User login
Infection ups mortality risk in patients with dementia
Infection increases mortality risk among patients with dementia, new research suggests. A large, registry-based cohort study showed that
“This is the first study to our knowledge to show that increased mortality is observed across all infection types in people with dementia and that increased mortality is seen both short and long term,” said coinvestigator Janet Janbek, a PhD student at the Danish Dementia Research Center, Rigshospitalet, University of Copenhagen.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Large Danish cohort
The investigators analyzed data from Danish national health registries for nearly 1.5 million individuals aged 65 years and older who had visited the hospital with an infection. There were 575,260 deaths during more than 12.7 million person-years of follow-up.
Patients with dementia who also had a hospital visit for infection died at a 6.5 times higher rate than participants without dementia or an infection. Those with either dementia alone or infection-related contacts alone had a threefold increased rate of death.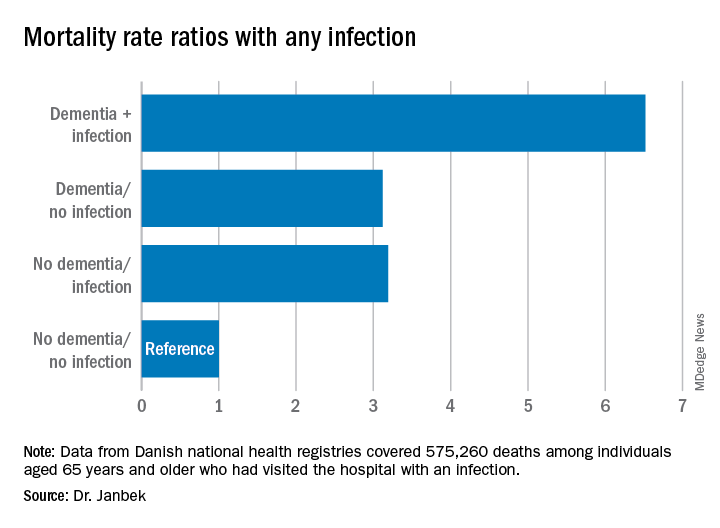
The mortality rate was highest within the first 30 days following the hospital visit for infection. However, the rate remained elevated for 10 years after the initial infection-related hospital visit.
Mortality rates from all infections, including major infections, such as sepsis, down to minor ear infections were elevated in patients with dementia, compared with people who did not have dementia or an infection-related hospital visit.
Ms. Janbek said there are several possible explanations for the association of infection and increased mortality risk in those with dementia. “After a hospital contact with a severe infection, people with dementia may become more reliant on external care, become more frail, and have declined functional levels, which might explain the observed association.”
It might also be that patients with dementia have more severe infections than those without dementia at the time of hospital contact, possibly because of delayed diagnosis, which could explain the higher mortality rates, said Ms. Janbek.
“It is also plausible that infections play a role in worsening dementia and subsequently lead to increased mortality,” she noted.
“Clinicians and health care personnel need to pay closer attention to infections of all types in people with dementia, and steps toward better clinical management and improved posthospital care need to be explored and undertaken. We need to identify possible preventive measures and targeted interventions in people with dementia and infections,” Ms. Janbek said.
‘Interesting observation’
Commenting on the study, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said it presents “an interesting observation.” However, “we can’t make any direct assumptions from this research per se about infections and dementia and whether they are causative in any way,” noted Dr. Edelmayer, who was not involved with the study.
Instead, the study highlighted the importance of “taking care of our overall health and making sure that individuals that might be vulnerable to infection, like those who are already living with dementia, are getting the best care possible,” she said.
Ms. Janbek and Dr. Edelmayer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Infection increases mortality risk among patients with dementia, new research suggests. A large, registry-based cohort study showed that
“This is the first study to our knowledge to show that increased mortality is observed across all infection types in people with dementia and that increased mortality is seen both short and long term,” said coinvestigator Janet Janbek, a PhD student at the Danish Dementia Research Center, Rigshospitalet, University of Copenhagen.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Large Danish cohort
The investigators analyzed data from Danish national health registries for nearly 1.5 million individuals aged 65 years and older who had visited the hospital with an infection. There were 575,260 deaths during more than 12.7 million person-years of follow-up.
Patients with dementia who also had a hospital visit for infection died at a 6.5 times higher rate than participants without dementia or an infection. Those with either dementia alone or infection-related contacts alone had a threefold increased rate of death.
The mortality rate was highest within the first 30 days following the hospital visit for infection. However, the rate remained elevated for 10 years after the initial infection-related hospital visit.
Mortality rates from all infections, including major infections, such as sepsis, down to minor ear infections were elevated in patients with dementia, compared with people who did not have dementia or an infection-related hospital visit.
Ms. Janbek said there are several possible explanations for the association of infection and increased mortality risk in those with dementia. “After a hospital contact with a severe infection, people with dementia may become more reliant on external care, become more frail, and have declined functional levels, which might explain the observed association.”
It might also be that patients with dementia have more severe infections than those without dementia at the time of hospital contact, possibly because of delayed diagnosis, which could explain the higher mortality rates, said Ms. Janbek.
“It is also plausible that infections play a role in worsening dementia and subsequently lead to increased mortality,” she noted.
“Clinicians and health care personnel need to pay closer attention to infections of all types in people with dementia, and steps toward better clinical management and improved posthospital care need to be explored and undertaken. We need to identify possible preventive measures and targeted interventions in people with dementia and infections,” Ms. Janbek said.
‘Interesting observation’
Commenting on the study, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said it presents “an interesting observation.” However, “we can’t make any direct assumptions from this research per se about infections and dementia and whether they are causative in any way,” noted Dr. Edelmayer, who was not involved with the study.
Instead, the study highlighted the importance of “taking care of our overall health and making sure that individuals that might be vulnerable to infection, like those who are already living with dementia, are getting the best care possible,” she said.
Ms. Janbek and Dr. Edelmayer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Infection increases mortality risk among patients with dementia, new research suggests. A large, registry-based cohort study showed that
“This is the first study to our knowledge to show that increased mortality is observed across all infection types in people with dementia and that increased mortality is seen both short and long term,” said coinvestigator Janet Janbek, a PhD student at the Danish Dementia Research Center, Rigshospitalet, University of Copenhagen.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Large Danish cohort
The investigators analyzed data from Danish national health registries for nearly 1.5 million individuals aged 65 years and older who had visited the hospital with an infection. There were 575,260 deaths during more than 12.7 million person-years of follow-up.
Patients with dementia who also had a hospital visit for infection died at a 6.5 times higher rate than participants without dementia or an infection. Those with either dementia alone or infection-related contacts alone had a threefold increased rate of death.
The mortality rate was highest within the first 30 days following the hospital visit for infection. However, the rate remained elevated for 10 years after the initial infection-related hospital visit.
Mortality rates from all infections, including major infections, such as sepsis, down to minor ear infections were elevated in patients with dementia, compared with people who did not have dementia or an infection-related hospital visit.
Ms. Janbek said there are several possible explanations for the association of infection and increased mortality risk in those with dementia. “After a hospital contact with a severe infection, people with dementia may become more reliant on external care, become more frail, and have declined functional levels, which might explain the observed association.”
It might also be that patients with dementia have more severe infections than those without dementia at the time of hospital contact, possibly because of delayed diagnosis, which could explain the higher mortality rates, said Ms. Janbek.
“It is also plausible that infections play a role in worsening dementia and subsequently lead to increased mortality,” she noted.
“Clinicians and health care personnel need to pay closer attention to infections of all types in people with dementia, and steps toward better clinical management and improved posthospital care need to be explored and undertaken. We need to identify possible preventive measures and targeted interventions in people with dementia and infections,” Ms. Janbek said.
‘Interesting observation’
Commenting on the study, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said it presents “an interesting observation.” However, “we can’t make any direct assumptions from this research per se about infections and dementia and whether they are causative in any way,” noted Dr. Edelmayer, who was not involved with the study.
Instead, the study highlighted the importance of “taking care of our overall health and making sure that individuals that might be vulnerable to infection, like those who are already living with dementia, are getting the best care possible,” she said.
Ms. Janbek and Dr. Edelmayer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
P-tau217 differentiates Alzheimer’s disease from other neurodegenerative conditions
new research suggests.
Results from a large multinational study showed that the level of P-tau217 in blood collected during life was an accurate predictor of tau brain changes seen in brain tissue after death. In addition, increasing blood P-tau217 levels can be detected in some individuals up to 20 years before the average age of onset of the early cognitive decline that signals Alzheimer’s disease, researchers reported.
“While there is still more work to be done, this biomarker has the potential to have a transformational impact on research, treatment, prevention, and therapy development, and in the clinical setting,” said senior author Eric M. Reiman, MD, executive director of Banner Alzheimer’s Institute in Phoenix.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and simultaneously published online July 28 in JAMA.
Three cohorts
The international team of researchers evaluated the P-tau217 blood test in 1,402 adults from three cohorts. The first cohort was made up of 81 individuals in the Arizona (Banner Sun Health Research Institute) Brain Donation program and included clinical, blood, and neuropathologic data. The second cohort included 699 individuals in the Swedish BioFINDER-2 study and provided clinical, brain imaging, cerebrospinal fluid (CSF), and blood data. The third cohort was made up of 522 participants from the Columbian autosomal-dominant Alzheimer’s disease kindred, including 365 PSEN1 E280A mutation carriers and 257 mutation noncarriers.
In the Arizona cohort, plasma P-tau217 discriminated neuropathologically defined Alzheimer’s disease from non-Alzheimer’s disease (area under the curve, 0.89; 95% CI, 0.81-0.97) with significantly higher accuracy than plasma P-tau181 and neurofilament light chain (NfL) (AUC range, 0.50-0.72; P < .05).
In the Swedish BioFINDER-2 cohort, the discriminative accuracy of plasma P-tau217 for clinical Alzheimer’s disease dementia versus other neurodegenerative diseases was 96% (AUC, 0.96; 95% CI, 0.93-0.98).
This was significantly higher than plasma P-tau181, plasma NfL, and MRI measures (AUC range, 0.50-0.81; P < .001), but was not significantly different than CSF P-tau217, CSF P-tau181, and tau-PET (AUC range, 0.90-0.99; P > .15).
In the Colombian cohort, plasma P-tau217 levels were significantly greater among PSEN1 mutation carriers than noncarriers starting at around age 25 years, which is 20 years prior to the estimated onset of mild cognitive impairment among mutation carriers.
Additionally, plasma P-tau217 levels correlated with cerebral tau tangles, and discriminated abnormal versus normal tau-PET scans with significantly higher accuracy than plasma P-tau181, plasma NfL, CSF P-tau181, CSF Abeta42:Abeta40 ratio, and MRI measures.
The blood test “opens the possibility of early diagnosis of Alzheimer’s disease before the dementia stage, which is very important for clinical trials evaluating novel therapies that might stop or slow down the disease process,” presenting author Oskar Hansson, MD, PhD, of Lund (Sweden) University, said in a statement.
Further research is now needed to optimize the P-tau217 blood test, validate the findings in unselected and diverse populations, and determine its potential role in the clinic, the investigators noted.
Potential game changer?
Commenting on the study, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, noted his enthusiasm for the test. “This tau blood test will be a real game changer, advancing clinical care and research,” said Dr. Fillit, who was not involved in the research.
“This is a real breakthrough: a simple and accessible blood test that can diagnose Alzheimer’s disease better than the more costly and invasive methods currently available like PET scans and cerebrospinal fluid biomarkers,” he said.
The P-tau217 blood test “is like the equivalent of the cholesterol test for heart disease, but for Alzheimer’s disease,” Dr. Fillit added.
As previously reported, another study presented at AAIC 2020 compared P-tau217 with P-tau181 to determine which could best identify individuals with Alzheimer’s disease. Results showed that, although the two biomarkers were similar overall, P-tau217 had a slight edge in terms of accuracy.
The study by Reiman et al. was funded by the Swedish Research Council, the Knut and Alice Wallenberg Foundation, and the Swedish Alzheimer Foundation. Dr. Hansson reported receiving grants from Roche, Biogen, and Pfizer, and receiving nonfinancial support from GE Healthcare, AVID Radiopharmaceuticals, and Euroimmun. Dr. Reiman has received grants from Roche/Roche Diagnostics and received personal fees from Alkahest, Alzheon, Aural Analytics, Denali, Green Valley, MagQ, Takeda/Zinfandel, and United Neuroscience. He is also a cofounder of AlzPath, which aims to further develop P-tau217 and fluid biomarkers; holds a patent owned by Banner Health for a strategy to use biomarkers to accelerate evaluation of Alzheimer prevention therapies; and is a principal investigator of prevention trials that include research agreements with Genentech/Roche and Novartis/Amgen, PET studies that include research agreements with Avid/Lilly, and several National Institute of Health–supported research studies. Dr. Fillit reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a large multinational study showed that the level of P-tau217 in blood collected during life was an accurate predictor of tau brain changes seen in brain tissue after death. In addition, increasing blood P-tau217 levels can be detected in some individuals up to 20 years before the average age of onset of the early cognitive decline that signals Alzheimer’s disease, researchers reported.
“While there is still more work to be done, this biomarker has the potential to have a transformational impact on research, treatment, prevention, and therapy development, and in the clinical setting,” said senior author Eric M. Reiman, MD, executive director of Banner Alzheimer’s Institute in Phoenix.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and simultaneously published online July 28 in JAMA.
Three cohorts
The international team of researchers evaluated the P-tau217 blood test in 1,402 adults from three cohorts. The first cohort was made up of 81 individuals in the Arizona (Banner Sun Health Research Institute) Brain Donation program and included clinical, blood, and neuropathologic data. The second cohort included 699 individuals in the Swedish BioFINDER-2 study and provided clinical, brain imaging, cerebrospinal fluid (CSF), and blood data. The third cohort was made up of 522 participants from the Columbian autosomal-dominant Alzheimer’s disease kindred, including 365 PSEN1 E280A mutation carriers and 257 mutation noncarriers.
In the Arizona cohort, plasma P-tau217 discriminated neuropathologically defined Alzheimer’s disease from non-Alzheimer’s disease (area under the curve, 0.89; 95% CI, 0.81-0.97) with significantly higher accuracy than plasma P-tau181 and neurofilament light chain (NfL) (AUC range, 0.50-0.72; P < .05).
In the Swedish BioFINDER-2 cohort, the discriminative accuracy of plasma P-tau217 for clinical Alzheimer’s disease dementia versus other neurodegenerative diseases was 96% (AUC, 0.96; 95% CI, 0.93-0.98).
This was significantly higher than plasma P-tau181, plasma NfL, and MRI measures (AUC range, 0.50-0.81; P < .001), but was not significantly different than CSF P-tau217, CSF P-tau181, and tau-PET (AUC range, 0.90-0.99; P > .15).
In the Colombian cohort, plasma P-tau217 levels were significantly greater among PSEN1 mutation carriers than noncarriers starting at around age 25 years, which is 20 years prior to the estimated onset of mild cognitive impairment among mutation carriers.
Additionally, plasma P-tau217 levels correlated with cerebral tau tangles, and discriminated abnormal versus normal tau-PET scans with significantly higher accuracy than plasma P-tau181, plasma NfL, CSF P-tau181, CSF Abeta42:Abeta40 ratio, and MRI measures.
The blood test “opens the possibility of early diagnosis of Alzheimer’s disease before the dementia stage, which is very important for clinical trials evaluating novel therapies that might stop or slow down the disease process,” presenting author Oskar Hansson, MD, PhD, of Lund (Sweden) University, said in a statement.
Further research is now needed to optimize the P-tau217 blood test, validate the findings in unselected and diverse populations, and determine its potential role in the clinic, the investigators noted.
Potential game changer?
Commenting on the study, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, noted his enthusiasm for the test. “This tau blood test will be a real game changer, advancing clinical care and research,” said Dr. Fillit, who was not involved in the research.
“This is a real breakthrough: a simple and accessible blood test that can diagnose Alzheimer’s disease better than the more costly and invasive methods currently available like PET scans and cerebrospinal fluid biomarkers,” he said.
The P-tau217 blood test “is like the equivalent of the cholesterol test for heart disease, but for Alzheimer’s disease,” Dr. Fillit added.
As previously reported, another study presented at AAIC 2020 compared P-tau217 with P-tau181 to determine which could best identify individuals with Alzheimer’s disease. Results showed that, although the two biomarkers were similar overall, P-tau217 had a slight edge in terms of accuracy.
The study by Reiman et al. was funded by the Swedish Research Council, the Knut and Alice Wallenberg Foundation, and the Swedish Alzheimer Foundation. Dr. Hansson reported receiving grants from Roche, Biogen, and Pfizer, and receiving nonfinancial support from GE Healthcare, AVID Radiopharmaceuticals, and Euroimmun. Dr. Reiman has received grants from Roche/Roche Diagnostics and received personal fees from Alkahest, Alzheon, Aural Analytics, Denali, Green Valley, MagQ, Takeda/Zinfandel, and United Neuroscience. He is also a cofounder of AlzPath, which aims to further develop P-tau217 and fluid biomarkers; holds a patent owned by Banner Health for a strategy to use biomarkers to accelerate evaluation of Alzheimer prevention therapies; and is a principal investigator of prevention trials that include research agreements with Genentech/Roche and Novartis/Amgen, PET studies that include research agreements with Avid/Lilly, and several National Institute of Health–supported research studies. Dr. Fillit reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a large multinational study showed that the level of P-tau217 in blood collected during life was an accurate predictor of tau brain changes seen in brain tissue after death. In addition, increasing blood P-tau217 levels can be detected in some individuals up to 20 years before the average age of onset of the early cognitive decline that signals Alzheimer’s disease, researchers reported.
“While there is still more work to be done, this biomarker has the potential to have a transformational impact on research, treatment, prevention, and therapy development, and in the clinical setting,” said senior author Eric M. Reiman, MD, executive director of Banner Alzheimer’s Institute in Phoenix.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and simultaneously published online July 28 in JAMA.
Three cohorts
The international team of researchers evaluated the P-tau217 blood test in 1,402 adults from three cohorts. The first cohort was made up of 81 individuals in the Arizona (Banner Sun Health Research Institute) Brain Donation program and included clinical, blood, and neuropathologic data. The second cohort included 699 individuals in the Swedish BioFINDER-2 study and provided clinical, brain imaging, cerebrospinal fluid (CSF), and blood data. The third cohort was made up of 522 participants from the Columbian autosomal-dominant Alzheimer’s disease kindred, including 365 PSEN1 E280A mutation carriers and 257 mutation noncarriers.
In the Arizona cohort, plasma P-tau217 discriminated neuropathologically defined Alzheimer’s disease from non-Alzheimer’s disease (area under the curve, 0.89; 95% CI, 0.81-0.97) with significantly higher accuracy than plasma P-tau181 and neurofilament light chain (NfL) (AUC range, 0.50-0.72; P < .05).
In the Swedish BioFINDER-2 cohort, the discriminative accuracy of plasma P-tau217 for clinical Alzheimer’s disease dementia versus other neurodegenerative diseases was 96% (AUC, 0.96; 95% CI, 0.93-0.98).
This was significantly higher than plasma P-tau181, plasma NfL, and MRI measures (AUC range, 0.50-0.81; P < .001), but was not significantly different than CSF P-tau217, CSF P-tau181, and tau-PET (AUC range, 0.90-0.99; P > .15).
In the Colombian cohort, plasma P-tau217 levels were significantly greater among PSEN1 mutation carriers than noncarriers starting at around age 25 years, which is 20 years prior to the estimated onset of mild cognitive impairment among mutation carriers.
Additionally, plasma P-tau217 levels correlated with cerebral tau tangles, and discriminated abnormal versus normal tau-PET scans with significantly higher accuracy than plasma P-tau181, plasma NfL, CSF P-tau181, CSF Abeta42:Abeta40 ratio, and MRI measures.
The blood test “opens the possibility of early diagnosis of Alzheimer’s disease before the dementia stage, which is very important for clinical trials evaluating novel therapies that might stop or slow down the disease process,” presenting author Oskar Hansson, MD, PhD, of Lund (Sweden) University, said in a statement.
Further research is now needed to optimize the P-tau217 blood test, validate the findings in unselected and diverse populations, and determine its potential role in the clinic, the investigators noted.
Potential game changer?
Commenting on the study, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, noted his enthusiasm for the test. “This tau blood test will be a real game changer, advancing clinical care and research,” said Dr. Fillit, who was not involved in the research.
“This is a real breakthrough: a simple and accessible blood test that can diagnose Alzheimer’s disease better than the more costly and invasive methods currently available like PET scans and cerebrospinal fluid biomarkers,” he said.
The P-tau217 blood test “is like the equivalent of the cholesterol test for heart disease, but for Alzheimer’s disease,” Dr. Fillit added.
As previously reported, another study presented at AAIC 2020 compared P-tau217 with P-tau181 to determine which could best identify individuals with Alzheimer’s disease. Results showed that, although the two biomarkers were similar overall, P-tau217 had a slight edge in terms of accuracy.
The study by Reiman et al. was funded by the Swedish Research Council, the Knut and Alice Wallenberg Foundation, and the Swedish Alzheimer Foundation. Dr. Hansson reported receiving grants from Roche, Biogen, and Pfizer, and receiving nonfinancial support from GE Healthcare, AVID Radiopharmaceuticals, and Euroimmun. Dr. Reiman has received grants from Roche/Roche Diagnostics and received personal fees from Alkahest, Alzheon, Aural Analytics, Denali, Green Valley, MagQ, Takeda/Zinfandel, and United Neuroscience. He is also a cofounder of AlzPath, which aims to further develop P-tau217 and fluid biomarkers; holds a patent owned by Banner Health for a strategy to use biomarkers to accelerate evaluation of Alzheimer prevention therapies; and is a principal investigator of prevention trials that include research agreements with Genentech/Roche and Novartis/Amgen, PET studies that include research agreements with Avid/Lilly, and several National Institute of Health–supported research studies. Dr. Fillit reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
Microbiome research ‘opening doors’ to new Alzheimer’s disease treatments
Research into the microbiome is yielding some positive new potential treatment options for Alzheimer’s disease, according to George T. Grossberg, MD.
“I think the growing focus on the gut-brain axis is opening doors to new Alzheimer’s disease and other brain disorders, and I think the first of a possible future generation of compounds for prevention or treatment of Alzheimer’s disease may indeed be emerging,” Dr. Grossberg said at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Focus on the microbiome and microbiota is “a really hot, really new, really emerging area,” said Dr. Grossberg, professor in the department of psychiatry & behavioral neuroscience at Saint Louis University. But the microbiota, which is the microorganisms within a specific organ such as the colon, is sometimes confused with the microbiome – which is defined as all of the bacteria, viruses, fungi and other microorganisms within a habitat as well as their genomes and the environment around them. “These are often used interchangeably, but they’re not the same,” Dr. Grossberg said at the meeting, presented by Global Academy for Medical Education.
A person’s microbiome is unique to them, and nearly all of the microbiome is contained in the gut. A reduction in diversity of the microbiota in the digestive system has been linked to a wide variety of diseases, Dr. Grossberg explained. Conversely, a microbial imbalance or dysbiosis has been implicated in anxiety and/or depression, dementia, and certain cancers, he noted.
Bacteria that positively affect the microbiome come from two main genera: Lactobacillus and Bifidobacterium. Factors such as diet, medications, geography, stage of life, birthing process, infant feeding method, and stress can all affect a person’s microbiome. “We’re all beginning to understand that trying to manage or trying to diversify, trying to manipulate the microbiota may have a lot of remote effects – even effects on weight or diabetes, or other disorders,” Dr. Grossberg said.
Fecal microbiota transplantation (FMT), or the process of administering a donor’s fecal matter into a recipient’s intestinal tract, has proved beneficial in improving the health of patients suffering from recurrent Clostridioides difficile infection. A recent Harvard Health Letter, written by Jessica Allegretti, MD, MPH, observed that FMT is standard of care for patients with C. diff, and the procedure has a success rate of between 80% and 90%.
“It shows us very directly, in a very practical way, how addressing the dysbiosis – the imbalance of the gut microbiome – by infusing healthy bacteria may make a potential lifesaving difference,” Dr. Grossberg said.
Research is beginning to show that the link between gut microbiota and health extends to Alzheimer’s disease as well. Within the last few years, “we’ve started to understand that the microbial diversity in Alzheimer’s disease versus healthy age-matched controls is decreased,” Dr. Grossberg said.
In a study published by Nicholas M. Vogt and colleagues, there was decreased fecal microbial diversity among individuals with Alzheimer’s, compared with healthy individuals matched for age. Another study by Ping Liu, PhD, and colleagues found that patients with Alzheimer’s disease had decreased fecal microbial diversity, compared with individuals who had pre-onset amnestic mild cognitive impairment and normal cognition.
Dr. Grossberg noted that, while these studies do not prove that less fecal microbial diversity is responsible for mild cognitive impairment or Alzheimer’s disease, “it makes us think that, maybe, there’s a contributing factor.”
“What happens with the dysbiosis of the gut microbiome is increased permeability of the epithelial area of the gut, which can then lead to the gut-brain axis dysregulation and may in fact allow the selective entry of bacteria into the central nervous system because the blood-brain barrier comes to be dysfunctional,” he said.
Early evidence suggests that the gut-brain axis can affect cognition. In an animal model study, transferring the microbiota of a mouse with Alzheimer’s disease to one that had been bred to be germ-free resulted in cognitive decline – but there was no cognitive decline for germ-free mice that received a microbiota transplant from a mouse in a healthy control group. Results from another animal study showed that transferring healthy microbiota from a mouse model into a mouse with Alzheimer’s disease reduces amyloid and tau pathology. “The conclusions of these studies seems to be that microbiota mediated intestinal and systemic immune changes or aberrations seem to contribute to the pathogenesis of Alzheimer’s disease in these mouse models,” Dr. Grossberg said. “Consequently, restoring the gut microbial homeostasis may have beneficial effects on Alzheimer’s disease treatment.”
Periodontal disease also might be linked to Alzheimer’s disease, Dr. Grossberg said. Several studies have shown gingipains secreted from Porphyromonas gingivalis, which contribute to inflammation in the brain, have been found in cadavers of patients with Alzheimer’s disease (Sci Adv. 2019 Jan 23;5[1]:eaau3333). “There’s reason to think that the same changes may be occurring in the human brain with periodontal disease,” he said.
The relationship also might extend to the gut microbiota and the central nervous system. “There seems to be a direct communication, a direct relationship between normal gut physiology and healthy central nervous system functioning, and then, when you have abnormal gut function, it may result in a variety of abnormal central nervous system functions,” Dr. Grossberg said.
Studies that have examined a relationship between Alzheimer’s disease and gut microbiota have highlighted the potential of probiotics and prebiotics as a method of restoring the gut microbiota (Aging [Albany NY]. 2020 Mar 31; 12[6]:5539-50). Probiotics are popularly sold in health food aisles of grocery stores, and prebiotics are available in foods such as yogurts, tempeh, sauerkraut, and kimchi, as well as in drinks such as Kombucha tea. The effectiveness of probiotics and prebiotics also are being examined in randomized, controlled trials in patients with mild cognitive decline and mild Alzheimer’s disease, Dr. Grossberg said. One therapy, Sodium oligomannate, a marine algae–derived oral oligosaccharide, has shown effectiveness in remodeling gut microbiota and has been approved in China to treat patients with mild or moderate Alzheimer’s disease. Currently, no approved gut microbiota therapies are approved in the United States to treat Alzheimer’s disease; however, encouraging use of a prebiotic, a probiotic, or a Mediterranean diet is something clinicians might want to consider for their patients.
“The fact that we’re studying these things has really led to the notion that it may not be a bad idea for people to consume these healthy bacteria in later life, either as a way to prevent or delay, or to treat Alzheimer’s disease,” Dr. Grossberg said. “There’s really no downside.”
Global Academy and this news organization are owned by the same parent company. Dr. Grossberg reported that he is a consultant for Acadia, Alkahest, Avanir, Axsome, Biogen, BioXcel, Karuna, Lundbeck, Otsuka, Roche, and Takeda; receives research support from the National Institute on Aging, Janssen, and Roche; performs safety monitoring for EryDel, Merck, and Newron; and serves on data monitoring committees for Avanex and ITI Therapeutics.
Research into the microbiome is yielding some positive new potential treatment options for Alzheimer’s disease, according to George T. Grossberg, MD.
“I think the growing focus on the gut-brain axis is opening doors to new Alzheimer’s disease and other brain disorders, and I think the first of a possible future generation of compounds for prevention or treatment of Alzheimer’s disease may indeed be emerging,” Dr. Grossberg said at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Focus on the microbiome and microbiota is “a really hot, really new, really emerging area,” said Dr. Grossberg, professor in the department of psychiatry & behavioral neuroscience at Saint Louis University. But the microbiota, which is the microorganisms within a specific organ such as the colon, is sometimes confused with the microbiome – which is defined as all of the bacteria, viruses, fungi and other microorganisms within a habitat as well as their genomes and the environment around them. “These are often used interchangeably, but they’re not the same,” Dr. Grossberg said at the meeting, presented by Global Academy for Medical Education.
A person’s microbiome is unique to them, and nearly all of the microbiome is contained in the gut. A reduction in diversity of the microbiota in the digestive system has been linked to a wide variety of diseases, Dr. Grossberg explained. Conversely, a microbial imbalance or dysbiosis has been implicated in anxiety and/or depression, dementia, and certain cancers, he noted.
Bacteria that positively affect the microbiome come from two main genera: Lactobacillus and Bifidobacterium. Factors such as diet, medications, geography, stage of life, birthing process, infant feeding method, and stress can all affect a person’s microbiome. “We’re all beginning to understand that trying to manage or trying to diversify, trying to manipulate the microbiota may have a lot of remote effects – even effects on weight or diabetes, or other disorders,” Dr. Grossberg said.
Fecal microbiota transplantation (FMT), or the process of administering a donor’s fecal matter into a recipient’s intestinal tract, has proved beneficial in improving the health of patients suffering from recurrent Clostridioides difficile infection. A recent Harvard Health Letter, written by Jessica Allegretti, MD, MPH, observed that FMT is standard of care for patients with C. diff, and the procedure has a success rate of between 80% and 90%.
“It shows us very directly, in a very practical way, how addressing the dysbiosis – the imbalance of the gut microbiome – by infusing healthy bacteria may make a potential lifesaving difference,” Dr. Grossberg said.
Research is beginning to show that the link between gut microbiota and health extends to Alzheimer’s disease as well. Within the last few years, “we’ve started to understand that the microbial diversity in Alzheimer’s disease versus healthy age-matched controls is decreased,” Dr. Grossberg said.
In a study published by Nicholas M. Vogt and colleagues, there was decreased fecal microbial diversity among individuals with Alzheimer’s, compared with healthy individuals matched for age. Another study by Ping Liu, PhD, and colleagues found that patients with Alzheimer’s disease had decreased fecal microbial diversity, compared with individuals who had pre-onset amnestic mild cognitive impairment and normal cognition.
Dr. Grossberg noted that, while these studies do not prove that less fecal microbial diversity is responsible for mild cognitive impairment or Alzheimer’s disease, “it makes us think that, maybe, there’s a contributing factor.”
“What happens with the dysbiosis of the gut microbiome is increased permeability of the epithelial area of the gut, which can then lead to the gut-brain axis dysregulation and may in fact allow the selective entry of bacteria into the central nervous system because the blood-brain barrier comes to be dysfunctional,” he said.
Early evidence suggests that the gut-brain axis can affect cognition. In an animal model study, transferring the microbiota of a mouse with Alzheimer’s disease to one that had been bred to be germ-free resulted in cognitive decline – but there was no cognitive decline for germ-free mice that received a microbiota transplant from a mouse in a healthy control group. Results from another animal study showed that transferring healthy microbiota from a mouse model into a mouse with Alzheimer’s disease reduces amyloid and tau pathology. “The conclusions of these studies seems to be that microbiota mediated intestinal and systemic immune changes or aberrations seem to contribute to the pathogenesis of Alzheimer’s disease in these mouse models,” Dr. Grossberg said. “Consequently, restoring the gut microbial homeostasis may have beneficial effects on Alzheimer’s disease treatment.”
Periodontal disease also might be linked to Alzheimer’s disease, Dr. Grossberg said. Several studies have shown gingipains secreted from Porphyromonas gingivalis, which contribute to inflammation in the brain, have been found in cadavers of patients with Alzheimer’s disease (Sci Adv. 2019 Jan 23;5[1]:eaau3333). “There’s reason to think that the same changes may be occurring in the human brain with periodontal disease,” he said.
The relationship also might extend to the gut microbiota and the central nervous system. “There seems to be a direct communication, a direct relationship between normal gut physiology and healthy central nervous system functioning, and then, when you have abnormal gut function, it may result in a variety of abnormal central nervous system functions,” Dr. Grossberg said.
Studies that have examined a relationship between Alzheimer’s disease and gut microbiota have highlighted the potential of probiotics and prebiotics as a method of restoring the gut microbiota (Aging [Albany NY]. 2020 Mar 31; 12[6]:5539-50). Probiotics are popularly sold in health food aisles of grocery stores, and prebiotics are available in foods such as yogurts, tempeh, sauerkraut, and kimchi, as well as in drinks such as Kombucha tea. The effectiveness of probiotics and prebiotics also are being examined in randomized, controlled trials in patients with mild cognitive decline and mild Alzheimer’s disease, Dr. Grossberg said. One therapy, Sodium oligomannate, a marine algae–derived oral oligosaccharide, has shown effectiveness in remodeling gut microbiota and has been approved in China to treat patients with mild or moderate Alzheimer’s disease. Currently, no approved gut microbiota therapies are approved in the United States to treat Alzheimer’s disease; however, encouraging use of a prebiotic, a probiotic, or a Mediterranean diet is something clinicians might want to consider for their patients.
“The fact that we’re studying these things has really led to the notion that it may not be a bad idea for people to consume these healthy bacteria in later life, either as a way to prevent or delay, or to treat Alzheimer’s disease,” Dr. Grossberg said. “There’s really no downside.”
Global Academy and this news organization are owned by the same parent company. Dr. Grossberg reported that he is a consultant for Acadia, Alkahest, Avanir, Axsome, Biogen, BioXcel, Karuna, Lundbeck, Otsuka, Roche, and Takeda; receives research support from the National Institute on Aging, Janssen, and Roche; performs safety monitoring for EryDel, Merck, and Newron; and serves on data monitoring committees for Avanex and ITI Therapeutics.
Research into the microbiome is yielding some positive new potential treatment options for Alzheimer’s disease, according to George T. Grossberg, MD.
“I think the growing focus on the gut-brain axis is opening doors to new Alzheimer’s disease and other brain disorders, and I think the first of a possible future generation of compounds for prevention or treatment of Alzheimer’s disease may indeed be emerging,” Dr. Grossberg said at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Focus on the microbiome and microbiota is “a really hot, really new, really emerging area,” said Dr. Grossberg, professor in the department of psychiatry & behavioral neuroscience at Saint Louis University. But the microbiota, which is the microorganisms within a specific organ such as the colon, is sometimes confused with the microbiome – which is defined as all of the bacteria, viruses, fungi and other microorganisms within a habitat as well as their genomes and the environment around them. “These are often used interchangeably, but they’re not the same,” Dr. Grossberg said at the meeting, presented by Global Academy for Medical Education.
A person’s microbiome is unique to them, and nearly all of the microbiome is contained in the gut. A reduction in diversity of the microbiota in the digestive system has been linked to a wide variety of diseases, Dr. Grossberg explained. Conversely, a microbial imbalance or dysbiosis has been implicated in anxiety and/or depression, dementia, and certain cancers, he noted.
Bacteria that positively affect the microbiome come from two main genera: Lactobacillus and Bifidobacterium. Factors such as diet, medications, geography, stage of life, birthing process, infant feeding method, and stress can all affect a person’s microbiome. “We’re all beginning to understand that trying to manage or trying to diversify, trying to manipulate the microbiota may have a lot of remote effects – even effects on weight or diabetes, or other disorders,” Dr. Grossberg said.
Fecal microbiota transplantation (FMT), or the process of administering a donor’s fecal matter into a recipient’s intestinal tract, has proved beneficial in improving the health of patients suffering from recurrent Clostridioides difficile infection. A recent Harvard Health Letter, written by Jessica Allegretti, MD, MPH, observed that FMT is standard of care for patients with C. diff, and the procedure has a success rate of between 80% and 90%.
“It shows us very directly, in a very practical way, how addressing the dysbiosis – the imbalance of the gut microbiome – by infusing healthy bacteria may make a potential lifesaving difference,” Dr. Grossberg said.
Research is beginning to show that the link between gut microbiota and health extends to Alzheimer’s disease as well. Within the last few years, “we’ve started to understand that the microbial diversity in Alzheimer’s disease versus healthy age-matched controls is decreased,” Dr. Grossberg said.
In a study published by Nicholas M. Vogt and colleagues, there was decreased fecal microbial diversity among individuals with Alzheimer’s, compared with healthy individuals matched for age. Another study by Ping Liu, PhD, and colleagues found that patients with Alzheimer’s disease had decreased fecal microbial diversity, compared with individuals who had pre-onset amnestic mild cognitive impairment and normal cognition.
Dr. Grossberg noted that, while these studies do not prove that less fecal microbial diversity is responsible for mild cognitive impairment or Alzheimer’s disease, “it makes us think that, maybe, there’s a contributing factor.”
“What happens with the dysbiosis of the gut microbiome is increased permeability of the epithelial area of the gut, which can then lead to the gut-brain axis dysregulation and may in fact allow the selective entry of bacteria into the central nervous system because the blood-brain barrier comes to be dysfunctional,” he said.
Early evidence suggests that the gut-brain axis can affect cognition. In an animal model study, transferring the microbiota of a mouse with Alzheimer’s disease to one that had been bred to be germ-free resulted in cognitive decline – but there was no cognitive decline for germ-free mice that received a microbiota transplant from a mouse in a healthy control group. Results from another animal study showed that transferring healthy microbiota from a mouse model into a mouse with Alzheimer’s disease reduces amyloid and tau pathology. “The conclusions of these studies seems to be that microbiota mediated intestinal and systemic immune changes or aberrations seem to contribute to the pathogenesis of Alzheimer’s disease in these mouse models,” Dr. Grossberg said. “Consequently, restoring the gut microbial homeostasis may have beneficial effects on Alzheimer’s disease treatment.”
Periodontal disease also might be linked to Alzheimer’s disease, Dr. Grossberg said. Several studies have shown gingipains secreted from Porphyromonas gingivalis, which contribute to inflammation in the brain, have been found in cadavers of patients with Alzheimer’s disease (Sci Adv. 2019 Jan 23;5[1]:eaau3333). “There’s reason to think that the same changes may be occurring in the human brain with periodontal disease,” he said.
The relationship also might extend to the gut microbiota and the central nervous system. “There seems to be a direct communication, a direct relationship between normal gut physiology and healthy central nervous system functioning, and then, when you have abnormal gut function, it may result in a variety of abnormal central nervous system functions,” Dr. Grossberg said.
Studies that have examined a relationship between Alzheimer’s disease and gut microbiota have highlighted the potential of probiotics and prebiotics as a method of restoring the gut microbiota (Aging [Albany NY]. 2020 Mar 31; 12[6]:5539-50). Probiotics are popularly sold in health food aisles of grocery stores, and prebiotics are available in foods such as yogurts, tempeh, sauerkraut, and kimchi, as well as in drinks such as Kombucha tea. The effectiveness of probiotics and prebiotics also are being examined in randomized, controlled trials in patients with mild cognitive decline and mild Alzheimer’s disease, Dr. Grossberg said. One therapy, Sodium oligomannate, a marine algae–derived oral oligosaccharide, has shown effectiveness in remodeling gut microbiota and has been approved in China to treat patients with mild or moderate Alzheimer’s disease. Currently, no approved gut microbiota therapies are approved in the United States to treat Alzheimer’s disease; however, encouraging use of a prebiotic, a probiotic, or a Mediterranean diet is something clinicians might want to consider for their patients.
“The fact that we’re studying these things has really led to the notion that it may not be a bad idea for people to consume these healthy bacteria in later life, either as a way to prevent or delay, or to treat Alzheimer’s disease,” Dr. Grossberg said. “There’s really no downside.”
Global Academy and this news organization are owned by the same parent company. Dr. Grossberg reported that he is a consultant for Acadia, Alkahest, Avanir, Axsome, Biogen, BioXcel, Karuna, Lundbeck, Otsuka, Roche, and Takeda; receives research support from the National Institute on Aging, Janssen, and Roche; performs safety monitoring for EryDel, Merck, and Newron; and serves on data monitoring committees for Avanex and ITI Therapeutics.
FROM CP/AACP PSYCHIATRY UPDATE
A better tau blood test for diagnosing Alzheimer’s disease?
.
In one new development, experts at the University of California, San Francisco (UCSF) compared phosphorylated-tau181 (P-tau181) to a related form of tau called P-tau217 to determine which can best identify individuals with Alzheimer’s disease.
Results showed that the two biomarkers were similar overall, but P-tau 217 had a slight edge in terms of accuracy. Importantly, both tau isoforms distinguished frontotemporal lobar degeneration (FTLD).
“These new blood tests for P-tau are going to be really exciting because they will improve our ability to simply and inexpensively assess whether someone is at high risk for having Alzheimer’s disease,” said study author Adam L. Boxer, MD, PhD, professor in UCSF’s department of neurology.
With the approval of the first disease-modifying therapy for Alzheimer’s disease possibly around the corner, developing an accurate diagnostic blood test for this condition is even more urgent, added Dr. Boxer, who is also director of UCSF’s Neurosciences Clinical Research Unit and AD and FTD Clinical Trials Program.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Important implications
Currently, the only approved Alzheimer’s disease biomarkers are expensive positron emission tomography (PET) scans using agents that detect tau or amyloid, another hallmark Alzheimer’s disease protein, and cerebrospinal fluid levels of amyloid and tau, the measurement of which entails invasive lumbar puncture procedures. This limits the ability to easily confirm the underlying cause of dementia or cognitive impairment, which “obviously has important prognostic and therapeutic implications,” said Dr. Boxer.
Having a plasma biomarker, especially for tau, would be extremely useful. Patients with increased tau in the brain tend to exhibit Alzheimer’s disease symptoms while those with amyloid plaques do not always have clear signs, at least not immediately. “We think that P-tau is probably a better measure because it is much more closely related to symptoms of disease,” said Dr. Boxer.
Earlier this year, he and colleagues published a study in Nature Medicine showing that P-tau181 is more than three times as high in individuals with Alzheimer’s disease compared with healthy elderly people. It also differentiated Alzheimer’s disease from frontotemporal dementia (FTD). “We found that P-tau 181 was almost as good as a PET scan or lumbar puncture at identifying individuals with Alzheimer’s disease pathology in the brain,” said Dr. Boxer.
They next wanted to assess how well P-tau 217 held up as a possible biomarker.
The new retrospective study was composed of 210 participants: 37 who acted as healthy controls, 99 who had FTLD, 39 who had Alzheimer’s disease, and 35 who had mild cognitive impairment.
More accurate test
Results showed that plasma P-tau217 was increased 5.7-fold in the participants with Alzheimer’s disease compared with the healthy controls group, and increased fivefold compared with those who had FTLD (both comparisons, P < .001).
The increase in plasma P-tau181 was lower. It was increased only 4.5-times in participants with Alzheimer’s disease compared with the healthy controls and 3.8-times relative to those with FTLD (both, P < .001). In addition, P-tau217 was potentially superior in predicting whether a person had a tau positive FTP-PET brain scan.
“This newer P-tau 217 test produces very similar results to the previous test we published [on P-tau181], but might be incrementally better or slightly more accurate, and even more closely related to the signal you get with a tau PET scan,” Dr. Boxer said.
The researchers are now examining these issues in a larger group of participants (N = 617). Results for those analyses are expected to be published soon. In addition to tau and amyloid markers, the researchers are examining another potential biomarker of neurodegeneration: the triple protein neurofilament light chain.
It’s too early to say which biomarker or biomarkers will prove to be the most useful in diagnosing Alzheimer’s disease, Dr. Boxer noted. “It’s an open question whether it will be necessary to measure multiple P-taus plus beta amyloid plus neurofilament, or maybe just measuring one P-tau level will be sufficient,” he said.
Upcoming therapy?
Having a test that verifies Alzheimer’s disease is becoming all the more important now that a therapy might soon be available. Massachusetts-based biotech company Biogen has submitted aducanumab, a monoclonal antibody that targets amyloid-beta (Abeta), to the Food and Drug Administration for approval. Should that move forward, aducanumab would be the first disease-modifying therapy for Alzheimer’s disease.
“If that’s the case, it will be even more important to have simple ways to screen people, to see if they might eventually be eligible for treatment,” said Dr. Boxer. Even if the drug isn’t approved, many patients simply want to know what is causing their cognitive problems, he added. Knowing they have Alzheimer’s disease might impact their life planning. If they have mild symptoms, interventions such as exercise and reducing cardiovascular risk could improve their overall health and quality of life, he said.
If individuals have another type of dementia, such as FTLD, that, too, might determine a different approach. Some forms of FTLD are caused by “completely different biological processes,” which are now being studied, Dr. Boxer said. So knowing that patients have this condition would allow them to participate in relevant clinical trials.
Exciting aspect
Having a tau blood test will also help those in underserviced and minority communities who can’t easily access memory specialists, Dr. Boxer noted. “It might allow them to access care, and get help much more easily, and that is a really exciting aspect of this new technology,” he said. It’s not clear when such blood tests will be on the market, although many companies are “scrambling” to make them available, said Dr. Boxer.
P-tau217 also holds promise as a marker for early Alzheimer’s disease pathology, according to another study presented at AAIC 2020. A Swedish research team measured P-tau217 in more than 1,000 participants, including those who were unimpaired and those with mild cognitive impairment, Alzheimer’s disease dementia, or non-Alzheimer’s disease neurodegenerative diseases.
Results showed that plasma P-tau217 levels increase in early stages of Alzheimer’s disease when insoluble tau aggregates are not yet detectable with PET. They also predict subsequent increases in tau-PET, as well as conversion to Alzheimer’s disease dementia.
‘Incredible breakthrough’
Commenting on the research, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, called the study amazing and “an incredible breakthrough.
“Researchers are able to detect disease up to 20 years before symptoms. The blood test has very good characteristics in terms of sensitivity and specificity. It correlates with the spinal fluid, it’s better than the PET imaging, it correlates with the amyloid test, and the results are being confirmed in many different cohorts,” said Dr. Fillit, who was not involved with the research.
A tau blood test, especially for P-tau 217, has the potential to be as important to determining dementia risk as cholesterol is to gauging heart disease risk, he added.
Having a tau blood test will “make our clinical trials much more precise and more efficient and reduce costs tremendously,” Dr. Fillit said, adding that he thinks tau blood tests might come to market as early as within a year.
Also commenting on the research, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said the new studies illustrate the rapid progress being made “in the blood biomarker space.”
Even 5 years ago, researchers would “never have thought” that blood biomarkers could be used as a tool to detect brain changes related to Alzheimer’s disease, said Dr. Edelmayer.
These new studies are “filling a gap in our understanding around tau” in Alzheimer’s disease and other neurodegenerative diseases, she said. “Being able to distinguish between diseases is going to be very, very crucial for clinicians in the future,” she added.
Dr. Edelmayer foresees that in the future there will be a panel of blood biomarkers in addition to imaging tests to help clinicians make an accurate diagnosis.
The study was supported by the National Institutes of Health and the Tau Research Consortium. Dr. Boxer disclosed that the blood p-tau test was done as part of a research collaboration between UCSF and Eli Lilly. Dr. Fillit and Dr. Edelmayer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In one new development, experts at the University of California, San Francisco (UCSF) compared phosphorylated-tau181 (P-tau181) to a related form of tau called P-tau217 to determine which can best identify individuals with Alzheimer’s disease.
Results showed that the two biomarkers were similar overall, but P-tau 217 had a slight edge in terms of accuracy. Importantly, both tau isoforms distinguished frontotemporal lobar degeneration (FTLD).
“These new blood tests for P-tau are going to be really exciting because they will improve our ability to simply and inexpensively assess whether someone is at high risk for having Alzheimer’s disease,” said study author Adam L. Boxer, MD, PhD, professor in UCSF’s department of neurology.
With the approval of the first disease-modifying therapy for Alzheimer’s disease possibly around the corner, developing an accurate diagnostic blood test for this condition is even more urgent, added Dr. Boxer, who is also director of UCSF’s Neurosciences Clinical Research Unit and AD and FTD Clinical Trials Program.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Important implications
Currently, the only approved Alzheimer’s disease biomarkers are expensive positron emission tomography (PET) scans using agents that detect tau or amyloid, another hallmark Alzheimer’s disease protein, and cerebrospinal fluid levels of amyloid and tau, the measurement of which entails invasive lumbar puncture procedures. This limits the ability to easily confirm the underlying cause of dementia or cognitive impairment, which “obviously has important prognostic and therapeutic implications,” said Dr. Boxer.
Having a plasma biomarker, especially for tau, would be extremely useful. Patients with increased tau in the brain tend to exhibit Alzheimer’s disease symptoms while those with amyloid plaques do not always have clear signs, at least not immediately. “We think that P-tau is probably a better measure because it is much more closely related to symptoms of disease,” said Dr. Boxer.
Earlier this year, he and colleagues published a study in Nature Medicine showing that P-tau181 is more than three times as high in individuals with Alzheimer’s disease compared with healthy elderly people. It also differentiated Alzheimer’s disease from frontotemporal dementia (FTD). “We found that P-tau 181 was almost as good as a PET scan or lumbar puncture at identifying individuals with Alzheimer’s disease pathology in the brain,” said Dr. Boxer.
They next wanted to assess how well P-tau 217 held up as a possible biomarker.
The new retrospective study was composed of 210 participants: 37 who acted as healthy controls, 99 who had FTLD, 39 who had Alzheimer’s disease, and 35 who had mild cognitive impairment.
More accurate test
Results showed that plasma P-tau217 was increased 5.7-fold in the participants with Alzheimer’s disease compared with the healthy controls group, and increased fivefold compared with those who had FTLD (both comparisons, P < .001).
The increase in plasma P-tau181 was lower. It was increased only 4.5-times in participants with Alzheimer’s disease compared with the healthy controls and 3.8-times relative to those with FTLD (both, P < .001). In addition, P-tau217 was potentially superior in predicting whether a person had a tau positive FTP-PET brain scan.
“This newer P-tau 217 test produces very similar results to the previous test we published [on P-tau181], but might be incrementally better or slightly more accurate, and even more closely related to the signal you get with a tau PET scan,” Dr. Boxer said.
The researchers are now examining these issues in a larger group of participants (N = 617). Results for those analyses are expected to be published soon. In addition to tau and amyloid markers, the researchers are examining another potential biomarker of neurodegeneration: the triple protein neurofilament light chain.
It’s too early to say which biomarker or biomarkers will prove to be the most useful in diagnosing Alzheimer’s disease, Dr. Boxer noted. “It’s an open question whether it will be necessary to measure multiple P-taus plus beta amyloid plus neurofilament, or maybe just measuring one P-tau level will be sufficient,” he said.
Upcoming therapy?
Having a test that verifies Alzheimer’s disease is becoming all the more important now that a therapy might soon be available. Massachusetts-based biotech company Biogen has submitted aducanumab, a monoclonal antibody that targets amyloid-beta (Abeta), to the Food and Drug Administration for approval. Should that move forward, aducanumab would be the first disease-modifying therapy for Alzheimer’s disease.
“If that’s the case, it will be even more important to have simple ways to screen people, to see if they might eventually be eligible for treatment,” said Dr. Boxer. Even if the drug isn’t approved, many patients simply want to know what is causing their cognitive problems, he added. Knowing they have Alzheimer’s disease might impact their life planning. If they have mild symptoms, interventions such as exercise and reducing cardiovascular risk could improve their overall health and quality of life, he said.
If individuals have another type of dementia, such as FTLD, that, too, might determine a different approach. Some forms of FTLD are caused by “completely different biological processes,” which are now being studied, Dr. Boxer said. So knowing that patients have this condition would allow them to participate in relevant clinical trials.
Exciting aspect
Having a tau blood test will also help those in underserviced and minority communities who can’t easily access memory specialists, Dr. Boxer noted. “It might allow them to access care, and get help much more easily, and that is a really exciting aspect of this new technology,” he said. It’s not clear when such blood tests will be on the market, although many companies are “scrambling” to make them available, said Dr. Boxer.
P-tau217 also holds promise as a marker for early Alzheimer’s disease pathology, according to another study presented at AAIC 2020. A Swedish research team measured P-tau217 in more than 1,000 participants, including those who were unimpaired and those with mild cognitive impairment, Alzheimer’s disease dementia, or non-Alzheimer’s disease neurodegenerative diseases.
Results showed that plasma P-tau217 levels increase in early stages of Alzheimer’s disease when insoluble tau aggregates are not yet detectable with PET. They also predict subsequent increases in tau-PET, as well as conversion to Alzheimer’s disease dementia.
‘Incredible breakthrough’
Commenting on the research, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, called the study amazing and “an incredible breakthrough.
“Researchers are able to detect disease up to 20 years before symptoms. The blood test has very good characteristics in terms of sensitivity and specificity. It correlates with the spinal fluid, it’s better than the PET imaging, it correlates with the amyloid test, and the results are being confirmed in many different cohorts,” said Dr. Fillit, who was not involved with the research.
A tau blood test, especially for P-tau 217, has the potential to be as important to determining dementia risk as cholesterol is to gauging heart disease risk, he added.
Having a tau blood test will “make our clinical trials much more precise and more efficient and reduce costs tremendously,” Dr. Fillit said, adding that he thinks tau blood tests might come to market as early as within a year.
Also commenting on the research, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said the new studies illustrate the rapid progress being made “in the blood biomarker space.”
Even 5 years ago, researchers would “never have thought” that blood biomarkers could be used as a tool to detect brain changes related to Alzheimer’s disease, said Dr. Edelmayer.
These new studies are “filling a gap in our understanding around tau” in Alzheimer’s disease and other neurodegenerative diseases, she said. “Being able to distinguish between diseases is going to be very, very crucial for clinicians in the future,” she added.
Dr. Edelmayer foresees that in the future there will be a panel of blood biomarkers in addition to imaging tests to help clinicians make an accurate diagnosis.
The study was supported by the National Institutes of Health and the Tau Research Consortium. Dr. Boxer disclosed that the blood p-tau test was done as part of a research collaboration between UCSF and Eli Lilly. Dr. Fillit and Dr. Edelmayer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In one new development, experts at the University of California, San Francisco (UCSF) compared phosphorylated-tau181 (P-tau181) to a related form of tau called P-tau217 to determine which can best identify individuals with Alzheimer’s disease.
Results showed that the two biomarkers were similar overall, but P-tau 217 had a slight edge in terms of accuracy. Importantly, both tau isoforms distinguished frontotemporal lobar degeneration (FTLD).
“These new blood tests for P-tau are going to be really exciting because they will improve our ability to simply and inexpensively assess whether someone is at high risk for having Alzheimer’s disease,” said study author Adam L. Boxer, MD, PhD, professor in UCSF’s department of neurology.
With the approval of the first disease-modifying therapy for Alzheimer’s disease possibly around the corner, developing an accurate diagnostic blood test for this condition is even more urgent, added Dr. Boxer, who is also director of UCSF’s Neurosciences Clinical Research Unit and AD and FTD Clinical Trials Program.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Important implications
Currently, the only approved Alzheimer’s disease biomarkers are expensive positron emission tomography (PET) scans using agents that detect tau or amyloid, another hallmark Alzheimer’s disease protein, and cerebrospinal fluid levels of amyloid and tau, the measurement of which entails invasive lumbar puncture procedures. This limits the ability to easily confirm the underlying cause of dementia or cognitive impairment, which “obviously has important prognostic and therapeutic implications,” said Dr. Boxer.
Having a plasma biomarker, especially for tau, would be extremely useful. Patients with increased tau in the brain tend to exhibit Alzheimer’s disease symptoms while those with amyloid plaques do not always have clear signs, at least not immediately. “We think that P-tau is probably a better measure because it is much more closely related to symptoms of disease,” said Dr. Boxer.
Earlier this year, he and colleagues published a study in Nature Medicine showing that P-tau181 is more than three times as high in individuals with Alzheimer’s disease compared with healthy elderly people. It also differentiated Alzheimer’s disease from frontotemporal dementia (FTD). “We found that P-tau 181 was almost as good as a PET scan or lumbar puncture at identifying individuals with Alzheimer’s disease pathology in the brain,” said Dr. Boxer.
They next wanted to assess how well P-tau 217 held up as a possible biomarker.
The new retrospective study was composed of 210 participants: 37 who acted as healthy controls, 99 who had FTLD, 39 who had Alzheimer’s disease, and 35 who had mild cognitive impairment.
More accurate test
Results showed that plasma P-tau217 was increased 5.7-fold in the participants with Alzheimer’s disease compared with the healthy controls group, and increased fivefold compared with those who had FTLD (both comparisons, P < .001).
The increase in plasma P-tau181 was lower. It was increased only 4.5-times in participants with Alzheimer’s disease compared with the healthy controls and 3.8-times relative to those with FTLD (both, P < .001). In addition, P-tau217 was potentially superior in predicting whether a person had a tau positive FTP-PET brain scan.
“This newer P-tau 217 test produces very similar results to the previous test we published [on P-tau181], but might be incrementally better or slightly more accurate, and even more closely related to the signal you get with a tau PET scan,” Dr. Boxer said.
The researchers are now examining these issues in a larger group of participants (N = 617). Results for those analyses are expected to be published soon. In addition to tau and amyloid markers, the researchers are examining another potential biomarker of neurodegeneration: the triple protein neurofilament light chain.
It’s too early to say which biomarker or biomarkers will prove to be the most useful in diagnosing Alzheimer’s disease, Dr. Boxer noted. “It’s an open question whether it will be necessary to measure multiple P-taus plus beta amyloid plus neurofilament, or maybe just measuring one P-tau level will be sufficient,” he said.
Upcoming therapy?
Having a test that verifies Alzheimer’s disease is becoming all the more important now that a therapy might soon be available. Massachusetts-based biotech company Biogen has submitted aducanumab, a monoclonal antibody that targets amyloid-beta (Abeta), to the Food and Drug Administration for approval. Should that move forward, aducanumab would be the first disease-modifying therapy for Alzheimer’s disease.
“If that’s the case, it will be even more important to have simple ways to screen people, to see if they might eventually be eligible for treatment,” said Dr. Boxer. Even if the drug isn’t approved, many patients simply want to know what is causing their cognitive problems, he added. Knowing they have Alzheimer’s disease might impact their life planning. If they have mild symptoms, interventions such as exercise and reducing cardiovascular risk could improve their overall health and quality of life, he said.
If individuals have another type of dementia, such as FTLD, that, too, might determine a different approach. Some forms of FTLD are caused by “completely different biological processes,” which are now being studied, Dr. Boxer said. So knowing that patients have this condition would allow them to participate in relevant clinical trials.
Exciting aspect
Having a tau blood test will also help those in underserviced and minority communities who can’t easily access memory specialists, Dr. Boxer noted. “It might allow them to access care, and get help much more easily, and that is a really exciting aspect of this new technology,” he said. It’s not clear when such blood tests will be on the market, although many companies are “scrambling” to make them available, said Dr. Boxer.
P-tau217 also holds promise as a marker for early Alzheimer’s disease pathology, according to another study presented at AAIC 2020. A Swedish research team measured P-tau217 in more than 1,000 participants, including those who were unimpaired and those with mild cognitive impairment, Alzheimer’s disease dementia, or non-Alzheimer’s disease neurodegenerative diseases.
Results showed that plasma P-tau217 levels increase in early stages of Alzheimer’s disease when insoluble tau aggregates are not yet detectable with PET. They also predict subsequent increases in tau-PET, as well as conversion to Alzheimer’s disease dementia.
‘Incredible breakthrough’
Commenting on the research, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, called the study amazing and “an incredible breakthrough.
“Researchers are able to detect disease up to 20 years before symptoms. The blood test has very good characteristics in terms of sensitivity and specificity. It correlates with the spinal fluid, it’s better than the PET imaging, it correlates with the amyloid test, and the results are being confirmed in many different cohorts,” said Dr. Fillit, who was not involved with the research.
A tau blood test, especially for P-tau 217, has the potential to be as important to determining dementia risk as cholesterol is to gauging heart disease risk, he added.
Having a tau blood test will “make our clinical trials much more precise and more efficient and reduce costs tremendously,” Dr. Fillit said, adding that he thinks tau blood tests might come to market as early as within a year.
Also commenting on the research, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said the new studies illustrate the rapid progress being made “in the blood biomarker space.”
Even 5 years ago, researchers would “never have thought” that blood biomarkers could be used as a tool to detect brain changes related to Alzheimer’s disease, said Dr. Edelmayer.
These new studies are “filling a gap in our understanding around tau” in Alzheimer’s disease and other neurodegenerative diseases, she said. “Being able to distinguish between diseases is going to be very, very crucial for clinicians in the future,” she added.
Dr. Edelmayer foresees that in the future there will be a panel of blood biomarkers in addition to imaging tests to help clinicians make an accurate diagnosis.
The study was supported by the National Institutes of Health and the Tau Research Consortium. Dr. Boxer disclosed that the blood p-tau test was done as part of a research collaboration between UCSF and Eli Lilly. Dr. Fillit and Dr. Edelmayer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
Flu and pneumonia vaccination tied to lower dementia risk
In a cohort study of more than 9,000 older adults, receiving a single influenza vaccination was associated with a 17% lower prevalence of Alzheimer’s disease compared with not receiving the vaccine. In addition, for those who were vaccinated more than once over the years, there was an additional 13% reduction in Alzheimer’s disease incidence.
In another study, which included more than 5,000 older participants, being vaccinated against pneumonia between the ages of 65 and 75 reduced the risk of developing Alzheimer’s disease by 30%.
The subject of vaccines “is obviously very topical with the COVID-19 pandemic,” said Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association. “While these are very preliminary data, these studies do suggest that with vaccination against both respiratory illnesses, there is the potential to lower risk for developing cognitive decline and dementia,” said Dr. Edelmayer, who was not involved in the research.
The findings of both studies were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Lower Alzheimer’s disease prevalence
The influenza vaccine study was presented by Albert Amran, a fourth-year medical student at McGovern Medical School at the University of Texas Health Science Center at Houston. The researchers used electronic health record data to create a propensity-matched cohort of 9,066 vaccinated and unvaccinated adults ages 60 and older.
Influenza vaccination, increased frequency of administration, and younger age at time of vaccination were all associated with reduced incidence of Alzheimer’s disease, Mr. Amran reported.
Being vaccinated for influenza was significantly linked to a lower prevalence of Alzheimer’s disease (odds ratio [OR], 0.83; P < .0001) in comparison with not being vaccinated. Receiving more than one vaccination over the years was associated with an additional reduction in AD incidence (OR, 0.87; P = .0342). The protection appeared to be strongest for those who received their first vaccination at a younger age, for example, at age 60 versus 70.
Mr. Amran and research colleagues have two theories as to why influenza vaccination may protect the brain.
One is that vaccination may aid the immune system as people age. “As people get older, their immune systems become less able to control infection. We’ve seen this with the ongoing pandemic, with older people at much higher risk for dying. Giving people the vaccine once a year may help keep the immune system in shape,” Mr. Amran said.
Another theory is that the prevention of influenza itself may be relevant. “Flu infections can be extremely deadly in older patients. Maybe the results of our study will give another reason for people to get vaccinated,” Mr. Amran said.
Pneumonia vaccine
The other study was presented by Svetlana Ukraintseva, PhD, of Duke University, Durham, N.C.
Dr. Ukraintseva and colleagues investigated associations between pneumococcal vaccine, with and without an accompanying influenza vaccine, and the risk for Alzheimer’s disease among 5,146 participants in the Cardiovascular Health Study. Covariates included sex, race, birth cohort, education, smoking, and a known genetic risk factor for Alzheimer’s disease: the rs2075650 G allele in the TOMM40 gene.
In a logistic model with all covariates, vaccination against pneumonia between ages 65 and 75 was significantly associated with reduced risk of developing AD (OR, 0.70; P < .04). The largest reduction in Alzheimer’s disease risk (OR, 0.62; P < .04) was among those vaccinated against pneumonia who were noncarriers of the rs2075650 G allele.
Total number of vaccinations against pneumonia and influenza between ages 65 and 75 was also associated with a lower risk for Alzheimer’s disease (OR, 0.88; P < .01). However, the effect was not evident for the influenza vaccination alone.
“The fact that very different pathogens – viral, bacterial, fungal – have been linked to Alzheimer’s disease indicates a possibility that compromised host immunity may play a role in Alzheimer’s disease through increasing overall brain’s vulnerability to various microbes,” said Dr. Ukraintseva.
The current findings support further investigation of pneumococcal vaccine as a “reasonable candidate for repurposing in personalized AD prevention,” she noted. “These results also support the important role of boosting overall immune robustness/resilience in preventing Alzheimer’s disease,” Dr. Ukraintseva added.
Her group is currently working on confirming the findings in another population.
Brain protective?
“Neither study can prove that the benefit is directly related to the vaccine itself, but what they can indicate is that potentially, vaccines are a way to protect your health and brain,” Dr. Edelmayer said.
In a statement, Maria Carrillo, PhD, chief science officer for the Alzheimer’s Association, noted that more research is needed.
The new data call “for further studies in large, diverse clinical trials to inform whether vaccinations as a public health strategy decrease our risk for developing dementia as we age,” Dr. Carillo said.
Funding for the influenza vaccine study was provided by the Christopher Sarofim Family Professorship in Biomedical Informatics and Bioengineering, a UT STARs Award, the Cancer Prevention and Research Institute of Texas, and the National Institutes of Health. Funding for the pneumonia study was provided by the National Institute on Aging. Dr. Amran, Dr. Ukraintseva, Dr. Edelmayer, and Dr. Carrillo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a cohort study of more than 9,000 older adults, receiving a single influenza vaccination was associated with a 17% lower prevalence of Alzheimer’s disease compared with not receiving the vaccine. In addition, for those who were vaccinated more than once over the years, there was an additional 13% reduction in Alzheimer’s disease incidence.
In another study, which included more than 5,000 older participants, being vaccinated against pneumonia between the ages of 65 and 75 reduced the risk of developing Alzheimer’s disease by 30%.
The subject of vaccines “is obviously very topical with the COVID-19 pandemic,” said Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association. “While these are very preliminary data, these studies do suggest that with vaccination against both respiratory illnesses, there is the potential to lower risk for developing cognitive decline and dementia,” said Dr. Edelmayer, who was not involved in the research.
The findings of both studies were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Lower Alzheimer’s disease prevalence
The influenza vaccine study was presented by Albert Amran, a fourth-year medical student at McGovern Medical School at the University of Texas Health Science Center at Houston. The researchers used electronic health record data to create a propensity-matched cohort of 9,066 vaccinated and unvaccinated adults ages 60 and older.
Influenza vaccination, increased frequency of administration, and younger age at time of vaccination were all associated with reduced incidence of Alzheimer’s disease, Mr. Amran reported.
Being vaccinated for influenza was significantly linked to a lower prevalence of Alzheimer’s disease (odds ratio [OR], 0.83; P < .0001) in comparison with not being vaccinated. Receiving more than one vaccination over the years was associated with an additional reduction in AD incidence (OR, 0.87; P = .0342). The protection appeared to be strongest for those who received their first vaccination at a younger age, for example, at age 60 versus 70.
Mr. Amran and research colleagues have two theories as to why influenza vaccination may protect the brain.
One is that vaccination may aid the immune system as people age. “As people get older, their immune systems become less able to control infection. We’ve seen this with the ongoing pandemic, with older people at much higher risk for dying. Giving people the vaccine once a year may help keep the immune system in shape,” Mr. Amran said.
Another theory is that the prevention of influenza itself may be relevant. “Flu infections can be extremely deadly in older patients. Maybe the results of our study will give another reason for people to get vaccinated,” Mr. Amran said.
Pneumonia vaccine
The other study was presented by Svetlana Ukraintseva, PhD, of Duke University, Durham, N.C.
Dr. Ukraintseva and colleagues investigated associations between pneumococcal vaccine, with and without an accompanying influenza vaccine, and the risk for Alzheimer’s disease among 5,146 participants in the Cardiovascular Health Study. Covariates included sex, race, birth cohort, education, smoking, and a known genetic risk factor for Alzheimer’s disease: the rs2075650 G allele in the TOMM40 gene.
In a logistic model with all covariates, vaccination against pneumonia between ages 65 and 75 was significantly associated with reduced risk of developing AD (OR, 0.70; P < .04). The largest reduction in Alzheimer’s disease risk (OR, 0.62; P < .04) was among those vaccinated against pneumonia who were noncarriers of the rs2075650 G allele.
Total number of vaccinations against pneumonia and influenza between ages 65 and 75 was also associated with a lower risk for Alzheimer’s disease (OR, 0.88; P < .01). However, the effect was not evident for the influenza vaccination alone.
“The fact that very different pathogens – viral, bacterial, fungal – have been linked to Alzheimer’s disease indicates a possibility that compromised host immunity may play a role in Alzheimer’s disease through increasing overall brain’s vulnerability to various microbes,” said Dr. Ukraintseva.
The current findings support further investigation of pneumococcal vaccine as a “reasonable candidate for repurposing in personalized AD prevention,” she noted. “These results also support the important role of boosting overall immune robustness/resilience in preventing Alzheimer’s disease,” Dr. Ukraintseva added.
Her group is currently working on confirming the findings in another population.
Brain protective?
“Neither study can prove that the benefit is directly related to the vaccine itself, but what they can indicate is that potentially, vaccines are a way to protect your health and brain,” Dr. Edelmayer said.
In a statement, Maria Carrillo, PhD, chief science officer for the Alzheimer’s Association, noted that more research is needed.
The new data call “for further studies in large, diverse clinical trials to inform whether vaccinations as a public health strategy decrease our risk for developing dementia as we age,” Dr. Carillo said.
Funding for the influenza vaccine study was provided by the Christopher Sarofim Family Professorship in Biomedical Informatics and Bioengineering, a UT STARs Award, the Cancer Prevention and Research Institute of Texas, and the National Institutes of Health. Funding for the pneumonia study was provided by the National Institute on Aging. Dr. Amran, Dr. Ukraintseva, Dr. Edelmayer, and Dr. Carrillo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a cohort study of more than 9,000 older adults, receiving a single influenza vaccination was associated with a 17% lower prevalence of Alzheimer’s disease compared with not receiving the vaccine. In addition, for those who were vaccinated more than once over the years, there was an additional 13% reduction in Alzheimer’s disease incidence.
In another study, which included more than 5,000 older participants, being vaccinated against pneumonia between the ages of 65 and 75 reduced the risk of developing Alzheimer’s disease by 30%.
The subject of vaccines “is obviously very topical with the COVID-19 pandemic,” said Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association. “While these are very preliminary data, these studies do suggest that with vaccination against both respiratory illnesses, there is the potential to lower risk for developing cognitive decline and dementia,” said Dr. Edelmayer, who was not involved in the research.
The findings of both studies were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Lower Alzheimer’s disease prevalence
The influenza vaccine study was presented by Albert Amran, a fourth-year medical student at McGovern Medical School at the University of Texas Health Science Center at Houston. The researchers used electronic health record data to create a propensity-matched cohort of 9,066 vaccinated and unvaccinated adults ages 60 and older.
Influenza vaccination, increased frequency of administration, and younger age at time of vaccination were all associated with reduced incidence of Alzheimer’s disease, Mr. Amran reported.
Being vaccinated for influenza was significantly linked to a lower prevalence of Alzheimer’s disease (odds ratio [OR], 0.83; P < .0001) in comparison with not being vaccinated. Receiving more than one vaccination over the years was associated with an additional reduction in AD incidence (OR, 0.87; P = .0342). The protection appeared to be strongest for those who received their first vaccination at a younger age, for example, at age 60 versus 70.
Mr. Amran and research colleagues have two theories as to why influenza vaccination may protect the brain.
One is that vaccination may aid the immune system as people age. “As people get older, their immune systems become less able to control infection. We’ve seen this with the ongoing pandemic, with older people at much higher risk for dying. Giving people the vaccine once a year may help keep the immune system in shape,” Mr. Amran said.
Another theory is that the prevention of influenza itself may be relevant. “Flu infections can be extremely deadly in older patients. Maybe the results of our study will give another reason for people to get vaccinated,” Mr. Amran said.
Pneumonia vaccine
The other study was presented by Svetlana Ukraintseva, PhD, of Duke University, Durham, N.C.
Dr. Ukraintseva and colleagues investigated associations between pneumococcal vaccine, with and without an accompanying influenza vaccine, and the risk for Alzheimer’s disease among 5,146 participants in the Cardiovascular Health Study. Covariates included sex, race, birth cohort, education, smoking, and a known genetic risk factor for Alzheimer’s disease: the rs2075650 G allele in the TOMM40 gene.
In a logistic model with all covariates, vaccination against pneumonia between ages 65 and 75 was significantly associated with reduced risk of developing AD (OR, 0.70; P < .04). The largest reduction in Alzheimer’s disease risk (OR, 0.62; P < .04) was among those vaccinated against pneumonia who were noncarriers of the rs2075650 G allele.
Total number of vaccinations against pneumonia and influenza between ages 65 and 75 was also associated with a lower risk for Alzheimer’s disease (OR, 0.88; P < .01). However, the effect was not evident for the influenza vaccination alone.
“The fact that very different pathogens – viral, bacterial, fungal – have been linked to Alzheimer’s disease indicates a possibility that compromised host immunity may play a role in Alzheimer’s disease through increasing overall brain’s vulnerability to various microbes,” said Dr. Ukraintseva.
The current findings support further investigation of pneumococcal vaccine as a “reasonable candidate for repurposing in personalized AD prevention,” she noted. “These results also support the important role of boosting overall immune robustness/resilience in preventing Alzheimer’s disease,” Dr. Ukraintseva added.
Her group is currently working on confirming the findings in another population.
Brain protective?
“Neither study can prove that the benefit is directly related to the vaccine itself, but what they can indicate is that potentially, vaccines are a way to protect your health and brain,” Dr. Edelmayer said.
In a statement, Maria Carrillo, PhD, chief science officer for the Alzheimer’s Association, noted that more research is needed.
The new data call “for further studies in large, diverse clinical trials to inform whether vaccinations as a public health strategy decrease our risk for developing dementia as we age,” Dr. Carillo said.
Funding for the influenza vaccine study was provided by the Christopher Sarofim Family Professorship in Biomedical Informatics and Bioengineering, a UT STARs Award, the Cancer Prevention and Research Institute of Texas, and the National Institutes of Health. Funding for the pneumonia study was provided by the National Institute on Aging. Dr. Amran, Dr. Ukraintseva, Dr. Edelmayer, and Dr. Carrillo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
Cardiovascular risk factors tied to midlife cognitive decline
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Still no clear answer on intranasal insulin for MCI and Alzheimer’s disease
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
Move over supplements, here come medical foods
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
Does moderate drinking slow cognitive decline?
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
Cognitive deficits complex in youths with type 2 diabetes
Teens and young adults with diabetes have cognitive deficits that vary by diabetes type and could negatively impact their medical literacy and self-care, an investigator reported at the virtual annual scientific sessions of the American Diabetes Association.
Individuals with youth-onset type 1 or 2 diabetes all performed below average on tests that measure flexible thinking and problem solving, according to the investigator, who reported an analysis including 1,380 individuals enrolled in the SEARCH for Diabetes in Youth study.
That finding suggests that diabetes diagnosed before age 20 contributes to poor fluid cognitive function, which consists of skills that facilitate goal-directed behaviors, according to investigator Allison Shapiro, MPH, PhD, of the University of Colorado at Denver, Aurora.
However, individuals with type 2 diabetes (T2D) performed even worse than those with type 1 diabetes (T1D) on the fluid cognitive function tests, even after adjustment for demographic factors and other confounders, Dr. Shapiro said in her presentation.
Further analysis revealed that individuals with T2D performed significantly worse on measures of crystallized cognition, a domain that includes skills such as vocabulary and language. That suggests the poor fluid cognitive abilities in youths with diabetes may in fact be a result of poor crystallized cognitive development, according to the investigator.
“Among adolescents and young adults with youth-onset type 2 diabetes specifically, intervention should focus on developing both fluid cognitive skills and crystallized cognitive skills,” Dr. Shapiro said.
Deficits in fluid cognitive function (such as reasoning or processing speed) can negatively affect diabetes self-care, thereby potentially increasing the risk of diabetes-related complications, while deficits in crystallized cognitive function (such as vocabulary and understanding of language) could impact medical literacy further compounding the self-care issues.
The study is believed to be one of the first to compare cognitive function deficits in youths with type 1 or 2 diabetes. Although studies in adults clearly show a detrimental relationship between diabetes and cognitive function, according to Dr. Shapiro, the bulk of the research in youths has focused on T1D.
“While limited work has been done in youth-onset type 2 diabetes, cognitive deficits are consistently observed, compared to youth without diabetes,” she said.
Results of this study emphasize the importance of dietary changes and other lifestyle interventions in young patients with diabetes, according to David Della-Morte, MD, PhD, associate professor of neurology at the University of Miami.
“Even the youngest patients may develop cognitive dysfunction,” Dr. Della-Morte said in an interview. “That means that lifestyle is very important, especially in obese patients that are prone to develop type 2 diabetes.”
The analysis by Dr. Shapiro and coinvestigators included 1,095 youths and young adults with T1D and 285 with T2D who had undergone a cognition assessment as part of a study visit. They were aged an average of 22 years, and had an average diabetes duration of 11 years.
The overall fluid cognition score was significantly lower in those individuals with T2D, compared with those with T1D, investigators found. Compared with the national average score of 100, the T2D group scored 84.7, or a full standard deviation below that average, said Dr. Shapiro, while those with T1D scored 95.5 (P < .001).
Participants with T2D also scored significantly lower in individual measures of fluid cognition, including processing speed, inhibitory control and attention, working memory, and episodic memory, she reported. At first glance, that suggested youth-onset T2D has a specific effect on fluid cognition; however, the story remains incomplete without looking at crystallized cognition markers such as vocabulary and language.
Toward that end, a picture vocabulary test conducted as part of the cognitive assessment showed a significant difference between those with T2D, who on average scored 91.5, and those with T1D, who scored 103.6 (P < .001). Accounting for those picture vocabulary scores attenuated the differences between groups in fluid cognitive scores, suggesting that differences in crystallized cognitive function underly the observed differences in fluid cognitive function between groups, Dr. Shapiro said.
Skills such as vocabulary and language are thought to be stable and not influenced by neurologic changes brought on by disease processes such as youth-onset diabetes, but rather, influenced by factors such as childcare and education, according to Dr. Shapiro.
“Crystallized cognition therefore provides a window into an individual’s cognitive functioning, independent of their disease or premorbid to the onset of their disease,” she said.
Dr. Shapiro said she had no conflicts of interest to disclose.
SOURCE: Shapiro A et al. ADA 2020, Abstract 279-OR.
Teens and young adults with diabetes have cognitive deficits that vary by diabetes type and could negatively impact their medical literacy and self-care, an investigator reported at the virtual annual scientific sessions of the American Diabetes Association.
Individuals with youth-onset type 1 or 2 diabetes all performed below average on tests that measure flexible thinking and problem solving, according to the investigator, who reported an analysis including 1,380 individuals enrolled in the SEARCH for Diabetes in Youth study.
That finding suggests that diabetes diagnosed before age 20 contributes to poor fluid cognitive function, which consists of skills that facilitate goal-directed behaviors, according to investigator Allison Shapiro, MPH, PhD, of the University of Colorado at Denver, Aurora.
However, individuals with type 2 diabetes (T2D) performed even worse than those with type 1 diabetes (T1D) on the fluid cognitive function tests, even after adjustment for demographic factors and other confounders, Dr. Shapiro said in her presentation.
Further analysis revealed that individuals with T2D performed significantly worse on measures of crystallized cognition, a domain that includes skills such as vocabulary and language. That suggests the poor fluid cognitive abilities in youths with diabetes may in fact be a result of poor crystallized cognitive development, according to the investigator.
“Among adolescents and young adults with youth-onset type 2 diabetes specifically, intervention should focus on developing both fluid cognitive skills and crystallized cognitive skills,” Dr. Shapiro said.
Deficits in fluid cognitive function (such as reasoning or processing speed) can negatively affect diabetes self-care, thereby potentially increasing the risk of diabetes-related complications, while deficits in crystallized cognitive function (such as vocabulary and understanding of language) could impact medical literacy further compounding the self-care issues.
The study is believed to be one of the first to compare cognitive function deficits in youths with type 1 or 2 diabetes. Although studies in adults clearly show a detrimental relationship between diabetes and cognitive function, according to Dr. Shapiro, the bulk of the research in youths has focused on T1D.
“While limited work has been done in youth-onset type 2 diabetes, cognitive deficits are consistently observed, compared to youth without diabetes,” she said.
Results of this study emphasize the importance of dietary changes and other lifestyle interventions in young patients with diabetes, according to David Della-Morte, MD, PhD, associate professor of neurology at the University of Miami.
“Even the youngest patients may develop cognitive dysfunction,” Dr. Della-Morte said in an interview. “That means that lifestyle is very important, especially in obese patients that are prone to develop type 2 diabetes.”
The analysis by Dr. Shapiro and coinvestigators included 1,095 youths and young adults with T1D and 285 with T2D who had undergone a cognition assessment as part of a study visit. They were aged an average of 22 years, and had an average diabetes duration of 11 years.
The overall fluid cognition score was significantly lower in those individuals with T2D, compared with those with T1D, investigators found. Compared with the national average score of 100, the T2D group scored 84.7, or a full standard deviation below that average, said Dr. Shapiro, while those with T1D scored 95.5 (P < .001).
Participants with T2D also scored significantly lower in individual measures of fluid cognition, including processing speed, inhibitory control and attention, working memory, and episodic memory, she reported. At first glance, that suggested youth-onset T2D has a specific effect on fluid cognition; however, the story remains incomplete without looking at crystallized cognition markers such as vocabulary and language.
Toward that end, a picture vocabulary test conducted as part of the cognitive assessment showed a significant difference between those with T2D, who on average scored 91.5, and those with T1D, who scored 103.6 (P < .001). Accounting for those picture vocabulary scores attenuated the differences between groups in fluid cognitive scores, suggesting that differences in crystallized cognitive function underly the observed differences in fluid cognitive function between groups, Dr. Shapiro said.
Skills such as vocabulary and language are thought to be stable and not influenced by neurologic changes brought on by disease processes such as youth-onset diabetes, but rather, influenced by factors such as childcare and education, according to Dr. Shapiro.
“Crystallized cognition therefore provides a window into an individual’s cognitive functioning, independent of their disease or premorbid to the onset of their disease,” she said.
Dr. Shapiro said she had no conflicts of interest to disclose.
SOURCE: Shapiro A et al. ADA 2020, Abstract 279-OR.
Teens and young adults with diabetes have cognitive deficits that vary by diabetes type and could negatively impact their medical literacy and self-care, an investigator reported at the virtual annual scientific sessions of the American Diabetes Association.
Individuals with youth-onset type 1 or 2 diabetes all performed below average on tests that measure flexible thinking and problem solving, according to the investigator, who reported an analysis including 1,380 individuals enrolled in the SEARCH for Diabetes in Youth study.
That finding suggests that diabetes diagnosed before age 20 contributes to poor fluid cognitive function, which consists of skills that facilitate goal-directed behaviors, according to investigator Allison Shapiro, MPH, PhD, of the University of Colorado at Denver, Aurora.
However, individuals with type 2 diabetes (T2D) performed even worse than those with type 1 diabetes (T1D) on the fluid cognitive function tests, even after adjustment for demographic factors and other confounders, Dr. Shapiro said in her presentation.
Further analysis revealed that individuals with T2D performed significantly worse on measures of crystallized cognition, a domain that includes skills such as vocabulary and language. That suggests the poor fluid cognitive abilities in youths with diabetes may in fact be a result of poor crystallized cognitive development, according to the investigator.
“Among adolescents and young adults with youth-onset type 2 diabetes specifically, intervention should focus on developing both fluid cognitive skills and crystallized cognitive skills,” Dr. Shapiro said.
Deficits in fluid cognitive function (such as reasoning or processing speed) can negatively affect diabetes self-care, thereby potentially increasing the risk of diabetes-related complications, while deficits in crystallized cognitive function (such as vocabulary and understanding of language) could impact medical literacy further compounding the self-care issues.
The study is believed to be one of the first to compare cognitive function deficits in youths with type 1 or 2 diabetes. Although studies in adults clearly show a detrimental relationship between diabetes and cognitive function, according to Dr. Shapiro, the bulk of the research in youths has focused on T1D.
“While limited work has been done in youth-onset type 2 diabetes, cognitive deficits are consistently observed, compared to youth without diabetes,” she said.
Results of this study emphasize the importance of dietary changes and other lifestyle interventions in young patients with diabetes, according to David Della-Morte, MD, PhD, associate professor of neurology at the University of Miami.
“Even the youngest patients may develop cognitive dysfunction,” Dr. Della-Morte said in an interview. “That means that lifestyle is very important, especially in obese patients that are prone to develop type 2 diabetes.”
The analysis by Dr. Shapiro and coinvestigators included 1,095 youths and young adults with T1D and 285 with T2D who had undergone a cognition assessment as part of a study visit. They were aged an average of 22 years, and had an average diabetes duration of 11 years.
The overall fluid cognition score was significantly lower in those individuals with T2D, compared with those with T1D, investigators found. Compared with the national average score of 100, the T2D group scored 84.7, or a full standard deviation below that average, said Dr. Shapiro, while those with T1D scored 95.5 (P < .001).
Participants with T2D also scored significantly lower in individual measures of fluid cognition, including processing speed, inhibitory control and attention, working memory, and episodic memory, she reported. At first glance, that suggested youth-onset T2D has a specific effect on fluid cognition; however, the story remains incomplete without looking at crystallized cognition markers such as vocabulary and language.
Toward that end, a picture vocabulary test conducted as part of the cognitive assessment showed a significant difference between those with T2D, who on average scored 91.5, and those with T1D, who scored 103.6 (P < .001). Accounting for those picture vocabulary scores attenuated the differences between groups in fluid cognitive scores, suggesting that differences in crystallized cognitive function underly the observed differences in fluid cognitive function between groups, Dr. Shapiro said.
Skills such as vocabulary and language are thought to be stable and not influenced by neurologic changes brought on by disease processes such as youth-onset diabetes, but rather, influenced by factors such as childcare and education, according to Dr. Shapiro.
“Crystallized cognition therefore provides a window into an individual’s cognitive functioning, independent of their disease or premorbid to the onset of their disease,” she said.
Dr. Shapiro said she had no conflicts of interest to disclose.
SOURCE: Shapiro A et al. ADA 2020, Abstract 279-OR.
FROM ADA 2020



