User login
IVC filter complications common, retrieval rare
CHICAGO – Penetration of the inferior vena cava and adjacent organs occurred with 46% of IVC filters placed among 262 patients, an award-winning analysis shows.
Grade 2 or 3 penetration was significantly associated with filter type (49% temporary vs. 5.3% permanent; P = .0001) and length of time in place (18.2% less than 30 days vs. 57.3% 30 days or more; P less than .0001).
"The majority of filters were placed for prophylaxis or relative indications and were temporary," Dr. Michael Go said at the annual meeting of the Midwestern Vascular Surgical Society.
The filter penetrated the aorta in 12 cases; duodenum in 26; and spine, colon, or kidney in 6; and simultaneously penetrated two organs in 7. Another 100 filters had struts immediately adjacent to the external aspect of the IVC, possibly indicating tenting of the cava.
Only 1.6% of temporary filters, however, were retrieved during the 3-year study period, he said.
A filter retrieval rate between 1.2% and 5.1% was cited in a recent Medicare data analysis, which reported an alarming 111% increase in the rate of IVC filter placement from 1999 through 2008 (J. Am. Coll. Radio. 2011;8:483-9).
"IVC filter placement is an epidemic because of the ever-increasing number of risk factors being identified for VTE [venous thromboembolism]," said Dr. Go, with the division of vascular diseases and surgery, Ohio State University Medical Center, Columbus.
Other culprits behind the explosive growth of IVC filters are that angiography suites and catheter labs are now commonplace, the skill needed to insert a filter is easily disseminated, and temporary filters have decreased the threshold for placement.
"Concern over filter complications is increasing, as are referrals for removal, but little long-term data exist," he remarked.
A total of 591 patients had an IVC filter placed at Ohio State University between January 2006 and December 2009, with an adequate postfilter computed tomography (CT) scan available in 262. CT findings were graded, based on a modified, previously published scale (J. Vasc. Interv. Radiol. 2011;22:70-4), ranging from 0 (struts confined entirely within the IVC) to 3 (strut interacts with aorta, duodenum, or other organs).
Indications for filter placement were prophylaxis in 16.4% and VTE in 83.6%. Among the filters placed for VTE, 44.7% were for absolute indications (inability/failure of anticoagulation) and 55.3% for relative indications.
Grade 0 penetration occurred in 42 filters, grade 1 in 100, grade 2 in 83, and grade 3 in 37, Dr. Go said.
Grade 2 or 3 penetration was present in 44.6% of Tulip filters, 74.4% of Celect, 5.3% of Greenfield, and 0% of Optease (P = .0000), according to the analysis, which won this year’s Pfeifer Venous Award from the society.
There was a trend toward increased grade 2 or 3 penetration with uniconical filters vs. biconical filters (46.7% vs. 0%; P = .0645).
In all, 32 patients sought clinical follow-up for abdominal or back pain, but none were conclusively tied to filter problems or penetration.
"It remains unclear if most penetrations caused clinically significant problems," he said. "Monitoring of penetrations with CT, or some other follow-up, may be important to understand the natural history of this condition."
Audience members remarked that the retrieval rate in the series was extremely low and asked whether efforts, such as percutaneous retrieval, were being undertaken.
"We have attempted to remove some of these filters, sometimes successfully, sometimes not," Dr. Go responded. "As far as our retrieval rate, I agree 1.6% is dismal. Vascular surgery put in 19% of the filters. We don’t have a specific protocol in place, other than hyperawareness amongst all of us partners about who has a filter in place and to bring them back when appropriate."
He also observed that even when retrieval is undertaken, technical failure rates are high at about 8.5% in the recent literature (Eur. J. Vasc. Endovasc. Surg. 2013;46:353-9).
Dr. Go and his coauthors reported no financial disclosures.
The results of this study speak for themselves – only a very small proportion of temporary filters are actually removed, and penetration of the inferior vena cava and adjacent structures by filter components is relatively common. It is particularly noteworthy that penetration was significantly more common with temporary filters vs. permanent filters, and the risk of penetration increased with time in place. These observations alone should inspire efforts to remove temporary filters as soon as clinically possible. The data presented also indicate that a large proportion of filters are being placed for prophylaxis or relative indications, suggesting that current evidence-based guidelines are not being followed.
However, in spite of the high observed penetration rate, this study does not provide much information on the clinical significance of this finding. In addition, this study represents a highly selected patient population, since a postfilter CT scan was available on less than half of all patients who received inferior vena cava filters during the study period. So while these results may not be completely generalizable, they certainly suggest that the majority of temporary filters become permanent filters, and a critical reappraisal of filter use is warranted.
Dr. R. Eugene Zierler is a professor of surgery at the University of Washington, Seattle, and an associate medical editor of Vascular Specialist.
The results of this study speak for themselves – only a very small proportion of temporary filters are actually removed, and penetration of the inferior vena cava and adjacent structures by filter components is relatively common. It is particularly noteworthy that penetration was significantly more common with temporary filters vs. permanent filters, and the risk of penetration increased with time in place. These observations alone should inspire efforts to remove temporary filters as soon as clinically possible. The data presented also indicate that a large proportion of filters are being placed for prophylaxis or relative indications, suggesting that current evidence-based guidelines are not being followed.
However, in spite of the high observed penetration rate, this study does not provide much information on the clinical significance of this finding. In addition, this study represents a highly selected patient population, since a postfilter CT scan was available on less than half of all patients who received inferior vena cava filters during the study period. So while these results may not be completely generalizable, they certainly suggest that the majority of temporary filters become permanent filters, and a critical reappraisal of filter use is warranted.
Dr. R. Eugene Zierler is a professor of surgery at the University of Washington, Seattle, and an associate medical editor of Vascular Specialist.
The results of this study speak for themselves – only a very small proportion of temporary filters are actually removed, and penetration of the inferior vena cava and adjacent structures by filter components is relatively common. It is particularly noteworthy that penetration was significantly more common with temporary filters vs. permanent filters, and the risk of penetration increased with time in place. These observations alone should inspire efforts to remove temporary filters as soon as clinically possible. The data presented also indicate that a large proportion of filters are being placed for prophylaxis or relative indications, suggesting that current evidence-based guidelines are not being followed.
However, in spite of the high observed penetration rate, this study does not provide much information on the clinical significance of this finding. In addition, this study represents a highly selected patient population, since a postfilter CT scan was available on less than half of all patients who received inferior vena cava filters during the study period. So while these results may not be completely generalizable, they certainly suggest that the majority of temporary filters become permanent filters, and a critical reappraisal of filter use is warranted.
Dr. R. Eugene Zierler is a professor of surgery at the University of Washington, Seattle, and an associate medical editor of Vascular Specialist.
CHICAGO – Penetration of the inferior vena cava and adjacent organs occurred with 46% of IVC filters placed among 262 patients, an award-winning analysis shows.
Grade 2 or 3 penetration was significantly associated with filter type (49% temporary vs. 5.3% permanent; P = .0001) and length of time in place (18.2% less than 30 days vs. 57.3% 30 days or more; P less than .0001).
"The majority of filters were placed for prophylaxis or relative indications and were temporary," Dr. Michael Go said at the annual meeting of the Midwestern Vascular Surgical Society.
The filter penetrated the aorta in 12 cases; duodenum in 26; and spine, colon, or kidney in 6; and simultaneously penetrated two organs in 7. Another 100 filters had struts immediately adjacent to the external aspect of the IVC, possibly indicating tenting of the cava.
Only 1.6% of temporary filters, however, were retrieved during the 3-year study period, he said.
A filter retrieval rate between 1.2% and 5.1% was cited in a recent Medicare data analysis, which reported an alarming 111% increase in the rate of IVC filter placement from 1999 through 2008 (J. Am. Coll. Radio. 2011;8:483-9).
"IVC filter placement is an epidemic because of the ever-increasing number of risk factors being identified for VTE [venous thromboembolism]," said Dr. Go, with the division of vascular diseases and surgery, Ohio State University Medical Center, Columbus.
Other culprits behind the explosive growth of IVC filters are that angiography suites and catheter labs are now commonplace, the skill needed to insert a filter is easily disseminated, and temporary filters have decreased the threshold for placement.
"Concern over filter complications is increasing, as are referrals for removal, but little long-term data exist," he remarked.
A total of 591 patients had an IVC filter placed at Ohio State University between January 2006 and December 2009, with an adequate postfilter computed tomography (CT) scan available in 262. CT findings were graded, based on a modified, previously published scale (J. Vasc. Interv. Radiol. 2011;22:70-4), ranging from 0 (struts confined entirely within the IVC) to 3 (strut interacts with aorta, duodenum, or other organs).
Indications for filter placement were prophylaxis in 16.4% and VTE in 83.6%. Among the filters placed for VTE, 44.7% were for absolute indications (inability/failure of anticoagulation) and 55.3% for relative indications.
Grade 0 penetration occurred in 42 filters, grade 1 in 100, grade 2 in 83, and grade 3 in 37, Dr. Go said.
Grade 2 or 3 penetration was present in 44.6% of Tulip filters, 74.4% of Celect, 5.3% of Greenfield, and 0% of Optease (P = .0000), according to the analysis, which won this year’s Pfeifer Venous Award from the society.
There was a trend toward increased grade 2 or 3 penetration with uniconical filters vs. biconical filters (46.7% vs. 0%; P = .0645).
In all, 32 patients sought clinical follow-up for abdominal or back pain, but none were conclusively tied to filter problems or penetration.
"It remains unclear if most penetrations caused clinically significant problems," he said. "Monitoring of penetrations with CT, or some other follow-up, may be important to understand the natural history of this condition."
Audience members remarked that the retrieval rate in the series was extremely low and asked whether efforts, such as percutaneous retrieval, were being undertaken.
"We have attempted to remove some of these filters, sometimes successfully, sometimes not," Dr. Go responded. "As far as our retrieval rate, I agree 1.6% is dismal. Vascular surgery put in 19% of the filters. We don’t have a specific protocol in place, other than hyperawareness amongst all of us partners about who has a filter in place and to bring them back when appropriate."
He also observed that even when retrieval is undertaken, technical failure rates are high at about 8.5% in the recent literature (Eur. J. Vasc. Endovasc. Surg. 2013;46:353-9).
Dr. Go and his coauthors reported no financial disclosures.
CHICAGO – Penetration of the inferior vena cava and adjacent organs occurred with 46% of IVC filters placed among 262 patients, an award-winning analysis shows.
Grade 2 or 3 penetration was significantly associated with filter type (49% temporary vs. 5.3% permanent; P = .0001) and length of time in place (18.2% less than 30 days vs. 57.3% 30 days or more; P less than .0001).
"The majority of filters were placed for prophylaxis or relative indications and were temporary," Dr. Michael Go said at the annual meeting of the Midwestern Vascular Surgical Society.
The filter penetrated the aorta in 12 cases; duodenum in 26; and spine, colon, or kidney in 6; and simultaneously penetrated two organs in 7. Another 100 filters had struts immediately adjacent to the external aspect of the IVC, possibly indicating tenting of the cava.
Only 1.6% of temporary filters, however, were retrieved during the 3-year study period, he said.
A filter retrieval rate between 1.2% and 5.1% was cited in a recent Medicare data analysis, which reported an alarming 111% increase in the rate of IVC filter placement from 1999 through 2008 (J. Am. Coll. Radio. 2011;8:483-9).
"IVC filter placement is an epidemic because of the ever-increasing number of risk factors being identified for VTE [venous thromboembolism]," said Dr. Go, with the division of vascular diseases and surgery, Ohio State University Medical Center, Columbus.
Other culprits behind the explosive growth of IVC filters are that angiography suites and catheter labs are now commonplace, the skill needed to insert a filter is easily disseminated, and temporary filters have decreased the threshold for placement.
"Concern over filter complications is increasing, as are referrals for removal, but little long-term data exist," he remarked.
A total of 591 patients had an IVC filter placed at Ohio State University between January 2006 and December 2009, with an adequate postfilter computed tomography (CT) scan available in 262. CT findings were graded, based on a modified, previously published scale (J. Vasc. Interv. Radiol. 2011;22:70-4), ranging from 0 (struts confined entirely within the IVC) to 3 (strut interacts with aorta, duodenum, or other organs).
Indications for filter placement were prophylaxis in 16.4% and VTE in 83.6%. Among the filters placed for VTE, 44.7% were for absolute indications (inability/failure of anticoagulation) and 55.3% for relative indications.
Grade 0 penetration occurred in 42 filters, grade 1 in 100, grade 2 in 83, and grade 3 in 37, Dr. Go said.
Grade 2 or 3 penetration was present in 44.6% of Tulip filters, 74.4% of Celect, 5.3% of Greenfield, and 0% of Optease (P = .0000), according to the analysis, which won this year’s Pfeifer Venous Award from the society.
There was a trend toward increased grade 2 or 3 penetration with uniconical filters vs. biconical filters (46.7% vs. 0%; P = .0645).
In all, 32 patients sought clinical follow-up for abdominal or back pain, but none were conclusively tied to filter problems or penetration.
"It remains unclear if most penetrations caused clinically significant problems," he said. "Monitoring of penetrations with CT, or some other follow-up, may be important to understand the natural history of this condition."
Audience members remarked that the retrieval rate in the series was extremely low and asked whether efforts, such as percutaneous retrieval, were being undertaken.
"We have attempted to remove some of these filters, sometimes successfully, sometimes not," Dr. Go responded. "As far as our retrieval rate, I agree 1.6% is dismal. Vascular surgery put in 19% of the filters. We don’t have a specific protocol in place, other than hyperawareness amongst all of us partners about who has a filter in place and to bring them back when appropriate."
He also observed that even when retrieval is undertaken, technical failure rates are high at about 8.5% in the recent literature (Eur. J. Vasc. Endovasc. Surg. 2013;46:353-9).
Dr. Go and his coauthors reported no financial disclosures.
AT THE MVSS ANNUAL MEETING
Major finding: Penetration of the inferior vena cava and adjacent organs occurred with 46% of IVC filters placed.
Data source: Retrospective study of 262 patients receiving inferior vena cava filters between January 2006 and December 2009.
Disclosures: Dr. Go and his coauthors reported no financial disclosures.
CABG edges PCI in quality-of-life measures for diabetes patients
For diabetic patients who have multivessel coronary artery disease, bypass surgery provides slightly better quality of life and cardiovascular-related health status than does stenting for roughly 2 years, according to a substudy of the FREEDOM trial reported online Oct. 15 in JAMA.
Beyond 2 years, there are no significant differences between the two approaches regarding health status and quality of life in this patient group, said Dr. Mouin S. Abdallah of St. Luke’s Mid America Heart Institute, Kansas City, Mo., and his associates.
Both revascularization strategies yield substantial and sustained improvements for patients who have concomitant multivessel coronary artery disease (CAD) and diabetes, but coronary artery bypass graft surgery generally is preferred because it has a small but significant edge in reducing morbidity and mortality, is less expensive, and produces markedly more durable results. However, the risk of stroke is higher with CABG, and it requires a longer recovery period because it is more invasive, "which may be particularly relevant to patients who are more concerned about quality rather than duration of life," the investigators noted.
"For such patients, our study provides reassurance that there are not major differences in long-term health status and quality of life between the two treatment strategies," they said.
Dr. Abdallah and his colleagues performed a prospective substudy of quality-of-life issues alongside the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) clinical trial. In FREEDOM, 1,900 patients from 18 countries were randomly assigned to undergo either CABG or percutaneous coronary intervention with drug-eluting stents during 2005-2010.
For their substudy, Dr. Abdallah and his associates assessed 935 participants who were assigned to CABG and 945 assigned to PCI. The mean patient age was 63 years, and 72% were men. Median follow-up was 44-47 months.
Patients in both study groups reported substantial and long-lasting improvements in cardiovascular-specific health status, as measured by the Seattle Angina Questionnaire and the Rose Dyspnea Scale. Scores on these instruments improved markedly within 1 month of both procedures and remained high throughout follow-up.
Patients in the PCI group showed more rapid improvement following the procedure, "but these benefits were transient and largely restricted to the first month of follow-up," the researchers said.
"Between 6 months and 2 years, health status was slightly better with CABG across a range of cardiac-specific domains including angina relief, physical function, and overall quality of life. Beyond 2 years, there were no consistent differences in any health status or quality-of-life domains between the CABG and PCI strategies," they reported (JAMA 2013;310:1581-90).
For example, the proportion of angina-free patients was slightly but significantly greater with CABG than with PCI at 6 months (83.7% vs. 78.1%) and at 12 months (83.5% vs. 79.5%), but was not significantly different thereafter.
Similarly, measures of physical limitations imposed by CAD were "modestly" higher with CABG than with PCI for 3 years after the procedure, but there were no significant between-group differences after year 3.
And dyspnea improved faster after PCI than after CABG, but by 6 months this difference had disappeared. By 1 year, the proportion of patients who reported moderate dyspnea was only 9% in both groups, and that proportion stayed fairly steady at 10%-12% in both groups for the remainder of follow-up.
The findings were similar in a sensitivity analysis and in a further analysis restricted only to patients who had reported daily or weekly angina at baseline.
In the FREEDOM trial, CABG showed a clear benefit over PCI for the composite endpoint of death, myocardial infarction, or stroke in patients with concomitant multivessel CAD and diabetes. CABG also afforded slightly better angina relief, especially in patients who had the most severe angina at baseline.
Moreover, patients in the PCI group were more likely to require continuing antianginal medication and twice as likely to undergo repeat revascularization procedures than those in the CABG group.
However, the study findings demonstrate that PCI is clearly beneficial for patients who want to avoid the acute risks of CABG surgery, and is an excellent alternative for those who want a less invasive treatment, Dr. Abdallah and his associates said.
This study was supported by the National Heart, Lung, and Blood Institute. Cordis and Boston Scientific provided the drug-eluting stents, Eli Lilly provided abciximab and research funds, and Sanofi-Aventis and Bristol-Myers Squibb provided clopidogrel. Dr. Abdallah reported no relevant financial conflicts of interest; his associates reported numerous ties to industry sources.
For diabetic patients who have multivessel coronary artery disease, bypass surgery provides slightly better quality of life and cardiovascular-related health status than does stenting for roughly 2 years, according to a substudy of the FREEDOM trial reported online Oct. 15 in JAMA.
Beyond 2 years, there are no significant differences between the two approaches regarding health status and quality of life in this patient group, said Dr. Mouin S. Abdallah of St. Luke’s Mid America Heart Institute, Kansas City, Mo., and his associates.
Both revascularization strategies yield substantial and sustained improvements for patients who have concomitant multivessel coronary artery disease (CAD) and diabetes, but coronary artery bypass graft surgery generally is preferred because it has a small but significant edge in reducing morbidity and mortality, is less expensive, and produces markedly more durable results. However, the risk of stroke is higher with CABG, and it requires a longer recovery period because it is more invasive, "which may be particularly relevant to patients who are more concerned about quality rather than duration of life," the investigators noted.
"For such patients, our study provides reassurance that there are not major differences in long-term health status and quality of life between the two treatment strategies," they said.
Dr. Abdallah and his colleagues performed a prospective substudy of quality-of-life issues alongside the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) clinical trial. In FREEDOM, 1,900 patients from 18 countries were randomly assigned to undergo either CABG or percutaneous coronary intervention with drug-eluting stents during 2005-2010.
For their substudy, Dr. Abdallah and his associates assessed 935 participants who were assigned to CABG and 945 assigned to PCI. The mean patient age was 63 years, and 72% were men. Median follow-up was 44-47 months.
Patients in both study groups reported substantial and long-lasting improvements in cardiovascular-specific health status, as measured by the Seattle Angina Questionnaire and the Rose Dyspnea Scale. Scores on these instruments improved markedly within 1 month of both procedures and remained high throughout follow-up.
Patients in the PCI group showed more rapid improvement following the procedure, "but these benefits were transient and largely restricted to the first month of follow-up," the researchers said.
"Between 6 months and 2 years, health status was slightly better with CABG across a range of cardiac-specific domains including angina relief, physical function, and overall quality of life. Beyond 2 years, there were no consistent differences in any health status or quality-of-life domains between the CABG and PCI strategies," they reported (JAMA 2013;310:1581-90).
For example, the proportion of angina-free patients was slightly but significantly greater with CABG than with PCI at 6 months (83.7% vs. 78.1%) and at 12 months (83.5% vs. 79.5%), but was not significantly different thereafter.
Similarly, measures of physical limitations imposed by CAD were "modestly" higher with CABG than with PCI for 3 years after the procedure, but there were no significant between-group differences after year 3.
And dyspnea improved faster after PCI than after CABG, but by 6 months this difference had disappeared. By 1 year, the proportion of patients who reported moderate dyspnea was only 9% in both groups, and that proportion stayed fairly steady at 10%-12% in both groups for the remainder of follow-up.
The findings were similar in a sensitivity analysis and in a further analysis restricted only to patients who had reported daily or weekly angina at baseline.
In the FREEDOM trial, CABG showed a clear benefit over PCI for the composite endpoint of death, myocardial infarction, or stroke in patients with concomitant multivessel CAD and diabetes. CABG also afforded slightly better angina relief, especially in patients who had the most severe angina at baseline.
Moreover, patients in the PCI group were more likely to require continuing antianginal medication and twice as likely to undergo repeat revascularization procedures than those in the CABG group.
However, the study findings demonstrate that PCI is clearly beneficial for patients who want to avoid the acute risks of CABG surgery, and is an excellent alternative for those who want a less invasive treatment, Dr. Abdallah and his associates said.
This study was supported by the National Heart, Lung, and Blood Institute. Cordis and Boston Scientific provided the drug-eluting stents, Eli Lilly provided abciximab and research funds, and Sanofi-Aventis and Bristol-Myers Squibb provided clopidogrel. Dr. Abdallah reported no relevant financial conflicts of interest; his associates reported numerous ties to industry sources.
For diabetic patients who have multivessel coronary artery disease, bypass surgery provides slightly better quality of life and cardiovascular-related health status than does stenting for roughly 2 years, according to a substudy of the FREEDOM trial reported online Oct. 15 in JAMA.
Beyond 2 years, there are no significant differences between the two approaches regarding health status and quality of life in this patient group, said Dr. Mouin S. Abdallah of St. Luke’s Mid America Heart Institute, Kansas City, Mo., and his associates.
Both revascularization strategies yield substantial and sustained improvements for patients who have concomitant multivessel coronary artery disease (CAD) and diabetes, but coronary artery bypass graft surgery generally is preferred because it has a small but significant edge in reducing morbidity and mortality, is less expensive, and produces markedly more durable results. However, the risk of stroke is higher with CABG, and it requires a longer recovery period because it is more invasive, "which may be particularly relevant to patients who are more concerned about quality rather than duration of life," the investigators noted.
"For such patients, our study provides reassurance that there are not major differences in long-term health status and quality of life between the two treatment strategies," they said.
Dr. Abdallah and his colleagues performed a prospective substudy of quality-of-life issues alongside the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) clinical trial. In FREEDOM, 1,900 patients from 18 countries were randomly assigned to undergo either CABG or percutaneous coronary intervention with drug-eluting stents during 2005-2010.
For their substudy, Dr. Abdallah and his associates assessed 935 participants who were assigned to CABG and 945 assigned to PCI. The mean patient age was 63 years, and 72% were men. Median follow-up was 44-47 months.
Patients in both study groups reported substantial and long-lasting improvements in cardiovascular-specific health status, as measured by the Seattle Angina Questionnaire and the Rose Dyspnea Scale. Scores on these instruments improved markedly within 1 month of both procedures and remained high throughout follow-up.
Patients in the PCI group showed more rapid improvement following the procedure, "but these benefits were transient and largely restricted to the first month of follow-up," the researchers said.
"Between 6 months and 2 years, health status was slightly better with CABG across a range of cardiac-specific domains including angina relief, physical function, and overall quality of life. Beyond 2 years, there were no consistent differences in any health status or quality-of-life domains between the CABG and PCI strategies," they reported (JAMA 2013;310:1581-90).
For example, the proportion of angina-free patients was slightly but significantly greater with CABG than with PCI at 6 months (83.7% vs. 78.1%) and at 12 months (83.5% vs. 79.5%), but was not significantly different thereafter.
Similarly, measures of physical limitations imposed by CAD were "modestly" higher with CABG than with PCI for 3 years after the procedure, but there were no significant between-group differences after year 3.
And dyspnea improved faster after PCI than after CABG, but by 6 months this difference had disappeared. By 1 year, the proportion of patients who reported moderate dyspnea was only 9% in both groups, and that proportion stayed fairly steady at 10%-12% in both groups for the remainder of follow-up.
The findings were similar in a sensitivity analysis and in a further analysis restricted only to patients who had reported daily or weekly angina at baseline.
In the FREEDOM trial, CABG showed a clear benefit over PCI for the composite endpoint of death, myocardial infarction, or stroke in patients with concomitant multivessel CAD and diabetes. CABG also afforded slightly better angina relief, especially in patients who had the most severe angina at baseline.
Moreover, patients in the PCI group were more likely to require continuing antianginal medication and twice as likely to undergo repeat revascularization procedures than those in the CABG group.
However, the study findings demonstrate that PCI is clearly beneficial for patients who want to avoid the acute risks of CABG surgery, and is an excellent alternative for those who want a less invasive treatment, Dr. Abdallah and his associates said.
This study was supported by the National Heart, Lung, and Blood Institute. Cordis and Boston Scientific provided the drug-eluting stents, Eli Lilly provided abciximab and research funds, and Sanofi-Aventis and Bristol-Myers Squibb provided clopidogrel. Dr. Abdallah reported no relevant financial conflicts of interest; his associates reported numerous ties to industry sources.
FROM JAMA
Major finding: The proportion of angina-free patients was slightly but significantly greater with CABG than with PCI at 6 months (83.7% vs. 78.1%) and 12 months (83.5% vs. 79.5%), but was not significantly different thereafter.
Data source: A prospective substudy of the international FREEDOM clinical trial comparing quality of life and CAD-related health status between 935 patients assigned to CABG and 945 assigned to PCI who were followed for a median of 4 years.
Disclosures: This study was supported by the National Heart, Lung, and Blood Institute. Cordis and Boston Scientific provided the drug-eluting stents, Eli Lilly provided abciximab and research funds, and Sanofi-Aventis and Bristol-Myers Squibb provided clopidogrel. Dr. Abdallah reported no relevant financial conflicts of interest; his associates reported numerous ties to industry sources.
VTE rate does not accurately measure quality of care
Postoperative venous thromboembolism rates may not be an effective way of measuring hospital quality, according to Dr. Karl Y. Bilimoria and his colleagues.
The investigators calculated patient-level rates of venous thromboembolism as well as rates of imaging for VTE using data from the American Hospital Association and Medicare Compare from 2009-2010 from nearly 1 million patients discharged from 2,786 hospitals after a major surgery.
They sought to determine the association between hospital adherence to VTE reduction protocols (Surgical Care Improvement Project for VTE or SCIP-VTE-2) and risk-adjusted rates of VTE as measured by Patient Safety Indicator 12 (PSI-12) from the Agency for Healthcare Research and Quality. They also looked at how overall hospital quality scores correlated with VTE prophylaxis and risk-adjusted VTE scores.
Their findings were presented at the annual clinical congress of the American College of Surgeons and simultaneously published Oct. 7 in JAMA (2013 Oct. 7 [doi:10.1001/jama.2013.280048]).
Hospitals that adhered consistently to VTE reduction protocols paradoxically had higher PSI-12 scores, although not significantly so (P = .03). Hospitals with higher overall quality scores also adhered to VTE reduction protocols at a higher rate (93.3% in the lowest quartile vs. 95.5% in the highest) and had significantly higher risk-adjusted VTE event scores (P less than .001).
"Most important, hospital VTE rates were associated with the intensity of detecting VTE with imaging studies," the investigators said. Mean VTE diagnostic imaging rates ranged from 32/1,000 in the lowest quartile to 167/1,000 in the highest.
Hospitals with the lowest imaging rates diagnosed 5.0 VTEs per 1,000 discharges, compared with hospitals with the highest imaging rates diagnosing 13.5 VTEs per 1,000 discharges.
In effect, PSI-12 scores the use of VTE imaging by hospitals instead of the quality of care provided, the investigators said. Further, surveillance bias impedes quality performance improvements; thus, decision making becomes more difficult for "patients seeking to identify a high-quality hospital."
In an accompanying editorial, Dr. Edwin H. Livingston, deputy editor of JAMA, noted that hypervigilance of VTEs might further worsen care in that "the very high compliance rate with VTE prophylaxis might result from many patients receiving treatments from which they are not likely to benefit. This is because current process measures were based on older guidelines that overestimated the benefits of VTE prophylaxis" (JAMA 2013 Oct. 7 [doi:10.1001/jama.2013.280049]).
For that reason, Dr. Livingston recommended that public reporting of VTEs be "reconsidered or curtailed because few hospitals have sufficient numbers of patients to show statistically significant effects of prophylactic measures on VTE rates."
The study was funded by the AHRQ and Northwestern University. Dr. Bilimoria has received honoraria from hospitals, professional societies, and continuing medical education companies for presentation on quality improvement.
Measuring outcomes in general and safety events in particular is a complex proposition. This is particularly true when using patient safety indicators (PSIs) and hospital-acquired conditions (HACs) as outcome metrics to compare performance across organizations.

|
| Dr. Robert Pendleton |
In addition to the usual challenging nuances such as severity of illness adjustment, these indicators rely on accurate documentation and coding and as the Agency for Healthcare Research and Quality states: PSIs identify "potential in-hospital complications and adverse events following surgeries, procedures, and childbirth."
This is well meaning when an analytic team uses these metrics as part of a comprehensive quality and patient safety program to identify potential internal improvement opportunities. However, there are real limitations when using these metrics as outcomes that are tied to public reporting initiatives, payment incentives, and rankings.
The study in this weeks JAMA by Bilimoria and colleagues highlights another limitation of some of these metrics- that of surveillance bias. Using PSI-12 (postoperative venous thromboembolism) risk-adjusted VTE rates were shown to correlate positively with intensity of imaging use (surveillance) and inversely with other measures of quality such as structure or process.
Thus, those with the highest VTE rates did everything right, but also looked for events more often.
This finding complicates the use of PSI-12 as an indicator to compare outcomes across healthcare systems. However, when used as an internal driver in the context of other local metrics of quality and safety, the original intent of PSI-12 as an indicator of potential hospital complications does not change.
This highlights the importance of health care systems in
understanding the strengths and limitations of quality and safety metrics and
in developing the analytic capabilities to turn data points into real
opportunities to deliver better care, rather than going down the proverbial
rabbit hole.
Yet, regulatory agencies should also recognize that using imperfect metrics as a part of payment-reform initiatives needs to be done with extreme caution or there will be unintended consequences that do not lead to our collective goal of exceptional value in healthcare for our patients.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City.
Measuring outcomes in general and safety events in particular is a complex proposition. This is particularly true when using patient safety indicators (PSIs) and hospital-acquired conditions (HACs) as outcome metrics to compare performance across organizations.

|
| Dr. Robert Pendleton |
In addition to the usual challenging nuances such as severity of illness adjustment, these indicators rely on accurate documentation and coding and as the Agency for Healthcare Research and Quality states: PSIs identify "potential in-hospital complications and adverse events following surgeries, procedures, and childbirth."
This is well meaning when an analytic team uses these metrics as part of a comprehensive quality and patient safety program to identify potential internal improvement opportunities. However, there are real limitations when using these metrics as outcomes that are tied to public reporting initiatives, payment incentives, and rankings.
The study in this weeks JAMA by Bilimoria and colleagues highlights another limitation of some of these metrics- that of surveillance bias. Using PSI-12 (postoperative venous thromboembolism) risk-adjusted VTE rates were shown to correlate positively with intensity of imaging use (surveillance) and inversely with other measures of quality such as structure or process.
Thus, those with the highest VTE rates did everything right, but also looked for events more often.
This finding complicates the use of PSI-12 as an indicator to compare outcomes across healthcare systems. However, when used as an internal driver in the context of other local metrics of quality and safety, the original intent of PSI-12 as an indicator of potential hospital complications does not change.
This highlights the importance of health care systems in
understanding the strengths and limitations of quality and safety metrics and
in developing the analytic capabilities to turn data points into real
opportunities to deliver better care, rather than going down the proverbial
rabbit hole.
Yet, regulatory agencies should also recognize that using imperfect metrics as a part of payment-reform initiatives needs to be done with extreme caution or there will be unintended consequences that do not lead to our collective goal of exceptional value in healthcare for our patients.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City.
Measuring outcomes in general and safety events in particular is a complex proposition. This is particularly true when using patient safety indicators (PSIs) and hospital-acquired conditions (HACs) as outcome metrics to compare performance across organizations.

|
| Dr. Robert Pendleton |
In addition to the usual challenging nuances such as severity of illness adjustment, these indicators rely on accurate documentation and coding and as the Agency for Healthcare Research and Quality states: PSIs identify "potential in-hospital complications and adverse events following surgeries, procedures, and childbirth."
This is well meaning when an analytic team uses these metrics as part of a comprehensive quality and patient safety program to identify potential internal improvement opportunities. However, there are real limitations when using these metrics as outcomes that are tied to public reporting initiatives, payment incentives, and rankings.
The study in this weeks JAMA by Bilimoria and colleagues highlights another limitation of some of these metrics- that of surveillance bias. Using PSI-12 (postoperative venous thromboembolism) risk-adjusted VTE rates were shown to correlate positively with intensity of imaging use (surveillance) and inversely with other measures of quality such as structure or process.
Thus, those with the highest VTE rates did everything right, but also looked for events more often.
This finding complicates the use of PSI-12 as an indicator to compare outcomes across healthcare systems. However, when used as an internal driver in the context of other local metrics of quality and safety, the original intent of PSI-12 as an indicator of potential hospital complications does not change.
This highlights the importance of health care systems in
understanding the strengths and limitations of quality and safety metrics and
in developing the analytic capabilities to turn data points into real
opportunities to deliver better care, rather than going down the proverbial
rabbit hole.
Yet, regulatory agencies should also recognize that using imperfect metrics as a part of payment-reform initiatives needs to be done with extreme caution or there will be unintended consequences that do not lead to our collective goal of exceptional value in healthcare for our patients.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City.
Postoperative venous thromboembolism rates may not be an effective way of measuring hospital quality, according to Dr. Karl Y. Bilimoria and his colleagues.
The investigators calculated patient-level rates of venous thromboembolism as well as rates of imaging for VTE using data from the American Hospital Association and Medicare Compare from 2009-2010 from nearly 1 million patients discharged from 2,786 hospitals after a major surgery.
They sought to determine the association between hospital adherence to VTE reduction protocols (Surgical Care Improvement Project for VTE or SCIP-VTE-2) and risk-adjusted rates of VTE as measured by Patient Safety Indicator 12 (PSI-12) from the Agency for Healthcare Research and Quality. They also looked at how overall hospital quality scores correlated with VTE prophylaxis and risk-adjusted VTE scores.
Their findings were presented at the annual clinical congress of the American College of Surgeons and simultaneously published Oct. 7 in JAMA (2013 Oct. 7 [doi:10.1001/jama.2013.280048]).
Hospitals that adhered consistently to VTE reduction protocols paradoxically had higher PSI-12 scores, although not significantly so (P = .03). Hospitals with higher overall quality scores also adhered to VTE reduction protocols at a higher rate (93.3% in the lowest quartile vs. 95.5% in the highest) and had significantly higher risk-adjusted VTE event scores (P less than .001).
"Most important, hospital VTE rates were associated with the intensity of detecting VTE with imaging studies," the investigators said. Mean VTE diagnostic imaging rates ranged from 32/1,000 in the lowest quartile to 167/1,000 in the highest.
Hospitals with the lowest imaging rates diagnosed 5.0 VTEs per 1,000 discharges, compared with hospitals with the highest imaging rates diagnosing 13.5 VTEs per 1,000 discharges.
In effect, PSI-12 scores the use of VTE imaging by hospitals instead of the quality of care provided, the investigators said. Further, surveillance bias impedes quality performance improvements; thus, decision making becomes more difficult for "patients seeking to identify a high-quality hospital."
In an accompanying editorial, Dr. Edwin H. Livingston, deputy editor of JAMA, noted that hypervigilance of VTEs might further worsen care in that "the very high compliance rate with VTE prophylaxis might result from many patients receiving treatments from which they are not likely to benefit. This is because current process measures were based on older guidelines that overestimated the benefits of VTE prophylaxis" (JAMA 2013 Oct. 7 [doi:10.1001/jama.2013.280049]).
For that reason, Dr. Livingston recommended that public reporting of VTEs be "reconsidered or curtailed because few hospitals have sufficient numbers of patients to show statistically significant effects of prophylactic measures on VTE rates."
The study was funded by the AHRQ and Northwestern University. Dr. Bilimoria has received honoraria from hospitals, professional societies, and continuing medical education companies for presentation on quality improvement.
Postoperative venous thromboembolism rates may not be an effective way of measuring hospital quality, according to Dr. Karl Y. Bilimoria and his colleagues.
The investigators calculated patient-level rates of venous thromboembolism as well as rates of imaging for VTE using data from the American Hospital Association and Medicare Compare from 2009-2010 from nearly 1 million patients discharged from 2,786 hospitals after a major surgery.
They sought to determine the association between hospital adherence to VTE reduction protocols (Surgical Care Improvement Project for VTE or SCIP-VTE-2) and risk-adjusted rates of VTE as measured by Patient Safety Indicator 12 (PSI-12) from the Agency for Healthcare Research and Quality. They also looked at how overall hospital quality scores correlated with VTE prophylaxis and risk-adjusted VTE scores.
Their findings were presented at the annual clinical congress of the American College of Surgeons and simultaneously published Oct. 7 in JAMA (2013 Oct. 7 [doi:10.1001/jama.2013.280048]).
Hospitals that adhered consistently to VTE reduction protocols paradoxically had higher PSI-12 scores, although not significantly so (P = .03). Hospitals with higher overall quality scores also adhered to VTE reduction protocols at a higher rate (93.3% in the lowest quartile vs. 95.5% in the highest) and had significantly higher risk-adjusted VTE event scores (P less than .001).
"Most important, hospital VTE rates were associated with the intensity of detecting VTE with imaging studies," the investigators said. Mean VTE diagnostic imaging rates ranged from 32/1,000 in the lowest quartile to 167/1,000 in the highest.
Hospitals with the lowest imaging rates diagnosed 5.0 VTEs per 1,000 discharges, compared with hospitals with the highest imaging rates diagnosing 13.5 VTEs per 1,000 discharges.
In effect, PSI-12 scores the use of VTE imaging by hospitals instead of the quality of care provided, the investigators said. Further, surveillance bias impedes quality performance improvements; thus, decision making becomes more difficult for "patients seeking to identify a high-quality hospital."
In an accompanying editorial, Dr. Edwin H. Livingston, deputy editor of JAMA, noted that hypervigilance of VTEs might further worsen care in that "the very high compliance rate with VTE prophylaxis might result from many patients receiving treatments from which they are not likely to benefit. This is because current process measures were based on older guidelines that overestimated the benefits of VTE prophylaxis" (JAMA 2013 Oct. 7 [doi:10.1001/jama.2013.280049]).
For that reason, Dr. Livingston recommended that public reporting of VTEs be "reconsidered or curtailed because few hospitals have sufficient numbers of patients to show statistically significant effects of prophylactic measures on VTE rates."
The study was funded by the AHRQ and Northwestern University. Dr. Bilimoria has received honoraria from hospitals, professional societies, and continuing medical education companies for presentation on quality improvement.
FROM THE ACS CLINICAL CONGRESS
Major finding: Hospitals that adhered consistently to VTE reduction protocols had higher rates of VTE, although not significantly so (P = .03).
Data source: Study of hospital risk-adjusted VTE prophylaxis adherence rates to postoperative VTE event rates in 2,786 hospitals.
Disclosures: The study was funded by the AHRQ and Northwestern University. Dr. Bilimoria has received honoraria from hospitals, professional societies, and continuing medical education companies for presentation on quality improvement.
TAVR shows large survival benefit in diabetes patients
AMSTERDAM – Transcatheter aortic valve replacement produced dramatically better 1-year survival, compared with surgical valve replacement, among high-risk patients with diabetes in a new, post hoc analysis of data collected in the first PARTNER trial.
The 145 patients with any type of diabetes who underwent transcatheter aortic valve replacement (TAVR) in the operable, cohort A of the first Placement of Aortic Transcatheter Valves (PARTNER) trial had an 18% all-cause mortality rate during 1-year follow-up, compared with a 27% rate among the 130 patients who underwent surgical aortic valve replacement (SAVR), a difference in this post hoc analysis that reached statistical significance.
The finding of a 9-percentage-point difference in mortality in the diabetic subgroup treated with TAVR, a 40% relative risk reduction compared with SAVR, contrasts with the overall, primary finding of the PARTNER I cohort A trial, which showed that TAVR and SAVR produced similar mortality rates in high surgical-risk patients after 1 year (N. Engl. J. Med. 2011;364:2187-98).
TAVR use in patients with diabetes also resulted in a statistically significant reduction in the 1-year incidence of renal failure requiring dialysis, a 4% rate compared with an 11% among the SAVR patients, Dr. Brian R. Lindman reported at the annual congress of the European Society of Cardiology. This also contrasted with the overall PARTNER results when patients without diabetes were included, which showed no difference in renal outcomes between TAVR and SAVR.
The results "raise the possibility that TAVR may be the preferred approach for patients with diabetes," said Dr. Lindman. But he cautioned that because this was a post hoc analysis, the results need confirmation in a prospective study.
Despite this caveat, Dr. Lindman said that the finding can’t be completely ignored when treatment options are discussed with patients who have severe aortic stenosis. "Going forward, I think this is something we’ll need to think about, and we might lean more toward TAVR for patients with diabetes. But we’d like to see some confirmatory evidence in the PARTNER II cohort A trial," he said in an interview. Investigators from the PARTNER II trial, which is randomizing patients with moderate surgical risk, have said that the cohort A results are expected in 2015.
"Although hypothesis-generating only, this result is good news for patients with diabetes," said Dr. William Wijns, codirector of the cardiovascular center at O.L.V. Hospital in Aalst, Belgium.
Dr. Lindman also cautioned that investigators collected limited data on patients’ diabetes status in PARTNER I. The data did not include information on diabetes type, hemoglobin A1c or blood glucose levels, or treatment received. He also acknowledged that the 42% prevalence of diabetes in the study cohort was unexpectedly high, but noted that if this meant that some patients with a questionable diabetes diagnosis entered the analysis, this should have diminished the mortality difference between TAVR and SAVR.
The 275 patients with diabetes in PARTNER I cohort A averaged 82 years of age, and just over a third were women. The subgroups of patients with diabetes randomized to TAVR and to SAVR showed no significant differences for any physiologic or cardiovascular measure or in the prevalence of various comorbidities.
The analysis also showed a statistically significant reduced rate of 1-year mortality in the subgroup treated with transfemoral TAVR compared with SAVR, and in the subgroup of patients with diabetes treated with transapical TAVR compared with SAVR. The 1-year rate of stroke was an identical 3.5% in the patients with diabetes treated with TAVR and in those treated with SAVR. Patients treated with SAVR had a higher incidence of a major bleeding event during follow-up compared with the TAVR patients, while the TAVR patients had a significantly higher rate of major vascular complications during follow-up. Both findings were consistent with the overall PARTNER I cohort A results, said Dr. Lindman, a cardiologist at Washington University in St. Louis.
The diabetes patients who underwent TAVR showed their striking reduction in 1-year mortality despite having the same problem with postprocedural aortic regurgitation as seen in the overall PARTNER I trial. At 6 months after treatment, 9% of the TAVR patients had moderate or severe aortic regurgitation, and 54% had mild regurgitation, compared with rates of 1% moderate or severe and 6% mild in the SAVR patients.
Dr. Lindman speculated that increased inflammation and oxidative stress in patients with diabetes may interact with the stresses of heart surgery to produce the excess mortality seen after SAVR in patients with diabetes. Results from prior studies had documented worsened survival in patients with diabetes who undergo heart surgery, he said.
The PARTNER trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER and said that he had no disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Transcatheter aortic valve replacement produced dramatically better 1-year survival, compared with surgical valve replacement, among high-risk patients with diabetes in a new, post hoc analysis of data collected in the first PARTNER trial.
The 145 patients with any type of diabetes who underwent transcatheter aortic valve replacement (TAVR) in the operable, cohort A of the first Placement of Aortic Transcatheter Valves (PARTNER) trial had an 18% all-cause mortality rate during 1-year follow-up, compared with a 27% rate among the 130 patients who underwent surgical aortic valve replacement (SAVR), a difference in this post hoc analysis that reached statistical significance.
The finding of a 9-percentage-point difference in mortality in the diabetic subgroup treated with TAVR, a 40% relative risk reduction compared with SAVR, contrasts with the overall, primary finding of the PARTNER I cohort A trial, which showed that TAVR and SAVR produced similar mortality rates in high surgical-risk patients after 1 year (N. Engl. J. Med. 2011;364:2187-98).
TAVR use in patients with diabetes also resulted in a statistically significant reduction in the 1-year incidence of renal failure requiring dialysis, a 4% rate compared with an 11% among the SAVR patients, Dr. Brian R. Lindman reported at the annual congress of the European Society of Cardiology. This also contrasted with the overall PARTNER results when patients without diabetes were included, which showed no difference in renal outcomes between TAVR and SAVR.
The results "raise the possibility that TAVR may be the preferred approach for patients with diabetes," said Dr. Lindman. But he cautioned that because this was a post hoc analysis, the results need confirmation in a prospective study.
Despite this caveat, Dr. Lindman said that the finding can’t be completely ignored when treatment options are discussed with patients who have severe aortic stenosis. "Going forward, I think this is something we’ll need to think about, and we might lean more toward TAVR for patients with diabetes. But we’d like to see some confirmatory evidence in the PARTNER II cohort A trial," he said in an interview. Investigators from the PARTNER II trial, which is randomizing patients with moderate surgical risk, have said that the cohort A results are expected in 2015.
"Although hypothesis-generating only, this result is good news for patients with diabetes," said Dr. William Wijns, codirector of the cardiovascular center at O.L.V. Hospital in Aalst, Belgium.
Dr. Lindman also cautioned that investigators collected limited data on patients’ diabetes status in PARTNER I. The data did not include information on diabetes type, hemoglobin A1c or blood glucose levels, or treatment received. He also acknowledged that the 42% prevalence of diabetes in the study cohort was unexpectedly high, but noted that if this meant that some patients with a questionable diabetes diagnosis entered the analysis, this should have diminished the mortality difference between TAVR and SAVR.
The 275 patients with diabetes in PARTNER I cohort A averaged 82 years of age, and just over a third were women. The subgroups of patients with diabetes randomized to TAVR and to SAVR showed no significant differences for any physiologic or cardiovascular measure or in the prevalence of various comorbidities.
The analysis also showed a statistically significant reduced rate of 1-year mortality in the subgroup treated with transfemoral TAVR compared with SAVR, and in the subgroup of patients with diabetes treated with transapical TAVR compared with SAVR. The 1-year rate of stroke was an identical 3.5% in the patients with diabetes treated with TAVR and in those treated with SAVR. Patients treated with SAVR had a higher incidence of a major bleeding event during follow-up compared with the TAVR patients, while the TAVR patients had a significantly higher rate of major vascular complications during follow-up. Both findings were consistent with the overall PARTNER I cohort A results, said Dr. Lindman, a cardiologist at Washington University in St. Louis.
The diabetes patients who underwent TAVR showed their striking reduction in 1-year mortality despite having the same problem with postprocedural aortic regurgitation as seen in the overall PARTNER I trial. At 6 months after treatment, 9% of the TAVR patients had moderate or severe aortic regurgitation, and 54% had mild regurgitation, compared with rates of 1% moderate or severe and 6% mild in the SAVR patients.
Dr. Lindman speculated that increased inflammation and oxidative stress in patients with diabetes may interact with the stresses of heart surgery to produce the excess mortality seen after SAVR in patients with diabetes. Results from prior studies had documented worsened survival in patients with diabetes who undergo heart surgery, he said.
The PARTNER trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER and said that he had no disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Transcatheter aortic valve replacement produced dramatically better 1-year survival, compared with surgical valve replacement, among high-risk patients with diabetes in a new, post hoc analysis of data collected in the first PARTNER trial.
The 145 patients with any type of diabetes who underwent transcatheter aortic valve replacement (TAVR) in the operable, cohort A of the first Placement of Aortic Transcatheter Valves (PARTNER) trial had an 18% all-cause mortality rate during 1-year follow-up, compared with a 27% rate among the 130 patients who underwent surgical aortic valve replacement (SAVR), a difference in this post hoc analysis that reached statistical significance.
The finding of a 9-percentage-point difference in mortality in the diabetic subgroup treated with TAVR, a 40% relative risk reduction compared with SAVR, contrasts with the overall, primary finding of the PARTNER I cohort A trial, which showed that TAVR and SAVR produced similar mortality rates in high surgical-risk patients after 1 year (N. Engl. J. Med. 2011;364:2187-98).
TAVR use in patients with diabetes also resulted in a statistically significant reduction in the 1-year incidence of renal failure requiring dialysis, a 4% rate compared with an 11% among the SAVR patients, Dr. Brian R. Lindman reported at the annual congress of the European Society of Cardiology. This also contrasted with the overall PARTNER results when patients without diabetes were included, which showed no difference in renal outcomes between TAVR and SAVR.
The results "raise the possibility that TAVR may be the preferred approach for patients with diabetes," said Dr. Lindman. But he cautioned that because this was a post hoc analysis, the results need confirmation in a prospective study.
Despite this caveat, Dr. Lindman said that the finding can’t be completely ignored when treatment options are discussed with patients who have severe aortic stenosis. "Going forward, I think this is something we’ll need to think about, and we might lean more toward TAVR for patients with diabetes. But we’d like to see some confirmatory evidence in the PARTNER II cohort A trial," he said in an interview. Investigators from the PARTNER II trial, which is randomizing patients with moderate surgical risk, have said that the cohort A results are expected in 2015.
"Although hypothesis-generating only, this result is good news for patients with diabetes," said Dr. William Wijns, codirector of the cardiovascular center at O.L.V. Hospital in Aalst, Belgium.
Dr. Lindman also cautioned that investigators collected limited data on patients’ diabetes status in PARTNER I. The data did not include information on diabetes type, hemoglobin A1c or blood glucose levels, or treatment received. He also acknowledged that the 42% prevalence of diabetes in the study cohort was unexpectedly high, but noted that if this meant that some patients with a questionable diabetes diagnosis entered the analysis, this should have diminished the mortality difference between TAVR and SAVR.
The 275 patients with diabetes in PARTNER I cohort A averaged 82 years of age, and just over a third were women. The subgroups of patients with diabetes randomized to TAVR and to SAVR showed no significant differences for any physiologic or cardiovascular measure or in the prevalence of various comorbidities.
The analysis also showed a statistically significant reduced rate of 1-year mortality in the subgroup treated with transfemoral TAVR compared with SAVR, and in the subgroup of patients with diabetes treated with transapical TAVR compared with SAVR. The 1-year rate of stroke was an identical 3.5% in the patients with diabetes treated with TAVR and in those treated with SAVR. Patients treated with SAVR had a higher incidence of a major bleeding event during follow-up compared with the TAVR patients, while the TAVR patients had a significantly higher rate of major vascular complications during follow-up. Both findings were consistent with the overall PARTNER I cohort A results, said Dr. Lindman, a cardiologist at Washington University in St. Louis.
The diabetes patients who underwent TAVR showed their striking reduction in 1-year mortality despite having the same problem with postprocedural aortic regurgitation as seen in the overall PARTNER I trial. At 6 months after treatment, 9% of the TAVR patients had moderate or severe aortic regurgitation, and 54% had mild regurgitation, compared with rates of 1% moderate or severe and 6% mild in the SAVR patients.
Dr. Lindman speculated that increased inflammation and oxidative stress in patients with diabetes may interact with the stresses of heart surgery to produce the excess mortality seen after SAVR in patients with diabetes. Results from prior studies had documented worsened survival in patients with diabetes who undergo heart surgery, he said.
The PARTNER trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER and said that he had no disclosures.
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2013
Major finding: In patients with diabetes, 1-year mortality was 18% after TAVR and 27% after SAVR in a post hoc analysis.
Data source: The PARTNER I cohort A study, which enrolled 699 high-risk patients with severe aortic stenosis to treatment with aortic valve replacement by the transcatheter or surgical approach, including 275 patients with diabetes.
Disclosures: The trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER, and said that he had no disclosures.
FDA drops access points from Sapien transcatheter valve label
The labeling for the Sapien transcatheter heart valve no longer includes references to specific access points, which will expand the group of patients who can be treated with the device, the Food and Drug Administration announced.
Approval has been for use via a transfemoral or transapical approach. But revised labeling for the device has been approved, which removes references to specific access points, according to the FDA statement issued on Sept. 23. Spokespersons for the FDA and for Edwards Lifesciences, the manufacturer of the valve, confirmed that the references to specific access points have been dropped for both inoperable and high-risk patients.
The approval for this change was based on data from patient registries in the United States and Europe and other sources, according to the announcement.
The percutaneous valve was approved in 2011 for treating patients with severe aortic stenosis who are considered inoperable, making it the first artificial heart valve that could be used to replace a damaged valve without open heart surgery. That approval was expanded in October 2012 to include patients who are operable but at high risk.
Edwards submitted data to the FDA for the labeling change from the Transcatheter Valve Therapy Registry (TVTR) in the United States, transcatheter heart valve (THV) device registries in Europe, as well as FDA-approved clinical studies and peer-reviewed medical journals. Data from the TVTR, which is managed by the American College of Cardiology (ACC) and the Society of Thoracic Surgeons, came from "several thousand procedures performed on patients using an alternative access point, and showed no evidence that the device performs differently or has a different benefit-risk profile based on the access point," the statement said.
"Just 2 years after the THV entered the market for a specific patient population, data from the TVTR was used to support FDA approval that expands patient access to a lifesaving therapy," Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, said in the statement. "Medical device registries like the TVTR not only play an important role in the FDA’s postmarket surveillance system, they also collect robust and timely data that can be used to identify additional patient populations that benefit from the therapy," he added.
"Leveraging clinical research inside the framework of a device registry to expand access to therapy for more patients is a new paradigm for the FDA, researchers, registry sponsors, and the medical device industry," Dr. Shuren noted.
Since 2012, the TVTR has collected data on all transcatheter aortic valve replacements performed in the United States. The manufacturer will use these data to evaluate short- and long-term outcomes in patients who receive the Sapien valve through an alternative access site, the FDA statement said.
ACC President John G. Harold, M.D., said in a statement that "the FDA’s decision is a true testament to the efficiency of rigorous clinical registries, which allowed FDA to make a prompt decision that will impact thousands of patients who previously would have not had access to this procedure."
The labeling for the Sapien transcatheter heart valve no longer includes references to specific access points, which will expand the group of patients who can be treated with the device, the Food and Drug Administration announced.
Approval has been for use via a transfemoral or transapical approach. But revised labeling for the device has been approved, which removes references to specific access points, according to the FDA statement issued on Sept. 23. Spokespersons for the FDA and for Edwards Lifesciences, the manufacturer of the valve, confirmed that the references to specific access points have been dropped for both inoperable and high-risk patients.
The approval for this change was based on data from patient registries in the United States and Europe and other sources, according to the announcement.
The percutaneous valve was approved in 2011 for treating patients with severe aortic stenosis who are considered inoperable, making it the first artificial heart valve that could be used to replace a damaged valve without open heart surgery. That approval was expanded in October 2012 to include patients who are operable but at high risk.
Edwards submitted data to the FDA for the labeling change from the Transcatheter Valve Therapy Registry (TVTR) in the United States, transcatheter heart valve (THV) device registries in Europe, as well as FDA-approved clinical studies and peer-reviewed medical journals. Data from the TVTR, which is managed by the American College of Cardiology (ACC) and the Society of Thoracic Surgeons, came from "several thousand procedures performed on patients using an alternative access point, and showed no evidence that the device performs differently or has a different benefit-risk profile based on the access point," the statement said.
"Just 2 years after the THV entered the market for a specific patient population, data from the TVTR was used to support FDA approval that expands patient access to a lifesaving therapy," Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, said in the statement. "Medical device registries like the TVTR not only play an important role in the FDA’s postmarket surveillance system, they also collect robust and timely data that can be used to identify additional patient populations that benefit from the therapy," he added.
"Leveraging clinical research inside the framework of a device registry to expand access to therapy for more patients is a new paradigm for the FDA, researchers, registry sponsors, and the medical device industry," Dr. Shuren noted.
Since 2012, the TVTR has collected data on all transcatheter aortic valve replacements performed in the United States. The manufacturer will use these data to evaluate short- and long-term outcomes in patients who receive the Sapien valve through an alternative access site, the FDA statement said.
ACC President John G. Harold, M.D., said in a statement that "the FDA’s decision is a true testament to the efficiency of rigorous clinical registries, which allowed FDA to make a prompt decision that will impact thousands of patients who previously would have not had access to this procedure."
The labeling for the Sapien transcatheter heart valve no longer includes references to specific access points, which will expand the group of patients who can be treated with the device, the Food and Drug Administration announced.
Approval has been for use via a transfemoral or transapical approach. But revised labeling for the device has been approved, which removes references to specific access points, according to the FDA statement issued on Sept. 23. Spokespersons for the FDA and for Edwards Lifesciences, the manufacturer of the valve, confirmed that the references to specific access points have been dropped for both inoperable and high-risk patients.
The approval for this change was based on data from patient registries in the United States and Europe and other sources, according to the announcement.
The percutaneous valve was approved in 2011 for treating patients with severe aortic stenosis who are considered inoperable, making it the first artificial heart valve that could be used to replace a damaged valve without open heart surgery. That approval was expanded in October 2012 to include patients who are operable but at high risk.
Edwards submitted data to the FDA for the labeling change from the Transcatheter Valve Therapy Registry (TVTR) in the United States, transcatheter heart valve (THV) device registries in Europe, as well as FDA-approved clinical studies and peer-reviewed medical journals. Data from the TVTR, which is managed by the American College of Cardiology (ACC) and the Society of Thoracic Surgeons, came from "several thousand procedures performed on patients using an alternative access point, and showed no evidence that the device performs differently or has a different benefit-risk profile based on the access point," the statement said.
"Just 2 years after the THV entered the market for a specific patient population, data from the TVTR was used to support FDA approval that expands patient access to a lifesaving therapy," Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, said in the statement. "Medical device registries like the TVTR not only play an important role in the FDA’s postmarket surveillance system, they also collect robust and timely data that can be used to identify additional patient populations that benefit from the therapy," he added.
"Leveraging clinical research inside the framework of a device registry to expand access to therapy for more patients is a new paradigm for the FDA, researchers, registry sponsors, and the medical device industry," Dr. Shuren noted.
Since 2012, the TVTR has collected data on all transcatheter aortic valve replacements performed in the United States. The manufacturer will use these data to evaluate short- and long-term outcomes in patients who receive the Sapien valve through an alternative access site, the FDA statement said.
ACC President John G. Harold, M.D., said in a statement that "the FDA’s decision is a true testament to the efficiency of rigorous clinical registries, which allowed FDA to make a prompt decision that will impact thousands of patients who previously would have not had access to this procedure."
How to foil post-CABG aspirin resistance
AMSTERDAM – Giving low-dose aspirin four times per day in the first days after coronary artery bypass graft surgery suppresses serum thromboxane levels far more effectively than does conventional once-daily dosing at 325 mg, according to a randomized trial.
The clinical implication of this finding is that more frequent dosing of aspirin may prevent the serious problem of premature vein graft failure from the development of aspirin resistance in the postoperative period, although at this point this is a hypothesis that requires testing in a future study, Dr. Jeremy S. Paikin said at the annual congress of the European Society of Cardiology.
He reported on 110 on-pump coronary artery bypass graft (CABG) patients randomized on postoperative day 1 to aspirin either at 81 mg four times daily, the standard 325 mg once daily, or to 81 mg once daily.
The primary study endpoint was the serum thromboxane level on the morning of postoperative day 4. The median level was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
"With 81 mg QD [four times daily], there’s almost complete suppression of serum thromboxane throughout the course of the hospital stay," according to Dr. Paikin of McMaster University, Hamilton, Ont.
Aspirin is known to prevent CABG graft failure, but its effectiveness is limited by the not-infrequent development of aspirin hyporesponsiveness in the postoperative period. The underlying mechanism involved in this aspirin resistance was previously unknown; however, in their randomized trial Dr. Paikin and coinvestigators established that the hyporesponsiveness is caused at least in part by increased platelet turnover in the postoperative period. The investigators showed that platelet turnover per day was increased two- to threefold in the week after CABG, compared with presurgical levels, a finding Dr. Paikin termed "quite exciting."
Recognizing that administration of any drug four times daily raises formidable adherence obstacles, he and his coworkers are just about to start a clinical trial looking at twice-daily aspirin dosing post CABG. They’re also interested in drawing a firm evidentiary connection between serum thromboxane levels and risk of premature graft failure.
Dr. Paikin reported having no financial conflicts of interest.
AMSTERDAM – Giving low-dose aspirin four times per day in the first days after coronary artery bypass graft surgery suppresses serum thromboxane levels far more effectively than does conventional once-daily dosing at 325 mg, according to a randomized trial.
The clinical implication of this finding is that more frequent dosing of aspirin may prevent the serious problem of premature vein graft failure from the development of aspirin resistance in the postoperative period, although at this point this is a hypothesis that requires testing in a future study, Dr. Jeremy S. Paikin said at the annual congress of the European Society of Cardiology.
He reported on 110 on-pump coronary artery bypass graft (CABG) patients randomized on postoperative day 1 to aspirin either at 81 mg four times daily, the standard 325 mg once daily, or to 81 mg once daily.
The primary study endpoint was the serum thromboxane level on the morning of postoperative day 4. The median level was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
"With 81 mg QD [four times daily], there’s almost complete suppression of serum thromboxane throughout the course of the hospital stay," according to Dr. Paikin of McMaster University, Hamilton, Ont.
Aspirin is known to prevent CABG graft failure, but its effectiveness is limited by the not-infrequent development of aspirin hyporesponsiveness in the postoperative period. The underlying mechanism involved in this aspirin resistance was previously unknown; however, in their randomized trial Dr. Paikin and coinvestigators established that the hyporesponsiveness is caused at least in part by increased platelet turnover in the postoperative period. The investigators showed that platelet turnover per day was increased two- to threefold in the week after CABG, compared with presurgical levels, a finding Dr. Paikin termed "quite exciting."
Recognizing that administration of any drug four times daily raises formidable adherence obstacles, he and his coworkers are just about to start a clinical trial looking at twice-daily aspirin dosing post CABG. They’re also interested in drawing a firm evidentiary connection between serum thromboxane levels and risk of premature graft failure.
Dr. Paikin reported having no financial conflicts of interest.
AMSTERDAM – Giving low-dose aspirin four times per day in the first days after coronary artery bypass graft surgery suppresses serum thromboxane levels far more effectively than does conventional once-daily dosing at 325 mg, according to a randomized trial.
The clinical implication of this finding is that more frequent dosing of aspirin may prevent the serious problem of premature vein graft failure from the development of aspirin resistance in the postoperative period, although at this point this is a hypothesis that requires testing in a future study, Dr. Jeremy S. Paikin said at the annual congress of the European Society of Cardiology.
He reported on 110 on-pump coronary artery bypass graft (CABG) patients randomized on postoperative day 1 to aspirin either at 81 mg four times daily, the standard 325 mg once daily, or to 81 mg once daily.
The primary study endpoint was the serum thromboxane level on the morning of postoperative day 4. The median level was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
"With 81 mg QD [four times daily], there’s almost complete suppression of serum thromboxane throughout the course of the hospital stay," according to Dr. Paikin of McMaster University, Hamilton, Ont.
Aspirin is known to prevent CABG graft failure, but its effectiveness is limited by the not-infrequent development of aspirin hyporesponsiveness in the postoperative period. The underlying mechanism involved in this aspirin resistance was previously unknown; however, in their randomized trial Dr. Paikin and coinvestigators established that the hyporesponsiveness is caused at least in part by increased platelet turnover in the postoperative period. The investigators showed that platelet turnover per day was increased two- to threefold in the week after CABG, compared with presurgical levels, a finding Dr. Paikin termed "quite exciting."
Recognizing that administration of any drug four times daily raises formidable adherence obstacles, he and his coworkers are just about to start a clinical trial looking at twice-daily aspirin dosing post CABG. They’re also interested in drawing a firm evidentiary connection between serum thromboxane levels and risk of premature graft failure.
Dr. Paikin reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2013
Major finding: The median serum thromboxane level on the morning of post CABG day 4 was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
Data source: A randomized clinical trial in which 110 patients who underwent on-pump CABG surgery were randomized on postoperative day 1 to aspirin at either 81 mg four times daily, 325 mg once daily, or 81 mg once daily.
Disclosures: The study presenter reported having no financial conflicts.
Prophylactic beta-blockers and noncardiac surgery: It's complicated!
AMSTERDAM – Results of a new Danish national study suggest the effects of prophylactic beta-blocker therapy in patients with ischemic heart disease undergoing noncardiac surgery are considerably more heterogeneous than portrayed in current pro-prophylaxis practice guidelines or, at the opposite extreme, in a recent highly critical meta-analysis.
"This is an extraordinarily confusing area at the moment," Dr. Charlotte Andersson observed in presenting the Danish national registry findings at the annual congress of the European Society of Cardiology.
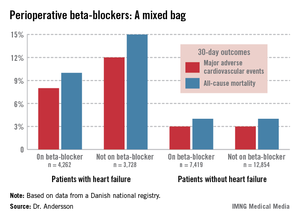
She reported on 28,263 adults with ischemic heart disease who underwent noncardiac surgery during 2004-2009. Hip or knee replacements were the most common operations, accounting for roughly one-third of the total. Patients were followed for 30 days postoperatively for the composite endpoint of acute myocardial infarction, ischemic stroke, or cardiovascular death, as well as for 30-day all-cause mortality.
In short, the effects of prophylactic beta-blocker therapy depended upon the type of background ischemic heart disease a surgical patient had.
"Our data suggest a beneficial effect of beta-blockers among patients with heart failure, perhaps a beneficial effect as well among patients with an MI within the previous 2 years, but no beneficial effect among patients with a more distant MI, and perhaps even harm associated with beta-blocker therapy among patients with neither heart failure nor a history of MI," according to Dr. Andersson of the University of Copenhagen.
The study population included 7,990 patients with heart failure, 53% of whom were on beta-blockers when they underwent noncardiac surgery. Those on beta-blockers fared significantly better in terms of the study endpoints (see graphic).
In contrast, 30-day outcomes in the 37% of patients without heart failure were identical regardless of whether or not they were on beta-blockers at surgery.
In a multivariate analysis, the use of beta-blockers in noncardiac surgery patients with heart failure was associated with a 22% reduction in major adverse cardiovascular events (MACEs) and an 18% reduction in all-cause mortality compared with no use of beta-blockers, both of which were statistically significant advantages. The analysis was adjusted for patient demographics, acute versus elective surgery, chronic obstructive pulmonary disease, diabetes, atrial fibrillation, peripheral artery disease, cancer, anemia, smoking, alcohol consumption, cerebrovascular disease, and American Society of Anesthesiologists score.
Among the 1,664 patients with an MI within the past 2 years, being on a beta-blocker at the time of surgery was associated with an adjusted highly significant 46% reduction in MACE and a 20% decrease in all-cause mortality, compared with no use of beta-blockers.
For the 1,679 patients with an MI 2-5 years prior to surgery, being on a beta-blocker was associated with a 29% reduction in the risk of MACE and a 26% reduction in 30-day all-cause mortality.
Among the 5,018 patients with an MI more than 5 years earlier, the use of beta-blockers at surgery was associated with a 35% greater risk of MACE than in nonusers of beta-blockers as well as a 33% increase in all-cause mortality. These differences in adverse outcomes rates barely missed achieving statistical significance.
Perhaps the most striking study finding was that patients with no prior MI or heart failure who were on a beta-blocker at the time of noncardiac surgery had a 44% increased risk of 30-day MACE and a 30% higher all-cause mortality, compared with those not on a beta-blocker, with both differences being significant.
Session cochair Dr. Elmir Omerovic thanked Dr. Andersson for a presentation that "really adds important new information" and asked whether she had been surprised by the findings.
"Yes, I have to say I was surprised by the increased risk in patients without prior MI or heart failure, because the ESC [European Society of Cardiology] guidelines state as a class I recommendation that all patients with ischemic heart disease undergoing noncardiac surgery should be on a beta-blocker. Perhaps we should reevaluate beta-blockers in noncardiac surgery," Dr. Andersson replied.
Current American College of Cardiology/American Heart Association guidelines also endorse perioperative beta-blockade in patients with coronary artery disease undergoing vascular or intermediate-risk noncardiac surgery.
She noted that in drawing up the current guidelines, the ESC and ACC/AHA committees relied heavily on strongly positive randomized clinical trials whose validity has recently been called into question in a major research scandal. Indeed, the lead investigator in those studies, Dr. Don Poldermans – who also happened to be chairperson of the ESC guidelines-writing task force – has been dismissed from the faculty at Erasmus University in Rotterdam.
Dr. Omerovic, of Sahlgrenska University, Gothenburg, Sweden, asked for Dr. Andersson’s thoughts regarding a new meta-analysis by investigators at Imperial College London which excluded the suspect Dutch clinical trials. The investigators concluded that initiation of perioperative beta-blocker therapy was associated with a 27% increase in 30-day all-cause mortality, a 27% reduction in nonfatal MI, a 73% increase in stroke, and a 51% increase in hypotension.
"Patient safety being paramount, guidelines for perioperative beta-blocker initiation should be retracted without further delay," the meta-analysts argued (Heart 2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
"I read that meta-analysis with great interest," Dr. Andersson said. "I think there definitely is a heterogeneity in the effects of perioperative beta-blockers, and it depends on your baseline risk. Most of the studies in the meta-analysis included many patients at lower risk."
Dr. Andersson’s study was funded by the Danish Medical Research Foundation. She reported having no financial conflicts of interest.
Dr. Jun Chiong, FCCP, comments: There are similar studies published regarding preoperative beta-blockers. Most studies are from large databases and not randomized. The results are varied. Important factors such as severity of comorbid conditions prior to surgery (eg, uncontrolled diabetic, noncompliance), amount of blood loss, and surgical technique (not just the organ involved) are often not factored in. I applaud the authors for reporting a significant finding from such a large database. I hope this will encourage investigators to initiate a large, well-controlled, randomized study.
Dr. Jun Chiong, FCCP, comments: There are similar studies published regarding preoperative beta-blockers. Most studies are from large databases and not randomized. The results are varied. Important factors such as severity of comorbid conditions prior to surgery (eg, uncontrolled diabetic, noncompliance), amount of blood loss, and surgical technique (not just the organ involved) are often not factored in. I applaud the authors for reporting a significant finding from such a large database. I hope this will encourage investigators to initiate a large, well-controlled, randomized study.
Dr. Jun Chiong, FCCP, comments: There are similar studies published regarding preoperative beta-blockers. Most studies are from large databases and not randomized. The results are varied. Important factors such as severity of comorbid conditions prior to surgery (eg, uncontrolled diabetic, noncompliance), amount of blood loss, and surgical technique (not just the organ involved) are often not factored in. I applaud the authors for reporting a significant finding from such a large database. I hope this will encourage investigators to initiate a large, well-controlled, randomized study.
AMSTERDAM – Results of a new Danish national study suggest the effects of prophylactic beta-blocker therapy in patients with ischemic heart disease undergoing noncardiac surgery are considerably more heterogeneous than portrayed in current pro-prophylaxis practice guidelines or, at the opposite extreme, in a recent highly critical meta-analysis.
"This is an extraordinarily confusing area at the moment," Dr. Charlotte Andersson observed in presenting the Danish national registry findings at the annual congress of the European Society of Cardiology.

She reported on 28,263 adults with ischemic heart disease who underwent noncardiac surgery during 2004-2009. Hip or knee replacements were the most common operations, accounting for roughly one-third of the total. Patients were followed for 30 days postoperatively for the composite endpoint of acute myocardial infarction, ischemic stroke, or cardiovascular death, as well as for 30-day all-cause mortality.
In short, the effects of prophylactic beta-blocker therapy depended upon the type of background ischemic heart disease a surgical patient had.
"Our data suggest a beneficial effect of beta-blockers among patients with heart failure, perhaps a beneficial effect as well among patients with an MI within the previous 2 years, but no beneficial effect among patients with a more distant MI, and perhaps even harm associated with beta-blocker therapy among patients with neither heart failure nor a history of MI," according to Dr. Andersson of the University of Copenhagen.
The study population included 7,990 patients with heart failure, 53% of whom were on beta-blockers when they underwent noncardiac surgery. Those on beta-blockers fared significantly better in terms of the study endpoints (see graphic).
In contrast, 30-day outcomes in the 37% of patients without heart failure were identical regardless of whether or not they were on beta-blockers at surgery.
In a multivariate analysis, the use of beta-blockers in noncardiac surgery patients with heart failure was associated with a 22% reduction in major adverse cardiovascular events (MACEs) and an 18% reduction in all-cause mortality compared with no use of beta-blockers, both of which were statistically significant advantages. The analysis was adjusted for patient demographics, acute versus elective surgery, chronic obstructive pulmonary disease, diabetes, atrial fibrillation, peripheral artery disease, cancer, anemia, smoking, alcohol consumption, cerebrovascular disease, and American Society of Anesthesiologists score.
Among the 1,664 patients with an MI within the past 2 years, being on a beta-blocker at the time of surgery was associated with an adjusted highly significant 46% reduction in MACE and a 20% decrease in all-cause mortality, compared with no use of beta-blockers.
For the 1,679 patients with an MI 2-5 years prior to surgery, being on a beta-blocker was associated with a 29% reduction in the risk of MACE and a 26% reduction in 30-day all-cause mortality.
Among the 5,018 patients with an MI more than 5 years earlier, the use of beta-blockers at surgery was associated with a 35% greater risk of MACE than in nonusers of beta-blockers as well as a 33% increase in all-cause mortality. These differences in adverse outcomes rates barely missed achieving statistical significance.
Perhaps the most striking study finding was that patients with no prior MI or heart failure who were on a beta-blocker at the time of noncardiac surgery had a 44% increased risk of 30-day MACE and a 30% higher all-cause mortality, compared with those not on a beta-blocker, with both differences being significant.
Session cochair Dr. Elmir Omerovic thanked Dr. Andersson for a presentation that "really adds important new information" and asked whether she had been surprised by the findings.
"Yes, I have to say I was surprised by the increased risk in patients without prior MI or heart failure, because the ESC [European Society of Cardiology] guidelines state as a class I recommendation that all patients with ischemic heart disease undergoing noncardiac surgery should be on a beta-blocker. Perhaps we should reevaluate beta-blockers in noncardiac surgery," Dr. Andersson replied.
Current American College of Cardiology/American Heart Association guidelines also endorse perioperative beta-blockade in patients with coronary artery disease undergoing vascular or intermediate-risk noncardiac surgery.
She noted that in drawing up the current guidelines, the ESC and ACC/AHA committees relied heavily on strongly positive randomized clinical trials whose validity has recently been called into question in a major research scandal. Indeed, the lead investigator in those studies, Dr. Don Poldermans – who also happened to be chairperson of the ESC guidelines-writing task force – has been dismissed from the faculty at Erasmus University in Rotterdam.
Dr. Omerovic, of Sahlgrenska University, Gothenburg, Sweden, asked for Dr. Andersson’s thoughts regarding a new meta-analysis by investigators at Imperial College London which excluded the suspect Dutch clinical trials. The investigators concluded that initiation of perioperative beta-blocker therapy was associated with a 27% increase in 30-day all-cause mortality, a 27% reduction in nonfatal MI, a 73% increase in stroke, and a 51% increase in hypotension.
"Patient safety being paramount, guidelines for perioperative beta-blocker initiation should be retracted without further delay," the meta-analysts argued (Heart 2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
"I read that meta-analysis with great interest," Dr. Andersson said. "I think there definitely is a heterogeneity in the effects of perioperative beta-blockers, and it depends on your baseline risk. Most of the studies in the meta-analysis included many patients at lower risk."
Dr. Andersson’s study was funded by the Danish Medical Research Foundation. She reported having no financial conflicts of interest.
AMSTERDAM – Results of a new Danish national study suggest the effects of prophylactic beta-blocker therapy in patients with ischemic heart disease undergoing noncardiac surgery are considerably more heterogeneous than portrayed in current pro-prophylaxis practice guidelines or, at the opposite extreme, in a recent highly critical meta-analysis.
"This is an extraordinarily confusing area at the moment," Dr. Charlotte Andersson observed in presenting the Danish national registry findings at the annual congress of the European Society of Cardiology.

She reported on 28,263 adults with ischemic heart disease who underwent noncardiac surgery during 2004-2009. Hip or knee replacements were the most common operations, accounting for roughly one-third of the total. Patients were followed for 30 days postoperatively for the composite endpoint of acute myocardial infarction, ischemic stroke, or cardiovascular death, as well as for 30-day all-cause mortality.
In short, the effects of prophylactic beta-blocker therapy depended upon the type of background ischemic heart disease a surgical patient had.
"Our data suggest a beneficial effect of beta-blockers among patients with heart failure, perhaps a beneficial effect as well among patients with an MI within the previous 2 years, but no beneficial effect among patients with a more distant MI, and perhaps even harm associated with beta-blocker therapy among patients with neither heart failure nor a history of MI," according to Dr. Andersson of the University of Copenhagen.
The study population included 7,990 patients with heart failure, 53% of whom were on beta-blockers when they underwent noncardiac surgery. Those on beta-blockers fared significantly better in terms of the study endpoints (see graphic).
In contrast, 30-day outcomes in the 37% of patients without heart failure were identical regardless of whether or not they were on beta-blockers at surgery.
In a multivariate analysis, the use of beta-blockers in noncardiac surgery patients with heart failure was associated with a 22% reduction in major adverse cardiovascular events (MACEs) and an 18% reduction in all-cause mortality compared with no use of beta-blockers, both of which were statistically significant advantages. The analysis was adjusted for patient demographics, acute versus elective surgery, chronic obstructive pulmonary disease, diabetes, atrial fibrillation, peripheral artery disease, cancer, anemia, smoking, alcohol consumption, cerebrovascular disease, and American Society of Anesthesiologists score.
Among the 1,664 patients with an MI within the past 2 years, being on a beta-blocker at the time of surgery was associated with an adjusted highly significant 46% reduction in MACE and a 20% decrease in all-cause mortality, compared with no use of beta-blockers.
For the 1,679 patients with an MI 2-5 years prior to surgery, being on a beta-blocker was associated with a 29% reduction in the risk of MACE and a 26% reduction in 30-day all-cause mortality.
Among the 5,018 patients with an MI more than 5 years earlier, the use of beta-blockers at surgery was associated with a 35% greater risk of MACE than in nonusers of beta-blockers as well as a 33% increase in all-cause mortality. These differences in adverse outcomes rates barely missed achieving statistical significance.
Perhaps the most striking study finding was that patients with no prior MI or heart failure who were on a beta-blocker at the time of noncardiac surgery had a 44% increased risk of 30-day MACE and a 30% higher all-cause mortality, compared with those not on a beta-blocker, with both differences being significant.
Session cochair Dr. Elmir Omerovic thanked Dr. Andersson for a presentation that "really adds important new information" and asked whether she had been surprised by the findings.
"Yes, I have to say I was surprised by the increased risk in patients without prior MI or heart failure, because the ESC [European Society of Cardiology] guidelines state as a class I recommendation that all patients with ischemic heart disease undergoing noncardiac surgery should be on a beta-blocker. Perhaps we should reevaluate beta-blockers in noncardiac surgery," Dr. Andersson replied.
Current American College of Cardiology/American Heart Association guidelines also endorse perioperative beta-blockade in patients with coronary artery disease undergoing vascular or intermediate-risk noncardiac surgery.
She noted that in drawing up the current guidelines, the ESC and ACC/AHA committees relied heavily on strongly positive randomized clinical trials whose validity has recently been called into question in a major research scandal. Indeed, the lead investigator in those studies, Dr. Don Poldermans – who also happened to be chairperson of the ESC guidelines-writing task force – has been dismissed from the faculty at Erasmus University in Rotterdam.
Dr. Omerovic, of Sahlgrenska University, Gothenburg, Sweden, asked for Dr. Andersson’s thoughts regarding a new meta-analysis by investigators at Imperial College London which excluded the suspect Dutch clinical trials. The investigators concluded that initiation of perioperative beta-blocker therapy was associated with a 27% increase in 30-day all-cause mortality, a 27% reduction in nonfatal MI, a 73% increase in stroke, and a 51% increase in hypotension.
"Patient safety being paramount, guidelines for perioperative beta-blocker initiation should be retracted without further delay," the meta-analysts argued (Heart 2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
"I read that meta-analysis with great interest," Dr. Andersson said. "I think there definitely is a heterogeneity in the effects of perioperative beta-blockers, and it depends on your baseline risk. Most of the studies in the meta-analysis included many patients at lower risk."
Dr. Andersson’s study was funded by the Danish Medical Research Foundation. She reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2013
Major finding: Patients with heart failure who were on beta-blocker therapy at the time of noncardiac surgery had a 22% reduction in 30-day major adverse cardiovascular events, compared with those not on a beta-blocker perioperatively. In stark contrast, patients with ischemic heart disease but no history of heart failure or MI had a 44% greater risk of such events if they were on a perioperative beta-blocker.
Data source: This was a Danish national registry study that included more than 28,000 patients with ischemic heart disease who underwent noncardiac surgery.
Disclosures: Dr. Andersson’s study was funded by the Danish Medical Research Foundation. She reported having no financial conflicts of interest.
CABG mortality goal elusive without process improvements
MINNEAPOLIS – Achieving a 1% or less operative mortality for primary, isolated coronary artery bypass grafting is feasible only in highly selected patients, according to a multicenter Society for Thoracic Surgery database analysis.
"This goal may only be achievable in less than 60% of CABG patients without other improvements in processes of care," said Dr. Damien LaPar, chief resident in the department of surgery, University of Virginia, Charlottesville.
Dr. Michael J. Mack threw down the gauntlet in his presidential address at the STS annual meeting last year, challenging the surgical community to achieve a CABG mortality rate of 1% or less nationwide in the next 5 years (Ann. Thor. Surg. 2012;94:1044-52).
Operative mortality currently stands at about 2% for CABG versus about 1% for percutaneous coronary intervention (PCI). Use of CABG has fallen off as first-line treatment for coronary artery disease with advances in PCI technology.
Dr. LaPar and his associates used the Society of Thoracic Surgery (STS) database for adult cardiac surgery to analyze the records of 34,416 patients who had undergone CABG from 2001 to 2011 at 17 cardiac surgery centers in Virginia, representing 99% of all cardiac surgeries performed in the state. Multiple logistic regression modeling was used to identify patient populations in which the 1% mortality goal was achievable, relative to the STS Predicted Risk of Mortality (PROM) score.
The patients’ average age was 64 years and 27% were female. The median number of CABG operations performed over the 10-year study period was 544, with an operative mortality of 1.87% (644 deaths), Dr. LaPar reported at the annual meeting of the American Association for Thoracic Surgery.
The STS PROM was highly associated with CABG mortality in both linear (odds ratio 1.89; P less than .0001) and nonlinear (OR 6.59; P less than .0001) models, after adjustment for operative year and surgeon volume.
Upon closer inspection, an STS PROM score of 1.27% or less was found to correlate with a probability of CABG death of 1% or less, he said. The predictive ability of the STS scoring tool appeared to wane, however, for those patients with an estimated risk exceeding 25%.
The investigators then evaluated the risk-adjusted association between mortality and 30 variables used to calculate the STS PROM and process-of-care measures such as internal mammary artery grafting and perioperative/discharge medications.
Several process-of-care, surgeon, and operative factors were correlated with CABG death among all patients, although the relationship was stronger in those at lower risk with an STS PROM score of 1.27% or less, Dr. LaPar said.
Among the 14,687 higher-risk patients with an STS score exceeding 1.27%, the median STS score was significantly higher among decedents than survivors (4.6% vs. 2.4%; P less than .001).
Higher-risk patients who died during CABG were significantly more likely to be older (72.3 years vs. 70.4 years), to have renal dysfunction/dialysis (11.3% vs. 5.3%), peripheral vascular disease (32% vs. 24%), heart failure (37.3% vs. 21%), New York Heart Association class IV (41.6% vs. 26%), and atrial fibrillation (11% vs. 7%), and to have undergone emergent surgery (17% vs. 7.3; all P less than .001).
Higher-risk decedents were also less likely to be on such process measures at discharge as beta-blocker therapy (20% vs. 86%), anti-platelets (21% vs. 94.5%), and lipid-lowering medications (19.5% vs. 85%; all P less than .001), Dr. LaPar said.
"The STS Predicted Risk of Mortality score can be used to strongly identify patients with a threshold value of estimated mortality risk of less than or equal to 1.27% to achieve this [1% mortality] goal," he noted.
During a discussion of the results, some members of the audience raised concerns about whether patients continued on aspirin therapy until the day of surgery or were using tranexamic acid, as these agents could impact interpretation of the results. Dr. LaPar said that aspirin was allowed up to the day of surgery, but that the analyses corrected for this, and that all patients received epsilon-aminocaproic acid (Amicar), not tranexamic acid.
Dr. LaPar reported having no financial disclosures.
MINNEAPOLIS – Achieving a 1% or less operative mortality for primary, isolated coronary artery bypass grafting is feasible only in highly selected patients, according to a multicenter Society for Thoracic Surgery database analysis.
"This goal may only be achievable in less than 60% of CABG patients without other improvements in processes of care," said Dr. Damien LaPar, chief resident in the department of surgery, University of Virginia, Charlottesville.
Dr. Michael J. Mack threw down the gauntlet in his presidential address at the STS annual meeting last year, challenging the surgical community to achieve a CABG mortality rate of 1% or less nationwide in the next 5 years (Ann. Thor. Surg. 2012;94:1044-52).
Operative mortality currently stands at about 2% for CABG versus about 1% for percutaneous coronary intervention (PCI). Use of CABG has fallen off as first-line treatment for coronary artery disease with advances in PCI technology.
Dr. LaPar and his associates used the Society of Thoracic Surgery (STS) database for adult cardiac surgery to analyze the records of 34,416 patients who had undergone CABG from 2001 to 2011 at 17 cardiac surgery centers in Virginia, representing 99% of all cardiac surgeries performed in the state. Multiple logistic regression modeling was used to identify patient populations in which the 1% mortality goal was achievable, relative to the STS Predicted Risk of Mortality (PROM) score.
The patients’ average age was 64 years and 27% were female. The median number of CABG operations performed over the 10-year study period was 544, with an operative mortality of 1.87% (644 deaths), Dr. LaPar reported at the annual meeting of the American Association for Thoracic Surgery.
The STS PROM was highly associated with CABG mortality in both linear (odds ratio 1.89; P less than .0001) and nonlinear (OR 6.59; P less than .0001) models, after adjustment for operative year and surgeon volume.
Upon closer inspection, an STS PROM score of 1.27% or less was found to correlate with a probability of CABG death of 1% or less, he said. The predictive ability of the STS scoring tool appeared to wane, however, for those patients with an estimated risk exceeding 25%.
The investigators then evaluated the risk-adjusted association between mortality and 30 variables used to calculate the STS PROM and process-of-care measures such as internal mammary artery grafting and perioperative/discharge medications.
Several process-of-care, surgeon, and operative factors were correlated with CABG death among all patients, although the relationship was stronger in those at lower risk with an STS PROM score of 1.27% or less, Dr. LaPar said.
Among the 14,687 higher-risk patients with an STS score exceeding 1.27%, the median STS score was significantly higher among decedents than survivors (4.6% vs. 2.4%; P less than .001).
Higher-risk patients who died during CABG were significantly more likely to be older (72.3 years vs. 70.4 years), to have renal dysfunction/dialysis (11.3% vs. 5.3%), peripheral vascular disease (32% vs. 24%), heart failure (37.3% vs. 21%), New York Heart Association class IV (41.6% vs. 26%), and atrial fibrillation (11% vs. 7%), and to have undergone emergent surgery (17% vs. 7.3; all P less than .001).
Higher-risk decedents were also less likely to be on such process measures at discharge as beta-blocker therapy (20% vs. 86%), anti-platelets (21% vs. 94.5%), and lipid-lowering medications (19.5% vs. 85%; all P less than .001), Dr. LaPar said.
"The STS Predicted Risk of Mortality score can be used to strongly identify patients with a threshold value of estimated mortality risk of less than or equal to 1.27% to achieve this [1% mortality] goal," he noted.
During a discussion of the results, some members of the audience raised concerns about whether patients continued on aspirin therapy until the day of surgery or were using tranexamic acid, as these agents could impact interpretation of the results. Dr. LaPar said that aspirin was allowed up to the day of surgery, but that the analyses corrected for this, and that all patients received epsilon-aminocaproic acid (Amicar), not tranexamic acid.
Dr. LaPar reported having no financial disclosures.
MINNEAPOLIS – Achieving a 1% or less operative mortality for primary, isolated coronary artery bypass grafting is feasible only in highly selected patients, according to a multicenter Society for Thoracic Surgery database analysis.
"This goal may only be achievable in less than 60% of CABG patients without other improvements in processes of care," said Dr. Damien LaPar, chief resident in the department of surgery, University of Virginia, Charlottesville.
Dr. Michael J. Mack threw down the gauntlet in his presidential address at the STS annual meeting last year, challenging the surgical community to achieve a CABG mortality rate of 1% or less nationwide in the next 5 years (Ann. Thor. Surg. 2012;94:1044-52).
Operative mortality currently stands at about 2% for CABG versus about 1% for percutaneous coronary intervention (PCI). Use of CABG has fallen off as first-line treatment for coronary artery disease with advances in PCI technology.
Dr. LaPar and his associates used the Society of Thoracic Surgery (STS) database for adult cardiac surgery to analyze the records of 34,416 patients who had undergone CABG from 2001 to 2011 at 17 cardiac surgery centers in Virginia, representing 99% of all cardiac surgeries performed in the state. Multiple logistic regression modeling was used to identify patient populations in which the 1% mortality goal was achievable, relative to the STS Predicted Risk of Mortality (PROM) score.
The patients’ average age was 64 years and 27% were female. The median number of CABG operations performed over the 10-year study period was 544, with an operative mortality of 1.87% (644 deaths), Dr. LaPar reported at the annual meeting of the American Association for Thoracic Surgery.
The STS PROM was highly associated with CABG mortality in both linear (odds ratio 1.89; P less than .0001) and nonlinear (OR 6.59; P less than .0001) models, after adjustment for operative year and surgeon volume.
Upon closer inspection, an STS PROM score of 1.27% or less was found to correlate with a probability of CABG death of 1% or less, he said. The predictive ability of the STS scoring tool appeared to wane, however, for those patients with an estimated risk exceeding 25%.
The investigators then evaluated the risk-adjusted association between mortality and 30 variables used to calculate the STS PROM and process-of-care measures such as internal mammary artery grafting and perioperative/discharge medications.
Several process-of-care, surgeon, and operative factors were correlated with CABG death among all patients, although the relationship was stronger in those at lower risk with an STS PROM score of 1.27% or less, Dr. LaPar said.
Among the 14,687 higher-risk patients with an STS score exceeding 1.27%, the median STS score was significantly higher among decedents than survivors (4.6% vs. 2.4%; P less than .001).
Higher-risk patients who died during CABG were significantly more likely to be older (72.3 years vs. 70.4 years), to have renal dysfunction/dialysis (11.3% vs. 5.3%), peripheral vascular disease (32% vs. 24%), heart failure (37.3% vs. 21%), New York Heart Association class IV (41.6% vs. 26%), and atrial fibrillation (11% vs. 7%), and to have undergone emergent surgery (17% vs. 7.3; all P less than .001).
Higher-risk decedents were also less likely to be on such process measures at discharge as beta-blocker therapy (20% vs. 86%), anti-platelets (21% vs. 94.5%), and lipid-lowering medications (19.5% vs. 85%; all P less than .001), Dr. LaPar said.
"The STS Predicted Risk of Mortality score can be used to strongly identify patients with a threshold value of estimated mortality risk of less than or equal to 1.27% to achieve this [1% mortality] goal," he noted.
During a discussion of the results, some members of the audience raised concerns about whether patients continued on aspirin therapy until the day of surgery or were using tranexamic acid, as these agents could impact interpretation of the results. Dr. LaPar said that aspirin was allowed up to the day of surgery, but that the analyses corrected for this, and that all patients received epsilon-aminocaproic acid (Amicar), not tranexamic acid.
Dr. LaPar reported having no financial disclosures.
AT THE AATS ANNUAL MEETING
Major finding: The STS PROM was significantly correlated with coronary artery bypass grafting mortality in both linear (odds ratio 1.89; P less than .0001) and nonlinear (OR 6.59; P less than .0001) models, after adjustment for operative year and surgeon volume.
Data source: Retrospective analysis of 34,416 patients who underwent CABG from 2001 to 2011 in STS database.
Disclosures: Dr. LaPar reported having no financial disclosures.
Early surgery vs. watchful waiting for flail mitral leaflets
In patients who have asymptomatic or minimally symptomatic mitral valve regurgitation as a result of flail mitral leaflets, early surgery yields markedly better survival, lower risk of heart failure, and equivalent rates of atrial fibrillation, compared with watchful waiting, according to a report published online August 13 in JAMA.
Overall long-term mortality is approximately 40% lower and HF risk is approximately 60% lower for early surgery than for watchful waiting. Moreover, these benefits persist for up to 20 years and are seen across every important subgroup of patients, said Dr. Rakesh M. Suri of the Mayo Clinic, Rochester, Minn., and his associates.
These are the findings of a series of analyses of data from an international registry of consecutive patients diagnosed in routine clinical practice – the largest study in the world of the comparative effectiveness of early surgery vs. watchful waiting in patients without traditional indications for immediate surgery. The study results "emanate from institutions that together provide a very high rate of mitral valve repair (more than 90%) with low operative mortality, emphasizing that such results might also be achieved in routine practice at many advanced repair centers," the investigators noted.
Despite the safety and efficacy of current surgical correction of flail mitral leaflets, clinicians disagree as to the best approach for patients who have no or minimal HF symptoms, a left ventricular ejection fraction of 60% or more, and a left ventricular end-systolic diameter of 40 mm or more. Those who support watchful waiting consider the consequences of uncorrected mitral regurgitation to be benign, especially when weighed against the potential morbidity and mortality of early surgical intervention. In particular, North American guidelines favor early surgery while European guidelines favor watchful waiting.
The Mitral Regurgitation International Database (MIDA) – a registry of patients at two tertiary care centers in France, two in Italy, one in Belgium, and one in the United States – provided an ideal study population to compare the two approaches. For their study, Dr. Suri and his associates examined data for 1,021 of these patients who had been diagnosed in 1980-2004 and followed for up to 25 years (mean follow-up, 10.3 years).
This study included only patients who had no ischemic mitral regurgitation and no significant concomitant aortic valve disease, congenital heart disease, mitral stenosis, or previous valve surgery.
A total of 446 of these patients underwent early surgery (within 30 days of diagnosis) and 575 had watchful waiting at the discretion of their treating physicians. Importantly, 339 (59%) of the watchful-waiting group eventually were advised by their cardiologists to undergo valve repair, at a median of 1.65 years after diagnosis.
Overall, there were 319 deaths during follow-up.
The primary end points of this study were all-cause mortality at 5, 10, and 20 years.
In the initial, unadjusted analysis, survival was 95%, 86%, and 63%, respectively, with early surgery. In contrast, survival was significantly lower with watchful waiting, at 84% at 5 years, 69% at 10 years, and 41% at 20 years, the researchers said (JAMA 2013; 310:609-16 [doi:10.1001/jama.2013.8643]).
The large survival benefit with early surgery was confirmed in a multivariable analysis that adjusted for patient age, sex, comorbidities, and the presence of subtle symptoms.
To account for differences between the two study groups in the propensity to undergo surgery, the investigators performed an analysis of the data in a set of 648 patients who were matched for age, comorbidities, and other factors. This analysis also showed a similar and distinct survival advantage with early surgery. Several subgroup analyses also confirmed the results.
Secondary end points were the incidence of heart failure and the onset of new atrial fibrillation during follow-up.
A total of 167 patients had at least one episode of HF. The rates were 7% with early surgery and 23% with watchful waiting at 10 years, and 10% vs. 35% at 20 years, showing a clear advantage for early surgery.
This strong advantage persisted in further analyses of the propensity-matched patients and in all other subgroups examined, at all time points examined.
New-onset AF developed in 227 patients overall. The rate was slightly higher in the early-surgery group during the immediate postoperative period but decreased thereafter and was equivalent between the two study groups at 5, 10, and 20 years.
"Long-term, the results are coherent by all methods used (direct comparison, adjusted comparison, propensity score matching, inverse probability weighing) that early surgical correction of mitral valve regurgitation was associated with a significant survival benefit (total mortality decrement of approximately 40%) and diminished HF risk (reduction of approximately 60%)," Dr. Suri and his associates wrote.
Dr. Suri reported ties to Edwards Lifesciences, Sorin Group, St. Jude Medical, and Abbott. His associates reported ties to Edwards Lifesciences, Valtech, and Abbott Vascular.

|
|
The findings by Dr. Suri and his associates indicate that it may be more beneficial to offer early surgery rather than wait for symptom onset or classic indications for intervention, said Dr. Catherine M. Otto.
"However, if surgical risk is high or if the likelihood of valve repair is low, it remains uncertain whether early surgical intervention is appropriate in the asymptomatic patient with severe mitral regurgitation due to a flail leaflet when LV size and systolic function are normal. Although the majority of these patients will develop clear indications for valve surgery within 2 years, it may be reasonable to postpone the risks of having an intervention and having a prosthetic valve as long as possible," she said.
Catherine M. Otto. M.D., is with the division of cardiology at the University of Washington, Seattle. She reported no financial conflicts of interest. These remarks were taken from her editorial (JAMA 2013;310:587-8) accompanying Dr. Suri’s report.

|
|
The findings by Dr. Suri and his associates indicate that it may be more beneficial to offer early surgery rather than wait for symptom onset or classic indications for intervention, said Dr. Catherine M. Otto.
"However, if surgical risk is high or if the likelihood of valve repair is low, it remains uncertain whether early surgical intervention is appropriate in the asymptomatic patient with severe mitral regurgitation due to a flail leaflet when LV size and systolic function are normal. Although the majority of these patients will develop clear indications for valve surgery within 2 years, it may be reasonable to postpone the risks of having an intervention and having a prosthetic valve as long as possible," she said.
Catherine M. Otto. M.D., is with the division of cardiology at the University of Washington, Seattle. She reported no financial conflicts of interest. These remarks were taken from her editorial (JAMA 2013;310:587-8) accompanying Dr. Suri’s report.

|
|
The findings by Dr. Suri and his associates indicate that it may be more beneficial to offer early surgery rather than wait for symptom onset or classic indications for intervention, said Dr. Catherine M. Otto.
"However, if surgical risk is high or if the likelihood of valve repair is low, it remains uncertain whether early surgical intervention is appropriate in the asymptomatic patient with severe mitral regurgitation due to a flail leaflet when LV size and systolic function are normal. Although the majority of these patients will develop clear indications for valve surgery within 2 years, it may be reasonable to postpone the risks of having an intervention and having a prosthetic valve as long as possible," she said.
Catherine M. Otto. M.D., is with the division of cardiology at the University of Washington, Seattle. She reported no financial conflicts of interest. These remarks were taken from her editorial (JAMA 2013;310:587-8) accompanying Dr. Suri’s report.
In patients who have asymptomatic or minimally symptomatic mitral valve regurgitation as a result of flail mitral leaflets, early surgery yields markedly better survival, lower risk of heart failure, and equivalent rates of atrial fibrillation, compared with watchful waiting, according to a report published online August 13 in JAMA.
Overall long-term mortality is approximately 40% lower and HF risk is approximately 60% lower for early surgery than for watchful waiting. Moreover, these benefits persist for up to 20 years and are seen across every important subgroup of patients, said Dr. Rakesh M. Suri of the Mayo Clinic, Rochester, Minn., and his associates.
These are the findings of a series of analyses of data from an international registry of consecutive patients diagnosed in routine clinical practice – the largest study in the world of the comparative effectiveness of early surgery vs. watchful waiting in patients without traditional indications for immediate surgery. The study results "emanate from institutions that together provide a very high rate of mitral valve repair (more than 90%) with low operative mortality, emphasizing that such results might also be achieved in routine practice at many advanced repair centers," the investigators noted.
Despite the safety and efficacy of current surgical correction of flail mitral leaflets, clinicians disagree as to the best approach for patients who have no or minimal HF symptoms, a left ventricular ejection fraction of 60% or more, and a left ventricular end-systolic diameter of 40 mm or more. Those who support watchful waiting consider the consequences of uncorrected mitral regurgitation to be benign, especially when weighed against the potential morbidity and mortality of early surgical intervention. In particular, North American guidelines favor early surgery while European guidelines favor watchful waiting.
The Mitral Regurgitation International Database (MIDA) – a registry of patients at two tertiary care centers in France, two in Italy, one in Belgium, and one in the United States – provided an ideal study population to compare the two approaches. For their study, Dr. Suri and his associates examined data for 1,021 of these patients who had been diagnosed in 1980-2004 and followed for up to 25 years (mean follow-up, 10.3 years).
This study included only patients who had no ischemic mitral regurgitation and no significant concomitant aortic valve disease, congenital heart disease, mitral stenosis, or previous valve surgery.
A total of 446 of these patients underwent early surgery (within 30 days of diagnosis) and 575 had watchful waiting at the discretion of their treating physicians. Importantly, 339 (59%) of the watchful-waiting group eventually were advised by their cardiologists to undergo valve repair, at a median of 1.65 years after diagnosis.
Overall, there were 319 deaths during follow-up.
The primary end points of this study were all-cause mortality at 5, 10, and 20 years.
In the initial, unadjusted analysis, survival was 95%, 86%, and 63%, respectively, with early surgery. In contrast, survival was significantly lower with watchful waiting, at 84% at 5 years, 69% at 10 years, and 41% at 20 years, the researchers said (JAMA 2013; 310:609-16 [doi:10.1001/jama.2013.8643]).
The large survival benefit with early surgery was confirmed in a multivariable analysis that adjusted for patient age, sex, comorbidities, and the presence of subtle symptoms.
To account for differences between the two study groups in the propensity to undergo surgery, the investigators performed an analysis of the data in a set of 648 patients who were matched for age, comorbidities, and other factors. This analysis also showed a similar and distinct survival advantage with early surgery. Several subgroup analyses also confirmed the results.
Secondary end points were the incidence of heart failure and the onset of new atrial fibrillation during follow-up.
A total of 167 patients had at least one episode of HF. The rates were 7% with early surgery and 23% with watchful waiting at 10 years, and 10% vs. 35% at 20 years, showing a clear advantage for early surgery.
This strong advantage persisted in further analyses of the propensity-matched patients and in all other subgroups examined, at all time points examined.
New-onset AF developed in 227 patients overall. The rate was slightly higher in the early-surgery group during the immediate postoperative period but decreased thereafter and was equivalent between the two study groups at 5, 10, and 20 years.
"Long-term, the results are coherent by all methods used (direct comparison, adjusted comparison, propensity score matching, inverse probability weighing) that early surgical correction of mitral valve regurgitation was associated with a significant survival benefit (total mortality decrement of approximately 40%) and diminished HF risk (reduction of approximately 60%)," Dr. Suri and his associates wrote.
Dr. Suri reported ties to Edwards Lifesciences, Sorin Group, St. Jude Medical, and Abbott. His associates reported ties to Edwards Lifesciences, Valtech, and Abbott Vascular.
In patients who have asymptomatic or minimally symptomatic mitral valve regurgitation as a result of flail mitral leaflets, early surgery yields markedly better survival, lower risk of heart failure, and equivalent rates of atrial fibrillation, compared with watchful waiting, according to a report published online August 13 in JAMA.
Overall long-term mortality is approximately 40% lower and HF risk is approximately 60% lower for early surgery than for watchful waiting. Moreover, these benefits persist for up to 20 years and are seen across every important subgroup of patients, said Dr. Rakesh M. Suri of the Mayo Clinic, Rochester, Minn., and his associates.
These are the findings of a series of analyses of data from an international registry of consecutive patients diagnosed in routine clinical practice – the largest study in the world of the comparative effectiveness of early surgery vs. watchful waiting in patients without traditional indications for immediate surgery. The study results "emanate from institutions that together provide a very high rate of mitral valve repair (more than 90%) with low operative mortality, emphasizing that such results might also be achieved in routine practice at many advanced repair centers," the investigators noted.
Despite the safety and efficacy of current surgical correction of flail mitral leaflets, clinicians disagree as to the best approach for patients who have no or minimal HF symptoms, a left ventricular ejection fraction of 60% or more, and a left ventricular end-systolic diameter of 40 mm or more. Those who support watchful waiting consider the consequences of uncorrected mitral regurgitation to be benign, especially when weighed against the potential morbidity and mortality of early surgical intervention. In particular, North American guidelines favor early surgery while European guidelines favor watchful waiting.
The Mitral Regurgitation International Database (MIDA) – a registry of patients at two tertiary care centers in France, two in Italy, one in Belgium, and one in the United States – provided an ideal study population to compare the two approaches. For their study, Dr. Suri and his associates examined data for 1,021 of these patients who had been diagnosed in 1980-2004 and followed for up to 25 years (mean follow-up, 10.3 years).
This study included only patients who had no ischemic mitral regurgitation and no significant concomitant aortic valve disease, congenital heart disease, mitral stenosis, or previous valve surgery.
A total of 446 of these patients underwent early surgery (within 30 days of diagnosis) and 575 had watchful waiting at the discretion of their treating physicians. Importantly, 339 (59%) of the watchful-waiting group eventually were advised by their cardiologists to undergo valve repair, at a median of 1.65 years after diagnosis.
Overall, there were 319 deaths during follow-up.
The primary end points of this study were all-cause mortality at 5, 10, and 20 years.
In the initial, unadjusted analysis, survival was 95%, 86%, and 63%, respectively, with early surgery. In contrast, survival was significantly lower with watchful waiting, at 84% at 5 years, 69% at 10 years, and 41% at 20 years, the researchers said (JAMA 2013; 310:609-16 [doi:10.1001/jama.2013.8643]).
The large survival benefit with early surgery was confirmed in a multivariable analysis that adjusted for patient age, sex, comorbidities, and the presence of subtle symptoms.
To account for differences between the two study groups in the propensity to undergo surgery, the investigators performed an analysis of the data in a set of 648 patients who were matched for age, comorbidities, and other factors. This analysis also showed a similar and distinct survival advantage with early surgery. Several subgroup analyses also confirmed the results.
Secondary end points were the incidence of heart failure and the onset of new atrial fibrillation during follow-up.
A total of 167 patients had at least one episode of HF. The rates were 7% with early surgery and 23% with watchful waiting at 10 years, and 10% vs. 35% at 20 years, showing a clear advantage for early surgery.
This strong advantage persisted in further analyses of the propensity-matched patients and in all other subgroups examined, at all time points examined.
New-onset AF developed in 227 patients overall. The rate was slightly higher in the early-surgery group during the immediate postoperative period but decreased thereafter and was equivalent between the two study groups at 5, 10, and 20 years.
"Long-term, the results are coherent by all methods used (direct comparison, adjusted comparison, propensity score matching, inverse probability weighing) that early surgical correction of mitral valve regurgitation was associated with a significant survival benefit (total mortality decrement of approximately 40%) and diminished HF risk (reduction of approximately 60%)," Dr. Suri and his associates wrote.
Dr. Suri reported ties to Edwards Lifesciences, Sorin Group, St. Jude Medical, and Abbott. His associates reported ties to Edwards Lifesciences, Valtech, and Abbott Vascular.
FROM JAMA
Major finding: Survival at 5, 10, and 20 years was 95%, 86%, and 63%, respectively, with early surgery, compared with 84%, 69%, and 41%, respectively, with watchful waiting.
Data source: Serial analyses of data on 1,021 patients with asymptomatic or minimally symptomatic mitral valve regurgitation caused by flail mitral leaflets who were advised to undergo early repair (446 subjects) or watchful waiting (575 subjects) and were followed for up to 25 years.
Disclosures: Dr. Suri reported ties to Edwards Lifesciences, Sorin Group, St. Jude Medical, and Abbott. His associates reported ties to Edwards Lifesciences, Valtech, and Abbott Vascular.
Edwards to start U.S. trial of SAPIEN 3
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller