User login
Platelet-rich plasma for hair regrowth requires art and science
or administer the highly technique-dependent treatment, which creates plenty of room for suboptimal results, according to several experts at the annual meeting of the American Academy of Dermatology.
“The process is the product,” emphasized Terrence Keaney, MD, clinical associate professor at George Washington University, Washington, as well as cofounder of SkinDC, a private practice in Arlington, Va. He characterized PRP as a “growth factor cytokine cocktail,” for which relative benefits are fully dependent on the ingredients.
In other words, the efficacy of PRP is mostly dependent on the multiple steps in which blood drawn from a patient is separated into its components, processed to create a platelet-rich product, and then administered to the patient by injection or in conjunction with microneedles. While the goal is a platelet concentration two- to fivefold greater than that found in whole blood, this is not as straightforward as it sounds.
Many PRP device kits available
“There are a ton of [centrifuge] devices on the market and a lot of differences in the methodology in optimizing the platelet concentration,” Dr. Keaney explained. In addition, there are numerous proprietary collection tubes using different types of anticoagulants and different separator gels that also play a role in the goal of optimizing a platelet-rich and readily activated product.
“Recognize that each step in the preparation of PRP introduces a source of variation that affects the composition and efficacy of the final product,” said Steven Krueger, MD, who is completing his residency in dermatology at the University of Massachusetts, Worcester, but who has become an expert in the field. He contributed a chapter on this topic in the recently published book, Aesthetic Clinician’s Guide to Platelet Rich Plasma.
The importance of technique is reflected in inconsistent results from published controlled trials. Unfortunately, the authors of many studies have failed to provide details of their protocol. Ultimately, Dr. Krueger said this lack of clarity among available protocols has created a serious obstacle for establishing which steps are important and how to move the field forward.
Dr. Keaney agreed. Because of the frequent lack of details about how PRP was processed in available studies, the effort to draw conclusions about the experiences at different centers is like “comparing apples to oranges.”
“What is the ideal dose and concentrate? We don’t know,” Dr. Keaney said.
The first centrifuge device to receive regulatory approval was developed for orthopedic indications more than 20 years ago. There are now at least 20 centrifuge devices with 510K Food and Drug Administration clearance for separating blood components to produce PRP. The 510K designation means that they are “substantially equivalent” to an already approved device, but Dr. Krueger cautioned that their use in preparing PRP for treatment of hair loss remains off label.
Substandard devices are marketed
In the rapidly expanding world of PRP, there is also a growing array of PRP kits. Some of these kits have been cleared by the FDA but others have not. Dr. Krueger warned that collection tubes are being marketed that are substandard imitations of better-established products. He specifically cautioned against do-it-yourself PRP kits, which are likely to be less effective for isolating platelets and can also be contaminated with pyogenes that cause infection.
“Please use an FDA-cleared kit,” he said, warning that the risk of failing to do so is not just associated with lack of efficacy but also a significant risk of serious adverse events.
Of the centrifuge devices, both Dr. Krueger and Dr. Keaney generally recommend single-spin over double-spin devices, particularly at centers with a limited volume of PRP-based hair loss interventions. These are generally simpler.
Once the PRP has been properly prepared, the efficacy of PRP upon application can also be influenced by strategies for activation. Although the exact mechanism of PRP in stimulating hair growth is incompletely defined, the role of platelets in releasing growth factors is believed to be critical. There are a number of methods to stimulate platelets upon administration, such as exposure to endogenous collagen or thrombin or exogenous chemicals, such as calcium chloride, but again, techniques differ and the optimal approach is unknown.
One concern is the recent and largely unregulated growth of regenerative cell and tissue products for treating a large array of clinical disorders or cosmetic issues, according to Dr. Keaney. He warned of a “wild, wild west mentality” that has attracted providers with inadequate training and experience. In turn, this is now attracting the attention of the FDA as well as those involved in enforcing FDA directives.
“There is definitely more scrutiny of regenerative products,” he said, noting that he is careful about how he markets PRP. While it is reasonable to offer this off-label treatment as an in-office procedure, he noted that it is illegal to advertise off-label products. He reported that he has become more prudent when including this option among hair regrowth services provided in his practice.
Omer E. Ibrahim, MD, a dermatologist affiliated with Chicago Cosmetic Surgery and Dermatology, agreed. While he also feels there is good evidence to support PRP as a hair loss treatment option, particularly for androgenic alopecia, he also expressed caution about promoting this approach in exclusion of other options.
“Patients ask me for a PRP consultation, but there is no such thing as a PRP consultation in my practice,” Dr. Ibrahim said. He incorporates PRP into other strategies. “I stress that it is one part of a multipronged approach,” he added.
Dr. Ibrahim has reported financial relationships with Alastin Skincare, Allergan, Eclipse Medical, Galderma USA, and Revision Skincare. Dr. Keaney has reported financial relationships with Allergan, DermTech, Evolus, Galderma USA, Merz Aesthetics, Revance Therapeutics, and Syneron Candela. Dr. Krueger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or administer the highly technique-dependent treatment, which creates plenty of room for suboptimal results, according to several experts at the annual meeting of the American Academy of Dermatology.
“The process is the product,” emphasized Terrence Keaney, MD, clinical associate professor at George Washington University, Washington, as well as cofounder of SkinDC, a private practice in Arlington, Va. He characterized PRP as a “growth factor cytokine cocktail,” for which relative benefits are fully dependent on the ingredients.
In other words, the efficacy of PRP is mostly dependent on the multiple steps in which blood drawn from a patient is separated into its components, processed to create a platelet-rich product, and then administered to the patient by injection or in conjunction with microneedles. While the goal is a platelet concentration two- to fivefold greater than that found in whole blood, this is not as straightforward as it sounds.
Many PRP device kits available
“There are a ton of [centrifuge] devices on the market and a lot of differences in the methodology in optimizing the platelet concentration,” Dr. Keaney explained. In addition, there are numerous proprietary collection tubes using different types of anticoagulants and different separator gels that also play a role in the goal of optimizing a platelet-rich and readily activated product.
“Recognize that each step in the preparation of PRP introduces a source of variation that affects the composition and efficacy of the final product,” said Steven Krueger, MD, who is completing his residency in dermatology at the University of Massachusetts, Worcester, but who has become an expert in the field. He contributed a chapter on this topic in the recently published book, Aesthetic Clinician’s Guide to Platelet Rich Plasma.
The importance of technique is reflected in inconsistent results from published controlled trials. Unfortunately, the authors of many studies have failed to provide details of their protocol. Ultimately, Dr. Krueger said this lack of clarity among available protocols has created a serious obstacle for establishing which steps are important and how to move the field forward.
Dr. Keaney agreed. Because of the frequent lack of details about how PRP was processed in available studies, the effort to draw conclusions about the experiences at different centers is like “comparing apples to oranges.”
“What is the ideal dose and concentrate? We don’t know,” Dr. Keaney said.
The first centrifuge device to receive regulatory approval was developed for orthopedic indications more than 20 years ago. There are now at least 20 centrifuge devices with 510K Food and Drug Administration clearance for separating blood components to produce PRP. The 510K designation means that they are “substantially equivalent” to an already approved device, but Dr. Krueger cautioned that their use in preparing PRP for treatment of hair loss remains off label.
Substandard devices are marketed
In the rapidly expanding world of PRP, there is also a growing array of PRP kits. Some of these kits have been cleared by the FDA but others have not. Dr. Krueger warned that collection tubes are being marketed that are substandard imitations of better-established products. He specifically cautioned against do-it-yourself PRP kits, which are likely to be less effective for isolating platelets and can also be contaminated with pyogenes that cause infection.
“Please use an FDA-cleared kit,” he said, warning that the risk of failing to do so is not just associated with lack of efficacy but also a significant risk of serious adverse events.
Of the centrifuge devices, both Dr. Krueger and Dr. Keaney generally recommend single-spin over double-spin devices, particularly at centers with a limited volume of PRP-based hair loss interventions. These are generally simpler.
Once the PRP has been properly prepared, the efficacy of PRP upon application can also be influenced by strategies for activation. Although the exact mechanism of PRP in stimulating hair growth is incompletely defined, the role of platelets in releasing growth factors is believed to be critical. There are a number of methods to stimulate platelets upon administration, such as exposure to endogenous collagen or thrombin or exogenous chemicals, such as calcium chloride, but again, techniques differ and the optimal approach is unknown.
One concern is the recent and largely unregulated growth of regenerative cell and tissue products for treating a large array of clinical disorders or cosmetic issues, according to Dr. Keaney. He warned of a “wild, wild west mentality” that has attracted providers with inadequate training and experience. In turn, this is now attracting the attention of the FDA as well as those involved in enforcing FDA directives.
“There is definitely more scrutiny of regenerative products,” he said, noting that he is careful about how he markets PRP. While it is reasonable to offer this off-label treatment as an in-office procedure, he noted that it is illegal to advertise off-label products. He reported that he has become more prudent when including this option among hair regrowth services provided in his practice.
Omer E. Ibrahim, MD, a dermatologist affiliated with Chicago Cosmetic Surgery and Dermatology, agreed. While he also feels there is good evidence to support PRP as a hair loss treatment option, particularly for androgenic alopecia, he also expressed caution about promoting this approach in exclusion of other options.
“Patients ask me for a PRP consultation, but there is no such thing as a PRP consultation in my practice,” Dr. Ibrahim said. He incorporates PRP into other strategies. “I stress that it is one part of a multipronged approach,” he added.
Dr. Ibrahim has reported financial relationships with Alastin Skincare, Allergan, Eclipse Medical, Galderma USA, and Revision Skincare. Dr. Keaney has reported financial relationships with Allergan, DermTech, Evolus, Galderma USA, Merz Aesthetics, Revance Therapeutics, and Syneron Candela. Dr. Krueger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or administer the highly technique-dependent treatment, which creates plenty of room for suboptimal results, according to several experts at the annual meeting of the American Academy of Dermatology.
“The process is the product,” emphasized Terrence Keaney, MD, clinical associate professor at George Washington University, Washington, as well as cofounder of SkinDC, a private practice in Arlington, Va. He characterized PRP as a “growth factor cytokine cocktail,” for which relative benefits are fully dependent on the ingredients.
In other words, the efficacy of PRP is mostly dependent on the multiple steps in which blood drawn from a patient is separated into its components, processed to create a platelet-rich product, and then administered to the patient by injection or in conjunction with microneedles. While the goal is a platelet concentration two- to fivefold greater than that found in whole blood, this is not as straightforward as it sounds.
Many PRP device kits available
“There are a ton of [centrifuge] devices on the market and a lot of differences in the methodology in optimizing the platelet concentration,” Dr. Keaney explained. In addition, there are numerous proprietary collection tubes using different types of anticoagulants and different separator gels that also play a role in the goal of optimizing a platelet-rich and readily activated product.
“Recognize that each step in the preparation of PRP introduces a source of variation that affects the composition and efficacy of the final product,” said Steven Krueger, MD, who is completing his residency in dermatology at the University of Massachusetts, Worcester, but who has become an expert in the field. He contributed a chapter on this topic in the recently published book, Aesthetic Clinician’s Guide to Platelet Rich Plasma.
The importance of technique is reflected in inconsistent results from published controlled trials. Unfortunately, the authors of many studies have failed to provide details of their protocol. Ultimately, Dr. Krueger said this lack of clarity among available protocols has created a serious obstacle for establishing which steps are important and how to move the field forward.
Dr. Keaney agreed. Because of the frequent lack of details about how PRP was processed in available studies, the effort to draw conclusions about the experiences at different centers is like “comparing apples to oranges.”
“What is the ideal dose and concentrate? We don’t know,” Dr. Keaney said.
The first centrifuge device to receive regulatory approval was developed for orthopedic indications more than 20 years ago. There are now at least 20 centrifuge devices with 510K Food and Drug Administration clearance for separating blood components to produce PRP. The 510K designation means that they are “substantially equivalent” to an already approved device, but Dr. Krueger cautioned that their use in preparing PRP for treatment of hair loss remains off label.
Substandard devices are marketed
In the rapidly expanding world of PRP, there is also a growing array of PRP kits. Some of these kits have been cleared by the FDA but others have not. Dr. Krueger warned that collection tubes are being marketed that are substandard imitations of better-established products. He specifically cautioned against do-it-yourself PRP kits, which are likely to be less effective for isolating platelets and can also be contaminated with pyogenes that cause infection.
“Please use an FDA-cleared kit,” he said, warning that the risk of failing to do so is not just associated with lack of efficacy but also a significant risk of serious adverse events.
Of the centrifuge devices, both Dr. Krueger and Dr. Keaney generally recommend single-spin over double-spin devices, particularly at centers with a limited volume of PRP-based hair loss interventions. These are generally simpler.
Once the PRP has been properly prepared, the efficacy of PRP upon application can also be influenced by strategies for activation. Although the exact mechanism of PRP in stimulating hair growth is incompletely defined, the role of platelets in releasing growth factors is believed to be critical. There are a number of methods to stimulate platelets upon administration, such as exposure to endogenous collagen or thrombin or exogenous chemicals, such as calcium chloride, but again, techniques differ and the optimal approach is unknown.
One concern is the recent and largely unregulated growth of regenerative cell and tissue products for treating a large array of clinical disorders or cosmetic issues, according to Dr. Keaney. He warned of a “wild, wild west mentality” that has attracted providers with inadequate training and experience. In turn, this is now attracting the attention of the FDA as well as those involved in enforcing FDA directives.
“There is definitely more scrutiny of regenerative products,” he said, noting that he is careful about how he markets PRP. While it is reasonable to offer this off-label treatment as an in-office procedure, he noted that it is illegal to advertise off-label products. He reported that he has become more prudent when including this option among hair regrowth services provided in his practice.
Omer E. Ibrahim, MD, a dermatologist affiliated with Chicago Cosmetic Surgery and Dermatology, agreed. While he also feels there is good evidence to support PRP as a hair loss treatment option, particularly for androgenic alopecia, he also expressed caution about promoting this approach in exclusion of other options.
“Patients ask me for a PRP consultation, but there is no such thing as a PRP consultation in my practice,” Dr. Ibrahim said. He incorporates PRP into other strategies. “I stress that it is one part of a multipronged approach,” he added.
Dr. Ibrahim has reported financial relationships with Alastin Skincare, Allergan, Eclipse Medical, Galderma USA, and Revision Skincare. Dr. Keaney has reported financial relationships with Allergan, DermTech, Evolus, Galderma USA, Merz Aesthetics, Revance Therapeutics, and Syneron Candela. Dr. Krueger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Mercury and other risks of cosmetic skin lighteners
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
The science of clean skin care and the clean beauty movement
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).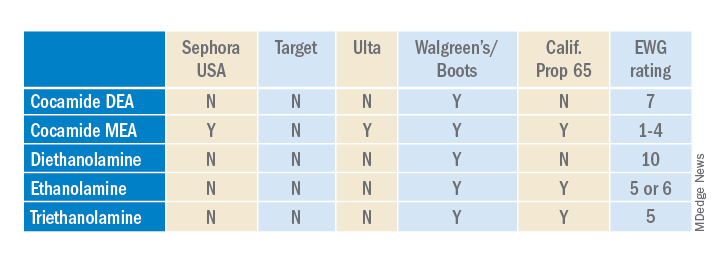
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
FDA warns about off-label use of laparoscopic device for aesthetic procedures
The .
The device is cleared by the FDA for “general use of cutting, coagulation, and ablation of soft tissue during open and laparoscopic surgical procedures” but it “has not been determined to be safe or effective for any procedure intended to improve the appearance of the skin,” according to the March 14 statement from the FDA. The statement adds that the agency has received reports describing “serious and potentially life-threatening adverse events with use of this device for certain aesthetic procedures,” including some that have required treatment in an intensive care unit. The statement does not mention whether any cases were fatal.
Adverse events that have been reported include second- and third-degree burns, infections, changes in skin color, scars, nerve damage, “significant bleeding,” and “air or gas accumulation under the skin, in body cavities, and in blood vessels.”
Manufactured by Apyx medical, the device includes a hand piece and generator and uses radiofrequency energy and helium to generate plasma, which is used to “cut, coagulate ... and eliminate soft tissue with heat during surgery,” according to the FDA.
The FDA is advising health care providers not to use the device for dermal resurfacing or skin contraction “alone or in combination with liposuction.”
The statement also advises consumers who are considering an aesthetic skin treatment with this device to consult their health care providers regarding its use – and if they have any problems or are concerned after being treated with this device, to “seek care from a licensed health care provider.”
The FDA is working with Apyx to evaluate information about the use of the device for aesthetic skin procedures and to inform consumers and health care providers about the warning.
Health care providers and consumers should report problems or complications associated with the Renuvion/J-Plasma device to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
The .
The device is cleared by the FDA for “general use of cutting, coagulation, and ablation of soft tissue during open and laparoscopic surgical procedures” but it “has not been determined to be safe or effective for any procedure intended to improve the appearance of the skin,” according to the March 14 statement from the FDA. The statement adds that the agency has received reports describing “serious and potentially life-threatening adverse events with use of this device for certain aesthetic procedures,” including some that have required treatment in an intensive care unit. The statement does not mention whether any cases were fatal.
Adverse events that have been reported include second- and third-degree burns, infections, changes in skin color, scars, nerve damage, “significant bleeding,” and “air or gas accumulation under the skin, in body cavities, and in blood vessels.”
Manufactured by Apyx medical, the device includes a hand piece and generator and uses radiofrequency energy and helium to generate plasma, which is used to “cut, coagulate ... and eliminate soft tissue with heat during surgery,” according to the FDA.
The FDA is advising health care providers not to use the device for dermal resurfacing or skin contraction “alone or in combination with liposuction.”
The statement also advises consumers who are considering an aesthetic skin treatment with this device to consult their health care providers regarding its use – and if they have any problems or are concerned after being treated with this device, to “seek care from a licensed health care provider.”
The FDA is working with Apyx to evaluate information about the use of the device for aesthetic skin procedures and to inform consumers and health care providers about the warning.
Health care providers and consumers should report problems or complications associated with the Renuvion/J-Plasma device to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
The .
The device is cleared by the FDA for “general use of cutting, coagulation, and ablation of soft tissue during open and laparoscopic surgical procedures” but it “has not been determined to be safe or effective for any procedure intended to improve the appearance of the skin,” according to the March 14 statement from the FDA. The statement adds that the agency has received reports describing “serious and potentially life-threatening adverse events with use of this device for certain aesthetic procedures,” including some that have required treatment in an intensive care unit. The statement does not mention whether any cases were fatal.
Adverse events that have been reported include second- and third-degree burns, infections, changes in skin color, scars, nerve damage, “significant bleeding,” and “air or gas accumulation under the skin, in body cavities, and in blood vessels.”
Manufactured by Apyx medical, the device includes a hand piece and generator and uses radiofrequency energy and helium to generate plasma, which is used to “cut, coagulate ... and eliminate soft tissue with heat during surgery,” according to the FDA.
The FDA is advising health care providers not to use the device for dermal resurfacing or skin contraction “alone or in combination with liposuction.”
The statement also advises consumers who are considering an aesthetic skin treatment with this device to consult their health care providers regarding its use – and if they have any problems or are concerned after being treated with this device, to “seek care from a licensed health care provider.”
The FDA is working with Apyx to evaluate information about the use of the device for aesthetic skin procedures and to inform consumers and health care providers about the warning.
Health care providers and consumers should report problems or complications associated with the Renuvion/J-Plasma device to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Mandelic acid
Acids peels are used to elicit a chemical exfoliation of the skin by hydrolyzing amide bonds between keratinocytes, reducing corneocyte adhesion, as well as inducing an inflammatory reaction stimulating tissue remodeling. Release of cytokines such as interleukin (IL)-1 and IL-6 by keratinocytes activates fibroblasts to increase the production of matrix metalloproteinases. These are involved in the production of hyaluronic acid and new collagen formation.
Mandelic acid was derived from bitter almonds (mandel is the German word for almond). It is a white powder originally used as an antibiotic for the treatment of urinary tract infections. Its antibacterial properties make it an excellent product for the topical treatment of acne, as well as for use in topical preparations to treat hyperpigmentation and photoaging. In cosmetic use, mandelic acid is a slow acting chemical peel that can be used in all skin types, including sensitive and rosacea-prone skin, as well as skin of color. Its large molecular size allows for the slow penetration of the acid on the skin and thus it can be carefully titrated.
Studies have shown its efficacy in reducing sebum content, acne, acne scarring, and hyperpigmentation. In clinical practice however, the most effective use of this acid is on sensitive skin. It is a great tool for clinicians to use as an effective exfoliant in less acid tolerant skin types. In commercially available concentrations of 5%-45%, mandelic acid can be used alone or in combination with other beta hydroxy peels, depending on the indication.
Most dermatologists and patients prefer in-office peels that induce noticeable peeling and resurfacing of the skin. Mandelic acid is one of the largest alpha hydroxy acids, a lipophilic acid that penetrates the skin slowly and uniformly, making it an ideal peel in sensitive or aging and thin skin types. Although many mandelic acid peels are available, however, there is a paucity of studies comparing their benefits and efficacies.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
1. Wójcik A et al. Dermatol Alergol. 2013 Jun;30(3):140-5.
2. Soleymani T et al. J Clin Aesthet Dermatol. 2018;11(8):21-8.
Acids peels are used to elicit a chemical exfoliation of the skin by hydrolyzing amide bonds between keratinocytes, reducing corneocyte adhesion, as well as inducing an inflammatory reaction stimulating tissue remodeling. Release of cytokines such as interleukin (IL)-1 and IL-6 by keratinocytes activates fibroblasts to increase the production of matrix metalloproteinases. These are involved in the production of hyaluronic acid and new collagen formation.
Mandelic acid was derived from bitter almonds (mandel is the German word for almond). It is a white powder originally used as an antibiotic for the treatment of urinary tract infections. Its antibacterial properties make it an excellent product for the topical treatment of acne, as well as for use in topical preparations to treat hyperpigmentation and photoaging. In cosmetic use, mandelic acid is a slow acting chemical peel that can be used in all skin types, including sensitive and rosacea-prone skin, as well as skin of color. Its large molecular size allows for the slow penetration of the acid on the skin and thus it can be carefully titrated.
Studies have shown its efficacy in reducing sebum content, acne, acne scarring, and hyperpigmentation. In clinical practice however, the most effective use of this acid is on sensitive skin. It is a great tool for clinicians to use as an effective exfoliant in less acid tolerant skin types. In commercially available concentrations of 5%-45%, mandelic acid can be used alone or in combination with other beta hydroxy peels, depending on the indication.
Most dermatologists and patients prefer in-office peels that induce noticeable peeling and resurfacing of the skin. Mandelic acid is one of the largest alpha hydroxy acids, a lipophilic acid that penetrates the skin slowly and uniformly, making it an ideal peel in sensitive or aging and thin skin types. Although many mandelic acid peels are available, however, there is a paucity of studies comparing their benefits and efficacies.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
1. Wójcik A et al. Dermatol Alergol. 2013 Jun;30(3):140-5.
2. Soleymani T et al. J Clin Aesthet Dermatol. 2018;11(8):21-8.
Acids peels are used to elicit a chemical exfoliation of the skin by hydrolyzing amide bonds between keratinocytes, reducing corneocyte adhesion, as well as inducing an inflammatory reaction stimulating tissue remodeling. Release of cytokines such as interleukin (IL)-1 and IL-6 by keratinocytes activates fibroblasts to increase the production of matrix metalloproteinases. These are involved in the production of hyaluronic acid and new collagen formation.
Mandelic acid was derived from bitter almonds (mandel is the German word for almond). It is a white powder originally used as an antibiotic for the treatment of urinary tract infections. Its antibacterial properties make it an excellent product for the topical treatment of acne, as well as for use in topical preparations to treat hyperpigmentation and photoaging. In cosmetic use, mandelic acid is a slow acting chemical peel that can be used in all skin types, including sensitive and rosacea-prone skin, as well as skin of color. Its large molecular size allows for the slow penetration of the acid on the skin and thus it can be carefully titrated.
Studies have shown its efficacy in reducing sebum content, acne, acne scarring, and hyperpigmentation. In clinical practice however, the most effective use of this acid is on sensitive skin. It is a great tool for clinicians to use as an effective exfoliant in less acid tolerant skin types. In commercially available concentrations of 5%-45%, mandelic acid can be used alone or in combination with other beta hydroxy peels, depending on the indication.
Most dermatologists and patients prefer in-office peels that induce noticeable peeling and resurfacing of the skin. Mandelic acid is one of the largest alpha hydroxy acids, a lipophilic acid that penetrates the skin slowly and uniformly, making it an ideal peel in sensitive or aging and thin skin types. Although many mandelic acid peels are available, however, there is a paucity of studies comparing their benefits and efficacies.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
1. Wójcik A et al. Dermatol Alergol. 2013 Jun;30(3):140-5.
2. Soleymani T et al. J Clin Aesthet Dermatol. 2018;11(8):21-8.
The gap in cosmeceuticals education
Starting this month, I will be joining Dr. Leslie S. Baumann as a cocontributor to the Cosmeceutical Critique column, and since this is my first column, I would like to formally introduce myself. I am a cosmetic and general dermatologist in private practice in Miami and a longtime skin care enthusiast. My path toward becoming a dermatologist began when I was working in New York City, my hometown, as a scientific researcher, fulfilling my passion for scientific inquiry. After realizing that I most enjoyed applying discoveries made in the lab directly to patient care, I decided to pursue medical school at New York University before completing a dermatology residency at the University of Miami, serving as Chief Resident during my final year. Although I was born and raised in New York, staying in Miami was an obvious decision for me. In addition to the tropical weather and amazing lifestyle, the medical community in Miami supports adventure, creativity, and innovation, which are key aspects that drew me to the University of Miami and continue to drive my personal evolution in private practice.
I now practice at Baumann Cosmetic & Research Institute alongside my mentor, Dr. Baumann. I truly have my dream job – I get to talk skin care and do a wide array of cosmetics procedures, perform skin surgeries, and solve complex medical dermatology cases all in a day’s work. My career sits at the intersection of my passions for science, critical thinking, beauty, aesthetics, and most importantly, engaging with patients.
For my first column, I want to , and I will provide a simple framework to approach the design of skin care regimens and utilization of cosmeceuticals in practice.
The focus of a dermatology residency is on medical and surgical skills. We become experts in diagnosing and treating conditions ranging from life-threatening drug reactions like Stevens-Johnson Syndrome to complex diseases like dermatomyositis, utilizing medications and treatments ranging from cyclosporine and methotrexate to biologics and intravenous immunoglobulin, and performing advanced skin surgeries utilizing flaps and grafts to repair defects.
The discipline of cosmetic dermatology, let alone cosmeceuticals, accounts for a fraction of our didactic and hands-on training. I completed a top dermatology residency program that prepared me to treat any dermatologic condition; however, I honestly felt like I didn’t have a strong understanding of cosmeceuticals and skin care and how to integrate them with prescription therapies when I completed residency, which is a sentiment shared by residents across the country. I remember a study break while preparing for my final board exam when I went into a tailspin for an entire day trying to decode an ingredient list of a new “antiaging serum” and researching its mechanisms of action and the clinical data supporting the active ingredients in the serum, which included bakuchiol and a blend of peptides. As a dermatologist who likes to treat and provide recommendations based on scientific rationale and data to deliver the highest level of care, I admit that I felt insecure not being as knowledgeable about cosmeceuticals as I was about more complex dermatology treatments. As both a cosmetic and general dermatologist, discussing skin care and cosmeceuticals independent of or in conjunction with medical management occurs daily, and I recognized that becoming an expert in this area is essential to becoming a top, well-rounded dermatologist.
A gap in cosmeceutical education in dermatology residency
Multiple studies have established that the field of cosmetic dermatology comprises a fraction of dermatology residency training. In 2013, Kirby et al. published a survey of dermatology instructors and chief residents across the country and found that only 67% of responders reported having received formal lectures on cosmetic dermatology.1 In 2014, Bauer et al. published a survey of dermatology program directors assessing attitudes toward cosmetic dermatology and reported that only 38% of program directors believed that cosmetic dermatology should be a necessary aspect of residency training.2 A survey sent to dermatology residents published in 2012 found that among respondents, more than 58% of residency programs have an “encouraging or somewhat encouraging” attitude toward teaching cosmetic dermatology, yet 22% of programs had a “somewhat discouraging” or “discouraging” attitude.3 While these noted studies have focused on procedural aspects of cosmetic dermatology training, Feetham et al. surveyed dermatology residents and faculty to assess attitudes toward and training on skin care and cosmeceuticals specifically. Among resident respondents, most (74.5%) reported their education on skin care and cosmeceuticals has been “too little or nonexistent” during residency and 76.5% “agree or strongly agree” that it should be part of their education.4 In contrast, 60% of faculty reported resident education on skin care and cosmeceuticals is “just the right amount or too much” (P < .001).
In my personal experience as a resident, discussing skin care was emphasized when treating patients with eczema, contact dermatitis, acne, and hair disorders, but otherwise, the majority of skin care discussions relied on having a stock list of recommended cleansers, moisturizers, and sunscreens. In regards to cosmeceuticals for facial skin specifically, there were only a handful of instances in which alternative ingredients, such as vitamin C for hyperpigmentation, were discussed and specific brands were mentioned. Upon reflection, I wish I had more opportunity to see the clinical benefits of cosmeceuticals first hand, just like when I observe dupilumab clear patients with severe atopic dermatitis, rather than reading about it in textbooks and journals.
While one hypothesis for programs’ limited attention given to cosmetic training may be that it detracts from medical training, the survey by Bauer et al. found that residents did not feel less prepared (94.9%) or less interested (97.4%) in medical dermatology as a result of their cosmetic training.2 In addition, providers in an academic dermatology residency may limit discussions of skin care because of the high patient volume and because extensive skin care discussions will not impact insurance billings. Academic dermatology programs often service patients with more financial constraints, which further limits OTC cosmeceutical discussions. In my residency experience, I had the opportunity to regularly treat more severe and rare dermatologic cases than those I encounter in private practice; therefore, I spent more time focusing on systemic therapies, with fewer opportunities to dedicate time to cosmeceuticals.
Why skin care and cosmeceuticals should be an essential aspect of residency training
Discussing skin care and cosmeceuticals is a valuable aspect of medical and general dermatology, not just aesthetic dermatology. When treating general dermatologic conditions, guidance on proper skin care can improve both adherence and efficacy of medical treatments. For example, an acne study by de Lucas et al. demonstrated that adherence to adjuvant treatment of acne (such as the use of moisturizers) was associated not only with a 2.4-fold increase in the probability of adherence to pharmacological treatment, but also with a significant reduction in acne severity.5 Aside from skin care, cosmeceuticals themselves have efficacy in treating general dermatologic conditions. In the treatment of acne, topical niacinamide, a popular cosmeceutical ingredient, has been shown to have sebosuppressive and anti-inflammatory effects, addressing key aspects of acne pathogenesis.6 A double-blind study by Draelos et al. reported topical 2% niacinamide was effective in reducing the rate of sebum excretion in 50 Japanese patients over 4 weeks.6 In several double-blind studies that have compared twice daily application of 4% nicotinamide gel with the same application of 1% clindamycin gel in moderate inflammatory acne over 8 weeks, nicotinamide gel reduced the number of inflammatory papules and acne lesions to a level comparable with clindamycin gel.6 These studies support the use of niacinamide cosmeceutical products as an adjunctive treatment for acne.
With increased clinical data supporting cosmeceuticals, it can be expected that some cosmeceuticals will substitute traditional prescription medications in the dermatologists’ arsenal. For example, hydroquinone – both prescription strength and OTC 2% – is a workhorse in treating melasma; however, there is increasing interest in hydroquinone-free treatments, especially since OTC cosmeceuticals containing 2% hydroquinone were banned in 2020 because of safety concerns. Dermatologists will therefore need to provide guidance about hydroquinone alternatives for skin lightening, including soy, licorice extracts, kojic acid, arbutin, niacinamide, N-acetylglucosamine, and vitamin C, among others.7 Utilizing knowledge of a cosmeceutical’s mechanisms of action and clinical data, the dermatologist is in the best position to guide patients toward optimal ingredients and dispel cosmeceutical myths. Given that cosmeceuticals are not regulated by the Food and Drug Administration, it is even more important that the dermatologist serves as an authority on cosmeceuticals.
How to become a master skin care and cosmeceutical prescriber
A common pitfall I have observed among practitioners less experienced with aesthetic-focused skin care and cosmeceuticals is adapting a one-size-fits-all approach. In the one-size-fits-all approach, every patient concerned about aging gets the same vitamin C serum and retinoid, and every patient with hyperpigmentation gets the same hydroquinone prescription, for example. This approach, however, does not take into account unique differences in patients’ skin. Below
is the basic skin care framework that I follow, taught to me by Dr. Baumann. It utilizes an individualized approach based on the patient’s skin qualities to achieve optimal results.
Determine the patient’s skin type (dry vs. oily; sensitive vs. not sensitive; pigmentation issues vs. no hyperpigmentation; wrinkled and mature vs. nonwrinkled) and identify concerns (e.g., dark spots, redness, acne, dehydration).
Separate products into categories of cleansers, eye creams, moisturizers, sun protection, and treatments. Treatments refers to any additional products in a skin care regimen intended to ameliorate a particular condition (e.g., vitamin C for hyperpigmentation, retinoids for fine lines).
Choose products for each category in step 2 (cleansers, eye creams, moisturizers, sun protection, treatments) that are complementary to the patient’s skin type (determined in step 1) and aid the patient in meeting their particular skin goals. For example, a salicylic acid cleanser would be beneficial for a patient with oily skin and acne, but this same cleanser may be too drying and irritating for an acne patient with dry skin.
Ensure that chosen ingredients and products work together harmoniously. For example, while the acne patient may benefit from a salicylic acid cleanser and retinoid cream, using them in succession initially may be overly drying for some patients.
Spend the time to make sure patients understand the appropriate order of application and recognize when efficacy of a product is impacted by another product in the regimen. For example, a low pH cleanser can increase penetration of an ascorbic acid product that follows it in the regimen.
After establishing a basic skin care framework, the next step for beginners is learning about ingredients and their mechanisms of action and familiarizing themselves with scientific and clinical studies. Until cosmeceuticals become an integral part of the training curriculum, dermatologists can gain knowledge independently by reading literature and studies on cosmeceutical active ingredients and experimenting with consumer products. I look forward to regularly contributing to this column to further our awareness and understanding of the mechanisms of and data supporting cosmeceuticals so that we can better guide our patients.
Please feel free to email me at [email protected] or message me on Instagram @DrChloeGoldman with ideas that you would like me to address in this column.
Dr. Goldman is a dermatologist in private practice in Miami, and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a new general dermatology practice. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other relevant disclosures.
References
1. Kirby JS et al. J Am Acad Dermatol. 2013;68(2):e23-8.
2. Bauer et al. JAMA Dermatol. 2014;150(2):125-9.
3. Group A et al. Dermatol Surg. 2012;38(12):1975-80.
4. Feetham HJ et al. J Cosmet Dermatol. 2018;17(2):220-6.
5. de Lucas R et al. BMC Dermatol. 2015;15:17.
6. Araviiskaia E and Dreno BJ. Eur Acad Dermatol Venereol. 2016;30(6):926-35.
7. Leyden JJ et al. J Eur Acad Dermatol Venereol. 2011;25(10):1140-5.
Starting this month, I will be joining Dr. Leslie S. Baumann as a cocontributor to the Cosmeceutical Critique column, and since this is my first column, I would like to formally introduce myself. I am a cosmetic and general dermatologist in private practice in Miami and a longtime skin care enthusiast. My path toward becoming a dermatologist began when I was working in New York City, my hometown, as a scientific researcher, fulfilling my passion for scientific inquiry. After realizing that I most enjoyed applying discoveries made in the lab directly to patient care, I decided to pursue medical school at New York University before completing a dermatology residency at the University of Miami, serving as Chief Resident during my final year. Although I was born and raised in New York, staying in Miami was an obvious decision for me. In addition to the tropical weather and amazing lifestyle, the medical community in Miami supports adventure, creativity, and innovation, which are key aspects that drew me to the University of Miami and continue to drive my personal evolution in private practice.
I now practice at Baumann Cosmetic & Research Institute alongside my mentor, Dr. Baumann. I truly have my dream job – I get to talk skin care and do a wide array of cosmetics procedures, perform skin surgeries, and solve complex medical dermatology cases all in a day’s work. My career sits at the intersection of my passions for science, critical thinking, beauty, aesthetics, and most importantly, engaging with patients.
For my first column, I want to , and I will provide a simple framework to approach the design of skin care regimens and utilization of cosmeceuticals in practice.
The focus of a dermatology residency is on medical and surgical skills. We become experts in diagnosing and treating conditions ranging from life-threatening drug reactions like Stevens-Johnson Syndrome to complex diseases like dermatomyositis, utilizing medications and treatments ranging from cyclosporine and methotrexate to biologics and intravenous immunoglobulin, and performing advanced skin surgeries utilizing flaps and grafts to repair defects.
The discipline of cosmetic dermatology, let alone cosmeceuticals, accounts for a fraction of our didactic and hands-on training. I completed a top dermatology residency program that prepared me to treat any dermatologic condition; however, I honestly felt like I didn’t have a strong understanding of cosmeceuticals and skin care and how to integrate them with prescription therapies when I completed residency, which is a sentiment shared by residents across the country. I remember a study break while preparing for my final board exam when I went into a tailspin for an entire day trying to decode an ingredient list of a new “antiaging serum” and researching its mechanisms of action and the clinical data supporting the active ingredients in the serum, which included bakuchiol and a blend of peptides. As a dermatologist who likes to treat and provide recommendations based on scientific rationale and data to deliver the highest level of care, I admit that I felt insecure not being as knowledgeable about cosmeceuticals as I was about more complex dermatology treatments. As both a cosmetic and general dermatologist, discussing skin care and cosmeceuticals independent of or in conjunction with medical management occurs daily, and I recognized that becoming an expert in this area is essential to becoming a top, well-rounded dermatologist.
A gap in cosmeceutical education in dermatology residency
Multiple studies have established that the field of cosmetic dermatology comprises a fraction of dermatology residency training. In 2013, Kirby et al. published a survey of dermatology instructors and chief residents across the country and found that only 67% of responders reported having received formal lectures on cosmetic dermatology.1 In 2014, Bauer et al. published a survey of dermatology program directors assessing attitudes toward cosmetic dermatology and reported that only 38% of program directors believed that cosmetic dermatology should be a necessary aspect of residency training.2 A survey sent to dermatology residents published in 2012 found that among respondents, more than 58% of residency programs have an “encouraging or somewhat encouraging” attitude toward teaching cosmetic dermatology, yet 22% of programs had a “somewhat discouraging” or “discouraging” attitude.3 While these noted studies have focused on procedural aspects of cosmetic dermatology training, Feetham et al. surveyed dermatology residents and faculty to assess attitudes toward and training on skin care and cosmeceuticals specifically. Among resident respondents, most (74.5%) reported their education on skin care and cosmeceuticals has been “too little or nonexistent” during residency and 76.5% “agree or strongly agree” that it should be part of their education.4 In contrast, 60% of faculty reported resident education on skin care and cosmeceuticals is “just the right amount or too much” (P < .001).
In my personal experience as a resident, discussing skin care was emphasized when treating patients with eczema, contact dermatitis, acne, and hair disorders, but otherwise, the majority of skin care discussions relied on having a stock list of recommended cleansers, moisturizers, and sunscreens. In regards to cosmeceuticals for facial skin specifically, there were only a handful of instances in which alternative ingredients, such as vitamin C for hyperpigmentation, were discussed and specific brands were mentioned. Upon reflection, I wish I had more opportunity to see the clinical benefits of cosmeceuticals first hand, just like when I observe dupilumab clear patients with severe atopic dermatitis, rather than reading about it in textbooks and journals.
While one hypothesis for programs’ limited attention given to cosmetic training may be that it detracts from medical training, the survey by Bauer et al. found that residents did not feel less prepared (94.9%) or less interested (97.4%) in medical dermatology as a result of their cosmetic training.2 In addition, providers in an academic dermatology residency may limit discussions of skin care because of the high patient volume and because extensive skin care discussions will not impact insurance billings. Academic dermatology programs often service patients with more financial constraints, which further limits OTC cosmeceutical discussions. In my residency experience, I had the opportunity to regularly treat more severe and rare dermatologic cases than those I encounter in private practice; therefore, I spent more time focusing on systemic therapies, with fewer opportunities to dedicate time to cosmeceuticals.
Why skin care and cosmeceuticals should be an essential aspect of residency training
Discussing skin care and cosmeceuticals is a valuable aspect of medical and general dermatology, not just aesthetic dermatology. When treating general dermatologic conditions, guidance on proper skin care can improve both adherence and efficacy of medical treatments. For example, an acne study by de Lucas et al. demonstrated that adherence to adjuvant treatment of acne (such as the use of moisturizers) was associated not only with a 2.4-fold increase in the probability of adherence to pharmacological treatment, but also with a significant reduction in acne severity.5 Aside from skin care, cosmeceuticals themselves have efficacy in treating general dermatologic conditions. In the treatment of acne, topical niacinamide, a popular cosmeceutical ingredient, has been shown to have sebosuppressive and anti-inflammatory effects, addressing key aspects of acne pathogenesis.6 A double-blind study by Draelos et al. reported topical 2% niacinamide was effective in reducing the rate of sebum excretion in 50 Japanese patients over 4 weeks.6 In several double-blind studies that have compared twice daily application of 4% nicotinamide gel with the same application of 1% clindamycin gel in moderate inflammatory acne over 8 weeks, nicotinamide gel reduced the number of inflammatory papules and acne lesions to a level comparable with clindamycin gel.6 These studies support the use of niacinamide cosmeceutical products as an adjunctive treatment for acne.
With increased clinical data supporting cosmeceuticals, it can be expected that some cosmeceuticals will substitute traditional prescription medications in the dermatologists’ arsenal. For example, hydroquinone – both prescription strength and OTC 2% – is a workhorse in treating melasma; however, there is increasing interest in hydroquinone-free treatments, especially since OTC cosmeceuticals containing 2% hydroquinone were banned in 2020 because of safety concerns. Dermatologists will therefore need to provide guidance about hydroquinone alternatives for skin lightening, including soy, licorice extracts, kojic acid, arbutin, niacinamide, N-acetylglucosamine, and vitamin C, among others.7 Utilizing knowledge of a cosmeceutical’s mechanisms of action and clinical data, the dermatologist is in the best position to guide patients toward optimal ingredients and dispel cosmeceutical myths. Given that cosmeceuticals are not regulated by the Food and Drug Administration, it is even more important that the dermatologist serves as an authority on cosmeceuticals.
How to become a master skin care and cosmeceutical prescriber
A common pitfall I have observed among practitioners less experienced with aesthetic-focused skin care and cosmeceuticals is adapting a one-size-fits-all approach. In the one-size-fits-all approach, every patient concerned about aging gets the same vitamin C serum and retinoid, and every patient with hyperpigmentation gets the same hydroquinone prescription, for example. This approach, however, does not take into account unique differences in patients’ skin. Below
is the basic skin care framework that I follow, taught to me by Dr. Baumann. It utilizes an individualized approach based on the patient’s skin qualities to achieve optimal results.
Determine the patient’s skin type (dry vs. oily; sensitive vs. not sensitive; pigmentation issues vs. no hyperpigmentation; wrinkled and mature vs. nonwrinkled) and identify concerns (e.g., dark spots, redness, acne, dehydration).
Separate products into categories of cleansers, eye creams, moisturizers, sun protection, and treatments. Treatments refers to any additional products in a skin care regimen intended to ameliorate a particular condition (e.g., vitamin C for hyperpigmentation, retinoids for fine lines).
Choose products for each category in step 2 (cleansers, eye creams, moisturizers, sun protection, treatments) that are complementary to the patient’s skin type (determined in step 1) and aid the patient in meeting their particular skin goals. For example, a salicylic acid cleanser would be beneficial for a patient with oily skin and acne, but this same cleanser may be too drying and irritating for an acne patient with dry skin.
Ensure that chosen ingredients and products work together harmoniously. For example, while the acne patient may benefit from a salicylic acid cleanser and retinoid cream, using them in succession initially may be overly drying for some patients.
Spend the time to make sure patients understand the appropriate order of application and recognize when efficacy of a product is impacted by another product in the regimen. For example, a low pH cleanser can increase penetration of an ascorbic acid product that follows it in the regimen.
After establishing a basic skin care framework, the next step for beginners is learning about ingredients and their mechanisms of action and familiarizing themselves with scientific and clinical studies. Until cosmeceuticals become an integral part of the training curriculum, dermatologists can gain knowledge independently by reading literature and studies on cosmeceutical active ingredients and experimenting with consumer products. I look forward to regularly contributing to this column to further our awareness and understanding of the mechanisms of and data supporting cosmeceuticals so that we can better guide our patients.
Please feel free to email me at [email protected] or message me on Instagram @DrChloeGoldman with ideas that you would like me to address in this column.
Dr. Goldman is a dermatologist in private practice in Miami, and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a new general dermatology practice. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other relevant disclosures.
References
1. Kirby JS et al. J Am Acad Dermatol. 2013;68(2):e23-8.
2. Bauer et al. JAMA Dermatol. 2014;150(2):125-9.
3. Group A et al. Dermatol Surg. 2012;38(12):1975-80.
4. Feetham HJ et al. J Cosmet Dermatol. 2018;17(2):220-6.
5. de Lucas R et al. BMC Dermatol. 2015;15:17.
6. Araviiskaia E and Dreno BJ. Eur Acad Dermatol Venereol. 2016;30(6):926-35.
7. Leyden JJ et al. J Eur Acad Dermatol Venereol. 2011;25(10):1140-5.
Starting this month, I will be joining Dr. Leslie S. Baumann as a cocontributor to the Cosmeceutical Critique column, and since this is my first column, I would like to formally introduce myself. I am a cosmetic and general dermatologist in private practice in Miami and a longtime skin care enthusiast. My path toward becoming a dermatologist began when I was working in New York City, my hometown, as a scientific researcher, fulfilling my passion for scientific inquiry. After realizing that I most enjoyed applying discoveries made in the lab directly to patient care, I decided to pursue medical school at New York University before completing a dermatology residency at the University of Miami, serving as Chief Resident during my final year. Although I was born and raised in New York, staying in Miami was an obvious decision for me. In addition to the tropical weather and amazing lifestyle, the medical community in Miami supports adventure, creativity, and innovation, which are key aspects that drew me to the University of Miami and continue to drive my personal evolution in private practice.
I now practice at Baumann Cosmetic & Research Institute alongside my mentor, Dr. Baumann. I truly have my dream job – I get to talk skin care and do a wide array of cosmetics procedures, perform skin surgeries, and solve complex medical dermatology cases all in a day’s work. My career sits at the intersection of my passions for science, critical thinking, beauty, aesthetics, and most importantly, engaging with patients.
For my first column, I want to , and I will provide a simple framework to approach the design of skin care regimens and utilization of cosmeceuticals in practice.
The focus of a dermatology residency is on medical and surgical skills. We become experts in diagnosing and treating conditions ranging from life-threatening drug reactions like Stevens-Johnson Syndrome to complex diseases like dermatomyositis, utilizing medications and treatments ranging from cyclosporine and methotrexate to biologics and intravenous immunoglobulin, and performing advanced skin surgeries utilizing flaps and grafts to repair defects.
The discipline of cosmetic dermatology, let alone cosmeceuticals, accounts for a fraction of our didactic and hands-on training. I completed a top dermatology residency program that prepared me to treat any dermatologic condition; however, I honestly felt like I didn’t have a strong understanding of cosmeceuticals and skin care and how to integrate them with prescription therapies when I completed residency, which is a sentiment shared by residents across the country. I remember a study break while preparing for my final board exam when I went into a tailspin for an entire day trying to decode an ingredient list of a new “antiaging serum” and researching its mechanisms of action and the clinical data supporting the active ingredients in the serum, which included bakuchiol and a blend of peptides. As a dermatologist who likes to treat and provide recommendations based on scientific rationale and data to deliver the highest level of care, I admit that I felt insecure not being as knowledgeable about cosmeceuticals as I was about more complex dermatology treatments. As both a cosmetic and general dermatologist, discussing skin care and cosmeceuticals independent of or in conjunction with medical management occurs daily, and I recognized that becoming an expert in this area is essential to becoming a top, well-rounded dermatologist.
A gap in cosmeceutical education in dermatology residency
Multiple studies have established that the field of cosmetic dermatology comprises a fraction of dermatology residency training. In 2013, Kirby et al. published a survey of dermatology instructors and chief residents across the country and found that only 67% of responders reported having received formal lectures on cosmetic dermatology.1 In 2014, Bauer et al. published a survey of dermatology program directors assessing attitudes toward cosmetic dermatology and reported that only 38% of program directors believed that cosmetic dermatology should be a necessary aspect of residency training.2 A survey sent to dermatology residents published in 2012 found that among respondents, more than 58% of residency programs have an “encouraging or somewhat encouraging” attitude toward teaching cosmetic dermatology, yet 22% of programs had a “somewhat discouraging” or “discouraging” attitude.3 While these noted studies have focused on procedural aspects of cosmetic dermatology training, Feetham et al. surveyed dermatology residents and faculty to assess attitudes toward and training on skin care and cosmeceuticals specifically. Among resident respondents, most (74.5%) reported their education on skin care and cosmeceuticals has been “too little or nonexistent” during residency and 76.5% “agree or strongly agree” that it should be part of their education.4 In contrast, 60% of faculty reported resident education on skin care and cosmeceuticals is “just the right amount or too much” (P < .001).
In my personal experience as a resident, discussing skin care was emphasized when treating patients with eczema, contact dermatitis, acne, and hair disorders, but otherwise, the majority of skin care discussions relied on having a stock list of recommended cleansers, moisturizers, and sunscreens. In regards to cosmeceuticals for facial skin specifically, there were only a handful of instances in which alternative ingredients, such as vitamin C for hyperpigmentation, were discussed and specific brands were mentioned. Upon reflection, I wish I had more opportunity to see the clinical benefits of cosmeceuticals first hand, just like when I observe dupilumab clear patients with severe atopic dermatitis, rather than reading about it in textbooks and journals.
While one hypothesis for programs’ limited attention given to cosmetic training may be that it detracts from medical training, the survey by Bauer et al. found that residents did not feel less prepared (94.9%) or less interested (97.4%) in medical dermatology as a result of their cosmetic training.2 In addition, providers in an academic dermatology residency may limit discussions of skin care because of the high patient volume and because extensive skin care discussions will not impact insurance billings. Academic dermatology programs often service patients with more financial constraints, which further limits OTC cosmeceutical discussions. In my residency experience, I had the opportunity to regularly treat more severe and rare dermatologic cases than those I encounter in private practice; therefore, I spent more time focusing on systemic therapies, with fewer opportunities to dedicate time to cosmeceuticals.
Why skin care and cosmeceuticals should be an essential aspect of residency training
Discussing skin care and cosmeceuticals is a valuable aspect of medical and general dermatology, not just aesthetic dermatology. When treating general dermatologic conditions, guidance on proper skin care can improve both adherence and efficacy of medical treatments. For example, an acne study by de Lucas et al. demonstrated that adherence to adjuvant treatment of acne (such as the use of moisturizers) was associated not only with a 2.4-fold increase in the probability of adherence to pharmacological treatment, but also with a significant reduction in acne severity.5 Aside from skin care, cosmeceuticals themselves have efficacy in treating general dermatologic conditions. In the treatment of acne, topical niacinamide, a popular cosmeceutical ingredient, has been shown to have sebosuppressive and anti-inflammatory effects, addressing key aspects of acne pathogenesis.6 A double-blind study by Draelos et al. reported topical 2% niacinamide was effective in reducing the rate of sebum excretion in 50 Japanese patients over 4 weeks.6 In several double-blind studies that have compared twice daily application of 4% nicotinamide gel with the same application of 1% clindamycin gel in moderate inflammatory acne over 8 weeks, nicotinamide gel reduced the number of inflammatory papules and acne lesions to a level comparable with clindamycin gel.6 These studies support the use of niacinamide cosmeceutical products as an adjunctive treatment for acne.
With increased clinical data supporting cosmeceuticals, it can be expected that some cosmeceuticals will substitute traditional prescription medications in the dermatologists’ arsenal. For example, hydroquinone – both prescription strength and OTC 2% – is a workhorse in treating melasma; however, there is increasing interest in hydroquinone-free treatments, especially since OTC cosmeceuticals containing 2% hydroquinone were banned in 2020 because of safety concerns. Dermatologists will therefore need to provide guidance about hydroquinone alternatives for skin lightening, including soy, licorice extracts, kojic acid, arbutin, niacinamide, N-acetylglucosamine, and vitamin C, among others.7 Utilizing knowledge of a cosmeceutical’s mechanisms of action and clinical data, the dermatologist is in the best position to guide patients toward optimal ingredients and dispel cosmeceutical myths. Given that cosmeceuticals are not regulated by the Food and Drug Administration, it is even more important that the dermatologist serves as an authority on cosmeceuticals.
How to become a master skin care and cosmeceutical prescriber
A common pitfall I have observed among practitioners less experienced with aesthetic-focused skin care and cosmeceuticals is adapting a one-size-fits-all approach. In the one-size-fits-all approach, every patient concerned about aging gets the same vitamin C serum and retinoid, and every patient with hyperpigmentation gets the same hydroquinone prescription, for example. This approach, however, does not take into account unique differences in patients’ skin. Below
is the basic skin care framework that I follow, taught to me by Dr. Baumann. It utilizes an individualized approach based on the patient’s skin qualities to achieve optimal results.
Determine the patient’s skin type (dry vs. oily; sensitive vs. not sensitive; pigmentation issues vs. no hyperpigmentation; wrinkled and mature vs. nonwrinkled) and identify concerns (e.g., dark spots, redness, acne, dehydration).
Separate products into categories of cleansers, eye creams, moisturizers, sun protection, and treatments. Treatments refers to any additional products in a skin care regimen intended to ameliorate a particular condition (e.g., vitamin C for hyperpigmentation, retinoids for fine lines).
Choose products for each category in step 2 (cleansers, eye creams, moisturizers, sun protection, treatments) that are complementary to the patient’s skin type (determined in step 1) and aid the patient in meeting their particular skin goals. For example, a salicylic acid cleanser would be beneficial for a patient with oily skin and acne, but this same cleanser may be too drying and irritating for an acne patient with dry skin.
Ensure that chosen ingredients and products work together harmoniously. For example, while the acne patient may benefit from a salicylic acid cleanser and retinoid cream, using them in succession initially may be overly drying for some patients.
Spend the time to make sure patients understand the appropriate order of application and recognize when efficacy of a product is impacted by another product in the regimen. For example, a low pH cleanser can increase penetration of an ascorbic acid product that follows it in the regimen.
After establishing a basic skin care framework, the next step for beginners is learning about ingredients and their mechanisms of action and familiarizing themselves with scientific and clinical studies. Until cosmeceuticals become an integral part of the training curriculum, dermatologists can gain knowledge independently by reading literature and studies on cosmeceutical active ingredients and experimenting with consumer products. I look forward to regularly contributing to this column to further our awareness and understanding of the mechanisms of and data supporting cosmeceuticals so that we can better guide our patients.
Please feel free to email me at [email protected] or message me on Instagram @DrChloeGoldman with ideas that you would like me to address in this column.
Dr. Goldman is a dermatologist in private practice in Miami, and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a new general dermatology practice. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other relevant disclosures.
References
1. Kirby JS et al. J Am Acad Dermatol. 2013;68(2):e23-8.
2. Bauer et al. JAMA Dermatol. 2014;150(2):125-9.
3. Group A et al. Dermatol Surg. 2012;38(12):1975-80.
4. Feetham HJ et al. J Cosmet Dermatol. 2018;17(2):220-6.
5. de Lucas R et al. BMC Dermatol. 2015;15:17.
6. Araviiskaia E and Dreno BJ. Eur Acad Dermatol Venereol. 2016;30(6):926-35.
7. Leyden JJ et al. J Eur Acad Dermatol Venereol. 2011;25(10):1140-5.
Clay minerals and the skin
Natural clay from the earth and its minerals, imperative for survival of life on our planet, have been used for medicinal purposes for thousands of years. , and for some, the environmental and sustainability viewpoint that minerals will not harm the environment after disposal.
Clay minerals namely consist of silica, alumina, and/or magnesia, and sometimes varying degrees of iron, sodium, potassium, calcium, and water. Depending on the type of clay, as many as 75 trace minerals may be present.
Ochre
The first uses of ochre, or natural clay earth pigment, are thought to be by Homo erectus and Homo neanderthalensis who used ochre with water to soothe irritations, heal wounds, and clean skin. The theory is that they mimicked some animals who instinctively used clay/mud/minerals in this manner.
The first recorded use of medicinal clay is on Mesopotamian clay tablets, dating to about 2500 B.C. The ancient Egyptian physicians used clays as anti-inflammatory agents and antiseptics. Clay was also used as a preservative during mummification.
Throughout history, clay has been used for dermatologic purposes. Aristotle (384-322 BC) made one of the first references to deliberately eating clay, earth, or soil by humans for therapeutic and religious purposes. Later, Marco Polo described in his travels seeing Muslim pilgrims cure fevers by ingesting “pink earth.”
The ochres have also long been found in indigenous and aboriginal art, and in current day Namibia, the Himba tribe have used Otjize paste (bright red clay consisting of butterfat, red ochre, and sometimes herbs) for their characteristic hairstyles and makeup, as well as for skin protection and as a soap replacement. Otjize is sacred to the culture and ethnic identity, signifying the beauty of their hair and skin and a sense of oneness with their surroundings (the earth). There are also many instances of religious, folklore, or mythological references of creation of life or creation of humankind from clay.
Dermatologic uses
The most common uses of clay in dermatology are for treatment of acne and in spa or cosmeceutical preparations to purportedly draw out dirt, impurities, or toxins.
Clay minerals are most commonly formed from prolonged chemical weathering of silicate-bearing rocks. Clay can also be formed from hydrothermal or volcanic activity. Chemical weathering takes place mainly by acid hydrolysis resulting from low concentrations of carbonic acid, dissolved in rainwater or released by plant roots. Clays differ in composition and structure depending upon the source. Simplistically, clay is structured in two layers, organized in various shapes, with varying minerals and electrical charges. The electric charge of clay allows the adsorption of various minerals, water, heavy and radioactive metals, free radicals, and other potentially unwanted byproducts of metabolic activity. With antibacterial properties and adsorptive properties, clay is often used to dry out acne or oily skin and/or to improve the appearance of large pores.
Bentonite clay
Bentonite clay is one of the most common forms of clay used in topical skin products. Bentonite clay, formed after volcanic ash has weathered and aged in the presence of water, is named after a formation called Benton Shale in the western United States. Bentonite has a strong negative electromagnetic charge and when mixed with water it swells like a sponge and can absorb 40-50 times its weight.
There are several types of Bentonite clay, named from the dominant element found within: Sodium bentonite, calcium bentonite, aluminum bentonite, and potassium bentonite are the most common. Bentonite clay is most commonly found in off-white or green colors.
Kaolin and red clay
Typically white or nearly white to sometimes gray in color, kaolin clay is one of the other most common types of clay used in skin care. While the minerals of the kaolin group display a relatively small specific surface area, compared with those of other clay groups, they can still adsorb small molecules, proteins, bacteria, and viruses on the surface of their particles.
Red clay, also sometimes seen in skin care, takes on its color because of a higher content of iron oxides.
In a 2011 study, Valenti et al. evaluated the impact of daily application of clay and retinoic acid 0.025% on the skin of rats.After 7 days, skin where clay had been applied showed a significant increase in collagen fibers, compared with control skin, while areas where retinoic acid had been applied did not show a significant increase in collagen fibers, compared with control skin.2
A recently published study claims that pH and its interaction with the clay particle surface charge may neutralize and impact properties – including antibacterial properties – of clay and is more significant than previously thought.3 The authors emphasize the dangers of this possibility with unregulated marketing and unsubstantiated bioceutical claims of products that contain clay. Many clay-based skin care products on the market today include other ingredients such as acids (for example salicylic acid, lactic acid, and malic acid) that may potentially counteract this issue and help enhance the targeted efficacy of the product.
The types and characteristics of all types of clay go beyond the scope of this column, but as demonstrated throughout history, clay may have a role in medicinal and dermatologic care, the research of which is still ongoing and is important in our understanding of how this earthly compound can affect our bodies.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
1: Moraes JDD et al. Int J Pharm. 2017 Dec 20;534(1-2):213-219. doi: 10.1016/j.ijpharm.2017.10.031.
2: Valenti DMZ et al. Clin Exp Dermatol. 2012 Mar;37(2):164-8. doi: 10.1111/j.1365-2230.2011.04216.x
3. Incledion A et al. Biomolecules. 2021 Jan 5;11(1):58. doi: 10.3390/biom11010058.
Natural clay from the earth and its minerals, imperative for survival of life on our planet, have been used for medicinal purposes for thousands of years. , and for some, the environmental and sustainability viewpoint that minerals will not harm the environment after disposal.
Clay minerals namely consist of silica, alumina, and/or magnesia, and sometimes varying degrees of iron, sodium, potassium, calcium, and water. Depending on the type of clay, as many as 75 trace minerals may be present.
Ochre
The first uses of ochre, or natural clay earth pigment, are thought to be by Homo erectus and Homo neanderthalensis who used ochre with water to soothe irritations, heal wounds, and clean skin. The theory is that they mimicked some animals who instinctively used clay/mud/minerals in this manner.
The first recorded use of medicinal clay is on Mesopotamian clay tablets, dating to about 2500 B.C. The ancient Egyptian physicians used clays as anti-inflammatory agents and antiseptics. Clay was also used as a preservative during mummification.
Throughout history, clay has been used for dermatologic purposes. Aristotle (384-322 BC) made one of the first references to deliberately eating clay, earth, or soil by humans for therapeutic and religious purposes. Later, Marco Polo described in his travels seeing Muslim pilgrims cure fevers by ingesting “pink earth.”
The ochres have also long been found in indigenous and aboriginal art, and in current day Namibia, the Himba tribe have used Otjize paste (bright red clay consisting of butterfat, red ochre, and sometimes herbs) for their characteristic hairstyles and makeup, as well as for skin protection and as a soap replacement. Otjize is sacred to the culture and ethnic identity, signifying the beauty of their hair and skin and a sense of oneness with their surroundings (the earth). There are also many instances of religious, folklore, or mythological references of creation of life or creation of humankind from clay.
Dermatologic uses
The most common uses of clay in dermatology are for treatment of acne and in spa or cosmeceutical preparations to purportedly draw out dirt, impurities, or toxins.
Clay minerals are most commonly formed from prolonged chemical weathering of silicate-bearing rocks. Clay can also be formed from hydrothermal or volcanic activity. Chemical weathering takes place mainly by acid hydrolysis resulting from low concentrations of carbonic acid, dissolved in rainwater or released by plant roots. Clays differ in composition and structure depending upon the source. Simplistically, clay is structured in two layers, organized in various shapes, with varying minerals and electrical charges. The electric charge of clay allows the adsorption of various minerals, water, heavy and radioactive metals, free radicals, and other potentially unwanted byproducts of metabolic activity. With antibacterial properties and adsorptive properties, clay is often used to dry out acne or oily skin and/or to improve the appearance of large pores.
Bentonite clay
Bentonite clay is one of the most common forms of clay used in topical skin products. Bentonite clay, formed after volcanic ash has weathered and aged in the presence of water, is named after a formation called Benton Shale in the western United States. Bentonite has a strong negative electromagnetic charge and when mixed with water it swells like a sponge and can absorb 40-50 times its weight.
There are several types of Bentonite clay, named from the dominant element found within: Sodium bentonite, calcium bentonite, aluminum bentonite, and potassium bentonite are the most common. Bentonite clay is most commonly found in off-white or green colors.
Kaolin and red clay
Typically white or nearly white to sometimes gray in color, kaolin clay is one of the other most common types of clay used in skin care. While the minerals of the kaolin group display a relatively small specific surface area, compared with those of other clay groups, they can still adsorb small molecules, proteins, bacteria, and viruses on the surface of their particles.
Red clay, also sometimes seen in skin care, takes on its color because of a higher content of iron oxides.
In a 2011 study, Valenti et al. evaluated the impact of daily application of clay and retinoic acid 0.025% on the skin of rats.After 7 days, skin where clay had been applied showed a significant increase in collagen fibers, compared with control skin, while areas where retinoic acid had been applied did not show a significant increase in collagen fibers, compared with control skin.2
A recently published study claims that pH and its interaction with the clay particle surface charge may neutralize and impact properties – including antibacterial properties – of clay and is more significant than previously thought.3 The authors emphasize the dangers of this possibility with unregulated marketing and unsubstantiated bioceutical claims of products that contain clay. Many clay-based skin care products on the market today include other ingredients such as acids (for example salicylic acid, lactic acid, and malic acid) that may potentially counteract this issue and help enhance the targeted efficacy of the product.
The types and characteristics of all types of clay go beyond the scope of this column, but as demonstrated throughout history, clay may have a role in medicinal and dermatologic care, the research of which is still ongoing and is important in our understanding of how this earthly compound can affect our bodies.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
1: Moraes JDD et al. Int J Pharm. 2017 Dec 20;534(1-2):213-219. doi: 10.1016/j.ijpharm.2017.10.031.
2: Valenti DMZ et al. Clin Exp Dermatol. 2012 Mar;37(2):164-8. doi: 10.1111/j.1365-2230.2011.04216.x
3. Incledion A et al. Biomolecules. 2021 Jan 5;11(1):58. doi: 10.3390/biom11010058.
Natural clay from the earth and its minerals, imperative for survival of life on our planet, have been used for medicinal purposes for thousands of years. , and for some, the environmental and sustainability viewpoint that minerals will not harm the environment after disposal.
Clay minerals namely consist of silica, alumina, and/or magnesia, and sometimes varying degrees of iron, sodium, potassium, calcium, and water. Depending on the type of clay, as many as 75 trace minerals may be present.
Ochre
The first uses of ochre, or natural clay earth pigment, are thought to be by Homo erectus and Homo neanderthalensis who used ochre with water to soothe irritations, heal wounds, and clean skin. The theory is that they mimicked some animals who instinctively used clay/mud/minerals in this manner.
The first recorded use of medicinal clay is on Mesopotamian clay tablets, dating to about 2500 B.C. The ancient Egyptian physicians used clays as anti-inflammatory agents and antiseptics. Clay was also used as a preservative during mummification.
Throughout history, clay has been used for dermatologic purposes. Aristotle (384-322 BC) made one of the first references to deliberately eating clay, earth, or soil by humans for therapeutic and religious purposes. Later, Marco Polo described in his travels seeing Muslim pilgrims cure fevers by ingesting “pink earth.”
The ochres have also long been found in indigenous and aboriginal art, and in current day Namibia, the Himba tribe have used Otjize paste (bright red clay consisting of butterfat, red ochre, and sometimes herbs) for their characteristic hairstyles and makeup, as well as for skin protection and as a soap replacement. Otjize is sacred to the culture and ethnic identity, signifying the beauty of their hair and skin and a sense of oneness with their surroundings (the earth). There are also many instances of religious, folklore, or mythological references of creation of life or creation of humankind from clay.
Dermatologic uses
The most common uses of clay in dermatology are for treatment of acne and in spa or cosmeceutical preparations to purportedly draw out dirt, impurities, or toxins.
Clay minerals are most commonly formed from prolonged chemical weathering of silicate-bearing rocks. Clay can also be formed from hydrothermal or volcanic activity. Chemical weathering takes place mainly by acid hydrolysis resulting from low concentrations of carbonic acid, dissolved in rainwater or released by plant roots. Clays differ in composition and structure depending upon the source. Simplistically, clay is structured in two layers, organized in various shapes, with varying minerals and electrical charges. The electric charge of clay allows the adsorption of various minerals, water, heavy and radioactive metals, free radicals, and other potentially unwanted byproducts of metabolic activity. With antibacterial properties and adsorptive properties, clay is often used to dry out acne or oily skin and/or to improve the appearance of large pores.
Bentonite clay
Bentonite clay is one of the most common forms of clay used in topical skin products. Bentonite clay, formed after volcanic ash has weathered and aged in the presence of water, is named after a formation called Benton Shale in the western United States. Bentonite has a strong negative electromagnetic charge and when mixed with water it swells like a sponge and can absorb 40-50 times its weight.
There are several types of Bentonite clay, named from the dominant element found within: Sodium bentonite, calcium bentonite, aluminum bentonite, and potassium bentonite are the most common. Bentonite clay is most commonly found in off-white or green colors.
Kaolin and red clay
Typically white or nearly white to sometimes gray in color, kaolin clay is one of the other most common types of clay used in skin care. While the minerals of the kaolin group display a relatively small specific surface area, compared with those of other clay groups, they can still adsorb small molecules, proteins, bacteria, and viruses on the surface of their particles.
Red clay, also sometimes seen in skin care, takes on its color because of a higher content of iron oxides.
In a 2011 study, Valenti et al. evaluated the impact of daily application of clay and retinoic acid 0.025% on the skin of rats.After 7 days, skin where clay had been applied showed a significant increase in collagen fibers, compared with control skin, while areas where retinoic acid had been applied did not show a significant increase in collagen fibers, compared with control skin.2
A recently published study claims that pH and its interaction with the clay particle surface charge may neutralize and impact properties – including antibacterial properties – of clay and is more significant than previously thought.3 The authors emphasize the dangers of this possibility with unregulated marketing and unsubstantiated bioceutical claims of products that contain clay. Many clay-based skin care products on the market today include other ingredients such as acids (for example salicylic acid, lactic acid, and malic acid) that may potentially counteract this issue and help enhance the targeted efficacy of the product.
The types and characteristics of all types of clay go beyond the scope of this column, but as demonstrated throughout history, clay may have a role in medicinal and dermatologic care, the research of which is still ongoing and is important in our understanding of how this earthly compound can affect our bodies.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
1: Moraes JDD et al. Int J Pharm. 2017 Dec 20;534(1-2):213-219. doi: 10.1016/j.ijpharm.2017.10.031.
2: Valenti DMZ et al. Clin Exp Dermatol. 2012 Mar;37(2):164-8. doi: 10.1111/j.1365-2230.2011.04216.x
3. Incledion A et al. Biomolecules. 2021 Jan 5;11(1):58. doi: 10.3390/biom11010058.
Buccal Fat Pad Reduction With Intraoperative Fat Transfer to the Temple
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
Practice Points
- Buccal fat pad reduction is an increasingly popular procedure for facial shaping.
- Buccal fat pad reduction in addition to natural aging can result in volume depletion of the temporal fossae.
- Removed buccal fat can be transferred to the temples for increased volume.
Increased AD severity linked to more frequent baths and showers, but not with duration
of showers or baths, results from a prospective observational study found.
“Patients may benefit most from counseling on showering or bathing once daily and regularly applying moisturizer after showering or bathing,” one of the study authors, Uros Rakita, MSc, told this news organization. “Recommending less than daily shower frequencies or counseling on specific shower durations may not be necessary.”
During a late-breaking abstract session at the Revolutionizing Atopic Dermatitis symposium, Mr. Rakita, a fourth-year student at Chicago Medical School, North Chicago, presented findings from a prospective, practice-based dermatology study that investigated the longitudinal relationship between different bathing practices and AD severity to help inform patient counseling about optimal bathing practices.
“AD is a chronic, inflammatory skin condition with a diverse set of environmental triggers and exacerbating factors,” Mr. Rakita said during the meeting. “Maintaining adequate skin hydration, skin hygiene, and avoiding triggers are key aspects of AD management across all disease severities. Therefore, understanding optimal shower or bath and moisturizing practices is essential.” In fact, he added, “bathing has been shown to not only hydrate the skin, but also to improve symptoms, remove allergens, and decrease [Staphylococcus] aureus colonization. However, at the same time, concern exists for the potential of inappropriate shower or bathing frequency or durations, as well as inconsistent moisturizer application to worsen disease severity and potentially compromise disease management.”
He noted that current guidelines on bathing frequency and duration among AD patients lack consensus, are limited, and are largely based on studies of pediatric populations.
Mr. Rakita, along with primary study author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, and Trisha Kaundinya, a medical student at Northwestern University, Chicago, prospectively evaluated 509 adults with AD who made an average of 2.3 visits at a single dermatology clinic between 2013 and 2020. At each visit, severity of AD signs and symptoms, as well as bathing and moisturizing practices, were assessed.
AD severity was assessed using the objective component of Scoring Atopic Dermatitis (o-SCORAD), intensity of pruritus in the past 3 days (SCORAD-itch), Eczema Area and Severity Index (EASI), Patient-Oriented Eczema Measure (POEM), and Dermatology Life Quality Index (DLQI). The researchers constructed repeated measures regression models to examine associations of bathing and moisturizing practices with change in AD severity outcome measure scores over time. Multivariable models controlled for age, sex, and race.
In adjusted linear regression models, showering or bathing more than once a day versus once daily was associated with significantly higher scores for SCORAD-itch (0.74; P = .0456), o-SCORAD (4.27; P = .0171), EASI (4.20; P = .0028), POEM (2.61; P = .0021), and DLQI (2.77; P = .0004).
The researchers also found that consistent application of moisturizer after the shower or bath was associated with significantly lower scores for o-SCORAD (–7.22; P < .0001), EASI (–3.91; P = .001) and POEM (–2.68; P = .0002), compared against not applying moisturizer after a shower or bath. However, shower or bath duration of more than, compared against fewer than, 15 minutes was not associated with significantly lower scores for o-SCORAD (1.26; P = .2868), SCORAD-itch (0.17; P = .4987), EASI (0.85; P = .3454), POEM (0.24; P = .6627) or DLQI (–0.40; P = .4318).
“Interestingly, this pattern was present when the reference shower or bath durations were under 10 minutes as well as under 5 minutes,” Mr. Rakita said. “Also, shower or bath frequencies of less than daily, relative to daily frequencies, were not significantly related to longitudinal AD severity.”
Mr. Rakita acknowledged certain limitations of the study, including the fact that the researchers did not examine the potential influence of specific soap and moisturizing products, water hardness, or other bathing features such as water temperature and bath additives.
Lawrence J. Green, MD, who was asked to comment on the study, said that he was not surprised by the finding that moisturizing after bathing improved AD signs and symptoms. “On the other hand, a long-held belief that longer duration of shower/bath time worsens AD was not found to be true,” said Dr. Green, a dermatologist who practices in Rockville, Md., and is also clinical professor of dermatology at George Washington University.
“This provides useful information for practicing dermatologists who wish to provide evidenced-based education about moisturizing and bathing to their AD patients,” he said.
The study was supported by the Agency for Healthcare Research and Quality and the Dermatology Foundation. Dr. Silverberg disclosed that he is a consultant to numerous pharmaceutical companies, receives fees for non-CME/CE services from Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, as well as contracted research fees from Galderma. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies. There were no other disclosures.
A version of this article first appeared on Medscape.com.
of showers or baths, results from a prospective observational study found.
“Patients may benefit most from counseling on showering or bathing once daily and regularly applying moisturizer after showering or bathing,” one of the study authors, Uros Rakita, MSc, told this news organization. “Recommending less than daily shower frequencies or counseling on specific shower durations may not be necessary.”
During a late-breaking abstract session at the Revolutionizing Atopic Dermatitis symposium, Mr. Rakita, a fourth-year student at Chicago Medical School, North Chicago, presented findings from a prospective, practice-based dermatology study that investigated the longitudinal relationship between different bathing practices and AD severity to help inform patient counseling about optimal bathing practices.
“AD is a chronic, inflammatory skin condition with a diverse set of environmental triggers and exacerbating factors,” Mr. Rakita said during the meeting. “Maintaining adequate skin hydration, skin hygiene, and avoiding triggers are key aspects of AD management across all disease severities. Therefore, understanding optimal shower or bath and moisturizing practices is essential.” In fact, he added, “bathing has been shown to not only hydrate the skin, but also to improve symptoms, remove allergens, and decrease [Staphylococcus] aureus colonization. However, at the same time, concern exists for the potential of inappropriate shower or bathing frequency or durations, as well as inconsistent moisturizer application to worsen disease severity and potentially compromise disease management.”
He noted that current guidelines on bathing frequency and duration among AD patients lack consensus, are limited, and are largely based on studies of pediatric populations.
Mr. Rakita, along with primary study author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, and Trisha Kaundinya, a medical student at Northwestern University, Chicago, prospectively evaluated 509 adults with AD who made an average of 2.3 visits at a single dermatology clinic between 2013 and 2020. At each visit, severity of AD signs and symptoms, as well as bathing and moisturizing practices, were assessed.
AD severity was assessed using the objective component of Scoring Atopic Dermatitis (o-SCORAD), intensity of pruritus in the past 3 days (SCORAD-itch), Eczema Area and Severity Index (EASI), Patient-Oriented Eczema Measure (POEM), and Dermatology Life Quality Index (DLQI). The researchers constructed repeated measures regression models to examine associations of bathing and moisturizing practices with change in AD severity outcome measure scores over time. Multivariable models controlled for age, sex, and race.
In adjusted linear regression models, showering or bathing more than once a day versus once daily was associated with significantly higher scores for SCORAD-itch (0.74; P = .0456), o-SCORAD (4.27; P = .0171), EASI (4.20; P = .0028), POEM (2.61; P = .0021), and DLQI (2.77; P = .0004).
The researchers also found that consistent application of moisturizer after the shower or bath was associated with significantly lower scores for o-SCORAD (–7.22; P < .0001), EASI (–3.91; P = .001) and POEM (–2.68; P = .0002), compared against not applying moisturizer after a shower or bath. However, shower or bath duration of more than, compared against fewer than, 15 minutes was not associated with significantly lower scores for o-SCORAD (1.26; P = .2868), SCORAD-itch (0.17; P = .4987), EASI (0.85; P = .3454), POEM (0.24; P = .6627) or DLQI (–0.40; P = .4318).
“Interestingly, this pattern was present when the reference shower or bath durations were under 10 minutes as well as under 5 minutes,” Mr. Rakita said. “Also, shower or bath frequencies of less than daily, relative to daily frequencies, were not significantly related to longitudinal AD severity.”
Mr. Rakita acknowledged certain limitations of the study, including the fact that the researchers did not examine the potential influence of specific soap and moisturizing products, water hardness, or other bathing features such as water temperature and bath additives.
Lawrence J. Green, MD, who was asked to comment on the study, said that he was not surprised by the finding that moisturizing after bathing improved AD signs and symptoms. “On the other hand, a long-held belief that longer duration of shower/bath time worsens AD was not found to be true,” said Dr. Green, a dermatologist who practices in Rockville, Md., and is also clinical professor of dermatology at George Washington University.
“This provides useful information for practicing dermatologists who wish to provide evidenced-based education about moisturizing and bathing to their AD patients,” he said.
The study was supported by the Agency for Healthcare Research and Quality and the Dermatology Foundation. Dr. Silverberg disclosed that he is a consultant to numerous pharmaceutical companies, receives fees for non-CME/CE services from Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, as well as contracted research fees from Galderma. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies. There were no other disclosures.
A version of this article first appeared on Medscape.com.
of showers or baths, results from a prospective observational study found.
“Patients may benefit most from counseling on showering or bathing once daily and regularly applying moisturizer after showering or bathing,” one of the study authors, Uros Rakita, MSc, told this news organization. “Recommending less than daily shower frequencies or counseling on specific shower durations may not be necessary.”
During a late-breaking abstract session at the Revolutionizing Atopic Dermatitis symposium, Mr. Rakita, a fourth-year student at Chicago Medical School, North Chicago, presented findings from a prospective, practice-based dermatology study that investigated the longitudinal relationship between different bathing practices and AD severity to help inform patient counseling about optimal bathing practices.
“AD is a chronic, inflammatory skin condition with a diverse set of environmental triggers and exacerbating factors,” Mr. Rakita said during the meeting. “Maintaining adequate skin hydration, skin hygiene, and avoiding triggers are key aspects of AD management across all disease severities. Therefore, understanding optimal shower or bath and moisturizing practices is essential.” In fact, he added, “bathing has been shown to not only hydrate the skin, but also to improve symptoms, remove allergens, and decrease [Staphylococcus] aureus colonization. However, at the same time, concern exists for the potential of inappropriate shower or bathing frequency or durations, as well as inconsistent moisturizer application to worsen disease severity and potentially compromise disease management.”
He noted that current guidelines on bathing frequency and duration among AD patients lack consensus, are limited, and are largely based on studies of pediatric populations.
Mr. Rakita, along with primary study author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, and Trisha Kaundinya, a medical student at Northwestern University, Chicago, prospectively evaluated 509 adults with AD who made an average of 2.3 visits at a single dermatology clinic between 2013 and 2020. At each visit, severity of AD signs and symptoms, as well as bathing and moisturizing practices, were assessed.
AD severity was assessed using the objective component of Scoring Atopic Dermatitis (o-SCORAD), intensity of pruritus in the past 3 days (SCORAD-itch), Eczema Area and Severity Index (EASI), Patient-Oriented Eczema Measure (POEM), and Dermatology Life Quality Index (DLQI). The researchers constructed repeated measures regression models to examine associations of bathing and moisturizing practices with change in AD severity outcome measure scores over time. Multivariable models controlled for age, sex, and race.
In adjusted linear regression models, showering or bathing more than once a day versus once daily was associated with significantly higher scores for SCORAD-itch (0.74; P = .0456), o-SCORAD (4.27; P = .0171), EASI (4.20; P = .0028), POEM (2.61; P = .0021), and DLQI (2.77; P = .0004).
The researchers also found that consistent application of moisturizer after the shower or bath was associated with significantly lower scores for o-SCORAD (–7.22; P < .0001), EASI (–3.91; P = .001) and POEM (–2.68; P = .0002), compared against not applying moisturizer after a shower or bath. However, shower or bath duration of more than, compared against fewer than, 15 minutes was not associated with significantly lower scores for o-SCORAD (1.26; P = .2868), SCORAD-itch (0.17; P = .4987), EASI (0.85; P = .3454), POEM (0.24; P = .6627) or DLQI (–0.40; P = .4318).
“Interestingly, this pattern was present when the reference shower or bath durations were under 10 minutes as well as under 5 minutes,” Mr. Rakita said. “Also, shower or bath frequencies of less than daily, relative to daily frequencies, were not significantly related to longitudinal AD severity.”
Mr. Rakita acknowledged certain limitations of the study, including the fact that the researchers did not examine the potential influence of specific soap and moisturizing products, water hardness, or other bathing features such as water temperature and bath additives.
Lawrence J. Green, MD, who was asked to comment on the study, said that he was not surprised by the finding that moisturizing after bathing improved AD signs and symptoms. “On the other hand, a long-held belief that longer duration of shower/bath time worsens AD was not found to be true,” said Dr. Green, a dermatologist who practices in Rockville, Md., and is also clinical professor of dermatology at George Washington University.
“This provides useful information for practicing dermatologists who wish to provide evidenced-based education about moisturizing and bathing to their AD patients,” he said.
The study was supported by the Agency for Healthcare Research and Quality and the Dermatology Foundation. Dr. Silverberg disclosed that he is a consultant to numerous pharmaceutical companies, receives fees for non-CME/CE services from Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, as well as contracted research fees from Galderma. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies. There were no other disclosures.
A version of this article first appeared on Medscape.com.
FROM REVOLUTIONIZING AD 2021
Is it safe to pair low-power fractional diode lasers with cosmetic injectables in a single session?
, results from a 6-year, single-center review showed.
“These treatments can be complementary in single-session treatments and can offer increased convenience for both patients and physicians,” primary study author Jordan V. Wang, MD, MBE, MBA, said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery.
To date, limited studies have demonstrated the safety of pairing botulinum neurotoxin type A and soft-tissue fillers with laser and other energy-based devices during the same treatment session on the same day, said Dr. Wang, medical research director at the Laser & Skin Surgery Center of New York. “Some concerns remain, though, regarding patient safety and efficacy,” he said. “Data on single-session treatment with low-power, low-density 1,927-nm and 1,440-nm fractional diode lasers and either botulinum neurotoxin or fillers are lacking.”
In a retrospective review of electronic medical records conducted from May 2015 to April 2021, Dr. Wang, Roy G. Geronemus, MD, and Carolyn Kushner, MD evaluated patients who received a single-session facial treatment with either BoNT-A or soft-tissue fillers and the low-power, low-density 1,927-nm and 1,440-nm fractional diode lasers (Clear+Brilliant Perméa and Original, Solta, Pleasanton, Calif.). Safety was assessed by documenting adverse events related to the spread of BoNT-A and fillers or laser treatment of filled areas within 4 weeks.
Adverse events they looked for related to botulinum neurotoxin use included eyelid ptosis; neck weakness or spasms; impairments in chewing, swallowing, speech, and respiration; and prescriptions of apraclonidine eye drops. Filler-related adverse events they looked for included product migration, unexpected loss of filler volume, vascular occlusion, acute pain, necrosis, blindness, and burn. “For both, we looked at hospital or emergency room transfers or admissions and referrals to ENT or ophthalmology,” Dr. Wang said.
During the 6-year study period, 525 patients had 1,562 single-session laser treatments with a mean 46.4 units of BoNT-A, and 398 patients had 1,237 single-session treatments with a mean 1.6 soft-tissue filler syringes. Among those who received BoNT-A, most (93%) were female, their mean age was 51 years, and 99% were treated with a 1,927-nm wavelength at a medium setting in 87% of cases. The top five injection sites were glabella (82%), forehead (69%), periorbital area (64%), neck (40%), and jawline and/or masseters (13%).
The researchers noted one case (0.06%) where apraclonidine eye drops were prescribed for ptosis. The patient had undergone eight other single-session treatments without issue. There were no other documented adverse events directly related to spread of BoNT-A. According to Dr. Wang, this rate of ptosis is lower than the incidence with BoNT-A alone in two landmark trials studying its effects on glabellar lines, which was reported as 5.4% and 1.0%.
Among the 398 patients who received soft-tissue fillers, most (94%) were female, their mean age was 54 years, and 99% were treated with a 1927nm wavelength at a medium setting in 97% of cases. The top five injection sites were cheeks and/or tear troughs (89%), perioral area and/or marionette lines (77%), lips (34%), nasolabial folds (19%), and temples (11%), and the mean number of filler syringes per treatment was 1.6. Slightly more than half (51%) had 1 session, while the remainder had 2 to greater than 10 sessions. The researchers observed no documented adverse events related to spread of fillers or laser treatment of filled areas.
“This laser is a low-powered device that creates small, superficial, and transient microchannels, which likely contributes to the safety of single-session treatments with cosmetic injectables,” Dr. Wang said. However, prospective studies are needed to further validate these results, he added.
“With this very mild laser, it is not surprising that combined treatment had no effect,” said Eric F. Bernstein, MD, MSE, director of the Main Line Center for Laser Surgery in Ardmore, Pa., who was asked to comment on the study results. “There have been numerous anecdotal reports of spreading of botulinum toxin effect to areas not in the target area for treatment following a variety of lasers, including the more powerful version of the laser used in this study. In addition, spread following vascular and other lasers has been reported,” he noted
The laser used in this study, Dr. Bernstein continued, “is low powered and emits a wavelength that is very superficially absorbed, resulting in injury to the stratum corneum, superficial epidermis, or possibly the very superficial dermis, and is often used by physician extenders and not physicians – although I suspect this is not the case in the current study. One can have a reasonable degree of confidence when combining this laser with injectables, but these results cannot be extrapolated to other devices.”
The abstract received the annual ASDS Carruthers Award during the meeting. Dr. Wang reported that he is a consultant or advisor to Allergan, Alastin, AVAVA, Cynosure, Lutronic, Novoxel, Sofwave, and Solta. Dr. Bernstein reported having received research funding from Cynosure, Candela, and Acclaro. He also has received consulting fees from Cynosure and holds ownership interest in Candela, Novoxel, OnSite, Joylux, and Acclaro and has served on the advisory board for Novoxel, Cynosure, and Acclaro.
, results from a 6-year, single-center review showed.
“These treatments can be complementary in single-session treatments and can offer increased convenience for both patients and physicians,” primary study author Jordan V. Wang, MD, MBE, MBA, said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery.
To date, limited studies have demonstrated the safety of pairing botulinum neurotoxin type A and soft-tissue fillers with laser and other energy-based devices during the same treatment session on the same day, said Dr. Wang, medical research director at the Laser & Skin Surgery Center of New York. “Some concerns remain, though, regarding patient safety and efficacy,” he said. “Data on single-session treatment with low-power, low-density 1,927-nm and 1,440-nm fractional diode lasers and either botulinum neurotoxin or fillers are lacking.”
In a retrospective review of electronic medical records conducted from May 2015 to April 2021, Dr. Wang, Roy G. Geronemus, MD, and Carolyn Kushner, MD evaluated patients who received a single-session facial treatment with either BoNT-A or soft-tissue fillers and the low-power, low-density 1,927-nm and 1,440-nm fractional diode lasers (Clear+Brilliant Perméa and Original, Solta, Pleasanton, Calif.). Safety was assessed by documenting adverse events related to the spread of BoNT-A and fillers or laser treatment of filled areas within 4 weeks.
Adverse events they looked for related to botulinum neurotoxin use included eyelid ptosis; neck weakness or spasms; impairments in chewing, swallowing, speech, and respiration; and prescriptions of apraclonidine eye drops. Filler-related adverse events they looked for included product migration, unexpected loss of filler volume, vascular occlusion, acute pain, necrosis, blindness, and burn. “For both, we looked at hospital or emergency room transfers or admissions and referrals to ENT or ophthalmology,” Dr. Wang said.
During the 6-year study period, 525 patients had 1,562 single-session laser treatments with a mean 46.4 units of BoNT-A, and 398 patients had 1,237 single-session treatments with a mean 1.6 soft-tissue filler syringes. Among those who received BoNT-A, most (93%) were female, their mean age was 51 years, and 99% were treated with a 1,927-nm wavelength at a medium setting in 87% of cases. The top five injection sites were glabella (82%), forehead (69%), periorbital area (64%), neck (40%), and jawline and/or masseters (13%).
The researchers noted one case (0.06%) where apraclonidine eye drops were prescribed for ptosis. The patient had undergone eight other single-session treatments without issue. There were no other documented adverse events directly related to spread of BoNT-A. According to Dr. Wang, this rate of ptosis is lower than the incidence with BoNT-A alone in two landmark trials studying its effects on glabellar lines, which was reported as 5.4% and 1.0%.
Among the 398 patients who received soft-tissue fillers, most (94%) were female, their mean age was 54 years, and 99% were treated with a 1927nm wavelength at a medium setting in 97% of cases. The top five injection sites were cheeks and/or tear troughs (89%), perioral area and/or marionette lines (77%), lips (34%), nasolabial folds (19%), and temples (11%), and the mean number of filler syringes per treatment was 1.6. Slightly more than half (51%) had 1 session, while the remainder had 2 to greater than 10 sessions. The researchers observed no documented adverse events related to spread of fillers or laser treatment of filled areas.
“This laser is a low-powered device that creates small, superficial, and transient microchannels, which likely contributes to the safety of single-session treatments with cosmetic injectables,” Dr. Wang said. However, prospective studies are needed to further validate these results, he added.
“With this very mild laser, it is not surprising that combined treatment had no effect,” said Eric F. Bernstein, MD, MSE, director of the Main Line Center for Laser Surgery in Ardmore, Pa., who was asked to comment on the study results. “There have been numerous anecdotal reports of spreading of botulinum toxin effect to areas not in the target area for treatment following a variety of lasers, including the more powerful version of the laser used in this study. In addition, spread following vascular and other lasers has been reported,” he noted
The laser used in this study, Dr. Bernstein continued, “is low powered and emits a wavelength that is very superficially absorbed, resulting in injury to the stratum corneum, superficial epidermis, or possibly the very superficial dermis, and is often used by physician extenders and not physicians – although I suspect this is not the case in the current study. One can have a reasonable degree of confidence when combining this laser with injectables, but these results cannot be extrapolated to other devices.”
The abstract received the annual ASDS Carruthers Award during the meeting. Dr. Wang reported that he is a consultant or advisor to Allergan, Alastin, AVAVA, Cynosure, Lutronic, Novoxel, Sofwave, and Solta. Dr. Bernstein reported having received research funding from Cynosure, Candela, and Acclaro. He also has received consulting fees from Cynosure and holds ownership interest in Candela, Novoxel, OnSite, Joylux, and Acclaro and has served on the advisory board for Novoxel, Cynosure, and Acclaro.
, results from a 6-year, single-center review showed.
“These treatments can be complementary in single-session treatments and can offer increased convenience for both patients and physicians,” primary study author Jordan V. Wang, MD, MBE, MBA, said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery.
To date, limited studies have demonstrated the safety of pairing botulinum neurotoxin type A and soft-tissue fillers with laser and other energy-based devices during the same treatment session on the same day, said Dr. Wang, medical research director at the Laser & Skin Surgery Center of New York. “Some concerns remain, though, regarding patient safety and efficacy,” he said. “Data on single-session treatment with low-power, low-density 1,927-nm and 1,440-nm fractional diode lasers and either botulinum neurotoxin or fillers are lacking.”
In a retrospective review of electronic medical records conducted from May 2015 to April 2021, Dr. Wang, Roy G. Geronemus, MD, and Carolyn Kushner, MD evaluated patients who received a single-session facial treatment with either BoNT-A or soft-tissue fillers and the low-power, low-density 1,927-nm and 1,440-nm fractional diode lasers (Clear+Brilliant Perméa and Original, Solta, Pleasanton, Calif.). Safety was assessed by documenting adverse events related to the spread of BoNT-A and fillers or laser treatment of filled areas within 4 weeks.
Adverse events they looked for related to botulinum neurotoxin use included eyelid ptosis; neck weakness or spasms; impairments in chewing, swallowing, speech, and respiration; and prescriptions of apraclonidine eye drops. Filler-related adverse events they looked for included product migration, unexpected loss of filler volume, vascular occlusion, acute pain, necrosis, blindness, and burn. “For both, we looked at hospital or emergency room transfers or admissions and referrals to ENT or ophthalmology,” Dr. Wang said.
During the 6-year study period, 525 patients had 1,562 single-session laser treatments with a mean 46.4 units of BoNT-A, and 398 patients had 1,237 single-session treatments with a mean 1.6 soft-tissue filler syringes. Among those who received BoNT-A, most (93%) were female, their mean age was 51 years, and 99% were treated with a 1,927-nm wavelength at a medium setting in 87% of cases. The top five injection sites were glabella (82%), forehead (69%), periorbital area (64%), neck (40%), and jawline and/or masseters (13%).
The researchers noted one case (0.06%) where apraclonidine eye drops were prescribed for ptosis. The patient had undergone eight other single-session treatments without issue. There were no other documented adverse events directly related to spread of BoNT-A. According to Dr. Wang, this rate of ptosis is lower than the incidence with BoNT-A alone in two landmark trials studying its effects on glabellar lines, which was reported as 5.4% and 1.0%.
Among the 398 patients who received soft-tissue fillers, most (94%) were female, their mean age was 54 years, and 99% were treated with a 1927nm wavelength at a medium setting in 97% of cases. The top five injection sites were cheeks and/or tear troughs (89%), perioral area and/or marionette lines (77%), lips (34%), nasolabial folds (19%), and temples (11%), and the mean number of filler syringes per treatment was 1.6. Slightly more than half (51%) had 1 session, while the remainder had 2 to greater than 10 sessions. The researchers observed no documented adverse events related to spread of fillers or laser treatment of filled areas.
“This laser is a low-powered device that creates small, superficial, and transient microchannels, which likely contributes to the safety of single-session treatments with cosmetic injectables,” Dr. Wang said. However, prospective studies are needed to further validate these results, he added.
“With this very mild laser, it is not surprising that combined treatment had no effect,” said Eric F. Bernstein, MD, MSE, director of the Main Line Center for Laser Surgery in Ardmore, Pa., who was asked to comment on the study results. “There have been numerous anecdotal reports of spreading of botulinum toxin effect to areas not in the target area for treatment following a variety of lasers, including the more powerful version of the laser used in this study. In addition, spread following vascular and other lasers has been reported,” he noted
The laser used in this study, Dr. Bernstein continued, “is low powered and emits a wavelength that is very superficially absorbed, resulting in injury to the stratum corneum, superficial epidermis, or possibly the very superficial dermis, and is often used by physician extenders and not physicians – although I suspect this is not the case in the current study. One can have a reasonable degree of confidence when combining this laser with injectables, but these results cannot be extrapolated to other devices.”
The abstract received the annual ASDS Carruthers Award during the meeting. Dr. Wang reported that he is a consultant or advisor to Allergan, Alastin, AVAVA, Cynosure, Lutronic, Novoxel, Sofwave, and Solta. Dr. Bernstein reported having received research funding from Cynosure, Candela, and Acclaro. He also has received consulting fees from Cynosure and holds ownership interest in Candela, Novoxel, OnSite, Joylux, and Acclaro and has served on the advisory board for Novoxel, Cynosure, and Acclaro.
FROM ASDS 2021