User login
How physicians can provide better care to transgender patients
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
Gastrointestinal Symptoms and Lactic Acidosis in a Chronic Marijuana User
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
Urine drug screening: A guide to monitoring Tx with controlled substances
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4
A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15
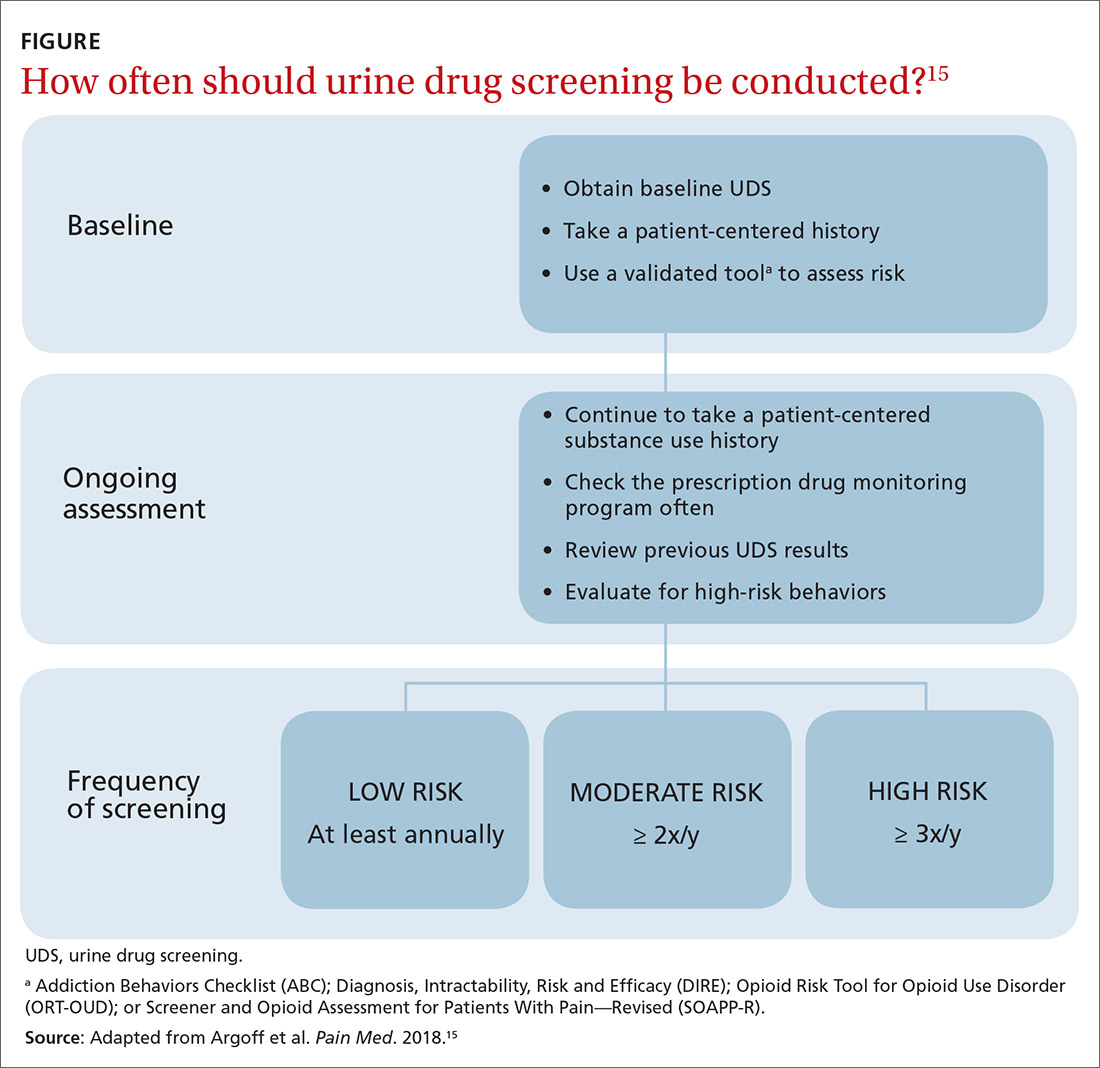
Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
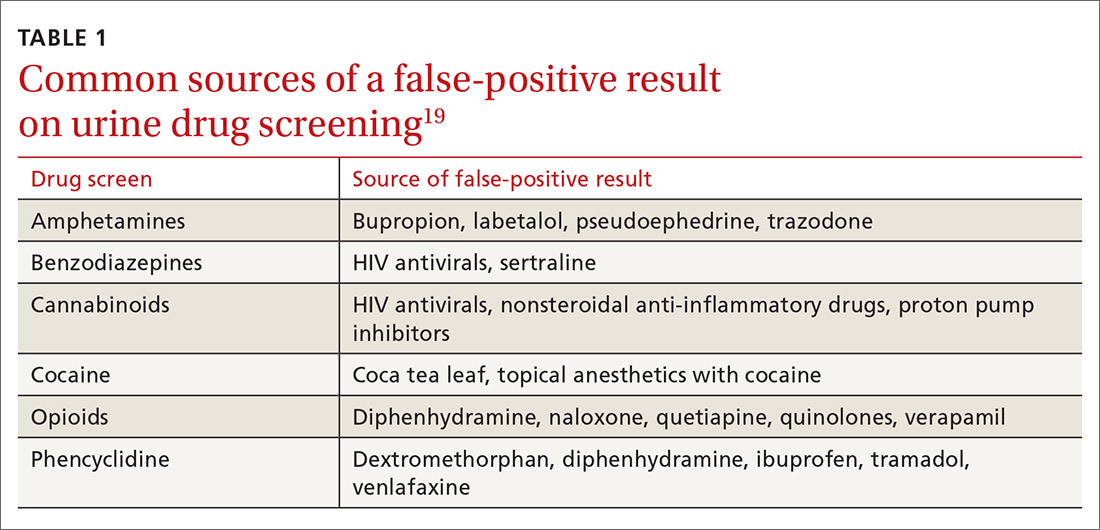
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19
Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20
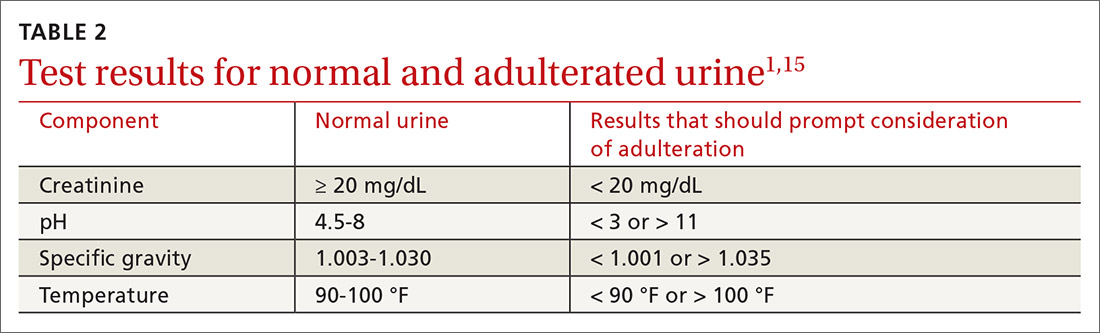
Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).
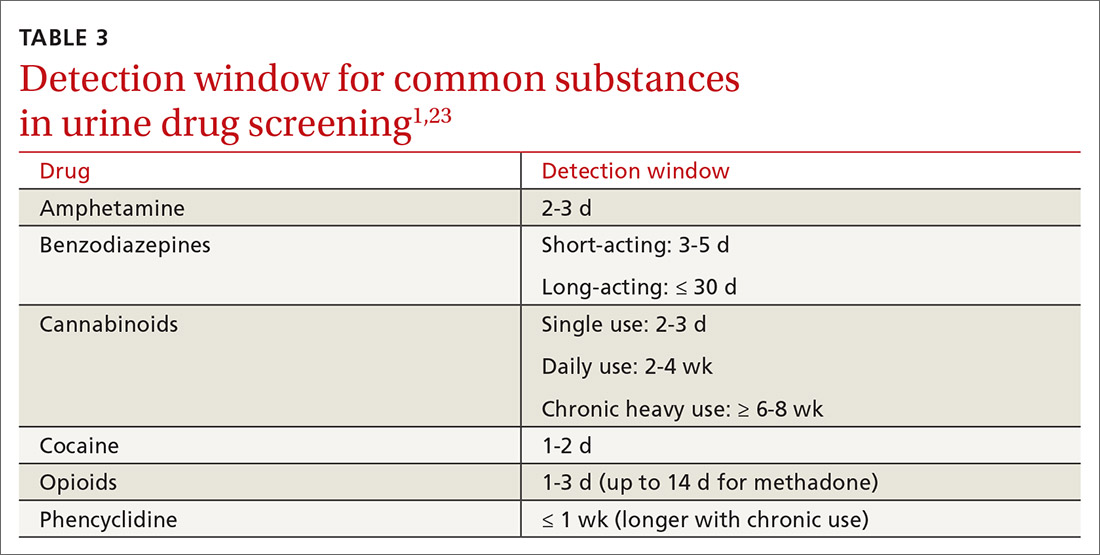
Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4

A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15

Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19

Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20

Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).

Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4

A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15

Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19

Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20

Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).

Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
PRACTICE RECOMMENDATIONS
› Consider developing a risk-based urine drug testing protocol for all patients who are on chronic opioid therapy. C
› Consider urine drug testing to augment a thorough history when identifying and offering treatment to patients with a substance use disorder. A
› Do not change your management plan based on results of a single screening urine test. Revisit unexpected positive or negative results with a thorough history or confirmatory testing. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An international trip: Global experts weigh in on psychedelics
In 1967, when the United Nations Convention on Drugs classified psychedelics as schedule I substances, it effectively ended research into these agents as potential therapeutics for psychiatric disorders.
Psychedelics induce altered states of perception. They bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include psilocybin, which is derived from “magic mushrooms”; N,N-dimethyltryptamine (DMT), a component of ayahuasca and mescaline (peyote cactus); and the synthesized compound D-lysergic acid diethylamide (LSD). Other agents, such as ketamine and 3,4-methylenedioxymethamphetamine (MDMA), also known as ecstasy, are sometimes considered psychedelics as well.
Before they were classified as schedule I agents, psychedelics had been shown to be particularly beneficial for patients with treatment-resistant conditions, including depression and posttraumatic stress disorder (PTSD), especially when administered in a supportive, therapeutic setting.
Now, after a hiatus of almost 50 years, there is renewed global interest in the scientific investigation of psychedelics. The attention was spurred in part by several exploratory studies of DMT in humans conducted in the 1990s by Rick Strassman, MD, and colleagues at the University of New Mexico, Albuquerque.
Around the same time, Franz X. Vollenweider, MD, and colleagues at the University of Zürich began researching psilocybin and its effects on human behavior. However, it was a 2006 study of psilocybin by a team of researchers at Johns Hopkins University, Baltimore, that is widely cited as a catalyst for the current renaissance in psychedelic research.
To provide a broad-based, international perspective on these agents, including their current legal status and indications, treatment regimens, safety, efficacy, and future considerations, this news organization interviewed nine expert researchers from around the globe.
Global legal status
In most, if not all, countries, it is still illegal to prescribe psychedelics in other than a research setting.
They can be used in research, but only with approval from the Food and Drug Administration under licensure from the Drug Enforcement Administration.
France lists all synthetic hallucinogens and hallucinogenic mushrooms as narcotic. As a result, possession, use, transportation, and collection are subject to criminal sanctions.
In France, NMDA antagonists such as ketamine and nitrous oxide are regarded as psychedelic molecules and can be used off label for various conditions or as part of research protocols authorized by the French public health code.
Although psychedelics are illegal under Mexican law, they are commonly used in indigenous communities as part of traditional rituals.
“The line between traditional consumption and psychedelic tourism is very thin,” José J. Mendoza Velásquez, MD, professor in the department of mental health, National Autonomous University of Mexico, Mexico City, said in an interview.
Psychedelics also are illegal in the United Kingdom, although government agencies have recently allowed research groups to investigate them. Psychedelics cannot be prescribed in Germany, Spain, or Italy. However, investigators in these countries can request permission from regulatory agencies to conduct research.
Brazil allows psychedelic substances to be researched, particularly ayahuasca, which has long traditional and religious roots in the country.
However, as in other countries, none of the classic psychedelics is regulated for therapeutic use in Brazil. It is widely expected that the Brazilian government will approve MDMA sometime in 2024 for use in the treatment of PTSD.
Potential indications
Psychedelics are currently under investigation as potential treatments for major depression, treatment-resistant depression, PTSD, pain management, and anorexia, among other conditions.
In France, Florian Ferreri, MD, PhD, at Hospital Saint-Antoine, Paris, is researching ketamine for treatment of patients with suicidal crisis/ideation and treatment-resistant depression.
In the United Kingdom, David Nutt, FMedSci, Edmond J. Safra Professor of Neuropsychopharmacology at Imperial College London, and his team have conducted studies of the use of psychedelics in conjunction with psychological support for patients with treatment-resistant depression, and they are currently exploring their use in the treatment of anorexia and various pain syndromes.
In Germany, Gerhard Gründer, MD, professor of psychiatry at the Central Institute of Mental Health, in Mannheim, noted that a study of psilocybin for treatment-resistant depression will launch sometime in 2021. In Italy, current research is focusing on MDMA and ketamine in the laboratory environment and in animal models for treating depression and drug abuse.
Researcher Helen Dolengevich-Segal, MD, a psychiatrist at Hospital Universitario del Henares, Madrid, noted that although research on esketamine for the treatment of severe depressive disorder with suicidal thoughts is underway, there is very limited published research from that country into the use of classic psychedelics for various psychiatric disorders, given their current illegal status.
Mexico’s Dr. Velásquez noted that although he is prohibited from prescribing psychedelics, he does have patients who take the drugs to augment medical treatment. For instance, he said, his patients frequently use psilocybin to help with severe depression, pain, and insomnia.
Environment is key
Most researchers agree that for psychedelics to be safe and effective, patient education and administration in a controlled environment by experienced clinicians are key to successful treatment.
Roland R. Griffiths, PhD, director of the Center for Psychedelic and Consciousness Research at Johns Hopkins, said that ongoing U.S. psilocybin research – primarily in major depressive disorder and psychological distress associated with life-threatening illness, drug addiction, anorexia nervosa, obsessive-compulsive disorder, and headache – generally includes one or two treatment sessions, each of which lasts 6-8 hours.
Such sessions typically involve oral administration of a moderately high dose of a psychedelic under what he characterizes as “psychologically supported conditions.”
For Dr. Griffiths, there are serious potential risks associated with the use of psilocybin and other psychedelics outside such environments.
“When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others, including life-threatening risk,” he said.
Dr. Gründer agreed.
“At the moment, I cannot imagine that you would go to the pharmacy with a prescription for psilocybin and get yourself a pill and then take it in a quiet little room,” he said. Dr. Dolengevich-Segal and Dr. Velásquez echoed these sentiments, noting the optimal location for administration is one that is quiet and secure and where patients feel safe.
Luís Fernando Tófoli, MD, PhD, professor of medical psychology and psychiatry at the University of Campinas, and Eduardo Schenberg, PhD, founder and CEO of Instituto Phaneros in São Paulo, Brazil, said more research is needed to determine the optimal therapeutic environment for individual agents.
“Most studies have a low number of participants (around 20 or 30), especially in neuroimaging, with high unblinding rates,” Dr. Schenberg said. “Therefore, novel methodological approaches are also necessary, as these substances do not easily fit into the traditional pharmacology epistemic model.”
Risks, abuse potential
The abuse potential of psychedelics is an ongoing concern for the public, researchers, and regulators, but the consensus among nearly all of these experts is that when administered by medical professionals in controlled settings, these drugs are associated with extremely low risk.
It is recreational use that presents an abuse concern, said Dr. Ferreri, but with the low doses used in psychiatry, the risk is “very limited or even nonexistent.”
Dr. Nutt said the abuse potential of psychedelics is so low that they can be used to treat addiction.
“Functionally, psychedelics are antiaddictive,” Dr. Nutt said. “The fact is, if you take them repeatedly, you develop tolerance, and the effect disappears. You can’t overcome it. But everyone believes they’re addictive because they’re scheduled drugs.”
Dr. Velásquez is something of an outlier. He believes the abuse potential with psychedelics is poorly understood and that some patients may develop tolerance, which is a potential gateway to dependence.
“Such is the case with LSD,” he said, “where this substance also favors tolerance to other psychedelic drugs such as psilocybin.”
Dosing also seems to play a key role in mitigating potential abuse, said Luca Pani, MD, professor of pharmacology and psychiatry at the University of Modena, Italy. Dr. Pani explained that with low doses and microdoses of psychedelics, the potential for abuse is eliminated.
Dr. Nutt, Dr. Pani, and Dr. Ferreri also noted the importance of medical supervision. For instance, said Dr. Ferreri, when administering ketamine, his team closely monitors both mental and physical parameters – heart rate and blood pressure, in particular – because the drug can have hypertensive effects.
Dr. Schenberg noted that ibogaine, a naturally occurring psychedelic frequently used by traditional communities in Africa in rituals and for healing purposes, could cause potentially fatal arrhythmias, so it’s critical that the treatment is administered in a hospital setting that has a cardiac unit.
Dr. Pani said there is a need for more research, especially regarding the molecular mechanisms behind the behavioral effects of low-dose psychedelic therapy and the potential risks of multiple treatments with the drugs.
“Although extensive toxicology has been conducted on a single active dose of psilocybin, which has been proven to be safe, further research is required to understand better the possible health risks, especially in relation to cardiac and lung tissue,” he said.
Psychologically challenging
The experts note that given the relative lack of experience with psychedelic therapy, preparing patients for potential adverse effects is paramount. This is particularly relevant in the research setting and highlights the need for adequate patient screening and aftercare.
Dr. Gründer and Dr. Dolengevich-Segal emphasized the importance of having qualified personnel available in the event that patients experience adverse psychological events during treatment.
For Dr. Gründer, the potential for psilocybin to cause patients to lose control, experience psychotic symptoms, or become paranoid warrants considerable preparation by treating physicians.
Patients occasionally experience fear and anxiety during treatment, though it’s usually short-lived, said Dr. Griffiths. Nevertheless, these experiences may open the door to greater insight. “A number of people report that these psychologically challenging states are a valuable part of the overall experience,” he said.
The situation is similar in Spain, where Dr. Dolengevich-Segal noted that typical treatment regimens have a strong focus on the patient’s experience as a therapeutic tool. As in the United Kingdom and the United States, her team guides patients to what they call a “peak experience,” which allows them to gain a better understanding of the trauma underlying their mental health problems.
Dr. Nutt said that in the United Kingdom, they haven’t seen adverse reactions in patients receiving psychedelic therapy, although sedatives such as benzodiazepines could be used to manage them. He added that at his center, two therapists are present at every treatment session, and all personnel are “trained medics or psychologists.”
Patient education
Preparing and educating patients about the therapy are critical, said Dr. Gründer, especially given the intense response psychedelic treatment often invokes.
Echoing Dr. Gründer, Dr. Tófoli said explaining the nature of psychedelic treatment to potential patients helps ease anxiety.
Dr. Griffiths noted that in the United States, study participants are not only educated about the potential effects of psychedelic agents but also undergo several hours of psychological preparation in advance of their first treatment session and are provided with psychological support after treatment.
There is also a strong emphasis on patient preparation and education in the United Kingdom, where patients meet with therapists before and after treatment. During these posttreatment debriefings, clinicians use the patients’ experience with psychedelics to help them gain insight into the underlying cause of their depression.
Dr. Schenberg noted that at his institution in São Paulo, there are online courses to teach clinicians about psychedelic therapy for psychiatric disorders. Next year, he added, a new training program in MDMA-assisted psychotherapy will begin.
Working out treatment protocols
Treatment protocols for psychedelics vary by agent and indication from country to country. For instance, Dr. Pani noted that current psychedelic research in Italy predominantly focuses more on microdosing, which involves administering 1% of the pharmacologically active dose to a maximum of 100 mcg, in contrast to low dosing or full dosing.
Therapeutic regimens in Brazil, said Dr. Schenberg, also differ by agent but share common elements. For instance, psychedelics are always administered in a research setting, and sessions include concomitant psychotherapy.
In Germany, investigators are working to determine optimal treatment regimen for psilocybin for resistant depression in a randomized three-arm study planned for 2021.
For Mexico’s Dr. Velásquez, treatment regimens are complex and varied. Either way, he said, patients always require long-term follow-up.
With ketamine therapy, Dr. Ferreri said his team administers the drug in 45- to 60-minute intravenous infusion sessions in a hospital room without light or sound stimulation. Regardless of the drug’s immediate effect, he said, the protocol is repeated within a 6-month period.
The question of the duration of treatment effect is important. Dr. Griffiths said research suggests that the positive effects of psilocybin are long lasting and that most individuals report positive changes in mood, attitude, and behavior that endure for months or even years after the session.
“Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experiences of their lives,” said Dr. Griffiths.
Dr. Nutt agreed, noting that a single intense “trip” can improve mood for weeks, months, or even years. Nevertheless, he said, in his experience, approximately three-quarters of patients treated with psychedelics for major depression relapse within 3-9 months.
“Most get better,” he said, “but the majority of depression comes back over a period of months.”
Given the current illegal status of the drugs, he said it’s nearly impossible to provide patients with regular, subsequent treatment with psychedelics over time.
“My suspicion is that you might well have to dose four or five times over a couple of years to get people to escape from very severe depression,” said Dr. Nutt. “The longer they’ve been depressed, the harder it is for them to make a full recovery, because it’s more entrenched in the brain.”
All experts agree that exciting times are ahead for psychedelics as therapeutics for a wide range of psychiatric disorders.
“We can look forward to continued growth and expansion of this research,” said Dr. Griffiths, “including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.”
A version of this article first appeared on Medscape.com.
In 1967, when the United Nations Convention on Drugs classified psychedelics as schedule I substances, it effectively ended research into these agents as potential therapeutics for psychiatric disorders.
Psychedelics induce altered states of perception. They bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include psilocybin, which is derived from “magic mushrooms”; N,N-dimethyltryptamine (DMT), a component of ayahuasca and mescaline (peyote cactus); and the synthesized compound D-lysergic acid diethylamide (LSD). Other agents, such as ketamine and 3,4-methylenedioxymethamphetamine (MDMA), also known as ecstasy, are sometimes considered psychedelics as well.
Before they were classified as schedule I agents, psychedelics had been shown to be particularly beneficial for patients with treatment-resistant conditions, including depression and posttraumatic stress disorder (PTSD), especially when administered in a supportive, therapeutic setting.
Now, after a hiatus of almost 50 years, there is renewed global interest in the scientific investigation of psychedelics. The attention was spurred in part by several exploratory studies of DMT in humans conducted in the 1990s by Rick Strassman, MD, and colleagues at the University of New Mexico, Albuquerque.
Around the same time, Franz X. Vollenweider, MD, and colleagues at the University of Zürich began researching psilocybin and its effects on human behavior. However, it was a 2006 study of psilocybin by a team of researchers at Johns Hopkins University, Baltimore, that is widely cited as a catalyst for the current renaissance in psychedelic research.
To provide a broad-based, international perspective on these agents, including their current legal status and indications, treatment regimens, safety, efficacy, and future considerations, this news organization interviewed nine expert researchers from around the globe.
Global legal status
In most, if not all, countries, it is still illegal to prescribe psychedelics in other than a research setting.
They can be used in research, but only with approval from the Food and Drug Administration under licensure from the Drug Enforcement Administration.
France lists all synthetic hallucinogens and hallucinogenic mushrooms as narcotic. As a result, possession, use, transportation, and collection are subject to criminal sanctions.
In France, NMDA antagonists such as ketamine and nitrous oxide are regarded as psychedelic molecules and can be used off label for various conditions or as part of research protocols authorized by the French public health code.
Although psychedelics are illegal under Mexican law, they are commonly used in indigenous communities as part of traditional rituals.
“The line between traditional consumption and psychedelic tourism is very thin,” José J. Mendoza Velásquez, MD, professor in the department of mental health, National Autonomous University of Mexico, Mexico City, said in an interview.
Psychedelics also are illegal in the United Kingdom, although government agencies have recently allowed research groups to investigate them. Psychedelics cannot be prescribed in Germany, Spain, or Italy. However, investigators in these countries can request permission from regulatory agencies to conduct research.
Brazil allows psychedelic substances to be researched, particularly ayahuasca, which has long traditional and religious roots in the country.
However, as in other countries, none of the classic psychedelics is regulated for therapeutic use in Brazil. It is widely expected that the Brazilian government will approve MDMA sometime in 2024 for use in the treatment of PTSD.
Potential indications
Psychedelics are currently under investigation as potential treatments for major depression, treatment-resistant depression, PTSD, pain management, and anorexia, among other conditions.
In France, Florian Ferreri, MD, PhD, at Hospital Saint-Antoine, Paris, is researching ketamine for treatment of patients with suicidal crisis/ideation and treatment-resistant depression.
In the United Kingdom, David Nutt, FMedSci, Edmond J. Safra Professor of Neuropsychopharmacology at Imperial College London, and his team have conducted studies of the use of psychedelics in conjunction with psychological support for patients with treatment-resistant depression, and they are currently exploring their use in the treatment of anorexia and various pain syndromes.
In Germany, Gerhard Gründer, MD, professor of psychiatry at the Central Institute of Mental Health, in Mannheim, noted that a study of psilocybin for treatment-resistant depression will launch sometime in 2021. In Italy, current research is focusing on MDMA and ketamine in the laboratory environment and in animal models for treating depression and drug abuse.
Researcher Helen Dolengevich-Segal, MD, a psychiatrist at Hospital Universitario del Henares, Madrid, noted that although research on esketamine for the treatment of severe depressive disorder with suicidal thoughts is underway, there is very limited published research from that country into the use of classic psychedelics for various psychiatric disorders, given their current illegal status.
Mexico’s Dr. Velásquez noted that although he is prohibited from prescribing psychedelics, he does have patients who take the drugs to augment medical treatment. For instance, he said, his patients frequently use psilocybin to help with severe depression, pain, and insomnia.
Environment is key
Most researchers agree that for psychedelics to be safe and effective, patient education and administration in a controlled environment by experienced clinicians are key to successful treatment.
Roland R. Griffiths, PhD, director of the Center for Psychedelic and Consciousness Research at Johns Hopkins, said that ongoing U.S. psilocybin research – primarily in major depressive disorder and psychological distress associated with life-threatening illness, drug addiction, anorexia nervosa, obsessive-compulsive disorder, and headache – generally includes one or two treatment sessions, each of which lasts 6-8 hours.
Such sessions typically involve oral administration of a moderately high dose of a psychedelic under what he characterizes as “psychologically supported conditions.”
For Dr. Griffiths, there are serious potential risks associated with the use of psilocybin and other psychedelics outside such environments.
“When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others, including life-threatening risk,” he said.
Dr. Gründer agreed.
“At the moment, I cannot imagine that you would go to the pharmacy with a prescription for psilocybin and get yourself a pill and then take it in a quiet little room,” he said. Dr. Dolengevich-Segal and Dr. Velásquez echoed these sentiments, noting the optimal location for administration is one that is quiet and secure and where patients feel safe.
Luís Fernando Tófoli, MD, PhD, professor of medical psychology and psychiatry at the University of Campinas, and Eduardo Schenberg, PhD, founder and CEO of Instituto Phaneros in São Paulo, Brazil, said more research is needed to determine the optimal therapeutic environment for individual agents.
“Most studies have a low number of participants (around 20 or 30), especially in neuroimaging, with high unblinding rates,” Dr. Schenberg said. “Therefore, novel methodological approaches are also necessary, as these substances do not easily fit into the traditional pharmacology epistemic model.”
Risks, abuse potential
The abuse potential of psychedelics is an ongoing concern for the public, researchers, and regulators, but the consensus among nearly all of these experts is that when administered by medical professionals in controlled settings, these drugs are associated with extremely low risk.
It is recreational use that presents an abuse concern, said Dr. Ferreri, but with the low doses used in psychiatry, the risk is “very limited or even nonexistent.”
Dr. Nutt said the abuse potential of psychedelics is so low that they can be used to treat addiction.
“Functionally, psychedelics are antiaddictive,” Dr. Nutt said. “The fact is, if you take them repeatedly, you develop tolerance, and the effect disappears. You can’t overcome it. But everyone believes they’re addictive because they’re scheduled drugs.”
Dr. Velásquez is something of an outlier. He believes the abuse potential with psychedelics is poorly understood and that some patients may develop tolerance, which is a potential gateway to dependence.
“Such is the case with LSD,” he said, “where this substance also favors tolerance to other psychedelic drugs such as psilocybin.”
Dosing also seems to play a key role in mitigating potential abuse, said Luca Pani, MD, professor of pharmacology and psychiatry at the University of Modena, Italy. Dr. Pani explained that with low doses and microdoses of psychedelics, the potential for abuse is eliminated.
Dr. Nutt, Dr. Pani, and Dr. Ferreri also noted the importance of medical supervision. For instance, said Dr. Ferreri, when administering ketamine, his team closely monitors both mental and physical parameters – heart rate and blood pressure, in particular – because the drug can have hypertensive effects.
Dr. Schenberg noted that ibogaine, a naturally occurring psychedelic frequently used by traditional communities in Africa in rituals and for healing purposes, could cause potentially fatal arrhythmias, so it’s critical that the treatment is administered in a hospital setting that has a cardiac unit.
Dr. Pani said there is a need for more research, especially regarding the molecular mechanisms behind the behavioral effects of low-dose psychedelic therapy and the potential risks of multiple treatments with the drugs.
“Although extensive toxicology has been conducted on a single active dose of psilocybin, which has been proven to be safe, further research is required to understand better the possible health risks, especially in relation to cardiac and lung tissue,” he said.
Psychologically challenging
The experts note that given the relative lack of experience with psychedelic therapy, preparing patients for potential adverse effects is paramount. This is particularly relevant in the research setting and highlights the need for adequate patient screening and aftercare.
Dr. Gründer and Dr. Dolengevich-Segal emphasized the importance of having qualified personnel available in the event that patients experience adverse psychological events during treatment.
For Dr. Gründer, the potential for psilocybin to cause patients to lose control, experience psychotic symptoms, or become paranoid warrants considerable preparation by treating physicians.
Patients occasionally experience fear and anxiety during treatment, though it’s usually short-lived, said Dr. Griffiths. Nevertheless, these experiences may open the door to greater insight. “A number of people report that these psychologically challenging states are a valuable part of the overall experience,” he said.
The situation is similar in Spain, where Dr. Dolengevich-Segal noted that typical treatment regimens have a strong focus on the patient’s experience as a therapeutic tool. As in the United Kingdom and the United States, her team guides patients to what they call a “peak experience,” which allows them to gain a better understanding of the trauma underlying their mental health problems.
Dr. Nutt said that in the United Kingdom, they haven’t seen adverse reactions in patients receiving psychedelic therapy, although sedatives such as benzodiazepines could be used to manage them. He added that at his center, two therapists are present at every treatment session, and all personnel are “trained medics or psychologists.”
Patient education
Preparing and educating patients about the therapy are critical, said Dr. Gründer, especially given the intense response psychedelic treatment often invokes.
Echoing Dr. Gründer, Dr. Tófoli said explaining the nature of psychedelic treatment to potential patients helps ease anxiety.
Dr. Griffiths noted that in the United States, study participants are not only educated about the potential effects of psychedelic agents but also undergo several hours of psychological preparation in advance of their first treatment session and are provided with psychological support after treatment.
There is also a strong emphasis on patient preparation and education in the United Kingdom, where patients meet with therapists before and after treatment. During these posttreatment debriefings, clinicians use the patients’ experience with psychedelics to help them gain insight into the underlying cause of their depression.
Dr. Schenberg noted that at his institution in São Paulo, there are online courses to teach clinicians about psychedelic therapy for psychiatric disorders. Next year, he added, a new training program in MDMA-assisted psychotherapy will begin.
Working out treatment protocols
Treatment protocols for psychedelics vary by agent and indication from country to country. For instance, Dr. Pani noted that current psychedelic research in Italy predominantly focuses more on microdosing, which involves administering 1% of the pharmacologically active dose to a maximum of 100 mcg, in contrast to low dosing or full dosing.
Therapeutic regimens in Brazil, said Dr. Schenberg, also differ by agent but share common elements. For instance, psychedelics are always administered in a research setting, and sessions include concomitant psychotherapy.
In Germany, investigators are working to determine optimal treatment regimen for psilocybin for resistant depression in a randomized three-arm study planned for 2021.
For Mexico’s Dr. Velásquez, treatment regimens are complex and varied. Either way, he said, patients always require long-term follow-up.
With ketamine therapy, Dr. Ferreri said his team administers the drug in 45- to 60-minute intravenous infusion sessions in a hospital room without light or sound stimulation. Regardless of the drug’s immediate effect, he said, the protocol is repeated within a 6-month period.
The question of the duration of treatment effect is important. Dr. Griffiths said research suggests that the positive effects of psilocybin are long lasting and that most individuals report positive changes in mood, attitude, and behavior that endure for months or even years after the session.
“Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experiences of their lives,” said Dr. Griffiths.
Dr. Nutt agreed, noting that a single intense “trip” can improve mood for weeks, months, or even years. Nevertheless, he said, in his experience, approximately three-quarters of patients treated with psychedelics for major depression relapse within 3-9 months.
“Most get better,” he said, “but the majority of depression comes back over a period of months.”
Given the current illegal status of the drugs, he said it’s nearly impossible to provide patients with regular, subsequent treatment with psychedelics over time.
“My suspicion is that you might well have to dose four or five times over a couple of years to get people to escape from very severe depression,” said Dr. Nutt. “The longer they’ve been depressed, the harder it is for them to make a full recovery, because it’s more entrenched in the brain.”
All experts agree that exciting times are ahead for psychedelics as therapeutics for a wide range of psychiatric disorders.
“We can look forward to continued growth and expansion of this research,” said Dr. Griffiths, “including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.”
A version of this article first appeared on Medscape.com.
In 1967, when the United Nations Convention on Drugs classified psychedelics as schedule I substances, it effectively ended research into these agents as potential therapeutics for psychiatric disorders.
Psychedelics induce altered states of perception. They bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include psilocybin, which is derived from “magic mushrooms”; N,N-dimethyltryptamine (DMT), a component of ayahuasca and mescaline (peyote cactus); and the synthesized compound D-lysergic acid diethylamide (LSD). Other agents, such as ketamine and 3,4-methylenedioxymethamphetamine (MDMA), also known as ecstasy, are sometimes considered psychedelics as well.
Before they were classified as schedule I agents, psychedelics had been shown to be particularly beneficial for patients with treatment-resistant conditions, including depression and posttraumatic stress disorder (PTSD), especially when administered in a supportive, therapeutic setting.
Now, after a hiatus of almost 50 years, there is renewed global interest in the scientific investigation of psychedelics. The attention was spurred in part by several exploratory studies of DMT in humans conducted in the 1990s by Rick Strassman, MD, and colleagues at the University of New Mexico, Albuquerque.
Around the same time, Franz X. Vollenweider, MD, and colleagues at the University of Zürich began researching psilocybin and its effects on human behavior. However, it was a 2006 study of psilocybin by a team of researchers at Johns Hopkins University, Baltimore, that is widely cited as a catalyst for the current renaissance in psychedelic research.
To provide a broad-based, international perspective on these agents, including their current legal status and indications, treatment regimens, safety, efficacy, and future considerations, this news organization interviewed nine expert researchers from around the globe.
Global legal status
In most, if not all, countries, it is still illegal to prescribe psychedelics in other than a research setting.
They can be used in research, but only with approval from the Food and Drug Administration under licensure from the Drug Enforcement Administration.
France lists all synthetic hallucinogens and hallucinogenic mushrooms as narcotic. As a result, possession, use, transportation, and collection are subject to criminal sanctions.
In France, NMDA antagonists such as ketamine and nitrous oxide are regarded as psychedelic molecules and can be used off label for various conditions or as part of research protocols authorized by the French public health code.
Although psychedelics are illegal under Mexican law, they are commonly used in indigenous communities as part of traditional rituals.
“The line between traditional consumption and psychedelic tourism is very thin,” José J. Mendoza Velásquez, MD, professor in the department of mental health, National Autonomous University of Mexico, Mexico City, said in an interview.
Psychedelics also are illegal in the United Kingdom, although government agencies have recently allowed research groups to investigate them. Psychedelics cannot be prescribed in Germany, Spain, or Italy. However, investigators in these countries can request permission from regulatory agencies to conduct research.
Brazil allows psychedelic substances to be researched, particularly ayahuasca, which has long traditional and religious roots in the country.
However, as in other countries, none of the classic psychedelics is regulated for therapeutic use in Brazil. It is widely expected that the Brazilian government will approve MDMA sometime in 2024 for use in the treatment of PTSD.
Potential indications
Psychedelics are currently under investigation as potential treatments for major depression, treatment-resistant depression, PTSD, pain management, and anorexia, among other conditions.
In France, Florian Ferreri, MD, PhD, at Hospital Saint-Antoine, Paris, is researching ketamine for treatment of patients with suicidal crisis/ideation and treatment-resistant depression.
In the United Kingdom, David Nutt, FMedSci, Edmond J. Safra Professor of Neuropsychopharmacology at Imperial College London, and his team have conducted studies of the use of psychedelics in conjunction with psychological support for patients with treatment-resistant depression, and they are currently exploring their use in the treatment of anorexia and various pain syndromes.
In Germany, Gerhard Gründer, MD, professor of psychiatry at the Central Institute of Mental Health, in Mannheim, noted that a study of psilocybin for treatment-resistant depression will launch sometime in 2021. In Italy, current research is focusing on MDMA and ketamine in the laboratory environment and in animal models for treating depression and drug abuse.
Researcher Helen Dolengevich-Segal, MD, a psychiatrist at Hospital Universitario del Henares, Madrid, noted that although research on esketamine for the treatment of severe depressive disorder with suicidal thoughts is underway, there is very limited published research from that country into the use of classic psychedelics for various psychiatric disorders, given their current illegal status.
Mexico’s Dr. Velásquez noted that although he is prohibited from prescribing psychedelics, he does have patients who take the drugs to augment medical treatment. For instance, he said, his patients frequently use psilocybin to help with severe depression, pain, and insomnia.
Environment is key
Most researchers agree that for psychedelics to be safe and effective, patient education and administration in a controlled environment by experienced clinicians are key to successful treatment.
Roland R. Griffiths, PhD, director of the Center for Psychedelic and Consciousness Research at Johns Hopkins, said that ongoing U.S. psilocybin research – primarily in major depressive disorder and psychological distress associated with life-threatening illness, drug addiction, anorexia nervosa, obsessive-compulsive disorder, and headache – generally includes one or two treatment sessions, each of which lasts 6-8 hours.
Such sessions typically involve oral administration of a moderately high dose of a psychedelic under what he characterizes as “psychologically supported conditions.”
For Dr. Griffiths, there are serious potential risks associated with the use of psilocybin and other psychedelics outside such environments.
“When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others, including life-threatening risk,” he said.
Dr. Gründer agreed.
“At the moment, I cannot imagine that you would go to the pharmacy with a prescription for psilocybin and get yourself a pill and then take it in a quiet little room,” he said. Dr. Dolengevich-Segal and Dr. Velásquez echoed these sentiments, noting the optimal location for administration is one that is quiet and secure and where patients feel safe.
Luís Fernando Tófoli, MD, PhD, professor of medical psychology and psychiatry at the University of Campinas, and Eduardo Schenberg, PhD, founder and CEO of Instituto Phaneros in São Paulo, Brazil, said more research is needed to determine the optimal therapeutic environment for individual agents.
“Most studies have a low number of participants (around 20 or 30), especially in neuroimaging, with high unblinding rates,” Dr. Schenberg said. “Therefore, novel methodological approaches are also necessary, as these substances do not easily fit into the traditional pharmacology epistemic model.”
Risks, abuse potential
The abuse potential of psychedelics is an ongoing concern for the public, researchers, and regulators, but the consensus among nearly all of these experts is that when administered by medical professionals in controlled settings, these drugs are associated with extremely low risk.
It is recreational use that presents an abuse concern, said Dr. Ferreri, but with the low doses used in psychiatry, the risk is “very limited or even nonexistent.”
Dr. Nutt said the abuse potential of psychedelics is so low that they can be used to treat addiction.
“Functionally, psychedelics are antiaddictive,” Dr. Nutt said. “The fact is, if you take them repeatedly, you develop tolerance, and the effect disappears. You can’t overcome it. But everyone believes they’re addictive because they’re scheduled drugs.”
Dr. Velásquez is something of an outlier. He believes the abuse potential with psychedelics is poorly understood and that some patients may develop tolerance, which is a potential gateway to dependence.
“Such is the case with LSD,” he said, “where this substance also favors tolerance to other psychedelic drugs such as psilocybin.”
Dosing also seems to play a key role in mitigating potential abuse, said Luca Pani, MD, professor of pharmacology and psychiatry at the University of Modena, Italy. Dr. Pani explained that with low doses and microdoses of psychedelics, the potential for abuse is eliminated.
Dr. Nutt, Dr. Pani, and Dr. Ferreri also noted the importance of medical supervision. For instance, said Dr. Ferreri, when administering ketamine, his team closely monitors both mental and physical parameters – heart rate and blood pressure, in particular – because the drug can have hypertensive effects.
Dr. Schenberg noted that ibogaine, a naturally occurring psychedelic frequently used by traditional communities in Africa in rituals and for healing purposes, could cause potentially fatal arrhythmias, so it’s critical that the treatment is administered in a hospital setting that has a cardiac unit.
Dr. Pani said there is a need for more research, especially regarding the molecular mechanisms behind the behavioral effects of low-dose psychedelic therapy and the potential risks of multiple treatments with the drugs.
“Although extensive toxicology has been conducted on a single active dose of psilocybin, which has been proven to be safe, further research is required to understand better the possible health risks, especially in relation to cardiac and lung tissue,” he said.
Psychologically challenging
The experts note that given the relative lack of experience with psychedelic therapy, preparing patients for potential adverse effects is paramount. This is particularly relevant in the research setting and highlights the need for adequate patient screening and aftercare.
Dr. Gründer and Dr. Dolengevich-Segal emphasized the importance of having qualified personnel available in the event that patients experience adverse psychological events during treatment.
For Dr. Gründer, the potential for psilocybin to cause patients to lose control, experience psychotic symptoms, or become paranoid warrants considerable preparation by treating physicians.
Patients occasionally experience fear and anxiety during treatment, though it’s usually short-lived, said Dr. Griffiths. Nevertheless, these experiences may open the door to greater insight. “A number of people report that these psychologically challenging states are a valuable part of the overall experience,” he said.
The situation is similar in Spain, where Dr. Dolengevich-Segal noted that typical treatment regimens have a strong focus on the patient’s experience as a therapeutic tool. As in the United Kingdom and the United States, her team guides patients to what they call a “peak experience,” which allows them to gain a better understanding of the trauma underlying their mental health problems.
Dr. Nutt said that in the United Kingdom, they haven’t seen adverse reactions in patients receiving psychedelic therapy, although sedatives such as benzodiazepines could be used to manage them. He added that at his center, two therapists are present at every treatment session, and all personnel are “trained medics or psychologists.”
Patient education
Preparing and educating patients about the therapy are critical, said Dr. Gründer, especially given the intense response psychedelic treatment often invokes.
Echoing Dr. Gründer, Dr. Tófoli said explaining the nature of psychedelic treatment to potential patients helps ease anxiety.
Dr. Griffiths noted that in the United States, study participants are not only educated about the potential effects of psychedelic agents but also undergo several hours of psychological preparation in advance of their first treatment session and are provided with psychological support after treatment.
There is also a strong emphasis on patient preparation and education in the United Kingdom, where patients meet with therapists before and after treatment. During these posttreatment debriefings, clinicians use the patients’ experience with psychedelics to help them gain insight into the underlying cause of their depression.
Dr. Schenberg noted that at his institution in São Paulo, there are online courses to teach clinicians about psychedelic therapy for psychiatric disorders. Next year, he added, a new training program in MDMA-assisted psychotherapy will begin.
Working out treatment protocols
Treatment protocols for psychedelics vary by agent and indication from country to country. For instance, Dr. Pani noted that current psychedelic research in Italy predominantly focuses more on microdosing, which involves administering 1% of the pharmacologically active dose to a maximum of 100 mcg, in contrast to low dosing or full dosing.
Therapeutic regimens in Brazil, said Dr. Schenberg, also differ by agent but share common elements. For instance, psychedelics are always administered in a research setting, and sessions include concomitant psychotherapy.
In Germany, investigators are working to determine optimal treatment regimen for psilocybin for resistant depression in a randomized three-arm study planned for 2021.
For Mexico’s Dr. Velásquez, treatment regimens are complex and varied. Either way, he said, patients always require long-term follow-up.
With ketamine therapy, Dr. Ferreri said his team administers the drug in 45- to 60-minute intravenous infusion sessions in a hospital room without light or sound stimulation. Regardless of the drug’s immediate effect, he said, the protocol is repeated within a 6-month period.
The question of the duration of treatment effect is important. Dr. Griffiths said research suggests that the positive effects of psilocybin are long lasting and that most individuals report positive changes in mood, attitude, and behavior that endure for months or even years after the session.
“Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experiences of their lives,” said Dr. Griffiths.
Dr. Nutt agreed, noting that a single intense “trip” can improve mood for weeks, months, or even years. Nevertheless, he said, in his experience, approximately three-quarters of patients treated with psychedelics for major depression relapse within 3-9 months.
“Most get better,” he said, “but the majority of depression comes back over a period of months.”
Given the current illegal status of the drugs, he said it’s nearly impossible to provide patients with regular, subsequent treatment with psychedelics over time.
“My suspicion is that you might well have to dose four or five times over a couple of years to get people to escape from very severe depression,” said Dr. Nutt. “The longer they’ve been depressed, the harder it is for them to make a full recovery, because it’s more entrenched in the brain.”
All experts agree that exciting times are ahead for psychedelics as therapeutics for a wide range of psychiatric disorders.
“We can look forward to continued growth and expansion of this research,” said Dr. Griffiths, “including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.”
A version of this article first appeared on Medscape.com.
Black nonsmokers still at high risk for secondhand smoke exposure
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.
While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
THC persists in breast milk 6 weeks after quitting cannabis
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Buprenorphine for OUD may also mitigate risk with concomitant benzo, Z-drug use
Buprenorphine for the treatment of opioid-use disorder (OUD) may also mitigate the risk associated with concomitant benzodiazepine and Z-drug use, which is frequent in this patient population, new research suggests.
A case-crossover study of more than 20,000 participants with OUD showed that drug treatment days in which benzodiazepines and Z-drugs were taken were associated with an 88% increase in nonfatal overdose; buprenorphine appeared to reduce this risk by almost 40%.
“One of our two primary findings is that patients with opioid use disorder can still benefit substantially from buprenorphine treatment, even if they have benzodiazepines on board,” lead author Kevin Xu, MD, a resident at the Washington University, St. Louis, told this news organization.
The other key finding was that “not all benzodiazepines are equal” and that some are associated with higher risk than others, Dr. Xu added.
“If anything, patients who are on buprenorphine and benzodiazepines do not necessarily need to be abruptly tapered off their benzodiazepines. Our data actually demonstrate that there are safe avenues for them,” he added.
The findings were published online March 3 in the American Journal of Psychiatry.
Cloudy relationship
Buprenorphine is commonly used to treat patients with OUD because of its ability to decrease all-cause mortality. However,
In addition, recent research shows that benzodiazepine/Z-drug use is associated with a variety of potential adverse effects, including respiratory depression, overdose, and addiction risk.
The relationship between benzodiazepine use and buprenorphine treatment outcomes is poorly characterized in individuals with OUD. Although some studies suggest benzodiazepines may enhance retention in buprenorphine maintenance treatment, others suggest a link to increased adverse events, including all-cause mortality, drug-related poisonings, and accidental injury–related emergency department visits.
In addition, there has been little research on the potential adverse effects associated with use of selective benzodiazepine receptor modulators in patients with OUD. These so-called Z-drugs include zolpidem, zaleplon, and eszopiclone.
Nevertheless, previous research in the general population shows that these medications have a range of adverse effects similar to those of benzodiazepines, with comparable dose-response effects on all-cause mortality.
“The challenge for any clinician is that many patients who are addicted to opioids are also polysubstance users,” said Dr. Xu. “There are so many hopeful articles regarding the benefits of buprenorphine treatment in opioid use disorder patients, but it seems like the individuals with polysubstance use are largely ignored in the setting of the opioid epidemic.”
“And this is really the back story that got me inspired to study this particular topic,” he added.
Improve, nullify, or reverse?
Given these questions, the researchers set out to quantify the odds of nonfatal drug-related poisoning, including overdoses, associated with benzodiazepine or Z-drug use by patients with OUD who were also taking buprenorphine.
“While the drug-related poisoning variable encompasses opioid overdoses, we used a broad definition per CDC guidelines to also include other types of drug overdoses – including poisoning events involving stimulants, overdoses involving sedatives, and overdoses involving psychotropic prescription drugs” that are commonly used by patients with OUD, said Dr. Xu.
They also wanted to determine whether the use of benzodiazepines or Z-drugs would improve, nullify, or reverse the protective effect of buprenorphine. The researchers also evaluated whether different sedative and hypnotic subtypes of these drugs were associated with different poisoning risks.
The researchers analyzed pharmaceutical claims data from 304,676 individuals (aged 12-64 years) in the IBM MarketScan Commercial and Multi-State Medicaid Databases. All had received buprenorphine treatment for OUD between Jan. 1, 2006, and Dec. 31, 2016.
Buprenorphine use was converted to a daily milligram dose and was classified as either greater than 12 mg or less than or equal to 12 mg, because previous research suggests there may be differences in treatment retention associated with this dose. Given the case-control nature of the investigation, patients who did not experience a drug-related poisoning were excluded from the analysis.
The study’s primary unit of observation was person-days, which were those days during which patients were enrolled in a health insurance plan. Participants were evaluated for 1 year before their first drug-related poisoning and 1 year after their first such poisoning. The primary outcome was nonfatal drug-related poisonings, including overdoses. The primary exposure was determined on the basis of benzodiazepine or Z-drug prescriptions.
The daily dose of benzodiazepines or Z-drugs was standardized as a function of diazepam-equivalent milligrams. Doses were classified as either high dose (diazepam-equivalent mg dose >30 mg) or low dose (≤30 mg). The drugs were also distinguished on the basis of their pharmacologic properties, such as whether they were short-acting or long-acting.
37% risk reduction
Of the original cohort of 304,676 patients with OUD, the study’s final analytic sample included 23,036 patients (mean age, 30 years; 51% men), representing 14,213,075 person-days of insurance coverage. Of these, 2,210,927 person-days (15.6%) entailed claims for buprenorphine (mean daily dose, 15.4 mg; SD, 7.31 mg).
A total of 474,181 person-days included claims for benzodiazepines or Z-drugs with concurrent buprenorphine treatment. The mean daily dose of any benzodiazepine or Z-drug was 23.4 diazepam-milligram equivalents. The mean daily dose of short-acting benzodiazepines, long-acting benzodiazepines, and Z-drugs was 25.3, 31.3, and 4.9 diazepam-milligram equivalents, respectively.
Buprenorphine treatment days were associated with a 37% lower chance of drug-related poisoning (95% confidence interval, 0.60-0.66) in comparison with nontreatment days. On the other hand, the odds of poisoning increased by 81% on days on which patients were treated with Z-drugs or benzodiazepines (95% CI, 1.73-1.91).
Interestingly, individual analyses showed that benzodiazepine and Z-drug treatment days were associated with increased odds of poisoning events (odds ratio, 1.29; 95% CI, 1.19-1.39). Odds of poisoning events on benzodiazepine-only treatment days, on the other hand, were markedly lower (OR, 1.88; 95% CI, 1.78-1.98).
Subgroup analyses revealed that both short-acting and long-acting benzodiazepine treatment days were associated with comparably elevated odds of drug-related poisoning (OR, 1.86 and 1.68, respectively). High-dose benzodiazepine treatment days were associated with higher increased odds of a poisoning event (122%) in comparison with low-dose treatment days (78%).
High-dose, but not low-dose, benzodiazepine or Z-drug treatment was linked to increased poisonings when the drug was taken concurrently with buprenorphine (OR, 1.64; 95% CI, 1.39-1.93). However, the risk was still lower than the risk associated with taking the agents without concurrent treatment with buprenorphine (low-dose OR, 1.69; high-dose OR, 2.23).
‘Not all benzodiazepines are bad’
Dr. Xu noted that the findings have potentially important implications for clinical practice, beginning with the dose-dependent relationship between benzodiazepine/Z-drug use and drug-related poisonings among individuals with OUD. This indicates that lowering doses or shortening treatment duration may reduce risk, he said.
Similarly, the lower risk associated with long-acting benzodiazepines relative to short-acting beonzodiazepines – as well as the substantially lower risk associated with Z-drugs, compared with either short- or long-acting benzodiazepines – suggests that switching from benzodiazepines to long-acting agents or Z-drugs may lower the risk for overdose, he added.
“Clinicians are often challenged by patients with opioid use disorder who are also on benzodiazepines or Z-drugs. There’s an inclination to say no to them, because they’re too high risk to start buprenorphine maintenance, or abruptly taper the benzodiazepines, which can be very destabilizing,” he noted.
“Our data show that people on benzodiazepines can absolutely receive buprenorphine and still get some benefit,” Dr. Xu said. “In addition, not all benzodiazepines are bad for these individuals. There are safer formulations and safer doses, too.”
However, he added, he would not initiate benzodiazepine treatment if he didn’t have to, especially long-term treatment.
“One of the messages from our data is that this clearly contributes to higher overdose risk. But we often inherit patients who already have benzodiazepines on board, so we need to figure out what to do. That is the question that nobody had really clearly addressed prior to this study,” Dr. Xu concluded.
Vigilance needed
Commenting on the findings for this news organization, Jerrold F. Rosenbaum, MD, Stanley Cobb Professor of Psychiatry, Harvard Medical School, Boston, urged caution when combining benzodiazepines with opioids.
“There are situations where you need to be circumspect about the use of benzodiazepines, and that’s clearly when people are being prescribed them in combination with other drugs that could be either sedating or respiratory depressant,” said Dr. Rosenbaum, who was not involved with the research.
“This paper reminds us that physicians need to be particularly vigilant about situations where patients might be combining the two agents,” he added.
Dr. Rosenbaum noted that patients who are using more medication than prescribed are at risk “for not appreciating the synergy” between the two treatments in terms of adverse events such as respiratory depression.
In addition, “if they’re intending to do themselves harm, the lethality of an overdose will be certainly far more than the benzodiazepines or opiates alone,” he said.
Another potential challenge for clinicians are situations in which patients are taking benzodiazepines for preexisting conditions that also require opiates. “Then you have to use special vigilance and try to use lowest doses to reduce the total burden of medication to minimize the potential risk,” said Dr. Rosenbaum.
The study was funded by the National Institutes of Health. Dr. Xu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Buprenorphine for the treatment of opioid-use disorder (OUD) may also mitigate the risk associated with concomitant benzodiazepine and Z-drug use, which is frequent in this patient population, new research suggests.
A case-crossover study of more than 20,000 participants with OUD showed that drug treatment days in which benzodiazepines and Z-drugs were taken were associated with an 88% increase in nonfatal overdose; buprenorphine appeared to reduce this risk by almost 40%.
“One of our two primary findings is that patients with opioid use disorder can still benefit substantially from buprenorphine treatment, even if they have benzodiazepines on board,” lead author Kevin Xu, MD, a resident at the Washington University, St. Louis, told this news organization.
The other key finding was that “not all benzodiazepines are equal” and that some are associated with higher risk than others, Dr. Xu added.
“If anything, patients who are on buprenorphine and benzodiazepines do not necessarily need to be abruptly tapered off their benzodiazepines. Our data actually demonstrate that there are safe avenues for them,” he added.
The findings were published online March 3 in the American Journal of Psychiatry.
Cloudy relationship
Buprenorphine is commonly used to treat patients with OUD because of its ability to decrease all-cause mortality. However,
In addition, recent research shows that benzodiazepine/Z-drug use is associated with a variety of potential adverse effects, including respiratory depression, overdose, and addiction risk.
The relationship between benzodiazepine use and buprenorphine treatment outcomes is poorly characterized in individuals with OUD. Although some studies suggest benzodiazepines may enhance retention in buprenorphine maintenance treatment, others suggest a link to increased adverse events, including all-cause mortality, drug-related poisonings, and accidental injury–related emergency department visits.
In addition, there has been little research on the potential adverse effects associated with use of selective benzodiazepine receptor modulators in patients with OUD. These so-called Z-drugs include zolpidem, zaleplon, and eszopiclone.
Nevertheless, previous research in the general population shows that these medications have a range of adverse effects similar to those of benzodiazepines, with comparable dose-response effects on all-cause mortality.
“The challenge for any clinician is that many patients who are addicted to opioids are also polysubstance users,” said Dr. Xu. “There are so many hopeful articles regarding the benefits of buprenorphine treatment in opioid use disorder patients, but it seems like the individuals with polysubstance use are largely ignored in the setting of the opioid epidemic.”
“And this is really the back story that got me inspired to study this particular topic,” he added.
Improve, nullify, or reverse?
Given these questions, the researchers set out to quantify the odds of nonfatal drug-related poisoning, including overdoses, associated with benzodiazepine or Z-drug use by patients with OUD who were also taking buprenorphine.
“While the drug-related poisoning variable encompasses opioid overdoses, we used a broad definition per CDC guidelines to also include other types of drug overdoses – including poisoning events involving stimulants, overdoses involving sedatives, and overdoses involving psychotropic prescription drugs” that are commonly used by patients with OUD, said Dr. Xu.
They also wanted to determine whether the use of benzodiazepines or Z-drugs would improve, nullify, or reverse the protective effect of buprenorphine. The researchers also evaluated whether different sedative and hypnotic subtypes of these drugs were associated with different poisoning risks.
The researchers analyzed pharmaceutical claims data from 304,676 individuals (aged 12-64 years) in the IBM MarketScan Commercial and Multi-State Medicaid Databases. All had received buprenorphine treatment for OUD between Jan. 1, 2006, and Dec. 31, 2016.
Buprenorphine use was converted to a daily milligram dose and was classified as either greater than 12 mg or less than or equal to 12 mg, because previous research suggests there may be differences in treatment retention associated with this dose. Given the case-control nature of the investigation, patients who did not experience a drug-related poisoning were excluded from the analysis.
The study’s primary unit of observation was person-days, which were those days during which patients were enrolled in a health insurance plan. Participants were evaluated for 1 year before their first drug-related poisoning and 1 year after their first such poisoning. The primary outcome was nonfatal drug-related poisonings, including overdoses. The primary exposure was determined on the basis of benzodiazepine or Z-drug prescriptions.
The daily dose of benzodiazepines or Z-drugs was standardized as a function of diazepam-equivalent milligrams. Doses were classified as either high dose (diazepam-equivalent mg dose >30 mg) or low dose (≤30 mg). The drugs were also distinguished on the basis of their pharmacologic properties, such as whether they were short-acting or long-acting.
37% risk reduction
Of the original cohort of 304,676 patients with OUD, the study’s final analytic sample included 23,036 patients (mean age, 30 years; 51% men), representing 14,213,075 person-days of insurance coverage. Of these, 2,210,927 person-days (15.6%) entailed claims for buprenorphine (mean daily dose, 15.4 mg; SD, 7.31 mg).
A total of 474,181 person-days included claims for benzodiazepines or Z-drugs with concurrent buprenorphine treatment. The mean daily dose of any benzodiazepine or Z-drug was 23.4 diazepam-milligram equivalents. The mean daily dose of short-acting benzodiazepines, long-acting benzodiazepines, and Z-drugs was 25.3, 31.3, and 4.9 diazepam-milligram equivalents, respectively.
Buprenorphine treatment days were associated with a 37% lower chance of drug-related poisoning (95% confidence interval, 0.60-0.66) in comparison with nontreatment days. On the other hand, the odds of poisoning increased by 81% on days on which patients were treated with Z-drugs or benzodiazepines (95% CI, 1.73-1.91).
Interestingly, individual analyses showed that benzodiazepine and Z-drug treatment days were associated with increased odds of poisoning events (odds ratio, 1.29; 95% CI, 1.19-1.39). Odds of poisoning events on benzodiazepine-only treatment days, on the other hand, were markedly lower (OR, 1.88; 95% CI, 1.78-1.98).
Subgroup analyses revealed that both short-acting and long-acting benzodiazepine treatment days were associated with comparably elevated odds of drug-related poisoning (OR, 1.86 and 1.68, respectively). High-dose benzodiazepine treatment days were associated with higher increased odds of a poisoning event (122%) in comparison with low-dose treatment days (78%).
High-dose, but not low-dose, benzodiazepine or Z-drug treatment was linked to increased poisonings when the drug was taken concurrently with buprenorphine (OR, 1.64; 95% CI, 1.39-1.93). However, the risk was still lower than the risk associated with taking the agents without concurrent treatment with buprenorphine (low-dose OR, 1.69; high-dose OR, 2.23).
‘Not all benzodiazepines are bad’
Dr. Xu noted that the findings have potentially important implications for clinical practice, beginning with the dose-dependent relationship between benzodiazepine/Z-drug use and drug-related poisonings among individuals with OUD. This indicates that lowering doses or shortening treatment duration may reduce risk, he said.
Similarly, the lower risk associated with long-acting benzodiazepines relative to short-acting beonzodiazepines – as well as the substantially lower risk associated with Z-drugs, compared with either short- or long-acting benzodiazepines – suggests that switching from benzodiazepines to long-acting agents or Z-drugs may lower the risk for overdose, he added.
“Clinicians are often challenged by patients with opioid use disorder who are also on benzodiazepines or Z-drugs. There’s an inclination to say no to them, because they’re too high risk to start buprenorphine maintenance, or abruptly taper the benzodiazepines, which can be very destabilizing,” he noted.
“Our data show that people on benzodiazepines can absolutely receive buprenorphine and still get some benefit,” Dr. Xu said. “In addition, not all benzodiazepines are bad for these individuals. There are safer formulations and safer doses, too.”
However, he added, he would not initiate benzodiazepine treatment if he didn’t have to, especially long-term treatment.
“One of the messages from our data is that this clearly contributes to higher overdose risk. But we often inherit patients who already have benzodiazepines on board, so we need to figure out what to do. That is the question that nobody had really clearly addressed prior to this study,” Dr. Xu concluded.
Vigilance needed
Commenting on the findings for this news organization, Jerrold F. Rosenbaum, MD, Stanley Cobb Professor of Psychiatry, Harvard Medical School, Boston, urged caution when combining benzodiazepines with opioids.
“There are situations where you need to be circumspect about the use of benzodiazepines, and that’s clearly when people are being prescribed them in combination with other drugs that could be either sedating or respiratory depressant,” said Dr. Rosenbaum, who was not involved with the research.
“This paper reminds us that physicians need to be particularly vigilant about situations where patients might be combining the two agents,” he added.
Dr. Rosenbaum noted that patients who are using more medication than prescribed are at risk “for not appreciating the synergy” between the two treatments in terms of adverse events such as respiratory depression.
In addition, “if they’re intending to do themselves harm, the lethality of an overdose will be certainly far more than the benzodiazepines or opiates alone,” he said.
Another potential challenge for clinicians are situations in which patients are taking benzodiazepines for preexisting conditions that also require opiates. “Then you have to use special vigilance and try to use lowest doses to reduce the total burden of medication to minimize the potential risk,” said Dr. Rosenbaum.
The study was funded by the National Institutes of Health. Dr. Xu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Buprenorphine for the treatment of opioid-use disorder (OUD) may also mitigate the risk associated with concomitant benzodiazepine and Z-drug use, which is frequent in this patient population, new research suggests.
A case-crossover study of more than 20,000 participants with OUD showed that drug treatment days in which benzodiazepines and Z-drugs were taken were associated with an 88% increase in nonfatal overdose; buprenorphine appeared to reduce this risk by almost 40%.
“One of our two primary findings is that patients with opioid use disorder can still benefit substantially from buprenorphine treatment, even if they have benzodiazepines on board,” lead author Kevin Xu, MD, a resident at the Washington University, St. Louis, told this news organization.
The other key finding was that “not all benzodiazepines are equal” and that some are associated with higher risk than others, Dr. Xu added.
“If anything, patients who are on buprenorphine and benzodiazepines do not necessarily need to be abruptly tapered off their benzodiazepines. Our data actually demonstrate that there are safe avenues for them,” he added.
The findings were published online March 3 in the American Journal of Psychiatry.
Cloudy relationship
Buprenorphine is commonly used to treat patients with OUD because of its ability to decrease all-cause mortality. However,
In addition, recent research shows that benzodiazepine/Z-drug use is associated with a variety of potential adverse effects, including respiratory depression, overdose, and addiction risk.
The relationship between benzodiazepine use and buprenorphine treatment outcomes is poorly characterized in individuals with OUD. Although some studies suggest benzodiazepines may enhance retention in buprenorphine maintenance treatment, others suggest a link to increased adverse events, including all-cause mortality, drug-related poisonings, and accidental injury–related emergency department visits.
In addition, there has been little research on the potential adverse effects associated with use of selective benzodiazepine receptor modulators in patients with OUD. These so-called Z-drugs include zolpidem, zaleplon, and eszopiclone.
Nevertheless, previous research in the general population shows that these medications have a range of adverse effects similar to those of benzodiazepines, with comparable dose-response effects on all-cause mortality.
“The challenge for any clinician is that many patients who are addicted to opioids are also polysubstance users,” said Dr. Xu. “There are so many hopeful articles regarding the benefits of buprenorphine treatment in opioid use disorder patients, but it seems like the individuals with polysubstance use are largely ignored in the setting of the opioid epidemic.”
“And this is really the back story that got me inspired to study this particular topic,” he added.
Improve, nullify, or reverse?
Given these questions, the researchers set out to quantify the odds of nonfatal drug-related poisoning, including overdoses, associated with benzodiazepine or Z-drug use by patients with OUD who were also taking buprenorphine.
“While the drug-related poisoning variable encompasses opioid overdoses, we used a broad definition per CDC guidelines to also include other types of drug overdoses – including poisoning events involving stimulants, overdoses involving sedatives, and overdoses involving psychotropic prescription drugs” that are commonly used by patients with OUD, said Dr. Xu.
They also wanted to determine whether the use of benzodiazepines or Z-drugs would improve, nullify, or reverse the protective effect of buprenorphine. The researchers also evaluated whether different sedative and hypnotic subtypes of these drugs were associated with different poisoning risks.
The researchers analyzed pharmaceutical claims data from 304,676 individuals (aged 12-64 years) in the IBM MarketScan Commercial and Multi-State Medicaid Databases. All had received buprenorphine treatment for OUD between Jan. 1, 2006, and Dec. 31, 2016.
Buprenorphine use was converted to a daily milligram dose and was classified as either greater than 12 mg or less than or equal to 12 mg, because previous research suggests there may be differences in treatment retention associated with this dose. Given the case-control nature of the investigation, patients who did not experience a drug-related poisoning were excluded from the analysis.
The study’s primary unit of observation was person-days, which were those days during which patients were enrolled in a health insurance plan. Participants were evaluated for 1 year before their first drug-related poisoning and 1 year after their first such poisoning. The primary outcome was nonfatal drug-related poisonings, including overdoses. The primary exposure was determined on the basis of benzodiazepine or Z-drug prescriptions.
The daily dose of benzodiazepines or Z-drugs was standardized as a function of diazepam-equivalent milligrams. Doses were classified as either high dose (diazepam-equivalent mg dose >30 mg) or low dose (≤30 mg). The drugs were also distinguished on the basis of their pharmacologic properties, such as whether they were short-acting or long-acting.
37% risk reduction
Of the original cohort of 304,676 patients with OUD, the study’s final analytic sample included 23,036 patients (mean age, 30 years; 51% men), representing 14,213,075 person-days of insurance coverage. Of these, 2,210,927 person-days (15.6%) entailed claims for buprenorphine (mean daily dose, 15.4 mg; SD, 7.31 mg).
A total of 474,181 person-days included claims for benzodiazepines or Z-drugs with concurrent buprenorphine treatment. The mean daily dose of any benzodiazepine or Z-drug was 23.4 diazepam-milligram equivalents. The mean daily dose of short-acting benzodiazepines, long-acting benzodiazepines, and Z-drugs was 25.3, 31.3, and 4.9 diazepam-milligram equivalents, respectively.
Buprenorphine treatment days were associated with a 37% lower chance of drug-related poisoning (95% confidence interval, 0.60-0.66) in comparison with nontreatment days. On the other hand, the odds of poisoning increased by 81% on days on which patients were treated with Z-drugs or benzodiazepines (95% CI, 1.73-1.91).
Interestingly, individual analyses showed that benzodiazepine and Z-drug treatment days were associated with increased odds of poisoning events (odds ratio, 1.29; 95% CI, 1.19-1.39). Odds of poisoning events on benzodiazepine-only treatment days, on the other hand, were markedly lower (OR, 1.88; 95% CI, 1.78-1.98).
Subgroup analyses revealed that both short-acting and long-acting benzodiazepine treatment days were associated with comparably elevated odds of drug-related poisoning (OR, 1.86 and 1.68, respectively). High-dose benzodiazepine treatment days were associated with higher increased odds of a poisoning event (122%) in comparison with low-dose treatment days (78%).
High-dose, but not low-dose, benzodiazepine or Z-drug treatment was linked to increased poisonings when the drug was taken concurrently with buprenorphine (OR, 1.64; 95% CI, 1.39-1.93). However, the risk was still lower than the risk associated with taking the agents without concurrent treatment with buprenorphine (low-dose OR, 1.69; high-dose OR, 2.23).
‘Not all benzodiazepines are bad’
Dr. Xu noted that the findings have potentially important implications for clinical practice, beginning with the dose-dependent relationship between benzodiazepine/Z-drug use and drug-related poisonings among individuals with OUD. This indicates that lowering doses or shortening treatment duration may reduce risk, he said.
Similarly, the lower risk associated with long-acting benzodiazepines relative to short-acting beonzodiazepines – as well as the substantially lower risk associated with Z-drugs, compared with either short- or long-acting benzodiazepines – suggests that switching from benzodiazepines to long-acting agents or Z-drugs may lower the risk for overdose, he added.
“Clinicians are often challenged by patients with opioid use disorder who are also on benzodiazepines or Z-drugs. There’s an inclination to say no to them, because they’re too high risk to start buprenorphine maintenance, or abruptly taper the benzodiazepines, which can be very destabilizing,” he noted.
“Our data show that people on benzodiazepines can absolutely receive buprenorphine and still get some benefit,” Dr. Xu said. “In addition, not all benzodiazepines are bad for these individuals. There are safer formulations and safer doses, too.”
However, he added, he would not initiate benzodiazepine treatment if he didn’t have to, especially long-term treatment.
“One of the messages from our data is that this clearly contributes to higher overdose risk. But we often inherit patients who already have benzodiazepines on board, so we need to figure out what to do. That is the question that nobody had really clearly addressed prior to this study,” Dr. Xu concluded.
Vigilance needed
Commenting on the findings for this news organization, Jerrold F. Rosenbaum, MD, Stanley Cobb Professor of Psychiatry, Harvard Medical School, Boston, urged caution when combining benzodiazepines with opioids.
“There are situations where you need to be circumspect about the use of benzodiazepines, and that’s clearly when people are being prescribed them in combination with other drugs that could be either sedating or respiratory depressant,” said Dr. Rosenbaum, who was not involved with the research.
“This paper reminds us that physicians need to be particularly vigilant about situations where patients might be combining the two agents,” he added.
Dr. Rosenbaum noted that patients who are using more medication than prescribed are at risk “for not appreciating the synergy” between the two treatments in terms of adverse events such as respiratory depression.
In addition, “if they’re intending to do themselves harm, the lethality of an overdose will be certainly far more than the benzodiazepines or opiates alone,” he said.
Another potential challenge for clinicians are situations in which patients are taking benzodiazepines for preexisting conditions that also require opiates. “Then you have to use special vigilance and try to use lowest doses to reduce the total burden of medication to minimize the potential risk,” said Dr. Rosenbaum.
The study was funded by the National Institutes of Health. Dr. Xu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fauci worries about possible post–COVID-19 ‘mental health pandemic’
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Smartphone ‘addiction’ tied to poor sleep in young adults
Smartphone “addiction” may explain poor sleep quality in a significant proportion of young adults, new research suggests.
Investigators found that almost 40% of adults aged 18-30 years who self-reported excessive smartphone use also reported poor sleep.
“Our study provides further support to the growing body of evidence that smartphone addiction has a deleterious impact on sleep,” wrote the researchers.
The study was published online March 2 in Frontiers of Psychiatry.
Not a clinical diagnosis
Smartphone addiction is not formally recognized as a clinical diagnosis, but it’s an “active” area of research, lead investigator Ben Carter, PhD, King’s College London, noted in the report.
In a cross-sectional survey, 1,043 college students (aged 18-30 years, 73% women) completed the 10-question validated Smartphone Addiction Scale Short Version (SAS-SV) and the adapted Pittsburgh Sleep Quality Score Index.
On the SAS-SV, 406 students (38.9%) reported “addiction” to their smartphones. This estimated prevalence is consistent with other reported studies in young adult populations globally, which is in the range of 30%-45%, the researchers noted.
Overall, 61.6% of participants surveyed reported poor sleep; among those who reported smartphone addiction, 68.7% had poor sleep quality, vs. 57.1% of those who did not report smartphone addiction.
In multivariable analysis that adjusted for a variety of relevant factors, among those for whom there was evidence of smartphone addiction, the odds of poor sleep were increased by 41% (adjusted odds ratio [aOR] = 1.41; 95% confidence interval, 1.06-1.87, P = .018).
The findings also suggest that a greater amount of time spent using the phone and greater use late at night can raise the risk for smartphone addiction.
“Should smartphone addiction become firmly established as a focus of clinical concern, those using their phones after midnight or using their phones for four or more hours per day are likely to be at high risk, and should guide administration of the SAS-SV,” the researchers wrote.
Caveats, cautions, and concerns
Reached for comment, Paul Weigle, MD, psychiatrist with Hartford HealthCare and Hartford (Conn.) Hospital, and member of the American Academy of Child and Adolescent Psychiatry, said the finding of a relationship between addictive smartphone usage and poor sleep quality is not surprising.
“Great increases in adolescent screen media habits in recent decades have seen a concurrent increase in rates of insomnia among this population,” he said in an interview.
Dr. Weigle also noted that young people who use the phone excessively often do so in bed, “which decreases sleep onset by disrupting conditioning (the tendency for our bodies to relate bed with sleep) and by increasing physiological arousal, which makes it more difficult to fall asleep. The blue light from smartphones used at night disrupts our body’s natural circadian rhythms, confusing our brains regarding whether it is night or day, and further worsens sleep.”
Dr. Weigle said in an interview that some of his patients come to him seeking sleep medications, although the best treatment is to perform a “smartphone-ectomy” every evening.
Teenage patients will “beg, borrow, or steal” to be allowed to keep their phones by the bed with the promise not to use them overnight. Three-quarters of the time, when the parents are able to charge the phone in another room, “the sleep problem resolves,” Dr. Weigle said.
One caveat, he said, is that it’s “somewhat unclear whether this is best classified as an addiction or simply a seriously problematic habit. Either way, this type of habit causes a great deal of distress and dysfunction in the lives of those it affects, so it is important to understand,” he said.
In a statement, Bob Patton, PhD, lecturer in clinical psychology, University of Surrey, Guildford, England, noted that this is a cross-sectional study “and as such cannot lead to any firm conclusions about phone usage as the cause of reduced sleep quality.
“It does, however, provide some compelling evidence,” Dr. Patton said, “that the nature of smartphone usage and its related consequences are important considerations in addressing the emerging phenomenon of ‘smartphone addiction.’ ”
Also weighing in, Andrew Przybylski, PhD, director of research, Oxford (England) Internet Institute, University of Oxford, said by any global health body and is not a psychiatric disorder.
“The study is a correlational analysis of a sample of participants recruited on university campuses and therefore only reflects the experiences of those who had the purpose of the study explained to them. It can say nothing about behaviors in the general population,” Dr. Przybylski said in a statement.
“Readers should be cautious of making any firm conclusions about the impact of smartphone use in the general population, or the idea that they’re addictive in any objective sense, on the basis of this work,” he added. The study had no specific funding. Dr. Carter, Dr. Weigle, Dr. Patton, and Dr. Przybylski have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Smartphone “addiction” may explain poor sleep quality in a significant proportion of young adults, new research suggests.
Investigators found that almost 40% of adults aged 18-30 years who self-reported excessive smartphone use also reported poor sleep.
“Our study provides further support to the growing body of evidence that smartphone addiction has a deleterious impact on sleep,” wrote the researchers.
The study was published online March 2 in Frontiers of Psychiatry.
Not a clinical diagnosis
Smartphone addiction is not formally recognized as a clinical diagnosis, but it’s an “active” area of research, lead investigator Ben Carter, PhD, King’s College London, noted in the report.
In a cross-sectional survey, 1,043 college students (aged 18-30 years, 73% women) completed the 10-question validated Smartphone Addiction Scale Short Version (SAS-SV) and the adapted Pittsburgh Sleep Quality Score Index.
On the SAS-SV, 406 students (38.9%) reported “addiction” to their smartphones. This estimated prevalence is consistent with other reported studies in young adult populations globally, which is in the range of 30%-45%, the researchers noted.
Overall, 61.6% of participants surveyed reported poor sleep; among those who reported smartphone addiction, 68.7% had poor sleep quality, vs. 57.1% of those who did not report smartphone addiction.
In multivariable analysis that adjusted for a variety of relevant factors, among those for whom there was evidence of smartphone addiction, the odds of poor sleep were increased by 41% (adjusted odds ratio [aOR] = 1.41; 95% confidence interval, 1.06-1.87, P = .018).
The findings also suggest that a greater amount of time spent using the phone and greater use late at night can raise the risk for smartphone addiction.
“Should smartphone addiction become firmly established as a focus of clinical concern, those using their phones after midnight or using their phones for four or more hours per day are likely to be at high risk, and should guide administration of the SAS-SV,” the researchers wrote.
Caveats, cautions, and concerns
Reached for comment, Paul Weigle, MD, psychiatrist with Hartford HealthCare and Hartford (Conn.) Hospital, and member of the American Academy of Child and Adolescent Psychiatry, said the finding of a relationship between addictive smartphone usage and poor sleep quality is not surprising.
“Great increases in adolescent screen media habits in recent decades have seen a concurrent increase in rates of insomnia among this population,” he said in an interview.
Dr. Weigle also noted that young people who use the phone excessively often do so in bed, “which decreases sleep onset by disrupting conditioning (the tendency for our bodies to relate bed with sleep) and by increasing physiological arousal, which makes it more difficult to fall asleep. The blue light from smartphones used at night disrupts our body’s natural circadian rhythms, confusing our brains regarding whether it is night or day, and further worsens sleep.”
Dr. Weigle said in an interview that some of his patients come to him seeking sleep medications, although the best treatment is to perform a “smartphone-ectomy” every evening.
Teenage patients will “beg, borrow, or steal” to be allowed to keep their phones by the bed with the promise not to use them overnight. Three-quarters of the time, when the parents are able to charge the phone in another room, “the sleep problem resolves,” Dr. Weigle said.
One caveat, he said, is that it’s “somewhat unclear whether this is best classified as an addiction or simply a seriously problematic habit. Either way, this type of habit causes a great deal of distress and dysfunction in the lives of those it affects, so it is important to understand,” he said.
In a statement, Bob Patton, PhD, lecturer in clinical psychology, University of Surrey, Guildford, England, noted that this is a cross-sectional study “and as such cannot lead to any firm conclusions about phone usage as the cause of reduced sleep quality.
“It does, however, provide some compelling evidence,” Dr. Patton said, “that the nature of smartphone usage and its related consequences are important considerations in addressing the emerging phenomenon of ‘smartphone addiction.’ ”
Also weighing in, Andrew Przybylski, PhD, director of research, Oxford (England) Internet Institute, University of Oxford, said by any global health body and is not a psychiatric disorder.
“The study is a correlational analysis of a sample of participants recruited on university campuses and therefore only reflects the experiences of those who had the purpose of the study explained to them. It can say nothing about behaviors in the general population,” Dr. Przybylski said in a statement.
“Readers should be cautious of making any firm conclusions about the impact of smartphone use in the general population, or the idea that they’re addictive in any objective sense, on the basis of this work,” he added. The study had no specific funding. Dr. Carter, Dr. Weigle, Dr. Patton, and Dr. Przybylski have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Smartphone “addiction” may explain poor sleep quality in a significant proportion of young adults, new research suggests.
Investigators found that almost 40% of adults aged 18-30 years who self-reported excessive smartphone use also reported poor sleep.
“Our study provides further support to the growing body of evidence that smartphone addiction has a deleterious impact on sleep,” wrote the researchers.
The study was published online March 2 in Frontiers of Psychiatry.
Not a clinical diagnosis
Smartphone addiction is not formally recognized as a clinical diagnosis, but it’s an “active” area of research, lead investigator Ben Carter, PhD, King’s College London, noted in the report.
In a cross-sectional survey, 1,043 college students (aged 18-30 years, 73% women) completed the 10-question validated Smartphone Addiction Scale Short Version (SAS-SV) and the adapted Pittsburgh Sleep Quality Score Index.
On the SAS-SV, 406 students (38.9%) reported “addiction” to their smartphones. This estimated prevalence is consistent with other reported studies in young adult populations globally, which is in the range of 30%-45%, the researchers noted.
Overall, 61.6% of participants surveyed reported poor sleep; among those who reported smartphone addiction, 68.7% had poor sleep quality, vs. 57.1% of those who did not report smartphone addiction.
In multivariable analysis that adjusted for a variety of relevant factors, among those for whom there was evidence of smartphone addiction, the odds of poor sleep were increased by 41% (adjusted odds ratio [aOR] = 1.41; 95% confidence interval, 1.06-1.87, P = .018).
The findings also suggest that a greater amount of time spent using the phone and greater use late at night can raise the risk for smartphone addiction.
“Should smartphone addiction become firmly established as a focus of clinical concern, those using their phones after midnight or using their phones for four or more hours per day are likely to be at high risk, and should guide administration of the SAS-SV,” the researchers wrote.
Caveats, cautions, and concerns
Reached for comment, Paul Weigle, MD, psychiatrist with Hartford HealthCare and Hartford (Conn.) Hospital, and member of the American Academy of Child and Adolescent Psychiatry, said the finding of a relationship between addictive smartphone usage and poor sleep quality is not surprising.
“Great increases in adolescent screen media habits in recent decades have seen a concurrent increase in rates of insomnia among this population,” he said in an interview.
Dr. Weigle also noted that young people who use the phone excessively often do so in bed, “which decreases sleep onset by disrupting conditioning (the tendency for our bodies to relate bed with sleep) and by increasing physiological arousal, which makes it more difficult to fall asleep. The blue light from smartphones used at night disrupts our body’s natural circadian rhythms, confusing our brains regarding whether it is night or day, and further worsens sleep.”
Dr. Weigle said in an interview that some of his patients come to him seeking sleep medications, although the best treatment is to perform a “smartphone-ectomy” every evening.
Teenage patients will “beg, borrow, or steal” to be allowed to keep their phones by the bed with the promise not to use them overnight. Three-quarters of the time, when the parents are able to charge the phone in another room, “the sleep problem resolves,” Dr. Weigle said.
One caveat, he said, is that it’s “somewhat unclear whether this is best classified as an addiction or simply a seriously problematic habit. Either way, this type of habit causes a great deal of distress and dysfunction in the lives of those it affects, so it is important to understand,” he said.
In a statement, Bob Patton, PhD, lecturer in clinical psychology, University of Surrey, Guildford, England, noted that this is a cross-sectional study “and as such cannot lead to any firm conclusions about phone usage as the cause of reduced sleep quality.
“It does, however, provide some compelling evidence,” Dr. Patton said, “that the nature of smartphone usage and its related consequences are important considerations in addressing the emerging phenomenon of ‘smartphone addiction.’ ”
Also weighing in, Andrew Przybylski, PhD, director of research, Oxford (England) Internet Institute, University of Oxford, said by any global health body and is not a psychiatric disorder.
“The study is a correlational analysis of a sample of participants recruited on university campuses and therefore only reflects the experiences of those who had the purpose of the study explained to them. It can say nothing about behaviors in the general population,” Dr. Przybylski said in a statement.
“Readers should be cautious of making any firm conclusions about the impact of smartphone use in the general population, or the idea that they’re addictive in any objective sense, on the basis of this work,” he added. The study had no specific funding. Dr. Carter, Dr. Weigle, Dr. Patton, and Dr. Przybylski have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Which detoxification regimens are effective for alcohol withdrawal syndrome?
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE-BASED ANSWER:
Benzodiazepines remain the first-line regimen for alcohol withdrawal syndrome (AWS) and are the only class more effective than placebo for reducing seizure (strength of recommendation [SOR]: B, based on 3 medium-quality randomized controlled trials [RCTs]). Anticonvulsants are no more effective than placebo at reducing seizures (SOR: B, based on 10 moderate-quality RCTs). Gabapentin reduces withdrawal symptoms and is less sedating than benzodiazepines (SOR: B, based on 1 medium-quality RCT). Carbamazepine also reduces withdrawal symptoms (SOR: B, based on 3 RCTs). Evidence of benzodiazepine superiority to other drugs with respect to safety is lacking (SOR: A, based on a meta-analysis).