User login
Deaths Linked to Substance Use, CVD on the Rise
TOPLINE:
, with the most pronounced rise among women, American Indians, younger people, rural residents, and users of cannabis and psychostimulants, results of new research suggest.
METHODOLOGY:
- From the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database and using International Classification of Diseases (ICD) codes, researchers collected data on deaths within the United States where both SU and CVD (SU+CVD) were a contributing or an underlying cause and gathered information on location of death (medical facility, home, hospice, nursing home/long-term care facility), demographics (sex, race/ethnicity, age), and region (urban-rural, state).
- Researchers determined crude and age-adjusted mortality rates (AAMRs) per 100,000 population, identified trends in AAMR using annual percent change (APC) and calculated the weighted average of APCs (AAPCs).
- Between 1999 and 2019, there were 636,572 deaths related to CVD+SU, 75.6% of which were among men and 70.6% among non-Hispanic White individuals, with 65% related to alcohol, and where location of death was available, 47.7% occurred in medical facilities.
TAKEAWAY:
- The overall SU+CVD-related AAMR from 1999 to 2019 was 14.3 (95% CI, 14.3-14.3) per 100,000 individuals, with the rate being higher in men (22.5) than in women (6.8) and highest in American Indians or Alaska Natives (37.7) compared with other races/ethnicities.
- Rural areas had higher SU+CVD-related AAMR (15.2; 95% CI, 15.1-15.3) than urban areas, with the District of Columbia having the highest AAMR geographically (25.4), individuals aged 55-69 years having the highest rate agewise (25.1), and alcohol accounting for the highest rate (9.09) among substance types.
- Temporal trends show that the overall SU+CVD-related AAMR increased from 9.9 in 1999 to 21.4 in 2019, a rate that started accelerating in 2012, with an AAPC of 4.0% (95% CI, 3.7-4.3); increases were across all ethnicities and age groups and were particularly pronounced among women (4.8%; 95% CI, 4.5-5.1).
- Cannabis had the highest AAPC of all substances (12.7%), but stimulants had an APC of 21.4 (95% CI, 20.0-22.8) from 2009 to 2019, a period during which stimulants were the fastest-growing substance abuse category.
IN PRACTICE:
These new results identify high-risk groups, which “is crucial for prioritizing preventive measures aiming to reduce substance use and cardiovascular disease-related mortality in these populations,” the researchers wrote.
SOURCE:
Abdul Mannan Khan Minhas, MD, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, and Jakrin Kewcharoen, MD, Division of Cardiology, Loma Linda University Medical Center, Loma Linda, California, were co-first authors of the study, which was published online in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
TOPLINE:
, with the most pronounced rise among women, American Indians, younger people, rural residents, and users of cannabis and psychostimulants, results of new research suggest.
METHODOLOGY:
- From the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database and using International Classification of Diseases (ICD) codes, researchers collected data on deaths within the United States where both SU and CVD (SU+CVD) were a contributing or an underlying cause and gathered information on location of death (medical facility, home, hospice, nursing home/long-term care facility), demographics (sex, race/ethnicity, age), and region (urban-rural, state).
- Researchers determined crude and age-adjusted mortality rates (AAMRs) per 100,000 population, identified trends in AAMR using annual percent change (APC) and calculated the weighted average of APCs (AAPCs).
- Between 1999 and 2019, there were 636,572 deaths related to CVD+SU, 75.6% of which were among men and 70.6% among non-Hispanic White individuals, with 65% related to alcohol, and where location of death was available, 47.7% occurred in medical facilities.
TAKEAWAY:
- The overall SU+CVD-related AAMR from 1999 to 2019 was 14.3 (95% CI, 14.3-14.3) per 100,000 individuals, with the rate being higher in men (22.5) than in women (6.8) and highest in American Indians or Alaska Natives (37.7) compared with other races/ethnicities.
- Rural areas had higher SU+CVD-related AAMR (15.2; 95% CI, 15.1-15.3) than urban areas, with the District of Columbia having the highest AAMR geographically (25.4), individuals aged 55-69 years having the highest rate agewise (25.1), and alcohol accounting for the highest rate (9.09) among substance types.
- Temporal trends show that the overall SU+CVD-related AAMR increased from 9.9 in 1999 to 21.4 in 2019, a rate that started accelerating in 2012, with an AAPC of 4.0% (95% CI, 3.7-4.3); increases were across all ethnicities and age groups and were particularly pronounced among women (4.8%; 95% CI, 4.5-5.1).
- Cannabis had the highest AAPC of all substances (12.7%), but stimulants had an APC of 21.4 (95% CI, 20.0-22.8) from 2009 to 2019, a period during which stimulants were the fastest-growing substance abuse category.
IN PRACTICE:
These new results identify high-risk groups, which “is crucial for prioritizing preventive measures aiming to reduce substance use and cardiovascular disease-related mortality in these populations,” the researchers wrote.
SOURCE:
Abdul Mannan Khan Minhas, MD, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, and Jakrin Kewcharoen, MD, Division of Cardiology, Loma Linda University Medical Center, Loma Linda, California, were co-first authors of the study, which was published online in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
TOPLINE:
, with the most pronounced rise among women, American Indians, younger people, rural residents, and users of cannabis and psychostimulants, results of new research suggest.
METHODOLOGY:
- From the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database and using International Classification of Diseases (ICD) codes, researchers collected data on deaths within the United States where both SU and CVD (SU+CVD) were a contributing or an underlying cause and gathered information on location of death (medical facility, home, hospice, nursing home/long-term care facility), demographics (sex, race/ethnicity, age), and region (urban-rural, state).
- Researchers determined crude and age-adjusted mortality rates (AAMRs) per 100,000 population, identified trends in AAMR using annual percent change (APC) and calculated the weighted average of APCs (AAPCs).
- Between 1999 and 2019, there were 636,572 deaths related to CVD+SU, 75.6% of which were among men and 70.6% among non-Hispanic White individuals, with 65% related to alcohol, and where location of death was available, 47.7% occurred in medical facilities.
TAKEAWAY:
- The overall SU+CVD-related AAMR from 1999 to 2019 was 14.3 (95% CI, 14.3-14.3) per 100,000 individuals, with the rate being higher in men (22.5) than in women (6.8) and highest in American Indians or Alaska Natives (37.7) compared with other races/ethnicities.
- Rural areas had higher SU+CVD-related AAMR (15.2; 95% CI, 15.1-15.3) than urban areas, with the District of Columbia having the highest AAMR geographically (25.4), individuals aged 55-69 years having the highest rate agewise (25.1), and alcohol accounting for the highest rate (9.09) among substance types.
- Temporal trends show that the overall SU+CVD-related AAMR increased from 9.9 in 1999 to 21.4 in 2019, a rate that started accelerating in 2012, with an AAPC of 4.0% (95% CI, 3.7-4.3); increases were across all ethnicities and age groups and were particularly pronounced among women (4.8%; 95% CI, 4.5-5.1).
- Cannabis had the highest AAPC of all substances (12.7%), but stimulants had an APC of 21.4 (95% CI, 20.0-22.8) from 2009 to 2019, a period during which stimulants were the fastest-growing substance abuse category.
IN PRACTICE:
These new results identify high-risk groups, which “is crucial for prioritizing preventive measures aiming to reduce substance use and cardiovascular disease-related mortality in these populations,” the researchers wrote.
SOURCE:
Abdul Mannan Khan Minhas, MD, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, and Jakrin Kewcharoen, MD, Division of Cardiology, Loma Linda University Medical Center, Loma Linda, California, were co-first authors of the study, which was published online in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
Buprenorphine Slightly Less Risky than Methadone for Fetal Malformation
Buprenorphine use, compared with methadone use, in pregnancy has been linked with a slightly lower risk of major congenital malformations in a new study of medications for opioid use disorder (OUD).
Elizabeth A. Suarez, PhD, MPH, with the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues published the findings in JAMA Internal Medicine.
The lower risk for buprenorphine was small (risk ratio, 0.82; 95% confidence interval [CI], 0.69-0.97), and methadone use should not be ruled out on that basis, the authors wrote. For some women, particularly those on stable treatment before pregnancy or women who do not respond well to buprenorphine, methadone may be the better choice, they explained.
Either Medication Better Than Not Treating
The authors noted that either medication “is strongly recommended over untreated OUD during pregnancy.”
JAMA Internal Medicine Deputy Editor Deborah Grady, MD, MPH, with the Department of Medicine, University of California, San Francisco, emphasized that recommendation in an editor’s note, highlighting that treatment for OUD is critical to prevent infections, overdose, and death in pregnant women as well as neonatal opioid withdrawal syndrome and fetal death.
She stressed that internists and other primary care physicians have a key role in ensuring pregnant women with OUD receive appropriate treatment.
Given the importance of the issue, she wrote, “we have taken the unusual step of publishing two accompanying invited commentaries.”
Two developments may help increase use of buprenorphine, the study authors wrote. One is a recent study showing lower risk of adverse neonatal outcomes when buprenorphine is used during pregnancy compared with methadone. Another is the removal last year of the prescribing waiver for buprenorphine.
Study Included Medicaid Data Over 18 Years
The population-based cohort study used data from publicly insured Medicaid beneficiaries from 2000 to 2018. Pregnancies with enrollment from 90 days before pregnancy through 1 month after delivery and first-trimester use of buprenorphine or methadone were included (n = 13,360). The data were linked with infants’ health data.
The study group included 9,514 pregnancies with first-trimester buprenorphine exposure and 3,846 with methadone exposure. The risk of malformations overall was 50.9 (95% CI, 46.5-55.3) per 1000 pregnancies for buprenorphine and 60.6 (95% CI, 53.0-68.1) per 1000 pregnancies for methadone.
Major malformations were any cardiac malformations, ventricular septal defect, secundum atrial septal defect/nonprematurity-related patent foramen ovale, neural tube defects, oral clefts, and clubfoot.
Two Invited Commentaries Urge Caution in Interpretation
The two invited commentaries Dr. Grady mentioned in her editor’s note point both to the importance of the team’s findings and the need for better understanding of factors that may affect the choice of which OUD medication to use.
A commentary by Max Jordan Nguemeni Tiako, MD, MS, with the Department of Medicine, Brigham and Women’s Hospital, and colleagues, said that while the Suarez et al. data are important to share with patients, “the ultimate treatment decision must be the result of shared decision-making between a knowledgeable clinician and the patient, rather than promoting one medication over another.”
They urge putting the findings in context given the study population, which comprises a relatively stable group of women with OUD, most of whom were taking OUD medications before they got pregnant. The study sample excludes a substantial number of women who are chronically underinsured or uninsured, Dr. Tiako’s team wrote, because those included were enrolled in Medicaid for 3 consecutive months before pregnancy.
“We urge caution when extrapolating these findings to newly pregnant individuals with untreated OUD,” they wrote.
Both Medications are Safe
Cara Poland, MD, MEd, with the Henry Ford Health + Michigan State University Health Sciences in Grand Rapids, and coauthors, added in another commentary that Suarez et al. didn’t include a comparison between the population-level congenital defect rate and the defect rate for people using medications for OUD in pregnancy.
That comparison, they wrote, would have better illustrated the safety of medications for OUD “instead of simply comparing two medications with long-standing safety data.”
When a clinician starts a woman on medication for OUD in pregnancy, it’s important to understand several factors, including individual access to and comfort with different treatment approaches, they noted. It’s also important to weigh whether changing medications is worth the potential drawbacks of disrupting their well-managed care.
They wrote that the paper by Suarez et al. does not make the case for switching medications based on their findings.
Internists, they added, are ideal experts to explain risk of fetal abnormalities in the wider context of supporting engagement with continuous medication for OUD.
“In the absence of other concerns, switching medications (methadone to buprenorphine) or — worse — discontinuing [medication for] OUD because of this study runs counter to the substantial evidence regarding the safety of these medications during pregnancy,” Dr. Poland’s team wrote. “No treatment is without risk in pregnancy.”
This study was supported by the National Institute on Drug Abuse. In the Suarez et al. study, coauthors Dr. Hernández-Díaz, Dr. Gray, Dr. Connery, Dr. Zhu, and Dr. Huybrechts reported grants, personal fees and consulting payments from several pharmaceutical companies. Dr. Grady reports no relevant financial relationships in her editor’s note. No relevant financial relationships were reported by authors of the Tiako et al. commentary.
Regarding the commentary by Poland et al., grants were reported from the Michigan Health Endowment Fund, the Michigan Department of Health and Human Services, the National Institute on Drug Abuse and Blue Cross Blue Shield of Michigan outside the submitted work. No other disclosures were reported.
Buprenorphine use, compared with methadone use, in pregnancy has been linked with a slightly lower risk of major congenital malformations in a new study of medications for opioid use disorder (OUD).
Elizabeth A. Suarez, PhD, MPH, with the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues published the findings in JAMA Internal Medicine.
The lower risk for buprenorphine was small (risk ratio, 0.82; 95% confidence interval [CI], 0.69-0.97), and methadone use should not be ruled out on that basis, the authors wrote. For some women, particularly those on stable treatment before pregnancy or women who do not respond well to buprenorphine, methadone may be the better choice, they explained.
Either Medication Better Than Not Treating
The authors noted that either medication “is strongly recommended over untreated OUD during pregnancy.”
JAMA Internal Medicine Deputy Editor Deborah Grady, MD, MPH, with the Department of Medicine, University of California, San Francisco, emphasized that recommendation in an editor’s note, highlighting that treatment for OUD is critical to prevent infections, overdose, and death in pregnant women as well as neonatal opioid withdrawal syndrome and fetal death.
She stressed that internists and other primary care physicians have a key role in ensuring pregnant women with OUD receive appropriate treatment.
Given the importance of the issue, she wrote, “we have taken the unusual step of publishing two accompanying invited commentaries.”
Two developments may help increase use of buprenorphine, the study authors wrote. One is a recent study showing lower risk of adverse neonatal outcomes when buprenorphine is used during pregnancy compared with methadone. Another is the removal last year of the prescribing waiver for buprenorphine.
Study Included Medicaid Data Over 18 Years
The population-based cohort study used data from publicly insured Medicaid beneficiaries from 2000 to 2018. Pregnancies with enrollment from 90 days before pregnancy through 1 month after delivery and first-trimester use of buprenorphine or methadone were included (n = 13,360). The data were linked with infants’ health data.
The study group included 9,514 pregnancies with first-trimester buprenorphine exposure and 3,846 with methadone exposure. The risk of malformations overall was 50.9 (95% CI, 46.5-55.3) per 1000 pregnancies for buprenorphine and 60.6 (95% CI, 53.0-68.1) per 1000 pregnancies for methadone.
Major malformations were any cardiac malformations, ventricular septal defect, secundum atrial septal defect/nonprematurity-related patent foramen ovale, neural tube defects, oral clefts, and clubfoot.
Two Invited Commentaries Urge Caution in Interpretation
The two invited commentaries Dr. Grady mentioned in her editor’s note point both to the importance of the team’s findings and the need for better understanding of factors that may affect the choice of which OUD medication to use.
A commentary by Max Jordan Nguemeni Tiako, MD, MS, with the Department of Medicine, Brigham and Women’s Hospital, and colleagues, said that while the Suarez et al. data are important to share with patients, “the ultimate treatment decision must be the result of shared decision-making between a knowledgeable clinician and the patient, rather than promoting one medication over another.”
They urge putting the findings in context given the study population, which comprises a relatively stable group of women with OUD, most of whom were taking OUD medications before they got pregnant. The study sample excludes a substantial number of women who are chronically underinsured or uninsured, Dr. Tiako’s team wrote, because those included were enrolled in Medicaid for 3 consecutive months before pregnancy.
“We urge caution when extrapolating these findings to newly pregnant individuals with untreated OUD,” they wrote.
Both Medications are Safe
Cara Poland, MD, MEd, with the Henry Ford Health + Michigan State University Health Sciences in Grand Rapids, and coauthors, added in another commentary that Suarez et al. didn’t include a comparison between the population-level congenital defect rate and the defect rate for people using medications for OUD in pregnancy.
That comparison, they wrote, would have better illustrated the safety of medications for OUD “instead of simply comparing two medications with long-standing safety data.”
When a clinician starts a woman on medication for OUD in pregnancy, it’s important to understand several factors, including individual access to and comfort with different treatment approaches, they noted. It’s also important to weigh whether changing medications is worth the potential drawbacks of disrupting their well-managed care.
They wrote that the paper by Suarez et al. does not make the case for switching medications based on their findings.
Internists, they added, are ideal experts to explain risk of fetal abnormalities in the wider context of supporting engagement with continuous medication for OUD.
“In the absence of other concerns, switching medications (methadone to buprenorphine) or — worse — discontinuing [medication for] OUD because of this study runs counter to the substantial evidence regarding the safety of these medications during pregnancy,” Dr. Poland’s team wrote. “No treatment is without risk in pregnancy.”
This study was supported by the National Institute on Drug Abuse. In the Suarez et al. study, coauthors Dr. Hernández-Díaz, Dr. Gray, Dr. Connery, Dr. Zhu, and Dr. Huybrechts reported grants, personal fees and consulting payments from several pharmaceutical companies. Dr. Grady reports no relevant financial relationships in her editor’s note. No relevant financial relationships were reported by authors of the Tiako et al. commentary.
Regarding the commentary by Poland et al., grants were reported from the Michigan Health Endowment Fund, the Michigan Department of Health and Human Services, the National Institute on Drug Abuse and Blue Cross Blue Shield of Michigan outside the submitted work. No other disclosures were reported.
Buprenorphine use, compared with methadone use, in pregnancy has been linked with a slightly lower risk of major congenital malformations in a new study of medications for opioid use disorder (OUD).
Elizabeth A. Suarez, PhD, MPH, with the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues published the findings in JAMA Internal Medicine.
The lower risk for buprenorphine was small (risk ratio, 0.82; 95% confidence interval [CI], 0.69-0.97), and methadone use should not be ruled out on that basis, the authors wrote. For some women, particularly those on stable treatment before pregnancy or women who do not respond well to buprenorphine, methadone may be the better choice, they explained.
Either Medication Better Than Not Treating
The authors noted that either medication “is strongly recommended over untreated OUD during pregnancy.”
JAMA Internal Medicine Deputy Editor Deborah Grady, MD, MPH, with the Department of Medicine, University of California, San Francisco, emphasized that recommendation in an editor’s note, highlighting that treatment for OUD is critical to prevent infections, overdose, and death in pregnant women as well as neonatal opioid withdrawal syndrome and fetal death.
She stressed that internists and other primary care physicians have a key role in ensuring pregnant women with OUD receive appropriate treatment.
Given the importance of the issue, she wrote, “we have taken the unusual step of publishing two accompanying invited commentaries.”
Two developments may help increase use of buprenorphine, the study authors wrote. One is a recent study showing lower risk of adverse neonatal outcomes when buprenorphine is used during pregnancy compared with methadone. Another is the removal last year of the prescribing waiver for buprenorphine.
Study Included Medicaid Data Over 18 Years
The population-based cohort study used data from publicly insured Medicaid beneficiaries from 2000 to 2018. Pregnancies with enrollment from 90 days before pregnancy through 1 month after delivery and first-trimester use of buprenorphine or methadone were included (n = 13,360). The data were linked with infants’ health data.
The study group included 9,514 pregnancies with first-trimester buprenorphine exposure and 3,846 with methadone exposure. The risk of malformations overall was 50.9 (95% CI, 46.5-55.3) per 1000 pregnancies for buprenorphine and 60.6 (95% CI, 53.0-68.1) per 1000 pregnancies for methadone.
Major malformations were any cardiac malformations, ventricular septal defect, secundum atrial septal defect/nonprematurity-related patent foramen ovale, neural tube defects, oral clefts, and clubfoot.
Two Invited Commentaries Urge Caution in Interpretation
The two invited commentaries Dr. Grady mentioned in her editor’s note point both to the importance of the team’s findings and the need for better understanding of factors that may affect the choice of which OUD medication to use.
A commentary by Max Jordan Nguemeni Tiako, MD, MS, with the Department of Medicine, Brigham and Women’s Hospital, and colleagues, said that while the Suarez et al. data are important to share with patients, “the ultimate treatment decision must be the result of shared decision-making between a knowledgeable clinician and the patient, rather than promoting one medication over another.”
They urge putting the findings in context given the study population, which comprises a relatively stable group of women with OUD, most of whom were taking OUD medications before they got pregnant. The study sample excludes a substantial number of women who are chronically underinsured or uninsured, Dr. Tiako’s team wrote, because those included were enrolled in Medicaid for 3 consecutive months before pregnancy.
“We urge caution when extrapolating these findings to newly pregnant individuals with untreated OUD,” they wrote.
Both Medications are Safe
Cara Poland, MD, MEd, with the Henry Ford Health + Michigan State University Health Sciences in Grand Rapids, and coauthors, added in another commentary that Suarez et al. didn’t include a comparison between the population-level congenital defect rate and the defect rate for people using medications for OUD in pregnancy.
That comparison, they wrote, would have better illustrated the safety of medications for OUD “instead of simply comparing two medications with long-standing safety data.”
When a clinician starts a woman on medication for OUD in pregnancy, it’s important to understand several factors, including individual access to and comfort with different treatment approaches, they noted. It’s also important to weigh whether changing medications is worth the potential drawbacks of disrupting their well-managed care.
They wrote that the paper by Suarez et al. does not make the case for switching medications based on their findings.
Internists, they added, are ideal experts to explain risk of fetal abnormalities in the wider context of supporting engagement with continuous medication for OUD.
“In the absence of other concerns, switching medications (methadone to buprenorphine) or — worse — discontinuing [medication for] OUD because of this study runs counter to the substantial evidence regarding the safety of these medications during pregnancy,” Dr. Poland’s team wrote. “No treatment is without risk in pregnancy.”
This study was supported by the National Institute on Drug Abuse. In the Suarez et al. study, coauthors Dr. Hernández-Díaz, Dr. Gray, Dr. Connery, Dr. Zhu, and Dr. Huybrechts reported grants, personal fees and consulting payments from several pharmaceutical companies. Dr. Grady reports no relevant financial relationships in her editor’s note. No relevant financial relationships were reported by authors of the Tiako et al. commentary.
Regarding the commentary by Poland et al., grants were reported from the Michigan Health Endowment Fund, the Michigan Department of Health and Human Services, the National Institute on Drug Abuse and Blue Cross Blue Shield of Michigan outside the submitted work. No other disclosures were reported.
FROM JAMA INTERNAL MEDICINE
Smoking and Drinking Up the Risk for Diverticulitis
TOPLINE:
New data link smoking and heavy drinking with an increased risk for diverticulitis, with the greatest risk seen in adults who smoke and consume two or more drinks daily.
METHODOLOGY:
- Researchers studied 84,232 women in the Nurses’ Health Study II who were 39-52 years old and without known diverticulitis at baseline in 2003.
- In 2015 and 2017, participants were asked via questionnaire whether they had been diagnosed with diverticulitis requiring antibiotic therapy or hospitalization. Diverticulitis was defined as a computed tomography scan or pathology report of diverticulitis or a provider diagnosis with a clinical presentation consistent with diverticulitis.
- Smoking was assessed every 2 years and alcohol consumption every 4 years using standard questionnaires.
- Consistent with prior studies on risk factors for diverticulitis, multivariable models adjusted for age, menopausal hormone status and hormone use, body mass index, physical activity, aspirin/nonsteroidal anti-inflammatory drug use, intake of fiber and red/processed meat, and other factors were used.
TAKEAWAY:
- During more than 1 million person-years of follow-up, 3018 incident cases of diverticulitis were identified.
- Both current and past smoking were associated with increased risk for diverticulitis (hazard ratio [HR], 1.2) compared with never smoking, although no dose-response relationship was evident. In an analysis restricted to participants who had surgery for diverticulitis, the magnitude of the association was strengthened (HR, 1.48 for current smokers and 1.46 for past smokers vs never smokers).
- Consumption of ≥ 30 g/d of alcohol (2+ drinks/day) was associated with an increased risk for incident diverticulitis (HR, 1.26) compared with not drinking.
- A joint analysis of smoking and alcohol found that individuals who ever smoked and consumed ≥ 30 g/d of alcohol were at the highest risk for diverticulitis (multivariate HR, 1.53) compared with individuals who never smoked and reported no alcohol use.
IN PRACTICE:
“As there are currently no medical means to prevent diverticulitis other than dietary and lifestyle interventions, counseling patients about the avoidance of smoking and alcohol may help lower the risk for developing diverticulitis,” the authors concluded.
SOURCE:
The study, with first author Sara Gunby, MD, University of Washington School of Medicine, Seattle, was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
Diverticulitis diagnoses were self-reported, although a review of a subset of medical records confirmed the diagnosis in more than 90% of cases establishing the validity of self-report in this population. The study was limited to female nurses, so it is possible the findings may not be generalizable to men or other populations. Residual confounding may have impacted the results.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health. The authors declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
New data link smoking and heavy drinking with an increased risk for diverticulitis, with the greatest risk seen in adults who smoke and consume two or more drinks daily.
METHODOLOGY:
- Researchers studied 84,232 women in the Nurses’ Health Study II who were 39-52 years old and without known diverticulitis at baseline in 2003.
- In 2015 and 2017, participants were asked via questionnaire whether they had been diagnosed with diverticulitis requiring antibiotic therapy or hospitalization. Diverticulitis was defined as a computed tomography scan or pathology report of diverticulitis or a provider diagnosis with a clinical presentation consistent with diverticulitis.
- Smoking was assessed every 2 years and alcohol consumption every 4 years using standard questionnaires.
- Consistent with prior studies on risk factors for diverticulitis, multivariable models adjusted for age, menopausal hormone status and hormone use, body mass index, physical activity, aspirin/nonsteroidal anti-inflammatory drug use, intake of fiber and red/processed meat, and other factors were used.
TAKEAWAY:
- During more than 1 million person-years of follow-up, 3018 incident cases of diverticulitis were identified.
- Both current and past smoking were associated with increased risk for diverticulitis (hazard ratio [HR], 1.2) compared with never smoking, although no dose-response relationship was evident. In an analysis restricted to participants who had surgery for diverticulitis, the magnitude of the association was strengthened (HR, 1.48 for current smokers and 1.46 for past smokers vs never smokers).
- Consumption of ≥ 30 g/d of alcohol (2+ drinks/day) was associated with an increased risk for incident diverticulitis (HR, 1.26) compared with not drinking.
- A joint analysis of smoking and alcohol found that individuals who ever smoked and consumed ≥ 30 g/d of alcohol were at the highest risk for diverticulitis (multivariate HR, 1.53) compared with individuals who never smoked and reported no alcohol use.
IN PRACTICE:
“As there are currently no medical means to prevent diverticulitis other than dietary and lifestyle interventions, counseling patients about the avoidance of smoking and alcohol may help lower the risk for developing diverticulitis,” the authors concluded.
SOURCE:
The study, with first author Sara Gunby, MD, University of Washington School of Medicine, Seattle, was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
Diverticulitis diagnoses were self-reported, although a review of a subset of medical records confirmed the diagnosis in more than 90% of cases establishing the validity of self-report in this population. The study was limited to female nurses, so it is possible the findings may not be generalizable to men or other populations. Residual confounding may have impacted the results.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health. The authors declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
New data link smoking and heavy drinking with an increased risk for diverticulitis, with the greatest risk seen in adults who smoke and consume two or more drinks daily.
METHODOLOGY:
- Researchers studied 84,232 women in the Nurses’ Health Study II who were 39-52 years old and without known diverticulitis at baseline in 2003.
- In 2015 and 2017, participants were asked via questionnaire whether they had been diagnosed with diverticulitis requiring antibiotic therapy or hospitalization. Diverticulitis was defined as a computed tomography scan or pathology report of diverticulitis or a provider diagnosis with a clinical presentation consistent with diverticulitis.
- Smoking was assessed every 2 years and alcohol consumption every 4 years using standard questionnaires.
- Consistent with prior studies on risk factors for diverticulitis, multivariable models adjusted for age, menopausal hormone status and hormone use, body mass index, physical activity, aspirin/nonsteroidal anti-inflammatory drug use, intake of fiber and red/processed meat, and other factors were used.
TAKEAWAY:
- During more than 1 million person-years of follow-up, 3018 incident cases of diverticulitis were identified.
- Both current and past smoking were associated with increased risk for diverticulitis (hazard ratio [HR], 1.2) compared with never smoking, although no dose-response relationship was evident. In an analysis restricted to participants who had surgery for diverticulitis, the magnitude of the association was strengthened (HR, 1.48 for current smokers and 1.46 for past smokers vs never smokers).
- Consumption of ≥ 30 g/d of alcohol (2+ drinks/day) was associated with an increased risk for incident diverticulitis (HR, 1.26) compared with not drinking.
- A joint analysis of smoking and alcohol found that individuals who ever smoked and consumed ≥ 30 g/d of alcohol were at the highest risk for diverticulitis (multivariate HR, 1.53) compared with individuals who never smoked and reported no alcohol use.
IN PRACTICE:
“As there are currently no medical means to prevent diverticulitis other than dietary and lifestyle interventions, counseling patients about the avoidance of smoking and alcohol may help lower the risk for developing diverticulitis,” the authors concluded.
SOURCE:
The study, with first author Sara Gunby, MD, University of Washington School of Medicine, Seattle, was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
Diverticulitis diagnoses were self-reported, although a review of a subset of medical records confirmed the diagnosis in more than 90% of cases establishing the validity of self-report in this population. The study was limited to female nurses, so it is possible the findings may not be generalizable to men or other populations. Residual confounding may have impacted the results.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health. The authors declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Dopamine Fasting: Some MDs Are Prescribing It. Should You?
It’s an appealing concept: Stop addictive behaviors for a while — think social media, video games, gambling, porn, junk food, drugs, alcohol (dry January, anyone?) — to reset your brain’s reward circuitry, so you can feel great minus the bad habits.
TikTok influencers and Silicon Valley execs seem to think so. But so do some physicians.
Prominent among the proponents is Anna Lembke, MD, professor of psychiatry at Stanford University School of Medicine and chief of the Stanford Addiction Medicine Dual Diagnosis Clinic. There, the dopamine fast is an early intervention framework for many of her patients.
“What we have seen in those patients is that not only does craving begin to subside in about 4 weeks, but that mood and anxiety and sleep and all these other parameters and markers of good mental health also improve,” Dr. Lembke said.
Any clinician, regardless of background, can adopt this framework, the Dopamine Nation author said during her talk at the American College of Lifestyle Medicine (ACLM) conference last fall. “There is this idea in medicine that we have to leave addiction to the Betty Ford Clinic or to an addiction psychiatrist,” she told the gathering. “But there’s so much that we can do, no matter what our training and no matter our treatment setting.”
But is dopamine fasting right for your patients? Some experts said it’s an oversimplified or even dangerous approach. Here’s what to know.
Dopamine and the Brain
From the prefrontal cortex — your brain’s control center — to the nucleus accumbens and ventral tegmental area located deep in your limbic system, dopamine bridges gaps between neurons to deliver critical messages about pleasure, reward, and motivation.
We all have a baseline level of dopamine. Substances and behaviors we like — everything from chocolate and sex to cocaine and amphetamines — increase dopamine firing.
“When we seek healthy rewards, like a good meal out in a restaurant or having a nice chat with friends, dopaminergic neurons fire, and dopamine is released,” said Birgitta Dresp, PhD, a cognitive psychologist and research director with the Centre National de la Recherche Scientifique in Paris. “That gives us a good feeling.”
But over time, with chronic exposure to hyperpleasurable stimuli, your brain adapts. Dopamine receptors downregulate and shrink, and your “hedonic setpoint,” or baseline happiness level, drops. You now need more of your favorite stimuli to feel as good as you did before.
This primitive brain wiring served evolutionary purposes, helping our ancestors relentlessly pursue scarce resources like food. But in our modern world full of easily accessible, novel, potent, and stimulating activities, our brains are constantly trying to compensate. Paradoxically, this constant “self-titillation” may be contributing to our national and global mental health crisis, Dr. Lembke suggested.
“Human activity has changed the world we live in,” said Dr. Lembke, “and now this ancient mechanistic structure has become a liability of sorts.”
The Dopamine Fast in Action
To reset this wiring, Dr. Lembke recommended a 4-week fast from a person’s “drug of choice.” But this isn’t the trendy tech-bro quick cure-all where you abstain from everything that brings you joy. It’s a targeted intervention usually aimed at one behavior or substance at a time. The fast allows a person to understand “the nature of the hijacked brain,” and breaking free motivates them to change habits long term, said Dr. Lembke.
Although the first 2 weeks are difficult, she found that many patients feel better and more motivated after 4 weeks.
How do you identify patients who might benefit from a dopamine fast? Start with “how much” and proceed to “why.” Instead of asking how much of a substance or behavior they indulge in per week, which can be inaccurate, Dr. Lembke uses a “timeline follow-back” technique — how much yesterday, the day before that, and so on. This can lead to an “aha” moment when they see the week’s true total, she told the ACLM conference.
She also explored why they do it. Often patients say they are self-medicating or that the substance helps with their anxiety or depression. When people are compulsively continuing to use despite negative consequences, she might recommend a 4-week reset.
Important exceptions: Dr. Lembke did not recommend dopamine fasting to anyone who has repeatedly and unsuccessfully tried to quit a drug on their own nor anyone for whom withdrawal is life-threatening.
For people who can safely try the dopamine fast, she recommended “self-binding” strategies to help them stay the course. Consider the people, places, and things that encourage you to use, and try to avoid them. For example, delete your social media apps if you’re trying to detox from social media. Put physical distance between you and your phone. For foods and substances, keep them out of the house.
Dr. Lembke also recommended “hormesis,” painful but productive activities like exercise. Your brain’s system for pleasure and pain are closely related, so these activities affect reward circuitry.
“You’re intentionally doing things that are hard, which doesn’t initially release dopamine, in contrast to intoxicants, but you get a gradual increase that remains elevated even after that activity is stopped, which is a nice way to get dopamine indirectly,” she said.
If patients plan to resume their “drug of choice” after the dopamine fast, Dr. Lembke helps them plan how much they will consume and when. For some, this works. Others, unfortunately, go back to using as much or more than they did before. But in many cases, she said, patients feel better and find that their “drug of choice” wasn’t serving them as well as they thought.
Critiques of Dopamine Fasting
Dopamine fasting isn’t for everyone, and experts debate its safety and effectiveness. Here are some common concerns:
It’s too simplistic. Peter Grinspoon, MD, a primary care physician at Massachusetts General Hospital and instructor at Harvard Medical School, said dopamine fasting isn’t really fasting — you don’t have a finite store of dopamine to conserve or deplete in a fixed amount of time. Even if you abstain from certain pleasures, your brain will still produce some dopamine.
What makes more sense, he said, is gradual “dopamine retargeting,” seeking rewards from healthy pleasurable activities.
“Addiction is a disease of isolation, and learning to take pleasure in the healthy things in life, like a nice home-cooked meal or a walk in the woods or a hug or a swim in the ocean, is exactly what addiction recovery is about,” he said. “Because once you learn to do that and to be happy, there’s no longer any room for the drug and you’re not nearly as susceptible to relapse.”
A related concern is that the dopamine system isn’t the only part of your brain that matters in addiction. “There are other bits of the brain which are much more important for controlling temptation,” said Trevor W. Robbins, PhD, professor of cognitive neuroscience and director of research at the Behavioural and Clinical Neuroscience Institute at the University of Cambridge. Dopamine plays an important role in addiction and recovery, “but to call this a dopamine fast, it’s just a trendy saying to make it sound exciting,” he said.
Empirical evidence is lacking. Without clinical trials to back it up, dopamine fasting lacks evidence on safety and effectiveness, said David Tzall, PsyD, a psychologist practicing in Brooklyn. “It sounds kind of fun, right? To think like, oh, I’ll just stop doing this for a while, and my body will correct itself,” said Dr. Tzall. “I think that’s a very dangerous thing because we don’t have enough evidence on it to think of how it can be effective or how it can be dangerous.”
Dr. Lembke “would like to see more evidence, too,” beyond clinical observation and expert consensus. Future research could also reveal who is most likely to benefit and how long the fast should last for maximum benefit.
It’s too much a one-size-fits-all approach. “Stopping a drug of choice is going to look different for a lot of people,” said Dr. Tzall. Some people can quit smoking cold turkey; others need to phase it out. Some need nicotine patches; some don’t. Some can do it alone; others need help.
The individual’s why behind addiction is also crucial. Without their drug or habit, can they “cope with the stressors of life?” Dr. Tzall asked. They may need new strategies. And if they quit before they are ready and fail, they could end up feeling even worse than they did before.
Experts do agree on one thing: We can do more to help people who are struggling. “It’s very good that people are having discussions around tempering consumption because we clearly have a serious drug and alcohol addiction, obesity, and digital media problem,” said Dr. Lembke.
A version of this article appeared on Medscape.com.
It’s an appealing concept: Stop addictive behaviors for a while — think social media, video games, gambling, porn, junk food, drugs, alcohol (dry January, anyone?) — to reset your brain’s reward circuitry, so you can feel great minus the bad habits.
TikTok influencers and Silicon Valley execs seem to think so. But so do some physicians.
Prominent among the proponents is Anna Lembke, MD, professor of psychiatry at Stanford University School of Medicine and chief of the Stanford Addiction Medicine Dual Diagnosis Clinic. There, the dopamine fast is an early intervention framework for many of her patients.
“What we have seen in those patients is that not only does craving begin to subside in about 4 weeks, but that mood and anxiety and sleep and all these other parameters and markers of good mental health also improve,” Dr. Lembke said.
Any clinician, regardless of background, can adopt this framework, the Dopamine Nation author said during her talk at the American College of Lifestyle Medicine (ACLM) conference last fall. “There is this idea in medicine that we have to leave addiction to the Betty Ford Clinic or to an addiction psychiatrist,” she told the gathering. “But there’s so much that we can do, no matter what our training and no matter our treatment setting.”
But is dopamine fasting right for your patients? Some experts said it’s an oversimplified or even dangerous approach. Here’s what to know.
Dopamine and the Brain
From the prefrontal cortex — your brain’s control center — to the nucleus accumbens and ventral tegmental area located deep in your limbic system, dopamine bridges gaps between neurons to deliver critical messages about pleasure, reward, and motivation.
We all have a baseline level of dopamine. Substances and behaviors we like — everything from chocolate and sex to cocaine and amphetamines — increase dopamine firing.
“When we seek healthy rewards, like a good meal out in a restaurant or having a nice chat with friends, dopaminergic neurons fire, and dopamine is released,” said Birgitta Dresp, PhD, a cognitive psychologist and research director with the Centre National de la Recherche Scientifique in Paris. “That gives us a good feeling.”
But over time, with chronic exposure to hyperpleasurable stimuli, your brain adapts. Dopamine receptors downregulate and shrink, and your “hedonic setpoint,” or baseline happiness level, drops. You now need more of your favorite stimuli to feel as good as you did before.
This primitive brain wiring served evolutionary purposes, helping our ancestors relentlessly pursue scarce resources like food. But in our modern world full of easily accessible, novel, potent, and stimulating activities, our brains are constantly trying to compensate. Paradoxically, this constant “self-titillation” may be contributing to our national and global mental health crisis, Dr. Lembke suggested.
“Human activity has changed the world we live in,” said Dr. Lembke, “and now this ancient mechanistic structure has become a liability of sorts.”
The Dopamine Fast in Action
To reset this wiring, Dr. Lembke recommended a 4-week fast from a person’s “drug of choice.” But this isn’t the trendy tech-bro quick cure-all where you abstain from everything that brings you joy. It’s a targeted intervention usually aimed at one behavior or substance at a time. The fast allows a person to understand “the nature of the hijacked brain,” and breaking free motivates them to change habits long term, said Dr. Lembke.
Although the first 2 weeks are difficult, she found that many patients feel better and more motivated after 4 weeks.
How do you identify patients who might benefit from a dopamine fast? Start with “how much” and proceed to “why.” Instead of asking how much of a substance or behavior they indulge in per week, which can be inaccurate, Dr. Lembke uses a “timeline follow-back” technique — how much yesterday, the day before that, and so on. This can lead to an “aha” moment when they see the week’s true total, she told the ACLM conference.
She also explored why they do it. Often patients say they are self-medicating or that the substance helps with their anxiety or depression. When people are compulsively continuing to use despite negative consequences, she might recommend a 4-week reset.
Important exceptions: Dr. Lembke did not recommend dopamine fasting to anyone who has repeatedly and unsuccessfully tried to quit a drug on their own nor anyone for whom withdrawal is life-threatening.
For people who can safely try the dopamine fast, she recommended “self-binding” strategies to help them stay the course. Consider the people, places, and things that encourage you to use, and try to avoid them. For example, delete your social media apps if you’re trying to detox from social media. Put physical distance between you and your phone. For foods and substances, keep them out of the house.
Dr. Lembke also recommended “hormesis,” painful but productive activities like exercise. Your brain’s system for pleasure and pain are closely related, so these activities affect reward circuitry.
“You’re intentionally doing things that are hard, which doesn’t initially release dopamine, in contrast to intoxicants, but you get a gradual increase that remains elevated even after that activity is stopped, which is a nice way to get dopamine indirectly,” she said.
If patients plan to resume their “drug of choice” after the dopamine fast, Dr. Lembke helps them plan how much they will consume and when. For some, this works. Others, unfortunately, go back to using as much or more than they did before. But in many cases, she said, patients feel better and find that their “drug of choice” wasn’t serving them as well as they thought.
Critiques of Dopamine Fasting
Dopamine fasting isn’t for everyone, and experts debate its safety and effectiveness. Here are some common concerns:
It’s too simplistic. Peter Grinspoon, MD, a primary care physician at Massachusetts General Hospital and instructor at Harvard Medical School, said dopamine fasting isn’t really fasting — you don’t have a finite store of dopamine to conserve or deplete in a fixed amount of time. Even if you abstain from certain pleasures, your brain will still produce some dopamine.
What makes more sense, he said, is gradual “dopamine retargeting,” seeking rewards from healthy pleasurable activities.
“Addiction is a disease of isolation, and learning to take pleasure in the healthy things in life, like a nice home-cooked meal or a walk in the woods or a hug or a swim in the ocean, is exactly what addiction recovery is about,” he said. “Because once you learn to do that and to be happy, there’s no longer any room for the drug and you’re not nearly as susceptible to relapse.”
A related concern is that the dopamine system isn’t the only part of your brain that matters in addiction. “There are other bits of the brain which are much more important for controlling temptation,” said Trevor W. Robbins, PhD, professor of cognitive neuroscience and director of research at the Behavioural and Clinical Neuroscience Institute at the University of Cambridge. Dopamine plays an important role in addiction and recovery, “but to call this a dopamine fast, it’s just a trendy saying to make it sound exciting,” he said.
Empirical evidence is lacking. Without clinical trials to back it up, dopamine fasting lacks evidence on safety and effectiveness, said David Tzall, PsyD, a psychologist practicing in Brooklyn. “It sounds kind of fun, right? To think like, oh, I’ll just stop doing this for a while, and my body will correct itself,” said Dr. Tzall. “I think that’s a very dangerous thing because we don’t have enough evidence on it to think of how it can be effective or how it can be dangerous.”
Dr. Lembke “would like to see more evidence, too,” beyond clinical observation and expert consensus. Future research could also reveal who is most likely to benefit and how long the fast should last for maximum benefit.
It’s too much a one-size-fits-all approach. “Stopping a drug of choice is going to look different for a lot of people,” said Dr. Tzall. Some people can quit smoking cold turkey; others need to phase it out. Some need nicotine patches; some don’t. Some can do it alone; others need help.
The individual’s why behind addiction is also crucial. Without their drug or habit, can they “cope with the stressors of life?” Dr. Tzall asked. They may need new strategies. And if they quit before they are ready and fail, they could end up feeling even worse than they did before.
Experts do agree on one thing: We can do more to help people who are struggling. “It’s very good that people are having discussions around tempering consumption because we clearly have a serious drug and alcohol addiction, obesity, and digital media problem,” said Dr. Lembke.
A version of this article appeared on Medscape.com.
It’s an appealing concept: Stop addictive behaviors for a while — think social media, video games, gambling, porn, junk food, drugs, alcohol (dry January, anyone?) — to reset your brain’s reward circuitry, so you can feel great minus the bad habits.
TikTok influencers and Silicon Valley execs seem to think so. But so do some physicians.
Prominent among the proponents is Anna Lembke, MD, professor of psychiatry at Stanford University School of Medicine and chief of the Stanford Addiction Medicine Dual Diagnosis Clinic. There, the dopamine fast is an early intervention framework for many of her patients.
“What we have seen in those patients is that not only does craving begin to subside in about 4 weeks, but that mood and anxiety and sleep and all these other parameters and markers of good mental health also improve,” Dr. Lembke said.
Any clinician, regardless of background, can adopt this framework, the Dopamine Nation author said during her talk at the American College of Lifestyle Medicine (ACLM) conference last fall. “There is this idea in medicine that we have to leave addiction to the Betty Ford Clinic or to an addiction psychiatrist,” she told the gathering. “But there’s so much that we can do, no matter what our training and no matter our treatment setting.”
But is dopamine fasting right for your patients? Some experts said it’s an oversimplified or even dangerous approach. Here’s what to know.
Dopamine and the Brain
From the prefrontal cortex — your brain’s control center — to the nucleus accumbens and ventral tegmental area located deep in your limbic system, dopamine bridges gaps between neurons to deliver critical messages about pleasure, reward, and motivation.
We all have a baseline level of dopamine. Substances and behaviors we like — everything from chocolate and sex to cocaine and amphetamines — increase dopamine firing.
“When we seek healthy rewards, like a good meal out in a restaurant or having a nice chat with friends, dopaminergic neurons fire, and dopamine is released,” said Birgitta Dresp, PhD, a cognitive psychologist and research director with the Centre National de la Recherche Scientifique in Paris. “That gives us a good feeling.”
But over time, with chronic exposure to hyperpleasurable stimuli, your brain adapts. Dopamine receptors downregulate and shrink, and your “hedonic setpoint,” or baseline happiness level, drops. You now need more of your favorite stimuli to feel as good as you did before.
This primitive brain wiring served evolutionary purposes, helping our ancestors relentlessly pursue scarce resources like food. But in our modern world full of easily accessible, novel, potent, and stimulating activities, our brains are constantly trying to compensate. Paradoxically, this constant “self-titillation” may be contributing to our national and global mental health crisis, Dr. Lembke suggested.
“Human activity has changed the world we live in,” said Dr. Lembke, “and now this ancient mechanistic structure has become a liability of sorts.”
The Dopamine Fast in Action
To reset this wiring, Dr. Lembke recommended a 4-week fast from a person’s “drug of choice.” But this isn’t the trendy tech-bro quick cure-all where you abstain from everything that brings you joy. It’s a targeted intervention usually aimed at one behavior or substance at a time. The fast allows a person to understand “the nature of the hijacked brain,” and breaking free motivates them to change habits long term, said Dr. Lembke.
Although the first 2 weeks are difficult, she found that many patients feel better and more motivated after 4 weeks.
How do you identify patients who might benefit from a dopamine fast? Start with “how much” and proceed to “why.” Instead of asking how much of a substance or behavior they indulge in per week, which can be inaccurate, Dr. Lembke uses a “timeline follow-back” technique — how much yesterday, the day before that, and so on. This can lead to an “aha” moment when they see the week’s true total, she told the ACLM conference.
She also explored why they do it. Often patients say they are self-medicating or that the substance helps with their anxiety or depression. When people are compulsively continuing to use despite negative consequences, she might recommend a 4-week reset.
Important exceptions: Dr. Lembke did not recommend dopamine fasting to anyone who has repeatedly and unsuccessfully tried to quit a drug on their own nor anyone for whom withdrawal is life-threatening.
For people who can safely try the dopamine fast, she recommended “self-binding” strategies to help them stay the course. Consider the people, places, and things that encourage you to use, and try to avoid them. For example, delete your social media apps if you’re trying to detox from social media. Put physical distance between you and your phone. For foods and substances, keep them out of the house.
Dr. Lembke also recommended “hormesis,” painful but productive activities like exercise. Your brain’s system for pleasure and pain are closely related, so these activities affect reward circuitry.
“You’re intentionally doing things that are hard, which doesn’t initially release dopamine, in contrast to intoxicants, but you get a gradual increase that remains elevated even after that activity is stopped, which is a nice way to get dopamine indirectly,” she said.
If patients plan to resume their “drug of choice” after the dopamine fast, Dr. Lembke helps them plan how much they will consume and when. For some, this works. Others, unfortunately, go back to using as much or more than they did before. But in many cases, she said, patients feel better and find that their “drug of choice” wasn’t serving them as well as they thought.
Critiques of Dopamine Fasting
Dopamine fasting isn’t for everyone, and experts debate its safety and effectiveness. Here are some common concerns:
It’s too simplistic. Peter Grinspoon, MD, a primary care physician at Massachusetts General Hospital and instructor at Harvard Medical School, said dopamine fasting isn’t really fasting — you don’t have a finite store of dopamine to conserve or deplete in a fixed amount of time. Even if you abstain from certain pleasures, your brain will still produce some dopamine.
What makes more sense, he said, is gradual “dopamine retargeting,” seeking rewards from healthy pleasurable activities.
“Addiction is a disease of isolation, and learning to take pleasure in the healthy things in life, like a nice home-cooked meal or a walk in the woods or a hug or a swim in the ocean, is exactly what addiction recovery is about,” he said. “Because once you learn to do that and to be happy, there’s no longer any room for the drug and you’re not nearly as susceptible to relapse.”
A related concern is that the dopamine system isn’t the only part of your brain that matters in addiction. “There are other bits of the brain which are much more important for controlling temptation,” said Trevor W. Robbins, PhD, professor of cognitive neuroscience and director of research at the Behavioural and Clinical Neuroscience Institute at the University of Cambridge. Dopamine plays an important role in addiction and recovery, “but to call this a dopamine fast, it’s just a trendy saying to make it sound exciting,” he said.
Empirical evidence is lacking. Without clinical trials to back it up, dopamine fasting lacks evidence on safety and effectiveness, said David Tzall, PsyD, a psychologist practicing in Brooklyn. “It sounds kind of fun, right? To think like, oh, I’ll just stop doing this for a while, and my body will correct itself,” said Dr. Tzall. “I think that’s a very dangerous thing because we don’t have enough evidence on it to think of how it can be effective or how it can be dangerous.”
Dr. Lembke “would like to see more evidence, too,” beyond clinical observation and expert consensus. Future research could also reveal who is most likely to benefit and how long the fast should last for maximum benefit.
It’s too much a one-size-fits-all approach. “Stopping a drug of choice is going to look different for a lot of people,” said Dr. Tzall. Some people can quit smoking cold turkey; others need to phase it out. Some need nicotine patches; some don’t. Some can do it alone; others need help.
The individual’s why behind addiction is also crucial. Without their drug or habit, can they “cope with the stressors of life?” Dr. Tzall asked. They may need new strategies. And if they quit before they are ready and fail, they could end up feeling even worse than they did before.
Experts do agree on one thing: We can do more to help people who are struggling. “It’s very good that people are having discussions around tempering consumption because we clearly have a serious drug and alcohol addiction, obesity, and digital media problem,” said Dr. Lembke.
A version of this article appeared on Medscape.com.
SUDs rates highest in head, neck, and gastric cancer survivors
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
FROM JAMA ONCOLOGY
‘Fake Xanax’ Tied to Seizures, Coma Is Resistant to Naloxone
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
FROM THE MORBIDITY AND MORTALITY WEEKLY REPORT
How an Obesity Drug Could Help Alcohol Use Disorder
The glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide has made headlines as a US Food and Drug Administration (FDA)–approved treatment for type 2 diabetes (Ozempic) and obesity (Wegovy).
Recently,
“There is some really interesting preclinical research in rodents and monkeys that shows that GLP-1 agonist molecules, like semaglutide, have the effect of reducing the consumption of not just food, but also alcohol, nicotine, cocaine and amphetamines,” Kyle Simmons, PhD, professor of pharmacology and physiology at Oklahoma State University Center for Health Sciences in Tulsa, said in an interview.
Some of that early research was conducted by Elisabet Jerlhag Holm, PhD, and colleagues at University of Gothenburg, Sweden.
“We have worked on GLP-1 and alcohol since 2012, and observe promising effects,” Holm told this news organization.
Her team published two studies earlier this year — one in one in Frontiers in Pharmacology and the other in eBioMedicine — demonstrating that semaglutide, in low doses, reduces alcohol intake in male and female rats.
“We have shown that semaglutide binds to the nucleus accumbens — an area of the brain associated with reward. We have also shown that semaglutide alters the dopamine metabolism when alcohol is on board. This provides a tentative mechanism,” Dr. Holm said.
First Human Data
The preclinical data fueled interest in testing the value of the GLP-1 agonist in patient populations with addiction.
Dr. Simmons and colleagues have now published what is believed to be the first evidence in humans that semaglutide specifically reduces the symptoms of alcohol use disorder (AUD).
In a report published online on November 27 in The Journal of Clinical Psychiatry, they describe six patients (of whom five are female; mean age, 43 years) who received semaglutide treatment in the course of pharmacotherapy for weight loss.
All six screened positive for AUD on the Alcohol Use Disorders Identification Test (AUDIT), and all six showed significant improvement in their alcohol-related symptoms after starting semaglutide.
An AUDIT score > 8 is considered positive. The mean AUDIT score at baseline was 14. It fell to 4.5 on average after semaglutide treatment. The mean 9.5-point decrease in AUDIT scores with semaglutide was statistically significant (P < .001).
The patients were followed up from a few weeks to almost 9 months, and all of them had a reduction in AUD symptoms. At the various follow-up time points, all six patients had AUDIT scores consistent with “low-risk” drinking.
Strong Response at Low Doses
“There was a very strong response, even at a very low dose,” lead author Jesse Richards, DO, director of obesity medicine and assistant professor of medicine University of Oklahoma School of Community Medicine, Tulsa, said in an interview.
Three patients were treated with 0.5 mg of semaglutide weekly, two with 0.25 mg weekly, and one with 1 mg weekly. These doses are lower than those currently approved for treatment of type 2 diabetes and obesity.
Dr. Holm is not surprised by the results in these six patients. “Based on our preclinical data, this outcome is expected. The data are promising and bigger studies needed,” she said.
Simmons is currently leading a randomized placebo-controlled trial to further test the impact of semaglutide on AUD.
The STAR (Semaglutide Therapy for Alcohol Reduction) study is funded by the Hardesty Family Foundation and Oklahoma State University Center for Health Sciences.
A sister study is also currently underway in Baltimore, funded by the National Institute on Drug Abuse.
Hopefully, these studies will be able to “definitively tell us whether semaglutide is safe and effective for treatment” for AUD, Dr. Simmons said in a statement.
Despite being a major cause of preventable death worldwide, AUD currently has only three FDA-approved pharmacotherapies. However, there has been limited uptake of these drugs.
“There remains a significant treatment gap and need for new and novel or perhaps better tolerated or different mechanism treatment options for patients,” Dr. Richards said.
The preclinical and early clinical data provide a “signal” of a treatment effect for semaglutide in AUD, Dr. Richards said. The randomized controlled trials now underway should be concluding in the next 1-2 years, “at which point we’ll have a much better sense of the safety and efficacy of this drug for AUD,” he said.
The case series had no specific funding. Dr. Richards is on speakers bureaus for Rhythm Pharmaceuticals and Novo Nordisk and is on an advisory board for Rhythm Pharmaceuticals. Simmons is the recipient of a grant from the Hardesty Family Foundation to support an ongoing clinical trial of semaglutide in the treatment of AUD. Dr. Holm has no relevant disclosures.
A version of this article appeared on Medscape.com.
The glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide has made headlines as a US Food and Drug Administration (FDA)–approved treatment for type 2 diabetes (Ozempic) and obesity (Wegovy).
Recently,
“There is some really interesting preclinical research in rodents and monkeys that shows that GLP-1 agonist molecules, like semaglutide, have the effect of reducing the consumption of not just food, but also alcohol, nicotine, cocaine and amphetamines,” Kyle Simmons, PhD, professor of pharmacology and physiology at Oklahoma State University Center for Health Sciences in Tulsa, said in an interview.
Some of that early research was conducted by Elisabet Jerlhag Holm, PhD, and colleagues at University of Gothenburg, Sweden.
“We have worked on GLP-1 and alcohol since 2012, and observe promising effects,” Holm told this news organization.
Her team published two studies earlier this year — one in one in Frontiers in Pharmacology and the other in eBioMedicine — demonstrating that semaglutide, in low doses, reduces alcohol intake in male and female rats.
“We have shown that semaglutide binds to the nucleus accumbens — an area of the brain associated with reward. We have also shown that semaglutide alters the dopamine metabolism when alcohol is on board. This provides a tentative mechanism,” Dr. Holm said.
First Human Data
The preclinical data fueled interest in testing the value of the GLP-1 agonist in patient populations with addiction.
Dr. Simmons and colleagues have now published what is believed to be the first evidence in humans that semaglutide specifically reduces the symptoms of alcohol use disorder (AUD).
In a report published online on November 27 in The Journal of Clinical Psychiatry, they describe six patients (of whom five are female; mean age, 43 years) who received semaglutide treatment in the course of pharmacotherapy for weight loss.
All six screened positive for AUD on the Alcohol Use Disorders Identification Test (AUDIT), and all six showed significant improvement in their alcohol-related symptoms after starting semaglutide.
An AUDIT score > 8 is considered positive. The mean AUDIT score at baseline was 14. It fell to 4.5 on average after semaglutide treatment. The mean 9.5-point decrease in AUDIT scores with semaglutide was statistically significant (P < .001).
The patients were followed up from a few weeks to almost 9 months, and all of them had a reduction in AUD symptoms. At the various follow-up time points, all six patients had AUDIT scores consistent with “low-risk” drinking.
Strong Response at Low Doses
“There was a very strong response, even at a very low dose,” lead author Jesse Richards, DO, director of obesity medicine and assistant professor of medicine University of Oklahoma School of Community Medicine, Tulsa, said in an interview.
Three patients were treated with 0.5 mg of semaglutide weekly, two with 0.25 mg weekly, and one with 1 mg weekly. These doses are lower than those currently approved for treatment of type 2 diabetes and obesity.
Dr. Holm is not surprised by the results in these six patients. “Based on our preclinical data, this outcome is expected. The data are promising and bigger studies needed,” she said.
Simmons is currently leading a randomized placebo-controlled trial to further test the impact of semaglutide on AUD.
The STAR (Semaglutide Therapy for Alcohol Reduction) study is funded by the Hardesty Family Foundation and Oklahoma State University Center for Health Sciences.
A sister study is also currently underway in Baltimore, funded by the National Institute on Drug Abuse.
Hopefully, these studies will be able to “definitively tell us whether semaglutide is safe and effective for treatment” for AUD, Dr. Simmons said in a statement.
Despite being a major cause of preventable death worldwide, AUD currently has only three FDA-approved pharmacotherapies. However, there has been limited uptake of these drugs.
“There remains a significant treatment gap and need for new and novel or perhaps better tolerated or different mechanism treatment options for patients,” Dr. Richards said.
The preclinical and early clinical data provide a “signal” of a treatment effect for semaglutide in AUD, Dr. Richards said. The randomized controlled trials now underway should be concluding in the next 1-2 years, “at which point we’ll have a much better sense of the safety and efficacy of this drug for AUD,” he said.
The case series had no specific funding. Dr. Richards is on speakers bureaus for Rhythm Pharmaceuticals and Novo Nordisk and is on an advisory board for Rhythm Pharmaceuticals. Simmons is the recipient of a grant from the Hardesty Family Foundation to support an ongoing clinical trial of semaglutide in the treatment of AUD. Dr. Holm has no relevant disclosures.
A version of this article appeared on Medscape.com.
The glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide has made headlines as a US Food and Drug Administration (FDA)–approved treatment for type 2 diabetes (Ozempic) and obesity (Wegovy).
Recently,
“There is some really interesting preclinical research in rodents and monkeys that shows that GLP-1 agonist molecules, like semaglutide, have the effect of reducing the consumption of not just food, but also alcohol, nicotine, cocaine and amphetamines,” Kyle Simmons, PhD, professor of pharmacology and physiology at Oklahoma State University Center for Health Sciences in Tulsa, said in an interview.
Some of that early research was conducted by Elisabet Jerlhag Holm, PhD, and colleagues at University of Gothenburg, Sweden.
“We have worked on GLP-1 and alcohol since 2012, and observe promising effects,” Holm told this news organization.
Her team published two studies earlier this year — one in one in Frontiers in Pharmacology and the other in eBioMedicine — demonstrating that semaglutide, in low doses, reduces alcohol intake in male and female rats.
“We have shown that semaglutide binds to the nucleus accumbens — an area of the brain associated with reward. We have also shown that semaglutide alters the dopamine metabolism when alcohol is on board. This provides a tentative mechanism,” Dr. Holm said.
First Human Data
The preclinical data fueled interest in testing the value of the GLP-1 agonist in patient populations with addiction.
Dr. Simmons and colleagues have now published what is believed to be the first evidence in humans that semaglutide specifically reduces the symptoms of alcohol use disorder (AUD).
In a report published online on November 27 in The Journal of Clinical Psychiatry, they describe six patients (of whom five are female; mean age, 43 years) who received semaglutide treatment in the course of pharmacotherapy for weight loss.
All six screened positive for AUD on the Alcohol Use Disorders Identification Test (AUDIT), and all six showed significant improvement in their alcohol-related symptoms after starting semaglutide.
An AUDIT score > 8 is considered positive. The mean AUDIT score at baseline was 14. It fell to 4.5 on average after semaglutide treatment. The mean 9.5-point decrease in AUDIT scores with semaglutide was statistically significant (P < .001).
The patients were followed up from a few weeks to almost 9 months, and all of them had a reduction in AUD symptoms. At the various follow-up time points, all six patients had AUDIT scores consistent with “low-risk” drinking.
Strong Response at Low Doses
“There was a very strong response, even at a very low dose,” lead author Jesse Richards, DO, director of obesity medicine and assistant professor of medicine University of Oklahoma School of Community Medicine, Tulsa, said in an interview.
Three patients were treated with 0.5 mg of semaglutide weekly, two with 0.25 mg weekly, and one with 1 mg weekly. These doses are lower than those currently approved for treatment of type 2 diabetes and obesity.
Dr. Holm is not surprised by the results in these six patients. “Based on our preclinical data, this outcome is expected. The data are promising and bigger studies needed,” she said.
Simmons is currently leading a randomized placebo-controlled trial to further test the impact of semaglutide on AUD.
The STAR (Semaglutide Therapy for Alcohol Reduction) study is funded by the Hardesty Family Foundation and Oklahoma State University Center for Health Sciences.
A sister study is also currently underway in Baltimore, funded by the National Institute on Drug Abuse.
Hopefully, these studies will be able to “definitively tell us whether semaglutide is safe and effective for treatment” for AUD, Dr. Simmons said in a statement.
Despite being a major cause of preventable death worldwide, AUD currently has only three FDA-approved pharmacotherapies. However, there has been limited uptake of these drugs.
“There remains a significant treatment gap and need for new and novel or perhaps better tolerated or different mechanism treatment options for patients,” Dr. Richards said.
The preclinical and early clinical data provide a “signal” of a treatment effect for semaglutide in AUD, Dr. Richards said. The randomized controlled trials now underway should be concluding in the next 1-2 years, “at which point we’ll have a much better sense of the safety and efficacy of this drug for AUD,” he said.
The case series had no specific funding. Dr. Richards is on speakers bureaus for Rhythm Pharmaceuticals and Novo Nordisk and is on an advisory board for Rhythm Pharmaceuticals. Simmons is the recipient of a grant from the Hardesty Family Foundation to support an ongoing clinical trial of semaglutide in the treatment of AUD. Dr. Holm has no relevant disclosures.
A version of this article appeared on Medscape.com.
Screening for alcohol use disorder cuts hospital readmission
Actively drinking patients who undergo screening, a brief intervention, and referral to treatment (SBIRT) for alcohol use disorder during hospital admission for alcohol-related conditions have fewer 30- and 90-day readmissions for alcohol-related liver disease, a new study suggests.
Nevertheless, SBIRT was administered to only 51.7% of patients admitted for alcohol-associated hepatitis (AAH) and 23.7% of patients admitted for decompensated alcohol-related cirrhosis (DARLC).
“Not only did conducting SBIRT with patients admitted for AAH reduce 30-day and 90-day liver-related readmissions, but even just being offered SBIRT reduced readmissions, too,” study author Dennis Wang, MD, of the adult gastroenterology residency program at McMaster University in Hamilton, Ontario, told this news organization. “The exact reason for this effect is unclear, but one can speculate that offering SBIRT to AAH patients may trigger them to consider abstaining from alcohol.”
By contrast, receiving or being offered SBIRT had no effect on readmissions for patients with DARLC.
The findings were published online on November 30 in the Journal of the Canadian Association of Gastroenterology.
Readmissions Significantly Reduced
The researchers retrospectively reviewed the electronic medical records of patients with AAH or DARLC who were admitted to Hamilton Health Sciences hospitals in Ontario from January 2017 to December 2021. Eligible patients were aged ≥ 18 years and actively drinking.
The study’s primary outcomes were the proportion of admissions in which SBIRT was conducted and the association between conducting SBIRT and 30- and 90-day readmissions for recurrent AAH or DARLC.
There were 120 admissions for AAH, representing 95 patients, 95 index admissions, 18 patients with 30-day readmissions, and 26 patients with 90-day readmissions. The sum of the index AAH admissions and 90-day readmissions was greater than the total number of AAH admissions because readmissions where patients were no longer actively drinking alcohol were included.
There were 177 admissions for DARLC, representing 132 patients, 132 index admissions, 13 30-day readmissions, and 31 90-day readmissions.
The mean age of patients admitted with AAH (47.7 years) was significantly lower than that of patients admitted with DARLC (58.2 years). Fewer men were admitted with AAH (59.2%) than with DARLC (73.4%).
There was no significant difference between AAH admissions and DARLC admissions in hospital length of stay, Model for End-Stage Liver Disease on admission, same-admission mortality, and 30- or 90-day readmissions.
SBIRT was conducted in 62 of 120 AAH admissions (51.7%) and 42 of 177 DARLC admissions (23.7%), mainly by social workers and addiction counselors and occasionally by physicians alone.
“Sometimes patients with AAH or DARLC can become so ill that they cannot participate in SBIRT,” noted Dr. Wang. “In addition, there may not be enough health care providers, resources, or time available to conduct high-quality SBIRT with all patients admitted to hospital.”
For patients with AAH, SBIRT was associated with significantly reduced 30-day (odds ratio [OR], 0.098) and 90-day (OR, 0.166) likelihood of readmission for recurrent AAH. However, there was no association with readmissions for patients with DARLC.
Liver Scarring Persists
“We suspect that DARLC patients do not see the same improvement in liver-related readmissions after receiving SBIRT because the liver scarring typically persists even with alcohol abstinence, and this scarring causes further decompensations,” said Dr. Wang.
“Physicians, social workers, addiction counselors, and other allied health providers should collaborate to conduct SBIRT for all actively drinking patients admitted for AAH or DARLC,” wrote the authors.
The researchers acknowledged that their study was limited by its inclusion of data from only a single center. The admissions for AAH and DARLC had a higher proportion of male patients than female patients, thus limiting the generalizability of the findings. In addition, there was a lack of data on ethnicity and socioeconomic status, which could affect readmissions.
Dr. Wang advises clinicians to “seek out and connect with other healthcare providers in their local and regional community, such as addiction counselors or psychologists, to build a robust referral network for patients wanting to reduce their alcohol use.”
In addition, “providers should become comfortable with asking patients nonjudgmentally about alcohol use, as this builds the initial rapport that lays the foundation for ongoing care,” he said. “Every interaction with a patient is a new opportunity to guide interested patients towards alcohol cessation.”
Multidisciplinary Team Essential
Commenting on the findings, Meena B. Bansal, MD, professor of medicine and director of translational research in liver diseases at the Icahn School of Medicine at Mount Sinai in New York, said that they reflect clinical experience in US hospitals. “Physicians are so busy handling the acute medical situation posed by the admission that while they do certainly tell the patient they should stop drinking, full discussion, intervention, and linkage to outpatient programs is often led by the social worker,” she said. Dr. Bansal was not involved in the study.
“Many alcohol use disorder therapies are not tested in extremely ill patients, and thus, pharmacotherapy is often reserved for outpatient management, when patients are more clinically stable,” she said. Yet, as mentioned by the authors, a recent study “showed that 71% of providers never prescribed pharmacotherapy for alcohol use disorder, with the most common reason being low comfort with the medications. We need to increase education around pharmacotherapy for alcohol use disorder to increase the comfort level of practicing gastroenterologists and hepatologists.”
Furthermore, she said, clinicians need to intervene and provide guidance to patients “wherever and whenever they touch our system, whether that be in the inpatient or outpatient setting, [and] provide SBIRT during inpatient admissions but then follow patients longitudinally in a multidisciplinary team to achieve long-term results.”
The study was conducted without external funding. Dr. Wang had no relevant conflicts to disclose. A coauthor acts as a consultant, clinical trial investigator, speaker, and member of the advisory board for AbbVie, Gilead, Intercept, and Novo Nordisk. He also acts as a speaker and member of the advisory board for Eisai and Lupin and as a clinical trial investigator for Madrigal.
A version of this article appeared on Medscape.com.
Actively drinking patients who undergo screening, a brief intervention, and referral to treatment (SBIRT) for alcohol use disorder during hospital admission for alcohol-related conditions have fewer 30- and 90-day readmissions for alcohol-related liver disease, a new study suggests.
Nevertheless, SBIRT was administered to only 51.7% of patients admitted for alcohol-associated hepatitis (AAH) and 23.7% of patients admitted for decompensated alcohol-related cirrhosis (DARLC).
“Not only did conducting SBIRT with patients admitted for AAH reduce 30-day and 90-day liver-related readmissions, but even just being offered SBIRT reduced readmissions, too,” study author Dennis Wang, MD, of the adult gastroenterology residency program at McMaster University in Hamilton, Ontario, told this news organization. “The exact reason for this effect is unclear, but one can speculate that offering SBIRT to AAH patients may trigger them to consider abstaining from alcohol.”
By contrast, receiving or being offered SBIRT had no effect on readmissions for patients with DARLC.
The findings were published online on November 30 in the Journal of the Canadian Association of Gastroenterology.
Readmissions Significantly Reduced
The researchers retrospectively reviewed the electronic medical records of patients with AAH or DARLC who were admitted to Hamilton Health Sciences hospitals in Ontario from January 2017 to December 2021. Eligible patients were aged ≥ 18 years and actively drinking.
The study’s primary outcomes were the proportion of admissions in which SBIRT was conducted and the association between conducting SBIRT and 30- and 90-day readmissions for recurrent AAH or DARLC.
There were 120 admissions for AAH, representing 95 patients, 95 index admissions, 18 patients with 30-day readmissions, and 26 patients with 90-day readmissions. The sum of the index AAH admissions and 90-day readmissions was greater than the total number of AAH admissions because readmissions where patients were no longer actively drinking alcohol were included.
There were 177 admissions for DARLC, representing 132 patients, 132 index admissions, 13 30-day readmissions, and 31 90-day readmissions.
The mean age of patients admitted with AAH (47.7 years) was significantly lower than that of patients admitted with DARLC (58.2 years). Fewer men were admitted with AAH (59.2%) than with DARLC (73.4%).
There was no significant difference between AAH admissions and DARLC admissions in hospital length of stay, Model for End-Stage Liver Disease on admission, same-admission mortality, and 30- or 90-day readmissions.
SBIRT was conducted in 62 of 120 AAH admissions (51.7%) and 42 of 177 DARLC admissions (23.7%), mainly by social workers and addiction counselors and occasionally by physicians alone.
“Sometimes patients with AAH or DARLC can become so ill that they cannot participate in SBIRT,” noted Dr. Wang. “In addition, there may not be enough health care providers, resources, or time available to conduct high-quality SBIRT with all patients admitted to hospital.”
For patients with AAH, SBIRT was associated with significantly reduced 30-day (odds ratio [OR], 0.098) and 90-day (OR, 0.166) likelihood of readmission for recurrent AAH. However, there was no association with readmissions for patients with DARLC.
Liver Scarring Persists
“We suspect that DARLC patients do not see the same improvement in liver-related readmissions after receiving SBIRT because the liver scarring typically persists even with alcohol abstinence, and this scarring causes further decompensations,” said Dr. Wang.
“Physicians, social workers, addiction counselors, and other allied health providers should collaborate to conduct SBIRT for all actively drinking patients admitted for AAH or DARLC,” wrote the authors.
The researchers acknowledged that their study was limited by its inclusion of data from only a single center. The admissions for AAH and DARLC had a higher proportion of male patients than female patients, thus limiting the generalizability of the findings. In addition, there was a lack of data on ethnicity and socioeconomic status, which could affect readmissions.
Dr. Wang advises clinicians to “seek out and connect with other healthcare providers in their local and regional community, such as addiction counselors or psychologists, to build a robust referral network for patients wanting to reduce their alcohol use.”
In addition, “providers should become comfortable with asking patients nonjudgmentally about alcohol use, as this builds the initial rapport that lays the foundation for ongoing care,” he said. “Every interaction with a patient is a new opportunity to guide interested patients towards alcohol cessation.”
Multidisciplinary Team Essential
Commenting on the findings, Meena B. Bansal, MD, professor of medicine and director of translational research in liver diseases at the Icahn School of Medicine at Mount Sinai in New York, said that they reflect clinical experience in US hospitals. “Physicians are so busy handling the acute medical situation posed by the admission that while they do certainly tell the patient they should stop drinking, full discussion, intervention, and linkage to outpatient programs is often led by the social worker,” she said. Dr. Bansal was not involved in the study.
“Many alcohol use disorder therapies are not tested in extremely ill patients, and thus, pharmacotherapy is often reserved for outpatient management, when patients are more clinically stable,” she said. Yet, as mentioned by the authors, a recent study “showed that 71% of providers never prescribed pharmacotherapy for alcohol use disorder, with the most common reason being low comfort with the medications. We need to increase education around pharmacotherapy for alcohol use disorder to increase the comfort level of practicing gastroenterologists and hepatologists.”
Furthermore, she said, clinicians need to intervene and provide guidance to patients “wherever and whenever they touch our system, whether that be in the inpatient or outpatient setting, [and] provide SBIRT during inpatient admissions but then follow patients longitudinally in a multidisciplinary team to achieve long-term results.”
The study was conducted without external funding. Dr. Wang had no relevant conflicts to disclose. A coauthor acts as a consultant, clinical trial investigator, speaker, and member of the advisory board for AbbVie, Gilead, Intercept, and Novo Nordisk. He also acts as a speaker and member of the advisory board for Eisai and Lupin and as a clinical trial investigator for Madrigal.
A version of this article appeared on Medscape.com.
Actively drinking patients who undergo screening, a brief intervention, and referral to treatment (SBIRT) for alcohol use disorder during hospital admission for alcohol-related conditions have fewer 30- and 90-day readmissions for alcohol-related liver disease, a new study suggests.
Nevertheless, SBIRT was administered to only 51.7% of patients admitted for alcohol-associated hepatitis (AAH) and 23.7% of patients admitted for decompensated alcohol-related cirrhosis (DARLC).
“Not only did conducting SBIRT with patients admitted for AAH reduce 30-day and 90-day liver-related readmissions, but even just being offered SBIRT reduced readmissions, too,” study author Dennis Wang, MD, of the adult gastroenterology residency program at McMaster University in Hamilton, Ontario, told this news organization. “The exact reason for this effect is unclear, but one can speculate that offering SBIRT to AAH patients may trigger them to consider abstaining from alcohol.”
By contrast, receiving or being offered SBIRT had no effect on readmissions for patients with DARLC.
The findings were published online on November 30 in the Journal of the Canadian Association of Gastroenterology.
Readmissions Significantly Reduced
The researchers retrospectively reviewed the electronic medical records of patients with AAH or DARLC who were admitted to Hamilton Health Sciences hospitals in Ontario from January 2017 to December 2021. Eligible patients were aged ≥ 18 years and actively drinking.
The study’s primary outcomes were the proportion of admissions in which SBIRT was conducted and the association between conducting SBIRT and 30- and 90-day readmissions for recurrent AAH or DARLC.
There were 120 admissions for AAH, representing 95 patients, 95 index admissions, 18 patients with 30-day readmissions, and 26 patients with 90-day readmissions. The sum of the index AAH admissions and 90-day readmissions was greater than the total number of AAH admissions because readmissions where patients were no longer actively drinking alcohol were included.
There were 177 admissions for DARLC, representing 132 patients, 132 index admissions, 13 30-day readmissions, and 31 90-day readmissions.
The mean age of patients admitted with AAH (47.7 years) was significantly lower than that of patients admitted with DARLC (58.2 years). Fewer men were admitted with AAH (59.2%) than with DARLC (73.4%).
There was no significant difference between AAH admissions and DARLC admissions in hospital length of stay, Model for End-Stage Liver Disease on admission, same-admission mortality, and 30- or 90-day readmissions.
SBIRT was conducted in 62 of 120 AAH admissions (51.7%) and 42 of 177 DARLC admissions (23.7%), mainly by social workers and addiction counselors and occasionally by physicians alone.
“Sometimes patients with AAH or DARLC can become so ill that they cannot participate in SBIRT,” noted Dr. Wang. “In addition, there may not be enough health care providers, resources, or time available to conduct high-quality SBIRT with all patients admitted to hospital.”
For patients with AAH, SBIRT was associated with significantly reduced 30-day (odds ratio [OR], 0.098) and 90-day (OR, 0.166) likelihood of readmission for recurrent AAH. However, there was no association with readmissions for patients with DARLC.
Liver Scarring Persists
“We suspect that DARLC patients do not see the same improvement in liver-related readmissions after receiving SBIRT because the liver scarring typically persists even with alcohol abstinence, and this scarring causes further decompensations,” said Dr. Wang.
“Physicians, social workers, addiction counselors, and other allied health providers should collaborate to conduct SBIRT for all actively drinking patients admitted for AAH or DARLC,” wrote the authors.
The researchers acknowledged that their study was limited by its inclusion of data from only a single center. The admissions for AAH and DARLC had a higher proportion of male patients than female patients, thus limiting the generalizability of the findings. In addition, there was a lack of data on ethnicity and socioeconomic status, which could affect readmissions.
Dr. Wang advises clinicians to “seek out and connect with other healthcare providers in their local and regional community, such as addiction counselors or psychologists, to build a robust referral network for patients wanting to reduce their alcohol use.”
In addition, “providers should become comfortable with asking patients nonjudgmentally about alcohol use, as this builds the initial rapport that lays the foundation for ongoing care,” he said. “Every interaction with a patient is a new opportunity to guide interested patients towards alcohol cessation.”
Multidisciplinary Team Essential
Commenting on the findings, Meena B. Bansal, MD, professor of medicine and director of translational research in liver diseases at the Icahn School of Medicine at Mount Sinai in New York, said that they reflect clinical experience in US hospitals. “Physicians are so busy handling the acute medical situation posed by the admission that while they do certainly tell the patient they should stop drinking, full discussion, intervention, and linkage to outpatient programs is often led by the social worker,” she said. Dr. Bansal was not involved in the study.
“Many alcohol use disorder therapies are not tested in extremely ill patients, and thus, pharmacotherapy is often reserved for outpatient management, when patients are more clinically stable,” she said. Yet, as mentioned by the authors, a recent study “showed that 71% of providers never prescribed pharmacotherapy for alcohol use disorder, with the most common reason being low comfort with the medications. We need to increase education around pharmacotherapy for alcohol use disorder to increase the comfort level of practicing gastroenterologists and hepatologists.”
Furthermore, she said, clinicians need to intervene and provide guidance to patients “wherever and whenever they touch our system, whether that be in the inpatient or outpatient setting, [and] provide SBIRT during inpatient admissions but then follow patients longitudinally in a multidisciplinary team to achieve long-term results.”
The study was conducted without external funding. Dr. Wang had no relevant conflicts to disclose. A coauthor acts as a consultant, clinical trial investigator, speaker, and member of the advisory board for AbbVie, Gilead, Intercept, and Novo Nordisk. He also acts as a speaker and member of the advisory board for Eisai and Lupin and as a clinical trial investigator for Madrigal.
A version of this article appeared on Medscape.com.
Opioid use disorder in pregnancy: A strategy for using methadone
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.
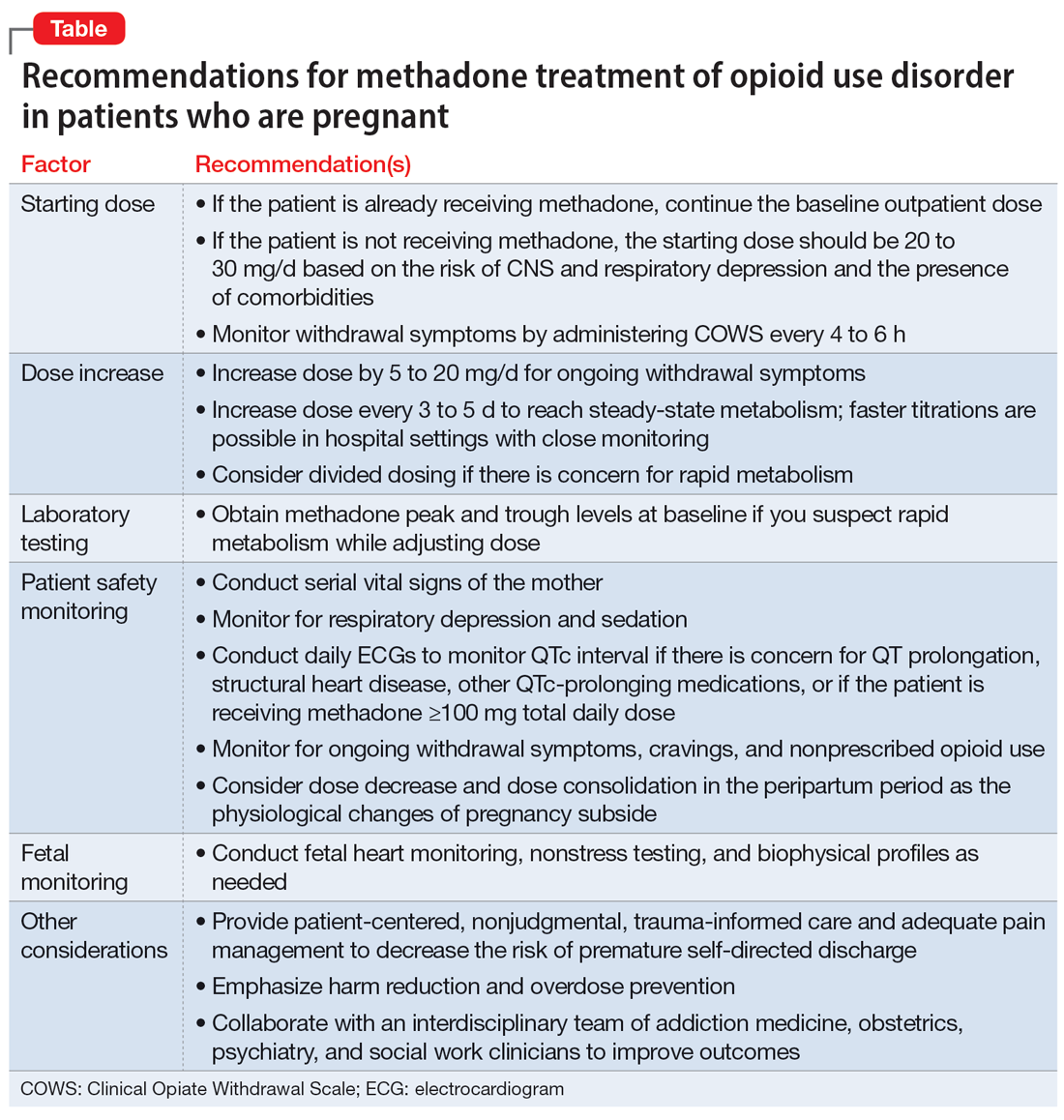
Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
Mass shooters and mental illness: Reexamining the connection
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.



