User login
Anxiety, depression prevalent in children with comorbid autism and ADHD
Children with comorbid autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of anxiety and mood disorders, a cross-sectional analysis has shown.
“Our study supports that anxiety and mood disorders, although highly prevalent in those with ASD alone, are even more prevalent in individuals who have ADHD,” wrote Eliza Gordon-Lipkin, MD, of the Kennedy Krieger Institute, Baltimore, and her associates. ”The identification of psychiatric conditions in children with ASD is important because these disorders are treatable and affect quality of life.”
The study was published in Pediatrics.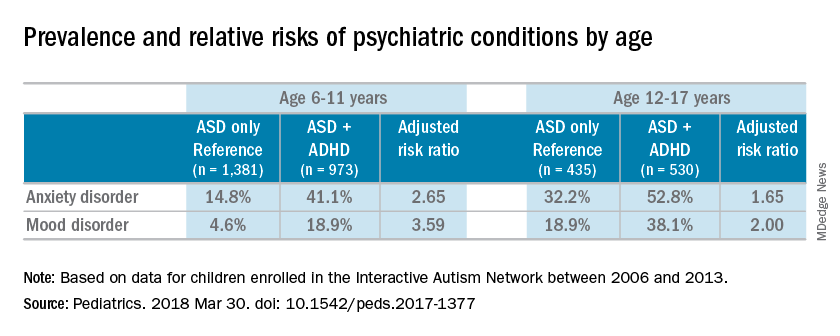
Most of the children were male (83%), white (87%), and non-Hispanic (92%); the mean age of the children was 10 years. Almost half of the children in the study had parent-reported ADHD (45%). Almost one-third of patients were diagnosed with an anxiety disorder (31%), and many also were reported to have been diagnosed with a mood disorder (16%). An increased risk of reported anxiety disorder was found in patients with both ADHD and ASD (adjusted relative risk, 2.20; 95% confidence interval, 1.97-2.46).
The researchers also found an increased risk of mood disorders (aRR, 2.72; 95% CI, 2.28-3.24) among children with comorbid conditions. Those risks increased with age (both P less than .001). An increased prevalence of anxiety and mood disorders was found in adolescents, compared with school-aged children with both ASD and ADHD or ASD alone. But higher relative risk ratios were found for the younger children, compared with the adolescents for those in the ADHD/ASD group and the ASD alone group.
“This suggests that or more likely to exhibit detectable symptoms at an earlier age,” reported Dr. Gordon-Lipkin, also with the department of pediatrics at Johns Hopkins University, in Baltimore.
The research team cited several limitations. For example, patient-reported data might be subject to recall or reporting biases. Also, computer and Internet access was required to complete the IAN questionnaires, which means that the findings could be biased toward people of higher socioeconomic status.
Nevertheless, the researchers wrote, their study is the largest to compare comorbidities in patients with ASD and ADHD, or ASD alone.
Further research is needed to better understand the relationship between ASD and ADHD. “ADHD affects nearly half of the children with ASD. This subgroup of individuals with ASD may represent a distinct clinical phenotype, with different diagnostic and therapeutic implications,” Dr. Gordon-Lipkin and her associates wrote. “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
None of the study authors had relevant financial disclosures to report. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
SOURCE: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
The work of Gordon-Lipkin et al. is one of the largest studies analyzing the relationships between autism, ADHD, and anxiety and mood disorders. But because of the inherent behavioral and biological complexity of autism, changes in the diagnostic criteria, and the use of parent-reported data, the current study might not reflect what is truly occurring in patients with autism, Christopher J. McDougle, MD, said in an interview.
“There are a number of things to say about [the study]. [One] of the strengths of the paper [is] the sample size,” Dr. McDougle said.“It’s always good to have a big sample size. The downside to having informant-databased information is that it is exactly what it is. This is fine, but the information may be inaccurate.”
In addition to parent-reported data, physicians are dealing with the relatively new diagnostic criteria. The May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders to the DSM-5 brought with it the ability to diagnose ADHD with autism, when just the day before the DSM-5 was released, this differential diagnosis was not listed in the manual, Dr. McDougle said. “If something that important can change with the strike of the clock, it makes me concerned.” He also said listing the differential diagnosis in the diagnostic manual underscored the uncertainty of medicine’s understanding of comorbid autism and ADHD.
“That’s reflective of the field’s lack of knowledge. Sometimes I think we like to portray things as though we understand what’s going on, when I think it’s better to be honest and say we really don’t; we are just doing our best.”
Dr. McDougle is the director of the Lurie Center for Autism at Massachusetts General Hospital and is the Nancy Lurie Marks Professor of Psychiatry at Harvard Medical Center, both in Boston. He treats children, adolescents, and adults with autism spectrum disorder and other neurodevelopmental disorders. He was asked to comment on this study.
The work of Gordon-Lipkin et al. is one of the largest studies analyzing the relationships between autism, ADHD, and anxiety and mood disorders. But because of the inherent behavioral and biological complexity of autism, changes in the diagnostic criteria, and the use of parent-reported data, the current study might not reflect what is truly occurring in patients with autism, Christopher J. McDougle, MD, said in an interview.
“There are a number of things to say about [the study]. [One] of the strengths of the paper [is] the sample size,” Dr. McDougle said.“It’s always good to have a big sample size. The downside to having informant-databased information is that it is exactly what it is. This is fine, but the information may be inaccurate.”
In addition to parent-reported data, physicians are dealing with the relatively new diagnostic criteria. The May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders to the DSM-5 brought with it the ability to diagnose ADHD with autism, when just the day before the DSM-5 was released, this differential diagnosis was not listed in the manual, Dr. McDougle said. “If something that important can change with the strike of the clock, it makes me concerned.” He also said listing the differential diagnosis in the diagnostic manual underscored the uncertainty of medicine’s understanding of comorbid autism and ADHD.
“That’s reflective of the field’s lack of knowledge. Sometimes I think we like to portray things as though we understand what’s going on, when I think it’s better to be honest and say we really don’t; we are just doing our best.”
Dr. McDougle is the director of the Lurie Center for Autism at Massachusetts General Hospital and is the Nancy Lurie Marks Professor of Psychiatry at Harvard Medical Center, both in Boston. He treats children, adolescents, and adults with autism spectrum disorder and other neurodevelopmental disorders. He was asked to comment on this study.
The work of Gordon-Lipkin et al. is one of the largest studies analyzing the relationships between autism, ADHD, and anxiety and mood disorders. But because of the inherent behavioral and biological complexity of autism, changes in the diagnostic criteria, and the use of parent-reported data, the current study might not reflect what is truly occurring in patients with autism, Christopher J. McDougle, MD, said in an interview.
“There are a number of things to say about [the study]. [One] of the strengths of the paper [is] the sample size,” Dr. McDougle said.“It’s always good to have a big sample size. The downside to having informant-databased information is that it is exactly what it is. This is fine, but the information may be inaccurate.”
In addition to parent-reported data, physicians are dealing with the relatively new diagnostic criteria. The May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders to the DSM-5 brought with it the ability to diagnose ADHD with autism, when just the day before the DSM-5 was released, this differential diagnosis was not listed in the manual, Dr. McDougle said. “If something that important can change with the strike of the clock, it makes me concerned.” He also said listing the differential diagnosis in the diagnostic manual underscored the uncertainty of medicine’s understanding of comorbid autism and ADHD.
“That’s reflective of the field’s lack of knowledge. Sometimes I think we like to portray things as though we understand what’s going on, when I think it’s better to be honest and say we really don’t; we are just doing our best.”
Dr. McDougle is the director of the Lurie Center for Autism at Massachusetts General Hospital and is the Nancy Lurie Marks Professor of Psychiatry at Harvard Medical Center, both in Boston. He treats children, adolescents, and adults with autism spectrum disorder and other neurodevelopmental disorders. He was asked to comment on this study.
Children with comorbid autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of anxiety and mood disorders, a cross-sectional analysis has shown.
“Our study supports that anxiety and mood disorders, although highly prevalent in those with ASD alone, are even more prevalent in individuals who have ADHD,” wrote Eliza Gordon-Lipkin, MD, of the Kennedy Krieger Institute, Baltimore, and her associates. ”The identification of psychiatric conditions in children with ASD is important because these disorders are treatable and affect quality of life.”
The study was published in Pediatrics.
Most of the children were male (83%), white (87%), and non-Hispanic (92%); the mean age of the children was 10 years. Almost half of the children in the study had parent-reported ADHD (45%). Almost one-third of patients were diagnosed with an anxiety disorder (31%), and many also were reported to have been diagnosed with a mood disorder (16%). An increased risk of reported anxiety disorder was found in patients with both ADHD and ASD (adjusted relative risk, 2.20; 95% confidence interval, 1.97-2.46).
The researchers also found an increased risk of mood disorders (aRR, 2.72; 95% CI, 2.28-3.24) among children with comorbid conditions. Those risks increased with age (both P less than .001). An increased prevalence of anxiety and mood disorders was found in adolescents, compared with school-aged children with both ASD and ADHD or ASD alone. But higher relative risk ratios were found for the younger children, compared with the adolescents for those in the ADHD/ASD group and the ASD alone group.
“This suggests that or more likely to exhibit detectable symptoms at an earlier age,” reported Dr. Gordon-Lipkin, also with the department of pediatrics at Johns Hopkins University, in Baltimore.
The research team cited several limitations. For example, patient-reported data might be subject to recall or reporting biases. Also, computer and Internet access was required to complete the IAN questionnaires, which means that the findings could be biased toward people of higher socioeconomic status.
Nevertheless, the researchers wrote, their study is the largest to compare comorbidities in patients with ASD and ADHD, or ASD alone.
Further research is needed to better understand the relationship between ASD and ADHD. “ADHD affects nearly half of the children with ASD. This subgroup of individuals with ASD may represent a distinct clinical phenotype, with different diagnostic and therapeutic implications,” Dr. Gordon-Lipkin and her associates wrote. “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
None of the study authors had relevant financial disclosures to report. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
SOURCE: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
Children with comorbid autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of anxiety and mood disorders, a cross-sectional analysis has shown.
“Our study supports that anxiety and mood disorders, although highly prevalent in those with ASD alone, are even more prevalent in individuals who have ADHD,” wrote Eliza Gordon-Lipkin, MD, of the Kennedy Krieger Institute, Baltimore, and her associates. ”The identification of psychiatric conditions in children with ASD is important because these disorders are treatable and affect quality of life.”
The study was published in Pediatrics.
Most of the children were male (83%), white (87%), and non-Hispanic (92%); the mean age of the children was 10 years. Almost half of the children in the study had parent-reported ADHD (45%). Almost one-third of patients were diagnosed with an anxiety disorder (31%), and many also were reported to have been diagnosed with a mood disorder (16%). An increased risk of reported anxiety disorder was found in patients with both ADHD and ASD (adjusted relative risk, 2.20; 95% confidence interval, 1.97-2.46).
The researchers also found an increased risk of mood disorders (aRR, 2.72; 95% CI, 2.28-3.24) among children with comorbid conditions. Those risks increased with age (both P less than .001). An increased prevalence of anxiety and mood disorders was found in adolescents, compared with school-aged children with both ASD and ADHD or ASD alone. But higher relative risk ratios were found for the younger children, compared with the adolescents for those in the ADHD/ASD group and the ASD alone group.
“This suggests that or more likely to exhibit detectable symptoms at an earlier age,” reported Dr. Gordon-Lipkin, also with the department of pediatrics at Johns Hopkins University, in Baltimore.
The research team cited several limitations. For example, patient-reported data might be subject to recall or reporting biases. Also, computer and Internet access was required to complete the IAN questionnaires, which means that the findings could be biased toward people of higher socioeconomic status.
Nevertheless, the researchers wrote, their study is the largest to compare comorbidities in patients with ASD and ADHD, or ASD alone.
Further research is needed to better understand the relationship between ASD and ADHD. “ADHD affects nearly half of the children with ASD. This subgroup of individuals with ASD may represent a distinct clinical phenotype, with different diagnostic and therapeutic implications,” Dr. Gordon-Lipkin and her associates wrote. “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
None of the study authors had relevant financial disclosures to report. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
SOURCE: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
FROM PEDIATRICS
Key clinical point: “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
Major finding: Sixteen percent of the children with autistic spectrum disorder had a mood disorder, and 31% had an anxiety disorder.
Study details: A cross-sectional analysis of information on 3,319 patients, obtained between 2006 and 2013 in the Interactive Autism Network (IAN), an online autism research registry that uses parent report information.
Disclosures: None of the study authors reported relevant financial disclosures. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
Source: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
Early reading aloud, play reduced hyperactivity at school entry
and had sustained behavioral effects after the program was completed, according to results of a randomized clinical trial.
The Video Interaction Project (VIP), in which parents review and reflect upon recordings of themselves interacting with their children, is a low-cost, scalable intervention that has a “high potential” for enhancing social and emotional development by reducing disruptive behaviors, the study authors reported in Pediatrics.
The study included 675 parent-child dyads enrolled post partum at an urban public hospital serving low-income families. Of that group, 450 families were randomized to the VIP program from 0 to 3 years of age, a control group, or a third group that included a different intervention called Building Blocks that incorporates parenting education newsletters, learning materials, and parent questionnaires.
In the VIP intervention, parent-child dyads participated in up to 15 one-on-one sessions from 2 weeks of age to 3 years. In each 30-minute session, the parent and child were video recorded for 5 minutes of play or shared reading; immediately afterward, the parent would review the video with a bilingual facilitator to identify positive interactions and reflect on them.
As previously reported, the VIP intervention had enhanced children’s social and emotional development. Compared with controls, children in the VIP group had higher scores in imitation/play and attention at the end of the program and lower scores in separation distress, hyperactivity, and externalizing problems, according to investigators.
Now, investigators are reporting results that include a second phase of random assignment to VIP from 3-5 years or a control group. The second-phase VIP intervention included nine 30- to 45-minute sessions enhanced with new strategies designed to support the rapidly emerging developmental capacities of preschoolers, Dr. Mendelsohn and associates said. Ultimately, 252 families completed the 4.5 year assessment.
Those new strategies included building sessions around themes (such as birthday party), incorporation of writing into play (such as party invitations), focusing on story characters’ feelings, and video recording both reading and play, with the story serving as the basis for the play.
The initial VIP 0-3 year intervention and the VIP 3-5 year intervention were both independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with Cohen’s d effect sizes ranging from approximately –0.25 to –0.30, according to investigators.
Participating in both VIP interventions was associated with a significant reduction in hyperactivity (effect size, –0.63; P = 0.001), Dr. Mendelsohn and his associates also reported.
Moreover, participation in the first VIP session was associated with a reduction in clinically significant hyperactivity (relative risk reduction, 69%; P = .03), they added.
The cost of the VIP program for 0-3 years is approximately $175-$200 per child per year, including staff, equipment, rent, and other expenses, according to the report, which notes that one interventionist can provide services for 400-500 families.
Taken together, these findings suggest the VIP intervention is a low-cost intervention that may prevent poverty-related disparities, investigators said.
“In this study, we provide strong support for the use of pediatric primary care to promote positive parenting activities such as reading aloud and play and the potential for such programs to promote social-emotional development as reflected through reductions in disruptive behaviors,” they wrote.
Dr. Mendelsohn and his coauthors reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
SOURCE: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
and had sustained behavioral effects after the program was completed, according to results of a randomized clinical trial.
The Video Interaction Project (VIP), in which parents review and reflect upon recordings of themselves interacting with their children, is a low-cost, scalable intervention that has a “high potential” for enhancing social and emotional development by reducing disruptive behaviors, the study authors reported in Pediatrics.
The study included 675 parent-child dyads enrolled post partum at an urban public hospital serving low-income families. Of that group, 450 families were randomized to the VIP program from 0 to 3 years of age, a control group, or a third group that included a different intervention called Building Blocks that incorporates parenting education newsletters, learning materials, and parent questionnaires.
In the VIP intervention, parent-child dyads participated in up to 15 one-on-one sessions from 2 weeks of age to 3 years. In each 30-minute session, the parent and child were video recorded for 5 minutes of play or shared reading; immediately afterward, the parent would review the video with a bilingual facilitator to identify positive interactions and reflect on them.
As previously reported, the VIP intervention had enhanced children’s social and emotional development. Compared with controls, children in the VIP group had higher scores in imitation/play and attention at the end of the program and lower scores in separation distress, hyperactivity, and externalizing problems, according to investigators.
Now, investigators are reporting results that include a second phase of random assignment to VIP from 3-5 years or a control group. The second-phase VIP intervention included nine 30- to 45-minute sessions enhanced with new strategies designed to support the rapidly emerging developmental capacities of preschoolers, Dr. Mendelsohn and associates said. Ultimately, 252 families completed the 4.5 year assessment.
Those new strategies included building sessions around themes (such as birthday party), incorporation of writing into play (such as party invitations), focusing on story characters’ feelings, and video recording both reading and play, with the story serving as the basis for the play.
The initial VIP 0-3 year intervention and the VIP 3-5 year intervention were both independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with Cohen’s d effect sizes ranging from approximately –0.25 to –0.30, according to investigators.
Participating in both VIP interventions was associated with a significant reduction in hyperactivity (effect size, –0.63; P = 0.001), Dr. Mendelsohn and his associates also reported.
Moreover, participation in the first VIP session was associated with a reduction in clinically significant hyperactivity (relative risk reduction, 69%; P = .03), they added.
The cost of the VIP program for 0-3 years is approximately $175-$200 per child per year, including staff, equipment, rent, and other expenses, according to the report, which notes that one interventionist can provide services for 400-500 families.
Taken together, these findings suggest the VIP intervention is a low-cost intervention that may prevent poverty-related disparities, investigators said.
“In this study, we provide strong support for the use of pediatric primary care to promote positive parenting activities such as reading aloud and play and the potential for such programs to promote social-emotional development as reflected through reductions in disruptive behaviors,” they wrote.
Dr. Mendelsohn and his coauthors reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
SOURCE: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
and had sustained behavioral effects after the program was completed, according to results of a randomized clinical trial.
The Video Interaction Project (VIP), in which parents review and reflect upon recordings of themselves interacting with their children, is a low-cost, scalable intervention that has a “high potential” for enhancing social and emotional development by reducing disruptive behaviors, the study authors reported in Pediatrics.
The study included 675 parent-child dyads enrolled post partum at an urban public hospital serving low-income families. Of that group, 450 families were randomized to the VIP program from 0 to 3 years of age, a control group, or a third group that included a different intervention called Building Blocks that incorporates parenting education newsletters, learning materials, and parent questionnaires.
In the VIP intervention, parent-child dyads participated in up to 15 one-on-one sessions from 2 weeks of age to 3 years. In each 30-minute session, the parent and child were video recorded for 5 minutes of play or shared reading; immediately afterward, the parent would review the video with a bilingual facilitator to identify positive interactions and reflect on them.
As previously reported, the VIP intervention had enhanced children’s social and emotional development. Compared with controls, children in the VIP group had higher scores in imitation/play and attention at the end of the program and lower scores in separation distress, hyperactivity, and externalizing problems, according to investigators.
Now, investigators are reporting results that include a second phase of random assignment to VIP from 3-5 years or a control group. The second-phase VIP intervention included nine 30- to 45-minute sessions enhanced with new strategies designed to support the rapidly emerging developmental capacities of preschoolers, Dr. Mendelsohn and associates said. Ultimately, 252 families completed the 4.5 year assessment.
Those new strategies included building sessions around themes (such as birthday party), incorporation of writing into play (such as party invitations), focusing on story characters’ feelings, and video recording both reading and play, with the story serving as the basis for the play.
The initial VIP 0-3 year intervention and the VIP 3-5 year intervention were both independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with Cohen’s d effect sizes ranging from approximately –0.25 to –0.30, according to investigators.
Participating in both VIP interventions was associated with a significant reduction in hyperactivity (effect size, –0.63; P = 0.001), Dr. Mendelsohn and his associates also reported.
Moreover, participation in the first VIP session was associated with a reduction in clinically significant hyperactivity (relative risk reduction, 69%; P = .03), they added.
The cost of the VIP program for 0-3 years is approximately $175-$200 per child per year, including staff, equipment, rent, and other expenses, according to the report, which notes that one interventionist can provide services for 400-500 families.
Taken together, these findings suggest the VIP intervention is a low-cost intervention that may prevent poverty-related disparities, investigators said.
“In this study, we provide strong support for the use of pediatric primary care to promote positive parenting activities such as reading aloud and play and the potential for such programs to promote social-emotional development as reflected through reductions in disruptive behaviors,” they wrote.
Dr. Mendelsohn and his coauthors reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
SOURCE: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
FROM PEDIATRICS
Key clinical point: A video-based intervention designed to promote parent-child reading aloud and play reduced hyperactivity at school entry and had sustained behavioral effects over time.
Major finding: Parent-child participation in the Video Interaction Project (VIP) was independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with effect sizes ranging from approximately –0.25 to –0.30.
Study details: A randomized controlled trial including 450 families enrolled at an urban public hospital that serves low-income families.
Disclosures: The researchers reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
Source: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
Children, adolescents with TBI at risk of secondary ADHD
FROM JAMA PEDIATRICS
Children and adolescents with traumatic brain injury (TBI) might be at increased risk of developing attention-deficit/hyperactivity disorder (ADHD) years after the injury, a prospective cohort study published March 19 shows.
Severe TBI was associated with significantly increased risk of new onset ADHD versus controls in the study, which was based on parent-completed assessments done as late as 6.8 years after the initial injury, according to results presented in JAMA Pediatrics.
Although children with severe TBI were at highest risk, those with less severe TBI had about twice the risk of developing ADHD, compared with control subjects who had no brain injury, the study results suggest.
Taken together, the findings suggest a need for long-term monitoring for attention problems, wrote investigator Megan E. Narad, PhD, of Cincinnati Children’s Hospital Medical Center, and her co-authors.
“Physicians and other clinicians should continue to be vigilant in monitoring attention problems in patients with a history of brain injury, even if it has been a number of years since the injury, the injury was moderate in nature, or the patient experienced a predominantly positive recovery,” Dr. Narad and her colleagues wrote.
The results were based on long-term analysis of 187 children who were hospitalized for TBI or orthopedic injury between the ages of 3 and 7 years. That group included 81 children with TBI and 106 with orthopedic injury.
Parents completed assessments soon after the injury, then again at 6 months, 12 months, 18 months, 3.4 years, and 6.8 years afterward, according to the study.
Over the full follow-up period, 48 children (25.7%) met the investigators’ definition of “secondary ADHD,” or onset of ADHD symptoms after an injury. They found that compared with orthopedic injury, the severe TBI was associated with new ADHD (hazard ratio, 3.62; 95% confidence interval, 1.59-8.26), the investigators reported.
In patients with mild or moderate TBI, associations with new onset ADHD did not meet the statistical significance threshol. However, compared with the orthopedic injury group, the risk for ADHD in TBI severity subgroups were up to 4 times higher.
This is not the first study showing an elevated risk of ADHD in TBI patients, but .
“Although most children with severe TBI who developed secondary ADHD did so within the first 18 months after injury, a portion of those with complicated mild and moderate TBI demonstrated new onset of secondary ADHD at the final two assessments, highlighting the importance of continued monitoring even years after TBI,” Dr. Narad and her colleagues wrote.
The study was funded by several sources, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the state of Ohio’s Emergency Medical Services.
Dr. Narad reported no relevant disclosures. Other study authors reported disclosures related to Akili Interactive Labs, Multi-Health Systems, Optimal Medicine, and IXICO.
SOURCE: Narad ME et al. JAMA Pediatr. 2018 Mar 19. doi: 10.1001/jamapediatrics.2017.5746.
FROM JAMA PEDIATRICS
Children and adolescents with traumatic brain injury (TBI) might be at increased risk of developing attention-deficit/hyperactivity disorder (ADHD) years after the injury, a prospective cohort study published March 19 shows.
Severe TBI was associated with significantly increased risk of new onset ADHD versus controls in the study, which was based on parent-completed assessments done as late as 6.8 years after the initial injury, according to results presented in JAMA Pediatrics.
Although children with severe TBI were at highest risk, those with less severe TBI had about twice the risk of developing ADHD, compared with control subjects who had no brain injury, the study results suggest.
Taken together, the findings suggest a need for long-term monitoring for attention problems, wrote investigator Megan E. Narad, PhD, of Cincinnati Children’s Hospital Medical Center, and her co-authors.
“Physicians and other clinicians should continue to be vigilant in monitoring attention problems in patients with a history of brain injury, even if it has been a number of years since the injury, the injury was moderate in nature, or the patient experienced a predominantly positive recovery,” Dr. Narad and her colleagues wrote.
The results were based on long-term analysis of 187 children who were hospitalized for TBI or orthopedic injury between the ages of 3 and 7 years. That group included 81 children with TBI and 106 with orthopedic injury.
Parents completed assessments soon after the injury, then again at 6 months, 12 months, 18 months, 3.4 years, and 6.8 years afterward, according to the study.
Over the full follow-up period, 48 children (25.7%) met the investigators’ definition of “secondary ADHD,” or onset of ADHD symptoms after an injury. They found that compared with orthopedic injury, the severe TBI was associated with new ADHD (hazard ratio, 3.62; 95% confidence interval, 1.59-8.26), the investigators reported.
In patients with mild or moderate TBI, associations with new onset ADHD did not meet the statistical significance threshol. However, compared with the orthopedic injury group, the risk for ADHD in TBI severity subgroups were up to 4 times higher.
This is not the first study showing an elevated risk of ADHD in TBI patients, but .
“Although most children with severe TBI who developed secondary ADHD did so within the first 18 months after injury, a portion of those with complicated mild and moderate TBI demonstrated new onset of secondary ADHD at the final two assessments, highlighting the importance of continued monitoring even years after TBI,” Dr. Narad and her colleagues wrote.
The study was funded by several sources, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the state of Ohio’s Emergency Medical Services.
Dr. Narad reported no relevant disclosures. Other study authors reported disclosures related to Akili Interactive Labs, Multi-Health Systems, Optimal Medicine, and IXICO.
SOURCE: Narad ME et al. JAMA Pediatr. 2018 Mar 19. doi: 10.1001/jamapediatrics.2017.5746.
FROM JAMA PEDIATRICS
Children and adolescents with traumatic brain injury (TBI) might be at increased risk of developing attention-deficit/hyperactivity disorder (ADHD) years after the injury, a prospective cohort study published March 19 shows.
Severe TBI was associated with significantly increased risk of new onset ADHD versus controls in the study, which was based on parent-completed assessments done as late as 6.8 years after the initial injury, according to results presented in JAMA Pediatrics.
Although children with severe TBI were at highest risk, those with less severe TBI had about twice the risk of developing ADHD, compared with control subjects who had no brain injury, the study results suggest.
Taken together, the findings suggest a need for long-term monitoring for attention problems, wrote investigator Megan E. Narad, PhD, of Cincinnati Children’s Hospital Medical Center, and her co-authors.
“Physicians and other clinicians should continue to be vigilant in monitoring attention problems in patients with a history of brain injury, even if it has been a number of years since the injury, the injury was moderate in nature, or the patient experienced a predominantly positive recovery,” Dr. Narad and her colleagues wrote.
The results were based on long-term analysis of 187 children who were hospitalized for TBI or orthopedic injury between the ages of 3 and 7 years. That group included 81 children with TBI and 106 with orthopedic injury.
Parents completed assessments soon after the injury, then again at 6 months, 12 months, 18 months, 3.4 years, and 6.8 years afterward, according to the study.
Over the full follow-up period, 48 children (25.7%) met the investigators’ definition of “secondary ADHD,” or onset of ADHD symptoms after an injury. They found that compared with orthopedic injury, the severe TBI was associated with new ADHD (hazard ratio, 3.62; 95% confidence interval, 1.59-8.26), the investigators reported.
In patients with mild or moderate TBI, associations with new onset ADHD did not meet the statistical significance threshol. However, compared with the orthopedic injury group, the risk for ADHD in TBI severity subgroups were up to 4 times higher.
This is not the first study showing an elevated risk of ADHD in TBI patients, but .
“Although most children with severe TBI who developed secondary ADHD did so within the first 18 months after injury, a portion of those with complicated mild and moderate TBI demonstrated new onset of secondary ADHD at the final two assessments, highlighting the importance of continued monitoring even years after TBI,” Dr. Narad and her colleagues wrote.
The study was funded by several sources, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the state of Ohio’s Emergency Medical Services.
Dr. Narad reported no relevant disclosures. Other study authors reported disclosures related to Akili Interactive Labs, Multi-Health Systems, Optimal Medicine, and IXICO.
SOURCE: Narad ME et al. JAMA Pediatr. 2018 Mar 19. doi: 10.1001/jamapediatrics.2017.5746.
Key clinical point: Children and adolescents with traumatic brain injury (TBI) should continue to be monitored for possible attention problems many years after the injury.
Major finding: In assessments taken up to 6.8 years after injury, severe TBI was associated with secondary ADHD, compared with a control group (hazard ratio, 3.62; 95% confidence interval, 1.59-8.26).
Study details: Analysis of a prospective concurrent cohort study including 187 children aged 3 to 7 years who were hospitalized for TBI or orthopedic injury.
Disclosures: The study was funded by several sources, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the state of Ohio’s Emergency Medical Services. The authors reported conflict of interest disclosures related to Akili Interactive Labs, Multi-Health Systems, Optimal Medicine, and IXICO.
Source: Narad ME et al. JAMA Pediatr. 2018 Mar 19. doi:10.1001/jamapediatrics.2017.5746.
Interventions ‘key’ when ADHD, conduct disorder, and delinquency overlap
LAS VEGAS – The overlap of ADHD, conduct disorder, substance use disorder, and criminality likely reflect related underlying mechanisms, which may elucidate different developmental pathways of offending.
“Early interventions are key,” Praveen R. Kambam, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
According to Dr. Kambam, a clinical and forensic psychiatrist at the University of California, Los Angeles, ADHD is overrepresented in correctional settings worldwide, especially the hyperactive-impulsive subtype. “In juvenile settings, ADHD rates are 3-4 times higher than rates in the general population,” he said. “If you combine juvenile and adult prison populations worldwide, the rates are about 2-5 times higher than the general population.”
The risks are increased for comorbid oppositional defiant disorder (ODD) and conduct disorder. In fact, ADHD and conduct disorder co-occur in about 50% of cases. In girls, the prevalence rate of conduct disorder is steady at 0.8% around age 5 years and increases to 2.8% around age 15 years, while in boys, conduct disorder is steady at 2.1% around age 5 years and rises to 5.5% at age 15 years.
According to a literature review of 18 prospective studies, 13 retrospective studies, and four reviews, individuals with ADHD plus or minus conduct disorder had an increased the risk of antisocial personality disorder, and those with ADHD plus conduct disorder had an increased risk of criminality (J Atten Disord. 2016;20[10]:815-24). “So it’s a subtle difference, where antisocial personality disorder and criminality are slightly different,” Dr. Kambam said. “It could be that the diagnostic criteria are catching the same thing. However, the added [conduct disorder] suggests that there may be subpopulations that are vulnerable.”
He went on to note that individuals with ADHD and delinquency tend to have more learning problems, poor academic achievement, peer relationship problems, and risk of social rejection, while individuals with oppositional defiant disorder and delinquency tend to have peer relationship problems, a negative parent-child relationship, and increased risk of developing conduct disorder.
ADHD is associated with alcohol and drug use in adulthood and nicotine use in adolescence. “Comorbidity between ADHD and ODD/[conduct disorder] is robustly related to substance outcomes,” Dr. Kambam said. “However, both initiation and continuation of substance use disorder are more likely when ADHD symptoms are present, even when controlling for ODD/[conduct disorder]. As for substance use disorder [SUD] and delinquency, the onset of delinquency is more likely in children with onset of SUD by age 11, and SUDs are closely linked with criminality in both juveniles and adults.”
Comorbidity of SUD with conduct disorder and ADHD likely reflects multifactorial mechanisms, he said, such as inherent novelty seeking or school failure leading to association with antisocial peers. Risk factors for chronic offending include early onset of criminal behaviors, ADHD plus conduct disorder, and ODD. ADHD has an independent yet weaker relationship with antisocial behaviors as well, while ADHD, conduct disorder, and SUD are independently associated with increased recidivism.
Environmental factors for chronic offending include the home environment, peer response, parenting skills, and in utero exposures and perinatal complications. “Whether ADHD develops into more severe conduct problems depends considerably on exposure to potentiating environmental factors,” Dr. Kambam said. “The converse is also true: Low-risk environments promote desistance from this pathway in impulsive boys.” He added that the chronic offenders/criminality pathway likely stems from underlying mechanisms, such as impulsivity, low self-control, and executive dysfunction.
If left untreated, ADHD is associated with poor academic and employment outcomes, SUDs, depression, bipolar disorder, suicide attempts, vehicular accidents, and use of mental health services. “The economic costs are estimated to be $42.5 billion annually, so it has a large impact,” he said.
Limited evidence exists to support pharmacological treatments for conduct disorder, although stimulants/alpha-agonists, antipsychotics, lithium, and mood stabilizers may offer some benefit for target symptoms. “Most of the treatment data center around multisystemic therapy, including behavioral modification/parent management training, and functional family training,” Dr. Kambam said. “Treating disruptive behavior disorders and SUDs are
likely to reduce criminality and recidivism, particularly if started early. There are many beneficial economic impacts. Think about the cost of having youth detained in the criminal justice systems. In Los Angeles County, that cost is about $230,000 per year per kid. That money can probably be better spent somewhere else.”
Numerous studies show that the nonmedical use of stimulants ranges from 25%-40%. “They’re mostly used to enhance academic and/or work performance, but some are used for euphoric effect,” he said. “Individuals in college and just out of college seem to be at the highest risk. There is a strong relationship between [conduct disorder]/[antisocial personality disorder] or SUDs and nonmedical use.”
Treatment with stimulants in correctional settings is controversial. “Some say try after failure of nonstimulants, while others say never use them due to substance abuse, misuse, intimidation of patients to surrender medication, and security/costs,” Dr. Kambam said. “The protocol for ADHD treatment in Massachusetts prisons calls for use of nonstimulants first, followed by ‘crushable’ stimulants if indicated.” The methylphenidate patch and lisdexamfetamine also can be effective in the incarcerated population.
Dr. Kambam reported having no financial disclosures.
SOURCE: Kambam PR. NPA 2018.
LAS VEGAS – The overlap of ADHD, conduct disorder, substance use disorder, and criminality likely reflect related underlying mechanisms, which may elucidate different developmental pathways of offending.
“Early interventions are key,” Praveen R. Kambam, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
According to Dr. Kambam, a clinical and forensic psychiatrist at the University of California, Los Angeles, ADHD is overrepresented in correctional settings worldwide, especially the hyperactive-impulsive subtype. “In juvenile settings, ADHD rates are 3-4 times higher than rates in the general population,” he said. “If you combine juvenile and adult prison populations worldwide, the rates are about 2-5 times higher than the general population.”
The risks are increased for comorbid oppositional defiant disorder (ODD) and conduct disorder. In fact, ADHD and conduct disorder co-occur in about 50% of cases. In girls, the prevalence rate of conduct disorder is steady at 0.8% around age 5 years and increases to 2.8% around age 15 years, while in boys, conduct disorder is steady at 2.1% around age 5 years and rises to 5.5% at age 15 years.
According to a literature review of 18 prospective studies, 13 retrospective studies, and four reviews, individuals with ADHD plus or minus conduct disorder had an increased the risk of antisocial personality disorder, and those with ADHD plus conduct disorder had an increased risk of criminality (J Atten Disord. 2016;20[10]:815-24). “So it’s a subtle difference, where antisocial personality disorder and criminality are slightly different,” Dr. Kambam said. “It could be that the diagnostic criteria are catching the same thing. However, the added [conduct disorder] suggests that there may be subpopulations that are vulnerable.”
He went on to note that individuals with ADHD and delinquency tend to have more learning problems, poor academic achievement, peer relationship problems, and risk of social rejection, while individuals with oppositional defiant disorder and delinquency tend to have peer relationship problems, a negative parent-child relationship, and increased risk of developing conduct disorder.
ADHD is associated with alcohol and drug use in adulthood and nicotine use in adolescence. “Comorbidity between ADHD and ODD/[conduct disorder] is robustly related to substance outcomes,” Dr. Kambam said. “However, both initiation and continuation of substance use disorder are more likely when ADHD symptoms are present, even when controlling for ODD/[conduct disorder]. As for substance use disorder [SUD] and delinquency, the onset of delinquency is more likely in children with onset of SUD by age 11, and SUDs are closely linked with criminality in both juveniles and adults.”
Comorbidity of SUD with conduct disorder and ADHD likely reflects multifactorial mechanisms, he said, such as inherent novelty seeking or school failure leading to association with antisocial peers. Risk factors for chronic offending include early onset of criminal behaviors, ADHD plus conduct disorder, and ODD. ADHD has an independent yet weaker relationship with antisocial behaviors as well, while ADHD, conduct disorder, and SUD are independently associated with increased recidivism.
Environmental factors for chronic offending include the home environment, peer response, parenting skills, and in utero exposures and perinatal complications. “Whether ADHD develops into more severe conduct problems depends considerably on exposure to potentiating environmental factors,” Dr. Kambam said. “The converse is also true: Low-risk environments promote desistance from this pathway in impulsive boys.” He added that the chronic offenders/criminality pathway likely stems from underlying mechanisms, such as impulsivity, low self-control, and executive dysfunction.
If left untreated, ADHD is associated with poor academic and employment outcomes, SUDs, depression, bipolar disorder, suicide attempts, vehicular accidents, and use of mental health services. “The economic costs are estimated to be $42.5 billion annually, so it has a large impact,” he said.
Limited evidence exists to support pharmacological treatments for conduct disorder, although stimulants/alpha-agonists, antipsychotics, lithium, and mood stabilizers may offer some benefit for target symptoms. “Most of the treatment data center around multisystemic therapy, including behavioral modification/parent management training, and functional family training,” Dr. Kambam said. “Treating disruptive behavior disorders and SUDs are
likely to reduce criminality and recidivism, particularly if started early. There are many beneficial economic impacts. Think about the cost of having youth detained in the criminal justice systems. In Los Angeles County, that cost is about $230,000 per year per kid. That money can probably be better spent somewhere else.”
Numerous studies show that the nonmedical use of stimulants ranges from 25%-40%. “They’re mostly used to enhance academic and/or work performance, but some are used for euphoric effect,” he said. “Individuals in college and just out of college seem to be at the highest risk. There is a strong relationship between [conduct disorder]/[antisocial personality disorder] or SUDs and nonmedical use.”
Treatment with stimulants in correctional settings is controversial. “Some say try after failure of nonstimulants, while others say never use them due to substance abuse, misuse, intimidation of patients to surrender medication, and security/costs,” Dr. Kambam said. “The protocol for ADHD treatment in Massachusetts prisons calls for use of nonstimulants first, followed by ‘crushable’ stimulants if indicated.” The methylphenidate patch and lisdexamfetamine also can be effective in the incarcerated population.
Dr. Kambam reported having no financial disclosures.
SOURCE: Kambam PR. NPA 2018.
LAS VEGAS – The overlap of ADHD, conduct disorder, substance use disorder, and criminality likely reflect related underlying mechanisms, which may elucidate different developmental pathways of offending.
“Early interventions are key,” Praveen R. Kambam, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
According to Dr. Kambam, a clinical and forensic psychiatrist at the University of California, Los Angeles, ADHD is overrepresented in correctional settings worldwide, especially the hyperactive-impulsive subtype. “In juvenile settings, ADHD rates are 3-4 times higher than rates in the general population,” he said. “If you combine juvenile and adult prison populations worldwide, the rates are about 2-5 times higher than the general population.”
The risks are increased for comorbid oppositional defiant disorder (ODD) and conduct disorder. In fact, ADHD and conduct disorder co-occur in about 50% of cases. In girls, the prevalence rate of conduct disorder is steady at 0.8% around age 5 years and increases to 2.8% around age 15 years, while in boys, conduct disorder is steady at 2.1% around age 5 years and rises to 5.5% at age 15 years.
According to a literature review of 18 prospective studies, 13 retrospective studies, and four reviews, individuals with ADHD plus or minus conduct disorder had an increased the risk of antisocial personality disorder, and those with ADHD plus conduct disorder had an increased risk of criminality (J Atten Disord. 2016;20[10]:815-24). “So it’s a subtle difference, where antisocial personality disorder and criminality are slightly different,” Dr. Kambam said. “It could be that the diagnostic criteria are catching the same thing. However, the added [conduct disorder] suggests that there may be subpopulations that are vulnerable.”
He went on to note that individuals with ADHD and delinquency tend to have more learning problems, poor academic achievement, peer relationship problems, and risk of social rejection, while individuals with oppositional defiant disorder and delinquency tend to have peer relationship problems, a negative parent-child relationship, and increased risk of developing conduct disorder.
ADHD is associated with alcohol and drug use in adulthood and nicotine use in adolescence. “Comorbidity between ADHD and ODD/[conduct disorder] is robustly related to substance outcomes,” Dr. Kambam said. “However, both initiation and continuation of substance use disorder are more likely when ADHD symptoms are present, even when controlling for ODD/[conduct disorder]. As for substance use disorder [SUD] and delinquency, the onset of delinquency is more likely in children with onset of SUD by age 11, and SUDs are closely linked with criminality in both juveniles and adults.”
Comorbidity of SUD with conduct disorder and ADHD likely reflects multifactorial mechanisms, he said, such as inherent novelty seeking or school failure leading to association with antisocial peers. Risk factors for chronic offending include early onset of criminal behaviors, ADHD plus conduct disorder, and ODD. ADHD has an independent yet weaker relationship with antisocial behaviors as well, while ADHD, conduct disorder, and SUD are independently associated with increased recidivism.
Environmental factors for chronic offending include the home environment, peer response, parenting skills, and in utero exposures and perinatal complications. “Whether ADHD develops into more severe conduct problems depends considerably on exposure to potentiating environmental factors,” Dr. Kambam said. “The converse is also true: Low-risk environments promote desistance from this pathway in impulsive boys.” He added that the chronic offenders/criminality pathway likely stems from underlying mechanisms, such as impulsivity, low self-control, and executive dysfunction.
If left untreated, ADHD is associated with poor academic and employment outcomes, SUDs, depression, bipolar disorder, suicide attempts, vehicular accidents, and use of mental health services. “The economic costs are estimated to be $42.5 billion annually, so it has a large impact,” he said.
Limited evidence exists to support pharmacological treatments for conduct disorder, although stimulants/alpha-agonists, antipsychotics, lithium, and mood stabilizers may offer some benefit for target symptoms. “Most of the treatment data center around multisystemic therapy, including behavioral modification/parent management training, and functional family training,” Dr. Kambam said. “Treating disruptive behavior disorders and SUDs are
likely to reduce criminality and recidivism, particularly if started early. There are many beneficial economic impacts. Think about the cost of having youth detained in the criminal justice systems. In Los Angeles County, that cost is about $230,000 per year per kid. That money can probably be better spent somewhere else.”
Numerous studies show that the nonmedical use of stimulants ranges from 25%-40%. “They’re mostly used to enhance academic and/or work performance, but some are used for euphoric effect,” he said. “Individuals in college and just out of college seem to be at the highest risk. There is a strong relationship between [conduct disorder]/[antisocial personality disorder] or SUDs and nonmedical use.”
Treatment with stimulants in correctional settings is controversial. “Some say try after failure of nonstimulants, while others say never use them due to substance abuse, misuse, intimidation of patients to surrender medication, and security/costs,” Dr. Kambam said. “The protocol for ADHD treatment in Massachusetts prisons calls for use of nonstimulants first, followed by ‘crushable’ stimulants if indicated.” The methylphenidate patch and lisdexamfetamine also can be effective in the incarcerated population.
Dr. Kambam reported having no financial disclosures.
SOURCE: Kambam PR. NPA 2018.
REPORTING FROM NPA 2018
Rapid weight loss, irritability, and nausea after restarting ADHD treatment
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
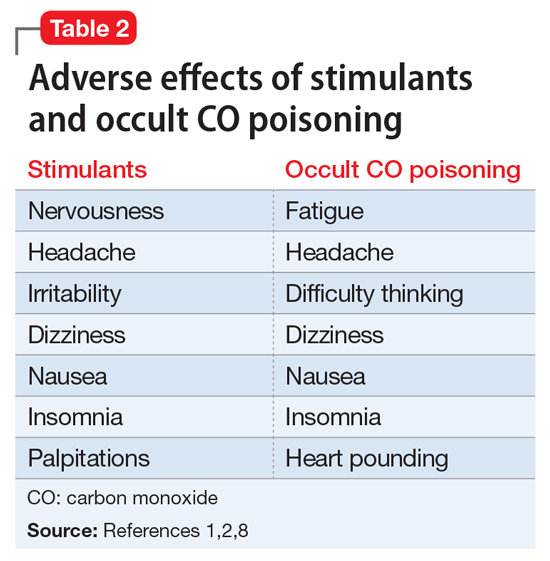
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.
[polldaddy:9928298]
The author’s observations
Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
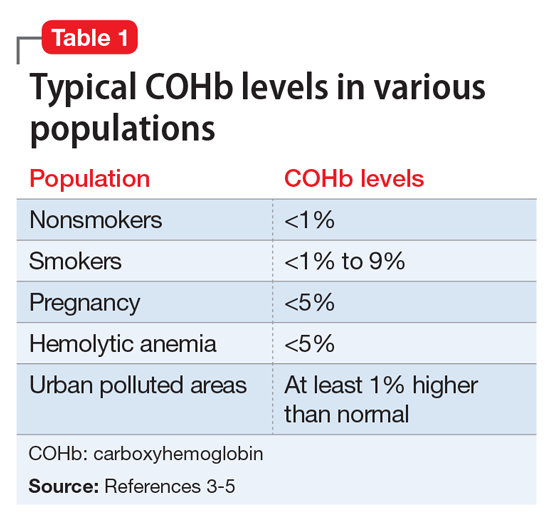
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.

[polldaddy:9928298]
The author’s observations

Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.

[polldaddy:9928298]
The author’s observations

Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
Reactive aggressive disorder in children with ADHD is looking for a name
NEW YORK – according to Robert L. Findling, MD.
Emphasizing the reactive component to this behavioral problem, he said: “They look okay until someone bumps into them at school. They do not have a mood disorder. They have a disorder of reactivity.”
The hurdle is that there is no accepted terminology to encourage clinicians to identify and initiate treatment in children with this behavior. The term conduct disorder has been used in the past, but Dr. Findling said that care delivered for conduct disorder is not reimbursable. This may be among the reasons that aggressive reactive behavior of ADHD is overlooked – even though treatment is likely to improve long-term outcome.
“I wish I had a magic label for this, but I don’t,” Dr. Findling said. However, he maintained that most clinicians who work with ADHD children are familiar with this type of behavior. Indeed, clinicians “grapple with this day to day. We all see these kids, and they are oftentimes the most impaired kids in our practices,” he said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
This behavior should not be confused with the aggression associated with mood disorders, such as disruptive mood dysregulation disorder (DMDD) or bipolar disease, according to Dr. Findling. Children with DMDD, for example, are chronically irritable or angry. Although bipolar disorder patients with aggressive behavior are not necessarily angry between episodes, they also have persistent mood disturbances.
In contrast, preadolescent children with ADHD who have episodes of aggression, a symptom far more common among males than females, do not otherwise exhibit disturbances in mood. In addition, the episodes of impulsive, reactive aggression are provoked. They require a perceived insult, threat, or similar trigger.
While many of these children continue to have episodes of impulsive aggressive behavior even on treatment effective for other ADHD symptoms, Dr. Findling said, “The good news is that there are treatments for aggression.” In addition to psychosocial support aimed at reducing aggressive behavior, once the diagnosis has been made, these include adjusting ADHD treatments to better target symptoms of episodic aggression. If needed, therapies known to treat aggression, such as atypical antipsychotics, anticonvulsants, or lithium also are options.
Dr. Findling did review one older double-blind study that associated methylphenidate with a reduction in aggression in children with conduct disorder, but said he believes that there is no guarantee for a response from any treatment. Rather, he recommended empirical strategies for symptom management and keeping in mind the benefit-to-risk relationship when considering treatments that impose a high burden of adverse events.
However, the first step to treatment is recognizing the problem.
“In my opinion, what is missing is the nosology for these kids,” Dr. Findling said. An evidence-based label will help increase awareness of the problem and encourage more extensive clinical study, he said.
“These children are not rare and they are really impaired. It is heartbreaking, because when you talk to them when they are still little, they know what people think of them. They know their teachers don’t like them. They know their parents think they’re bad. They know their peers are scared of them, and they cannot make friends,” he said. However, there is a potential for reversing these problems if treatment is initiated early.
“As you watch them get older, you watch them scarring over,” he added.
Dr. Findling reported financial ties with numerous pharmaceutical companies.
SOURCE: Findling RL. Psychopharmacology Update Institute
NEW YORK – according to Robert L. Findling, MD.
Emphasizing the reactive component to this behavioral problem, he said: “They look okay until someone bumps into them at school. They do not have a mood disorder. They have a disorder of reactivity.”
The hurdle is that there is no accepted terminology to encourage clinicians to identify and initiate treatment in children with this behavior. The term conduct disorder has been used in the past, but Dr. Findling said that care delivered for conduct disorder is not reimbursable. This may be among the reasons that aggressive reactive behavior of ADHD is overlooked – even though treatment is likely to improve long-term outcome.
“I wish I had a magic label for this, but I don’t,” Dr. Findling said. However, he maintained that most clinicians who work with ADHD children are familiar with this type of behavior. Indeed, clinicians “grapple with this day to day. We all see these kids, and they are oftentimes the most impaired kids in our practices,” he said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
This behavior should not be confused with the aggression associated with mood disorders, such as disruptive mood dysregulation disorder (DMDD) or bipolar disease, according to Dr. Findling. Children with DMDD, for example, are chronically irritable or angry. Although bipolar disorder patients with aggressive behavior are not necessarily angry between episodes, they also have persistent mood disturbances.
In contrast, preadolescent children with ADHD who have episodes of aggression, a symptom far more common among males than females, do not otherwise exhibit disturbances in mood. In addition, the episodes of impulsive, reactive aggression are provoked. They require a perceived insult, threat, or similar trigger.
While many of these children continue to have episodes of impulsive aggressive behavior even on treatment effective for other ADHD symptoms, Dr. Findling said, “The good news is that there are treatments for aggression.” In addition to psychosocial support aimed at reducing aggressive behavior, once the diagnosis has been made, these include adjusting ADHD treatments to better target symptoms of episodic aggression. If needed, therapies known to treat aggression, such as atypical antipsychotics, anticonvulsants, or lithium also are options.
Dr. Findling did review one older double-blind study that associated methylphenidate with a reduction in aggression in children with conduct disorder, but said he believes that there is no guarantee for a response from any treatment. Rather, he recommended empirical strategies for symptom management and keeping in mind the benefit-to-risk relationship when considering treatments that impose a high burden of adverse events.
However, the first step to treatment is recognizing the problem.
“In my opinion, what is missing is the nosology for these kids,” Dr. Findling said. An evidence-based label will help increase awareness of the problem and encourage more extensive clinical study, he said.
“These children are not rare and they are really impaired. It is heartbreaking, because when you talk to them when they are still little, they know what people think of them. They know their teachers don’t like them. They know their parents think they’re bad. They know their peers are scared of them, and they cannot make friends,” he said. However, there is a potential for reversing these problems if treatment is initiated early.
“As you watch them get older, you watch them scarring over,” he added.
Dr. Findling reported financial ties with numerous pharmaceutical companies.
SOURCE: Findling RL. Psychopharmacology Update Institute
NEW YORK – according to Robert L. Findling, MD.
Emphasizing the reactive component to this behavioral problem, he said: “They look okay until someone bumps into them at school. They do not have a mood disorder. They have a disorder of reactivity.”
The hurdle is that there is no accepted terminology to encourage clinicians to identify and initiate treatment in children with this behavior. The term conduct disorder has been used in the past, but Dr. Findling said that care delivered for conduct disorder is not reimbursable. This may be among the reasons that aggressive reactive behavior of ADHD is overlooked – even though treatment is likely to improve long-term outcome.
“I wish I had a magic label for this, but I don’t,” Dr. Findling said. However, he maintained that most clinicians who work with ADHD children are familiar with this type of behavior. Indeed, clinicians “grapple with this day to day. We all see these kids, and they are oftentimes the most impaired kids in our practices,” he said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
This behavior should not be confused with the aggression associated with mood disorders, such as disruptive mood dysregulation disorder (DMDD) or bipolar disease, according to Dr. Findling. Children with DMDD, for example, are chronically irritable or angry. Although bipolar disorder patients with aggressive behavior are not necessarily angry between episodes, they also have persistent mood disturbances.
In contrast, preadolescent children with ADHD who have episodes of aggression, a symptom far more common among males than females, do not otherwise exhibit disturbances in mood. In addition, the episodes of impulsive, reactive aggression are provoked. They require a perceived insult, threat, or similar trigger.
While many of these children continue to have episodes of impulsive aggressive behavior even on treatment effective for other ADHD symptoms, Dr. Findling said, “The good news is that there are treatments for aggression.” In addition to psychosocial support aimed at reducing aggressive behavior, once the diagnosis has been made, these include adjusting ADHD treatments to better target symptoms of episodic aggression. If needed, therapies known to treat aggression, such as atypical antipsychotics, anticonvulsants, or lithium also are options.
Dr. Findling did review one older double-blind study that associated methylphenidate with a reduction in aggression in children with conduct disorder, but said he believes that there is no guarantee for a response from any treatment. Rather, he recommended empirical strategies for symptom management and keeping in mind the benefit-to-risk relationship when considering treatments that impose a high burden of adverse events.
However, the first step to treatment is recognizing the problem.
“In my opinion, what is missing is the nosology for these kids,” Dr. Findling said. An evidence-based label will help increase awareness of the problem and encourage more extensive clinical study, he said.
“These children are not rare and they are really impaired. It is heartbreaking, because when you talk to them when they are still little, they know what people think of them. They know their teachers don’t like them. They know their parents think they’re bad. They know their peers are scared of them, and they cannot make friends,” he said. However, there is a potential for reversing these problems if treatment is initiated early.
“As you watch them get older, you watch them scarring over,” he added.
Dr. Findling reported financial ties with numerous pharmaceutical companies.
SOURCE: Findling RL. Psychopharmacology Update Institute
REPORTING FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Characterize duration when seeking etiology of tantrums in children
NEW YORK – Although explosive outbursts or tantrums accompany nearly every psychiatric illness that affects children, the specific features may help identify an etiology, according to Gabrielle A. Carlson, MD.
“There are two components of irritability,” explained Dr. Carlson, professor of psychiatry and pediatrics, Stony Brook (N.Y.) University Medical Center. “One is how often the child loses his or her temper, and the other is what they do when they lose their temper.”
To be useful in identifying the source, the characterization of explosive outbursts must be undertaken in the context of the patient’s history and the duration and types of tantrum-related behaviors, particularly aggressive behavior toward others, according to Dr. Carlson.
Presenting a diagnostic algorithm relevant to children with frequent explosive outbursts, Dr. Carlson suggested that pathways differ for young children and adolescents. Yet, the first step – which is evaluating whether or not irritability is a feature of the patient’s disposition when not in the midst of a tantrum – is common to both groups.
In young children with new onset of explosive outbursts, stressors in school, such as bullying, or family, such as abuse, represent an appropriate initial focus. In adolescents, initial attention should be paid to the potential role of mood disorders, particularly depression, mania, or anxiety, according to Dr. Carlson.
For most patients and most etiologies, tantrums follow a trigger and then resolve quickly. When tantrums do not resolve quickly in patients who remain generally irritable even when they are not having a tantrum, there is an increased likelihood of disruptive mood dysregulation disorder (DMDD).
Relative to tantrums associated with attention deficit hyperactive disorder (ADHD), oppositional defiant disorder (ODD), or affective disorders, explosive outbursts associated with DMDD are also more likely to include aggression toward others.
Physical restraint to safeguard the patient or others during a tantrum is uncommon in most conditions associated with tantrums, with the exception of DMDD. Greater aggression tracks with greater DMDD severity. According to data presented by Dr. Carlson, 92% of a clinical sample of DMDD patients exhibited physical aggression, compared with none of those in a community sample.
Tantrums lasting more than 30 minutes were observed in 60% of the clinic sample, versus only 12.5% of the community sample.
Explosive outbursts “are not an uncommon or trivial problem,” according to Dr. Carlson, who cited data suggesting that 70% of children between the ages of 5 and 12 years hospitalized for a psychiatric diseases are referred for an explosive outburst.
She believes that a systematic approach toward characterizing the tantrum will be helpful in understanding the underlying etiology and appropriate treatment. Using such tools as the Irritability and Rages Inventory or the Affective Reactivity Index Child Form, clinicians should seek to evaluate the frequency of tantrums, the duration, and the patient’s symptom burden between tantrums.
If explosive outbursts are rare, they are unlikely to be due to DMDD or affective disorders, such as bipolar disease. If frequent in a patient with chronic psychopathology, those who are generally “fine until frustrated” are the ones more likely to have ADHD or even oppositional defiant disorder (ODD).
The less common profile, which is rage that does not completely resolve, suggests DMDD, a condition that Dr. Carlson described with the mnemonic OI VEY to convey key features. The letters stand for Outbursts that are frequent, Irritable mood in the absence of an outburst, Very chronic (more than 1 per year), Explained by other co-existing conditions, such as mania, and Young (starts between ages 6 and 10 years).
Although tantrums are the way in which children with a broad array of psychiatric conditions express frustration, Dr. Carlson said it is not clear if the mechanisms for irritability and explosive outbursts are shared across conditions. Despite the guidance she offered for linking specific tantrum features with DMDD, she also reiterated that tantrums cannot be considered a symptom specific to any single etiology. The difference between etiologies for irritable children having a tantrum “is not how they feel, the difference is what they do,” Dr. Carlson suggested.
Dr. Carlson reported no relevant financial relationships.
NEW YORK – Although explosive outbursts or tantrums accompany nearly every psychiatric illness that affects children, the specific features may help identify an etiology, according to Gabrielle A. Carlson, MD.
“There are two components of irritability,” explained Dr. Carlson, professor of psychiatry and pediatrics, Stony Brook (N.Y.) University Medical Center. “One is how often the child loses his or her temper, and the other is what they do when they lose their temper.”
To be useful in identifying the source, the characterization of explosive outbursts must be undertaken in the context of the patient’s history and the duration and types of tantrum-related behaviors, particularly aggressive behavior toward others, according to Dr. Carlson.
Presenting a diagnostic algorithm relevant to children with frequent explosive outbursts, Dr. Carlson suggested that pathways differ for young children and adolescents. Yet, the first step – which is evaluating whether or not irritability is a feature of the patient’s disposition when not in the midst of a tantrum – is common to both groups.
In young children with new onset of explosive outbursts, stressors in school, such as bullying, or family, such as abuse, represent an appropriate initial focus. In adolescents, initial attention should be paid to the potential role of mood disorders, particularly depression, mania, or anxiety, according to Dr. Carlson.
For most patients and most etiologies, tantrums follow a trigger and then resolve quickly. When tantrums do not resolve quickly in patients who remain generally irritable even when they are not having a tantrum, there is an increased likelihood of disruptive mood dysregulation disorder (DMDD).
Relative to tantrums associated with attention deficit hyperactive disorder (ADHD), oppositional defiant disorder (ODD), or affective disorders, explosive outbursts associated with DMDD are also more likely to include aggression toward others.
Physical restraint to safeguard the patient or others during a tantrum is uncommon in most conditions associated with tantrums, with the exception of DMDD. Greater aggression tracks with greater DMDD severity. According to data presented by Dr. Carlson, 92% of a clinical sample of DMDD patients exhibited physical aggression, compared with none of those in a community sample.
Tantrums lasting more than 30 minutes were observed in 60% of the clinic sample, versus only 12.5% of the community sample.
Explosive outbursts “are not an uncommon or trivial problem,” according to Dr. Carlson, who cited data suggesting that 70% of children between the ages of 5 and 12 years hospitalized for a psychiatric diseases are referred for an explosive outburst.
She believes that a systematic approach toward characterizing the tantrum will be helpful in understanding the underlying etiology and appropriate treatment. Using such tools as the Irritability and Rages Inventory or the Affective Reactivity Index Child Form, clinicians should seek to evaluate the frequency of tantrums, the duration, and the patient’s symptom burden between tantrums.
If explosive outbursts are rare, they are unlikely to be due to DMDD or affective disorders, such as bipolar disease. If frequent in a patient with chronic psychopathology, those who are generally “fine until frustrated” are the ones more likely to have ADHD or even oppositional defiant disorder (ODD).
The less common profile, which is rage that does not completely resolve, suggests DMDD, a condition that Dr. Carlson described with the mnemonic OI VEY to convey key features. The letters stand for Outbursts that are frequent, Irritable mood in the absence of an outburst, Very chronic (more than 1 per year), Explained by other co-existing conditions, such as mania, and Young (starts between ages 6 and 10 years).
Although tantrums are the way in which children with a broad array of psychiatric conditions express frustration, Dr. Carlson said it is not clear if the mechanisms for irritability and explosive outbursts are shared across conditions. Despite the guidance she offered for linking specific tantrum features with DMDD, she also reiterated that tantrums cannot be considered a symptom specific to any single etiology. The difference between etiologies for irritable children having a tantrum “is not how they feel, the difference is what they do,” Dr. Carlson suggested.
Dr. Carlson reported no relevant financial relationships.
NEW YORK – Although explosive outbursts or tantrums accompany nearly every psychiatric illness that affects children, the specific features may help identify an etiology, according to Gabrielle A. Carlson, MD.
“There are two components of irritability,” explained Dr. Carlson, professor of psychiatry and pediatrics, Stony Brook (N.Y.) University Medical Center. “One is how often the child loses his or her temper, and the other is what they do when they lose their temper.”
To be useful in identifying the source, the characterization of explosive outbursts must be undertaken in the context of the patient’s history and the duration and types of tantrum-related behaviors, particularly aggressive behavior toward others, according to Dr. Carlson.
Presenting a diagnostic algorithm relevant to children with frequent explosive outbursts, Dr. Carlson suggested that pathways differ for young children and adolescents. Yet, the first step – which is evaluating whether or not irritability is a feature of the patient’s disposition when not in the midst of a tantrum – is common to both groups.
In young children with new onset of explosive outbursts, stressors in school, such as bullying, or family, such as abuse, represent an appropriate initial focus. In adolescents, initial attention should be paid to the potential role of mood disorders, particularly depression, mania, or anxiety, according to Dr. Carlson.
For most patients and most etiologies, tantrums follow a trigger and then resolve quickly. When tantrums do not resolve quickly in patients who remain generally irritable even when they are not having a tantrum, there is an increased likelihood of disruptive mood dysregulation disorder (DMDD).
Relative to tantrums associated with attention deficit hyperactive disorder (ADHD), oppositional defiant disorder (ODD), or affective disorders, explosive outbursts associated with DMDD are also more likely to include aggression toward others.
Physical restraint to safeguard the patient or others during a tantrum is uncommon in most conditions associated with tantrums, with the exception of DMDD. Greater aggression tracks with greater DMDD severity. According to data presented by Dr. Carlson, 92% of a clinical sample of DMDD patients exhibited physical aggression, compared with none of those in a community sample.
Tantrums lasting more than 30 minutes were observed in 60% of the clinic sample, versus only 12.5% of the community sample.
Explosive outbursts “are not an uncommon or trivial problem,” according to Dr. Carlson, who cited data suggesting that 70% of children between the ages of 5 and 12 years hospitalized for a psychiatric diseases are referred for an explosive outburst.
She believes that a systematic approach toward characterizing the tantrum will be helpful in understanding the underlying etiology and appropriate treatment. Using such tools as the Irritability and Rages Inventory or the Affective Reactivity Index Child Form, clinicians should seek to evaluate the frequency of tantrums, the duration, and the patient’s symptom burden between tantrums.
If explosive outbursts are rare, they are unlikely to be due to DMDD or affective disorders, such as bipolar disease. If frequent in a patient with chronic psychopathology, those who are generally “fine until frustrated” are the ones more likely to have ADHD or even oppositional defiant disorder (ODD).
The less common profile, which is rage that does not completely resolve, suggests DMDD, a condition that Dr. Carlson described with the mnemonic OI VEY to convey key features. The letters stand for Outbursts that are frequent, Irritable mood in the absence of an outburst, Very chronic (more than 1 per year), Explained by other co-existing conditions, such as mania, and Young (starts between ages 6 and 10 years).
Although tantrums are the way in which children with a broad array of psychiatric conditions express frustration, Dr. Carlson said it is not clear if the mechanisms for irritability and explosive outbursts are shared across conditions. Despite the guidance she offered for linking specific tantrum features with DMDD, she also reiterated that tantrums cannot be considered a symptom specific to any single etiology. The difference between etiologies for irritable children having a tantrum “is not how they feel, the difference is what they do,” Dr. Carlson suggested.
Dr. Carlson reported no relevant financial relationships.
REPORTING FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Women filling more ADHD prescriptions
, according to the Centers for Disease Control and Prevention.
In 2003, 0.9% of women aged 15-44 years with private employer–sponsored insurance filled a prescription for an ADHD medication. By 2015, that figure had gone up to 4.0% for an increase of 344% that was unevenly split by medication class: prescriptions for stimulants were up by 388%, but nonstimulants had no change, wrote Kayla N. Anderson, PhD, and her associates in the Morbidity and Mortality Weekly Report.
“The substantial increase in the percentage of reproductive-aged women filling ADHD medication prescriptions from 2003 to 2015 ... is of public health concern given the high percentage of unintended pregnancies and uncertainty concerning the safety of ADHD medication exposure before and during pregnancy,” they wrote.
This analysis was restricted to women with at least 11 months of enrollment in a private health insurance plan that included prescription drug coverage during the year of interest. The sample included a median of 4.6 million women each year.
SOURCE: Anderson K et al. MMWR. 2018 Jan 19;76(2):66-70.
, according to the Centers for Disease Control and Prevention.
In 2003, 0.9% of women aged 15-44 years with private employer–sponsored insurance filled a prescription for an ADHD medication. By 2015, that figure had gone up to 4.0% for an increase of 344% that was unevenly split by medication class: prescriptions for stimulants were up by 388%, but nonstimulants had no change, wrote Kayla N. Anderson, PhD, and her associates in the Morbidity and Mortality Weekly Report.
“The substantial increase in the percentage of reproductive-aged women filling ADHD medication prescriptions from 2003 to 2015 ... is of public health concern given the high percentage of unintended pregnancies and uncertainty concerning the safety of ADHD medication exposure before and during pregnancy,” they wrote.
This analysis was restricted to women with at least 11 months of enrollment in a private health insurance plan that included prescription drug coverage during the year of interest. The sample included a median of 4.6 million women each year.
SOURCE: Anderson K et al. MMWR. 2018 Jan 19;76(2):66-70.
, according to the Centers for Disease Control and Prevention.
In 2003, 0.9% of women aged 15-44 years with private employer–sponsored insurance filled a prescription for an ADHD medication. By 2015, that figure had gone up to 4.0% for an increase of 344% that was unevenly split by medication class: prescriptions for stimulants were up by 388%, but nonstimulants had no change, wrote Kayla N. Anderson, PhD, and her associates in the Morbidity and Mortality Weekly Report.
“The substantial increase in the percentage of reproductive-aged women filling ADHD medication prescriptions from 2003 to 2015 ... is of public health concern given the high percentage of unintended pregnancies and uncertainty concerning the safety of ADHD medication exposure before and during pregnancy,” they wrote.
This analysis was restricted to women with at least 11 months of enrollment in a private health insurance plan that included prescription drug coverage during the year of interest. The sample included a median of 4.6 million women each year.
SOURCE: Anderson K et al. MMWR. 2018 Jan 19;76(2):66-70.
FROM MMWR
U.S. autism rates edge up from 2014-2016
The prevalence of autism spectrum disorder (ASD) in U.S. children and adolescents climbed to 2.41% from 2014 to 2016, Guifeng Xu, MD, and her associates, wrote in a research letter. However, the increased prevalence of ASD over the 3-year period was not statistically significant.
Males were far more likely to be diagnosed with ASD than females, with respective prevalence rates of 3.54% and 1.22%, from 2014 to 2016.
using data from the Autism and Developmental Disabilities Monitoring Network. The ADDM reported a rate of 1.46%, compared with the NHIS rate of 2.41%. Differences in study design and participant characteristics are likely the cause of this difference; the ADDM is conducted at specific sites, while the NHIS is considered to be a nationally representative sample.
“Changes in nonetiologic factors (such as diagnostic criteria, public awareness, and referral), as well as in etiologic factors (including genetic and environmental risk factors), have been postulated to account for the previously observed increase in ASD prevalence,” concluded Dr. Xu, of the department of epidemiology at the University of Iowa, Iowa City, and her associates. “Continued monitoring of the prevalence and investigation of changes in risk factors are warranted.”
Find the full research letter in JAMA (2018;319[1]:81-2. doi: 10.1001/jama.2017.17812).
The prevalence of autism spectrum disorder (ASD) in U.S. children and adolescents climbed to 2.41% from 2014 to 2016, Guifeng Xu, MD, and her associates, wrote in a research letter. However, the increased prevalence of ASD over the 3-year period was not statistically significant.
Males were far more likely to be diagnosed with ASD than females, with respective prevalence rates of 3.54% and 1.22%, from 2014 to 2016.
using data from the Autism and Developmental Disabilities Monitoring Network. The ADDM reported a rate of 1.46%, compared with the NHIS rate of 2.41%. Differences in study design and participant characteristics are likely the cause of this difference; the ADDM is conducted at specific sites, while the NHIS is considered to be a nationally representative sample.
“Changes in nonetiologic factors (such as diagnostic criteria, public awareness, and referral), as well as in etiologic factors (including genetic and environmental risk factors), have been postulated to account for the previously observed increase in ASD prevalence,” concluded Dr. Xu, of the department of epidemiology at the University of Iowa, Iowa City, and her associates. “Continued monitoring of the prevalence and investigation of changes in risk factors are warranted.”
Find the full research letter in JAMA (2018;319[1]:81-2. doi: 10.1001/jama.2017.17812).
The prevalence of autism spectrum disorder (ASD) in U.S. children and adolescents climbed to 2.41% from 2014 to 2016, Guifeng Xu, MD, and her associates, wrote in a research letter. However, the increased prevalence of ASD over the 3-year period was not statistically significant.
Males were far more likely to be diagnosed with ASD than females, with respective prevalence rates of 3.54% and 1.22%, from 2014 to 2016.
using data from the Autism and Developmental Disabilities Monitoring Network. The ADDM reported a rate of 1.46%, compared with the NHIS rate of 2.41%. Differences in study design and participant characteristics are likely the cause of this difference; the ADDM is conducted at specific sites, while the NHIS is considered to be a nationally representative sample.
“Changes in nonetiologic factors (such as diagnostic criteria, public awareness, and referral), as well as in etiologic factors (including genetic and environmental risk factors), have been postulated to account for the previously observed increase in ASD prevalence,” concluded Dr. Xu, of the department of epidemiology at the University of Iowa, Iowa City, and her associates. “Continued monitoring of the prevalence and investigation of changes in risk factors are warranted.”
Find the full research letter in JAMA (2018;319[1]:81-2. doi: 10.1001/jama.2017.17812).
FROM JAMA
MRI-guided neurofeedback improves ADHD long term in adolescent boys
PARIS – Neurofeedback based upon real-time functional magnetic resonance imaging resulted in long-term reduction in attention-deficit/hyperactivity disorder symptoms in adolescents in a randomized controlled proof-of-concept study, Katya Rubia, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The effect size of the improvement when measured at follow-up 11 months after completing the functional MRI-based neurofeedback (fMRI-NF) training exercises was moderate to large and comparable to that of psychostimulant medication in published placebo-controlled clinical trials. But the effects of the medications last only 24 hours after administration, and the drugs have side effects.
Neurofeedback is an operant conditioning procedure, which, through trial and error, teaches patients to self-regulate specific areas of the brain involved in psychopathology. EEG-based neurofeedback for ADHD has been extensively studied, with generally small to medium effect sizes being reported. Morever, patients need to be very highly motivated in order to succeed at EEG-NF: It takes 30-40 EEG-NF sessions, each an hour long, in order to learn targeted brain self-control in ADHD, whereas in Dr. Rubia’s study, patients learned to self-regulate brain activity in an average of eight fMRI sessions, each lasting 8.5 minutes, over the course of 2 weeks. The far speedier learning curve is probably tied to the superior specificity of spatial localization afforded by fMRI neurofeedback, according to the neuroscientist.
Also, fMRI-NF can reach certain key regions of the brain involved in ADHD that EEG-NF cannot, most notably the inferior frontal cortex (IFC) and basal ganglia, she added.
The target region in the proof-of-concept study was the right IFC, an area important for cognitive control, attention, and timing. Functional neuroimaging studies consistently have shown that the right IFC is underactive in ADHD, and that psychostimulant medications upregulate this area. A dysfunctional right IFC is an ADHD-specific abnormality not present in children with obessive-compulsive disorder (JAMA Psychiatry. 2016 Aug 1;73[8]:815-25), conduct disorder, or autism.
“The IFC seems to be a very good functional biomarker for ADHD,” Dr. Rubia said.
The proof-of-concept study, published in Human Brain Mapping, included 31 boys with a DSM-5 diagnosis of ADHD, aged 12-17, who were randomized to fMRI-NF of the right IFC or, as a control condition, to fMRI-NF targeting the left parahippocampal gyrus. Two patients had the inattentive subtype of ADHD; the rest had the combined hyperactive/inattentive form. Parents and patients were blinded as to their study arm.
So this program uses neuroimaging as a treatment. It is neuroimaging employed as neurotherapy. To make the training experience more attractive to young patients, it was presented as a computer game: By making progress in controlling their brain activity, patients could launch a rocket ship on the screen. With further progress, they could send the rocket through the atmosphere into space and eventually land it on another planet.
The primary study endpoint was change in the ADHD Rating Scale. The group that targeted self-upregulation of right IFC activity showed roughly a 20% improvement in scores, from a baseline mean total score of 36.7 to 30.2 immediately post treatment, further improving to a score of 26.7 at roughly 11 months of follow-up. Mean scores on the inattention subscale improved from 19.8 to 15.9 immediately post treatment and 15.3 at follow-up. Scores on the hyperactivity/impulsivity subscale went from 16.9 before treatment to 14.2 after treatment and 11.5 at follow-up.
There were no side effects of fMRI-NF in either study arm.
However, a degree of uncertainty exists regarding the clinical significance of the results, Dr. Rubia said. That’s because the control group showed a similar degree of improvement in ADHD symptoms immediately after learning to upregulate the left parahippocampal gyrus, although their scores did backslide modestly during 11 months of follow-up, while the IFC group continued to improve.
Dr. Rubia acknowledged that this raises the possibility that the observed improvement in clinical symptoms achieved through fMRI-NF could be attributable to a placebo effect. However, she said she believes this is unlikely for several reasons. For one, brain scans showed that targeting either the right IFC or the left parahippocampal gyrus not only resulted in upregulation of activity in those specific regions, but throughout the broader neural networks of which they are a part. The right IFC upregulators showed activation of a bilateral dorsolateral prefrontal cortex/IFC-insular-striato-cerebellar cognitive control network. In contrast, the boys who targeted the left parahippocampal gyrus experienced activation of associated posterior visual-spatial attention regions, which are relevant to ADHD. This made for a far from ideal control group.
Also, the amount of improvement in ADHD symptoms in the right IFC-targeted group correlated with the degree of activation of that region, indicative of a brain-behavior correlation that speaks against a nonspecific effect.
Because this was a small, unpowered pilot study and interest remains intense in potential nonpharmacologic treatments for ADHD, the U.K. Medical Research Council is funding Dr. Rubia and her colleagues for a new 100-patient study – including a sham fMRI-NF arm – in order to definitively address the possibility of a placebo effect. The study also will attempt to pin down the patient population most likely to benefit from fMRI-NF. “It’s possible that the inattentive subtype of ADHD will respond best. Neurofeedback is, after all, a form of attention training,” she noted.
While real-time fMRI-NF might sound prohibitively expensive for widespread use in clinical practice for a disorder as common as ADHD, which has an estimated prevalence of about 7%, it might actually stack up reasonably well in a cost-benefit analysis, compared with ongoing medication costs and side effects or with a year’s worth of weekly psychotherapy, according to Dr. Rubia.
In parallel with the ongoing sham-controlled fMRI-NF study, Dr. Rubia also is conducting a clinical trial of transcranial direct current stimulation of the right IFC in combination with cognitive training. The idea is to study the clinical impact of directly upregulating activity in this area of the brain, bypassing the added step of training patients to gain self-control over this dysregulated region. The early findings, she said, look promising.
The fMRI-NF study (Hum Brain Mapp. 2017 Jun;38[6]:3190-209) was sponsored by the U.K. National Institute for Health Research and the Maudsley NHS Foundation Trust. Dr. Rubia reported receiving speakers honoraria from Lilly, Shire, and Medice.
Source: Rubia K et al. European College of Neuropsychopharmacology.
PARIS – Neurofeedback based upon real-time functional magnetic resonance imaging resulted in long-term reduction in attention-deficit/hyperactivity disorder symptoms in adolescents in a randomized controlled proof-of-concept study, Katya Rubia, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The effect size of the improvement when measured at follow-up 11 months after completing the functional MRI-based neurofeedback (fMRI-NF) training exercises was moderate to large and comparable to that of psychostimulant medication in published placebo-controlled clinical trials. But the effects of the medications last only 24 hours after administration, and the drugs have side effects.
Neurofeedback is an operant conditioning procedure, which, through trial and error, teaches patients to self-regulate specific areas of the brain involved in psychopathology. EEG-based neurofeedback for ADHD has been extensively studied, with generally small to medium effect sizes being reported. Morever, patients need to be very highly motivated in order to succeed at EEG-NF: It takes 30-40 EEG-NF sessions, each an hour long, in order to learn targeted brain self-control in ADHD, whereas in Dr. Rubia’s study, patients learned to self-regulate brain activity in an average of eight fMRI sessions, each lasting 8.5 minutes, over the course of 2 weeks. The far speedier learning curve is probably tied to the superior specificity of spatial localization afforded by fMRI neurofeedback, according to the neuroscientist.
Also, fMRI-NF can reach certain key regions of the brain involved in ADHD that EEG-NF cannot, most notably the inferior frontal cortex (IFC) and basal ganglia, she added.
The target region in the proof-of-concept study was the right IFC, an area important for cognitive control, attention, and timing. Functional neuroimaging studies consistently have shown that the right IFC is underactive in ADHD, and that psychostimulant medications upregulate this area. A dysfunctional right IFC is an ADHD-specific abnormality not present in children with obessive-compulsive disorder (JAMA Psychiatry. 2016 Aug 1;73[8]:815-25), conduct disorder, or autism.
“The IFC seems to be a very good functional biomarker for ADHD,” Dr. Rubia said.
The proof-of-concept study, published in Human Brain Mapping, included 31 boys with a DSM-5 diagnosis of ADHD, aged 12-17, who were randomized to fMRI-NF of the right IFC or, as a control condition, to fMRI-NF targeting the left parahippocampal gyrus. Two patients had the inattentive subtype of ADHD; the rest had the combined hyperactive/inattentive form. Parents and patients were blinded as to their study arm.
So this program uses neuroimaging as a treatment. It is neuroimaging employed as neurotherapy. To make the training experience more attractive to young patients, it was presented as a computer game: By making progress in controlling their brain activity, patients could launch a rocket ship on the screen. With further progress, they could send the rocket through the atmosphere into space and eventually land it on another planet.
The primary study endpoint was change in the ADHD Rating Scale. The group that targeted self-upregulation of right IFC activity showed roughly a 20% improvement in scores, from a baseline mean total score of 36.7 to 30.2 immediately post treatment, further improving to a score of 26.7 at roughly 11 months of follow-up. Mean scores on the inattention subscale improved from 19.8 to 15.9 immediately post treatment and 15.3 at follow-up. Scores on the hyperactivity/impulsivity subscale went from 16.9 before treatment to 14.2 after treatment and 11.5 at follow-up.
There were no side effects of fMRI-NF in either study arm.
However, a degree of uncertainty exists regarding the clinical significance of the results, Dr. Rubia said. That’s because the control group showed a similar degree of improvement in ADHD symptoms immediately after learning to upregulate the left parahippocampal gyrus, although their scores did backslide modestly during 11 months of follow-up, while the IFC group continued to improve.
Dr. Rubia acknowledged that this raises the possibility that the observed improvement in clinical symptoms achieved through fMRI-NF could be attributable to a placebo effect. However, she said she believes this is unlikely for several reasons. For one, brain scans showed that targeting either the right IFC or the left parahippocampal gyrus not only resulted in upregulation of activity in those specific regions, but throughout the broader neural networks of which they are a part. The right IFC upregulators showed activation of a bilateral dorsolateral prefrontal cortex/IFC-insular-striato-cerebellar cognitive control network. In contrast, the boys who targeted the left parahippocampal gyrus experienced activation of associated posterior visual-spatial attention regions, which are relevant to ADHD. This made for a far from ideal control group.
Also, the amount of improvement in ADHD symptoms in the right IFC-targeted group correlated with the degree of activation of that region, indicative of a brain-behavior correlation that speaks against a nonspecific effect.
Because this was a small, unpowered pilot study and interest remains intense in potential nonpharmacologic treatments for ADHD, the U.K. Medical Research Council is funding Dr. Rubia and her colleagues for a new 100-patient study – including a sham fMRI-NF arm – in order to definitively address the possibility of a placebo effect. The study also will attempt to pin down the patient population most likely to benefit from fMRI-NF. “It’s possible that the inattentive subtype of ADHD will respond best. Neurofeedback is, after all, a form of attention training,” she noted.
While real-time fMRI-NF might sound prohibitively expensive for widespread use in clinical practice for a disorder as common as ADHD, which has an estimated prevalence of about 7%, it might actually stack up reasonably well in a cost-benefit analysis, compared with ongoing medication costs and side effects or with a year’s worth of weekly psychotherapy, according to Dr. Rubia.
In parallel with the ongoing sham-controlled fMRI-NF study, Dr. Rubia also is conducting a clinical trial of transcranial direct current stimulation of the right IFC in combination with cognitive training. The idea is to study the clinical impact of directly upregulating activity in this area of the brain, bypassing the added step of training patients to gain self-control over this dysregulated region. The early findings, she said, look promising.
The fMRI-NF study (Hum Brain Mapp. 2017 Jun;38[6]:3190-209) was sponsored by the U.K. National Institute for Health Research and the Maudsley NHS Foundation Trust. Dr. Rubia reported receiving speakers honoraria from Lilly, Shire, and Medice.
Source: Rubia K et al. European College of Neuropsychopharmacology.
PARIS – Neurofeedback based upon real-time functional magnetic resonance imaging resulted in long-term reduction in attention-deficit/hyperactivity disorder symptoms in adolescents in a randomized controlled proof-of-concept study, Katya Rubia, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The effect size of the improvement when measured at follow-up 11 months after completing the functional MRI-based neurofeedback (fMRI-NF) training exercises was moderate to large and comparable to that of psychostimulant medication in published placebo-controlled clinical trials. But the effects of the medications last only 24 hours after administration, and the drugs have side effects.
Neurofeedback is an operant conditioning procedure, which, through trial and error, teaches patients to self-regulate specific areas of the brain involved in psychopathology. EEG-based neurofeedback for ADHD has been extensively studied, with generally small to medium effect sizes being reported. Morever, patients need to be very highly motivated in order to succeed at EEG-NF: It takes 30-40 EEG-NF sessions, each an hour long, in order to learn targeted brain self-control in ADHD, whereas in Dr. Rubia’s study, patients learned to self-regulate brain activity in an average of eight fMRI sessions, each lasting 8.5 minutes, over the course of 2 weeks. The far speedier learning curve is probably tied to the superior specificity of spatial localization afforded by fMRI neurofeedback, according to the neuroscientist.
Also, fMRI-NF can reach certain key regions of the brain involved in ADHD that EEG-NF cannot, most notably the inferior frontal cortex (IFC) and basal ganglia, she added.
The target region in the proof-of-concept study was the right IFC, an area important for cognitive control, attention, and timing. Functional neuroimaging studies consistently have shown that the right IFC is underactive in ADHD, and that psychostimulant medications upregulate this area. A dysfunctional right IFC is an ADHD-specific abnormality not present in children with obessive-compulsive disorder (JAMA Psychiatry. 2016 Aug 1;73[8]:815-25), conduct disorder, or autism.
“The IFC seems to be a very good functional biomarker for ADHD,” Dr. Rubia said.
The proof-of-concept study, published in Human Brain Mapping, included 31 boys with a DSM-5 diagnosis of ADHD, aged 12-17, who were randomized to fMRI-NF of the right IFC or, as a control condition, to fMRI-NF targeting the left parahippocampal gyrus. Two patients had the inattentive subtype of ADHD; the rest had the combined hyperactive/inattentive form. Parents and patients were blinded as to their study arm.
So this program uses neuroimaging as a treatment. It is neuroimaging employed as neurotherapy. To make the training experience more attractive to young patients, it was presented as a computer game: By making progress in controlling their brain activity, patients could launch a rocket ship on the screen. With further progress, they could send the rocket through the atmosphere into space and eventually land it on another planet.
The primary study endpoint was change in the ADHD Rating Scale. The group that targeted self-upregulation of right IFC activity showed roughly a 20% improvement in scores, from a baseline mean total score of 36.7 to 30.2 immediately post treatment, further improving to a score of 26.7 at roughly 11 months of follow-up. Mean scores on the inattention subscale improved from 19.8 to 15.9 immediately post treatment and 15.3 at follow-up. Scores on the hyperactivity/impulsivity subscale went from 16.9 before treatment to 14.2 after treatment and 11.5 at follow-up.
There were no side effects of fMRI-NF in either study arm.
However, a degree of uncertainty exists regarding the clinical significance of the results, Dr. Rubia said. That’s because the control group showed a similar degree of improvement in ADHD symptoms immediately after learning to upregulate the left parahippocampal gyrus, although their scores did backslide modestly during 11 months of follow-up, while the IFC group continued to improve.
Dr. Rubia acknowledged that this raises the possibility that the observed improvement in clinical symptoms achieved through fMRI-NF could be attributable to a placebo effect. However, she said she believes this is unlikely for several reasons. For one, brain scans showed that targeting either the right IFC or the left parahippocampal gyrus not only resulted in upregulation of activity in those specific regions, but throughout the broader neural networks of which they are a part. The right IFC upregulators showed activation of a bilateral dorsolateral prefrontal cortex/IFC-insular-striato-cerebellar cognitive control network. In contrast, the boys who targeted the left parahippocampal gyrus experienced activation of associated posterior visual-spatial attention regions, which are relevant to ADHD. This made for a far from ideal control group.
Also, the amount of improvement in ADHD symptoms in the right IFC-targeted group correlated with the degree of activation of that region, indicative of a brain-behavior correlation that speaks against a nonspecific effect.
Because this was a small, unpowered pilot study and interest remains intense in potential nonpharmacologic treatments for ADHD, the U.K. Medical Research Council is funding Dr. Rubia and her colleagues for a new 100-patient study – including a sham fMRI-NF arm – in order to definitively address the possibility of a placebo effect. The study also will attempt to pin down the patient population most likely to benefit from fMRI-NF. “It’s possible that the inattentive subtype of ADHD will respond best. Neurofeedback is, after all, a form of attention training,” she noted.
While real-time fMRI-NF might sound prohibitively expensive for widespread use in clinical practice for a disorder as common as ADHD, which has an estimated prevalence of about 7%, it might actually stack up reasonably well in a cost-benefit analysis, compared with ongoing medication costs and side effects or with a year’s worth of weekly psychotherapy, according to Dr. Rubia.
In parallel with the ongoing sham-controlled fMRI-NF study, Dr. Rubia also is conducting a clinical trial of transcranial direct current stimulation of the right IFC in combination with cognitive training. The idea is to study the clinical impact of directly upregulating activity in this area of the brain, bypassing the added step of training patients to gain self-control over this dysregulated region. The early findings, she said, look promising.
The fMRI-NF study (Hum Brain Mapp. 2017 Jun;38[6]:3190-209) was sponsored by the U.K. National Institute for Health Research and the Maudsley NHS Foundation Trust. Dr. Rubia reported receiving speakers honoraria from Lilly, Shire, and Medice.
Source: Rubia K et al. European College of Neuropsychopharmacology.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Neuroimaging can be employed as neurotherapy to improve ADHD nonpharmacologically.
Major finding: Adolescents with ADHD who learned via functional MRI neurofeedback to upregulate activity in their right inferior frontal cortex showed significant improvement in scores on the ADHD Rating Scale, from a baseline mean total score of 36.7 to 30.2 immediately after the training program, further improving to 26.7 at roughly 11 months of follow-up.
Study details: A prospective, randomized, single-blind study of 31 boys aged 12-17 with ADHD.
Disclosures: The study was sponsored by the U.K. National Institute for Health Research and the Maudsley NHS Foundation Trust. The presenter reported receiving speakers honoraria from Lilly, Shire, and Medice.
Source: Rubia K et al. European College of Neuropsychopharmacology.











