User login
Similar brain atrophy in obesity and Alzheimer’s disease
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Can a ‘smart’ skin patch detect early neurodegenerative diseases?
A new “smart patch” composed of microneedles that can detect proinflammatory markers via simulated skin interstitial fluid (ISF) may help diagnose neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease very early on.
Originally developed to deliver medications and vaccines via the skin in a minimally invasive manner, the microneedle arrays were fitted with molecular sensors that, when placed on the skin, detect neuroinflammatory biomarkers such as interleukin-6 in as little as 6 minutes.
The literature suggests that these biomarkers of neurodegenerative disease are present years before patients become symptomatic, said study investigator Sanjiv Sharma, PhD.
“Neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease are [characterized by] progressive loss in nerve cell and brain cells, which leads to memory problems and a loss of mental ability. That is why early diagnosis is key to preventing the loss of brain tissue in dementia, which can go undetected for years,” added Dr. Sharma, who is a lecturer in medical engineering at Swansea (Wales) University.
Dr. Sharma developed the patch with scientists at the Polytechnic of Porto (Portugal) School of Engineering in Portugal. In 2022, they designed, and are currently testing, a microneedle patch that will deliver the COVID vaccine.
The investigators describe their research on the patch’s ability to detect IL-6 in an article published in ACS Omega.
At-home diagnosis?
“The skin is the largest organ in the body – it contains more skin interstitial fluid than the total blood volume,” Dr. Sharma noted. “This fluid is an ultrafiltrate of blood and holds biomarkers that complement other biofluids, such as sweat, saliva, and urine. It can be sampled in a minimally invasive manner and used either for point-of-care testing or real-time using microneedle devices.”
Dr. Sharma and associates tested the microneedle patch in artificial ISF that contained the inflammatory cytokine IL-6. They found that the patch accurately detected IL-6 concentrations as low as 1 pg/mL in the fabricated ISF solution.
“In general, the transdermal sensor presented here showed simplicity in designing, short measuring time, high accuracy, and low detection limit. This approach seems a successful tool for the screening of inflammatory biomarkers in point of care testing wherein the skin acts as a window to the body,” the investigators reported.
Dr. Sharma noted that early detection of neurodegenerative diseases is crucial, as once symptoms appear, the disease may have already progressed significantly, and meaningful intervention is challenging.
The device has yet to be tested in humans, which is the next step, said Dr. Sharma.
“We will have to test the hypothesis through extensive preclinical and clinical studies to determine if bloodless, transdermal (skin) diagnostics can offer a cost-effective device that could allow testing in simpler settings such as a clinician’s practice or even home settings,” he noted.
Early days
Commenting on the research, David K. Simon, MD, PhD, professor of neurology at Harvard Medical School, Boston, said it is “a promising step regarding validation of a potentially beneficial method for rapidly and accurately measuring IL-6.”
However, he added, “many additional steps are needed to validate the method in actual human skin and to determine whether or not measuring these biomarkers in skin will be useful in studies of neurodegenerative diseases.”
He noted that one study limitation is that inflammatory cytokines such as IL-6 are highly nonspecific, and levels are elevated in various diseases associated with inflammation.
“It is highly unlikely that measuring IL-6 will be useful as a diagnostic tool. However, it does have potential as a biomarker for measuring the impact of treatments aimed at reducing inflammation. As the authors point out, it’s more likely that clinicians will require a panel of biomarkers rather than only measuring IL-6,” he said.
The study was funded by Fundação para a Ciência e Tecnologia. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new “smart patch” composed of microneedles that can detect proinflammatory markers via simulated skin interstitial fluid (ISF) may help diagnose neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease very early on.
Originally developed to deliver medications and vaccines via the skin in a minimally invasive manner, the microneedle arrays were fitted with molecular sensors that, when placed on the skin, detect neuroinflammatory biomarkers such as interleukin-6 in as little as 6 minutes.
The literature suggests that these biomarkers of neurodegenerative disease are present years before patients become symptomatic, said study investigator Sanjiv Sharma, PhD.
“Neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease are [characterized by] progressive loss in nerve cell and brain cells, which leads to memory problems and a loss of mental ability. That is why early diagnosis is key to preventing the loss of brain tissue in dementia, which can go undetected for years,” added Dr. Sharma, who is a lecturer in medical engineering at Swansea (Wales) University.
Dr. Sharma developed the patch with scientists at the Polytechnic of Porto (Portugal) School of Engineering in Portugal. In 2022, they designed, and are currently testing, a microneedle patch that will deliver the COVID vaccine.
The investigators describe their research on the patch’s ability to detect IL-6 in an article published in ACS Omega.
At-home diagnosis?
“The skin is the largest organ in the body – it contains more skin interstitial fluid than the total blood volume,” Dr. Sharma noted. “This fluid is an ultrafiltrate of blood and holds biomarkers that complement other biofluids, such as sweat, saliva, and urine. It can be sampled in a minimally invasive manner and used either for point-of-care testing or real-time using microneedle devices.”
Dr. Sharma and associates tested the microneedle patch in artificial ISF that contained the inflammatory cytokine IL-6. They found that the patch accurately detected IL-6 concentrations as low as 1 pg/mL in the fabricated ISF solution.
“In general, the transdermal sensor presented here showed simplicity in designing, short measuring time, high accuracy, and low detection limit. This approach seems a successful tool for the screening of inflammatory biomarkers in point of care testing wherein the skin acts as a window to the body,” the investigators reported.
Dr. Sharma noted that early detection of neurodegenerative diseases is crucial, as once symptoms appear, the disease may have already progressed significantly, and meaningful intervention is challenging.
The device has yet to be tested in humans, which is the next step, said Dr. Sharma.
“We will have to test the hypothesis through extensive preclinical and clinical studies to determine if bloodless, transdermal (skin) diagnostics can offer a cost-effective device that could allow testing in simpler settings such as a clinician’s practice or even home settings,” he noted.
Early days
Commenting on the research, David K. Simon, MD, PhD, professor of neurology at Harvard Medical School, Boston, said it is “a promising step regarding validation of a potentially beneficial method for rapidly and accurately measuring IL-6.”
However, he added, “many additional steps are needed to validate the method in actual human skin and to determine whether or not measuring these biomarkers in skin will be useful in studies of neurodegenerative diseases.”
He noted that one study limitation is that inflammatory cytokines such as IL-6 are highly nonspecific, and levels are elevated in various diseases associated with inflammation.
“It is highly unlikely that measuring IL-6 will be useful as a diagnostic tool. However, it does have potential as a biomarker for measuring the impact of treatments aimed at reducing inflammation. As the authors point out, it’s more likely that clinicians will require a panel of biomarkers rather than only measuring IL-6,” he said.
The study was funded by Fundação para a Ciência e Tecnologia. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new “smart patch” composed of microneedles that can detect proinflammatory markers via simulated skin interstitial fluid (ISF) may help diagnose neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease very early on.
Originally developed to deliver medications and vaccines via the skin in a minimally invasive manner, the microneedle arrays were fitted with molecular sensors that, when placed on the skin, detect neuroinflammatory biomarkers such as interleukin-6 in as little as 6 minutes.
The literature suggests that these biomarkers of neurodegenerative disease are present years before patients become symptomatic, said study investigator Sanjiv Sharma, PhD.
“Neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease are [characterized by] progressive loss in nerve cell and brain cells, which leads to memory problems and a loss of mental ability. That is why early diagnosis is key to preventing the loss of brain tissue in dementia, which can go undetected for years,” added Dr. Sharma, who is a lecturer in medical engineering at Swansea (Wales) University.
Dr. Sharma developed the patch with scientists at the Polytechnic of Porto (Portugal) School of Engineering in Portugal. In 2022, they designed, and are currently testing, a microneedle patch that will deliver the COVID vaccine.
The investigators describe their research on the patch’s ability to detect IL-6 in an article published in ACS Omega.
At-home diagnosis?
“The skin is the largest organ in the body – it contains more skin interstitial fluid than the total blood volume,” Dr. Sharma noted. “This fluid is an ultrafiltrate of blood and holds biomarkers that complement other biofluids, such as sweat, saliva, and urine. It can be sampled in a minimally invasive manner and used either for point-of-care testing or real-time using microneedle devices.”
Dr. Sharma and associates tested the microneedle patch in artificial ISF that contained the inflammatory cytokine IL-6. They found that the patch accurately detected IL-6 concentrations as low as 1 pg/mL in the fabricated ISF solution.
“In general, the transdermal sensor presented here showed simplicity in designing, short measuring time, high accuracy, and low detection limit. This approach seems a successful tool for the screening of inflammatory biomarkers in point of care testing wherein the skin acts as a window to the body,” the investigators reported.
Dr. Sharma noted that early detection of neurodegenerative diseases is crucial, as once symptoms appear, the disease may have already progressed significantly, and meaningful intervention is challenging.
The device has yet to be tested in humans, which is the next step, said Dr. Sharma.
“We will have to test the hypothesis through extensive preclinical and clinical studies to determine if bloodless, transdermal (skin) diagnostics can offer a cost-effective device that could allow testing in simpler settings such as a clinician’s practice or even home settings,” he noted.
Early days
Commenting on the research, David K. Simon, MD, PhD, professor of neurology at Harvard Medical School, Boston, said it is “a promising step regarding validation of a potentially beneficial method for rapidly and accurately measuring IL-6.”
However, he added, “many additional steps are needed to validate the method in actual human skin and to determine whether or not measuring these biomarkers in skin will be useful in studies of neurodegenerative diseases.”
He noted that one study limitation is that inflammatory cytokines such as IL-6 are highly nonspecific, and levels are elevated in various diseases associated with inflammation.
“It is highly unlikely that measuring IL-6 will be useful as a diagnostic tool. However, it does have potential as a biomarker for measuring the impact of treatments aimed at reducing inflammation. As the authors point out, it’s more likely that clinicians will require a panel of biomarkers rather than only measuring IL-6,” he said.
The study was funded by Fundação para a Ciência e Tecnologia. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACS OMEGA
Six healthy lifestyle habits linked to slowed memory decline
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.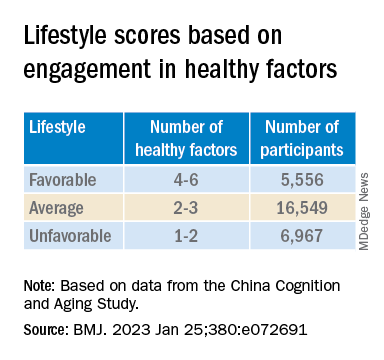
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.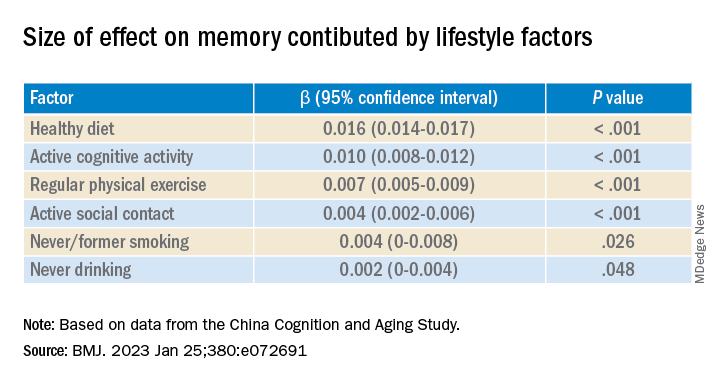
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Nine more minutes a day of vigorous exercise tied to better cognition
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF EPIDEMIOLOGY AND COMMUNITY HEALTH
Geriatrician advises on use of vitamin D supplementation, lecanemab, and texting for her patients
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Kids with concussions may benefit from early return to school
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Social isolation hikes dementia risk in older adults
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HRT may prevent Alzheimer’s in high-risk women
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S RESEARCH AND THERAPY
Nearly 50% of patients with dementia experience falls
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
Hearing loss strongly tied to increased dementia risk
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.



