therapy had a higher rate of response, a higher rate of progression free survival, and an improved overall survival. The main issue with obinutuzumab is the fact that the infusion reactions are much greater than what we traditionally see with rituximab. Severe and life-threatening infusion reactions have been reported. The reactions can also be more abrupt, although they typically occur very early in infusion, so they are more predictable. If the patient develops an infusion reaction or can’t tolerate the drug, the infusion needs to be interrupted. If the patient does not experience any further infusion reaction symptoms, the infusion may be restarted at a lower rate. I believe grade 3 to grade 4 events were higher than 10% in the registration trial, with infusion reactions of any grade seen in 50%–70%, so it can be common—usually within the first day. By the third infusion, the rate of reaction decreases significantly. Most of the time after that third infusion, most patients won’t have any more issues with tolerability.
Who are the patients that develop these infusion reactions? It has been noted that the level of interleukin 6 is elevated in patients that develop an infusion reaction. That’s the reason why all patients should be premedicated with potent steroids (methylprednisolone or dexamethasone, not hydrocortisone). In addition, patients need to be premedicated with acetaminophen and an antihistamine. In the future hopefully we will be able to use other agents like tocilizumab to lessen the risk of infusion reactions, this is currently being tested in clinical trials as its use is theoretical at this point based on the observation of the elevated interleukin 6 levels.
There are other important side effects with this combination regimen that were noted in the registration trial. There was a higher rate of neutropenia in the patients receiving obinutuzumab and chlorambucil, although this did not correlate with a higher rate of grade 3 or grade 4 infections. The rate of grade 3 or 4 infections was the same all across the board in patients that received chlorambucil, chlorambucil in combination with rituximab, or chlorambucil in combination with obinutuzumab.
DR. BROWN: Are you using much obinutuzumab chlorambucil in your practice?
DR. BARRIENTOS: In select patients, yes. For untreated patients with comorbidities that are not participating in a clinical trial, we discuss with them data from the frontline bendamustine and rituximab combination and obinutuzumab and chlorambucil combination. For the most part, most patients prefer obinutuzumab with chlorambucil because the obinutuzumab chlorambucil combination might be better tolerated and possibly less myelosuppressive than the bendamustine rituximab combination. Unfortunately, most of my patients have already been treated by the time we see them. We have a minority of patients that come recently diagnosed, we just don’t see that many untreated patients.
DR. BROWN: How about you, Jeff? Are you using it?
DR. JONES: Yes, it is a consideration for frontline therapy in patients who don’t have deletion 17p. As we discussed before, most of us have already adopted ibrutinib as our first choice in that 17p deleted population outside of clinical trial. For the remainder of patients, I think the first question remains whether their age and health are permissive to safely give FCR, since that regimen has been associated with the best survival outcomes, even some really long survival, in a group of patients with IgVH mutated, favorable cytogenetic risk disease.
For patients who are not eligible or willing to receive FCR, I think the choice between bendumustine and rituximab (BR) and chlorambucil and obinutuzumab is a relatively challenging one. Part of the reason is that while the overall response rates and complete response rates are lower with obinutuzumab and chlorambucil, the toxicity is also a bit lower. That makes it an appealing choice, particularly when we have the availability of drugs like ibrutinib and idelalisib in the second line. For older patients with comorbid medical illnesses in particular, it may be that the duration of first remission after chemotherapy may not matter as much when we have more effective second line options.
DR. BROWN: Yes, I think that’s definitely true. I just want to highlight two points. Your point about the long-term efficacy of FCR, particularly in the IgVH mutated patients—it is important to note that we now have data from both MD Anderson and the German CLL Study Group. The MD Anderson data with 10 year follow up, 60% of that genetic subgroup are progression free after FCR suggesting that a subset of them may in fact be cured. We don’t want to forget that with the excitement of the new inhibitors. I would second your point also about the potential toxicities of BR which can be as myelosuppressive as FCR even though it is not in every case. Again, it’s very important to assess the comorbidities of the patient not just for FCR but also for BR, particularly when FCR has this chance of very long-term remission which is not seen with BR.
DR. JONES: Yes, and there’s also a risk for opportunistic infections with both regimens. Like fludarabine-treated patients, there are patients treated with bendumustine who experience pneumocystis pneumonia or viral reactivation from immune suppression beyond just the neutropenia.
DR. BROWN: Yes, absolutely. Let’s talk briefly about where we see CLL therapy going in the next few years given these exciting new drugs. I’ll just leave that open and see what you have to say. Jacquie?
DR. BARRIENTOS: Some of the possible developments that we may see over the next couple of years are the use of these targeted agents or small molecules as initial therapy either as monotherapy or in combination regimens. We are expecting to see the data of the clinical trial of frontline ibrutinib against chlorambucil in patients that are older than age 65. Idelalisib has other ongoing clinical trials in the frontline setting as monotherapy and in combination therapy. Data have been presented of idelalisib in combination with rituximab as frontline therapy. It was interesting to note that some of these side effects that we saw in the relapsed or refractory setting occurred more often in patients in the frontline setting, although efficacy was very high. These promising data may eventually lead to a change in the way that we treat patients in the frontline, not only as monotherapy. There are several clinical trials that incorporate chemoimmunotherapy with these new targeted agents to see if maybe we will obtain deeper remissions or longer duration of response.
DR. JONES: What preliminary data exist in small phase 1 or phase 2 studies suggest that the new agents may be even more effective in previously untreated disease, with higher overall response rates, higher complete response rates, and more durable remissions than observed among patients with relapsed and refractory disease.9,10 These results underscore that the individual agents are among the most effective drugs that have been developed for CLL in terms of their single-agent activity. If you include the oral BCL-2 inhibitor in development, venetoclax, these drugs have really had remarkable single-agent efficacy. If these newer agents are like older cytotoxic chemotherapy agents, like fludarabine, they may become superstars when used in combination. While we will soon see these drugs move into the frontline setting as single agents, I think the real potential for magic is when they get combined. There we may see the kinds of deep remissions that we only achieve now with chemoimmunotherapy, remissions that will allow similar long-term treatment-free survival without cytotoxic chemotherapy. I’d like nothing more than to see a 60% 10 year survival after a nonchemotherapy-containing combination that emerges when we use these new drugs in ways that maximize their benefit in combination.
DR. BROWN: I would certainly agree. I think that although we have remarkable single-agent activity of these drugs, we know that in the context of single-agent activity, resistance is likely to develop over time. For a subset of patients that may not matter. If they’re older and have comorbidities, they may get enough durability of response from their first single agent that it doesn’t matter, particularly the patients with lower risk CLL. For our younger patients, I think the combinations will have the opportunity to minimize the development of resistance and also allow shorter courses of therapy so that patients can be off treatment still with deep remissions. That is what most excites me about the future of these agents.
Let’s just talk about the future of watch and wait. We now have great drugs and great therapies. Are you considering treatment earlier in any of your patients at this point, Jacquie?
DR. BARRIENTOS: I have been very hesitant to start our patients on any drug before they develop symptoms from the disease. I still wait to initiate therapy according to the International Workshop on CLL (IWCLL) guidelines.11 The reason is that anytime that we start a new agent, the patients may develop some mutation that is driven by these new agents. At this point, there are no data for us to start therapy before symptoms develop. The German CLL study group is currently doing a high risk study in patients that are asymptomatic but have a high risk profile like 17p deletion to see if maybe a drug like ibrutinib could have a benefit. I think that will be very interesting once the data come out. There are certain patients with whom you are always wondering, “Am I doing more harm by withholding therapy at this moment?” So far, early intervention with chemotherapy before symptoms has not shown any additional benefit. We still do the watch and wait for the time being, but this may change in the future for certain patients with certain high-risk characteristics.
DR. BROWN: Yes, I share your concerns about the possibility of evolution of the disease in the context of any treatment. Even though we hope that there will be less clonal evolution with these targeted inhibitors, there is some increasing evidence that some adverse clones like TP53 mutated or 17p deleted clones are preexisting in many cases. Then, under the influence of treatment, these mutations become more evident, ie a higher percentage of the disease. Personally, I would like to see overall survival data before we start treating patients earlier.
DR. JONES: I would absolutely agree. I think if you want to undertake the systematic treatment of patients before they actually progress clinically, those are the kind of data that you want. You want to know whether you are impacting the natural history of the disease. I’ll take a slightly contrarian point of view in talking about elderly patients in particular. Some of our colleagues who treat low-grade lymphoma—where watch and wait is often employed in the initial asymptomatic setting—have argued that there is a strong rationale to treat earlier rather than later because you may find that toxicity becomes more prohibitive if you wait until the patients become ill. There’s a somewhat perverse logic underlying our current approach to therapy—we don’t treat to maintain health, we treat when patients become sick. I think there is room for a slightly different approach still operating within current consensus guidelines. There is a group of elderly patients with comorbid medical illnesses that as it seems their disease is starting to progress, I am inclined to consider—at least discuss—the feasibility of treatment then as a way of limiting both the morbidity from the disease, as well as the morbidity of treatment. When the only available treatments were chemotherapy drugs like fludarabine, which has not clearly resulted in survival benefits for elderly patients, that was as feasible as when the treatment is perhaps obinutuzumab and chlorambucil, or maybe in the near future drugs like ibrutinib and idelalisib. Therefore I think we may all want to start rethinking our approach, cautiously. Ultimately, this is a research question.
DR. BROWN: That’s interesting. I certainly agree that in the setting of chemotherapy or chemoimmunotherapy patients with a higher disease burden have a lot harder time getting started on therapy. If in fact the targeted inhibitors move to upfront therapy, it’s not so clear to me that those drugs have more initial toxicity in patients with a greater disease burden—at least for ibrutinib. Do you disagree?
DR. JONES: No, I think that’s true. You will even hear an argument sometimes that a single-agent rituximab for follicular lymphoma or obinutuzumab and chlorambucil would be better tolerated, and you have more room for management of toxicity when you give them to patients who are healthier at baseline. Part of that is with less extensive disease, but you’re right. I agree that there is no indication right now that the novel, targeted agents are more toxic in older patients. However, I will say that our own retrospective analysis from Ohio State suggested that age was one of the factors associated with early discontinuation among our patients.4
DR. BROWN: Right, but to me, the fact that age is a predictor of less tolerability of therapy suggests that maybe we should save the therapy until the patient really needs it. The toxicities of ibrutinib are not as clearly disease-burden related necessarily.
DR. JONES: Yeah, I think that our disagreement really suggests that it’s a question to study.
As the treatment becomes more manageable and potentially more effective, you start to question whether our goal is to treat patients as they become ill, or to prevent them from ever becoming ill in the first place.DR. BROWN: Oh, absolutely.
DR. JONES: These are important questions that we will necessarily revisit. As the treatment becomes more manageable and potentially more effective, you start to question whether our goal is to treat patients as they become ill, or to prevent them from ever becoming ill in the first place.
DR. BROWN: Right, absolutely. I would say that I feel that we don’t always let the patients become symptomatically ill even in following IWCLL criteria. For example, their counts may be relatively poor, requiring treatment, but the patients are not yet suffering from that.
DR. JONES: Right.
DR. BROWN: I think this was a great discussion. It’s obviously an extremely exciting time in CLL research as we learn how to use our targeted inhibitors, our new antibodies, and hopefully soon we’ll have another targeted inhibitor with ABT199 the BCL-2 inhibitor. Jacquie or Jeff, do you have any points you would like to add before we wrap up?
DR. BARRIENTOS: No. I think we covered most of the important concepts.
DR. JONES: I will just say that with analogy to a cousin disease, chronic myeloid leukemia, after imatinib and the subsequent oral kinase inhibitors were introduced in that disease people thought that the final chapter of the story had been. I think we’re going to find the same thing in CLL medicine. These phenomenally effective agents, safer than the ones we have had available to employ before, are going to open up a whole new range of investigations that we will continue innovating over the next decade.
DR. BROWN: To summarize, in 2014 we saw four new drug approvals for CLL, including two new antibodies for upfront therapy, obinutuzumab and ofatumumab, and two new targeted inhibitors for relapsed therapy, ibrutinib and idelalisib. These innovations are starting to revolutionize the treatment of CLL for the benefit of our patients. However, many questions remain about how best to use each of these drugs, about toxicity, and about resistance. The next 5 years in CLL research will be a very exciting time as we start to answer these questions. Hopefully, ultimately, we will cure more and more of our patients, maybe eventually all of them.
References
1. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
2. Byrd JC, Brown JR, O’Brien S, et al. for the RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223.
3. Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067.
4. Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80–87.
5. Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397.
6. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed in chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007.
7. Coutre S, Leonard J, Flowers C, et al. Idelalisib monotherapy results in durable responses in patients with relapsed or refractory Waldenstrom’s macroglobulinemia (WM). Poster presented at: 20th Congress of European Hematology Association; June 11–14, 2015; Vienna, Austria. Abstract P690.
8. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110.
9. O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58.
10. O’Brien S, Lamanna N, Kipps TJ, et al. Update of a phase 2 study of idelalisib in combination with rituximab in treatment-naïve patients ≥65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, CA. Abstract 1994.
11. Hallek M, Cheson BD, Catovsky D, et al. for the International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456.
Moderated by: Jennifer R. Brown, MD, PhD1
Discussants: Jeffrey A. Jones, MD, MPH2; Jacqueline C. Barrientos, MD3
From the Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA1; Ohio State University, Columbus, OH2; Hofstra North Shore-LIJ School of Medicine, Lake Success, NY
Address for correspondence: Jennifer R. Brown, MD, PhD, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215
E-mail: [email protected]
Biographical Sketch
From Dana-Farber Cancer Institute and Harvard Medical School:
Jennifer R. Brown, MD, PhD is the Director of the CLL Center of the Division of Hematologic Malignancies at Dana-Farber Cancer Institute and an Associate Professor of Medicine at Harvard Medical School in Boston, Massachusetts. Dr. Brown completed a BS and MS simultaneously in molecular biophysics and biochemistry (MB&B) at Yale, graduating summa cum laude with distinction in MB&B. She proceeded to Harvard Medical School where she received her MD and PhD in molecular genetics in 1998 and was awarded the James Tolbert Shipley Prize for research accomplishment in the graduating class. She then served as an intern and resident in Internal Medicine at Massachusetts General Hospital followed by fellowship in Hematology and Medical Oncology at the Dana-Farber Cancer Institute. Dr. Brown joined the faculty of DFCI and Harvard Medical School in 2004, where she has an active clinical-translational research program in CLL.
Her particular interests include the development of novel targeted therapeutics for CLL, as well as the genomics of CLL. She has been instrumental in the clinical development of both idelalisib and ibrutinib, leading to their regulatory approvals in CLL.
In the area of genomics she has been instrumental in the description of the somatic mutation profile of CLL, and is now particularly interested in the implementation of genomic technology in the clinic, including for prognosis and targeted therapy. She also has a longstanding research interest and focus on the inherited predisposition to CLL.
To date she has published over 130 papers in the scientific literature, predominantly in CLL. She is an active member of the CLL Research Consortium and serves on the Alliance Leukemia and Leukemia Correlative Science Committees as well as the NIH Cancer Biomarkers Study Section. In 2014 she was the recipient of two awards from Dana-Farber Cancer Institute, the Clinical Innovation Award, as well as the George Canellos Award for Excellence in Clinical Investigation and Patient Care. She enjoys a worldwide reputation as a CLL expert and is in much demand as an international speaker.
From Hofstra North Shore-LIJ School of Medicine:
Jacqueline C. Barrientos, MD, is Attending Physician at the Chronic Lymphocytic Leukemia (CLL) Research & Treatment Program of the Division of Hematology and Medical Oncology, Department of Medicine, in the North Shore – LIJ Cancer Institute in Lake Success, New York. She is also Assistant Professor of Medicine at the Hofstra North Shore-LIJ School of Medicine. Dr. Barrientos works in close collaboration with her mentors, Dr. Kanti R. Rai and Dr. Nicholas Chiorazzi of the Feinstein Institute for Medical Research.
Dr. Barrientos received her medical degree at the Ponce School of Medicine in Puerto Rico, where she was elected vice-president of Alpha Omega Alpha Honor Medical Society. During her medical studies, she was the recipient of two Research Fellowship Awards from the Howard Hughes Medical Institute. She completed her internship and residency in internal medicine at Yale-New Haven Hospital of the Yale School of Medicine, and her fellowship in Hematology/Oncology at New York Presbyterian Hospital of Weill Cornell Medical College in New York City, where she also served as Chief Fellow. She is board certified in internal medicine, hematology and oncology.
Dr. Barrientos’ research focus is on chronic lymphocytic leukemia and lymphoma. She has extensive experience with the new promising agents targeting the B-cell receptor signaling pathway in B-cell malignancies, serving as Principal Investigator on several phase I-III clinical trials.
Dr. Barrientos actively participates in multi-institutional clinical trials with the Chronic Lymphocytic Leukemia Research Consortium (CRC) and the Alliance for Clinical Trials in Oncology. She is a cadre member of the Leukemia Committee of the Alliance for Clinical Trials in Oncology and in this capacity is co-chair of a study comparing chemoimmunotherapy against a combination of targeted agents. She is a member of the American Society of Clinical Oncology (ASCO) and the American Society of Hematology (ASH).
She has been an invited speaker for ASCO University “CLL Tumor Board”, ASH “State of the Art Symposium”, and “Highlights of ASH in Latin America”. Dr. Barrientos is the recipient of a 2015 American Society of Hematology-Harold Amos Medical Faculty Development Program (ASH-AMFDP) Fellowship award.
DR. BROWN: I am Jennifer Brown, Director of the Chronic Lymphocytic Leukemia (CLL) Center at Dana-Farber Cancer Institute, and Associate Professor of Medicine at Harvard Medical School. Today, I will be speaking with two of my esteemed CLL colleagues, Drs. Jeffrey Jones and Jacqueline Barrientos, about the new drug approvals in CLL.
DR. BARRIENTOS: I’m Jacqueline Barrientos, Assistant Professor of Hematology/Oncology at the Hofstra North Shore-LIJ School of Medicine, and Attending Hematologist at the CLL Research and Treatment Program in Long Island, NY. Our center participates in clinical trials and we perform correlative basic research. I’m very happy to participate in this expert roundtable discussion.
DR. JONES: I’m Dr. Jeffrey Jones, Associate Professor of Internal Medicine and Section Chief for CLL in the Division of Hematology at The Ohio State University.
DR. BROWN: Thank you Jeff and Jacquie for joining me today. I think we’re all aware what an exciting time this is in CLL with the approvals last year of the targeted inhibitors ibrutinib and idelalisib as well as the new antibody approval obinutuzumab as well as the additional indication for ofatumumab. Let’s start our discussion with ibrutinib and idelalisib. Jeff, please introduce the approvals that these inhibitors received and get us started.
DR. JONES: February 2014 marked a really important time in CLL medicine with the approval of the first oral kinase inhibitor, ibrutinib, for the treatment of CLL after one prior therapy.1,2 This ushered in an entirely new era of molecularly-targeted therapy for CLL. Later that year, ibrutinib received approval for deletion 17p CLL, the highest risk genetic subtype of CLL, whether previously untreated or relapsed disease. The drug has rapidly entered the clinic, although I think most of us are still trying to determine how best to incorporate them into our practice.
DR. BROWN: Jacquie, please comment on how you’re using ibrutinib now in your practice.
DR. BARRIENTOS: In CLL patients with the presence of a mutation of TP53 or deletion 17p, we use ibrutinib. We essentially do not use chemotherapy on this particular set of patients. If, for any reason, they are not able to tolerate the drug, then we consider idelalisib, which is not approved separately for this 17p deletion indication. Idelalisib is approved for use in combination with rituximab for the treatment of relapsed or refractory CLL patients. Idelalisib has shown clinical activity in several clinical trials in patients with deletion 17p.
At this moment, we mainly are using ibrutinib or idelalisib for our relapsed or refractory CLL patients. Clinical trials are underway in the frontline setting and we hope to see the results of the frontline use of ibrutinib in elderly patients soon. As of right now, we don’t use ibrutinib as a frontline therapy unless there is a reason, and usually it’s that they carry the 17p deletion or they are participating in a clinical trial.
DR. JONES: Outside of clinical trials our practice has really been to follow the label indications for ibrutinib. For previously untreated patients, our use has been limited to patients with deletion 17p or TP53 mutated disease, as Jacquie said, since that is the group for which the drug has been approved in the frontline.
DR. BROWN: I would agree. That’s been my practice as well. We should perhaps review the data from the registration trial that led to the ibrutinib approval for relapsed refractory CLL. The initial approval was from the stage IB2 study and was an accelerated approval.1 The confirmatory registration trial, RESONATE, randomized relapsed refractory CLL patients to ibrutinib versus the anti-CD20 antibody ofatumumab.2 Ibrutinib was found to be significantly better in improving both progression free and overall survival, although there was crossover later. As a result, this has moved into our relapse refractory use very rapidly. Although we still use chemoimmunotherapy for upfront therapy for patients without 17p deletion, for those in relapse we have moved entirely to targeted inhibitors. Would you both agree?
DR. JONES: For sure. I think it is very hard in 2015 to think of the patient for whom chemo-immunotherapy is the better choice than ibrutinib for relapsed disease.I think it is very hard in 2015 to think of the patient for whom chemoimmunotherapy is the better choice than ibrutinib for relapsed disease. The benefit is most marked for the group with higher-risk disease as characterized by genetic risk features, not just deletion 17p, but patients with complex abnormal karyotype or deletions of chromosome 11q. All of these patients particularly benefit from treatment with ibrutinib in the second line vs chemoimmunotherapy, as do patients who had either a suboptimal response to frontline chemoimmunotherapy or a brief duration of first remission. All of us are sometimes asked, “Well, who is the patient with relapsed CLL for whom ibrutinib is the best choice?” Right now, in most clinical situations, my response is, “For which patient is ibrutinib not the best choice in first relapse?”
DR. BROWN: That’s actually a good question. Jacquie, how would you answer that? Are there patients for whom you would not choose ibrutinib in first relapse?
DR. BARRIENTOS: I feel a hesitant to use ibrutinib in some patients with a particular comorbidity or medical history. For example, patients with a previous intracranial bleed or a recent history of bleeding, I would prefer to avoid using ibrutinib because there have been rare cases of spontaneous intracranial bleed or severe bleeding after trauma. The other type of patient where I would be cautious is a patient with uncontrolled atrial fibrillation because there are data that in the minority of patients (up to 10% of patients), atrial fibrillation has been an issue. We have some patients that are so frail that they couldn’t tolerate another episode of uncontrolled atrial fibrillation and as such they would not be ideal candidates for the drug. For that type of patient, I would probably abstain from using ibrutinib and consider the use of another therapy. Finally, I would be careful in patients on antiplatelet and anticoagulation therapy because ibrutinib affects platelet functions increasing the risk of bleeding. The bleeding events seen with ibrutinib are mostly grade 1 or grade 2. If the patients have had a serious bleed or serious gastrointestinal bleed or a recent surgery, then I would preferably use another agent.
DR. BROWN:Yes, so that gets to the toxicities of ibrutinib. The more medically significant ones do include perhaps a 5% to 10% risk of atrial fibrillation as well as bleeding risks, which as Jacquie points out are low and usually low grade, but there are occasional higher-risk bleeds. I personally still try to avoid combining anticoagulation with ibrutinib, as we don’t fully understand the mechanism or the risk factors for the more serious bleeds. Jeff, please comment.
DR. JONES: I think the data from the randomized study are actually the most helpful since, as you say, mild bleeding events (grade 1 or 2) were indeed more common amongst the group of patients who were treated with ibrutinib.2 Major bleeding events—which are typically defined as intracranial hemorrhage, bleeding requiring transfusion, or inpatient management—were actually similar between the two arms of the trial. An important caveat in interpreting these data is to know that patients in this trial were excluded if they were anticoagulated with warfarin, if they had an antecedent history of intracranial hemorrhage or recent bleeding, or recent surgery. In line with those exclusions, we will often consider other options. If there is any specific concern for bleeding, such as a patient who has experienced bleeding complications during routine anticoagulation, which is also a patient for whom ibrutinib may not be the best choice. In these clinical situations, it is important to involve the patient in discussing the balance of risks and benefits.
DR. BROWN: Yes. Jacquie, please comment on some of the side effects the patients on ibrutinib have, and how you manage those.
DR. BARRIENTOS: I usually mention to my patients that over the first 2 or 3 months about half of them will have a possible change in their bowel movements. Usually they report some diarrhea or loose stools. Usually these episodes are mild, nothing that requires hospitalization. In any case, if it becomes severe, I definitely make sure that it’s not an infection. We all know that our patients with CLL are prone to infections. The other thing I tell the patients is that in some cases patients may develop a rash on the skin. Many times it may look like a rash, but it’s actually ecchymosis—an effect from the drug on the platelets. Essentially, they are grade 1 and don’t require intervention. I just tell them that eventually they will go away. It can be scary for the patients if they are not expecting these. We have had patients with large areas of hematomas in the arms or in the legs. That is unexpected with a drug that they are taking by mouth. They usually expect that with other drugs like warfarin, but not with ibrutinib, so it is important to mention before they start the drug.
Last but not least, I mention the fact that they may get arthralgias—joint pain—in different areas of their bodies. I would say that I see that in about 20% to 30% of patients. Usually it’s very mild, but on occasion I’ve had patients with arthritis so severe that we’ve had to hold the drug and give them some steroids to help them improve their ability to maneuver their hands or move their joints. I’m sure you have seen some of those same side effects.
DR. BROWN: Yes, definitely. In general, it’s pretty well tolerated but it’s best to warn the patients, then there are no surprises. Let’s turn our attention for a moment back to the highest risk genetic subgroup, the 17p deleted patients—which Jeff had mentioned get particularly strong benefit from ibrutinib. This is certainly true, although it’s also the case that it appears, depending on the data set you look at, that they may relapse earlier than other patients on ibrutinib. In the original phase IB2, the median progression survival for the 17p deleted patients was 28 months. More recent data from Ohio State and MD Anderson suggest that complex karyotype may be a risk factor.3,4 Given these data, how are you two handling the question of allogeneic stem cell transplantation for these patients in this new era?
DR. BARRIENTOS: At our center, if the patient is young and they have access and are fit to tolerate a reduced-intensity allogeneic transplant, we recommend that they be evaluated for a transplant. Unfortunately, if they lose the response to the best drug available for their particular genetic mutation, then we have limited options of salvage therapy. It’s risky to think that they will not relapse at some point, and then what do we do at the time of relapse? We can use other targeted agents that are available, like idelalisib, with the knowledge that they may not always respond to the salvage therapy. Promising clinical activity has been reported for patients with 17p deletion treated with venetoclax in clinical trials. Venetoclax is a new targeted agent in development stages but the drug is only available in clinical trials.Promising clinical activity has been reported for patients with 17p deletion treated with venetoclax in clinical trials. Venetoclax is a new targeted agent in development stages but the drug is only available in clinical trials. One problem is that in order to participate in a clinical trial the patient needs to be able to get to the center to get the drug. Additionally, the patient needs to satisfy certain eligibility criteria for study entry. For these patients that stop responding to ibrutinib, the options of care are very limited at this time. This is the reason why I send all my young patients with a 17p deletion for a transplant evaluation.
At the end of the day it is tough to convince the patients to go for a transplant when they’re feeling in excellent shape. It’s still difficult to make a case to go for a procedure that may have its complications on its own. It is well known that there are some increased mortality risks and infection risks that can arise as a result of a transplant. They may not want to do it because they are feeling so great with their routine. I still sit down and have a long frank talk with the patients, especially if they have complex karyotype and 17p deletion. I am concerned that at some point they’re going to stop responding to ibrutinib.
DR. BROWN: That’s generally my practice as well. What about you, Jeff?
DR. JONES: Until there is greater clarity regarding which of the newer agents can salvage patients progressing after ibrutinib, I think it is still important for younger, transplant eligible patients with deletion 17p disease to undergo evaluation for allograft. It remains potentially curative therapy, and I think the availability of ibrutinib has not really changed the importance of that evaluation.
DR. BROWN: Yes, I would agree. I think that was a good discussion on ibrutinib. Why don’t we turn our attention now to idelalisib, the phosphoinositide 3-kinase (PI3K) inhibitor. How are you using idelalisib in your practices? Is this after ibrutinib in general?
DR. JONES: Published data regarding the sequencing of the new agents are relatively limited since all of the registration trials for idelalisib excluded patients who had received prior therapy with an inhibitor of B-cell receptor signaling, including Bruton’s tyrosine kinase inhibitors like ibrutinib.5,6 A small number of patients enrolled on the phase IB2 trial of ibrutinib, as well as the subsequent randomized trial, had received prior therapy with idelalisib and responded similarly to patients who had not received prior idelalisib.1,2 In our practice, the use of idelalisib has pretty much been limited to patients who have either received prior ibrutinib or patients who are not eligible to receive ibrutinib because of some important contraindication, such as an inherited bleeding defect, perceived increased bleeding, or history of difficult to control atrial fibrillation, since that event also seems to be more likely among patients treated with ibrutinib.
DR. BROWN: How about you, Jacquie?
DR. BARRIENTOS: The same type of patient with the addition of patients with kidney disease. The rationale for this is based on the phase III trial for idelalisib and rituximab, the enrollment allowed participation of patients with decreased renal function, that was one of the entry criteria for eligibility to participate in the trial.6 In most of the ibrutinib trials the creatinine clearance needed to be adequate, whereas this was allowed to be lower on the idelalisib trials. For those patients with severe renal impairment, I tend to prefer idelalisib rather than ibrutinib—only because I feel more comfortable and have more experience treating patients with impaired kidney function with idelalisib.
DR. BROWN: I have seen some episodic elevations in creatinine in patients on ibrutinib, but they’re fairly sporadic and it’s a little hard to assess the direct drug relationship. It is true that the patients in the idelalisib studies had a high level of comorbidity deliberately on the initial registration trial and generally did reasonably well with idelalisib. The toxicity profile of idelalisib is pretty characteristic, and is potentially harder to manage than that of ibrutinib. I think it also dictates some of how it’s being used in later line therapy. Does one of you wish to comment on the pattern of the key toxicities?
DR. BARRIENTOS: One key toxicity that is very particular to this drug that may happen overnight and is very striking is transaminitis. It usually happens more with non-Hodgkin lymphoma patients compared to relapsed CLL patients, but transaminitis can still be very severe. Patients can develop transaminitis even after more than a cycle on therapy even if they were tolerating the drug well without other issues. It’s very important to educate physicians and healthcare providers about the need to monitor the liver function tests, at least every 2 weeks for the first 2 months. Transaminitis events can be very prompt, very rapid, and usually asymptomatic. My patients that developed transaminitis never complained and had we not been cautious about it, we may have missed it.
DR. BROWN: Yes, I even check weekly. The recent safety analysis said the overall incidence of grade 3 to 4 transaminitis is about 15% in relapse patients.7 That’s pretty significant.
DR. JONES: I think it’s important to know that the transaminitis, if monitored carefully and managed with drug interruption and/or dose reduction upon reintroduction, need not lead to discontinuation. Discontinuations for transaminitis are actually the minority of patients who experience the side effect.
DR. BROWN: Absolutely. Do you want to comment on some of the other side effects that may more often lead to discontinuation?
DR. JONES: We should mention that there are some preclinical animal data suggesting that the molecular target of idelalisib, the PI3K delta isoform, is an important signaling molecule in regulatory T cells important for self-tolerance. While it has efficacy in treating B-cell disorders, inhibiting PI3K-delta may also be impairing T regulatory cell function. That may be what leads to the more characteristic later side effects of idelalisib, including pneumonitis and colitis. Pneumonitis is relatively rare, but because it can masquerade as other respiratory ailments in an older patient population with comorbid medical illnesses like chronic obstructive pulmonary disease and preexisting immune dysfunction because of CLL or prior therapy, inflammatory pneumonitis can be misdiagnosed. This rare but potentially life-threatening complication of idelalisib treatment requires prompt recognition, discontinuation of the drug, and appears to be most effectively managed with corticosteroids.
The other commonly occurring late toxicity, colitis, is often one that also eludes prompt recognition since many times patients are seen by primary care practitioners between oncology visits, and these doctors may not yet be aware that colitis can occur as a late side effect of idelalisib. Sometimes the colitis is misdiagnosed as gastroenteritis or Clostridium difficile colitis and eludes initial management. Like the pneumonitis, this problem, which may occur in more than a quarter of patients, is really best managed by prompt recognition and, in many cases, interruption of the drug. In some cases, patients have been managed with interruption of the drug and perhaps rechallenge at a lower dose, but in many other cases, colitis has been a treatment-limiting side effect and is a leading cause of drug discontinuation for toxicity.
DR. BROWN: Yes, I would agree. It can occur even at much later times in people who have tolerated the drug for even a couple of years, which is surprising compared to typical drug-related diarrhea.
DR. JONES: Right. With many other drugs, a patient starts taking the drug and expects the treatment-related side effects to become manifest very early. The diarrhea and rash associated with ibrutinib, for instance, are really timed very close to drug initiation, similar to antibiotics and other medications that we commonly prescribe. When side effects occur late in the course of treatment, I think it is just not on anyone’s radar to suspect that they could be related to a drug that they have been receiving for some time. That is an important message to communicate to patients, as well as to doctors who are just beginning to prescribe these new drugs for the first time.
DR. BROWN: Exactly. Why don’t we turn our attention now to the approval of obinutuzumab, and review the registration trial data there and then how you’re using that in practice. Jacquie?
DR. BARRIENTOS: Obinutuzumab is a third generation monoclonal antibody targeting the CD20 receptor on B cells. It was approved in November of 2013 by the US Food and Drug Administration for use in combination with chlorambucil to treat patients with previously untreated CLL.8 The trial enrolled patients with comorbidities as measured by the Cumulative Index Rating Scale, the scale helps define fitness. The patients that participated in the registration trial were patients that due to their comorbidities would not tolerate well a chemoimmunotherapy regimen like fludarabine, cyclophosphamide, and rituximab (FCR), and possibly the combination of bendamustine and rituximab. In patients older than age 65 with multiple comorbidities, chlorambucil monotherapy is widely used worldwide due to concerns of complications from the use of other chemoimmunotherapy regimens like the ones mentioned above. In the United States, we usually see that physicians prefer to use rituximab as a single agent in frail patients with multiple comorbidities.
The combination of obinutuzumab with chlorambucil compared to chlorambucil as a single agent showed that the patients treated with the combination therapy had a higher rate of response, a higher rate of progression free survival, and an improved overall survival. The main issue with obinutuzumab is the fact that the infusion reactions are much greater than what we traditionally see with rituximab. Severe and life-threatening infusion reactions have been reported. The reactions can also be more abrupt, although they typically occur very early in infusion, so they are more predictable. If the patient develops an infusion reaction or can’t tolerate the drug, the infusion needs to be interrupted. If the patient does not experience any further infusion reaction symptoms, the infusion may be restarted at a lower rate. I believe grade 3 to grade 4 events were higher than 10% in the registration trial, with infusion reactions of any grade seen in 50%–70%, so it can be common—usually within the first day. By the third infusion, the rate of reaction decreases significantly. Most of the time after that third infusion, most patients won’t have any more issues with tolerability.
Who are the patients that develop these infusion reactions? It has been noted that the level of interleukin 6 is elevated in patients that develop an infusion reaction. That’s the reason why all patients should be premedicated with potent steroids (methylprednisolone or dexamethasone, not hydrocortisone). In addition, patients need to be premedicated with acetaminophen and an antihistamine. In the future hopefully we will be able to use other agents like tocilizumab to lessen the risk of infusion reactions, this is currently being tested in clinical trials as its use is theoretical at this point based on the observation of the elevated interleukin 6 levels.
There are other important side effects with this combination regimen that were noted in the registration trial. There was a higher rate of neutropenia in the patients receiving obinutuzumab and chlorambucil, although this did not correlate with a higher rate of grade 3 or grade 4 infections. The rate of grade 3 or 4 infections was the same all across the board in patients that received chlorambucil, chlorambucil in combination with rituximab, or chlorambucil in combination with obinutuzumab.
DR. BROWN: Are you using much obinutuzumab chlorambucil in your practice?
DR. BARRIENTOS: In select patients, yes. For untreated patients with comorbidities that are not participating in a clinical trial, we discuss with them data from the frontline bendamustine and rituximab combination and obinutuzumab and chlorambucil combination. For the most part, most patients prefer obinutuzumab with chlorambucil because the obinutuzumab chlorambucil combination might be better tolerated and possibly less myelosuppressive than the bendamustine rituximab combination. Unfortunately, most of my patients have already been treated by the time we see them. We have a minority of patients that come recently diagnosed, we just don’t see that many untreated patients.
DR. BROWN: How about you, Jeff? Are you using it?
DR. JONES: Yes, it is a consideration for frontline therapy in patients who don’t have deletion 17p. As we discussed before, most of us have already adopted ibrutinib as our first choice in that 17p deleted population outside of clinical trial. For the remainder of patients, I think the first question remains whether their age and health are permissive to safely give FCR, since that regimen has been associated with the best survival outcomes, even some really long survival, in a group of patients with IgVH mutated, favorable cytogenetic risk disease.
For patients who are not eligible or willing to receive FCR, I think the choice between bendumustine and rituximab (BR) and chlorambucil and obinutuzumab is a relatively challenging one. Part of the reason is that while the overall response rates and complete response rates are lower with obinutuzumab and chlorambucil, the toxicity is also a bit lower. That makes it an appealing choice, particularly when we have the availability of drugs like ibrutinib and idelalisib in the second line. For older patients with comorbid medical illnesses in particular, it may be that the duration of first remission after chemotherapy may not matter as much when we have more effective second line options.
DR. BROWN: Yes, I think that’s definitely true. I just want to highlight two points. Your point about the long-term efficacy of FCR, particularly in the IgVH mutated patients—it is important to note that we now have data from both MD Anderson and the German CLL Study Group. The MD Anderson data with 10 year follow up, 60% of that genetic subgroup are progression free after FCR suggesting that a subset of them may in fact be cured. We don’t want to forget that with the excitement of the new inhibitors. I would second your point also about the potential toxicities of BR which can be as myelosuppressive as FCR even though it is not in every case. Again, it’s very important to assess the comorbidities of the patient not just for FCR but also for BR, particularly when FCR has this chance of very long-term remission which is not seen with BR.
DR. JONES: Yes, and there’s also a risk for opportunistic infections with both regimens. Like fludarabine-treated patients, there are patients treated with bendumustine who experience pneumocystis pneumonia or viral reactivation from immune suppression beyond just the neutropenia.
DR. BROWN: Yes, absolutely. Let’s talk briefly about where we see CLL therapy going in the next few years given these exciting new drugs. I’ll just leave that open and see what you have to say. Jacquie?
DR. BARRIENTOS: Some of the possible developments that we may see over the next couple of years are the use of these targeted agents or small molecules as initial therapy either as monotherapy or in combination regimens. We are expecting to see the data of the clinical trial of frontline ibrutinib against chlorambucil in patients that are older than age 65. Idelalisib has other ongoing clinical trials in the frontline setting as monotherapy and in combination therapy. Data have been presented of idelalisib in combination with rituximab as frontline therapy. It was interesting to note that some of these side effects that we saw in the relapsed or refractory setting occurred more often in patients in the frontline setting, although efficacy was very high. These promising data may eventually lead to a change in the way that we treat patients in the frontline, not only as monotherapy. There are several clinical trials that incorporate chemoimmunotherapy with these new targeted agents to see if maybe we will obtain deeper remissions or longer duration of response.
DR. JONES: What preliminary data exist in small phase 1 or phase 2 studies suggest that the new agents may be even more effective in previously untreated disease, with higher overall response rates, higher complete response rates, and more durable remissions than observed among patients with relapsed and refractory disease.9,10 These results underscore that the individual agents are among the most effective drugs that have been developed for CLL in terms of their single-agent activity. If you include the oral BCL-2 inhibitor in development, venetoclax, these drugs have really had remarkable single-agent efficacy. If these newer agents are like older cytotoxic chemotherapy agents, like fludarabine, they may become superstars when used in combination. While we will soon see these drugs move into the frontline setting as single agents, I think the real potential for magic is when they get combined. There we may see the kinds of deep remissions that we only achieve now with chemoimmunotherapy, remissions that will allow similar long-term treatment-free survival without cytotoxic chemotherapy. I’d like nothing more than to see a 60% 10 year survival after a nonchemotherapy-containing combination that emerges when we use these new drugs in ways that maximize their benefit in combination.
DR. BROWN: I would certainly agree. I think that although we have remarkable single-agent activity of these drugs, we know that in the context of single-agent activity, resistance is likely to develop over time. For a subset of patients that may not matter. If they’re older and have comorbidities, they may get enough durability of response from their first single agent that it doesn’t matter, particularly the patients with lower risk CLL. For our younger patients, I think the combinations will have the opportunity to minimize the development of resistance and also allow shorter courses of therapy so that patients can be off treatment still with deep remissions. That is what most excites me about the future of these agents.
Let’s just talk about the future of watch and wait. We now have great drugs and great therapies. Are you considering treatment earlier in any of your patients at this point, Jacquie?
DR. BARRIENTOS: I have been very hesitant to start our patients on any drug before they develop symptoms from the disease. I still wait to initiate therapy according to the International Workshop on CLL (IWCLL) guidelines.11 The reason is that anytime that we start a new agent, the patients may develop some mutation that is driven by these new agents. At this point, there are no data for us to start therapy before symptoms develop. The German CLL study group is currently doing a high risk study in patients that are asymptomatic but have a high risk profile like 17p deletion to see if maybe a drug like ibrutinib could have a benefit. I think that will be very interesting once the data come out. There are certain patients with whom you are always wondering, “Am I doing more harm by withholding therapy at this moment?” So far, early intervention with chemotherapy before symptoms has not shown any additional benefit. We still do the watch and wait for the time being, but this may change in the future for certain patients with certain high-risk characteristics.
DR. BROWN: Yes, I share your concerns about the possibility of evolution of the disease in the context of any treatment. Even though we hope that there will be less clonal evolution with these targeted inhibitors, there is some increasing evidence that some adverse clones like TP53 mutated or 17p deleted clones are preexisting in many cases. Then, under the influence of treatment, these mutations become more evident, ie a higher percentage of the disease. Personally, I would like to see overall survival data before we start treating patients earlier.
DR. JONES: I would absolutely agree. I think if you want to undertake the systematic treatment of patients before they actually progress clinically, those are the kind of data that you want. You want to know whether you are impacting the natural history of the disease. I’ll take a slightly contrarian point of view in talking about elderly patients in particular. Some of our colleagues who treat low-grade lymphoma—where watch and wait is often employed in the initial asymptomatic setting—have argued that there is a strong rationale to treat earlier rather than later because you may find that toxicity becomes more prohibitive if you wait until the patients become ill. There’s a somewhat perverse logic underlying our current approach to therapy—we don’t treat to maintain health, we treat when patients become sick. I think there is room for a slightly different approach still operating within current consensus guidelines. There is a group of elderly patients with comorbid medical illnesses that as it seems their disease is starting to progress, I am inclined to consider—at least discuss—the feasibility of treatment then as a way of limiting both the morbidity from the disease, as well as the morbidity of treatment. When the only available treatments were chemotherapy drugs like fludarabine, which has not clearly resulted in survival benefits for elderly patients, that was as feasible as when the treatment is perhaps obinutuzumab and chlorambucil, or maybe in the near future drugs like ibrutinib and idelalisib. Therefore I think we may all want to start rethinking our approach, cautiously. Ultimately, this is a research question.
DR. BROWN: That’s interesting. I certainly agree that in the setting of chemotherapy or chemoimmunotherapy patients with a higher disease burden have a lot harder time getting started on therapy. If in fact the targeted inhibitors move to upfront therapy, it’s not so clear to me that those drugs have more initial toxicity in patients with a greater disease burden—at least for ibrutinib. Do you disagree?
DR. JONES: No, I think that’s true. You will even hear an argument sometimes that a single-agent rituximab for follicular lymphoma or obinutuzumab and chlorambucil would be better tolerated, and you have more room for management of toxicity when you give them to patients who are healthier at baseline. Part of that is with less extensive disease, but you’re right. I agree that there is no indication right now that the novel, targeted agents are more toxic in older patients. However, I will say that our own retrospective analysis from Ohio State suggested that age was one of the factors associated with early discontinuation among our patients.4
DR. BROWN: Right, but to me, the fact that age is a predictor of less tolerability of therapy suggests that maybe we should save the therapy until the patient really needs it. The toxicities of ibrutinib are not as clearly disease-burden related necessarily.
DR. JONES: Yeah, I think that our disagreement really suggests that it’s a question to study.
As the treatment becomes more manageable and potentially more effective, you start to question whether our goal is to treat patients as they become ill, or to prevent them from ever becoming ill in the first place.DR. BROWN: Oh, absolutely.
DR. JONES: These are important questions that we will necessarily revisit. As the treatment becomes more manageable and potentially more effective, you start to question whether our goal is to treat patients as they become ill, or to prevent them from ever becoming ill in the first place.
DR. BROWN: Right, absolutely. I would say that I feel that we don’t always let the patients become symptomatically ill even in following IWCLL criteria. For example, their counts may be relatively poor, requiring treatment, but the patients are not yet suffering from that.
DR. JONES: Right.
DR. BROWN: I think this was a great discussion. It’s obviously an extremely exciting time in CLL research as we learn how to use our targeted inhibitors, our new antibodies, and hopefully soon we’ll have another targeted inhibitor with ABT199 the BCL-2 inhibitor. Jacquie or Jeff, do you have any points you would like to add before we wrap up?
DR. BARRIENTOS: No. I think we covered most of the important concepts.
DR. JONES: I will just say that with analogy to a cousin disease, chronic myeloid leukemia, after imatinib and the subsequent oral kinase inhibitors were introduced in that disease people thought that the final chapter of the story had been. I think we’re going to find the same thing in CLL medicine. These phenomenally effective agents, safer than the ones we have had available to employ before, are going to open up a whole new range of investigations that we will continue innovating over the next decade.
DR. BROWN: To summarize, in 2014 we saw four new drug approvals for CLL, including two new antibodies for upfront therapy, obinutuzumab and ofatumumab, and two new targeted inhibitors for relapsed therapy, ibrutinib and idelalisib. These innovations are starting to revolutionize the treatment of CLL for the benefit of our patients. However, many questions remain about how best to use each of these drugs, about toxicity, and about resistance. The next 5 years in CLL research will be a very exciting time as we start to answer these questions. Hopefully, ultimately, we will cure more and more of our patients, maybe eventually all of them.
References
1. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
2. Byrd JC, Brown JR, O’Brien S, et al. for the RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223.
3. Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067.
4. Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80–87.
5. Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397.
6. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed in chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007.
7. Coutre S, Leonard J, Flowers C, et al. Idelalisib monotherapy results in durable responses in patients with relapsed or refractory Waldenstrom’s macroglobulinemia (WM). Poster presented at: 20th Congress of European Hematology Association; June 11–14, 2015; Vienna, Austria. Abstract P690.
8. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110.
9. O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58.
10. O’Brien S, Lamanna N, Kipps TJ, et al. Update of a phase 2 study of idelalisib in combination with rituximab in treatment-naïve patients ≥65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, CA. Abstract 1994.
11. Hallek M, Cheson BD, Catovsky D, et al. for the International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456.
Five epigenetic biomarkers define three CLL subgroups
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
FROM LEUKEMIA
Key clinical point: A new strategy allows CLL patients to be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes.
Major finding: Epigenetic classification was the strongest predictor of time to treatment (P <.001), along with Binet stage (P <.001); these findings were corroborated in a validation series (n = 97).
Data source: A prediction model formulated using five epigenetic biomarkers that was able to classify CLL patients accurately into the three subgroups.
Disclosures: The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
IgA increase linked to fewer infections in CLL patients on ibrutinib
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
FROM BLOOD