User login
Treatment for a tobacco-dependent adult
Applying American Thoracic Society’s new clinical practice guideline
Complications from tobacco use are the most common preventable cause of death, disability, and disease in the United States. Tobacco use causes 480,000 premature deaths every year. In pregnancy, tobacco use causes complications such as premature birth, intrauterine growth restriction, and placental abruption. In the perinatal period, it is associated with sudden infant death syndrome. While cigarette smoking is decreasing in adolescents, e-cigarette use in on the rise. Approximately 1,600 children aged 12-17 smoke their first cigarette every day and it is estimated that 5.6 million children and adolescents will die of a tobacco use–related death.1 For these reasons it is important to address tobacco use and cessation with patients whenever it is possible.
Case
A forty-five-year-old male who rarely comes to the office is here today for a physical exam at the urging of his partner. He has been smoking a pack a day since age 17. You have tried at past visits to discuss quitting, but he had been in the precontemplative stage and had been unwilling to consider any change. This visit, however, he is ready to try to quit. What can you offer him?
Core recommendations from ATS guidelines
This patient can be offered varenicline plus nicotine replacement therapy rather than nicotine replacement therapy, bupropion, e-cigarettes, or varenicline alone. His course of therapy should extend beyond 12 weeks instead of the standard 6- to 12-week therapy. Alternatively, he could be offered varenicline alone, rather than nicotine replacement.2
A change from previous guidelines
What makes this recommendation so interesting and new is the emphasis it places on varenicline. The United States Preventive Services Task Force released a recommendation statement in 2015 that stressed a combination of pharmacological and behavioral interventions. It discussed nicotine replacement therapy, bupropion, and varenicline, but did not recommend any one over any of the others.3 The new recommendation from the American Thoracic Society favors varenicline over other pharmacologic interventions. It is based on an independent systematic review of the literature that showed higher rates of tobacco use abstinence at the 6-month follow-up with varenicline alone versus nicotine replacement therapy alone, bupropion alone, or e-cigarette use only.
A review of 14 randomized controlled trials showed that varenicline improves abstinence rates during treatment by approximately 40% compared with nicotine replacement, and by 20% at the end of 6 months of treatment. The review found that varenicline plus nicotine replacement therapy is more effective than varenicline alone. In this comparison, based on three trials, there was a 36% higher abstinence rate at 6 months using varenicline plus nicotine replacement. When varenicline use was compared with use of a nicotine patch, bupropion, or e-cigarettes, there was a reduction in serious adverse events – changes in mood, suicidal ideation, and neurological side effects such as seizures.2 Clinicians may remember a black box warning on the varenicline label citing neuropsychiatric effects and it is important to note that the Food and Drug Administration removed this boxed warning in 2016.4
Opinion
This recommendation represents an important, evidence-based change from previous guidelines. It presents the opportunity for better outcomes, but will likely take a while to filter into practice, as clinicians need to become more comfortable with the use of varenicline and insurance supports the cost of varenicline.
The average cost of varenicline for 12 weeks is between $1,220 and $1,584. For comparison, nicotine replacement therapy costs $170 to $240 for the same number of weeks. To put those costs in perspective, the 12-week cost of cigarettes for a two-pack-a-day smoker is approximately $1,000.
For some patients, the motivation to quit smoking comes from the realization of how much they are spending on cigarettes each month. That said, if a patient does not have insurance or their insurance does not cover the cost of varenicline, nicotine replacement therapy might be more appealing. It should be noted that better abstinence rates have been seen in patients taking varenicline plus nicotine replacement therapy versus varenicline alone.
Suggested treatment
Based on a systematic review of randomized controlled trials, the American Thoracic Society’s guideline on pharmacological treatment in tobacco-dependent adults concludes that varenicline plus nicotine patch is the preferred pharmacological treatment for tobacco cessation when compared with varenicline alone, bupropion alone, nicotine replacement therapy alone, and e-cigarettes alone. If the patient does not want to start two medicines at once, then varenicline alone would be the preferred choice.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. U.S. Preventive Services Task Force. Primary care interventions for prevention and cessation of tobacco use in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. JAMA.2020;323(16):1590-8. doi: 10.1001/jama.2020.4679.
2. Leone FT et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31.
3. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. U.S. Preventive Services Task Force 2015 Sep 21.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. 2016 Dec. 16.
Applying American Thoracic Society’s new clinical practice guideline
Applying American Thoracic Society’s new clinical practice guideline
Complications from tobacco use are the most common preventable cause of death, disability, and disease in the United States. Tobacco use causes 480,000 premature deaths every year. In pregnancy, tobacco use causes complications such as premature birth, intrauterine growth restriction, and placental abruption. In the perinatal period, it is associated with sudden infant death syndrome. While cigarette smoking is decreasing in adolescents, e-cigarette use in on the rise. Approximately 1,600 children aged 12-17 smoke their first cigarette every day and it is estimated that 5.6 million children and adolescents will die of a tobacco use–related death.1 For these reasons it is important to address tobacco use and cessation with patients whenever it is possible.
Case
A forty-five-year-old male who rarely comes to the office is here today for a physical exam at the urging of his partner. He has been smoking a pack a day since age 17. You have tried at past visits to discuss quitting, but he had been in the precontemplative stage and had been unwilling to consider any change. This visit, however, he is ready to try to quit. What can you offer him?
Core recommendations from ATS guidelines
This patient can be offered varenicline plus nicotine replacement therapy rather than nicotine replacement therapy, bupropion, e-cigarettes, or varenicline alone. His course of therapy should extend beyond 12 weeks instead of the standard 6- to 12-week therapy. Alternatively, he could be offered varenicline alone, rather than nicotine replacement.2
A change from previous guidelines
What makes this recommendation so interesting and new is the emphasis it places on varenicline. The United States Preventive Services Task Force released a recommendation statement in 2015 that stressed a combination of pharmacological and behavioral interventions. It discussed nicotine replacement therapy, bupropion, and varenicline, but did not recommend any one over any of the others.3 The new recommendation from the American Thoracic Society favors varenicline over other pharmacologic interventions. It is based on an independent systematic review of the literature that showed higher rates of tobacco use abstinence at the 6-month follow-up with varenicline alone versus nicotine replacement therapy alone, bupropion alone, or e-cigarette use only.
A review of 14 randomized controlled trials showed that varenicline improves abstinence rates during treatment by approximately 40% compared with nicotine replacement, and by 20% at the end of 6 months of treatment. The review found that varenicline plus nicotine replacement therapy is more effective than varenicline alone. In this comparison, based on three trials, there was a 36% higher abstinence rate at 6 months using varenicline plus nicotine replacement. When varenicline use was compared with use of a nicotine patch, bupropion, or e-cigarettes, there was a reduction in serious adverse events – changes in mood, suicidal ideation, and neurological side effects such as seizures.2 Clinicians may remember a black box warning on the varenicline label citing neuropsychiatric effects and it is important to note that the Food and Drug Administration removed this boxed warning in 2016.4
Opinion
This recommendation represents an important, evidence-based change from previous guidelines. It presents the opportunity for better outcomes, but will likely take a while to filter into practice, as clinicians need to become more comfortable with the use of varenicline and insurance supports the cost of varenicline.
The average cost of varenicline for 12 weeks is between $1,220 and $1,584. For comparison, nicotine replacement therapy costs $170 to $240 for the same number of weeks. To put those costs in perspective, the 12-week cost of cigarettes for a two-pack-a-day smoker is approximately $1,000.
For some patients, the motivation to quit smoking comes from the realization of how much they are spending on cigarettes each month. That said, if a patient does not have insurance or their insurance does not cover the cost of varenicline, nicotine replacement therapy might be more appealing. It should be noted that better abstinence rates have been seen in patients taking varenicline plus nicotine replacement therapy versus varenicline alone.
Suggested treatment
Based on a systematic review of randomized controlled trials, the American Thoracic Society’s guideline on pharmacological treatment in tobacco-dependent adults concludes that varenicline plus nicotine patch is the preferred pharmacological treatment for tobacco cessation when compared with varenicline alone, bupropion alone, nicotine replacement therapy alone, and e-cigarettes alone. If the patient does not want to start two medicines at once, then varenicline alone would be the preferred choice.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. U.S. Preventive Services Task Force. Primary care interventions for prevention and cessation of tobacco use in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. JAMA.2020;323(16):1590-8. doi: 10.1001/jama.2020.4679.
2. Leone FT et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31.
3. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. U.S. Preventive Services Task Force 2015 Sep 21.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. 2016 Dec. 16.
Complications from tobacco use are the most common preventable cause of death, disability, and disease in the United States. Tobacco use causes 480,000 premature deaths every year. In pregnancy, tobacco use causes complications such as premature birth, intrauterine growth restriction, and placental abruption. In the perinatal period, it is associated with sudden infant death syndrome. While cigarette smoking is decreasing in adolescents, e-cigarette use in on the rise. Approximately 1,600 children aged 12-17 smoke their first cigarette every day and it is estimated that 5.6 million children and adolescents will die of a tobacco use–related death.1 For these reasons it is important to address tobacco use and cessation with patients whenever it is possible.
Case
A forty-five-year-old male who rarely comes to the office is here today for a physical exam at the urging of his partner. He has been smoking a pack a day since age 17. You have tried at past visits to discuss quitting, but he had been in the precontemplative stage and had been unwilling to consider any change. This visit, however, he is ready to try to quit. What can you offer him?
Core recommendations from ATS guidelines
This patient can be offered varenicline plus nicotine replacement therapy rather than nicotine replacement therapy, bupropion, e-cigarettes, or varenicline alone. His course of therapy should extend beyond 12 weeks instead of the standard 6- to 12-week therapy. Alternatively, he could be offered varenicline alone, rather than nicotine replacement.2
A change from previous guidelines
What makes this recommendation so interesting and new is the emphasis it places on varenicline. The United States Preventive Services Task Force released a recommendation statement in 2015 that stressed a combination of pharmacological and behavioral interventions. It discussed nicotine replacement therapy, bupropion, and varenicline, but did not recommend any one over any of the others.3 The new recommendation from the American Thoracic Society favors varenicline over other pharmacologic interventions. It is based on an independent systematic review of the literature that showed higher rates of tobacco use abstinence at the 6-month follow-up with varenicline alone versus nicotine replacement therapy alone, bupropion alone, or e-cigarette use only.
A review of 14 randomized controlled trials showed that varenicline improves abstinence rates during treatment by approximately 40% compared with nicotine replacement, and by 20% at the end of 6 months of treatment. The review found that varenicline plus nicotine replacement therapy is more effective than varenicline alone. In this comparison, based on three trials, there was a 36% higher abstinence rate at 6 months using varenicline plus nicotine replacement. When varenicline use was compared with use of a nicotine patch, bupropion, or e-cigarettes, there was a reduction in serious adverse events – changes in mood, suicidal ideation, and neurological side effects such as seizures.2 Clinicians may remember a black box warning on the varenicline label citing neuropsychiatric effects and it is important to note that the Food and Drug Administration removed this boxed warning in 2016.4
Opinion
This recommendation represents an important, evidence-based change from previous guidelines. It presents the opportunity for better outcomes, but will likely take a while to filter into practice, as clinicians need to become more comfortable with the use of varenicline and insurance supports the cost of varenicline.
The average cost of varenicline for 12 weeks is between $1,220 and $1,584. For comparison, nicotine replacement therapy costs $170 to $240 for the same number of weeks. To put those costs in perspective, the 12-week cost of cigarettes for a two-pack-a-day smoker is approximately $1,000.
For some patients, the motivation to quit smoking comes from the realization of how much they are spending on cigarettes each month. That said, if a patient does not have insurance or their insurance does not cover the cost of varenicline, nicotine replacement therapy might be more appealing. It should be noted that better abstinence rates have been seen in patients taking varenicline plus nicotine replacement therapy versus varenicline alone.
Suggested treatment
Based on a systematic review of randomized controlled trials, the American Thoracic Society’s guideline on pharmacological treatment in tobacco-dependent adults concludes that varenicline plus nicotine patch is the preferred pharmacological treatment for tobacco cessation when compared with varenicline alone, bupropion alone, nicotine replacement therapy alone, and e-cigarettes alone. If the patient does not want to start two medicines at once, then varenicline alone would be the preferred choice.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. U.S. Preventive Services Task Force. Primary care interventions for prevention and cessation of tobacco use in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. JAMA.2020;323(16):1590-8. doi: 10.1001/jama.2020.4679.
2. Leone FT et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31.
3. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. U.S. Preventive Services Task Force 2015 Sep 21.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. 2016 Dec. 16.
COVID-19: Optimizing therapeutic strategies for children, adolescents with ADHD
Recently, the Yakima Health District (YHD), in collaboration with the Washington State Department of Health, issued dramatic revisions to its educational curriculum, opting for exclusively remote learning as an important next step in COVID-19 containment measures.
The newly implemented “enhanced” distance-learning paradigm has garnered considerable national attention. Even more noteworthy is how YHD addressed those with language barriers and learning differences such as ADHD as a “priority group”; these individuals are exempt from the newly implemented measures, and small instructional groups of no more than five “at-risk” students will be directly supervised by specialized educators.1,2 To overcome these new unprecedented challenges from the coronavirus pandemic, especially from the perspective of distance education and mental health for susceptible groups such as those with ADHD, it is of utmost importance to explore various programs of interest, as well as the targeted therapies being considered during this crisis.
From a therapeutic standpoint, individuals with learning differences are more likely to play catch-up with their age-matched peers. This puts them at significant risk for developmental delays with symptoms manifesting as disruptive behavioral issues. This is why ongoing parental guidance, coupled with a paradoxically stimulating environment, is critical for children and adolescents with ADHD.3 Accumulating evidence, based on a myriad of studies, demonstrates that childhood treatment with ADHD stimulants reduces the incidence of future substance use, as well as that of other negative outcomes.4,5
Therapeutic strategies that work
“The new normal” has forced unique challenges on clinicians for mitigating distress by novel means of health care delivery. Given the paucity of research exploring the interactions of individuals with ADHD within the context of COVID-19, Take for example, the suggested guidelines from the European ADHD Guidelines Group (EAGG) – such as the following:
- Telecommunications in general, and telepsychiatry in particular, should function as the primary mode of health care delivery to fulfill societal standards of physical distancing.
- Children and adolescents with ADHD should be designated as a “priority group” with respect to monitoring initiatives by educators in a school setting, be it virtual or otherwise.
- Implementation of behavioral strategies by parent or guardian to address psychological well-being and reduce the presence of comorbid behavioral conditions (such as oppositional defiant disorder).
In addition to the aforementioned guidance, EAGG maintains that individuals with ADHD may be initiated on medications after the completion of a baseline examination; if the patients in question are already on a treatment regimen, they should proceed with it as indicated. Interruptions to therapy are not ideal because patients are then subjected to health-related stressors of COVID-19. Reasonable regulations concerning access to medications, without unnecessary delays, undoubtedly will facilitate patient needs, allowing for a smooth transition in day-to-day activities. The family, as a cohesive unit, may benefit from reeducation because it contributes toward the therapeutic process. Neurofeedback, coping skills, and cognitive restructuring training are potential modalities that can augment medications.
Although it may seem counterintuitive, parents or caregivers should resist the urge to increase the medication dose during an outbreak with the intended goal of diminishing the psychosocial burden of ADHD symptomatology. Likewise, unless indicated by a specialist, antipsychotics and/or hypnotics should not be introduced for addressing behavioral dysregulation (such as agitation) during the confinement period.
Historically, numerous clinicians have suggested that patients undergo a routine cardiovascular examination and EKG before being prescribed psychostimulants (the rationale for this recommendation is that sympathomimetics unduly affect blood pressure and heart rate).6,7 However, the American Academy of Pediatrics (AAP) and the American Heart Association (AHA) eventually amended their previous stance by releasing a joint statement in which they deemed a baseline EKG necessary only in ADHD patients with preexisting cardiac risk. For all other patients, the use of EKGs was entirely contingent on physician discretion. However, given the nature of safety precautions for COVID-19, it is prudent to discourage or delay in-person cardiovascular examination/monitoring protocols altogether, especially in those patients without known heart conditions.
Another area of concern is sleep dysfunction, which might exist as an untoward effect of ADHD medication intake or because of the presence of COVID-19 psychosocial stressors. However, clinicians advise that unnecessary psychopharmacology (such as hypnotics or melatonin) be avoided. Instead, conservative lifestyle measures should be enacted, emphasizing the role of proper sleep hygiene in maintaining optimal behavioral health. Despite setbacks to in-person appointments, patients are expected to continue their pharmacotherapy with “parent-focused” ADHD interventions taking a primary role in facilitating compliance through remote monitoring.
ADMiRE, a tertiary-level, dedicated ADHD intervention program from South Dublin, Ireland, has identified several roadblocks with respect to streamlining health care for individuals with ADHD during the confinement period. The proposed resolution to these issues, some of which are derived from EAGG guidelines, might have universal applications elsewhere, thereby facilitating the development of therapeutic services of interest. ADMiRE has noted a correspondence between the guidelines established by EAGG and that of the Canadian ADHD Resource Alliance (CADDRA), including minimal in-person interactions (in favor of virtual teleconferencing) and a cardiovascular screen can be performed in lieu of baseline cardiac auscultation. Moreover, in the event that the patient is a low cardiac risk candidate for ADHD treatment, monitoring protocols may be continued from a home setting. However, if a physical examination is indicated, CADDRA recommends the use of precautionary PPE before commencing ADHD pharmacotherapy.
One of the most significant hurdles is that of school closures because teacher feedback for baseline behavior was traditionally instrumental in dictating patient medical management (for example, for titration schedule). It is expected that, for the time being, this role will be supplanted by parental reports. As well as disclosing information on behavioral dysregulation, family members should be trained to relay critical information about the development of stimulant-induced cardiovascular symptoms – namely, dyspnea, chest pain, and/or palpitations. Furthermore, as primary caregivers, parents should harbor a certain degree of emotional sensitivity because their mood state may influence the child’s overall behavioral course in terms of symptom exacerbation.8
Toward adopting an integrated model for care
Developing an effective assessment plan for patients with ADHD often proves to be a challenging task for clinicians, perhaps even more so in environments that enforce social distancing and limited physical contact by default. As a neurodevelopmental disorder from childhood, the symptoms (including inattention, hyperactivity, and/or impulsivity) of ADHD do not arise in a vacuum – comorbid conditions include mood and anxiety disorders, which are complicated further by a background risk for substance use and self-medicating tendencies.9 Unfortunately, the pandemic has limited the breadth of non-COVID doctors visits, which hinders the overall diagnostic and monitoring process for identifiable comorbid conditions, such as autism spectrum disorder, intellectual disability, oppositional defiant and conduct disorders, and so on.10 Since ADHD symptoms cannot be treated by pharmacotherapy or behavioral interventions alone, our team advocates that families provide additional emotional support and continuous encouragement during these uncertain times.
ADHD and the self-medication hypothesis
The Khantzian self-medication hypothesis posits that a drug seeker may subconsciously gravitate toward a particular agent only to discover a sense of relief concerning inner turmoil or restlessness after use. Observations support the notion that individuals with undiagnosed ADHD have sought cocaine or even recreational designer drugs (such as methylenedioxypyrovalerone, or “bath salts”).11 Given the similar mechanism of action between cocaine, methylenedioxypyrovalerone, and prescribed psychostimulants such as methylphenidate, the results are hardly surprising because these agents all work on the brain’s “reward center” (for example, the nucleus accumbens) by invoking dopamine release. Aside from the aforementioned self-medication hypothesis, “downers” such as Xanax recently have experienced a prescription spike during the outbreak. While there isn’t an immediate cause for concern of Xanax abuse in ADHD individuals, the potential for addiction is certainly real, especially when taking into account comorbid anxiety disorder or sleep dysfunction.
Because of limited resources and precautionary guidelines, clinicians are at a considerable disadvantage in terms of formulating a comprehensive diagnostic and treatment plan for children and adolescents with ADHD. This situation is further compounded by the recent closure of schools and the lack of feedback with respect to baseline behavior from teachers and specialized educators. This is why it is imperative for primary caregivers to closely monitor children with ADHD for developing changes in behavioral patterns (for example, mood or anxiety issues and drug-seeking or disruptive behavior) and work with health care professionals.
References
1. “Distance learning strongly recommended for all Yakima county schools.” NBC Right Now. 2020 Aug 5.
2. Retka J. “Enhanced” remote learning in Yakima county schools? What that means for students this fall. Yakima Herald-Republic. 2020 Aug 8.
3. Armstrong T. “To empower! Not Control! A holistic approach to ADHD.” American Institute for Learning and Development. 1998.
4. J Child Psychol Psychiatry. 2014 Aug;55(8):878-85.
5. Ir J Psychol Med. 2020 May 21:1-22.
6. Lancet Child Adolesc Health. 2020 Jun;4(6):412-4.
7. O’Keefe L. AAP News. 2008 Jun;29(6):1.
8. Asian J Psychiatr. 2020 Jun;51:102077.
9. Current Psychiatry. 2015 Dec;14(12):e3-4.
10. Encephale. 2020 Jun 7;46(3S):S85-92.
11. Current Psychiatry. 2014 Dec; 3(12): e3-4.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clini-cal research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
Recently, the Yakima Health District (YHD), in collaboration with the Washington State Department of Health, issued dramatic revisions to its educational curriculum, opting for exclusively remote learning as an important next step in COVID-19 containment measures.
The newly implemented “enhanced” distance-learning paradigm has garnered considerable national attention. Even more noteworthy is how YHD addressed those with language barriers and learning differences such as ADHD as a “priority group”; these individuals are exempt from the newly implemented measures, and small instructional groups of no more than five “at-risk” students will be directly supervised by specialized educators.1,2 To overcome these new unprecedented challenges from the coronavirus pandemic, especially from the perspective of distance education and mental health for susceptible groups such as those with ADHD, it is of utmost importance to explore various programs of interest, as well as the targeted therapies being considered during this crisis.
From a therapeutic standpoint, individuals with learning differences are more likely to play catch-up with their age-matched peers. This puts them at significant risk for developmental delays with symptoms manifesting as disruptive behavioral issues. This is why ongoing parental guidance, coupled with a paradoxically stimulating environment, is critical for children and adolescents with ADHD.3 Accumulating evidence, based on a myriad of studies, demonstrates that childhood treatment with ADHD stimulants reduces the incidence of future substance use, as well as that of other negative outcomes.4,5
Therapeutic strategies that work
“The new normal” has forced unique challenges on clinicians for mitigating distress by novel means of health care delivery. Given the paucity of research exploring the interactions of individuals with ADHD within the context of COVID-19, Take for example, the suggested guidelines from the European ADHD Guidelines Group (EAGG) – such as the following:
- Telecommunications in general, and telepsychiatry in particular, should function as the primary mode of health care delivery to fulfill societal standards of physical distancing.
- Children and adolescents with ADHD should be designated as a “priority group” with respect to monitoring initiatives by educators in a school setting, be it virtual or otherwise.
- Implementation of behavioral strategies by parent or guardian to address psychological well-being and reduce the presence of comorbid behavioral conditions (such as oppositional defiant disorder).
In addition to the aforementioned guidance, EAGG maintains that individuals with ADHD may be initiated on medications after the completion of a baseline examination; if the patients in question are already on a treatment regimen, they should proceed with it as indicated. Interruptions to therapy are not ideal because patients are then subjected to health-related stressors of COVID-19. Reasonable regulations concerning access to medications, without unnecessary delays, undoubtedly will facilitate patient needs, allowing for a smooth transition in day-to-day activities. The family, as a cohesive unit, may benefit from reeducation because it contributes toward the therapeutic process. Neurofeedback, coping skills, and cognitive restructuring training are potential modalities that can augment medications.
Although it may seem counterintuitive, parents or caregivers should resist the urge to increase the medication dose during an outbreak with the intended goal of diminishing the psychosocial burden of ADHD symptomatology. Likewise, unless indicated by a specialist, antipsychotics and/or hypnotics should not be introduced for addressing behavioral dysregulation (such as agitation) during the confinement period.
Historically, numerous clinicians have suggested that patients undergo a routine cardiovascular examination and EKG before being prescribed psychostimulants (the rationale for this recommendation is that sympathomimetics unduly affect blood pressure and heart rate).6,7 However, the American Academy of Pediatrics (AAP) and the American Heart Association (AHA) eventually amended their previous stance by releasing a joint statement in which they deemed a baseline EKG necessary only in ADHD patients with preexisting cardiac risk. For all other patients, the use of EKGs was entirely contingent on physician discretion. However, given the nature of safety precautions for COVID-19, it is prudent to discourage or delay in-person cardiovascular examination/monitoring protocols altogether, especially in those patients without known heart conditions.
Another area of concern is sleep dysfunction, which might exist as an untoward effect of ADHD medication intake or because of the presence of COVID-19 psychosocial stressors. However, clinicians advise that unnecessary psychopharmacology (such as hypnotics or melatonin) be avoided. Instead, conservative lifestyle measures should be enacted, emphasizing the role of proper sleep hygiene in maintaining optimal behavioral health. Despite setbacks to in-person appointments, patients are expected to continue their pharmacotherapy with “parent-focused” ADHD interventions taking a primary role in facilitating compliance through remote monitoring.
ADMiRE, a tertiary-level, dedicated ADHD intervention program from South Dublin, Ireland, has identified several roadblocks with respect to streamlining health care for individuals with ADHD during the confinement period. The proposed resolution to these issues, some of which are derived from EAGG guidelines, might have universal applications elsewhere, thereby facilitating the development of therapeutic services of interest. ADMiRE has noted a correspondence between the guidelines established by EAGG and that of the Canadian ADHD Resource Alliance (CADDRA), including minimal in-person interactions (in favor of virtual teleconferencing) and a cardiovascular screen can be performed in lieu of baseline cardiac auscultation. Moreover, in the event that the patient is a low cardiac risk candidate for ADHD treatment, monitoring protocols may be continued from a home setting. However, if a physical examination is indicated, CADDRA recommends the use of precautionary PPE before commencing ADHD pharmacotherapy.
One of the most significant hurdles is that of school closures because teacher feedback for baseline behavior was traditionally instrumental in dictating patient medical management (for example, for titration schedule). It is expected that, for the time being, this role will be supplanted by parental reports. As well as disclosing information on behavioral dysregulation, family members should be trained to relay critical information about the development of stimulant-induced cardiovascular symptoms – namely, dyspnea, chest pain, and/or palpitations. Furthermore, as primary caregivers, parents should harbor a certain degree of emotional sensitivity because their mood state may influence the child’s overall behavioral course in terms of symptom exacerbation.8
Toward adopting an integrated model for care
Developing an effective assessment plan for patients with ADHD often proves to be a challenging task for clinicians, perhaps even more so in environments that enforce social distancing and limited physical contact by default. As a neurodevelopmental disorder from childhood, the symptoms (including inattention, hyperactivity, and/or impulsivity) of ADHD do not arise in a vacuum – comorbid conditions include mood and anxiety disorders, which are complicated further by a background risk for substance use and self-medicating tendencies.9 Unfortunately, the pandemic has limited the breadth of non-COVID doctors visits, which hinders the overall diagnostic and monitoring process for identifiable comorbid conditions, such as autism spectrum disorder, intellectual disability, oppositional defiant and conduct disorders, and so on.10 Since ADHD symptoms cannot be treated by pharmacotherapy or behavioral interventions alone, our team advocates that families provide additional emotional support and continuous encouragement during these uncertain times.
ADHD and the self-medication hypothesis
The Khantzian self-medication hypothesis posits that a drug seeker may subconsciously gravitate toward a particular agent only to discover a sense of relief concerning inner turmoil or restlessness after use. Observations support the notion that individuals with undiagnosed ADHD have sought cocaine or even recreational designer drugs (such as methylenedioxypyrovalerone, or “bath salts”).11 Given the similar mechanism of action between cocaine, methylenedioxypyrovalerone, and prescribed psychostimulants such as methylphenidate, the results are hardly surprising because these agents all work on the brain’s “reward center” (for example, the nucleus accumbens) by invoking dopamine release. Aside from the aforementioned self-medication hypothesis, “downers” such as Xanax recently have experienced a prescription spike during the outbreak. While there isn’t an immediate cause for concern of Xanax abuse in ADHD individuals, the potential for addiction is certainly real, especially when taking into account comorbid anxiety disorder or sleep dysfunction.
Because of limited resources and precautionary guidelines, clinicians are at a considerable disadvantage in terms of formulating a comprehensive diagnostic and treatment plan for children and adolescents with ADHD. This situation is further compounded by the recent closure of schools and the lack of feedback with respect to baseline behavior from teachers and specialized educators. This is why it is imperative for primary caregivers to closely monitor children with ADHD for developing changes in behavioral patterns (for example, mood or anxiety issues and drug-seeking or disruptive behavior) and work with health care professionals.
References
1. “Distance learning strongly recommended for all Yakima county schools.” NBC Right Now. 2020 Aug 5.
2. Retka J. “Enhanced” remote learning in Yakima county schools? What that means for students this fall. Yakima Herald-Republic. 2020 Aug 8.
3. Armstrong T. “To empower! Not Control! A holistic approach to ADHD.” American Institute for Learning and Development. 1998.
4. J Child Psychol Psychiatry. 2014 Aug;55(8):878-85.
5. Ir J Psychol Med. 2020 May 21:1-22.
6. Lancet Child Adolesc Health. 2020 Jun;4(6):412-4.
7. O’Keefe L. AAP News. 2008 Jun;29(6):1.
8. Asian J Psychiatr. 2020 Jun;51:102077.
9. Current Psychiatry. 2015 Dec;14(12):e3-4.
10. Encephale. 2020 Jun 7;46(3S):S85-92.
11. Current Psychiatry. 2014 Dec; 3(12): e3-4.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clini-cal research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
Recently, the Yakima Health District (YHD), in collaboration with the Washington State Department of Health, issued dramatic revisions to its educational curriculum, opting for exclusively remote learning as an important next step in COVID-19 containment measures.
The newly implemented “enhanced” distance-learning paradigm has garnered considerable national attention. Even more noteworthy is how YHD addressed those with language barriers and learning differences such as ADHD as a “priority group”; these individuals are exempt from the newly implemented measures, and small instructional groups of no more than five “at-risk” students will be directly supervised by specialized educators.1,2 To overcome these new unprecedented challenges from the coronavirus pandemic, especially from the perspective of distance education and mental health for susceptible groups such as those with ADHD, it is of utmost importance to explore various programs of interest, as well as the targeted therapies being considered during this crisis.
From a therapeutic standpoint, individuals with learning differences are more likely to play catch-up with their age-matched peers. This puts them at significant risk for developmental delays with symptoms manifesting as disruptive behavioral issues. This is why ongoing parental guidance, coupled with a paradoxically stimulating environment, is critical for children and adolescents with ADHD.3 Accumulating evidence, based on a myriad of studies, demonstrates that childhood treatment with ADHD stimulants reduces the incidence of future substance use, as well as that of other negative outcomes.4,5
Therapeutic strategies that work
“The new normal” has forced unique challenges on clinicians for mitigating distress by novel means of health care delivery. Given the paucity of research exploring the interactions of individuals with ADHD within the context of COVID-19, Take for example, the suggested guidelines from the European ADHD Guidelines Group (EAGG) – such as the following:
- Telecommunications in general, and telepsychiatry in particular, should function as the primary mode of health care delivery to fulfill societal standards of physical distancing.
- Children and adolescents with ADHD should be designated as a “priority group” with respect to monitoring initiatives by educators in a school setting, be it virtual or otherwise.
- Implementation of behavioral strategies by parent or guardian to address psychological well-being and reduce the presence of comorbid behavioral conditions (such as oppositional defiant disorder).
In addition to the aforementioned guidance, EAGG maintains that individuals with ADHD may be initiated on medications after the completion of a baseline examination; if the patients in question are already on a treatment regimen, they should proceed with it as indicated. Interruptions to therapy are not ideal because patients are then subjected to health-related stressors of COVID-19. Reasonable regulations concerning access to medications, without unnecessary delays, undoubtedly will facilitate patient needs, allowing for a smooth transition in day-to-day activities. The family, as a cohesive unit, may benefit from reeducation because it contributes toward the therapeutic process. Neurofeedback, coping skills, and cognitive restructuring training are potential modalities that can augment medications.
Although it may seem counterintuitive, parents or caregivers should resist the urge to increase the medication dose during an outbreak with the intended goal of diminishing the psychosocial burden of ADHD symptomatology. Likewise, unless indicated by a specialist, antipsychotics and/or hypnotics should not be introduced for addressing behavioral dysregulation (such as agitation) during the confinement period.
Historically, numerous clinicians have suggested that patients undergo a routine cardiovascular examination and EKG before being prescribed psychostimulants (the rationale for this recommendation is that sympathomimetics unduly affect blood pressure and heart rate).6,7 However, the American Academy of Pediatrics (AAP) and the American Heart Association (AHA) eventually amended their previous stance by releasing a joint statement in which they deemed a baseline EKG necessary only in ADHD patients with preexisting cardiac risk. For all other patients, the use of EKGs was entirely contingent on physician discretion. However, given the nature of safety precautions for COVID-19, it is prudent to discourage or delay in-person cardiovascular examination/monitoring protocols altogether, especially in those patients without known heart conditions.
Another area of concern is sleep dysfunction, which might exist as an untoward effect of ADHD medication intake or because of the presence of COVID-19 psychosocial stressors. However, clinicians advise that unnecessary psychopharmacology (such as hypnotics or melatonin) be avoided. Instead, conservative lifestyle measures should be enacted, emphasizing the role of proper sleep hygiene in maintaining optimal behavioral health. Despite setbacks to in-person appointments, patients are expected to continue their pharmacotherapy with “parent-focused” ADHD interventions taking a primary role in facilitating compliance through remote monitoring.
ADMiRE, a tertiary-level, dedicated ADHD intervention program from South Dublin, Ireland, has identified several roadblocks with respect to streamlining health care for individuals with ADHD during the confinement period. The proposed resolution to these issues, some of which are derived from EAGG guidelines, might have universal applications elsewhere, thereby facilitating the development of therapeutic services of interest. ADMiRE has noted a correspondence between the guidelines established by EAGG and that of the Canadian ADHD Resource Alliance (CADDRA), including minimal in-person interactions (in favor of virtual teleconferencing) and a cardiovascular screen can be performed in lieu of baseline cardiac auscultation. Moreover, in the event that the patient is a low cardiac risk candidate for ADHD treatment, monitoring protocols may be continued from a home setting. However, if a physical examination is indicated, CADDRA recommends the use of precautionary PPE before commencing ADHD pharmacotherapy.
One of the most significant hurdles is that of school closures because teacher feedback for baseline behavior was traditionally instrumental in dictating patient medical management (for example, for titration schedule). It is expected that, for the time being, this role will be supplanted by parental reports. As well as disclosing information on behavioral dysregulation, family members should be trained to relay critical information about the development of stimulant-induced cardiovascular symptoms – namely, dyspnea, chest pain, and/or palpitations. Furthermore, as primary caregivers, parents should harbor a certain degree of emotional sensitivity because their mood state may influence the child’s overall behavioral course in terms of symptom exacerbation.8
Toward adopting an integrated model for care
Developing an effective assessment plan for patients with ADHD often proves to be a challenging task for clinicians, perhaps even more so in environments that enforce social distancing and limited physical contact by default. As a neurodevelopmental disorder from childhood, the symptoms (including inattention, hyperactivity, and/or impulsivity) of ADHD do not arise in a vacuum – comorbid conditions include mood and anxiety disorders, which are complicated further by a background risk for substance use and self-medicating tendencies.9 Unfortunately, the pandemic has limited the breadth of non-COVID doctors visits, which hinders the overall diagnostic and monitoring process for identifiable comorbid conditions, such as autism spectrum disorder, intellectual disability, oppositional defiant and conduct disorders, and so on.10 Since ADHD symptoms cannot be treated by pharmacotherapy or behavioral interventions alone, our team advocates that families provide additional emotional support and continuous encouragement during these uncertain times.
ADHD and the self-medication hypothesis
The Khantzian self-medication hypothesis posits that a drug seeker may subconsciously gravitate toward a particular agent only to discover a sense of relief concerning inner turmoil or restlessness after use. Observations support the notion that individuals with undiagnosed ADHD have sought cocaine or even recreational designer drugs (such as methylenedioxypyrovalerone, or “bath salts”).11 Given the similar mechanism of action between cocaine, methylenedioxypyrovalerone, and prescribed psychostimulants such as methylphenidate, the results are hardly surprising because these agents all work on the brain’s “reward center” (for example, the nucleus accumbens) by invoking dopamine release. Aside from the aforementioned self-medication hypothesis, “downers” such as Xanax recently have experienced a prescription spike during the outbreak. While there isn’t an immediate cause for concern of Xanax abuse in ADHD individuals, the potential for addiction is certainly real, especially when taking into account comorbid anxiety disorder or sleep dysfunction.
Because of limited resources and precautionary guidelines, clinicians are at a considerable disadvantage in terms of formulating a comprehensive diagnostic and treatment plan for children and adolescents with ADHD. This situation is further compounded by the recent closure of schools and the lack of feedback with respect to baseline behavior from teachers and specialized educators. This is why it is imperative for primary caregivers to closely monitor children with ADHD for developing changes in behavioral patterns (for example, mood or anxiety issues and drug-seeking or disruptive behavior) and work with health care professionals.
References
1. “Distance learning strongly recommended for all Yakima county schools.” NBC Right Now. 2020 Aug 5.
2. Retka J. “Enhanced” remote learning in Yakima county schools? What that means for students this fall. Yakima Herald-Republic. 2020 Aug 8.
3. Armstrong T. “To empower! Not Control! A holistic approach to ADHD.” American Institute for Learning and Development. 1998.
4. J Child Psychol Psychiatry. 2014 Aug;55(8):878-85.
5. Ir J Psychol Med. 2020 May 21:1-22.
6. Lancet Child Adolesc Health. 2020 Jun;4(6):412-4.
7. O’Keefe L. AAP News. 2008 Jun;29(6):1.
8. Asian J Psychiatr. 2020 Jun;51:102077.
9. Current Psychiatry. 2015 Dec;14(12):e3-4.
10. Encephale. 2020 Jun 7;46(3S):S85-92.
11. Current Psychiatry. 2014 Dec; 3(12): e3-4.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clini-cal research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
Frequent cannabis use in depression tripled over past decade
Not only are individuals with depression at significantly higher risk for cannabis use, compared with those without depression, this trend has increased dramatically over the last decade, new research shows.
Investigators analyzed data from more than 16,000 U.S. adults between the ages of 20 and 59 years and found that those with depression had almost twice the odds of any past-month cannabis use compared with those without depression. Odds rose from 1.5 in the 2005-2006 period to 2.3 in the 2015-2016 period.
Moreover, the odds ratio for daily or near-daily use almost tripled for those with versus without depression between the two periods.
“Clinicians should screen their depressed patients for cannabis use, since this is becoming more common and could actually make their depressive symptoms worse rather than better,” senior author Deborah Hasin, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York City, told Medscape Medical News.
The results were published online August 18 in JAMA Network Open.
Misleading advertising
“Cannabis use is increasing in the U.S. and the potency of cannabis products is increasing as well,” Dr. Hasin said.
“Misleading media information and advertising suggests that cannabis is a good treatment for depression, although studies show that cannabis use may actually worsen depression symptoms, [so] we were interested in whether U.S. adults were increasingly likely to be cannabis users if they were depressed,” she reported.
To investigate, the researchers assessed data from the National Health and Nutrition Examination Survey (NHANES), with a final study sample consisting of 16,216 U.S. adults. The mean age was 39.12 years, 48.9% were men, 66.4% were non-Hispanic White, 65.6% had at least some college education, and 62.4% had an annual family income of less than $75,000.
Of these participants, 7.5% had “probable depression,” based on the Patient Health Questionnaire–9, the investigators report.
Past-month cannabis use was defined as using cannabis at least once during the past 20 days. Daily or near-daily past-month use was defined as using cannabis at least 20 times in the past 30 days.
Covariates included age, gender, race, education, marital status, annual family income, and past-year use of other substances, such as alcohol, heroin, and methamphetamine.
The researchers note that because the NHANES data were divided into six survey years (2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, and 2015-2016), their analysis was based on a “new sample weight” that combined the datasets.
Especially pronounced
Results showed that the prevalence of any past-month cannabis use in the overall sample group increased from 12.2% in the 2005-2006 period to 17.3% in the 2015-2016 period (P < .001).
The investigators characterized this change as “significant,” adding that the estimated odds of cannabis use increased by approximately 9% between every 2-year time period.
The change was even more dramatic when the increase was examined across survey time periods (OR, 1.12; P < .001). The estimated odds of daily or near-daily use increased by approximately 12% between every 2-year period.
Interestingly, however, there were no significant changes in odds for depression when consecutive survey years were compared.
When the researchers specifically focused on the association between any past-month cannabis use and depression versus no depression, they found an adjusted OR of 1.90 (95% CI, 1.62-2.12; P < .001).
Individuals with depression also had 2.29 (95% CI, 1.80-2.92) times the odds for daily or near-daily cannabis use, compared with those without depression.
A post-hoc analysis looked at time trends in a sample group that included those missing information on at least one covariate (n = 17,724 participants). It showed similar results to those in the final sample that included no missing data.
People with depression have increased risk of using “most substances that can be abused,” Dr. Hasin said. “However, with the overall rates of cannabis use increasing in the general population, this is becoming especially pronounced for cannabis.”
Clear implications
Commenting on the findings for Medscape Medical News, Deepak D’Souza, MD, professor of psychiatry, Yale University, New Haven, Conn., said there is “concern about the unsubstantiated claims of cannabis having a beneficial effect in psychiatric disorders, the most common being depression.”
Dr. D’Souza, who was not involved with the study, called it “yet another piece of evidence suggesting that over the period of time during which cannabis laws have been liberalized, rates of past-month and daily cannabis use have increased, whereas rates of other substances, including alcohol, have remained stable.”
He suggested that a common limitation of epidemiological studies is that it is difficult to tell the direction of the association, “and it could be bidirectional.”
Nevertheless, there are clear implications for the practicing clinician, he added.
“If people have a history of depression, one should ask patients about the use of cannabis and also remind them about potential psychiatric negative effects of use,” Dr. D’Souza noted.
For the general public, “the point is that there is no good evidence to support cannabis use in depression treatment and, in fact, people with depression might be more likely to use it in problematic way,” he said.
Dr. Hasin agreed that it is “certainly possible that the relationship between cannabis use and depression is bidirectional, but the mechanism of this association requires more study.”
The study was supported by a grant from the National Institute on Drug Abuse to Dr. Hasin and by the New York State Psychiatric Institute. The study authors and Dr. D’Souza disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Not only are individuals with depression at significantly higher risk for cannabis use, compared with those without depression, this trend has increased dramatically over the last decade, new research shows.
Investigators analyzed data from more than 16,000 U.S. adults between the ages of 20 and 59 years and found that those with depression had almost twice the odds of any past-month cannabis use compared with those without depression. Odds rose from 1.5 in the 2005-2006 period to 2.3 in the 2015-2016 period.
Moreover, the odds ratio for daily or near-daily use almost tripled for those with versus without depression between the two periods.
“Clinicians should screen their depressed patients for cannabis use, since this is becoming more common and could actually make their depressive symptoms worse rather than better,” senior author Deborah Hasin, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York City, told Medscape Medical News.
The results were published online August 18 in JAMA Network Open.
Misleading advertising
“Cannabis use is increasing in the U.S. and the potency of cannabis products is increasing as well,” Dr. Hasin said.
“Misleading media information and advertising suggests that cannabis is a good treatment for depression, although studies show that cannabis use may actually worsen depression symptoms, [so] we were interested in whether U.S. adults were increasingly likely to be cannabis users if they were depressed,” she reported.
To investigate, the researchers assessed data from the National Health and Nutrition Examination Survey (NHANES), with a final study sample consisting of 16,216 U.S. adults. The mean age was 39.12 years, 48.9% were men, 66.4% were non-Hispanic White, 65.6% had at least some college education, and 62.4% had an annual family income of less than $75,000.
Of these participants, 7.5% had “probable depression,” based on the Patient Health Questionnaire–9, the investigators report.
Past-month cannabis use was defined as using cannabis at least once during the past 20 days. Daily or near-daily past-month use was defined as using cannabis at least 20 times in the past 30 days.
Covariates included age, gender, race, education, marital status, annual family income, and past-year use of other substances, such as alcohol, heroin, and methamphetamine.
The researchers note that because the NHANES data were divided into six survey years (2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, and 2015-2016), their analysis was based on a “new sample weight” that combined the datasets.
Especially pronounced
Results showed that the prevalence of any past-month cannabis use in the overall sample group increased from 12.2% in the 2005-2006 period to 17.3% in the 2015-2016 period (P < .001).
The investigators characterized this change as “significant,” adding that the estimated odds of cannabis use increased by approximately 9% between every 2-year time period.
The change was even more dramatic when the increase was examined across survey time periods (OR, 1.12; P < .001). The estimated odds of daily or near-daily use increased by approximately 12% between every 2-year period.
Interestingly, however, there were no significant changes in odds for depression when consecutive survey years were compared.
When the researchers specifically focused on the association between any past-month cannabis use and depression versus no depression, they found an adjusted OR of 1.90 (95% CI, 1.62-2.12; P < .001).
Individuals with depression also had 2.29 (95% CI, 1.80-2.92) times the odds for daily or near-daily cannabis use, compared with those without depression.
A post-hoc analysis looked at time trends in a sample group that included those missing information on at least one covariate (n = 17,724 participants). It showed similar results to those in the final sample that included no missing data.
People with depression have increased risk of using “most substances that can be abused,” Dr. Hasin said. “However, with the overall rates of cannabis use increasing in the general population, this is becoming especially pronounced for cannabis.”
Clear implications
Commenting on the findings for Medscape Medical News, Deepak D’Souza, MD, professor of psychiatry, Yale University, New Haven, Conn., said there is “concern about the unsubstantiated claims of cannabis having a beneficial effect in psychiatric disorders, the most common being depression.”
Dr. D’Souza, who was not involved with the study, called it “yet another piece of evidence suggesting that over the period of time during which cannabis laws have been liberalized, rates of past-month and daily cannabis use have increased, whereas rates of other substances, including alcohol, have remained stable.”
He suggested that a common limitation of epidemiological studies is that it is difficult to tell the direction of the association, “and it could be bidirectional.”
Nevertheless, there are clear implications for the practicing clinician, he added.
“If people have a history of depression, one should ask patients about the use of cannabis and also remind them about potential psychiatric negative effects of use,” Dr. D’Souza noted.
For the general public, “the point is that there is no good evidence to support cannabis use in depression treatment and, in fact, people with depression might be more likely to use it in problematic way,” he said.
Dr. Hasin agreed that it is “certainly possible that the relationship between cannabis use and depression is bidirectional, but the mechanism of this association requires more study.”
The study was supported by a grant from the National Institute on Drug Abuse to Dr. Hasin and by the New York State Psychiatric Institute. The study authors and Dr. D’Souza disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Not only are individuals with depression at significantly higher risk for cannabis use, compared with those without depression, this trend has increased dramatically over the last decade, new research shows.
Investigators analyzed data from more than 16,000 U.S. adults between the ages of 20 and 59 years and found that those with depression had almost twice the odds of any past-month cannabis use compared with those without depression. Odds rose from 1.5 in the 2005-2006 period to 2.3 in the 2015-2016 period.
Moreover, the odds ratio for daily or near-daily use almost tripled for those with versus without depression between the two periods.
“Clinicians should screen their depressed patients for cannabis use, since this is becoming more common and could actually make their depressive symptoms worse rather than better,” senior author Deborah Hasin, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York City, told Medscape Medical News.
The results were published online August 18 in JAMA Network Open.
Misleading advertising
“Cannabis use is increasing in the U.S. and the potency of cannabis products is increasing as well,” Dr. Hasin said.
“Misleading media information and advertising suggests that cannabis is a good treatment for depression, although studies show that cannabis use may actually worsen depression symptoms, [so] we were interested in whether U.S. adults were increasingly likely to be cannabis users if they were depressed,” she reported.
To investigate, the researchers assessed data from the National Health and Nutrition Examination Survey (NHANES), with a final study sample consisting of 16,216 U.S. adults. The mean age was 39.12 years, 48.9% were men, 66.4% were non-Hispanic White, 65.6% had at least some college education, and 62.4% had an annual family income of less than $75,000.
Of these participants, 7.5% had “probable depression,” based on the Patient Health Questionnaire–9, the investigators report.
Past-month cannabis use was defined as using cannabis at least once during the past 20 days. Daily or near-daily past-month use was defined as using cannabis at least 20 times in the past 30 days.
Covariates included age, gender, race, education, marital status, annual family income, and past-year use of other substances, such as alcohol, heroin, and methamphetamine.
The researchers note that because the NHANES data were divided into six survey years (2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, and 2015-2016), their analysis was based on a “new sample weight” that combined the datasets.
Especially pronounced
Results showed that the prevalence of any past-month cannabis use in the overall sample group increased from 12.2% in the 2005-2006 period to 17.3% in the 2015-2016 period (P < .001).
The investigators characterized this change as “significant,” adding that the estimated odds of cannabis use increased by approximately 9% between every 2-year time period.
The change was even more dramatic when the increase was examined across survey time periods (OR, 1.12; P < .001). The estimated odds of daily or near-daily use increased by approximately 12% between every 2-year period.
Interestingly, however, there were no significant changes in odds for depression when consecutive survey years were compared.
When the researchers specifically focused on the association between any past-month cannabis use and depression versus no depression, they found an adjusted OR of 1.90 (95% CI, 1.62-2.12; P < .001).
Individuals with depression also had 2.29 (95% CI, 1.80-2.92) times the odds for daily or near-daily cannabis use, compared with those without depression.
A post-hoc analysis looked at time trends in a sample group that included those missing information on at least one covariate (n = 17,724 participants). It showed similar results to those in the final sample that included no missing data.
People with depression have increased risk of using “most substances that can be abused,” Dr. Hasin said. “However, with the overall rates of cannabis use increasing in the general population, this is becoming especially pronounced for cannabis.”
Clear implications
Commenting on the findings for Medscape Medical News, Deepak D’Souza, MD, professor of psychiatry, Yale University, New Haven, Conn., said there is “concern about the unsubstantiated claims of cannabis having a beneficial effect in psychiatric disorders, the most common being depression.”
Dr. D’Souza, who was not involved with the study, called it “yet another piece of evidence suggesting that over the period of time during which cannabis laws have been liberalized, rates of past-month and daily cannabis use have increased, whereas rates of other substances, including alcohol, have remained stable.”
He suggested that a common limitation of epidemiological studies is that it is difficult to tell the direction of the association, “and it could be bidirectional.”
Nevertheless, there are clear implications for the practicing clinician, he added.
“If people have a history of depression, one should ask patients about the use of cannabis and also remind them about potential psychiatric negative effects of use,” Dr. D’Souza noted.
For the general public, “the point is that there is no good evidence to support cannabis use in depression treatment and, in fact, people with depression might be more likely to use it in problematic way,” he said.
Dr. Hasin agreed that it is “certainly possible that the relationship between cannabis use and depression is bidirectional, but the mechanism of this association requires more study.”
The study was supported by a grant from the National Institute on Drug Abuse to Dr. Hasin and by the New York State Psychiatric Institute. The study authors and Dr. D’Souza disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
CDC data confirm mental health is suffering during COVID-19
The ongoing COVID-19 pandemic continues to exact a huge toll on mental health in the United States, according to results of a survey released Aug. 13 by the Centers for Disease Control and Prevention.
During late June, about two in five U.S. adults surveyed said they were struggling with mental health or substance use. Younger adults, racial/ethnic minorities, essential workers, and those with preexisting psychiatric conditions were suffering the most.
“Addressing mental health disparities and preparing support systems to mitigate mental health consequences as the pandemic evolves will continue to be needed urgently,” write Rashon Lane, with the CDC COVID-19 Response Team, and colleagues in an article published online in the CDC’s Morbidity and Mortality Weekly Report.
During the period of June 24-30, 2020, 5,412 U.S. adults aged 18 and older completed online surveys that gauged mental health, substance use, and suicidal ideation.
Overall, 40.9% of respondents reported having at least one adverse mental or behavioral health condition; 31% reported symptoms of anxiety or depressive disorder; and 26% reported symptoms of a trauma- and stressor-related disorder related to the pandemic.
The prevalence of symptoms of anxiety disorder alone was roughly three times that reported in the second quarter of 2019, the authors noted.
In addition, , and nearly 11% reported having seriously considered suicide in the preceding 30 days.
Approximately twice as many respondents reported seriously considering suicide in the prior month compared with adults in the United States in 2018 (referring to the previous 12 months), the authors noted.
Suicidal ideation was significantly higher among younger respondents (aged 18-24 years, 26%), Hispanic persons (19%), non-Hispanic Black persons (15%), unpaid caregivers for adults (31%), and essential workers (22%).
The survey results are in line with recent data from Mental Health America, which indicate dramatic increases in depression, anxiety, and suicidality since the start of the COVID-19 pandemic.
The “markedly elevated” prevalence of adverse mental and behavioral health conditions associated with the COVID-19 pandemic highlights the “broad impact of the pandemic and the need to prevent and treat these conditions,” the researchers wrote.
The survey also highlights populations at increased risk for psychological distress and unhealthy coping.
“Future studies should identify drivers of adverse mental and behavioral health during the COVID-19 pandemic and whether factors such as social isolation, absence of school structure, unemployment and other financial worries, and various forms of violence (e.g., physical, emotional, mental, or sexual abuse) serve as additional stressors,” they suggested.
A version of this article originally appeared on Medscape.com.
The ongoing COVID-19 pandemic continues to exact a huge toll on mental health in the United States, according to results of a survey released Aug. 13 by the Centers for Disease Control and Prevention.
During late June, about two in five U.S. adults surveyed said they were struggling with mental health or substance use. Younger adults, racial/ethnic minorities, essential workers, and those with preexisting psychiatric conditions were suffering the most.
“Addressing mental health disparities and preparing support systems to mitigate mental health consequences as the pandemic evolves will continue to be needed urgently,” write Rashon Lane, with the CDC COVID-19 Response Team, and colleagues in an article published online in the CDC’s Morbidity and Mortality Weekly Report.
During the period of June 24-30, 2020, 5,412 U.S. adults aged 18 and older completed online surveys that gauged mental health, substance use, and suicidal ideation.
Overall, 40.9% of respondents reported having at least one adverse mental or behavioral health condition; 31% reported symptoms of anxiety or depressive disorder; and 26% reported symptoms of a trauma- and stressor-related disorder related to the pandemic.
The prevalence of symptoms of anxiety disorder alone was roughly three times that reported in the second quarter of 2019, the authors noted.
In addition, , and nearly 11% reported having seriously considered suicide in the preceding 30 days.
Approximately twice as many respondents reported seriously considering suicide in the prior month compared with adults in the United States in 2018 (referring to the previous 12 months), the authors noted.
Suicidal ideation was significantly higher among younger respondents (aged 18-24 years, 26%), Hispanic persons (19%), non-Hispanic Black persons (15%), unpaid caregivers for adults (31%), and essential workers (22%).
The survey results are in line with recent data from Mental Health America, which indicate dramatic increases in depression, anxiety, and suicidality since the start of the COVID-19 pandemic.
The “markedly elevated” prevalence of adverse mental and behavioral health conditions associated with the COVID-19 pandemic highlights the “broad impact of the pandemic and the need to prevent and treat these conditions,” the researchers wrote.
The survey also highlights populations at increased risk for psychological distress and unhealthy coping.
“Future studies should identify drivers of adverse mental and behavioral health during the COVID-19 pandemic and whether factors such as social isolation, absence of school structure, unemployment and other financial worries, and various forms of violence (e.g., physical, emotional, mental, or sexual abuse) serve as additional stressors,” they suggested.
A version of this article originally appeared on Medscape.com.
The ongoing COVID-19 pandemic continues to exact a huge toll on mental health in the United States, according to results of a survey released Aug. 13 by the Centers for Disease Control and Prevention.
During late June, about two in five U.S. adults surveyed said they were struggling with mental health or substance use. Younger adults, racial/ethnic minorities, essential workers, and those with preexisting psychiatric conditions were suffering the most.
“Addressing mental health disparities and preparing support systems to mitigate mental health consequences as the pandemic evolves will continue to be needed urgently,” write Rashon Lane, with the CDC COVID-19 Response Team, and colleagues in an article published online in the CDC’s Morbidity and Mortality Weekly Report.
During the period of June 24-30, 2020, 5,412 U.S. adults aged 18 and older completed online surveys that gauged mental health, substance use, and suicidal ideation.
Overall, 40.9% of respondents reported having at least one adverse mental or behavioral health condition; 31% reported symptoms of anxiety or depressive disorder; and 26% reported symptoms of a trauma- and stressor-related disorder related to the pandemic.
The prevalence of symptoms of anxiety disorder alone was roughly three times that reported in the second quarter of 2019, the authors noted.
In addition, , and nearly 11% reported having seriously considered suicide in the preceding 30 days.
Approximately twice as many respondents reported seriously considering suicide in the prior month compared with adults in the United States in 2018 (referring to the previous 12 months), the authors noted.
Suicidal ideation was significantly higher among younger respondents (aged 18-24 years, 26%), Hispanic persons (19%), non-Hispanic Black persons (15%), unpaid caregivers for adults (31%), and essential workers (22%).
The survey results are in line with recent data from Mental Health America, which indicate dramatic increases in depression, anxiety, and suicidality since the start of the COVID-19 pandemic.
The “markedly elevated” prevalence of adverse mental and behavioral health conditions associated with the COVID-19 pandemic highlights the “broad impact of the pandemic and the need to prevent and treat these conditions,” the researchers wrote.
The survey also highlights populations at increased risk for psychological distress and unhealthy coping.
“Future studies should identify drivers of adverse mental and behavioral health during the COVID-19 pandemic and whether factors such as social isolation, absence of school structure, unemployment and other financial worries, and various forms of violence (e.g., physical, emotional, mental, or sexual abuse) serve as additional stressors,” they suggested.
A version of this article originally appeared on Medscape.com.
Deaths, despair tied to drug dependence are accelerating amid COVID-19
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
Patients with OUDs need assistance now more than ever.
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
ED visits for mental health, substance use doubled in 1 decade
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).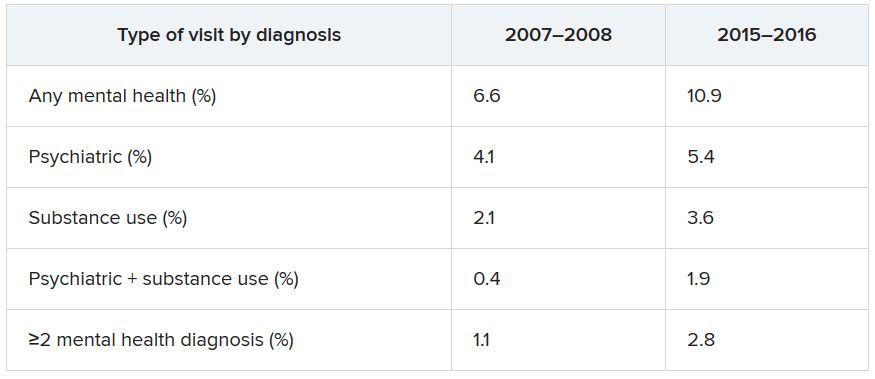
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Exploring cannabis use by older adults
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
A doctor conquers his demons
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Addiction specialist charged in $681 million treatment fraud case
The federal government has charged a Florida addiction medicine specialist in what it says was a scheme to defraud Medicare and private insurers, charging them roughly $681 million over about a decade for lab tests, office visits, therapy sessions, and other services that were either unnecessary or never delivered.
The Department of Justice and the Attorney for the Southern District of Florida are prosecuting Michael Ligotti, DO, 46, saying that he preyed on individuals seeking substance abuse treatment. They have yet to issue a formal indictment.
“The substance abuse treatment fraud allegedly perpetrated by the defendant sacrificed the genuine care of vulnerable patients at a time when they urgently needed a trusted health care provider,” U.S. Attorney Ariana Fajardo Orshan, Southern District of Florida, said in a statement.
“Health care providers who allow greed to take precedence over their Hippocratic Oath and participate in these schemes are criminals and will be held accountable for their unscrupulous conduct,” she added.
Dr. Ligotti was charged July 31. The prosecutors were seeking to detain him until trial. However, a judge approved his bond today and he is free on bond, according to Ligotti’s attorney, Ben Curtis.
“As is always the case with any criminal matter, the burden of proof rests entirely with the government,” Mr. Curtis said in an interview.
“In this instance, we do not believe the US Department of Justice’s claims – and that is exactly what they are at this point, just one-sided claims – will reconcile with actual evidence at a future trial,” he said.
Mr. Curtis added that Dr. Ligotti “looks forward to establishing his innocence.”
Unnecessary urine tests
The government alleges that Dr. Ligotti played a central role in a scheme in which Medicare and private insurers paid about $121 million to cover some $680 million in charges from 2011 to 2020.
According to the prosecutors, Dr. Ligotti received a fee for becoming a “purported” medical director of about 50 addiction treatment facilities and sober homes – and that he issued 136 separate standing orders for medically unnecessary urinalysis (UA) tests.
The labs allegedly paid occasional kickbacks to the facilities and homes, and those facilities in turn were required to have their patients treated by Dr. Ligotti’s clinic, Whole Health, which is based in Delray Beach, Fla.
This allowed Dr. Ligotti to “bill hundreds of millions of dollars in additional fraudulent treatments, including unnecessary and expensive UAs, costly blood tests, nonexistent therapy sessions, office visits, and other unnecessary services, regardless of whether such treatment and testing were medically necessary and/or actually provided,” alleges the government.
Urine tests have been exploited before by addiction treatment clinics as a revenue generator. Kaiser Health News reported in 2017 that a single nurse practitioner at one pain clinic in Tennessee generated $1 million in billings to Medicare for drug-related urine tests in a single year.
Also in 2017, The New York Times reported that a single patient had been billed $260,000 for urine tests by his treatment center.
The federal government alleges that Dr. Ligotti also “billed for psychiatric services and therapy sessions that never happened, and that he and his staff were not qualified to conduct.”
In addition, they assert that Dr. Ligotti improperly prescribed controlled substances, including large quantities of buprenorphine/Suboxone, often exceeding the number of patients he was legally authorized to treat or giving it to patients who did not require the medications.
Dr. Ligotti may not be practicing any longer. His clinic’s website features a message that the practice will be closed as of Aug. 7.
This article first appeared on Medscape.com.
The federal government has charged a Florida addiction medicine specialist in what it says was a scheme to defraud Medicare and private insurers, charging them roughly $681 million over about a decade for lab tests, office visits, therapy sessions, and other services that were either unnecessary or never delivered.
The Department of Justice and the Attorney for the Southern District of Florida are prosecuting Michael Ligotti, DO, 46, saying that he preyed on individuals seeking substance abuse treatment. They have yet to issue a formal indictment.
“The substance abuse treatment fraud allegedly perpetrated by the defendant sacrificed the genuine care of vulnerable patients at a time when they urgently needed a trusted health care provider,” U.S. Attorney Ariana Fajardo Orshan, Southern District of Florida, said in a statement.
“Health care providers who allow greed to take precedence over their Hippocratic Oath and participate in these schemes are criminals and will be held accountable for their unscrupulous conduct,” she added.
Dr. Ligotti was charged July 31. The prosecutors were seeking to detain him until trial. However, a judge approved his bond today and he is free on bond, according to Ligotti’s attorney, Ben Curtis.
“As is always the case with any criminal matter, the burden of proof rests entirely with the government,” Mr. Curtis said in an interview.
“In this instance, we do not believe the US Department of Justice’s claims – and that is exactly what they are at this point, just one-sided claims – will reconcile with actual evidence at a future trial,” he said.
Mr. Curtis added that Dr. Ligotti “looks forward to establishing his innocence.”
Unnecessary urine tests
The government alleges that Dr. Ligotti played a central role in a scheme in which Medicare and private insurers paid about $121 million to cover some $680 million in charges from 2011 to 2020.
According to the prosecutors, Dr. Ligotti received a fee for becoming a “purported” medical director of about 50 addiction treatment facilities and sober homes – and that he issued 136 separate standing orders for medically unnecessary urinalysis (UA) tests.
The labs allegedly paid occasional kickbacks to the facilities and homes, and those facilities in turn were required to have their patients treated by Dr. Ligotti’s clinic, Whole Health, which is based in Delray Beach, Fla.
This allowed Dr. Ligotti to “bill hundreds of millions of dollars in additional fraudulent treatments, including unnecessary and expensive UAs, costly blood tests, nonexistent therapy sessions, office visits, and other unnecessary services, regardless of whether such treatment and testing were medically necessary and/or actually provided,” alleges the government.
Urine tests have been exploited before by addiction treatment clinics as a revenue generator. Kaiser Health News reported in 2017 that a single nurse practitioner at one pain clinic in Tennessee generated $1 million in billings to Medicare for drug-related urine tests in a single year.
Also in 2017, The New York Times reported that a single patient had been billed $260,000 for urine tests by his treatment center.
The federal government alleges that Dr. Ligotti also “billed for psychiatric services and therapy sessions that never happened, and that he and his staff were not qualified to conduct.”
In addition, they assert that Dr. Ligotti improperly prescribed controlled substances, including large quantities of buprenorphine/Suboxone, often exceeding the number of patients he was legally authorized to treat or giving it to patients who did not require the medications.
Dr. Ligotti may not be practicing any longer. His clinic’s website features a message that the practice will be closed as of Aug. 7.
This article first appeared on Medscape.com.
The federal government has charged a Florida addiction medicine specialist in what it says was a scheme to defraud Medicare and private insurers, charging them roughly $681 million over about a decade for lab tests, office visits, therapy sessions, and other services that were either unnecessary or never delivered.
The Department of Justice and the Attorney for the Southern District of Florida are prosecuting Michael Ligotti, DO, 46, saying that he preyed on individuals seeking substance abuse treatment. They have yet to issue a formal indictment.
“The substance abuse treatment fraud allegedly perpetrated by the defendant sacrificed the genuine care of vulnerable patients at a time when they urgently needed a trusted health care provider,” U.S. Attorney Ariana Fajardo Orshan, Southern District of Florida, said in a statement.
“Health care providers who allow greed to take precedence over their Hippocratic Oath and participate in these schemes are criminals and will be held accountable for their unscrupulous conduct,” she added.
Dr. Ligotti was charged July 31. The prosecutors were seeking to detain him until trial. However, a judge approved his bond today and he is free on bond, according to Ligotti’s attorney, Ben Curtis.
“As is always the case with any criminal matter, the burden of proof rests entirely with the government,” Mr. Curtis said in an interview.
“In this instance, we do not believe the US Department of Justice’s claims – and that is exactly what they are at this point, just one-sided claims – will reconcile with actual evidence at a future trial,” he said.
Mr. Curtis added that Dr. Ligotti “looks forward to establishing his innocence.”
Unnecessary urine tests
The government alleges that Dr. Ligotti played a central role in a scheme in which Medicare and private insurers paid about $121 million to cover some $680 million in charges from 2011 to 2020.
According to the prosecutors, Dr. Ligotti received a fee for becoming a “purported” medical director of about 50 addiction treatment facilities and sober homes – and that he issued 136 separate standing orders for medically unnecessary urinalysis (UA) tests.
The labs allegedly paid occasional kickbacks to the facilities and homes, and those facilities in turn were required to have their patients treated by Dr. Ligotti’s clinic, Whole Health, which is based in Delray Beach, Fla.
This allowed Dr. Ligotti to “bill hundreds of millions of dollars in additional fraudulent treatments, including unnecessary and expensive UAs, costly blood tests, nonexistent therapy sessions, office visits, and other unnecessary services, regardless of whether such treatment and testing were medically necessary and/or actually provided,” alleges the government.
Urine tests have been exploited before by addiction treatment clinics as a revenue generator. Kaiser Health News reported in 2017 that a single nurse practitioner at one pain clinic in Tennessee generated $1 million in billings to Medicare for drug-related urine tests in a single year.
Also in 2017, The New York Times reported that a single patient had been billed $260,000 for urine tests by his treatment center.
The federal government alleges that Dr. Ligotti also “billed for psychiatric services and therapy sessions that never happened, and that he and his staff were not qualified to conduct.”
In addition, they assert that Dr. Ligotti improperly prescribed controlled substances, including large quantities of buprenorphine/Suboxone, often exceeding the number of patients he was legally authorized to treat or giving it to patients who did not require the medications.
Dr. Ligotti may not be practicing any longer. His clinic’s website features a message that the practice will be closed as of Aug. 7.
This article first appeared on Medscape.com.
CYP450 interactions between illicit substances and prescription medications
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
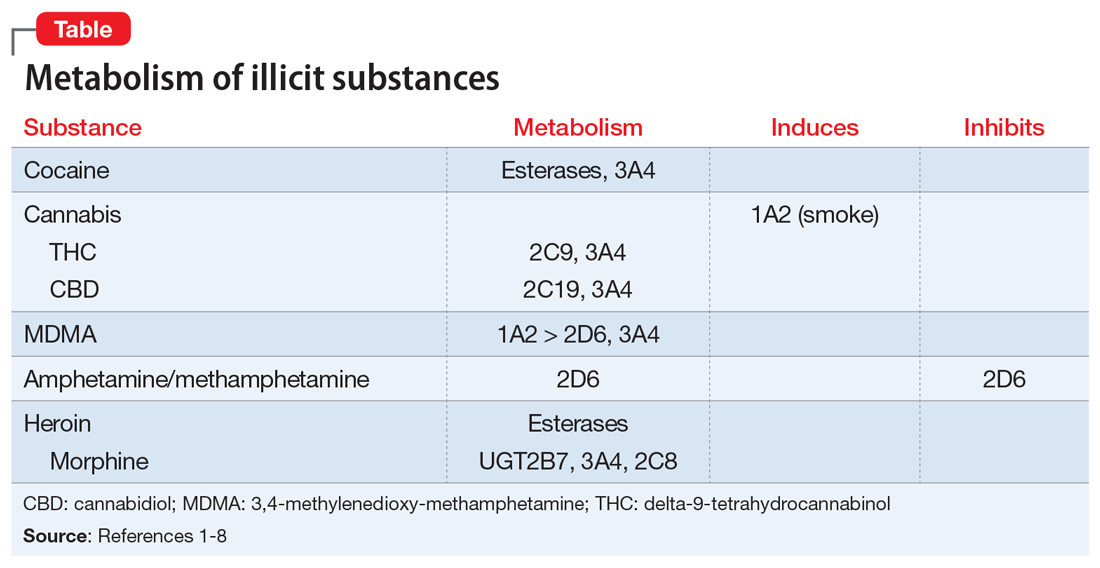
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.

Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.

Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.