User login
Nonpharmacologic strategies for helping children with ADHD
Attention-deficit/hyperactivity disorder (ADHD) affects 5% of children and adolescents worldwide.1 Children with ADHD commonly have trouble with attention, hyperactivity, impulsivity, organization, and emotional reactivity, and these difficulties can result in behaviors that frustrate, worry, and overwhelm parents, teachers, and other caregivers.
Extensive evidence supports stimulants as a first-line treatment. However, nonpharmacologic interventions are important, yet often overlooked, adjuncts that can be helpful for children who have a partial response to stimulants or are not prescribed medication. Teaching caregivers to use the following interventions will allow them to help children better navigate situations that require managing their symptoms, such as in a classroom setting.2
Attention. Children with ADHD typically find it challenging to prioritize what to focus on, sustain that focus, and switch between tasks. Shouting instructions often is unproductive. Therefore, encourage parents and teachers to use clear and concise instructions with supplementary visual tools to aid these children. When providing instructions in classrooms, teachers should look directly at the student and call him (her) by name. It also can be helpful to have the student repeat the instructions. Seating students with ADHD near the front of the classroom, close to the teacher and away from other distracting students, can improve their focus and allow the teacher to more easily give nonverbal cues, such as tapping on the student’s desk if his attention is waning.
Hyperactivity. Children with ADHD are prone to excessive talkativeness and continuous motor movement; therefore, sitting still for long periods can be exceptionally difficult. Teachers and caregivers should keep assignments short. For students whose primary manifestation of ADHD is hyperactivity, sitting near the back of the classroom will allow them to stand and stretch without disrupting the class. Occasionally giving these students a time-limited, acceptable outlet for their urge to move may be beneficial.
Impulsivity. Children who exhibit this symptom are more focused on the present and have difficulty weighing the consequences of their actions. Allowing these children to take frequent breaks (eg, more play time) will let their brains rest and recharge so that they can take a step back to evaluate the outcomes of their actions. Instruct parents and teachers to give children with ADHD regular verbal or written feedback to monitor and modify behaviors over time. Consequences for not following the rules should be immediate and consistent.
Organization. School assignments require sequencing, planning, and time management. Therefore, having daily visual reminders of prioritized assignments and schedules is helpful for children with ADHD, both at school and at home. Teachers and parents can help children stay organized by checking and reviewing the child’s agenda with him several times a day; this will allow him more time to think about what he needs to do to complete assignments.Emotional reactivity. Children with ADHD become frustrated easily and often are particularly sensitive to disappointment because of the continuous redirection they receive. Normalizing their mistakes by reinforcing that everyone makes mistakes and teaching them to learn from their mistakes can help reduce their embarrassment.
It also can be helpful to identify triggers for emotional reactivity. Parents and teachers should minimize the amount of talking when a child is unable to control his emotions. Helping children label their emotions, developing strategies for when they become upset, and outlining clear consequences for unacceptable behaviors can help modify their reactions.
1. Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20.
2. Barkley RA. Classroom accommodations for children with ADHD. The ADHD Report. 2008;16(4):7-10.
Attention-deficit/hyperactivity disorder (ADHD) affects 5% of children and adolescents worldwide.1 Children with ADHD commonly have trouble with attention, hyperactivity, impulsivity, organization, and emotional reactivity, and these difficulties can result in behaviors that frustrate, worry, and overwhelm parents, teachers, and other caregivers.
Extensive evidence supports stimulants as a first-line treatment. However, nonpharmacologic interventions are important, yet often overlooked, adjuncts that can be helpful for children who have a partial response to stimulants or are not prescribed medication. Teaching caregivers to use the following interventions will allow them to help children better navigate situations that require managing their symptoms, such as in a classroom setting.2
Attention. Children with ADHD typically find it challenging to prioritize what to focus on, sustain that focus, and switch between tasks. Shouting instructions often is unproductive. Therefore, encourage parents and teachers to use clear and concise instructions with supplementary visual tools to aid these children. When providing instructions in classrooms, teachers should look directly at the student and call him (her) by name. It also can be helpful to have the student repeat the instructions. Seating students with ADHD near the front of the classroom, close to the teacher and away from other distracting students, can improve their focus and allow the teacher to more easily give nonverbal cues, such as tapping on the student’s desk if his attention is waning.
Hyperactivity. Children with ADHD are prone to excessive talkativeness and continuous motor movement; therefore, sitting still for long periods can be exceptionally difficult. Teachers and caregivers should keep assignments short. For students whose primary manifestation of ADHD is hyperactivity, sitting near the back of the classroom will allow them to stand and stretch without disrupting the class. Occasionally giving these students a time-limited, acceptable outlet for their urge to move may be beneficial.
Impulsivity. Children who exhibit this symptom are more focused on the present and have difficulty weighing the consequences of their actions. Allowing these children to take frequent breaks (eg, more play time) will let their brains rest and recharge so that they can take a step back to evaluate the outcomes of their actions. Instruct parents and teachers to give children with ADHD regular verbal or written feedback to monitor and modify behaviors over time. Consequences for not following the rules should be immediate and consistent.
Organization. School assignments require sequencing, planning, and time management. Therefore, having daily visual reminders of prioritized assignments and schedules is helpful for children with ADHD, both at school and at home. Teachers and parents can help children stay organized by checking and reviewing the child’s agenda with him several times a day; this will allow him more time to think about what he needs to do to complete assignments.Emotional reactivity. Children with ADHD become frustrated easily and often are particularly sensitive to disappointment because of the continuous redirection they receive. Normalizing their mistakes by reinforcing that everyone makes mistakes and teaching them to learn from their mistakes can help reduce their embarrassment.
It also can be helpful to identify triggers for emotional reactivity. Parents and teachers should minimize the amount of talking when a child is unable to control his emotions. Helping children label their emotions, developing strategies for when they become upset, and outlining clear consequences for unacceptable behaviors can help modify their reactions.
Attention-deficit/hyperactivity disorder (ADHD) affects 5% of children and adolescents worldwide.1 Children with ADHD commonly have trouble with attention, hyperactivity, impulsivity, organization, and emotional reactivity, and these difficulties can result in behaviors that frustrate, worry, and overwhelm parents, teachers, and other caregivers.
Extensive evidence supports stimulants as a first-line treatment. However, nonpharmacologic interventions are important, yet often overlooked, adjuncts that can be helpful for children who have a partial response to stimulants or are not prescribed medication. Teaching caregivers to use the following interventions will allow them to help children better navigate situations that require managing their symptoms, such as in a classroom setting.2
Attention. Children with ADHD typically find it challenging to prioritize what to focus on, sustain that focus, and switch between tasks. Shouting instructions often is unproductive. Therefore, encourage parents and teachers to use clear and concise instructions with supplementary visual tools to aid these children. When providing instructions in classrooms, teachers should look directly at the student and call him (her) by name. It also can be helpful to have the student repeat the instructions. Seating students with ADHD near the front of the classroom, close to the teacher and away from other distracting students, can improve their focus and allow the teacher to more easily give nonverbal cues, such as tapping on the student’s desk if his attention is waning.
Hyperactivity. Children with ADHD are prone to excessive talkativeness and continuous motor movement; therefore, sitting still for long periods can be exceptionally difficult. Teachers and caregivers should keep assignments short. For students whose primary manifestation of ADHD is hyperactivity, sitting near the back of the classroom will allow them to stand and stretch without disrupting the class. Occasionally giving these students a time-limited, acceptable outlet for their urge to move may be beneficial.
Impulsivity. Children who exhibit this symptom are more focused on the present and have difficulty weighing the consequences of their actions. Allowing these children to take frequent breaks (eg, more play time) will let their brains rest and recharge so that they can take a step back to evaluate the outcomes of their actions. Instruct parents and teachers to give children with ADHD regular verbal or written feedback to monitor and modify behaviors over time. Consequences for not following the rules should be immediate and consistent.
Organization. School assignments require sequencing, planning, and time management. Therefore, having daily visual reminders of prioritized assignments and schedules is helpful for children with ADHD, both at school and at home. Teachers and parents can help children stay organized by checking and reviewing the child’s agenda with him several times a day; this will allow him more time to think about what he needs to do to complete assignments.Emotional reactivity. Children with ADHD become frustrated easily and often are particularly sensitive to disappointment because of the continuous redirection they receive. Normalizing their mistakes by reinforcing that everyone makes mistakes and teaching them to learn from their mistakes can help reduce their embarrassment.
It also can be helpful to identify triggers for emotional reactivity. Parents and teachers should minimize the amount of talking when a child is unable to control his emotions. Helping children label their emotions, developing strategies for when they become upset, and outlining clear consequences for unacceptable behaviors can help modify their reactions.
1. Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20.
2. Barkley RA. Classroom accommodations for children with ADHD. The ADHD Report. 2008;16(4):7-10.
1. Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20.
2. Barkley RA. Classroom accommodations for children with ADHD. The ADHD Report. 2008;16(4):7-10.
Intrauterine exposure to methylphenidate tied to increased cardiac risk
The use of methylphenidate by pregnant women is associated with a small increased risk of congenital cardiac malformations in newborns. However, a comparable increased risk is not found with intrauterine exposure to stimulants, according to a population-based cohort study published Dec. 13.
Krista F. Huybrechts, PhD, and her associates analyzed data from more than 1 million pregnancies in the United States. They found that the overall incidence of congenital malformations among the 1,813,894 pregnancies was 35 per 1,000 control infants, compared with 45.9 per 1,000 infants whose mothers used methylphenidate and 45.4 per 1,000 infants whose mothers used amphetamines.
For the subset of infants with cardiac malformations, the risk per 1,000 infants was 12.7 for controls, 18.8 for methylphenidate exposure, and 15.4 for amphetamine exposure. The researchers identified an adjusted relative risk of 1.11 for overall congenital abnormalities and 1.28 for cardiac abnormalities with methylphenidate exposure, compared with a relative risk of 1.05 for overall congenital abnormalities and 0.96 for cardiac abnormalities with stimulant exposure.
An analysis among 2,560,069 pregnancies in Denmark, Finland, Iceland, Norway, and Sweden yielded a similarly significant relative risk of 1.28 for cardiac malformations associated with methylphenidate exposure (JAMA Psychiatry 2017. doi: 10.1001/jamapsychiatry.2017.3644).
“We found a 28% increased prevalence of cardiac malformations after first-trimester exposure to methylphenidate,” wrote Dr. Huybrechts of Brigham and Women’s Hospital, Boston, and her associates. “Although the absolute risk is small, it is nevertheless important evidence to consider when weighing the potential risks and benefits of different treatment strategies for [attention-deficit/hyperactivity disorder] in young women of reproductive age and in pregnant women.”
The researchers had no financial conflicts to disclose. The study was supported in part by grants from the National Institutes of Mental Health, the Eunice Kennedy Shriver National Institute for Child Health & Human Development, and the Söderström König Foundation.
The use of methylphenidate by pregnant women is associated with a small increased risk of congenital cardiac malformations in newborns. However, a comparable increased risk is not found with intrauterine exposure to stimulants, according to a population-based cohort study published Dec. 13.
Krista F. Huybrechts, PhD, and her associates analyzed data from more than 1 million pregnancies in the United States. They found that the overall incidence of congenital malformations among the 1,813,894 pregnancies was 35 per 1,000 control infants, compared with 45.9 per 1,000 infants whose mothers used methylphenidate and 45.4 per 1,000 infants whose mothers used amphetamines.
For the subset of infants with cardiac malformations, the risk per 1,000 infants was 12.7 for controls, 18.8 for methylphenidate exposure, and 15.4 for amphetamine exposure. The researchers identified an adjusted relative risk of 1.11 for overall congenital abnormalities and 1.28 for cardiac abnormalities with methylphenidate exposure, compared with a relative risk of 1.05 for overall congenital abnormalities and 0.96 for cardiac abnormalities with stimulant exposure.
An analysis among 2,560,069 pregnancies in Denmark, Finland, Iceland, Norway, and Sweden yielded a similarly significant relative risk of 1.28 for cardiac malformations associated with methylphenidate exposure (JAMA Psychiatry 2017. doi: 10.1001/jamapsychiatry.2017.3644).
“We found a 28% increased prevalence of cardiac malformations after first-trimester exposure to methylphenidate,” wrote Dr. Huybrechts of Brigham and Women’s Hospital, Boston, and her associates. “Although the absolute risk is small, it is nevertheless important evidence to consider when weighing the potential risks and benefits of different treatment strategies for [attention-deficit/hyperactivity disorder] in young women of reproductive age and in pregnant women.”
The researchers had no financial conflicts to disclose. The study was supported in part by grants from the National Institutes of Mental Health, the Eunice Kennedy Shriver National Institute for Child Health & Human Development, and the Söderström König Foundation.
The use of methylphenidate by pregnant women is associated with a small increased risk of congenital cardiac malformations in newborns. However, a comparable increased risk is not found with intrauterine exposure to stimulants, according to a population-based cohort study published Dec. 13.
Krista F. Huybrechts, PhD, and her associates analyzed data from more than 1 million pregnancies in the United States. They found that the overall incidence of congenital malformations among the 1,813,894 pregnancies was 35 per 1,000 control infants, compared with 45.9 per 1,000 infants whose mothers used methylphenidate and 45.4 per 1,000 infants whose mothers used amphetamines.
For the subset of infants with cardiac malformations, the risk per 1,000 infants was 12.7 for controls, 18.8 for methylphenidate exposure, and 15.4 for amphetamine exposure. The researchers identified an adjusted relative risk of 1.11 for overall congenital abnormalities and 1.28 for cardiac abnormalities with methylphenidate exposure, compared with a relative risk of 1.05 for overall congenital abnormalities and 0.96 for cardiac abnormalities with stimulant exposure.
An analysis among 2,560,069 pregnancies in Denmark, Finland, Iceland, Norway, and Sweden yielded a similarly significant relative risk of 1.28 for cardiac malformations associated with methylphenidate exposure (JAMA Psychiatry 2017. doi: 10.1001/jamapsychiatry.2017.3644).
“We found a 28% increased prevalence of cardiac malformations after first-trimester exposure to methylphenidate,” wrote Dr. Huybrechts of Brigham and Women’s Hospital, Boston, and her associates. “Although the absolute risk is small, it is nevertheless important evidence to consider when weighing the potential risks and benefits of different treatment strategies for [attention-deficit/hyperactivity disorder] in young women of reproductive age and in pregnant women.”
The researchers had no financial conflicts to disclose. The study was supported in part by grants from the National Institutes of Mental Health, the Eunice Kennedy Shriver National Institute for Child Health & Human Development, and the Söderström König Foundation.
FROM JAMA PSYCHIATRY
ADHD and insomnia appear intertwined
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Scandinavian registries answer key questions about ADHD
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
expert analysis from THE ECNP CONGRESS
Pediatric seclusion and restraint increases with ADHD, decreases with PTSD
NEW ORLEANS – Attention-deficit/hyperactivity disorder and non-suicidal self-harm – cutting and head banging, for instance – strongly predicted longer seclusion and restraint episodes, sometimes past 2 hours, in a review at the University of Missouri, Columbia, pediatric inpatient psychiatric unit.
Meanwhile, children with histories of physical abuse, post-traumatic stress disorder (PTSD), or out-of-home placement were less likely to have multiple seclusion and restraint (SR) episodes during an admission, and had lower numbers of SR events overall. Perhaps hyper-vigilance due to past traumas helped them avoid situations that led to problems. Staff might also have used a lighter touch given the children’s histories.
The investigators reviewed 305 SR episodes from 2011-2014 among 92 children aged 5-18 years old. They plan to expand their study to 2009-2017 and add a prospective arm.
It’s well known that SR, a last-ditch effort to prevent physical harm, traumatizes patients, but research on how to avoid it has mostly focused on adults. The Missouri team wants to change that by identifying the children most at risk, so that something can be done beforehand to prevent it. Maybe extra one-on-one care would help, Dr. Badawy said.
The children in the review were an average of 10.5 years old, and most were admitted for 3-7 days. Fifty-five (60%) had multiple SR episodes, 34 in a single admission and 21 across multiple admissions; 71% of the episodes were in boys, 58% in white children, 27.7% in black children, and the rest in multiracial children. Twenty-one percent of the episodes were in children with intellectual problems. ADHD and oppositional defiant disorder were the most common diagnoses, each diagnosed in more than half of the subjects.
When asked how a 5-year-old child can end up in restraints, Dr. Badawy agreed that bad parenting is a factor. Parents who don’t know any better might escalate normal behavior, and others might simply not care that much about their kids, or have much empathy. “In the 5-10 year-olds, I do think a lot of it is parenting. Neglect is the number one form of abuse,” she said, “and it causes instability in children.”
But parents aren’t always the problem. Dr. Badawy mentioned an “extremely depressed” boy who tried to hang himself again and again, at 8 years old. “His parents were supportive and focused on getting him better,” she said. It’s unknown if anything happened to him when they weren’t around.
If SR is related to children’s own behavior, and if they are able to listen to staff afterward and control it, kids seem able to avoid another episode. Those who have PTSD, or who have been through physical abuse or out-of-home placement, seemed particularly adept in the review.
Avoidance seems less likely, however, when children are set off by other kids.
Sometimes there are clues of impending trouble, like the boy who walked around whistling before he lashed out. Staff at the university quickly learned to swoop in and calm him when they heard the whistling.
The investigators had no industry disclosures.
NEW ORLEANS – Attention-deficit/hyperactivity disorder and non-suicidal self-harm – cutting and head banging, for instance – strongly predicted longer seclusion and restraint episodes, sometimes past 2 hours, in a review at the University of Missouri, Columbia, pediatric inpatient psychiatric unit.
Meanwhile, children with histories of physical abuse, post-traumatic stress disorder (PTSD), or out-of-home placement were less likely to have multiple seclusion and restraint (SR) episodes during an admission, and had lower numbers of SR events overall. Perhaps hyper-vigilance due to past traumas helped them avoid situations that led to problems. Staff might also have used a lighter touch given the children’s histories.
The investigators reviewed 305 SR episodes from 2011-2014 among 92 children aged 5-18 years old. They plan to expand their study to 2009-2017 and add a prospective arm.
It’s well known that SR, a last-ditch effort to prevent physical harm, traumatizes patients, but research on how to avoid it has mostly focused on adults. The Missouri team wants to change that by identifying the children most at risk, so that something can be done beforehand to prevent it. Maybe extra one-on-one care would help, Dr. Badawy said.
The children in the review were an average of 10.5 years old, and most were admitted for 3-7 days. Fifty-five (60%) had multiple SR episodes, 34 in a single admission and 21 across multiple admissions; 71% of the episodes were in boys, 58% in white children, 27.7% in black children, and the rest in multiracial children. Twenty-one percent of the episodes were in children with intellectual problems. ADHD and oppositional defiant disorder were the most common diagnoses, each diagnosed in more than half of the subjects.
When asked how a 5-year-old child can end up in restraints, Dr. Badawy agreed that bad parenting is a factor. Parents who don’t know any better might escalate normal behavior, and others might simply not care that much about their kids, or have much empathy. “In the 5-10 year-olds, I do think a lot of it is parenting. Neglect is the number one form of abuse,” she said, “and it causes instability in children.”
But parents aren’t always the problem. Dr. Badawy mentioned an “extremely depressed” boy who tried to hang himself again and again, at 8 years old. “His parents were supportive and focused on getting him better,” she said. It’s unknown if anything happened to him when they weren’t around.
If SR is related to children’s own behavior, and if they are able to listen to staff afterward and control it, kids seem able to avoid another episode. Those who have PTSD, or who have been through physical abuse or out-of-home placement, seemed particularly adept in the review.
Avoidance seems less likely, however, when children are set off by other kids.
Sometimes there are clues of impending trouble, like the boy who walked around whistling before he lashed out. Staff at the university quickly learned to swoop in and calm him when they heard the whistling.
The investigators had no industry disclosures.
NEW ORLEANS – Attention-deficit/hyperactivity disorder and non-suicidal self-harm – cutting and head banging, for instance – strongly predicted longer seclusion and restraint episodes, sometimes past 2 hours, in a review at the University of Missouri, Columbia, pediatric inpatient psychiatric unit.
Meanwhile, children with histories of physical abuse, post-traumatic stress disorder (PTSD), or out-of-home placement were less likely to have multiple seclusion and restraint (SR) episodes during an admission, and had lower numbers of SR events overall. Perhaps hyper-vigilance due to past traumas helped them avoid situations that led to problems. Staff might also have used a lighter touch given the children’s histories.
The investigators reviewed 305 SR episodes from 2011-2014 among 92 children aged 5-18 years old. They plan to expand their study to 2009-2017 and add a prospective arm.
It’s well known that SR, a last-ditch effort to prevent physical harm, traumatizes patients, but research on how to avoid it has mostly focused on adults. The Missouri team wants to change that by identifying the children most at risk, so that something can be done beforehand to prevent it. Maybe extra one-on-one care would help, Dr. Badawy said.
The children in the review were an average of 10.5 years old, and most were admitted for 3-7 days. Fifty-five (60%) had multiple SR episodes, 34 in a single admission and 21 across multiple admissions; 71% of the episodes were in boys, 58% in white children, 27.7% in black children, and the rest in multiracial children. Twenty-one percent of the episodes were in children with intellectual problems. ADHD and oppositional defiant disorder were the most common diagnoses, each diagnosed in more than half of the subjects.
When asked how a 5-year-old child can end up in restraints, Dr. Badawy agreed that bad parenting is a factor. Parents who don’t know any better might escalate normal behavior, and others might simply not care that much about their kids, or have much empathy. “In the 5-10 year-olds, I do think a lot of it is parenting. Neglect is the number one form of abuse,” she said, “and it causes instability in children.”
But parents aren’t always the problem. Dr. Badawy mentioned an “extremely depressed” boy who tried to hang himself again and again, at 8 years old. “His parents were supportive and focused on getting him better,” she said. It’s unknown if anything happened to him when they weren’t around.
If SR is related to children’s own behavior, and if they are able to listen to staff afterward and control it, kids seem able to avoid another episode. Those who have PTSD, or who have been through physical abuse or out-of-home placement, seemed particularly adept in the review.
Avoidance seems less likely, however, when children are set off by other kids.
Sometimes there are clues of impending trouble, like the boy who walked around whistling before he lashed out. Staff at the university quickly learned to swoop in and calm him when they heard the whistling.
The investigators had no industry disclosures.
AT IPS 2017
Key clinical point:
Major finding: ADHD and non-suicidal self-harm – cutting and head banging – strongly predict longer seclusion and restraint episodes, sometimes past 2 hours.
Data source: A University of Missouri, Columbia, review of 305 seclusion and restraint episodes from 2011-2014 among 92 children aged 5-18 years old.
Disclosures: The investigators had no industry disclosures.
Methylphenidate shows enduring sleep benefits in pediatric ADHD
PARIS – Methylphenidate therapy for attention-deficit/hyperactivity disorder in medication-naive boys significantly improved their sleep quality, timing, and duration in a double-blind randomized trial, Michelle M. Solleveld, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Moreover, these salutary effects on sleep persisted for at least 1 week after methylphenidate was stopped at the end of the 16-week study, added Dr. Solleveld of the University of Amsterdam.

Indeed, while parents embrace the improvement in behavioral symptoms of ADHD provided by methylphenidate, they often express concern about the possible adverse effects of stimulant medication on their child’s sleep. The new study findings are reassuring on that score.
Sleep difficulties are a major problem in patients with ADHD: They tend to fall asleep later and have more frequent awakenings during the night, which results in decreased total sleep time and sleep efficiency, Dr. Solleveld noted.
Prior studies of methylphenidate’s effects on sleep in pediatric ADHD have yielded mixed results. The negative studies were too brief to provide meaningful results, according to Dr. Solleveld, who said at least 8 weeks of treatment are required in order to evaluate the drug’s effect on sleep problems properly.
She presented a randomized, double-blind, 16-week, placebo-controlled clinical trial involving 50 medication-naive boys with ADHD who were 10-12 years old. Their sleep was assessed via actigraphy measurements taken over 5 consecutive nights, keeping a sleep diary, and answering questionnaires, including the Epworth Sleepiness Scale, at three time points: prior to randomization, 8 weeks into the trial, and finally 1 week after the study ended.
Sleep efficiency – the primary study outcome – showed a strong 5% improvement in the methylphenidate group but was unchanged from baseline in placebo-treated controls. The boys who received methylphenidate fell asleep earlier, had a shorter latency of sleep onset, and slept for longer, compared with their baseline measures or with the sleep results in controls.
The finding that the methylphenidate-induced improvements in sleep persisted for a week after drug clearance is consistent with brain imaging studies carried out by Dr. Solleveld and her coinvestigators. They believe that the effects of stimulant therapy may be age dependent. The investigators previously have shown that adults with ADHD who began treatment with stimulants before age 16 years – when brain development is still ongoing – had lower levels of basal gamma-aminobutyric acid (GABA) and higher GABA response to an oral methylphenidate than did those who began treatment with stimulants after age 23 years. This is thought to be attributable to prolonged reductions in dopamine turnover induced by methylphenidate in the developing brain (Neuroimage Clin. 2017 Jun 2;15:812-8).
The Dutch investigators also have reported that methylphenidate therapy in children with ADHD – but not in affected adults – increased the cerebral blood flow response within the thalamus to a dopamine challenge (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
The clinical ramifications of these apparently long-lasting, drug-related alterations in GABA neurotransmission are the subject of ongoing research.
Dr. Solleveld reported having no financial conflicts of interest.
PARIS – Methylphenidate therapy for attention-deficit/hyperactivity disorder in medication-naive boys significantly improved their sleep quality, timing, and duration in a double-blind randomized trial, Michelle M. Solleveld, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Moreover, these salutary effects on sleep persisted for at least 1 week after methylphenidate was stopped at the end of the 16-week study, added Dr. Solleveld of the University of Amsterdam.

Indeed, while parents embrace the improvement in behavioral symptoms of ADHD provided by methylphenidate, they often express concern about the possible adverse effects of stimulant medication on their child’s sleep. The new study findings are reassuring on that score.
Sleep difficulties are a major problem in patients with ADHD: They tend to fall asleep later and have more frequent awakenings during the night, which results in decreased total sleep time and sleep efficiency, Dr. Solleveld noted.
Prior studies of methylphenidate’s effects on sleep in pediatric ADHD have yielded mixed results. The negative studies were too brief to provide meaningful results, according to Dr. Solleveld, who said at least 8 weeks of treatment are required in order to evaluate the drug’s effect on sleep problems properly.
She presented a randomized, double-blind, 16-week, placebo-controlled clinical trial involving 50 medication-naive boys with ADHD who were 10-12 years old. Their sleep was assessed via actigraphy measurements taken over 5 consecutive nights, keeping a sleep diary, and answering questionnaires, including the Epworth Sleepiness Scale, at three time points: prior to randomization, 8 weeks into the trial, and finally 1 week after the study ended.
Sleep efficiency – the primary study outcome – showed a strong 5% improvement in the methylphenidate group but was unchanged from baseline in placebo-treated controls. The boys who received methylphenidate fell asleep earlier, had a shorter latency of sleep onset, and slept for longer, compared with their baseline measures or with the sleep results in controls.
The finding that the methylphenidate-induced improvements in sleep persisted for a week after drug clearance is consistent with brain imaging studies carried out by Dr. Solleveld and her coinvestigators. They believe that the effects of stimulant therapy may be age dependent. The investigators previously have shown that adults with ADHD who began treatment with stimulants before age 16 years – when brain development is still ongoing – had lower levels of basal gamma-aminobutyric acid (GABA) and higher GABA response to an oral methylphenidate than did those who began treatment with stimulants after age 23 years. This is thought to be attributable to prolonged reductions in dopamine turnover induced by methylphenidate in the developing brain (Neuroimage Clin. 2017 Jun 2;15:812-8).
The Dutch investigators also have reported that methylphenidate therapy in children with ADHD – but not in affected adults – increased the cerebral blood flow response within the thalamus to a dopamine challenge (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
The clinical ramifications of these apparently long-lasting, drug-related alterations in GABA neurotransmission are the subject of ongoing research.
Dr. Solleveld reported having no financial conflicts of interest.
PARIS – Methylphenidate therapy for attention-deficit/hyperactivity disorder in medication-naive boys significantly improved their sleep quality, timing, and duration in a double-blind randomized trial, Michelle M. Solleveld, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Moreover, these salutary effects on sleep persisted for at least 1 week after methylphenidate was stopped at the end of the 16-week study, added Dr. Solleveld of the University of Amsterdam.

Indeed, while parents embrace the improvement in behavioral symptoms of ADHD provided by methylphenidate, they often express concern about the possible adverse effects of stimulant medication on their child’s sleep. The new study findings are reassuring on that score.
Sleep difficulties are a major problem in patients with ADHD: They tend to fall asleep later and have more frequent awakenings during the night, which results in decreased total sleep time and sleep efficiency, Dr. Solleveld noted.
Prior studies of methylphenidate’s effects on sleep in pediatric ADHD have yielded mixed results. The negative studies were too brief to provide meaningful results, according to Dr. Solleveld, who said at least 8 weeks of treatment are required in order to evaluate the drug’s effect on sleep problems properly.
She presented a randomized, double-blind, 16-week, placebo-controlled clinical trial involving 50 medication-naive boys with ADHD who were 10-12 years old. Their sleep was assessed via actigraphy measurements taken over 5 consecutive nights, keeping a sleep diary, and answering questionnaires, including the Epworth Sleepiness Scale, at three time points: prior to randomization, 8 weeks into the trial, and finally 1 week after the study ended.
Sleep efficiency – the primary study outcome – showed a strong 5% improvement in the methylphenidate group but was unchanged from baseline in placebo-treated controls. The boys who received methylphenidate fell asleep earlier, had a shorter latency of sleep onset, and slept for longer, compared with their baseline measures or with the sleep results in controls.
The finding that the methylphenidate-induced improvements in sleep persisted for a week after drug clearance is consistent with brain imaging studies carried out by Dr. Solleveld and her coinvestigators. They believe that the effects of stimulant therapy may be age dependent. The investigators previously have shown that adults with ADHD who began treatment with stimulants before age 16 years – when brain development is still ongoing – had lower levels of basal gamma-aminobutyric acid (GABA) and higher GABA response to an oral methylphenidate than did those who began treatment with stimulants after age 23 years. This is thought to be attributable to prolonged reductions in dopamine turnover induced by methylphenidate in the developing brain (Neuroimage Clin. 2017 Jun 2;15:812-8).
The Dutch investigators also have reported that methylphenidate therapy in children with ADHD – but not in affected adults – increased the cerebral blood flow response within the thalamus to a dopamine challenge (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
The clinical ramifications of these apparently long-lasting, drug-related alterations in GABA neurotransmission are the subject of ongoing research.
Dr. Solleveld reported having no financial conflicts of interest.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Sleep efficiency in boys with ADHD improved significantly by 5% in response to methylphenidate therapy.
Data source: This randomized, double-blind, placebo-controlled clinical trial included 50 medication-naive boys aged 10-12 years with ADHD.
Disclosures: The study presenter reported having no financial conflicts of interest.
ADHD meds don’t raise seizure risk in epilepsy patients
PARIS – The use of attention-deficit/hyperactivity disorder medications is not associated with increased risk of epileptic seizures in patients with both disorders, according to an analysis of Swedish national registry data.
“Seizure history should not exempt patients from ADHD medication treatment,” Isabell Brikell stated at the annual congress of the European College of Neuropsychopharmacology.

“That’s why it’s such an important question, whether ADHD medications increase the risk of seizures,” observed Ms. Brikell, a PhD candidate in psychiatric genetics and epidemiology at the Karolinska Institute in Stockholm.
Swedish health care registries are famously comprehensive. For example, the Swedish prescription medication registry that Ms. Brikell and her coinvestigators tapped into for their ADHD/epilepsy study contains information on 99% of all prescriptions ordered in the country since 2005.
She reported on 38,247 Swedish patients with epilepsy born during 1976-2008 and followed during 2006-2013. Forty-eight percent were female. They collectively experienced 30,093 acute epileptic seizures of sufficient severity that they presented to a hospital for an unplanned visit. When the investigators compared the rate of seizures while the patients with ADHD were on a collective 4,248 ADHD medication exposure periods to that of epilepsy patients without ADHD, they found that the seizure risk was actually 17% lower in ADHD patients while on medication. This difference fell just shy of statistical significance. The analysis was adjusted for gender, age, and time on ADHD medications.
However, Ms. Brikell and her coworkers also performed a separate analysis for each individual with ADHD in which they compared seizure rates when a given patient was on ADHD medication versus off medication, a design that controls for many of the potential confounding factors that can occur with observational data. The seizure risk proved to be 19% lower while an individual was on ADHD medication – and this difference was indeed statistically significant.
In an interview, Ms. Brikell noted that the Swedish data are confirmed by a much larger National Institute of Mental Health–sponsored American study she was involved with that is now under review for publication. The U.S. study, which used the enormous MarketScan private health insurance database, demonstrated with the power provided by very large patient numbers that the seizure risk was convincingly lower while dual-diagnosis patients were on ADHD medication than when they were off.
“It’s reassuring to see the same effect across two countries with such different health care systems,” she commented.
Epilepsy is known to be inherently associated with a threefold increased prevalence of ADHD.
Ms. Brikell’s study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. She reported having no financial conflicts of interest.
PARIS – The use of attention-deficit/hyperactivity disorder medications is not associated with increased risk of epileptic seizures in patients with both disorders, according to an analysis of Swedish national registry data.
“Seizure history should not exempt patients from ADHD medication treatment,” Isabell Brikell stated at the annual congress of the European College of Neuropsychopharmacology.

“That’s why it’s such an important question, whether ADHD medications increase the risk of seizures,” observed Ms. Brikell, a PhD candidate in psychiatric genetics and epidemiology at the Karolinska Institute in Stockholm.
Swedish health care registries are famously comprehensive. For example, the Swedish prescription medication registry that Ms. Brikell and her coinvestigators tapped into for their ADHD/epilepsy study contains information on 99% of all prescriptions ordered in the country since 2005.
She reported on 38,247 Swedish patients with epilepsy born during 1976-2008 and followed during 2006-2013. Forty-eight percent were female. They collectively experienced 30,093 acute epileptic seizures of sufficient severity that they presented to a hospital for an unplanned visit. When the investigators compared the rate of seizures while the patients with ADHD were on a collective 4,248 ADHD medication exposure periods to that of epilepsy patients without ADHD, they found that the seizure risk was actually 17% lower in ADHD patients while on medication. This difference fell just shy of statistical significance. The analysis was adjusted for gender, age, and time on ADHD medications.
However, Ms. Brikell and her coworkers also performed a separate analysis for each individual with ADHD in which they compared seizure rates when a given patient was on ADHD medication versus off medication, a design that controls for many of the potential confounding factors that can occur with observational data. The seizure risk proved to be 19% lower while an individual was on ADHD medication – and this difference was indeed statistically significant.
In an interview, Ms. Brikell noted that the Swedish data are confirmed by a much larger National Institute of Mental Health–sponsored American study she was involved with that is now under review for publication. The U.S. study, which used the enormous MarketScan private health insurance database, demonstrated with the power provided by very large patient numbers that the seizure risk was convincingly lower while dual-diagnosis patients were on ADHD medication than when they were off.
“It’s reassuring to see the same effect across two countries with such different health care systems,” she commented.
Epilepsy is known to be inherently associated with a threefold increased prevalence of ADHD.
Ms. Brikell’s study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. She reported having no financial conflicts of interest.
PARIS – The use of attention-deficit/hyperactivity disorder medications is not associated with increased risk of epileptic seizures in patients with both disorders, according to an analysis of Swedish national registry data.
“Seizure history should not exempt patients from ADHD medication treatment,” Isabell Brikell stated at the annual congress of the European College of Neuropsychopharmacology.

“That’s why it’s such an important question, whether ADHD medications increase the risk of seizures,” observed Ms. Brikell, a PhD candidate in psychiatric genetics and epidemiology at the Karolinska Institute in Stockholm.
Swedish health care registries are famously comprehensive. For example, the Swedish prescription medication registry that Ms. Brikell and her coinvestigators tapped into for their ADHD/epilepsy study contains information on 99% of all prescriptions ordered in the country since 2005.
She reported on 38,247 Swedish patients with epilepsy born during 1976-2008 and followed during 2006-2013. Forty-eight percent were female. They collectively experienced 30,093 acute epileptic seizures of sufficient severity that they presented to a hospital for an unplanned visit. When the investigators compared the rate of seizures while the patients with ADHD were on a collective 4,248 ADHD medication exposure periods to that of epilepsy patients without ADHD, they found that the seizure risk was actually 17% lower in ADHD patients while on medication. This difference fell just shy of statistical significance. The analysis was adjusted for gender, age, and time on ADHD medications.
However, Ms. Brikell and her coworkers also performed a separate analysis for each individual with ADHD in which they compared seizure rates when a given patient was on ADHD medication versus off medication, a design that controls for many of the potential confounding factors that can occur with observational data. The seizure risk proved to be 19% lower while an individual was on ADHD medication – and this difference was indeed statistically significant.
In an interview, Ms. Brikell noted that the Swedish data are confirmed by a much larger National Institute of Mental Health–sponsored American study she was involved with that is now under review for publication. The U.S. study, which used the enormous MarketScan private health insurance database, demonstrated with the power provided by very large patient numbers that the seizure risk was convincingly lower while dual-diagnosis patients were on ADHD medication than when they were off.
“It’s reassuring to see the same effect across two countries with such different health care systems,” she commented.
Epilepsy is known to be inherently associated with a threefold increased prevalence of ADHD.
Ms. Brikell’s study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. She reported having no financial conflicts of interest.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Patients with ADHD and a history of epilepsy were at 19% lower risk of experiencing seizures while on ADHD medication than when off it.
Data source: This was an observational study of prospectively collected data on all of the nearly 40,000 Swedish patients with epilepsy born during 1976-2008.
Disclosures: The study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. The presenter reported having no financial conflicts of interest.
Alternative therapies
Alternative therapies, from vitamins and supplements to meditation and acupuncture, have become increasingly popular treatments in the United States for many medical problems in the past few decades. In 2008, the National Institutes of Health reported that nearly 40% of adults and 12% of children had used “complementary or alternative medicine” (CAM) in the preceding year. Other surveys have suggested that closer to 30% of general pediatric patients and as many as 75% of adolescent patients have used CAM at least once. These treatments are especially popular for chronic conditions that are managed but not usually cured with current evidence-based treatments. Psychiatric conditions in childhood sometimes have a long course, and have effective but controversial treatments, as with stimulants for ADHD. Parents sometimes feel guilty about their child’s problem and want to use “natural” methods or deny the accepted understanding of their child’s illness. So it is not surprising that families may investigate alternative treatments, and such treatments have multiplied.
While there is evidence that parents and patients rarely discuss these treatments with their physicians, it is critical that you know what therapies your patients are using. You should focus on tolerance in the context of protecting the child from harm and improving the child’s functioning. If you have ever recommended chicken soup for a cold, then you have prescribed complementary medicine, so it is not a stretch for you to offer some input about the other alternative therapies your patients may be considering.
It is important to note that rigorous, case-controlled studies of efficacy of most alternative therapies are few in number and usually small in size (so any evidence of efficacy is weaker), and that the products themselves are not regulated by the Food and Drug Administration or other public body. This means that the family (and you) will have to do some homework to ensure that the therapy they purchase comes from a reputable source and is what it purports to be.
Many of the alternative therapies patients are investigating will be herbs or supplements. Omega-3 fatty acids are critical to multiple essential body functions, and are taken in primarily via certain foods, primarily fish and certain seeds and nuts. A deficiency in certain omega-3 fatty acids can cause problems in infant neurological development and put one at risk for heart disease, rheumatologic illness, and depression. Supplementation with Omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA], specifically) has a solid evidence base as an effective adjunctive treatment for depression and bipolar disorder in adults. In addition, randomized, placebo-controlled, double-blind studies have demonstrated efficacy in treatment of children with mild to moderate ADHD at doses of 1,200 mg/day. There are some studies that have demonstrated improvement in hyperactivity in children with autism with supplementation at similar doses. These supplements have very low risk of side effects. They are a reasonable recommendation to your patients whose children have mild to moderate ADHD, and they want to manage it without stimulants.
Families also may be considering physical or mechanical treatments. Acupuncture has demonstrated efficacy in the treatment of fatigue and pain, migraines, and addiction, although there are very few studies in children and adolescents. There is some evidence for its efficacy in treatment of mild to moderate depression and anxiety in adults, but again no research has been done in youth. Hypnotherapy has shown modest efficacy in treatment of anticipatory anxiety symptoms, headache, chronic pain, nausea and vomiting, migraines, hair-pulling and skin picking as well as compulsive eating and smoking cessation in adults. There is some clinical evidence for its efficacy in children and adolescents, and its safety is well established. Massage therapy has shown value in improving mood and behavior in children with ADHD, but not efficacy as a first-line treatment for ADHD symptoms. Chiropractic care, which is among the most commonly used alternative therapies, claims to be effective for the treatment of anxiety, depression, ADHD, behavioral problems of autism and even schizophrenia and bipolar disorder, but there is no significant scientific evidence to support these claims. And neurofeedback, which is a variant of biofeedback in which patients practice calming themselves or improving focus while watching an EEG has shown modest efficacy in the treatment of ADHD in children in early studies. It is worth noting that all of these therapies may be costly and not covered by insurance.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Additional readings
1. Child Adolesc Psychiatr Clin N Am. 2013 Jul;22(3):375-80.
2. J Am Acad Child Adolesc Psychiatry. 2008;47(4):364-8.
3. J Am Acad Child Adolesc Psychiatry. 2011;50(10):991-1000.
4. J Am Acad Child Adolesc Psychiatry. 2014 Jun; 53(6):658-66.
5. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):S168-9.
Alternative therapies, from vitamins and supplements to meditation and acupuncture, have become increasingly popular treatments in the United States for many medical problems in the past few decades. In 2008, the National Institutes of Health reported that nearly 40% of adults and 12% of children had used “complementary or alternative medicine” (CAM) in the preceding year. Other surveys have suggested that closer to 30% of general pediatric patients and as many as 75% of adolescent patients have used CAM at least once. These treatments are especially popular for chronic conditions that are managed but not usually cured with current evidence-based treatments. Psychiatric conditions in childhood sometimes have a long course, and have effective but controversial treatments, as with stimulants for ADHD. Parents sometimes feel guilty about their child’s problem and want to use “natural” methods or deny the accepted understanding of their child’s illness. So it is not surprising that families may investigate alternative treatments, and such treatments have multiplied.
While there is evidence that parents and patients rarely discuss these treatments with their physicians, it is critical that you know what therapies your patients are using. You should focus on tolerance in the context of protecting the child from harm and improving the child’s functioning. If you have ever recommended chicken soup for a cold, then you have prescribed complementary medicine, so it is not a stretch for you to offer some input about the other alternative therapies your patients may be considering.
It is important to note that rigorous, case-controlled studies of efficacy of most alternative therapies are few in number and usually small in size (so any evidence of efficacy is weaker), and that the products themselves are not regulated by the Food and Drug Administration or other public body. This means that the family (and you) will have to do some homework to ensure that the therapy they purchase comes from a reputable source and is what it purports to be.
Many of the alternative therapies patients are investigating will be herbs or supplements. Omega-3 fatty acids are critical to multiple essential body functions, and are taken in primarily via certain foods, primarily fish and certain seeds and nuts. A deficiency in certain omega-3 fatty acids can cause problems in infant neurological development and put one at risk for heart disease, rheumatologic illness, and depression. Supplementation with Omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA], specifically) has a solid evidence base as an effective adjunctive treatment for depression and bipolar disorder in adults. In addition, randomized, placebo-controlled, double-blind studies have demonstrated efficacy in treatment of children with mild to moderate ADHD at doses of 1,200 mg/day. There are some studies that have demonstrated improvement in hyperactivity in children with autism with supplementation at similar doses. These supplements have very low risk of side effects. They are a reasonable recommendation to your patients whose children have mild to moderate ADHD, and they want to manage it without stimulants.
Families also may be considering physical or mechanical treatments. Acupuncture has demonstrated efficacy in the treatment of fatigue and pain, migraines, and addiction, although there are very few studies in children and adolescents. There is some evidence for its efficacy in treatment of mild to moderate depression and anxiety in adults, but again no research has been done in youth. Hypnotherapy has shown modest efficacy in treatment of anticipatory anxiety symptoms, headache, chronic pain, nausea and vomiting, migraines, hair-pulling and skin picking as well as compulsive eating and smoking cessation in adults. There is some clinical evidence for its efficacy in children and adolescents, and its safety is well established. Massage therapy has shown value in improving mood and behavior in children with ADHD, but not efficacy as a first-line treatment for ADHD symptoms. Chiropractic care, which is among the most commonly used alternative therapies, claims to be effective for the treatment of anxiety, depression, ADHD, behavioral problems of autism and even schizophrenia and bipolar disorder, but there is no significant scientific evidence to support these claims. And neurofeedback, which is a variant of biofeedback in which patients practice calming themselves or improving focus while watching an EEG has shown modest efficacy in the treatment of ADHD in children in early studies. It is worth noting that all of these therapies may be costly and not covered by insurance.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Additional readings
1. Child Adolesc Psychiatr Clin N Am. 2013 Jul;22(3):375-80.
2. J Am Acad Child Adolesc Psychiatry. 2008;47(4):364-8.
3. J Am Acad Child Adolesc Psychiatry. 2011;50(10):991-1000.
4. J Am Acad Child Adolesc Psychiatry. 2014 Jun; 53(6):658-66.
5. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):S168-9.
Alternative therapies, from vitamins and supplements to meditation and acupuncture, have become increasingly popular treatments in the United States for many medical problems in the past few decades. In 2008, the National Institutes of Health reported that nearly 40% of adults and 12% of children had used “complementary or alternative medicine” (CAM) in the preceding year. Other surveys have suggested that closer to 30% of general pediatric patients and as many as 75% of adolescent patients have used CAM at least once. These treatments are especially popular for chronic conditions that are managed but not usually cured with current evidence-based treatments. Psychiatric conditions in childhood sometimes have a long course, and have effective but controversial treatments, as with stimulants for ADHD. Parents sometimes feel guilty about their child’s problem and want to use “natural” methods or deny the accepted understanding of their child’s illness. So it is not surprising that families may investigate alternative treatments, and such treatments have multiplied.
While there is evidence that parents and patients rarely discuss these treatments with their physicians, it is critical that you know what therapies your patients are using. You should focus on tolerance in the context of protecting the child from harm and improving the child’s functioning. If you have ever recommended chicken soup for a cold, then you have prescribed complementary medicine, so it is not a stretch for you to offer some input about the other alternative therapies your patients may be considering.
It is important to note that rigorous, case-controlled studies of efficacy of most alternative therapies are few in number and usually small in size (so any evidence of efficacy is weaker), and that the products themselves are not regulated by the Food and Drug Administration or other public body. This means that the family (and you) will have to do some homework to ensure that the therapy they purchase comes from a reputable source and is what it purports to be.
Many of the alternative therapies patients are investigating will be herbs or supplements. Omega-3 fatty acids are critical to multiple essential body functions, and are taken in primarily via certain foods, primarily fish and certain seeds and nuts. A deficiency in certain omega-3 fatty acids can cause problems in infant neurological development and put one at risk for heart disease, rheumatologic illness, and depression. Supplementation with Omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA], specifically) has a solid evidence base as an effective adjunctive treatment for depression and bipolar disorder in adults. In addition, randomized, placebo-controlled, double-blind studies have demonstrated efficacy in treatment of children with mild to moderate ADHD at doses of 1,200 mg/day. There are some studies that have demonstrated improvement in hyperactivity in children with autism with supplementation at similar doses. These supplements have very low risk of side effects. They are a reasonable recommendation to your patients whose children have mild to moderate ADHD, and they want to manage it without stimulants.
Families also may be considering physical or mechanical treatments. Acupuncture has demonstrated efficacy in the treatment of fatigue and pain, migraines, and addiction, although there are very few studies in children and adolescents. There is some evidence for its efficacy in treatment of mild to moderate depression and anxiety in adults, but again no research has been done in youth. Hypnotherapy has shown modest efficacy in treatment of anticipatory anxiety symptoms, headache, chronic pain, nausea and vomiting, migraines, hair-pulling and skin picking as well as compulsive eating and smoking cessation in adults. There is some clinical evidence for its efficacy in children and adolescents, and its safety is well established. Massage therapy has shown value in improving mood and behavior in children with ADHD, but not efficacy as a first-line treatment for ADHD symptoms. Chiropractic care, which is among the most commonly used alternative therapies, claims to be effective for the treatment of anxiety, depression, ADHD, behavioral problems of autism and even schizophrenia and bipolar disorder, but there is no significant scientific evidence to support these claims. And neurofeedback, which is a variant of biofeedback in which patients practice calming themselves or improving focus while watching an EEG has shown modest efficacy in the treatment of ADHD in children in early studies. It is worth noting that all of these therapies may be costly and not covered by insurance.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Additional readings
1. Child Adolesc Psychiatr Clin N Am. 2013 Jul;22(3):375-80.
2. J Am Acad Child Adolesc Psychiatry. 2008;47(4):364-8.
3. J Am Acad Child Adolesc Psychiatry. 2011;50(10):991-1000.
4. J Am Acad Child Adolesc Psychiatry. 2014 Jun; 53(6):658-66.
5. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):S168-9.
AMA’s stance on choline, prenatal vitamins could bring ‘staggering’ results
For quite some time now, I’ve been urging my colleagues to follow the science on the powerful impact of choline on the brain.
In May 2017, based on studies using genetically altered mice that show the developmental changes of Down syndrome and Alzheimer’s disease at 6 months, I raised the question of whether prenatal choline could lead to the prevention of Alzheimer’s.
Thanks to the leadership of Niva Lubin-Johnson, MD, now president-elect of the National Medical Association, while a member and immediate past chair of the American Medical Association’s minority affairs section governing council*, the AMA ’s delegates passed a resolution to support an increase in choline in prenatal vitamins.
If the prenatal vitamin companies take the AMA’s resolution to heart and put more choline in their prenatal vitamins or if physicians in the United States pay attention to the AMA’s action and recommend pregnant women ensure they get adequate choline in their diets, the benefit to Americans’ public health could be staggering. Currently, it is known that choline deficiency – usually brought about by fetal alcohol exposure – is a public health problem, and choline deficiency is the leading preventable cause of intellectual disability. Public health efforts aimed at preventing intellectual disabilities from fetal alcohol exposure are designed to warn women about the risks of drinking during pregnancy; while this effort is commendable, it does not solve a very common problem – namely, women’s engaging in social drinking before they realize they are pregnant. (Psychiatric Serv. 2015 66[5]:539-42).
The late Julius B. Richmond, MD, former director of the Institute for Juvenile Research, surgeon general under former President Jimmy Carter, and one of the founders of Head Start under former President Lyndon B. Johnson, used to say that, in order to institutionalize a public policy, you need a solid scientific basis for the policy, a mechanism to actualize the policy, and the “political will” to do so. The AMA’s recommendation has the Institute of Medicine’s science behind it, so putting choline in prenatal vitamins or having physicians recommend that pregnant women get adequate doses of choline should be pretty easy to actualize. The political will to do this extremely important, biotechnical preventive intervention should be a no-brainer.
Should this AMA recommendation gain the traction it deserves, the American people might see a substantial decrease in the prevalence of premature and low-birth-weight infants, intellectual disability, ADHD, speech and language difficulties, epilepsy, heart defects, schizophrenia, Alzheimer’s disease, depression, school failure, juvenile delinquency, violence, and suicide – all of which seem to be tied to choline deficiency.
*This story was updated August 17, 2017.
For quite some time now, I’ve been urging my colleagues to follow the science on the powerful impact of choline on the brain.
In May 2017, based on studies using genetically altered mice that show the developmental changes of Down syndrome and Alzheimer’s disease at 6 months, I raised the question of whether prenatal choline could lead to the prevention of Alzheimer’s.
Thanks to the leadership of Niva Lubin-Johnson, MD, now president-elect of the National Medical Association, while a member and immediate past chair of the American Medical Association’s minority affairs section governing council*, the AMA ’s delegates passed a resolution to support an increase in choline in prenatal vitamins.
If the prenatal vitamin companies take the AMA’s resolution to heart and put more choline in their prenatal vitamins or if physicians in the United States pay attention to the AMA’s action and recommend pregnant women ensure they get adequate choline in their diets, the benefit to Americans’ public health could be staggering. Currently, it is known that choline deficiency – usually brought about by fetal alcohol exposure – is a public health problem, and choline deficiency is the leading preventable cause of intellectual disability. Public health efforts aimed at preventing intellectual disabilities from fetal alcohol exposure are designed to warn women about the risks of drinking during pregnancy; while this effort is commendable, it does not solve a very common problem – namely, women’s engaging in social drinking before they realize they are pregnant. (Psychiatric Serv. 2015 66[5]:539-42).
The late Julius B. Richmond, MD, former director of the Institute for Juvenile Research, surgeon general under former President Jimmy Carter, and one of the founders of Head Start under former President Lyndon B. Johnson, used to say that, in order to institutionalize a public policy, you need a solid scientific basis for the policy, a mechanism to actualize the policy, and the “political will” to do so. The AMA’s recommendation has the Institute of Medicine’s science behind it, so putting choline in prenatal vitamins or having physicians recommend that pregnant women get adequate doses of choline should be pretty easy to actualize. The political will to do this extremely important, biotechnical preventive intervention should be a no-brainer.
Should this AMA recommendation gain the traction it deserves, the American people might see a substantial decrease in the prevalence of premature and low-birth-weight infants, intellectual disability, ADHD, speech and language difficulties, epilepsy, heart defects, schizophrenia, Alzheimer’s disease, depression, school failure, juvenile delinquency, violence, and suicide – all of which seem to be tied to choline deficiency.
*This story was updated August 17, 2017.
For quite some time now, I’ve been urging my colleagues to follow the science on the powerful impact of choline on the brain.
In May 2017, based on studies using genetically altered mice that show the developmental changes of Down syndrome and Alzheimer’s disease at 6 months, I raised the question of whether prenatal choline could lead to the prevention of Alzheimer’s.
Thanks to the leadership of Niva Lubin-Johnson, MD, now president-elect of the National Medical Association, while a member and immediate past chair of the American Medical Association’s minority affairs section governing council*, the AMA ’s delegates passed a resolution to support an increase in choline in prenatal vitamins.
If the prenatal vitamin companies take the AMA’s resolution to heart and put more choline in their prenatal vitamins or if physicians in the United States pay attention to the AMA’s action and recommend pregnant women ensure they get adequate choline in their diets, the benefit to Americans’ public health could be staggering. Currently, it is known that choline deficiency – usually brought about by fetal alcohol exposure – is a public health problem, and choline deficiency is the leading preventable cause of intellectual disability. Public health efforts aimed at preventing intellectual disabilities from fetal alcohol exposure are designed to warn women about the risks of drinking during pregnancy; while this effort is commendable, it does not solve a very common problem – namely, women’s engaging in social drinking before they realize they are pregnant. (Psychiatric Serv. 2015 66[5]:539-42).
The late Julius B. Richmond, MD, former director of the Institute for Juvenile Research, surgeon general under former President Jimmy Carter, and one of the founders of Head Start under former President Lyndon B. Johnson, used to say that, in order to institutionalize a public policy, you need a solid scientific basis for the policy, a mechanism to actualize the policy, and the “political will” to do so. The AMA’s recommendation has the Institute of Medicine’s science behind it, so putting choline in prenatal vitamins or having physicians recommend that pregnant women get adequate doses of choline should be pretty easy to actualize. The political will to do this extremely important, biotechnical preventive intervention should be a no-brainer.
Should this AMA recommendation gain the traction it deserves, the American people might see a substantial decrease in the prevalence of premature and low-birth-weight infants, intellectual disability, ADHD, speech and language difficulties, epilepsy, heart defects, schizophrenia, Alzheimer’s disease, depression, school failure, juvenile delinquency, violence, and suicide – all of which seem to be tied to choline deficiency.
*This story was updated August 17, 2017.
Triple-bead mixed amphetamine salt for ADHD
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
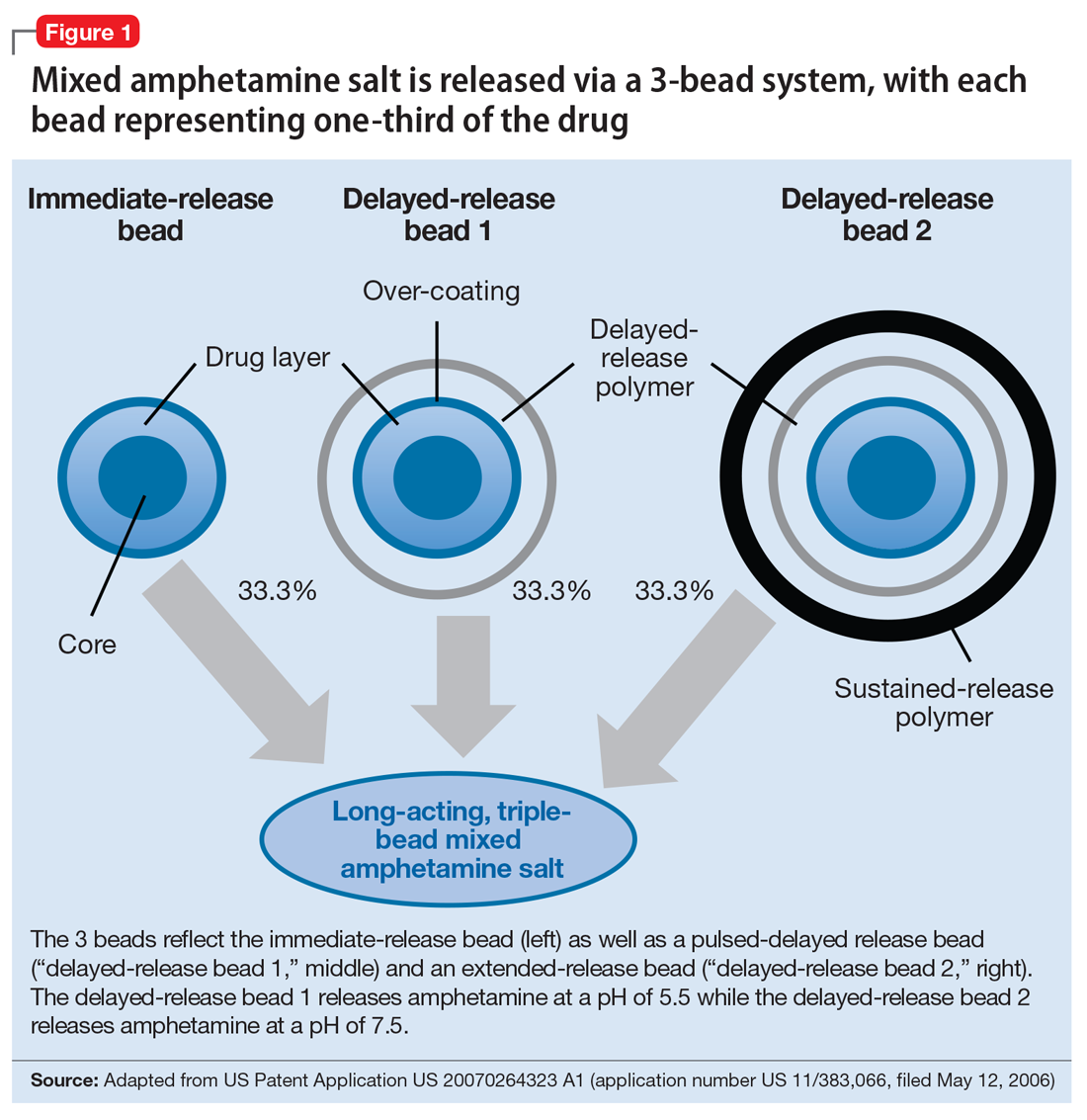
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
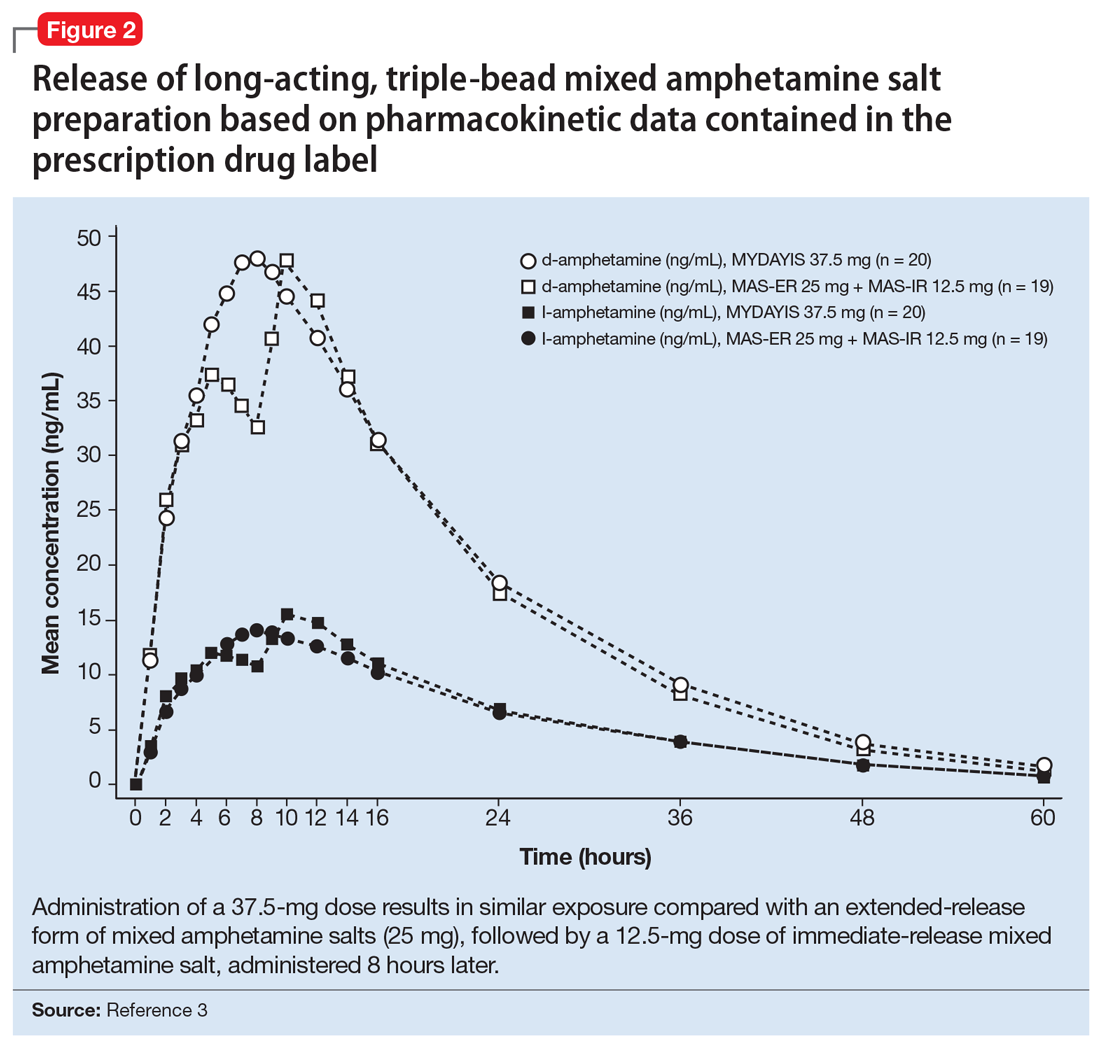
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3

In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3

Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3

Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3

In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3

Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3

Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3

In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.















