User login
Depression, or something else?
CASE Suicidal behavior, severe headaches
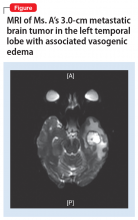
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
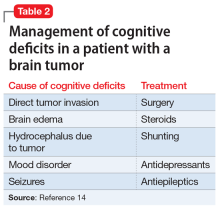
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
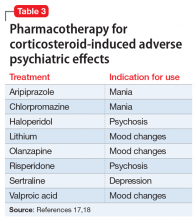
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
USPSTF again deems evidence insufficient to recommend cognitive impairment screening in older adults
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
FROM JAMA
As costs for neurologic drugs rise, adherence to therapy drops
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
FROM NEUROLOGY
Seminal, highly anticipated Alzheimer’s trial falters
DIAN-TU top-line results negative
Top-line results from the seminal phase 2/3 Dominantly Inherited Alzheimer’s Network–Trials Unit (DIAN-TU) study show that the novel drugs gantenerumab (Roche) and solanezumab (Lilly) did not meet the primary endpoint in patients with early-stage, dominantly inherited Alzheimer’s disease (AD), investigators have announced.
In the international trial, which included almost 200 participants, the two experimental agents were evaluated separately. However, initial analyses showed that neither significantly slowed cognitive decline, the primary outcome measure, nor memory loss.
Still, the researchers noted that they will continue exploring data from DIAN-TU’s cognitive and clinical outcomes and are awaiting analyses of various biomarkers.
“Although the drugs we evaluated were not successful, the trial will move us forward in understanding Alzheimer’s,” principal investigator Randall J. Bateman, MD, of Washington University, St. Louis, said in a news release.
Funders for the trial included the National Institute on Aging and the Alzheimer’s Association.
“While the top-line data fell short, the Alzheimer’s Association looks forward to a more complete report at upcoming scientific conferences. We learn from every trial,” Maria Carrillo, PhD, chief scientific officer at the Alzheimer’s Association, noted in the organization’s own release.
Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, agreed with Dr. Carrillo that, although the results were disappointing, the data will be beneficial for the field.
“It’s always a difficult day when we get news like this,” Dr. Edelmayer said in an interview. However, “this research is going to absolutely provide valuable information once we can really pick through all of the data.”
Rare condition
Dominantly inherited AD, also known as familial AD or autosomal dominant AD, is rare but can affect memory and cognitive skills in individuals as young as age 30. It is caused by mutations on chromosomes 21, 14, and/or 1 that play a part in the breakdown of amyloid proteins and formation of amyloid plaques.
Both gantenerumab and solanezumab were created to target and neutralize amyloid-beta, albeit through different mechanisms. Both are also being assessed in other trials as treatment for more common forms of AD.
As reported by Medscape Medical News, results of the phase 3 EXPEDITION3 trial of solanezumab in patients with mild AD were negative, as were two other phase 3 trials. The drug is now being evaluated in the ongoing solanezumab Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study.
Although the phase 3 SCARLET ROAD trial of gantenerumab for mild AD was stopped early for futility in 2014, it was continued as an open-label extension at the high dose for 2 years. During that period, follow-up analyses showed a dramatic decline in amyloid-beta deposition in the participants – leading to the launch of the phase 3 GRADUATE 1 and GRADUATE 2 trials.
Starting in 2012, DIAN-TU was conducted at 24 sites in the United States, the United Kingdom, Canada, Europe, and Australia. It followed 194 adult patients for up to 7 years (average duration, 5 years). Its original estimated completion date was December 2020, as stated on clinicaltrials.gov.
All participants had family members with a genetic mutation that causes early-onset Alzheimer’s disease. They already had very mild symptoms of cognitive decline and memory loss at the start of the trial or were expected to develop symptoms within 15 years of enrollment.
“People who inherit the mutation are all but guaranteed to develop symptoms at about the same age their parents did,” the release noted.
“While devastating for families, such mutations allow researchers to identify people in the early stages of the disease before their behavior and memory begin to change,” it added.
The Alzheimer’s Association noted in its release that a child of a parent with the mutation has a 50/50 chance of inheriting the disease. “This form affects less than 1% of the individuals living with Alzheimer’s disease today,” Dr. Edelmayer noted.
Detailed data coming soon
Trial participants were randomly assigned to receive either solanezumab, gantenerumab, or matching placebo. To act as a comparator group, family members without the AD mutation were also included.
The primary measure was change from baseline in the DIAN-TU cognitive composite score. Secondary measures included changes on the Mini-Mental State Examination, the Functional Assessment Scale, the Neuropsychiatric Inventory Questionnaire, the 12-item International Shopping List Test, the Memory Complaint Questionnaire, and the Wechsler Memory Scale Logical Memory/Paragraph Memory test.
The researchers also conducted imaging scans and collected samples of blood and cerebrospinal fluid.
Along with announcing the negative top-line results for the trial, the investigators noted that “a more detailed analysis of the trial’s data” will be presented at the Advances in Alzheimer’s and Parkinson’s Therapies in Vienna on April 2, 2020, and at the Alzheimer’s Association International Conference in Amsterdam in July.
The researchers will continue to explore all data gathered – but already new insights have been discovered into the development and progression of AD, Dr. Bateman noted.
Included among these discoveries is that brain changes that occur as the disease progresses are similar among those with the inherited, early-onset form of AD and the late-onset form.
“The trial’s innovative design ... will make advances for future Alzheimer’s trials. Ongoing and continued research and trials will bring us closer to our goal to stop Alzheimer’s,” Dr. Bateman said. “We will continue until we are successful.”
“These results reflect the difficult nature of treating [AD] and the great need for continued research,” said Daniel Skovronsky, MD, PhD, chief scientific officer and president of Lilly Research Labs.
“If we have learned one thing after more than 30 years of Alzheimer’s research, it is that even negative results propel the science forward,” he added.
Lilly noted in a statement that the DIAN-TU top-line results will not affect its ongoing A4 study of solanezumab. Roche noted in its own statement that the findings also will not affect the company’s ongoing GRADUATE studies of gantenerumab.
“The work doesn’t stop here”
Richard J. Hodes, MD, director of the National Institute on Aging, said that DIAN-TU will advance the field’s knowledge about a complex disease.
“We look forward to learning more through the published, peer-reviewed data, which will provide a broad range of scientists with crucial information and guidance for future research,” he said.
Howard Fillit, MD, founding executive director and chief science officer at the Alzheimer’s Drug Discovery Foundation (ADDF), agreed.
“While we are disappointed that patients in this study did not see a benefit, we need to keep in mind that Alzheimer’s is a complicated disease due to complex, multifactorial causes,” he said in a statement.
“ADDF has long supported a broader approach that moves past targeting beta-amyloid and advances a diverse pipeline of drugs addressing multiple targets” in AD, Dr. Fillit added. “We need multiple ‘shots on goals’ to discover effective drugs.”
Dr. Edelmayer said the results emphasize that “this story isn’t yet completely told” and that there is still a lot to learn from the data, especially regarding the biomarkers that were tested.
“With that information, we will gain valuable insight into the outcomes that have been released but will also probably better understand where we should be putting our energies and focus moving forward,” she said.
Going forward, “we will continue this fight until we have an effective treatment for all individuals living with Alzheimer’s, whether it’s dominantly inherited [AD] or the more common version, which is the late-onset or sporadic form of the disease,” said Dr. Edelmayer.
“We have to stay optimistic. The work doesn’t stop here.”
The trial was funded by Eli Lilly, Roche, the Alzheimer’s Association, the National Institute on Aging, the GHR Foundation, and FBRI.
This article first appeared on Medscape.com.
DIAN-TU top-line results negative
DIAN-TU top-line results negative
Top-line results from the seminal phase 2/3 Dominantly Inherited Alzheimer’s Network–Trials Unit (DIAN-TU) study show that the novel drugs gantenerumab (Roche) and solanezumab (Lilly) did not meet the primary endpoint in patients with early-stage, dominantly inherited Alzheimer’s disease (AD), investigators have announced.
In the international trial, which included almost 200 participants, the two experimental agents were evaluated separately. However, initial analyses showed that neither significantly slowed cognitive decline, the primary outcome measure, nor memory loss.
Still, the researchers noted that they will continue exploring data from DIAN-TU’s cognitive and clinical outcomes and are awaiting analyses of various biomarkers.
“Although the drugs we evaluated were not successful, the trial will move us forward in understanding Alzheimer’s,” principal investigator Randall J. Bateman, MD, of Washington University, St. Louis, said in a news release.
Funders for the trial included the National Institute on Aging and the Alzheimer’s Association.
“While the top-line data fell short, the Alzheimer’s Association looks forward to a more complete report at upcoming scientific conferences. We learn from every trial,” Maria Carrillo, PhD, chief scientific officer at the Alzheimer’s Association, noted in the organization’s own release.
Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, agreed with Dr. Carrillo that, although the results were disappointing, the data will be beneficial for the field.
“It’s always a difficult day when we get news like this,” Dr. Edelmayer said in an interview. However, “this research is going to absolutely provide valuable information once we can really pick through all of the data.”
Rare condition
Dominantly inherited AD, also known as familial AD or autosomal dominant AD, is rare but can affect memory and cognitive skills in individuals as young as age 30. It is caused by mutations on chromosomes 21, 14, and/or 1 that play a part in the breakdown of amyloid proteins and formation of amyloid plaques.
Both gantenerumab and solanezumab were created to target and neutralize amyloid-beta, albeit through different mechanisms. Both are also being assessed in other trials as treatment for more common forms of AD.
As reported by Medscape Medical News, results of the phase 3 EXPEDITION3 trial of solanezumab in patients with mild AD were negative, as were two other phase 3 trials. The drug is now being evaluated in the ongoing solanezumab Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study.
Although the phase 3 SCARLET ROAD trial of gantenerumab for mild AD was stopped early for futility in 2014, it was continued as an open-label extension at the high dose for 2 years. During that period, follow-up analyses showed a dramatic decline in amyloid-beta deposition in the participants – leading to the launch of the phase 3 GRADUATE 1 and GRADUATE 2 trials.
Starting in 2012, DIAN-TU was conducted at 24 sites in the United States, the United Kingdom, Canada, Europe, and Australia. It followed 194 adult patients for up to 7 years (average duration, 5 years). Its original estimated completion date was December 2020, as stated on clinicaltrials.gov.
All participants had family members with a genetic mutation that causes early-onset Alzheimer’s disease. They already had very mild symptoms of cognitive decline and memory loss at the start of the trial or were expected to develop symptoms within 15 years of enrollment.
“People who inherit the mutation are all but guaranteed to develop symptoms at about the same age their parents did,” the release noted.
“While devastating for families, such mutations allow researchers to identify people in the early stages of the disease before their behavior and memory begin to change,” it added.
The Alzheimer’s Association noted in its release that a child of a parent with the mutation has a 50/50 chance of inheriting the disease. “This form affects less than 1% of the individuals living with Alzheimer’s disease today,” Dr. Edelmayer noted.
Detailed data coming soon
Trial participants were randomly assigned to receive either solanezumab, gantenerumab, or matching placebo. To act as a comparator group, family members without the AD mutation were also included.
The primary measure was change from baseline in the DIAN-TU cognitive composite score. Secondary measures included changes on the Mini-Mental State Examination, the Functional Assessment Scale, the Neuropsychiatric Inventory Questionnaire, the 12-item International Shopping List Test, the Memory Complaint Questionnaire, and the Wechsler Memory Scale Logical Memory/Paragraph Memory test.
The researchers also conducted imaging scans and collected samples of blood and cerebrospinal fluid.
Along with announcing the negative top-line results for the trial, the investigators noted that “a more detailed analysis of the trial’s data” will be presented at the Advances in Alzheimer’s and Parkinson’s Therapies in Vienna on April 2, 2020, and at the Alzheimer’s Association International Conference in Amsterdam in July.
The researchers will continue to explore all data gathered – but already new insights have been discovered into the development and progression of AD, Dr. Bateman noted.
Included among these discoveries is that brain changes that occur as the disease progresses are similar among those with the inherited, early-onset form of AD and the late-onset form.
“The trial’s innovative design ... will make advances for future Alzheimer’s trials. Ongoing and continued research and trials will bring us closer to our goal to stop Alzheimer’s,” Dr. Bateman said. “We will continue until we are successful.”
“These results reflect the difficult nature of treating [AD] and the great need for continued research,” said Daniel Skovronsky, MD, PhD, chief scientific officer and president of Lilly Research Labs.
“If we have learned one thing after more than 30 years of Alzheimer’s research, it is that even negative results propel the science forward,” he added.
Lilly noted in a statement that the DIAN-TU top-line results will not affect its ongoing A4 study of solanezumab. Roche noted in its own statement that the findings also will not affect the company’s ongoing GRADUATE studies of gantenerumab.
“The work doesn’t stop here”
Richard J. Hodes, MD, director of the National Institute on Aging, said that DIAN-TU will advance the field’s knowledge about a complex disease.
“We look forward to learning more through the published, peer-reviewed data, which will provide a broad range of scientists with crucial information and guidance for future research,” he said.
Howard Fillit, MD, founding executive director and chief science officer at the Alzheimer’s Drug Discovery Foundation (ADDF), agreed.
“While we are disappointed that patients in this study did not see a benefit, we need to keep in mind that Alzheimer’s is a complicated disease due to complex, multifactorial causes,” he said in a statement.
“ADDF has long supported a broader approach that moves past targeting beta-amyloid and advances a diverse pipeline of drugs addressing multiple targets” in AD, Dr. Fillit added. “We need multiple ‘shots on goals’ to discover effective drugs.”
Dr. Edelmayer said the results emphasize that “this story isn’t yet completely told” and that there is still a lot to learn from the data, especially regarding the biomarkers that were tested.
“With that information, we will gain valuable insight into the outcomes that have been released but will also probably better understand where we should be putting our energies and focus moving forward,” she said.
Going forward, “we will continue this fight until we have an effective treatment for all individuals living with Alzheimer’s, whether it’s dominantly inherited [AD] or the more common version, which is the late-onset or sporadic form of the disease,” said Dr. Edelmayer.
“We have to stay optimistic. The work doesn’t stop here.”
The trial was funded by Eli Lilly, Roche, the Alzheimer’s Association, the National Institute on Aging, the GHR Foundation, and FBRI.
This article first appeared on Medscape.com.
Top-line results from the seminal phase 2/3 Dominantly Inherited Alzheimer’s Network–Trials Unit (DIAN-TU) study show that the novel drugs gantenerumab (Roche) and solanezumab (Lilly) did not meet the primary endpoint in patients with early-stage, dominantly inherited Alzheimer’s disease (AD), investigators have announced.
In the international trial, which included almost 200 participants, the two experimental agents were evaluated separately. However, initial analyses showed that neither significantly slowed cognitive decline, the primary outcome measure, nor memory loss.
Still, the researchers noted that they will continue exploring data from DIAN-TU’s cognitive and clinical outcomes and are awaiting analyses of various biomarkers.
“Although the drugs we evaluated were not successful, the trial will move us forward in understanding Alzheimer’s,” principal investigator Randall J. Bateman, MD, of Washington University, St. Louis, said in a news release.
Funders for the trial included the National Institute on Aging and the Alzheimer’s Association.
“While the top-line data fell short, the Alzheimer’s Association looks forward to a more complete report at upcoming scientific conferences. We learn from every trial,” Maria Carrillo, PhD, chief scientific officer at the Alzheimer’s Association, noted in the organization’s own release.
Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, agreed with Dr. Carrillo that, although the results were disappointing, the data will be beneficial for the field.
“It’s always a difficult day when we get news like this,” Dr. Edelmayer said in an interview. However, “this research is going to absolutely provide valuable information once we can really pick through all of the data.”
Rare condition
Dominantly inherited AD, also known as familial AD or autosomal dominant AD, is rare but can affect memory and cognitive skills in individuals as young as age 30. It is caused by mutations on chromosomes 21, 14, and/or 1 that play a part in the breakdown of amyloid proteins and formation of amyloid plaques.
Both gantenerumab and solanezumab were created to target and neutralize amyloid-beta, albeit through different mechanisms. Both are also being assessed in other trials as treatment for more common forms of AD.
As reported by Medscape Medical News, results of the phase 3 EXPEDITION3 trial of solanezumab in patients with mild AD were negative, as were two other phase 3 trials. The drug is now being evaluated in the ongoing solanezumab Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study.
Although the phase 3 SCARLET ROAD trial of gantenerumab for mild AD was stopped early for futility in 2014, it was continued as an open-label extension at the high dose for 2 years. During that period, follow-up analyses showed a dramatic decline in amyloid-beta deposition in the participants – leading to the launch of the phase 3 GRADUATE 1 and GRADUATE 2 trials.
Starting in 2012, DIAN-TU was conducted at 24 sites in the United States, the United Kingdom, Canada, Europe, and Australia. It followed 194 adult patients for up to 7 years (average duration, 5 years). Its original estimated completion date was December 2020, as stated on clinicaltrials.gov.
All participants had family members with a genetic mutation that causes early-onset Alzheimer’s disease. They already had very mild symptoms of cognitive decline and memory loss at the start of the trial or were expected to develop symptoms within 15 years of enrollment.
“People who inherit the mutation are all but guaranteed to develop symptoms at about the same age their parents did,” the release noted.
“While devastating for families, such mutations allow researchers to identify people in the early stages of the disease before their behavior and memory begin to change,” it added.
The Alzheimer’s Association noted in its release that a child of a parent with the mutation has a 50/50 chance of inheriting the disease. “This form affects less than 1% of the individuals living with Alzheimer’s disease today,” Dr. Edelmayer noted.
Detailed data coming soon
Trial participants were randomly assigned to receive either solanezumab, gantenerumab, or matching placebo. To act as a comparator group, family members without the AD mutation were also included.
The primary measure was change from baseline in the DIAN-TU cognitive composite score. Secondary measures included changes on the Mini-Mental State Examination, the Functional Assessment Scale, the Neuropsychiatric Inventory Questionnaire, the 12-item International Shopping List Test, the Memory Complaint Questionnaire, and the Wechsler Memory Scale Logical Memory/Paragraph Memory test.
The researchers also conducted imaging scans and collected samples of blood and cerebrospinal fluid.
Along with announcing the negative top-line results for the trial, the investigators noted that “a more detailed analysis of the trial’s data” will be presented at the Advances in Alzheimer’s and Parkinson’s Therapies in Vienna on April 2, 2020, and at the Alzheimer’s Association International Conference in Amsterdam in July.
The researchers will continue to explore all data gathered – but already new insights have been discovered into the development and progression of AD, Dr. Bateman noted.
Included among these discoveries is that brain changes that occur as the disease progresses are similar among those with the inherited, early-onset form of AD and the late-onset form.
“The trial’s innovative design ... will make advances for future Alzheimer’s trials. Ongoing and continued research and trials will bring us closer to our goal to stop Alzheimer’s,” Dr. Bateman said. “We will continue until we are successful.”
“These results reflect the difficult nature of treating [AD] and the great need for continued research,” said Daniel Skovronsky, MD, PhD, chief scientific officer and president of Lilly Research Labs.
“If we have learned one thing after more than 30 years of Alzheimer’s research, it is that even negative results propel the science forward,” he added.
Lilly noted in a statement that the DIAN-TU top-line results will not affect its ongoing A4 study of solanezumab. Roche noted in its own statement that the findings also will not affect the company’s ongoing GRADUATE studies of gantenerumab.
“The work doesn’t stop here”
Richard J. Hodes, MD, director of the National Institute on Aging, said that DIAN-TU will advance the field’s knowledge about a complex disease.
“We look forward to learning more through the published, peer-reviewed data, which will provide a broad range of scientists with crucial information and guidance for future research,” he said.
Howard Fillit, MD, founding executive director and chief science officer at the Alzheimer’s Drug Discovery Foundation (ADDF), agreed.
“While we are disappointed that patients in this study did not see a benefit, we need to keep in mind that Alzheimer’s is a complicated disease due to complex, multifactorial causes,” he said in a statement.
“ADDF has long supported a broader approach that moves past targeting beta-amyloid and advances a diverse pipeline of drugs addressing multiple targets” in AD, Dr. Fillit added. “We need multiple ‘shots on goals’ to discover effective drugs.”
Dr. Edelmayer said the results emphasize that “this story isn’t yet completely told” and that there is still a lot to learn from the data, especially regarding the biomarkers that were tested.
“With that information, we will gain valuable insight into the outcomes that have been released but will also probably better understand where we should be putting our energies and focus moving forward,” she said.
Going forward, “we will continue this fight until we have an effective treatment for all individuals living with Alzheimer’s, whether it’s dominantly inherited [AD] or the more common version, which is the late-onset or sporadic form of the disease,” said Dr. Edelmayer.
“We have to stay optimistic. The work doesn’t stop here.”
The trial was funded by Eli Lilly, Roche, the Alzheimer’s Association, the National Institute on Aging, the GHR Foundation, and FBRI.
This article first appeared on Medscape.com.
APOE genotype directly regulates alpha-synuclein accumulation
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
FROM SCIENCE TRANSLATIONAL MEDICINE
Dietary flavonol intake linked to reduced risk of Alzheimer’s
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
FROM NEUROLOGY
Genetic factor linked to impaired memory after heading many soccer balls
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
FROM JAMA Neurology
Cognitive screening of older physicians: What’s fair?
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
FROM JAMA
Could preventing dementia be as simple as following your mom’s advice?
SAN DIEGO – To prevent dementia, follow Mom’s advice: Get up off the couch, go play with your friends, and eat your vegetables.
After 15 years of disappointing drug trials, strong new evidence says the best way to attack Alzheimer’s disease is not to treat it once it develops, but to prevent it in the first place, Laura D. Baker, PhD, said at the Clinical Trials on Alzheimer’s Disease conference. Studies of exercise, cognitive and social stimulation, and diet show that each one can reduce the risk of dementia, and that a combination of all three may have even a more powerful and synergistic effect.
“We have become absolutely phobic of exercise,” said Dr. Baker of Wake Forest University, Winston-Salem, N.C. And it’s not just structured exercise we shirk. “We take the closest parking space, sit for hours on end, don’t even take the stairs. Yet we know from years of work that exercise has a powerful benefit on cardiovascular disease, lipid profiles, metabolic disease, stress, and mood. Now we are seeing that exercise also promotes brain health in normal aging and protects against cognitive decline and prevention.”
Get off the couch
The general benefits of exercise – chiefly aerobic exercise – are myriad, Dr. Baker said.
“Exercise increases effective neurorepair. It reduces oxidative stress. It improves insulin sensitivity and helps with maintaining normal weight. It reduces inflammation and increases normal clearance of amyloid-beta.”
A 2017 meta-analysis reviewed some of these findings. “The current review [of 16 studies] suggests that aerobic exercise may have positive effects on the right hippocampus and potentially beneficial effects on the overall and other parts of the hippocampus, the cingulate cortex, and the medial temporal areas. ... Moreover, aerobic exercise may increase functional connectivity or activation in the hippocampus, cingulate cortex, and parahippocampal gyrus regions,” wrote Mo-yi Li, PhD, of Fujian University of Traditional Chinese Medicine, Fuzhou, China, and colleagues.
Exercise increases brain-derived neurotrophic factor (BDNF), which in turn increases neuronal potentiation and synaptic plasticity. BDNF is also important in hippocampal neurogenesis; mice, after just one aerobic session, showed dramatic boosts in BDNF. A 2018 review elaborates on these findings.
Eat right
Diet mediates dementia risk through less direct, but very effective, pathways, Dr. Baker said. Diets rich in vegetables, berries, nuts, fish, lean proteins, and healthy fats improves virtually all metabolic measures. These, in turn, reduce the risk cerebrovascular disease – an important driver of vascular dementias and a contributor to Alzheimer’s disease risk as well.
The MIND diet study (Mediterranean-DASH Diet Intervention for Neurodegenerative Delay), reported in 2015 was a very successful demonstration of this concept. A combination of the Mediterranean diet and the DASH diet (Dietary Approaches to Stop Hypertension), the MIND diet stresses frequent consumption of vegetables – especially leafy greens – as well as nuts, berries, whole grains, fish, poultry, and wine or grape juice. In the large, nearly 5-year study of 923 subjects aged 58-98 years, the MIND diet was associated with significant gains in cognition – equivalent to a 7-year reversal of age. After 4.5 years, those who strictly adhered to the diet had a 53% reduction in risk for Alzheimer’s disease, and those who adhered moderately had a 35% reduction. And in a more recent Australian longitudinal study, the MIND diet was associated with a 53% reduced risk of cognitive impairment over 12 years.
Ketogenic diets also may exert a benefit. Theoretically, a state of ketosis forces the brain to burn ketones as an alternative fuel to glucose, thus boosting brain function in glucose-starved brains. A small pilot study with exploratory cognitive endpoints determined that diet-compliant subjects with mild to moderate Alzheimer’s experienced a mean 5-point improvement in the Alzheimer’s Disease Assessment Score–Cognition. They reverted to baseline scores within a month of ending the study.
Recent initial work into the gut microbiome provides some additional speculative, but interesting, data. A dysregulated microbiome can shift microbial populations toward a more inflammatory profile. Some work suggests that inflammatory cytokines then travel to the brain and induce a hyperresponse of neuron-damaging immune cells. A comprehensive review article discusses the complicated mechanisms that may be in play.
Play with your friends
Cognitive stimulation and social interaction also appear to modify dementia risk, although the data are a little more limited. But personal interaction is a key element of Dr. Baker’s ongoing EXERT trial.
The ongoing phase 3 trial randomized 300 adults with amnestic mild cognitive impairment to moderate to high intensity aerobic exercise plus one-on-one support at local YMCA gyms or a low-intensity stretching, balance, and range of motion program. In additional to cognitive testing, the trial includes brain imaging, cerebrospinal fluid sampling for biomarkers of Alzheimer’s disease, and a sleep study.
A key component is personal interaction with a trainer. “They spend a lot of one-on-one time with each person,” Dr. Baker said. “For me, that’s the crucial ingredient – that personal touch. It’s what helps people move from Point A to Point B in their behaviors.”
Virtual cognitive stimulation is also a burgeoning area of dementia prevention research right now. Numerous studies are ongoing to test whether virtual reality or other computer-based games might keep the mind sharp or even improve cognition in people at risk.
The power of three
If one lifestyle change can reduce dementia risk, what happens when all three work together?
That’s the newest question, first successfully explored in the mid-2000s, with the FINGER study (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability). In FINGER, the triad of exercise, personal support at the gym, and a modified Mediterranean diet reduced Alzheimer’s disease risk and improved cognition relative to the control group.
FINGER showed that the intervention was feasible and that it was associated with cognitive preservation and reduced Alzheimer’s disease risk in a group of at-risk subjects. The active group also had a 25% greater improvement on a neuropsychological test battery relative to the control group. They also performed 150% better in processing speed, 83% better in executive function, and 40% better in short-term memory. They showed no increased risk of cognitive decline relative to the control group, which experienced a 30% increase in risk, according to lead investigator Miia Kivipelto, PhD, of the Karolinska Institute, Stockholm.
So successful was FINGER that it launched a global consortium of related studies called World Wide FINGERS. Active in six countries now, including the United States, the studies aim to discover whether such combinations of lifestyle interventions are workable across countries and cultures. World Wide FINGERS is largely supported by the Alzheimer’s Association.
Global enthusiasm for lifestyle interventions
In recognition of the importance of lifestyle changes for dementia prevention, the World Health Organization recently published “Risk reduction of cognitive decline and dementia.” The document reviews many studies and makes recommendations regarding not only exercise, diet, and cognitive stimulation, but also smoking and alcohol.
Research interest in these areas is surging, Dr. Baker said. “The [U.S.] National Institute on Aging now has 29 ongoing trials. There’s a strong commitment to investigations into how lifestyle interventions could protect brain health as we get older. Certainly, many fit and healthy people do develop Alzheimer’s. But for some, it could be medicine.”
But no matter how compliant people are, lifestyle changes will never completely rid the world of Alzheimer’s and other dementias. The view of Dr. Baker – and most other Alzheimer’s researchers – is to employ lifestyle changes to reduce risk as much as possible and not to stop when cognitive problems do present.
“We need to understand how lifestyle interventions might work in combination with pharmaceuticals,” she said. “If we can support the health of the body and the health of the mind, lifestyle interventions can be the fertilizer that would help drug therapy have its maximum effect.”
SAN DIEGO – To prevent dementia, follow Mom’s advice: Get up off the couch, go play with your friends, and eat your vegetables.
After 15 years of disappointing drug trials, strong new evidence says the best way to attack Alzheimer’s disease is not to treat it once it develops, but to prevent it in the first place, Laura D. Baker, PhD, said at the Clinical Trials on Alzheimer’s Disease conference. Studies of exercise, cognitive and social stimulation, and diet show that each one can reduce the risk of dementia, and that a combination of all three may have even a more powerful and synergistic effect.
“We have become absolutely phobic of exercise,” said Dr. Baker of Wake Forest University, Winston-Salem, N.C. And it’s not just structured exercise we shirk. “We take the closest parking space, sit for hours on end, don’t even take the stairs. Yet we know from years of work that exercise has a powerful benefit on cardiovascular disease, lipid profiles, metabolic disease, stress, and mood. Now we are seeing that exercise also promotes brain health in normal aging and protects against cognitive decline and prevention.”
Get off the couch
The general benefits of exercise – chiefly aerobic exercise – are myriad, Dr. Baker said.
“Exercise increases effective neurorepair. It reduces oxidative stress. It improves insulin sensitivity and helps with maintaining normal weight. It reduces inflammation and increases normal clearance of amyloid-beta.”
A 2017 meta-analysis reviewed some of these findings. “The current review [of 16 studies] suggests that aerobic exercise may have positive effects on the right hippocampus and potentially beneficial effects on the overall and other parts of the hippocampus, the cingulate cortex, and the medial temporal areas. ... Moreover, aerobic exercise may increase functional connectivity or activation in the hippocampus, cingulate cortex, and parahippocampal gyrus regions,” wrote Mo-yi Li, PhD, of Fujian University of Traditional Chinese Medicine, Fuzhou, China, and colleagues.
Exercise increases brain-derived neurotrophic factor (BDNF), which in turn increases neuronal potentiation and synaptic plasticity. BDNF is also important in hippocampal neurogenesis; mice, after just one aerobic session, showed dramatic boosts in BDNF. A 2018 review elaborates on these findings.
Eat right
Diet mediates dementia risk through less direct, but very effective, pathways, Dr. Baker said. Diets rich in vegetables, berries, nuts, fish, lean proteins, and healthy fats improves virtually all metabolic measures. These, in turn, reduce the risk cerebrovascular disease – an important driver of vascular dementias and a contributor to Alzheimer’s disease risk as well.
The MIND diet study (Mediterranean-DASH Diet Intervention for Neurodegenerative Delay), reported in 2015 was a very successful demonstration of this concept. A combination of the Mediterranean diet and the DASH diet (Dietary Approaches to Stop Hypertension), the MIND diet stresses frequent consumption of vegetables – especially leafy greens – as well as nuts, berries, whole grains, fish, poultry, and wine or grape juice. In the large, nearly 5-year study of 923 subjects aged 58-98 years, the MIND diet was associated with significant gains in cognition – equivalent to a 7-year reversal of age. After 4.5 years, those who strictly adhered to the diet had a 53% reduction in risk for Alzheimer’s disease, and those who adhered moderately had a 35% reduction. And in a more recent Australian longitudinal study, the MIND diet was associated with a 53% reduced risk of cognitive impairment over 12 years.
Ketogenic diets also may exert a benefit. Theoretically, a state of ketosis forces the brain to burn ketones as an alternative fuel to glucose, thus boosting brain function in glucose-starved brains. A small pilot study with exploratory cognitive endpoints determined that diet-compliant subjects with mild to moderate Alzheimer’s experienced a mean 5-point improvement in the Alzheimer’s Disease Assessment Score–Cognition. They reverted to baseline scores within a month of ending the study.
Recent initial work into the gut microbiome provides some additional speculative, but interesting, data. A dysregulated microbiome can shift microbial populations toward a more inflammatory profile. Some work suggests that inflammatory cytokines then travel to the brain and induce a hyperresponse of neuron-damaging immune cells. A comprehensive review article discusses the complicated mechanisms that may be in play.
Play with your friends
Cognitive stimulation and social interaction also appear to modify dementia risk, although the data are a little more limited. But personal interaction is a key element of Dr. Baker’s ongoing EXERT trial.
The ongoing phase 3 trial randomized 300 adults with amnestic mild cognitive impairment to moderate to high intensity aerobic exercise plus one-on-one support at local YMCA gyms or a low-intensity stretching, balance, and range of motion program. In additional to cognitive testing, the trial includes brain imaging, cerebrospinal fluid sampling for biomarkers of Alzheimer’s disease, and a sleep study.
A key component is personal interaction with a trainer. “They spend a lot of one-on-one time with each person,” Dr. Baker said. “For me, that’s the crucial ingredient – that personal touch. It’s what helps people move from Point A to Point B in their behaviors.”
Virtual cognitive stimulation is also a burgeoning area of dementia prevention research right now. Numerous studies are ongoing to test whether virtual reality or other computer-based games might keep the mind sharp or even improve cognition in people at risk.
The power of three
If one lifestyle change can reduce dementia risk, what happens when all three work together?
That’s the newest question, first successfully explored in the mid-2000s, with the FINGER study (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability). In FINGER, the triad of exercise, personal support at the gym, and a modified Mediterranean diet reduced Alzheimer’s disease risk and improved cognition relative to the control group.
FINGER showed that the intervention was feasible and that it was associated with cognitive preservation and reduced Alzheimer’s disease risk in a group of at-risk subjects. The active group also had a 25% greater improvement on a neuropsychological test battery relative to the control group. They also performed 150% better in processing speed, 83% better in executive function, and 40% better in short-term memory. They showed no increased risk of cognitive decline relative to the control group, which experienced a 30% increase in risk, according to lead investigator Miia Kivipelto, PhD, of the Karolinska Institute, Stockholm.
So successful was FINGER that it launched a global consortium of related studies called World Wide FINGERS. Active in six countries now, including the United States, the studies aim to discover whether such combinations of lifestyle interventions are workable across countries and cultures. World Wide FINGERS is largely supported by the Alzheimer’s Association.
Global enthusiasm for lifestyle interventions
In recognition of the importance of lifestyle changes for dementia prevention, the World Health Organization recently published “Risk reduction of cognitive decline and dementia.” The document reviews many studies and makes recommendations regarding not only exercise, diet, and cognitive stimulation, but also smoking and alcohol.
Research interest in these areas is surging, Dr. Baker said. “The [U.S.] National Institute on Aging now has 29 ongoing trials. There’s a strong commitment to investigations into how lifestyle interventions could protect brain health as we get older. Certainly, many fit and healthy people do develop Alzheimer’s. But for some, it could be medicine.”
But no matter how compliant people are, lifestyle changes will never completely rid the world of Alzheimer’s and other dementias. The view of Dr. Baker – and most other Alzheimer’s researchers – is to employ lifestyle changes to reduce risk as much as possible and not to stop when cognitive problems do present.
“We need to understand how lifestyle interventions might work in combination with pharmaceuticals,” she said. “If we can support the health of the body and the health of the mind, lifestyle interventions can be the fertilizer that would help drug therapy have its maximum effect.”
SAN DIEGO – To prevent dementia, follow Mom’s advice: Get up off the couch, go play with your friends, and eat your vegetables.
After 15 years of disappointing drug trials, strong new evidence says the best way to attack Alzheimer’s disease is not to treat it once it develops, but to prevent it in the first place, Laura D. Baker, PhD, said at the Clinical Trials on Alzheimer’s Disease conference. Studies of exercise, cognitive and social stimulation, and diet show that each one can reduce the risk of dementia, and that a combination of all three may have even a more powerful and synergistic effect.
“We have become absolutely phobic of exercise,” said Dr. Baker of Wake Forest University, Winston-Salem, N.C. And it’s not just structured exercise we shirk. “We take the closest parking space, sit for hours on end, don’t even take the stairs. Yet we know from years of work that exercise has a powerful benefit on cardiovascular disease, lipid profiles, metabolic disease, stress, and mood. Now we are seeing that exercise also promotes brain health in normal aging and protects against cognitive decline and prevention.”
Get off the couch
The general benefits of exercise – chiefly aerobic exercise – are myriad, Dr. Baker said.
“Exercise increases effective neurorepair. It reduces oxidative stress. It improves insulin sensitivity and helps with maintaining normal weight. It reduces inflammation and increases normal clearance of amyloid-beta.”
A 2017 meta-analysis reviewed some of these findings. “The current review [of 16 studies] suggests that aerobic exercise may have positive effects on the right hippocampus and potentially beneficial effects on the overall and other parts of the hippocampus, the cingulate cortex, and the medial temporal areas. ... Moreover, aerobic exercise may increase functional connectivity or activation in the hippocampus, cingulate cortex, and parahippocampal gyrus regions,” wrote Mo-yi Li, PhD, of Fujian University of Traditional Chinese Medicine, Fuzhou, China, and colleagues.
Exercise increases brain-derived neurotrophic factor (BDNF), which in turn increases neuronal potentiation and synaptic plasticity. BDNF is also important in hippocampal neurogenesis; mice, after just one aerobic session, showed dramatic boosts in BDNF. A 2018 review elaborates on these findings.
Eat right
Diet mediates dementia risk through less direct, but very effective, pathways, Dr. Baker said. Diets rich in vegetables, berries, nuts, fish, lean proteins, and healthy fats improves virtually all metabolic measures. These, in turn, reduce the risk cerebrovascular disease – an important driver of vascular dementias and a contributor to Alzheimer’s disease risk as well.
The MIND diet study (Mediterranean-DASH Diet Intervention for Neurodegenerative Delay), reported in 2015 was a very successful demonstration of this concept. A combination of the Mediterranean diet and the DASH diet (Dietary Approaches to Stop Hypertension), the MIND diet stresses frequent consumption of vegetables – especially leafy greens – as well as nuts, berries, whole grains, fish, poultry, and wine or grape juice. In the large, nearly 5-year study of 923 subjects aged 58-98 years, the MIND diet was associated with significant gains in cognition – equivalent to a 7-year reversal of age. After 4.5 years, those who strictly adhered to the diet had a 53% reduction in risk for Alzheimer’s disease, and those who adhered moderately had a 35% reduction. And in a more recent Australian longitudinal study, the MIND diet was associated with a 53% reduced risk of cognitive impairment over 12 years.
Ketogenic diets also may exert a benefit. Theoretically, a state of ketosis forces the brain to burn ketones as an alternative fuel to glucose, thus boosting brain function in glucose-starved brains. A small pilot study with exploratory cognitive endpoints determined that diet-compliant subjects with mild to moderate Alzheimer’s experienced a mean 5-point improvement in the Alzheimer’s Disease Assessment Score–Cognition. They reverted to baseline scores within a month of ending the study.
Recent initial work into the gut microbiome provides some additional speculative, but interesting, data. A dysregulated microbiome can shift microbial populations toward a more inflammatory profile. Some work suggests that inflammatory cytokines then travel to the brain and induce a hyperresponse of neuron-damaging immune cells. A comprehensive review article discusses the complicated mechanisms that may be in play.
Play with your friends
Cognitive stimulation and social interaction also appear to modify dementia risk, although the data are a little more limited. But personal interaction is a key element of Dr. Baker’s ongoing EXERT trial.
The ongoing phase 3 trial randomized 300 adults with amnestic mild cognitive impairment to moderate to high intensity aerobic exercise plus one-on-one support at local YMCA gyms or a low-intensity stretching, balance, and range of motion program. In additional to cognitive testing, the trial includes brain imaging, cerebrospinal fluid sampling for biomarkers of Alzheimer’s disease, and a sleep study.
A key component is personal interaction with a trainer. “They spend a lot of one-on-one time with each person,” Dr. Baker said. “For me, that’s the crucial ingredient – that personal touch. It’s what helps people move from Point A to Point B in their behaviors.”
Virtual cognitive stimulation is also a burgeoning area of dementia prevention research right now. Numerous studies are ongoing to test whether virtual reality or other computer-based games might keep the mind sharp or even improve cognition in people at risk.
The power of three
If one lifestyle change can reduce dementia risk, what happens when all three work together?
That’s the newest question, first successfully explored in the mid-2000s, with the FINGER study (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability). In FINGER, the triad of exercise, personal support at the gym, and a modified Mediterranean diet reduced Alzheimer’s disease risk and improved cognition relative to the control group.
FINGER showed that the intervention was feasible and that it was associated with cognitive preservation and reduced Alzheimer’s disease risk in a group of at-risk subjects. The active group also had a 25% greater improvement on a neuropsychological test battery relative to the control group. They also performed 150% better in processing speed, 83% better in executive function, and 40% better in short-term memory. They showed no increased risk of cognitive decline relative to the control group, which experienced a 30% increase in risk, according to lead investigator Miia Kivipelto, PhD, of the Karolinska Institute, Stockholm.
So successful was FINGER that it launched a global consortium of related studies called World Wide FINGERS. Active in six countries now, including the United States, the studies aim to discover whether such combinations of lifestyle interventions are workable across countries and cultures. World Wide FINGERS is largely supported by the Alzheimer’s Association.
Global enthusiasm for lifestyle interventions
In recognition of the importance of lifestyle changes for dementia prevention, the World Health Organization recently published “Risk reduction of cognitive decline and dementia.” The document reviews many studies and makes recommendations regarding not only exercise, diet, and cognitive stimulation, but also smoking and alcohol.
Research interest in these areas is surging, Dr. Baker said. “The [U.S.] National Institute on Aging now has 29 ongoing trials. There’s a strong commitment to investigations into how lifestyle interventions could protect brain health as we get older. Certainly, many fit and healthy people do develop Alzheimer’s. But for some, it could be medicine.”
But no matter how compliant people are, lifestyle changes will never completely rid the world of Alzheimer’s and other dementias. The view of Dr. Baker – and most other Alzheimer’s researchers – is to employ lifestyle changes to reduce risk as much as possible and not to stop when cognitive problems do present.
“We need to understand how lifestyle interventions might work in combination with pharmaceuticals,” she said. “If we can support the health of the body and the health of the mind, lifestyle interventions can be the fertilizer that would help drug therapy have its maximum effect.”
EXPERT ANALYSIS FROM CTAD 2019
Pimavanserin reduced dementia-related psychotic symptoms without affecting cognition
SAN DIEGO – Pimavanserin, a second-generation antipsychotic approved for hallucinations and delusions in patients with Parkinson’s disease, may also be helpful for psychotic symptoms in other dementia patients, Erin P. Foff, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
In fact, the phase 3 HARMONY trial was stopped early, after an interim efficacy analysis determined that treatment with pimavanserin (Nuplazid) had achieved its primary endpoint – a statistically significant threefold reduction in the risk of relapse (P less than .0033).
Importantly, pimavanserin didn’t significantly affect cognition nor, at least in this controlled setting, did it appear to increase falls or other adverse events often seen with antipsychotic use in elderly patients, said Dr. Foff, clinical lead for the dementia-related psychosis program at Acadia Pharmaceuticals, which makes the drug and sponsored the study.
Based on the positive results, Acadia intends to submit a supplemental new drug application for this indication, according to an investor presentation posted on the company website.
“There is a critical need for an intervention [for psychosis symptoms] in this population,” Dr. Foff said. “We saw a robust response that was well tolerated and well maintained with no negative impact on cognitive scores.”
The second-generation antipsychotic was approved in 2016 for treating hallucinations and delusions in patients with Parkinson’s disease.
The drug is a selective antagonist of 5-HT2 receptors, with low affinity for dopamine receptors. This slightly differentiates it from other second-generation antipsychotics that affect dopamine receptors as well as 5-HT2 receptors.
HARMONY was not a typical placebo-controlled, randomized efficacy trial. Rather, it employed a two-phase design: an open-label treatment response period followed by a placebo-controlled randomization limited to open-label responders. Overall, HARMONY involved 392 patients with mild to severe dementia of numerous etiologies, including Alzheimer’s disease (66.8%), Parkinson’s disease dementia (14.3%), frontotemporal dementia (1.8%), vascular dementia (9.7%), and dementia with Lewy bodies (7.4%). All patients entered a 12-week, open-label period during which they received pimavanserin 34 mg daily. The primary endpoint was a combination of least a 30% reduction on the total Scale for the Assessment of Positive Symptom–Hallucinations and Delusions (SAPS-HD) scale plus a score of 1-2 on the Clinical Global Impressions–Improvement (CGI-I) scale, meaning better or very much better.
At 12 weeks, all responders were then randomized to placebo or continued therapy for 26 weeks. The primary endpoint was relapse, defined as at least a 30% worsening of the SAPS-HD relative to open-label baseline, plus a CGI-I score of 6-7 (worse or very much worse).
Patients were aged a mean of 74 years. Most (about 90%) were living at home. Visual hallucinations occurred in 80% and delusions in 83%. At baseline, the mean SAPS-HD score was 24.4, and the mean CGI-Severity score was 4.7. The mean Mini-Mental State Exam (MMSE) score was 16.7.
In the open-label period, pimavanserin reduced the SAPS-HD score at 12 weeks by a mean of 75%. Symptoms began to decline in the first week of treatment, with continuing improvement throughout the treatment period. By week 4, 30% had hit the response target. This number increased steadily, with 51% responding by week 4, 75% by week 8, and 88% by week 12.
By probable diagnosis, response rates were 59.8% in Alzheimer’s patients, 45.5% for those with Lewy body dementia, 71.2% among patients with Parkinson’s disease, 71% in patients with vascular dementia, and 50% in patients with frontotemporal dementia. In the final analysis, 80% of patients overall were considered responders.
The randomized potion began immediately thereafter with no washout period. About 62% (194) of the entire cohort – all responders – entered into the placebo-controlled phase. The remaining patients were either not responders (20%), dropped out because of an adverse event (7.7%), or left the study for unspecified reasons (10%). There was one death, which was not related to the study medication. A total of 41 patients were still being treated when the study was discontinued, and they were excluded from the final analysis.
When the randomized study ended, relapses had occurred in 28.3% of those taking placebo and in 12.6% of those taking pimavanserin – a statistically significant difference (hazard ratio, 0.353). This translated to a 180% reduction in relapse.
The rate of adverse events was similar in both active and placebo groups (41% vs. 36.6%). Serious adverse events occurred in 4.8% and 3.6%, respectively. The most commonly reported adverse events were headache (9.5% vs. 4.5%) and urinary tract infection (6.7% vs. 3.6%). Asthenia occurred in 2.9% of treated patients and 0.9% of placebo patients, but no falls were reported. Anxiety and dizziness were also reported in three patients taking the study medication.
Three patients (2.9%) experienced a prolonged QT phase on ECG, with a mean delay of 5.4 milliseconds from baseline. “Pimavanserin is known to have this effect of QT prolongation,” Dr. Foff said. “This 5.4-ms change is exactly in line with what we already know about pimavanserin and is not clinically significant. We saw no effect on motor function, consistent with the mechanism of action, and very low levels of agitation or aggression.”
Pimavanserin didn’t significantly change cognition from baseline in the open-label period, and in the randomized period, MMSE never differed significantly between groups.
The company also conducted an exploratory subgroup analysis that looked at placebo versus pimavanserin relapse by probable clinical diagnosis. Among the types of dementia, relapse rates for placebo versus pimavanserin were 23% versus 13% among Alzheimer’s patients, 67% versus 0% in Lewy body dementia patients, 50% versus 7% in patients with Parkinson’s, and 17% each among vascular dementia patients. Only one patient in the randomized period had frontotemporal dementia, and that patient relapsed on treatment.
Whether pimavanserin is effective specifically for psychosis in Alzheimer’s disease patients, however, remains in question. In 2018, Acadia published a negative phase 2 trial in a targeted group of 181 Alzheimer’s patients. The primary outcome in each study was mean change on the Neuropsychiatric Inventory–Nursing Home Version psychosis score (NPI-NH-PS). Clive Ballard, MD, of the University of Exeter (England), was the primary investigator.
After 6 weeks, those taking pimavanserin had a 3.76-point change in the NPI-NH-PS, compared with a 1.93-point change in the placebo group. The mean 1.84-point difference was not statistically significant.
This Alzheimer’s-only cohort group also experienced more adverse events than the HARMONY mixed-diagnosis cohort did, although the differences between pimavanserin and placebo groups were not significant. Adverse events included falls (23% of each group) and agitation (21% with pimavanserin vs. 14% with placebo). Cognition was unaffected.
Later that year, Acadia published a subgroup analysis of the same cohort parsing response by symptom severity, again with Dr. Ballard as the lead investigator.
The analysis focused on 57 patients with a baseline NPI-NH-PS of at least 12, indicating severe symptoms of psychosis.
Treatment effects were more pronounced in this group, significantly favoring pimavanserin. On the NPI-NH-PS, 88.9% of the pimavanserin group and 43.3% of the placebo group had at least a 30% improvement; 77.8% and 43.3% experienced at least a 50% improvement. The rate of serious adverse events was similar (18% with pimavanserin and 17% with placebo) and cognition was unaffected. Falls occurred in 14% of the treated group and 20% of the placebo group.
“These findings coupled with the results from other studies of pimavanserin suggest a potential role for pimavanserin in treating psychosis in patients across a range of neuropsychiatric conditions,” Dr. Ballard wrote.
SOURCE: Foff EP et al. CTAD 2019, Late-breaker 1
SAN DIEGO – Pimavanserin, a second-generation antipsychotic approved for hallucinations and delusions in patients with Parkinson’s disease, may also be helpful for psychotic symptoms in other dementia patients, Erin P. Foff, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
In fact, the phase 3 HARMONY trial was stopped early, after an interim efficacy analysis determined that treatment with pimavanserin (Nuplazid) had achieved its primary endpoint – a statistically significant threefold reduction in the risk of relapse (P less than .0033).
Importantly, pimavanserin didn’t significantly affect cognition nor, at least in this controlled setting, did it appear to increase falls or other adverse events often seen with antipsychotic use in elderly patients, said Dr. Foff, clinical lead for the dementia-related psychosis program at Acadia Pharmaceuticals, which makes the drug and sponsored the study.
Based on the positive results, Acadia intends to submit a supplemental new drug application for this indication, according to an investor presentation posted on the company website.
“There is a critical need for an intervention [for psychosis symptoms] in this population,” Dr. Foff said. “We saw a robust response that was well tolerated and well maintained with no negative impact on cognitive scores.”
The second-generation antipsychotic was approved in 2016 for treating hallucinations and delusions in patients with Parkinson’s disease.
The drug is a selective antagonist of 5-HT2 receptors, with low affinity for dopamine receptors. This slightly differentiates it from other second-generation antipsychotics that affect dopamine receptors as well as 5-HT2 receptors.
HARMONY was not a typical placebo-controlled, randomized efficacy trial. Rather, it employed a two-phase design: an open-label treatment response period followed by a placebo-controlled randomization limited to open-label responders. Overall, HARMONY involved 392 patients with mild to severe dementia of numerous etiologies, including Alzheimer’s disease (66.8%), Parkinson’s disease dementia (14.3%), frontotemporal dementia (1.8%), vascular dementia (9.7%), and dementia with Lewy bodies (7.4%). All patients entered a 12-week, open-label period during which they received pimavanserin 34 mg daily. The primary endpoint was a combination of least a 30% reduction on the total Scale for the Assessment of Positive Symptom–Hallucinations and Delusions (SAPS-HD) scale plus a score of 1-2 on the Clinical Global Impressions–Improvement (CGI-I) scale, meaning better or very much better.
At 12 weeks, all responders were then randomized to placebo or continued therapy for 26 weeks. The primary endpoint was relapse, defined as at least a 30% worsening of the SAPS-HD relative to open-label baseline, plus a CGI-I score of 6-7 (worse or very much worse).
Patients were aged a mean of 74 years. Most (about 90%) were living at home. Visual hallucinations occurred in 80% and delusions in 83%. At baseline, the mean SAPS-HD score was 24.4, and the mean CGI-Severity score was 4.7. The mean Mini-Mental State Exam (MMSE) score was 16.7.
In the open-label period, pimavanserin reduced the SAPS-HD score at 12 weeks by a mean of 75%. Symptoms began to decline in the first week of treatment, with continuing improvement throughout the treatment period. By week 4, 30% had hit the response target. This number increased steadily, with 51% responding by week 4, 75% by week 8, and 88% by week 12.
By probable diagnosis, response rates were 59.8% in Alzheimer’s patients, 45.5% for those with Lewy body dementia, 71.2% among patients with Parkinson’s disease, 71% in patients with vascular dementia, and 50% in patients with frontotemporal dementia. In the final analysis, 80% of patients overall were considered responders.
The randomized potion began immediately thereafter with no washout period. About 62% (194) of the entire cohort – all responders – entered into the placebo-controlled phase. The remaining patients were either not responders (20%), dropped out because of an adverse event (7.7%), or left the study for unspecified reasons (10%). There was one death, which was not related to the study medication. A total of 41 patients were still being treated when the study was discontinued, and they were excluded from the final analysis.
When the randomized study ended, relapses had occurred in 28.3% of those taking placebo and in 12.6% of those taking pimavanserin – a statistically significant difference (hazard ratio, 0.353). This translated to a 180% reduction in relapse.
The rate of adverse events was similar in both active and placebo groups (41% vs. 36.6%). Serious adverse events occurred in 4.8% and 3.6%, respectively. The most commonly reported adverse events were headache (9.5% vs. 4.5%) and urinary tract infection (6.7% vs. 3.6%). Asthenia occurred in 2.9% of treated patients and 0.9% of placebo patients, but no falls were reported. Anxiety and dizziness were also reported in three patients taking the study medication.
Three patients (2.9%) experienced a prolonged QT phase on ECG, with a mean delay of 5.4 milliseconds from baseline. “Pimavanserin is known to have this effect of QT prolongation,” Dr. Foff said. “This 5.4-ms change is exactly in line with what we already know about pimavanserin and is not clinically significant. We saw no effect on motor function, consistent with the mechanism of action, and very low levels of agitation or aggression.”
Pimavanserin didn’t significantly change cognition from baseline in the open-label period, and in the randomized period, MMSE never differed significantly between groups.
The company also conducted an exploratory subgroup analysis that looked at placebo versus pimavanserin relapse by probable clinical diagnosis. Among the types of dementia, relapse rates for placebo versus pimavanserin were 23% versus 13% among Alzheimer’s patients, 67% versus 0% in Lewy body dementia patients, 50% versus 7% in patients with Parkinson’s, and 17% each among vascular dementia patients. Only one patient in the randomized period had frontotemporal dementia, and that patient relapsed on treatment.
Whether pimavanserin is effective specifically for psychosis in Alzheimer’s disease patients, however, remains in question. In 2018, Acadia published a negative phase 2 trial in a targeted group of 181 Alzheimer’s patients. The primary outcome in each study was mean change on the Neuropsychiatric Inventory–Nursing Home Version psychosis score (NPI-NH-PS). Clive Ballard, MD, of the University of Exeter (England), was the primary investigator.
After 6 weeks, those taking pimavanserin had a 3.76-point change in the NPI-NH-PS, compared with a 1.93-point change in the placebo group. The mean 1.84-point difference was not statistically significant.
This Alzheimer’s-only cohort group also experienced more adverse events than the HARMONY mixed-diagnosis cohort did, although the differences between pimavanserin and placebo groups were not significant. Adverse events included falls (23% of each group) and agitation (21% with pimavanserin vs. 14% with placebo). Cognition was unaffected.
Later that year, Acadia published a subgroup analysis of the same cohort parsing response by symptom severity, again with Dr. Ballard as the lead investigator.
The analysis focused on 57 patients with a baseline NPI-NH-PS of at least 12, indicating severe symptoms of psychosis.
Treatment effects were more pronounced in this group, significantly favoring pimavanserin. On the NPI-NH-PS, 88.9% of the pimavanserin group and 43.3% of the placebo group had at least a 30% improvement; 77.8% and 43.3% experienced at least a 50% improvement. The rate of serious adverse events was similar (18% with pimavanserin and 17% with placebo) and cognition was unaffected. Falls occurred in 14% of the treated group and 20% of the placebo group.
“These findings coupled with the results from other studies of pimavanserin suggest a potential role for pimavanserin in treating psychosis in patients across a range of neuropsychiatric conditions,” Dr. Ballard wrote.
SOURCE: Foff EP et al. CTAD 2019, Late-breaker 1
SAN DIEGO – Pimavanserin, a second-generation antipsychotic approved for hallucinations and delusions in patients with Parkinson’s disease, may also be helpful for psychotic symptoms in other dementia patients, Erin P. Foff, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
In fact, the phase 3 HARMONY trial was stopped early, after an interim efficacy analysis determined that treatment with pimavanserin (Nuplazid) had achieved its primary endpoint – a statistically significant threefold reduction in the risk of relapse (P less than .0033).
Importantly, pimavanserin didn’t significantly affect cognition nor, at least in this controlled setting, did it appear to increase falls or other adverse events often seen with antipsychotic use in elderly patients, said Dr. Foff, clinical lead for the dementia-related psychosis program at Acadia Pharmaceuticals, which makes the drug and sponsored the study.
Based on the positive results, Acadia intends to submit a supplemental new drug application for this indication, according to an investor presentation posted on the company website.
“There is a critical need for an intervention [for psychosis symptoms] in this population,” Dr. Foff said. “We saw a robust response that was well tolerated and well maintained with no negative impact on cognitive scores.”
The second-generation antipsychotic was approved in 2016 for treating hallucinations and delusions in patients with Parkinson’s disease.
The drug is a selective antagonist of 5-HT2 receptors, with low affinity for dopamine receptors. This slightly differentiates it from other second-generation antipsychotics that affect dopamine receptors as well as 5-HT2 receptors.
HARMONY was not a typical placebo-controlled, randomized efficacy trial. Rather, it employed a two-phase design: an open-label treatment response period followed by a placebo-controlled randomization limited to open-label responders. Overall, HARMONY involved 392 patients with mild to severe dementia of numerous etiologies, including Alzheimer’s disease (66.8%), Parkinson’s disease dementia (14.3%), frontotemporal dementia (1.8%), vascular dementia (9.7%), and dementia with Lewy bodies (7.4%). All patients entered a 12-week, open-label period during which they received pimavanserin 34 mg daily. The primary endpoint was a combination of least a 30% reduction on the total Scale for the Assessment of Positive Symptom–Hallucinations and Delusions (SAPS-HD) scale plus a score of 1-2 on the Clinical Global Impressions–Improvement (CGI-I) scale, meaning better or very much better.
At 12 weeks, all responders were then randomized to placebo or continued therapy for 26 weeks. The primary endpoint was relapse, defined as at least a 30% worsening of the SAPS-HD relative to open-label baseline, plus a CGI-I score of 6-7 (worse or very much worse).
Patients were aged a mean of 74 years. Most (about 90%) were living at home. Visual hallucinations occurred in 80% and delusions in 83%. At baseline, the mean SAPS-HD score was 24.4, and the mean CGI-Severity score was 4.7. The mean Mini-Mental State Exam (MMSE) score was 16.7.
In the open-label period, pimavanserin reduced the SAPS-HD score at 12 weeks by a mean of 75%. Symptoms began to decline in the first week of treatment, with continuing improvement throughout the treatment period. By week 4, 30% had hit the response target. This number increased steadily, with 51% responding by week 4, 75% by week 8, and 88% by week 12.
By probable diagnosis, response rates were 59.8% in Alzheimer’s patients, 45.5% for those with Lewy body dementia, 71.2% among patients with Parkinson’s disease, 71% in patients with vascular dementia, and 50% in patients with frontotemporal dementia. In the final analysis, 80% of patients overall were considered responders.
The randomized potion began immediately thereafter with no washout period. About 62% (194) of the entire cohort – all responders – entered into the placebo-controlled phase. The remaining patients were either not responders (20%), dropped out because of an adverse event (7.7%), or left the study for unspecified reasons (10%). There was one death, which was not related to the study medication. A total of 41 patients were still being treated when the study was discontinued, and they were excluded from the final analysis.
When the randomized study ended, relapses had occurred in 28.3% of those taking placebo and in 12.6% of those taking pimavanserin – a statistically significant difference (hazard ratio, 0.353). This translated to a 180% reduction in relapse.
The rate of adverse events was similar in both active and placebo groups (41% vs. 36.6%). Serious adverse events occurred in 4.8% and 3.6%, respectively. The most commonly reported adverse events were headache (9.5% vs. 4.5%) and urinary tract infection (6.7% vs. 3.6%). Asthenia occurred in 2.9% of treated patients and 0.9% of placebo patients, but no falls were reported. Anxiety and dizziness were also reported in three patients taking the study medication.
Three patients (2.9%) experienced a prolonged QT phase on ECG, with a mean delay of 5.4 milliseconds from baseline. “Pimavanserin is known to have this effect of QT prolongation,” Dr. Foff said. “This 5.4-ms change is exactly in line with what we already know about pimavanserin and is not clinically significant. We saw no effect on motor function, consistent with the mechanism of action, and very low levels of agitation or aggression.”
Pimavanserin didn’t significantly change cognition from baseline in the open-label period, and in the randomized period, MMSE never differed significantly between groups.
The company also conducted an exploratory subgroup analysis that looked at placebo versus pimavanserin relapse by probable clinical diagnosis. Among the types of dementia, relapse rates for placebo versus pimavanserin were 23% versus 13% among Alzheimer’s patients, 67% versus 0% in Lewy body dementia patients, 50% versus 7% in patients with Parkinson’s, and 17% each among vascular dementia patients. Only one patient in the randomized period had frontotemporal dementia, and that patient relapsed on treatment.
Whether pimavanserin is effective specifically for psychosis in Alzheimer’s disease patients, however, remains in question. In 2018, Acadia published a negative phase 2 trial in a targeted group of 181 Alzheimer’s patients. The primary outcome in each study was mean change on the Neuropsychiatric Inventory–Nursing Home Version psychosis score (NPI-NH-PS). Clive Ballard, MD, of the University of Exeter (England), was the primary investigator.
After 6 weeks, those taking pimavanserin had a 3.76-point change in the NPI-NH-PS, compared with a 1.93-point change in the placebo group. The mean 1.84-point difference was not statistically significant.
This Alzheimer’s-only cohort group also experienced more adverse events than the HARMONY mixed-diagnosis cohort did, although the differences between pimavanserin and placebo groups were not significant. Adverse events included falls (23% of each group) and agitation (21% with pimavanserin vs. 14% with placebo). Cognition was unaffected.
Later that year, Acadia published a subgroup analysis of the same cohort parsing response by symptom severity, again with Dr. Ballard as the lead investigator.
The analysis focused on 57 patients with a baseline NPI-NH-PS of at least 12, indicating severe symptoms of psychosis.
Treatment effects were more pronounced in this group, significantly favoring pimavanserin. On the NPI-NH-PS, 88.9% of the pimavanserin group and 43.3% of the placebo group had at least a 30% improvement; 77.8% and 43.3% experienced at least a 50% improvement. The rate of serious adverse events was similar (18% with pimavanserin and 17% with placebo) and cognition was unaffected. Falls occurred in 14% of the treated group and 20% of the placebo group.
“These findings coupled with the results from other studies of pimavanserin suggest a potential role for pimavanserin in treating psychosis in patients across a range of neuropsychiatric conditions,” Dr. Ballard wrote.
SOURCE: Foff EP et al. CTAD 2019, Late-breaker 1
REPORTING FROM CTAD 2019