User login
COVID affects executive functioning in young to middle-age adults: Study
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
New data support a causal role for depression in Alzheimer’s
Researchers have known for some time that depression is associated with Alzheimer’s disease (AD), but a causal link has been elusive. Now, using newly available data, they have uncovered genetic evidence of a causal role for depression in AD.
As depression typically affects those in early or midlife and dementia often occurs in later life, “it’s fascinating to see a connection between the two brain illnesses that manifest in different time windows,” coinvestigator Aliza P. Wingo, MD, associate professor of psychiatry and behavioral science, Emory University, Atlanta, said in an interview.
“If we can treat the depression early on, we may help reduce risk for dementia for our patients later in life,” Dr. Wingo said.
The findings were published online Dec. 16, 2021, in Biological Psychiatry.
Postmortem data
The investigators, who are all from the Emory University Center for Neurodegenerative Disease, wanted to clarify the genetic basis underlying the association between the established link between depression and dementia risk.
They used data from the largest and most recent genomewide association studies (GWAS). These included a 2019 analysis of depression among 807,553 individuals and a 2019 study of AD among 455,258 individuals, all of European ancestry. For sensitivity analyses, they used results from two additional AD GWAS.
The researchers also accessed postmortem brain samples from participants in the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). These participants were cognitively normal at enrollment, underwent annual clinical evaluations, and agreed to donate their brains.
They also assessed brain samples donated by participants in the Banner Sun Health Research Institute longitudinal study of healthy aging, Alzheimer’s, and Parkinson’s disease.
The brain samples allowed researchers to use deep brain proteomic data to help determine molecular links between depression and AD.
After quality control, the analysis included 8,356 proteins in 391 ROS/MAP participants and 7,854 proteins in 196 Banner participants.
suggesting the two conditions have a shared genetic basis.
The investigators also applied a framework called “Mendelian randomization” to determine causality between depression and AD.
After assessing the effect of 115 independent single-nucleotide polymorphisms (SNPs) from the GWAS of depression, they uncovered significant evidence “that the SNPs cause depression, which in turn cause AD,” said Dr. Wingo.
One-way relationship
The researchers conducted the same analysis on 61 significant SNPs from the GWAS of AD but did not find evidence to conclude AD causes depression.
“We found genetic evidence supporting a causal role of depression in AD but not vice versa,” Dr. Wingo said.
In addition, the investigators identified 75 brain transcripts (messenger RNA) and 28 brain proteins regulated by the depression-predisposing genetic variants. Of these, 46 brain transcripts and seven proteins were significantly associated with at least one AD feature – for example, beta-amyloid, tau tangles, and cognitive trajectory.
“These findings support the notion that the depression risk variants contribute to AD via regulating expression of their corresponding transcripts in the brain,” the investigators wrote.
It is only recently that large enough studies have allowed researchers sufficient power to reach these conclusions, coinvestigator Thomas Wingo, MD, said in an interview.
These additional “insights” into the relationship between depression and AD might “motivate” clinicians more to screen for and treat depressive symptoms, Dr. Aliza Wingo noted.
The new results also have implications for developing therapeutics to treat depression, she said. “If we target the genes, the brain proteins, that are shared risk between depression and AD, the medications that target that gene might mitigate risk for AD later on.”
However, the investigators advised caution. “A lot of this is still unknown,” said Dr. Thomas Wingo.
For example, it is not clear whether successfully treating depression mitigates the eventual risk of dementia, which is “a very important topic of inquiry and one we continue to work on,” he said, adding that a significant number of patients do not respond well to existing antidepressants such as SSRIs.
Need for further research
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach, Alzheimer’s Association, said the study contributes to the debate about whether depression increases risk for AD, whether AD increases risk for depression, or both.
“These newly published findings strengthen our understanding of the role of depression as a risk factor for Alzheimer’s dementia,” said Dr. Sexton, who was not involved with the research.
While experts do not yet fully understand the impact of treating depression on dementia risk, “the findings emphasize the importance of assessing mental health status, particularly depression, and getting it properly diagnosed and treated in a timely manner,” she said.
However, she agreed more research in this area is needed. “Importantly, these findings need replication in broader, more diverse study populations,” Dr. Sexton said.
A study funded by the Alzheimer’s Association may provide more information on the link between depression and AD. It will investigate whether machine learning, an advanced computer science technique, can better predict cognitive decline, compared with traditional methods.
Over a period of 6 months, researchers will collect smartphone conversations from 225 older adults with dementia, mild cognitive impairment, or no cognitive impairment. They will also have data from cognitive tests, brain scans, and biomarkers such as cerebrospinal fluid samples to study brain changes associated with AD.
The novel method of analysis should be able to identify subtle differences in speech quality to indicate which depressive symptoms an individual might be experiencing.
“The study could help us further understand the potential impact of depression in the risk of developing dementia,” said Dr. Sexton.
Dr. Aliza Wingo and Dr. Thomas Wingo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have known for some time that depression is associated with Alzheimer’s disease (AD), but a causal link has been elusive. Now, using newly available data, they have uncovered genetic evidence of a causal role for depression in AD.
As depression typically affects those in early or midlife and dementia often occurs in later life, “it’s fascinating to see a connection between the two brain illnesses that manifest in different time windows,” coinvestigator Aliza P. Wingo, MD, associate professor of psychiatry and behavioral science, Emory University, Atlanta, said in an interview.
“If we can treat the depression early on, we may help reduce risk for dementia for our patients later in life,” Dr. Wingo said.
The findings were published online Dec. 16, 2021, in Biological Psychiatry.
Postmortem data
The investigators, who are all from the Emory University Center for Neurodegenerative Disease, wanted to clarify the genetic basis underlying the association between the established link between depression and dementia risk.
They used data from the largest and most recent genomewide association studies (GWAS). These included a 2019 analysis of depression among 807,553 individuals and a 2019 study of AD among 455,258 individuals, all of European ancestry. For sensitivity analyses, they used results from two additional AD GWAS.
The researchers also accessed postmortem brain samples from participants in the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). These participants were cognitively normal at enrollment, underwent annual clinical evaluations, and agreed to donate their brains.
They also assessed brain samples donated by participants in the Banner Sun Health Research Institute longitudinal study of healthy aging, Alzheimer’s, and Parkinson’s disease.
The brain samples allowed researchers to use deep brain proteomic data to help determine molecular links between depression and AD.
After quality control, the analysis included 8,356 proteins in 391 ROS/MAP participants and 7,854 proteins in 196 Banner participants.
suggesting the two conditions have a shared genetic basis.
The investigators also applied a framework called “Mendelian randomization” to determine causality between depression and AD.
After assessing the effect of 115 independent single-nucleotide polymorphisms (SNPs) from the GWAS of depression, they uncovered significant evidence “that the SNPs cause depression, which in turn cause AD,” said Dr. Wingo.
One-way relationship
The researchers conducted the same analysis on 61 significant SNPs from the GWAS of AD but did not find evidence to conclude AD causes depression.
“We found genetic evidence supporting a causal role of depression in AD but not vice versa,” Dr. Wingo said.
In addition, the investigators identified 75 brain transcripts (messenger RNA) and 28 brain proteins regulated by the depression-predisposing genetic variants. Of these, 46 brain transcripts and seven proteins were significantly associated with at least one AD feature – for example, beta-amyloid, tau tangles, and cognitive trajectory.
“These findings support the notion that the depression risk variants contribute to AD via regulating expression of their corresponding transcripts in the brain,” the investigators wrote.
It is only recently that large enough studies have allowed researchers sufficient power to reach these conclusions, coinvestigator Thomas Wingo, MD, said in an interview.
These additional “insights” into the relationship between depression and AD might “motivate” clinicians more to screen for and treat depressive symptoms, Dr. Aliza Wingo noted.
The new results also have implications for developing therapeutics to treat depression, she said. “If we target the genes, the brain proteins, that are shared risk between depression and AD, the medications that target that gene might mitigate risk for AD later on.”
However, the investigators advised caution. “A lot of this is still unknown,” said Dr. Thomas Wingo.
For example, it is not clear whether successfully treating depression mitigates the eventual risk of dementia, which is “a very important topic of inquiry and one we continue to work on,” he said, adding that a significant number of patients do not respond well to existing antidepressants such as SSRIs.
Need for further research
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach, Alzheimer’s Association, said the study contributes to the debate about whether depression increases risk for AD, whether AD increases risk for depression, or both.
“These newly published findings strengthen our understanding of the role of depression as a risk factor for Alzheimer’s dementia,” said Dr. Sexton, who was not involved with the research.
While experts do not yet fully understand the impact of treating depression on dementia risk, “the findings emphasize the importance of assessing mental health status, particularly depression, and getting it properly diagnosed and treated in a timely manner,” she said.
However, she agreed more research in this area is needed. “Importantly, these findings need replication in broader, more diverse study populations,” Dr. Sexton said.
A study funded by the Alzheimer’s Association may provide more information on the link between depression and AD. It will investigate whether machine learning, an advanced computer science technique, can better predict cognitive decline, compared with traditional methods.
Over a period of 6 months, researchers will collect smartphone conversations from 225 older adults with dementia, mild cognitive impairment, or no cognitive impairment. They will also have data from cognitive tests, brain scans, and biomarkers such as cerebrospinal fluid samples to study brain changes associated with AD.
The novel method of analysis should be able to identify subtle differences in speech quality to indicate which depressive symptoms an individual might be experiencing.
“The study could help us further understand the potential impact of depression in the risk of developing dementia,” said Dr. Sexton.
Dr. Aliza Wingo and Dr. Thomas Wingo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have known for some time that depression is associated with Alzheimer’s disease (AD), but a causal link has been elusive. Now, using newly available data, they have uncovered genetic evidence of a causal role for depression in AD.
As depression typically affects those in early or midlife and dementia often occurs in later life, “it’s fascinating to see a connection between the two brain illnesses that manifest in different time windows,” coinvestigator Aliza P. Wingo, MD, associate professor of psychiatry and behavioral science, Emory University, Atlanta, said in an interview.
“If we can treat the depression early on, we may help reduce risk for dementia for our patients later in life,” Dr. Wingo said.
The findings were published online Dec. 16, 2021, in Biological Psychiatry.
Postmortem data
The investigators, who are all from the Emory University Center for Neurodegenerative Disease, wanted to clarify the genetic basis underlying the association between the established link between depression and dementia risk.
They used data from the largest and most recent genomewide association studies (GWAS). These included a 2019 analysis of depression among 807,553 individuals and a 2019 study of AD among 455,258 individuals, all of European ancestry. For sensitivity analyses, they used results from two additional AD GWAS.
The researchers also accessed postmortem brain samples from participants in the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). These participants were cognitively normal at enrollment, underwent annual clinical evaluations, and agreed to donate their brains.
They also assessed brain samples donated by participants in the Banner Sun Health Research Institute longitudinal study of healthy aging, Alzheimer’s, and Parkinson’s disease.
The brain samples allowed researchers to use deep brain proteomic data to help determine molecular links between depression and AD.
After quality control, the analysis included 8,356 proteins in 391 ROS/MAP participants and 7,854 proteins in 196 Banner participants.
suggesting the two conditions have a shared genetic basis.
The investigators also applied a framework called “Mendelian randomization” to determine causality between depression and AD.
After assessing the effect of 115 independent single-nucleotide polymorphisms (SNPs) from the GWAS of depression, they uncovered significant evidence “that the SNPs cause depression, which in turn cause AD,” said Dr. Wingo.
One-way relationship
The researchers conducted the same analysis on 61 significant SNPs from the GWAS of AD but did not find evidence to conclude AD causes depression.
“We found genetic evidence supporting a causal role of depression in AD but not vice versa,” Dr. Wingo said.
In addition, the investigators identified 75 brain transcripts (messenger RNA) and 28 brain proteins regulated by the depression-predisposing genetic variants. Of these, 46 brain transcripts and seven proteins were significantly associated with at least one AD feature – for example, beta-amyloid, tau tangles, and cognitive trajectory.
“These findings support the notion that the depression risk variants contribute to AD via regulating expression of their corresponding transcripts in the brain,” the investigators wrote.
It is only recently that large enough studies have allowed researchers sufficient power to reach these conclusions, coinvestigator Thomas Wingo, MD, said in an interview.
These additional “insights” into the relationship between depression and AD might “motivate” clinicians more to screen for and treat depressive symptoms, Dr. Aliza Wingo noted.
The new results also have implications for developing therapeutics to treat depression, she said. “If we target the genes, the brain proteins, that are shared risk between depression and AD, the medications that target that gene might mitigate risk for AD later on.”
However, the investigators advised caution. “A lot of this is still unknown,” said Dr. Thomas Wingo.
For example, it is not clear whether successfully treating depression mitigates the eventual risk of dementia, which is “a very important topic of inquiry and one we continue to work on,” he said, adding that a significant number of patients do not respond well to existing antidepressants such as SSRIs.
Need for further research
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach, Alzheimer’s Association, said the study contributes to the debate about whether depression increases risk for AD, whether AD increases risk for depression, or both.
“These newly published findings strengthen our understanding of the role of depression as a risk factor for Alzheimer’s dementia,” said Dr. Sexton, who was not involved with the research.
While experts do not yet fully understand the impact of treating depression on dementia risk, “the findings emphasize the importance of assessing mental health status, particularly depression, and getting it properly diagnosed and treated in a timely manner,” she said.
However, she agreed more research in this area is needed. “Importantly, these findings need replication in broader, more diverse study populations,” Dr. Sexton said.
A study funded by the Alzheimer’s Association may provide more information on the link between depression and AD. It will investigate whether machine learning, an advanced computer science technique, can better predict cognitive decline, compared with traditional methods.
Over a period of 6 months, researchers will collect smartphone conversations from 225 older adults with dementia, mild cognitive impairment, or no cognitive impairment. They will also have data from cognitive tests, brain scans, and biomarkers such as cerebrospinal fluid samples to study brain changes associated with AD.
The novel method of analysis should be able to identify subtle differences in speech quality to indicate which depressive symptoms an individual might be experiencing.
“The study could help us further understand the potential impact of depression in the risk of developing dementia,” said Dr. Sexton.
Dr. Aliza Wingo and Dr. Thomas Wingo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BIOLOGICAL PSYCHIATRY
Formaldehyde exposure tied to cognitive impairment
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Last call? Moderate alcohol’s health benefits look increasingly doubtful
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
Even light physical activity linked to lower dementia risk
Older adults who participate in even light physical activity (LPA) may have a lower risk of developing dementia, new research suggests.
In a retrospective analysis of more than 62,000 individuals aged 65 or older without preexisting dementia, 6% developed dementia.
Compared with inactive individuals, “insufficiently active,” “active,” and “highly active” individuals all had a 10%, 20%, and 28% lower risk for dementia, respectively. And this association was consistent regardless of age, sex, other comorbidities, or after the researchers censored for stroke.
Even the lowest amount of LPA was associated with reduced dementia risk, investigators noted.
“In older adults, an increased physical activity level, including a low amount of LPA, was associated with a reduced risk of dementia,” Minjae Yoon, MD, division of cardiology, Severance Cardiovascular Hospital, Yonsei University, Seoul, South Korea, and colleagues wrote.
“Promotion of LPA might reduce the risk of dementia in older adults,” they added.
The findings were published online in JAMA Network Open.
Reverse causation?
Physical activity has been shown previously to be associated with reduced dementia risk. Current World Health Organization guidelines recommend that adults with normal cognition should engage in PA to reduce their risk for cognitive decline.
However, some studies have not yielded this result, “suggesting that previous findings showing a lower risk of dementia in physically active people could be attributed to reverse causation,” the investigators noted. Additionally, previous research regarding exercise intensity has been “inconsistent” concerning the role of LPA in reducing dementia risk.
Many older adults with frailty and comorbidity cannot perform intense or even moderate PA, therefore “these adults would have to gain the benefits of physical activity from LPA,” the researchers noted.
To clarify the potential association between PA and new-onset dementia, they focused specifically on the “dose-response association” between PA and dementia – especially LPA.
Between 2009 and 2012, the investigators enrolled 62,286 older individuals (60.4% women; mean age, 73.2 years) with available health checkup data from the National Health Insurance Service–Senior Database of Korea. All had no history of dementia.
Leisure-time PA was assessed with self-report questionnaires that used a 7-day recall method and included three questions regarding usual frequency (in days per week):
- Vigorous PA (VPA) for at least 20 minutes
- Moderate-intensity PA (MPA) for at least 30 minutes
- LPA for at least 30 minutes
VPA was defined as “intense exercise that caused severe shortness of breath, MPA was defined as activity causing mild shortness of breath, and LPA was defined as “walking at a slow or leisurely pace.”
PA-related energy expenditure was also calculated in metabolic equivalent (MET) minutes per week by “summing the product of frequency, intensity, and duration,” the investigators noted.
Participants were stratified on the basis of their weekly total PA levels into the following groups:
- Inactive (no LPA beyond basic movements)
- Insufficiently active (less than the recommended target range of 1-499 MET-min/wk)
- Active (meeting the recommended target range of 500-999 MET-min/wk)
- Highly active (exceeding the recommended target range of at least 1,000 MET-min/wk)
Of all participants, 35% were categorized as inactive, 25% were insufficiently active, 24.4% were active, and 15.2% were highly active.
Controversy remains
During the total median follow-up of 42 months, 6% of participants had all-cause dementia. After the researchers excluded the first 2 years, incidence of dementia was 21.6 per 1000 person-years during follow-up.
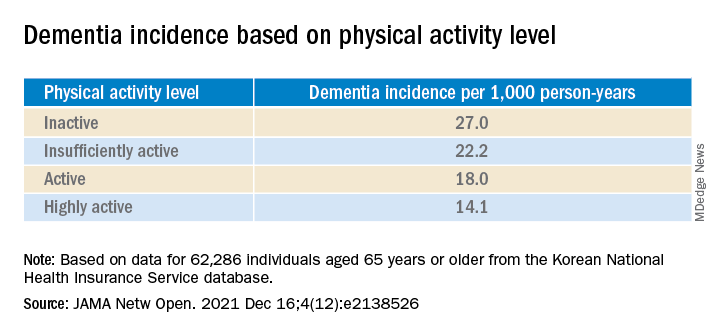
“The cumulative incidence of dementia was associated with a progressively decreasing trend with increasing physical activity” (P = .001 for trend), the investigators reported.
When using a competing-risk multivariable regression model, they found that higher levels of PA were associated with lower risk for dementia, compared with the inactive group.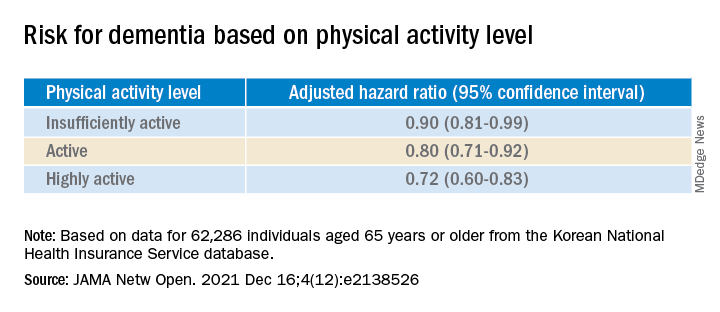
Similar findings were obtained after censoring for stroke, and were consistent for all follow-up periods. In subgroup analysis, the association between PA level and dementia risk remained consistent, regardless of age, sex, and comorbidities.
Even a low amount of LPA (1-299 MET-min/wk) was linked to reduced risk for dementia versus total sedentary behavior (adjusted HR, 0.86; 95% CI, 0.74-0.99).
The investigators noted that some “controversy” remains regarding the possibility of reverse causation and, because their study was observational in nature, “it cannot be used to establish causal relationship.”
Nevertheless, the study had important strengths, including the large number of older adults with available data, the assessment of dose-response association between PA and dementia, and the sensitivity analyses they performed, the researchers added.
Piece of important evidence
Commenting on the findings, Takashi Tarumi, PhD, senior research investigator, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, said previous studies have suggested “an inverse association between physical activity and dementia risk, such that older adults performing a higher dose of exercise may have a greater benefit for reducing the dementia risk.”
Dr. Tarumi, an associate editor at the Journal of Alzheimer’s Disease, added the current study “significantly extends our knowledge by showing that dementia risk can also be reduced by light physical activities when they are performed for longer hours.”
This provides “another piece of important evidence” to support clinicians recommending regular physical activity for the prevention of dementia in later life, said Dr. Tarumi, who was not involved with the research.
Also commenting, Martin Underwood, MD, Warwick Medical School, Coventry, England, described the association between reduced physical inactivity and dementia as well established – and noted the current study “appears to confirm earlier observational data showing this relationship.”
The current results have “still not been able to fully exclude the possibility of reverse causation,” said Dr. Underwood, who was also not associated with the study.
However, the finding that more physically active individuals are less likely to develop dementia “only becomes of real interest if we can show that increased physical activity prevents the onset, or slows the progression, of dementia,” he noted.
“To my knowledge this has not yet been established” in randomized clinical trials, Dr. Underwood added.
The study was supported by grants from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health & Welfare, Republic of Korea; and by a research grant from Yonsei University. One coauthor reported serving as a speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, and Daiichi-Sankyo, and receiving research funds from Medtronic and Abbott. No other author disclosures were reported. Dr. Tarumi and Dr. Underwood have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults who participate in even light physical activity (LPA) may have a lower risk of developing dementia, new research suggests.
In a retrospective analysis of more than 62,000 individuals aged 65 or older without preexisting dementia, 6% developed dementia.
Compared with inactive individuals, “insufficiently active,” “active,” and “highly active” individuals all had a 10%, 20%, and 28% lower risk for dementia, respectively. And this association was consistent regardless of age, sex, other comorbidities, or after the researchers censored for stroke.
Even the lowest amount of LPA was associated with reduced dementia risk, investigators noted.
“In older adults, an increased physical activity level, including a low amount of LPA, was associated with a reduced risk of dementia,” Minjae Yoon, MD, division of cardiology, Severance Cardiovascular Hospital, Yonsei University, Seoul, South Korea, and colleagues wrote.
“Promotion of LPA might reduce the risk of dementia in older adults,” they added.
The findings were published online in JAMA Network Open.
Reverse causation?
Physical activity has been shown previously to be associated with reduced dementia risk. Current World Health Organization guidelines recommend that adults with normal cognition should engage in PA to reduce their risk for cognitive decline.
However, some studies have not yielded this result, “suggesting that previous findings showing a lower risk of dementia in physically active people could be attributed to reverse causation,” the investigators noted. Additionally, previous research regarding exercise intensity has been “inconsistent” concerning the role of LPA in reducing dementia risk.
Many older adults with frailty and comorbidity cannot perform intense or even moderate PA, therefore “these adults would have to gain the benefits of physical activity from LPA,” the researchers noted.
To clarify the potential association between PA and new-onset dementia, they focused specifically on the “dose-response association” between PA and dementia – especially LPA.
Between 2009 and 2012, the investigators enrolled 62,286 older individuals (60.4% women; mean age, 73.2 years) with available health checkup data from the National Health Insurance Service–Senior Database of Korea. All had no history of dementia.
Leisure-time PA was assessed with self-report questionnaires that used a 7-day recall method and included three questions regarding usual frequency (in days per week):
- Vigorous PA (VPA) for at least 20 minutes
- Moderate-intensity PA (MPA) for at least 30 minutes
- LPA for at least 30 minutes
VPA was defined as “intense exercise that caused severe shortness of breath, MPA was defined as activity causing mild shortness of breath, and LPA was defined as “walking at a slow or leisurely pace.”
PA-related energy expenditure was also calculated in metabolic equivalent (MET) minutes per week by “summing the product of frequency, intensity, and duration,” the investigators noted.
Participants were stratified on the basis of their weekly total PA levels into the following groups:
- Inactive (no LPA beyond basic movements)
- Insufficiently active (less than the recommended target range of 1-499 MET-min/wk)
- Active (meeting the recommended target range of 500-999 MET-min/wk)
- Highly active (exceeding the recommended target range of at least 1,000 MET-min/wk)
Of all participants, 35% were categorized as inactive, 25% were insufficiently active, 24.4% were active, and 15.2% were highly active.
Controversy remains
During the total median follow-up of 42 months, 6% of participants had all-cause dementia. After the researchers excluded the first 2 years, incidence of dementia was 21.6 per 1000 person-years during follow-up.

“The cumulative incidence of dementia was associated with a progressively decreasing trend with increasing physical activity” (P = .001 for trend), the investigators reported.
When using a competing-risk multivariable regression model, they found that higher levels of PA were associated with lower risk for dementia, compared with the inactive group.
Similar findings were obtained after censoring for stroke, and were consistent for all follow-up periods. In subgroup analysis, the association between PA level and dementia risk remained consistent, regardless of age, sex, and comorbidities.
Even a low amount of LPA (1-299 MET-min/wk) was linked to reduced risk for dementia versus total sedentary behavior (adjusted HR, 0.86; 95% CI, 0.74-0.99).
The investigators noted that some “controversy” remains regarding the possibility of reverse causation and, because their study was observational in nature, “it cannot be used to establish causal relationship.”
Nevertheless, the study had important strengths, including the large number of older adults with available data, the assessment of dose-response association between PA and dementia, and the sensitivity analyses they performed, the researchers added.
Piece of important evidence
Commenting on the findings, Takashi Tarumi, PhD, senior research investigator, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, said previous studies have suggested “an inverse association between physical activity and dementia risk, such that older adults performing a higher dose of exercise may have a greater benefit for reducing the dementia risk.”
Dr. Tarumi, an associate editor at the Journal of Alzheimer’s Disease, added the current study “significantly extends our knowledge by showing that dementia risk can also be reduced by light physical activities when they are performed for longer hours.”
This provides “another piece of important evidence” to support clinicians recommending regular physical activity for the prevention of dementia in later life, said Dr. Tarumi, who was not involved with the research.
Also commenting, Martin Underwood, MD, Warwick Medical School, Coventry, England, described the association between reduced physical inactivity and dementia as well established – and noted the current study “appears to confirm earlier observational data showing this relationship.”
The current results have “still not been able to fully exclude the possibility of reverse causation,” said Dr. Underwood, who was also not associated with the study.
However, the finding that more physically active individuals are less likely to develop dementia “only becomes of real interest if we can show that increased physical activity prevents the onset, or slows the progression, of dementia,” he noted.
“To my knowledge this has not yet been established” in randomized clinical trials, Dr. Underwood added.
The study was supported by grants from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health & Welfare, Republic of Korea; and by a research grant from Yonsei University. One coauthor reported serving as a speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, and Daiichi-Sankyo, and receiving research funds from Medtronic and Abbott. No other author disclosures were reported. Dr. Tarumi and Dr. Underwood have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults who participate in even light physical activity (LPA) may have a lower risk of developing dementia, new research suggests.
In a retrospective analysis of more than 62,000 individuals aged 65 or older without preexisting dementia, 6% developed dementia.
Compared with inactive individuals, “insufficiently active,” “active,” and “highly active” individuals all had a 10%, 20%, and 28% lower risk for dementia, respectively. And this association was consistent regardless of age, sex, other comorbidities, or after the researchers censored for stroke.
Even the lowest amount of LPA was associated with reduced dementia risk, investigators noted.
“In older adults, an increased physical activity level, including a low amount of LPA, was associated with a reduced risk of dementia,” Minjae Yoon, MD, division of cardiology, Severance Cardiovascular Hospital, Yonsei University, Seoul, South Korea, and colleagues wrote.
“Promotion of LPA might reduce the risk of dementia in older adults,” they added.
The findings were published online in JAMA Network Open.
Reverse causation?
Physical activity has been shown previously to be associated with reduced dementia risk. Current World Health Organization guidelines recommend that adults with normal cognition should engage in PA to reduce their risk for cognitive decline.
However, some studies have not yielded this result, “suggesting that previous findings showing a lower risk of dementia in physically active people could be attributed to reverse causation,” the investigators noted. Additionally, previous research regarding exercise intensity has been “inconsistent” concerning the role of LPA in reducing dementia risk.
Many older adults with frailty and comorbidity cannot perform intense or even moderate PA, therefore “these adults would have to gain the benefits of physical activity from LPA,” the researchers noted.
To clarify the potential association between PA and new-onset dementia, they focused specifically on the “dose-response association” between PA and dementia – especially LPA.
Between 2009 and 2012, the investigators enrolled 62,286 older individuals (60.4% women; mean age, 73.2 years) with available health checkup data from the National Health Insurance Service–Senior Database of Korea. All had no history of dementia.
Leisure-time PA was assessed with self-report questionnaires that used a 7-day recall method and included three questions regarding usual frequency (in days per week):
- Vigorous PA (VPA) for at least 20 minutes
- Moderate-intensity PA (MPA) for at least 30 minutes
- LPA for at least 30 minutes
VPA was defined as “intense exercise that caused severe shortness of breath, MPA was defined as activity causing mild shortness of breath, and LPA was defined as “walking at a slow or leisurely pace.”
PA-related energy expenditure was also calculated in metabolic equivalent (MET) minutes per week by “summing the product of frequency, intensity, and duration,” the investigators noted.
Participants were stratified on the basis of their weekly total PA levels into the following groups:
- Inactive (no LPA beyond basic movements)
- Insufficiently active (less than the recommended target range of 1-499 MET-min/wk)
- Active (meeting the recommended target range of 500-999 MET-min/wk)
- Highly active (exceeding the recommended target range of at least 1,000 MET-min/wk)
Of all participants, 35% were categorized as inactive, 25% were insufficiently active, 24.4% were active, and 15.2% were highly active.
Controversy remains
During the total median follow-up of 42 months, 6% of participants had all-cause dementia. After the researchers excluded the first 2 years, incidence of dementia was 21.6 per 1000 person-years during follow-up.

“The cumulative incidence of dementia was associated with a progressively decreasing trend with increasing physical activity” (P = .001 for trend), the investigators reported.
When using a competing-risk multivariable regression model, they found that higher levels of PA were associated with lower risk for dementia, compared with the inactive group.
Similar findings were obtained after censoring for stroke, and were consistent for all follow-up periods. In subgroup analysis, the association between PA level and dementia risk remained consistent, regardless of age, sex, and comorbidities.
Even a low amount of LPA (1-299 MET-min/wk) was linked to reduced risk for dementia versus total sedentary behavior (adjusted HR, 0.86; 95% CI, 0.74-0.99).
The investigators noted that some “controversy” remains regarding the possibility of reverse causation and, because their study was observational in nature, “it cannot be used to establish causal relationship.”
Nevertheless, the study had important strengths, including the large number of older adults with available data, the assessment of dose-response association between PA and dementia, and the sensitivity analyses they performed, the researchers added.
Piece of important evidence
Commenting on the findings, Takashi Tarumi, PhD, senior research investigator, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, said previous studies have suggested “an inverse association between physical activity and dementia risk, such that older adults performing a higher dose of exercise may have a greater benefit for reducing the dementia risk.”
Dr. Tarumi, an associate editor at the Journal of Alzheimer’s Disease, added the current study “significantly extends our knowledge by showing that dementia risk can also be reduced by light physical activities when they are performed for longer hours.”
This provides “another piece of important evidence” to support clinicians recommending regular physical activity for the prevention of dementia in later life, said Dr. Tarumi, who was not involved with the research.
Also commenting, Martin Underwood, MD, Warwick Medical School, Coventry, England, described the association between reduced physical inactivity and dementia as well established – and noted the current study “appears to confirm earlier observational data showing this relationship.”
The current results have “still not been able to fully exclude the possibility of reverse causation,” said Dr. Underwood, who was also not associated with the study.
However, the finding that more physically active individuals are less likely to develop dementia “only becomes of real interest if we can show that increased physical activity prevents the onset, or slows the progression, of dementia,” he noted.
“To my knowledge this has not yet been established” in randomized clinical trials, Dr. Underwood added.
The study was supported by grants from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health & Welfare, Republic of Korea; and by a research grant from Yonsei University. One coauthor reported serving as a speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, and Daiichi-Sankyo, and receiving research funds from Medtronic and Abbott. No other author disclosures were reported. Dr. Tarumi and Dr. Underwood have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Califf plans work on opioids, accelerated approvals on return to FDA
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
When the benchwarmer is a slugger
I still, on occasion, use Felbatol (felbamate).
Thirty years since its explosive entrance to the market, then even more explosive collapse, it remains, in my opinion, the most effective of the second generation of anti-seizure medications. Arguably, even more effective than any of the third generation, too.
That’s not to say I use a lot of it. I don’t. It’s like handling unstable dynamite. Tremendous power, but also an above-average degree of risk. Even after things hit the fan with it in the mid-90s, I remember one of my epilepsy clinic attendings telling me, “This is a home-run drug. In refractory patients you might see some benefit by adding another agent, but with this one, you could stop their seizures and hit it out of the park.”
Like most neurologists, I use other epilepsy options first and second line. But sometimes you get the patient who’s failed the usual ones. Then I start to think about Felbatol. I explain the situation to the patients and their families and let them make the final decision. I worry and watch labs very closely for a while. I probably have no more than three to five patients on it in the practice. But when it works, it’s amazing stuff.
Now, let’s jump ahead to 2021. The year of Aduhelm (and several similar agents racing up behind it).
None of these drugs are even close to hitting home runs. For that matter, I’m not convinced they’re even able to get a man on base. To stretch my baseball analogy a bit, imagine watching a game by looking only at the RBI and ERA stats changing. The numbers change slightly, but you have no evidence that either team is winning. Which is, after all, the whole point.
And, to some extent, that’s the basis of Aduhelm’s approval, and likely the same standards its competitors will be held to.
Although they treat different conditions, and are chemically unrelated, the similarities between Felbatol and the currently advancing bunch of monoclonal antibody (MAB) agents for Alzheimer’s disease make an interesting contrast.
Unlike Felbatol’s proven efficacy for epilepsy, the current MABs offer minimal statistically significant clinical benefit for Alzheimer’s disease. At the same time the risk of amyloid-related imaging abnormalities (ARIA) and its complications with them is significantly higher than that of either of Felbatol’s known, potentially lethal, idiosyncratic effects.
With those odds, In medicine, every day is an exercise in working through the risks and benefits of each patient’s individual situation.
As I’ve stated before, I’m not in the grandstand rooting for these Alzheimer’s drugs to fail. I’ve lost a few family members, and certainly my share of patients, to dementia. I’d be thrilled, and more than willing to prescribe it, if something truly effective came along for it.
Nor do I take any kind of pleasure in the recent news that, because of Aduhelm’s failings, around 1,000 Biogen employees will lose their jobs. I feel terrible for them, as most had nothing to do with the decision to forge ahead with the product. More may soon follow at other companies working with similar agents.
Here we are, though, going into 2022. I’m still, albeit rarely, writing for Felbatol 30 years after it came to market for one reason: It works. But it seems pretty unlikely that future neurologists in 2052 will say the same about the current crops of MABs for Alzheimer’s disease.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I still, on occasion, use Felbatol (felbamate).
Thirty years since its explosive entrance to the market, then even more explosive collapse, it remains, in my opinion, the most effective of the second generation of anti-seizure medications. Arguably, even more effective than any of the third generation, too.
That’s not to say I use a lot of it. I don’t. It’s like handling unstable dynamite. Tremendous power, but also an above-average degree of risk. Even after things hit the fan with it in the mid-90s, I remember one of my epilepsy clinic attendings telling me, “This is a home-run drug. In refractory patients you might see some benefit by adding another agent, but with this one, you could stop their seizures and hit it out of the park.”
Like most neurologists, I use other epilepsy options first and second line. But sometimes you get the patient who’s failed the usual ones. Then I start to think about Felbatol. I explain the situation to the patients and their families and let them make the final decision. I worry and watch labs very closely for a while. I probably have no more than three to five patients on it in the practice. But when it works, it’s amazing stuff.
Now, let’s jump ahead to 2021. The year of Aduhelm (and several similar agents racing up behind it).
None of these drugs are even close to hitting home runs. For that matter, I’m not convinced they’re even able to get a man on base. To stretch my baseball analogy a bit, imagine watching a game by looking only at the RBI and ERA stats changing. The numbers change slightly, but you have no evidence that either team is winning. Which is, after all, the whole point.
And, to some extent, that’s the basis of Aduhelm’s approval, and likely the same standards its competitors will be held to.
Although they treat different conditions, and are chemically unrelated, the similarities between Felbatol and the currently advancing bunch of monoclonal antibody (MAB) agents for Alzheimer’s disease make an interesting contrast.
Unlike Felbatol’s proven efficacy for epilepsy, the current MABs offer minimal statistically significant clinical benefit for Alzheimer’s disease. At the same time the risk of amyloid-related imaging abnormalities (ARIA) and its complications with them is significantly higher than that of either of Felbatol’s known, potentially lethal, idiosyncratic effects.
With those odds, In medicine, every day is an exercise in working through the risks and benefits of each patient’s individual situation.
As I’ve stated before, I’m not in the grandstand rooting for these Alzheimer’s drugs to fail. I’ve lost a few family members, and certainly my share of patients, to dementia. I’d be thrilled, and more than willing to prescribe it, if something truly effective came along for it.
Nor do I take any kind of pleasure in the recent news that, because of Aduhelm’s failings, around 1,000 Biogen employees will lose their jobs. I feel terrible for them, as most had nothing to do with the decision to forge ahead with the product. More may soon follow at other companies working with similar agents.
Here we are, though, going into 2022. I’m still, albeit rarely, writing for Felbatol 30 years after it came to market for one reason: It works. But it seems pretty unlikely that future neurologists in 2052 will say the same about the current crops of MABs for Alzheimer’s disease.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I still, on occasion, use Felbatol (felbamate).
Thirty years since its explosive entrance to the market, then even more explosive collapse, it remains, in my opinion, the most effective of the second generation of anti-seizure medications. Arguably, even more effective than any of the third generation, too.
That’s not to say I use a lot of it. I don’t. It’s like handling unstable dynamite. Tremendous power, but also an above-average degree of risk. Even after things hit the fan with it in the mid-90s, I remember one of my epilepsy clinic attendings telling me, “This is a home-run drug. In refractory patients you might see some benefit by adding another agent, but with this one, you could stop their seizures and hit it out of the park.”
Like most neurologists, I use other epilepsy options first and second line. But sometimes you get the patient who’s failed the usual ones. Then I start to think about Felbatol. I explain the situation to the patients and their families and let them make the final decision. I worry and watch labs very closely for a while. I probably have no more than three to five patients on it in the practice. But when it works, it’s amazing stuff.
Now, let’s jump ahead to 2021. The year of Aduhelm (and several similar agents racing up behind it).
None of these drugs are even close to hitting home runs. For that matter, I’m not convinced they’re even able to get a man on base. To stretch my baseball analogy a bit, imagine watching a game by looking only at the RBI and ERA stats changing. The numbers change slightly, but you have no evidence that either team is winning. Which is, after all, the whole point.
And, to some extent, that’s the basis of Aduhelm’s approval, and likely the same standards its competitors will be held to.
Although they treat different conditions, and are chemically unrelated, the similarities between Felbatol and the currently advancing bunch of monoclonal antibody (MAB) agents for Alzheimer’s disease make an interesting contrast.
Unlike Felbatol’s proven efficacy for epilepsy, the current MABs offer minimal statistically significant clinical benefit for Alzheimer’s disease. At the same time the risk of amyloid-related imaging abnormalities (ARIA) and its complications with them is significantly higher than that of either of Felbatol’s known, potentially lethal, idiosyncratic effects.
With those odds, In medicine, every day is an exercise in working through the risks and benefits of each patient’s individual situation.
As I’ve stated before, I’m not in the grandstand rooting for these Alzheimer’s drugs to fail. I’ve lost a few family members, and certainly my share of patients, to dementia. I’d be thrilled, and more than willing to prescribe it, if something truly effective came along for it.
Nor do I take any kind of pleasure in the recent news that, because of Aduhelm’s failings, around 1,000 Biogen employees will lose their jobs. I feel terrible for them, as most had nothing to do with the decision to forge ahead with the product. More may soon follow at other companies working with similar agents.
Here we are, though, going into 2022. I’m still, albeit rarely, writing for Felbatol 30 years after it came to market for one reason: It works. But it seems pretty unlikely that future neurologists in 2052 will say the same about the current crops of MABs for Alzheimer’s disease.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A very strange place to find a tooth
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
Could Viagra help prevent Alzheimer’s?
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
FROM NATURE AGING
Higher resting heart rate tied to increased dementia risk
independent of the presence of cardiovascular disease (CVD) risk factors, new research shows.
“RHR is easy to measure and might be used to identify older people potentially at high risk of dementia and cognitive decline for early interventions,” Yume Imahori, MD, PhD, with the Aging Research Center, Karolinska Institutet, Stockholm, said in an interview.
“Health care professionals should be aware of potential cognitive consequences associated with elevated RHR in older people and may advise older people with high RHR to have a follow-up assessment of cognitive function,” Dr. Imahori said.
The study was published online Dec. 3, 2021, in Alzheimer’s & Dementia.
Heart-brain connection
The findings are based on 2,147 adults (62% women) aged 60 years and older (mean age, 70.6 years) from the population-based Swedish National Aging and Care in Kungsholmen (SNAC-K) study. All were free of dementia at baseline and were followed regularly from 2001-2004 to 2013-2016.
The average RHR at baseline was 65.7 bpm. Individuals in higher RHR groups were older, less educated, and were more likely to be smokers and sedentary and to have hypertension. There were no differences among RHR groups in the prevalence of CVD at baseline.
During a median follow-up of 11.4 years, 289 participants were diagnosed with dementia.
In the fully adjusted model, participants with RHR of 80 bpm or higher had a 55% increased risk of developing dementia, compared with peers with lower RHR of 60 to 69 bpm (hazard ratio, 1.55; 95% CI, 1.06-2.27).
“This association was not due to underlying cardiovascular diseases such as atrial fibrillation and heart failure, which is important because elevated RHR is often related to heart disease,” Dr. Imahori said in an interview.
Regarding cognitive function, Mini-Mental State Examination scores declined over time during the follow-up period in all RHR groups, but participants with RHR 70-79 and 80+ bpm had a greater decline, compared with those with lower RHR of 60-69 bpm.
Dr. Imahori said these findings are in line with data from the U.S. Atherosclerosis Risk in Communities study linking elevated RHR of 80+ bpm in midlife to dementia and cognitive decline in late life.
Public health implications
Reached for comment, Claire Sexton, DPhil, Alzheimer’s Association director of scientific programs and outreach, said this study adds to the “growing body of research showing the health of the heart and brain are closely connected. However, this study only shows a correlation between resting heart rate and cognition, not causation. More research is needed.
“Evidence shows that other risk factors for cardiovascular disease and stroke – obesity, high blood pressure, and diabetes – negatively impact your cognitive health,” Dr. Sexton said in an interview.
“The Alzheimer’s Association believes the conversation about heart health management is something everyone should be having with their doctor,” she said.
“There are things you can do today to lower your risk for cardiovascular disease, including regular exercise and maintaining a healthy diet. Improving your heart health is an important step to maintaining your brain health as you age,” Dr. Sexton added.
SNAC-K is supported by the Swedish Ministry of Health and Social Affairs and the participating county councils and municipalities and in part by additional grants from the Swedish Research Council and the Swedish Research Council for Health, Working Life and Welfare. Dr. Imahori and Dr. Sexton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
independent of the presence of cardiovascular disease (CVD) risk factors, new research shows.
“RHR is easy to measure and might be used to identify older people potentially at high risk of dementia and cognitive decline for early interventions,” Yume Imahori, MD, PhD, with the Aging Research Center, Karolinska Institutet, Stockholm, said in an interview.
“Health care professionals should be aware of potential cognitive consequences associated with elevated RHR in older people and may advise older people with high RHR to have a follow-up assessment of cognitive function,” Dr. Imahori said.
The study was published online Dec. 3, 2021, in Alzheimer’s & Dementia.
Heart-brain connection
The findings are based on 2,147 adults (62% women) aged 60 years and older (mean age, 70.6 years) from the population-based Swedish National Aging and Care in Kungsholmen (SNAC-K) study. All were free of dementia at baseline and were followed regularly from 2001-2004 to 2013-2016.
The average RHR at baseline was 65.7 bpm. Individuals in higher RHR groups were older, less educated, and were more likely to be smokers and sedentary and to have hypertension. There were no differences among RHR groups in the prevalence of CVD at baseline.
During a median follow-up of 11.4 years, 289 participants were diagnosed with dementia.
In the fully adjusted model, participants with RHR of 80 bpm or higher had a 55% increased risk of developing dementia, compared with peers with lower RHR of 60 to 69 bpm (hazard ratio, 1.55; 95% CI, 1.06-2.27).
“This association was not due to underlying cardiovascular diseases such as atrial fibrillation and heart failure, which is important because elevated RHR is often related to heart disease,” Dr. Imahori said in an interview.
Regarding cognitive function, Mini-Mental State Examination scores declined over time during the follow-up period in all RHR groups, but participants with RHR 70-79 and 80+ bpm had a greater decline, compared with those with lower RHR of 60-69 bpm.
Dr. Imahori said these findings are in line with data from the U.S. Atherosclerosis Risk in Communities study linking elevated RHR of 80+ bpm in midlife to dementia and cognitive decline in late life.
Public health implications
Reached for comment, Claire Sexton, DPhil, Alzheimer’s Association director of scientific programs and outreach, said this study adds to the “growing body of research showing the health of the heart and brain are closely connected. However, this study only shows a correlation between resting heart rate and cognition, not causation. More research is needed.
“Evidence shows that other risk factors for cardiovascular disease and stroke – obesity, high blood pressure, and diabetes – negatively impact your cognitive health,” Dr. Sexton said in an interview.
“The Alzheimer’s Association believes the conversation about heart health management is something everyone should be having with their doctor,” she said.
“There are things you can do today to lower your risk for cardiovascular disease, including regular exercise and maintaining a healthy diet. Improving your heart health is an important step to maintaining your brain health as you age,” Dr. Sexton added.
SNAC-K is supported by the Swedish Ministry of Health and Social Affairs and the participating county councils and municipalities and in part by additional grants from the Swedish Research Council and the Swedish Research Council for Health, Working Life and Welfare. Dr. Imahori and Dr. Sexton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
independent of the presence of cardiovascular disease (CVD) risk factors, new research shows.
“RHR is easy to measure and might be used to identify older people potentially at high risk of dementia and cognitive decline for early interventions,” Yume Imahori, MD, PhD, with the Aging Research Center, Karolinska Institutet, Stockholm, said in an interview.
“Health care professionals should be aware of potential cognitive consequences associated with elevated RHR in older people and may advise older people with high RHR to have a follow-up assessment of cognitive function,” Dr. Imahori said.
The study was published online Dec. 3, 2021, in Alzheimer’s & Dementia.
Heart-brain connection
The findings are based on 2,147 adults (62% women) aged 60 years and older (mean age, 70.6 years) from the population-based Swedish National Aging and Care in Kungsholmen (SNAC-K) study. All were free of dementia at baseline and were followed regularly from 2001-2004 to 2013-2016.
The average RHR at baseline was 65.7 bpm. Individuals in higher RHR groups were older, less educated, and were more likely to be smokers and sedentary and to have hypertension. There were no differences among RHR groups in the prevalence of CVD at baseline.
During a median follow-up of 11.4 years, 289 participants were diagnosed with dementia.
In the fully adjusted model, participants with RHR of 80 bpm or higher had a 55% increased risk of developing dementia, compared with peers with lower RHR of 60 to 69 bpm (hazard ratio, 1.55; 95% CI, 1.06-2.27).
“This association was not due to underlying cardiovascular diseases such as atrial fibrillation and heart failure, which is important because elevated RHR is often related to heart disease,” Dr. Imahori said in an interview.
Regarding cognitive function, Mini-Mental State Examination scores declined over time during the follow-up period in all RHR groups, but participants with RHR 70-79 and 80+ bpm had a greater decline, compared with those with lower RHR of 60-69 bpm.
Dr. Imahori said these findings are in line with data from the U.S. Atherosclerosis Risk in Communities study linking elevated RHR of 80+ bpm in midlife to dementia and cognitive decline in late life.
Public health implications
Reached for comment, Claire Sexton, DPhil, Alzheimer’s Association director of scientific programs and outreach, said this study adds to the “growing body of research showing the health of the heart and brain are closely connected. However, this study only shows a correlation between resting heart rate and cognition, not causation. More research is needed.
“Evidence shows that other risk factors for cardiovascular disease and stroke – obesity, high blood pressure, and diabetes – negatively impact your cognitive health,” Dr. Sexton said in an interview.
“The Alzheimer’s Association believes the conversation about heart health management is something everyone should be having with their doctor,” she said.
“There are things you can do today to lower your risk for cardiovascular disease, including regular exercise and maintaining a healthy diet. Improving your heart health is an important step to maintaining your brain health as you age,” Dr. Sexton added.
SNAC-K is supported by the Swedish Ministry of Health and Social Affairs and the participating county councils and municipalities and in part by additional grants from the Swedish Research Council and the Swedish Research Council for Health, Working Life and Welfare. Dr. Imahori and Dr. Sexton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA