User login
Positive functional results reported for aducanumab in a pooled, post hoc analysis
SAN DIEGO – Positive findings from a post hoc subanalysis of two unsuccessful studies represent “a major step forward in Alzheimer’s disease research” and could set the antiamyloid antibody up as a “foothold” in slowing disease progression, study investigators said at the Clinical Trials on Alzheimer’s Disease conference.
After full follow-up of 78 weeks, patients with mild Alzheimer’s disease (AD) who took the highest 10-mg/kg dose for a full 14 doses experienced up to a 53% slowing of functional decline on the Clinical Dementia Rating–Sum of Boxes (CDR-SB) in one study and a 48% slowing in the other study – relative to placebo – a result that might give them “an extra year or 2” of independence; they might perhaps retain the ability to drive and even stay employed, said Sharon Cohen, MD, a panelist at the meeting’s aducanumab presentation session and a clinical investigator in EMERGE, one of two phase 3 studies from which the data were derived.
Samantha Budd Haeberlein, PhD, Biogen’s vice president and head of late-stage clinical development in Alzheimer’s disease, presented the new data. They “are complex” and require much more study before investigators, clinicians, and federal regulators can fully embrace them, said the panelists who discussed the results. Nevertheless, Biogen, which is codeveloping the antibody with partner Eisai, said in October it will put aducanumab forward to the Food and Drug Administration in a new drug application for the first-ever AD disease-modifying agent. FDA regulators have said they will review the data.
The new subanalysis comprised 570 of 3,285 patients in two identical studies with negative primary endpoint results. One, ENGAGE, failed to reach both its primary and secondary endpoints; the other, EMERGE, was halted last spring after a futility analysis determined that aducanumab was unlikely to confer significant benefit. The post hoc subanalysis looked at a combined subset of those who received the highest 10-mg/kg dose for the full 78 weeks of each trial. The statistically significant functional endpoints occurred in this group, comprised largely of apolipoprotein E epsilon-4 (APOE4) allele carriers.
“The futility analysis of EMERGE was highly unfortunate,” said panelist Paul Aisen, MD, founding director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles. “Clearly in the final analysis, EMERGE was positive in the primary endpoints, and now the secondary analysis of both studies is positive and consistent.” The diverging trajectory of placebo and treatment groups continued to the end of follow-up in both studies, a finding that at least suggests continuing improvement, he added.
Biogen undertook the pooled analysis after ENGAGE’s futility analysis. Early in the development program, concern about amyloid-related imaging abnormalities (ARIA) in APOE4 carriers led Biogen to stratify doses in that group.
“When we started [creating aducanumab trials], we stratified the dose so that e4 carriers had the lowest dose, but in PRIME [the phase 1b study], we saw the best result from the 10-mg/kg dose, so we believed that was important for efficacy. However, we didn’t have sufficient evidence to believe that it was safe to put carriers on that dose. In EMERGE, we saw that carriers could safely take it until the end of the study.”
Since the trials were running almost synchronously, a new version of randomization ensued. This allowed more e4 carriers to go forward on the 10-mg/kg dose.
“I would not normally recommend changing dose in the middle of a phase 3 trial, but it did have a real impact in the high-dose group,” Dr. Haeberlein said. Additionally, by the time of data lock after the futility analysis, more patients had completed the entire 78 weeks at the 10-mg/kg dose. Cumulative dosing ended up being quite different in the APOE4 carriers after this new version ensued. Before, the median cumulative dose for both carriers and noncarriers was 116 mg/kg. After the change, the median cumulative dose was 153 mg/kg. And before the alteration, 21% in EMERGE and 15% in ENGAGE received the full 14 possible 10-mg/kg doses. After the change, 51% in EMERGE and 47% in ENGAGE received the full 14 doses of 10 mg/kg.
The pooled analysis comprised this combined group, which was then largely composed of APOE4 carriers.
Imaging confirmed such dose-driven reductions in both brain amyloid plaques and phosphorylated tau. Although amyloid reduction has never been tied to cognitive or functional benefits, tau reduction has been associated with nonsignificant cognitive benefits in prior studies.
In the primary analysis of ENGAGE, aducanumab conferred no cognitive or functional benefit. In EMERGE, there were significant cognitive improvements on both the Mini Mental State Exam score (an 18% slowing of decline relative to placebo) and the Alzheimer’s Disease Assessment Scale cognitive portion (a 27% slowing).
However, the functional improvements seen in the pooled post hoc data “are a big deal,” and probably more meaningful to patients and families than the memory improvements, Dr. Cohen said.
“Those of us who know this disease well know what it means to lose yourself slice by slice, and anything you can hang onto is a triumph,” said Dr. Cohen, medical director and principal investigator of the Toronto Memory Program, an independent medical facility for dementia care and research. “I am pleased with a 27% slowing of cognitive decline, but a 40% slowing of functional decline is what will be really meaningful to patients. This is a long, slow disease, and if we can slow it at all, we’re winning out.”
Safety endpoints, especially ARIA, were not unexpected considering past studies. ARIA occurred in 41% of patients treated with the high aducanumab dose in EMERGE and in 40% in ENGAGE. It was largely asymptomatic (80% in EMERGE and 71% in ENGAGE). Headache was the next most common adverse event, followed by dizziness, visual disturbance, and nausea and vomiting. ARIA generally resolved within 4-6 weeks, and most patients continued their 10-mg/kg dose.
Biogen intends to begin a new study, an open-label nonrandomized trial that will offer the 10-mg/kg dose to all patients in both trials, including those who took placebo. This may provide interesting data regarding redosing patients who were off their successful 10-mg/kg dose for an extended period of time, said Laurie Ryan, PhD, chief of the Dementias of Aging Branch in the Division of Neuroscience at the National Institute on Aging.
“If those in the high-dose group had a regression of their improvements and then improved again when restarted, that would certainly tell us something,” she said in an interview. Likewise, researchers will be carefully looking at any placebo group response. “But we have to remember that this will not be a randomized study,” and will bring with it all the issues that such a study typically carries.
“I agree it’s unfortunate that they had to stop the EMERGE trial,” she said. “It really did complicate the results, even though they are certainly trending in the right way. But we have had a number of post hoc analyses that show APOE4-positive benefiting, or e4-negative benefiting, and these haven’t panned out.”
SAN DIEGO – Positive findings from a post hoc subanalysis of two unsuccessful studies represent “a major step forward in Alzheimer’s disease research” and could set the antiamyloid antibody up as a “foothold” in slowing disease progression, study investigators said at the Clinical Trials on Alzheimer’s Disease conference.
After full follow-up of 78 weeks, patients with mild Alzheimer’s disease (AD) who took the highest 10-mg/kg dose for a full 14 doses experienced up to a 53% slowing of functional decline on the Clinical Dementia Rating–Sum of Boxes (CDR-SB) in one study and a 48% slowing in the other study – relative to placebo – a result that might give them “an extra year or 2” of independence; they might perhaps retain the ability to drive and even stay employed, said Sharon Cohen, MD, a panelist at the meeting’s aducanumab presentation session and a clinical investigator in EMERGE, one of two phase 3 studies from which the data were derived.
Samantha Budd Haeberlein, PhD, Biogen’s vice president and head of late-stage clinical development in Alzheimer’s disease, presented the new data. They “are complex” and require much more study before investigators, clinicians, and federal regulators can fully embrace them, said the panelists who discussed the results. Nevertheless, Biogen, which is codeveloping the antibody with partner Eisai, said in October it will put aducanumab forward to the Food and Drug Administration in a new drug application for the first-ever AD disease-modifying agent. FDA regulators have said they will review the data.
The new subanalysis comprised 570 of 3,285 patients in two identical studies with negative primary endpoint results. One, ENGAGE, failed to reach both its primary and secondary endpoints; the other, EMERGE, was halted last spring after a futility analysis determined that aducanumab was unlikely to confer significant benefit. The post hoc subanalysis looked at a combined subset of those who received the highest 10-mg/kg dose for the full 78 weeks of each trial. The statistically significant functional endpoints occurred in this group, comprised largely of apolipoprotein E epsilon-4 (APOE4) allele carriers.
“The futility analysis of EMERGE was highly unfortunate,” said panelist Paul Aisen, MD, founding director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles. “Clearly in the final analysis, EMERGE was positive in the primary endpoints, and now the secondary analysis of both studies is positive and consistent.” The diverging trajectory of placebo and treatment groups continued to the end of follow-up in both studies, a finding that at least suggests continuing improvement, he added.
Biogen undertook the pooled analysis after ENGAGE’s futility analysis. Early in the development program, concern about amyloid-related imaging abnormalities (ARIA) in APOE4 carriers led Biogen to stratify doses in that group.
“When we started [creating aducanumab trials], we stratified the dose so that e4 carriers had the lowest dose, but in PRIME [the phase 1b study], we saw the best result from the 10-mg/kg dose, so we believed that was important for efficacy. However, we didn’t have sufficient evidence to believe that it was safe to put carriers on that dose. In EMERGE, we saw that carriers could safely take it until the end of the study.”
Since the trials were running almost synchronously, a new version of randomization ensued. This allowed more e4 carriers to go forward on the 10-mg/kg dose.
“I would not normally recommend changing dose in the middle of a phase 3 trial, but it did have a real impact in the high-dose group,” Dr. Haeberlein said. Additionally, by the time of data lock after the futility analysis, more patients had completed the entire 78 weeks at the 10-mg/kg dose. Cumulative dosing ended up being quite different in the APOE4 carriers after this new version ensued. Before, the median cumulative dose for both carriers and noncarriers was 116 mg/kg. After the change, the median cumulative dose was 153 mg/kg. And before the alteration, 21% in EMERGE and 15% in ENGAGE received the full 14 possible 10-mg/kg doses. After the change, 51% in EMERGE and 47% in ENGAGE received the full 14 doses of 10 mg/kg.
The pooled analysis comprised this combined group, which was then largely composed of APOE4 carriers.
Imaging confirmed such dose-driven reductions in both brain amyloid plaques and phosphorylated tau. Although amyloid reduction has never been tied to cognitive or functional benefits, tau reduction has been associated with nonsignificant cognitive benefits in prior studies.
In the primary analysis of ENGAGE, aducanumab conferred no cognitive or functional benefit. In EMERGE, there were significant cognitive improvements on both the Mini Mental State Exam score (an 18% slowing of decline relative to placebo) and the Alzheimer’s Disease Assessment Scale cognitive portion (a 27% slowing).
However, the functional improvements seen in the pooled post hoc data “are a big deal,” and probably more meaningful to patients and families than the memory improvements, Dr. Cohen said.
“Those of us who know this disease well know what it means to lose yourself slice by slice, and anything you can hang onto is a triumph,” said Dr. Cohen, medical director and principal investigator of the Toronto Memory Program, an independent medical facility for dementia care and research. “I am pleased with a 27% slowing of cognitive decline, but a 40% slowing of functional decline is what will be really meaningful to patients. This is a long, slow disease, and if we can slow it at all, we’re winning out.”
Safety endpoints, especially ARIA, were not unexpected considering past studies. ARIA occurred in 41% of patients treated with the high aducanumab dose in EMERGE and in 40% in ENGAGE. It was largely asymptomatic (80% in EMERGE and 71% in ENGAGE). Headache was the next most common adverse event, followed by dizziness, visual disturbance, and nausea and vomiting. ARIA generally resolved within 4-6 weeks, and most patients continued their 10-mg/kg dose.
Biogen intends to begin a new study, an open-label nonrandomized trial that will offer the 10-mg/kg dose to all patients in both trials, including those who took placebo. This may provide interesting data regarding redosing patients who were off their successful 10-mg/kg dose for an extended period of time, said Laurie Ryan, PhD, chief of the Dementias of Aging Branch in the Division of Neuroscience at the National Institute on Aging.
“If those in the high-dose group had a regression of their improvements and then improved again when restarted, that would certainly tell us something,” she said in an interview. Likewise, researchers will be carefully looking at any placebo group response. “But we have to remember that this will not be a randomized study,” and will bring with it all the issues that such a study typically carries.
“I agree it’s unfortunate that they had to stop the EMERGE trial,” she said. “It really did complicate the results, even though they are certainly trending in the right way. But we have had a number of post hoc analyses that show APOE4-positive benefiting, or e4-negative benefiting, and these haven’t panned out.”
SAN DIEGO – Positive findings from a post hoc subanalysis of two unsuccessful studies represent “a major step forward in Alzheimer’s disease research” and could set the antiamyloid antibody up as a “foothold” in slowing disease progression, study investigators said at the Clinical Trials on Alzheimer’s Disease conference.
After full follow-up of 78 weeks, patients with mild Alzheimer’s disease (AD) who took the highest 10-mg/kg dose for a full 14 doses experienced up to a 53% slowing of functional decline on the Clinical Dementia Rating–Sum of Boxes (CDR-SB) in one study and a 48% slowing in the other study – relative to placebo – a result that might give them “an extra year or 2” of independence; they might perhaps retain the ability to drive and even stay employed, said Sharon Cohen, MD, a panelist at the meeting’s aducanumab presentation session and a clinical investigator in EMERGE, one of two phase 3 studies from which the data were derived.
Samantha Budd Haeberlein, PhD, Biogen’s vice president and head of late-stage clinical development in Alzheimer’s disease, presented the new data. They “are complex” and require much more study before investigators, clinicians, and federal regulators can fully embrace them, said the panelists who discussed the results. Nevertheless, Biogen, which is codeveloping the antibody with partner Eisai, said in October it will put aducanumab forward to the Food and Drug Administration in a new drug application for the first-ever AD disease-modifying agent. FDA regulators have said they will review the data.
The new subanalysis comprised 570 of 3,285 patients in two identical studies with negative primary endpoint results. One, ENGAGE, failed to reach both its primary and secondary endpoints; the other, EMERGE, was halted last spring after a futility analysis determined that aducanumab was unlikely to confer significant benefit. The post hoc subanalysis looked at a combined subset of those who received the highest 10-mg/kg dose for the full 78 weeks of each trial. The statistically significant functional endpoints occurred in this group, comprised largely of apolipoprotein E epsilon-4 (APOE4) allele carriers.
“The futility analysis of EMERGE was highly unfortunate,” said panelist Paul Aisen, MD, founding director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles. “Clearly in the final analysis, EMERGE was positive in the primary endpoints, and now the secondary analysis of both studies is positive and consistent.” The diverging trajectory of placebo and treatment groups continued to the end of follow-up in both studies, a finding that at least suggests continuing improvement, he added.
Biogen undertook the pooled analysis after ENGAGE’s futility analysis. Early in the development program, concern about amyloid-related imaging abnormalities (ARIA) in APOE4 carriers led Biogen to stratify doses in that group.
“When we started [creating aducanumab trials], we stratified the dose so that e4 carriers had the lowest dose, but in PRIME [the phase 1b study], we saw the best result from the 10-mg/kg dose, so we believed that was important for efficacy. However, we didn’t have sufficient evidence to believe that it was safe to put carriers on that dose. In EMERGE, we saw that carriers could safely take it until the end of the study.”
Since the trials were running almost synchronously, a new version of randomization ensued. This allowed more e4 carriers to go forward on the 10-mg/kg dose.
“I would not normally recommend changing dose in the middle of a phase 3 trial, but it did have a real impact in the high-dose group,” Dr. Haeberlein said. Additionally, by the time of data lock after the futility analysis, more patients had completed the entire 78 weeks at the 10-mg/kg dose. Cumulative dosing ended up being quite different in the APOE4 carriers after this new version ensued. Before, the median cumulative dose for both carriers and noncarriers was 116 mg/kg. After the change, the median cumulative dose was 153 mg/kg. And before the alteration, 21% in EMERGE and 15% in ENGAGE received the full 14 possible 10-mg/kg doses. After the change, 51% in EMERGE and 47% in ENGAGE received the full 14 doses of 10 mg/kg.
The pooled analysis comprised this combined group, which was then largely composed of APOE4 carriers.
Imaging confirmed such dose-driven reductions in both brain amyloid plaques and phosphorylated tau. Although amyloid reduction has never been tied to cognitive or functional benefits, tau reduction has been associated with nonsignificant cognitive benefits in prior studies.
In the primary analysis of ENGAGE, aducanumab conferred no cognitive or functional benefit. In EMERGE, there were significant cognitive improvements on both the Mini Mental State Exam score (an 18% slowing of decline relative to placebo) and the Alzheimer’s Disease Assessment Scale cognitive portion (a 27% slowing).
However, the functional improvements seen in the pooled post hoc data “are a big deal,” and probably more meaningful to patients and families than the memory improvements, Dr. Cohen said.
“Those of us who know this disease well know what it means to lose yourself slice by slice, and anything you can hang onto is a triumph,” said Dr. Cohen, medical director and principal investigator of the Toronto Memory Program, an independent medical facility for dementia care and research. “I am pleased with a 27% slowing of cognitive decline, but a 40% slowing of functional decline is what will be really meaningful to patients. This is a long, slow disease, and if we can slow it at all, we’re winning out.”
Safety endpoints, especially ARIA, were not unexpected considering past studies. ARIA occurred in 41% of patients treated with the high aducanumab dose in EMERGE and in 40% in ENGAGE. It was largely asymptomatic (80% in EMERGE and 71% in ENGAGE). Headache was the next most common adverse event, followed by dizziness, visual disturbance, and nausea and vomiting. ARIA generally resolved within 4-6 weeks, and most patients continued their 10-mg/kg dose.
Biogen intends to begin a new study, an open-label nonrandomized trial that will offer the 10-mg/kg dose to all patients in both trials, including those who took placebo. This may provide interesting data regarding redosing patients who were off their successful 10-mg/kg dose for an extended period of time, said Laurie Ryan, PhD, chief of the Dementias of Aging Branch in the Division of Neuroscience at the National Institute on Aging.
“If those in the high-dose group had a regression of their improvements and then improved again when restarted, that would certainly tell us something,” she said in an interview. Likewise, researchers will be carefully looking at any placebo group response. “But we have to remember that this will not be a randomized study,” and will bring with it all the issues that such a study typically carries.
“I agree it’s unfortunate that they had to stop the EMERGE trial,” she said. “It really did complicate the results, even though they are certainly trending in the right way. But we have had a number of post hoc analyses that show APOE4-positive benefiting, or e4-negative benefiting, and these haven’t panned out.”
REPORTING FROM CTAD 2019
Key clinical point: A pooled posthoc subanalysis of two unsuccessful phase 3 trials, found that the antiamyloid antibody aducanumab conferred significant functional benefits in patients with mild Alzheimer’s disease who took the highest 10-mg/kg dose for a full 78 weeks.
Major finding: Aducanumab conferred a 53% slowing of functional decline on the Clinical Dementia Rating–Sum of Boxes (CDR-SB) in one study, ENGAGE, and a 48% slowing in the other, EMERGE, relative to placebo.
Study details: The pooled group comprised 570 of 3,285 patients in the two identical ENGAGE and EMERGE studies.
Disclosures: Biogen and Eisai sponsored the studies and are codeveloping aducanumab.
Source: Budd SH et al. CTAD 2019, OC 1-4.
Intensive BP control reduced dementia but increased brain atrophy and hurt cognition
SAN DIEGO – Intensive blood pressure control over 4 years reduced the overall risk of all-cause dementia by 17%, compared with standard care, but in subanalyses of the Systolic Blood Pressure Intervention Trial (SPRINT) it was also associated with significant decreases in cognitive function and total brain volume, researchers said at the Clinical Trials on Alzheimer’s Disease conference.
Whether these between-group differences were clinically meaningful was the topic of some debate, but they were enough to prompt Mary Sano, PhD, to strongly state her reservations.
“The cardiovascular effects of SPRINT were impressive, but I am concerned about minimizing the potentially negative effect on cognition,” said Dr. Sano, professor of psychiatry and director of the Alzheimer’s Disease Research Center at the Icahn School of Medicine at Mount Sinai, New York. “Do I really want to treat a healthy, nonimpaired patient like this if I have to warn them that their cognition might actually get worse? We just cannot minimize this risk. There is very strong evidence that [intensive treatment of blood pressure] might be a step backward in cognition. Would you lower your own blood pressure at a risk of losing some points on your cognition?”
The subanalyses were conducted as part of the SPRINT Memory and Cognition In Decreased Hypertension (SPRINT MIND) substudy, which looked at cardiovascular and mortality outcomes in 9,361 subjects whose hypertension was managed intensively or by standard care (target systolic blood pressure less than 120 mm Hg vs. less than 140 mm Hg). The trial was stopped early because of a 25% reduction in the primary composite cardiovascular disease endpoint and a 27% reduction in all-cause mortality in the intensive-treatment group.
SPRINT MIND examined the risks of incident probable dementia, mild cognitive impairment (MCI), and a composite outcome of both. Intensive control reduced the risk of MCI by 19% and the combined outcome by 15%.
At the conference, SPRINT MIND investigators presented three long-term subanalyses with a median intervention and follow-up time of about 4 years.
Sarah Gaussoin of Wake Forest University, Winston-Salem, N.C., presented unpublished data detailing the effects of intensive control on several dementia subtypes: nonamnestic single domain, nonamnestic multidomain, amnestic single domain, and amnestic multidomain. There were 640 subjects in this analysis.
After a median of 3.3 years of intervention and 5 years of follow-up, there were no differences in the rate of incident probable dementia between the single- and multidomain nonamnestic groups. “We did see a strong 22% decreased risk in single-domain versus multidomain amnestic MCI, however,” she said.
Nicholas Pajewski, PhD, also of Wake Forest University, discussed more detailed cognitive outcomes in SPRINT MIND among 2,900 subjects who had a full battery of cognitive testing at every assessment over 5 years. The outcomes included memory deficit and processing speed.
Dr. Pajewski reported finding no significant difference between the groups in the rates of memory decline in either outcome. But there was a greater rate of decline in processing speed in the intensively treated group, he added. The difference was small but statistically significant.
The difference was largely driven by results of a single cognitive test – the Trail Making Test Part A. “It corresponded to about a 1.25-second increase over 4 years,” in processing speed on this test, Dr. Pajewski said.
There were no between-group differences in any of the other domains explored, including language, executive function, global cognitive function, or the Montreal Cognitive Assessment.
“Obviously, these results are perplexing,” given the overall positive results of SPRINT MIND, he said. “Intensive blood pressure control is a beneficial thing, and we expected to see an effect on memory, or a blunting of decline, and instead we saw some small decrements going the other way. This led us to speculate about what’s going on.”
The trial relied on a narrow definition of MCI that might have affected the outcomes. There was also a very broad range of ages in the study, ranging from 53 to 86 years. More importantly, he said, the original SPRINT study didn’t collect cognitive data at baseline, so there was no way to know how many subjects already might have had MCI when they entered the trial.
Ilya Nasrallah, MD, PhD, of the University of Pennsylvania, Philadelphia, presented MRI data on white-matter lesions, hippocampal volume fractional anisotropy in the cingulum, and cerebral blood flow. The median time between scans was 4 years, with a median treatment time of 3.4 years.
The standard-care group showed a significantly greater increase in white-matter lesion volume at the follow-up scan than did the intensive-treatment group (1.45 cm3 vs. 0.92 cm3). But the intensively treated group had significantly more brain atrophy, losing a median of 30.6 cm3, compared with a loss of 26.9 cm3 in the standard-treatment group.
“It was a very small difference amounting to less than 1% of the total brain volume, but it was still statistically significant,” Dr. Nasrallah said.
Loss of gray-matter volume drove about two-thirds of the difference in the intensively treated group. There was a corresponding increase in cerebrospinal fluid volume that was driven by differences in the ventricles and the subarachnoid space.
However, there were no significant differences in right, left, or total hippocampal volume. There also were no differences in cingulate bundle anisotropy or cerebral blood flow.
SPRINT was funded by the National Institutes of Health. None of the investigators reported having financial conflicts of interest.
SAN DIEGO – Intensive blood pressure control over 4 years reduced the overall risk of all-cause dementia by 17%, compared with standard care, but in subanalyses of the Systolic Blood Pressure Intervention Trial (SPRINT) it was also associated with significant decreases in cognitive function and total brain volume, researchers said at the Clinical Trials on Alzheimer’s Disease conference.
Whether these between-group differences were clinically meaningful was the topic of some debate, but they were enough to prompt Mary Sano, PhD, to strongly state her reservations.
“The cardiovascular effects of SPRINT were impressive, but I am concerned about minimizing the potentially negative effect on cognition,” said Dr. Sano, professor of psychiatry and director of the Alzheimer’s Disease Research Center at the Icahn School of Medicine at Mount Sinai, New York. “Do I really want to treat a healthy, nonimpaired patient like this if I have to warn them that their cognition might actually get worse? We just cannot minimize this risk. There is very strong evidence that [intensive treatment of blood pressure] might be a step backward in cognition. Would you lower your own blood pressure at a risk of losing some points on your cognition?”
The subanalyses were conducted as part of the SPRINT Memory and Cognition In Decreased Hypertension (SPRINT MIND) substudy, which looked at cardiovascular and mortality outcomes in 9,361 subjects whose hypertension was managed intensively or by standard care (target systolic blood pressure less than 120 mm Hg vs. less than 140 mm Hg). The trial was stopped early because of a 25% reduction in the primary composite cardiovascular disease endpoint and a 27% reduction in all-cause mortality in the intensive-treatment group.
SPRINT MIND examined the risks of incident probable dementia, mild cognitive impairment (MCI), and a composite outcome of both. Intensive control reduced the risk of MCI by 19% and the combined outcome by 15%.
At the conference, SPRINT MIND investigators presented three long-term subanalyses with a median intervention and follow-up time of about 4 years.
Sarah Gaussoin of Wake Forest University, Winston-Salem, N.C., presented unpublished data detailing the effects of intensive control on several dementia subtypes: nonamnestic single domain, nonamnestic multidomain, amnestic single domain, and amnestic multidomain. There were 640 subjects in this analysis.
After a median of 3.3 years of intervention and 5 years of follow-up, there were no differences in the rate of incident probable dementia between the single- and multidomain nonamnestic groups. “We did see a strong 22% decreased risk in single-domain versus multidomain amnestic MCI, however,” she said.
Nicholas Pajewski, PhD, also of Wake Forest University, discussed more detailed cognitive outcomes in SPRINT MIND among 2,900 subjects who had a full battery of cognitive testing at every assessment over 5 years. The outcomes included memory deficit and processing speed.
Dr. Pajewski reported finding no significant difference between the groups in the rates of memory decline in either outcome. But there was a greater rate of decline in processing speed in the intensively treated group, he added. The difference was small but statistically significant.
The difference was largely driven by results of a single cognitive test – the Trail Making Test Part A. “It corresponded to about a 1.25-second increase over 4 years,” in processing speed on this test, Dr. Pajewski said.
There were no between-group differences in any of the other domains explored, including language, executive function, global cognitive function, or the Montreal Cognitive Assessment.
“Obviously, these results are perplexing,” given the overall positive results of SPRINT MIND, he said. “Intensive blood pressure control is a beneficial thing, and we expected to see an effect on memory, or a blunting of decline, and instead we saw some small decrements going the other way. This led us to speculate about what’s going on.”
The trial relied on a narrow definition of MCI that might have affected the outcomes. There was also a very broad range of ages in the study, ranging from 53 to 86 years. More importantly, he said, the original SPRINT study didn’t collect cognitive data at baseline, so there was no way to know how many subjects already might have had MCI when they entered the trial.
Ilya Nasrallah, MD, PhD, of the University of Pennsylvania, Philadelphia, presented MRI data on white-matter lesions, hippocampal volume fractional anisotropy in the cingulum, and cerebral blood flow. The median time between scans was 4 years, with a median treatment time of 3.4 years.
The standard-care group showed a significantly greater increase in white-matter lesion volume at the follow-up scan than did the intensive-treatment group (1.45 cm3 vs. 0.92 cm3). But the intensively treated group had significantly more brain atrophy, losing a median of 30.6 cm3, compared with a loss of 26.9 cm3 in the standard-treatment group.
“It was a very small difference amounting to less than 1% of the total brain volume, but it was still statistically significant,” Dr. Nasrallah said.
Loss of gray-matter volume drove about two-thirds of the difference in the intensively treated group. There was a corresponding increase in cerebrospinal fluid volume that was driven by differences in the ventricles and the subarachnoid space.
However, there were no significant differences in right, left, or total hippocampal volume. There also were no differences in cingulate bundle anisotropy or cerebral blood flow.
SPRINT was funded by the National Institutes of Health. None of the investigators reported having financial conflicts of interest.
SAN DIEGO – Intensive blood pressure control over 4 years reduced the overall risk of all-cause dementia by 17%, compared with standard care, but in subanalyses of the Systolic Blood Pressure Intervention Trial (SPRINT) it was also associated with significant decreases in cognitive function and total brain volume, researchers said at the Clinical Trials on Alzheimer’s Disease conference.
Whether these between-group differences were clinically meaningful was the topic of some debate, but they were enough to prompt Mary Sano, PhD, to strongly state her reservations.
“The cardiovascular effects of SPRINT were impressive, but I am concerned about minimizing the potentially negative effect on cognition,” said Dr. Sano, professor of psychiatry and director of the Alzheimer’s Disease Research Center at the Icahn School of Medicine at Mount Sinai, New York. “Do I really want to treat a healthy, nonimpaired patient like this if I have to warn them that their cognition might actually get worse? We just cannot minimize this risk. There is very strong evidence that [intensive treatment of blood pressure] might be a step backward in cognition. Would you lower your own blood pressure at a risk of losing some points on your cognition?”
The subanalyses were conducted as part of the SPRINT Memory and Cognition In Decreased Hypertension (SPRINT MIND) substudy, which looked at cardiovascular and mortality outcomes in 9,361 subjects whose hypertension was managed intensively or by standard care (target systolic blood pressure less than 120 mm Hg vs. less than 140 mm Hg). The trial was stopped early because of a 25% reduction in the primary composite cardiovascular disease endpoint and a 27% reduction in all-cause mortality in the intensive-treatment group.
SPRINT MIND examined the risks of incident probable dementia, mild cognitive impairment (MCI), and a composite outcome of both. Intensive control reduced the risk of MCI by 19% and the combined outcome by 15%.
At the conference, SPRINT MIND investigators presented three long-term subanalyses with a median intervention and follow-up time of about 4 years.
Sarah Gaussoin of Wake Forest University, Winston-Salem, N.C., presented unpublished data detailing the effects of intensive control on several dementia subtypes: nonamnestic single domain, nonamnestic multidomain, amnestic single domain, and amnestic multidomain. There were 640 subjects in this analysis.
After a median of 3.3 years of intervention and 5 years of follow-up, there were no differences in the rate of incident probable dementia between the single- and multidomain nonamnestic groups. “We did see a strong 22% decreased risk in single-domain versus multidomain amnestic MCI, however,” she said.
Nicholas Pajewski, PhD, also of Wake Forest University, discussed more detailed cognitive outcomes in SPRINT MIND among 2,900 subjects who had a full battery of cognitive testing at every assessment over 5 years. The outcomes included memory deficit and processing speed.
Dr. Pajewski reported finding no significant difference between the groups in the rates of memory decline in either outcome. But there was a greater rate of decline in processing speed in the intensively treated group, he added. The difference was small but statistically significant.
The difference was largely driven by results of a single cognitive test – the Trail Making Test Part A. “It corresponded to about a 1.25-second increase over 4 years,” in processing speed on this test, Dr. Pajewski said.
There were no between-group differences in any of the other domains explored, including language, executive function, global cognitive function, or the Montreal Cognitive Assessment.
“Obviously, these results are perplexing,” given the overall positive results of SPRINT MIND, he said. “Intensive blood pressure control is a beneficial thing, and we expected to see an effect on memory, or a blunting of decline, and instead we saw some small decrements going the other way. This led us to speculate about what’s going on.”
The trial relied on a narrow definition of MCI that might have affected the outcomes. There was also a very broad range of ages in the study, ranging from 53 to 86 years. More importantly, he said, the original SPRINT study didn’t collect cognitive data at baseline, so there was no way to know how many subjects already might have had MCI when they entered the trial.
Ilya Nasrallah, MD, PhD, of the University of Pennsylvania, Philadelphia, presented MRI data on white-matter lesions, hippocampal volume fractional anisotropy in the cingulum, and cerebral blood flow. The median time between scans was 4 years, with a median treatment time of 3.4 years.
The standard-care group showed a significantly greater increase in white-matter lesion volume at the follow-up scan than did the intensive-treatment group (1.45 cm3 vs. 0.92 cm3). But the intensively treated group had significantly more brain atrophy, losing a median of 30.6 cm3, compared with a loss of 26.9 cm3 in the standard-treatment group.
“It was a very small difference amounting to less than 1% of the total brain volume, but it was still statistically significant,” Dr. Nasrallah said.
Loss of gray-matter volume drove about two-thirds of the difference in the intensively treated group. There was a corresponding increase in cerebrospinal fluid volume that was driven by differences in the ventricles and the subarachnoid space.
However, there were no significant differences in right, left, or total hippocampal volume. There also were no differences in cingulate bundle anisotropy or cerebral blood flow.
SPRINT was funded by the National Institutes of Health. None of the investigators reported having financial conflicts of interest.
REPORTING FROM CTAD 2019
‘Brain enhancement’ supplements sold online may illegally contain piracetam
, according to an analysis of products sold online.
Sales of so-called ‘brain enhancement’ supplements exceeded $640 million in 2015 in the United States alone, but little is known about the risks of these dietary supplements, Pieter A. Cohen, MD, of the Cambridge Health Alliance in Somerville, Mass., and his coauthors wrote in a research letter published online Nov. 25 in JAMA Internal Medicine.
Piracetam is prescribed in many European countries for cognitive impairment and other disorders, the authors said. There is limited evidence for its efficacy, and the United States does not permit its sale as a dietary supplement.
Using the search terms “piracetam” and “dietary supplement,” researchers identified five brands of supplements sold online and analyzed 10 samples from these. Their chemical analysis revealed that eight samples from four brands contained piracetam, ranging from 831 mg to 1,452 mg per recommended serving size, and 85%-118% of the amount on the product’s label.
“Our findings demonstrate that, even after the FDA rejected an application to market piracetam as a new supplement ingredient, the drug was nevertheless introduced into the marketplace,” the authors wrote.
The authors calculated that, if consumers followed the recommended dosage on the labels of these products, they could be exposed to up to 11,283 mg of piracetam per day.
For comparison, prescription piracetam in Europe is commonly found in 800-mg and 1,200-mg tablets, and the recommended daily dose for cognitive disorders ranges from 2,400 to 4,800 mg per day, adjusted for renal function.
The authors commented that piracetam is associated with side effects at pharmaceutical dosages, including anxiety, insomnia, agitation, depression, drowsiness, and weight gain. However, the risk associated with higher doses, particularly in the elderly and those with renal insufficiency, are unknown.
“Until the law governing supplements is reformed such that products adulterated with drugs can be effectively removed from the market, clinicians should advise patients that supplements marketed as cognitive enhancers may contain prohibited drugs at supratherapeutic doses,” the authors wrote.
One author declared research support from two organizations unrelated to the study. No conflicts of interest were declared.
SOURCE: Cohen P et al. JAMA Int Med. 2019 Nov 25. doi: 10.1001/jamainternmed.2019.5507.
, according to an analysis of products sold online.
Sales of so-called ‘brain enhancement’ supplements exceeded $640 million in 2015 in the United States alone, but little is known about the risks of these dietary supplements, Pieter A. Cohen, MD, of the Cambridge Health Alliance in Somerville, Mass., and his coauthors wrote in a research letter published online Nov. 25 in JAMA Internal Medicine.
Piracetam is prescribed in many European countries for cognitive impairment and other disorders, the authors said. There is limited evidence for its efficacy, and the United States does not permit its sale as a dietary supplement.
Using the search terms “piracetam” and “dietary supplement,” researchers identified five brands of supplements sold online and analyzed 10 samples from these. Their chemical analysis revealed that eight samples from four brands contained piracetam, ranging from 831 mg to 1,452 mg per recommended serving size, and 85%-118% of the amount on the product’s label.
“Our findings demonstrate that, even after the FDA rejected an application to market piracetam as a new supplement ingredient, the drug was nevertheless introduced into the marketplace,” the authors wrote.
The authors calculated that, if consumers followed the recommended dosage on the labels of these products, they could be exposed to up to 11,283 mg of piracetam per day.
For comparison, prescription piracetam in Europe is commonly found in 800-mg and 1,200-mg tablets, and the recommended daily dose for cognitive disorders ranges from 2,400 to 4,800 mg per day, adjusted for renal function.
The authors commented that piracetam is associated with side effects at pharmaceutical dosages, including anxiety, insomnia, agitation, depression, drowsiness, and weight gain. However, the risk associated with higher doses, particularly in the elderly and those with renal insufficiency, are unknown.
“Until the law governing supplements is reformed such that products adulterated with drugs can be effectively removed from the market, clinicians should advise patients that supplements marketed as cognitive enhancers may contain prohibited drugs at supratherapeutic doses,” the authors wrote.
One author declared research support from two organizations unrelated to the study. No conflicts of interest were declared.
SOURCE: Cohen P et al. JAMA Int Med. 2019 Nov 25. doi: 10.1001/jamainternmed.2019.5507.
, according to an analysis of products sold online.
Sales of so-called ‘brain enhancement’ supplements exceeded $640 million in 2015 in the United States alone, but little is known about the risks of these dietary supplements, Pieter A. Cohen, MD, of the Cambridge Health Alliance in Somerville, Mass., and his coauthors wrote in a research letter published online Nov. 25 in JAMA Internal Medicine.
Piracetam is prescribed in many European countries for cognitive impairment and other disorders, the authors said. There is limited evidence for its efficacy, and the United States does not permit its sale as a dietary supplement.
Using the search terms “piracetam” and “dietary supplement,” researchers identified five brands of supplements sold online and analyzed 10 samples from these. Their chemical analysis revealed that eight samples from four brands contained piracetam, ranging from 831 mg to 1,452 mg per recommended serving size, and 85%-118% of the amount on the product’s label.
“Our findings demonstrate that, even after the FDA rejected an application to market piracetam as a new supplement ingredient, the drug was nevertheless introduced into the marketplace,” the authors wrote.
The authors calculated that, if consumers followed the recommended dosage on the labels of these products, they could be exposed to up to 11,283 mg of piracetam per day.
For comparison, prescription piracetam in Europe is commonly found in 800-mg and 1,200-mg tablets, and the recommended daily dose for cognitive disorders ranges from 2,400 to 4,800 mg per day, adjusted for renal function.
The authors commented that piracetam is associated with side effects at pharmaceutical dosages, including anxiety, insomnia, agitation, depression, drowsiness, and weight gain. However, the risk associated with higher doses, particularly in the elderly and those with renal insufficiency, are unknown.
“Until the law governing supplements is reformed such that products adulterated with drugs can be effectively removed from the market, clinicians should advise patients that supplements marketed as cognitive enhancers may contain prohibited drugs at supratherapeutic doses,” the authors wrote.
One author declared research support from two organizations unrelated to the study. No conflicts of interest were declared.
SOURCE: Cohen P et al. JAMA Int Med. 2019 Nov 25. doi: 10.1001/jamainternmed.2019.5507.
FROM JAMA INTERNAL MEDICINE
Alzheimer’s disease subtypes follow neuropathologic patterns seen in the nucleus basalis of Meynert
Cholinergic neurons in the nucleus basalis of Meynert appear more susceptible to neurofibrillary tangles and neuronal destruction in women, patients carrying the apolipoprotein E–epsilon 4 (APOE4) allele, and people with hippocampal-sparing Alzheimer’s disease, a subtype characterized by early onset and rapid cognitive decline.
Those findings and others from a postmortem study published in JAMA Neurology also suggests that the nucleus basalis of Meynert (nbM) could be the first place that neuronal damage appears in Alzheimer’s disease (AD), according to first author Fadi S. Hanna Al-Shaikh and colleagues.
The study also confirmed the authors’ previous categorization of three AD subtypes: early-onset, rapidly declining hippocampal-sparing AD (HpSp), typical sporadic AD, and limbic predominant AD, a later-onset form with a slower rate of decline.
“We observed a wave of vulnerability in which the exacerbation of nbM neurofibrillary tangles [NFTs] in HpSp AD may leave the cortex more vulnerable to [tangle] accumulation, perhaps via a biologically accelerated process or through a mechanism of disinhibition,” wrote Mr. Al-Shaikh, of the Mayo Clinic, Jacksonville, Fla., and colleagues. “By contrast, the limbic predominant AD cases had an exacerbation of areas vulnerable early in the Braak-like pattern of NFT accumulation, perhaps via a biologically restrictive process that relatively confines pathology to limbic areas.”
The nbM is of interest to researchers because 90% of its neurons are cholinergic with cortical penetration. “Postmortem studies of AD and more recent neuroimaging studies provide evidence that involvement of the nucleus basalis of Meynert may be critical and early in the molecular cascade of events,” the authors said. “The accumulation of NFTs in the nbM may precede entorhinal cortex and locus coeruleus involvement, making the nbM potentially one of the earliest sites where NFT accumulation occurs.”
Previously, this team had identified three AD subtypes based on patterns of corticolimbic neurofibrillary tangling. In HpSp, the hippocampus is relatively spared, while the cortex has a greater number of tangles. In limbic predominant AD, the cortex is relatively spared, and the hippocampus is severely involved. Typical AD shows the expected patterns of hippocampal and cortical tangling.
Cases in this study came from the Florida Autopsied Multi-Ethnic (FLAME) cohort, comprising 1,361 brain tissue samples from confirmed AD cases and 103 nondemented controls. The investigators sought to understand the patterns of neuronal demise in the nbM, and any associations with clinical signs, demographics, and the recently described three subtypes.
In the cohort, AD subtypes included 175 with HpSp, 1,014 with typical AD, and 172 with limbic predominant AD. Patients with HpSp were the youngest, with a median disease onset age of 65 years, compared with 71 years in typical AD and 78 in limbic predominant. There were fewer women in the HpSp group (35%), compared with the typical AD group (54%) and the limbic group (70%). More patients with HpSp had atypical presentation (38%) in comparison with typical (11%) and limbic predominant AD (2%). But patients with HpSp were less likely to be APOE4 positive (46%), whereas those with limbic predominant AD were most likely to be APOE4 positive (72%).
Cognitively, HpSp patients declined more rapidly, losing a median of 4 points per year on the Mini Mental State Exam (MMSE), compared with 2 and 1 points in those with typical and limbic predominant AD. At death, the HpSp patients had a median MMSE score of 7, versus 13 in the typical AD group and 18 in the limbic group.
Patients with HpSp had the highest concentration of tangles and the lowest neuronal density in the nbM. Limbic predominant cases had the lowest tangle burden and the highest neuronal density. Typical AD cases lay between these extremes on both measures.
A multivariate regression analysis determined the overlap of neuronal findings and AD subtypes. A younger age at symptom onset was significantly associated with higher tangle counts in the nbM regions among patients with HpSp. In women with typical AD, there were 2.5 times more tangles than in men. APOE4 carriers had 1.3 times more tangles than did noncarriers.
There were also associations with cognition. “For every 10-point decrease in final MMSE of typical AD cases, the number of nbM NFTs was expected to increase by 1.8,” the authors wrote.
Although limbic predominant AD wasn’t associated with any clinical or demographic variables in this analysis, it was associated with neuronal changes in the nbM. “For every 10 years’ younger age at onset, the number of neurons was expected to be lower by 4.6 [per mm2]. … In addition, limbic predominant cases were observed to have 4.3 [per mm2] fewer neurons for every 10-point decrease in MMSE,” the authors said.
This study was supported by the National Institute on Aging, the Florida Department of Health, the Ed and Ethel Moore Alzheimer’s Disease Research Program, a Gerstner Family Career Development Award, and the Alzheimer’s Association. Two authors reported financial relationships with industry outside the submitted work.
SOURCE: Al Shaikh FSH et al. JAMA Neurol. 2019 Oct 28. doi: 10.1001/jamaneurol.2019.3606.
Cholinergic neurons in the nucleus basalis of Meynert appear more susceptible to neurofibrillary tangles and neuronal destruction in women, patients carrying the apolipoprotein E–epsilon 4 (APOE4) allele, and people with hippocampal-sparing Alzheimer’s disease, a subtype characterized by early onset and rapid cognitive decline.
Those findings and others from a postmortem study published in JAMA Neurology also suggests that the nucleus basalis of Meynert (nbM) could be the first place that neuronal damage appears in Alzheimer’s disease (AD), according to first author Fadi S. Hanna Al-Shaikh and colleagues.
The study also confirmed the authors’ previous categorization of three AD subtypes: early-onset, rapidly declining hippocampal-sparing AD (HpSp), typical sporadic AD, and limbic predominant AD, a later-onset form with a slower rate of decline.
“We observed a wave of vulnerability in which the exacerbation of nbM neurofibrillary tangles [NFTs] in HpSp AD may leave the cortex more vulnerable to [tangle] accumulation, perhaps via a biologically accelerated process or through a mechanism of disinhibition,” wrote Mr. Al-Shaikh, of the Mayo Clinic, Jacksonville, Fla., and colleagues. “By contrast, the limbic predominant AD cases had an exacerbation of areas vulnerable early in the Braak-like pattern of NFT accumulation, perhaps via a biologically restrictive process that relatively confines pathology to limbic areas.”
The nbM is of interest to researchers because 90% of its neurons are cholinergic with cortical penetration. “Postmortem studies of AD and more recent neuroimaging studies provide evidence that involvement of the nucleus basalis of Meynert may be critical and early in the molecular cascade of events,” the authors said. “The accumulation of NFTs in the nbM may precede entorhinal cortex and locus coeruleus involvement, making the nbM potentially one of the earliest sites where NFT accumulation occurs.”
Previously, this team had identified three AD subtypes based on patterns of corticolimbic neurofibrillary tangling. In HpSp, the hippocampus is relatively spared, while the cortex has a greater number of tangles. In limbic predominant AD, the cortex is relatively spared, and the hippocampus is severely involved. Typical AD shows the expected patterns of hippocampal and cortical tangling.
Cases in this study came from the Florida Autopsied Multi-Ethnic (FLAME) cohort, comprising 1,361 brain tissue samples from confirmed AD cases and 103 nondemented controls. The investigators sought to understand the patterns of neuronal demise in the nbM, and any associations with clinical signs, demographics, and the recently described three subtypes.
In the cohort, AD subtypes included 175 with HpSp, 1,014 with typical AD, and 172 with limbic predominant AD. Patients with HpSp were the youngest, with a median disease onset age of 65 years, compared with 71 years in typical AD and 78 in limbic predominant. There were fewer women in the HpSp group (35%), compared with the typical AD group (54%) and the limbic group (70%). More patients with HpSp had atypical presentation (38%) in comparison with typical (11%) and limbic predominant AD (2%). But patients with HpSp were less likely to be APOE4 positive (46%), whereas those with limbic predominant AD were most likely to be APOE4 positive (72%).
Cognitively, HpSp patients declined more rapidly, losing a median of 4 points per year on the Mini Mental State Exam (MMSE), compared with 2 and 1 points in those with typical and limbic predominant AD. At death, the HpSp patients had a median MMSE score of 7, versus 13 in the typical AD group and 18 in the limbic group.
Patients with HpSp had the highest concentration of tangles and the lowest neuronal density in the nbM. Limbic predominant cases had the lowest tangle burden and the highest neuronal density. Typical AD cases lay between these extremes on both measures.
A multivariate regression analysis determined the overlap of neuronal findings and AD subtypes. A younger age at symptom onset was significantly associated with higher tangle counts in the nbM regions among patients with HpSp. In women with typical AD, there were 2.5 times more tangles than in men. APOE4 carriers had 1.3 times more tangles than did noncarriers.
There were also associations with cognition. “For every 10-point decrease in final MMSE of typical AD cases, the number of nbM NFTs was expected to increase by 1.8,” the authors wrote.
Although limbic predominant AD wasn’t associated with any clinical or demographic variables in this analysis, it was associated with neuronal changes in the nbM. “For every 10 years’ younger age at onset, the number of neurons was expected to be lower by 4.6 [per mm2]. … In addition, limbic predominant cases were observed to have 4.3 [per mm2] fewer neurons for every 10-point decrease in MMSE,” the authors said.
This study was supported by the National Institute on Aging, the Florida Department of Health, the Ed and Ethel Moore Alzheimer’s Disease Research Program, a Gerstner Family Career Development Award, and the Alzheimer’s Association. Two authors reported financial relationships with industry outside the submitted work.
SOURCE: Al Shaikh FSH et al. JAMA Neurol. 2019 Oct 28. doi: 10.1001/jamaneurol.2019.3606.
Cholinergic neurons in the nucleus basalis of Meynert appear more susceptible to neurofibrillary tangles and neuronal destruction in women, patients carrying the apolipoprotein E–epsilon 4 (APOE4) allele, and people with hippocampal-sparing Alzheimer’s disease, a subtype characterized by early onset and rapid cognitive decline.
Those findings and others from a postmortem study published in JAMA Neurology also suggests that the nucleus basalis of Meynert (nbM) could be the first place that neuronal damage appears in Alzheimer’s disease (AD), according to first author Fadi S. Hanna Al-Shaikh and colleagues.
The study also confirmed the authors’ previous categorization of three AD subtypes: early-onset, rapidly declining hippocampal-sparing AD (HpSp), typical sporadic AD, and limbic predominant AD, a later-onset form with a slower rate of decline.
“We observed a wave of vulnerability in which the exacerbation of nbM neurofibrillary tangles [NFTs] in HpSp AD may leave the cortex more vulnerable to [tangle] accumulation, perhaps via a biologically accelerated process or through a mechanism of disinhibition,” wrote Mr. Al-Shaikh, of the Mayo Clinic, Jacksonville, Fla., and colleagues. “By contrast, the limbic predominant AD cases had an exacerbation of areas vulnerable early in the Braak-like pattern of NFT accumulation, perhaps via a biologically restrictive process that relatively confines pathology to limbic areas.”
The nbM is of interest to researchers because 90% of its neurons are cholinergic with cortical penetration. “Postmortem studies of AD and more recent neuroimaging studies provide evidence that involvement of the nucleus basalis of Meynert may be critical and early in the molecular cascade of events,” the authors said. “The accumulation of NFTs in the nbM may precede entorhinal cortex and locus coeruleus involvement, making the nbM potentially one of the earliest sites where NFT accumulation occurs.”
Previously, this team had identified three AD subtypes based on patterns of corticolimbic neurofibrillary tangling. In HpSp, the hippocampus is relatively spared, while the cortex has a greater number of tangles. In limbic predominant AD, the cortex is relatively spared, and the hippocampus is severely involved. Typical AD shows the expected patterns of hippocampal and cortical tangling.
Cases in this study came from the Florida Autopsied Multi-Ethnic (FLAME) cohort, comprising 1,361 brain tissue samples from confirmed AD cases and 103 nondemented controls. The investigators sought to understand the patterns of neuronal demise in the nbM, and any associations with clinical signs, demographics, and the recently described three subtypes.
In the cohort, AD subtypes included 175 with HpSp, 1,014 with typical AD, and 172 with limbic predominant AD. Patients with HpSp were the youngest, with a median disease onset age of 65 years, compared with 71 years in typical AD and 78 in limbic predominant. There were fewer women in the HpSp group (35%), compared with the typical AD group (54%) and the limbic group (70%). More patients with HpSp had atypical presentation (38%) in comparison with typical (11%) and limbic predominant AD (2%). But patients with HpSp were less likely to be APOE4 positive (46%), whereas those with limbic predominant AD were most likely to be APOE4 positive (72%).
Cognitively, HpSp patients declined more rapidly, losing a median of 4 points per year on the Mini Mental State Exam (MMSE), compared with 2 and 1 points in those with typical and limbic predominant AD. At death, the HpSp patients had a median MMSE score of 7, versus 13 in the typical AD group and 18 in the limbic group.
Patients with HpSp had the highest concentration of tangles and the lowest neuronal density in the nbM. Limbic predominant cases had the lowest tangle burden and the highest neuronal density. Typical AD cases lay between these extremes on both measures.
A multivariate regression analysis determined the overlap of neuronal findings and AD subtypes. A younger age at symptom onset was significantly associated with higher tangle counts in the nbM regions among patients with HpSp. In women with typical AD, there were 2.5 times more tangles than in men. APOE4 carriers had 1.3 times more tangles than did noncarriers.
There were also associations with cognition. “For every 10-point decrease in final MMSE of typical AD cases, the number of nbM NFTs was expected to increase by 1.8,” the authors wrote.
Although limbic predominant AD wasn’t associated with any clinical or demographic variables in this analysis, it was associated with neuronal changes in the nbM. “For every 10 years’ younger age at onset, the number of neurons was expected to be lower by 4.6 [per mm2]. … In addition, limbic predominant cases were observed to have 4.3 [per mm2] fewer neurons for every 10-point decrease in MMSE,” the authors said.
This study was supported by the National Institute on Aging, the Florida Department of Health, the Ed and Ethel Moore Alzheimer’s Disease Research Program, a Gerstner Family Career Development Award, and the Alzheimer’s Association. Two authors reported financial relationships with industry outside the submitted work.
SOURCE: Al Shaikh FSH et al. JAMA Neurol. 2019 Oct 28. doi: 10.1001/jamaneurol.2019.3606.
FROM JAMA NEUROLOGY
Seaweed floats to the top of Alzheimer’s news
China’s National Medical Products Administration has approved a new therapy for patients with mild to moderate Alzheimer’s disease – a seaweed extract thought to alter the gut microbiome profile and subsequently decrease microbiome-driven neuroinflammation.
Sodium oligomannate – dubbed GV-971 – won approval based on a 36-week, placebo-controlled, phase 3 study of 818 patients with mild to moderate Alzheimer’s disease (AD). The study hit its primary endpoint of change on the Alzheimer’s Disease Assessment Scale cognitive portion (ADAS-cog12). It did not meet any of the trial’s other cognitive or functional secondary endpoints.
A portion of the data were presented last year at the Clinical Trials on Alzheimer’s Disease meeting in Barcelona. But the full study has never appeared in a peer-reviewed journal. A truncated version is publicly available on the website of Shanghai Green Valley Pharmaceuticals, the company developing the molecule.
Shanghai Green Valley contends that it reduces neuroinflammation by improving a proinflammatory microbiome profile that it says is characteristic of AD. However, the mechanism by which GV-971 alters intestinal bacterial composition is unclear – or at least it is not fully described in the public literature.
In the United States, some key researchers appraised the news with a cautiously optimistic eye, while others pointed noted that the AD-microbiome link is an unproven concept, and that it was evaluated in a study of questionable worth.
“The company has presented data that suggest there is a modest cognitive benefit to this treatment,” Paul S. Aisen, MD, said in an interview. “The key secondary endpoint was missed, and the other secondary endpoints showed no benefit. It’s a single trial and the mechanism is still unclear.”
“We do need to pursue all possible leads, and I’m glad the company is pursuing additional studies, but I wouldn’t draw a firm conclusion from these data. And they certainly would not be enough to win approval in the U.S.,” said Dr. Aisen, founding director Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
Preclinical findings on GV-971
In commenting on preclinical findings of GV-971 published in Cell Research in September 2019, David Holtzman, MD, associate director of the Alzheimer’s disease research center at Washington University, St. Louis, and coauthors observed that the data support research exploring treatments that modulate the gut microbiome but leave it unclear as to whether GV-971 has AD-specific effects.
“[The company shows] that GV-971 decreases amyloid beta-related pathologies by reconditioning the gut microbiota, providing further evidence that gut-targeted interventions may serve as novel strategies to tackle AD,” Dr. Holtzman and coauthors wrote. “Whether this potential mechanism represents an AD-specific process is not clear, since there is great overlap in immunological changes and gut dysbiosis with other diseases. … In addition, although this study reveals that gut reconditioning may be one mechanism of action of the drug GV-971, it does not rule out other possible mechanisms. For example, GV-971 may attenuate AD pathogenesis by directly inhibiting neuroinflammation or amyloid-beta fibril formation. However, there is no question that [these] data further [support] the emerging idea that modulation of the gut microbiome via treatments such as GV-971 or other strategies should be further explored as novel strategies to slow the progression of AD.”
Sodium oligomannate is a long-chain saccharide extracted from brown sea algae and consists of acidic linear oligosaccharides with structures ranging from dimers to decamers. Related molecules without the sugar backbone were inactive, suggesting that the saccharides are the active portion, Xinyi Wang of Shanghai Green Valley and colleagues wrote in the Cell Research paper.
Based on these studies, the company contends that Alzheimer’s progression is accompanied by a characteristic microbiome change to a proinflammatory profile. And indeed, two transgenic Alzheimer’s mouse models – one with five familial AD mutations (5xFAD) and one with mutations of amyloid precursor protein and presenilin 1 (APP/PS1) – showed similar gradual age- and progression-related decreases of Bacteroides and Verrucomicrobia, two components of a normal microbiome. Bacteroides species perform key functions necessary for survival, including sensing and adapting to nutrient variability, expelling toxins, and stimulating the immune system). Species of the Verrucomicrobia phylum are important in glucose homeostasis. The decline in Bacteroides and Verrucomicrobia species is accompanied by an increase in concomitant proinflammatory species.
The investigators then explored the relationship between the microbiome composition and cognitive function in both transgenic models and a wild-type mouse.
First, they showed that the bacterial populations shifted as the mice aged and their AD pathology developed. This was accompanied by an uptick in activated microglia and, in turn, proinflammatory T1 helper cells that migrated through the intestinal membranes and into the periphery, then cross the blood-brain barrier to enter the brain.
Then the investigators used a cocktail of powerful antibiotics to disturb the intestinal flora in both transgenic and wild-type mice. After this, the 5xFAD mice showed fewer activated microglia and fewer infiltrating T cells. Later, they gave wild-type mice a fecal transplant from the 5xFAD mice. The wild-type mice developed more activated and infiltrating cells and their microbiome began to resemble that of the transgenic mice. Conversely, when the transgenic mice received a transplant from the wild-type mice, their microbiome changed to resemble the donors’, and their activated and infiltrating cells declined.
After this, the team gave GV-971 to the mice. The APP/PS1 mice improved cognitively, and the 5xFAD mice had fewer activated and infiltrating cells, fewer amyloid brain plaques, and less tau phosphorylation. These changes were accompanied by higher levels of two amino acids, phenylalanine and isoleucine. These proteins appear to act on T-cell proliferation and differentiation, they said.
“Taken together, these analyses suggest the idea that gut dysbiosis contributes to [phenylalanine and isoleucine] elevation, which drives the proliferation/differentiation and brain infiltration of [T1 helper] cells,” Dr. Holtzman and coauthors wrote. “These infiltrating Th1 cells may then further activate microglia and contribute to amyloid-related pathogenesis.”
The phase 3 study
The approval of GV-971 was based on the subsequent 36-week phase 3, placebo-controlled study of 818 patients with mild to moderate AD, which was reported last year. Patients were randomized to placebo or to GV-971 450 mg twice daily. Amyloid PET imaging was not required at entrance to the study, so there was no measure of baseline amyloid load. However, all patients showed MRI evidence of cortical atrophy. The Mini Mental State Exam (MMSE) ranged from 11 to 26, indicating mild to moderate AD.
Secondary endpoints included change on the Alzheimer’s Disease Cooperative Study Activities of Daily Living, the Clinician’s Interview-Based Impression of Change–Plus, and the Neuropsychiatric Index.
Patients taking GV-971 experienced a statistically significant 2.54-point difference on the ADAS-cog12, compared with placebo. The difference was apparent by week 4 and was seen at every clinical visit. When patients were grouped according to baseline MMSE (11-14, 15-19, and 20-26), the drug performed similarly.
But none of the secondary endpoints significantly favored of GV-971. And the placebo group behaved in an unexpected way, which could throw the data interpretation off-kilter somewhat, David Knopman, MD, said in an interview.
“It was a very weird and unusual-looking trajectory of the placebo and treated groups,” said Dr. Knopman of the Mayo Clinic, Rochester, Minn., with those taking placebo staying relatively stable for some time before the cognitive scores dropped precipitously. “The data were unconvincing to me.”
He also pointed out that although the extensive preclinical data appeared in the recent peer-reviewed Cell Research paper, the phase 3 data has not appeared in any peer-reviewed forum, “even though the trial has been completed for some time now. Furthermore, the duration of the study was inadequate.”
Finally, none of the study subjects were taking the standard-of-care cholinesterase inhibitors, which virtually every AD patient in the United States does take.
“This makes it almost completely inapplicable to the U.S.,” he said. “It’s not bad news. I’m just not convinced.”
Dr. Holtzman is a cofounder of C2N Diagnostics. He is on the scientific advisory board of Genentech, Denali, and C2N Diagnostics. He consults for Idorsia. Dr. Knopman is a consultant for the Bluefield Project to Cure Frontotemporal Dementia and for Lundbeck.
China’s National Medical Products Administration has approved a new therapy for patients with mild to moderate Alzheimer’s disease – a seaweed extract thought to alter the gut microbiome profile and subsequently decrease microbiome-driven neuroinflammation.
Sodium oligomannate – dubbed GV-971 – won approval based on a 36-week, placebo-controlled, phase 3 study of 818 patients with mild to moderate Alzheimer’s disease (AD). The study hit its primary endpoint of change on the Alzheimer’s Disease Assessment Scale cognitive portion (ADAS-cog12). It did not meet any of the trial’s other cognitive or functional secondary endpoints.
A portion of the data were presented last year at the Clinical Trials on Alzheimer’s Disease meeting in Barcelona. But the full study has never appeared in a peer-reviewed journal. A truncated version is publicly available on the website of Shanghai Green Valley Pharmaceuticals, the company developing the molecule.
Shanghai Green Valley contends that it reduces neuroinflammation by improving a proinflammatory microbiome profile that it says is characteristic of AD. However, the mechanism by which GV-971 alters intestinal bacterial composition is unclear – or at least it is not fully described in the public literature.
In the United States, some key researchers appraised the news with a cautiously optimistic eye, while others pointed noted that the AD-microbiome link is an unproven concept, and that it was evaluated in a study of questionable worth.
“The company has presented data that suggest there is a modest cognitive benefit to this treatment,” Paul S. Aisen, MD, said in an interview. “The key secondary endpoint was missed, and the other secondary endpoints showed no benefit. It’s a single trial and the mechanism is still unclear.”
“We do need to pursue all possible leads, and I’m glad the company is pursuing additional studies, but I wouldn’t draw a firm conclusion from these data. And they certainly would not be enough to win approval in the U.S.,” said Dr. Aisen, founding director Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
Preclinical findings on GV-971
In commenting on preclinical findings of GV-971 published in Cell Research in September 2019, David Holtzman, MD, associate director of the Alzheimer’s disease research center at Washington University, St. Louis, and coauthors observed that the data support research exploring treatments that modulate the gut microbiome but leave it unclear as to whether GV-971 has AD-specific effects.
“[The company shows] that GV-971 decreases amyloid beta-related pathologies by reconditioning the gut microbiota, providing further evidence that gut-targeted interventions may serve as novel strategies to tackle AD,” Dr. Holtzman and coauthors wrote. “Whether this potential mechanism represents an AD-specific process is not clear, since there is great overlap in immunological changes and gut dysbiosis with other diseases. … In addition, although this study reveals that gut reconditioning may be one mechanism of action of the drug GV-971, it does not rule out other possible mechanisms. For example, GV-971 may attenuate AD pathogenesis by directly inhibiting neuroinflammation or amyloid-beta fibril formation. However, there is no question that [these] data further [support] the emerging idea that modulation of the gut microbiome via treatments such as GV-971 or other strategies should be further explored as novel strategies to slow the progression of AD.”
Sodium oligomannate is a long-chain saccharide extracted from brown sea algae and consists of acidic linear oligosaccharides with structures ranging from dimers to decamers. Related molecules without the sugar backbone were inactive, suggesting that the saccharides are the active portion, Xinyi Wang of Shanghai Green Valley and colleagues wrote in the Cell Research paper.
Based on these studies, the company contends that Alzheimer’s progression is accompanied by a characteristic microbiome change to a proinflammatory profile. And indeed, two transgenic Alzheimer’s mouse models – one with five familial AD mutations (5xFAD) and one with mutations of amyloid precursor protein and presenilin 1 (APP/PS1) – showed similar gradual age- and progression-related decreases of Bacteroides and Verrucomicrobia, two components of a normal microbiome. Bacteroides species perform key functions necessary for survival, including sensing and adapting to nutrient variability, expelling toxins, and stimulating the immune system). Species of the Verrucomicrobia phylum are important in glucose homeostasis. The decline in Bacteroides and Verrucomicrobia species is accompanied by an increase in concomitant proinflammatory species.
The investigators then explored the relationship between the microbiome composition and cognitive function in both transgenic models and a wild-type mouse.
First, they showed that the bacterial populations shifted as the mice aged and their AD pathology developed. This was accompanied by an uptick in activated microglia and, in turn, proinflammatory T1 helper cells that migrated through the intestinal membranes and into the periphery, then cross the blood-brain barrier to enter the brain.
Then the investigators used a cocktail of powerful antibiotics to disturb the intestinal flora in both transgenic and wild-type mice. After this, the 5xFAD mice showed fewer activated microglia and fewer infiltrating T cells. Later, they gave wild-type mice a fecal transplant from the 5xFAD mice. The wild-type mice developed more activated and infiltrating cells and their microbiome began to resemble that of the transgenic mice. Conversely, when the transgenic mice received a transplant from the wild-type mice, their microbiome changed to resemble the donors’, and their activated and infiltrating cells declined.
After this, the team gave GV-971 to the mice. The APP/PS1 mice improved cognitively, and the 5xFAD mice had fewer activated and infiltrating cells, fewer amyloid brain plaques, and less tau phosphorylation. These changes were accompanied by higher levels of two amino acids, phenylalanine and isoleucine. These proteins appear to act on T-cell proliferation and differentiation, they said.
“Taken together, these analyses suggest the idea that gut dysbiosis contributes to [phenylalanine and isoleucine] elevation, which drives the proliferation/differentiation and brain infiltration of [T1 helper] cells,” Dr. Holtzman and coauthors wrote. “These infiltrating Th1 cells may then further activate microglia and contribute to amyloid-related pathogenesis.”
The phase 3 study
The approval of GV-971 was based on the subsequent 36-week phase 3, placebo-controlled study of 818 patients with mild to moderate AD, which was reported last year. Patients were randomized to placebo or to GV-971 450 mg twice daily. Amyloid PET imaging was not required at entrance to the study, so there was no measure of baseline amyloid load. However, all patients showed MRI evidence of cortical atrophy. The Mini Mental State Exam (MMSE) ranged from 11 to 26, indicating mild to moderate AD.
Secondary endpoints included change on the Alzheimer’s Disease Cooperative Study Activities of Daily Living, the Clinician’s Interview-Based Impression of Change–Plus, and the Neuropsychiatric Index.
Patients taking GV-971 experienced a statistically significant 2.54-point difference on the ADAS-cog12, compared with placebo. The difference was apparent by week 4 and was seen at every clinical visit. When patients were grouped according to baseline MMSE (11-14, 15-19, and 20-26), the drug performed similarly.
But none of the secondary endpoints significantly favored of GV-971. And the placebo group behaved in an unexpected way, which could throw the data interpretation off-kilter somewhat, David Knopman, MD, said in an interview.
“It was a very weird and unusual-looking trajectory of the placebo and treated groups,” said Dr. Knopman of the Mayo Clinic, Rochester, Minn., with those taking placebo staying relatively stable for some time before the cognitive scores dropped precipitously. “The data were unconvincing to me.”
He also pointed out that although the extensive preclinical data appeared in the recent peer-reviewed Cell Research paper, the phase 3 data has not appeared in any peer-reviewed forum, “even though the trial has been completed for some time now. Furthermore, the duration of the study was inadequate.”
Finally, none of the study subjects were taking the standard-of-care cholinesterase inhibitors, which virtually every AD patient in the United States does take.
“This makes it almost completely inapplicable to the U.S.,” he said. “It’s not bad news. I’m just not convinced.”
Dr. Holtzman is a cofounder of C2N Diagnostics. He is on the scientific advisory board of Genentech, Denali, and C2N Diagnostics. He consults for Idorsia. Dr. Knopman is a consultant for the Bluefield Project to Cure Frontotemporal Dementia and for Lundbeck.
China’s National Medical Products Administration has approved a new therapy for patients with mild to moderate Alzheimer’s disease – a seaweed extract thought to alter the gut microbiome profile and subsequently decrease microbiome-driven neuroinflammation.
Sodium oligomannate – dubbed GV-971 – won approval based on a 36-week, placebo-controlled, phase 3 study of 818 patients with mild to moderate Alzheimer’s disease (AD). The study hit its primary endpoint of change on the Alzheimer’s Disease Assessment Scale cognitive portion (ADAS-cog12). It did not meet any of the trial’s other cognitive or functional secondary endpoints.
A portion of the data were presented last year at the Clinical Trials on Alzheimer’s Disease meeting in Barcelona. But the full study has never appeared in a peer-reviewed journal. A truncated version is publicly available on the website of Shanghai Green Valley Pharmaceuticals, the company developing the molecule.
Shanghai Green Valley contends that it reduces neuroinflammation by improving a proinflammatory microbiome profile that it says is characteristic of AD. However, the mechanism by which GV-971 alters intestinal bacterial composition is unclear – or at least it is not fully described in the public literature.
In the United States, some key researchers appraised the news with a cautiously optimistic eye, while others pointed noted that the AD-microbiome link is an unproven concept, and that it was evaluated in a study of questionable worth.
“The company has presented data that suggest there is a modest cognitive benefit to this treatment,” Paul S. Aisen, MD, said in an interview. “The key secondary endpoint was missed, and the other secondary endpoints showed no benefit. It’s a single trial and the mechanism is still unclear.”
“We do need to pursue all possible leads, and I’m glad the company is pursuing additional studies, but I wouldn’t draw a firm conclusion from these data. And they certainly would not be enough to win approval in the U.S.,” said Dr. Aisen, founding director Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
Preclinical findings on GV-971
In commenting on preclinical findings of GV-971 published in Cell Research in September 2019, David Holtzman, MD, associate director of the Alzheimer’s disease research center at Washington University, St. Louis, and coauthors observed that the data support research exploring treatments that modulate the gut microbiome but leave it unclear as to whether GV-971 has AD-specific effects.
“[The company shows] that GV-971 decreases amyloid beta-related pathologies by reconditioning the gut microbiota, providing further evidence that gut-targeted interventions may serve as novel strategies to tackle AD,” Dr. Holtzman and coauthors wrote. “Whether this potential mechanism represents an AD-specific process is not clear, since there is great overlap in immunological changes and gut dysbiosis with other diseases. … In addition, although this study reveals that gut reconditioning may be one mechanism of action of the drug GV-971, it does not rule out other possible mechanisms. For example, GV-971 may attenuate AD pathogenesis by directly inhibiting neuroinflammation or amyloid-beta fibril formation. However, there is no question that [these] data further [support] the emerging idea that modulation of the gut microbiome via treatments such as GV-971 or other strategies should be further explored as novel strategies to slow the progression of AD.”
Sodium oligomannate is a long-chain saccharide extracted from brown sea algae and consists of acidic linear oligosaccharides with structures ranging from dimers to decamers. Related molecules without the sugar backbone were inactive, suggesting that the saccharides are the active portion, Xinyi Wang of Shanghai Green Valley and colleagues wrote in the Cell Research paper.
Based on these studies, the company contends that Alzheimer’s progression is accompanied by a characteristic microbiome change to a proinflammatory profile. And indeed, two transgenic Alzheimer’s mouse models – one with five familial AD mutations (5xFAD) and one with mutations of amyloid precursor protein and presenilin 1 (APP/PS1) – showed similar gradual age- and progression-related decreases of Bacteroides and Verrucomicrobia, two components of a normal microbiome. Bacteroides species perform key functions necessary for survival, including sensing and adapting to nutrient variability, expelling toxins, and stimulating the immune system). Species of the Verrucomicrobia phylum are important in glucose homeostasis. The decline in Bacteroides and Verrucomicrobia species is accompanied by an increase in concomitant proinflammatory species.
The investigators then explored the relationship between the microbiome composition and cognitive function in both transgenic models and a wild-type mouse.
First, they showed that the bacterial populations shifted as the mice aged and their AD pathology developed. This was accompanied by an uptick in activated microglia and, in turn, proinflammatory T1 helper cells that migrated through the intestinal membranes and into the periphery, then cross the blood-brain barrier to enter the brain.
Then the investigators used a cocktail of powerful antibiotics to disturb the intestinal flora in both transgenic and wild-type mice. After this, the 5xFAD mice showed fewer activated microglia and fewer infiltrating T cells. Later, they gave wild-type mice a fecal transplant from the 5xFAD mice. The wild-type mice developed more activated and infiltrating cells and their microbiome began to resemble that of the transgenic mice. Conversely, when the transgenic mice received a transplant from the wild-type mice, their microbiome changed to resemble the donors’, and their activated and infiltrating cells declined.
After this, the team gave GV-971 to the mice. The APP/PS1 mice improved cognitively, and the 5xFAD mice had fewer activated and infiltrating cells, fewer amyloid brain plaques, and less tau phosphorylation. These changes were accompanied by higher levels of two amino acids, phenylalanine and isoleucine. These proteins appear to act on T-cell proliferation and differentiation, they said.
“Taken together, these analyses suggest the idea that gut dysbiosis contributes to [phenylalanine and isoleucine] elevation, which drives the proliferation/differentiation and brain infiltration of [T1 helper] cells,” Dr. Holtzman and coauthors wrote. “These infiltrating Th1 cells may then further activate microglia and contribute to amyloid-related pathogenesis.”
The phase 3 study
The approval of GV-971 was based on the subsequent 36-week phase 3, placebo-controlled study of 818 patients with mild to moderate AD, which was reported last year. Patients were randomized to placebo or to GV-971 450 mg twice daily. Amyloid PET imaging was not required at entrance to the study, so there was no measure of baseline amyloid load. However, all patients showed MRI evidence of cortical atrophy. The Mini Mental State Exam (MMSE) ranged from 11 to 26, indicating mild to moderate AD.
Secondary endpoints included change on the Alzheimer’s Disease Cooperative Study Activities of Daily Living, the Clinician’s Interview-Based Impression of Change–Plus, and the Neuropsychiatric Index.
Patients taking GV-971 experienced a statistically significant 2.54-point difference on the ADAS-cog12, compared with placebo. The difference was apparent by week 4 and was seen at every clinical visit. When patients were grouped according to baseline MMSE (11-14, 15-19, and 20-26), the drug performed similarly.
But none of the secondary endpoints significantly favored of GV-971. And the placebo group behaved in an unexpected way, which could throw the data interpretation off-kilter somewhat, David Knopman, MD, said in an interview.
“It was a very weird and unusual-looking trajectory of the placebo and treated groups,” said Dr. Knopman of the Mayo Clinic, Rochester, Minn., with those taking placebo staying relatively stable for some time before the cognitive scores dropped precipitously. “The data were unconvincing to me.”
He also pointed out that although the extensive preclinical data appeared in the recent peer-reviewed Cell Research paper, the phase 3 data has not appeared in any peer-reviewed forum, “even though the trial has been completed for some time now. Furthermore, the duration of the study was inadequate.”
Finally, none of the study subjects were taking the standard-of-care cholinesterase inhibitors, which virtually every AD patient in the United States does take.
“This makes it almost completely inapplicable to the U.S.,” he said. “It’s not bad news. I’m just not convinced.”
Dr. Holtzman is a cofounder of C2N Diagnostics. He is on the scientific advisory board of Genentech, Denali, and C2N Diagnostics. He consults for Idorsia. Dr. Knopman is a consultant for the Bluefield Project to Cure Frontotemporal Dementia and for Lundbeck.
Survey asks adults: How likely are you to develop dementia?
Donovan T. Maust, MD, and colleagues reported in a research letter published in JAMA Neurology.
More than half of study participants used crossword puzzles as a memory exercise, but only 5% said they spoke to their physician about how to reduce risk. Ironically, this lack of communication was also associated with buying unproven over-the-counter memory supplements, while still remaining ignorant of proven ways to head off dementia and other contributing chronic conditions, wrote Dr. Maust of the University of Michigan, Ann Arbor, and coauthors.
Their analysis of the Michigan National Poll on Healthy Aging found that close to half of respondents (48.5%) reported that they were at least somewhat likely to develop dementia. Another 4.2% thought dementia was “very likely” in their future.
The study comprised survey responses from 1,019 adults aged 50-64 years. Most rated their physical health either excellent (445 respondents) or good (413 respondents). Most also reported excellent or very good mental health (721 respondents); 234 reported good mental health. Many (678) were affluent, with annual incomes of $60,000 or higher. They tended to be well educated; only 337 were without at least some college education. More than half were white (753); there were 101 Hispanic respondents and 93 black respondents. Other groups made up the remainder.
A multivariate analysis found that black respondents were about half as likely to believe they would develop dementia, compared with whites – an assumption contrary to epidemiologic findings that blacks are more likely than whites to develop dementia.
People who reported fair or poor mental health were more than twice as likely to feel dementia was in their future (odds ratio, 2.3). But fair or poor physical health was not significantly associated with that concern.
“Those with fair to poor physical health did not accurately perceive that their likelihood of developing dementia was potentially higher than respondents with very good or excellent physical health,” the authors wrote. “In contrast, fair to poor mental health had the largest association with perceived likelihood of dementia, even though less evidence suggests that poor mental health is causally linked with dementia.”
Despite the concerns, just 5% of respondents said that they had spoken to their physician. Those who believed they had a high likelihood of dementia were more likely to talk with their clinician (7.1%) than those who believed they had a low risk (3.6%).
Many more, however, were using non–evidence-based compounds touted as memory supporting. These included fish oil or omega-3 fatty acids (31.6%) and vitamins or supplements (32.9%). Crossword puzzles were a very popular prevention strategy, employed by about 55% in both belief groups.
“While managing chronic medical conditions, such as diabetes or cardiovascular disease, could reduce dementia risk, few respondents appear to have discussed this with their physician. Given repeated failures of disease-preventing or disease-modifying treatments for dementia, interest in treatment and prevention has shifted earlier in the disease process. Adults in middle age may not accurately estimate their risk of developing dementia, which could lead to both overuse and underuse if preclinical dementia treatments become available. Policy and physicians should emphasize current evidence-based strategies of managing lifestyle and chronic medical conditions to reduce the risk of dementia,” the investigators wrote.
Dr. Maust had no financial disclosures.
SOURCE: Maust D et al. JAMA Neurol. 2019 Nov 15. doi: 10.1001/jamaneurol.2019.3946
I do not find it surprising that older adults fear dementia. Since they correctly perceive that there is no disease-modifying therapy (and maybe also that “getting caught with memory loss” would lead to a loss of driving privileges and other restrictions), they may be trying not to focus on it. As for asking about strategies to “prevent” dementia, that question implies unwarranted optimism about the effectiveness of any such strategy, especially in an older adult. I think we can say that a lifetime of healthy habits (regular physical exercise and careful control of any chronic conditions like diabetes being particularly important) may reduce our risk of dementia a bit, but the idea that anything a 75-year-old does is going to prevent it at that point is probably wishful thinking. Supplements and the like seem to have their own followers. It amazes me how many people suspect what they are taking probably does no good but they do it anyway out of blind hope. Sometimes we can talk them out of spending their money on such things – but not always.
Richard Caselli, MD, is associate director and clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Scottsdale, Ariz.
I do not find it surprising that older adults fear dementia. Since they correctly perceive that there is no disease-modifying therapy (and maybe also that “getting caught with memory loss” would lead to a loss of driving privileges and other restrictions), they may be trying not to focus on it. As for asking about strategies to “prevent” dementia, that question implies unwarranted optimism about the effectiveness of any such strategy, especially in an older adult. I think we can say that a lifetime of healthy habits (regular physical exercise and careful control of any chronic conditions like diabetes being particularly important) may reduce our risk of dementia a bit, but the idea that anything a 75-year-old does is going to prevent it at that point is probably wishful thinking. Supplements and the like seem to have their own followers. It amazes me how many people suspect what they are taking probably does no good but they do it anyway out of blind hope. Sometimes we can talk them out of spending their money on such things – but not always.
Richard Caselli, MD, is associate director and clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Scottsdale, Ariz.
I do not find it surprising that older adults fear dementia. Since they correctly perceive that there is no disease-modifying therapy (and maybe also that “getting caught with memory loss” would lead to a loss of driving privileges and other restrictions), they may be trying not to focus on it. As for asking about strategies to “prevent” dementia, that question implies unwarranted optimism about the effectiveness of any such strategy, especially in an older adult. I think we can say that a lifetime of healthy habits (regular physical exercise and careful control of any chronic conditions like diabetes being particularly important) may reduce our risk of dementia a bit, but the idea that anything a 75-year-old does is going to prevent it at that point is probably wishful thinking. Supplements and the like seem to have their own followers. It amazes me how many people suspect what they are taking probably does no good but they do it anyway out of blind hope. Sometimes we can talk them out of spending their money on such things – but not always.
Richard Caselli, MD, is associate director and clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Scottsdale, Ariz.
Donovan T. Maust, MD, and colleagues reported in a research letter published in JAMA Neurology.
More than half of study participants used crossword puzzles as a memory exercise, but only 5% said they spoke to their physician about how to reduce risk. Ironically, this lack of communication was also associated with buying unproven over-the-counter memory supplements, while still remaining ignorant of proven ways to head off dementia and other contributing chronic conditions, wrote Dr. Maust of the University of Michigan, Ann Arbor, and coauthors.
Their analysis of the Michigan National Poll on Healthy Aging found that close to half of respondents (48.5%) reported that they were at least somewhat likely to develop dementia. Another 4.2% thought dementia was “very likely” in their future.
The study comprised survey responses from 1,019 adults aged 50-64 years. Most rated their physical health either excellent (445 respondents) or good (413 respondents). Most also reported excellent or very good mental health (721 respondents); 234 reported good mental health. Many (678) were affluent, with annual incomes of $60,000 or higher. They tended to be well educated; only 337 were without at least some college education. More than half were white (753); there were 101 Hispanic respondents and 93 black respondents. Other groups made up the remainder.
A multivariate analysis found that black respondents were about half as likely to believe they would develop dementia, compared with whites – an assumption contrary to epidemiologic findings that blacks are more likely than whites to develop dementia.
People who reported fair or poor mental health were more than twice as likely to feel dementia was in their future (odds ratio, 2.3). But fair or poor physical health was not significantly associated with that concern.
“Those with fair to poor physical health did not accurately perceive that their likelihood of developing dementia was potentially higher than respondents with very good or excellent physical health,” the authors wrote. “In contrast, fair to poor mental health had the largest association with perceived likelihood of dementia, even though less evidence suggests that poor mental health is causally linked with dementia.”
Despite the concerns, just 5% of respondents said that they had spoken to their physician. Those who believed they had a high likelihood of dementia were more likely to talk with their clinician (7.1%) than those who believed they had a low risk (3.6%).
Many more, however, were using non–evidence-based compounds touted as memory supporting. These included fish oil or omega-3 fatty acids (31.6%) and vitamins or supplements (32.9%). Crossword puzzles were a very popular prevention strategy, employed by about 55% in both belief groups.
“While managing chronic medical conditions, such as diabetes or cardiovascular disease, could reduce dementia risk, few respondents appear to have discussed this with their physician. Given repeated failures of disease-preventing or disease-modifying treatments for dementia, interest in treatment and prevention has shifted earlier in the disease process. Adults in middle age may not accurately estimate their risk of developing dementia, which could lead to both overuse and underuse if preclinical dementia treatments become available. Policy and physicians should emphasize current evidence-based strategies of managing lifestyle and chronic medical conditions to reduce the risk of dementia,” the investigators wrote.
Dr. Maust had no financial disclosures.
SOURCE: Maust D et al. JAMA Neurol. 2019 Nov 15. doi: 10.1001/jamaneurol.2019.3946
Donovan T. Maust, MD, and colleagues reported in a research letter published in JAMA Neurology.
More than half of study participants used crossword puzzles as a memory exercise, but only 5% said they spoke to their physician about how to reduce risk. Ironically, this lack of communication was also associated with buying unproven over-the-counter memory supplements, while still remaining ignorant of proven ways to head off dementia and other contributing chronic conditions, wrote Dr. Maust of the University of Michigan, Ann Arbor, and coauthors.
Their analysis of the Michigan National Poll on Healthy Aging found that close to half of respondents (48.5%) reported that they were at least somewhat likely to develop dementia. Another 4.2% thought dementia was “very likely” in their future.
The study comprised survey responses from 1,019 adults aged 50-64 years. Most rated their physical health either excellent (445 respondents) or good (413 respondents). Most also reported excellent or very good mental health (721 respondents); 234 reported good mental health. Many (678) were affluent, with annual incomes of $60,000 or higher. They tended to be well educated; only 337 were without at least some college education. More than half were white (753); there were 101 Hispanic respondents and 93 black respondents. Other groups made up the remainder.
A multivariate analysis found that black respondents were about half as likely to believe they would develop dementia, compared with whites – an assumption contrary to epidemiologic findings that blacks are more likely than whites to develop dementia.
People who reported fair or poor mental health were more than twice as likely to feel dementia was in their future (odds ratio, 2.3). But fair or poor physical health was not significantly associated with that concern.
“Those with fair to poor physical health did not accurately perceive that their likelihood of developing dementia was potentially higher than respondents with very good or excellent physical health,” the authors wrote. “In contrast, fair to poor mental health had the largest association with perceived likelihood of dementia, even though less evidence suggests that poor mental health is causally linked with dementia.”
Despite the concerns, just 5% of respondents said that they had spoken to their physician. Those who believed they had a high likelihood of dementia were more likely to talk with their clinician (7.1%) than those who believed they had a low risk (3.6%).
Many more, however, were using non–evidence-based compounds touted as memory supporting. These included fish oil or omega-3 fatty acids (31.6%) and vitamins or supplements (32.9%). Crossword puzzles were a very popular prevention strategy, employed by about 55% in both belief groups.
“While managing chronic medical conditions, such as diabetes or cardiovascular disease, could reduce dementia risk, few respondents appear to have discussed this with their physician. Given repeated failures of disease-preventing or disease-modifying treatments for dementia, interest in treatment and prevention has shifted earlier in the disease process. Adults in middle age may not accurately estimate their risk of developing dementia, which could lead to both overuse and underuse if preclinical dementia treatments become available. Policy and physicians should emphasize current evidence-based strategies of managing lifestyle and chronic medical conditions to reduce the risk of dementia,” the investigators wrote.
Dr. Maust had no financial disclosures.
SOURCE: Maust D et al. JAMA Neurol. 2019 Nov 15. doi: 10.1001/jamaneurol.2019.3946
FROM JAMA NEUROLOGY
Medicare beneficiaries pay most for Alzheimer’s
according to the Kaiser Family Foundation.
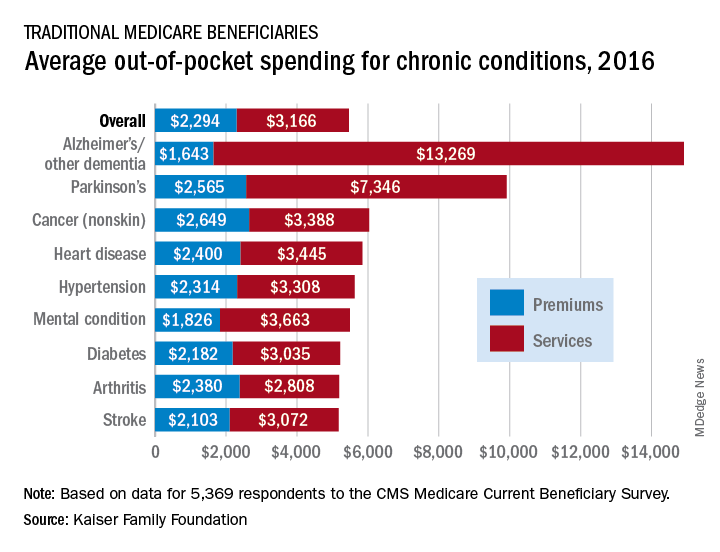
Out-of-pocket spending for Alzheimer’s disease or other dementia was higher than any other chronic condition, averaging $14,913 in 2016 (the latest year for which data are available), compared with $5,460 for all beneficiaries in traditional Medicare, Kaiser investigators said in a recent report based on data for 5,369 respondents to the Medicare Current Beneficiary Survey.
Those totals were divided between services – including long-term care facilities, medical providers and supplies, and prescription drugs – and premiums for Medicare and other types of supplemental insurance. The premium associated with Alzheimer’s, $1,643, was the lowest of any major chronic condition, but the average cost for services, $13,269, was almost twice as high as the next most expensive condition, Parkinson’s disease, and more than four times higher than the overall Medicare average, Juliette Cubanski, PhD, and associates said.
Out-of-pocket costs are higher for patients with Alzheimer’s and Parkinson’s because “these beneficiaries are more likely to reside in a long-term care facility than those with other conditions,” they said. In 2016, out-of-pocket spending on long-term care facility services averaged over $27,000 for Medicare beneficiaries with Alzheimer’s and other dementia and over $28,000 for those with Parkinson’s disease. For all traditional Medicare beneficiaries, average out-of-pocket spending on such services was $1,014.
“The fact that traditional Medicare does not have an annual out-of-pocket limit and does not cover certain services that older adults are more likely to need may undermine the financial security that Medicare provides, especially for people with significant needs and limited incomes. Addressing these gaps would help to alleviate the financial burden of health care for people with Medicare, although doing so would also increase federal spending and taxes,” Dr. Cubanski and associates wrote.
according to the Kaiser Family Foundation.

Out-of-pocket spending for Alzheimer’s disease or other dementia was higher than any other chronic condition, averaging $14,913 in 2016 (the latest year for which data are available), compared with $5,460 for all beneficiaries in traditional Medicare, Kaiser investigators said in a recent report based on data for 5,369 respondents to the Medicare Current Beneficiary Survey.
Those totals were divided between services – including long-term care facilities, medical providers and supplies, and prescription drugs – and premiums for Medicare and other types of supplemental insurance. The premium associated with Alzheimer’s, $1,643, was the lowest of any major chronic condition, but the average cost for services, $13,269, was almost twice as high as the next most expensive condition, Parkinson’s disease, and more than four times higher than the overall Medicare average, Juliette Cubanski, PhD, and associates said.
Out-of-pocket costs are higher for patients with Alzheimer’s and Parkinson’s because “these beneficiaries are more likely to reside in a long-term care facility than those with other conditions,” they said. In 2016, out-of-pocket spending on long-term care facility services averaged over $27,000 for Medicare beneficiaries with Alzheimer’s and other dementia and over $28,000 for those with Parkinson’s disease. For all traditional Medicare beneficiaries, average out-of-pocket spending on such services was $1,014.
“The fact that traditional Medicare does not have an annual out-of-pocket limit and does not cover certain services that older adults are more likely to need may undermine the financial security that Medicare provides, especially for people with significant needs and limited incomes. Addressing these gaps would help to alleviate the financial burden of health care for people with Medicare, although doing so would also increase federal spending and taxes,” Dr. Cubanski and associates wrote.
according to the Kaiser Family Foundation.

Out-of-pocket spending for Alzheimer’s disease or other dementia was higher than any other chronic condition, averaging $14,913 in 2016 (the latest year for which data are available), compared with $5,460 for all beneficiaries in traditional Medicare, Kaiser investigators said in a recent report based on data for 5,369 respondents to the Medicare Current Beneficiary Survey.
Those totals were divided between services – including long-term care facilities, medical providers and supplies, and prescription drugs – and premiums for Medicare and other types of supplemental insurance. The premium associated with Alzheimer’s, $1,643, was the lowest of any major chronic condition, but the average cost for services, $13,269, was almost twice as high as the next most expensive condition, Parkinson’s disease, and more than four times higher than the overall Medicare average, Juliette Cubanski, PhD, and associates said.
Out-of-pocket costs are higher for patients with Alzheimer’s and Parkinson’s because “these beneficiaries are more likely to reside in a long-term care facility than those with other conditions,” they said. In 2016, out-of-pocket spending on long-term care facility services averaged over $27,000 for Medicare beneficiaries with Alzheimer’s and other dementia and over $28,000 for those with Parkinson’s disease. For all traditional Medicare beneficiaries, average out-of-pocket spending on such services was $1,014.
“The fact that traditional Medicare does not have an annual out-of-pocket limit and does not cover certain services that older adults are more likely to need may undermine the financial security that Medicare provides, especially for people with significant needs and limited incomes. Addressing these gaps would help to alleviate the financial burden of health care for people with Medicare, although doing so would also increase federal spending and taxes,” Dr. Cubanski and associates wrote.
Neuropsychological testing: A useful but underutilized resource
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
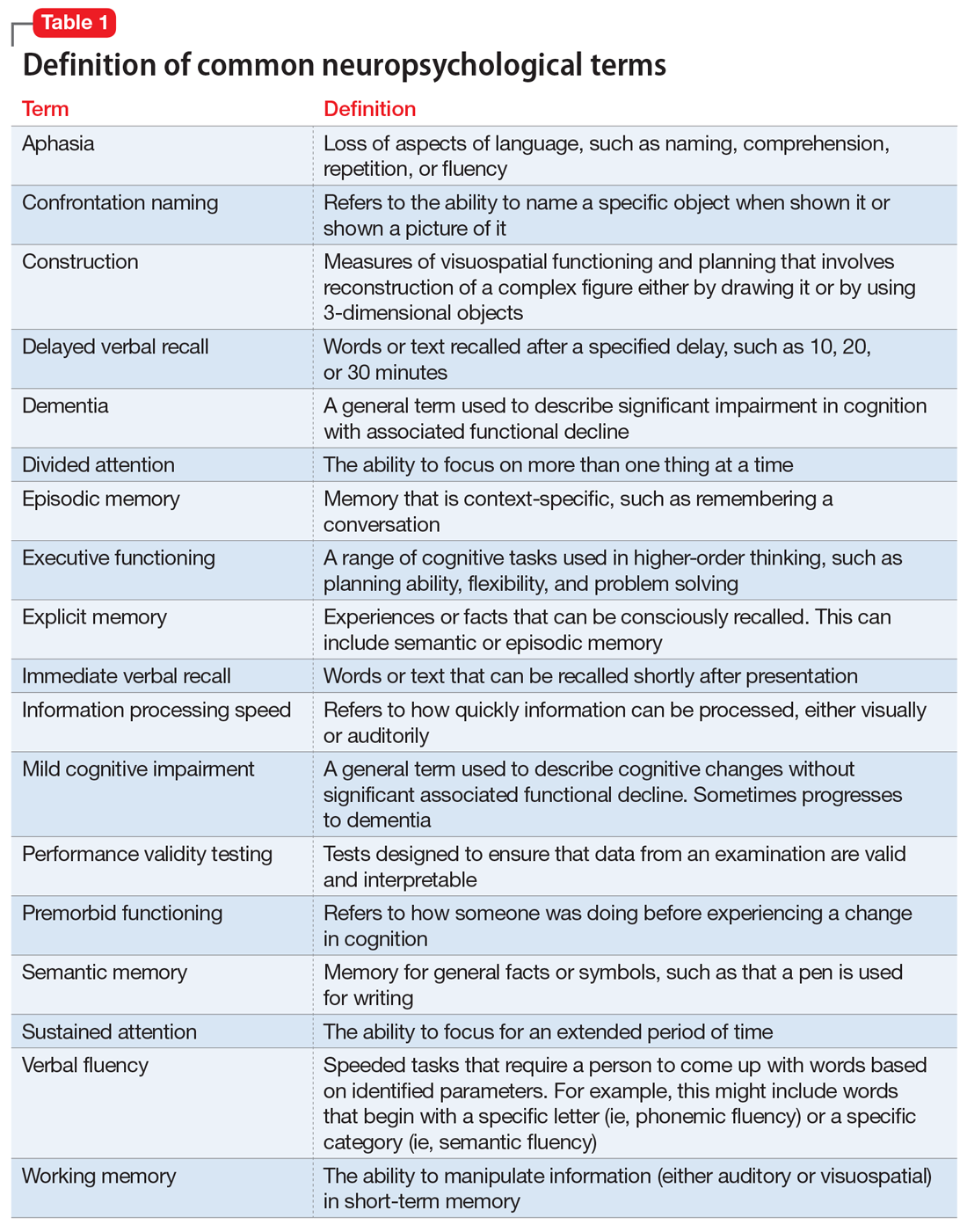
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
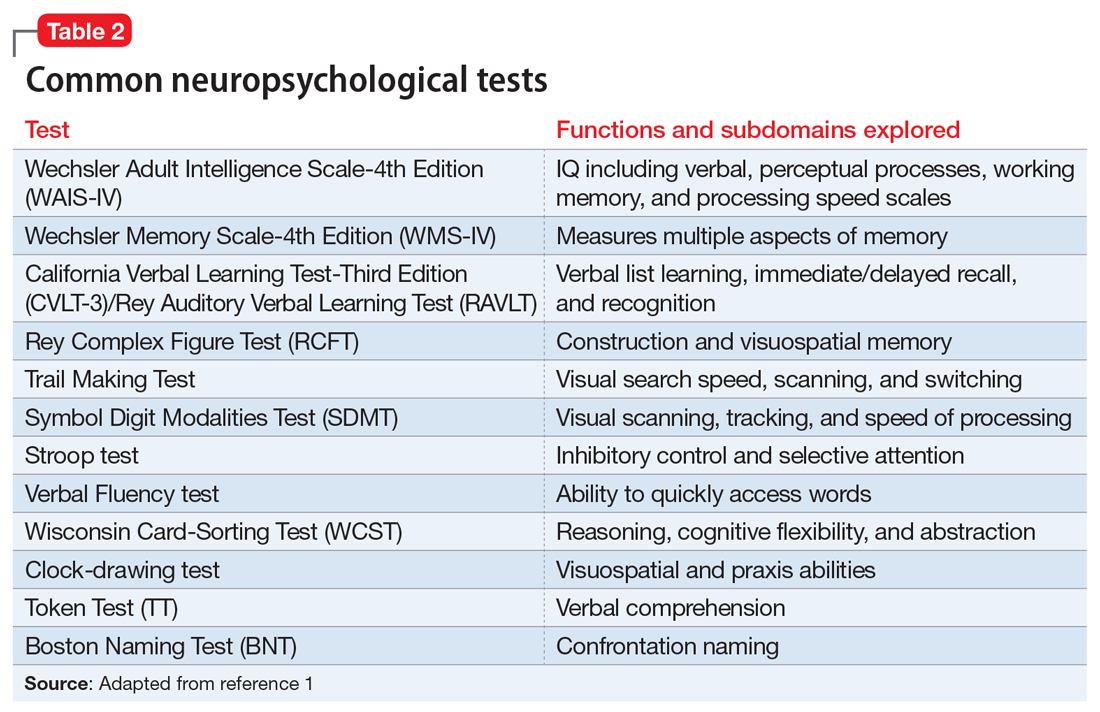
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.

The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1

Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.

The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1

Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
Blood test might rival PET scan for detecting brain amyloidosis
ST. LOUIS – according to a report at the annual meeting of the American Neurological Association. The research was also published in Neurology (2019 Oct 22;93[17]:e1647-59).
Investigators at Washington University, St. Louis, found that, among 158 mostly cognitively normal people in their 60s and 70s, the plasma ratio of amyloid-beta 42 peptide to amyloid-beta 40 peptide identified people who were PET positive and PET negative for amyloid with an area under the curve of 0.88 (95% confidence interval, 0.82-0.93) and climbed to 0.94 when combined with age and Apolipoprotein E epsilon 4 status (95% CI, 0.90-0.97), “which is really quite spectacular for a blood test,” said study lead Suzanne Schindler, MD, PhD, who is affiliated with the university.
People who had a positive blood test – a ratio below .1281 – but a negative PET scan were 15 times more likely to convert to a positive scan at an average of 4 years than subjects with a negative test. “The blood test [detected] brain changes of Alzheimer’s disease before the amyloid PET scan,” Dr. Schindler said.
Amyloid-beta 42 – the number refers to how many amino acids are in the peptide chain – is much stickier and more prone to aggregate in plaques than amyloid-beta 40. The ratio of the two falls as the 42 form is sequestered preferentially into amyloid plaques while the level of amyloid-beta 40 remains more constant, she explained at the meeting.
The team concluded that the test accurately “predicts current and future brain amyloidosis” and “could be used in prevention trials to screen for individuals likely to be amyloid PET-positive and at risk for Alzheimer disease dementia.”
“We are really excited about it. I think there’s been recognition for a long time that a blood test would really be a game changer. We still have a little bit more work to do, but I don’t think it’s that far away,” Dr. Schindler said in an interview after her presentation.
The goal of Alzheimer’s research is to slow, reverse, or even prevent brain pathology before symptoms set in, at which point damage is likely irreversible. For that to happen, plaques need to be detected early.
Currently there are two ways to do that, both with difficulties: PET scans, which are expensive, expose people to radiation, and of limited availability, and spinal fluid analysis, which involves a lumbar puncture that “not many people want to undergo.” The problems slow down enrollment for prevention trials, Dr. Schindler said.
The blood test, which the Food and Drug Administration granted breakthrough status in January 2019, could offer a much easier and less expensive way to identify subjects and monitor outcomes. It could “really speed up enrollment and help us get to effective drugs faster,” she said.
Beyond that, clinicians could use it to help figure out what’s going on in older people with cognitive issues. If a drug or some other way is ever found to prevent Alzheimer’s, there’s even the possibility of screening patients for amyloidosis during routine exams. Potentially, “I think the market is huge,” she said.
The test is being developed by a company, C2N diagnostics, founded by Dr. Schindler’s colleagues at the university, and could be available commercially in 2-3 years. It involves high precision immunoprecipitation and liquid chromatography/mass spectrometry, so “it isn’t something your general lab is going to do. It’s probably going to be a couple centers that have this test, and everybody mails their samples in, which we do for a lot of different tests,” she said.
Several companies are working on similar assays.
Dr. Schindler said she has no financial stake in the blood test.
SOURCE: Schindler S et al. Neurology. 2019 Oct 22;93(17):e1647-59.
ST. LOUIS – according to a report at the annual meeting of the American Neurological Association. The research was also published in Neurology (2019 Oct 22;93[17]:e1647-59).
Investigators at Washington University, St. Louis, found that, among 158 mostly cognitively normal people in their 60s and 70s, the plasma ratio of amyloid-beta 42 peptide to amyloid-beta 40 peptide identified people who were PET positive and PET negative for amyloid with an area under the curve of 0.88 (95% confidence interval, 0.82-0.93) and climbed to 0.94 when combined with age and Apolipoprotein E epsilon 4 status (95% CI, 0.90-0.97), “which is really quite spectacular for a blood test,” said study lead Suzanne Schindler, MD, PhD, who is affiliated with the university.
People who had a positive blood test – a ratio below .1281 – but a negative PET scan were 15 times more likely to convert to a positive scan at an average of 4 years than subjects with a negative test. “The blood test [detected] brain changes of Alzheimer’s disease before the amyloid PET scan,” Dr. Schindler said.
Amyloid-beta 42 – the number refers to how many amino acids are in the peptide chain – is much stickier and more prone to aggregate in plaques than amyloid-beta 40. The ratio of the two falls as the 42 form is sequestered preferentially into amyloid plaques while the level of amyloid-beta 40 remains more constant, she explained at the meeting.
The team concluded that the test accurately “predicts current and future brain amyloidosis” and “could be used in prevention trials to screen for individuals likely to be amyloid PET-positive and at risk for Alzheimer disease dementia.”
“We are really excited about it. I think there’s been recognition for a long time that a blood test would really be a game changer. We still have a little bit more work to do, but I don’t think it’s that far away,” Dr. Schindler said in an interview after her presentation.
The goal of Alzheimer’s research is to slow, reverse, or even prevent brain pathology before symptoms set in, at which point damage is likely irreversible. For that to happen, plaques need to be detected early.
Currently there are two ways to do that, both with difficulties: PET scans, which are expensive, expose people to radiation, and of limited availability, and spinal fluid analysis, which involves a lumbar puncture that “not many people want to undergo.” The problems slow down enrollment for prevention trials, Dr. Schindler said.
The blood test, which the Food and Drug Administration granted breakthrough status in January 2019, could offer a much easier and less expensive way to identify subjects and monitor outcomes. It could “really speed up enrollment and help us get to effective drugs faster,” she said.
Beyond that, clinicians could use it to help figure out what’s going on in older people with cognitive issues. If a drug or some other way is ever found to prevent Alzheimer’s, there’s even the possibility of screening patients for amyloidosis during routine exams. Potentially, “I think the market is huge,” she said.
The test is being developed by a company, C2N diagnostics, founded by Dr. Schindler’s colleagues at the university, and could be available commercially in 2-3 years. It involves high precision immunoprecipitation and liquid chromatography/mass spectrometry, so “it isn’t something your general lab is going to do. It’s probably going to be a couple centers that have this test, and everybody mails their samples in, which we do for a lot of different tests,” she said.
Several companies are working on similar assays.
Dr. Schindler said she has no financial stake in the blood test.
SOURCE: Schindler S et al. Neurology. 2019 Oct 22;93(17):e1647-59.
ST. LOUIS – according to a report at the annual meeting of the American Neurological Association. The research was also published in Neurology (2019 Oct 22;93[17]:e1647-59).
Investigators at Washington University, St. Louis, found that, among 158 mostly cognitively normal people in their 60s and 70s, the plasma ratio of amyloid-beta 42 peptide to amyloid-beta 40 peptide identified people who were PET positive and PET negative for amyloid with an area under the curve of 0.88 (95% confidence interval, 0.82-0.93) and climbed to 0.94 when combined with age and Apolipoprotein E epsilon 4 status (95% CI, 0.90-0.97), “which is really quite spectacular for a blood test,” said study lead Suzanne Schindler, MD, PhD, who is affiliated with the university.
People who had a positive blood test – a ratio below .1281 – but a negative PET scan were 15 times more likely to convert to a positive scan at an average of 4 years than subjects with a negative test. “The blood test [detected] brain changes of Alzheimer’s disease before the amyloid PET scan,” Dr. Schindler said.
Amyloid-beta 42 – the number refers to how many amino acids are in the peptide chain – is much stickier and more prone to aggregate in plaques than amyloid-beta 40. The ratio of the two falls as the 42 form is sequestered preferentially into amyloid plaques while the level of amyloid-beta 40 remains more constant, she explained at the meeting.
The team concluded that the test accurately “predicts current and future brain amyloidosis” and “could be used in prevention trials to screen for individuals likely to be amyloid PET-positive and at risk for Alzheimer disease dementia.”
“We are really excited about it. I think there’s been recognition for a long time that a blood test would really be a game changer. We still have a little bit more work to do, but I don’t think it’s that far away,” Dr. Schindler said in an interview after her presentation.
The goal of Alzheimer’s research is to slow, reverse, or even prevent brain pathology before symptoms set in, at which point damage is likely irreversible. For that to happen, plaques need to be detected early.
Currently there are two ways to do that, both with difficulties: PET scans, which are expensive, expose people to radiation, and of limited availability, and spinal fluid analysis, which involves a lumbar puncture that “not many people want to undergo.” The problems slow down enrollment for prevention trials, Dr. Schindler said.
The blood test, which the Food and Drug Administration granted breakthrough status in January 2019, could offer a much easier and less expensive way to identify subjects and monitor outcomes. It could “really speed up enrollment and help us get to effective drugs faster,” she said.
Beyond that, clinicians could use it to help figure out what’s going on in older people with cognitive issues. If a drug or some other way is ever found to prevent Alzheimer’s, there’s even the possibility of screening patients for amyloidosis during routine exams. Potentially, “I think the market is huge,” she said.
The test is being developed by a company, C2N diagnostics, founded by Dr. Schindler’s colleagues at the university, and could be available commercially in 2-3 years. It involves high precision immunoprecipitation and liquid chromatography/mass spectrometry, so “it isn’t something your general lab is going to do. It’s probably going to be a couple centers that have this test, and everybody mails their samples in, which we do for a lot of different tests,” she said.
Several companies are working on similar assays.
Dr. Schindler said she has no financial stake in the blood test.
SOURCE: Schindler S et al. Neurology. 2019 Oct 22;93(17):e1647-59.
REPORTING FROM ANA 2019
Dr. Paul Aisen Q&A: Aducanumab for Alzheimer’s
In the wake of Biogen and Eisai’s Oct. 22 announcement about plans to apply to the Food and Drug Administration next year for the regulatory approval of the investigational monoclonal antibody aducanumab as a treatment for Alzheimer’s disease, we spoke with Paul Aisen, MD, the founding director of the Alzheimer’s Therapy Research Institute at the University of Southern California, Los Angeles, for his views on the news. He has been a consultant for Biogen and is a member of the aducanumab steering committee.
Q: What was your first reaction when you heard about the plan to submit an application for aducanumab to the FDA?
A: My initial reaction is that this provides terrific support for the amyloid hypothesis, and is consistent with the early aducanumab studies showing significant reductions in brain amyloid with resulting clinical improvement.
My next thought was that these data are going to be very, very challenging to analyze because both of these trials were stopped early, and one was clearly negative. We really need to scrutinize the data, but even at this point I would say this strongly supports targeting amyloid. The scrutiny will begin in detail at the Clinical Trials in Alzheimer’s Disease conference in December, when Biogen will likely release detailed data. A lot of people will analyze it, and I think that’s great. It’s beneficial to bring different perspectives.
We have had a terribly frustrating series of disappointments in the field. After the futility analysis of aducanumab and the multiple failures of BACE [beta-secretase] inhibitors, many were convinced we were barking up the wrong tree. I think these results, although complicated, should resurrect the enthusiasm for targeting amyloid.
Q: What is different about aducanumab from other antibodies tested – and rejected – in Alzheimer’s drug development?
A: There are lots of antibodies that have been tested in clinical trials. They all differ in terms of their affinity for amyloid beta. Some target monomers of the protein. Some target dimers. Some target fibrils. Some tie up amyloid and some reduce it. Aducanumab directly attacks brain plaques, reducing the plaque load in the brain. It carries a liability of amyloid-related imaging abnormalities [ARIA], but it also allows us to assess the impact that removing plaques might have on downstream events, including biomarkers. Overall, these data show that aducanumab did remove brain plaques and that removing them had a beneficial effect on cognition and function, and also a favorable effect on downstream biomarkers.
But again, we must be cautious because this is a complex data set taken from a post hoc analysis of two different terminated trials.
Q: We see some statistically significant differences in cognitive and functional outcomes. What would that mean for patients on an everyday basis?
A: Well, everyone is different, so that’s hard to say. A 25% slowing of functional decline on the Clinical Dementia Rating Scale sum of boxes (CDR-SB) might mean that, at the end of a year, there’s not a significant change in memory, or that there’s better social function. If both trials had been completed and if people had 18 months of high-dose aducanumab, the slowing of functional decline on the CDR-SB might in fact be greater than reported. Again, we’re having to draw conclusions from interrupted trials.
Q: This suggestion you make of a potentially continuous slowing of decline – are you suggesting that aducanumab might slow decline to the point of stopping it altogether? If an elderly patient has little or no progression until death would that, in effect, be considered a “cure?”
A: I don’t think it is possible to cure AD once the disease is clinically evident. These are studies of people with early AD, late mild cognitive impairment, and mild dementia. At that stage, there’s already a loss of synapses that won’t come back, and these studies don’t suggest that aducanumab can cure that. But what if people took it earlier, when the brain is still functioning normally? Some of us have argued for many years that earlier intervention is the way to go. And since we can now identify people [with brain plaques] before they become symptomatic, there is the possibility that if we removed them, we could stop progression.
Q: Are there any plans to study aducanumab as a preventive agent?
A: A grant has been awarded for this, but it was put on hold after the futility analysis. I don’t know when or if that will go forward.
(Editor’s note: The National Institutes of Health previously awarded Banner Health a $32 million, 5-year grant to examine this. The 2-year prevention study of aducanumab is aimed at cognitively unimpaired 65- to 80-year-old patients with PET-confirmed amyloid brain plaques. It was to be a multicenter, double-blind, placebo-controlled trial using Alzheimer’s biomarker endpoints as primary outcomes, along with cognitive and clinical changes, safety, and tolerability. The study was put on hold after Biogen discontinued the aducanumab development program in March. Investigators are considering whether to resurrect plans considering the new data. The study is intended to be a public-private partnership, with additional unspecified funding from Biogen plus $10 million from philanthropic sources. It has three intended goals: To find an approved prevention therapy as early as 2023, ahead of the National Plan to Address Alzheimer’s Disease’s goal of an effective prevention strategy by 2025; to advance the use of surrogate biomarkers to rapidly test and support accelerated approval of prevention therapies in almost everyone at biomarker or genetic risk, even in earlier preclinical Alzheimer’s stages when some treatments may have their greatest benefit; and to help make it possible to conduct prevention trials in at-risk persons even before they have extensive amyloid plaques, when some treatments may have their greatest benefit.)
Q: It seems like rolling this out to an enormous population of patients is going to be difficult, if not impossible. Are people really going to be able to commit to what could be a lifetime of monthly intravenous infusions of a medicine that could be expensive, as therapeutic antibodies generally are?
A: I would say, nothing about this disease is easy. It’s devastating and horrible. And if someone is diagnosed at this stage, I would think that individual would embrace any opportunity to treat it. My hope is that we will be able to prescreen people with an effective blood test for amyloid that would be part of a regular testing protocol once they reach a certain age. Those with positive results would be referred for more testing, including amyloid brain imaging.
In the wake of Biogen and Eisai’s Oct. 22 announcement about plans to apply to the Food and Drug Administration next year for the regulatory approval of the investigational monoclonal antibody aducanumab as a treatment for Alzheimer’s disease, we spoke with Paul Aisen, MD, the founding director of the Alzheimer’s Therapy Research Institute at the University of Southern California, Los Angeles, for his views on the news. He has been a consultant for Biogen and is a member of the aducanumab steering committee.
Q: What was your first reaction when you heard about the plan to submit an application for aducanumab to the FDA?
A: My initial reaction is that this provides terrific support for the amyloid hypothesis, and is consistent with the early aducanumab studies showing significant reductions in brain amyloid with resulting clinical improvement.
My next thought was that these data are going to be very, very challenging to analyze because both of these trials were stopped early, and one was clearly negative. We really need to scrutinize the data, but even at this point I would say this strongly supports targeting amyloid. The scrutiny will begin in detail at the Clinical Trials in Alzheimer’s Disease conference in December, when Biogen will likely release detailed data. A lot of people will analyze it, and I think that’s great. It’s beneficial to bring different perspectives.
We have had a terribly frustrating series of disappointments in the field. After the futility analysis of aducanumab and the multiple failures of BACE [beta-secretase] inhibitors, many were convinced we were barking up the wrong tree. I think these results, although complicated, should resurrect the enthusiasm for targeting amyloid.
Q: What is different about aducanumab from other antibodies tested – and rejected – in Alzheimer’s drug development?
A: There are lots of antibodies that have been tested in clinical trials. They all differ in terms of their affinity for amyloid beta. Some target monomers of the protein. Some target dimers. Some target fibrils. Some tie up amyloid and some reduce it. Aducanumab directly attacks brain plaques, reducing the plaque load in the brain. It carries a liability of amyloid-related imaging abnormalities [ARIA], but it also allows us to assess the impact that removing plaques might have on downstream events, including biomarkers. Overall, these data show that aducanumab did remove brain plaques and that removing them had a beneficial effect on cognition and function, and also a favorable effect on downstream biomarkers.
But again, we must be cautious because this is a complex data set taken from a post hoc analysis of two different terminated trials.
Q: We see some statistically significant differences in cognitive and functional outcomes. What would that mean for patients on an everyday basis?
A: Well, everyone is different, so that’s hard to say. A 25% slowing of functional decline on the Clinical Dementia Rating Scale sum of boxes (CDR-SB) might mean that, at the end of a year, there’s not a significant change in memory, or that there’s better social function. If both trials had been completed and if people had 18 months of high-dose aducanumab, the slowing of functional decline on the CDR-SB might in fact be greater than reported. Again, we’re having to draw conclusions from interrupted trials.
Q: This suggestion you make of a potentially continuous slowing of decline – are you suggesting that aducanumab might slow decline to the point of stopping it altogether? If an elderly patient has little or no progression until death would that, in effect, be considered a “cure?”
A: I don’t think it is possible to cure AD once the disease is clinically evident. These are studies of people with early AD, late mild cognitive impairment, and mild dementia. At that stage, there’s already a loss of synapses that won’t come back, and these studies don’t suggest that aducanumab can cure that. But what if people took it earlier, when the brain is still functioning normally? Some of us have argued for many years that earlier intervention is the way to go. And since we can now identify people [with brain plaques] before they become symptomatic, there is the possibility that if we removed them, we could stop progression.
Q: Are there any plans to study aducanumab as a preventive agent?
A: A grant has been awarded for this, but it was put on hold after the futility analysis. I don’t know when or if that will go forward.
(Editor’s note: The National Institutes of Health previously awarded Banner Health a $32 million, 5-year grant to examine this. The 2-year prevention study of aducanumab is aimed at cognitively unimpaired 65- to 80-year-old patients with PET-confirmed amyloid brain plaques. It was to be a multicenter, double-blind, placebo-controlled trial using Alzheimer’s biomarker endpoints as primary outcomes, along with cognitive and clinical changes, safety, and tolerability. The study was put on hold after Biogen discontinued the aducanumab development program in March. Investigators are considering whether to resurrect plans considering the new data. The study is intended to be a public-private partnership, with additional unspecified funding from Biogen plus $10 million from philanthropic sources. It has three intended goals: To find an approved prevention therapy as early as 2023, ahead of the National Plan to Address Alzheimer’s Disease’s goal of an effective prevention strategy by 2025; to advance the use of surrogate biomarkers to rapidly test and support accelerated approval of prevention therapies in almost everyone at biomarker or genetic risk, even in earlier preclinical Alzheimer’s stages when some treatments may have their greatest benefit; and to help make it possible to conduct prevention trials in at-risk persons even before they have extensive amyloid plaques, when some treatments may have their greatest benefit.)
Q: It seems like rolling this out to an enormous population of patients is going to be difficult, if not impossible. Are people really going to be able to commit to what could be a lifetime of monthly intravenous infusions of a medicine that could be expensive, as therapeutic antibodies generally are?
A: I would say, nothing about this disease is easy. It’s devastating and horrible. And if someone is diagnosed at this stage, I would think that individual would embrace any opportunity to treat it. My hope is that we will be able to prescreen people with an effective blood test for amyloid that would be part of a regular testing protocol once they reach a certain age. Those with positive results would be referred for more testing, including amyloid brain imaging.
In the wake of Biogen and Eisai’s Oct. 22 announcement about plans to apply to the Food and Drug Administration next year for the regulatory approval of the investigational monoclonal antibody aducanumab as a treatment for Alzheimer’s disease, we spoke with Paul Aisen, MD, the founding director of the Alzheimer’s Therapy Research Institute at the University of Southern California, Los Angeles, for his views on the news. He has been a consultant for Biogen and is a member of the aducanumab steering committee.
Q: What was your first reaction when you heard about the plan to submit an application for aducanumab to the FDA?
A: My initial reaction is that this provides terrific support for the amyloid hypothesis, and is consistent with the early aducanumab studies showing significant reductions in brain amyloid with resulting clinical improvement.
My next thought was that these data are going to be very, very challenging to analyze because both of these trials were stopped early, and one was clearly negative. We really need to scrutinize the data, but even at this point I would say this strongly supports targeting amyloid. The scrutiny will begin in detail at the Clinical Trials in Alzheimer’s Disease conference in December, when Biogen will likely release detailed data. A lot of people will analyze it, and I think that’s great. It’s beneficial to bring different perspectives.
We have had a terribly frustrating series of disappointments in the field. After the futility analysis of aducanumab and the multiple failures of BACE [beta-secretase] inhibitors, many were convinced we were barking up the wrong tree. I think these results, although complicated, should resurrect the enthusiasm for targeting amyloid.
Q: What is different about aducanumab from other antibodies tested – and rejected – in Alzheimer’s drug development?
A: There are lots of antibodies that have been tested in clinical trials. They all differ in terms of their affinity for amyloid beta. Some target monomers of the protein. Some target dimers. Some target fibrils. Some tie up amyloid and some reduce it. Aducanumab directly attacks brain plaques, reducing the plaque load in the brain. It carries a liability of amyloid-related imaging abnormalities [ARIA], but it also allows us to assess the impact that removing plaques might have on downstream events, including biomarkers. Overall, these data show that aducanumab did remove brain plaques and that removing them had a beneficial effect on cognition and function, and also a favorable effect on downstream biomarkers.
But again, we must be cautious because this is a complex data set taken from a post hoc analysis of two different terminated trials.
Q: We see some statistically significant differences in cognitive and functional outcomes. What would that mean for patients on an everyday basis?
A: Well, everyone is different, so that’s hard to say. A 25% slowing of functional decline on the Clinical Dementia Rating Scale sum of boxes (CDR-SB) might mean that, at the end of a year, there’s not a significant change in memory, or that there’s better social function. If both trials had been completed and if people had 18 months of high-dose aducanumab, the slowing of functional decline on the CDR-SB might in fact be greater than reported. Again, we’re having to draw conclusions from interrupted trials.
Q: This suggestion you make of a potentially continuous slowing of decline – are you suggesting that aducanumab might slow decline to the point of stopping it altogether? If an elderly patient has little or no progression until death would that, in effect, be considered a “cure?”
A: I don’t think it is possible to cure AD once the disease is clinically evident. These are studies of people with early AD, late mild cognitive impairment, and mild dementia. At that stage, there’s already a loss of synapses that won’t come back, and these studies don’t suggest that aducanumab can cure that. But what if people took it earlier, when the brain is still functioning normally? Some of us have argued for many years that earlier intervention is the way to go. And since we can now identify people [with brain plaques] before they become symptomatic, there is the possibility that if we removed them, we could stop progression.
Q: Are there any plans to study aducanumab as a preventive agent?
A: A grant has been awarded for this, but it was put on hold after the futility analysis. I don’t know when or if that will go forward.
(Editor’s note: The National Institutes of Health previously awarded Banner Health a $32 million, 5-year grant to examine this. The 2-year prevention study of aducanumab is aimed at cognitively unimpaired 65- to 80-year-old patients with PET-confirmed amyloid brain plaques. It was to be a multicenter, double-blind, placebo-controlled trial using Alzheimer’s biomarker endpoints as primary outcomes, along with cognitive and clinical changes, safety, and tolerability. The study was put on hold after Biogen discontinued the aducanumab development program in March. Investigators are considering whether to resurrect plans considering the new data. The study is intended to be a public-private partnership, with additional unspecified funding from Biogen plus $10 million from philanthropic sources. It has three intended goals: To find an approved prevention therapy as early as 2023, ahead of the National Plan to Address Alzheimer’s Disease’s goal of an effective prevention strategy by 2025; to advance the use of surrogate biomarkers to rapidly test and support accelerated approval of prevention therapies in almost everyone at biomarker or genetic risk, even in earlier preclinical Alzheimer’s stages when some treatments may have their greatest benefit; and to help make it possible to conduct prevention trials in at-risk persons even before they have extensive amyloid plaques, when some treatments may have their greatest benefit.)
Q: It seems like rolling this out to an enormous population of patients is going to be difficult, if not impossible. Are people really going to be able to commit to what could be a lifetime of monthly intravenous infusions of a medicine that could be expensive, as therapeutic antibodies generally are?
A: I would say, nothing about this disease is easy. It’s devastating and horrible. And if someone is diagnosed at this stage, I would think that individual would embrace any opportunity to treat it. My hope is that we will be able to prescreen people with an effective blood test for amyloid that would be part of a regular testing protocol once they reach a certain age. Those with positive results would be referred for more testing, including amyloid brain imaging.













