User login
COPD and asthma: Diagnostic accuracy requires spirometry
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
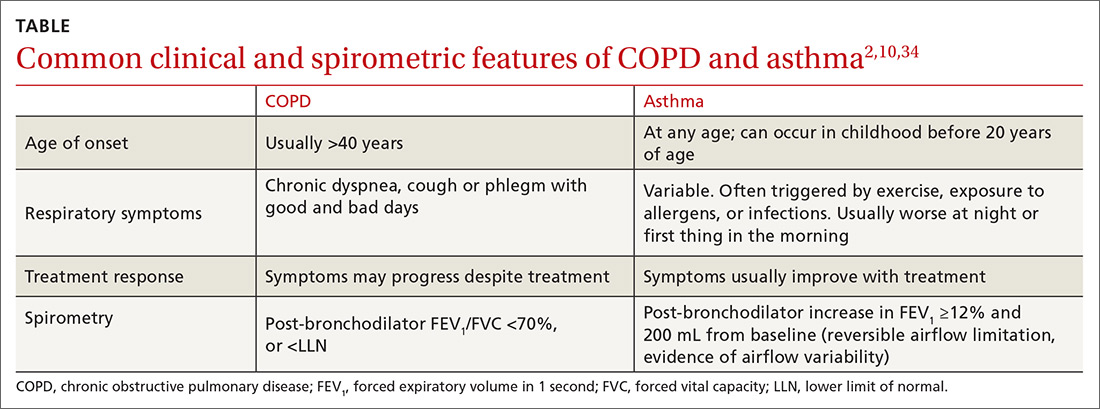
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; [email protected].
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.

In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics

Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; [email protected].
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.

In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics

Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; [email protected].
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
PRACTICE RECOMMENDATIONS
› Perform spirometry in all patients with symptoms and risk factors suggestive of chronic obstructive pulmonary disease (COPD) or asthma. B
› Consider having a patient use a peak flow meter to support a diagnosis of asthma if spirometry is unavailable. B
› Consider the possibility of a diagnostic error if COPD or asthma is unresponsive to treatment and the initial diagnosis was made without spirometry. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Atopic dermatitis at 1 year links with persistent food allergies
SAN FRANCISCO – Children diagnosed with atopic dermatitis when they were 1 year old were significantly more likely to have active food allergies and to have those allergies persist throughout childhood to age 18 years, based on findings from a prospective, longitudinal study of 287 Wisconsin children.
The link between atopic dermatitis (AD) and food allergy was especially strong in children who displayed early and recurrent AD; the link was weaker or essentially nonexistent for children with early transient AD or AD that first appeared later in childhood, Anne Marie Singh, MD, said while presenting a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The results also showed that even mild AD linked with an increased prevalence of food allergy when it appeared early and persisted, but more severe AD with this onset and recurrence pattern led to an even greater prevalence of food allergy, said Dr. Singh, a pediatric allergist and asthma specialist at the University of Wisconsin–Madison.
“The data suggest that something about early, recurrent AD increases the risk for food allergy throughout childhood,” Dr. Singh said in an interview. The findings suggest that surveillance for food allergies need to be intensified in infants who present with AD by the time they’re 1 year old and that food allergy surveillance should continue as these children age as long as their AD recurs.
The results also hint that these children might potentially benefit from steps aimed at desensitizing the allergy, although this must be proven in a future intervention study, she said.
The results suggest that a food allergy prevention regimen like the one used in the Learning Early About Peanut Allergy (LEAP) trial (New Engl J Med. 2015 Feb 26;372[9]:803-13) to prevent peanut allergy may be appropriate for selected, high-risk children with early AD, but this hypothesis needs testing, Dr. Singh said. She noted that some important differences exist between the patients enrolled in LEAP and the children studied in the current report: In LEAP, all enrolled children had severe eczema, an established egg allergy, or both. The findings reported by Dr. Singh came from children with AD, but only about 30% had moderate or severe eczema, and her analysis did not subdivide the observed food allergies by the type of food that caused a reaction.
She and her associates used data collected in the Childhood Origins of Asthma (COAST) study, begun in 1998, which enrolled 287 infants prior to birth who had at least one parent who was allergic, asthmatic, or both (Pediatr Allergy Immunol. 2002 Dec;13[s15]:38-43). The data showed that 62% of the infants had either no AD or transient AD, 14% had late onset AD, and 24% had early, recurrent AD. Although the data showed a statistically significant link between AD at 1 year old and food allergies throughout childhood, further analysis that broke the population into three different patterns of AD showed that the link with food allergy primarily existed among children with the early, recurrent form. Children with early, recurrent atopic dermatitis had a food allergy prevalence of 12%-27% annually through the age of 18 years.
“The data suggest that immunologic changes early in life are critical to food allergy development and that these changes have long-lasting effects throughout childhood,” Dr. Singh concluded. “The immunologic mechanisms by which early AD affects food allergy development and disease expression require further investigation.”
COAST received no commercial funding. Dr. Singh reported no relevant financial disclosures.
SOURCE: Singh AM et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB125.
SAN FRANCISCO – Children diagnosed with atopic dermatitis when they were 1 year old were significantly more likely to have active food allergies and to have those allergies persist throughout childhood to age 18 years, based on findings from a prospective, longitudinal study of 287 Wisconsin children.
The link between atopic dermatitis (AD) and food allergy was especially strong in children who displayed early and recurrent AD; the link was weaker or essentially nonexistent for children with early transient AD or AD that first appeared later in childhood, Anne Marie Singh, MD, said while presenting a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The results also showed that even mild AD linked with an increased prevalence of food allergy when it appeared early and persisted, but more severe AD with this onset and recurrence pattern led to an even greater prevalence of food allergy, said Dr. Singh, a pediatric allergist and asthma specialist at the University of Wisconsin–Madison.
“The data suggest that something about early, recurrent AD increases the risk for food allergy throughout childhood,” Dr. Singh said in an interview. The findings suggest that surveillance for food allergies need to be intensified in infants who present with AD by the time they’re 1 year old and that food allergy surveillance should continue as these children age as long as their AD recurs.
The results also hint that these children might potentially benefit from steps aimed at desensitizing the allergy, although this must be proven in a future intervention study, she said.
The results suggest that a food allergy prevention regimen like the one used in the Learning Early About Peanut Allergy (LEAP) trial (New Engl J Med. 2015 Feb 26;372[9]:803-13) to prevent peanut allergy may be appropriate for selected, high-risk children with early AD, but this hypothesis needs testing, Dr. Singh said. She noted that some important differences exist between the patients enrolled in LEAP and the children studied in the current report: In LEAP, all enrolled children had severe eczema, an established egg allergy, or both. The findings reported by Dr. Singh came from children with AD, but only about 30% had moderate or severe eczema, and her analysis did not subdivide the observed food allergies by the type of food that caused a reaction.
She and her associates used data collected in the Childhood Origins of Asthma (COAST) study, begun in 1998, which enrolled 287 infants prior to birth who had at least one parent who was allergic, asthmatic, or both (Pediatr Allergy Immunol. 2002 Dec;13[s15]:38-43). The data showed that 62% of the infants had either no AD or transient AD, 14% had late onset AD, and 24% had early, recurrent AD. Although the data showed a statistically significant link between AD at 1 year old and food allergies throughout childhood, further analysis that broke the population into three different patterns of AD showed that the link with food allergy primarily existed among children with the early, recurrent form. Children with early, recurrent atopic dermatitis had a food allergy prevalence of 12%-27% annually through the age of 18 years.
“The data suggest that immunologic changes early in life are critical to food allergy development and that these changes have long-lasting effects throughout childhood,” Dr. Singh concluded. “The immunologic mechanisms by which early AD affects food allergy development and disease expression require further investigation.”
COAST received no commercial funding. Dr. Singh reported no relevant financial disclosures.
SOURCE: Singh AM et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB125.
SAN FRANCISCO – Children diagnosed with atopic dermatitis when they were 1 year old were significantly more likely to have active food allergies and to have those allergies persist throughout childhood to age 18 years, based on findings from a prospective, longitudinal study of 287 Wisconsin children.
The link between atopic dermatitis (AD) and food allergy was especially strong in children who displayed early and recurrent AD; the link was weaker or essentially nonexistent for children with early transient AD or AD that first appeared later in childhood, Anne Marie Singh, MD, said while presenting a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The results also showed that even mild AD linked with an increased prevalence of food allergy when it appeared early and persisted, but more severe AD with this onset and recurrence pattern led to an even greater prevalence of food allergy, said Dr. Singh, a pediatric allergist and asthma specialist at the University of Wisconsin–Madison.
“The data suggest that something about early, recurrent AD increases the risk for food allergy throughout childhood,” Dr. Singh said in an interview. The findings suggest that surveillance for food allergies need to be intensified in infants who present with AD by the time they’re 1 year old and that food allergy surveillance should continue as these children age as long as their AD recurs.
The results also hint that these children might potentially benefit from steps aimed at desensitizing the allergy, although this must be proven in a future intervention study, she said.
The results suggest that a food allergy prevention regimen like the one used in the Learning Early About Peanut Allergy (LEAP) trial (New Engl J Med. 2015 Feb 26;372[9]:803-13) to prevent peanut allergy may be appropriate for selected, high-risk children with early AD, but this hypothesis needs testing, Dr. Singh said. She noted that some important differences exist between the patients enrolled in LEAP and the children studied in the current report: In LEAP, all enrolled children had severe eczema, an established egg allergy, or both. The findings reported by Dr. Singh came from children with AD, but only about 30% had moderate or severe eczema, and her analysis did not subdivide the observed food allergies by the type of food that caused a reaction.
She and her associates used data collected in the Childhood Origins of Asthma (COAST) study, begun in 1998, which enrolled 287 infants prior to birth who had at least one parent who was allergic, asthmatic, or both (Pediatr Allergy Immunol. 2002 Dec;13[s15]:38-43). The data showed that 62% of the infants had either no AD or transient AD, 14% had late onset AD, and 24% had early, recurrent AD. Although the data showed a statistically significant link between AD at 1 year old and food allergies throughout childhood, further analysis that broke the population into three different patterns of AD showed that the link with food allergy primarily existed among children with the early, recurrent form. Children with early, recurrent atopic dermatitis had a food allergy prevalence of 12%-27% annually through the age of 18 years.
“The data suggest that immunologic changes early in life are critical to food allergy development and that these changes have long-lasting effects throughout childhood,” Dr. Singh concluded. “The immunologic mechanisms by which early AD affects food allergy development and disease expression require further investigation.”
COAST received no commercial funding. Dr. Singh reported no relevant financial disclosures.
SOURCE: Singh AM et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB125.
REPORTING FROM AAAAI 2019
Don’t discount sleep disturbance for children with atopic dermatitis
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
FROM JAMA PEDIATRICS
Age 1 food allergies often disappear by age 6
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
[email protected]
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
[email protected]
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
[email protected]
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
REPORTING FROM AAAAI
Dupilumab relieves severe sinusitis with polyposis
SAN FRANCISCO – Dupilumab, an anti-inflammatory drug already approved for use in the United States, met its efficacy endpoints for treating chronic rhinosinusitis with nasal polyps in a pivotal trial with 276 patients.
The results make it likely that dupilumab (Dupixent) will receive a new indication from the Food and Drug Administration, pending similar results in a second pivotal trial for nasal polyps that researchers will report soon. Dupilumab, which works by blocking a receptor for both interleukin 4 and interleukin 13 and thereby shutting down type 2 inflammation, is already approved in the United States for treating atopic dermatitis and asthma.
Type 2 inflammation drives polyp formation in patients with chronic rhinosinusitis that can produce severe nasal congestion, breathing difficulty, and substantially reduced quality of life.
In the new trial, the drug showed efficacy by significantly improving both the nasal congestion score reported by patients and the nasal polyp score measured by sinus endoscopy after 24 weeks on treatment, when compared with control patients on placebo, Joseph K. Han, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients enrolled in the study had chronic, severe sinusitis and nasal polyps that remained uncontrolled despite prior surgery, for 75% of enrolled patients, or treatment with systemic corticosteroids, used on about 90% of the patients within the prior 2 years.
During the 24 weeks of treatment, 23% of patients in the control arm had to restart systemic corticosteroid treatment or have surgery, compared with 7% of patients on dupilumab treatment, a statistically significant difference.
The new drug is a “game changer,” for these patients, Dr. Han said in a video interview.
In some patients, treatment produced complete polyp resolution. He and his colleagues in the otolaryngology field are now trying to decide exactly which patients with polyps secondary to sinusitis will be good candidates for dupilumab after it receives an expected indication for shrinking nasal polyps.
Roughly 4% of the adult population has chronic rhinosinusitis that generates polyps. How many of these patients are affected severely enough to warrant dupilumab treatment is not clear, but will likely include several hundreds of thousands of U.S. adults, said Dr. Han, professor of otolaryngology and chief of the division of allergy at Eastern Virginia Medical School in Norfolk.
The SINUS-24 (A Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps) trial enrolled patients at 76 sites in the United States and in several European countries. The study randomized 143 patients who received standard treatment plus a 300-mg dupilumab subcutaneous injection every 2 weeks, and 133 patients who received standard treatment plus placebo injections. Standard treatment included a nasal corticosteroid spray.
After 24 weeks of treatment, the endoscopically-measured nasal polyp score, which averaged about 6 at baseline on a scale of 0-8, fell by an average of 2.06 points, compared with controls, which was a statistically significant and clinically meaningful change, said Dr. Han.
The second primary endpoint, patient self-assessment of nasal congestion on a scale of 0-3, showed an average 0.89 improvement, compared with controls, which was also a statistically significant and meaningful change from the average baseline score of about 2.4.
Other efficacy measures also showed benefits from treatment, including a substantial improvement compared with controls in a quality-of-life measure. The safety profile was benign compared with placebo, and consistent with existing safety data for the drug.SINUS-24 was funded by Regeneron and Sanofi, the companies that market dupilumab. Dr. Han has been an adviser to Regeneron and Sanofi.
SOURCE: Han JK et al. AAAAI 2019, Abstract L4.
SAN FRANCISCO – Dupilumab, an anti-inflammatory drug already approved for use in the United States, met its efficacy endpoints for treating chronic rhinosinusitis with nasal polyps in a pivotal trial with 276 patients.
The results make it likely that dupilumab (Dupixent) will receive a new indication from the Food and Drug Administration, pending similar results in a second pivotal trial for nasal polyps that researchers will report soon. Dupilumab, which works by blocking a receptor for both interleukin 4 and interleukin 13 and thereby shutting down type 2 inflammation, is already approved in the United States for treating atopic dermatitis and asthma.
Type 2 inflammation drives polyp formation in patients with chronic rhinosinusitis that can produce severe nasal congestion, breathing difficulty, and substantially reduced quality of life.
In the new trial, the drug showed efficacy by significantly improving both the nasal congestion score reported by patients and the nasal polyp score measured by sinus endoscopy after 24 weeks on treatment, when compared with control patients on placebo, Joseph K. Han, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients enrolled in the study had chronic, severe sinusitis and nasal polyps that remained uncontrolled despite prior surgery, for 75% of enrolled patients, or treatment with systemic corticosteroids, used on about 90% of the patients within the prior 2 years.
During the 24 weeks of treatment, 23% of patients in the control arm had to restart systemic corticosteroid treatment or have surgery, compared with 7% of patients on dupilumab treatment, a statistically significant difference.
The new drug is a “game changer,” for these patients, Dr. Han said in a video interview.
In some patients, treatment produced complete polyp resolution. He and his colleagues in the otolaryngology field are now trying to decide exactly which patients with polyps secondary to sinusitis will be good candidates for dupilumab after it receives an expected indication for shrinking nasal polyps.
Roughly 4% of the adult population has chronic rhinosinusitis that generates polyps. How many of these patients are affected severely enough to warrant dupilumab treatment is not clear, but will likely include several hundreds of thousands of U.S. adults, said Dr. Han, professor of otolaryngology and chief of the division of allergy at Eastern Virginia Medical School in Norfolk.
The SINUS-24 (A Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps) trial enrolled patients at 76 sites in the United States and in several European countries. The study randomized 143 patients who received standard treatment plus a 300-mg dupilumab subcutaneous injection every 2 weeks, and 133 patients who received standard treatment plus placebo injections. Standard treatment included a nasal corticosteroid spray.
After 24 weeks of treatment, the endoscopically-measured nasal polyp score, which averaged about 6 at baseline on a scale of 0-8, fell by an average of 2.06 points, compared with controls, which was a statistically significant and clinically meaningful change, said Dr. Han.
The second primary endpoint, patient self-assessment of nasal congestion on a scale of 0-3, showed an average 0.89 improvement, compared with controls, which was also a statistically significant and meaningful change from the average baseline score of about 2.4.
Other efficacy measures also showed benefits from treatment, including a substantial improvement compared with controls in a quality-of-life measure. The safety profile was benign compared with placebo, and consistent with existing safety data for the drug.SINUS-24 was funded by Regeneron and Sanofi, the companies that market dupilumab. Dr. Han has been an adviser to Regeneron and Sanofi.
SOURCE: Han JK et al. AAAAI 2019, Abstract L4.
SAN FRANCISCO – Dupilumab, an anti-inflammatory drug already approved for use in the United States, met its efficacy endpoints for treating chronic rhinosinusitis with nasal polyps in a pivotal trial with 276 patients.
The results make it likely that dupilumab (Dupixent) will receive a new indication from the Food and Drug Administration, pending similar results in a second pivotal trial for nasal polyps that researchers will report soon. Dupilumab, which works by blocking a receptor for both interleukin 4 and interleukin 13 and thereby shutting down type 2 inflammation, is already approved in the United States for treating atopic dermatitis and asthma.
Type 2 inflammation drives polyp formation in patients with chronic rhinosinusitis that can produce severe nasal congestion, breathing difficulty, and substantially reduced quality of life.
In the new trial, the drug showed efficacy by significantly improving both the nasal congestion score reported by patients and the nasal polyp score measured by sinus endoscopy after 24 weeks on treatment, when compared with control patients on placebo, Joseph K. Han, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients enrolled in the study had chronic, severe sinusitis and nasal polyps that remained uncontrolled despite prior surgery, for 75% of enrolled patients, or treatment with systemic corticosteroids, used on about 90% of the patients within the prior 2 years.
During the 24 weeks of treatment, 23% of patients in the control arm had to restart systemic corticosteroid treatment or have surgery, compared with 7% of patients on dupilumab treatment, a statistically significant difference.
The new drug is a “game changer,” for these patients, Dr. Han said in a video interview.
In some patients, treatment produced complete polyp resolution. He and his colleagues in the otolaryngology field are now trying to decide exactly which patients with polyps secondary to sinusitis will be good candidates for dupilumab after it receives an expected indication for shrinking nasal polyps.
Roughly 4% of the adult population has chronic rhinosinusitis that generates polyps. How many of these patients are affected severely enough to warrant dupilumab treatment is not clear, but will likely include several hundreds of thousands of U.S. adults, said Dr. Han, professor of otolaryngology and chief of the division of allergy at Eastern Virginia Medical School in Norfolk.
The SINUS-24 (A Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps) trial enrolled patients at 76 sites in the United States and in several European countries. The study randomized 143 patients who received standard treatment plus a 300-mg dupilumab subcutaneous injection every 2 weeks, and 133 patients who received standard treatment plus placebo injections. Standard treatment included a nasal corticosteroid spray.
After 24 weeks of treatment, the endoscopically-measured nasal polyp score, which averaged about 6 at baseline on a scale of 0-8, fell by an average of 2.06 points, compared with controls, which was a statistically significant and clinically meaningful change, said Dr. Han.
The second primary endpoint, patient self-assessment of nasal congestion on a scale of 0-3, showed an average 0.89 improvement, compared with controls, which was also a statistically significant and meaningful change from the average baseline score of about 2.4.
Other efficacy measures also showed benefits from treatment, including a substantial improvement compared with controls in a quality-of-life measure. The safety profile was benign compared with placebo, and consistent with existing safety data for the drug.SINUS-24 was funded by Regeneron and Sanofi, the companies that market dupilumab. Dr. Han has been an adviser to Regeneron and Sanofi.
SOURCE: Han JK et al. AAAAI 2019, Abstract L4.
REPORTING FROM AAAAI
Severe, uncontrolled asthma patients must avoid subcutaneous immunotherapy
SAN FRANCISCO – appears to be the “major factor” causing higher-grade systemic reactions or death from this treatment, David I. Bernstein, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
While that was Dr. Bernstein’s top take-home message on how to optimize tolerability of subcutaneous immunotherapy (SCIT), a few other empiric rules have also emerged from his ongoing analysis of survey results from the AAAAI/American College of Allergy, Asthma, and Immunology SCIT surveillance study. The study began tracking the safety of SCIT in 2008 through annual surveys sent to members of either of these two allergy societies. By early 2019, the surveys had gathered data from more than 55 million office visits for SCIT, with responses from roughly 200-500 allergy practices annually, said Dr. Bernstein, professor of medicine at the University of Cincinnati.
The survey results identified seven SCIT-related fatalities over about a decade of surveillance. The most common risk factor among these cases was severe, uncontrolled asthma, prompting Dr. Bernstein to conclude that these patients should not receive SCIT. “If the asthma is well controlled, then SCIT is fine,” even if it had been severe before treatment, he said in an interview.
Other factors affecting SCIT safety based on the survey results included:
- Screening patients with an asthma history for current asthma symptoms and lung function before each injection. Survey results showed that while 86% of respondents screened for symptoms, only a third also checked lung function.
- Modifying the dose or stopping SCIT injections after a severe systemic reaction. Survey results showed that more than a quarter of all systemic reactions and more than a third of grade 3 systemic reactions (severe anaphylaxis) happened following a prior systemic reaction. Dr. Bernstein called this “an important, modifiable risk factor.”
- Administering SCIT only in a setting staffed to manage a possible anaphylaxis episode, and adhere to at least a 30-minute observation period. “A key step is observing for at least 30 minutes, and giving epinephrine promptly when needed; the sooner the better,” Dr. Bernstein said. Although the percentage of practices that observe patients for at least 30 minutes has steadily improved during the decade that the survey has run, in 2016 a quarter of responding practices continued to not observe patients for at least 30 minutes.
- Modifying the SCIT dose in high-risk patients during the peak season for aeroallergens like pollen. Survey results showed that practices that did not adjust their SCIT dosages during peak pollen seasons had about double the rate of grade 3 or 4 systemic reactions, compared with practices that dialed down their dosages.
- Reducing SCIT dosages during an accelerated cluster buildup, a treatment approach that in general increases the risk for systemic reactions.
Survey results also showed that sublingual immunotherapy, available in U.S. practice since 2014, has been very safe, with no reported associated deaths and only rare reports of anaphylactic episodes, Dr. Bernstein said. The most recent published report from the surveillance study appeared online a few days before Dr. Bernstein spoke (J Allergy Clin Immunol Pract. 2019 Feb 15. doi: 10.1016/j.jaip.2019.01.058).
Dr. Bernstein had no relevant disclosures.
SAN FRANCISCO – appears to be the “major factor” causing higher-grade systemic reactions or death from this treatment, David I. Bernstein, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
While that was Dr. Bernstein’s top take-home message on how to optimize tolerability of subcutaneous immunotherapy (SCIT), a few other empiric rules have also emerged from his ongoing analysis of survey results from the AAAAI/American College of Allergy, Asthma, and Immunology SCIT surveillance study. The study began tracking the safety of SCIT in 2008 through annual surveys sent to members of either of these two allergy societies. By early 2019, the surveys had gathered data from more than 55 million office visits for SCIT, with responses from roughly 200-500 allergy practices annually, said Dr. Bernstein, professor of medicine at the University of Cincinnati.
The survey results identified seven SCIT-related fatalities over about a decade of surveillance. The most common risk factor among these cases was severe, uncontrolled asthma, prompting Dr. Bernstein to conclude that these patients should not receive SCIT. “If the asthma is well controlled, then SCIT is fine,” even if it had been severe before treatment, he said in an interview.
Other factors affecting SCIT safety based on the survey results included:
- Screening patients with an asthma history for current asthma symptoms and lung function before each injection. Survey results showed that while 86% of respondents screened for symptoms, only a third also checked lung function.
- Modifying the dose or stopping SCIT injections after a severe systemic reaction. Survey results showed that more than a quarter of all systemic reactions and more than a third of grade 3 systemic reactions (severe anaphylaxis) happened following a prior systemic reaction. Dr. Bernstein called this “an important, modifiable risk factor.”
- Administering SCIT only in a setting staffed to manage a possible anaphylaxis episode, and adhere to at least a 30-minute observation period. “A key step is observing for at least 30 minutes, and giving epinephrine promptly when needed; the sooner the better,” Dr. Bernstein said. Although the percentage of practices that observe patients for at least 30 minutes has steadily improved during the decade that the survey has run, in 2016 a quarter of responding practices continued to not observe patients for at least 30 minutes.
- Modifying the SCIT dose in high-risk patients during the peak season for aeroallergens like pollen. Survey results showed that practices that did not adjust their SCIT dosages during peak pollen seasons had about double the rate of grade 3 or 4 systemic reactions, compared with practices that dialed down their dosages.
- Reducing SCIT dosages during an accelerated cluster buildup, a treatment approach that in general increases the risk for systemic reactions.
Survey results also showed that sublingual immunotherapy, available in U.S. practice since 2014, has been very safe, with no reported associated deaths and only rare reports of anaphylactic episodes, Dr. Bernstein said. The most recent published report from the surveillance study appeared online a few days before Dr. Bernstein spoke (J Allergy Clin Immunol Pract. 2019 Feb 15. doi: 10.1016/j.jaip.2019.01.058).
Dr. Bernstein had no relevant disclosures.
SAN FRANCISCO – appears to be the “major factor” causing higher-grade systemic reactions or death from this treatment, David I. Bernstein, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
While that was Dr. Bernstein’s top take-home message on how to optimize tolerability of subcutaneous immunotherapy (SCIT), a few other empiric rules have also emerged from his ongoing analysis of survey results from the AAAAI/American College of Allergy, Asthma, and Immunology SCIT surveillance study. The study began tracking the safety of SCIT in 2008 through annual surveys sent to members of either of these two allergy societies. By early 2019, the surveys had gathered data from more than 55 million office visits for SCIT, with responses from roughly 200-500 allergy practices annually, said Dr. Bernstein, professor of medicine at the University of Cincinnati.
The survey results identified seven SCIT-related fatalities over about a decade of surveillance. The most common risk factor among these cases was severe, uncontrolled asthma, prompting Dr. Bernstein to conclude that these patients should not receive SCIT. “If the asthma is well controlled, then SCIT is fine,” even if it had been severe before treatment, he said in an interview.
Other factors affecting SCIT safety based on the survey results included:
- Screening patients with an asthma history for current asthma symptoms and lung function before each injection. Survey results showed that while 86% of respondents screened for symptoms, only a third also checked lung function.
- Modifying the dose or stopping SCIT injections after a severe systemic reaction. Survey results showed that more than a quarter of all systemic reactions and more than a third of grade 3 systemic reactions (severe anaphylaxis) happened following a prior systemic reaction. Dr. Bernstein called this “an important, modifiable risk factor.”
- Administering SCIT only in a setting staffed to manage a possible anaphylaxis episode, and adhere to at least a 30-minute observation period. “A key step is observing for at least 30 minutes, and giving epinephrine promptly when needed; the sooner the better,” Dr. Bernstein said. Although the percentage of practices that observe patients for at least 30 minutes has steadily improved during the decade that the survey has run, in 2016 a quarter of responding practices continued to not observe patients for at least 30 minutes.
- Modifying the SCIT dose in high-risk patients during the peak season for aeroallergens like pollen. Survey results showed that practices that did not adjust their SCIT dosages during peak pollen seasons had about double the rate of grade 3 or 4 systemic reactions, compared with practices that dialed down their dosages.
- Reducing SCIT dosages during an accelerated cluster buildup, a treatment approach that in general increases the risk for systemic reactions.
Survey results also showed that sublingual immunotherapy, available in U.S. practice since 2014, has been very safe, with no reported associated deaths and only rare reports of anaphylactic episodes, Dr. Bernstein said. The most recent published report from the surveillance study appeared online a few days before Dr. Bernstein spoke (J Allergy Clin Immunol Pract. 2019 Feb 15. doi: 10.1016/j.jaip.2019.01.058).
Dr. Bernstein had no relevant disclosures.
REPORTING FROM AAAAI
When to “Undiagnose” Asthma

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
Years in practice, burnout risk linked in otolaryngology
CORONADO, CALIF. – Otolaryngologists and otolaryngology nurse practitioners at the Cleveland Clinic who have been practicing for 6-10 years are at the highest risk for burnout, while those who have been practicing for more than 10 are at the lowest risk.
The finding comes from a cross-sectional survey published in Otolaryngology–Head and Neck Surgery designed to evaluate the presence of burnout among 52 otolaryngology clinicians and to compare results among faculty, trainees, and advanced practice practitioners.
“Other studies have shown that work-life balance can contribute to burnout symptoms, including low spouse support, having young children at home, and a decreased satisfaction with work-life balance,” Michael S. Benninger, MD, said at the Triological Society’s Combined Sections Meeting. “We wanted to know if there was difference within our group among people at different points in their career.”
In a study led by Katie Geelan-Hansen, MD, Dr. Benninger, who chairs the Head and Neck Institute at the Cleveland Clinic, and his colleagues administered the Maslach Burnout Inventory (MBI) and questions regarding work stressors specific to that department to 52 employees (Otolaryngol Head Neck Surg. 2018;159[2]:254-7). The questions focused on domains of emotional exhaustion, depersonalization, and a sense of personal accomplishment.
Of the 52 surveys distributed, 42 participants (85%) completed the survey. The researchers found that respondents who had worked for 6-10 years had higher MBI scores on emotional exhaustion, compared with their peers who had worked for 5 years or fewer, and those who had worked for more than 10 years (18.18, compared with 15.78 and 14.68, respectively; P = .63). A similar association was observed for MBI scores on depersonalization (15.14, compared with 14.72 and 9.68; P = .07). MBI scores on personal accomplishment were similar between the two groups (39, compared with 38.33 and 40.84; P = .5).
“People who are more mature in their practice tend to have less burnout,” Dr. Benninger said. “That may be because they’ve found a place of homeostasis. They’ve figured out how to maximize their efficiency, and they may have more support.
“The people who tend to be the biggest concern are those 6 -10 years into the field. I recommend that you focus on that group. It’s a transitional time in their careers. It’s a time when there’s some insecurity; they’re being asked to do a lot more.” It remains unclear if male or female respondents had a higher level of burnout, he added, although other surveys have suggested that female physicians have a higher level of burnout, compared with male physicians.
“Our overall evaluation of burnout was lower than what you see from national statistics,” Dr. Benninger said at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “We have had a wellness officer [at Cleveland Clinic] for a long time. We have a group of people on our clinic’s board of governors who any staff can go to in order to vent issues on a private basis. All of those things help, but I am seeing an escalating unsatisfaction with the workload and the work environment. We’re looking at other things. Expectation setting and rewarding people are also important.”
He reported having no relevant financial disclosures.
SOURCE: Benninger MS et al. Triological CSM, Abstracts.
CORONADO, CALIF. – Otolaryngologists and otolaryngology nurse practitioners at the Cleveland Clinic who have been practicing for 6-10 years are at the highest risk for burnout, while those who have been practicing for more than 10 are at the lowest risk.
The finding comes from a cross-sectional survey published in Otolaryngology–Head and Neck Surgery designed to evaluate the presence of burnout among 52 otolaryngology clinicians and to compare results among faculty, trainees, and advanced practice practitioners.
“Other studies have shown that work-life balance can contribute to burnout symptoms, including low spouse support, having young children at home, and a decreased satisfaction with work-life balance,” Michael S. Benninger, MD, said at the Triological Society’s Combined Sections Meeting. “We wanted to know if there was difference within our group among people at different points in their career.”
In a study led by Katie Geelan-Hansen, MD, Dr. Benninger, who chairs the Head and Neck Institute at the Cleveland Clinic, and his colleagues administered the Maslach Burnout Inventory (MBI) and questions regarding work stressors specific to that department to 52 employees (Otolaryngol Head Neck Surg. 2018;159[2]:254-7). The questions focused on domains of emotional exhaustion, depersonalization, and a sense of personal accomplishment.
Of the 52 surveys distributed, 42 participants (85%) completed the survey. The researchers found that respondents who had worked for 6-10 years had higher MBI scores on emotional exhaustion, compared with their peers who had worked for 5 years or fewer, and those who had worked for more than 10 years (18.18, compared with 15.78 and 14.68, respectively; P = .63). A similar association was observed for MBI scores on depersonalization (15.14, compared with 14.72 and 9.68; P = .07). MBI scores on personal accomplishment were similar between the two groups (39, compared with 38.33 and 40.84; P = .5).
“People who are more mature in their practice tend to have less burnout,” Dr. Benninger said. “That may be because they’ve found a place of homeostasis. They’ve figured out how to maximize their efficiency, and they may have more support.
“The people who tend to be the biggest concern are those 6 -10 years into the field. I recommend that you focus on that group. It’s a transitional time in their careers. It’s a time when there’s some insecurity; they’re being asked to do a lot more.” It remains unclear if male or female respondents had a higher level of burnout, he added, although other surveys have suggested that female physicians have a higher level of burnout, compared with male physicians.
“Our overall evaluation of burnout was lower than what you see from national statistics,” Dr. Benninger said at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “We have had a wellness officer [at Cleveland Clinic] for a long time. We have a group of people on our clinic’s board of governors who any staff can go to in order to vent issues on a private basis. All of those things help, but I am seeing an escalating unsatisfaction with the workload and the work environment. We’re looking at other things. Expectation setting and rewarding people are also important.”
He reported having no relevant financial disclosures.
SOURCE: Benninger MS et al. Triological CSM, Abstracts.
CORONADO, CALIF. – Otolaryngologists and otolaryngology nurse practitioners at the Cleveland Clinic who have been practicing for 6-10 years are at the highest risk for burnout, while those who have been practicing for more than 10 are at the lowest risk.
The finding comes from a cross-sectional survey published in Otolaryngology–Head and Neck Surgery designed to evaluate the presence of burnout among 52 otolaryngology clinicians and to compare results among faculty, trainees, and advanced practice practitioners.
“Other studies have shown that work-life balance can contribute to burnout symptoms, including low spouse support, having young children at home, and a decreased satisfaction with work-life balance,” Michael S. Benninger, MD, said at the Triological Society’s Combined Sections Meeting. “We wanted to know if there was difference within our group among people at different points in their career.”
In a study led by Katie Geelan-Hansen, MD, Dr. Benninger, who chairs the Head and Neck Institute at the Cleveland Clinic, and his colleagues administered the Maslach Burnout Inventory (MBI) and questions regarding work stressors specific to that department to 52 employees (Otolaryngol Head Neck Surg. 2018;159[2]:254-7). The questions focused on domains of emotional exhaustion, depersonalization, and a sense of personal accomplishment.
Of the 52 surveys distributed, 42 participants (85%) completed the survey. The researchers found that respondents who had worked for 6-10 years had higher MBI scores on emotional exhaustion, compared with their peers who had worked for 5 years or fewer, and those who had worked for more than 10 years (18.18, compared with 15.78 and 14.68, respectively; P = .63). A similar association was observed for MBI scores on depersonalization (15.14, compared with 14.72 and 9.68; P = .07). MBI scores on personal accomplishment were similar between the two groups (39, compared with 38.33 and 40.84; P = .5).
“People who are more mature in their practice tend to have less burnout,” Dr. Benninger said. “That may be because they’ve found a place of homeostasis. They’ve figured out how to maximize their efficiency, and they may have more support.
“The people who tend to be the biggest concern are those 6 -10 years into the field. I recommend that you focus on that group. It’s a transitional time in their careers. It’s a time when there’s some insecurity; they’re being asked to do a lot more.” It remains unclear if male or female respondents had a higher level of burnout, he added, although other surveys have suggested that female physicians have a higher level of burnout, compared with male physicians.
“Our overall evaluation of burnout was lower than what you see from national statistics,” Dr. Benninger said at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “We have had a wellness officer [at Cleveland Clinic] for a long time. We have a group of people on our clinic’s board of governors who any staff can go to in order to vent issues on a private basis. All of those things help, but I am seeing an escalating unsatisfaction with the workload and the work environment. We’re looking at other things. Expectation setting and rewarding people are also important.”
He reported having no relevant financial disclosures.
SOURCE: Benninger MS et al. Triological CSM, Abstracts.
REPORTING FROM TRIOLOGICAL CSM
Asthma, obesity, and the risk for severe sleep apnea in children
CORONADO, CALIF. –
“We have a good idea that obesity and asthma independently increase the risk of OSA, but a lot of the time in the pediatric population, these risk factors are found comorbid,” Ajay Narayanan at the Triological Society’s Combined Sections Meeting. “For this study we asked, how does the presence of asthma change the likelihood of having severe OSA in a cohort of obese patients? Knowing that both asthma and obesity independently increase the risk for OSA, we hypothesized that when they were comorbid, asthma would have a synergistic effect with obesity, causing severe OSA.”
Mr. Narayanan, a third-year student at the University of Texas Southwestern Medical Center, Dallas, and his colleagues performed a retrospective chart review of 367 children aged 9-17 years referred for a full-night polysomnography (PSG) for suspicion of having OSA. Demographic variables recorded included race, body mass index, rhinitis, gastroesophageal reflux disease, and tonsillar hypertrophy. Sleep variables recorded included apnea hypopnea index (AHI), sleep efficiency, rapid eye movement, and the peripheral capillary oxygen saturation (SpO2) nadir. The primary outcome was severe OSA defined as an AHI of 10 or greater on the PSG. They used logistic modeling to determine the association between asthma, obesity, and severe OSA.
The mean age of the study population was 14 years, 56% were male, and 43% were Hispanic. Of the 367 patients, 77 were neither obese nor asthmatic, 93 were nonobese but were asthmatic, 102 were obese but were nonasthmatic, and 95 were both obese and asthmatic. PSG results confirmed that obesity was associated with more signs of sleep apnea. For example, the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, while the obese, nonasthmatic group had a mean AHI of 19 events per hour. “We observed a similar trend amongst our asthmatic population,” Mr. Narayanan said. “We observed an increase in the mean AHI amongst our asthmatic kids when we added obesity to the picture. Surprisingly, we found that asthma was associated with having fewer signs of sleep apnea.” Specifically, while the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, those in the nonobese, asthmatic group had a mean of 5.6 events per hour (P = .005). “The finding was similar amongst our obese kids,” he said. “We saw a decrease in the mean AHI of our obese kids when we added asthma to the picture.”
On logistic regression analysis using obesity and asthma as independent variables, the researchers found that obesity increased the risk of severe OSA by 2.4-fold, but asthma decreased the odds of having severe OSA by about half (0.55). On multiple logistic regression controlling for commonly associated factors such as tonsillar hypertrophy, black race, and Hispanic ethnicity, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half (0.51).
“In trying to explain this finding, we can turn to how these diseases are treated,” Mr. Narayanan said. “I say this because of the proven association between preexisting asthma and new onset OSA. Some of the reasons for this association include the tendency for airway collapsibility and systemwide inflammation seen in asthma, which then might contribute to the development of OSA. If we treat asthma symptoms early on, it might prevent the progression to sleep apnea down the line.”
Considering how prevalent comorbid asthma and OSA is, he continued, “we need to confirm that it is in fact well-controlled asthma that is associated with lowering the risk of severe OSA. Once we do this, we can ask the question: Can we use asthma pharmacotherapy to treat OSA? Some studies have shown that inhaled corticosteroids and montelukast (Singulair) may be effective treatment options for kids with OSA, but there’s definitely room for more research in this field, [such as determining] which patients would most benefit from this pharmacotherapy.” The researchers reported having no financial disclosures.
SOURCE: Narayanan A et al. Triological CSM, Abstracts.
CORONADO, CALIF. –
“We have a good idea that obesity and asthma independently increase the risk of OSA, but a lot of the time in the pediatric population, these risk factors are found comorbid,” Ajay Narayanan at the Triological Society’s Combined Sections Meeting. “For this study we asked, how does the presence of asthma change the likelihood of having severe OSA in a cohort of obese patients? Knowing that both asthma and obesity independently increase the risk for OSA, we hypothesized that when they were comorbid, asthma would have a synergistic effect with obesity, causing severe OSA.”
Mr. Narayanan, a third-year student at the University of Texas Southwestern Medical Center, Dallas, and his colleagues performed a retrospective chart review of 367 children aged 9-17 years referred for a full-night polysomnography (PSG) for suspicion of having OSA. Demographic variables recorded included race, body mass index, rhinitis, gastroesophageal reflux disease, and tonsillar hypertrophy. Sleep variables recorded included apnea hypopnea index (AHI), sleep efficiency, rapid eye movement, and the peripheral capillary oxygen saturation (SpO2) nadir. The primary outcome was severe OSA defined as an AHI of 10 or greater on the PSG. They used logistic modeling to determine the association between asthma, obesity, and severe OSA.
The mean age of the study population was 14 years, 56% were male, and 43% were Hispanic. Of the 367 patients, 77 were neither obese nor asthmatic, 93 were nonobese but were asthmatic, 102 were obese but were nonasthmatic, and 95 were both obese and asthmatic. PSG results confirmed that obesity was associated with more signs of sleep apnea. For example, the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, while the obese, nonasthmatic group had a mean AHI of 19 events per hour. “We observed a similar trend amongst our asthmatic population,” Mr. Narayanan said. “We observed an increase in the mean AHI amongst our asthmatic kids when we added obesity to the picture. Surprisingly, we found that asthma was associated with having fewer signs of sleep apnea.” Specifically, while the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, those in the nonobese, asthmatic group had a mean of 5.6 events per hour (P = .005). “The finding was similar amongst our obese kids,” he said. “We saw a decrease in the mean AHI of our obese kids when we added asthma to the picture.”
On logistic regression analysis using obesity and asthma as independent variables, the researchers found that obesity increased the risk of severe OSA by 2.4-fold, but asthma decreased the odds of having severe OSA by about half (0.55). On multiple logistic regression controlling for commonly associated factors such as tonsillar hypertrophy, black race, and Hispanic ethnicity, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half (0.51).
“In trying to explain this finding, we can turn to how these diseases are treated,” Mr. Narayanan said. “I say this because of the proven association between preexisting asthma and new onset OSA. Some of the reasons for this association include the tendency for airway collapsibility and systemwide inflammation seen in asthma, which then might contribute to the development of OSA. If we treat asthma symptoms early on, it might prevent the progression to sleep apnea down the line.”
Considering how prevalent comorbid asthma and OSA is, he continued, “we need to confirm that it is in fact well-controlled asthma that is associated with lowering the risk of severe OSA. Once we do this, we can ask the question: Can we use asthma pharmacotherapy to treat OSA? Some studies have shown that inhaled corticosteroids and montelukast (Singulair) may be effective treatment options for kids with OSA, but there’s definitely room for more research in this field, [such as determining] which patients would most benefit from this pharmacotherapy.” The researchers reported having no financial disclosures.
SOURCE: Narayanan A et al. Triological CSM, Abstracts.
CORONADO, CALIF. –
“We have a good idea that obesity and asthma independently increase the risk of OSA, but a lot of the time in the pediatric population, these risk factors are found comorbid,” Ajay Narayanan at the Triological Society’s Combined Sections Meeting. “For this study we asked, how does the presence of asthma change the likelihood of having severe OSA in a cohort of obese patients? Knowing that both asthma and obesity independently increase the risk for OSA, we hypothesized that when they were comorbid, asthma would have a synergistic effect with obesity, causing severe OSA.”
Mr. Narayanan, a third-year student at the University of Texas Southwestern Medical Center, Dallas, and his colleagues performed a retrospective chart review of 367 children aged 9-17 years referred for a full-night polysomnography (PSG) for suspicion of having OSA. Demographic variables recorded included race, body mass index, rhinitis, gastroesophageal reflux disease, and tonsillar hypertrophy. Sleep variables recorded included apnea hypopnea index (AHI), sleep efficiency, rapid eye movement, and the peripheral capillary oxygen saturation (SpO2) nadir. The primary outcome was severe OSA defined as an AHI of 10 or greater on the PSG. They used logistic modeling to determine the association between asthma, obesity, and severe OSA.
The mean age of the study population was 14 years, 56% were male, and 43% were Hispanic. Of the 367 patients, 77 were neither obese nor asthmatic, 93 were nonobese but were asthmatic, 102 were obese but were nonasthmatic, and 95 were both obese and asthmatic. PSG results confirmed that obesity was associated with more signs of sleep apnea. For example, the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, while the obese, nonasthmatic group had a mean AHI of 19 events per hour. “We observed a similar trend amongst our asthmatic population,” Mr. Narayanan said. “We observed an increase in the mean AHI amongst our asthmatic kids when we added obesity to the picture. Surprisingly, we found that asthma was associated with having fewer signs of sleep apnea.” Specifically, while the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, those in the nonobese, asthmatic group had a mean of 5.6 events per hour (P = .005). “The finding was similar amongst our obese kids,” he said. “We saw a decrease in the mean AHI of our obese kids when we added asthma to the picture.”
On logistic regression analysis using obesity and asthma as independent variables, the researchers found that obesity increased the risk of severe OSA by 2.4-fold, but asthma decreased the odds of having severe OSA by about half (0.55). On multiple logistic regression controlling for commonly associated factors such as tonsillar hypertrophy, black race, and Hispanic ethnicity, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half (0.51).
“In trying to explain this finding, we can turn to how these diseases are treated,” Mr. Narayanan said. “I say this because of the proven association between preexisting asthma and new onset OSA. Some of the reasons for this association include the tendency for airway collapsibility and systemwide inflammation seen in asthma, which then might contribute to the development of OSA. If we treat asthma symptoms early on, it might prevent the progression to sleep apnea down the line.”
Considering how prevalent comorbid asthma and OSA is, he continued, “we need to confirm that it is in fact well-controlled asthma that is associated with lowering the risk of severe OSA. Once we do this, we can ask the question: Can we use asthma pharmacotherapy to treat OSA? Some studies have shown that inhaled corticosteroids and montelukast (Singulair) may be effective treatment options for kids with OSA, but there’s definitely room for more research in this field, [such as determining] which patients would most benefit from this pharmacotherapy.” The researchers reported having no financial disclosures.
SOURCE: Narayanan A et al. Triological CSM, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: In children, having asthma could decrease the risk of having severe obstructive sleep apnea, regardless of their obesity status.
Major finding: On multiple logistic regression, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half.
Study details: A retrospective review of 367 children referred for a full-night polysomnography for suspicion of having OSA.
Disclosures: The researchers reported having no financial disclosures.
Source: Narayanan A et al. Triological CSM, Abstracts.
FDA approves generic Advair Diskus
The Food and Drug Administration has approved a generic version of the Advair Diskus, a complex device-drug combination containing fluticasone propionate and salmeterol inhalation powder.
The generic device will be available in three strengths: fluticasone propionate 100 mcg/ salmeterol 50 mcg, fluticasone propionate 250 mcg/ salmeterol 50 mcg and fluticasone propionate 500 mcg/ salmeterol 50 mcg, according to the FDA announcement. It will be marketed by Mylan as Wixela Inhub and will launch in late February, according to a statement from Mylan.
Advair Diskus is among the most commonly used treatments for asthma and for chronic obstructive pulmonary disease (COPD), so it’s hoped this approval will increase access to the therapy, FDA officials said in a statement.
This approval is part of the FDA’s “longstanding commitment to advance access to lower cost, high quality generic alternatives,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a statement. “People living with asthma and COPD know too well the critical importance of having access to the treatment they need to feel better. Today’s approval will bring more competition to the market which will ultimately benefit the patients who rely on this drug.”
Wixela Inhub is indicated for twice-daily treatment of asthma in patients aged 4 years and older who are not adequately controlled by long-term asthma control treatments or whose disease warrants treatment with a combination of inhaled corticosteroids and long-acting beta agonists. It also is indicated for maintenance of COPD and reduction of COPD exacerbations.
The Food and Drug Administration has approved a generic version of the Advair Diskus, a complex device-drug combination containing fluticasone propionate and salmeterol inhalation powder.
The generic device will be available in three strengths: fluticasone propionate 100 mcg/ salmeterol 50 mcg, fluticasone propionate 250 mcg/ salmeterol 50 mcg and fluticasone propionate 500 mcg/ salmeterol 50 mcg, according to the FDA announcement. It will be marketed by Mylan as Wixela Inhub and will launch in late February, according to a statement from Mylan.
Advair Diskus is among the most commonly used treatments for asthma and for chronic obstructive pulmonary disease (COPD), so it’s hoped this approval will increase access to the therapy, FDA officials said in a statement.
This approval is part of the FDA’s “longstanding commitment to advance access to lower cost, high quality generic alternatives,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a statement. “People living with asthma and COPD know too well the critical importance of having access to the treatment they need to feel better. Today’s approval will bring more competition to the market which will ultimately benefit the patients who rely on this drug.”
Wixela Inhub is indicated for twice-daily treatment of asthma in patients aged 4 years and older who are not adequately controlled by long-term asthma control treatments or whose disease warrants treatment with a combination of inhaled corticosteroids and long-acting beta agonists. It also is indicated for maintenance of COPD and reduction of COPD exacerbations.
The Food and Drug Administration has approved a generic version of the Advair Diskus, a complex device-drug combination containing fluticasone propionate and salmeterol inhalation powder.
The generic device will be available in three strengths: fluticasone propionate 100 mcg/ salmeterol 50 mcg, fluticasone propionate 250 mcg/ salmeterol 50 mcg and fluticasone propionate 500 mcg/ salmeterol 50 mcg, according to the FDA announcement. It will be marketed by Mylan as Wixela Inhub and will launch in late February, according to a statement from Mylan.
Advair Diskus is among the most commonly used treatments for asthma and for chronic obstructive pulmonary disease (COPD), so it’s hoped this approval will increase access to the therapy, FDA officials said in a statement.
This approval is part of the FDA’s “longstanding commitment to advance access to lower cost, high quality generic alternatives,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a statement. “People living with asthma and COPD know too well the critical importance of having access to the treatment they need to feel better. Today’s approval will bring more competition to the market which will ultimately benefit the patients who rely on this drug.”
Wixela Inhub is indicated for twice-daily treatment of asthma in patients aged 4 years and older who are not adequately controlled by long-term asthma control treatments or whose disease warrants treatment with a combination of inhaled corticosteroids and long-acting beta agonists. It also is indicated for maintenance of COPD and reduction of COPD exacerbations.