User login
Heart attack care not equal for women and people of color
Radiating chest pain, shortness of breath, nausea, lightheadedness. Everyone knows the telltale signs of a myocardial infarction. Yet a new study shows that despite this widespread recognition, heart attacks aren’t attended to quickly across the board. Historically, the study says, women and people of color wait longer to access emergency care for a heart attack.
Researchers from the University of California, San Francisco published these findings in the Annals of Emergency Medicine. The study used the Office of Statewide Health Planning and Development dataset to gather information on 453,136 cases of heart attack in California between 2005 and 2015. They found that over time, differences in timely treatment between the demographics narrowed, but the gap still existed.
The study defined timely treatment as receiving care for a heart attack within 3 days of admission to a hospital. Women and people of color were found to wait 3 days or more to receive care than their White male counterparts. A disparity of this sort can cause ripples of health effects across society, ripples that doctors should be aware of, says lead author Juan Carlos Montoy, MD. Dr. Montoy was “sadly surprised by our findings that disparities for women and for Black patients only decreased slightly or not at all over time.”
In the study, the team separated the dataset between the two primary types of heart attack: ST-segment elevation myocardial infarction (STEMI), caused by blood vessel blockage, and non–ST-segment elevation myocardial infarction (NSTEMI), caused by a narrowing or temporary blockage of the artery.
Regardless of the type of heart attack, the standard first step in treatment is a coronary angiogram. After finding out where blood flow is disrupted using the angiogram, a physician can proceed with treatment.
But when looking back, the team found that it took a while for many patients to receive this first step in treatment. In 2005, 50% of men and 35.7% of women with STEMI and 45% of men and 33.1% of women with NSTEMI had a timely angiography. In the same year, 46% of White patients and 31.2% of Black patients with STEMI underwent timely angiography.
By 2015, timely treatment increased across the board, but there were still discrepancies, with 76.7% of men and 66.8% of women with STEMI undergoing timely angiography and 56.3% of men and 45.9% of women with NSTEMI undergoing timely angiography. Also in 2015, 75.2% of White patients and 69.2% of Black patients underwent timely angiography for STEMI.
Although differences in care decreased between the demographics, the gap still exists. Whereas this dataset only extends to 2015, this trend may still persist today, says Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, who was not involved in the study. Therefore, physicians need to consider this bias when treating patients. “The bottom line is that we continue to have much work to do to achieve equality in managing not only medical conditions but treating people who have them equally,” Dr. Glatter said.
“Raising awareness of ongoing inequality in care related to gender and ethnic disparities is critical to drive change in our institutions,” he emphasized. “We simply cannot accept the status quo.”
The study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Glatter and the authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Radiating chest pain, shortness of breath, nausea, lightheadedness. Everyone knows the telltale signs of a myocardial infarction. Yet a new study shows that despite this widespread recognition, heart attacks aren’t attended to quickly across the board. Historically, the study says, women and people of color wait longer to access emergency care for a heart attack.
Researchers from the University of California, San Francisco published these findings in the Annals of Emergency Medicine. The study used the Office of Statewide Health Planning and Development dataset to gather information on 453,136 cases of heart attack in California between 2005 and 2015. They found that over time, differences in timely treatment between the demographics narrowed, but the gap still existed.
The study defined timely treatment as receiving care for a heart attack within 3 days of admission to a hospital. Women and people of color were found to wait 3 days or more to receive care than their White male counterparts. A disparity of this sort can cause ripples of health effects across society, ripples that doctors should be aware of, says lead author Juan Carlos Montoy, MD. Dr. Montoy was “sadly surprised by our findings that disparities for women and for Black patients only decreased slightly or not at all over time.”
In the study, the team separated the dataset between the two primary types of heart attack: ST-segment elevation myocardial infarction (STEMI), caused by blood vessel blockage, and non–ST-segment elevation myocardial infarction (NSTEMI), caused by a narrowing or temporary blockage of the artery.
Regardless of the type of heart attack, the standard first step in treatment is a coronary angiogram. After finding out where blood flow is disrupted using the angiogram, a physician can proceed with treatment.
But when looking back, the team found that it took a while for many patients to receive this first step in treatment. In 2005, 50% of men and 35.7% of women with STEMI and 45% of men and 33.1% of women with NSTEMI had a timely angiography. In the same year, 46% of White patients and 31.2% of Black patients with STEMI underwent timely angiography.
By 2015, timely treatment increased across the board, but there were still discrepancies, with 76.7% of men and 66.8% of women with STEMI undergoing timely angiography and 56.3% of men and 45.9% of women with NSTEMI undergoing timely angiography. Also in 2015, 75.2% of White patients and 69.2% of Black patients underwent timely angiography for STEMI.
Although differences in care decreased between the demographics, the gap still exists. Whereas this dataset only extends to 2015, this trend may still persist today, says Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, who was not involved in the study. Therefore, physicians need to consider this bias when treating patients. “The bottom line is that we continue to have much work to do to achieve equality in managing not only medical conditions but treating people who have them equally,” Dr. Glatter said.
“Raising awareness of ongoing inequality in care related to gender and ethnic disparities is critical to drive change in our institutions,” he emphasized. “We simply cannot accept the status quo.”
The study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Glatter and the authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Radiating chest pain, shortness of breath, nausea, lightheadedness. Everyone knows the telltale signs of a myocardial infarction. Yet a new study shows that despite this widespread recognition, heart attacks aren’t attended to quickly across the board. Historically, the study says, women and people of color wait longer to access emergency care for a heart attack.
Researchers from the University of California, San Francisco published these findings in the Annals of Emergency Medicine. The study used the Office of Statewide Health Planning and Development dataset to gather information on 453,136 cases of heart attack in California between 2005 and 2015. They found that over time, differences in timely treatment between the demographics narrowed, but the gap still existed.
The study defined timely treatment as receiving care for a heart attack within 3 days of admission to a hospital. Women and people of color were found to wait 3 days or more to receive care than their White male counterparts. A disparity of this sort can cause ripples of health effects across society, ripples that doctors should be aware of, says lead author Juan Carlos Montoy, MD. Dr. Montoy was “sadly surprised by our findings that disparities for women and for Black patients only decreased slightly or not at all over time.”
In the study, the team separated the dataset between the two primary types of heart attack: ST-segment elevation myocardial infarction (STEMI), caused by blood vessel blockage, and non–ST-segment elevation myocardial infarction (NSTEMI), caused by a narrowing or temporary blockage of the artery.
Regardless of the type of heart attack, the standard first step in treatment is a coronary angiogram. After finding out where blood flow is disrupted using the angiogram, a physician can proceed with treatment.
But when looking back, the team found that it took a while for many patients to receive this first step in treatment. In 2005, 50% of men and 35.7% of women with STEMI and 45% of men and 33.1% of women with NSTEMI had a timely angiography. In the same year, 46% of White patients and 31.2% of Black patients with STEMI underwent timely angiography.
By 2015, timely treatment increased across the board, but there were still discrepancies, with 76.7% of men and 66.8% of women with STEMI undergoing timely angiography and 56.3% of men and 45.9% of women with NSTEMI undergoing timely angiography. Also in 2015, 75.2% of White patients and 69.2% of Black patients underwent timely angiography for STEMI.
Although differences in care decreased between the demographics, the gap still exists. Whereas this dataset only extends to 2015, this trend may still persist today, says Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, who was not involved in the study. Therefore, physicians need to consider this bias when treating patients. “The bottom line is that we continue to have much work to do to achieve equality in managing not only medical conditions but treating people who have them equally,” Dr. Glatter said.
“Raising awareness of ongoing inequality in care related to gender and ethnic disparities is critical to drive change in our institutions,” he emphasized. “We simply cannot accept the status quo.”
The study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Glatter and the authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF EMERGENCY MEDICINE
Early cardiac rehab as effective as later start after sternotomy
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
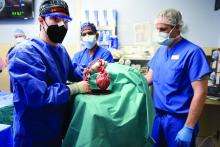
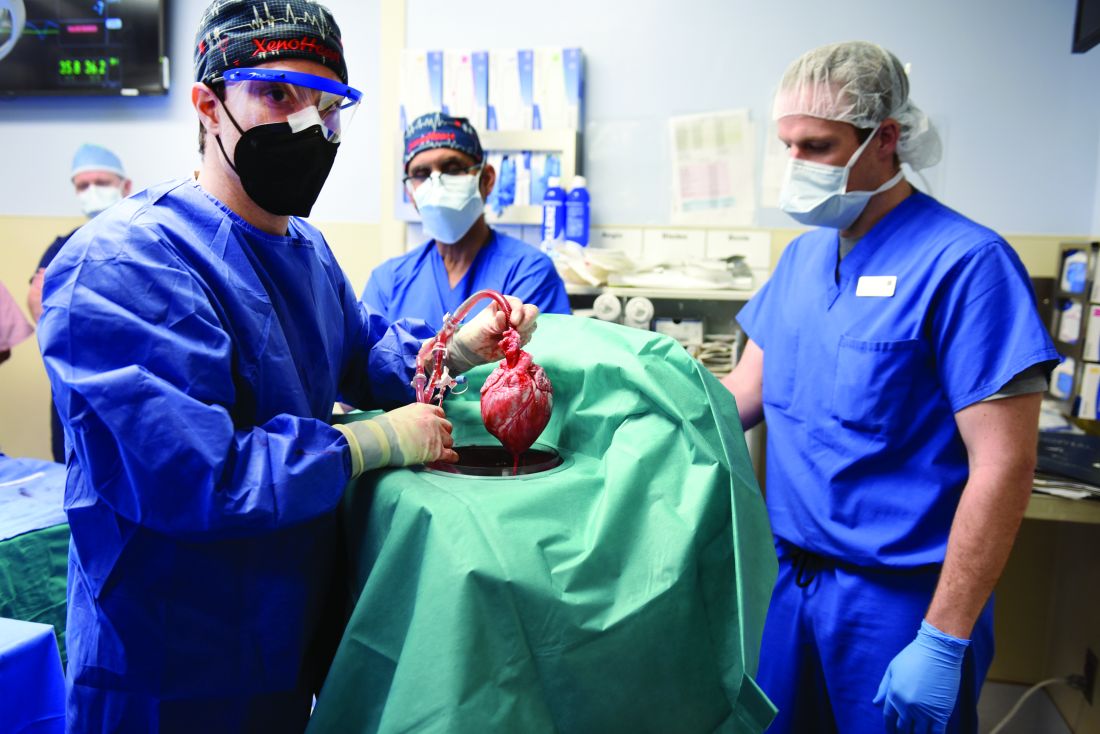
Pig-heart transplant case published with new details, insights
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
Class I recall for Medtronic’s HeartWare HVAD batteries
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to [email protected].
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to [email protected].
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to [email protected].
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cause of death in pig heart recipient: New clues
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
Harmony pulmonary valve update: Regurgitation resolved 1 year out
The 1-year results of the Harmony transcatheter pulmonary valve to treat severe pulmonary regurgitation have shown a high rate of eliminating or reducing the degree of symptoms as well as freedom from endocarditis, sustained ventricular tachycardia, and the need for further interventions.
“Simply put, the good news is no endocarditis,” said Daniel S. Levi, MD, in presenting results from three different studies with 108 patients who received three different iterations of the device at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“Endocarditis has been an issue for us in the pulmonary position; we have yet to have an endocarditis in these patients in 1 year,” he stressed.
The studies evaluated three different versions of the Harmony valve: TPV22 (42 patients), the first version with a 22-mm diameter; the Clinical TPV25 (17 patients), the first iteration of a 25 mm–wide device that has since been discontinued; and the modified TPV25 (45 patients), the second version of the 25-mm valve. The three studies are the early feasibility study of the TPV22, the continued-access study of the TPV22 and the mTPV25, and the pivotal study that included all three versions.
At baseline, 89% of patients had severe and 11% had moderate pulmonary regurgitation (PR). At 1 year, 92% had none or trace PR, 3% had mild PR, and 4% moderate disease.
Dr. Levi said the device “speaks for itself” in the results he presented. They include no deaths, no heart attacks, and no pulmonary thromboembolism. Other key outcomes include:
- One major stent fracture in one of the early feasibility study patients at 1-month follow-up.
- Four explants, with two in the discontinued cTPV25 and two with the TPV22 in the early-feasibility study.
- Four reinterventions, two with the discontinued cTPV25 and two valve-in-valve procedures with the mTPV25 in the continued-access study, one with stent placement in the right ventricular outflow tract.
Dr. Levi and coinvestigators also performed a breakdown of 1-year outcomes – freedom from PR, stenosis, and interventions – by device: 95.1% for TPV22; 89.7% for mTPV25; and 73.3% for the discontinued cTPV25.
Although the valve is indicated for adolescents and adults, most of the patients in the three studies were adults, with an average weight of 165 pounds (75 kg) who have had PR for decades, said Dr. Levi, an interventional pediatric cardiologist at the University of California, Los Angeles. “With a device like this we are hopefully shifting to treating that a little bit earlier, but fortunately we don’t usually need to treat it before puberty.” The 25-mm TPV gives “a really nice landing zone” for future valve placement. “The goal is to keep patients out of the operating room for at least a few decades if not their whole lives,” he said.
Dr. Levi said the Harmony investigators will follow outcomes with the 22- and modified 25-mm Harmony valves, both of which remain commercially available, out to 10 years.
The study represents the first collective cohort evaluating the Harmony device across the early feasibility, continued access and pivotal studies, said Brian Morray, MD. “It’s important that people understand that evolution and how that impacts the way we look at outcomes, because when you aggregate the data, particularly for the TPV25, some of the procedural outcomes and the adverse events are no longer really reflective in the current time frame.”
These Harmony results “represent another big step in the evolution of interventional cardiology and will be up there with development of the Melody valve and the utility and the use of the Sapien valve in the pulmonary position,” said Dr. Morray, an associate professor of pediatrics at the University of Washington, Seattle, and an interventional cardiologist at Seattle Children’s Hospital.
Dr. Levi disclosed he is a consultant to Medtronic and Edwards Lifesciences. Dr. Morray disclosed he is a clinical proctor for Abbott and a consultant to Medtronic, but not for the Harmony device.
The 1-year results of the Harmony transcatheter pulmonary valve to treat severe pulmonary regurgitation have shown a high rate of eliminating or reducing the degree of symptoms as well as freedom from endocarditis, sustained ventricular tachycardia, and the need for further interventions.
“Simply put, the good news is no endocarditis,” said Daniel S. Levi, MD, in presenting results from three different studies with 108 patients who received three different iterations of the device at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“Endocarditis has been an issue for us in the pulmonary position; we have yet to have an endocarditis in these patients in 1 year,” he stressed.
The studies evaluated three different versions of the Harmony valve: TPV22 (42 patients), the first version with a 22-mm diameter; the Clinical TPV25 (17 patients), the first iteration of a 25 mm–wide device that has since been discontinued; and the modified TPV25 (45 patients), the second version of the 25-mm valve. The three studies are the early feasibility study of the TPV22, the continued-access study of the TPV22 and the mTPV25, and the pivotal study that included all three versions.
At baseline, 89% of patients had severe and 11% had moderate pulmonary regurgitation (PR). At 1 year, 92% had none or trace PR, 3% had mild PR, and 4% moderate disease.
Dr. Levi said the device “speaks for itself” in the results he presented. They include no deaths, no heart attacks, and no pulmonary thromboembolism. Other key outcomes include:
- One major stent fracture in one of the early feasibility study patients at 1-month follow-up.
- Four explants, with two in the discontinued cTPV25 and two with the TPV22 in the early-feasibility study.
- Four reinterventions, two with the discontinued cTPV25 and two valve-in-valve procedures with the mTPV25 in the continued-access study, one with stent placement in the right ventricular outflow tract.
Dr. Levi and coinvestigators also performed a breakdown of 1-year outcomes – freedom from PR, stenosis, and interventions – by device: 95.1% for TPV22; 89.7% for mTPV25; and 73.3% for the discontinued cTPV25.
Although the valve is indicated for adolescents and adults, most of the patients in the three studies were adults, with an average weight of 165 pounds (75 kg) who have had PR for decades, said Dr. Levi, an interventional pediatric cardiologist at the University of California, Los Angeles. “With a device like this we are hopefully shifting to treating that a little bit earlier, but fortunately we don’t usually need to treat it before puberty.” The 25-mm TPV gives “a really nice landing zone” for future valve placement. “The goal is to keep patients out of the operating room for at least a few decades if not their whole lives,” he said.
Dr. Levi said the Harmony investigators will follow outcomes with the 22- and modified 25-mm Harmony valves, both of which remain commercially available, out to 10 years.
The study represents the first collective cohort evaluating the Harmony device across the early feasibility, continued access and pivotal studies, said Brian Morray, MD. “It’s important that people understand that evolution and how that impacts the way we look at outcomes, because when you aggregate the data, particularly for the TPV25, some of the procedural outcomes and the adverse events are no longer really reflective in the current time frame.”
These Harmony results “represent another big step in the evolution of interventional cardiology and will be up there with development of the Melody valve and the utility and the use of the Sapien valve in the pulmonary position,” said Dr. Morray, an associate professor of pediatrics at the University of Washington, Seattle, and an interventional cardiologist at Seattle Children’s Hospital.
Dr. Levi disclosed he is a consultant to Medtronic and Edwards Lifesciences. Dr. Morray disclosed he is a clinical proctor for Abbott and a consultant to Medtronic, but not for the Harmony device.
The 1-year results of the Harmony transcatheter pulmonary valve to treat severe pulmonary regurgitation have shown a high rate of eliminating or reducing the degree of symptoms as well as freedom from endocarditis, sustained ventricular tachycardia, and the need for further interventions.
“Simply put, the good news is no endocarditis,” said Daniel S. Levi, MD, in presenting results from three different studies with 108 patients who received three different iterations of the device at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“Endocarditis has been an issue for us in the pulmonary position; we have yet to have an endocarditis in these patients in 1 year,” he stressed.
The studies evaluated three different versions of the Harmony valve: TPV22 (42 patients), the first version with a 22-mm diameter; the Clinical TPV25 (17 patients), the first iteration of a 25 mm–wide device that has since been discontinued; and the modified TPV25 (45 patients), the second version of the 25-mm valve. The three studies are the early feasibility study of the TPV22, the continued-access study of the TPV22 and the mTPV25, and the pivotal study that included all three versions.
At baseline, 89% of patients had severe and 11% had moderate pulmonary regurgitation (PR). At 1 year, 92% had none or trace PR, 3% had mild PR, and 4% moderate disease.
Dr. Levi said the device “speaks for itself” in the results he presented. They include no deaths, no heart attacks, and no pulmonary thromboembolism. Other key outcomes include:
- One major stent fracture in one of the early feasibility study patients at 1-month follow-up.
- Four explants, with two in the discontinued cTPV25 and two with the TPV22 in the early-feasibility study.
- Four reinterventions, two with the discontinued cTPV25 and two valve-in-valve procedures with the mTPV25 in the continued-access study, one with stent placement in the right ventricular outflow tract.
Dr. Levi and coinvestigators also performed a breakdown of 1-year outcomes – freedom from PR, stenosis, and interventions – by device: 95.1% for TPV22; 89.7% for mTPV25; and 73.3% for the discontinued cTPV25.
Although the valve is indicated for adolescents and adults, most of the patients in the three studies were adults, with an average weight of 165 pounds (75 kg) who have had PR for decades, said Dr. Levi, an interventional pediatric cardiologist at the University of California, Los Angeles. “With a device like this we are hopefully shifting to treating that a little bit earlier, but fortunately we don’t usually need to treat it before puberty.” The 25-mm TPV gives “a really nice landing zone” for future valve placement. “The goal is to keep patients out of the operating room for at least a few decades if not their whole lives,” he said.
Dr. Levi said the Harmony investigators will follow outcomes with the 22- and modified 25-mm Harmony valves, both of which remain commercially available, out to 10 years.
The study represents the first collective cohort evaluating the Harmony device across the early feasibility, continued access and pivotal studies, said Brian Morray, MD. “It’s important that people understand that evolution and how that impacts the way we look at outcomes, because when you aggregate the data, particularly for the TPV25, some of the procedural outcomes and the adverse events are no longer really reflective in the current time frame.”
These Harmony results “represent another big step in the evolution of interventional cardiology and will be up there with development of the Melody valve and the utility and the use of the Sapien valve in the pulmonary position,” said Dr. Morray, an associate professor of pediatrics at the University of Washington, Seattle, and an interventional cardiologist at Seattle Children’s Hospital.
Dr. Levi disclosed he is a consultant to Medtronic and Edwards Lifesciences. Dr. Morray disclosed he is a clinical proctor for Abbott and a consultant to Medtronic, but not for the Harmony device.
FROM SCAI 2022
Data concerns mount despite ISCHEMIA substudy correction
A long-standing request to clarify data irregularities in a 2021 ISCHEMIA substudy resulted in the publication of one correction, with a second correction in the works.
Further, the lone cardiac surgeon on the ISCHEMIA trial steering committee, T. Bruce Ferguson, MD, has resigned from the committee, citing a series of factors, including an inability to reconcile data in the substudy and two additional ISCHEMIA papers currently under review.
As previously reported, cardiac surgeons Faisal Bakaeen, MD, and Joseph Sabik III, MD, notified the journal Circulation in March that the Dr. Reynolds et al. substudy had inconsistencies between data in the main paper and supplemental tables detailing patients’ coronary artery disease (CAD) and ischemia severity.
The substudy found that CAD severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the landmark trial.
Circulation published a correction for the substudy on May 20, explaining that a “formatting error” resulted in data being incorrectly presented in two supplemental tables. It does not mention the surgeons’ letter to the editor, which can be found by clicking the “Q” icon below the paper.
Dr. Bakaeen, from the Cleveland Clinic, and Dr. Sabik, from University Hospitals Cleveland Medical Center, told this news organization that they submitted a second letter to editor on May 23 stating that “significant discrepancies” persist.
For example, 7.2% of participants (179/2,475) had moderate stenosis in one coronary vessel in the corrected Reynolds paper (Supplemental Tables I and II) versus 23.3% (697/2,986) in the primary ISCHEMIA manuscript published in the New England Journal of Medicine (Table S5).
The number of patients with left main ≥ 50% stenosis is, surprisingly, identical in both manuscripts, at 40, they said, despite the denominator dropping from 3,845 participants in the primary study to 2,475 participants with an evaluable modified Duke Prognostic Index score in the substudy.
The number of participants with previous coronary artery bypass surgery (CABG) is also hard to reconcile between manuscripts and, importantly, the substudy doesn’t distinguish between lesions bypassed with patent grafts and unbypassed grafts or those with occluded grafts.
“The fact that the authors are working on a second correction is appreciated, but with such numerous inconsistencies, at some point you reach the conclusion that an independent review of the data is the right thing to do for such a high-profile study that received over $100 million of National Institutes of Health support,” Dr. Bakaeen said. “No one should be satisfied or happy if there is any shadow of doubt here regarding the accuracy of the data.”
Speaking to this news organization prior to the first correction, lead substudy author Harmony Reynolds, MD, NYU Langone Health, detailed in depth how the formatting glitch inadvertently upgraded the number of diseased vessels and lesion severity in two supplemental tables.
She noted, as does the correction, that the data were correctly reported in the main manuscript tables and figures and in the remainder of the supplement.
Dr. Reynolds also said they’re in the process of preparing the data for “public sharing soon,” including the Duke Prognostic score at all levels. Dr. Reynolds had not responded by the time of this publication to a request for further details or a timeline.
The surgeons’ first letter to the editor was rejected because it was submitted outside the journal’s 6-week window for letters and was posted as a public comment April 18 via the research platform, Remarq.
Dr. Bakaeen said they were told their second letter was rejected because of Circulation’s “long standing policy” not to publish letters to the editor regarding manuscript corrections but that a correction is being issued.
Circulation editor-in-chief Joseph A. Hill, MD, PhD, UT Southwestern Medical Center, Dallas, said via email that the journal will update its online policies to more clearly state its requirements for publication and that it has been fully transparent with Dr. Bakaeen and Dr. Sabik regarding where it is in the current process.
He confirmed the surgeons were told June 1 that “after additional review, the authors have determined that whereas there are no errors, an additional minor correction is warranted to clarify the description of the study population and sample size. This correction will be published soon.”
Dr. Hill thanked Dr. Bakaeen and Dr. Sabik for bringing the matter to their attention and said, “It is also important to note that both updates to the Dr. Reynolds et al. paper are published as corrections. However, the results and conclusions of the paper remain unchanged.”
The bigger issue
Importantly, the recent AHA/ACC/SCAI coronary revascularization guidelines used ISCHEMIA data to support downgrading the CABG recommendation from class 1 to class 2B in 3-vessel CAD with normal left ventricular function and from class 1 to 2a in 3-vessel CAD with mild to moderate left ventricular dysfunction.
The trial reported no significant benefit with an initial invasive strategy over medical therapy in stable patients with moderate or severe CAD. European guidelines, however, give CABG a class I recommendation for severe three- or two-vessel disease with proximal left anterior descending (LAD) involvement.
Dr. Sabik and Dr. Bakaeen say patients with severe three- or two-vessel disease with proximal LAD involvement were underrepresented in the randomized trials cited by the guidelines but are the typical CABG patients in modern-day practice.
“That is why it is important to determine the severity of CAD accurately and definitively in ISCHEMIA,” Dr. Bakaeen said. “But the more we look at the data, the more errors we encounter.”
Two U.S. surgical groups that were part of the writing process withdrew support for the revascularization guidelines, as did several international surgical societies, citing the data used to support the changes as well as the makeup of the writing committee.
Dr. Ferguson, now with the medical device manufacturer Perfusio, said he resigned from the ISCHEMIA steering committee on May 8 after being unable to accurately reconcile the ISCHEMIA surgical subset data with the Reynolds substudy and two other ISCHEMIA papers on the CABG subset. At least one of those papers, he noted, was being hurriedly pushed through the review process to counter concerns raised by surgeons regarding interpretation of ISCHEMIA.
“This is the first time in my lengthy career in medicine where a level of political agendaism was actually driving the truck,” he said. “It was appalling to me, and I would have said that if I was an interventional cardiologist looking at the results.”
ISCHEMIA results have also been touted as representing state-of-the-art care around the world, but that didn’t appear to be the case for the surgical subset where, for example, China and India performed most CABGs off pump, and globally there was considerable variation in how surgeons approached surgical revascularization strategies, Dr. Ferguson said. “Whether this variability might impact the guideline discussion and these papers coming out remains to be determined.”
He noted that the study protocol allowed for the ISCHEMIA investigators to evaluate whether the variability in the surgical subset influenced the results by comparing the data to that in the Society of Thoracic Surgeons registry, but this option was never acted upon despite being brought to their attention.
“Something political between 2020 and 2022 has crept into the ISCHEMIA trial mindset gestalt, and I don’t like it,” Dr. Ferguson said. “And this can have enormous consequences.”
Asked whether their letters to Circulation are being used to undermine confidence in the ISCHEMIA findings, Dr. Sabik replied, “It is not about undermining ISCHEMIA, but understanding how applicable ISCHEMIA is to patients having CABG today. Understanding the severity of the CAD in patients enrolled in ISCHEMIA is, therefore, necessary.”
“The authors and Circulation have admitted to errors,” he said. “We want to be sure we understand how severe the errors are.”
“This is just about accuracy in a manuscript that may affect patient treatment and therefore patient lives. We want to make sure it is correct,” Dr. Sabik added.
A version of this article first appeared on Medscape.com.
A long-standing request to clarify data irregularities in a 2021 ISCHEMIA substudy resulted in the publication of one correction, with a second correction in the works.
Further, the lone cardiac surgeon on the ISCHEMIA trial steering committee, T. Bruce Ferguson, MD, has resigned from the committee, citing a series of factors, including an inability to reconcile data in the substudy and two additional ISCHEMIA papers currently under review.
As previously reported, cardiac surgeons Faisal Bakaeen, MD, and Joseph Sabik III, MD, notified the journal Circulation in March that the Dr. Reynolds et al. substudy had inconsistencies between data in the main paper and supplemental tables detailing patients’ coronary artery disease (CAD) and ischemia severity.
The substudy found that CAD severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the landmark trial.
Circulation published a correction for the substudy on May 20, explaining that a “formatting error” resulted in data being incorrectly presented in two supplemental tables. It does not mention the surgeons’ letter to the editor, which can be found by clicking the “Q” icon below the paper.
Dr. Bakaeen, from the Cleveland Clinic, and Dr. Sabik, from University Hospitals Cleveland Medical Center, told this news organization that they submitted a second letter to editor on May 23 stating that “significant discrepancies” persist.
For example, 7.2% of participants (179/2,475) had moderate stenosis in one coronary vessel in the corrected Reynolds paper (Supplemental Tables I and II) versus 23.3% (697/2,986) in the primary ISCHEMIA manuscript published in the New England Journal of Medicine (Table S5).
The number of patients with left main ≥ 50% stenosis is, surprisingly, identical in both manuscripts, at 40, they said, despite the denominator dropping from 3,845 participants in the primary study to 2,475 participants with an evaluable modified Duke Prognostic Index score in the substudy.
The number of participants with previous coronary artery bypass surgery (CABG) is also hard to reconcile between manuscripts and, importantly, the substudy doesn’t distinguish between lesions bypassed with patent grafts and unbypassed grafts or those with occluded grafts.
“The fact that the authors are working on a second correction is appreciated, but with such numerous inconsistencies, at some point you reach the conclusion that an independent review of the data is the right thing to do for such a high-profile study that received over $100 million of National Institutes of Health support,” Dr. Bakaeen said. “No one should be satisfied or happy if there is any shadow of doubt here regarding the accuracy of the data.”
Speaking to this news organization prior to the first correction, lead substudy author Harmony Reynolds, MD, NYU Langone Health, detailed in depth how the formatting glitch inadvertently upgraded the number of diseased vessels and lesion severity in two supplemental tables.
She noted, as does the correction, that the data were correctly reported in the main manuscript tables and figures and in the remainder of the supplement.
Dr. Reynolds also said they’re in the process of preparing the data for “public sharing soon,” including the Duke Prognostic score at all levels. Dr. Reynolds had not responded by the time of this publication to a request for further details or a timeline.
The surgeons’ first letter to the editor was rejected because it was submitted outside the journal’s 6-week window for letters and was posted as a public comment April 18 via the research platform, Remarq.
Dr. Bakaeen said they were told their second letter was rejected because of Circulation’s “long standing policy” not to publish letters to the editor regarding manuscript corrections but that a correction is being issued.
Circulation editor-in-chief Joseph A. Hill, MD, PhD, UT Southwestern Medical Center, Dallas, said via email that the journal will update its online policies to more clearly state its requirements for publication and that it has been fully transparent with Dr. Bakaeen and Dr. Sabik regarding where it is in the current process.
He confirmed the surgeons were told June 1 that “after additional review, the authors have determined that whereas there are no errors, an additional minor correction is warranted to clarify the description of the study population and sample size. This correction will be published soon.”
Dr. Hill thanked Dr. Bakaeen and Dr. Sabik for bringing the matter to their attention and said, “It is also important to note that both updates to the Dr. Reynolds et al. paper are published as corrections. However, the results and conclusions of the paper remain unchanged.”
The bigger issue
Importantly, the recent AHA/ACC/SCAI coronary revascularization guidelines used ISCHEMIA data to support downgrading the CABG recommendation from class 1 to class 2B in 3-vessel CAD with normal left ventricular function and from class 1 to 2a in 3-vessel CAD with mild to moderate left ventricular dysfunction.
The trial reported no significant benefit with an initial invasive strategy over medical therapy in stable patients with moderate or severe CAD. European guidelines, however, give CABG a class I recommendation for severe three- or two-vessel disease with proximal left anterior descending (LAD) involvement.
Dr. Sabik and Dr. Bakaeen say patients with severe three- or two-vessel disease with proximal LAD involvement were underrepresented in the randomized trials cited by the guidelines but are the typical CABG patients in modern-day practice.
“That is why it is important to determine the severity of CAD accurately and definitively in ISCHEMIA,” Dr. Bakaeen said. “But the more we look at the data, the more errors we encounter.”
Two U.S. surgical groups that were part of the writing process withdrew support for the revascularization guidelines, as did several international surgical societies, citing the data used to support the changes as well as the makeup of the writing committee.
Dr. Ferguson, now with the medical device manufacturer Perfusio, said he resigned from the ISCHEMIA steering committee on May 8 after being unable to accurately reconcile the ISCHEMIA surgical subset data with the Reynolds substudy and two other ISCHEMIA papers on the CABG subset. At least one of those papers, he noted, was being hurriedly pushed through the review process to counter concerns raised by surgeons regarding interpretation of ISCHEMIA.
“This is the first time in my lengthy career in medicine where a level of political agendaism was actually driving the truck,” he said. “It was appalling to me, and I would have said that if I was an interventional cardiologist looking at the results.”
ISCHEMIA results have also been touted as representing state-of-the-art care around the world, but that didn’t appear to be the case for the surgical subset where, for example, China and India performed most CABGs off pump, and globally there was considerable variation in how surgeons approached surgical revascularization strategies, Dr. Ferguson said. “Whether this variability might impact the guideline discussion and these papers coming out remains to be determined.”
He noted that the study protocol allowed for the ISCHEMIA investigators to evaluate whether the variability in the surgical subset influenced the results by comparing the data to that in the Society of Thoracic Surgeons registry, but this option was never acted upon despite being brought to their attention.
“Something political between 2020 and 2022 has crept into the ISCHEMIA trial mindset gestalt, and I don’t like it,” Dr. Ferguson said. “And this can have enormous consequences.”
Asked whether their letters to Circulation are being used to undermine confidence in the ISCHEMIA findings, Dr. Sabik replied, “It is not about undermining ISCHEMIA, but understanding how applicable ISCHEMIA is to patients having CABG today. Understanding the severity of the CAD in patients enrolled in ISCHEMIA is, therefore, necessary.”
“The authors and Circulation have admitted to errors,” he said. “We want to be sure we understand how severe the errors are.”
“This is just about accuracy in a manuscript that may affect patient treatment and therefore patient lives. We want to make sure it is correct,” Dr. Sabik added.
A version of this article first appeared on Medscape.com.
A long-standing request to clarify data irregularities in a 2021 ISCHEMIA substudy resulted in the publication of one correction, with a second correction in the works.
Further, the lone cardiac surgeon on the ISCHEMIA trial steering committee, T. Bruce Ferguson, MD, has resigned from the committee, citing a series of factors, including an inability to reconcile data in the substudy and two additional ISCHEMIA papers currently under review.
As previously reported, cardiac surgeons Faisal Bakaeen, MD, and Joseph Sabik III, MD, notified the journal Circulation in March that the Dr. Reynolds et al. substudy had inconsistencies between data in the main paper and supplemental tables detailing patients’ coronary artery disease (CAD) and ischemia severity.
The substudy found that CAD severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the landmark trial.
Circulation published a correction for the substudy on May 20, explaining that a “formatting error” resulted in data being incorrectly presented in two supplemental tables. It does not mention the surgeons’ letter to the editor, which can be found by clicking the “Q” icon below the paper.
Dr. Bakaeen, from the Cleveland Clinic, and Dr. Sabik, from University Hospitals Cleveland Medical Center, told this news organization that they submitted a second letter to editor on May 23 stating that “significant discrepancies” persist.
For example, 7.2% of participants (179/2,475) had moderate stenosis in one coronary vessel in the corrected Reynolds paper (Supplemental Tables I and II) versus 23.3% (697/2,986) in the primary ISCHEMIA manuscript published in the New England Journal of Medicine (Table S5).
The number of patients with left main ≥ 50% stenosis is, surprisingly, identical in both manuscripts, at 40, they said, despite the denominator dropping from 3,845 participants in the primary study to 2,475 participants with an evaluable modified Duke Prognostic Index score in the substudy.
The number of participants with previous coronary artery bypass surgery (CABG) is also hard to reconcile between manuscripts and, importantly, the substudy doesn’t distinguish between lesions bypassed with patent grafts and unbypassed grafts or those with occluded grafts.
“The fact that the authors are working on a second correction is appreciated, but with such numerous inconsistencies, at some point you reach the conclusion that an independent review of the data is the right thing to do for such a high-profile study that received over $100 million of National Institutes of Health support,” Dr. Bakaeen said. “No one should be satisfied or happy if there is any shadow of doubt here regarding the accuracy of the data.”
Speaking to this news organization prior to the first correction, lead substudy author Harmony Reynolds, MD, NYU Langone Health, detailed in depth how the formatting glitch inadvertently upgraded the number of diseased vessels and lesion severity in two supplemental tables.
She noted, as does the correction, that the data were correctly reported in the main manuscript tables and figures and in the remainder of the supplement.
Dr. Reynolds also said they’re in the process of preparing the data for “public sharing soon,” including the Duke Prognostic score at all levels. Dr. Reynolds had not responded by the time of this publication to a request for further details or a timeline.
The surgeons’ first letter to the editor was rejected because it was submitted outside the journal’s 6-week window for letters and was posted as a public comment April 18 via the research platform, Remarq.
Dr. Bakaeen said they were told their second letter was rejected because of Circulation’s “long standing policy” not to publish letters to the editor regarding manuscript corrections but that a correction is being issued.
Circulation editor-in-chief Joseph A. Hill, MD, PhD, UT Southwestern Medical Center, Dallas, said via email that the journal will update its online policies to more clearly state its requirements for publication and that it has been fully transparent with Dr. Bakaeen and Dr. Sabik regarding where it is in the current process.
He confirmed the surgeons were told June 1 that “after additional review, the authors have determined that whereas there are no errors, an additional minor correction is warranted to clarify the description of the study population and sample size. This correction will be published soon.”
Dr. Hill thanked Dr. Bakaeen and Dr. Sabik for bringing the matter to their attention and said, “It is also important to note that both updates to the Dr. Reynolds et al. paper are published as corrections. However, the results and conclusions of the paper remain unchanged.”
The bigger issue
Importantly, the recent AHA/ACC/SCAI coronary revascularization guidelines used ISCHEMIA data to support downgrading the CABG recommendation from class 1 to class 2B in 3-vessel CAD with normal left ventricular function and from class 1 to 2a in 3-vessel CAD with mild to moderate left ventricular dysfunction.
The trial reported no significant benefit with an initial invasive strategy over medical therapy in stable patients with moderate or severe CAD. European guidelines, however, give CABG a class I recommendation for severe three- or two-vessel disease with proximal left anterior descending (LAD) involvement.
Dr. Sabik and Dr. Bakaeen say patients with severe three- or two-vessel disease with proximal LAD involvement were underrepresented in the randomized trials cited by the guidelines but are the typical CABG patients in modern-day practice.
“That is why it is important to determine the severity of CAD accurately and definitively in ISCHEMIA,” Dr. Bakaeen said. “But the more we look at the data, the more errors we encounter.”
Two U.S. surgical groups that were part of the writing process withdrew support for the revascularization guidelines, as did several international surgical societies, citing the data used to support the changes as well as the makeup of the writing committee.
Dr. Ferguson, now with the medical device manufacturer Perfusio, said he resigned from the ISCHEMIA steering committee on May 8 after being unable to accurately reconcile the ISCHEMIA surgical subset data with the Reynolds substudy and two other ISCHEMIA papers on the CABG subset. At least one of those papers, he noted, was being hurriedly pushed through the review process to counter concerns raised by surgeons regarding interpretation of ISCHEMIA.
“This is the first time in my lengthy career in medicine where a level of political agendaism was actually driving the truck,” he said. “It was appalling to me, and I would have said that if I was an interventional cardiologist looking at the results.”
ISCHEMIA results have also been touted as representing state-of-the-art care around the world, but that didn’t appear to be the case for the surgical subset where, for example, China and India performed most CABGs off pump, and globally there was considerable variation in how surgeons approached surgical revascularization strategies, Dr. Ferguson said. “Whether this variability might impact the guideline discussion and these papers coming out remains to be determined.”
He noted that the study protocol allowed for the ISCHEMIA investigators to evaluate whether the variability in the surgical subset influenced the results by comparing the data to that in the Society of Thoracic Surgeons registry, but this option was never acted upon despite being brought to their attention.
“Something political between 2020 and 2022 has crept into the ISCHEMIA trial mindset gestalt, and I don’t like it,” Dr. Ferguson said. “And this can have enormous consequences.”
Asked whether their letters to Circulation are being used to undermine confidence in the ISCHEMIA findings, Dr. Sabik replied, “It is not about undermining ISCHEMIA, but understanding how applicable ISCHEMIA is to patients having CABG today. Understanding the severity of the CAD in patients enrolled in ISCHEMIA is, therefore, necessary.”
“The authors and Circulation have admitted to errors,” he said. “We want to be sure we understand how severe the errors are.”
“This is just about accuracy in a manuscript that may affect patient treatment and therefore patient lives. We want to make sure it is correct,” Dr. Sabik added.
A version of this article first appeared on Medscape.com.
CTO PCI success rates rising, with blip during COVID-19, registry shows
Technical and procedural success rates for chronic total occlusion percutaneous coronary intervention (CTO PCI) have increased steadily over the past 6 years, with rates of in-hospital major adverse cardiac events (MACE) declining to the 2%-or-lower range in that time.
“CTO PCI technical and procedural success rates are high and continue to increase over time,” Spyridon Kostantinis, MD said in presenting updated results from the international PROGRESS-CTO registry at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“The overall success rate increased from 81.6% in 2018 to 88.1% in 2021,” he added. The overall incidence of in-hospital MACE in that time was “an acceptable” 2.1% without significant changes over that period.
The analysis examined clinical, angiographic and procedural outcomes of 10,249 CTO PCIs performed on 10,019 patients from 63 centers in nine countries during 2016-2021. PROGRESS-CTO stands for Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
The target CTOs were highly complex, he said, with an average J-CTO (multicenter CTO registry in Japan) score of 2.4 ± 1.3 and PROGRESS-CTO score of 1.3 ± 1. The most common CTO target vessel was the right coronary artery (53%), followed by the left anterior descending artery (26%) and the circumflex artery (19%).
The registry also tracked how characteristics of the CTO PCI procedures themselves changed over time. “The septal and the epicardial collaterals were the most common collaterals used for retrograde crossing, with a decreasing trend for epicardial collaterals over time,” said Dr. Kostantinis, a research fellow at the Minneapolis Heart Institute.
Septal collateral use varied between 64% and 69% of cases from 2016 to 2021, but the share of epicardial collaterals declined from 35% to 22% in that time.
“Over time, the range of antegrade wiring as the final successfully crossing strategy increased from 46% in 2016 to 61% in 2021, with a decrease in antegrade dissection and re-entry (ADR) and no change in the retrograde approach,” Dr. Kostantinis said. The percentage of procedures using ADR as the final crossing strategy declined from 18% in 2016 to 12% in 2021, with the rate of retrograde crossings peaking at 21% in 2016 but leveling off to 18% or 19% in the subsequent years.
“An increasing use in the efficiency of antegrade wiring may reflect an improvement in guidewire retrograde crossing as well as the increasing operator expertise,” Dr. Kostantinis said.
The study also found that contrast volume, air kerma radiation dose, fluoroscopy time, and procedure time declined steadily over time. “The potential explanations for these are using new x-ray systems as well as the use of intravascular imaging,” Dr. Kostantinis said.
In 2020, the rates of technical and procedural success, as well as the number of overall procedures, declined from 2019, while MACE rates ticked upward that year, probably because of the COVID-19 pandemic, Dr. Kostantinis said.
“It is true that we noticed a rise in MACE rate from 1.6% in 2019 to 2.7% in 2020, but in 2021 that decreased again to 1.7%,” he said in an interview. “Another potential explanation is the higher angiographic complexity of CTOs treated during that year (2020) that resulted in more adverse events.”
Previous results from the PROGRESS-CTO registry reported the difference in MACE between 2019 and 2020 was significant (P = .01). “So, yes, the difference between those 2 years is significant,” Dr. Kostantinis said. However, he noted, the overall trend was not significant, with a P value of .194.
The risk profile of CTO PCI has improved “slowly” over time, said Kirk N. Garratt, MD, but “it’s not yet were it needs to be.”
He added, “Undoubtedly we’ve learned that, without any question, one method for minimizing the risk is to concentrate these cases in the hands of those that do many of them.” As the number of procedures fell – an “embedded” pandemic impact –“I worry that it’s inevitable that complication rates will tick up a bit,” said Dr. Garratt, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del.
By the same token, he added, this situation with regard to CTOs “parallels what’s happening elsewhere in interventional medicine and medicine broadly; numbers are increasing and we’re busy again. In most domains we’re not as busy as we had been prepandemic, and time will allow us to catch up.”
PROGRESS-CTO has received funding from the Joseph F. and Mary M. Fleischhacker Foundation and the Abbott Northwestern Hospital Foundation Innovation Grant.
Dr. Kostantinis has no disclosures. Dr. Garratt is an advisory board member for Abbott.
Technical and procedural success rates for chronic total occlusion percutaneous coronary intervention (CTO PCI) have increased steadily over the past 6 years, with rates of in-hospital major adverse cardiac events (MACE) declining to the 2%-or-lower range in that time.
“CTO PCI technical and procedural success rates are high and continue to increase over time,” Spyridon Kostantinis, MD said in presenting updated results from the international PROGRESS-CTO registry at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“The overall success rate increased from 81.6% in 2018 to 88.1% in 2021,” he added. The overall incidence of in-hospital MACE in that time was “an acceptable” 2.1% without significant changes over that period.
The analysis examined clinical, angiographic and procedural outcomes of 10,249 CTO PCIs performed on 10,019 patients from 63 centers in nine countries during 2016-2021. PROGRESS-CTO stands for Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
The target CTOs were highly complex, he said, with an average J-CTO (multicenter CTO registry in Japan) score of 2.4 ± 1.3 and PROGRESS-CTO score of 1.3 ± 1. The most common CTO target vessel was the right coronary artery (53%), followed by the left anterior descending artery (26%) and the circumflex artery (19%).
The registry also tracked how characteristics of the CTO PCI procedures themselves changed over time. “The septal and the epicardial collaterals were the most common collaterals used for retrograde crossing, with a decreasing trend for epicardial collaterals over time,” said Dr. Kostantinis, a research fellow at the Minneapolis Heart Institute.
Septal collateral use varied between 64% and 69% of cases from 2016 to 2021, but the share of epicardial collaterals declined from 35% to 22% in that time.
“Over time, the range of antegrade wiring as the final successfully crossing strategy increased from 46% in 2016 to 61% in 2021, with a decrease in antegrade dissection and re-entry (ADR) and no change in the retrograde approach,” Dr. Kostantinis said. The percentage of procedures using ADR as the final crossing strategy declined from 18% in 2016 to 12% in 2021, with the rate of retrograde crossings peaking at 21% in 2016 but leveling off to 18% or 19% in the subsequent years.
“An increasing use in the efficiency of antegrade wiring may reflect an improvement in guidewire retrograde crossing as well as the increasing operator expertise,” Dr. Kostantinis said.
The study also found that contrast volume, air kerma radiation dose, fluoroscopy time, and procedure time declined steadily over time. “The potential explanations for these are using new x-ray systems as well as the use of intravascular imaging,” Dr. Kostantinis said.
In 2020, the rates of technical and procedural success, as well as the number of overall procedures, declined from 2019, while MACE rates ticked upward that year, probably because of the COVID-19 pandemic, Dr. Kostantinis said.
“It is true that we noticed a rise in MACE rate from 1.6% in 2019 to 2.7% in 2020, but in 2021 that decreased again to 1.7%,” he said in an interview. “Another potential explanation is the higher angiographic complexity of CTOs treated during that year (2020) that resulted in more adverse events.”
Previous results from the PROGRESS-CTO registry reported the difference in MACE between 2019 and 2020 was significant (P = .01). “So, yes, the difference between those 2 years is significant,” Dr. Kostantinis said. However, he noted, the overall trend was not significant, with a P value of .194.
The risk profile of CTO PCI has improved “slowly” over time, said Kirk N. Garratt, MD, but “it’s not yet were it needs to be.”
He added, “Undoubtedly we’ve learned that, without any question, one method for minimizing the risk is to concentrate these cases in the hands of those that do many of them.” As the number of procedures fell – an “embedded” pandemic impact –“I worry that it’s inevitable that complication rates will tick up a bit,” said Dr. Garratt, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del.
By the same token, he added, this situation with regard to CTOs “parallels what’s happening elsewhere in interventional medicine and medicine broadly; numbers are increasing and we’re busy again. In most domains we’re not as busy as we had been prepandemic, and time will allow us to catch up.”
PROGRESS-CTO has received funding from the Joseph F. and Mary M. Fleischhacker Foundation and the Abbott Northwestern Hospital Foundation Innovation Grant.
Dr. Kostantinis has no disclosures. Dr. Garratt is an advisory board member for Abbott.
Technical and procedural success rates for chronic total occlusion percutaneous coronary intervention (CTO PCI) have increased steadily over the past 6 years, with rates of in-hospital major adverse cardiac events (MACE) declining to the 2%-or-lower range in that time.
“CTO PCI technical and procedural success rates are high and continue to increase over time,” Spyridon Kostantinis, MD said in presenting updated results from the international PROGRESS-CTO registry at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“The overall success rate increased from 81.6% in 2018 to 88.1% in 2021,” he added. The overall incidence of in-hospital MACE in that time was “an acceptable” 2.1% without significant changes over that period.
The analysis examined clinical, angiographic and procedural outcomes of 10,249 CTO PCIs performed on 10,019 patients from 63 centers in nine countries during 2016-2021. PROGRESS-CTO stands for Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
The target CTOs were highly complex, he said, with an average J-CTO (multicenter CTO registry in Japan) score of 2.4 ± 1.3 and PROGRESS-CTO score of 1.3 ± 1. The most common CTO target vessel was the right coronary artery (53%), followed by the left anterior descending artery (26%) and the circumflex artery (19%).
The registry also tracked how characteristics of the CTO PCI procedures themselves changed over time. “The septal and the epicardial collaterals were the most common collaterals used for retrograde crossing, with a decreasing trend for epicardial collaterals over time,” said Dr. Kostantinis, a research fellow at the Minneapolis Heart Institute.
Septal collateral use varied between 64% and 69% of cases from 2016 to 2021, but the share of epicardial collaterals declined from 35% to 22% in that time.
“Over time, the range of antegrade wiring as the final successfully crossing strategy increased from 46% in 2016 to 61% in 2021, with a decrease in antegrade dissection and re-entry (ADR) and no change in the retrograde approach,” Dr. Kostantinis said. The percentage of procedures using ADR as the final crossing strategy declined from 18% in 2016 to 12% in 2021, with the rate of retrograde crossings peaking at 21% in 2016 but leveling off to 18% or 19% in the subsequent years.
“An increasing use in the efficiency of antegrade wiring may reflect an improvement in guidewire retrograde crossing as well as the increasing operator expertise,” Dr. Kostantinis said.
The study also found that contrast volume, air kerma radiation dose, fluoroscopy time, and procedure time declined steadily over time. “The potential explanations for these are using new x-ray systems as well as the use of intravascular imaging,” Dr. Kostantinis said.
In 2020, the rates of technical and procedural success, as well as the number of overall procedures, declined from 2019, while MACE rates ticked upward that year, probably because of the COVID-19 pandemic, Dr. Kostantinis said.
“It is true that we noticed a rise in MACE rate from 1.6% in 2019 to 2.7% in 2020, but in 2021 that decreased again to 1.7%,” he said in an interview. “Another potential explanation is the higher angiographic complexity of CTOs treated during that year (2020) that resulted in more adverse events.”
Previous results from the PROGRESS-CTO registry reported the difference in MACE between 2019 and 2020 was significant (P = .01). “So, yes, the difference between those 2 years is significant,” Dr. Kostantinis said. However, he noted, the overall trend was not significant, with a P value of .194.
The risk profile of CTO PCI has improved “slowly” over time, said Kirk N. Garratt, MD, but “it’s not yet were it needs to be.”
He added, “Undoubtedly we’ve learned that, without any question, one method for minimizing the risk is to concentrate these cases in the hands of those that do many of them.” As the number of procedures fell – an “embedded” pandemic impact –“I worry that it’s inevitable that complication rates will tick up a bit,” said Dr. Garratt, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del.
By the same token, he added, this situation with regard to CTOs “parallels what’s happening elsewhere in interventional medicine and medicine broadly; numbers are increasing and we’re busy again. In most domains we’re not as busy as we had been prepandemic, and time will allow us to catch up.”
PROGRESS-CTO has received funding from the Joseph F. and Mary M. Fleischhacker Foundation and the Abbott Northwestern Hospital Foundation Innovation Grant.
Dr. Kostantinis has no disclosures. Dr. Garratt is an advisory board member for Abbott.
FROM SCAI 2022
No-implant interatrial shunt remains patent at a year
The first in-human trials of a no-implant approach to interatrial shunting to alleviate heart failure symptoms have shown a signal that the procedure reduces peak exercise wedge pressure in recipients a month afterward, according to early trial results.
Colin M. Barker, MD, reported 30-day results of 31 patients who had no-implant interatrial shunting for heart failure across three studies, at the Society for Cardiovascular Angiography & Interventions scientific sessions. The studies included patients with HF with preserved and reduced ejection fraction (HFpEF and HFrEF).
“At 30 days, there was a response with a decrease in the wedge pressures both at rest and at peak exercise, and that was consistent through all three of these initial trials,” Dr. Barker said. In all 33 patients who have been treated to date, there were no major adverse cardiac and cerebrovascular or thromboembolic events through 1 month. (Two of the patients weren’t included in the results Dr. Barker presented.)
The three studies he reported on were the Alleviate-HF-1 (n = 15), Alleviate-HF-2 (n = 11) for patients with HFpEF, and Alleviate-HFrEF (n = 5). The average patient age was 67 years, and all were New York Heart Association class II, III, or IV with elevated peak pulmonary capillary wedge pressure (PCWP).
The device that creates the no-implant shunt as “not very exotic, but it is very effective, and what it does is create a very predictable, reproducible atrial septostomy” between the left and right atria. The device obtains “almost a biopsy” that’s 7 mm in diameter. “There’s no hardware or foreign bodies left inside the patient,” said Dr. Barker, director of interventional cardiology at Vanderbilt University in Nashville, Tenn. “There’s a natural healing process at the rims after the radiofrequency ablation has been done.” Femoral access was used.
Study participants were also asked to complete the Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline and at 1 and 3 months across all three studies, and at 6 months in the Alleviate-HF-1 study. “Just as important is how patients feel,” Dr. Barker said. KCCQ overall summary scores increased at each time interval across all three studies.
“Durability has been proven with multiple different imaging modalities,” Dr. Barker added, explaining that CT scans in 10 of 10 shunts demonstrated patency through 12 months, and 15 of 15 at 6 months. He noted that none of the created shunts have closed yet. At 6 months, the average shunt measured 7.5 mm (± 1.1 mm, n = 22), left atrial diameter decreased 2.4 mm (P = .031) in HFpEF patients, and no significant changes were observed in right ventricular fractional area change or right atrial volume index.
None of the septostomies have had to be closed or enlarged to date, Dr. Barker said. “We are creating an atrial septal defect that we have a lot of comfort and experience with closing with other devices if need be, but that hasn’t been an issue,” he said. “As of now, it’s one size, but as you can imagine, one-size-fits-all is not the way this will go, and this does allow for variations in size ultimately.”
Kirk N. Garratt, MD, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del., noted that the approach to unload the left atrium “is novel, but I think is becoming well accepted in the advanced HF population. There remain questions about long-term consequences of an intentional interatrial shunt – what happens to pulmonary flow dynamics and the like – but to date the impact of this approach has been favorable.
“The liabilities that come with an implanted device in the septal space, both in terms of the durability of the shunt and the impact that it would have on the ability to perform other transseptal procedures, is overcome with this approach,” he added.
Dr. Barker disclosed he is an advisory board member and consultant to Alleviant Medical. Dr. Garratt is an advisory board member for Abbott.
The first in-human trials of a no-implant approach to interatrial shunting to alleviate heart failure symptoms have shown a signal that the procedure reduces peak exercise wedge pressure in recipients a month afterward, according to early trial results.
Colin M. Barker, MD, reported 30-day results of 31 patients who had no-implant interatrial shunting for heart failure across three studies, at the Society for Cardiovascular Angiography & Interventions scientific sessions. The studies included patients with HF with preserved and reduced ejection fraction (HFpEF and HFrEF).
“At 30 days, there was a response with a decrease in the wedge pressures both at rest and at peak exercise, and that was consistent through all three of these initial trials,” Dr. Barker said. In all 33 patients who have been treated to date, there were no major adverse cardiac and cerebrovascular or thromboembolic events through 1 month. (Two of the patients weren’t included in the results Dr. Barker presented.)
The three studies he reported on were the Alleviate-HF-1 (n = 15), Alleviate-HF-2 (n = 11) for patients with HFpEF, and Alleviate-HFrEF (n = 5). The average patient age was 67 years, and all were New York Heart Association class II, III, or IV with elevated peak pulmonary capillary wedge pressure (PCWP).
The device that creates the no-implant shunt as “not very exotic, but it is very effective, and what it does is create a very predictable, reproducible atrial septostomy” between the left and right atria. The device obtains “almost a biopsy” that’s 7 mm in diameter. “There’s no hardware or foreign bodies left inside the patient,” said Dr. Barker, director of interventional cardiology at Vanderbilt University in Nashville, Tenn. “There’s a natural healing process at the rims after the radiofrequency ablation has been done.” Femoral access was used.
Study participants were also asked to complete the Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline and at 1 and 3 months across all three studies, and at 6 months in the Alleviate-HF-1 study. “Just as important is how patients feel,” Dr. Barker said. KCCQ overall summary scores increased at each time interval across all three studies.
“Durability has been proven with multiple different imaging modalities,” Dr. Barker added, explaining that CT scans in 10 of 10 shunts demonstrated patency through 12 months, and 15 of 15 at 6 months. He noted that none of the created shunts have closed yet. At 6 months, the average shunt measured 7.5 mm (± 1.1 mm, n = 22), left atrial diameter decreased 2.4 mm (P = .031) in HFpEF patients, and no significant changes were observed in right ventricular fractional area change or right atrial volume index.
None of the septostomies have had to be closed or enlarged to date, Dr. Barker said. “We are creating an atrial septal defect that we have a lot of comfort and experience with closing with other devices if need be, but that hasn’t been an issue,” he said. “As of now, it’s one size, but as you can imagine, one-size-fits-all is not the way this will go, and this does allow for variations in size ultimately.”
Kirk N. Garratt, MD, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del., noted that the approach to unload the left atrium “is novel, but I think is becoming well accepted in the advanced HF population. There remain questions about long-term consequences of an intentional interatrial shunt – what happens to pulmonary flow dynamics and the like – but to date the impact of this approach has been favorable.
“The liabilities that come with an implanted device in the septal space, both in terms of the durability of the shunt and the impact that it would have on the ability to perform other transseptal procedures, is overcome with this approach,” he added.
Dr. Barker disclosed he is an advisory board member and consultant to Alleviant Medical. Dr. Garratt is an advisory board member for Abbott.
The first in-human trials of a no-implant approach to interatrial shunting to alleviate heart failure symptoms have shown a signal that the procedure reduces peak exercise wedge pressure in recipients a month afterward, according to early trial results.
Colin M. Barker, MD, reported 30-day results of 31 patients who had no-implant interatrial shunting for heart failure across three studies, at the Society for Cardiovascular Angiography & Interventions scientific sessions. The studies included patients with HF with preserved and reduced ejection fraction (HFpEF and HFrEF).
“At 30 days, there was a response with a decrease in the wedge pressures both at rest and at peak exercise, and that was consistent through all three of these initial trials,” Dr. Barker said. In all 33 patients who have been treated to date, there were no major adverse cardiac and cerebrovascular or thromboembolic events through 1 month. (Two of the patients weren’t included in the results Dr. Barker presented.)
The three studies he reported on were the Alleviate-HF-1 (n = 15), Alleviate-HF-2 (n = 11) for patients with HFpEF, and Alleviate-HFrEF (n = 5). The average patient age was 67 years, and all were New York Heart Association class II, III, or IV with elevated peak pulmonary capillary wedge pressure (PCWP).
The device that creates the no-implant shunt as “not very exotic, but it is very effective, and what it does is create a very predictable, reproducible atrial septostomy” between the left and right atria. The device obtains “almost a biopsy” that’s 7 mm in diameter. “There’s no hardware or foreign bodies left inside the patient,” said Dr. Barker, director of interventional cardiology at Vanderbilt University in Nashville, Tenn. “There’s a natural healing process at the rims after the radiofrequency ablation has been done.” Femoral access was used.
Study participants were also asked to complete the Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline and at 1 and 3 months across all three studies, and at 6 months in the Alleviate-HF-1 study. “Just as important is how patients feel,” Dr. Barker said. KCCQ overall summary scores increased at each time interval across all three studies.
“Durability has been proven with multiple different imaging modalities,” Dr. Barker added, explaining that CT scans in 10 of 10 shunts demonstrated patency through 12 months, and 15 of 15 at 6 months. He noted that none of the created shunts have closed yet. At 6 months, the average shunt measured 7.5 mm (± 1.1 mm, n = 22), left atrial diameter decreased 2.4 mm (P = .031) in HFpEF patients, and no significant changes were observed in right ventricular fractional area change or right atrial volume index.
None of the septostomies have had to be closed or enlarged to date, Dr. Barker said. “We are creating an atrial septal defect that we have a lot of comfort and experience with closing with other devices if need be, but that hasn’t been an issue,” he said. “As of now, it’s one size, but as you can imagine, one-size-fits-all is not the way this will go, and this does allow for variations in size ultimately.”
Kirk N. Garratt, MD, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del., noted that the approach to unload the left atrium “is novel, but I think is becoming well accepted in the advanced HF population. There remain questions about long-term consequences of an intentional interatrial shunt – what happens to pulmonary flow dynamics and the like – but to date the impact of this approach has been favorable.
“The liabilities that come with an implanted device in the septal space, both in terms of the durability of the shunt and the impact that it would have on the ability to perform other transseptal procedures, is overcome with this approach,” he added.
Dr. Barker disclosed he is an advisory board member and consultant to Alleviant Medical. Dr. Garratt is an advisory board member for Abbott.
FROM SCAI 2022