User login
Adult with ADHD? Try medication + psychotherapy
Mr. B, age 50, dreams of becoming a computer programmer but fears he will embarrass himself—as he has in many classrooms before. He is seeking evaluation because his teenage son was recently diagnosed with attention-deficit/hyperactivity disorder (ADHD), and he recognizes similar symptoms in himself.
Mr. B received a college degree with great difficulty, putting off assignments until the last minute and “squeaking by.” For years he has changed occupations often, never progressing beyond entry level, and now works as a personal care provider and limousine driver. He reports problems keeping up with work and managing time.
His history includes early childhood hyperactivity, difficulty sitting through classes, sloppy handwriting, disorganization, short attention span, and distractibility. He is restless, fidgety, and has trouble staying on topic. His disorganization has caused marital difficulties, for which he has sought counseling.
After careful evaluation, you determine that Mr. B meets criteria for ADHD, combined type, and for anxiety disorder not otherwise specified. His treatment goals are to increase his ability to focus; procrastinate less; improve his planning, prioritizing, and self-esteem; and to become less sensitive to criticism and less anxious about handling work demands.
Like Mr. B, adults with ADHD need treatment for the disorder’s core symptoms as well as its psychiatric comorbidities and psychosocial consequences. Comprehensive treatment with medications, cognitive-behavioral therapy (CBT), and environmental adaptations is usually recommended.
Comorbidity rules
Core symptoms. ADHD is a lifespan disorder with multiple behavioral, cognitive, and emotional manifestations that impair relationships and academic and vocational functioning. ADHD-like symptoms are seen in other conditions such as mood disorders or substance abuse, but complaints of inattention, distractibility, procrastination, restlessness, and impulsivity—particularly when pervasive and chronic—are highly indicative of ADHD.
In treating adults with ADHD, we have noticed common behavioral patterns that contribute to their psychosocial problems (Table 1). Dysfunctional coping behaviors have short-term advantages, but patients readily admit they would rather accomplish tasks through greater thought and planning.
Chronic frustrations—often associated with deep shame—are typical of adult ADHD. Many patients have maladaptive core beliefs of failure, self-mistrust, and inadequacy (Table 2).
Table 1
Common dysfunctional behavioral patterns in adults with ADHD
| Behavior | Description | Short-term gain/long-term loss |
|---|---|---|
| Anticipatory avoidance | Magnifying the difficulty of a pending task and doubts about being able to complete it; results in rationalizations to justify procrastination | Defers short-term stress, but often creates a self-fulfilling prophecy because the task looms and may seem overwhelming when facing a deadline |
| Brinksmanship | Waiting until the last moment (eg, the night before) to complete a task, often when facing an impending deadline | Deadline-associated stress can be focusing, but this tactic leaves little room for error and may yield a substandard result |
| Pseudoefficiency | Completing several low-priority, manageable tasks (eg, checking e-mail) but avoiding high-priority tasks (eg, a project for work) | Creates sense of productivity by reducing items on to-do list but defers a more difficult project |
| Juggling | Taking on new, exciting projects and feeling ‘busy’ without completing projects already started | It is easier to become motivated to start a novel project than to complete an ongoing one; pattern usually results in several incomplete projects |
Table 2
5 common maladaptive core beliefs of adults with ADHD
| Self-mistrust | ‘I cannot rely on myself to do what I need to do. I let myself down’ |
| Failure | ‘I always have failed and always will fail at what I set out to do.’ |
| Inadequacy | ‘I am basically a bad and defective person.’ |
| Incompetence | ‘I am too inept to handle life’s basic demands.’ |
| Instability | ‘My life will always be chaotic and in turmoil.’ |
Psychiatric comorbidity is the rule in adults with ADHD (Table 3). For example, among 43 patients who received combined medication and CBT at the University of Pennsylvania Adult ADHD Treatment and Research Program, 75% reported at least one comorbid condition, including:
- 27 (63%) with mood disorder
- 23 (54%) with anxiety disorder
- 5 (12%) with substance abuse.1
Other treatment studies have reported similar comorbidity rates in adults with ADHD.2-4
Table 3
Psychiatric comorbidity in adult ADHD
| Disorder | Prevalence |
|---|---|
| Mood disorders | 50% to 65% |
| Recurrent depression | |
| Bipolar disorder | |
| Cyclothymia | |
| Dysthymia | |
| Depressive disorder NOS | |
| Anxiety disorders | 40% to 55% |
| Generalized anxiety disorder | |
| Anxiety disorder NOS | |
| Others | Various |
| Substance use disorder | |
| Learning disabilities | |
| Intermittent explosive disorder | |
| Tourette syndrome | |
| Antisocial personality | |
| Borderline personality disorder | |
| Dependent personality | |
| NOS: Not otherwise specified | |
Making the diagnosis
Diagnosis of adult ADHD is based on a comprehensive assessment, including:
- careful history of presenting complaints
- thorough review of educational, occupational, and family history
- standardized rating scales (such as the Barkley ADHD Behavior Checklists, the Conners’ Adult ADHD Rating Scale, or the Brown Attention Deficit Disorder Scales)
- collateral information
- assessment of mood, anxiety, substance use, and learning/organizational skills. For details, consult references on adult ADHD.5-8
Case continued: Self-fulfilling prophesies
On standardized rating scales, Mr. B meets criteria for combined ADHD for childhood and current symptoms. Information from his wife and brother also confirms the ADHD diagnosis.
He is motivated, resilient, optimistic, and has a good support system. However, his negative automatic thoughts about his ability to succeed in school and to handle increasing time demands suggest deeper beliefs of inadequacy and failure.
Mr. B struggled academically. Without guidance about how to change his approach to difficult situations, he has repeated old thinking and behavior patterns. Believing he will embarrass himself and fail to learn required material, Mr. B procrastinates and avoids doing assignments. In class, his feelings of inadequacy make him self-conscious, which causes him to lose focus and have trouble concentrating.
See the world through the patient’s eyes
Understanding your patient. Before you start treatment, we recommend that you conceptualize how ADHD has influenced your patient’s life, including:
- developmental experiences
- family-of-origin issues, such as conflicts with parents stemming from ADHD symptoms or reciprocal interactions with an ADHD parent
- world view (“schemata”)
- patterns of coping with (or avoiding) stress
- attitudes toward self and important others
- readiness to change.
Developing a working case conceptualization is a dynamic, collaborative process. You talk with patients, and encourage them to reflect on how ADHD affects their view of themselves and their important relationships. The conceptualization takes shape as you:
- observe patients’ behaviors
- elicit how they think and feel
- assess with them the relevance and accuracies of their belief systems and response patterns.
Seeing the world “through their eyes” prepares you to help them accept the diagnosis and learn to manage ADHD symptoms. Then, by providing a blueprint to manage what patients may see as uncontrollable responses, you can help them take charge of their automatic reactions.
Psychoeducation. To set the stage for treatment, encourage patients to learn about ADHD by reading articles and books and consulting Web sites for adults with ADHD (see Related resources). Psychoeducation helps patients:
- review possible treatment approaches, including organizational (environmental) management, medication, and psychotherapy (individual or group)
- become informed participants in setting treatment goals.
Explain the relative contribution of each treatment component. For example, medications can reduce distractibility and improve attention, organizational strategies can reduce disorganization and improve time management, and structured psychotherapy can help the patient develop more effective coping skills.
Case continued: Planning combined treatment
You discuss diagnosis and treatment options with Mr. B, and he agrees to start the methylphenidate compound Concerta, initially at 18 mg/d, and weekly CBT sessions. You recommended a stimulant based on efficacy studies and your clinical experience in treating adults with ADHD. Mr. B wants a medication that will help him focus while working or studying, and he says Concerta has improved his son’s ADHD symptoms.
You instruct Mr. B to increase the dosage by 18 mg each week until he reaches 72 mg/d. You also tell him to keep a medication response log and to note any positive changes and side effects.
If an adult with ADHD expresses preference for a particular medication, we usually prescribe that one first. Most patients to whom we offer both medication and psychotherapy agree to this “top-down” and “bottom-up” approach. “Top down” means giving patients new ways of thinking to help them understand and modify their responses. “Bottom up” refers to the medication reducing their impulsivity, distractibility, and inattentiveness.
CBT for adult ADHD
Medications can ameliorate key symptoms of adult ADHD, but adjunctive interventions are needed to improve functioning and quality of life. Evidence supporting psychosocial treatment for adults with ADHD is limited, but CBT has been studied the most.1,9-13 Safren et al13 found a four-fold greater therapeutic response when patients received adjunctive CBT for residual ADHD symptoms, compared with patients who received medication alone.
We usually provide CBT weekly for 12 weeks and then taper to 8 additional sessions over 3 months (total 20 sessions). We may extend CBT with additional sessions to address complicated issues. CBT helps adults with ADHD to:
- identify dysfunctional thinking, feeling, and behaving patterns
- recognize contexts in which patterns arise
- systematically change these patterns.
CBT can reduce ADHD-associated anxiety and depression and improve coping skills and sense of well-being.1,9,11 Its flexibility allows you to address family issues with patients’ partners, children and other relatives to improve communication, reduce conflict, and develop healthier interactions.
We focus CBT sessions on finding alternate coping strategies. We might try role playing, rehearsing, creating “thought experiments,” and anticipating and preparing to modify typical patterns of avoidance. These approaches have been described elsewhere.10,11,14
We adopt an active stance during therapy to keep ADHD patients’ distractibility from disrupting our conversation. For example, we set the therapeutic agenda, provide feedback about patients’ behaviors, and encourage them to clarify rewards and consequences of using (or avoiding) problem-solving strategies.
Although we typically assign between-session homework, we expect patients to have difficulty completing it. We remain nonjudgmental and collaborative, viewing incomplete assignments as opportunities to learn about patients’ unproductive problem solving and to help them develop more-effective patterns.
Challenging maladaptive beliefs. A strong therapeutic relationship allows adults with ADHD to discuss their chronic frustrations, which often are associated with deep shame. We then shift CBT’s focus to deeper ADHD-related schemata that perpetuate dysfunctional patterns.
We work with patients to elucidate and challenge their maladaptive core beliefs and encourage new ways to view themselves and others. Allowing patients to grieve about the limitations ADHD imposes on their lives also helps to reduce chronic negative self-esteem.
Case continued: ‘less frenetic’
Mr. B achieves good results within 3 weeks of an increasing titration of stimulant medication, reporting significantly less restlessness and greater concentration without significant side effects. His wife confirms that he is less frenetic, can converse without interruptions, and is better at managing his complicated work schedule.
Which medications?
Drug therapy for adult ADHD is not as well-studied as in children and adolescents, but American Academy of Child and Adolescent Psychiatry guidelines and others15-18 recommend stimulant and nonstimulant medications. Your choice depends on the patient’s clinical profile (including risk factors and comorbid conditions), past medication use, treatment goals, preferred medication effects and dosing patterns (once-daily versus multiple times), and potential side effects. Stimulants or atomoxetine are first-line choices for adult ADHD without psychiatric comorbidity.
Stimulants work quickly and are cleared relatively rapidly from the brain without causing euphoria or dependency. They are effective (80% to 90% response rate) and well-tolerated, though long-term effects have not been studied in adults (Table 4).
Stimulants’ effect size of 0.9 is considered substantial. Effect size—a statistical method of reporting an intervention’s effect across different studies—is typically rated as:
- <0.32 very small
- 0.33 to 0.54, moderate
- >0.55, significant or very strong.
When choosing a medication, we usually try methylphenidate and amphetamine first, one after the other. We explain to the patient how stimulants work in the brain and the need for a comparative trial to determine which might work best for him or her. If the patient has tried a stimulant and found it helpful, we start with that class. Similarly, if he/she has not had good results with one type, we start with the other. Approximately one-third of our patients respond equally well to methylphenidate or amphetamine, one-third respond better to methylphenidate, and one-third respond better to amphetamine.
To determine the optimal dosage, we usually titrate up from 10 to 30 mg per dose of an immediate-release preparation. We begin with this form to help patients notice the medication’s onset and duration of action. After we find the optimal dosage, we switch to a longer-acting preparation.
Insomnia, mood instability, and euphoria are unacceptable stimulant side effects, although many patients welcome others such as appetite suppression and weight loss. Closely monitor cardiovascular effects, and review potential interactions with other medications, such as antihypertensives or bronchodilators. Because sudden death has been reported with stimulants in persons with structural cardiac lesions,19 obtain a cardiology consultation for patients with a history of heart disease.
We encourage patients to keep daily medication logs (Box), which we review at each visit and use to make dosing or medication changes. Dosing guidelines resemble those used for children and adolescents, although adults usually tolerate higher maximum dosages (such as methylphenidate, 80 to 100 mg/d).
Because of stimulants’ potential for recreational misuse and abuse, remain wary about choosing stimulants for patients with whom you lack a solid doctor-patient relationship.
Table 4
Stimulant dosages used in treating adult ADHD
| Class (brand name) | Daily dosing | Typical dosing schedule |
|---|---|---|
| Methylphenidate | ||
| Short-acting (Metadate, Ritadex, Ritalin) | Two to four times | 10 to 40 mg bid to qid |
| Intermediate-acting (Metadate SR, Ritalin SR) | Once or twice | 20 to 60 mg qd to bid |
| Extended-release (Concerta, Metadate CD, Ritalin LA) | Once or twice | 18 to 108 mg qd (Concerta) 20 to 40 mg bid (Ritalin LA, Metadate CD) |
| Dextromethylphenidate | ||
| Short-acting (Focalin) | Two to four times | 5 to 20 mg bid to qid |
| Long-acting (Focalin XR) | Once or twice | 10 to 20 mg qd or bid |
| Dextroamphetamine | ||
| Short-acting (Dexedrine) | Twice or three times | 10 to 30 mg bid or tid |
| Intermediate-acting (Dexedrine spansules) | Once or twice | 10 to 30 mg bid |
| Mixed amphetamine salts | ||
| Intermediate-acting (Adderall) | Once or twice | 10 to 30 mg bid or tid |
| Extended-release (Adderall XR) | Once or twice | 10 to 40 mg qd or bid |
Atomoxetine, a nonstimulant, norepinephrine re-uptake inhibitor, is approved for ADHD in adults.20-22 In two double-blind, controlled, randomized trials totalling 536 adults, Michaelson et al20 found significantly reduced ADHD symptoms after 10 weeks of atomoxetine treatment. Effect sizes of 0.35 and 0.40 were reported, with 10% of patients discontinuing because of side effects.
Atomoxetine has a long duration of action (>12 hours) but a more gradual onset (4 to 6 weeks) than that of stimulants. Approximately 60% of patients respond to atomoxetine, though effect sizes are less than those of stimulants. We have found atomoxetine works well for patients who:
- do not tolerate or are uncomfortable with taking stimulants
- are highly anxious
- report emotional dysregulation as a major target symptom.
To reduce risk of common side effects (nausea, GI upset, headache, sedation, reduced sex drive), we start with low dosages (such as 25 mg bid) and increase weekly by 25 mg to a target of 80 to 100 mg/d.
Treating complicated ADHD
Bupropion or tricyclic antidepressants are reasonable options for ADHD with depression. Atomoxetine, a tricyclic, or a stimulant plus a selective serotonin reuptake inhibitor (SSRI) can provide good symptom relief for adults with ADHD and comorbid anxiety and/or depression.
Bupropion. Approximately 50% of adults with ADHD respond to bupropion,23,24 with a treatment effect size of 0.6. Bupropion’s efficacy in smoking cessation adds value for those trying to quit.
We usually start extended-release bupropion at 150 mg/d and increase after 2 weeks to 300 mg/d if response is suboptimal. Headache, dry mouth, insomnia, and nausea are the most common adverse effects. Agitation or irritability is sometimes serious enough to warrant stopping bupropion.
Combining medications. Using SSRIs with stimulants can help adults with ADHD and comorbid anxiety or depression. Any SSRI can be safely combined with stimulants, though we tend to pick:
- more-sedating agents such as paroxetine or sertraline when patients report difficulty with insomnia or overactivation
- less-sedating compounds such as fluoxetine or citalopram when patients complain of being too tired or underactive.
When patients taking SSRIs seek help for ADHD, adding a stimulant usually reduces inattention, distractibility, impulsivity, and/or subjective feelings of restlessness. We prescribe usual dosages because stimulants and SSRIs do not interact. We have not seen serious side effects, but some patients report feeling oversedated.
Tricyclics. We use tricyclics when a stimulant/SSRI combination does not relieve symptoms satisfactorily or a patient complains of side effects. We usually have good results with desipramine or imipramine, 150 to 300 mg/d, or nortriptyline, 50 to 150 mg/d. Spencer et al have reported a response rate of 68% with nortriptyline or desipramine in a retrospective chart review25 and a prospective placebo-controlled trial26 of adults with ADHD.
Case continued: Closer to dream job
After 6 months of combined treatment, Mr. B reports much-improved ADHD symptoms, with minimal stimulant-related side effects. He has made some realistic plans for computer programming school and is taking preliminary courses. Keeping a schedule book has reduced his tardiness and tendency to procrastinate.
He is more comfortable in the classroom and better able to challenge self-critical thinking. When routine difficulties arise, he is using more-adaptive coping strategies. To maintain gains achieved in therapy, he chooses to continue periodic CBT booster sessions.
Long-term treatment
Even with medication and CBT, patients may require referral for organizational coaching, academic counseling, school or workplace accommodations, vocational counseling, cognitive remediation, group therapy, or social skills classes. You can help them obtain quality adjunctive care by collaborating with professionals who offer these services.
No studies have examined long-term care of adults with ADHD. In our experience, ongoing medication and intermittent therapy can sustain symptom control and coping skills for years. Most patients are initially skeptical about staying on medication, but after they experience the benefits most seem willing to continue as long as the medication helps.
Most of our patients sustain changes in thinking, feeling, and behaving that they learn through BT. They may seek additional sessions to meet a challenge, such as a new job or starting a family.
Books
- Kolberg J, Nadeau K. ADD-friendly ways to organize your life. New York: Brunner-Routledge; 2002.
- Hallowell EM, Ratey JJ. Driven to distraction. New York: Touchstone; 1994.
- Hallowell E, Ratey J. Delivered from distraction. New York: Ballantine Books; 2005.
Organizations
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD). National Resource Center on AD/HD. www.chadd.org.
- Attention Deficit Disorder Association (ADDA). Resources and membership organization for adults with ADHD. www.add.org.
Drug brand names
- Amphetamine • Adderall, Dexedrine
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Imipramine • Tofranil
- Methylphenidate • Concerta, Focalin, Metadate, Ritalin
- Nortriptyline • Aventyl, Pamelor
Disclosures
Dr. Rostain is a consultant to Shire Pharamaceuticals Group and a speaker for Eli Lilly & Co. and Ortho-McNeil Pharmaceutical
Dr. Ramsay reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rostain AL, Ramsay JR. A combined treatment approach for adults with attention-deficit/hyperactivity disorder. Results of an open study of 43 patients J Attention Disorders. In press.
2. Shekim WO, Asarnow RF, Hess E, et al. A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Comp Psychiatry 1990;31:416-25.
3. Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993;150:1792-8.
4. Wilens TE, Biederman J, Spencer T. Attention-deficit/hyperactivity disorder across the lifespan. Ann Rev Medicine 2002;53:113-31.
5. Barkley RA. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford Press; 1998.
6. Wender PH. ADHD: Attention-deficit hyperactivity disorder in children and adults. New York: Oxford University Press; 2000.
7. Goldstein S, Ellison AT. Clinician’s guide to adult ADHD. San Diego: Academic Press; 2000.
8. Brown TE. Attention-deficit disorder: the unfocused mind in children and adults. New Haven, CT: Yale University Press; 2005.
9. Wilens TE, McDermott SP, Biederman J, et al. Cognitive therapy in the treatment of adults with ADHD: a systematic chart review of 26 cases. J Cogn Ther 1999;13:215-26.
10. Ramsay JR, Rostain AL. A cognitive therapy approach for adult attention-deficit/hyperactivity disorder. J Cogn Psychother 2003;17:319-34.
11. Safren SA, Sprich S, Chulvick S, Otto MW. Psychosocial treatments for adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2004;27:349-60.
12. Ramsay JR, Rostain AL. Adapting psychotherapy to meet the needs of adults with attention-deficit/hyperactivity disorder. Psychotherapy: Theory, Research, Practice, Training 2005;42:72-84.
13. Safren SA, Otto MW, Sprich S, et al. Cognitive-behavior therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther 2005;43:831-42.
14. Ramsay JR, Rostain AL. Girl, repeatedly interrupted: The case of a young adult woman with ADHD. Clinical Case Studies 2005;4:329-46.
15. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;42(suppl 2):26S-49S.
16. Weiss M, Murray C, Weiss G. Adults with attention-deficit/hyperactivity disorder: Current concepts. J Psychiatr Pract 2002;8:99-111.
17. Wilens TE. Drug therapy for adults with attention-deficit hyperactivity disorder. Drugs 2003;63:2395-411.
18. Dodson WW. Pharmacotherapy of adult ADHD. J Clin Psychol 2005;61:589-606.
19. Francis PD. Effects of psychotropic medications on the pediatric electrocardiogram and recommendations for monitoring. Curr Opin Pediatr 2002;14(2):224-30
20. Michaelson D, Adler L, Spencer T. Atomoxetine in adults: Two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
21. Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 2004;64:205-22.
22. Reimherr FW, Marchant BK, Strong RE, et al. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005;58:125-31.
23. Wilens TE, Spencer T, Biederman J. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
24. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 2005;57:793-801.
25. Wilens TE, Biederman JB, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention deficit/hyperactivity disorder. J Nerv Ment Dis 1995;183:48-50.
26. Wilens TE, Biederman JB, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
Mr. B, age 50, dreams of becoming a computer programmer but fears he will embarrass himself—as he has in many classrooms before. He is seeking evaluation because his teenage son was recently diagnosed with attention-deficit/hyperactivity disorder (ADHD), and he recognizes similar symptoms in himself.
Mr. B received a college degree with great difficulty, putting off assignments until the last minute and “squeaking by.” For years he has changed occupations often, never progressing beyond entry level, and now works as a personal care provider and limousine driver. He reports problems keeping up with work and managing time.
His history includes early childhood hyperactivity, difficulty sitting through classes, sloppy handwriting, disorganization, short attention span, and distractibility. He is restless, fidgety, and has trouble staying on topic. His disorganization has caused marital difficulties, for which he has sought counseling.
After careful evaluation, you determine that Mr. B meets criteria for ADHD, combined type, and for anxiety disorder not otherwise specified. His treatment goals are to increase his ability to focus; procrastinate less; improve his planning, prioritizing, and self-esteem; and to become less sensitive to criticism and less anxious about handling work demands.
Like Mr. B, adults with ADHD need treatment for the disorder’s core symptoms as well as its psychiatric comorbidities and psychosocial consequences. Comprehensive treatment with medications, cognitive-behavioral therapy (CBT), and environmental adaptations is usually recommended.
Comorbidity rules
Core symptoms. ADHD is a lifespan disorder with multiple behavioral, cognitive, and emotional manifestations that impair relationships and academic and vocational functioning. ADHD-like symptoms are seen in other conditions such as mood disorders or substance abuse, but complaints of inattention, distractibility, procrastination, restlessness, and impulsivity—particularly when pervasive and chronic—are highly indicative of ADHD.
In treating adults with ADHD, we have noticed common behavioral patterns that contribute to their psychosocial problems (Table 1). Dysfunctional coping behaviors have short-term advantages, but patients readily admit they would rather accomplish tasks through greater thought and planning.
Chronic frustrations—often associated with deep shame—are typical of adult ADHD. Many patients have maladaptive core beliefs of failure, self-mistrust, and inadequacy (Table 2).
Table 1
Common dysfunctional behavioral patterns in adults with ADHD
| Behavior | Description | Short-term gain/long-term loss |
|---|---|---|
| Anticipatory avoidance | Magnifying the difficulty of a pending task and doubts about being able to complete it; results in rationalizations to justify procrastination | Defers short-term stress, but often creates a self-fulfilling prophecy because the task looms and may seem overwhelming when facing a deadline |
| Brinksmanship | Waiting until the last moment (eg, the night before) to complete a task, often when facing an impending deadline | Deadline-associated stress can be focusing, but this tactic leaves little room for error and may yield a substandard result |
| Pseudoefficiency | Completing several low-priority, manageable tasks (eg, checking e-mail) but avoiding high-priority tasks (eg, a project for work) | Creates sense of productivity by reducing items on to-do list but defers a more difficult project |
| Juggling | Taking on new, exciting projects and feeling ‘busy’ without completing projects already started | It is easier to become motivated to start a novel project than to complete an ongoing one; pattern usually results in several incomplete projects |
Table 2
5 common maladaptive core beliefs of adults with ADHD
| Self-mistrust | ‘I cannot rely on myself to do what I need to do. I let myself down’ |
| Failure | ‘I always have failed and always will fail at what I set out to do.’ |
| Inadequacy | ‘I am basically a bad and defective person.’ |
| Incompetence | ‘I am too inept to handle life’s basic demands.’ |
| Instability | ‘My life will always be chaotic and in turmoil.’ |
Psychiatric comorbidity is the rule in adults with ADHD (Table 3). For example, among 43 patients who received combined medication and CBT at the University of Pennsylvania Adult ADHD Treatment and Research Program, 75% reported at least one comorbid condition, including:
- 27 (63%) with mood disorder
- 23 (54%) with anxiety disorder
- 5 (12%) with substance abuse.1
Other treatment studies have reported similar comorbidity rates in adults with ADHD.2-4
Table 3
Psychiatric comorbidity in adult ADHD
| Disorder | Prevalence |
|---|---|
| Mood disorders | 50% to 65% |
| Recurrent depression | |
| Bipolar disorder | |
| Cyclothymia | |
| Dysthymia | |
| Depressive disorder NOS | |
| Anxiety disorders | 40% to 55% |
| Generalized anxiety disorder | |
| Anxiety disorder NOS | |
| Others | Various |
| Substance use disorder | |
| Learning disabilities | |
| Intermittent explosive disorder | |
| Tourette syndrome | |
| Antisocial personality | |
| Borderline personality disorder | |
| Dependent personality | |
| NOS: Not otherwise specified | |
Making the diagnosis
Diagnosis of adult ADHD is based on a comprehensive assessment, including:
- careful history of presenting complaints
- thorough review of educational, occupational, and family history
- standardized rating scales (such as the Barkley ADHD Behavior Checklists, the Conners’ Adult ADHD Rating Scale, or the Brown Attention Deficit Disorder Scales)
- collateral information
- assessment of mood, anxiety, substance use, and learning/organizational skills. For details, consult references on adult ADHD.5-8
Case continued: Self-fulfilling prophesies
On standardized rating scales, Mr. B meets criteria for combined ADHD for childhood and current symptoms. Information from his wife and brother also confirms the ADHD diagnosis.
He is motivated, resilient, optimistic, and has a good support system. However, his negative automatic thoughts about his ability to succeed in school and to handle increasing time demands suggest deeper beliefs of inadequacy and failure.
Mr. B struggled academically. Without guidance about how to change his approach to difficult situations, he has repeated old thinking and behavior patterns. Believing he will embarrass himself and fail to learn required material, Mr. B procrastinates and avoids doing assignments. In class, his feelings of inadequacy make him self-conscious, which causes him to lose focus and have trouble concentrating.
See the world through the patient’s eyes
Understanding your patient. Before you start treatment, we recommend that you conceptualize how ADHD has influenced your patient’s life, including:
- developmental experiences
- family-of-origin issues, such as conflicts with parents stemming from ADHD symptoms or reciprocal interactions with an ADHD parent
- world view (“schemata”)
- patterns of coping with (or avoiding) stress
- attitudes toward self and important others
- readiness to change.
Developing a working case conceptualization is a dynamic, collaborative process. You talk with patients, and encourage them to reflect on how ADHD affects their view of themselves and their important relationships. The conceptualization takes shape as you:
- observe patients’ behaviors
- elicit how they think and feel
- assess with them the relevance and accuracies of their belief systems and response patterns.
Seeing the world “through their eyes” prepares you to help them accept the diagnosis and learn to manage ADHD symptoms. Then, by providing a blueprint to manage what patients may see as uncontrollable responses, you can help them take charge of their automatic reactions.
Psychoeducation. To set the stage for treatment, encourage patients to learn about ADHD by reading articles and books and consulting Web sites for adults with ADHD (see Related resources). Psychoeducation helps patients:
- review possible treatment approaches, including organizational (environmental) management, medication, and psychotherapy (individual or group)
- become informed participants in setting treatment goals.
Explain the relative contribution of each treatment component. For example, medications can reduce distractibility and improve attention, organizational strategies can reduce disorganization and improve time management, and structured psychotherapy can help the patient develop more effective coping skills.
Case continued: Planning combined treatment
You discuss diagnosis and treatment options with Mr. B, and he agrees to start the methylphenidate compound Concerta, initially at 18 mg/d, and weekly CBT sessions. You recommended a stimulant based on efficacy studies and your clinical experience in treating adults with ADHD. Mr. B wants a medication that will help him focus while working or studying, and he says Concerta has improved his son’s ADHD symptoms.
You instruct Mr. B to increase the dosage by 18 mg each week until he reaches 72 mg/d. You also tell him to keep a medication response log and to note any positive changes and side effects.
If an adult with ADHD expresses preference for a particular medication, we usually prescribe that one first. Most patients to whom we offer both medication and psychotherapy agree to this “top-down” and “bottom-up” approach. “Top down” means giving patients new ways of thinking to help them understand and modify their responses. “Bottom up” refers to the medication reducing their impulsivity, distractibility, and inattentiveness.
CBT for adult ADHD
Medications can ameliorate key symptoms of adult ADHD, but adjunctive interventions are needed to improve functioning and quality of life. Evidence supporting psychosocial treatment for adults with ADHD is limited, but CBT has been studied the most.1,9-13 Safren et al13 found a four-fold greater therapeutic response when patients received adjunctive CBT for residual ADHD symptoms, compared with patients who received medication alone.
We usually provide CBT weekly for 12 weeks and then taper to 8 additional sessions over 3 months (total 20 sessions). We may extend CBT with additional sessions to address complicated issues. CBT helps adults with ADHD to:
- identify dysfunctional thinking, feeling, and behaving patterns
- recognize contexts in which patterns arise
- systematically change these patterns.
CBT can reduce ADHD-associated anxiety and depression and improve coping skills and sense of well-being.1,9,11 Its flexibility allows you to address family issues with patients’ partners, children and other relatives to improve communication, reduce conflict, and develop healthier interactions.
We focus CBT sessions on finding alternate coping strategies. We might try role playing, rehearsing, creating “thought experiments,” and anticipating and preparing to modify typical patterns of avoidance. These approaches have been described elsewhere.10,11,14
We adopt an active stance during therapy to keep ADHD patients’ distractibility from disrupting our conversation. For example, we set the therapeutic agenda, provide feedback about patients’ behaviors, and encourage them to clarify rewards and consequences of using (or avoiding) problem-solving strategies.
Although we typically assign between-session homework, we expect patients to have difficulty completing it. We remain nonjudgmental and collaborative, viewing incomplete assignments as opportunities to learn about patients’ unproductive problem solving and to help them develop more-effective patterns.
Challenging maladaptive beliefs. A strong therapeutic relationship allows adults with ADHD to discuss their chronic frustrations, which often are associated with deep shame. We then shift CBT’s focus to deeper ADHD-related schemata that perpetuate dysfunctional patterns.
We work with patients to elucidate and challenge their maladaptive core beliefs and encourage new ways to view themselves and others. Allowing patients to grieve about the limitations ADHD imposes on their lives also helps to reduce chronic negative self-esteem.
Case continued: ‘less frenetic’
Mr. B achieves good results within 3 weeks of an increasing titration of stimulant medication, reporting significantly less restlessness and greater concentration without significant side effects. His wife confirms that he is less frenetic, can converse without interruptions, and is better at managing his complicated work schedule.
Which medications?
Drug therapy for adult ADHD is not as well-studied as in children and adolescents, but American Academy of Child and Adolescent Psychiatry guidelines and others15-18 recommend stimulant and nonstimulant medications. Your choice depends on the patient’s clinical profile (including risk factors and comorbid conditions), past medication use, treatment goals, preferred medication effects and dosing patterns (once-daily versus multiple times), and potential side effects. Stimulants or atomoxetine are first-line choices for adult ADHD without psychiatric comorbidity.
Stimulants work quickly and are cleared relatively rapidly from the brain without causing euphoria or dependency. They are effective (80% to 90% response rate) and well-tolerated, though long-term effects have not been studied in adults (Table 4).
Stimulants’ effect size of 0.9 is considered substantial. Effect size—a statistical method of reporting an intervention’s effect across different studies—is typically rated as:
- <0.32 very small
- 0.33 to 0.54, moderate
- >0.55, significant or very strong.
When choosing a medication, we usually try methylphenidate and amphetamine first, one after the other. We explain to the patient how stimulants work in the brain and the need for a comparative trial to determine which might work best for him or her. If the patient has tried a stimulant and found it helpful, we start with that class. Similarly, if he/she has not had good results with one type, we start with the other. Approximately one-third of our patients respond equally well to methylphenidate or amphetamine, one-third respond better to methylphenidate, and one-third respond better to amphetamine.
To determine the optimal dosage, we usually titrate up from 10 to 30 mg per dose of an immediate-release preparation. We begin with this form to help patients notice the medication’s onset and duration of action. After we find the optimal dosage, we switch to a longer-acting preparation.
Insomnia, mood instability, and euphoria are unacceptable stimulant side effects, although many patients welcome others such as appetite suppression and weight loss. Closely monitor cardiovascular effects, and review potential interactions with other medications, such as antihypertensives or bronchodilators. Because sudden death has been reported with stimulants in persons with structural cardiac lesions,19 obtain a cardiology consultation for patients with a history of heart disease.
We encourage patients to keep daily medication logs (Box), which we review at each visit and use to make dosing or medication changes. Dosing guidelines resemble those used for children and adolescents, although adults usually tolerate higher maximum dosages (such as methylphenidate, 80 to 100 mg/d).
Because of stimulants’ potential for recreational misuse and abuse, remain wary about choosing stimulants for patients with whom you lack a solid doctor-patient relationship.
Table 4
Stimulant dosages used in treating adult ADHD
| Class (brand name) | Daily dosing | Typical dosing schedule |
|---|---|---|
| Methylphenidate | ||
| Short-acting (Metadate, Ritadex, Ritalin) | Two to four times | 10 to 40 mg bid to qid |
| Intermediate-acting (Metadate SR, Ritalin SR) | Once or twice | 20 to 60 mg qd to bid |
| Extended-release (Concerta, Metadate CD, Ritalin LA) | Once or twice | 18 to 108 mg qd (Concerta) 20 to 40 mg bid (Ritalin LA, Metadate CD) |
| Dextromethylphenidate | ||
| Short-acting (Focalin) | Two to four times | 5 to 20 mg bid to qid |
| Long-acting (Focalin XR) | Once or twice | 10 to 20 mg qd or bid |
| Dextroamphetamine | ||
| Short-acting (Dexedrine) | Twice or three times | 10 to 30 mg bid or tid |
| Intermediate-acting (Dexedrine spansules) | Once or twice | 10 to 30 mg bid |
| Mixed amphetamine salts | ||
| Intermediate-acting (Adderall) | Once or twice | 10 to 30 mg bid or tid |
| Extended-release (Adderall XR) | Once or twice | 10 to 40 mg qd or bid |
Atomoxetine, a nonstimulant, norepinephrine re-uptake inhibitor, is approved for ADHD in adults.20-22 In two double-blind, controlled, randomized trials totalling 536 adults, Michaelson et al20 found significantly reduced ADHD symptoms after 10 weeks of atomoxetine treatment. Effect sizes of 0.35 and 0.40 were reported, with 10% of patients discontinuing because of side effects.
Atomoxetine has a long duration of action (>12 hours) but a more gradual onset (4 to 6 weeks) than that of stimulants. Approximately 60% of patients respond to atomoxetine, though effect sizes are less than those of stimulants. We have found atomoxetine works well for patients who:
- do not tolerate or are uncomfortable with taking stimulants
- are highly anxious
- report emotional dysregulation as a major target symptom.
To reduce risk of common side effects (nausea, GI upset, headache, sedation, reduced sex drive), we start with low dosages (such as 25 mg bid) and increase weekly by 25 mg to a target of 80 to 100 mg/d.
Treating complicated ADHD
Bupropion or tricyclic antidepressants are reasonable options for ADHD with depression. Atomoxetine, a tricyclic, or a stimulant plus a selective serotonin reuptake inhibitor (SSRI) can provide good symptom relief for adults with ADHD and comorbid anxiety and/or depression.
Bupropion. Approximately 50% of adults with ADHD respond to bupropion,23,24 with a treatment effect size of 0.6. Bupropion’s efficacy in smoking cessation adds value for those trying to quit.
We usually start extended-release bupropion at 150 mg/d and increase after 2 weeks to 300 mg/d if response is suboptimal. Headache, dry mouth, insomnia, and nausea are the most common adverse effects. Agitation or irritability is sometimes serious enough to warrant stopping bupropion.
Combining medications. Using SSRIs with stimulants can help adults with ADHD and comorbid anxiety or depression. Any SSRI can be safely combined with stimulants, though we tend to pick:
- more-sedating agents such as paroxetine or sertraline when patients report difficulty with insomnia or overactivation
- less-sedating compounds such as fluoxetine or citalopram when patients complain of being too tired or underactive.
When patients taking SSRIs seek help for ADHD, adding a stimulant usually reduces inattention, distractibility, impulsivity, and/or subjective feelings of restlessness. We prescribe usual dosages because stimulants and SSRIs do not interact. We have not seen serious side effects, but some patients report feeling oversedated.
Tricyclics. We use tricyclics when a stimulant/SSRI combination does not relieve symptoms satisfactorily or a patient complains of side effects. We usually have good results with desipramine or imipramine, 150 to 300 mg/d, or nortriptyline, 50 to 150 mg/d. Spencer et al have reported a response rate of 68% with nortriptyline or desipramine in a retrospective chart review25 and a prospective placebo-controlled trial26 of adults with ADHD.
Case continued: Closer to dream job
After 6 months of combined treatment, Mr. B reports much-improved ADHD symptoms, with minimal stimulant-related side effects. He has made some realistic plans for computer programming school and is taking preliminary courses. Keeping a schedule book has reduced his tardiness and tendency to procrastinate.
He is more comfortable in the classroom and better able to challenge self-critical thinking. When routine difficulties arise, he is using more-adaptive coping strategies. To maintain gains achieved in therapy, he chooses to continue periodic CBT booster sessions.
Long-term treatment
Even with medication and CBT, patients may require referral for organizational coaching, academic counseling, school or workplace accommodations, vocational counseling, cognitive remediation, group therapy, or social skills classes. You can help them obtain quality adjunctive care by collaborating with professionals who offer these services.
No studies have examined long-term care of adults with ADHD. In our experience, ongoing medication and intermittent therapy can sustain symptom control and coping skills for years. Most patients are initially skeptical about staying on medication, but after they experience the benefits most seem willing to continue as long as the medication helps.
Most of our patients sustain changes in thinking, feeling, and behaving that they learn through BT. They may seek additional sessions to meet a challenge, such as a new job or starting a family.
Books
- Kolberg J, Nadeau K. ADD-friendly ways to organize your life. New York: Brunner-Routledge; 2002.
- Hallowell EM, Ratey JJ. Driven to distraction. New York: Touchstone; 1994.
- Hallowell E, Ratey J. Delivered from distraction. New York: Ballantine Books; 2005.
Organizations
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD). National Resource Center on AD/HD. www.chadd.org.
- Attention Deficit Disorder Association (ADDA). Resources and membership organization for adults with ADHD. www.add.org.
Drug brand names
- Amphetamine • Adderall, Dexedrine
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Imipramine • Tofranil
- Methylphenidate • Concerta, Focalin, Metadate, Ritalin
- Nortriptyline • Aventyl, Pamelor
Disclosures
Dr. Rostain is a consultant to Shire Pharamaceuticals Group and a speaker for Eli Lilly & Co. and Ortho-McNeil Pharmaceutical
Dr. Ramsay reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. B, age 50, dreams of becoming a computer programmer but fears he will embarrass himself—as he has in many classrooms before. He is seeking evaluation because his teenage son was recently diagnosed with attention-deficit/hyperactivity disorder (ADHD), and he recognizes similar symptoms in himself.
Mr. B received a college degree with great difficulty, putting off assignments until the last minute and “squeaking by.” For years he has changed occupations often, never progressing beyond entry level, and now works as a personal care provider and limousine driver. He reports problems keeping up with work and managing time.
His history includes early childhood hyperactivity, difficulty sitting through classes, sloppy handwriting, disorganization, short attention span, and distractibility. He is restless, fidgety, and has trouble staying on topic. His disorganization has caused marital difficulties, for which he has sought counseling.
After careful evaluation, you determine that Mr. B meets criteria for ADHD, combined type, and for anxiety disorder not otherwise specified. His treatment goals are to increase his ability to focus; procrastinate less; improve his planning, prioritizing, and self-esteem; and to become less sensitive to criticism and less anxious about handling work demands.
Like Mr. B, adults with ADHD need treatment for the disorder’s core symptoms as well as its psychiatric comorbidities and psychosocial consequences. Comprehensive treatment with medications, cognitive-behavioral therapy (CBT), and environmental adaptations is usually recommended.
Comorbidity rules
Core symptoms. ADHD is a lifespan disorder with multiple behavioral, cognitive, and emotional manifestations that impair relationships and academic and vocational functioning. ADHD-like symptoms are seen in other conditions such as mood disorders or substance abuse, but complaints of inattention, distractibility, procrastination, restlessness, and impulsivity—particularly when pervasive and chronic—are highly indicative of ADHD.
In treating adults with ADHD, we have noticed common behavioral patterns that contribute to their psychosocial problems (Table 1). Dysfunctional coping behaviors have short-term advantages, but patients readily admit they would rather accomplish tasks through greater thought and planning.
Chronic frustrations—often associated with deep shame—are typical of adult ADHD. Many patients have maladaptive core beliefs of failure, self-mistrust, and inadequacy (Table 2).
Table 1
Common dysfunctional behavioral patterns in adults with ADHD
| Behavior | Description | Short-term gain/long-term loss |
|---|---|---|
| Anticipatory avoidance | Magnifying the difficulty of a pending task and doubts about being able to complete it; results in rationalizations to justify procrastination | Defers short-term stress, but often creates a self-fulfilling prophecy because the task looms and may seem overwhelming when facing a deadline |
| Brinksmanship | Waiting until the last moment (eg, the night before) to complete a task, often when facing an impending deadline | Deadline-associated stress can be focusing, but this tactic leaves little room for error and may yield a substandard result |
| Pseudoefficiency | Completing several low-priority, manageable tasks (eg, checking e-mail) but avoiding high-priority tasks (eg, a project for work) | Creates sense of productivity by reducing items on to-do list but defers a more difficult project |
| Juggling | Taking on new, exciting projects and feeling ‘busy’ without completing projects already started | It is easier to become motivated to start a novel project than to complete an ongoing one; pattern usually results in several incomplete projects |
Table 2
5 common maladaptive core beliefs of adults with ADHD
| Self-mistrust | ‘I cannot rely on myself to do what I need to do. I let myself down’ |
| Failure | ‘I always have failed and always will fail at what I set out to do.’ |
| Inadequacy | ‘I am basically a bad and defective person.’ |
| Incompetence | ‘I am too inept to handle life’s basic demands.’ |
| Instability | ‘My life will always be chaotic and in turmoil.’ |
Psychiatric comorbidity is the rule in adults with ADHD (Table 3). For example, among 43 patients who received combined medication and CBT at the University of Pennsylvania Adult ADHD Treatment and Research Program, 75% reported at least one comorbid condition, including:
- 27 (63%) with mood disorder
- 23 (54%) with anxiety disorder
- 5 (12%) with substance abuse.1
Other treatment studies have reported similar comorbidity rates in adults with ADHD.2-4
Table 3
Psychiatric comorbidity in adult ADHD
| Disorder | Prevalence |
|---|---|
| Mood disorders | 50% to 65% |
| Recurrent depression | |
| Bipolar disorder | |
| Cyclothymia | |
| Dysthymia | |
| Depressive disorder NOS | |
| Anxiety disorders | 40% to 55% |
| Generalized anxiety disorder | |
| Anxiety disorder NOS | |
| Others | Various |
| Substance use disorder | |
| Learning disabilities | |
| Intermittent explosive disorder | |
| Tourette syndrome | |
| Antisocial personality | |
| Borderline personality disorder | |
| Dependent personality | |
| NOS: Not otherwise specified | |
Making the diagnosis
Diagnosis of adult ADHD is based on a comprehensive assessment, including:
- careful history of presenting complaints
- thorough review of educational, occupational, and family history
- standardized rating scales (such as the Barkley ADHD Behavior Checklists, the Conners’ Adult ADHD Rating Scale, or the Brown Attention Deficit Disorder Scales)
- collateral information
- assessment of mood, anxiety, substance use, and learning/organizational skills. For details, consult references on adult ADHD.5-8
Case continued: Self-fulfilling prophesies
On standardized rating scales, Mr. B meets criteria for combined ADHD for childhood and current symptoms. Information from his wife and brother also confirms the ADHD diagnosis.
He is motivated, resilient, optimistic, and has a good support system. However, his negative automatic thoughts about his ability to succeed in school and to handle increasing time demands suggest deeper beliefs of inadequacy and failure.
Mr. B struggled academically. Without guidance about how to change his approach to difficult situations, he has repeated old thinking and behavior patterns. Believing he will embarrass himself and fail to learn required material, Mr. B procrastinates and avoids doing assignments. In class, his feelings of inadequacy make him self-conscious, which causes him to lose focus and have trouble concentrating.
See the world through the patient’s eyes
Understanding your patient. Before you start treatment, we recommend that you conceptualize how ADHD has influenced your patient’s life, including:
- developmental experiences
- family-of-origin issues, such as conflicts with parents stemming from ADHD symptoms or reciprocal interactions with an ADHD parent
- world view (“schemata”)
- patterns of coping with (or avoiding) stress
- attitudes toward self and important others
- readiness to change.
Developing a working case conceptualization is a dynamic, collaborative process. You talk with patients, and encourage them to reflect on how ADHD affects their view of themselves and their important relationships. The conceptualization takes shape as you:
- observe patients’ behaviors
- elicit how they think and feel
- assess with them the relevance and accuracies of their belief systems and response patterns.
Seeing the world “through their eyes” prepares you to help them accept the diagnosis and learn to manage ADHD symptoms. Then, by providing a blueprint to manage what patients may see as uncontrollable responses, you can help them take charge of their automatic reactions.
Psychoeducation. To set the stage for treatment, encourage patients to learn about ADHD by reading articles and books and consulting Web sites for adults with ADHD (see Related resources). Psychoeducation helps patients:
- review possible treatment approaches, including organizational (environmental) management, medication, and psychotherapy (individual or group)
- become informed participants in setting treatment goals.
Explain the relative contribution of each treatment component. For example, medications can reduce distractibility and improve attention, organizational strategies can reduce disorganization and improve time management, and structured psychotherapy can help the patient develop more effective coping skills.
Case continued: Planning combined treatment
You discuss diagnosis and treatment options with Mr. B, and he agrees to start the methylphenidate compound Concerta, initially at 18 mg/d, and weekly CBT sessions. You recommended a stimulant based on efficacy studies and your clinical experience in treating adults with ADHD. Mr. B wants a medication that will help him focus while working or studying, and he says Concerta has improved his son’s ADHD symptoms.
You instruct Mr. B to increase the dosage by 18 mg each week until he reaches 72 mg/d. You also tell him to keep a medication response log and to note any positive changes and side effects.
If an adult with ADHD expresses preference for a particular medication, we usually prescribe that one first. Most patients to whom we offer both medication and psychotherapy agree to this “top-down” and “bottom-up” approach. “Top down” means giving patients new ways of thinking to help them understand and modify their responses. “Bottom up” refers to the medication reducing their impulsivity, distractibility, and inattentiveness.
CBT for adult ADHD
Medications can ameliorate key symptoms of adult ADHD, but adjunctive interventions are needed to improve functioning and quality of life. Evidence supporting psychosocial treatment for adults with ADHD is limited, but CBT has been studied the most.1,9-13 Safren et al13 found a four-fold greater therapeutic response when patients received adjunctive CBT for residual ADHD symptoms, compared with patients who received medication alone.
We usually provide CBT weekly for 12 weeks and then taper to 8 additional sessions over 3 months (total 20 sessions). We may extend CBT with additional sessions to address complicated issues. CBT helps adults with ADHD to:
- identify dysfunctional thinking, feeling, and behaving patterns
- recognize contexts in which patterns arise
- systematically change these patterns.
CBT can reduce ADHD-associated anxiety and depression and improve coping skills and sense of well-being.1,9,11 Its flexibility allows you to address family issues with patients’ partners, children and other relatives to improve communication, reduce conflict, and develop healthier interactions.
We focus CBT sessions on finding alternate coping strategies. We might try role playing, rehearsing, creating “thought experiments,” and anticipating and preparing to modify typical patterns of avoidance. These approaches have been described elsewhere.10,11,14
We adopt an active stance during therapy to keep ADHD patients’ distractibility from disrupting our conversation. For example, we set the therapeutic agenda, provide feedback about patients’ behaviors, and encourage them to clarify rewards and consequences of using (or avoiding) problem-solving strategies.
Although we typically assign between-session homework, we expect patients to have difficulty completing it. We remain nonjudgmental and collaborative, viewing incomplete assignments as opportunities to learn about patients’ unproductive problem solving and to help them develop more-effective patterns.
Challenging maladaptive beliefs. A strong therapeutic relationship allows adults with ADHD to discuss their chronic frustrations, which often are associated with deep shame. We then shift CBT’s focus to deeper ADHD-related schemata that perpetuate dysfunctional patterns.
We work with patients to elucidate and challenge their maladaptive core beliefs and encourage new ways to view themselves and others. Allowing patients to grieve about the limitations ADHD imposes on their lives also helps to reduce chronic negative self-esteem.
Case continued: ‘less frenetic’
Mr. B achieves good results within 3 weeks of an increasing titration of stimulant medication, reporting significantly less restlessness and greater concentration without significant side effects. His wife confirms that he is less frenetic, can converse without interruptions, and is better at managing his complicated work schedule.
Which medications?
Drug therapy for adult ADHD is not as well-studied as in children and adolescents, but American Academy of Child and Adolescent Psychiatry guidelines and others15-18 recommend stimulant and nonstimulant medications. Your choice depends on the patient’s clinical profile (including risk factors and comorbid conditions), past medication use, treatment goals, preferred medication effects and dosing patterns (once-daily versus multiple times), and potential side effects. Stimulants or atomoxetine are first-line choices for adult ADHD without psychiatric comorbidity.
Stimulants work quickly and are cleared relatively rapidly from the brain without causing euphoria or dependency. They are effective (80% to 90% response rate) and well-tolerated, though long-term effects have not been studied in adults (Table 4).
Stimulants’ effect size of 0.9 is considered substantial. Effect size—a statistical method of reporting an intervention’s effect across different studies—is typically rated as:
- <0.32 very small
- 0.33 to 0.54, moderate
- >0.55, significant or very strong.
When choosing a medication, we usually try methylphenidate and amphetamine first, one after the other. We explain to the patient how stimulants work in the brain and the need for a comparative trial to determine which might work best for him or her. If the patient has tried a stimulant and found it helpful, we start with that class. Similarly, if he/she has not had good results with one type, we start with the other. Approximately one-third of our patients respond equally well to methylphenidate or amphetamine, one-third respond better to methylphenidate, and one-third respond better to amphetamine.
To determine the optimal dosage, we usually titrate up from 10 to 30 mg per dose of an immediate-release preparation. We begin with this form to help patients notice the medication’s onset and duration of action. After we find the optimal dosage, we switch to a longer-acting preparation.
Insomnia, mood instability, and euphoria are unacceptable stimulant side effects, although many patients welcome others such as appetite suppression and weight loss. Closely monitor cardiovascular effects, and review potential interactions with other medications, such as antihypertensives or bronchodilators. Because sudden death has been reported with stimulants in persons with structural cardiac lesions,19 obtain a cardiology consultation for patients with a history of heart disease.
We encourage patients to keep daily medication logs (Box), which we review at each visit and use to make dosing or medication changes. Dosing guidelines resemble those used for children and adolescents, although adults usually tolerate higher maximum dosages (such as methylphenidate, 80 to 100 mg/d).
Because of stimulants’ potential for recreational misuse and abuse, remain wary about choosing stimulants for patients with whom you lack a solid doctor-patient relationship.
Table 4
Stimulant dosages used in treating adult ADHD
| Class (brand name) | Daily dosing | Typical dosing schedule |
|---|---|---|
| Methylphenidate | ||
| Short-acting (Metadate, Ritadex, Ritalin) | Two to four times | 10 to 40 mg bid to qid |
| Intermediate-acting (Metadate SR, Ritalin SR) | Once or twice | 20 to 60 mg qd to bid |
| Extended-release (Concerta, Metadate CD, Ritalin LA) | Once or twice | 18 to 108 mg qd (Concerta) 20 to 40 mg bid (Ritalin LA, Metadate CD) |
| Dextromethylphenidate | ||
| Short-acting (Focalin) | Two to four times | 5 to 20 mg bid to qid |
| Long-acting (Focalin XR) | Once or twice | 10 to 20 mg qd or bid |
| Dextroamphetamine | ||
| Short-acting (Dexedrine) | Twice or three times | 10 to 30 mg bid or tid |
| Intermediate-acting (Dexedrine spansules) | Once or twice | 10 to 30 mg bid |
| Mixed amphetamine salts | ||
| Intermediate-acting (Adderall) | Once or twice | 10 to 30 mg bid or tid |
| Extended-release (Adderall XR) | Once or twice | 10 to 40 mg qd or bid |
Atomoxetine, a nonstimulant, norepinephrine re-uptake inhibitor, is approved for ADHD in adults.20-22 In two double-blind, controlled, randomized trials totalling 536 adults, Michaelson et al20 found significantly reduced ADHD symptoms after 10 weeks of atomoxetine treatment. Effect sizes of 0.35 and 0.40 were reported, with 10% of patients discontinuing because of side effects.
Atomoxetine has a long duration of action (>12 hours) but a more gradual onset (4 to 6 weeks) than that of stimulants. Approximately 60% of patients respond to atomoxetine, though effect sizes are less than those of stimulants. We have found atomoxetine works well for patients who:
- do not tolerate or are uncomfortable with taking stimulants
- are highly anxious
- report emotional dysregulation as a major target symptom.
To reduce risk of common side effects (nausea, GI upset, headache, sedation, reduced sex drive), we start with low dosages (such as 25 mg bid) and increase weekly by 25 mg to a target of 80 to 100 mg/d.
Treating complicated ADHD
Bupropion or tricyclic antidepressants are reasonable options for ADHD with depression. Atomoxetine, a tricyclic, or a stimulant plus a selective serotonin reuptake inhibitor (SSRI) can provide good symptom relief for adults with ADHD and comorbid anxiety and/or depression.
Bupropion. Approximately 50% of adults with ADHD respond to bupropion,23,24 with a treatment effect size of 0.6. Bupropion’s efficacy in smoking cessation adds value for those trying to quit.
We usually start extended-release bupropion at 150 mg/d and increase after 2 weeks to 300 mg/d if response is suboptimal. Headache, dry mouth, insomnia, and nausea are the most common adverse effects. Agitation or irritability is sometimes serious enough to warrant stopping bupropion.
Combining medications. Using SSRIs with stimulants can help adults with ADHD and comorbid anxiety or depression. Any SSRI can be safely combined with stimulants, though we tend to pick:
- more-sedating agents such as paroxetine or sertraline when patients report difficulty with insomnia or overactivation
- less-sedating compounds such as fluoxetine or citalopram when patients complain of being too tired or underactive.
When patients taking SSRIs seek help for ADHD, adding a stimulant usually reduces inattention, distractibility, impulsivity, and/or subjective feelings of restlessness. We prescribe usual dosages because stimulants and SSRIs do not interact. We have not seen serious side effects, but some patients report feeling oversedated.
Tricyclics. We use tricyclics when a stimulant/SSRI combination does not relieve symptoms satisfactorily or a patient complains of side effects. We usually have good results with desipramine or imipramine, 150 to 300 mg/d, or nortriptyline, 50 to 150 mg/d. Spencer et al have reported a response rate of 68% with nortriptyline or desipramine in a retrospective chart review25 and a prospective placebo-controlled trial26 of adults with ADHD.
Case continued: Closer to dream job
After 6 months of combined treatment, Mr. B reports much-improved ADHD symptoms, with minimal stimulant-related side effects. He has made some realistic plans for computer programming school and is taking preliminary courses. Keeping a schedule book has reduced his tardiness and tendency to procrastinate.
He is more comfortable in the classroom and better able to challenge self-critical thinking. When routine difficulties arise, he is using more-adaptive coping strategies. To maintain gains achieved in therapy, he chooses to continue periodic CBT booster sessions.
Long-term treatment
Even with medication and CBT, patients may require referral for organizational coaching, academic counseling, school or workplace accommodations, vocational counseling, cognitive remediation, group therapy, or social skills classes. You can help them obtain quality adjunctive care by collaborating with professionals who offer these services.
No studies have examined long-term care of adults with ADHD. In our experience, ongoing medication and intermittent therapy can sustain symptom control and coping skills for years. Most patients are initially skeptical about staying on medication, but after they experience the benefits most seem willing to continue as long as the medication helps.
Most of our patients sustain changes in thinking, feeling, and behaving that they learn through BT. They may seek additional sessions to meet a challenge, such as a new job or starting a family.
Books
- Kolberg J, Nadeau K. ADD-friendly ways to organize your life. New York: Brunner-Routledge; 2002.
- Hallowell EM, Ratey JJ. Driven to distraction. New York: Touchstone; 1994.
- Hallowell E, Ratey J. Delivered from distraction. New York: Ballantine Books; 2005.
Organizations
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD). National Resource Center on AD/HD. www.chadd.org.
- Attention Deficit Disorder Association (ADDA). Resources and membership organization for adults with ADHD. www.add.org.
Drug brand names
- Amphetamine • Adderall, Dexedrine
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Imipramine • Tofranil
- Methylphenidate • Concerta, Focalin, Metadate, Ritalin
- Nortriptyline • Aventyl, Pamelor
Disclosures
Dr. Rostain is a consultant to Shire Pharamaceuticals Group and a speaker for Eli Lilly & Co. and Ortho-McNeil Pharmaceutical
Dr. Ramsay reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rostain AL, Ramsay JR. A combined treatment approach for adults with attention-deficit/hyperactivity disorder. Results of an open study of 43 patients J Attention Disorders. In press.
2. Shekim WO, Asarnow RF, Hess E, et al. A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Comp Psychiatry 1990;31:416-25.
3. Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993;150:1792-8.
4. Wilens TE, Biederman J, Spencer T. Attention-deficit/hyperactivity disorder across the lifespan. Ann Rev Medicine 2002;53:113-31.
5. Barkley RA. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford Press; 1998.
6. Wender PH. ADHD: Attention-deficit hyperactivity disorder in children and adults. New York: Oxford University Press; 2000.
7. Goldstein S, Ellison AT. Clinician’s guide to adult ADHD. San Diego: Academic Press; 2000.
8. Brown TE. Attention-deficit disorder: the unfocused mind in children and adults. New Haven, CT: Yale University Press; 2005.
9. Wilens TE, McDermott SP, Biederman J, et al. Cognitive therapy in the treatment of adults with ADHD: a systematic chart review of 26 cases. J Cogn Ther 1999;13:215-26.
10. Ramsay JR, Rostain AL. A cognitive therapy approach for adult attention-deficit/hyperactivity disorder. J Cogn Psychother 2003;17:319-34.
11. Safren SA, Sprich S, Chulvick S, Otto MW. Psychosocial treatments for adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2004;27:349-60.
12. Ramsay JR, Rostain AL. Adapting psychotherapy to meet the needs of adults with attention-deficit/hyperactivity disorder. Psychotherapy: Theory, Research, Practice, Training 2005;42:72-84.
13. Safren SA, Otto MW, Sprich S, et al. Cognitive-behavior therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther 2005;43:831-42.
14. Ramsay JR, Rostain AL. Girl, repeatedly interrupted: The case of a young adult woman with ADHD. Clinical Case Studies 2005;4:329-46.
15. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;42(suppl 2):26S-49S.
16. Weiss M, Murray C, Weiss G. Adults with attention-deficit/hyperactivity disorder: Current concepts. J Psychiatr Pract 2002;8:99-111.
17. Wilens TE. Drug therapy for adults with attention-deficit hyperactivity disorder. Drugs 2003;63:2395-411.
18. Dodson WW. Pharmacotherapy of adult ADHD. J Clin Psychol 2005;61:589-606.
19. Francis PD. Effects of psychotropic medications on the pediatric electrocardiogram and recommendations for monitoring. Curr Opin Pediatr 2002;14(2):224-30
20. Michaelson D, Adler L, Spencer T. Atomoxetine in adults: Two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
21. Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 2004;64:205-22.
22. Reimherr FW, Marchant BK, Strong RE, et al. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005;58:125-31.
23. Wilens TE, Spencer T, Biederman J. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
24. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 2005;57:793-801.
25. Wilens TE, Biederman JB, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention deficit/hyperactivity disorder. J Nerv Ment Dis 1995;183:48-50.
26. Wilens TE, Biederman JB, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
1. Rostain AL, Ramsay JR. A combined treatment approach for adults with attention-deficit/hyperactivity disorder. Results of an open study of 43 patients J Attention Disorders. In press.
2. Shekim WO, Asarnow RF, Hess E, et al. A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Comp Psychiatry 1990;31:416-25.
3. Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993;150:1792-8.
4. Wilens TE, Biederman J, Spencer T. Attention-deficit/hyperactivity disorder across the lifespan. Ann Rev Medicine 2002;53:113-31.
5. Barkley RA. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford Press; 1998.
6. Wender PH. ADHD: Attention-deficit hyperactivity disorder in children and adults. New York: Oxford University Press; 2000.
7. Goldstein S, Ellison AT. Clinician’s guide to adult ADHD. San Diego: Academic Press; 2000.
8. Brown TE. Attention-deficit disorder: the unfocused mind in children and adults. New Haven, CT: Yale University Press; 2005.
9. Wilens TE, McDermott SP, Biederman J, et al. Cognitive therapy in the treatment of adults with ADHD: a systematic chart review of 26 cases. J Cogn Ther 1999;13:215-26.
10. Ramsay JR, Rostain AL. A cognitive therapy approach for adult attention-deficit/hyperactivity disorder. J Cogn Psychother 2003;17:319-34.
11. Safren SA, Sprich S, Chulvick S, Otto MW. Psychosocial treatments for adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2004;27:349-60.
12. Ramsay JR, Rostain AL. Adapting psychotherapy to meet the needs of adults with attention-deficit/hyperactivity disorder. Psychotherapy: Theory, Research, Practice, Training 2005;42:72-84.
13. Safren SA, Otto MW, Sprich S, et al. Cognitive-behavior therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther 2005;43:831-42.
14. Ramsay JR, Rostain AL. Girl, repeatedly interrupted: The case of a young adult woman with ADHD. Clinical Case Studies 2005;4:329-46.
15. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;42(suppl 2):26S-49S.
16. Weiss M, Murray C, Weiss G. Adults with attention-deficit/hyperactivity disorder: Current concepts. J Psychiatr Pract 2002;8:99-111.
17. Wilens TE. Drug therapy for adults with attention-deficit hyperactivity disorder. Drugs 2003;63:2395-411.
18. Dodson WW. Pharmacotherapy of adult ADHD. J Clin Psychol 2005;61:589-606.
19. Francis PD. Effects of psychotropic medications on the pediatric electrocardiogram and recommendations for monitoring. Curr Opin Pediatr 2002;14(2):224-30
20. Michaelson D, Adler L, Spencer T. Atomoxetine in adults: Two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
21. Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 2004;64:205-22.
22. Reimherr FW, Marchant BK, Strong RE, et al. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005;58:125-31.
23. Wilens TE, Spencer T, Biederman J. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
24. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 2005;57:793-801.
25. Wilens TE, Biederman JB, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention deficit/hyperactivity disorder. J Nerv Ment Dis 1995;183:48-50.
26. Wilens TE, Biederman JB, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
Reduce appetite suppression, insomnia in ADHD treatment
Appetite suppression and insomnia—both common, dose-related side effects of psychostimulants—can jeopardize treatment adherence for patients with attention-deficit/hyperactivity disorder (ADHD). The following strategies can minimize these effects.
First, wait and see
For most patients, the optimal psychostimulant dosage produces few or no side effects. Those that occur are usually minor, transient, and disappear as patients develop tolerance within days of starting medication.
The two most commonly used stimulants—methylphenidate and amphetamine—cause similar side effects.1 No evidence suggests either is more effective or less tolerable than the other.
Fine-tune psychostimulants to the lowest dosage that produces maximum benefit and minimum side effects. If side effects persist beyond 7 to 10 days, the dosage is probably too high or the patient is taking another stimulating medication. Before you attribute insomnia or appetite suppression to psychostimulants, ask the patient if he or she is using a decongestant, caffeine, diet pills, systemic corticosteroids, systemic albuterol, or theophylline.
Countering appetite suppression
Approximately one-third of adult and pediatric ADHD patients report appetite suppression at therapeutic psychostimulant dosages, but in most patients this effect is transient or clinically insignificant. If a child taking psychostimulants is not eating or gaining weight appropriately:
- suggest that parents plan mealtimes before the patient’s next dose or give high-calorie snacks throughout the day. (This strategy, although recommended by the American Academy of Pediatrics, can be cumbersome and has limited long-term efficacy.)
- switch from amphetamine to methylphenidate or vice versa.
- add the antihistamine cyproheptadine, 4 mg, with morning and evening meals
- add mirtazapine, one-half of a 15-mg tablet at bedtime to stimulate appetite and initiate sleep.
If none of these interventions work, recommend drug holidays from ADHD medications as a last resort when impairment is lowest, such as during weekends, holidays, or summers.
Curbing insomnia
About 20% of prepubertal children and 75% to 80% of adults have difficulty falling asleep while taking ADHD medications.2 For many patients it is not the medications but the mental and physical restlessness of ADHD that disturbs sleep. Take a careful baseline sleep history before starting psychostimulants to help you determine later if they are causing insomnia.
Avoid benzodiazepines, which may promote tolerance and dependence. I discourage using any hypnotic to treat insomnia that occurs as a side effect. Also avoid antihistamines (Benedryl, trazodone) that may leave the patient sedated the next day.
Try a trial nap. After fine-tuning the psychostimulant to the lowest optimal dosage, ask the patient to test his ability to sleep while on that dose by taking an afternoon nap. Most patients discover they can sleep well, proving to both patient and doctor that ADHD medications usually help sleep initiation or are sleep-neutral. A successful nap can ease a patient’s fear that her medication will keep her awake.
Even the longest extended-release psychostimulant formulations do not last the 14 to 16 hours of a typical waking day. This no-risk trial nap reassures patients that they can take supplemental doses as prescribed to assist them through even the longest workdays, without fear of sleep disruption.
Time-release formulations smooth the abrupt kinetics and rebound activation seen with immediate-release psychostimulants. But for patients taking immediate-release formulations, reducing the day’s last dose or taking the last dose earlier can often prevent medication-associated insomnia.
If insomnia persists, try:
- melatonin, 0.5 to 1.0 mg, at bedtime, 1 hour before bedtime, at sunset, or 6 hours before anticipated bedtime. I try to mimic the natural release of melatonin triggered by sunset, but no definitive data prove the most effective dosing time.
- alpha agonists such as clonidine, 0.1 to 0.2 mg at bedtime, or guanfacine, 1 to 2 mg at bedtime. These agents have proven efficacy for treating hyperactivity and sleep disturbances without causing tolerance but may be associated with nightmares in some children.3
- mirtazapine, one-half of a 15-mg tablet at bedtime.
1. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35(10):1304-13.
2. Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1998;37:637-46.
3. Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsycho-pharmacol 2004;7(1):77-97.
Dr. Dodson is a board-certified psychiatrist specializing in adult ADHD. He is director of the Attention Disorders Treatment Center, Denver, CO.
Appetite suppression and insomnia—both common, dose-related side effects of psychostimulants—can jeopardize treatment adherence for patients with attention-deficit/hyperactivity disorder (ADHD). The following strategies can minimize these effects.
First, wait and see
For most patients, the optimal psychostimulant dosage produces few or no side effects. Those that occur are usually minor, transient, and disappear as patients develop tolerance within days of starting medication.
The two most commonly used stimulants—methylphenidate and amphetamine—cause similar side effects.1 No evidence suggests either is more effective or less tolerable than the other.
Fine-tune psychostimulants to the lowest dosage that produces maximum benefit and minimum side effects. If side effects persist beyond 7 to 10 days, the dosage is probably too high or the patient is taking another stimulating medication. Before you attribute insomnia or appetite suppression to psychostimulants, ask the patient if he or she is using a decongestant, caffeine, diet pills, systemic corticosteroids, systemic albuterol, or theophylline.
Countering appetite suppression
Approximately one-third of adult and pediatric ADHD patients report appetite suppression at therapeutic psychostimulant dosages, but in most patients this effect is transient or clinically insignificant. If a child taking psychostimulants is not eating or gaining weight appropriately:
- suggest that parents plan mealtimes before the patient’s next dose or give high-calorie snacks throughout the day. (This strategy, although recommended by the American Academy of Pediatrics, can be cumbersome and has limited long-term efficacy.)
- switch from amphetamine to methylphenidate or vice versa.
- add the antihistamine cyproheptadine, 4 mg, with morning and evening meals
- add mirtazapine, one-half of a 15-mg tablet at bedtime to stimulate appetite and initiate sleep.
If none of these interventions work, recommend drug holidays from ADHD medications as a last resort when impairment is lowest, such as during weekends, holidays, or summers.
Curbing insomnia
About 20% of prepubertal children and 75% to 80% of adults have difficulty falling asleep while taking ADHD medications.2 For many patients it is not the medications but the mental and physical restlessness of ADHD that disturbs sleep. Take a careful baseline sleep history before starting psychostimulants to help you determine later if they are causing insomnia.
Avoid benzodiazepines, which may promote tolerance and dependence. I discourage using any hypnotic to treat insomnia that occurs as a side effect. Also avoid antihistamines (Benedryl, trazodone) that may leave the patient sedated the next day.
Try a trial nap. After fine-tuning the psychostimulant to the lowest optimal dosage, ask the patient to test his ability to sleep while on that dose by taking an afternoon nap. Most patients discover they can sleep well, proving to both patient and doctor that ADHD medications usually help sleep initiation or are sleep-neutral. A successful nap can ease a patient’s fear that her medication will keep her awake.
Even the longest extended-release psychostimulant formulations do not last the 14 to 16 hours of a typical waking day. This no-risk trial nap reassures patients that they can take supplemental doses as prescribed to assist them through even the longest workdays, without fear of sleep disruption.
Time-release formulations smooth the abrupt kinetics and rebound activation seen with immediate-release psychostimulants. But for patients taking immediate-release formulations, reducing the day’s last dose or taking the last dose earlier can often prevent medication-associated insomnia.
If insomnia persists, try:
- melatonin, 0.5 to 1.0 mg, at bedtime, 1 hour before bedtime, at sunset, or 6 hours before anticipated bedtime. I try to mimic the natural release of melatonin triggered by sunset, but no definitive data prove the most effective dosing time.
- alpha agonists such as clonidine, 0.1 to 0.2 mg at bedtime, or guanfacine, 1 to 2 mg at bedtime. These agents have proven efficacy for treating hyperactivity and sleep disturbances without causing tolerance but may be associated with nightmares in some children.3
- mirtazapine, one-half of a 15-mg tablet at bedtime.
Appetite suppression and insomnia—both common, dose-related side effects of psychostimulants—can jeopardize treatment adherence for patients with attention-deficit/hyperactivity disorder (ADHD). The following strategies can minimize these effects.
First, wait and see
For most patients, the optimal psychostimulant dosage produces few or no side effects. Those that occur are usually minor, transient, and disappear as patients develop tolerance within days of starting medication.
The two most commonly used stimulants—methylphenidate and amphetamine—cause similar side effects.1 No evidence suggests either is more effective or less tolerable than the other.
Fine-tune psychostimulants to the lowest dosage that produces maximum benefit and minimum side effects. If side effects persist beyond 7 to 10 days, the dosage is probably too high or the patient is taking another stimulating medication. Before you attribute insomnia or appetite suppression to psychostimulants, ask the patient if he or she is using a decongestant, caffeine, diet pills, systemic corticosteroids, systemic albuterol, or theophylline.
Countering appetite suppression
Approximately one-third of adult and pediatric ADHD patients report appetite suppression at therapeutic psychostimulant dosages, but in most patients this effect is transient or clinically insignificant. If a child taking psychostimulants is not eating or gaining weight appropriately:
- suggest that parents plan mealtimes before the patient’s next dose or give high-calorie snacks throughout the day. (This strategy, although recommended by the American Academy of Pediatrics, can be cumbersome and has limited long-term efficacy.)
- switch from amphetamine to methylphenidate or vice versa.
- add the antihistamine cyproheptadine, 4 mg, with morning and evening meals
- add mirtazapine, one-half of a 15-mg tablet at bedtime to stimulate appetite and initiate sleep.
If none of these interventions work, recommend drug holidays from ADHD medications as a last resort when impairment is lowest, such as during weekends, holidays, or summers.
Curbing insomnia
About 20% of prepubertal children and 75% to 80% of adults have difficulty falling asleep while taking ADHD medications.2 For many patients it is not the medications but the mental and physical restlessness of ADHD that disturbs sleep. Take a careful baseline sleep history before starting psychostimulants to help you determine later if they are causing insomnia.
Avoid benzodiazepines, which may promote tolerance and dependence. I discourage using any hypnotic to treat insomnia that occurs as a side effect. Also avoid antihistamines (Benedryl, trazodone) that may leave the patient sedated the next day.
Try a trial nap. After fine-tuning the psychostimulant to the lowest optimal dosage, ask the patient to test his ability to sleep while on that dose by taking an afternoon nap. Most patients discover they can sleep well, proving to both patient and doctor that ADHD medications usually help sleep initiation or are sleep-neutral. A successful nap can ease a patient’s fear that her medication will keep her awake.
Even the longest extended-release psychostimulant formulations do not last the 14 to 16 hours of a typical waking day. This no-risk trial nap reassures patients that they can take supplemental doses as prescribed to assist them through even the longest workdays, without fear of sleep disruption.
Time-release formulations smooth the abrupt kinetics and rebound activation seen with immediate-release psychostimulants. But for patients taking immediate-release formulations, reducing the day’s last dose or taking the last dose earlier can often prevent medication-associated insomnia.
If insomnia persists, try:
- melatonin, 0.5 to 1.0 mg, at bedtime, 1 hour before bedtime, at sunset, or 6 hours before anticipated bedtime. I try to mimic the natural release of melatonin triggered by sunset, but no definitive data prove the most effective dosing time.
- alpha agonists such as clonidine, 0.1 to 0.2 mg at bedtime, or guanfacine, 1 to 2 mg at bedtime. These agents have proven efficacy for treating hyperactivity and sleep disturbances without causing tolerance but may be associated with nightmares in some children.3
- mirtazapine, one-half of a 15-mg tablet at bedtime.
1. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35(10):1304-13.
2. Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1998;37:637-46.
3. Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsycho-pharmacol 2004;7(1):77-97.
Dr. Dodson is a board-certified psychiatrist specializing in adult ADHD. He is director of the Attention Disorders Treatment Center, Denver, CO.
1. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35(10):1304-13.
2. Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1998;37:637-46.
3. Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsycho-pharmacol 2004;7(1):77-97.
Dr. Dodson is a board-certified psychiatrist specializing in adult ADHD. He is director of the Attention Disorders Treatment Center, Denver, CO.
ADHD or bipolar, but not both
“What’s the best treatment for comorbid ADHD/bipolar mania?” by Drs. Nick C. Patel and Floyd R. Sallee (Current Psychiatry, April 2005) was well-written and offers excellent treatment guidelines. However, the idea that patients can have comorbid bipolar disorder and attention-deficit/hyperactivity disorder (ADHD) is a fallacy.
I challenge any colleague, from the leading expert to the most recent graduate, to present a bona fide case of “comorbid” ADHD/bipolar disorder. I can prove that only one diagnosis is correct because:
- Bipolar disorder is more heritable than other psychiatric illnesses. Many patients labeled as having “comorbid” bipolar disorder and ADHD have parents with bipolar disorder or schizophrenia or are in foster care and their biological parents’ histories are unknown.
- I’ve seen hundreds of patients enter full-blown psychosis after another clinician put them on amphetamines or antidepressants while being treated for ADHD.
- Bipolar disorder can explain any so-called ADHD symptom.
- ADHD does not include moodiness or predatory aggression.
Over 10 years, I have diagnosed three or four patients as having comorbid bipolar disorder and ADHD. After a few years and inpatient treatments, these patients proved the second diagnosis wrong. We can decrease costs and avoid patients’ suffering by refining diagnostic criteria.
Manuel Mota-Castillo, MD, medical director
The Grove Academy, Sanford, FL
and Lake Mary Psychiatric Services
Lake Mary, FL
Drs. Patel and Sallee respond
Dr. Mota-Castillo’s argument is most often stated from the opposite point of view that bipolar symptoms, particularly in patients age <10, are almost indistinguishable from those of ADHD. Our article did not—and cannot—address this controversy.
Because the evidence has been inconclusive, it is unclear if comorbid bipolar disorder and ADHD result from overlapping DSM-IV-TR diagnostic criteria, or whether two concurrent disorders exist. Suffice it to say that ADHD and bipolar disorder have many phenotypes and are both highly—but distinctly—heritable.
Overlapping symptoms may confound clinical diagnosis and result in “false positives” but may not account for most bipolar youths with comorbid ADHD. In one study,1 56% of subjects with both disorders maintained a bipolar disorder diagnosis when overlapping ADHD symptoms were subtracted.
Combination pharmacotherapy is needed because mood stabilizers do not treat attention and neurocognitive problems associated with ADHD. Therefore, a psychostimulant trial may help euthymic bipolar children and adolescents. In a recent placebo-controlled study by Scheffer et al,2 ADHD symptoms—as measured with the Clinical Global Impression of Improvement scale and based upon Conners’ Teachers and Parent Ratings—significantly improved among divalproex sodium responders receiving mixed amphetamine salts.
Dr. Mota-Castillo, however, brings up two important questions:
- Are childhood symptoms that result in ADHD diagnosis a prodromal manifestation of bipolar disorder in some patients? Data from the first 1,000 STEP-BD participants suggest that ADHD may be part of the developmental phenotype of bipolar disorder comorbidity. Participants with mood symptom onset before age 13 had higher rates of comorbid ADHD than did those whose mood symptoms surfaced later on.3
- Do psychostimulants hasten mood disorder onset in a child diagnosed with ADHD who has a high familial risk of a mood disorder? How these agents influence the course of bipolar disorder is unclear. DelBello et al4 reported that psychostimulant exposure may be a stressor in youths at risk for bipolar disorder, may progressively worsen affective symptoms over time, and may lead to earlier mood symptom onset.
Both questions need further exploration as the implications for clinical practice may be tremendous.
Results from numerous independent studies consistently suggest that patients can be diagnosed with comorbid bipolar disorder and ADHD. More research is needed, however, to solve this diagnostic conundrum.
Nick C. Patel, PharmD, PhD
Assistant professor
Departments of pharmacy practice and psychiatry
Floyd R. Sallee, MD, PhD
Professor, department of psychiatry
University of Cincinnati
1. Milberger S, Biederman J, Faraone SV, et al. Attention deficit hyperactivity disorder and comorbid disorders: issues of overlapping symptoms. Am J Psychiatry 1995;152:1793-9.
2. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
3. Perlis RH, Miyahara S, Marangell LB, et al. for the STEP-BD Investigators. Long-term implications of early onset in bipolar disorder: data from the first 1,000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004;55:875-81.
4. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
“What’s the best treatment for comorbid ADHD/bipolar mania?” by Drs. Nick C. Patel and Floyd R. Sallee (Current Psychiatry, April 2005) was well-written and offers excellent treatment guidelines. However, the idea that patients can have comorbid bipolar disorder and attention-deficit/hyperactivity disorder (ADHD) is a fallacy.
I challenge any colleague, from the leading expert to the most recent graduate, to present a bona fide case of “comorbid” ADHD/bipolar disorder. I can prove that only one diagnosis is correct because:
- Bipolar disorder is more heritable than other psychiatric illnesses. Many patients labeled as having “comorbid” bipolar disorder and ADHD have parents with bipolar disorder or schizophrenia or are in foster care and their biological parents’ histories are unknown.
- I’ve seen hundreds of patients enter full-blown psychosis after another clinician put them on amphetamines or antidepressants while being treated for ADHD.
- Bipolar disorder can explain any so-called ADHD symptom.
- ADHD does not include moodiness or predatory aggression.
Over 10 years, I have diagnosed three or four patients as having comorbid bipolar disorder and ADHD. After a few years and inpatient treatments, these patients proved the second diagnosis wrong. We can decrease costs and avoid patients’ suffering by refining diagnostic criteria.
Manuel Mota-Castillo, MD, medical director
The Grove Academy, Sanford, FL
and Lake Mary Psychiatric Services
Lake Mary, FL
Drs. Patel and Sallee respond
Dr. Mota-Castillo’s argument is most often stated from the opposite point of view that bipolar symptoms, particularly in patients age <10, are almost indistinguishable from those of ADHD. Our article did not—and cannot—address this controversy.
Because the evidence has been inconclusive, it is unclear if comorbid bipolar disorder and ADHD result from overlapping DSM-IV-TR diagnostic criteria, or whether two concurrent disorders exist. Suffice it to say that ADHD and bipolar disorder have many phenotypes and are both highly—but distinctly—heritable.
Overlapping symptoms may confound clinical diagnosis and result in “false positives” but may not account for most bipolar youths with comorbid ADHD. In one study,1 56% of subjects with both disorders maintained a bipolar disorder diagnosis when overlapping ADHD symptoms were subtracted.
Combination pharmacotherapy is needed because mood stabilizers do not treat attention and neurocognitive problems associated with ADHD. Therefore, a psychostimulant trial may help euthymic bipolar children and adolescents. In a recent placebo-controlled study by Scheffer et al,2 ADHD symptoms—as measured with the Clinical Global Impression of Improvement scale and based upon Conners’ Teachers and Parent Ratings—significantly improved among divalproex sodium responders receiving mixed amphetamine salts.
Dr. Mota-Castillo, however, brings up two important questions:
- Are childhood symptoms that result in ADHD diagnosis a prodromal manifestation of bipolar disorder in some patients? Data from the first 1,000 STEP-BD participants suggest that ADHD may be part of the developmental phenotype of bipolar disorder comorbidity. Participants with mood symptom onset before age 13 had higher rates of comorbid ADHD than did those whose mood symptoms surfaced later on.3
- Do psychostimulants hasten mood disorder onset in a child diagnosed with ADHD who has a high familial risk of a mood disorder? How these agents influence the course of bipolar disorder is unclear. DelBello et al4 reported that psychostimulant exposure may be a stressor in youths at risk for bipolar disorder, may progressively worsen affective symptoms over time, and may lead to earlier mood symptom onset.
Both questions need further exploration as the implications for clinical practice may be tremendous.
Results from numerous independent studies consistently suggest that patients can be diagnosed with comorbid bipolar disorder and ADHD. More research is needed, however, to solve this diagnostic conundrum.
Nick C. Patel, PharmD, PhD
Assistant professor
Departments of pharmacy practice and psychiatry
Floyd R. Sallee, MD, PhD
Professor, department of psychiatry
University of Cincinnati
“What’s the best treatment for comorbid ADHD/bipolar mania?” by Drs. Nick C. Patel and Floyd R. Sallee (Current Psychiatry, April 2005) was well-written and offers excellent treatment guidelines. However, the idea that patients can have comorbid bipolar disorder and attention-deficit/hyperactivity disorder (ADHD) is a fallacy.
I challenge any colleague, from the leading expert to the most recent graduate, to present a bona fide case of “comorbid” ADHD/bipolar disorder. I can prove that only one diagnosis is correct because:
- Bipolar disorder is more heritable than other psychiatric illnesses. Many patients labeled as having “comorbid” bipolar disorder and ADHD have parents with bipolar disorder or schizophrenia or are in foster care and their biological parents’ histories are unknown.
- I’ve seen hundreds of patients enter full-blown psychosis after another clinician put them on amphetamines or antidepressants while being treated for ADHD.
- Bipolar disorder can explain any so-called ADHD symptom.
- ADHD does not include moodiness or predatory aggression.
Over 10 years, I have diagnosed three or four patients as having comorbid bipolar disorder and ADHD. After a few years and inpatient treatments, these patients proved the second diagnosis wrong. We can decrease costs and avoid patients’ suffering by refining diagnostic criteria.
Manuel Mota-Castillo, MD, medical director
The Grove Academy, Sanford, FL
and Lake Mary Psychiatric Services
Lake Mary, FL
Drs. Patel and Sallee respond
Dr. Mota-Castillo’s argument is most often stated from the opposite point of view that bipolar symptoms, particularly in patients age <10, are almost indistinguishable from those of ADHD. Our article did not—and cannot—address this controversy.
Because the evidence has been inconclusive, it is unclear if comorbid bipolar disorder and ADHD result from overlapping DSM-IV-TR diagnostic criteria, or whether two concurrent disorders exist. Suffice it to say that ADHD and bipolar disorder have many phenotypes and are both highly—but distinctly—heritable.
Overlapping symptoms may confound clinical diagnosis and result in “false positives” but may not account for most bipolar youths with comorbid ADHD. In one study,1 56% of subjects with both disorders maintained a bipolar disorder diagnosis when overlapping ADHD symptoms were subtracted.
Combination pharmacotherapy is needed because mood stabilizers do not treat attention and neurocognitive problems associated with ADHD. Therefore, a psychostimulant trial may help euthymic bipolar children and adolescents. In a recent placebo-controlled study by Scheffer et al,2 ADHD symptoms—as measured with the Clinical Global Impression of Improvement scale and based upon Conners’ Teachers and Parent Ratings—significantly improved among divalproex sodium responders receiving mixed amphetamine salts.
Dr. Mota-Castillo, however, brings up two important questions:
- Are childhood symptoms that result in ADHD diagnosis a prodromal manifestation of bipolar disorder in some patients? Data from the first 1,000 STEP-BD participants suggest that ADHD may be part of the developmental phenotype of bipolar disorder comorbidity. Participants with mood symptom onset before age 13 had higher rates of comorbid ADHD than did those whose mood symptoms surfaced later on.3
- Do psychostimulants hasten mood disorder onset in a child diagnosed with ADHD who has a high familial risk of a mood disorder? How these agents influence the course of bipolar disorder is unclear. DelBello et al4 reported that psychostimulant exposure may be a stressor in youths at risk for bipolar disorder, may progressively worsen affective symptoms over time, and may lead to earlier mood symptom onset.
Both questions need further exploration as the implications for clinical practice may be tremendous.
Results from numerous independent studies consistently suggest that patients can be diagnosed with comorbid bipolar disorder and ADHD. More research is needed, however, to solve this diagnostic conundrum.
Nick C. Patel, PharmD, PhD
Assistant professor
Departments of pharmacy practice and psychiatry
Floyd R. Sallee, MD, PhD
Professor, department of psychiatry
University of Cincinnati
1. Milberger S, Biederman J, Faraone SV, et al. Attention deficit hyperactivity disorder and comorbid disorders: issues of overlapping symptoms. Am J Psychiatry 1995;152:1793-9.
2. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
3. Perlis RH, Miyahara S, Marangell LB, et al. for the STEP-BD Investigators. Long-term implications of early onset in bipolar disorder: data from the first 1,000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004;55:875-81.
4. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
1. Milberger S, Biederman J, Faraone SV, et al. Attention deficit hyperactivity disorder and comorbid disorders: issues of overlapping symptoms. Am J Psychiatry 1995;152:1793-9.
2. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
3. Perlis RH, Miyahara S, Marangell LB, et al. for the STEP-BD Investigators. Long-term implications of early onset in bipolar disorder: data from the first 1,000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004;55:875-81.
4. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
ADHD or bipolar disorder? Age-specific manic symptoms are key
Knowing what to look for can help you differentiate between pediatric bipolar disorder and attention-deficit/hyperactivity disorder (ADHD):
- Bipolar disorder is a problem with mood. Children with bipolar mania are elated and/or irritable and experience mood states that appear uncontrollable.
- ADHD is a problem with cognitive functioning, including attention, distractibility, and energy level.
Mood and cognitive symptoms may overlap,1,2 but recognizing manic features is the key to distinguishing between these disorders—even when they co-occur.
We offer tips from our experience and a recent clinical trial to help you sort out the core symptoms that point to bipolar mania.
BIPOLAR CORE SYMPTOMS
Pediatric bipolar disorder is relatively rare, but children with it can experience substantial impairment and developmental delay. Intervening early with effective treatment3 can improve their quality of life, function, and prognosis.
Diagnostic criteria for type I bipolar disorder require at least one manic episode and are the same for all ages. Many clinicians and researchers have advocated adapting DSM-IV criteria for children, but we believe separate adult and pediatric criteria would confuse discussions about the same phenomena. We do agree that symptoms should be evaluated in a developmentally appropriate context, as mania can present differently across the ages (Table 1).
Mania in children and young adolescents tends to present with rapid cycling and a primarily irritable mood.4 Older adolescents and adults may present with more-distinct mood changes, with a primarily euphoric mood. Euphoric mania is less common in adults than previously thought. Forty percent to 60% of adults with bipolar disorder experience a chronic course, rather than more-discrete mood episodes.
A manic episode is an abnormally and persistently elevated (euphoria) or irritable mood that lasts at least 1 week. To satisfy DSM-IV-TR diagnostic criteria for a manic episode:
- patients with euphoria require three additional symptoms
- those who are irritable (and not euphoric) require another four symptoms.5
These symptoms must significantly impair several areas of functioning and not be caused by other mental or physical illness, including substance use or abuse. When depressive symptoms occur in the same week as mania, the mixed mania modifier is used.
Table 1
Diagnostic features of bipolar mania in adolescents vs adults
| Feature | Prepubertal and early adolescent | Older adolescent and adult |
|---|---|---|
| Initial episode | Mixed presentations predominate | Mania is more balanced between mixed and euphoric |
| Episode type | More consistently ill | Persistent/distinct episodes |
| Primary mood | Irritable | Euphoric |
| Duration | Chronic, continuous course | Weeks |
| Inter-episode functioning | Less distinct episodes | May return to baseline or deteriorate over time |
| Reality testing | Delusions (grandiosity) is common; hallucinations | More variable |
Disruptive and aggressive behavior are common and are what usually prompts parents to bring children to psychiatrists. These behaviors are not diagnostic of mania, however, and aggression has many other causes.
The threshold between a variant of normal and pathologic disruptive behavior can be difficult to establish and varies from culture to culture. Some families, for example, would allow a child to tell the parents what to do, whereas other families consider this a serious boundary violation.
Prolonged rages have been used as a proxy for mood swings. Although we agree that rages lasting >15 minutes and out-of-proportion to the circumstances may signal bipolar disorder, they are not diagnostic.
Other symptoms. Psychotic symptoms (hallucinations, delusions, disorganization) can occur in youths with bipolar disorder. Evaluation often reveals impaired social and cognitive development. Keep in mind that a child’s developmental level can affect symptom expression.
ADHD CORE SYMPTOMS
Children with ADHD often present with hyperactive, uncontrollable behaviors and academic failure. To meet DSM-IV-TR diagnostic criteria, they must show symptoms before age 7. Primary symptoms may be inattention, hyperactivity and impulsivity, or both.
ADHD is a disorder of attention and the cognitive skills related to attention, rather than a mood disorder. Children with ADHD show substantially impaired function in at least two settings (such as at home and in school), and—unlike bipolar disorder—their symptoms are persistent rather than episodic.
DIFFERENTIATING BY SYMPTOMS
When differentiating between ADHD and bipolar disorder in children, remain focused on both diagnoses’ core symptoms.
Euphoria, or elation, is a key distinguishing factor in bipolar disorder.6 Although all children are at times giddy or silly in appropriate environments—such as during slumber parties—consider a threshold of appropriateness when making a bipolar diagnosis. Families perceive the giddiness, inappropriate laughter, and elevated mood of children with mania as disturbing and inappropriate, not funny or endearing. They are often annoyed and concerned.
Children with primary ADHD do not show inappropriately elevated mood. In fact, their failures often make these children dysphoric.
Irritability is common in children with psychiatric illnesses. Manic youngsters can be very irritable most of the time. Families describe “walking on eggshells” because of these children’s touchiness. Unpredictable triggers set off explosive, prolonged tantrums that may be associated with aggression, and their mood swings are almost constant.
Children with ADHD can be irritable, but their irritability is less severe and intense than that seen in bipolar disorder. Stimulant medication “wear-off” can cause irritability in ADHD, so consider this possibility if symptoms occur mostly in the evening.
Grandiosity can be confusing to evaluate in children but is often a core symptom in bipolar disorder. All children sometimes say self-inflating things, but those with pathologic grandiosity cross the threshold into the dysfunctional belief that they are better, stronger, smarter, or more talented than others.
For example, a 7-year-old patient insisted he was the world’s best chess player and could beat anyone, including Russian chess masters. When the therapist asked him about chess, he did not know the names of the pieces or how they moved. Yet despite facing these contradictory facts, he continued to insist that he was the best.
Children with grandiosity may act inappropriately on their beliefs, such as by telling adults what to do or engaging in risky, daredevil acts with no concern for their safety or the law.
Children with ADHD are not usually grandiose. Instead, they often become demoralized and develop poor self-esteem from negative feedback about their behavior.
Decreased need for sleep is the hallmark symptom of mania that is absent in other psychiatric disorders. A true decreased need for sleep is only indicated in someone sleeping less than his or her usual cumulative hours each day, without fatigue or recuperative sleep.
Children with bipolar disorder may need 1 or more hours less sleep or deny needing sleep at all. Use age-appropriate amounts of sleep as a standard. A school age child usually averages 9 to 11 hours of sleep per night. If the patient is getting only 6 hours and is not tired, this would be a decreased need for sleep. A 24-hour sleep history can easily assess decreased need for sleep (Box).
Determine daytime fatigue by self-report or observation by parents or teachers. Then ascertain if there are periods of days with less fatigue. Many bipolar youth have a nearly continuous decreased need for sleep.
Children with ADHD often have difficulty settling at night, which delays their falling asleep. The sleep history will likely show that—once asleep—they sleep well for an appropriate amount of time or are fatigued during the day.
Decreased need for sleep is a hallmark symptom of bipolar mania. A 24-hour sleep history may help determine if a child’s irregular sleep patterns signal ADHD or bipolar disorder.
To perform the sleep history, collect time in bed and time asleep over several days, or ask the patient,
- On a typical night, not your best, not your worst:”
- When do you go to bed?
- How long does it take to fall asleep?
- Once asleep, do you wake up?
- How long are you awake?
- When do you arise in the morning?
- What is the total amount of time you are asleep?
- Do you take naps?
- Are you rested or fatigued during the day?
Pressured speech, or the need to talk excessively, is a relatively straightforward symptom. Children experiencing mania often speak so quickly and excessively that others cannot understand or interrupt them. Flight of ideas and racing thoughts are reflected in their speech.
By contrast, rapid speech by children with ADHD is related to hyperactivity. They speak too fast and often become distracted from the topic.
Racing thoughts. Children with bipolar disorder may report that their thoughts come so quickly they cannot get them out fast enough. The idea that their thoughts “need a stop-sign” suggests racing thoughts, a core bipolar symptom. Their speech can be unintelligible, with rapid changes in thought patterns, flight of ideas, and sentence fragments. Children with ADHD are energetic and quick but do not report racing thoughts.
LESS-HELPFUL SYMPTOMS
Distractibility—a core symptom of both inattentive ADHD and manic episodes—does not help differentiate the two diagnoses. The high comorbidity of ADHD with bipolar disorder increases the likelihood that the child will be easily distracted.
Multi-tasking. Increased goal-directed activity is often associated with the high energy of children with mania. They have more energy than most people, are always on the go, and engage in multiple projects or activities that may be markedly creative or unrealistic. This increased energy—combined with other hallmark manic symptoms—can lead to high-risk behaviors.
Hyperactivity in ADHD can appear similar to agitation in bipolar disorder. In both disorders, children may engage in many tasks—not finishing any of them—or appear to move quickly from one task to another.
High-risk behaviors. Parents often report their children with bipolar disorder have tried to jump from moving vehicles, “fly” off of roofs, and jump their bicycles or skateboards over impossible distances. These children behave as if the laws of nature do not apply to them. Children with ADHD behave impulsively but are not always “daredevils.” Their activities appear more impulsive and feature high activity in inappropriate situations, rather than distinctly high-risk activities.
RESPONSE TO THERAPY
Our group7 showed that pediatric manic and ADHD symptoms respond differently to mood-stabilizer treatment (Table 2).
We first used open-label divalproex sodium to treat manic symptoms in 40 children ages 6 to 17 with bipolar I or II disorder and concurrent ADHD. Serum valproic acid levels averaged 82 μg/mL. Manic symptoms improved in 80% of patients, whereas ADHD symptoms improved in <10%. Most children’s symptoms still met severity criteria for ADHD.
In a subsequent double-blind, crossover trial, we compared the effects of mixed amphetamine salts (MAS) or placebo on ADHD symptoms in 30 children whose manic symptoms stabilized on divalproex. MAS showed a significant, independent effect on ADHD symptoms One patient’s manic symptoms recurred during stimulant therapy and subsided with MAS discontinuation.
In this trial, mania symptoms responded to divalproex, whereas ADHD symptoms did not. MAS treatment showed a specific effect on ADHD symptoms of inattention, impulsivity, and hyperactivity. The shared symptoms of mania and ADHD (impulsivity and hyperactivity) decreased with divalproex to some extent.
Table 2
Pediatric mania and ADHD
respond differently to mood-stabilizer therapy
| Children with bipolar I or II disorder and concurrent ADHD | Treatment with divalproex, 8 weeks (N = 40)a | Subjects enter double-blind, crossover treatment with MAS and placebo, 2 weeks each (N = 30) | |
|---|---|---|---|
| Manic symptomsb | 32 of 40 (80%) improved; significant (P <0.0001) | MAS | Placebo |
| No significant change in manic symptoms (P = 0.17) | |||
| ADHD symptomsc | 3 of 40 (7.5%) improved; not significant (P = 0.96) | 26 of 30 (87%) improved | 3 of 30 (10%) improved |
| CGI scores improved 1.9 points more on MAS than on placebo, a significant difference (P <0.0001) | |||
| a Average divalproex blood levels = 82 μg/mL | |||
| b Manic symptom improvement defined as >50% decrease in baseline Young Mania Rating Scale scores | |||
| c ADHD symptom improvement defined as Clinical Global Impression (CGI)–Improvement scores of 1 or 2 | |||
| MAS: Mixed amphetamine salts | |||
| Source: Reference 7 | |||
WHEN MANIA/ADHD CO-OCCUR
ADHD and bipolar disorder symptoms overlap to a great extent, and the disorders can co-occur:
- Up to 20% of children diagnosed with ADHD also meet bipolar criteria.
- Two-thirds of children with bipolar disorder may also meet criteria for ADHD, with reports ranging from 29% to 98%.1,2
When trying to differentiate ADHD and bipolar disorder in children, consider the core symptoms of each diagnosis (Table 3).
Table 3
Core symptoms: Pediatric bipolar disorder vs ADHD
| Symptom | Bipolar disorder | ADHD |
|---|---|---|
| Euphoria/giddiness | Excessive | Appropriate to situations |
| Irritability | Severe and intense, often accompanied by tantrums | Occasional, may be caused by medication “wear-off” |
| Self-esteem | Grandiose | Demoralized |
| Sleep patterns | Decreased need for sleep | Difficulty settling at night |
| Speech patterns | Pressured, fragmented, with flight of ideas | Energetic and quick |
| Thought processes | Racing thoughts | Patients do not report racing thoughts |
| Psychosis can occur at times | ||
| Attention | Distractible | Distractible |
| Activity level | High energy, on-the-go, multiple projects, creative | Hyperactive, multiple projects |
| High-risk behaviors | Impulsive | |
| Disruptive behaviors | Can become aggressive | Intrusive and active |
Related resources
- Geller B, DelBello MP (eds). Bipolar disorder in childhood and early adolescence. New York: Guilford Press, 2003.
- Papolos DF, Papolos J. Bipolar child: The definitive and reassuring guide to childhood’s most misunderstood disorder. New York: Broadway Books, 2002.
- Fristad MA, Goldberg Arnold JS. Raising a moody child: How to cope with depression and bipolar disorder. New York: Guilford Press, 2003.
- Child and Adolescent Bipolar Foundation. Available at www.cabf.org. Accessed March 4, 2005.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Scheffer receives research support from and is a speaker for Abbott Laboratories.
Dr. Apps reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
2. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35(8):997-1008.
3. Patel NC, Sallee FR. What’s the best treatment for ADHD/bipolar mania? Current Psychiatry 2005;3(3):27-37.
4. Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004;61:459-67.
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., rev). Washington, DC: American Psychiatric Association, 2000.
6. Geller B, Williams M, Zimerman B, et al. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord 1998;51(2):81-91.
7. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
Knowing what to look for can help you differentiate between pediatric bipolar disorder and attention-deficit/hyperactivity disorder (ADHD):
- Bipolar disorder is a problem with mood. Children with bipolar mania are elated and/or irritable and experience mood states that appear uncontrollable.
- ADHD is a problem with cognitive functioning, including attention, distractibility, and energy level.
Mood and cognitive symptoms may overlap,1,2 but recognizing manic features is the key to distinguishing between these disorders—even when they co-occur.
We offer tips from our experience and a recent clinical trial to help you sort out the core symptoms that point to bipolar mania.
BIPOLAR CORE SYMPTOMS
Pediatric bipolar disorder is relatively rare, but children with it can experience substantial impairment and developmental delay. Intervening early with effective treatment3 can improve their quality of life, function, and prognosis.
Diagnostic criteria for type I bipolar disorder require at least one manic episode and are the same for all ages. Many clinicians and researchers have advocated adapting DSM-IV criteria for children, but we believe separate adult and pediatric criteria would confuse discussions about the same phenomena. We do agree that symptoms should be evaluated in a developmentally appropriate context, as mania can present differently across the ages (Table 1).
Mania in children and young adolescents tends to present with rapid cycling and a primarily irritable mood.4 Older adolescents and adults may present with more-distinct mood changes, with a primarily euphoric mood. Euphoric mania is less common in adults than previously thought. Forty percent to 60% of adults with bipolar disorder experience a chronic course, rather than more-discrete mood episodes.
A manic episode is an abnormally and persistently elevated (euphoria) or irritable mood that lasts at least 1 week. To satisfy DSM-IV-TR diagnostic criteria for a manic episode:
- patients with euphoria require three additional symptoms
- those who are irritable (and not euphoric) require another four symptoms.5
These symptoms must significantly impair several areas of functioning and not be caused by other mental or physical illness, including substance use or abuse. When depressive symptoms occur in the same week as mania, the mixed mania modifier is used.
Table 1
Diagnostic features of bipolar mania in adolescents vs adults
| Feature | Prepubertal and early adolescent | Older adolescent and adult |
|---|---|---|
| Initial episode | Mixed presentations predominate | Mania is more balanced between mixed and euphoric |
| Episode type | More consistently ill | Persistent/distinct episodes |
| Primary mood | Irritable | Euphoric |
| Duration | Chronic, continuous course | Weeks |
| Inter-episode functioning | Less distinct episodes | May return to baseline or deteriorate over time |
| Reality testing | Delusions (grandiosity) is common; hallucinations | More variable |
Disruptive and aggressive behavior are common and are what usually prompts parents to bring children to psychiatrists. These behaviors are not diagnostic of mania, however, and aggression has many other causes.
The threshold between a variant of normal and pathologic disruptive behavior can be difficult to establish and varies from culture to culture. Some families, for example, would allow a child to tell the parents what to do, whereas other families consider this a serious boundary violation.
Prolonged rages have been used as a proxy for mood swings. Although we agree that rages lasting >15 minutes and out-of-proportion to the circumstances may signal bipolar disorder, they are not diagnostic.
Other symptoms. Psychotic symptoms (hallucinations, delusions, disorganization) can occur in youths with bipolar disorder. Evaluation often reveals impaired social and cognitive development. Keep in mind that a child’s developmental level can affect symptom expression.
ADHD CORE SYMPTOMS
Children with ADHD often present with hyperactive, uncontrollable behaviors and academic failure. To meet DSM-IV-TR diagnostic criteria, they must show symptoms before age 7. Primary symptoms may be inattention, hyperactivity and impulsivity, or both.
ADHD is a disorder of attention and the cognitive skills related to attention, rather than a mood disorder. Children with ADHD show substantially impaired function in at least two settings (such as at home and in school), and—unlike bipolar disorder—their symptoms are persistent rather than episodic.
DIFFERENTIATING BY SYMPTOMS
When differentiating between ADHD and bipolar disorder in children, remain focused on both diagnoses’ core symptoms.
Euphoria, or elation, is a key distinguishing factor in bipolar disorder.6 Although all children are at times giddy or silly in appropriate environments—such as during slumber parties—consider a threshold of appropriateness when making a bipolar diagnosis. Families perceive the giddiness, inappropriate laughter, and elevated mood of children with mania as disturbing and inappropriate, not funny or endearing. They are often annoyed and concerned.
Children with primary ADHD do not show inappropriately elevated mood. In fact, their failures often make these children dysphoric.
Irritability is common in children with psychiatric illnesses. Manic youngsters can be very irritable most of the time. Families describe “walking on eggshells” because of these children’s touchiness. Unpredictable triggers set off explosive, prolonged tantrums that may be associated with aggression, and their mood swings are almost constant.
Children with ADHD can be irritable, but their irritability is less severe and intense than that seen in bipolar disorder. Stimulant medication “wear-off” can cause irritability in ADHD, so consider this possibility if symptoms occur mostly in the evening.
Grandiosity can be confusing to evaluate in children but is often a core symptom in bipolar disorder. All children sometimes say self-inflating things, but those with pathologic grandiosity cross the threshold into the dysfunctional belief that they are better, stronger, smarter, or more talented than others.
For example, a 7-year-old patient insisted he was the world’s best chess player and could beat anyone, including Russian chess masters. When the therapist asked him about chess, he did not know the names of the pieces or how they moved. Yet despite facing these contradictory facts, he continued to insist that he was the best.
Children with grandiosity may act inappropriately on their beliefs, such as by telling adults what to do or engaging in risky, daredevil acts with no concern for their safety or the law.
Children with ADHD are not usually grandiose. Instead, they often become demoralized and develop poor self-esteem from negative feedback about their behavior.
Decreased need for sleep is the hallmark symptom of mania that is absent in other psychiatric disorders. A true decreased need for sleep is only indicated in someone sleeping less than his or her usual cumulative hours each day, without fatigue or recuperative sleep.
Children with bipolar disorder may need 1 or more hours less sleep or deny needing sleep at all. Use age-appropriate amounts of sleep as a standard. A school age child usually averages 9 to 11 hours of sleep per night. If the patient is getting only 6 hours and is not tired, this would be a decreased need for sleep. A 24-hour sleep history can easily assess decreased need for sleep (Box).
Determine daytime fatigue by self-report or observation by parents or teachers. Then ascertain if there are periods of days with less fatigue. Many bipolar youth have a nearly continuous decreased need for sleep.
Children with ADHD often have difficulty settling at night, which delays their falling asleep. The sleep history will likely show that—once asleep—they sleep well for an appropriate amount of time or are fatigued during the day.
Decreased need for sleep is a hallmark symptom of bipolar mania. A 24-hour sleep history may help determine if a child’s irregular sleep patterns signal ADHD or bipolar disorder.
To perform the sleep history, collect time in bed and time asleep over several days, or ask the patient,
- On a typical night, not your best, not your worst:”
- When do you go to bed?
- How long does it take to fall asleep?
- Once asleep, do you wake up?
- How long are you awake?
- When do you arise in the morning?
- What is the total amount of time you are asleep?
- Do you take naps?
- Are you rested or fatigued during the day?
Pressured speech, or the need to talk excessively, is a relatively straightforward symptom. Children experiencing mania often speak so quickly and excessively that others cannot understand or interrupt them. Flight of ideas and racing thoughts are reflected in their speech.
By contrast, rapid speech by children with ADHD is related to hyperactivity. They speak too fast and often become distracted from the topic.
Racing thoughts. Children with bipolar disorder may report that their thoughts come so quickly they cannot get them out fast enough. The idea that their thoughts “need a stop-sign” suggests racing thoughts, a core bipolar symptom. Their speech can be unintelligible, with rapid changes in thought patterns, flight of ideas, and sentence fragments. Children with ADHD are energetic and quick but do not report racing thoughts.
LESS-HELPFUL SYMPTOMS
Distractibility—a core symptom of both inattentive ADHD and manic episodes—does not help differentiate the two diagnoses. The high comorbidity of ADHD with bipolar disorder increases the likelihood that the child will be easily distracted.
Multi-tasking. Increased goal-directed activity is often associated with the high energy of children with mania. They have more energy than most people, are always on the go, and engage in multiple projects or activities that may be markedly creative or unrealistic. This increased energy—combined with other hallmark manic symptoms—can lead to high-risk behaviors.
Hyperactivity in ADHD can appear similar to agitation in bipolar disorder. In both disorders, children may engage in many tasks—not finishing any of them—or appear to move quickly from one task to another.
High-risk behaviors. Parents often report their children with bipolar disorder have tried to jump from moving vehicles, “fly” off of roofs, and jump their bicycles or skateboards over impossible distances. These children behave as if the laws of nature do not apply to them. Children with ADHD behave impulsively but are not always “daredevils.” Their activities appear more impulsive and feature high activity in inappropriate situations, rather than distinctly high-risk activities.
RESPONSE TO THERAPY
Our group7 showed that pediatric manic and ADHD symptoms respond differently to mood-stabilizer treatment (Table 2).
We first used open-label divalproex sodium to treat manic symptoms in 40 children ages 6 to 17 with bipolar I or II disorder and concurrent ADHD. Serum valproic acid levels averaged 82 μg/mL. Manic symptoms improved in 80% of patients, whereas ADHD symptoms improved in <10%. Most children’s symptoms still met severity criteria for ADHD.
In a subsequent double-blind, crossover trial, we compared the effects of mixed amphetamine salts (MAS) or placebo on ADHD symptoms in 30 children whose manic symptoms stabilized on divalproex. MAS showed a significant, independent effect on ADHD symptoms One patient’s manic symptoms recurred during stimulant therapy and subsided with MAS discontinuation.
In this trial, mania symptoms responded to divalproex, whereas ADHD symptoms did not. MAS treatment showed a specific effect on ADHD symptoms of inattention, impulsivity, and hyperactivity. The shared symptoms of mania and ADHD (impulsivity and hyperactivity) decreased with divalproex to some extent.
Table 2
Pediatric mania and ADHD
respond differently to mood-stabilizer therapy
| Children with bipolar I or II disorder and concurrent ADHD | Treatment with divalproex, 8 weeks (N = 40)a | Subjects enter double-blind, crossover treatment with MAS and placebo, 2 weeks each (N = 30) | |
|---|---|---|---|
| Manic symptomsb | 32 of 40 (80%) improved; significant (P <0.0001) | MAS | Placebo |
| No significant change in manic symptoms (P = 0.17) | |||
| ADHD symptomsc | 3 of 40 (7.5%) improved; not significant (P = 0.96) | 26 of 30 (87%) improved | 3 of 30 (10%) improved |
| CGI scores improved 1.9 points more on MAS than on placebo, a significant difference (P <0.0001) | |||
| a Average divalproex blood levels = 82 μg/mL | |||
| b Manic symptom improvement defined as >50% decrease in baseline Young Mania Rating Scale scores | |||
| c ADHD symptom improvement defined as Clinical Global Impression (CGI)–Improvement scores of 1 or 2 | |||
| MAS: Mixed amphetamine salts | |||
| Source: Reference 7 | |||
WHEN MANIA/ADHD CO-OCCUR
ADHD and bipolar disorder symptoms overlap to a great extent, and the disorders can co-occur:
- Up to 20% of children diagnosed with ADHD also meet bipolar criteria.
- Two-thirds of children with bipolar disorder may also meet criteria for ADHD, with reports ranging from 29% to 98%.1,2
When trying to differentiate ADHD and bipolar disorder in children, consider the core symptoms of each diagnosis (Table 3).
Table 3
Core symptoms: Pediatric bipolar disorder vs ADHD
| Symptom | Bipolar disorder | ADHD |
|---|---|---|
| Euphoria/giddiness | Excessive | Appropriate to situations |
| Irritability | Severe and intense, often accompanied by tantrums | Occasional, may be caused by medication “wear-off” |
| Self-esteem | Grandiose | Demoralized |
| Sleep patterns | Decreased need for sleep | Difficulty settling at night |
| Speech patterns | Pressured, fragmented, with flight of ideas | Energetic and quick |
| Thought processes | Racing thoughts | Patients do not report racing thoughts |
| Psychosis can occur at times | ||
| Attention | Distractible | Distractible |
| Activity level | High energy, on-the-go, multiple projects, creative | Hyperactive, multiple projects |
| High-risk behaviors | Impulsive | |
| Disruptive behaviors | Can become aggressive | Intrusive and active |
Related resources
- Geller B, DelBello MP (eds). Bipolar disorder in childhood and early adolescence. New York: Guilford Press, 2003.
- Papolos DF, Papolos J. Bipolar child: The definitive and reassuring guide to childhood’s most misunderstood disorder. New York: Broadway Books, 2002.
- Fristad MA, Goldberg Arnold JS. Raising a moody child: How to cope with depression and bipolar disorder. New York: Guilford Press, 2003.
- Child and Adolescent Bipolar Foundation. Available at www.cabf.org. Accessed March 4, 2005.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Scheffer receives research support from and is a speaker for Abbott Laboratories.
Dr. Apps reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Knowing what to look for can help you differentiate between pediatric bipolar disorder and attention-deficit/hyperactivity disorder (ADHD):
- Bipolar disorder is a problem with mood. Children with bipolar mania are elated and/or irritable and experience mood states that appear uncontrollable.
- ADHD is a problem with cognitive functioning, including attention, distractibility, and energy level.
Mood and cognitive symptoms may overlap,1,2 but recognizing manic features is the key to distinguishing between these disorders—even when they co-occur.
We offer tips from our experience and a recent clinical trial to help you sort out the core symptoms that point to bipolar mania.
BIPOLAR CORE SYMPTOMS
Pediatric bipolar disorder is relatively rare, but children with it can experience substantial impairment and developmental delay. Intervening early with effective treatment3 can improve their quality of life, function, and prognosis.
Diagnostic criteria for type I bipolar disorder require at least one manic episode and are the same for all ages. Many clinicians and researchers have advocated adapting DSM-IV criteria for children, but we believe separate adult and pediatric criteria would confuse discussions about the same phenomena. We do agree that symptoms should be evaluated in a developmentally appropriate context, as mania can present differently across the ages (Table 1).
Mania in children and young adolescents tends to present with rapid cycling and a primarily irritable mood.4 Older adolescents and adults may present with more-distinct mood changes, with a primarily euphoric mood. Euphoric mania is less common in adults than previously thought. Forty percent to 60% of adults with bipolar disorder experience a chronic course, rather than more-discrete mood episodes.
A manic episode is an abnormally and persistently elevated (euphoria) or irritable mood that lasts at least 1 week. To satisfy DSM-IV-TR diagnostic criteria for a manic episode:
- patients with euphoria require three additional symptoms
- those who are irritable (and not euphoric) require another four symptoms.5
These symptoms must significantly impair several areas of functioning and not be caused by other mental or physical illness, including substance use or abuse. When depressive symptoms occur in the same week as mania, the mixed mania modifier is used.
Table 1
Diagnostic features of bipolar mania in adolescents vs adults
| Feature | Prepubertal and early adolescent | Older adolescent and adult |
|---|---|---|
| Initial episode | Mixed presentations predominate | Mania is more balanced between mixed and euphoric |
| Episode type | More consistently ill | Persistent/distinct episodes |
| Primary mood | Irritable | Euphoric |
| Duration | Chronic, continuous course | Weeks |
| Inter-episode functioning | Less distinct episodes | May return to baseline or deteriorate over time |
| Reality testing | Delusions (grandiosity) is common; hallucinations | More variable |
Disruptive and aggressive behavior are common and are what usually prompts parents to bring children to psychiatrists. These behaviors are not diagnostic of mania, however, and aggression has many other causes.
The threshold between a variant of normal and pathologic disruptive behavior can be difficult to establish and varies from culture to culture. Some families, for example, would allow a child to tell the parents what to do, whereas other families consider this a serious boundary violation.
Prolonged rages have been used as a proxy for mood swings. Although we agree that rages lasting >15 minutes and out-of-proportion to the circumstances may signal bipolar disorder, they are not diagnostic.
Other symptoms. Psychotic symptoms (hallucinations, delusions, disorganization) can occur in youths with bipolar disorder. Evaluation often reveals impaired social and cognitive development. Keep in mind that a child’s developmental level can affect symptom expression.
ADHD CORE SYMPTOMS
Children with ADHD often present with hyperactive, uncontrollable behaviors and academic failure. To meet DSM-IV-TR diagnostic criteria, they must show symptoms before age 7. Primary symptoms may be inattention, hyperactivity and impulsivity, or both.
ADHD is a disorder of attention and the cognitive skills related to attention, rather than a mood disorder. Children with ADHD show substantially impaired function in at least two settings (such as at home and in school), and—unlike bipolar disorder—their symptoms are persistent rather than episodic.
DIFFERENTIATING BY SYMPTOMS
When differentiating between ADHD and bipolar disorder in children, remain focused on both diagnoses’ core symptoms.
Euphoria, or elation, is a key distinguishing factor in bipolar disorder.6 Although all children are at times giddy or silly in appropriate environments—such as during slumber parties—consider a threshold of appropriateness when making a bipolar diagnosis. Families perceive the giddiness, inappropriate laughter, and elevated mood of children with mania as disturbing and inappropriate, not funny or endearing. They are often annoyed and concerned.
Children with primary ADHD do not show inappropriately elevated mood. In fact, their failures often make these children dysphoric.
Irritability is common in children with psychiatric illnesses. Manic youngsters can be very irritable most of the time. Families describe “walking on eggshells” because of these children’s touchiness. Unpredictable triggers set off explosive, prolonged tantrums that may be associated with aggression, and their mood swings are almost constant.
Children with ADHD can be irritable, but their irritability is less severe and intense than that seen in bipolar disorder. Stimulant medication “wear-off” can cause irritability in ADHD, so consider this possibility if symptoms occur mostly in the evening.
Grandiosity can be confusing to evaluate in children but is often a core symptom in bipolar disorder. All children sometimes say self-inflating things, but those with pathologic grandiosity cross the threshold into the dysfunctional belief that they are better, stronger, smarter, or more talented than others.
For example, a 7-year-old patient insisted he was the world’s best chess player and could beat anyone, including Russian chess masters. When the therapist asked him about chess, he did not know the names of the pieces or how they moved. Yet despite facing these contradictory facts, he continued to insist that he was the best.
Children with grandiosity may act inappropriately on their beliefs, such as by telling adults what to do or engaging in risky, daredevil acts with no concern for their safety or the law.
Children with ADHD are not usually grandiose. Instead, they often become demoralized and develop poor self-esteem from negative feedback about their behavior.
Decreased need for sleep is the hallmark symptom of mania that is absent in other psychiatric disorders. A true decreased need for sleep is only indicated in someone sleeping less than his or her usual cumulative hours each day, without fatigue or recuperative sleep.
Children with bipolar disorder may need 1 or more hours less sleep or deny needing sleep at all. Use age-appropriate amounts of sleep as a standard. A school age child usually averages 9 to 11 hours of sleep per night. If the patient is getting only 6 hours and is not tired, this would be a decreased need for sleep. A 24-hour sleep history can easily assess decreased need for sleep (Box).
Determine daytime fatigue by self-report or observation by parents or teachers. Then ascertain if there are periods of days with less fatigue. Many bipolar youth have a nearly continuous decreased need for sleep.
Children with ADHD often have difficulty settling at night, which delays their falling asleep. The sleep history will likely show that—once asleep—they sleep well for an appropriate amount of time or are fatigued during the day.
Decreased need for sleep is a hallmark symptom of bipolar mania. A 24-hour sleep history may help determine if a child’s irregular sleep patterns signal ADHD or bipolar disorder.
To perform the sleep history, collect time in bed and time asleep over several days, or ask the patient,
- On a typical night, not your best, not your worst:”
- When do you go to bed?
- How long does it take to fall asleep?
- Once asleep, do you wake up?
- How long are you awake?
- When do you arise in the morning?
- What is the total amount of time you are asleep?
- Do you take naps?
- Are you rested or fatigued during the day?
Pressured speech, or the need to talk excessively, is a relatively straightforward symptom. Children experiencing mania often speak so quickly and excessively that others cannot understand or interrupt them. Flight of ideas and racing thoughts are reflected in their speech.
By contrast, rapid speech by children with ADHD is related to hyperactivity. They speak too fast and often become distracted from the topic.
Racing thoughts. Children with bipolar disorder may report that their thoughts come so quickly they cannot get them out fast enough. The idea that their thoughts “need a stop-sign” suggests racing thoughts, a core bipolar symptom. Their speech can be unintelligible, with rapid changes in thought patterns, flight of ideas, and sentence fragments. Children with ADHD are energetic and quick but do not report racing thoughts.
LESS-HELPFUL SYMPTOMS
Distractibility—a core symptom of both inattentive ADHD and manic episodes—does not help differentiate the two diagnoses. The high comorbidity of ADHD with bipolar disorder increases the likelihood that the child will be easily distracted.
Multi-tasking. Increased goal-directed activity is often associated with the high energy of children with mania. They have more energy than most people, are always on the go, and engage in multiple projects or activities that may be markedly creative or unrealistic. This increased energy—combined with other hallmark manic symptoms—can lead to high-risk behaviors.
Hyperactivity in ADHD can appear similar to agitation in bipolar disorder. In both disorders, children may engage in many tasks—not finishing any of them—or appear to move quickly from one task to another.
High-risk behaviors. Parents often report their children with bipolar disorder have tried to jump from moving vehicles, “fly” off of roofs, and jump their bicycles or skateboards over impossible distances. These children behave as if the laws of nature do not apply to them. Children with ADHD behave impulsively but are not always “daredevils.” Their activities appear more impulsive and feature high activity in inappropriate situations, rather than distinctly high-risk activities.
RESPONSE TO THERAPY
Our group7 showed that pediatric manic and ADHD symptoms respond differently to mood-stabilizer treatment (Table 2).
We first used open-label divalproex sodium to treat manic symptoms in 40 children ages 6 to 17 with bipolar I or II disorder and concurrent ADHD. Serum valproic acid levels averaged 82 μg/mL. Manic symptoms improved in 80% of patients, whereas ADHD symptoms improved in <10%. Most children’s symptoms still met severity criteria for ADHD.
In a subsequent double-blind, crossover trial, we compared the effects of mixed amphetamine salts (MAS) or placebo on ADHD symptoms in 30 children whose manic symptoms stabilized on divalproex. MAS showed a significant, independent effect on ADHD symptoms One patient’s manic symptoms recurred during stimulant therapy and subsided with MAS discontinuation.
In this trial, mania symptoms responded to divalproex, whereas ADHD symptoms did not. MAS treatment showed a specific effect on ADHD symptoms of inattention, impulsivity, and hyperactivity. The shared symptoms of mania and ADHD (impulsivity and hyperactivity) decreased with divalproex to some extent.
Table 2
Pediatric mania and ADHD
respond differently to mood-stabilizer therapy
| Children with bipolar I or II disorder and concurrent ADHD | Treatment with divalproex, 8 weeks (N = 40)a | Subjects enter double-blind, crossover treatment with MAS and placebo, 2 weeks each (N = 30) | |
|---|---|---|---|
| Manic symptomsb | 32 of 40 (80%) improved; significant (P <0.0001) | MAS | Placebo |
| No significant change in manic symptoms (P = 0.17) | |||
| ADHD symptomsc | 3 of 40 (7.5%) improved; not significant (P = 0.96) | 26 of 30 (87%) improved | 3 of 30 (10%) improved |
| CGI scores improved 1.9 points more on MAS than on placebo, a significant difference (P <0.0001) | |||
| a Average divalproex blood levels = 82 μg/mL | |||
| b Manic symptom improvement defined as >50% decrease in baseline Young Mania Rating Scale scores | |||
| c ADHD symptom improvement defined as Clinical Global Impression (CGI)–Improvement scores of 1 or 2 | |||
| MAS: Mixed amphetamine salts | |||
| Source: Reference 7 | |||
WHEN MANIA/ADHD CO-OCCUR
ADHD and bipolar disorder symptoms overlap to a great extent, and the disorders can co-occur:
- Up to 20% of children diagnosed with ADHD also meet bipolar criteria.
- Two-thirds of children with bipolar disorder may also meet criteria for ADHD, with reports ranging from 29% to 98%.1,2
When trying to differentiate ADHD and bipolar disorder in children, consider the core symptoms of each diagnosis (Table 3).
Table 3
Core symptoms: Pediatric bipolar disorder vs ADHD
| Symptom | Bipolar disorder | ADHD |
|---|---|---|
| Euphoria/giddiness | Excessive | Appropriate to situations |
| Irritability | Severe and intense, often accompanied by tantrums | Occasional, may be caused by medication “wear-off” |
| Self-esteem | Grandiose | Demoralized |
| Sleep patterns | Decreased need for sleep | Difficulty settling at night |
| Speech patterns | Pressured, fragmented, with flight of ideas | Energetic and quick |
| Thought processes | Racing thoughts | Patients do not report racing thoughts |
| Psychosis can occur at times | ||
| Attention | Distractible | Distractible |
| Activity level | High energy, on-the-go, multiple projects, creative | Hyperactive, multiple projects |
| High-risk behaviors | Impulsive | |
| Disruptive behaviors | Can become aggressive | Intrusive and active |
Related resources
- Geller B, DelBello MP (eds). Bipolar disorder in childhood and early adolescence. New York: Guilford Press, 2003.
- Papolos DF, Papolos J. Bipolar child: The definitive and reassuring guide to childhood’s most misunderstood disorder. New York: Broadway Books, 2002.
- Fristad MA, Goldberg Arnold JS. Raising a moody child: How to cope with depression and bipolar disorder. New York: Guilford Press, 2003.
- Child and Adolescent Bipolar Foundation. Available at www.cabf.org. Accessed March 4, 2005.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Scheffer receives research support from and is a speaker for Abbott Laboratories.
Dr. Apps reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
2. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35(8):997-1008.
3. Patel NC, Sallee FR. What’s the best treatment for ADHD/bipolar mania? Current Psychiatry 2005;3(3):27-37.
4. Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004;61:459-67.
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., rev). Washington, DC: American Psychiatric Association, 2000.
6. Geller B, Williams M, Zimerman B, et al. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord 1998;51(2):81-91.
7. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
1. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
2. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35(8):997-1008.
3. Patel NC, Sallee FR. What’s the best treatment for ADHD/bipolar mania? Current Psychiatry 2005;3(3):27-37.
4. Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry 2004;61:459-67.
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., rev). Washington, DC: American Psychiatric Association, 2000.
6. Geller B, Williams M, Zimerman B, et al. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord 1998;51(2):81-91.
7. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
Are psychostimulants useful in pervasive developmental disorders?
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs
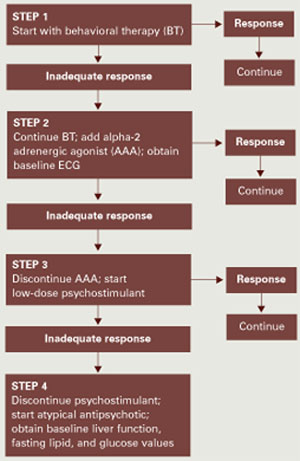
To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs

To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs

To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
5 fundamentals of managing adult ADHD
As a psychiatrist specializing in college health, I see 40 to 50 young adults yearly with undiagnosed attention-deficit/hyperactivity disorder (ADHD). I have found that understanding five fundamentals of ADHD is key to recognizing this disorder in adults.
- There is no “adult onset” ADHD. Although ADHD may manifest itself differently in adults than in children, studies indicate that the disorder is a continuation of childhood ADHD rather than a discrete adult disorder. Clinicians thus need to establish that adult patients exhibited symptomatic and functional impairment before age 7 (as per DSM-IV), although some experts suggest preadolescence as a cutoff.1
- Most people do not “outgrow” ADHD. We once assumed that most patients with ADHD became asymptomatic as they matured from adolescence into adulthood. Research reveals that hyperactivity and impulsivity decline over time but inattention and executive dysfunction usually persist into adulthood.2 These residual deficits cause continued vocational, academic, and interpersonal difficulties.
- ADHD can mimic other psychiatric disorders. The hyperkinesis, impulsivity, and inattention that are the essence of ADHD are also commonly observed in adults with anxiety disorders, mood disorders, substance abuse problems, and learning disorders. Patients who present with atypical affective or anxiety symptoms or learning problems, or who do not respond to conventional treatments, should be screened for ADHD.
- The genetic apple does not fall far from the tree in ADHD. Many adults with ADHD are identified in middle age after their children are diagnosed. Adoption data and multiple twin studies have placed the heritability of ADHD at approximately 75%,3 putting first-degree relatives at fairly high predisposition.
- Stimulant medications do not promote substance abuse in ADHD patients. Stimulant medication is more likely to reduce the risk of substance abuse in ADHD than enhance it.4 For patients at high-risk for substance abuse disorders, however, atomoxetine and bupropion offer nonstimulant alternatives. Also, the newer, longer-acting dextroamphetamine/amphetamine and methylphenidate preparations are more difficult to abuse because of their slow-release mechanisms.
1. Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criteria for attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1997;36:1204-10.
2. Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into adulthood as a function of reporting sources and definition of disorder. J Abnorm Psychol 2002;111:279-89.
3. Sprich S, Biederman J, Crawford MH, et al. Adoptive and biological families of children and adolescents with ADHD. J Am Acad. Child Adolesc Psychiatry 2000;143:1432-7.
4. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
Dr. Anders is clinical assistant professor of psychiatry, University Health Services, University of Wisconsin, Madison.
As a psychiatrist specializing in college health, I see 40 to 50 young adults yearly with undiagnosed attention-deficit/hyperactivity disorder (ADHD). I have found that understanding five fundamentals of ADHD is key to recognizing this disorder in adults.
- There is no “adult onset” ADHD. Although ADHD may manifest itself differently in adults than in children, studies indicate that the disorder is a continuation of childhood ADHD rather than a discrete adult disorder. Clinicians thus need to establish that adult patients exhibited symptomatic and functional impairment before age 7 (as per DSM-IV), although some experts suggest preadolescence as a cutoff.1
- Most people do not “outgrow” ADHD. We once assumed that most patients with ADHD became asymptomatic as they matured from adolescence into adulthood. Research reveals that hyperactivity and impulsivity decline over time but inattention and executive dysfunction usually persist into adulthood.2 These residual deficits cause continued vocational, academic, and interpersonal difficulties.
- ADHD can mimic other psychiatric disorders. The hyperkinesis, impulsivity, and inattention that are the essence of ADHD are also commonly observed in adults with anxiety disorders, mood disorders, substance abuse problems, and learning disorders. Patients who present with atypical affective or anxiety symptoms or learning problems, or who do not respond to conventional treatments, should be screened for ADHD.
- The genetic apple does not fall far from the tree in ADHD. Many adults with ADHD are identified in middle age after their children are diagnosed. Adoption data and multiple twin studies have placed the heritability of ADHD at approximately 75%,3 putting first-degree relatives at fairly high predisposition.
- Stimulant medications do not promote substance abuse in ADHD patients. Stimulant medication is more likely to reduce the risk of substance abuse in ADHD than enhance it.4 For patients at high-risk for substance abuse disorders, however, atomoxetine and bupropion offer nonstimulant alternatives. Also, the newer, longer-acting dextroamphetamine/amphetamine and methylphenidate preparations are more difficult to abuse because of their slow-release mechanisms.
As a psychiatrist specializing in college health, I see 40 to 50 young adults yearly with undiagnosed attention-deficit/hyperactivity disorder (ADHD). I have found that understanding five fundamentals of ADHD is key to recognizing this disorder in adults.
- There is no “adult onset” ADHD. Although ADHD may manifest itself differently in adults than in children, studies indicate that the disorder is a continuation of childhood ADHD rather than a discrete adult disorder. Clinicians thus need to establish that adult patients exhibited symptomatic and functional impairment before age 7 (as per DSM-IV), although some experts suggest preadolescence as a cutoff.1
- Most people do not “outgrow” ADHD. We once assumed that most patients with ADHD became asymptomatic as they matured from adolescence into adulthood. Research reveals that hyperactivity and impulsivity decline over time but inattention and executive dysfunction usually persist into adulthood.2 These residual deficits cause continued vocational, academic, and interpersonal difficulties.
- ADHD can mimic other psychiatric disorders. The hyperkinesis, impulsivity, and inattention that are the essence of ADHD are also commonly observed in adults with anxiety disorders, mood disorders, substance abuse problems, and learning disorders. Patients who present with atypical affective or anxiety symptoms or learning problems, or who do not respond to conventional treatments, should be screened for ADHD.
- The genetic apple does not fall far from the tree in ADHD. Many adults with ADHD are identified in middle age after their children are diagnosed. Adoption data and multiple twin studies have placed the heritability of ADHD at approximately 75%,3 putting first-degree relatives at fairly high predisposition.
- Stimulant medications do not promote substance abuse in ADHD patients. Stimulant medication is more likely to reduce the risk of substance abuse in ADHD than enhance it.4 For patients at high-risk for substance abuse disorders, however, atomoxetine and bupropion offer nonstimulant alternatives. Also, the newer, longer-acting dextroamphetamine/amphetamine and methylphenidate preparations are more difficult to abuse because of their slow-release mechanisms.
1. Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criteria for attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1997;36:1204-10.
2. Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into adulthood as a function of reporting sources and definition of disorder. J Abnorm Psychol 2002;111:279-89.
3. Sprich S, Biederman J, Crawford MH, et al. Adoptive and biological families of children and adolescents with ADHD. J Am Acad. Child Adolesc Psychiatry 2000;143:1432-7.
4. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
Dr. Anders is clinical assistant professor of psychiatry, University Health Services, University of Wisconsin, Madison.
1. Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criteria for attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1997;36:1204-10.
2. Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into adulthood as a function of reporting sources and definition of disorder. J Abnorm Psychol 2002;111:279-89.
3. Sprich S, Biederman J, Crawford MH, et al. Adoptive and biological families of children and adolescents with ADHD. J Am Acad. Child Adolesc Psychiatry 2000;143:1432-7.
4. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
Dr. Anders is clinical assistant professor of psychiatry, University Health Services, University of Wisconsin, Madison.
Atomoxetine: A different approach to ADHD
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
ADHD and substance abuse: 4 therapeutic options for patients with addictions
Should you prescribe a stimulant to treat attention and hyperactivity problems in teenagers and adults with a history of substance abuse? Evidence suggests that using a stimulant to treat attention-deficit/hyperactivity disorder (ADHD) may place such patients at risk for stimulant abuse or for relapse into abuse of other substances. But a stimulant may be the only option for patients whose ADHD symptoms do not respond to alternate medications, such as antidepressants.
Growing numbers of adults are being treated for ADHD. Because substance abuse problems are common in adults with ADHD (Box 1 ),1-3 prescribing an antidepressant instead of a stimulant in some cases may be prudent. Consider the following factors when choosing ADHD therapy for patients with a history of substance abuse.
Prevalence of stimulant use and abuse
In the United States, more than 95% of medications prescribed for children and adults with ADHD are stimulants—usually methylphenidate.4 Stimulant use has increased as more children and adults are diagnosed with ADHD. Methylphenidate prescriptions increased five-fold from 1990 to 1995.5 Visits to psychiatrists and physicians that included stimulant prescriptions grew from 570,000 to 2.86 million from 1985 to 1994, with most of that increase occurring during visits to primary care and other physicians.6
When used as prescribed, methylphenidate is safe and effective for treating most children and adults with ADHD. Methylphenidate’s pharmacologic properties, however, are similar to those of amphetamines and cocaine (Box 2, Figure 1),7,8 which is why methylphenidate is a schedule-II controlled substance.
Published data. Fifteen reports of methylphenidate abuse were published in the medical literature between 1960 and 1999,7 but little is known about the prevalence of stimulant abuse among patients with ADHD. Banov and colleagues recently published what may be the only data available, when they reported that 3 of 37 (8%) patients abused the stimulants they were prescribed for ADHD.9 The three patients who abused stimulants had histories of drug and alcohol abuse at study entry. In all three cases, stimulant abuse did not develop immediately but became apparent within 6 months after the study began.
In a study of 651 students ages 11 to 18 in Wisconsin and Minnesota, more than one-third of those taking stimulants reported being asked to sell or trade their medications. More than one-half of those not taking ADHD medications said they knew someone who sold or gave away his or her medication.10
Stimulant theft, recreational use. Methylphenidate has been identified as the third most abused prescribed substance in the United States.11 It was the 10th most frequently stolen controlled drug from pharmacies between 1990 and 1995, and 700,000 dosage units were reported stolen in 1996 and 1997.12
As many as 50% of adults with ADHD have substance abuse problems (including alcohol, cocaine, and marijuana), and as many as 30% have antisocial personality disorder (with increased potential for drug-seeking behaviors).1 Compared with the general population, persons with ADHD have an earlier onset of substance abuse that is less responsive to treatment and more likely to progress from alcohol to other drugs.2
The elevated risk of substance abuse in ADHD may be related to a subtle lack of response to normal positive and negative reinforcements. Hunt has outlined four neurobehavioral deficits that define ADHD.3 Besides inattention, hyperarousal, and impulsiveness, he proposes that persons with ADHD have a reward system deficit. They may gravitate toward substance abuse because drugs, alcohol, and nicotine provide stronger rewards than life’s more subtle social interactions.
The popular media have reported recreational use of methylphenidate—with street names such as “R-Ball” and “Vitamin R”—among teens and college students.13 Illegal stimulants are perceived to be easily accessible on college campuses, but no data have been reported.
The use of stimulant medication for ADHD patients with substance abuse problems remains controversial. For such patients, this author reserves stimulant medication for those:
- whose ADHD symptoms have not responded adequately to alternate treatments
- who have been reliable with prescription medications
- and whose functional level is seriously impaired by their ADHD.
Antidepressants vs. stimulants
Although few well-designed controlled studies have been published, four antidepressants appear to be reasonably equivalent in effectiveness for adults with ADHD and do not carry potential for stimulant abuse.14
Desipraime, bupropion, venlafaxine, and the experimental drug atomoxetine (Table 1) all increase norepinephrine at the synapse by inhibiting presynaptic reuptake. Though dopamine has traditionally been considered the neurotransmitter of choice for ADHD treatment, norepinephrine may be equally potent.
Impulse control center. Several lines of research have recently established a connection between the prefrontal cortex, norepinephrine, and ADHD.15 This evidence suggests that the prefrontal cortex plays a major role in inhibiting impulses and responses to distractions:
Figure 1
PET scans of the brain using carbon 11 (11C)-labeled cocaine and methylphenidate HCl show similar distributions in the striatium when the drugs are administered intravenously.
Source: Reproduced with permission from Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
Stimulants are classified as schedule-II drugs because they produce powerful reinforcing effects by increasing synaptic dopamine.7 Positron-emission tomography (PET) scans using carbon labeling have shown similar distributions of methylphenidate and cocaine in the brain (Figure 1).8 When administered intravenously, both drugs occupy the same receptors in the striatum and produce a “high” that parallels rapid neuronal uptake. Methylphenidate and cocaine similarly increase stimulation of the postsynaptic neuron by blocking the dopamine reuptake pump (dopamine transporter).
How a substance gets to the brain’s euphoric receptors greatly affects its addictive properties. Delivery systems with rapid onset—smoking, “snorting,” or IV injection—have much greater ability to produce a “high” than do oral or transdermal routes. The greater the “high,” the greater the potential for abuse.
Because methylphenidate is prescribed for oral use, the potential for abuse is minimal. However, we need to be extremely cautious when giving methylphenidate or similar stimulant medications to patients who have shown they are unable to control their abuse of other substances.
Stimulants can also re-ignite a dormant substance abuse problem. Though little has been written about this in the medical literature, Elizabeth Wurtzel, author of the controversial Prozac Nation, chronicles the resumption of her cocaine abuse in More, Now, Again: A Memoir of Addiction. She contends that after her doctor added methylphenidate to augment treatment of partially remitting depression, she began abusing it and eventually was using 40 tablets per day before slipping back into cocaine dependence.
- Patients with prefrontal cortex deficits can have problems with inattention and poor impulse control.
- Patients with ADHD have frontal lobe impairments, as neuropsychological testing and imaging studies have shown.
- Norepinephrine neurons, with cell bodies in the locus coeruleus, have projections that terminate in the prefrontal cortex.
- Agents with norepinephrine activity, but without mood-altering properties (e.g., clonidine), have been shown to improve ADHD symptoms.
Table 1
ADHD IN ADULTS: ANTIDEPRESSANT DOSAGES AND SIDE EFFECTS
| Medication | Class | Effective dosage | Side effects |
|---|---|---|---|
| Desipramine | Tricyclic | 100 to 200 mg/d | Sedation, weight gain, dry mouth, constipation, orthostatic hypotension, prolonged cardiac conduction time; may be lethal in overdose |
| Bupropion SR | Norepinephrine and dopamine reuptake inhibitor | 150 mg bid to 200 mg bid | Headaches, insomnia, agitation, increased risk of seizures |
| Venlafaxine XR | Serotonin and norepinephrine reuptake inhibitor | 75 to 225 mg/d | Nausea, sexual side effects, agitation, increased blood pressure at higher dosages |
| Atomoxetine* | Norepinephrine reuptake inhibitor | To be determined | To be determined |
| * Investigational agent; not FDA-approved | |||
Additional evidence suggests that the prefrontal cortex has projections back to the locus coeruleus, which may explain the relationship between the two areas. It may be that the brain’s higher-functioning areas, such as the prefrontal cortex, provide intelligent screening of impulses from the brain’s older areas, such as the locus coeruleus. Therefore, increased prefrontal cortex activity may modulate some impulses that ADHD patients cannot control otherwise.

No ‘high’ with antidepressants. Patients with ADHD who have experienced the powerful effects of street drugs such as cocaine, methamphetamine, or even alcohol may report that antidepressants do not provide the effect they desire. It is difficult to know if these patients are reporting a lack of benefit or simply the absence of a euphoric “high” they are used to experiencing with substances of abuse. The newer antidepressants do not activate the brain’s euphoric receptors to an appreciable degree.
Patients who take stimulants as prescribed also do not report a “high” but can detect the medication’s presence and absence. Most do not crave this feeling, but substance abusers tend to like it. A patient recently told me he didn’t think stimulants improved his ADHD, but said, “I just liked the way they made me feel.”
Desipramine
The tricyclic antidepressant desipramine is a potent norepinephrine reuptake inhibitor that is effective in treating ADHD in children and adults. In a double-blind, placebo-controlled study of adults with ADHD, subjects receiving desipramine showed robust improvement in symptom scores on the ADHD Rating Scale,16 compared with those receiving the placebo (Figure 2).17
During the 6-week trial, 41 adults with ADHD received desipramine, 200 mg/d, or a placebo. Those receiving desipramine showed significant improvement in 12 of 14 ADHD symptoms and less hyperactivity, impulsivity, and inattentiveness, whereas those receiving the placebo showed no improvement. According to the study criteria, 68% of those who received desipramine and none who received the placebo were considered positive responders.
Though no head-to-head studies have compared desipramine with methylphenidate, the same researchers conducted a similar placebo-controlled study with methylphenidate. The 6-week symptom score on the ADHD Rating Scale was 12.5 for methylphenidate, compared with a score of 12 for desipramine.18
Recommendation. Desipramine may be the most effective of the antidepressant treatments for patients with ADHD. Because of its side effects, however, it is not this author’s first choice and is usually reserved for patients whose symptoms fail to respond to other antidepressants. Desipramine can cause sedation, dry mouth, and constipation, which are related to blockade of adrenergic, histamine, and muscarinic cholinergic receptors. It also can be lethal in overdose.
Some substance abusers lose confidence in a medication that they cannot feel working. The side effects of desipramine, which can be intolerable for some patients, can reassure others with a history of substance abuse that they are being medicated.
Bupropion
Bupropion is a unique antidepressant that inhibits the presynaptic reuptake of dopamine and norepinephrine. Open-label studies demonstrate good responses to bupropion by adults with ADHD. One placebo-controlled, double-blind study found improved ADHD symptoms in 76% of patients receiving bupropion SR, compared with 37% of those receiving a placebo; the difference was statistically significant.19
Figure 2 IMPROVED ADHD SYMPTOMS WITH DESIPRAMINE
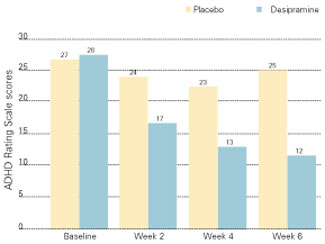
Adults with ADHD who received desipramine, 200 mg/d, in a double-blind trial showed significantly less hyperactivity, impulsivity, and inattentiveness after 6 weeks of therapy than a control group that received a placebo.
Source: Adapted with permission from Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.Another study of adults with ADHD compared bupropion SR to methylphenidate and a placebo.20 Using a primary outcome of the Clinical Global Impression (CGI) scale, response rates were 64% for bupropion SR, 50% for methylphenidate, and 27% for the placebo. The difference in response rates between the two agents was not statistically significant (p = 0.14).
Recommendation. The risk of seizures with bupropion is about 1 in 1,000. Therefore, bupropion should not be given to patients with a seizure disorder or to those with conditions that alter the seizure threshold (e.g., eating disorders, recent head trauma, or benzodiazepine withdrawal).21
This author uses bupropion as first-line treatment for appropriate patients with ADHD and a substance abuse history. Bupropion’s mild benefit with smoking cessation may provide some crossover effect for other substances of abuse. The low incidence of sexual side effects is another benefit. Drawbacks include twice-daily dosing and lack of a robust effect on attention and concentration.
Venlafaxine
Venlafaxine is a potent inhibitor of serotonin reuptake, a moderate inhibitor of norepinephrine, and a mild inhibitor of dopamine. Venlafaxine has displayed response rates similar to those of desipramine and bupropion in open-label studies in adults with ADHD,14 but no placebo-controlled studies exist.
As noted above, these antidepressants are believed to improve ADHD symptoms by making norepinephrine more available at the synapse. Hypothetically, then, one would need to administer venlafaxine at dosages that adequately inhibit the norepinephrine reuptake receptor. Venlafaxine XR, 150 mg/d, provides significant norepinephrine activity, according to several lines of evidence.
Recently, Upadhyaya et al reported the use of venlafaxine in one of the few treatment studies of patients with ADHD and comorbid alcohol/cocaine abuse. In an open-label trial, 10 subjects received venlafaxine, up to 300 mg/d, along with psychotherapy and attendance at Alcoholics Anonymous meetings. The nine who completed 4 weeks of treatment showed significantly improved ADHD symptoms and decreased alcohol craving.22
Recommendation. Venlafaxine is this author’s second choice for patients with ADHD and substance abuse problems. Sexual side effects that some patients experience with venlafaxine can limit its use. Some clinicians are concerned about increases in blood pressure associated with venlafaxine, although significant changes do not seem to occur at dosages below 300 mg/d.23
Atomoxetine
Atomoxetine is an investigational antidepressant in phase-III trials as a treatment for ADHD. Evidence shows atomoxetine to be a potent inhibitor of the presynaptic norepinephrine transporter, with minimal affinity for other neurotransmitter receptors. Initial studies suggest that atomoxetine is effective for adults and children with ADHD:
- In a small, double-blind, placebo-controlled, crossover trial, 11 of 20 adults showed improvement in ADHD symptoms within 3 weeks of starting atomoxetine.24
- In 297 children and adolescents, atomoxetine at dosages averaging approximately 1.2 mg/kg/d was more effective than a placebo in reducing ADHD symptoms and improving social and family functioning. Treatment was well tolerated and without significant side effects.25
- In a randomized open-label trial, 228 children received atomoxetine or methylphenidate. Both treatments significantly reduced inattention and hyperactive/impulsive symptoms.26
Related resources
- Arnsten AF. Genetics of childhood disorders (XVIII). ADHD, Part 2: Norepinephrine has a critical modulatory influence on prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 2000;39:1201-3.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention-deficit/hyperactivity disorder. Am J Psychiatry. 1993;150:885-90.
- Research Report: Prescription Drugs—abuse and addiction. National Institute on Drug Abuse, Substance Abuse and Mental Health Services Administration.
Drug brand names
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Ritalin, Concerta
- Venlafaxine • Effexor
Disclosure
Dr. Higgins reports that he is on the speakers’ bureaus for Wyeth-Ayerst Pharmaceuticals and Eli Lilly and Co.
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993;50:565-76.
2. Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann NY Acad Sci 2001;931:251-70.
3. Hunt RD. Nosology, neurobiology, and clinical patterns of ADHD in adults. Psychiatric Ann 1997;27:572-81.
4. Taylor MA. Evaluation and management of attention-deficit hyperactivity disorder. Am Fam Phys 1997;55:887-904.
5. Diller LH. The run on Ritalin. Attention deficit disorder and stimulant treatment in the 1990s. Hasting Center Report 1996;26:12-18.
6. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotrophic medications: primary care, psychiatry, and other medical specialties. JAMA 1998;279:526-31.
7. Mortan WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. J Clin Psychiatry (Primary Care) 2000;2:159-64.
8. Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
9. Banov MD, Palmer T, Brody E. Antidepressants are as effective as stimulants in the long-term treatment of ADHD in Adults. Primary Psychiatry 2001;8:54-7.
10. Moline S, Frankenberger W. Use of stimulant medication for treatment of attention-deficit/hyperactivity disorder. A survey of middle and high school students attitudes. Psychology in the Schools 2001;38:569-84.
11. Prescription drugs: abuse and addiction. National Institute on Drug Abuse Research Report Series. NIH publication number 01-4881, July 2001.
12. Mann A. Illicit methylphenidate trade appears widespread. Clinical Psychiatry News June 2000;28(6):5-
13. ABCNews.com. http://more/abcnews.go.com/sections/living/DailyNews/ritalin0505.html
14. Higgins ES. A comparative analysis of antidepressants and stimulants for the treatment of adults with attention-deficit hyperactivity disorder. J Fam Pract 1999;48:15-20.
15. Arnsten AF, Steere JC, Hunt RD. The contribution of alpha2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry 1996;53:448-55.
16. DuPaul G. The ADHD Rating Scale: normative data, reliability, and validity. Worcester, MA: University of Massachusetts Medical School, 1990.
17. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
18. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
19. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
20. Kuperman S, Perry PJ, Gaffney GR, et al. Bupropion SR vs. methylphenidate vs. placebo for attention-deficit/hyperactivity disorder in adults. Ann Clin Psychiatry 2001;13:129-34.
21. Wooltorton E. Bupropion (Zyban, Wellbutrin SR): reports of death, seizures, serum sickness. Can Med J 2002;166:68.-
22. Upadhyaya HP, Brady KT, Sethuraman G, et al. Venlafaxine treatment of patients with comorbid alcohol/cocaine abuse and attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol 2001;21:116-8.
23. Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry 1998;59:502-8.
24. Spencer T, Biederman J, Wilens T, Prince J, Hatch M. Effectiveness and tolerability of tomoxetine in adults with attention-deficit/hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
25. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108:E83.-
26. Kratochvil C, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in ADHD children: a randomized, open-label trial (presentation). Honolulu: American Academy of Child and Adolescent Psychiatry, October, 2001.
Should you prescribe a stimulant to treat attention and hyperactivity problems in teenagers and adults with a history of substance abuse? Evidence suggests that using a stimulant to treat attention-deficit/hyperactivity disorder (ADHD) may place such patients at risk for stimulant abuse or for relapse into abuse of other substances. But a stimulant may be the only option for patients whose ADHD symptoms do not respond to alternate medications, such as antidepressants.
Growing numbers of adults are being treated for ADHD. Because substance abuse problems are common in adults with ADHD (Box 1 ),1-3 prescribing an antidepressant instead of a stimulant in some cases may be prudent. Consider the following factors when choosing ADHD therapy for patients with a history of substance abuse.
Prevalence of stimulant use and abuse
In the United States, more than 95% of medications prescribed for children and adults with ADHD are stimulants—usually methylphenidate.4 Stimulant use has increased as more children and adults are diagnosed with ADHD. Methylphenidate prescriptions increased five-fold from 1990 to 1995.5 Visits to psychiatrists and physicians that included stimulant prescriptions grew from 570,000 to 2.86 million from 1985 to 1994, with most of that increase occurring during visits to primary care and other physicians.6
When used as prescribed, methylphenidate is safe and effective for treating most children and adults with ADHD. Methylphenidate’s pharmacologic properties, however, are similar to those of amphetamines and cocaine (Box 2, Figure 1),7,8 which is why methylphenidate is a schedule-II controlled substance.
Published data. Fifteen reports of methylphenidate abuse were published in the medical literature between 1960 and 1999,7 but little is known about the prevalence of stimulant abuse among patients with ADHD. Banov and colleagues recently published what may be the only data available, when they reported that 3 of 37 (8%) patients abused the stimulants they were prescribed for ADHD.9 The three patients who abused stimulants had histories of drug and alcohol abuse at study entry. In all three cases, stimulant abuse did not develop immediately but became apparent within 6 months after the study began.
In a study of 651 students ages 11 to 18 in Wisconsin and Minnesota, more than one-third of those taking stimulants reported being asked to sell or trade their medications. More than one-half of those not taking ADHD medications said they knew someone who sold or gave away his or her medication.10
Stimulant theft, recreational use. Methylphenidate has been identified as the third most abused prescribed substance in the United States.11 It was the 10th most frequently stolen controlled drug from pharmacies between 1990 and 1995, and 700,000 dosage units were reported stolen in 1996 and 1997.12
As many as 50% of adults with ADHD have substance abuse problems (including alcohol, cocaine, and marijuana), and as many as 30% have antisocial personality disorder (with increased potential for drug-seeking behaviors).1 Compared with the general population, persons with ADHD have an earlier onset of substance abuse that is less responsive to treatment and more likely to progress from alcohol to other drugs.2
The elevated risk of substance abuse in ADHD may be related to a subtle lack of response to normal positive and negative reinforcements. Hunt has outlined four neurobehavioral deficits that define ADHD.3 Besides inattention, hyperarousal, and impulsiveness, he proposes that persons with ADHD have a reward system deficit. They may gravitate toward substance abuse because drugs, alcohol, and nicotine provide stronger rewards than life’s more subtle social interactions.
The popular media have reported recreational use of methylphenidate—with street names such as “R-Ball” and “Vitamin R”—among teens and college students.13 Illegal stimulants are perceived to be easily accessible on college campuses, but no data have been reported.
The use of stimulant medication for ADHD patients with substance abuse problems remains controversial. For such patients, this author reserves stimulant medication for those:
- whose ADHD symptoms have not responded adequately to alternate treatments
- who have been reliable with prescription medications
- and whose functional level is seriously impaired by their ADHD.
Antidepressants vs. stimulants
Although few well-designed controlled studies have been published, four antidepressants appear to be reasonably equivalent in effectiveness for adults with ADHD and do not carry potential for stimulant abuse.14
Desipraime, bupropion, venlafaxine, and the experimental drug atomoxetine (Table 1) all increase norepinephrine at the synapse by inhibiting presynaptic reuptake. Though dopamine has traditionally been considered the neurotransmitter of choice for ADHD treatment, norepinephrine may be equally potent.
Impulse control center. Several lines of research have recently established a connection between the prefrontal cortex, norepinephrine, and ADHD.15 This evidence suggests that the prefrontal cortex plays a major role in inhibiting impulses and responses to distractions:
Figure 1

PET scans of the brain using carbon 11 (11C)-labeled cocaine and methylphenidate HCl show similar distributions in the striatium when the drugs are administered intravenously.
Source: Reproduced with permission from Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
Stimulants are classified as schedule-II drugs because they produce powerful reinforcing effects by increasing synaptic dopamine.7 Positron-emission tomography (PET) scans using carbon labeling have shown similar distributions of methylphenidate and cocaine in the brain (Figure 1).8 When administered intravenously, both drugs occupy the same receptors in the striatum and produce a “high” that parallels rapid neuronal uptake. Methylphenidate and cocaine similarly increase stimulation of the postsynaptic neuron by blocking the dopamine reuptake pump (dopamine transporter).
How a substance gets to the brain’s euphoric receptors greatly affects its addictive properties. Delivery systems with rapid onset—smoking, “snorting,” or IV injection—have much greater ability to produce a “high” than do oral or transdermal routes. The greater the “high,” the greater the potential for abuse.
Because methylphenidate is prescribed for oral use, the potential for abuse is minimal. However, we need to be extremely cautious when giving methylphenidate or similar stimulant medications to patients who have shown they are unable to control their abuse of other substances.
Stimulants can also re-ignite a dormant substance abuse problem. Though little has been written about this in the medical literature, Elizabeth Wurtzel, author of the controversial Prozac Nation, chronicles the resumption of her cocaine abuse in More, Now, Again: A Memoir of Addiction. She contends that after her doctor added methylphenidate to augment treatment of partially remitting depression, she began abusing it and eventually was using 40 tablets per day before slipping back into cocaine dependence.
- Patients with prefrontal cortex deficits can have problems with inattention and poor impulse control.
- Patients with ADHD have frontal lobe impairments, as neuropsychological testing and imaging studies have shown.
- Norepinephrine neurons, with cell bodies in the locus coeruleus, have projections that terminate in the prefrontal cortex.
- Agents with norepinephrine activity, but without mood-altering properties (e.g., clonidine), have been shown to improve ADHD symptoms.
Table 1
ADHD IN ADULTS: ANTIDEPRESSANT DOSAGES AND SIDE EFFECTS
| Medication | Class | Effective dosage | Side effects |
|---|---|---|---|
| Desipramine | Tricyclic | 100 to 200 mg/d | Sedation, weight gain, dry mouth, constipation, orthostatic hypotension, prolonged cardiac conduction time; may be lethal in overdose |
| Bupropion SR | Norepinephrine and dopamine reuptake inhibitor | 150 mg bid to 200 mg bid | Headaches, insomnia, agitation, increased risk of seizures |
| Venlafaxine XR | Serotonin and norepinephrine reuptake inhibitor | 75 to 225 mg/d | Nausea, sexual side effects, agitation, increased blood pressure at higher dosages |
| Atomoxetine* | Norepinephrine reuptake inhibitor | To be determined | To be determined |
| * Investigational agent; not FDA-approved | |||
Additional evidence suggests that the prefrontal cortex has projections back to the locus coeruleus, which may explain the relationship between the two areas. It may be that the brain’s higher-functioning areas, such as the prefrontal cortex, provide intelligent screening of impulses from the brain’s older areas, such as the locus coeruleus. Therefore, increased prefrontal cortex activity may modulate some impulses that ADHD patients cannot control otherwise.

No ‘high’ with antidepressants. Patients with ADHD who have experienced the powerful effects of street drugs such as cocaine, methamphetamine, or even alcohol may report that antidepressants do not provide the effect they desire. It is difficult to know if these patients are reporting a lack of benefit or simply the absence of a euphoric “high” they are used to experiencing with substances of abuse. The newer antidepressants do not activate the brain’s euphoric receptors to an appreciable degree.
Patients who take stimulants as prescribed also do not report a “high” but can detect the medication’s presence and absence. Most do not crave this feeling, but substance abusers tend to like it. A patient recently told me he didn’t think stimulants improved his ADHD, but said, “I just liked the way they made me feel.”
Desipramine
The tricyclic antidepressant desipramine is a potent norepinephrine reuptake inhibitor that is effective in treating ADHD in children and adults. In a double-blind, placebo-controlled study of adults with ADHD, subjects receiving desipramine showed robust improvement in symptom scores on the ADHD Rating Scale,16 compared with those receiving the placebo (Figure 2).17
During the 6-week trial, 41 adults with ADHD received desipramine, 200 mg/d, or a placebo. Those receiving desipramine showed significant improvement in 12 of 14 ADHD symptoms and less hyperactivity, impulsivity, and inattentiveness, whereas those receiving the placebo showed no improvement. According to the study criteria, 68% of those who received desipramine and none who received the placebo were considered positive responders.
Though no head-to-head studies have compared desipramine with methylphenidate, the same researchers conducted a similar placebo-controlled study with methylphenidate. The 6-week symptom score on the ADHD Rating Scale was 12.5 for methylphenidate, compared with a score of 12 for desipramine.18
Recommendation. Desipramine may be the most effective of the antidepressant treatments for patients with ADHD. Because of its side effects, however, it is not this author’s first choice and is usually reserved for patients whose symptoms fail to respond to other antidepressants. Desipramine can cause sedation, dry mouth, and constipation, which are related to blockade of adrenergic, histamine, and muscarinic cholinergic receptors. It also can be lethal in overdose.
Some substance abusers lose confidence in a medication that they cannot feel working. The side effects of desipramine, which can be intolerable for some patients, can reassure others with a history of substance abuse that they are being medicated.
Bupropion
Bupropion is a unique antidepressant that inhibits the presynaptic reuptake of dopamine and norepinephrine. Open-label studies demonstrate good responses to bupropion by adults with ADHD. One placebo-controlled, double-blind study found improved ADHD symptoms in 76% of patients receiving bupropion SR, compared with 37% of those receiving a placebo; the difference was statistically significant.19
Figure 2 IMPROVED ADHD SYMPTOMS WITH DESIPRAMINE

Adults with ADHD who received desipramine, 200 mg/d, in a double-blind trial showed significantly less hyperactivity, impulsivity, and inattentiveness after 6 weeks of therapy than a control group that received a placebo.
Source: Adapted with permission from Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.Another study of adults with ADHD compared bupropion SR to methylphenidate and a placebo.20 Using a primary outcome of the Clinical Global Impression (CGI) scale, response rates were 64% for bupropion SR, 50% for methylphenidate, and 27% for the placebo. The difference in response rates between the two agents was not statistically significant (p = 0.14).
Recommendation. The risk of seizures with bupropion is about 1 in 1,000. Therefore, bupropion should not be given to patients with a seizure disorder or to those with conditions that alter the seizure threshold (e.g., eating disorders, recent head trauma, or benzodiazepine withdrawal).21
This author uses bupropion as first-line treatment for appropriate patients with ADHD and a substance abuse history. Bupropion’s mild benefit with smoking cessation may provide some crossover effect for other substances of abuse. The low incidence of sexual side effects is another benefit. Drawbacks include twice-daily dosing and lack of a robust effect on attention and concentration.
Venlafaxine
Venlafaxine is a potent inhibitor of serotonin reuptake, a moderate inhibitor of norepinephrine, and a mild inhibitor of dopamine. Venlafaxine has displayed response rates similar to those of desipramine and bupropion in open-label studies in adults with ADHD,14 but no placebo-controlled studies exist.
As noted above, these antidepressants are believed to improve ADHD symptoms by making norepinephrine more available at the synapse. Hypothetically, then, one would need to administer venlafaxine at dosages that adequately inhibit the norepinephrine reuptake receptor. Venlafaxine XR, 150 mg/d, provides significant norepinephrine activity, according to several lines of evidence.
Recently, Upadhyaya et al reported the use of venlafaxine in one of the few treatment studies of patients with ADHD and comorbid alcohol/cocaine abuse. In an open-label trial, 10 subjects received venlafaxine, up to 300 mg/d, along with psychotherapy and attendance at Alcoholics Anonymous meetings. The nine who completed 4 weeks of treatment showed significantly improved ADHD symptoms and decreased alcohol craving.22
Recommendation. Venlafaxine is this author’s second choice for patients with ADHD and substance abuse problems. Sexual side effects that some patients experience with venlafaxine can limit its use. Some clinicians are concerned about increases in blood pressure associated with venlafaxine, although significant changes do not seem to occur at dosages below 300 mg/d.23
Atomoxetine
Atomoxetine is an investigational antidepressant in phase-III trials as a treatment for ADHD. Evidence shows atomoxetine to be a potent inhibitor of the presynaptic norepinephrine transporter, with minimal affinity for other neurotransmitter receptors. Initial studies suggest that atomoxetine is effective for adults and children with ADHD:
- In a small, double-blind, placebo-controlled, crossover trial, 11 of 20 adults showed improvement in ADHD symptoms within 3 weeks of starting atomoxetine.24
- In 297 children and adolescents, atomoxetine at dosages averaging approximately 1.2 mg/kg/d was more effective than a placebo in reducing ADHD symptoms and improving social and family functioning. Treatment was well tolerated and without significant side effects.25
- In a randomized open-label trial, 228 children received atomoxetine or methylphenidate. Both treatments significantly reduced inattention and hyperactive/impulsive symptoms.26
Related resources
- Arnsten AF. Genetics of childhood disorders (XVIII). ADHD, Part 2: Norepinephrine has a critical modulatory influence on prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 2000;39:1201-3.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention-deficit/hyperactivity disorder. Am J Psychiatry. 1993;150:885-90.
- Research Report: Prescription Drugs—abuse and addiction. National Institute on Drug Abuse, Substance Abuse and Mental Health Services Administration.
Drug brand names
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Ritalin, Concerta
- Venlafaxine • Effexor
Disclosure
Dr. Higgins reports that he is on the speakers’ bureaus for Wyeth-Ayerst Pharmaceuticals and Eli Lilly and Co.
Should you prescribe a stimulant to treat attention and hyperactivity problems in teenagers and adults with a history of substance abuse? Evidence suggests that using a stimulant to treat attention-deficit/hyperactivity disorder (ADHD) may place such patients at risk for stimulant abuse or for relapse into abuse of other substances. But a stimulant may be the only option for patients whose ADHD symptoms do not respond to alternate medications, such as antidepressants.
Growing numbers of adults are being treated for ADHD. Because substance abuse problems are common in adults with ADHD (Box 1 ),1-3 prescribing an antidepressant instead of a stimulant in some cases may be prudent. Consider the following factors when choosing ADHD therapy for patients with a history of substance abuse.
Prevalence of stimulant use and abuse
In the United States, more than 95% of medications prescribed for children and adults with ADHD are stimulants—usually methylphenidate.4 Stimulant use has increased as more children and adults are diagnosed with ADHD. Methylphenidate prescriptions increased five-fold from 1990 to 1995.5 Visits to psychiatrists and physicians that included stimulant prescriptions grew from 570,000 to 2.86 million from 1985 to 1994, with most of that increase occurring during visits to primary care and other physicians.6
When used as prescribed, methylphenidate is safe and effective for treating most children and adults with ADHD. Methylphenidate’s pharmacologic properties, however, are similar to those of amphetamines and cocaine (Box 2, Figure 1),7,8 which is why methylphenidate is a schedule-II controlled substance.
Published data. Fifteen reports of methylphenidate abuse were published in the medical literature between 1960 and 1999,7 but little is known about the prevalence of stimulant abuse among patients with ADHD. Banov and colleagues recently published what may be the only data available, when they reported that 3 of 37 (8%) patients abused the stimulants they were prescribed for ADHD.9 The three patients who abused stimulants had histories of drug and alcohol abuse at study entry. In all three cases, stimulant abuse did not develop immediately but became apparent within 6 months after the study began.
In a study of 651 students ages 11 to 18 in Wisconsin and Minnesota, more than one-third of those taking stimulants reported being asked to sell or trade their medications. More than one-half of those not taking ADHD medications said they knew someone who sold or gave away his or her medication.10
Stimulant theft, recreational use. Methylphenidate has been identified as the third most abused prescribed substance in the United States.11 It was the 10th most frequently stolen controlled drug from pharmacies between 1990 and 1995, and 700,000 dosage units were reported stolen in 1996 and 1997.12
As many as 50% of adults with ADHD have substance abuse problems (including alcohol, cocaine, and marijuana), and as many as 30% have antisocial personality disorder (with increased potential for drug-seeking behaviors).1 Compared with the general population, persons with ADHD have an earlier onset of substance abuse that is less responsive to treatment and more likely to progress from alcohol to other drugs.2
The elevated risk of substance abuse in ADHD may be related to a subtle lack of response to normal positive and negative reinforcements. Hunt has outlined four neurobehavioral deficits that define ADHD.3 Besides inattention, hyperarousal, and impulsiveness, he proposes that persons with ADHD have a reward system deficit. They may gravitate toward substance abuse because drugs, alcohol, and nicotine provide stronger rewards than life’s more subtle social interactions.
The popular media have reported recreational use of methylphenidate—with street names such as “R-Ball” and “Vitamin R”—among teens and college students.13 Illegal stimulants are perceived to be easily accessible on college campuses, but no data have been reported.
The use of stimulant medication for ADHD patients with substance abuse problems remains controversial. For such patients, this author reserves stimulant medication for those:
- whose ADHD symptoms have not responded adequately to alternate treatments
- who have been reliable with prescription medications
- and whose functional level is seriously impaired by their ADHD.
Antidepressants vs. stimulants
Although few well-designed controlled studies have been published, four antidepressants appear to be reasonably equivalent in effectiveness for adults with ADHD and do not carry potential for stimulant abuse.14
Desipraime, bupropion, venlafaxine, and the experimental drug atomoxetine (Table 1) all increase norepinephrine at the synapse by inhibiting presynaptic reuptake. Though dopamine has traditionally been considered the neurotransmitter of choice for ADHD treatment, norepinephrine may be equally potent.
Impulse control center. Several lines of research have recently established a connection between the prefrontal cortex, norepinephrine, and ADHD.15 This evidence suggests that the prefrontal cortex plays a major role in inhibiting impulses and responses to distractions:
Figure 1

PET scans of the brain using carbon 11 (11C)-labeled cocaine and methylphenidate HCl show similar distributions in the striatium when the drugs are administered intravenously.
Source: Reproduced with permission from Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
Stimulants are classified as schedule-II drugs because they produce powerful reinforcing effects by increasing synaptic dopamine.7 Positron-emission tomography (PET) scans using carbon labeling have shown similar distributions of methylphenidate and cocaine in the brain (Figure 1).8 When administered intravenously, both drugs occupy the same receptors in the striatum and produce a “high” that parallels rapid neuronal uptake. Methylphenidate and cocaine similarly increase stimulation of the postsynaptic neuron by blocking the dopamine reuptake pump (dopamine transporter).
How a substance gets to the brain’s euphoric receptors greatly affects its addictive properties. Delivery systems with rapid onset—smoking, “snorting,” or IV injection—have much greater ability to produce a “high” than do oral or transdermal routes. The greater the “high,” the greater the potential for abuse.
Because methylphenidate is prescribed for oral use, the potential for abuse is minimal. However, we need to be extremely cautious when giving methylphenidate or similar stimulant medications to patients who have shown they are unable to control their abuse of other substances.
Stimulants can also re-ignite a dormant substance abuse problem. Though little has been written about this in the medical literature, Elizabeth Wurtzel, author of the controversial Prozac Nation, chronicles the resumption of her cocaine abuse in More, Now, Again: A Memoir of Addiction. She contends that after her doctor added methylphenidate to augment treatment of partially remitting depression, she began abusing it and eventually was using 40 tablets per day before slipping back into cocaine dependence.
- Patients with prefrontal cortex deficits can have problems with inattention and poor impulse control.
- Patients with ADHD have frontal lobe impairments, as neuropsychological testing and imaging studies have shown.
- Norepinephrine neurons, with cell bodies in the locus coeruleus, have projections that terminate in the prefrontal cortex.
- Agents with norepinephrine activity, but without mood-altering properties (e.g., clonidine), have been shown to improve ADHD symptoms.
Table 1
ADHD IN ADULTS: ANTIDEPRESSANT DOSAGES AND SIDE EFFECTS
| Medication | Class | Effective dosage | Side effects |
|---|---|---|---|
| Desipramine | Tricyclic | 100 to 200 mg/d | Sedation, weight gain, dry mouth, constipation, orthostatic hypotension, prolonged cardiac conduction time; may be lethal in overdose |
| Bupropion SR | Norepinephrine and dopamine reuptake inhibitor | 150 mg bid to 200 mg bid | Headaches, insomnia, agitation, increased risk of seizures |
| Venlafaxine XR | Serotonin and norepinephrine reuptake inhibitor | 75 to 225 mg/d | Nausea, sexual side effects, agitation, increased blood pressure at higher dosages |
| Atomoxetine* | Norepinephrine reuptake inhibitor | To be determined | To be determined |
| * Investigational agent; not FDA-approved | |||
Additional evidence suggests that the prefrontal cortex has projections back to the locus coeruleus, which may explain the relationship between the two areas. It may be that the brain’s higher-functioning areas, such as the prefrontal cortex, provide intelligent screening of impulses from the brain’s older areas, such as the locus coeruleus. Therefore, increased prefrontal cortex activity may modulate some impulses that ADHD patients cannot control otherwise.

No ‘high’ with antidepressants. Patients with ADHD who have experienced the powerful effects of street drugs such as cocaine, methamphetamine, or even alcohol may report that antidepressants do not provide the effect they desire. It is difficult to know if these patients are reporting a lack of benefit or simply the absence of a euphoric “high” they are used to experiencing with substances of abuse. The newer antidepressants do not activate the brain’s euphoric receptors to an appreciable degree.
Patients who take stimulants as prescribed also do not report a “high” but can detect the medication’s presence and absence. Most do not crave this feeling, but substance abusers tend to like it. A patient recently told me he didn’t think stimulants improved his ADHD, but said, “I just liked the way they made me feel.”
Desipramine
The tricyclic antidepressant desipramine is a potent norepinephrine reuptake inhibitor that is effective in treating ADHD in children and adults. In a double-blind, placebo-controlled study of adults with ADHD, subjects receiving desipramine showed robust improvement in symptom scores on the ADHD Rating Scale,16 compared with those receiving the placebo (Figure 2).17
During the 6-week trial, 41 adults with ADHD received desipramine, 200 mg/d, or a placebo. Those receiving desipramine showed significant improvement in 12 of 14 ADHD symptoms and less hyperactivity, impulsivity, and inattentiveness, whereas those receiving the placebo showed no improvement. According to the study criteria, 68% of those who received desipramine and none who received the placebo were considered positive responders.
Though no head-to-head studies have compared desipramine with methylphenidate, the same researchers conducted a similar placebo-controlled study with methylphenidate. The 6-week symptom score on the ADHD Rating Scale was 12.5 for methylphenidate, compared with a score of 12 for desipramine.18
Recommendation. Desipramine may be the most effective of the antidepressant treatments for patients with ADHD. Because of its side effects, however, it is not this author’s first choice and is usually reserved for patients whose symptoms fail to respond to other antidepressants. Desipramine can cause sedation, dry mouth, and constipation, which are related to blockade of adrenergic, histamine, and muscarinic cholinergic receptors. It also can be lethal in overdose.
Some substance abusers lose confidence in a medication that they cannot feel working. The side effects of desipramine, which can be intolerable for some patients, can reassure others with a history of substance abuse that they are being medicated.
Bupropion
Bupropion is a unique antidepressant that inhibits the presynaptic reuptake of dopamine and norepinephrine. Open-label studies demonstrate good responses to bupropion by adults with ADHD. One placebo-controlled, double-blind study found improved ADHD symptoms in 76% of patients receiving bupropion SR, compared with 37% of those receiving a placebo; the difference was statistically significant.19
Figure 2 IMPROVED ADHD SYMPTOMS WITH DESIPRAMINE

Adults with ADHD who received desipramine, 200 mg/d, in a double-blind trial showed significantly less hyperactivity, impulsivity, and inattentiveness after 6 weeks of therapy than a control group that received a placebo.
Source: Adapted with permission from Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.Another study of adults with ADHD compared bupropion SR to methylphenidate and a placebo.20 Using a primary outcome of the Clinical Global Impression (CGI) scale, response rates were 64% for bupropion SR, 50% for methylphenidate, and 27% for the placebo. The difference in response rates between the two agents was not statistically significant (p = 0.14).
Recommendation. The risk of seizures with bupropion is about 1 in 1,000. Therefore, bupropion should not be given to patients with a seizure disorder or to those with conditions that alter the seizure threshold (e.g., eating disorders, recent head trauma, or benzodiazepine withdrawal).21
This author uses bupropion as first-line treatment for appropriate patients with ADHD and a substance abuse history. Bupropion’s mild benefit with smoking cessation may provide some crossover effect for other substances of abuse. The low incidence of sexual side effects is another benefit. Drawbacks include twice-daily dosing and lack of a robust effect on attention and concentration.
Venlafaxine
Venlafaxine is a potent inhibitor of serotonin reuptake, a moderate inhibitor of norepinephrine, and a mild inhibitor of dopamine. Venlafaxine has displayed response rates similar to those of desipramine and bupropion in open-label studies in adults with ADHD,14 but no placebo-controlled studies exist.
As noted above, these antidepressants are believed to improve ADHD symptoms by making norepinephrine more available at the synapse. Hypothetically, then, one would need to administer venlafaxine at dosages that adequately inhibit the norepinephrine reuptake receptor. Venlafaxine XR, 150 mg/d, provides significant norepinephrine activity, according to several lines of evidence.
Recently, Upadhyaya et al reported the use of venlafaxine in one of the few treatment studies of patients with ADHD and comorbid alcohol/cocaine abuse. In an open-label trial, 10 subjects received venlafaxine, up to 300 mg/d, along with psychotherapy and attendance at Alcoholics Anonymous meetings. The nine who completed 4 weeks of treatment showed significantly improved ADHD symptoms and decreased alcohol craving.22
Recommendation. Venlafaxine is this author’s second choice for patients with ADHD and substance abuse problems. Sexual side effects that some patients experience with venlafaxine can limit its use. Some clinicians are concerned about increases in blood pressure associated with venlafaxine, although significant changes do not seem to occur at dosages below 300 mg/d.23
Atomoxetine
Atomoxetine is an investigational antidepressant in phase-III trials as a treatment for ADHD. Evidence shows atomoxetine to be a potent inhibitor of the presynaptic norepinephrine transporter, with minimal affinity for other neurotransmitter receptors. Initial studies suggest that atomoxetine is effective for adults and children with ADHD:
- In a small, double-blind, placebo-controlled, crossover trial, 11 of 20 adults showed improvement in ADHD symptoms within 3 weeks of starting atomoxetine.24
- In 297 children and adolescents, atomoxetine at dosages averaging approximately 1.2 mg/kg/d was more effective than a placebo in reducing ADHD symptoms and improving social and family functioning. Treatment was well tolerated and without significant side effects.25
- In a randomized open-label trial, 228 children received atomoxetine or methylphenidate. Both treatments significantly reduced inattention and hyperactive/impulsive symptoms.26
Related resources
- Arnsten AF. Genetics of childhood disorders (XVIII). ADHD, Part 2: Norepinephrine has a critical modulatory influence on prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 2000;39:1201-3.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention-deficit/hyperactivity disorder. Am J Psychiatry. 1993;150:885-90.
- Research Report: Prescription Drugs—abuse and addiction. National Institute on Drug Abuse, Substance Abuse and Mental Health Services Administration.
Drug brand names
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Ritalin, Concerta
- Venlafaxine • Effexor
Disclosure
Dr. Higgins reports that he is on the speakers’ bureaus for Wyeth-Ayerst Pharmaceuticals and Eli Lilly and Co.
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993;50:565-76.
2. Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann NY Acad Sci 2001;931:251-70.
3. Hunt RD. Nosology, neurobiology, and clinical patterns of ADHD in adults. Psychiatric Ann 1997;27:572-81.
4. Taylor MA. Evaluation and management of attention-deficit hyperactivity disorder. Am Fam Phys 1997;55:887-904.
5. Diller LH. The run on Ritalin. Attention deficit disorder and stimulant treatment in the 1990s. Hasting Center Report 1996;26:12-18.
6. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotrophic medications: primary care, psychiatry, and other medical specialties. JAMA 1998;279:526-31.
7. Mortan WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. J Clin Psychiatry (Primary Care) 2000;2:159-64.
8. Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
9. Banov MD, Palmer T, Brody E. Antidepressants are as effective as stimulants in the long-term treatment of ADHD in Adults. Primary Psychiatry 2001;8:54-7.
10. Moline S, Frankenberger W. Use of stimulant medication for treatment of attention-deficit/hyperactivity disorder. A survey of middle and high school students attitudes. Psychology in the Schools 2001;38:569-84.
11. Prescription drugs: abuse and addiction. National Institute on Drug Abuse Research Report Series. NIH publication number 01-4881, July 2001.
12. Mann A. Illicit methylphenidate trade appears widespread. Clinical Psychiatry News June 2000;28(6):5-
13. ABCNews.com. http://more/abcnews.go.com/sections/living/DailyNews/ritalin0505.html
14. Higgins ES. A comparative analysis of antidepressants and stimulants for the treatment of adults with attention-deficit hyperactivity disorder. J Fam Pract 1999;48:15-20.
15. Arnsten AF, Steere JC, Hunt RD. The contribution of alpha2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry 1996;53:448-55.
16. DuPaul G. The ADHD Rating Scale: normative data, reliability, and validity. Worcester, MA: University of Massachusetts Medical School, 1990.
17. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
18. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
19. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
20. Kuperman S, Perry PJ, Gaffney GR, et al. Bupropion SR vs. methylphenidate vs. placebo for attention-deficit/hyperactivity disorder in adults. Ann Clin Psychiatry 2001;13:129-34.
21. Wooltorton E. Bupropion (Zyban, Wellbutrin SR): reports of death, seizures, serum sickness. Can Med J 2002;166:68.-
22. Upadhyaya HP, Brady KT, Sethuraman G, et al. Venlafaxine treatment of patients with comorbid alcohol/cocaine abuse and attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol 2001;21:116-8.
23. Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry 1998;59:502-8.
24. Spencer T, Biederman J, Wilens T, Prince J, Hatch M. Effectiveness and tolerability of tomoxetine in adults with attention-deficit/hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
25. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108:E83.-
26. Kratochvil C, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in ADHD children: a randomized, open-label trial (presentation). Honolulu: American Academy of Child and Adolescent Psychiatry, October, 2001.
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993;50:565-76.
2. Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann NY Acad Sci 2001;931:251-70.
3. Hunt RD. Nosology, neurobiology, and clinical patterns of ADHD in adults. Psychiatric Ann 1997;27:572-81.
4. Taylor MA. Evaluation and management of attention-deficit hyperactivity disorder. Am Fam Phys 1997;55:887-904.
5. Diller LH. The run on Ritalin. Attention deficit disorder and stimulant treatment in the 1990s. Hasting Center Report 1996;26:12-18.
6. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotrophic medications: primary care, psychiatry, and other medical specialties. JAMA 1998;279:526-31.
7. Mortan WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. J Clin Psychiatry (Primary Care) 2000;2:159-64.
8. Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
9. Banov MD, Palmer T, Brody E. Antidepressants are as effective as stimulants in the long-term treatment of ADHD in Adults. Primary Psychiatry 2001;8:54-7.
10. Moline S, Frankenberger W. Use of stimulant medication for treatment of attention-deficit/hyperactivity disorder. A survey of middle and high school students attitudes. Psychology in the Schools 2001;38:569-84.
11. Prescription drugs: abuse and addiction. National Institute on Drug Abuse Research Report Series. NIH publication number 01-4881, July 2001.
12. Mann A. Illicit methylphenidate trade appears widespread. Clinical Psychiatry News June 2000;28(6):5-
13. ABCNews.com. http://more/abcnews.go.com/sections/living/DailyNews/ritalin0505.html
14. Higgins ES. A comparative analysis of antidepressants and stimulants for the treatment of adults with attention-deficit hyperactivity disorder. J Fam Pract 1999;48:15-20.
15. Arnsten AF, Steere JC, Hunt RD. The contribution of alpha2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry 1996;53:448-55.
16. DuPaul G. The ADHD Rating Scale: normative data, reliability, and validity. Worcester, MA: University of Massachusetts Medical School, 1990.
17. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
18. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
19. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
20. Kuperman S, Perry PJ, Gaffney GR, et al. Bupropion SR vs. methylphenidate vs. placebo for attention-deficit/hyperactivity disorder in adults. Ann Clin Psychiatry 2001;13:129-34.
21. Wooltorton E. Bupropion (Zyban, Wellbutrin SR): reports of death, seizures, serum sickness. Can Med J 2002;166:68.-
22. Upadhyaya HP, Brady KT, Sethuraman G, et al. Venlafaxine treatment of patients with comorbid alcohol/cocaine abuse and attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol 2001;21:116-8.
23. Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry 1998;59:502-8.
24. Spencer T, Biederman J, Wilens T, Prince J, Hatch M. Effectiveness and tolerability of tomoxetine in adults with attention-deficit/hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
25. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108:E83.-
26. Kratochvil C, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in ADHD children: a randomized, open-label trial (presentation). Honolulu: American Academy of Child and Adolescent Psychiatry, October, 2001.
Adult ADHD: Less hyperactivity, but lingering inattention and distress
Attention-deficit/hyperactivity disorder (ADHD) may be the only mental disorder that was discovered in children and later acknowledged in adults. Although controlled studies of adults with ADHD are few, we know that ADHD is common in adults, it can be diagnosed reliably, and 75% of those treated respond to treatment.1
The hallmark symptom of ADHD in children—hyperactivity—is usually attenuated in adults. In fact, some adults prefer the term ADD to ADHD because they are not hyperactive. This may be especially true of women, as their attention problems during childhood often were not recognized as ADHD (Box 1).
In childhood, girls with ADHD typically present with attention problems and over-talkativeness, rather than hyperactivity. Talking too much does not disrupt the classroom as much as the larger-scale misbehavior of boys with ADHD, so the diagnosis is often missed in these girls. Overtalkativeness was added to the DSM-III-R criteria for ADHD in 1987, after it was recognized as a symptom of overactivity.
Now in midlife, many women with undiagnosed ADHD have children with ADHD. As they bring their children to treatment, these women are recognizing similar attention deficit symptoms from their own childhoods and are getting the help they need. As adults, many have low self-esteem, low energy, and weight problems. Among adults with ADHD, these women may be the most underdiagnosed.
Characteristics of adult ADHD
Adults with ADHD visit a psychiatrist for a variety of reasons. Often they are parents of children diagnosed with ADHD, and the possibility that they are similarly affected has arisen during their children’s evaluation and treatment. Sometimes they have recognized themselves in consumer articles about ADHD, or others have seen them in this light.
Adults with ADHD continue to experience their childhood difficulties in sustaining attention, listening, following instructions, and organizing tasks; inattention to details; lack of sustained mental effort; losing things; distractibility, and forgetfulness. Typical complaints include underachievement and poor adjustment at work or home. Comorbid ADHD may also be identified in patients who present with depression, anxiety, substance misuse, and mood swings.
The cognitive impairment of ADHD continues into adulthood, even in adults without hyperactive symptoms. It may be that adults are not hyperactive because the basal ganglia, which control motor activity in the brain, have over the years accommodated the problem through behavior modification or neurodevelopmental changes in late adolescence.2
Children with ADHD have abnormal cerebrospinal fluid (CSF) and blood levels of the dopaminergic metabolite homovanillic acid (HVA), but adults with ADHD may not. The primary origin for CSF HVA is the nigrostriatum, which suggests that subcortical dopaminergic nuclei are more often affected in children than adults.2 This may mean that compensatory changes occur as persons with ADHD mature, or perhaps the forms of ADHD that persist into adulthood have a different pathology or pathophysiology.
Comorbidities with ADHD
Rarely does one see pure ADHD; comorbidity is the rule. ADHD can be diagnosed quickly if you know what to look for. But a facile diagnosis may overlook a comorbidity that must be treated first—especially if you plan to use stimulants. Many patients with ADHD also have bipolar disorder, and a smaller proportion of patients with bipolar disorder have undetected ADHD. Placing a patient with undetected bipolar disorder on a stimulant could precipitate mania.
Table 1
COMMON COMORBIDITIES WITH ADHD
| Bipolar disorder |
| Anxiety disorder |
| Depression |
| Drug dependence |
| Personality disorders |
| Somatoform disorders |
| Tourette’s disorder |
| Obsessive-compulsive disorder |
| Intermittent explosive disorder |
| Impulse control problems |
| Addictive behaviors |
| Sexual problems |
| Compulsive gambling |
| Learning disabilities |
| Asperger’s syndrome |
From the initial assessment, your treatment plan must address comorbid conditions (Table 1). This means taking a good history that includes corroborating information from relatives and data from the past, if possible. The case will then be much easier to manage, and quality of care greatly enhanced.
Stimulants: Usual first-choice therapy
In most cases, adult ADHD responds well to stimulant medications, although most available evidence is limited to studies in children. Several nonstimulant medications are also available, and the FDA is considering a new-drug application for a medication indicated for adult ADHD. Stimulants produce significant improvement in 30% of patients and mixed results in another 40%. Comorbidities may account for the 10 to 30% of patients who do not respond to stimulant therapy.
Methylphenidate, taken multiple times daily, is the most common treatment for ADHD. Dextroamphetamine and mixed salts of amphetamine also are used (Table 2).3 Patients usually respond to either methylphenidate or an amphetamine, and typically 25% of those who do not respond to one will respond to the other. When the clinical efficacy of amphetamines diminishes over time, many psychiatrists rotate medications. Replacing one amphetamine with another often eliminates the need to slowly increase the dosage and allows the clinician to maintain a relatively stable regimen.
When administering stimulants to adults, consider the individual’s total dosage requirement and daily schedule. Will he or she fare better with multiple daily dosing or a sustained-release form? How long is his or her average day? Does the patient have to be alert for 12 hours—or longer?
Some patients cannot sleep unless they take their last stimulant dose at bedtime. Others will have insomnia if a last dose is taken too late in the afternoon, especially with a sustained-release formulation.
When starting a patient on stimulants, begin with a 12-hour day and titrate the dosage—usually up, sometimes down—depending on response and side effects. Educating patients about their medications enables them to participate in decision-making.
Common side effects of stimulants include insomnia, decreased appetite, upset stomach, headache, anxiety, agitation, and increased pulse rate and blood pressure. The increase in blood pressure is usually less than 10%, but patients with poorly controlled hypertension should not be treated with stimulants until their blood pressure is well controlled. Until more is known about long-term effects, periodic assessment of blood pressure may be warranted.
Table 2
STIMULANT THERAPY FOR ADULTS WITH ADHD
| Stimulants | Starting dosage | Titration rate | Usual dosing interval | Maximum dosage in adults | |
|---|---|---|---|---|---|
| Methylphenidate | |||||
| Short-acting | |||||
| d, l-methylphenidate (Ritalin, Methylin) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 3 to 4 hours Usually bid-tid | Average oral dosage 0.92 mg/kg/d; best response to 1.0 mg/kg/d16 | |
| Intermediate-acting | |||||
| d, l-methylphenidate (Ritalin SR, Metadate ER, Methylin ER) | 20 mg Ritalin SR; 10 mg Methylin ER or Metadate ER | 10 to 20 mg per week | qd to bid | ||
| d-methylphenidate (Focalin) | 2.5 mg bid | 2.5 to 5 mg per week | bid, at least 4 hours apart | ||
| Long-acting | |||||
| d, l-methylphenidate (Concerta) | 18 mg qd | 18 mg every 3 to 5 days | 12+ hours, usually qd | ||
| d, l-methylphenidate (Metadate CD) | 20 mg qd | 20 mg per week | qd | ||
| Amphetamine | |||||
| Short-acting | |||||
| (Dexedrine, Dextrostat) | 2.5 to 5 mg qd | 2.5 to 5 mg every 3 to 5 days | Every 4 to 6 hours Usually bid-tid | ||
| Intermediate-acting | |||||
| Mixed salts (Adderall) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 4 to 6 hours Usually qd to bid | Average dosage 54 mg/d divided in two doses; maximum 30 mg bid | |
| (Dexedrine Spansule) | 5 or 10 mg qd | 5 mg per week | qd | ||
| Long-acting | |||||
| (Adderall XR) | 10 mg qd | 10 mg per week | qd | ||
| Stimulant | |||||
| Pemoline (Cylert) | 37.5 mg qd | 18.75 mg per week | qd; typical range 56.25 to 75 mg qd | Maximum dosage 112.5 mg/d | |
- Organized and orderly home and working environment
- Designated work/study space at home
- Designated coach to supervise work/study
- Healthy meals at regularly scheduled times
- Regular exercise
Adults with ADHD have been treated with mixed amphetamine salts with positive results. In a 7-week controlled, crossover study, 27 adults with ADHD received an average of 54 mg/d administered in two doses. Symptoms improved significantly—a 42% decrease on the ADHD Rating Scale. The medication was well-tolerated, and 70% of those receiving mixed amphetamine salts improved, compared with 7% of those who received a placebo.4
Duration of action of mixed salts of amphetamine has been measured at 3.5 hours with a 5-mg dose and 6.4 hours with a 20-mg dose.5 With methylphenidate, a dose of 12.5 mg worked for 4 hours. The maximum recommended dosage of mixed salts of amphetamine is 40 mg/d in divided doses.
Stimulant medications are well-tolerated. Addiction and the need for increased dosages can occur over long-term use (months to years). Reducing the dosage or switching from methylphenidate to an amphetamine variant can usually prevent these problems.
The FDA recently approved a single-enantiomer form of methylphenidate. It contains only the active “d” enantiomer, whereas the racemic mixture contains both the “d” and “l” enantiomers. Because the “l” enantiomer is inert, the resulting medication is more potent and may be prescribed at half the dosage of the racemic mixture.
Pemoline, a once-daily stimulant, is considered a second-line treatment because of reports of hepatic failure in some patients. Its use requires written informed consent and liver function tests at baseline and every 2 weeks. In a controlled trial, pemoline at high dosages (120 to 160 mg/d) was found moderately effective in adults with ADHD.6
Newer options: Longer-acting stimulants
Newer forms of slow-release methylphenidate and mixed amphetamine salts with sophisticated delivery systems are available.
Metadate CD is delivered in capsules containing beads with polymer coatings that dissolve and release their contents at different times. The capsules contain a 30:70 ratio of immediate- and extended-release beads.
Metadate CD has not been tested for adults in controlled clinical trials. In children ages 6 to 15, a single morning dose has been shown to be clinically effective in the morning and afternoon. A supplemental immediate-release capsule can be given in the morning if a patient’s medication levels need to be increased quickly. Dosage supplementation may also be required later in the day.
Concerta is delivered in 18-mg and 36-mg tablets. The immediate-release coating on the tablets delivers medication within the first hour. The drug inside then dissolves in the GI tract and is released at a controlled rate by osmotic pressure. The indigestible tablet is passed in the stool.
Concerta was investigated in children ages 6 to 12 and provides 10 to 12 hours of sustained medication. From child studies, we know that when a patient takes a 36-mg tablet at 6 AM, blood levels decline in late afternoon. An 18-mg dose at noon covers the 4 to 6 hours needed for evening chores.
Adderall XR is an extended-release, once-daily form of mixed amphetamine salts. No controlled trials of this formulation are available in adults with ADHD. Its efficacy was established after two clinical trials of children aged 6 to 12 who met DSM-IV criteria for ADHD.
Individualized and flexible dosing improves symptom control and compliance when treating adults with ADHD. For some patients, once-daily dosing is more convenient than multiple doses, while others prefer the immediate-release form because they like its midday “pause” and bid dosing. The immediate-release tablet allows the flexibility of bid or tid dosing, depending on the day’s requirements.
Antidepressants: Another choice
Antidepressants are usually considered second-line treatment for ADHD because of concerns about efficacy and side effects. The few available studies show antidepressants work as well as stimulants but more slowly. It is good practice, therefore, to advise patients that—unlike feeling the effect of a stimulant in 60 minutes—they will not feel an effect from an antidepressant for days or weeks, and that achieving an optimal effect may take 4 to 6 weeks.
Antidepressants have several advantages over stimulants. They are not classified as narcotics, work without the on-off effects of stimulants, and can treat comorbid depression and anxiety. For adult ADHD, the most effective agents work on the catecholamine systems—norepinephrine and/or dopamine. This includes the tricyclic antidepressants, MAO inhibitors, bupropion, and venlafaxine. The serotonin reuptake inhibitors have not shown promise in ADHD, nor have mirtazapine or nefazodone demonstrated much effect.
Desipramine, a tricyclic antidepressant, is a strong inhibitor of norepinephrine reuptake. In a double-blind, controlled study in 41 adults with ADHD, 68% of patients receiving desipramine, 200 mg/d, responded positively, compared with no patients who took a placebo.7
When venlafaxine was given in standard dosages to 10 adults with ADHD in an open, 8-week clinical trial, an effect was seen by week two. Of the nine patients who completed the study, seven were considered responders. Symptoms were reduced significantly with venlafaxine treatment, and most side effects were mild.8
In an open study, bupropion treatment resulted in moderate to marked response in 74% of 19 patients. Ten of those patients who responded chose to continue bupropion rather than their previous medication.9 In a 6-week controlled study of 40 patients, bupropion use was associated with a 42% reduction in ADHD symptoms in the 38 patients who completed the study. Patients who received a placebo showed only a 24% reduction in symptoms. According to the CGI, 52% of patients who received bupropion reported being “much improved” or “very improved” compared with 11% of those receiving a placebo.10
Other treatment options that have shown mixed results include modafinil, alpha-2a agonists, acetylcholinesterase inhibitors, and the histaminergic agents.
Managing adverse effects
Substance abuse Stimulant abuse has been a concern, but it has not become the problem many feared. In fact, some studies have found that methylphenidate may help stanch the craving for cocaine in adults with ADHD.11,12 Treating ADHD with pharmacotherapy also has been shown to reduce the risk for substance abuse in adolescence by 85%.13
With careful screening, you can usually identify drug-seeking behavior in adult patients. For patients with substance abuse problems, you can prescribe the nonstimulants.
Tics that can occur with stimulant medications usually can be suppressed by reducing the dosage, being vigilant, and waiting it out. Tics may ameliorate over weeks to months.
Cardiac and cognitive effects Long-term use of stimulant medications at high dosages has been associated with cardiac and cognitive toxicity, as noted in the 1998 NIH consensus statement on diagnosis and treatment of ADHD. It is important to provide patients with this information as part of their informed-consent briefing. (See “Related resources,” to view the consensus statement.)
Nonpharmacologic management
Nonpharmacologic treatments such as EEG biofeedback; psychoeducational approaches; and individual, family, and group psychotherapy are widely used to treat adults with ADHD. Clinicians and patients often perceive these interventions as beneficial, although none have been tested in randomized, placebo-controlled studies.
Patients often function better when their home and work environments are thoughtfully organized, with a designated work/study space and regularly scheduled times for meals, sleep, and exercise. An ADHD coach may facilitate such structure and discipline (Box 2).
New agents in the pipeline
Efforts are being made to increase awareness of adult ADHD and to improve its treatment. For example, the National Institute of Mental Health is funding research on adult ADHD and displays on its Web site a PET scan of an adult brain with ADHD (see “Related resources”).14 Several medications also are being developed to treat ADHD.
Atomoxetine, a nonstimulant medication awaiting FDA approval for adult ADHD, is a selective norepinephrine reuptake inhibitor. In a double-blind, placebo-controlled, crossover study of adults with well-characterized ADHD, 11 of 21 patients improved with use of atomoxetine, compared with 2 of 21 who improved with use of a placebo. The average dosage of 76 mg/d was well-tolerated.15 The 52% response rate is similar to the 54% average improvement rate reported for methylphenidate in previous studies of adult ADHD.
In clinical trials, atomoxetine was given bid. Insomnia was not a side effect, so bid dosing does not interfere with sleep. Approximately 10 to 15% of patients experienced weight loss as a side effect.
Other treatment options under development include:
- a transdermal system for delivery of methylphenidate16
- a novel nicotinic analogue
- glutamate AMPA receptor modulation
- omega-3 fatty acids.
- Hallowell EM, Ratey JJ. Driven to distraction: Recognizing and coping with attention deficit disorder from childhood through adulthood. New York: Simon and Schuster; Reprint edition 1995.
- Solanto MV, Arnstein AFT, Castellanos FX, eds. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001.
- Weiss M, Trokenberg-Hechtman L, Weiss G. ADHD in adulthood: A guide to current theory, diagnosis, and treatment. Baltimore: Johns Hopkins University Press; 1999.
- National Institute of Mental Health. http://www.nimh.nih.gov
Drug brand names
- Atomoxetine • (investigational)
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Methamphetamine • Desoxyn
- Methylphenidate • Focalin, Ritalin
- Methylphenidate SR • Concerta, Metadate CD, Metadate ER, Methylin ER, Ritalin SR
- Mixed salts of amphetamine • Adderall, Adderall XR
- Modafinil • Provigil
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
The author reports that he serves as a consultant to Eli Lilly and Company and is on the speaker’s bureaus of Wyeth Pharmaceuticals and AstraZeneca.
1. Gadrow KD, Weiss M. Attention-deficit/hyperactivity disorder in adults: beyond controversy. Arch Gen Psychiatry 2001;58(8):784-5.
2. Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci 1998;18(15):5901-7.
3. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
4. Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001;58(8):775-82.
5. Swanson J, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 1998;34(1):55-60.
6. Wilens TE, Biederman J, Spencer TJ, et al. Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999;19(3):257-64.
7. Wilens TE, Biederman J, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention-deficit hyperactivity disorder. J Nerv Ment Dis 1995;183(1):48-50.
8. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57(5):184-9.
9. Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry 1990;147(8):1018-20.
10. Wilens TE, Spencer TJ, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
11. Grabowski J, Roache JD, Schmitz JM, Rhoades H, et al. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 1997;17(6):485-8.
12. Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry 1998;59(6):300-5.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104(2):e20.-
14. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990;323(20):1361-6.
15. Spencer T, Biederman J, Wilens T. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155(5):693-5.
16. Noven Pharmaceuticals. The Science of Noven. Research and development. Transdermal technology. Available at: http://www.noven.com/research.htm.
Attention-deficit/hyperactivity disorder (ADHD) may be the only mental disorder that was discovered in children and later acknowledged in adults. Although controlled studies of adults with ADHD are few, we know that ADHD is common in adults, it can be diagnosed reliably, and 75% of those treated respond to treatment.1
The hallmark symptom of ADHD in children—hyperactivity—is usually attenuated in adults. In fact, some adults prefer the term ADD to ADHD because they are not hyperactive. This may be especially true of women, as their attention problems during childhood often were not recognized as ADHD (Box 1).
In childhood, girls with ADHD typically present with attention problems and over-talkativeness, rather than hyperactivity. Talking too much does not disrupt the classroom as much as the larger-scale misbehavior of boys with ADHD, so the diagnosis is often missed in these girls. Overtalkativeness was added to the DSM-III-R criteria for ADHD in 1987, after it was recognized as a symptom of overactivity.
Now in midlife, many women with undiagnosed ADHD have children with ADHD. As they bring their children to treatment, these women are recognizing similar attention deficit symptoms from their own childhoods and are getting the help they need. As adults, many have low self-esteem, low energy, and weight problems. Among adults with ADHD, these women may be the most underdiagnosed.
Characteristics of adult ADHD
Adults with ADHD visit a psychiatrist for a variety of reasons. Often they are parents of children diagnosed with ADHD, and the possibility that they are similarly affected has arisen during their children’s evaluation and treatment. Sometimes they have recognized themselves in consumer articles about ADHD, or others have seen them in this light.
Adults with ADHD continue to experience their childhood difficulties in sustaining attention, listening, following instructions, and organizing tasks; inattention to details; lack of sustained mental effort; losing things; distractibility, and forgetfulness. Typical complaints include underachievement and poor adjustment at work or home. Comorbid ADHD may also be identified in patients who present with depression, anxiety, substance misuse, and mood swings.
The cognitive impairment of ADHD continues into adulthood, even in adults without hyperactive symptoms. It may be that adults are not hyperactive because the basal ganglia, which control motor activity in the brain, have over the years accommodated the problem through behavior modification or neurodevelopmental changes in late adolescence.2
Children with ADHD have abnormal cerebrospinal fluid (CSF) and blood levels of the dopaminergic metabolite homovanillic acid (HVA), but adults with ADHD may not. The primary origin for CSF HVA is the nigrostriatum, which suggests that subcortical dopaminergic nuclei are more often affected in children than adults.2 This may mean that compensatory changes occur as persons with ADHD mature, or perhaps the forms of ADHD that persist into adulthood have a different pathology or pathophysiology.
Comorbidities with ADHD
Rarely does one see pure ADHD; comorbidity is the rule. ADHD can be diagnosed quickly if you know what to look for. But a facile diagnosis may overlook a comorbidity that must be treated first—especially if you plan to use stimulants. Many patients with ADHD also have bipolar disorder, and a smaller proportion of patients with bipolar disorder have undetected ADHD. Placing a patient with undetected bipolar disorder on a stimulant could precipitate mania.
Table 1
COMMON COMORBIDITIES WITH ADHD
| Bipolar disorder |
| Anxiety disorder |
| Depression |
| Drug dependence |
| Personality disorders |
| Somatoform disorders |
| Tourette’s disorder |
| Obsessive-compulsive disorder |
| Intermittent explosive disorder |
| Impulse control problems |
| Addictive behaviors |
| Sexual problems |
| Compulsive gambling |
| Learning disabilities |
| Asperger’s syndrome |
From the initial assessment, your treatment plan must address comorbid conditions (Table 1). This means taking a good history that includes corroborating information from relatives and data from the past, if possible. The case will then be much easier to manage, and quality of care greatly enhanced.
Stimulants: Usual first-choice therapy
In most cases, adult ADHD responds well to stimulant medications, although most available evidence is limited to studies in children. Several nonstimulant medications are also available, and the FDA is considering a new-drug application for a medication indicated for adult ADHD. Stimulants produce significant improvement in 30% of patients and mixed results in another 40%. Comorbidities may account for the 10 to 30% of patients who do not respond to stimulant therapy.
Methylphenidate, taken multiple times daily, is the most common treatment for ADHD. Dextroamphetamine and mixed salts of amphetamine also are used (Table 2).3 Patients usually respond to either methylphenidate or an amphetamine, and typically 25% of those who do not respond to one will respond to the other. When the clinical efficacy of amphetamines diminishes over time, many psychiatrists rotate medications. Replacing one amphetamine with another often eliminates the need to slowly increase the dosage and allows the clinician to maintain a relatively stable regimen.
When administering stimulants to adults, consider the individual’s total dosage requirement and daily schedule. Will he or she fare better with multiple daily dosing or a sustained-release form? How long is his or her average day? Does the patient have to be alert for 12 hours—or longer?
Some patients cannot sleep unless they take their last stimulant dose at bedtime. Others will have insomnia if a last dose is taken too late in the afternoon, especially with a sustained-release formulation.
When starting a patient on stimulants, begin with a 12-hour day and titrate the dosage—usually up, sometimes down—depending on response and side effects. Educating patients about their medications enables them to participate in decision-making.
Common side effects of stimulants include insomnia, decreased appetite, upset stomach, headache, anxiety, agitation, and increased pulse rate and blood pressure. The increase in blood pressure is usually less than 10%, but patients with poorly controlled hypertension should not be treated with stimulants until their blood pressure is well controlled. Until more is known about long-term effects, periodic assessment of blood pressure may be warranted.
Table 2
STIMULANT THERAPY FOR ADULTS WITH ADHD
| Stimulants | Starting dosage | Titration rate | Usual dosing interval | Maximum dosage in adults | |
|---|---|---|---|---|---|
| Methylphenidate | |||||
| Short-acting | |||||
| d, l-methylphenidate (Ritalin, Methylin) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 3 to 4 hours Usually bid-tid | Average oral dosage 0.92 mg/kg/d; best response to 1.0 mg/kg/d16 | |
| Intermediate-acting | |||||
| d, l-methylphenidate (Ritalin SR, Metadate ER, Methylin ER) | 20 mg Ritalin SR; 10 mg Methylin ER or Metadate ER | 10 to 20 mg per week | qd to bid | ||
| d-methylphenidate (Focalin) | 2.5 mg bid | 2.5 to 5 mg per week | bid, at least 4 hours apart | ||
| Long-acting | |||||
| d, l-methylphenidate (Concerta) | 18 mg qd | 18 mg every 3 to 5 days | 12+ hours, usually qd | ||
| d, l-methylphenidate (Metadate CD) | 20 mg qd | 20 mg per week | qd | ||
| Amphetamine | |||||
| Short-acting | |||||
| (Dexedrine, Dextrostat) | 2.5 to 5 mg qd | 2.5 to 5 mg every 3 to 5 days | Every 4 to 6 hours Usually bid-tid | ||
| Intermediate-acting | |||||
| Mixed salts (Adderall) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 4 to 6 hours Usually qd to bid | Average dosage 54 mg/d divided in two doses; maximum 30 mg bid | |
| (Dexedrine Spansule) | 5 or 10 mg qd | 5 mg per week | qd | ||
| Long-acting | |||||
| (Adderall XR) | 10 mg qd | 10 mg per week | qd | ||
| Stimulant | |||||
| Pemoline (Cylert) | 37.5 mg qd | 18.75 mg per week | qd; typical range 56.25 to 75 mg qd | Maximum dosage 112.5 mg/d | |
- Organized and orderly home and working environment
- Designated work/study space at home
- Designated coach to supervise work/study
- Healthy meals at regularly scheduled times
- Regular exercise
Adults with ADHD have been treated with mixed amphetamine salts with positive results. In a 7-week controlled, crossover study, 27 adults with ADHD received an average of 54 mg/d administered in two doses. Symptoms improved significantly—a 42% decrease on the ADHD Rating Scale. The medication was well-tolerated, and 70% of those receiving mixed amphetamine salts improved, compared with 7% of those who received a placebo.4
Duration of action of mixed salts of amphetamine has been measured at 3.5 hours with a 5-mg dose and 6.4 hours with a 20-mg dose.5 With methylphenidate, a dose of 12.5 mg worked for 4 hours. The maximum recommended dosage of mixed salts of amphetamine is 40 mg/d in divided doses.
Stimulant medications are well-tolerated. Addiction and the need for increased dosages can occur over long-term use (months to years). Reducing the dosage or switching from methylphenidate to an amphetamine variant can usually prevent these problems.
The FDA recently approved a single-enantiomer form of methylphenidate. It contains only the active “d” enantiomer, whereas the racemic mixture contains both the “d” and “l” enantiomers. Because the “l” enantiomer is inert, the resulting medication is more potent and may be prescribed at half the dosage of the racemic mixture.
Pemoline, a once-daily stimulant, is considered a second-line treatment because of reports of hepatic failure in some patients. Its use requires written informed consent and liver function tests at baseline and every 2 weeks. In a controlled trial, pemoline at high dosages (120 to 160 mg/d) was found moderately effective in adults with ADHD.6
Newer options: Longer-acting stimulants
Newer forms of slow-release methylphenidate and mixed amphetamine salts with sophisticated delivery systems are available.
Metadate CD is delivered in capsules containing beads with polymer coatings that dissolve and release their contents at different times. The capsules contain a 30:70 ratio of immediate- and extended-release beads.
Metadate CD has not been tested for adults in controlled clinical trials. In children ages 6 to 15, a single morning dose has been shown to be clinically effective in the morning and afternoon. A supplemental immediate-release capsule can be given in the morning if a patient’s medication levels need to be increased quickly. Dosage supplementation may also be required later in the day.
Concerta is delivered in 18-mg and 36-mg tablets. The immediate-release coating on the tablets delivers medication within the first hour. The drug inside then dissolves in the GI tract and is released at a controlled rate by osmotic pressure. The indigestible tablet is passed in the stool.
Concerta was investigated in children ages 6 to 12 and provides 10 to 12 hours of sustained medication. From child studies, we know that when a patient takes a 36-mg tablet at 6 AM, blood levels decline in late afternoon. An 18-mg dose at noon covers the 4 to 6 hours needed for evening chores.
Adderall XR is an extended-release, once-daily form of mixed amphetamine salts. No controlled trials of this formulation are available in adults with ADHD. Its efficacy was established after two clinical trials of children aged 6 to 12 who met DSM-IV criteria for ADHD.
Individualized and flexible dosing improves symptom control and compliance when treating adults with ADHD. For some patients, once-daily dosing is more convenient than multiple doses, while others prefer the immediate-release form because they like its midday “pause” and bid dosing. The immediate-release tablet allows the flexibility of bid or tid dosing, depending on the day’s requirements.
Antidepressants: Another choice
Antidepressants are usually considered second-line treatment for ADHD because of concerns about efficacy and side effects. The few available studies show antidepressants work as well as stimulants but more slowly. It is good practice, therefore, to advise patients that—unlike feeling the effect of a stimulant in 60 minutes—they will not feel an effect from an antidepressant for days or weeks, and that achieving an optimal effect may take 4 to 6 weeks.
Antidepressants have several advantages over stimulants. They are not classified as narcotics, work without the on-off effects of stimulants, and can treat comorbid depression and anxiety. For adult ADHD, the most effective agents work on the catecholamine systems—norepinephrine and/or dopamine. This includes the tricyclic antidepressants, MAO inhibitors, bupropion, and venlafaxine. The serotonin reuptake inhibitors have not shown promise in ADHD, nor have mirtazapine or nefazodone demonstrated much effect.
Desipramine, a tricyclic antidepressant, is a strong inhibitor of norepinephrine reuptake. In a double-blind, controlled study in 41 adults with ADHD, 68% of patients receiving desipramine, 200 mg/d, responded positively, compared with no patients who took a placebo.7
When venlafaxine was given in standard dosages to 10 adults with ADHD in an open, 8-week clinical trial, an effect was seen by week two. Of the nine patients who completed the study, seven were considered responders. Symptoms were reduced significantly with venlafaxine treatment, and most side effects were mild.8
In an open study, bupropion treatment resulted in moderate to marked response in 74% of 19 patients. Ten of those patients who responded chose to continue bupropion rather than their previous medication.9 In a 6-week controlled study of 40 patients, bupropion use was associated with a 42% reduction in ADHD symptoms in the 38 patients who completed the study. Patients who received a placebo showed only a 24% reduction in symptoms. According to the CGI, 52% of patients who received bupropion reported being “much improved” or “very improved” compared with 11% of those receiving a placebo.10
Other treatment options that have shown mixed results include modafinil, alpha-2a agonists, acetylcholinesterase inhibitors, and the histaminergic agents.
Managing adverse effects
Substance abuse Stimulant abuse has been a concern, but it has not become the problem many feared. In fact, some studies have found that methylphenidate may help stanch the craving for cocaine in adults with ADHD.11,12 Treating ADHD with pharmacotherapy also has been shown to reduce the risk for substance abuse in adolescence by 85%.13
With careful screening, you can usually identify drug-seeking behavior in adult patients. For patients with substance abuse problems, you can prescribe the nonstimulants.
Tics that can occur with stimulant medications usually can be suppressed by reducing the dosage, being vigilant, and waiting it out. Tics may ameliorate over weeks to months.
Cardiac and cognitive effects Long-term use of stimulant medications at high dosages has been associated with cardiac and cognitive toxicity, as noted in the 1998 NIH consensus statement on diagnosis and treatment of ADHD. It is important to provide patients with this information as part of their informed-consent briefing. (See “Related resources,” to view the consensus statement.)
Nonpharmacologic management
Nonpharmacologic treatments such as EEG biofeedback; psychoeducational approaches; and individual, family, and group psychotherapy are widely used to treat adults with ADHD. Clinicians and patients often perceive these interventions as beneficial, although none have been tested in randomized, placebo-controlled studies.
Patients often function better when their home and work environments are thoughtfully organized, with a designated work/study space and regularly scheduled times for meals, sleep, and exercise. An ADHD coach may facilitate such structure and discipline (Box 2).
New agents in the pipeline
Efforts are being made to increase awareness of adult ADHD and to improve its treatment. For example, the National Institute of Mental Health is funding research on adult ADHD and displays on its Web site a PET scan of an adult brain with ADHD (see “Related resources”).14 Several medications also are being developed to treat ADHD.
Atomoxetine, a nonstimulant medication awaiting FDA approval for adult ADHD, is a selective norepinephrine reuptake inhibitor. In a double-blind, placebo-controlled, crossover study of adults with well-characterized ADHD, 11 of 21 patients improved with use of atomoxetine, compared with 2 of 21 who improved with use of a placebo. The average dosage of 76 mg/d was well-tolerated.15 The 52% response rate is similar to the 54% average improvement rate reported for methylphenidate in previous studies of adult ADHD.
In clinical trials, atomoxetine was given bid. Insomnia was not a side effect, so bid dosing does not interfere with sleep. Approximately 10 to 15% of patients experienced weight loss as a side effect.
Other treatment options under development include:
- a transdermal system for delivery of methylphenidate16
- a novel nicotinic analogue
- glutamate AMPA receptor modulation
- omega-3 fatty acids.
- Hallowell EM, Ratey JJ. Driven to distraction: Recognizing and coping with attention deficit disorder from childhood through adulthood. New York: Simon and Schuster; Reprint edition 1995.
- Solanto MV, Arnstein AFT, Castellanos FX, eds. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001.
- Weiss M, Trokenberg-Hechtman L, Weiss G. ADHD in adulthood: A guide to current theory, diagnosis, and treatment. Baltimore: Johns Hopkins University Press; 1999.
- National Institute of Mental Health. http://www.nimh.nih.gov
Drug brand names
- Atomoxetine • (investigational)
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Methamphetamine • Desoxyn
- Methylphenidate • Focalin, Ritalin
- Methylphenidate SR • Concerta, Metadate CD, Metadate ER, Methylin ER, Ritalin SR
- Mixed salts of amphetamine • Adderall, Adderall XR
- Modafinil • Provigil
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
The author reports that he serves as a consultant to Eli Lilly and Company and is on the speaker’s bureaus of Wyeth Pharmaceuticals and AstraZeneca.
Attention-deficit/hyperactivity disorder (ADHD) may be the only mental disorder that was discovered in children and later acknowledged in adults. Although controlled studies of adults with ADHD are few, we know that ADHD is common in adults, it can be diagnosed reliably, and 75% of those treated respond to treatment.1
The hallmark symptom of ADHD in children—hyperactivity—is usually attenuated in adults. In fact, some adults prefer the term ADD to ADHD because they are not hyperactive. This may be especially true of women, as their attention problems during childhood often were not recognized as ADHD (Box 1).
In childhood, girls with ADHD typically present with attention problems and over-talkativeness, rather than hyperactivity. Talking too much does not disrupt the classroom as much as the larger-scale misbehavior of boys with ADHD, so the diagnosis is often missed in these girls. Overtalkativeness was added to the DSM-III-R criteria for ADHD in 1987, after it was recognized as a symptom of overactivity.
Now in midlife, many women with undiagnosed ADHD have children with ADHD. As they bring their children to treatment, these women are recognizing similar attention deficit symptoms from their own childhoods and are getting the help they need. As adults, many have low self-esteem, low energy, and weight problems. Among adults with ADHD, these women may be the most underdiagnosed.
Characteristics of adult ADHD
Adults with ADHD visit a psychiatrist for a variety of reasons. Often they are parents of children diagnosed with ADHD, and the possibility that they are similarly affected has arisen during their children’s evaluation and treatment. Sometimes they have recognized themselves in consumer articles about ADHD, or others have seen them in this light.
Adults with ADHD continue to experience their childhood difficulties in sustaining attention, listening, following instructions, and organizing tasks; inattention to details; lack of sustained mental effort; losing things; distractibility, and forgetfulness. Typical complaints include underachievement and poor adjustment at work or home. Comorbid ADHD may also be identified in patients who present with depression, anxiety, substance misuse, and mood swings.
The cognitive impairment of ADHD continues into adulthood, even in adults without hyperactive symptoms. It may be that adults are not hyperactive because the basal ganglia, which control motor activity in the brain, have over the years accommodated the problem through behavior modification or neurodevelopmental changes in late adolescence.2
Children with ADHD have abnormal cerebrospinal fluid (CSF) and blood levels of the dopaminergic metabolite homovanillic acid (HVA), but adults with ADHD may not. The primary origin for CSF HVA is the nigrostriatum, which suggests that subcortical dopaminergic nuclei are more often affected in children than adults.2 This may mean that compensatory changes occur as persons with ADHD mature, or perhaps the forms of ADHD that persist into adulthood have a different pathology or pathophysiology.
Comorbidities with ADHD
Rarely does one see pure ADHD; comorbidity is the rule. ADHD can be diagnosed quickly if you know what to look for. But a facile diagnosis may overlook a comorbidity that must be treated first—especially if you plan to use stimulants. Many patients with ADHD also have bipolar disorder, and a smaller proportion of patients with bipolar disorder have undetected ADHD. Placing a patient with undetected bipolar disorder on a stimulant could precipitate mania.
Table 1
COMMON COMORBIDITIES WITH ADHD
| Bipolar disorder |
| Anxiety disorder |
| Depression |
| Drug dependence |
| Personality disorders |
| Somatoform disorders |
| Tourette’s disorder |
| Obsessive-compulsive disorder |
| Intermittent explosive disorder |
| Impulse control problems |
| Addictive behaviors |
| Sexual problems |
| Compulsive gambling |
| Learning disabilities |
| Asperger’s syndrome |
From the initial assessment, your treatment plan must address comorbid conditions (Table 1). This means taking a good history that includes corroborating information from relatives and data from the past, if possible. The case will then be much easier to manage, and quality of care greatly enhanced.
Stimulants: Usual first-choice therapy
In most cases, adult ADHD responds well to stimulant medications, although most available evidence is limited to studies in children. Several nonstimulant medications are also available, and the FDA is considering a new-drug application for a medication indicated for adult ADHD. Stimulants produce significant improvement in 30% of patients and mixed results in another 40%. Comorbidities may account for the 10 to 30% of patients who do not respond to stimulant therapy.
Methylphenidate, taken multiple times daily, is the most common treatment for ADHD. Dextroamphetamine and mixed salts of amphetamine also are used (Table 2).3 Patients usually respond to either methylphenidate or an amphetamine, and typically 25% of those who do not respond to one will respond to the other. When the clinical efficacy of amphetamines diminishes over time, many psychiatrists rotate medications. Replacing one amphetamine with another often eliminates the need to slowly increase the dosage and allows the clinician to maintain a relatively stable regimen.
When administering stimulants to adults, consider the individual’s total dosage requirement and daily schedule. Will he or she fare better with multiple daily dosing or a sustained-release form? How long is his or her average day? Does the patient have to be alert for 12 hours—or longer?
Some patients cannot sleep unless they take their last stimulant dose at bedtime. Others will have insomnia if a last dose is taken too late in the afternoon, especially with a sustained-release formulation.
When starting a patient on stimulants, begin with a 12-hour day and titrate the dosage—usually up, sometimes down—depending on response and side effects. Educating patients about their medications enables them to participate in decision-making.
Common side effects of stimulants include insomnia, decreased appetite, upset stomach, headache, anxiety, agitation, and increased pulse rate and blood pressure. The increase in blood pressure is usually less than 10%, but patients with poorly controlled hypertension should not be treated with stimulants until their blood pressure is well controlled. Until more is known about long-term effects, periodic assessment of blood pressure may be warranted.
Table 2
STIMULANT THERAPY FOR ADULTS WITH ADHD
| Stimulants | Starting dosage | Titration rate | Usual dosing interval | Maximum dosage in adults | |
|---|---|---|---|---|---|
| Methylphenidate | |||||
| Short-acting | |||||
| d, l-methylphenidate (Ritalin, Methylin) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 3 to 4 hours Usually bid-tid | Average oral dosage 0.92 mg/kg/d; best response to 1.0 mg/kg/d16 | |
| Intermediate-acting | |||||
| d, l-methylphenidate (Ritalin SR, Metadate ER, Methylin ER) | 20 mg Ritalin SR; 10 mg Methylin ER or Metadate ER | 10 to 20 mg per week | qd to bid | ||
| d-methylphenidate (Focalin) | 2.5 mg bid | 2.5 to 5 mg per week | bid, at least 4 hours apart | ||
| Long-acting | |||||
| d, l-methylphenidate (Concerta) | 18 mg qd | 18 mg every 3 to 5 days | 12+ hours, usually qd | ||
| d, l-methylphenidate (Metadate CD) | 20 mg qd | 20 mg per week | qd | ||
| Amphetamine | |||||
| Short-acting | |||||
| (Dexedrine, Dextrostat) | 2.5 to 5 mg qd | 2.5 to 5 mg every 3 to 5 days | Every 4 to 6 hours Usually bid-tid | ||
| Intermediate-acting | |||||
| Mixed salts (Adderall) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 4 to 6 hours Usually qd to bid | Average dosage 54 mg/d divided in two doses; maximum 30 mg bid | |
| (Dexedrine Spansule) | 5 or 10 mg qd | 5 mg per week | qd | ||
| Long-acting | |||||
| (Adderall XR) | 10 mg qd | 10 mg per week | qd | ||
| Stimulant | |||||
| Pemoline (Cylert) | 37.5 mg qd | 18.75 mg per week | qd; typical range 56.25 to 75 mg qd | Maximum dosage 112.5 mg/d | |
- Organized and orderly home and working environment
- Designated work/study space at home
- Designated coach to supervise work/study
- Healthy meals at regularly scheduled times
- Regular exercise
Adults with ADHD have been treated with mixed amphetamine salts with positive results. In a 7-week controlled, crossover study, 27 adults with ADHD received an average of 54 mg/d administered in two doses. Symptoms improved significantly—a 42% decrease on the ADHD Rating Scale. The medication was well-tolerated, and 70% of those receiving mixed amphetamine salts improved, compared with 7% of those who received a placebo.4
Duration of action of mixed salts of amphetamine has been measured at 3.5 hours with a 5-mg dose and 6.4 hours with a 20-mg dose.5 With methylphenidate, a dose of 12.5 mg worked for 4 hours. The maximum recommended dosage of mixed salts of amphetamine is 40 mg/d in divided doses.
Stimulant medications are well-tolerated. Addiction and the need for increased dosages can occur over long-term use (months to years). Reducing the dosage or switching from methylphenidate to an amphetamine variant can usually prevent these problems.
The FDA recently approved a single-enantiomer form of methylphenidate. It contains only the active “d” enantiomer, whereas the racemic mixture contains both the “d” and “l” enantiomers. Because the “l” enantiomer is inert, the resulting medication is more potent and may be prescribed at half the dosage of the racemic mixture.
Pemoline, a once-daily stimulant, is considered a second-line treatment because of reports of hepatic failure in some patients. Its use requires written informed consent and liver function tests at baseline and every 2 weeks. In a controlled trial, pemoline at high dosages (120 to 160 mg/d) was found moderately effective in adults with ADHD.6
Newer options: Longer-acting stimulants
Newer forms of slow-release methylphenidate and mixed amphetamine salts with sophisticated delivery systems are available.
Metadate CD is delivered in capsules containing beads with polymer coatings that dissolve and release their contents at different times. The capsules contain a 30:70 ratio of immediate- and extended-release beads.
Metadate CD has not been tested for adults in controlled clinical trials. In children ages 6 to 15, a single morning dose has been shown to be clinically effective in the morning and afternoon. A supplemental immediate-release capsule can be given in the morning if a patient’s medication levels need to be increased quickly. Dosage supplementation may also be required later in the day.
Concerta is delivered in 18-mg and 36-mg tablets. The immediate-release coating on the tablets delivers medication within the first hour. The drug inside then dissolves in the GI tract and is released at a controlled rate by osmotic pressure. The indigestible tablet is passed in the stool.
Concerta was investigated in children ages 6 to 12 and provides 10 to 12 hours of sustained medication. From child studies, we know that when a patient takes a 36-mg tablet at 6 AM, blood levels decline in late afternoon. An 18-mg dose at noon covers the 4 to 6 hours needed for evening chores.
Adderall XR is an extended-release, once-daily form of mixed amphetamine salts. No controlled trials of this formulation are available in adults with ADHD. Its efficacy was established after two clinical trials of children aged 6 to 12 who met DSM-IV criteria for ADHD.
Individualized and flexible dosing improves symptom control and compliance when treating adults with ADHD. For some patients, once-daily dosing is more convenient than multiple doses, while others prefer the immediate-release form because they like its midday “pause” and bid dosing. The immediate-release tablet allows the flexibility of bid or tid dosing, depending on the day’s requirements.
Antidepressants: Another choice
Antidepressants are usually considered second-line treatment for ADHD because of concerns about efficacy and side effects. The few available studies show antidepressants work as well as stimulants but more slowly. It is good practice, therefore, to advise patients that—unlike feeling the effect of a stimulant in 60 minutes—they will not feel an effect from an antidepressant for days or weeks, and that achieving an optimal effect may take 4 to 6 weeks.
Antidepressants have several advantages over stimulants. They are not classified as narcotics, work without the on-off effects of stimulants, and can treat comorbid depression and anxiety. For adult ADHD, the most effective agents work on the catecholamine systems—norepinephrine and/or dopamine. This includes the tricyclic antidepressants, MAO inhibitors, bupropion, and venlafaxine. The serotonin reuptake inhibitors have not shown promise in ADHD, nor have mirtazapine or nefazodone demonstrated much effect.
Desipramine, a tricyclic antidepressant, is a strong inhibitor of norepinephrine reuptake. In a double-blind, controlled study in 41 adults with ADHD, 68% of patients receiving desipramine, 200 mg/d, responded positively, compared with no patients who took a placebo.7
When venlafaxine was given in standard dosages to 10 adults with ADHD in an open, 8-week clinical trial, an effect was seen by week two. Of the nine patients who completed the study, seven were considered responders. Symptoms were reduced significantly with venlafaxine treatment, and most side effects were mild.8
In an open study, bupropion treatment resulted in moderate to marked response in 74% of 19 patients. Ten of those patients who responded chose to continue bupropion rather than their previous medication.9 In a 6-week controlled study of 40 patients, bupropion use was associated with a 42% reduction in ADHD symptoms in the 38 patients who completed the study. Patients who received a placebo showed only a 24% reduction in symptoms. According to the CGI, 52% of patients who received bupropion reported being “much improved” or “very improved” compared with 11% of those receiving a placebo.10
Other treatment options that have shown mixed results include modafinil, alpha-2a agonists, acetylcholinesterase inhibitors, and the histaminergic agents.
Managing adverse effects
Substance abuse Stimulant abuse has been a concern, but it has not become the problem many feared. In fact, some studies have found that methylphenidate may help stanch the craving for cocaine in adults with ADHD.11,12 Treating ADHD with pharmacotherapy also has been shown to reduce the risk for substance abuse in adolescence by 85%.13
With careful screening, you can usually identify drug-seeking behavior in adult patients. For patients with substance abuse problems, you can prescribe the nonstimulants.
Tics that can occur with stimulant medications usually can be suppressed by reducing the dosage, being vigilant, and waiting it out. Tics may ameliorate over weeks to months.
Cardiac and cognitive effects Long-term use of stimulant medications at high dosages has been associated with cardiac and cognitive toxicity, as noted in the 1998 NIH consensus statement on diagnosis and treatment of ADHD. It is important to provide patients with this information as part of their informed-consent briefing. (See “Related resources,” to view the consensus statement.)
Nonpharmacologic management
Nonpharmacologic treatments such as EEG biofeedback; psychoeducational approaches; and individual, family, and group psychotherapy are widely used to treat adults with ADHD. Clinicians and patients often perceive these interventions as beneficial, although none have been tested in randomized, placebo-controlled studies.
Patients often function better when their home and work environments are thoughtfully organized, with a designated work/study space and regularly scheduled times for meals, sleep, and exercise. An ADHD coach may facilitate such structure and discipline (Box 2).
New agents in the pipeline
Efforts are being made to increase awareness of adult ADHD and to improve its treatment. For example, the National Institute of Mental Health is funding research on adult ADHD and displays on its Web site a PET scan of an adult brain with ADHD (see “Related resources”).14 Several medications also are being developed to treat ADHD.
Atomoxetine, a nonstimulant medication awaiting FDA approval for adult ADHD, is a selective norepinephrine reuptake inhibitor. In a double-blind, placebo-controlled, crossover study of adults with well-characterized ADHD, 11 of 21 patients improved with use of atomoxetine, compared with 2 of 21 who improved with use of a placebo. The average dosage of 76 mg/d was well-tolerated.15 The 52% response rate is similar to the 54% average improvement rate reported for methylphenidate in previous studies of adult ADHD.
In clinical trials, atomoxetine was given bid. Insomnia was not a side effect, so bid dosing does not interfere with sleep. Approximately 10 to 15% of patients experienced weight loss as a side effect.
Other treatment options under development include:
- a transdermal system for delivery of methylphenidate16
- a novel nicotinic analogue
- glutamate AMPA receptor modulation
- omega-3 fatty acids.
- Hallowell EM, Ratey JJ. Driven to distraction: Recognizing and coping with attention deficit disorder from childhood through adulthood. New York: Simon and Schuster; Reprint edition 1995.
- Solanto MV, Arnstein AFT, Castellanos FX, eds. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001.
- Weiss M, Trokenberg-Hechtman L, Weiss G. ADHD in adulthood: A guide to current theory, diagnosis, and treatment. Baltimore: Johns Hopkins University Press; 1999.
- National Institute of Mental Health. http://www.nimh.nih.gov
Drug brand names
- Atomoxetine • (investigational)
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Methamphetamine • Desoxyn
- Methylphenidate • Focalin, Ritalin
- Methylphenidate SR • Concerta, Metadate CD, Metadate ER, Methylin ER, Ritalin SR
- Mixed salts of amphetamine • Adderall, Adderall XR
- Modafinil • Provigil
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
The author reports that he serves as a consultant to Eli Lilly and Company and is on the speaker’s bureaus of Wyeth Pharmaceuticals and AstraZeneca.
1. Gadrow KD, Weiss M. Attention-deficit/hyperactivity disorder in adults: beyond controversy. Arch Gen Psychiatry 2001;58(8):784-5.
2. Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci 1998;18(15):5901-7.
3. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
4. Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001;58(8):775-82.
5. Swanson J, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 1998;34(1):55-60.
6. Wilens TE, Biederman J, Spencer TJ, et al. Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999;19(3):257-64.
7. Wilens TE, Biederman J, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention-deficit hyperactivity disorder. J Nerv Ment Dis 1995;183(1):48-50.
8. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57(5):184-9.
9. Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry 1990;147(8):1018-20.
10. Wilens TE, Spencer TJ, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
11. Grabowski J, Roache JD, Schmitz JM, Rhoades H, et al. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 1997;17(6):485-8.
12. Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry 1998;59(6):300-5.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104(2):e20.-
14. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990;323(20):1361-6.
15. Spencer T, Biederman J, Wilens T. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155(5):693-5.
16. Noven Pharmaceuticals. The Science of Noven. Research and development. Transdermal technology. Available at: http://www.noven.com/research.htm.
1. Gadrow KD, Weiss M. Attention-deficit/hyperactivity disorder in adults: beyond controversy. Arch Gen Psychiatry 2001;58(8):784-5.
2. Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci 1998;18(15):5901-7.
3. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
4. Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001;58(8):775-82.
5. Swanson J, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 1998;34(1):55-60.
6. Wilens TE, Biederman J, Spencer TJ, et al. Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999;19(3):257-64.
7. Wilens TE, Biederman J, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention-deficit hyperactivity disorder. J Nerv Ment Dis 1995;183(1):48-50.
8. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57(5):184-9.
9. Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry 1990;147(8):1018-20.
10. Wilens TE, Spencer TJ, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
11. Grabowski J, Roache JD, Schmitz JM, Rhoades H, et al. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 1997;17(6):485-8.
12. Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry 1998;59(6):300-5.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104(2):e20.-
14. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990;323(20):1361-6.
15. Spencer T, Biederman J, Wilens T. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155(5):693-5.
16. Noven Pharmaceuticals. The Science of Noven. Research and development. Transdermal technology. Available at: http://www.noven.com/research.htm.
Adult ADHD: Less hyperactivity, but lingering inattention and distress
Attention-deficit/hyperactivity disorder (ADHD) may be the only mental disorder that was discovered in children and later acknowledged in adults. Although controlled studies of adults with ADHD are few, we know that ADHD is common in adults, it can be diagnosed reliably, and 75% of those treated respond to treatment.1
The hallmark symptom of ADHD in children—hyperactivity—is usually attenuated in adults. In fact, some adults prefer the term ADD to ADHD because they are not hyperactive. This may be especially true of women, as their attention problems during childhood often were not recognized as ADHD (Box 1).
In childhood, girls with ADHD typically present with attention problems and over-talkativeness, rather than hyperactivity. Talking too much does not disrupt the classroom as much as the larger-scale misbehavior of boys with ADHD, so the diagnosis is often missed in these girls. Overtalkativeness was added to the DSM-III-R criteria for ADHD in 1987, after it was recognized as a symptom of overactivity.
Now in midlife, many women with undiagnosed ADHD have children with ADHD. As they bring their children to treatment, these women are recognizing similar attention deficit symptoms from their own childhoods and are getting the help they need. As adults, many have low self-esteem, low energy, and weight problems. Among adults with ADHD, these women may be the most underdiagnosed.
Characteristics of adult ADHD
Adults with ADHD visit a psychiatrist for a variety of reasons. Often they are parents of children diagnosed with ADHD, and the possibility that they are similarly affected has arisen during their children’s evaluation and treatment. Sometimes they have recognized themselves in consumer articles about ADHD, or others have seen them in this light.
Adults with ADHD continue to experience their childhood difficulties in sustaining attention, listening, following instructions, and organizing tasks; inattention to details; lack of sustained mental effort; losing things; distractibility, and forgetfulness. Typical complaints include underachievement and poor adjustment at work or home. Comorbid ADHD may also be identified in patients who present with depression, anxiety, substance misuse, and mood swings.
The cognitive impairment of ADHD continues into adulthood, even in adults without hyperactive symptoms. It may be that adults are not hyperactive because the basal ganglia, which control motor activity in the brain, have over the years accommodated the problem through behavior modification or neurodevelopmental changes in late adolescence.2
Children with ADHD have abnormal cerebrospinal fluid (CSF) and blood levels of the dopaminergic metabolite homovanillic acid (HVA), but adults with ADHD may not. The primary origin for CSF HVA is the nigrostriatum, which suggests that subcortical dopaminergic nuclei are more often affected in children than adults.2 This may mean that compensatory changes occur as persons with ADHD mature, or perhaps the forms of ADHD that persist into adulthood have a different pathology or pathophysiology.
Comorbidities with ADHD
Rarely does one see pure ADHD; comorbidity is the rule. ADHD can be diagnosed quickly if you know what to look for. But a facile diagnosis may overlook a comorbidity that must be treated first—especially if you plan to use stimulants. Many patients with ADHD also have bipolar disorder, and a smaller proportion of patients with bipolar disorder have undetected ADHD. Placing a patient with undetected bipolar disorder on a stimulant could precipitate mania.
Table 1
COMMON COMORBIDITIES WITH ADHD
| Bipolar disorder |
| Anxiety disorder |
| Depression |
| Drug dependence |
| Personality disorders |
| Somatoform disorders |
| Tourette’s disorder |
| Obsessive-compulsive disorder |
| Intermittent explosive disorder |
| Impulse control problems |
| Addictive behaviors |
| Sexual problems |
| Compulsive gambling |
| Learning disabilities |
| Asperger’s syndrome |
From the initial assessment, your treatment plan must address comorbid conditions (Table 1). This means taking a good history that includes corroborating information from relatives and data from the past, if possible. The case will then be much easier to manage, and quality of care greatly enhanced.
Stimulants: Usual first-choice therapy
In most cases, adult ADHD responds well to stimulant medications, although most available evidence is limited to studies in children. Several nonstimulant medications are also available, and the FDA is considering a new-drug application for a medication indicated for adult ADHD. Stimulants produce significant improvement in 30% of patients and mixed results in another 40%. Comorbidities may account for the 10 to 30% of patients who do not respond to stimulant therapy.
Methylphenidate, taken multiple times daily, is the most common treatment for ADHD. Dextroamphetamine and mixed salts of amphetamine also are used (Table 2).3 Patients usually respond to either methylphenidate or an amphetamine, and typically 25% of those who do not respond to one will respond to the other. When the clinical efficacy of amphetamines diminishes over time, many psychiatrists rotate medications. Replacing one amphetamine with another often eliminates the need to slowly increase the dosage and allows the clinician to maintain a relatively stable regimen.
When administering stimulants to adults, consider the individual’s total dosage requirement and daily schedule. Will he or she fare better with multiple daily dosing or a sustained-release form? How long is his or her average day? Does the patient have to be alert for 12 hours—or longer?
Some patients cannot sleep unless they take their last stimulant dose at bedtime. Others will have insomnia if a last dose is taken too late in the afternoon, especially with a sustained-release formulation.
When starting a patient on stimulants, begin with a 12-hour day and titrate the dosage—usually up, sometimes down—depending on response and side effects. Educating patients about their medications enables them to participate in decision-making.
Common side effects of stimulants include insomnia, decreased appetite, upset stomach, headache, anxiety, agitation, and increased pulse rate and blood pressure. The increase in blood pressure is usually less than 10%, but patients with poorly controlled hypertension should not be treated with stimulants until their blood pressure is well controlled. Until more is known about long-term effects, periodic assessment of blood pressure may be warranted.
Table 2
STIMULANT THERAPY FOR ADULTS WITH ADHD
| Stimulants | Starting dosage | Titration rate | Usual dosing interval | Maximum dosage in adults | |
|---|---|---|---|---|---|
| Methylphenidate | |||||
| Short-acting | |||||
| d, l-methylphenidate (Ritalin, Methylin) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 3 to 4 hours Usually bid-tid | Average oral dosage 0.92 mg/kg/d; best response to 1.0 mg/kg/d16 | |
| Intermediate-acting | |||||
| d, l-methylphenidate (Ritalin SR, Metadate ER, Methylin ER) | 20 mg Ritalin SR; 10 mg Methylin ER or Metadate ER | 10 to 20 mg per week | qd to bid | ||
| d-methylphenidate (Focalin) | 2.5 mg bid | 2.5 to 5 mg per week | bid, at least 4 hours apart | ||
| Long-acting | |||||
| d, l-methylphenidate (Concerta) | 18 mg qd | 18 mg every 3 to 5 days | 12+ hours, usually qd | ||
| d, l-methylphenidate (Metadate CD) | 20 mg qd | 20 mg per week | qd | ||
| Amphetamine | |||||
| Short-acting | |||||
| (Dexedrine, Dextrostat) | 2.5 to 5 mg qd | 2.5 to 5 mg every 3 to 5 days | Every 4 to 6 hours Usually bid-tid | ||
| Intermediate-acting | |||||
| Mixed salts (Adderall) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 4 to 6 hours Usually qd to bid | Average dosage 54 mg/d divided in two doses; maximum 30 mg bid | |
| (Dexedrine Spansule) | 5 or 10 mg qd | 5 mg per week | qd | ||
| Long-acting | |||||
| (Adderall XR) | 10 mg qd | 10 mg per week | qd | ||
| Stimulant | |||||
| Pemoline (Cylert) | 37.5 mg qd | 18.75 mg per week | qd; typical range 56.25 to 75 mg qd | Maximum dosage 112.5 mg/d | |
- Organized and orderly home and working environment
- Designated work/study space at home
- Designated coach to supervise work/study
- Healthy meals at regularly scheduled times
- Regular exercise
Adults with ADHD have been treated with mixed amphetamine salts with positive results. In a 7-week controlled, crossover study, 27 adults with ADHD received an average of 54 mg/d administered in two doses. Symptoms improved significantly—a 42% decrease on the ADHD Rating Scale. The medication was well-tolerated, and 70% of those receiving mixed amphetamine salts improved, compared with 7% of those who received a placebo.4
Duration of action of mixed salts of amphetamine has been measured at 3.5 hours with a 5-mg dose and 6.4 hours with a 20-mg dose.5 With methylphenidate, a dose of 12.5 mg worked for 4 hours. The maximum recommended dosage of mixed salts of amphetamine is 40 mg/d in divided doses.
Stimulant medications are well-tolerated. Addiction and the need for increased dosages can occur over long-term use (months to years). Reducing the dosage or switching from methylphenidate to an amphetamine variant can usually prevent these problems.
The FDA recently approved a single-enantiomer form of methylphenidate. It contains only the active “d” enantiomer, whereas the racemic mixture contains both the “d” and “l” enantiomers. Because the “l” enantiomer is inert, the resulting medication is more potent and may be prescribed at half the dosage of the racemic mixture.
Pemoline, a once-daily stimulant, is considered a second-line treatment because of reports of hepatic failure in some patients. Its use requires written informed consent and liver function tests at baseline and every 2 weeks. In a controlled trial, pemoline at high dosages (120 to 160 mg/d) was found moderately effective in adults with ADHD.6
Newer options: Longer-acting stimulants
Newer forms of slow-release methylphenidate and mixed amphetamine salts with sophisticated delivery systems are available.
Metadate CD is delivered in capsules containing beads with polymer coatings that dissolve and release their contents at different times. The capsules contain a 30:70 ratio of immediate- and extended-release beads.
Metadate CD has not been tested for adults in controlled clinical trials. In children ages 6 to 15, a single morning dose has been shown to be clinically effective in the morning and afternoon. A supplemental immediate-release capsule can be given in the morning if a patient’s medication levels need to be increased quickly. Dosage supplementation may also be required later in the day.
Concerta is delivered in 18-mg and 36-mg tablets. The immediate-release coating on the tablets delivers medication within the first hour. The drug inside then dissolves in the GI tract and is released at a controlled rate by osmotic pressure. The indigestible tablet is passed in the stool.
Concerta was investigated in children ages 6 to 12 and provides 10 to 12 hours of sustained medication. From child studies, we know that when a patient takes a 36-mg tablet at 6 AM, blood levels decline in late afternoon. An 18-mg dose at noon covers the 4 to 6 hours needed for evening chores.
Adderall XR is an extended-release, once-daily form of mixed amphetamine salts. No controlled trials of this formulation are available in adults with ADHD. Its efficacy was established after two clinical trials of children aged 6 to 12 who met DSM-IV criteria for ADHD.
Individualized and flexible dosing improves symptom control and compliance when treating adults with ADHD. For some patients, once-daily dosing is more convenient than multiple doses, while others prefer the immediate-release form because they like its midday “pause” and bid dosing. The immediate-release tablet allows the flexibility of bid or tid dosing, depending on the day’s requirements.
Antidepressants: Another choice
Antidepressants are usually considered second-line treatment for ADHD because of concerns about efficacy and side effects. The few available studies show antidepressants work as well as stimulants but more slowly. It is good practice, therefore, to advise patients that—unlike feeling the effect of a stimulant in 60 minutes—they will not feel an effect from an antidepressant for days or weeks, and that achieving an optimal effect may take 4 to 6 weeks.
Antidepressants have several advantages over stimulants. They are not classified as narcotics, work without the on-off effects of stimulants, and can treat comorbid depression and anxiety. For adult ADHD, the most effective agents work on the catecholamine systems—norepinephrine and/or dopamine. This includes the tricyclic antidepressants, MAO inhibitors, bupropion, and venlafaxine. The serotonin reuptake inhibitors have not shown promise in ADHD, nor have mirtazapine or nefazodone demonstrated much effect.
Desipramine, a tricyclic antidepressant, is a strong inhibitor of norepinephrine reuptake. In a double-blind, controlled study in 41 adults with ADHD, 68% of patients receiving desipramine, 200 mg/d, responded positively, compared with no patients who took a placebo.7
When venlafaxine was given in standard dosages to 10 adults with ADHD in an open, 8-week clinical trial, an effect was seen by week two. Of the nine patients who completed the study, seven were considered responders. Symptoms were reduced significantly with venlafaxine treatment, and most side effects were mild.8
In an open study, bupropion treatment resulted in moderate to marked response in 74% of 19 patients. Ten of those patients who responded chose to continue bupropion rather than their previous medication.9 In a 6-week controlled study of 40 patients, bupropion use was associated with a 42% reduction in ADHD symptoms in the 38 patients who completed the study. Patients who received a placebo showed only a 24% reduction in symptoms. According to the CGI, 52% of patients who received bupropion reported being “much improved” or “very improved” compared with 11% of those receiving a placebo.10
Other treatment options that have shown mixed results include modafinil, alpha-2a agonists, acetylcholinesterase inhibitors, and the histaminergic agents.
Managing adverse effects
Substance abuse Stimulant abuse has been a concern, but it has not become the problem many feared. In fact, some studies have found that methylphenidate may help stanch the craving for cocaine in adults with ADHD.11,12 Treating ADHD with pharmacotherapy also has been shown to reduce the risk for substance abuse in adolescence by 85%.13
With careful screening, you can usually identify drug-seeking behavior in adult patients. For patients with substance abuse problems, you can prescribe the nonstimulants.
Tics that can occur with stimulant medications usually can be suppressed by reducing the dosage, being vigilant, and waiting it out. Tics may ameliorate over weeks to months.
Cardiac and cognitive effects Long-term use of stimulant medications at high dosages has been associated with cardiac and cognitive toxicity, as noted in the 1998 NIH consensus statement on diagnosis and treatment of ADHD. It is important to provide patients with this information as part of their informed-consent briefing. (See “Related resources,” to view the consensus statement.)
Nonpharmacologic management
Nonpharmacologic treatments such as EEG biofeedback; psychoeducational approaches; and individual, family, and group psychotherapy are widely used to treat adults with ADHD. Clinicians and patients often perceive these interventions as beneficial, although none have been tested in randomized, placebo-controlled studies.
Patients often function better when their home and work environments are thoughtfully organized, with a designated work/study space and regularly scheduled times for meals, sleep, and exercise. An ADHD coach may facilitate such structure and discipline (Box 2).
New agents in the pipeline
Efforts are being made to increase awareness of adult ADHD and to improve its treatment. For example, the National Institute of Mental Health is funding research on adult ADHD and displays on its Web site a PET scan of an adult brain with ADHD (see “Related resources”).14 Several medications also are being developed to treat ADHD.
Atomoxetine, a nonstimulant medication awaiting FDA approval for adult ADHD, is a selective norepinephrine reuptake inhibitor. In a double-blind, placebo-controlled, crossover study of adults with well-characterized ADHD, 11 of 21 patients improved with use of atomoxetine, compared with 2 of 21 who improved with use of a placebo. The average dosage of 76 mg/d was well-tolerated.15 The 52% response rate is similar to the 54% average improvement rate reported for methylphenidate in previous studies of adult ADHD.
In clinical trials, atomoxetine was given bid. Insomnia was not a side effect, so bid dosing does not interfere with sleep. Approximately 10 to 15% of patients experienced weight loss as a side effect.
Other treatment options under development include:
- a transdermal system for delivery of methylphenidate16
- a novel nicotinic analogue
- glutamate AMPA receptor modulation
- omega-3 fatty acids.
- Hallowell EM, Ratey JJ. Driven to distraction: Recognizing and coping with attention deficit disorder from childhood through adulthood. New York: Simon and Schuster; Reprint edition 1995.
- Solanto MV, Arnstein AFT, Castellanos FX, eds. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001.
- Weiss M, Trokenberg-Hechtman L, Weiss G. ADHD in adulthood: A guide to current theory, diagnosis, and treatment. Baltimore: Johns Hopkins University Press; 1999.
- National Institute of Mental Health. http://www.nimh.nih.gov
Drug brand names
- Atomoxetine • (investigational)
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Methamphetamine • Desoxyn
- Methylphenidate • Focalin, Ritalin
- Methylphenidate SR • Concerta, Metadate CD, Metadate ER, Methylin ER, Ritalin SR
- Mixed salts of amphetamine • Adderall, Adderall XR
- Modafinil • Provigil
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
The author reports that he serves as a consultant to Eli Lilly and Company and is on the speaker’s bureaus of Wyeth Pharmaceuticals and AstraZeneca.
1. Gadrow KD, Weiss M. Attention-deficit/hyperactivity disorder in adults: beyond controversy. Arch Gen Psychiatry 2001;58(8):784-5.
2. Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci 1998;18(15):5901-7.
3. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
4. Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001;58(8):775-82.
5. Swanson J, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 1998;34(1):55-60.
6. Wilens TE, Biederman J, Spencer TJ, et al. Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999;19(3):257-64.
7. Wilens TE, Biederman J, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention-deficit hyperactivity disorder. J Nerv Ment Dis 1995;183(1):48-50.
8. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57(5):184-9.
9. Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry 1990;147(8):1018-20.
10. Wilens TE, Spencer TJ, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
11. Grabowski J, Roache JD, Schmitz JM, Rhoades H, et al. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 1997;17(6):485-8.
12. Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry 1998;59(6):300-5.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104(2):e20.-
14. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990;323(20):1361-6.
15. Spencer T, Biederman J, Wilens T. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155(5):693-5.
16. Noven Pharmaceuticals. The Science of Noven. Research and development. Transdermal technology. Available at: http://www.noven.com/research.htm.
Attention-deficit/hyperactivity disorder (ADHD) may be the only mental disorder that was discovered in children and later acknowledged in adults. Although controlled studies of adults with ADHD are few, we know that ADHD is common in adults, it can be diagnosed reliably, and 75% of those treated respond to treatment.1
The hallmark symptom of ADHD in children—hyperactivity—is usually attenuated in adults. In fact, some adults prefer the term ADD to ADHD because they are not hyperactive. This may be especially true of women, as their attention problems during childhood often were not recognized as ADHD (Box 1).
In childhood, girls with ADHD typically present with attention problems and over-talkativeness, rather than hyperactivity. Talking too much does not disrupt the classroom as much as the larger-scale misbehavior of boys with ADHD, so the diagnosis is often missed in these girls. Overtalkativeness was added to the DSM-III-R criteria for ADHD in 1987, after it was recognized as a symptom of overactivity.
Now in midlife, many women with undiagnosed ADHD have children with ADHD. As they bring their children to treatment, these women are recognizing similar attention deficit symptoms from their own childhoods and are getting the help they need. As adults, many have low self-esteem, low energy, and weight problems. Among adults with ADHD, these women may be the most underdiagnosed.
Characteristics of adult ADHD
Adults with ADHD visit a psychiatrist for a variety of reasons. Often they are parents of children diagnosed with ADHD, and the possibility that they are similarly affected has arisen during their children’s evaluation and treatment. Sometimes they have recognized themselves in consumer articles about ADHD, or others have seen them in this light.
Adults with ADHD continue to experience their childhood difficulties in sustaining attention, listening, following instructions, and organizing tasks; inattention to details; lack of sustained mental effort; losing things; distractibility, and forgetfulness. Typical complaints include underachievement and poor adjustment at work or home. Comorbid ADHD may also be identified in patients who present with depression, anxiety, substance misuse, and mood swings.
The cognitive impairment of ADHD continues into adulthood, even in adults without hyperactive symptoms. It may be that adults are not hyperactive because the basal ganglia, which control motor activity in the brain, have over the years accommodated the problem through behavior modification or neurodevelopmental changes in late adolescence.2
Children with ADHD have abnormal cerebrospinal fluid (CSF) and blood levels of the dopaminergic metabolite homovanillic acid (HVA), but adults with ADHD may not. The primary origin for CSF HVA is the nigrostriatum, which suggests that subcortical dopaminergic nuclei are more often affected in children than adults.2 This may mean that compensatory changes occur as persons with ADHD mature, or perhaps the forms of ADHD that persist into adulthood have a different pathology or pathophysiology.
Comorbidities with ADHD
Rarely does one see pure ADHD; comorbidity is the rule. ADHD can be diagnosed quickly if you know what to look for. But a facile diagnosis may overlook a comorbidity that must be treated first—especially if you plan to use stimulants. Many patients with ADHD also have bipolar disorder, and a smaller proportion of patients with bipolar disorder have undetected ADHD. Placing a patient with undetected bipolar disorder on a stimulant could precipitate mania.
Table 1
COMMON COMORBIDITIES WITH ADHD
| Bipolar disorder |
| Anxiety disorder |
| Depression |
| Drug dependence |
| Personality disorders |
| Somatoform disorders |
| Tourette’s disorder |
| Obsessive-compulsive disorder |
| Intermittent explosive disorder |
| Impulse control problems |
| Addictive behaviors |
| Sexual problems |
| Compulsive gambling |
| Learning disabilities |
| Asperger’s syndrome |
From the initial assessment, your treatment plan must address comorbid conditions (Table 1). This means taking a good history that includes corroborating information from relatives and data from the past, if possible. The case will then be much easier to manage, and quality of care greatly enhanced.
Stimulants: Usual first-choice therapy
In most cases, adult ADHD responds well to stimulant medications, although most available evidence is limited to studies in children. Several nonstimulant medications are also available, and the FDA is considering a new-drug application for a medication indicated for adult ADHD. Stimulants produce significant improvement in 30% of patients and mixed results in another 40%. Comorbidities may account for the 10 to 30% of patients who do not respond to stimulant therapy.
Methylphenidate, taken multiple times daily, is the most common treatment for ADHD. Dextroamphetamine and mixed salts of amphetamine also are used (Table 2).3 Patients usually respond to either methylphenidate or an amphetamine, and typically 25% of those who do not respond to one will respond to the other. When the clinical efficacy of amphetamines diminishes over time, many psychiatrists rotate medications. Replacing one amphetamine with another often eliminates the need to slowly increase the dosage and allows the clinician to maintain a relatively stable regimen.
When administering stimulants to adults, consider the individual’s total dosage requirement and daily schedule. Will he or she fare better with multiple daily dosing or a sustained-release form? How long is his or her average day? Does the patient have to be alert for 12 hours—or longer?
Some patients cannot sleep unless they take their last stimulant dose at bedtime. Others will have insomnia if a last dose is taken too late in the afternoon, especially with a sustained-release formulation.
When starting a patient on stimulants, begin with a 12-hour day and titrate the dosage—usually up, sometimes down—depending on response and side effects. Educating patients about their medications enables them to participate in decision-making.
Common side effects of stimulants include insomnia, decreased appetite, upset stomach, headache, anxiety, agitation, and increased pulse rate and blood pressure. The increase in blood pressure is usually less than 10%, but patients with poorly controlled hypertension should not be treated with stimulants until their blood pressure is well controlled. Until more is known about long-term effects, periodic assessment of blood pressure may be warranted.
Table 2
STIMULANT THERAPY FOR ADULTS WITH ADHD
| Stimulants | Starting dosage | Titration rate | Usual dosing interval | Maximum dosage in adults | |
|---|---|---|---|---|---|
| Methylphenidate | |||||
| Short-acting | |||||
| d, l-methylphenidate (Ritalin, Methylin) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 3 to 4 hours Usually bid-tid | Average oral dosage 0.92 mg/kg/d; best response to 1.0 mg/kg/d16 | |
| Intermediate-acting | |||||
| d, l-methylphenidate (Ritalin SR, Metadate ER, Methylin ER) | 20 mg Ritalin SR; 10 mg Methylin ER or Metadate ER | 10 to 20 mg per week | qd to bid | ||
| d-methylphenidate (Focalin) | 2.5 mg bid | 2.5 to 5 mg per week | bid, at least 4 hours apart | ||
| Long-acting | |||||
| d, l-methylphenidate (Concerta) | 18 mg qd | 18 mg every 3 to 5 days | 12+ hours, usually qd | ||
| d, l-methylphenidate (Metadate CD) | 20 mg qd | 20 mg per week | qd | ||
| Amphetamine | |||||
| Short-acting | |||||
| (Dexedrine, Dextrostat) | 2.5 to 5 mg qd | 2.5 to 5 mg every 3 to 5 days | Every 4 to 6 hours Usually bid-tid | ||
| Intermediate-acting | |||||
| Mixed salts (Adderall) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 4 to 6 hours Usually qd to bid | Average dosage 54 mg/d divided in two doses; maximum 30 mg bid | |
| (Dexedrine Spansule) | 5 or 10 mg qd | 5 mg per week | qd | ||
| Long-acting | |||||
| (Adderall XR) | 10 mg qd | 10 mg per week | qd | ||
| Stimulant | |||||
| Pemoline (Cylert) | 37.5 mg qd | 18.75 mg per week | qd; typical range 56.25 to 75 mg qd | Maximum dosage 112.5 mg/d | |
- Organized and orderly home and working environment
- Designated work/study space at home
- Designated coach to supervise work/study
- Healthy meals at regularly scheduled times
- Regular exercise
Adults with ADHD have been treated with mixed amphetamine salts with positive results. In a 7-week controlled, crossover study, 27 adults with ADHD received an average of 54 mg/d administered in two doses. Symptoms improved significantly—a 42% decrease on the ADHD Rating Scale. The medication was well-tolerated, and 70% of those receiving mixed amphetamine salts improved, compared with 7% of those who received a placebo.4
Duration of action of mixed salts of amphetamine has been measured at 3.5 hours with a 5-mg dose and 6.4 hours with a 20-mg dose.5 With methylphenidate, a dose of 12.5 mg worked for 4 hours. The maximum recommended dosage of mixed salts of amphetamine is 40 mg/d in divided doses.
Stimulant medications are well-tolerated. Addiction and the need for increased dosages can occur over long-term use (months to years). Reducing the dosage or switching from methylphenidate to an amphetamine variant can usually prevent these problems.
The FDA recently approved a single-enantiomer form of methylphenidate. It contains only the active “d” enantiomer, whereas the racemic mixture contains both the “d” and “l” enantiomers. Because the “l” enantiomer is inert, the resulting medication is more potent and may be prescribed at half the dosage of the racemic mixture.
Pemoline, a once-daily stimulant, is considered a second-line treatment because of reports of hepatic failure in some patients. Its use requires written informed consent and liver function tests at baseline and every 2 weeks. In a controlled trial, pemoline at high dosages (120 to 160 mg/d) was found moderately effective in adults with ADHD.6
Newer options: Longer-acting stimulants
Newer forms of slow-release methylphenidate and mixed amphetamine salts with sophisticated delivery systems are available.
Metadate CD is delivered in capsules containing beads with polymer coatings that dissolve and release their contents at different times. The capsules contain a 30:70 ratio of immediate- and extended-release beads.
Metadate CD has not been tested for adults in controlled clinical trials. In children ages 6 to 15, a single morning dose has been shown to be clinically effective in the morning and afternoon. A supplemental immediate-release capsule can be given in the morning if a patient’s medication levels need to be increased quickly. Dosage supplementation may also be required later in the day.
Concerta is delivered in 18-mg and 36-mg tablets. The immediate-release coating on the tablets delivers medication within the first hour. The drug inside then dissolves in the GI tract and is released at a controlled rate by osmotic pressure. The indigestible tablet is passed in the stool.
Concerta was investigated in children ages 6 to 12 and provides 10 to 12 hours of sustained medication. From child studies, we know that when a patient takes a 36-mg tablet at 6 AM, blood levels decline in late afternoon. An 18-mg dose at noon covers the 4 to 6 hours needed for evening chores.
Adderall XR is an extended-release, once-daily form of mixed amphetamine salts. No controlled trials of this formulation are available in adults with ADHD. Its efficacy was established after two clinical trials of children aged 6 to 12 who met DSM-IV criteria for ADHD.
Individualized and flexible dosing improves symptom control and compliance when treating adults with ADHD. For some patients, once-daily dosing is more convenient than multiple doses, while others prefer the immediate-release form because they like its midday “pause” and bid dosing. The immediate-release tablet allows the flexibility of bid or tid dosing, depending on the day’s requirements.
Antidepressants: Another choice
Antidepressants are usually considered second-line treatment for ADHD because of concerns about efficacy and side effects. The few available studies show antidepressants work as well as stimulants but more slowly. It is good practice, therefore, to advise patients that—unlike feeling the effect of a stimulant in 60 minutes—they will not feel an effect from an antidepressant for days or weeks, and that achieving an optimal effect may take 4 to 6 weeks.
Antidepressants have several advantages over stimulants. They are not classified as narcotics, work without the on-off effects of stimulants, and can treat comorbid depression and anxiety. For adult ADHD, the most effective agents work on the catecholamine systems—norepinephrine and/or dopamine. This includes the tricyclic antidepressants, MAO inhibitors, bupropion, and venlafaxine. The serotonin reuptake inhibitors have not shown promise in ADHD, nor have mirtazapine or nefazodone demonstrated much effect.
Desipramine, a tricyclic antidepressant, is a strong inhibitor of norepinephrine reuptake. In a double-blind, controlled study in 41 adults with ADHD, 68% of patients receiving desipramine, 200 mg/d, responded positively, compared with no patients who took a placebo.7
When venlafaxine was given in standard dosages to 10 adults with ADHD in an open, 8-week clinical trial, an effect was seen by week two. Of the nine patients who completed the study, seven were considered responders. Symptoms were reduced significantly with venlafaxine treatment, and most side effects were mild.8
In an open study, bupropion treatment resulted in moderate to marked response in 74% of 19 patients. Ten of those patients who responded chose to continue bupropion rather than their previous medication.9 In a 6-week controlled study of 40 patients, bupropion use was associated with a 42% reduction in ADHD symptoms in the 38 patients who completed the study. Patients who received a placebo showed only a 24% reduction in symptoms. According to the CGI, 52% of patients who received bupropion reported being “much improved” or “very improved” compared with 11% of those receiving a placebo.10
Other treatment options that have shown mixed results include modafinil, alpha-2a agonists, acetylcholinesterase inhibitors, and the histaminergic agents.
Managing adverse effects
Substance abuse Stimulant abuse has been a concern, but it has not become the problem many feared. In fact, some studies have found that methylphenidate may help stanch the craving for cocaine in adults with ADHD.11,12 Treating ADHD with pharmacotherapy also has been shown to reduce the risk for substance abuse in adolescence by 85%.13
With careful screening, you can usually identify drug-seeking behavior in adult patients. For patients with substance abuse problems, you can prescribe the nonstimulants.
Tics that can occur with stimulant medications usually can be suppressed by reducing the dosage, being vigilant, and waiting it out. Tics may ameliorate over weeks to months.
Cardiac and cognitive effects Long-term use of stimulant medications at high dosages has been associated with cardiac and cognitive toxicity, as noted in the 1998 NIH consensus statement on diagnosis and treatment of ADHD. It is important to provide patients with this information as part of their informed-consent briefing. (See “Related resources,” to view the consensus statement.)
Nonpharmacologic management
Nonpharmacologic treatments such as EEG biofeedback; psychoeducational approaches; and individual, family, and group psychotherapy are widely used to treat adults with ADHD. Clinicians and patients often perceive these interventions as beneficial, although none have been tested in randomized, placebo-controlled studies.
Patients often function better when their home and work environments are thoughtfully organized, with a designated work/study space and regularly scheduled times for meals, sleep, and exercise. An ADHD coach may facilitate such structure and discipline (Box 2).
New agents in the pipeline
Efforts are being made to increase awareness of adult ADHD and to improve its treatment. For example, the National Institute of Mental Health is funding research on adult ADHD and displays on its Web site a PET scan of an adult brain with ADHD (see “Related resources”).14 Several medications also are being developed to treat ADHD.
Atomoxetine, a nonstimulant medication awaiting FDA approval for adult ADHD, is a selective norepinephrine reuptake inhibitor. In a double-blind, placebo-controlled, crossover study of adults with well-characterized ADHD, 11 of 21 patients improved with use of atomoxetine, compared with 2 of 21 who improved with use of a placebo. The average dosage of 76 mg/d was well-tolerated.15 The 52% response rate is similar to the 54% average improvement rate reported for methylphenidate in previous studies of adult ADHD.
In clinical trials, atomoxetine was given bid. Insomnia was not a side effect, so bid dosing does not interfere with sleep. Approximately 10 to 15% of patients experienced weight loss as a side effect.
Other treatment options under development include:
- a transdermal system for delivery of methylphenidate16
- a novel nicotinic analogue
- glutamate AMPA receptor modulation
- omega-3 fatty acids.
- Hallowell EM, Ratey JJ. Driven to distraction: Recognizing and coping with attention deficit disorder from childhood through adulthood. New York: Simon and Schuster; Reprint edition 1995.
- Solanto MV, Arnstein AFT, Castellanos FX, eds. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001.
- Weiss M, Trokenberg-Hechtman L, Weiss G. ADHD in adulthood: A guide to current theory, diagnosis, and treatment. Baltimore: Johns Hopkins University Press; 1999.
- National Institute of Mental Health. http://www.nimh.nih.gov
Drug brand names
- Atomoxetine • (investigational)
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Methamphetamine • Desoxyn
- Methylphenidate • Focalin, Ritalin
- Methylphenidate SR • Concerta, Metadate CD, Metadate ER, Methylin ER, Ritalin SR
- Mixed salts of amphetamine • Adderall, Adderall XR
- Modafinil • Provigil
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
The author reports that he serves as a consultant to Eli Lilly and Company and is on the speaker’s bureaus of Wyeth Pharmaceuticals and AstraZeneca.
Attention-deficit/hyperactivity disorder (ADHD) may be the only mental disorder that was discovered in children and later acknowledged in adults. Although controlled studies of adults with ADHD are few, we know that ADHD is common in adults, it can be diagnosed reliably, and 75% of those treated respond to treatment.1
The hallmark symptom of ADHD in children—hyperactivity—is usually attenuated in adults. In fact, some adults prefer the term ADD to ADHD because they are not hyperactive. This may be especially true of women, as their attention problems during childhood often were not recognized as ADHD (Box 1).
In childhood, girls with ADHD typically present with attention problems and over-talkativeness, rather than hyperactivity. Talking too much does not disrupt the classroom as much as the larger-scale misbehavior of boys with ADHD, so the diagnosis is often missed in these girls. Overtalkativeness was added to the DSM-III-R criteria for ADHD in 1987, after it was recognized as a symptom of overactivity.
Now in midlife, many women with undiagnosed ADHD have children with ADHD. As they bring their children to treatment, these women are recognizing similar attention deficit symptoms from their own childhoods and are getting the help they need. As adults, many have low self-esteem, low energy, and weight problems. Among adults with ADHD, these women may be the most underdiagnosed.
Characteristics of adult ADHD
Adults with ADHD visit a psychiatrist for a variety of reasons. Often they are parents of children diagnosed with ADHD, and the possibility that they are similarly affected has arisen during their children’s evaluation and treatment. Sometimes they have recognized themselves in consumer articles about ADHD, or others have seen them in this light.
Adults with ADHD continue to experience their childhood difficulties in sustaining attention, listening, following instructions, and organizing tasks; inattention to details; lack of sustained mental effort; losing things; distractibility, and forgetfulness. Typical complaints include underachievement and poor adjustment at work or home. Comorbid ADHD may also be identified in patients who present with depression, anxiety, substance misuse, and mood swings.
The cognitive impairment of ADHD continues into adulthood, even in adults without hyperactive symptoms. It may be that adults are not hyperactive because the basal ganglia, which control motor activity in the brain, have over the years accommodated the problem through behavior modification or neurodevelopmental changes in late adolescence.2
Children with ADHD have abnormal cerebrospinal fluid (CSF) and blood levels of the dopaminergic metabolite homovanillic acid (HVA), but adults with ADHD may not. The primary origin for CSF HVA is the nigrostriatum, which suggests that subcortical dopaminergic nuclei are more often affected in children than adults.2 This may mean that compensatory changes occur as persons with ADHD mature, or perhaps the forms of ADHD that persist into adulthood have a different pathology or pathophysiology.
Comorbidities with ADHD
Rarely does one see pure ADHD; comorbidity is the rule. ADHD can be diagnosed quickly if you know what to look for. But a facile diagnosis may overlook a comorbidity that must be treated first—especially if you plan to use stimulants. Many patients with ADHD also have bipolar disorder, and a smaller proportion of patients with bipolar disorder have undetected ADHD. Placing a patient with undetected bipolar disorder on a stimulant could precipitate mania.
Table 1
COMMON COMORBIDITIES WITH ADHD
| Bipolar disorder |
| Anxiety disorder |
| Depression |
| Drug dependence |
| Personality disorders |
| Somatoform disorders |
| Tourette’s disorder |
| Obsessive-compulsive disorder |
| Intermittent explosive disorder |
| Impulse control problems |
| Addictive behaviors |
| Sexual problems |
| Compulsive gambling |
| Learning disabilities |
| Asperger’s syndrome |
From the initial assessment, your treatment plan must address comorbid conditions (Table 1). This means taking a good history that includes corroborating information from relatives and data from the past, if possible. The case will then be much easier to manage, and quality of care greatly enhanced.
Stimulants: Usual first-choice therapy
In most cases, adult ADHD responds well to stimulant medications, although most available evidence is limited to studies in children. Several nonstimulant medications are also available, and the FDA is considering a new-drug application for a medication indicated for adult ADHD. Stimulants produce significant improvement in 30% of patients and mixed results in another 40%. Comorbidities may account for the 10 to 30% of patients who do not respond to stimulant therapy.
Methylphenidate, taken multiple times daily, is the most common treatment for ADHD. Dextroamphetamine and mixed salts of amphetamine also are used (Table 2).3 Patients usually respond to either methylphenidate or an amphetamine, and typically 25% of those who do not respond to one will respond to the other. When the clinical efficacy of amphetamines diminishes over time, many psychiatrists rotate medications. Replacing one amphetamine with another often eliminates the need to slowly increase the dosage and allows the clinician to maintain a relatively stable regimen.
When administering stimulants to adults, consider the individual’s total dosage requirement and daily schedule. Will he or she fare better with multiple daily dosing or a sustained-release form? How long is his or her average day? Does the patient have to be alert for 12 hours—or longer?
Some patients cannot sleep unless they take their last stimulant dose at bedtime. Others will have insomnia if a last dose is taken too late in the afternoon, especially with a sustained-release formulation.
When starting a patient on stimulants, begin with a 12-hour day and titrate the dosage—usually up, sometimes down—depending on response and side effects. Educating patients about their medications enables them to participate in decision-making.
Common side effects of stimulants include insomnia, decreased appetite, upset stomach, headache, anxiety, agitation, and increased pulse rate and blood pressure. The increase in blood pressure is usually less than 10%, but patients with poorly controlled hypertension should not be treated with stimulants until their blood pressure is well controlled. Until more is known about long-term effects, periodic assessment of blood pressure may be warranted.
Table 2
STIMULANT THERAPY FOR ADULTS WITH ADHD
| Stimulants | Starting dosage | Titration rate | Usual dosing interval | Maximum dosage in adults | |
|---|---|---|---|---|---|
| Methylphenidate | |||||
| Short-acting | |||||
| d, l-methylphenidate (Ritalin, Methylin) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 3 to 4 hours Usually bid-tid | Average oral dosage 0.92 mg/kg/d; best response to 1.0 mg/kg/d16 | |
| Intermediate-acting | |||||
| d, l-methylphenidate (Ritalin SR, Metadate ER, Methylin ER) | 20 mg Ritalin SR; 10 mg Methylin ER or Metadate ER | 10 to 20 mg per week | qd to bid | ||
| d-methylphenidate (Focalin) | 2.5 mg bid | 2.5 to 5 mg per week | bid, at least 4 hours apart | ||
| Long-acting | |||||
| d, l-methylphenidate (Concerta) | 18 mg qd | 18 mg every 3 to 5 days | 12+ hours, usually qd | ||
| d, l-methylphenidate (Metadate CD) | 20 mg qd | 20 mg per week | qd | ||
| Amphetamine | |||||
| Short-acting | |||||
| (Dexedrine, Dextrostat) | 2.5 to 5 mg qd | 2.5 to 5 mg every 3 to 5 days | Every 4 to 6 hours Usually bid-tid | ||
| Intermediate-acting | |||||
| Mixed salts (Adderall) | 5 mg qd or 5 mg bid | 5 to 10 mg every 3 to 5 days | Every 4 to 6 hours Usually qd to bid | Average dosage 54 mg/d divided in two doses; maximum 30 mg bid | |
| (Dexedrine Spansule) | 5 or 10 mg qd | 5 mg per week | qd | ||
| Long-acting | |||||
| (Adderall XR) | 10 mg qd | 10 mg per week | qd | ||
| Stimulant | |||||
| Pemoline (Cylert) | 37.5 mg qd | 18.75 mg per week | qd; typical range 56.25 to 75 mg qd | Maximum dosage 112.5 mg/d | |
- Organized and orderly home and working environment
- Designated work/study space at home
- Designated coach to supervise work/study
- Healthy meals at regularly scheduled times
- Regular exercise
Adults with ADHD have been treated with mixed amphetamine salts with positive results. In a 7-week controlled, crossover study, 27 adults with ADHD received an average of 54 mg/d administered in two doses. Symptoms improved significantly—a 42% decrease on the ADHD Rating Scale. The medication was well-tolerated, and 70% of those receiving mixed amphetamine salts improved, compared with 7% of those who received a placebo.4
Duration of action of mixed salts of amphetamine has been measured at 3.5 hours with a 5-mg dose and 6.4 hours with a 20-mg dose.5 With methylphenidate, a dose of 12.5 mg worked for 4 hours. The maximum recommended dosage of mixed salts of amphetamine is 40 mg/d in divided doses.
Stimulant medications are well-tolerated. Addiction and the need for increased dosages can occur over long-term use (months to years). Reducing the dosage or switching from methylphenidate to an amphetamine variant can usually prevent these problems.
The FDA recently approved a single-enantiomer form of methylphenidate. It contains only the active “d” enantiomer, whereas the racemic mixture contains both the “d” and “l” enantiomers. Because the “l” enantiomer is inert, the resulting medication is more potent and may be prescribed at half the dosage of the racemic mixture.
Pemoline, a once-daily stimulant, is considered a second-line treatment because of reports of hepatic failure in some patients. Its use requires written informed consent and liver function tests at baseline and every 2 weeks. In a controlled trial, pemoline at high dosages (120 to 160 mg/d) was found moderately effective in adults with ADHD.6
Newer options: Longer-acting stimulants
Newer forms of slow-release methylphenidate and mixed amphetamine salts with sophisticated delivery systems are available.
Metadate CD is delivered in capsules containing beads with polymer coatings that dissolve and release their contents at different times. The capsules contain a 30:70 ratio of immediate- and extended-release beads.
Metadate CD has not been tested for adults in controlled clinical trials. In children ages 6 to 15, a single morning dose has been shown to be clinically effective in the morning and afternoon. A supplemental immediate-release capsule can be given in the morning if a patient’s medication levels need to be increased quickly. Dosage supplementation may also be required later in the day.
Concerta is delivered in 18-mg and 36-mg tablets. The immediate-release coating on the tablets delivers medication within the first hour. The drug inside then dissolves in the GI tract and is released at a controlled rate by osmotic pressure. The indigestible tablet is passed in the stool.
Concerta was investigated in children ages 6 to 12 and provides 10 to 12 hours of sustained medication. From child studies, we know that when a patient takes a 36-mg tablet at 6 AM, blood levels decline in late afternoon. An 18-mg dose at noon covers the 4 to 6 hours needed for evening chores.
Adderall XR is an extended-release, once-daily form of mixed amphetamine salts. No controlled trials of this formulation are available in adults with ADHD. Its efficacy was established after two clinical trials of children aged 6 to 12 who met DSM-IV criteria for ADHD.
Individualized and flexible dosing improves symptom control and compliance when treating adults with ADHD. For some patients, once-daily dosing is more convenient than multiple doses, while others prefer the immediate-release form because they like its midday “pause” and bid dosing. The immediate-release tablet allows the flexibility of bid or tid dosing, depending on the day’s requirements.
Antidepressants: Another choice
Antidepressants are usually considered second-line treatment for ADHD because of concerns about efficacy and side effects. The few available studies show antidepressants work as well as stimulants but more slowly. It is good practice, therefore, to advise patients that—unlike feeling the effect of a stimulant in 60 minutes—they will not feel an effect from an antidepressant for days or weeks, and that achieving an optimal effect may take 4 to 6 weeks.
Antidepressants have several advantages over stimulants. They are not classified as narcotics, work without the on-off effects of stimulants, and can treat comorbid depression and anxiety. For adult ADHD, the most effective agents work on the catecholamine systems—norepinephrine and/or dopamine. This includes the tricyclic antidepressants, MAO inhibitors, bupropion, and venlafaxine. The serotonin reuptake inhibitors have not shown promise in ADHD, nor have mirtazapine or nefazodone demonstrated much effect.
Desipramine, a tricyclic antidepressant, is a strong inhibitor of norepinephrine reuptake. In a double-blind, controlled study in 41 adults with ADHD, 68% of patients receiving desipramine, 200 mg/d, responded positively, compared with no patients who took a placebo.7
When venlafaxine was given in standard dosages to 10 adults with ADHD in an open, 8-week clinical trial, an effect was seen by week two. Of the nine patients who completed the study, seven were considered responders. Symptoms were reduced significantly with venlafaxine treatment, and most side effects were mild.8
In an open study, bupropion treatment resulted in moderate to marked response in 74% of 19 patients. Ten of those patients who responded chose to continue bupropion rather than their previous medication.9 In a 6-week controlled study of 40 patients, bupropion use was associated with a 42% reduction in ADHD symptoms in the 38 patients who completed the study. Patients who received a placebo showed only a 24% reduction in symptoms. According to the CGI, 52% of patients who received bupropion reported being “much improved” or “very improved” compared with 11% of those receiving a placebo.10
Other treatment options that have shown mixed results include modafinil, alpha-2a agonists, acetylcholinesterase inhibitors, and the histaminergic agents.
Managing adverse effects
Substance abuse Stimulant abuse has been a concern, but it has not become the problem many feared. In fact, some studies have found that methylphenidate may help stanch the craving for cocaine in adults with ADHD.11,12 Treating ADHD with pharmacotherapy also has been shown to reduce the risk for substance abuse in adolescence by 85%.13
With careful screening, you can usually identify drug-seeking behavior in adult patients. For patients with substance abuse problems, you can prescribe the nonstimulants.
Tics that can occur with stimulant medications usually can be suppressed by reducing the dosage, being vigilant, and waiting it out. Tics may ameliorate over weeks to months.
Cardiac and cognitive effects Long-term use of stimulant medications at high dosages has been associated with cardiac and cognitive toxicity, as noted in the 1998 NIH consensus statement on diagnosis and treatment of ADHD. It is important to provide patients with this information as part of their informed-consent briefing. (See “Related resources,” to view the consensus statement.)
Nonpharmacologic management
Nonpharmacologic treatments such as EEG biofeedback; psychoeducational approaches; and individual, family, and group psychotherapy are widely used to treat adults with ADHD. Clinicians and patients often perceive these interventions as beneficial, although none have been tested in randomized, placebo-controlled studies.
Patients often function better when their home and work environments are thoughtfully organized, with a designated work/study space and regularly scheduled times for meals, sleep, and exercise. An ADHD coach may facilitate such structure and discipline (Box 2).
New agents in the pipeline
Efforts are being made to increase awareness of adult ADHD and to improve its treatment. For example, the National Institute of Mental Health is funding research on adult ADHD and displays on its Web site a PET scan of an adult brain with ADHD (see “Related resources”).14 Several medications also are being developed to treat ADHD.
Atomoxetine, a nonstimulant medication awaiting FDA approval for adult ADHD, is a selective norepinephrine reuptake inhibitor. In a double-blind, placebo-controlled, crossover study of adults with well-characterized ADHD, 11 of 21 patients improved with use of atomoxetine, compared with 2 of 21 who improved with use of a placebo. The average dosage of 76 mg/d was well-tolerated.15 The 52% response rate is similar to the 54% average improvement rate reported for methylphenidate in previous studies of adult ADHD.
In clinical trials, atomoxetine was given bid. Insomnia was not a side effect, so bid dosing does not interfere with sleep. Approximately 10 to 15% of patients experienced weight loss as a side effect.
Other treatment options under development include:
- a transdermal system for delivery of methylphenidate16
- a novel nicotinic analogue
- glutamate AMPA receptor modulation
- omega-3 fatty acids.
- Hallowell EM, Ratey JJ. Driven to distraction: Recognizing and coping with attention deficit disorder from childhood through adulthood. New York: Simon and Schuster; Reprint edition 1995.
- Solanto MV, Arnstein AFT, Castellanos FX, eds. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001.
- Weiss M, Trokenberg-Hechtman L, Weiss G. ADHD in adulthood: A guide to current theory, diagnosis, and treatment. Baltimore: Johns Hopkins University Press; 1999.
- National Institute of Mental Health. http://www.nimh.nih.gov
Drug brand names
- Atomoxetine • (investigational)
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Methamphetamine • Desoxyn
- Methylphenidate • Focalin, Ritalin
- Methylphenidate SR • Concerta, Metadate CD, Metadate ER, Methylin ER, Ritalin SR
- Mixed salts of amphetamine • Adderall, Adderall XR
- Modafinil • Provigil
- Pemoline • Cylert
- Venlafaxine • Effexor
Disclosure
The author reports that he serves as a consultant to Eli Lilly and Company and is on the speaker’s bureaus of Wyeth Pharmaceuticals and AstraZeneca.
1. Gadrow KD, Weiss M. Attention-deficit/hyperactivity disorder in adults: beyond controversy. Arch Gen Psychiatry 2001;58(8):784-5.
2. Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci 1998;18(15):5901-7.
3. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
4. Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001;58(8):775-82.
5. Swanson J, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 1998;34(1):55-60.
6. Wilens TE, Biederman J, Spencer TJ, et al. Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999;19(3):257-64.
7. Wilens TE, Biederman J, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention-deficit hyperactivity disorder. J Nerv Ment Dis 1995;183(1):48-50.
8. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57(5):184-9.
9. Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry 1990;147(8):1018-20.
10. Wilens TE, Spencer TJ, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
11. Grabowski J, Roache JD, Schmitz JM, Rhoades H, et al. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 1997;17(6):485-8.
12. Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry 1998;59(6):300-5.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104(2):e20.-
14. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990;323(20):1361-6.
15. Spencer T, Biederman J, Wilens T. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155(5):693-5.
16. Noven Pharmaceuticals. The Science of Noven. Research and development. Transdermal technology. Available at: http://www.noven.com/research.htm.
1. Gadrow KD, Weiss M. Attention-deficit/hyperactivity disorder in adults: beyond controversy. Arch Gen Psychiatry 2001;58(8):784-5.
2. Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci 1998;18(15):5901-7.
3. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
4. Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001;58(8):775-82.
5. Swanson J, Wigal S, Greenhill L, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 1998;34(1):55-60.
6. Wilens TE, Biederman J, Spencer TJ, et al. Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999;19(3):257-64.
7. Wilens TE, Biederman J, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention-deficit hyperactivity disorder. J Nerv Ment Dis 1995;183(1):48-50.
8. Findling RL, Schwartz MA, Flannery DJ, Manos MJ. Venlafaxine in adults with attention-deficit/hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1996;57(5):184-9.
9. Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry 1990;147(8):1018-20.
10. Wilens TE, Spencer TJ, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
11. Grabowski J, Roache JD, Schmitz JM, Rhoades H, et al. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 1997;17(6):485-8.
12. Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry 1998;59(6):300-5.
13. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104(2):e20.-
14. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990;323(20):1361-6.
15. Spencer T, Biederman J, Wilens T. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155(5):693-5.
16. Noven Pharmaceuticals. The Science of Noven. Research and development. Transdermal technology. Available at: http://www.noven.com/research.htm.