User login
How should you use the lab to monitor patients taking a mood stabilizer?
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
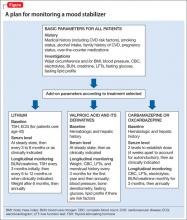
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
Four questions address stigma
Naomi is a 61-year-old woman who has lived with bipolar disorder and its stigma for 30 years. After a major manic episode and hospitalization, she entered into family treatment at the urging of her three daughters. Previously, her husband had been the primary force in guiding her psychiatric care, and she had been in treatment with a psychiatrist who is his professional colleague.
The patient’s first depressive episode began in the postpartum period, but she did not seek help at that time because she thought that her feelings were normal for a new mother. She did not receive any psychiatric attention until she cycled into mania and called the police for fear her child was being poisoned by neighbors. Her most recent manic episode occurred after she stopped her medications because of concerns about side effects. She was too embarrassed to tell her husband or doctor. She routinely fails to tell her other medical doctors that she is on mood stabilizers, because she does not want them to know she has bipolar disorder.
As Naomi recovers from the most recent manic episode and settles into family treatment, she is struggling with the consequences of her actions to her family. In family therapy in the past, her husband has revealed his belief that he has been protecting the family from Naomi’s mania and protecting Naomi from "embarrassing herself." This is difficult for Naomi to hear as she has always prided herself on being a good mother and protecting her daughters. Naomi’s situation illustrates the difficulty of coping with a diagnosis of bipolar disorder, the consequences of the illness on the family, and the importance of addressing stigma.
How stigma gets in the way
As discussed previously by Dr. Alison M. Heru ("Mental illness stigma is a family affair," Clinical Psychiatry News, April 2014, p. 8), stigma, when internalized or self-directed, can lead to psychological distress, decreased self-esteem and life satisfaction, and increased depression and suicidality (Compr. Psychiatry 2010;51:603-6). Close family members of those with mental disorders are affected by stigma, commonly referred to as "stigma by association" or "courtesy stigma."
Up to 92% of caregivers of people with psychiatric disorders have reported internalized stigma (J. Psychiatr. Ment. Health Nurs. 2012;19:665-71). These family members become distant and avoidant, resulting in a reduced quality of life and an impaired ability to provide critical support for their loved ones. Caregiver anxiety is inversely related to patient anxiety, stigma, and poor alliance (J. Nerv. Ment. Disease 2011;199:18-24).
As a result of these factors, while people with psychiatric disorders have to cope with their own mental illness as well as the public and self-stigma that alienate them from society, they also are at risk of losing their family connections.
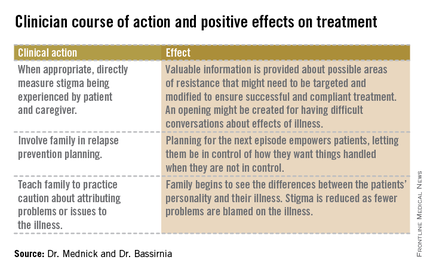
In order to confront stigma, the Family Center for Bipolar Disorder in New York City, for example, uses a Family Inclusive Treatment (FIT) model. The FIT model includes an engagement period at the initiation of treatment that is focused on psychoeducation and relapse prevention planning. FIT is unique in that every patient is required to sign a release of information giving permission for full, open communication at all times between the patient’s clinician and a treatment partner of their choice. After the initial engagement period, there are quarterly family visits to supplement regular individual treatment sessions. FIT treatment promotes open communication about symptoms and medications. FIT strives to minimize patient isolation from families; they can talk openly with one another and their clinician.
After seeing many families enter treatment, FIT staff noticed the prominence of stigma.
We have begun to ask about stigma directly. Do people with more stigma do worse in treatment? Do they adhere more poorly to treatment? Do their families tend to become less involved over time? To begin, Dr. Mednick and staff examined demographic data looking for factors that might predispose a person to experience increased stigma.
In terms of diagnosis, people with more internalizing disorders such as depression and anxiety disorders tend to experience more stigma. Distress is experienced internally. As Dr. Bassirnia and her colleagues wrote in a poster presented at the recent American Psychiatric Association meeting, people with externalizing disorders, such as substance abuse and antisocial disorders, are more likely to express their distress outwardly and are less likely to suffer from stigma ("The relationship between personality traits and perceived internalized stigma in bipolar patients and their caregivers," 2014).
Meanwhile, two systematic review studies have reported moderate to high levels of internalized stigma in people with bipolar disorder. In these studies, a higher level of internalized stigma had a negative correlation with self-esteem, social adjustment, and perceived social support, and positive correlation with severity of symptoms, functional impairment, and rehospitalization. In spite of having more severe symptoms; people with higher levels of self-stigma are less likely to seek professional help and adhere to their treatment. Stigma by association and its negative consequences in caregivers of people with mental disorders also have been reported (J. Affect. Disord. 2013;150:181-91).
A useful and easy to administer scale that helps to identify stigma is the "Perceived Criticism Scale" (J. Abnorm. Psychol. 1989;98:225-35). By asking four questions, the clinician can get a good sense of family dynamics and can monitor the progress and change over time. The questions rate perception on a scale of 1-10, where "X" is the other person involved in treatment, either patient or caregiver. Here are the questions:
1. How critical do you think you are of X?
2. How critical do you think X is of you?
3. When X criticizes you, how upset do you get?
4. When you criticize X, how upset does he/she get?
For families with high scores, follow-up is needed. The Internalized Stigma of Mental Illness (ISMI) scale (Psychiatry Res. 2003;121:31-49) can be used. The ISMI scale makes statements about stigma for which participants rate their agreement on a Likert scale, such as:
• I don’t talk about myself much because I don’t want to burden others with my mental illness.
• Being around people who don’t have a mental illness makes me feel out of place or inadequate.
• People can tell that I have a mental illness by the way I look.
• Mentally ill people tend to be violent.
• I feel out of place in the world because I have a mental illness.
The ISMI scale contains 29 short, simple statements like the ones above and can be completed in less than 10 minutes. The statements are designed to avoid hypothetical situations, stay focused in the present, and address the participant’s own identity and experience.
Using the tools in practice
Naomi entered family treatment with her husband and daughters. Using the ISMI to measure the stigma of mental illness that each family member was experiencing, Naomi was shocked to see that her daughters felt far less stigma about having a mother with mental illness than she had assumed. In turn, her daughters were shocked at how much stigma Naomi was experiencing. Naomi’s husband scored between them. This data paved the way for an open family conversation about how Naomi’s illness had affected their lives, and especially how Naomi’s husband and his perceptions of her illness had affected her treatment course.
Caregivers play a very important role in bipolar disorder. After all, the illness can lead to difficulty functioning and can threaten the family’s stability. Sometimes caregivers can serve as a source of strength and a beacon of stability in the occasional storm. It is hard for the family between the storms, when the same flashing beacon can be a constant reminder to the patient of their illness. Often, well intentioned concerns become constant checking up, making the patient feel stigmatized and expected to fail.
"Good" caregivers will be aware of the stigma and the impact it has on their loved one and on themselves, without becoming a source of stigma.
Dr. Mednick is an attending psychiatrist at the Family Center for Bipolar at Mount Sinai Beth Israel in New York City. Dr. Bassirnia is a second-year psychiatry resident at Mount Sinai Beth Israel. Scan the QR code to read more Families in Psychiatry columns at clinicalpsychiatrynews.com.
Naomi is a 61-year-old woman who has lived with bipolar disorder and its stigma for 30 years. After a major manic episode and hospitalization, she entered into family treatment at the urging of her three daughters. Previously, her husband had been the primary force in guiding her psychiatric care, and she had been in treatment with a psychiatrist who is his professional colleague.
The patient’s first depressive episode began in the postpartum period, but she did not seek help at that time because she thought that her feelings were normal for a new mother. She did not receive any psychiatric attention until she cycled into mania and called the police for fear her child was being poisoned by neighbors. Her most recent manic episode occurred after she stopped her medications because of concerns about side effects. She was too embarrassed to tell her husband or doctor. She routinely fails to tell her other medical doctors that she is on mood stabilizers, because she does not want them to know she has bipolar disorder.

As Naomi recovers from the most recent manic episode and settles into family treatment, she is struggling with the consequences of her actions to her family. In family therapy in the past, her husband has revealed his belief that he has been protecting the family from Naomi’s mania and protecting Naomi from "embarrassing herself." This is difficult for Naomi to hear as she has always prided herself on being a good mother and protecting her daughters. Naomi’s situation illustrates the difficulty of coping with a diagnosis of bipolar disorder, the consequences of the illness on the family, and the importance of addressing stigma.
How stigma gets in the way
As discussed previously by Dr. Alison M. Heru ("Mental illness stigma is a family affair," Clinical Psychiatry News, April 2014, p. 8), stigma, when internalized or self-directed, can lead to psychological distress, decreased self-esteem and life satisfaction, and increased depression and suicidality (Compr. Psychiatry 2010;51:603-6). Close family members of those with mental disorders are affected by stigma, commonly referred to as "stigma by association" or "courtesy stigma."
Up to 92% of caregivers of people with psychiatric disorders have reported internalized stigma (J. Psychiatr. Ment. Health Nurs. 2012;19:665-71). These family members become distant and avoidant, resulting in a reduced quality of life and an impaired ability to provide critical support for their loved ones. Caregiver anxiety is inversely related to patient anxiety, stigma, and poor alliance (J. Nerv. Ment. Disease 2011;199:18-24).
As a result of these factors, while people with psychiatric disorders have to cope with their own mental illness as well as the public and self-stigma that alienate them from society, they also are at risk of losing their family connections.
In order to confront stigma, the Family Center for Bipolar Disorder in New York City, for example, uses a Family Inclusive Treatment (FIT) model. The FIT model includes an engagement period at the initiation of treatment that is focused on psychoeducation and relapse prevention planning. FIT is unique in that every patient is required to sign a release of information giving permission for full, open communication at all times between the patient’s clinician and a treatment partner of their choice. After the initial engagement period, there are quarterly family visits to supplement regular individual treatment sessions. FIT treatment promotes open communication about symptoms and medications. FIT strives to minimize patient isolation from families; they can talk openly with one another and their clinician.
After seeing many families enter treatment, FIT staff noticed the prominence of stigma.
We have begun to ask about stigma directly. Do people with more stigma do worse in treatment? Do they adhere more poorly to treatment? Do their families tend to become less involved over time? To begin, Dr. Mednick and staff examined demographic data looking for factors that might predispose a person to experience increased stigma.
In terms of diagnosis, people with more internalizing disorders such as depression and anxiety disorders tend to experience more stigma. Distress is experienced internally. As Dr. Bassirnia and her colleagues wrote in a poster presented at the recent American Psychiatric Association meeting, people with externalizing disorders, such as substance abuse and antisocial disorders, are more likely to express their distress outwardly and are less likely to suffer from stigma ("The relationship between personality traits and perceived internalized stigma in bipolar patients and their caregivers," 2014).
Meanwhile, two systematic review studies have reported moderate to high levels of internalized stigma in people with bipolar disorder. In these studies, a higher level of internalized stigma had a negative correlation with self-esteem, social adjustment, and perceived social support, and positive correlation with severity of symptoms, functional impairment, and rehospitalization. In spite of having more severe symptoms; people with higher levels of self-stigma are less likely to seek professional help and adhere to their treatment. Stigma by association and its negative consequences in caregivers of people with mental disorders also have been reported (J. Affect. Disord. 2013;150:181-91).
A useful and easy to administer scale that helps to identify stigma is the "Perceived Criticism Scale" (J. Abnorm. Psychol. 1989;98:225-35). By asking four questions, the clinician can get a good sense of family dynamics and can monitor the progress and change over time. The questions rate perception on a scale of 1-10, where "X" is the other person involved in treatment, either patient or caregiver. Here are the questions:
1. How critical do you think you are of X?
2. How critical do you think X is of you?
3. When X criticizes you, how upset do you get?
4. When you criticize X, how upset does he/she get?
For families with high scores, follow-up is needed. The Internalized Stigma of Mental Illness (ISMI) scale (Psychiatry Res. 2003;121:31-49) can be used. The ISMI scale makes statements about stigma for which participants rate their agreement on a Likert scale, such as:
• I don’t talk about myself much because I don’t want to burden others with my mental illness.
• Being around people who don’t have a mental illness makes me feel out of place or inadequate.
• People can tell that I have a mental illness by the way I look.
• Mentally ill people tend to be violent.
• I feel out of place in the world because I have a mental illness.
The ISMI scale contains 29 short, simple statements like the ones above and can be completed in less than 10 minutes. The statements are designed to avoid hypothetical situations, stay focused in the present, and address the participant’s own identity and experience.
Using the tools in practice
Naomi entered family treatment with her husband and daughters. Using the ISMI to measure the stigma of mental illness that each family member was experiencing, Naomi was shocked to see that her daughters felt far less stigma about having a mother with mental illness than she had assumed. In turn, her daughters were shocked at how much stigma Naomi was experiencing. Naomi’s husband scored between them. This data paved the way for an open family conversation about how Naomi’s illness had affected their lives, and especially how Naomi’s husband and his perceptions of her illness had affected her treatment course.
Caregivers play a very important role in bipolar disorder. After all, the illness can lead to difficulty functioning and can threaten the family’s stability. Sometimes caregivers can serve as a source of strength and a beacon of stability in the occasional storm. It is hard for the family between the storms, when the same flashing beacon can be a constant reminder to the patient of their illness. Often, well intentioned concerns become constant checking up, making the patient feel stigmatized and expected to fail.
"Good" caregivers will be aware of the stigma and the impact it has on their loved one and on themselves, without becoming a source of stigma.
Dr. Mednick is an attending psychiatrist at the Family Center for Bipolar at Mount Sinai Beth Israel in New York City. Dr. Bassirnia is a second-year psychiatry resident at Mount Sinai Beth Israel. Scan the QR code to read more Families in Psychiatry columns at clinicalpsychiatrynews.com.
Naomi is a 61-year-old woman who has lived with bipolar disorder and its stigma for 30 years. After a major manic episode and hospitalization, she entered into family treatment at the urging of her three daughters. Previously, her husband had been the primary force in guiding her psychiatric care, and she had been in treatment with a psychiatrist who is his professional colleague.
The patient’s first depressive episode began in the postpartum period, but she did not seek help at that time because she thought that her feelings were normal for a new mother. She did not receive any psychiatric attention until she cycled into mania and called the police for fear her child was being poisoned by neighbors. Her most recent manic episode occurred after she stopped her medications because of concerns about side effects. She was too embarrassed to tell her husband or doctor. She routinely fails to tell her other medical doctors that she is on mood stabilizers, because she does not want them to know she has bipolar disorder.

As Naomi recovers from the most recent manic episode and settles into family treatment, she is struggling with the consequences of her actions to her family. In family therapy in the past, her husband has revealed his belief that he has been protecting the family from Naomi’s mania and protecting Naomi from "embarrassing herself." This is difficult for Naomi to hear as she has always prided herself on being a good mother and protecting her daughters. Naomi’s situation illustrates the difficulty of coping with a diagnosis of bipolar disorder, the consequences of the illness on the family, and the importance of addressing stigma.
How stigma gets in the way
As discussed previously by Dr. Alison M. Heru ("Mental illness stigma is a family affair," Clinical Psychiatry News, April 2014, p. 8), stigma, when internalized or self-directed, can lead to psychological distress, decreased self-esteem and life satisfaction, and increased depression and suicidality (Compr. Psychiatry 2010;51:603-6). Close family members of those with mental disorders are affected by stigma, commonly referred to as "stigma by association" or "courtesy stigma."
Up to 92% of caregivers of people with psychiatric disorders have reported internalized stigma (J. Psychiatr. Ment. Health Nurs. 2012;19:665-71). These family members become distant and avoidant, resulting in a reduced quality of life and an impaired ability to provide critical support for their loved ones. Caregiver anxiety is inversely related to patient anxiety, stigma, and poor alliance (J. Nerv. Ment. Disease 2011;199:18-24).
As a result of these factors, while people with psychiatric disorders have to cope with their own mental illness as well as the public and self-stigma that alienate them from society, they also are at risk of losing their family connections.
In order to confront stigma, the Family Center for Bipolar Disorder in New York City, for example, uses a Family Inclusive Treatment (FIT) model. The FIT model includes an engagement period at the initiation of treatment that is focused on psychoeducation and relapse prevention planning. FIT is unique in that every patient is required to sign a release of information giving permission for full, open communication at all times between the patient’s clinician and a treatment partner of their choice. After the initial engagement period, there are quarterly family visits to supplement regular individual treatment sessions. FIT treatment promotes open communication about symptoms and medications. FIT strives to minimize patient isolation from families; they can talk openly with one another and their clinician.
After seeing many families enter treatment, FIT staff noticed the prominence of stigma.
We have begun to ask about stigma directly. Do people with more stigma do worse in treatment? Do they adhere more poorly to treatment? Do their families tend to become less involved over time? To begin, Dr. Mednick and staff examined demographic data looking for factors that might predispose a person to experience increased stigma.
In terms of diagnosis, people with more internalizing disorders such as depression and anxiety disorders tend to experience more stigma. Distress is experienced internally. As Dr. Bassirnia and her colleagues wrote in a poster presented at the recent American Psychiatric Association meeting, people with externalizing disorders, such as substance abuse and antisocial disorders, are more likely to express their distress outwardly and are less likely to suffer from stigma ("The relationship between personality traits and perceived internalized stigma in bipolar patients and their caregivers," 2014).
Meanwhile, two systematic review studies have reported moderate to high levels of internalized stigma in people with bipolar disorder. In these studies, a higher level of internalized stigma had a negative correlation with self-esteem, social adjustment, and perceived social support, and positive correlation with severity of symptoms, functional impairment, and rehospitalization. In spite of having more severe symptoms; people with higher levels of self-stigma are less likely to seek professional help and adhere to their treatment. Stigma by association and its negative consequences in caregivers of people with mental disorders also have been reported (J. Affect. Disord. 2013;150:181-91).
A useful and easy to administer scale that helps to identify stigma is the "Perceived Criticism Scale" (J. Abnorm. Psychol. 1989;98:225-35). By asking four questions, the clinician can get a good sense of family dynamics and can monitor the progress and change over time. The questions rate perception on a scale of 1-10, where "X" is the other person involved in treatment, either patient or caregiver. Here are the questions:
1. How critical do you think you are of X?
2. How critical do you think X is of you?
3. When X criticizes you, how upset do you get?
4. When you criticize X, how upset does he/she get?
For families with high scores, follow-up is needed. The Internalized Stigma of Mental Illness (ISMI) scale (Psychiatry Res. 2003;121:31-49) can be used. The ISMI scale makes statements about stigma for which participants rate their agreement on a Likert scale, such as:
• I don’t talk about myself much because I don’t want to burden others with my mental illness.
• Being around people who don’t have a mental illness makes me feel out of place or inadequate.
• People can tell that I have a mental illness by the way I look.
• Mentally ill people tend to be violent.
• I feel out of place in the world because I have a mental illness.
The ISMI scale contains 29 short, simple statements like the ones above and can be completed in less than 10 minutes. The statements are designed to avoid hypothetical situations, stay focused in the present, and address the participant’s own identity and experience.
Using the tools in practice
Naomi entered family treatment with her husband and daughters. Using the ISMI to measure the stigma of mental illness that each family member was experiencing, Naomi was shocked to see that her daughters felt far less stigma about having a mother with mental illness than she had assumed. In turn, her daughters were shocked at how much stigma Naomi was experiencing. Naomi’s husband scored between them. This data paved the way for an open family conversation about how Naomi’s illness had affected their lives, and especially how Naomi’s husband and his perceptions of her illness had affected her treatment course.
Caregivers play a very important role in bipolar disorder. After all, the illness can lead to difficulty functioning and can threaten the family’s stability. Sometimes caregivers can serve as a source of strength and a beacon of stability in the occasional storm. It is hard for the family between the storms, when the same flashing beacon can be a constant reminder to the patient of their illness. Often, well intentioned concerns become constant checking up, making the patient feel stigmatized and expected to fail.
"Good" caregivers will be aware of the stigma and the impact it has on their loved one and on themselves, without becoming a source of stigma.
Dr. Mednick is an attending psychiatrist at the Family Center for Bipolar at Mount Sinai Beth Israel in New York City. Dr. Bassirnia is a second-year psychiatry resident at Mount Sinai Beth Israel. Scan the QR code to read more Families in Psychiatry columns at clinicalpsychiatrynews.com.
Lithium for bipolar disorder
Lithium for bipolar disorder: A re-emerging treatment for mood instability
Lithium is among the most effective therapies for bipolar disorder (BD), and enthusiasm for this simple molecule is waxing. The history of lithium is fascinating,1 and recent considerations include that this element, the third on the periodic table, has few, if any, industry champions. The recent renaissance is caused by a groundswell of appreciation for the clinical efficacy of lithium and an increasing number of providers who are willing to manage patients with lithium.
Target: Bipolar disorder
The target illness for lithium is BD, a spectrum of mood disorders with characteristic features of unstable mood and affect. Shifts in mood include recurrent episodes of mania, which are pathologically energized states with misguided volition and behavior with intoxicating euphoria (or irritability).2 Psychomotor activity is elevated and out of character; speech and body movements are revved up, with a diminished need for sleep. The social, personal, and vocational consequences often are disastrous.
The most common mood state of BD is depression. Depressive episodes consist of pathologically compromised energy and volition with a slowing of bodily functions, most prominently cognition and concentration; a pervasive depressed or sad mood is common but not always present. Presence of mixed states, when features of depression and mania are present simultaneously, is one of the many challenges of treating BD; an elevated volitional or energized state may occur with a depressed, dysphoric mood.
Evidence for lithium
Efficacy studies of lithium have focused on managing mood disorders, treating mania and depression, and prevention or maintenance care.3 Most were performed during the 1970s and 1980s,3 but recent studies have been comparing lithium with other mood stabilizers4-7 and searching for a genetic basis for lithium response.8-10 Other researchers have examined the use of lithium to prevent suicide.11 Some have suggested a neuroprotective effect of lithium, which may have profound implications for neuropsychiatry if valid.12-14 Results of additional studies, which are at different stages of completion, will clarify lithium use,15,16 and characterize the genetic makeup of individuals who respond to lithium.17 The primary evidence for lithium, however, is for maintenance treatment of BD and for preventing manic and depressive episodes.
Biochemistry and physiology of lithium. The biochemical and physiological effects of lithium are complex, wide-ranging, and likely to affect hundreds, if not thousands, of genes and gene products. The mechanisms of action remain a focus of academic pursuit (for a review of hypotheses related to these mechanisms see Goodwin and Jamison2 and Can et al18) Lithium is involved in cell signaling pathways that involve complex molecular mechanisms of inter- and intracellular communication19; some neural receptors are down-regulated20 and others show inhibition,21 which is thought to be a mechanism of lithium. The hypothesized neuroprotective effect of lithium22 may be mediated through an increased level of brain-derived neurotrophic factor in brain tissue.14 Recently, investigators using induced pluripotent stem cell derived neurons have shown that patterns of calcium-related cell signaling in bipolar neurons are affected specifically by lithium in the culture media.23 There likely are many mechanisms through which lithium’s effects are mediated, including a series of dynamic pathways that vary over time and in reaction to the internal and external environments of the cell and person.
The lithium renaissance
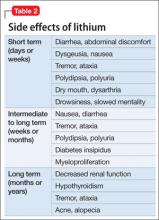
In the past decade, there has been an increase in interest and use of lithium because clinicians recognize its efficacy and advantages and can monitor serum levels and gauge the patient’s response and side effects24 against the lithium level. This is important because balancing effi cacy and side effects depends on the serum level. Efficacy often is not immediate, although side effects may emerge early. All systems of the body may show effects that could be related to lithium use. It is helpful to be aware of the side effects in chronological order, because some immediate effects may be associated with starting at higher dosages (Table 1). Common side effects in the short term include:
• GI distress, such as nausea, vomiting, diarrhea, and abdominal discomfort
• a fine neurologic tremor, which may be seen with accentuation upon deliberate movement
• prominent thirst with polyuria
• drowsiness and clouded thinking, which can be upsetting to the patient and family.
In the longer term, adverse effects on kidney and thyroid function are common. Management must include monitoring of the serum level.
Lithium is FDA-approved for acute and maintenance treatment of mania in BD. There are reports that discuss most variants of mood disorders, including BD I, BD II, unipolar depression, rapid cycling, and even alcohol abuse.25-29 Lithium could help manage mood dysregulation in the context of temperament and personality.30 There is evidence that lithium has an antidepressant effect31-33 and has shown efficacy as an adjunctive treatment for depression.31-33 There are data that suggest that lithium, with its neuroprotective mechanisms, may prevent progression of mild cognitive impairment.34
Is there an ideal lithium candidate?
Mood instability is the characteristic feature of a lithium responder. The instability may be over the course of the day, such as a dysregulated temperament that often is associated with DSM-IV personality categories, shorter-term fluctuations (within days with BD II), or in the context of episodic shifts of mood states over weeks and months, which are characteristic of BD I. The hallmark of mood instability is fluctuation from depression to elevated mood states and charged emotions with increased energy.
The patient considered ideal for lithium treatment has BD I with recurrent severe euphoric manic episodes, absence of significant comorbid disorders such as substance abuse, and a family history of lithium response. However, any patient with a clinically significant and unstable mood disorder, regardless of the DSM diagnosis, should be considered for lithium treatment.
When considering a lithium trial for a patient with significant mood instability, it is critical to establish the target symptoms and behavior that will help you gauge the efficacy of the intervention. Measurement-based care utilizes clinician and self-report instruments to provide data on the illness course and response to intervention. Commonly used clinician driven assessments include the Young Mania Rating Scale35 and the Quick Inventory of Depressive Symptoms,36 while the self-report assessments are the Patient Health Questionnaire37 and the Altman Self- Rating Mania Scale.38
During acute mania or depression, lithium often is used in combination with another medications such as an antipsychotic or antidepressant. Used in the outpatient and non-acute setting, lithium may be an “add-on” or monotherapy for preventing recurrence of episodes. Response in early acute manic symptoms are predictive of later response and remission.39
Dosing strategies
An initial problem with lithium is side effects that emerge when beginning treatment, which may discourage the patient and family from using this agent. Starting with 150 mg/d for the first 2 or 3 doses is unlikely to produce any adverse effects and can show the patient that there is a high likelihood that he will be able to tolerate the medication. Gradual titration over several days—or even weeks—to the target dosage and serum levels will enhance patient compliance. Rate of dosage increase is best guided by tolerance to the medication. The general consensus is that lithium is most effective at levels of 0.6 to 0.8 mEq/L,40 although a lower level (0.5 mEq/L) over a 2-year period also can be effective.41 Lithium may be used in to treat acute mania at higher serum levels (0.8 to 1.2 mEq/L), however, the acute phase often requires urgent management, usually with an antipsychotic.
Emerging consensus
Although there is a need to gather and analyze longer observational periods to clarify the clinical and biological characteristics of persons who respond to lithium, there are several points of consensus. Management will be guided by patient characteristics such as age, comorbidities, and other therapies. Most studies that address the effect of lithium level focus on high vs low serum levels. There are 3 categories of lithium serum levels, low (<0.6 mEq/L), mid-range (0.6 to 0.8 mEq/L), and high (>0.8 mEq/L), each has risk-benefit considerations.
The LiTMUS study42 compared low-level lithium augmentation with optimized personal treatment without lithium. Both groups had similar outcomes but the lithium-treated group had significantly lower use of atypical antipsychotics. This may be important when considering the long-term risk of the metabolic syndrome because the tolerability and side-effect profile of lithium at lower levels is more favorable than that of atypical antipsychotics. As lithium levels increase, there seems to be concomitant increase in efficacy and side effects. Many patients will benefit with low-level lithium use; yet clearly some individuals require higher dosages for effective maintenance therapy.
Dosing and monitoring. In patients age >50 or those with comorbid medical conditions, use a lower level of lithium (<0.6 mEq/L). Most individuals with BD likely will benefit from the mid-range level strategy (0.6 to 0.8 mEq/L); however, there will be those who require a higher level. When beginning lithium, start at a low dosage (150 mg/d) and increase as tolerated to the desired serum level. With acute mania, temporary use of an antipsychotic will be required.
There are no tests available to determine whether a patient will do well at any of these lithium serum levels. Breakthrough mania in an adherent patient with a serum lithium level of 0.7 mEq/L indicates the need to obtain a higher lithium level. A major deficit in lithium research is the lack of long-term data (>5 years) on outcomes, clinical and biological features with lithium levels because of a lack of pharmaceutical company support.3,17 Monitoring mood symptoms using detailed mood charts, whether clinician-administered or self-reported, is an effective way to monitor outcomes, provided the clinician uses the same scales or methods to record a patient’s moods. If a patient wants to discontinue lithium, taper the drug over an extended period (months) to minimize the likelihood of emerging manic or depressive episodes related to drug discontinuation.
Managing side effects
Consider lithium’s side effects in the context of their short-, intermediate-, and long-term presence (Table 2). Gradually increasing the lithium dosage often will prevent side effects that manifest in the short term. If side effects emerge at low dosages, proceed slowly with lithium and manage symptoms with other medications. When a patient shows a change in side effects, obtain lithium and electrolytes levels; a change in mental status with confusion will require an acute lithium level.
A diary of symptoms or clinically relevant matters such as fluid intake or frequency of GI- or neurological-related events will help the clinician monitor the frequency and severity of side effects. The patient and clinician should not be discouraged by emerging side effects in the short term, because they may dissipate or become minimally intrusive.
Several strategies can alleviate immediate GI effects, such as dosing with meals, enteric-coated formulations, multiple dose strategies, and short-term use of antidiarrheal medicine as needed. Side effects that disrupt a patient’s fluid and electrolyte balance (diabetes insipidus) to the point of clouding mental status will require discontinuing the medication until mental status improves, then reconsideration of the treatment regime, which will include managing diabetes insipidus with amiloride. Managing side effects may require consultation with specialty services. Likewise, some patients might experience neurologic side effects, such as profound tremor, that interferes with their ability to function. However, many side effects can be managed symptomatically with practical strategies (eg, a sugar-free lozenge for dry mouth or dysgeusia). Consider lower lithium dosages and serum levels because patients may experience benefits with lower therapeutic levels.
Long-term side effects include decreased renal function, hypothyroidism, persistent tremor, and dermatologic effects of acne and alopecia. Monitor renal and thyroid function annually in stable patients and more frequently when making changes in the treatment plan.
Before discontinuing lithium, consider discussing the medical issues with a specialist who has experience with complications of lithium.
Bottom Line
Lithium is an effective and under used medication for managing bipolar disorder. Initial prejudices and side effects often deter patients and prescribers from proceeding with a therapeutic trial of lithium. Although the mid-range lithium level of 0.6 to 0.8 mEq/L is desirable, many patients will experience significant benefits with lower levels. Initial strategies include the use of low-dose preparations that are unlikely to have uncomfortable side effects.
Related Resources
• Andreasen A, Ellingrod VL. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
• Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646.
Drug Brand Names
Amiloride • Midamor Lithium • Eskalith, Lithobid
Disclosure
Dr. McInnis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(11 suppl 2):4-9.
2. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
3. Burgess S, Geddes J, Hawton K, et al. Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev. 2001:CD003013.
4. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex maintenance study group. Arch Gen Psychiatry. 2000;57(5):481-489.
5. Bowden CL, Calabrese JR, Sachs G, et al; Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
6. Swann AC, Bowden CL, Calabrese JR, et al. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26(4):530-536.
7. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
8. Perlis RH, Smoller JW, Ferreira MA, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009; 166(6):718-725.
9. Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10): 942-947.
10. Duffy A, Alda M, Kutcher S, et al. A prospective study of the offspring of bipolar parents responsive and nonresponsive to lithium treatment. J Clin Psychiatry. 2002;63(12): 1171-1178.
11. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
12. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010; 62(1):50-60.
13. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
14. de Sousa RT, van de Bilt MT, Diniz BS, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494(1):54-56.
15. Nierenberg AA, Sylvia LG, Leon AC, et al; LiTMUS Study Group. Lithium treatment–moderate dose use study (LiTMUS) for bipolar disorder: rationale and design. Clin Trials. 2009;6(6):637-648.
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Methods to limit attrition in longitudinal comparative effectiveness trials: lessons from the Lithium Treatment - Moderate dose Use Study (LiTMUS) for bipolar disorder. Clin Trials. 2012;9(1):94-101.
17. McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11(10):1439-1465.
18. Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy [published online February 15, 2014]. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2014.02.004.
19. Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1-21.
20. Devaki R, Shankar Rao S, Nadgir SM. The effect of lithium on the adrenoceptor-mediated second messenger system in the rat brain. J Psychiatry Neurosci. 2006;31(4):246-252.
21. Pan JQ, Lewis MC, Ketterman JK, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36(7):1397-1411.
22. Hu LW, Kawamoto EM, Brietzke E, et al. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):11-17.
23. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients [published online March 25, 2014]. Transl Psychiatry. doi:10.1038/tp.2014.12.
24. Jefferson JW. Lithium. In: Aronson JK, ed. Side effects of drugs annual, volume 26. Amsterdam, The Netherlands: Elsevier Science; 2003:19-29.
25. Baldessarini RJ, Tondo L, Floris G, et al. Effects of rapid cycling on response to lithium maintenance treatment in 360 bipolar I and II disorder patients. J Affect Disord. 2000;61(2):13-22.
26. Baldessarini RJ, Tondo L, Hennen J, et al. Latency and episodes before treatment: response to lithium maintenance in bipolar I and II disorders. Bipolar Disord. 1999;1(2): 91-97.
27. Fieve RR, Kumbaraci T, Dunner DL. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry. 1976;133(8):925-929.
28. Peck CC, Pond SM, Becker CE, et al. An evaluation of the effects of lithium in the treatment of chronic alcoholism. II. Assessment of the two-period crossover design. Alcohol Clin Exp Res. 1981;5(2):252-255.
29. Peselow ED, Dunner DL, Fieve RR, et al. Lithium prophylaxis of depression in unipolar, bipolar II, and cyclothymic patients. Am J Psychiatry. 1982;139(6):747-752.
30. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
31. Alevizos B, Alevizos E, Leonardou A, et al. Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study. Psychiatrike. 2012;23(2):143-148.
32. Goldberg JF, Sacks MH, Kocsis JH. Low-dose lithium augmentation of divalproex in geriatric mania. J Clin Psychiatry. 2000;61(4):304.
33. Saunders KE, Goodwin GM. New approaches in the treatment of bipolar depression. Curr Top Behav Neurosci. 2013;14:291-307.
34. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
35. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435.
36. Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Altman EG, Hedeker D, Peterson JL, et al. The Altman Self- Rating Mania Scale. Biol Psychiatry. 1997;42(10):948-955.
39. Machado-Vieira R, Luckenbaugh DA, Soeiro-de-Souza MG, et al. Early improvement with lithium in classic mania and its association with later response. J Affect Disord. 2013;144(1-2):160-164.
40. Severus WE, Lipkovich IA, Licht RW, et al. In search of optimal lithium levels and olanzapine doses in the long-term treatment of bipolar I disorder. A post-hoc analysis of the maintenance study by Tohen et al. 2005. Eur Psychiatry. 2010;25(8):443-449.
41. Vestergaard P, Licht RW, Brodersen A, et al. Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum lithium levels. Acta Psychiatr Scand. 1998;98(4):310-315.
42. Nierenberg AA, Friedman ES, Bowden CL, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102-111.
Lithium is among the most effective therapies for bipolar disorder (BD), and enthusiasm for this simple molecule is waxing. The history of lithium is fascinating,1 and recent considerations include that this element, the third on the periodic table, has few, if any, industry champions. The recent renaissance is caused by a groundswell of appreciation for the clinical efficacy of lithium and an increasing number of providers who are willing to manage patients with lithium.
Target: Bipolar disorder
The target illness for lithium is BD, a spectrum of mood disorders with characteristic features of unstable mood and affect. Shifts in mood include recurrent episodes of mania, which are pathologically energized states with misguided volition and behavior with intoxicating euphoria (or irritability).2 Psychomotor activity is elevated and out of character; speech and body movements are revved up, with a diminished need for sleep. The social, personal, and vocational consequences often are disastrous.
The most common mood state of BD is depression. Depressive episodes consist of pathologically compromised energy and volition with a slowing of bodily functions, most prominently cognition and concentration; a pervasive depressed or sad mood is common but not always present. Presence of mixed states, when features of depression and mania are present simultaneously, is one of the many challenges of treating BD; an elevated volitional or energized state may occur with a depressed, dysphoric mood.
Evidence for lithium
Efficacy studies of lithium have focused on managing mood disorders, treating mania and depression, and prevention or maintenance care.3 Most were performed during the 1970s and 1980s,3 but recent studies have been comparing lithium with other mood stabilizers4-7 and searching for a genetic basis for lithium response.8-10 Other researchers have examined the use of lithium to prevent suicide.11 Some have suggested a neuroprotective effect of lithium, which may have profound implications for neuropsychiatry if valid.12-14 Results of additional studies, which are at different stages of completion, will clarify lithium use,15,16 and characterize the genetic makeup of individuals who respond to lithium.17 The primary evidence for lithium, however, is for maintenance treatment of BD and for preventing manic and depressive episodes.
Biochemistry and physiology of lithium. The biochemical and physiological effects of lithium are complex, wide-ranging, and likely to affect hundreds, if not thousands, of genes and gene products. The mechanisms of action remain a focus of academic pursuit (for a review of hypotheses related to these mechanisms see Goodwin and Jamison2 and Can et al18) Lithium is involved in cell signaling pathways that involve complex molecular mechanisms of inter- and intracellular communication19; some neural receptors are down-regulated20 and others show inhibition,21 which is thought to be a mechanism of lithium. The hypothesized neuroprotective effect of lithium22 may be mediated through an increased level of brain-derived neurotrophic factor in brain tissue.14 Recently, investigators using induced pluripotent stem cell derived neurons have shown that patterns of calcium-related cell signaling in bipolar neurons are affected specifically by lithium in the culture media.23 There likely are many mechanisms through which lithium’s effects are mediated, including a series of dynamic pathways that vary over time and in reaction to the internal and external environments of the cell and person.
The lithium renaissance
In the past decade, there has been an increase in interest and use of lithium because clinicians recognize its efficacy and advantages and can monitor serum levels and gauge the patient’s response and side effects24 against the lithium level. This is important because balancing effi cacy and side effects depends on the serum level. Efficacy often is not immediate, although side effects may emerge early. All systems of the body may show effects that could be related to lithium use. It is helpful to be aware of the side effects in chronological order, because some immediate effects may be associated with starting at higher dosages (Table 1). Common side effects in the short term include:
• GI distress, such as nausea, vomiting, diarrhea, and abdominal discomfort
• a fine neurologic tremor, which may be seen with accentuation upon deliberate movement
• prominent thirst with polyuria
• drowsiness and clouded thinking, which can be upsetting to the patient and family.
In the longer term, adverse effects on kidney and thyroid function are common. Management must include monitoring of the serum level.
Lithium is FDA-approved for acute and maintenance treatment of mania in BD. There are reports that discuss most variants of mood disorders, including BD I, BD II, unipolar depression, rapid cycling, and even alcohol abuse.25-29 Lithium could help manage mood dysregulation in the context of temperament and personality.30 There is evidence that lithium has an antidepressant effect31-33 and has shown efficacy as an adjunctive treatment for depression.31-33 There are data that suggest that lithium, with its neuroprotective mechanisms, may prevent progression of mild cognitive impairment.34
Is there an ideal lithium candidate?
Mood instability is the characteristic feature of a lithium responder. The instability may be over the course of the day, such as a dysregulated temperament that often is associated with DSM-IV personality categories, shorter-term fluctuations (within days with BD II), or in the context of episodic shifts of mood states over weeks and months, which are characteristic of BD I. The hallmark of mood instability is fluctuation from depression to elevated mood states and charged emotions with increased energy.
The patient considered ideal for lithium treatment has BD I with recurrent severe euphoric manic episodes, absence of significant comorbid disorders such as substance abuse, and a family history of lithium response. However, any patient with a clinically significant and unstable mood disorder, regardless of the DSM diagnosis, should be considered for lithium treatment.
When considering a lithium trial for a patient with significant mood instability, it is critical to establish the target symptoms and behavior that will help you gauge the efficacy of the intervention. Measurement-based care utilizes clinician and self-report instruments to provide data on the illness course and response to intervention. Commonly used clinician driven assessments include the Young Mania Rating Scale35 and the Quick Inventory of Depressive Symptoms,36 while the self-report assessments are the Patient Health Questionnaire37 and the Altman Self- Rating Mania Scale.38
During acute mania or depression, lithium often is used in combination with another medications such as an antipsychotic or antidepressant. Used in the outpatient and non-acute setting, lithium may be an “add-on” or monotherapy for preventing recurrence of episodes. Response in early acute manic symptoms are predictive of later response and remission.39
Dosing strategies
An initial problem with lithium is side effects that emerge when beginning treatment, which may discourage the patient and family from using this agent. Starting with 150 mg/d for the first 2 or 3 doses is unlikely to produce any adverse effects and can show the patient that there is a high likelihood that he will be able to tolerate the medication. Gradual titration over several days—or even weeks—to the target dosage and serum levels will enhance patient compliance. Rate of dosage increase is best guided by tolerance to the medication. The general consensus is that lithium is most effective at levels of 0.6 to 0.8 mEq/L,40 although a lower level (0.5 mEq/L) over a 2-year period also can be effective.41 Lithium may be used in to treat acute mania at higher serum levels (0.8 to 1.2 mEq/L), however, the acute phase often requires urgent management, usually with an antipsychotic.
Emerging consensus
Although there is a need to gather and analyze longer observational periods to clarify the clinical and biological characteristics of persons who respond to lithium, there are several points of consensus. Management will be guided by patient characteristics such as age, comorbidities, and other therapies. Most studies that address the effect of lithium level focus on high vs low serum levels. There are 3 categories of lithium serum levels, low (<0.6 mEq/L), mid-range (0.6 to 0.8 mEq/L), and high (>0.8 mEq/L), each has risk-benefit considerations.
The LiTMUS study42 compared low-level lithium augmentation with optimized personal treatment without lithium. Both groups had similar outcomes but the lithium-treated group had significantly lower use of atypical antipsychotics. This may be important when considering the long-term risk of the metabolic syndrome because the tolerability and side-effect profile of lithium at lower levels is more favorable than that of atypical antipsychotics. As lithium levels increase, there seems to be concomitant increase in efficacy and side effects. Many patients will benefit with low-level lithium use; yet clearly some individuals require higher dosages for effective maintenance therapy.
Dosing and monitoring. In patients age >50 or those with comorbid medical conditions, use a lower level of lithium (<0.6 mEq/L). Most individuals with BD likely will benefit from the mid-range level strategy (0.6 to 0.8 mEq/L); however, there will be those who require a higher level. When beginning lithium, start at a low dosage (150 mg/d) and increase as tolerated to the desired serum level. With acute mania, temporary use of an antipsychotic will be required.
There are no tests available to determine whether a patient will do well at any of these lithium serum levels. Breakthrough mania in an adherent patient with a serum lithium level of 0.7 mEq/L indicates the need to obtain a higher lithium level. A major deficit in lithium research is the lack of long-term data (>5 years) on outcomes, clinical and biological features with lithium levels because of a lack of pharmaceutical company support.3,17 Monitoring mood symptoms using detailed mood charts, whether clinician-administered or self-reported, is an effective way to monitor outcomes, provided the clinician uses the same scales or methods to record a patient’s moods. If a patient wants to discontinue lithium, taper the drug over an extended period (months) to minimize the likelihood of emerging manic or depressive episodes related to drug discontinuation.
Managing side effects
Consider lithium’s side effects in the context of their short-, intermediate-, and long-term presence (Table 2). Gradually increasing the lithium dosage often will prevent side effects that manifest in the short term. If side effects emerge at low dosages, proceed slowly with lithium and manage symptoms with other medications. When a patient shows a change in side effects, obtain lithium and electrolytes levels; a change in mental status with confusion will require an acute lithium level.
A diary of symptoms or clinically relevant matters such as fluid intake or frequency of GI- or neurological-related events will help the clinician monitor the frequency and severity of side effects. The patient and clinician should not be discouraged by emerging side effects in the short term, because they may dissipate or become minimally intrusive.
Several strategies can alleviate immediate GI effects, such as dosing with meals, enteric-coated formulations, multiple dose strategies, and short-term use of antidiarrheal medicine as needed. Side effects that disrupt a patient’s fluid and electrolyte balance (diabetes insipidus) to the point of clouding mental status will require discontinuing the medication until mental status improves, then reconsideration of the treatment regime, which will include managing diabetes insipidus with amiloride. Managing side effects may require consultation with specialty services. Likewise, some patients might experience neurologic side effects, such as profound tremor, that interferes with their ability to function. However, many side effects can be managed symptomatically with practical strategies (eg, a sugar-free lozenge for dry mouth or dysgeusia). Consider lower lithium dosages and serum levels because patients may experience benefits with lower therapeutic levels.
Long-term side effects include decreased renal function, hypothyroidism, persistent tremor, and dermatologic effects of acne and alopecia. Monitor renal and thyroid function annually in stable patients and more frequently when making changes in the treatment plan.
Before discontinuing lithium, consider discussing the medical issues with a specialist who has experience with complications of lithium.
Bottom Line
Lithium is an effective and under used medication for managing bipolar disorder. Initial prejudices and side effects often deter patients and prescribers from proceeding with a therapeutic trial of lithium. Although the mid-range lithium level of 0.6 to 0.8 mEq/L is desirable, many patients will experience significant benefits with lower levels. Initial strategies include the use of low-dose preparations that are unlikely to have uncomfortable side effects.
Related Resources
• Andreasen A, Ellingrod VL. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
• Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646.
Drug Brand Names
Amiloride • Midamor Lithium • Eskalith, Lithobid
Disclosure
Dr. McInnis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Lithium is among the most effective therapies for bipolar disorder (BD), and enthusiasm for this simple molecule is waxing. The history of lithium is fascinating,1 and recent considerations include that this element, the third on the periodic table, has few, if any, industry champions. The recent renaissance is caused by a groundswell of appreciation for the clinical efficacy of lithium and an increasing number of providers who are willing to manage patients with lithium.
Target: Bipolar disorder
The target illness for lithium is BD, a spectrum of mood disorders with characteristic features of unstable mood and affect. Shifts in mood include recurrent episodes of mania, which are pathologically energized states with misguided volition and behavior with intoxicating euphoria (or irritability).2 Psychomotor activity is elevated and out of character; speech and body movements are revved up, with a diminished need for sleep. The social, personal, and vocational consequences often are disastrous.
The most common mood state of BD is depression. Depressive episodes consist of pathologically compromised energy and volition with a slowing of bodily functions, most prominently cognition and concentration; a pervasive depressed or sad mood is common but not always present. Presence of mixed states, when features of depression and mania are present simultaneously, is one of the many challenges of treating BD; an elevated volitional or energized state may occur with a depressed, dysphoric mood.
Evidence for lithium
Efficacy studies of lithium have focused on managing mood disorders, treating mania and depression, and prevention or maintenance care.3 Most were performed during the 1970s and 1980s,3 but recent studies have been comparing lithium with other mood stabilizers4-7 and searching for a genetic basis for lithium response.8-10 Other researchers have examined the use of lithium to prevent suicide.11 Some have suggested a neuroprotective effect of lithium, which may have profound implications for neuropsychiatry if valid.12-14 Results of additional studies, which are at different stages of completion, will clarify lithium use,15,16 and characterize the genetic makeup of individuals who respond to lithium.17 The primary evidence for lithium, however, is for maintenance treatment of BD and for preventing manic and depressive episodes.
Biochemistry and physiology of lithium. The biochemical and physiological effects of lithium are complex, wide-ranging, and likely to affect hundreds, if not thousands, of genes and gene products. The mechanisms of action remain a focus of academic pursuit (for a review of hypotheses related to these mechanisms see Goodwin and Jamison2 and Can et al18) Lithium is involved in cell signaling pathways that involve complex molecular mechanisms of inter- and intracellular communication19; some neural receptors are down-regulated20 and others show inhibition,21 which is thought to be a mechanism of lithium. The hypothesized neuroprotective effect of lithium22 may be mediated through an increased level of brain-derived neurotrophic factor in brain tissue.14 Recently, investigators using induced pluripotent stem cell derived neurons have shown that patterns of calcium-related cell signaling in bipolar neurons are affected specifically by lithium in the culture media.23 There likely are many mechanisms through which lithium’s effects are mediated, including a series of dynamic pathways that vary over time and in reaction to the internal and external environments of the cell and person.
The lithium renaissance
In the past decade, there has been an increase in interest and use of lithium because clinicians recognize its efficacy and advantages and can monitor serum levels and gauge the patient’s response and side effects24 against the lithium level. This is important because balancing effi cacy and side effects depends on the serum level. Efficacy often is not immediate, although side effects may emerge early. All systems of the body may show effects that could be related to lithium use. It is helpful to be aware of the side effects in chronological order, because some immediate effects may be associated with starting at higher dosages (Table 1). Common side effects in the short term include:
• GI distress, such as nausea, vomiting, diarrhea, and abdominal discomfort
• a fine neurologic tremor, which may be seen with accentuation upon deliberate movement
• prominent thirst with polyuria
• drowsiness and clouded thinking, which can be upsetting to the patient and family.
In the longer term, adverse effects on kidney and thyroid function are common. Management must include monitoring of the serum level.
Lithium is FDA-approved for acute and maintenance treatment of mania in BD. There are reports that discuss most variants of mood disorders, including BD I, BD II, unipolar depression, rapid cycling, and even alcohol abuse.25-29 Lithium could help manage mood dysregulation in the context of temperament and personality.30 There is evidence that lithium has an antidepressant effect31-33 and has shown efficacy as an adjunctive treatment for depression.31-33 There are data that suggest that lithium, with its neuroprotective mechanisms, may prevent progression of mild cognitive impairment.34
Is there an ideal lithium candidate?
Mood instability is the characteristic feature of a lithium responder. The instability may be over the course of the day, such as a dysregulated temperament that often is associated with DSM-IV personality categories, shorter-term fluctuations (within days with BD II), or in the context of episodic shifts of mood states over weeks and months, which are characteristic of BD I. The hallmark of mood instability is fluctuation from depression to elevated mood states and charged emotions with increased energy.
The patient considered ideal for lithium treatment has BD I with recurrent severe euphoric manic episodes, absence of significant comorbid disorders such as substance abuse, and a family history of lithium response. However, any patient with a clinically significant and unstable mood disorder, regardless of the DSM diagnosis, should be considered for lithium treatment.
When considering a lithium trial for a patient with significant mood instability, it is critical to establish the target symptoms and behavior that will help you gauge the efficacy of the intervention. Measurement-based care utilizes clinician and self-report instruments to provide data on the illness course and response to intervention. Commonly used clinician driven assessments include the Young Mania Rating Scale35 and the Quick Inventory of Depressive Symptoms,36 while the self-report assessments are the Patient Health Questionnaire37 and the Altman Self- Rating Mania Scale.38
During acute mania or depression, lithium often is used in combination with another medications such as an antipsychotic or antidepressant. Used in the outpatient and non-acute setting, lithium may be an “add-on” or monotherapy for preventing recurrence of episodes. Response in early acute manic symptoms are predictive of later response and remission.39
Dosing strategies
An initial problem with lithium is side effects that emerge when beginning treatment, which may discourage the patient and family from using this agent. Starting with 150 mg/d for the first 2 or 3 doses is unlikely to produce any adverse effects and can show the patient that there is a high likelihood that he will be able to tolerate the medication. Gradual titration over several days—or even weeks—to the target dosage and serum levels will enhance patient compliance. Rate of dosage increase is best guided by tolerance to the medication. The general consensus is that lithium is most effective at levels of 0.6 to 0.8 mEq/L,40 although a lower level (0.5 mEq/L) over a 2-year period also can be effective.41 Lithium may be used in to treat acute mania at higher serum levels (0.8 to 1.2 mEq/L), however, the acute phase often requires urgent management, usually with an antipsychotic.
Emerging consensus
Although there is a need to gather and analyze longer observational periods to clarify the clinical and biological characteristics of persons who respond to lithium, there are several points of consensus. Management will be guided by patient characteristics such as age, comorbidities, and other therapies. Most studies that address the effect of lithium level focus on high vs low serum levels. There are 3 categories of lithium serum levels, low (<0.6 mEq/L), mid-range (0.6 to 0.8 mEq/L), and high (>0.8 mEq/L), each has risk-benefit considerations.
The LiTMUS study42 compared low-level lithium augmentation with optimized personal treatment without lithium. Both groups had similar outcomes but the lithium-treated group had significantly lower use of atypical antipsychotics. This may be important when considering the long-term risk of the metabolic syndrome because the tolerability and side-effect profile of lithium at lower levels is more favorable than that of atypical antipsychotics. As lithium levels increase, there seems to be concomitant increase in efficacy and side effects. Many patients will benefit with low-level lithium use; yet clearly some individuals require higher dosages for effective maintenance therapy.
Dosing and monitoring. In patients age >50 or those with comorbid medical conditions, use a lower level of lithium (<0.6 mEq/L). Most individuals with BD likely will benefit from the mid-range level strategy (0.6 to 0.8 mEq/L); however, there will be those who require a higher level. When beginning lithium, start at a low dosage (150 mg/d) and increase as tolerated to the desired serum level. With acute mania, temporary use of an antipsychotic will be required.
There are no tests available to determine whether a patient will do well at any of these lithium serum levels. Breakthrough mania in an adherent patient with a serum lithium level of 0.7 mEq/L indicates the need to obtain a higher lithium level. A major deficit in lithium research is the lack of long-term data (>5 years) on outcomes, clinical and biological features with lithium levels because of a lack of pharmaceutical company support.3,17 Monitoring mood symptoms using detailed mood charts, whether clinician-administered or self-reported, is an effective way to monitor outcomes, provided the clinician uses the same scales or methods to record a patient’s moods. If a patient wants to discontinue lithium, taper the drug over an extended period (months) to minimize the likelihood of emerging manic or depressive episodes related to drug discontinuation.
Managing side effects
Consider lithium’s side effects in the context of their short-, intermediate-, and long-term presence (Table 2). Gradually increasing the lithium dosage often will prevent side effects that manifest in the short term. If side effects emerge at low dosages, proceed slowly with lithium and manage symptoms with other medications. When a patient shows a change in side effects, obtain lithium and electrolytes levels; a change in mental status with confusion will require an acute lithium level.
A diary of symptoms or clinically relevant matters such as fluid intake or frequency of GI- or neurological-related events will help the clinician monitor the frequency and severity of side effects. The patient and clinician should not be discouraged by emerging side effects in the short term, because they may dissipate or become minimally intrusive.
Several strategies can alleviate immediate GI effects, such as dosing with meals, enteric-coated formulations, multiple dose strategies, and short-term use of antidiarrheal medicine as needed. Side effects that disrupt a patient’s fluid and electrolyte balance (diabetes insipidus) to the point of clouding mental status will require discontinuing the medication until mental status improves, then reconsideration of the treatment regime, which will include managing diabetes insipidus with amiloride. Managing side effects may require consultation with specialty services. Likewise, some patients might experience neurologic side effects, such as profound tremor, that interferes with their ability to function. However, many side effects can be managed symptomatically with practical strategies (eg, a sugar-free lozenge for dry mouth or dysgeusia). Consider lower lithium dosages and serum levels because patients may experience benefits with lower therapeutic levels.
Long-term side effects include decreased renal function, hypothyroidism, persistent tremor, and dermatologic effects of acne and alopecia. Monitor renal and thyroid function annually in stable patients and more frequently when making changes in the treatment plan.
Before discontinuing lithium, consider discussing the medical issues with a specialist who has experience with complications of lithium.
Bottom Line
Lithium is an effective and under used medication for managing bipolar disorder. Initial prejudices and side effects often deter patients and prescribers from proceeding with a therapeutic trial of lithium. Although the mid-range lithium level of 0.6 to 0.8 mEq/L is desirable, many patients will experience significant benefits with lower levels. Initial strategies include the use of low-dose preparations that are unlikely to have uncomfortable side effects.
Related Resources
• Andreasen A, Ellingrod VL. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
• Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646.
Drug Brand Names
Amiloride • Midamor Lithium • Eskalith, Lithobid
Disclosure
Dr. McInnis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(11 suppl 2):4-9.
2. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
3. Burgess S, Geddes J, Hawton K, et al. Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev. 2001:CD003013.
4. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex maintenance study group. Arch Gen Psychiatry. 2000;57(5):481-489.
5. Bowden CL, Calabrese JR, Sachs G, et al; Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
6. Swann AC, Bowden CL, Calabrese JR, et al. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26(4):530-536.
7. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
8. Perlis RH, Smoller JW, Ferreira MA, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009; 166(6):718-725.
9. Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10): 942-947.
10. Duffy A, Alda M, Kutcher S, et al. A prospective study of the offspring of bipolar parents responsive and nonresponsive to lithium treatment. J Clin Psychiatry. 2002;63(12): 1171-1178.
11. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
12. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010; 62(1):50-60.
13. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
14. de Sousa RT, van de Bilt MT, Diniz BS, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494(1):54-56.
15. Nierenberg AA, Sylvia LG, Leon AC, et al; LiTMUS Study Group. Lithium treatment–moderate dose use study (LiTMUS) for bipolar disorder: rationale and design. Clin Trials. 2009;6(6):637-648.
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Methods to limit attrition in longitudinal comparative effectiveness trials: lessons from the Lithium Treatment - Moderate dose Use Study (LiTMUS) for bipolar disorder. Clin Trials. 2012;9(1):94-101.
17. McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11(10):1439-1465.
18. Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy [published online February 15, 2014]. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2014.02.004.
19. Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1-21.
20. Devaki R, Shankar Rao S, Nadgir SM. The effect of lithium on the adrenoceptor-mediated second messenger system in the rat brain. J Psychiatry Neurosci. 2006;31(4):246-252.
21. Pan JQ, Lewis MC, Ketterman JK, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36(7):1397-1411.
22. Hu LW, Kawamoto EM, Brietzke E, et al. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):11-17.
23. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients [published online March 25, 2014]. Transl Psychiatry. doi:10.1038/tp.2014.12.
24. Jefferson JW. Lithium. In: Aronson JK, ed. Side effects of drugs annual, volume 26. Amsterdam, The Netherlands: Elsevier Science; 2003:19-29.
25. Baldessarini RJ, Tondo L, Floris G, et al. Effects of rapid cycling on response to lithium maintenance treatment in 360 bipolar I and II disorder patients. J Affect Disord. 2000;61(2):13-22.
26. Baldessarini RJ, Tondo L, Hennen J, et al. Latency and episodes before treatment: response to lithium maintenance in bipolar I and II disorders. Bipolar Disord. 1999;1(2): 91-97.
27. Fieve RR, Kumbaraci T, Dunner DL. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry. 1976;133(8):925-929.
28. Peck CC, Pond SM, Becker CE, et al. An evaluation of the effects of lithium in the treatment of chronic alcoholism. II. Assessment of the two-period crossover design. Alcohol Clin Exp Res. 1981;5(2):252-255.
29. Peselow ED, Dunner DL, Fieve RR, et al. Lithium prophylaxis of depression in unipolar, bipolar II, and cyclothymic patients. Am J Psychiatry. 1982;139(6):747-752.
30. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
31. Alevizos B, Alevizos E, Leonardou A, et al. Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study. Psychiatrike. 2012;23(2):143-148.
32. Goldberg JF, Sacks MH, Kocsis JH. Low-dose lithium augmentation of divalproex in geriatric mania. J Clin Psychiatry. 2000;61(4):304.
33. Saunders KE, Goodwin GM. New approaches in the treatment of bipolar depression. Curr Top Behav Neurosci. 2013;14:291-307.
34. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
35. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435.
36. Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Altman EG, Hedeker D, Peterson JL, et al. The Altman Self- Rating Mania Scale. Biol Psychiatry. 1997;42(10):948-955.
39. Machado-Vieira R, Luckenbaugh DA, Soeiro-de-Souza MG, et al. Early improvement with lithium in classic mania and its association with later response. J Affect Disord. 2013;144(1-2):160-164.
40. Severus WE, Lipkovich IA, Licht RW, et al. In search of optimal lithium levels and olanzapine doses in the long-term treatment of bipolar I disorder. A post-hoc analysis of the maintenance study by Tohen et al. 2005. Eur Psychiatry. 2010;25(8):443-449.
41. Vestergaard P, Licht RW, Brodersen A, et al. Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum lithium levels. Acta Psychiatr Scand. 1998;98(4):310-315.
42. Nierenberg AA, Friedman ES, Bowden CL, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102-111.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(11 suppl 2):4-9.
2. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
3. Burgess S, Geddes J, Hawton K, et al. Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev. 2001:CD003013.
4. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex maintenance study group. Arch Gen Psychiatry. 2000;57(5):481-489.
5. Bowden CL, Calabrese JR, Sachs G, et al; Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
6. Swann AC, Bowden CL, Calabrese JR, et al. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26(4):530-536.
7. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
8. Perlis RH, Smoller JW, Ferreira MA, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009; 166(6):718-725.
9. Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10): 942-947.
10. Duffy A, Alda M, Kutcher S, et al. A prospective study of the offspring of bipolar parents responsive and nonresponsive to lithium treatment. J Clin Psychiatry. 2002;63(12): 1171-1178.
11. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
12. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010; 62(1):50-60.
13. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
14. de Sousa RT, van de Bilt MT, Diniz BS, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494(1):54-56.
15. Nierenberg AA, Sylvia LG, Leon AC, et al; LiTMUS Study Group. Lithium treatment–moderate dose use study (LiTMUS) for bipolar disorder: rationale and design. Clin Trials. 2009;6(6):637-648.
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Methods to limit attrition in longitudinal comparative effectiveness trials: lessons from the Lithium Treatment - Moderate dose Use Study (LiTMUS) for bipolar disorder. Clin Trials. 2012;9(1):94-101.
17. McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11(10):1439-1465.
18. Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy [published online February 15, 2014]. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2014.02.004.
19. Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1-21.
20. Devaki R, Shankar Rao S, Nadgir SM. The effect of lithium on the adrenoceptor-mediated second messenger system in the rat brain. J Psychiatry Neurosci. 2006;31(4):246-252.
21. Pan JQ, Lewis MC, Ketterman JK, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36(7):1397-1411.
22. Hu LW, Kawamoto EM, Brietzke E, et al. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):11-17.
23. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients [published online March 25, 2014]. Transl Psychiatry. doi:10.1038/tp.2014.12.
24. Jefferson JW. Lithium. In: Aronson JK, ed. Side effects of drugs annual, volume 26. Amsterdam, The Netherlands: Elsevier Science; 2003:19-29.
25. Baldessarini RJ, Tondo L, Floris G, et al. Effects of rapid cycling on response to lithium maintenance treatment in 360 bipolar I and II disorder patients. J Affect Disord. 2000;61(2):13-22.
26. Baldessarini RJ, Tondo L, Hennen J, et al. Latency and episodes before treatment: response to lithium maintenance in bipolar I and II disorders. Bipolar Disord. 1999;1(2): 91-97.
27. Fieve RR, Kumbaraci T, Dunner DL. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry. 1976;133(8):925-929.
28. Peck CC, Pond SM, Becker CE, et al. An evaluation of the effects of lithium in the treatment of chronic alcoholism. II. Assessment of the two-period crossover design. Alcohol Clin Exp Res. 1981;5(2):252-255.
29. Peselow ED, Dunner DL, Fieve RR, et al. Lithium prophylaxis of depression in unipolar, bipolar II, and cyclothymic patients. Am J Psychiatry. 1982;139(6):747-752.
30. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
31. Alevizos B, Alevizos E, Leonardou A, et al. Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study. Psychiatrike. 2012;23(2):143-148.
32. Goldberg JF, Sacks MH, Kocsis JH. Low-dose lithium augmentation of divalproex in geriatric mania. J Clin Psychiatry. 2000;61(4):304.
33. Saunders KE, Goodwin GM. New approaches in the treatment of bipolar depression. Curr Top Behav Neurosci. 2013;14:291-307.
34. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
35. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435.
36. Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Altman EG, Hedeker D, Peterson JL, et al. The Altman Self- Rating Mania Scale. Biol Psychiatry. 1997;42(10):948-955.
39. Machado-Vieira R, Luckenbaugh DA, Soeiro-de-Souza MG, et al. Early improvement with lithium in classic mania and its association with later response. J Affect Disord. 2013;144(1-2):160-164.
40. Severus WE, Lipkovich IA, Licht RW, et al. In search of optimal lithium levels and olanzapine doses in the long-term treatment of bipolar I disorder. A post-hoc analysis of the maintenance study by Tohen et al. 2005. Eur Psychiatry. 2010;25(8):443-449.
41. Vestergaard P, Licht RW, Brodersen A, et al. Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum lithium levels. Acta Psychiatr Scand. 1998;98(4):310-315.
42. Nierenberg AA, Friedman ES, Bowden CL, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102-111.
BPD and the broader landscape of neuropsychiatric illness
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
CBT effective for social anxiety disorder even with comorbidities
Group cognitive-behavioral therapy, often recommended as first-line therapy for social anxiety disorder, is effective even when patients have comorbid major depressive or anxiety disorders, a recent report shows.
Few previous studies have examined whether coexisting depression or anxiety influence the outcome of psychological treatment for social anxiety, and their findings have been conflicting. In this study, researchers assessed pre- and posttreatment results on the Social Phobia Inventory and the Depression Anxiety Stress Scales for 163 adults treated with group CBT at a single community hospital outpatient clinic over a 10-year period. Forty-one of these participants had social anxiety disorder with no comorbid diagnoses, 76 had comorbid major depressive disorder, 19 had comorbid bipolar disorder, and 27 had additional comorbid anxiety disorders.
The average age of onset of social anxiety disorder was 12 years in the 77 men and 86 women. A total of 85% were white, said Katie Fracalanza, a doctoral student in the department of psychology, Ryerson University, Toronto, and her associates.
All patients showed significant improvement in social anxiety symptoms after treatment, regardless of their comorbidities. Also, small to moderate improvements were found in depressive symptoms, the investigators reported (J. Affect. Disord. 2014;162:61-6).
Patients with comorbid disorders presented with more severe symptoms than did those who only had social anxiety disorder, and they completed treatment with greater residual symptoms, though still significantly improved. Additional sessions might be helpful for further symptom remission in such patients, Ms. Fracalanza and her associates said.
Limitations of the study include the self-report nature of social anxiety and depressive symptoms, and the relatively small sample size. Nevertheless, these findings are in line with those of previous studies reporting that depressive symptoms "do not hinder the effectiveness of CBT for social anxiety disorder" and might improve even though they are not specifically targeted by the treatment, the investigators noted.
Group cognitive-behavioral therapy, often recommended as first-line therapy for social anxiety disorder, is effective even when patients have comorbid major depressive or anxiety disorders, a recent report shows.
Few previous studies have examined whether coexisting depression or anxiety influence the outcome of psychological treatment for social anxiety, and their findings have been conflicting. In this study, researchers assessed pre- and posttreatment results on the Social Phobia Inventory and the Depression Anxiety Stress Scales for 163 adults treated with group CBT at a single community hospital outpatient clinic over a 10-year period. Forty-one of these participants had social anxiety disorder with no comorbid diagnoses, 76 had comorbid major depressive disorder, 19 had comorbid bipolar disorder, and 27 had additional comorbid anxiety disorders.
The average age of onset of social anxiety disorder was 12 years in the 77 men and 86 women. A total of 85% were white, said Katie Fracalanza, a doctoral student in the department of psychology, Ryerson University, Toronto, and her associates.
All patients showed significant improvement in social anxiety symptoms after treatment, regardless of their comorbidities. Also, small to moderate improvements were found in depressive symptoms, the investigators reported (J. Affect. Disord. 2014;162:61-6).
Patients with comorbid disorders presented with more severe symptoms than did those who only had social anxiety disorder, and they completed treatment with greater residual symptoms, though still significantly improved. Additional sessions might be helpful for further symptom remission in such patients, Ms. Fracalanza and her associates said.
Limitations of the study include the self-report nature of social anxiety and depressive symptoms, and the relatively small sample size. Nevertheless, these findings are in line with those of previous studies reporting that depressive symptoms "do not hinder the effectiveness of CBT for social anxiety disorder" and might improve even though they are not specifically targeted by the treatment, the investigators noted.
Group cognitive-behavioral therapy, often recommended as first-line therapy for social anxiety disorder, is effective even when patients have comorbid major depressive or anxiety disorders, a recent report shows.
Few previous studies have examined whether coexisting depression or anxiety influence the outcome of psychological treatment for social anxiety, and their findings have been conflicting. In this study, researchers assessed pre- and posttreatment results on the Social Phobia Inventory and the Depression Anxiety Stress Scales for 163 adults treated with group CBT at a single community hospital outpatient clinic over a 10-year period. Forty-one of these participants had social anxiety disorder with no comorbid diagnoses, 76 had comorbid major depressive disorder, 19 had comorbid bipolar disorder, and 27 had additional comorbid anxiety disorders.
The average age of onset of social anxiety disorder was 12 years in the 77 men and 86 women. A total of 85% were white, said Katie Fracalanza, a doctoral student in the department of psychology, Ryerson University, Toronto, and her associates.
All patients showed significant improvement in social anxiety symptoms after treatment, regardless of their comorbidities. Also, small to moderate improvements were found in depressive symptoms, the investigators reported (J. Affect. Disord. 2014;162:61-6).
Patients with comorbid disorders presented with more severe symptoms than did those who only had social anxiety disorder, and they completed treatment with greater residual symptoms, though still significantly improved. Additional sessions might be helpful for further symptom remission in such patients, Ms. Fracalanza and her associates said.
Limitations of the study include the self-report nature of social anxiety and depressive symptoms, and the relatively small sample size. Nevertheless, these findings are in line with those of previous studies reporting that depressive symptoms "do not hinder the effectiveness of CBT for social anxiety disorder" and might improve even though they are not specifically targeted by the treatment, the investigators noted.
FROM JOURNAL OF AFFECTIVE DISORDERS
Key clinical point: Twelve 2-hour manualized group CBT sessions can bring positive results for patients with comorbid SAD and bipolar disorder, SAD and depression, and SAD and other anxiety disorder.
Major finding: All patients showed significant improvement in social anxiety symptoms after group CBT, regardless of their comorbidities; there also were small to moderate improvements in depressive symptoms.
Data source: A single-center cohort study involving adults with social anxiety disorder alone (41 patients), SAD plus comorbid major depressive disorder (76 patients), SAD plus comorbid bipolar disorder (19), or SAD plus one or more comorbid anxiety disorders (27) who were treated with group CBT over a 10-year period.
Disclosures: This study was supported in part by McMaster University. Ms. Fracalanza and her associates reported no financial conflicts of interest.
Suicidal acts rise with longer duration of high-risk mood disorder states
NEW YORK – The likelihood that patients with major mood disorders will attempt or complete suicide appears to be related to the amount of time they spend in high-risk states of illness, results of longitudinal studies suggest.
"Among mood disorder patients, in any setting, there is a very strong association between suicidal acts and mood state," said Dr. Erkki T. Isometsä, professor of psychiatry at the University of Helsinki.
"Time spent in high-risk illness states is a major determinant of overall risk. Thus, for prevention of suicidal acts, reducing time in high-risk illness states is essential," he said at the American Psychiatric Association annual meeting.
Providing effective acute therapies and maintenance-phase treatment and improving continuity of care has the potential to significantly decrease suicidal acts, Dr. Isometsä said.
He noted that half of all suicides are committed by people who suffer from major mood disorders such as depression and bipolar disorder. A recently published meta-analysis and systematic review found that factors significantly associated with suicide were male sex (odds ratio, 1.76); a family history of psychiatric disorder (OR, 1.41); previous suicide attempt (OR, 4.84); more severe depression (OR, 2.20); hopelessness (OR, 2.20); and comorbid disorders, including anxiety (OR, 1.59) and substance misuse (OR, 2.17) (J. Affect. Disord. 2013;147:17-28).
Dr. Isometsä pointed to an International Society for Bipolar Disorders (ISBD) task force on suicide, whose members identified risk variables associated with suicide attempts and suicide deaths.
Suicide attempts were more likely in patients with bipolar disorder who had depressive polarity of their first, current, or most recent illness episode; comorbid cluster/borderline personality disorder; any comorbid anxiety or substance use disorder; history of suicide in a first-degree relative; female sex; and younger age of illness at onset.
However, only suicide of a first-degree relative and male sex were significantly associated with suicide deaths.
Longitudinal studies
Dr. Isometsä was the lead investigator for two longitudinal studies of Finnish patients with mood disorders: the Vantaa Primary Care Depression Study and the Jorvi Bipolar Study. Both studies looked at the incidence of suicide attempts over time across variable mood states.
In the depression study, the investigators compared the incidence of suicide attempts among depressed patients during major depressive episodes, partial remissions, and full remissions over 5 years of follow-up and found that 78% of the attempts (adjusted population attributable fraction [PAF]) occurred during major depressive episodes.
Factors significantly associated with suicide attempts were a prior attempt (OR, 4.39), partial vs. full remission (OR, 4.20), and major depressive episode (OR, 7.74).
Protective factors included age (OR, 0.94), married or cohabiting (OR, 0.43), intermediate social support (OR, 0.36), and high social support (OR, 0.28).
In the Jorvi Bipolar Study, which looked at patients with both bipolar disorders I and II, the incidence was of suicide attempts was 37 times higher when patients were in combined mixed and depressive mixed states and 18-fold higher during major depressive phases, compared with other illness states. In this study, the PAF for time spent in high-risk illness phase was 86%.
In each study, suicidal acts during mood episodes also was more likely among patients who had hopelessness, a history of abuse in childhood, poor social support, concurrent substance use, cluster B personality disorders, and those with impulsive-aggressive traits.
But of all of the associated factors, time spent in high-risk phases of illness appeared to be the predominant, modifiable driver of suicidal acts, Dr. Isometsä concluded.
The Vantaa and Jorvi studies were supported by the National Institute for Health and Welfare, Finland. Dr. Isometsä reported having no conflicts of interest.
NEW YORK – The likelihood that patients with major mood disorders will attempt or complete suicide appears to be related to the amount of time they spend in high-risk states of illness, results of longitudinal studies suggest.
"Among mood disorder patients, in any setting, there is a very strong association between suicidal acts and mood state," said Dr. Erkki T. Isometsä, professor of psychiatry at the University of Helsinki.
"Time spent in high-risk illness states is a major determinant of overall risk. Thus, for prevention of suicidal acts, reducing time in high-risk illness states is essential," he said at the American Psychiatric Association annual meeting.
Providing effective acute therapies and maintenance-phase treatment and improving continuity of care has the potential to significantly decrease suicidal acts, Dr. Isometsä said.
He noted that half of all suicides are committed by people who suffer from major mood disorders such as depression and bipolar disorder. A recently published meta-analysis and systematic review found that factors significantly associated with suicide were male sex (odds ratio, 1.76); a family history of psychiatric disorder (OR, 1.41); previous suicide attempt (OR, 4.84); more severe depression (OR, 2.20); hopelessness (OR, 2.20); and comorbid disorders, including anxiety (OR, 1.59) and substance misuse (OR, 2.17) (J. Affect. Disord. 2013;147:17-28).
Dr. Isometsä pointed to an International Society for Bipolar Disorders (ISBD) task force on suicide, whose members identified risk variables associated with suicide attempts and suicide deaths.
Suicide attempts were more likely in patients with bipolar disorder who had depressive polarity of their first, current, or most recent illness episode; comorbid cluster/borderline personality disorder; any comorbid anxiety or substance use disorder; history of suicide in a first-degree relative; female sex; and younger age of illness at onset.
However, only suicide of a first-degree relative and male sex were significantly associated with suicide deaths.
Longitudinal studies
Dr. Isometsä was the lead investigator for two longitudinal studies of Finnish patients with mood disorders: the Vantaa Primary Care Depression Study and the Jorvi Bipolar Study. Both studies looked at the incidence of suicide attempts over time across variable mood states.
In the depression study, the investigators compared the incidence of suicide attempts among depressed patients during major depressive episodes, partial remissions, and full remissions over 5 years of follow-up and found that 78% of the attempts (adjusted population attributable fraction [PAF]) occurred during major depressive episodes.
Factors significantly associated with suicide attempts were a prior attempt (OR, 4.39), partial vs. full remission (OR, 4.20), and major depressive episode (OR, 7.74).
Protective factors included age (OR, 0.94), married or cohabiting (OR, 0.43), intermediate social support (OR, 0.36), and high social support (OR, 0.28).
In the Jorvi Bipolar Study, which looked at patients with both bipolar disorders I and II, the incidence was of suicide attempts was 37 times higher when patients were in combined mixed and depressive mixed states and 18-fold higher during major depressive phases, compared with other illness states. In this study, the PAF for time spent in high-risk illness phase was 86%.
In each study, suicidal acts during mood episodes also was more likely among patients who had hopelessness, a history of abuse in childhood, poor social support, concurrent substance use, cluster B personality disorders, and those with impulsive-aggressive traits.
But of all of the associated factors, time spent in high-risk phases of illness appeared to be the predominant, modifiable driver of suicidal acts, Dr. Isometsä concluded.
The Vantaa and Jorvi studies were supported by the National Institute for Health and Welfare, Finland. Dr. Isometsä reported having no conflicts of interest.
NEW YORK – The likelihood that patients with major mood disorders will attempt or complete suicide appears to be related to the amount of time they spend in high-risk states of illness, results of longitudinal studies suggest.
"Among mood disorder patients, in any setting, there is a very strong association between suicidal acts and mood state," said Dr. Erkki T. Isometsä, professor of psychiatry at the University of Helsinki.
"Time spent in high-risk illness states is a major determinant of overall risk. Thus, for prevention of suicidal acts, reducing time in high-risk illness states is essential," he said at the American Psychiatric Association annual meeting.
Providing effective acute therapies and maintenance-phase treatment and improving continuity of care has the potential to significantly decrease suicidal acts, Dr. Isometsä said.
He noted that half of all suicides are committed by people who suffer from major mood disorders such as depression and bipolar disorder. A recently published meta-analysis and systematic review found that factors significantly associated with suicide were male sex (odds ratio, 1.76); a family history of psychiatric disorder (OR, 1.41); previous suicide attempt (OR, 4.84); more severe depression (OR, 2.20); hopelessness (OR, 2.20); and comorbid disorders, including anxiety (OR, 1.59) and substance misuse (OR, 2.17) (J. Affect. Disord. 2013;147:17-28).
Dr. Isometsä pointed to an International Society for Bipolar Disorders (ISBD) task force on suicide, whose members identified risk variables associated with suicide attempts and suicide deaths.
Suicide attempts were more likely in patients with bipolar disorder who had depressive polarity of their first, current, or most recent illness episode; comorbid cluster/borderline personality disorder; any comorbid anxiety or substance use disorder; history of suicide in a first-degree relative; female sex; and younger age of illness at onset.
However, only suicide of a first-degree relative and male sex were significantly associated with suicide deaths.
Longitudinal studies
Dr. Isometsä was the lead investigator for two longitudinal studies of Finnish patients with mood disorders: the Vantaa Primary Care Depression Study and the Jorvi Bipolar Study. Both studies looked at the incidence of suicide attempts over time across variable mood states.
In the depression study, the investigators compared the incidence of suicide attempts among depressed patients during major depressive episodes, partial remissions, and full remissions over 5 years of follow-up and found that 78% of the attempts (adjusted population attributable fraction [PAF]) occurred during major depressive episodes.
Factors significantly associated with suicide attempts were a prior attempt (OR, 4.39), partial vs. full remission (OR, 4.20), and major depressive episode (OR, 7.74).
Protective factors included age (OR, 0.94), married or cohabiting (OR, 0.43), intermediate social support (OR, 0.36), and high social support (OR, 0.28).
In the Jorvi Bipolar Study, which looked at patients with both bipolar disorders I and II, the incidence was of suicide attempts was 37 times higher when patients were in combined mixed and depressive mixed states and 18-fold higher during major depressive phases, compared with other illness states. In this study, the PAF for time spent in high-risk illness phase was 86%.
In each study, suicidal acts during mood episodes also was more likely among patients who had hopelessness, a history of abuse in childhood, poor social support, concurrent substance use, cluster B personality disorders, and those with impulsive-aggressive traits.
But of all of the associated factors, time spent in high-risk phases of illness appeared to be the predominant, modifiable driver of suicidal acts, Dr. Isometsä concluded.
The Vantaa and Jorvi studies were supported by the National Institute for Health and Welfare, Finland. Dr. Isometsä reported having no conflicts of interest.
AT THE APA ANNUAL MEETING
Key clinical point: Effective acute therapies have the potential to significantly decrease suicide attempts among mood disorder patients.
Major finding: In a longitudinal study of patients with bipolar disorder types I and II, the population-attributable fraction of suicide attempts by time spent in high-risk illness phase was 86%.
Data source: Two longitudinal studies of patients in Finland: the Vantaa Depression Study of 269 patients and the Jorvi Bipolar Study of 191 patients.
Disclosures: The Vantaa and Jorvi studies were supported by the National Institute for Health and Welfare, Finland. Dr. Isometsä reported having no conflicts of interest.
N-acetylcysteine may calm hair-pulling, skin-picking disorders
NEW YORK – The safe, cheap, readily available medication N-acetylcysteine may be effective against difficult-to-treat compulsive grooming disorders, a trans-Atlantic study showed.
In a case series of six patients treated for trichotillomania (hair pulling) and/or skin excoriation (skin-picking disorder, or SPD) as comorbidities to an impulse control or affective disorder, treatment with N-acetylcysteine (NAC) 1,200-1,800 mg/day resulted in either complete abstinence or great improvement in self-damaging habits, said Dr. Gustavo Jesus of the Central Psychiatric Hospital of Lisbon.
Most cases show very good results of the use of NAC in difficult-to-treat grooming disorders, he said at the American Psychiatric Association annual meeting.
Although support for the use of NAC in grooming disorders comes largely from case reports, the available evidence supports his team’s findings, he added.
NAC is a precursor to cysteine, an amino acid that protects cells from oxidative stress, interacts with inflammatory mediators, and is a modulator of the glutaminergic system of neurotransmission.
The medication historically has been used to counteract acetaminophen overdose, as a mucolytic agent for the treatment of respiratory diseases, and in the treatment of contrast-induced nephropathy, polycystic ovary syndrome, and more recently, psychiatric disorders, because of its antioxidative and anti-inflammatory properties.
The agent has been studied most in schizophrenia, Dr. Jesus said, but has also been investigated in bipolar and depressive disorders, substance use problems, obsessive-compulsive disorders and impulse control disorders, including grooming disorders.
Few options
Dr. Jesus’s team, with colleagues in São Paulo, Brazil, reviewed the literature for drug treatment of trichotillomania, and found varying evidence for the use of clomipramine, desipramine, fluoxetine, olanzapine, naltrexone, and citalopram.
"Still, we are short of evidence for which medications can be used to treat grooming disorders," Dr. Jesus said.
He presented data on a case series of three Brazilian and three Portuguese patients, four of whom had SPD, one with trichotillomania, and one with both disorders secondary to conditions that included bipolar disorder, major depressive disorder, generalized anxiety disorder, and dysthymia.
Previous medications tried for their compulsive behaviors included lithium, quetiapine, fluoxetine, venlafaxine, oxcarbazepine, and gabapentin.
Two patients had complete abstinence from SPD for 10-12 months while taking NAC 1,200 mg daily. One of these patients had a relapse after 2 weeks off NAC and improved after restarting the drug. The second patient had a relapse 1 month after stopping the drug and also had improvement after restarting it, this time at a dose of 1,800 mg/day. This patient also had trichotillomania that was considered "greatly improved" with NAC 1,200 mg/day.
The remaining patients all had "great improvement" of SPD, and three had improvement of symptoms when they restarted the drug after hiatuses ranging from 2 weeks to 3 months.
Dr. Jesus emphasized that NAC treated the compulsive grooming symptoms only and not the underlying disorder. For example, one patient with SPD secondary to major depressive disorder also had pathologic jealousy and an addiction to the Internet; NAC did not diminish symptoms of either the jealousy or the addiction.
There has been only one small, randomized, double-blind placebo-controlled trial of NAC in trichotillomania to date, Dr. Jesus noted. The investigators of that study found that patients in the active treatment group had significant improvement of symptoms on both the Massachusetts General Hospital Hair Pulling Scale and the Psychiatric Institute Trichotillomania Scale (P = .001). They also found that more than half of the 50 patients were "much" or "very much" improved with NAC, compared with patients on placebo (P = .003).
Dr. Jesus did not disclose the funding source for the study but reported having no significant financial disclosures or relationships with the manufacturers of any products he discussed.
NEW YORK – The safe, cheap, readily available medication N-acetylcysteine may be effective against difficult-to-treat compulsive grooming disorders, a trans-Atlantic study showed.
In a case series of six patients treated for trichotillomania (hair pulling) and/or skin excoriation (skin-picking disorder, or SPD) as comorbidities to an impulse control or affective disorder, treatment with N-acetylcysteine (NAC) 1,200-1,800 mg/day resulted in either complete abstinence or great improvement in self-damaging habits, said Dr. Gustavo Jesus of the Central Psychiatric Hospital of Lisbon.
Most cases show very good results of the use of NAC in difficult-to-treat grooming disorders, he said at the American Psychiatric Association annual meeting.
Although support for the use of NAC in grooming disorders comes largely from case reports, the available evidence supports his team’s findings, he added.
NAC is a precursor to cysteine, an amino acid that protects cells from oxidative stress, interacts with inflammatory mediators, and is a modulator of the glutaminergic system of neurotransmission.
The medication historically has been used to counteract acetaminophen overdose, as a mucolytic agent for the treatment of respiratory diseases, and in the treatment of contrast-induced nephropathy, polycystic ovary syndrome, and more recently, psychiatric disorders, because of its antioxidative and anti-inflammatory properties.
The agent has been studied most in schizophrenia, Dr. Jesus said, but has also been investigated in bipolar and depressive disorders, substance use problems, obsessive-compulsive disorders and impulse control disorders, including grooming disorders.
Few options
Dr. Jesus’s team, with colleagues in São Paulo, Brazil, reviewed the literature for drug treatment of trichotillomania, and found varying evidence for the use of clomipramine, desipramine, fluoxetine, olanzapine, naltrexone, and citalopram.
"Still, we are short of evidence for which medications can be used to treat grooming disorders," Dr. Jesus said.
He presented data on a case series of three Brazilian and three Portuguese patients, four of whom had SPD, one with trichotillomania, and one with both disorders secondary to conditions that included bipolar disorder, major depressive disorder, generalized anxiety disorder, and dysthymia.
Previous medications tried for their compulsive behaviors included lithium, quetiapine, fluoxetine, venlafaxine, oxcarbazepine, and gabapentin.
Two patients had complete abstinence from SPD for 10-12 months while taking NAC 1,200 mg daily. One of these patients had a relapse after 2 weeks off NAC and improved after restarting the drug. The second patient had a relapse 1 month after stopping the drug and also had improvement after restarting it, this time at a dose of 1,800 mg/day. This patient also had trichotillomania that was considered "greatly improved" with NAC 1,200 mg/day.
The remaining patients all had "great improvement" of SPD, and three had improvement of symptoms when they restarted the drug after hiatuses ranging from 2 weeks to 3 months.
Dr. Jesus emphasized that NAC treated the compulsive grooming symptoms only and not the underlying disorder. For example, one patient with SPD secondary to major depressive disorder also had pathologic jealousy and an addiction to the Internet; NAC did not diminish symptoms of either the jealousy or the addiction.
There has been only one small, randomized, double-blind placebo-controlled trial of NAC in trichotillomania to date, Dr. Jesus noted. The investigators of that study found that patients in the active treatment group had significant improvement of symptoms on both the Massachusetts General Hospital Hair Pulling Scale and the Psychiatric Institute Trichotillomania Scale (P = .001). They also found that more than half of the 50 patients were "much" or "very much" improved with NAC, compared with patients on placebo (P = .003).
Dr. Jesus did not disclose the funding source for the study but reported having no significant financial disclosures or relationships with the manufacturers of any products he discussed.
NEW YORK – The safe, cheap, readily available medication N-acetylcysteine may be effective against difficult-to-treat compulsive grooming disorders, a trans-Atlantic study showed.
In a case series of six patients treated for trichotillomania (hair pulling) and/or skin excoriation (skin-picking disorder, or SPD) as comorbidities to an impulse control or affective disorder, treatment with N-acetylcysteine (NAC) 1,200-1,800 mg/day resulted in either complete abstinence or great improvement in self-damaging habits, said Dr. Gustavo Jesus of the Central Psychiatric Hospital of Lisbon.
Most cases show very good results of the use of NAC in difficult-to-treat grooming disorders, he said at the American Psychiatric Association annual meeting.
Although support for the use of NAC in grooming disorders comes largely from case reports, the available evidence supports his team’s findings, he added.
NAC is a precursor to cysteine, an amino acid that protects cells from oxidative stress, interacts with inflammatory mediators, and is a modulator of the glutaminergic system of neurotransmission.
The medication historically has been used to counteract acetaminophen overdose, as a mucolytic agent for the treatment of respiratory diseases, and in the treatment of contrast-induced nephropathy, polycystic ovary syndrome, and more recently, psychiatric disorders, because of its antioxidative and anti-inflammatory properties.
The agent has been studied most in schizophrenia, Dr. Jesus said, but has also been investigated in bipolar and depressive disorders, substance use problems, obsessive-compulsive disorders and impulse control disorders, including grooming disorders.
Few options
Dr. Jesus’s team, with colleagues in São Paulo, Brazil, reviewed the literature for drug treatment of trichotillomania, and found varying evidence for the use of clomipramine, desipramine, fluoxetine, olanzapine, naltrexone, and citalopram.
"Still, we are short of evidence for which medications can be used to treat grooming disorders," Dr. Jesus said.
He presented data on a case series of three Brazilian and three Portuguese patients, four of whom had SPD, one with trichotillomania, and one with both disorders secondary to conditions that included bipolar disorder, major depressive disorder, generalized anxiety disorder, and dysthymia.
Previous medications tried for their compulsive behaviors included lithium, quetiapine, fluoxetine, venlafaxine, oxcarbazepine, and gabapentin.
Two patients had complete abstinence from SPD for 10-12 months while taking NAC 1,200 mg daily. One of these patients had a relapse after 2 weeks off NAC and improved after restarting the drug. The second patient had a relapse 1 month after stopping the drug and also had improvement after restarting it, this time at a dose of 1,800 mg/day. This patient also had trichotillomania that was considered "greatly improved" with NAC 1,200 mg/day.
The remaining patients all had "great improvement" of SPD, and three had improvement of symptoms when they restarted the drug after hiatuses ranging from 2 weeks to 3 months.
Dr. Jesus emphasized that NAC treated the compulsive grooming symptoms only and not the underlying disorder. For example, one patient with SPD secondary to major depressive disorder also had pathologic jealousy and an addiction to the Internet; NAC did not diminish symptoms of either the jealousy or the addiction.
There has been only one small, randomized, double-blind placebo-controlled trial of NAC in trichotillomania to date, Dr. Jesus noted. The investigators of that study found that patients in the active treatment group had significant improvement of symptoms on both the Massachusetts General Hospital Hair Pulling Scale and the Psychiatric Institute Trichotillomania Scale (P = .001). They also found that more than half of the 50 patients were "much" or "very much" improved with NAC, compared with patients on placebo (P = .003).
Dr. Jesus did not disclose the funding source for the study but reported having no significant financial disclosures or relationships with the manufacturers of any products he discussed.
AT APA 2014
Key clinical point: Finding medication with a proven track record of treating grooming disorders is difficult.
Major finding: N-acetylcysteine was effective at treating skin-picking disorder and trichotillomania in patients with compulsive behaviors secondary to other disorders.
Data source: A literature review and case series involving six patients.
Disclosures: Dr. Jesus did not disclose the funding source for the study but reported having no significant financial disclosures or relationships with the manufacturers of any products he discussed.
Speech comprehension worse in bipolar mania
Bipolar disorder patients perform worse than do their counterparts without-bipolar disorder at language comprehension tests at the behavioral level, but not the physiological level, according to findings from a small-scale study.
A team of researchers from CHU Sainte Marguerite and Aix-Marseille Université, Marseille, France, examined behavioral and electrophysiological responses to speech by measuring the "N400 effect," an ERP (event-related brain potential) observed in patients with bipolar disorder and schizophrenia that typically is provoked via the subjects’ response to unexpected or incongruous words at the end of a sentence.
Led by Dr. Michel Cermolacce of CHU Sainte Marguerite, the team compared responses from 38 participants, including 19 bipolar type I patients and 19 healthy comparison subjects. The patients with bipolar disorder were recruited from the Marseille University Department of Psychiatry while presenting a mild to severe manic episode and did not have concurrent neurological disorders (J. Affect. Disord. 2014;158:161-71).
The participants in the study were asked to listen to a series of congruous and incongruous complete sentences and judge whether the last word of each sentence was congruous or incongruous. The subjects’ brain waves were measured throughout the test with an electroencephalogram.
The study participants with bipolar disorder exhibited a lower rate of correct responses for both congruous endings (76.7%, compared to 80% from healthy subjects) and incongruous endings (75% in patients with bipolar disorder, and 80% from healthy subjects). In addition, bipolar patients had longer response times when looking for congruous endings than their peers did (1,120 ms compared to 970 ms for healthy subjects.)
However, EEG readings showed preserved amplitude but delayed latency in difference waves, suggesting no significant disruption of brain waves through the N400. The authors noted that the findings contrast with the only previous N400 study, which showed a disruption of brain waves that is in line with similar results found in patients with schizophrenia (Prog. Neuropsychopharmacol. Biol. Psychiatry 2012;38:194-200). The previous study used visually presented word pairs rather than verbal word pairs, an approach the authors contend does not reflect a natural language setting.
Dr. Cermolacce and his colleagues cited several limitations, including the study’s small sample size and the absence of a group of patients with schizophrenia.
However, the findings suggest that specificity and adherence to natural speech patterns should be taken into account when examining speech disruptions in patients with mental disorders in a research setting, they noted.
"The discrepancy in manic patients between (i) preserved N400 and (ii) delayed [Late Positive Component] and impaired behavioral performances under natural speech conditions can be interpreted as reflecting non-specific cognitive rather than primary language alterations in line with previous behavioral findings," they wrote.
The authors report no conflict of interest.
Bipolar disorder patients perform worse than do their counterparts without-bipolar disorder at language comprehension tests at the behavioral level, but not the physiological level, according to findings from a small-scale study.
A team of researchers from CHU Sainte Marguerite and Aix-Marseille Université, Marseille, France, examined behavioral and electrophysiological responses to speech by measuring the "N400 effect," an ERP (event-related brain potential) observed in patients with bipolar disorder and schizophrenia that typically is provoked via the subjects’ response to unexpected or incongruous words at the end of a sentence.
Led by Dr. Michel Cermolacce of CHU Sainte Marguerite, the team compared responses from 38 participants, including 19 bipolar type I patients and 19 healthy comparison subjects. The patients with bipolar disorder were recruited from the Marseille University Department of Psychiatry while presenting a mild to severe manic episode and did not have concurrent neurological disorders (J. Affect. Disord. 2014;158:161-71).
The participants in the study were asked to listen to a series of congruous and incongruous complete sentences and judge whether the last word of each sentence was congruous or incongruous. The subjects’ brain waves were measured throughout the test with an electroencephalogram.
The study participants with bipolar disorder exhibited a lower rate of correct responses for both congruous endings (76.7%, compared to 80% from healthy subjects) and incongruous endings (75% in patients with bipolar disorder, and 80% from healthy subjects). In addition, bipolar patients had longer response times when looking for congruous endings than their peers did (1,120 ms compared to 970 ms for healthy subjects.)
However, EEG readings showed preserved amplitude but delayed latency in difference waves, suggesting no significant disruption of brain waves through the N400. The authors noted that the findings contrast with the only previous N400 study, which showed a disruption of brain waves that is in line with similar results found in patients with schizophrenia (Prog. Neuropsychopharmacol. Biol. Psychiatry 2012;38:194-200). The previous study used visually presented word pairs rather than verbal word pairs, an approach the authors contend does not reflect a natural language setting.
Dr. Cermolacce and his colleagues cited several limitations, including the study’s small sample size and the absence of a group of patients with schizophrenia.
However, the findings suggest that specificity and adherence to natural speech patterns should be taken into account when examining speech disruptions in patients with mental disorders in a research setting, they noted.
"The discrepancy in manic patients between (i) preserved N400 and (ii) delayed [Late Positive Component] and impaired behavioral performances under natural speech conditions can be interpreted as reflecting non-specific cognitive rather than primary language alterations in line with previous behavioral findings," they wrote.
The authors report no conflict of interest.
Bipolar disorder patients perform worse than do their counterparts without-bipolar disorder at language comprehension tests at the behavioral level, but not the physiological level, according to findings from a small-scale study.
A team of researchers from CHU Sainte Marguerite and Aix-Marseille Université, Marseille, France, examined behavioral and electrophysiological responses to speech by measuring the "N400 effect," an ERP (event-related brain potential) observed in patients with bipolar disorder and schizophrenia that typically is provoked via the subjects’ response to unexpected or incongruous words at the end of a sentence.
Led by Dr. Michel Cermolacce of CHU Sainte Marguerite, the team compared responses from 38 participants, including 19 bipolar type I patients and 19 healthy comparison subjects. The patients with bipolar disorder were recruited from the Marseille University Department of Psychiatry while presenting a mild to severe manic episode and did not have concurrent neurological disorders (J. Affect. Disord. 2014;158:161-71).
The participants in the study were asked to listen to a series of congruous and incongruous complete sentences and judge whether the last word of each sentence was congruous or incongruous. The subjects’ brain waves were measured throughout the test with an electroencephalogram.
The study participants with bipolar disorder exhibited a lower rate of correct responses for both congruous endings (76.7%, compared to 80% from healthy subjects) and incongruous endings (75% in patients with bipolar disorder, and 80% from healthy subjects). In addition, bipolar patients had longer response times when looking for congruous endings than their peers did (1,120 ms compared to 970 ms for healthy subjects.)
However, EEG readings showed preserved amplitude but delayed latency in difference waves, suggesting no significant disruption of brain waves through the N400. The authors noted that the findings contrast with the only previous N400 study, which showed a disruption of brain waves that is in line with similar results found in patients with schizophrenia (Prog. Neuropsychopharmacol. Biol. Psychiatry 2012;38:194-200). The previous study used visually presented word pairs rather than verbal word pairs, an approach the authors contend does not reflect a natural language setting.
Dr. Cermolacce and his colleagues cited several limitations, including the study’s small sample size and the absence of a group of patients with schizophrenia.
However, the findings suggest that specificity and adherence to natural speech patterns should be taken into account when examining speech disruptions in patients with mental disorders in a research setting, they noted.
"The discrepancy in manic patients between (i) preserved N400 and (ii) delayed [Late Positive Component] and impaired behavioral performances under natural speech conditions can be interpreted as reflecting non-specific cognitive rather than primary language alterations in line with previous behavioral findings," they wrote.
The authors report no conflict of interest.
FROM JOURNAL OF AFFECTIVE DISORDERS
Key clinical point: Specificity and adherence to natural speech patterns should be taken into account when examining speech disruptions in patients with bipolar disorder in a research setting.
Major finding: Patients with bipolar disorder showed worst performances in speech cognitions compared with healthy participants on a behavioral level but not necessarily at the electrophysiological level.
Data source: A study of 19 subjects with bipolar 1 disorder and 19 control subjects.
Disclosures: The authors had no relevant disclosures.
EEG anomaly is pronounced in alcohol misuse, bipolar disorder
Mismatch negativity on EEG examination is altered among young adults who engage in risky drinking behavior, compared with nondrinkers, and is even more pronounced among people who report risky alcohol use and also have bipolar disorder, a recently published report shows.
Mismatch negativity refers to the automatic electrical activity that occurs in the brain in response to auditory stimulation that incorporates a deviation in a sequence of sounds, and reductions in mismatch negativity are a marker for impairment of NMDA (N-methyl-D-aspartate)-receptor activation. NMDA receptors are thought to be compromised already by alcohol use and in patients with bipolar disorder.
To examine the effects of alcohol use and bipolar disorder on mismatch negativity, EEG with auditory stimuli was performed on 42 bipolar disorder patients and 34 control subjects aged 16-30 years, reported Kate M. Chitty, a PhD candidate at the University of Sydney, Australia, and her associates.
Sixteen of the bipolar disorder patients and 14 of the control subjects engaged in high-risk drinking, while 26 of the patients with bipolar disorder and 20 of the control subjects did not. Alcohol misuse was found to be a strong predictor of attenuations in mismatch negativity, and alcohol’s effect was even more pronounced in bipolar disorder patients than in control subjects. "The attenuated mismatch negativity may reflect an additive effect of alcohol’s antagonistic actions on an already perturbed NMDA/glutamatergic system," the investigators said (Biol. Psychol. 2014;99:60-8).
"Just as glutamatergic agents are becoming increasingly popular in the treatment of bipolar disease, our results suggest that limiting alcohol use could help to control glutamatergic regulation and may be a crucial first step prior to initiating such treatment," they added.
Youths with bipolar disorder have reported lifetime rates of alcohol misuse of up to 70%, and one recent study found that the demographic group most likely to participate in weekly substance use was 20- to 30-year-old males with bipolar disorder, reported Ms. Chitty, who is affiliated with the university’s clinical research unit at the Brain and Mind Research Institute.
Ms. Chitty and her associates cited several limitations of their study. For example, because participants under age 18 years are unable to buy alcohol legally in Australia, it is possible those minors might have been inclined to underreport their alcohol use. Also, the assessment of alcohol use was based on self-report answers to the AUDIT (alcohol use disorder identification test), which is designed to access alcohol use over the last year. "Therefore, information regarding the duration and intensity of the drinking habits beyond this timeframe is limited," they wrote.
Nevertheless, the study suggests that limiting alcohol use might help control glutamatergic regulation and "may be a crucial first step prior to initiating" the treatment of bipolar disorder, Ms. Chitty and her associates wrote.
This study was funded by Australia’s National Health and Medical Research Council; and the New South Wales Health, Mental Health and Drug & Alcohol Office. Ms. Chitty and her associates reported no financial conflicts of interest.
Mismatch negativity on EEG examination is altered among young adults who engage in risky drinking behavior, compared with nondrinkers, and is even more pronounced among people who report risky alcohol use and also have bipolar disorder, a recently published report shows.
Mismatch negativity refers to the automatic electrical activity that occurs in the brain in response to auditory stimulation that incorporates a deviation in a sequence of sounds, and reductions in mismatch negativity are a marker for impairment of NMDA (N-methyl-D-aspartate)-receptor activation. NMDA receptors are thought to be compromised already by alcohol use and in patients with bipolar disorder.
To examine the effects of alcohol use and bipolar disorder on mismatch negativity, EEG with auditory stimuli was performed on 42 bipolar disorder patients and 34 control subjects aged 16-30 years, reported Kate M. Chitty, a PhD candidate at the University of Sydney, Australia, and her associates.
Sixteen of the bipolar disorder patients and 14 of the control subjects engaged in high-risk drinking, while 26 of the patients with bipolar disorder and 20 of the control subjects did not. Alcohol misuse was found to be a strong predictor of attenuations in mismatch negativity, and alcohol’s effect was even more pronounced in bipolar disorder patients than in control subjects. "The attenuated mismatch negativity may reflect an additive effect of alcohol’s antagonistic actions on an already perturbed NMDA/glutamatergic system," the investigators said (Biol. Psychol. 2014;99:60-8).
"Just as glutamatergic agents are becoming increasingly popular in the treatment of bipolar disease, our results suggest that limiting alcohol use could help to control glutamatergic regulation and may be a crucial first step prior to initiating such treatment," they added.
Youths with bipolar disorder have reported lifetime rates of alcohol misuse of up to 70%, and one recent study found that the demographic group most likely to participate in weekly substance use was 20- to 30-year-old males with bipolar disorder, reported Ms. Chitty, who is affiliated with the university’s clinical research unit at the Brain and Mind Research Institute.
Ms. Chitty and her associates cited several limitations of their study. For example, because participants under age 18 years are unable to buy alcohol legally in Australia, it is possible those minors might have been inclined to underreport their alcohol use. Also, the assessment of alcohol use was based on self-report answers to the AUDIT (alcohol use disorder identification test), which is designed to access alcohol use over the last year. "Therefore, information regarding the duration and intensity of the drinking habits beyond this timeframe is limited," they wrote.
Nevertheless, the study suggests that limiting alcohol use might help control glutamatergic regulation and "may be a crucial first step prior to initiating" the treatment of bipolar disorder, Ms. Chitty and her associates wrote.
This study was funded by Australia’s National Health and Medical Research Council; and the New South Wales Health, Mental Health and Drug & Alcohol Office. Ms. Chitty and her associates reported no financial conflicts of interest.
Mismatch negativity on EEG examination is altered among young adults who engage in risky drinking behavior, compared with nondrinkers, and is even more pronounced among people who report risky alcohol use and also have bipolar disorder, a recently published report shows.
Mismatch negativity refers to the automatic electrical activity that occurs in the brain in response to auditory stimulation that incorporates a deviation in a sequence of sounds, and reductions in mismatch negativity are a marker for impairment of NMDA (N-methyl-D-aspartate)-receptor activation. NMDA receptors are thought to be compromised already by alcohol use and in patients with bipolar disorder.
To examine the effects of alcohol use and bipolar disorder on mismatch negativity, EEG with auditory stimuli was performed on 42 bipolar disorder patients and 34 control subjects aged 16-30 years, reported Kate M. Chitty, a PhD candidate at the University of Sydney, Australia, and her associates.
Sixteen of the bipolar disorder patients and 14 of the control subjects engaged in high-risk drinking, while 26 of the patients with bipolar disorder and 20 of the control subjects did not. Alcohol misuse was found to be a strong predictor of attenuations in mismatch negativity, and alcohol’s effect was even more pronounced in bipolar disorder patients than in control subjects. "The attenuated mismatch negativity may reflect an additive effect of alcohol’s antagonistic actions on an already perturbed NMDA/glutamatergic system," the investigators said (Biol. Psychol. 2014;99:60-8).
"Just as glutamatergic agents are becoming increasingly popular in the treatment of bipolar disease, our results suggest that limiting alcohol use could help to control glutamatergic regulation and may be a crucial first step prior to initiating such treatment," they added.
Youths with bipolar disorder have reported lifetime rates of alcohol misuse of up to 70%, and one recent study found that the demographic group most likely to participate in weekly substance use was 20- to 30-year-old males with bipolar disorder, reported Ms. Chitty, who is affiliated with the university’s clinical research unit at the Brain and Mind Research Institute.
Ms. Chitty and her associates cited several limitations of their study. For example, because participants under age 18 years are unable to buy alcohol legally in Australia, it is possible those minors might have been inclined to underreport their alcohol use. Also, the assessment of alcohol use was based on self-report answers to the AUDIT (alcohol use disorder identification test), which is designed to access alcohol use over the last year. "Therefore, information regarding the duration and intensity of the drinking habits beyond this timeframe is limited," they wrote.
Nevertheless, the study suggests that limiting alcohol use might help control glutamatergic regulation and "may be a crucial first step prior to initiating" the treatment of bipolar disorder, Ms. Chitty and her associates wrote.
This study was funded by Australia’s National Health and Medical Research Council; and the New South Wales Health, Mental Health and Drug & Alcohol Office. Ms. Chitty and her associates reported no financial conflicts of interest.
FROM BIOLOGICAL PSYCHOLOGY
Major finding: Alcohol misuse was found to be a strong predictor of attenuations in mismatch negativity, and alcohol’s effect was even more pronounced in bipolar disorder patients than in control subjects.
Data source: The findings are based on analysis of EEG responses to auditory stimulation among 42 patients with bipolar disorder and 34 control subjects, all aged 16-30 years.
Disclosures: This study was funded by Australia’s National Health and Medical Research Council; and the New South Wales Health, Mental Health and Drug & Alcohol Office. Ms. Chitty and her associates reported no financial conflicts of interest.