User login
Statin use linked to less depression, anxiety in ACOS patients
based on data from approximately 9,000 patients.
Although asthma–COPD overlap syndrome (ACOS) has been associated with depression, the effects of oral and inhaled corticosteroids on anxiety and depression in these patients have not been well investigated, wrote Jun-Jun Yeh, MD, of Ditmanson Medical Foundation Chia-Yi (Taiwan) Christian Hospital, and colleagues.
In a study published in the Journal of Affective Disorders, the researchers analyzed 9,139 ACOS patients including 1,252 statin users and 7,887 nonstatin users; 62% were male.
The statin users had significantly lower risk of both anxiety and depression than did the nonstatin users (adjusted hazard ratio 0.34 for anxiety and 0.36 for depression) after researchers controlled for factors including age, sex, comorbidities, and medications. Statin users experienced a total of 109 anxiety or depression events over an average of 8 years’ follow-up, while nonstatin users experienced a total of 1,333 anxiety or depression events over an average of 5 years’ follow-up.
The incidence density rate of anxiety was 11/1,000 person-years for statin users and 33/1,000 person-years for nonstatin users. The incidence density rate of depression was 3/1,000 person-years for statin users and 9/1,000 person-years for nonstatin users.
Significantly lower risk of anxiety and depression also were observed in statin users, compared with nonstatin users, in subgroups of men, women, patients younger than 50 years, and patients aged 50 years and older. The risks of anxiety and depression were lower in statin users versus nonstatin users across all subgroups with or without inhaled or oral corticosteroids.
Overall, the statin users were significantly younger, had more comorbidities, and were more likely to use inhaled or oral corticosteroids than were the nonstatin users.
The findings were limited by several factors including the retrospective nature of the study and a lack of information on prescribed daily doses of medication, the researchers noted. However, the results support those from previous studies and suggest that “the anti-inflammatory effect of statins may attenuate anxiety and depression in ACOS patients, even in the late stages of the disease,” although the exact mechanism of action remains unknown and larger, randomized, controlled trials are needed, they said.
The study was supported by grants from a variety of organizations in Taiwan, China, and Japan. The researchers had no financial conflicts to disclose.
SOURCE: Yeh JJ et al. J Affect Disord. 2019 Jun 15; 253:277-84.
based on data from approximately 9,000 patients.
Although asthma–COPD overlap syndrome (ACOS) has been associated with depression, the effects of oral and inhaled corticosteroids on anxiety and depression in these patients have not been well investigated, wrote Jun-Jun Yeh, MD, of Ditmanson Medical Foundation Chia-Yi (Taiwan) Christian Hospital, and colleagues.
In a study published in the Journal of Affective Disorders, the researchers analyzed 9,139 ACOS patients including 1,252 statin users and 7,887 nonstatin users; 62% were male.
The statin users had significantly lower risk of both anxiety and depression than did the nonstatin users (adjusted hazard ratio 0.34 for anxiety and 0.36 for depression) after researchers controlled for factors including age, sex, comorbidities, and medications. Statin users experienced a total of 109 anxiety or depression events over an average of 8 years’ follow-up, while nonstatin users experienced a total of 1,333 anxiety or depression events over an average of 5 years’ follow-up.
The incidence density rate of anxiety was 11/1,000 person-years for statin users and 33/1,000 person-years for nonstatin users. The incidence density rate of depression was 3/1,000 person-years for statin users and 9/1,000 person-years for nonstatin users.
Significantly lower risk of anxiety and depression also were observed in statin users, compared with nonstatin users, in subgroups of men, women, patients younger than 50 years, and patients aged 50 years and older. The risks of anxiety and depression were lower in statin users versus nonstatin users across all subgroups with or without inhaled or oral corticosteroids.
Overall, the statin users were significantly younger, had more comorbidities, and were more likely to use inhaled or oral corticosteroids than were the nonstatin users.
The findings were limited by several factors including the retrospective nature of the study and a lack of information on prescribed daily doses of medication, the researchers noted. However, the results support those from previous studies and suggest that “the anti-inflammatory effect of statins may attenuate anxiety and depression in ACOS patients, even in the late stages of the disease,” although the exact mechanism of action remains unknown and larger, randomized, controlled trials are needed, they said.
The study was supported by grants from a variety of organizations in Taiwan, China, and Japan. The researchers had no financial conflicts to disclose.
SOURCE: Yeh JJ et al. J Affect Disord. 2019 Jun 15; 253:277-84.
based on data from approximately 9,000 patients.
Although asthma–COPD overlap syndrome (ACOS) has been associated with depression, the effects of oral and inhaled corticosteroids on anxiety and depression in these patients have not been well investigated, wrote Jun-Jun Yeh, MD, of Ditmanson Medical Foundation Chia-Yi (Taiwan) Christian Hospital, and colleagues.
In a study published in the Journal of Affective Disorders, the researchers analyzed 9,139 ACOS patients including 1,252 statin users and 7,887 nonstatin users; 62% were male.
The statin users had significantly lower risk of both anxiety and depression than did the nonstatin users (adjusted hazard ratio 0.34 for anxiety and 0.36 for depression) after researchers controlled for factors including age, sex, comorbidities, and medications. Statin users experienced a total of 109 anxiety or depression events over an average of 8 years’ follow-up, while nonstatin users experienced a total of 1,333 anxiety or depression events over an average of 5 years’ follow-up.
The incidence density rate of anxiety was 11/1,000 person-years for statin users and 33/1,000 person-years for nonstatin users. The incidence density rate of depression was 3/1,000 person-years for statin users and 9/1,000 person-years for nonstatin users.
Significantly lower risk of anxiety and depression also were observed in statin users, compared with nonstatin users, in subgroups of men, women, patients younger than 50 years, and patients aged 50 years and older. The risks of anxiety and depression were lower in statin users versus nonstatin users across all subgroups with or without inhaled or oral corticosteroids.
Overall, the statin users were significantly younger, had more comorbidities, and were more likely to use inhaled or oral corticosteroids than were the nonstatin users.
The findings were limited by several factors including the retrospective nature of the study and a lack of information on prescribed daily doses of medication, the researchers noted. However, the results support those from previous studies and suggest that “the anti-inflammatory effect of statins may attenuate anxiety and depression in ACOS patients, even in the late stages of the disease,” although the exact mechanism of action remains unknown and larger, randomized, controlled trials are needed, they said.
The study was supported by grants from a variety of organizations in Taiwan, China, and Japan. The researchers had no financial conflicts to disclose.
SOURCE: Yeh JJ et al. J Affect Disord. 2019 Jun 15; 253:277-84.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
LAIV doesn’t up asthmatic children’s risk of lower respiratory events
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
FROM VACCINE
Inhaler technique not to blame for uncontrolled asthma in inner-city study
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Key clinical point: Factors other than inhaler technique should be considered to explain uncontrolled asthma in a low-income, inner-city population.
Major finding: In the study, 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers were using their devices correctly.
Study details: In all, 586 patients were observed using their inhalers, and their technique was scored by way of a checklist developed for the study.
Disclosures: The National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute supported the study. Coinvestigator Andrea J. Apter, MD, consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
Source: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
Tailored intervention improves asthma self-management for older patients
A needs- and barriers-based intervention that addressed psychosocial, physical, cognitive, and environmental barriers to self-management of asthma for older adults was successful in improving asthma outcomes and management, a recent trial has shown.
“This study demonstrates the value of patient centeredness and care coaching in supporting older adults with asthma and for ongoing efforts to engage patients in care delivery design and personalization,” Alex D. Federman, MD, of the division of general internal medicine at Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote in their study, which was published in JAMA Internal Medicine. “It also highlights the challenges of engaging vulnerable populations in self-management support, including modest retention rates and reduced impact over time despite repeated encounters designed to sustain its effects.”
The researchers said older adults often have difficulty with self-management tasks like inhaler technique and use of inhaled corticosteroids, which can be caused by various psychosocial, physical, cognitive, or environmental barriers. However, an attempt at creating self-management tools around specific problems, rather than generalized training, has not been traditionally attempted, they noted.
For the SAMBA trial, Dr. Federman and colleagues enrolled 391 patients who were randomized to receive a home-based intervention, clinic-based intervention, or usual care, where an asthma care coach would identify the barriers to asthma control, train the patient in areas of improvement, and provide reinforcement when necessary. Patients were at least age 60 years (15.1% men) with uncontrolled asthma in New York City and were enrolled between February 2014 and December 2017. Researchers used the Mini Asthma Quality of Life Questionnaire, Asthma Control Test, metered dose inhaler technique, Medication Adherence Rating Scale, and visits to the emergency room to assess outcomes between interventions and usual care, and between home and clinic care. The data was analyzed using the ‘difference in differences’ statistical technique to compare the change differential between the groups.
They found significantly better asthma control scores between the intervention group and the control groups at 3 months (difference-in-differences, 1.2; 95% confidence interval, 0.2-2.2; P = .02), 6 months (D-in-Ds, 1.0; 95% CI, 0.0-2.1; P = .049), and 12 months (D-inDs, 0.6; 95% CI, −0.5 to 1.8; P = .28). Quality of life was significantly improved in the intervention group, compared with control patients (overall effect, chi-squared = 10.5; with 4 degrees of freedom; P = .01), as was adherence to medication (overall effect, chi-squared = 9.5, with 4 degrees of freedom; P = .049), and inhaler technique as measured by correctly completed steps at 12 months (75% vs. 58%). Visits to the emergency room were also lower in the intervention group, compared with the control group (6.2% vs. 12.7%; adjusted odds ratio, 0.8; 95% CI, 0.6-0.99; both P = .03). The researchers noted there were no significant differences between home care and clinic care.
Potential limitations in the study included a lower-than-planned statistical power, 70% retention in the intervention arms, low generalizability of the findings, and lack of blinding on the part of research assistants as well as some improvement in asthma control and outcomes in the control group.
This study was funded in part by the Patient-Centered Outcomes Research Institute. Coauthors Nandini Shroff reported grants from the Patient-Centered Outcomes Research Institute; Michael S. Wolf reported grants from Eli Lilly; and Juan P. Wisnivesky reported personal fees from Sanofi, Quintiles, and Banook, and grants from Sanofi and Quorum. The other authors reported no relevant conflicts of interest.
SOURCE: Federman AD et al. JAMA Intern Med. 2019; doi: 10.1001/jamainternmed.2019.1201.
A needs- and barriers-based intervention that addressed psychosocial, physical, cognitive, and environmental barriers to self-management of asthma for older adults was successful in improving asthma outcomes and management, a recent trial has shown.
“This study demonstrates the value of patient centeredness and care coaching in supporting older adults with asthma and for ongoing efforts to engage patients in care delivery design and personalization,” Alex D. Federman, MD, of the division of general internal medicine at Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote in their study, which was published in JAMA Internal Medicine. “It also highlights the challenges of engaging vulnerable populations in self-management support, including modest retention rates and reduced impact over time despite repeated encounters designed to sustain its effects.”
The researchers said older adults often have difficulty with self-management tasks like inhaler technique and use of inhaled corticosteroids, which can be caused by various psychosocial, physical, cognitive, or environmental barriers. However, an attempt at creating self-management tools around specific problems, rather than generalized training, has not been traditionally attempted, they noted.
For the SAMBA trial, Dr. Federman and colleagues enrolled 391 patients who were randomized to receive a home-based intervention, clinic-based intervention, or usual care, where an asthma care coach would identify the barriers to asthma control, train the patient in areas of improvement, and provide reinforcement when necessary. Patients were at least age 60 years (15.1% men) with uncontrolled asthma in New York City and were enrolled between February 2014 and December 2017. Researchers used the Mini Asthma Quality of Life Questionnaire, Asthma Control Test, metered dose inhaler technique, Medication Adherence Rating Scale, and visits to the emergency room to assess outcomes between interventions and usual care, and between home and clinic care. The data was analyzed using the ‘difference in differences’ statistical technique to compare the change differential between the groups.
They found significantly better asthma control scores between the intervention group and the control groups at 3 months (difference-in-differences, 1.2; 95% confidence interval, 0.2-2.2; P = .02), 6 months (D-in-Ds, 1.0; 95% CI, 0.0-2.1; P = .049), and 12 months (D-inDs, 0.6; 95% CI, −0.5 to 1.8; P = .28). Quality of life was significantly improved in the intervention group, compared with control patients (overall effect, chi-squared = 10.5; with 4 degrees of freedom; P = .01), as was adherence to medication (overall effect, chi-squared = 9.5, with 4 degrees of freedom; P = .049), and inhaler technique as measured by correctly completed steps at 12 months (75% vs. 58%). Visits to the emergency room were also lower in the intervention group, compared with the control group (6.2% vs. 12.7%; adjusted odds ratio, 0.8; 95% CI, 0.6-0.99; both P = .03). The researchers noted there were no significant differences between home care and clinic care.
Potential limitations in the study included a lower-than-planned statistical power, 70% retention in the intervention arms, low generalizability of the findings, and lack of blinding on the part of research assistants as well as some improvement in asthma control and outcomes in the control group.
This study was funded in part by the Patient-Centered Outcomes Research Institute. Coauthors Nandini Shroff reported grants from the Patient-Centered Outcomes Research Institute; Michael S. Wolf reported grants from Eli Lilly; and Juan P. Wisnivesky reported personal fees from Sanofi, Quintiles, and Banook, and grants from Sanofi and Quorum. The other authors reported no relevant conflicts of interest.
SOURCE: Federman AD et al. JAMA Intern Med. 2019; doi: 10.1001/jamainternmed.2019.1201.
A needs- and barriers-based intervention that addressed psychosocial, physical, cognitive, and environmental barriers to self-management of asthma for older adults was successful in improving asthma outcomes and management, a recent trial has shown.
“This study demonstrates the value of patient centeredness and care coaching in supporting older adults with asthma and for ongoing efforts to engage patients in care delivery design and personalization,” Alex D. Federman, MD, of the division of general internal medicine at Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote in their study, which was published in JAMA Internal Medicine. “It also highlights the challenges of engaging vulnerable populations in self-management support, including modest retention rates and reduced impact over time despite repeated encounters designed to sustain its effects.”
The researchers said older adults often have difficulty with self-management tasks like inhaler technique and use of inhaled corticosteroids, which can be caused by various psychosocial, physical, cognitive, or environmental barriers. However, an attempt at creating self-management tools around specific problems, rather than generalized training, has not been traditionally attempted, they noted.
For the SAMBA trial, Dr. Federman and colleagues enrolled 391 patients who were randomized to receive a home-based intervention, clinic-based intervention, or usual care, where an asthma care coach would identify the barriers to asthma control, train the patient in areas of improvement, and provide reinforcement when necessary. Patients were at least age 60 years (15.1% men) with uncontrolled asthma in New York City and were enrolled between February 2014 and December 2017. Researchers used the Mini Asthma Quality of Life Questionnaire, Asthma Control Test, metered dose inhaler technique, Medication Adherence Rating Scale, and visits to the emergency room to assess outcomes between interventions and usual care, and between home and clinic care. The data was analyzed using the ‘difference in differences’ statistical technique to compare the change differential between the groups.
They found significantly better asthma control scores between the intervention group and the control groups at 3 months (difference-in-differences, 1.2; 95% confidence interval, 0.2-2.2; P = .02), 6 months (D-in-Ds, 1.0; 95% CI, 0.0-2.1; P = .049), and 12 months (D-inDs, 0.6; 95% CI, −0.5 to 1.8; P = .28). Quality of life was significantly improved in the intervention group, compared with control patients (overall effect, chi-squared = 10.5; with 4 degrees of freedom; P = .01), as was adherence to medication (overall effect, chi-squared = 9.5, with 4 degrees of freedom; P = .049), and inhaler technique as measured by correctly completed steps at 12 months (75% vs. 58%). Visits to the emergency room were also lower in the intervention group, compared with the control group (6.2% vs. 12.7%; adjusted odds ratio, 0.8; 95% CI, 0.6-0.99; both P = .03). The researchers noted there were no significant differences between home care and clinic care.
Potential limitations in the study included a lower-than-planned statistical power, 70% retention in the intervention arms, low generalizability of the findings, and lack of blinding on the part of research assistants as well as some improvement in asthma control and outcomes in the control group.
This study was funded in part by the Patient-Centered Outcomes Research Institute. Coauthors Nandini Shroff reported grants from the Patient-Centered Outcomes Research Institute; Michael S. Wolf reported grants from Eli Lilly; and Juan P. Wisnivesky reported personal fees from Sanofi, Quintiles, and Banook, and grants from Sanofi and Quorum. The other authors reported no relevant conflicts of interest.
SOURCE: Federman AD et al. JAMA Intern Med. 2019; doi: 10.1001/jamainternmed.2019.1201.
FROM JAMA INTERNAL MEDICINE
Allergy immunotherapy: Who, what, when … and how safe?
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
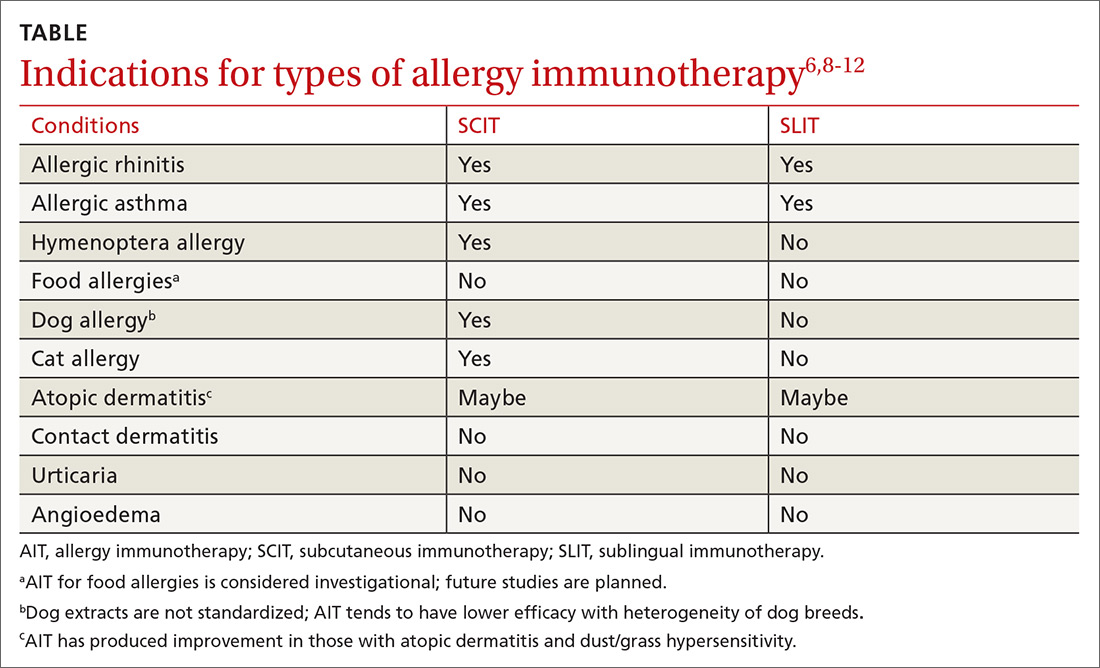
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
PRACTICE RECOMMENDATIONS
› Diagnose allergies that are amenable to allergy immunotherapy (AIT) using skin prick/puncture allergy testing in conjunction with clinical symptoms, triggers, and exposure. A
› Do not use AIT for urticaria, angioedema, drug hypersensitivity, or latex allergy. A
› Do not initiate AIT during pregnancy or in patients with acquired immune deficiency syndrome or severe asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FDA approves Nucala’s new at-home formulations
, according to a press release from the drug’s developer. The biologic will now be available as an autoinjector and as a prefilled safety syringe.
The 100-mg subcutaneous mepolizumab injection is indicated as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and the three-dose 100-mg subcutaneous injections are indicated for the rare eosinophilic granulomatosis and polyangiitis, with the biologic administered every 4 weeks in either context. The release emphasizes that mepolizumab is not approved for acute bronchospasm or status asthmaticus. Health care professionals should first determine whether self-assisted administration or administration provided by a caregiver is appropriate, and then they should provide patients and/or caregivers with proper training in how to do so.
The approval is based on two open-label, single-arm, phase 3a studies that demonstrated successful administration was possible with these options among patients with severe eosinophilic asthma, at rates of 89%-95% in one study and 100% in the other. These results were followed by those of an open-label, parallel group, single-dose study that confirmed the pharmacokinetic and pharmacodynamic profiles of these new means of administration were comparable with those currently approved.
Mepolizumab is not indicated for those with a history of hypersensitivity to either mepolizumab or to the formulation’s excipients, such as anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, or rash. Any reductions of inhaled corticosteroids after initiation of mepolizumab should be gradual and under the supervision of a health care professional. Some infections by herpes zoster have been observed. The most common adverse reactions (occurring in 3% or more of patients and more often than with placebo) during the first 24 weeks of treatment were headache (19%), injection site reaction (8%), back pain (5%), fatigue (5%), influenza (3%), urinary tract infection (3%), abdominal pain upper (3%), pruritus (3%), eczema (3%), and muscle spasm (3%). Full prescribing information can be found on the FDA website.
, according to a press release from the drug’s developer. The biologic will now be available as an autoinjector and as a prefilled safety syringe.
The 100-mg subcutaneous mepolizumab injection is indicated as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and the three-dose 100-mg subcutaneous injections are indicated for the rare eosinophilic granulomatosis and polyangiitis, with the biologic administered every 4 weeks in either context. The release emphasizes that mepolizumab is not approved for acute bronchospasm or status asthmaticus. Health care professionals should first determine whether self-assisted administration or administration provided by a caregiver is appropriate, and then they should provide patients and/or caregivers with proper training in how to do so.
The approval is based on two open-label, single-arm, phase 3a studies that demonstrated successful administration was possible with these options among patients with severe eosinophilic asthma, at rates of 89%-95% in one study and 100% in the other. These results were followed by those of an open-label, parallel group, single-dose study that confirmed the pharmacokinetic and pharmacodynamic profiles of these new means of administration were comparable with those currently approved.
Mepolizumab is not indicated for those with a history of hypersensitivity to either mepolizumab or to the formulation’s excipients, such as anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, or rash. Any reductions of inhaled corticosteroids after initiation of mepolizumab should be gradual and under the supervision of a health care professional. Some infections by herpes zoster have been observed. The most common adverse reactions (occurring in 3% or more of patients and more often than with placebo) during the first 24 weeks of treatment were headache (19%), injection site reaction (8%), back pain (5%), fatigue (5%), influenza (3%), urinary tract infection (3%), abdominal pain upper (3%), pruritus (3%), eczema (3%), and muscle spasm (3%). Full prescribing information can be found on the FDA website.
, according to a press release from the drug’s developer. The biologic will now be available as an autoinjector and as a prefilled safety syringe.
The 100-mg subcutaneous mepolizumab injection is indicated as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and the three-dose 100-mg subcutaneous injections are indicated for the rare eosinophilic granulomatosis and polyangiitis, with the biologic administered every 4 weeks in either context. The release emphasizes that mepolizumab is not approved for acute bronchospasm or status asthmaticus. Health care professionals should first determine whether self-assisted administration or administration provided by a caregiver is appropriate, and then they should provide patients and/or caregivers with proper training in how to do so.
The approval is based on two open-label, single-arm, phase 3a studies that demonstrated successful administration was possible with these options among patients with severe eosinophilic asthma, at rates of 89%-95% in one study and 100% in the other. These results were followed by those of an open-label, parallel group, single-dose study that confirmed the pharmacokinetic and pharmacodynamic profiles of these new means of administration were comparable with those currently approved.
Mepolizumab is not indicated for those with a history of hypersensitivity to either mepolizumab or to the formulation’s excipients, such as anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, or rash. Any reductions of inhaled corticosteroids after initiation of mepolizumab should be gradual and under the supervision of a health care professional. Some infections by herpes zoster have been observed. The most common adverse reactions (occurring in 3% or more of patients and more often than with placebo) during the first 24 weeks of treatment were headache (19%), injection site reaction (8%), back pain (5%), fatigue (5%), influenza (3%), urinary tract infection (3%), abdominal pain upper (3%), pruritus (3%), eczema (3%), and muscle spasm (3%). Full prescribing information can be found on the FDA website.
Lack of inhaler at school a major barrier to asthma care
BALTIMORE – frequently because the parent did not provide an inhaler or did not provide a written order for one, according to new research. Only seven U.S. states have laws allowing schools to stock albuterol for students.
“Most students only have access to this lifesaving medication when they bring a personal inhaler,” Alexandra M. Sims, MD, of Children’s National Hospital in Washington and colleagues wrote in their abstract at the annual meeting of Pediatric Academic Societies. “Interventions that address medication availability may be an important step in removing obstacles to asthma care in school.”
One such option is a stock inhaler available for any students to use. National guidelines from the Centers for Disease Control and Prevention recommend that students with asthma have access to inhaled albuterol at school, yet most states do not have legislation related to albuterol stocking in schools, according to the Asthma and Allergy Foundation of America.
Not having access to rescue inhaler medication at school contributes to lost class time and referrals to the emergency department, the authors note in their background information. Yet, “in most U.S. jurisdictions, including the school district we examined, students need both a personal albuterol inhaler and a physician order to receive medication at school.”
To determine what barriers exist regarding students’ asthma care in schools, the authors sent 166 school nurses in an urban school district an anonymous survey during the 2015-2016 school year. The survey asked about 21 factors that could delay or prevent students from returning to class and asked nurses’ agreement or disagreement with 25 additional statements.
The 130 respondents made up a 78% response rate. The institutions represented by the nurses included 44% elementary schools, 9% middle schools, 16% high schools, and 32% other (such as those who may serve multiple schools).
The majority of respondents (72%) agreed that asthma is one of the biggest health problems students face, particularly among middle and high school students (P less than .05). Most (74%) also said an albuterol inhaler at school could reduce the likelihood of students with asthma needing to leave school early.
The largest barrier to students returning to class was parents not providing an albuterol inhaler and/or a written order for an inhaler despite a request from the nurse, according to 69% of the respondents (P less than .05). In high schools in particular, another barrier was students simply not bringing their inhaler to school even though they usually carry one (P less than .01).
Only 15% of nurses saw disease severity as a significant barrier, and 17% cited the staff not adequately recognizing a student’s symptoms.
The researchers did not note use of external funding or author disclosures.
BALTIMORE – frequently because the parent did not provide an inhaler or did not provide a written order for one, according to new research. Only seven U.S. states have laws allowing schools to stock albuterol for students.
“Most students only have access to this lifesaving medication when they bring a personal inhaler,” Alexandra M. Sims, MD, of Children’s National Hospital in Washington and colleagues wrote in their abstract at the annual meeting of Pediatric Academic Societies. “Interventions that address medication availability may be an important step in removing obstacles to asthma care in school.”
One such option is a stock inhaler available for any students to use. National guidelines from the Centers for Disease Control and Prevention recommend that students with asthma have access to inhaled albuterol at school, yet most states do not have legislation related to albuterol stocking in schools, according to the Asthma and Allergy Foundation of America.
Not having access to rescue inhaler medication at school contributes to lost class time and referrals to the emergency department, the authors note in their background information. Yet, “in most U.S. jurisdictions, including the school district we examined, students need both a personal albuterol inhaler and a physician order to receive medication at school.”
To determine what barriers exist regarding students’ asthma care in schools, the authors sent 166 school nurses in an urban school district an anonymous survey during the 2015-2016 school year. The survey asked about 21 factors that could delay or prevent students from returning to class and asked nurses’ agreement or disagreement with 25 additional statements.
The 130 respondents made up a 78% response rate. The institutions represented by the nurses included 44% elementary schools, 9% middle schools, 16% high schools, and 32% other (such as those who may serve multiple schools).
The majority of respondents (72%) agreed that asthma is one of the biggest health problems students face, particularly among middle and high school students (P less than .05). Most (74%) also said an albuterol inhaler at school could reduce the likelihood of students with asthma needing to leave school early.
The largest barrier to students returning to class was parents not providing an albuterol inhaler and/or a written order for an inhaler despite a request from the nurse, according to 69% of the respondents (P less than .05). In high schools in particular, another barrier was students simply not bringing their inhaler to school even though they usually carry one (P less than .01).
Only 15% of nurses saw disease severity as a significant barrier, and 17% cited the staff not adequately recognizing a student’s symptoms.
The researchers did not note use of external funding or author disclosures.
BALTIMORE – frequently because the parent did not provide an inhaler or did not provide a written order for one, according to new research. Only seven U.S. states have laws allowing schools to stock albuterol for students.
“Most students only have access to this lifesaving medication when they bring a personal inhaler,” Alexandra M. Sims, MD, of Children’s National Hospital in Washington and colleagues wrote in their abstract at the annual meeting of Pediatric Academic Societies. “Interventions that address medication availability may be an important step in removing obstacles to asthma care in school.”
One such option is a stock inhaler available for any students to use. National guidelines from the Centers for Disease Control and Prevention recommend that students with asthma have access to inhaled albuterol at school, yet most states do not have legislation related to albuterol stocking in schools, according to the Asthma and Allergy Foundation of America.
Not having access to rescue inhaler medication at school contributes to lost class time and referrals to the emergency department, the authors note in their background information. Yet, “in most U.S. jurisdictions, including the school district we examined, students need both a personal albuterol inhaler and a physician order to receive medication at school.”
To determine what barriers exist regarding students’ asthma care in schools, the authors sent 166 school nurses in an urban school district an anonymous survey during the 2015-2016 school year. The survey asked about 21 factors that could delay or prevent students from returning to class and asked nurses’ agreement or disagreement with 25 additional statements.
The 130 respondents made up a 78% response rate. The institutions represented by the nurses included 44% elementary schools, 9% middle schools, 16% high schools, and 32% other (such as those who may serve multiple schools).
The majority of respondents (72%) agreed that asthma is one of the biggest health problems students face, particularly among middle and high school students (P less than .05). Most (74%) also said an albuterol inhaler at school could reduce the likelihood of students with asthma needing to leave school early.
The largest barrier to students returning to class was parents not providing an albuterol inhaler and/or a written order for an inhaler despite a request from the nurse, according to 69% of the respondents (P less than .05). In high schools in particular, another barrier was students simply not bringing their inhaler to school even though they usually carry one (P less than .01).
Only 15% of nurses saw disease severity as a significant barrier, and 17% cited the staff not adequately recognizing a student’s symptoms.
The researchers did not note use of external funding or author disclosures.
REPORTING FROM PAS 2019
Peanut desensitization comes at cost of anaphylaxis
based on a meta-analysis from more than 1,000 patients published in the Lancet.
In the Peanut Allergen immunotherapy, Clarifying the Evidence (PACE) systematic review and meta-analysis, Derek K. Chu, MD, of McMaster University, Hamilton, Ont., and colleagues reviewed 12 trials conducted between 2011 and 2018 with a total of 1,041 patients (median age, 9 years).
Overall, the risk of anaphylaxis was significantly higher among children who received oral immunotherapy, compared with no therapy (risk ratio, 3.12) as was anaphylaxis frequency (incidence rate ratio, 2.72) and use of epinephrine (RR, 2.21).
In addition, oral immunotherapy increased serious adverse events, compared with no therapy (RR, 1.92). Nonanaphylactic reactions also went up among oral immunotherapy patients, with increased risk for vomiting (RR, 1.79), angioedema (RR, 2.25), upper respiratory tract reactions (RR, 1.36), and lower respiratory tract infections (RR, 1.55).
Quality of life scores were not significantly different between patients who did and did not receive oral immunotherapy, the researchers noted.
The oral immunotherapy consisted of defatted, lightly roasted peanut flour in 10 studies, and a combination of peanut paste, peanut extract, or ground and defatted peanut in the other studies.
The oral immunotherapy did induce desensitization to peanuts in support of earlier studies including the subcutaneous immunotherapy trial, but “this outcome does not translate into achieving the clinical and patient-desired aim of less allergic reactions and anaphylaxis,” Dr. Chu and associates wrote.
However, “rather than take the view that these data denounce current research in oral immunotherapy as not successful, we instead suggest that this research has reached an important milestone in mechanistic but not clinical efficacy. From a clinical or biological perspective, the apparently paradoxical desensitization versus longitudinal clinical findings show the lability and unreliability of allergen thresholds identified during oral food challenges because patients often unpredictably reacted to previously tolerated doses outside of clinic,” they emphasized.
The findings were limited by several factors including the small sample size, compared with similar studies for asthma or cardiovascular conditions, and by incomplete or inconsistent data reporting, the researchers noted. However, the results are the most comprehensive to date, and support the need for food allergy treatments with better safety profiles, using peanut allergy immunotherapy as a model for other food allergies.
Dr. Chu and two other authors reported being investigators on a federally funded ongoing peanut oral immunotherapy trial. Two authors reported receiving a variety of grants from organizations such as the National Institutes of Health; the American Academy of Allergy, Asthma, & Immunology; or pharmaceutical companies.
SOURCE: Chu DK et al. Lancet. 2019 June 1;393:2222-32.
“The key criticism of this systematic review is inherent in its method because studies with different designs were grouped together,” Graham Roberts, MD, and Elizabeth Angier, MD, wrote in an accompanying editorial. In addition, the studies chosen did not account for the development of long-term peanut tolerance after the therapy was discontinued.
Also, the researchers did not factor in the variation in patterns of anaphylactic events, with patients in the treatment groups having events at home in conjunction with daily peanut doses, while the control patients would have had events mainly away from home.
“Unfortunately, the trials have not provided information about which participants benefited most from the intervention,” they wrote.
“Trading treatment-related side effects at home for allergic reactions to accidental exposures out of the house [i.e., in social situations] might beneficial for some patients,” they added. However, more research is needed to determine which patients would benefit from different treatment options at home and outside the home. The less effective but safer option of epicutaneous immunotherapy might be preferred by some patients. And early introduction of peanut products during infancy may prevent many cases of peanut allergy.
Dr. Roberts and Dr. Angier are at the University of Southampton (England). Both are members of the European Academy of Allergy and Clinical Immunology Allergen Immunotherapy Guidelines Group, which has recently published guidelines on immunotherapy. They wrote an editorial to accompany the article by Chu et al (Lancet. 2019 June 1;393:2180-1). They had no financial conflicts to disclose.
“The key criticism of this systematic review is inherent in its method because studies with different designs were grouped together,” Graham Roberts, MD, and Elizabeth Angier, MD, wrote in an accompanying editorial. In addition, the studies chosen did not account for the development of long-term peanut tolerance after the therapy was discontinued.
Also, the researchers did not factor in the variation in patterns of anaphylactic events, with patients in the treatment groups having events at home in conjunction with daily peanut doses, while the control patients would have had events mainly away from home.
“Unfortunately, the trials have not provided information about which participants benefited most from the intervention,” they wrote.
“Trading treatment-related side effects at home for allergic reactions to accidental exposures out of the house [i.e., in social situations] might beneficial for some patients,” they added. However, more research is needed to determine which patients would benefit from different treatment options at home and outside the home. The less effective but safer option of epicutaneous immunotherapy might be preferred by some patients. And early introduction of peanut products during infancy may prevent many cases of peanut allergy.
Dr. Roberts and Dr. Angier are at the University of Southampton (England). Both are members of the European Academy of Allergy and Clinical Immunology Allergen Immunotherapy Guidelines Group, which has recently published guidelines on immunotherapy. They wrote an editorial to accompany the article by Chu et al (Lancet. 2019 June 1;393:2180-1). They had no financial conflicts to disclose.
“The key criticism of this systematic review is inherent in its method because studies with different designs were grouped together,” Graham Roberts, MD, and Elizabeth Angier, MD, wrote in an accompanying editorial. In addition, the studies chosen did not account for the development of long-term peanut tolerance after the therapy was discontinued.
Also, the researchers did not factor in the variation in patterns of anaphylactic events, with patients in the treatment groups having events at home in conjunction with daily peanut doses, while the control patients would have had events mainly away from home.
“Unfortunately, the trials have not provided information about which participants benefited most from the intervention,” they wrote.
“Trading treatment-related side effects at home for allergic reactions to accidental exposures out of the house [i.e., in social situations] might beneficial for some patients,” they added. However, more research is needed to determine which patients would benefit from different treatment options at home and outside the home. The less effective but safer option of epicutaneous immunotherapy might be preferred by some patients. And early introduction of peanut products during infancy may prevent many cases of peanut allergy.
Dr. Roberts and Dr. Angier are at the University of Southampton (England). Both are members of the European Academy of Allergy and Clinical Immunology Allergen Immunotherapy Guidelines Group, which has recently published guidelines on immunotherapy. They wrote an editorial to accompany the article by Chu et al (Lancet. 2019 June 1;393:2180-1). They had no financial conflicts to disclose.
based on a meta-analysis from more than 1,000 patients published in the Lancet.
In the Peanut Allergen immunotherapy, Clarifying the Evidence (PACE) systematic review and meta-analysis, Derek K. Chu, MD, of McMaster University, Hamilton, Ont., and colleagues reviewed 12 trials conducted between 2011 and 2018 with a total of 1,041 patients (median age, 9 years).
Overall, the risk of anaphylaxis was significantly higher among children who received oral immunotherapy, compared with no therapy (risk ratio, 3.12) as was anaphylaxis frequency (incidence rate ratio, 2.72) and use of epinephrine (RR, 2.21).
In addition, oral immunotherapy increased serious adverse events, compared with no therapy (RR, 1.92). Nonanaphylactic reactions also went up among oral immunotherapy patients, with increased risk for vomiting (RR, 1.79), angioedema (RR, 2.25), upper respiratory tract reactions (RR, 1.36), and lower respiratory tract infections (RR, 1.55).
Quality of life scores were not significantly different between patients who did and did not receive oral immunotherapy, the researchers noted.
The oral immunotherapy consisted of defatted, lightly roasted peanut flour in 10 studies, and a combination of peanut paste, peanut extract, or ground and defatted peanut in the other studies.
The oral immunotherapy did induce desensitization to peanuts in support of earlier studies including the subcutaneous immunotherapy trial, but “this outcome does not translate into achieving the clinical and patient-desired aim of less allergic reactions and anaphylaxis,” Dr. Chu and associates wrote.
However, “rather than take the view that these data denounce current research in oral immunotherapy as not successful, we instead suggest that this research has reached an important milestone in mechanistic but not clinical efficacy. From a clinical or biological perspective, the apparently paradoxical desensitization versus longitudinal clinical findings show the lability and unreliability of allergen thresholds identified during oral food challenges because patients often unpredictably reacted to previously tolerated doses outside of clinic,” they emphasized.
The findings were limited by several factors including the small sample size, compared with similar studies for asthma or cardiovascular conditions, and by incomplete or inconsistent data reporting, the researchers noted. However, the results are the most comprehensive to date, and support the need for food allergy treatments with better safety profiles, using peanut allergy immunotherapy as a model for other food allergies.
Dr. Chu and two other authors reported being investigators on a federally funded ongoing peanut oral immunotherapy trial. Two authors reported receiving a variety of grants from organizations such as the National Institutes of Health; the American Academy of Allergy, Asthma, & Immunology; or pharmaceutical companies.
SOURCE: Chu DK et al. Lancet. 2019 June 1;393:2222-32.
based on a meta-analysis from more than 1,000 patients published in the Lancet.
In the Peanut Allergen immunotherapy, Clarifying the Evidence (PACE) systematic review and meta-analysis, Derek K. Chu, MD, of McMaster University, Hamilton, Ont., and colleagues reviewed 12 trials conducted between 2011 and 2018 with a total of 1,041 patients (median age, 9 years).
Overall, the risk of anaphylaxis was significantly higher among children who received oral immunotherapy, compared with no therapy (risk ratio, 3.12) as was anaphylaxis frequency (incidence rate ratio, 2.72) and use of epinephrine (RR, 2.21).
In addition, oral immunotherapy increased serious adverse events, compared with no therapy (RR, 1.92). Nonanaphylactic reactions also went up among oral immunotherapy patients, with increased risk for vomiting (RR, 1.79), angioedema (RR, 2.25), upper respiratory tract reactions (RR, 1.36), and lower respiratory tract infections (RR, 1.55).
Quality of life scores were not significantly different between patients who did and did not receive oral immunotherapy, the researchers noted.
The oral immunotherapy consisted of defatted, lightly roasted peanut flour in 10 studies, and a combination of peanut paste, peanut extract, or ground and defatted peanut in the other studies.
The oral immunotherapy did induce desensitization to peanuts in support of earlier studies including the subcutaneous immunotherapy trial, but “this outcome does not translate into achieving the clinical and patient-desired aim of less allergic reactions and anaphylaxis,” Dr. Chu and associates wrote.
However, “rather than take the view that these data denounce current research in oral immunotherapy as not successful, we instead suggest that this research has reached an important milestone in mechanistic but not clinical efficacy. From a clinical or biological perspective, the apparently paradoxical desensitization versus longitudinal clinical findings show the lability and unreliability of allergen thresholds identified during oral food challenges because patients often unpredictably reacted to previously tolerated doses outside of clinic,” they emphasized.
The findings were limited by several factors including the small sample size, compared with similar studies for asthma or cardiovascular conditions, and by incomplete or inconsistent data reporting, the researchers noted. However, the results are the most comprehensive to date, and support the need for food allergy treatments with better safety profiles, using peanut allergy immunotherapy as a model for other food allergies.
Dr. Chu and two other authors reported being investigators on a federally funded ongoing peanut oral immunotherapy trial. Two authors reported receiving a variety of grants from organizations such as the National Institutes of Health; the American Academy of Allergy, Asthma, & Immunology; or pharmaceutical companies.
SOURCE: Chu DK et al. Lancet. 2019 June 1;393:2222-32.
FROM THE LANCET
Children’s anxiety during asthma exacerbations linked to better outcomes
BALTIMORE – according to new research.
“When kids are anxious specifically during their asthma attacks, that can be a good thing because it means that they’re more vigilant,” lead author Jonathan M. Feldman, PhD, of the Albert Einstein College of Medicine’s Children’s Hospital at Montefiore and of Yeshiva University in the New York said in an interview. “They may be more likely to react during the early stages of an attack, and they may be more likely to be using self-management strategies at home and using their controller medications on a daily basis.”
He said pediatric providers can ask their patients with asthma how they feel during asthma attacks, such as whether they ever feel scared or worried.
“If a kid says no, not at all, then I would be concerned as a provider because they may not be paying attention to their asthma symptoms and they may not be taking it seriously,” Dr. Feldman said.
Past research has suggested that “illness-specific panic-fear” – the amount of anxiety someone experiences during asthma exacerbations – helps adults develop adaptive asthma management strategies, so Dr. Feldman and his colleagues examined the phenomenon as a potential protective factor in children. They shared their findings at the annual meeting of the Pediatric Academic Societies.
The research focused on Puerto Rican (n = 79) and Mexican (n = 188) children because of the substantial disparity in asthma prevalence and control between these two different Latino populations. Puerto Rican children have the highest asthma prevalence and morbidity among American children, whereas Mexican children have the lowest rates.
The 267 participants, aged 5-12 years, included 110 children from two inner-city hospitals in the New York and 157 children from two school-based health clinics and a Breathmobile in Phoenix. Nearly all the Arizona children were Mexican, and most (71%) of the Bronx children were Puerto Rican.
The authors collected the following measures at baseline and at 3, 6, 9, and 12 months follow-up: spirometry (forced expiratory volume in 1 second [FEV1]), Childhood Asthma Control Test (CACT) for children 5-11 years old, the Asthma Control Test (ACT) for 12-year-olds, adherence to inhaled corticosteroids (ICS), and acute health care utilizations (clinic sick visits, ED visits, and hospitalizations).
The authors also queried patients on four illness-specific panic-fear measures from the Childhood Asthma Symptoms Checklist: how often they felt frightened, panicky, afraid of being alone, and afraid of dying during an asthma attack (Likert 1-5 scale).
Mexican children reported higher levels of illness-specific panic-fear at the start of the study. They also tended to have lower severity of asthma, better asthma control, and better adherence to ICS, compared with Puerto Rican children.
Also at baseline, the Mexican children’s caregivers tended to be younger, poorer, and more likely to be married and to speak Spanish. The Puerto Rican caregivers, on the other hand, had a higher educational level, including 61% high school graduates, and had more depressive symptoms on the Center for Epidemiologic Studies Depression Scale (CES-D).
One-year data revealed several links between baseline reports of panic-fear and better outcomes. Mexican children who reported experiencing panic-fear at baseline were more likely to have higher FEV1 measures at 1 year of follow-up than were those who didn’t experience panic-fear (P = .02). Similarly, Puerto Rican children initially reporting panic-fear had better asthma control at 1 year, compared with those who didn’t report panic-fear (P = .007).
The researchers reported their effect sizes in terms of predicted variance in a model that accounted for the child’s age, sex, asthma duration, asthma severity, social support, acculturation, health care provider relationship, and number of family members with asthma. The model also factored in the caregiver’s age, sex, marital status, poverty level, education, and depressive symptoms.
For example, in their model, experiencing panic-fear accounted for 67% of the variance in FEV1 levels in Mexican children and 53% of the variance in asthma control in Puerto Rican children.
Less acute health care utilization also was associated with children’s baseline levels of illness-specific panic-fear. In the model, 12% of the variance in acute health care utilization among Mexican children (P = .03) and 41% of the variance among Puerto Rican children (P = .02) was explained by child-reported panic-fear. No association was seen with medication adherence.
Although caregivers’ reports of children feeling panic-fear were linked to better FEV1 outcomes in Mexican children (P = .02), the association was only slightly significant in Puerto Rican children (P = .05). Caregiver reports of children’s panic-fear were not associated with asthma control, acute health care utilization, or medication adherence.
“Providers should be aware that anxiety focused on asthma may be beneficial and facilitate adaptive asthma management strategies,” the authors concluded.
The research was funded by the National Institutes of Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to new research.
“When kids are anxious specifically during their asthma attacks, that can be a good thing because it means that they’re more vigilant,” lead author Jonathan M. Feldman, PhD, of the Albert Einstein College of Medicine’s Children’s Hospital at Montefiore and of Yeshiva University in the New York said in an interview. “They may be more likely to react during the early stages of an attack, and they may be more likely to be using self-management strategies at home and using their controller medications on a daily basis.”
He said pediatric providers can ask their patients with asthma how they feel during asthma attacks, such as whether they ever feel scared or worried.
“If a kid says no, not at all, then I would be concerned as a provider because they may not be paying attention to their asthma symptoms and they may not be taking it seriously,” Dr. Feldman said.
Past research has suggested that “illness-specific panic-fear” – the amount of anxiety someone experiences during asthma exacerbations – helps adults develop adaptive asthma management strategies, so Dr. Feldman and his colleagues examined the phenomenon as a potential protective factor in children. They shared their findings at the annual meeting of the Pediatric Academic Societies.
The research focused on Puerto Rican (n = 79) and Mexican (n = 188) children because of the substantial disparity in asthma prevalence and control between these two different Latino populations. Puerto Rican children have the highest asthma prevalence and morbidity among American children, whereas Mexican children have the lowest rates.
The 267 participants, aged 5-12 years, included 110 children from two inner-city hospitals in the New York and 157 children from two school-based health clinics and a Breathmobile in Phoenix. Nearly all the Arizona children were Mexican, and most (71%) of the Bronx children were Puerto Rican.
The authors collected the following measures at baseline and at 3, 6, 9, and 12 months follow-up: spirometry (forced expiratory volume in 1 second [FEV1]), Childhood Asthma Control Test (CACT) for children 5-11 years old, the Asthma Control Test (ACT) for 12-year-olds, adherence to inhaled corticosteroids (ICS), and acute health care utilizations (clinic sick visits, ED visits, and hospitalizations).
The authors also queried patients on four illness-specific panic-fear measures from the Childhood Asthma Symptoms Checklist: how often they felt frightened, panicky, afraid of being alone, and afraid of dying during an asthma attack (Likert 1-5 scale).
Mexican children reported higher levels of illness-specific panic-fear at the start of the study. They also tended to have lower severity of asthma, better asthma control, and better adherence to ICS, compared with Puerto Rican children.
Also at baseline, the Mexican children’s caregivers tended to be younger, poorer, and more likely to be married and to speak Spanish. The Puerto Rican caregivers, on the other hand, had a higher educational level, including 61% high school graduates, and had more depressive symptoms on the Center for Epidemiologic Studies Depression Scale (CES-D).
One-year data revealed several links between baseline reports of panic-fear and better outcomes. Mexican children who reported experiencing panic-fear at baseline were more likely to have higher FEV1 measures at 1 year of follow-up than were those who didn’t experience panic-fear (P = .02). Similarly, Puerto Rican children initially reporting panic-fear had better asthma control at 1 year, compared with those who didn’t report panic-fear (P = .007).
The researchers reported their effect sizes in terms of predicted variance in a model that accounted for the child’s age, sex, asthma duration, asthma severity, social support, acculturation, health care provider relationship, and number of family members with asthma. The model also factored in the caregiver’s age, sex, marital status, poverty level, education, and depressive symptoms.
For example, in their model, experiencing panic-fear accounted for 67% of the variance in FEV1 levels in Mexican children and 53% of the variance in asthma control in Puerto Rican children.
Less acute health care utilization also was associated with children’s baseline levels of illness-specific panic-fear. In the model, 12% of the variance in acute health care utilization among Mexican children (P = .03) and 41% of the variance among Puerto Rican children (P = .02) was explained by child-reported panic-fear. No association was seen with medication adherence.
Although caregivers’ reports of children feeling panic-fear were linked to better FEV1 outcomes in Mexican children (P = .02), the association was only slightly significant in Puerto Rican children (P = .05). Caregiver reports of children’s panic-fear were not associated with asthma control, acute health care utilization, or medication adherence.
“Providers should be aware that anxiety focused on asthma may be beneficial and facilitate adaptive asthma management strategies,” the authors concluded.
The research was funded by the National Institutes of Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to new research.
“When kids are anxious specifically during their asthma attacks, that can be a good thing because it means that they’re more vigilant,” lead author Jonathan M. Feldman, PhD, of the Albert Einstein College of Medicine’s Children’s Hospital at Montefiore and of Yeshiva University in the New York said in an interview. “They may be more likely to react during the early stages of an attack, and they may be more likely to be using self-management strategies at home and using their controller medications on a daily basis.”
He said pediatric providers can ask their patients with asthma how they feel during asthma attacks, such as whether they ever feel scared or worried.
“If a kid says no, not at all, then I would be concerned as a provider because they may not be paying attention to their asthma symptoms and they may not be taking it seriously,” Dr. Feldman said.
Past research has suggested that “illness-specific panic-fear” – the amount of anxiety someone experiences during asthma exacerbations – helps adults develop adaptive asthma management strategies, so Dr. Feldman and his colleagues examined the phenomenon as a potential protective factor in children. They shared their findings at the annual meeting of the Pediatric Academic Societies.
The research focused on Puerto Rican (n = 79) and Mexican (n = 188) children because of the substantial disparity in asthma prevalence and control between these two different Latino populations. Puerto Rican children have the highest asthma prevalence and morbidity among American children, whereas Mexican children have the lowest rates.
The 267 participants, aged 5-12 years, included 110 children from two inner-city hospitals in the New York and 157 children from two school-based health clinics and a Breathmobile in Phoenix. Nearly all the Arizona children were Mexican, and most (71%) of the Bronx children were Puerto Rican.
The authors collected the following measures at baseline and at 3, 6, 9, and 12 months follow-up: spirometry (forced expiratory volume in 1 second [FEV1]), Childhood Asthma Control Test (CACT) for children 5-11 years old, the Asthma Control Test (ACT) for 12-year-olds, adherence to inhaled corticosteroids (ICS), and acute health care utilizations (clinic sick visits, ED visits, and hospitalizations).
The authors also queried patients on four illness-specific panic-fear measures from the Childhood Asthma Symptoms Checklist: how often they felt frightened, panicky, afraid of being alone, and afraid of dying during an asthma attack (Likert 1-5 scale).
Mexican children reported higher levels of illness-specific panic-fear at the start of the study. They also tended to have lower severity of asthma, better asthma control, and better adherence to ICS, compared with Puerto Rican children.
Also at baseline, the Mexican children’s caregivers tended to be younger, poorer, and more likely to be married and to speak Spanish. The Puerto Rican caregivers, on the other hand, had a higher educational level, including 61% high school graduates, and had more depressive symptoms on the Center for Epidemiologic Studies Depression Scale (CES-D).
One-year data revealed several links between baseline reports of panic-fear and better outcomes. Mexican children who reported experiencing panic-fear at baseline were more likely to have higher FEV1 measures at 1 year of follow-up than were those who didn’t experience panic-fear (P = .02). Similarly, Puerto Rican children initially reporting panic-fear had better asthma control at 1 year, compared with those who didn’t report panic-fear (P = .007).
The researchers reported their effect sizes in terms of predicted variance in a model that accounted for the child’s age, sex, asthma duration, asthma severity, social support, acculturation, health care provider relationship, and number of family members with asthma. The model also factored in the caregiver’s age, sex, marital status, poverty level, education, and depressive symptoms.
For example, in their model, experiencing panic-fear accounted for 67% of the variance in FEV1 levels in Mexican children and 53% of the variance in asthma control in Puerto Rican children.
Less acute health care utilization also was associated with children’s baseline levels of illness-specific panic-fear. In the model, 12% of the variance in acute health care utilization among Mexican children (P = .03) and 41% of the variance among Puerto Rican children (P = .02) was explained by child-reported panic-fear. No association was seen with medication adherence.
Although caregivers’ reports of children feeling panic-fear were linked to better FEV1 outcomes in Mexican children (P = .02), the association was only slightly significant in Puerto Rican children (P = .05). Caregiver reports of children’s panic-fear were not associated with asthma control, acute health care utilization, or medication adherence.
“Providers should be aware that anxiety focused on asthma may be beneficial and facilitate adaptive asthma management strategies,” the authors concluded.
The research was funded by the National Institutes of Health. The authors reported no relevant financial disclosures.
REPORTING FROM PAS 2019
Stock inhalers at school effectively meet students’ rescue medication needs
DALLAS – Allowing public and private schools to store multiuse stock albuterol inhalers for students with asthma is a legally and medically feasible way to provide students with rescue medication without their need to leave school, according to a recent study.
“Stakeholder coalitions can facilitate the large-scale adoption of stock inhaler programs in schools,” concluded Ashley A. Lowe, MSPH, a senior research specialist and PhD candidate at the University of Arizona, Tucson, and colleagues in a poster at the American Thoracic Society’s international conference.“These programs improve access to rescue medication while returning students back to their classroom.”
The Arizona legislature passed H.B. 2208, “Stock Inhalers for Schools” in March 2017 to allow schools to store and administer albuterol sulfate while indemnifying trained staff against liability when they allowed students to use the inhaler in good faith. A stock inhaler can used by different students because of its disposable valved-holding chambers.
“Such laws allow schools to overcome the legal obstacles that make it difficult for them to ensure such medication is readily available to all children experiencing respiratory distress,” the authors wrote. They assessed the use and outcomes of schools’ storage of stock inhalers during the 2017-2018 school year in Pima County, Arizona.
Of the 213 public, 90 charter, and 61 private/parochial schools in Pima County, 246 (67%) total schools participated, including nearly all of the public schools (93%), nearly half the private/parochial schools (49%), and 17% of the charter schools. A total of 134,251 students had access to a stock inhaler at school.
Each participating school received a kit containing a 60-dose albuterol sulfate inhaler, 10 valved-holding chambers, a signed standing medical order, a standardized emergency protocol for albuterol use, access to an online training curriculum and template resources, along with technical support.
Each time a school used the stock inhaler, they documented whether an asthma diagnosis was known or not, total puffs administered and where the student went next – returned to class, sent home with caregiver, 911 call without transport, or 911 call with EMS transport.
Based on data analyzed from 240 schools, the stock inhalers were used 1,032 times at 152 schools during the study period, predominantly at public schools (97%) and by students with a known asthma diagnosis (82%). In 12.2% of cases, the student did not have a known asthma diagnosis, and 5.8% of the time, asthma diagnosis status was unknown. The students received a mean 2.7 puffs at each use.
Ethnicity and race data of those students who used the inhalers was not complete. Most of the students for whom ethnicity data were available (n = 343) and who used the inhaler were Hispanic/Latino (69.8%) independent of race. Based only on the 437 students for whom data on race were available, students using the inhaler included 41% white, 11.7% black, 3.1% Native American/Alaskan Native, 1% Asian and 0.6% Native Hawaiian/Pacific Islander.
Among the 915 uses of the inhaler for which subsequent student location was available, the majority of students (84%) returned to their classroom after using the inhaler. Only five were transported to a medical facility via EMS following a 911 call, and 911 was called for one student who did not receive EMS transport.
According to the Allergy & Asthma Network, the following states have school stock albuterol laws: Arizona, Colorado, Georgia, Illinois, Missouri, New Hampshire, New Mexico, Oklahoma, Ohio, Texas, Utah, and West Virginia.*
The research was funded by Banner–University Medical Center Tucson, Thayer Medical Corporation, and the Asthma & Airway Disease Research Center. The authors had no disclosures.
SOURCE: Lowe AA et al. ATS 2019, Abstract A4070.
* This article was updated on July 15, 2019.
DALLAS – Allowing public and private schools to store multiuse stock albuterol inhalers for students with asthma is a legally and medically feasible way to provide students with rescue medication without their need to leave school, according to a recent study.
“Stakeholder coalitions can facilitate the large-scale adoption of stock inhaler programs in schools,” concluded Ashley A. Lowe, MSPH, a senior research specialist and PhD candidate at the University of Arizona, Tucson, and colleagues in a poster at the American Thoracic Society’s international conference.“These programs improve access to rescue medication while returning students back to their classroom.”
The Arizona legislature passed H.B. 2208, “Stock Inhalers for Schools” in March 2017 to allow schools to store and administer albuterol sulfate while indemnifying trained staff against liability when they allowed students to use the inhaler in good faith. A stock inhaler can used by different students because of its disposable valved-holding chambers.
“Such laws allow schools to overcome the legal obstacles that make it difficult for them to ensure such medication is readily available to all children experiencing respiratory distress,” the authors wrote. They assessed the use and outcomes of schools’ storage of stock inhalers during the 2017-2018 school year in Pima County, Arizona.
Of the 213 public, 90 charter, and 61 private/parochial schools in Pima County, 246 (67%) total schools participated, including nearly all of the public schools (93%), nearly half the private/parochial schools (49%), and 17% of the charter schools. A total of 134,251 students had access to a stock inhaler at school.
Each participating school received a kit containing a 60-dose albuterol sulfate inhaler, 10 valved-holding chambers, a signed standing medical order, a standardized emergency protocol for albuterol use, access to an online training curriculum and template resources, along with technical support.
Each time a school used the stock inhaler, they documented whether an asthma diagnosis was known or not, total puffs administered and where the student went next – returned to class, sent home with caregiver, 911 call without transport, or 911 call with EMS transport.
Based on data analyzed from 240 schools, the stock inhalers were used 1,032 times at 152 schools during the study period, predominantly at public schools (97%) and by students with a known asthma diagnosis (82%). In 12.2% of cases, the student did not have a known asthma diagnosis, and 5.8% of the time, asthma diagnosis status was unknown. The students received a mean 2.7 puffs at each use.
Ethnicity and race data of those students who used the inhalers was not complete. Most of the students for whom ethnicity data were available (n = 343) and who used the inhaler were Hispanic/Latino (69.8%) independent of race. Based only on the 437 students for whom data on race were available, students using the inhaler included 41% white, 11.7% black, 3.1% Native American/Alaskan Native, 1% Asian and 0.6% Native Hawaiian/Pacific Islander.
Among the 915 uses of the inhaler for which subsequent student location was available, the majority of students (84%) returned to their classroom after using the inhaler. Only five were transported to a medical facility via EMS following a 911 call, and 911 was called for one student who did not receive EMS transport.
According to the Allergy & Asthma Network, the following states have school stock albuterol laws: Arizona, Colorado, Georgia, Illinois, Missouri, New Hampshire, New Mexico, Oklahoma, Ohio, Texas, Utah, and West Virginia.*
The research was funded by Banner–University Medical Center Tucson, Thayer Medical Corporation, and the Asthma & Airway Disease Research Center. The authors had no disclosures.
SOURCE: Lowe AA et al. ATS 2019, Abstract A4070.
* This article was updated on July 15, 2019.
DALLAS – Allowing public and private schools to store multiuse stock albuterol inhalers for students with asthma is a legally and medically feasible way to provide students with rescue medication without their need to leave school, according to a recent study.
“Stakeholder coalitions can facilitate the large-scale adoption of stock inhaler programs in schools,” concluded Ashley A. Lowe, MSPH, a senior research specialist and PhD candidate at the University of Arizona, Tucson, and colleagues in a poster at the American Thoracic Society’s international conference.“These programs improve access to rescue medication while returning students back to their classroom.”
The Arizona legislature passed H.B. 2208, “Stock Inhalers for Schools” in March 2017 to allow schools to store and administer albuterol sulfate while indemnifying trained staff against liability when they allowed students to use the inhaler in good faith. A stock inhaler can used by different students because of its disposable valved-holding chambers.
“Such laws allow schools to overcome the legal obstacles that make it difficult for them to ensure such medication is readily available to all children experiencing respiratory distress,” the authors wrote. They assessed the use and outcomes of schools’ storage of stock inhalers during the 2017-2018 school year in Pima County, Arizona.
Of the 213 public, 90 charter, and 61 private/parochial schools in Pima County, 246 (67%) total schools participated, including nearly all of the public schools (93%), nearly half the private/parochial schools (49%), and 17% of the charter schools. A total of 134,251 students had access to a stock inhaler at school.
Each participating school received a kit containing a 60-dose albuterol sulfate inhaler, 10 valved-holding chambers, a signed standing medical order, a standardized emergency protocol for albuterol use, access to an online training curriculum and template resources, along with technical support.
Each time a school used the stock inhaler, they documented whether an asthma diagnosis was known or not, total puffs administered and where the student went next – returned to class, sent home with caregiver, 911 call without transport, or 911 call with EMS transport.
Based on data analyzed from 240 schools, the stock inhalers were used 1,032 times at 152 schools during the study period, predominantly at public schools (97%) and by students with a known asthma diagnosis (82%). In 12.2% of cases, the student did not have a known asthma diagnosis, and 5.8% of the time, asthma diagnosis status was unknown. The students received a mean 2.7 puffs at each use.
Ethnicity and race data of those students who used the inhalers was not complete. Most of the students for whom ethnicity data were available (n = 343) and who used the inhaler were Hispanic/Latino (69.8%) independent of race. Based only on the 437 students for whom data on race were available, students using the inhaler included 41% white, 11.7% black, 3.1% Native American/Alaskan Native, 1% Asian and 0.6% Native Hawaiian/Pacific Islander.
Among the 915 uses of the inhaler for which subsequent student location was available, the majority of students (84%) returned to their classroom after using the inhaler. Only five were transported to a medical facility via EMS following a 911 call, and 911 was called for one student who did not receive EMS transport.
According to the Allergy & Asthma Network, the following states have school stock albuterol laws: Arizona, Colorado, Georgia, Illinois, Missouri, New Hampshire, New Mexico, Oklahoma, Ohio, Texas, Utah, and West Virginia.*
The research was funded by Banner–University Medical Center Tucson, Thayer Medical Corporation, and the Asthma & Airway Disease Research Center. The authors had no disclosures.
SOURCE: Lowe AA et al. ATS 2019, Abstract A4070.
* This article was updated on July 15, 2019.
REPORTING FROM ATS 2019