User login
Clinical pearls for administering cognitive exams during the pandemic
Patients have often been labeled as “poor historians” if they are not able to recollect their own medical history, whether through illness or difficulties in communication. But Fred Ovsiew, MD, speaking at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sees that label as an excuse on the part of the clinician.
“I strongly advise you to drop that phrase from your vocabulary if you do use it, because the patient is not the historian. The doctor, the clinician is the historian,” Dr. Ovsiew said at the meeting, presented by Global Academy for Medical Education. “It is the clinician’s job to put the story together using the account by the patient as one source, but [also] interviewing a collateral informant and/or reviewing records, which is necessary in almost every case of a neuropsychiatric illness.”
Rather, clinicians taking history at the bedside should focus on why the patients cannot give a narrative account of their illness. Patients can have narrative incapacity on a psychogenic basis, such as in patients with conversion or somatoform disorder, he explained. “I think this is a result of the narrative incapacity that develops in people who have had trauma or adverse experiences in childhood and insecure attachment. This is shown on the adult attachment interview as a disorganized account of their childhoods.”
Other patients might not be able to recount their medical history because they are amnestic, which leaves their account vague because of a lack of access to information. “It may be frozen in time in the sense that, up to a certain point in their life, they can recount the history,” Dr. Ovsiew said. “But in recent years, their account becomes vague.”
Patients with right hemisphere lesions might not know that their account has incongruity and is implausible, while patients with dorsolateral prefrontal lesions might be aspontaneous, use few words to describe their situation, and have poor insight. Those with ventromedial prefrontal lesions can be impulsive and have poor insight, not considering alternative possibilities, Dr. Ovsiew noted.
Asking open-ended questions of the patient is the first step to identifying any potential narrative incapacity, followed by a detailed medical history by the clinician. When taking a medical history, try avoiding what Dr. Ovsiew calls the “anything like that?” problem, where a clinician asks a question about a cluster of symptoms that would make sense to a doctor, but not a patient. For example, a doctor might ask whether a patient is experiencing “chest pain or leg swelling – anything like that?” because he or she knows what those symptoms have in common, but the patient might not know the relationship between those symptoms. “You can’t count on the patient to tell you all the relevant information,” he said. “You have to know what to ask about.”
“Patients with brain disease have subtle personality changes, sometimes more obvious personality changes. These need to be inquired about,” Dr. Ovsiew said. “The patient with apathy has reduced negative as well as positive emotions. The patient with depression has reduced positive emotions, but often tells you very clearly about the negative emotions of sadness, guilt. The patient with depression has diurnal variation in mood, a very telling symptom, especially when it’s disclosed spontaneously,” Dr. Ovsiew explained. “The point is, you need to know to ask about it.”
When taking a sleep history, clinicians should be aware of sleep disturbances apart from insomnia and early waking. REM sleep behavior disorder is a condition that should be inquired about. Obstructive sleep apnea is a condition that might not be immediately apparent to the patient, but a bed partner can identify whether a patient has problems breathing throughout the night.
“This is an important condition to uncover for the neuropsychiatrist because it contributes to treatment resistance and depression, and it contributes to cognitive impairment,” Dr. Ovsiew said. “These patients commonly have mild difficulties with attention and concentration.”
Always ask about head injury in every history, which can be relevant to later onset depression, PTSD, and cognitive impairment. Every head injury follows a trajectory of retrograde amnesia and altered state of consciousness (including coma), followed by a period of posttraumatic amnesia. Duration of these states can be used to assess the severity of brain injury, but the 15-point Glasgow Coma Scale is another way to assess injury severity, Dr. Ovsiew explained.
However, the two do not always overlap, he noted. “Someone may have a Glasgow Coma Scale score that is 9-12, predicting moderate brain injury, but they may have a short duration of amnesia. These don’t always follow the same path. There are many different ways of classifying how severe the brain injury is.”
Keep probes brief, straightforward
Cognitive exams of patients with suspected psychiatric disorders should be simple, easy to administer and focused on a single domain of cognition. “Probes should be brief. They should not require specialized equipment. The Purdue Pegboard Test might be a great neuropsychological instrument, but very few of us carry a pegboard around in our medical bags,” Dr. Ovsiew said.
The probe administered should also be accessible to the patient. The serial sevens clinical test, where a patient is asked to repeatedly subtract 7 from 100, is only effective at testing concentration if the patient is capable of completing the test. “There are going to be patients who can’t do the task, but it’s not because of concentration failure, it’s because of subtraction failure,” he said.
When assessing attention, effective tasks include having the patient perform the digit span test forward and backward, count backward from 20 to 1, listing the months of the year in reverse, and performing the Mental Alternation Test. However, Dr. Ovsiew explained there may be some barriers for patients in completing these tasks. “The person may be aphasic and not know the alphabet. The person may have English as a second language and not be skilled at giving the alphabet in English. In some cases, you may want to check and not assume that the patient can count and does know the alphabet.”
In assessing language, listen for aphasic abnormalities. “The patient, of course, is speaking throughout the interview, but you need to take a moment to listen for prosody, to listen to rate of speech, to listen for paraphasic errors or word-finding problems,” Dr. Ovsiew said. Any abnormalities should be probed further through confrontation naming tasks, which can be done in person and with some success through video, but not by phone. Naming to definition (“What do you call the part of a shirt that covers the arm?”) is one way of administering the test over the phone.
Visuospatial function can be assessed by clock drawing but also carries problems. Patients who do not plan their clock before beginning to draw, for example, may have an executive function problem instead of a visuospatial problem, Dr. Ovsiew noted. Patients in whom a clinician suspects hemineglect should be given a visual search task or line by section task. “I like doing clock drawing. It’s a nice screening test. It’s becoming, I think, less useful as people count on digital clocks and have trouble even imagining what an analog clock looks like.”
An approach that is better suited to in-person assessment, but also works by video, is the Poppelreuter figure visual perceptual function test, which is a prompt for the patient that involves common household items overlaying one another “in atypical positions and atypical configurations” where the patient is instructed to describe the items they see on the card. Another approach that works over video is the interlocking finger test, where the patient is asked to copy the hand positions made by the clinician.
Dr. Ovsiew admitted that visuospatial function is nearly impossible to assess over the phone. Asking topographical questions (“If you’re driving from Chicago to Los Angeles, is the Pacific Ocean in front of you, behind you, to your left, or to your right?”) may help judge visuospatial function, but this relies on the patient having the topographic knowledge to answer the questions. Some patients who are topographically disoriented can’t do them at all,” Dr. Ovsiew said.
Bedside neuropsychiatry assesses encoding of a memory, its retention and its retrieval as well as verbal and visual cues. Each one of these aspects of memory can be impaired on its own and should be explored separately, Dr. Ovsiew explained. “Neuropsychiatric clinicians have a rough-and-ready, seat-of-the-pants way of approaching this that wouldn’t pass muster if you’re a psychologist, but is the best we can do at the bedside.”
To test retrieval and retention, the Three Words–Three Shapes test works well in person, with some difficulty by video, and is not possible to administer over the phone. In lieu of that test, giving the patient a simple word list and asking them to repeat the list in order. Using the word list, “these different stages of memory function can be parsed out pretty well at the bedside or chairside, and even by the phone. Figuring out where the memory failure is diagnostically important,” Dr. Ovsiew said.
Executive function, which involves activation, planning, sequencing, maintaining, self-monitoring, and flexible employment of action and attention, is “complicated to evaluate because there are multiple aspects of executive function, multiple deficits that can be seen with executive dysfunction, and they don’t all correlate with each other.”
Within executive function evaluation, the Mental Alternation Test can assess working memory, motor sequencing can be assessed through the ring/fist, fist/edge/palm, alternating fist, and rampart tests. The Go/No-Go test can be used to assess response inhibition. For effortful retrieval evaluation, spontaneous word-list generation – such as thinking of all the items one can buy at a supermarket– can test category fluency, while a task to name all the words starting with a certain letter can assess letter stimulus.
Executive function “is of crucial importance in the neuropsychiatric evaluation because it’s strongly correlated with how well the person functions outside the office,” Dr. Ovsiew said.
Global Academy and this news organization are owned by the same parent company. Dr. Ovsiew reported relationships with Wolters Kluwer Health in the form of consulting, receiving royalty payments, and related activities.
Patients have often been labeled as “poor historians” if they are not able to recollect their own medical history, whether through illness or difficulties in communication. But Fred Ovsiew, MD, speaking at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sees that label as an excuse on the part of the clinician.
“I strongly advise you to drop that phrase from your vocabulary if you do use it, because the patient is not the historian. The doctor, the clinician is the historian,” Dr. Ovsiew said at the meeting, presented by Global Academy for Medical Education. “It is the clinician’s job to put the story together using the account by the patient as one source, but [also] interviewing a collateral informant and/or reviewing records, which is necessary in almost every case of a neuropsychiatric illness.”
Rather, clinicians taking history at the bedside should focus on why the patients cannot give a narrative account of their illness. Patients can have narrative incapacity on a psychogenic basis, such as in patients with conversion or somatoform disorder, he explained. “I think this is a result of the narrative incapacity that develops in people who have had trauma or adverse experiences in childhood and insecure attachment. This is shown on the adult attachment interview as a disorganized account of their childhoods.”
Other patients might not be able to recount their medical history because they are amnestic, which leaves their account vague because of a lack of access to information. “It may be frozen in time in the sense that, up to a certain point in their life, they can recount the history,” Dr. Ovsiew said. “But in recent years, their account becomes vague.”
Patients with right hemisphere lesions might not know that their account has incongruity and is implausible, while patients with dorsolateral prefrontal lesions might be aspontaneous, use few words to describe their situation, and have poor insight. Those with ventromedial prefrontal lesions can be impulsive and have poor insight, not considering alternative possibilities, Dr. Ovsiew noted.
Asking open-ended questions of the patient is the first step to identifying any potential narrative incapacity, followed by a detailed medical history by the clinician. When taking a medical history, try avoiding what Dr. Ovsiew calls the “anything like that?” problem, where a clinician asks a question about a cluster of symptoms that would make sense to a doctor, but not a patient. For example, a doctor might ask whether a patient is experiencing “chest pain or leg swelling – anything like that?” because he or she knows what those symptoms have in common, but the patient might not know the relationship between those symptoms. “You can’t count on the patient to tell you all the relevant information,” he said. “You have to know what to ask about.”
“Patients with brain disease have subtle personality changes, sometimes more obvious personality changes. These need to be inquired about,” Dr. Ovsiew said. “The patient with apathy has reduced negative as well as positive emotions. The patient with depression has reduced positive emotions, but often tells you very clearly about the negative emotions of sadness, guilt. The patient with depression has diurnal variation in mood, a very telling symptom, especially when it’s disclosed spontaneously,” Dr. Ovsiew explained. “The point is, you need to know to ask about it.”
When taking a sleep history, clinicians should be aware of sleep disturbances apart from insomnia and early waking. REM sleep behavior disorder is a condition that should be inquired about. Obstructive sleep apnea is a condition that might not be immediately apparent to the patient, but a bed partner can identify whether a patient has problems breathing throughout the night.
“This is an important condition to uncover for the neuropsychiatrist because it contributes to treatment resistance and depression, and it contributes to cognitive impairment,” Dr. Ovsiew said. “These patients commonly have mild difficulties with attention and concentration.”
Always ask about head injury in every history, which can be relevant to later onset depression, PTSD, and cognitive impairment. Every head injury follows a trajectory of retrograde amnesia and altered state of consciousness (including coma), followed by a period of posttraumatic amnesia. Duration of these states can be used to assess the severity of brain injury, but the 15-point Glasgow Coma Scale is another way to assess injury severity, Dr. Ovsiew explained.
However, the two do not always overlap, he noted. “Someone may have a Glasgow Coma Scale score that is 9-12, predicting moderate brain injury, but they may have a short duration of amnesia. These don’t always follow the same path. There are many different ways of classifying how severe the brain injury is.”
Keep probes brief, straightforward
Cognitive exams of patients with suspected psychiatric disorders should be simple, easy to administer and focused on a single domain of cognition. “Probes should be brief. They should not require specialized equipment. The Purdue Pegboard Test might be a great neuropsychological instrument, but very few of us carry a pegboard around in our medical bags,” Dr. Ovsiew said.
The probe administered should also be accessible to the patient. The serial sevens clinical test, where a patient is asked to repeatedly subtract 7 from 100, is only effective at testing concentration if the patient is capable of completing the test. “There are going to be patients who can’t do the task, but it’s not because of concentration failure, it’s because of subtraction failure,” he said.
When assessing attention, effective tasks include having the patient perform the digit span test forward and backward, count backward from 20 to 1, listing the months of the year in reverse, and performing the Mental Alternation Test. However, Dr. Ovsiew explained there may be some barriers for patients in completing these tasks. “The person may be aphasic and not know the alphabet. The person may have English as a second language and not be skilled at giving the alphabet in English. In some cases, you may want to check and not assume that the patient can count and does know the alphabet.”
In assessing language, listen for aphasic abnormalities. “The patient, of course, is speaking throughout the interview, but you need to take a moment to listen for prosody, to listen to rate of speech, to listen for paraphasic errors or word-finding problems,” Dr. Ovsiew said. Any abnormalities should be probed further through confrontation naming tasks, which can be done in person and with some success through video, but not by phone. Naming to definition (“What do you call the part of a shirt that covers the arm?”) is one way of administering the test over the phone.
Visuospatial function can be assessed by clock drawing but also carries problems. Patients who do not plan their clock before beginning to draw, for example, may have an executive function problem instead of a visuospatial problem, Dr. Ovsiew noted. Patients in whom a clinician suspects hemineglect should be given a visual search task or line by section task. “I like doing clock drawing. It’s a nice screening test. It’s becoming, I think, less useful as people count on digital clocks and have trouble even imagining what an analog clock looks like.”
An approach that is better suited to in-person assessment, but also works by video, is the Poppelreuter figure visual perceptual function test, which is a prompt for the patient that involves common household items overlaying one another “in atypical positions and atypical configurations” where the patient is instructed to describe the items they see on the card. Another approach that works over video is the interlocking finger test, where the patient is asked to copy the hand positions made by the clinician.
Dr. Ovsiew admitted that visuospatial function is nearly impossible to assess over the phone. Asking topographical questions (“If you’re driving from Chicago to Los Angeles, is the Pacific Ocean in front of you, behind you, to your left, or to your right?”) may help judge visuospatial function, but this relies on the patient having the topographic knowledge to answer the questions. Some patients who are topographically disoriented can’t do them at all,” Dr. Ovsiew said.
Bedside neuropsychiatry assesses encoding of a memory, its retention and its retrieval as well as verbal and visual cues. Each one of these aspects of memory can be impaired on its own and should be explored separately, Dr. Ovsiew explained. “Neuropsychiatric clinicians have a rough-and-ready, seat-of-the-pants way of approaching this that wouldn’t pass muster if you’re a psychologist, but is the best we can do at the bedside.”
To test retrieval and retention, the Three Words–Three Shapes test works well in person, with some difficulty by video, and is not possible to administer over the phone. In lieu of that test, giving the patient a simple word list and asking them to repeat the list in order. Using the word list, “these different stages of memory function can be parsed out pretty well at the bedside or chairside, and even by the phone. Figuring out where the memory failure is diagnostically important,” Dr. Ovsiew said.
Executive function, which involves activation, planning, sequencing, maintaining, self-monitoring, and flexible employment of action and attention, is “complicated to evaluate because there are multiple aspects of executive function, multiple deficits that can be seen with executive dysfunction, and they don’t all correlate with each other.”
Within executive function evaluation, the Mental Alternation Test can assess working memory, motor sequencing can be assessed through the ring/fist, fist/edge/palm, alternating fist, and rampart tests. The Go/No-Go test can be used to assess response inhibition. For effortful retrieval evaluation, spontaneous word-list generation – such as thinking of all the items one can buy at a supermarket– can test category fluency, while a task to name all the words starting with a certain letter can assess letter stimulus.
Executive function “is of crucial importance in the neuropsychiatric evaluation because it’s strongly correlated with how well the person functions outside the office,” Dr. Ovsiew said.
Global Academy and this news organization are owned by the same parent company. Dr. Ovsiew reported relationships with Wolters Kluwer Health in the form of consulting, receiving royalty payments, and related activities.
Patients have often been labeled as “poor historians” if they are not able to recollect their own medical history, whether through illness or difficulties in communication. But Fred Ovsiew, MD, speaking at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sees that label as an excuse on the part of the clinician.
“I strongly advise you to drop that phrase from your vocabulary if you do use it, because the patient is not the historian. The doctor, the clinician is the historian,” Dr. Ovsiew said at the meeting, presented by Global Academy for Medical Education. “It is the clinician’s job to put the story together using the account by the patient as one source, but [also] interviewing a collateral informant and/or reviewing records, which is necessary in almost every case of a neuropsychiatric illness.”
Rather, clinicians taking history at the bedside should focus on why the patients cannot give a narrative account of their illness. Patients can have narrative incapacity on a psychogenic basis, such as in patients with conversion or somatoform disorder, he explained. “I think this is a result of the narrative incapacity that develops in people who have had trauma or adverse experiences in childhood and insecure attachment. This is shown on the adult attachment interview as a disorganized account of their childhoods.”
Other patients might not be able to recount their medical history because they are amnestic, which leaves their account vague because of a lack of access to information. “It may be frozen in time in the sense that, up to a certain point in their life, they can recount the history,” Dr. Ovsiew said. “But in recent years, their account becomes vague.”
Patients with right hemisphere lesions might not know that their account has incongruity and is implausible, while patients with dorsolateral prefrontal lesions might be aspontaneous, use few words to describe their situation, and have poor insight. Those with ventromedial prefrontal lesions can be impulsive and have poor insight, not considering alternative possibilities, Dr. Ovsiew noted.
Asking open-ended questions of the patient is the first step to identifying any potential narrative incapacity, followed by a detailed medical history by the clinician. When taking a medical history, try avoiding what Dr. Ovsiew calls the “anything like that?” problem, where a clinician asks a question about a cluster of symptoms that would make sense to a doctor, but not a patient. For example, a doctor might ask whether a patient is experiencing “chest pain or leg swelling – anything like that?” because he or she knows what those symptoms have in common, but the patient might not know the relationship between those symptoms. “You can’t count on the patient to tell you all the relevant information,” he said. “You have to know what to ask about.”
“Patients with brain disease have subtle personality changes, sometimes more obvious personality changes. These need to be inquired about,” Dr. Ovsiew said. “The patient with apathy has reduced negative as well as positive emotions. The patient with depression has reduced positive emotions, but often tells you very clearly about the negative emotions of sadness, guilt. The patient with depression has diurnal variation in mood, a very telling symptom, especially when it’s disclosed spontaneously,” Dr. Ovsiew explained. “The point is, you need to know to ask about it.”
When taking a sleep history, clinicians should be aware of sleep disturbances apart from insomnia and early waking. REM sleep behavior disorder is a condition that should be inquired about. Obstructive sleep apnea is a condition that might not be immediately apparent to the patient, but a bed partner can identify whether a patient has problems breathing throughout the night.
“This is an important condition to uncover for the neuropsychiatrist because it contributes to treatment resistance and depression, and it contributes to cognitive impairment,” Dr. Ovsiew said. “These patients commonly have mild difficulties with attention and concentration.”
Always ask about head injury in every history, which can be relevant to later onset depression, PTSD, and cognitive impairment. Every head injury follows a trajectory of retrograde amnesia and altered state of consciousness (including coma), followed by a period of posttraumatic amnesia. Duration of these states can be used to assess the severity of brain injury, but the 15-point Glasgow Coma Scale is another way to assess injury severity, Dr. Ovsiew explained.
However, the two do not always overlap, he noted. “Someone may have a Glasgow Coma Scale score that is 9-12, predicting moderate brain injury, but they may have a short duration of amnesia. These don’t always follow the same path. There are many different ways of classifying how severe the brain injury is.”
Keep probes brief, straightforward
Cognitive exams of patients with suspected psychiatric disorders should be simple, easy to administer and focused on a single domain of cognition. “Probes should be brief. They should not require specialized equipment. The Purdue Pegboard Test might be a great neuropsychological instrument, but very few of us carry a pegboard around in our medical bags,” Dr. Ovsiew said.
The probe administered should also be accessible to the patient. The serial sevens clinical test, where a patient is asked to repeatedly subtract 7 from 100, is only effective at testing concentration if the patient is capable of completing the test. “There are going to be patients who can’t do the task, but it’s not because of concentration failure, it’s because of subtraction failure,” he said.
When assessing attention, effective tasks include having the patient perform the digit span test forward and backward, count backward from 20 to 1, listing the months of the year in reverse, and performing the Mental Alternation Test. However, Dr. Ovsiew explained there may be some barriers for patients in completing these tasks. “The person may be aphasic and not know the alphabet. The person may have English as a second language and not be skilled at giving the alphabet in English. In some cases, you may want to check and not assume that the patient can count and does know the alphabet.”
In assessing language, listen for aphasic abnormalities. “The patient, of course, is speaking throughout the interview, but you need to take a moment to listen for prosody, to listen to rate of speech, to listen for paraphasic errors or word-finding problems,” Dr. Ovsiew said. Any abnormalities should be probed further through confrontation naming tasks, which can be done in person and with some success through video, but not by phone. Naming to definition (“What do you call the part of a shirt that covers the arm?”) is one way of administering the test over the phone.
Visuospatial function can be assessed by clock drawing but also carries problems. Patients who do not plan their clock before beginning to draw, for example, may have an executive function problem instead of a visuospatial problem, Dr. Ovsiew noted. Patients in whom a clinician suspects hemineglect should be given a visual search task or line by section task. “I like doing clock drawing. It’s a nice screening test. It’s becoming, I think, less useful as people count on digital clocks and have trouble even imagining what an analog clock looks like.”
An approach that is better suited to in-person assessment, but also works by video, is the Poppelreuter figure visual perceptual function test, which is a prompt for the patient that involves common household items overlaying one another “in atypical positions and atypical configurations” where the patient is instructed to describe the items they see on the card. Another approach that works over video is the interlocking finger test, where the patient is asked to copy the hand positions made by the clinician.
Dr. Ovsiew admitted that visuospatial function is nearly impossible to assess over the phone. Asking topographical questions (“If you’re driving from Chicago to Los Angeles, is the Pacific Ocean in front of you, behind you, to your left, or to your right?”) may help judge visuospatial function, but this relies on the patient having the topographic knowledge to answer the questions. Some patients who are topographically disoriented can’t do them at all,” Dr. Ovsiew said.
Bedside neuropsychiatry assesses encoding of a memory, its retention and its retrieval as well as verbal and visual cues. Each one of these aspects of memory can be impaired on its own and should be explored separately, Dr. Ovsiew explained. “Neuropsychiatric clinicians have a rough-and-ready, seat-of-the-pants way of approaching this that wouldn’t pass muster if you’re a psychologist, but is the best we can do at the bedside.”
To test retrieval and retention, the Three Words–Three Shapes test works well in person, with some difficulty by video, and is not possible to administer over the phone. In lieu of that test, giving the patient a simple word list and asking them to repeat the list in order. Using the word list, “these different stages of memory function can be parsed out pretty well at the bedside or chairside, and even by the phone. Figuring out where the memory failure is diagnostically important,” Dr. Ovsiew said.
Executive function, which involves activation, planning, sequencing, maintaining, self-monitoring, and flexible employment of action and attention, is “complicated to evaluate because there are multiple aspects of executive function, multiple deficits that can be seen with executive dysfunction, and they don’t all correlate with each other.”
Within executive function evaluation, the Mental Alternation Test can assess working memory, motor sequencing can be assessed through the ring/fist, fist/edge/palm, alternating fist, and rampart tests. The Go/No-Go test can be used to assess response inhibition. For effortful retrieval evaluation, spontaneous word-list generation – such as thinking of all the items one can buy at a supermarket– can test category fluency, while a task to name all the words starting with a certain letter can assess letter stimulus.
Executive function “is of crucial importance in the neuropsychiatric evaluation because it’s strongly correlated with how well the person functions outside the office,” Dr. Ovsiew said.
Global Academy and this news organization are owned by the same parent company. Dr. Ovsiew reported relationships with Wolters Kluwer Health in the form of consulting, receiving royalty payments, and related activities.
FROM FOCUS ON NEUROPSYCHIATRY 2020
More evidence links gum disease and dementia risk
especially in those with severe gum inflammation and edentulism, new research suggests.
Over a 20-year period, investigators prospectively followed more than 8,000 individuals aged around 63 years who did not have cognitive impairment or dementia at baseline, grouping them based on the extent and severity of their periodontal disease and number of lost teeth.
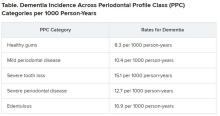
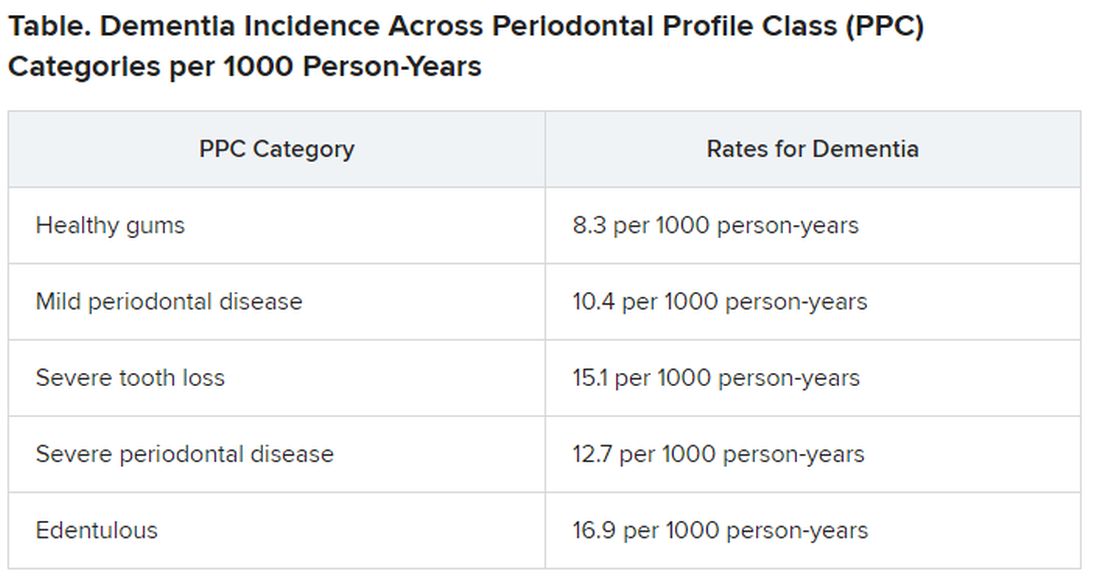
Results showed that 14% of participants with healthy gums and all their teeth at baseline developed dementia, compared with 18% of those with mild periodontal disease and 22% who had severe periodontal disease. The highest percentage (23%) of participants who developed dementia was found in those who were edentulous.
After accounting for comorbidities that might affect dementia risk, edentulous participants had a 20% higher risk for developing MCI or dementia, compared with the healthy group.
Because the study was observational, “we don’t have knowledge of causality so we cannot state that if you treat periodontal disease you can prevent or treat dementia,” said lead author Ryan T. Demmer, PhD, MPH, associate professor, division of epidemiology and community health, University of Minnesota, Minneapolis. However, “the take-home message from this paper is that it further supports the possibility that oral infections could be a risk factor for dementia.”
The study was published online July 29 in Neurology.
The ARIC trial
Prior studies have “described the interrelation of tooth loss or periodontal disease and cognitive outcomes, although many reports were cross-sectional or case-control … and often lacked robust confounder adjustment,” the investigators noted. Additionally, lack of longitudinal data impedes the “potential for baseline periodontal status to predict incident MCI.”
To explore the associations between periodontal status and incident MCI and dementia, the researchers studied participants in the ARIC study, a community-based longitudinal cohort consisting of 15,792 predominantly Black and White participants aged 45-64 years. The current analysis included 8,275 individuals (55% women; 21% black; mean age, 63 years) who at baseline did not meet criteria for dementia or MCI.
A full-mouth periodontal examination was conducted at baseline and participants were categorized according to the severity and extent of gingival inflammation and tooth attachment loss based on the Periodontal Profile Class (PPC) seven-category model. Potential confounding variables included age, race, education level, physical activity, smoking status, oral hygiene and access to care, plasma lipid levels, APOE genotype, body mass index, blood pressure, type 2 diabetes, and heart failure.
Based on PPC categorization, 22% of the patients had healthy gums, 12% had mild periodontal disease, 8% had a high gingival inflammation index, and 12% had posterior disease (with 6% having severe disease). In addition, 9% had tooth loss, 11% had severe tooth loss, and 20% were edentulous.
Infection hypothesis
Results showed that participants with worse periodontal status were more likely to have risk factors for vascular disease and dementia, such as smoking, hypertension, diabetes, and coronary heart disease. During median follow-up of 18.4 years, 19% of participants overall (n = 1,569) developed dementia, translating into 11.8 cases per 1,000 person-years. There were notable differences between the PPC categories in rates of incident dementia, with edentulous participants at twice the risk for developing dementia, compared with those who had healthy gums.
For participants with severe PPC, including severe tooth loss and severe disease, the multivariable-adjusted hazard ratio for incident dementia was 1.22 (95% confidence interval, 1.01-1.47) versus those who were periodontally healthy. For participants with edentulism, the HR was 1.21 (95% CI, 0.99-1.48). The adjusted risk ratios for the combined dementia/MCI outcome among participants with mild to intermediate PPC, severe PPC, or edentulism versus the periodontal healthy group were 1.22 (95% CI, 1.00-1.48), 1.15 (95% CI, 0.88-1.51), and 1.90 (95% CI, 1.40-2.58), respectively.
These findings were most pronounced among younger (median age at dental exam, younger than 62) versus older (62 years and older) participants (P = .02). Severe disease or total tooth loss were associated with an approximately 20% greater dementia incidence during the follow-up period, compared with healthy gums.
The investigators noted that the findings were “generally consistent” when considering the combined outcome of MCI and dementia. However, they noted that the association between edentulism and MCI was “markedly stronger,” with an approximate 100% increase in MCI or MCI plus dementia.
The association between periodontal disease and MCI or dementia “is rooted in the infection hypothesis, meaning adverse microbial exposures in the mucosal surfaces of the mouth, especially the subgingival space,” Dr. Demmer said. “One notion is that there could somehow be a direct infection of the brain with oral organisms, which posits that the oral organism could travel to the brain, colonize there, and cause damage that impairs cognition.”
Another possible mechanism is that chronic systemic inflammation in response to oral infections can eventually lead to vascular disease which, in turn, is a known risk factor for future dementia, he noted.
“Brush and floss”
Commenting on the research findings, James M. Noble, MD, associate professor of neurology, Taub Institute for Research on Alzheimer’s and the Aging Brain, Columbia University, New York, called the study “well characterized both by whole-mouth assessments and cognitive assessments performed in a standardized manner.” Moreover, “the study was sufficiently sized to allow for exploration of age and suggests that oral health may be a more important factor earlier in the course of aging, in late adulthood,” said Dr. Noble, who was not involved with the research.
The study also “makes an important contribution to this field through a rigorously followed cohort and robust design for both periodontal predictor and cognitive outcome assessments,” he said, noting that, “as always, the take-home message is ‘brush and floss.’
“Although we don’t know if treating periodontal disease can help treat dementia, this study suggests that we have to pay attention to good oral hygiene and make referrals to dentists when appropriate,” Dr. Demmer added.
The ARIC trial is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute. Dr. Demmer, the study coauthors, and Dr. Noble have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
especially in those with severe gum inflammation and edentulism, new research suggests.
Over a 20-year period, investigators prospectively followed more than 8,000 individuals aged around 63 years who did not have cognitive impairment or dementia at baseline, grouping them based on the extent and severity of their periodontal disease and number of lost teeth.
Results showed that 14% of participants with healthy gums and all their teeth at baseline developed dementia, compared with 18% of those with mild periodontal disease and 22% who had severe periodontal disease. The highest percentage (23%) of participants who developed dementia was found in those who were edentulous.
After accounting for comorbidities that might affect dementia risk, edentulous participants had a 20% higher risk for developing MCI or dementia, compared with the healthy group.
Because the study was observational, “we don’t have knowledge of causality so we cannot state that if you treat periodontal disease you can prevent or treat dementia,” said lead author Ryan T. Demmer, PhD, MPH, associate professor, division of epidemiology and community health, University of Minnesota, Minneapolis. However, “the take-home message from this paper is that it further supports the possibility that oral infections could be a risk factor for dementia.”
The study was published online July 29 in Neurology.
The ARIC trial
Prior studies have “described the interrelation of tooth loss or periodontal disease and cognitive outcomes, although many reports were cross-sectional or case-control … and often lacked robust confounder adjustment,” the investigators noted. Additionally, lack of longitudinal data impedes the “potential for baseline periodontal status to predict incident MCI.”
To explore the associations between periodontal status and incident MCI and dementia, the researchers studied participants in the ARIC study, a community-based longitudinal cohort consisting of 15,792 predominantly Black and White participants aged 45-64 years. The current analysis included 8,275 individuals (55% women; 21% black; mean age, 63 years) who at baseline did not meet criteria for dementia or MCI.
A full-mouth periodontal examination was conducted at baseline and participants were categorized according to the severity and extent of gingival inflammation and tooth attachment loss based on the Periodontal Profile Class (PPC) seven-category model. Potential confounding variables included age, race, education level, physical activity, smoking status, oral hygiene and access to care, plasma lipid levels, APOE genotype, body mass index, blood pressure, type 2 diabetes, and heart failure.
Based on PPC categorization, 22% of the patients had healthy gums, 12% had mild periodontal disease, 8% had a high gingival inflammation index, and 12% had posterior disease (with 6% having severe disease). In addition, 9% had tooth loss, 11% had severe tooth loss, and 20% were edentulous.
Infection hypothesis
Results showed that participants with worse periodontal status were more likely to have risk factors for vascular disease and dementia, such as smoking, hypertension, diabetes, and coronary heart disease. During median follow-up of 18.4 years, 19% of participants overall (n = 1,569) developed dementia, translating into 11.8 cases per 1,000 person-years. There were notable differences between the PPC categories in rates of incident dementia, with edentulous participants at twice the risk for developing dementia, compared with those who had healthy gums.
For participants with severe PPC, including severe tooth loss and severe disease, the multivariable-adjusted hazard ratio for incident dementia was 1.22 (95% confidence interval, 1.01-1.47) versus those who were periodontally healthy. For participants with edentulism, the HR was 1.21 (95% CI, 0.99-1.48). The adjusted risk ratios for the combined dementia/MCI outcome among participants with mild to intermediate PPC, severe PPC, or edentulism versus the periodontal healthy group were 1.22 (95% CI, 1.00-1.48), 1.15 (95% CI, 0.88-1.51), and 1.90 (95% CI, 1.40-2.58), respectively.
These findings were most pronounced among younger (median age at dental exam, younger than 62) versus older (62 years and older) participants (P = .02). Severe disease or total tooth loss were associated with an approximately 20% greater dementia incidence during the follow-up period, compared with healthy gums.
The investigators noted that the findings were “generally consistent” when considering the combined outcome of MCI and dementia. However, they noted that the association between edentulism and MCI was “markedly stronger,” with an approximate 100% increase in MCI or MCI plus dementia.
The association between periodontal disease and MCI or dementia “is rooted in the infection hypothesis, meaning adverse microbial exposures in the mucosal surfaces of the mouth, especially the subgingival space,” Dr. Demmer said. “One notion is that there could somehow be a direct infection of the brain with oral organisms, which posits that the oral organism could travel to the brain, colonize there, and cause damage that impairs cognition.”
Another possible mechanism is that chronic systemic inflammation in response to oral infections can eventually lead to vascular disease which, in turn, is a known risk factor for future dementia, he noted.
“Brush and floss”
Commenting on the research findings, James M. Noble, MD, associate professor of neurology, Taub Institute for Research on Alzheimer’s and the Aging Brain, Columbia University, New York, called the study “well characterized both by whole-mouth assessments and cognitive assessments performed in a standardized manner.” Moreover, “the study was sufficiently sized to allow for exploration of age and suggests that oral health may be a more important factor earlier in the course of aging, in late adulthood,” said Dr. Noble, who was not involved with the research.
The study also “makes an important contribution to this field through a rigorously followed cohort and robust design for both periodontal predictor and cognitive outcome assessments,” he said, noting that, “as always, the take-home message is ‘brush and floss.’
“Although we don’t know if treating periodontal disease can help treat dementia, this study suggests that we have to pay attention to good oral hygiene and make referrals to dentists when appropriate,” Dr. Demmer added.
The ARIC trial is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute. Dr. Demmer, the study coauthors, and Dr. Noble have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
especially in those with severe gum inflammation and edentulism, new research suggests.
Over a 20-year period, investigators prospectively followed more than 8,000 individuals aged around 63 years who did not have cognitive impairment or dementia at baseline, grouping them based on the extent and severity of their periodontal disease and number of lost teeth.
Results showed that 14% of participants with healthy gums and all their teeth at baseline developed dementia, compared with 18% of those with mild periodontal disease and 22% who had severe periodontal disease. The highest percentage (23%) of participants who developed dementia was found in those who were edentulous.
After accounting for comorbidities that might affect dementia risk, edentulous participants had a 20% higher risk for developing MCI or dementia, compared with the healthy group.
Because the study was observational, “we don’t have knowledge of causality so we cannot state that if you treat periodontal disease you can prevent or treat dementia,” said lead author Ryan T. Demmer, PhD, MPH, associate professor, division of epidemiology and community health, University of Minnesota, Minneapolis. However, “the take-home message from this paper is that it further supports the possibility that oral infections could be a risk factor for dementia.”
The study was published online July 29 in Neurology.
The ARIC trial
Prior studies have “described the interrelation of tooth loss or periodontal disease and cognitive outcomes, although many reports were cross-sectional or case-control … and often lacked robust confounder adjustment,” the investigators noted. Additionally, lack of longitudinal data impedes the “potential for baseline periodontal status to predict incident MCI.”
To explore the associations between periodontal status and incident MCI and dementia, the researchers studied participants in the ARIC study, a community-based longitudinal cohort consisting of 15,792 predominantly Black and White participants aged 45-64 years. The current analysis included 8,275 individuals (55% women; 21% black; mean age, 63 years) who at baseline did not meet criteria for dementia or MCI.
A full-mouth periodontal examination was conducted at baseline and participants were categorized according to the severity and extent of gingival inflammation and tooth attachment loss based on the Periodontal Profile Class (PPC) seven-category model. Potential confounding variables included age, race, education level, physical activity, smoking status, oral hygiene and access to care, plasma lipid levels, APOE genotype, body mass index, blood pressure, type 2 diabetes, and heart failure.
Based on PPC categorization, 22% of the patients had healthy gums, 12% had mild periodontal disease, 8% had a high gingival inflammation index, and 12% had posterior disease (with 6% having severe disease). In addition, 9% had tooth loss, 11% had severe tooth loss, and 20% were edentulous.
Infection hypothesis
Results showed that participants with worse periodontal status were more likely to have risk factors for vascular disease and dementia, such as smoking, hypertension, diabetes, and coronary heart disease. During median follow-up of 18.4 years, 19% of participants overall (n = 1,569) developed dementia, translating into 11.8 cases per 1,000 person-years. There were notable differences between the PPC categories in rates of incident dementia, with edentulous participants at twice the risk for developing dementia, compared with those who had healthy gums.
For participants with severe PPC, including severe tooth loss and severe disease, the multivariable-adjusted hazard ratio for incident dementia was 1.22 (95% confidence interval, 1.01-1.47) versus those who were periodontally healthy. For participants with edentulism, the HR was 1.21 (95% CI, 0.99-1.48). The adjusted risk ratios for the combined dementia/MCI outcome among participants with mild to intermediate PPC, severe PPC, or edentulism versus the periodontal healthy group were 1.22 (95% CI, 1.00-1.48), 1.15 (95% CI, 0.88-1.51), and 1.90 (95% CI, 1.40-2.58), respectively.
These findings were most pronounced among younger (median age at dental exam, younger than 62) versus older (62 years and older) participants (P = .02). Severe disease or total tooth loss were associated with an approximately 20% greater dementia incidence during the follow-up period, compared with healthy gums.
The investigators noted that the findings were “generally consistent” when considering the combined outcome of MCI and dementia. However, they noted that the association between edentulism and MCI was “markedly stronger,” with an approximate 100% increase in MCI or MCI plus dementia.
The association between periodontal disease and MCI or dementia “is rooted in the infection hypothesis, meaning adverse microbial exposures in the mucosal surfaces of the mouth, especially the subgingival space,” Dr. Demmer said. “One notion is that there could somehow be a direct infection of the brain with oral organisms, which posits that the oral organism could travel to the brain, colonize there, and cause damage that impairs cognition.”
Another possible mechanism is that chronic systemic inflammation in response to oral infections can eventually lead to vascular disease which, in turn, is a known risk factor for future dementia, he noted.
“Brush and floss”
Commenting on the research findings, James M. Noble, MD, associate professor of neurology, Taub Institute for Research on Alzheimer’s and the Aging Brain, Columbia University, New York, called the study “well characterized both by whole-mouth assessments and cognitive assessments performed in a standardized manner.” Moreover, “the study was sufficiently sized to allow for exploration of age and suggests that oral health may be a more important factor earlier in the course of aging, in late adulthood,” said Dr. Noble, who was not involved with the research.
The study also “makes an important contribution to this field through a rigorously followed cohort and robust design for both periodontal predictor and cognitive outcome assessments,” he said, noting that, “as always, the take-home message is ‘brush and floss.’
“Although we don’t know if treating periodontal disease can help treat dementia, this study suggests that we have to pay attention to good oral hygiene and make referrals to dentists when appropriate,” Dr. Demmer added.
The ARIC trial is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute. Dr. Demmer, the study coauthors, and Dr. Noble have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Are aging physicians a burden?
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
The evaluation of physicians with alleged cognitive decline
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
Impaired senses, especially smell, linked to dementia
new research suggests. The study, which included almost 1,800 participants, adds to emerging evidence that even mild levels of multisensory impairment are associated with accelerated cognitive aging, the researchers noted.
Clinicians should be aware of this link between sensory impairment and dementia risk, said lead author Willa Brenowitz, PhD, assistant professor, department of psychiatry and behavioral sciences, University of California, San Francisco. “Many of these impairments are treatable, or at least physicians can monitor them; and this can improve quality of life, even if it doesn’t improve dementia risk.”
The findings were published online July 12 in Alzheimer’s and Dementia.
Additive effects
Previous research has focused on the link between dementia and individual senses, but this new work is unique in that it focuses on the additive effects of multiple impairments in sensory function, said Dr. Brenowitz. The study included 1,794 dementia-free participants in their 70s from the Health, Aging and Body Composition study, a prospective cohort study of healthy Black and White men and women.
Researchers tested participants’ hearing using a pure tone average without hearing aids and vision using contrast sensitivity with glasses permitted. They also measured vibrations in the big toe to assess touch and had participants identify distinctive odors such as paint thinner, roses, lemons, and onions to assess smell.
A score of 0-3 was assigned based on sample quartiles for each of the four sensory functions. Individuals with the best quartile were assigned a score of 0 and those with the worst were assigned a score of 3.
The investigators added scores across all senses to create a summary score of multisensory function (0-12) and classified the participants into tertiles of good, medium, and poor. Individuals with a score of 0 would have good function in all senses, whereas those with 12 would have poor function in all senses. Those with medium scores could have a mix of impairments.
Participants with good multisensory function were more likely to be healthier than those with poor function. They were also significantly more likely to have completed high school (85.0% vs. 72.1%), were significantly less likely to have diabetes (16.9% vs. 27.9%), and were marginally less likely to have cardiovascular disease, high blood pressure, and history of stroke.
Investigators measured cognition using the Modified Mini-Mental State (3MS) examination, a test of global cognitive function, and the Digit Symbol Substitution Test (DSST), a measure of cognitive processing speed. Cognitive testing was carried out at the beginning of the study and repeated every other year.
Dementia was defined as the use of dementia medication, being hospitalized with dementia as a primary or secondary diagnosis, or having a 3MS score 1.5 standard deviations lower than the race-stratified Health ABC study baseline mean.
Over an average follow-up of 6.3 years, 18% of participants developed dementia.
Dose-response increase
Results showed that, with worsening multisensory function score, the risk for dementia increased in a dose-response manner. In models adjusted for demographics and health conditions, participants with a poor multisensory function score were more than twice as likely to develop dementia than those with a good score (hazard ratio, 2.05; 95% confidence interval, 1.50-2.81; P < .001). Those with a middle multisensory function score were 1.45 times more likely to develop dementia (HR, 1.45; 95% CI, 1.09-1.91; P < .001).
Even a 1-point worse multisensory function score was associated with a 14% higher risk for dementia (95% CI, 8%-21%), while a 4-point worse score was associated with 71% higher risk for dementia (95% CI, 38%-211%).
Smell was the sensory function most strongly associated with dementia risk. Participants whose sense of smell declined by 10% had a 19% higher risk for dementia versus a 1%-3% higher risk for declines in vision, hearing, and touch.
It is not clear why smell was a stronger determinant of dementia risk. However, loss of this sense is often considered to be a marker for Alzheimer’s disease “because it is closely linked with brain regions that are affected” in that disease, said Dr. Brenowitz.
However, that does not necessarily mean smell is more important than vision or hearing, she added. “Even if hearing and vision have a smaller contribution to dementia, they have a stronger potential for intervention.” The findings suggest “some additive or cumulative” effects for loss of the different senses. “There’s an association above and beyond those which can be attributed to individual sensory domains,” she said.
Frailty link
After including mobility, which is a potential mediator, estimates for the multisensory function score were slightly lower. “Walking speed is pretty strongly associated with dementia risk,” Dr. Brenowitz noted. Physical frailty might help explain the link between sensory impairment and dementia risk. “It’s not clear if that’s because people with dementia are declining or because people with frailty are especially vulnerable to dementia,” she said.
The researchers also assessed the role of social support, another potential mechanism by which sensory decline, especially in hearing and vision, could influence dementia risk. Although the study did not find substantial differences in social support measures, the investigators noted that questions assessing social support were limited in scope.
Interactions between multisensory function score and race, APOE e4 allele status, and sex were not significant.
Worsening multisensory function was also linked to faster annual rates of cognitive decline as measured by both the 3MS and DSST. Each 1-point worse score was associated with faster decline (P < .05), even after adjustment for demographics and health conditions.
Possible mechanisms
A number of possible mechanisms may explain the link between poor sensory function and dementia. It could be that neurodegeneration underlying dementia affects the senses, or vision and/or hearing loss leads to social isolation and poor mental health, which in turn could affect dementia risk, the researchers wrote. It also is possible that cardiovascular disease or diabetes affect both dementia risk and sensory impairment.
Dr. Brenowitz noted that, because cognitive tests rely on a certain degree of vision and hearing, impairment of these senses may complicate such tests. Still to be determined is whether correcting sensory impairments, such as wearing corrective lenses or hearing aids, affects dementia risk.
Meanwhile, it might be a good idea to more regularly check sensory function, especially vision and hearing, the researchers suggested. These functions affect various aspects of health and can be assessed rather easily. However, because smell is so strongly associated with dementia risk, Dr. Brenowitz said she would like to see it also become “part of a screening tool.”
A possible study limitation cited was that the researchers checked sensory function only once. “Most likely, some of these would change over time, but at least it captured sensory function at one point,” Dr. Brenowitz said.
“Sheds further light”
Commenting on the study, Jo V. Rushworth, PhD, associate professor and national teaching fellow, De Montfort University Leicester (England), said it “sheds further light on the emerging links” between multisensory impairment and cognitive decline leading to dementia. “The authors show that people with even mild loss of function in various senses are more likely to develop cognitive impairment.”
Dr. Rushworth was not involved with the study but has done research in the area.
The current results suggest that measuring patients’ hearing, vision, sense of smell, and touch might “flag at-risk groups” who could be targeted for dementia prevention strategies, Dr. Rushworth noted. Such tests are noninvasive and potentially less distressing than other methods of diagnosing dementia. “Importantly, the relatively low cost and simplicity of sensory tests offer the potential for more frequent testing and the use of these methods in areas of the world where medical facilities and resources are limited.”
This new study raises the question of whether the observed sensory impairments are a cause or an effect of dementia, Dr. Rushworth noted. “As the authors suggest, decreased sensory function can lead to a decrease in social engagement, mobility, and other factors which would usually contribute to counteracting cognitive decline.”
The study raises other questions, too, said Dr. Rushworth. She noted that the participants who experienced more severe sensory impairments were, on average, 2 years older than those with the least impairments. “To what degree were the observed sensory deficits linked to normal aging rather than dementia?”
As well, Dr. Rushworth pointed out that the molecular mechanisms that “kick-start” dementia are believed to occur in midlife – so possibly at an age younger than the study participants. “Do younger people of a ‘predementia’ age range display multisensory impairments?”
Because study participants could wear glasses during vision tests but were not allowed to wear hearing aids for the hearing tests, further standardization of sensory impairment is required, Dr. Rushworth said.
“Future studies will be essential in determining the value of clinical measurement of multisensory impairment as a possible dementia indicator and prevention strategy,” she concluded.
The study was funded by the National Institute on Aging, the National Institute of Nursing Research, and the Alzheimer’s Association. Dr. Brenowitz and Dr. Rushworth have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. The study, which included almost 1,800 participants, adds to emerging evidence that even mild levels of multisensory impairment are associated with accelerated cognitive aging, the researchers noted.
Clinicians should be aware of this link between sensory impairment and dementia risk, said lead author Willa Brenowitz, PhD, assistant professor, department of psychiatry and behavioral sciences, University of California, San Francisco. “Many of these impairments are treatable, or at least physicians can monitor them; and this can improve quality of life, even if it doesn’t improve dementia risk.”
The findings were published online July 12 in Alzheimer’s and Dementia.
Additive effects
Previous research has focused on the link between dementia and individual senses, but this new work is unique in that it focuses on the additive effects of multiple impairments in sensory function, said Dr. Brenowitz. The study included 1,794 dementia-free participants in their 70s from the Health, Aging and Body Composition study, a prospective cohort study of healthy Black and White men and women.
Researchers tested participants’ hearing using a pure tone average without hearing aids and vision using contrast sensitivity with glasses permitted. They also measured vibrations in the big toe to assess touch and had participants identify distinctive odors such as paint thinner, roses, lemons, and onions to assess smell.
A score of 0-3 was assigned based on sample quartiles for each of the four sensory functions. Individuals with the best quartile were assigned a score of 0 and those with the worst were assigned a score of 3.
The investigators added scores across all senses to create a summary score of multisensory function (0-12) and classified the participants into tertiles of good, medium, and poor. Individuals with a score of 0 would have good function in all senses, whereas those with 12 would have poor function in all senses. Those with medium scores could have a mix of impairments.
Participants with good multisensory function were more likely to be healthier than those with poor function. They were also significantly more likely to have completed high school (85.0% vs. 72.1%), were significantly less likely to have diabetes (16.9% vs. 27.9%), and were marginally less likely to have cardiovascular disease, high blood pressure, and history of stroke.
Investigators measured cognition using the Modified Mini-Mental State (3MS) examination, a test of global cognitive function, and the Digit Symbol Substitution Test (DSST), a measure of cognitive processing speed. Cognitive testing was carried out at the beginning of the study and repeated every other year.
Dementia was defined as the use of dementia medication, being hospitalized with dementia as a primary or secondary diagnosis, or having a 3MS score 1.5 standard deviations lower than the race-stratified Health ABC study baseline mean.
Over an average follow-up of 6.3 years, 18% of participants developed dementia.
Dose-response increase
Results showed that, with worsening multisensory function score, the risk for dementia increased in a dose-response manner. In models adjusted for demographics and health conditions, participants with a poor multisensory function score were more than twice as likely to develop dementia than those with a good score (hazard ratio, 2.05; 95% confidence interval, 1.50-2.81; P < .001). Those with a middle multisensory function score were 1.45 times more likely to develop dementia (HR, 1.45; 95% CI, 1.09-1.91; P < .001).
Even a 1-point worse multisensory function score was associated with a 14% higher risk for dementia (95% CI, 8%-21%), while a 4-point worse score was associated with 71% higher risk for dementia (95% CI, 38%-211%).
Smell was the sensory function most strongly associated with dementia risk. Participants whose sense of smell declined by 10% had a 19% higher risk for dementia versus a 1%-3% higher risk for declines in vision, hearing, and touch.
It is not clear why smell was a stronger determinant of dementia risk. However, loss of this sense is often considered to be a marker for Alzheimer’s disease “because it is closely linked with brain regions that are affected” in that disease, said Dr. Brenowitz.
However, that does not necessarily mean smell is more important than vision or hearing, she added. “Even if hearing and vision have a smaller contribution to dementia, they have a stronger potential for intervention.” The findings suggest “some additive or cumulative” effects for loss of the different senses. “There’s an association above and beyond those which can be attributed to individual sensory domains,” she said.
Frailty link
After including mobility, which is a potential mediator, estimates for the multisensory function score were slightly lower. “Walking speed is pretty strongly associated with dementia risk,” Dr. Brenowitz noted. Physical frailty might help explain the link between sensory impairment and dementia risk. “It’s not clear if that’s because people with dementia are declining or because people with frailty are especially vulnerable to dementia,” she said.
The researchers also assessed the role of social support, another potential mechanism by which sensory decline, especially in hearing and vision, could influence dementia risk. Although the study did not find substantial differences in social support measures, the investigators noted that questions assessing social support were limited in scope.
Interactions between multisensory function score and race, APOE e4 allele status, and sex were not significant.
Worsening multisensory function was also linked to faster annual rates of cognitive decline as measured by both the 3MS and DSST. Each 1-point worse score was associated with faster decline (P < .05), even after adjustment for demographics and health conditions.
Possible mechanisms
A number of possible mechanisms may explain the link between poor sensory function and dementia. It could be that neurodegeneration underlying dementia affects the senses, or vision and/or hearing loss leads to social isolation and poor mental health, which in turn could affect dementia risk, the researchers wrote. It also is possible that cardiovascular disease or diabetes affect both dementia risk and sensory impairment.
Dr. Brenowitz noted that, because cognitive tests rely on a certain degree of vision and hearing, impairment of these senses may complicate such tests. Still to be determined is whether correcting sensory impairments, such as wearing corrective lenses or hearing aids, affects dementia risk.
Meanwhile, it might be a good idea to more regularly check sensory function, especially vision and hearing, the researchers suggested. These functions affect various aspects of health and can be assessed rather easily. However, because smell is so strongly associated with dementia risk, Dr. Brenowitz said she would like to see it also become “part of a screening tool.”
A possible study limitation cited was that the researchers checked sensory function only once. “Most likely, some of these would change over time, but at least it captured sensory function at one point,” Dr. Brenowitz said.
“Sheds further light”
Commenting on the study, Jo V. Rushworth, PhD, associate professor and national teaching fellow, De Montfort University Leicester (England), said it “sheds further light on the emerging links” between multisensory impairment and cognitive decline leading to dementia. “The authors show that people with even mild loss of function in various senses are more likely to develop cognitive impairment.”
Dr. Rushworth was not involved with the study but has done research in the area.
The current results suggest that measuring patients’ hearing, vision, sense of smell, and touch might “flag at-risk groups” who could be targeted for dementia prevention strategies, Dr. Rushworth noted. Such tests are noninvasive and potentially less distressing than other methods of diagnosing dementia. “Importantly, the relatively low cost and simplicity of sensory tests offer the potential for more frequent testing and the use of these methods in areas of the world where medical facilities and resources are limited.”
This new study raises the question of whether the observed sensory impairments are a cause or an effect of dementia, Dr. Rushworth noted. “As the authors suggest, decreased sensory function can lead to a decrease in social engagement, mobility, and other factors which would usually contribute to counteracting cognitive decline.”
The study raises other questions, too, said Dr. Rushworth. She noted that the participants who experienced more severe sensory impairments were, on average, 2 years older than those with the least impairments. “To what degree were the observed sensory deficits linked to normal aging rather than dementia?”
As well, Dr. Rushworth pointed out that the molecular mechanisms that “kick-start” dementia are believed to occur in midlife – so possibly at an age younger than the study participants. “Do younger people of a ‘predementia’ age range display multisensory impairments?”
Because study participants could wear glasses during vision tests but were not allowed to wear hearing aids for the hearing tests, further standardization of sensory impairment is required, Dr. Rushworth said.
“Future studies will be essential in determining the value of clinical measurement of multisensory impairment as a possible dementia indicator and prevention strategy,” she concluded.
The study was funded by the National Institute on Aging, the National Institute of Nursing Research, and the Alzheimer’s Association. Dr. Brenowitz and Dr. Rushworth have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. The study, which included almost 1,800 participants, adds to emerging evidence that even mild levels of multisensory impairment are associated with accelerated cognitive aging, the researchers noted.
Clinicians should be aware of this link between sensory impairment and dementia risk, said lead author Willa Brenowitz, PhD, assistant professor, department of psychiatry and behavioral sciences, University of California, San Francisco. “Many of these impairments are treatable, or at least physicians can monitor them; and this can improve quality of life, even if it doesn’t improve dementia risk.”
The findings were published online July 12 in Alzheimer’s and Dementia.
Additive effects
Previous research has focused on the link between dementia and individual senses, but this new work is unique in that it focuses on the additive effects of multiple impairments in sensory function, said Dr. Brenowitz. The study included 1,794 dementia-free participants in their 70s from the Health, Aging and Body Composition study, a prospective cohort study of healthy Black and White men and women.
Researchers tested participants’ hearing using a pure tone average without hearing aids and vision using contrast sensitivity with glasses permitted. They also measured vibrations in the big toe to assess touch and had participants identify distinctive odors such as paint thinner, roses, lemons, and onions to assess smell.
A score of 0-3 was assigned based on sample quartiles for each of the four sensory functions. Individuals with the best quartile were assigned a score of 0 and those with the worst were assigned a score of 3.
The investigators added scores across all senses to create a summary score of multisensory function (0-12) and classified the participants into tertiles of good, medium, and poor. Individuals with a score of 0 would have good function in all senses, whereas those with 12 would have poor function in all senses. Those with medium scores could have a mix of impairments.
Participants with good multisensory function were more likely to be healthier than those with poor function. They were also significantly more likely to have completed high school (85.0% vs. 72.1%), were significantly less likely to have diabetes (16.9% vs. 27.9%), and were marginally less likely to have cardiovascular disease, high blood pressure, and history of stroke.
Investigators measured cognition using the Modified Mini-Mental State (3MS) examination, a test of global cognitive function, and the Digit Symbol Substitution Test (DSST), a measure of cognitive processing speed. Cognitive testing was carried out at the beginning of the study and repeated every other year.
Dementia was defined as the use of dementia medication, being hospitalized with dementia as a primary or secondary diagnosis, or having a 3MS score 1.5 standard deviations lower than the race-stratified Health ABC study baseline mean.
Over an average follow-up of 6.3 years, 18% of participants developed dementia.
Dose-response increase
Results showed that, with worsening multisensory function score, the risk for dementia increased in a dose-response manner. In models adjusted for demographics and health conditions, participants with a poor multisensory function score were more than twice as likely to develop dementia than those with a good score (hazard ratio, 2.05; 95% confidence interval, 1.50-2.81; P < .001). Those with a middle multisensory function score were 1.45 times more likely to develop dementia (HR, 1.45; 95% CI, 1.09-1.91; P < .001).
Even a 1-point worse multisensory function score was associated with a 14% higher risk for dementia (95% CI, 8%-21%), while a 4-point worse score was associated with 71% higher risk for dementia (95% CI, 38%-211%).
Smell was the sensory function most strongly associated with dementia risk. Participants whose sense of smell declined by 10% had a 19% higher risk for dementia versus a 1%-3% higher risk for declines in vision, hearing, and touch.
It is not clear why smell was a stronger determinant of dementia risk. However, loss of this sense is often considered to be a marker for Alzheimer’s disease “because it is closely linked with brain regions that are affected” in that disease, said Dr. Brenowitz.
However, that does not necessarily mean smell is more important than vision or hearing, she added. “Even if hearing and vision have a smaller contribution to dementia, they have a stronger potential for intervention.” The findings suggest “some additive or cumulative” effects for loss of the different senses. “There’s an association above and beyond those which can be attributed to individual sensory domains,” she said.
Frailty link
After including mobility, which is a potential mediator, estimates for the multisensory function score were slightly lower. “Walking speed is pretty strongly associated with dementia risk,” Dr. Brenowitz noted. Physical frailty might help explain the link between sensory impairment and dementia risk. “It’s not clear if that’s because people with dementia are declining or because people with frailty are especially vulnerable to dementia,” she said.
The researchers also assessed the role of social support, another potential mechanism by which sensory decline, especially in hearing and vision, could influence dementia risk. Although the study did not find substantial differences in social support measures, the investigators noted that questions assessing social support were limited in scope.
Interactions between multisensory function score and race, APOE e4 allele status, and sex were not significant.
Worsening multisensory function was also linked to faster annual rates of cognitive decline as measured by both the 3MS and DSST. Each 1-point worse score was associated with faster decline (P < .05), even after adjustment for demographics and health conditions.
Possible mechanisms
A number of possible mechanisms may explain the link between poor sensory function and dementia. It could be that neurodegeneration underlying dementia affects the senses, or vision and/or hearing loss leads to social isolation and poor mental health, which in turn could affect dementia risk, the researchers wrote. It also is possible that cardiovascular disease or diabetes affect both dementia risk and sensory impairment.
Dr. Brenowitz noted that, because cognitive tests rely on a certain degree of vision and hearing, impairment of these senses may complicate such tests. Still to be determined is whether correcting sensory impairments, such as wearing corrective lenses or hearing aids, affects dementia risk.
Meanwhile, it might be a good idea to more regularly check sensory function, especially vision and hearing, the researchers suggested. These functions affect various aspects of health and can be assessed rather easily. However, because smell is so strongly associated with dementia risk, Dr. Brenowitz said she would like to see it also become “part of a screening tool.”
A possible study limitation cited was that the researchers checked sensory function only once. “Most likely, some of these would change over time, but at least it captured sensory function at one point,” Dr. Brenowitz said.
“Sheds further light”
Commenting on the study, Jo V. Rushworth, PhD, associate professor and national teaching fellow, De Montfort University Leicester (England), said it “sheds further light on the emerging links” between multisensory impairment and cognitive decline leading to dementia. “The authors show that people with even mild loss of function in various senses are more likely to develop cognitive impairment.”
Dr. Rushworth was not involved with the study but has done research in the area.
The current results suggest that measuring patients’ hearing, vision, sense of smell, and touch might “flag at-risk groups” who could be targeted for dementia prevention strategies, Dr. Rushworth noted. Such tests are noninvasive and potentially less distressing than other methods of diagnosing dementia. “Importantly, the relatively low cost and simplicity of sensory tests offer the potential for more frequent testing and the use of these methods in areas of the world where medical facilities and resources are limited.”
This new study raises the question of whether the observed sensory impairments are a cause or an effect of dementia, Dr. Rushworth noted. “As the authors suggest, decreased sensory function can lead to a decrease in social engagement, mobility, and other factors which would usually contribute to counteracting cognitive decline.”
The study raises other questions, too, said Dr. Rushworth. She noted that the participants who experienced more severe sensory impairments were, on average, 2 years older than those with the least impairments. “To what degree were the observed sensory deficits linked to normal aging rather than dementia?”
As well, Dr. Rushworth pointed out that the molecular mechanisms that “kick-start” dementia are believed to occur in midlife – so possibly at an age younger than the study participants. “Do younger people of a ‘predementia’ age range display multisensory impairments?”
Because study participants could wear glasses during vision tests but were not allowed to wear hearing aids for the hearing tests, further standardization of sensory impairment is required, Dr. Rushworth said.
“Future studies will be essential in determining the value of clinical measurement of multisensory impairment as a possible dementia indicator and prevention strategy,” she concluded.
The study was funded by the National Institute on Aging, the National Institute of Nursing Research, and the Alzheimer’s Association. Dr. Brenowitz and Dr. Rushworth have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Concussion linked to risk for dementia, Parkinson’s disease, and ADHD
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Family Medicine and Community Health
Consensus document reviews determination of brain death
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
Twelve risk factors linked to 40% of world’s dementia cases
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
From AAIC 2020
Cognitive impairment in 9/11 responders tied to brain atrophy
, suggest results from the first structural neuroimaging study conducted in this population. The study clarifies that a neurodegenerative condition is present in first responders who experience cognitive impairment in midlife, which “is incredibly important to know,” said lead author Sean Clouston, PhD, of Stony Brook (N.Y.) University.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were published online in Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring.
Brain atrophy in midlife
During the 9/11 attack and in its aftermath, WTC responders were exposed to a range of inhaled neurotoxicants, as well as extreme psychosocial stressors. A growing number of WTC responders who are now in their 50s and early 60s are experiencing early cognitive impairment.
Using MRI, the investigators examined cortical thickness (CTX), a surrogate marker for neurodegeneration, in 99 mostly male WTC responders; 48 had cognitive impairment, and 51 did not. The age range of the participants was 45 to 65 years, a range during which cortical atrophy is uncommon in the general population, the researchers noted.
Compared with cognitively normal responders, those with cognitive impairment were found to have reductions in CTX across the whole brain and across 21 of 34 cortical regions, including frontal, temporal, and occipital lobes.
In both cognitively impaired and cognitively unimpaired WTC responders, CTX was reduced in the entorhinal and temporal cortices compared with normative data, but reductions were greater with cognitive impairment. Posttraumatic distress disorder (PTSD) status was not predictive of a reduction in CTX across groups.
Dr. Clouston said the level of reduction in CTX in many responders is similar to that commonly found in patients with dementia and may reflect early-stage dementia occurring in midlife.
Limitations of the study include the small sample size, the cross-sectional design, the unique nature of the exposure, and a lack of a non-WTC external control group.
‘Illuminating’ study
Keith N. Fargo, PhD, director of scientific engagement for the Alzheimer’s Association, called the findings “interesting and illuminating” but cautioned that it is not possible to show cause and effect with this type of study.
“We also don’t know when cortical thinning might have started or how quickly it might be progressing,” Dr. Fargo said in an interview.
He noted that the pattern of cortical thinning is “somewhat consistent with what we see among people who live with high levels of air pollution, which is an emerging risk factor for Alzheimer’s disease and other dementias.”
The Lancet Commission on Dementia Prevention, Intervention, and Care added air pollution to its list of modifiable risk factors for dementia, which was recently updated.
Clinicians “need to be aware that their middle-aged 9/11 first responders are at a higher risk level for cognitive impairment, as well as PTSD and depression,” Dr. Fargo said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Aging. Dr. Clouston and Dr. Fargo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, suggest results from the first structural neuroimaging study conducted in this population. The study clarifies that a neurodegenerative condition is present in first responders who experience cognitive impairment in midlife, which “is incredibly important to know,” said lead author Sean Clouston, PhD, of Stony Brook (N.Y.) University.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were published online in Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring.
Brain atrophy in midlife
During the 9/11 attack and in its aftermath, WTC responders were exposed to a range of inhaled neurotoxicants, as well as extreme psychosocial stressors. A growing number of WTC responders who are now in their 50s and early 60s are experiencing early cognitive impairment.
Using MRI, the investigators examined cortical thickness (CTX), a surrogate marker for neurodegeneration, in 99 mostly male WTC responders; 48 had cognitive impairment, and 51 did not. The age range of the participants was 45 to 65 years, a range during which cortical atrophy is uncommon in the general population, the researchers noted.
Compared with cognitively normal responders, those with cognitive impairment were found to have reductions in CTX across the whole brain and across 21 of 34 cortical regions, including frontal, temporal, and occipital lobes.
In both cognitively impaired and cognitively unimpaired WTC responders, CTX was reduced in the entorhinal and temporal cortices compared with normative data, but reductions were greater with cognitive impairment. Posttraumatic distress disorder (PTSD) status was not predictive of a reduction in CTX across groups.
Dr. Clouston said the level of reduction in CTX in many responders is similar to that commonly found in patients with dementia and may reflect early-stage dementia occurring in midlife.
Limitations of the study include the small sample size, the cross-sectional design, the unique nature of the exposure, and a lack of a non-WTC external control group.
‘Illuminating’ study
Keith N. Fargo, PhD, director of scientific engagement for the Alzheimer’s Association, called the findings “interesting and illuminating” but cautioned that it is not possible to show cause and effect with this type of study.
“We also don’t know when cortical thinning might have started or how quickly it might be progressing,” Dr. Fargo said in an interview.
He noted that the pattern of cortical thinning is “somewhat consistent with what we see among people who live with high levels of air pollution, which is an emerging risk factor for Alzheimer’s disease and other dementias.”
The Lancet Commission on Dementia Prevention, Intervention, and Care added air pollution to its list of modifiable risk factors for dementia, which was recently updated.
Clinicians “need to be aware that their middle-aged 9/11 first responders are at a higher risk level for cognitive impairment, as well as PTSD and depression,” Dr. Fargo said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Aging. Dr. Clouston and Dr. Fargo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, suggest results from the first structural neuroimaging study conducted in this population. The study clarifies that a neurodegenerative condition is present in first responders who experience cognitive impairment in midlife, which “is incredibly important to know,” said lead author Sean Clouston, PhD, of Stony Brook (N.Y.) University.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were published online in Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring.
Brain atrophy in midlife
During the 9/11 attack and in its aftermath, WTC responders were exposed to a range of inhaled neurotoxicants, as well as extreme psychosocial stressors. A growing number of WTC responders who are now in their 50s and early 60s are experiencing early cognitive impairment.
Using MRI, the investigators examined cortical thickness (CTX), a surrogate marker for neurodegeneration, in 99 mostly male WTC responders; 48 had cognitive impairment, and 51 did not. The age range of the participants was 45 to 65 years, a range during which cortical atrophy is uncommon in the general population, the researchers noted.
Compared with cognitively normal responders, those with cognitive impairment were found to have reductions in CTX across the whole brain and across 21 of 34 cortical regions, including frontal, temporal, and occipital lobes.
In both cognitively impaired and cognitively unimpaired WTC responders, CTX was reduced in the entorhinal and temporal cortices compared with normative data, but reductions were greater with cognitive impairment. Posttraumatic distress disorder (PTSD) status was not predictive of a reduction in CTX across groups.
Dr. Clouston said the level of reduction in CTX in many responders is similar to that commonly found in patients with dementia and may reflect early-stage dementia occurring in midlife.
Limitations of the study include the small sample size, the cross-sectional design, the unique nature of the exposure, and a lack of a non-WTC external control group.
‘Illuminating’ study
Keith N. Fargo, PhD, director of scientific engagement for the Alzheimer’s Association, called the findings “interesting and illuminating” but cautioned that it is not possible to show cause and effect with this type of study.
“We also don’t know when cortical thinning might have started or how quickly it might be progressing,” Dr. Fargo said in an interview.
He noted that the pattern of cortical thinning is “somewhat consistent with what we see among people who live with high levels of air pollution, which is an emerging risk factor for Alzheimer’s disease and other dementias.”
The Lancet Commission on Dementia Prevention, Intervention, and Care added air pollution to its list of modifiable risk factors for dementia, which was recently updated.
Clinicians “need to be aware that their middle-aged 9/11 first responders are at a higher risk level for cognitive impairment, as well as PTSD and depression,” Dr. Fargo said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Aging. Dr. Clouston and Dr. Fargo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From AAIC 2020
Plasma exchange is ‘encouraging’ as a novel Alzheimer’s disease treatment
Results from the phase 2b/3 AMBAR study showed that the treatment, which aims to remove amyloid-beta (Abeta) from plasma, was associated with a 60% decrease in functional and cognitive decline in patients with moderate Alzheimer’s disease.
The reduction in cognitive decline uncovered by the study is more striking than that reported for other investigational treatments targeting Abeta, such as monoclonal antibodies, said coinvestigator Antonio Páez, MD, medical director of the AMBAR program, Alzheimer’s Research Group, Grifols, Barcelona.
The results “open a new path for the development of plasma protein replacement therapies not only in Alzheimer’s disease but also in other degenerative diseases that we are planning to investigate,” Dr. Páez said.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were simultaneously published in Alzheimer’s and Dementia.
Removing amyloid
Plasma exchange treatments, which have been available for several decades, are used to treat a range of neurologic, immunologic, and metabolic disorders. The treatment involves plasmapheresis, whereby plasma is separated from blood cells (red blood cells, white blood cells, platelets, etc) and toxic substances are removed. The albumin in plasma, to which plasma Abeta is bound, is replaced with a fresh commercial albumin product made from plasma from healthy donors.
“Our initial hypothesis was that, by removing albumin together with Abeta and substituting it with newer albumin periodically, we may be removing Abeta from the cerebrospinal fluid and eventually from the brain,” Dr. Páez said.
The AMBAR study included 347 men and women aged 55-85 years with probable Alzheimer’s disease dementia who were enrolled at 41 sites in Spain and the United States. All were diagnosed with mild Alzheimer’s disease, as shown by a baseline Mini-Mental State Examination score of 22-26, or moderate Alzheimer’s disease, having a baseline MMSE score of 18-21.
Investigators randomly assigned the participants to four groups; one group received placebo, and each of the other three treatment arms received different doses of albumin and intravenous immunoglobulin (IVIg) replacement.
During the first 6-week study phase, patients received weekly sham or conventional plasma exchange treatments of 2.5-3 liters of plasma, which Dr. Páez referred to as the “intensive-treatment phase to remove as much Abeta as possible.”
This was followed by a 12-month maintenance phase, which involved monthly low-volume (700-800 mL) plasma exchange or sham treatments.
Although the volume of plasma removed was the same in all three active-treatment groups, the amount of albumin and IVIg that was subsequently replaced varied. In one group, the same amount of albumin and IVIg that was removed was replaced; in another, half the amount removed was replaced; and in the third, only albumin was replaced.
The researchers collected cerebrospinal fluid (CSF) samples at baseline and after each treatment period. They assessed Abeta40, Abeta42, total tau, and phosphorylated-tau biomarkers.
The two primary outcomes were change from baseline to 14 months in scores on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) scale and the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog).
Symptom reduction
Results showed a reduction in the progression of symptoms in the plasma exchange–treated patients for both primary endpoints.
The ADCS-ADL showed 52% less decline in the plasma exchange–treated group, compared with the placebo group (P = .03); the ADAS-Cog showed 66% less decline (P = 0.06). In the moderate group, both endpoints showed 61% less decline (P = .002 and .05, respectively).
There were no clear differences between the three active-treatment groups, “suggesting that any of them could be considered for further investigation,” said Dr. Páez.
Differences in baseline demographic characteristics did not appear to have an influence on the outcomes. ADAS-Cog was more than twice as effective as some candidate monoclonal antibodies targeting Abeta that are being investigated for Alzheimer’s disease, Dr. Páez noted.
Although the plasma exchange approach is relatively invasive, so too are monoclonal antibody therapies that are infused intravenously through a pump, he said. In addition, a low-volume plasma exchange maintenance treatment takes less than 2 hours, which is on a par with some monoclonal antibody treatments.
Key secondary outcomes
For both primary outcomes, changes were found in those with moderate but not mild Alzheimer’s disease, possibly because the ADAS-Cog was designed for patients with more severe symptoms and may not be sensitive enough for patients with better cognitive performance, said Dr. Páez.
However, the difference between mild and moderate Alzheimer’s disease did not hold up in post hoc analyses that included additional baseline characteristics, including amyloid and APOE e4 status.
“We observed that both mild and moderate subjects performed better than placebo even in the two coprimary endpoints,” Dr. Páez said. “It suggested that the differences between mild and moderate patients was not so apparent.”
The study’s key secondary outcomes included scores on the Clinical Dementia Rating Sum of Boxes (CDR-sb) and the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change (ADCS-CGIC) scales. Treated patients scored better than the placebo group on both the CDR-sb (71% less decline, P = .002) and the ADCS-CGIC (100% less decline, P < .0001) scales.
For disease biomarkers in the moderate Alzheimer’s disease study population, levels of CSF Abeta42 and tau protein remained stable in the treated patients. In the placebo group, Abeta42 was decreased and tau protein increased. Dr. Páez explained that, if amyloid in the brain comes from the CSF, this process may take some time.
The findings suggest that more than one mechanism may be involved in the plasma exchange approach, such as changes in oxidation status and inflammatory mediators, the investigators noted.
Safety profile
About 28% of the participants dropped out of the study, which the researchers note is a rate similar to that reported in studies of solanezumab and other treatments in patients with Alzheimer’s disease. “The high percentage (72%) of patients who completed the study further supports that this procedure is feasible in mild to moderate Alzheimer’s disease,” the investigators wrote.
Overall, adverse events were similar to the known safety profile of plasma exchange procedures for other indications. The two most common adverse events were catheter local reactions and hypotension.
Almost 90% of the apheresis procedures were “uneventful,” the researchers reported. Two patients (0.6%) died during the study, which is similar to the low mortality rates reported elsewhere.
However, the investigators stressed that, because many patients with Alzheimer’s disease are in fragile health, plasma exchange treatments should be undertaken with caution, because of its invasive nature.
Dr. Páez noted that a possible limitation of this treatment approach is the availability of plasma for manufacturing plasma products. In the future, this plasma exchange approach might be combined with current and future Alzheimer’s disease therapies.
They are currently in discussions with the American Society for Apheresis, which develops guidelines for plasma exchange. After additional research, the investigators hope to eventually receive Food and Drug Administration approval of plasma exchange with albumin replacement as a treatment for Alzheimer’s disease.
Speculative, yet reasonable approach
Commenting on the research findings, Pierre N. Tariot, MD, director of Banner Alzheimer’s Institute and research professor of psychiatry at the University of Arizona, both in Phoenix, said the study is “meaningful and large enough” to “come close” to determining whether the therapy is safe and effective. “The fundamental rationale for this experimental approach, while speculative, is reasonable and certainly seems to be worth testing,” said Dr. Tariot, who was not involved with the research.
However, “there’s a decent chance” that not all trial participants had Alzheimer’s disease. Although some CSF amyloid measures suggest levels consistent with AD, “this is not conclusive,” he said.
In addition, “there’s a slightly low rate of apolipoprotein E4 allele carriage [in the current study], compared with most Alzheimer’s disease trials,” Dr. Tariot said.
He also pointed out that the trial failed to show statistical significance on both coprimary outcomes. “It’s unclear what health authorities, if presented with these data, would decide to do with the file.”
Although it was “encouraging” that secondary endpoints were supportive, the fact that they had greater statistical significance than some of the other objective measures “raises at least the potential for partial unblinding as a result of side effects,” said Dr. Tariot. It is also unclear why changes would be more evident in the moderate subpopulation.
The study was funded by Grifols. Dr. Páez is an employee of Grifols. Dr. Tariot reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Results from the phase 2b/3 AMBAR study showed that the treatment, which aims to remove amyloid-beta (Abeta) from plasma, was associated with a 60% decrease in functional and cognitive decline in patients with moderate Alzheimer’s disease.
The reduction in cognitive decline uncovered by the study is more striking than that reported for other investigational treatments targeting Abeta, such as monoclonal antibodies, said coinvestigator Antonio Páez, MD, medical director of the AMBAR program, Alzheimer’s Research Group, Grifols, Barcelona.
The results “open a new path for the development of plasma protein replacement therapies not only in Alzheimer’s disease but also in other degenerative diseases that we are planning to investigate,” Dr. Páez said.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were simultaneously published in Alzheimer’s and Dementia.
Removing amyloid
Plasma exchange treatments, which have been available for several decades, are used to treat a range of neurologic, immunologic, and metabolic disorders. The treatment involves plasmapheresis, whereby plasma is separated from blood cells (red blood cells, white blood cells, platelets, etc) and toxic substances are removed. The albumin in plasma, to which plasma Abeta is bound, is replaced with a fresh commercial albumin product made from plasma from healthy donors.
“Our initial hypothesis was that, by removing albumin together with Abeta and substituting it with newer albumin periodically, we may be removing Abeta from the cerebrospinal fluid and eventually from the brain,” Dr. Páez said.
The AMBAR study included 347 men and women aged 55-85 years with probable Alzheimer’s disease dementia who were enrolled at 41 sites in Spain and the United States. All were diagnosed with mild Alzheimer’s disease, as shown by a baseline Mini-Mental State Examination score of 22-26, or moderate Alzheimer’s disease, having a baseline MMSE score of 18-21.
Investigators randomly assigned the participants to four groups; one group received placebo, and each of the other three treatment arms received different doses of albumin and intravenous immunoglobulin (IVIg) replacement.
During the first 6-week study phase, patients received weekly sham or conventional plasma exchange treatments of 2.5-3 liters of plasma, which Dr. Páez referred to as the “intensive-treatment phase to remove as much Abeta as possible.”
This was followed by a 12-month maintenance phase, which involved monthly low-volume (700-800 mL) plasma exchange or sham treatments.
Although the volume of plasma removed was the same in all three active-treatment groups, the amount of albumin and IVIg that was subsequently replaced varied. In one group, the same amount of albumin and IVIg that was removed was replaced; in another, half the amount removed was replaced; and in the third, only albumin was replaced.
The researchers collected cerebrospinal fluid (CSF) samples at baseline and after each treatment period. They assessed Abeta40, Abeta42, total tau, and phosphorylated-tau biomarkers.
The two primary outcomes were change from baseline to 14 months in scores on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) scale and the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog).
Symptom reduction
Results showed a reduction in the progression of symptoms in the plasma exchange–treated patients for both primary endpoints.
The ADCS-ADL showed 52% less decline in the plasma exchange–treated group, compared with the placebo group (P = .03); the ADAS-Cog showed 66% less decline (P = 0.06). In the moderate group, both endpoints showed 61% less decline (P = .002 and .05, respectively).
There were no clear differences between the three active-treatment groups, “suggesting that any of them could be considered for further investigation,” said Dr. Páez.
Differences in baseline demographic characteristics did not appear to have an influence on the outcomes. ADAS-Cog was more than twice as effective as some candidate monoclonal antibodies targeting Abeta that are being investigated for Alzheimer’s disease, Dr. Páez noted.
Although the plasma exchange approach is relatively invasive, so too are monoclonal antibody therapies that are infused intravenously through a pump, he said. In addition, a low-volume plasma exchange maintenance treatment takes less than 2 hours, which is on a par with some monoclonal antibody treatments.
Key secondary outcomes
For both primary outcomes, changes were found in those with moderate but not mild Alzheimer’s disease, possibly because the ADAS-Cog was designed for patients with more severe symptoms and may not be sensitive enough for patients with better cognitive performance, said Dr. Páez.
However, the difference between mild and moderate Alzheimer’s disease did not hold up in post hoc analyses that included additional baseline characteristics, including amyloid and APOE e4 status.
“We observed that both mild and moderate subjects performed better than placebo even in the two coprimary endpoints,” Dr. Páez said. “It suggested that the differences between mild and moderate patients was not so apparent.”
The study’s key secondary outcomes included scores on the Clinical Dementia Rating Sum of Boxes (CDR-sb) and the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change (ADCS-CGIC) scales. Treated patients scored better than the placebo group on both the CDR-sb (71% less decline, P = .002) and the ADCS-CGIC (100% less decline, P < .0001) scales.
For disease biomarkers in the moderate Alzheimer’s disease study population, levels of CSF Abeta42 and tau protein remained stable in the treated patients. In the placebo group, Abeta42 was decreased and tau protein increased. Dr. Páez explained that, if amyloid in the brain comes from the CSF, this process may take some time.
The findings suggest that more than one mechanism may be involved in the plasma exchange approach, such as changes in oxidation status and inflammatory mediators, the investigators noted.
Safety profile
About 28% of the participants dropped out of the study, which the researchers note is a rate similar to that reported in studies of solanezumab and other treatments in patients with Alzheimer’s disease. “The high percentage (72%) of patients who completed the study further supports that this procedure is feasible in mild to moderate Alzheimer’s disease,” the investigators wrote.
Overall, adverse events were similar to the known safety profile of plasma exchange procedures for other indications. The two most common adverse events were catheter local reactions and hypotension.
Almost 90% of the apheresis procedures were “uneventful,” the researchers reported. Two patients (0.6%) died during the study, which is similar to the low mortality rates reported elsewhere.
However, the investigators stressed that, because many patients with Alzheimer’s disease are in fragile health, plasma exchange treatments should be undertaken with caution, because of its invasive nature.
Dr. Páez noted that a possible limitation of this treatment approach is the availability of plasma for manufacturing plasma products. In the future, this plasma exchange approach might be combined with current and future Alzheimer’s disease therapies.
They are currently in discussions with the American Society for Apheresis, which develops guidelines for plasma exchange. After additional research, the investigators hope to eventually receive Food and Drug Administration approval of plasma exchange with albumin replacement as a treatment for Alzheimer’s disease.
Speculative, yet reasonable approach
Commenting on the research findings, Pierre N. Tariot, MD, director of Banner Alzheimer’s Institute and research professor of psychiatry at the University of Arizona, both in Phoenix, said the study is “meaningful and large enough” to “come close” to determining whether the therapy is safe and effective. “The fundamental rationale for this experimental approach, while speculative, is reasonable and certainly seems to be worth testing,” said Dr. Tariot, who was not involved with the research.
However, “there’s a decent chance” that not all trial participants had Alzheimer’s disease. Although some CSF amyloid measures suggest levels consistent with AD, “this is not conclusive,” he said.
In addition, “there’s a slightly low rate of apolipoprotein E4 allele carriage [in the current study], compared with most Alzheimer’s disease trials,” Dr. Tariot said.
He also pointed out that the trial failed to show statistical significance on both coprimary outcomes. “It’s unclear what health authorities, if presented with these data, would decide to do with the file.”
Although it was “encouraging” that secondary endpoints were supportive, the fact that they had greater statistical significance than some of the other objective measures “raises at least the potential for partial unblinding as a result of side effects,” said Dr. Tariot. It is also unclear why changes would be more evident in the moderate subpopulation.
The study was funded by Grifols. Dr. Páez is an employee of Grifols. Dr. Tariot reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Results from the phase 2b/3 AMBAR study showed that the treatment, which aims to remove amyloid-beta (Abeta) from plasma, was associated with a 60% decrease in functional and cognitive decline in patients with moderate Alzheimer’s disease.
The reduction in cognitive decline uncovered by the study is more striking than that reported for other investigational treatments targeting Abeta, such as monoclonal antibodies, said coinvestigator Antonio Páez, MD, medical director of the AMBAR program, Alzheimer’s Research Group, Grifols, Barcelona.
The results “open a new path for the development of plasma protein replacement therapies not only in Alzheimer’s disease but also in other degenerative diseases that we are planning to investigate,” Dr. Páez said.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were simultaneously published in Alzheimer’s and Dementia.
Removing amyloid
Plasma exchange treatments, which have been available for several decades, are used to treat a range of neurologic, immunologic, and metabolic disorders. The treatment involves plasmapheresis, whereby plasma is separated from blood cells (red blood cells, white blood cells, platelets, etc) and toxic substances are removed. The albumin in plasma, to which plasma Abeta is bound, is replaced with a fresh commercial albumin product made from plasma from healthy donors.
“Our initial hypothesis was that, by removing albumin together with Abeta and substituting it with newer albumin periodically, we may be removing Abeta from the cerebrospinal fluid and eventually from the brain,” Dr. Páez said.
The AMBAR study included 347 men and women aged 55-85 years with probable Alzheimer’s disease dementia who were enrolled at 41 sites in Spain and the United States. All were diagnosed with mild Alzheimer’s disease, as shown by a baseline Mini-Mental State Examination score of 22-26, or moderate Alzheimer’s disease, having a baseline MMSE score of 18-21.
Investigators randomly assigned the participants to four groups; one group received placebo, and each of the other three treatment arms received different doses of albumin and intravenous immunoglobulin (IVIg) replacement.
During the first 6-week study phase, patients received weekly sham or conventional plasma exchange treatments of 2.5-3 liters of plasma, which Dr. Páez referred to as the “intensive-treatment phase to remove as much Abeta as possible.”
This was followed by a 12-month maintenance phase, which involved monthly low-volume (700-800 mL) plasma exchange or sham treatments.
Although the volume of plasma removed was the same in all three active-treatment groups, the amount of albumin and IVIg that was subsequently replaced varied. In one group, the same amount of albumin and IVIg that was removed was replaced; in another, half the amount removed was replaced; and in the third, only albumin was replaced.
The researchers collected cerebrospinal fluid (CSF) samples at baseline and after each treatment period. They assessed Abeta40, Abeta42, total tau, and phosphorylated-tau biomarkers.
The two primary outcomes were change from baseline to 14 months in scores on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) scale and the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog).
Symptom reduction
Results showed a reduction in the progression of symptoms in the plasma exchange–treated patients for both primary endpoints.
The ADCS-ADL showed 52% less decline in the plasma exchange–treated group, compared with the placebo group (P = .03); the ADAS-Cog showed 66% less decline (P = 0.06). In the moderate group, both endpoints showed 61% less decline (P = .002 and .05, respectively).
There were no clear differences between the three active-treatment groups, “suggesting that any of them could be considered for further investigation,” said Dr. Páez.
Differences in baseline demographic characteristics did not appear to have an influence on the outcomes. ADAS-Cog was more than twice as effective as some candidate monoclonal antibodies targeting Abeta that are being investigated for Alzheimer’s disease, Dr. Páez noted.
Although the plasma exchange approach is relatively invasive, so too are monoclonal antibody therapies that are infused intravenously through a pump, he said. In addition, a low-volume plasma exchange maintenance treatment takes less than 2 hours, which is on a par with some monoclonal antibody treatments.
Key secondary outcomes
For both primary outcomes, changes were found in those with moderate but not mild Alzheimer’s disease, possibly because the ADAS-Cog was designed for patients with more severe symptoms and may not be sensitive enough for patients with better cognitive performance, said Dr. Páez.
However, the difference between mild and moderate Alzheimer’s disease did not hold up in post hoc analyses that included additional baseline characteristics, including amyloid and APOE e4 status.
“We observed that both mild and moderate subjects performed better than placebo even in the two coprimary endpoints,” Dr. Páez said. “It suggested that the differences between mild and moderate patients was not so apparent.”
The study’s key secondary outcomes included scores on the Clinical Dementia Rating Sum of Boxes (CDR-sb) and the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change (ADCS-CGIC) scales. Treated patients scored better than the placebo group on both the CDR-sb (71% less decline, P = .002) and the ADCS-CGIC (100% less decline, P < .0001) scales.
For disease biomarkers in the moderate Alzheimer’s disease study population, levels of CSF Abeta42 and tau protein remained stable in the treated patients. In the placebo group, Abeta42 was decreased and tau protein increased. Dr. Páez explained that, if amyloid in the brain comes from the CSF, this process may take some time.
The findings suggest that more than one mechanism may be involved in the plasma exchange approach, such as changes in oxidation status and inflammatory mediators, the investigators noted.
Safety profile
About 28% of the participants dropped out of the study, which the researchers note is a rate similar to that reported in studies of solanezumab and other treatments in patients with Alzheimer’s disease. “The high percentage (72%) of patients who completed the study further supports that this procedure is feasible in mild to moderate Alzheimer’s disease,” the investigators wrote.
Overall, adverse events were similar to the known safety profile of plasma exchange procedures for other indications. The two most common adverse events were catheter local reactions and hypotension.
Almost 90% of the apheresis procedures were “uneventful,” the researchers reported. Two patients (0.6%) died during the study, which is similar to the low mortality rates reported elsewhere.
However, the investigators stressed that, because many patients with Alzheimer’s disease are in fragile health, plasma exchange treatments should be undertaken with caution, because of its invasive nature.
Dr. Páez noted that a possible limitation of this treatment approach is the availability of plasma for manufacturing plasma products. In the future, this plasma exchange approach might be combined with current and future Alzheimer’s disease therapies.
They are currently in discussions with the American Society for Apheresis, which develops guidelines for plasma exchange. After additional research, the investigators hope to eventually receive Food and Drug Administration approval of plasma exchange with albumin replacement as a treatment for Alzheimer’s disease.
Speculative, yet reasonable approach
Commenting on the research findings, Pierre N. Tariot, MD, director of Banner Alzheimer’s Institute and research professor of psychiatry at the University of Arizona, both in Phoenix, said the study is “meaningful and large enough” to “come close” to determining whether the therapy is safe and effective. “The fundamental rationale for this experimental approach, while speculative, is reasonable and certainly seems to be worth testing,” said Dr. Tariot, who was not involved with the research.
However, “there’s a decent chance” that not all trial participants had Alzheimer’s disease. Although some CSF amyloid measures suggest levels consistent with AD, “this is not conclusive,” he said.
In addition, “there’s a slightly low rate of apolipoprotein E4 allele carriage [in the current study], compared with most Alzheimer’s disease trials,” Dr. Tariot said.
He also pointed out that the trial failed to show statistical significance on both coprimary outcomes. “It’s unclear what health authorities, if presented with these data, would decide to do with the file.”
Although it was “encouraging” that secondary endpoints were supportive, the fact that they had greater statistical significance than some of the other objective measures “raises at least the potential for partial unblinding as a result of side effects,” said Dr. Tariot. It is also unclear why changes would be more evident in the moderate subpopulation.
The study was funded by Grifols. Dr. Páez is an employee of Grifols. Dr. Tariot reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
‘Long sleep’ or apnea in middle age double risk for Alzheimer’s disease
new research suggests. A U.K. Biobank study of more than 500,000 individuals also showed that excessive daytime sleepiness was associated with increased risk for Alzheimer’s disease.
“Addressing sleep problems in middle-age may play a role in improving brain health,” said lead author Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School and associate scientist in the division of sleep and circadian disorders at Brigham and Women’s Hospital, both in Boston.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
Intricately linked
Sleep disturbances are common and on the rise around the world. In recent years, researchers have become increasingly aware of the intricate link between sleep health and brain health, Dr. Gao noted.
The current study included 502,538 individuals from the U.K. Biobank (mean age, 57 years) who were free from Alzheimer’s disease at baseline. They were followed for up to 12 years. The participants self-reported sleep traits, including hours of nighttime sleep, daytime sleepiness, sleep apnea diagnosis, snoring, and napping. Researchers determined Alzheimer’s disease diagnoses from hospital admissions and from death registries.
In addition to adjusting for age, sex, education, and ethnicity, the full model adjusted for socioeconomic status, body mass index, physical activity, smoking and alcohol use, cardiovascular diseases and risk factors, neurological diseases, respiratory diseases, depression/anxiety, and medication use. Over the course of a mean follow-up of 6.4 years, 932 participants developed Alzheimer’s disease.
Complex disorder
Compared with those who got an average of 6-9 hours of sleep per night, those getting more than 9 hours had a higher risk for Alzheimer’s disease (hazard ratio, 2.04; 95% confidence interval, 1.56-2.67; P < .001). Having sleep apnea also raised the risk significantly (HR, 2.05; 95% CI, 1.23-3.42; P = .006), as did daytime sleepiness (HR, 1.56; 95% CI, 1.18-2.03; P = .001).
Dr. Gao noted that daytime sleepiness and sleep apnea remained predictive after controlling for sleep duration. “In fact, all three sleep traits remained associated with Alzheimer’s disease within the same model, suggesting some degree of independence.”
Interestingly, snoring, which is a common symptom of sleep apnea, was not linked to Alzheimer’s disease risk. The “vast majority” of people who snore don’t meet criteria for a diagnosis of sleep apnea, which was particularly true for this large cohort of relatively healthy study participants, Dr. Gao noted.
“Sleep apnea is a complex, multisystemic sleep disorder associated with obesity, high blood pressure, and often other heart problems,” he said.
He added that, as an anesthesiologist, he is particularly wary if patients have this condition, “given their increased risk for airway difficulties, adverse cardiac events, postoperative respiratory complications, and confusion or delirium, which is also associated with higher risk for eventual Alzheimer’s disease and death.”
These multisystemic factors may be driving the link to Alzheimer’s disease. “We certainly need to address this better as the population ages and obesity rates rise,” Dr. Gao said.
No association with napping
Unlike another of Dr. Gao’s studies that was conducted in a much older population, napping was not a risk factor for Alzheimer’s disease in the current study’s younger participants. It could be that the impacts of different sleep traits on health outcome change with age, Dr. Gao said, or this could represent a limitation of using self-reported sleep measures as opposed to objective and/or quantitative measures, such as actigraphy. The reasons for napping, which differ around the world with the habit being common in certain parts, may also help explain differences in observed associations.
Although the investigators tried to control for comorbidities and medication use, there “most certainly” could be a reverse causation at work. For example, sleeping too much could be both a cause and a symptom of dementia. Dr. Gao noted that sleep disturbances often become more prevalent with dementia, and sleeping too much or complaining of daytime sleepiness may be a result of preclinical Alzheimer’s disease. Even if there is a reverse causation, however, the average time to Alzheimer’s disease diagnosis was over 6 years in this study. “This may be a significant window of time to intervene,” he said.
To improve sleep health, he recommends going to bed and waking at similar times every day, avoiding caffeine or alcohol close to bedtime, limiting screen time before bed, dimming lights, and reducing noise.
It’s also important to have sleep apnea treated. “While more studies are needed, it’s generally believed that addressing the pauses in breathing, the apnea episodes, will help reduce cardiovascular health risks such as obesity, high blood pressure and heart failure. All are known to be strongly linked to dementia risk,” Dr. Gao said.
Results from an assessment of 100,000 actigraphy records from a subset of the same population are expected soon and will add objective confirmation of these self-reported results, he added.
Unique, powerful
Commenting on the findings, Alberto Ramos, MD, associate professor of clinical neurology and research director of the sleep medicine program at the University of Miami, called the study “unique” and “powerful” because of its prospective design and large sample size.
“Another strength of the study was that it included a population-based sample as opposed to one from a memory or sleep clinic where people already have symptoms or are already sick,” said Dr. Ramos, who was not involved with the research.
In addition, while most studies that have linked sleep disturbances with dementia risk have been in older adults, this study’s population was middle-aged to start out, he noted.
Dr. Gao and Dr. Ramos reported no relevant financial relationships. Although Dr. Gao’s lab receives funding from the National Institutes of Health, the BrightFocus Foundation, the University of Manchester, the Medical Biodynamics Program, Brigham and Women’s Hospital, and the Broad Institute, the study itself does not have its own specific funding.
A version of this article originally appeared on Medscape.com.
new research suggests. A U.K. Biobank study of more than 500,000 individuals also showed that excessive daytime sleepiness was associated with increased risk for Alzheimer’s disease.
“Addressing sleep problems in middle-age may play a role in improving brain health,” said lead author Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School and associate scientist in the division of sleep and circadian disorders at Brigham and Women’s Hospital, both in Boston.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
Intricately linked
Sleep disturbances are common and on the rise around the world. In recent years, researchers have become increasingly aware of the intricate link between sleep health and brain health, Dr. Gao noted.
The current study included 502,538 individuals from the U.K. Biobank (mean age, 57 years) who were free from Alzheimer’s disease at baseline. They were followed for up to 12 years. The participants self-reported sleep traits, including hours of nighttime sleep, daytime sleepiness, sleep apnea diagnosis, snoring, and napping. Researchers determined Alzheimer’s disease diagnoses from hospital admissions and from death registries.
In addition to adjusting for age, sex, education, and ethnicity, the full model adjusted for socioeconomic status, body mass index, physical activity, smoking and alcohol use, cardiovascular diseases and risk factors, neurological diseases, respiratory diseases, depression/anxiety, and medication use. Over the course of a mean follow-up of 6.4 years, 932 participants developed Alzheimer’s disease.
Complex disorder
Compared with those who got an average of 6-9 hours of sleep per night, those getting more than 9 hours had a higher risk for Alzheimer’s disease (hazard ratio, 2.04; 95% confidence interval, 1.56-2.67; P < .001). Having sleep apnea also raised the risk significantly (HR, 2.05; 95% CI, 1.23-3.42; P = .006), as did daytime sleepiness (HR, 1.56; 95% CI, 1.18-2.03; P = .001).
Dr. Gao noted that daytime sleepiness and sleep apnea remained predictive after controlling for sleep duration. “In fact, all three sleep traits remained associated with Alzheimer’s disease within the same model, suggesting some degree of independence.”
Interestingly, snoring, which is a common symptom of sleep apnea, was not linked to Alzheimer’s disease risk. The “vast majority” of people who snore don’t meet criteria for a diagnosis of sleep apnea, which was particularly true for this large cohort of relatively healthy study participants, Dr. Gao noted.
“Sleep apnea is a complex, multisystemic sleep disorder associated with obesity, high blood pressure, and often other heart problems,” he said.
He added that, as an anesthesiologist, he is particularly wary if patients have this condition, “given their increased risk for airway difficulties, adverse cardiac events, postoperative respiratory complications, and confusion or delirium, which is also associated with higher risk for eventual Alzheimer’s disease and death.”
These multisystemic factors may be driving the link to Alzheimer’s disease. “We certainly need to address this better as the population ages and obesity rates rise,” Dr. Gao said.
No association with napping
Unlike another of Dr. Gao’s studies that was conducted in a much older population, napping was not a risk factor for Alzheimer’s disease in the current study’s younger participants. It could be that the impacts of different sleep traits on health outcome change with age, Dr. Gao said, or this could represent a limitation of using self-reported sleep measures as opposed to objective and/or quantitative measures, such as actigraphy. The reasons for napping, which differ around the world with the habit being common in certain parts, may also help explain differences in observed associations.
Although the investigators tried to control for comorbidities and medication use, there “most certainly” could be a reverse causation at work. For example, sleeping too much could be both a cause and a symptom of dementia. Dr. Gao noted that sleep disturbances often become more prevalent with dementia, and sleeping too much or complaining of daytime sleepiness may be a result of preclinical Alzheimer’s disease. Even if there is a reverse causation, however, the average time to Alzheimer’s disease diagnosis was over 6 years in this study. “This may be a significant window of time to intervene,” he said.
To improve sleep health, he recommends going to bed and waking at similar times every day, avoiding caffeine or alcohol close to bedtime, limiting screen time before bed, dimming lights, and reducing noise.
It’s also important to have sleep apnea treated. “While more studies are needed, it’s generally believed that addressing the pauses in breathing, the apnea episodes, will help reduce cardiovascular health risks such as obesity, high blood pressure and heart failure. All are known to be strongly linked to dementia risk,” Dr. Gao said.
Results from an assessment of 100,000 actigraphy records from a subset of the same population are expected soon and will add objective confirmation of these self-reported results, he added.
Unique, powerful
Commenting on the findings, Alberto Ramos, MD, associate professor of clinical neurology and research director of the sleep medicine program at the University of Miami, called the study “unique” and “powerful” because of its prospective design and large sample size.
“Another strength of the study was that it included a population-based sample as opposed to one from a memory or sleep clinic where people already have symptoms or are already sick,” said Dr. Ramos, who was not involved with the research.
In addition, while most studies that have linked sleep disturbances with dementia risk have been in older adults, this study’s population was middle-aged to start out, he noted.
Dr. Gao and Dr. Ramos reported no relevant financial relationships. Although Dr. Gao’s lab receives funding from the National Institutes of Health, the BrightFocus Foundation, the University of Manchester, the Medical Biodynamics Program, Brigham and Women’s Hospital, and the Broad Institute, the study itself does not have its own specific funding.
A version of this article originally appeared on Medscape.com.
new research suggests. A U.K. Biobank study of more than 500,000 individuals also showed that excessive daytime sleepiness was associated with increased risk for Alzheimer’s disease.
“Addressing sleep problems in middle-age may play a role in improving brain health,” said lead author Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School and associate scientist in the division of sleep and circadian disorders at Brigham and Women’s Hospital, both in Boston.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
Intricately linked
Sleep disturbances are common and on the rise around the world. In recent years, researchers have become increasingly aware of the intricate link between sleep health and brain health, Dr. Gao noted.
The current study included 502,538 individuals from the U.K. Biobank (mean age, 57 years) who were free from Alzheimer’s disease at baseline. They were followed for up to 12 years. The participants self-reported sleep traits, including hours of nighttime sleep, daytime sleepiness, sleep apnea diagnosis, snoring, and napping. Researchers determined Alzheimer’s disease diagnoses from hospital admissions and from death registries.
In addition to adjusting for age, sex, education, and ethnicity, the full model adjusted for socioeconomic status, body mass index, physical activity, smoking and alcohol use, cardiovascular diseases and risk factors, neurological diseases, respiratory diseases, depression/anxiety, and medication use. Over the course of a mean follow-up of 6.4 years, 932 participants developed Alzheimer’s disease.
Complex disorder
Compared with those who got an average of 6-9 hours of sleep per night, those getting more than 9 hours had a higher risk for Alzheimer’s disease (hazard ratio, 2.04; 95% confidence interval, 1.56-2.67; P < .001). Having sleep apnea also raised the risk significantly (HR, 2.05; 95% CI, 1.23-3.42; P = .006), as did daytime sleepiness (HR, 1.56; 95% CI, 1.18-2.03; P = .001).
Dr. Gao noted that daytime sleepiness and sleep apnea remained predictive after controlling for sleep duration. “In fact, all three sleep traits remained associated with Alzheimer’s disease within the same model, suggesting some degree of independence.”
Interestingly, snoring, which is a common symptom of sleep apnea, was not linked to Alzheimer’s disease risk. The “vast majority” of people who snore don’t meet criteria for a diagnosis of sleep apnea, which was particularly true for this large cohort of relatively healthy study participants, Dr. Gao noted.
“Sleep apnea is a complex, multisystemic sleep disorder associated with obesity, high blood pressure, and often other heart problems,” he said.
He added that, as an anesthesiologist, he is particularly wary if patients have this condition, “given their increased risk for airway difficulties, adverse cardiac events, postoperative respiratory complications, and confusion or delirium, which is also associated with higher risk for eventual Alzheimer’s disease and death.”
These multisystemic factors may be driving the link to Alzheimer’s disease. “We certainly need to address this better as the population ages and obesity rates rise,” Dr. Gao said.
No association with napping
Unlike another of Dr. Gao’s studies that was conducted in a much older population, napping was not a risk factor for Alzheimer’s disease in the current study’s younger participants. It could be that the impacts of different sleep traits on health outcome change with age, Dr. Gao said, or this could represent a limitation of using self-reported sleep measures as opposed to objective and/or quantitative measures, such as actigraphy. The reasons for napping, which differ around the world with the habit being common in certain parts, may also help explain differences in observed associations.
Although the investigators tried to control for comorbidities and medication use, there “most certainly” could be a reverse causation at work. For example, sleeping too much could be both a cause and a symptom of dementia. Dr. Gao noted that sleep disturbances often become more prevalent with dementia, and sleeping too much or complaining of daytime sleepiness may be a result of preclinical Alzheimer’s disease. Even if there is a reverse causation, however, the average time to Alzheimer’s disease diagnosis was over 6 years in this study. “This may be a significant window of time to intervene,” he said.
To improve sleep health, he recommends going to bed and waking at similar times every day, avoiding caffeine or alcohol close to bedtime, limiting screen time before bed, dimming lights, and reducing noise.
It’s also important to have sleep apnea treated. “While more studies are needed, it’s generally believed that addressing the pauses in breathing, the apnea episodes, will help reduce cardiovascular health risks such as obesity, high blood pressure and heart failure. All are known to be strongly linked to dementia risk,” Dr. Gao said.
Results from an assessment of 100,000 actigraphy records from a subset of the same population are expected soon and will add objective confirmation of these self-reported results, he added.
Unique, powerful
Commenting on the findings, Alberto Ramos, MD, associate professor of clinical neurology and research director of the sleep medicine program at the University of Miami, called the study “unique” and “powerful” because of its prospective design and large sample size.
“Another strength of the study was that it included a population-based sample as opposed to one from a memory or sleep clinic where people already have symptoms or are already sick,” said Dr. Ramos, who was not involved with the research.
In addition, while most studies that have linked sleep disturbances with dementia risk have been in older adults, this study’s population was middle-aged to start out, he noted.
Dr. Gao and Dr. Ramos reported no relevant financial relationships. Although Dr. Gao’s lab receives funding from the National Institutes of Health, the BrightFocus Foundation, the University of Manchester, the Medical Biodynamics Program, Brigham and Women’s Hospital, and the Broad Institute, the study itself does not have its own specific funding.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020