User login
Guanfacine extended release for ADHD
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants
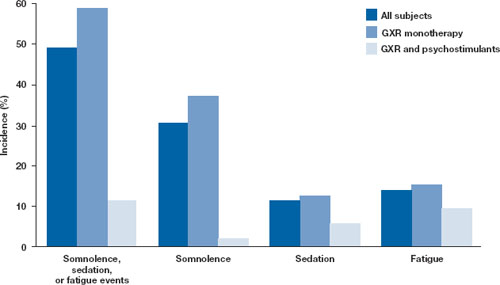
In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
What’s the best treatment for comorbid ADHD/bipolar mania?
Comorbid attention-deficit/hyperactivity disorder (ADHD) is nearly universal in youths with bipolar disorder (BPD),1 and comorbid mania has been noted in 16% of children with ADHD.2 Choosing medication for these complex patients is difficult because psychostimulants may worsen mania and mood stabilizers may not resolve ADHD symptoms. Yet, very little information exists on combining psychostimulants with mood stabilizers or atypical antipsychotics.
This article offers evidence to help you decide:
- which to treat first—ADHD or BPD
- how to individualize combination therapy.
CHALLENGES OF COMORBIDITY
Differential diagnosis. ADHD and bipolar disorder (BPD) symptoms overlap, and experts disagree on which symptoms indicate co-existing ADHD and BPD. Multiple daily mood swings and irritability are commonly found in prepubertal BPD.3 Recent reviews address differential diagnosis and specific assessment tools;3-5 after careful evaluation, then focus on treatment.
Treating comorbid ADHD and BPD usually requires more than one medication, and use of multiple drugs in children and adolescents is becoming increasingly common.6,7
PSYCHOSTIMULANTS AND MOOD STABILIZERS
Small, uncontrolled studies of children and adolescents with comorbid ADHD and BPD have shown that treatment with a mood stabilizer and a psychostimulant can control both sets of symptoms. For example:
- Lithium (serum levels 0.7 to 1.1 mEq/L) plus methylphenidate (10 to 20 mg/d) improved attention and hyperactivity symptoms more effectively than either agent alone in 7 children (6 boys, 1 girl) ages 6 to 10 hospitalized with disruptive behavioral disorders and BPD or major depression.8
- A retrospective analysis of 38 children (ages 3 to 16; 84% male) with BPD found that ADHD symptoms were 7.5 times more likely to improve if mood was stabilized before rather than after ADHD treatment with tricyclic antidepressants.9
The efficacy of combining a mood stabilizer and psychostimulant has been confirmed by only one controlled study—a randomized, placebo-controlled trial of mixed amphetamine salts in divalproex-treated patients.10 Forty patients (ages 6 to 17; 83% male) with BPD and ADHD received open-label divalproex (median dosage 750 mg/d) for 8 weeks. Thirty patients whose manic symptoms were significantly reduced entered a 4-week, double-blind, crossover trial of mixed amphetamine salts, 10 mg/d, or placebo.
Following this double-blind phase, 23 patients received open-label divalproex plus mixed amphetamine salts for 12 weeks. The Young Mania Rating Scale and Clinical Global Impression-Improvement scale were used to assess manic and ADHD symptoms during all three study phases.
Manic symptoms in patients treated with divalproex monotherapy improved significantly, but ADHD symptoms did not. ADHD symptoms improved more with divalproex plus mixed amphetamine salts than with divalproex plus placebo. One patient experienced manic symptom exacerbation with combination therapy.
PSYCHOSTIMULANTS AND ANTIPSYCHOTICS
Combinations of psychostimulants and atypical antipsychotics are commonly used in children and adolescents with comorbid psychiatric and behavioral disorders, such as ADHD and disruptive behavioral disorders (oppositional defiant disorder, conduct disorder). In 78 children ages 5 to 12 (83% male) with comorbid ADHD and a disruptive behavioral disorder, disruptive behavior and hyperactivity improved significantly with risperidone alone or with a psychostimulant.11
Combined psychostimulant/atypical antipsychotic therapy may help youths with comorbid ADHD and Tourette syndrome. Methylphenidate can reduce ADHD symptoms without exacerbating tics,12 and risperidone can treat tic disorders, even in patients with comorbid ADHD.13,14 No controlled trials have examined psychostimulant and atypical antipsychotic combinations in these patients, however.
Atypical antipsychotics have been shown to be effective in treating adult BPD, and limited data suggest the same to be true in pediatric patients. Olanzapine, quetiapine, and risperidone have been shown to reduce manic symptoms in children and adolescents (Table 1).15-17 Atypical antipsychotics, however, have been associated with metabolic side effects, including weight gain, hyperglycemia, hyperlipidemia, and hyperprolactinemia.
To date, no study has systematically evaluated combination psychostimulant and atypical antipsychotic treatment in comorbid ADHD and BPD. In the olanzapine and risperidone studies,15,17 concomitant psychostimulant use was permitted and did not affect manic symptom response.
Table 1
Atypical antipsychotic studies in pediatric bipolar disorder
| Drug and mean dosage | Study design | Sample characteristics | Efficacy measures | Results |
|---|---|---|---|---|
| Olanzapine15 9.6±4.3 mg/d | 8-week, open-label monotherapy | 23 patients, mean age 10±3 yrs, 57% male | ≥30% decrease on YMRS | Response rate 61% |
| Quetiapine16 432 mg/d | 6-week, randomized, placebo-controlled, adjunctive (+DVP) | 30 patients, mean age 14±2 yrs, 53% male | ≥50% decrease on YMRS | Response rates: DVP + placebo 53% DVP + quetiapine 87% |
| Risperidone17 1.7±1.3 mg/d | Retrospective, adjunctive | 28 patients, mean age 10±4 yrs, 97% male | ≤2 on CGI-I | Response rate 82% |
| CGI-I: Clinical Global Impressions-Improvement scale | ||||
| DVP: divalproex | ||||
| YMRS: Young Mania Rating Scale | ||||
WHICH COMBINATION?
Which combination treatment—psychostimulant plus mood stabilizer, psychostimulant plus atypical antipsychotic, or psychostimulant plus both mood stabilizer and atypical antipsychotic—is most appropriate for a child or adolescent with comorbid ADHD and BPD? Recommended treatment strategies are based on studies of pediatric and adult BPD and expert consensus.18,19
Consider the type of bipolar episode (Table 2).
For initial treatment of youths with BPD manic or mixed without psychosis, recent guidelines by Kowatch et al suggest using mood-stabilizer or atypical antipsychotic monotherapy. Youths who are more severely ill or present with psychosis may respond more favorably to a mood stabilizer plus an atypical antipsychotic.16,19
Individual patient traits will also determine whether a mood stabilizer or atypical antipsychotic is used and which agent within either medication class is chosen. For example:
- If the patient is aggressive, risperidone may reduce aggression and manic symptoms. Among the atypicals, risperidone has the most evidence suggesting efficacy for aggressive behaviors in youths across psychiatric conditions.20
- If an atypical antipsychotic is warranted and the patient’s weight is an issue, ziprasidone or aripiprazole would be preferred. These agents are considered weight-neutral compared with other atypicals.20
Other factors to consider include medication side effects, interactions, adherence, and cost.
Table 2
Mood stabilizer, atypical antipsychotic, or both with ADHD therapy?
| Type of bipolar episode | Recommended psychotropics |
|---|---|
| Manic or mixed episode with psychosis | Mood stabilizer + atypical antipsychotic |
| Manic or mixed episode without psychosis | Mood stabilizer or atypical antipsychotic monotherapy first
|
| Prominent irritability without psychosis | Atypical antipsychotic |
| Source: Adapted from references 18 and 19 | |
WHICH TO TREAT FIRST?
If the child or adolescent with comorbid ADHD and BPD has acute manic symptoms, available data and expert opinion recommend starting treatment with a mood stabilizer or atypical antipsychotic.9,19,21 If ADHD symptoms persist after mood stabilization, a psychostimulant trial is warranted.
In practice, however, youngsters usually present with ADHD symptoms first. Psychostimulant treatment is initiated, ADHD symptoms are controlled, and the child’s academic and social functioning improve. Bipolar symptoms emerge later, often heralded by a depressive or mixed episode. Is it necessary to discontinue the psychostimulant and risk worsening ADHD symptoms before starting a mood stabilizer or atypical antipsychotic?
Clinical lore and one case report suggest that psychostimulants may destabilize mood.22,23 A 10-year-old boy with severe hyperactivity and family history of BPD experienced manic symptoms—rapid and pressured speech, grandiose delusions of identity, and tangentiality of thought processes—during methylphenidate treatment.22
Conversely, an analysis24 of children ages 7 to 10 from the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD contradicts these assumptions. Although a clinical diagnosis of BPD was not assigned, 29 children (83% male) met the Diagnostic Interview Schedule for Children proxy for mania, 32 (88% male) met the Child Behavior Checklist proxy, and 7 met both proxies for mania.
The first month of methylphenidate treatment did not increase irritability, mood symptoms, or mania in the 54 children with ADHD and manic symptoms, compared with children with ADHD alone. The authors concluded that clinicians should not categorically avoid using stimulants in children with ADHD and some manic symptoms.
In a study by Pavuluri et al18 of pediatric bipolar type I disorder, 17 patients (mean age 11±4 years) received mood stabilizers—following a drug therapy algorithm that included risperidone—and typically received a psychostimulant after mood stabilization. This group was compared with 17 patients receiving “treatment as usual.”
The usual-treatment group remained on psychostimulant therapy after BPD intervention with a mood stabilizer and was less likely to receive an atypical antipsychotic. The algorithm treatment group showed better outcomes overall, specifically for mania and aggression.
Clearly, more studies are needed to determine the optimum treatment sequence with psychostimulants and mood stabilizers in youths with comorbid ADHD and BPD. With either approach, routinely monitor patients treated with psychostimulants for emerging or worsening bipolar symptoms.
LESSONS FROM CLINICAL EXPERIENCE
Nonstimulants. Using psychostimulants is appropriate for ADHD in patients with stable bipolar symptoms. Evidence for using nonstimulants such as clonidine, guanfacine, or atomoxetine is less clear.
In a naturalistic study of 153 children and adolescent outpatients treated with atomoxetine, 51 (33%) experienced irritability, aggression, mania, or hypomania. Of these patients, 31 (61%) had a family history of a mood disorder, and 41 (80%) had a personal history of mood symptoms.25 Although these findings suggest that atomoxetine may be associated with mood exacerbation and hypomania, additional data are needed to determine whether atomoxetine may be used for ADHD symptoms in youths with comorbid BPD.
Atypical antipsychotics. Mood stabilization—particularly with atypical antipsychotics—often can address comorbid disruptive behaviors and aggressive symptoms. Combinations of atypical antipsychotics with psychostimulants are largely devoid of drug-drug interactions and metabolic interference, making them uncomplicated to use.
Though published studies of pediatric BPD have focused on three atypical antipsychotics—olanzapine, quetiapine, and risperidone—any agent in this class can be used in this population, with the choice often depending on how side effects are likely to affect individual patients (Table 3 ).
Pharmacologic attributes may also determine which atypical antipsychotic is used. For example, ziprasidone’s serotonergic profile—with serotonin-1A receptor agonism and serotonin-1D antagonism—may make it useful for patients with mixed states and bipolar depression.26 Aripiprazole offers potential synergism of dopamine agonism with psychostimulant therapy, which could be useful for treating both disruptive behaviors and ADHD.
Table 3
Using atypical antipsychotics to treat comorbid ADHD/bipolar disorder
| Drug | Target dosage (mg/d) | Side effects | Useful in… |
|---|---|---|---|
| Aripiprazole | 10 to 15 | Nausea, vomiting | Comorbid disruptive behavioral disorders, maintenance stabilization |
| Olanzapine | 10 to 20 | Weight gain, hyperlipidemia, hyperglycemia, sedation | Maintenance stabilization |
| Quetiapine | 400 to 600 | Weight gain, sedation | Mixed states, bipolar depression |
| Risperidone | 1 to 2 | Weight gain, hyperprolactinemia, extrapyramidal symptoms | Comorbid disruptive behavioral disorders, including aggression |
| Ziprasidone | 80 to 120 | Cardiac abnormalities, akathisia | Mixed states, bipolar depression |
Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org
- Children and Adults with Attention-Deficit Hyperactivity Disorder (CHADD). www.chadd.org
- Findling RF, Kowatch RA, Post RM. P ediatric bipolar disorders: a handbook for clinicians. London: Martin Dunitz Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Aripiprazole • Abilify
- Divalproex • Depakote
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Patel is a consultant to Eli Lilly and Co. and a speaker for Eli Lilly and Co. and Pfizer Inc.
Dr. Sallee receives research support from Otsuka America Pharmaceutical, Pfizer Inc., and Bristol-Myers Squibb Co. and is a consultant or speaker for Eli Lilly and Co, Otsuka America Pharmaceutical, and Pfizer Inc.
1. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998;37(2):171-8.
2. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
3. Kowatch RA, DelBello MP. Pediatric bipolar disorder: mood swings, irritability are diagnostic cues. Current Psychiatry 2003;2(3):40-7.
4. Quinn CA, Fristad MA. Defining and identifying early onset bipolar spectrum disorder. Curr Psychiatry Rep 2004;6(2):101-7.
5. Bhatara VS, Feil M, Hoagwood K, et al. Trends in combined pharmacotherapy with stimulants for children. Psychiatr Serv 2002;53(3):244.-
6. Zito JM, Safer DJ, dosReis S, et al. Psychotherapeutic medication patterns for youths with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 1999;153(12):1257-63.
7. Wolf DV, Wagner KD. Bipolar disorder in children and adolescents. CNS Spectr 2003;8(12):954-9.
8. Carlson GA, Rapport MD, Kelly KL, Pataki CS. The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992;31(2):262-70.
9. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
10. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
11. Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavioral disorders, and subaverage IQ. J Child Adolesc Psychopharmacology 2004;14(2):243-54.
12. Gadow KD, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56(4):330-6.
13. Gaffney GR, Perry PJ, Lund BC, et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002;41(3):330-6.
14. Lombroso PJ, Scahill L, King RA, et al. Risperidone treatment of children and adolescents with chronic tic disorders: a preliminary report. J Am Acad Child Adolesc Psychiatry 1995;34(9):1147-52.
15. Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2001;11(3):239-50.
16. DelBello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002;41(10):1216-23.
17. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
18. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2004;43(7):859-67.
19. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44(3):213-35.
20. Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry 2004;65(suppl 6):30-44.
21. Kowatch RA, Sethuraman G, Hume JH, et al. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry 2003;53(11):978-84.
22. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
23. Craney J, Geller B. Clinical implications of antidepressant and stimulant use on switching from depression to mania in children. J Child Adolesc Psychopharmacol 2003;13(2):201-4.
24. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13(2):123-36.
25. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114(3):895-6.
26. Sallee FR, Gilbert DL, Vinks AA, et al. Pharmacodynamics of ziprasidone in children and adolescents: impact on dopamine transmission. J Am Acad Child Adolesc Psychiatry 2003;42(8):902-7.
Comorbid attention-deficit/hyperactivity disorder (ADHD) is nearly universal in youths with bipolar disorder (BPD),1 and comorbid mania has been noted in 16% of children with ADHD.2 Choosing medication for these complex patients is difficult because psychostimulants may worsen mania and mood stabilizers may not resolve ADHD symptoms. Yet, very little information exists on combining psychostimulants with mood stabilizers or atypical antipsychotics.
This article offers evidence to help you decide:
- which to treat first—ADHD or BPD
- how to individualize combination therapy.
CHALLENGES OF COMORBIDITY
Differential diagnosis. ADHD and bipolar disorder (BPD) symptoms overlap, and experts disagree on which symptoms indicate co-existing ADHD and BPD. Multiple daily mood swings and irritability are commonly found in prepubertal BPD.3 Recent reviews address differential diagnosis and specific assessment tools;3-5 after careful evaluation, then focus on treatment.
Treating comorbid ADHD and BPD usually requires more than one medication, and use of multiple drugs in children and adolescents is becoming increasingly common.6,7
PSYCHOSTIMULANTS AND MOOD STABILIZERS
Small, uncontrolled studies of children and adolescents with comorbid ADHD and BPD have shown that treatment with a mood stabilizer and a psychostimulant can control both sets of symptoms. For example:
- Lithium (serum levels 0.7 to 1.1 mEq/L) plus methylphenidate (10 to 20 mg/d) improved attention and hyperactivity symptoms more effectively than either agent alone in 7 children (6 boys, 1 girl) ages 6 to 10 hospitalized with disruptive behavioral disorders and BPD or major depression.8
- A retrospective analysis of 38 children (ages 3 to 16; 84% male) with BPD found that ADHD symptoms were 7.5 times more likely to improve if mood was stabilized before rather than after ADHD treatment with tricyclic antidepressants.9
The efficacy of combining a mood stabilizer and psychostimulant has been confirmed by only one controlled study—a randomized, placebo-controlled trial of mixed amphetamine salts in divalproex-treated patients.10 Forty patients (ages 6 to 17; 83% male) with BPD and ADHD received open-label divalproex (median dosage 750 mg/d) for 8 weeks. Thirty patients whose manic symptoms were significantly reduced entered a 4-week, double-blind, crossover trial of mixed amphetamine salts, 10 mg/d, or placebo.
Following this double-blind phase, 23 patients received open-label divalproex plus mixed amphetamine salts for 12 weeks. The Young Mania Rating Scale and Clinical Global Impression-Improvement scale were used to assess manic and ADHD symptoms during all three study phases.
Manic symptoms in patients treated with divalproex monotherapy improved significantly, but ADHD symptoms did not. ADHD symptoms improved more with divalproex plus mixed amphetamine salts than with divalproex plus placebo. One patient experienced manic symptom exacerbation with combination therapy.
PSYCHOSTIMULANTS AND ANTIPSYCHOTICS
Combinations of psychostimulants and atypical antipsychotics are commonly used in children and adolescents with comorbid psychiatric and behavioral disorders, such as ADHD and disruptive behavioral disorders (oppositional defiant disorder, conduct disorder). In 78 children ages 5 to 12 (83% male) with comorbid ADHD and a disruptive behavioral disorder, disruptive behavior and hyperactivity improved significantly with risperidone alone or with a psychostimulant.11
Combined psychostimulant/atypical antipsychotic therapy may help youths with comorbid ADHD and Tourette syndrome. Methylphenidate can reduce ADHD symptoms without exacerbating tics,12 and risperidone can treat tic disorders, even in patients with comorbid ADHD.13,14 No controlled trials have examined psychostimulant and atypical antipsychotic combinations in these patients, however.
Atypical antipsychotics have been shown to be effective in treating adult BPD, and limited data suggest the same to be true in pediatric patients. Olanzapine, quetiapine, and risperidone have been shown to reduce manic symptoms in children and adolescents (Table 1).15-17 Atypical antipsychotics, however, have been associated with metabolic side effects, including weight gain, hyperglycemia, hyperlipidemia, and hyperprolactinemia.
To date, no study has systematically evaluated combination psychostimulant and atypical antipsychotic treatment in comorbid ADHD and BPD. In the olanzapine and risperidone studies,15,17 concomitant psychostimulant use was permitted and did not affect manic symptom response.
Table 1
Atypical antipsychotic studies in pediatric bipolar disorder
| Drug and mean dosage | Study design | Sample characteristics | Efficacy measures | Results |
|---|---|---|---|---|
| Olanzapine15 9.6±4.3 mg/d | 8-week, open-label monotherapy | 23 patients, mean age 10±3 yrs, 57% male | ≥30% decrease on YMRS | Response rate 61% |
| Quetiapine16 432 mg/d | 6-week, randomized, placebo-controlled, adjunctive (+DVP) | 30 patients, mean age 14±2 yrs, 53% male | ≥50% decrease on YMRS | Response rates: DVP + placebo 53% DVP + quetiapine 87% |
| Risperidone17 1.7±1.3 mg/d | Retrospective, adjunctive | 28 patients, mean age 10±4 yrs, 97% male | ≤2 on CGI-I | Response rate 82% |
| CGI-I: Clinical Global Impressions-Improvement scale | ||||
| DVP: divalproex | ||||
| YMRS: Young Mania Rating Scale | ||||
WHICH COMBINATION?
Which combination treatment—psychostimulant plus mood stabilizer, psychostimulant plus atypical antipsychotic, or psychostimulant plus both mood stabilizer and atypical antipsychotic—is most appropriate for a child or adolescent with comorbid ADHD and BPD? Recommended treatment strategies are based on studies of pediatric and adult BPD and expert consensus.18,19
Consider the type of bipolar episode (Table 2).
For initial treatment of youths with BPD manic or mixed without psychosis, recent guidelines by Kowatch et al suggest using mood-stabilizer or atypical antipsychotic monotherapy. Youths who are more severely ill or present with psychosis may respond more favorably to a mood stabilizer plus an atypical antipsychotic.16,19
Individual patient traits will also determine whether a mood stabilizer or atypical antipsychotic is used and which agent within either medication class is chosen. For example:
- If the patient is aggressive, risperidone may reduce aggression and manic symptoms. Among the atypicals, risperidone has the most evidence suggesting efficacy for aggressive behaviors in youths across psychiatric conditions.20
- If an atypical antipsychotic is warranted and the patient’s weight is an issue, ziprasidone or aripiprazole would be preferred. These agents are considered weight-neutral compared with other atypicals.20
Other factors to consider include medication side effects, interactions, adherence, and cost.
Table 2
Mood stabilizer, atypical antipsychotic, or both with ADHD therapy?
| Type of bipolar episode | Recommended psychotropics |
|---|---|
| Manic or mixed episode with psychosis | Mood stabilizer + atypical antipsychotic |
| Manic or mixed episode without psychosis | Mood stabilizer or atypical antipsychotic monotherapy first
|
| Prominent irritability without psychosis | Atypical antipsychotic |
| Source: Adapted from references 18 and 19 | |
WHICH TO TREAT FIRST?
If the child or adolescent with comorbid ADHD and BPD has acute manic symptoms, available data and expert opinion recommend starting treatment with a mood stabilizer or atypical antipsychotic.9,19,21 If ADHD symptoms persist after mood stabilization, a psychostimulant trial is warranted.
In practice, however, youngsters usually present with ADHD symptoms first. Psychostimulant treatment is initiated, ADHD symptoms are controlled, and the child’s academic and social functioning improve. Bipolar symptoms emerge later, often heralded by a depressive or mixed episode. Is it necessary to discontinue the psychostimulant and risk worsening ADHD symptoms before starting a mood stabilizer or atypical antipsychotic?
Clinical lore and one case report suggest that psychostimulants may destabilize mood.22,23 A 10-year-old boy with severe hyperactivity and family history of BPD experienced manic symptoms—rapid and pressured speech, grandiose delusions of identity, and tangentiality of thought processes—during methylphenidate treatment.22
Conversely, an analysis24 of children ages 7 to 10 from the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD contradicts these assumptions. Although a clinical diagnosis of BPD was not assigned, 29 children (83% male) met the Diagnostic Interview Schedule for Children proxy for mania, 32 (88% male) met the Child Behavior Checklist proxy, and 7 met both proxies for mania.
The first month of methylphenidate treatment did not increase irritability, mood symptoms, or mania in the 54 children with ADHD and manic symptoms, compared with children with ADHD alone. The authors concluded that clinicians should not categorically avoid using stimulants in children with ADHD and some manic symptoms.
In a study by Pavuluri et al18 of pediatric bipolar type I disorder, 17 patients (mean age 11±4 years) received mood stabilizers—following a drug therapy algorithm that included risperidone—and typically received a psychostimulant after mood stabilization. This group was compared with 17 patients receiving “treatment as usual.”
The usual-treatment group remained on psychostimulant therapy after BPD intervention with a mood stabilizer and was less likely to receive an atypical antipsychotic. The algorithm treatment group showed better outcomes overall, specifically for mania and aggression.
Clearly, more studies are needed to determine the optimum treatment sequence with psychostimulants and mood stabilizers in youths with comorbid ADHD and BPD. With either approach, routinely monitor patients treated with psychostimulants for emerging or worsening bipolar symptoms.
LESSONS FROM CLINICAL EXPERIENCE
Nonstimulants. Using psychostimulants is appropriate for ADHD in patients with stable bipolar symptoms. Evidence for using nonstimulants such as clonidine, guanfacine, or atomoxetine is less clear.
In a naturalistic study of 153 children and adolescent outpatients treated with atomoxetine, 51 (33%) experienced irritability, aggression, mania, or hypomania. Of these patients, 31 (61%) had a family history of a mood disorder, and 41 (80%) had a personal history of mood symptoms.25 Although these findings suggest that atomoxetine may be associated with mood exacerbation and hypomania, additional data are needed to determine whether atomoxetine may be used for ADHD symptoms in youths with comorbid BPD.
Atypical antipsychotics. Mood stabilization—particularly with atypical antipsychotics—often can address comorbid disruptive behaviors and aggressive symptoms. Combinations of atypical antipsychotics with psychostimulants are largely devoid of drug-drug interactions and metabolic interference, making them uncomplicated to use.
Though published studies of pediatric BPD have focused on three atypical antipsychotics—olanzapine, quetiapine, and risperidone—any agent in this class can be used in this population, with the choice often depending on how side effects are likely to affect individual patients (Table 3 ).
Pharmacologic attributes may also determine which atypical antipsychotic is used. For example, ziprasidone’s serotonergic profile—with serotonin-1A receptor agonism and serotonin-1D antagonism—may make it useful for patients with mixed states and bipolar depression.26 Aripiprazole offers potential synergism of dopamine agonism with psychostimulant therapy, which could be useful for treating both disruptive behaviors and ADHD.
Table 3
Using atypical antipsychotics to treat comorbid ADHD/bipolar disorder
| Drug | Target dosage (mg/d) | Side effects | Useful in… |
|---|---|---|---|
| Aripiprazole | 10 to 15 | Nausea, vomiting | Comorbid disruptive behavioral disorders, maintenance stabilization |
| Olanzapine | 10 to 20 | Weight gain, hyperlipidemia, hyperglycemia, sedation | Maintenance stabilization |
| Quetiapine | 400 to 600 | Weight gain, sedation | Mixed states, bipolar depression |
| Risperidone | 1 to 2 | Weight gain, hyperprolactinemia, extrapyramidal symptoms | Comorbid disruptive behavioral disorders, including aggression |
| Ziprasidone | 80 to 120 | Cardiac abnormalities, akathisia | Mixed states, bipolar depression |
Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org
- Children and Adults with Attention-Deficit Hyperactivity Disorder (CHADD). www.chadd.org
- Findling RF, Kowatch RA, Post RM. P ediatric bipolar disorders: a handbook for clinicians. London: Martin Dunitz Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Aripiprazole • Abilify
- Divalproex • Depakote
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Patel is a consultant to Eli Lilly and Co. and a speaker for Eli Lilly and Co. and Pfizer Inc.
Dr. Sallee receives research support from Otsuka America Pharmaceutical, Pfizer Inc., and Bristol-Myers Squibb Co. and is a consultant or speaker for Eli Lilly and Co, Otsuka America Pharmaceutical, and Pfizer Inc.
Comorbid attention-deficit/hyperactivity disorder (ADHD) is nearly universal in youths with bipolar disorder (BPD),1 and comorbid mania has been noted in 16% of children with ADHD.2 Choosing medication for these complex patients is difficult because psychostimulants may worsen mania and mood stabilizers may not resolve ADHD symptoms. Yet, very little information exists on combining psychostimulants with mood stabilizers or atypical antipsychotics.
This article offers evidence to help you decide:
- which to treat first—ADHD or BPD
- how to individualize combination therapy.
CHALLENGES OF COMORBIDITY
Differential diagnosis. ADHD and bipolar disorder (BPD) symptoms overlap, and experts disagree on which symptoms indicate co-existing ADHD and BPD. Multiple daily mood swings and irritability are commonly found in prepubertal BPD.3 Recent reviews address differential diagnosis and specific assessment tools;3-5 after careful evaluation, then focus on treatment.
Treating comorbid ADHD and BPD usually requires more than one medication, and use of multiple drugs in children and adolescents is becoming increasingly common.6,7
PSYCHOSTIMULANTS AND MOOD STABILIZERS
Small, uncontrolled studies of children and adolescents with comorbid ADHD and BPD have shown that treatment with a mood stabilizer and a psychostimulant can control both sets of symptoms. For example:
- Lithium (serum levels 0.7 to 1.1 mEq/L) plus methylphenidate (10 to 20 mg/d) improved attention and hyperactivity symptoms more effectively than either agent alone in 7 children (6 boys, 1 girl) ages 6 to 10 hospitalized with disruptive behavioral disorders and BPD or major depression.8
- A retrospective analysis of 38 children (ages 3 to 16; 84% male) with BPD found that ADHD symptoms were 7.5 times more likely to improve if mood was stabilized before rather than after ADHD treatment with tricyclic antidepressants.9
The efficacy of combining a mood stabilizer and psychostimulant has been confirmed by only one controlled study—a randomized, placebo-controlled trial of mixed amphetamine salts in divalproex-treated patients.10 Forty patients (ages 6 to 17; 83% male) with BPD and ADHD received open-label divalproex (median dosage 750 mg/d) for 8 weeks. Thirty patients whose manic symptoms were significantly reduced entered a 4-week, double-blind, crossover trial of mixed amphetamine salts, 10 mg/d, or placebo.
Following this double-blind phase, 23 patients received open-label divalproex plus mixed amphetamine salts for 12 weeks. The Young Mania Rating Scale and Clinical Global Impression-Improvement scale were used to assess manic and ADHD symptoms during all three study phases.
Manic symptoms in patients treated with divalproex monotherapy improved significantly, but ADHD symptoms did not. ADHD symptoms improved more with divalproex plus mixed amphetamine salts than with divalproex plus placebo. One patient experienced manic symptom exacerbation with combination therapy.
PSYCHOSTIMULANTS AND ANTIPSYCHOTICS
Combinations of psychostimulants and atypical antipsychotics are commonly used in children and adolescents with comorbid psychiatric and behavioral disorders, such as ADHD and disruptive behavioral disorders (oppositional defiant disorder, conduct disorder). In 78 children ages 5 to 12 (83% male) with comorbid ADHD and a disruptive behavioral disorder, disruptive behavior and hyperactivity improved significantly with risperidone alone or with a psychostimulant.11
Combined psychostimulant/atypical antipsychotic therapy may help youths with comorbid ADHD and Tourette syndrome. Methylphenidate can reduce ADHD symptoms without exacerbating tics,12 and risperidone can treat tic disorders, even in patients with comorbid ADHD.13,14 No controlled trials have examined psychostimulant and atypical antipsychotic combinations in these patients, however.
Atypical antipsychotics have been shown to be effective in treating adult BPD, and limited data suggest the same to be true in pediatric patients. Olanzapine, quetiapine, and risperidone have been shown to reduce manic symptoms in children and adolescents (Table 1).15-17 Atypical antipsychotics, however, have been associated with metabolic side effects, including weight gain, hyperglycemia, hyperlipidemia, and hyperprolactinemia.
To date, no study has systematically evaluated combination psychostimulant and atypical antipsychotic treatment in comorbid ADHD and BPD. In the olanzapine and risperidone studies,15,17 concomitant psychostimulant use was permitted and did not affect manic symptom response.
Table 1
Atypical antipsychotic studies in pediatric bipolar disorder
| Drug and mean dosage | Study design | Sample characteristics | Efficacy measures | Results |
|---|---|---|---|---|
| Olanzapine15 9.6±4.3 mg/d | 8-week, open-label monotherapy | 23 patients, mean age 10±3 yrs, 57% male | ≥30% decrease on YMRS | Response rate 61% |
| Quetiapine16 432 mg/d | 6-week, randomized, placebo-controlled, adjunctive (+DVP) | 30 patients, mean age 14±2 yrs, 53% male | ≥50% decrease on YMRS | Response rates: DVP + placebo 53% DVP + quetiapine 87% |
| Risperidone17 1.7±1.3 mg/d | Retrospective, adjunctive | 28 patients, mean age 10±4 yrs, 97% male | ≤2 on CGI-I | Response rate 82% |
| CGI-I: Clinical Global Impressions-Improvement scale | ||||
| DVP: divalproex | ||||
| YMRS: Young Mania Rating Scale | ||||
WHICH COMBINATION?
Which combination treatment—psychostimulant plus mood stabilizer, psychostimulant plus atypical antipsychotic, or psychostimulant plus both mood stabilizer and atypical antipsychotic—is most appropriate for a child or adolescent with comorbid ADHD and BPD? Recommended treatment strategies are based on studies of pediatric and adult BPD and expert consensus.18,19
Consider the type of bipolar episode (Table 2).
For initial treatment of youths with BPD manic or mixed without psychosis, recent guidelines by Kowatch et al suggest using mood-stabilizer or atypical antipsychotic monotherapy. Youths who are more severely ill or present with psychosis may respond more favorably to a mood stabilizer plus an atypical antipsychotic.16,19
Individual patient traits will also determine whether a mood stabilizer or atypical antipsychotic is used and which agent within either medication class is chosen. For example:
- If the patient is aggressive, risperidone may reduce aggression and manic symptoms. Among the atypicals, risperidone has the most evidence suggesting efficacy for aggressive behaviors in youths across psychiatric conditions.20
- If an atypical antipsychotic is warranted and the patient’s weight is an issue, ziprasidone or aripiprazole would be preferred. These agents are considered weight-neutral compared with other atypicals.20
Other factors to consider include medication side effects, interactions, adherence, and cost.
Table 2
Mood stabilizer, atypical antipsychotic, or both with ADHD therapy?
| Type of bipolar episode | Recommended psychotropics |
|---|---|
| Manic or mixed episode with psychosis | Mood stabilizer + atypical antipsychotic |
| Manic or mixed episode without psychosis | Mood stabilizer or atypical antipsychotic monotherapy first
|
| Prominent irritability without psychosis | Atypical antipsychotic |
| Source: Adapted from references 18 and 19 | |
WHICH TO TREAT FIRST?
If the child or adolescent with comorbid ADHD and BPD has acute manic symptoms, available data and expert opinion recommend starting treatment with a mood stabilizer or atypical antipsychotic.9,19,21 If ADHD symptoms persist after mood stabilization, a psychostimulant trial is warranted.
In practice, however, youngsters usually present with ADHD symptoms first. Psychostimulant treatment is initiated, ADHD symptoms are controlled, and the child’s academic and social functioning improve. Bipolar symptoms emerge later, often heralded by a depressive or mixed episode. Is it necessary to discontinue the psychostimulant and risk worsening ADHD symptoms before starting a mood stabilizer or atypical antipsychotic?
Clinical lore and one case report suggest that psychostimulants may destabilize mood.22,23 A 10-year-old boy with severe hyperactivity and family history of BPD experienced manic symptoms—rapid and pressured speech, grandiose delusions of identity, and tangentiality of thought processes—during methylphenidate treatment.22
Conversely, an analysis24 of children ages 7 to 10 from the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD contradicts these assumptions. Although a clinical diagnosis of BPD was not assigned, 29 children (83% male) met the Diagnostic Interview Schedule for Children proxy for mania, 32 (88% male) met the Child Behavior Checklist proxy, and 7 met both proxies for mania.
The first month of methylphenidate treatment did not increase irritability, mood symptoms, or mania in the 54 children with ADHD and manic symptoms, compared with children with ADHD alone. The authors concluded that clinicians should not categorically avoid using stimulants in children with ADHD and some manic symptoms.
In a study by Pavuluri et al18 of pediatric bipolar type I disorder, 17 patients (mean age 11±4 years) received mood stabilizers—following a drug therapy algorithm that included risperidone—and typically received a psychostimulant after mood stabilization. This group was compared with 17 patients receiving “treatment as usual.”
The usual-treatment group remained on psychostimulant therapy after BPD intervention with a mood stabilizer and was less likely to receive an atypical antipsychotic. The algorithm treatment group showed better outcomes overall, specifically for mania and aggression.
Clearly, more studies are needed to determine the optimum treatment sequence with psychostimulants and mood stabilizers in youths with comorbid ADHD and BPD. With either approach, routinely monitor patients treated with psychostimulants for emerging or worsening bipolar symptoms.
LESSONS FROM CLINICAL EXPERIENCE
Nonstimulants. Using psychostimulants is appropriate for ADHD in patients with stable bipolar symptoms. Evidence for using nonstimulants such as clonidine, guanfacine, or atomoxetine is less clear.
In a naturalistic study of 153 children and adolescent outpatients treated with atomoxetine, 51 (33%) experienced irritability, aggression, mania, or hypomania. Of these patients, 31 (61%) had a family history of a mood disorder, and 41 (80%) had a personal history of mood symptoms.25 Although these findings suggest that atomoxetine may be associated with mood exacerbation and hypomania, additional data are needed to determine whether atomoxetine may be used for ADHD symptoms in youths with comorbid BPD.
Atypical antipsychotics. Mood stabilization—particularly with atypical antipsychotics—often can address comorbid disruptive behaviors and aggressive symptoms. Combinations of atypical antipsychotics with psychostimulants are largely devoid of drug-drug interactions and metabolic interference, making them uncomplicated to use.
Though published studies of pediatric BPD have focused on three atypical antipsychotics—olanzapine, quetiapine, and risperidone—any agent in this class can be used in this population, with the choice often depending on how side effects are likely to affect individual patients (Table 3 ).
Pharmacologic attributes may also determine which atypical antipsychotic is used. For example, ziprasidone’s serotonergic profile—with serotonin-1A receptor agonism and serotonin-1D antagonism—may make it useful for patients with mixed states and bipolar depression.26 Aripiprazole offers potential synergism of dopamine agonism with psychostimulant therapy, which could be useful for treating both disruptive behaviors and ADHD.
Table 3
Using atypical antipsychotics to treat comorbid ADHD/bipolar disorder
| Drug | Target dosage (mg/d) | Side effects | Useful in… |
|---|---|---|---|
| Aripiprazole | 10 to 15 | Nausea, vomiting | Comorbid disruptive behavioral disorders, maintenance stabilization |
| Olanzapine | 10 to 20 | Weight gain, hyperlipidemia, hyperglycemia, sedation | Maintenance stabilization |
| Quetiapine | 400 to 600 | Weight gain, sedation | Mixed states, bipolar depression |
| Risperidone | 1 to 2 | Weight gain, hyperprolactinemia, extrapyramidal symptoms | Comorbid disruptive behavioral disorders, including aggression |
| Ziprasidone | 80 to 120 | Cardiac abnormalities, akathisia | Mixed states, bipolar depression |
Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org
- Children and Adults with Attention-Deficit Hyperactivity Disorder (CHADD). www.chadd.org
- Findling RF, Kowatch RA, Post RM. P ediatric bipolar disorders: a handbook for clinicians. London: Martin Dunitz Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Aripiprazole • Abilify
- Divalproex • Depakote
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Patel is a consultant to Eli Lilly and Co. and a speaker for Eli Lilly and Co. and Pfizer Inc.
Dr. Sallee receives research support from Otsuka America Pharmaceutical, Pfizer Inc., and Bristol-Myers Squibb Co. and is a consultant or speaker for Eli Lilly and Co, Otsuka America Pharmaceutical, and Pfizer Inc.
1. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998;37(2):171-8.
2. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
3. Kowatch RA, DelBello MP. Pediatric bipolar disorder: mood swings, irritability are diagnostic cues. Current Psychiatry 2003;2(3):40-7.
4. Quinn CA, Fristad MA. Defining and identifying early onset bipolar spectrum disorder. Curr Psychiatry Rep 2004;6(2):101-7.
5. Bhatara VS, Feil M, Hoagwood K, et al. Trends in combined pharmacotherapy with stimulants for children. Psychiatr Serv 2002;53(3):244.-
6. Zito JM, Safer DJ, dosReis S, et al. Psychotherapeutic medication patterns for youths with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 1999;153(12):1257-63.
7. Wolf DV, Wagner KD. Bipolar disorder in children and adolescents. CNS Spectr 2003;8(12):954-9.
8. Carlson GA, Rapport MD, Kelly KL, Pataki CS. The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992;31(2):262-70.
9. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
10. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
11. Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavioral disorders, and subaverage IQ. J Child Adolesc Psychopharmacology 2004;14(2):243-54.
12. Gadow KD, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56(4):330-6.
13. Gaffney GR, Perry PJ, Lund BC, et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002;41(3):330-6.
14. Lombroso PJ, Scahill L, King RA, et al. Risperidone treatment of children and adolescents with chronic tic disorders: a preliminary report. J Am Acad Child Adolesc Psychiatry 1995;34(9):1147-52.
15. Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2001;11(3):239-50.
16. DelBello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002;41(10):1216-23.
17. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
18. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2004;43(7):859-67.
19. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44(3):213-35.
20. Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry 2004;65(suppl 6):30-44.
21. Kowatch RA, Sethuraman G, Hume JH, et al. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry 2003;53(11):978-84.
22. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
23. Craney J, Geller B. Clinical implications of antidepressant and stimulant use on switching from depression to mania in children. J Child Adolesc Psychopharmacol 2003;13(2):201-4.
24. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13(2):123-36.
25. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114(3):895-6.
26. Sallee FR, Gilbert DL, Vinks AA, et al. Pharmacodynamics of ziprasidone in children and adolescents: impact on dopamine transmission. J Am Acad Child Adolesc Psychiatry 2003;42(8):902-7.
1. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998;37(2):171-8.
2. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
3. Kowatch RA, DelBello MP. Pediatric bipolar disorder: mood swings, irritability are diagnostic cues. Current Psychiatry 2003;2(3):40-7.
4. Quinn CA, Fristad MA. Defining and identifying early onset bipolar spectrum disorder. Curr Psychiatry Rep 2004;6(2):101-7.
5. Bhatara VS, Feil M, Hoagwood K, et al. Trends in combined pharmacotherapy with stimulants for children. Psychiatr Serv 2002;53(3):244.-
6. Zito JM, Safer DJ, dosReis S, et al. Psychotherapeutic medication patterns for youths with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 1999;153(12):1257-63.
7. Wolf DV, Wagner KD. Bipolar disorder in children and adolescents. CNS Spectr 2003;8(12):954-9.
8. Carlson GA, Rapport MD, Kelly KL, Pataki CS. The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992;31(2):262-70.
9. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
10. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
11. Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavioral disorders, and subaverage IQ. J Child Adolesc Psychopharmacology 2004;14(2):243-54.
12. Gadow KD, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56(4):330-6.
13. Gaffney GR, Perry PJ, Lund BC, et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002;41(3):330-6.
14. Lombroso PJ, Scahill L, King RA, et al. Risperidone treatment of children and adolescents with chronic tic disorders: a preliminary report. J Am Acad Child Adolesc Psychiatry 1995;34(9):1147-52.
15. Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2001;11(3):239-50.
16. DelBello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002;41(10):1216-23.
17. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
18. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2004;43(7):859-67.
19. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44(3):213-35.
20. Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry 2004;65(suppl 6):30-44.
21. Kowatch RA, Sethuraman G, Hume JH, et al. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry 2003;53(11):978-84.
22. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
23. Craney J, Geller B. Clinical implications of antidepressant and stimulant use on switching from depression to mania in children. J Child Adolesc Psychopharmacol 2003;13(2):201-4.
24. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13(2):123-36.
25. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114(3):895-6.
26. Sallee FR, Gilbert DL, Vinks AA, et al. Pharmacodynamics of ziprasidone in children and adolescents: impact on dopamine transmission. J Am Acad Child Adolesc Psychiatry 2003;42(8):902-7.
Atomoxetine: A different approach to ADHD
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.