User login
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs
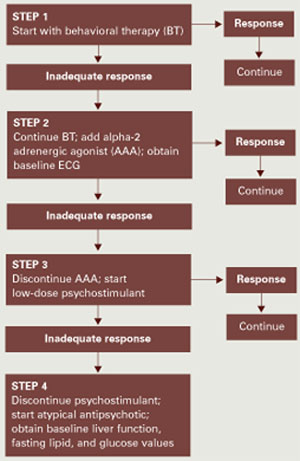
To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs

To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
Psychostimulants benefit many patients with attention-deficit/hyperactivity disorder (ADHD)1 and thus might seem a logical choice to manage hyperactivity and inattention in youths with a pervasive developmental disorder (PDD). Some PDD patients do respond to psychostimulant therapy, but others worsen—and side effects are common.
Youths with PDDs often exhibit maladaptive behaviors—aggression, self-injury, irritability, hyperactivity, inattention—with repetitive activity patterns and fundamentally impaired social interaction and communication.2 To help you treat youths with PDD, we draw on the evidence, clinical experience, and our research to suggest psychostimulants’ role in a multimodal approach.
Targeting hyperactivity and inattentions
Step 1. Our approach begins with behavioral therapy (Figure), which includes identifying situations that trigger maladaptive behavior and environments that yield optimum behavior. The therapist assesses the child’s baseline attention and works with him or her to gradually increase it, using reinforcement and visual token boards.
Algorithm Suggested approach to hyperactivity and/or inattention in patients with PDDs

To set limits and expectations, the therapist introduces structure such as designating work and break areas and using visual schedules and timers to indicate activity duration. Minimizing distractions and understanding the child’s sensory needs may increase motivation and attention. Initially, the therapist allows numerous breaks and then may slowly decrease them as the child progresses. Tailoring work and play materials to the child’s interests can also help increase attention.
Step 2. Many patients will not respond to behavior therapy alone and will require added drug therapy. Based on evidence, we suggest starting with an alpha-2 adrenergic agonist. Guanfacine may be considered the drug of choice because of clonidine’s higher risk of adverse effects, such as hypotension and sedation. Obtain a baseline ECG with either agent, as clonidine has been associated with rare cardiovascular events.
Clonidine. Two small studies showed that clonidine may be of some benefit to patients with PDDs:
- Results were mixed in a 6-week, double-blind, placebo-controlled, crossover study of clonidine (4 to 10 μg/kg/d) in 8 autistic children ages 5 to 13.3 Teacher and parent rating instruments reflected significantly improved hyperactivity, irritability, and oppositional behavior. Clinician ratings, however, showed no significant difference between clonidine and placebo. Adverse effects with clonidine included hypotension, sedation, and decreased activity.
- In a 4-week, double-blind, placebo-con-trolled, crossover study of transdermal clonidine (0.16 to 0.48 mg/kg/d; mean: 3.6 μg/kg/d), clinician ratings showed significantly decreased hyperactivity, impulsivity, and anxiety in 9 autistic males ages 5 to 33. Sedation and fatigue were common adverse effects.
Guanfacine. In a recent retrospective review,5 we examined outcomes of 80 PDD patients ages 3 to 18 who received guanfacine (0.25 to 9 mg/d; mean: 2.6). Hyperactivity, inattention, and tics decreased in 19 patients (24%) treated for a mean 10 months.
Step 3. If clonidine or guanfacine fails to reduce hyperactivity and inattention, discontinue it and consider a psychostimulant trial.
Because psychostimulants’ efficacy in PDDs remains inconclusive, we suggest beginning with a low dosage and carefully monitoring the patient for worsening target symptoms and activation, such as emerging aggression or irritability.
Step 4. If hyperactivity and inattention remain prominent and treatment-refractory, we suggest that you discontinue the stimulant and consider an atypical antipsychotic trial. With the atypicals, monitor patients closely for adverse effects, including weight gain, extrapyramidal symptoms, and tardive dyskinesia. Fasting serum glucose and lipid profiles and liver function tests are recommended at least every 6 months and more often in individuals at risk for diabetes or hepatic disease.
Two studies provide evidence of atypicals’ efficacy in PDDs:
- In a 6-week open-label comparison,6 olanzapine significantly reduced hyperactivity and anger or uncooperativeness in 12 children with autistic disorder, but haloperidol did not. Average weight gain was 9 lbs in patients receiving olanzapine vs 3.2 lbs in those receiving haloperidol.
- An 8-week, double-blind study7 compared risperidone (0.5 to 3.5 mg/d; mean: 1.8) with placebo in 101 children and adolescents with autistic disorder. Response rates were 69% in the risperidone group and 12% in the control group. Risperidone reduced hyperactivity, aggression, agitation, and repetitive behavior. Adverse drug effects included weight gain (2.7 kg vs. 0.8 kg with placebo), increased appetite, and sedation.
Psychostimulant use in PDDs
Evidence is conflicting on psychostimulant use in patients with PDDs (Table). Early reviews suggested that stimulants were ineffective in PDDs and associated with adverse effects.8,9 Some preliminary studies supported that view, but recent reports have been mixed.
Dextroamphetamine. Campbell et al10 published a placebo-controlled study comparing triiodothyronine and dextroamphetamine (mean dosage, 4.8 mg/d; range 1.25 to 10 mg/d) in 16 children ages 3 to 6 (mean, 4.3 years) with diagnoses of autism, schizophrenia, and organic brain syndrome. All diagnostic groups worsened clinically with dextroamphetamine, and adverse effects—hyperactivity, worsened stereotypy, irritability, and decreased appetite—were common.
A subsequent case report11 found dex-troamphetamine effective when 2 patients ages 9 and 12 with PDD were treated with 10 and 5 mg/d, respectively. Hyperactivity, inattention, and impulsivity improved in both patients, and core PDD features did not worsen.
Levoamphetamine. In an 8-week, double-blind, crossover comparison with levodopa,12 levoamphetamine, 3.5 to 42 mg/d (mean, 13.4), worsened symptoms in 12 children ages 3 to 12 who had schizophrenia with autistic features. stereotypy emerged or increased in 9 of the 11 patients (82%) available for follow-up, and levoamphetamine was poorly tolerated.
Methylphenidate. In an early report, methylphenidate decreased hyperactivity and impulsivity in 9 of 15 children (60%) ages 2 to 13 with infantile autism.13 Dosages of 5 to 10 mg/d or 0.3 to 1 mg/kg/d were given for 2 to 60 weeks (mean, 26). Adverse effects included irritability, insomnia, and anorexia.
Table
Selected reports of stimulant use in pervasive developmental disorders
| Medication | Type of report | Dosage (mg/d); duration | Outcome | Adverse effects |
|---|---|---|---|---|
| Dextroamphetamine | Placebo-controlled10 (N=16) Case report11 (N=2) | Mean 4.8; N/A Mean 7.5; N/A | Clinical worsening Improved hyperactivity,inattention,impulsivity | Hyperactivity, irritability, decreased appetite, worsened stereotypy N/A |
| Levoamphetamine | Double-blind12 (N=12) | Mean 13.4 | Clinical worsening | Stereotypy emerged or worsened |
| Methylphenidate | Retrospective13 (N=15) Open-label14 (N=9) Case report15 (N=1) Double-blind, placebo-controlled, crossover16 (N=10) Double-blind, placebo-controlled, crossover17 (N=13) | 5 to 10; 26 weeks 10 to 50; 2 weeks 20; 4 weeks 20 mg/d for 2 weeks, 40 mg/d for 2 weeks 0.3 mg/kg and 0.6 mg/kg | Improved hyperactivity, impulsivity Improved hyperactivity Improved hyperactivity, concentration Modest benefit over placebo Improved hyperactivity, inattention | Irritability, insomnia, anorexia Initial mild insomnia Dysphoria, angry outbursts Statistically similar to placebo Social withdrawal, irritability |
| Methylphenidate, levoamphetamine, dextroamphetamine, or pemoline | Retrospective18 (N=195) | Various dosages, durations | Patients with, Asperger’s disorder were significantly more likely to respond | Agitation, dysphoria, irritability |
| N/A: not available | ||||
A subsequent open-label study and a case report also indicated that methylphenidate improved hyperactivity in patients with autistic disorder:
- In the 2-week, open-label study,14 9 patients ages 4 to 16 received methylphenidate, 10 to 50 mg/d. Two patients also received haloperidol, 4 and 5 mg/d. Hyperactivity improved significantly, as measured by the Conners Teacher Questionnaire.
- In the case report,15 one child, age 6, was. treated with methylphenidate, 10 mg bid, for 31 days. The drug significantly alleviated hyperactivity and improved concentration. Adverse effects included dysphoria and outbursts of anger.
Atomoxetine—a nonstimulant, selective norepinephrine reuptake inhibitor—has been approved to treat hyperactivity and inattention in ADHD, but no evidence has been published on its use in PDDs. A study of desipramine19 —also a norepinephrine reuptake inhibitor—may offer some insight into the possible efficacy and tolerability of atomoxetine in PDDs.
Desipramine (mean, 127 mg/d) was compared with the serotonin reuptake inhibitor clomipramine (mean, 153 mg/d) in a 10-week, double-blind, crossover study of 24 autistic patients ages 6 to 23. The agents were equally effective and superior to placebo in decreasing hyperactivity, although desipramine was associated with increased aggression and irritability.
Despite these results with desipramine, research is needed to understand atomoxetine’s potential role in treating hyperactivity and inattention in youths with PDDs.
Controlled trials. These early reports were followed by two double-blind, placebo-controlled, crossover studies of methylphenidate in children with autistic disorder.
- In the first trial,16 methylphenidate, 10 or 20 mg/d, improved irritability and hyperactivity in 10 children ages 7 to 11 but was only modestly more beneficial than placebo. Side-effect incidence—including decreased appetite, irritability, and insomnia—was similar during active and placebo treatments. Two patients required adjunctive haloperidol for prevailing behavioral problems.
- In the second trial,17 8 of 13 children (62%) ages 5 to 11 responded to methylphenidate, 0.3 and 0.6 mg/kg per dose. Hyperactivity and inattention improved significantly, as measured by a minimum 50% decrease in Conners Hyperactivity Index score. Ratings of stereotypy and inappropriate speech also decreased, but no changes were seen in the Child Autism Rating Scale. Adverse effects, which were more common with the 0.6 mg/kg dose, included social withdrawal and irritability.
Retrospective trial. Our group recently completed a retrospective study of 195 youth (mean age, 7.3 years; range, 2 to 19 years) with PDDs treated with a stimulant medication.18 As a whole, stimulants appeared ineffective.
Analysis of response by PDD subtype found that individuals with Asperger’s disorder—in contrast to those with autistic disorder or PDD not otherwise specified—were significantly more likely to respond to a stimulant medication. Gender, intelligence quotient (IQ), type of stimulant, and dosage did not significantly affect response. Adverse effects—including agitation, dysphoria, and irritability—occurred in 57.5% of the trials.
Atomoxetine. This nonstimulant medication has been approved for treating ADHD. However, research is needed to understand its use in patients with PDDs (Box)19
Summary. These mixed findings—combined with anecdotal reports from physicians describing the onset or exacerbation of hyperactivity, irritability, and aggression—indicate that much more evidence is needed regarding psychostimulant use in patients with PDDs.
To help meet this need, the National Institutes of Mental Health’s Research Units on Pediatric Psychopharmacology (RUPP) autism network recently completed a large, double-blind, placebo-controlled study to investigate methylphenidate’s efficacy and tolerability in PDDs. It is anticipated that the results will help us discern whether factors such as PDD subtype, patient age, dosage, or degree of mental retardation are associated with response.
Related resources
- Autism Society of America. www.autism-society.org
- McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002.
- McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, Charney DS, Leckman JF (eds). Pediatric psychopharmacology: Principles and practice.New York: Oxford University Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Clomipramine • Anafranil
- Clonidine • Catapres
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine, Dextrostat
- Guanfacine • Tenex
- Haloperidol • Haldol
- Levoamphetamine • Adderall
- Levodopa • Dopar, Laradopa
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Pemoline • Cylert
- Risperidone • Risperdal
Disclosure
Dr. Stigler reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Posey receives research support from Janssen Pharmaceutica and Eli Lilly and Co. and is a speaker for Janssen Pharmaceutica.
Dr. McDougle receives research support from Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., and Bristol-Myers Squibb Co. He is a consultant to or speaker for Janssen Pharmaceutica, Pfizer Inc., Eli Lilly and Co., RepliGen Corp., and Bristol-Myers Squibb Co.
Acknowledgments
This work was supported in part by a Daniel X. Freedman Psychiatric Research Fellowship Award (Dr. Posey), a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Posey), a Research Units on Pediatric Psychopharmacology Grant (U10MH66766-02) from the National Institute of Mental Health (NIMH) to Indiana University (Dr. McDougle, Dr. Stigler, and Dr. Posey), a Research Career Development Award (K23-MH068627-01) from the NIMH (Dr. Posey), a National Institutes of Health Clinical Research Center grant to Indiana University (M01-RR00750), and a Department of Housing and Urban Development (HUD) grant (B-01-SP-IN-0200) (Dr. McDougle).
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(2 suppl):26S-49S.
2. Posey DJ, McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry 2000;8(2):45-63.
3. Jaselskis CA, Cook EH Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J Clin Psychopharmacol 1992;12(5):322-7.
4. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 1992;53(3):77-82.
5. Posey DJ, Decker J, Sasher TM, et al. A retrospective analysis of guanfacine in the treatment of autism. J Child Adolesc.
6. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001;40(8):887-94.
7. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
8. Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry 1975;10(4):399-423.
9. Aman MG. Stimulant drug effects in developmental disorders and hyperactivity—toward a resolution of disparate findings. J Autism Dev Disord 1982;12(4):385-98.
10. Campbell M, Fish B, David R, et al. Response to triiodothyronine and dextroamphetamine: a study of preschool schizophrenic children. J Autism Child Schizophr 1972;2(4):343-58.
11. Geller B, Guttmacher LB, Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry 1981;138(3):388-9.
12. Campbell M, Small AM, Collins PJ, et al. Levodopa and levoamphetamine: a crossover study in young schizophrenic children. Curr Ther Res Clin Exp 1976;19(1):70-86.
13. Hoshino Y, Kumashiro H, Kaneko M, Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn 1977;31(4):605-14.
14. Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry 1988;27(2):248-51.
15. Strayhorn JM Jr, Rapp N, Donina W, Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry 1988;27(2):244-7.
16. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord 1995;25(3):283-94.
17. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 2000;30(3):245-55.
18. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
19. Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50(6):441-7.