User login
Psychiatric manifestations of sport-related concussion
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
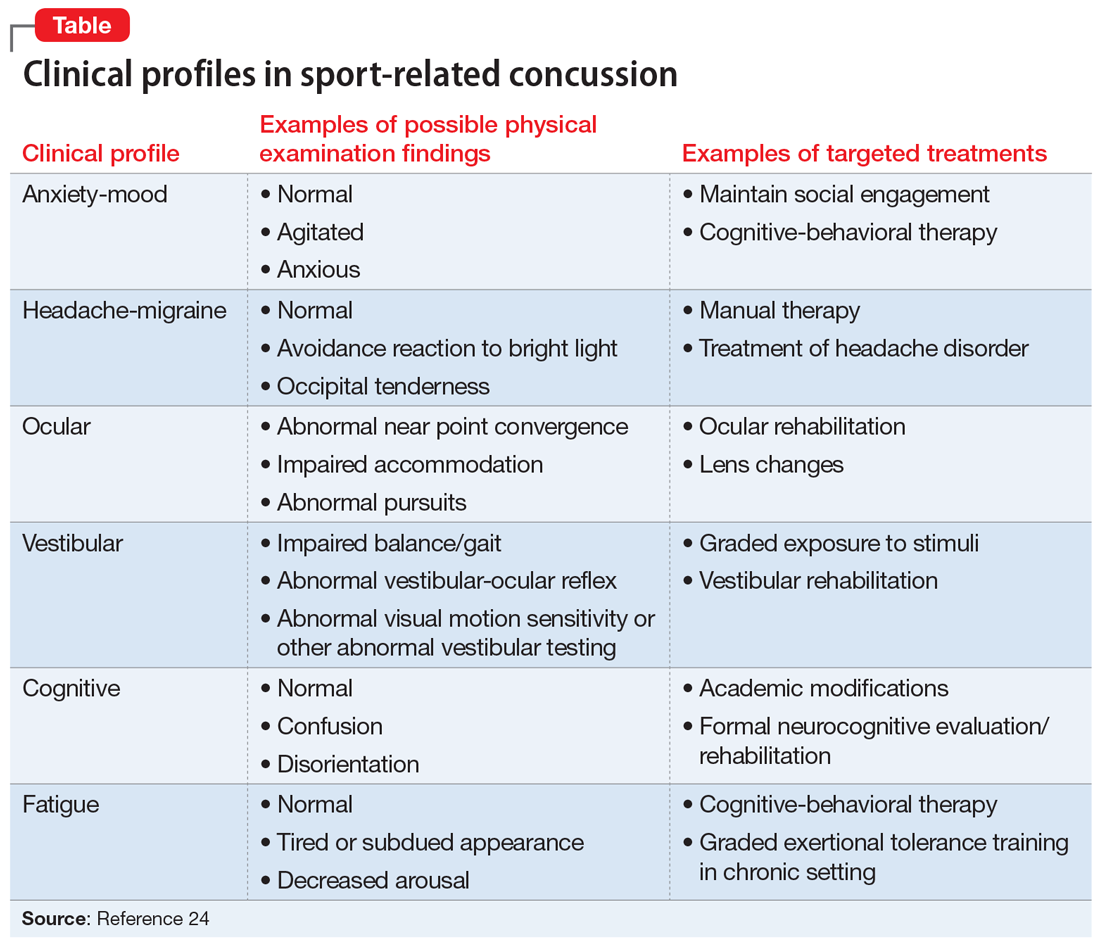
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.
Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
Five healthy lifestyle choices tied to dramatic cut in dementia risk
“I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, department of internal medicine, Rush University Medical Center, Chicago, said in an interview.
The study was published online June 17 in Neurology.
Risk-modifying behaviors
To help quantify the impact of a healthy life on risk for Alzheimer’s dementia, Dr. Dhana and colleagues reviewed data from two longitudinal study populations: the Chicago Health and Aging Project (CHAP), with 1,845 participants, and the Memory and Aging Project (MAP), with 920 participants.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise of moderate to vigorous intensity; light to moderate alcohol consumption (between 1 and less than 15 g/day for women and between 1 and less than 30 g/day for men); consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities (upper 40%). The overall score ranged from 0 to 5.
At baseline, the mean age of participants was 73.2 years in the CHAP study and 81.1 years in the MAP study; 62.4% of the CHAP participants and 75.2% of the MAP participants were women.
During a median follow-up of 5.8 years in CHAP and 6.0 years in MAP, a total of 379 and 229 participants, respectively, developed Alzheimer’s dementia. Rates of dementia decreased with an increasing number of healthy lifestyle behaviors.
In multivariable-adjusted models across the two cohorts, the risk for Alzheimer’s dementia was 27% lower with each additional healthy lifestyle factor (pooled hazard ratio, 0.73; 95% confidence interval, 0.66-0.80).
Compared with individuals with a healthy lifestyle score of 0-1, the risk was 37% lower (pooled HR, 0.63; 95% CI, 0.47-0.84) for those with two or three healthy lifestyle factors and 60% lower (pooled HR, 0.40; 95% CI, 0.28-0.56) for those with four or five healthy lifestyle factors.
“From these findings and the fact that the lifestyle factors we studied are modifiable and in direct control of the individual, it is imperative to promote them concurrently among older adults as a strategy to delay or prevent Alzheimer’s dementia,” Dr. Dhana and colleagues concluded.
In a statement, Dallas Anderson, PhD, program director, division of neuroscience, National Institute on Aging, said the findings help “paint the picture of how multiple factors are likely playing parts in Alzheimer’s disease risk.”
“It’s not a clear cause-and-effect result, but a strong finding because of the dual data sets and combination of modifiable lifestyle factors that appear to lead to risk reduction,” Dr. Anderson added.
Essential questions remain
Commenting on the new study, Luca Giliberto, MD, PhD, neurologist with the Litwin-Zucker Research Center for Alzheimer’s Disease and Memory Disorders at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said this analysis is “further demonstration that a healthy lifestyle is essential to overcome or curb” the risk for Alzheimer’s disease.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Dr. Giliberto.
Of note, he added, although addressing vascular risk factors such as hypertension, hyperlipidemia, and diabetes “may require an extensive mindful and logistic effort, a healthy diet is effortlessly achieved in some countries, where both the DASH and MIND diets do not need to be ‘prescribed’ but are rather culturally engraved in the population.
“This is, in part, related to the wide availability of high-quality food in these countries, which is not the same in the U.S. This work is one more demonstration of the need to revisit our take on quality of food in the U.S.,” said Dr. Giliberto.
Numerous clinical trials testing lifestyle interventions for dementia prevention are currently underway. The MIND Diet Intervention to Prevent Alzheimer’s Disease, for example, is an interventional clinical trial comparing parallel groups with two different diets. MIND has enrolled more than 600 participants and is ongoing. The anticipated completion date is 2021. Another is the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), a multisite randomized clinical trial evaluating whether lifestyle interventions – including exercise, cognitively stimulating activities, and the MIND diet – may protect cognitive function in older adults who are at increased risk for cognitive decline.
Funding for the current study was provided by the National Institutes of Health and the National Institute on Aging. Dr. Dhana and Dr. Giliberto have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, department of internal medicine, Rush University Medical Center, Chicago, said in an interview.
The study was published online June 17 in Neurology.
Risk-modifying behaviors
To help quantify the impact of a healthy life on risk for Alzheimer’s dementia, Dr. Dhana and colleagues reviewed data from two longitudinal study populations: the Chicago Health and Aging Project (CHAP), with 1,845 participants, and the Memory and Aging Project (MAP), with 920 participants.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise of moderate to vigorous intensity; light to moderate alcohol consumption (between 1 and less than 15 g/day for women and between 1 and less than 30 g/day for men); consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities (upper 40%). The overall score ranged from 0 to 5.
At baseline, the mean age of participants was 73.2 years in the CHAP study and 81.1 years in the MAP study; 62.4% of the CHAP participants and 75.2% of the MAP participants were women.
During a median follow-up of 5.8 years in CHAP and 6.0 years in MAP, a total of 379 and 229 participants, respectively, developed Alzheimer’s dementia. Rates of dementia decreased with an increasing number of healthy lifestyle behaviors.
In multivariable-adjusted models across the two cohorts, the risk for Alzheimer’s dementia was 27% lower with each additional healthy lifestyle factor (pooled hazard ratio, 0.73; 95% confidence interval, 0.66-0.80).
Compared with individuals with a healthy lifestyle score of 0-1, the risk was 37% lower (pooled HR, 0.63; 95% CI, 0.47-0.84) for those with two or three healthy lifestyle factors and 60% lower (pooled HR, 0.40; 95% CI, 0.28-0.56) for those with four or five healthy lifestyle factors.
“From these findings and the fact that the lifestyle factors we studied are modifiable and in direct control of the individual, it is imperative to promote them concurrently among older adults as a strategy to delay or prevent Alzheimer’s dementia,” Dr. Dhana and colleagues concluded.
In a statement, Dallas Anderson, PhD, program director, division of neuroscience, National Institute on Aging, said the findings help “paint the picture of how multiple factors are likely playing parts in Alzheimer’s disease risk.”
“It’s not a clear cause-and-effect result, but a strong finding because of the dual data sets and combination of modifiable lifestyle factors that appear to lead to risk reduction,” Dr. Anderson added.
Essential questions remain
Commenting on the new study, Luca Giliberto, MD, PhD, neurologist with the Litwin-Zucker Research Center for Alzheimer’s Disease and Memory Disorders at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said this analysis is “further demonstration that a healthy lifestyle is essential to overcome or curb” the risk for Alzheimer’s disease.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Dr. Giliberto.
Of note, he added, although addressing vascular risk factors such as hypertension, hyperlipidemia, and diabetes “may require an extensive mindful and logistic effort, a healthy diet is effortlessly achieved in some countries, where both the DASH and MIND diets do not need to be ‘prescribed’ but are rather culturally engraved in the population.
“This is, in part, related to the wide availability of high-quality food in these countries, which is not the same in the U.S. This work is one more demonstration of the need to revisit our take on quality of food in the U.S.,” said Dr. Giliberto.
Numerous clinical trials testing lifestyle interventions for dementia prevention are currently underway. The MIND Diet Intervention to Prevent Alzheimer’s Disease, for example, is an interventional clinical trial comparing parallel groups with two different diets. MIND has enrolled more than 600 participants and is ongoing. The anticipated completion date is 2021. Another is the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), a multisite randomized clinical trial evaluating whether lifestyle interventions – including exercise, cognitively stimulating activities, and the MIND diet – may protect cognitive function in older adults who are at increased risk for cognitive decline.
Funding for the current study was provided by the National Institutes of Health and the National Institute on Aging. Dr. Dhana and Dr. Giliberto have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, department of internal medicine, Rush University Medical Center, Chicago, said in an interview.
The study was published online June 17 in Neurology.
Risk-modifying behaviors
To help quantify the impact of a healthy life on risk for Alzheimer’s dementia, Dr. Dhana and colleagues reviewed data from two longitudinal study populations: the Chicago Health and Aging Project (CHAP), with 1,845 participants, and the Memory and Aging Project (MAP), with 920 participants.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise of moderate to vigorous intensity; light to moderate alcohol consumption (between 1 and less than 15 g/day for women and between 1 and less than 30 g/day for men); consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities (upper 40%). The overall score ranged from 0 to 5.
At baseline, the mean age of participants was 73.2 years in the CHAP study and 81.1 years in the MAP study; 62.4% of the CHAP participants and 75.2% of the MAP participants were women.
During a median follow-up of 5.8 years in CHAP and 6.0 years in MAP, a total of 379 and 229 participants, respectively, developed Alzheimer’s dementia. Rates of dementia decreased with an increasing number of healthy lifestyle behaviors.
In multivariable-adjusted models across the two cohorts, the risk for Alzheimer’s dementia was 27% lower with each additional healthy lifestyle factor (pooled hazard ratio, 0.73; 95% confidence interval, 0.66-0.80).
Compared with individuals with a healthy lifestyle score of 0-1, the risk was 37% lower (pooled HR, 0.63; 95% CI, 0.47-0.84) for those with two or three healthy lifestyle factors and 60% lower (pooled HR, 0.40; 95% CI, 0.28-0.56) for those with four or five healthy lifestyle factors.
“From these findings and the fact that the lifestyle factors we studied are modifiable and in direct control of the individual, it is imperative to promote them concurrently among older adults as a strategy to delay or prevent Alzheimer’s dementia,” Dr. Dhana and colleagues concluded.
In a statement, Dallas Anderson, PhD, program director, division of neuroscience, National Institute on Aging, said the findings help “paint the picture of how multiple factors are likely playing parts in Alzheimer’s disease risk.”
“It’s not a clear cause-and-effect result, but a strong finding because of the dual data sets and combination of modifiable lifestyle factors that appear to lead to risk reduction,” Dr. Anderson added.
Essential questions remain
Commenting on the new study, Luca Giliberto, MD, PhD, neurologist with the Litwin-Zucker Research Center for Alzheimer’s Disease and Memory Disorders at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said this analysis is “further demonstration that a healthy lifestyle is essential to overcome or curb” the risk for Alzheimer’s disease.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Dr. Giliberto.
Of note, he added, although addressing vascular risk factors such as hypertension, hyperlipidemia, and diabetes “may require an extensive mindful and logistic effort, a healthy diet is effortlessly achieved in some countries, where both the DASH and MIND diets do not need to be ‘prescribed’ but are rather culturally engraved in the population.
“This is, in part, related to the wide availability of high-quality food in these countries, which is not the same in the U.S. This work is one more demonstration of the need to revisit our take on quality of food in the U.S.,” said Dr. Giliberto.
Numerous clinical trials testing lifestyle interventions for dementia prevention are currently underway. The MIND Diet Intervention to Prevent Alzheimer’s Disease, for example, is an interventional clinical trial comparing parallel groups with two different diets. MIND has enrolled more than 600 participants and is ongoing. The anticipated completion date is 2021. Another is the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), a multisite randomized clinical trial evaluating whether lifestyle interventions – including exercise, cognitively stimulating activities, and the MIND diet – may protect cognitive function in older adults who are at increased risk for cognitive decline.
Funding for the current study was provided by the National Institutes of Health and the National Institute on Aging. Dr. Dhana and Dr. Giliberto have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY
Population study supports migraine–dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
“The findings of this study emphasize the need for studies in the migraine-dementia pathophysiology, in particular in migraine cases with aura,” said Sabrina Islamoska, MSc, PhD, a postdoctoral researcher in the department of public health at the University of Copenhagen. “This study highlights the importance of monitoring severe migraine to potentially prevent dementia.”
A national register-based study
The study used Danish national register–based data from 1988 to 2017 of 1.66 million individuals born between 1935 and 1956, retrieving exposure information until age 59 years and following individuals for dementia after age 60. The matched analysis included 18,135 people registered with migraine before age 59 and 1.38 million without migraine. The matched study population was 62,578.
A diagnosis of dementia or use of dementia medications after age 60 years was the main outcome. Covariates included socioeconomic factors, psychiatric comorbidities and other headache diagnoses.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Islamoska said.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“We also found a 20% higher but nonsignificant dementia rate in individuals who had migraine without aura,” she said. However, when the migraine-with-aura population was evaluated, it was found to have a dementia rate two times higher than people with no migraine. “The dementia rate was higher if individuals had more frequent hospital contacts with migraine.”
The findings support the hypothesis that migraine is a midlife risk factor for dementia later in life, she said.
“The findings underline the value of investigating the effect of migraine medications in dementia risk to assess the impact of mild to moderate migraines,” Dr. Islamoska said. “Therefore, the next step is to investigate the risk of dementia among users of migraine medications who are not diagnosed with migraines at hospitals.”
Strengths of the study, Dr. Islamoska noted, were its size and national nature of its population, that it included all migraine diagnoses at hospitals over a 29-year period, that it made adjustments for confounding of well-established dementia risk factors, and that it validated dementia diagnoses after age 60 years.
One limitation was that the study only included hospital-based diagnoses of dementia while 60% of cases in Denmark are undiagnosed, “thus our results only apply to migraine that is severe enough to require a hospital contact,” Dr. Islamoska said, while most migraine cases are treated in the primary care setting.
Also, the young study population may have a lower dementia risk. “We also know that age of migraine registration may not corresponded with the actual onset, since migraine is a complex disorder with individual variation in patient’s burden and course of disease,” Dr. Islamoska said.
“Future studies are needed to understand the pathological mechanisms underlying the relationship between migraine and dementia and to investigate whether proper prophylactic treatment of migraine can potentially prevent dementia,” Dr. Islamoska said. “In addition, when investigating the association between these two prevalent neurological disorders, the timing of migraine diagnosis and dementia onset is important to ensure temporality. We took this into account in our study to strengthen the validity of our results.”
‘Surprising’ findings
Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles, said the Danish study makes an important contribution to the literature on dementia risk factors. “Vanishingly small amounts of attention have been paid to migraine as a potential risk factor,” he said. However, he called the results “surprising” based on his own clinical experience. “I actually had a sense that migraine was somehow protective against Alzheimer’s or other kinds of dementias.”
He questioned if the migraine-dementia link could be a “reporting artifact” of migraine sufferers merely going to the neurologist, raising the likelihood of a positive migraine diagnosis. Nonetheless, the results are “intriguing” and raise important questions about migraine therapy and dementia risk.
“If it holds up, it really is something that behooves us to understand whether intervening in terms of therapy for migraine has even more consequences beyond just the immediate relief of symptoms,” Dr. Charles said. “It’s something we should be thinking about in terms of preventing longer-term consequences of this disorder.”
Dr. Islamoska disclosed that Veluxfondent funded the study as part of her PhD project. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
SOURCE: Islamoska S et al. AHS 2020, Submission 846214.
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
“The findings of this study emphasize the need for studies in the migraine-dementia pathophysiology, in particular in migraine cases with aura,” said Sabrina Islamoska, MSc, PhD, a postdoctoral researcher in the department of public health at the University of Copenhagen. “This study highlights the importance of monitoring severe migraine to potentially prevent dementia.”
A national register-based study
The study used Danish national register–based data from 1988 to 2017 of 1.66 million individuals born between 1935 and 1956, retrieving exposure information until age 59 years and following individuals for dementia after age 60. The matched analysis included 18,135 people registered with migraine before age 59 and 1.38 million without migraine. The matched study population was 62,578.
A diagnosis of dementia or use of dementia medications after age 60 years was the main outcome. Covariates included socioeconomic factors, psychiatric comorbidities and other headache diagnoses.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Islamoska said.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“We also found a 20% higher but nonsignificant dementia rate in individuals who had migraine without aura,” she said. However, when the migraine-with-aura population was evaluated, it was found to have a dementia rate two times higher than people with no migraine. “The dementia rate was higher if individuals had more frequent hospital contacts with migraine.”
The findings support the hypothesis that migraine is a midlife risk factor for dementia later in life, she said.
“The findings underline the value of investigating the effect of migraine medications in dementia risk to assess the impact of mild to moderate migraines,” Dr. Islamoska said. “Therefore, the next step is to investigate the risk of dementia among users of migraine medications who are not diagnosed with migraines at hospitals.”
Strengths of the study, Dr. Islamoska noted, were its size and national nature of its population, that it included all migraine diagnoses at hospitals over a 29-year period, that it made adjustments for confounding of well-established dementia risk factors, and that it validated dementia diagnoses after age 60 years.
One limitation was that the study only included hospital-based diagnoses of dementia while 60% of cases in Denmark are undiagnosed, “thus our results only apply to migraine that is severe enough to require a hospital contact,” Dr. Islamoska said, while most migraine cases are treated in the primary care setting.
Also, the young study population may have a lower dementia risk. “We also know that age of migraine registration may not corresponded with the actual onset, since migraine is a complex disorder with individual variation in patient’s burden and course of disease,” Dr. Islamoska said.
“Future studies are needed to understand the pathological mechanisms underlying the relationship between migraine and dementia and to investigate whether proper prophylactic treatment of migraine can potentially prevent dementia,” Dr. Islamoska said. “In addition, when investigating the association between these two prevalent neurological disorders, the timing of migraine diagnosis and dementia onset is important to ensure temporality. We took this into account in our study to strengthen the validity of our results.”
‘Surprising’ findings
Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles, said the Danish study makes an important contribution to the literature on dementia risk factors. “Vanishingly small amounts of attention have been paid to migraine as a potential risk factor,” he said. However, he called the results “surprising” based on his own clinical experience. “I actually had a sense that migraine was somehow protective against Alzheimer’s or other kinds of dementias.”
He questioned if the migraine-dementia link could be a “reporting artifact” of migraine sufferers merely going to the neurologist, raising the likelihood of a positive migraine diagnosis. Nonetheless, the results are “intriguing” and raise important questions about migraine therapy and dementia risk.
“If it holds up, it really is something that behooves us to understand whether intervening in terms of therapy for migraine has even more consequences beyond just the immediate relief of symptoms,” Dr. Charles said. “It’s something we should be thinking about in terms of preventing longer-term consequences of this disorder.”
Dr. Islamoska disclosed that Veluxfondent funded the study as part of her PhD project. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
SOURCE: Islamoska S et al. AHS 2020, Submission 846214.
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
“The findings of this study emphasize the need for studies in the migraine-dementia pathophysiology, in particular in migraine cases with aura,” said Sabrina Islamoska, MSc, PhD, a postdoctoral researcher in the department of public health at the University of Copenhagen. “This study highlights the importance of monitoring severe migraine to potentially prevent dementia.”
A national register-based study
The study used Danish national register–based data from 1988 to 2017 of 1.66 million individuals born between 1935 and 1956, retrieving exposure information until age 59 years and following individuals for dementia after age 60. The matched analysis included 18,135 people registered with migraine before age 59 and 1.38 million without migraine. The matched study population was 62,578.
A diagnosis of dementia or use of dementia medications after age 60 years was the main outcome. Covariates included socioeconomic factors, psychiatric comorbidities and other headache diagnoses.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Islamoska said.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“We also found a 20% higher but nonsignificant dementia rate in individuals who had migraine without aura,” she said. However, when the migraine-with-aura population was evaluated, it was found to have a dementia rate two times higher than people with no migraine. “The dementia rate was higher if individuals had more frequent hospital contacts with migraine.”
The findings support the hypothesis that migraine is a midlife risk factor for dementia later in life, she said.
“The findings underline the value of investigating the effect of migraine medications in dementia risk to assess the impact of mild to moderate migraines,” Dr. Islamoska said. “Therefore, the next step is to investigate the risk of dementia among users of migraine medications who are not diagnosed with migraines at hospitals.”
Strengths of the study, Dr. Islamoska noted, were its size and national nature of its population, that it included all migraine diagnoses at hospitals over a 29-year period, that it made adjustments for confounding of well-established dementia risk factors, and that it validated dementia diagnoses after age 60 years.
One limitation was that the study only included hospital-based diagnoses of dementia while 60% of cases in Denmark are undiagnosed, “thus our results only apply to migraine that is severe enough to require a hospital contact,” Dr. Islamoska said, while most migraine cases are treated in the primary care setting.
Also, the young study population may have a lower dementia risk. “We also know that age of migraine registration may not corresponded with the actual onset, since migraine is a complex disorder with individual variation in patient’s burden and course of disease,” Dr. Islamoska said.
“Future studies are needed to understand the pathological mechanisms underlying the relationship between migraine and dementia and to investigate whether proper prophylactic treatment of migraine can potentially prevent dementia,” Dr. Islamoska said. “In addition, when investigating the association between these two prevalent neurological disorders, the timing of migraine diagnosis and dementia onset is important to ensure temporality. We took this into account in our study to strengthen the validity of our results.”
‘Surprising’ findings
Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles, said the Danish study makes an important contribution to the literature on dementia risk factors. “Vanishingly small amounts of attention have been paid to migraine as a potential risk factor,” he said. However, he called the results “surprising” based on his own clinical experience. “I actually had a sense that migraine was somehow protective against Alzheimer’s or other kinds of dementias.”
He questioned if the migraine-dementia link could be a “reporting artifact” of migraine sufferers merely going to the neurologist, raising the likelihood of a positive migraine diagnosis. Nonetheless, the results are “intriguing” and raise important questions about migraine therapy and dementia risk.
“If it holds up, it really is something that behooves us to understand whether intervening in terms of therapy for migraine has even more consequences beyond just the immediate relief of symptoms,” Dr. Charles said. “It’s something we should be thinking about in terms of preventing longer-term consequences of this disorder.”
Dr. Islamoska disclosed that Veluxfondent funded the study as part of her PhD project. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
SOURCE: Islamoska S et al. AHS 2020, Submission 846214.
FROM AHS 2020
FDA okays first tau radiotracer to aid Alzheimer’s disease diagnosis
to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs) in adults with cognitive impairment who are being evaluated for Alzheimer disease.
“While there are FDA-approved imaging drugs for amyloid pathology, this is the first drug approved for imaging tau pathology, one of the two neuropathological hallmarks of Alzheimer’s disease, and represents a major advance for patients with cognitive impairment being evaluated for the condition,” Charles Ganley, MD, director of the Office of Specialty Medicine at the Center for Drug Evaluation and Research, said in an FDA news release.
“The use of diagnostic imaging can help patients and their families plan for the future and make informed choices about their health and well-being, in addition to facilitating appropriate patient management for physicians,” Reisa Sperling, MD, director of the Center for Alzheimer Research and Treatment at Brigham and Women’s Hospital and Massachusetts General Hospital, Boston, said in a company news release.
“Determining the anatomic distribution and density of tau NFTs in the brain was previously possible only at autopsy. Now we have a way to obtain this important information in patients,” said Dr. Sperling.
Clinical trial results
Following intravenous administration, flortaucipir F18 binds to tau pathology in the brain and can be seen on a PET scan.
The safety and effectiveness of the tau tracer were demonstrated in two clinical studies. In each study, five evaluators, blinded to clinical information, interpreted the flortaucipir F18 PET scan results as positive or negative.
The first study included 156 terminally ill patients who agreed to undergo flortaucipir F18 PET imaging and to donate their brains after death. Of these patients, 64 died within 9 months of undergoing brain scanning. The evaluators’ readings of these scans were compared with postmortem readings from independent pathologists blinded to scan results.
Evaluators reading the flortaucipir F18 PET scans had a “high probability” of correctly evaluating patients with tau pathology and had an “average to high probability” of correctly evaluating patients without tau pathology, the FDA said in the release.
According to the company, reader sensitivity ranged from 92% (95% confidence interval, 80%-97%) to 100% (95% CI, 91%-100%). Specificity ranged from 52% (95% CI, 34%-70%) to 92% (95% CI, 75%-98%).
Initial limited availability
The second study included the same patients with terminal illness as the first study, plus 18 additional patients who had terminal illness and 159 patients who had cognitive impairment and were being evaluated for Alzheimer’s disease (the indicated population).
The study gauged how well evaluators’ readings of flortaucipir F18 PET scans agreed with each other’s assessments of the readings. In this study, reader agreement was 0.87 (perfect agreement was indicated as 1) across all 241 patients.
In a separate subgroup analysis that included the 82 terminally ill patients who were diagnosed after death and the 159 patients with cognitive impairment, reader agreement was 0.90 for the patients in the indicated population and 0.82 in the terminally ill patients.
The FDA noted that the ability of flortaucipir F18 PET scans to detect tau pathology was assessed in patients with generally severe stages of dementia and may be lower in patients with cognitive decline of earlier stages.
The most common adverse reactions among patients who received flortaucipir F18 injection were headache, injection site pain, and an increase in blood pressure. The tau radiotracer is not indicated for use in the evaluation of patients for chronic traumatic encephalopathy.
The FDA granted flortaucipir F18 priority review, in which the FDA aims to take action on an application within 6 months of the time the agency determines that the drug, if approved, would significantly improve the safety or effectiveness of treating, diagnosing, or preventing a serious condition.
The company said that the availability of flortaucipir F18 will initially be “limited and will expand in response to commercial demand and payor reimbursement.”
Alzheimer’s disease is among the top 10 leading causes of death in the United States. In 2014, 5 million Americans were living with the disease, according to federal health officials. That number is projected to nearly triple to 14 million by 2060.
A version of this article originally appeared on Medscape.com.
to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs) in adults with cognitive impairment who are being evaluated for Alzheimer disease.
“While there are FDA-approved imaging drugs for amyloid pathology, this is the first drug approved for imaging tau pathology, one of the two neuropathological hallmarks of Alzheimer’s disease, and represents a major advance for patients with cognitive impairment being evaluated for the condition,” Charles Ganley, MD, director of the Office of Specialty Medicine at the Center for Drug Evaluation and Research, said in an FDA news release.
“The use of diagnostic imaging can help patients and their families plan for the future and make informed choices about their health and well-being, in addition to facilitating appropriate patient management for physicians,” Reisa Sperling, MD, director of the Center for Alzheimer Research and Treatment at Brigham and Women’s Hospital and Massachusetts General Hospital, Boston, said in a company news release.
“Determining the anatomic distribution and density of tau NFTs in the brain was previously possible only at autopsy. Now we have a way to obtain this important information in patients,” said Dr. Sperling.
Clinical trial results
Following intravenous administration, flortaucipir F18 binds to tau pathology in the brain and can be seen on a PET scan.
The safety and effectiveness of the tau tracer were demonstrated in two clinical studies. In each study, five evaluators, blinded to clinical information, interpreted the flortaucipir F18 PET scan results as positive or negative.
The first study included 156 terminally ill patients who agreed to undergo flortaucipir F18 PET imaging and to donate their brains after death. Of these patients, 64 died within 9 months of undergoing brain scanning. The evaluators’ readings of these scans were compared with postmortem readings from independent pathologists blinded to scan results.
Evaluators reading the flortaucipir F18 PET scans had a “high probability” of correctly evaluating patients with tau pathology and had an “average to high probability” of correctly evaluating patients without tau pathology, the FDA said in the release.
According to the company, reader sensitivity ranged from 92% (95% confidence interval, 80%-97%) to 100% (95% CI, 91%-100%). Specificity ranged from 52% (95% CI, 34%-70%) to 92% (95% CI, 75%-98%).
Initial limited availability
The second study included the same patients with terminal illness as the first study, plus 18 additional patients who had terminal illness and 159 patients who had cognitive impairment and were being evaluated for Alzheimer’s disease (the indicated population).
The study gauged how well evaluators’ readings of flortaucipir F18 PET scans agreed with each other’s assessments of the readings. In this study, reader agreement was 0.87 (perfect agreement was indicated as 1) across all 241 patients.
In a separate subgroup analysis that included the 82 terminally ill patients who were diagnosed after death and the 159 patients with cognitive impairment, reader agreement was 0.90 for the patients in the indicated population and 0.82 in the terminally ill patients.
The FDA noted that the ability of flortaucipir F18 PET scans to detect tau pathology was assessed in patients with generally severe stages of dementia and may be lower in patients with cognitive decline of earlier stages.
The most common adverse reactions among patients who received flortaucipir F18 injection were headache, injection site pain, and an increase in blood pressure. The tau radiotracer is not indicated for use in the evaluation of patients for chronic traumatic encephalopathy.
The FDA granted flortaucipir F18 priority review, in which the FDA aims to take action on an application within 6 months of the time the agency determines that the drug, if approved, would significantly improve the safety or effectiveness of treating, diagnosing, or preventing a serious condition.
The company said that the availability of flortaucipir F18 will initially be “limited and will expand in response to commercial demand and payor reimbursement.”
Alzheimer’s disease is among the top 10 leading causes of death in the United States. In 2014, 5 million Americans were living with the disease, according to federal health officials. That number is projected to nearly triple to 14 million by 2060.
A version of this article originally appeared on Medscape.com.
to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs) in adults with cognitive impairment who are being evaluated for Alzheimer disease.
“While there are FDA-approved imaging drugs for amyloid pathology, this is the first drug approved for imaging tau pathology, one of the two neuropathological hallmarks of Alzheimer’s disease, and represents a major advance for patients with cognitive impairment being evaluated for the condition,” Charles Ganley, MD, director of the Office of Specialty Medicine at the Center for Drug Evaluation and Research, said in an FDA news release.
“The use of diagnostic imaging can help patients and their families plan for the future and make informed choices about their health and well-being, in addition to facilitating appropriate patient management for physicians,” Reisa Sperling, MD, director of the Center for Alzheimer Research and Treatment at Brigham and Women’s Hospital and Massachusetts General Hospital, Boston, said in a company news release.
“Determining the anatomic distribution and density of tau NFTs in the brain was previously possible only at autopsy. Now we have a way to obtain this important information in patients,” said Dr. Sperling.
Clinical trial results
Following intravenous administration, flortaucipir F18 binds to tau pathology in the brain and can be seen on a PET scan.
The safety and effectiveness of the tau tracer were demonstrated in two clinical studies. In each study, five evaluators, blinded to clinical information, interpreted the flortaucipir F18 PET scan results as positive or negative.
The first study included 156 terminally ill patients who agreed to undergo flortaucipir F18 PET imaging and to donate their brains after death. Of these patients, 64 died within 9 months of undergoing brain scanning. The evaluators’ readings of these scans were compared with postmortem readings from independent pathologists blinded to scan results.
Evaluators reading the flortaucipir F18 PET scans had a “high probability” of correctly evaluating patients with tau pathology and had an “average to high probability” of correctly evaluating patients without tau pathology, the FDA said in the release.
According to the company, reader sensitivity ranged from 92% (95% confidence interval, 80%-97%) to 100% (95% CI, 91%-100%). Specificity ranged from 52% (95% CI, 34%-70%) to 92% (95% CI, 75%-98%).
Initial limited availability
The second study included the same patients with terminal illness as the first study, plus 18 additional patients who had terminal illness and 159 patients who had cognitive impairment and were being evaluated for Alzheimer’s disease (the indicated population).
The study gauged how well evaluators’ readings of flortaucipir F18 PET scans agreed with each other’s assessments of the readings. In this study, reader agreement was 0.87 (perfect agreement was indicated as 1) across all 241 patients.
In a separate subgroup analysis that included the 82 terminally ill patients who were diagnosed after death and the 159 patients with cognitive impairment, reader agreement was 0.90 for the patients in the indicated population and 0.82 in the terminally ill patients.
The FDA noted that the ability of flortaucipir F18 PET scans to detect tau pathology was assessed in patients with generally severe stages of dementia and may be lower in patients with cognitive decline of earlier stages.
The most common adverse reactions among patients who received flortaucipir F18 injection were headache, injection site pain, and an increase in blood pressure. The tau radiotracer is not indicated for use in the evaluation of patients for chronic traumatic encephalopathy.
The FDA granted flortaucipir F18 priority review, in which the FDA aims to take action on an application within 6 months of the time the agency determines that the drug, if approved, would significantly improve the safety or effectiveness of treating, diagnosing, or preventing a serious condition.
The company said that the availability of flortaucipir F18 will initially be “limited and will expand in response to commercial demand and payor reimbursement.”
Alzheimer’s disease is among the top 10 leading causes of death in the United States. In 2014, 5 million Americans were living with the disease, according to federal health officials. That number is projected to nearly triple to 14 million by 2060.
A version of this article originally appeared on Medscape.com.
Mixed results for aducanumab in two phase 3 trials for Alzheimer’s disease
Aducanumab was associated with favorable changes in activities of daily living and in Alzheimer’s disease biomarkers.
The EMERGE and ENGAGE studies compared low-dose and high-dose aducanumab and placebo over 78 weeks. The high-dose EMERGE cohort experienced a 22% improvement in the primary outcome – adjusted mean Clinical Dementia Rating Sum of Box (CDR-SB) scores – compared with baseline.
“We have with EMERGE, in the high-dose group, a positive result,” said lead author Samantha Budd Haeberlein, PhD, who presented this research online as part of the 2020 American Academy of Neurology Science Highlights.
In contrast, the low-dose EMERGE group, as well as the low-dose and high-dose cohorts in the ENGAGE study, experienced no statistically significant change in CDR-SB outcomes.
Clinical benefit was associated with the degree of exposure to aducanumab. For example, a protocol adjustment during the study increased the mean dose of aducanumab, a move associated with better outcomes.
“We believe that the difference between the results was largely due to patients’ greater exposure to the high dose of aducanumab,” Dr. Haerberlein, senior vice president and head of the neurodegeneration development unit at Biogen in Cambridge, Mass., said in an interview.
Although the studies shared an identical design, “because ENGAGE began enrolling first and recruitment remained ahead of EMERGE, more patients in EMERGE were impacted by the protocol amendments, which we believe resulted in a higher number of patients exposed to the highest dose in EMERGE versus ENGAGE,” Dr. Haerberlein added.
The EMERGE and ENGAGE studies were conducted at 348 sites in 20 countries. The research included a total of 3,285 participants with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
The mean age was 70 years, about 52% were women, and slightly more than half had a history of taking medication for Alzheimer’s disease. The mean Mini-Mental State Exam (MMSE) score was 26 at baseline.
Key findings
Dr. Haerberlein and colleagues reported that the 22% decrease in CDR-SB scores in the high-dose EMERGE participants was significant (P = .01). No significant difference emerged, however, in the ENGAGE study, where high-dose participants had a 2% decrease at 78 weeks in CDR-SB scores (P = .83).
The high-dose EMERGE regimen was also associated with an 18% improvement in MMSE scores (P < .05). In the ENGAGE study, the high-dose MMSE scores increased a nonsignificant 3% (P = .81).
The researchers reported no significant differences in the low-dose cohorts in both studies regarding CDR-SB or MMSE scores at week 78, compared with baseline.
They also assessed amyloid using PET scans. Levels remained essentially the same throughout both studies in the placebo participants. In contrast, there was a statistically significant, dose- and time-dependent reduction associated with both low- and high-dose aducanumab.
Aducanumab treatment was associated with significant benefits on measures of cognition and function such as memory, orientation, and language, Dr. Haeberlein said. “Patients also experienced benefits on activities of daily living including conducting personal finances; performing household chores such as cleaning, shopping, and doing laundry; and independently traveling out of the home.”
Furthermore, reductions in the CSF biomarker phospho-tau in the high-dose EMERGE and ENGAGE cohorts were statistically significant. In contrast, changes in total tau were not significant.
The proportion of patients who experienced an adverse event during EMERGE was similar across groups – 92% of the high-dose group, 88% of the low-dose group, and 87% of the placebo cohort. Similar rates were reported in the ENGAGE high-dose, 90%; low-dose, 90%; and placebo cohorts, 86%.
Adverse events reported in more than 10% of participants included headache, nasopharyngitis, and two forms of amyloid-related imaging abnormalities (ARIA), one of which related to edema (ARIA-E) and the other to hemosiderosis (ARIA-H).
Future plans
Going forward, the researchers are conducting a redosing study to offer aducanumab to all participants in the clinical trials. Also, Biogen is completing the filing of a Biologics License Application with the Food and Drug Administration and with regulatory agencies in other countries.
Early identification and treatment of Alzheimer’s disease remains a priority, Dr. Haeberlein said, because it offers an opportunity to begin health measures like exercise, mental activity, and social engagement; allows people more time to plan for the future; and gives families and loved ones’ time to prepare and support each other. From a research perspective, early identification of this population can maximize chances of participation in a clinical trial as well.
Unanswered questions
“Briefly, while both [studies] were looking at aducanumab’s effect on rate of decline across a variety of measures, one statistically showed a positive impact in a subset and the other did not,” Richard J. Caselli, MD, said when asked to comment on the EMERGE and ENGAGE findings. “The subset were the mildest affected patients on the highest dose for the longest time.”
The main difference between the two studies was that one was adequately powered for this subanalysis and the other was not. Even the underpowered subanalysis showed a beneficial trend, added Dr. Caselli, a neurologist at the Mayo Clinic in Phoenix, Arizona.
Dr. Caselli said these findings raise a number of unanswered questions. For example, is a subanalysis valid? Is the degree of improvement clinically meaningful or meaningful enough to justify the anticipated cost of the drug itself – “likely to be very expensive” plus the “cost and hassle” of monthly IV infusions? Is there enough provider and infusion center capacity going forward? What will the reimbursement from third party payers be like?
Biogen sponsored the EMERGE and ENGAGE studies. Dr. Haeberlein is a Biogen employee. Dr. Caselli had no relevant disclosures.
SOURCE: Haeberlein SB et al. AAN 2020, Abstract 46977.
Aducanumab was associated with favorable changes in activities of daily living and in Alzheimer’s disease biomarkers.
The EMERGE and ENGAGE studies compared low-dose and high-dose aducanumab and placebo over 78 weeks. The high-dose EMERGE cohort experienced a 22% improvement in the primary outcome – adjusted mean Clinical Dementia Rating Sum of Box (CDR-SB) scores – compared with baseline.
“We have with EMERGE, in the high-dose group, a positive result,” said lead author Samantha Budd Haeberlein, PhD, who presented this research online as part of the 2020 American Academy of Neurology Science Highlights.
In contrast, the low-dose EMERGE group, as well as the low-dose and high-dose cohorts in the ENGAGE study, experienced no statistically significant change in CDR-SB outcomes.
Clinical benefit was associated with the degree of exposure to aducanumab. For example, a protocol adjustment during the study increased the mean dose of aducanumab, a move associated with better outcomes.
“We believe that the difference between the results was largely due to patients’ greater exposure to the high dose of aducanumab,” Dr. Haerberlein, senior vice president and head of the neurodegeneration development unit at Biogen in Cambridge, Mass., said in an interview.
Although the studies shared an identical design, “because ENGAGE began enrolling first and recruitment remained ahead of EMERGE, more patients in EMERGE were impacted by the protocol amendments, which we believe resulted in a higher number of patients exposed to the highest dose in EMERGE versus ENGAGE,” Dr. Haerberlein added.
The EMERGE and ENGAGE studies were conducted at 348 sites in 20 countries. The research included a total of 3,285 participants with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
The mean age was 70 years, about 52% were women, and slightly more than half had a history of taking medication for Alzheimer’s disease. The mean Mini-Mental State Exam (MMSE) score was 26 at baseline.
Key findings
Dr. Haerberlein and colleagues reported that the 22% decrease in CDR-SB scores in the high-dose EMERGE participants was significant (P = .01). No significant difference emerged, however, in the ENGAGE study, where high-dose participants had a 2% decrease at 78 weeks in CDR-SB scores (P = .83).
The high-dose EMERGE regimen was also associated with an 18% improvement in MMSE scores (P < .05). In the ENGAGE study, the high-dose MMSE scores increased a nonsignificant 3% (P = .81).
The researchers reported no significant differences in the low-dose cohorts in both studies regarding CDR-SB or MMSE scores at week 78, compared with baseline.
They also assessed amyloid using PET scans. Levels remained essentially the same throughout both studies in the placebo participants. In contrast, there was a statistically significant, dose- and time-dependent reduction associated with both low- and high-dose aducanumab.
Aducanumab treatment was associated with significant benefits on measures of cognition and function such as memory, orientation, and language, Dr. Haeberlein said. “Patients also experienced benefits on activities of daily living including conducting personal finances; performing household chores such as cleaning, shopping, and doing laundry; and independently traveling out of the home.”
Furthermore, reductions in the CSF biomarker phospho-tau in the high-dose EMERGE and ENGAGE cohorts were statistically significant. In contrast, changes in total tau were not significant.
The proportion of patients who experienced an adverse event during EMERGE was similar across groups – 92% of the high-dose group, 88% of the low-dose group, and 87% of the placebo cohort. Similar rates were reported in the ENGAGE high-dose, 90%; low-dose, 90%; and placebo cohorts, 86%.
Adverse events reported in more than 10% of participants included headache, nasopharyngitis, and two forms of amyloid-related imaging abnormalities (ARIA), one of which related to edema (ARIA-E) and the other to hemosiderosis (ARIA-H).
Future plans
Going forward, the researchers are conducting a redosing study to offer aducanumab to all participants in the clinical trials. Also, Biogen is completing the filing of a Biologics License Application with the Food and Drug Administration and with regulatory agencies in other countries.
Early identification and treatment of Alzheimer’s disease remains a priority, Dr. Haeberlein said, because it offers an opportunity to begin health measures like exercise, mental activity, and social engagement; allows people more time to plan for the future; and gives families and loved ones’ time to prepare and support each other. From a research perspective, early identification of this population can maximize chances of participation in a clinical trial as well.
Unanswered questions
“Briefly, while both [studies] were looking at aducanumab’s effect on rate of decline across a variety of measures, one statistically showed a positive impact in a subset and the other did not,” Richard J. Caselli, MD, said when asked to comment on the EMERGE and ENGAGE findings. “The subset were the mildest affected patients on the highest dose for the longest time.”
The main difference between the two studies was that one was adequately powered for this subanalysis and the other was not. Even the underpowered subanalysis showed a beneficial trend, added Dr. Caselli, a neurologist at the Mayo Clinic in Phoenix, Arizona.
Dr. Caselli said these findings raise a number of unanswered questions. For example, is a subanalysis valid? Is the degree of improvement clinically meaningful or meaningful enough to justify the anticipated cost of the drug itself – “likely to be very expensive” plus the “cost and hassle” of monthly IV infusions? Is there enough provider and infusion center capacity going forward? What will the reimbursement from third party payers be like?
Biogen sponsored the EMERGE and ENGAGE studies. Dr. Haeberlein is a Biogen employee. Dr. Caselli had no relevant disclosures.
SOURCE: Haeberlein SB et al. AAN 2020, Abstract 46977.
Aducanumab was associated with favorable changes in activities of daily living and in Alzheimer’s disease biomarkers.
The EMERGE and ENGAGE studies compared low-dose and high-dose aducanumab and placebo over 78 weeks. The high-dose EMERGE cohort experienced a 22% improvement in the primary outcome – adjusted mean Clinical Dementia Rating Sum of Box (CDR-SB) scores – compared with baseline.
“We have with EMERGE, in the high-dose group, a positive result,” said lead author Samantha Budd Haeberlein, PhD, who presented this research online as part of the 2020 American Academy of Neurology Science Highlights.
In contrast, the low-dose EMERGE group, as well as the low-dose and high-dose cohorts in the ENGAGE study, experienced no statistically significant change in CDR-SB outcomes.
Clinical benefit was associated with the degree of exposure to aducanumab. For example, a protocol adjustment during the study increased the mean dose of aducanumab, a move associated with better outcomes.
“We believe that the difference between the results was largely due to patients’ greater exposure to the high dose of aducanumab,” Dr. Haerberlein, senior vice president and head of the neurodegeneration development unit at Biogen in Cambridge, Mass., said in an interview.
Although the studies shared an identical design, “because ENGAGE began enrolling first and recruitment remained ahead of EMERGE, more patients in EMERGE were impacted by the protocol amendments, which we believe resulted in a higher number of patients exposed to the highest dose in EMERGE versus ENGAGE,” Dr. Haerberlein added.
The EMERGE and ENGAGE studies were conducted at 348 sites in 20 countries. The research included a total of 3,285 participants with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
The mean age was 70 years, about 52% were women, and slightly more than half had a history of taking medication for Alzheimer’s disease. The mean Mini-Mental State Exam (MMSE) score was 26 at baseline.
Key findings
Dr. Haerberlein and colleagues reported that the 22% decrease in CDR-SB scores in the high-dose EMERGE participants was significant (P = .01). No significant difference emerged, however, in the ENGAGE study, where high-dose participants had a 2% decrease at 78 weeks in CDR-SB scores (P = .83).
The high-dose EMERGE regimen was also associated with an 18% improvement in MMSE scores (P < .05). In the ENGAGE study, the high-dose MMSE scores increased a nonsignificant 3% (P = .81).
The researchers reported no significant differences in the low-dose cohorts in both studies regarding CDR-SB or MMSE scores at week 78, compared with baseline.
They also assessed amyloid using PET scans. Levels remained essentially the same throughout both studies in the placebo participants. In contrast, there was a statistically significant, dose- and time-dependent reduction associated with both low- and high-dose aducanumab.
Aducanumab treatment was associated with significant benefits on measures of cognition and function such as memory, orientation, and language, Dr. Haeberlein said. “Patients also experienced benefits on activities of daily living including conducting personal finances; performing household chores such as cleaning, shopping, and doing laundry; and independently traveling out of the home.”
Furthermore, reductions in the CSF biomarker phospho-tau in the high-dose EMERGE and ENGAGE cohorts were statistically significant. In contrast, changes in total tau were not significant.
The proportion of patients who experienced an adverse event during EMERGE was similar across groups – 92% of the high-dose group, 88% of the low-dose group, and 87% of the placebo cohort. Similar rates were reported in the ENGAGE high-dose, 90%; low-dose, 90%; and placebo cohorts, 86%.
Adverse events reported in more than 10% of participants included headache, nasopharyngitis, and two forms of amyloid-related imaging abnormalities (ARIA), one of which related to edema (ARIA-E) and the other to hemosiderosis (ARIA-H).
Future plans
Going forward, the researchers are conducting a redosing study to offer aducanumab to all participants in the clinical trials. Also, Biogen is completing the filing of a Biologics License Application with the Food and Drug Administration and with regulatory agencies in other countries.
Early identification and treatment of Alzheimer’s disease remains a priority, Dr. Haeberlein said, because it offers an opportunity to begin health measures like exercise, mental activity, and social engagement; allows people more time to plan for the future; and gives families and loved ones’ time to prepare and support each other. From a research perspective, early identification of this population can maximize chances of participation in a clinical trial as well.
Unanswered questions
“Briefly, while both [studies] were looking at aducanumab’s effect on rate of decline across a variety of measures, one statistically showed a positive impact in a subset and the other did not,” Richard J. Caselli, MD, said when asked to comment on the EMERGE and ENGAGE findings. “The subset were the mildest affected patients on the highest dose for the longest time.”
The main difference between the two studies was that one was adequately powered for this subanalysis and the other was not. Even the underpowered subanalysis showed a beneficial trend, added Dr. Caselli, a neurologist at the Mayo Clinic in Phoenix, Arizona.
Dr. Caselli said these findings raise a number of unanswered questions. For example, is a subanalysis valid? Is the degree of improvement clinically meaningful or meaningful enough to justify the anticipated cost of the drug itself – “likely to be very expensive” plus the “cost and hassle” of monthly IV infusions? Is there enough provider and infusion center capacity going forward? What will the reimbursement from third party payers be like?
Biogen sponsored the EMERGE and ENGAGE studies. Dr. Haeberlein is a Biogen employee. Dr. Caselli had no relevant disclosures.
SOURCE: Haeberlein SB et al. AAN 2020, Abstract 46977.
FROM AAN 2020
COVID-19: Psychiatrists assess geriatric harm from social distancing
One of the greatest tragedies of the first wave of the COVID-19 pandemic has been the failure of health policy makers to anticipate and mitigate the enormous havoc the policy of social distancing would wreak on mental health and cognitive function in older persons, speakers agreed at a webinar on COVID-19, social distancing, and its impact on social and mental health in the elderly hosted by the International Psychogeriatric Association in collaboration with INTERDEM.
“Social distancing” is a two-edged sword: It is for now and the foreseeable future the only available effective strategy for protecting against infection in the older population most vulnerable to severe forms of COVID-19. Yet social distancing also has caused many elderly – particularly those in nursing homes and other long-term care facilities – to plunge into a profound experience of loneliness, isolation, distress, feelings of abandonment, anxiety, depression, and accelerated cognitive deterioration. And this needn’t have happened, the mental health professionals asserted.
“When are we going to get rid of the term ‘social distancing?’ ” asked IPA President William E. Reichman, MD. “Many have appreciated – including the World Health Organization – that the real issue is physical distancing to prevent contagion. And physical distancing doesn’t have to mean social distancing.”
Social connectedness between elderly persons and their peers and family members can be maintained and should be emphatically encouraged during the physical distancing required by the pandemic, said Myrra Vernooij-Dassen, PhD, of Radboud University in Nigmegen, the Netherlands, and chair of INTERDEM, a pan-European network of dementia researchers.
This can be achieved using readily available technologies, including the telephone and videoconferencing, as well as by creating opportunities for supervised masked visits between a family member and an elderly loved one in outdoor courtyards or gardens within long-term care facilities. And yet, as the pandemic seized hold in many parts of the world, family members were blocked from entry to these facilities, she observed.
Impact on mental health, cognition
Dr. Vernooij-Dassen noted that studies of previous quarantine periods as well as preliminary findings during the COVID-19 pandemic demonstrate an inverse relationship between social isolation measures and cognitive functioning in the elderly.
“ Conversely, epidemiologic data indicate that a socially integrated lifestyle had a favorable influence on cognitive functioning and could even delay onset of dementia,” she said.
INTERDEM is backing two ongoing studies evaluating the hypothesis that interventions fostering increased social interaction among elderly individuals can delay onset of dementia or favorably affect its course. The proposed mechanism of benefit is stimulation of brain plasticity to enhance cognitive reserve.
“This is a hypothesis of hope. We know that social interaction for humans is like water to plants – we really, really need it,” she explained.
Diego de Leo, MD, PhD, emeritus professor of psychiatry and former director of the Australian Institute for Suicide Research and Prevention at Griffith University in Brisbane, was living in hard-hit Padua, Italy, during the first surge of COVID-19. He described his anecdotal experience.
“What I hear from many Italian colleagues and friends and directors of mental health services is that emergency admissions related to mental disorders declined during the first wave of the COVID pandemic. For example, not many people attended emergency departments due to suicide attempts; there was a very marked decrease in the number of suicide attempts during the worst days of the pandemic,” he said.
People with psychiatric conditions were afraid to go to the hospital because they thought they would contract the infection and die there. That’s changing now, however.
“Now there is an increased number of admissions to mental health units. A new wave. It has been a U-shaped curve. And we’re now witnessing an increasing number of fatal suicides due to persistent fears, due to people imagining that there is no more room for them, and no more future for them from a financial point of view – which is the major negative outcome of this crisis. It will be a disaster for many families,” the psychiatrist continued.
A noteworthy phenomenon in northern Italy was that, when tablets were made available to nursing home residents in an effort to enhance their connectedness to the outside world, those with dementia often became so frustrated and confused by their difficulty in using the devices that they developed a hypokinetic delirium marked by refusal to eat or leave their bed, he reported.
It’s far too early to have reliable data on suicide trends in response to the pandemic, according to Dr. de Leo. But one thing is for sure: The strategy of social distancing employed to curb COVID-19 has increased the prevalence of known risk factors for suicide in older individuals, including loneliness, anxiety, and depression; increased alcohol use; and a perception of being a burden on society. Dr. de Leo directs a foundation dedicated to helping people experiencing traumatic bereavement, and in one recent week, the foundation was contacted by eight families in the province of Padua with a recent death by suicide apparently related to fallout from the COVID-19 pandemic. That’s an unusually high spike in suicide in a province with a population of 1 million.
“People probably preferred to end the agitation, the fear, the extreme anxiety about their destiny by deciding to prematurely truncate their life. That has been reported by nursing staff,” he said.
The Italian government has determined that, to date, 36% of all COVID-related deaths have occurred in people aged 85 years or older, and 84% of deaths were in individuals aged at least 70 years. And in Milan and the surrounding province of Lombardy, it’s estimated that COVID-19 has taken the lives of 25% of all nursing home residents. The North American experience has been uncomfortably similar.
“Almost 80% of COVID deaths in Canada have occurred in congregate settings,” observed Dr. Reichman, professor of psychiatry at the University of Toronto, and president and CEO of Baycrest Health Sciences, a geriatric research center.
“Certainly, the appalling number of deaths in nursing homes is the No. 1 horror of the pandemic,” declared Carmelle Peisah, MBBS, MD, a psychiatrist at the University of New South Wales in Kensington, Australia.
The fire next time
The conventional wisdom holds that COVID-19 has caused all sorts of mayhem in the delivery of elder care. Not so, in Dr. Reichman’s view.
“I would suggest that the pandemic has not caused many of the problems we talk about, it’s actually revealed problems that have always been there under the surface. For example, many older people, even before COVID-19, were socially isolated, socially distant. They had difficulty connecting with their relatives, difficulty accessing transportation to get to the store to buy food and see their doctors, and to interact with other older people,” the psychiatrist said.
“I would say as well that the pandemic didn’t cause the problems we’ve seen in long-term congregate senior care. The pandemic revealed them. We’ve had facilities where older people were severely crowded together, which compromises their quality of life, even when there’s not a pandemic. We’ve had difficulty staffing these kinds of environments with people that are paid an honest wage for the very hard work that they do. In many of these settings they’re inadequately trained, not only in infection prevention and control but in all other aspects of care. And the pandemic has revealed that many of these organizations are not properly funded. The government doesn’t support them well enough across jurisdictions, and they can’t raise enough philanthropic funds to provide the kind of quality of life that residents demand,” Dr. Reichman continued.
Could the pandemic spur improved elder care? His hope is that health care professionals, politicians, and society at large will learn from the devastation left by the first surge of the pandemic and will lobby for the resources necessary for much-needed improvements in geriatric care.
“We need to be better prepared should there be not only a second wave of this pandemic, but for other pandemics to come,” Dr. Reichman concluded.
The speakers indicated they had no financial conflicts regarding their presentations.
One of the greatest tragedies of the first wave of the COVID-19 pandemic has been the failure of health policy makers to anticipate and mitigate the enormous havoc the policy of social distancing would wreak on mental health and cognitive function in older persons, speakers agreed at a webinar on COVID-19, social distancing, and its impact on social and mental health in the elderly hosted by the International Psychogeriatric Association in collaboration with INTERDEM.
“Social distancing” is a two-edged sword: It is for now and the foreseeable future the only available effective strategy for protecting against infection in the older population most vulnerable to severe forms of COVID-19. Yet social distancing also has caused many elderly – particularly those in nursing homes and other long-term care facilities – to plunge into a profound experience of loneliness, isolation, distress, feelings of abandonment, anxiety, depression, and accelerated cognitive deterioration. And this needn’t have happened, the mental health professionals asserted.
“When are we going to get rid of the term ‘social distancing?’ ” asked IPA President William E. Reichman, MD. “Many have appreciated – including the World Health Organization – that the real issue is physical distancing to prevent contagion. And physical distancing doesn’t have to mean social distancing.”
Social connectedness between elderly persons and their peers and family members can be maintained and should be emphatically encouraged during the physical distancing required by the pandemic, said Myrra Vernooij-Dassen, PhD, of Radboud University in Nigmegen, the Netherlands, and chair of INTERDEM, a pan-European network of dementia researchers.
This can be achieved using readily available technologies, including the telephone and videoconferencing, as well as by creating opportunities for supervised masked visits between a family member and an elderly loved one in outdoor courtyards or gardens within long-term care facilities. And yet, as the pandemic seized hold in many parts of the world, family members were blocked from entry to these facilities, she observed.
Impact on mental health, cognition
Dr. Vernooij-Dassen noted that studies of previous quarantine periods as well as preliminary findings during the COVID-19 pandemic demonstrate an inverse relationship between social isolation measures and cognitive functioning in the elderly.
“ Conversely, epidemiologic data indicate that a socially integrated lifestyle had a favorable influence on cognitive functioning and could even delay onset of dementia,” she said.
INTERDEM is backing two ongoing studies evaluating the hypothesis that interventions fostering increased social interaction among elderly individuals can delay onset of dementia or favorably affect its course. The proposed mechanism of benefit is stimulation of brain plasticity to enhance cognitive reserve.
“This is a hypothesis of hope. We know that social interaction for humans is like water to plants – we really, really need it,” she explained.
Diego de Leo, MD, PhD, emeritus professor of psychiatry and former director of the Australian Institute for Suicide Research and Prevention at Griffith University in Brisbane, was living in hard-hit Padua, Italy, during the first surge of COVID-19. He described his anecdotal experience.
“What I hear from many Italian colleagues and friends and directors of mental health services is that emergency admissions related to mental disorders declined during the first wave of the COVID pandemic. For example, not many people attended emergency departments due to suicide attempts; there was a very marked decrease in the number of suicide attempts during the worst days of the pandemic,” he said.
People with psychiatric conditions were afraid to go to the hospital because they thought they would contract the infection and die there. That’s changing now, however.
“Now there is an increased number of admissions to mental health units. A new wave. It has been a U-shaped curve. And we’re now witnessing an increasing number of fatal suicides due to persistent fears, due to people imagining that there is no more room for them, and no more future for them from a financial point of view – which is the major negative outcome of this crisis. It will be a disaster for many families,” the psychiatrist continued.
A noteworthy phenomenon in northern Italy was that, when tablets were made available to nursing home residents in an effort to enhance their connectedness to the outside world, those with dementia often became so frustrated and confused by their difficulty in using the devices that they developed a hypokinetic delirium marked by refusal to eat or leave their bed, he reported.
It’s far too early to have reliable data on suicide trends in response to the pandemic, according to Dr. de Leo. But one thing is for sure: The strategy of social distancing employed to curb COVID-19 has increased the prevalence of known risk factors for suicide in older individuals, including loneliness, anxiety, and depression; increased alcohol use; and a perception of being a burden on society. Dr. de Leo directs a foundation dedicated to helping people experiencing traumatic bereavement, and in one recent week, the foundation was contacted by eight families in the province of Padua with a recent death by suicide apparently related to fallout from the COVID-19 pandemic. That’s an unusually high spike in suicide in a province with a population of 1 million.
“People probably preferred to end the agitation, the fear, the extreme anxiety about their destiny by deciding to prematurely truncate their life. That has been reported by nursing staff,” he said.
The Italian government has determined that, to date, 36% of all COVID-related deaths have occurred in people aged 85 years or older, and 84% of deaths were in individuals aged at least 70 years. And in Milan and the surrounding province of Lombardy, it’s estimated that COVID-19 has taken the lives of 25% of all nursing home residents. The North American experience has been uncomfortably similar.
“Almost 80% of COVID deaths in Canada have occurred in congregate settings,” observed Dr. Reichman, professor of psychiatry at the University of Toronto, and president and CEO of Baycrest Health Sciences, a geriatric research center.
“Certainly, the appalling number of deaths in nursing homes is the No. 1 horror of the pandemic,” declared Carmelle Peisah, MBBS, MD, a psychiatrist at the University of New South Wales in Kensington, Australia.
The fire next time
The conventional wisdom holds that COVID-19 has caused all sorts of mayhem in the delivery of elder care. Not so, in Dr. Reichman’s view.
“I would suggest that the pandemic has not caused many of the problems we talk about, it’s actually revealed problems that have always been there under the surface. For example, many older people, even before COVID-19, were socially isolated, socially distant. They had difficulty connecting with their relatives, difficulty accessing transportation to get to the store to buy food and see their doctors, and to interact with other older people,” the psychiatrist said.
“I would say as well that the pandemic didn’t cause the problems we’ve seen in long-term congregate senior care. The pandemic revealed them. We’ve had facilities where older people were severely crowded together, which compromises their quality of life, even when there’s not a pandemic. We’ve had difficulty staffing these kinds of environments with people that are paid an honest wage for the very hard work that they do. In many of these settings they’re inadequately trained, not only in infection prevention and control but in all other aspects of care. And the pandemic has revealed that many of these organizations are not properly funded. The government doesn’t support them well enough across jurisdictions, and they can’t raise enough philanthropic funds to provide the kind of quality of life that residents demand,” Dr. Reichman continued.
Could the pandemic spur improved elder care? His hope is that health care professionals, politicians, and society at large will learn from the devastation left by the first surge of the pandemic and will lobby for the resources necessary for much-needed improvements in geriatric care.
“We need to be better prepared should there be not only a second wave of this pandemic, but for other pandemics to come,” Dr. Reichman concluded.
The speakers indicated they had no financial conflicts regarding their presentations.
One of the greatest tragedies of the first wave of the COVID-19 pandemic has been the failure of health policy makers to anticipate and mitigate the enormous havoc the policy of social distancing would wreak on mental health and cognitive function in older persons, speakers agreed at a webinar on COVID-19, social distancing, and its impact on social and mental health in the elderly hosted by the International Psychogeriatric Association in collaboration with INTERDEM.
“Social distancing” is a two-edged sword: It is for now and the foreseeable future the only available effective strategy for protecting against infection in the older population most vulnerable to severe forms of COVID-19. Yet social distancing also has caused many elderly – particularly those in nursing homes and other long-term care facilities – to plunge into a profound experience of loneliness, isolation, distress, feelings of abandonment, anxiety, depression, and accelerated cognitive deterioration. And this needn’t have happened, the mental health professionals asserted.
“When are we going to get rid of the term ‘social distancing?’ ” asked IPA President William E. Reichman, MD. “Many have appreciated – including the World Health Organization – that the real issue is physical distancing to prevent contagion. And physical distancing doesn’t have to mean social distancing.”
Social connectedness between elderly persons and their peers and family members can be maintained and should be emphatically encouraged during the physical distancing required by the pandemic, said Myrra Vernooij-Dassen, PhD, of Radboud University in Nigmegen, the Netherlands, and chair of INTERDEM, a pan-European network of dementia researchers.
This can be achieved using readily available technologies, including the telephone and videoconferencing, as well as by creating opportunities for supervised masked visits between a family member and an elderly loved one in outdoor courtyards or gardens within long-term care facilities. And yet, as the pandemic seized hold in many parts of the world, family members were blocked from entry to these facilities, she observed.
Impact on mental health, cognition
Dr. Vernooij-Dassen noted that studies of previous quarantine periods as well as preliminary findings during the COVID-19 pandemic demonstrate an inverse relationship between social isolation measures and cognitive functioning in the elderly.
“ Conversely, epidemiologic data indicate that a socially integrated lifestyle had a favorable influence on cognitive functioning and could even delay onset of dementia,” she said.
INTERDEM is backing two ongoing studies evaluating the hypothesis that interventions fostering increased social interaction among elderly individuals can delay onset of dementia or favorably affect its course. The proposed mechanism of benefit is stimulation of brain plasticity to enhance cognitive reserve.
“This is a hypothesis of hope. We know that social interaction for humans is like water to plants – we really, really need it,” she explained.
Diego de Leo, MD, PhD, emeritus professor of psychiatry and former director of the Australian Institute for Suicide Research and Prevention at Griffith University in Brisbane, was living in hard-hit Padua, Italy, during the first surge of COVID-19. He described his anecdotal experience.
“What I hear from many Italian colleagues and friends and directors of mental health services is that emergency admissions related to mental disorders declined during the first wave of the COVID pandemic. For example, not many people attended emergency departments due to suicide attempts; there was a very marked decrease in the number of suicide attempts during the worst days of the pandemic,” he said.
People with psychiatric conditions were afraid to go to the hospital because they thought they would contract the infection and die there. That’s changing now, however.
“Now there is an increased number of admissions to mental health units. A new wave. It has been a U-shaped curve. And we’re now witnessing an increasing number of fatal suicides due to persistent fears, due to people imagining that there is no more room for them, and no more future for them from a financial point of view – which is the major negative outcome of this crisis. It will be a disaster for many families,” the psychiatrist continued.
A noteworthy phenomenon in northern Italy was that, when tablets were made available to nursing home residents in an effort to enhance their connectedness to the outside world, those with dementia often became so frustrated and confused by their difficulty in using the devices that they developed a hypokinetic delirium marked by refusal to eat or leave their bed, he reported.
It’s far too early to have reliable data on suicide trends in response to the pandemic, according to Dr. de Leo. But one thing is for sure: The strategy of social distancing employed to curb COVID-19 has increased the prevalence of known risk factors for suicide in older individuals, including loneliness, anxiety, and depression; increased alcohol use; and a perception of being a burden on society. Dr. de Leo directs a foundation dedicated to helping people experiencing traumatic bereavement, and in one recent week, the foundation was contacted by eight families in the province of Padua with a recent death by suicide apparently related to fallout from the COVID-19 pandemic. That’s an unusually high spike in suicide in a province with a population of 1 million.
“People probably preferred to end the agitation, the fear, the extreme anxiety about their destiny by deciding to prematurely truncate their life. That has been reported by nursing staff,” he said.
The Italian government has determined that, to date, 36% of all COVID-related deaths have occurred in people aged 85 years or older, and 84% of deaths were in individuals aged at least 70 years. And in Milan and the surrounding province of Lombardy, it’s estimated that COVID-19 has taken the lives of 25% of all nursing home residents. The North American experience has been uncomfortably similar.
“Almost 80% of COVID deaths in Canada have occurred in congregate settings,” observed Dr. Reichman, professor of psychiatry at the University of Toronto, and president and CEO of Baycrest Health Sciences, a geriatric research center.
“Certainly, the appalling number of deaths in nursing homes is the No. 1 horror of the pandemic,” declared Carmelle Peisah, MBBS, MD, a psychiatrist at the University of New South Wales in Kensington, Australia.
The fire next time
The conventional wisdom holds that COVID-19 has caused all sorts of mayhem in the delivery of elder care. Not so, in Dr. Reichman’s view.
“I would suggest that the pandemic has not caused many of the problems we talk about, it’s actually revealed problems that have always been there under the surface. For example, many older people, even before COVID-19, were socially isolated, socially distant. They had difficulty connecting with their relatives, difficulty accessing transportation to get to the store to buy food and see their doctors, and to interact with other older people,” the psychiatrist said.
“I would say as well that the pandemic didn’t cause the problems we’ve seen in long-term congregate senior care. The pandemic revealed them. We’ve had facilities where older people were severely crowded together, which compromises their quality of life, even when there’s not a pandemic. We’ve had difficulty staffing these kinds of environments with people that are paid an honest wage for the very hard work that they do. In many of these settings they’re inadequately trained, not only in infection prevention and control but in all other aspects of care. And the pandemic has revealed that many of these organizations are not properly funded. The government doesn’t support them well enough across jurisdictions, and they can’t raise enough philanthropic funds to provide the kind of quality of life that residents demand,” Dr. Reichman continued.
Could the pandemic spur improved elder care? His hope is that health care professionals, politicians, and society at large will learn from the devastation left by the first surge of the pandemic and will lobby for the resources necessary for much-needed improvements in geriatric care.
“We need to be better prepared should there be not only a second wave of this pandemic, but for other pandemics to come,” Dr. Reichman concluded.
The speakers indicated they had no financial conflicts regarding their presentations.
Neuropsychiatric manifestations of COVID-19
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
Framingham risk score may also predict cognitive decline
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Blood pressure lowering lessens risk of dementia, cognitive decline
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
FROM JAMA
Incidental finding on brain MRI seen in 5% of older patients
New research shows that Knowing the expected prevalence of such incidental findings in the older general population is “extremely useful” for both researchers and clinicians, said study co-author Sarah Elisabeth Keuss, MBChB, clinical research associate, Dementia Research Centre, UCL Queen Square Institute of Neurology, London, UK.
“In research, the knowledge helps to inform study protocols regarding how to manage incidental findings and enables study participants to be appropriately informed,” said Dr. Keuss. Greater awareness also helps clinicians make decisions about whether or not to scan a patient, she said, adding that imaging is increasingly available to them. It allows clinicians to counsel patients regarding the probability of an incidental finding and balance that risk against the potential benefits of having a test.
The research is being presented online as part of the American Academy of Neurology 2020 Science Highlights. The incidental findings also were published last year in BMJ Open.
The new findings are from the first wave of data collection for the Insight 46 study, a neuroimaging substudy of the MRC National Survey of Health and Development (NSHD) 1946 British birth cohort, a broadly representative sample of the population born in mainland Britain during 1946. The research uses detailed brain imaging, cognitive testing, and blood and other biomarkers to investigate genetic and life-course factors associated with Alzheimer’s disease and cerebrovascular disease.
The current study included 502 individuals, aged about 71 years at the time of the analysis, and 49% were women. Almost all (93.8%) participants underwent 1-day MRI scans. Some 4.5% of these participants had an incidental finding of brain abnormality as per a prespecified standardized protocol.
Suspected vascular malformations were present in 1.9%, and suspected intracranial mass lesions were present in 1.5%. The single most common vascular abnormality was a suspected cerebral aneurysm, which affected 1.1% of participants.
Suspected meningiomas were the most common intracranial lesion, affecting 0.6% of study participants.
Action plan
Participants and their primary care provider were informed of findings “that were deemed to be potentially serious, or life-threatening, or could have a major impact on quality of life,” said Dr. Keuss. Relevant experts “came up with a recommended clinical action plan to help the primary care provider decide what should be the next course of action with regard to investigation or referral to another specialist,” said Dr. Keuss.
The new results are important for clinical decision-making, said Dr. Keuss. “Clinicians should consider the possibility of detecting an incidental finding whenever they’re requesting a brain scan. They should balance that risk against the possible benefits of recommending a test.”
The prevalence of incidental findings on MRI reported in the literature varies because of different methods used to review scans. “However, comparing our study with similar studies, the prevalence of the key findings with regard to aneurysms and intracranial mass lesions are very similar,” said Dr. Keuss.
Dr. Keuss and colleagues do not recommend all elderly patients get a brain scan.
“We don’t know what the long-term consequences are of being informed you have an incidental finding of an abnormality; we don’t know if it improves their outcome, and it potentially could cause anxiety,” said Dr. Keuss.
Psychological impact
The researchers have not looked at the psychological impact of negative findings on study participants, but they could do so at a later date.
“It would be very important to look into that given the potential to cause anxiety,” said Dr. Keuss. “It’s important to find out the potential negative consequences to inform researchers in future about how best to manage these findings.”
From blood tests, the analysis found that more than a third (34.6%) of participants had at least one related abnormality. The most common of these were kidney impairment (about 9%), thyroid function abnormalities (between 4% and 5%), anemia (about 4%), and low vitamin B12 levels (about 3%).
However, few of these reached the prespecified threshold for urgent action, and Dr. Keuss noted these findings were not the focus of her AAN presentation.
A strength of the study was that participants were almost the exact same age.
Important issue
Commenting on the research, David S. Liebeskind, MD, professor of neurology and director, Neurovascular Imaging Research Core, University of California, Los Angeles, said it raises “a very interesting” and “important” public health issue.
“The question is whether we do things based around individual symptomatic status, or at a larger level in terms of public health, screening the larger population to figure out who is at risk for any particular disease or disorder.”
From the standpoint of imaging technologies like MRI that show details about brain structures, experts debate whether the population should be screened “before something occurs,” said Dr. Liebeskind. “Imaging has the capacity to tell us a tremendous amount; whether this implies we should therefore image everybody is a larger public health question.”
The issue is “fraught with a lot of difficulty and complexity” as treatment paradigms tend to be “built around symptomatic status,” he said. “When we sit in the office or with a patient at the bedside, we usually focus on that individual patient and not necessarily on the larger public.”
Dr. Liebeskind noted that the question of whether to put the emphasis on the individual patient or the public at large is also being discussed during the current COVID-19 pandemic.
He wasn’t surprised that the study uncovered incidental findings in almost 5% of the sample. “If you take an 80-year-old and study their brain, a good chunk, if not half or more, will have some abnormality,” he said.
Drs. Keuss and Liebeskind have reported no relevant financial relationships.
This article first appeared on Medscape.com.
New research shows that Knowing the expected prevalence of such incidental findings in the older general population is “extremely useful” for both researchers and clinicians, said study co-author Sarah Elisabeth Keuss, MBChB, clinical research associate, Dementia Research Centre, UCL Queen Square Institute of Neurology, London, UK.
“In research, the knowledge helps to inform study protocols regarding how to manage incidental findings and enables study participants to be appropriately informed,” said Dr. Keuss. Greater awareness also helps clinicians make decisions about whether or not to scan a patient, she said, adding that imaging is increasingly available to them. It allows clinicians to counsel patients regarding the probability of an incidental finding and balance that risk against the potential benefits of having a test.
The research is being presented online as part of the American Academy of Neurology 2020 Science Highlights. The incidental findings also were published last year in BMJ Open.
The new findings are from the first wave of data collection for the Insight 46 study, a neuroimaging substudy of the MRC National Survey of Health and Development (NSHD) 1946 British birth cohort, a broadly representative sample of the population born in mainland Britain during 1946. The research uses detailed brain imaging, cognitive testing, and blood and other biomarkers to investigate genetic and life-course factors associated with Alzheimer’s disease and cerebrovascular disease.
The current study included 502 individuals, aged about 71 years at the time of the analysis, and 49% were women. Almost all (93.8%) participants underwent 1-day MRI scans. Some 4.5% of these participants had an incidental finding of brain abnormality as per a prespecified standardized protocol.
Suspected vascular malformations were present in 1.9%, and suspected intracranial mass lesions were present in 1.5%. The single most common vascular abnormality was a suspected cerebral aneurysm, which affected 1.1% of participants.
Suspected meningiomas were the most common intracranial lesion, affecting 0.6% of study participants.
Action plan
Participants and their primary care provider were informed of findings “that were deemed to be potentially serious, or life-threatening, or could have a major impact on quality of life,” said Dr. Keuss. Relevant experts “came up with a recommended clinical action plan to help the primary care provider decide what should be the next course of action with regard to investigation or referral to another specialist,” said Dr. Keuss.
The new results are important for clinical decision-making, said Dr. Keuss. “Clinicians should consider the possibility of detecting an incidental finding whenever they’re requesting a brain scan. They should balance that risk against the possible benefits of recommending a test.”
The prevalence of incidental findings on MRI reported in the literature varies because of different methods used to review scans. “However, comparing our study with similar studies, the prevalence of the key findings with regard to aneurysms and intracranial mass lesions are very similar,” said Dr. Keuss.
Dr. Keuss and colleagues do not recommend all elderly patients get a brain scan.
“We don’t know what the long-term consequences are of being informed you have an incidental finding of an abnormality; we don’t know if it improves their outcome, and it potentially could cause anxiety,” said Dr. Keuss.
Psychological impact
The researchers have not looked at the psychological impact of negative findings on study participants, but they could do so at a later date.
“It would be very important to look into that given the potential to cause anxiety,” said Dr. Keuss. “It’s important to find out the potential negative consequences to inform researchers in future about how best to manage these findings.”
From blood tests, the analysis found that more than a third (34.6%) of participants had at least one related abnormality. The most common of these were kidney impairment (about 9%), thyroid function abnormalities (between 4% and 5%), anemia (about 4%), and low vitamin B12 levels (about 3%).
However, few of these reached the prespecified threshold for urgent action, and Dr. Keuss noted these findings were not the focus of her AAN presentation.
A strength of the study was that participants were almost the exact same age.
Important issue
Commenting on the research, David S. Liebeskind, MD, professor of neurology and director, Neurovascular Imaging Research Core, University of California, Los Angeles, said it raises “a very interesting” and “important” public health issue.
“The question is whether we do things based around individual symptomatic status, or at a larger level in terms of public health, screening the larger population to figure out who is at risk for any particular disease or disorder.”
From the standpoint of imaging technologies like MRI that show details about brain structures, experts debate whether the population should be screened “before something occurs,” said Dr. Liebeskind. “Imaging has the capacity to tell us a tremendous amount; whether this implies we should therefore image everybody is a larger public health question.”
The issue is “fraught with a lot of difficulty and complexity” as treatment paradigms tend to be “built around symptomatic status,” he said. “When we sit in the office or with a patient at the bedside, we usually focus on that individual patient and not necessarily on the larger public.”
Dr. Liebeskind noted that the question of whether to put the emphasis on the individual patient or the public at large is also being discussed during the current COVID-19 pandemic.
He wasn’t surprised that the study uncovered incidental findings in almost 5% of the sample. “If you take an 80-year-old and study their brain, a good chunk, if not half or more, will have some abnormality,” he said.
Drs. Keuss and Liebeskind have reported no relevant financial relationships.
This article first appeared on Medscape.com.
New research shows that Knowing the expected prevalence of such incidental findings in the older general population is “extremely useful” for both researchers and clinicians, said study co-author Sarah Elisabeth Keuss, MBChB, clinical research associate, Dementia Research Centre, UCL Queen Square Institute of Neurology, London, UK.
“In research, the knowledge helps to inform study protocols regarding how to manage incidental findings and enables study participants to be appropriately informed,” said Dr. Keuss. Greater awareness also helps clinicians make decisions about whether or not to scan a patient, she said, adding that imaging is increasingly available to them. It allows clinicians to counsel patients regarding the probability of an incidental finding and balance that risk against the potential benefits of having a test.
The research is being presented online as part of the American Academy of Neurology 2020 Science Highlights. The incidental findings also were published last year in BMJ Open.
The new findings are from the first wave of data collection for the Insight 46 study, a neuroimaging substudy of the MRC National Survey of Health and Development (NSHD) 1946 British birth cohort, a broadly representative sample of the population born in mainland Britain during 1946. The research uses detailed brain imaging, cognitive testing, and blood and other biomarkers to investigate genetic and life-course factors associated with Alzheimer’s disease and cerebrovascular disease.
The current study included 502 individuals, aged about 71 years at the time of the analysis, and 49% were women. Almost all (93.8%) participants underwent 1-day MRI scans. Some 4.5% of these participants had an incidental finding of brain abnormality as per a prespecified standardized protocol.
Suspected vascular malformations were present in 1.9%, and suspected intracranial mass lesions were present in 1.5%. The single most common vascular abnormality was a suspected cerebral aneurysm, which affected 1.1% of participants.
Suspected meningiomas were the most common intracranial lesion, affecting 0.6% of study participants.
Action plan
Participants and their primary care provider were informed of findings “that were deemed to be potentially serious, or life-threatening, or could have a major impact on quality of life,” said Dr. Keuss. Relevant experts “came up with a recommended clinical action plan to help the primary care provider decide what should be the next course of action with regard to investigation or referral to another specialist,” said Dr. Keuss.
The new results are important for clinical decision-making, said Dr. Keuss. “Clinicians should consider the possibility of detecting an incidental finding whenever they’re requesting a brain scan. They should balance that risk against the possible benefits of recommending a test.”
The prevalence of incidental findings on MRI reported in the literature varies because of different methods used to review scans. “However, comparing our study with similar studies, the prevalence of the key findings with regard to aneurysms and intracranial mass lesions are very similar,” said Dr. Keuss.
Dr. Keuss and colleagues do not recommend all elderly patients get a brain scan.
“We don’t know what the long-term consequences are of being informed you have an incidental finding of an abnormality; we don’t know if it improves their outcome, and it potentially could cause anxiety,” said Dr. Keuss.
Psychological impact
The researchers have not looked at the psychological impact of negative findings on study participants, but they could do so at a later date.
“It would be very important to look into that given the potential to cause anxiety,” said Dr. Keuss. “It’s important to find out the potential negative consequences to inform researchers in future about how best to manage these findings.”
From blood tests, the analysis found that more than a third (34.6%) of participants had at least one related abnormality. The most common of these were kidney impairment (about 9%), thyroid function abnormalities (between 4% and 5%), anemia (about 4%), and low vitamin B12 levels (about 3%).
However, few of these reached the prespecified threshold for urgent action, and Dr. Keuss noted these findings were not the focus of her AAN presentation.
A strength of the study was that participants were almost the exact same age.
Important issue
Commenting on the research, David S. Liebeskind, MD, professor of neurology and director, Neurovascular Imaging Research Core, University of California, Los Angeles, said it raises “a very interesting” and “important” public health issue.
“The question is whether we do things based around individual symptomatic status, or at a larger level in terms of public health, screening the larger population to figure out who is at risk for any particular disease or disorder.”
From the standpoint of imaging technologies like MRI that show details about brain structures, experts debate whether the population should be screened “before something occurs,” said Dr. Liebeskind. “Imaging has the capacity to tell us a tremendous amount; whether this implies we should therefore image everybody is a larger public health question.”
The issue is “fraught with a lot of difficulty and complexity” as treatment paradigms tend to be “built around symptomatic status,” he said. “When we sit in the office or with a patient at the bedside, we usually focus on that individual patient and not necessarily on the larger public.”
Dr. Liebeskind noted that the question of whether to put the emphasis on the individual patient or the public at large is also being discussed during the current COVID-19 pandemic.
He wasn’t surprised that the study uncovered incidental findings in almost 5% of the sample. “If you take an 80-year-old and study their brain, a good chunk, if not half or more, will have some abnormality,” he said.
Drs. Keuss and Liebeskind have reported no relevant financial relationships.
This article first appeared on Medscape.com.