User login
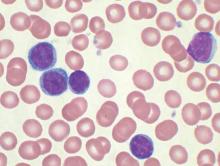
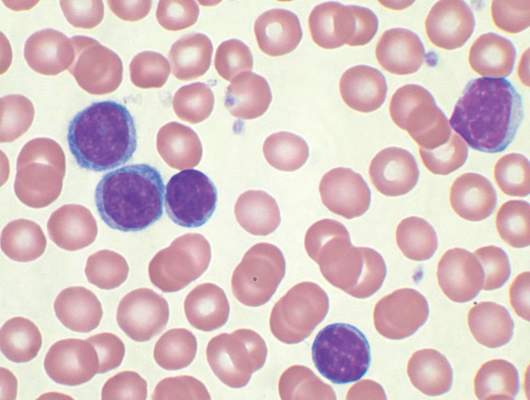
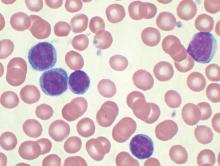
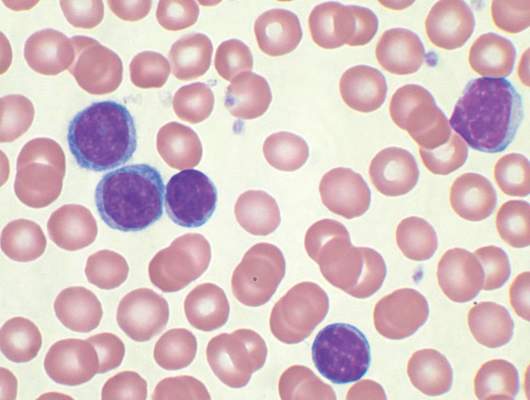
MHM: Novel agents, combos show promise for relapsed/refractory CLL
CHICAGO – Consider using novel agents in chronic lymphocytic leukemia (CLL) patients who are refractory to treatment or who relapse within 2 years of first-line therapy, Dr. John G. Gribben said.
Ibrutinib or idelalisib/rituximab, or other novel agent combinations within clinical trials are his choice in these patients, thus fitness for therapy becomes irrelevant, he said at the American Society of Hematology Meeting on Hematologic Malignancies.
“Of course, I’m particularly excited by the results in CLL of ABT-199. We saw at [the International Workshop on CLL] last week that there are increasing numbers of patients who are on this therapy in combination with anti-CD20 monoclonal antibodies who are achieving minimal residual disease (MRD) eradication and are able to stop that therapy, unlike what we’ve been seeing,” he said. “I have a patient at my own center now who was treated with ABT-199 plus obinutuzumab within a clinical trial – a 17p deletion patient refractory to seven previous lines of therapy. Had a donor, I was trying to get him to transplant, and now he’s MRD-negative and off therapy, having had 6 months of therapy with ABT-199 and obinutuzumab. [This is] a very exciting combination.”
Other novel-novel agent combinations are also being looked at, he noted.
In CLL patients without 17p deletions who progress later than 2 years after initial chemotherapy, novel agents can be considered, as could alternative approaches with chemotherapy.
“But of course, in the setting of p53 deletions or mutations, then ibrutinib or idelalisib/rituximab, or novel agents like ABT-199 within clinical trials become the treatment approach to be thinking about,” he said.
CHICAGO – Consider using novel agents in chronic lymphocytic leukemia (CLL) patients who are refractory to treatment or who relapse within 2 years of first-line therapy, Dr. John G. Gribben said.
Ibrutinib or idelalisib/rituximab, or other novel agent combinations within clinical trials are his choice in these patients, thus fitness for therapy becomes irrelevant, he said at the American Society of Hematology Meeting on Hematologic Malignancies.
“Of course, I’m particularly excited by the results in CLL of ABT-199. We saw at [the International Workshop on CLL] last week that there are increasing numbers of patients who are on this therapy in combination with anti-CD20 monoclonal antibodies who are achieving minimal residual disease (MRD) eradication and are able to stop that therapy, unlike what we’ve been seeing,” he said. “I have a patient at my own center now who was treated with ABT-199 plus obinutuzumab within a clinical trial – a 17p deletion patient refractory to seven previous lines of therapy. Had a donor, I was trying to get him to transplant, and now he’s MRD-negative and off therapy, having had 6 months of therapy with ABT-199 and obinutuzumab. [This is] a very exciting combination.”
Other novel-novel agent combinations are also being looked at, he noted.
In CLL patients without 17p deletions who progress later than 2 years after initial chemotherapy, novel agents can be considered, as could alternative approaches with chemotherapy.
“But of course, in the setting of p53 deletions or mutations, then ibrutinib or idelalisib/rituximab, or novel agents like ABT-199 within clinical trials become the treatment approach to be thinking about,” he said.
CHICAGO – Consider using novel agents in chronic lymphocytic leukemia (CLL) patients who are refractory to treatment or who relapse within 2 years of first-line therapy, Dr. John G. Gribben said.
Ibrutinib or idelalisib/rituximab, or other novel agent combinations within clinical trials are his choice in these patients, thus fitness for therapy becomes irrelevant, he said at the American Society of Hematology Meeting on Hematologic Malignancies.
“Of course, I’m particularly excited by the results in CLL of ABT-199. We saw at [the International Workshop on CLL] last week that there are increasing numbers of patients who are on this therapy in combination with anti-CD20 monoclonal antibodies who are achieving minimal residual disease (MRD) eradication and are able to stop that therapy, unlike what we’ve been seeing,” he said. “I have a patient at my own center now who was treated with ABT-199 plus obinutuzumab within a clinical trial – a 17p deletion patient refractory to seven previous lines of therapy. Had a donor, I was trying to get him to transplant, and now he’s MRD-negative and off therapy, having had 6 months of therapy with ABT-199 and obinutuzumab. [This is] a very exciting combination.”
Other novel-novel agent combinations are also being looked at, he noted.
In CLL patients without 17p deletions who progress later than 2 years after initial chemotherapy, novel agents can be considered, as could alternative approaches with chemotherapy.
“But of course, in the setting of p53 deletions or mutations, then ibrutinib or idelalisib/rituximab, or novel agents like ABT-199 within clinical trials become the treatment approach to be thinking about,” he said.
EXPERT ANALYSIS FROM MHM 2015
MHM: Novel agents, combos show promise for relapsed/refractory CLL
CHICAGO – Consider using novel agents in chronic lymphocytic leukemia (CLL) patients who are refractory to treatment or who relapse within 2 years of first-line therapy, Dr. John G. Gribben said.
Ibrutinib or idelalisib/rituximab, or other novel agent combinations within clinical trials are his choice in these patients, thus fitness for therapy becomes irrelevant, he said at the American Society of Hematology Meeting on Hematologic Malignancies.
“Of course, I’m particularly excited by the results in CLL of ABT-199. We saw at [the International Workshop on CLL] last week that there are increasing numbers of patients who are on this therapy in combination with anti-CD20 monoclonal antibodies who are achieving minimal residual disease (MRD) eradication and are able to stop that therapy, unlike what we’ve been seeing,” he said. “I have a patient at my own center now who was treated with ABT-199 plus obinutuzumab within a clinical trial – a 17p deletion patient refractory to seven previous lines of therapy. Had a donor, I was trying to get him to transplant, and now he’s MRD-negative and off therapy, having had 6 months of therapy with ABT-199 and obinutuzumab. [This is] a very exciting combination.”
Other novel-novel agent combinations are also being looked at, he noted.
In CLL patients without 17p deletions who progress later than 2 years after initial chemotherapy, novel agents can be considered, as could alternative approaches with chemotherapy.
“But of course, in the setting of p53 deletions or mutations, then ibrutinib or idelalisib/rituximab, or novel agents like ABT-199 within clinical trials become the treatment approach to be thinking about,” he said.
CHICAGO – Consider using novel agents in chronic lymphocytic leukemia (CLL) patients who are refractory to treatment or who relapse within 2 years of first-line therapy, Dr. John G. Gribben said.
Ibrutinib or idelalisib/rituximab, or other novel agent combinations within clinical trials are his choice in these patients, thus fitness for therapy becomes irrelevant, he said at the American Society of Hematology Meeting on Hematologic Malignancies.
“Of course, I’m particularly excited by the results in CLL of ABT-199. We saw at [the International Workshop on CLL] last week that there are increasing numbers of patients who are on this therapy in combination with anti-CD20 monoclonal antibodies who are achieving minimal residual disease (MRD) eradication and are able to stop that therapy, unlike what we’ve been seeing,” he said. “I have a patient at my own center now who was treated with ABT-199 plus obinutuzumab within a clinical trial – a 17p deletion patient refractory to seven previous lines of therapy. Had a donor, I was trying to get him to transplant, and now he’s MRD-negative and off therapy, having had 6 months of therapy with ABT-199 and obinutuzumab. [This is] a very exciting combination.”
Other novel-novel agent combinations are also being looked at, he noted.
In CLL patients without 17p deletions who progress later than 2 years after initial chemotherapy, novel agents can be considered, as could alternative approaches with chemotherapy.
“But of course, in the setting of p53 deletions or mutations, then ibrutinib or idelalisib/rituximab, or novel agents like ABT-199 within clinical trials become the treatment approach to be thinking about,” he said.
CHICAGO – Consider using novel agents in chronic lymphocytic leukemia (CLL) patients who are refractory to treatment or who relapse within 2 years of first-line therapy, Dr. John G. Gribben said.
Ibrutinib or idelalisib/rituximab, or other novel agent combinations within clinical trials are his choice in these patients, thus fitness for therapy becomes irrelevant, he said at the American Society of Hematology Meeting on Hematologic Malignancies.
“Of course, I’m particularly excited by the results in CLL of ABT-199. We saw at [the International Workshop on CLL] last week that there are increasing numbers of patients who are on this therapy in combination with anti-CD20 monoclonal antibodies who are achieving minimal residual disease (MRD) eradication and are able to stop that therapy, unlike what we’ve been seeing,” he said. “I have a patient at my own center now who was treated with ABT-199 plus obinutuzumab within a clinical trial – a 17p deletion patient refractory to seven previous lines of therapy. Had a donor, I was trying to get him to transplant, and now he’s MRD-negative and off therapy, having had 6 months of therapy with ABT-199 and obinutuzumab. [This is] a very exciting combination.”
Other novel-novel agent combinations are also being looked at, he noted.
In CLL patients without 17p deletions who progress later than 2 years after initial chemotherapy, novel agents can be considered, as could alternative approaches with chemotherapy.
“But of course, in the setting of p53 deletions or mutations, then ibrutinib or idelalisib/rituximab, or novel agents like ABT-199 within clinical trials become the treatment approach to be thinking about,” he said.
EXPERT ANALYSIS FROM MHM 2015
CLL Therapy: Focus on comorbidities, not age
CHICAGO – The majority of patients with chronic lymphocytic leukemia (CLL) are elderly patients over age 65 years, which underscores the need for a careful assessment of fitness for therapy – not necessarily because of age, but because of comorbidity burden, according to Dr. John G. Gribben.
In fact, 68% of CLL patients are over age 65 years (median, 71 years), and 41% are over age 75 years. Perhaps more importantly, 89% of elderly CLL patients have one or more comorbidities, and 46% have at least one major comorbidity, said Dr. Gribben of Barts Cancer Institute, Queen Mary University of London.
Conventional wisdom has long suggested that CLL shortens the life span only in younger patients; older patients were thought to be more likely “to die with CLL rather than of CLL,” he said at the American Society of Hematology Meeting on Hematologic Malignancies.
However, recent findings suggest that CLL shortens the life span of elderly patients as well, he noted.
“I think we probably have been undertreating and underthinking about the impact that CLL can have on these more elderly patients, and I think it does represent an area of unmet need,” he said.
Treatment options in the elderly include FCR (fludarabine, cyclophosphamide, rituximab) in those deemed fit enough to tolerate the regimen, he said, adding, “if you are concerned about neutropenia associated with FCR, there are those who use rituximab-fludarabine [RF], and that’s certainly a good option.”
However, in those with an 11q abnormality, good data show that the addition of the alkylator does add benefit. “I do think that FCR is worthwhile pushing [in those cases],” he said.
Bendamustine-rituximab is also an attractive option, as demonstrated in the CLL10 trial, but it is important to remember that patients in that trial were “fit, healthy patients” based on Clinical Illness Rating Scale (CIRS) scores of less than 6; they were patients who were deemed fit to be randomized to receive FCR.
Chlorambucil-based therapies administered with anti-CD20 monoclonal antibodies are also an option, as are novel agents in those with 17p deletions or a P53 mutation, he said.
When it comes to assessing elderly patients’ fitness for therapy, comorbidities play a more important role than age, he said, explaining that many patients over age 65 are very fit and would do well with therapies such as FCR.
For this reason, comorbidities should be the determining factor in treatment selection, he said.
No standard criteria for assessing fitness exist, but there are a few tools that can help.
Eastern Cooperative Oncology Group performance status and organ function (for example, creatinine clearance) can be helpful and often are used in trial settings, as are criteria for excluding patients from participation, but CIRS, used by the German CLL study group, is a more formal tool for assessing comorbidity.
The German group is not the first to use the tool – CIRS is a widely validated test that provides an objective measurement of fitness for more aggressive chemotherapy regimens – but the group did demonstrate in CLL11 that it could be used to enroll more elderly patients with comorbidities into clinical trials, Dr. Gribben said.
A CIRS score of 6 or lower indicates fitness, whereas increasing scores indicate an increasing lack of fitness, he explained, noting that “like every scoring system there are some issues … somebody could easily have a score higher than 6 with comorbidities that really don’t impact on chemotherapy tolerability.
“But in general terms, this is a good way to be making these sorts of assessments,” he said.
Dr. Gribben has received research funding from the National Institutes of Health, Cancer Research UK, MRC, and Wellcome Trust. He has received honoraria from Roche/Genentech, Celgene, Janssen, Pharmacyclics, Gilead, Mundipharma, Infinity, TG Therapeutics, and Ascerta, and he has a patent or receives royalties from Celgene. He also has been the principal investigator on a clinical trial for Roche, Takeda, Pharmacyclics, Gilead, and Infinity.
CHICAGO – The majority of patients with chronic lymphocytic leukemia (CLL) are elderly patients over age 65 years, which underscores the need for a careful assessment of fitness for therapy – not necessarily because of age, but because of comorbidity burden, according to Dr. John G. Gribben.
In fact, 68% of CLL patients are over age 65 years (median, 71 years), and 41% are over age 75 years. Perhaps more importantly, 89% of elderly CLL patients have one or more comorbidities, and 46% have at least one major comorbidity, said Dr. Gribben of Barts Cancer Institute, Queen Mary University of London.
Conventional wisdom has long suggested that CLL shortens the life span only in younger patients; older patients were thought to be more likely “to die with CLL rather than of CLL,” he said at the American Society of Hematology Meeting on Hematologic Malignancies.
However, recent findings suggest that CLL shortens the life span of elderly patients as well, he noted.
“I think we probably have been undertreating and underthinking about the impact that CLL can have on these more elderly patients, and I think it does represent an area of unmet need,” he said.
Treatment options in the elderly include FCR (fludarabine, cyclophosphamide, rituximab) in those deemed fit enough to tolerate the regimen, he said, adding, “if you are concerned about neutropenia associated with FCR, there are those who use rituximab-fludarabine [RF], and that’s certainly a good option.”
However, in those with an 11q abnormality, good data show that the addition of the alkylator does add benefit. “I do think that FCR is worthwhile pushing [in those cases],” he said.
Bendamustine-rituximab is also an attractive option, as demonstrated in the CLL10 trial, but it is important to remember that patients in that trial were “fit, healthy patients” based on Clinical Illness Rating Scale (CIRS) scores of less than 6; they were patients who were deemed fit to be randomized to receive FCR.
Chlorambucil-based therapies administered with anti-CD20 monoclonal antibodies are also an option, as are novel agents in those with 17p deletions or a P53 mutation, he said.
When it comes to assessing elderly patients’ fitness for therapy, comorbidities play a more important role than age, he said, explaining that many patients over age 65 are very fit and would do well with therapies such as FCR.
For this reason, comorbidities should be the determining factor in treatment selection, he said.
No standard criteria for assessing fitness exist, but there are a few tools that can help.
Eastern Cooperative Oncology Group performance status and organ function (for example, creatinine clearance) can be helpful and often are used in trial settings, as are criteria for excluding patients from participation, but CIRS, used by the German CLL study group, is a more formal tool for assessing comorbidity.
The German group is not the first to use the tool – CIRS is a widely validated test that provides an objective measurement of fitness for more aggressive chemotherapy regimens – but the group did demonstrate in CLL11 that it could be used to enroll more elderly patients with comorbidities into clinical trials, Dr. Gribben said.
A CIRS score of 6 or lower indicates fitness, whereas increasing scores indicate an increasing lack of fitness, he explained, noting that “like every scoring system there are some issues … somebody could easily have a score higher than 6 with comorbidities that really don’t impact on chemotherapy tolerability.
“But in general terms, this is a good way to be making these sorts of assessments,” he said.
Dr. Gribben has received research funding from the National Institutes of Health, Cancer Research UK, MRC, and Wellcome Trust. He has received honoraria from Roche/Genentech, Celgene, Janssen, Pharmacyclics, Gilead, Mundipharma, Infinity, TG Therapeutics, and Ascerta, and he has a patent or receives royalties from Celgene. He also has been the principal investigator on a clinical trial for Roche, Takeda, Pharmacyclics, Gilead, and Infinity.
CHICAGO – The majority of patients with chronic lymphocytic leukemia (CLL) are elderly patients over age 65 years, which underscores the need for a careful assessment of fitness for therapy – not necessarily because of age, but because of comorbidity burden, according to Dr. John G. Gribben.
In fact, 68% of CLL patients are over age 65 years (median, 71 years), and 41% are over age 75 years. Perhaps more importantly, 89% of elderly CLL patients have one or more comorbidities, and 46% have at least one major comorbidity, said Dr. Gribben of Barts Cancer Institute, Queen Mary University of London.
Conventional wisdom has long suggested that CLL shortens the life span only in younger patients; older patients were thought to be more likely “to die with CLL rather than of CLL,” he said at the American Society of Hematology Meeting on Hematologic Malignancies.
However, recent findings suggest that CLL shortens the life span of elderly patients as well, he noted.
“I think we probably have been undertreating and underthinking about the impact that CLL can have on these more elderly patients, and I think it does represent an area of unmet need,” he said.
Treatment options in the elderly include FCR (fludarabine, cyclophosphamide, rituximab) in those deemed fit enough to tolerate the regimen, he said, adding, “if you are concerned about neutropenia associated with FCR, there are those who use rituximab-fludarabine [RF], and that’s certainly a good option.”
However, in those with an 11q abnormality, good data show that the addition of the alkylator does add benefit. “I do think that FCR is worthwhile pushing [in those cases],” he said.
Bendamustine-rituximab is also an attractive option, as demonstrated in the CLL10 trial, but it is important to remember that patients in that trial were “fit, healthy patients” based on Clinical Illness Rating Scale (CIRS) scores of less than 6; they were patients who were deemed fit to be randomized to receive FCR.
Chlorambucil-based therapies administered with anti-CD20 monoclonal antibodies are also an option, as are novel agents in those with 17p deletions or a P53 mutation, he said.
When it comes to assessing elderly patients’ fitness for therapy, comorbidities play a more important role than age, he said, explaining that many patients over age 65 are very fit and would do well with therapies such as FCR.
For this reason, comorbidities should be the determining factor in treatment selection, he said.
No standard criteria for assessing fitness exist, but there are a few tools that can help.
Eastern Cooperative Oncology Group performance status and organ function (for example, creatinine clearance) can be helpful and often are used in trial settings, as are criteria for excluding patients from participation, but CIRS, used by the German CLL study group, is a more formal tool for assessing comorbidity.
The German group is not the first to use the tool – CIRS is a widely validated test that provides an objective measurement of fitness for more aggressive chemotherapy regimens – but the group did demonstrate in CLL11 that it could be used to enroll more elderly patients with comorbidities into clinical trials, Dr. Gribben said.
A CIRS score of 6 or lower indicates fitness, whereas increasing scores indicate an increasing lack of fitness, he explained, noting that “like every scoring system there are some issues … somebody could easily have a score higher than 6 with comorbidities that really don’t impact on chemotherapy tolerability.
“But in general terms, this is a good way to be making these sorts of assessments,” he said.
Dr. Gribben has received research funding from the National Institutes of Health, Cancer Research UK, MRC, and Wellcome Trust. He has received honoraria from Roche/Genentech, Celgene, Janssen, Pharmacyclics, Gilead, Mundipharma, Infinity, TG Therapeutics, and Ascerta, and he has a patent or receives royalties from Celgene. He also has been the principal investigator on a clinical trial for Roche, Takeda, Pharmacyclics, Gilead, and Infinity.
AT MHM 2015
CLL: No symptoms, no treatment still appropriate
CHICAGO – Despite exciting new advances in the understanding of chronic lymphocytic leukemia, particularly with respect to prognostic features that predict risk for relapse, a watch-and-wait approach remains appropriate for asymptomatic disease pending outcomes data for newer approaches, according to Dr. John G. Gribben.
“When the disease is diagnosed, if it is asymptomatic, the correct approach – of course – is to continue to watch and wait,” Dr. Gribben of Barts Cancer Institute, Queen Mary University of London, said at the American Society of Hematology Meeting on Hematologic Malignancies.
Numerous clinical trials have demonstrated no advantage of early treatment vs. watch and wait, he said, adding that all of the trials published to date have used treatment of all-comers, and have used chlorambucil (CLB) as the treatment.
“There has been a whole variety of more modern trials that have used select prognostic features to identify subgroups of people who are at higher risk of relapse, who then go on to receive earlier treatment with either FCR [fludarabine, cyclophosphamide, rituximab], or more recently, ibrutinib,” he said.
These treatments are interesting, and the trials have demonstrated that prognostic features can identify patients who will progress more rapidly, but none have reported, he explained.
“In the absence of any published trial, I continue to ‘watch and wait’ patients, and there are no high-risk features which will make me alter that approach. Even the highest-risk features of complex karyotype and p53 abnormalities are not indications to treat patients until they become symptomatic,” he said.
It is striking how white counts vary widely in both asymptomatic and symptomatic patients, he noted.
“I don’t personally have any particular white count which is the number at which I’ll treat a patient. I don’t treat white counts, I treat patients,” he said.
When patients become symptomatic, the treatment of choice is now immunochemotherapies, irrespective of performance status, he said.
“Within the past year we have seen approval of obinutuzumab and ofatumumab for treatment of previously untreated CLL, as well as ibrutinib and idelalisib plus rituximab for treatment of both previously untreated CLL and those with 17p deletions for relapsed/refractory disease, as well as for up-front treatment,” he said, adding that these new agents greatly increase the available options for treating CLL.
Dr. Gribben said he considers these questions when it comes to treating CLL:
• Does the patient require treatment, or is watching and waiting appropriate?
• What is the goal of therapy? This is determined through conversation with the patient and the patient’s family regarding the side-effect profile they are willing to tolerate vs. the potential longer duration of response.
• What comorbidities are present to determine “fitness” for specific immunochemotherapy? Specifically, is the patient fit for an FCR-type approach, or is an alternative more appropriate?
• Is there a 17p deletion or P53 mutation that would make chemotherapy a less attractive option, and use of novel agents a more attractive option?
His approach, based on the answers to these questions, is as follows:
• In Rai stage 0-II patients with inactive disease, fitness and 17p deletion or p53 mutation status is irrelevant; no therapy is given.
• For active disease or Rai III-IV disease, a “go-go” patient, (or patient in good physical condition) is treated based on the presence or absence of 17p deletion or p53 mutation status. Those without 17p deletions or p53 mutations can be treated with FCR (his preference), or fludarabine-rituximab (FR). Bendamustine-rituximab (BR) is also an attractive option in certain cases, he said.
• For patients with active disease or Rai stage III-IV disease who do have a 17p deletion, his treatment of choice is either ibrutinib or idelalisib plus rituximab, depending on the patient.
• In “slow-go” patients (those with poorer physical condition) treatment is again based on mutational analysis. Those without mutation receive either FR, BR, or CLB plus obinutuzumab. These are very good options, and represent a spectrum to choose from based on the patient’s core abilities and ability to withstand particular types of treatments, he said.
“If they do have a 17p deletion, these patients are just as eligible for ibrutinib or idelalisib plus rituximab as the younger patients,” he noted.
His choices are based largely on the findings from the CLL8 trial (Lancet 2010 Oct;376[9747]:1164-74) which demonstrated an overall survival advantage with chemoimmunotherapy for front-line therapy vs. chemotherapy alone (hazard ratio, 0.68).
Over time, the advantage has become even more pronounced, according to follow-up data.
Starting with something “gentle” and saving the best treatment for later in the event of relapse was recently considered a reasonable approach, but in the wake of the CLL8 findings, this is no longer an acceptable plan, Dr. Gribben said.
“That’s why for my choice, FCR remains the treatment of choice for those patients who are fit enough to tolerate this type of approach,” he said.
In those with 17p deletions or P53 mutations, the CLL8 trial showed poor outcomes with FCR.
“This is a group of patients whom I believe chemoimmunotherapy would no longer be the treatment of choice,” he said, adding that newer findings suggest outcomes in these patients are better with novel agents.
He also noted that patients with 11q abnormalities, which were previously associated with a poor prognosis, were found in CLL8 to respond well to chemoimmunotherapy when used front line.
While there are special considerations in the elderly, and different strategies in relapsed and refractory disease, the future of CLL treatment is promising. The benefit of adding rituximab to combination chemotherapy is well established, the benefit of novel agents is also now established, and the future likely involves combining targeted therapies with each other and with immunochemotherapies, and combining targeted therapies across different pathways.
“And of course the hope is that we’re going to use the biology of the disease to decide what specific therapy is ideal for that patient. Better understanding of biology and genetics is facilitating rational development of new treatments,” he said, adding that whenever possible, patients should be treated within clinical trials.
Dr. Gribben has received research funding from the NIH, Cancer Research UK, MRC, and Wellcome Trust. He has received honoraria from Roche/Genentech, Celgene, Janssen, Pharmacyclics, Gilead, Mundipharma, Infinity, TG Therapeutics, and Ascerta, and he has a patent or receives royalties from Celgene. He also has been the principal investigator on a clinical trial for Roche, Takeda, Pharmacyclics, Gilead, and Infinity.
CHICAGO – Despite exciting new advances in the understanding of chronic lymphocytic leukemia, particularly with respect to prognostic features that predict risk for relapse, a watch-and-wait approach remains appropriate for asymptomatic disease pending outcomes data for newer approaches, according to Dr. John G. Gribben.
“When the disease is diagnosed, if it is asymptomatic, the correct approach – of course – is to continue to watch and wait,” Dr. Gribben of Barts Cancer Institute, Queen Mary University of London, said at the American Society of Hematology Meeting on Hematologic Malignancies.
Numerous clinical trials have demonstrated no advantage of early treatment vs. watch and wait, he said, adding that all of the trials published to date have used treatment of all-comers, and have used chlorambucil (CLB) as the treatment.
“There has been a whole variety of more modern trials that have used select prognostic features to identify subgroups of people who are at higher risk of relapse, who then go on to receive earlier treatment with either FCR [fludarabine, cyclophosphamide, rituximab], or more recently, ibrutinib,” he said.
These treatments are interesting, and the trials have demonstrated that prognostic features can identify patients who will progress more rapidly, but none have reported, he explained.
“In the absence of any published trial, I continue to ‘watch and wait’ patients, and there are no high-risk features which will make me alter that approach. Even the highest-risk features of complex karyotype and p53 abnormalities are not indications to treat patients until they become symptomatic,” he said.
It is striking how white counts vary widely in both asymptomatic and symptomatic patients, he noted.
“I don’t personally have any particular white count which is the number at which I’ll treat a patient. I don’t treat white counts, I treat patients,” he said.
When patients become symptomatic, the treatment of choice is now immunochemotherapies, irrespective of performance status, he said.
“Within the past year we have seen approval of obinutuzumab and ofatumumab for treatment of previously untreated CLL, as well as ibrutinib and idelalisib plus rituximab for treatment of both previously untreated CLL and those with 17p deletions for relapsed/refractory disease, as well as for up-front treatment,” he said, adding that these new agents greatly increase the available options for treating CLL.
Dr. Gribben said he considers these questions when it comes to treating CLL:
• Does the patient require treatment, or is watching and waiting appropriate?
• What is the goal of therapy? This is determined through conversation with the patient and the patient’s family regarding the side-effect profile they are willing to tolerate vs. the potential longer duration of response.
• What comorbidities are present to determine “fitness” for specific immunochemotherapy? Specifically, is the patient fit for an FCR-type approach, or is an alternative more appropriate?
• Is there a 17p deletion or P53 mutation that would make chemotherapy a less attractive option, and use of novel agents a more attractive option?
His approach, based on the answers to these questions, is as follows:
• In Rai stage 0-II patients with inactive disease, fitness and 17p deletion or p53 mutation status is irrelevant; no therapy is given.
• For active disease or Rai III-IV disease, a “go-go” patient, (or patient in good physical condition) is treated based on the presence or absence of 17p deletion or p53 mutation status. Those without 17p deletions or p53 mutations can be treated with FCR (his preference), or fludarabine-rituximab (FR). Bendamustine-rituximab (BR) is also an attractive option in certain cases, he said.
• For patients with active disease or Rai stage III-IV disease who do have a 17p deletion, his treatment of choice is either ibrutinib or idelalisib plus rituximab, depending on the patient.
• In “slow-go” patients (those with poorer physical condition) treatment is again based on mutational analysis. Those without mutation receive either FR, BR, or CLB plus obinutuzumab. These are very good options, and represent a spectrum to choose from based on the patient’s core abilities and ability to withstand particular types of treatments, he said.
“If they do have a 17p deletion, these patients are just as eligible for ibrutinib or idelalisib plus rituximab as the younger patients,” he noted.
His choices are based largely on the findings from the CLL8 trial (Lancet 2010 Oct;376[9747]:1164-74) which demonstrated an overall survival advantage with chemoimmunotherapy for front-line therapy vs. chemotherapy alone (hazard ratio, 0.68).
Over time, the advantage has become even more pronounced, according to follow-up data.
Starting with something “gentle” and saving the best treatment for later in the event of relapse was recently considered a reasonable approach, but in the wake of the CLL8 findings, this is no longer an acceptable plan, Dr. Gribben said.
“That’s why for my choice, FCR remains the treatment of choice for those patients who are fit enough to tolerate this type of approach,” he said.
In those with 17p deletions or P53 mutations, the CLL8 trial showed poor outcomes with FCR.
“This is a group of patients whom I believe chemoimmunotherapy would no longer be the treatment of choice,” he said, adding that newer findings suggest outcomes in these patients are better with novel agents.
He also noted that patients with 11q abnormalities, which were previously associated with a poor prognosis, were found in CLL8 to respond well to chemoimmunotherapy when used front line.
While there are special considerations in the elderly, and different strategies in relapsed and refractory disease, the future of CLL treatment is promising. The benefit of adding rituximab to combination chemotherapy is well established, the benefit of novel agents is also now established, and the future likely involves combining targeted therapies with each other and with immunochemotherapies, and combining targeted therapies across different pathways.
“And of course the hope is that we’re going to use the biology of the disease to decide what specific therapy is ideal for that patient. Better understanding of biology and genetics is facilitating rational development of new treatments,” he said, adding that whenever possible, patients should be treated within clinical trials.
Dr. Gribben has received research funding from the NIH, Cancer Research UK, MRC, and Wellcome Trust. He has received honoraria from Roche/Genentech, Celgene, Janssen, Pharmacyclics, Gilead, Mundipharma, Infinity, TG Therapeutics, and Ascerta, and he has a patent or receives royalties from Celgene. He also has been the principal investigator on a clinical trial for Roche, Takeda, Pharmacyclics, Gilead, and Infinity.
CHICAGO – Despite exciting new advances in the understanding of chronic lymphocytic leukemia, particularly with respect to prognostic features that predict risk for relapse, a watch-and-wait approach remains appropriate for asymptomatic disease pending outcomes data for newer approaches, according to Dr. John G. Gribben.
“When the disease is diagnosed, if it is asymptomatic, the correct approach – of course – is to continue to watch and wait,” Dr. Gribben of Barts Cancer Institute, Queen Mary University of London, said at the American Society of Hematology Meeting on Hematologic Malignancies.
Numerous clinical trials have demonstrated no advantage of early treatment vs. watch and wait, he said, adding that all of the trials published to date have used treatment of all-comers, and have used chlorambucil (CLB) as the treatment.
“There has been a whole variety of more modern trials that have used select prognostic features to identify subgroups of people who are at higher risk of relapse, who then go on to receive earlier treatment with either FCR [fludarabine, cyclophosphamide, rituximab], or more recently, ibrutinib,” he said.
These treatments are interesting, and the trials have demonstrated that prognostic features can identify patients who will progress more rapidly, but none have reported, he explained.
“In the absence of any published trial, I continue to ‘watch and wait’ patients, and there are no high-risk features which will make me alter that approach. Even the highest-risk features of complex karyotype and p53 abnormalities are not indications to treat patients until they become symptomatic,” he said.
It is striking how white counts vary widely in both asymptomatic and symptomatic patients, he noted.
“I don’t personally have any particular white count which is the number at which I’ll treat a patient. I don’t treat white counts, I treat patients,” he said.
When patients become symptomatic, the treatment of choice is now immunochemotherapies, irrespective of performance status, he said.
“Within the past year we have seen approval of obinutuzumab and ofatumumab for treatment of previously untreated CLL, as well as ibrutinib and idelalisib plus rituximab for treatment of both previously untreated CLL and those with 17p deletions for relapsed/refractory disease, as well as for up-front treatment,” he said, adding that these new agents greatly increase the available options for treating CLL.
Dr. Gribben said he considers these questions when it comes to treating CLL:
• Does the patient require treatment, or is watching and waiting appropriate?
• What is the goal of therapy? This is determined through conversation with the patient and the patient’s family regarding the side-effect profile they are willing to tolerate vs. the potential longer duration of response.
• What comorbidities are present to determine “fitness” for specific immunochemotherapy? Specifically, is the patient fit for an FCR-type approach, or is an alternative more appropriate?
• Is there a 17p deletion or P53 mutation that would make chemotherapy a less attractive option, and use of novel agents a more attractive option?
His approach, based on the answers to these questions, is as follows:
• In Rai stage 0-II patients with inactive disease, fitness and 17p deletion or p53 mutation status is irrelevant; no therapy is given.
• For active disease or Rai III-IV disease, a “go-go” patient, (or patient in good physical condition) is treated based on the presence or absence of 17p deletion or p53 mutation status. Those without 17p deletions or p53 mutations can be treated with FCR (his preference), or fludarabine-rituximab (FR). Bendamustine-rituximab (BR) is also an attractive option in certain cases, he said.
• For patients with active disease or Rai stage III-IV disease who do have a 17p deletion, his treatment of choice is either ibrutinib or idelalisib plus rituximab, depending on the patient.
• In “slow-go” patients (those with poorer physical condition) treatment is again based on mutational analysis. Those without mutation receive either FR, BR, or CLB plus obinutuzumab. These are very good options, and represent a spectrum to choose from based on the patient’s core abilities and ability to withstand particular types of treatments, he said.
“If they do have a 17p deletion, these patients are just as eligible for ibrutinib or idelalisib plus rituximab as the younger patients,” he noted.
His choices are based largely on the findings from the CLL8 trial (Lancet 2010 Oct;376[9747]:1164-74) which demonstrated an overall survival advantage with chemoimmunotherapy for front-line therapy vs. chemotherapy alone (hazard ratio, 0.68).
Over time, the advantage has become even more pronounced, according to follow-up data.
Starting with something “gentle” and saving the best treatment for later in the event of relapse was recently considered a reasonable approach, but in the wake of the CLL8 findings, this is no longer an acceptable plan, Dr. Gribben said.
“That’s why for my choice, FCR remains the treatment of choice for those patients who are fit enough to tolerate this type of approach,” he said.
In those with 17p deletions or P53 mutations, the CLL8 trial showed poor outcomes with FCR.
“This is a group of patients whom I believe chemoimmunotherapy would no longer be the treatment of choice,” he said, adding that newer findings suggest outcomes in these patients are better with novel agents.
He also noted that patients with 11q abnormalities, which were previously associated with a poor prognosis, were found in CLL8 to respond well to chemoimmunotherapy when used front line.
While there are special considerations in the elderly, and different strategies in relapsed and refractory disease, the future of CLL treatment is promising. The benefit of adding rituximab to combination chemotherapy is well established, the benefit of novel agents is also now established, and the future likely involves combining targeted therapies with each other and with immunochemotherapies, and combining targeted therapies across different pathways.
“And of course the hope is that we’re going to use the biology of the disease to decide what specific therapy is ideal for that patient. Better understanding of biology and genetics is facilitating rational development of new treatments,” he said, adding that whenever possible, patients should be treated within clinical trials.
Dr. Gribben has received research funding from the NIH, Cancer Research UK, MRC, and Wellcome Trust. He has received honoraria from Roche/Genentech, Celgene, Janssen, Pharmacyclics, Gilead, Mundipharma, Infinity, TG Therapeutics, and Ascerta, and he has a patent or receives royalties from Celgene. He also has been the principal investigator on a clinical trial for Roche, Takeda, Pharmacyclics, Gilead, and Infinity.
EXPERT ANALYSIS FROM MHM 2015
HELIOS trial: Ibrutinib safely boosts survival in CLL/SLL
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
AT MHM 2015
Key clinical point: Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Major finding: The overall infection rate was lower in the ibrutinib group than in the placebo group (10.3/100 vs. 11.2/100 patient months).
Data source: The phase III HELIOS study involving 578 patients.
Disclosures: Janssen Pharmaceuticals sponsored the study. Dr. Chanan-Khan reported having no disclosures.
No evidence for CLL transmission via blood transfusion
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
FROM BLOOD
Key clinical point: There is no evidence for higher risk of chronic lymphocytic leukemia (CLL) among recipients of blood products from donors who subsequently were diagnosed with CLL.
Major finding: Among exposed recipients (7,413 who received blood from 796 donors who subsequently developed CLL), 12 were diagnosed with CLL. Among unexposed recipients (80,431 who received blood from 7,477 donors free of CLL), 107 were diagnosed with CLL, for an incidence rate ratio of 0.94 (95% CI, 0.52-1.71).
Data source: The Scandinavian Donations and Transfusions (SCANDAT2) database comprises information, including donor and recipient health outcomes, for more than 20 million blood products handled by blood banks from 1968 to 2010.
Disclosures: Dr. Hjalgrim and his coauthors reported having no disclosures.
CLL exosomes promote stromal cell transition into cancer-associated fibroblasts
Exosomes released by chronic lymphocytic leukemia (CLL) cells induce stromal cells to adopt a cancer-associated fibroblast phenotype, thereby creating a microenvironment conducive to CLL cell adhesion, survival, and growth.
Although the role of exosomes in other cancers has been well studied, their role in hematologic malignancies has not been well characterized. Also, this study confirmed that exosomes are present in CLL lymph nodes and promote tumor growth in vivo.
“Our in vitro and in vivo data show that CLL exosomes harbor an oncogenic potential by stimulating stromal cells to induce an inflammatory and protumorigenic milieu, including increased angiogenesis, thus supporting the survival and outgrowth of CLL cells,” wrote Jerome Paggetti, Ph.D., of the laboratory of experimental hemato-oncology, Luxembourg Institute of Health (Blood 2015 Aug 27. doi:10.1182/blood-2014-12-618025).
Cells were obtained from 21 CLL patients; all patients had an absolute lymphocyte count of more than 30,000/mcL and were untreated for 3 months. The researchers established 30-day cocultures of bone marrow mesenchymal stem cells with primary CLL cells in culture inserts or they treated bone marrow mesenchymal stem cells weekly with exosomes. Similar experiments were performed with the Burkitt lymphoma cell line Namalwa to investigate whether the impact on stromal cells is CLL specific.
Based on gene expression analysis, CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells, highlighting the relevance of exosomes for microenvironment changes. “Importantly, lymphoma cells induced a distinct gene expression pattern in bone marrow mesenchymal stem cells, suggesting a specific response to CLL exosomes,” wrote Dr. Paggetti and coauthors.
The impact of CLL exosomes on tumor growth was studied in vivo by subcutaneously injecting cells with and without CLL exosomes into immunocompromised mice. Cells supplemented with exosomes resulted in an increased tumor size compared with tumor cells injected without additional exosomes. Also, the cells supplemented with exosomes accumulated in mice kidneys, confirming the renal involvement observed in CLL patients. “Our data demonstrate a protumorigenic effect of CLL-derived exosomes in vivo and their importance in the early onset of the disease when tumor cells impact the microenvironment to proliferate and promote angiogenesis,” the researchers concluded.
Chronic lymphocytic leukemia results in clonal expansion and invasive migration of cells that infiltrate the lymph nodes and bone marrow. Understanding the tumor microenvironment and the communication that occurs between malignant cells and their surroundings is imperative to improving cancer therapies.
Alongside well-studied signaling mechanisms involving cytokines, growth factors, and receptors, exosome shedding has emerged recently as a key player in cancer signaling. Paggetti et al. comprehensively analyzed CLL-derived exosomes and provided functional data illustrating the impact of exosomes on the tumor microenvironment by reprogramming healthy stromal cells into cancer-associated fibroblasts.
The RNA and proteins delivered by exosomes to stromal cells induce an inflammatory phenotype characteristic of cancer-associated fibroblasts.
The work supports the theory that tumor cell induction of cancer-associated fibroblasts is a universal feature of progression in both solid and blood cancers. Continued research may identify novel therapies that reconfigure the tumor microenvironment for antitumorigenic effect.
Dr. Benedetta Apollonio is a researcher and Dr. Alan Ramsey is a senior lecturer in lymphoma biology at King’s College, London. Their remarks were part of an editorial accompanying the report (Blood 2015 Aug 27. doi:10.1182/blood-2015-07-655233). The authors had no disclosures to report.
Chronic lymphocytic leukemia results in clonal expansion and invasive migration of cells that infiltrate the lymph nodes and bone marrow. Understanding the tumor microenvironment and the communication that occurs between malignant cells and their surroundings is imperative to improving cancer therapies.
Alongside well-studied signaling mechanisms involving cytokines, growth factors, and receptors, exosome shedding has emerged recently as a key player in cancer signaling. Paggetti et al. comprehensively analyzed CLL-derived exosomes and provided functional data illustrating the impact of exosomes on the tumor microenvironment by reprogramming healthy stromal cells into cancer-associated fibroblasts.
The RNA and proteins delivered by exosomes to stromal cells induce an inflammatory phenotype characteristic of cancer-associated fibroblasts.
The work supports the theory that tumor cell induction of cancer-associated fibroblasts is a universal feature of progression in both solid and blood cancers. Continued research may identify novel therapies that reconfigure the tumor microenvironment for antitumorigenic effect.
Dr. Benedetta Apollonio is a researcher and Dr. Alan Ramsey is a senior lecturer in lymphoma biology at King’s College, London. Their remarks were part of an editorial accompanying the report (Blood 2015 Aug 27. doi:10.1182/blood-2015-07-655233). The authors had no disclosures to report.
Chronic lymphocytic leukemia results in clonal expansion and invasive migration of cells that infiltrate the lymph nodes and bone marrow. Understanding the tumor microenvironment and the communication that occurs between malignant cells and their surroundings is imperative to improving cancer therapies.
Alongside well-studied signaling mechanisms involving cytokines, growth factors, and receptors, exosome shedding has emerged recently as a key player in cancer signaling. Paggetti et al. comprehensively analyzed CLL-derived exosomes and provided functional data illustrating the impact of exosomes on the tumor microenvironment by reprogramming healthy stromal cells into cancer-associated fibroblasts.
The RNA and proteins delivered by exosomes to stromal cells induce an inflammatory phenotype characteristic of cancer-associated fibroblasts.
The work supports the theory that tumor cell induction of cancer-associated fibroblasts is a universal feature of progression in both solid and blood cancers. Continued research may identify novel therapies that reconfigure the tumor microenvironment for antitumorigenic effect.
Dr. Benedetta Apollonio is a researcher and Dr. Alan Ramsey is a senior lecturer in lymphoma biology at King’s College, London. Their remarks were part of an editorial accompanying the report (Blood 2015 Aug 27. doi:10.1182/blood-2015-07-655233). The authors had no disclosures to report.
Exosomes released by chronic lymphocytic leukemia (CLL) cells induce stromal cells to adopt a cancer-associated fibroblast phenotype, thereby creating a microenvironment conducive to CLL cell adhesion, survival, and growth.
Although the role of exosomes in other cancers has been well studied, their role in hematologic malignancies has not been well characterized. Also, this study confirmed that exosomes are present in CLL lymph nodes and promote tumor growth in vivo.
“Our in vitro and in vivo data show that CLL exosomes harbor an oncogenic potential by stimulating stromal cells to induce an inflammatory and protumorigenic milieu, including increased angiogenesis, thus supporting the survival and outgrowth of CLL cells,” wrote Jerome Paggetti, Ph.D., of the laboratory of experimental hemato-oncology, Luxembourg Institute of Health (Blood 2015 Aug 27. doi:10.1182/blood-2014-12-618025).
Cells were obtained from 21 CLL patients; all patients had an absolute lymphocyte count of more than 30,000/mcL and were untreated for 3 months. The researchers established 30-day cocultures of bone marrow mesenchymal stem cells with primary CLL cells in culture inserts or they treated bone marrow mesenchymal stem cells weekly with exosomes. Similar experiments were performed with the Burkitt lymphoma cell line Namalwa to investigate whether the impact on stromal cells is CLL specific.
Based on gene expression analysis, CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells, highlighting the relevance of exosomes for microenvironment changes. “Importantly, lymphoma cells induced a distinct gene expression pattern in bone marrow mesenchymal stem cells, suggesting a specific response to CLL exosomes,” wrote Dr. Paggetti and coauthors.
The impact of CLL exosomes on tumor growth was studied in vivo by subcutaneously injecting cells with and without CLL exosomes into immunocompromised mice. Cells supplemented with exosomes resulted in an increased tumor size compared with tumor cells injected without additional exosomes. Also, the cells supplemented with exosomes accumulated in mice kidneys, confirming the renal involvement observed in CLL patients. “Our data demonstrate a protumorigenic effect of CLL-derived exosomes in vivo and their importance in the early onset of the disease when tumor cells impact the microenvironment to proliferate and promote angiogenesis,” the researchers concluded.
Exosomes released by chronic lymphocytic leukemia (CLL) cells induce stromal cells to adopt a cancer-associated fibroblast phenotype, thereby creating a microenvironment conducive to CLL cell adhesion, survival, and growth.
Although the role of exosomes in other cancers has been well studied, their role in hematologic malignancies has not been well characterized. Also, this study confirmed that exosomes are present in CLL lymph nodes and promote tumor growth in vivo.
“Our in vitro and in vivo data show that CLL exosomes harbor an oncogenic potential by stimulating stromal cells to induce an inflammatory and protumorigenic milieu, including increased angiogenesis, thus supporting the survival and outgrowth of CLL cells,” wrote Jerome Paggetti, Ph.D., of the laboratory of experimental hemato-oncology, Luxembourg Institute of Health (Blood 2015 Aug 27. doi:10.1182/blood-2014-12-618025).
Cells were obtained from 21 CLL patients; all patients had an absolute lymphocyte count of more than 30,000/mcL and were untreated for 3 months. The researchers established 30-day cocultures of bone marrow mesenchymal stem cells with primary CLL cells in culture inserts or they treated bone marrow mesenchymal stem cells weekly with exosomes. Similar experiments were performed with the Burkitt lymphoma cell line Namalwa to investigate whether the impact on stromal cells is CLL specific.
Based on gene expression analysis, CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells, highlighting the relevance of exosomes for microenvironment changes. “Importantly, lymphoma cells induced a distinct gene expression pattern in bone marrow mesenchymal stem cells, suggesting a specific response to CLL exosomes,” wrote Dr. Paggetti and coauthors.
The impact of CLL exosomes on tumor growth was studied in vivo by subcutaneously injecting cells with and without CLL exosomes into immunocompromised mice. Cells supplemented with exosomes resulted in an increased tumor size compared with tumor cells injected without additional exosomes. Also, the cells supplemented with exosomes accumulated in mice kidneys, confirming the renal involvement observed in CLL patients. “Our data demonstrate a protumorigenic effect of CLL-derived exosomes in vivo and their importance in the early onset of the disease when tumor cells impact the microenvironment to proliferate and promote angiogenesis,” the researchers concluded.
FROM BLOOD
Key clinical point: Exosomes derived from chronic lymphocytic leukemia (CLL) cells induce stromal cell transition to cancer-associated fibroblasts.
Major finding: CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells.
Data source: In vitro and in vivo studies that used cells obtained from 21 CLL patients.
Disclosures: Jerome Paggetti, Ph.D., and coauthors reported having no disclosures.
Reovirus ready for clinical testing in CLL
Reovirus, a naturally occurring oncolytic virus, appears to exert direct cytotoxic activity against chronic lymphocytic leukemia (CLL) and “phenotypically and functionally” activates patient natural killer cells using a monocyte-derived interferon alpha–dependent mechanism, according to preclinical data published in Leukemia.
Reovirus also enhances antibody-dependent cellular cytotoxicity – mediated killing of CLL cells in combination with anti-CD20 antibodies.
“Reovirus together with anti-CD20 antibodies represents a promising combination strategy for the treatment of CLL … and now warrants clinical evaluation,” wrote Christopher Parrish, Ph.D., of the Leeds (England) Institute of Cancer and Pathology and his colleagues (Leukemia. 2015;29:1799-1810. doi: 10.1038/leu.2015.88).Reovirus is a naturally occurring double-stranded RNA virus, and it exerts its effects against cancer cells by direct oncolysis and activation of antitumor immunity. The researchers investigated the efficacy of reovirus for the treatment of CLL, both as a direct cytotoxic agent and as an immunomodulator, using CLL cell lines and primary CLL cells from 24 patients.
Dr. Parrish and his team treated human CLL cells with live or UV-inactivated reovirus for 7 days. They assessed the ability of reovirus to stimulate immune-mediated killing of CLL using peripheral blood mononuclear cells from healthy volunteers and CLL patients. Reovirus activated natural killer (NK) cells from CLL patients, as well as stimulating innate antitumor immunity.
Rituximab, which is believed to act in part via NK cell–mediated antibody-dependent cellular cytotoxicity, was added to peripheral blood mononuclear cells that were treated with reovirus. Compared with rituximab alone, reovirus treatment significantly increased NK-cell CD107a/b degranulation. Reovirus was then paired with ofatumumab and GA101 to see if the effects observed with reovirus/rituximab could be translated to other anti-CD20 antibodies in which NK cells also play a role. The results were similar.
Absolute monocyte count and the type 1 interferon-alpha response could be used to predict the generation of antitumor innate immunity by reovirus. In cells from 24 CLL patients, about 75% responded to reovirus, with NK-cell activation. Further, NK-cell activation correlated with absolute monocyte count. The role of interferon-alpha is further supported by identification of an interferon gene signature within NK cells from reovirus-treated patients, the researchers said.
The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
Reovirus, a naturally occurring oncolytic virus, appears to exert direct cytotoxic activity against chronic lymphocytic leukemia (CLL) and “phenotypically and functionally” activates patient natural killer cells using a monocyte-derived interferon alpha–dependent mechanism, according to preclinical data published in Leukemia.
Reovirus also enhances antibody-dependent cellular cytotoxicity – mediated killing of CLL cells in combination with anti-CD20 antibodies.
“Reovirus together with anti-CD20 antibodies represents a promising combination strategy for the treatment of CLL … and now warrants clinical evaluation,” wrote Christopher Parrish, Ph.D., of the Leeds (England) Institute of Cancer and Pathology and his colleagues (Leukemia. 2015;29:1799-1810. doi: 10.1038/leu.2015.88).Reovirus is a naturally occurring double-stranded RNA virus, and it exerts its effects against cancer cells by direct oncolysis and activation of antitumor immunity. The researchers investigated the efficacy of reovirus for the treatment of CLL, both as a direct cytotoxic agent and as an immunomodulator, using CLL cell lines and primary CLL cells from 24 patients.
Dr. Parrish and his team treated human CLL cells with live or UV-inactivated reovirus for 7 days. They assessed the ability of reovirus to stimulate immune-mediated killing of CLL using peripheral blood mononuclear cells from healthy volunteers and CLL patients. Reovirus activated natural killer (NK) cells from CLL patients, as well as stimulating innate antitumor immunity.
Rituximab, which is believed to act in part via NK cell–mediated antibody-dependent cellular cytotoxicity, was added to peripheral blood mononuclear cells that were treated with reovirus. Compared with rituximab alone, reovirus treatment significantly increased NK-cell CD107a/b degranulation. Reovirus was then paired with ofatumumab and GA101 to see if the effects observed with reovirus/rituximab could be translated to other anti-CD20 antibodies in which NK cells also play a role. The results were similar.
Absolute monocyte count and the type 1 interferon-alpha response could be used to predict the generation of antitumor innate immunity by reovirus. In cells from 24 CLL patients, about 75% responded to reovirus, with NK-cell activation. Further, NK-cell activation correlated with absolute monocyte count. The role of interferon-alpha is further supported by identification of an interferon gene signature within NK cells from reovirus-treated patients, the researchers said.
The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
Reovirus, a naturally occurring oncolytic virus, appears to exert direct cytotoxic activity against chronic lymphocytic leukemia (CLL) and “phenotypically and functionally” activates patient natural killer cells using a monocyte-derived interferon alpha–dependent mechanism, according to preclinical data published in Leukemia.
Reovirus also enhances antibody-dependent cellular cytotoxicity – mediated killing of CLL cells in combination with anti-CD20 antibodies.
“Reovirus together with anti-CD20 antibodies represents a promising combination strategy for the treatment of CLL … and now warrants clinical evaluation,” wrote Christopher Parrish, Ph.D., of the Leeds (England) Institute of Cancer and Pathology and his colleagues (Leukemia. 2015;29:1799-1810. doi: 10.1038/leu.2015.88).Reovirus is a naturally occurring double-stranded RNA virus, and it exerts its effects against cancer cells by direct oncolysis and activation of antitumor immunity. The researchers investigated the efficacy of reovirus for the treatment of CLL, both as a direct cytotoxic agent and as an immunomodulator, using CLL cell lines and primary CLL cells from 24 patients.
Dr. Parrish and his team treated human CLL cells with live or UV-inactivated reovirus for 7 days. They assessed the ability of reovirus to stimulate immune-mediated killing of CLL using peripheral blood mononuclear cells from healthy volunteers and CLL patients. Reovirus activated natural killer (NK) cells from CLL patients, as well as stimulating innate antitumor immunity.
Rituximab, which is believed to act in part via NK cell–mediated antibody-dependent cellular cytotoxicity, was added to peripheral blood mononuclear cells that were treated with reovirus. Compared with rituximab alone, reovirus treatment significantly increased NK-cell CD107a/b degranulation. Reovirus was then paired with ofatumumab and GA101 to see if the effects observed with reovirus/rituximab could be translated to other anti-CD20 antibodies in which NK cells also play a role. The results were similar.
Absolute monocyte count and the type 1 interferon-alpha response could be used to predict the generation of antitumor innate immunity by reovirus. In cells from 24 CLL patients, about 75% responded to reovirus, with NK-cell activation. Further, NK-cell activation correlated with absolute monocyte count. The role of interferon-alpha is further supported by identification of an interferon gene signature within NK cells from reovirus-treated patients, the researchers said.
The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
FROM LEUKEMIA
Key clinical point: Reovirus, a naturally occurring oncolytic virus, in combination with anti-CD20 immunotherapy, may have benefit for the treatment of chronic lymphocytic leukemia.
Major finding: In cells from 24 CLL patients, about 75% responded to reovirus, with natural killer cell activation.
Data source: Preclinical trial that used human CLL lines and murine L929 cells.
Disclosures: The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
CLL patients achieve remission with CAR-modified T-cells
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: CAR-modified T cell therapy lacks the toxicities and limitations of allogeneic stem cell transplantation and may be an effective treatment for chronic lymphocytic leukemia.
Major finding: CAR-modified T cell therapy elicited a response in 8 of 14 patients (57%) with relapsed and refractory chronic lymphocytic leukemia, and 4 patients (29%) achieved a complete remission.
Data source: Mature results from a pilot clinical trial.
Disclosures: Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Melanoma twice as likely after CLL/SLL than other types of NHL
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma.
Major finding: A total of 202 melanomas developed during 5.5 years of follow-up, and nearly half occurred in patients with CLL/SLL rather than other types of NHL.
Data source: A large population-based study using SEER data to assess melanoma risk in 44,870 older survivors of non-Hodgkin lymphoma.
Disclosures: The National Cancer Institute supported the study. Dr. Lam and her associates reported having no relevant financial disclosures.