User login
Obsessive and inattentive
CASE: Perfect breath
Mr. C, a 20-year-old college student, is diagnosed with obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), and tic disorder (TD). His obsessions consist of a persistent sense that he is not breathing “correctly” or “perfectly.” He compulsively holds his breath to “rush blood to my head” until “the pressure feels just right.” Mr. C says that his OCD has had longstanding, significant negative impact on his academic performance and capacity to engage in other activities. Tics have been present for years and manifest as coughing and throat-clearing. After multiple syncopal epi-sodes from breath-holding with Valsalva maneuver—some of which caused falls and head injury—Mr. C is admitted to a residential psychiatric unit specializing in treating OCD. At the time of his admission, his Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores1,2 are 23 total, 12 on the obsessions subscale, and 11 on the compulsions subscale, indicating moderate to severe illness. Cognitive-behavioral therapy (CBT) is offered, along with a combination of escitalopram, 60 mg/d, and quetiapine, 50 mg/d. Quetiapine is over-sedating at subtherapeutic doses and Mr. C’s compulsions worsen. He reports that “[it] took longer and longer to get the ‘just right’ feeling.’” Quetiapine is discontinued and risperidone, 0.5 mg/d, is started, which decreases the frequency of his tics. When he is discharged after a 36-day stay, Mr. C’s Y-BOCS scores are greatly improved at 13 total, 7 on the obsessions subscale, and 3 on the compulsions subscale.
Mr. C’s psychologist refers him to our outpatient clinic for continued psychiatric evaluation and treatment of his OCD, ADHD, and TD. At this time, he is prescribed escitalopram, 60 mg/d, and risperidone, 0.5 mg/d, along with CBT with his psychologist. We do not readminister the Y-BOCS at this time, but Mr. C reports that his OCD is “60% improved.” However, he describes prominent obsessive thoughts regarding his breathing similar to those he experienced before residential treatment. These obsessive thoughts arise in the context of specific environmental “triggers,” such as other people coughing or his own tics. The obsessions lead to compulsive urges to engage in breath-holding rituals. Mr. C experiences the thoughts and compulsions as deeply troubling and they consume 5 to 6 hours each day. Mr. C reports impaired concentration in class and during studying: “I can focus for 5 minutes, then not for 2 minutes, then for 3 minutes… I can never stay focused for more than a couple minutes,” before becoming distracted “by my OCD” or other environmental stimuli. We note on exam prominent breath-holding occurring several times per minute. Mr. C says his OCD has not impaired his ability to socialize.
Mr. C notes that he has been exposed to an array of CBT techniques, but he has difficulty using these techniques because his “mind wanders” or he lacks “motivation.” He admits he occasionally has taken a classmate’s ADHD medication (mixed amphetamine salts [MAS], dose unspecified) and found it improved his ability to focus on his academic work.
The authors’ observations
Researchers have established a relationship among OCD, ADHD, and TD across all combinations of comorbidity (OCD and ADHD,3 ADHD and TD,4 OCD and TD,5,6 and all 3 entities7). Data suggests a poorer prognosis for OCD when comorbid with either or both of these conditions.8 Researchers have raised concerns that psychostimulants could exacerbate or potentiate tic behaviors in patients with ADHD,9,10 although safe and effective use of these medications has been documented in controlled trials of patients with comorbid ADHD and tics.11-13 Furthermore, tic suppression has been reported with psychostimulants,14 as well as a differential effect of stimulants on motor vs vocal tics.15 Despite these data (Table 1),9-15 the FDA regards using psychostimulants in patients with TD as a contraindication,16 although clinicians often recognize that this practice may be unavoidable in some circumstances because of high comorbidity rates. Psychostimulants could exacerbate obsessions or compulsions in some patients because of their dopaminergic properties or through mitigation of the purported anti-obsessional properties of dopamine antagonists.17
Although there is evidence that the prevalence of prescribed psychostimulant abuse is low among ADHD patients,18 diversion of prescribed medication is a risk inherent in the use of these agents, particularly among college-age patients.19,20
Table 1
Evidence of effect of psychostimulants on tics
| Study/disorder(s) | Medication and study design | Relevant findings |
|---|---|---|
| Lipkin et al, 19949; ADHD without TD | Chart review (N = 122) to determine the incidence of tics or dyskinesias in children treated with stimulants | Approximately 9% of children developed tics or dyskinesias, which predominantly were transient, with <1% developing chronic tics or Tourette’s syndrome. Personal or family tic history and medication selection or dosage were not related to onset of tics or dyskinesias |
| Gadow et al, 199515; ADHD with TD | Methylphenidate variable dose, placebo-controlled, 2-week trials (N = 24) | All children’s ADHD symptoms improved. At a 0.1 mg/kg dose, motor tics observed in the classroom increased, but there were fewer vocal tics observed in the lunchroom |
| Castellanos et al, 199710; ADHD with Tourette’s syndrome | Methylphenidate, dextroamphetamine, variable-dose, double-blind, placebo-controlled, 9-week crossover (N = 20) | 3 patients had consistent worsening of tics while taking stimulants. Stimulants reduced hyperactivity rates compared with placebo (P = .03). Stimulants improved ADHD symptoms and had acceptable effects on tics. Methylphenidate was better tolerated than dextroamphetamine |
| Gadow et al, 199911; ADHD with TD | 34 methylphenidate-treated children, followed at 6-month intervals for 2 years | No evidence that frequency or severity of motor or vocal tics changed during maintenance therapy |
| Tourette Syndrome Study Group, 200213; ADHD with TD | Clonidine alone, methylphenidate alone, clonidine plus methylphenidate, or placebo | Worsening of tics was not reported in any group at a rate significantly higher than placebo. Tic severity was more reduced in the 2 clonidine groups than in the methylphenidate group |
| Lyon et al, 201014; ADHD with Tourette’s syndrome | Dexmethylphenidate, single-dose challenge. Ten patients with or without TSP | Acute dexmethylphenidate administration resulted in tic suppression but did not augment TSP |
| Gadow et al, 200712; ADHD with TD | Double-blind, placebo-controlled, 2-week trials each of 3 doses of methylphenidate and placebo (N = 71) | MPH-IR did not alter the overall severity of TD or OCD behaviors. Teacher ratings indicated that MPH-IR therapy decreased tic frequency and severity |
| ADHD: attention-deficit/hyperactivity disorder; MPH-IR: methylphenidate immediate release; OCD: obsessive-compulsive disorder; TD: tic disorder; TSP: tic suppression protocol | ||
TREATMENT: Weighing options
To manage impaired attention and executive function difficulties secondary to ADHD, we offer Mr. C several options, including bupropion, modafinil, and memantine augmentation. Mr. C asks for a psychostimulant because exam week is approaching and he wants a treatment with quick therapeutic effect. We discuss with Mr. C the potential for dopaminergic agents, such as psychostimulants, to exacerbate tics or OCD symptoms. Ultimately, we prescribe immediate-release MAS, 20 mg/d.
Two days later, Mr. C says he has taken 3 MAS doses and describes a marked reduction in obsessions, significant decrease in frequency of “triggers,” and greater capacity to use CBT saying, “when I am [triggered], I am able to move past the urges without doing any compulsions.” Daily time spent “stuck on” obsessions or compulsions decreases from 5 to 6 hours per day to “about 2 and a half minutes.”
Mr. C reports a modest increase in the prevalence of tics, experienced as “little throat clears and quick stuttering of breath.” He notes that, although in the past such tics would be followed by urges for “perfecting the tic and making it feel just right,” he presently “had no desire to do so.”
OUTCOME: Sharper focus
Increasing MAS immediate release from 20 mg/d to 30 mg/d suppresses Mr. C’s obsessions and compulsions for 8 hours. On the 19th day of treatment, MAS immediate release was replaced with an extended release formulation, 30 mg/d, which preserves therapeutic effect and tolerability for 16 weeks. Repeat Y-BOCS yields 9 total, 3 on obsessions subscale, and 6 on compulsions subscale scores.
One month later, Mr. C reports that his symptoms have been “improving ever since” the previous appointment. He continues to be able to access skills for managing his OCD and is doing well in his 2 accelerated summer courses, saying “I focus really well” in 3-hour class sessions. On exam, tic behaviors are nearly absent. Mr. C describes occasional bouts of anxiety associated with urges to engage in tic behaviors, in turn arising from fear of symptomatic recurrence as he worked toward stopping smoking as advised by his primary care physician and psychiatrist.
The authors’ observations
The results of the repeat Y-BOCS are consistent with improvement in obsessions but possible worsening of compulsions since Mr. C was discharged from residential treatment. Alternatively, compulsions may have worsened immediately after discharge and declined again with introduction of MAS.
A substantial body of literature describes the challenges associated with treating ADHD with comorbid tics, including the relative degree of risk of tic exacerbation associated with treating ADHD with psychostimulants. The range of FDA-approved pharmacologic options for treatment of this comorbidity is limited (Table 2),21 particularly given the risk for tardive dyskinesia associated with the typical antipsychotics haloperidol and chlorpromazine. Data support using the α-2 agonist clonidine to treat hyperactivity associated with ADHD22 and TD23 and an extended-release preparation of this medication is FDA-approved for the former but not the latter indication (an α-2A receptor subtype agonist, guanfacine, also is FDA-approved for ADHD in pediatric patients). Mr. C’s experience of robust, sustained reduction in obsessions, if not compulsions, after treatment with MAS is consistent with the few studies of stimulant use in ADHD with comorbid OCD.24,25
Effective treatment of ADHD may help Mr. C better access CBT strategies and thereby potentiate treatment of comorbid OCD.
Table 2
FDA-approved medications for ADHD, OCD, and TD
| Disorder | Medications |
|---|---|
| ADHD | Amphetamine (racemic), atomoxetine, chlorpromazine (hyperactivity), clonidine extended release, dexmethylphenidate, dextroamphetamine, guanfacine extended release, haloperidol (hyperactivity, second-line), lisdexamfetamine, methylphenidate (racemic) |
| OCD | Clomipramine, fluoxetine, fluvoxamine, paroxetine, sertraline |
| TD/Tourette’s syndrome | Haloperidol (Tourette’s), pimozide (Tourette’s) |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder; TD: tic disorder Source: Reference 21 | |
Related Resources
- Pliszka SR. Treating ADHD and comorbid disorders: psychosocial and psychopharmacological interventions. New York, NY: The Guilford Press; 2011.
- Pollak Y, Benarroch F, Kanengisser L, et al. Tourette syndrome-associated psychopathology: roles of comorbid attention-deficit hyperactivity disorder and obsessive-compulsive disorder. J Dev Behav Pediatr. 2009;30(5):413-419.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clonidine extended release • Kapvay
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Guanfacine • Intuniv, Tenex
- Haloperidol • Haldol
- Lisdexamfetamine • Vyvanse
- Memantine • Namenda
- Methylphenidate • Methylin, Ritalin
- Modafinil • Provigil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use and reliability. Arch Gen Psych. 1989;46(11):1006-1011.
2. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: II. Validity. Arch Gen Psych. 1989;46(11):1012-1016.
3. Geller DA, Biederman J, Faraone S, et al. Re-examining comorbidity of obsessive compulsive and attention-deficit hyperactivity disorder using an empirically derived taxonomy. Eur Child Adolesc Psychiatry. 2004;13(2):83-91.
4. Freeman RD. Attention deficit hyperactivity disorder in the presence of Tourette syndrome. Neurol Clin. 1997;15(2):411-420.
5. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin North Am. 2006;29(2):353-370.
6. Eapen V, Fox-Hiley P, Banerjee S, et al. Clinical features and associated psychopathology in a Tourette syndrome cohort. Acta Neurol Scand. 2004;109(4):255-260.
7. Kano Y, Ohta M, Nagai Y, et al. Association between Tourette syndrome and comorbidities in Japan. Brain Dev. 2010;32(3):201-207.
8. Grados M, Riddle M. Do all obsessive-compulsive disorder subtypes respond to medication? Int Rev Psychiatry. 2008;20(2):189-193.
9. Lipkin PH, Goldstein IH, Adesman AR. Tics and dyskinesias associated with stimulant treatment in attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 1994;148(8):859-861.
10. Castellanos FX, Giedd JN, Elia J, et al. Controlled stimulant treatment of ADHD and comorbid Tourette’s syndrome: effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry. 1997;36(5):589-596.
11. Gadow K, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry. 1999;56(4):330-333.
12. Gadow KD, Sverd J, Nolan EE, et al. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):840-848.
13. Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527-536.
14. Lyon GJ, Samar SM, Conelea C, et al. Testing tic suppression: comparing the effects of dexmethylphenidate to no mediation in children and adolescents with attention-deficit/hyperactivity disorder and Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):283-289.
15. Gadow KD, Sverd J, Sprafkin J, et al. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry. 1995;52(6):444-455.
16. Bloch MH, Panza KE, Landerso-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
17. McDougle CJ, Goodman WK, Price LH. Dopamine antagonists in tic-related and psychotic spectrum obsessive compulsive disorder. J Clin Psychiatry. 1994;55(suppl):24-31.
18. Wilens TE, Morrison NR. The intersection of attention-deficit/hyperactivity disorder and substance abuse. Curr Opin Psychiatry. 2011;24(4):280-285.
19. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
20. Schubiner H. Substance abuse in patients with attention-deficit hyperactivity disorder: therapeutic implications. CNS Drugs. 2005;19(8):643-655.
21. Stahl SM. The prescriber’s guide. Stahl’s essential psychopharmacology. 3rd ed. New York NY: Cambridge University Press; 2009.
22. Jain R, Segal S, Kollins SH, et al. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(2):171-179.
23. Hedderick EF, Morris CM, Singer HS. Double-blind crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol. 2009;40(6):420-425.
24. Joffe RT, Swinson RP, Levitt AJ. Acute psychostimulant challenge in primary obsessive-compulsive disorder. J Clin Psychopharmacol. 1991;11(4):237-241.
25. Insel TR, Hamilton JA, Guttmacher LB, et al. D-amphetamine in obsessive-compulsive disorder. Psychopharmacology (Berl). 1983;80(3):231-235.
CASE: Perfect breath
Mr. C, a 20-year-old college student, is diagnosed with obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), and tic disorder (TD). His obsessions consist of a persistent sense that he is not breathing “correctly” or “perfectly.” He compulsively holds his breath to “rush blood to my head” until “the pressure feels just right.” Mr. C says that his OCD has had longstanding, significant negative impact on his academic performance and capacity to engage in other activities. Tics have been present for years and manifest as coughing and throat-clearing. After multiple syncopal epi-sodes from breath-holding with Valsalva maneuver—some of which caused falls and head injury—Mr. C is admitted to a residential psychiatric unit specializing in treating OCD. At the time of his admission, his Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores1,2 are 23 total, 12 on the obsessions subscale, and 11 on the compulsions subscale, indicating moderate to severe illness. Cognitive-behavioral therapy (CBT) is offered, along with a combination of escitalopram, 60 mg/d, and quetiapine, 50 mg/d. Quetiapine is over-sedating at subtherapeutic doses and Mr. C’s compulsions worsen. He reports that “[it] took longer and longer to get the ‘just right’ feeling.’” Quetiapine is discontinued and risperidone, 0.5 mg/d, is started, which decreases the frequency of his tics. When he is discharged after a 36-day stay, Mr. C’s Y-BOCS scores are greatly improved at 13 total, 7 on the obsessions subscale, and 3 on the compulsions subscale.
Mr. C’s psychologist refers him to our outpatient clinic for continued psychiatric evaluation and treatment of his OCD, ADHD, and TD. At this time, he is prescribed escitalopram, 60 mg/d, and risperidone, 0.5 mg/d, along with CBT with his psychologist. We do not readminister the Y-BOCS at this time, but Mr. C reports that his OCD is “60% improved.” However, he describes prominent obsessive thoughts regarding his breathing similar to those he experienced before residential treatment. These obsessive thoughts arise in the context of specific environmental “triggers,” such as other people coughing or his own tics. The obsessions lead to compulsive urges to engage in breath-holding rituals. Mr. C experiences the thoughts and compulsions as deeply troubling and they consume 5 to 6 hours each day. Mr. C reports impaired concentration in class and during studying: “I can focus for 5 minutes, then not for 2 minutes, then for 3 minutes… I can never stay focused for more than a couple minutes,” before becoming distracted “by my OCD” or other environmental stimuli. We note on exam prominent breath-holding occurring several times per minute. Mr. C says his OCD has not impaired his ability to socialize.
Mr. C notes that he has been exposed to an array of CBT techniques, but he has difficulty using these techniques because his “mind wanders” or he lacks “motivation.” He admits he occasionally has taken a classmate’s ADHD medication (mixed amphetamine salts [MAS], dose unspecified) and found it improved his ability to focus on his academic work.
The authors’ observations
Researchers have established a relationship among OCD, ADHD, and TD across all combinations of comorbidity (OCD and ADHD,3 ADHD and TD,4 OCD and TD,5,6 and all 3 entities7). Data suggests a poorer prognosis for OCD when comorbid with either or both of these conditions.8 Researchers have raised concerns that psychostimulants could exacerbate or potentiate tic behaviors in patients with ADHD,9,10 although safe and effective use of these medications has been documented in controlled trials of patients with comorbid ADHD and tics.11-13 Furthermore, tic suppression has been reported with psychostimulants,14 as well as a differential effect of stimulants on motor vs vocal tics.15 Despite these data (Table 1),9-15 the FDA regards using psychostimulants in patients with TD as a contraindication,16 although clinicians often recognize that this practice may be unavoidable in some circumstances because of high comorbidity rates. Psychostimulants could exacerbate obsessions or compulsions in some patients because of their dopaminergic properties or through mitigation of the purported anti-obsessional properties of dopamine antagonists.17
Although there is evidence that the prevalence of prescribed psychostimulant abuse is low among ADHD patients,18 diversion of prescribed medication is a risk inherent in the use of these agents, particularly among college-age patients.19,20
Table 1
Evidence of effect of psychostimulants on tics
| Study/disorder(s) | Medication and study design | Relevant findings |
|---|---|---|
| Lipkin et al, 19949; ADHD without TD | Chart review (N = 122) to determine the incidence of tics or dyskinesias in children treated with stimulants | Approximately 9% of children developed tics or dyskinesias, which predominantly were transient, with <1% developing chronic tics or Tourette’s syndrome. Personal or family tic history and medication selection or dosage were not related to onset of tics or dyskinesias |
| Gadow et al, 199515; ADHD with TD | Methylphenidate variable dose, placebo-controlled, 2-week trials (N = 24) | All children’s ADHD symptoms improved. At a 0.1 mg/kg dose, motor tics observed in the classroom increased, but there were fewer vocal tics observed in the lunchroom |
| Castellanos et al, 199710; ADHD with Tourette’s syndrome | Methylphenidate, dextroamphetamine, variable-dose, double-blind, placebo-controlled, 9-week crossover (N = 20) | 3 patients had consistent worsening of tics while taking stimulants. Stimulants reduced hyperactivity rates compared with placebo (P = .03). Stimulants improved ADHD symptoms and had acceptable effects on tics. Methylphenidate was better tolerated than dextroamphetamine |
| Gadow et al, 199911; ADHD with TD | 34 methylphenidate-treated children, followed at 6-month intervals for 2 years | No evidence that frequency or severity of motor or vocal tics changed during maintenance therapy |
| Tourette Syndrome Study Group, 200213; ADHD with TD | Clonidine alone, methylphenidate alone, clonidine plus methylphenidate, or placebo | Worsening of tics was not reported in any group at a rate significantly higher than placebo. Tic severity was more reduced in the 2 clonidine groups than in the methylphenidate group |
| Lyon et al, 201014; ADHD with Tourette’s syndrome | Dexmethylphenidate, single-dose challenge. Ten patients with or without TSP | Acute dexmethylphenidate administration resulted in tic suppression but did not augment TSP |
| Gadow et al, 200712; ADHD with TD | Double-blind, placebo-controlled, 2-week trials each of 3 doses of methylphenidate and placebo (N = 71) | MPH-IR did not alter the overall severity of TD or OCD behaviors. Teacher ratings indicated that MPH-IR therapy decreased tic frequency and severity |
| ADHD: attention-deficit/hyperactivity disorder; MPH-IR: methylphenidate immediate release; OCD: obsessive-compulsive disorder; TD: tic disorder; TSP: tic suppression protocol | ||
TREATMENT: Weighing options
To manage impaired attention and executive function difficulties secondary to ADHD, we offer Mr. C several options, including bupropion, modafinil, and memantine augmentation. Mr. C asks for a psychostimulant because exam week is approaching and he wants a treatment with quick therapeutic effect. We discuss with Mr. C the potential for dopaminergic agents, such as psychostimulants, to exacerbate tics or OCD symptoms. Ultimately, we prescribe immediate-release MAS, 20 mg/d.
Two days later, Mr. C says he has taken 3 MAS doses and describes a marked reduction in obsessions, significant decrease in frequency of “triggers,” and greater capacity to use CBT saying, “when I am [triggered], I am able to move past the urges without doing any compulsions.” Daily time spent “stuck on” obsessions or compulsions decreases from 5 to 6 hours per day to “about 2 and a half minutes.”
Mr. C reports a modest increase in the prevalence of tics, experienced as “little throat clears and quick stuttering of breath.” He notes that, although in the past such tics would be followed by urges for “perfecting the tic and making it feel just right,” he presently “had no desire to do so.”
OUTCOME: Sharper focus
Increasing MAS immediate release from 20 mg/d to 30 mg/d suppresses Mr. C’s obsessions and compulsions for 8 hours. On the 19th day of treatment, MAS immediate release was replaced with an extended release formulation, 30 mg/d, which preserves therapeutic effect and tolerability for 16 weeks. Repeat Y-BOCS yields 9 total, 3 on obsessions subscale, and 6 on compulsions subscale scores.
One month later, Mr. C reports that his symptoms have been “improving ever since” the previous appointment. He continues to be able to access skills for managing his OCD and is doing well in his 2 accelerated summer courses, saying “I focus really well” in 3-hour class sessions. On exam, tic behaviors are nearly absent. Mr. C describes occasional bouts of anxiety associated with urges to engage in tic behaviors, in turn arising from fear of symptomatic recurrence as he worked toward stopping smoking as advised by his primary care physician and psychiatrist.
The authors’ observations
The results of the repeat Y-BOCS are consistent with improvement in obsessions but possible worsening of compulsions since Mr. C was discharged from residential treatment. Alternatively, compulsions may have worsened immediately after discharge and declined again with introduction of MAS.
A substantial body of literature describes the challenges associated with treating ADHD with comorbid tics, including the relative degree of risk of tic exacerbation associated with treating ADHD with psychostimulants. The range of FDA-approved pharmacologic options for treatment of this comorbidity is limited (Table 2),21 particularly given the risk for tardive dyskinesia associated with the typical antipsychotics haloperidol and chlorpromazine. Data support using the α-2 agonist clonidine to treat hyperactivity associated with ADHD22 and TD23 and an extended-release preparation of this medication is FDA-approved for the former but not the latter indication (an α-2A receptor subtype agonist, guanfacine, also is FDA-approved for ADHD in pediatric patients). Mr. C’s experience of robust, sustained reduction in obsessions, if not compulsions, after treatment with MAS is consistent with the few studies of stimulant use in ADHD with comorbid OCD.24,25
Effective treatment of ADHD may help Mr. C better access CBT strategies and thereby potentiate treatment of comorbid OCD.
Table 2
FDA-approved medications for ADHD, OCD, and TD
| Disorder | Medications |
|---|---|
| ADHD | Amphetamine (racemic), atomoxetine, chlorpromazine (hyperactivity), clonidine extended release, dexmethylphenidate, dextroamphetamine, guanfacine extended release, haloperidol (hyperactivity, second-line), lisdexamfetamine, methylphenidate (racemic) |
| OCD | Clomipramine, fluoxetine, fluvoxamine, paroxetine, sertraline |
| TD/Tourette’s syndrome | Haloperidol (Tourette’s), pimozide (Tourette’s) |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder; TD: tic disorder Source: Reference 21 | |
Related Resources
- Pliszka SR. Treating ADHD and comorbid disorders: psychosocial and psychopharmacological interventions. New York, NY: The Guilford Press; 2011.
- Pollak Y, Benarroch F, Kanengisser L, et al. Tourette syndrome-associated psychopathology: roles of comorbid attention-deficit hyperactivity disorder and obsessive-compulsive disorder. J Dev Behav Pediatr. 2009;30(5):413-419.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clonidine extended release • Kapvay
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Guanfacine • Intuniv, Tenex
- Haloperidol • Haldol
- Lisdexamfetamine • Vyvanse
- Memantine • Namenda
- Methylphenidate • Methylin, Ritalin
- Modafinil • Provigil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Perfect breath
Mr. C, a 20-year-old college student, is diagnosed with obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), and tic disorder (TD). His obsessions consist of a persistent sense that he is not breathing “correctly” or “perfectly.” He compulsively holds his breath to “rush blood to my head” until “the pressure feels just right.” Mr. C says that his OCD has had longstanding, significant negative impact on his academic performance and capacity to engage in other activities. Tics have been present for years and manifest as coughing and throat-clearing. After multiple syncopal epi-sodes from breath-holding with Valsalva maneuver—some of which caused falls and head injury—Mr. C is admitted to a residential psychiatric unit specializing in treating OCD. At the time of his admission, his Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores1,2 are 23 total, 12 on the obsessions subscale, and 11 on the compulsions subscale, indicating moderate to severe illness. Cognitive-behavioral therapy (CBT) is offered, along with a combination of escitalopram, 60 mg/d, and quetiapine, 50 mg/d. Quetiapine is over-sedating at subtherapeutic doses and Mr. C’s compulsions worsen. He reports that “[it] took longer and longer to get the ‘just right’ feeling.’” Quetiapine is discontinued and risperidone, 0.5 mg/d, is started, which decreases the frequency of his tics. When he is discharged after a 36-day stay, Mr. C’s Y-BOCS scores are greatly improved at 13 total, 7 on the obsessions subscale, and 3 on the compulsions subscale.
Mr. C’s psychologist refers him to our outpatient clinic for continued psychiatric evaluation and treatment of his OCD, ADHD, and TD. At this time, he is prescribed escitalopram, 60 mg/d, and risperidone, 0.5 mg/d, along with CBT with his psychologist. We do not readminister the Y-BOCS at this time, but Mr. C reports that his OCD is “60% improved.” However, he describes prominent obsessive thoughts regarding his breathing similar to those he experienced before residential treatment. These obsessive thoughts arise in the context of specific environmental “triggers,” such as other people coughing or his own tics. The obsessions lead to compulsive urges to engage in breath-holding rituals. Mr. C experiences the thoughts and compulsions as deeply troubling and they consume 5 to 6 hours each day. Mr. C reports impaired concentration in class and during studying: “I can focus for 5 minutes, then not for 2 minutes, then for 3 minutes… I can never stay focused for more than a couple minutes,” before becoming distracted “by my OCD” or other environmental stimuli. We note on exam prominent breath-holding occurring several times per minute. Mr. C says his OCD has not impaired his ability to socialize.
Mr. C notes that he has been exposed to an array of CBT techniques, but he has difficulty using these techniques because his “mind wanders” or he lacks “motivation.” He admits he occasionally has taken a classmate’s ADHD medication (mixed amphetamine salts [MAS], dose unspecified) and found it improved his ability to focus on his academic work.
The authors’ observations
Researchers have established a relationship among OCD, ADHD, and TD across all combinations of comorbidity (OCD and ADHD,3 ADHD and TD,4 OCD and TD,5,6 and all 3 entities7). Data suggests a poorer prognosis for OCD when comorbid with either or both of these conditions.8 Researchers have raised concerns that psychostimulants could exacerbate or potentiate tic behaviors in patients with ADHD,9,10 although safe and effective use of these medications has been documented in controlled trials of patients with comorbid ADHD and tics.11-13 Furthermore, tic suppression has been reported with psychostimulants,14 as well as a differential effect of stimulants on motor vs vocal tics.15 Despite these data (Table 1),9-15 the FDA regards using psychostimulants in patients with TD as a contraindication,16 although clinicians often recognize that this practice may be unavoidable in some circumstances because of high comorbidity rates. Psychostimulants could exacerbate obsessions or compulsions in some patients because of their dopaminergic properties or through mitigation of the purported anti-obsessional properties of dopamine antagonists.17
Although there is evidence that the prevalence of prescribed psychostimulant abuse is low among ADHD patients,18 diversion of prescribed medication is a risk inherent in the use of these agents, particularly among college-age patients.19,20
Table 1
Evidence of effect of psychostimulants on tics
| Study/disorder(s) | Medication and study design | Relevant findings |
|---|---|---|
| Lipkin et al, 19949; ADHD without TD | Chart review (N = 122) to determine the incidence of tics or dyskinesias in children treated with stimulants | Approximately 9% of children developed tics or dyskinesias, which predominantly were transient, with <1% developing chronic tics or Tourette’s syndrome. Personal or family tic history and medication selection or dosage were not related to onset of tics or dyskinesias |
| Gadow et al, 199515; ADHD with TD | Methylphenidate variable dose, placebo-controlled, 2-week trials (N = 24) | All children’s ADHD symptoms improved. At a 0.1 mg/kg dose, motor tics observed in the classroom increased, but there were fewer vocal tics observed in the lunchroom |
| Castellanos et al, 199710; ADHD with Tourette’s syndrome | Methylphenidate, dextroamphetamine, variable-dose, double-blind, placebo-controlled, 9-week crossover (N = 20) | 3 patients had consistent worsening of tics while taking stimulants. Stimulants reduced hyperactivity rates compared with placebo (P = .03). Stimulants improved ADHD symptoms and had acceptable effects on tics. Methylphenidate was better tolerated than dextroamphetamine |
| Gadow et al, 199911; ADHD with TD | 34 methylphenidate-treated children, followed at 6-month intervals for 2 years | No evidence that frequency or severity of motor or vocal tics changed during maintenance therapy |
| Tourette Syndrome Study Group, 200213; ADHD with TD | Clonidine alone, methylphenidate alone, clonidine plus methylphenidate, or placebo | Worsening of tics was not reported in any group at a rate significantly higher than placebo. Tic severity was more reduced in the 2 clonidine groups than in the methylphenidate group |
| Lyon et al, 201014; ADHD with Tourette’s syndrome | Dexmethylphenidate, single-dose challenge. Ten patients with or without TSP | Acute dexmethylphenidate administration resulted in tic suppression but did not augment TSP |
| Gadow et al, 200712; ADHD with TD | Double-blind, placebo-controlled, 2-week trials each of 3 doses of methylphenidate and placebo (N = 71) | MPH-IR did not alter the overall severity of TD or OCD behaviors. Teacher ratings indicated that MPH-IR therapy decreased tic frequency and severity |
| ADHD: attention-deficit/hyperactivity disorder; MPH-IR: methylphenidate immediate release; OCD: obsessive-compulsive disorder; TD: tic disorder; TSP: tic suppression protocol | ||
TREATMENT: Weighing options
To manage impaired attention and executive function difficulties secondary to ADHD, we offer Mr. C several options, including bupropion, modafinil, and memantine augmentation. Mr. C asks for a psychostimulant because exam week is approaching and he wants a treatment with quick therapeutic effect. We discuss with Mr. C the potential for dopaminergic agents, such as psychostimulants, to exacerbate tics or OCD symptoms. Ultimately, we prescribe immediate-release MAS, 20 mg/d.
Two days later, Mr. C says he has taken 3 MAS doses and describes a marked reduction in obsessions, significant decrease in frequency of “triggers,” and greater capacity to use CBT saying, “when I am [triggered], I am able to move past the urges without doing any compulsions.” Daily time spent “stuck on” obsessions or compulsions decreases from 5 to 6 hours per day to “about 2 and a half minutes.”
Mr. C reports a modest increase in the prevalence of tics, experienced as “little throat clears and quick stuttering of breath.” He notes that, although in the past such tics would be followed by urges for “perfecting the tic and making it feel just right,” he presently “had no desire to do so.”
OUTCOME: Sharper focus
Increasing MAS immediate release from 20 mg/d to 30 mg/d suppresses Mr. C’s obsessions and compulsions for 8 hours. On the 19th day of treatment, MAS immediate release was replaced with an extended release formulation, 30 mg/d, which preserves therapeutic effect and tolerability for 16 weeks. Repeat Y-BOCS yields 9 total, 3 on obsessions subscale, and 6 on compulsions subscale scores.
One month later, Mr. C reports that his symptoms have been “improving ever since” the previous appointment. He continues to be able to access skills for managing his OCD and is doing well in his 2 accelerated summer courses, saying “I focus really well” in 3-hour class sessions. On exam, tic behaviors are nearly absent. Mr. C describes occasional bouts of anxiety associated with urges to engage in tic behaviors, in turn arising from fear of symptomatic recurrence as he worked toward stopping smoking as advised by his primary care physician and psychiatrist.
The authors’ observations
The results of the repeat Y-BOCS are consistent with improvement in obsessions but possible worsening of compulsions since Mr. C was discharged from residential treatment. Alternatively, compulsions may have worsened immediately after discharge and declined again with introduction of MAS.
A substantial body of literature describes the challenges associated with treating ADHD with comorbid tics, including the relative degree of risk of tic exacerbation associated with treating ADHD with psychostimulants. The range of FDA-approved pharmacologic options for treatment of this comorbidity is limited (Table 2),21 particularly given the risk for tardive dyskinesia associated with the typical antipsychotics haloperidol and chlorpromazine. Data support using the α-2 agonist clonidine to treat hyperactivity associated with ADHD22 and TD23 and an extended-release preparation of this medication is FDA-approved for the former but not the latter indication (an α-2A receptor subtype agonist, guanfacine, also is FDA-approved for ADHD in pediatric patients). Mr. C’s experience of robust, sustained reduction in obsessions, if not compulsions, after treatment with MAS is consistent with the few studies of stimulant use in ADHD with comorbid OCD.24,25
Effective treatment of ADHD may help Mr. C better access CBT strategies and thereby potentiate treatment of comorbid OCD.
Table 2
FDA-approved medications for ADHD, OCD, and TD
| Disorder | Medications |
|---|---|
| ADHD | Amphetamine (racemic), atomoxetine, chlorpromazine (hyperactivity), clonidine extended release, dexmethylphenidate, dextroamphetamine, guanfacine extended release, haloperidol (hyperactivity, second-line), lisdexamfetamine, methylphenidate (racemic) |
| OCD | Clomipramine, fluoxetine, fluvoxamine, paroxetine, sertraline |
| TD/Tourette’s syndrome | Haloperidol (Tourette’s), pimozide (Tourette’s) |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder; TD: tic disorder Source: Reference 21 | |
Related Resources
- Pliszka SR. Treating ADHD and comorbid disorders: psychosocial and psychopharmacological interventions. New York, NY: The Guilford Press; 2011.
- Pollak Y, Benarroch F, Kanengisser L, et al. Tourette syndrome-associated psychopathology: roles of comorbid attention-deficit hyperactivity disorder and obsessive-compulsive disorder. J Dev Behav Pediatr. 2009;30(5):413-419.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clonidine extended release • Kapvay
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Guanfacine • Intuniv, Tenex
- Haloperidol • Haldol
- Lisdexamfetamine • Vyvanse
- Memantine • Namenda
- Methylphenidate • Methylin, Ritalin
- Modafinil • Provigil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use and reliability. Arch Gen Psych. 1989;46(11):1006-1011.
2. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: II. Validity. Arch Gen Psych. 1989;46(11):1012-1016.
3. Geller DA, Biederman J, Faraone S, et al. Re-examining comorbidity of obsessive compulsive and attention-deficit hyperactivity disorder using an empirically derived taxonomy. Eur Child Adolesc Psychiatry. 2004;13(2):83-91.
4. Freeman RD. Attention deficit hyperactivity disorder in the presence of Tourette syndrome. Neurol Clin. 1997;15(2):411-420.
5. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin North Am. 2006;29(2):353-370.
6. Eapen V, Fox-Hiley P, Banerjee S, et al. Clinical features and associated psychopathology in a Tourette syndrome cohort. Acta Neurol Scand. 2004;109(4):255-260.
7. Kano Y, Ohta M, Nagai Y, et al. Association between Tourette syndrome and comorbidities in Japan. Brain Dev. 2010;32(3):201-207.
8. Grados M, Riddle M. Do all obsessive-compulsive disorder subtypes respond to medication? Int Rev Psychiatry. 2008;20(2):189-193.
9. Lipkin PH, Goldstein IH, Adesman AR. Tics and dyskinesias associated with stimulant treatment in attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 1994;148(8):859-861.
10. Castellanos FX, Giedd JN, Elia J, et al. Controlled stimulant treatment of ADHD and comorbid Tourette’s syndrome: effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry. 1997;36(5):589-596.
11. Gadow K, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry. 1999;56(4):330-333.
12. Gadow KD, Sverd J, Nolan EE, et al. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):840-848.
13. Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527-536.
14. Lyon GJ, Samar SM, Conelea C, et al. Testing tic suppression: comparing the effects of dexmethylphenidate to no mediation in children and adolescents with attention-deficit/hyperactivity disorder and Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):283-289.
15. Gadow KD, Sverd J, Sprafkin J, et al. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry. 1995;52(6):444-455.
16. Bloch MH, Panza KE, Landerso-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
17. McDougle CJ, Goodman WK, Price LH. Dopamine antagonists in tic-related and psychotic spectrum obsessive compulsive disorder. J Clin Psychiatry. 1994;55(suppl):24-31.
18. Wilens TE, Morrison NR. The intersection of attention-deficit/hyperactivity disorder and substance abuse. Curr Opin Psychiatry. 2011;24(4):280-285.
19. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
20. Schubiner H. Substance abuse in patients with attention-deficit hyperactivity disorder: therapeutic implications. CNS Drugs. 2005;19(8):643-655.
21. Stahl SM. The prescriber’s guide. Stahl’s essential psychopharmacology. 3rd ed. New York NY: Cambridge University Press; 2009.
22. Jain R, Segal S, Kollins SH, et al. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(2):171-179.
23. Hedderick EF, Morris CM, Singer HS. Double-blind crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol. 2009;40(6):420-425.
24. Joffe RT, Swinson RP, Levitt AJ. Acute psychostimulant challenge in primary obsessive-compulsive disorder. J Clin Psychopharmacol. 1991;11(4):237-241.
25. Insel TR, Hamilton JA, Guttmacher LB, et al. D-amphetamine in obsessive-compulsive disorder. Psychopharmacology (Berl). 1983;80(3):231-235.
1. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use and reliability. Arch Gen Psych. 1989;46(11):1006-1011.
2. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: II. Validity. Arch Gen Psych. 1989;46(11):1012-1016.
3. Geller DA, Biederman J, Faraone S, et al. Re-examining comorbidity of obsessive compulsive and attention-deficit hyperactivity disorder using an empirically derived taxonomy. Eur Child Adolesc Psychiatry. 2004;13(2):83-91.
4. Freeman RD. Attention deficit hyperactivity disorder in the presence of Tourette syndrome. Neurol Clin. 1997;15(2):411-420.
5. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin North Am. 2006;29(2):353-370.
6. Eapen V, Fox-Hiley P, Banerjee S, et al. Clinical features and associated psychopathology in a Tourette syndrome cohort. Acta Neurol Scand. 2004;109(4):255-260.
7. Kano Y, Ohta M, Nagai Y, et al. Association between Tourette syndrome and comorbidities in Japan. Brain Dev. 2010;32(3):201-207.
8. Grados M, Riddle M. Do all obsessive-compulsive disorder subtypes respond to medication? Int Rev Psychiatry. 2008;20(2):189-193.
9. Lipkin PH, Goldstein IH, Adesman AR. Tics and dyskinesias associated with stimulant treatment in attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 1994;148(8):859-861.
10. Castellanos FX, Giedd JN, Elia J, et al. Controlled stimulant treatment of ADHD and comorbid Tourette’s syndrome: effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry. 1997;36(5):589-596.
11. Gadow K, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry. 1999;56(4):330-333.
12. Gadow KD, Sverd J, Nolan EE, et al. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):840-848.
13. Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527-536.
14. Lyon GJ, Samar SM, Conelea C, et al. Testing tic suppression: comparing the effects of dexmethylphenidate to no mediation in children and adolescents with attention-deficit/hyperactivity disorder and Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):283-289.
15. Gadow KD, Sverd J, Sprafkin J, et al. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry. 1995;52(6):444-455.
16. Bloch MH, Panza KE, Landerso-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
17. McDougle CJ, Goodman WK, Price LH. Dopamine antagonists in tic-related and psychotic spectrum obsessive compulsive disorder. J Clin Psychiatry. 1994;55(suppl):24-31.
18. Wilens TE, Morrison NR. The intersection of attention-deficit/hyperactivity disorder and substance abuse. Curr Opin Psychiatry. 2011;24(4):280-285.
19. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
20. Schubiner H. Substance abuse in patients with attention-deficit hyperactivity disorder: therapeutic implications. CNS Drugs. 2005;19(8):643-655.
21. Stahl SM. The prescriber’s guide. Stahl’s essential psychopharmacology. 3rd ed. New York NY: Cambridge University Press; 2009.
22. Jain R, Segal S, Kollins SH, et al. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(2):171-179.
23. Hedderick EF, Morris CM, Singer HS. Double-blind crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol. 2009;40(6):420-425.
24. Joffe RT, Swinson RP, Levitt AJ. Acute psychostimulant challenge in primary obsessive-compulsive disorder. J Clin Psychopharmacol. 1991;11(4):237-241.
25. Insel TR, Hamilton JA, Guttmacher LB, et al. D-amphetamine in obsessive-compulsive disorder. Psychopharmacology (Berl). 1983;80(3):231-235.
Mood instability in ADHD
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Do stimulants for ADHD increase the risk of substance use disorders?
Discuss this article at www.facebook.com/CurrentPsychiatry
Does prescribing stimulants to patients with attention-deficit/hyperactivity disorder (ADHD) increase their risk of future substance abuse? Because ADHD is a common pediatric condition with symptoms that often persist into adulthood, and stimulants are an efficacious first-line therapy, this possible association is a concern for psychiatrists whether they treat children or adults.
Some researchers have expressed concerns that stimulant exposure could predispose patients to future substance abuse.1 Proponents of the biologic model of “kindling” hypothesize early exposure to stimulants could increase the risk of later substance use disorders (SUDs) by modifying or “priming” the brain, which then becomes more receptive to illicit drug exposure. Although there is some evidence that stimulant use does increase SUD risk, other evidence suggests stimulant use does not increase susceptibility to SUDs2,3 and some studies have suggested stimulant use in ADHD patients may protect against SUDs.4,5
This article reviews shared characteristics of ADHD and SUDs and the latest research on the association between the clinical use of stimulants and later development of SUDs. We also offer clinical recommendations for assessing and treating ADHD and comorbid SUD.
ADHD/SUD overlap
Compared with those without the disorder, patients with ADHD have a 6.2 times higher risk of developing an SUD.6 Individuals with ADHD experience an earlier age of onset and a longer duration of SUDs.7 Several retrospective and prospective studies reveal ADHD is a risk factor for SUDs.8 A longitudinal study that tracked teenage males with or without ADHD into young adulthood found SUDs were 4 times more common among those with ADHD.9 Up to 45% of adults with ADHD have a history of alcohol abuse or dependence, and up to 30% have a history of illegal drug abuse or dependence.10
Conversely, an estimated 35% to 71% of alcohol abusers and 15% to 25% of substance-dependent patients have ADHD.11 Adults with ADHD and comorbid SUD report earlier onset12 and greater severity13 of substance abuse than adults without ADHD. Patients with ADHD experience earlier onset and higher rates of tobacco smoking by mid-adolescence.14
Developmental psychopathology. Longitudinal studies have suggested certain psychopathologic characteristics of ADHD can predispose an individual to SUDs independent of stimulant exposure. For example, inattention, impulsivity, and hyperactivity predispose an individual to develop an SUD and also are core symptoms of ADHD.15 Another study found impulsivity, impersistence, and difficulty sitting still at age 3 predicted alcohol abuse at age 21.16 A different longitudinal study found novelty-seeking behavior (restlessness, running/jumping and not keeping still, being squirmy and fidgety) between age 6 to 10 predicted adolescent drug abuse and cigarette smoking.17 Poor response inhibition is a key characteristic of ADHD and has been linked to adolescent drinking.18
ADHD may be an independent risk factor for SUD because a common neurobiologic psychopathology may predispose an individual to develop both conditions. The dopamine system has been implicated in SUD, and dysfunction in the dopaminergic circuits—mostly in basal and frontal cortex with consequent defects in executive function and reward system—also has been found in ADHD.19 Cognitive dysfunction associated with ADHD may decrease a patient’s ability to estimate the negative consequences of substance abuse and to delay immediate gratification from drug or alcohol use.
ADHD patients are more vulnerable to SUDs if they have a comorbid condition, such as oppositional defiant disorder,13,20 bipolar disorder,20,21 or conduct disorder (CD).20,22 Patients with ADHD and comorbid CD are estimated to be 8.8 times more likely to have an SUD before age 18 compared with those with ADHD alone.23 Comorbid ADHD and CD may increase patients’ predisposition to develop dependence on highly addictive drugs, such as cocaine or methamphetamine.24 Impaired executive function, behavioral dyscontrol, impulsivity, and peer rejection are common in both ADHD and CD and may increase the risk of developing SUDs in individuals who have both conditions.25 Other risk factors for SUDs in patients with ADHD are listed in Table 1.26
Table 1
Risk factors for SUDs in patients with ADHD
| Presence of comorbid conditions (ie, oppositional defiant disorder, conduct disorder, bipolar disorder, eating disorder) |
| White or Hispanic race |
| Partially treated or residual ADHD symptoms |
| Attending a competitive college program |
| College youth who had late onset of stimulant treatment |
| Member of a college sorority/fraternity |
| ADHD: attention-deficit/hyperactivity disorder; SUDs: substance use disorders Source: Reference 26 |
Stimulants’ affect on SUD risk
Increased risk. Limited studies suggest exposure to stimulants is a risk factor for developing SUDs. In a longitudinal study, Lambert et al27 followed 218 patients with ADHD and 182 without ADHD into adulthood and found a linear trend between duration of stimulant treatment and prevalence of cocaine dependence. ADHD patients exposed to stimulants for >1 year had the highest prevalence of cocaine abuse (27%), compared with untreated subjects (15%), or those treated with stimulants for <1 year (18%). However, the study did not control for comorbid contributing factors, such as CD.
No change. In a 10-year naturalistic study, Biederman et al28 followed 109 children with ADHD age 7 to 12 into adulthood. These children had a developmental reading disorder but no other psychiatric comorbidities. When comparing patients who were treated with methylphenidate (n = 43) with those who did not receive stimulants (n = 66), Bierderman et al found no significant difference between the 2 groups in the prevalence of SUD for any of the 7 drug categories studied.
Decreased risk. Two meta-analyses found children with ADHD who were treated with stimulants and followed until adolescence were 5.8 times less likely to develop SUDs compared with those who did not receive stimulants.28,29 This protective effect diminished when patients were followed into adulthood, but individuals treated with stimulants were 1.4 times less likely to develop SUDs than those not treated with stimulants.30 In a prospective case-control, 5-year follow-up study of 114 patients with ADHD treated with stimulants, Wilens et al31 found significant protective effects of stimulant treatment on the development of any SUD. They found no effects from time of onset or duration of stimulant therapy on subsequent risk of SUDs or cigarette smoking.
One possible explanation for stimulants’ apparently reduced protective effect among adults is for patients with ADHD, stimulant use might delay but not prevent SUDs. It also is likely that by adulthood, loss of parental supervision leads to poor medication adherence and increased susceptibility to SUDs.30
Other studies have found exposure to stimulants may protect against SUDs. Katusic et al23 reviewed medical records for documented SUDs in 295 adults with ADHD treated with stimulants and 84 who did not receive stimulants. They found 20% of patients who received stimulants had a documented SUD compared with 27% of those not treated with stimulants. Barkely et al32 followed 98 stimulant-treated and 21 untreated ADHD patients with a mean age of 15 and 21, respectively. They found stimulant treatment did not increase the risk for substance use or abuse in either group.
ADHD and stimulant abuse
The prevalence of stimulant misuse is as high as 9% in patients in grade school and high school and up to 35% in college-age individuals.33 ADHD patients who misuse stimulants (eg, escalating dose without authorization) or skip stimulant doses to use illicit drugs or alcohol are more likely to sell their medication.34 Immediate-release stimulant formulations are more liable to be abused than extended-release drugs because they achieve earlier peak drug concentrations and dopamine blockade, indicating rapid drug absorption and central drug activity. Close monitoring and use of extended-release formulations are useful deterrents against stimulant abuse.
Clinical recommendations
Detecting and treating SUDs in patients with ADHD can be challenging. Ideally, the best time to assess for ADHD symptoms is after a prolonged abstinence from any influencing substance. However, in most clinical situations this is not practical. A better approach is a longitudinal assessment for ADHD symptoms. Detecting evidence of early childhood onset of ADHD symptoms before the patient began using substances can be helpful in conducting a proper differential diagnosis. Assessing for symptoms of SUDs in early adolescence, along with serial assessment of ADHD symptoms, also can be helpful. Symptoms secondary to ADHD are likely to show a consistent pattern, whereas symptoms secondary to an SUD may be sporadic.
When assessing SUD risk, consider the patient’s clinical condition, history of comorbidities that suggest SUDs, and overall functional status. Collateral information about the patient’s behavior and substance abuse from family members is important. A history of CD, bipolar disorder, or antisocial personality disorder should raise concerns about potential future stimulant abuse or diversion. Close monitoring of patients suspected of having an SUD is essential to detect stimulant abuse or diversion, which often manifests as weight loss, requests for higher doses, requests to switch from long-acting or extended-release formulations to immediate-release formulations, and repeated and suspicious “lost prescriptions.” Close observation for other subtle signs—such as changes in personality or mood and unexplained accidents or injuries—also may be needed.35
Challenges of treating ADHD and co-occurring SUD include poor medication adherence, need for a higher therapeutic stimulant dose, and difficulty in assessing the therapeutic benefit of pharmacotherapy in the presence of an SUD.36 Treating ADHD comorbid with SUD requires a collaborative approach that involves a psychiatrist, family members, and a behavioral care provider in addition to frequent monitoring.34
In the absence of treatment guidelines for treating ADHD with comorbid SUDs, some clinicians prefer to stabilize the SUD before initiating stimulants. Others prefer to use nonstimulants (such as atomoxetine, guanfacine, bupropion, venlafaxine, tricyclic antidepressants, or modafinil) as a first-line treatment. However, nonstimulants have not demonstrated efficacy comparable to that of stimulants for ADHD.35
Table 2 offers clinical recommendations to minimize the risk of SUDs when treating ADHD patients with stimulants. Long-acting stimulant formulations are preferred over short-acting medications because they are less likely to be abused. Psychosocial interventions for treating ADHD and co-occurring SUD disorder include cognitive-behavioral therapy with emphasis on structured skills training and cognitive remediation.
Table 2
Minimizing SUD risk when treating ADHD patients with stimulants
| Assess symptom burden and psychosocial impairment |
| Establish a treatment contract and boundaries at the onset of treatment, including your right to terminate treatment if you suspect stimulant misuse |
| Assess for comorbidities that may increase your patient’s SUD risk (see Table 1) |
| Emphasize strict adherence to treatment recommendations |
| Involve the patient’s family as much as possible |
| Obtain collateral information on the patient’s history of ADHD-related symptoms from parents, siblings, significant others, etc. |
| Distinguish between patients with substance use vs an SUD or a history of an SUD |
| Obtain urine toxicology screening as appropriate |
| Carefully document dispensed stimulants– strength of medication, number of capsules, pills, patches, etc. Note date of dispensation and refill dates |
| Select delayed- or extended-release stimulant formulations |
| Consider prescribing nonstimulants if appropriate |
| Use rating scales such as Conners Adult ADHD Rating Scale to monitor ADHD symptom severity and response to treatment |
| Schedule frequent, face-to-face clinical monitoring visits |
| ADHD: attention-deficit/hyperactivity disorder; SUD: substance use disorder |
Related Resource
- Faraone SV, Wilens T. Does stimulant treatment lead to substance use disorders? J Clin Psychiatry. 2003;64(suppl 11):9-13.
- Upadhyaya HP, Rose K, Wang W, et al. Attention deficit hyperactivity disorder medication and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15:799-809.
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;16(suppl 1):45-56.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Guanfacine • Tenex, Intuniv
- Methylphenidate • Ritalin
- Modafinil • Provigil
- Venlafaxine • Effexor
Disclosures
Dr. Shailesh Jain and Dr. Islam report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rakesh Jain has received research support from, is a consultant to, and/or is a speaker for Addrenex Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Forest Pharmaceuticals, Merck, Pamlab, Pfizer Inc., Shionogi Inc., Shire, and Sunovion Pharmaceuticals.
1. Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713-725.
2. Mannuzza S, Klein RG, Moulton JL. Does stimulant treatment place children at risk for adult substance abuse? A controlled prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13(3):273-282.
3. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
4. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
5. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20.-
6. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
7. Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. J Clin Psychiatry. 2004;65(suppl 3):38-45.
8. Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45(2):195-221.
9. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565-576.
10. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
11. Wilens TE. AOD use and attention deficit/hyperactivity disorder. Alcohol Health Res World. 1998;22(2):127-130.
12. Wilens TE, Biederman J, Abrantes AM, et al. Clinical characteristics of psychiatrically referred adolescent outpatients with substance use disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(7):941-947.
13. Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61(4):244-251.
14. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
15. Zucker RA. Alcohol use and the alcohol use disorders: a developmental biopsychosocial systems formulation covering the life course. In: Cicchetti D Cohen D, eds. Developmental psychopathology. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2006;620-656.
16. Caspi A, Moffitt TE, Newman DL, et al. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53(11):1033-1039.
17. Màsse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54(1):62-68.
18. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468-475.
19. Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit hyperactivity disorder. Biol Psychiatry. 2005;57(11):1263-1272.
20. Biederman J, Wilens T, Mick E, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36(1):21-29.
21. Wilens TE, Biederman J, Millstein RB, et al. Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(6):680-685.
22. Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286-294.
23. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
24. Flory K, Milich R, Lynam DR, et al. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17(2):151-158.
25. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
26. Wilson JJ. ADHD and substance use disorders: developmental aspects and the impact of stimulant treatment. Am J Addict. 2007;16(suppl 1):5-11.
27. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
28. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597-603.
29. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
30. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse abuse, and diversion. J Clin Psychiatry. 2007;68(suppl 11):15-22.
31. Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162(10):916-921.
32. Barkley RA, Fischer M, Smallish L, et al. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111(1):97-109.
33. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
34. Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799-809.
35. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
36. Faraone SV, Biederman J, Wilens TE, et al. A naturalistic study of the effects of pharmacotherapy on substance use disorders among ADHD adults. Psychol Med. 2007;37(12):1743-1752.
Discuss this article at www.facebook.com/CurrentPsychiatry
Does prescribing stimulants to patients with attention-deficit/hyperactivity disorder (ADHD) increase their risk of future substance abuse? Because ADHD is a common pediatric condition with symptoms that often persist into adulthood, and stimulants are an efficacious first-line therapy, this possible association is a concern for psychiatrists whether they treat children or adults.
Some researchers have expressed concerns that stimulant exposure could predispose patients to future substance abuse.1 Proponents of the biologic model of “kindling” hypothesize early exposure to stimulants could increase the risk of later substance use disorders (SUDs) by modifying or “priming” the brain, which then becomes more receptive to illicit drug exposure. Although there is some evidence that stimulant use does increase SUD risk, other evidence suggests stimulant use does not increase susceptibility to SUDs2,3 and some studies have suggested stimulant use in ADHD patients may protect against SUDs.4,5
This article reviews shared characteristics of ADHD and SUDs and the latest research on the association between the clinical use of stimulants and later development of SUDs. We also offer clinical recommendations for assessing and treating ADHD and comorbid SUD.
ADHD/SUD overlap
Compared with those without the disorder, patients with ADHD have a 6.2 times higher risk of developing an SUD.6 Individuals with ADHD experience an earlier age of onset and a longer duration of SUDs.7 Several retrospective and prospective studies reveal ADHD is a risk factor for SUDs.8 A longitudinal study that tracked teenage males with or without ADHD into young adulthood found SUDs were 4 times more common among those with ADHD.9 Up to 45% of adults with ADHD have a history of alcohol abuse or dependence, and up to 30% have a history of illegal drug abuse or dependence.10
Conversely, an estimated 35% to 71% of alcohol abusers and 15% to 25% of substance-dependent patients have ADHD.11 Adults with ADHD and comorbid SUD report earlier onset12 and greater severity13 of substance abuse than adults without ADHD. Patients with ADHD experience earlier onset and higher rates of tobacco smoking by mid-adolescence.14
Developmental psychopathology. Longitudinal studies have suggested certain psychopathologic characteristics of ADHD can predispose an individual to SUDs independent of stimulant exposure. For example, inattention, impulsivity, and hyperactivity predispose an individual to develop an SUD and also are core symptoms of ADHD.15 Another study found impulsivity, impersistence, and difficulty sitting still at age 3 predicted alcohol abuse at age 21.16 A different longitudinal study found novelty-seeking behavior (restlessness, running/jumping and not keeping still, being squirmy and fidgety) between age 6 to 10 predicted adolescent drug abuse and cigarette smoking.17 Poor response inhibition is a key characteristic of ADHD and has been linked to adolescent drinking.18
ADHD may be an independent risk factor for SUD because a common neurobiologic psychopathology may predispose an individual to develop both conditions. The dopamine system has been implicated in SUD, and dysfunction in the dopaminergic circuits—mostly in basal and frontal cortex with consequent defects in executive function and reward system—also has been found in ADHD.19 Cognitive dysfunction associated with ADHD may decrease a patient’s ability to estimate the negative consequences of substance abuse and to delay immediate gratification from drug or alcohol use.
ADHD patients are more vulnerable to SUDs if they have a comorbid condition, such as oppositional defiant disorder,13,20 bipolar disorder,20,21 or conduct disorder (CD).20,22 Patients with ADHD and comorbid CD are estimated to be 8.8 times more likely to have an SUD before age 18 compared with those with ADHD alone.23 Comorbid ADHD and CD may increase patients’ predisposition to develop dependence on highly addictive drugs, such as cocaine or methamphetamine.24 Impaired executive function, behavioral dyscontrol, impulsivity, and peer rejection are common in both ADHD and CD and may increase the risk of developing SUDs in individuals who have both conditions.25 Other risk factors for SUDs in patients with ADHD are listed in Table 1.26
Table 1
Risk factors for SUDs in patients with ADHD
| Presence of comorbid conditions (ie, oppositional defiant disorder, conduct disorder, bipolar disorder, eating disorder) |
| White or Hispanic race |
| Partially treated or residual ADHD symptoms |
| Attending a competitive college program |
| College youth who had late onset of stimulant treatment |
| Member of a college sorority/fraternity |
| ADHD: attention-deficit/hyperactivity disorder; SUDs: substance use disorders Source: Reference 26 |
Stimulants’ affect on SUD risk
Increased risk. Limited studies suggest exposure to stimulants is a risk factor for developing SUDs. In a longitudinal study, Lambert et al27 followed 218 patients with ADHD and 182 without ADHD into adulthood and found a linear trend between duration of stimulant treatment and prevalence of cocaine dependence. ADHD patients exposed to stimulants for >1 year had the highest prevalence of cocaine abuse (27%), compared with untreated subjects (15%), or those treated with stimulants for <1 year (18%). However, the study did not control for comorbid contributing factors, such as CD.
No change. In a 10-year naturalistic study, Biederman et al28 followed 109 children with ADHD age 7 to 12 into adulthood. These children had a developmental reading disorder but no other psychiatric comorbidities. When comparing patients who were treated with methylphenidate (n = 43) with those who did not receive stimulants (n = 66), Bierderman et al found no significant difference between the 2 groups in the prevalence of SUD for any of the 7 drug categories studied.
Decreased risk. Two meta-analyses found children with ADHD who were treated with stimulants and followed until adolescence were 5.8 times less likely to develop SUDs compared with those who did not receive stimulants.28,29 This protective effect diminished when patients were followed into adulthood, but individuals treated with stimulants were 1.4 times less likely to develop SUDs than those not treated with stimulants.30 In a prospective case-control, 5-year follow-up study of 114 patients with ADHD treated with stimulants, Wilens et al31 found significant protective effects of stimulant treatment on the development of any SUD. They found no effects from time of onset or duration of stimulant therapy on subsequent risk of SUDs or cigarette smoking.
One possible explanation for stimulants’ apparently reduced protective effect among adults is for patients with ADHD, stimulant use might delay but not prevent SUDs. It also is likely that by adulthood, loss of parental supervision leads to poor medication adherence and increased susceptibility to SUDs.30
Other studies have found exposure to stimulants may protect against SUDs. Katusic et al23 reviewed medical records for documented SUDs in 295 adults with ADHD treated with stimulants and 84 who did not receive stimulants. They found 20% of patients who received stimulants had a documented SUD compared with 27% of those not treated with stimulants. Barkely et al32 followed 98 stimulant-treated and 21 untreated ADHD patients with a mean age of 15 and 21, respectively. They found stimulant treatment did not increase the risk for substance use or abuse in either group.
ADHD and stimulant abuse
The prevalence of stimulant misuse is as high as 9% in patients in grade school and high school and up to 35% in college-age individuals.33 ADHD patients who misuse stimulants (eg, escalating dose without authorization) or skip stimulant doses to use illicit drugs or alcohol are more likely to sell their medication.34 Immediate-release stimulant formulations are more liable to be abused than extended-release drugs because they achieve earlier peak drug concentrations and dopamine blockade, indicating rapid drug absorption and central drug activity. Close monitoring and use of extended-release formulations are useful deterrents against stimulant abuse.
Clinical recommendations
Detecting and treating SUDs in patients with ADHD can be challenging. Ideally, the best time to assess for ADHD symptoms is after a prolonged abstinence from any influencing substance. However, in most clinical situations this is not practical. A better approach is a longitudinal assessment for ADHD symptoms. Detecting evidence of early childhood onset of ADHD symptoms before the patient began using substances can be helpful in conducting a proper differential diagnosis. Assessing for symptoms of SUDs in early adolescence, along with serial assessment of ADHD symptoms, also can be helpful. Symptoms secondary to ADHD are likely to show a consistent pattern, whereas symptoms secondary to an SUD may be sporadic.
When assessing SUD risk, consider the patient’s clinical condition, history of comorbidities that suggest SUDs, and overall functional status. Collateral information about the patient’s behavior and substance abuse from family members is important. A history of CD, bipolar disorder, or antisocial personality disorder should raise concerns about potential future stimulant abuse or diversion. Close monitoring of patients suspected of having an SUD is essential to detect stimulant abuse or diversion, which often manifests as weight loss, requests for higher doses, requests to switch from long-acting or extended-release formulations to immediate-release formulations, and repeated and suspicious “lost prescriptions.” Close observation for other subtle signs—such as changes in personality or mood and unexplained accidents or injuries—also may be needed.35
Challenges of treating ADHD and co-occurring SUD include poor medication adherence, need for a higher therapeutic stimulant dose, and difficulty in assessing the therapeutic benefit of pharmacotherapy in the presence of an SUD.36 Treating ADHD comorbid with SUD requires a collaborative approach that involves a psychiatrist, family members, and a behavioral care provider in addition to frequent monitoring.34
In the absence of treatment guidelines for treating ADHD with comorbid SUDs, some clinicians prefer to stabilize the SUD before initiating stimulants. Others prefer to use nonstimulants (such as atomoxetine, guanfacine, bupropion, venlafaxine, tricyclic antidepressants, or modafinil) as a first-line treatment. However, nonstimulants have not demonstrated efficacy comparable to that of stimulants for ADHD.35
Table 2 offers clinical recommendations to minimize the risk of SUDs when treating ADHD patients with stimulants. Long-acting stimulant formulations are preferred over short-acting medications because they are less likely to be abused. Psychosocial interventions for treating ADHD and co-occurring SUD disorder include cognitive-behavioral therapy with emphasis on structured skills training and cognitive remediation.
Table 2
Minimizing SUD risk when treating ADHD patients with stimulants
| Assess symptom burden and psychosocial impairment |
| Establish a treatment contract and boundaries at the onset of treatment, including your right to terminate treatment if you suspect stimulant misuse |
| Assess for comorbidities that may increase your patient’s SUD risk (see Table 1) |
| Emphasize strict adherence to treatment recommendations |
| Involve the patient’s family as much as possible |
| Obtain collateral information on the patient’s history of ADHD-related symptoms from parents, siblings, significant others, etc. |
| Distinguish between patients with substance use vs an SUD or a history of an SUD |
| Obtain urine toxicology screening as appropriate |
| Carefully document dispensed stimulants– strength of medication, number of capsules, pills, patches, etc. Note date of dispensation and refill dates |
| Select delayed- or extended-release stimulant formulations |
| Consider prescribing nonstimulants if appropriate |
| Use rating scales such as Conners Adult ADHD Rating Scale to monitor ADHD symptom severity and response to treatment |
| Schedule frequent, face-to-face clinical monitoring visits |
| ADHD: attention-deficit/hyperactivity disorder; SUD: substance use disorder |
Related Resource
- Faraone SV, Wilens T. Does stimulant treatment lead to substance use disorders? J Clin Psychiatry. 2003;64(suppl 11):9-13.
- Upadhyaya HP, Rose K, Wang W, et al. Attention deficit hyperactivity disorder medication and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15:799-809.
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;16(suppl 1):45-56.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Guanfacine • Tenex, Intuniv
- Methylphenidate • Ritalin
- Modafinil • Provigil
- Venlafaxine • Effexor
Disclosures
Dr. Shailesh Jain and Dr. Islam report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rakesh Jain has received research support from, is a consultant to, and/or is a speaker for Addrenex Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Forest Pharmaceuticals, Merck, Pamlab, Pfizer Inc., Shionogi Inc., Shire, and Sunovion Pharmaceuticals.
Discuss this article at www.facebook.com/CurrentPsychiatry
Does prescribing stimulants to patients with attention-deficit/hyperactivity disorder (ADHD) increase their risk of future substance abuse? Because ADHD is a common pediatric condition with symptoms that often persist into adulthood, and stimulants are an efficacious first-line therapy, this possible association is a concern for psychiatrists whether they treat children or adults.
Some researchers have expressed concerns that stimulant exposure could predispose patients to future substance abuse.1 Proponents of the biologic model of “kindling” hypothesize early exposure to stimulants could increase the risk of later substance use disorders (SUDs) by modifying or “priming” the brain, which then becomes more receptive to illicit drug exposure. Although there is some evidence that stimulant use does increase SUD risk, other evidence suggests stimulant use does not increase susceptibility to SUDs2,3 and some studies have suggested stimulant use in ADHD patients may protect against SUDs.4,5
This article reviews shared characteristics of ADHD and SUDs and the latest research on the association between the clinical use of stimulants and later development of SUDs. We also offer clinical recommendations for assessing and treating ADHD and comorbid SUD.
ADHD/SUD overlap
Compared with those without the disorder, patients with ADHD have a 6.2 times higher risk of developing an SUD.6 Individuals with ADHD experience an earlier age of onset and a longer duration of SUDs.7 Several retrospective and prospective studies reveal ADHD is a risk factor for SUDs.8 A longitudinal study that tracked teenage males with or without ADHD into young adulthood found SUDs were 4 times more common among those with ADHD.9 Up to 45% of adults with ADHD have a history of alcohol abuse or dependence, and up to 30% have a history of illegal drug abuse or dependence.10
Conversely, an estimated 35% to 71% of alcohol abusers and 15% to 25% of substance-dependent patients have ADHD.11 Adults with ADHD and comorbid SUD report earlier onset12 and greater severity13 of substance abuse than adults without ADHD. Patients with ADHD experience earlier onset and higher rates of tobacco smoking by mid-adolescence.14
Developmental psychopathology. Longitudinal studies have suggested certain psychopathologic characteristics of ADHD can predispose an individual to SUDs independent of stimulant exposure. For example, inattention, impulsivity, and hyperactivity predispose an individual to develop an SUD and also are core symptoms of ADHD.15 Another study found impulsivity, impersistence, and difficulty sitting still at age 3 predicted alcohol abuse at age 21.16 A different longitudinal study found novelty-seeking behavior (restlessness, running/jumping and not keeping still, being squirmy and fidgety) between age 6 to 10 predicted adolescent drug abuse and cigarette smoking.17 Poor response inhibition is a key characteristic of ADHD and has been linked to adolescent drinking.18
ADHD may be an independent risk factor for SUD because a common neurobiologic psychopathology may predispose an individual to develop both conditions. The dopamine system has been implicated in SUD, and dysfunction in the dopaminergic circuits—mostly in basal and frontal cortex with consequent defects in executive function and reward system—also has been found in ADHD.19 Cognitive dysfunction associated with ADHD may decrease a patient’s ability to estimate the negative consequences of substance abuse and to delay immediate gratification from drug or alcohol use.
ADHD patients are more vulnerable to SUDs if they have a comorbid condition, such as oppositional defiant disorder,13,20 bipolar disorder,20,21 or conduct disorder (CD).20,22 Patients with ADHD and comorbid CD are estimated to be 8.8 times more likely to have an SUD before age 18 compared with those with ADHD alone.23 Comorbid ADHD and CD may increase patients’ predisposition to develop dependence on highly addictive drugs, such as cocaine or methamphetamine.24 Impaired executive function, behavioral dyscontrol, impulsivity, and peer rejection are common in both ADHD and CD and may increase the risk of developing SUDs in individuals who have both conditions.25 Other risk factors for SUDs in patients with ADHD are listed in Table 1.26
Table 1
Risk factors for SUDs in patients with ADHD
| Presence of comorbid conditions (ie, oppositional defiant disorder, conduct disorder, bipolar disorder, eating disorder) |
| White or Hispanic race |
| Partially treated or residual ADHD symptoms |
| Attending a competitive college program |
| College youth who had late onset of stimulant treatment |
| Member of a college sorority/fraternity |
| ADHD: attention-deficit/hyperactivity disorder; SUDs: substance use disorders Source: Reference 26 |
Stimulants’ affect on SUD risk
Increased risk. Limited studies suggest exposure to stimulants is a risk factor for developing SUDs. In a longitudinal study, Lambert et al27 followed 218 patients with ADHD and 182 without ADHD into adulthood and found a linear trend between duration of stimulant treatment and prevalence of cocaine dependence. ADHD patients exposed to stimulants for >1 year had the highest prevalence of cocaine abuse (27%), compared with untreated subjects (15%), or those treated with stimulants for <1 year (18%). However, the study did not control for comorbid contributing factors, such as CD.
No change. In a 10-year naturalistic study, Biederman et al28 followed 109 children with ADHD age 7 to 12 into adulthood. These children had a developmental reading disorder but no other psychiatric comorbidities. When comparing patients who were treated with methylphenidate (n = 43) with those who did not receive stimulants (n = 66), Bierderman et al found no significant difference between the 2 groups in the prevalence of SUD for any of the 7 drug categories studied.
Decreased risk. Two meta-analyses found children with ADHD who were treated with stimulants and followed until adolescence were 5.8 times less likely to develop SUDs compared with those who did not receive stimulants.28,29 This protective effect diminished when patients were followed into adulthood, but individuals treated with stimulants were 1.4 times less likely to develop SUDs than those not treated with stimulants.30 In a prospective case-control, 5-year follow-up study of 114 patients with ADHD treated with stimulants, Wilens et al31 found significant protective effects of stimulant treatment on the development of any SUD. They found no effects from time of onset or duration of stimulant therapy on subsequent risk of SUDs or cigarette smoking.
One possible explanation for stimulants’ apparently reduced protective effect among adults is for patients with ADHD, stimulant use might delay but not prevent SUDs. It also is likely that by adulthood, loss of parental supervision leads to poor medication adherence and increased susceptibility to SUDs.30
Other studies have found exposure to stimulants may protect against SUDs. Katusic et al23 reviewed medical records for documented SUDs in 295 adults with ADHD treated with stimulants and 84 who did not receive stimulants. They found 20% of patients who received stimulants had a documented SUD compared with 27% of those not treated with stimulants. Barkely et al32 followed 98 stimulant-treated and 21 untreated ADHD patients with a mean age of 15 and 21, respectively. They found stimulant treatment did not increase the risk for substance use or abuse in either group.
ADHD and stimulant abuse
The prevalence of stimulant misuse is as high as 9% in patients in grade school and high school and up to 35% in college-age individuals.33 ADHD patients who misuse stimulants (eg, escalating dose without authorization) or skip stimulant doses to use illicit drugs or alcohol are more likely to sell their medication.34 Immediate-release stimulant formulations are more liable to be abused than extended-release drugs because they achieve earlier peak drug concentrations and dopamine blockade, indicating rapid drug absorption and central drug activity. Close monitoring and use of extended-release formulations are useful deterrents against stimulant abuse.
Clinical recommendations
Detecting and treating SUDs in patients with ADHD can be challenging. Ideally, the best time to assess for ADHD symptoms is after a prolonged abstinence from any influencing substance. However, in most clinical situations this is not practical. A better approach is a longitudinal assessment for ADHD symptoms. Detecting evidence of early childhood onset of ADHD symptoms before the patient began using substances can be helpful in conducting a proper differential diagnosis. Assessing for symptoms of SUDs in early adolescence, along with serial assessment of ADHD symptoms, also can be helpful. Symptoms secondary to ADHD are likely to show a consistent pattern, whereas symptoms secondary to an SUD may be sporadic.
When assessing SUD risk, consider the patient’s clinical condition, history of comorbidities that suggest SUDs, and overall functional status. Collateral information about the patient’s behavior and substance abuse from family members is important. A history of CD, bipolar disorder, or antisocial personality disorder should raise concerns about potential future stimulant abuse or diversion. Close monitoring of patients suspected of having an SUD is essential to detect stimulant abuse or diversion, which often manifests as weight loss, requests for higher doses, requests to switch from long-acting or extended-release formulations to immediate-release formulations, and repeated and suspicious “lost prescriptions.” Close observation for other subtle signs—such as changes in personality or mood and unexplained accidents or injuries—also may be needed.35
Challenges of treating ADHD and co-occurring SUD include poor medication adherence, need for a higher therapeutic stimulant dose, and difficulty in assessing the therapeutic benefit of pharmacotherapy in the presence of an SUD.36 Treating ADHD comorbid with SUD requires a collaborative approach that involves a psychiatrist, family members, and a behavioral care provider in addition to frequent monitoring.34
In the absence of treatment guidelines for treating ADHD with comorbid SUDs, some clinicians prefer to stabilize the SUD before initiating stimulants. Others prefer to use nonstimulants (such as atomoxetine, guanfacine, bupropion, venlafaxine, tricyclic antidepressants, or modafinil) as a first-line treatment. However, nonstimulants have not demonstrated efficacy comparable to that of stimulants for ADHD.35
Table 2 offers clinical recommendations to minimize the risk of SUDs when treating ADHD patients with stimulants. Long-acting stimulant formulations are preferred over short-acting medications because they are less likely to be abused. Psychosocial interventions for treating ADHD and co-occurring SUD disorder include cognitive-behavioral therapy with emphasis on structured skills training and cognitive remediation.
Table 2
Minimizing SUD risk when treating ADHD patients with stimulants
| Assess symptom burden and psychosocial impairment |
| Establish a treatment contract and boundaries at the onset of treatment, including your right to terminate treatment if you suspect stimulant misuse |
| Assess for comorbidities that may increase your patient’s SUD risk (see Table 1) |
| Emphasize strict adherence to treatment recommendations |
| Involve the patient’s family as much as possible |
| Obtain collateral information on the patient’s history of ADHD-related symptoms from parents, siblings, significant others, etc. |
| Distinguish between patients with substance use vs an SUD or a history of an SUD |
| Obtain urine toxicology screening as appropriate |
| Carefully document dispensed stimulants– strength of medication, number of capsules, pills, patches, etc. Note date of dispensation and refill dates |
| Select delayed- or extended-release stimulant formulations |
| Consider prescribing nonstimulants if appropriate |
| Use rating scales such as Conners Adult ADHD Rating Scale to monitor ADHD symptom severity and response to treatment |
| Schedule frequent, face-to-face clinical monitoring visits |
| ADHD: attention-deficit/hyperactivity disorder; SUD: substance use disorder |
Related Resource
- Faraone SV, Wilens T. Does stimulant treatment lead to substance use disorders? J Clin Psychiatry. 2003;64(suppl 11):9-13.
- Upadhyaya HP, Rose K, Wang W, et al. Attention deficit hyperactivity disorder medication and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15:799-809.
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;16(suppl 1):45-56.
Drug Brand Names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin, Zyban
- Guanfacine • Tenex, Intuniv
- Methylphenidate • Ritalin
- Modafinil • Provigil
- Venlafaxine • Effexor
Disclosures
Dr. Shailesh Jain and Dr. Islam report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rakesh Jain has received research support from, is a consultant to, and/or is a speaker for Addrenex Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Forest Pharmaceuticals, Merck, Pamlab, Pfizer Inc., Shionogi Inc., Shire, and Sunovion Pharmaceuticals.
1. Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713-725.
2. Mannuzza S, Klein RG, Moulton JL. Does stimulant treatment place children at risk for adult substance abuse? A controlled prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13(3):273-282.
3. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
4. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
5. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20.-
6. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
7. Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. J Clin Psychiatry. 2004;65(suppl 3):38-45.
8. Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45(2):195-221.
9. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565-576.
10. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
11. Wilens TE. AOD use and attention deficit/hyperactivity disorder. Alcohol Health Res World. 1998;22(2):127-130.
12. Wilens TE, Biederman J, Abrantes AM, et al. Clinical characteristics of psychiatrically referred adolescent outpatients with substance use disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(7):941-947.
13. Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61(4):244-251.
14. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
15. Zucker RA. Alcohol use and the alcohol use disorders: a developmental biopsychosocial systems formulation covering the life course. In: Cicchetti D Cohen D, eds. Developmental psychopathology. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2006;620-656.
16. Caspi A, Moffitt TE, Newman DL, et al. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53(11):1033-1039.
17. Màsse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54(1):62-68.
18. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468-475.
19. Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit hyperactivity disorder. Biol Psychiatry. 2005;57(11):1263-1272.
20. Biederman J, Wilens T, Mick E, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36(1):21-29.
21. Wilens TE, Biederman J, Millstein RB, et al. Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(6):680-685.
22. Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286-294.
23. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
24. Flory K, Milich R, Lynam DR, et al. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17(2):151-158.
25. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
26. Wilson JJ. ADHD and substance use disorders: developmental aspects and the impact of stimulant treatment. Am J Addict. 2007;16(suppl 1):5-11.
27. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
28. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597-603.
29. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
30. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse abuse, and diversion. J Clin Psychiatry. 2007;68(suppl 11):15-22.
31. Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162(10):916-921.
32. Barkley RA, Fischer M, Smallish L, et al. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111(1):97-109.
33. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
34. Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799-809.
35. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
36. Faraone SV, Biederman J, Wilens TE, et al. A naturalistic study of the effects of pharmacotherapy on substance use disorders among ADHD adults. Psychol Med. 2007;37(12):1743-1752.
1. Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713-725.
2. Mannuzza S, Klein RG, Moulton JL. Does stimulant treatment place children at risk for adult substance abuse? A controlled prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13(3):273-282.
3. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
4. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
5. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20.-
6. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
7. Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. J Clin Psychiatry. 2004;65(suppl 3):38-45.
8. Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45(2):195-221.
9. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565-576.
10. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
11. Wilens TE. AOD use and attention deficit/hyperactivity disorder. Alcohol Health Res World. 1998;22(2):127-130.
12. Wilens TE, Biederman J, Abrantes AM, et al. Clinical characteristics of psychiatrically referred adolescent outpatients with substance use disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(7):941-947.
13. Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61(4):244-251.
14. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
15. Zucker RA. Alcohol use and the alcohol use disorders: a developmental biopsychosocial systems formulation covering the life course. In: Cicchetti D Cohen D, eds. Developmental psychopathology. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2006;620-656.
16. Caspi A, Moffitt TE, Newman DL, et al. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53(11):1033-1039.
17. Màsse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54(1):62-68.
18. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468-475.
19. Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit hyperactivity disorder. Biol Psychiatry. 2005;57(11):1263-1272.
20. Biederman J, Wilens T, Mick E, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36(1):21-29.
21. Wilens TE, Biederman J, Millstein RB, et al. Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(6):680-685.
22. Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286-294.
23. Katusic SK, Barbaresi WJ, Colligan RC, et al. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764-776.
24. Flory K, Milich R, Lynam DR, et al. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17(2):151-158.
25. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27(2):283-301.
26. Wilson JJ. ADHD and substance use disorders: developmental aspects and the impact of stimulant treatment. Am J Addict. 2007;16(suppl 1):5-11.
27. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533-544.
28. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597-603.
29. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179-185.
30. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse abuse, and diversion. J Clin Psychiatry. 2007;68(suppl 11):15-22.
31. Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162(10):916-921.
32. Barkley RA, Fischer M, Smallish L, et al. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111(1):97-109.
33. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
34. Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799-809.
35. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24(5):1345-1357.
36. Faraone SV, Biederman J, Wilens TE, et al. A naturalistic study of the effects of pharmacotherapy on substance use disorders among ADHD adults. Psychol Med. 2007;37(12):1743-1752.
Guanfacine extended release for ADHD
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants
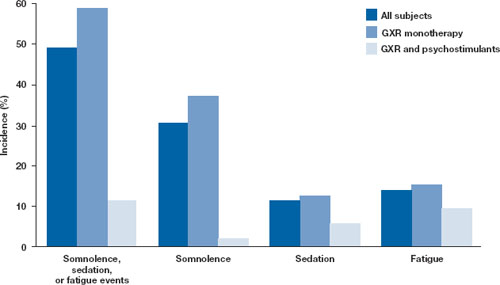
In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
ADHD in adults: Matching therapies with patients’ needs
Mr. Z, age 42, is referred by his primary care physician with symptoms suggesting attention-deficit/hyperactivity disorder (ADHD). Mr. Z has seen his physician sporadically for 10 years and acknowledges not following dietary and exercise advice. He has had intermittent “minor” depression, is overweight, and is a smoker with a family history of cardiovascular disease and diabetes.
A salesman, Mr. Z recently was promoted to an administrative position that substantially increased his paperwork. He is having difficulty performing his job because of longstanding forgetfulness and disorganization. He says he feels “like I’m in grade school again, lost in paperwork.” He also describes a recent educational assessment for his son, age 7, who may have ADHD. Similarities between Mr. Z’s and his son’s early childhood academic struggles are striking.
Like Mr. Z, adults with ADHD commonly seek treatment when increasing stressors and demands overwhelm their cognitive-attentional abilities. Some may be “healthy” men and women without psychiatric histories, whose disorganization, forgetfulness, or impulsivity contributes to functional impairment, including nonadherence with medical advice. For others, such as those with known psychiatric disorders, ADHD may be a hidden comorbidity contributing to seemingly refractory depression or anxiety disorder.
Despite growing evidence related to adult ADHD, individualizing and maintaining treatment over time can be challenging for clinicians and patients. Fortunately, new tools and multiple stimulant and nonstimulant medications can help you screen for, assess, and treat adult ADHD.
ADHD diagnosis
To diagnose ADHD in an adult patient, first establish that symptoms have existed from childhood to adulthood. One approach is to review DSM-IV-TR criteria for ADHD with your patient and ask him or her to reflect on childhood symptoms and dysfunction. Begin with orienting questions, such as “Do you remember your first grade teacher, your school, where you lived?” ADHD symptoms might have been present even if the patient maintained acceptable grades, particularly in elementary school, as dedicated parents or teachers might have contributed to early academic success.
Next, turn to diagnostic language that captures ADHD symptoms in adults. For example, the 18-item World Health Organization Adult ADHD Self-Report Scale (ASRS-v1.1) prompts individuals to self-report DSM-IV ADHD symptoms, and a 6-item subset (Table 1) is a highly specific screener (see Related Resources). The ASRS is most reliable in adults with limited psychiatric comorbidity.1
Adults often describe fluctuations in symptom severity over time. Symptoms may have less impact with more physically demanding work—such as sales—and greater impact with organizationally demanding work—such as administration.
Base your summary ADHD diagnosis on DSM-IV-TR criteria, including:
- lifetime persistence of symptoms, beginning before age 7
- functional impairment in ≥2 life settings, such as work, school, or home
- lack of another medical or psychiatric condition sufficient to explain the symptoms.
Table 1
Adult Self-Report Scale-v1.1 WHO 6-question screening tool for ADHD*
| Check the box that best describes how you have felt and conducted yourself over the past 6 months. Please give the completed questionnaire to your healthcare professional during your next appointment to discuss the results | Never | Rarely | Sometimes | Often | Very often |
|---|---|---|---|---|---|
| 1. How often do you have trouble wrapping up the final details of a project, once the challenging parts have been done? | |||||
| 2. How often do you have difficulty getting things in order when you have to do a task that requires organization? | |||||
| 3. How often do you have problems remembering appointments or obligations? | |||||
| 4. When you have a task that requires a lot of thought, how often do you avoid or delay getting started? | |||||
| 5. How often do you fidget or squirm with your hands or feet when you have to sit down for a long time? | |||||
| 6. How often do you feel overly active and compelled to do things, like you were driven by a motor? | |||||
| Add the number of checkmarks that appear in the darkly shaded area. Four (4) or more checkmarks indicate that your symptoms may be consistent with adult ADHD. It may be beneficial for you to talk with your healthcare provider about an evaluation. | |||||
| * Intended for use by persons age 18 and older ADHD: attention-deficit/hyperactivity disorder; WHO: World Health Organization | |||||
| Source: Reprinted with permission. World Health Organization Copyright 2003. All rights reserved | |||||
CASE CONTINUED: ‘All the time, every day’
Mr. Z completes the ASRS self-report symptom checklist and brings his wife to the next appointment. He rated all 6 screening symptoms and most others as occurring “often” or “very often.” He describes functional impairments “essentially all the time, basically every day” at work, home, and socially. His wife confirms these symptoms and the frustrations and conflicts they have caused.
Mr. Z describes ADHD symptoms from early elementary school to college. He was held back in kindergarten for being “immature,” his academic performance was inconsistent, and he “just got by…by cramming” in high school and college. His school performance pattern does not suggest a learning disability; he did not need special help in 1 subject more than others, and under pressure he could achieve average grades.
Medical review excludes explanations other than ADHD for his inattention, restlessness, and impulsivity. You conclude that Mr. Z meets criteria for ADHD, combined subtype, and discuss medication treatment.
FDA-approved medications
Medication for ADHD is appropriate only if symptoms are impairing. Five effective and generally well-tolerated medications are FDA-approved for adults with ADHD (Table 2):
- extended-release mixed amphetamine (Adderall XR)
- extended-release OROS methylphenidate (Concerta)
- extended-release dexmethylphenidate (Focalin XR)
- atomoxetine (Strattera)
- lisdexamfetamine (Vyvanse).
Efficacy. A meta-analysis of 29 pediatric ADHD trials across 30 years demonstrated greater effect size for stimulant class medications (immediate- and long-acting), compared with nonstimulant medications (including bupropion, atomoxetine, and modafinil).2 This finding is consistent with the American Academy of Child and Adolescent Psychiatry’s recommendation of stimulant medications as first-line agents for pediatric ADHD.3 A similar meta-analysis of 6 controlled studies of methylphenidate-class medications in adults found a large mean effect size (0.9), with greater effects associated with higher doses.4
Atomoxetine, a norepinephrine reuptake inhibitor, is the only nonstimulant medication FDA-approved for ADHD in adults. More than 6,000 children, adolescents, and adults have taken atomoxetine in clinical trials for ADHD (Lilly, prescribing information), with 4 years of open treatment data showing benefit being maintained over time.5
Tolerability. Although ADHD medications are generally well-tolerated by healthy adults, assess for a history of potential contraindications:
- unstable medical condition, hyperthyroidism, glaucoma
- treatment with a monoamine oxidase inhibitor or other pressor agents because of possible effects on blood pressure and heart rate
- use of cytochrome P450 2D6 inhibitors, which may increase atomoxetine steady-state plasma concentrations
- cardiovascular disease or family history of early cardiac disease (Box 1)6,7
- history of or active substance use disorder, such as alcohol dependence, cocaine or heroin abuse
- history of psychosis, bipolar disorder, or an active clinically significant psychiatric comorbidity (major depression, agitated state, suicidality).
Clinically, some patients appear to tolerate 1 class of stimulant (such as methylphenidate or amphetamine) over another. Consider switching to an alternate stimulant if your patient has bothersome side effects—mild low appetite, insomnia, tension, or jitteriness—or has received limited or partial benefit during an initial stimulant trial.
Serious cardiovascular events and sudden death have occurred in adults and children treated with stimulants.6 Agents used for attention-deficit/hyperactivity disorder (ADHD) have not been shown to cause sudden cardiac death, but the FDA requires stimulants’ labeling to warn about this risk in patients with structural cardiac abnormalities. The warning advises against using stimulants in adults with cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease.
When treating adults with ADHD, look to advisories about cardiovascular monitoring in children with ADHD. Before initiating medications, do a physical exam focused on cardiovascular disease risk factors and obtain a patient and family health history of:
- fainting or dizziness
- sudden or unexplained death in someone young
- sudden cardiac death or “heart attack” in family members age <35 years.
The American Academy of Pediatrics, American Academy of Child and Adolescent Psychiatry, and American Heart Association concur that electrocardiography (ECG) is not mandatory in cardiovascular assessment and monitoring during ADHD pharmacotherapy.7 This author (PH) refers cardiovascular questions to a primary care physician or cardiologist.
During ADHD treatment, monitor vital signs and refer patients with emergent cardiac symptoms or concerns to a cardiologist. Expect increases in blood pressure (1 to 4 mm Hg) and heart rate (2 to 6 bpm) during treatment with methylphenidate and amphetamine-class stimulants as well as with atomoxetine. Do not expect significant changes in ECG parameters (PR, QRS, and QTC intervals).
Extended-release formulations. Early adult studies demonstrated the efficacy of immediate-release stimulants, but adults with ADHD’s inherent deficits in organization and memory may have higher adherence rates and greater success with once-daily, extended-release formulations.8-11 Unless your patient wants to begin with small, short-acting dosages (5 to 10 mg) or desires to target treatment to specific times of day (such as in the morning for administrative work only), many appreciate once-daily formulations. Extended-release formulations also may be the simplest stimulants with which to begin ADHD treatment.
Over time, patients may benefit from an immediate-release form:
- added for certain times of day—such as in late afternoon, when the morning extended-release dose has worn off (Box 2)12,13
- to use as an alternative to extended-release formulations when more or less flexibly is desired, such as on weekends.
Table 2
Administering medications approved for adult ADHD
| Drug | Recommended dosage* | Comments |
|---|---|---|
| Stimulants | ||
| Extended-release mixed amphetamine (Adderall XR) | 20 mg | Initial prescription of 10-mg XR capsules allows gradual titration |
| Extended-release OROS methylphenidate (Concerta) | 18 to 72 mg/d | Initial prescription of 18-mg OROS MPH capsules allows gradual titration |
| Extended-release dexmethylphenidate (Focalin XR) | 10 mg/d; maximum 20 mg/d | Dosing is one-half the typical dosing of racemic MPH |
| Lisdexamfetamine (Vyvanse) | 30 mg/d; maximum 70 mg/d | May be adjusted weekly in 10-mg or 20-mg increments |
| Nonstimulant | ||
| Atomoxetine (Strattera) | 80 mg/d; maximum 100 mg/d | Initial dosage of 40 mg/d can be increased to target dosage after a minimum of 3 days; can be given as a morning dose or divided evenly between morning and evening doses |
| * FDA-approved dosages as listed in the package inserts of these medications ADHD: attention-deficit/hyperactivity disorder; MPH: methylphenidate; OROS: osmotic release oral system; XR: extended-release formulation | ||
CASE CONTINUED: Feeling ‘calm, less frenetic’
During the next 6 months, you start Mr. Z on stimulant treatment at robust dosing consistent with his weight (90 kg). He complains that extended-duration methylphenidate (MPH)—titrated to 90 mg/d—doesn’t last into the late afternoon, and he feels mildly tense with a low appetite. Because of an apparent partial response and relatively mild adverse effects, you discontinue MPH and try an extended-duration amphetamine, titrated to 60 mg.
Mr. Z’s blood pressure and heart rate remain stable. He begins to exercise regularly and reduce his use of tobacco and caffeine drinks, as you recommend. He says he feels “calm, less frenetic.” He reports no tension on this medication and only mild reduced appetite. With a plan to continue taking the stimulant medication with regular monitoring, he then disappears from treatment.
Promoting adherence
Treatment nonadherence is an issue throughout medicine, and individuals with disorganization, forgetfulness, and impulsivity may be at higher-than-usual risk of not following through on medication regimens.
Combining short- and long-acting stimulants may cover hours when attention-deficit/hyperactivity (ADHD) symptoms emerge despite therapy with a long-acting agent.12,13 Ask patients who report lack of full-day coverage if the once-daily, extended-duration formulation they are taking works well until a certain time of day. Then consider adding a similar-class immediate-release stimulant at this time to cover the later hours.
If a patient reports partial response throughout the day—such as early in treatment—begin by optimizing the long-acting agent’s dosage. Keep a target daily dose in mind, based on FDA recommendations and clinical trial data. For example, an adult weighing 80 kg may respond optimally to a combination of 60 mg of a long-acting methylphenidate (MPH) in the morning, followed by 10 to 20 mg of an immediate-release MPH in mid-afternoon.
The later stimulants are taken in the day, the more likely insomnia may emerge as an adverse effect. Some patients adjust to this problem within the first weeks of treatment. If insomnia remains impairing, reduce the stimulant dose or consider switching to a shorter duration medication or to the nonstimulant atomoxetine.
In addition, restrictions on stimulant-class medications do not permit multiple-month prescribing (refills), as is allowed with non-scheduled medications such as atomoxetine. Discuss with patients how they will obtain stimulant medications on a regular, monthly or bimonthly basis. In our experience, the practical challenges of remaining in treatment at times may limit patients’ adherence to ADHD medications more than a lack of response or tolerability concerns.
Explain to patients early in treatment that they might need to try several different medications before settling on 1 that is optimally tolerated and efficacious. Because stimulants are generally quite effective for ADHD symptoms, set your goal to identify adverse effects and aim for a patient response of “this works well, and I don’t feel any different on it.”
CASE CONTINUED: Ready to try again
Three years later, Mr. Z returns and reports gradually discontinuing the stimulant because he “wanted to go it on my own.” He functioned relatively well at first, but errors and conflicts at his job led to his dismissal.
Since then, he has been unemployed. He is increasingly depressed and reports drinking and smoking “more heavily than in college.” He asks about resuming ADHD treatment.
Mr. Z does not meet DSM-IV-TR criteria for major depressive disorder or alcohol abuse/dependence. His depressed mood appears to be linked to his marked ADHD symptoms. Mr. Z agrees to a new treatment plan that includes starting atomoxetine at 25 mg to allow for flexible titration and psychotherapy to monitor his mood and achieve sobriety.
ADHD and substance abuse
Clinical judgment determines whether an adult with ADHD and a history of substance use disorders may safely benefit from treatment with a stimulant. The relationship between ADHD and substance use disorders is of clinical concern, but ADHD medications have not been shown to increase risk for later substance use disorders in children.14 Conversely, effective ADHD treatment appears to reduce later cigarette and substance use.15
Consider using a nonstimulant-class medication in adults with ADHD and active substance use disorders. In a 12-week, double-blind, controlled trial, atomoxetine improved ADHD symptoms significantly more than placebo in adults meeting DS-MIV-TR criteria for comorbid alcohol use disorders. After 4 to 30 days of alcohol abstinence, 72 patients were randomly assigned to atomoxetine, 25 to 100 mg/d (mean final dose 90 mg/d), and 75 patients to placebo. Although estimated times to initial relapse to heavy drinking did not differ:
- atomoxetine-treated subjects had 26% fewer cumulative heavy drinking days than placebo-treated subjects (P=0.023)
- the difference in cumulative heavy drinking days between the atomoxetine and placebo groups became statistically significant after 55 days of treatment.16
- World Health Organization Adult Self-Report Scale (ASRS) 18-item instrument and 6-item screener. www.med.nyu.edu/psych/psychiatrist/adhd.html.
- Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 2008;165:553-5.
- Adler LA, Spencer TJ, Levine LR, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord 2008; 11:720-7.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Extended-release mixed amphetamine • Adderall XR
- Extended duration OROS methylphenidate • Concerta
- Extended-release dexmethylphenidate • Focalin XR
- Lisdexamfetamine • Vyvanse
- Modafinil • Provigil
Disclosure
Dr. Hammerness has received research support from and is on the speakers bureau for Shire Pharmaceuticals. He has received support for CME activities and talks from Shire Pharmaceuticals, Ortho-McNeil, and Abbott Laboratories.
Dr. Surman receives research support and/or is a speaker for Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, Ortho-McNeil, Merck, New River Pharmaceuticals, Novartis, Pfizer Inc., Shire Pharmaceuticals, and Takeda Pharmaceutical Company.
Dr. Sassi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Clinical research assistant Katherine Miller, BA, contributed to the literature review for this article and assisted in preparing the manuscript.
1. Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005;35:245-56.
2. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed 2006;8(4):4.-
3. Greenhill L, Pliszka S, Dulcan M, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2001;40(11):1352-5.
4. Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2004;24:24-9.
5. Adler LA, Spencer TJ, Williams DW, et al. Long-term, open-label safety and efficacy of atomoxetine in adults with ADHD: final report of a 4-year study. J Atten Disord Epub 2008 April 30.
6. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med 2006;354:1445-8.
7. American Academy of Pediatrics/American Heart Association clarification of statement on cardiovascular evaluation and monitoring of children and adolescents with heart disease receiving medications for ADHD May 16, 2008. Available at: http://www.aap.org/pressroom/aap-ahastatement.htm. Accessed August 14, 2008.
8. Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2006;59(9):829-35.
9. Biederman J, Mick E, Surman C, et al. Comparative acute efficacy and tolerability of OROS and immediate release formulations of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2007;7:49.-
10. Mick E, Spencer TJ, Surman C, et al. Randomized single-blind substitution study of methylphenidate in ADHD adults receiving immediate-release methylphenidate. NR357. Poster presented at: Annual Meeting of the American Psychiatric Association; May 19-24, 2007; San Diego, CA.
11. Capone N, McDonnel T. Medication persistence among agents used to treat attention-deficit/hyperactivity disorder, diabetes, and elevated serum cholesterol. NR 639. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
12. Adler L, Morrill M, Reingold B. d-methylphenidate augmentation of extended-release stimulant therapy in ADHD. NR 619. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
13. Adler L, Reingold LS, Morrill MS, Wilens TE. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep 2006;8:409-15.
14. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry 2008;165:597-603.
15. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 2007;68(suppl 11):15-22.
16. Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 2008;96:145-54.
Mr. Z, age 42, is referred by his primary care physician with symptoms suggesting attention-deficit/hyperactivity disorder (ADHD). Mr. Z has seen his physician sporadically for 10 years and acknowledges not following dietary and exercise advice. He has had intermittent “minor” depression, is overweight, and is a smoker with a family history of cardiovascular disease and diabetes.
A salesman, Mr. Z recently was promoted to an administrative position that substantially increased his paperwork. He is having difficulty performing his job because of longstanding forgetfulness and disorganization. He says he feels “like I’m in grade school again, lost in paperwork.” He also describes a recent educational assessment for his son, age 7, who may have ADHD. Similarities between Mr. Z’s and his son’s early childhood academic struggles are striking.
Like Mr. Z, adults with ADHD commonly seek treatment when increasing stressors and demands overwhelm their cognitive-attentional abilities. Some may be “healthy” men and women without psychiatric histories, whose disorganization, forgetfulness, or impulsivity contributes to functional impairment, including nonadherence with medical advice. For others, such as those with known psychiatric disorders, ADHD may be a hidden comorbidity contributing to seemingly refractory depression or anxiety disorder.
Despite growing evidence related to adult ADHD, individualizing and maintaining treatment over time can be challenging for clinicians and patients. Fortunately, new tools and multiple stimulant and nonstimulant medications can help you screen for, assess, and treat adult ADHD.
ADHD diagnosis
To diagnose ADHD in an adult patient, first establish that symptoms have existed from childhood to adulthood. One approach is to review DSM-IV-TR criteria for ADHD with your patient and ask him or her to reflect on childhood symptoms and dysfunction. Begin with orienting questions, such as “Do you remember your first grade teacher, your school, where you lived?” ADHD symptoms might have been present even if the patient maintained acceptable grades, particularly in elementary school, as dedicated parents or teachers might have contributed to early academic success.
Next, turn to diagnostic language that captures ADHD symptoms in adults. For example, the 18-item World Health Organization Adult ADHD Self-Report Scale (ASRS-v1.1) prompts individuals to self-report DSM-IV ADHD symptoms, and a 6-item subset (Table 1) is a highly specific screener (see Related Resources). The ASRS is most reliable in adults with limited psychiatric comorbidity.1
Adults often describe fluctuations in symptom severity over time. Symptoms may have less impact with more physically demanding work—such as sales—and greater impact with organizationally demanding work—such as administration.
Base your summary ADHD diagnosis on DSM-IV-TR criteria, including:
- lifetime persistence of symptoms, beginning before age 7
- functional impairment in ≥2 life settings, such as work, school, or home
- lack of another medical or psychiatric condition sufficient to explain the symptoms.
Table 1
Adult Self-Report Scale-v1.1 WHO 6-question screening tool for ADHD*
| Check the box that best describes how you have felt and conducted yourself over the past 6 months. Please give the completed questionnaire to your healthcare professional during your next appointment to discuss the results | Never | Rarely | Sometimes | Often | Very often |
|---|---|---|---|---|---|
| 1. How often do you have trouble wrapping up the final details of a project, once the challenging parts have been done? | |||||
| 2. How often do you have difficulty getting things in order when you have to do a task that requires organization? | |||||
| 3. How often do you have problems remembering appointments or obligations? | |||||
| 4. When you have a task that requires a lot of thought, how often do you avoid or delay getting started? | |||||
| 5. How often do you fidget or squirm with your hands or feet when you have to sit down for a long time? | |||||
| 6. How often do you feel overly active and compelled to do things, like you were driven by a motor? | |||||
| Add the number of checkmarks that appear in the darkly shaded area. Four (4) or more checkmarks indicate that your symptoms may be consistent with adult ADHD. It may be beneficial for you to talk with your healthcare provider about an evaluation. | |||||
| * Intended for use by persons age 18 and older ADHD: attention-deficit/hyperactivity disorder; WHO: World Health Organization | |||||
| Source: Reprinted with permission. World Health Organization Copyright 2003. All rights reserved | |||||
CASE CONTINUED: ‘All the time, every day’
Mr. Z completes the ASRS self-report symptom checklist and brings his wife to the next appointment. He rated all 6 screening symptoms and most others as occurring “often” or “very often.” He describes functional impairments “essentially all the time, basically every day” at work, home, and socially. His wife confirms these symptoms and the frustrations and conflicts they have caused.
Mr. Z describes ADHD symptoms from early elementary school to college. He was held back in kindergarten for being “immature,” his academic performance was inconsistent, and he “just got by…by cramming” in high school and college. His school performance pattern does not suggest a learning disability; he did not need special help in 1 subject more than others, and under pressure he could achieve average grades.
Medical review excludes explanations other than ADHD for his inattention, restlessness, and impulsivity. You conclude that Mr. Z meets criteria for ADHD, combined subtype, and discuss medication treatment.
FDA-approved medications
Medication for ADHD is appropriate only if symptoms are impairing. Five effective and generally well-tolerated medications are FDA-approved for adults with ADHD (Table 2):
- extended-release mixed amphetamine (Adderall XR)
- extended-release OROS methylphenidate (Concerta)
- extended-release dexmethylphenidate (Focalin XR)
- atomoxetine (Strattera)
- lisdexamfetamine (Vyvanse).
Efficacy. A meta-analysis of 29 pediatric ADHD trials across 30 years demonstrated greater effect size for stimulant class medications (immediate- and long-acting), compared with nonstimulant medications (including bupropion, atomoxetine, and modafinil).2 This finding is consistent with the American Academy of Child and Adolescent Psychiatry’s recommendation of stimulant medications as first-line agents for pediatric ADHD.3 A similar meta-analysis of 6 controlled studies of methylphenidate-class medications in adults found a large mean effect size (0.9), with greater effects associated with higher doses.4
Atomoxetine, a norepinephrine reuptake inhibitor, is the only nonstimulant medication FDA-approved for ADHD in adults. More than 6,000 children, adolescents, and adults have taken atomoxetine in clinical trials for ADHD (Lilly, prescribing information), with 4 years of open treatment data showing benefit being maintained over time.5
Tolerability. Although ADHD medications are generally well-tolerated by healthy adults, assess for a history of potential contraindications:
- unstable medical condition, hyperthyroidism, glaucoma
- treatment with a monoamine oxidase inhibitor or other pressor agents because of possible effects on blood pressure and heart rate
- use of cytochrome P450 2D6 inhibitors, which may increase atomoxetine steady-state plasma concentrations
- cardiovascular disease or family history of early cardiac disease (Box 1)6,7
- history of or active substance use disorder, such as alcohol dependence, cocaine or heroin abuse
- history of psychosis, bipolar disorder, or an active clinically significant psychiatric comorbidity (major depression, agitated state, suicidality).
Clinically, some patients appear to tolerate 1 class of stimulant (such as methylphenidate or amphetamine) over another. Consider switching to an alternate stimulant if your patient has bothersome side effects—mild low appetite, insomnia, tension, or jitteriness—or has received limited or partial benefit during an initial stimulant trial.
Serious cardiovascular events and sudden death have occurred in adults and children treated with stimulants.6 Agents used for attention-deficit/hyperactivity disorder (ADHD) have not been shown to cause sudden cardiac death, but the FDA requires stimulants’ labeling to warn about this risk in patients with structural cardiac abnormalities. The warning advises against using stimulants in adults with cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease.
When treating adults with ADHD, look to advisories about cardiovascular monitoring in children with ADHD. Before initiating medications, do a physical exam focused on cardiovascular disease risk factors and obtain a patient and family health history of:
- fainting or dizziness
- sudden or unexplained death in someone young
- sudden cardiac death or “heart attack” in family members age <35 years.
The American Academy of Pediatrics, American Academy of Child and Adolescent Psychiatry, and American Heart Association concur that electrocardiography (ECG) is not mandatory in cardiovascular assessment and monitoring during ADHD pharmacotherapy.7 This author (PH) refers cardiovascular questions to a primary care physician or cardiologist.
During ADHD treatment, monitor vital signs and refer patients with emergent cardiac symptoms or concerns to a cardiologist. Expect increases in blood pressure (1 to 4 mm Hg) and heart rate (2 to 6 bpm) during treatment with methylphenidate and amphetamine-class stimulants as well as with atomoxetine. Do not expect significant changes in ECG parameters (PR, QRS, and QTC intervals).
Extended-release formulations. Early adult studies demonstrated the efficacy of immediate-release stimulants, but adults with ADHD’s inherent deficits in organization and memory may have higher adherence rates and greater success with once-daily, extended-release formulations.8-11 Unless your patient wants to begin with small, short-acting dosages (5 to 10 mg) or desires to target treatment to specific times of day (such as in the morning for administrative work only), many appreciate once-daily formulations. Extended-release formulations also may be the simplest stimulants with which to begin ADHD treatment.
Over time, patients may benefit from an immediate-release form:
- added for certain times of day—such as in late afternoon, when the morning extended-release dose has worn off (Box 2)12,13
- to use as an alternative to extended-release formulations when more or less flexibly is desired, such as on weekends.
Table 2
Administering medications approved for adult ADHD
| Drug | Recommended dosage* | Comments |
|---|---|---|
| Stimulants | ||
| Extended-release mixed amphetamine (Adderall XR) | 20 mg | Initial prescription of 10-mg XR capsules allows gradual titration |
| Extended-release OROS methylphenidate (Concerta) | 18 to 72 mg/d | Initial prescription of 18-mg OROS MPH capsules allows gradual titration |
| Extended-release dexmethylphenidate (Focalin XR) | 10 mg/d; maximum 20 mg/d | Dosing is one-half the typical dosing of racemic MPH |
| Lisdexamfetamine (Vyvanse) | 30 mg/d; maximum 70 mg/d | May be adjusted weekly in 10-mg or 20-mg increments |
| Nonstimulant | ||
| Atomoxetine (Strattera) | 80 mg/d; maximum 100 mg/d | Initial dosage of 40 mg/d can be increased to target dosage after a minimum of 3 days; can be given as a morning dose or divided evenly between morning and evening doses |
| * FDA-approved dosages as listed in the package inserts of these medications ADHD: attention-deficit/hyperactivity disorder; MPH: methylphenidate; OROS: osmotic release oral system; XR: extended-release formulation | ||
CASE CONTINUED: Feeling ‘calm, less frenetic’
During the next 6 months, you start Mr. Z on stimulant treatment at robust dosing consistent with his weight (90 kg). He complains that extended-duration methylphenidate (MPH)—titrated to 90 mg/d—doesn’t last into the late afternoon, and he feels mildly tense with a low appetite. Because of an apparent partial response and relatively mild adverse effects, you discontinue MPH and try an extended-duration amphetamine, titrated to 60 mg.
Mr. Z’s blood pressure and heart rate remain stable. He begins to exercise regularly and reduce his use of tobacco and caffeine drinks, as you recommend. He says he feels “calm, less frenetic.” He reports no tension on this medication and only mild reduced appetite. With a plan to continue taking the stimulant medication with regular monitoring, he then disappears from treatment.
Promoting adherence
Treatment nonadherence is an issue throughout medicine, and individuals with disorganization, forgetfulness, and impulsivity may be at higher-than-usual risk of not following through on medication regimens.
Combining short- and long-acting stimulants may cover hours when attention-deficit/hyperactivity (ADHD) symptoms emerge despite therapy with a long-acting agent.12,13 Ask patients who report lack of full-day coverage if the once-daily, extended-duration formulation they are taking works well until a certain time of day. Then consider adding a similar-class immediate-release stimulant at this time to cover the later hours.
If a patient reports partial response throughout the day—such as early in treatment—begin by optimizing the long-acting agent’s dosage. Keep a target daily dose in mind, based on FDA recommendations and clinical trial data. For example, an adult weighing 80 kg may respond optimally to a combination of 60 mg of a long-acting methylphenidate (MPH) in the morning, followed by 10 to 20 mg of an immediate-release MPH in mid-afternoon.
The later stimulants are taken in the day, the more likely insomnia may emerge as an adverse effect. Some patients adjust to this problem within the first weeks of treatment. If insomnia remains impairing, reduce the stimulant dose or consider switching to a shorter duration medication or to the nonstimulant atomoxetine.
In addition, restrictions on stimulant-class medications do not permit multiple-month prescribing (refills), as is allowed with non-scheduled medications such as atomoxetine. Discuss with patients how they will obtain stimulant medications on a regular, monthly or bimonthly basis. In our experience, the practical challenges of remaining in treatment at times may limit patients’ adherence to ADHD medications more than a lack of response or tolerability concerns.
Explain to patients early in treatment that they might need to try several different medications before settling on 1 that is optimally tolerated and efficacious. Because stimulants are generally quite effective for ADHD symptoms, set your goal to identify adverse effects and aim for a patient response of “this works well, and I don’t feel any different on it.”
CASE CONTINUED: Ready to try again
Three years later, Mr. Z returns and reports gradually discontinuing the stimulant because he “wanted to go it on my own.” He functioned relatively well at first, but errors and conflicts at his job led to his dismissal.
Since then, he has been unemployed. He is increasingly depressed and reports drinking and smoking “more heavily than in college.” He asks about resuming ADHD treatment.
Mr. Z does not meet DSM-IV-TR criteria for major depressive disorder or alcohol abuse/dependence. His depressed mood appears to be linked to his marked ADHD symptoms. Mr. Z agrees to a new treatment plan that includes starting atomoxetine at 25 mg to allow for flexible titration and psychotherapy to monitor his mood and achieve sobriety.
ADHD and substance abuse
Clinical judgment determines whether an adult with ADHD and a history of substance use disorders may safely benefit from treatment with a stimulant. The relationship between ADHD and substance use disorders is of clinical concern, but ADHD medications have not been shown to increase risk for later substance use disorders in children.14 Conversely, effective ADHD treatment appears to reduce later cigarette and substance use.15
Consider using a nonstimulant-class medication in adults with ADHD and active substance use disorders. In a 12-week, double-blind, controlled trial, atomoxetine improved ADHD symptoms significantly more than placebo in adults meeting DS-MIV-TR criteria for comorbid alcohol use disorders. After 4 to 30 days of alcohol abstinence, 72 patients were randomly assigned to atomoxetine, 25 to 100 mg/d (mean final dose 90 mg/d), and 75 patients to placebo. Although estimated times to initial relapse to heavy drinking did not differ:
- atomoxetine-treated subjects had 26% fewer cumulative heavy drinking days than placebo-treated subjects (P=0.023)
- the difference in cumulative heavy drinking days between the atomoxetine and placebo groups became statistically significant after 55 days of treatment.16
- World Health Organization Adult Self-Report Scale (ASRS) 18-item instrument and 6-item screener. www.med.nyu.edu/psych/psychiatrist/adhd.html.
- Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 2008;165:553-5.
- Adler LA, Spencer TJ, Levine LR, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord 2008; 11:720-7.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Extended-release mixed amphetamine • Adderall XR
- Extended duration OROS methylphenidate • Concerta
- Extended-release dexmethylphenidate • Focalin XR
- Lisdexamfetamine • Vyvanse
- Modafinil • Provigil
Disclosure
Dr. Hammerness has received research support from and is on the speakers bureau for Shire Pharmaceuticals. He has received support for CME activities and talks from Shire Pharmaceuticals, Ortho-McNeil, and Abbott Laboratories.
Dr. Surman receives research support and/or is a speaker for Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, Ortho-McNeil, Merck, New River Pharmaceuticals, Novartis, Pfizer Inc., Shire Pharmaceuticals, and Takeda Pharmaceutical Company.
Dr. Sassi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Clinical research assistant Katherine Miller, BA, contributed to the literature review for this article and assisted in preparing the manuscript.
Mr. Z, age 42, is referred by his primary care physician with symptoms suggesting attention-deficit/hyperactivity disorder (ADHD). Mr. Z has seen his physician sporadically for 10 years and acknowledges not following dietary and exercise advice. He has had intermittent “minor” depression, is overweight, and is a smoker with a family history of cardiovascular disease and diabetes.
A salesman, Mr. Z recently was promoted to an administrative position that substantially increased his paperwork. He is having difficulty performing his job because of longstanding forgetfulness and disorganization. He says he feels “like I’m in grade school again, lost in paperwork.” He also describes a recent educational assessment for his son, age 7, who may have ADHD. Similarities between Mr. Z’s and his son’s early childhood academic struggles are striking.
Like Mr. Z, adults with ADHD commonly seek treatment when increasing stressors and demands overwhelm their cognitive-attentional abilities. Some may be “healthy” men and women without psychiatric histories, whose disorganization, forgetfulness, or impulsivity contributes to functional impairment, including nonadherence with medical advice. For others, such as those with known psychiatric disorders, ADHD may be a hidden comorbidity contributing to seemingly refractory depression or anxiety disorder.
Despite growing evidence related to adult ADHD, individualizing and maintaining treatment over time can be challenging for clinicians and patients. Fortunately, new tools and multiple stimulant and nonstimulant medications can help you screen for, assess, and treat adult ADHD.
ADHD diagnosis
To diagnose ADHD in an adult patient, first establish that symptoms have existed from childhood to adulthood. One approach is to review DSM-IV-TR criteria for ADHD with your patient and ask him or her to reflect on childhood symptoms and dysfunction. Begin with orienting questions, such as “Do you remember your first grade teacher, your school, where you lived?” ADHD symptoms might have been present even if the patient maintained acceptable grades, particularly in elementary school, as dedicated parents or teachers might have contributed to early academic success.
Next, turn to diagnostic language that captures ADHD symptoms in adults. For example, the 18-item World Health Organization Adult ADHD Self-Report Scale (ASRS-v1.1) prompts individuals to self-report DSM-IV ADHD symptoms, and a 6-item subset (Table 1) is a highly specific screener (see Related Resources). The ASRS is most reliable in adults with limited psychiatric comorbidity.1
Adults often describe fluctuations in symptom severity over time. Symptoms may have less impact with more physically demanding work—such as sales—and greater impact with organizationally demanding work—such as administration.
Base your summary ADHD diagnosis on DSM-IV-TR criteria, including:
- lifetime persistence of symptoms, beginning before age 7
- functional impairment in ≥2 life settings, such as work, school, or home
- lack of another medical or psychiatric condition sufficient to explain the symptoms.
Table 1
Adult Self-Report Scale-v1.1 WHO 6-question screening tool for ADHD*
| Check the box that best describes how you have felt and conducted yourself over the past 6 months. Please give the completed questionnaire to your healthcare professional during your next appointment to discuss the results | Never | Rarely | Sometimes | Often | Very often |
|---|---|---|---|---|---|
| 1. How often do you have trouble wrapping up the final details of a project, once the challenging parts have been done? | |||||
| 2. How often do you have difficulty getting things in order when you have to do a task that requires organization? | |||||
| 3. How often do you have problems remembering appointments or obligations? | |||||
| 4. When you have a task that requires a lot of thought, how often do you avoid or delay getting started? | |||||
| 5. How often do you fidget or squirm with your hands or feet when you have to sit down for a long time? | |||||
| 6. How often do you feel overly active and compelled to do things, like you were driven by a motor? | |||||
| Add the number of checkmarks that appear in the darkly shaded area. Four (4) or more checkmarks indicate that your symptoms may be consistent with adult ADHD. It may be beneficial for you to talk with your healthcare provider about an evaluation. | |||||
| * Intended for use by persons age 18 and older ADHD: attention-deficit/hyperactivity disorder; WHO: World Health Organization | |||||
| Source: Reprinted with permission. World Health Organization Copyright 2003. All rights reserved | |||||
CASE CONTINUED: ‘All the time, every day’
Mr. Z completes the ASRS self-report symptom checklist and brings his wife to the next appointment. He rated all 6 screening symptoms and most others as occurring “often” or “very often.” He describes functional impairments “essentially all the time, basically every day” at work, home, and socially. His wife confirms these symptoms and the frustrations and conflicts they have caused.
Mr. Z describes ADHD symptoms from early elementary school to college. He was held back in kindergarten for being “immature,” his academic performance was inconsistent, and he “just got by…by cramming” in high school and college. His school performance pattern does not suggest a learning disability; he did not need special help in 1 subject more than others, and under pressure he could achieve average grades.
Medical review excludes explanations other than ADHD for his inattention, restlessness, and impulsivity. You conclude that Mr. Z meets criteria for ADHD, combined subtype, and discuss medication treatment.
FDA-approved medications
Medication for ADHD is appropriate only if symptoms are impairing. Five effective and generally well-tolerated medications are FDA-approved for adults with ADHD (Table 2):
- extended-release mixed amphetamine (Adderall XR)
- extended-release OROS methylphenidate (Concerta)
- extended-release dexmethylphenidate (Focalin XR)
- atomoxetine (Strattera)
- lisdexamfetamine (Vyvanse).
Efficacy. A meta-analysis of 29 pediatric ADHD trials across 30 years demonstrated greater effect size for stimulant class medications (immediate- and long-acting), compared with nonstimulant medications (including bupropion, atomoxetine, and modafinil).2 This finding is consistent with the American Academy of Child and Adolescent Psychiatry’s recommendation of stimulant medications as first-line agents for pediatric ADHD.3 A similar meta-analysis of 6 controlled studies of methylphenidate-class medications in adults found a large mean effect size (0.9), with greater effects associated with higher doses.4
Atomoxetine, a norepinephrine reuptake inhibitor, is the only nonstimulant medication FDA-approved for ADHD in adults. More than 6,000 children, adolescents, and adults have taken atomoxetine in clinical trials for ADHD (Lilly, prescribing information), with 4 years of open treatment data showing benefit being maintained over time.5
Tolerability. Although ADHD medications are generally well-tolerated by healthy adults, assess for a history of potential contraindications:
- unstable medical condition, hyperthyroidism, glaucoma
- treatment with a monoamine oxidase inhibitor or other pressor agents because of possible effects on blood pressure and heart rate
- use of cytochrome P450 2D6 inhibitors, which may increase atomoxetine steady-state plasma concentrations
- cardiovascular disease or family history of early cardiac disease (Box 1)6,7
- history of or active substance use disorder, such as alcohol dependence, cocaine or heroin abuse
- history of psychosis, bipolar disorder, or an active clinically significant psychiatric comorbidity (major depression, agitated state, suicidality).
Clinically, some patients appear to tolerate 1 class of stimulant (such as methylphenidate or amphetamine) over another. Consider switching to an alternate stimulant if your patient has bothersome side effects—mild low appetite, insomnia, tension, or jitteriness—or has received limited or partial benefit during an initial stimulant trial.
Serious cardiovascular events and sudden death have occurred in adults and children treated with stimulants.6 Agents used for attention-deficit/hyperactivity disorder (ADHD) have not been shown to cause sudden cardiac death, but the FDA requires stimulants’ labeling to warn about this risk in patients with structural cardiac abnormalities. The warning advises against using stimulants in adults with cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease.
When treating adults with ADHD, look to advisories about cardiovascular monitoring in children with ADHD. Before initiating medications, do a physical exam focused on cardiovascular disease risk factors and obtain a patient and family health history of:
- fainting or dizziness
- sudden or unexplained death in someone young
- sudden cardiac death or “heart attack” in family members age <35 years.
The American Academy of Pediatrics, American Academy of Child and Adolescent Psychiatry, and American Heart Association concur that electrocardiography (ECG) is not mandatory in cardiovascular assessment and monitoring during ADHD pharmacotherapy.7 This author (PH) refers cardiovascular questions to a primary care physician or cardiologist.
During ADHD treatment, monitor vital signs and refer patients with emergent cardiac symptoms or concerns to a cardiologist. Expect increases in blood pressure (1 to 4 mm Hg) and heart rate (2 to 6 bpm) during treatment with methylphenidate and amphetamine-class stimulants as well as with atomoxetine. Do not expect significant changes in ECG parameters (PR, QRS, and QTC intervals).
Extended-release formulations. Early adult studies demonstrated the efficacy of immediate-release stimulants, but adults with ADHD’s inherent deficits in organization and memory may have higher adherence rates and greater success with once-daily, extended-release formulations.8-11 Unless your patient wants to begin with small, short-acting dosages (5 to 10 mg) or desires to target treatment to specific times of day (such as in the morning for administrative work only), many appreciate once-daily formulations. Extended-release formulations also may be the simplest stimulants with which to begin ADHD treatment.
Over time, patients may benefit from an immediate-release form:
- added for certain times of day—such as in late afternoon, when the morning extended-release dose has worn off (Box 2)12,13
- to use as an alternative to extended-release formulations when more or less flexibly is desired, such as on weekends.
Table 2
Administering medications approved for adult ADHD
| Drug | Recommended dosage* | Comments |
|---|---|---|
| Stimulants | ||
| Extended-release mixed amphetamine (Adderall XR) | 20 mg | Initial prescription of 10-mg XR capsules allows gradual titration |
| Extended-release OROS methylphenidate (Concerta) | 18 to 72 mg/d | Initial prescription of 18-mg OROS MPH capsules allows gradual titration |
| Extended-release dexmethylphenidate (Focalin XR) | 10 mg/d; maximum 20 mg/d | Dosing is one-half the typical dosing of racemic MPH |
| Lisdexamfetamine (Vyvanse) | 30 mg/d; maximum 70 mg/d | May be adjusted weekly in 10-mg or 20-mg increments |
| Nonstimulant | ||
| Atomoxetine (Strattera) | 80 mg/d; maximum 100 mg/d | Initial dosage of 40 mg/d can be increased to target dosage after a minimum of 3 days; can be given as a morning dose or divided evenly between morning and evening doses |
| * FDA-approved dosages as listed in the package inserts of these medications ADHD: attention-deficit/hyperactivity disorder; MPH: methylphenidate; OROS: osmotic release oral system; XR: extended-release formulation | ||
CASE CONTINUED: Feeling ‘calm, less frenetic’
During the next 6 months, you start Mr. Z on stimulant treatment at robust dosing consistent with his weight (90 kg). He complains that extended-duration methylphenidate (MPH)—titrated to 90 mg/d—doesn’t last into the late afternoon, and he feels mildly tense with a low appetite. Because of an apparent partial response and relatively mild adverse effects, you discontinue MPH and try an extended-duration amphetamine, titrated to 60 mg.
Mr. Z’s blood pressure and heart rate remain stable. He begins to exercise regularly and reduce his use of tobacco and caffeine drinks, as you recommend. He says he feels “calm, less frenetic.” He reports no tension on this medication and only mild reduced appetite. With a plan to continue taking the stimulant medication with regular monitoring, he then disappears from treatment.
Promoting adherence
Treatment nonadherence is an issue throughout medicine, and individuals with disorganization, forgetfulness, and impulsivity may be at higher-than-usual risk of not following through on medication regimens.
Combining short- and long-acting stimulants may cover hours when attention-deficit/hyperactivity (ADHD) symptoms emerge despite therapy with a long-acting agent.12,13 Ask patients who report lack of full-day coverage if the once-daily, extended-duration formulation they are taking works well until a certain time of day. Then consider adding a similar-class immediate-release stimulant at this time to cover the later hours.
If a patient reports partial response throughout the day—such as early in treatment—begin by optimizing the long-acting agent’s dosage. Keep a target daily dose in mind, based on FDA recommendations and clinical trial data. For example, an adult weighing 80 kg may respond optimally to a combination of 60 mg of a long-acting methylphenidate (MPH) in the morning, followed by 10 to 20 mg of an immediate-release MPH in mid-afternoon.
The later stimulants are taken in the day, the more likely insomnia may emerge as an adverse effect. Some patients adjust to this problem within the first weeks of treatment. If insomnia remains impairing, reduce the stimulant dose or consider switching to a shorter duration medication or to the nonstimulant atomoxetine.
In addition, restrictions on stimulant-class medications do not permit multiple-month prescribing (refills), as is allowed with non-scheduled medications such as atomoxetine. Discuss with patients how they will obtain stimulant medications on a regular, monthly or bimonthly basis. In our experience, the practical challenges of remaining in treatment at times may limit patients’ adherence to ADHD medications more than a lack of response or tolerability concerns.
Explain to patients early in treatment that they might need to try several different medications before settling on 1 that is optimally tolerated and efficacious. Because stimulants are generally quite effective for ADHD symptoms, set your goal to identify adverse effects and aim for a patient response of “this works well, and I don’t feel any different on it.”
CASE CONTINUED: Ready to try again
Three years later, Mr. Z returns and reports gradually discontinuing the stimulant because he “wanted to go it on my own.” He functioned relatively well at first, but errors and conflicts at his job led to his dismissal.
Since then, he has been unemployed. He is increasingly depressed and reports drinking and smoking “more heavily than in college.” He asks about resuming ADHD treatment.
Mr. Z does not meet DSM-IV-TR criteria for major depressive disorder or alcohol abuse/dependence. His depressed mood appears to be linked to his marked ADHD symptoms. Mr. Z agrees to a new treatment plan that includes starting atomoxetine at 25 mg to allow for flexible titration and psychotherapy to monitor his mood and achieve sobriety.
ADHD and substance abuse
Clinical judgment determines whether an adult with ADHD and a history of substance use disorders may safely benefit from treatment with a stimulant. The relationship between ADHD and substance use disorders is of clinical concern, but ADHD medications have not been shown to increase risk for later substance use disorders in children.14 Conversely, effective ADHD treatment appears to reduce later cigarette and substance use.15
Consider using a nonstimulant-class medication in adults with ADHD and active substance use disorders. In a 12-week, double-blind, controlled trial, atomoxetine improved ADHD symptoms significantly more than placebo in adults meeting DS-MIV-TR criteria for comorbid alcohol use disorders. After 4 to 30 days of alcohol abstinence, 72 patients were randomly assigned to atomoxetine, 25 to 100 mg/d (mean final dose 90 mg/d), and 75 patients to placebo. Although estimated times to initial relapse to heavy drinking did not differ:
- atomoxetine-treated subjects had 26% fewer cumulative heavy drinking days than placebo-treated subjects (P=0.023)
- the difference in cumulative heavy drinking days between the atomoxetine and placebo groups became statistically significant after 55 days of treatment.16
- World Health Organization Adult Self-Report Scale (ASRS) 18-item instrument and 6-item screener. www.med.nyu.edu/psych/psychiatrist/adhd.html.
- Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 2008;165:553-5.
- Adler LA, Spencer TJ, Levine LR, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord 2008; 11:720-7.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Extended-release mixed amphetamine • Adderall XR
- Extended duration OROS methylphenidate • Concerta
- Extended-release dexmethylphenidate • Focalin XR
- Lisdexamfetamine • Vyvanse
- Modafinil • Provigil
Disclosure
Dr. Hammerness has received research support from and is on the speakers bureau for Shire Pharmaceuticals. He has received support for CME activities and talks from Shire Pharmaceuticals, Ortho-McNeil, and Abbott Laboratories.
Dr. Surman receives research support and/or is a speaker for Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, Ortho-McNeil, Merck, New River Pharmaceuticals, Novartis, Pfizer Inc., Shire Pharmaceuticals, and Takeda Pharmaceutical Company.
Dr. Sassi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Clinical research assistant Katherine Miller, BA, contributed to the literature review for this article and assisted in preparing the manuscript.
1. Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005;35:245-56.
2. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed 2006;8(4):4.-
3. Greenhill L, Pliszka S, Dulcan M, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2001;40(11):1352-5.
4. Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2004;24:24-9.
5. Adler LA, Spencer TJ, Williams DW, et al. Long-term, open-label safety and efficacy of atomoxetine in adults with ADHD: final report of a 4-year study. J Atten Disord Epub 2008 April 30.
6. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med 2006;354:1445-8.
7. American Academy of Pediatrics/American Heart Association clarification of statement on cardiovascular evaluation and monitoring of children and adolescents with heart disease receiving medications for ADHD May 16, 2008. Available at: http://www.aap.org/pressroom/aap-ahastatement.htm. Accessed August 14, 2008.
8. Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2006;59(9):829-35.
9. Biederman J, Mick E, Surman C, et al. Comparative acute efficacy and tolerability of OROS and immediate release formulations of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2007;7:49.-
10. Mick E, Spencer TJ, Surman C, et al. Randomized single-blind substitution study of methylphenidate in ADHD adults receiving immediate-release methylphenidate. NR357. Poster presented at: Annual Meeting of the American Psychiatric Association; May 19-24, 2007; San Diego, CA.
11. Capone N, McDonnel T. Medication persistence among agents used to treat attention-deficit/hyperactivity disorder, diabetes, and elevated serum cholesterol. NR 639. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
12. Adler L, Morrill M, Reingold B. d-methylphenidate augmentation of extended-release stimulant therapy in ADHD. NR 619. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
13. Adler L, Reingold LS, Morrill MS, Wilens TE. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep 2006;8:409-15.
14. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry 2008;165:597-603.
15. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 2007;68(suppl 11):15-22.
16. Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 2008;96:145-54.
1. Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005;35:245-56.
2. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed 2006;8(4):4.-
3. Greenhill L, Pliszka S, Dulcan M, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2001;40(11):1352-5.
4. Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2004;24:24-9.
5. Adler LA, Spencer TJ, Williams DW, et al. Long-term, open-label safety and efficacy of atomoxetine in adults with ADHD: final report of a 4-year study. J Atten Disord Epub 2008 April 30.
6. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med 2006;354:1445-8.
7. American Academy of Pediatrics/American Heart Association clarification of statement on cardiovascular evaluation and monitoring of children and adolescents with heart disease receiving medications for ADHD May 16, 2008. Available at: http://www.aap.org/pressroom/aap-ahastatement.htm. Accessed August 14, 2008.
8. Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2006;59(9):829-35.
9. Biederman J, Mick E, Surman C, et al. Comparative acute efficacy and tolerability of OROS and immediate release formulations of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2007;7:49.-
10. Mick E, Spencer TJ, Surman C, et al. Randomized single-blind substitution study of methylphenidate in ADHD adults receiving immediate-release methylphenidate. NR357. Poster presented at: Annual Meeting of the American Psychiatric Association; May 19-24, 2007; San Diego, CA.
11. Capone N, McDonnel T. Medication persistence among agents used to treat attention-deficit/hyperactivity disorder, diabetes, and elevated serum cholesterol. NR 639. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
12. Adler L, Morrill M, Reingold B. d-methylphenidate augmentation of extended-release stimulant therapy in ADHD. NR 619. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
13. Adler L, Reingold LS, Morrill MS, Wilens TE. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep 2006;8:409-15.
14. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry 2008;165:597-603.
15. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 2007;68(suppl 11):15-22.
16. Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 2008;96:145-54.
Double jeopardy: How to treat kids with comorbid anxiety and ADHD
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
Lisdexamfetamine for ADHD
Lisdexamfetamine—FDA-approved to treat attention-deficit/hyperactivity disorder (ADHD) in children ages 6 to 12 (Table 1)—reduces ADHD symptoms during and after school and may be less likely to be abused than other psychostimulants, particularly immediate-release preparations, clinical data suggest.
Table 1
Lisdexamfetamine: Fast facts
| Brand name: Vyvanse |
| Indication: ADHD in children ages 6 to 12 |
| Approval date: February 23, 2007 |
| Manufacturers: New River Pharmaceuticals and Shire |
| Dosing forms: 30-, 50-, and 70-mg capsules |
| Recommended dosage: Start at 30 mg/d. If necessary, titrate by 20 mg every 3 to 7 days to a maximum 70 mg/d. |
Clinical implications
Because it is effective for about 12 hours, lisdexamfetamine might improve the child’s ability to complete homework and participate in extracurricular activities, which in turn might enhance academic performance and/or socialization skills.
Lisdexamfetamine could help the child with ADHD who shows no contraindications to the drug —particularly if he or she needs daylong coverage.
How it works
Lisdexamfetamine—a dextroamphetamine derivative—is rapidly absorbed and converted to dextroamphetamine, which is believed to exert therapeutic effect by:
- blocking norepinephrine and dopamine reuptake into presynaptic neurons
- increasing the neurotransmitters’ release into the extraneuronal space.
The medication’s amphetamine release is highly predictable, which contributes to its therapeutic benefit in ADHD. Amphetamine is released through GI metabolism of lisdexamfetamine, which produces the active d-amphetamine moiety that reaches the bloodstream. The medication is derived from d-amphetamine, with negligible amounts of lysine cleaved.
Lisdexamfetamine requires in vivo metabolism (in the GI tract) to its active constituent d-amphetamine. As a result, the medication will not produce high d-amphetamine blood levels—and should not cause euphoria or other reinforcing effects—if injected or snorted. Its abuse potential is lower overall compared with immediate-release psychostimulant formulations.
Pharmacokinetics
Dextroamphetamine’s plasma elimination half-life is approximately 9½ hours—which accounts for lisdexamfetamine’s extended action. The drug reaches steady-state concentrations in 2 to 3 days.
Food does not affect absorption and delays maximum concentration by 1 hour or less, so taking lisdexamfetamine during breakfast should not slow its therapeutic effect. Because dextroamphetamine reaches maximum concentration in approximately 3½ hours, the medication should take effect by the time the child gets to school. In one randomized, phase-2 trial, children with ADHD who received lisdexamfetamine, 30 to 70 mg/d, showed overall improvement within 2 hours after dosing.1
Efficacy
Lisdexamfetamine reduced ADHD symptoms in 2 double-blind studies: a phase-2 crossover study and a phase-3 random-dose trial.
Phase-2 crossover study.2 Fifty-two children ages 6 to 12 with combined or hyperactive-impulsive type ADHD received extended-release mixed amphetamine salts (MAS) for 3 weeks. Subjects received 10 mg/d or dosages titrated to 20 or 30 mg/d based on response to medication.
The youths then were divided into 3 groups based on optimal MAS dosage and received 3 treatments for 1 week each:
- group 1: placebo; MAS, 10 mg/d; lisdexamfetamine, 30 mg/d
- group 2: placebo; MAS, 20 mg/d; lisdexamfetamine, 50 mg/d
- group 3: placebo; MAS, 30 mg/d; lisdexamfetamine, 70 mg/d.
While taking lisdexamfetamine or MAS, subjects showed similar improvement in behavior, based on Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scores, and inattention, based on SKAMP and Permanent Product Measure of Performance scores.
Both psychostimulants outperformed placebo in both measures, and both improved behavior more decisively than inattention. Based on post-hoc analysis, improvement 12 hours after dosing was more substantial with lisdexamfetamine than with MAS.
Phase-3 random-dose trial.3 A total of 290 children ages 6 to 12 with combined or hyperactive-impulsive type ADHD were “washed out” from prior medications over 1 week, then received lisdexamfetamine or placebo for 4 weeks. Treatment-group children were started at 30 mg/d; some received dosages titrated at random to 50 or 70 mg/d in weekly 20-mg increments.
Over 4 weeks, ADHD Rating Scale Version IV (ADHD-RS-IV) scores fell 50% to 59% among the 3 lisdexamfetamine dosage groups, compared with a 15% reduction in the placebo group. Substantial ADHD-RS-IV score improvements after 1 week of lisdexamfetamine were maintained throughout the trial, suggesting the medication sustains ADHD symptom improvement. Controlled trials have not addressed lisdexamfetamine use >4 weeks, however.
Based on parents’ and guardians’ reports, treatment-group patients’ ADHD symptoms were notably less severe at 10 AM, 2 PM, and 6 PM compared with placebo-group children.3 This suggests that lisdexamfetamine offers a daylong therapeutic effect.
Tolerability
In the phase-3 study,3 162 of 218 (74%) children receiving any dosage of lisdexamfetamine reported an adverse event, compared with 34 of 72 (47%) children in the placebo group. Overall, 39% of lisdexamfetamine-group patients reported decreased appetite. Also common were insomnia, headaches, irritability, upper abdominal pain, vomiting, and weight loss (Table 2).
Although most adverse events were mild to moderate, 9.2% of treatment-group children dropped out because of intolerability, compared with 1.4% of the placebo group. The investigators increased dosages quickly, regardless of efficacy or tolerability,3 which might have increased side-effect incidence among the treatment groups.
In the phase-2 crossover trial,2 adverse event rates were similar among the lisdexamfetamine, extended-release MAS, and placebo groups (15% to 18%). Among youths receiving lisdexamfetamine, 8% reported insomnia and 6% reported appetite loss, compared with 2% and 4% of the MAS group, respectively.
Table 2
Rates of commonly reported adverse effects
during phase-3 lisdexamfetamine (LDX) study
| Adverse effect | LDX 30 mg/d | LDX 50 mg/d* | LDX 70 mg/d* | LDX all dosages | Placebo |
|---|---|---|---|---|---|
| All adverse effects | 72% | 68% | 84% | 74% | 47% |
| Decreased appetite | 37% | 31% | 49% | 39% | 4% |
| Insomnia | 16% | 16% | 25% | 19% | 3% |
| Upper abdominal pain | 14% | 7% | 15% | 12% | 6% |
| Headache | 10% | 10% | 16% | 12% | 10% |
| Irritability | 11% | 8% | 10% | 10% | 0% |
| Vomiting | 7% | 5% | 14% | 9% | 4% |
| Weight loss | 6% | 3% | 19% | 9% | 1% |
| *Dosages were randomly titrated regardless of efficacy or tolerability. | |||||
| Source: Reference 3 | |||||
Safety
Findling et al4 found a larger change in corrected QT interval with lisdexamfetamine (7 to 14 msec) than with extended-release MAS (5 to 10 msec) 5 and 10½ hours after dosing. The authors reasoned that these findings are atypical, and no children suffered serious adverse events during the trial. Nonetheless, more research on whether lisdexamfetamine increases cardiac risk is needed.
In a lethal-dose study in rats,5 oral lisdexamfetamine doses up to 1,000 mg/kg did not result in death, suggesting the medication might undergo saturation kinetics in the GI tract that may protect against overdose or abuse at higher dosages. By comparison, the median lethal oral dosage of d-amphetamine in rats was 96.8 mg/kg.5
Abuse potential
As with other psychostimulants indicated for ADHD, the Drug Enforcement Administration has classified lisdexamfetamine as a schedule II drug, which applies to addictive prescription-only medications with an accepted medical use.
Clinical data suggest, however, that lisdexamfetamine might be less “enjoyable”—and less likely to be abused intravenously, orally, or intranasally—than equipotent d-amphetamine. In an abuse liability study,6 12 adults with histories of stimulant abuse received intravenous immediate-release (IR) d-amphetamine, 10 or 20 mg. Two days later, they received a comparable dose of IV lisdexamfetamine, 25 or 50 mg. The researchers found that:
- Plasma d-amphetamine peaked within 5 minutes after injection, compared with 2 to 3 hours after lisdexamfetamine dosing.
- Subjects who received IR d-amphetamine said they felt euphoria within 15 minutes of injection. By contrast, no one reported euphoria or amphetamine-like subjective effects after receiving lisdexamfetamine.
When asked which medication they would try again, 9 of 12 subjects chose IR d-amphetamine and 1 chose lisdexamfetamine.
In a double-blind, randomized, placebo-controlled study,7 oral lisdexamfetamine, 50 or 100 mg, was not more “likeable” than placebo. Subjects reported “liking” effects with 150 mg of lisdexamfetamine, however, suggesting the medication could be misused or abused at higher-than-therapeutic dosages.
As with other psychostimulants, do not give lisdexamfetamine to youths with preexisting serious structural cardiac abnormalities or other heart problems. Assess patient and family history of heart disease before prescribing this medication.
Do not prescribe lisdexamfetamine to patients taking a monoamine oxidase inhibitor (MAOI). By slowing amphetamine metabolism, these antidepressants intensify amphetamines’ effect on monoamine release, which can cause headaches and lead to hypertensive crisis. Before starting lisdexamfetamine, ask if the patient is taking an MAOI or has taken one within 2 weeks of presentation.
Use caution when prescribing lisdexamfetamine to patients with:
- a comorbid eating disorder or sleep disturbance. Determine whether to address the comorbidity before treating ADHD symptoms, and make sure lisdexamfetamine is not worsening the comorbid symptoms.
- untreated hypertension or other cardiovascular conditions, as stimulant medications can increase blood pressure and heart rate. Watch for significant heart rate and blood pressure changes in patients taking lisdexamfetamine, which probably would not cause sustained blood pressure increase in patients taking antihypertensives.8
Related resources
- Lisdexamfetamine Web site. www.vyvanse.com.
Drug brand names
- Extended-release mixed amphetamine salts • Adderall XR
- Lisdexamfetamine • Vyvanse
Disclosure
Dr. Wilens receives research/grant support from Abbott Laboratories, Eli Lilly and Company, National Institute on Drug Abuse, NeuroSearch, Ortho-McNeil, and Shire; is a speaker for Novartis Pharmaceuticals Corp., Ortho-McNeil, and Shire; and is a consultant to Abbott Laboratories, Eli Lilly and Company, GlaxoSmithKline, National Institute on Drug Abuse, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire.
1. Lopez FA, Boellner SW, Childress A, et al. ADHD symptom improvement in children treated with lisdexamfetamine dimesylate (LDX). Poster presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 24-29, 2006; San Diego, CA.
2. Biederman J, Boellner SW, Childress A, et al. Improvements in symptoms of attention-deficit/hyperactivity disorder in school-aged children with lisdexamfetamine (NRP 104) and mixed amphetamine salts, extended-release versus placebo. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Canada.
3. Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP 104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 2007;29:450-63.
4. Findling FL, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr 2005;147:348-54.
5. Krishnan S. Toxicity profile of lisdexamfetamine dimesylate (LDX NRP104) in three independent rat toxicology studies. Basic Clin Phamacol Toxicol. In press.
6. Jasinski DR. Abuse liability of intravenous L-lysine-d-amphetamine (NRP 104). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=4234033. Accessed April 5, 2007.
7. Jasinski D, Krishnan S. A double-blind, randomized, placebo and active-controlled, six-period crossover study to evaluate the likeability, safety, and abuse liability of NRP 104 in healthy adult volunteers with histories of stimulant abuse (NRP104. A03). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://www.secinfo.com/d12Pk6.v9Ac.d.htm. Accessed May 14, 2007.
8. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67:696-702.
Lisdexamfetamine—FDA-approved to treat attention-deficit/hyperactivity disorder (ADHD) in children ages 6 to 12 (Table 1)—reduces ADHD symptoms during and after school and may be less likely to be abused than other psychostimulants, particularly immediate-release preparations, clinical data suggest.
Table 1
Lisdexamfetamine: Fast facts
| Brand name: Vyvanse |
| Indication: ADHD in children ages 6 to 12 |
| Approval date: February 23, 2007 |
| Manufacturers: New River Pharmaceuticals and Shire |
| Dosing forms: 30-, 50-, and 70-mg capsules |
| Recommended dosage: Start at 30 mg/d. If necessary, titrate by 20 mg every 3 to 7 days to a maximum 70 mg/d. |
Clinical implications
Because it is effective for about 12 hours, lisdexamfetamine might improve the child’s ability to complete homework and participate in extracurricular activities, which in turn might enhance academic performance and/or socialization skills.
Lisdexamfetamine could help the child with ADHD who shows no contraindications to the drug —particularly if he or she needs daylong coverage.
How it works
Lisdexamfetamine—a dextroamphetamine derivative—is rapidly absorbed and converted to dextroamphetamine, which is believed to exert therapeutic effect by:
- blocking norepinephrine and dopamine reuptake into presynaptic neurons
- increasing the neurotransmitters’ release into the extraneuronal space.
The medication’s amphetamine release is highly predictable, which contributes to its therapeutic benefit in ADHD. Amphetamine is released through GI metabolism of lisdexamfetamine, which produces the active d-amphetamine moiety that reaches the bloodstream. The medication is derived from d-amphetamine, with negligible amounts of lysine cleaved.
Lisdexamfetamine requires in vivo metabolism (in the GI tract) to its active constituent d-amphetamine. As a result, the medication will not produce high d-amphetamine blood levels—and should not cause euphoria or other reinforcing effects—if injected or snorted. Its abuse potential is lower overall compared with immediate-release psychostimulant formulations.
Pharmacokinetics
Dextroamphetamine’s plasma elimination half-life is approximately 9½ hours—which accounts for lisdexamfetamine’s extended action. The drug reaches steady-state concentrations in 2 to 3 days.
Food does not affect absorption and delays maximum concentration by 1 hour or less, so taking lisdexamfetamine during breakfast should not slow its therapeutic effect. Because dextroamphetamine reaches maximum concentration in approximately 3½ hours, the medication should take effect by the time the child gets to school. In one randomized, phase-2 trial, children with ADHD who received lisdexamfetamine, 30 to 70 mg/d, showed overall improvement within 2 hours after dosing.1
Efficacy
Lisdexamfetamine reduced ADHD symptoms in 2 double-blind studies: a phase-2 crossover study and a phase-3 random-dose trial.
Phase-2 crossover study.2 Fifty-two children ages 6 to 12 with combined or hyperactive-impulsive type ADHD received extended-release mixed amphetamine salts (MAS) for 3 weeks. Subjects received 10 mg/d or dosages titrated to 20 or 30 mg/d based on response to medication.
The youths then were divided into 3 groups based on optimal MAS dosage and received 3 treatments for 1 week each:
- group 1: placebo; MAS, 10 mg/d; lisdexamfetamine, 30 mg/d
- group 2: placebo; MAS, 20 mg/d; lisdexamfetamine, 50 mg/d
- group 3: placebo; MAS, 30 mg/d; lisdexamfetamine, 70 mg/d.
While taking lisdexamfetamine or MAS, subjects showed similar improvement in behavior, based on Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scores, and inattention, based on SKAMP and Permanent Product Measure of Performance scores.
Both psychostimulants outperformed placebo in both measures, and both improved behavior more decisively than inattention. Based on post-hoc analysis, improvement 12 hours after dosing was more substantial with lisdexamfetamine than with MAS.
Phase-3 random-dose trial.3 A total of 290 children ages 6 to 12 with combined or hyperactive-impulsive type ADHD were “washed out” from prior medications over 1 week, then received lisdexamfetamine or placebo for 4 weeks. Treatment-group children were started at 30 mg/d; some received dosages titrated at random to 50 or 70 mg/d in weekly 20-mg increments.
Over 4 weeks, ADHD Rating Scale Version IV (ADHD-RS-IV) scores fell 50% to 59% among the 3 lisdexamfetamine dosage groups, compared with a 15% reduction in the placebo group. Substantial ADHD-RS-IV score improvements after 1 week of lisdexamfetamine were maintained throughout the trial, suggesting the medication sustains ADHD symptom improvement. Controlled trials have not addressed lisdexamfetamine use >4 weeks, however.
Based on parents’ and guardians’ reports, treatment-group patients’ ADHD symptoms were notably less severe at 10 AM, 2 PM, and 6 PM compared with placebo-group children.3 This suggests that lisdexamfetamine offers a daylong therapeutic effect.
Tolerability
In the phase-3 study,3 162 of 218 (74%) children receiving any dosage of lisdexamfetamine reported an adverse event, compared with 34 of 72 (47%) children in the placebo group. Overall, 39% of lisdexamfetamine-group patients reported decreased appetite. Also common were insomnia, headaches, irritability, upper abdominal pain, vomiting, and weight loss (Table 2).
Although most adverse events were mild to moderate, 9.2% of treatment-group children dropped out because of intolerability, compared with 1.4% of the placebo group. The investigators increased dosages quickly, regardless of efficacy or tolerability,3 which might have increased side-effect incidence among the treatment groups.
In the phase-2 crossover trial,2 adverse event rates were similar among the lisdexamfetamine, extended-release MAS, and placebo groups (15% to 18%). Among youths receiving lisdexamfetamine, 8% reported insomnia and 6% reported appetite loss, compared with 2% and 4% of the MAS group, respectively.
Table 2
Rates of commonly reported adverse effects
during phase-3 lisdexamfetamine (LDX) study
| Adverse effect | LDX 30 mg/d | LDX 50 mg/d* | LDX 70 mg/d* | LDX all dosages | Placebo |
|---|---|---|---|---|---|
| All adverse effects | 72% | 68% | 84% | 74% | 47% |
| Decreased appetite | 37% | 31% | 49% | 39% | 4% |
| Insomnia | 16% | 16% | 25% | 19% | 3% |
| Upper abdominal pain | 14% | 7% | 15% | 12% | 6% |
| Headache | 10% | 10% | 16% | 12% | 10% |
| Irritability | 11% | 8% | 10% | 10% | 0% |
| Vomiting | 7% | 5% | 14% | 9% | 4% |
| Weight loss | 6% | 3% | 19% | 9% | 1% |
| *Dosages were randomly titrated regardless of efficacy or tolerability. | |||||
| Source: Reference 3 | |||||
Safety
Findling et al4 found a larger change in corrected QT interval with lisdexamfetamine (7 to 14 msec) than with extended-release MAS (5 to 10 msec) 5 and 10½ hours after dosing. The authors reasoned that these findings are atypical, and no children suffered serious adverse events during the trial. Nonetheless, more research on whether lisdexamfetamine increases cardiac risk is needed.
In a lethal-dose study in rats,5 oral lisdexamfetamine doses up to 1,000 mg/kg did not result in death, suggesting the medication might undergo saturation kinetics in the GI tract that may protect against overdose or abuse at higher dosages. By comparison, the median lethal oral dosage of d-amphetamine in rats was 96.8 mg/kg.5
Abuse potential
As with other psychostimulants indicated for ADHD, the Drug Enforcement Administration has classified lisdexamfetamine as a schedule II drug, which applies to addictive prescription-only medications with an accepted medical use.
Clinical data suggest, however, that lisdexamfetamine might be less “enjoyable”—and less likely to be abused intravenously, orally, or intranasally—than equipotent d-amphetamine. In an abuse liability study,6 12 adults with histories of stimulant abuse received intravenous immediate-release (IR) d-amphetamine, 10 or 20 mg. Two days later, they received a comparable dose of IV lisdexamfetamine, 25 or 50 mg. The researchers found that:
- Plasma d-amphetamine peaked within 5 minutes after injection, compared with 2 to 3 hours after lisdexamfetamine dosing.
- Subjects who received IR d-amphetamine said they felt euphoria within 15 minutes of injection. By contrast, no one reported euphoria or amphetamine-like subjective effects after receiving lisdexamfetamine.
When asked which medication they would try again, 9 of 12 subjects chose IR d-amphetamine and 1 chose lisdexamfetamine.
In a double-blind, randomized, placebo-controlled study,7 oral lisdexamfetamine, 50 or 100 mg, was not more “likeable” than placebo. Subjects reported “liking” effects with 150 mg of lisdexamfetamine, however, suggesting the medication could be misused or abused at higher-than-therapeutic dosages.
As with other psychostimulants, do not give lisdexamfetamine to youths with preexisting serious structural cardiac abnormalities or other heart problems. Assess patient and family history of heart disease before prescribing this medication.
Do not prescribe lisdexamfetamine to patients taking a monoamine oxidase inhibitor (MAOI). By slowing amphetamine metabolism, these antidepressants intensify amphetamines’ effect on monoamine release, which can cause headaches and lead to hypertensive crisis. Before starting lisdexamfetamine, ask if the patient is taking an MAOI or has taken one within 2 weeks of presentation.
Use caution when prescribing lisdexamfetamine to patients with:
- a comorbid eating disorder or sleep disturbance. Determine whether to address the comorbidity before treating ADHD symptoms, and make sure lisdexamfetamine is not worsening the comorbid symptoms.
- untreated hypertension or other cardiovascular conditions, as stimulant medications can increase blood pressure and heart rate. Watch for significant heart rate and blood pressure changes in patients taking lisdexamfetamine, which probably would not cause sustained blood pressure increase in patients taking antihypertensives.8
Related resources
- Lisdexamfetamine Web site. www.vyvanse.com.
Drug brand names
- Extended-release mixed amphetamine salts • Adderall XR
- Lisdexamfetamine • Vyvanse
Disclosure
Dr. Wilens receives research/grant support from Abbott Laboratories, Eli Lilly and Company, National Institute on Drug Abuse, NeuroSearch, Ortho-McNeil, and Shire; is a speaker for Novartis Pharmaceuticals Corp., Ortho-McNeil, and Shire; and is a consultant to Abbott Laboratories, Eli Lilly and Company, GlaxoSmithKline, National Institute on Drug Abuse, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire.
Lisdexamfetamine—FDA-approved to treat attention-deficit/hyperactivity disorder (ADHD) in children ages 6 to 12 (Table 1)—reduces ADHD symptoms during and after school and may be less likely to be abused than other psychostimulants, particularly immediate-release preparations, clinical data suggest.
Table 1
Lisdexamfetamine: Fast facts
| Brand name: Vyvanse |
| Indication: ADHD in children ages 6 to 12 |
| Approval date: February 23, 2007 |
| Manufacturers: New River Pharmaceuticals and Shire |
| Dosing forms: 30-, 50-, and 70-mg capsules |
| Recommended dosage: Start at 30 mg/d. If necessary, titrate by 20 mg every 3 to 7 days to a maximum 70 mg/d. |
Clinical implications
Because it is effective for about 12 hours, lisdexamfetamine might improve the child’s ability to complete homework and participate in extracurricular activities, which in turn might enhance academic performance and/or socialization skills.
Lisdexamfetamine could help the child with ADHD who shows no contraindications to the drug —particularly if he or she needs daylong coverage.
How it works
Lisdexamfetamine—a dextroamphetamine derivative—is rapidly absorbed and converted to dextroamphetamine, which is believed to exert therapeutic effect by:
- blocking norepinephrine and dopamine reuptake into presynaptic neurons
- increasing the neurotransmitters’ release into the extraneuronal space.
The medication’s amphetamine release is highly predictable, which contributes to its therapeutic benefit in ADHD. Amphetamine is released through GI metabolism of lisdexamfetamine, which produces the active d-amphetamine moiety that reaches the bloodstream. The medication is derived from d-amphetamine, with negligible amounts of lysine cleaved.
Lisdexamfetamine requires in vivo metabolism (in the GI tract) to its active constituent d-amphetamine. As a result, the medication will not produce high d-amphetamine blood levels—and should not cause euphoria or other reinforcing effects—if injected or snorted. Its abuse potential is lower overall compared with immediate-release psychostimulant formulations.
Pharmacokinetics
Dextroamphetamine’s plasma elimination half-life is approximately 9½ hours—which accounts for lisdexamfetamine’s extended action. The drug reaches steady-state concentrations in 2 to 3 days.
Food does not affect absorption and delays maximum concentration by 1 hour or less, so taking lisdexamfetamine during breakfast should not slow its therapeutic effect. Because dextroamphetamine reaches maximum concentration in approximately 3½ hours, the medication should take effect by the time the child gets to school. In one randomized, phase-2 trial, children with ADHD who received lisdexamfetamine, 30 to 70 mg/d, showed overall improvement within 2 hours after dosing.1
Efficacy
Lisdexamfetamine reduced ADHD symptoms in 2 double-blind studies: a phase-2 crossover study and a phase-3 random-dose trial.
Phase-2 crossover study.2 Fifty-two children ages 6 to 12 with combined or hyperactive-impulsive type ADHD received extended-release mixed amphetamine salts (MAS) for 3 weeks. Subjects received 10 mg/d or dosages titrated to 20 or 30 mg/d based on response to medication.
The youths then were divided into 3 groups based on optimal MAS dosage and received 3 treatments for 1 week each:
- group 1: placebo; MAS, 10 mg/d; lisdexamfetamine, 30 mg/d
- group 2: placebo; MAS, 20 mg/d; lisdexamfetamine, 50 mg/d
- group 3: placebo; MAS, 30 mg/d; lisdexamfetamine, 70 mg/d.
While taking lisdexamfetamine or MAS, subjects showed similar improvement in behavior, based on Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scores, and inattention, based on SKAMP and Permanent Product Measure of Performance scores.
Both psychostimulants outperformed placebo in both measures, and both improved behavior more decisively than inattention. Based on post-hoc analysis, improvement 12 hours after dosing was more substantial with lisdexamfetamine than with MAS.
Phase-3 random-dose trial.3 A total of 290 children ages 6 to 12 with combined or hyperactive-impulsive type ADHD were “washed out” from prior medications over 1 week, then received lisdexamfetamine or placebo for 4 weeks. Treatment-group children were started at 30 mg/d; some received dosages titrated at random to 50 or 70 mg/d in weekly 20-mg increments.
Over 4 weeks, ADHD Rating Scale Version IV (ADHD-RS-IV) scores fell 50% to 59% among the 3 lisdexamfetamine dosage groups, compared with a 15% reduction in the placebo group. Substantial ADHD-RS-IV score improvements after 1 week of lisdexamfetamine were maintained throughout the trial, suggesting the medication sustains ADHD symptom improvement. Controlled trials have not addressed lisdexamfetamine use >4 weeks, however.
Based on parents’ and guardians’ reports, treatment-group patients’ ADHD symptoms were notably less severe at 10 AM, 2 PM, and 6 PM compared with placebo-group children.3 This suggests that lisdexamfetamine offers a daylong therapeutic effect.
Tolerability
In the phase-3 study,3 162 of 218 (74%) children receiving any dosage of lisdexamfetamine reported an adverse event, compared with 34 of 72 (47%) children in the placebo group. Overall, 39% of lisdexamfetamine-group patients reported decreased appetite. Also common were insomnia, headaches, irritability, upper abdominal pain, vomiting, and weight loss (Table 2).
Although most adverse events were mild to moderate, 9.2% of treatment-group children dropped out because of intolerability, compared with 1.4% of the placebo group. The investigators increased dosages quickly, regardless of efficacy or tolerability,3 which might have increased side-effect incidence among the treatment groups.
In the phase-2 crossover trial,2 adverse event rates were similar among the lisdexamfetamine, extended-release MAS, and placebo groups (15% to 18%). Among youths receiving lisdexamfetamine, 8% reported insomnia and 6% reported appetite loss, compared with 2% and 4% of the MAS group, respectively.
Table 2
Rates of commonly reported adverse effects
during phase-3 lisdexamfetamine (LDX) study
| Adverse effect | LDX 30 mg/d | LDX 50 mg/d* | LDX 70 mg/d* | LDX all dosages | Placebo |
|---|---|---|---|---|---|
| All adverse effects | 72% | 68% | 84% | 74% | 47% |
| Decreased appetite | 37% | 31% | 49% | 39% | 4% |
| Insomnia | 16% | 16% | 25% | 19% | 3% |
| Upper abdominal pain | 14% | 7% | 15% | 12% | 6% |
| Headache | 10% | 10% | 16% | 12% | 10% |
| Irritability | 11% | 8% | 10% | 10% | 0% |
| Vomiting | 7% | 5% | 14% | 9% | 4% |
| Weight loss | 6% | 3% | 19% | 9% | 1% |
| *Dosages were randomly titrated regardless of efficacy or tolerability. | |||||
| Source: Reference 3 | |||||
Safety
Findling et al4 found a larger change in corrected QT interval with lisdexamfetamine (7 to 14 msec) than with extended-release MAS (5 to 10 msec) 5 and 10½ hours after dosing. The authors reasoned that these findings are atypical, and no children suffered serious adverse events during the trial. Nonetheless, more research on whether lisdexamfetamine increases cardiac risk is needed.
In a lethal-dose study in rats,5 oral lisdexamfetamine doses up to 1,000 mg/kg did not result in death, suggesting the medication might undergo saturation kinetics in the GI tract that may protect against overdose or abuse at higher dosages. By comparison, the median lethal oral dosage of d-amphetamine in rats was 96.8 mg/kg.5
Abuse potential
As with other psychostimulants indicated for ADHD, the Drug Enforcement Administration has classified lisdexamfetamine as a schedule II drug, which applies to addictive prescription-only medications with an accepted medical use.
Clinical data suggest, however, that lisdexamfetamine might be less “enjoyable”—and less likely to be abused intravenously, orally, or intranasally—than equipotent d-amphetamine. In an abuse liability study,6 12 adults with histories of stimulant abuse received intravenous immediate-release (IR) d-amphetamine, 10 or 20 mg. Two days later, they received a comparable dose of IV lisdexamfetamine, 25 or 50 mg. The researchers found that:
- Plasma d-amphetamine peaked within 5 minutes after injection, compared with 2 to 3 hours after lisdexamfetamine dosing.
- Subjects who received IR d-amphetamine said they felt euphoria within 15 minutes of injection. By contrast, no one reported euphoria or amphetamine-like subjective effects after receiving lisdexamfetamine.
When asked which medication they would try again, 9 of 12 subjects chose IR d-amphetamine and 1 chose lisdexamfetamine.
In a double-blind, randomized, placebo-controlled study,7 oral lisdexamfetamine, 50 or 100 mg, was not more “likeable” than placebo. Subjects reported “liking” effects with 150 mg of lisdexamfetamine, however, suggesting the medication could be misused or abused at higher-than-therapeutic dosages.
As with other psychostimulants, do not give lisdexamfetamine to youths with preexisting serious structural cardiac abnormalities or other heart problems. Assess patient and family history of heart disease before prescribing this medication.
Do not prescribe lisdexamfetamine to patients taking a monoamine oxidase inhibitor (MAOI). By slowing amphetamine metabolism, these antidepressants intensify amphetamines’ effect on monoamine release, which can cause headaches and lead to hypertensive crisis. Before starting lisdexamfetamine, ask if the patient is taking an MAOI or has taken one within 2 weeks of presentation.
Use caution when prescribing lisdexamfetamine to patients with:
- a comorbid eating disorder or sleep disturbance. Determine whether to address the comorbidity before treating ADHD symptoms, and make sure lisdexamfetamine is not worsening the comorbid symptoms.
- untreated hypertension or other cardiovascular conditions, as stimulant medications can increase blood pressure and heart rate. Watch for significant heart rate and blood pressure changes in patients taking lisdexamfetamine, which probably would not cause sustained blood pressure increase in patients taking antihypertensives.8
Related resources
- Lisdexamfetamine Web site. www.vyvanse.com.
Drug brand names
- Extended-release mixed amphetamine salts • Adderall XR
- Lisdexamfetamine • Vyvanse
Disclosure
Dr. Wilens receives research/grant support from Abbott Laboratories, Eli Lilly and Company, National Institute on Drug Abuse, NeuroSearch, Ortho-McNeil, and Shire; is a speaker for Novartis Pharmaceuticals Corp., Ortho-McNeil, and Shire; and is a consultant to Abbott Laboratories, Eli Lilly and Company, GlaxoSmithKline, National Institute on Drug Abuse, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire.
1. Lopez FA, Boellner SW, Childress A, et al. ADHD symptom improvement in children treated with lisdexamfetamine dimesylate (LDX). Poster presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 24-29, 2006; San Diego, CA.
2. Biederman J, Boellner SW, Childress A, et al. Improvements in symptoms of attention-deficit/hyperactivity disorder in school-aged children with lisdexamfetamine (NRP 104) and mixed amphetamine salts, extended-release versus placebo. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Canada.
3. Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP 104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 2007;29:450-63.
4. Findling FL, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr 2005;147:348-54.
5. Krishnan S. Toxicity profile of lisdexamfetamine dimesylate (LDX NRP104) in three independent rat toxicology studies. Basic Clin Phamacol Toxicol. In press.
6. Jasinski DR. Abuse liability of intravenous L-lysine-d-amphetamine (NRP 104). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=4234033. Accessed April 5, 2007.
7. Jasinski D, Krishnan S. A double-blind, randomized, placebo and active-controlled, six-period crossover study to evaluate the likeability, safety, and abuse liability of NRP 104 in healthy adult volunteers with histories of stimulant abuse (NRP104. A03). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://www.secinfo.com/d12Pk6.v9Ac.d.htm. Accessed May 14, 2007.
8. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67:696-702.
1. Lopez FA, Boellner SW, Childress A, et al. ADHD symptom improvement in children treated with lisdexamfetamine dimesylate (LDX). Poster presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 24-29, 2006; San Diego, CA.
2. Biederman J, Boellner SW, Childress A, et al. Improvements in symptoms of attention-deficit/hyperactivity disorder in school-aged children with lisdexamfetamine (NRP 104) and mixed amphetamine salts, extended-release versus placebo. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Canada.
3. Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP 104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 2007;29:450-63.
4. Findling FL, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr 2005;147:348-54.
5. Krishnan S. Toxicity profile of lisdexamfetamine dimesylate (LDX NRP104) in three independent rat toxicology studies. Basic Clin Phamacol Toxicol. In press.
6. Jasinski DR. Abuse liability of intravenous L-lysine-d-amphetamine (NRP 104). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://xml.10kwizard.com/filing_raw.php?repo=tenk&ipage=4234033. Accessed April 5, 2007.
7. Jasinski D, Krishnan S. A double-blind, randomized, placebo and active-controlled, six-period crossover study to evaluate the likeability, safety, and abuse liability of NRP 104 in healthy adult volunteers with histories of stimulant abuse (NRP104. A03). Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; June 17-22, 2006; Scottsdale, AZ. Available at: http://www.secinfo.com/d12Pk6.v9Ac.d.htm. Accessed May 14, 2007.
8. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67:696-702.
ADHD: Only half the diagnosis in an adult with inattention?
Overlapping symptoms may obscure comorbid bipolar illness
An adult with function-impairing inattention could have attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), or both. Comorbid ADHD and BD often is unrecognized, however, because patients are more likely to report ADHD-related symptoms than manic symptoms.1
To help you recognize comorbid ADHD/BD—and protect adults who might switch into mania if given stimulants or antidepressants—this article describes a hierarchy to diagnose and treat this comorbidity. Based on the evidence and our experience, we:
- discuss how to differentiate between these disorders with overlapping symptoms
- provide tools and suggestions to screen for BD and adult ADHD
- offer 3 algorithms to guide your diagnosis and choice of medications.
Clinical challenges
Prevalence is unclear. Adult ADHD—with an estimated prevalence of 4.4%2—is more common than BD. Lifetime prevalences of BD types I and II are 1.6% and 0.5%, respectively.3 Studies of ADHD/BD comorbidity suggest wide-ranging prevalence rates:
Underdiagnosis. Adult ADHD/BD is a more severe illness than ADHD or BD alone and is highly comorbid with agoraphobia, social phobia, posttraumatic stress disorder, and alcohol or drug addiction. Adults with ADHD/BD have more frequent affective episodes, suicide attempts, violence, and legal problems.4 Diagnosing this comorbidity remains a challenge, however, because:
- identifying which symptoms are caused by which disorder can be difficult
- BD tends to be underdiagnosed9
- patients often misidentify, underreport, or deny manic symptoms1,10,11
- if a patient presents with active bipolar symptoms, DSM-IV-TR criteria require that ADHD not be diagnosed until mood symptoms are resolved.
Overlapping symptoms. ADHD and bipolar mania share some DSM-IV-TR diagnostic criteria, including talkativeness, distractibility, increased activity or physical restlessness, and loss of social inhibitions (Table 1).12 Overlapping symptoms also are notable within ADHD diagnostic criteria (Table 2). In the inattention category, for example, “easily distracted by extraneous stimuli,” “difficulty sustaining attention in tasks,” and “fails to give close attention to details” are considered 3 separate symptoms. In the hyperactivity category, “often leaves seat,” “often runs about or climbs excessively,” and “often on the go, or often acts as if driven by a motor” are 3 separate symptoms.
Given ADHD’s relatively loose diagnostic criteria and high comorbidity in adults with mood disorders, the question of whether adult ADHD/BD represents comorbidity or diagnostic overlap remains unresolved. For the clinician, the disorders’ nonoverlapping features (Table 1) can assist with the differential diagnosis. For example:
- ADHD symptoms tend to be chronic and BD symptoms episodic.
- ADHD patients may have high energy but lack increased productivity seen in BD patients.
- ADHD patients do not need less sleep or have inflated self-esteem like symptomatic BD patients.
- Psychotic symptoms such as hallucinations or delusions might be present in severe BD but are absent in ADHD.
Table 1
Overlap between DSM-IV-TR diagnostic criteria for ADHD and bipolar mania
| Overlapping symptoms | |
|---|---|
| ADHD | Bipolar mania |
| Talks excessively | More talkative than usual |
| Easily distracted/jumps from one activity to the next | Distractibility or constant changes in activity or plans |
| Fidgets Difficulty remaining seated Runs or climbs about inappropriately Difficulty playing quietly On the go as if driven by a motor | Increased activity or physical restlessness |
| Interrupts or butts in uninvited Blurts out answers | Loss of normal social inhibitions |
| Nonoverlapping symptoms | |
| ADHD Forgetful in daily activities Difficulty awaiting turn Difficulty organizing self Loses things Avoids sustained mental effort Does not seem to listen Difficulty following through on instructions/fails to finish work Difficulty sustaining attention Fails to give close attention to details/makes careless mistakes | |
| Bipolar mania Inflated self-esteem/grandiosity Increase in goal-directed activity Flight of ideas Decreased need for sleep Excessive involvement in pleasurable activities with disregard for potential adverse consequences Marked sexual energy or sexual indiscretions | |
| ADHD: attention-deficit/hyperactivity disorder | |
| Source: Adapted and reprinted with permission from reference 12 | |
Table 2
DSM-IV-TR diagnostic criteria for attention-deficit/ hyperactivity disorder
| Inattention |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Hyperactivity/impulsivity |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Diagnosis requires evidence of inattention or hyperactivity/impulsivity or both |
| Some hyperactive/impulsive or inattentive symptoms that caused impairment were present before age 7 |
| Some impairment from symptoms is present in ≥2 settings (such as at school, work, or home) |
| Symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder (mood disorder, anxiety disorder, dissociative disorder, or a personality disorder) |
| Source: DSM-IV-TR |
Mood symptoms first
A diagnostic hierarchy is implicit in DSM-IV-TR; anxiety disorders are not diagnosed during an active major depressive or manic episode, and schizophrenia is not diagnosed on the basis of psychotic symptoms during an active major depressive or manic episode. Mood disorders sit atop this implied diagnostic hierarchy and must be ruled out before psychotic or anxiety disorders are diagnosed. Similarly, most personality disorders are not diagnosed during an active mood or psychotic episode.
Diagnosing adult ADHD when a patient is actively depressed or manic is inconsistent with this hierarchy and conflicts with extensive nosologic literature.13 We suggest that ADHD—a cognitive-behavioral problem—not be diagnosed solely on symptoms observed when a patient is experiencing a mood episode or psychotic illness.
Bipolar disorder. Two useful mnemonics (Table 3) assist in screening for DSM-IV-TR symptoms of BD type I:
- Pure mania consists of euphoric mood and ≥3 of 7 DIGFAST criteria, or irritable mood and ≥4 of 7 DIGFAST criteria
- Mixed mania consists of depressed mood with ≥4 of 7 DIGFAST criteria and ≥4 of 8 SIGECAPS criteria.
To be diagnostic, these symptoms must cause substantial social or occupational dysfunction and be present at least 1 week. Diagnose BD type I if a patient has experienced a single pure or mixed manic episode at any time, unless the episode had a medical cause such as hyperthyroidism or antidepressant use. Because patients with mixed episodes experience depressed mood, assess any patient with clinical depression for manic symptoms. Otherwise, a patient with a mixed episode could be misdiagnosed as having unipolar depression instead of BD type I.14
BD type II also has been observed in patients with comorbid adult ADHD/BD.4,6 The main difference between BD types I and II is that manic symptoms in type II are not severe enough to cause functional impairment or psychotic symptoms.15
Adult ADHD. The clinical interview seeking evidence of inattention and hyperactivity/impulsivity remains the basis of adult ADHD diagnosis (Table 2). Key areas are:
- the patient’s past and current functional impairment
- whether substantial impairment occurs in at least 2 areas of life (such as school, work, or home).
Take medical, educational, social, psychological, and vocational histories, and rule out other conditions before concluding that adult ADHD is the appropriate diagnosis.16 In adult ADHD, inattentive symptoms become far more prominent, about twice as common as hyperactive symptoms.17 Inattentive symptoms may manifest as neglect, poor time management, motivational deficits, or poor concentration that results in forgetfulness, distractibility, item misplacement, or excessive mistakes in paperwork.18 When impulsive symptoms persist in adults, they may manifest as automobile accidents or low tolerance for frustration, which may lead to frequent job changes and unstable, interrupted interpersonal relationships.18
Neuropsychological testing is not required to make an adult ADHD diagnosis but can help establish the breadth of symptoms or comorbidity.17 Rating scales can screen, gather data (including presence and severity of symptoms), and measure treatment response.16 Commonly used rating scales include:
- Conners’ Adult ADHD Rating Scales19
- Brown Attention Deficit Disorder Rating Scale for Adults20
- Adult ADHD Self-Report Scale.21
When using rating scales, remember that adult psychopathology can distort perceptions, and some self-report scales have questionable reliability.16
Table 3
Mnemonics for diagnostic symptoms of pure and mixed bipolar mania
| DIGFAST* for bipolar mania symptoms | SIGECAPS† bipolar depression symptoms |
|---|---|
| Distractibility Insomnia Grandiosity Flight of ideas Activities Speech Thoughtlessness | Sleep Interest Guilt Energy Concentration Appetite Psychomotor Suicide |
| Pure mania: Euphoric mood with ≥3 DIGFAST criteria or irritable mood with ≥4 DIGFAST criteria. | |
| Mixed mania: Depressed mood with ≥4 DIGFAST criteria and ≥4 SIGECAPS criteria. | |
| * Developed by William Falk, MD | |
| †Developed by Carey Gross, MD | |
| Source: Adapted from Ghaemi SN. Mood disorders. New York: Lippincott, Williams, & Wilkins; 2003 | |
Treatment recommendations
Limited data. We found only 1 study on adult ADHD/BD treatment. In this open trial,22 36 adults with comorbid ADHD and BD received bupropion SR, up to 200 mg bid, for ADHD symptoms while maintained on mood stabilizers, antipsychotics, or both. Improvement was defined as ≥30% reduction in ADHD Symptom Checklist Scale scores, without concurrent mania. After 6 weeks, 82% of patients had improved; 1 dropped out at week 2 because of hypomanic activation. Methodologic limitations included trial design (non-randomized, nonblinded, short duration) and patient selection (90% of subjects had BD type II).
In the absence of adequate data on adult ADHD/BD, studies in children suggest:
- stimulants may not be effective for ADHD symptoms in patients with active manic or depressive symptoms
- mood stabilization is a prerequisite for successful pharmacologic treatment of ADHD in patients with both ADHD and manic or depressive symptoms.23,24
Follow the hierarchy. First treat acute mood symptoms, then reevaluate and possibly treat ADHD symptoms if they persist during euthymia (Algorithm 1). When a patient meets criteria for adult ADHD/BD, first stabilize bipolar manic or depressive symptoms (Algorithm 2). For acute mania, treat with standard mood stabilizers (lithium, valproate, lamotrigine, or carbamazepine) with or without a second-generation antipsychotic.25 Starting stimulants for ADHD when patients have active mood symptoms is sub-optimal and potentially harmful because of the risk of inducing mania. For acute bipolar depression, adjunctive antidepressant treatment has been found to be no more effective than a mood stabilizer alone.26
After bipolar symptoms respond or remit, reassess for adult ADHD. If ADHD symptoms persist during euthymia, additional treatment may be indicated.
Very little evidence exists on treating adult ADHD/BD; as mentioned, bupropion is the only medication studied in this population. For adult ADHD alone, clinical trials have showed varying efficacy with bupropion,27,28 atomoxetine,29 venlafaxine,30,31 desipramine,32 methylphenidate,33 mixed amphetamine salts,34 and guanfacine.35 Whether these treatments can be generalized as safe and efficacious for comorbid adult ADHD/BD is unclear. Nonetheless, we suggest using bupropion first, followed by atomoxetine or guanfacine before you consider amphetamine stimulants (Algorithm 3).
Algorithm 1
Hierarchy for diagnosis and treatment of adult ADHD/BD
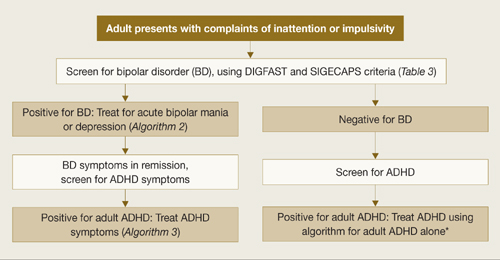
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
*Adler LA, Chua HC. Management of ADHD in adults. J Clin Psychiatry 2002;63(suppl 12):29-35.
Algorithm 2
Treating acute episodes of bipolar disorder
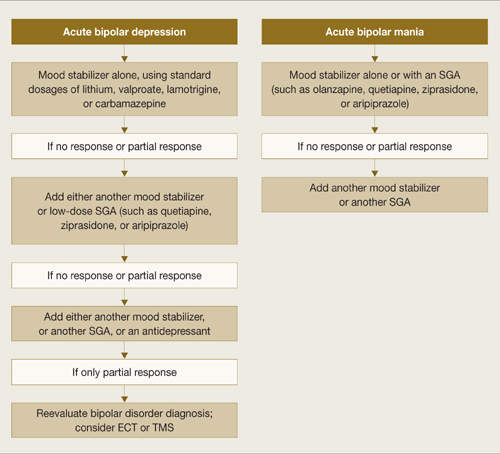
ECT: electroconvulsive therapy; SGA: second-generation antipsychotic; TMS: transcranial magnetic stimulation
Algorithm 3
Suggested approach to adult ADHD with comorbid BD*
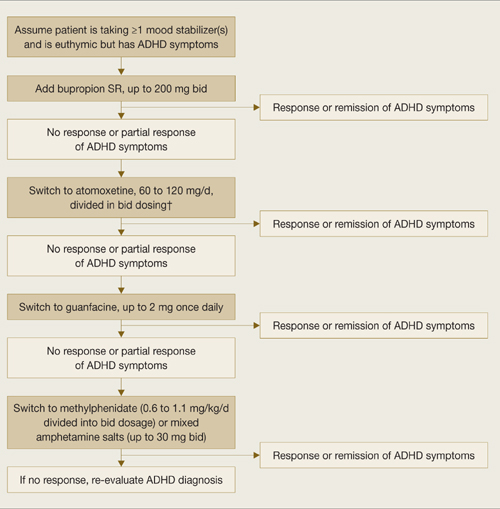
* Based on data extrapolated from samples of patients with ADHD alone because of very limited data in ADHD/BD samples.
† We recommend against combining antidepressants and stimulants because of additive risks of mania in BD. Discontinue stimulant or antidepressant if manic symptoms appear or rapid cycling emerges.
Reducing mania risk
Antidepressants and stimulants may help adults with ADHD alone, but risks of mania and rapid cycling limit their use in adults with ADHD/BD.
Stimulants and mania. One study found a 17% manic switch rate when methylphenidate (≤10 mg bid) was given to 14 bipolar depressed adults (10 BD type I, 2 BD type II, and 2 with secondary mania) taking mood stabilizers.36 A chart review of 82 bipolar children not taking mood stabilizers found an 18% switch rate with methylphenidate or amphetamine.37 Another chart review of 80 children with BD type I found that past amphetamine treatment (but not history of ADHD diagnosis or antidepressant treatment) was associated with more severe bipolar illness.38
No studies have examined predictors of amphetamine-induced mania. In our clinical experience, triggers are similar to those that can cause antidepressant-induced mania, such as:
- recent manic episodes
- current rapid cycling
- past antidepressant-induced mania.
Antidepressants and mania. When 64 patients with acute bipolar depression received both antidepressants and mood stabilizers in a randomized, double-blind trial, switch rates into mania or hypomania were 10% for bupropion, 9% for sertraline, and 29% for venlafaxine.39 In a meta analysis of clinical trials using selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs), the manic switch rate was threefold higher with TCAs than SSRIs.40 Antidepressant use in bipolar patients was associated with rapid cycling in the only randomized study of this topic.41
Insufficient data exist to clarify whether mania induction with antidepressants is dose-dependent.42 Factors associated with antidepressant-induced mania include:
- previous antidepressant-induced mania
- family history of BD
- exposure to multiple antidepressant trials42
- history of substance abuse and/or dependence.43
Related resources
- Bipolar disorder information and resources. www.psycheducation.org.
- ADHD Information and resources. www.adhdnews.com.
- Phelps J. Why am I still depressed? Recognizing and managing the ups and downs of bipolar II and soft bipolar disorder. New York: McGraw-Hill; 2006.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Wingo reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Ghaemi receives research grants from GlaxoSmithKline and Pfizer and is a speaker for GlaxoSmithKline, AstraZeneca, Pfizer, and Abbott Laboratories. Neither he nor his family hold equity positions in pharmaceutical companies.
1. Ghaemi SN, Stoll AL, Pope HG, Jr, et al. Lack of insight in bipolar disorder. The acute manic episode. J Nerv Ment Dis 1995;183(7):464-7.
2. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
3. Sadock BJ, Sadock VA, eds. Synopsis of psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.
4. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
5. Tamam L, Tuglu C, Karatas G, et al. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
6. Wilens TE, Biederman J, Wozniak J, et al. Can adults with attention-deficit/hyperactivity disorder be distinguished from those with comorbid bipolar disorder? Findings from a sample of clinically referred adults. Biol Psychiatry 2003;54(1):1-8.
7. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry 2005;162(9):1621-7.
8. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006;163(10):1720-9.
9. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999;52(1-3):135-44.
10. Keitner GI, Solomon DA, Ryan CE, et al. Prodromal and residual symptoms in bipolar I disorder. Compr Psychiatry 1996;37(5):362-7.
11. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv 2001;52(1):51-5.
12. Kent L, Craddock N. Is there a relationship between attention deficit hyperactivity disorder and bipolar disorder? J Affect Disord 2003;73(3):211-21.
13. Surtees PG, Kendell RE. The hierarchy model of psychiatric symptomatology: an investigation based on present state examination ratings. Br J Psychiatry 1979;135:438-43.
14. Benazzi F. Symptoms of depression as possible markers of bipolar II disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):471-7.
15. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
16. Murphy KR, Adler LA. Assessing attention-deficit hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry 2004;65(suppl 3):12-17.
17. Adler LA. Diagnosing adult attention deficit hyperactivity disorder. Primary Psychiatry 2006;13(suppl 3):9-10.
18. Montano B. Diagnosis and treatment of ADHD in adults in primary care. J Clin Psychiatry 2004;65(suppl 3):18-21.
19. Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS). North Tonawanda, NY: Multi-Health Systems; 1999.
20. Brown TE. Brown Attention Deficit Disorder Scales. San Antonio, TX: The Psychological Corporation; 1996.
21. Adler LA, Kessler RC, Spencer T. Adult ADHD Self-report Scale v1.1 (ASRS-v1.1) Symptom Checklist. World Health Organization. Available at: http://www.med.nyu.edu/psych/assets/adhdscreen18.pdf. Accessed May 7, 2007.
22. Wilens TE, Prince JB, Spencer T, et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry 2003;54(1):9-16.
23. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
24. Daviss WB, Bentivoglio P, Racusin R, et al. Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry 2001;40(3):307-14.
25. Scherk H, Pajonk FG, Leucht SL. Second-generation antipsychotic agents in the treatment of acute mania. Arch Gen Psychiatry 2007;64:442-55.
26. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007:356:(17):1711-22.
27. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention deficit hyperactivity disorder: a randomized, placebo controlled study. Biol Psychiatry 2005;57:793-801.
28. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
29. Michelson D, Adler LA, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo controlled studies. Biol Psychiatry 2003;53:112-20.
30. Adler LA, Resnick S, Kunz M, Devinsky O. Open-label trial of venlafaxine in adults with attention deficit disorder. Psychopharmacol Bull 1995;31(4):785-8.
31. Hedges D, Reimherr FW, Rogers A, et al. An open trial of venlafaxine in adult patients with attention deficit hyperactivity disorder. Psychopharmacol Bull 1995;31(4):779-83.
32. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153(9):1147-53.
33. Faraone SV, Spencer T, Aleardi M, et al. Meta analysis of the efficacy of methylphenidate for treating adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2004;24(1):24-8.
34. Spencer T, Biederman J, Wilens TE, et al. Efficacy of a mixed amphetamine salts compound in adults with attention deficit hyperactivity disorder. Arch Gen Psychiatry 2001;58:775-82.
35. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2000;21(2):223-8.
36. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
37. Faedda GL, Baldessarini RJ, Glovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord 2004;82(1):149-58.
38. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70(3):323-7.
39. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006;189:124-31.
40. Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994;164(4):549-50.
41. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145(2):179-84.
42. Goldberg JF. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract. 2003;9(3):181-94.
43. Goldberg JF, Whiteside JE. The association between substance abuse and antidepressant-induced mania in bipolar disorder: a preliminary study. J Clin Psychiatry 2002;63(9):791-5.
Overlapping symptoms may obscure comorbid bipolar illness
An adult with function-impairing inattention could have attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), or both. Comorbid ADHD and BD often is unrecognized, however, because patients are more likely to report ADHD-related symptoms than manic symptoms.1
To help you recognize comorbid ADHD/BD—and protect adults who might switch into mania if given stimulants or antidepressants—this article describes a hierarchy to diagnose and treat this comorbidity. Based on the evidence and our experience, we:
- discuss how to differentiate between these disorders with overlapping symptoms
- provide tools and suggestions to screen for BD and adult ADHD
- offer 3 algorithms to guide your diagnosis and choice of medications.
Clinical challenges
Prevalence is unclear. Adult ADHD—with an estimated prevalence of 4.4%2—is more common than BD. Lifetime prevalences of BD types I and II are 1.6% and 0.5%, respectively.3 Studies of ADHD/BD comorbidity suggest wide-ranging prevalence rates:
Underdiagnosis. Adult ADHD/BD is a more severe illness than ADHD or BD alone and is highly comorbid with agoraphobia, social phobia, posttraumatic stress disorder, and alcohol or drug addiction. Adults with ADHD/BD have more frequent affective episodes, suicide attempts, violence, and legal problems.4 Diagnosing this comorbidity remains a challenge, however, because:
- identifying which symptoms are caused by which disorder can be difficult
- BD tends to be underdiagnosed9
- patients often misidentify, underreport, or deny manic symptoms1,10,11
- if a patient presents with active bipolar symptoms, DSM-IV-TR criteria require that ADHD not be diagnosed until mood symptoms are resolved.
Overlapping symptoms. ADHD and bipolar mania share some DSM-IV-TR diagnostic criteria, including talkativeness, distractibility, increased activity or physical restlessness, and loss of social inhibitions (Table 1).12 Overlapping symptoms also are notable within ADHD diagnostic criteria (Table 2). In the inattention category, for example, “easily distracted by extraneous stimuli,” “difficulty sustaining attention in tasks,” and “fails to give close attention to details” are considered 3 separate symptoms. In the hyperactivity category, “often leaves seat,” “often runs about or climbs excessively,” and “often on the go, or often acts as if driven by a motor” are 3 separate symptoms.
Given ADHD’s relatively loose diagnostic criteria and high comorbidity in adults with mood disorders, the question of whether adult ADHD/BD represents comorbidity or diagnostic overlap remains unresolved. For the clinician, the disorders’ nonoverlapping features (Table 1) can assist with the differential diagnosis. For example:
- ADHD symptoms tend to be chronic and BD symptoms episodic.
- ADHD patients may have high energy but lack increased productivity seen in BD patients.
- ADHD patients do not need less sleep or have inflated self-esteem like symptomatic BD patients.
- Psychotic symptoms such as hallucinations or delusions might be present in severe BD but are absent in ADHD.
Table 1
Overlap between DSM-IV-TR diagnostic criteria for ADHD and bipolar mania
| Overlapping symptoms | |
|---|---|
| ADHD | Bipolar mania |
| Talks excessively | More talkative than usual |
| Easily distracted/jumps from one activity to the next | Distractibility or constant changes in activity or plans |
| Fidgets Difficulty remaining seated Runs or climbs about inappropriately Difficulty playing quietly On the go as if driven by a motor | Increased activity or physical restlessness |
| Interrupts or butts in uninvited Blurts out answers | Loss of normal social inhibitions |
| Nonoverlapping symptoms | |
| ADHD Forgetful in daily activities Difficulty awaiting turn Difficulty organizing self Loses things Avoids sustained mental effort Does not seem to listen Difficulty following through on instructions/fails to finish work Difficulty sustaining attention Fails to give close attention to details/makes careless mistakes | |
| Bipolar mania Inflated self-esteem/grandiosity Increase in goal-directed activity Flight of ideas Decreased need for sleep Excessive involvement in pleasurable activities with disregard for potential adverse consequences Marked sexual energy or sexual indiscretions | |
| ADHD: attention-deficit/hyperactivity disorder | |
| Source: Adapted and reprinted with permission from reference 12 | |
Table 2
DSM-IV-TR diagnostic criteria for attention-deficit/ hyperactivity disorder
| Inattention |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Hyperactivity/impulsivity |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Diagnosis requires evidence of inattention or hyperactivity/impulsivity or both |
| Some hyperactive/impulsive or inattentive symptoms that caused impairment were present before age 7 |
| Some impairment from symptoms is present in ≥2 settings (such as at school, work, or home) |
| Symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder (mood disorder, anxiety disorder, dissociative disorder, or a personality disorder) |
| Source: DSM-IV-TR |
Mood symptoms first
A diagnostic hierarchy is implicit in DSM-IV-TR; anxiety disorders are not diagnosed during an active major depressive or manic episode, and schizophrenia is not diagnosed on the basis of psychotic symptoms during an active major depressive or manic episode. Mood disorders sit atop this implied diagnostic hierarchy and must be ruled out before psychotic or anxiety disorders are diagnosed. Similarly, most personality disorders are not diagnosed during an active mood or psychotic episode.
Diagnosing adult ADHD when a patient is actively depressed or manic is inconsistent with this hierarchy and conflicts with extensive nosologic literature.13 We suggest that ADHD—a cognitive-behavioral problem—not be diagnosed solely on symptoms observed when a patient is experiencing a mood episode or psychotic illness.
Bipolar disorder. Two useful mnemonics (Table 3) assist in screening for DSM-IV-TR symptoms of BD type I:
- Pure mania consists of euphoric mood and ≥3 of 7 DIGFAST criteria, or irritable mood and ≥4 of 7 DIGFAST criteria
- Mixed mania consists of depressed mood with ≥4 of 7 DIGFAST criteria and ≥4 of 8 SIGECAPS criteria.
To be diagnostic, these symptoms must cause substantial social or occupational dysfunction and be present at least 1 week. Diagnose BD type I if a patient has experienced a single pure or mixed manic episode at any time, unless the episode had a medical cause such as hyperthyroidism or antidepressant use. Because patients with mixed episodes experience depressed mood, assess any patient with clinical depression for manic symptoms. Otherwise, a patient with a mixed episode could be misdiagnosed as having unipolar depression instead of BD type I.14
BD type II also has been observed in patients with comorbid adult ADHD/BD.4,6 The main difference between BD types I and II is that manic symptoms in type II are not severe enough to cause functional impairment or psychotic symptoms.15
Adult ADHD. The clinical interview seeking evidence of inattention and hyperactivity/impulsivity remains the basis of adult ADHD diagnosis (Table 2). Key areas are:
- the patient’s past and current functional impairment
- whether substantial impairment occurs in at least 2 areas of life (such as school, work, or home).
Take medical, educational, social, psychological, and vocational histories, and rule out other conditions before concluding that adult ADHD is the appropriate diagnosis.16 In adult ADHD, inattentive symptoms become far more prominent, about twice as common as hyperactive symptoms.17 Inattentive symptoms may manifest as neglect, poor time management, motivational deficits, or poor concentration that results in forgetfulness, distractibility, item misplacement, or excessive mistakes in paperwork.18 When impulsive symptoms persist in adults, they may manifest as automobile accidents or low tolerance for frustration, which may lead to frequent job changes and unstable, interrupted interpersonal relationships.18
Neuropsychological testing is not required to make an adult ADHD diagnosis but can help establish the breadth of symptoms or comorbidity.17 Rating scales can screen, gather data (including presence and severity of symptoms), and measure treatment response.16 Commonly used rating scales include:
- Conners’ Adult ADHD Rating Scales19
- Brown Attention Deficit Disorder Rating Scale for Adults20
- Adult ADHD Self-Report Scale.21
When using rating scales, remember that adult psychopathology can distort perceptions, and some self-report scales have questionable reliability.16
Table 3
Mnemonics for diagnostic symptoms of pure and mixed bipolar mania
| DIGFAST* for bipolar mania symptoms | SIGECAPS† bipolar depression symptoms |
|---|---|
| Distractibility Insomnia Grandiosity Flight of ideas Activities Speech Thoughtlessness | Sleep Interest Guilt Energy Concentration Appetite Psychomotor Suicide |
| Pure mania: Euphoric mood with ≥3 DIGFAST criteria or irritable mood with ≥4 DIGFAST criteria. | |
| Mixed mania: Depressed mood with ≥4 DIGFAST criteria and ≥4 SIGECAPS criteria. | |
| * Developed by William Falk, MD | |
| †Developed by Carey Gross, MD | |
| Source: Adapted from Ghaemi SN. Mood disorders. New York: Lippincott, Williams, & Wilkins; 2003 | |
Treatment recommendations
Limited data. We found only 1 study on adult ADHD/BD treatment. In this open trial,22 36 adults with comorbid ADHD and BD received bupropion SR, up to 200 mg bid, for ADHD symptoms while maintained on mood stabilizers, antipsychotics, or both. Improvement was defined as ≥30% reduction in ADHD Symptom Checklist Scale scores, without concurrent mania. After 6 weeks, 82% of patients had improved; 1 dropped out at week 2 because of hypomanic activation. Methodologic limitations included trial design (non-randomized, nonblinded, short duration) and patient selection (90% of subjects had BD type II).
In the absence of adequate data on adult ADHD/BD, studies in children suggest:
- stimulants may not be effective for ADHD symptoms in patients with active manic or depressive symptoms
- mood stabilization is a prerequisite for successful pharmacologic treatment of ADHD in patients with both ADHD and manic or depressive symptoms.23,24
Follow the hierarchy. First treat acute mood symptoms, then reevaluate and possibly treat ADHD symptoms if they persist during euthymia (Algorithm 1). When a patient meets criteria for adult ADHD/BD, first stabilize bipolar manic or depressive symptoms (Algorithm 2). For acute mania, treat with standard mood stabilizers (lithium, valproate, lamotrigine, or carbamazepine) with or without a second-generation antipsychotic.25 Starting stimulants for ADHD when patients have active mood symptoms is sub-optimal and potentially harmful because of the risk of inducing mania. For acute bipolar depression, adjunctive antidepressant treatment has been found to be no more effective than a mood stabilizer alone.26
After bipolar symptoms respond or remit, reassess for adult ADHD. If ADHD symptoms persist during euthymia, additional treatment may be indicated.
Very little evidence exists on treating adult ADHD/BD; as mentioned, bupropion is the only medication studied in this population. For adult ADHD alone, clinical trials have showed varying efficacy with bupropion,27,28 atomoxetine,29 venlafaxine,30,31 desipramine,32 methylphenidate,33 mixed amphetamine salts,34 and guanfacine.35 Whether these treatments can be generalized as safe and efficacious for comorbid adult ADHD/BD is unclear. Nonetheless, we suggest using bupropion first, followed by atomoxetine or guanfacine before you consider amphetamine stimulants (Algorithm 3).
Algorithm 1
Hierarchy for diagnosis and treatment of adult ADHD/BD

ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
*Adler LA, Chua HC. Management of ADHD in adults. J Clin Psychiatry 2002;63(suppl 12):29-35.
Algorithm 2
Treating acute episodes of bipolar disorder

ECT: electroconvulsive therapy; SGA: second-generation antipsychotic; TMS: transcranial magnetic stimulation
Algorithm 3
Suggested approach to adult ADHD with comorbid BD*

* Based on data extrapolated from samples of patients with ADHD alone because of very limited data in ADHD/BD samples.
† We recommend against combining antidepressants and stimulants because of additive risks of mania in BD. Discontinue stimulant or antidepressant if manic symptoms appear or rapid cycling emerges.
Reducing mania risk
Antidepressants and stimulants may help adults with ADHD alone, but risks of mania and rapid cycling limit their use in adults with ADHD/BD.
Stimulants and mania. One study found a 17% manic switch rate when methylphenidate (≤10 mg bid) was given to 14 bipolar depressed adults (10 BD type I, 2 BD type II, and 2 with secondary mania) taking mood stabilizers.36 A chart review of 82 bipolar children not taking mood stabilizers found an 18% switch rate with methylphenidate or amphetamine.37 Another chart review of 80 children with BD type I found that past amphetamine treatment (but not history of ADHD diagnosis or antidepressant treatment) was associated with more severe bipolar illness.38
No studies have examined predictors of amphetamine-induced mania. In our clinical experience, triggers are similar to those that can cause antidepressant-induced mania, such as:
- recent manic episodes
- current rapid cycling
- past antidepressant-induced mania.
Antidepressants and mania. When 64 patients with acute bipolar depression received both antidepressants and mood stabilizers in a randomized, double-blind trial, switch rates into mania or hypomania were 10% for bupropion, 9% for sertraline, and 29% for venlafaxine.39 In a meta analysis of clinical trials using selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs), the manic switch rate was threefold higher with TCAs than SSRIs.40 Antidepressant use in bipolar patients was associated with rapid cycling in the only randomized study of this topic.41
Insufficient data exist to clarify whether mania induction with antidepressants is dose-dependent.42 Factors associated with antidepressant-induced mania include:
- previous antidepressant-induced mania
- family history of BD
- exposure to multiple antidepressant trials42
- history of substance abuse and/or dependence.43
Related resources
- Bipolar disorder information and resources. www.psycheducation.org.
- ADHD Information and resources. www.adhdnews.com.
- Phelps J. Why am I still depressed? Recognizing and managing the ups and downs of bipolar II and soft bipolar disorder. New York: McGraw-Hill; 2006.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Wingo reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Ghaemi receives research grants from GlaxoSmithKline and Pfizer and is a speaker for GlaxoSmithKline, AstraZeneca, Pfizer, and Abbott Laboratories. Neither he nor his family hold equity positions in pharmaceutical companies.
Overlapping symptoms may obscure comorbid bipolar illness
An adult with function-impairing inattention could have attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), or both. Comorbid ADHD and BD often is unrecognized, however, because patients are more likely to report ADHD-related symptoms than manic symptoms.1
To help you recognize comorbid ADHD/BD—and protect adults who might switch into mania if given stimulants or antidepressants—this article describes a hierarchy to diagnose and treat this comorbidity. Based on the evidence and our experience, we:
- discuss how to differentiate between these disorders with overlapping symptoms
- provide tools and suggestions to screen for BD and adult ADHD
- offer 3 algorithms to guide your diagnosis and choice of medications.
Clinical challenges
Prevalence is unclear. Adult ADHD—with an estimated prevalence of 4.4%2—is more common than BD. Lifetime prevalences of BD types I and II are 1.6% and 0.5%, respectively.3 Studies of ADHD/BD comorbidity suggest wide-ranging prevalence rates:
Underdiagnosis. Adult ADHD/BD is a more severe illness than ADHD or BD alone and is highly comorbid with agoraphobia, social phobia, posttraumatic stress disorder, and alcohol or drug addiction. Adults with ADHD/BD have more frequent affective episodes, suicide attempts, violence, and legal problems.4 Diagnosing this comorbidity remains a challenge, however, because:
- identifying which symptoms are caused by which disorder can be difficult
- BD tends to be underdiagnosed9
- patients often misidentify, underreport, or deny manic symptoms1,10,11
- if a patient presents with active bipolar symptoms, DSM-IV-TR criteria require that ADHD not be diagnosed until mood symptoms are resolved.
Overlapping symptoms. ADHD and bipolar mania share some DSM-IV-TR diagnostic criteria, including talkativeness, distractibility, increased activity or physical restlessness, and loss of social inhibitions (Table 1).12 Overlapping symptoms also are notable within ADHD diagnostic criteria (Table 2). In the inattention category, for example, “easily distracted by extraneous stimuli,” “difficulty sustaining attention in tasks,” and “fails to give close attention to details” are considered 3 separate symptoms. In the hyperactivity category, “often leaves seat,” “often runs about or climbs excessively,” and “often on the go, or often acts as if driven by a motor” are 3 separate symptoms.
Given ADHD’s relatively loose diagnostic criteria and high comorbidity in adults with mood disorders, the question of whether adult ADHD/BD represents comorbidity or diagnostic overlap remains unresolved. For the clinician, the disorders’ nonoverlapping features (Table 1) can assist with the differential diagnosis. For example:
- ADHD symptoms tend to be chronic and BD symptoms episodic.
- ADHD patients may have high energy but lack increased productivity seen in BD patients.
- ADHD patients do not need less sleep or have inflated self-esteem like symptomatic BD patients.
- Psychotic symptoms such as hallucinations or delusions might be present in severe BD but are absent in ADHD.
Table 1
Overlap between DSM-IV-TR diagnostic criteria for ADHD and bipolar mania
| Overlapping symptoms | |
|---|---|
| ADHD | Bipolar mania |
| Talks excessively | More talkative than usual |
| Easily distracted/jumps from one activity to the next | Distractibility or constant changes in activity or plans |
| Fidgets Difficulty remaining seated Runs or climbs about inappropriately Difficulty playing quietly On the go as if driven by a motor | Increased activity or physical restlessness |
| Interrupts or butts in uninvited Blurts out answers | Loss of normal social inhibitions |
| Nonoverlapping symptoms | |
| ADHD Forgetful in daily activities Difficulty awaiting turn Difficulty organizing self Loses things Avoids sustained mental effort Does not seem to listen Difficulty following through on instructions/fails to finish work Difficulty sustaining attention Fails to give close attention to details/makes careless mistakes | |
| Bipolar mania Inflated self-esteem/grandiosity Increase in goal-directed activity Flight of ideas Decreased need for sleep Excessive involvement in pleasurable activities with disregard for potential adverse consequences Marked sexual energy or sexual indiscretions | |
| ADHD: attention-deficit/hyperactivity disorder | |
| Source: Adapted and reprinted with permission from reference 12 | |
Table 2
DSM-IV-TR diagnostic criteria for attention-deficit/ hyperactivity disorder
| Inattention |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Hyperactivity/impulsivity |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Diagnosis requires evidence of inattention or hyperactivity/impulsivity or both |
| Some hyperactive/impulsive or inattentive symptoms that caused impairment were present before age 7 |
| Some impairment from symptoms is present in ≥2 settings (such as at school, work, or home) |
| Symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder (mood disorder, anxiety disorder, dissociative disorder, or a personality disorder) |
| Source: DSM-IV-TR |
Mood symptoms first
A diagnostic hierarchy is implicit in DSM-IV-TR; anxiety disorders are not diagnosed during an active major depressive or manic episode, and schizophrenia is not diagnosed on the basis of psychotic symptoms during an active major depressive or manic episode. Mood disorders sit atop this implied diagnostic hierarchy and must be ruled out before psychotic or anxiety disorders are diagnosed. Similarly, most personality disorders are not diagnosed during an active mood or psychotic episode.
Diagnosing adult ADHD when a patient is actively depressed or manic is inconsistent with this hierarchy and conflicts with extensive nosologic literature.13 We suggest that ADHD—a cognitive-behavioral problem—not be diagnosed solely on symptoms observed when a patient is experiencing a mood episode or psychotic illness.
Bipolar disorder. Two useful mnemonics (Table 3) assist in screening for DSM-IV-TR symptoms of BD type I:
- Pure mania consists of euphoric mood and ≥3 of 7 DIGFAST criteria, or irritable mood and ≥4 of 7 DIGFAST criteria
- Mixed mania consists of depressed mood with ≥4 of 7 DIGFAST criteria and ≥4 of 8 SIGECAPS criteria.
To be diagnostic, these symptoms must cause substantial social or occupational dysfunction and be present at least 1 week. Diagnose BD type I if a patient has experienced a single pure or mixed manic episode at any time, unless the episode had a medical cause such as hyperthyroidism or antidepressant use. Because patients with mixed episodes experience depressed mood, assess any patient with clinical depression for manic symptoms. Otherwise, a patient with a mixed episode could be misdiagnosed as having unipolar depression instead of BD type I.14
BD type II also has been observed in patients with comorbid adult ADHD/BD.4,6 The main difference between BD types I and II is that manic symptoms in type II are not severe enough to cause functional impairment or psychotic symptoms.15
Adult ADHD. The clinical interview seeking evidence of inattention and hyperactivity/impulsivity remains the basis of adult ADHD diagnosis (Table 2). Key areas are:
- the patient’s past and current functional impairment
- whether substantial impairment occurs in at least 2 areas of life (such as school, work, or home).
Take medical, educational, social, psychological, and vocational histories, and rule out other conditions before concluding that adult ADHD is the appropriate diagnosis.16 In adult ADHD, inattentive symptoms become far more prominent, about twice as common as hyperactive symptoms.17 Inattentive symptoms may manifest as neglect, poor time management, motivational deficits, or poor concentration that results in forgetfulness, distractibility, item misplacement, or excessive mistakes in paperwork.18 When impulsive symptoms persist in adults, they may manifest as automobile accidents or low tolerance for frustration, which may lead to frequent job changes and unstable, interrupted interpersonal relationships.18
Neuropsychological testing is not required to make an adult ADHD diagnosis but can help establish the breadth of symptoms or comorbidity.17 Rating scales can screen, gather data (including presence and severity of symptoms), and measure treatment response.16 Commonly used rating scales include:
- Conners’ Adult ADHD Rating Scales19
- Brown Attention Deficit Disorder Rating Scale for Adults20
- Adult ADHD Self-Report Scale.21
When using rating scales, remember that adult psychopathology can distort perceptions, and some self-report scales have questionable reliability.16
Table 3
Mnemonics for diagnostic symptoms of pure and mixed bipolar mania
| DIGFAST* for bipolar mania symptoms | SIGECAPS† bipolar depression symptoms |
|---|---|
| Distractibility Insomnia Grandiosity Flight of ideas Activities Speech Thoughtlessness | Sleep Interest Guilt Energy Concentration Appetite Psychomotor Suicide |
| Pure mania: Euphoric mood with ≥3 DIGFAST criteria or irritable mood with ≥4 DIGFAST criteria. | |
| Mixed mania: Depressed mood with ≥4 DIGFAST criteria and ≥4 SIGECAPS criteria. | |
| * Developed by William Falk, MD | |
| †Developed by Carey Gross, MD | |
| Source: Adapted from Ghaemi SN. Mood disorders. New York: Lippincott, Williams, & Wilkins; 2003 | |
Treatment recommendations
Limited data. We found only 1 study on adult ADHD/BD treatment. In this open trial,22 36 adults with comorbid ADHD and BD received bupropion SR, up to 200 mg bid, for ADHD symptoms while maintained on mood stabilizers, antipsychotics, or both. Improvement was defined as ≥30% reduction in ADHD Symptom Checklist Scale scores, without concurrent mania. After 6 weeks, 82% of patients had improved; 1 dropped out at week 2 because of hypomanic activation. Methodologic limitations included trial design (non-randomized, nonblinded, short duration) and patient selection (90% of subjects had BD type II).
In the absence of adequate data on adult ADHD/BD, studies in children suggest:
- stimulants may not be effective for ADHD symptoms in patients with active manic or depressive symptoms
- mood stabilization is a prerequisite for successful pharmacologic treatment of ADHD in patients with both ADHD and manic or depressive symptoms.23,24
Follow the hierarchy. First treat acute mood symptoms, then reevaluate and possibly treat ADHD symptoms if they persist during euthymia (Algorithm 1). When a patient meets criteria for adult ADHD/BD, first stabilize bipolar manic or depressive symptoms (Algorithm 2). For acute mania, treat with standard mood stabilizers (lithium, valproate, lamotrigine, or carbamazepine) with or without a second-generation antipsychotic.25 Starting stimulants for ADHD when patients have active mood symptoms is sub-optimal and potentially harmful because of the risk of inducing mania. For acute bipolar depression, adjunctive antidepressant treatment has been found to be no more effective than a mood stabilizer alone.26
After bipolar symptoms respond or remit, reassess for adult ADHD. If ADHD symptoms persist during euthymia, additional treatment may be indicated.
Very little evidence exists on treating adult ADHD/BD; as mentioned, bupropion is the only medication studied in this population. For adult ADHD alone, clinical trials have showed varying efficacy with bupropion,27,28 atomoxetine,29 venlafaxine,30,31 desipramine,32 methylphenidate,33 mixed amphetamine salts,34 and guanfacine.35 Whether these treatments can be generalized as safe and efficacious for comorbid adult ADHD/BD is unclear. Nonetheless, we suggest using bupropion first, followed by atomoxetine or guanfacine before you consider amphetamine stimulants (Algorithm 3).
Algorithm 1
Hierarchy for diagnosis and treatment of adult ADHD/BD

ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
*Adler LA, Chua HC. Management of ADHD in adults. J Clin Psychiatry 2002;63(suppl 12):29-35.
Algorithm 2
Treating acute episodes of bipolar disorder

ECT: electroconvulsive therapy; SGA: second-generation antipsychotic; TMS: transcranial magnetic stimulation
Algorithm 3
Suggested approach to adult ADHD with comorbid BD*

* Based on data extrapolated from samples of patients with ADHD alone because of very limited data in ADHD/BD samples.
† We recommend against combining antidepressants and stimulants because of additive risks of mania in BD. Discontinue stimulant or antidepressant if manic symptoms appear or rapid cycling emerges.
Reducing mania risk
Antidepressants and stimulants may help adults with ADHD alone, but risks of mania and rapid cycling limit their use in adults with ADHD/BD.
Stimulants and mania. One study found a 17% manic switch rate when methylphenidate (≤10 mg bid) was given to 14 bipolar depressed adults (10 BD type I, 2 BD type II, and 2 with secondary mania) taking mood stabilizers.36 A chart review of 82 bipolar children not taking mood stabilizers found an 18% switch rate with methylphenidate or amphetamine.37 Another chart review of 80 children with BD type I found that past amphetamine treatment (but not history of ADHD diagnosis or antidepressant treatment) was associated with more severe bipolar illness.38
No studies have examined predictors of amphetamine-induced mania. In our clinical experience, triggers are similar to those that can cause antidepressant-induced mania, such as:
- recent manic episodes
- current rapid cycling
- past antidepressant-induced mania.
Antidepressants and mania. When 64 patients with acute bipolar depression received both antidepressants and mood stabilizers in a randomized, double-blind trial, switch rates into mania or hypomania were 10% for bupropion, 9% for sertraline, and 29% for venlafaxine.39 In a meta analysis of clinical trials using selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs), the manic switch rate was threefold higher with TCAs than SSRIs.40 Antidepressant use in bipolar patients was associated with rapid cycling in the only randomized study of this topic.41
Insufficient data exist to clarify whether mania induction with antidepressants is dose-dependent.42 Factors associated with antidepressant-induced mania include:
- previous antidepressant-induced mania
- family history of BD
- exposure to multiple antidepressant trials42
- history of substance abuse and/or dependence.43
Related resources
- Bipolar disorder information and resources. www.psycheducation.org.
- ADHD Information and resources. www.adhdnews.com.
- Phelps J. Why am I still depressed? Recognizing and managing the ups and downs of bipolar II and soft bipolar disorder. New York: McGraw-Hill; 2006.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Wingo reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Ghaemi receives research grants from GlaxoSmithKline and Pfizer and is a speaker for GlaxoSmithKline, AstraZeneca, Pfizer, and Abbott Laboratories. Neither he nor his family hold equity positions in pharmaceutical companies.
1. Ghaemi SN, Stoll AL, Pope HG, Jr, et al. Lack of insight in bipolar disorder. The acute manic episode. J Nerv Ment Dis 1995;183(7):464-7.
2. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
3. Sadock BJ, Sadock VA, eds. Synopsis of psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.
4. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
5. Tamam L, Tuglu C, Karatas G, et al. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
6. Wilens TE, Biederman J, Wozniak J, et al. Can adults with attention-deficit/hyperactivity disorder be distinguished from those with comorbid bipolar disorder? Findings from a sample of clinically referred adults. Biol Psychiatry 2003;54(1):1-8.
7. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry 2005;162(9):1621-7.
8. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006;163(10):1720-9.
9. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999;52(1-3):135-44.
10. Keitner GI, Solomon DA, Ryan CE, et al. Prodromal and residual symptoms in bipolar I disorder. Compr Psychiatry 1996;37(5):362-7.
11. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv 2001;52(1):51-5.
12. Kent L, Craddock N. Is there a relationship between attention deficit hyperactivity disorder and bipolar disorder? J Affect Disord 2003;73(3):211-21.
13. Surtees PG, Kendell RE. The hierarchy model of psychiatric symptomatology: an investigation based on present state examination ratings. Br J Psychiatry 1979;135:438-43.
14. Benazzi F. Symptoms of depression as possible markers of bipolar II disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):471-7.
15. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
16. Murphy KR, Adler LA. Assessing attention-deficit hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry 2004;65(suppl 3):12-17.
17. Adler LA. Diagnosing adult attention deficit hyperactivity disorder. Primary Psychiatry 2006;13(suppl 3):9-10.
18. Montano B. Diagnosis and treatment of ADHD in adults in primary care. J Clin Psychiatry 2004;65(suppl 3):18-21.
19. Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS). North Tonawanda, NY: Multi-Health Systems; 1999.
20. Brown TE. Brown Attention Deficit Disorder Scales. San Antonio, TX: The Psychological Corporation; 1996.
21. Adler LA, Kessler RC, Spencer T. Adult ADHD Self-report Scale v1.1 (ASRS-v1.1) Symptom Checklist. World Health Organization. Available at: http://www.med.nyu.edu/psych/assets/adhdscreen18.pdf. Accessed May 7, 2007.
22. Wilens TE, Prince JB, Spencer T, et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry 2003;54(1):9-16.
23. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
24. Daviss WB, Bentivoglio P, Racusin R, et al. Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry 2001;40(3):307-14.
25. Scherk H, Pajonk FG, Leucht SL. Second-generation antipsychotic agents in the treatment of acute mania. Arch Gen Psychiatry 2007;64:442-55.
26. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007:356:(17):1711-22.
27. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention deficit hyperactivity disorder: a randomized, placebo controlled study. Biol Psychiatry 2005;57:793-801.
28. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
29. Michelson D, Adler LA, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo controlled studies. Biol Psychiatry 2003;53:112-20.
30. Adler LA, Resnick S, Kunz M, Devinsky O. Open-label trial of venlafaxine in adults with attention deficit disorder. Psychopharmacol Bull 1995;31(4):785-8.
31. Hedges D, Reimherr FW, Rogers A, et al. An open trial of venlafaxine in adult patients with attention deficit hyperactivity disorder. Psychopharmacol Bull 1995;31(4):779-83.
32. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153(9):1147-53.
33. Faraone SV, Spencer T, Aleardi M, et al. Meta analysis of the efficacy of methylphenidate for treating adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2004;24(1):24-8.
34. Spencer T, Biederman J, Wilens TE, et al. Efficacy of a mixed amphetamine salts compound in adults with attention deficit hyperactivity disorder. Arch Gen Psychiatry 2001;58:775-82.
35. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2000;21(2):223-8.
36. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
37. Faedda GL, Baldessarini RJ, Glovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord 2004;82(1):149-58.
38. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70(3):323-7.
39. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006;189:124-31.
40. Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994;164(4):549-50.
41. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145(2):179-84.
42. Goldberg JF. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract. 2003;9(3):181-94.
43. Goldberg JF, Whiteside JE. The association between substance abuse and antidepressant-induced mania in bipolar disorder: a preliminary study. J Clin Psychiatry 2002;63(9):791-5.
1. Ghaemi SN, Stoll AL, Pope HG, Jr, et al. Lack of insight in bipolar disorder. The acute manic episode. J Nerv Ment Dis 1995;183(7):464-7.
2. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
3. Sadock BJ, Sadock VA, eds. Synopsis of psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.
4. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
5. Tamam L, Tuglu C, Karatas G, et al. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
6. Wilens TE, Biederman J, Wozniak J, et al. Can adults with attention-deficit/hyperactivity disorder be distinguished from those with comorbid bipolar disorder? Findings from a sample of clinically referred adults. Biol Psychiatry 2003;54(1):1-8.
7. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry 2005;162(9):1621-7.
8. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006;163(10):1720-9.
9. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999;52(1-3):135-44.
10. Keitner GI, Solomon DA, Ryan CE, et al. Prodromal and residual symptoms in bipolar I disorder. Compr Psychiatry 1996;37(5):362-7.
11. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv 2001;52(1):51-5.
12. Kent L, Craddock N. Is there a relationship between attention deficit hyperactivity disorder and bipolar disorder? J Affect Disord 2003;73(3):211-21.
13. Surtees PG, Kendell RE. The hierarchy model of psychiatric symptomatology: an investigation based on present state examination ratings. Br J Psychiatry 1979;135:438-43.
14. Benazzi F. Symptoms of depression as possible markers of bipolar II disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):471-7.
15. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
16. Murphy KR, Adler LA. Assessing attention-deficit hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry 2004;65(suppl 3):12-17.
17. Adler LA. Diagnosing adult attention deficit hyperactivity disorder. Primary Psychiatry 2006;13(suppl 3):9-10.
18. Montano B. Diagnosis and treatment of ADHD in adults in primary care. J Clin Psychiatry 2004;65(suppl 3):18-21.
19. Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS). North Tonawanda, NY: Multi-Health Systems; 1999.
20. Brown TE. Brown Attention Deficit Disorder Scales. San Antonio, TX: The Psychological Corporation; 1996.
21. Adler LA, Kessler RC, Spencer T. Adult ADHD Self-report Scale v1.1 (ASRS-v1.1) Symptom Checklist. World Health Organization. Available at: http://www.med.nyu.edu/psych/assets/adhdscreen18.pdf. Accessed May 7, 2007.
22. Wilens TE, Prince JB, Spencer T, et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry 2003;54(1):9-16.
23. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
24. Daviss WB, Bentivoglio P, Racusin R, et al. Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry 2001;40(3):307-14.
25. Scherk H, Pajonk FG, Leucht SL. Second-generation antipsychotic agents in the treatment of acute mania. Arch Gen Psychiatry 2007;64:442-55.
26. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007:356:(17):1711-22.
27. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention deficit hyperactivity disorder: a randomized, placebo controlled study. Biol Psychiatry 2005;57:793-801.
28. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
29. Michelson D, Adler LA, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo controlled studies. Biol Psychiatry 2003;53:112-20.
30. Adler LA, Resnick S, Kunz M, Devinsky O. Open-label trial of venlafaxine in adults with attention deficit disorder. Psychopharmacol Bull 1995;31(4):785-8.
31. Hedges D, Reimherr FW, Rogers A, et al. An open trial of venlafaxine in adult patients with attention deficit hyperactivity disorder. Psychopharmacol Bull 1995;31(4):779-83.
32. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153(9):1147-53.
33. Faraone SV, Spencer T, Aleardi M, et al. Meta analysis of the efficacy of methylphenidate for treating adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2004;24(1):24-8.
34. Spencer T, Biederman J, Wilens TE, et al. Efficacy of a mixed amphetamine salts compound in adults with attention deficit hyperactivity disorder. Arch Gen Psychiatry 2001;58:775-82.
35. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2000;21(2):223-8.
36. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
37. Faedda GL, Baldessarini RJ, Glovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord 2004;82(1):149-58.
38. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70(3):323-7.
39. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006;189:124-31.
40. Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994;164(4):549-50.
41. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145(2):179-84.
42. Goldberg JF. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract. 2003;9(3):181-94.
43. Goldberg JF, Whiteside JE. The association between substance abuse and antidepressant-induced mania in bipolar disorder: a preliminary study. J Clin Psychiatry 2002;63(9):791-5.
Make ADHD treatment as effective as possible
Clinical practice guidelines (CPGs) for the diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) in children and adults represent a consensus on the minimal standards and most reasonable, evidence-based practices.1-3 ADHD is too complex for any set of guidelines to address every situation, but CPGs are an excellent starting point for the conscientious practitioner who wants to make ADHD treatment as effective as possible.
Obtain a copy of the CPG that best fits your patients. Several are available for free at www.pediatrics.org/cgi/content/full/105/5/1158 (children) and www.aacap.org/galleries/PracticeParameters/New_ADHD_Parameter.pdf (children, adolescents, and adults).
Use a validated rating scale to confirm your clinical judgment and monitor treatment progress. Several rating scales for childhood psychiatric conditions are available at www.massgeneral.org/schoolpsychiatry/screeningtools_table.asp.
For adults with suspected ADHD, consider asking those who knew the patient as a child to fill out the Adult ADHD Self-Report Scale—available at www.med.nyu.edu/psych/assets/adhdscreen18.pdf—and corroborate the patient’s memory of childhood symptoms. This step is not always necessary, however, because adults with ADHD have been shown to adequately report childhood impairment.4
Start treatment with stimulant medications unless there are clinical reasons to avoid them, such as active substance abuse, glaucoma, or unstablized bipolar disorder. CPGs note that many FDA contraindications for stimulants have little basis in practice or research. These drugs therefore can be used as first-line treatment of ADHD in patients with comorbid tics, anxiety disorders, seizures, stabilized bipolar disorder, carefully monitored substance abuse, and during pregnancy.
Nineteen medications are FDA-approved for ADHD, and 18 are delivery systems of amphetamine or methylphenidate. In large groups, both chemicals have:
- similar effect size (about 0.95)
- the same side effects
- a response rate of 70% to 75%, which increases to 80% to 90% when both are tried.5
Although studies do not show either molecule to be more effective, individuals usually have a clear preference based on how well the medication manages their target symptoms.
Adjust medication according to the patient’s target symptoms. This process educates the patient about why he or she should take the medication. Remember that the patient with ADHD rarely seeks treatment; the primary motivation usually comes from parents or significant others.
Asking “What bothers you the most about your ADHD, and what do you want to get fixed today?” speaks to how the patient can benefit from therapy and indicates what symptoms he or she should look for. Remember, these patients always have had ADHD; they do not know what is possible with treatment.
This answer also tells you what the patient—as opposed to the family—defines as success and reveals his or her motivation to adhere to the medication. Particularly when treating adolescents, get a list of target symptoms from them and their parents because the lists may be different. Unless both the parents and adolescent are satisfied, one might sabotage therapy.
Fine-tune the medication for optimal relief of target symptoms. Although this seems obvious, the prevailing practice pattern is to increase the dosage until the first sign of improvement and then stop. This practice forfeits many potential benefits of medication. Instead, increase the dosage by the lowest increment available as long as the patient:
- reports clear improvement of his or her target symptoms with each dosage increase
- experiences no side effects other than a mild loss of appetite.
When the patient no longer sees improvement, the lowest dose that resolved the target symptoms will be that individual’s optimal dose.
1. Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158-70.
2. Dulcan M, Dunne JE, Ayres W, et al. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997;(suppl 10):S85-S121.
3. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;(suppl 2):S26-S49.
4. Murphy P, Schachar R. Uses of self-ratings in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry 2000;157:1156-9.
5. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-13.
Dr. Dodson is in private practice in Denver, CO.
Clinical practice guidelines (CPGs) for the diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) in children and adults represent a consensus on the minimal standards and most reasonable, evidence-based practices.1-3 ADHD is too complex for any set of guidelines to address every situation, but CPGs are an excellent starting point for the conscientious practitioner who wants to make ADHD treatment as effective as possible.
Obtain a copy of the CPG that best fits your patients. Several are available for free at www.pediatrics.org/cgi/content/full/105/5/1158 (children) and www.aacap.org/galleries/PracticeParameters/New_ADHD_Parameter.pdf (children, adolescents, and adults).
Use a validated rating scale to confirm your clinical judgment and monitor treatment progress. Several rating scales for childhood psychiatric conditions are available at www.massgeneral.org/schoolpsychiatry/screeningtools_table.asp.
For adults with suspected ADHD, consider asking those who knew the patient as a child to fill out the Adult ADHD Self-Report Scale—available at www.med.nyu.edu/psych/assets/adhdscreen18.pdf—and corroborate the patient’s memory of childhood symptoms. This step is not always necessary, however, because adults with ADHD have been shown to adequately report childhood impairment.4
Start treatment with stimulant medications unless there are clinical reasons to avoid them, such as active substance abuse, glaucoma, or unstablized bipolar disorder. CPGs note that many FDA contraindications for stimulants have little basis in practice or research. These drugs therefore can be used as first-line treatment of ADHD in patients with comorbid tics, anxiety disorders, seizures, stabilized bipolar disorder, carefully monitored substance abuse, and during pregnancy.
Nineteen medications are FDA-approved for ADHD, and 18 are delivery systems of amphetamine or methylphenidate. In large groups, both chemicals have:
- similar effect size (about 0.95)
- the same side effects
- a response rate of 70% to 75%, which increases to 80% to 90% when both are tried.5
Although studies do not show either molecule to be more effective, individuals usually have a clear preference based on how well the medication manages their target symptoms.
Adjust medication according to the patient’s target symptoms. This process educates the patient about why he or she should take the medication. Remember that the patient with ADHD rarely seeks treatment; the primary motivation usually comes from parents or significant others.
Asking “What bothers you the most about your ADHD, and what do you want to get fixed today?” speaks to how the patient can benefit from therapy and indicates what symptoms he or she should look for. Remember, these patients always have had ADHD; they do not know what is possible with treatment.
This answer also tells you what the patient—as opposed to the family—defines as success and reveals his or her motivation to adhere to the medication. Particularly when treating adolescents, get a list of target symptoms from them and their parents because the lists may be different. Unless both the parents and adolescent are satisfied, one might sabotage therapy.
Fine-tune the medication for optimal relief of target symptoms. Although this seems obvious, the prevailing practice pattern is to increase the dosage until the first sign of improvement and then stop. This practice forfeits many potential benefits of medication. Instead, increase the dosage by the lowest increment available as long as the patient:
- reports clear improvement of his or her target symptoms with each dosage increase
- experiences no side effects other than a mild loss of appetite.
When the patient no longer sees improvement, the lowest dose that resolved the target symptoms will be that individual’s optimal dose.
Clinical practice guidelines (CPGs) for the diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) in children and adults represent a consensus on the minimal standards and most reasonable, evidence-based practices.1-3 ADHD is too complex for any set of guidelines to address every situation, but CPGs are an excellent starting point for the conscientious practitioner who wants to make ADHD treatment as effective as possible.
Obtain a copy of the CPG that best fits your patients. Several are available for free at www.pediatrics.org/cgi/content/full/105/5/1158 (children) and www.aacap.org/galleries/PracticeParameters/New_ADHD_Parameter.pdf (children, adolescents, and adults).
Use a validated rating scale to confirm your clinical judgment and monitor treatment progress. Several rating scales for childhood psychiatric conditions are available at www.massgeneral.org/schoolpsychiatry/screeningtools_table.asp.
For adults with suspected ADHD, consider asking those who knew the patient as a child to fill out the Adult ADHD Self-Report Scale—available at www.med.nyu.edu/psych/assets/adhdscreen18.pdf—and corroborate the patient’s memory of childhood symptoms. This step is not always necessary, however, because adults with ADHD have been shown to adequately report childhood impairment.4
Start treatment with stimulant medications unless there are clinical reasons to avoid them, such as active substance abuse, glaucoma, or unstablized bipolar disorder. CPGs note that many FDA contraindications for stimulants have little basis in practice or research. These drugs therefore can be used as first-line treatment of ADHD in patients with comorbid tics, anxiety disorders, seizures, stabilized bipolar disorder, carefully monitored substance abuse, and during pregnancy.
Nineteen medications are FDA-approved for ADHD, and 18 are delivery systems of amphetamine or methylphenidate. In large groups, both chemicals have:
- similar effect size (about 0.95)
- the same side effects
- a response rate of 70% to 75%, which increases to 80% to 90% when both are tried.5
Although studies do not show either molecule to be more effective, individuals usually have a clear preference based on how well the medication manages their target symptoms.
Adjust medication according to the patient’s target symptoms. This process educates the patient about why he or she should take the medication. Remember that the patient with ADHD rarely seeks treatment; the primary motivation usually comes from parents or significant others.
Asking “What bothers you the most about your ADHD, and what do you want to get fixed today?” speaks to how the patient can benefit from therapy and indicates what symptoms he or she should look for. Remember, these patients always have had ADHD; they do not know what is possible with treatment.
This answer also tells you what the patient—as opposed to the family—defines as success and reveals his or her motivation to adhere to the medication. Particularly when treating adolescents, get a list of target symptoms from them and their parents because the lists may be different. Unless both the parents and adolescent are satisfied, one might sabotage therapy.
Fine-tune the medication for optimal relief of target symptoms. Although this seems obvious, the prevailing practice pattern is to increase the dosage until the first sign of improvement and then stop. This practice forfeits many potential benefits of medication. Instead, increase the dosage by the lowest increment available as long as the patient:
- reports clear improvement of his or her target symptoms with each dosage increase
- experiences no side effects other than a mild loss of appetite.
When the patient no longer sees improvement, the lowest dose that resolved the target symptoms will be that individual’s optimal dose.
1. Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158-70.
2. Dulcan M, Dunne JE, Ayres W, et al. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997;(suppl 10):S85-S121.
3. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;(suppl 2):S26-S49.
4. Murphy P, Schachar R. Uses of self-ratings in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry 2000;157:1156-9.
5. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-13.
Dr. Dodson is in private practice in Denver, CO.
1. Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158-70.
2. Dulcan M, Dunne JE, Ayres W, et al. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997;(suppl 10):S85-S121.
3. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;(suppl 2):S26-S49.
4. Murphy P, Schachar R. Uses of self-ratings in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry 2000;157:1156-9.
5. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-13.
Dr. Dodson is in private practice in Denver, CO.
New warnings on stimulants for ADHD: Cause for alarm?
In August the FDA called for new warnings on stimulants used for attention-deficit/hyperactivity disorder (ADHD). Amphetamines now carry black box warnings that say, “Misuse of amphetamines may cause sudden death and serious cardiovascular adverse events.” Amphetamines and methylphenidates used for ADHD include expanded information about cardiovascular risks at usual dosages for patients with heart conditions.
To examine the clinical implications of these warnings, Current Psychiatry hosted a conversation between ADHD experts Anthony Rostain, MD, MA, and Lenard Adler, MD.
Dr. Rostain: Changes to warnings on ADHD medications have many psychiatrists looking for guidance on using stimulants. Can you give us some background and discuss the labeling changes?
Dr. Adler: Stimulants have been used for more than 40 years as ADHD treatments, and they’ve been shown to be highly effective. The FDA, which monitors issues of cardiovascular safety and stimulants in an ongoing way, examined specific isolated cases and changed some of the warnings as a result.
Dr. Rostain: What should practicing psychiatrists be concerned about if they’re thinking of prescribing stimulants for an ADHD patient?
Dr. Adler: The take-home point is that stimulants—because of the way they work—have been known to have minor effects of increasing blood pressure and pulse (Box 1).1-3 Clinicians have known about issues regarding stimulant use by patients with pre-existing cardiovascular conditions, but now the warnings are more formal for the methylphenidate and amphetamine products.
Dr. Rostain: An FDA committee recommended black box warnings on all stimulants used for ADHD, but the FDA decided instead to clarify warnings in prescription information for some medications. What was the FDA process?
Dr. Adler: The discussion was internal at the FDA, so I can’t say what their thinking was. The black box warning on amphetamines notes two issues. One is the potential for abuse and diversion, and the other warns of potential for sudden death and serious cardiovascular effects if the drug is misused. A warning has also been placed on all methylphenidate products regarding cardiovascular risk for patients with pre-existing cardiovascular conditions, but it is not a black box warning.
in healthy children and adults
Researchers at Massachusetts General Hospital have examined the effects of ADHD medications on blood pressure and heart rate in children and adults.
Children and adolescents. The first study1 was a 1-year extension of an open-label trial of once-daily, osmotic-release methylphenidate (MPH) in 432 children (age 6 to 13) with ADHD. Their blood pressure and heart rate were recorded at baseline and monthly.
At 12 months, MPH use at 18 to 54 mg/d was associated with minor but statistically significant mean increases in:
- systolic blood pressure (3.3 mm Hg [P<0.001])
- diastolic blood pressure (1.5 mm Hg [P<0.001])
- heart rate (3.9 bpm [P<0.0001]).
Adults. In a 24-month study,2 223 healthy adults with ADHD (age≥18) received mixed amphetamine salts extended-release (MAS XR) in an open-label extension of a 4-week, double-blind, placebo-controlled trial. MAS XR was started at 20 mg/d for 1 week, then increased up to 60 mg/d based on therapeutic effect, as measured by the ADHD Rating Scale IV.
Blood pressure and pulse were measured at baseline, weekly, then monthly, and 12-lead ECGs were obtained at baseline, weekly, then at 3- and 6-month intervals. Changes after 2 years were small and not statistically significant:
- systolic blood pressure (2.3±12.5 mm Hg)
- diastolic blood pressure (1.3±9.2 mm Hg)
- pulse (2.1±13.4 bpm).
A clinically insignificant increase was observed in the mean QTcB interval (7.2 msec; P<0.001), although no patient’s QTcB interval exceeded 480 msec. Seven patients dropped out because of cardiovascular side effects (5 with hypertension, and 2 with palpitation/tachycardia), which were not reported as being serious.
Stimulants and nonstimulants. In another study,3 the same researchers analyzed the cardiovascular effects of three stimulants (methylphenidate, amphetamine compounds, and pemoline) and two nonstimulants (bupropion and desipramine) used to treat ADHD in adults. Data on a total of 125 patients (mean age 39±9 years) from three previous placebo-controlled studies were re-examined for the medications’ effects on blood pressure and heart rate.
Minor but statistically significant changes in blood pressure and heart rate were found to be associated with both stimulant and nonstimulant medications:
- systolic blood pressure (bupropion, +5.9 mm Hg [P<0.05]; amphetamine, +5.4 mm Hg [P<0.05])
- diastolic blood pressure (desipramine, +7.1 mm Hg [P<0.05])
- heart rate (bupropion, +6.9 bpm [P<0.05]; amphetamine, +7.3 bpm [P<0.05]; methylphenidate, +4.5 bpm [P<0.05]).
In the last two studies, the authors concluded that although the cardiovascular effects of ADHD medications in healthy adults were minimal, clinicians should monitor vital signs at baseline and periodically during treatment.
Dr. Rostain: How were the warnings clarified?
Dr. Adler: The FDA has changed the language. Now physicians are warned that sudden death can occur at usual doses in patients with a pre-existing structural cardiac abnormality or other serious heart problem. So, stimulants generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug.
Dr. Rostain: What about adults?
Dr. Adler: The language is the same for adults. Adults have a greater likelihood than children of having a history of serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other cardiac problems. Adults with such abnormalities generally should not be treated with stimulant drugs.
Dr. Rostain: What’s the impact for clinicians?
Dr. Adler: Clinicians have known that stimulants should not be used in patients with significant pre-existing cardiovascular conditions. That generally includes structural abnormalities such as serious heart murmurs and abnormalities of the electro-conduction of the impulse through the heart. When patients present with a history of cardiac abnormalities, clinicians should speak to the pediatrician, primary care physician (PCP), or cardiologist, go over the risk factors, and decide whether these medications can be prescribed for the patient.
Dr. Rostain: Should psychiatrists perform screening tests before prescribing stimulants? When should they consult with a specialist?
Dr. Adler: There is no recommendation in the prescribing information. But clearly a clinician should determine whether a patient has structural cardiac abnormalities or serious heart problems. That means taking a history about heart murmur, syncope, or other serious heart problems. Also, you want to know if the patient is hypertensive. The burden is on the prescribing clinician.
Dr. Rostain: Suppose you have a patient with hypertension or a history of a heart condition, should that patient first be evaluated by a cardiologist? What about a screening ECG?
Dr. Adler: There are no specific recommendations. If clinicians have questions about prescribing the medication, they should consult with the patient’s PCP or cardiologist.
Dr. Rostain: Let’s say the patient has some heart issues, but the PCP or pediatrician gives the goahead to prescribe stimulants. What sort of monitoring do you recommend?
Dr. Adler: I can’t answer that directly. Clearly, you’re going to want to partner with the PCP to establish a plan of how to carefully monitor this patient. FDA guidelines recommend ongoing blood pressure monitoring, especially if the patient is hypertensive, but do not specify how often.
Dr. Rostain: What alternatives do psychiatrists have when treating ADHD in patients in whom stimulants may pose some risk?
Dr. Adler: The only approved nonstimulant ADHD medication is atomoxetine, the labeling of which carries language about possible effects on blood pressure. The FDA warning about structural cardiac abnormalities has not been extended to atomoxetine, but blood pressure needs to be monitored. Whether our medical colleagues feel comfortable using a nonstimulant in patients with structural cardiac abnormalities has not been determined.
Dr. Rostain: In the absence of guidelines in the new warnings on stimulants, are there any studies to help clinicians with treatment and monitoring?
Dr. Adler: There’s very little data. A group at Massachusetts General Hospital has been studying the effects of ADHD medication on adults with hypertension (Box 2).4 That’s a different issue than a structural cardiac abnormality, but at least we have some data. This group found that you can safely give stimulants to hypertensive patients by partnering with medical colleagues and monitoring the patient carefully. Antihypertensive dosages may need to be adjusted during psychostimulant treatment.
Dr. Rostain: How do you choose a medication if your patient has a structural heart abnormality?
Dr. Adler: Again, we don’t have a lot of data. The decision would depend on the cardiac abnormality and the consulting physician’s comfort level. Keep in mind that psychostimulants have a short duration of effect, so the effects of the medication can dissipate fairly quickly. Again, the decision to medicate a patient with pre-existing cardiac abnormalities must be done with medical guidance.
In a short-term, open-label trial by Wilens et al,4 13 adults with ADHD and hypertension received mixed amphetamine salts extended-release (MAS-XR), up to 60 mg/d, for 6 weeks (phase 1), then discontinued MAS-XR for 2 weeks (phase 2). All patients had normal blood pressure (<135/85 mm Hg) for at least 4 weeks before entering the study and received a comprehensive clinical assessment, including ECG. Blood pressure was measured manually at each clinic visit.
Single episodes of hypertension (>140/90 mm Hg) occurred at similar rates in each phase, but these episodes were not sustained at any two consecutive visits. Group mean systolic and diastolic blood pressures and pulse did not increase during stimulant treatment. No clinically significant ECG changes were observed, and no serious adverse events occurred.
The authors concluded that this preliminary trial suggests that adults with ADHD and controlled hypertension can be safely treated with stimulant medications.
Dr. Rostain: So are you saying clinicians should make decisions about prescribing stimulants for patients with ADHD on a case-by-case basis?
Dr. Adler: Exactly.
Dr. Rostain: What about children and adolescents who have unknown structural heart defects? A lot of parents are concerned about reports of sudden cardiac death in young athletes, such as when playing soccer or basketball. Is there any way for practitioners to protect children with ADHD from an unexpected event?
Dr. Adler: In general, stimulants are safe medications, but we don’t have guidelines to help us determine who will need an ECG and who will not. Children are less likely to have had an ECG in the past than an adult, so it’s important to do a history, obtain input from the pediatrician or PCP, and clearly review the risks and benefits of medication therapy with the patient’s family.
Dr. Rostain: What would you advise clinicians to tell parents of children with ADHD or adult patients who have concerns about the new labeling on stimulants?
Dr. Adler: It would be a shame if patients were not receiving treatment for ADHD because of unfounded medical concerns. When these medications are used appropriately, they have dramatic and positive affects on ADHD.
ADHD is common and highly impairing. Deciding not to treat it has serious consequences in terms of divorce, separation, underperformance in school and on the job, unemployment, smoking, substance use, and issues with motor vehicle accidents and driving.
The goal of treatment is for our patients to get better, and ADHD is highly treatable with medication. But we must be cognizant of the warnings and prescribe medications appropriately. The message is that we’ve got to work collaboratively with our partners in medicine and, in the absence of guidelines, use good common sense.
Related resources
- Wilens TE, Hammerness PG, Biederman J, et al. Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2005;66:253-9.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Concerta, Ritalin
- Mixed amphetamine salts • Adderall
- Pemoline • Cylert
Disclosures
Dr. Adler is a consultant to and receives grant/research support from Abbott Laboratories, Cephalon, Cortex Pharmaceuticals, Eli Lilly and Company, New River Pharmaceuticals, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire. He also receives grant/research support from Bristol-Myers Squibb and Merck and Co., and is a speaker for Eli Lilly and Company.
Dr. Rostain is a consultant to Shire and a speaker for Eli Lilly and Company and Ortho-McNeil.
1. Wilens TE, Biederman J, Lerner M. Concerta Study Group. Effects of once-daily osmotic-release methylphenidate on blood pressure and heart rate in children with attention-deficit/hyperactivity disorder: results from a one-year follow-up study. J Clin Psychopharmacol 2004;24(1):36-41.
2. Biederman J, Spencer TJ, Wilens TE, et al. Long-term safety and effectiveness of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr 2005;10(12 suppl 20):16-25.
3. Wilens TE, Hammerness PG, Biederman J, et al. Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2005;66(2):253-9.
4. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/ hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67(5):696-702.
Dr. Adler is associate professor of psychiatry and director of the adult ADHD program at New York University Medical Center. He recently published a book for patients, Scattered Minds: Hope and Help for Adults with ADHD.
Dr. Rostain is associate professor of psychiatry and pediatrics and director of education, department of psychiatry, University of Pennsylvania School of Medicine, Philadelphia.
In August the FDA called for new warnings on stimulants used for attention-deficit/hyperactivity disorder (ADHD). Amphetamines now carry black box warnings that say, “Misuse of amphetamines may cause sudden death and serious cardiovascular adverse events.” Amphetamines and methylphenidates used for ADHD include expanded information about cardiovascular risks at usual dosages for patients with heart conditions.
To examine the clinical implications of these warnings, Current Psychiatry hosted a conversation between ADHD experts Anthony Rostain, MD, MA, and Lenard Adler, MD.
Dr. Rostain: Changes to warnings on ADHD medications have many psychiatrists looking for guidance on using stimulants. Can you give us some background and discuss the labeling changes?
Dr. Adler: Stimulants have been used for more than 40 years as ADHD treatments, and they’ve been shown to be highly effective. The FDA, which monitors issues of cardiovascular safety and stimulants in an ongoing way, examined specific isolated cases and changed some of the warnings as a result.
Dr. Rostain: What should practicing psychiatrists be concerned about if they’re thinking of prescribing stimulants for an ADHD patient?
Dr. Adler: The take-home point is that stimulants—because of the way they work—have been known to have minor effects of increasing blood pressure and pulse (Box 1).1-3 Clinicians have known about issues regarding stimulant use by patients with pre-existing cardiovascular conditions, but now the warnings are more formal for the methylphenidate and amphetamine products.
Dr. Rostain: An FDA committee recommended black box warnings on all stimulants used for ADHD, but the FDA decided instead to clarify warnings in prescription information for some medications. What was the FDA process?
Dr. Adler: The discussion was internal at the FDA, so I can’t say what their thinking was. The black box warning on amphetamines notes two issues. One is the potential for abuse and diversion, and the other warns of potential for sudden death and serious cardiovascular effects if the drug is misused. A warning has also been placed on all methylphenidate products regarding cardiovascular risk for patients with pre-existing cardiovascular conditions, but it is not a black box warning.
in healthy children and adults
Researchers at Massachusetts General Hospital have examined the effects of ADHD medications on blood pressure and heart rate in children and adults.
Children and adolescents. The first study1 was a 1-year extension of an open-label trial of once-daily, osmotic-release methylphenidate (MPH) in 432 children (age 6 to 13) with ADHD. Their blood pressure and heart rate were recorded at baseline and monthly.
At 12 months, MPH use at 18 to 54 mg/d was associated with minor but statistically significant mean increases in:
- systolic blood pressure (3.3 mm Hg [P<0.001])
- diastolic blood pressure (1.5 mm Hg [P<0.001])
- heart rate (3.9 bpm [P<0.0001]).
Adults. In a 24-month study,2 223 healthy adults with ADHD (age≥18) received mixed amphetamine salts extended-release (MAS XR) in an open-label extension of a 4-week, double-blind, placebo-controlled trial. MAS XR was started at 20 mg/d for 1 week, then increased up to 60 mg/d based on therapeutic effect, as measured by the ADHD Rating Scale IV.
Blood pressure and pulse were measured at baseline, weekly, then monthly, and 12-lead ECGs were obtained at baseline, weekly, then at 3- and 6-month intervals. Changes after 2 years were small and not statistically significant:
- systolic blood pressure (2.3±12.5 mm Hg)
- diastolic blood pressure (1.3±9.2 mm Hg)
- pulse (2.1±13.4 bpm).
A clinically insignificant increase was observed in the mean QTcB interval (7.2 msec; P<0.001), although no patient’s QTcB interval exceeded 480 msec. Seven patients dropped out because of cardiovascular side effects (5 with hypertension, and 2 with palpitation/tachycardia), which were not reported as being serious.
Stimulants and nonstimulants. In another study,3 the same researchers analyzed the cardiovascular effects of three stimulants (methylphenidate, amphetamine compounds, and pemoline) and two nonstimulants (bupropion and desipramine) used to treat ADHD in adults. Data on a total of 125 patients (mean age 39±9 years) from three previous placebo-controlled studies were re-examined for the medications’ effects on blood pressure and heart rate.
Minor but statistically significant changes in blood pressure and heart rate were found to be associated with both stimulant and nonstimulant medications:
- systolic blood pressure (bupropion, +5.9 mm Hg [P<0.05]; amphetamine, +5.4 mm Hg [P<0.05])
- diastolic blood pressure (desipramine, +7.1 mm Hg [P<0.05])
- heart rate (bupropion, +6.9 bpm [P<0.05]; amphetamine, +7.3 bpm [P<0.05]; methylphenidate, +4.5 bpm [P<0.05]).
In the last two studies, the authors concluded that although the cardiovascular effects of ADHD medications in healthy adults were minimal, clinicians should monitor vital signs at baseline and periodically during treatment.
Dr. Rostain: How were the warnings clarified?
Dr. Adler: The FDA has changed the language. Now physicians are warned that sudden death can occur at usual doses in patients with a pre-existing structural cardiac abnormality or other serious heart problem. So, stimulants generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug.
Dr. Rostain: What about adults?
Dr. Adler: The language is the same for adults. Adults have a greater likelihood than children of having a history of serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other cardiac problems. Adults with such abnormalities generally should not be treated with stimulant drugs.
Dr. Rostain: What’s the impact for clinicians?
Dr. Adler: Clinicians have known that stimulants should not be used in patients with significant pre-existing cardiovascular conditions. That generally includes structural abnormalities such as serious heart murmurs and abnormalities of the electro-conduction of the impulse through the heart. When patients present with a history of cardiac abnormalities, clinicians should speak to the pediatrician, primary care physician (PCP), or cardiologist, go over the risk factors, and decide whether these medications can be prescribed for the patient.
Dr. Rostain: Should psychiatrists perform screening tests before prescribing stimulants? When should they consult with a specialist?
Dr. Adler: There is no recommendation in the prescribing information. But clearly a clinician should determine whether a patient has structural cardiac abnormalities or serious heart problems. That means taking a history about heart murmur, syncope, or other serious heart problems. Also, you want to know if the patient is hypertensive. The burden is on the prescribing clinician.
Dr. Rostain: Suppose you have a patient with hypertension or a history of a heart condition, should that patient first be evaluated by a cardiologist? What about a screening ECG?
Dr. Adler: There are no specific recommendations. If clinicians have questions about prescribing the medication, they should consult with the patient’s PCP or cardiologist.
Dr. Rostain: Let’s say the patient has some heart issues, but the PCP or pediatrician gives the goahead to prescribe stimulants. What sort of monitoring do you recommend?
Dr. Adler: I can’t answer that directly. Clearly, you’re going to want to partner with the PCP to establish a plan of how to carefully monitor this patient. FDA guidelines recommend ongoing blood pressure monitoring, especially if the patient is hypertensive, but do not specify how often.
Dr. Rostain: What alternatives do psychiatrists have when treating ADHD in patients in whom stimulants may pose some risk?
Dr. Adler: The only approved nonstimulant ADHD medication is atomoxetine, the labeling of which carries language about possible effects on blood pressure. The FDA warning about structural cardiac abnormalities has not been extended to atomoxetine, but blood pressure needs to be monitored. Whether our medical colleagues feel comfortable using a nonstimulant in patients with structural cardiac abnormalities has not been determined.
Dr. Rostain: In the absence of guidelines in the new warnings on stimulants, are there any studies to help clinicians with treatment and monitoring?
Dr. Adler: There’s very little data. A group at Massachusetts General Hospital has been studying the effects of ADHD medication on adults with hypertension (Box 2).4 That’s a different issue than a structural cardiac abnormality, but at least we have some data. This group found that you can safely give stimulants to hypertensive patients by partnering with medical colleagues and monitoring the patient carefully. Antihypertensive dosages may need to be adjusted during psychostimulant treatment.
Dr. Rostain: How do you choose a medication if your patient has a structural heart abnormality?
Dr. Adler: Again, we don’t have a lot of data. The decision would depend on the cardiac abnormality and the consulting physician’s comfort level. Keep in mind that psychostimulants have a short duration of effect, so the effects of the medication can dissipate fairly quickly. Again, the decision to medicate a patient with pre-existing cardiac abnormalities must be done with medical guidance.
In a short-term, open-label trial by Wilens et al,4 13 adults with ADHD and hypertension received mixed amphetamine salts extended-release (MAS-XR), up to 60 mg/d, for 6 weeks (phase 1), then discontinued MAS-XR for 2 weeks (phase 2). All patients had normal blood pressure (<135/85 mm Hg) for at least 4 weeks before entering the study and received a comprehensive clinical assessment, including ECG. Blood pressure was measured manually at each clinic visit.
Single episodes of hypertension (>140/90 mm Hg) occurred at similar rates in each phase, but these episodes were not sustained at any two consecutive visits. Group mean systolic and diastolic blood pressures and pulse did not increase during stimulant treatment. No clinically significant ECG changes were observed, and no serious adverse events occurred.
The authors concluded that this preliminary trial suggests that adults with ADHD and controlled hypertension can be safely treated with stimulant medications.
Dr. Rostain: So are you saying clinicians should make decisions about prescribing stimulants for patients with ADHD on a case-by-case basis?
Dr. Adler: Exactly.
Dr. Rostain: What about children and adolescents who have unknown structural heart defects? A lot of parents are concerned about reports of sudden cardiac death in young athletes, such as when playing soccer or basketball. Is there any way for practitioners to protect children with ADHD from an unexpected event?
Dr. Adler: In general, stimulants are safe medications, but we don’t have guidelines to help us determine who will need an ECG and who will not. Children are less likely to have had an ECG in the past than an adult, so it’s important to do a history, obtain input from the pediatrician or PCP, and clearly review the risks and benefits of medication therapy with the patient’s family.
Dr. Rostain: What would you advise clinicians to tell parents of children with ADHD or adult patients who have concerns about the new labeling on stimulants?
Dr. Adler: It would be a shame if patients were not receiving treatment for ADHD because of unfounded medical concerns. When these medications are used appropriately, they have dramatic and positive affects on ADHD.
ADHD is common and highly impairing. Deciding not to treat it has serious consequences in terms of divorce, separation, underperformance in school and on the job, unemployment, smoking, substance use, and issues with motor vehicle accidents and driving.
The goal of treatment is for our patients to get better, and ADHD is highly treatable with medication. But we must be cognizant of the warnings and prescribe medications appropriately. The message is that we’ve got to work collaboratively with our partners in medicine and, in the absence of guidelines, use good common sense.
Related resources
- Wilens TE, Hammerness PG, Biederman J, et al. Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2005;66:253-9.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Concerta, Ritalin
- Mixed amphetamine salts • Adderall
- Pemoline • Cylert
Disclosures
Dr. Adler is a consultant to and receives grant/research support from Abbott Laboratories, Cephalon, Cortex Pharmaceuticals, Eli Lilly and Company, New River Pharmaceuticals, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire. He also receives grant/research support from Bristol-Myers Squibb and Merck and Co., and is a speaker for Eli Lilly and Company.
Dr. Rostain is a consultant to Shire and a speaker for Eli Lilly and Company and Ortho-McNeil.
In August the FDA called for new warnings on stimulants used for attention-deficit/hyperactivity disorder (ADHD). Amphetamines now carry black box warnings that say, “Misuse of amphetamines may cause sudden death and serious cardiovascular adverse events.” Amphetamines and methylphenidates used for ADHD include expanded information about cardiovascular risks at usual dosages for patients with heart conditions.
To examine the clinical implications of these warnings, Current Psychiatry hosted a conversation between ADHD experts Anthony Rostain, MD, MA, and Lenard Adler, MD.
Dr. Rostain: Changes to warnings on ADHD medications have many psychiatrists looking for guidance on using stimulants. Can you give us some background and discuss the labeling changes?
Dr. Adler: Stimulants have been used for more than 40 years as ADHD treatments, and they’ve been shown to be highly effective. The FDA, which monitors issues of cardiovascular safety and stimulants in an ongoing way, examined specific isolated cases and changed some of the warnings as a result.
Dr. Rostain: What should practicing psychiatrists be concerned about if they’re thinking of prescribing stimulants for an ADHD patient?
Dr. Adler: The take-home point is that stimulants—because of the way they work—have been known to have minor effects of increasing blood pressure and pulse (Box 1).1-3 Clinicians have known about issues regarding stimulant use by patients with pre-existing cardiovascular conditions, but now the warnings are more formal for the methylphenidate and amphetamine products.
Dr. Rostain: An FDA committee recommended black box warnings on all stimulants used for ADHD, but the FDA decided instead to clarify warnings in prescription information for some medications. What was the FDA process?
Dr. Adler: The discussion was internal at the FDA, so I can’t say what their thinking was. The black box warning on amphetamines notes two issues. One is the potential for abuse and diversion, and the other warns of potential for sudden death and serious cardiovascular effects if the drug is misused. A warning has also been placed on all methylphenidate products regarding cardiovascular risk for patients with pre-existing cardiovascular conditions, but it is not a black box warning.
in healthy children and adults
Researchers at Massachusetts General Hospital have examined the effects of ADHD medications on blood pressure and heart rate in children and adults.
Children and adolescents. The first study1 was a 1-year extension of an open-label trial of once-daily, osmotic-release methylphenidate (MPH) in 432 children (age 6 to 13) with ADHD. Their blood pressure and heart rate were recorded at baseline and monthly.
At 12 months, MPH use at 18 to 54 mg/d was associated with minor but statistically significant mean increases in:
- systolic blood pressure (3.3 mm Hg [P<0.001])
- diastolic blood pressure (1.5 mm Hg [P<0.001])
- heart rate (3.9 bpm [P<0.0001]).
Adults. In a 24-month study,2 223 healthy adults with ADHD (age≥18) received mixed amphetamine salts extended-release (MAS XR) in an open-label extension of a 4-week, double-blind, placebo-controlled trial. MAS XR was started at 20 mg/d for 1 week, then increased up to 60 mg/d based on therapeutic effect, as measured by the ADHD Rating Scale IV.
Blood pressure and pulse were measured at baseline, weekly, then monthly, and 12-lead ECGs were obtained at baseline, weekly, then at 3- and 6-month intervals. Changes after 2 years were small and not statistically significant:
- systolic blood pressure (2.3±12.5 mm Hg)
- diastolic blood pressure (1.3±9.2 mm Hg)
- pulse (2.1±13.4 bpm).
A clinically insignificant increase was observed in the mean QTcB interval (7.2 msec; P<0.001), although no patient’s QTcB interval exceeded 480 msec. Seven patients dropped out because of cardiovascular side effects (5 with hypertension, and 2 with palpitation/tachycardia), which were not reported as being serious.
Stimulants and nonstimulants. In another study,3 the same researchers analyzed the cardiovascular effects of three stimulants (methylphenidate, amphetamine compounds, and pemoline) and two nonstimulants (bupropion and desipramine) used to treat ADHD in adults. Data on a total of 125 patients (mean age 39±9 years) from three previous placebo-controlled studies were re-examined for the medications’ effects on blood pressure and heart rate.
Minor but statistically significant changes in blood pressure and heart rate were found to be associated with both stimulant and nonstimulant medications:
- systolic blood pressure (bupropion, +5.9 mm Hg [P<0.05]; amphetamine, +5.4 mm Hg [P<0.05])
- diastolic blood pressure (desipramine, +7.1 mm Hg [P<0.05])
- heart rate (bupropion, +6.9 bpm [P<0.05]; amphetamine, +7.3 bpm [P<0.05]; methylphenidate, +4.5 bpm [P<0.05]).
In the last two studies, the authors concluded that although the cardiovascular effects of ADHD medications in healthy adults were minimal, clinicians should monitor vital signs at baseline and periodically during treatment.
Dr. Rostain: How were the warnings clarified?
Dr. Adler: The FDA has changed the language. Now physicians are warned that sudden death can occur at usual doses in patients with a pre-existing structural cardiac abnormality or other serious heart problem. So, stimulants generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug.
Dr. Rostain: What about adults?
Dr. Adler: The language is the same for adults. Adults have a greater likelihood than children of having a history of serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other cardiac problems. Adults with such abnormalities generally should not be treated with stimulant drugs.
Dr. Rostain: What’s the impact for clinicians?
Dr. Adler: Clinicians have known that stimulants should not be used in patients with significant pre-existing cardiovascular conditions. That generally includes structural abnormalities such as serious heart murmurs and abnormalities of the electro-conduction of the impulse through the heart. When patients present with a history of cardiac abnormalities, clinicians should speak to the pediatrician, primary care physician (PCP), or cardiologist, go over the risk factors, and decide whether these medications can be prescribed for the patient.
Dr. Rostain: Should psychiatrists perform screening tests before prescribing stimulants? When should they consult with a specialist?
Dr. Adler: There is no recommendation in the prescribing information. But clearly a clinician should determine whether a patient has structural cardiac abnormalities or serious heart problems. That means taking a history about heart murmur, syncope, or other serious heart problems. Also, you want to know if the patient is hypertensive. The burden is on the prescribing clinician.
Dr. Rostain: Suppose you have a patient with hypertension or a history of a heart condition, should that patient first be evaluated by a cardiologist? What about a screening ECG?
Dr. Adler: There are no specific recommendations. If clinicians have questions about prescribing the medication, they should consult with the patient’s PCP or cardiologist.
Dr. Rostain: Let’s say the patient has some heart issues, but the PCP or pediatrician gives the goahead to prescribe stimulants. What sort of monitoring do you recommend?
Dr. Adler: I can’t answer that directly. Clearly, you’re going to want to partner with the PCP to establish a plan of how to carefully monitor this patient. FDA guidelines recommend ongoing blood pressure monitoring, especially if the patient is hypertensive, but do not specify how often.
Dr. Rostain: What alternatives do psychiatrists have when treating ADHD in patients in whom stimulants may pose some risk?
Dr. Adler: The only approved nonstimulant ADHD medication is atomoxetine, the labeling of which carries language about possible effects on blood pressure. The FDA warning about structural cardiac abnormalities has not been extended to atomoxetine, but blood pressure needs to be monitored. Whether our medical colleagues feel comfortable using a nonstimulant in patients with structural cardiac abnormalities has not been determined.
Dr. Rostain: In the absence of guidelines in the new warnings on stimulants, are there any studies to help clinicians with treatment and monitoring?
Dr. Adler: There’s very little data. A group at Massachusetts General Hospital has been studying the effects of ADHD medication on adults with hypertension (Box 2).4 That’s a different issue than a structural cardiac abnormality, but at least we have some data. This group found that you can safely give stimulants to hypertensive patients by partnering with medical colleagues and monitoring the patient carefully. Antihypertensive dosages may need to be adjusted during psychostimulant treatment.
Dr. Rostain: How do you choose a medication if your patient has a structural heart abnormality?
Dr. Adler: Again, we don’t have a lot of data. The decision would depend on the cardiac abnormality and the consulting physician’s comfort level. Keep in mind that psychostimulants have a short duration of effect, so the effects of the medication can dissipate fairly quickly. Again, the decision to medicate a patient with pre-existing cardiac abnormalities must be done with medical guidance.
In a short-term, open-label trial by Wilens et al,4 13 adults with ADHD and hypertension received mixed amphetamine salts extended-release (MAS-XR), up to 60 mg/d, for 6 weeks (phase 1), then discontinued MAS-XR for 2 weeks (phase 2). All patients had normal blood pressure (<135/85 mm Hg) for at least 4 weeks before entering the study and received a comprehensive clinical assessment, including ECG. Blood pressure was measured manually at each clinic visit.
Single episodes of hypertension (>140/90 mm Hg) occurred at similar rates in each phase, but these episodes were not sustained at any two consecutive visits. Group mean systolic and diastolic blood pressures and pulse did not increase during stimulant treatment. No clinically significant ECG changes were observed, and no serious adverse events occurred.
The authors concluded that this preliminary trial suggests that adults with ADHD and controlled hypertension can be safely treated with stimulant medications.
Dr. Rostain: So are you saying clinicians should make decisions about prescribing stimulants for patients with ADHD on a case-by-case basis?
Dr. Adler: Exactly.
Dr. Rostain: What about children and adolescents who have unknown structural heart defects? A lot of parents are concerned about reports of sudden cardiac death in young athletes, such as when playing soccer or basketball. Is there any way for practitioners to protect children with ADHD from an unexpected event?
Dr. Adler: In general, stimulants are safe medications, but we don’t have guidelines to help us determine who will need an ECG and who will not. Children are less likely to have had an ECG in the past than an adult, so it’s important to do a history, obtain input from the pediatrician or PCP, and clearly review the risks and benefits of medication therapy with the patient’s family.
Dr. Rostain: What would you advise clinicians to tell parents of children with ADHD or adult patients who have concerns about the new labeling on stimulants?
Dr. Adler: It would be a shame if patients were not receiving treatment for ADHD because of unfounded medical concerns. When these medications are used appropriately, they have dramatic and positive affects on ADHD.
ADHD is common and highly impairing. Deciding not to treat it has serious consequences in terms of divorce, separation, underperformance in school and on the job, unemployment, smoking, substance use, and issues with motor vehicle accidents and driving.
The goal of treatment is for our patients to get better, and ADHD is highly treatable with medication. But we must be cognizant of the warnings and prescribe medications appropriately. The message is that we’ve got to work collaboratively with our partners in medicine and, in the absence of guidelines, use good common sense.
Related resources
- Wilens TE, Hammerness PG, Biederman J, et al. Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2005;66:253-9.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Concerta, Ritalin
- Mixed amphetamine salts • Adderall
- Pemoline • Cylert
Disclosures
Dr. Adler is a consultant to and receives grant/research support from Abbott Laboratories, Cephalon, Cortex Pharmaceuticals, Eli Lilly and Company, New River Pharmaceuticals, Novartis Pharmaceuticals Corp., Ortho-McNeil, Pfizer, and Shire. He also receives grant/research support from Bristol-Myers Squibb and Merck and Co., and is a speaker for Eli Lilly and Company.
Dr. Rostain is a consultant to Shire and a speaker for Eli Lilly and Company and Ortho-McNeil.
1. Wilens TE, Biederman J, Lerner M. Concerta Study Group. Effects of once-daily osmotic-release methylphenidate on blood pressure and heart rate in children with attention-deficit/hyperactivity disorder: results from a one-year follow-up study. J Clin Psychopharmacol 2004;24(1):36-41.
2. Biederman J, Spencer TJ, Wilens TE, et al. Long-term safety and effectiveness of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr 2005;10(12 suppl 20):16-25.
3. Wilens TE, Hammerness PG, Biederman J, et al. Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2005;66(2):253-9.
4. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/ hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67(5):696-702.
Dr. Adler is associate professor of psychiatry and director of the adult ADHD program at New York University Medical Center. He recently published a book for patients, Scattered Minds: Hope and Help for Adults with ADHD.
Dr. Rostain is associate professor of psychiatry and pediatrics and director of education, department of psychiatry, University of Pennsylvania School of Medicine, Philadelphia.
1. Wilens TE, Biederman J, Lerner M. Concerta Study Group. Effects of once-daily osmotic-release methylphenidate on blood pressure and heart rate in children with attention-deficit/hyperactivity disorder: results from a one-year follow-up study. J Clin Psychopharmacol 2004;24(1):36-41.
2. Biederman J, Spencer TJ, Wilens TE, et al. Long-term safety and effectiveness of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr 2005;10(12 suppl 20):16-25.
3. Wilens TE, Hammerness PG, Biederman J, et al. Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2005;66(2):253-9.
4. Wilens TE, Zusman RM, Hammerness PG, et al. An open-label study of the tolerability of mixed amphetamine salts in adults with attention-deficit/ hyperactivity disorder and treated primary essential hypertension. J Clin Psychiatry 2006;67(5):696-702.
Dr. Adler is associate professor of psychiatry and director of the adult ADHD program at New York University Medical Center. He recently published a book for patients, Scattered Minds: Hope and Help for Adults with ADHD.
Dr. Rostain is associate professor of psychiatry and pediatrics and director of education, department of psychiatry, University of Pennsylvania School of Medicine, Philadelphia.